ABSTRACT
Background
The first step in evaluating a patient with suspected stable coronary artery disease (CAD) is the determination of the pretest probability. The European Society of Cardiology guidelines recommend the use of the CAD Consortium 1 score (CAD1), which contrary to CAD Consortium 2 (CAD2) score and Duke Clinical Score (DCS), does not include modifiable cardiovascular risk factors.
Hypothesis
Using scores that include modifiable risk factors (DCS and CAD2) enhances prediction of CAD.
Methods
We retrospectively included all patients referred to invasive coronary angiography for suspected CAD from January/2008–December/2012 (N = 2234). Pretest probability was calculated using 3 models (CAD1, DCS, and CAD2), and they were compared using the net reclassification improvement.
Results
Mean patient age was 63.7 years, 67.5% were male, and the majority (66.9%) had typical angina. Coronary artery disease was diagnosed in 58.5%, and the area under the curve was 0.685 for DCS, 0.664 for CAD1, and 0.683 for CAD2, with a statistically significant difference between CAD1 and the others (P < 0.001). The net reclassification improvement was 20% for DCS, related to adequate reclassification of 32% of patients with CAD to a higher risk category, and 5% for CAD2, at the cost of adequate reclassification of 34% of patients without CAD to a lower risk category.
Conclusions
Prediction of CAD using scores that include modifiable cardiovascular risk factors seems to improve accuracy. Our results suggest that, in high‐prevalence populations, DCS may better identify patients at higher risk and CAD2 those at lower risk for CAD.
Introduction
The initial evaluation of a patient with suspected stable obstructive coronary artery disease (CAD) includes the clinical assessment of the pretest probability (PTP).1 This step is of major importance because it influences further diagnostic management.2 Although invasive coronary angiography (ICA) remains the gold standard for diagnosis of CAD, Patel et al reported a diagnostic yield of only 41%, concluding that better stratification tools were needed.3 The Diamond‐Forrester score (DF) was introduced in 1979 and it includes age, sex, and type of chest pain for the calculation of PTP.4 This method overestimates the probability of CAD, especially in low‐risk populations5 and in women.6 Recently, it was updated using contemporary cohorts and extended to include patients age >70 years.7 This model was designated CAD Consortium 1 (CAD1) and recommended by the most recent European Society of Cardiology (ESC) guidelines.2 The American Heart Association/American College of Cardiology (AHA/ACC) guidelines recommend the use of DF or the Duke Clinical Score (DCS).1 The DCS was first described in 1983, based on a large cohort of patients referred to ICA, and it includes modifiable cardiovascular risk factors.8, 9 Although DCS seems to improve prediction of CAD in patients referred to coronary computed tomographic angiography (CCTA) as compared with DF,10 it still appears to overestimate PTP.11, 12 Recently, CAD Consortium 2 (CAD2), which also includes modifiable cardiovascular risk factors, was created and demonstrated accuracy for the prediction of CAD in low‐risk populations.11 The accuracy of this method in patients referred to ICA is not known. We aimed to assess whether the use of scores that include modifiable risk factors (DCS and CAD2) enhances the prediction of CAD in a contemporary population referred to ICA.
Methods
Study Design
This retrospective, single‐center study included consecutively all patients with chest pain and suspected CAD referred to ICA between January 2008 and December 2012 in a country in southwestern Europe. Data were collected from patient medical records. Patients with a history of CAD, acute coronary syndrome, or coronary revascularization were excluded.
Definitions
Angina pectoris was classified as typical angina if the following criteria were present: substernal chest pain, provoked by exertion or emotional stress, and relieved by rest and/or nitroglycerin. Atypical angina was defined by 2 of those criteria, and nonanginal chest pain if only 1 or none of those characteristics were present. The presence of the following cardiovascular risk factors was collected: hypertension (blood pressure >140/90 mm Hg or antihypertensive medication), diabetes mellitus (DM; fasting plasma glucose >126 mg/dL, postprandial plasma glucose >200 mg/dL, glycated hemoglobin >6.5%, or antidiabetic agents), dyslipidemia (total cholesterol >220 mg/dL, low‐density lipoprotein cholesterol >140 mg/dL, fasting triglycerides >150 mg/dL, high‐density lipoprotein cholesterol <40 mg/dL, or lipid‐lowering agents), and smoking (patients who had smoked during the past 1 year). The presence of obstructive CAD was defined as a stenosis of >50% in at ≥1 major epicardial vessel.
Pretest Probability Calculation
Pretest probability of CAD was calculated using 3 scores: CAD1, based on age, sex, and type of chest pain7; DCS, based on sex, age, smoking, DM, history of myocardial infarction, type of chest pain, dyslipidemia, and electrocardiogram changes (ST‐segment and Q‐wave changes)8; and the CAD2 clinical model, which used age, sex, type of chest pain, DM, hypertension, dyslipidemia, and smoking. For DCS calculation, we assumed normal resting electrocardiogram because that information was unavailable and patients with evidence of previous CAD were excluded. The population was classified according to the risk of having obstructive CAD: low (<15%), intermediate (15%–85%), and high (>85%), in line with the ESC guidelines.
Statistical Analysis
Continuous variables are presented as mean ± SD and categorical variables are presented as absolute numbers and percentages. Continuous data were compared using Student t tests or Mann‐Whitney U tests, as appropriate, and categorical variables were compared using the χ2 test or Fisher exact test, as appropriate. Area under the receiver operating characteristic curve (ROC) was used to compare the scores' performances for the diagnosis of CAD. Reclassification tables were constructed to evaluate how many patients changed risk category after using a score with modifiable cardiovascular risk factors (CAD1 vs CAD2 and DCS). To assess the appropriateness of the risk category change, the net reclassification improvement (NRI) was calculated.13, 14 A 2‐tailed test with P < 0.05 was considered statistically significant. Data analysis was performed using SPSS version 22 (SPSS Inc., Chicago, IL) and Stata/IC version 13.1 (StataCorp LP, College Station, TX).
Results
Patient Characteristics
The study population included 2234 patients (male, 67.5%; mean age, 63.7 ± 9.7 years). Most were referred with typical angina (66.9%). The overall prevalence of obstructive CAD was 58.5% (n = 1308). Men presented CAD in 67.4% of cases and women in 40.1% of cases. As expected, patients with CAD were older (64.4% vs 62.6%; P < 0.001), mostly male (77.8% vs 53%; P < 0.001), and with higher frequency of smoking (15% vs 9.4%; P < 0.001), dyslipidemia (74% vs 63.9%; P = 0.017), and DM (34.3% vs 23.9%; P < 0.001). There were no statistical differences in the prevalence of arterial hypertension (75.1% vs 72.4%; P = 0.163) and family history of premature coronary disease (11.8% vs 9.8%; P = 0.165). Baseline characteristics are detailed in Table 1.
Table 1.
Baseline Characteristics and Pretest Probability of CAD
| Characteristic | All Patients, N = 2234 | With CAD, n = 1308 | Without CAD, n = 926 | P Value |
|---|---|---|---|---|
| Age, y | 63.7 ± 9.7 | 64.4 ± 9.5 | 62.6 ± 9.9 | <0.001 |
| Male sex | 1508 (67.5) | 1017 (77.8) | 491 (53) | <0.001 |
| Arterial hypertension | 1652 (73.9) | 982 (75.1) | 670 (72.4) | 0.163 |
| Smoking | 283 (12.7) | 196 (15) | 87 (9.4) | <0.001 |
| Dyslipidemia | 1610 (72.1) | 968 (74) | 642 (63.9) | 0.017 |
| DM | 669 (29.9) | 448 (34.3) | 221 (23.9) | <0.001 |
| Family history (premature CHD) | 245 (11) | 154 (11.8) | 91 (9.8) | 0.165 |
| Typical angina | 1495 (66.9) | 954 (72.9) | 541 (58.4) | <0.001 |
| Atypical angina | 306 (13.7) | 152 (11.6) | 154 (16.6) | <0.001 |
| Nonanginal chest pain | 433 (19.4) | 202 (15.4) | 231 (24.9) | <0.001 |
| CAD1, % | 63.5 ± 21.8 | 68.7 ± 19.3 | 56.1 ± 22.9 | <0.001 |
| DCS, % | 71.1 ± 26.6 | 78.4 ± 22 | 60.9 ± 29 | <0.001 |
| CAD2, % | 41.5 ± 23.2 | 47.6 ± 21.9 | 32.9 ± 22.3 | <0.001 |
Abbreviations: CAD, coronary artery disease; CAD1, Coronary Artery Disease Consortium 1 score; CAD2, Coronary Artery Disease Consortium 2 score; CHD, coronary heart disease; DCS, Duke Clinical Score; DM, diabetes mellitus; SD, standard deviation.
Data are presented as n (%) or mean ± SD.
Pretest Probability Estimation and Risk Classification
The expected prevalence of CAD was underestimated by CAD2 (41.5%) and overestimated by CAD1 (63.5%) and DCS (71.1%), as seen in Table 1. All scores showed significantly superior PTP in patients with CAD. In those patients (Figure 1, left side), DCS classified correctly more than half of patients as high risk, whereas only 2% were incorrectly classified as low risk. On the contrary, in patients without CAD (Figure 1, right side), CAD2 classified correctly almost one‐third of patients as low risk and only 0.5% as high risk.
Figure 1.
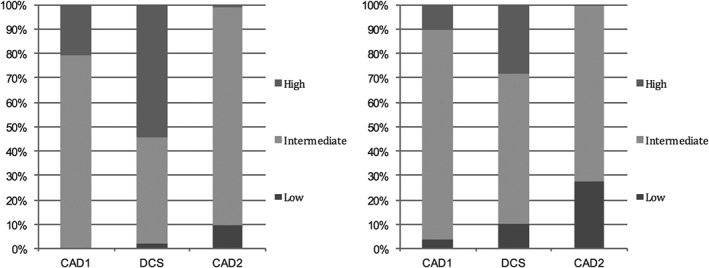
Risk classification in patients with CAD (left) and without CAD (right). Abbreviations: CAD, coronary artery disease; CAD1, Coronary Artery Disease Consortium 1 score; CAD2, Coronary Artery Disease Consortium 2 score; DCS, Duke clinical score.
Predictive Performance of the Risk Scores
For the prediction of obstructive CAD, the DCS score had superior area under the curve (AUC: 0.685, 95% confidence interval [CI]: 0.663‐0.708, P < 0.001), followed by CAD2 (AUC: 0.683, 95% CI: 0.661‐0.706, P < 0.001) and at last CAD1 (AUC: 0.664, 95% CI: 0.641‐0.687, P < 0.001), as shown in Figure 2. Compared with CAD1, the DCS and CAD2 areas under the curve were significantly different (P < 0.001) and there was no statistically significant difference between CAD2 and DCS (P = 0.52).
Figure 2.
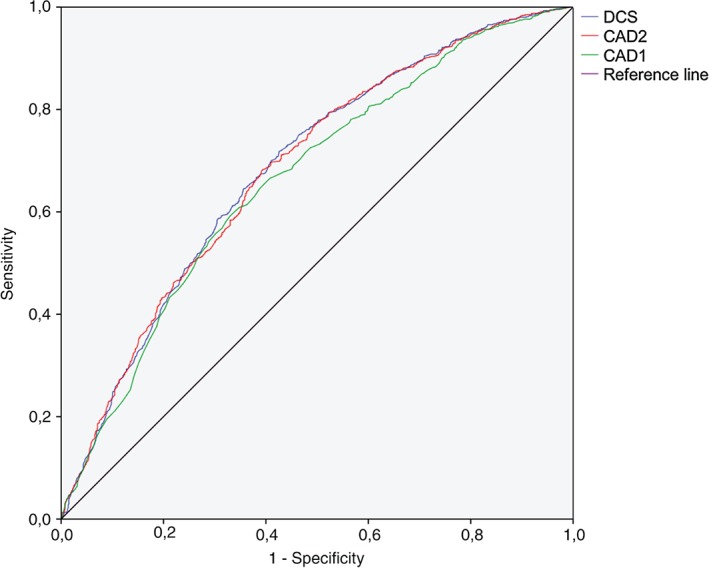
ROC analysis of CAD1, DCS, and CAD2 for the prediction of CAD. Abbreviations: CAD, coronary artery disease; CAD1, Coronary Artery Disease Consortium 1 score; CAD2, Coronary Artery Disease Consortium 2 score; DCS, Duke clinical score; PTP, pretest probability; ROC, receiver operating characteristic.
Reclassification of Risk
Given the small and non–statistically significant difference between the performances of CAD2 and DCS evaluated by the area under the ROC curve, we decided to construct reclassification tables to assess the improvement in reclassification of the risk category of each score compared with CAD1.
Using DCS (Table 2), 434 patients with CAD were reclassified from an intermediate risk with CAD1 to a higher risk category, and 20 were reclassified to a lower risk category, resulting in 32% (414/1308) of patients being correctly reclassified. However, in patients without CAD, DCS inadequately reclassified 165 patients from intermediate risk with CAD1 to high‐risk category, whereas only 57 were correctly stratified to low‐risk category, resulting in 12% (108/926) of patients incorrectly classified. The total net reclassification improvement of DCS was 20% (continuous NRI: 0.18, P < 0.001). The CAD2 model showed better risk reclassification in patients without CAD (Table 3), correctly reclassifying 314 of 926 patients to a lower risk category (34%). On the other hand, in patients with CAD, 379 of 1308 patients were incorrectly reclassified to lower risk categories (29%). Total NRI was 5% (continuous NRI: 0.23, P < 0.001).
Table 2.
Reclassification Tables of DCS in Patients with CAD and Without CAD
| DCS | Total | |||
|---|---|---|---|---|
| Low | Intermediate | High | ||
| With CAD | ||||
| CAD1 | ||||
| Low | 6 | 0 | 0 | 6 |
| Intermediate | 20a | 572 | 434b | 1026 |
| High | 0 | 0 | 276 | 276 |
| Total | 26 | 572 | 710 | 1308 |
| Without CAD | ||||
| CAD1 | ||||
| Low | 35 | 0 | 0 | 35 |
| Intermediate | 57a | 572 | 165b | 794 |
| High | 0 | 0 | 97 | 97 |
| Total | 92 | 572 | 262 | 926 |
Abbreviations: CAD, coronary artery disease; CAD1, Coronary Artery Disease Consortium 1 score; DCS, Duke Clinical Score.
Inadequate reclassification.
Correct reclassification.
Table 3.
Reclassification Tables of CAD2 in Patients With CAD and Without CAD
| CAD2 | Total | |||
|---|---|---|---|---|
| Low | Intermediate | High | ||
| With CAD | ||||
| CAD1 | ||||
| Low | 6 | 0 | 0 | 6 |
| Intermediate | 121a | 905 | 0 | 1026 |
| High | 0 | 258a | 18 | 276 |
| Total | 127 | 1163 | 18 | 1308 |
| Without CAD | ||||
| CAD1 | ||||
| Low | 35 | 0 | 0 | 35 |
| Intermediate | 222b | 572 | 0 | 794 |
| High | 0 | 92b | 5 | 97 |
| Total | 257 | 664 | 5 | 926 |
Abbreviations: CAD, coronary artery disease; CAD1, Coronary Artery Disease Consortium 1 score; CAD2, Coronary Artery Disease Consortium 2 score; DCS, Duke Clinical Score.
Inadequate reclassification.
Correct reclassification.
Discussion
Performance and Comparison of Pretest Probability Scores
Overall, the discriminatory power of the 3 scores was modest for the prediction of angiographically significant CAD in our cohort of patients (all with AUC <0.7). In this specific population of patients referred to ICA, DCS and CAD1 scores overestimated the prevalence of obstructive CAD. This was previously demonstrated for DCS score in patients referred to CCTA.12, 15 It may be related to a contemporary lower prevalence of CAD as compared with the historic cohorts that served as the base for the development of that model.8 Even though CAD1 is an updated version of the original DF method, which was shown to overestimate the probability of CAD in patients referred to ICA3, 7, 16 or noninvasive tests,5, 10, 17, 18 it still overestimated the prevalence of CAD in our population. Recently, the same was shown by Zorlak et al in patients referred to ICA or noninvasive testing.19 As expected, CAD2 underestimated the actual prevalence of CAD, because this model was created for the estimation of the PTP of CAD in low‐prevalence populations.
Modifiable cardiovascular risk factors such as smoking, DM, and dyslipidemia seem to have a predictive effect inferior to sex, age, and chest pain.7 Nevertheless, the use of models that include those risk factors appears to increase performance in the prediction of CAD, as demonstrated by the superior AUC of CAD2 and DCS compared with CAD1.
Clinical Implications
According to the most recent ESC guidelines for the diagnosis of CAD, in patients with high risk (PTP >85%) or low risk (PTP <15%), no further diagnostic tests are needed, because in the first case the clinical diagnosis of CAD can be made, whereas in the second, other causes of chest pain should be investigated.2 Despite a small but statistically significant difference in AUC of DCS and CAD2 compared with CAD1, the evaluation of the NRI showed that both improved the predictability of CAD in our cohort of patients. The reclassification improvement was 20% for DCS and 5% for CAD2; therefore, the stratification of risk categories was significantly enhanced by the application of models that included modifiable cardiovascular risk factors. As mentioned earlier, because each risk category has a distinct clinical meaning, the improvement in correct classification has a direct impact on clinical decisions. The reclassification of risk using DCS showed improved discrimination of the high‐risk category in patients with CAD. Therefore, that model performed better for the identification of patients in whom it was safe to assume obstructive CAD, requiring optimal medical treatment and risk stratification of events. On the other side, CAD2 more accurately classified patients without CAD as low risk, predicting those who would not benefit from further diagnostic testing. Although DCS had superior NRI over CAD2, these results have to be interpreted in the context of high prevalence of CAD (almost 60%). Indeed, DCS was developed on the basis of patients referred to ICA, and CAD2 mostly with low‐prevalence populations. Possibly, CAD2 would perform even better in more heterogeneous populations, including patients referred to ICA and CCTA, a hypothesis that needs to be tested in future studies.
Study Limitations
The studied patients represent a highly selected population, because only patients from a single institution who underwent ICA were retrospectively included. Therefore, the PTP is inherently higher. Although DCS was validated for the prediction of ≥1 stenosis >75%, in this study it was tested for the prediction of a different cutoff (>50%), and CAD2, as previously stated, was validated for low‐risk populations. Also, no information regarding previous noninvasive testing or follow‐up was obtained.
Conclusion
The estimation of likelihood pretest of CAD using scores that include modifiable cardiovascular risk factors (CAD2 and DCS) seems to improve the accuracy of CAD prediction. Our results suggest that, in high‐risk populations, DCS may better select patients at higher risk and CAD2 may better predict those at lower risk for CAD. Developing better models, probably with the inclusion of those risk factors, will enhance the pretest likelihood prediction and improve clinical decisions, because it will help the clinician to decide whether further diagnostic testing is needed.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Fihn SD, Gardin JM, Abrams J, et al. 2012. ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons [published correction appears in Circulation. 2014;129:e463]. Circulation. 2012;126:e354–e471. [DOI] [PubMed] [Google Scholar]
- 2. Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the Management of Stable Coronary Artery Disease of the European Society of Cardiology [published correction appears in Eur Heart J. 2014;35:2260–2261]. Eur Heart J. 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 3. Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography [published correction appears in N Engl J Med. 2010;363:498]. N Engl J Med. 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary‐artery disease. N Engl J Med. 1979;300:1350–1358. [DOI] [PubMed] [Google Scholar]
- 5. Pickett CA, Hulten EA, Goyal M, et al. Accuracy of traditional age, gender and symptom based pre‐test estimation of angiographically significant coronary artery disease in patients referred for coronary computed tomographic angiography. Am J Cardiol. 2013;112:208–211. [DOI] [PubMed] [Google Scholar]
- 6. Rademaker AA, Danad I, Groothuis JG, et al. Comparison of different cardiac risk scores for coronary artery disease in symptomatic women: do female‐specific risk factors matter? Eur J Prev Cardiol. 2014;21:1443–1450. [DOI] [PubMed] [Google Scholar]
- 7. Genders TS, Steyerberg EW, Alkadhi H, et al; CAD Consortium . A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur Heart J. 2011;32:1316–1330. [DOI] [PubMed] [Google Scholar]
- 8. Pryor DB, Harrell FE Jr, Lee KL, et al. Estimating the likelihood of significant coronary artery disease. Am J Med. 1983;75:771–780. [DOI] [PubMed] [Google Scholar]
- 9. Pryor DB, Shaw L, McCants CB, et al. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med. 1993;118:81–90. [DOI] [PubMed] [Google Scholar]
- 10. Wasfy MM, Brady TJ, Abbara S, et al. Comparison of the Diamond‐Forrester method and Duke Clinical Score to predict obstructive coronary artery disease by computed tomographic angiography. Am J Cardiol. 2012;109:998–1004. [DOI] [PubMed] [Google Scholar]
- 11. Genders TS, Steyerberg EW, Hunink MG, et al. Prediction model to estimate presence of coronary artery disease: retrospective pooled analysis of existing cohorts. BMJ. 2012;344:e3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumamaru KK, Arai T, Morita H, et al. Overestimation of pretest probability of coronary artery disease by Duke clinical score in patients undergoing coronary CT angiography in a Japanese population. J Cardiovasc Comput Tomogr. 2014;8:198–204. [DOI] [PubMed] [Google Scholar]
- 13. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207–212. [DOI] [PubMed] [Google Scholar]
- 14. Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bom MJ, van der Zee PM, Cornel JH, et al. Diagnostic and therapeutic usefulness of coronary computed tomography angiography in out‐clinic patients referred for chest pain. Am J Cardiol. 2015;116:30–36. [DOI] [PubMed] [Google Scholar]
- 16. Høilund‐Carlsen PF, Johansen A, Vach W, et al. High probability of disease in angina pectoris patients: is clinical estimation reliable? Can J Cardiol. 2007;23:641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng VY, Berman DS, Rozanski A, et al. Performance of the traditional age, sex, and angina typicality‐based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM). Circulation. 2011;124:2423–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jensen JM, Voss M, Hansen VB, et al. Risk stratification of patients suspected of coronary artery disease: comparison of five different models. Atherosclerosis. 220:557–562. [DOI] [PubMed] [Google Scholar]
- 19. Zorlak A, Thomassen A, Gerke O, et al. Patients with suspected coronary artery disease referred for examinations in the era of coronary computed tomography angiography. Am J Cardiol. 2015;116:344–349. [DOI] [PubMed] [Google Scholar]


