ABSTRACT
Paravalvular regurgitation (PVR) remains one of the drawbacks of transcatheter aortic valve implantation (TAVI). Details of percutaneous closure (PCC) of PVR after TAVI remain obscure. We aimed to explore the patient characteristics, procedural details, closure devices used, and outcomes of PCC after TAVI. A systematic search of the MEDLINE/PubMed and Embase databases from January 2002 to September 2015 was conducted. Reports considered to include same patient were excluded and only the studies with largest cohorts were included. A total of 14 studies including 58 patients (61 cases) were included in the study. A balloon‐expandable (BE) valve was used more frequently compared with a self‐expandable (SE) valve (72.6% vs 27.4%, respectively). The mean success rate was 86.9% (100% and 77.8%, respectively; P = 0.097). The median number of closure devices used was 1 (range, 1–4) and did not differ between SE and BE valves (P = 0.71). Mean time from index procedure to PCC did not differ between SE and BE valves (295 ± 380 days vs 379 ± 353 days; P = 0.71). Seven patients had history of valve‐in‐valve and 6 patients had procedural success. Among the patients with available follow‐up data (94.8%), there were 15 deaths (27.3%). Percutaneous closure of PVR after TAVI had a high success rate in selected patients in both BE and SE valves. The success rate, timing, and number of closure devices were similar between BE and SE valves. However, prognosis remains fairly poor.
Introduction
Paravalvular regurgitation (PVR) is one of the main drawbacks of transcatheter aortic valve implantation (TAVI) as compared with surgical aortic valve replacement (SAVR). Previous articles have reported its negative prognostic impact following TAVI in both midterm and long‐term follow‐up.1, 2 Some reports suggest that even mild PVR could affect the prognosis.3 Even though the second‐generation TAVI prosthetic valves are promising in decreasing the PVR rate, their long‐term valve durability has not yet been proven.4
Revision cardiac surgery in TAVI patients is less desirable, as these patients were deemed high or inoperable surgical risks prior to TAVI. Postdilation (PD) and valve‐in‐valve (ViV) have been widely performed for the treatment of PVR after TAVI. However, the treatment strategy for PVR after TAVI is not standardized, and it remains largely dependent on institutions and operators. In addition, these 2 commonly utilized treatment modalities are not free from complications.5, 6
Percutaneous closure (PCC), although it appears to be an attractive additional treatment option, has not been studied in detail compared with the aforementioned 2 treatment modalities. Past reports have been restricted only to case reports and case series with small numbers of patients.7, 8 Concerns regarding its technical difficulty and safety could be one of the reasons for underutilization of this method.
Therefore, we aimed to systematically review the current published articles to elucidate patient characteristics, procedural details, closure devices used, and outcomes in PCC after TAVI.
Methods
Search Strategy
A systematic literature search was conducted through MEDLINE/PubMed and Embase databases from January 1, 2002, to March 17, 2016. Observational studies and case series/reports were searched with the following search terms: (1) “TAVI” or “TAVR” or “transcatheter aortic valve replacement” or “transcatheter aortic valve implantation” or “CoreValve” or “Sapien” or “balloon expandable” or “self expandable”; and (2) “closure” or “paravalvular” or “perivalvular” or “periprosthetic” or “paraprosthetic.”
Abstracts/titles were screened for relevant articles, and if they were considered to include information related to the study's purpose, full manuscripts were obtained and reviewed. References of manuscripts included for full review were also manually reviewed to minimize missing relevant articles. Conference abstracts included in the Ovid database were also reviewed. Screening and retrieval of the studies and data were performed by 2 independent reviewers on the basis of inclusion and exclusion criteria (T.A. and T.H.). There was no language restriction. When ≥2 studies were considered to contain overlapping patient cohorts, only the study with the largest number of patients was included. Studies were included when PCC was performed for PVR following TAVI and the study included at least procedural or patient outcomes. Studies were excluded when PCC was performed for PVR following SAVR. Author, year of publication, study location, cohort number, patient characteristics, procedural success/failure, implanted prosthetic valve, deployed PCC device, and in‐hospital or follow‐up outcomes were abstracted when available. Procedural success was defined as “PCC device was successfully deployed and residual PVR < moderate, grade ≤2 or clinical improvement was achieved.” The time from the TAVI procedure to PCC was abstracted. When >1 PCC procedures were performed in the same patient, the day from the TAVI to the latest PCC procedure was abstracted and used to calculate the time from TAVI to PCC procedure, regardless of success or failure. The present study was reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.9
Statistical Analysis
Categorical variables are expressed as number and percentage; continuous variables are expressed as mean ± SD or median (interquartile range). The χ2 test or Fisher exact test was used to evaluate categorical variables, as appropriate. Continuous variables were analyzed with the t test or Mann–Whitney U test, as appropriate. A P value <0.05 was considered significant. Statistical analysis was performed with the EZR software program (Saitama Medical Center, Jichi Medical University, Japan).10
Results
A total of 14 studies were identified and included in this systematic review.7, 8, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 All studies were either case reports or case series. Included studies are summarized in Table 1. (For a flow diagram of study selection, see Supporting Information, Figure, in the online version of this article.) The reported literature was from North America and Europe.
Table 1.
Characteristics of Included Studies
| Author | Year | Study Location(s) | Study Design | Cohort N | No. of Cases |
|---|---|---|---|---|---|
| Cruz‐González7 | 2015 | Spain | Case report | 1 | 1 |
| Okuyama8 | 2015 | United States | Case series | 10 | 10 |
| Rossi11 | 2015 | Italy | Case report | 1 | 1 |
| Saia12 | 2015 | Italy, United States, Germany, Canada | Case series | 24 | 27 |
| White13 | 2015 | United States | Case report | 1 | 1 |
| Cockburn14 | 2016 | United Kingdom | Case report | 1 | 1 |
| Arri15 | 2015 | United Kingdom | Case series | 5 | 5 |
| Saireddy16 | 2014 | Australia | Case report | 1 | 1 |
| Citro17 | 2014 | Italy | Case report | 1 | 1 |
| Luu18 | 2013 | United States | Case series | 2 | 2 |
| Estévez‐Loureiro19 | 2013 | Spain | Case series | 2 | 2 |
| Whisenant20 | 2013 | United States | Case series | 2 | 2 |
| Sinning21 | 2012 | Germany | Case report | 1 | 1 |
| Feldman22 | 2014 | Germany | Case series | 6 | 6 |
A total of 58 patients (male, 77.6%; 1 patient had a total of 3 PCC and another had 2 PCC procedures, making total of 61 cases) were included, and the mean patient age was 81.7 ± 7.9 years. Among the included studies, balloon‐expandable valves (BE; Sapien or Sapien XT; Edwards Lifesciences LLC, Irvine, CA) were utilized in 72.4% of patients (42/58) and self‐expandable valves (SE; CoreValve; Medtronic Inc., Minneapolis, MN) were utilized in 27.6% (16/58). Patients were mostly elderly, with high surgical risk. Only 1 study reported the incidence of PCC performed out of the entire cohort, and it was 1.5% (10/657).8
The overall success rate was 83.6%. The success rate was 100% (16/16 cases) in the SE valve group and 77.8% (35/45 cases) in the BE valve group (P = 0.095). (Reasons for failure are listed in Supporting Information, Table, in the online version of this article.) The success rate was compared between patients with and without previous SAVR or ViV procedures in 12 studies including 28 patients.7, 8, 11, 13, 14, 15, 16, 17, 18, 19, 20, 21 The overall success rate was 82.1% and did not differ between patients with and without previous SAVR/ViV (85.7% vs 81.0%; P = 1.00). There was 1 serious procedural complication of valve embolization.12 Patient characteristics and outcomes are summarized in Table 2.
Table 2.
Patient and Procedural Characteristics and Outcomes
| Author | Age, y/Sex | Surgical Risk, % | Valve Type and Size, mm | Successes/Cases | No. of Devices for PVR Closure | Outcome |
|---|---|---|---|---|---|---|
| Cruz‐González | 86/F | LE 22 | CV 26 | 1/1 | 2 | Asymptomatic 1 month later |
| Rossi | 82/F | LE 32 | SXT 23 | 0/1 (residual severe PVR) | 1 | Patient admitted for CHF at 3 months, residual severe PVR treated with V‐in‐V, stable after 1 year (NYHA class I–II) |
| Okuyama | 81 (70–92)/M 60% | STS 7.1 (4.0–13.1) | SP 23, 26; SXT 23, 29 | 6/10 (all 4 cases unable to pass the sheath) | 1 each for 6 patients | 2 patients died at day 38 |
| Saia | 80.6 ± 7.1/M 75% | STS 6.6 ± 3.9; LE 23.5 ± 20.1 | CV 26, 27, 29, 31; SP 23, 26; SXT 20, 26 | 24/27 (1 case unable to pass the wire through the leak; prosthetic valve embolized in 1 case; 1 case had grade 3 residual PVR) | 1 for 18 patients; 2 for 6 patients | 11 patients died during 12.3 ± 11.4 months follow‐up |
| White | 86/M | NR | SP 26 | 1/1 | 1 | Discharged on day 3; no outpatient follow‐up data available |
| Cockburn | 86/M | LE 33 | SXT (size NR) | 1/1 | 1 | Symptomatic improvement in subsequent follow‐up |
| Arri | 77–85/M 100% | Case 1, LE 10; NR for the other 4 cases | CV 26, 29, 31 | 5/5 | 1 for 4 cases; 2 for 1 case | 4 patients have outpatient clinical follow‐up and are stable; 1 patient was safely discharged from hospital but no outpatient follow‐up data available |
| Saireddy | 75/F | LE 35, STS 15 | SXT 23 | 1/1 | 1 | Death on day 14 |
| Citro | 71/M | NR | SXT 23 | 1/1 | 2 | Discharged on day 5 |
| Luu | 86/M; 90/M | NR; NR | SP 26; SP 26 | 2/2 | 1; 1 | NR; NR |
| Estévez‐Loureiro | 85/M; 84/M | LE 17.7; LE 19.9 | SP 26; SP 23 | 2/2 | 4; 1 | Stable at 1 year; stable at 3 months |
| Whisenant | 88/M; 83/M | NR; NR | SP 26; SP 23 | 2/2 | 2; 2 | NYHA class II at 1‐year follow‐up; V‐in‐V was performed to close central aortic regurgitation after PVR closure, stable at 16 months since V‐in‐V |
| Sinning | 83/F | NR | SXT 23 | 1/1 | 1 | Discharged on day 5 |
| Feldman | 78–91; M 83.3% | STS 5.2–15.9 | SP 23, 26 | 4/6 (2 cases had moderate residual PVR) | 1 for 5 cases; 2 for 1 case | 1 patient died at 1 month; during follow‐up (range, 1–11 months), the remaining 5 patients were free of symptoms |
Abbreviations: CHF, congestive heart failure; CV, CoreValve; EuroSCORE, European System for Cardiac Operative Risk Evaluation; F, female; IQR, interquartile range; LE, logistic EuroSCORE; M, male; NR, not reported; NYHA, New York Heart Association; PVR, paravalvular regurgitation; SD, standard deviation; SP, Sapien; STS, Society of Thoracic Surgeons; SXT, Sapien XT; V‐in‐V, valve‐in‐valve.
Data are expressed as mean ± SD or median (IQR).
The vast majority of the deployed closure devices were Amplatzer vascular plugs (II, III or IV; Figure 1). Other devices used were the Amplatzer septal occluder and ventricular septal occluder in a total of 5 cases (Figure 2).11, 12 Only 1 case was performed under transthoracic echocardiography plus fluoroscopy guidance14; other cases were performed under fluoroscopy with transesophageal echocardiography (TEE).7, 8, 11, 13, 15, 16, 17, 18, 19, 20, 21, 22 The imaging modality was not described in other cases.12 After excluding patients who had deployment failure of PCC devices (3 cases from Saia et al, 4 cases from Okuyama), there was no difference in the average number of devices used between the BE and SE valve groups (1.11 ± 0.73 vs 1.31 ± 0.48; P = 0.14).
Figure 1.
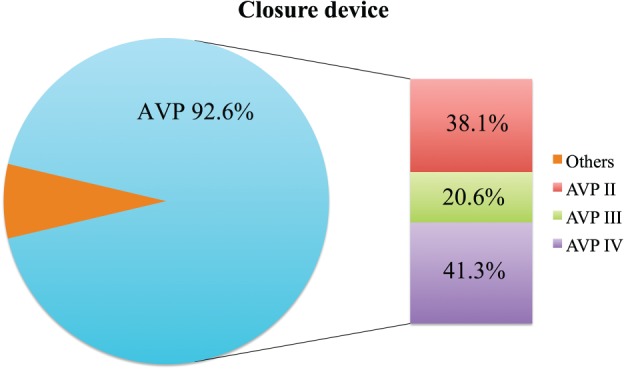
Details of deployed closure devices. Abbreviation: AVP, Amplatzer vascular plug.
Figure 2.
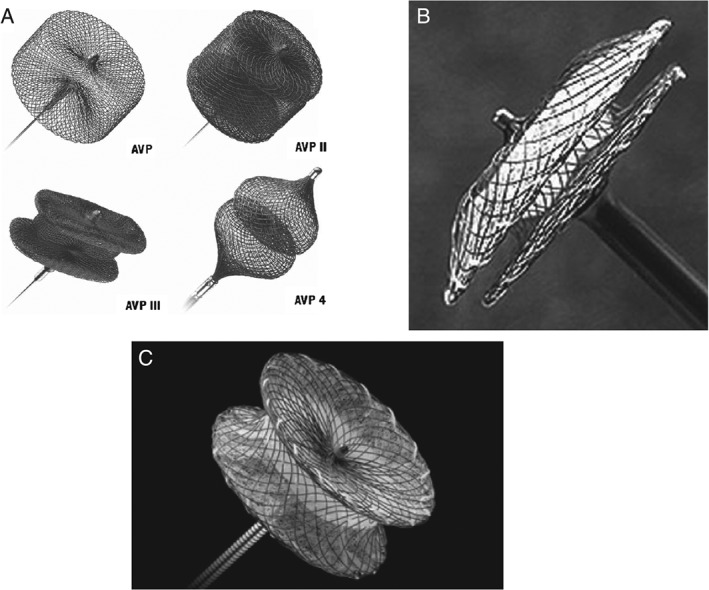
(A) AVP and AVP II, III, and IV; reproduced with permission from Weber C et al.42 (B) Amplatzer septal occluder; reproduced with permission from Cubeddu RJ et al.43 (C) Amplatzer ventricular septal occluder; reproduced with permission from Springer. The AVP was not used for percutaneous paravalvular closure. Figure reproduced with permission from Wang W et al.44 Abbreviation: AVP, Amplatzer vascular plug.
The time from TAVI to PCC procedure was assessed in 52 patients. Three patients who had the same‐day procedure13, 14, 16 and those with no description of the time from TAVI to PCC procedure17, 18 were removed from analysis. Overall, the time from TAVI to PCC procedure was 321 ± 371 days (range, 3–1506 days). There was no difference between the BE and SE valve groups regarding duration from initial TAVI to PCC (295 ± 380 days vs 379 ± 353 days; P = 0.71; Figure 3).
Figure 3.
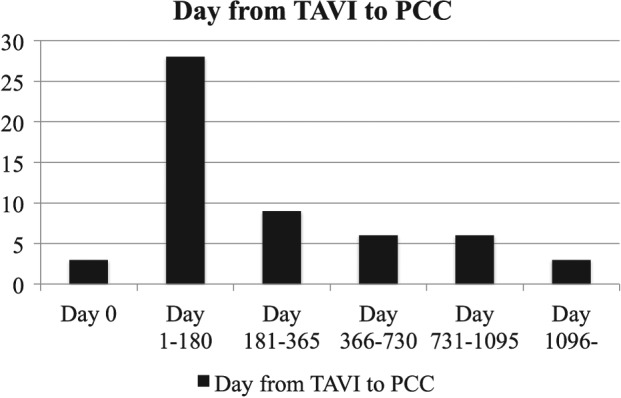
Time from TAVI to PCC. Abbreviations: PCC, percutaneous closure; TAVI, transcatheter aortic valve implantation.
In 5 cases8, 11, 15, 16, 6 ViV procedures were performed during the clinical course. In 4 of these cases, 4 ViV procedures were performed in order to decrease PVR after TAVI11, 15, 16, whereas in 1 case, 2 VIV procedures were performed because of PCC failure (severe residual PVR)8.
Finally, follow‐up data including both inpatient and outpatient data were available in 55 patients.7, 8, 11, 12, 13, 14, 15, 16, 17, 19, 20, 21, 22 The reported mortality rate was 27.3% (15/55).
Discussion
This is the first systematic review of PCC of PVR following TAVI. In addition, we conducted statistical analyses of patient‐level data, when possible. The main findings were: (1) the success rate was high and did not differ between SE and BE; (2) the Amplatzer vascular plug was used in majority of the cases; and (3) survival remained relatively poor in this analysis of only published data.
Percutaneous Closure as a Treatment Option for Paravalvular Regurgitation After Transcatheter Aortic Valve Implantation
The success rate was high and did not differ between the SE and BE valve groups. The success rate of PVR closure after surgical valve replacement (both aortic and mitral) varied from 75% to 90% and numerically approximates the success rate of our study.23, 24, 25, 26 The only serious procedural complication was valve embolization during an attempt to cross the leak, reported by Saia et al.12 Complications of PVR closure after aortic or mitral valve surgery included emergent surgery (2.9%) and closure‐device embolization (1.6%).7, 24, 25, 26
The indications for PCC were mainly shortness of breath or symptomatic heart failure. None of the studies included in our systematic review clearly indicated that PCC was performed for the treatment of intractable hemolysis following TAVI. There are limited data regarding hemolysis after TAVI. Laflamme et al reported incidence of hemolysis after TAVI at 14.8%, but no patient required transfusion.27 There has been no report assessing the clinical impact of hemolysis after TAVI.
One of the major reasons for underutilization of this procedure is considered to be its technical difficulty. Indeed, this procedure would be challenging to accurately identify the culprit area of PVR and secure the passage of wire into it. Sorajja et al reported a learning curve of PCC in mitral and aortic PVR in 243 patients. With increased operator experience, they demonstrated shorter procedural and fluoroscopy time, lower administered contrast volume, and shorter length of stay—and, more important, fewer major adverse cardiovascular events in 30 days.28 This result may imply that in PCC for the treatment of PVR after TAVI, outcomes may improve with more experience.
Postdilation is commonly used as the first option because it is considered an easy and effective measure in treating PVR. Past studies have reported the usage of PD in treating PVR from 12.4% to 28.0%, but the success rate in reducing the PVR to less than moderate was limited to 54% to 63%.5, 29, 30 In addition, few studies have reported increased death or stroke at 1 year in the PD group.5, 31 Furthermore, Watanabe et al reported numerically higher aortic‐root rupture rates (4.1% vs 1.7%) in the PD group.32 Percutaneous closure was able to offer higher success rates compared with PD. One of the potential explanations is that if PVR is caused by the presence of focal and bulky calcification, PD may unlikely be able to further expand the implanted valve. In such a case, PCC will have an advantage over PD and may be able to achieve higher success rates because the vascular plug or other devices are able to close the leakage area without forceful expansion of the valve adjacent to the bulky calcification. The degree of calcification and balloon PD was an independent risk factor for aortic‐root rupture, and therefore PCC may be safer in such a case.33
Valve‐in‐valve is another strategy for the treatment of post‐TAVI PVR. Toggweiler et al have reported 21 patients who underwent ViV after TAVI. They reported the technical success rate of 90%. At 1‐year follow up, only 1 patient had moderate PVR, and the mortality rate was 24.3%.34 Makkar et al reported on their series of 63 patients who underwent ViV after TAVI and revealed that ViV was associated with increased 1‐year cardiovascular mortality.35 In our systematic review, there were 4 cases in which ViV did not resolve the PVR, requiring PCC, which may be useful in such cases.
Percutaneous Closure Device
The Amplatzer vascular plug (St. Jude Medical, St. Paul, MN) was used in the majority of the cases. The vascular plug was not initially invented specifically for PVR closure, but it remains useful in off‐label fashion in the absence of a specific PVR closure device. However, recently Burriesci et al examined the effectiveness of Amplatzer vascular plugs II and III for efficacy on the reduction of PVR in Sapien XT valves. They reported a maximum efficiency of <50% and concluded that specifically designed devices are warranted.36
Paravalvular regurgitation closure has been usually performed under fluoroscopy and TEE guidance.24, 26 The majority of PVR after TAVI procedures were performed under fluoroscopy and TEE guidance in our review. Transcatheter aortic valve implantation generally has been performed under TEE and fluoroscopy guidance in the early experiences; however, transfemoral TAVI has also been shown to be feasible and safe under fluoroscopic (but without TEE) guidance.37 Further study is needed to access the feasibility and safety of PVR closure after TAVI under fluoroscopic guidance.
Mortality After Percutaneous Closure
The patient prognosis remained relatively poor despite the high success rate. Percutaneous closure has been utilized to treat PVR after surgical valve replacement. Ruiz et al reported the high survival rate of 86.5% at 18 months.38 According to Sorrajja, 23.0% of patients died during the median follow‐up of 11.0 months after catheter‐based treatment of prosthetic PVR.26 It may not be fair to compare these mortality rates with those of PCC after TAVI, as TAVI patients are at higher risk than patients undergoing surgical valve replacement. Although the success rate was high in the included studies, there may be publication bias as a cause of the discordance between the high success rate and the relatively high mortality rate. Indeed, the largest series by Saia et al showed a 1‐year all‐cause mortality rate of 38.5%, and 45.8% of patients expired during the total follow‐up.12 These rates were numerically higher than 1‐year mortality in the first large trial of TAVI, which was the Placement of Aortic Transcatheter (PARTNER) trial (24.2%–30.7%).39, 40 However, most of the deaths reported by Saia et al were noncardiac (8/11, 72.7%), which was different from the PARTNER trial. In the PARTNER trial, the cardiovascular mortality rate was 56.0% to 63.6%.39, 40 This may imply that successful PCC of PVR may be associated with decreased cardiac mortality. This hypothesis may be supported by a meta‐analysis by Millán et al, which showed that successful PVR reduction was associated with decreased cardiac mortality.41 However, further clinical trials are warranted to prove this hypothesis.
Study Limitations
There are several limitations that require attention to interpret the data presented in this manuscript. First, the search result only yielded case reports or case series, and therefore the result may be subject to significant publication bias. Especially, unsuccessful cases or cases with serious complications would be less likely to be reported; therefore, the data in the published‐only articles may result in a higher success rate and lower mortality rate than the actual rate. Second, although we vigorously searched for published articles based on inclusion and exclusion criteria, the total patient cohort was limited. Third, because of the limited available data, the statistical results would have limited power.
Conclusion
Percutaneous closure for PVR after TAVI was feasible, safe, and effective in this systematic review of published data. However, mortality remains high, and establishment of a treatment strategy for PVR after TAVI, including PCC as well as specific closure devices, is warranted in the future.
Supporting information
Figure S1 Flow diagram of study selection.
Table S1 Reasons for failure
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Schewel D, Frerker C, Schewel J, et al. Clinical impact of paravalvular leaks on biomarkers and survival after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2015;85:502–514. [DOI] [PubMed] [Google Scholar]
- 2. Ruparelia N, Latib A, Buzzatti N, et al. Long‐term outcomes after transcatheter aortic valve implantation from a single high‐volume center (the Milan experience). Am J Cardiol. 2016;117:813–819. [DOI] [PubMed] [Google Scholar]
- 3. Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta‐analysis and systematic review of literature. J Am Coll Cardiol. 2013;61:1585–1595. [DOI] [PubMed] [Google Scholar]
- 4. Athappan G, Gajulapalli RD, Tuzcu ME, et al. A systematic review on the safety of second‐generation transcatheter aortic valves. EuroIntervention. 2016;11:1034–1043. [DOI] [PubMed] [Google Scholar]
- 5. Hahn RT, Pibarot P, Webb J, et al. Outcomes with post‐dilation following transcatheter aortic valve replacement: the PARTNER I trial (Placement of Aortic Transcatheter Valve). JACC Cardiovasc Interv. 2014;7:781–789. [DOI] [PubMed] [Google Scholar]
- 6. Dvir D, Webb J, Brecker S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the Global Valve‐in‐Valve Registry. Circulation. 2012;126:2335–2344. [DOI] [PubMed] [Google Scholar]
- 7. Cruz‐González I, Rama‐Merchan JC, Rodríguez‐Collado J, et al. Paravalvular leak closure after transcatheter aortic valve implantation simultaneously using Amplatzer vascular plug III and IV devices. Rev Esp Cardiol (Engl Ed). 2015;68:1035–1036. [DOI] [PubMed] [Google Scholar]
- 8. Okuyama K, Jilaihawi H, Kashif M, et al. Percutaneous paravalvular leak closure for balloon‐expandable transcatheter aortic valve replacement: a comparison with surgical aortic valve replacement paravalvular leak closure. J Invasive Cardiol. 2015;27:284–290. [PubMed] [Google Scholar]
- 9. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 10. Kanda Y. Investigation of the freely available, easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rossi ML, Belli G, Pagnotta P, et al. Paravalvular leak leading to severe aortic valve regurgitation after TAVI: percutaneous closure strategy. Heart Lung Circ. 2015;24:936–939. [DOI] [PubMed] [Google Scholar]
- 12. Saia F, Martinez C, Gafoor S, et al. Long‐term outcomes of percutaneous paravalvular regurgitation closure after transcatheter aortic valve replacement: a multicenter experience. JACC Cardiovasc Interv. 2015;8:681–688. [DOI] [PubMed] [Google Scholar]
- 13. White JM, Khalique OK, Kodali SK. Immediate, same‐setting paravalvular leak closure following transcatheter aortic valve replacement. Int J Cardiol. 2015;189:235–237. [DOI] [PubMed] [Google Scholar]
- 14. Cockburn J, Charlton T, Gomes A, et al. Transcatheter aortic valve implantation and simultaneous closure of associated paravalvular leak. Cardiovasc Interv Ther. 2016;31:56–60. [DOI] [PubMed] [Google Scholar]
- 15. Arri SS, Poliacikova P, Hildick‐Smith D. Percutaneous paravalvular leak closure for symptomatic aortic regurgitation after CoreValve transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2015;85:657–664. [DOI] [PubMed] [Google Scholar]
- 16. Saireddy R, Subban V, Lamana A, et al. Immediate closure of paravalvular leak after transcatheter aortic valve implantation. Heart Lung Circ. 2014;23:e251–e253. [DOI] [PubMed] [Google Scholar]
- 17. Citro R, Attisano T, Vigorito F, et al. Combined percutaneous closure of paravalvular leaks and intraprosthetic regurgitation after transcatheter aortic valve implantation. Int J Cardiol. 2014;175:e48–e51. [DOI] [PubMed] [Google Scholar]
- 18. Luu J, Ali O, Feldman TE, et al. Percutaneous closure of paravalvular leak after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2013;6:e6–e8. [DOI] [PubMed] [Google Scholar]
- 19. Estévez‐Loureiro R, Salgado‐Fernández J, Vázquez‐González N, et al. Percutaneous closure of paravalvular leaks after transcatheter aortic valve implantation with Edwards Sapien prosthesis: a report of two cases. J Invasive Cardiol. 2013;25:92–95. [PubMed] [Google Scholar]
- 20. Whisenant B, Jones K, Horton KD, et al. Device closure of paravalvular defects following transcatheter aortic valve replacement with the Edwards Sapien valve. Catheter Cardiovasc Interv. 2013;81:901–905. [DOI] [PubMed] [Google Scholar]
- 21. Sinning JM, Vasa‐Nicotera M, Werner N, et al. Interventional closure of paravalvular leakage after transcatheter aortic valve implantation. Eur Heart J. 2012;33:2498. [DOI] [PubMed] [Google Scholar]
- 22. Feldman T, Salinger MH, Levisay JP, et al. Low‐profile vascular plugs for paravalvular leaks after TAVR. Catheter Cardiovasc Interv. 2014;83:280–288. [DOI] [PubMed] [Google Scholar]
- 23. Cruz‐González I, Rama‐Merchan JC, Arribas‐Jiménez A, et al. Paravalvular leak closure with the Amplatzer vascular plug III device: immediate and short‐term results. Rev Esp Cardiol (Engl Ed). 2014;67:608–614. [DOI] [PubMed] [Google Scholar]
- 24. Noble S, Jolicoeur EM, Basmadjian A, et al. Percutaneous paravalvular leak reduction: procedural and long‐term clinical outcomes. Can J Cardiol. 2013;29:1422–1428. [DOI] [PubMed] [Google Scholar]
- 25. Smolka G, Pysz P, Wojakowski W, et al. Clinical manifestations of heart failure abate with transcatheter aortic paravalvular leak closure using Amplatzer vascular plug II and III devices. J Invasive Cardiol. 2013;25:226–231. [PubMed] [Google Scholar]
- 26. Sorajja P, Cabalka AK, Hagler DJ, et al. Long‐term follow‐up of percutaneous repair of paravalvular prosthetic regurgitation. J Am Coll Cardiol. 2011;58:2218–2224. [DOI] [PubMed] [Google Scholar]
- 27. Laflamme J, Puri R, Urena M, et al. Incidence and risk factors of hemolysis after transcatheter aortic valve implantation with a balloon‐expandable valve. Am J Cardiol. 2015;115:1574–1579. [DOI] [PubMed] [Google Scholar]
- 28. Sorajja P, Cabalka AK, Hagler DJ, et al. The learning curve in percutaneous repair of paravalvular prosthetic regurgitation: an analysis of 200 cases. JACC Cardiovasc Interv. 2014;7:521–529. [DOI] [PubMed] [Google Scholar]
- 29. Barbanti M, Petronio AS, Capodanno D, et al. Impact of balloon post‐dilation on clinical outcomes after transcatheter aortic valve replacement with the self‐expanding CoreValve prosthesis. JACC Cardiovasc Interv. 2014;7:1014–1021. [DOI] [PubMed] [Google Scholar]
- 30. Nombela‐Franco L, Rodés‐Cabau J, DeLarochellière R, et al. Predictive factors, efficacy, and safety of balloon post‐dilation after transcatheter aortic valve implantation with a balloon‐expandable valve. JACC Cardiovasc Interv. 2012;5:499–512. [DOI] [PubMed] [Google Scholar]
- 31. Mastoris I, Schoos MM, Dangas GD, et al. Stroke after transcatheter aortic valve replacement: incidence, risk factors, prognosis, and preventive strategies. Clin Cardiol. 2014;37:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Watanabe Y, Hayashida K, Lefévre T, et al. Is postdilatation useful after implantation of the Edwards valve? Catheter Cardiovasc Interv. 2015;85:667–676. [DOI] [PubMed] [Google Scholar]
- 33. Barbanti M, Yang TH, Rodés‐Cabau J, et al. Anatomical and procedural features associated with aortic root rupture during balloon‐expandable transcatheter aortic valve replacement. Circulation. 2013;128:244–253. [DOI] [PubMed] [Google Scholar]
- 34. Toggweiler S, Wood DA, Rodés‐Cabau J, et al. Transcatheter valve‐in‐valve implantation for failed balloon‐expandable transcatheter aortic valves. JACC Cardiovasc Interv. 2012;5:571–577. [DOI] [PubMed] [Google Scholar]
- 35. Makkar RR, Jilaihawi H, Chakravarty T, et al. Determinants and outcomes of acute transcatheter valve‐in‐valve therapy or embolization: a study of multiple valve implants in the US PARTNER trial (Placement of Aortic Transcatheter Valve Trial Edwards SAPIEN Transcatheter Heart Valve). J Am Coll Cardiol. 2013;62:418–430. [DOI] [PubMed] [Google Scholar]
- 36. Burriesci G, Peruzzo P, Susin FM, et al. In vitro hemodynamic testing of Amplatzer plugs for paravalvular leak occlusion after transcatheter aortic valve implantation. Int J Cardiol. 2016;203:1093–1099. [DOI] [PubMed] [Google Scholar]
- 37. Attizzani GF, Ohno Y, Latib A, et al. Transcatheter aortic valve implantation under angiographic guidance with and without adjunctive transesophageal echocardiography. Am J Cardiol. 2015;116:604–611. [DOI] [PubMed] [Google Scholar]
- 38. Ruiz CE, Jelnin V, Kronzon I, et al. Clinical outcomes in patients undergoing percutaneous closure of periprosthetic paravalvular leaks. J Am Coll Cardiol. 2011;58:2210–2217. [DOI] [PubMed] [Google Scholar]
- 39. Leon MB, Smith CR, Mack MJ, et al.; PARTNER Trial Investigators. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 40. Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 41. Millán X, Skaf S, Joseph L, et al. Transcatheter reduction of paravalvular leaks: a systematic review and meta‐analysis. Can J Cardiol. 2015;31:260–269. [DOI] [PubMed] [Google Scholar]
- 42. Weber C, Weber M, Ekinci O, et al. Atrial septal defects type II: noninvasive evaluation of patients before implantation of an Amplatzer Septal Occluder and on follow‐up by magnetic resonance imaging compared with TEE and invasive measurement. Eur Radiol. 2008;18:2406–2413. [DOI] [PubMed] [Google Scholar]
- 43. Cubeddu RJ, Babin I, Inglessis I. The off‐label use of the Amplatzer muscular VSD occluder for large patent ductus arteriosus: a case report and review. Cardiovasc Interv Ther. 2014;29:256–260. [DOI] [PubMed] [Google Scholar]
- 44. Wang W, Li H, Tam MD, et al. The Amplatzer vascular plug: a review of the device and its clinical applications. Cardiovasc Intervent Radiol. 2012;35:725–740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Flow diagram of study selection.
Table S1 Reasons for failure


