ABSTRACT
Background
The Global Registry of Acute Coronary Events (GRACE) risk score has been extensively validated to predict risk during hospitalization in patients with acute coronary syndrome (ACS). Recently, serum calcium has been suggested as an independent predictor for in‐hospital mortality in patients with ST‐segment elevation myocardial infarction; however, the relationship between the 2 has not been evaluated.
Hypothesis
The combination of GRACE risk score and serum calcium could provide better performance in risk prediction.
Methods
The study enrolled 2229 consecutive patients with ACS. Independent predictors were identified by a multivariate logistic regression model. The incremental prognostic value added by serum calcium to the GRACE score was evaluated by receiver operating characteristic, net reclassification improvement (NRI), and integrated discrimination improvement (IDI).
Results
Patients in the upper quartiles of serum calcium presented with lower in‐hospital mortality (odds ratios for 3 upper quartiles vs lowest quartile, respectively: 0.443, 95% confidence interval [CI]: 0.206‐0.953; 0.243, 95% CI: 0.090‐0.654; and 0.210, 95% CI: 0.082‐0.538). Area under the curve increased significantly after adding serum calcium to the GRACE score (0.685 vs 0.746; Z = 2.617, P = 0.009). Furthermore, inclusion of serum calcium in the GRACE score enhanced NRI (0.524; P = 0.009) and IDI (0.011; P = 0.003).
Conclusions
Lower serum calcium level on admission is a possible indicator of increased risk of in‐hospital mortality in ACS patients. Inclusion of serum calcium in the GRACE score may lead to a more accurate prediction of this risk. Large prospective studies are needed to confirm this finding.
Introduction
Acute coronary syndromes (ACS), ranging from unstable angina (UA) to myocardial infarction, represent a life‐threatening manifestation of atherosclerotic progression in the coronary district. Risk stratification is crucial for appropriate therapeutic decision‐making in these patients. As a widely used risk‐evaluating score from a large, multinational, prospective registry, the Global Registry of Acute Coronary Events (GRACE) risk score has been extensively validated and proven to have an excellent ability to predict in‐hospital mortality across the spectrum of patients with ACS.1, 2, 3 Some clinical parameters not incorporated in the GRACE risk score, such as C‐reactive protein, platelet volume, and glycated hemoglobin, have been reported to further improve the prognostic performance of the GRACE score in ACS patients.4, 5, 6
Extraskeletal calcium, which is widely distributed throughout the organs and tissues, plays a critical role in a range of biological processes related to cardiovascular disease, including platelet adhesion, blood coagulation, cardiac contraction, cardiomyocyte apoptosis, and electrophysiology of the heart. Serum calcium is one of the main components of extraskeletal calcium and is also a widely applied biochemical index in clinical practice.7, 8 Recently, hypocalcemia has been reported to be a predictor of increased in‐hospital mortality in patients with severe coronary artery disease (CAD).9 However, the association between serum calcium and the GRACE score remains unclear to date.
In the present study, we sought to investigate the relationship between serum calcium at admission and the GRACE score and to identify the incremental prognostic value added by serum calcium when included in the GRACE score in patients with ACS.
Methods
Study Population
The data source for this study was the West China Hospital Coronary Artery Disease Database, which includes all CAD patients undergoing angiography in the West China Hospital of Sichuan University. We retrospectively recruited 2229 consecutive patients with angiography‐confirmed ACS by cardiologists in our group from July 2008 to September 2012. Patients with ACS were eligible for inclusion if they met all 3 of the following criteria: (1) >50% stenosis in ≥1 epicardial coronary artery, confirmed by coronary angiography; (2) symptoms of ischemia that increased or occurred at rest; and (3) elevated cardiac troponin T levels (0.03 mg/L) or new electrocardiographic deviation in ≥2 contiguous leads (either pathologic Q waves [≥0.04 s in duration], ST‐segment dynamic horizontal/downsloping depression ≥0.05 mV, or persistent ST‐segment elevation ≥0.1 mV in ≥2 contiguous precordial leads or ≥2 adjacent limb leads, or new left bundle branch block). Patients were excluded for the following reasons: hemodynamic instability, malignancies, active bleeding, pregnancy, severe liver or hematological disorders, and missing laboratory value or measuring time of serum calcium on admission. Patients received care according to the current practice guidelines; treatment and management of the patients was not affected by participation in this study. The study protocol was approved by the local institutional review boards in accordance with the Declaration of Helsinki. All subjects provided written informed consent before enrollment.
Demographic and Clinical Data
All data entered into the computerized database were collected from the patient charts and by bedside inquiry and physical examination. Hypertension was defined as blood pressure >140/90 mm Hg on ≥2 independent readings, or the current use of antihypertensive medication. Diabetes mellitus was diagnosed in patients who had previously undergone dietary treatment, had received additional oral antidiabetic or insulin medication, or had a current fasting blood glucose level of >7.0 mmol/L in 2 blood samples.
Determination of Serum Calcium and GRACE Risk Score
Admission serum calcium level was defined as the first peripheral venous serum calcium level obtained during hospitalization and was measured by the laboratory medicine department as per usual practice.
Variables included in the GRACE risk score model were readily available at hospital admission (age, heart rate, systolic blood pressure, serum creatinine (SCr) concentration, Killip class, ST‐segment deviation, elevated cardiac enzymes, and cardiac arrest). The GRACE risk score were calculated by software affiliated to the database as previously described2 and in accordance with GRACE risk calculator (http://www.outcomes‐umassmed.org/grace).
Statistical Analysis
Continuous variables are expressed as mean ± SD or as median (interquartile range), and categorical variables are reported as counts and percentages. Independent samples t test and Kruskal‐Wallis test were applied, respectively, to assess normally distributed and non–normally distributed continuous and categorical variables. To assess categorical variables, χ2 tests were applied. Pearson rank correlation was used to assess the relationship between serum calcium and the GRACE risk score. Univariate logistic regression was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for in‐hospital mortality. All significant variables (P < 0.1) were tested in a multivariate logistic regression model to identify independent predictors.
Serum calcium was performed as a continuous variable when it was included into the GRACE risk score. Two logistic regression models were constructed: one with GRACE risk score alone and the other with the combination of serum calcium. Receiver operating characteristic analysis was used to calculate the C statistic, and the increase in the area under curve (AUC) was evaluated and tested for significance using the test proposed by Hanley and McNeil.10 Net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were performed to analyze the degree to which the addition of serum calcium to the GRACE risk score model improved predictive ability, as previously described.11 Two‐sided P values <0.05 indicated statistical significance. All analyses were performed with SPSS software version 22.0 (IBM Corp., Armonk, NY), MedCalc software version 15.10 (MedCalc Software, Ostend, Belgium), and STATA software version 13.0 (StataCorp LP, College Station, TX).
Results
Population Characteristics
The baseline characteristics of the patients are listed in Table 1. A total of 2229 patients with angiography confirmed ACS were enrolled in this study in accordance with the inclusion criteria, and in‐hospital mortality occurred in 56 patients. The mean age was 64.55 ± 10.66 years, 78.9% were men, 21.1% presented with ST‐segment elevation myocardial infarction (STEMI), and 78.1% underwent percutaneous coronary intervention. The admission serum calcium levels were normally distributed (Figure 1), with a mean admission calcium level of 2.20 ± 0.15 mmol/L.
Table 1.
Baseline Characteristics of Overall Patients and Grouped Patients Stratified by Serum Calcium at Admission
| Serum Calcium, mmol/L | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Overall | Q1, <2.12 | Q2, 2.12– < 2.21 | Q3, 2.21– < 2.28 | Q4, ≥2.28 | P Value |
| No. of patients, n | 2229 | 534 | 601 | 491 | 603 | |
| In‐hospital death | 56 (2.5) | 31 (5.8) | 12 (2.0) | 6 (1.2) | 7 (1.2) | <0.001 |
| Mean age, y | 64.55 ± 10.66 | 66.34 ± 10.11 | 65.16 ± 10.32 | 63.87 ± 10.78 | 62.92 ± 11.11 | <0.001 |
| Male sex | 1759 (78.9) | 438 (82.0) | 487 (81.0) | 377 (76.8) | 457 (75.8) | 0.022 |
| Serum calcium, mmol/L | 2.20 ± 0.15 | 2.02 ± 0.13 | 2.16 ± 0.025 | 2.23 ± 0.02 | 2.36 ± 0.15 | <0.001 |
| Calcium measuring time from admission, h | 2 (0.5–15) | 1.5 (0.5–13.5) | 2 (0.5–15) | 3 (0.5–15) | 2 (0.5–15) | 0.025 |
| Calcium measuring time of day | ||||||
| 6 am to 11 am | 1129 (50.6) | 267 (50.0) | 320 (53.2) | 251 (51.1) | 290 (48.1) | 0.344 |
| 12 pm to 5 pm | 388 (17.4) | 99 (18.5) | 90 (15.0) | 79 (16.1) | 120 (19.9) | 0.105 |
| 6 pm to 11 pm | 484 (21.7) | 108 (20.2) | 128 (21.3) | 115 (23.4) | 133 (22.1) | 0.650 |
| 00 am to 5 am | 229 (10.3) | 60 (11.2) | 63 (10.5) | 46 (9.4) | 60 (10.0) | 0.784 |
| GRACE risk score | 92.80 ± 26.27 | 98.23 ± 26.07 | 92.78 ± 25.97 | 89.49 ± 26.08 | 90.65 ± 26.20 | <0.001 |
| Previous or current smoker | 1307 (58.6) | 325 (60.9) | 365 (60.7) | 293 (59.7) | 324 (53.7) | <0.001 |
| HTN | 1217 (54.6) | 281 (52.6) | 308 (51.2) | 278 (56.6) | 350 (58.0) | 0.064 |
| DM | 554 (24.9) | 133 (24.9) | 135 (22.5) | 119 (24.2) | 167 (27.7) | 0.208 |
| Previous PCI or CABG | ||||||
| PCI | 162 (7.3) | 40 (7.5) | 47 (7.8) | 41 (8.4) | 34 (5.6) | 0.316 |
| CABG | 22 (1.0) | 5 (0.9) | 5 (0.8) | 4 (0.8) | 8 (1.3) | 0.796 |
| Previous drug treatment | ||||||
| Aspirin | 724 (32.5) | 139 (26.0) | 203 (33.8) | 175 (35.6) | 207 (34.3) | 0.003 |
| Statin | 460 (20.6) | 87 (16.3) | 124 (20.6) | 124 (25.3) | 125 (20.7) | 0.006 |
| ACEI/ARB | 420 (18.8) | 83 (15.5) | 119 (19.8) | 91 (18.5) | 127 (21.1) | 0.105 |
| β‐Blocker | 531 (23.8) | 92 (17.2) | 149 (24.8) | 132 (26.9) | 158 (26.2) | 0.001 |
| BMI, kg/m2 | 24.21 ± 2.94 | 24.06 ± 2.89 | 24.02 ± 2.75 | 24.27 ± 2.90 | 24.48 ± 3.17 | 0.028 |
| BP at admission, mm Hg | ||||||
| SBP | 130.27 ± 22.08 | 125.69 ± 23.82 | 129.46 ± 20.50 | 132.97 ± 21.64 | 132.93 ± 21.68 | <0.001 |
| DBP | 76.45 ± 12.84 | 73.90 ± 13.52 | 75.61 ± 11.98 | 77.55 ± 12.82 | 78.64 ± 12.64 | <0.001 |
| Heart rate at admission, bpm | 74.54 ± 14.38 | 76.21 ± 16.74 | 73.59 ± 13.39 | 73.44 ± 12.52 | 74.92 ± 14.39 | 0.005 |
| SCr, mmol/L | 90.79 ± 28.68 | 92.74 ± 33.71 | 89.00 ± 24.96 | 90.08 ± 26.76 | 91.43 ± 28.80 | 0.146 |
| Albumin, g/dL | 40.45 ± 4.25 | 37.75 ± 4.17 | 40.32 ± 3.51 | 41.48 ± 3.66 | 42.09 ± 4.25 | <0.001 |
| Lipids, mmol/L | ||||||
| TC | 4.14 ± 1.08 | 3.82 ± 0.96 | 4.08 ± 1.11 | 4.23 ± 0.99 | 4.39 ± 1.15 | <0.001 |
| LDL‐C | 2.45 ± 0.94 | 2.28 ± 0.81 | 2.37 ± 0.95 | 2.49 ± 0.87 | 2.62 ± 1.04 | <0.001 |
| HDL‐C | 1.15 ± 0.37 | 1.12 ± 0.46 | 1.13 ± 0.37 | 1.17 ± 0.34 | 1.16 ± 0.30 | 0.077 |
| TG | 1.79 ± 1.17 | 1.50 ± 0.81 | 1.75 ± 1.25 | 1.78 ± 1.16 | 2.07 ± 1.28 | <0.001 |
| WBC count, ×109/L | 7.87 ± 3.42 | 8.49 ± 3.88 | 7.70 ± 3.47 | 7.48 ± 3.13 | 7.83 ± 3.10 | <0.001 |
| Hgb, g/L | 133.87 ± 18.09 | 126.70 ± 19.21 | 133.56 ± 17.36 | 136.43 ± 16.20 | 138.48 ± 17.24 | <0.001 |
| Platelet count, ×109/L | 162.88 ± 61.52 | 162.00 ± 63.39 | 158.19 ± 61.83 | 165.74 ± 65.09 | 166.01 ± 56.16 | 0.101 |
| Serum potassium, mmol/L | 3.94 ± 0.47 | 3.94 ± 0.53 | 3.93 ± 0.43 | 3.98 ± 0.44 | 3.99 ± 0.45 | <0.001 |
| Serum sodium, mmol/L | 140.81 ± 3.73 | 139.91 ± 4.70 | 140.94 ± 3.34 | 141.17 ± 3.67 | 141.18 ± 2.98 | <0.001 |
| Serum chloride, mmol/L | 104.73 ± 5.74 | 105.07 ± 7.32 | 105.30 ± 3.31 | 104.71 ± 4.84 | 103.87 ± 6.59 | <0.001 |
| Lesion characteristic | ||||||
| LM disease | 221 (9.9) | 54 (10.1) | 63 (10.5) | 46 (9.4) | 58 (9.6) | 0.927 |
| 3‐vessel disease | 529 (23.7) | 119 (22.3) | 136 (22.6) | 132 (26.9) | 142 (23.5) | 0.292 |
| PCI | 1741 (78.1) | 420 (78.7) | 472 (78.5) | 380 (77.4) | 469 (77.8) | 0.952 |
| Clinical diagnosis | ||||||
| STEMI | 471 (21.1) | 137 (25.7) | 116 (19.3) | 92 (18.7) | 127 (21.0) | 0.023 |
| NSTEMI | 230 (10.3) | 61 (11.4) | 62 (10.3) | 40 (8.1) | 67 (11.1) | 0.304 |
| UA | 1528 (68.6) | 336 (62.9) | 434 (72.1) | 370 (75.2) | 411 (67.9) | <0.001 |
| Drug treatment in hospital | ||||||
| ASA | 2151 (96.5) | 510 (95.5) | 585 (97.3) | 472 (96.1) | 584 (96.8) | 0.358 |
| Clopidogrel | 2137 (95.9) | 507 (94.9) | 576 (95.8) | 472 (96.1) | 582 (96.5) | 0.597 |
| Statin | 2089 (93.7) | 502 (94.2) | 559 (93.0) | 463 (94.3) | 565 (93.7) | 0.805 |
| ACEI/ARB | 1318 (59.1) | 297 (55.6) | 351 (58.5) | 292 (59.5) | 378 (62.7) | 0.111 |
| β‐Blocker | 1523 (68.3) | 325 (60.9) | 398 (66.3) | 345 (70.3) | 455 (75.5) | <0.001 |
| Nitrated derivative | 1025 (46.0) | 212 (39.7) | 290 (48.3) | 237 (48.3) | 286 (47.4) | 0.10 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; ASA, aspirin; BP, blood pressure; CABG, coronary artery bypass grafting; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; Hgb, hemoglobin; HTN, hypertension; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; LM, left main; PCI, percutaneous coronary intervention; Q, quartile; SBP, systolic blood pressure; SCr, serum creatinine; SD, standard deviation; STEMI, ST‐segment elevation myocardial infarction; TC, total cholesterol; TG, triglycerides; UA, unstable angina; WBC, white blood cell.
Data are expressed as n (%), mean ± SD, or median (IQR), as appropriate.
Figure 1.
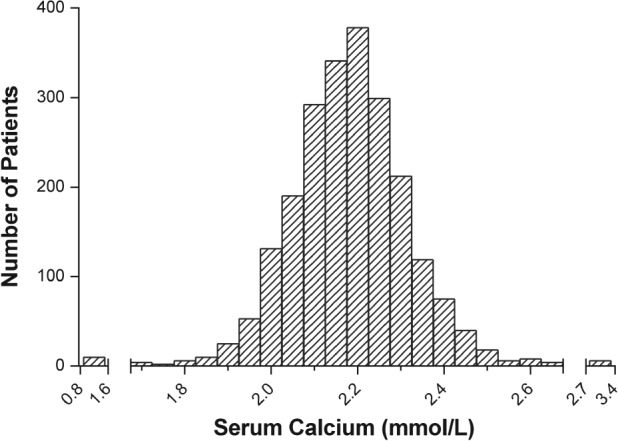
The distribution of admission serum calcium in overall participants.
There were no significant differences in past medical history of hypertension or diabetes mellitus, arterial lesion characteristics, percutaneous coronary intervention, and primary drug treatment between the 4 groups of patients stratified by serum calcium level. Patients with lower baseline serum calcium levels were older, more likely to present with STEMI, and had higher GRACE risk score, higher heart rate, lower blood pressure, higher SCr levels, higher white blood cell counts, lower hemoglobin levels, and a higher frequency of smoking history.
Baseline Serum Calcium as an Independent Predictor
Admission serum calcium was stratified into quartiles (Q1: <2.12 mmol/L; Q2: 2.12– < 2.21 mmol/L; Q3: 2.21– < 2.28 mmol/L; and Q4: >2.28 mmol/L) to analyze its predictive value. Table 2 summarizes the results of the univariate and multivariate logistic regression analyses of the factors associated with in‐hospital mortality. After adjustment for all potentially confounding variables (P < 0.1), including albumin, a low level of serum calcium (OR: 0.443, 95% CI: 0.206‐0.953; OR: 0.243, 95% CI: 0.090‐0.654; and OR: 0.210, 95% CI: 0.082‐0.538) for the second, third, and fourth quartiles, respectively, compared with the lowest quartile) was still an independent predictor of in‐hospital mortality in the multivariate logistic regression analysis.
Table 2.
Univariate and Multivariate Logistic Regression Analysis for Predictors of In‐hospital Mortality
| Variables | Unadjusted OR | P Value | Adjusted OR | P Value |
|---|---|---|---|---|
| Serum calcium | ||||
| Q1 | Ref | — | Ref | — |
| Q2 | 0.330 | 0.001 | 0.443 | 0.037 |
| Q3 | 0.200 | <0.001 | 0.243 | 0.005 |
| Q4 | 0.190 | <0.001 | 0.210 | 0.001 |
| Calcium measuring time from admission | 0.943 | 0.010 | 0.953 | 0.059 |
| Calcium measuring time of day | ||||
| 6 am to 11 pm | Ref | — | ||
| 12 pm to 5 pm | 1.092 | 0.823 | ||
| 6 pm to 11 pm | 1.370 | 0.355 | ||
| 00 am to 5 am | 1.882 | 0.112 | ||
| GRACE risk score | 1.025 | <0.001 | 1.017 | 0.017 |
| Age | 1.043 | <0.001 | 1.010 | 0.701 |
| Male sex | 1.804 | 0.043 | 2.236 | 0.037 |
| Smoking history | 0.699 | 0.186 | ||
| HTN | 1.112 | 0.699 | ||
| DM | 2.155 | 0.005 | 1.585 | 0.158 |
| Previous PCI | 0.466 | 0.292 | ||
| Previous CABG | <0.001 | 0.998 | ||
| Previous ASA | 0.560 | 0.078 | 1.423 | 0.421 |
| Previous statin | 0.454 | 0.070 | 0.609 | 0.367 |
| Previous ACEI/ARB | 0.712 | 0.379 | ||
| Previous β‐Blocker | 0.377 | 0.025 | 0.459 | 0.138 |
| BMI | 0.955 | 0.332 | ||
| SBP | 0.982 | 0.006 | 0.996 | 0.688 |
| DBP | 0.977 | 0.031 | 0.996 | 0.806 |
| Heart rate | 1.044 | <0.001 | 1.020 | 0.023 |
| SCr | 1.017 | <0.001 | 1.010 | 0.013 |
| Albumin | 0.925 | 0.007 | 1.071 | 0.063 |
| TC | 1.009 | 0.947 | ||
| LDL‐C | 0.844 | 0.276 | ||
| HDL‐C | 0.749 | 0.526 | ||
| TG | 0.844 | 0.276 | ||
| WBC | 1.203 | <0.001 | 1.139 | <0.001 |
| Hgb | 0.982 | 0.007 | 0.998 | 0.816 |
| Platelet counts | 0.999 | 0.805 | ||
| Serum potassium | 1.485 | 0.140 | ||
| Serum sodium | 0.954 | 0.107 | ||
| Serum chloride | 0.971 | 0.010 | 0.979 | 0.162 |
| LM disease | 2.285 | 0.016 | 3.124 | 0.011 |
| 3‐vessel disease | 2.296 | 0.003 | 2.870 | 0.002 |
| PCI | 1.297 | 0.461 | ||
| STEMI | 2.297 | 0.003 | 1.480 | 0.447 |
| NSTEMI | 1.249 | 0.588 | ||
| UA | 0.449 | 0.003 | 1.640 | 0.335 |
| ASA | 0.001 | 0.997 | ||
| Clopidogrel | 0.001 | 0.997 | ||
| Statin | 0.001 | 0.996 | ||
| ACEI/ARB | 0.590 | 0.052 | 0.837 | 0.582 |
| β‐Blocker | 1.273 | 0.430 | ||
| Nitrated derivative | 1.180 | 0.542 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, aspirin; BMI, body mass index; CABG, coronary artery bypass grafting; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; Hgb, hemoglobin; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; LM, left main; NSTEMI, non–ST‐segment elevation myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; Q, quartile; Ref, reference; SBP, systolic blood pressure; SCr, serum creatinine; STEMI, ST‐segment elevation myocardial infarction; TC, total cholesterol; TG, triglycerides; WBC, white blood cells.
Association and Combination of the GRACE Risk Score With Admission Serum Calcium
The correlation between serum calcium and the GRACE risk score was assessed by Pearson correlation. The result showed that there was a very weak negative, though significant, correlation between serum calcium and the GRACE risk score (r = −0.089, P = 0.001; Figure 2).
Figure 2.
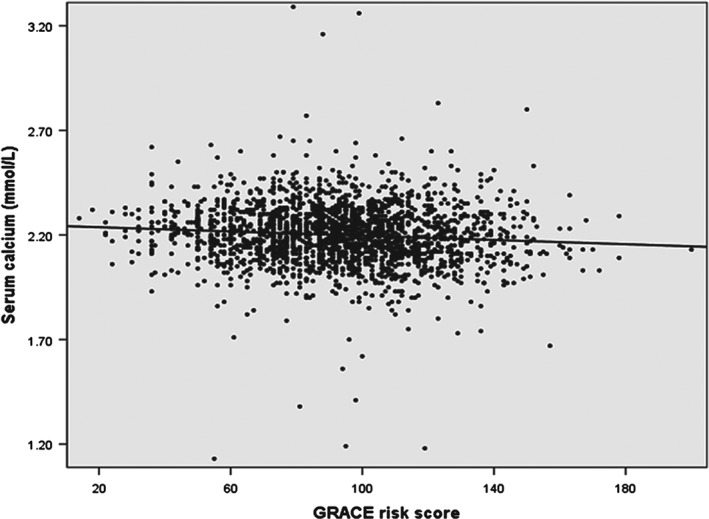
Correlation between serum calcium concentration and the GRACE risk score. Abbreviations: GRACE, Global Registry of Acute Coronary Events.
The receiver operating characteristic analysis was performed to assess whether a combination of serum calcium and the GRACE score could improve predictive ability compared with the GRACE score alone. The results showed that the AUC increased significantly after the addition of serum calcium to the GRACE risk score (0.685 vs 0.746; Z = 2.617, P = 0.009; Figure 3A). Moreover, the inclusion of serum calcium into the GRACE risk score model was associated with a NRI of 52.4% (P < 0.001), suggesting an effective reclassification. The IDI again showed that the diagnostic performance of the model was significantly improved by adding serum calcium to the GRACE risk score (IDI: 0.011, P = 0.003; Figure 3A).
Figure 3.
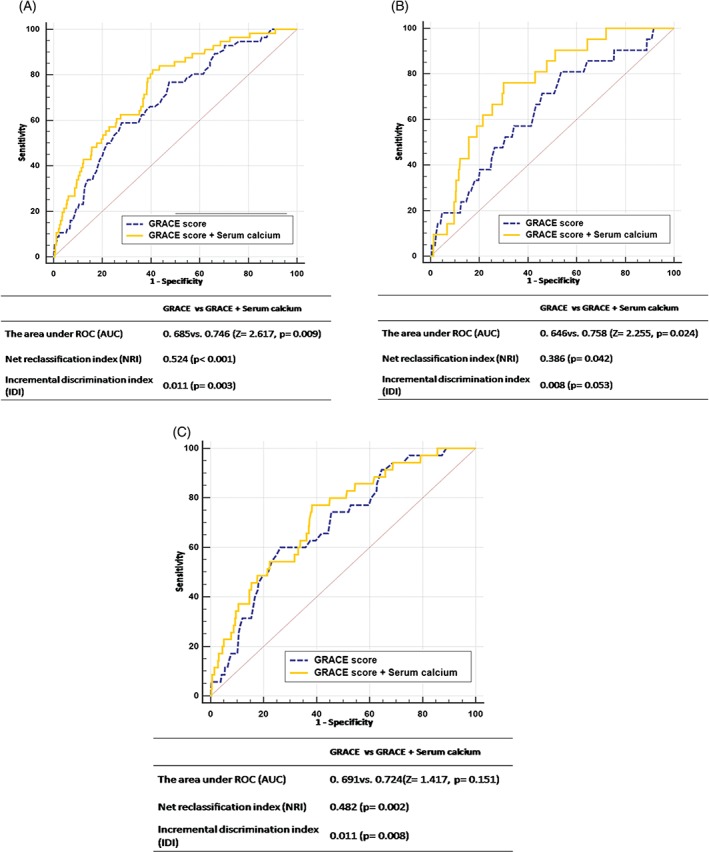
The ROC curves of the GRACE risk score and its combination with admission serum calcium in (A) overall participants and subgroups of patients with (B) STEMI or (C) UA/NSTEMI. Abbreviations: AUC, area under the curve; GRACE, Global Registry of Acute Coronary Events; IDI, integrated discrimination improvement; NRI, net reclassification improvement; NSTEMI, non–ST‐segment elevation myocardial infarction; ROC, receiver operating characteristic; UA, unstable angina.
In the subgroup of patients with STEMI, serum calcium also significantly increased the AUC and led to a similar improvement in reclassification and discrimination when added to the GRACE risk score (AUC: 0.646 vs 0.758, Z = 2.255, P = 0.024; NRI: 0.386, P = 0.042; and IDI: 0.008, P = 0.053; Figure 3B). Serum calcium showed a tendency to increase the AUC, but this relationship did not reach significance (AUC: 0.691 vs 0.724, Z = 1.417, P = 0.151; Figure 3C) in patients with UA or non–ST‐elevation myocardial infarction (UA/NSTEMI).
Discussion
The results of this study showed that a decreased baseline serum calcium level measured at admission in patients with ACS was an independent predictor of the in‐hospital mortality after adjusting for the potential confounding predictors. Moreover, serum calcium was weakly correlated with the GRACE risk score and added incremental predictive value when combined with the GRACE score. The effectiveness of serum calcium in increasing the predictive value of the GRACE risk score was attenuated in the UA/NSTEMI group, which may be due to the relatively lower ratio of events, and suggests that serum calcium may be more influential in STEMI. Thus, a larger multicenter study is warranted to better evaluate the effects of baseline serum calcium in ACS patients.
The use of various albumin adjustment formulas has been suggested to assess serum calcium, but no clear consensus has been reached12, 13, 14; in this study, albumin was included in the multivariable regression model but was not used directly to correct serum calcium. Hypocalcemia is prevalent in critically ill patients and has been shown to be associated with increased mortality in a considerable number of clinical studies.15, 16, 17, 18 Our results were consistent with those of a recent study by Xin Lu et al9 conducted on patients with STEMI. Although a lower level of serum calcium was independently associated with in‐hospital mortality, the mechanism that may account for this association was not clear. Possible mechanisms of the association between serum calcium and in‐hospital mortality in patients with severe CAD have been suggested. First, low levels of serum calcium may prolong the plateau phase of the cardiac action potential following the delayed closure of calcium channel on the membrane of cardiomyocyte. And a prolonged plateau phase has been widely recognized as an independent high‐risk factor for mortality. Second, hypocalcemia could reduce renal sodium excretion, thus contributing to fluid overload19 and diminished myocardial contractility, as demonstrated by decreased left ventricular work index.20, 21
To our knowledge, this is the first study to evaluate the association between serum calcium and the GRACE risk score. The current GRACE risk score only includes 2 laboratory‐based biomarkers: SCr and troponin. Therefore, it is conceivable that variables that reflect other pathophysiological aspects of ACS could provide additional information. In this study, both serum calcium and the GRACE risk score were significantly different in 2 groups of patients with or without in‐hospital mortality, but there was only a very weak correlation between them. Furthermore, the addition of serum calcium to the GRACE risk score could effectively improve the predictive power of the scoring system for the risk of hospitalization. All these results suggest that serum calcium offers independent information in addition to that provided by the GRACE risk score.
Although previous studies concerning calcium supplements have been conflicting,22, 23 some recent evidence suggests that the beneficial effects of calcium supplementation could be population‐dependent, such as in patients with calcium deficiency.24, 25 A more recent study showed that calcium supplementation improves short‐term outcomes in intensive care unit patients with hypocalcemia.26 Thus, further investigations are needed to determine whether calcium‐supplementation therapy in ACS patients with low serum calcium could improve their prognosis.
Study Limitations
Despite consecutive patient recruitment and relatively comprehensive clinical data collection, there are still some limitations in our present study. First, this investigation was a single‐center retrospective study and recruited exclusively from a Chinese population; therefore, our findings should be further validated in prospective studies recruiting other ethnic groups. Second, this investigation was a single‐center observational study, and the lack of validation cohorts was another weakness.
Conclusion
Serum calcium level at admission was an independent predictor of in‐hospital mortality in ACS patients. The inclusion of serum calcium into the GRACE risk score could lead to a more accurate prediction. We suggest considering low serum calcium level at admission as a possible indicator of increased risk of in‐hospital mortality while awaiting more data to confirm this finding.
Shao‐di Yan and Xiao‐jing Liu are equally contributing authors. This work was funded by the National High‐Tech Research and Development Program of China (grant number: 2012AA02A510, Beijing, China), the National Natural Science Foundation of China (grant numbers: 81370219 and 81400267, Beijing, China), and the General Financial Grant from the China Postdoctoral Science Foundation (grant number: 2015 M582559, Beijing, China).
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 2. Granger CB, Goldberg RJ, Dabbous O, et al; GRACE Investigators . Predictors of hospital mortality in the Global Registry of Acute Coronary Events. Arch Intern Med. 2003;163:2345–2353. [DOI] [PubMed] [Google Scholar]
- 3. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non–ST‐Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2014;64:2713–2714]. J Am Coll Cardiol. 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 4. Correia LC, Souza AC, Sabino M, et al. Hemoglobin level adds prognostic value to the Global Registry of Acute Coronary Events score in non–ST‐elevation acute coronary syndromes. Cardiology. 2012;121:213–219. [DOI] [PubMed] [Google Scholar]
- 5. Schiele F, Meneveau N, Seronde MF, et al. C‐reactive protein improves risk prediction in patients with acute coronary syndromes. Eur Heart J. 2010;31:290–297. [DOI] [PubMed] [Google Scholar]
- 6. Niu X, Yang C, Zhang Y, et al. Mean platelet volume on admission improves risk prediction in patients with acute coronary syndromes. Angiology. 2015;66:456–463. [DOI] [PubMed] [Google Scholar]
- 7. Kraft MD. Phosphorus and calcium: a review for the adult nutrition support clinician. Nutr Clin Pract. 2015;30:21–33. [DOI] [PubMed] [Google Scholar]
- 8. Berridge MJ. Calcium signalling remodelling and disease. Biochem Soc Trans. 2012;40:297–309. [DOI] [PubMed] [Google Scholar]
- 9. Lu X, Wang Y, Meng H, et al. Association of admission serum calcium levels and in‐hospital mortality in patients with acute ST‐elevation myocardial infarction: an eight‐year, single‐center study in China. PLoS One. 2014;9:e99895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. [DOI] [PubMed] [Google Scholar]
- 11. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Slomp J, van der Voort PH, Gerritsen RT, et al. Albumin‐adjusted calcium is not suitable for diagnosis of hyper‐ and hypocalcemia in the critically ill. Crit Care Med. 2003;31:1389–1393. [DOI] [PubMed] [Google Scholar]
- 13. Björkman MP, Sorva AJ, Tilvis RS. Calculated serum calcium is an insufficient surrogate for measured ionized calcium. Arch Gerontol Geriatr. 2009;49:348–350. [DOI] [PubMed] [Google Scholar]
- 14. Steele T, Kolamunnage‐Dona R, Downey C, et al. Albumin‐adjusted calcium concentration should not be used to identify hypocalcaemia in critical illness. Crit Care. 2013;17(suppl 2):446–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miura S, Yoshihisa A, Takiguchi M, et al. Association of hypocalcemia with mortality in hospitalized patients with heart failure and chronic kidney disease. J Card Fail. 2015;21:621–627. [DOI] [PubMed] [Google Scholar]
- 16. Müller B, Becker KL, Kranzlin M, et al. Disordered calcium homeostasis of sepsis: association with calcitonin precursors. Eur J Clin Invest. 2000;30:823–831. [DOI] [PubMed] [Google Scholar]
- 17. Sauter TC, Lindner G, Ahmad SS, et al. Calcium disorders in the emergency department: independent risk factors for mortality. PLoS One. 2015;10:e0132788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Z, Xu X, Ni H, et al. Predictive value of ionized calcium in critically ill patients: an analysis of a large clinical database MIMIC II. PLoS One. 2014;9:e95204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levine SN, Rheams CN. Hypocalcemic heart failure. Am J Med. 1985;78(6 part 1):1033–1035. [DOI] [PubMed] [Google Scholar]
- 20. Hurley K, Baggs D. Hypocalcemic cardiac failure in the emergency department. J Emerg Med. 2005;28:155–159. [DOI] [PubMed] [Google Scholar]
- 21. Suzuki T, Ikeda U, Fujikawa H, et al. Hypocalcemic heart failure: a reversible form of heart muscle disease. Clin Cardiol. 1998;21:227–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bostick RM, Kushi LH, Wu Y, et al. Relation of calcium, vitamin D, and dairy food intake to ischemic heart disease mortality among postmenopausal women. Am J Epidemiol. 1999;149:151–161. [DOI] [PubMed] [Google Scholar]
- 23. Bolland MJ, Grey A, Avenell A, et al. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta‐analysis. BMJ. 2011;342:d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larsson SC, Orsini N, Wolk A. Dietary calcium intake and risk of stroke: a dose–response meta‐analysis. Am J Clin Nutr. 2013;97:951–957. [DOI] [PubMed] [Google Scholar]
- 25. Michaëlsson K, Melhus H, Warensjö Lemming E, et al. Long‐term calcium intake and rates of all cause and cardiovascular mortality: community‐based prospective longitudinal cohort study. BMJ. 2013;346:f228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Z, Chen K, Ni H. Calcium supplementation improves clinical outcome in intensive care unit patients: a propensity score matched analysis of a large clinical database MIMIC‐II. Springerplus. 2015;4:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]


