Abstract
Background
Chronic inflammation promotes atherosclerosis and is a prognostic factor in coronary artery disease (CAD). Patients with type 2 diabetes mellitus (DM2) are at risk for progressive atherosclerosis. Macrophage migration inhibitory factor (MIF) is a key player in atherosclerosis, mediating pro‐inflammatory responses. Its endogenous antagonist Gremlin‐1 inhibits foam‐cell formation and atheroprogression by binding MIF, neutralizing its proatherosclerotic functions.
Hypothesis
Plasma levels of MIF and Gremlin‐1 correlate with the stability of CAD in patients with DM2.
Method
We assessed plasma levels of Gremlin‐1 and MIF in 198 nondiabetic and 88 diabetic patients with symptomatic CAD using enzyme‐linked immunosorbent assays.
Results
Plasma levels of Gremlin‐1 were higher DM2 patients (278.8 ± 16.6 vs 224.7 ± 6.7 ng/mL; P = 0.001). MIF levels were elevated but not significantly increased in DM2 (P = 0.098). Interestingly, we found that Gremlin‐1 plasma levels were significantly higher in diabetic patients with stable angina pectoris (SAP; n = 53) or acute coronary syndrome (ACS; n = 35) compared with nondiabetic patients with SAP (P = 0.008 and P = 0.011, respectively). MIF levels were significantly higher in diabetic patients with ACS compared with SAP (P < 0.001). Although the single plasma parameters showed an association with DM2 and CAD status, we could not confirm that the Gremlin‐1/MIF ratio is significantly different in patients stratified by DM2 and CAD (P = 0.072). Hence, Gremlin‐1/MIF ratio was significantly lower in patients with ACS compared with SAP (1.1 ± 0.1 vs 4.4 ± 1.1; P = 0.003).
Conclusions
Diabetic patients with ACS show increased levels of Gremlin‐1 and MIF, leading to unfavorable Gremlin‐1/MIF ratios. However, DM2 alone is not associated with low Gremlin‐1/MIF ratios.
Introduction
Atherosclerosis is characterized by endothelial dysfunction and vascular inflammation.1, 2, 3 Sustained inflammation and cholesterol accumulation result in monocyte migration and platelet activation, as well as alterations of inflammatory mediators such as C‐reactive protein (CRP), pro‐inflammatory cytokines, adhesion molecules, and metalloproteinases.4 They play a crucial role in upholding a pro‐inflammatory and prothrombotic milieu, leading to plaque progression and instability with subsequent atherothrombotic events.5, 6, 7, 8
Especially in subjects at risk for atherosclerosis, such as patients with type 2 diabetes mellitus (DM2), inflammatory components seem to play an important role besides metabolic influences and platelet activation. These patients are prone to adverse cardiovascular events during their course of the disease.9, 10 In this context, DM2 is associated with chronic inflammatory reactions characterized by an increase of cytokines and acute‐phase proteins. Hyperglycemia induces the expression of inflammatory cytokines and leads to their activation.11 Furthermore, higher levels of CRP and interleukin‐6 correlate with an increased risk for DM2.
The chemokine‐like cytokine macrophage migration inhibitory factor (MIF) is a component of the stress response and the host antimicrobial alarm system that promotes pro‐inflammatory functions of immune cells. MIF is involved in pathomechanisms of sepsis and inflammatory and autoimmune diseases, and it plays an important role in atherosclerosis.12, 13, 14 MIF binds to chemokine receptors and regulates monocyte recruitment toward atherosclerotic lesions.15 If MIF‐mediated signaling is inhibited, the number of macrophages in atherosclerotic plaques is significantly reduced, suggesting a slower atheroprogression.16, 17 In particular, signs of plaque instability in progressive atherosclerosis have been associated with increased MIF levels.18 In our previous work, we found that MIF levels were significantly elevated in patients with acute coronary syndrome (ACS). MIF correlated with the degree of inflammation and myocardial necrosis markers. Patients with signs of plaque thrombosis showed significantly higher MIF levels.19 Hence, MIF is a potential biomarker to predict the severity and the risk of ACS and plaque stability.20
Furthermore, we could demonstrate that Gremlin‐1 is an endogenous antagonist of MIF that binds MIF with high affinity and leads to a decrease of MIF‐induced foam‐cell formation in vitro.16 Administration of a recombinant fusion protein mGremlin‐1‐Fc reduced the content of macrophages in atherosclerotic plaques and limited atheroprogression in apolipoprotein E (ApoE)−/− mice in vivo.21
As we observed these regulatory functions of Gremlin‐1 and MIF in atherosclerosis, we hypothesized that plasma levels of Gremlin‐1 and MIF in the peripheral blood might also be associated with the stability of coronary artery disease (CAD) and with an increased risk for acute cardiovascular events in patients with DM2.
Methods
Study Population and Data Acquisition
The investigation was designed as a pilot study of 286 consecutive, unselected patients with symptomatic CAD undergoing percutaneous coronary intervention (PCI). We evaluated the association of Gremlin‐1 and MIF plasma levels with a higher risk for progressive CAD in a single‐center registry at the University Hospital Tübingen.
All patients, who were naïve to clopidogrel, received a 600‐mg loading dose before undergoing PCI. Patients already on chronic clopidogrel therapy were treated with an additional 300‐mg loading dose prior to PCI. A standard maintenance dose of 75 mg/d clopidogrel and aspirin 100 mg/d was prescribed for all patients after PCI. All patients underwent coronary stent implantation (bare‐metal or drug‐eluting stents). Acute coronary syndrome was diagnosed in the presence of one of the following criteria: unstable angina (clinical symptoms and new electrocardiographic changes, but no markers of myocardial necrosis) and acute myocardial infarction (MI) with elevated markers of myocardial necrosis (troponin I), including ST‐elevation myocardial infarction (STEMI) and non–ST‐elevation myocardial infarction (NSTEMI). Inclusion criteria comprised written informed consent and patient age >18 years. Patients with increased risk for bleeding, indication for oral anticoagulation, or patients receiving glycoprotein IIb/IIIa inhibitor treatment ≤5 days prior to analysis of Gremlin‐1 and MIF plasma levels were excluded from the study. Type 2 diabetes mellitus was defined according to current diagnostic criteria of the American Diabetes Association.22 The study protocol was approved by the local ethics committee.
Blood Samples and Biochemical Measurements
Peripheral venous blood samples were obtained in EDTA collection tubes at the time of PCI, centrifuged at room temperature (1500g) for 15 minutes, and the supernatants were stored as EDTA‐plasma at −80°C, until MIF and Gremlin‐1 levels and inflammatory markers were analyzed. To assess MIF and Gremlin‐1 concentrations in the obtained plasma samples, we used antihuman MIF and antihuman Gremlin‐1 enzyme‐linked immunosorbent assay (R&D Systems, Inc., Minneapolis, MN). The assay was performed according to the instruction booklet provided by R&D Systems, Inc. Samples were tested for CRP using an immunoturbidimetric assay (ADVIA 1800 chemistry analyzer; Siemens Medical Solutions, Germany).
Statistical Analysis
A Shapiro‐Wilk test was used to assess for normality of continuous variables. Normally distributed continuous data are expressed as mean ± SD or SEM where applicable. For these variables, means between 2 categories/groups were compared with a 2‐tailed unpaired t test for dichotomous analysis. A χ2 test was performed to evaluate the distribution of categorical data. Categorical data are presented as proportions or scores. For the comparison of ≥2 groups, analysis of variance (ANOVA) was performed. All analyses were 2‐sided, and a P value ≤0.05 was considered statistically significant. Statistical analysis was performed with SPSS version 21.0 for Windows (IBM Corp., Armonk, NY).
Results
Study Cohort and Association of Diabetes With Demographic Factors
Baseline demographics of the study cohort (N = 286) are shown in Table 1 and are stratified by DM2 status (n = 88 diabetic and n = 198 nondiabetic patients). Fifty‐three (31.9%) patients presented with DM2 within the group of patients with SAP (n = 166), whereas 35 (29.2%) diabetic patients were among the ACS group (n = 120).
Table 1.
Baseline Characteristics of the Study Population
| Characteristics | Total, N = 286 | Nondiabetics, N = 198 | Diabetics, N = 88 | P Value |
|---|---|---|---|---|
| Age, y | 68.6 ± 10.9 | 66.6 ± 14.1 | 70.7 ± 9.5 | <0.001 |
| Sex | ||||
| M | 202 (70.6) | 138 (69.7) | 64 (72.7) | 0.60 |
| F | 84 (29.4) | 60 (30.3) | 24 (27.3) | |
| CV risk factors | ||||
| Arterial hypertension | 221 (77.3) | 140 (70.7) | 81 (92.0) | <0.001 |
| Hyperlipidemia | 168 (58.7) | 104 (52.5) | 64 (72.7) | 0.001 |
| Renal insufficiency | 75 (26.2) | 41 (20.7) | 34 (38.6) | 0.001 |
| Tobacco use | 105 (36.7) | 71 (35.9) | 34 (38.6) | 0.65 |
| Family history | 55 (19.2) | 44 (22.2) | 11 (12.5) | 0.54 |
| History | ||||
| ACS | 166 (58.0) | 113 (57.1) | 53 (60.2) | 0.62 |
| SAP | 120 (42.0) | 85 (42.9) | 35 (39.8) | 0.62 |
| CAD type | ||||
| 1 vessel | 62 (21.7) | 51 (25.8) | 11 (12.5) | 0.02 |
| 2 vessels | 94 (32.9) | 57 (28.8) | 37 (42.0) | |
| 3 vessels | 130 (45.5) | 90 (45.5) | 40 (45.5) | |
| Previous MI | 87 (30.4) | 57 (28.8) | 30 (34.1) | 0.37 |
| Medications | ||||
| ACEIs | 177 (61.9) | 121 (61.1) | 56 (63.6) | 0.69 |
| ARBs | 36 (12.6) | 21 (10.6) | 15 (17.0) | 0.13 |
| β‐Blockers | 214 (74.8) | 145 (73.2) | 69 (78.4) | 0.35 |
| CCBs | 48 (16.8) | 25 (12.6) | 23 (26.1) | 0.005 |
| Diuretics | 153 (53.5) | 101 (51.0) | 52 (59.1) | 0.21 |
| Statins | 140 (49.0) | 93 (47.0) | 47 (53.4) | 0.32 |
| Insulin | 28 (9.8) | N/A | 28 (31.8) | <0.0001 |
| Clopidogrel | 87 (30.4) | 59 (29.8) | 28 (31.8) | 0.73 |
| ASA | 189 (66.1) | 126 (63.6) | 63 (71.6) | 0.19 |
| Laboratory parameters | ||||
| GFRa, mL/min/1.73 m2 | 36.7 ± 17.7 | 40.7 ± 19.7 | 32.7 ± 15.7 | 0.010 |
| CRP, mg/L | 2.48 ± 3.8 | 2.06 ± 3.3 | 2.9 ± 4.2 | 0.008 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; ASA, aspirin; CAD, coronary artery disease; CCB, calcium channel blocker; CRP, C‐reactive protein; CV, cardiovascular; F, female; GFR, glomerular filtration rate; M, male; MI, myocardial infarction; N/A, not applicable; SAP, stable angina pectoris; SD, standard deviation.
Values are given as n (%) or mean ± SD.
Calculated according to Modification of Diet in Renal Disease equation.
Patients with DM2 suffered more often from arterial hypertension, hyperlipidemia, and renal insufficiency (Table 1). Diabetic patients were more often treated with aspirin, calcium channel blockers, and insulin. They showed a significantly reduced glomerular filtration rate compared with nondiabetic patients (32.7 ± 15.7 mL/min/1.73 m2 in nondiabetic patients vs 40.7 ± 19.7 mL/min/1.73 m2 in diabetic patients; P = 0.010). Patients with DM2 showed significantly higher levels of CRP (2.85 ± 0.46 mg/dL in diabetic patients vs 2.05 ± 0.24 mg/dL in nondiabetic patients; P = 0.008; Table 1).
Gremlin‐1 and MIF Plasma Levels Are Increased in Diabetic Patients
Plasma levels of Gremlin‐1 were significantly higher in DM2 patients (278.8 ± 16.6 ng/mL in diabetic patients vs 224.7 ± 6.7 ng/mL in nondiabetic patients; P = 0.001). MIF levels were increased in the plasma of diabetic patients (2.8 ± 0.2 ng/mL in diabetic patients vs 2.4 ± 0.1 ng/mL in nondiabetic patients; P = 0.098; Figure 1, Table 2), but the difference between the 2 patient groups was not significant.
Figure 1.
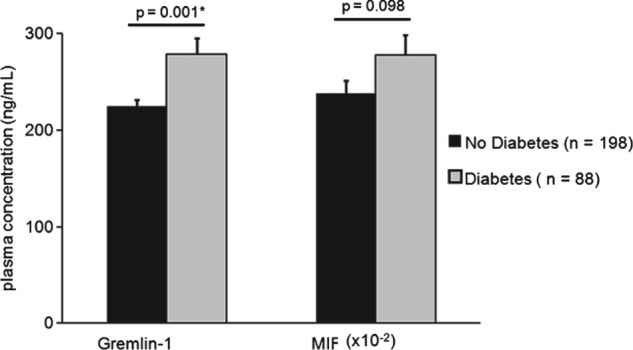
Plasma levels of Gremlin‐1 and MIF and Gremlin‐1/MIF plasma ratio in diabetic and nondiabetic patients. The t test was performed for comparison of the 2 groups. Values are presented as mean ± SEM. A P value ≤0.05 was considered significant. Abbreviations: MIF, macrophage migration inhibitory factor; SEM, standard error of the mean.
Table 2.
Plasma Levels of Gremlin‐1 and MIF Stratified by CAD and DM2
| Plasma Marker | CAD | P Value | DM2 | P Value | ||
|---|---|---|---|---|---|---|
| SAP, n = 166 | ACS, n = 120 | Negative, n = 198 | Positive, n = 88 | |||
| Gremlin‐1 | 245.3 ± 9.8 | 236.0 ± 10.0 | 0.517 | 224.7 ± 6.7 | 278.8 ± 16.6 | 0.001 |
| MIF | 2.1 ± 0.1 | 3.1 ± 0.2 | <0.001 | 2.4 ± 0.1 | 2.8 ± 0.2 | 0.098 |
| Gremlin‐1/MIF ratio | 4.4 ± 1.1 | 1.1 ± 0.1 | 0.003 | 3.2 ± 0.9 | 2.6 ± 0.5 | 0.70 |
Abbreviations: ACS, acute coronary syndrome; CAD, coronary artery disease; DM2, type 2 diabetes mellitus; MIF, macrophage migration inhibitory factor; SAP, stable angina pectoris; SEM, standard error of the mean.
Values are given as mean ± SEM.
Gremlin‐1 and MIF Levels Are Associated With Acute Coronary Syndrome and Gremlin‐1/MIF Plasma Ratios Are Lower in Diabetic Patients With Acute Coronary Syndrome Compared With Stable Angina Pectoris
We could show recently that the Gremlin‐1/MIF ratio was independently associated with the occurrence of ACS, whereas the single parameters MIF and Gremlin‐1 both were not associated with the presence of ACS.20 Furthermore, the Gremlin‐1/MIF ratio was associated with angiographic signs of intracoronary thrombi and severity of thrombus burden.20
In our present study on patients with DM2, we found that the Gremlin‐1/MIF plasma ratio was lower in diabetic patients than in nondiabetic patients, but this difference was not significant (2.6 ± 0.5 in diabetic patients vs 3.2 ± 0.9 in nondiabetic patients; P = 0.7; Figure 2, Table 2).
Figure 2.
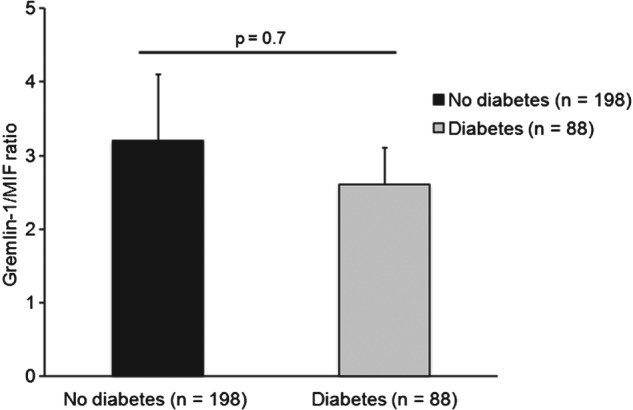
Gremlin‐1/MIF plasma ratios for patients with and without DM2. The t test was performed for comparison of the 2 groups. Values are presented as mean ± SEM. A P value ≤0.05 was considered significant. Abbreviations: DM2, type 2 diabetes mellitus; MIF, macrophage migration inhibitory factor; SEM, standard error of the mean.
For further evaluation in the ANOVA analysis, we stratified the patient groups further by DM2 and CAD status (Figure 3A–C).
Figure 3.
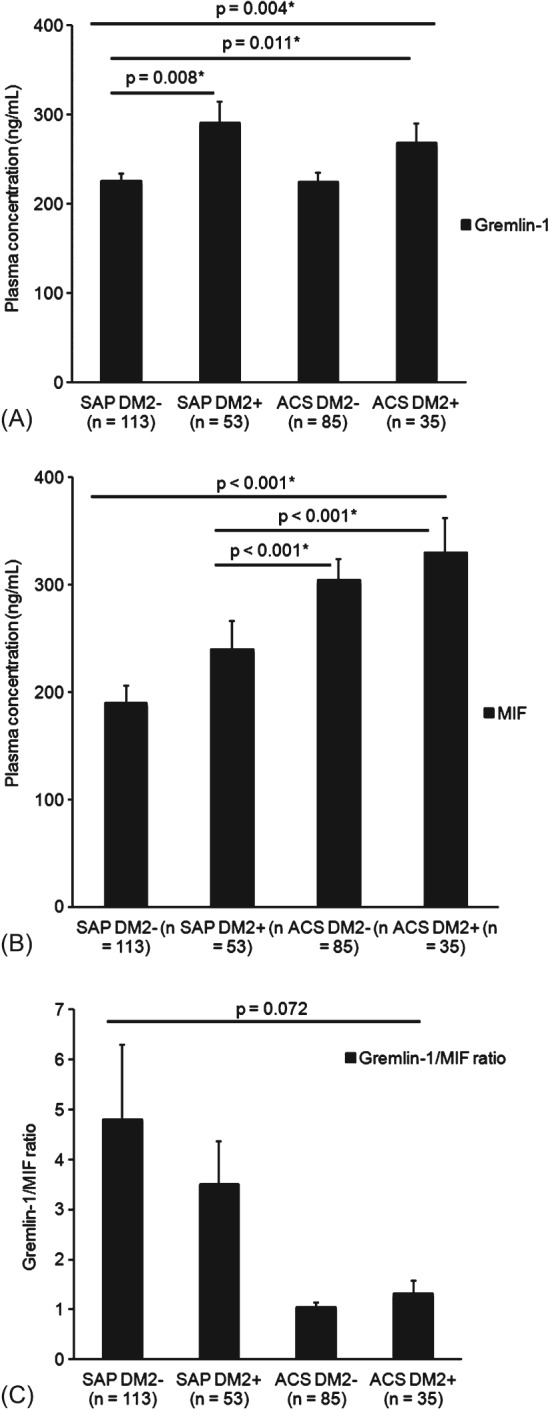
Plasma levels of Gremlin‐1 and MIF and Gremlin‐1/MIF plasma ratio in diabetic and nondiabetic patients stratified by CAD. (A) Gremlin‐1 plasma levels are significantly higher in diabetic patients with SAP and ACS compared with nondiabetic patients with SAP (P = 0.008 and P = 0.011, respectively). (B) MIF levels were significantly higher in diabetic patients with ACS compared with SAP (P < 0.001). (C) Diabetic patients with ACS (n = 35) showed a lower Gremlin‐1/MIF plasma ratio than diabetic patients with stable CAD (n = 53; P = 0.072), but these findings were not statistically significant. For comparison of the 4 groups, ANOVA analysis was performed; patients were stratified by DM2 and CAD status. Values are presented as mean ± SEM. A P value ≤0.05 was considered significant. Abbreviations: ACS, acute coronary syndrome; ANOVA, analysis of variance; CAD, coronary artery disease; DM2, type 2 diabetes mellitus; MIF, macrophage migration inhibitory factor; SAP, stable angina pectoris; SEM, standard error of the mean.
Interestingly, we found that Gremlin‐1 plasma levels were significantly higher in diabetic patients with SAP (n = 53) or ACS (n = 35) compared with nondiabetic patients with SAP (P = 0.008 and P = 0.011, respectively; Figure 3A). Hence, MIF levels were significantly higher in diabetic patients with ACS compared with SAP (P < 0.001; Figure 3B).
Although the single plasma parameters showed an association with DM2 and CAD status, we could not confirm that the Gremlin‐1/MIF ratio is significantly different in patients stratified by DM2 and CAD. Hence, diabetic patients with ACS (n = 35) showed a lower Gremlin‐1/MIF plasma ratio than diabetic patients with stable CAD (n = 53; P = 0.072; Figure 3C), but this was not statistically significant.
Table 2 illustrates that when only stratified by CAD status, MIF levels are significantly higher in patients with ACS compared with SAP (3.1 ± 0.2 vs 2.1 ± 0.1; P < 0.001). As described previously, the Gremlin‐1/MIF ratio is significantly lower in patients with ACS compared with SAP (1.1 ± 0.1 vs 4.4 ± 1.1; P = 0.003), but not when additionally stratified by DM2 status.
Discussion
Patients with DM2 show a significantly elevated inflammatory response, which is of clinical importance, as a linkage of pro‐inflammatory conditions and adverse cardiovascular outcome has been described previously.9, 10, 23 MIF is a proatherosclerotic and pro‐inflammatory factor that induces foam‐cell formation, atheroprogression, and plaque instability.20 Unstable or vulnerable plaques are characterized by a high inflammatory activity reflected by a high content of inflammatory cells such as monocytes and macrophages.24 Gremlin‐1 is expressed in monocytes and macrophages.5 Recently, we could demonstrate that Gremlin‐1 blocks MIF‐induced foam‐cell formation and substantially reduces plaque size and plaque foam‐cell content in ApoE−/− mice in vivo.16, 21
As Gremlin‐1 inhibits the proatherosclerotic effects of MIF, a favorable ratio of Gremlin‐1 and MIF with relatively higher amounts of Gremlin‐1 in the peripheral blood might support a slower progression of atherosclerosis, as well as the reduction of acute cardiovascular events, due to a possible plaque stabilization and inhibition of plaque growth. We could show recently that the Gremlin‐1/MIF ratio was independently associated with the occurrence of ACS, whereas the single parameters MIF and Gremlin‐1 both were not associated with the presence of ACS.20 Furthermore, the Gremlin‐1/MIF ratio was associated with angiographic signs of intracoronary thrombi and severity of thrombus burden.20
In the present study on patients with DM2, we found that total plasma amounts of Gremlin‐1 are significantly higher in diabetic patients than in nondiabetic patients. MIF levels were increased in patients with DM2 but not significantly different from nondiabetic patients with CAD. Although not significant, the Gremlin‐1/MIF ratio was lower in diabetic patients compared with nondiabetic patients, indicating an unfavorable proportion of Gremlin‐1 and MIF in these patients. Comparing diabetic patients with ACS to diabetic patients with stable CAD/SAP, we found that Gremlin‐1 and MIF levels were significantly increased in patients with DM2 and ACS, underscoring the previously described interactions of Gremlin‐1 and MIF.20
Although the single plasma parameters showed an association with DM2 and CAD status, we could not confirm that the Gremlin‐1/MIF ratio was significantly different in patients stratified by DM2 and CAD, even though we could observe that diabetic patients with ACS presented with the lowest Gremlin‐1/MIF plasma ratios.
However, Gremlin‐1/MIF ratios were significantly lower in patients with ACS compared with SAP (1.1 ± 0.1 vs 4.4 ± 1.1; P = 0.003) when not further stratified by DM2 status.
Our findings suggest that Gremlin‐1/MIF ratio might serve as a new additional marker for the risk assessment in patients with CAD and ACS, but its impact in diabetic patients regarding the progression of CAD and the occurrence of acute cardiovascular events remains unclear. As patients with DM2 are at risk for developing adverse cardiovascular events such as MI or stroke, an additional evaluation of the Gremlin‐1/MIF ratio in their plasma might be helpful for the individual risk management in the clinical routine, along with consequent treatment of concomitant cardiovascular risk factors such as arterial hypertension and dyslipidemia,10, 11, 25 but larger randomized studies are needed to further clarify the relevance of the Gremlin‐1/MIF ratio in DM2.
Study Limitations
We are aware that our findings are rather observational and only hypothesis generating, as no mechanistic experiments have been undertaken to prove our hypothesis so far. Furthermore, it is a cross‐sectional study and we did not perform repeated measurements to investigate the stability and time course of MIF and Gremlin‐1 plasma concentrations. Second, we investigated neither other markers of plaque instability nor the prognostic impact of MIF and Gremlin‐1‐levels. Thus, multimodal strategies are necessary to address the question of whether anti‐inflammatory and plaque‐stabilizing approaches may improve the prognosis of patients with diabetes and symptomatic CAD or ACS undergoing PCI.
Conclusion
The present data underscore the necessity to further characterize the role of MIF and Gremlin‐1 in an intensified risk assessment and its prognostic impact in diabetic patients with symptomatic CAD undergoing PCI.
Acknowledgments
The authors thank L. Laptev, D. Lombardi, and A. Hoffmann for the excellent support in data collection.
K.M. performed experiments, researched and analyzed data, wrote the manuscript, and outlined the project. M.C. performed experiments. D.R. researched data and reviewed the manuscript. M.S. collected and researched clinical data. H.S. performed the enzyme‐linked immunosorbent assays. M.G. reviewed and edited the manuscript. T.G. analyzed data, reviewed and edited the manuscript, and contributed to the discussion. I.M. wrote the manuscript, analyzed data, and outlined the project.
This study was supported by the Klinische Forschergruppe “Platelets–Molecular Mechanisms and Translational Implications” (KFO 274), restricted grants (DFG, Iris Müller, MU 2928/2‐1; University of Tübingen, Iris Müller, Fortüne), and the TÜFF Frauenförderungsprogramm (Karin Müller, 2241‐0‐0) of the University Tübingen.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138(5 part 2):S419–S420. [DOI] [PubMed] [Google Scholar]
- 3. Hansson GK, Robertson AK, Söderberg‐Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. [DOI] [PubMed] [Google Scholar]
- 4. Geisler T, Bhatt DL. The role of inflammation in atherothrombosis: current and future strategies of medical treatment. Med Sci Monit. 2004;10:RA308–RA316. [PubMed] [Google Scholar]
- 5. Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. May AE, Kälsch T, Massberg S, et al. Engagement of glycoprotein IIb/IIIa (α(IIb)β3) on platelets upregulates CD40L and triggers CD40L‐dependent matrix degradation by endothelial cells. Circulation. 2002;106:2111–2117. [DOI] [PubMed] [Google Scholar]
- 7. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. [DOI] [PubMed] [Google Scholar]
- 8. Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. [DOI] [PubMed] [Google Scholar]
- 9. Tadic M, Cuspidi C. The influence of type 2 diabetes on left atrial remodeling. Clin Cardiol. 2015;38:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quinones A, Lobach I, Maduro GA Jr, et al. Diabetes and ischemic heart disease death in people age 25–54: a multiple‐cause‐of‐death analysis based on over 400 000 deaths from 1990 to 2008 in New York City. Clin Cardiol. 2015;38:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6:1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roger T, David J, Glauser MP, et al. MIF regulates innate immune responses through modulation of toll‐like receptor 4. Nature. 2001;414:920–924. [DOI] [PubMed] [Google Scholar]
- 13. Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lue H, Kleemann R, Calandra T, et al. Macrophage migration inhibitory factor (MIF): mechanisms of action and role in disease. Microbes Infect. 2002;4:449–460. [DOI] [PubMed] [Google Scholar]
- 15. Bernhagen J, Krohn R, Lue H, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–596. [DOI] [PubMed] [Google Scholar]
- 16. Müller II, Chatterjee M, Schneider M, et al. Gremlin‐1 inhibits macrophage migration inhibitory factor–dependent monocyte function and survival. Int J Cardiol. 2014;176:923–929. [DOI] [PubMed] [Google Scholar]
- 17. Weber C, Kraemer S, Drechsler M, et al. Structural determinants of MIF functions in CXCR2‐mediated inflammatory and atherogenic leukocyte recruitment. Proc Natl Acad Sci U S A. 2008;105:16278–16283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmeisser A, Marquetant R, Illmer T, et al. The expression of macrophage migration inhibitory factor 1α (MIF 1α) in human atherosclerotic plaques is induced by different proatherogenic stimuli and associated with plaque instability. Atherosclerosis. 2005;178:83–94. [DOI] [PubMed] [Google Scholar]
- 19. Müller II, Müller KA, Schonleber H, et al. Macrophage migration inhibitory factor is enhanced in acute coronary syndromes and is associated with the inflammatory response. PloS One. 2012;7:e38376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Müller II, Müller KA, Karathanos A, et al. Impact of counterbalance between macrophage migration inhibitory factor and its inhibitor Gremlin‐1 in patients with coronary artery disease. Atherosclerosis. 2014;237:426–432. [DOI] [PubMed] [Google Scholar]
- 21. Müller II, Schonberger T, Schneider M, et al. Gremlin‐1 is an inhibitor of macrophage migration inhibitory factor and attenuates atherosclerotic plaque growth in ApoE−/− mice. J Biol Chem. 2013;288:31635–31645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rydén L, Grant PJ, Anker SD, et al; Authors/Task Force Members . ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on Diabetes, Pre‐diabetes, and Cardiovascular Diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) [published correction appears in Eur Heart J. 2014;35:1824]. Eur Heart J. 2013;34:3035–3087. [DOI] [PubMed] [Google Scholar]
- 23. Müller K, Aichele S, Herkommer M, et al. Impact of inflammatory markers on platelet inhibition and cardiovascular outcome including stent thrombosis in patients with symptomatic coronary artery disease. Atherosclerosis. 2010;213:256–262. [DOI] [PubMed] [Google Scholar]
- 24. Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk‐assessment strategies: part I. Circulation. 2003;108:1664–1672. [DOI] [PubMed] [Google Scholar]
- 25. Snipelisky D, Waldo O, Burton MC. Clinical Diagnosis and management of hypertension compared with the Joint National Committee 8 panelists' recommendations. Clin Cardiol. 2015;38:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]


