ABSTRACT
Ranolazine utilization in the management of refractory angina has been established by multiple randomized clinical studies. However, there is growing evidence showing an evolving role in the field of cardiac arrhythmias. Multiple experimental and clinical studies have evaluated the role of ranolazine in prevention and management of atrial fibrillation, with ongoing studies on its role in ventricular arrhythmias. In this review, we will discuss the pharmacological, experimental, and clinical evidence behind ranolazine use in the management of various cardiac arrhythmias.
Introduction
Ranolazine is a medication approved by the US Food and Drug Administration (FDA) for angina refractory to conventional anti‐ischemic therapy.1 It exerts its action mainly through inhibition of peak and late Na+ currents, as well as rapidly activating delayed‐rectifier K+ current.2 Recently, studies have shown an emerging role for ranolazine in the prevention and management of various atrial and ventricular arrhythmias. In this review, we will discuss the mechanism of action of ranolazine, as well as clinical evidence for its use in various cardiac arrhythmias.
Biology of the Sodium Channels
The cardiac action potential is generated by the movement of different ions across the ion channels creating a change in the transmembrane potentials. Five phases of the cardiac action potential exist in nonpacemaker cells (Figure 1). Phase 0, the depolarization phase, is mainly generated by the influx of Na+ inside the myocardial cells through fast Na+ channels. This transient rise in the intracellular Na+ in turn leads to influx of Ca2+ via L‐type voltage‐gated channels with subsequent release of Ca2+ from the sarcoplasmic reticulum required for the initiation of myocardial contractility.3
Figure 1.
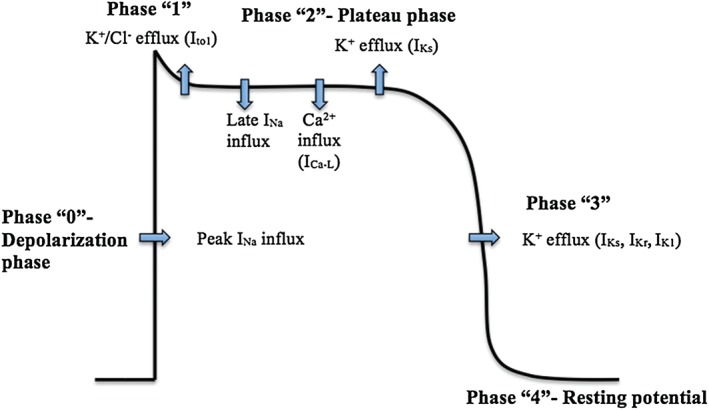
Phases of cardiac action potential in nonpacemaker cells. Abbreviations: ICa‐L, L‐type Ca2+ channels; IK1, inwardly rectifier K+ channels; IKr, rapid delayed rectifier K+ channels; IKs, slow delayed rectifier K+ channels; INa, sodium current; Ito1, cardiac transient outward potassium current.
The cardiac voltage‐gated Na+ channel consists of 4 domains; each is formed of 6 spanning segments (S1–S6). The S4 segment represents the voltage sensor, and the loop between S5 and S6 in each domain forms the pore of the channel (P loop).4 The activity of Na+ channels has 3 modes: transient mode, burst mode, and late‐scattered mode.5 The transient mode is responsible for the peak Na+ current (INa) during phase 0 and lasts for about 1 ms.5, 6 After the peak INa, Na+ channels quickly become inactivated, resulting in the burst and late‐scattered modes responsible for a sustained current component that lasts up to 100 ms during the plateau phase of action potential and is referred to as “late INa” (Figure 1).7 The amplitude of late INa represents 0.1% to 1% of that of peak INa; however, because of the relatively longer duration of plateau phase 2 compared with phase 0, the net late Na+ influx is comparable with the peak Na+ influx.8, 9
Role of Late INa Current in Atrial and Ventricular Arrhythmias
Enhanced late INa in atrial myocytes has been shown to lower the threshold of action potential firing, initiate diastolic depolarization, and increase excitability, and hence the risk of atrial arrhythmias, mainly atrial fibrillation (AF; Figure 2).7
Figure 2.
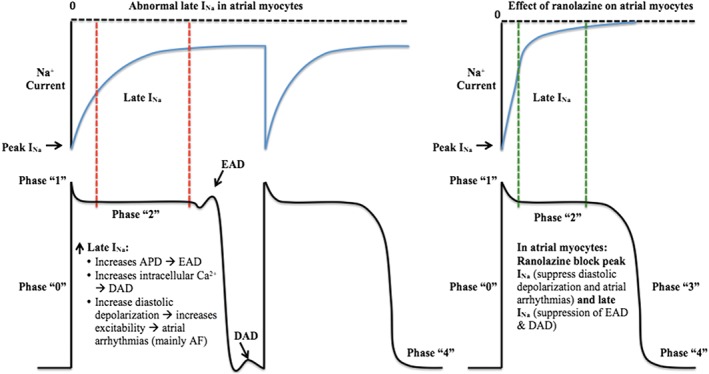
Effect of ranolazine on atrial myocytes. Ranolazine suppresses diastolic depolarization, atrial myocyte excitement, as well as EAD and DAD, and results in decreased risk of atrial arrhythmias such as AF. Abbreviations: AF, atrial fibrillation; APD, action potential duration; DAD, delayed after depolarization; EAD, early after depolarization; late INa, late Na+ current; peak INa, peak Na+ current.
On the other side, several electrophysiological mechanisms result in the initiation of ventricular arrhythmias, including triggered activity (which is either early after depolarization [EAD] or delayed after depolarization [DAD]), abnormal automaticity, and reentry.10 Patients with cardiomyopathies may have increased transmural dispersion of repolarization (TDR), which is considered one of the mechanisms behind torsade de pointes (TdP).8 Increased late INa plays a major role in all of the above mechanisms (Figure 3).
Figure 3.
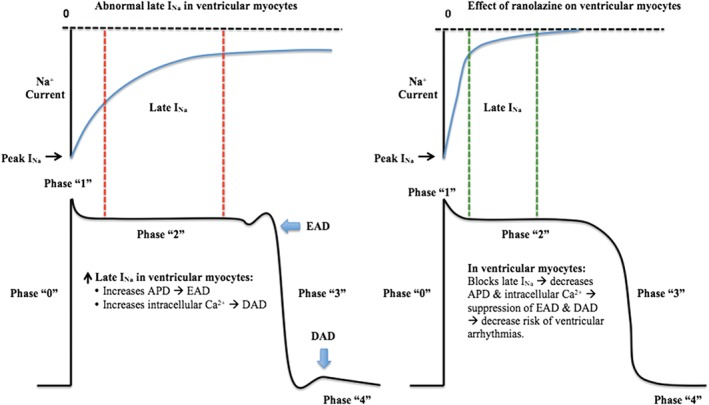
Effect of ranolazine on ventricular myocytes. Ranolazine blocks late INa, causing decrease in APD and intracellular Ca2+ with suppression of EAD and DAD and resulting in decrease risk of ventricular arrhythmias. Abbreviations: AF, atrial fibrillation; APD, action potential duration; DAD, delayed after depolarization; EAD, early after depolarization; late INa, late Na+ current; peak INa, peak Na+ current.
Pharmacology of Ranolazine
Pharmacodynamics
Ranolazine inhibits various cardiac ion channels with different potencies acting mainly on peak and late INa, with some effect on rapidly activating delayed‐rectifier K+ current (IKr) and L‐type Ca2+ (ICa‐L) channels.2, 11
Ranolazine and INa:
The potency of ranolazine to inhibit peak and late INa is tissue‐specific, indicating different effects on atrial vs ventricular Na+ channels.2 Ranolazine inhibits late INa at concentrations that are 30‐fold to 38‐fold lower than that required to inhibit peak INa in ventricular myocytes.9 The same is not true with the atria, as ranolazine has increased potency in inhibiting peak INa, leading to depression of the rate of upstroke of phase 0 of action potential, decreased conduction velocity, as well as enhancing post‐repolarization refractoriness.9 The effects of ranolazine in inhibiting peak and late INa is frequency‐ and voltage‐dependent, being more potent in the settings of tachycardia rather than normal heart rate.9
Ranolazine and Rapidly Activating Delayed‐Rectifier K+ Current:
IKr is an important regulator of the cardiac repolarization. In a normal heart, ranolazine exerts a concentration‐dependent inhibition of IKr with half maximal inhibitory concentration of 11.5 μM, resulting in prolonged TDR and QTc.12, 13, 14, 15 Despite this effect, ranolazine does not induce ventricular arrhythmias or TdP, as this effect is opposed by its potent inhibition of late INa.2 In patients with long QT3 syndrome (LQT3), where the underlying electrophysiological mechanism of ventricular arrhythmias is secondary to increase in late INa, ranolazine was found to suppress ventricular arrhythmias by shortening QTc in a dose‐dependent pattern.16 Ranolazine abbreviates QTc by 22 ms to 40 ms from baseline at plasma concentration of ∼ 4 μM, with more decrease in QTc duration by 24 ms with every ∼ 2 μM increase in plasma concentration.16 This effect of ranolazine was also seen in patients with LQT1 and LQT2.17
Ranolazine and L‐Type Calcium Channel:
The inhibitory effect of ranolazine on ICa‐L is minimal at concentrations ≤10 μM and mainly affects late rather than peak ICa‐L, thus ranolazine is a weak direct vasodilator and has minimal direct effect on atrioventricular nodal conduction.2, 17 Thus, with current FDA‐approved doses of ranolazine, the late INa and IKr currents will be predominantly inhibited in the ventricular myocytes, with minimal effect on late ICa‐L, whereas in the atrial myocytes, ranolazine will inhibit peak INa as well as IKr, with less effect on late INa.17
Ranolazine and Cardiac Action Potential:
Ranolazine exerts minimal effect on the resting membrane potential of both atrial and ventricular myocytes due to the lack of effect on inwardly rectifying K+ current (IK1).9, 17 In atrial myocytes, ranolazine causes significant depression of the action potential amplitude as well as the action potential upstroke (Vmax), whereas it requires higher concentrations to exert the same effect in ventricular myocytes.9
Late INa and IKr have opposite effects on action potential duration (APD) in ventricular myocytes, where late INa causes its prolongation, whereas IKr shortens it. As ranolazine inhibits both currents, the net effect of ranolazine on APD depends on the cell type, as well as the degree of contribution of these currents in repolarization at any given time.2 Late INa is increased in M cells and Purkinje fibers compared with epicardial cells, causing relatively prolonged APD in the former. By inhibiting this increased late INa, ranolazine causes concentration‐dependent shortening of APD in M cells and Purkinje fibers. On the other side, ranolazine causes prolonged APD in epicardial cells. This unique action of prolonging APD in epicardial cells but abbreviating it in M cells and Purkinje fibers helps in its protective effect against ventricular arrhythmias through reducing TDR.2, 17
Pharmacokinetics
Ranolazine is mainly excreted through the kidneys. It undergoes extensive metabolism with cytochrome P (CYP)450 prior to excretion, mainly through the CYP3A4 enzyme with a minor role for the CYP2D6 enzyme. Both ketoconazole and diltiazem increase ranolazine plasma concentrations by inhibiting the CYP3A4 enzyme, whereas paroxetine, which is a selective serotonin reuptake inhibitor, increases its concentrations by inhibiting the CYP2D6 enzyme.18
Simvastatin has a minor inhibitory effect to CYP3A4 enzyme; thus, it can be safely administered with ranolazine 1000 mg twice daily. Some cases of rhabdomyolysis were reported after adding ranolazine to atorvastatin therapy through impaired clearance of atorvastatin by sharing CYP450 biotransformation pathway.19, 20 Pravastatin and rosuvastatin are mainly excreted unchanged21; thus, ranolazine is less likely to affect their plasma concentrations. Verapamil inhibits gut P‐glycoprotein, which increases ranolazine average plasma concentrations at steady state. Ranolazine was reported to increase digoxin concentrations by inhibiting P‐glycoprotein at the level of the gut and the renal tubules.18
Ranolazine in the Management of Ischemic Heart Disease
In contrast to all anti‐ischemic medications used in treatment of angina, ranolazine acts through blocking the late INa, thus decreasing the intracellular Na+ and Ca2+ load, resulting in a decrease in myocardial stiffness and contractility and improving myocardial energetics.6 Ranolazine, registered in the United States as Ranexa since 2006, is currently approved in patients with chronic stable angina for symptomatic relief if initial therapy with β‐blockers is unsatisfactory or contraindicated as recommended by current guidelines (class IIA).1 Thus, if ischemia is the cause of arrhythmia, an anti‐ischemic medication may prevent or diminish the arrhythmia.
Ranolazine in the Management of Atrial Fibrillation
Experimental Evidence: Animal Models
In an experimental study on canine pulmonary vein sleeve preparations, ranolazine suppressed late phase 3 EAD‐ and DAD‐mediated triggered activity, indicating a beneficial role of ranolazine in suppressing AF triggers from pulmonary vein sleeves.22 Another study tested ranolazine with dronedarone in treatment of AF in animal models and found that the combined use results in better rhythm control.23 Moreover, ranolazine helped with dronedarone to decrease ischemia‐induced vulnerability to AF as well as ventricular arrhythmias.24
Clinical Evidence: Role of Ranolazine in Primary Prevention of Atrial Fibrillation
Ranolazine in Prevention of Atrial Fibrillation in Patients With Acute Coronary Syndrome:
In a substudy of the Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST‐Segment Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN‐TIMI 36) trial, patients randomized to ranolazine showed a trend toward lower incidence of new‐onset AF at 7 days after randomization (risk reduction [RR]: 0.74, 95% confidence interval [CI]: 0.52‐1.05, P = 0.08) without an increase in the incidence of pro‐arrhythmias.25 At 1‐year follow up, ranolazine showed decrease in AF burden in the paroxysmal AF category (P = 0.015) as well as clinical AF‐related events compared with placebo (RR: 0.71, P = 0.01).26
Ranolazine in Prevention of Postoperative Atrial Fibrillation in Patients Undergoing Cardiac Surgeries:
In a study done by Tagarakis et al,27 102 patients undergoing on‐pump coronary artery bypass grafting (CABG) were randomized to a moderate dose of ranolazine (375 mg twice daily) for 3 days preoperatively and postoperatively until discharge vs usual care. The incidence of postoperative AF (POAF) was significantly lower in the ranolazine group compared with the control group (8.8% vs 30.8%, respectively; P < 0.001). Miles et al randomized 393 patients planned for CABG to either ranolazine 1500 mg preoperatively followed by 1000 mg twice daily for 10 to 14 days (n = 182) or amiodarone 400 mg preoperatively followed by 200 mg twice daily for 10 to 14 days (n = 211). Ranolazine significantly decreased the incidence of POAF compared with amiodarone (17.5% vs 26.5%, respectively; P = 0.035).28 Similar results were demonstrated on postoperative patients after CABG, valvular, or combined surgeries, with the majority of patients receiving preoperative β‐blockers. Ranolazine led to a significant decrease in the incidence of POAF compared with the control group (10.1% vs 41.9%; odds ratio: 0.16, 95% CI: 0.07‐0.37, P < 0.0001).29 However, there is not enough evidence to support the benefit of combining ranolazine and amiodarone in prevention of POAF.
Role of Ranolazine in Pharmacological Cardioversion of Atrial Fibrillation:
In a prospective randomized pilot study, 51 patients with recent‐onset (<48 hours) AF eligible for pharmacologic cardioversion were randomized to a combined therapy of intravenous (IV) amiodarone plus a single oral dose of ranolazine 1500 mg given at the time of randomization (n = 25) vs IV amiodarone alone (n = 26). Atrial fibrillation cardioversion was achieved in 88% of the patients in the combined amiodarone/ranolazine group, compared with 65% in the amiodarone‐only group (P = 0.056), with a significant shortening of the time to cardioversion (9.8 ± 4.1 vs 14.6 ± 5.3 hours; P = 0.002). In this study, combined amiodarone/ranolazine therapy was independently associated with time to AF conversion (hazard ratio: 0.81, 95% CI: 0.74‐0.88, P < 0.001), suggesting a potential synergistic effect of ranolazine when added to amiodarone in the conversion of AF to sinus rhythm (SR).30 Another study demonstrated that ranolazine shortened the time to cardioversion of POAF after elective on‐pump CABG when combined with amiodarone (19.9 ± 3.2 hours vs 37.2 ± 3.9 hours when amiodarone was used alone; P < 0.001).31
In another study, 18 patients with either new (n = 11) or paroxysmal (n = 7) AF were given a single oral dose of ranolazine 2000 mg for cardioversion. Seventeen patients had underlying structural heart disease. Ranolazine resulted in a 72% conversion rate to SR within 6 hours of administration, with absence of pro‐arrhythmias or significant side effects. The authors of this study considered high‐dose ranolazine a novel “pill in the pocket” medication for conversion of AF in patients with structural heart disease.32 In a case series of 25 patients who failed attempts of electric cardioversion of AF, a single oral dose of ranolazine 2000 mg, followed by another electric cardioversion attempt after 3.5 to 4 hours, facilitated the restoration of SR in 19 (76%) of the 25 patients.33
A Study to Evaluate the Effect of Ranolazine and Dronedarone When Given Alone and in Combination in Patients With Paroxysmal Atrial Fibrillation (HARMONY Trial),34 a randomized, double‐blind, placebo‐controlled, parallel‐arm study, included 134 patients with paroxysmal AF. Patients were given either ranolazine 750 mg twice daily alone, dronedarone 225 mg twice daily alone, ranolazine 750 mg twice daily plus dronedarone 225 mg twice daily, ranolazine 750 mg twice daily plus dronedarone 150 mg twice daily, or placebo. At 12 weeks' follow‐up, patients on the ranolazine 750 mg/dronedarone 150 mg combination, and those on the ranolazine 750 mg/dronedarone 225 mg combination, showed significant reductions of 45% and 59%, respectively, in the AF burden from baseline vs placebo (P = 0.072 and P = 0.008, respectively). Neither ranolazine nor dronedarone alone significantly reduced the AF burden.34
Ranolazine in the Maintenance of Sinus Rhythm After Atrial Fibrillation Direct Current Cardioversion:
The Ranolazine in Atrial Fibrillation Following an Electrical Cardioversion (RAFFAELLO) study was a prospective, multicenter, randomized, double‐blind trial that evaluated the safety and efficacy of ranolazine in maintaining SR after successful electrical cardioversion. Two hundred forty‐one patients with persistent AF (7 days to 6 months) were randomized to either placebo or ranolazine, with doses ranging from 375 to 750 mg twice daily, 2 hours after successful electrical cardioversion. The study showed that AF recurred in 56.4% in the placebo group compared with 56.9% in the ranolazine 375‐mg group, 41.7% in the ranolazine 500‐mg group, and 39.7% in the ranolazine 750‐mg group. However, none of the doses used in that study significantly prolonged the time to first AF recurrence.35 Table 1 summarizes the clinical evidence for the role of ranolazine in AF.
Table 1.
Clinical Evidence for the Role of Ranolazine in AF
| Study | Design and aim | Methods | Results |
|---|---|---|---|
| Scirica et al25, 26 | A substudy of the MERLIN‐TIMI 36 randomized controlled trial | 6560 patients with NSTE‐ACS received ranolazine vs placebo in addition to the standard therapy. | Ranolazine showed a trend toward lower incidence of new‐onset AF at 7 d after randomization (RR: 0.74, 95% CI: 0.52‐1.05, P = 0.08). |
| Role of ranolazine in the prevention of arrhythmias in post–NSTE‐ACS patients | Holter ECG monitoring for the first 7 d after randomization | No increase in the incidence of pro‐arrhythmia | |
| At 1‐year follow‐up, ranolazine showed decrease in AF burden in the paroxysmal AF category (P = 0.015) as well as clinical AF‐related events compared with placebo (RR: 0.71, P = 0.01). | |||
| Tagarakis et al27 | Prospective, randomized, single‐blind, clinical trial | 102 patients received either ranolazine 375 mg bid for 3 d prior to surgery and until discharge, or usual care. | Ranolazine significantly lowered the incidence of POAF compared with the control group (8.8% vs 30.8%; P < 0.001). |
| Role of ranolazine in the prevention of POAF after on‐pump CABG surgery | Patients were monitored for the development of POAF. | ||
| Miles et al28 | Retrospective cohort study | 393 patients undergoing CABG received ranolazine 1500 mg preoperatively followed by 1000 mg bid for 10 to 14 d vs amiodarone 400 mg preoperatively followed by 200 mg bid for 10 to 14 d | Ranolazine significantly decreased the incidence of POAF compared with amiodarone (17.5% vs 26.5%; P = 0.035). |
| Ranolazine vs amiodarone for the prevention of POAF after CABG | |||
| Hammond et al29 | A single‐center, retrospective cohort study: | 205 patients received ranolazine 1000 mg preoperatively, then 1000 mg bid for 7 d or until discharge vs standard therapy only. | Ranolazine led to significant decrease in the incidence of POAF compared with control group (10.1% vs 41.9%; OR: 0.157, 95% CI: 0.067‐0.367, P < 0.0001). |
| Role of ranolazine in prevention of POAF after CABG, valvular, or combined surgeries | After propensity‐score matched‐pair analysis and conditional logistic regression, ranolazine was an independent predictor of preventing POAF (P < 0.0001). | ||
| Fragakis et al30 | Prospective randomized pilot study | 51 patients with recent‐onset (<48 h) AF eligible for pharmacologic cardioversion were randomized to a combined therapy of IV amiodarone plus single oral dose of ranolazine 1500 mg given at the time of randomization vs IV amiodarone alone. | Combined amiodarone/ranolazine therapy resulted in increased success of AF cardioversion (88% vs 65% in amiodarone‐only group; P = 0.056); and significant shortening of time to cardioversion (9.8 ± 4.1 vs 14.6 ± 5.3 h in amiodarone‐only group; P = 0.002). |
| Safety and efficacy of ranolazine plus amiodarone vs amiodarone alone for the conversion of recent‐onset AF | |||
| Simopoulos et al31 | Prospective, randomized, single‐blind, single‐site clinical trial | 41 patients were randomized to receive either ranolazine 375 mg bid orally plus IV amiodarone or IV amiodarone alone | Ranolazine shortened time to cardioversion of POAF when combined with amiodarone (19.9 ± 3.2 vs 37.2 ± 3.9 hours in amiodarone only group, P < 0.001). |
| Role of adding ranolazine to amiodarone in shortening time to conversion of POAF after CABG surgery | |||
| Murdock et al32 | Prospective cohort study | 18 patients with either new AF (n = 11) or paroxysmal AF (n = 7) received 2000 mg of ranolazine. 17 patients had underlying structural heart disease. | Ranolazine resulted in 72% conversion rate to SR within 6 h of administration. |
| Safety and efficacy of ranolazine as “pill in the pocket” therapy for cardioversion of new or paroxysmal AF | No pro‐arrhythmias or significant side effects reported. | ||
| Murdock et al33 | 3‐year retrospective analysis | 25 patients with persistent or permanent AF who failed either elective or emergent electric cardioversion and had been administered a single oral dose of 2000 mg ranolazine followed by another electric cardioversion attempt after 3.5 to 4 h | Ranolazine facilitated the restoration of SR in 19 of the 25 patients (76%). |
| Role of ranolazine in facilitating restoration of SR in patients with failed electric cardioversion. | 5 out of the 6 patients who were refractory to repeat electric cardioversion remained in permanent AF. | ||
| No adverse effects were reported. | |||
| Kowey et al34 (HARMONY trial) | Randomized, double‐blind, placebo‐controlled, parallel‐arm study | 134 patients with paroxysmal AF randomized into 5 groups to receive either ranolazine 750 mg bid alone, dronedarone 225 mg bid alone, ranolazine 750 mg bid plus dronedarone 225 mg bid, ranolazine 750 mg bid plus dronedarone 150 mg bid, or placebo. | The ranolazine 750 mg/dronedarone 150 mg combination decreased AF burden by 45% compared with placebo (P = 0.072). |
| Role of combination of ranolazine and dronedarone in reducing the burden of AF in patients with paroxysmal AF | 12‐wk follow‐up of the AF burden | The ranolazine 750 mg/dronedarone 225 mg combination decreased AF burden by 59% compared with placebo (P = 0.008). | |
| Neither ranolazine nor dronedarone alone significantly reduced the AF burden | |||
| De Ferrari et al35 (RAFFAELLO trial) | Prospective, multicenter, randomized, double‐blind, placebo‐control, parallel‐group phase 2 dose‐ranging trial | 241 patients with persistent AF were randomized to either placebo or ranolazine, with doses ranging from 375 to 750 mg bid, 2 h after successful electrical cardioversion. | AF recurred in 56.4%, 56.9%, 41.7%, and 39.7% in the placebo group, ranolazine 375‐mg group, ranolazine 500‐mg group, and ranolazine 750‐mg group, respectively. |
| Safety and efficacy of ranolazine in the prevention of AF recurrence after successful electrical cardioversion | No dose of ranolazine significantly prolonged time to AF recurrence; however, reduction in overall AF recurrence in combined 500‐mg and 750‐mg groups showed borderline significance compared with placebo (P = 0.053) and was significant compared with the 375‐mg group (P = 0.035). |
Abbreviations: AF, atrial fibrillation; bid, twice daily; CABG, coronary artery bypass grafting; CI, confidence interval; ECG, electrocardiogram; HARMONY, A Study to Evaluate the Effect of Ranolazine and Dronedarone When Given Alone and in Combination in Patients With Paroxysmal Atrial Fibrillation; IV, intravenous; MERLIN‐TIMI 36, Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST‐Segment Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36; NSTE‐ACS, non–ST‐segment elevation acute coronary syndrome; OR, odds ratio; POAF, postoperative atrial fibrillation; RAFFAELLO, Ranolazine in Atrial Fibrillation Following an Electrical Cardioversion; RR, risk reduction; SR, sinus rhythm.
Ranolazine in the Management of Ventricular Arrhythmias
Most of the evidence behind the beneficial role of ranolazine in the treatment of ventricular arrhythmias is mainly derived from experimental studies, with fewer clinical studies on humans.
Experimental Evidence
In the ventricular myocytes of guinea pigs, enhanced late INa by sea anemone toxin ATX‐II was counteracted by ranolazine (61% ± 8% reduction). Ranolazine also reduced the 13.6‐fold increase in APD variability by 89%.36 In an experimental study of its effect on ventricular vulnerability, ranolazine caused significant increase in the threshold of ventricular fibrillation and repetitive extrasystole, as well as reduction in TDR.37
In a head‐to‐head study, ranolazine was found to be as effective as sotalol and lidocaine in the prevention of ischemia/reperfusion induced arrhythmias in anesthetized rats (P = 0.01 in sotalol group vs control, P = 0.10 in lidocaine group vs control, and P = 0.048 in ranolazine group vs control).38 When combined with dronedarone in low doses, ranolazine led to blunting of ischemia‐induced T‐wave heterogeneity and ventricular tachycardia (VT) vulnerability in Yorkshire pigs.24 Furthermore, ranolazine was found to suppress TdP in various long‐QT syndromes.17
Clinical Evidence
Few clinical studies evaluated the role of ranolazine in ventricular arrhythmias. In 5 patients with LQT3, ranolazine succeeded to significantly shorten the QTc by 26 ± 3 ms (P < 0.0001).16 In another prospective cohort study done on 12 patients with drug‐refractory implantable cardioverter‐defibrillator (ICD) shocks, ranolazine reduced VT burden and ICD shocks significantly in 11 out of the 12 patients without significant increase in QTc interval or QRS duration.39
In a substudy of the MERLIN‐TIMI 36 trial, Holter electrocardiographic monitoring showed that the patients who received IV ranolazine in addition to the standard therapy showed significant reduction in VT lasting ≥8 beats compared with placebo at 24 hours (2.3% vs 3.4%; RR: 0.67, 95% CI: 0.50‐0.90, P = 0.008) and 48 hours (3.1% vs 4.7%; RR: 0.65; 95% CI: 0.51‐0.84, P < 0.001) after randomization.25 An ongoing randomized trial, the Ranolazine Implantable Cardioverter‐Defibrillator (RAID) trial, is currently evaluating the efficacy of ranolazine on top of standard therapy in reducing ventricular arrhythmia and death in patients with ICDs.40 Table 2 summarizes the clinical evidence for the role of ranolazine in ventricular arrhythmias.
Table 2.
Clinical Evidence for the Role of Ranolazine in Ventricular Arrhythmias
| Study | Design and aim | Methods | Results |
|---|---|---|---|
| Moss et al16 | Prospective cohort study | 5 patients with hereditary LQT3 syndrome due to SCN5A‐ΔKPQ mutation underwent an 8‐h IV ranolazine infusion (45 mg/h for 3 h followed by 90 mg/h for 5 h). | Ranolazine significantly shortened the QTc by 26 ± 3 ms (P < 0.0001). |
| Effect of IV ranolazine infusion on ventricular repolarization in LQT3 syndrome due to SCN5A‐ΔKPQ mutation | Evaluation of QTc before and during ranolazine infusion. | ||
| Bunch et al39 | Prospective cohort study | 12 patients with AAD‐refractory VT and recurrent ICD shocks were treated with ranolazine with mean follow‐up of 6 ± 6 mo | Ranolazine reduced VT burden and ICD shocks significantly in 11 of the 12 patients. |
| Effect of ranolazine in reducing VT burden and ICD shocks in patients with AAD refractory VT. | No significant increase in QTc interval or QRS duration | ||
| Scirica et al25 | A substudy of the MERLIN‐TIMI 36 randomized controlled trial | 6560 patients with NSTE‐ACS received ranolazine vs placebo in addition to the standard therapy. | Ranolazine significantly reduced the incidence of VT lasting for ≥8 beats compared with placebo (5.3% vs 8.3%; P < 0.001) |
| Role of ranolazine in the prevention of arrhythmias in post NSTE‐ACS patients | Holter ECG monitoring for the first 7 d after randomization |
Abbreviations: AAD, antiarrhythmic drugs; ECG, electrocardiogram; ICD, implantable cardioverter‐defibrillator; IV, intravenous; LQT3, long QT3 syndrome; MERLIN‐TIMI 36, Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST‐Segment Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36;QTc, corrected QT interval; NSTE‐ACS, non–ST‐segment elevation myocardial infarction; VT, ventricular tachycardia.
Conclusions and Future Directions
Ranolazine, although approved as a second‐line antianginal medicine, is not approved for use as an antiarrhythmic but has a potential antiarrhythmic role in the prevention as well as management of atrial and ventricular arrhythmias, with fewer side effects compared with currently available antiarrhythmic medications.
The role of ranolazine in preventing AF or ventricular tachyarrhythmias has not been well established in large‐population trials. The same is true for other endpoints, such as primary prevention of sudden cardiac death. Thus, the conduction of large randomized clinical trials is imperative to accurately establish the safety and efficacy of ranolazine use for such indications, especially in patients with acute coronary syndromes, structural heart diseases such as cardiomyopathies, and postcardiac surgeries.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. [DOI] [PubMed] [Google Scholar]
- 2. Antzelevitch C, Burashnikov A, Sicouri S, et al. Electrophysiologic basis for the antiarrhythmic actions of ranolazine. Heart Rhythm. 2011;8:1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Györke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77:245–255. [DOI] [PubMed] [Google Scholar]
- 4. Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol. 2009;2:185–194. [DOI] [PubMed] [Google Scholar]
- 5. Maltsev VA, Undrovinas AI. A multi‐modal composition of the late Na + current in human ventricular cardiomyocytes. Cardiovasc Res. 2006;69:116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noble D, Noble PJ. Late sodium current in the pathophysiology of cardiovascular disease: consequences of sodium‐calcium overload. Heart. 2006;92(suppl 4):iv1–iv5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Horvath B, Bers DM. The late sodium current in heart failure: pathophysiology and clinical relevance. ESC Heart Fail. 2014;1:26–40. [DOI] [PubMed] [Google Scholar]
- 8. Shryock JC, Song Y, Rajamani S, et al. The arrhythmogenic consequences of increasing late INa in the cardiomyocyte. Cardiovasc Res. 2013;99:600–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta T, Khera S, Kolte D, et al. Antiarrhythmic properties of ranolazine: a review of the current evidence. Int J Cardiol. 2015;187:66–74. [DOI] [PubMed] [Google Scholar]
- 10. Belardinelli L, Giles WR, Rajamani S, et al. Cardiac late Na+ current: proarrhythmic effects, roles in long QT syndromes, and pathological relationship to CaMKII and oxidative stress. Heart Rhythm. 2015;12:440–448. [DOI] [PubMed] [Google Scholar]
- 11. Hasenfuss G, Maier LS. Mechanism of action of the new anti‐ischemia drug ranolazine. Clin Res Cardiol. 2008;97:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stone PH, Gratsiansky NA, Blokhin A, et al. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol. 2006;48:566–575. [DOI] [PubMed] [Google Scholar]
- 13. Wilson SR, Scirica BM, Braunwald E, et al. Efficacy of ranolazine in patients with chronic angina: observations from the randomized, double‐blind, placebo‐controlled MERLIN‐TIMI (Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST‐Segment Elevation Acute Coronary Syndromes) 36 Trial. J Am Coll Cardiol. 2009;53:1510–1516. [DOI] [PubMed] [Google Scholar]
- 14. Kosiborod M, Arnold SV, Spertus JA, et al. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina: results from the TERISA randomized clinical trial (Type 2 Diabetes Evaluation of Ranolazine in Subjects With Chronic Stable Angina). J Am Coll Cardiol. 2013;61:2038–2045. [DOI] [PubMed] [Google Scholar]
- 15. Chaitman BR. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation. 2006;113:2462–2472. [DOI] [PubMed] [Google Scholar]
- 16. Moss AJ, Zareba W, Schwarz KQ, et al. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type‐3 long‐QT syndrome. J Cardiovasc Electrophysiol. 2008;19:1289–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antzelevitch C, Belardinelli L, Zygmunt AC, et al. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. CV Therapeutics . FDA Briefing Document for the Cardiovascular and Renal Drugs Advisory Committee: Ranexa (ranolazine), extended‐release tablets. http://www.fda.gov/ohrms/dockets/ac/03/briefing/4012B2_15_CV‐Therapeutics‐RANEXA.pdf. Published December 9, 2003. Accessed May 30, 2015.
- 19. Correa D, Landau M. Ranolazine‐induced myopathy in a patient on chronic statin therapy. J Clin Neuromuscul Dis. 2013;14:114–116. [DOI] [PubMed] [Google Scholar]
- 20. Ginanneschi F, Volpi N, Giannini F, et al. Rhabdomyolysis in an elderly multitreated patient: multiple drug interactions after statin withdrawal. J Neurol Sci. 2014;336:284–287. [DOI] [PubMed] [Google Scholar]
- 21. Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid‐lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–581. [DOI] [PubMed] [Google Scholar]
- 22. Sicouri S, Glass A, Belardinelli L, et al. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;5:1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Antzelevitch C, Belardinelli L, Burashnikov A, et al. Method of treating atrial fibrillation. US 8513254 B2, August 20, 2013.
- 24. Verrier RL, Pagotto VPF, Kanas AF, et al. Low doses of ranolazine and dronedarone in combination exert potent protection against atrial fibrillation and vulnerability to ventricular arrhythmias during acute myocardial ischemia. Heart Rhythm. 2013;10:121–127. [DOI] [PubMed] [Google Scholar]
- 25. Scirica BM, Morrow DA, Hod H, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non–ST‐segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST‐Segment Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN‐TIMI 36) randomized controlled trial. Circulation. 2007;116:1647–1652. [DOI] [PubMed] [Google Scholar]
- 26. Scirica BM, Belardinelli L, Chaitman BR, et al. Effect of ranolazine on atrial fibrillation in patients with non–ST‐elevation acute coronary syndromes: observations from the MERLIN‐TIMI 36 trial. Europace. 2015;17:32–37. [DOI] [PubMed] [Google Scholar]
- 27. Tagarakis GI, Aidonidis I, Daskalopoulou SS, et al. Effect of ranolazine in preventing postoperative atrial fibrillation in patients undergoing coronary revascularization surgery. Curr Vasc Pharmacol. 2013;11:988–991. [DOI] [PubMed] [Google Scholar]
- 28. Miles RH, Passman R, Murdock DK. Comparison of effectiveness and safety of ranolazine versus amiodarone for preventing atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2011;108:673–676. [DOI] [PubMed] [Google Scholar]
- 29. Hammond DA, Smotherman C, Jankowski CA, et al. Short‐course of ranolazine prevents postoperative atrial fibrillation following coronary artery bypass grafting and valve surgeries. Clin Res Cardiol. 2015;104:410–417. [DOI] [PubMed] [Google Scholar]
- 30. Fragakis N, Koskinas KC, Katritsis DG, et al. Comparison of effectiveness of ranolazine plus amiodarone versus amiodarone alone for conversion of recent‐onset atrial fibrillation. Am J Cardiol. 2012;110:673–677. [DOI] [PubMed] [Google Scholar]
- 31. Simopoulos V, Tagarakis GI, Daskalopoulou SS, et al. Ranolazine enhances the antiarrhythmic activity of amiodarone by accelerating conversion of new‐onset atrial fibrillation after cardiac surgery. Angiology. 2014;65:294–297. [DOI] [PubMed] [Google Scholar]
- 32. Murdock DK, Kersten M, Kaliebe J, et al. The use of oral ranolazine to convert new or paroxysmal atrial fibrillation: a review of experience with implications for possible “pill in the pocket” approach to atrial fibrillation. Indian Pacing Electrophysiol J. 2009;9:260–267. [PMC free article] [PubMed] [Google Scholar]
- 33. Murdock DK, Kaliebe J, Larrain G. The use of ranolazine to facilitate electrical cardioversion in cardioversion‐resistant patients: a case series. Pacing Clin Electrophysiol. 2012;35:302–307. [DOI] [PubMed] [Google Scholar]
- 34. Kowey PR. The effect of the combination of ranolazine and low‐dose dronedarone on atrial fibrillation burden in patients with paroxysmal atrial fibrillation (HARMONY trial). Heart Rhythm Society 2014 Scientific Sessions; May 10, 2014; San Francisco, CA. Abstract LB03‐05.
- 35. De Ferrari GM, Maier LS, Mont L, et al; RAFFAELLO Investigators . Ranolazine in the treatment of atrial fibrillation: Results of the dose‐ranging RAFFAELLO (Ranolazine in Atrial Fibrillation Following an Electrical Cardioversion) study. Heart Rhythm. 2015;12:872–878. [DOI] [PubMed] [Google Scholar]
- 36. Song Y, Shryock JC, Wu L, et al. Antagonism by ranolazine of the pro‐arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2004;44:192–199. [DOI] [PubMed] [Google Scholar]
- 37. Kumar K, Nearing BD, Bartoli CR, et al. Effect of ranolazine on ventricular vulnerability and defibrillation threshold in the intact porcine heart. J Cardiovasc Electrophysiol. 2008;19:1073–1079. [DOI] [PubMed] [Google Scholar]
- 38. Kloner RA, Dow JS, Bhandari A. First direct comparison of the late sodium current blocker ranolazine to established antiarrhythmic agents in an ischemia/reperfusion model. J Cardiovasc Pharmacol Ther. 2011;16:192–196. [DOI] [PubMed] [Google Scholar]
- 39. Bunch TJ, Mahapatra S, Murdock D, et al. Ranolazine reduces ventricular tachycardia burden and ICD shocks in patients with drug‐refractory ICD shocks. Pacing Clin Electrophysiol. 2011;34:1600–1606. [DOI] [PubMed] [Google Scholar]
- 40. US National Institutes of Health, ClinicalTrials.gov . Ranolazine Implantable Cardioverter‐Defibrillator Trial (RAID). http://clinicaltrials.gov/show/NCT01215253. Accessed May 30, 2015.


