ABSTRACT
Several studies have investigated the role of physical fitness in atrial fibrillation (AF), but the results remain controversial. We aimed to estimate the association between physical fitness and risk of AF. We comprehensively retrieved data from the Cochrane Library, PubMed, and Embase databases until February 29, 2016, for studies evaluating the association of physical fitness with the risk of AF. Data were abstracted from included studies, and effect estimates were pooled using a random‐effects model. Six studies with a total of 205 094 participants and 15 919 AF cases fulfilled the inclusion criteria. When physical fitness was assessed as a continuous variable, per incremental increase of physical fitness was associated with a 9% reduced risk of AF (risk ratio [RR]: 0.91, 95% confidence interval [CI]: 0.84‐1.00, P = 0.05). When physical fitness was assessed as a categorical variable, the risk of AF was significantly reduced (RR: 0.51, 95% CI: 0.28‐0.91, P = 0.02) in individuals with the highest level of physical fitness compared with those with the lowest level. The intermediate vs the lowest level of physical fitness was associated with a 28% reduced risk of AF (RR: 0.72, 95% CI: 0.56‐0.93, P = 0.01). The sensitivity analysis indicated that these results were stable. Notably, there was evidence of statistical heterogeneity across studies; therefore, we should interpret the results cautiously. In conclusion, published literature supports that a higher level of physical fitness is associated with a lower risk of AF. Further studies should be performed to confirm these findings.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, affecting approximately 33.5 million individuals worldwide.1 Atrial fibrillation could increase the risk of morbidity and mortality, as well as clinical and public health expenses. Thus, the management and prevention of AF are vitally important and mainly include anticoagulation therapy, rhythm control, and rate control. In addition, comprehensive risk‐factor modification, such as the management of modifiable cardiac risk factors, obstructive sleep apnea, obesity, and exercise, may also help prevent and treat AF.2, 3, 4 The 2008 Physical Activity Guidelines Advisory Committee Report recommends 150 min/wk of moderate‐intensity aerobic exercise or 75 min/wk of vigorous‐intensity aerobic exercise for the general population.5 Guidelines from the American College of Cardiology and American Heart Association also include specific recommendations for diverse populations of cardiac patients, such as those with congenital heart disease, heart failure (HF), acute coronary syndrome, and stable ischemic heart disease.6, 7, 8, 9, 10 However, the effect of exercise on the risk of AF is still unclear.
To explore this effect, both physical activity and fitness should be examined, because they have different definitions11 and may be different physiological markers of cardiovascular diseases.12 Although several previous meta‐analyses have indicated the inconsistent effects of physical activity on AF among different populations13, 14, 15 and have demonstrated an inverse association of physical fitness with all‐cause mortality, coronary heart disease, and HF,16, 17 little is known regarding the link between physical fitness and AF risk. Recently, epidemiological studies have explored the association between physical fitness and the risk of AF; however, they yielded conflicting findings. Specifically, higher exercise capacity estimated by the ergometer bicycle test seems to be associated with an increased risk of AF.18 In contrast, 5 previous studies have reported an inverse association between physical fitness and the risk of AF,19, 20, 21, 22, 23 and 2 of the 5 showed no statistically significant association.21, 22 To date, the corresponding quantitative association has not been established; therefore, we conducted a meta‐analysis to estimate the role of physical fitness on the risk of AF.
Methods
Inclusion and Exclusion Criteria
We included studies that (1) investigated the association of physical fitness with the risk of incident or recurrent AF, especially studies combining AF and atrial flutter as an outcome; (2) included population‐based or hospital‐based participants; (3) had a cohort study design; (4) used physical fitness as the exposure; (5) assessed physical fitness using an exercise tolerance test and expressed the maximal exercise capacity in units of metabolic equivalents (METs) or other measures; and (6) reported the risk ratios (RRs) and their corresponding 95% confidence intervals (CIs), or provided sufficient data to calculate them. Studies were excluded if (1) they reported individuals with postoperative AF; (2) the exposure of physical fitness was combined with another exposure; or (3) the studies were of certain publication types (eg, letters, case reports, and comments).
Literature‐Search Strategy
We comprehensively reviewed the Cochrane Library, PubMed, and Embase databases through February 29, 2016, for relevant studies evaluating the association between physical fitness and the risk of AF. We did not apply any language restrictions. Two groups of keywords were combined; the first keywords were linked to the exposure (“physical fitness” or “oxygen consumption” or “cardiorespiratory fitness” or “exercise test” or “exercise tolerance” or “exercise”). The second keywords were linked to the outcome (“atrial fibrillation” or “atrial flutter” or “atrial tachycardia” or “supraventricular tachycardia” or “arrhythmia”). Further searches were performed using reference lists, relevant journals, and conference abstracts.
Data Abstraction and Quality Assessment
Two reviewers (WZ and YS) independently abstracted data from the eligible studies and conducted the quality assessment. Discrepancies were resolved through discussion or consultation with a third reviewer (KH). We abstracted the following data from each study: name of the first author, year of publication, country of origin, study design, participants (sample size, sex, and age), follow‐up duration, method of physical‐fitness assessment, ascertainment of AF, adjusted covariates, and the RR values with 95% CIs. When several RRs were presented in 1 study, such as unadjusted and adjusted RRs, we abstracted the most completely adjusted RR. To judge the quality of the included studies, we used the validated Newcastle‐Ottawa Scale (NOS) items, involving the selection of cohorts, the comparability of cohorts, and the assessment of outcome.24 We defined studies with a NOS of ≥6 stars as moderate‐ to high‐quality studies and studies with a NOS of <6 stars as low‐quality studies.
Statistical Analysis
When physical fitness was assessed as a continuous variable, we performed a dose‐response analysis to examine how much risk reduction could be predicted per incremental increase of physical fitness. When physical fitness was assessed as a categorical variable, we compared the corresponding RR values between these groups as follows: (1) the highest vs the lowest category, and (2) the intermediate vs the lowest category. This method was based on a previous meta‐analysis evaluating the association between physical fitness and cancer mortality.21 We defined the intermediate category as the median of all categories. In addition, the sensitivity analysis was performed where appropriate.
The effect measures were transformed to their natural logarithm (log RR), and the standard error (SE) was calculated from the corresponding CI. Statistical heterogeneity was calculated by a consistency test using the Cochrane Q test complemented with the I 2 statistic.25 In this meta‐analysis, given that the populations were variable across studies, we chose a random‐effects model because it was better than the fixed‐effects model for explaining between‐study heterogeneity. All statistical analyses were performed using Review Manager version 5.3 software (the Nordic Cochrane Center, the Cochrane Collaboration, Copenhagen, Denmark). All tests were 2‐sided, and statistical significance was defined as P < 0.05.
Results
Study Selection
We initially identified 9633 studies through electronic searches (Figure 1). We found no additional studies via searching reference lists, relevant journals, and conference abstracts. After we screened the titles and abstracts, 1261 relevant studies remained. Then we screened the full text and excluded 1248 studies for not directly involving physical fitness and AF. The 13 remaining studies were reviewed in more detail, and we further excluded 7 studies with due to type of publication (1 letter, 1 review, and 5 comments). Finally, 6 studies18, 19, 20, 21, 22, 23 comprising 205 094 participants and 15 919 AF cases were included in the meta‐analysis (1 post‐hoc analysis of a randomized controlled trial,22 4 prospective cohort studies,18, 19, 20, 21, 22, 23 and 1 retrospective cohort study20).
Figure 1.
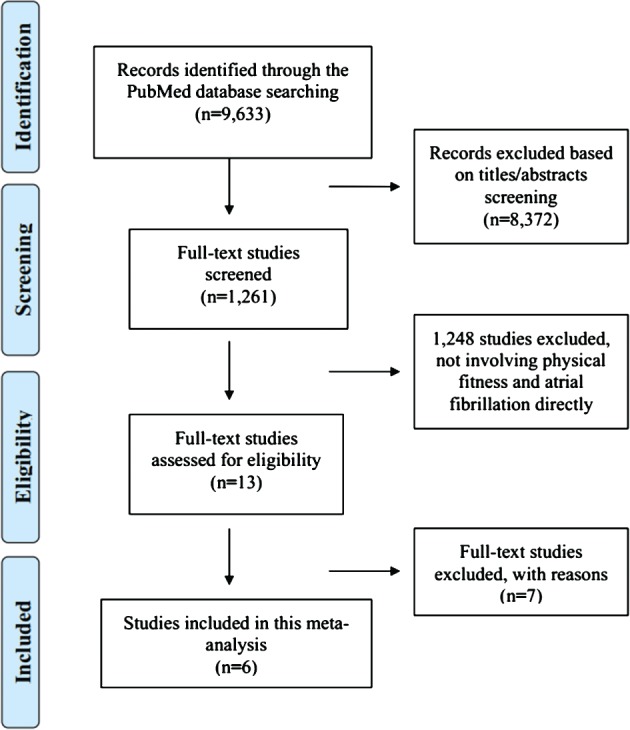
Flow diagram of literature search and study selection for the meta‐analysis.
Maximal exercise capacity was expressed in METs19, 20, 21, 22, 23 or watts (Table 1).18 Two studies assessed physical fitness using the cycle ergometer, both of which carried out a maximal exercise test.18, 21 Two studies assessed physical fitness using a maximal treadmill exercise test according to the standard Bruce protocol.19, 20 In the study of Alonso et al,22 a graded exercise treadmill test was applied, including a maximal test administered at the baseline and a submaximal test administered at years 1 and 4. In addition, the details of the exercise tolerance test were unclear in 1 study.23 All studies had a quality score ≥6 stars (Table 1).
Table 1.
Basic Characteristics of Studies Included in the Meta‐Analysis
| Study, by First Author and Year | Region | Design | Sex, Age | Participants (Sample Size, n) | New‐Onset or Recurrent AF (n) | Follow‐up Duration | Categorization of Physical Fitness | Method for Physical Fitness Assessment | Ascertainment of AF | Adjusted Covariates | NOS Items |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Andersen 201518 | Sweden | Prospective cohort | Males, median 18.2 years | Population‐based (1 126 899) | New‐onset (9668) | Median 23.6 years | 5 fitness categories (measured in watts): first, second, third, fourth, and fifth | Ergometer | AF hospitalization: ICD‐10 (I48.9), ICD‐9 (427D), ICD‐8 (427.92) | Age, conscription date, region, education level, height, muscle strength, SBP and DBP, weight, and ischemic heart disease | 8 stars |
| Pathak 201519 | Australia | Prospective cohort | Both, mean 60 years | Hospital‐based (308) | Recurrent (198) | Mean 2.0 years | 3 fitness categories (baseline): low (<85%), adequate (86% to 100%), and high (>100%) | Treadmill | ECG, Holter monitoring, and clinical review | Weight loss, baseline cardiorespiratory fitness | 8 stars |
| Qureshi 201520 | United States | Retrospective cohort | Both, mean 54.5 years | Hospital‐based (64 561) | New‐onset (4616) | Median 5.4 years | 4 fitness categories: <6 METs, 6 to 9 METs, 10 to 11 METs, and >11 METs | Treadmill | AF hospitalization: ICD‐9 427.31 | Age, sex, race, history of HTN, DM, sedentary habits, obesity, family history of CHD, smoking, hyperlipidemia, hypertensive response, chronotropic incompetence, thyroid medications, digoxin, treated lung disease, β‐blocker use, ACEI use, ARB use, CCB use, and all lipid‐lowering medications including statins | 8 stars |
| Khan 201521 | Finland | Prospective cohort | Males, mean 52.6 years | Population‐based (1950) | New‐onset (305) | Mean 19.5 years | 4 fitness categories: 20.3, 27.7, 32.6, and 40.6 mL/kg/min | Ergometer | Hospital discharge diagnoses, inpatient physician claims data, and study ECG | Age, SBP, BMI, history of CVD, history of DM, and resting heart rate, smoking status, HTN, fasting glucose, and LDL‐C | 9 stars |
| Alonso 201522 | United States | Post‐hoc analysis of RCT | Both, 45–76 years | Hospital‐based (5067) | New‐onset (294) | Mean 9 years | 5 fitness categories (% improved fitness): −52.9 to −6.9, −6.8 to 0.0, 0.1 to 13.5, 13.6 to 31.7, and 31.8 to 185.7 | Treadmill | ECG and hospitalization discharge diagnosis: ICD‐9 427.31/9 427.32 | Clinic, age, sex, race, intervention group, education, family income, smoking, BMI, height, SBP, DBP, use of antihypertensive medication, HbA1c, prevalent CHD, and prevalent HF | 8 stars |
| Pittaras 201523 | United States | Prospective cohort | Unclear | Population‐based (6309) | New‐onset (838) | Median 8.0 years | 4 fitness categories: least fit (4.9 ± 1.13 METs), low fit (6.7 ± 1.0 METs), moderate fit (7.9 ± 1.0 METs), and high fit (9.3 ± 1.2 METs) | Unclear | Unclear | Uncleara | 7 stars |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; CCB, calcium channel blocker; CHD, coronary heart disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; DM, diabetes mellitus; ECG, electrocardiogram; HbA1c, glycated hemoglobin; HF, heart failure; HTN, hypertension; ICD, International Classification of Diseases; LDL‐C, low‐density lipoprotein cholesterol; METs, metabolic equivalents of oxygen consumption; NOS, Newcastle‐Ottawa Scale; PAD, peripheral arterial disease; RCT, randomized controlled trial; SBP, systolic blood pressure.
Multivariable Cox models were performed, but the adjustments could not be reached.
Meta‐Analyses
When physical fitness was assessed as a continuous variable, the analysis comprised a total of 5 risk estimates. According to the dose‐response analysis, per incremental increase of physical fitness was associated with a 9% reduction in the risk of AF (RR: 0.91, 95% CI: 0.84‐1.00, P = 0.05; Figure 2). When physical fitness was assessed as a categorical variable, the analysis comprised a total of 6 risk estimates. There were 4 physical fitness categories in 3 studies20, 21, 23; therefore, we used the pooled RRs of the second and third categories as the intermediate category. As shown in Figure 3, the risk of AF was significantly reduced (RR: 0.51, 95% CI: 0.28‐0.91, P = 0.02) in individuals with the highest level of physical fitness compared with those with the lowest level. The intermediate vs the lowest level of physical fitness was associated with a 28% reduced risk of AF (RR: 0.72, 95% CI: 0.56‐0.93, P = 0.01). The sensitivity analysis indicated that these results were stable after we removed the included RR values one at a time. Notably, there was evidence of statistical heterogeneity across studies; therefore, the results should be interpreted cautiously.
Figure 2.
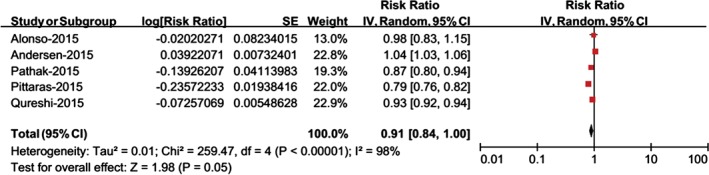
Meta‐analysis of the risk of AF per incremental increase of physical fitness. Abbreviations: AF, atrial fibrillation; CI, confidence interval; df, degrees of freedom; IV, inverse of the variance; SE, standard error.
Figure 3.
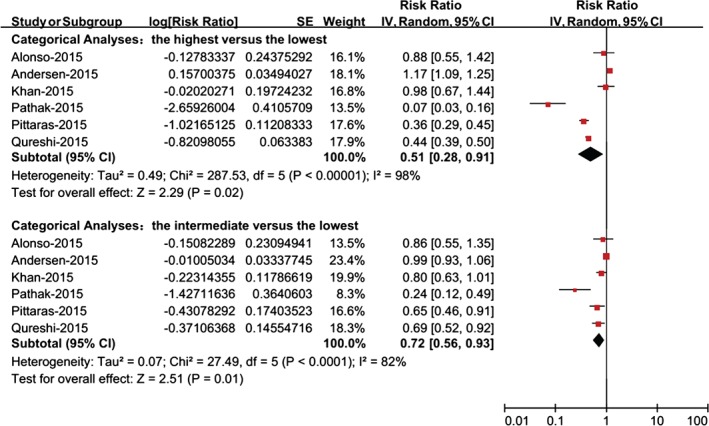
Meta‐analysis of the risk of AF for individuals with the highest vs the lowest physical fitness or the intermediate vs the lowest physical fitness. Abbreviations: AF, atrial fibrillation; CI, confidence interval; df, degrees of freedom; IV, inverse of the variance; SE, standard error.
Specifically, Pathak et al19 divided obese individuals with symptomatic AF into low (n = 95), adequate (n = 134), and high (n = 79) cardiorespiratory fitness groups based on their baseline exercise performance. At an average of a 4‐year follow‐up, the rate of AF recurrence was 88%, 65%, and 34% in the low, adequate, and high fitness groups, respectively. Each increase in METs of baseline cardiorespiratory fitness was associated with a 13% reduced risk of AF recurrence (RR: 0.87, 95% CI: 0.80‐0.94, P < 0.001). Therefore, baseline fitness could predict the risk of AF recurrence. In addition, increasing fitness via the study intervention was also associated with a reduced risk of AF recurrence. For every increase in METs, there was a 9% reduction in the risk of AF recurrence (RR: 0.90, 95% CI: 0.83‐1.00, P = 0.036). The rate of AF recurrence was 82% in the <2 METs gain group but only 39% in the >2 METs gain group.
Discussion
To the best of our knowledge, our meta‐analysis is the first to quantify the association between physical fitness and the risk of AF. According to the dose‐response analysis, per incremental increase of physical fitness was associated with a 9% reduced risk of AF. In the categorical analysis, when compared with the low level of physical fitness, both the intermediate and higher levels of physical fitness were associated with a lower risk of AF. The sensitivity analysis indicated that these results were stable. Specifically, a higher level of physical fitness was also associated with lower risk of AF recurrence.
Previously, several meta‐analytic studies have quantified the association between physical activity and the risk of AF.13, 14, 15 Long‐term vigorous physical training is associated with an increased risk of AF in athletes,13, 15 but this association does not exist in nonathletes.13, 14 Interestingly, there is a sex difference in the aforementioned association; in brief, an increased level of physical activity is most likely associated with an increased risk of AF in men and a reduction of AF incidence in women.26 These inconsistent results may be due to the imprecision of physical activity assessment via self‐reported rather than direct measurement. Although physical‐activity habits are considered the main determinant of physical fitness,27 we should not equate physical fitness with habitual physical activity. Physical activity and fitness may be different physiological markers of cardiovascular diseases,12 and physical fitness can be readily assessed by an exercise tolerance test. Higher level of physical fitness have been previously shown to reduce the risk of all‐cause mortality, coronary heart disease, and HF,16, 17 and our current meta‐analysis also indicated an inverse association between physical fitness and the risk of AF.
Because AF has multiple cardiovascular and noncardiovascular risk factors, the reduction in the risk of AF at a higher level of physical fitness may be partly mediated through the improvement of preexisting risk factors. A previous study showed that higher physical fitness is associated with a lower level of inflammatory markers (eg, C‐reactive protein).28, 29 Thus, it is likely that the reduction in the risk of AF at a higher level of physical fitness is partly mediated through reducing the incidence of inflammation. Overweight and obesity are also the important risk factors for AF30, 31 and may induce AF by increasing left atrial size and volume.32, 33 Whether the inverse association between physical fitness and AF is mediated through the regulation of body mass index (BMI, a measure of obesity) is not well established. In a cohort of healthy middle‐age men, a BMI >28 kg/m2 and weight gain >10 kg were associated with an increased risk of incident AF, but increasing fitness via the study intervention could attenuate this association.34 In the Henry Ford Exercise Testing (FIT) Project,20 there was an inverse association between cardiorespiratory fitness and the risk of AF, and the magnitude of this association was greater in obese individuals than in nonobese individuals. However, Khan and colleagues21 indicated that BMI did not play an important role in determining the association between cardiorespiratory fitness and the risk of AF. Overall, to examine the effect modification of the association between physical fitness and AF, further meta‐analysis should be conducted by strata of BMI and other possible confounders of AF, such as smoking, diabetes mellitus, and cardiovascular disease status at baseline.
Recently, clinicians have recommended their patients perform moderate‐intensity exercise training for significant cardiovascular improvements.35, 36 Although the etiologies of AF are not fully understood, multiple modifiable and nonmodifiable risk factors for AF have been discovered. There is growing evidence that potential lifestyle modifications, such as the management of exercise, may help prevent and treat AF.4 The Aggressive Risk Factor Reduction Study for Atrial Fibrillation and Implications for the Outcome of Ablation (ARREST‐AF) cohort study demonstrated that aggressive risk‐factor modifications, mainly including the management of blood pressure and glycemic control, weight management, and obstructive sleep apnea, could improve the long‐term success of AF ablation.37 Regular exercise for ≥20 min/d in conjunction with other lifestyle modifications could reduce the risk of AF by 50%.3 However, no study has evaluated the effects of physical‐fitness improvement, in conjunction with other risk modifications, on the prevention and treatment of AF. From a clinical viewpoint, the observations of our study may be valuable, because the predictive ability of AF risk could be improved when we comprehensively combined physical fitness with other risk‐factor modifications.
Notably, physical activity has a U‐shaped relationship with the risk of AF in both athletes and nonathletes,38, 39, 40 and a nonlinear relationship is observed between physical fitness and the risk of AF.21 Andersen and co‐workers18 investigated 1.1 million Swedish young men at a median follow‐up of 26.3 years and found that exercise capacity had a U‐shaped relationship with the risk of arrhythmia, driven by a direct association with risk of AF. Importantly, this study examined the association between exercise capacity and risk of arrhythmia in the young participants, which would minimize the risk of reverse causation caused by preexisting cardiac diseases. Therefore, it would not be surprising if there were adverse effects of physical fitness on AF in people with sustained exercise training. Before the upper limit of benefit is well established, we should not overinterpret the results and intensify the exercise prescription for our patients.
Study Limitations
Our study has several potential limitations. First, the populations were variable across studies, and the pooled RRs would be expected to represent a weighted average of association between physical fitness and the risk of AF. Although the random‐effects model could explain between‐study heterogeneity to a certain degree, we should interpret the results carefully and further confirm the causal association. Second, the physical‐fitness categories were variable across studies, and we may have misclassified them in our analysis. Third, although most studies additionally adjusted for potential risk factors of AF, it was possible that some unknown or residual confounding was not fully ruled out. Fourth, the methods of AF ascertainment were highly variable, and we did not differentiate the subtypes of AF. Additionally, a number of individuals with undiagnosed AF as well as those with asymptomatic paroxysmal AF may be missed. Finally, we did not determine the magnitude of the association between physical fitness and AF modulated by age, sex, ethnicity, study type, or other study characteristics.
Conclusion
Overall, a higher level of physical fitness was associated with a lower risk of AF. We suggest that physical fitness could be useful for predicting the risk of AF. Further study should confirm the causal association between physical fitness and the risk of AF.
WZ and YS are co–first authors. KH was in charge of the entire project and revised the draft; WZ and YS performed the systematic literature review, constructed the database, and analyzed the data. WZ and YS drafted the first version of the manuscript with the help of QZ, ZX, LH, and QC. All authors took part in the interpretation of the results and prepared the final version.
This work was supported by the National Natural Science Foundation of China (8153000545, 81530013, 81370288), National Basic Research Program of China (973 Program: 2013CB531103).
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller JD, Aronis KN, Chrispin J, et al. Obesity, exercise, obstructive sleep apnea, and modifiable atherosclerotic cardiovascular disease risk factors in atrial fibrillation. J Am Coll Cardiol. 2015;66:2899–2906. [DOI] [PubMed] [Google Scholar]
- 3. Larsson SC, Drca N, Jensen‐Urstad M, et al. Combined impact of healthy lifestyle factors on risk of atrial fibrillation: prospective study in men and women. Int J Cardiol. 2015;203:46–49. [DOI] [PubMed] [Google Scholar]
- 4. Menezes AR, Lavie CJ, De Schutter A, et al. Lifestyle modification in the prevention and treatment of atrial fibrillation. Prog Cardiovasc Dis. 2015;58:117–125. [DOI] [PubMed] [Google Scholar]
- 5. US Department of Health and Human Services . Physical Activity Guidelines Advisory Committee Report. http://health.gov/paguidelines/Report/pdf/CommitteeReport.pdf. Washington, DC: US Department of Health and Human Services; 2008. [Google Scholar]
- 6. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society [published correction appears in J Am Coll Cardiol. 2014;64:2305–2307]. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 7. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2014;64:2713–2714]. J Am Coll Cardiol. 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 8. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 9. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. [DOI] [PubMed] [Google Scholar]
- 10. Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143–e263. [DOI] [PubMed] [Google Scholar]
- 11. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health‐related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 12. Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta‐analysis. Med Sci Sports Exerc. 2001;33:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kwok CS, Anderson SG, Myint PK, et al. Physical activity and incidence of atrial fibrillation: a systematic review and meta‐analysis. Int J Cardiol. 2014;177:467–476. [DOI] [PubMed] [Google Scholar]
- 14. Ofman P, Khawaja O, Rahilly‐Tierney CR, et al. Regular physical activity and risk of atrial fibrillation: a systematic review and meta‐analysis. Circ Arrhythm Electrophysiol. 2013;6:252–256. [DOI] [PubMed] [Google Scholar]
- 15. Nielsen J, Wachtell K, Abdulla J. The relationship between physical activity and risk of atrial fibrillation: a systematic review and meta‐analysis. J Atr Fibrillation. 2013;5:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Echouffo‐Tcheugui JB, Butler J, Yancy CW, et al. Association of physical activity or fitness with incident heart failure: a systematic review and meta‐analysis. Circ Heart Fail. 2015;8:853–861. [DOI] [PubMed] [Google Scholar]
- 17. Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all‐cause mortality and cardiovascular events in healthy men and women: a meta‐analysis. JAMA. 2009;301:2024–2035. [DOI] [PubMed] [Google Scholar]
- 18. Andersen K, Rasmussen F, Held C, et al. Exercise capacity and muscle strength and risk of vascular disease and arrhythmia in 1.1 million young Swedish men: cohort study. BMJ. 2015;351:h4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pathak RK, Elliott A, Middeldorp ME, et al. Impact of Cardiorespiratory Fitness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation: the CARDIO‐FIT Study. J Am Coll Cardiol. 2015;66:985–996. [DOI] [PubMed] [Google Scholar]
- 20. Qureshi WT, Alirhayim Z, Blaha MJ, et al. Cardiorespiratory fitness and risk of incident atrial fibrillation: results from the Henry Ford Exercise Testing (FIT) Project. Circulation. 2015;131:1827–1834. [DOI] [PubMed] [Google Scholar]
- 21. Khan H, Kella D, Rauramaa R, et al. Cardiorespiratory fitness and atrial fibrillation: a population‐based follow‐up study. Heart Rhythm. 2015;12:1424–1430. [DOI] [PubMed] [Google Scholar]
- 22. Alonso A, Bahnson JL, Gaussoin SA, et al. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: the Look AHEAD randomized trial. Am Heart J. 2015;170:770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pittaras A, Faselis C, Doumas M, et al. 5A.02: Fitness status and risk for atrial fibrillation. J Hypertens. 2015;33(suppl 1):e64. [Google Scholar]
- 24. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 26. Zhu WG, Wan R, Din Y, et al. Sex differences in the association between regular physical activity and incident atrial fibrillation: a meta‐analysis of 13 prospective studies [published online ahead of print March 21, 2016]. Clin Cardiol. doi: 10.1002/clc.22531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ingelsson E, Larson MG, Vasan RS, et al. Heritability, linkage, and genetic associations of exercise treadmill test responses. Circulation. 2007;115:2917–2924. [DOI] [PubMed] [Google Scholar]
- 28. Church TS, Barlow CE, Earnest CP, et al. Associations between cardiorespiratory fitness and C‐reactive protein in men. Arterioscler Thromb Vasc Biol. 2002;22:1869–1876. [DOI] [PubMed] [Google Scholar]
- 29. Aronson D, Sheikh‐Ahmad M, Avizohar O, et al. C‐Reactive protein is inversely related to physical fitness in middle‐aged subjects. Atherosclerosis. 2004;176:173–179. [DOI] [PubMed] [Google Scholar]
- 30. Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118:489–495. [DOI] [PubMed] [Google Scholar]
- 31. Wanahita N, Messerli FH, Bangalore S, et al. Atrial fibrillation and obesity—results of a meta‐analysis. Am Heart J. 2008;155:310–315. [DOI] [PubMed] [Google Scholar]
- 32. Qureshi W, Soliman EZ, Solomon SD, et al. Risk factors for atrial fibrillation in patients with normal versus dilated left atrium (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2014;114:1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 34. Grundvold I, Skretteberg PT, Liestøl K, et al. Importance of physical fitness on predictive effect of body mass index and weight gain on incident atrial fibrillation in healthy middle‐age men. Am J Cardiol. 2012;110:425–432. [DOI] [PubMed] [Google Scholar]
- 35. Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation. 2003;107:3109–3116. [DOI] [PubMed] [Google Scholar]
- 36. Eijsvogels TM, Molossi S, Lee DC, et al. Exercise at the extremes: the amount of exercise to reduce cardiovascular events. J Am Coll Cardiol. 2016;67:316–329. [DOI] [PubMed] [Google Scholar]
- 37. Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST‐AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]
- 38. Mont L, Tamborero D, Elosua R, et al; GIRAFA Investigators . Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle‐aged healthy individuals. Europace. 2008;10:15–20. [DOI] [PubMed] [Google Scholar]
- 39. Molina L, Mont L, Marrugat J, et al. Long‐term endurance sport practice increases the incidence of lone atrial fibrillation in men: a follow‐up study. Europace. 2008;10:618–623. [DOI] [PubMed] [Google Scholar]
- 40. Mozaffarian D, Furberg CD, Psaty BM, et al. Physical activity and incidence of atrial fibrillation in older adults: the Cardiovascular Health Study. Circulation. 2008;118:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]


