ABSTRACT
Our objective was to compare the diagnostic accuracy between the HAS‐BLED score and any of HEMORR2HAGES, ATRIA, CHADS2, or CHA2DS2‐VASc scores in anticoagulated patients with atrial fibrillation. We systematically searched the Cochrane Library, MEDLINE, PubMed, and Embase databases for relevant studies. Data were extracted and analyzed according to predefined clinical endpoints. Eleven studies were identified. Discrimination analysis demonstrates that HAS‐BLED has no significant C‐statistic differences for bleeding risk prediction compared with ATRIA or HEMORR2HAGES, but it has significant differences compared with CHADS2 or CHA2DS2‐VASc. The significant positive net reclassification improvement and integrated discrimination improvement values also show that HAS‐BLED is superior to that of any of HEMORR2HAGES, ATRIA, CHADS2, or CHA2DS2‐VASc scores. According to calibration analysis of HAS‐BLED, it overpredicts the risk of bleeding in the low (risk ratio [RR]: 1.16, 95% confidence interval [CI]: 0.63‐2.13, P = 0.64) risk stratification but underpredicts that in the moderate (RR: 0.66, 95% CI: 0.51‐0.86, P = 0.002) and high (RR: 0.88, 95% CI: 0.70‐1.10, P = 0.27) risk stratifications. The HAS‐BLED score not only performs better than the HEMORR2HAGES and ATRIA bleeding scores, but it also is superior to the CHADS2 and CHA2DS2‐VASc stroke scores for bleeding prediction. The HAS‐BLED score should be the optimal choice to assess major bleeding risk in clinical practice.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia in clinical practice, and it is associated with an increased risk of stroke and thromboembolism.1, 2 Oral anticoagulation therapy can reduce these embolic risks; therefore, it is recommended in AF patients at high risk of cardiovascular events. Nonetheless, the bleeding risks due to long‐term anticoagulation therapy may be devastating to stroke prevention.3 Given that increasing numbers of AF patients are treated with anticoagulants, the risk evaluation of both stroke and bleeding events is vitally important to guide the selection of the most appropriate prophylactic measures.4 Although several bleeding scores for risk evaluation have been recently developed and employed, only 3 are specifically used for AF patients5, 6, 7: HAS‐BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio [INR], elderly [age ≥65 years], drugs/alcohol concomitantly); HEMORR2HAGES (hepatic or renal disease, ethanol abuse, malignancy, older age [≥75 years], reduced platelet count or function, re‐bleeding risk, hypertension [uncontrolled], anemia, genetic factors, excessive fall risk, stroke); and ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation: anemia, renal disease, elderly [age ≥75 years], any prior bleeding, hypertension).
The HAS‐BLED score, which was first proposed in 2010, used data from a real‐world cohort of 3450 anticoagulated patients who have AF6 and then recommended clinical guidelines to predict potential bleeding risks.8 According to the HAS‐BLED score, AF patients are subdivided into 3 risk stratifications, in which a score of 0 indicates low risk, 1–2 indicates moderate risk, and ≥3 indicates high risk. Recently, these bleeding risk scores have been validated in various cohort studies. Additionally, with overlap of some risk factors between stroke and bleeding risk scores, the CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack history) and CHA2DS2‐VASc (congestive heart failure/left ventricular ejection fraction ≤40%, hypertension, age ≥75 y, diabetes mellitus, stroke/transient ischemic attack/thromboembolism history, vascular disease, age 65–74 y, female sex) stroke scores may also be associated with an increased risk of bleeding. However, data regarding this risk are sparse. Therefore, we compared the diagnostic accuracy of the HAS‐BLED score with that of other risk scores (eg, HEMORR2HAGES, ATRIA, CHADS2, or CHA2DS2‐VASc) in anticoagulated patients with AF.
Methods
Inclusion and Exclusion Criteria
Studies were included based on the following inclusion criteria: (1) the studies reported the comparative diagnostic performance between the HAS‐BLED score and any of the HEMORR2HAGES, ATRIA, CHADS2, or CHA2DS2‐VASc scores; (2) the study type (prospective or retrospective); (3) the participants (adult AF patients with anticoagulants [vitamin K antagonists]); (4) the patient outcomes (major bleeding, defined as fatal bleeding requiring a transfusion of ≥2 units of whole blood or red cells, or hemorrhage into a critical area or organ [eg, intracranial, intraocular, pericardial], or an overt bleed causing a fall in hemoglobin level of ≥2 g/dL); (5) timing (the follow‐up time is not limited); and (6) the treatment setting (inpatient or outpatient).
The following exclusion criteria were applied: (1) studies including valvular AF patients or individuals who received ablation or percutaneous coronary intervention procedures; (2) clinically relevant non–major bleeding events (eg, ischemic stroke and minor bleed); (3) certain publication type (eg, review, letter, case report, comment); and (4) studies with duplicate or insufficient data.
Literature Search
We conducted a comprehensive electronic search of the Cochrane Library, MEDLINE, PubMed, and Embase databases in March 2015 to identify relevant English‐language literature published since January 2010; the first study concerning the HAS‐BLED score was published in 2010.6 We used the following words (restricted to human studies) as search terms: “HAS‐BLED,” “atrial fibrillation,” “risk,” “prediction,” and “major bleeding.” Further manual research was performed using reference lists, relevant journals, and conference abstracts.
Study Selection and Data Extraction
Using the predetermined criteria, the titles and abstracts of studies retrieved electronically and manually were screened independently by W.Z. and W.H. for potentially relevant studies. When the necessary information was not apparent, we comprehensively reviewed the full text. Disagreements were resolved through discussion or consultation with a third reviewer (K.H.). The characteristics extracted from the available studies included the study type, demographic data, mean patient age, sex ratio, and follow‐up time.
Quality Assessment of Individual Studies
We used the Diagnostic Accuracy Studies (QUADAS‐2) to perform a quality evaluation of individual studies.9 QUADAS‐2 comprises 4 domains of patient selection, index test, reference standard, and flow and timing. The questions in each domain are evaluated in terms of risk of bias, and the first 3 domains are also evaluated in terms of applicability concerns. Observational studies are separately graded as good, fair, or poor.
Statistical Analysis
All statistical analyses were performed using Review Manager software, version 5.2 (the Nordic Cochrane Centre, Copenhagen, Denmark; http://ims.cochrane.org/revman). A P value ≤0.05 indicated statistical significance.
Consistency Test of Individual Studies
The consistency of the included studies was evaluated using the Cochrane Q test complemented with the I 2 statistic, where I2 values ≤25% indicated low heterogeneity, 25% to ≤50% indicated moderate heterogeneity, and >50% indicated high heterogeneity. When I2 values were ≤50%, a fixed‐effects model was chosen.
Meta‐analysis
We are interested in the C statistic with 95% confidence interval (CI) for the discrimination analysis. A C statistic ≥0.5 indicates that the model performs significantly better than chance. A C statistic of 0.6 to 0.7 indicates a low value of risk prediction, 0.7 to 0.9 indicates a modest value, and ≥0.9 indicates a high value. We then compared the C statistic of 2 different risk scores by calculating the Z statistic as follows:
In this formula, AZ1 and AZ2 represent the C statistic of 2 different risk scores, respectively, and represent the corresponding SEs. The improvement in predictive accuracy was evaluated by calculating the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) values.10
Second, the calibration analysis was generally evaluated across 3 risk stratifications, according to the HAS‐BLED score. The data presented in the HAS‐BLED derivation study were used as the predictive model.6 To calculate the predicted number of major bleeding events, we applied the adjusted bleeding rate (events/100 patient‐years) from the HAS‐BLED derivation study across 3 risk stratifications (low, 1.13; moderate, 1.33; and high, 4.94). The observed number of major bleeding events was collected in each available study. The results are presented as the risk ratio (RR) with 95% CI for each risk stratification. RR = 1 indicates an accurate prediction of the risk of bleeding by the HAS‐BLED score, RR < 1 indicates underprediction, and RR > 1 indicates overprediction.
Results
Description of the Included Studies
The study flow through the literature search and screening process is shown in Figure 1. We initially retrieved 485 unique citations through the electronic database search, and we identified 5 additional citations in the manual search. After we read the titles and abstracts, 70 full‐text studies were included in the review. Eleven of these studies fulfilled the inclusion criteria (11 of good quality).6, 7, 11, 12, 13, 14, 15, 16, 17, 18, 19 Table 1 shows the basic characteristics of the 11 included studies.
Figure 1.
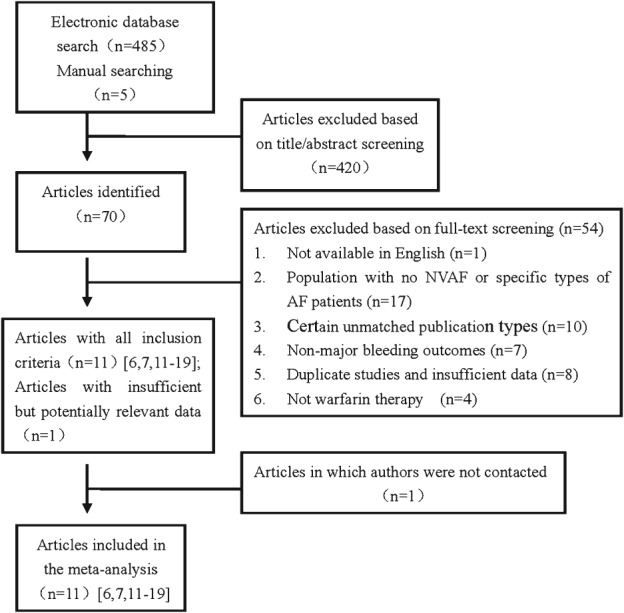
Flowchart showing number of articles included during each stage of the systematic review process. Abbreviations: AF, atrial fibrillation; NVAF, nonvalvular atrial fibrillation.
Table 1.
Basic Characteristics of All Included Studies
| Study | Type of Study | Participants With Anticoagulants, n | Follow‐up | Participant Age, y | F Ratio, % | Major Bleeding Definitions | Quality Rating |
|---|---|---|---|---|---|---|---|
| Pisters 20106 | Prospective | 2242 | Mean, 1 y | Mean, 67 | 41 | NA | Good |
| Fang 20117 | Prospective | 9186 | Mean, 3.5 y | NA | NA | ICD‐9 codes | Good |
| Lip 201111 | Prospective | 3665 | Mean, 499 d | Mean, 72 | 39 | NA | Good |
| Gallego 201212 | Prospective | 965 | Median, 861 d | Median, 76 | 50 | NA | Good |
| Apostolakis 201213 | Retrospective | 2293 | Mean, 429 d | Mean, 70 | 35 | 2005 ISTH criteria | Good |
| Naganuma 201214 | Retrospective | 845 | Median, 2.3 y | Median, 74 | 31 | NA | Good |
| Friberg 201215 | Prospective | 48 599 | Mean, 1.5 y | Mean, 76 | 47 | ICD‐10 codes | Good |
| Apostolakis 201316 | Retrospective | 2293 | Mean, 429 d | Mean, 70 | 35 | 2005 ISTH criteria | Good |
| Roldán 201317 | Prospective | 937 | Median, 952 d | Median, 76 | 51 | 2005 ISTH criteria | Good |
| Roldán 201318 | Retrospective | 1370 | Median, 996 d | Median, 76 | 53 | 2005 ISTH criteria | Good |
| Barnes 201419 | Retrospective | 2600 | Mean, 1 y | Mean, 70 | 42 | 2005 ISTH criteria | Good |
Abbreviations: F, female; ICD, International Classification of Diseases; ISTH, International Society on Thrombosis and Haemostasis; NA, not available.
Data Analysis
Discrimination Analysis of the HAS‐BLED Score
The C statistic with 95% CI is shown in Table 2. Heterogeneity was obvious in the global effect of the samples (I2 values, 24%–90%). Due to the inconsistent definitions of major bleeding and the complicated study participants, it is problematic for the diagnostic test accuracy, and it is difficult to find the main source of heterogeneity. Thus, we performed a Mantel‐Haenszel dichotomous‐weighted random‐effects model analysis. Thus, the results should be interpreted cautiously.
Table 2.
Summary of the Range and Synthesis of C Statistic (95% CI) Across Included Studiesa
| Score | No. of Studies | Range of C Statistic | Synthesis of C Statistic (95% CI) |
|---|---|---|---|
| HAS‐BLED | 7 | 0.60–0.69 (median, 0.66) | 0.65 (0.61‐0.69) |
| HEMORR2HAGES | 5 | 0.60–0.67 (median, 0.63) | 0.63 (0.61‐0.66) |
| ATRIA | 3 | 0.59–0.69 (median, 0.61) | 0.63 (0.56‐0.72) |
| CHADS2 | 3 | 0.51–0.59 (median, 0.53) | 0.55 (0.49‐0.61) |
| CHA2DS2‐VASc | 3 | 0.53–0.58 (median, 0.56) | 0.56 (0.53‐0.59) |
Abbreviations: ATRIA, Anticoagulation and Risk Factors in Atrial Fibrillation; CI, confidence interval; CHADS2, congestive heart failure, hypertension, age ≥75 years, DM, stroke/TIA history; CHA2DS2‐VASc, congestive heart failure/LVEF ≤40%, hypertension, age ≥75 y, DM, stroke/TIA/TE history, vascular disease, age 65–74 y, sex (F); DM, diabetes mellitus; F, female; HAS‐BLED, hypertension, abnormal liver/renal function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly; HEMORR2HAGES, hepatic or renal disease, ethanol abuse, malignancy, older age, reduced platelet count or function, re‐bleeding risk, hypertension (uncontrolled), anemia, genetic factors, excessive fall risk, stroke; INR, international normalized ratio; LVEF, left ventricular ejection fraction; TE, thromboembolism; TIA, transient ischemic attack.
Z statistic presented as follows: HAS‐BLED vs HEMORR2HAGES (Z statistic: 0.88, P > 0.05); HAS‐BLED vs ATRIA (Z statistic: 0.49, P > 0.05); HAS‐BLED vs CHADS2 (Z statistic: 2.72, P < 0.01); HAS‐BLED vs CHA2DS2‐VASc (Z statistic: 3.53, P < 0.01).
Among the included studies, only 1 study showed that the HAS‐BLED score had a significant higher C statistic than the ATRIA score.17 However, 3 studies showed that the HAS‐BLED score had a significant higher C statistic than the CHADS2 and CHA2DS2‐VASc stroke scores.16, 18, 19 In anticoagulated patients with AF, the C statistic ranged from 0.60 to 0.69 (median, 0.66) for HAS‐BLED; from 0.60 to 0.67 (median, 0.63) for HEMORR2HAGES; from 0.59 to 0.69 (median, 0.61) for ATRIA; from 0.51 to 0.59 (median, 0.53) for CHADS2; and from 0.53 to 0.58 (median, 0.56) for CHA2DS2‐VASc. This indicates that all risk scores of interest performed significantly better than chance. The pooled C statistics with 95% CIs were 0.65 (0.61‐0.69) for HAS‐BLED, 0.63 (0.61‐0.66) for HEMORR2HAGES, 0.63 (0.56‐0.72) for ATRIA, 0.55 (0.49‐0.61) for CHADS2, and 0.56 (0.53‐0.59) for CHA2DS2‐VASc, respectively. All 3 bleeding scores demonstrated similar discriminative values for major bleeding risk prediction, but they appeared to be more dominant than the CHADS2 and CHA2DS2‐VASc stroke scores. Then, we pooled and compared the C statistic of 2 different tested risk scores using the Z statistic. The comparison results were as follows: HAS‐BLED vs HEMORR2HAGES (Z statistic: 0.88, P > 0.05); HAS‐BLED vs ATRIA (Z statistic: 0.49, P > 0.05); HAS‐BLED vs CHADS2 (Z statistic: 2.72, P < 0.01); and HAS‐BLED vs CHA2DS2‐VASc (Z statistic: 3.53, P < 0.01). The HAS‐BLED score demonstrated no statistically significant C‐statistic differences when compared with the HEMORR2HAGES and ATRIA bleeding scores, but it demonstrated significant differences when compared with the CHADS2 and CHA2DS2‐VASc stroke scores. Therefore, all 3 bleeding scores demonstrated similar discriminative performance for major bleeding risk prediction, but the CHADS2 and CHA2DS2‐VASc stroke scores did not perform as well as the HAS‐BLED score for predicting major bleeding risk in anticoagulated patients with AF.
Net Reclassification Improvement and Integrated Discrimination Improvement Analysis of the HAS‐BLED Score
In the current meta‐analysis, 5 studies presented the mean values of NRI analyses to assess the improvement in predictive accuracy (Table 3).13, 16, 17, 18, 19 Among these studies, the HAS‐BLED score had significant positive NRI values for AF patients compared with the HEMORR2HAGES (+26.0%, P = 0.006)19 or ATRIA (+19.6%, P = 0.01917 and +31.0%, P = 0.00119) scores. The probability of correctly predicting major bleeding events using the HAS‐BLED score was reflected in the percentage of events correctly reclassified. Therefore, the HAS‐BLED score performed better than both the HEMORR2HAGES and ATRIA bleeding scores. In addition, the HAS‐BLED score also presented significant positive NRI values compared with the CHADS2 (+13.0%, P = 0.00116; +38.62%, P < 0.00118; and +58.0%, P < 0.00119) or CHA2DS2‐VASc (+10.0%, P = 0.0416; +37.6%, P < 0.00118; and +36.0%, P < 0.00119) stroke scores.16, 18, 19 Thus, the value of the HAS‐BLED score in predicting major bleeding risk was more dominant compared with the CHADS2 and CHA2DS2‐VASc scores. Furthermore, the improved predictive accuracy was also evaluated in terms of the IDI analysis. Similarly, the HAS‐BLED score demonstrated significant positive IDI values for AF patients compared with ATRIA (+7.0%, P = 0.001),17 CHADS2 (+10.0%, P < 0.001),18 or CHA2DS2‐VASc (+12.0%, P < 0.001).18 The predictive value of the HAS‐BLED score should be superior to that of other risk scores in anticoagulated patients with AF.
Table 3.
NRI and IDI Analysis for Predicting Major Bleeding Risk in Anticoagulated Patients With AFa
| Study | Contrast | NRI Analysis | IDI Analysis |
|---|---|---|---|
| Apostolakis 201213 | HAS‐BLED | vs HEMORR2HAGES: +6.8%, P = 0.42; vs ATRIA: +9.0%, P = 0.33 | NA |
| Apostolakis 201316 | HAS‐BLED | vs CHADS2: +13.0%, P = 0.001; vs CHA2DS2‐VASc: +10.0%, P = 0.04 | NA |
| Roldán 201317 | HAS‐BLED | vs ATRIA: +19.6%, P = 0.019 | vs ATRIA: +7.0%, P = 0.001 |
| Roldán 201318 | HAS‐BLED | vs CHADS2: +38.62%, P < 0.001; vs CHA2DS2‐VASc: +37.6%, P < 0.001 | vs CHADS2: +10.0%, P < 0.001; vs CHA2DS2‐VASc: +12.0%, P < 0.001 |
| Barnes 201419 | HAS‐BLED | vs HEMORR2HAGES: +26.0%, P = 0.006; vs ATRIA: +31.0%, P = 0.001; vs CHADS2: +58.0%, P < 0.001; vs CHA2DS2‐VASc: +36.0%, P < 0.001 | NA |
Abbreviations: AF, atrial fibrillation; ATRIA, Anticoagulation and Risk Factors in Atrial Fibrillation; CHADS2, congestive heart failure, hypertension, age ≥75 years, DM, stroke/TIA history; CHA2DS2‐VASc, congestive heart failure/LVEF ≤40%, hypertension, age ≥75 y, DM, stroke/TIA/TE history, vascular disease, age 65–74 y, sex (F); DM, diabetes mellitus; F, female; HAS‐BLED, hypertension, abnormal liver/renal function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly; HEMORR2HAGES, hepatic or renal disease, ethanol abuse, malignancy, older age, reduced platelet count or function, re‐bleeding risk, hypertension (uncontrolled), anemia, genetic factors, excessive fall risk, stroke; IDI, integrated discrimination improvement; INR, international normalized ratio; LVEF, left ventricular ejection fraction; NA, not available; NRI, net reclassification improvement; TE, thromboembolism; TIA, transient ischemic attack.
A P value <0.05 demonstrated that the HAS‐BLED score had statistically significant positive NRI or IDI values when compared with other risk scores.
Calibration Analysis of the HAS‐BLED Score
Six different studies were included to perform the calibration analysis.6, 11, 12, 13, 14, 17 Because of I2 values ≤50%, a fixed‐effects model was chosen for this analysis. A forest plot showed that the HAS‐BLED score overpredicts the risk of bleeding in the low (RR: 1.16, 95% CI: 0.63‐2.13, P = 0.64) risk stratification and underpredicts the risk of bleeding in the moderate (RR: 0.66, 95% CI: 0.51‐0.86, P = 0.002) and high (RR: 0.88, 95% CI: 0.70‐1.10, P = 0.27) risk stratifications (Figure 2). There were no significant differences between the predicted and observed bleeding events in the low‐risk and high‐risk stratifications, respectively. A P value ≥0.05 demonstrated adequate model calibration. Thus, there was good calibration in the low‐risk and high‐risk stratifications, according to the HAS‐BLED score. Note that these results should be interpreted with caution due to the limited amount of available studies in each group.
Figure 2.
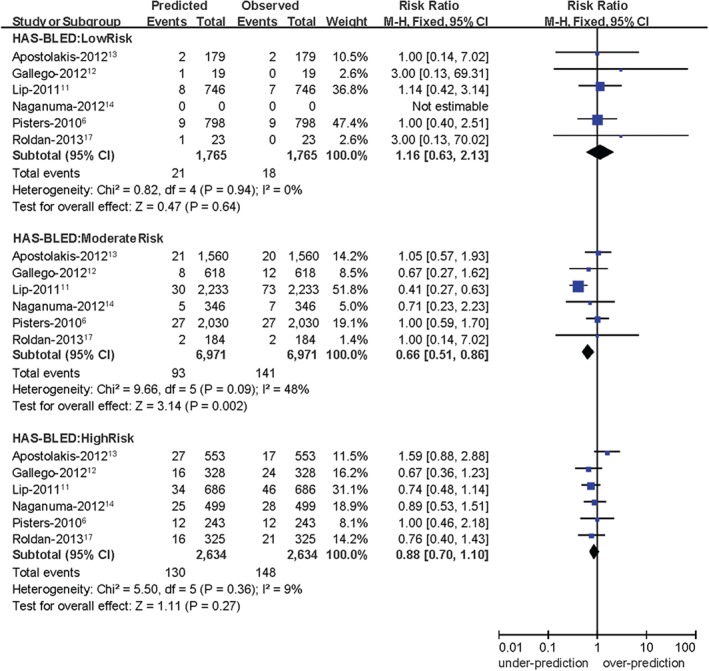
Forest plot showing calibration analysis of the HAS‐BLED score. Abbreviations: CI, confidence interval; df, degrees of freedom; HAS‐BLED, hypertension, abnormal liver/renal function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly; INR, international normalized ratio; M‐H, Mantel‐Haenszel.
Discussion
Although it is not immediately life‐threatening in clinical practice, AF is associated with a well‐known increase in embolic risk.20 Oral anticoagulation therapy is highly effective in reducing these embolic risks, and it is widely recommended in high‐risk patients with AF.2 However, anticoagulation therapy is also closely related to an increased risk of bleeding. Although new anticoagulants, particularly the IIa inhibitor (dabigatran) and the Xa inhibitors (rivaroxaban and apixaban), have been introduced into clinical practice, AF patients are still at high risk of bleeding. For this reason, the decision‐making process for the optimal use of anticoagulants should be based on a balanced assessment of the risks (eg, bleeding) and benefits (eg, stroke prevention) of the available treatment options. To date, 11 bleeding risk scores have been developed and employed in evaluating bleeding risk,21 3 of which (HAS‐BLED, HEMORR2HAGES, and ATRIA) have been specifically derived and validated exclusively in AF patients. The HAS‐BLED score originates from a 2010 European Heart Survey database, in which a score of ≥3 points is considered to be an indicator of high‐risk bleeding.
The HAS‐BLED score is regarded as the most commonly advocated scoring system, performing better in predicting bleeding risk than the HEMORR2HAGES and ATRIA scores in anticoagulated patients with AF.13, 17, 22 In this meta‐analysis, the HAS‐BLED score has a significantly higher C statistic in only 1 study compared with the ATRIA score.17 The remaining 10 studies show no significant differences in bleeding risk prediction among 3 bleeding risk scores. According to the pooled C statistics (0.65 for HAS‐BLED, 0.63 for HEMORR2HAGES, and 0.63 for ATRIA), all 3 bleeding risk scores demonstrate similar predictive values for major bleeding risk. Additionally, due to the overlap between some of the risk factors (eg, age, hypertension, previous stroke, and diabetes) for the bleeding and stroke scores, many risk factors for stroke scores are also closely associated with an increased risk of bleeding.23 Indeed, several studies have reported that the CHADS2 and CHA2DS2‐VASc scores are associated with an increased risk of both stroke and bleeding in anticoagulated patients with AF.16, 18, 19 In this meta‐analysis, in 3 different studies, the C statistic for the HAS‐BLED score is significantly higher than that for any of the CHADS2 and CHA2DS2‐VASc scores.16, 18, 19 The pooled C statistics are 0.55 for CHADS2 and 0.56 for CHA2DS2‐VASc, which demonstrates that both the CHADS2 and CHA2DS2‐VASc scores have lower predictive values for major bleeding risk compared with the HAS‐BLED score. Thus, the HAS‐BLED score demonstrates better discrimination for major bleeding risk prediction than the CHADS2 and CHA2DS2‐VASc stroke scores.
Although the C statistic can reflect the predictive ability of risk predictions quantitatively, the C statistic for each risk‐scoring system has always varied considerably in the study participants, which complicates the comparisons.24 Due to high heterogeneity of the diagnostic test accuracy, the role of the C statistic in the evaluation of the discriminative performance of risk scores has been questioned. Conventionally, the evaluation of a new scoring system is performed using the above‐mentioned discrimination measures, but most researchers are familiar with the classification measures such as sensitivity and specificity. An earlier meta‐analysis shows that, compared with the HEMORR2HAGES and ATRIA scores, the HAS‐BLED score is better for evaluating major bleeding risks in AF patients due to the higher sensitivity.25 Additionally, the improvement in predictive accuracy is also evaluated in terms of the NRI and IDI analysis. Our study indicates that the HAS‐BLED score performs better than any of the bleeding (HEMORR2HAGES and ATRIA) and stroke (CHADS2 and CHA2DS2‐VASc) risk scores, as reflected by the significant positive NRI and IDI values. Even in those AF patients without anticoagulants, when the HAS‐BLED score is compared with other bleeding risk scores (HEMORR2HAGES and ATRIA), the NRI values are significantly improved against all other scores tested.26 These results reveal that the HAS‐BLED score performs better in predicting bleeding complications than other risk scores, consistent with the findings of Kamran et al.27 Furthermore, there is good calibration between the predicted and observed bleeding events in the low‐risk and high‐risk stratifications of HAS‐BLED. Of note, these results should be interpreted with caution due to the limited available studies in each group.
Implications for Clinical Practice
In combination with NRI and IDI values in addition to the area under the curve (C statistic), our study shows that the HAS‐BLED score performs better than both the HEMORR2HAGES and ATRIA scores and certainly helps clinicians make informed clinical decisions. The HAS‐BLED score also indicates powerful predictive values for major bleeding risk prediction in high‐risk AF patients. However, given that AF is a leading cause of neurological disability and mortality depending on severity of cardioembolic stroke, oral anticoagulation therapy is critical for high‐risk AF patients. The HAS‐BLED score is therefore not used to help us exclude anticoagulation therapy in AF patients, but it allows clinicians to identify the potential bleeding risk factors (rather than relying on guesswork).2 We could search for appropriate measures (eg, correcting reversible risk factors and providing appropriate follow‐up services) to reduce the occurrence of major bleeding risk in anticoagulated patients with AF, particularly for patients with a HAS‐BLED score ≥3.
Our study also shows that the HAS‐BLED score performed better than the CHADS2 and CHA2DS2‐VASc stroke scores for predicting major bleeding risk in anticoagulated patients with AF. Therefore, we recommend that the HAS‐BLED score be used in AF patients who are considering anticoagulation therapy for stroke prevention, particularly for high‐risk patients.
Limitations of the Meta‐analysis
This meta‐analysis has several limitations. First, the high heterogeneity of the individual studies is observed in the discrimination analysis. Therefore, the results should be interpreted cautiously using the C statistic for comparisons, and the predictive value requires further evidence‐based assessment. Second, these tested risk scores are derived and validated in independent studies with different methods, ranging from highly selected clinical‐trial cohorts to real‐world populations.28 Therefore, different definitions of bleeding outcome and methodological differences of individual studies complicate the synthesis of the findings. Third, some studies come from the same team of professor Lip; perhaps this may lead to a certain degree of unknown bias, so it requires further cohort studies from different authors. Fourth, the follow‐up durations in each study are not clearly restricted during the collection of endpoint event samples. Finally, an INR level of 2–3 or time in therapeutic range (TTR) >70% provides the best tradeoff between preventing stroke events and causing bleeding.29, 30 The TTR is an independent risk factor for major bleeding in AF patients initiated with vitamin K antagonist therapy.31 Future studies should evaluate the predictive role of INR and TTR values and its association with bleeding risk.
Conclusion
The HAS‐BLED score performed better than both the HEMORR2HAGES and ATRIA scores, as reflected by the positive NRI and IDI values. Moreover, the discriminative performance of HAS‐BLED is superior to that of both the CHADS2 and CHA2DS2‐VASc stroke scores. It is appropriate to use the HAS‐BLED score for evaluating anticoagulation‐related major bleeding risk in everyday clinical practice.
Acknowledgments
The authors thank Huiqiong Yu. (School of Public Health, Nanchang University, Jiangxi, China) for help in designing the search strategy and for assistance in the consistency test for the included studies.
K.H. was in charge of the entire project and revised the draft; W.Z. and W.H. performed the systematic literature review, constructed the database, and analyzed the data with the help of X.W. and L.G.; W.Z. and W.H. drafted the first version of the manuscript. All authors took part in the interpretation of the results and prepared the final version.
Wengen Zhu, MD, and Wenfeng He, MD, are co–first authors.
The authors acknowledge support from the Ministry of Chinese Education Innovation Team Development Plan (IRT1141, HK), the National Basic Research Program of China (973 Program: 2013CB531103), and the National Natural Science Foundation of China (81160023, 81370288).
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Lloyd‐Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association [published correction appears in Circulation. 2010;121:e259]. Circulation. 2010;121:948–954. [DOI] [PubMed] [Google Scholar]
- 2. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385–1413. [DOI] [PubMed] [Google Scholar]
- 3. Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gorin L, Fauchier L, Nonin E, et al. Prognosis and guideline‐adherent antithrombotic treatment in patients with atrial fibrillation and atrial flutter: implications of undertreatment and overtreatment in real‐life clinical practice: the Loire Valley Atrial Fibrillation Project. Chest. 2011;140:911–917. [DOI] [PubMed] [Google Scholar]
- 5. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713–719. [DOI] [PubMed] [Google Scholar]
- 6. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 7. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin‐associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skanes AC, Healey JS, Cairns JA, et al. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control [published correction appears in Can J Cardiol. 2012;28:396]. Can J Cardiol. 2012;28:125–136. [DOI] [PubMed] [Google Scholar]
- 9. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS‐2 Group . QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. [DOI] [PubMed] [Google Scholar]
- 10. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172, 207–212. [DOI] [PubMed] [Google Scholar]
- 11. Lip GY, Frison L, Halperin JL, et al. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS‐BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol concomitantly) score. J Am Coll Cardiol. 2011;57:173–180. [DOI] [PubMed] [Google Scholar]
- 12. Gallego P, Roldán V, Torregrosa JM, et al. Relation of the HAS‐BLED bleeding risk score to major bleeding, cardiovascular events, and mortality in anticoagulated patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:312–318. [DOI] [PubMed] [Google Scholar]
- 13. Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR(2)HAGES, ATRIA, and HAS‐BLED bleeding risk‐prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol. 2012;60:861–867. [DOI] [PubMed] [Google Scholar]
- 14. Naganuma M, Shiga T, Sato K, et al. Clinical outcome in Japanese elderly patients with non‐valvular atrial fibrillation taking warfarin: a single‐center observational study. Thromb Res. 2012;130:21–26. [DOI] [PubMed] [Google Scholar]
- 15. Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. [DOI] [PubMed] [Google Scholar]
- 16. Apostolakis S, Lane DA, Buller H, et al. Comparison of the CHADS2, CHA2DS2‐VASc and HAS‐BLED scores for the prediction of clinically relevant bleeding in anticoagulated patients with atrial fibrillation: the AMADEUS trial. Thromb Haemost. 2013;110:1074–1079. [DOI] [PubMed] [Google Scholar]
- 17. Roldán V, Marín F, Fernández H, et al. Predictive value of the HAS‐BLED and ATRIA bleeding scores for the risk of serious bleeding in a “real‐world” population with atrial fibrillation receiving anticoagulant therapy. Chest. 2013;143:179–184. [DOI] [PubMed] [Google Scholar]
- 18. Roldán V, Marín F, Manzano‐Fernández S, et al. The HAS‐BLED score has better prediction accuracy for major bleeding than CHADS2 or CHA2DS2‐VASc scores in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2013;62:2199–2204. [DOI] [PubMed] [Google Scholar]
- 19. Barnes GD, Gu X, Haymart B, et al. The predictive ability of the CHADS2 and CHA2DS2‐VASc scores for bleeding risk in atrial fibrillation: the MAQI(2) experience. Thromb Res. 2014;134:294–299. [DOI] [PubMed] [Google Scholar]
- 20. Roger VL, Go AS, Lloyd‐Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association [published correction appears in Circulation. 2012;125:e1002]. Circulation. 2012;125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Bajorek B. Safe use of antithrombotics for stroke prevention in atrial fibrillation: consideration of risk assessment tools to support decision‐making. Ther Adv Drug Saf. 2014;5:21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR(2)HAGES, ATRIA, and HAS‐BLED bleeding risk‐prediction scores in nonwarfarin anticoagulated atrial fibrillation patients. J Am Coll Cardiol. 2013;61:386–387. [DOI] [PubMed] [Google Scholar]
- 23. Nieuwlaat R, Capucci A, Lip GY, et al. Euro Heart Survey Investigators. Antithrombotic treatment in real‐life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2006;27:3018–3026. [DOI] [PubMed] [Google Scholar]
- 24. Corbanese U. Assessing the performance of the HAS‐BLED score: is the C statistic sufficient? Chest. 2011;139:1247–1248. [DOI] [PubMed] [Google Scholar]
- 25. Caldeira D, Costa J, Fernandes RM, et al. Performance of the HAS‐BLED high bleeding‐risk category, compared to ATRIA and HEMORR2HAGES in patients with atrial fibrillation: a systematic review and meta‐analysis. J Interv Card Electrophysiol. 2014;40:277–284. [DOI] [PubMed] [Google Scholar]
- 26. Lip GY, Banerjee A, Lagrenade I, et al. Assessing the risk of bleeding in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Circ Arrhythm Electrophysiol. 2012;5:941–948. [DOI] [PubMed] [Google Scholar]
- 27. Kamran SH, Muzammil SM, Kamal AK. Is HAS‐BLED score better than CHADS2 and HEMOR2RHAGES schemes in assessing 1 year risk of major bleed in atrial fibrillation patients? J Pak Med Assoc. 2013;63:281–282. [PubMed] [Google Scholar]
- 28. Dzeshka MS, Lane DA, Lip GY. Stroke and bleeding risk in atrial fibrillation: navigating the alphabet soup of risk‐score acronyms (CHADS2, CHA2DS2‐VASc, R2 CHADS2, HAS‐BLED, ATRIA, and more). Clin Cardiol. 2014;37:634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Caterina R, Husted S, Wallentin L, et al. Vitamin K antagonists in heart disease: current status and perspectives (Section III). Position paper of the ESC Working Group on Thrombosis—Task Force on Anticoagulants in Heart Disease. Thromb Haemost. 2013;110:1087–1107. [DOI] [PubMed] [Google Scholar]
- 30. Thomas IC, Sorrentino MJ. Bleeding risk prediction models in atrial fibrillation. Curr Cardiol Rep. 2014;16:432. [DOI] [PubMed] [Google Scholar]
- 31. Gallego P, Roldán V, Marín F, et al. Cessation of oral anticoagulation in relation to mortality and the risk of thrombotic events in patients with atrial fibrillation. Thromb Haemost. 2013;110:1189–1198. [DOI] [PubMed] [Google Scholar]


