ABSTRACT
The heritability of coronary atherosclerotic plaque burden, coronary geometry, and phenotypes associated with increased cardiometabolic risk are largely unknown. The primary aim of the Burden of Atherosclerotic Plaques Study in Twins—Genetic Loci and the Burden of Atherosclerotic Lesions (BUDAPEST‐GLOBAL) study is to evaluate the influence of genetic and environmental factors on the burden of coronary artery disease. By design this is a prospective, single‐center, classical twin study. In total, 202 twins (61 monozygotic pairs, 40 dizygotic same‐sex pairs) were enrolled from the Hungarian Twin Registry database. All twins underwent non–contrast‐enhanced computed tomography (CT) for the detection and quantification of coronary artery calcium and for the measurement of epicardial fat volumes. In addition, a single non–contrast‐enhanced image slice was acquired at the level of L3‐L4 to assess abdominal fat distribution. Coronary CT angiography was used for the detection and quantification of plaque, stenosis, and overall coronary artery disease burden. For the primary analysis, we will assess the presence and volume of atherosclerotic plaques. Furthermore, the 3‐dimensional coronary geometry will be assessed based on the coronary CT angiography datasets. Additional phenotypic analyses will include per‐patient epicardial and abdominal fat quantity measurements. Measurements obtained from monozygotic and dizygotic twin pairs will be compared to evaluate the genetic or environmental effects of the given phenotype. The BUDAPEST‐GLOBAL study provides a unique framework to shed some light on the genetic and environmental influences of cardiometabolic disorders.
Background and Rationale
Modern personalized medicine aims to predict, diagnose, and treat diseases using multi‐omics data and genetic information. Coronary artery disease (CAD) is one of the leading causes of mortality and morbidity globally.1, 2 It is a complex disease influenced by multiple combinations of gene‐gene and gene‐environment interactions, and age is critically important for the onset and severity of the disease. Twins have a matching age and they share a wide range of environmental variables that contribute to the expression of CAD. These unique characteristics of twins provide a powerful tool for the study of genetic and environmental factors attributable to the development of complex diseases.3 Twin studies compare the concordance of a phenotype/disease between genetically identical or monozygotic (MZ) twin pairs, and nonidentical or dizygotic (DZ) twin pairs, who share approximately 50% of their genome. The traditional twin method is predicated on the equal‐environment assumption–that MZ and DZ twins are equally correlated in their exposure to environmental events of etiologic importance for the trait studied. Therefore, more similar MZ pairs than DZ pairs for a given phenotype/disease indicate genetic background.3, 4, 5 Landmark studies published in the 1990s demonstrated for the first time the strong genetic basis of CAD and myocardial infarction and their association with cardiovascular (CV) risk factors.6, 7, 8, 9
The distribution of various adipose tissue compartments is a heritable trait and a well‐established CV risk factor independent of overall adiposity.10, 11, 12, 13 The role of the abdominal obesity in the development of insulin resistance syndrome and cardiovascular diseases (CVD) is well characterized.14, 15 Nonalcoholic fatty liver disease (NAFLD) is a spectrum of liver disorders that includes isolated steatosis, characterized by ectopic fat accumulation in the liver, and nonalcoholic steatohepatitis, characterized by steatosis combined with additional features such as hepatocellular injury, inflammation, and fibrosis.16, 17 Nonalcoholic fatty liver disease is often regarded as the hepatic manifestation of the insulin resistance syndrome and is considered a strong predictor of CVD.18, 19 The epicardial fat is an adipose tissue layer between the myocardium and the visceral pericardium with unique characteristics and roles. It was recently suggested that it might locally contribute to the development of coronary atherosclerosis as well.20, 21, 22 Little is known about the genetic and environmental influences on coronary plaque distribution and morphology in association with various adipose tissue compartments and hepatic lipid accumulation. To the best of our knowledge, no classical twin study using advanced CAD phenotyping by coronary computed tomography (CT) angiography has been performed to date to evaluate the genetic and environmental interplay in the complex etiopathogenesis of CAD, fat accumulation, and NAFLD development.
Accordingly, we designed the Burden of Atherosclerotic Plaques Study in Twins—Genetic Loci and the Burden of Atherosclerotic Lesions (BUDAPEST‐GLOBAL) clinical study, and enrollment was recently completed. The primary aim of the BUDAPEST‐GLOBAL clinical study is to evaluate the influence of genetic and environmental factors on the burden of CAD. We hypothesize that the correlation of coronary plaque volume will be stronger between the MZ twins as compared with DZ twins, which may suggest that this CAD phenotype is mainly driven by genetic factors. The secondary aims of the study are to quantify the heritability of coronary artery geometry, and furthermore to assess the association between CAD heritability and the heritability of hepatic lipid accumulation, epicardial and abdominal adipose tissue quantity, carotid intima‐media thickness, and hemodynamic parameters. Classical and new CV risk factors will be measured and potential associations with CAD and adipose tissue compartments will be analyzed. This article describes the background, rationale, and design of the study, as well as the primary methods used.
Overall Study Design
The BUDAPEST‐GLOBAL study is a prospective, single‐center study, performed in twin subjects with self‐reported Caucasian ethnic background; the participants had been co‐enrolled with the large, international, multicenter Genetic Loci and the Burden of Atherosclerotic Lesions (GLOBAL) clinical study (http//:www.ClinicalTrials.gov: NCT01738828).23 The national ethics committee approved the BUDAPEST‐GLOBAL study (institutional review board number: 58401/2012/EKU [828/PI/12]; Amendment: 12292/2013/EKU), and all patients provided written, informed consent. Subjects were enrolled from the Hungarian Twin Registry on a voluntary basis.24 We searched the Hungarian Twin Registry database to identify adult MZ and same‐sex DZ twins whose previously registered disease history meets the inclusion criteria of the study. The aim was to balance the overall participation for 50% females and ≥50% DZ twins. These twins were contacted by phone or email and the study protocols were described in detail. Thereafter, detailed study description was sent by email or mail to twins, which included inclusion and exclusion criteria as well. The majority (90%) of the contacted twin pairs were willing to participate.
Inclusion and exclusion criteria are listed in Table 1. Of note, subjects with pregnancy, regular alcohol consumption (>2 units daily), conditions possibly interfering with compliance during CT scanning, and acute infection within 3 weeks were excluded from the study. All subjects were asked not to smoke and not to eat for 3 hours and not to drink alcohol and coffee for 10 hours prior to the examinations. During enrollment, the zygosity was assessed using a standardized questionnaire based on 7 self‐reported responses (for a timeline of study procedures, see Supporting Information, Appendix 1, in the online version of this article).25 The study enrolled a total of 202 twin subjects (101 twin pairs) prospectively between April 2013 and July 2014 (Table 2).
Table 1.
Enrollment Criteria
| Inclusion criteria |
| 1. MZ twins and same‐sex DZ twins |
| 2. Females age 40 to 75 years, males age 35 to 75 years |
| 3. A signed IRB/ethics committee–approved informed consent form |
| Exclusion criteria |
| 1. Subjects for whom coronary CTA is contraindicated per institutional standard of carea |
| 2. Subjects with previous coronary arterial revascularization (PCI or CABG) |
| 3. Subjects with AF/flutter or frequent irregular or rapid heart rhythms occurring within the past 3 months |
| 4. Subjects with a pacemaker or ICD implant |
| 5. Active CHF or the presence of known nonischemic cardiomyopathy |
| 6. Known genetic disorders of atherosclerosis, lipid, or lipoprotein metabolism |
Abbreviations: AF, atrial fibrillation; CABG, coronary artery bypass grafting; CHF, congestive heart failure; CTA, computed tomography angiography; DZ, dizygotic; ICD, implantable cardioverter‐defibrillator; IRB, institutional review board; MZ, monozygotic; PCI, percutaneous coronary intervention.
History of severe and/or anaphylactic contrast reaction, inability to cooperate with scan acquisition and/or breathhold instructions, pregnancy, clinical instability, and renal insufficiency.
Table 2.
Demographics and Twin Characteristics
| Characteristic | Full Cohort, N = 202 | MZ Twins, n = 122 | DZ Twins, n = 80 | P Valuea |
|---|---|---|---|---|
| Female sex | 130 (64.4) | 74 (60.7) | 56 (70.0) | 0.18 |
| Age, y | 56.2 ± 9.4 | 54.9 ± 9.7 | 58.3 ± 8.4 | 0.01 |
| Height, cm | 166.3 ± 9.6 | 166.2 ± 10.0 | 166.4 ± 9.0 | 0.87 |
| Weight, kga | 77.3 ± 17.2 | 77.4 ± 17.7 | 77.1 ± 16.4 | 0.92 |
| BMI, kg/m2 | 27.8 ± 5.3 | 27.8 ± 5.0 | 27.9 ± 5.8 | 0.94 |
| Waist circumference, cm | 97.1 ± 14.0 | 96.8 ± 14.2 | 97.5 ± 13.7 | 0.72 |
| HTN | 86 (42.6) | 49 (40.2) | 37 (46.3) | 0.39 |
| DM | 18 (8.9) | 12 (9.8) | 6 (7.5) | 0.57 |
| Dyslipidemia | 87 (43.1) | 48 (39.3) | 39 (48.8) | 0.19 |
| Current smoker | 31 (15.3) | 19 (15.6) | 12 (15.0) | 0.91 |
Abbreviations: BMI, body mass index; DM, diabetes mellitus; DZ, dizygotic; HTN, hypertension; MZ, monozygotic; SD, standard deviation.
Data are presented as n (%) or mean ± SD.
Difference between MZ and DZ twins: t test or χ2 as appropriate.
Methods
Anthropometric Data and Medical History
Complete physical examination was performed and anthropometric parameters (weight, height, and waist circumference) were recorded. Brachial blood pressure was measured prior to CT. A 12‐lead electrocardiogram (ECG) and echocardiographic evaluation were performed in each twin subject.
Smoking habit was assessed and smoking years recorded, and alcohol consumption was evaluated as units per week. Physical activity, diet, and socioeconomic status were assessed by using questionnaires. Prevalence of hypertension, diabetes mellitus, dyslipidemia, and cerebrovascular disease was documented based on the medical history of the participants.
Laboratory Parameters and Panomics Data
Enrolled twins underwent a peripheral blood draw, and blood was aliquoted and stored as whole blood, plasma, serum, and buffy coat. RNA was collected in RNA preservation tubes. All subjects will undergo whole‐genome sequencing, whole‐genome DNA methylation, whole‐blood‐based full transcriptome sequencing, as well as mass spectrometry–based unbiased, unlabeled proteomics, metabolomics, lipidomics, and lipoprotein proteomics according the protocol described in the GLOBAL study.23 Conventional biomarker testing was performed at Health Diagnostic Laboratory, Inc. (Richmond, VA). Fasting lipid profile was measured on an autoanalyzer using standard clinical methods (Beckman‐Coulter, Fullerton, CA). Glycated hemoglobin (HbA1c) was measured using Trinity Biotech reagents (Trinity Biotech USA Inc., Jamestown, NY). Low‐density lipoprotein particle size was measured using NMR technology (Numares Health, Regensburg, Germany). Whole‐genome sequencing is performed by Illumina (San Diego, CA) on the Illumina HiSeq2500 platform. DNA methylation is performed on an Illumina 450k array and RNA sequencing on the Illumina HiSeq2500 platform by Expression Analysis, Q2 Company (Raleigh‐Durham, NC). Metabolomics and lipidomics are performed by Metabolon (Raleigh‐Durham, NC). All “omics” analyses and CT data interpretations are performed independently and blinded to each other.
Echocardiography
The twin pairs underwent standard 2‐dimensional and Doppler echocardiographic examinations (iE33 system, S5‐1 transducer; Philips Healthcare, Best, The Netherlands). Chamber dimensions, left and right ventricular systolic and diastolic function, and presence and grade of valvular diseases were determined according to current guidelines. For advanced analysis, high‐quality recordings were also acquired optimized for speckle tracking. Measurements were performed offline (Image‐Arena; TomTec Imaging Systems GmbH, Unterschleissheim, Germany), by readers with ≥7 years of experience in echocardiography (A.A.M and A.K).
Vascular Ultrasonography
Carotid and femoral ultrasonographic examinations were performed in B‐mode and color Doppler mode, with linear array, high‐frequency (5–10 MHz) transducers (Philips HD15; Philips Healthcare). Carotid arteries were assessed on both sides between the origin of the common carotid and the proximal 3‐cm to 4‐cm segments of the internal and external carotid arteries. Femoral arteries were assessed from the level of inguinal ligament until their bifurcation. The visualizable portions of the deep femoral artery and the superficial femoral artery were also assessed bilaterally. Endoluminal protrusions of ≥1.5 mm or a focal thickening >50% of the intima‐media thickness relative to the adjacent wall segment were considered plaques.26 In case of a plaque, we measured the plaque‐free arterial wall adjacent to the plaque. All images were stored digitally for further analysis. All measurements and data analysis were performed by experts with ≥5 years of experience in vascular ultrasonography (D.L.T. and A.D.T.).
Hemodynamic Measurements
Hemodynamic variables such as brachial and central blood pressures; aortic, carotid‐femoral, carotid‐brachial, carotid‐radial and brachial‐radial pulse wave velocity values; along with central and brachial augmentation indices were assessed noninvasively by oscillometry (TensioMed Arteriograph; Medexpert Ltd., Budapest, Hungary) and applanation tonometry (Millar SPT‐301; Millar Inc., Houston, TX).27, 28 Tonometric data was evaluated with Biopac AcqKnowledge software version 3.7 and an in‐house–built software, using the method described by Kelly and Fitchett.29 Hemodynamic measurements were performed by an expert with 10 years of experience in the field (T.H.).
Cardiac Computed Tomography
The cardiac CT exam was performed subsequent to the hemodynamic measurements. We administered per os β‐blockers (metoprolol, maximum dose 100 mg) 1 hour before the CT scan if the heart rate was >65 bpm. Participants underwent non–contrast enhanced prospectively ECG triggered scan using a 256‐slice multidetector CT (Brilliance iCT; Philips HealthTech, Best, The Netherlands) for the quantification of coronary artery calcium (CAC) on a per‐patient and per‐vessel basis. Scans were acquired during a single inspiratory breathhold at 78% of the R‐R interval with a slice thickness of 2.0 mm. Tube voltage of 120 kVp was used with tube current of 20 to 50 mAs depending on body mass index. The mean effective radiation dose of the calcium score scans was 0.69 ± 0.43 mSv (dose length product: 49.1 ± 31.0 mGy × cm). The CAC was quantified using commercially available software (Extended Brilliance Workspace; Philips Healthcare). The CAC quantity was expressed in Agatston score.30 Subsequently, prospectively ECG triggered coronary CT angiography was performed using a 256‐slice multidetector CT (Brilliance iCT; Philips HealthTech). Intravenous β‐blocker (metoprolol) was administered (maximum cumulative dose, 20 mg) on the table if the heart rate was still >65 bpm. Sublingual nitroglycerin (0.8 mg) was administered on the table, maximum 2 minutes before the image acquisition. Images were acquired during a single inspiratory breathhold in axial mode with 270‐ms rotation time, 128 × 0.625 mm collimation, tube voltage of 100 to 120 kVp, maximum effective tube current‐time product of 200 to 300 mAs at 78% of the R‐R interval. Triphasic contrast injection protocol was used with 80 mL of iodinated contrast agent in average (Iomeprol 400 g/cm3; Iomeron, Bracco Imaging S.p.A., Milan, Italy); mixture of contrast agent and saline (10 mL contrast agent and 30 mL saline); and 40 mL saline solution, all injected at a rate of 4.5 to 5.5 mL/s. We reconstructed the minimum slice thickness (0.8 mm) available in prospective ECG triggered image acquisition with an increment of 0.4 mm, which resulted in an approximately 0.6 mm isotropic resolution. The mean effective radiation dose of the coronary CT angiography (CTA) scans was 3.64 ± 1.04 mSv (dose length product: 260.1 ± 74.5 mGy × cm). All image analyses were performed offline on dedicated cardiac workstations (Intellispace Portal, Philips Healthcare). All data acquisition and measurements were performed by readers who had ≥10 years of experience and a level III training in coronary CTA (P.M.‐H. and A.J.).
Coronary Plaque and Geometry Assessment
The coronary CTA data sets are analyzed on a qualitative and quantitative basis. Coronary segments with a minimum diameter of 2.0 mm are included in the analysis. Each coronary segment is assessed for presence of plaque, plaque type, degree of stenosis, plaque features, and plaque attenuation pattern. Coronary plaque is classified as noncalcified plaque (NCP), partially calcified plaque, or calcified plaque.31, 32 Plaque features are assessed according to Motoyama et al, and the presence of positive remodeling, low‐attenuation plaque, or both is recorded.33, 34 Plaque pattern is assessed as suggested by Maurovich‐Horvat et al, accordingly noncalcified components of NCP and partially calcified plaque are graded as homogenous, heterogeneous, or napkin‐ring sign pattern.35, 36 Stenosis is graded as none, minimal (<25%), mild (25%–49%), moderate (50%–69%), severe (70%–99%), or occlusion (100%), based on visual estimation of percent diameter stenosis.31 Segment involvement score and segment involvement score index will be used to provide a semiquantitative measurement of plaque burden.37 Quantitative CT analysis is performed using a dedicated software tool for automated plaque assessment (QAngioCT; Medis BV, Leiden, The Netherlands). For each coronary segment, we will measure the minimal luminal diameter, minimal luminal area, percent diameter stenosis, and percent area stenosis based on a proximal and distal reference segment, as well as percent atheroma volume and plaque burden.38, 39 Based on attenuation values, we also quantify volume and percent of calcified plaque, high and low CT attenuation NCP (Figure 1). Furthermore, we extracted the coronary artery centerlines for the twin siblings and compared their 3‐dimensional structure, using the Kabsch algorithm (Figure 2).40
Figure 1.
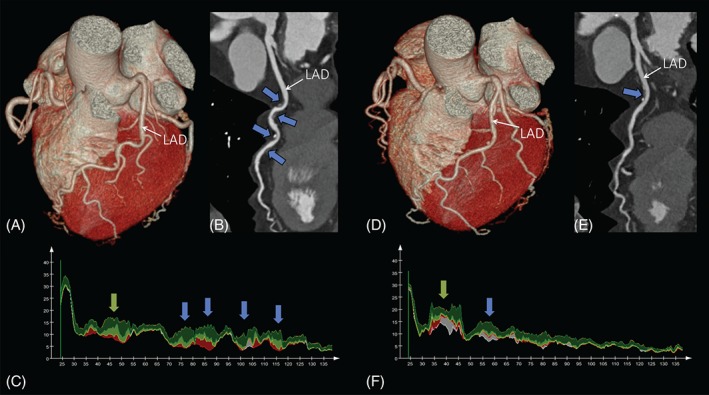
Coronary CTA images of a 58‐year‐old female monozygotic twin pair. Volume rendered reformations of the heart (A, D) of twin A and twin B, respectively. Curved multiplanar reconstructions (B, E). The blue arrows indicate coronary atherosclerotic plaques. The graphs on (C) and (F) illustrate the areas of different plaque components of twin A and twin B, respectively. The lipid‐rich (low‐CT attenuation) plaque components are shown in red. Fibro‐fatty tissue is shown in light green. Fibrous tissue is shown in dark green. Calcium is shown in white. The blue arrows indicate the corresponding plaques to panels B and E, whereas the green arrows indicate plaques that are not visible on panels B and E due to the viewing direction on the vessel. Abbreviations: CT, computed tomography; CTA, computed tomography angiography; LAD, left anterior descending artery.
Figure 2.
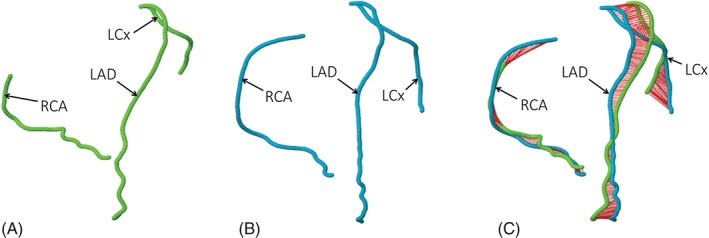
Coronary centerlines (A, B) of a monozygotic 46‐year‐old female twin pair. The distance between the superimposed coronary trees is indicated by the red cords on (C). Abbreviations: LAD, left anterior descending artery; LCx, left circumflex coronary artery; RCA, right coronary artery.
Epicardial Fat Volumetric Assessment
The pericardial space was manually traced in each CT‐slice in the non–contrast enhanced native cardiac CT data sets. The adipose tissue was defined as tissue in the attenuation range of −45 to −195 HU (Hounsfield units). Epicardial adipose tissue (EAT) was defined as any adipose tissue within the visceral pericardium from the level of the right pulmonary artery to the diaphragm.41, 42 The EAT segmentation was automatically interpolated within the manually traced region of interest (ROI), and the volume was calculated by using an offline workstation (Extended Brilliance Workspace; Philips Healthcare; Figure 3).
Figure 3.
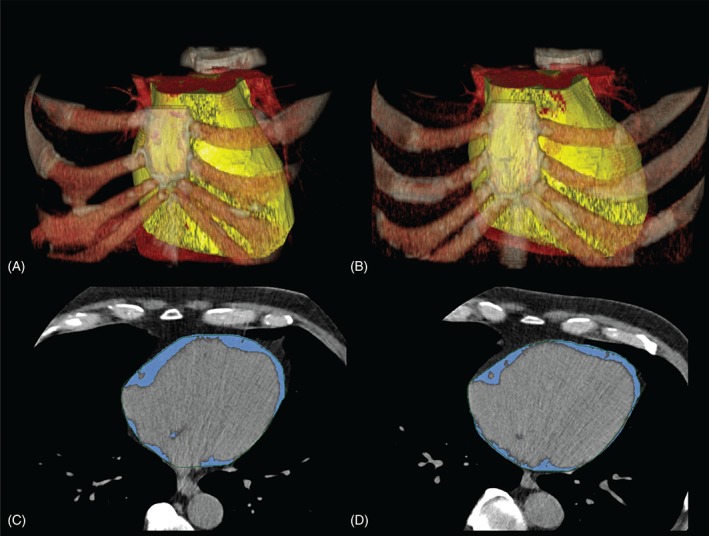
Volume rendered non–contrast‐enhanced native CT images of the chest of a 67‐year‐old male monozygotic twin pair (A, B). The yellow volume represents the epicardial adipose tissue compartment. The panels C and D are axial CT images of the same twin pair. The blue areas represent the epicardial adipose tissue compartments. Abbreviations: CT, computed tomography.
Assessment of Visceral Adipose Tissue and Subcutaneous Adipose Tissue
Subsequently, the non–contrast‐enhanced cardiac CT a single 5‐mm‐thick slice (120 kVp; 200 mA; gantry rotation time, 270 ms) was acquired at the level of L3‐L4. The mean effective radiation dose of the abdominal scan was 0.03 ± 0.01 mSv (dose length product: 2.3 ± 0.8 mGy × cm). The single CT slice was loaded onto an offline workstation and the subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) areas (cm2) were measured using a dedicated offline workstation (Extended Brilliance Workspace, Philips Healthcare). Semiautomated software tool identified the abdominal muscular wall separating the SAT and VAT compartments with the possibility of manual adjustment when needed. To identify pixels containing adipose tissue, an attenuation range of −45 to −195 HU was defined.43
Assessment of Hepatic Lipid Accumulation
The CT attenuation of the liver on CT when controlling for the penetrance of the scan using an internal control can be correlated to the amount of fat in the liver.44 The liver‐to‐spleen ratio (LSR; the CT HU of the liver divided by the CT HU of the spleen) of 1.1 and absolute liver CT attenuation <60 HU were described as the most reliable markers of 30% or more of liver fat.45 We determine the HU of the liver and spleen in the images acquired for coronary calcium measurement. We will use 3 circular ROI measurement of ≥300 mm2 in the liver and 2 ROIs with ≥150 mm2 in the spleen to assess the HU of these organs.45, 46
Statistical Analysis
In classical twin studies, greater levels of MZ than DZ within‐pair similarity indicate genetic influence on a phenotype, whereas similarity of co‐twin correlations suggests that the variance is a result of environmental influence. Similarity will be determined using within‐pair co‐twin correlations. Covariates for adjustment will be selected using linear regression, determining which factors individually contribute to the dependent variables.
Based on within‐twin correlations between MZ and DZ twins, structural equation modeling will carried out by R version 3.2.1 (R Foundation for Statistical Computing, http://www.r‐project.org) using the OpenMX (version 2.2.4) software package to break down the variance into environmental and genetic effects using the univariate and multivariate ACDE models.47, 48 The additive genetic component (A) measures the effect of genes being present at multiple loci or multiple alleles at 1 locus. The common environmental component (C) estimates the contribution of the mutual family environment in both twins. The dominant component (D) estimates the dominant interaction between alleles at the same locus or on different loci. The unique environmental component (E) estimates the effects that contribute only to each individual twin, and includes measurement error. All variance components (A, C, D, E) cannot be estimated simultaneously, because C and D are confounded; therefore, ACE and ADE models will be considered separately for a selected trait.49, 50 We will compare the fit of the saturated ACE or ADE models to their nested submodels on the basis of likelihood‐ratio test; P values <0.05 will be considered significant. Although data are sparse regarding the heritability of coronary arteriosclerotic plaque burden, we conducted a power analysis to establish the number of twin pairs to be investigated. Based on previously published data on the heritability of arterial calcification, we expected ≥0.35 differences between the correlations of MZ and DZ twins.51 With a 2‐sided P value of 0.05 and a power of 80%, the estimated total sample size is 200 to show difference in plaque volume correlation values between MZ and DZ twins. Due to uncertainty of our assumptions, we will perform bootstrapping for all our estimates to ensure robust results.
Discussion
The aim of the present clinical study is to further elucidate the effects of genetic and environmental influences on CAD using advanced coronary imaging by coronary CTA in a twin cohort. To the best of our knowledge, to date this is the largest prospective, classical twin study that utilizes coronary CTA for the detection and quantification of CAD with the assessment of coronary artery geometry.
The robust assessment of phenotype is crucial in studies aiming to assess genetic and environmental influences. In previously published twin studies, CAC scoring was used to characterize and quantify CAD burden.51 However, CAC score is an aggregate score and does not account for noncalcified plaque components. Therefore, studies utilizing calcium scoring for phenotyping CAD inevitably underestimate the burden of atherosclerotic plaques. In preliminary observations in the GLOBAL clinical study, approximately 22% of all enrolled patients with a CAC score of zero in fact had coronary atherosclerosis on the basis of the contrast‐enhanced CTA examination (unpublished data). Moreover, it has been recently demonstrated that the presence of nonobstructive plaques with high‐risk features and increased total coronary‐artery plaque burden have a strong prognostic value in predicting adverse CV events.52, 53, 54, 55, 56 Coronary CTA‐based plaque characterization and quantification methods have been validated against histology,57 intravascular ultrasonography with radiofrequency backscatter analysis,58, 59 fractional flow reserve,60 and near‐infrared spectroscopy.58 These studies have demonstrated that coronary CTA has a >90% sensitivity for plaque detection.61 We have demonstrated that coronary CTA allows for an accurate quantification of calcified and noncalcified plaque and plaque components with low CT numbers correspond to fibro‐fatty and fatty plaque components on intravascular ultrasound and intravascular ultrasound–virtual histology.23, 59, 62
Despite the systemic nature of CV risk factors, atherosclerosis is a geometrically focal disease that has a propensity to involve the inner curvatures of coronary arteries and outer edges of coronary bifurcations.63 In these susceptible areas, blood flow is slow and changes direction with the cardiac cycle, resulting in a weak net hemodynamic shear stress and endothelial damage.63, 64 In contrast, vessel regions that are exposed to steady blood flow and a higher magnitude of shear stress remain comparatively disease‐free.63 Therefore, the geometry of the coronary arterial system might be a potential risk factor for the onset of atherosclerosis.65 However, coronary arteries exhibit large variation in anatomic configuration, and little is known about the magnitude of genetic and environmental influences in the development of coronary geometry. Therefore, we use similar techniques for coronary geometry comparisons that have been used in the field of particle physics to assess the degree of similarity of 2 different 3‐dimensional molecular structures.40
Furthermore, we assess the metabolically active, ectopic adipose tissue compartments, which have been linked to CVD development. With the simultaneous quantification of epicardial and abdominal (subcutaneous and visceral) adipose tissue compartments, the association (possible genetic or environmental covariance) between CAD and various adipose tissue depots can be assessed. In addition, we measure the hepatic lipid accumulation, which has been recently identified as a strong, independent risk factor of coronary atherosclerosis and has been linked to the development of high‐risk coronary plaques.38 The heritability of hepatic fat accumulation was assessed in twins with ultrasound and a weak genetic influence was demonstrated.66 However, assessment of NAFLD can be measured more objectively and with higher precision using CT.67 The evaluation of genetic and environmental influences on these different adipose tissue compartments in association with the presence of CAD may lead to better understanding of the pathogenesis of CVD development. The genetic decomposition analysis between the coronary atherosclerosis and other investigated CV phenotypes, such as carotid and femoral intima‐media thickness, plaque characteristics, and arterial stiffness, could reveal the genetic or environmental interplay between these atherosclerotic markers.
Study Limitations
Although this is a prospective, single‐center, classical twin study of coronary atherosclerotic plaque burden, it has limitations. The sample size was seemingly modest but is comparable with other clinical studies with twins.27, 66, 68 During enrollment, the zygosity in our twin cohort was classified according to validated questionnaires; nevertheless, this method is widely accepted in clinically oriented twin studies.25 However, zygosity will be confirmed on the basis of the whole‐genome sequencing data. Furthermore, the aim was to balance the overall participation for 50% females and ≥50% DZ twins; however, 64.4% of the enrolled twins are females. This might be due to the well‐known phenomenon that females and monozygotic twins are more willing to participate in research than are males.69
Conclusion
There are multiple unique aspects to the BUDAPEST‐GLOBAL study. To the best of our knowledge, this is the single largest classical twin study of coronary CTA today. In addition, it is one of the largest prospective heritability study of CVD, coronary geometry, and abdominal, epicardial, and hepatic lipid accumulation. Furthermore, this is the first prospective twin study with whole‐genome sequencing and panomic analysis in a carefully phenotyped cohort. Therefore, the BUDAPEST‐GLOBAL study provides a unique framework to shed some light on the genetic and environmental influences of cardiometabolic disorders.
Supporting information
Appendix S1. Timeline of study procedures
György Jermendy, MD, and Béla Merkely, MD, contributed equally to this study.
The initial part of the study was supported by a grant from the EFSD New Horizons Program to György Jermendy, MD. Szilard Voros, MD, is a shareholder in Global Genomics Group, LLC, and receives salary from Global Genomics Group, LLC.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mangino M, Spector T. Understanding coronary artery disease using twin studies. Heart. 2013;99:373–375. [DOI] [PubMed] [Google Scholar]
- 4. Derks EM, Dolan CV, Boomsma DI. A test of the equal environment assumption (EEA) in multivariate twin studies. Twin Res Hum Genet. 2006;9:403–411. [DOI] [PubMed] [Google Scholar]
- 5. Kendler KS, Neale MC, Kessler RC, et al. A test of the equal‐environment assumption in twin studies of psychiatric illness. Behav Genet. 1993;23:21–27. [DOI] [PubMed] [Google Scholar]
- 6. Marenberg ME, Risch N, Berkman LF, et al. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. [DOI] [PubMed] [Google Scholar]
- 7. Samuels LE, Samuels FS, Thomas MP, et al. Coronary artery disease in identical twins. Ann Thorac Surg. 1999;68:594–600. [DOI] [PubMed] [Google Scholar]
- 8. Snieder H, van Doornen LJ, Boomsma DI. The age dependency of gene expression for plasma lipids, lipoproteins, and apolipoproteins. Am J Hum Genet. 1997;60:638–650. [PMC free article] [PubMed] [Google Scholar]
- 9. Roberts R, Stewart AF. Genetics of coronary artery disease in the 21st century. Clin Cardiol. 2012;35:536–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heid IM, Jackson AU, Randall JC, et al. Meta‐analysis identifies 13 new loci associated with waist‐hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution [published correction appears in Nat Genet. 2011;43:1164]. Nat Genet. 2010;42:949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Randall JC, Winkler TW, Kutalik Z, et al. Sex‐stratified genome‐wide association studies including 270 000 individuals show sexual dimorphism in genetic loci for anthropometric traits. PLoS Genet. 2013;9:e1003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fox CS, White CC, Lohman K, et al. Genome‐wide association of pericardial fat identifies a unique locus for ectopic fat. PLoS Genet. 2012;8:e1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Feyter PJ. Epicardial adipose tissue: an emerging role for the development of coronary atherosclerosis. Clin Cardiol. 2011;34:143–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10:293–302. [DOI] [PubMed] [Google Scholar]
- 15. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. [DOI] [PubMed] [Google Scholar]
- 16. Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. [DOI] [PubMed] [Google Scholar]
- 17. McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:521–533, viii. [DOI] [PubMed] [Google Scholar]
- 18. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease [published correction appears in N Engl J Med. 2014;371:2241]. N Engl J Med. 2014;371:1131–1141. [DOI] [PubMed] [Google Scholar]
- 19. Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119–2121. [DOI] [PubMed] [Google Scholar]
- 20. Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. [DOI] [PubMed] [Google Scholar]
- 21. Gorter PM, de Vos AM, van der Graaf Y, et al. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol. 2008;102:380–385. [DOI] [PubMed] [Google Scholar]
- 22. Maurovich‐Horvat P, Kallianos K, Engel LC, et al. Influence of pericoronary adipose tissue on local coronary atherosclerosis as assessed by a novel MDCT volumetric method. Atherosclerosis. 2011;219:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Voros S, Maurovich‐Horvat P, Marvasty IB, et al. Precision phenotyping, panomics, and system‐level bioinformatics to delineate complex biologies of atherosclerosis: rationale and design of the “Genetic Loci and the Burden of Atherosclerotic Lesions” study. J Cardiovasc Comput Tomogr. 2014;8:442–451. [DOI] [PubMed] [Google Scholar]
- 24. Littvay L, Métneki J, Tárnoki AD, et al. The Hungarian Twin Registry. Twin Res Hum Genet. 2013;16:185–189. [DOI] [PubMed] [Google Scholar]
- 25. Christiansen L, Frederiksen H, Schousboe K, et al. Age and sex differences in the validity of questionnaire‐based zygosity in twins. Twin Res. 2003;6:275–278. [DOI] [PubMed] [Google Scholar]
- 26. Kobayashi E, Ono J, Hirai S, et al. Detection of unstable plaques in patients with carotid stenosis using B‐mode ultrasonography. Interv Neuroradiol. 2000;6(suppl 1):165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tarnoki AD, Tarnoki DL, Stazi MA, et al. Heritability of central blood pressure and arterial stiffness: a twin study. J Hypertens. 2012;30:1564–1571. [DOI] [PubMed] [Google Scholar]
- 28. Laurent S, Cockcroft J, Van Bortel L, et al; European Network for Noninvasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 29. Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. [DOI] [PubMed] [Google Scholar]
- 30. Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 31. Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8:342–358. [DOI] [PubMed] [Google Scholar]
- 32. Maurovich‐Horvat P, Ferencik M, Bamberg F, et al. Methods of plaque quantification and characterization by cardiac computed tomography. J Cardiovasc Comput Tomogr. 2009;3(suppl 2):S91–S98. [DOI] [PubMed] [Google Scholar]
- 33. Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–326. [DOI] [PubMed] [Google Scholar]
- 34. Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. [DOI] [PubMed] [Google Scholar]
- 35. Maurovich‐Horvat P, Ferencik M, Voros S, et al. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol. 2014;11:390–402. [DOI] [PubMed] [Google Scholar]
- 36. Maurovich‐Horvat P, Schlett CL, Alkadhi H, et al. The napkin‐ring sign indicates advanced atherosclerotic lesions in coronary CT angiography. JACC Cardiovasc Imaging. 2012;5:1243–1252. [DOI] [PubMed] [Google Scholar]
- 37. Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all‐cause mortality. J Am Coll Cardiol. 2007;50:1161–1170. [DOI] [PubMed] [Google Scholar]
- 38. Boogers MJ, Broersen A, van Velzen JE, et al. Automated quantification of coronary plaque with computed tomography: comparison with intravascular ultrasound using a dedicated registration algorithm for fusion‐based quantification. Eur Heart J. 2012;33:1007–1016. [DOI] [PubMed] [Google Scholar]
- 39. Schlett CL, Ferencik M, Celeng C, et al. How to assess non‐calcified plaque in CT angiography: delineation methods affect diagnostic accuracy of low‐attenuation plaque by CT for lipid‐core plaque in histology. Eur Heart J Cardiovasc Imaging. 2013;14:1099–1105. [DOI] [PubMed] [Google Scholar]
- 40. Kabsch W. Integration, scaling, space‐group assignment and post‐refinement. Acta Crystallogr D Biol Crystallogr. 2010;66(part 2):133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community‐based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. [DOI] [PubMed] [Google Scholar]
- 43. Maurovich‐Horvat P, Massaro J, Fox CS, et al. Comparison of anthropometric, area‐ and volume‐based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi‐detector computed tomography. Int J Obes (Lond). 2007;31:500–506. [DOI] [PubMed] [Google Scholar]
- 44. Iwasaki M, Takada Y, Hayashi M, et al. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78:1501–1505. [DOI] [PubMed] [Google Scholar]
- 45. Speliotes EK, Massaro JM, Hoffmann U, et al. Liver fat is reproducibly measured using computed tomography in the Framingham Heart Study. J Gastroenterol Hepatol. 2008;23:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Puchner SB, Lu MT, Mayrhofer T, et al. High‐risk coronary plaque at coronary CT angiography is associated with nonalcoholic fatty liver disease, independent of coronary plaque and stenosis burden: results from the ROMICAT II trial. Radiology. 2015;274:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R‐project.org.
- 48. Neale MC, Hunter MD, Pritikin JN, et al. OpenMx 2.0: extended structural equation and statistical modeling [published online ahead of print January 27, 2015]. Psychometrika. doi: 10.1007/s11336-014-9435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Neale M, Cardon L. Methodology for Genetic Studies of Twins and Families. Dordrecht, Netherlands: Springer Science & Business Media; 1992. [Google Scholar]
- 50. Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3:119–133. [DOI] [PubMed] [Google Scholar]
- 51. Cecelja M, Jiang B, Bevan L, et al. Arterial stiffening relates to arterial calcification but not to noncalcified atheroma in women: a twin study. J Am Coll Cardiol. 2011;57:1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bittencourt MS, Hulten E, Ghoshhajra B, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7:282–291. [DOI] [PubMed] [Google Scholar]
- 53. Otsuka K, Fukuda S, Tanaka A, et al. Prognosis of vulnerable plaque on computed tomographic coronary angiography with normal myocardial perfusion image. Eur Heart J Cardiovasc Imaging. 2014;15:332–340. [DOI] [PubMed] [Google Scholar]
- 54. Saraste A, Knuuti J. Prognosis of non‐obstructive coronary plaques with high‐risk CT morphology. Eur Heart J Cardiovasc Imaging. 2014;15:255–256. [DOI] [PubMed] [Google Scholar]
- 55. Contractor T, Parekh M, Ahmed S, et al. Value of coronary computed tomography as a prognostic tool. Clin Cardiol. 2012;35:467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Celik O, Cakmak HA, Satilmis S, et al. The relationship between gamma‐glutamyl transferase levels and coronary plaque burdens and plaque structures in young adults with coronary atherosclerosis. Clin Cardiol. 2014;37:552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schlett CL, Maurovich‐Horvat P, Ferencik M, et al. Histogram analysis of lipid‐core plaques in coronary computed tomographic angiography: ex vivo validation against histology. Invest Radiol. 2013;48:646–653. [DOI] [PubMed] [Google Scholar]
- 58. Voros S, Rinehart S, Qian Z, et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta‐analysis. JACC Cardiovasc Imaging. 2011;4:537–548. [DOI] [PubMed] [Google Scholar]
- 59. Voros S, Rinehart S, Qian Z, et al. Prospective validation of standardized, 3‐dimensional, quantitative coronary computed tomographic plaque measurements using radiofrequency backscatter intravascular ultrasound as reference standard in intermediate coronary arterial lesions: results from the ATLANTA (Assessment of Tissue Characteristics, Lesion Morphology, and Hemodynamics by Angiography With Fractional Flow Reserve, Intravascular Ultrasound and Virtual Histology, and Noninvasive Computed Tomography in Atherosclerotic Plaques) I study. JACC Cardiovasc Interv. 2011;4:198–208. [DOI] [PubMed] [Google Scholar]
- 60. Voros S, Rinehart S, Vazquez‐Figueroa JG, et al. Prospective, head‐to‐head comparison of quantitative coronary angiography, quantitative computed tomography angiography, and intravascular ultrasound for the prediction of hemodynamic significance in intermediate and severe lesions, using fractional flow reserve as reference standard (from the ATLANTA I and II Study). Am J Cardiol. 2014;113:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fischer C, Hulten E, Belur P, et al. Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: a meta‐analysis. J Cardiovasc Comput Tomogr. 2013;7:256–266. [DOI] [PubMed] [Google Scholar]
- 62. van der Giessen AG, Toepker MH, Donelly PM, et al. Reproducibility, accuracy, and predictors of accuracy for the detection of coronary atherosclerotic plaque composition by computed tomography: an ex vivo comparison to intravascular ultrasound. Invest Radiol. 2010;45:693–701. [DOI] [PubMed] [Google Scholar]
- 63. Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. [DOI] [PubMed] [Google Scholar]
- 64. Hong SJ, Chang HJ, Park S, et al. Impact of atorvastatin treatment in first‐degree relatives of patients with premature coronary artery disease with endothelial dysfunction: a double‐blind, randomized, placebo‐controlled crossover trial. Clin Cardiol. 2013;36:480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wahle A, Lopez JJ, Olszewski ME, et al. Plaque development, vessel curvature, and wall shear stress in coronary arteries assessed by X‐ray angiography and intravascular ultrasound. Med Image Anal. 2006;10:615–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tarnoki AD, Tarnoki DL, Bata P, et al. Heritability of non‐alcoholic fatty liver disease and association with abnormal vascular parameters: a twin study. Liver Int. 2012;32:1287–1293. [DOI] [PubMed] [Google Scholar]
- 67. Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. [DOI] [PubMed] [Google Scholar]
- 68. Cecelja M, Hussain T, Greil G, et al. Multimodality imaging of subclinical aortic atherosclerosis: relation of aortic stiffness to calcification and plaque in female twins. Hypertension. 2013;61:609–614. [DOI] [PubMed] [Google Scholar]
- 69. Coccaro EF, Jacobson KC. PennTwins: a population‐based cohort for twin studies. Twin Res Hum Genet. 2006;9:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Timeline of study procedures


