Abstract
Background
Antianginal medications are a class I recommendation by the American College of Cardiology/American Heart Association guidelines for stable ischemic heart disease. We sought to better understand guidance in drug selection and real‐life outcomes of antianginal medication use.
Hypothesis
In patients with stable ischemic heart disease, antianginal medications lower mortality.
Methods
We evaluated 5608 patients with obstructive coronary artery disease (CAD) on elective cardiac catheterization with follow‐up through self‐administered questionnaires. Patients were classified as being prescribed a particular medication if they received that medication at index catheterization, or within 3 months postcatheterization. The association between antianginal medication use and outcomes was evaluated using Cox proportional hazards models.
Results
Compared with the 11% not prescribed any antianginal medication, patients prescribed antianginal medication were more likely to be older and female; have a history of hypertension, diabetes mellitus, peripheral vascular disease, or 3‐vessel CAD; have lower adjusted mortality (hazard ratio [HR]: 0.75, 95% confidence interval [CI]: 0.63‐0.89); and experience mortality or myocardial infarction (HR: 0.83, 95% CI: 0.71‐0.98). Compared with patients not taking β‐blockers (17%), those taking β‐blockers had a lower risk of mortality (HR: 0.76, 95% CI: 0.66‐0.88). Patients prescribed calcium channel blockers or long‐acting nitrates had a higher risk of mortality compared with nonusers (HR: 1.16, 95% CI: 1.04‐1.29; HR: 1.20, 95% CI: 1.08‐1.34; respectively).
Conclusions
Antianginal medications are not universally prescribed among obstructive CAD patients; nonuse was associated with higher mortality. For CAD patients with or without prior myocardial infarction, β‐blockers were associated with improved long‐term survival.
Keywords: angina, coronary disease, long‐term outcomes, medications
1. INTRODUCTION
The use of β‐blockers, calcium channel blockers (CCBs), and long‐acting nitrates as antianginal medications are class I recommendations according to the American College of Cardiology/American Heart Association (ACC/AHA) guidelines for stable ischemic heart disease (SIHD).1 β‐Blockers (ie, one class of antianginal medications) are recommended for ≥3 years of treatment for CAD patients following acute coronary syndrome or heart failure—even in asymptomatic individuals.1 Randomized clinical trials have demonstrated that optimal medical treatment alone is equivalent to or better than revascularization in improving symptoms and preventing events among SIHD patients,2, 3, 4 yet detailed recommendations are lacking with regard to which medications to use individually or in combination. Furthermore, in contrast to the numerous examinations of real‐life antianginal medication use and outcomes in ST‐segment elevation myocardial infarction patients, there have been few examinations of optimal antianginal medicine treatment in SIHD patients, particularly those taking CCBs and ranolazine.1 Consequently, guidance for clinical decision‐making regarding antianginal drug use is limited.
Using the Duke Databank for Cardiovascular Disease (DDCD), we examined the real‐world use of antianginal medications and the long‐term outcomes of their use on SIHD patients. First, we determined the type and frequency of antianginal medication prescribed in the 90 days post–elective catheterization demonstrating significant obstructive CAD. Then, we ascertained the patient characteristics associated with receiving a specific antianginal medication regimen vs the characteristics of those who did not, and the relationship of medication‐use differences with long‐term outcomes.
2. METHODS
Data were obtained from the DDCD, which is an ongoing database of all patients undergoing diagnostic cardiac catheterization at Duke University Medical Center; this databank has been previously described.5 We included patients if they underwent elective coronary angiography during a cardiac catheterization at Duke (January 2006–December 2010) for the evaluation of suspect or known SIHD, where obstructive CAD was found (defined as ≥1 stenosis ≥50% in a major epicardial vessel). We excluded patients if they had primary valve disease, congenital heart disease, a coexisting life‐threatening comorbidity, a myocardial infarction (MI) within 3 days of catheterization, or other characteristics (Figure 1). After exclusions, our final study population contained 5608 patients.
Figure 1.
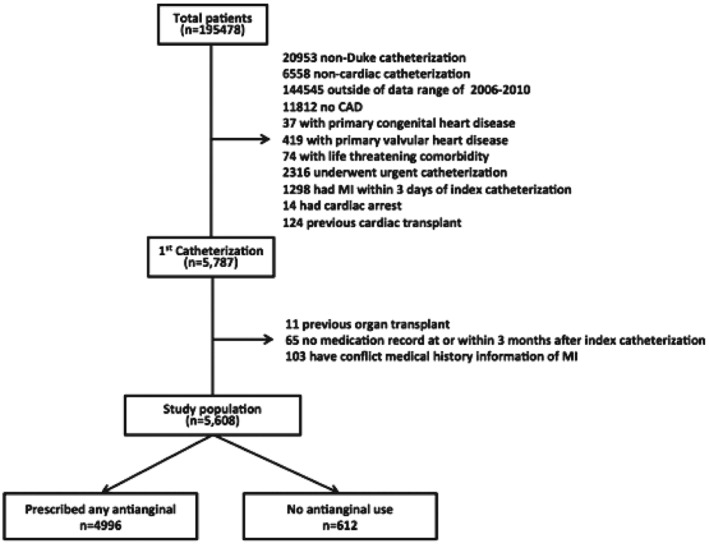
Patient flowchart displaying the final study cohort, from the initial study population through exclusions. Abbreviations: CAD, coronary artery disease; MI, myocardial infarction.
Data from the index catheterization were prospectively collected as part of routine patient care. Baseline clinical variables for each patient were stored in the DDCD using methods previously described.5 Follow‐up was obtained through Duke electronic medical records and self‐administered questionnaires, with telephone follow‐up to nonresponders. Patients without death information had vital status determined through a National Death Index search.6 Patients were classified as being prescribed a particular medication if there was record that they received that medication at or within 3 months after the index catheterization. Follow‐up began at 30 days after index catheterization and continued to death or to when the patient was last known alive. Median follow‐up time was 4.4 years (interquartile range, 3.1–6.1 years), with a maximum of 8.1 years. The primary clinical outcomes of interest were all‐cause mortality, nonfatal MI, and combined mortality and MI. We had a 97% follow‐up rate.
2.1. Statistical analysis
Various patient characteristics were summarized by whether or not specific individual treatment or different treatment combinations were prescribed. Continuous variables were summarized by their medians, along with 25th and 75th percentiles. Categorical variables were presented as frequencies and percentages. To determine which patient characteristics were associated with the receipt of a specific antianginal medication, we constructed unadjusted logistic regression models for each of the 4 medication classes (β‐blockers, CCBs, long‐acting nitrates, and ranolazine).
Multivariable Cox proportional hazard models were used to assess the association between different patterns of initial antianginal drug use and outcomes with adjustment for age, sex, diabetes mellitus (DM), left ventricular ejection fraction (LVEF), body mass index, CAD severity (defined as significant stenosis [≥50%] in 1, 2, or 3 major coronary arteries), and vascular disease index (defined as the total number of a history of peripheral vascular disease, history of cerebrovascular disease, and presence of bruits ranging from 0 to 3).7 We also tested whether the relationship between medication use and long‐term outcomes depended on whether a patient had a revascularization within 30 days after index catheterization.
The percentage of missing data was <5% for all variables except LVEF (~15% missing). All missing data were imputed using statistical multiple imputation techniques, which allowed the multivariable Cox proportional hazard model analysis to account for additional variation. Comparisons were 2‐sided; P < 0.05 was considered statistically significant. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina).
3. RESULTS
Of 5608 obstructive CAD patients identified with coronary angiography at elective catheterization, 11% had no record of being prescribed any antianginal medication within 3 months. At least 1 drug was prescribed in 89% of patients; β‐blockers in 83%, CCBs in 41%, long‐acting nitrates in 30%, and ranolazine in 1%. Among all patients, 38% were administered 1 class of antianginal medication, 36% received 2 classes, and 14% received 3 or 4 classes (see Supporting Information, Figure 1, in the online version of this article). In patients taking only 1 medication, the medication most often used was a β‐blocker (86%); in patients taking 2 classes of medications, the medications most often used were β‐blockers (97%) and CCBs (64%); and with 3 or 4 classes of medications, the medications most often used were β‐blockers (100%), CCBs (99%), and nitrates (99%; see Supporting Information, Figure 2, in the online version of this article).
Compared with patients with no antianginal medication prescription record, those taking antianginal medications were older, more likely to be female, and more likely to have comorbidities including hypertension (HTN), hyperlipidemia, DM, peripheral vascular disease (PVD), history of MI, typical chest pain, a positive stress test, and 3‐vessel CAD (Table 1). Similarly, among medication users, female sex and comorbidities such as HTN, DM, PVD, and history of MI were significantly associated with the use of 3 or 4 classes of medications, slightly associated with 2 classes of medication use, but were not associated with single medication use (Table 2; see Supporting Information, Figure 3, in the online version of this article).
Table 1.
Baseline characteristics of antianginal medication users and nonusers, by each medication group
| Any Kind of Drug | β‐Blocker | CCB | Long‐acting Nitrate | Ranolazine | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 4996) | No (n = 612) | Yes (n = 4629) | No (n = 979) | Yes (n = 2294) | No (n = 3314) | Yes (n = 1685) | No (n = 3923) | Yes (n = 65) | No (n = 5543) | |
| Age, y, median (IQR) | 65 (57–73) | 63 (56–71) | 65 (57–73) | 64 (57–72) | 66 (58–74) | 64 (56–72) | 66 (57–73) | 65 (57–72) | 69 (60–75) | 65 (57–73) |
| Female sex | 32 | 30 | 32 | 31 | 37 | 29 | 36 | 30 | 34 | 32 |
| HTN | 78 | 67 | 78 | 71 | 83 | 72 | 81 | 75 | 86 | 77 |
| Hyperlipidemia | 70 | 68 | 70 | 68 | 70 | 70 | 72 | 69 | 80 | 70 |
| DM | 35 | 31 | 36 | 31 | 39 | 32 | 38 | 33 | 37 | 35 |
| PVD | 12 | 9 | 12 | 10 | 14 | 10 | 14 | 11 | 22 | 11 |
| History of MI | 31 | 26 | 32 | 23 | 28 | 32 | 34 | 29 | 42 | 30 |
| Typical chest pain | 71 | 58 | 71 | 60 | 69 | 70 | 71 | 69 | 76 | 69 |
| Positive stress test | 59 | 56 | 59 | 57 | 57 | 60 | 57 | 60 | 46 | 59 |
| Number of 3‐vessel disease | 37 | 31 | 38 | 30 | 38 | 35 | 43 | 33 | 45 | 36 |
| Revascularization (PCI/CABG) within 30 days post‐catheterization | 59 | 23 | 60 | 34 | 62 | 51 | 52 | 57 | 49 | 55 |
Abbreviations: CABG, coronary artery bypass grafting; CCB, calcium channel blocker; DM, diabetes mellitus; HTN, hypertension; IQR, interquartile range; MI, myocardial infarction; NSTEMI, non–ST‐segment elevation myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; STEMI, ST‐segment elevation myocardial infarction.
All values are reported as % unless otherwise specified.
Table 2.
Baseline characteristics by number of categories of medications prescribed
| No Medications, n = 612 | 1 Class, n = 2145 | 2 Classes, n = 2043 | 3 or 4 Classes, n = 808 | |
|---|---|---|---|---|
| Age, y, median (IQR) | 63 (56–71) | 64 (57–72) | 66 (57–73) | 66 (58–74) |
| Female sex | 30 | 27 | 34 | 41 |
| HTN | 67 | 73 | 80 | 85 |
| DM | 31 | 32 | 36 | 43 |
| PVD | 9 | 10 | 12 | 17 |
| History of MI | 26 | 31 | 30 | 33 |
| History of HF | 23 | 27 | 29 | 34 |
| History of CVD | 8 | 9 | 12 | 13 |
| On dialysis | 3 | 2 | 3 | 5 |
| Renal disease | 4 | 3 | 3 | 5 |
| Typical chest pain | 58 | 70 | 71 | 70 |
| Positive stress test | 56 | 63 | 57 | 56 |
| Prior ACS category | ||||
| STEMI | 1 | 2 | 1 | 2 |
| NSTEMI | 3 | 4 | 4 | 7 |
| UA | 20 | 27 | 32 | 35 |
| No. of 3‐vessel disease | 31 | 33 | 38 | 46 |
| Revascularization (PCI/CABG) within 30 days post‐catheterization | 23 | 60 | 59 | 57 |
Abbreviations: ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; CVD, cerebrovascular disease; DM, diabetes mellitus; HF, heart failure; HTN, hypertension; IQR, interquartile range; MI, myocardial infarction; NSTEMI, non–ST‐segment elevation myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; STEMI, ST‐segment elevation myocardial infarction; UA, unstable angina.
All values reported as % unless otherwise specified.
Patients prescribed β‐blockers, CCBs, or nitrates were more likely to be older, female, and have HTN, DM, PVD, and 3‐vessel CAD (Table 1). Logistic regression model results indicate baseline characteristics were associated with the use of any antianginal medication. The association between some patient characteristics and antianginal medication use varied with medication choice (see Supporting Information, Figure 3, in the online version of this article).
Compared with patients not prescribed any antianginal medication, those taking any antianginal drug had a significantly lower adjusted mortality, as well as a lower rate of combined mortality or MI events; patients on 1 medication class had a significantly lower adjusted mortality and a lower rate of combined mortality or MI; patients on 2 medication classes had a significantly lower adjusted rate of mortality, but a higher adjusted MI rate; and those on 3 or 4 medication classes had similar mortality rates, but higher adjusted MI rates (Table 3).
Table 3.
Relationships of the number of classes of medications prescribed with long‐term outcomes, compared with patients using no anginal medications
| Mortality | MI | Mortality or MI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Event Rate1 | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Event Rate2 | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Event Rate1 | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Any vs 0 | 25 vs 27 | 0.94 (0.80‐1.11) | 0.75 (0.63‐0.89) | 7 vs 4 | 1.68 (1.11‐2.53) | 1.52 (1.00‐2.30) | 29 vs 30 | 1.02 (0.87‐1.20) | 0.83 (0.71‐0.98) |
| 1 class vs 0 | 21 vs 27 | 0.78 (0.65‐0.93) | 0.65 (0.54‐0.78) | 6 vs 4 | 1.47 (0.95‐2.27) | 1.42 (0.92‐2.20) | 26 vs 30 | 0.86 (0.73‐1.02) | 0.74 (0.62‐0.88) |
| 2 classes vs 0 | 25 vs 27 | 0.95 (0.80‐1.14) | 0.77 (0.64‐0.92 | 7 vs 4 | 1.75 (1.14‐2.70) | 1.57 (1.02‐2.43) | 30 vs 30 | 1.04 (0.88‐1.24) | 0.86 (0.72‐1.02) |
| 3 or 4 classes vs 0 | 34 vs 27 | 1.37 (1.13‐1.67) | 0.96 (0.79‐1.18) | 8 vs 4 | 2.06 (1.29‐3.29) | 1.65 (1.03‐2.66) | 38 vs 30 | 1.42 (1.18‐1.71) | 1.03 (0.85‐1.24) |
Abbreviations: CI, confidence interval; HR, hazard ratio; MI, myocardial infarction.
5‐year unadjusted Kaplan‐Meier event rate (%).
5‐year unadjusted cumulative incidence rate (%).
Patients on 3 or 4 medication classes had higher unadjusted mortality rates than untreated patients, except for β‐blockers users, who had similar unadjusted mortality rates as β‐blocker nonusers. After adjusting for baseline characteristics, β‐blocker users (compared with nonusers) had a lower risk of mortality, as well as a lower risk of mortality or MI (Table 4). β‐Blocker use was associated with lower long‐term mortality outcomes for patients with and without prior MI (see Supporting Information, Table 1, in the online version of this article).
Table 4.
Relationship between prescription of each different category of medication and long‐term outcomes
| Mortality | MI | Mortality or MI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Event Rate | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Event Rate1 | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Event Rate | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
| β‐Blocker | 25 vs 27 | 0.97 (0.84‐1.11) | 0.76 (0.66‐0.88) | 7 vs 5 | 1.37 (1.01‐1.86) | 1.20 (0.87‐1.64) | 29 vs 30 | 1.02 (0.89‐1.16) | 0.82 (0.72‐0.94) |
| CCB | 28 vs 23 | 1.26 (1.13‐1.39) | 1.16 (1.04‐1.29) | 7 vs 6 | 1.17 (0.95‐1.44) | 1.04 (0.84‐1.29) | 32 vs 27 | 1.23 (1.12‐1.36) | 1.13 (1.03‐1.25) |
| Long‐acting nitrates | 29 vs 23 | 1.35 (1.22‐1.51) | 1.20 (1.08‐1.34) | 8 vs 6 | 1.40 (1.13‐1.73) | 1.23 (0.99‐1.53) | 34 vs 27 | 1.34 (1.21‐1.48) | 1.19 (1.07‐1.32) |
| Ranolazine | 35 vs 25 | 1.51 (0.96‐2.37) | 1.29 (0.82‐2.04) | 5 vs 7 | 0.85 (0.27‐2.65) | 0.73 (0.23‐2.28) | 38 vs 29 | 1.38 (0.90‐2.12) | 1.19 (0.77‐1.83) |
Abbreviations: CCB, calcium channel blocker; CI, confidence interval; HR, hazard ratio; MI myocardial infarction.
5‐year unadjusted cumulative incidence rate (%).
In contrast, higher adjusted rates of mortality, and combined mortality or MI, were observed in long‐acting nitrate users when compared with nonusers. Similarly, a higher adjusted risk of mortality, and combined mortality or MI, was also present in CCB users when compared with nonusers (Table 4). The relationship between medication use and long‐term outcomes was not affected by whether a patient had revascularization within 30 days after the index catheterization.
4. DISCUSSION
In this large observational cohort of patients with SIHD, we found that (1) antianginal medication use was not universal among patients with obstructive CAD, with 11% of patients receiving no antianginal medication and 17% not being prescribed a β‐blocker; (2) increasing age, female sex, and higher comorbidity burden was associated with a greater likelihood of receiving any medication, as well as multiple classes of medications; (3) there was an overall association of medication use (vs nonuse) with reduced death and death or MI outcomes; and (4) the relationships between outcomes varied with individual classes of drugs.
To our knowledge, our study is the only detailed description of single or combined antianginal medication use in SIHD patients. To categorize medication use, we used medication records from the first 3 months post‐catheterization, because discharge medication use tends to determine long‐term use for CAD patients8 , 9; studies have shown that the prescription at discharge is strongly associated with patients being on an appropriate dose at follow‐up. For example, β‐blocker use at discharge was strongly associated with optimal β‐blocker use at follow‐up (adjusted odds ratio: 6.08, 95% confidence interval [CI]: 3.70‐10.01).8 Alternative approaches to characterizing medication use over time are cumbersome, may yield conflicting results, and may not provide additional information.10
Among all patients, 38% received 1 class of antianginal medication, 36% received 2 classes, and 14% received 3 or 4 classes. Similar to previous studies,2 , 11 we found that antianginal medications were underused; 11% of patients in our cohort did not receive any antianginal medication within 3 months after angiography. We were unable to assess symptoms related to medication use, yet guideline recommendations for SIHD patients suggest proactive use of anti‐ischemia therapy, regardless of symptoms,1 with β‐blockers being are recommended to be considered as chronic therapy for all patients with coronary vascular disease, even if symptoms are resolved by revascularization.1 In our study, almost 17% of SIHD patients did not receive β‐blocker treatment, despite guidelines recommending β‐blockers as the initial therapy for symptom relief (class Ib)1; we were unable to formally assess whether or not these patients had β‐blocker contraindications, but other studies have shown that 5% to 15% of CAD patients have contraindications.9 , 11 In SIHD patients, medication use is critical as it is associated with at least an equivalent (if not greater) improvement in patient symptoms and outcomes than revascularization.3 , 4 , 13 Yet a recent study demonstrated the underuse of medications,14 suggesting underuse is problematic in real life2 and, therefore, may be an actionable area to improve quality of care.
Compared with antianginal medication users, nonusers were more likely to be male and younger, have a lower prevalence of each cardiovascular risk factor, a lower likelihood of having a history of CAD, and less severe CAD. A history of MI was associated with higher β‐blocker and nitrate use, which is consistent with previous literature and guidelines15; however, CCB use was less likely to be observed in patients with a history of MI.1 Revascularization within 30 days of the index catheterization was positively associated with β‐blocker and CCB use, but negatively associated with nitrate use, which suggests medication use was symptom‐driven with a reduced requirement post‐revascularization.
Despite the greater burden of comorbidities, greater age, and more severe CAD in medication users, when compared with nonuse, antianginal medication use was associated with a 25% reduction in death and a 17% reduction in death or MI; improved outcomes were most apparent in those prescribed a single antianginal drug vs none (mortality: HR: 0.65, 95% CI: 0.54‐0.78; mortality or MI: HR: 0.74, 95% CI: 0.62‐0.88). The comparison of β‐blocker use vs nonuse across all patients demonstrated a 24% reduction in death and an 18% reduction in death or MI. Long‐term β‐blocker use has shown benefits in SIHD by reducing both ischemia burden and threshold, and improving survival in patients with left ventricular dysfunction or a history of MI.16, 17, 18
Our data differ from 2 other observational studies. Andersson et al found β‐blockers unhelpful in revascularized/unstable angina patients without prior MI.19 Similarly, Bangalore et al found that β‐blocker use was not associated with a reduced risk of composite cardiac events among a heterogeneous group including those with known prior MI, CAD without prior MI, or with CAD risk factors only.20 However, in the Bangalore study, the definition of β‐blocker use was documentation of β‐blocker at the time of enrollment. Differing from the Bangalore study, our study took into account the impact of invasive therapy by defining β‐blockers use as medication use at or within 3 months after the index catheterization. In our study, we found that among SIHD patients, β‐blocker prescription was associated with a lower risk of mortality for those with and without prior MI, regardless of the time duration since prior MI or revascularization, supporting the current 2012 ACC/AHA SIHD guideline recommendations for their use in patients with coronary vascular disease (class IIb, level of evidence C).1 Similarly, our findings suggest that there should be no time limit on the current class I recommendation for β‐blocker use after MI or with LVEF ≤40%; the recommended duration is now 3 years.1
A larger number of antianginal agents used correlated with a reduction in adjusted mortality benefit: there were lower odds of experiencing death in patients taking 2 classes of drugs, but not in those taking 3 or 4 classes, and MIs became more frequent in patients taking 2, 3, or 4 classes than in those not taking any antianginal agents. Examining the outcomes in users vs nonusers of specific drug classes is helpful in understanding this pattern since users of CCBs and nitrates each had greater mortality rates than nonusers despite adjustment, perhaps because CCBs and nitrates are mechanistically not thought to reduce events, but relieve symptoms.1 , 21 – 23 In conjunction with our findings, previous reports have demonstrated that long‐term nitrate therapy increases the risk of cardiac mortality in postinfarction patients.24 Importantly, we found that nitrates were most commonly used as add‐on drugs, rather than monotherapy; patients on multiple drugs had a higher baseline risk profile. Even after risk adjustment, there was residual increased mortality risk associated with comorbidities in these nitrate‐using patients; perhaps this risk profile contributes to the need for more medication rather than an independent negative effect of nitrates or CCBs. Further studies are needed to better understand the benefits and risks of long‐term nitrate or CCB use in this population.25
4.1. Study limitations
Our study had several limitations. First, our data were limited to prospectively collected information from this long‐term, single‐center registry. As a result, some outcomes‐influential data are unavailable, but because the category and discharge medication dose have been reported to be highly related to long‐term use,8 then our results would probably not change appreciably. Second, because short‐acting nitrates are often prescribed “as needed” rather than a fixed dose, we were unable to examine their use. Third, our data assess antianginal medication prescription after elective catheterization; we were unable to evaluate the adequacy of optimal medication, which is complex and may evolve over years of follow‐up. Finally, the limited number of ranolazine users means that this medication class is underpowered to fully capture patient characteristics and the related outcomes impact. Fortunately, additional data regarding post–percutaneous coronary intervention ranolazine use will soon be available from the Ranolazine for Incomplete Vessel Revascularization Post‐Percutaneous Coronary Intervention (RIVER‐PCI) trial.26
5. CONCLUSION
Among patients diagnosed with obstructive CAD at elective catheterization, a substantial minority (11%) did not receive a prescription for any antianginal medication, and nearly one‐fifth of patients were not prescribed β‐blockers. Patients who were prescribed medications were older, had a higher burden of risk factors, comorbidities, and more extensive CAD, but those prescribed β‐blockers had better outcomes. In contrast, those prescribed long‐acting nitrates and CCBs had higher mortality, and risk adjustment did not fully explain why. Further research is needed to definitively determine the value of β‐blockers in SIHD patients.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Appendix S1. Supplemental Material
Acknowledgments
The authors thank Erin Hanley, MS, for her editorial contributions to this article. Ms. Hanley did not receive compensation for her contributions, apart from her employment at the institution where this study was conducted.
Shen L, Vavalle JP, Broderick S, Shaw LK and Douglas PS. Antianginal medications and long‐term outcomes after elective catheterization in patients with coronary artery disease, Clin Cardiol 2016;39(12):721–727.
Author contributions: All authors had access to the data and a role in writing the manuscript.
REFERENCES
- 1. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. [DOI] [PubMed] [Google Scholar]
- 2. Peterson ED, Rumsfeld JS. Finding the courage to reconsider medical therapy for stable angina. N Engl J Med. 2008;359:751–753. [DOI] [PubMed] [Google Scholar]
- 3. Boden WE, O'Rourke RA, Teo KK, et al; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 4. Iqbal J, Zhang YJ, Holmes DR, et al. Optimal medical therapy improves clinical outcomes in patients undergoing revascularization with percutaneous coronary intervention or coronary artery bypass grafting: insights from the Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery (SYNTAX) trial at the 5‐year follow‐up. Circulation. 2015;131:1269–1277. [DOI] [PubMed] [Google Scholar]
- 5. Harris PJ, Lee KL, Harrell FE Jr, et al. Outcome in medically treated coronary artery disease. Ischemic events: nonfatal infarction and death. Circulation. 1980;62:718–726. [DOI] [PubMed] [Google Scholar]
- 6. Boyle CA, Decouflé P. National sources of vital status information: extent of coverage and possible selectivity in reporting. Am J Epidemiol. 1990;131:160–168. [DOI] [PubMed] [Google Scholar]
- 7. Alexander KP, Shaw LJ, Shaw LK, et al. Value of exercise treadmill testing in women [published correction appears in J Am Coll Cardiol. 1999;33:289]. J Am Coll Cardiol. 1998;32:1657–1664. [DOI] [PubMed] [Google Scholar]
- 8. Arnold SV, Spertus JA, Masoudi FA, et al. Beyond medication prescription as performance measures: optimal secondary prevention medication dosing after acute myocardial infarction [published correction appears in J Am Coll Cardiol. 2014;63:944]. J Am Coll Cardiol. 2013;62:1791–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldberger JJ, Bonow RO, Cuffe M, et al. β‐Blocker use following myocardial infarction: low prevalence of evidence‐based dosing. Am Heart J. 2010;160:435.e1–442.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. [DOI] [PubMed] [Google Scholar]
- 11. Tavazzi L. The need to identify new targets for therapeutic intervention in angina. Eur Heart J Suppl. 2006;8:A3–A5. [Google Scholar]
- 12. Goldberger JJ, Bonow RO, Cuffe M, et al. Post–myocardial infarction β‐blocker therapy: the bradycardia conundrum. Rationale and design for the Pacemaker and β‐blocker Therapy Post‐MI (PACE‐MI) trial. Am Heart J. 2008;155:455–464. [DOI] [PubMed] [Google Scholar]
- 13. Frye RL, August P, Brooks MM, et al; BARI 2D Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borden WB, Redberg RF, Mushlin AI, et al. Patterns and intensity of medical therapy in patients undergoing percutaneous coronary intervention [published correction appears in JAMA. 2011;305:2418]. JAMA. 2011;305:1882–1889. [DOI] [PubMed] [Google Scholar]
- 15. Drozda J Jr, Messer JV, Spertus J, et al. ACCF/AHA/AMA‐PCPI 2011 performance measures for adults with coronary artery disease and hypertension: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association‐Physician Consortium for Performance Improvement. J Am Coll Cardiol. 2011;58:316–336. [DOI] [PubMed] [Google Scholar]
- 16. Narahara KA; for Betaxolol Investigators Group. Double‐blind comparison of once daily betaxolol versus propranolol four times daily in stable angina pectoris. Am J Cardiol. 1990;65:577–582. [DOI] [PubMed] [Google Scholar]
- 17. Frishman WH, Heiman M, Soberman J, et al; Celiprolol International Angina Study Group. Comparison of celiprolol and propranolol in stable angina pectoris. Am J Cardiol. 1991;67:665–670. [DOI] [PubMed] [Google Scholar]
- 18. Kernis SJ, Harjai KJ, Stone GW, et al. Does β‐blocker therapy improve clinical outcomes of acute myocardial infarction after successful primary angioplasty? J Am Coll Cardiol. 2004;43:1773–1779. [DOI] [PubMed] [Google Scholar]
- 19. Andersson C, Shilane D, Go AS, et al. β‐Blocker therapy and cardiac events among patients with newly diagnosed coronary heart disease. J Am Coll Cardiol. 2014;64:247–252. [DOI] [PubMed] [Google Scholar]
- 20. Bangalore S, Steg G, Deedwania P, et al; REACH Registry Investigators. β‐Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308:1340–1349. [DOI] [PubMed] [Google Scholar]
- 21. Ezekowitz MD, Hossack K, Mehta JL, et al. Amlodipine in chronic stable angina: results of a multicenter double‐blind crossover trial. Am Heart J. 1995;129:527–535. [DOI] [PubMed] [Google Scholar]
- 22. Brogden RN, Benfield P. Verapamil: a review of its pharmacological properties and therapeutic use in coronary artery disease. Drugs. 1996;51:792–819. [DOI] [PubMed] [Google Scholar]
- 23. Heidenreich PA, McDonald KM, Hastie T, et al. Meta‐analysis of trials comparing β‐blockers, calcium antagonists, and nitrates for stable angina. JAMA. 1999;281:1927–1936. [DOI] [PubMed] [Google Scholar]
- 24. Nakamura Y, Moss AJ, Brown MW, et al. Long‐term nitrate use may be deleterious in ischemic heart disease: a study using the databases from two large‐scale postinfarction studies. Multicenter Myocardial Ischemia Research Group. Am Heart J. 1999;138(3 pt 1):577–585. [DOI] [PubMed] [Google Scholar]
- 25. Münzel T, Daiber A, Mülsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97:618–628. [DOI] [PubMed] [Google Scholar]
- 26. Weisz G, Farzaneh‐Far R, Ben‐Yehuda O, et al. Use of ranolazine in patients with incomplete revascularization after percutaneous coronary intervention: design and rationale of the Ranolazine for Incomplete Vessel Revascularization Post–Percutaneous Coronary Intervention (RIVER‐PCI) trial. Am Heart J. 2013;166:953.e3–959.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplemental Material


