ABSTRACT
Associations between atrial fibrillation (AF), outcomes, and response to antiplatelet therapies in patients with acute coronary syndrome (ACS) managed medically without revascularization remain uncertain. We examined these associations for medically managed ACS patients randomized to dual antiplatelet therapy (DAPT) using patient data from the TRILOGY ACS trial. DAPT included aspirin plus clopidogrel 75 mg/d or prasugrel 10 mg/d (5 mg/d for those <60 kg or age ≥75 years). Patients receiving oral anticoagulants were excluded. Cox proportional hazards regression modeling was used to characterize associations between patients with AF (AF+) vs those without (AF−) and risk of ischemic and bleeding events, and to explore effects of randomized treatment on outcomes. Among 9101 patients with baseline AF status, 710 (7.8%) had AF. AF+ patients were older and had more comorbidities. Unadjusted associations of the composite of cardiovascular death/myocardial infarction/stroke were significantly higher among AF patients at 30 months (31.1% vs 18.4%; HR: 1.61, 95% CI: 1.35‐1.92, P < 0.001), but differences did not persist after adjustment (HR: 1.16, 95% CI: 0.97‐1.39, P = 0.11). When individual components of the composite endpoint were evaluated, 30‐month risk of events in AF+ patients was significantly higher. Thirty‐month risk of all‐cause death was significantly higher in AF+ patients: 18.1% vs 11.1% (HR: 1.62, 95% CI: 1.30‐2.02, P < 0.001). There was no significant interaction with randomized treatment and AF for the primary endpoint. Among medically managed high‐risk ACS patients receiving DAPT, AF was associated with higher unadjusted risks of ischemic and bleeding outcomes that were similar by treatment group.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is a risk factor for ischemic stroke and cardiovascular mortality.1 In patients with stable coronary artery disease, the prevalence of AF is 12.5%2, 3, 4 and is associated with a higher risk of cardiovascular outcomes.5 Dual antiplatelet therapy (DAPT), comprising aspirin and a P2Y12 receptor inhibitor, is standard of care following percutaneous coronary intervention (PCI) for patients with acute coronary syndromes (ACS). However, a substantial proportion of these patients have AF, for which oral anticoagulants (OACs) provide superior prevention of stroke compared with antiplatelet agents; but, when combined with DAPT, they may significantly increase the risk of bleeding, specifically when prescribed with a newer P2Y12 receptor inhibitor such as prasugrel or ticagrelor.6, 7, 8, 9, 10, 11
Patients with both AF and ACS present a clinical conundrum, given uncertainty regarding the optimal antithrombotic strategy, as well as for those undergoing PCI (A Study Exploring Two Strategies of Rivaroxaban and One of Oral Vitamin K Antagonist in Patients With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention [PIONEER AF‐PCI; http://www.ClinicalTrials.gov NCT01830543] and Evaluation of Dual Therapy With Dabigatran vs Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting [REDUAL‐PCI; NCT02164864]). Currently, controversy exists regarding the prognostic role of AF in patients with ACS,12, 13 and to date there are no studies analyzing the association between AF and outcomes among ACS patients at highest risk—specifically, elderly patients managed medically without coronary revascularization. The randomized Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes (TRILOGY ACS; NCT00699998) trial included a prospective analysis plan to evaluate the prognostic role of AF in elderly patients with ACS. Accordingly, using the TRILOGY ACS study, we sought to (1) determine the risk of ischemic and bleeding outcomes in high‐risk patients with both AF and ACS who were medically managed with DAPT and without planned invasive revascularization; (2) evaluate the safety and efficacy of DAPT with prasugrel or clopidogrel among high‐risk elderly patients with AF and ACS; and (3) evaluate the magnitude of platelet aggregation in AF patients with recent ACS using platelet reactivity units (PRUs) obtained from the substudy of the TRILOGY ACS trial.
Methods
Trial Design
The design, methods, and primary results of TRILOGY ACS have been previously described.14, 15 Briefly, TRILOGY ACS was an international, randomized, active‐controlled, double‐blinded, double‐dummy trial that enrolled 9326 intention‐to‐treat (ITT) patients who were planned to undergo medical management without invasive revascularization for unstable angina (UA) or non–ST‐segment elevation myocardial infarction (NSTEMI). Study participants were randomly assigned in a 1:1 fashion to receive prasugrel 10 mg/d (5 mg/d in patients <60 kg body weight or age ≥75 years) or clopidogrel (75 mg/d), plus background low‐dose aspirin therapy.15 Participants for whom AF status at baseline was missing (n = 225, 2.4%) were excluded from the present analysis. A final cohort of 9101 participants was assessed for ischemic outcomes; for the analysis of safety outcomes, patients were assessed during the “at‐risk” interval of actual study‐drug treatment through 7 days after study‐drug discontinuation (n = 9022).
All appropriate national regulatory agencies and institutional review boards at each participating center approved the TRILOGY ACS study and all participants gave informed consent. An international multidisciplinary executive committee designed the study and takes responsibility for the accuracy and completeness of all data and subsequent analyses.
Clinical Outcomes
The primary efficacy endpoint was the composite of cardiovascular death, nonfatal myocardial infarction (MI), or nonfatal stroke. An independent cardiovascular adjudication committee whose members were blinded to study‐group assignments evaluated suspected ischemic and bleeding endpoints. Key bleeding and safety endpoints included Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) criteria for severe or life‐threatening bleeding unrelated to coronary artery bypass grafting and Thrombolysis in Myocardial Infarction (TIMI) criteria for major bleeding unrelated to coronary artery bypass grafting.16, 17
Platelet Function Substudy
A total of 2564 TRILOGY ACS subjects from participating sites were enrolled in a prospective platelet function substudy that evaluated differences in platelet reactivity over time for patients receiving prasugrel vs clopidogrel.18 Briefly, substudy participants underwent serial platelet reactivity assessments (as measured in P2Y12 PRUs) with the VerifyNow system (Accriva Diagnostics, San Diego, CA). The PRU measurements were performed at baseline, at 2 hours, and at 1, 3, 6, 12, 18, 24, and 30 months following randomization. Participants receiving prasugrel 10 mg/d exhibited significantly lower platelet reactivity vs those receiving clopidogrel, but no significant independent associations with ischemic endpoints were seen after adjustment.18
Atrial Fibrillation
Patients included in this secondary analysis had a history of AF prior to or during the index hospitalization and were not taking an OAC. In addition to identifying a history of prior or current AF, additional characteristics were collected, including duration of AF, a history of prior thromboembolic events associated with AF prior to randomization, the use of OACs prior to randomization, antiarrhythmic drug use within the previous 12 months prior to randomization, and discontinuation of OAC due to a bleeding complication. Site investigators were not queried as to the overall use of OAC in patients with AF and why patients were not treated with OAC prior to an ACS event.
Statistical Analysis
Baseline characteristics have been summarized according to AF status at baseline. This analysis analyzed patients with history of current or prior AF (AF+) vs those without history of AF (AF−). Continuous variables are presented as median (interquartile range [IQR]), and differences are compared using the Wilcoxon rank‐sum test. Categorical variables are presented as counts (percentages), and differences are compared using the Pearson χ2 test when cell frequencies are sufficient; otherwise, an exact test is used.
For patients with a history of AF (current or prior), the duration of AF (in months), stoppage of an OAC due to a bleeding event, and treatments received within the last 12 months are summarized according to treatment and for the entire ITT population. For continuous variables, descriptive statistics (ie, mean, SD, median, minimum, and maximum) are reported, and differences between treatments are compared using the Wilcoxon rank‐sum test. Categorical variables are presented as counts (percentages), and differences are compared using the Pearson χ2 test if cell frequencies are sufficient; otherwise, an exact test is used.
For each ischemic and bleeding endpoint, the total number of events and Kaplan‐Meier event rate estimate (95% confidence interval [CI]) through 30 months after randomization are shown separately for AF+ vs AF− patients. Events across the follow‐up period are compared by AF status using the log‐rank test. To examine the relationship between AF and clinical outcomes, unadjusted and adjusted Cox proportional hazards regression models were developed that tested the univariate and multivariate association of AF (AF+ vs AF−) and each clinical outcome. The TRILOGY ACS adjustment models have been built for each ischemic and bleeding outcome and control for baseline characteristics and risk factors.19 When fitting the adjustment model, the proportional hazards assumption was checked for each variable and the linearity assumption was checked for each continuous variable. If the proportional hazard assumption was violated, an interaction of the variable with log‐transformed time was included in the model. If the linearity assumption was violated, a restricted cubic spline was used to approximate the nonlinear relationship of the variable with the outcome. For a full description of the TRILOGY ACS adjustment models used in this analysis, see Supporting Information in the online version of this article.
Only patients enrolled in the platelet function substudy (n = 2564) were considered for the analysis comparing platelet function of medically managed ACS patients with/without a history of AF and treated with prasugrel or clopidogrel. For each treatment group, the Wilcoxon rank‐sum test was used to test whether the distribution of 30‐day PRU was different between patients with vs without AF. Results are presented as medians (IQR). To test for a difference across the 4 groups, the Kruskal‐Wallis test was used.
All statistical tests are 2‐sided, and a P value <0.05 was considered statistically significant. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC) and R version 3.0.3 (R Foundation, Vienna, Austria) by independent statisticians at the Duke Clinical Research Institute in Durham, North Carolina.
Results
A total of 9326 TRILOGY ACS participants underwent randomization according to the ITT definition. As previously noted, 225 (2.4%) participants who lacked data regarding baseline AF status were excluded from this analysis. Key clinical characteristics of AF+ patients are shown in Table 1. In this medically managed population, AF+ patients were older and more likely to have an increased number of medical comorbidities, as well as higher CHA2DS2‐VASc scores (congestive heart failure, hypertension, age ≥75 y, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65–74 y, sex category [women]) and higher Global Registry of Acute Coronary Events (GRACE) risk scores.
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | History of Prior or Current AF | P Value | |
|---|---|---|---|
| Yes, n = 710a | No, n = 8391b | ||
| Demographics | |||
| Sex | <0.001 | ||
| F | 323 (45.5) | 3237 (38.6) | |
| M | 387 (54.5) | 5154 (61.4) | |
| Median age, y | 72.0 (66.0–78.0) | 65.0 (58.0–73.0) | <0.001 |
| Age ≥75 y | 282 (39.7) | 1718 (20.5) | <0.001 |
| Median weight, kg | 77.0 (66.1–88.0) | 75.0 (65.0–86.0) | 0.008 |
| Weight <60 kg | 87/709 (12.3) | 1287/8385 (15.3) | 0.028 |
| Region | <0.001 | ||
| Central/Eastern Europe | 341 (48.0) | 2681 (32.0) | |
| East Asia | 57 (8.0) | 684 (8.2) | |
| Indian subcontinent | 17 (2.4) | 1114 (13.3) | |
| Latin America | 68 (9.6) | 1190 (14.2) | |
| Mediterranean basin | 33 (4.6) | 594 (7.1) | |
| North America | 104 (14.6) | 1129 (13.5) | |
| Western Europe/Scandinavia | 77 (10.8) | 876 (10.4) | |
| Rest of world | 13 (1.8) | 123 (1.5) | |
| Randomized treatment | 0.538 | ||
| Prasugrel | 347 (48.9) | 4202 (50.1) | |
| Clopidogrel | 363 (51.1) | 4189 (49.9) | |
| Prasugrel dose | <0.001 | ||
| 5 mg | 153/347 (44.1) | 1317/4202 (31.3) | |
| 10 mg | 194/347 (55.9) | 2885/4202 (68.7) | |
| Presentation characteristics | |||
| Median time from presentation until start of study drug, h | 117.3 (71.8–167.4) | 107.3 (61.5–158.9) | <0.001 |
| Killip class II–IV on presentation | 137/709 (19.3) | 965/8387 (11.5) | <0.001 |
| Disease classification | 0.881 | ||
| UA/unknown | 217 (30.6) | 2542 (30.3) | |
| NSTEMI | 493 (69.4) | 5849 (69.7) | |
| Cardiovascular risk factors | |||
| Family history of CAD | 186/595 (31.3) | 2269/7544 (30.1) | 0.545 |
| HTN | 636 (89.6) | 6802/8370 (81.3) | <0.001 |
| Hyperlipidemia | 428/671 (63.8) | 4672/7993 (58.5) | 0.007 |
| DM | 228 (32.1) | 3217/8374 (38.4) | <0.001 |
| Current/recent smokingc | 94/703 (13.4) | 1718/8312 (20.7) | <0.001 |
| CVD history | |||
| Prior MI | 292/706 (41.4) | 3582/8333 (43.0) | 0.402 |
| Prior PCI | 194/707 (27.4) | 2167/8351 (25.9) | 0.386 |
| Prior CABG | 134 (18.9) | 1265/8373 (15.1) | 0.008 |
| Prior PAD | 67/690 (9.7) | 592/8270 (7.2) | 0.014 |
| Prior HF | 238/707 (33.7) | 1348/8350 (16.1) | <0.001 |
| Baseline risk assessment | |||
| Median GRACE risk score | 138.0 (120.0–155.0) | 120.0 (104.0–138.0) | <0.001 |
| CHA2DS2‐VASc risk of stroke | |||
| Mean score | 3.7 ± 1.4 | 2.9 ± 1.4 | <0.001 |
| Score | <0.001 | ||
| 1 | 30 (4.2) | 1022 (12.2) | |
| 2 | 91 (12.8) | 2155 (25.7) | |
| 3 | 196 (27.6) | 2287 (27.3) | |
| 4 | 200 (28.2) | 1623 (19.3) | |
| 5 | 122 (17.2) | 784 (9.3) | |
| 6 | 49 (6.9) | 248 (3.0) | |
| 7 | 14 (2.0) | 62 (0.7) | |
| 8 | 3 (0.4) | 4 (0.0) | |
| 9 | 1 (0.1) | 2 (0.0) | |
| Baseline laboratory values | |||
| Median SBP, mm Hg | 130.0 (120.0–140.0) | 129.0 (119.0–139.0) | 0.054 |
| Median heart rate, bpm | 68.0 (61.0–76.0) | 69.0 (62.0–76.0) | 0.412 |
| Median Hgb, g/dL | 13.4 (12.4–14.5) | 13.6 (12.4–14.7) | 0.108 |
| Median CrCl, mL/min | 64.4 (46.5–84.2) | 73.8 (55.1–97.0) | <0.001 |
| Prerandomization procedures | |||
| Angiography performed | 263 (37.0) | 3464 (41.3) | 0.027 |
| Concomitant medications at randomization | |||
| ASA | |||
| Daily dose <100 mg | 246 (34.6) | 2808 (33.5) | 0.521 |
| Daily dose 100–250 mg | 369 (52.0) | 4460 (53.2) | 0.545 |
| Daily dose >250 mg | 54 (7.6) | 583 (6.9) | 0.510 |
| β‐Blocker | 551 (77.6) | 6530 (77.8) | 0.894 |
| ACEI/ARB | 577 (81.3) | 6284 (74.9) | <0.001 |
| Statin | 563 (79.3) | 7033 (83.8) | 0.002 |
| PPI | 185 (26.1) | 2099 (25.0) | 0.539 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid (aspirin); CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHA2DS2‐VASc, congestive heart failure, HTN, age ≥75 y, DM, stroke/TIA, vascular disease, age 65–74 y, sex category (women); CrCl, creatinine clearance; CVD, cardiovascular disease; DM, diabetes mellitus; F, female; GRACE, Global Registry of Acute Coronary Events; HF, heart failure; Hgb, hemoglobin; HTN, hypertension; IQR, interquartile range; M, male; MI, myocardial infarction; NSTEMI, non–ST‐segment elevation myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor; SBP, systolic blood pressure; SD, standard deviation; TIA, transient ischemic attack; UA, unstable angina.
Data are presented as n (%), mean ± SD, or median (IQR).
Total no. of patients is 710 unless otherwise noted. b Total no. of patients is 8391 unless otherwise noted. c Smoking within 30 days of randomization.
Within the ITT population, 4549 (50.0%) participants were randomly assigned to receive prasugrel and 4552 (50.0%) were randomly assigned to clopidogrel. The proportion of AF+ within the ITT population (n = 710, 7.8%) was balanced between treatment groups (7.6% vs 8.0%; P = 0.54). The median duration of AF for both treatment groups was 1 month (P = 0.81), with 64.5% (453/702) of AF+ patients reporting duration of AF ≤1 month. There was no significant difference between treatment groups in the proportion of AF+ patients who were treated with antiarrhythmic drug therapy in the previous 12 months (38.0% vs 37.7%; P = 0.97). Groups were balanced with respect to previous treatment with OACs (19.9% vs 22.3%; P = 0.72). There was no difference in previous thromboembolic events associated with AF between treatment groups (2.0% vs 2.2%; P = 0.41).
Ischemic Outcomes
The unadjusted association of the primary composite endpoint of cardiovascular death, MI, or stroke occurred with increased risk in AF+ patients at 30 months: 31.1% vs 18.4% (hazard ratio [HR]: 1.61, 95% CI: 1.35‐1.92, P < 0.001; Table 2/Figure 1). When all secondary outcomes were evaluated, there was a significantly increased risk of events, particularly stroke, in AF+ patients at 30 months, although the overall incidence of stroke was low (Table 2, Figure 2). The rate of all‐cause death was significantly higher in AF+ patients at 30 months: 18.1% vs 11.1% (HR: 1.62, 95% CI: 1.30‐2.02, P < 0.001). After adjustment, there was no difference in rates of the composite endpoint of cardiovascular death, MI, or stroke (HR: 1.16, 95% CI: 0.97‐1.39, P = 0.11). Rates of all‐cause death were also similar between AF+ and AF− patients (HR: 1.10, 95% CI: 0.87‐1.39, P = 0.42).
Table 2.
Association of AF on the Risk of Ischemic and Bleeding Outcomes
| Efficacy and Safetya Outcomes | AF+, N = 710 | AF−, N = 8391 | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Interaction P Valueb |
|---|---|---|---|---|---|
| Cardiovascular death, MI, or stroke | 1.61 (1.35‐1.92) | 1.16 (0.97‐1.39) | 0.067 | ||
| No. of events | 138 | 1085 | |||
| Event rate at 30 months (95% CI) | 31.1 (25.1‐37.2) | 18.4 (17.1‐19.6) | |||
| Cardiovascular death | 1.69 (1.32‐2.16) | 1.15 (0.89‐1.49) | 0.549 | ||
| No. of events | 73 | 537 | |||
| Event rate at 30 months (95% CI) | 16.7 (11.6‐21.7) | 9.3 (8.4‐10.2) | |||
| MI | 1.48 (1.16‐1.88) | 1.09 (0.85‐1.40) | 0.013 | ||
| No. of events | 75 | 640 | |||
| Event rate at 30 months (95% CI) | 16.3 (12.3‐20.3) | 11.1 (10.0‐12.2) | |||
| Stroke | 2.23 (1.37‐3.63) | 1.59 (0.96‐2.65) | 0.401 | ||
| No. of events | 19 | 107 | |||
| Event rate at 30 months (95% CI) | 6.6 (2.2‐11.0) | 2.0 (1.5‐2.4) | |||
| All‐cause death | 1.62 (1.30‐2.02) | 1.10 (0.87‐1.39) | 0.373 | ||
| No. of events | 88 | 672 | |||
| Event rate at 30 months (95% CI) | 18.1 (13.5‐22.7) | 11.1 (10.2‐12.1) | |||
| GUSTO severe/life‐threatening bleeding | 1.89 (0.80‐4.45) | 1.40 (0.58‐3.37) | 0.685 | ||
| No. of events | 6 | 42 | |||
| Event rate at 30 months (95% CI) | 3.4 (0.0‐7.3) | 0.9 (0.6‐1.2) | |||
| GUSTO severe/life‐threatening/moderate bleeding | 1.33 (0.77‐2.30) | 1.01 (0.58‐1.78) | 0.310 | ||
| No. of events | 14 | 139 | |||
| Event rate at 30 months (95% CI) | 6.8 (2.0‐11.7) | 2.8 (2.3‐3.3) | |||
| TIMI major bleeding | 0.96 (0.44‐2.06) | 0.76 (0.35‐1.65) | 0.716 | ||
| No. of events | 7 | 97 | |||
| Event rate at 30 months (95% CI) | 3.9 (0.0‐7.9) | 2.0 (1.5‐2.4) | |||
| TIMI major or minor bleeding | 1.76 (1.10‐2.81) | 1.46 (0.90‐2.36) | 0.189 | ||
| No. of events | 20 | 150 | |||
| Event rate at 30 months (95% CI) | 8.3 (3.4‐13.2) | 3.0 (2.5‐3.6) |
Abbreviations: AF, atrial fibrillation; CI, confidence interval; GUSTO, Global Use of Strategies to Open Occluded Coronary Arteries; HR, hazard ratio; MI, myocardial infarction; TIMI, Thrombolysis In Myocardial Infarction.
Safety outcomes computed on the population of patients who received study treatment (AF+: n = 702; AF−: n = 8320).
AF‐by‐treatment interaction P values are from the adjusted models.
Figure 1.
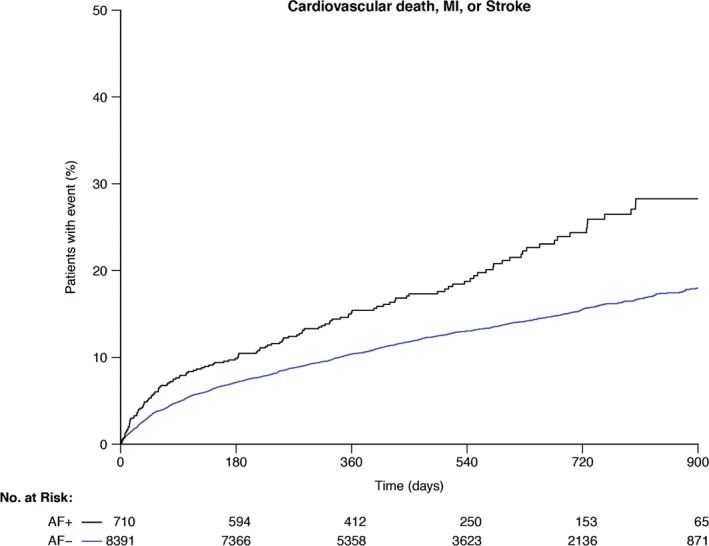
Kaplan‐Meier estimates of the composite of cardiovascular death, MI, or stroke through 30 months in patients with AF (black line) and without AF (blue line). Abbreviations: AF, atrial fibrillation; MI, myocardial infarction.
Figure 2.
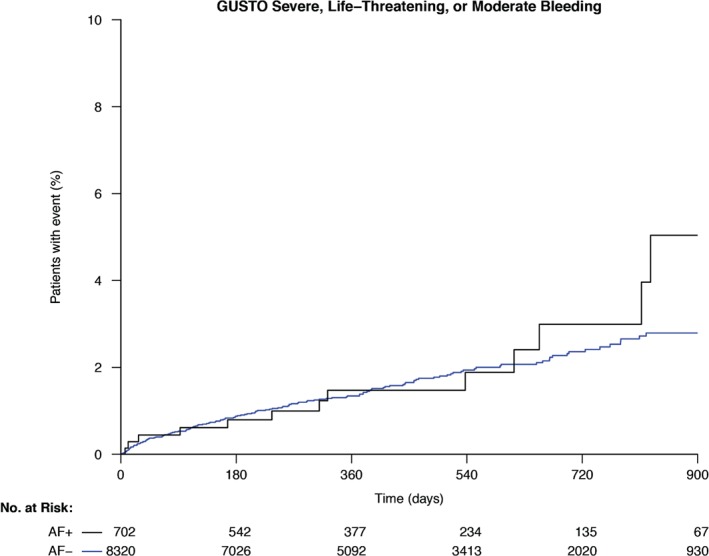
Kaplan‐Meier estimates of GUSTO severe/life‐threatening/moderate bleeding through 30 months in patients with AF (black line) and without AF (blue line). Abbreviations: AF, atrial fibrillation; GUSTO, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries.
Although no significant treatment interactions were found for either the primary composite endpoint or secondary component endpoints, a significant interaction was observed for AF+ patients treated with prasugrel and an increased risk of MI (P for interaction = 0.01).
Bleeding Outcomes
Rates of major bleeding (GUSTO severe or life‐threatening bleeding; GUSTO severe, life‐threatening, or moderate bleeding; TIMI major bleeding) at 30 months were similar between both AF groups. The TIMI major or minor bleeding was higher in patients with AF: 8.3% vs 3.0% (HR: 1.76, 95% CI: 1.10‐2.81, P = 0.016; Table 2). This effect, however, was attenuated after adjustment (HR: 1.46, 95% CI: 0.90‐2.36, P = 0.124). There was no significant interaction for AF by treatment for any bleeding endpoint.
Study‐Drug Discontinuation
Rates of study‐drug discontinuation were highest among AF+ patients. The primary indications for study‐drug discontinuation among AF+ patients include patient decision and adverse events (Table 3). Among AF+ patients, the rate of study‐drug discontinuation for the initiation of OAC therapy was low but significantly higher than in non‐AF patients. In addition, there were no significant differences in loss to follow‐up for either AF+ or AF− study participants (0.3% vs 0.2%; P = 0.34).
Table 3.
Primary Reason for Permanent Study‐Drug Discontinuation
| Disposition, n (%) | All Patients, N = 9022 | AF+, n = 702 | AF−, n = 8320 | P Valuea |
|---|---|---|---|---|
| Permanent study‐drug discontinuation | 2076 (23.0) | 221 (31.5) | 1855 (22.3) | <0.001 |
| Subject had a procedure | 21 (0.2) | 1 (0.1) | 20 (0.2) | 1.00 |
| Adverse event | 793 (8.8) | 91 (13.0) | 702 (8.4) | <0.001 |
| Hemorrhagic | 261 (2.9) | 24 (3.4) | 237 (2.8) | 0.39 |
| Nonhemorrhagic | 532 (5.9) | 67 (9.5) | 465 (5.6) | <0.001 |
| Need for OAC | 102 (1.1) | 25 (3.6) | 77 (0.9) | <0.001 |
| Investigator decision | 97 (1.1) | 7 (1.0) | 90 (1.1) | 0.84 |
| Subject decision | 981 (10.9) | 88 (12.5) | 893 (10.7) | 0.14 |
| Study drug unblinded | 3 (0.0) | 0 (0.0) | 3 (0.0) | 1.00 |
| Entry criteria not met | 62 (0.7) | 7 (1.0) | 55 (0.7) | 0.33 |
| Lost to follow‐up | 16 (0.2) | 2 (0.3) | 14 (0.2) | 0.34 |
Abbreviations: AF, atrial fibrillation; OAC, oral anticoagulation.
P value based on Fisher exact test.
Atrial Fibrillation and Platelet Function
Among patients randomized to clopidogrel, there was no statistically significant difference in the median PRU values between AF+ patients (212 PRU [IQR, 140–246]) and AF− patients (206 PRU [IQR, 143–263]; P = 0.63). However, among those randomized to prasugrel, AF+ patients had significantly higher median PRU values (131 PRU [IQR, 76–197]) than AF− patients (85 PRU [IQR, 42–154]); P < 0.001). In addition, AF+ patients receiving prasugrel had lower median PRU values than did those receiving clopidogrel.
Discussion
This study, which examined the association of AF with ischemic and bleeding outcomes in ACS patients treated medically with DAPT but without oral anticoagulation, yields several important findings. First, AF is accompanied by older age, an increased burden of comorbidities, as well as an increased risk of stroke/systemic embolism and death or death/MI, as evidenced by higher CHA2DS2‐VASc and GRACE risk scores, respectively. Second, the presence of AF is associated with significantly higher median PRU measurement values at 30 days among AF patients treated with the 5‐mg dose of prasugrel and with an increased risk of MI in this subgroup. Third, the presence of AF in study participants with UA/NSTEMI who were treated with DAPT was not associated with worse ischemic or bleeding outcomes after adjustment when compared with patients without AF.
Atrial fibrillation has been assumed to be a causal factor associated with increased morbidity and mortality in patients with MI. Potential mechanistic explanations include loss of atrial contraction, decreased atrioventricular synchrony, elevated filling pressures with enhanced remodeling and worsening left ventricular systolic dysfunction, and increased rates of ventricular arrhythmias and sudden cardiac death.20, 21, 22 Previous studies have shown an increased risk of major adverse cardiovascular events and all‐cause mortality in AF patients with ACS.23, 24, 25, 26, 27, 28, 29 However, controversy still exists as to whether AF plays a prognostic role in poor outcomes or is merely a marker of worsening risk due to an increased number of confounding comorbidities.
In our analysis from a large, randomized trial of medically managed patients with UA/NSTEMI, we demonstrated that patients with AF do not have an increased risk of ischemic or bleeding outcomes after covariate adjustment. This suggests that patients with AF and ACS within the TRILOGY ACS cohort who undergo medical management without oral anticoagulation or planned invasive revascularization represent a high‐risk, older population with a greater burden of confounding comorbid conditions that accompany AF.
To date, few randomized studies have examined outcomes from patients with AF and ACS by treatment strategy. Ruiz‐Nodar and colleagues have described the largest randomized cohort of patients with AF and ACS undergoing revascularization with PCI and subsequent outcomes based on antithrombotic therapy.30 In this 2‐center cohort of patients with AF and ACS who were treated with medical therapy and revascularization, those discharged on DAPT comprising aspirin and clopidogrel (n = 174, 40.8%) had increased mortality compared with patients receiving triple oral antithrombotic therapy (n = 213, 50%): 38.7% vs 26.5% (HR: 4.9, 95% CI: 1.61‐7.54, P = 0.002), as well as higher rates of major adverse cardiovascular events: 27.8% vs 17.8% (HR: 3.43, 95% CI: 2.17‐11.1, P < 0.01), but no differences in major or minor bleeding.30
In comparison, observational studies have demonstrated conflicting results with regard to outcomes for patients with AF and ACS based on antithrombotic drug regimens. Hansen et al showed that combination therapy with OACs and DAPT is associated with a significantly higher risk of bleeding compared with monotherapy.9 Conversely, Goa et al showed that combination therapy with warfarin and DAPT did not decrease the rates of MI or mortality but did increase major bleeding.31
Our analysis cohort is derived from a randomized population of elderly participants, who are at greater risk for developing AF. In addition, patients were balanced with respect to study‐drug treatment with a P2Y12 receptor inhibitor (prasugrel: n = 347, 48.9%; clopidogrel: n = 363, 51.1%) and were not treated with OACs. In this study, we observed significant increases in the incidence of primary‐endpoint events in participants with both ACS and AF. However, after adjusting for possible confounders such as age, sex, hypertension, history of diabetes mellitus, systolic blood pressure, heart rate, Killip class at presentation, and history of MI, the rates of primary‐endpoint events were not significantly different between study participants with vs without AF. These findings suggest that comorbidities and not AF play an increased prognostic role in outcomes for ACS patients treated primarily with medical therapy, and that AF is merely one marker of overall increased risk.
Although OAC has been proven to be superior to DAPT in reducing stroke, DAPT offers a reasonable option for reducing ischemic outcomes including both all‐cause and cardiac mortality. In our analysis, DAPT was chosen by treating physicians and was not an exclusion criterion in the trial. The results from our analysis are important given the documented suboptimal utilization of oral anticoagulation in patients with AF.32, 33 Dual antiplatelet therapy has been demonstrated to reduce the risk of ischemic endpoints including stroke and systemic embolism (the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events [ACTIVE W] trial, NCT00243178).34 Additionally, long‐term use of DAPT with a P2Y12 receptor inhibitor has been shown to reduce the risk ischemic endpoints including stroke (Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin [PEGASUS‐TIMI 54], NCT01225562).35
Atrial fibrillation is known to cause increased platelet activation. However, the effect of specific P2Y12 receptor inhibitors on PRU values in the setting of AF is unknown. In our study, the 5‐mg/d dose of prasugrel was used more frequently among elderly AF+ patients to decrease the risk of bleeding, which explains the difference in observed PRU values among patients taking prasugrel. In addition, among AF+ patients, the PRU value associated with prasugrel is lower than the PRU value associated with clopidogrel. This suggests that prasugrel did reduce platelet reactivity to a greater extent than clopidogrel for patients with and without AF. However, in patients with AF+ vs AF− on prasugrel, median PRU values were higher for those AF+ patients receiving prasugrel, suggesting that full suppression of platelets was not achieved. It is unclear from this study whether multiple factors including prasugrel dosing, comorbidities, higher percentage of elderly patients in the AF+ group, or AF type led to such a finding and whether the inability to fully suppress platelet aggregation for AF+ patients on prasugrel led to increased risk of MI in the prasugrel group.
Study Limitations
Our study has several limitations. First, the present investigation is a post hoc analysis of prospectively collected clinical trial data, and the findings are only hypothesis‐generating. Second, AF+ patients were defined as those with either current or prior AF as a clinical variable only, without detailed information or a confirmatory electrocardiogram regarding timing or type of AF or proof of AF, respectively. Third, patients receiving OAC therapy were excluded from this study, and the reasons for not using an OAC were not collected on the baseline case‐report form. In addition, patients were only initiated on OAC therapy after study‐drug discontinuation. Finally, there may be unmeasured confounders associated with AF that potentially could have influenced comparisons.
Conclusion
Among a randomized population of high‐risk elderly patients with ACS managed medically with DAPT and without OAC therapy or invasive revascularization, current or prior AF was not associated with higher adjusted risks of ischemic and bleeding outcomes. Further studies are needed to elucidate both the contribution of AF to the constellation of comorbid conditions affecting prognosis in this patient population as well as optimal therapeutic strategies for patients with both ACS and AF.
Supporting information
AppendixS1
Acknowledgments
The authors thank Karen Pieper, MS, of the Duke Clinical Research Institute, Durham, North Carolina, for management of the statistical analyses for the TRILOGY ACS study. The authors also thank Jonathan McCall, MS, of the Duke Clinical Research Institute, for editorial assistance in preparing this manuscript. Ms. Pieper and Mr. McCall received no compensation for their work other than their usual salaries.
Drs. Jackson, Cyr, Neely, and Corbalán have no conflicts of interest to disclose. Dr. Piccini receives consulting fees from Forest Laboratories, Janssen Pharmaceuticals, Medtronic, and Spectranetics. Dr. Roe receives research funding from Eli Lilly, Sanofi‐Aventis, Daiichi Sankyo, Janssen Pharmaceuticals, Ferring Pharmaceuticals, the American College of Cardiology, the American Heart Association, and the Familial Hypercholesterolemia Foundation. He also receives consulting payments or honoraria from AstraZeneca, Boehringer Ingelheim, Merck, Amgen, Pri‐Med, and Elsevier Publishers. All conflicts of interest are listed at https://www.dcri.org/about‐us/conflict‐of‐interest. Dr. Martinez reports receiving consulting fees/honoraria from Eli Lilly and Daiichi Sankyo. Dr. Lüscher has received research grants and honoraria from AstraZeneca Switzerland and Eli Lilly Switzerland, Indianapolis, and Japan. Dr. Lopes reports receiving research grants and consulting fees for Bristol‐Myers Squibb and GlaxoSmithKline, and consultancies for Bayer Corp. U.S., Boehringer Ingelheim, and Pfizer. Dr. Winters is an employee and minor shareholder of Eli Lilly and Company. Dr. White reports receiving grant support from Sanofi‐Aventis, Eli Lilly, The Medicines Company, the National Institutes of Health, Pfizer, Roche, Johnson & Johnson, Schering‐Plough, Merck Sharpe & Dohme, AstraZeneca, GlaxoSmithKline, Daiichi Sankyo Pharma Development, and Bristol‐Myers Squibb; he also participates in advisory boards for Merck Sharpe & Dohme, Roche, and Regado Biosciences. Dr. Armstrong reports receiving consulting fees from Eli Lilly, Hoffmann‐La Roche, Merck, Axio Research, and Orexigen; grant support from Boehringer Ingelheim, Hoffmann‐La Roche, Sanofi‐Aventis, Scios, Ortho Biotech, Johnson & Johnson, Janssen Pharmaceuticals, GlaxoSmithKline, Amylin Pharmaceuticals, and Merck; and payment for developing educational presentations from AstraZeneca and Eli Lilly and Company. Dr. Fox reports receiving research grants from Lilly, Bayer, Johnson & Johnson, and AstraZeneca; speakers bureau payments from Bayer, Johnson & Johnson, AstraZeneca, and Sanofi‐Aventis; and consulting/other payments from Lilly, Bayer, Johnson & Johnson, AstraZeneca, Sanofi‐Aventis, Boehringer Ingelheim, and Eli Lilly. Dr. Prabhakaran reports receiving research grants from Eli Lilly and the Medtronic Foundation and honoraria from Eli Lilly. Dr. Bhatt has served on the advisory board for Cardax, Elsevier PracticeUpdate Cardiology, Medscape Cardiology, and Regado Biosciences; has served on the board of directors for Boston VA Research Institute and the Society of Cardiovascular Patient Care; has been chair of the American Heart Association Get With The Guidelines Steering Committee; has served on data monitoring committees for Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, and Population Health Research Institute; has received honoraria from American College of Cardiology (senior associate editor, Clinical Trials and News, http://www.acc.org), Belvoir Publications (editor in chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including TRILOGY ACS), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (editor in chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (associate editor; section editor, Pharmacology), Population Health Research Institute (clinical trial steering committee), Slack Publications (chief medical editor, Cardiology Today's Intervention), and WebMD (CME steering committees); has served as deputy editor for Clinical Cardiology; has received research funding from Amarin, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Medtronic, Pfizer, Roche, Sanofi‐Aventis, and The Medicines Company; and reports unfunded research for FlowCo, PLx Pharma, and Takeda. Dr. Ohman reports receiving grant support and travel expenses from Daiichi Sankyo and Eli Lilly; consulting fees from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals, Liposcience, Merck, Pozen, Hoffmann‐La Roche, Sanofi‐Aventis, The Medicines Company, and WebMD; grant support from Gilead Sciences; and lecture fees from Gilead Sciences, Boehringer Ingelheim, and The Medicines Company.
The TRILOGY ACS study was funded by Daiichi Sankyo and Eli Lilly and Company. The study sponsors had no role in the design or conduct of this study. All statistical analyses were performed independently by the Duke Clinical Research Institute, Durham, North Carolina. An employee of Eli Lilly and Company (K.J. Winters) participated as an author on this study. https://clinicaltrials.gov/ct2/show/NCT00699998.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. McManus DD, Huang W, Domakonda KV, et al. Trends in atrial fibrillation in patients hospitalized with an acute coronary syndrome. Am J Med. 2012;125:1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 3. Feinberg WM, Blackshear JL, Laupacis A, et al. Prevalence, age distribution, and gender of patients with atrial fibrillation: analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 4. Goto S, Bhatt DL, Röther J, et al; REACH Registry Investigators. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J. 2008;156:855–863. [DOI] [PubMed] [Google Scholar]
- 5. Jabre P, Roger VL, Murad MH, et al. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta‐analysis. Circulation. 2011;123:1587–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Connolly S, Pogue J, Hart R, et al; ACTIVE Writing Group. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–1912. [DOI] [PubMed] [Google Scholar]
- 7. Reed GW, Cannon CP. Triple oral antithrombotic therapy in atrial fibrillation and coronary artery stenting. Clin Cardiol. 2013;36:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Depta JP, Cannon CP, Fonarow GC, et al; Get With The Guidelines Steering Committee and Investigators. Patient characteristics associated with the choice of triple antithrombotic therapy in acute coronary syndromes. Am J Cardiol. 2009;104:1171–1178. [DOI] [PubMed] [Google Scholar]
- 9. Hansen ML, Sørensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433–1441. [DOI] [PubMed] [Google Scholar]
- 10. Lopes RD, Starr A, Pieper CF, et al. Warfarin use and outcomes in patients with atrial fibrillation complicating acute coronary syndromes. Am J Med. 2010;123:134–140. [DOI] [PubMed] [Google Scholar]
- 11. Fu A, Singh K, Abunassar J, et al; CAPITAL Investigators. Ticagrelor in triple antithrombotic therapy: predictors of ischemic and bleeding complications. Clin Cardiol. 2016;39:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv. 2011;4:522–534. [DOI] [PubMed] [Google Scholar]
- 13. Potpara TS, Lip GY, Dagres N, et al. Management of acute coronary syndrome in patients with non‐valvular atrial fibrillation: results of the European Heart Rhythm Association Survey. Europace. 2014;16:293–298. [DOI] [PubMed] [Google Scholar]
- 14. Chin CT, Roe MT, Fox KA, et al; TRILOGY ACS Steering Committee . Study design and rationale of a comparison of prasugrel and clopidogrel in medically managed patients with unstable angina/non–ST‐segment elevation myocardial infarction: the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes (TRILOGY ACS) trial. Am Heart J. 2010;160:16e1–22.e1. [DOI] [PubMed] [Google Scholar]
- 15. Roe MT, Armstrong PW, Fox KA, et al; TRILOGY ACS Investigators . Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367:1297–1309. [DOI] [PubMed] [Google Scholar]
- 16. GUSTO Investigators . An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. [DOI] [PubMed] [Google Scholar]
- 17. Bovill EG, Terrin ML, Stump DC, et al. Hemorrhagic events during therapy with recombinant tissue‐type plasminogen activator, heparin, and aspirin for acute myocardial infarction: results of the Thrombolysis in Myocardial Infarction (TIMI), Phase II Trial. Ann Intern Med. 1991;115:256–265. [DOI] [PubMed] [Google Scholar]
- 18. Gurbel PA, Erlinge D, Ohman EM, et al. Platelet function during extended prasugrel and clopidogrel therapy for patients with ACS treated without revascularization: the TRILOGY ACS platelet function substudy. JAMA. 2012;308:1785–1794. [DOI] [PubMed] [Google Scholar]
- 19. Roy A, Roe MT, Neely ML, et al. Impact of Human Development Index on the profile and outcomes of patients with acute coronary syndrome. Heart. 2015;101:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clark DM, Plumb VJ, Epstein AE, et al. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol. 1997;30:1039–1045. [DOI] [PubMed] [Google Scholar]
- 21. Berton G, Cordiano R, Cucchini F, et al. Atrial fibrillation during acute myocardial infarction: association with all‐cause mortality and sudden death after 7‐year of follow‐up. Int J Clin Pract. 2009;63:712–721. [DOI] [PubMed] [Google Scholar]
- 22. Ehrlich JR, Nattel S, Hohnloser SH. Atrial fibrillation and congestive heart failure: specific considerations at the intersection of two common and important cardiac disease sets. J Cardiovasc Electrophysiol. 2002;13:399–405. [DOI] [PubMed] [Google Scholar]
- 23. Køber L, Swedberg K, McMurray JJ, et al. Previously known and newly diagnosed atrial fibrillation: a major risk indicator after a myocardial infarction complicated by heart failure or left ventricular dysfunction. Eur J Heart Fail. 2006;8:591–598. [DOI] [PubMed] [Google Scholar]
- 24. Pizzetti F, Turazza FM, Franzosi MG, et al. Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: the GISSI‐3 data. Heart. 2001;86:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wong CK, White HD, Wilcox RG, et al. New atrial fibrillation after acute myocardial infarction independently predicts death: the GUSTO‐III experience. Am Heart J. 2000;140:878–885. [DOI] [PubMed] [Google Scholar]
- 26. Crenshaw BS, Ward SR, Granger CB, et al. Atrial fibrillation in the setting of acute myocardial infarction: the GUSTO‐I experience. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J Am Coll Cardiol. 1997;30:406–413. [DOI] [PubMed] [Google Scholar]
- 27. Lopes RD, White JA, Atar D, et al. Incidence, treatment, and outcomes of atrial fibrillation complicating non‐ST‐segment elevation acute coronary syndromes. Int J Cardiol. 2013;168:2510–2517. [DOI] [PubMed] [Google Scholar]
- 28. Fosbol EL, Wang TY, Li S, et al. Safety and effectiveness of antithrombotic strategies in older adult patients with atrial fibrillation and non–ST‐elevation myocardial infarction. Am Heart J. 2012;163:720–728. [DOI] [PubMed] [Google Scholar]
- 29. Lopes RD, Elliott LE, White HD, et al. Antithrombotic therapy and outcomes of patients with atrial fibrillation following primary percutaneous coronary intervention: results from the APEX‐AMI trial. Eur Heart J. 2009;30:2019–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruiz‐Nodar JM, Marín F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol. 2008;51:818–825. [DOI] [PubMed] [Google Scholar]
- 31. Gao F, Zhou YJ, Wang ZJ, et al. Meta‐analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int J Cardiol. 2011;148:96–101. [DOI] [PubMed] [Google Scholar]
- 32. Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927–934. [DOI] [PubMed] [Google Scholar]
- 33. Reynolds MR, Shah J, Essebag V, et al. Patterns and predictors of warfarin use in patients with new‐onset atrial fibrillation from the FRACTAL Registry. Am J Cardiol. 2006;97:538–543. [DOI] [PubMed] [Google Scholar]
- 34. Hohnloser SH, Pajitnev D, Pogue J, et al. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W substudy. J Am Coll Cardiol. 2007;50:2156–2161. [DOI] [PubMed] [Google Scholar]
- 35. Bonaca MP, Bhatt DL, Cohen M, et al; PEGASUS‐TIMI 54 Steering Committee and Investigators . Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AppendixS1


