ABSTRACT
This analysis investigated the extent of different outcome reductions from low‐density lipoprotein cholesterol (LDL‐C) lowering following ezetimibe/simvastatin treatment and the proportionality of outcome to LDL‐C reductions. The authors searched PubMed between 1997 and mid‐June 2015 (any language) and the Cochrane Library to identify all randomized controlled trials comparing ezetimibe/simvastatin with placebo or less intensive LDL‐C lowering. Risk ratios (RR) and 95% confidence intervals (CIs), standardized to 20 mg/dL LDL‐C reduction, were calculated for 5 primary outcomes (fatal and nonfatal) and 4 secondary outcomes (non‐cardiovascular [CV] death, cancer, myopathy, and hepatopathy). Five ezetimibe/simvastatin RCTs (30 051 individuals) were eligible, 2 comparing ezetimibe/simvastatin vs placebo and 3 vs less intensive treatment. Outcomes reduced almost to the same extent were stroke (RR: −13%, 95% CI: −21% to −3%), coronary heart disease (CHD; RR: −12%, 95% CI: −19% to −5%), and composite of stroke and CHD (RR: −14%, 95% CI: −20% to −8%). Absolute risk reductions: 5 strokes, 10 CHD events, and 16 stroke and CHD events prevented for every 1000 patients treated for 5 years. Residual risk was almost 7× higher than absolute risk reduction for all the above outcomes. All death outcomes were not reduced, and secondary outcomes did not differ between groups. Logarithmic risk ratios were not associated with LDL‐C lowering. Our meta‐analysis provides evidence that, in patients with different CV disease burden, major CV events are safely reduced by LDL‐C lowering with ezetimibe/simvastatin, while raising the hypothesis that the extent of LDL‐C lowering might not be accompanied by incremental clinical‐event reduction.
Introduction
The lowering of low‐density lipoprotein cholesterol (LDL‐C) is accompanied by cardiovascular (CV) risk reduction.1 Beyond lifestyle changes, statins represent the more established option to reduce LDL‐C in patients with hypercholesterolemia, and the majority of randomized controlled trials (RCTs) so far investigated both their effects on hard endpoints and their long‐term safety. Although a great amount of evidence suggests that the beneficial effect of statins on CV outcomes is accomplished by LDL‐C lowering per se, the potential pleiotropic effects of statins as modulators of risk reduction could not be definitively excluded.2 In the usual clinical practice, the introduction of additional LDL‐C–lowering agents on top of statins for different clinical reasons (eg, patient intolerance of high statin doses, only a small additional reduction from doubling statin dose, LDL‐C target not achieved with high statin dose alone, taking advantage of a complementary mechanism of action to statins) may be considered, especially in high‐risk patients.3, 4
Although more intensive LDL‐C lowering (compared with less intensive LDL‐C lowering) based on statin monotherapy steadily reduced all types of clinical outcomes in a wide spectrum of patients with CV risk,1, 5 the use of different traditional lipid‐modifying agents such as niacin, fibrates, and cholesteryl ester transfer protein (CETP) inhibitors as an adjunct to statins was not accompanied by incremental CV event reduction. Ezetimibe (an enterocyte cholesterol transporter Niemann‐Pick C1‐like 1 protein inhibitor) in combination with simvastatin has been reported to effectively reduce LDL‐C levels by almost 23%,6 but whether this reduction, compared with less active treatment (placebo or simvastatin alone), is accompanied by a positive effect on different outcomes, and whether there is a proportional association between the LDL‐C lowering and reduced event rate, still remain unclear.4
Previous overviews and meta‐analyses have specifically focused on the effects of statins alone on clinical outcomes,1, 5 whereas pooled long‐term efficacy and safety results of LDL‐C lowering following the use of the ezetimibe/simvastatin combination are lacking. We have done a comprehensive overview of ezetimibe/simvastatin RCTs accompanied by a series of meta‐analyses and meta‐regression analyses to address these issues. We quantified the relative and absolute benefits of LDL‐C lowering following ezetimibe/simvastatin treatment in patients with different cardiovascular disease (CVD) burden on distinct types of CV and safety outcomes, and we investigated the effects of LDL‐C reductions of different extent. Finally, we calculated the residual risk in those randomized to ezetimibe/simvastatin treatment as a measure of inadequate or suboptimal treatment.
Methods
Trial Eligibility
The present overview intended to include RCTs of LDL‐C lowering with ezetimibe/simvastatin, in which (1) ezetimibe/simvastatin was compared with placebo with the intention to investigate the consequences of LDL‐C differences (intentional LDL‐C–lowering trials, placebo controlled); or (2) a more intensive LDL‐C lowering based on the combination of ezetimibe/simvastatin was compared with a less intensive one (intentional LDL‐C–lowering trials, more or less intensive).
In addition, trials had to meet the following predetermined criteria: (1) protocol including measurement of ≥1 type of CV event as a primary endpoint; (2) LDL‐C values measured at baseline and follow‐up; (3) follow‐up of ≥6 months; (4) a minimum of 5 events during follow‐up; (5) achieved LDL‐C difference among arms of ≥5 mg/dL; (6) randomized allocation to treatments; (7) publication before June 15, 2015. The database search was done by 2 of the authors (C.Th and G.S.) by consulting PubMed between 1997 and mid‐June 2015 (any language) and the Cochrane Library. Reference lists from retrieved articles and abstracts from international CV conferences were also sought. Recommendations of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement were adhered to.7
Outcomes
Data on 5 predetermined primary outcomes were extracted: (1) stroke (fatal and nonfatal); (2) coronary heart disease (CHD) events (coronary death and nonfatal myocardial infarction [MI]); (3) major CV events (composite of stroke and CHD); (4) CV death; (5) all‐cause death. The definition of outcomes reported in the original article was retained, but whenever possible transient ischemic attacks, angina, and revascularization procedures were excluded. Sudden death was added to CV death, but death of unknown origin was only considered in the all‐cause death outcome. Four predetermined secondary outcomes were also extracted: (1) non‐CV death; (2) cancer (fatal and nonfatal); (3) myopathy (creatine kinase >10× upper limit of normal with muscle symptoms); (4) hepatopathy (increases of alanine or aspartate transaminase >3× upper limit of normal). Two authors (C.Th. and G.S.) independently extracted the data, with differences resolved by discussion.
Quality Assessment
Selection, detection, and attrition bias were assessed based on randomization procedure, method of blinding, and combined evaluation of lost to follow‐up and therapy discontinuation ratio. Studies of higher quality were those reporting randomization generation sequence, with double blinding, and lost to follow‐up ratio <10%, accompanied by therapy discontinuation of <10% per year of follow‐up. We also arbitrarily assigned higher quality to studies with ≥5000 patient‐years. Moreover, we evaluated the number of primary outcomes reported in each individual trial, with those reporting ≥3 types of outcomes being of higher quality compared with those reporting <3 types of outcomes. The evaluation and scoring of these 5 criteria were based on a binomial integer scale ranging from 0 to 1, with 1 being better. The scores were summed up and reflected the overall study quality, with 5 being best. Trials scoring 4 or 5 were considered of higher quality than those scoring 3 or lower.
Statistical Analysis
All analyses were done using data as tabulated in the original publications. In each group, baseline patient characteristics and LDL‐C differences between randomized treatments were the means of all individual trial values weighted by number of patients and follow‐up duration. For every group of comparisons, the null hypothesis of no difference between randomized treatments (ezetimibe/simvastatin vs placebo or less active treatment) was tested for each of the outcomes. Relative risk estimates (with 95% confidence intervals [CI]) were combined using a random‐effects model, in which the log relative risk for every trial was weighted by the reciprocal of the variance of the log relative risk. The proportion of inconsistency across studies not explained by chance was quantified with the I2 statistic. Whenever no significant heterogeneity was detected by the χ2 Q statistics (P > 0.05), a fixed‐effect model was also implemented. The influence of individual trials on pooled effect sizes was tested by excluding one trial at a time (one‐study removed analysis): If the point estimate of the combined effect size with a given trial excluded lay outside the CI of the overall estimate risk with all available trials, the trial in question was considered to have an excessive influence.
Risk ratios (RR) and their 95% CIs were reported using the Mantel‐Haenszel method, and the effect of LDL‐C lowering on each outcome was illustrated with forest plots under the random‐effects model. Risk estimates were standardized to a difference of 20 mg/dL by multiplying the relative risk estimate in each trial by the appropriate factor after having considered the effect of the inverse variance of individual trials. Five‐year absolute risk reductions (weighted for follow‐up period inverse variance and sample size) of standardized LDL‐C–lowering treatment were also calculated, as well as numbers of patients needed to treat for 5 years to prevent 1 outcome event. Residual risk (remaining risk in the ezetimibe/simvastatin group) represented the difference between baseline outcome risk calculated in the placebo or less intensive LDL‐C–lowering group and the absolute risk reduction. Random‐effects meta‐regression models with inverse variance weighting were constructed to explore whether the achieved LDL‐C difference (independent variable) between randomized groups explained the variance of relative risk estimates for various outcomes. To correct for different levels of control LDL‐C, meta‐regressions were also calculated by expressing achieved LDL‐C reductions as percentages of respective ongoing LDL‐C values in the control groups. The presence of publication bias was investigated graphically by funnel plots of precision (random effects plotting) and the Duval and Tweedie trim‐and‐fill method. All statistical analyses were done using Comprehensive Meta‐Analysis software, version 3 (Biostat, Englewood, NJ). In each individual analysis, a P value <0.05 was considered to indicate statistical significance.
Results
Trials and Patients
Figure 1 illustrates the investigational steps to identify trials for inclusion in the meta‐analysis. For the search strategy and a list of excluded trials, see Supporting Information, tables S1 and S2, in the online version of this article.
Figure 1.
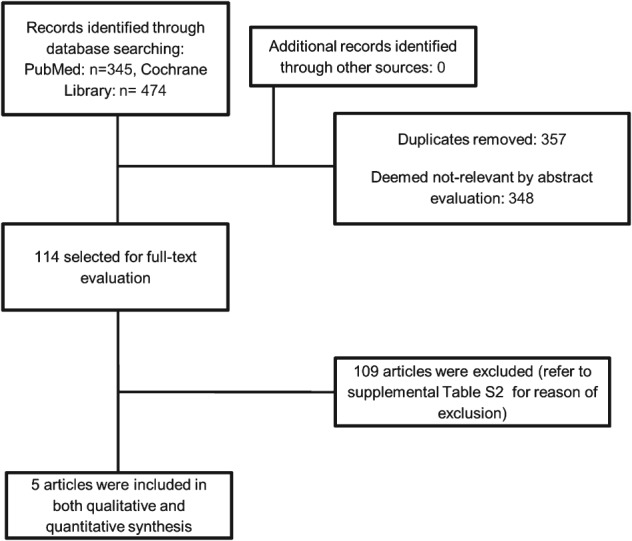
Identification process for inclusion of randomized controlled trials.
This procedure identified 5 eligible RCTs. Characteristics of the 5 included trials,8, 9, 10, 11, 12 with a total of 30 051 participants followed up for a mean of 5.5 years (163 778 patient‐years), are presented in Table 1. Eighty percent of RCTs (4/5) were of higher quality (scoring from 4 to 5). Two trials10, 11 (11 143 participants, 961 major CV events) were of intentional LDL‐C lowering vs placebo, and 3 trials8, 9, 12 (18 908 participants, 2751 major CV events) were of intentional more vs less intensive LDL‐C lowering. One study reported data only for major CV events and all‐cause death.12
Table 1.
Characteristics of Included Ezetimibe/Simvastatin Trials of LDL‐C Lowering
| Trial | Comparator | Clinical Setting | Statin Pretreatment % | Follow‐up, y | Participants, n | Age, y | Men, % | BMI, kg/m2 | Smoking, % | DM, % | HTN, % | 10‐y Risk CV Death, % | Baseline LDL‐C, EZE Group, mg/dL | Baseline LDL‐C, Non‐EZE Group, mg/dL | Achieved LDL‐C, EZE Group, mg/dL | Achieved LDL‐C, Non‐EZE Group, mg/dL | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ENHANCE, 20088 | Simvastatin | FH | 81 | 2 | 720 | 45.9 | 51.4 | 27 | 28.7 | 1.8 | 16 | 1.4 | 319 | 318 | 141.3 | 192.7 | 4/5 |
| SEAS, 200810 | Placebo | AoS | 0 | 4.4 | 1873 | 67.6 | 61.4 | 26.9 | 19 | 0 | 52 | 13.7 | 139 | 140 | 102 | 137.3 | 5/5 |
| SHARP, 201111 | Placebo | CKD | NR | 4.9 | 9270 | 62 | 63 | 27.1 | 13 | 23 | NR | 19.6 | 107.1 | 107.5 | 69 | 104.9 | 5/5 |
| IMPROVE‐IT, 20149 | Simvastatin | Post‐MI | 35 | 6 | 18144 | 64 | 76 | 28.3 | 33 | 27 | 61 | 11.5 | 94 | 94 | 53.7 | 69.5 | 5/5 |
| West et al, 201112 | Simvastatin | PAD | 0 | 2 | 44 | 62 | 55 | 29 | 50 | 30 | 78 | NR | 118 | 118 | 67.5 | 87 | 3/5 |
Abbreviations: AoS, mild to moderate asymptomatic aortic stenosis; BMI, body mass index; CKD, chronic kidney disease; CV, cardiovascular; DM, diabetes mellitus; ENHANCE, Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression; EZE, ezetimibe; FH, heterozygous familial hypercholesterolemia; HTN, hypertension; IMPROVE‐IT, Improved Reduction of Outcomes: Vytorin Efficacy International Trial; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; NR, not reported; PAD, peripheral arterial (lower limb) disease; SEAS, Simvastatin and Ezetimibe in Aortic Stenosis; SHARP, Study of Heart and Renal Protection.
Effects of Ezetimibe/Simvastatin LDL‐C–Lowering Treatment on Various Outcomes
Achieved LDL‐C differences between treatments were larger in placebo‐controlled trials10, 11 than in more vs less intensive LDL‐C–lowering trials8, 9, 12 (−35.8 vs −17.1 mg/dL), even after standardization to ongoing LDL‐C levels in the placebo or less active group (32.8% vs 22.8%). Ezetimibe‐based LDL‐C–lowering treatment reduced the risk of stroke, CHD, and their composite outcome but was not associated with better mortality outcomes (Figure 2A). A standardized LDL‐C reduction of 20 mg/dL was found to reduce the risk of CV events by 14%. In terms of absolute risk, the same LDL‐C reduction could prevent 5 strokes, 10 CHD, and 16 major CV events (composite of stroke and CHD) for every 1000 patients treated for 5 years (number needed to treat: 216, 102, and 63, respectively). The residual risk for all primary outcomes (Table 2) was higher the higher the baseline risk and the lower the absolute risk reduction. For example, residual risk of major CV events amounted to 115 events for each 1000 patients during 5 years, suggesting that suboptimal treatment was almost 7.2× greater (115/16) than absolute risk reduction (16%) from LDL‐C lowering. This “suboptimal to beneficial” treatment ratio following LDL‐C lowering, resulting from the same group of trials, was 6.8 (34/5) and 7.3 (73/10) for stroke and CHD, respectively.
Figure 2.
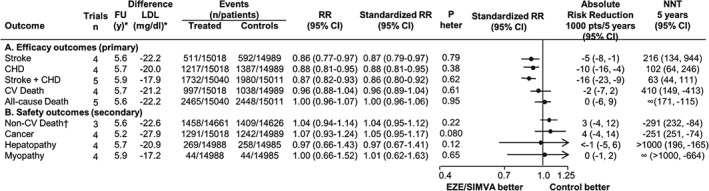
Relative and absolute risk reduction of various outcomes in EZE/SIMVA trials of LDL‐C lowering. Standardized RR is to an LDL‐C reduction of 20 mg/dL. Absolute risk reduction refers to the number (and 95% CI) of events prevented every 1000 patients treated for 5 years with a standardized RR. NNT is the numbers (and 95% CI) of patients needed to treat for 5 years to prevent 1 event. Abbreviations: CHD, coronary heart disease; CI, confidence interval; CV, cardiovascular; ENHANCE, Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression; EZE/SIMVA, ezetimibe/simvastatin; FU, follow‐up; LDL‐C, low‐density lipoprotein cholesterol; n, number; NNT, number needed to treat; P heter., P for heterogeneity; RR, Mantel‐Haenszel risk ratios. * Weighted for the inverse variance. † ENHANCE trial reported 0 non‐CV deaths.
Table 2.
Residual Riska in the Ezetimibe‐Treated Groups by Different Primary Outcomes
| Outcome | Baseline Outcome Risk, % | Residual Risk per 1000 Patients/5 Years (95% CI) |
|---|---|---|
| Stroke | 3.9 | 34 (31–38) |
| CHD | 8.3 | 73 (67–79) |
| Stroke + CHD | 13.1 | 115 (108–122) |
| CV death | 6.9 | 67 (62–71) |
| All‐cause death | 16.3 | 163 (157–172) |
Abbreviations: CI, confidence interval; CHD, coronary heart disease; CV, cardiovascular.
Residual risk refers to the residual events every 1000 patients treated for 5 years.
Lowering of LDL‐C by ezetimibe/simvastatin was not accompanied by different incident rates of non‐CV death, cancer, hepatopathy, and myopathy as compared with placebo or less active treatment (Figure 2B). In our analysis, baseline risk for non‐CV death, cancer, hepatopathy, and myopathy (9.6%, 8.3%, 1.7%, and 0.3%, respectively) remained almost unaltered after 5.5 years. By excluding the trial of lower quality,12 no different results for both primary and secondary outcomes were obtained (data not shown).
Outcome Reductions and Extent of LDL‐C Lowering by Ezetimibe/Simvastatin
Although the span of LDL‐C reductions was narrower for stroke and CHD compared with that observed for their composite outcome (9.8–51.4 mg/dL vs 15.8–51.4 mg/dL), the natural logarithm of the RR of stroke, CHD, and of their composite was not significantly related to the extent of LDL‐C lowering (Figure 3A). In other words, progressively lower LDL‐C levels were not accompanied by incremental lowering of clinical events. When meta‐regressions were calculated by using percentage changes in LDL‐C (maximum reduction of 34%), regression coefficients did not change for stroke and major CV events (composite stroke and CHD; Figure 3B). By contrast, although without statistical significance, the direction of slope for CHD was inversed.
Figure 3.
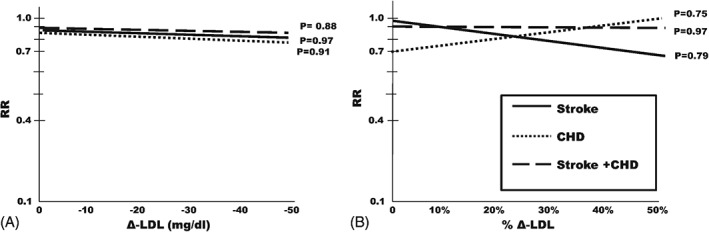
Relationships of (A) primary outcome reductions to the extent of LDL‐C reductions. Meta‐regressions of risk ratios on absolute LDL‐C differences (active treatment group minus placebo or less active treatment group). Relationships of (B) outcome reductions to percentage LDL reductions. The meta‐regressions of (A) are calculated on percentage LDL‐C differences (LDL‐C difference as percentage of on‐treatment LDL‐C in the control group). Regressions relative to stroke are in continuous lines; CHD, in dotted lines; composite of stroke and CHD, in dashed lines. Abbreviations: CHD, coronary heart disease; Δ‐LDL, LDL‐C difference; LDL‐C, low‐density lipoprotein cholesterol; RR, risk ratio.
Influence of Studies on Pooled Effect Sizes, Fixed‐Effect Models, and Publication Bias
Whenever a fixed‐effect model was implemented, RRs and their significance did not substantially change (data not shown). Also, by applying the one‐study removed analytical procedure, no trial had an excessive influence in any analysis. Although graphic representations could not exclude publication bias for all primary outcomes, significant bias was denied by the trim‐and‐fill method. For this assessment, see Supporting Information, Figure S1 and Table S3, in the online version of this article.
Discussion
This is the first overview to systematically address the extent of LDL‐C–lowering benefits following ezetimibe/simvastatin treatment and whether this extent is proportional to LDL‐C reduction in patients with different CVD burden. Additionally, over 5.5 years of follow‐up, we assessed whether the combination of ezetimibe/simvastatin is safe in terms of non‐CV death, cancer, myopathy, and hepatopathy incidence.
We observed that the relative risk reduction of stroke, CHD, and of their composite, standardized for achieved LDL‐C difference of 20 mg/dL (close to the mean observed in trials considered), was 13%, 12%, and 14%, respectively. However, the observed benefit might be inflated because we also included patients after acute MI,9 a condition in which ezetimibe/simvastatin treatment might have potentiated pleiotropic effects beyond LDL‐C lowering. For 20‐mg/dL LDL‐C lowering, 5 strokes were avoided for every 1000 patients treated for 5 years (this means that 216 patients had to be treated for 5 years to prevent 1 stroke), 10 CHD events were avoided (this means that 102 patients had to be treated to prevent 1 event), and 16 major CV events (stroke and CHD) were avoided (this means that 63 patients had to be treated to prevent 1 CV event). By contrast, both CV and all‐cause mortality were not associated with more intensive LDL‐C lowering following ezetimibe/simvastatin treatment. Although the span of the achieved difference in LDL‐C was relatively small (almost 50 mg/dL), the lack of relationship of LDL‐C reductions by ezetimibe/simvastatin with the logarithm of the outcome RRs suggests that progressively greater LDL‐C reductions do not result in progressively lower increments of risk reduction. Thus, based on the cross‐sectional nature of our meta‐regression data, we could only hypothesize that the reduction of major CV events following ezetimibe/simvastatin treatment might be independent of the LDL‐C–lowering extent, in contrast to the suggestion of the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE‐IT) of “the lower the better,” which, however, included only post‐MI patients.9
So far, in the larger meta‐analysis of LDL‐C lowering,1 in which the achieved LDL‐C difference was standardized by 38.7 mg/dL (1 mmol/L), statins alone reduced the incidence of CHD by 24% (compared with 22% in the present analysis), of stroke by 16% (compared with 23% in the present analysis), and of major vascular events, including revascularizations, by 22% (compared with 26% in the present analysis but excluding revascularizations). The difference in benefit of major CV events between the present analysis and the analysis of the Cholesterol Treatment Trialists' Collaboration (CTT)1 might be driven from the relative higher number of post‐MI participants in our case.9 Also, in another overview of the CTT including only primary‐prevention trials,5 the reduction of major coronary events was 2% higher than our estimates. Although the higher stroke benefit by 6% to 7% between the present overview and the 2 analyses of the CTT group1, 5 might not be explained by the baseline prevalence of hypertension (almost 60% in both the previous and the present analysis), ongoing blood‐pressure levels are lacking in all LDL‐C–lowering trials. A specific vascular protection on intermediate endpoints (carotid artery atherosclerotic changes) by the ezetimibe/simvastatin combination compared with simvastatin alone was rejected in previous studies, but their conclusions are limited, either because of nonrandomized allocation13 or because they included a very limited number of participants.12 We have resisted performing a differential analysis between ischemic and hemorrhagic stroke, because in previous observational studies14 and trials15, 16 not included in the CTT meta‐analysis,1 LDL‐C lowering has been associated with increased incidence of hemorrhagic stroke.
At variance with the integrated evidence based on statin LDL‐C–lowering trials,1, 5 ezetimibe/simvastatin treatment was not associated with CV death reduction, possibly because the prevention of occlusive cardiac death by statins at higher doses might have an incremental functional and/or structural beneficial effect on the vasculature, such as vasodilation and/or neoangiogenesis that in turn can reduce the extent of fatal arrhythmiogenesis.17 However, our meta‐analysis cannot suggest that for the same extent of LDL‐C lowering, mixed statin and nonstatin treatment could be less effective compared with more aggressive statin treatment alone to modulate pivotal mechanisms of coronary death such as fatal arrhythmias. A previous meta‐analysis,18 in which nonstatin LDL‐C lowering intervention by diet, bile‐acid sequestrants, and surgery was accompanied by the same extent of CV risk benefit compared with statins, should be interpreted with caution: first, ezetimibe was not included among the nonstatin treatments; second, LDL‐C levels were indirectly measured in two‐thirds of nonstatin treatment studies; third, women were excluded in >60% of studies; fourth, the sample size of nonstatin studies was quite limited; and, finally, the recruitment of participants of nonstatin trials was initiated almost 30 years before the availability of statins.
Our analysis highlights once again19, 20 the importance of residual risk in patients having received the more active LDL‐C–lowering treatment. Residual risk is a measure of inadequate or suboptimal treatment and should always be taken into account in relation to treatment benefit assessed by absolute risk reduction within a predetermined period of time under treatment. Our findings demonstrated that suboptimal treatment during 5 years with ezetimibe/simvastatin vs less active treatment including placebo was almost 7× higher compared with treatment benefit. Thus, to minimize “inadequate treatment” rate, more effective therapeutic strategies should be undertaken, such as even more aggressive LDL‐C lowering21, 22, 23 or implementation of optimal management of traditional and emerging CV risk factors (eg, hypertension control, smoking cessation, targeting on modulation of other lipid parameters). Lowering of LDL‐C by ezetimibe/simvastatin was not accompanied by changes in the rate of non‐CV death and any (fatal and nonfatal) cancer, in line with previous overviews but restricted to statins.1, 5 Additionally, incidence of clinically important side effects like myopathy and hepatopathy was quite small and not different from that reported with statin monotherapy.24
Study Strengths and Limitations
One of the strengths of our meta‐analysis is that we have investigated not only the effects of LDL‐C lowering on relative risk reductions following ezetimibe/simvastatin compared with less active treatment, but we have also estimated the residual risk (ie, suboptimal treatment) and its relation with absolute risk reduction (ie, treatment benefits), to provide a more integrated insight for clinical decision‐making. We standardized risk reductions to a 20‐mg/dL LDL‐C decrease (similar to the mean ongoing LDL‐C between arms), and not to 38.7 mg/dL (1 mmol/L), to avoid bias of extrapolation of risk estimates.
In the trials of ezetimibe/simvastatin considered in our analysis, the comparator arm was either placebo or simvastatin alone. Thus, risk reduction could not be assigned to ezetimibe alone, but only to LDL‐C lowering developed by the combined treatment. We have included a large post‐MI trial9 in which the incidence of clinical events may be independent of, and occasionally hindered by, LDL‐C lowering. In our analysis, we included different CVD burden patients (eg, valvular heart disease, post‐MI, chronic kidney disease) with potential high variability on outcomes' incidence. Despite the different extent of 10‐year CV death risk and statin pretreatment, we did not perform stratified analyses because the number of trials was quite small. Also, the limited number of trials did not allow us to perform analyses across different LDL‐C thresholds. Meta‐regression analyses, though instrumental at investigating quantitative relationships between risk and intervention, could not be seen as alternative to traditional meta‐analyses for the estimation of the mean effect for a given intervention. Therefore, the finding that major CV events, CHD, and stroke can be reduced by LDL‐C lowering should be considered stronger than the cross‐sectional evidence provided by our meta‐regressions that denied the association between this benefit and extent of LDL‐C lowering.
Conclusions
In our meta‐analysis, LDL‐C lowering following ezetimibe/simvastatin treatment was associated with reduced rate of CHD, stroke, and their composite outcome. Risk reductions were not proportional with the extent of LDL‐C lowering, raising the hypothesis that beneficial effects might be partially mediated by effects independent of LDL‐C lowering. Cardiovascular death was not reduced, and further studies should examine whether ezetimibe‐based treatment could modulate pivotal mechanisms of vascular death compared with statin monotherapy at the same extent of LDL‐C lowering. We also demonstrated that ezetimibe/simvastatin treatment is safe by means of non‐CV death, cancer incidence, and incidence of clinically important side effects such as myopathy and hepatopathy.
Supporting information
Supplemental References (for excluded randomized studies)
Figure S1. Publication bias for primary outcomes
TableS1. Searching strategy in Pubmed
TableS2. Studies excluded and reason for exclusion
TableS3. Publication bias for primary outcomes
The corresponding author (C.Th.) is responsible for the design of the study and preparation of the first draft of the manuscript; C.Th. and G.S. performed the systematic review of the literature and extracted data; C.Th. conducted the meta‐analyses; but all authors (C.Th., G.S., H.M., C.Ts., and T.M.) substantially contributed to interpretation of data and critical revision of the manuscript for important intellectual content and gave final approval for the manuscript to be published. The corresponding author (C.Th.) takes responsibility for the integrity of the analyses. No external funding was sought for this review.
C.Th. declares consultancy fees from AstraZeneca and lecture honoraria from Sanofi; C.Ts. served as consultant and received modest honoraria from St. Jude Medical, but he is not a stakeholder or shareholder of the company.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists' Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Napoli P, Taccardi AA, Oliver M, et al. Statins and stroke: evidence for cholesterol‐independent effects. Eur Heart J. 2002;23:1908–1921. [DOI] [PubMed] [Google Scholar]
- 3. Stone NJ, Robinson JG, Lichtenstein AH et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2014;63(25 part B):3024–3025]. J Am Coll Cardiol. 2014;63(25 part B):2889–2934. [DOI] [PubMed] [Google Scholar]
- 4. Morrone D, Weintraub WS, Toth PP, et al. Lipid‐altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: a pooled analysis of over 21 000 subjects from 27 clinical trials. Atherosclerosis. 2012;223:251–261. [DOI] [PubMed] [Google Scholar]
- 5. Mihaylova B, Emberson J, Blackwell L, et al; Cholesterol Treatment Trialists' Collaborators . The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Catapano AL, Farnier M, Foody JM, et al. Combination therapy in dyslipidemia: where are we now? Atherosclerosis. 2014;237:319–335. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kastelein JJ, Akdim F, Stroes ES, et al; ENHANCE Investigators . Simvastatin with or without ezetimibe in familial hypercholesterolemia [published correction appears in N Engl J Med. 2008;358:1977]. N Engl J Med. 2008;358:1431–1443. [DOI] [PubMed] [Google Scholar]
- 9. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 10. Rossebø AB, Pedersen TR, Boman K, et al; SEAS Investigators . Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. [DOI] [PubMed] [Google Scholar]
- 11. Baigent C, Landray MJ, Reith C, et al; SHARP Investigators . The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo‐controlled trial. Lancet. 2011;377:2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. West AM, Anderson JD, Meyer CH, et al. The effect of ezetimibe on peripheral arterial atherosclerosis depends upon statin use at baseline. Atherosclerosis. 2011;218:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fleg JL, Mete M, Howard BV, et al. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes: the SANDS (Stop Atherosclerosis in Native Diabetics Study) trial. J Am Coll Cardiol. 2008;52:2198–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lewington S, Whitlock G, Clarke R, et al; Prospective Studies Collaboration . Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta‐analysis of individual data from 61 prospective studies with 55 000 vascular deaths [published correction appears in Lancet. 2008;372:292]. Lancet. 2007;370:1829–1839. [DOI] [PubMed] [Google Scholar]
- 15. Kjekshus J, Apetrei E, Barrios V, et al; CORONA Group . Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. [DOI] [PubMed] [Google Scholar]
- 16. Amarenco P, Bogousslavsky J, Callahan A 3rd, et al; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators . High‐dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 17. Vedre A, Gurm HS, Froehlich JB et al; GRACE Investigators . Impact of prior statin therapy on arrhythmic events in patients with acute coronary syndromes (from the Global Registry of Acute Coronary Events [GRACE]). Am J Cardiol. 2009;104:1613–1617. [DOI] [PubMed] [Google Scholar]
- 18. Robinson JG, Smith B, Maheshwari N, et al. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta‐regression analysis. J Am Coll Cardiol. 2005;46:1855–1862. [DOI] [PubMed] [Google Scholar]
- 19. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 3. Effects in patients at different levels of cardiovascular risk—overview and meta‐analyses of randomized trials. J Hypertens. 2014;32:2305–2314. [DOI] [PubMed] [Google Scholar]
- 20. Stein EA, Raal FJ. Targeting LDL: is lower better and is it safe? Best Pract Res Clin Endocrinol Metab. 2014;28:309–324. [DOI] [PubMed] [Google Scholar]
- 21. Robinson JG, Colhoun HM, Bays HE, et al. Efficacy and safety of alirocumab as add‐on therapy in high‐cardiovascular‐risk patients with hypercholesterolemia not adequately controlled with atorvastatin (20 or 40 mg) or rosuvastatin (10 or 20 mg): design and rationale of the ODYSSEY OPTIONS studies. Clin Cardiol. 2014;37:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robinson JG, Rogers WJ, Nedergaard BS, et al. Rationale and design of LAPLACE‐2: a phase 3, randomized, double‐blind, placebo‐ and ezetimibe‐controlled trial evaluating the efficacy and safety of evolocumab in subjects with hypercholesterolemia on background statin therapy. Clin Cardiol. 2014;37:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cho L, Rocco M, Colquhoun D, et al. Design and rationale of the GAUSS‐2 study trial: a double‐blind, ezetimibe‐controlled phase 3 study of the efficacy and tolerability of evolocumab (AMG 145) in subjects with hypercholesterolemia who are intolerant of statin therapy. Clin Cardiol. 2014;37:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kashani A, Sallam T, Bheemreddy S, et al. Review of side‐effect profile of combination ezetimibe and statin therapy in randomized clinical trials. Am J Cardiol. 2008;101:1606–1613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental References (for excluded randomized studies)
Figure S1. Publication bias for primary outcomes
TableS1. Searching strategy in Pubmed
TableS2. Studies excluded and reason for exclusion
TableS3. Publication bias for primary outcomes


