Abstract
Background
Low bone mineral density (BMD) and left ventricular (LV) diastolic function are associated with heart failure. However, little is known about the association between BMD and LV diastolic function.
Hypothesis
BMD is independently related to LV diastolic function in women.
Methods
We conducted a cross‐sectional study of 432 women. Brachial‐ankle pulse wave velocity (baPWV) and BMD measurements were performed. LV diastolic function and structure were assessed by echocardiographic examination.
Results
BaPWV and the percentage of LV diastolic dysfunction increased with progressive bone loss. Moreover, partial correlation analysis demonstrated that BMD at spine L2–4 and at femoral neck were correlated with baPWV and LV diastolic function parameters after adjusting covariates. Multivariate logistic regression analysis revealed that osteoporosis was independently associated with LV diastolic dysfunction in women.
Conclusions
Osteoporosis is independently associated with LV diastolic dysfunction in women. A prospective study is needed to elucidate the effects of BMD on cardiac function in women.
Keywords: Imaging, echocardiography
1. INTRODUCTION
Osteoporosis has been suggested as an independent risk factor for cardiovascular disease (CVD).1 Low bone mineral density (BMD) is associated with increased cardiovascular mortality.2 Moreover, individuals with CVD have a higher risk of bone loss and fracture.3 Currently, there is growing evidence that vascular calcification and bone mineralization share a number of anatomical and pathophysiological common features.3 Some proteins, such as bone morphogenetic protein, alkaline phosphatase, and osteopontin, play key roles in bone and calcified vascular tissue.4, 5
Diastolic dysfunction is linked with occurrence of heart failure and is a predictor of cardiovascular morbidity and mortality.6 Several studies have documented that left ventricular (LV) diastolic dysfunction could be found in a variety of conditions, including aging, obesity, metabolic syndrome, nonalcoholic fatty liver disease, diabetes mellitus (DM), and hypertension.7, 8, 9, 10
More recently, literature has emerged that shows the relationship between BMD and cardiac function. A study reported that BMD is associated with left ventricular function.11 Another study found that BMD is an independent determinant of left ventricular mass index (LVMI) in general subjects.12 In addition, low BMD predicts incident heart failure in healthy individuals.13 On the basis of these observations, we hypothesized that low BMD might be independently associated with LV diastolic dysfunction. However, little research has been conducted to investigate the relationship.
The purpose of this investigation is to explore the relationship between BMD and LV diastolic function in women.
2. METHODS
2.1. Participants
Between January 2013 to December 2013, we enrolled into our study 432 women who received a general health examination. The exclusion criteria were thyroid disease, cancer, chronic obstructive pulmonary disease, chronic renal failure, autoimmune diseases, chronic heart failure, fractures, coronary heart disease, stroke, atrial fibrillation, and medical treatment with glucocorticoids, estrogen, bisphosphonates, vitamin D, or calcium. The study protocol was approved by our institutional ethics committee, and written informed consent was obtained from all women before participation.
2.2. Clinical Examination
Trained interviewers used a standardized questionnaire to obtain information about medical history and lifestyle. All the study subjects underwent physical examination, which included anthropometric and blood pressure measurements. The anthropometric measurements comprised height and body weight, and the body mass index (BMI) was calculated as weight/height2 (kg/m2). Blood pressure was measured using a mercury‐gravity sphygmomanometer in the sitting position after a 15‐minute rest. Systolic and diastolic blood pressures were determined twice, with a 10‐minute interval, and mean values were used. Cigarette smoking was defined as having smoked ≥100 cigarettes in one's lifetime. Alcohol drinking was defined as the consumption of ≥30 g of alcohol per week for ≥1 year. Regular leisure‐time physical activity was defined as participation in moderate or vigorous activity for ≥30 minutes per day, ≥3 days a week.
2.3. Biochemical Analyses
After overnight fasting, blood samples were taken for total cholesterol (TC), triglycerides (TG), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), and fasting plasma glucose (FPG). All assays were performed at the Laboratory of Analytical Biochemistry at the Second Hospital of Harbin Medical University using a biochemical analyzer (Modular Analytics; Roche Diagnostics, Mannheim, Germany). All measurements were conducted within 2 hours of sampling.
2.4. BMD Measurement
BMD at the lumbar spine (L2–L4) and femoral neck (FN) was measured using dual‐energy X‐ray absorptiometry (GE Lunar DPX‐MD; GE, Madison, WI). BMD was expressed as g/cm2 and as T‐score. Subjects were categorized as having osteoporosis (T‐score ≤ −2.5), osteopenia (T‐score between −1.0 and −2.5), or normal BMD (T‐score ≥ −1.0) by using the lowest reported T‐score. The method was validated in a previous report.14
2.5. Measurement of BaPWV
Before the subjects were tested, they abstained from caffeine and fasted for ≥4 hours. Brachial‐ankle pulse wave velocity (baPWV) was determined using an automatic device (model MB3000; M&B Electronic Instruments, Beijing, China). The baPWV was automatically calculated according to the following formula: baPWV = (Lb – La)/PTT, where La and Lb are defined as the distance from the aortic valve to the elbow and to the ankle, respectively, and PTT was defined as pulse transit time between the brachial and tibial arterial waveforms. The mean of the right and left baPWV was obtained for analysis. All measurements were conducted by a single examiner who was blinded to the clinical data. The reproducibility and validity of the baPWV measurement using this method have been previously reported.15
2.6. Echocardiographic Examination
Echocardiography was performed by standardized procedures with Philips iE33 (Philips Ultrasound, Bothell, WA). LV linear dimensions were measured according to American Society of Echocardiography recommendations.16 LV mass was calculated with a validated formula and indexed for height to the 2.7 power.17 LV ejection fraction was calculated by the modified Simpson biplane rule. The peak early diastolic transmitral flow velocity (E), peak late diastolic transmitral flow velocity (A), and E/A ratio were measured using pulsed‐wave Doppler imaging of the mitral valve inflow from the apical 4‐chamber view. Peak early diastolic mitral annular velocity (e′) was measured in the septal position using tissue Doppler imaging. The e′ wave velocities from the septal and lateral walls were averaged and the E/ e′ ratio was calculated as an indicator of LV filling pressure. Diastolic dysfunction was defined as: (1) E/A ≤ 0.7 (impaired relaxation, grade I); (2) 0.7 < E/A ≤ 1.5 and e′ < 7 cm/s (pseudo‐normalized pattern, grade II); or (3) E/A > 1.5 and e′ < 7 cm/s (restrictive pattern, grade III).18
2.7. Statistical Analysis
All data were expressed as mean ± SD or median (interquartile range) for continuous variables and percentages of the number for categorical variables. The χ2 statistical test was used for categorical variables, and 1‐way ANOVA or the Kruskal‐Wallis H was used for continuous variables. Categories of the participants comprised the following: normal, osteopenia, and osteoporosis. Partial correlation coefficients were used to determine the relationship between BMD and LV diastolic function parameters after adjustment for several confounding factors. The independence of the associations of variables with LV diastolic dysfunction, included as the dependent variable, were assessed by multivariate logistic regression analyses and expressed as odds ratios (OR). P values < 0.05 (2‐tailed) were considered statistically significant. Statistical analyses were performed using the SPSS software package, version 22.0 (IBM Corp., Armonk, NY).
3. RESULTS
The baseline characteristics of participants stratified by BMD status are shown in Table 1. Mean systolic blood pressure (SBP), diastolic blood pressure (DBP), TC, TG, LDL‐C, FPG, baPWV, and the percentage of hypertension increased as BMD decreased. L2–4 BMD, T‐score L2–4, FN BMD, and T‐score FN decreased as BMD reduced. However, age, BMI, HDL‐C, estimated glomerular filtration rate (eGFR), heart rate, and the percentage of smokers, drinking, physical activity, type 2 DM and the proportion using statins had no difference. The mean baPWV values in the control group, osteopenia group, and osteoporosis group were 1388.5 (145.2), 1421.5 (162.5), and 1446.4 (171.6) cm/s, respectively (P = 0.009).
Table 1.
Clinical characteristics of the participants
| Variable | Control | Osteopenia | Osteoporosis | P Value |
|---|---|---|---|---|
| N | 157 | 146 | 129 | — |
| Age, y | 67.9 (8.8) | 67.7 (9.1) | 68.4 (8.2) | 0.825 |
| Smoker, n (%) | 7 (4.5) | 3 (2.1) | 4 (3.1) | 0.507 |
| Drinking, n (%) | 58 (36.9) | 50 (34.2) | 53 (41.1) | 0.501 |
| Physical activity, n (%) | 33 (21.0) | 28 (19.2) | 23 (17.8) | 0.791 |
| BMI, kg/m2 | 25.2 (3.1) | 25.4 (2.8) | 25.0 (3.2) | 0.464 |
| SBP, mm Hg | 132.9 (8.4) | 134.9 (8.1) | 135.7 (8.0) | 0.009 |
| DBP, mm Hg | 73.9 (6.4) | 77.5 (7.4) | 77.6 (7.7) | <0.001 |
| TC, mmol/L | 4.86 (0.90) | 4.97 (1.11) | 5.19 (1.09) | 0.024 |
| TG, mmol/L | 3.98 (3.35–4.62) | 4.05 (3.51–4.65) | 4.68 (3.47–5.09) | 0.008 |
| HDL‐C, mmol/L | 1.37 (1.07–1.52) | 1.33 (1.16–1.46) | 1.31 (1.18–1.43) | 0.950 |
| LDL‐C, mmol/L | 2.50 (0.77) | 2.77 (0.84) | 3.02 (1.02) | <0.001 |
| FPG, mmol/L | 5.52 (5.08–5.98) | 5.60 (5.06–6.22) | 5.79 (5.45–6.30) | <0.001 |
| Heart rate, bpm | 76.2 (13.3) | 77.9 (14.0) | 77.5 (11.2) | 0.493 |
| L2–4 BMD, g/cm2 | 1.127 (0.169) | 0.996 (0.277) | 0.892 (0.165) | <0.001 |
| T‐score L2–4 (SD) | −0.274 (0.411) | −1.395 (0.683) | −2.471 (0.796) | <0.001 |
| FN BMD, g/cm2 | 0.806 (0.205) | 0.756 (0.198) | 0.523 (0.169) | <0.001 |
| T‐score FN (SD) | −0.403 (0.321) | −1.449 (0.778) | −2.802 (0.807) | <0.001 |
| BaPWV, cm/s | 1388.5 (145.2) | 1421.5 (162.5) | 1446.4 (171.6) | 0.009 |
| eGFR, mL/min/1.73 m2 | 73.6 (15.7) | 72.3 (14.5) | 69.5 (13.4) | 0.051 |
| HTN (n, %) | 39 (24.8) | 45 (30.8) | 53 (41.1) | 0.013 |
| Type 2 DM (n, %) | 34 (21.7) | 37 (25.3) | 42 (32.6) | 0.109 |
| Statins (n, %) | 44 (28.0) | 42 (28.8) | 37 (28.7) | 0.988 |
| LV diastolic dysfunction grade | ||||
| Normal | 92 (58.6) | 82 (56.2) | 55 (42.6) | 0.017 |
| I | 55 (35.0) | 52 (35.6) | 49 (38.0) | 0.865 |
| II | 10 (6.4) | 11 (7.5) | 23 (17.8) | 0.003 |
| III | 0 (0.0) | 1.0 (0.7) | 2.0 (1.6) | 0.291 |
Abbreviations: ANOVA, analysis of variance; baPWV, brachial‐ankle pulse wave velocity; BMD, bone mineral density; BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FN, femoral neck; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; HTN, hypertension; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; LV, left ventricular; SBP, systolic blood pressure; SD, standard deviation; T2DM, type 2 diabetes; TC, total cholesterol; TG, triglycerides.
Data are presented as mean (SD), median (IQR), or n (%). P values calculated by 1‐way ANOVA test, Kruskal‐Wallis H, or χ2 test.
The echocardiographic parameters of the subjects are shown in Table 2. In terms of cardiac structure, interventricular septal diameter, LV posterior wall thickness, LV wall thickness, LV relative wall thickness, LV mass, LVMI, and LA diameter increased gradually as BMD reduced. However, LV diameter in end‐diastole, LV diameter in end‐systole, and LV ejection fraction were not significantly different. In terms of LV diastolic function, the E′ and E/A ratio decreased gradually and A velocity and E/E′ ratio increased gradually as BMD reduced.
Table 2.
Echocardiographic features of the subjects
| Variable | Control | Osteopenia | Osteoporosis | P Value |
|---|---|---|---|---|
| N | 157 | 146 | 129 | — |
| Cardiac structure | ||||
| LVEDD, mm | 48.6 ± 5.4 | 49.1 ± 5.8 | 49.4 ± 5.5 | 0.472 |
| LVESD, mm | 27.1 ± 4.7 | 27.3 ± 5.0 | 27.8 ± 4.5 | 0.497 |
| IVSD, mm | 9.4 ± 1.2 | 9.5 ± 1.2 | 10.2 ± 1.1 | <0.001 |
| LVPWT, mm | 9.2 ± 1.1 | 9.3 ± 1.1 | 10.2 ± 1.2 | <0.001 |
| LVWT, mm | 18.6 ± 1.7 | 18.7 ± 1.6 | 20.4 ± 1.9 | <0.001 |
| LVRWT | 0.39 ± 0.06 | 0.39 ± 0.06 | 0.42 ± 0.06 | <0.001 |
| LV mass, g | 162.8 ± 35.3 | 167.6 ± 37.1 | 189.7 ± 43.0 | <0.001 |
| LV mass index, g/m2.7 | 45.7 ± 12.3 | 46.7 ± 12.8 | 51.6 ± 14.7 | <0.001 |
| LA diameter, mm | 33.6 ± 4.3 | 33.6 ± 4.6 | 36.0 ± 4.5 | <0.001 |
| LVEF, % | 62.4 ± 4.1 | 62.5 ± 3.4 | 63.0 ± 4.1 | 0.418 |
| LV diastolic function | ||||
| E velocity, cm/s | 63.1 ± 11.6 | 52.0 ± 9.8 | 53.7 ± 11.5 | 0.406 |
| A velocity, cm/s | 54.6 ± 10.8 | 72.3 ± 12.2 | 80.0 ± 16.3 | <0.001 |
| E/A ratio | 1.15 ± 0.15 | 0.89 ± 0.10 | 0.84 ± 0.18 | <0.001 |
| E′, cm/s | 7.6 ± 1.6 | 7.4 ± 1.6 | 7.1 ± 1.3 | 0.016 |
| E/E′ ratio | 8.6 ± 2.5 | 8.8 ± 1.8 | 9.4 ± 2.2 | 0.004 |
Abbreviations: A, peak late diastolic transmitral flow velocity; E, peak early diastolic transmitral flow velocity; E′, peak early diastolic mitral annular velocity; IVSD, interventricular septal diameter; LA, left atrial; LV, left ventricular; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; LVPWT, left ventricular posterior wall thickness; LVRWT, left ventricular relative wall thickness; LVWT, left ventricular wall thickness.
The prevalence of LV diastolic dysfunction is calculated in subjects with normal BMD, osteopenia, and osteoporosis (Figure 1). LV diastolic dysfunction was present in 21.8% of the normal BMD group, in 33.6% of the osteopenia group, and in 46.1% of the osteoporosis group (P < 0.001). The results indicated that the prevalence of LV diastolic dysfunction increased as BMD reduced.
Figure 1.
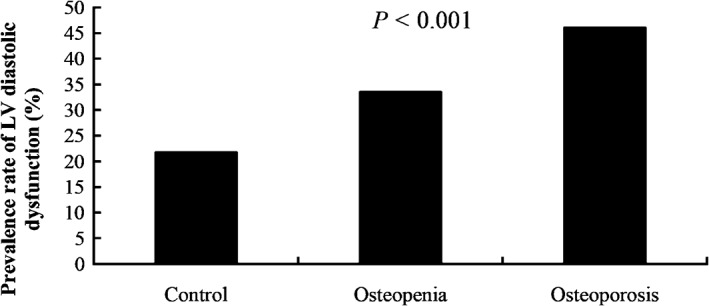
The association between BMD and prevalence rate of LV diastolic dysfunction (%). Participants were stratified into 3 groups: normal, osteopenia, and osteoporosis. Abbreviations: BMD, bone mineral density; LV, left ventricular.
Table 3 shows the partial correlation between BMD and baPWV and LV diastolic function parameters. Both L2–4 BMD and FN BMD were correlated with baPWV, peak E′, E/A ratio, and E/E′ ratio even after adjusting for age, BMI, drinking, smoking status, physical activity, SBP, DBP, FPG, TC, TG, HDL‐C, LDL‐C, eGFR, heart rate, and use of statins.
Table 3.
Partial correlation coefficient (r) for BMD in relation to baPWV and LV diastolic function parameters
| L2–4 BMD | FN BMD | |||
|---|---|---|---|---|
| r | P Value | r | P Value | |
| E/A ratio | 0.178 | <0.001 | 0.302 | <0.001 |
| Peak E′ | 0.058 | 0.240 | 0.117 | 0.017 |
| E/ E′ ratio | −0.126 | 0.010 | −0.120 | 0.014 |
| BaPWV | −0.114 | 0.020 | −0.157 | 0.001 |
Abbreviations: A, peak late diastolic transmitral flow velocity; baPWV, brachial‐ankle pulse wave velocity; BMD, bone mineral density; BMI, body mass index; DBP, diastolic blood pressure; E, peak early diastolic transmitral flow velocity; E′, peak early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; FN, femoral neck; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LV, left ventricular; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
Adjusted for age, BMI, drinking, smoking status, physical activity, SBP, DBP, FPG, TC, TG, HDL‐C, LDL‐C, eGFR, heart rate, and use of statins.
Variables such as TG, HDL‐C, and FPG were logarithmically transformed before statistical analysis.
In multivariate logistic regression analysis (Table 4), there was a significant association between osteoporosis and LV diastolic dysfunction after adjustment for age, BMI, drinking, smoking status, and physical activity (model 1). The association between osteoporosis and LV diastolic dysfunction was little affected after further adjustment for SBP, DBP, FPG, TC, TG, HDL‐C, and LDL‐C (model 2). The results did not substantially change after exclusion of eGFR, heart rate, type 2 DM, hypertension, and use of statins (model 3).
Table 4.
Multivariate logistic regression analysis for the risk of diastolic dysfunction
| β | OR (95% CI) | P Value | |
|---|---|---|---|
| Model 1 | |||
| Control | Ref | — | — |
| Osteopenia | 0.101 | 1.107 (0.700‐1.750) | 0.664 |
| Osteoporosis | 0.607 | 1.836 (1.144‐2.946) | 0.012 |
| Model 2 | |||
| Control | Ref | — | — |
| Osteopenia | 0.292 | 1.339 (0.818‐2.193) | 0.246 |
| Osteoporosis | 1.000 | 2.718 (1.579‐4.679) | <0.001 |
| Model 3 | |||
| Control | Ref | — | — |
| Osteopenia | 0.332 | 1.394 (0.843‐2.304) | 0.195 |
| Osteoporosis | 1.044 | 2.841 (1.626‐4.963) | <0.001 |
Abbreviations: BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; OR, odds ratio; Ref, reference; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
Model 1: adjusted for age, BMI, drinking, smoking status, and physical activity. Model 2: adjusted for age, BMI, drinking, smoking status, physical activity, SBP, DBP, FPG, TC, TG, HDL‐C, and LDL‐C. Model 3: adjusted for age, BMI, drinking, smoking status, physical activity, SBP, DBP, FPG, TC, TG, HDL‐C, LDL‐C, eGFR, heart rate, type 2 DM, HTN, and use of statins.
4. DISCUSSION
In this study, we found that baPWV and the percentage of LV diastolic dysfunction increased with progressive bone loss. Moreover, partial correlation analysis demonstrated that BMD at spine L2–4 and at the FN were correlated with baPWV and LV diastolic function parameters after adjusting covariates. Multivariable logistic regression analysis revealed that osteoporosis was independently associated with LV diastolic dysfunction in women.
The biological mechanisms by which reduced BMD could contribute to LV diastolic dysfunction are still poorly known. First, growing evidence indicates the existence of a correlation between CVD and osteoporosis. The calcification of the arterial tissue resembles the process of osteogenesis, involving various cells, proteins, and cytokines that result in tissue mineralization.19 Other involved mechanisms included oxidative stress, inflammation, free radicals, and lipids metabolism.3 Furthermore, some medications, such as statins and bisphosphonates, are effective in both osteoporosis and CVD, suggesting a common pathophysiological basis.3 Second, the association is related to abnormal ventricular‐vascular coupling due to arterial stiffness.20 Brachial‐ankle pulse wave velocity is a technique to assess arterial stiffness. Recent reports documented interplay between reduced BMD, elevated arterial stiffness, and LV diastolic dysfunction.21, 22, 23 Increased arterial stiffness raises the LV afterload by elevating the SBP and alters the coronary perfusion.24 In accordance with the results, we found that baPWV is increased and correlated with BMD. Third, estrogen deficiency is responsible for bone loss in women. The decline in production of estrogen causes secretion of pro‐inflammatory cytokines such as interleukin‐1, interleukin‐6, and tumor necrosis factor‐α. In addition, estrogen protects against cardiac remodeling and diastolic dysfunction in women by regulating the cardiac renin‐angiotensin‐aldosterone and nitric oxide synthase system.25
Our study has important clinical implications. Osteoporosis is an independent risk factor for CVD. Moreover, chronic heart failure was associated with an increased risk for osteoporotic fractures.26 Recently, a study confirmed that bisphosphonates, widely used as treatment of osteoporosis, also inhibit the atherosclerotic process.27 Our results revealed that BMD was correlated to arterial stiffness and independently associated with LV diastolic dysfunction. In agreement with our results, Russo et al showed that LV diastolic function abnormalities are associated with arterial stiffness and wave reflection.28 Moreover, Lim et al found that reduced BMD was associated with LVMI.12 LV diastolic dysfunction represents the earliest preclinical manifestation of heart failure. Therefore, early intervention may reduce the risk of LV diastolic dysfunction in patients with osteoporosis.
4.1. Study Limitations
The limitations of our study deserve comment. Firstly, a cross‐sectional study design tends to leave uncertainty regarding to inferring the causal relationship. Secondly, the findings were based on a sample of Chinese women. Thus, the results may not generalize to other ethnicities. Thirdly, it is not known whether baPWV is associated with well‐established indices of central arterial stiffness. Further study is needed to clarify the association. Fourthly, comparison of bone mineral density between patients’ groups with varying degrees of diastolic dysfunction, might provide additional information about the relationship between the parameters.
5. CONCLUSION
The present study showed that osteoporosis is independently associated with LV diastolic dysfunction. A prospective study is needed to elucidate the effects of BMD on cardiac function in women.
Conflicts of interest
The authors declare no potential conflicts of interest.
5.1. Author contributions
R.‐T.W. and X.‐S.L. participated in manuscript preparation, data analysis, and editing. J.‐R.Z. participated in data collection and data analysis. Y.S. participated in data analysis and manuscript revision. K.‐J.Y and T.L. participated in study design and manuscript preparation. All authors read and approved the final manuscript. R.‐T.W. and X.‐S.L. contributed equally to this work.
Wang R‐t, Li X‐s, Zhang J‐r, Sun Y, Yu K‐j and Liu T. Bone mineral density is associated with left ventricular diastolic function in women, Clin Cardiol 2016;39(12):709–714.
Funding Information This study was supported by Key Laboratory of Myocardial Ischemia, Harbin Medical University, Ministry of Education, Heilongjiang Province, China.
Contributor Information
Kai‐jiang Yu, Email: kaijiang_yu@yeah.net.
Tiemin Liu, Email: Tiemin.Liu@UTSouthwestern.edu.
References
- 1. Marcovitz PA, Tran HH, Franklin BA, et al. Usefulness of bone mineral density to predict significant coronary artery disease. Am J Cardiol. 2005;96:1059–1063. [DOI] [PubMed] [Google Scholar]
- 2. von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106:273–278. [DOI] [PubMed] [Google Scholar]
- 3. Sprini D, Rini GB, Di SL, et al. Correlation between osteoporosis and cardiovascular disease. Clin Cases Miner Bone Metab. 2014;11:117–119. [PMC free article] [PubMed] [Google Scholar]
- 4. Boström K, Watson KE, Horn S, et al. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berezin AE, Kremzer AA. Circulating osteopontin as a marker of early coronary vascular calcification in type two diabetes mellitus patients with known asymptomatic coronary artery disease. Atherosclerosis. 2013;229:475–481. [DOI] [PubMed] [Google Scholar]
- 6. Redfield MM, Jacobsen SJ, Burnett JC Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 7. Russo C, Jin Z, Homma S, et al. Effect of obesity and overweight on left ventricular diastolic function: a community‐based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wood P, Piran S, Liu PP. Diastolic heart failure: progress, treatment challenges, and prevention. Can J Cardiol. 2011;27:302–310. [DOI] [PubMed] [Google Scholar]
- 9. Hwang YC, Jee JH, Kang M, et al. Metabolic syndrome and insulin resistance are associated with abnormal left ventricular diastolic function and structure independent of blood pressure and fasting plasma glucose level. Int J Cardiol. 2012;159:107–111. [DOI] [PubMed] [Google Scholar]
- 10. Bonapace S, Perseghin G, Molon G, et al. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laudisio A, Marzetti E, Antonica L, et al. Association of left ventricular function with bone mineral density in older women: a population‐based study. Calcif Tissue Int. 2008;82:27–33. [DOI] [PubMed] [Google Scholar]
- 12. Lim YH, Shin J, Lee JU, et al. Bone mineral density is an independent determinant of left ventricular mass index in the general female population. Korean Circ J. 2010;40:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pfister R, Michels G, Sharp SJ, et al. Low bone mineral density predicts incident heart failure in men and women: the EPIC (European Prospective Investigation into Cancer and Nutrition)‐Norfolk prospective study. JACC Heart Fail. 2014;2:380–389. [DOI] [PubMed] [Google Scholar]
- 14. Li XS, Zhang JR, Meng SY, et al. Mean platelet volume is negatively associated with bone mineral density in postmenopausal women. J Bone Miner Metab . 2012;30:660–665. [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Meng SY, Meng CC, et al. Decreased serum bilirubin is associated with arterial stiffness in men. Nutr Metab Cardiovasc Dis. 2013;23:375–381. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 17. de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. [DOI] [PubMed] [Google Scholar]
- 18. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 19. Danilevicius CF, Lopes JB, Pereira RM. Bone metabolism and vascular calcification. Braz J Med Biol Res. 2007;40:435–442. [DOI] [PubMed] [Google Scholar]
- 20. Zito C, Mohammed M, Todaro MC, et al. Interplay between arterial stiffness and diastolic function: a marker of ventricular‐vascular coupling. J Cardiovasc Med (Hagerstown) . 2014;15:788–796. [DOI] [PubMed] [Google Scholar]
- 21. Cecelja M, Chowienczyk P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc Dis . doi:10.1258/cvd.2012.012016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lampropoulos CE, Papaioannou I, D'Cruz DP. Osteoporosis—a risk factor for cardiovascular disease? Nat Rev Rheumatol. 2012;8:587–598. [DOI] [PubMed] [Google Scholar]
- 23. Sumino H, Ichikawa S, Kasama S, et al. Elevated arterial stiffness in postmenopausal women with osteoporosis. Maturitas. 2006;55:212–218. [DOI] [PubMed] [Google Scholar]
- 24. London GM, Guerin AP. Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am Heart J . 1999;138(3 part 2):220–224. [DOI] [PubMed] [Google Scholar]
- 25. Zhao Z, Wang H, Jessup JA, et al. Role of estrogen in diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H628–H640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Diepen S, Majumdar SR, Bakal JA, et al. Heart failure is a risk factor for orthopedic fracture: a population‐based analysis of 16 ;294 patients. Circulation. 2008;118:1946–1952. [DOI] [PubMed] [Google Scholar]
- 27. Price PA, Faus SA, Williamson MK. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arterioscler Thromb Vasc Biol. 2001;21:817–824. [DOI] [PubMed] [Google Scholar]
- 28. Russo C, Jin Z, Palmieri V, et al. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]


