Abstract
Type 2 diabetes mellitus (DM) is a major risk factor for developing tuberculosis (TB). TB-DM comorbidity is expected to pose a serious future health problem due to the alarming rise in global DM incidence. At present, the causal underlying mechanisms linking DM and TB remain unclear. DM is associated with elevated levels of oxidized low-density lipoprotein (oxLDL), a pathologically modified lipoprotein which plays a key role during atherosclerosis development through the formation of lipid-loaded foamy macrophages, an event which also occurs during progression of the TB granuloma. We therefore hypothesized that oxLDL could be a common factor connecting DM to TB. To study this, we measured oxLDL levels in plasma samples of healthy controls, TB, DM and TB-DM patients, and subsequently investigated the effect of oxLDL treatment on human macrophage infection with Mycobacterium tuberculosis (Mtb). Plasma oxLDL levels were significantly elevated in DM patients and associated with high triglyceride levels in TB-DM. Strikingly, incubation with oxLDL strongly increased macrophage Mtb load compared to native or acetylated LDL (acLDL). Mechanistically, oxLDL -but not acLDL- treatment induced macrophage lysosomal cholesterol accumulation and increased protein levels of lysosomal and autophagy markers, while reducing Mtb colocalization with lysosomes. Importantly, combined treatment of acLDL and intracellular cholesterol transport inhibitor (U18666A) mimicked the oxLDL-induced lysosomal phenotype and impaired macrophage Mtb control, illustrating that the localization of lipid accumulation is critical. Collectively, these results demonstrate that oxLDL could be an important DM-associated TB-risk factor by causing lysosomal dysfunction and impaired control of Mtb infection in human macrophages.
Author summary
Tuberculosis (TB) is an infectious disease of the lungs caused by a bacterium, Mycobacterium tuberculosis (Mtb), and is responsible for over a million deaths per year worldwide. Population studies have demonstrated that type 2 diabetes mellitus (DM) is a risk factor for TB as it triples the risk of developing the disease. DM is a metabolic disorder which is generally associated with obesity, and is characterized by resistance to the pancreatic hormone insulin and high blood glucose and lipid levels. As the global incidence of DM is rising at an alarming rate, especially in regions where TB is common, it is important to understand precisely how DM increases the risk of developing TB. Both TB and DM are associated with the development of foamy macrophages, lipid-loaded white blood cells, which can be the result of a specific lipoprotein particle called oxidized low-density lipoprotein (oxLDL). Here, we demonstrated that DM patients have high blood levels of oxLDL, and generating foamy macrophages with oxLDL supported Mtb survival after infection as a result of faulty intracellular cholesterol accumulation. Our results propose a proof of concept for oxLDL as a risk factor for TB development, encouraging future studies on lipid-lowering therapies for TB-DM.
Introduction
Type 2 diabetes mellitus (DM) has been recognized as a major risk factor for tuberculosis (TB) for decades [1]. Recent epidemiological studies have demonstrated that DM triples the risk of developing active TB [2], and approximately 15% of global TB cases can be attributed to DM comorbidity [3]. The precise mechanisms through which DM enhances the risk of active TB disease progression are unknown, however it has been hypothesized that metabolic changes associated with DM attenuate the immune response towards Mycobacterium tuberculosis (Mtb), the causative pathogen of TB. As the global incidence of DM has been rising at an alarming rate [4], including more recently in TB endemic regions of African and Asia, it is of great importance to identify the molecular and cellular mechanisms underlying TB-DM comorbidity.
DM patients often suffer from dyslipidemia and oxidative stress, conditions which can contribute to the formation of oxidized low density lipoprotein (oxLDL) [5]. LDL can be oxidized by free radicals and reactive products of oxygenases, a process which has been mostly studied in the context of atherosclerosis during which oxLDL is generated in the subendothelial space of the arterial wall [6, 7]. High levels of circulating oxLDL were shown to be associated with DM, insulin resistance and decreased glucose tolerance [8–11]. oxLDL is recognized as a damage-associated molecular pattern (DAMP) by macrophages and is a ligand for various scavenger receptors on the cell surface, including CD36, scavenger receptor A (SR-A) and lectin-type oxidized LDL receptor 1 (LOX-1) [12]. The uptake of oxLDL by macrophages plays a major role during the pathophysiology of atherosclerosis as it leads to the generation of pro-inflammatory lipid-loaded foam cells in the arterial vessel wall [13, 14]. These macrophages exhibit increased scavenger receptor expression, cytokine secretion and production of oxidizing agents, supporting both immune cell infiltration and further generation of oxLDL which can culminate in atherosclerotic plaque formation [15].
Foamy macrophages also occur during TB progression and are thought to be of great importance for the development of TB granulomas and persisting Mtb infection, since the bacterium relies on host-derived lipids and cholesterol as a source of carbon for its survival [16–18]. Infection of alveolar macrophages with Mtb initiates the formation of the early TB granuloma, which progresses from a core of infected foam cells to an enclosed structure with a thick fibrous capsule and a lipid-rich caseous center of necrotic macrophages [16]. Various studies have demonstrated that Mtb and other mycobacteria are able to utilize host-derived lipids and even reprogram lipid metabolism in infected macrophages to induce foam cell formation, in part through the effects of mycobacterial cell wall lipids [19–24]. Interestingly, oxLDL was also found to accumulate in granulomas and alveolar macrophages of Mtb infected guinea pigs and to enhance bacterial replication [25], suggesting that local oxLDL production could play a role in foam cell formation and Mtb persistence during TB disease.
OxLDL-derived lipids have been demonstrated to be resistant to lysosomal esterases which are normally responsible for lipid breakdown. This results in lipid accumulation inside lysosomes after initial uptake by macrophages [26, 27], as well as to dysfunctions in the trafficking and efflux of intracellular cholesterol which mimic those observed in the lysosomal storage disorder Niemann Pick disease type C (NPC). During NPC disease, mutations in the lysosomal cholesterol transporters NPC1 or NPC2 result in severe neurological defects due to excessive intralysosomal storage of cholesterol and sphingolipids [28]. Cholesterol accumulation due to oxLDL uptake or NPC1-deficiency induces lysosomal dysfunction in macrophages, as it can interfere with phagolysosomal trafficking, maturation and fusion [29, 30]; inhibit autophagy [31, 32], an important cellular pathway which is simultaneously involved in lipid and cholesterol metabolism [33] and Mtb killing [34] in macrophages; increase lysosomal pH [35]; directly damage lysosomal membranes [36, 37]; and trigger various downstream inflammatory pathways such as formation of the NLRP3 inflammasome [38]. A recent paper demonstrated that both infection with live M. smegmatis or M. bovis BCG and treatment with mycobacterial cell wall lipids induced a NPC-like phenotype in macrophages with associated defects in lysosomal function [39], indicating that cholesterol accumulation could provide a permissive environment for mycobacteria in addition to being a nutritional source.
To investigate whether oxLDL is a molecular component in the interplay between TB and DM, we measured oxLDL concentrations in plasma samples of DM, TB and TB-DM patients and analyzed the effect of oxLDL on in vitro Mtb infection in primary human macrophages. We found that oxLDL is elevated in the plasma of DM patients and supported Mtb intracellular survival in vitro by inducing lysosomal dysfunction. Collectively, our findings provide a proof of concept for a contribution of oxLDL as a risk factor for TB during DM.
Results
Plasma oxLDL levels are increased in DM and TB-DM patients with dyslipidemia
First, we sought to confirm the presence of high levels of circulating oxLDL in DM patients from a TB endemic setting and to assess the relative impact of TB-DM comorbidity on circulating oxLDL levels. OxLDL concentrations were determined in plasma samples from healthy endemic controls (HC), TB, DM and TB-DM patients of a South-African cohort, previously used in a lipidomic biomarker analysis [40], by sandwich ELISA using a monoclonal antibody against a conformational epitope in oxidized ApoB-100 [41]. Patient characteristics are described in S1 Table.
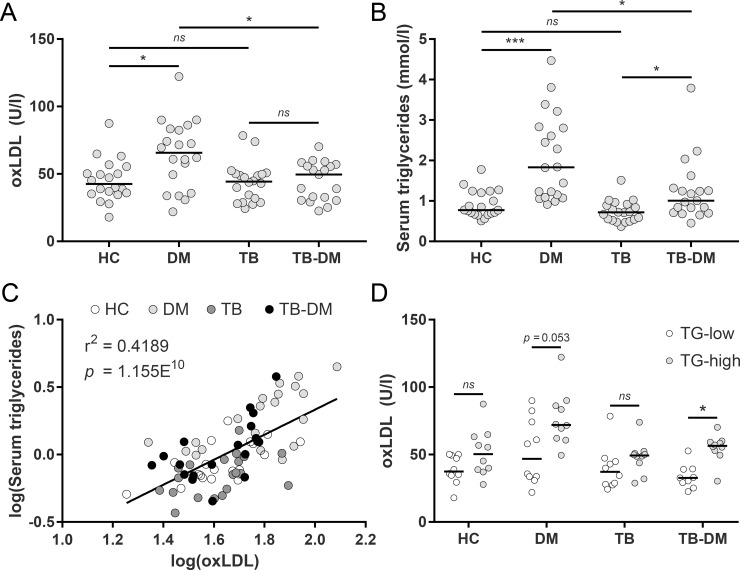
Plasma oxLDL levels were significantly higher in DM patients (median: 65.8 [interquartile range: 39.2–83.2] U/l) compared to both HC (42.3 [35.3–82.2] U/l, p < 0.05) and TB-DM patients (44.4 [30.3–56.7] U/l, p < 0.05) (Fig 1A), but not significantly different in patients with TB-DM compared to TB alone (44.3 [29.6–50.0] U/l). However, a clear dichotomy was distinguishable in the TB-DM patient group: our previous analysis of these samples [40] had demonstrated that both DM and TB-DM patients displayed characteristics of dyslipidemia, as evidenced by high levels of serum triglycerides (TG) (Fig 1B). Furthermore, serum triglyceride levels were positively correlated with oxLDL across all measured samples (r2: 0.4189, p = 1.155−10) (Fig 1C). To investigate whether oxLDL levels were related to the severity of dyslipidemia in TB-DM patients, we subdivided the groups according to serum TG-concentrations (TG-high and TG-low, Fig 1D). DM and TB-DM patients with TG-high had increased oxLDL levels compared to those with TG-low (DM: 72.0 [61.7–87.1] vs 46.8 [33.0–76.3] U/l, p = 0.053; TB-DM: 56.4 [52.1–59.4] vs 32.7 [27.2–39.2] U/l, p < 0.05). Taken together, the results validate that DM patients have increased levels of circulating oxLDL and that plasma oxLDL concentrations are elevated in DM and TB-DM patients with concomitant hypertriglyceridemia.
Fig 1. OxLDL levels are increased in DM and associated with triglyceride levels in TB-DM patients.
(A) OxLDL concentrations (U/l) were determined in plasma samples of healthy controls (HC) (n = 20), TB (n = 20), DM (n = 20) and TB-DM patients (n = 19) by ELISA. (B) Serum triglyceride (TG) levels (mmol/l) were determined by H+-NMR spectroscopy. (C) Linear correlation analysis of log-transformed serum triglyceride and oxLDL levels. (D) Plasma oxLDL concentrations in HC, TB, DM and TB-DM patients stratified by TG levels (TG-high vs TG-low; n = 10/group except for TB-DM + TG-low: n = 9). Individual patients are depicted as dots with group medians. Statistical significance was determined by Kruskal-Wallis test with post-hoc Dunn’s test. * = p < 0.05, *** = p < 0.001.
OxLDL treatment increases Mtb bacterial burden in infected human macrophages
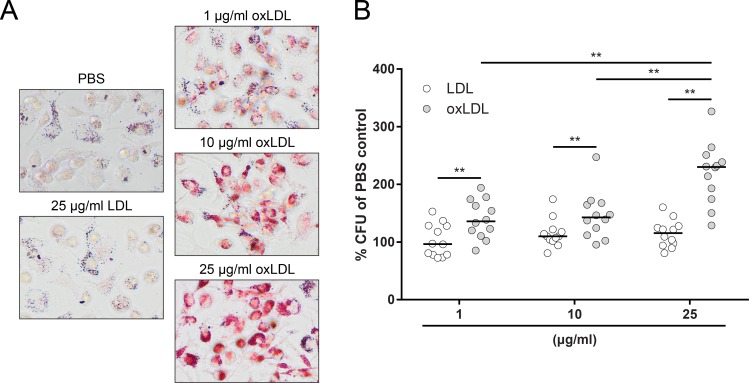
As oxLDL was clearly elevated in DM patients and has been described to have profound effects on macrophage function, we hypothesized that oxLDL treatment could compromise the capacity of macrophages to control Mtb infection. To investigate this, macrophages were treated with 1, 10 or 25 μg/ml oxLDL or native LDL overnight. Oil Red O staining indicated a dose-dependent increase in intracellular lipid levels after oxLDL treatment, while native LDL did not induce foam cells (Figs 2A and S1B). These macrophages were subsequently infected for 24 h with Mtb H37Rv and intracellular bacterial loads were assessed by bacterial colony forming unit (CFU) assay. OxLDL treatment significantly increased Mtb load compared to native LDL at all tested concentrations (1 μg/ml: 136% [113% - 171%] vs 97% [78% - 128%], p < 0.01; 10 μg/ml: 143% [115% - 167%] vs 110% [102% - 121%], p < 0.01; 25 μg/ml: 230% [179% - 248%] vs 115% [94.8% - 127%], p < 0.01), and this effect was dose-dependent (25 μg/ml oxLDL vs 1 μg/ml: p < 0.01; vs 10 μg/ml: p < 0.01) (Fig 2B). The magnitude of the increase in bacterial load was not correlated with small fluctuations in infectious load (MOI) (S1C Fig).
Fig 2. OxLDL-treated macrophages have an increased Mtb burden.
Primary human macrophages were treated with PBS control, native LDL or oxLDL (1, 10 or 25 μg/ml) overnight and subsequently infected with Mtb H37Rv at a MOI of 10:1. (A) Oil Red O staining of macrophages treated overnight with PBS, 25 μg/ml LDL or 1, 10 and 25 μg/ml oxLDL. Pictures were taken at a 20x magnification. (B) Macrophages were lysed at 24 h post-infection and bacterial load was determined by CFU assay. Results were normalized versus PBS control (n = 8). Individual donors are depicted as dots with group medians. Statistical significance was determined by Wilcoxon signed rank test with post-hoc FDR correction. ** = p < 0.01.
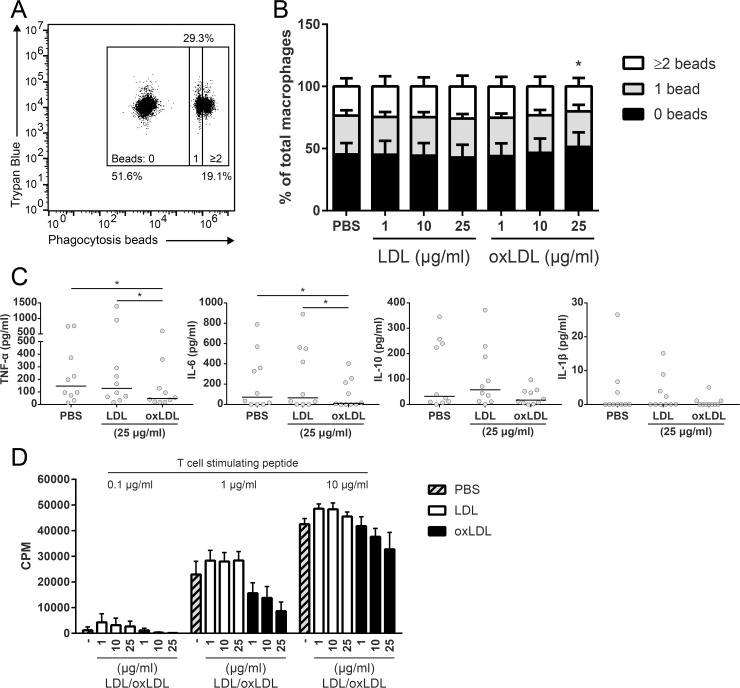
While these experiments demonstrated that oxLDL treatment supported Mtb persistence in human macrophages, it was unclear whether this was the result of increased phagocytosis,reduced intracellular mycobacterial control or enhanced replication. To gain a better understanding on the cellular processes affected by oxLDL treatment, we explored the functional consequences of oxLDL-induced foam cell formation. Firstly, the phagocytic capacity of oxLDL-treated macrophages was assessed to investigate whether the increased mycobacterial load might be related to enhanced Mtb uptake. Macrophages treated with either native LDL or oxLDL were incubated with fluorescent polystyrene beads and bead phagocytosis was quantified by flow cytometry (Fig 3A). Although a small but significant decrease in bead uptake was observed in macrophages incubated with 25 μg/ml oxLDL compared to LDL (p < 0.05) (Fig 3B), overall macrophage phagocytic capacity was unaffected by oxLDL treatment, indicating that the increased mycobacterial burden in oxLDL-derived foam cells was probably not the result of increased phagocytic uptake. To confirm this, we investigated the intracellular bacterial load of oxLDL-treated macrophages directly after 1 h of infection and found no significant differences compared to control conditions (S2C Fig).
Fig 3. Functional analysis of oxLDL-treated macrophages.
Primary human macrophages were treated with PBS control, native LDL or oxLDL (1, 10 or 25 μg/ml) overnight. (A) Macrophages were incubated with fluorescent phagocytosis beads at a MOI of 10:1 and subsequently analyzed by flow cytometry. Fluorescence of extracellular beads was quenched with Trypan Blue. (B) Percentage of macrophages with 0, 1 or ≥2 beads internalized beads (n = 6). Data is represented as means with standard deviations. (C) Macrophages were infected with Mtb H37Rv at a MOI of 10:1 for 1 h. Supernatants were harvested at 24 h post-infection and concentrations of TNF-α, IL-6, IL-10 and IL-1β were determined by ELISA (n = 10). Individual donors are depicted as dots with group medians. (D) Macrophages were co-cultured for four days with the HLA-DR2-restricted CD4+ T cell R2F10 at a ratio of 1:4 and 0.1, 1 or 10 μg/ml of its cognate peptide. T cell proliferation was measured by tritium-thymidine incorporation during the last 24 h (n = 3). Data is represented as means with standard deviations. Statistical significance was determined by Wilcoxon signed rank test with post-hoc FDR correction. * = p < 0.05.
Next, we explored the cytokine response of oxLDL-derived foam cells to Mtb-infection as earlier studies had reported potent oxLDL-induced pro-inflammatory cytokine production. In contrast to these studies, oxLDL-treatment in our experiments significantly decreased the secretion of TNF-α compared to treatment with LDL (47 [20 – 186] vs 128 [54 – 453] pg/ml, p < 0.05) or PBS (146 [62 – 466] pg/ml, p < 0.05). Similar results were obtained for IL-6 after oxLDL treatment versus LDL (11 [0–226] vs 66 [0–553] pg/ml, p < 0.05) or PBS (73 [0–412] pg/ml, p < 0.05), although some inter-individual variation was observed (Fig 3C). IL-10 levels were not significantly affected by oxLDL, while IL-1β levels were very low.
Finally, oxLDL-derived macrophages were co-cultured with a HLA-DR2-restricted CD4+ T cell clone (R2F10) and its cognate peptide (Mlep hsp65 p418–427) and T cell proliferation was measured to determine macrophage dependent antigen presentation. OxLDL treatment dose-dependently diminished the antigen presentation capacity of macrophages, especially at suboptimal peptide concentrations (Fig 3D). Similar results were obtained using a second, HLA-DR3-restricted CD4+ T cell clone (Rp15 1–1) (S2A Fig), both after loading with its cognate peptide or purified protein derivative (PPD). This diminished antigen presentation capacity was independent of cell surface expression of HLA-DR and co-stimulatory molecules CD80 and CD86 (S2B Fig). Taken together, oxLDL treatment impaired several macrophage functions, including antigen presentation and pro-inflammatory cytokine secretion, but not their phagocytic capacity.
OxLDL supports Mtb intracellular survival through lysosomal cholesterol accumulation
OxLDL-derived free and esterified cholesterol have been demonstrated to be sequestered in lysosomes in macrophages [26, 27], which potentially leads to lysosomal dysfunction. To investigate whether lysosomal localization of oxLDL lipids is required for its effect on Mtb load, oxLDL treatment was compared to acLDL, a non-naturally occurring modified lipoprotein which is endocytosed through identical scavenger receptor pathways as oxLDL, but does not induce lysosomal cholesterol accumulation [26, 42]. In resemblance to oxLDL, acLDL treatment of macrophages resulted in foam cell formation. However, while lipid staining intensities were similar (S1B Fig), clear differences in intracellular lipid localization and droplet structure were observed between both types of lipoproteins: in general, acLDL-induced intracellular lipid droplets were darker in color and appeared more granular than those resulting from oxLDL treatment (Fig 4A). Most importantly, however, acLDL did not affect macrophage Mtb load compared to untreated macrophages while oxLDL treatment significantly increased mycobacterial load (Fig 4B: oxLDL: 232% [194%– 278%] vs acLDL: 108% [88% - 126%]; p < 0.0001). This effect was not restricted to Mtb, as comparable results were obtained after macrophage infection with Salmonella enterica serovar Typhimurium (Stm) (Fig 4D: 179% [162% - 183%] vs 124% [88% - 136%]; p < 0.05) and M. bovis BCG (Fig 4C: 178% [133% - 254%] vs 97% [82% - 123%]; p < 0.05). To examine whether the observed difference between oxLDL and acLDL could be related to lysosomal function, their effect on lysosomal and autophagy markers during Mtb infection was analyzed by Western blot (Fig 4E). OxLDL treatment increased protein levels of lysosomal markers compared to PBS and acLDL, as demonstrated by higher levels of lysosomal membrane glycoproteins (LAMP1 & LAMP2) and proteases (Cathepsin D & L) (Fig 4F), also including the 48 kDa processing intermediate pro-cathepsin D (S3A Fig). Furthermore, oxLDL but not acLDL treatment led to an increased accumulation of LC3-II in the presence of vacuolar type H+-ATPase inhibitor bafilomycin A1 (10 nM) to block vesicle breakdown, indicative of increased autophagic flux. In contrast, levels of autophagosome cargo protein p62, a mediator of selective autophagy, were not elevated by oxLDL (Fig 4F). Collectively, these results indicate that oxLDL induces a general defect in macrophage antimicrobial function which is dependent on intracellular lipid localization.
Fig 4. Lysosomal cholesterol accumulation attenuates macrophage Mtb control.
Primary human macrophages were treated with PBS control, acLDL or oxLDL (25 μg/ml) overnight. (A) Oil Red O staining of macrophages treated overnight with 25 μg/ml acLDL or oxLDL. Pictures were taken at a 20x magnification. Macrophages were infected with Mtb H37Rv (B) (n = 32), M. bovis BCG (C) (n = 6) or Salmonella enterica serovar Typhimurium (D) (n = 6) at a MOI of 10:1. Cells were lysed at 24 h post-infection and bacterial load was determined by CFU assay. Results were normalized versus PBS control. (E) Western blot analysis of lysosomal and autophagy markers in macrophages treated with bafilomycin A1 (10 nM) or DMSO control during 24 h of H37Rv Mtb infection. Data shown is from one representative donor (n = 4). (F) Quantification of LAMP1, LAMP2, Cathepsin D, Cathepsin L (+ DMSO), p62 and LC3-II (+ bafilomycin A1) protein levels. Protein levels were first normalized to actin and subsequently versus PBS control (n = 4). (G) Macrophages were co-treated with U18666A (3 μg/ml) or DMSO control for 24 h. Cells were subsequently stained for neutral lipids (LipidTOX, green), lysosomes (Lysotracker, red) and cholesterol (filipin, blue) and analyzed by confocal microscopy. Pictures were taken at a 63x magnification. Scale bars represent 5 μM. (H) Macrophages were co-treated with U18666A (3 μg/ml) or DMSO control for 24 h pre- and post-infection with Mtb H37Rv at a MOI of 10:1. Cells were lysed at 24 h post-infection and bacterial load was determined by CFU assay. Results were normalized versus PBS control (+ DMSO). Individual donors are depicted as dots with group medians. Statistical significance was determined by Wilcoxon signed rank test. * = p < 0.05, **** = p < 0.0001.
To further substantiate this hypothesis, macrophages were treated with PBS, oxLDL or acLDL in the absence or presence of U18666A (3 μg/ml), an inhibitor of intracellular cholesterol transport [43]. Lysosomal cholesterol sequestration was visualized using confocal microscopy by staining with fluorescent probes for neutral lipids (LipidTOX), lysosomes (Lysotracker) and cholesterol (filipin) (Fig 4G). OxLDL treatment induced a marked accumulation of cholesterol inside lysosomal vesicles as indicated by filipin and Lysotracker colocalization, which was not observed in macrophages treated with PBS or acLDL. Strikingly, when combined with U18666A, acLDL-treated macrophages showed identical lysosomal cholesterol sequestration as oxLDL. The absence of an effect of acLDL treatment alone on Mtb load suggested that the localization of cholesterol inside lysosomes might be a causative factor in the increased Mtb growth phenotype of oxLDL-treated macrophages. To test this, we investigated whether combined treatment of acLDL with U18666A could mimic the effect of oxLDL on macrophage Mtb control. Indeed, while U18666A alone or in combination with oxLDL did not significantly alter macrophage phenotype (Fig 4G) and Mtb load, it increased mycobacterial burden when applied in conjunction with acLDL compared to DMSO control (Fig 4H: 169% ± 62% vs 107% ± 32%; p < 0.05). Similar to oxLDL, U18666A treatment alone and in combination with acLDL increased protein levels of lysosome and autophagy markers in Mtb-infected macrophages, most notably Cathepsin L and when combined with bafilomycin A1 (10 nM), p62 and LC3-II (S3B Fig). Macrophage viability was unaffected by oxLDL and/or U18666A treatment in combination with Mtb infection as determined by combined Hoechst/propidium iodide (PI) staining (S3C and S3D Fig). Collectively, these results indicate that not simply the presence, but the specific accumulation of cholesterol inside lysosomes is crucial for the oxLDL- and U18666A-induced increase in Mtb survival in human macrophages.
While the above model proposes that oxLDL can interfere with macrophage mycobacterial control, we could not yet exclude whether oxLDL-induced foam cell formation also supported Mtb replication, possibly by providing increased nutrients. To gain a better understanding of overall kinetics of oxLDL-induced increased Mtb load and its associated cytokine response, infected macrophages treated with PBS control, oxLDL or acLDL were infected with Mtb and the intracellular bacterial load and concentrations of 29 cytokines and chemokines in supernatants were determined at 0 (uptake control), 4, 24, 48, 72 and 144 h post-infection. OxLDL treatment showed increased Mtb survival compared to PBS as early as 4 h post-infection, and versus both PBS and acLDL at all later time points (24–144 h) (S4A Fig). For all treatment conditions the intracellular Mtb load decreased with time, ranging from 1.3 to 12.4% of original bacterial uptake after 144 h of infection, which is supportive of a model in which the effect of oxLDL is the result of inhibited bacterial killing and not of increased bacterial outgrowth.
The multiplex results were congruent with the ELISA data from Fig 3C, as oxLDL-treated macrophages produced significantly lower levels of TNF-α and IL-6 after 24 h of Mtb infection compared to PBS control (S4B Fig). Many cyto- and chemokine concentrations were lower in oxLDL-treated macrophages between 4–48 h of Mtb infection, while supernatants from acLDL-treated macrophages often showed intermediate levels compared to PBS and oxLDL (IL-10, IL-6, TNF-α, IL-8, CCL3, CCL4, G-CSF, GM-CSF). We did not find significant differences at 72 and 144 h post-infection after FDR correction. IL-1RA was the only cytokine which showed increased production as a result of oxLDL, although the magnitude of this response varied between donors. Concentrations of CXCL10, IFNα2, CCL2 and VEGF increased as a result of Mtb infection, however no differences were observed between treatment conditions for these factors. Levels of Epidermal Growth Factor (EGF), Eotaxin, IFNγ, IL-12p40, IL-12p70, IL-1β, IL-13, IL-15, IL-17A, IL-1α, IL-2, IL-3, IL-4, IL-5, IL-7 and TNF-β were measured but not shown as their concentrations were either very low in all samples (<100 pg/ml) or not detectable. Taken together, these experiments provide further evidence for an overall diminished cytokine response as a result of oxLDL treatment during Mtb infection.
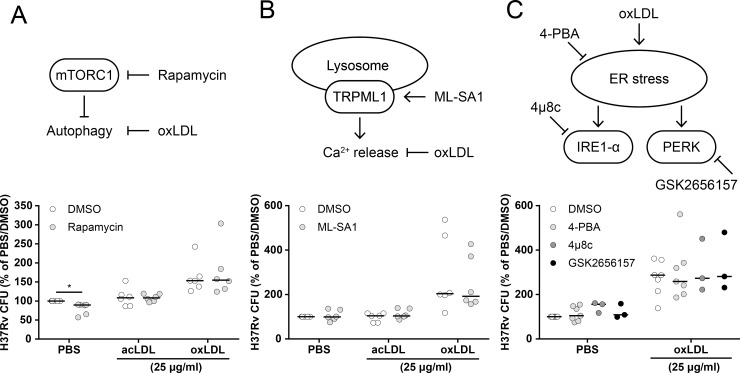
OxLDL-inhibited mycobacterial killing is not rescued by small-molecules targeting known downstream signaling pathways
To identify the relevant molecular processes which are deregulated by lysosomal cholesterol accumulation, oxLDL-treated macrophages infected with Mtb were treated with compounds targeting various cell signaling pathways which are known to be affected by oxLDL in an attempt to rescue their antimicrobial capacity. Firstly, infected foamy macrophages were treated with rapamycin, an inhibitor of mammalian target of rapamycin complex 1 (mTORC1). mTOR is a master regulator of various cellular pathways including autophagy, and rapamycin-induced autophagy was reported to ameliorate foam cell formation [44, 45]. Rapamycin (2 μM) slightly but significantly reduced Mtb load compared to DMSO in PBS-treated macrophages (80 ± 15% of PBS/DMSO, p < 0.05), but did not affect bacterial burden in either oxLDL or acLDL-induced foamy macrophages (Fig 5A). Secondly, lysosomal storage disorders such as NPC disease are associated with defects in lysosomal Ca2+ homeostasis [46], and activation of the lysosomal ion channel transient receptor potential channel 1 (TRPML1) by small-molecule activator ML-SA1 was shown to rescue lysosomal trafficking in NPC-/--macrophages [30]. However, ML-SA1 treatment (10 μM) did not affect Mtb infection in any of our conditions (Fig 5B). Finally, oxLDL can induce endoplasmic reticulum (ER) stress in macrophages [47], a state of disturbed ER homeostasis due to accumulation of unfolded proteins and/or disrupted Ca2+ handling which plays a role in the apoptotic response in atherosclerotic plaques and the TB granuloma [48, 49]. Treatment of Mtb-infected macrophages with three established reducers of the ER stress response, namely chemical chaperone 4-phenylbutyrate (4-PBA; 3 mM) and downstream kinase inhibitors 4μ8c (10 μM) and GSK2656157 (10 μM) (respectively targeting inositol-requiring enzyme 1-α (IRE1-α) and protein kinase RNA-like endoplasmic reticulum kinase (PERK)), did not alleviate the oxLDL-induced increase in mycobacterial survival (Fig 5C). In conclusion, chemical modulation of mTOR signaling, lysosomal Ca2+ homeostasis or ER stress did not reverse the oxLDL-induced increased mycobacterial load in human macrophages.
Fig 5. oxLDL-induced Mtb survival was not reversed by targeting known downstream pathways.
Primary human macrophages were treated with PBS control, acLDL or oxLDL (25 μg/ml) overnight and subsequently infected with Mtb H37Rv at a MOI of 10:1 and treated with compounds or DMSO control overnight. Cells were lysed at 24 h post-infection and bacterial load was determined by CFU assay. The following treatments were applied: (A) rapamycin (2 μM, n = 6) to induce mTORC1-regulated autophagy, (B) ML-SA1 (10 μM, n = 6) to stimulate lysosomal Ca2+ release and (C) 4-PBA (3 mM, n = 7), 4μ8c (10 μM, n = 3) and GSK2656157 (10 μM, n = 3) to inhibit the ER stress response. Individual donors are depicted as dots with group medians. Results were normalized versus PBS control (+ DMSO). Statistical significance was determined by Wilcoxon signed rank test. * = p < 0.05.
OxLDL inhibits Mtb localization to functional lysosomes in infected macrophages
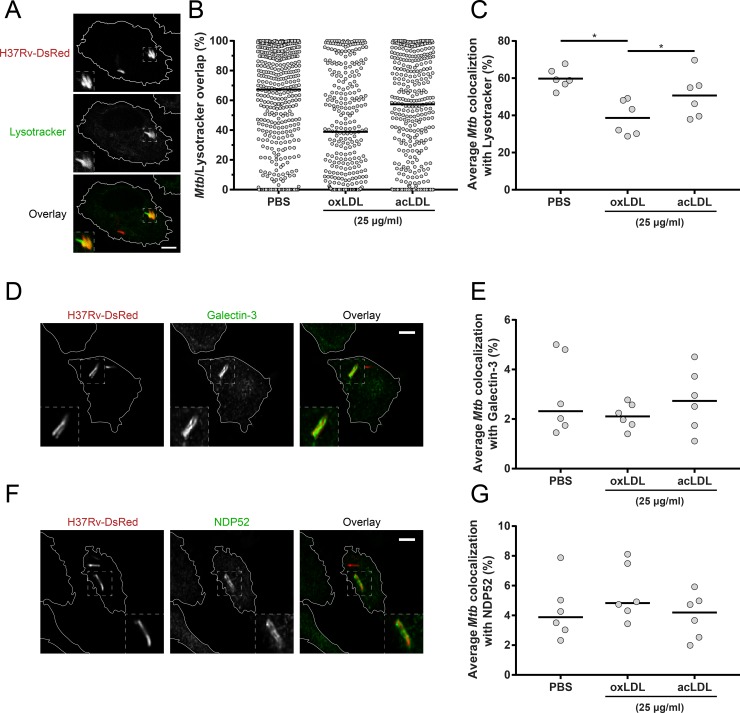
The above experiments demonstrated that the endolysosomal system is pivotal for oxLDL-induced increased mycobacterial survival. As earlier studies have reported that cholesterol accumulation impaired proper lysosomal trafficking [29, 30], we hypothesized that Mtb trafficking to functional lysosomes was inhibited by oxLDL treatment. To investigate this, oxLDL-treated macrophages infected with fluorescent DsRed-expressing H37Rv were stained for functional lysosomes with Lysotracker (Fig 6A), and lysosomal colocalization was determined for each intracellular mycobacterium individually (Fig 6B). OxLDL significantly decreased the average colocalization between Mtb and Lysotracker (39 ± 9%) compared to acLDL (51 ± 12%, p < 0.05) or PBS treatment (60 ± 6%, p < 0.05) (Fig 6C), indicating that oxLDL inhibits phagolysosomal fusion in Mtb-infected macrophages.
Fig 6. oxLDL impairs Mtb localization to lysosomes in macrophages.
Primary human macrophages were treated overnight with PBS control, acLDL or oxLDL (25 μg/ml) and subsequently infected with DsRed-Mtb H37Rv (red) at a MOI of 10:1. Cells were stained (green) for lysosomes (Lysotracker) (A), galectin-3 (D) or NDP52 (F) at 4 h post-infection and analyzed by confocal microscopy. Pictures were taken at a 63x magnification. Scale bars represent 5 μM. Percentage overlap of intracellular mycobacteria with staining was determined for 3 wells * 3 = 9 pictures per condition. (B) Results of a representative donor of Mtb overlap with Lysotracker. Individual mycobacteria are represented by dots with group medians. Average colocalization of Mtb with Lysotracker (C), galectin-3 (E) and NDP52 (G) are displayed for macrophages from six independent donors. Individual donors are depicted as dots with group medians. Statistical significance was determined by Wilcoxon signed rank test with post-hoc FDR correction. * = p < 0.05.
In an attempt to identify the specific lysosomal pathways affected by oxLDL treatment, we investigated colocalization of Mtb with galectin-3 and NDP52. Galectins are carbohydrate-binding proteins which play a role in targeting damaged endomembrane structures for autophagy [50], including phagolysosomes damaged by Stm or Mtb [51–53], and galectin-3 colocalization with lysosomes is an established measure of lysosomal damage [54]. NDP52 is an autophagy adaptor which has previously been implicated in the autophagic clearance of both Stm and Mtb [51, 55, 56]. Although colocalization events with Mtb were observed for both galectin-3 and NDP52, this occurred for a minority of intracellular bacteria (range 2–8% of bacteria) and no significant differences were found between oxLDL and control conditions (Fig 6D–6G).
Discussion
The looming epidemic of concurrent TB-DM poses a serious global health problem. Identification of the causal molecular and cellular mechanisms underlying the increased risk of TB in DM patients is paramount for adequate treatment. Previously, we have demonstrated that TB-DM patients have a blood lipid profile with pro-atherogenic properties [40], which could have implications for TB-DM pathogenesis. We now identify oxLDL as a potential risk factor for TB. OxLDL levels were found to be increased in plasma samples of DM patients from a TB endemic region, who represent the specific population at increased risk for disease. Although both triglyceride and oxLDL levels were lower in the TB-DM group compared to DM, this might well be related to the duration and severity of DM disease as the majority of TB-DM patients were recently diagnosed diabetics compared to the DM alone group (S1 Table). Furthermore, TB was associated with wasting syndrome and therefore with low levels of many circulating metabolites in this patient population, including LDL [40]. As these patients were not merely at increased risk of TB at the moment of blood collection but had already developed active disease, it is not unlikely that oxLDL levels are decreased since onset of TB. Nonetheless, a clear dichotomy in oxLDL concentrations was visible based on triglyceride-status in TB-DM patients, implying that diabetes-associated dyslipidemia was a factor associated with increased oxLDL levels in this population.
Importantly, oxLDL-, but not acLDL-, induced foamy macrophage formation supported intracellular Mtb survival through lysosomal cholesterol accumulation and subsequent dysfunction. This effect was not limited to Mtb as similarly enhanced bacterial loads were observed for Stm and M. bovis BCG, which reside in different intracellular compartments compared to Mtb [57]. Pharmacological manipulation of intracellular cholesterol transport with U18666A confirmed that subcellular localization of cholesterol to lysosomes was essential to lysosomal dysfunction. Since foamy macrophages play an important role during progression of the TB granuloma [16, 18], our results suggest that increased levels of oxLDL could contribute to the enhanced TB susceptibility in DM patients.
Our findings are in line with earlier studies that reported increased levels of oxLDL in DM patients [8–11]. Both hyperglycemia and dyslipidemia contribute to the generation of free radicals and oxidative stress during chronic DM [58, 59], which can lead to the pathological modification of proteins and lipids involved in foam cell formation and atherosclerosis, such as oxLDL. Additionally, DM and hyperglycemia are associated with increased expression of oxLDL scavenger receptors CD36 [60–62], SR-A [62, 63] and LOX-1 [62, 64], and macrophages from type 2 diabetics showed higher uptake of oxLDL [65]. Similar to DM, TB has been demonstrated to result in increased oxidative stress and a systemic decrease in antioxidant capacity, e.g. reduced levels of glutathione [66–69]. Mtb infection increased CD36 expression in vitro [19] and CD36-mediated uptake of surfactant lipids has been reported to support Mtb growth [20]. In contrast, a recent paper did not find a role for CD36-mediated macrophage lipid droplet formation in Mtb control [70], which could indicate that not simply the presence of lipid droplets but rather the specific composition and/or localization of the intracellular lipids is most important for their effect on Mtb intracellular survival, similar to what we observed here when comparing acLDL and oxLDL.
At the functional level, oxLDL treatment displayed potential to inhibit macrophage antigen presentation to CD4+ T cells, which could in principal lead to impaired activation of adaptive immune responses. While their phagocytic capacity was largely unaffected, oxLDL-treatment macrophages showed an overall decreased cytokine production in response to Mtb. These results were somewhat surprising, as oxLDL has been associated with increased inflammation during atherosclerosis [71] and non-alcoholic steatohepatitis (NASH) [72–74], including activation of the NLRP3 inflammasome and subsequent secretion of IL-1β by macrophages [38, 75]. However, in these studies oxLDL treatment was often accompanied by secondary factors which may be required for the observed pro-inflammatory responses, such as macrophage apoptosis, circulating anti-oxLDL immune complexes or the formation of intralysosomal cholesterol crystals. In agreement with our own observations, several studies reported diminished inflammatory responses of oxLDL-treated macrophages after stimulation with TLR ligands [76–78]. These divergent results could be related to study-specific differences in experimental setup, including variations in species, cell types, stimulations and degree of LDL oxidation. Additionally, oxLDL was reported to induce a long-lasting pro-inflammatory phenotype in monocytes through epigenetic changes, which possibly did not occur in our experiments due to their relatively short timeframe or lack of restimulation [79, 80].
Hypercholesterolemia has been implicated in increasing the risk of developing TB [81–83], and cholesterol catabolism is needed for mycobacterial persistence and growth [84, 85]. For this reason, most studies on foamy macrophage induction by mycobacteria have focused on the relatively long-term nutritional benefits of intracellular lipid accumulation [20, 86]. The results presented in this manuscript demonstrate that pathologically modified lipids also directly interfere with macrophage antimicrobial capacities, providing a novel perspective on the importance of foam cell formation during TB. These findings are corroborated by a study which demonstrated that M. smegmatis and M. bovis BCG blocked phagolysosomal fusion by inducing an NPC-like phenotype in infected macrophages [39]. Additionally, macrophage cholesterol depletion restored halted phagosome maturation during M. avium infection [87]. Drugs which target host cholesterol metabolism can therefore have potential for TB host directed treatment, and e.g. statins have shown promise as adjunctive anti-mycobacterial therapy both in vitro and in vivo [88–92]. Furthermore, our results suggest that oxLDL treatment supports mycobacterial survival through interference with phagolysosomal trafficking and/or fusion. Lysosomal lipid accumulation has been reported to influence these processes in several ways. Late endosomal transport is mediated by the lysosomal protein ORP1L, which modulates the interaction between Rab GTPases and their effectors, motor protein complexes and the ER through conformational changes induced by fluctuations in intraluminal cholesterol levels [93, 94]. Furthermore, abnormal sphingolipid storage due to NPC1-deficiency or U18666A treatment was shown to disrupt lysosomal Ca2+ homeostasis, blocking vesicle transport and fusion [30, 46]. Finally, several studies have reported that lysosomal storage disorders interfere with the autophagic system [31, 32], which might be reflected by the increased LC3-II levels detected in oxLDL- and U18666A-treated macrophages during Mtb infection. Although pharmacological modulation of these pathways did not ameliorate the oxLDL-induced effect on Mtb control, their involvement should not yet be excluded as the phenotype induced by oxLDL was practically irreversible in our experimental setup.
Our study might have had a number of limitations. Firstly, the oxLDL used throughout this manuscript was generated by copper-induced oxidation of native LDL, which is sometimes referred to as extensively oxidized LDL in literature due to its high oxidation grade [6]. It is generally believed that naturally occurring oxLDL is composed of less extensively oxidized variants as abundantly oxidized LDL would be rapidly cleared from the circulation. Therefore, it is possible that the phenotypes observed in our experiments are more extreme than would have occurred using naturally oxidized LDL. However, the precise composition of physiological oxLDL is still uncertain as accurate characterization of isolated oxLDL is technically challenging. As LDL oxidation mostly occurs in the subendothelial space during atherosclerosis, locally generated oxidized species might be of greater importance for disease than circulating oxLDL. Regardless, it would be of interest to investigate the effects of minimally modified LDL (mmLDL), a variant which is believed to be more similar to naturally occurring oxLDL [6], on macrophage Mtb infection. Secondly, oxLDL was applied at a concentration of 25 μg/ml for the majority of the experiments, which is at the high end of what has been physiologically observed [95–97]. However, oxLDL treatment times were relatively short compared to what can be expected in vivo, and low levels of oxLDL (1 μg/ml) were already sufficient to increase mycobacterial load during this period. Thirdly, oxLDL is a complex particle consisting of hundreds of phospholipids, triglycerides and cholesteryl esters, which vary in terms of composition and susceptibility to oxidation and therefore have different intracellular effects [12]. It would be of great interest to study whether specific oxidized lipids or proteins are required for the observed oxLDL phenotype. Finally, although not within the scope of this study and technically challenging, it would be important to validate these findings in a disease model for translation to in vivo settings, e.g. using monocytes isolated from DM patients.
In conclusion, oxLDL treatment of human macrophages supports Mtb intracellular survival as a result of lysosomal dysfunction, providing a proof of concept for a contribution of increased levels of oxLDL as a potential risk factor for TB development during DM. While we previously demonstrated that hyperglycemia alone did not directly influence outcome of macrophage Mtb infection [98], we postulate that elevated lipid levels, which are associated with DM, can be in involved in TB-DM pathogenesis [40]. These findings pave the way for further research, including the use of LDL-lowering drugs such as statins or antioxidant drugs as part of the DM-treatment regimen for the reduction of the risk of TB.
Materials and methods
Ethics statement/Patient population and plasma oxLDL measurements
The patient population was previously used in an extensive lipid profiling analysis using H+-NMR spectroscopy as part of an EU-funded collaborative project, TANDEM [99], of which details regarding patient inclusion were reported earlier [40]. From this population plasma samples of 20 healthy endemic controls, 20 TB patients, 20 DM patients and 20 TB-DM patients were selected at random for oxLDL determination. Plasma oxLDL levels were measured by sandwich ELISA according to manufacturer’s instructions (Mercodia AB, Uppsala, Sweden). One TB-DM patient was excluded post-hoc due to the presence of clinical evidence suggestive of type 1 diabetes, while all other DM patients suffered from type 2 diabetes. This study was approved by the Health Research Ethics Committee of the University of Stellenbosch, and conducted according to the Helsinki Declaration and International Conference of Harmonization guidelines. Written informed consent was obtained from all participants.
Reagents and antibodies
Primary antibodies against LAMP1, LAMP2, Cathepsin D, Cathepsin L, p62, galectin-3 and secondary goat anti-mouse IgG (Alexa Fluor 647) were purchased from Abcam (Cambridge, UK). LC3A/B was from Cell Signaling (Bioke, Leiden, The Netherlands), actin-HRP from Santa Cruz Biotechnology (Santa Cruz, CA, USA), CD86-Alexa700 and HLA-DR-PeCy5 from BD Biosciences (Erembodegem, Belgium) and CD80-BV650, CD14-FITC and CD163-Alexa647 were bought from Biolegend (ITK diagnostics, Uithoorn, The Netherlands). NDP52 (CALCOCO2), secondary goat anti-rabbit IgG (Alexa Fluor 647) and HRP-conjugated antibodies reactive with mouse and rabbit were purchased from Thermo Fisher Scientific (Merelbeke, Belgium).
LDL isolation
LDL was isolated from the serum of healthy volunteers by density gradient ultracentrifugation [100]. Blood was collected in clot activator tubes and clotted for 90 minutes at room temperature. Serum was obtained after 10 minutes of centrifugation at 1,500 g. EDTA was added to a final concentration of 1 mM, after which serum density was adjusted to 1.21 g/l by addition of solid potassium bromide and gentle stirring. The resulting serum solution was distributed over 13.7 ml UltraClear ultracentrifuge tubes (Beckman Coulter, Woerden, The Netherlands) and a density gradient was prepared by overlaying it with potassium bromide solutions of decreasing concentrations (1.063 g/l, 1.019 g/l, 1.0063 g/l) in PBS supplemented with 0.3 mM EDTA (pH 7.4) using a wide bore pipette tip. The serum was then centrifuged at 40,000 RPM for 20 h at 4°C in a SW41 Ti swinging bucket rotor (Optima LE-80K, Beckman Coulter). After centrifugation the tubes were carefully removed from the rotor and the LDL fraction was aspirated using a glass Pasteur pipette. The LDL was dialyzed against PBS at 4°C for 16 h during which the buffer was refreshed three times. The protein concentration of LDL was determined using a BCA kit according to the manufacturer’s instructions (Pierce, Thermo Fisher Scientific).
Generation of oxLDL and acetylated LDL (acLDL)
OxLDL was generated by copper oxidation of native LDL. Copper sulfate was added to 200 μg/ml LDL in PBS at a final concentration of 5 μM and incubated for 20 h at 37°C in the dark. The reaction was stopped by addition of 0.2 mM EDTA and oxLDL was then dialyzed against PBS containing 1 mM EDTA at 4°C for 24 h during which the buffer was refreshed three times. To produce acLDL, LDL was acetylated according to the protocol by Fraenkel-Conrat et al. [101]. An equal volume of saturated sodium acetate was added to 1 mg/ml of LDL and stirred at 4°C until cold. During the following hour acetic anhydride was added in 2 μl aliquots until 1.5x the mass of LDL was added in total. The mixture was stirred for another 30 minutes after the last aliquot was added. The acLDL was then dialyzed against PBS containing 1 mM EDTA at 4°C for 24 h during which the buffer was refreshed three times. Finally, the modified lipoproteins were concentrated to 1 mg/ml using 100 kDa Amicon Ultracel centrifugal filter units (Merck Millipore, Amsterdam, The Netherlands).
Macrophage differentiation and foam cell generation
CD14+ monocytes were isolated from buffy coats of healthy blood bank donors by positive selection using an autoMACS Pro Separator (Miltenyi Biotec BV, Leiden, The Netherlands). Donors were not part of an already-existing collection. Monocytes were differentiated into macrophages by addition of 50 ng/ml macrophage-colony stimulating factor (M-CSF) (Miltenyi Biotec) during culture for 6 days at 37°C/5% CO2 [102]. Cells were cultured in RPMI-1640 medium with L-glutamine, without glucose and sodium bicarbonate (Sigma-Aldrich Chemie BV, Zwijndrecht, the Netherlands), supplemented with 5 mM D-glucose, 2 g/l sodium bicarbonate, 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin. After differentiation macrophages were harvested by trypsinization and seeded in multi-well plates. As a quality control, macrophages were stained for surface expression of CD14 and CD163 and acquired on a BD LSRFortessa flow cytometer (BD Biosciences) (S1A Fig). To generate foam cells, macrophages were treated with various concentrations of oxLDL overnight. PBS, native LDL and/or acLDL were used as controls. Foam cell formation was confirmed by Oil Red O staining. Macrophages were fixed for 30 minutes in 4% paraformaldehyde and subsequently stained with a filtered work solution of Oil Red O (Sigma-Aldrich) in isopropanol (0.3% Oil Red O in 60% isopropanol) for 20 minutes. Afterwards, the red stain was dissolved in 4% NP-40 in isopropanol and quantified by measuring the optical density (OD) at 520 nm using a iMark Microplate Absorbance Reader (Bio-Rad, Veenendaal, The Netherlands).
Mtb H37Rv infection and cytokine measurements
Mtb H37Rv cultures were grown to mid-log phase in Middlebrook 7H9 liquid medium (Difco, BD Biosciences) supplemented with albumin/dextrose/catalase (ADC) (BBL, BD Biosciences). Bacterial concentrations were determined by measuring culture optical density at 600 nm. Macrophages were infected with H37Rv at a multiplicity of infection (MOI) of 10:1 for 1 h at 37°C, after which the cells were washed twice with medium containing 30 μg/ml gentamicin and cultured overnight in fresh medium containing 5 μg/ml gentamicin. Infected cells were lysed either directly after infection or at 4, 24, 48, 72 or 144 h post-infection using 0.05% Triton X-100 and a dilution series of the lysates was plated on 7H10 square agar plates (Difco, BD Biosciences) supplemented with oleate/albumin/dextrose/catalase (OADC) (BBL, BD Biosciences). Colony-forming units (CFU) were determined after 2–3 weeks of incubation at 37°C. From some experiments supernatants were harvested and filtered for determination of IL-1β, IL-6, TNF-α (Invitrogen, Thermo Fisher Scientific) and IL-10 (Sanquin, Amsterdam, The Netherlands) by ELISA or for testing using a Human Cytokine/Chemokine Immunology Multiplex Assay (Merck Millipore, Amsterdam, the Netherlands) according to their manufacturers’ instructions.
Phagocytosis assay
To quantify phagocytic capacity, fluorescent polystyrene particles (Fluoresbrite YG carboxylate microspheres) (Polysciences, Hirschberg an der Bergstrasse, Germany) were used as described by Leclerc et al [103]. Macrophages were incubated with fluorescent beads in a ratio of 10 beads to 1 cell for 90 min at 37°C. Cells were subsequently harvested by gentle scraping and resuspended in a 1:1 mixture of culture medium and Trypan Blue, and internalization of the beads was quantified by acquisition on a BD Accuri C6 flow cytometer (BD Biosciences). Non-internalized bead fluorescence was quenched by Trypan Blue and detected in the FL-3 channel (red), whereas internalized beads were detected in the FL-1 channel (green). Analysis was performed using Flowjo software (version 10.1, Tree Star Inc, Ashland, OR).
Antigen presentation assay
HLA-DR2/HLA-DR3-postive macrophages were harvested, seeded in 96-well plates at 2,500 cells/wells and treated with PBS, 25 μg/ml oxLDL or native LDL. The following day the cells were washed once in assay medium (IMDM with 10% human serum) and HLA class II restricted CD4+ T cell clones were added at a ratio of 4:1 together with a dilution series of their specific cognate peptide (R2F10 clone: HLA-DR2 restricted, reactive with Mycobacterium leprae (Mlep) hsp65; Rp15 1–1: HLA-DR3 restricted, reactive with Mtb and Mlep hsp65) or 1.25 μg/ml purified protein derivative (PPD) (Staten Serum Institute, Copenhagen, Denmmark) [104, 105]. Medium was used as negative control. Macrophages and T cells were co-cultured for 3 days at 37°C/5% CO2, and tritium-thymidine was added for the last 16 h of culture after which the cells were harvested and tritium-thymidine incorporation was measured using a Microbetaplate counter (Wallac, Turku, Finland). Furthermore, macrophages were stained for surface expression of CD86, CD80 and HLA-DR and analyzed on a BD LSRFortessa flow cytometer (BD Biosciences).
Western blotting
For analysis of lysosomal and autophagy-related proteins, (Mtb-infected) macrophages were lysed for 5 minutes using a buffer containing 3% SDS, 4 mm glycerol, 100 mM Tris-HCl (pH 6.8) containing protease inhibitors (Roche, Woerden, The Netherlands) and the resulting lysates were boiled for 10 min at 95°C. Protein concentrations were determined by bicinchoninic acid assay (Pierce, Thermo Fisher Scientific) and equal amounts were mixed with 4x Laemmli buffer before loading on a 4–20% Mini-PROTEAN TGX precast protein gel (Bio-Rad). After separation, proteins were transferred onto a polyvinylidene fluoride membrane and blocked for 1 h in Tris-buffered saline/2.5% Tween-20 containing 5% non-fat dry milk and subsequently probed with primary antibodies overnight at 4°C. Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (reactive against mouse or rabbit) for 2 h at room temperature before visualization by Amersham Enhanced Chemiluminescence Western Blotting Detection kit (GE Healthcare, Hoevelaken, The Netherlands). Blots were quantified using Image J (NIH, Bethesda, MD, USA) and proteins were normalized versus actin.
Confocal microscopy
For confocal microscopy, macrophages were seeded in black poly-d-lysine coated glass 96-well plates (MatTek Corporation, Ashland, MA, USA). To stain lysosomes, macrophages were incubated with 75 nM Lysotracker Red or Deep Red (Thermo Fisher Scientific) at 37°C/5%CO2 for 1 h before fixation. Cells were fixed for 1 h in 1% EM-grade formaldehyde, followed by quenching with PBS/1.5 mg/ml glycine for 10 min and blocking in 5% human serum for 45 min, all at room temperature. For immunostaining, cells were permeabilized for 10 minutes with 0.1% Triton X-100 before blocking and subsequently stained with primary and secondary antibodies for 30 minutes each in the dark at room temperature. Finally, cells were stained with phalloidin-Alexa488 (Thermo Fisher Scientific) and/or LipidTOX Green (Thermo Fisher Scientific) for 30 min according to the manufacturers’ instructions, and/or 50 μg/ml Filipin complex from Streptomyces filipinensis (Sigma-Aldrich) for 2 h at room temperature in the dark. Lysotracker and filipin pictures were taken using a SP8WLL confocal microscope (Leica, Amsterdam, The Netherlands). Galectin-3 and NDP52 colocalization was visualized using a Dragonfly spinning-disk confocal microscope (Andor Technologies, Belfast, UK) equipped with 405, 488, 561 and 640nm lasers and a Zyla 4.2 sCMOS camera.
Colocalization analysis
Macrophages were infected for 4 h with a DsRed-expressing Mtb H37Rv strain at a MOI of 10:1 and stained with Lysotracker Deep Red or primary antibodies for galectin 3 and NDP52 as described above. Lysotracker channel background was subtracted by rolling ball algorithm (20 pixel radius). All images were analyzed using CellProlifer 3.0.0 [106]. First, pictures were corrected for non-homogenous illumination if necessary. DsRed-Mtb were segmented by manual global thresholding with intensity-based declumping, and stained objects were segmented by adaptive two-class Otsu thresholding with upper and lower bounds to correct for individual cell-specific differences in background signal with intensity-based declumping. Then, the percentage of staining object overlap with individual DsRed-Mtb was calculated for each image and the average colocalization was calculated for each treatment condition.
Macrophage viability assay
To assess cell viability after treatment and infection with H37Rv Mtb, macrophages were stained with 2 μg/ml propidium iodide (PI) (Sigma-Aldrich) and 2 μg/ml Hoechst 33342 (Sigma-Aldrich) in RPMI without phenol red and FCS for 5 min in the dark. Cells were subsequently imaged on a AF6000 fluorescence microscope (Leica) and pictures were taken at a 20x magnification. Pictures were processed and analyzed in Image J. First, the background was subtracted by rolling ball algorithm (20 pixel radius). Then, Hoechst- or PI-positive nuclei were segmented by Otsu thresholding and counted, from which the percentages of viable macrophages were calculated. Staurosporin (5 μM) (Sigma-Aldrich) was used as a positive control for cell death.
Statistical analysis
Statistical significance was assessed by Kruskal-Wallis test with post-hoc Dunn’s test, or Wilcoxon signed rank test using GraphPad software (version 7.02, Prism, La Jolla, CA, USA) with post-hoc false discovery rate (FDR) correction for multiple comparisons when necessary. Statistical analysis of patients characteristics was performed in SPSS 23 (IBM, Armonk, NY, USA) by one-way ANOVA (reported p-values are the outcome of the F-test), independent samples t-test or chi-squared test.
Supporting information
Monocyte-derived macrophages were differentiated using M-CSF (50 ng/ml) for 6 days. (A) Histograms of the cell surface expression of CD14 and CD163 as determined by flow cytometry. Stained (blue) and unstained (blue) samples are displayed. Data shown are from one representative donor. (B) Macrophages were treated overnight with PBS control, LDL, acLDL or oxLDL at 25 μg/ml and stained for neutral lipids with Oil Red O. Staining was dissolved and quantified by measuring OD at 520 nm. Data are displayed as ΔOD520 versus PBS control. Individual donors are depicted as dots with group medians. Statistical significance was determined by Kruskal-Wallis test with post-hoc Dunn’s test. ** = p < 0.01. (C) Macrophages were treated with 25 μg/ml oxLDL or PBS control overnight and infected with Mtb H37Rv for 24 h. OxLDL-induced increased Mtb loads were normalized to PBS control and plotted versus the infectious load (MOI) as determined by CFU assay (n = 34; each dot represents one individual donor). Kendall tau correlation and associated two-sided p-value are displayed.
(TIF)
Primary human macrophages were treated with PBS control, native LDL or oxLDL (1, 10 or 25 μg/ml) overnight. (A) Macrophages were co-cultured for four days with the HLA-DR3-restricted CD4+ T cell Rp15 1–1 at a ratio of 1:4 and 0.1, 1 or 10 μg/ml of its cognate peptide or 1.25 μg/ml PPD. T cell proliferation was measured by tritium-thymidine incorporation during the last 24 h (n = 3). Data is represented as means with standard deviations. (B) Cell surface expression of CD86, CD80 and HLA-DR as determined by flow cytometry of macrophages treated overnight with PBS, native LDL or oxLDL (25 μg/ml). Stained (blue) and unstained (blue) samples are displayed. Data shown are from one representative donor (n = 3). (C) Primary human macrophages were treated overnight with PBS control (n = 15), acLDL (n = 9), oxLDL (n = 15) or native LDL (n = 6) (25 μg/ml) and subsequently infected with Mtb H37Rv at a MOI of 10:1. Cells were lysed directly after 1 h of infection and bacterial load was determined by CFU assay to determine Mtb uptake. Results were normalized versus PBS control and depicted as group medians with 95% confidence intervals.
(TIF)
Primary human macrophages were treated with PBS control, acLDL or oxLDL (25 μg/ml) for 24 h prior to infection with Mtb H37Rv at a MOI of 10:1. (A) Representative Western Blot result of Cathepsin D protein levels from macrophages treated with bafilomycin A1 (10 nM) or DMSO, showing protein bands of both the mature heavy chain (34 kDa) and the processing intermediate pro-cathepsin D (48 kDa) after 30 and 900 seconds of exposure time. Pro-cathepsin D levels were first normalized to actin and subsequently versus PBS control (n = 4). (B) Western blot analysis of lysosomal and autophagy markers in macrophages co-treated with PBS, oxLDL or acLDL (25 μg/ml) and U18666A (3 μg/ml), bafilomycin A1 (10 nM) or DMSO control during 24 h of H37Rv Mtb infection. Data shown is from one representative donor (n = 2). (C) Mtb-infected macrophages were stained with Hoechst and PI to determine cell viability. Staurosporin (5 μM) and PBS were used as positive and negative control for cell death. (D) Percentages of viable cells (Hoechts+/PI-). Data are displayed as means with standard deviations (n = 4).
(TIF)
Primary human macrophages were treated with PBS control, acLDL or oxLDL (25 μg/ml) overnight and subsequently infected with Mtb H37Rv at a MOI of 10:1. Cells were lysed at 0 (uptake), 4, 24, 48, 72 and 144 h post-infection for CFU analysis (n = 7). (A) Intracellular Mtb loads are depicted as fraction of uptake in Tukey’s boxplots for each time point and condition: PBS (white), acLDL (grey) and oxLDL (red). (B) Supernatants were harvested at each time point post-infection and cytokine concentrations were determined by multiplex assay. Levels of IL-10, IL-6, TNF-α, IL-8, IL-1RA, CXCL10, IFNα2, CCL2, CCL3, CCL4, G-CSF, VEGF and GM-CSF (pg/ml) are depicted in Tukey’s boxplots for each time point and condition: PBS (green), acLDL (purple) and oxLDL (red). Group medians are shown as dashed lines. Statistical significance was determined by Wilcoxon signed rank test with post-hoc FDR correction. p < 0.05 for * = PBS vs oxLDL, # = oxLDL vs acLDL, ‡ = PBS vs acLDL.
(TIF)
Data is presented as percentage of total (%) or mean ± SD, *Point-of-care measurements, †lab measurements, 1data available from 12/19 patients, 2data available from 12/20 patients, 3data available from 16/19 patients.
(DOCX)
Acknowledgments
We are grateful to Prof. dr. Ronit Shiri-Sverdlov and Dr. Sofie Walenbergh, Departments of Molecular Genetics, Human Biology and Surgery, School of Nutrition and Translational Research in Metabolism (NUTRIM), Maastricht University, for their helpful suggestions regarding acLDL treatment, to Dr. Yanan Wang, Department of Medicine, Division of Endocrinology, Leiden University Medical Center, for providing the relevant protocols for LDL isolation and modification, to the Molecular Biology Clinical Research team at Stellenbosch University for characterizing the participants and for collecting the samples and to all the study participants and blood donors.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by the TANDEM (Tuberculosis and Diabetes Mellitus) Grant of the ECFP7 (European Union’s Seventh Framework Programme) under Grant Agreement No. 305279 for patient recruitment, data collection and analysis, authors: FV, SAJ, THMO, LW, GW; and by TBVAC2020 Grant of EC HOR2020 (Grant Agreement No. 643381) for data collection and analysis, authors: FV, SAJ, THMO, LW, GW The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Morton R. Phthisiolgia: or a treatise of consumptions. London: Smith and Walford; 1694. [Google Scholar]
- 2.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoSMed. 2008;5(7):e152 10.1371/journal.pmed.0050152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Diabetes & TB—fact sheet. Geneva, Switzerland 2016
- 4.International Diabetes Federation. Diabetes Atlas 8th edn Brussels, Belgium: 2017 [Google Scholar]
- 5.Singh R, Devi S, Gollen R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: larger-than-life. Diabetes/metabolism research and reviews. 2015;31(2):113–26. 10.1002/dmrr.2558 [DOI] [PubMed] [Google Scholar]
- 6.Levitan I, Volkov S, Subbaiah PV. Oxidized LDL: diversity, patterns of recognition, and pathophysiology. Antioxidants & redox signaling. 2010;13(1):39–75. 10.1089/ars.2009.2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida H, Kisugi R. Mechanisms of LDL oxidation. Clin Chim Acta. 2010;411(23–24):1875–82. 10.1016/j.cca.2010.08.038 [DOI] [PubMed] [Google Scholar]
- 8.Toshima S, Hasegawa A, Kurabayashi M, Itabe H, Takano T, Sugano J, et al. Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arteriosclerosis, thrombosis, and vascular biology. 2000;20(10):2243–7. [DOI] [PubMed] [Google Scholar]
- 9.Park K, Gross M, Lee DH, Holvoet P, Himes JH, Shikany JM, et al. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. 2009;32(7):1302–7. 10.2337/dc09-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Njajou OT, Kanaya AM, Holvoet P, Connelly S, Strotmeyer ES, Harris TB, et al. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes/metabolism research and reviews. 2009;25(8):733–9. 10.1002/dmrr.1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marin MT, Dasari PS, Tryggestad JB, Aston CE, Teague AM, Short KR. Oxidized HDL and LDL in adolescents with type 2 diabetes compared to normal weight and obese peers. Journal of diabetes and its complications. 2015;29(5):679–85. 10.1016/j.jdiacomp.2015.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller YI, Shyy JY. Context-Dependent Role of Oxidized Lipids and Lipoproteins in Inflammation. Trends in endocrinology and metabolism: TEM. 2016. 10.1016/j.tem.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(8):1702–11. 10.1161/01.ATV.0000229218.97976.43 [DOI] [PubMed] [Google Scholar]
- 14.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circulation research. 2011;108(2):235–48. 10.1161/CIRCRESAHA.110.223875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park YM. CD36, a scavenger receptor implicated in atherosclerosis. Exp Mol Med. 2014;46:e99 10.1038/emm.2014.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nature immunology. 2009;10(9):943–8. 10.1038/ni.1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santucci P, Bouzid F, Smichi N, Poncin I, Kremer L, De Chastellier C, et al. Experimental Models of Foamy Macrophages and Approaches for Dissecting the Mechanisms of Lipid Accumulation and Consumption during Dormancy and Reactivation of Tuberculosis. Frontiers in cellular and infection microbiology. 2016;6:122 10.3389/fcimb.2016.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peyron P, Vaubourgeix J, Poquet Y, Levillain F, Botanch C, Bardou F, et al. Foamy macrophages from tuberculous patients' granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS pathogens. 2008;4(11):e1000204 10.1371/journal.ppat.1000204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahajan S, Dkhar HK, Chandra V, Dave S, Nanduri R, Janmeja AK, et al. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARgamma and TR4 for survival. JImmunol. 2012;188(11):5593–603. jimmunol.1103038 [pii]; 10.4049/jimmunol.1103038 [DOI] [PubMed] [Google Scholar]
- 20.Dodd CE, Pyle CJ, Glowinski R, Rajaram MV, Schlesinger LS. CD36-Mediated Uptake of Surfactant Lipids by Human Macrophages Promotes Intracellular Growth of Mycobacterium tuberculosis. Journal of immunology. 2016;197(12):4727–35. 10.4049/jimmunol.1600856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouimet M, Koster S, Sakowski E, Ramkhelawon B, van Solingen C, Oldebeken S, et al. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nature immunology. 2016;17(6):677–86. 10.1038/ni.3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh V, Jamwal S, Jain R, Verma P, Gokhale R, Rao KV. Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell host & microbe. 2012;12(5):669–81. 10.1016/j.chom.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 23.Mattos KA, Oliveira VC, Berredo-Pinho M, Amaral JJ, Antunes LC, Melo RC, et al. Mycobacterium leprae intracellular survival relies on cholesterol accumulation in infected macrophages: a potential target for new drugs for leprosy treatment. Cell Microbiol. 2014. 10.1111/cmi.12279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MJ, Wainwright HC, Locketz M, Bekker LG, Walther GB, Dittrich C, et al. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO molecular medicine. 2010;2(7):258–74. 10.1002/emmm.201000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palanisamy GS, Kirk NM, Ackart DF, Obregon-Henao A, Shanley CA, Orme IM, et al. Uptake and accumulation of oxidized low-density lipoprotein during Mycobacterium tuberculosis infection in guinea pigs. PLoSOne. 2012;7(3):e34148 10.1371/journal.pone.0034148 PONE-D-11-17086 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yancey PG, Jerome WG. Lysosomal sequestration of free and esterified cholesterol from oxidized low density lipoprotein in macrophages of different species. Journal of lipid research. 1998;39(7):1349–61. [PubMed] [Google Scholar]
- 27.Brown AJ, Mander EL, Gelissen IC, Kritharides L, Dean RT, Jessup W. Cholesterol and oxysterol metabolism and subcellular distribution in macrophage foam cells. Accumulation of oxidized esters in lysosomes. Journal of lipid research. 2000;41(2):226–37. [PubMed] [Google Scholar]
- 28.Platt N, Speak AO, Colaco A, Gray J, Smith DA, Williams IM, et al. Immune dysfunction in Niemann-Pick disease type C. Journal of neurochemistry. 2016;136 Suppl 1:74–80. 10.1111/jnc.13138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huynh KK, Gershenzon E, Grinstein S. Cholesterol accumulation by macrophages impairs phagosome maturation. The Journal of biological chemistry. 2008;283(51):35745–55. 10.1074/jbc.M806232200 [DOI] [PubMed] [Google Scholar]
- 30.Shen D, Wang X, Li X, Zhang X, Yao Z, Dibble S, et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nature communications. 2012;3:731 10.1038/ncomms1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwerd T, Pandey S, Yang HT, Bagola K, Jameson E, Jung J, et al. Impaired antibacterial autophagy links granulomatous intestinal inflammation in Niemann-Pick disease type C1 and XIAP deficiency with NOD2 variants in Crohn's disease. Gut. 2016. 10.1136/gutjnl-2015-310382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarkar S, Carroll B, Buganim Y, Maetzel D, Ng AH, Cassady JP, et al. Impaired autophagy in the lipid-storage disorder Niemann-Pick type C1 disease. Cell reports. 2013;5(5):1302–15. 10.1016/j.celrep.2013.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13(6):655–67. 10.1016/j.cmet.2011.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandra P, Kumar D. Selective autophagy gets more selective: Uncoupling of autophagy flux and xenophagy flux in Mycobacterium tuberculosis-infected macrophages. Autophagy. 2016;12(3):608–9. 10.1080/15548627.2016.1139263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emanuel R, Sergin I, Bhattacharya S, Turner JN, Epelman S, Settembre C, et al. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(9):1942–52. 10.1161/ATVBAHA.114.303342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W, Yuan XM, Olsson AG, Brunk UT. Uptake of oxidized LDL by macrophages results in partial lysosomal enzyme inactivation and relocation. Arteriosclerosis, thrombosis, and vascular biology. 1998;18(2):177–84. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Dalen H, Eaton JW, Yuan XM. Apoptotic death of inflammatory cells in human atheroma. Arteriosclerosis, thrombosis, and vascular biology. 2001;21(7):1124–30. [DOI] [PubMed] [Google Scholar]
- 38.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–61. 10.1038/nature08938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fineran P, Lloyd-Evans E, Lack NA, Platt N, Davis LC, Morgan AJ, et al. Pathogenic mycobacteria achieve cellular persistence by inhibiting the Niemann-Pick Type C disease cellular pathway. Wellcome open research. 2016;1:18 10.12688/wellcomeopenres.10036.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vrieling F, Ronacher K, Kleynhans L, van den Akker E, Walzl G, Ottenhoff THM, et al. Patients with Concurrent Tuberculosis and Diabetes Have a Pro-Atherogenic Plasma Lipid Profile. EBioMedicine. 2018. 10.1016/j.ebiom.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holvoet P, Donck J, Landeloos M, Brouwers E, Luijtens K, Arnout J, et al. Correlation between oxidized low density lipoproteins and von Willebrand factor in chronic renal failure. Thrombosis and haemostasis. 1996;76(5):663–9. [PubMed] [Google Scholar]
- 42.Lougheed M, Moore ED, Scriven DR, Steinbrecher UP. Uptake of oxidized LDL by macrophages differs from that of acetyl LDL and leads to expansion of an acidic endolysosomal compartment. Arteriosclerosis, thrombosis, and vascular biology. 1999;19(8):1881–90. [DOI] [PubMed] [Google Scholar]
- 43.Liscum L, Faust JR. The intracellular transport of low density lipoprotein-derived cholesterol is inhibited in Chinese hamster ovary cells cultured with 3-beta-[2-(diethylamino)ethoxy]androst-5-en-17-one. The Journal of biological chemistry. 1989;264(20):11796–806. [PubMed] [Google Scholar]
- 44.Zhang Y, Han Q, You S, Cao Y, Zhang X, Liu H, et al. Rapamycin Promotes the Autophagic Degradation of Oxidized Low-Density Lipoprotein in Human Umbilical Vein Endothelial Cells. J Vasc Res. 2015;52(3):210–9. 10.1159/000441143 [DOI] [PubMed] [Google Scholar]
- 45.Liu X, Tang Y, Cui Y, Zhang H, Zhang D. Autophagy is associated with cell fate in the process of macrophage-derived foam cells formation and progress. J Biomed Sci. 2016;23(1):57 10.1186/s12929-016-0274-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, Sillence DJ, et al. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nature medicine. 2008;14(11):1247–55. 10.1038/nm.1876 [DOI] [PubMed] [Google Scholar]
- 47.Yao S, Miao C, Tian H, Sang H, Yang N, Jiao P, et al. Endoplasmic reticulum stress promotes macrophage-derived foam cell formation by up-regulating cluster of differentiation 36 (CD36) expression. The Journal of biological chemistry. 2014;289(7):4032–42. 10.1074/jbc.M113.524512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116(11):1226–33. 10.1161/CIRCULATIONAHA.106.682054 [DOI] [PubMed] [Google Scholar]
- 49.Seimon TA, Kim MJ, Blumenthal A, Koo J, Ehrt S, Wainwright H, et al. Induction of ER stress in macrophages of tuberculosis granulomas. PloS one. 2010;5(9):e12772 10.1371/journal.pone.0012772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia J, Abudu YP, Claude-Taupin A, Gu Y, Kumar S, Choi SW, et al. Galectins Control mTOR in Response to Endomembrane Damage. Mol Cell. 2018;70(1):120–35 e8. 10.1016/j.molcel.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thurston TL, Wandel MP, von Muhlinen N, Foeglein A, Randow F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature. 2012;482(7385):414–8. 10.1038/nature10744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weng IC, Chen HL, Lo TH, Lin WH, Chen HY, Hsu DK, et al. Cytosolic galectin-3 and -8 regulate antibacterial autophagy through differential recognition of host glycans on damaged phagosomes. Glycobiology. 2018;28(6):392–405. 10.1093/glycob/cwy017 [DOI] [PubMed] [Google Scholar]
- 53.Mittal E, Skowyra ML, Uwase G, Tinaztepe E, Mehra A, Koster S, et al. Mycobacterium tuberculosis Type VII Secretion System Effectors Differentially Impact the ESCRT Endomembrane Damage Response. MBio. 2018;9(6). 10.1128/mBio.01765-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aits S, Kricker J, Liu B, Ellegaard AM, Hamalisto S, Tvingsholm S, et al. Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy. 2015;11(8):1408–24. 10.1080/15548627.2015.1063871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150(4):803–15. 10.1016/j.cell.2012.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, Rae CS, et al. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature. 2013;501(7468):512–6. 10.1038/nature12566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell G, Chen C, Portnoy DA. Strategies Used by Bacteria to Grow in Macrophages. Microbiol Spectr. 2016;4(3). 10.1128/microbiolspec.MCHD-0012-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ceriello A, Taboga C, Tonutti L, Quagliaro L, Piconi L, Bais B, et al. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation. 2002;106(10):1211–8. [DOI] [PubMed] [Google Scholar]
- 59.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circulation research. 2010;107(9):1058–70. 10.1161/CIRCRESAHA.110.223545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griffin E, Re A, Hamel N, Fu C, Bush H, McCaffrey T, et al. A link between diabetes and atherosclerosis: Glucose regulates expression of CD36 at the level of translation. Nature medicine. 2001;7(7):840–6. 10.1038/89969 [DOI] [PubMed] [Google Scholar]
- 61.Sampson MJ, Davies IR, Braschi S, Ivory K, Hughes DA. Increased expression of a scavenger receptor (CD36) in monocytes from subjects with Type 2 diabetes. Atherosclerosis. 2003;167(1):129–34. [DOI] [PubMed] [Google Scholar]
- 62.Lu H, Yao K, Huang D, Sun A, Zou Y, Qian J, et al. High glucose induces upregulation of scavenger receptors and promotes maturation of dendritic cells. Cardiovascular diabetology. 2013;12:80 10.1186/1475-2840-12-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fukuhara-Takaki K, Sakai M, Sakamoto Y, Takeya M, Horiuchi S. Expression of class A scavenger receptor is enhanced by high glucose in vitro and under diabetic conditions in vivo: one mechanism for an increased rate of atherosclerosis in diabetes. The Journal of biological chemistry. 2005;280(5):3355–64. 10.1074/jbc.M408715200 [DOI] [PubMed] [Google Scholar]
- 64.Chen M, Nagase M, Fujita T, Narumiya S, Masaki T, Sawamura T. Diabetes enhances lectin-like oxidized LDL receptor-1 (LOX-1) expression in the vascular endothelium: possible role of LOX-1 ligand and AGE. Biochem Biophys Res Commun. 2001;287(4):962–8. 10.1006/bbrc.2001.5674 [DOI] [PubMed] [Google Scholar]
- 65.Balderas FL, Quezada-Larios M, Garcia Latorre EA, Mendez JD. Increased uptake of oxidized LDL by macrophages from type 2 diabetics is inhibited by polyamines. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2016;77:59–64. 10.1016/j.biopha.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 66.Palanisamy GS, Kirk NM, Ackart DF, Shanley CA, Orme IM, Basaraba RJ. Evidence for oxidative stress and defective antioxidant response in guinea pigs with tuberculosis. PloS one. 2011;6(10):e26254 10.1371/journal.pone.0026254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torun E, Gedik AH, Cakir E, Umutoglu T, Gok O, Kilic U. Serum paraoxonase 1 activity and oxidative stress in pediatric patients with pulmonary tuberculosis. Medical principles and practice: international journal of the Kuwait University, Health Science Centre. 2014;23(5):426–31. 10.1159/000363700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jack CI, Jackson MJ, Hind CR. Circulating markers of free radical activity in patients with pulmonary tuberculosis. Tubercle and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 1994;75(2):132–7. 10.1016/0962-8479 [DOI] [PubMed] [Google Scholar]
- 69.Venketaraman V, Millman A, Salman M, Swaminathan S, Goetz M, Lardizabal A, et al. Glutathione levels and immune responses in tuberculosis patients. Microbial pathogenesis. 2008;44(3):255–61. 10.1016/j.micpath.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 70.Knight M, Braverman J, Asfaha K, Gronert K, Stanley S. Lipid droplet formation in Mycobacterium tuberculosis infected macrophages requires IFN-gamma/HIF-1alpha signaling and supports host defense. PLoS pathogens. 2018;14(1):e1006874 10.1371/journal.ppat.1006874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12(5):467–82. 10.1016/j.cmet.2010.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yimin, Furumaki H, Matsuoka S, Sakurai T, Kohanawa M, Zhao S, et al. A novel murine model for non-alcoholic steatohepatitis developed by combination of a high-fat diet and oxidized low-density lipoprotein. Lab Invest. 2012;92(2):265–81. 10.1038/labinvest.2011.159 [DOI] [PubMed] [Google Scholar]
- 73.Bieghs V, van Gorp PJ, Walenbergh SM, Gijbels MJ, Verheyen F, Buurman WA, et al. Specific immunization strategies against oxidized low-density lipoprotein: a novel way to reduce nonalcoholic steatohepatitis in mice. Hepatology. 2012;56(3):894–903. 10.1002/hep.25660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bieghs V, Walenbergh SM, Hendrikx T, van Gorp PJ, Verheyen F, Olde Damink SW, et al. Trapping of oxidized LDL in lysosomes of Kupffer cells is a trigger for hepatic inflammation. Liver international: official journal of the International Association for the Study of the Liver. 2013;33(7):1056–61. 10.1111/liv.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nature immunology. 2013;14(8):812–20. 10.1038/ni.2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohlsson BG, Englund MC, Karlsson AL, Knutsen E, Erixon C, Skribeck H, et al. Oxidized low density lipoprotein inhibits lipopolysaccharide-induced binding of nuclear factor-kappaB to DNA and the subsequent expression of tumor necrosis factor-alpha and interleukin-1beta in macrophages. The Journal of clinical investigation. 1996;98(1):78–89. 10.1172/JCI118780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chung SW, Kang BY, Kim SH, Pak YK, Cho D, Trinchieri G, et al. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-gamma and nuclear factor-kappa B. The Journal of biological chemistry. 2000;275(42):32681–7. 10.1074/jbc.M002577200 [DOI] [PubMed] [Google Scholar]
- 78.Jongstra-Bilen J, Zhang CX, Wisnicki T, Li MK, White-Alfred S, Ilaalagan R, et al. Oxidized Low-Density Lipoprotein Loading of Macrophages Downregulates TLR-Induced Proinflammatory Responses in a Gene-Specific and Temporal Manner through Transcriptional Control. Journal of immunology. 2017;199(6):2149–57. 10.4049/jimmunol.1601363 [DOI] [PubMed] [Google Scholar]
- 79.Bekkering S, Quintin J, Joosten LA, van der Meer JW, Netea MG, Riksen NP. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(8):1731–8. 10.1161/ATVBAHA.114.303887 [DOI] [PubMed] [Google Scholar]
- 80.Christ A, Gunther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell. 2018;172(1–2):162–75 e14. 10.1016/j.cell.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soh AZ, Chee CB, Wang YT, Yuan JM, Koh WP. Dietary Cholesterol Increases the Risk whereas PUFAs Reduce the Risk of Active Tuberculosis in Singapore Chinese. J Nutr. 2016;146(5):1093–100. 10.3945/jn.115.228049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martens GW, Arikan MC, Lee J, Ren F, Vallerskog T, Kornfeld H. Hypercholesterolemia impairs immunity to tuberculosis. Infection and immunity. 2008;76(8):3464–72. 10.1128/IAI.00037-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martens GW, Vallerskog T, Kornfeld H. Hypercholesterolemic LDL receptor-deficient mice mount a neutrophilic response to tuberculosis despite the timely expression of protective immunity. J Leukoc Biol. 2012;91(6):849–57. 10.1189/jlb.0311164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pandey AK, Sassetti CM. Mycobacterial persistence requires the utilization of host cholesterol. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(11):4376–80. 10.1073/pnas.0711159105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brzostek A, Pawelczyk J, Rumijowska-Galewicz A, Dziadek B, Dziadek J. Mycobacterium tuberculosis is able to accumulate and utilize cholesterol. J Bacteriol. 2009;191(21):6584–91. 10.1128/JB.00488-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Genoula M, Marin Franco JL, Dupont M, Kviatcovsky D, Milillo A, Schierloh P, et al. Formation of Foamy Macrophages by Tuberculous Pleural Effusions Is Triggered by the Interleukin-10/Signal Transducer and Activator of Transcription 3 Axis through ACAT Upregulation. Frontiers in immunology. 2018;9:459 10.3389/fimmu.2018.00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Chastellier C, Thilo L. Cholesterol depletion in Mycobacterium avium-infected macrophages overcomes the block in phagosome maturation and leads to the reversible sequestration of viable mycobacteria in phagolysosome-derived autophagic vacuoles. Cellular microbiology. 2006;8(2):242–56. 10.1111/j.1462-5822.2005.00617.x [DOI] [PubMed] [Google Scholar]
- 88.Parihar SP, Guler R, Khutlang R, Lang DM, Hurdayal R, Mhlanga MM, et al. Statin therapy reduces the mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J Infect Dis. 2014;209(5):754–63. 10.1093/infdis/jit550 [DOI] [PubMed] [Google Scholar]
- 89.Lai CC, Lee MT, Lee SH, Hsu WT, Chang SS, Chen SC, et al. Statin treatment is associated with a decreased risk of active tuberculosis: an analysis of a nationally representative cohort. Thorax. 2016;71(7):646–51. 10.1136/thoraxjnl-2015-207052 [DOI] [PubMed] [Google Scholar]
- 90.Dutta NK, Bruiners N, Pinn ML, Zimmerman MD, Prideaux B, Dartois V, et al. Statin adjunctive therapy shortens the duration of TB treatment in mice. J Antimicrob Chemother. 2016;71(6):1570–7. 10.1093/jac/dkw014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Su VY, Su WJ, Yen YF, Pan SW, Chuang PH, Feng JY, et al. Statin Use Is Associated With a Lower Risk of TB. Chest. 2017;152(3):598–606. 10.1016/j.chest.2017.04.170 [DOI] [PubMed] [Google Scholar]
- 92.Lobato LS, Rosa PS, Ferreira Jda S, Neumann Ada S, da Silva MG, do Nascimento DC, et al. Statins increase rifampin mycobactericidal effect. Antimicrob Agents Chemother. 2014;58(10):5766–74. 10.1128/AAC.01826-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rocha N, Kuijl C, van der Kant R, Janssen L, Houben D, Janssen H, et al. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol. 2009;185(7):1209–25. 10.1083/jcb.200811005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van der Kant R, Fish A, Janssen L, Janssen H, Krom S, Ho N, et al. Late endosomal transport and tethering are coupled processes controlled by RILP and the cholesterol sensor ORP1L. Journal of cell science. 2013;126(Pt 15):3462–74. 10.1242/jcs.129270 [DOI] [PubMed] [Google Scholar]
- 95.Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98(15):1487–94. [DOI] [PubMed] [Google Scholar]
- 96.Brinkley TE, Nicklas BJ, Kanaya AM, Satterfield S, Lakatta EG, Simonsick EM, et al. Plasma oxidized low-density lipoprotein levels and arterial stiffness in older adults: the health, aging, and body composition study. Hypertension. 2009;53(5):846–52. 10.1161/HYPERTENSIONAHA.108.127043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamed S, Brenner B, Abassi Z, Aharon A, Daoud D, Roguin A. Hyperglycemia and oxidized-LDL exert a deleterious effect on endothelial progenitor cell migration in type 2 diabetes mellitus. Thrombosis research. 2010;126(3):166–74. 10.1016/j.thromres.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 98.Lachmandas E, Vrieling F, Wilson LG, Joosten SA, Netea MG, Ottenhoff TH, et al. The effect of hyperglycaemia on in vitro cytokine production and macrophage infection with Mycobacterium tuberculosis. PLoSOne. 2015;10(2):e0117941 10.1371/journal.pone.0117941 PONE-D-14-29657 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Crevel R, Dockrell HM. TANDEM: understanding diabetes and tuberculosis. Lancet Diabetes Endocrinol. 2014;2(4):270–2. S2213-8587(14)70011-7 [pii]; 10.1016/S2213-8587(14)70011-7 [DOI] [PubMed] [Google Scholar]
- 100.Redgrave TG, Roberts DC, West CE. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975;65(1–2):42–9. [DOI] [PubMed] [Google Scholar]
- 101.Fraenkel-Conrat H. [11] Methods for investigating the essential groups for enzyme activity Methods in Enzymology. Volume 4: Academic Press; 1957. p. 247–69. [Google Scholar]
- 102.Verreck FA, de BT, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. ProcNatlAcadSciUSA. 2004;101(13):4560–5. 10.1073/pnas.0400983101 0400983101 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leclerc L, Boudard D, Pourchez J, Forest V, Sabido O, Bin V, et al. Quantification of microsized fluorescent particles phagocytosis to a better knowledge of toxicity mechanisms. InhalToxicol. 2010;22(13):1091–100. 10.3109/08958378.2010.522781 [DOI] [PubMed] [Google Scholar]
- 104.Haanen JB, de Waal Malefijt R, Res PC, Kraakman EM, Ottenhoff TH, de Vries RR, et al. Selection of a human T helper type 1-like T cell subset by mycobacteria. The Journal of experimental medicine. 1991;174(3):583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ottenhoff TH, Haanen JB, Geluk A, Mutis T, Ab BK, Thole JE, et al. Regulation of mycobacterial heat-shock protein-reactive T cells by HLA class II molecules: lessons from leprosy. Immunol Rev. 1991;121:171–91. [DOI] [PubMed] [Google Scholar]
- 106.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7(10):R100 10.1186/gb-2006-7-10-r100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Monocyte-derived macrophages were differentiated using M-CSF (50 ng/ml) for 6 days. (A) Histograms of the cell surface expression of CD14 and CD163 as determined by flow cytometry. Stained (blue) and unstained (blue) samples are displayed. Data shown are from one representative donor. (B) Macrophages were treated overnight with PBS control, LDL, acLDL or oxLDL at 25 μg/ml and stained for neutral lipids with Oil Red O. Staining was dissolved and quantified by measuring OD at 520 nm. Data are displayed as ΔOD520 versus PBS control. Individual donors are depicted as dots with group medians. Statistical significance was determined by Kruskal-Wallis test with post-hoc Dunn’s test. ** = p < 0.01. (C) Macrophages were treated with 25 μg/ml oxLDL or PBS control overnight and infected with Mtb H37Rv for 24 h. OxLDL-induced increased Mtb loads were normalized to PBS control and plotted versus the infectious load (MOI) as determined by CFU assay (n = 34; each dot represents one individual donor). Kendall tau correlation and associated two-sided p-value are displayed.
(TIF)
Primary human macrophages were treated with PBS control, native LDL or oxLDL (1, 10 or 25 μg/ml) overnight. (A) Macrophages were co-cultured for four days with the HLA-DR3-restricted CD4+ T cell Rp15 1–1 at a ratio of 1:4 and 0.1, 1 or 10 μg/ml of its cognate peptide or 1.25 μg/ml PPD. T cell proliferation was measured by tritium-thymidine incorporation during the last 24 h (n = 3). Data is represented as means with standard deviations. (B) Cell surface expression of CD86, CD80 and HLA-DR as determined by flow cytometry of macrophages treated overnight with PBS, native LDL or oxLDL (25 μg/ml). Stained (blue) and unstained (blue) samples are displayed. Data shown are from one representative donor (n = 3). (C) Primary human macrophages were treated overnight with PBS control (n = 15), acLDL (n = 9), oxLDL (n = 15) or native LDL (n = 6) (25 μg/ml) and subsequently infected with Mtb H37Rv at a MOI of 10:1. Cells were lysed directly after 1 h of infection and bacterial load was determined by CFU assay to determine Mtb uptake. Results were normalized versus PBS control and depicted as group medians with 95% confidence intervals.
(TIF)
Primary human macrophages were treated with PBS control, acLDL or oxLDL (25 μg/ml) for 24 h prior to infection with Mtb H37Rv at a MOI of 10:1. (A) Representative Western Blot result of Cathepsin D protein levels from macrophages treated with bafilomycin A1 (10 nM) or DMSO, showing protein bands of both the mature heavy chain (34 kDa) and the processing intermediate pro-cathepsin D (48 kDa) after 30 and 900 seconds of exposure time. Pro-cathepsin D levels were first normalized to actin and subsequently versus PBS control (n = 4). (B) Western blot analysis of lysosomal and autophagy markers in macrophages co-treated with PBS, oxLDL or acLDL (25 μg/ml) and U18666A (3 μg/ml), bafilomycin A1 (10 nM) or DMSO control during 24 h of H37Rv Mtb infection. Data shown is from one representative donor (n = 2). (C) Mtb-infected macrophages were stained with Hoechst and PI to determine cell viability. Staurosporin (5 μM) and PBS were used as positive and negative control for cell death. (D) Percentages of viable cells (Hoechts+/PI-). Data are displayed as means with standard deviations (n = 4).
(TIF)
Primary human macrophages were treated with PBS control, acLDL or oxLDL (25 μg/ml) overnight and subsequently infected with Mtb H37Rv at a MOI of 10:1. Cells were lysed at 0 (uptake), 4, 24, 48, 72 and 144 h post-infection for CFU analysis (n = 7). (A) Intracellular Mtb loads are depicted as fraction of uptake in Tukey’s boxplots for each time point and condition: PBS (white), acLDL (grey) and oxLDL (red). (B) Supernatants were harvested at each time point post-infection and cytokine concentrations were determined by multiplex assay. Levels of IL-10, IL-6, TNF-α, IL-8, IL-1RA, CXCL10, IFNα2, CCL2, CCL3, CCL4, G-CSF, VEGF and GM-CSF (pg/ml) are depicted in Tukey’s boxplots for each time point and condition: PBS (green), acLDL (purple) and oxLDL (red). Group medians are shown as dashed lines. Statistical significance was determined by Wilcoxon signed rank test with post-hoc FDR correction. p < 0.05 for * = PBS vs oxLDL, # = oxLDL vs acLDL, ‡ = PBS vs acLDL.
(TIF)
Data is presented as percentage of total (%) or mean ± SD, *Point-of-care measurements, †lab measurements, 1data available from 12/19 patients, 2data available from 12/20 patients, 3data available from 16/19 patients.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.