Abstract
Objective
To determine the association between SpO2 at 5 min and preterm infant outcomes.
Design
Data from 768 infants <32 weeks gestation from 8 randomised controlled trials (RCTs) of lower (≤0.3) versus higher (≥0.6) initial inspiratory fractions of oxygen (FiO2) for resuscitation, were examined.
Setting
Individual patient analysis of 8 RCTs
Interventions
Lower (≤0.3) versus higher (≥0.6) oxygen resuscitation strategies targeted to specific predefined SpO2 before 10 min of age.
Patients
Infants <32 weeks gestation.
Main outcome measures
Relationship between SpO2 at 5 min, death and intraventricular haemorrhage (IVH) >grade 3.
Results
5 min SpO2 data were obtained from 706 (92%) infants. Only 159 (23%) infants met SpO2 study targets and 323 (46%) did not reach SpO280%. Pooled data showed decreased likelihood of reaching SpO280% if resuscitation was initiated with FiO2 <0.3 (OR 2.63, 95% CI 1.21 to 5.74, p<0.05). SpO2 <80% was associated with lower heart rates (mean difference −8.37, 95% CI −15.73 to –1.01, *p<0.05) and after accounting for confounders, with IVH (OR 2.04, 95% CI 1.01 to 4.11, p<0.05). Bradycardia (heart rate <100 bpm) at 5 min increased risk of death (OR 4.57, 95% CI 1.62 to 13.98, p<0.05). Taking into account confounders including gestation, birth weight and 5 min bradycardia, risk of death was significantly increased with time taken to reach SpO280%.
Conclusion
Not reaching SpO280% at 5 min is associated with adverse outcomes, including IVH. Whether this is because of infant illness or the amount of oxygen that is administered during stabilisation is uncertain and needs to be examined in randomised trials
INTRODUCTION
Preductal pulse oximetry (SpO2) monitoring is now an integral component of delivery room stabilization of sick newborn infants.1 SpO2 is used to guide administration of fractional inspired oxygen (FiO2) to achieve SpO2 levels derived from healthy, spontaneously breathing full-term infants.2 This is a relatively new practice. Prior to 2005, pure oxygen (FiO2 1.0) was used for respiratory support of all newborn infants, regardless of gestation and SpO2 was not consistently monitored.3 Most delivery rooms, even in high resource countries, were not equipped to either blend oxygen or monitor SpO2.4
In the 1990s, the Resair studies demonstrated that air (FiO2 0.21) could be used instead of FiO2 1.0 for resuscitating hypoxic, mature infants.5,6 Using air, in fact, decreased oxidative stress, organ injury,7 death8 and encephalopathy.9 Major changes in the use of oxygen for newborn infant stabilization subsequently ensued. Expert committees first suggested in 2005 that air could10 or should11 be used to resuscitate full-term infants. With the acquisition of transitioning SpO2 data several years later from healthy term2 and preterm12 infants, guidelines then recommended that FiO2 should be, first, initiated at low levels (air for term infants and ‘judicious’ amounts for preterm infants) to prevent rapid rise of SpO2 and second, FiO2 should be adjusted to meet healthy term infant SpO2 thresholds.2,12
These SpO2 recommendations were mostly applied to all infants, regardless of gestation.13,14 However, the implications of these recommended SpO2 thresholds on preterm infant outcomes are unknown and this is consistently acknowledged as one of the biggest knowledge gaps in clinical care of the sick preterm infant.1,15–17 Prematurity is associated with both respiratory immaturity and anti-oxidant deficiency18 that makes ‘optimum’ oxygen requirements difficult to balance. Using just air may cause hypoxaemia but conversely, using only FiO2 1.0 will rapidly cause hyperoxaemia.19
Escrig et al first demonstrated that resuscitation with low levels of blended oxygen (eg, FiO2 0.3) was feasible.20 Vento et al then showed that this decreased biochemical oxidative stress when compared with the use of higher oxygen (eg, FiO2 0.6).21 In 2015, international committees that lower FiO2 (≤0.3%) should be used for preterm resuscitation and that higher oxygen levels >0.65 should be avoided.15–17 Over the last 10 years, a paradigm shift in the use of oxygen has evolved. In 2008, 50% of Australian and New Zealand perinatal centres used FiO2 1.0 to resuscitate preterm infants.4 In 2015, a survey of 630 clinicians from 25 countries found that only four clinicians would use FiO2 1.0 and >70% would use FiO2 <0.4. Furthermore, most would target SpO2 thresholds of ‘healthy term infants’ or those at the lower percentiles of healthy preterm infants.22
Prospective acquisition of oxygen data from randomised controlled trials (RCTs) would take many years. Eight existing studies have been conducted to determine outcomes of preterm infants after resuscitation with either lower (<0.3) or higher (>0.6) initial FiO2. A meta-analysis of this studies showed that FiO2 did not influence death, major intraventricular haemorrhage (IVH) or bronchopulmonary dysplasia (BPD),23 but the association between SpO2 and outcomes have not been examined even though the Resair 2 study showed >20 years ago that failure to achieve a 1 min SpO2 of 60% markedly increased the risk of death in asphyxiated term infants (OR 8.6).24
In this study, therefore, we obtained individual patient SpO2 data from the eight RCTs20,21,25–30 that have previously examined outcomes of premature infants <32 weeks gestation to compare outcomes of higher (≥0.6) versus lower (≤0.3) FiO2 resuscitation strategies. We hypothesised that infants not reaching SpO2 80% by 5 min would be at an increased risk of adverse outcomes, including death, major IVH or BPD, regardless of the initial level of FiO2.
METHODS
Individual patient data were obtained directly from the authors of eight RCTs that initiated delivery room resuscitation with higher (>0.6) and lower (<0.3) FiO2 in infants <32 weeks gestation.20,21,25–30 Four studies were excluded: authors uncontactable (1),31 FiO2 not titrated at birth (3).32–34 No study examined FiO2 between 0.31 and 0.59 (see table 1 and figure 1).
Table 1.
SpO2 targets and FiO2 strategies from individual studies
| Enrolment | Gestation | 5 min SpO2study targets |
||||||
|---|---|---|---|---|---|---|---|---|
| Study | period/location | (weeks) | FiO2 | SpO2targets | Detected | Not met | Met | Overshot |
| Wang et al26 | 2005–2007 USA |
23–32 | 0.21 vs 1.0 n=31 |
► FiO2↑ to aim for SpO2 <70% at 3 min or <85% at 5 min |
26 (84%) | 3 (10%) | 9 (29%) | 14 (45%) |
| ► FiO2↓ if SpO2 was >95% any time | ||||||||
| ► FiO21.0 given if heart rate was <100 bpm at 2 min or CM needed |
||||||||
| ► Time 0 not stated | ||||||||
| Escrig et al20 | 2005–2007 Spain |
≤28 | 0.3–0.9 n=42 |
► FiO2 adjusted by 0.1 every 30 s to aim for a SpO2 of 75% at 5 min and 85% at 10 min |
42 (100%) | 18 (43%) | 1 (2%) | 23 (55%) |
| ► FiO21.0 given if heart rate ≤60 bpm >30 s | ||||||||
| ► Time 0 not stated | ||||||||
| Vento et al21 | 2007–2008 Spain |
≤28 | 0.3–0.9 n=78 |
Procedure as per Escrig et al22 | 78 (100%) | 47 (60%) | 29 (37%) | 2 (3%) |
| Rabi27 | 2005–2007 Canada |
≤32 | 0.21 vs 1.0 n=26 |
► FiO2adjusted by 0.2 every 15 s to aim for SpO2 85%–92% |
26 (100%) | 13 (50%) | 6 (23%) | 7 (27%) |
| ► 5%–10% changes made to maintain SpO2 within target range |
||||||||
| ► FiO21.0 given if heart rate was <100 bpm for >30 s or CM needed |
||||||||
| Aguar et al28 | 2010–2012 Spain |
<30 | 0.3 vs 0.6 n=60 |
► FiO2adjusted according to internationally recommended SpO2 targets |
60 (100%) | 53 (88%) | 7 (12%) | 0 |
| ► Time 0 not stated | ||||||||
| Rook et al29 | 2008–2012 The Netherlands |
26+5–32 | 0.3 vs 0.6 n=193 |
► FiO2adjusted to target SpO of 88%–94% at 10 min of age |
149 (77%) | 87 (45%) | 39 (20%) | 23 (12%) |
| ► FiO2decreased if SpO2 was >94% | ||||||||
| ► Time 0=at cord clamping | ||||||||
| Kapadia et al30 | 2010–2011 USA |
24–34 | 0.21% vs 1.0 n=51 |
► Only infants <32 weeks included | 51 (100%) | 18 (35%) | 4 (8%) | 29 (57%) |
| ► Time 0 not stated | ||||||||
| ► FiO20.21 group given extra oxygen if HR <100 bpm after effective ventilation, lower limit of term infant SpO2 (5 min 80%–85%) not reached. FiO2 1.0 if HR <60 |
||||||||
| ► FiO2 adjusted 10% every 30 s to maintain SpO2 in IQR |
||||||||
| ► FiO21.0 group had oxygen reduced by 0.1 q30s to meet SpO2 85%–94% |
||||||||
| Oei et al25 | 2009–2014 Australia |
<31+6 | 0.21 vs 1.0 n=287 |
► FiO2increased by 0.1 if SpO2 was <65% before 5 min or <80% after 5 min |
274 (96%) | 104 (36%) | 64 (22%) | 106 (37% |
| Malaysia | ► FiO2decreased by 0.1 if SpO2 >95%. | |||||||
| Qatar | ► FiO2increased to 1.0 if heart rate <100 bpm or SpO2 <65% at 5 min or if CM was required |
|||||||
| ► Time 0=at cord clamping | ||||||||
| Total | 768 | 706 (92%) | 343 (49%) | 159 (23%) | 204 (29%) | |||
CC, cord clampling; CM, cardiac massage; FiO2, fraction of inspired oxygen; HR, heart rate; NS, not stated; SpO2, pulse oximetry.
Figure 1.
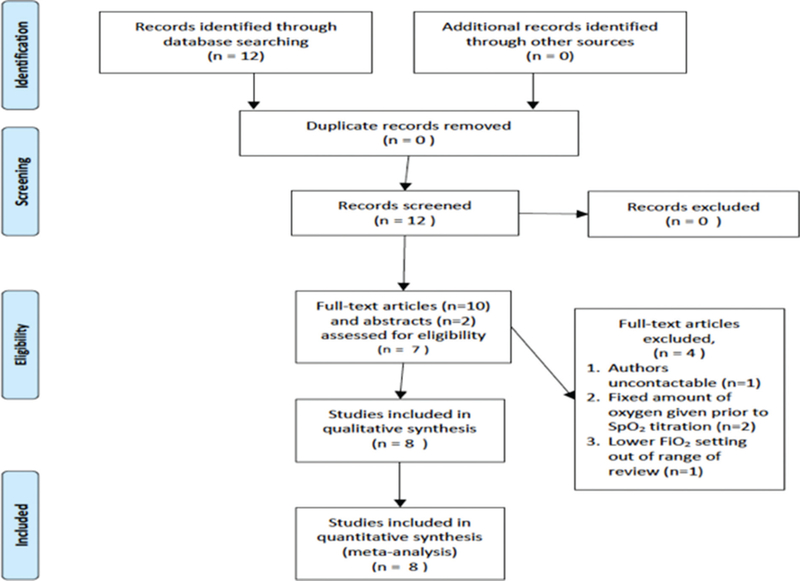
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
SEARCH STRATEGY AND DATA SOURCES
Databases (Medline/PubMed, EMBASE, ClinicalTrials.gov, Cochrane controlled trial registers) and meeting abstracts (Pediatric Academic Societies, European Society of Paediatric Research, European Association of Paediatric Societies) were searched from 1990 to 1 November 2016 using the index terms: preterm/resuscitation/oxygen. Reference lists of relevant articles were also scrutinised. Data were extracted and based on consensus between at least two investigators to resolve uncertainties. Studies published in abstract and full manuscript forms were included. Each study was cross-checked for duplication.
SPO2 TARGETS
Three studies used oximeter downloads to obtain or to verify SpO2 data25–27 and the rest obtained data manually. SpO2 targets were different for each study as were FiO2 titration protocols. SpO2 at 5 min was used as the primary outcome because earlier readings were not consistent. SpO2 above or below 80% (lower limit of the most common expert committee 5 min SpO2 recommendation (80%−85%)13 was used as a dichotomous variable to determine influence on the primary outcomes of death before hospital discharge, major IVH (>grade 3)35 and BPD (defined as the need for respiratory support or supplemental oxygen at 36 weeks postconceptional age).36
STATISTICAL ANALYSIS
Random effects models were used to account for variation within and between studies (heterogeneity) and to compute summary OR, mean differences (MD) and 95% CIs for dichotomous and continuous variables, where appropriate.37,38 Heterogeneity between studies was evaluated with I2 statistics.39 Publication bias was assessed with Egger’s test40,41 and by funnel plot inspection. Categorical data were examined by the χ2 test for proportions and expressed as number (%) and OR (95% CI). Parametric continuous data were examined with the two-sided Student’s t-test. Logistic regression analysis using factors associated with death, IVH and BPD, including gestation, birth weight, gender, FiO2, low heart rate (<100 bpm) were conducted to determine the influence of 5 min SpO2 </>80% on the primary outcomes.42–44 Cox regression analyses was used to determine HR to estimate the effect size of confounders between time to reach SpO2 80% and death in infants with and without bradycardia (heart rate >100 bpm) at 5 min, using model predictors of: gestation, birth weight, male gender, 5 min heart rate <100 bpm, starting FiO2 and mean SpO2 at 5 min.
Analyses were performed with RevMan, V5 and SPSS (IBM) V22. A p value of <0.05 was considered to be statistically significant.
Ethics board approval and registration with clinical trial registries
All studies had been approved from the relevant ethics boards and were registered in approved clinical trial registries (details available from individual studies).
RESULTS
Study and patient characteristics
Study and patient characteristics are presented in tables 1 and 2. Data from 768 infants (369, 52% male) enrolled in eight studies were suitable for analyses. Of these, 191 were randomised to FiO2 0.21, 189 to FiO2 0.3,120 to FiO2 0.6–0.65 and 268 to FiO2 1.0. 5 min SpO2 data were available from 706 (92%) infants. One study did not collect heart rate data.29
Table 2.
Patient demographics
| Gestation (weeks) |
n | 5 min SpO2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Detected | <80% | 80%–85% | >85% | Male gender | Died | IVH >grade 3* | BPD† | ||
| 23 | 2 | 2 (100%) | 2 (100%) | 0 | 0 | 1 (50%) | 1 (50%) | 0 | 1/1 (100%) |
| 24 | 47 | 44 (94%) | 30 (68%) | 7 (16%) | 7 (16%) | 19 (43%) | 14 (32%) | 6/43 (14%) | 14/49 (48%) |
| 25 | 80 | 73 (91%) | 42 (58%) | 10 (14%) | 21 (29%) | 40 (55%) | 17 (23%) | 13/70 (19%) | 33/53 (62%) |
| 26 | 112 | 105 (94%) | 55 (52%) | 17 (16%) | 33 (31%) | 57 (54%) | 13 (12%) | 14/101 (14%) | 43/88 (49%) |
| 27 | 103 | 99 (96%) | 52 (52%) | 13 (13%) | 34 (34%) | 53 (54%) | 5 (5%) | 11/94 (12%) | 24/90 (27%) |
| 28 | 148 | 135 (91%) | 72 (53%) | 12 (9%) | 51 (38%) | 67 (50%) | 5 (4%) | 6/121 (5%) | 18/117 (15%) |
| 29 | 94 | 89 (95%) | 25 (28%) | 11 (12%) | 53 (60%) | 53 (60%) | 4 (5%) | 2/86 (2%) | 14/85 (17%) |
| 30 | 84 | 75 (89%) | 18 (24%) | 7 (9%) | 50 (67%) | 37 (49%) | 0 | 0 | 3/75 (4%) |
| 31 | 98 | 84 (86%) | 27 (32%) | 9 (11%) | 48 (57%) | 38 (45%) | 0 | 0 | 3/84 (4%) |
| Total | 768 | 706 (92%) | 323 (46%) | 86 (12%) | 297 (42%) | 365 (52%) | 59 (8%) | 52/675 (8%) | 153/622 (25%) |
675 infants had a head ultrasound performed.
Excludes deceased infants.
BPD, bronchopulmonary dysplasia35; IVH, intraventricular haemorrhage; SpO2, pulse oximetry.
SpO2 targets
At 5 min, 159 (23%) of the 706 infants reached SpO2 targets for their individual study. Most infants either did not reach SpO2 80% (323, 46%) or exceeded SpO2 85% (297, 42%). SpO2 was between 80% and 85% in 86 (12%) of infants. Figure 2 illustrates differences in SpO2 for babies who were given either lower (≤0.3) or higher (≥0.6) initial FiO2. Babies who were given lower oxygen were more likely to not reach SpO2 80% (59.0% vs 32.2%, OR 2.63, 95% CI 1.21 to 5.74, I2 73%, p = 0.01, figure 2A), took longer to reach SpO2 ≥80% (MD 1.00, 95% CI 0.15 to 1.84), I2 90%, p = 0.02, figure 2B) and had significantly lower SpO2 at 5 min (MD −7.61, 95% CI −13.26 to −1.26, I2 = 89%, p = 0.008, figure 2C) than babies given higher oxygen.
Figure 2.
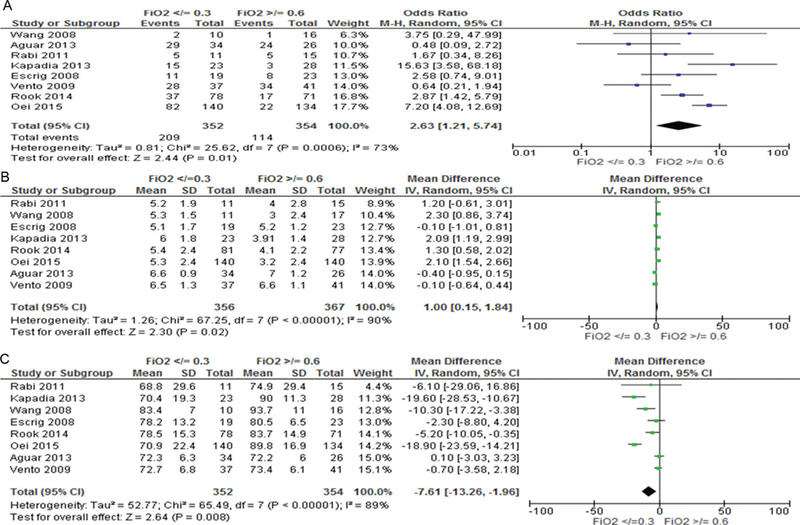
5 min SpO2 for infants given initial FiO2 <0.3 or >0.6. (A) Number of infants in each group with SpO280%, (B) time to reach SpO2 >80%, (C) mean difference in SpO2at 5 min.
Characteristics of infants with SpO2 below and above 80% at 5 min
Infants with SpO2 <80% at 5 min were more premature and had lower birth weights than infants with SpO2 >80%. They were also more likely to be given initial FiO2 <0.3 (OR 3.08, 95% CI 2.26 to 4.19). There was no difference in FiO2 administered at 5 min but mean SpO2 (63.7% vs 91.8%) and heart rates (142.0 vs 148.7 bpm) were significantly lower in infants with SpO2 <80%. Only two infants with SpO2 >80% were bradycardic at 5 min (see table 3).
Table 3.
Characteristics and outcomes of infants with 5 min SpO2 above or below 80%
| 5 min SpO2 <80% |
5 min SpO2≥80% |
OR or mean difference |
95% CI | |
|---|---|---|---|---|
| Gestation (weeks) | 27.1 (1.9) | 28.2 (1.9) | −1.03 | −1.37 to 0.74* |
| Birth weight (g) | 988 (297) | 1100 (325) | −111 | −157 to 65.2* |
| Male gender | 170 (47%) | 195 (53%) | 0.93 | 0.69 to 1.26 |
| Starting FiO2 <0.3 | 209 (59%) | 254 (41%) | 3.08 | 2.26 to 4.19* |
| 5 min status | ||||
| FiO2 | 0.56 (0.23) | 0.55 (0.26) | 1.28 | −2.42 to 4.99 |
| SpO2 (%) | 63.7 (15.2) | 91.8 (6.2) | −28.1 | −29.7 to 26.4 |
| HR (bpm) | 142.0 (30.5) | 148.7 (19.5) | −6.7 | |
| HR <100 bpm† | 25/278 (9.3%) | 2/263 (1%) | 12.8 | |
| Short-term outcomes | ||||
| Dead | 41/323 (13%) | 18/303 (5%) | 2.70 | 1.58 to 4.61* |
| IVH ≥grade 3‡ | 36/310 (12%) | 16/365 (4%) | 1.82 | 1.20 to 2.75* |
| BPD§ | 73/273 (27%) | 80/349 (23%) | 1.17 | 0.89 to 1.54 |
p<0.001,
p<0.05
557 infants had both SpO2 and heart rate detected at 5 min.
Head ultrasound data were obtained on 675 infants
Excludes deceased infants
BPD, bronchopulmonary dysplasia; FiO2, fractional inspired oxygen; HR, heart rate; IVH, intraventricular haemorrhage; SpO2, pulse oximetry.
Outcomes of infants with SpO2 </> 80% at 5 min
Infants with 5 min SpO2 <80% were more likely to die (OR 2.70, 95% CI 1.58 to 4.61) and develop IVH (OR 1.82, 95% CI 1.20 to 2.75) but there was no difference in BPD (OR 1.17, 95% CI 0.89 to 1.54), table 3. Logistic regression analysis was conducted to determine the association between SpO2 <80% in the development of IVH, BPD and death. After taking confounders including gestation, birth weight, gender, FiO2, low heart rate (<100 bpm) into account, SpO2 <80% was associated only with an increased risk of IVH (OR 2.04, 95% CI 1.01 to 4.11, p = 0.04). Increasing gestation decreased the risk of all three primary outcomes (death OR 0.6 5, IVH OR 0.70, BPD OR 0.77). Bradycardia at 5 min was associated with increased risk of death (OR 4.57, 95% CI 1.62 to 13.98), table 4. Pooled patient data accounting for study differences showed that failure to achieve SpO2 80% by 5 min increased the risk of bradycardia (8.9% vs 0.7%, OR 8.47, 95% CI 1.13 to 63.37, I2 50%, p = 0.04, figure 3A) and that babies with SpO2 <80% had significantly lower heart rates than those with higher SpO2 (MD −8.37, 95% CI −15.73 to −1.01, I2 77%, p = 0.03, figure 3B). Furthermore, babies with 5 min SpO2 <80% had an increased risk of death (OR 2.66, 95% CI 1.45 to 4.87, p = 0.02, I2 0% (figure 4A) but not IVH (figure 4B) or BPD (figure 4C).
Table 4.
Adjusted regression analysis of factors associated with death, severe (≥grade 3) intraventricular haemorrhage and bronchopulmonary dysplasia
| Factor |
Died |
IVH >grade 3 |
BPD |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Gestation | 0.65 | 0.49 to 0.86 | 0.02** | 0.70 | 0.52 to 0.93 | 0.01** | 0.77 | 0.64 to 0.94 | 0.01* |
| Birth weight | 0.99 | 0.99 to 1.00 | 0.03** | 0.99 | 0.99 to 1.00 | 0.62 | 0.99 | 0.99 to 0.99 | <0.001* |
| Male gender | 1.07 | 0.55 to 2.07 | 0.84 | 1.29 | 0.69 to 2.42 | 0.42 | 2.39 | 1.46 to 3.90 | 0.001* |
| 5 min SpO2 <80% | 1.57 | 0.74 to 3.34 | 0.24 | 2.04 | 1.01 to 4.11 | 0.04** | 1.55 | 0.93 to 2.57 | 0.09 |
| 5 min HR <100 | 4.57 | 1.62 to 13.98 | 0.005* | 0.69 | 0.14 to 3.29 | 0.9 | 0.88 | 0.19 to 4.08 | 0.87 |
| Starting FiO2 | 0.67 | 0.34 to 1.33 | 0.26 | 1.23 | 0.65 to 2.31 | 0.53 | 1.11 | 0.68 to 1.81 | 0.67 |
| Model | 0.09 | <0.001* | 0.10 | <0.001* | 0.35 | <0.001* | |||
Data are expressed as adjusted OR, 95% CI.
p<0.001.
p<0.05.
BPD, bronchopulmonary dysplasia; bpm, beats per minute; FiO2, fractional inspired oxygen; HR, heart rate; IVH, intraventricular haemorrhage; SpO2, pulse oximetry.
Figure 3.
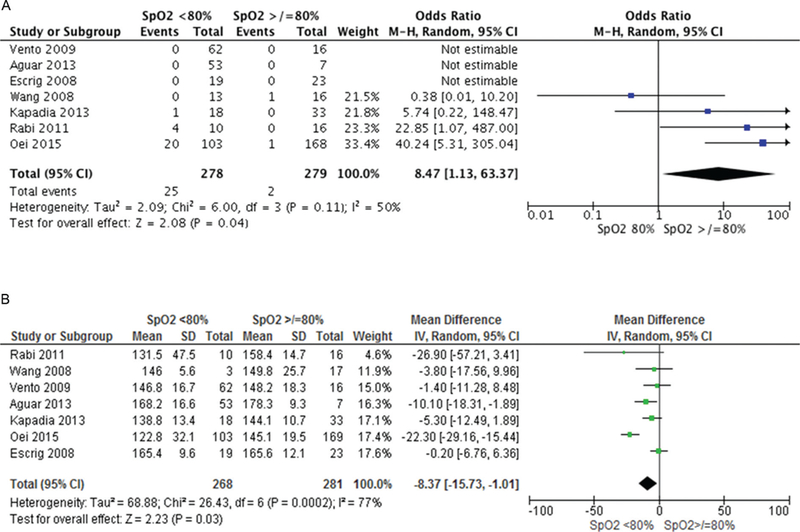
Heart rate (HR) differences between infants with SpO2 </>80% at 5 min. (A) Infants with HR <100 bpm at 5 min, (B) mean HR at 5 min.
Figure 4.
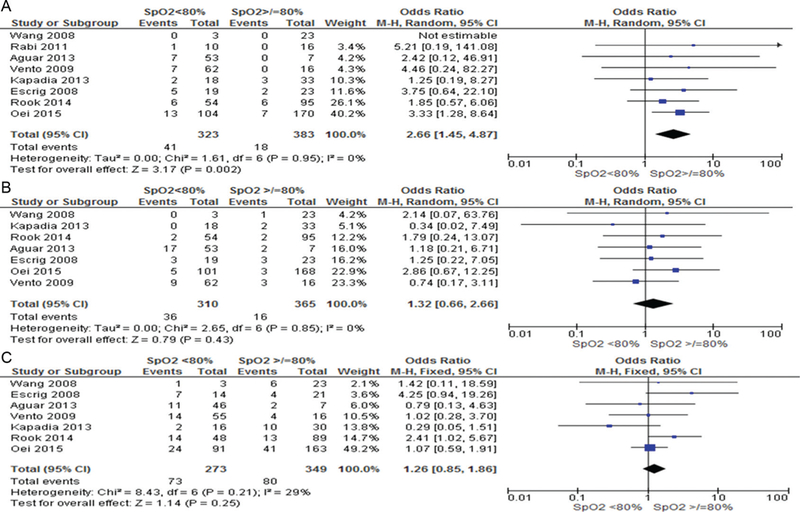
Risks of death, intraventricular haemorrhage and bronchopulmonary dysplasia in infants with 5 min SpO2</>80%.
Association between time to attain SpO2 >80% and risk of death
A Cox model of proportional hazards was developed to determine the relationship between time to reach SpO2 >80% and death. No infant died during resuscitation. Increasing gestation (HR 0.76) and birth weight (HR 0.99) were associated with a decreased risk of death but bradycardia at 5 min (HR 4.17) and lower 5 min SpO2 (HR 1.04) increased risk of death. Male gender and starting FiO2 levels did not influence risk of death (see figure 5 and table 5).
Figure 5.
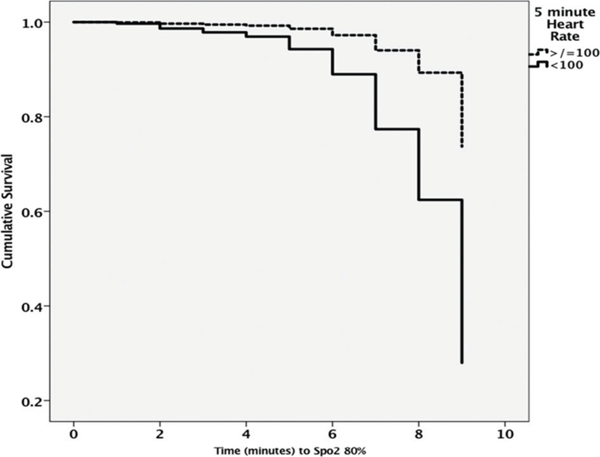
Cumulative risk of death with time taken to reach SpO2 80%. Note higher risk of death in infants with heart rates <100 bpm at 5 min (also see table 5 for HRs associated with each confounder).
Table 5.
HRs associated with risk of death, IVH and BPD, accounting for time taken to reach SpO2 >80% (see figure 5)
| Factor | Died | ||
|---|---|---|---|
| HR | 95% CI | p Value | |
| Gestation | 0.76 | 0.60 to 0.96 | 0.019** |
| Birth weight | 0.99 | 0.99 to 0.99 | 0.01** |
| Male gender | 1.01 | 0.55 to 1.86 | 0.97 |
| 5 min HR <100 bpm | 4.17 | 1.20 to 14.48 | 0.02** |
| Starting FiO2 | 0.67 | 0.35 to 1.28 | 0.22 |
| SpO2 5 min (%) | 1.04 | 1.01 to 1.07 | 0.004** |
Data are expressed as adjusted OR, 95% CI
p<0.001
p<0.05
BPD, bronchopulmonary dysplasia; bpm, beats per minute; FiO2, fractional inspired oxygen; HR, heart rate; IVH, intraventricular haemorrhage; SpO2, pulse oximetry.
DISCUSSION
In this study, we show that only 12% of preterm infants who were resuscitated with blended oxygen in eight RCTs reached the lower limit of expert committee SpO2 (80%) at 5 min of age.1 The implications of these recommendations on sick preterm newborn infants are unknown as they are predominantly derived from observational studies of healthy term and preterm infants.12 Our study showed that babies who did not reach SpO2 80% by 5 min were more premature, but also had lower heart rates. In univariate analyses, they were more likely to die before hospital discharge and to develop a major IVH. Risk of death was also increased with the time taken to attain SpO2 80% but whether this was iatrogenic or due to inherent illness (eg, more severe respiratory pathology) cannot be determined from this study.
Nevertheless, we show that the relatively new practice of using blended oxygen and targeting SpO2 requires urgent and careful evaluation in well-designed and sufficiently large randomised studies. Clinicians now accept lower oxygenation more readily than higher, primarily because of concerns of oxidative stress22 but again, the implications of this practice on both short-term and long-term outcomes is unknown. Boronat et al showed no difference in 2-year neurodevelopmental outcomes after resuscitation with either FiO2 0.3 or 0.645 and further evaluation of this concept is warranted after results from other studies, for example, the neurodevelopmental follow-up of babies enrolled in the Tor2pido study25 is available.
It must be acknowledged, however, that outcomes of sick newborn infants are influenced by multiple factors and not only oxygenation. After accounting for confounders, low SpO2 appeared to be important in increasing only the risk of IVH (OR 2.04) but not death (risk changed with gestation (OR 0.65), birth weight (OR 0.99) and bradycardia (OR 4.57)) or BPD (risk influenced by factors inherent to the patient, eg, gestation (OR 0.77), birth weight (OR 0.99) and male gender (OR 2.39)). Clinicians must therefore account for all these factors when stabilising preterm babies during the first critical few minutes of life. The practice of using blended FiO2 to target SpO2 is a relatively new method of resuscitation and definitive data about the outcomes of this practice are still being acquired. Adopting SpO2 recommendations requires significant infrastructure changes that may be unfeasible in resource-limited regions.46 Therefore, fall-back safety measures that do not necessitate the use of additional equipment, for example, clinical assessment and heart rate auscultation, must be evaluated to ensure best patient outcomes.
We also caution that none of the RCTs was designed to examine infant outcomes in relation to target SpO2. All but two studies29,30 were designed before the first recommended SpO2 targets were published in 2010.1,14 To date, there continues to be wide variability in SpO2 recommendations. No international expert committee differentiates between term and preterm infants for SpO2 recommendations and SpO2 recommendations may vary by >20% between countries.47 Further study to evaluate patient outcomes against differing SpO2 targets is, as mentioned, greatly needed, considering the now almost ubiquitous nature of oxygen blending.
Currently, the only certain data point for oxygen administration during resuscitation is starting FiO2. International expert committees recommend not using FiO2>0.65 and to use lower FiO2 (0.21–0.3) to initiate preterm resuscitation.17 Note that no study has examined outcomes for initiation of resuscitation with FiO2 0.4–0.58 or the impact of differing oxygen titration strategies. Pooled data from this study, however, shows that babies given lower (FiO2<0.3) were more likely to have SpO2 <80% (OR 2.63) and lower SpO2 (MD −7.61) at 5 min and also need 1 min more to reach SpO2 80% after birth. Lower SpO2 was associated with lower heart rates which in turn increased the risk of death (OR 4.57), even after accounting for gestation and birth weight differences.
Whether infants were unable to reach threshold SpO2 because of insufficient oxygen or illness cannot be determined by this study. Certainly, there was no difference in 5 min FiO2 in infants with SpO2 above or below 80% or in the starting FiO2 level between babies with and without adverse outcomes after adjustment of confounders. There is increasing evidence that premature infants may be more susceptible to poor short-term outcomes with lower oxygenation strategies. Rabi et al showed that extremely preterm infants were at increased risk of death or neurological injury after Canadian resuscitation policies were changed from FiO2 1.0 to titrated oxygen.48 In an unplanned, post hoc analysis, the recently closed To2rpido study showed a marginally statistically significant increased risk of death (OR 3.9, p = 0.01) in babies <28 weeks gestation after initiation of resuscitation with air instead of FiO2 1.0. Whether these strategies impact on long-term (including neurodevelopmental) outcomes are unclear and need evaluation.
The major limitations of our study were its observational nature and the prolonged duration over which the studies were conducted. Knowledge, opinion and the capability of clinicians in SpO2 targeting and FiO2 titration would undoubtedly have changed considerably and may now influence clinical outcomes. Nevertheless, theresuscitation. results of our study show that randomised trials are urgently needed to evaluate the relatively new practice of oxygen blending and SpO2 targeting in preterm infant stabilisation at birth.
What is already known on this topic?
-
►
Clinicians initiate preterm infant resuscitation with low levels of blended oxygen (FiO2 <0.4) that is manipulated to meet SpO2 derived from healthy term and preterm infants.
-
►
This is now almost standard practice but whether clinicians are able to achieve recommended SpO2 targets is unknown.
What this study adds?
-
►
Almost half of preterm infants enrolled in oxygen titration studies did not reach SpO2 80% at 5 min, and this was associated with increased risk of major intraventricular haemorrhage and bradycardia (heart rate <100 bpm).
-
►
Bradycardia at 5 min increased risk of death by almost five times, suggesting that randomized trials to determine the consequences of oxygen titration and SpO2 targeting strategies in preterm infants are urgently needed.
Funding
MV acknowledges RETICS funded by the PN 2018–201 1 (Spain), ISCIII- Sub-Directorate General for Research Assessment and Promotion and the European Regional Development Fund (FEDER), reference RD12/0026. VK acknowledges support by K23HD08351 1 grant by NIH.
Footnotes
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Additional pooled data that are not yet published are available to VK and JLO.
REFERENCES
- 1.Neonatal Resuscitation Program Part 13. Neonatal Resuscitation. https://eccguidelines.heart.org/wp-content/themes/eccstaging/dompdf-master/pdffiles/part-13-neonatal-resuscitation.pdf
- 2.Mariani G, Dik PB, Ezquer A, et al. Pre-ductal and post-ductal 02 saturation in healthy term neonates after birth. J Pediatr 2007;150:418–21. [DOI] [PubMed] [Google Scholar]
- 3.Whyte SD, Sinha AK, Wyllie JP. Neonatal resuscitation-a practical assessment. Resuscitation 1999;40:21–5. [DOI] [PubMed] [Google Scholar]
- 4.Clark R, Lui K, Oei J. The use of blended oxygen in the resuscitation of newborn infants in Australia and New Zealand - A suivey of current opinion and practice. J Paediatr Child Health 2009;45:31–5. [DOI] [PubMed] [Google Scholar]
- 5.Ramji S, Ahuja S, Thirupuram S, et al. Resuscitation of asphyxic newborn infants with room air or 100% oxygen. Pediatr Res 1993;34:809–12. [DOI] [PubMed] [Google Scholar]
- 6.Saugstad OD, Rootwelt T, Aalen 0. Resuscitation of asphyxiated newborn infants with room air or oxygen: an international controlled trial: the Resair 2 study. Pediatrics 1998;102:e1. [DOI] [PubMed] [Google Scholar]
- 7.Vento M, Sastre J, Asensi MA, et al. Room-air resuscitation causes less damage to heart and kidney than 100% oxygen. Am J Respir Crit Care Med 2005;172:1393–8. [DOI] [PubMed] [Google Scholar]
- 8.Davis PG, Tan A, O’Donnell CP, et al. Resuscitation of newborn infants with 100% oxygen or air: a systematic review and meta-analysis. Lancet 2004;364:1329–33. [DOI] [PubMed] [Google Scholar]
- 9.Saugstad OD, Ramji S, Soll RF, et al. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology 2008;94:176–82. [DOI] [PubMed] [Google Scholar]
- 10.American Heart Association; American Academy of Pediatrics. 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: neonatal resuscitation guidelines. Pediatrics 2006;117:e1029–38. [DOI] [PubMed] [Google Scholar]
- 11.Morley C New Australian Neonatal Resuscitation Guidelines. J Paediatr Child Health 2007;43:6–8. [DOI] [PubMed] [Google Scholar]
- 12.Dawson JA, Kamlin CO, Vento M, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics 2010;125:e1340–7. [DOI] [PubMed] [Google Scholar]
- 13.Wyllie J, Perlman JM, Kattwinkel J, et al. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2010;81(Suppl 1):e260–87. [DOI] [PubMed] [Google Scholar]
- 14.Australian Resuscitation Council; New Zealand Resuscitation Council. Assessment of the newborn infant. ARC and NZRC Guideline 2010. Emerg Med Australas 2011;23:426–7. [DOI] [PubMed] [Google Scholar]
- 15.Australian and New Zealand Committee on Resuscitation (ANZCOR) guidelines. 2016. http://resus.org.au/guidelines/anzcor-guidelines/ (accessed 15 Jun 2016).
- 16.European Resuscitation Council. 2015. https://cprguidelines.eu (accessed 16 Jun 2016). [Google Scholar]
- 17.Copublishing of the pediatric and neonatal portions of the 2015 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations and the 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics 2015;136(Suppl 2):S83–7. [DOI] [PubMed] [Google Scholar]
- 18.Vento M, Aguar M, Escobar J, et al. Antenatal steroids and antioxidant enzyme activity in preterm infants: influence of gender and timing. Antioxid Redox Signal 2009;11:2945–55. [DOI] [PubMed] [Google Scholar]
- 19.Dawson JA, Kamlin CO, Wong C, et al. Oxygen saturation and heart rate during delivery room resuscitation of infants <30 weeks’ gestation with air or 100% oxygen. Arch Dis Child Fetal Neonatal Ed 2009;94:F87–91. [DOI] [PubMed] [Google Scholar]
- 20.Escrig R, Arruza L, Izquierdo I, et al. Achievement of targeted saturation values in extremely low gestational age neonates resuscitated with low or high oxygen concentrations: a prospective, randomized trial. Pediatrics 2008;121:875–81. [DOI] [PubMed] [Google Scholar]
- 21.Vento M, Moro M, Escrig R, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 2009;124:e439–49. [DOI] [PubMed] [Google Scholar]
- 22.Oei JL, Ghadge A, Coates E, et al. Clinicians in 25 countries prefer to use lower levels of oxygen to resuscitate preterm infants at birth. Acta Paediatr 2016;105:1061–6. [DOI] [PubMed] [Google Scholar]
- 23.Oei JL, Vento M, Rabi Y, et al. Higher or lower oxygen for delivery room resuscitation of preterm infants below 28 completed weeks gestation: a meta-analysis. Arch Dis Child Fetal Neonatal Ed 2017;102:F24–F30. [DOI] [PubMed] [Google Scholar]
- 24.Saugstad OD, Ramji S, Rootwelt T, et al. Response to resuscitation of the newborn: early prognostic variables. Acta Paediatr 2005;94:890–5. [DOI] [PubMed] [Google Scholar]
- 25.Oei JL, Saugstad OD, Lui K, et al. Targeted oxygen in the resuscitation of preterm infants, a randomized clinical trial. Pediatrics 2017;139:e20161452. [DOI] [PubMed] [Google Scholar]
- 26.Wang CL, Anderson C, Leone TA, et al. Resuscitation of preterm neonates by using room air or 100% oxygen. Pediatrics 2008;121:1083–9. [DOI] [PubMed] [Google Scholar]
- 27.Rabi Y, Singhal N, Nettel-Aguirre A. Room-air versus oxygen administration for resuscitation of preterm infants: the ROAR study. Pediatrics 2011;128:e374–81. [DOI] [PubMed] [Google Scholar]
- 28.Aguar M, Izquierdo M, Brugada M, et al. Preterm babies randomly assigned to be blindly resuscitated with higher (60%) Vs. Lower (30%) initial FIO2: effects on oxidative stress and mortality. EPAS 2014;3843:540. [Google Scholar]
- 29.Rook D, Schierbeek H, Vento M, et al. Resuscitation of preterm infants with different inspired oxygen fractions. J Pediatr 2014;164:1322–6. [DOI] [PubMed] [Google Scholar]
- 30.Kapadia VS, Chalak LF, Sparks JE, et al. Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics 2013;132:1488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundstrom KE, Pryds O, Greisen G. Oxygen at birth and prolonged cerebral vasoconstriction in preterm infants. Arch Dis Child Fetal Neonatal Ed 1995;73:F81–F86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ezaki S, Suzuki K, Kurishima C, et al. Resuscitation of preterm infants with reduced oxygen results in less oxidative stress than resuscitation with 100% oxygen. J Clin Biochem Nutr 2009;44:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harling AE, Beresford MW, Vince GS, et al. Does the use of 50% oxygen at birth in preterm infants reduce lung injury? Arch Dis Child Fetal Neonatal Ed 2005;90:F401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar VHS, Carrion V, Wynn KA, et al. Oxygen resuscitation and oxidative-stress biomarkers in premature infants. Research and Reports in Neonatology 2014;4:91–9. [Google Scholar]
- 35.Shennan AT, Dunn MS, Ohlsson A, et al. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82:527–32. [PubMed] [Google Scholar]
- 36.Levene MI, de Crespigny LC. Classification of intraventricular hemorrhage. Lancet 1983;1:643. [DOI] [PubMed] [Google Scholar]
- 37.Thomas D, Radji S, Benedetti A. Systematic review of methods for individual patient data meta- analysis with binary outcomes. BMC Med Res Methodol 2014;14:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. StatMed 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 40.Dickersin K, Berlin JA, Meta-analysis: state of-the-science. Epidemiol Rev 1992;14:154–76. [DOI] [PubMed] [Google Scholar]
- 41.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kent AL, Wright IM, Abdel-Latif ME. New South Wales and Australian Capital Territory Neonatal Intensive Care Units Audit Group. Mortality and adverse neurologic outcomes are greater in preterm male infants. Pediatrics 2012;129:124–31. [DOI] [PubMed] [Google Scholar]
- 43.Nuytten A, Behal H, Duhamel A, et al. Evidence-based neonatal unit practices and determinants of postnatal corticosteroid-use in preterm births below 30 weeks GA in Europe. A population-based cohort study. PLoS One 2017;12:e0170234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Genzel-Boroviczeny O, Hempelman J, Zoppelli L, et al. Predictive value of the 1-min Apgar score for survival at 23–26 weeks gestational age. Acta Paediatr 2010;99:1790–4. [DOI] [PubMed] [Google Scholar]
- 45.Boronat N, Aguar M, Rook D, et al. Survival and neurodevelopmental outcomes of preterms resuscitated with different oxygen fractions. Pediatrics 2016;138:e20161405. [DOI] [PubMed] [Google Scholar]
- 46.Koh J, Yeo CL, Wright I, et al. The use of oxygen for delivery room resuscitation of newborn infants in non-Western countries. Early Hum Dev 2012;88:631–5. [DOI] [PubMed] [Google Scholar]
- 47.Wilson A, Vento M, Shah PS, et al. A review of international clinical practice guidelines for the use of oxygen in the delivery room resuscitation of preterm infants. Acta Paediatr 2017;91. [DOI] [PubMed] [Google Scholar]
- 48.Rabi Y, Lodha A, Soraisham A, et al. Outcomes of preterm infants following the introduction of room air resuscitation. Resuscitation 2015;96:252–9. [DOI] [PubMed] [Google Scholar]


