Abstract
Objective:
To evaluate relationships between frailty and cognition longitudinally in adults aged ≥50 with breast cancer receiving chemotherapy.
Design:
Secondary analysis of a prospective, longitudinal observational study.
Participants:
Patients with breast cancer aged ≥50 receiving adjuvant/neoadjuvant chemotherapy (n=376) and age-matched controls without cancer (n=234).
Setting:
University of Rochester NCI Community Oncology Research Program community oncology clinics.
Measures:
Frailty was assessed using a modified frailty score from self-reported assessments (weakness, exhaustion, physical activity, gait speed). Cognition was assessed by patient-report (Functional Assessment of Cancer Therapy-Cognition [FACT-Cog]) and objective measures. Frailty and cognition were measured at three time-points (pre-chemotherapy [A1], post-chemotherapy [A2], 6-months post-chemotherapy [A3]; similar time interval for controls). Linear regression models evaluated associations between frailty and cognition adjusting for covariates.
Results:
The average age was 59 years (SD 6.4 years). At baseline patients with cancer had higher mean frailty score (1.21 vs 0.73, p<0.001), and lower mean FACT-Cog score (158.4 vs 167.3, p<0.001) compared to controls, objective cognitive measures were not statistically different. Longitudinal decline in FACT-Cog between A1 and A2 (p<0.05) and between A1 and A3 (p<0.01) was associated with increased frailty score in patients compared to controls. Longitudinal worsening in Controlled Oral Word Association (p<0.05) and Trail Making Test (p<0.01) were associated with increase in frailty between A1 and A2 in patients compared to controls; longitudinal decline in Delayed Match Sample was associated with increase in frailty between A1 and A3 (p<0.05) in patients compared to controls. This finding remained significant for a subset analysis of those aged ≥65..
Conclusions:
In patients with breast cancer aged ≥50, longitudinal decline in FACT-Cog and objective measures of attention and memory were associated with increased frailty during treatment and up to 6 months post-treatment. Overall, our study suggests cognition and frailty are both important factors to assess in breast cancer patients.
Keywords: frailty, cognition, cognitive impairment, breast cancer, chemotherapy
INTRODUCTION
Frailty is a clinically recognizable syndrome of increased vulnerability associated with adverse clinical outcomes in older adults.1–3 The frailty phenotype is characterized by weakness, low physical activity, exhaustion, slow gait speed, and unintentional weight loss. In patients with cancer, the presence of frailty at the time of cancer diagnosis is predictive of all-cause mortality.4,5 Older adults with cancer that are frail or pre-frail are more likely to experience chemotherapy toxicity, to be hospitalized or discontinue chemotherapy treatment.6
Although frailty is typically considered in the older adult population, studies in oncology have examined the presence of frailty characteristics in younger cancer survivors due to the physiologic stress that cancer treatment induces.7,8 In fact, the prevalence of pre-frailty and frailty has been shown to be similar between younger cancer survivors and adults aged >65 years old who have not had cancer, despite the average age of survivors included in these studies being much younger.7,8 Additionally, frail adult survivors of childhood cancer had an increased risk of death and chronic condition onset, as compared to those that were not frail.7 Therefore the construct of frailty in cancer survivors who receive chemotherapy is important, even at ages younger than the typical geriatric population.
In the non-cancer setting, the relationship between frailty and cognition has been explored in multiple studies.2,9,10 In cross-sectional studies of older adults, higher odds of cognitive impairment have been observed in frail patients as compared to those that are not frail.9,10 In a longitudinal study, patients with a greater degree of frailty at baseline had a higher risk of subsequent development of mild cognitive impairment.11 Additionally, greater annual rate of change in frailty is associated with subsequent development of Alzheimer’s disease.12 Physical function and cognitive impairment have been shown to be independent predictors of incident disability and death in older adults without cancer, and when cognitive measures are added to frailty assessment, the combination was better at identifying vulnerable older adults at risk of future activities of daily living dependence.13
Given our aging population, the prevalence of cancer, and the association of cognitive impairment with frailty in older adults, improved understanding of the relationships between frailty and cognition in cancer is critical for managing these risk factors.14–17 This analysis evaluates frailty and its relation to cognition longitudinally over the course of adjuvant chemotherapy and up to 6-months post-chemotherapy, in patients with breast cancer aged ≥50 years old, including both subjective and objective assessments of cognition.18
METHODS
2.2. Study design and participants
This study is a secondary analysis of data from a nationwide, multicenter, prospective longitudinal study that examined whether chemotherapy is associated with worse cognition in female patients with non-metastatic breast cancer undergoing treatment at community oncology clinics associated with the University of Rochester Cancer Center (URCC) National Cancer Institute Community Oncology Research Program (NCORP).19,20 Eligible subjects had a diagnosis of invasive breast cancer, stage I to stage IIIC disease, were chemotherapy naïve, and were scheduled to begin adjuvant or neoadjuvant chemotherapy. Subjects were excluded if they had metastatic disease, a prior diagnosis of neurodegenerative disease, currently pregnant, hospitalized within the past year for psychiatric illness, or were receiving concurrent radiation therapy. Age-matched non-cancer control participants were also recruited from the same geographic location as patients.19 The current study evaluated participants from the parent study that were aged ≥50 years old with complete data for cognitive assessment and frailty characteristics. We focused our analysis on subjects aged 50 years and over to include mainly post-menopausal subjects who may be more vulnerable to frailty. Institutional review boards at the URCC NCORP Research Base and each of the NCORP Community Affiliates approved the study. All subjects provided informed consent before entering the study.
2.2. Measures
Clinical and demographic information was collected by research coordinators. Measures at three time points were assessed: 1) within seven days before chemotherapy (pre-chemotherapy baseline; assessment 1 [A1]); 2) within 4 weeks of completion of chemotherapy (post-chemotherapy; assessment 2 [A2]); and 3) six-months after A2 (six month follow-up; assessment 3 [A3]). Controls completed study assessments within the same time windows as the patients with breast cancer.
Frailty Assessment
The frailty assessment was based upon the Fried frailty phenotype, which has previously been described as a clinical syndrome involving self-reported exhaustion, weakness, slow walking speed, low physical activity, and/or unintentional weight loss.2 We approximated frailty by creating a modified Fried frailty score based on four of five self-report measures: 1) exhaustion and 2) weakness, were measured using the symptom inventory questionnaire (impaired defined as score ≥4 on scale 1–10); 3) walk speed and 4) physical activity were measured using the Aerobics Center Longitudinal Study (ACLS) questionnaire with impaired gait speed defined as “casual or strolling” (2 mph) and impaired physical activity defined as <150 minutes per week of physical activity. The frailty score was the sum of the 4 scales yielding (range 0–4), with higher scores reflecting a greater number of frailty characteristics. Prior studies investigating frailty have demonstrated the ability to assess frailty using self-report measures21,22 or modification of the traditional frailty assessment components.23,24 Unintentional weight loss was not consistently assessed at follow-up time points and could not be evaluated longitudinally; thus it was not included in the modified frailty score. Prior studies have demonstrated that patients with breast cancer receiving adjuvant chemotherapy do not experience significant change in weight over the course of treatment,25 thus it was felt that omitting weight from the frailty approximation would not influence the longitudinal change in frailty significantly.
Cognitive Assessment
Perceived cognition was assessed using the Functional Assessment of Cancer Therapy (FACT-Cog) version 2, a validated measure to evaluate cognitive complaints related to cancer therapy.26 The FACT-Cog consists of four subscales that assess different facets of self-reported cognitive function. An overall cognitive function score (total FACT-Cog) was calculated as the sum of the four subscales, with smaller values implying greater cognitive difficulties. For our analysis, we compared patients with a lower than median value (“worse” subjective cognitive assessment) versus greater than median value (“better” subjective cognitive assessment).
Objective cognitive function was assessed using computerized cognitive assessments as well as paper-based neuropsychological assessments. The Cambridge Neuropsychological Test Battery (CANTAB) software was used for computerized assessment in specific cognitive domains; tests included delayed match to sample test (DMS) at the 12-second delay, a measure of visual memory, and the Rapid Visual Processing (RVP), an evaluation of sustained attention.27 Paper-based assessments included the Controlled Oral Word Association test (COWA), a test assessing verbal fluency,28 the Trail Making Test (TMT), an assessment of attention (the CTMT trail 1 was used),29,30 and Hopkins Verbal Learning and Memory Test-Revised (HVLT-R), a measure of verbal memory.31,32
2.3. Statistical Analysis
The aim of this study was to assess longitudinal change in frailty in patients with breast cancer receiving curative intent chemotherapy, and whether baseline cognition, as well as longitudinal change in cognition, were associated with change in frailty post-treatment in patients with breast cancer receiving chemotherapy compared to controls.
Descriptive analyses
For comparison of baseline characteristics of patients with breast cancer and controls, t-tests were used for continuous variables and Chi square tests were used for categorical variables. Characteristics included age (50–64 versus ≥65 years), race (Caucasian versus other), marital status (married versus other), education (≥ some college versus ≤ high school), Karnofsky performance status (≥70 versus <70), frailty score (0–4, continuous), total FACT-Cog score (continuous), anxiety (State Trait Anxiety Inventory [STAI] continuous), depression (Multidimensional Fatigue Symptom Inventory [MFSI] question #21 “I feel depressed”, continuous with 0–4 indicating “not at all” to “very much”), and objective cognitive assessment measure scores (CANTAB DMS and RVP, COWA, TMT, HVLT-R; all continuous). Means and standard deviations were calculated for all continuous variables. Differences between cancer and control groups in dichotomous characteristics were compared using a Chi-square test; differences in continuous characteristics were compared using a t-test. Change in scores over time was assessed by unadjusted mean change analysis of the change in frailty and cognitive measures from A1 to A2 and A1 to A3 (similar time interval for controls) to show overall trajectories of the population in frailty and cognitive outcomes. Differences in the change over time between cancer and control groups were compared using a t-test.
Regression analysis
Linear regression models were used to evaluate the association between frailty and cognition, controlling for age, race, marital status, education, performance status, anxiety, depression and baseline frailty score. Separate models were utilized to assess each of the cognitive measures to evaluate the independent association with change in frailty. We explored the association of baseline cognition with change in frailty between A1 to A2 and A1 to A3. We also tested the association of change in cognition with change in frailty score between A1 to A2 and A1 to A3.
Computations were performed using SAS, version 9.4 (SAS Institute, Cary, NC). P<0.05 was used to assess statistical significance.
RESULTS
Baseline Characteristics
The parent study enrolled 964 subjects, of which 945 were evaluable. For this sub-study, we excluded subjects age <50 years, leaving 610 patients in our analysis (376 patients with breast cancer, 234 age-matched controls; Supplemental Figure 1)
The mean age of the study population was 59.36 (SD 6.42), and mean age was similar between cancer and control groups. There were more subjects in the control group with higher education (some college or above). The population included 7.9% non-Caucasian subjects, with more in the breast cancer group than in the control group (Table 1).
Table 1:
Baseline characteristics
| Characteristic | Statistic | All patients | Breast Cancer |
Non-Cancer Control |
P- value* |
|---|---|---|---|---|---|
| Age (range 50-81) | Mean (SD) |
59.36 (6.42) | 59.70 (6.48) | 58.82 (6.30) | 0.100 |
| 50-64 | N (%) | 471 (77.21) | 284 (75.53) | 187 (79.91) | 0.210 |
| ≥65 | N (%) | 139 (22.79) | 92 (24.47) | 47 (20.09) | |
| Race | 0.009 | ||||
| White | N (%) | 562 (92.13) | 338 (89.89) | 224 (95.73) | |
| Other | N (%) | 48 (7.87) | 38 (10.11) | 10 (4.27) | |
| Marital status | 0.113 | ||||
| Married | N (%) | 431 (70.66) | 257 (68.35) | 174 (74.36) | |
| Other | N (%) | 179 (29.34) | 119 (31.65) | 60 (25.64) | |
| Education | 0.001 | ||||
| ≥Some college | N (%) | 474 (77.70) | 276 (73.40) | 198 (84.62) | |
| ≤High school | N (%) | 136 (22.30) | 100 (26.60) | 36 (15.38) | |
| Karnofsky Performance Status | 0.526 | ||||
| ≥70 | N (%) | 606 (99.67) | 372 (99.47) | 234 (100) | |
| <70 | N (%) | 2 (0.33) | 2 (0.53) | 0 (0) | |
| Menopausal status | |||||
|
Post-menopausal or medically induced menopause |
N (%) | 527 (86.39) | 326 (86.70) | 201 (85.90) | 0.778 |
|
Pre-menopausal or peri-menopausal |
N (%) | 83 (13.61) | 50 (13.30) | 33 (14.10) | |
| Anxiety (STAI) | Mean (SD) |
32.02 (11.51) |
34.80 (12.10) |
27.59 (8.87) | <0.001 |
|
Depression (single item from MFSI) |
Mean (SD) |
0.56 (0.85) | 0.67 (0.89) | 0.37 (0.73) | <0.001 |
|
Frailty score (range 0-4) |
Mean (SD) |
1.02 (1.04) | 1.21 (1.10) | 0.73 (0.85) | <0.001 |
|
Total FACT-Cog score |
Mean (SD) |
161.8 (24.50) |
158.4 (26.32) |
167.3 (20.10) |
<0.001 |
| COWA | Mean (SD) |
13.68 (3.61) | 13.56 (3.65) | 13.86 (3.56) | 0.319 |
| CANTAB DMS | Mean (SD) |
83.31 (19.00) |
83.42 (19.12) |
83.12 (18.85) |
0.848 |
| CANTAB RVP | Mean (SD) |
248.2 (12.08) |
247.8 (12.07) |
248.9 (12.08) |
0.273 |
| TMT (CTMT 1) | Mean (SD) |
42.03 (16.06) |
42.76 (17.71) |
40.87 (12.92) |
0.129 |
|
HVLT-R immediate recall |
Mean (SD) |
8.85 (1.60) | 8.77 (1.72) | 8.98 (1.37) | 0.088 |
|
HVLT-R delayed recall |
Mean (SD) |
9.80 (2.27) | 9.69 (2.39) | 9.97 (2.06) | 0.132 |
Footnote:
The Chi-square test was used to compare the differences in age, race, marital status, education and menopausal status between cancer and control groups. Fisher’s Exact test was used to compare the differences in performance status between the cancer and control groups. A t-test was used to compare the differences in anxiety, depression, frailty score, total FACT-Cog score, COWA, CANTAB DMS, CANTAB RVP, TMT A, HVLT-R average, and HVLT-R delayed recall between cancer and control groups.
STAI, State Trait Anxiety Inventory; MFSI, Multidimensional Fatigue Symptom Inventory; FACT-Cog, Functional Assessment of Cancer Therapy; COWA, Controlled Oral Word Association test; CANTAB, Cambridge Neuropsychological Test Battery; DMS delayed match to sample test; RVP, Rapid Visual Processing; TMT, Trail Making Test; HVLT-R, Hopkins Verbal Learning and Memory Test-Revised.
Prior to chemotherapy, patients with breast cancer had a higher mean frailty score than controls (mean±SD: 1.21±1.10 versus 0.73±0.85, p<0.001). Also at baseline, FACT-Cog scores were lower in patients with breast cancer compared to controls (mean±SD: 158.4±26.3 versus 167.3±20.1, p<0.001), although there was no significant difference in objective neuropsychological measures (Table 1).
Unadjusted Longitudinal Analyses in Frailty and Cognition
In an unadjusted change score analysis to assess overall trends of the data in our sub-study of patients and controls ≥50, patients with breast cancer had an increase in mean frailty score between A1 to A2, whereas controls did not experience a change in frailty score across a similar time frame (p<0.001; Figure 1a). Mean frailty score for breast cancer patients improved at A3, although remained greater than control patients (1.34 in breast cancer patients versus 0.74 in controls, p=0.099 [Figure 1a]). Patients with breast cancer also reported worsening of perceived cognition between A1 and A2 as well as from A1 to A3, whereas controls did not experience a decline over the same timeframes (p<0.001 for both; Figure 1b). Patients with worse perceived cognition (e.g., those with FACT-Cog below the median) had higher frailty scores as compared to patients reporting better perceived cognition at A1, A2, and A3 (p<0.001 for all time points; Figure 2).
Figure 1: Pre- and post- treatment data for (a) frailty, (b) Functional Assessment of Cancer Therapy-Cognition (FACT-Cog) total score, (c) the Controlled Oral Word Association (COWA) test, (d) the Trail Making Test (TMT) A scores, and (e) Delayed Match to Sample (DMS) at the 12-second delay and (f) Rapid Visual Processing (RVP) in breast cancer patients (dark gray) age ≥50 years versus age-matched non-cancer controls (light gray).
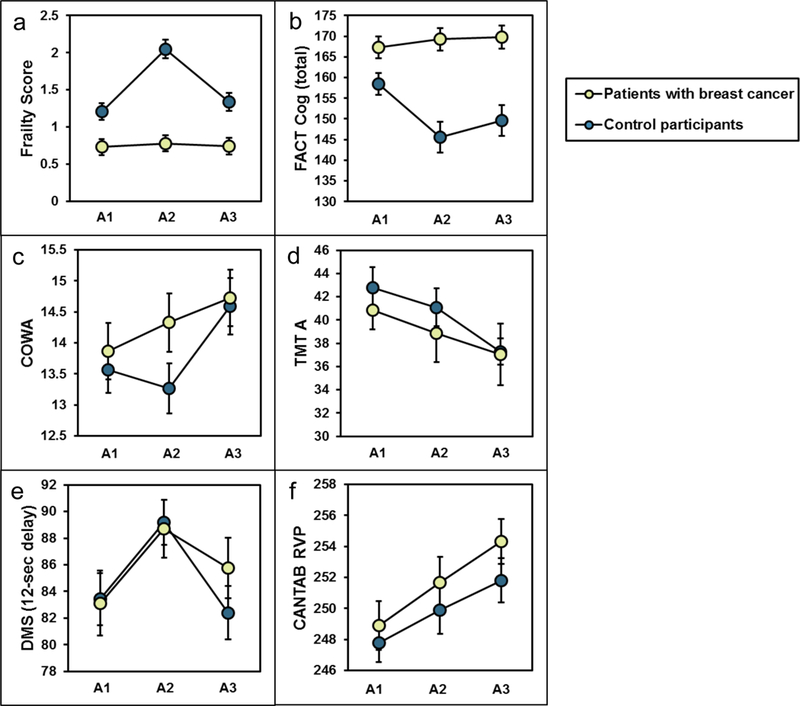
The data points show the unadjusted mean and the 95% confidence interval. A1: pre-chemotherapy, A2: post-chemotherapy, A3: six months post-chemotherapy. Lower scores indicate lower performance on FACT-Cog, COWA, DMS, and RVP. Higher scores indicate lower performance on the Frailty Score and TMT.
Figure 2: Follow-up frailty scores in subjects with greater than versus less than median baseline FACT-Cog score.
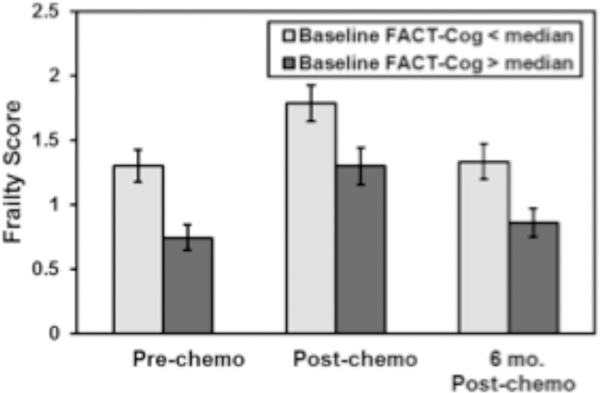
The values show the mean frailty score based on FACT-Cog above or below the median at each timepoint. Please note that a lower score on the FACT-Cog indicates worse perceived cognitive abilities.
In an unadjusted change score analysis to assess overall trends of the data in our cognitive sub-study of patients and controls ≥50, patients with cancer experienced a decline in COWA score as compared to controls at A2 (p<0.001); however at A3, scores were similar between the two groups (p=0.929; Figure 1c). On computerized neuropsychological testing, both the DMS and RVP were similar between patients with cancer at A2 (p=0.802 for DMS and p=0.191 for RVP), however began to diverge by A3, with patients having worse scores compared to controls compared to A1 (p≤0.05 for DMS [Figure 1e] and p≤0.01 for RVP [Figure 1f]). Overall, in unadjusted analyses, TMT and HVLT mean score changes from A1 to A2 and A1 to A3 did not differ between the two groups (Figure 1d).
Associations between baseline cognitive measures and longitudinal change in frailty: Adjusted Analyses
In linear regression models evaluating the association between baseline cognitive measures with the change in frailty score controlling for baseline covariates, lower baseline FACT-Cog was associated with increase in frailty score between A1 to A2 and A1 to A3 (p<0.05 for A1 to A2 and p<0.01 for A1 to A3, Supplemental Table 1 and Supplemental Table 2). For objective cognitive assessment measures using a linear regression model controlling for baseline covariates, there was no association between any of the baseline objective cognitive measures and change in frailty score between A1 to A2. Lower baseline CANTAB RVP score was significantly associated with increase in frailty score between A1 to A3 (p<0.01, Supplemental Table 1 and Supplemental Table 2)
Associations between longitudinal change in cognitive measures and longitudinal change in frailty: Adjusted Analyses
In a separate model, evaluating the association between longitudinal change in cognitive measures with longitudinal change in frailty score, also controlling for baseline covariates, change in FACT-Cog, change in COWA, and change in TMT were significantly associated with change in frailty score between A1 and A2 (p<0.05 for FACT-Cog, p<0.05 for COWA and p<0.01 for TMT) where greater perceived cognitive impairment and worse cognitive function on objective tests were observed with increasing frailty. Between A1 and A3, change in FACT-Cog and change in CANTAB DMS were significantly associated with change in frailty score (p<0.01 for FACT-Cog and p<=0.05 for DMS), where the association between change in frailty and change in COWA remained a trend (p=0.104). (Tables 2 and 3)
Table 2:
Association of Cognition and Frailty among Breast Cancer Patients and Controls: Change in Cognition and Change in Frailty Score between Baseline and Post-chemotherapy
| Variables | Change in Frailty Score between Baseline and Post-chemotherapy | ||||||
|---|---|---|---|---|---|---|---|
| Change in total FACT- Cog score |
−0.003** | ||||||
| (0.002) | |||||||
| Change in COWA | −0.037** | ||||||
| (0.017) | |||||||
| Change in CANTAB DMS | −0.000 | ||||||
| (0.002) | |||||||
| Baseline CANTAB RVP | 0.001 | ||||||
| (0.004) | |||||||
| Baseline TMT | 0.008*** | ||||||
| (0.003) | |||||||
| Baseline HVLT-R immediate |
−0.009 | ||||||
| (0.034) | |||||||
| Baseline HVLT-R delayed recall |
−0.000 | ||||||
| (0.025) | |||||||
| Baseline Frailty Score | −0.635*** | −0.647*** | −0.641*** | −0.649*** | −0.643*** | −0.643*** | −0.641*** |
| (0.044) | (0.044) | (0.044) | (0.045) | (0.044) | (0.044) | (0.044) | |
| Cancer Patient (Yes=1) | 1.034*** | 1.077*** | 1.095*** | 1.102*** | 1.089*** | 1.103*** | 1.112*** |
| (0.097) | (0.092) | (0.093) | (0.094) | (0.091) | (0.092) | (0.093) | |
| Age (>=65) | 0.125 | 0.080 | 0.096 | 0.086 | 0.068 | 0.089 | 0.070 |
| (0.104) | (0.103) | (0.104) | (0.104) | (0.102) | (0.103) | (0.104) | |
| Race (White=1) | 0.031 | 0.059 | 0.055 | 0.057 | 0.051 | 0.057 | 0.073 |
| (0.168) | (0.165) | (0.166) | (0.167) | (0.164) | (0.165) | (0.166) | |
| Marital Status (Married=1) | −0.198** | −0.212** | −0.227** | −0.244** | −0.237** | −0.219** | −0.218** |
| (0.096) | (0.095) | (0.096) | (0.098) | (0.095) | (0.095) | (0.096) | |
| Education (Some College or Above=1) |
0.123 | 0.160 | 0.162 | 0.177* | 0.139 | 0.159 | 0.126 |
| (0.106) | (0.104) | (0.106) | (0.107) | (0.104) | (0.105) | (0.105) | |
| KPS (>=70) | −0.700 | −0.564 | −0.655 | −0.657 | −0.620 | −0.650 | −0.674 |
| (0.719) | (0.718) | (0.725) | (0.726) | (0.715) | (0.720) | (0.716) | |
| Baseline anxiety (STAI) | −0.003 | −0.002 | −0.003 | −0.003 | −0.001 | −0.002 | −0.003 |
| (0.005) | (0.005) | (0.005) | (0.005) | (0.005) | (0.005) | (0.005) | |
| Baseline depression (MFSI) |
0.125* | 0.116* | 0.123* | 0.126* | 0.123* | 0.116* | 0.109* |
| (0.065) | (0.065) | (0.067) | (0.067) | (0.066) | (0.065) | (0.066) | |
| Constant | 1.243 | 1.070 | 1.171 | 1.190 | 1.143 | 1.144 | 1.190 |
| (0.774) | (0.772) | (0.780) | (0.782) | (0.770) | (0.776) | (0.770) | |
| Obs | 568 | 570 | 565 | 560 | 568 | 570 | 555 |
| R-squared | 0.370 | 0.374 | 0.364 | 0.367 | 0.376 | 0.369 | 0.373 |
Footnote: Linear regression models were used to evaluate the association between frailty and cognition, controlling for age (50–64 versus ≥65 years), race (Caucasian versus other), marital status (married versus other), education (≥ some college versus ≤ high school), performance status (≥70 versus <70), baseline anxiety (STAI), baseline depression (MSFI) and baseline frailty score. Standard errors are reported in brackets.
significant at 10%;
significant at 5%;
significant at 1%.
significant at 11%.
STAI, State Trait Anxiety Inventory; MFSI, Multidimensional Fatigue Symptom Inventory; FACT-Cog, Functional Assessment of Cancer Therapy; COWA, Controlled Oral Word Association test; CANTAB, Cambridge Neuropsychological Test Battery; DMS delayed match to sample test; RVP, Rapid Visual Processing; TMT, Trail Making Test; HVLT-R, Hopkins Verbal Learning and Memory Test-Revised.
Table 3:
Association of Cognition and Frailty among Breast Cancer Patients and Controls: Change in Cognition and Change in Frailty Score between Baseline and Six Months Post-chemotherapy
| Variables | Change in Frailty Score between Baseline and 6 Months Post-chemotherapy | ||||||
|---|---|---|---|---|---|---|---|
| Change in total FACT- Cog score |
-0.010*** | ||||||
| (0.002) | |||||||
| Change in COWA | −0.024† | ||||||
| (0.015) | |||||||
| Change in CANTAB DMS | −0.004** | ||||||
| (0.002) | |||||||
| Baseline CANTAB RVP | 0.001 | ||||||
| (0.004) | |||||||
| Baseline TMT | −0.002 | ||||||
| (0.003) | |||||||
| Baseline HVLT-R immediate | −0.037 | ||||||
| (0.032) | |||||||
| Baseline HVLT-R delayed recall |
−0.023 | ||||||
| (0.023) | |||||||
| Baseline Frailty Score | −0.584*** | −0.580*** | −0.582*** | −0.582*** | −0.579*** | −0.580*** | −0.574*** |
| (0.041) | (0.042) | (0.042) | (0.043) | (0.042) | (0.042) | (0.043) | |
| Cancer Patient (Yes=1) | 0.203** | 0.342*** | 0.305*** | 0.327*** | 0.340*** | 0.342*** | 0.349*** |
| (0.086) | (0.086) | (0.086) | (0.087) | (0.086) | (0.086) | (0.087) | |
| Age (>=65) | −0.037 | −0.100 | −0.120 | −0.100 | −0.086 | −0.098 | −0.101 |
| (0.094) | (0.097) | (0.098) | (0.099) | (0.097) | (0.097) | (0.099) | |
| Race (White=1) | −0.312* | −0.179 | −0.239 | −0.214 | −0.193 | −0.212 | −0.171 |
| (0.161) | (0.164) | (0.164) | (0.166) | (0.165) | (0.165) | (0.168) | |
| Marital Status (Married=1) | −0.131 | −0.200** | −0.172* | −0.189** | −0.201** | −0.197** | −0.213** |
| (0.088) | (0.090) | (0.091) | (0.093) | (0.090) | (0.090) | (0.092) | |
| Education (Some College or Above=1) |
−0.159* | −0.130 | −0.139 | −0.115 | −0.127 | −0.133 | −0.149 |
| (0.096) | (0.099) | (0.099) | (0.100) | (0.099) | (0.099) | (0.100) | |
| KPS (>=70) | 1.134* | 1.301** | 1.203* | 1.201* | 1.242* | 1.210* | 1.218* |
| (0.638) | (0.661) | (0.657) | (0.663) | (0.661) | (0.660) | (0.662) | |
| Baseline anxiety (STAI) | 0.006 | 0.004 | 0.003 | 0.002 | 0.003 | 0.004 | 0.004 |
| (0.005) | (0.005) | (0.005) | (0.005) | (0.005) | (0.005) | (0.005) | |
| Baseline depression (MFSI) | 0.100* | 0.089 | 0.105* | 0.110* | 0.096 | 0.094 | 0.073 |
| (0.059) | (0.062) | (0.062) | (0.063) | (0.062) | (0.062) | (0.062) | |
| Constant | −0.320 | −0.516 | −0.350 | −0.381 | −0.465 | −0.370 | −0.437 |
| (0.692) | (0.717) | (0.713) | (0.720) | (0.718) | (0.717) | (0.719) | |
| Obs | 542 | 545 | 538 | 533 | 544 | 545 | 530 |
| R-squared | 0.322 | 0.276 | 0.282 | 0.273 | 0.273 | 0.274 | 0.275 |
Footnote: Linear regression models were used to evaluate the association between frailty and cognition, controlling for age (50–64 versus ≥65 years), race (Caucasian versus other), marital status (married versus other), education (≥ some college versus ≤ high school), performance status (≥70 versus <70), baseline anxiety (STAI), baseline depression (MSFI) and baseline frailty score. Standard errors are reported in brackets.
significant at 10%;
significant at 5%;
significant at 1%.
significant at 11%.
STAI, State Trait Anxiety Inventory; MFSI, Multidimensional Fatigue Symptom Inventory; FACT-Cog, Functional Assessment of Cancer Therapy; COWA, Controlled Oral Word Association test; CANTAB, Cambridge Neuropsychological Test Battery; DMS delayed match to sample test; RVP, Rapid Visual Processing; TMT, Trail Making Test; HVLT-R, Hopkins Verbal Learning and Memory Test-Revised.
Additionally, we performed a subset analysis of patients aged ≥65 to evaluate the associations between longitudinal change in cognitive measures and frailty in this population specifically (N= 133). Between baseline and post-chemotherapy in patients aged ≥65, change in frailty was not associated with change in FACT-Cog score, however in this smaller group of patients association with multiple objective measures was observed at the p<0.1 level (COWA, CANTAB DMS, CANTAB RVP and TMT; data not shown). Between baseline and 6-months post-chemotherapy, the association between change in frailty and change in CANTAB DMS score remained (p<0.05).
DISCUSSION
Using a modified Fried frailty index, we found that frailty characteristics are common among patients with breast cancer aged ≥50 years even before receiving adjuvant chemotherapy, and mean frailty scores in this population were higher than age-matched controls. We also observed that frailty characteristics increased from pre- to post-chemotherapy in breast cancer patients, as compared to non-cancer controls. Although frailty scores in breast cancer patients improved at A3, mean frailty remained greater than age-matched non-cancer controls even six months after completion of chemotherapy compared to the control time-equivalent assessment. Within this cohort, subjects with worse perceived cognition at baseline had higher frailty scores at baseline and both follow-up time points when compared to patients with better subjective cognitive function at baseline. Baseline score on the CANTAB RVP was associated with change in frailty at 6-months post-treatment. Additionally, patients who experienced decline in FACT-Cog, as well as decline in COWA and TMT between A1 and A2 were more likely to develop increased frailty over the course of treatment. The association between change in FACT-Cog with change in frailty persisted even to 6-month post-chemotherapy completion, and decline in CANTAB DMS was also associated change frailty at 6-months post-chemotherapy completion. Although there was a more dramatic difference between patients with cancer and controls in perceived cognition as compared to objective measures, this is consistent with several prior studies33–36 and is likely because perceived cognition captures perceptions of global cognitive functioning whereas objective measures target functioning in specific cognitive domains. A subset analysis of patients aged ≥65 demonstrated that although perceived cognition was not associated with change in frailty in this group, there was a trend towards association between change in several objective cognitive measures and change in frailty. We have previously observed that younger age was predictive of problems with perceived cognition.19 This may be that patients <65 are more aware or more likely to report cognitive problems. Alternatively, the FACT-Cog was developed based upon qualitative work in a younger population of patients37 and thus may not fully capture cognitive complaints of older adults. This finding also emphasizes the potential importance of using objective cognitive assessments in this population, as opposed to relying on self-report measures alone. It should also be noted that the smaller number of patients aged ≥65 may limit the interpretation of this finding as well.
The relationship between frailty and cognition has been evaluated in breast cancer survivors in two prior studies.38,39 In the first, investigators used group-based trajectory modeling to identify the trajectories of cognitive and physical function and determined that patients who were frail were more likely to be in the accelerated cognitive decline or the phase shift group (declines that are shifted below but parallel to mild decline). Additionally, patients receiving chemotherapy were more likely to be in the accelerated cognitive decline group compared to survivors who did not receive chemotherapy.38 Investigators also observed that oncology patients who were frail at baseline were more likely to report subjective cognitive decline with longitudinal follow-up. Comparing this to our results, we identified that patients who had worse perceived cognition at baseline had greater frailty scores, further strengthening the connection between cognition and frailty in this population of patients. In the second study, also by Mandelblatt and colleagues,39 breast cancer patients aged ≥60 were compared to matched controls without cancer and assessment included perceived and objective cognitive measures at baseline and 12 and 24 months. Frailty was also assessed in this study at baseline using an adapted Searle’s deficits accumulation index. Investigators determined that, at baseline, frailty was associated with impairment on neuropsychological tests measuring attention, processing speed, and executive function as well as self-reported decline in cognition. In our study, we also observed an association between frailty and perceived cognition as well as an association between change in frailty and change in objective measures in TMT which assesses attention, and CANTAB RVP which assesses sustained attention. Taken together, this suggests that interventions focusing in the area of attention and memory may be potential considerations to mitigate the development of cognitive and frailty problems with cancer treatment.
Although frailty is typically studied in older adult populations, we elected to use a lower age cutoff for inclusion onto our study. Prior studies have demonstrated greater than expected rates of frailty in populations of adult survivors of childhood cancers7 and breast cancer survivors several years after treatment,40 thus we suspected that frailty may be a relevant issue in breast cancer patients younger than 65 years old. Indeed, we observed that despite including a “younger” population on average, frailty characteristics were prevalent and mean frailty scores were higher in cancer patients compared to controls, even prior to treatment. This finding reinforces that these issues of cognition and frailty, which are typically considered aging-related, can be reported by patients and identified objectively earlier in the lifespan for individuals receiving chemotherapy. This finding is relevant for geriatricians and other providers caring for patients with a prior history of cancer, particularly those who received chemotherapy, as they may exhibit accelerated aging phenotypes in the areas of frailty and cognition if these issues are manifesting earlier in their lifespan.
There are several strengths of this study. First, we included validated cognitive measures to provide a comprehensive assessment of cognition. Also, we utilized a large primary dataset that enrolled patients nationwide from community oncology practices, so our results are generalizable to other community oncology practices across the United States. Additionally, our study included a larger number of patients receiving chemotherapy compared to prior studies on frailty and cognition in the oncology setting, further adding to the evidence base on this issue.We also included assessments at multiple time points. Cognitive assessments may have practice effects with repeated administration20,41 and therefore comparing longitudinal change between patients and controls accounted for this effect.
There are limitations to this work. We developed a modified Fried frailty index which is not a validated measure of frailty and did not account for weight change as this information was not available in our dataset. Ideally, frailty evaluation would include this information, although we observed a significant degree of frailty characteristics in our population even without this metric. Additionally, we did not include an objective measure of frailty such as grip-strength, and reliance on self-report measures of frailty is a limitation of this study. The objective cognitive measures used in our study are not tools that are typically used by oncologists or geriatricians (such as the Montreal Cognitive Assessment or Mini-Mental Status Exam). However, perceived cognition could be assessed in a clinical setting and given that perceived cognition was more strongly associated with frailty in our study and others, assessing perceived cognition in clinical practice should be considered. Additionally, this study suggests that cognitive measures based in cognitive neuroscience and neuropsychological assessment may be useful for geriatricians and geriatric oncologists to consider.
In conclusion, in this secondary analysis of a large nationwide study of cognition in breast cancer patients, we determined that breast cancer patients age ≥50 years old had higher mean frailty scores compared to age-matched non-cancer controls both prior to and following adjuvant chemotherapy and up to six months post-chemotherapy. Within this cohort, patients with worse subjective cognition at baseline had a higher degree of frailty at pre- and both post-treatment time points. Additionally, longitudinal increase in frailty characteristics was associated with longitudinal decline in self-reported cognition, COWA, TMT and DMS tests over the same time frame.Given that frailty is a known risk factor for older patients, and our results showing that cognitive problems are associated with frailty, consideration of this phenotype developing earlier needs to be considered for all clinicians treating patients with breast cancer. Interventions are needed to minimize the progression of frailty and cognitive decline.
Supplementary Material
Consort Flow Diagram
Footnote: Linear regression models were used to evaluate the association between frailty and cognition, controlling for age (50–64 versus ≥65 years), race (Caucasian versus other), marital status (married versus other), education (≥ some college versus ≤ high school), performance status (≥70 versus <70), baseline anxiety (STAI), baseline depression (MSFI) and baseline frailty score. Standard errors are reported in brackets. *significant at 10%; **significant at 5%; ***significant at 1%.† significant at 11%. STAI, State Trait Anxiety Inventory; MFSI, Multidimensional Fatigue Symptom Inventory; FACT-Cog, Functional Assessment of Cancer Therapy; COWA, Controlled Oral Word Association test; CANTAB, Cambridge Neuropsychological Test Battery; DMS delayed match to sample test; RVP, Rapid Visual Processing; TMT, Trail Making Test; HVLT-R, Hopkins Verbal Learning and Memory Test-Revised.
Footnote: Linear regression models were used to evaluate the association between frailty and cognition, controlling for age (50–64 versus ≥65 years), race (Caucasian versus other), marital status (married versus other), education (≥ some college versus ≤ high school), performance status (≥70 versus <70), baseline anxiety (STAI), baseline depression (MSFI) and baseline frailty score. Standard errors are reported in brackets. *significant at 10%; **significant at 5%; ***significant at 1%.† significant at 11%. STAI, State Trait Anxiety Inventory; MFSI, Multidimensional Fatigue Symptom Inventory; FACT-Cog, Functional Assessment of Cancer Therapy; COWA, Controlled Oral Word Association test; CANTAB, Cambridge Neuropsychological Test Battery; DMS delayed match to sample test; RVP, Rapid Visual Processing; TMT, Trail Making Test; HVLT-R, Hopkins Verbal Learning and Memory Test-Revised.
ACKNOWLEDGMENTS
Dr. Hurria reports research funding from Celegene, Novartis, and GSK and served as a consultant for Boehringer Ingelheim Pharmaceuticals, Carevive, Sanofi, GTx, Inc., Pierian Biosciences, and MJH Healthcare Holdings, LLC outside the submitted work.
Sponsor’s role
This work was supported by the National Institutes of Health, National Cancer Institute (U10CA037420, UG1CA189961, K07CA168886, R21/R33AG059206, R25CA102618, and DP2CA195765). Dr. Magnuson is supported by the Empire Clinical Research Investigator Program (ECRIP) at the University of Rochester Medical Center, sponsored by the New York State Department of Health. The sponsor had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of the manuscript.
Footnotes
Conflict of interest
References
- 1.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 3.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54(6):975–979. [DOI] [PubMed] [Google Scholar]
- 4.Guerard EJ, Deal AM, Chang Y, et al. Frailty Index Developed From a Cancer-Specific Geriatric Assessment and the Association With Mortality Among Older Adults With Cancer. J Natl Compr Canc Netw. 2017;15(7):894–902. [DOI] [PubMed] [Google Scholar]
- 5.Mandelblatt JS, Cai L, Luta G, et al. Frailty and long-term mortality of older breast cancer patients: CALGB 369901 (Alliance). Breast Cancer Res Treat. 2017;164(1):107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen HJ, Smith D, Sun CL, et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122(24):3865–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31(36):4496–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arora M, Sun CL, Ness KK, et al. Physiologic Frailty in Nonelderly Hematopoietic Cell Transplantation Patients: Results From the Bone Marrow Transplant Survivor Study. JAMA Oncol. 2016;2(10):1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulmala J, Nykanen I, Manty M, Hartikainen S. Association between frailty and dementia: a population-based study. Gerontology. 2014;60(1):16–21. [DOI] [PubMed] [Google Scholar]
- 10.Han ES, Lee Y, Kim J. Association of cognitive impairment with frailty in community-dwelling older adults. Int Psychogeriatr. 2014;26(1):155–163. [DOI] [PubMed] [Google Scholar]
- 11.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58(2):248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom Med. 2007;69(5):483–489. [DOI] [PubMed] [Google Scholar]
- 13.Aliberti MJR, Cenzer IS, Smith AK, Lee SJ, Yaffe K, Covinsky KE. Assessing Risk for Adverse Outcomes in Older Adults: The Need to Include Both Physical Frailty and Cognition. J Am Geriatr Soc. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.65+ in the United States: 2010. US Department of commerce, economics and statistics administration, US census Bureau; 2014. [Google Scholar]
- 15.Fast Stats: An interactive tool for access to SEER cancer statistics. Surveillance Research Program, National Cancer Institute. https://seer.cancer.gov/faststats. (Accessed on 3-27-2018)
- 16.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–1492. [DOI] [PubMed] [Google Scholar]
- 18.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. The lancet oncology. 2011;12(7):703–708. [DOI] [PubMed] [Google Scholar]
- 19.Janelsins MC, Heckler CE, Peppone LJ, et al. Cognitive Complaints in Survivors of Breast Cancer After Chemotherapy Compared With Age-Matched Controls: An Analysis From a Nationwide, Multicenter, Prospective Longitudinal Study. J Clin Oncol. 2017;35(5):506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janelsins MC, Heckler CE, Peppone LJ, Ahles TA, Mohile SG, Mustian KM, Palesh O, O’Mara AM, Miasian LM, Williams AM, Magnuson A, Geer J, Dakhil SR, Hopkins JO, Morrow GR Longitudinal trajectory and characterization of cancer-related cognitive impairment in a nationwide cohort study. Journal of Clinical Oncology (en press). 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papachristou E, Wannamethee SG, Lennon LT, et al. Ability of Self-Reported Frailty Components to Predict Incident Disability, Falls, and All-Cause Mortality: Results From a Population-Based Study of Older British Men. J Am Med Dir Assoc. 2017;18(2):152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunes DP, Duarte YA, Santos JL, Lebrao ML. Screening for frailty in older adults using a self-reported instrument. Rev Saude Publica. 2015;49:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53(8):1321–1330. [DOI] [PubMed] [Google Scholar]
- 24.Widagdo IS, Pratt N, Russell M, Roughead EE. Predictive performance of four frailty measures in an older Australian population. Age Ageing. 2015;44(6):967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freedman RJ, Aziz N, Albanes D, et al. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. J Clin Endocrinol Metab. 2004;89(5):2248–2253. [DOI] [PubMed] [Google Scholar]
- 26.Wagner LSJ, Butt Z, Lai J, Cella D . Measuring patient self-reported cognitive function: Development of the Functional Assessment of Cancer Therapy-Cognitive Function Instrument. J Sup Oncol. 2009;7:W32–39. [Google Scholar]
- 27.Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–281. [DOI] [PubMed] [Google Scholar]
- 28.Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–338. [PubMed] [Google Scholar]
- 29.Goul WR, Brown M. Effects of age and intelligence on trail making test performance and validity. Percept Mot Skills. 1970;30(1):319–326. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds C Comprehensive Trail-Making Test professional manual. Austin, TX, Pro-ed; 2002. [Google Scholar]
- 31.Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13(3):348–358. [DOI] [PubMed] [Google Scholar]
- 32.Rasmusson DX, Bylsma FW, Brandt J. Stability of performance on the Hopkins Verbal Learning Test. Arch Clin Neuropsychol. 1995;10(1):21–26. [PubMed] [Google Scholar]
- 33.Middleton LS, Denney DR, Lynch SG, Parmenter B. The relationship between perceived and objective cognitive functioning in multiple sclerosis. Arch Clin Neuropsychol. 2006;21(5):487–494. [DOI] [PubMed] [Google Scholar]
- 34.Khatri P, Babyak M, Clancy C, et al. Perception of cognitive function in older adults following coronary artery bypass surgery. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 1999;18(3):301–306. [DOI] [PubMed] [Google Scholar]
- 35.Maor Y, Olmer L, Mozes B. The relation between objective and subjective impairment in cognitive function among multiple sclerosis patients--the role of depression. Mult Scler. 2001;7(2):131–135. [DOI] [PubMed] [Google Scholar]
- 36.Sawrie SM, Martin RC, Kuzniecky R, et al. Subjective versus objective memory change after temporal lobe epilepsy surgery. Neurology. 1999;53(7):1511–1517. [DOI] [PubMed] [Google Scholar]
- 37.Wagner L, Sweet J, Butt Z, Lai J., Cella D. Measuring Patient Self-Reported Cognitive Function: Development of the Functional Assessment of Cancer Therapy-Cognitive Function Instrument. Journal of Supportive Oncology. 2009;7:32–39. [Google Scholar]
- 38.Mandelblatt JS, Clapp JD, Luta G, et al. Long-term trajectories of self-reported cognitive function in a cohort of older survivors of breast cancer: CALGB 369901 (Alliance). Cancer. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandelblatt JS, Small BJ, Luta G, et al. Cancer-Related Cognitive Outcomes Among Older Breast Cancer Survivors in the Thinking and Living With Cancer Study. J Clin Oncol. 2018:JCO1800140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett JA, Winters-Stone KM, Dobek J, Nail LM. Frailty in older breast cancer survivors: age, prevalence, and associated factors. Oncol Nurs Forum. 2013;40(3):E126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartels C, Wegrzyn M, Wiedl A, Ackermann V, Ehrenreich H. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci. 2010;11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Consort Flow Diagram
Footnote: Linear regression models were used to evaluate the association between frailty and cognition, controlling for age (50–64 versus ≥65 years), race (Caucasian versus other), marital status (married versus other), education (≥ some college versus ≤ high school), performance status (≥70 versus <70), baseline anxiety (STAI), baseline depression (MSFI) and baseline frailty score. Standard errors are reported in brackets. *significant at 10%; **significant at 5%; ***significant at 1%.† significant at 11%. STAI, State Trait Anxiety Inventory; MFSI, Multidimensional Fatigue Symptom Inventory; FACT-Cog, Functional Assessment of Cancer Therapy; COWA, Controlled Oral Word Association test; CANTAB, Cambridge Neuropsychological Test Battery; DMS delayed match to sample test; RVP, Rapid Visual Processing; TMT, Trail Making Test; HVLT-R, Hopkins Verbal Learning and Memory Test-Revised.
Footnote: Linear regression models were used to evaluate the association between frailty and cognition, controlling for age (50–64 versus ≥65 years), race (Caucasian versus other), marital status (married versus other), education (≥ some college versus ≤ high school), performance status (≥70 versus <70), baseline anxiety (STAI), baseline depression (MSFI) and baseline frailty score. Standard errors are reported in brackets. *significant at 10%; **significant at 5%; ***significant at 1%.† significant at 11%. STAI, State Trait Anxiety Inventory; MFSI, Multidimensional Fatigue Symptom Inventory; FACT-Cog, Functional Assessment of Cancer Therapy; COWA, Controlled Oral Word Association test; CANTAB, Cambridge Neuropsychological Test Battery; DMS delayed match to sample test; RVP, Rapid Visual Processing; TMT, Trail Making Test; HVLT-R, Hopkins Verbal Learning and Memory Test-Revised.


