Abstract
Compared with neural biomarkers of risk for mental illness, little is known about biomarkers of resilience. We explore if greater executive control-related prefrontal activity may function as a resilience biomarker by “rescuing” risk associated with higher threat-related amygdala and lower reward-related ventral striatum activity. Functional MRI was used to assay baseline threat-related amygdala, reward-related ventral striatum, and executive control-related prefrontal activity in 120 young adult volunteers. Participants provided self-reported mood and anxiety ratings at baseline and follow-up. A moderation model revealed a significant three-way interaction wherein higher amygdala and lower ventral striatum activity predicted increases in anxiety in those with average or low but not high prefrontal activity. This effect was specific to anxiety, with the neural biomarkers explaining ~10% of the variance in change over time, above and beyond baseline symptoms, sex, age, IQ, presence or absence of DMS-IV diagnosis, and both early and recent stress. Our findings are consistent with the importance of top-down executive control in adaptive regulation of negative emotions, and highlight a unique combination of neural biomarkers that may identify at-risk individuals for whom the adoption of strategies to improve executive control of negative emotions may prove particularly beneficial.
Keywords: amygdala, biomarker, dorsolateral prefrontal cortex, resilience, ventral striatum
Introduction
The global public health impact of mood and anxiety disorders continues to rise but effective treatment remains lacking, leading to an increasing importance of identifying strategies for prevention (Craske et al. 2017). Here, translational neuroscience research has been particularly successful in revealing potential biomarkers of risk (Hariri and Holmes 2015). For example, relatively higher activity of the amygdala, which coordinates threat-related behavior, predicts risk for stress-related negative mood and anxiety up to 4 years later (Swartz et al. 2015), as well as the development of posttraumatic stress disorder (McLaughlin et al. 2014; Stevens et al. 2016). Likewise, relatively lower activity of the ventral striatum (VS), which coordinates goal-directed behavior, predicts lower positive affect in interaction with stressful life events (Nikolova et al. 2012), as well as future depression up to a year later (Telzer et al. 2014). Moreover, the combination of relatively high threat-related amygdala and low reward-related VS activity has been found to predict alcohol abuse and alcohol use disorder as a coping mechanism in response to stress (Corral-Frías et al. 2015; Nikolova et al. 2016).
In contrast to the growing literature on biomarkers of risk for mental illness, there are relatively few studies addressing possible biomarkers of resilience. We recently reported that greater activity of the dorsolateral prefrontal cortex (dlPFC), which coordinates executive control, is associated with decreased experience of stress-related negative mood and anxiety, in part, by supporting the use of cognitive reappraisal in regulating negative emotions (Scult et al. 2017). This observation is consistent with the proposed critical importance of the dlPFC in shaping the adaptive, contextually-appropriate expression of emotions by effecting top-down regulation of the amygdala and VS (Ochsner and Gross 2005; Heller 2016). Here, we examine the extent to which such dlPFC activity may act as a marker of resilience by rescuing the risk associated with higher threat-related amygdala and lower reward-related VS activity.
We employed 3 independent tasks during functional MRI to assay baseline threat-related amygdala, reward-related VS, and executive control-related dlPFC activity. This strategy allowed us to isolate activity in these target regions representing their core contributions to processing threat, reward, and executive control, respectively, and, subsequently, their independent and interactive contributions to future symptoms of mood and anxiety. In contrast, single tasks wherein explicit forms of executive control are required for regulating either positive or negative emotions do not readily afford isolation of the independent processing of threat, reward, or executive control. The 3 tasks were administered to 120 young adult university students who also provided self-reported experiences of stressful life events as well as symptoms of anxiety and depression as detailed previously (see Nikolova and Hariri 2012; Nikolova et al. 2012; Corral-Frías et al. 2015; Swartz et al. 2015; Scult et al. 2017 for details). These self-report measures were provided at baseline and through online follow-up surveys (mean time from baseline = 7 months; range 3–14 months). Based on prior literature (McLaughlin et al. 2014; Telzer et al. 2014; Corral-Frías et al. 2015; Swartz et al. 2015; Nikolova et al. 2016; Stevens et al. 2016), we predicted that the combination of relatively high threat-related amygdala and low reward-related VS activity would be associated with increases in mood and anxiety symptoms over time, but not for individuals with relatively high dlPFC activity.
Materials and Methods
Participants
Data were available from 120 young adult university students who successfully completed the Duke Neurogenetics Study (DNS) between 3 September 2014 and 16 February 2017. Informed consent was obtained for all participants in accordance with the Duke University School of Medicine Institutional Review Board. Exclusion criteria included: (1) medical diagnoses of cancer, stroke, head injury with loss of consciousness, untreated migraine headaches, diabetes requiring insulin treatment, chronic kidney or liver disease, or lifetime history of psychotic disorder; (2) use of psychotropic, glucocorticoid, or hypolipidemic medication; and (3) conditions affecting cerebral blood flow and metabolism (e.g., hypertension).
The DNS seeks to establish broad variability in multiple behavioral phenotypes related to psychopathology, so other than psychotic disorders, participants were not excluded based on diagnosis of past or current DSM-IV Axis I or select Axis II (borderline and antisocial personality) disorder. However, no participants were taking psychotropic medication at the time of or at least 10 days prior to study participation. Clinical interview identified 23 participants (19.2%) as having a DSM-IV diagnosis, the most common of which were current or past depression (8.3%) or alcohol abuse (5%).
Self-Report Questionnaires
The Mood and Anxiety Symptom Questionnaire – Short Form (MASQ-SF) is a 62-item self-report questionnaire designed to assess symptoms during the past week across 4 subscales: general distress depression, anhedonic depression, general distress anxiety, and anxious arousal (Watson et al. 1995a). Items are rated on a 5-point-Likert scale from “not at all” to “extremely.” One item from the anhedonic depression subscale that asked about suicidality was removed from the questionnaire in order to comply with IRB protocol. Items were summed to calculate each of the subscales, which have high internal consistency in multiple samples (Watson et al. 1995b).
The Life Events Scale for Students (LESS) is a 46-item self-report questionnaire that assesses the number of life events that occurred in the past 12 months (Clements and Turpin 1996). Participants rate the impact that the life event had on them on a 1–4 scale (4 = severe impact). The impact score for each event reported was summed to yield a LESS total impact score; higher values indicate both greater number and severity of life events.
The Childhood Trauma Questionnaire (CTQ), is a 28-item inventory that assesses frequency and severity of early stressful life events (Bernstein et al. 2003). This questionnaire asks participants to reflect on their upbringing and childhood experiences related to emotional, physical, and sexual abuse and emotional and physical neglect. For this study, the total CTQ score was used to capture overall levels of childhood trauma.
Clinical and Neuropsychological Interview
All participants were assessed for common psychiatric disorders using the electronic version of the M.I.N.I International Neuropsychiatric Interview (Sheehan et al. 1998), administered by trained staff under the supervision of a licensed clinical psychologist (BDB). The interview follows DSM-IV (American Psychiatric Association 1994) and ICD-10 (World Health Organization 2004) diagnostic criteria. IQ was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1999) by trained staff, also under the supervision of a licensed clinical psychologist (BDB).
Amygdala Paradigm
Our fMRI challenge paradigm has been used extensively to elicit a robust and replicable amygdala response across an array of experimental protocols and sample populations (Hariri 2002; Fisher et al. 2006; Manuck et al. 2007; Zhou et al. 2008). The experimental fMRI paradigm consists of 4 blocks of a perceptual face-matching task interleaved with 5 blocks of a sensorimotor control task. The DNS version of this paradigm consists of one block each of fearful, angry, surprised, and neutral facial expressions presented in a pseudorandom order across participants. During face-matching blocks, participants view a trio of faces and select one of two faces (on the bottom) identical to a target face (on the top). Each face processing block consists of 6 images, balanced for gender, all of which were derived from a standard set of pictures of facial affect (Ekman and Friesen 1976). During the sensorimotor control blocks, participants view a trio of simple geometric shapes (circles and vertical and horizontal ellipses) and select one of two shapes (bottom) that are identical to a target shape (top). Each sensorimotor control block consists of 6 different shape trios. All blocks are preceded by a brief instruction (“Match Faces” or “Match Shapes”) that lasts 2 s. In the task blocks, each of the 6 face trios is presented for 4 s with a variable interstimulus interval (ISI) of 2–6 s (mean = 4 s) for a total block length of 48 s. A variable ISI is used to minimize expectancy effects and resulting habituation, and maximize amygdala reactivity throughout the paradigm. In the control blocks, each of the 6 shape trios is presented for 4 s with a fixed ISI of 2 s for a total block length of 36 s. Total task time is 390 s.
Ventral Striatum Paradigm
As described previously (Forbes et al. 2009), our blocked-design number-guessing paradigm consists of a pseudorandom presentation of 3 blocks of predominantly positive feedback (80% correct guess), 3 blocks of predominantly negative feedback (20% correct guess) and 3 control blocks. There are 5 trials in 3 s to guess, via button press, whether the value of a visually presented card is lower or higher than 5 (index and middle finger, respectively). The numerical value of the card is then presented for 500 ms and followed by appropriate feedback (green upward-facing arrow for positive feedback; red downward-facing arrow for negative feedback) for an additional 500 ms. A crosshair is then presented for 3 s, for a total trial length of 7 s. Each block comprises 5 trails, with 3 blocks each of predominantly positive feedback (80% correct) and 3 of predominantly negative feedback (20% correct) interleaved with 3 control blocks. During control blocks, participants are instructed to simply make button presses during the presentation of an “x” (3 s), which is followed by an asterisk (500 ms) and a yellow circle (500 ms). Each block is preceded by an instruction of “Guess Number” (positive or negative feedback blocks) or “Press Button” (control blocks) for 2 s resulting in a total block length of 38 s and a total task length of 342 s. Participants were unaware of the fixed outcome probabilities associated with each block and were led to believe that their performance would determine a net monetary gain at the end of the scanning session. Instead, all participants received $10. We included one incongruent trial within each task block (e.g., one of five trials during positive feedback blocks was incorrect resulting in negative feedback) to prevent participants from anticipating the feedback for each trial and to maintain participants’ engagement and motivation to perform well.
Prefrontal Paradigm
Activity of the dlPFC was measured during BOLD fMRI using an event-related working memory paradigm adapted from Tan et al. (Tan et al. 2007), that has been used previously as a robust biomarker of dlPFC activity (Scult et al. 2017). The paradigm included 10 trials for each of 6 different conditions, including 3 control conditions, consisting only of a 3 s response phase, and 3 working memory (WM) conditions, consisting of a 0.5 s encoding phase followed by a 4 s maintenance interval and a 3 s response phase. Control and WM conditions were interleaved with jittered rest intervals lasting 4–8.5 s for a total scan length of 11 m 48 s. Responses were recorded via an MR-compatible button box using the index (left button) and middle (right button) fingers of the dominant hand.
During the control conditions, participants performed (1) a simple motor task (M) in which they pressed either the left or the right button according to a prompt, (2) a numerical size judgment task (J) in which they chose the number on the left or right based on an instruction to choose either the larger or the smaller number, and (3) a numerical computation and size judgment task (CJ) in which they performed a numerical subtraction of 2 or 3 from either the left or right number, and made a numerical size judgment as instructed. In the first WM condition, participants viewed 2 numbers during the brief encoding phase, then recalled the numbers and performed a numerical size judgment as instructed (E_RJ). In the second WM condition, the participants additionally performed subtraction of 2 or 3 from one of the remembered numbers as indicated before making the numerical size judgment during recall (E_RCJ). In the final WM condition, participants performed subtraction of 2 or 3 from one of the 2 numbers during the brief encoding phase, then recalled the resulting 2 numbers and performed a numerical size judgment as instructed during the response phase after the maintenance interval (EC_RJ). In each WM condition trial, all the numbers were single digits from 0 to 9; the 2 numbers on which the numerical size judgment was ultimately performed (after numerical computation if applicable) were equally balanced across 0 to 9, and equally likely to differ by either 1 or 3 units. Numerical computation was equally likely on the left or right number, with correct responses equally balanced on the left or right, and equally likely to be the larger or smaller number for each WM trial type. The trials were performed in an order that was optimized using a sequencing program (Wager and Nichols 2003). Incorrect responses were modeled as regressors of no interest.
A linear contrast employing the canonical hemodynamic response function was used to estimate main effects for each individual for the comparison of E_RCJ > EC_RJ in order to isolate the manipulation of information in working memory above and beyond basic computation and maintenance of information across a delay. Activity associated with this contrast was selected based on our prior demonstration that it is associated with the use of adaptive emotion regulation strategies in everyday life, as well as lower concurrent mood and anxiety symptoms when faced with stressful life events (Scult et al. 2017).
BOLD fMRI Data Acquisition
Each participant was scanned using one of two identical research-dedicated GE MR750 3 T scanners equipped with high-power high-duty-cycle 50-mT/m gradients at 200 T/m/s slew rate, and an eight-channel head coil for parallel imaging at high bandwidth up to 1 MHz at the Duke-UNC Brain Imaging and Analysis Center. A semi-automated high-order shimming program was used to ensure global field homogeneity. A series of 34 interleaved axial functional slices aligned with the anterior commissure–posterior commissure plane were acquired for full-brain coverage using an inverse-spiral pulse sequence to reduce susceptibility artifacts (TR/TE/flip angle = 2000 ms/30 ms/60; FOV = 240 mm; 3.75 × 3.75 × 4 mm3 voxels; interslice skip = 0). Four initial radiofrequency excitations were performed (and discarded) to achieve steady-state equilibrium. To allow for spatial registration of each participant’s data to a standard coordinate system, high-resolution three-dimensional structural images were acquired in 34 axial slices coplanar with the functional scans (TR/TE/flip angle = 7.7 s/3.0 ms/12; voxel size = 0.9 × 0.9 × 4 mm3; FOV = 240 mm, interslice skip = 0).
BOLD fMRI Data Preprocessing
Preprocessing was conducted using SPM8 (www.fil.ion.ucl.ac.uk/spm). Images for each subject were realigned to the first volume in the time series to correct for head motion, spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) via the co-registered grey matter segmentation of the anatomical coplanar scan using a 12-parameter affine model (final resolution of functional images = 2 mm isotropic voxels), and smoothed to minimize noise and residual difference in gyral anatomy with a Gaussian filter, set at 6-mm full-width at half-maximum. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean. Variability in single-subject whole-brain functional volumes was determined using the Artifact Recognition Toolbox (http://www.nitrc.org/projects/artifact_detect). Individual whole-brain BOLD fMRI volumes meeting at least one of two criteria were assigned a lower weight in determination of task-specific effects: (1) significant mean-volume signal intensity variation (i.e., within volume mean signal greater or less than 4 SD of mean signal of all volumes in time series) and (2) individual volumes where scan-to-scan movement exceeded 2 mm translation or 2° rotation in any direction.
fMRI Quality Assurance Criteria
Quality control criteria for inclusion of a participant’s imaging data were: <5% volumes exceed artifact detection criteria for motion or signal intensity outliers and ≥90% coverage of signal within the ROIs. Additionally, data were only included in further analyses if the participant demonstrated sufficient engagement with the task, defined as (A) for the faces matching task: 75% accuracy matching; (B) for the guessing game task: responding to and receiving positive or negative feedback on at least 60% of trials within win and loss blocks, respectively; (C) for the working memory task: at least 75% average accuracy across all trial types, and at least 50% accuracy within each trial type.
BOLD fMRI Data Analysis
Following preprocessing, linear contrasts employing canonical hemodynamic response functions were used to estimate effects of task condition for each a priori region of interest. The task conditions were selected based on our prior work establishing associations between these specific neural phenotypes and the experience of mood and anxiety (Nikolova et al. 2012; Swartz et al. 2015; Scult 2017). Threat-related amygdala activity was identified from the task contrast of fearful and angry facial expressions > shapes. Reward-related VS activity was identified from the task contrast of positive feedback > negative feedback. Executive control-related dlPFC activity was identified from the task contrast of computation in working memory > computation plus maintenance of information in working memory (i.e., E_RCJ > EC_RJ). Individual contrast images were then used in second-level random effects models to conduct group-level analyses. Mean task-specific statistically significant activation clusters (P < 0.05 FWE-corrected, k > 10) within a priori regions of interest were determined across all participants. The first eigenvariate within each task-specific mean activation cluster was subsequently extracted for each participant. These task-specific parameter estimates were then averaged across right and left hemispheres, and these values were entered into our statistical moderation models.
Hypothesis Testing
To assess the impact of brain-based biomarkers on change in mood and anxiety symptoms, extracted parameter estimates from the amygdala, VS, and dlPFC clusters exhibiting main effects of each task-specific contrast of interest were entered into moderation models using PROCESS for SPSS (Hayes 2012) with change in MASQ subscale scores as the dependent variable. The statistical significance threshold was set at P < 0.0125 to correct (Bonferroni) for multiple comparisons across the 4 MASQ subscales. The analyses controlled for the MASQ subscale scores at baseline and the number of days elapsed between scan and follow-up for each participant. Subsequent sensitivity analyses also controlled for sex, age, IQ, presence or absence of psychological diagnosis, stressful life events at baseline and follow-up (LESS) and childhood trauma (CTQ).
Results
Consistent with our prior work (e.g., Nikolova and Hariri 2012; Swartz et al. 2015; Scult et al. 2017), the 3 fMRI paradigms elicited significant task-specific activity in our a priori regions of interest (Fig. 1). Results from our moderation models revealed a significant (P = 0.0123, R2-change = 4.1%) three-way interaction between baseline amygdala, VS, and dlPFC activity predicting change in anxiety (MASQ anxious arousal) over 3–14 months (mean 7 months), controlling for days between assessments and baseline anxiety. There were no significant interaction effects for the other MASQ subscales (Ps > 0.1).
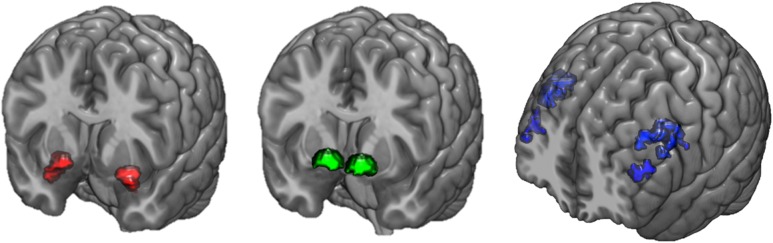
Figure 1.
Task-specific brain activity. The 3 fMRI tasks each led to robust activity in a priori regions of interest: threat-related activity in the left (–22, –6, –18) and right amygdala (28, –4, –20) for the contrast of fearful and angry faces > shapes (left), reward-related activity in the left (–12, 8, –8) and right ventral striatum (12, 10, –8) for the contrast of positive feedback > negative feedback (middle), and executive control-related activity in the left (–42, 2, 30) and right dorsolateral prefrontal cortex (44, 10, 30) for the contrast of computation in working memory > computation plus maintenance of information (right). All coordinates are reported in MNI space, and all activation clusters are from contrast-specific thresholds of P < 0.05, FWE-corrected.
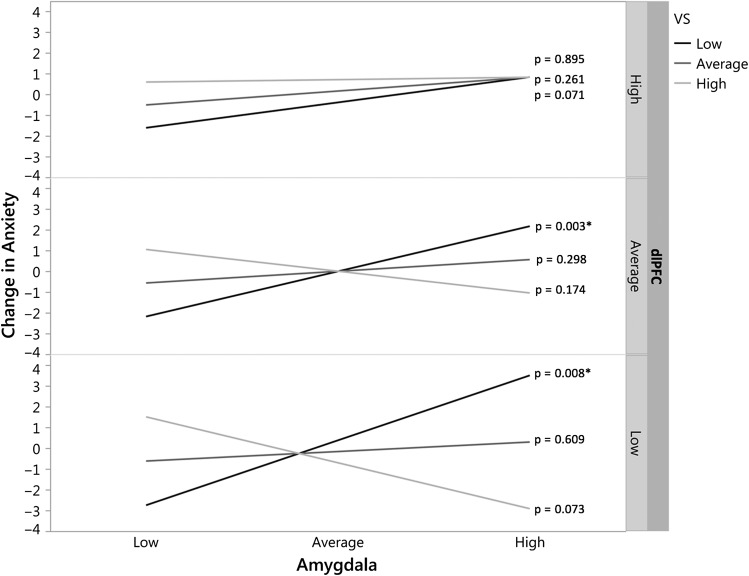
Post-hoc analyses of the two-way interactions revealed a significant interaction between reward-related VS activity and threat-related amygdala activity leading to changes in anxiety, at low dlPFC activity (b = –41.65, P < 0.001) or average dlPFC activity (b = –25.15, P = 0.002), but not at high dlPFC activity (b = –8.64, P = 0.278) (Fig. 2). Specifically, the Johnson-Neyman method indicated that the interaction between high amygdala and low VS activity was significantly associated with future anxiety only when dlPFC activity was less than 0.67 standard deviations above the mean.
Figure 2.
Neural biomarkers of change in anxiety. A significant three-way interaction between amygdala, VS, and dlPFC activity predicted change in anxiety over time (P = 0.0123, R2-change = 4.1%). Post hoc analyses of the two-way interactions revealed a significant interaction between reward-related VS activity and threat-related amygdala activity leading to changes in anxiety, at low dlPFC activity (bottom; b = –41.65, P < 0.001) or average dlPFC activity (middle; b = –25.15, P = 0.002), but not at high dlPFC activity (top; b = –8.64, P = 0.278).
This pattern was further robust to the inclusion of participant sex, age, IQ, recent life stress at baseline and follow-up, as well as early life stress and categorical diagnosis of a past or present DSM-IV disorder at baseline. A stepwise regression revealed that our 3 systems-level neural phenotypes explained approximately 10% of the variance in change in anxiety above and beyond all other model variables (R2-change = 0.094), and the total model collectively accounted for 42% of the variance in change in anxiety (R2 = 0.422).
Discussion
Our analyses provide initial evidence that relatively high dlPFC activity, supporting executive control, may protect against risk for future anxiety associated with relatively high threat-related amygdala activity and relatively low reward-related VS activity. Our current findings not only help establish a novel combination of brain biomarkers predicting change in anxiety symptoms over time but also highlight the importance of executive control in mood and anxiety disorders (Carver et al. 2017). These findings are consistent with the fact that commonly employed forms of psychotherapy encourage and train individuals to better regulate their negative emotional experiences through cognitive reappraisal strategies supported by the dlPFC. Thus, the combination of brain biomarkers described here could be leveraged to identify at-risk individuals who may particularly benefit from such strategies, thereby reducing their likelihood of future illness.
In particular, we found that for individuals with low-to-average dlPFC activity during our executive control task, relatively higher amygdala activity and lower VS activity was associated with greater increases in anxiety. This finding parallels our prior work reporting that a similar imbalance between amygdala and VS activity is related to problem drinking in response to stress (Nikolova et al. 2016) as well as sexual risk behavior in men (Victor et al. 2015), and may reflect reliance on a balance between these systems in order to produce flexible, adaptive functioning. The present findings extend this work by demonstrating that such an imbalance between amygdala and VS activity, at least with regards to the experience of anxiety, is most problematic for individuals with relatively poor executive dlPFC activity.
Prefrontal function has been linked with anxiety, with anxious individuals showing altered prefrontal activity during attentional control (Bishop 2009), working memory (Balderston et al. 2016b), and inhibitory control tasks (Basten et al. 2011). Moreover, targeting prefrontal executive control using either behavioral or stimulation protocols is associated with decreased anxiety (Balderston et al. 2016a; Diefenbach et al. 2016b) possibly through improvements in emotion regulation (Diefenbach et al. 2016a) or decreased attentional bias to threat-related cues (Heeren et al. 2016). Our current findings provide initial evidence that intrinsic prefrontal executive control function may further represent a prognostic biomarker of risk for future anxiety.
Unexpectedly, our observed interaction effects were specific to increases in anxiety as indexed by the anxious arousal subscale of the MASQ, which assays hypervigilance, frequent experience of fear, and heightened sympathetic nervous system activity to mild stressors that are specific to anxiety rather than symptoms that overlap with depression (Clark and Watson 1991; Conway et al. 2017). While it is unclear why the effects were not also seen for the other MASQ subscales, one possibility is that this combination of biomarkers is more sensitive to identifying physiological symptoms most commonly associated with anxiety. Future studies employing measures of physiological arousal (e.g., skin conductance response) as well as protocols specifically designed to test responses to stressful situations (e.g., Trier Social Stress Test) could address this conjecture. Alternatively, the specificity of the interaction effects for anxiety may reflect the nature of our sample as university students generally experience increases in anxiety more so than depression (Center for Collegiate Mental Health 2017), and represent a particularly vulnerable group for whom the development of anxiety disorders can perpetuate lifelong dysfunction (Craske et al. 2017). It is possible that a similar interaction effect may be present in samples wherein depression is a primary feature of negative mood.
An important limitation of our current study is that we did not ascertain mental health at follow-up using structured interviews. Therefore, while our findings could have important implications for the treatment of categorically diagnosed psychiatric disorders, they more directly address the experience of dimensional symptoms in non-clinical populations. Relatedly, the predictive function of our 3 biomarkers may not extend beyond relatively high functioning young adult university students or symptoms of anxiety. Studies in both patient and community samples are needed to evaluate the extent to which these biomarkers can predict risk broadly. A final consideration is our approach of explicitly examining activity of the amygdala, VS and dlPFC supporting core processing of threat, reward, and executive control, respectively. While we believe this strategy affords a unique opportunity to isolate the contributions of these neural phenotypes to relative risk and resilience, it is possible that the real-time dynamic regulation of negative emotions involves different patterns of interactions between these circuits. Nevertheless, our current work provides a foundation from which these neural phenotypes can be further examined as biomarkers of relative risk and resilience and, if replicated, begin to inform efforts to improve treatment targets and ultimately help prevent mental illness.
Notes
We thank all members of the Laboratory of NeuroGenetics for their assistance in conducting the Duke Neurogenetics Study. M.A.S. is supported by an NSF Graduate Research Fellowship. Conflict of Interest: The authors declare no competing financial or other conflicts of interest.
Funding
The Duke Neurogenetics Study was supported by Duke University and NIH grant R01DA033369. A.R.H. is further supported by NIH grant R01AG049789.
References
- American Psychiatric Association 1994. Diagnostic and statistical manual of mental disorders. 4th ed Washington, DC: APA. [Google Scholar]
- Balderston NL, Quispe-Escudero D, Hale E, Davis A, O’Connell K, Ernst M, Grillon C. 2016. a. Working memory maintenance is sufficient to reduce state anxiety. Psychophysiology. 53:1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderston NL, Vytal KE, O’Connell K, Torrisi S, Letkiewicz A, Ernst M, Grillon C. 2016. b. Anxiety patients show reduced working memory related dlpfc activation during safety and threat. Depress Anxiety. 36:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten U, Stelzel C, Fiebach CJ. 2011. Trait anxiety modulates the neural efficiency of inhibitory control. J Cogn Neurosci. 23:3132–3145. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, et al. 2003. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 27:169–190. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. 2009. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 12:92–98. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Timpano KR. 2017. Toward a functional view of the p factor in psychopathology. Clin Psychol Sci. 5:880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Collegiate Mental Health 2017. 2016 Annual Report. Publ No STA 17-74.
- Clark LA, Watson D. 1991. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol. 100:316–336. [DOI] [PubMed] [Google Scholar]
- Clements K, Turpin G. 1996. The life events scale for students: validation for use with British samples. Pers Individ Dif. 20:747–751. [Google Scholar]
- Conway CC, Zinbarg RE, Mineka S, Craske MG. 2017. Core dimensions of anxiety and depression change independently during adolescence. J Abnorm Psychol. 126:160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Frías NS, Nikolova YS, Michalski LJ, Baranger DAA, Hariri AR, Bogdan R. 2015. Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychol Med. 45:2605–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Stein MB, Eley TC, Milad MR, Holmes A, Rapee RM, Wittchen H-U. 2017. Anxiety disorders. Nat Rev Dis Primers. 3:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach GJ, Assaf M, Goethe JW, Gueorguieva R, Tolin DF. 2016. a. Improvements in emotion regulation following repetitive transcranial magnetic stimulation for generalized anxiety disorder. J Anxiety Disord. 43:1–7. [DOI] [PubMed] [Google Scholar]
- Diefenbach GJ, Bragdon LB, Zertuche L, Hyatt CJ, Hallion LS, Tolin DF, Goethe JW, Assaf M. 2016. b. Repetitive transcranial magnetic stimulation for generalised anxiety disorder: a pilot randomised, double-blind, sham-controlled trial. Br J Psychiatry. 209:222–228. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen W. 1976. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Fisher PM, Meltzer CC, Ziolko SK, Price JC, Moses-Kolko EL, Berga SL, Hariri AR. 2006. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nat Neurosci. 9:1362–1363. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. 2009. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 14:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. 2002. Serotonin transporter genetic variation and the response of the human amygdala. Science. 297:400–403. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. 2015. Finding translation in stress research. Nat Neurosci. 18:1347–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. 2012. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. [White Paper].
- Heeren A, Billieux J, Philippot P, de Raedt R, Baeken C, de Timary P, Maurage P, Vanderhasselt M-A. 2016. Impact of transcranial direct current stimulation on attentional bias for threat: a proof-of-concept study among individuals with social anxiety disorder. Soc Cogn Affect Neurosci. 12:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS. 2016. Cortical-subcortical interactions in depression: from animal models to human psychopathology. Front Syst Neurosci. 10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Brown SM, Forbes EE, Hariri AR. 2007. Temporal stability of individual differences in amygdala reactivity. Am J Psychiatry. 164:1613–1614. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, Sheridan MA. 2014. Amygdala response to negative stimuli predicts ptsd symptom onset following a terrorist attack. Depress Anxiety. 31:834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Bogdan R, Brigidi BD, Hariri AR. 2012. Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biol Psychiatry. 72:157–163. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Hariri AR. 2012. Neural responses to threat and reward interact to predict stress-related problem drinking: A novel protective role of the amygdala. Biol Mood Anxiety Disord. 2:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Knodt AR, Radtke SR, Hariri AR. 2016. Divergent responses of the amygdala and ventral striatum predict stress-related problem drinking in young adults: possible differential markers of affective and impulsive pathways of risk for alcohol use disorder. Mol Psychiatry. 21:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. 2005. The cognitive control of emotion. Trends Cogn Sci. 9:242–249. [DOI] [PubMed] [Google Scholar]
- Scult MA. 2017. Flexible adaptation of brain networks during stress. J Neurosci. 37:3992–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scult MA, Knodt AR, Swartz JR, Brigidi BD, Hariri AR. 2017. Thinking and feeling: individual differences in habitual emotion regulation and stress-related mood are associated with prefrontal executive control. Clin Psychol Sci. 5:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. 1998. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 59:22–33. [PubMed] [Google Scholar]
- Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, Hudak LA, Jovanovic T, Rothbaum BO, Ressler KJ. 2016. Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biol Psychiatry. 81:1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, Hariri AR. 2015. A neural biomarker of psychological vulnerability to future life stress. Neuron. 85:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H-Y, Chen Q, Goldberg TE, Mattay VS, Meyer-Lindenberg A, Weinberger DR, Callicott JH. 2007. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. J Neurosci. 27:13393–13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galvan A. 2014. Neural sensitivity to eudaimonic and hedonic rewards differentially predict adolescent depressive symptoms over time. Proc Natl Acad Sci. 111:6600–6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor EC, Sansosti AA, Bowman HC, Hariri AR. 2015. Differential patterns of amygdala and ventral striatum activation predict gender-specific changes in sexual risk behavior. J Neurosci. 35:8896–8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Nichols TE. 2003. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. Neuroimage. 18:293–309. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. 1995. a. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol. 104:15–25. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, Mccormick RA. 1995. b. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of Anxiety and Depression Symptom Scales. J Abnorm Psychol. 104:3–14. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1999. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- World Health Organization 2004. International statistical classification of diseases and health related problems (The) ICD-10.
- Zhou Z, Zhu G, Hariri AR, Enoch M-A, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu X-Z, et al. 2008. Genetic variation in human NPY expression affects stress response and emotion. Nature. 452:997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]