Abstract
Purpose of Review
Staphylococcus aureus (S. aureus) is correlated with the development of persistent severe inflammatory disease of the upper airway including chronic rhinosinusitis with nasal polyps (CRSwNP). The presence of S. aureus is associated with atopic disease including allergic rhinitis and atopic dermatitis and is associated with poor outcomes.
Recent Findings
Several different strains of S. aureus generate different toxins and gene products that can account for organism pathogenicity. S. aureus bacteria and its antigens shape the bacterial and fungal microbiome and the mucosal niche which generates host responses that can account for inflammation. The multiple disease phenotypes and molecular endotypes seen in CRSwNP can be characterized by T-helper cell environment within the inflammatory milieu, the presence of epithelial barrier dysfunction, aberrant eicosanoid metabolism, poor wound healing, and dysfunctional host-bacteria interactions which lead to recalcitrant disease and worse surgical outcomes.
Summary
Understanding the pathomechanisms that S. aureus utilizes to promote nasal polyp formation, prolonged tissue inflammation, and bacterial dysbiosis are essential in our efforts to identify new therapeutic approaches to resolve this chronic inflammatory process.
Keywords: Chronic rhinosinusitis, Nasal polyps, Staphylococcus aureus, Innate and adaptive immune system, Superantigens
Introduction
Chronic rhinosinusitis (CRS) continues to be a highly prevalent and morbid clinical syndrome characterized by persistent inflammation of the nasal cavity and paranasal sinuses that results in symptoms of nasal obstruction, rhinorrhea, facial pain, and anosmia. CRS is commonly classified by the presence or absence of nasal polyps on endoscopy or imaging as recommended by recent AAO-HNS guidelines [1]. CRS is widespread with a reported prevalence of 4.5–12% in the general population, and the subset of patients with nasal polyposis observed in population-based studies ranges from 0.5–4.3% with the higher prevalence observed in Western countries, male patients, the elderly, and asthmatics [2] [3].
Despite the higher incidence of polyposis in men, women with CRS and nasal polyposis (CRSwNP) have more severe disease features than men including higher rates of aspirin sensitivity and comorbid asthma [4]. The symptoms and clinical findings that define CRS represent the common endpoint for several discrete pathogenic processes, which collectively promote dysregulated innate and adaptive immune response, epithelial barrier dysfunction, and perturbations in host-microbe interactions. Understanding the host-specific and environmental factors that pre-dispose individuals to developing CRS, including the role of Staphylococcus aureus in the pathogenesis of CRSwNP is an active area of research [5••].
Etiology of CRS
The etiology of CRS is known to be multifactorial. Though both CRS without nasal polyps (CRSsNP) and with nasal polyps (CRSwNP) are characterized by persistent inflammation, the cytokine profile of the inflammatory milieu in patients with nasal polyps appears to be remarkably different. Historically, CRSsNP was thought to result from an incompletely resolved acute bacterial sinusitis with an inflammatory phenotype enriched in polymorphic neutrophils, with high levels of pro-inflammatory cytokines (IL-1, IL-6, IFNγ, and TNFα), and TH1 skewing of the T cell population. In contrast, CRSwNP is classically associated with eosinophilic inflammation typified by TH2 T cells, with abundant IgE, histamine, eosinophilic cationic protein (ECP), and type II inflammatory cytokines (IL-5, IL-13) [6–8]. CRSwNP is also associated with higher rates of upper airway colonization with Staphylococcus aureus (S. aureus) and biofilm formation which correlate with more severe disease phenotypes [9, 10].
In practice, CRSwNP is a heterogeneous entity with similar phenotypes having different immunologic cytokine and biomarker profiles that vary with geographic sampling. For example, a seminal study by Zhang et al. observed polyps from Western countries generated higher levels of TH2 cytokines, eosinophils, and corresponding transcription factors when compared to polyps from Southeast Asian patients which were found to have neutrophilic inflammatory signatures with elevated TH1/TH17 and elevated levels of myeloperoxidase [11]. This has been reiterated in a follow-up study demonstrating higher levels of IL-1β, IL-6, and IL-8 and lower levels of IL-5 in Southeast Asian polyps [12]. A multi-center study by Wang et al. found that greater than 50% of patients with CRSwNP in Europe, Australia, and Japan demonstrated a predominantly eosinophilic endotype compared with less than 30% patients in Southeast Asia as measured by the eosinophilic cationic protein/ myeloperoxidase ratio (ECP/MPO). This same study found levels of Staphylococcus aureus enterotoxin-specific IgE to be significantly greater in patients with CRSwNP from Europe, Australia, and Japan and significantly lower amounts in Southeast Asian cohorts suggesting a variable effect of Staphylococcus colonization in the pathogenesis of nasal polyps [13•].
Staphylococcal colonization of the nose is common with approximately 50% of the general population having intermittent nasal colonization with Staphylococcus aureus [14]. The role of nasal S. aureus colonization in the pathogenesis of CRSwNP is still being elucidated. The prevalence of sinus S. aureus colonization is not fully understood; however, 64% of patients with nasal polyps demonstrate nasal cavity colonization with S. aureus compared with only 33% and 20% of non-polyp CRS subjects and healthy controls respectively [10]. Patients colonized with specific pathogenic strains of S. aureus appear to retain the same strain over time instead of harboring different isolates over time, suggesting either a pathogenic resistance to therapeutic intervention or existence of a reservoir for recolonization [15]. Several unique virulence factors and immune-modulatory actions of S. aureus have been described which remain essential to the currently accepted theories about CRS pathogenesis. Historically, staphylococcal infection was thought to be a causative agent in the pathogenesis of CRS with recurrent infections selecting for increasingly virulent and antibiotic-resistant strains of S. aureus [16, 17]. Increasingly S. aureus colonization is appreciated to be a disease modifier that promotes immune dysregulation, barrier dysfunction, and bacterial dysbiosis leading to biofilm formation and recalcitrant disease.
Staphylococcal Toxins and CRS
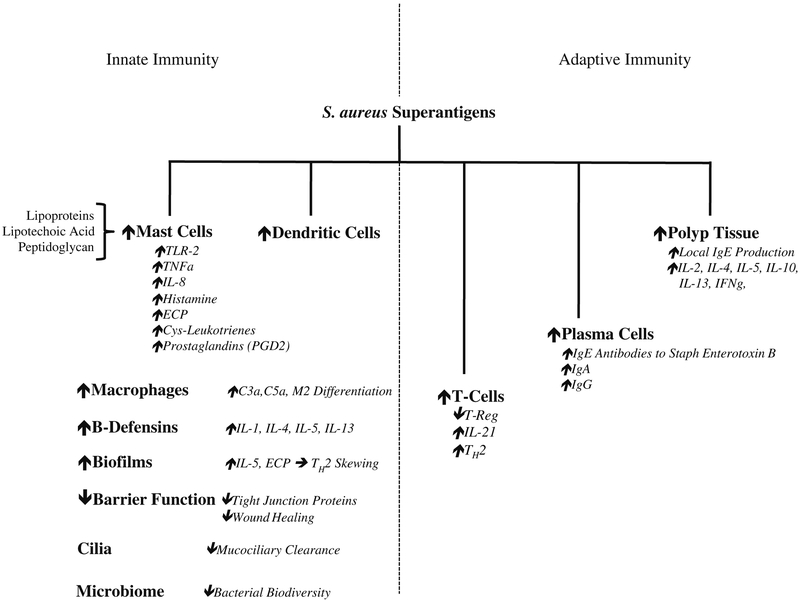
Staphylococcal strains are well known to produce many virulence factors and enterotoxins that promote inflammation including the staphylococcal superantigens. These protein toxins are potent activators of T cells which bind directly to the T cell receptor outside of the native antigen-binding site and bypass the human leukocyte antigen (HLA) class II major histocompatibility complex (MHC) of antigen presenting cells. This direct binding pathway results in excessive and uncoordinated T cell response with simultaneous B cell proliferation causing local production of polyclonal IgE and resultant eosinophil activation [18]. The massive B cell activation by superantigens is known to upregulate the production of IL-4, Il-5, and Il-13 leading to polyclonal IgE production and the release of histamine. IgE antibodies against staphylococcal superantigens are frequently identified and enriched in patients with CRSwNP and especially within the subset of patients with nasal polyposis with comorbid asthma and have been demonstrated to perpetuate eosinophilic TH2 inflammation [19]. Strains of S. aureus isolated from the middle meatus are more likely to possess accessory gene regulator (AGR) variants associated with enterotoxin-mediated disease [20]. Eicosanoid metabolism in nasal polyp tissue is regulated by the presence of staphylococcal superantigens which have dramatic effects on tissue inflammatory phenotypes. For example, S. aureus superantigens decrease the production of arachidonic acid metabolites such as prostaglandin E2 (PGE2) and the enzyme cyclooxygenase 2 (COX-2) in nasal epithelial cells cultured in vitro [21]. Conversely, higher levels of lipoxygenase pathway metabolites such as cys-leukotrienes, leukotriene B4, and lipoxin A4 in tissue homogenates from nasal polyps with high levels of IgE antibodies against staphylococcal enterotoxin B (SEB). These shifts in eicosanoid metabolism in polyp tissue with high levels of SEB suggest a pathway for innate immune dysregulation which may lead to persistent inflammation [22]. In similar experiments, Patou et al. stimulated tissue supernatants produced from inferior turbinate samples from CRS patients with SEB. In these experiments, they observed significant elevations in key pro-inflammatory cytokines, including IL1β, TNFα, IFNγ, IL-2, IL-4, IL-5, IL-10, and IL-13, indicative of a type 2-skewed inflammatory phenotype in CRSwNP samples. The frequently observed type 2-biased immune response promotes the differentiation of immunotolerant M2 macrophages which demonstrate decreased phagocytosis of S. aureus and may contribute to its persistence in CRSwNP (Fig. 1) [23].
Fig. 1.
Innate and adaptive immune responses to S. aureus superantigens that contribute to polyp formation and disease phenotypes in CRSwNP
Staphylococcal enterotoxin B has also been shown to regulate T follicular helper cells in nasal polyps by increasing the amount of IL-21 and IL-21 positive T cells in surgical sinus tissue stimulated with SEB ex vivo [24]. Takeda and colleagues have demonstrated that S. aureus is not the only bacteria that stimulate TH2 inflammation in nasal polyp tissue, however. This group isolated the specific monoclonal IgE antibody clones residing in nasal polyp plasmablasts and found specific antibodies against multiple bacteria including S. aureus, Streptococcus pyogenes, and Haemophilus influenzae. This study also provides evidence that the IgE antibodies possess a large number of mutations suggesting that somatic hypermutation plays a role in generating bacterial strain-specific antibodies. Additionally, the observed bacteria-specific IgE identified in nasal polyps share several nearly identical VH gene sequences with IgA and IgG subtypes, suggesting that the IgE may actually be derived from class-switching recombination from IgA and IgG antibodies [25•]. This observation suggests that multiple pathways contribute to IgE production and anti-staphylococcal antibody production in nasal polyp tissue. Furthermore, enterotoxins are not the only mechanism by which S. aureus exerts its TH2 skewing effect. Lan et al. demonstrated that S. aureus also directly binds to toll-like receptor 2 (TLR-2) in the absence of enterotoxin, leading to increased production of IL-5, IL-13, IL-33, and thymic stromal lymphopoietin (TSLP) in nasal polyp tissue [26].
S. aureus Effects on Sinus Epithelium
Staphylococcus genera, including coagulase-negative Staphylococci, are frequently observed to colonize the upper aerodigestive tract in health [27]. The specific interactions of the commensal bacteria and pathogenic strains such as S. aureus with the host mucosal surface in the pathogenic state are important to understand in order to delineate pathogen from bystander. In vitro studies using cultured epithelial cells from patients with CRSwNP exposed to S. aureus supernatants demonstrated significantly higher intracellular bacteria than non-polyp patients. The intracellular S. aureus also demonstrated prolonged intracellular survival as measure by an in vitro “persistence” study [28]. Taken together, these findings implicate evasion of homeostatic bacterial communities and potential local persistence by pathogenic genera suggesting local intracellular S. aureus as a reservoir for disease recurrence and/or recalcitrance. These observations need further study in the context of the host microbiome to further define the goals of treatment including better elucidating the utility of S. aureus eradication from the sinonasal tract and promoting homeostasis through restoring a diverse microbial community. Previous studies elaborating the “immune barrier hypothesis” suggested that Alternaria fungi and toxigenic strains of S. aureus facilitate immunologic and mechanical barrier disruption by promoting defective mucociliary clearance leading to bacterial invasion and degradation of intercellular tight junction proteins [29]. More recently, Altunbulaki and colleagues demonstrated that infection of healthy inferior turbinate with S. aureus upregulated the mRNA expression of several tight junction proteins including claudins, occludin, tricellulin, ZO-3, and E-cadherin whereas tissue from CRSwNP exposed to the same S. aureus infection demonstrated no change, or in some cases decreased levels of tight junction proteins [30]. This observation that staphylococcal infection can promote barrier function in homeostasis in healthy epithelium and innate immune dysfunction in patients CRSwNP exemplifies the potential importance of dysfunctional host-microbe interactions in our understanding of CRS. Wound healing is also known to be defective in CRSwNP. A recent study by Valera et al. demonstrated that S. aureus inhibited wound healing in primary epithelial cells derived from CRSwNP patients. This observation was characterized by decreased cytoskeleton organization and slower lamellipodial activity when exposed to S. aureus exoproducts in the culture medium. This effect was not observed when Rho-associated coiled-coil kinase (ROCK) inhibitors were introduced and co-incubated with the bacteria and CRSwNP cultures. This suggests that S. aureus plays a role in the poor wound healing observed in polyp patients [31•].
Biofilms Leading to CRSwNP
Biofilms form when planktonic microbes organize into threedimensional multilayered colonies wherein they release virulence factors, share nutrients, evade host defense mechanisms, and promote physical and chemical resistance to antibimicrobials [9]. The presence of bacterial biofilms in the paranasal sinuses is commonly observed in CRS with a reported prevalence of 42–80% and an even higher prevalence in CRSwNP [9, 32–35]. The most frequently reported organisms in CRS biofilms are S. aureus and Pseudomonas aeruginosa. Biofilms are known to be more prevalent in patients undergoing revision sinus surgery and thus are considered a marker of recalcitrant and severe disease [36, 32]. Foreman and colleagues demonstrated that S. aureus associated biofilms are associated with eosinophilic markers of inflammation including ECP and IL-5, suggesting a potential mechanism for Staphylococcus biofilms in the observed TH2 skewing found in CRSwNP [37].
Staphylococcus and the Microbiome
In addition to staphylococcal superantigens and biofilms, the role of staphylococci within the greater microbial community is a topic of great interest. With the widespread implementation of culture-independent methods to investigate the microbiome in health and disease, the presence of S. aureus in CRS patients is hypothesized to be a disease modifier as opposed to a primary etiologic agent [38•]. Increasingly, biodiversity (as measured by richness and evenness) is appreciated as a marker of health upper airway tissue. Using swabs from both CRS and non-CRS patients, Feazel et al. demonstrated that increased relative abundance of S. aureus correlates with decreased overall bacterial biodiversity. This study also showed that increasing antibiotic exposure correlated with lower diversity, potentially linking the two findings by suggesting that frequent antibiotic use contributed to a sustained microbiome disturbance resulting in chronic S. aureus colonization. Several studies have demonstrated that total bacterial load in patients with and without CRS is similar, but that CRS patients demonstrate expansion of pathogenic microbes—specifically S. aureus and anaerobes. Furthermore, CRSwNP—and especially the subset of these patients with comorbid asthma—have a higher relative abundance of S. aureus [39–41]. For instance, in a study by Ramakrishnan et al., 22% of healthy subjects demonstrate sinus colonization by S. aureus when compared to 15% and 24% of CRSsNP and CRSwNP patients, respectively. Interestingly, the presence of S. aureus in the patient’s with CRS and asthma was found to be 21%, suggesting similar rates of colonization rates in patients with nasal polyps and asthma. These studies also demonstrate that the increased relative abundance of Actinobacteria, specifically Propionibacterium acnes (P. acnes), is consistently associated with the healthy state. Expansion of P. acnes in sinonasal tissue is inversely correlated with S. aureus, with higher levels thought to play a role in the maintenance of a healthy microbial community while limiting the expansion of S. aureus through pathogen exclusion. A recent study by Chalermwatanachai and colleagues reproduced the earlier findings demonstrating an expansion of S. aureus in CRSwNP patients but also identified an increased abundance of the Proteobacteria and H. influenzae. These authors hypothesize that in addition to S. aureus, these Proteobacteria play an important role in establishing the type II inflammation that characterizes CRSwNP in Western countries [42].
Many hypothesize those unique core microbiome communities specific to individuals may fluctuate in their composition between the healthy, diseased, and post-surgical states. A recent long-term study of the microbiome in CRS by Koutsourelakis and colleagues demonstrates that ~25% of the sinonasal bacterial taxa remain stable over time including S. aureus. The so-called stably abundant species were present in both health and disease. However, these major species are accompanied by numerous lower prevalence taxa that are thought to alter the community dynamics of the microbial niche [43, 44]. Perhaps, the composition of these low prevalence bacteria is essential for homeostasis, and the abundance of Staphylococcus becomes clinically apparent in the setting of frequent steroids, antibiotics, and chronic inflammation.
Medical Treatment Options
Topical intranasal corticosteroids continue to be the mainstay of medical treatment CRSwNP with evidence demonstrating that it is safe and effective for improving symptoms, decreasing polyp size, and slowing polyp recurrence. Topical steroid sprays and steroid nasal rinses are also known to be more effective after endoscopic sinus surgery [45–47]. For moderate symptoms, oral corticosteroids are also indicated for the treatment of CRSwNP and have been shown in randomized, placebo-controlled trials to decreased nasal symptoms, and polyp size when taken in moderate doses [48]. S. aureus is notoriously difficult to eradicate from the sinonasal tract, and IV antibiotics have not been shown to be helpful for this purpose. Despite this, significant interest has emerged in the antimicrobial and the anti-inflammatory/immunomodulatory effects of doxycycline therapy as treatment for nasal polyps. Van Zele and colleagues performed a randomized, double-blinded, and placebo-controlled trial comparing the use of oral methylprednisone versus doxycycline in 47 CRSwNP patients followed over 12 weeks. This study found that both treatments were effective in reducing polyp size with the effect lasting slightly longer in the group treated with doxycycline when compared to methylprednisone (12 vs 8 weeks). Though this study did not look at the impact of doxycycline on S. aureus colonization, they did quantify Th2 inflammatory mediators in the nasal secretions of these patients and found lower levels of ECP, IL-5, and IgE in nasal secretions of the patients treated with methylprednisone whereas the cohort treated with doxycycline demonstrated reduced levels of ECP, myeloperoxidase, and matrix metalloproteinase 9 (MMP-9) [49]. Matrix metalloproteinases are zinc metalloproteinase enzymes that degrade extracellular matrix that may be elevated in CRSwNP and are hypothesized to cause excessive inflammation and edema contributing to polyp formation [50]. In vitro studies have demonstrated that doxycycline regulates transforming growth factor beta-1 (TGFβ-1) actions in nasal polyp tissues including the regulation of MMPs. In nasal polyp fibroblasts pretreated with TGFβ-1, the initially elevated expression MMP-2 was found to be lower with doxycycline treatment [51]. Similar studies using fibroblasts from nasal polyp patients pre-stimulated with TGFβ-1 and exposed to doxycycline had lower levels of α-smooth muscle actin (SMA) and fibronectin as measured by protein and gene expression. Additionally, doxycycline treatment reduced the total collagen expression. Taken together, these findings support the hypothesis that even if doxycycline does not fully eliminate S. aureus colonization, it likely plays a role in modulating fibroblast differentiation and extracellular matrix production in nasal polyp tissue [52].
Efforts to treat CRS using other anti-staph agents include studies of more targeted topical antibiotics such as mupirocin. Several systematic reviews have been performed to determine the efficacy of topical therapies in CRS without nasal polyps, but similar studies are lacking for CRSwNP [53]. Multiple randomized control trials have been performed to study topical antibiotic rinses for CRS but not specifically for nasal polyps. One study of recalcitrant S. aureus positive CRS demonstrated that patients receiving 1 month of large-volume mupirocin nasal rinses were less likely to have Staphpositive cultures when compared to controls at the end of the treatment period. This study also demonstrated a shortterm improvement in symptom severity scores [54]. Additionally, mupirocin rinses have been shown to be effective at treating methicillin resistant S. aureus (MRSA) detected during CRS flairs [55]. These effects are likely not permanent, but can be used in the treatment algorithm for acute infectious flare-ups as an alternative to oral antibiotics.
With the widespread availability of targeted antibody therapies for specific immune targets, new interest in the role of biologics for the treatment of CRS is emerging. Current studies of monoclonal antibody therapeutics utilize small sample sizes and specific guidelines for selecting an agent based on the endotype are lacking. However, early studies of monoclonal antibodies against TH2 inflammatory modulators show promise at reducing inflammatory phenotypes, improving symptom control, and decreasing steroid burden. The latest evidence on monoclonal antibodies the treatment of CRSwNP including anti-IgE (omalizumab), IL-4 (dupilumab), and IL-5 (reslizumab and mepolizumab) are summarized in a review by Lane and Halderman [56•]. Recent data has also shown the ability of monoclonal antibodies to reduce the need for surgery in patients with severe recurrent CRSwNP. A recent randomized, placebo-controlled trial of 105 patients by the Bachert group demonstrated that patients with severe CRSwNP receiving mepolizumab no longer required surgery as measured by the nasal polyposis severity visual analog scale (VAS) score after 4 weeks of treatment [57]. These studies did not include culture data to quantify the effects of immunotherapy treatment on S. aureus carriage. However, these early data demonstrating improvement in disease severity with immunotherapy reflects the nature of CRSwNP as an inflammatory disease characterized by immune dysregulation wherein S. aureus serves as an important disease modifier instead of the etiological agent.
Role of Surgery
Endoscopic sinus surgery remains an important treatment option for restoring patency and aeration to the sinuses and improving the delivery of topical therapies to the nasal epithelium. Surgery is more commonly performed for CRSwNP than CRSsNP. This may be due to the unfortunate circumstance of disease recurrence over time in CRSwNP, or owing to the observation of large quality of life gains in surgery for CRSwNP. Roughly, 30% of all sinus surgery is performed for CRSwNP. These surgeries are usually longer, more extensive, and more likely to use image guidance than for surgeries for non-polyp CRS [58]. Reporting of primary outcomes from sinus surgery is variable and relies on nasal endoscopy, radiographic findings, or self-reported symptom instruments such as the SNOT-22 survey. A recent systematic review by Le et al. considered 3048 patients with CRSwNP from 15 articles. In this meta-analysis, patient SNOT-22 scores improved significantly by a mean of 23 points with the greatest improvement observed in patients with older age, higher preoperative SNOT-22, prior sinus surgery, and asthma [59]. Recurrence rates of CRS have been reported in a large retrospective cohort study of 61,000 patients which found that the revision ESS rate is 6.65%. This same study used multivariable regression modeling to identify patients with CRSwNP as being at higher risk of having revision surgery [60]. The impact of S. aureus on the effectiveness of ESS has not been studied in detail, but variations in the nasal microbiome may impact the outcome of sinus surgery [61]. Exploratory studies demonstrate that increased biodiversity (as measured by richness, evenness, and complexity) portends better outcomes for patients undergoing ESS. The currently performed studies on the microbiome effects on surgical outcomes are not adequately powered to understand the exact role of staphylococcal expansion within the microbiome on surgical outcomes [41].
Discussion/Conclusion
S. aureus remains an important bacteriologic suspect in the pathogenesis of CRSwNP. S. aureus is known to regulate both innate and adaptive immunity by promoting type II inflammation, disrupt tissue barrier function, promote impaired mucociliary clearance, and drive polyp formation. Although there is a high prevalence of S. aureus in CRSwNP, not all patients with polyps are colonized with toxinogenic strains of Staphylococcus, and therefore its superantigen and barrier dysfunction contribution to CRSwNP are incomplete. Many questions persist about the exact importance of S. aureus in the nasal microbiome as it relates to severe recalcitrant disease as is frequently seen in nasal polyps. The lack of effect of anti-staphylococcal therapeutics suggests that eradicating S. aureus may not be a primary goal of CRSwNP treatment.
Though we now appreciate the inflammatory nature of CRSwNP, there still may be a large role for staphylococcal superinfections during acute exacerbations. Perhaps the presence of S. aureus in upper airway CRSwNP is a contributor to a larger eosinophilic state with chronic allergic and asthmatic disease representing a continuum of the unified upper and lower airways. Further efforts are needed understand the specific roles of S. aureus as an immune modulator, bacterial community member, and disease modifier for CRSwNP in order to understand its potential as a therapeutic target, reduce perioperative antibiotic burden, and understand the specific qualities that contribute to microbial homeostasis in the sinuses.
Acknowledgments
Funding Details This work was supported by the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD., USA, under grants K23DC014747 and T32DC012280. This funding organization did not contribute to the design or conduct of this study: preparation, review, approval, or decision to submit this manuscript for publication.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
••Of major importance
- 1.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Ashok Kumar K, Kramper M, et al. Clinical practice guideline (update): adult sinusitis executive summary. Otolaryngol Head Neck Surg. 2015;152:598–609. [DOI] [PubMed] [Google Scholar]
- 2.Fokkens WJ et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;3. Preceding table of contents–1–298 [PubMed] [Google Scholar]
- 3.DeConde AS, Soler ZM. Chronic rhinosinusitis: epidemiology and burden of disease. Am J Rhinol Allergy. 2016;30:134–9. [DOI] [PubMed] [Google Scholar]
- 4.Stevens WW, Peters AT, Suh L, Norton JE, Kern RC, Conley DB, et al. A retrospective, cross-sectional study reveals that women with CRSwNP have more severe disease than men. Immun Inflamm Dis. 2015;3:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.••.Derycke L, Perez-Novo C, Van Crombruggen K, Corriveau M-N, Bachert C. Staphylococcus aureus and chronic airway disease. World Allergy Organ J. 2010;3:223–8Comprehensive overview of the role of S. aureus. in upper airway disease.
- 6.Van Zele T, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–9. [DOI] [PubMed] [Google Scholar]
- 7.Mygind N, Dahl R, Bachert C. Nasal polyposis, eosinophil dominated inflammation, and allergy. Thorax. 2000;55(Suppl 2):S79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foreman A, Holtappels G, Psaltis AJ, Jervis-Bardy J, Field J, Wormald PJ, et al. Adaptive immune responses in Staphylococcus aureus biofilm-associated chronic rhinosinusitis. Allergy. 2011;66:1449–56. [DOI] [PubMed] [Google Scholar]
- 9.Suh JD, Ramakrishnan V, Palmer JN. Biofilms. Otolaryngol Clin N Am. 2010;43:521–30 viii. [DOI] [PubMed] [Google Scholar]
- 10.van Zele T, Gevaert P, Watelet JB, Claeys G, Holtappels G, Claeys C, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004;114:981–3. [DOI] [PubMed] [Google Scholar]
- 11.Zhang N, van Zele T, Perez-Novo C, van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–8. [DOI] [PubMed] [Google Scholar]
- 12.Ba L, Zhang N, Meng J, Zhang J, Lin P, Zhou P, et al. The association between bacterial colonization and inflammatory pattern in Chinese chronic rhinosinusitis patients with nasal polyps. Allergy. 2011;66:1296–303. [DOI] [PubMed] [Google Scholar]
- 13.•.Wang X, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138:1344–53.Multicenter study examining geographic variation of inflammatory cytokine profiles of CRS.
- 14.Frank DN, Feazel LM, Bessesen MT, Price CS, Janoff EN, Pace NR. The human nasal microbiota and Staphylococcus aureus carriage. PLoS One. 2010;5:e10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drilling A, Coombs GW, Tan HL, Pearson JC, Boase S, Psaltis A, et al. Cousins, siblings, or copies: the genomics of recurrent Staphylococcus aureus infections in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4:953–60. [DOI] [PubMed] [Google Scholar]
- 16.Doyle PW, Woodham JD. Evaluation of the microbiology of chronic ethmoid sinusitis. J Clin Microbiol. 1991;29:2396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manarey CRA, Anand VK, Huang C. Incidence of methicillin-resistant Staphylococcus aureus causing chronic rhinosinusitis. Laryngoscope. 2004;114:939–41. [DOI] [PubMed] [Google Scholar]
- 18.Schubert MS. A superantigen hypothesis for the pathogenesis of chronic hypertrophic rhinosinusitis, allergic fungal sinusitis, and related disorders. Ann Allergy Asthma Immunol. 2001;87:181–8. [DOI] [PubMed] [Google Scholar]
- 19.Zhang N, Gevaert P, van Zele T, Perez-Novo C, Patou J, Holtappels G, et al. An update on the impact of Staphylococcus aureus enterotoxins in chronic sinusitis with nasal polyposis. Rhinology. 2005;43:162–8. [PubMed] [Google Scholar]
- 20.van Zele T, Vaneechoutte M, Holtappels G, Gevaert P, van Cauwenberge P, Bachert C. Detection of enterotoxin DNA in Staphylococcus aureus strains obtained from the middle meatus in controls and nasal polyp patients. Am J Rhinol. 2008;22:223–7. [DOI] [PubMed] [Google Scholar]
- 21.Pérez Novo CA, Waeytens A, Claeys C, Cauwenberge PV, Bachert C. Staphylococcus aureus enterotoxin B regulates prostaglandin E2 synthesis, growth, and migration in nasal tissue fibroblasts. J Infect Dis. 2008;197:1036–43. [DOI] [PubMed] [Google Scholar]
- 22.Pérez-Novo CA, et al. Eicosanoid metabolism and eosinophilic inflammation in nasal polyp patients with immune response to Staphylococcus aureus enterotoxins. Am J Rhinol. 2006;20:456–60. [DOI] [PubMed] [Google Scholar]
- 23.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. [DOI] [PubMed] [Google Scholar]
- 24.Calus L, Derycke L, Dullaers M, van Zele T, de Ruyck N, Pérez-Novo C, et al. IL-21 is increased in nasal polyposis and after stimulation with Staphylococcus aureus enterotoxin B. Int Arch Allergy Immunol 2017;174:161–9. [DOI] [PubMed] [Google Scholar]
- 25.•.Takeda K, et al. Allergic conversion of protective mucosal immunity against nasal bacteria in patients with chronic rhinosinusitis with nasal polyposis. J Allergy Clin Immunol. 2018. 10.1016/j.jaci.2018.07.006.Study suggesting that immunoglobulin class switching of B cells (from IgA and IgG to IgE) during the mucosal response against nasal bacteria contributes to the IgE phenotyope often observed in CRSwNP.
- 26.Lan F, Zhang N, Holtappels G, de Ruyck N, Krysko O, van Crombruggen K, et al. Staphylococcus aureus induces a mucosal type 2 immune response via epithelial cell-derived cytokines. Am J Respir Crit Care Med. 2018;198:452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramakrishnan VR, Feazel LM, Gitomer SA, Ir D, Robertson CE, Frank DN. The microbiome of the middle meatus in healthy adults. PLoS One. 2013;8:e85507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachse F, Becker K, von Eiff C, Metze D, Rudack C. Staphylococcus aureus invades the epithelium in nasal polyposis and induces IL-6 in nasal epithelial cells in vitro. Allergy. 2010;65:1430–7. [DOI] [PubMed] [Google Scholar]
- 29.Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008;22:549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altunbulakli C, et al. Staphylococcus aureus enhances the tight junction barrier integrity in healthy nasal tissue, but not in nasal polyps. J Allergy Clin Immunol. 2018;142:665–668.e8. [DOI] [PubMed] [Google Scholar]
- 31.•.Valera FCP, et al. Staphylococcus aureus impairs sinonasal epithelial repair: effects in patients with chronic rhinosinusitis with nasal polyps and control subjects. J Allergy Clin Immunol. 2018. 10.1016/j.jaci.2018.05.035.Study demonstrating the negative impact of Staphylococcus on wound healing in CRSwNP.
- 32.Singh P, Mehta R, Agarwal S, Mishra P. Bacterial biofilm on the sinus mucosa of healthy subjects and patients with chronic rhinosinusitis (with or without nasal polyposis). J Laryngol Otol. 2015;129:46–9. [DOI] [PubMed] [Google Scholar]
- 33.Sanderson AR, Leid JG, Hunsaker D. Bacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitis. Laryngoscope. 2006;116:1121–6. [DOI] [PubMed] [Google Scholar]
- 34.Sanclement JA, Webster P, Thomas J, Ramadan HH. Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis. Laryngoscope. 2005;115:578–82. [DOI] [PubMed] [Google Scholar]
- 35.Dlugaszewska J, Leszczynska M, Lenkowski M, Tatarska A, Pastusiak T, Szyfter W. The pathophysiological role of bacterial biofilms in chronic sinusitis. Eur Arch Otorhinolaryngol. 2016;273:1989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prince AA, et al. Prevalence of biofilm-forming bacteria in chronic rhinosinusitis. Am J Rhinol. 2008;22:239–45. [DOI] [PubMed] [Google Scholar]
- 37.Foreman A, Holtappels G, Psaltis AJ, Jervis-Bardy J, Field J, Wormald PJ, et al. Adaptive immune responses in Staphylococcus aureus biofilm-associated chronic rhinosinusitis. Allergy. 2011;66:1449–56. [DOI] [PubMed] [Google Scholar]
- 38.•.Vickery TW, Ramakrishnan VR Bacterial pathogens and the microbiome. Otolaryngol Clin N Am. 2017;50:29–47.Review article summarizing the current understanding of host-microbe interactions and their role in CRS.
- 39.Abreu NA, et al. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Transl Med. 2012;4:151ra124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramakrishnan VR, Feazel LM, Abrass LJ, Frank DN. Prevalence and abundance of Staphylococcus aureus in the middle meatus of patients with chronic rhinosinusitis, nasal polyps, and asthma. Int Forum Allergy Rhinol. 2013;3:267–71. [DOI] [PubMed] [Google Scholar]
- 41.Ramakrishnan VR, et al. Sinus microbiota varies among chronic rhinosinusitis phenotypes and predicts surgical outcome. J Allergy Clin Immunol. 2015;136:334–42.e1. [DOI] [PubMed] [Google Scholar]
- 42.Chalermwatanachai T, Vilchez-Vargas R, Holtappels G, Lacoere T, Jáuregui R, Kerckhof FM, et al. Chronic rhinosinusitis with nasal polyps is characterized by dysbacteriosis of the nasal microbiota. Sci Rep. 2018;8:7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koutsourelakis I, Halderman A, Khalil S, Hittle LE, Mongodin EF, Lane AP Temporal instability of the post-surgical maxillary sinus microbiota. BMC Infect Dis. 2018;18:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frank DN, Zhu W, Sartor RB, Li E. Investigating the biological and clinical significance of human dysbioses. Trends Microbiol. 2011;19:427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chong LY, et al. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:CD011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marcela FC, Macdonald KI, Lee J, Witterick IJ. The use of postoperative topical corticosteroids in chronic rhinosinusitis with nasal polyps: a systematic review and meta-analysis. Am J Rhinol Allergy. 2013;27:e146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon HY, Lee HS, Kim IH, Hwang SH. Post-operative corticosteroid irrigation for chronic rhinosinusitis after endoscopic sinus surgery: a meta-analysis. Clin Otolaryngol. 2018;43:525–32. [DOI] [PubMed] [Google Scholar]
- 48.Hissaria P, Smith W, Wormald PJ, Taylor J, Vadas M, Gillis D, et al. Short course of systemic corticosteroids in sinonasal polyposis: a double-blind, randomized, placebo-controlled trial with evaluation of outcome measures. J Allergy Clin Immunol. 2006;118:128–33. [DOI] [PubMed] [Google Scholar]
- 49.van Zele T, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. J Allergy Clin Immunol. 2010;125:1069–1076.e4. [DOI] [PubMed] [Google Scholar]
- 50.Watelet JB, Bachert C, Claeys C, van Cauwenberge P. Matrix metalloproteinases MMP-7, MMP-9 and their tissue inhibitor TIMP-1: expression in chronic sinusitis vs nasal polyposis. Allergy. 2004;59:54–60. [DOI] [PubMed] [Google Scholar]
- 51.Shin J-M, et al. Effect of doxycycline on transforming growth factor-beta-1-induced matrix metalloproteinase 2 expression, migration, and collagen contraction in nasal polyp-derived fibroblasts. Am J Rhinol Allergy. 2016;30:385–90. [DOI] [PubMed] [Google Scholar]
- 52.Shin J-M, Park J-H, Park I-H, Lee H-M. Doxycycline inhibits TGF-β1-induced extracellular matrix production in nasal polyp-derived fibroblasts. Int Forum Allergy Rhinol. 2016;6:256–63. [DOI] [PubMed] [Google Scholar]
- 53.Rudmik L, Soler ZM. Medical therapies for adult chronic sinusitis: a systematic review. JAMA. 2015;314:926–39. [DOI] [PubMed] [Google Scholar]
- 54.Jervis-Bardy J, Boase S, Psaltis A, Foreman A, Wormald P-J. A randomized trial of mupirocin sinonasal rinses versus saline in surgically recalcitrant staphylococcal chronic rhinosinusitis. Laryngoscope. 2012;122:2148–53. [DOI] [PubMed] [Google Scholar]
- 55.Solares CA, Batra PS, Hall GS, Citardi MJ. Treatment of chronic rhinosinusitis exacerbations due to methicillin-resistant Staphylococcus aureus with mupirocin irrigations. Am J Otolaryngol. 2006;27:161–5. [DOI] [PubMed] [Google Scholar]
- 56.•.Halderman AA, Lane AP. Immunomodulators in the treatment of nasal polyposis. Adv Otorhinolaryngol. 2016;79:103–13.Comprehensive review on the current data utilizing biologic immunomodulators for the treatment of CRSwNP.
- 57.Bachert C, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. J Allergy Clin Immunol. 2017;140:1024–1031.e14. [DOI] [PubMed] [Google Scholar]
- 58.Ference EH, Suh JD, Tan BK, Smith SS. How often is sinus surgery performed for chronic rhinosinusitis with versus without nasal polyps? Am J Rhinol Allergy 2018;32:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le PT, et al. Systematic review and meta-analysis of SNOT-22 outcomes after surgery for chronic rhinosinusitis with nasal polyposis. Otolaryngol Head Neck Surg. 2018;159:414–23. [DOI] [PubMed] [Google Scholar]
- 60.Miglani A, Divekar RD, Azar A, Rank MA, Lal D. Revision endoscopic sinus surgery rates by chronic rhinosinusitis subtype. Int Forum Allergy Rhinol. 2018;8:1047–51. [DOI] [PubMed] [Google Scholar]
- 61.Singhal D, Foreman A, Jervis-Bardy J, Bardy J-J, Wormald P-J. Staphylococcus aureus biofilms: nemesis of endoscopic sinus surgery. Laryngoscope. 2011;121:1578–83. [DOI] [PubMed] [Google Scholar]