Abstract
Rationale: Honeycombing on chest computed tomography (CT) has been described in diverse forms of interstitial lung disease (ILD); however, its prevalence and association with mortality across the spectrum of ILD remains unclear.
Objective: To determine the prevalence and prognostic value of CT honeycombing and characterize associated mortality patterns across diverse ILD subtypes in a multicenter cohort.
Methods: This was an observational cohort study of adult participants with multidisciplinary or adjudicated ILD diagnosis and documentation of chest CT imaging at index diagnosis across five U.S. hospitals (one tertiary and four nontertiary medical centers). Participants were stratified based on presence or absence of CT honeycombing. Vital status was determined from review of medical records and social security death index. Transplant-free survival was analyzed using univariate and multivariable Cox regression.
Results: The sample comprised 1,330 participants (mean age, 66.8 yr; 50% men) with 4,831 person-years of follow-up. The prevalences of CT honeycombing were 42.0%, 41.9%, 37.6%, and 28.6% in chronic hypersensitivity pneumonitis, connective tissue disease–related ILD (CTD-ILD), idiopathic pulmonary fibrosis (IPF), and unclassifiable/other ILDs, respectively. Among those with CT honeycombing, cumulative mortality hazards were similar across ILD subtypes, except for CTD-ILD, which had a lower mortality hazard. Overall, the mean survival time was shorter among those with CT honeycombing (107 mo; 95% confidence interval [CI], 92–122 mo) than those without CT honeycombing (161 mo; 95% CI, 147–174 mo). CT honeycombing was associated with an increased mortality rate (hazard ratio, 1.72; 95% CI, 1.38–2.14) even after adjustment for center, sex, age, forced vital capacity, diffusing capacity, ILD subtype, and use of immunosuppressive therapy (hazard ratio, 1.62; 95% CI, 1.29–2.02). CT honeycombing was associated with an increased mortality rate within non-IPF ILD subgroups (chronic hypersensitivity pneumonitis, CTD-ILD, and unclassifiable/other ILD). In IPF, however, mortality rates were similar between those with and without CT honeycombing.
Conclusions: CT honeycombing is prevalent in diverse forms of ILD and uniquely identifies a progressive fibrotic ILD phenotype with a high mortality rate similar to IPF. CT honeycombing did not confer additional risk in IPF, which is already known to be a progressive fibrotic ILD phenotype regardless of the presence of CT honeycombing.
Keywords: interstitial lung disease, mortality, pulmonary fibrosis, CT, honeycombing
The interstitial lung diseases (ILD) are a group of heterogeneous complex lung disorders frequently characterized by parenchymal fibrosis (1, 2). The most common of these fibrotic lung diseases is idiopathic pulmonary fibrosis (IPF), a disorder in which lung function declines progressively, with death ensuing within 3 to 5 years (3, 4). Recognized features of pulmonary fibrosis on high-resolution computed tomography (CT) chest imaging include reticulation (short, irregular, linear opacities) (1), bronchiectasis/bronchiolectasis (ranges from subtle irregularity and nontapering of the bronchial/bronchiolar wall to marked airway distortion and varicosity) (5), and honeycombing (5). Honeycombing is a key CT characteristic in IPF and is defined as clustered cystic airspaces of comparable diameters (∼3–10 mm) that are usually subpleural with thick, well-defined walls (5). IPF, the most lethal form of pulmonary fibrosis, can be diagnosed in persons with no identifiable cause, when the high-resolution CT imaging shows predominantly peripheral and lower lobe bilateral reticulation and honeycombing in a pattern, termed “usual interstitial pneumonia” (UIP) (1, 5).
The 2011 consensus guidelines by the American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Association state that the extent of CT honeycombing independently predicts mortality in participants with IPF (2, 6, 7). Therefore, extent of honeycombing is frequently assessed on chest CT scans during evaluation for IPF; however, agreement on quantitative estimates is only moderate, even among expert thoracic radiologists (8). Paradoxically, although participants with IPF often have microscopic honeycombing on lung biopsy specimens (9), they may lack evidence of CT honeycombing, develop CT honeycombing at a later time (10), or even undergo diagnostic reclassification after additional workup (11). These phenotypic complexities further compound the practical utility of CT honeycombing in its diagnostic specificity for IPF.
Although reticulation with or without traction bronchiectasis is common in non-IPF ILDs (1), recent studies suggest that CT honeycombing may also be evident in these ILD subtypes (3, 12–18). However, the prevalence, prognostic impact, and associated mortality patterns of CT honeycombing across the diverse spectrum of ILD remain poorly understood. As diagnostic reclassification to alternate ILD subtypes is common in participants initially classified as IPF (11, 19), and different progressive fibrotic ILDs frequently share similar phenotypes (14), we hypothesized that, irrespective of ILD classification, the radiologic presence of CT honeycombing would be prevalent and predict reduced survival across diverse ILD subtypes. Thus, we assessed if CT honeycombing was associated with mortality independent of ILD subtype or clinical disease severity and characterized the mortality patterns in study participants with CT honeycombing. This work was presented at the American Thoracic Society 2018 International Conference (20).
Methods
We performed an observational cohort study of data from five U.S. hospitals (one tertiary medical center [University of Chicago Hospitals] and four nontertiary medical centers [Evanston Hospital, Highland Park Hospital, Glenbrook Hospital, and Skokie Hospital]; see Figure E1 in the online supplement). Local research ethics committees approved the study (#IRB16-1062 [tertiary medical center]; #EH17-025 [nontertiary medical centers]). Adult participants (≥18 yr) with International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes (ICD-9 codes 495.xx, 515.xx, 516.xx, or 517.xx) between January 2006 and July 2016 were identified. Cases were identified and pertinent clinical data extracted from reviewed electronic charts with the assistance of the Clinical Research Data Warehouse maintained by the Center for Research Informatics at University of Chicago (tertiary medical center), and NorthShore HealthSystem Clinical Research Data Analytics unit (nontertiary medical centers). Ascertainment of the extracted data was performed in conjunction with study pulmonologists as previously described (A.A., J.M.O., S.K.B., R.V., and I.N.) (21, 22). Participants with ICD-9 diagnosis codes for sarcoidosis were excluded. Participants with an eventual multidisciplinary diagnosis (MDD) of sarcoidosis were also excluded. All study participants underwent a multidisciplinary evaluation or independent adjudication of ILD diagnosis as previously described (21).
MDD was performed using available clinical data, pulmonary function tests, high-resolution computed tomography (HRCT) scans, and surgical lung biopsies according to current American Thoracic Society/European Respiratory Society criteria (2, 23–26). An assessment of multidisciplinary diagnosis of ILD (MDD-ILD) was performed by pulmonologists in conjunction with rheumatologists, dedicated thoracic radiologists, and a thoracic pathologist. As the four nontertiary hospitals are suburban community hospitals that do not document multidisciplinary discussions in a standardized fashion available to researchers, an independent adjudication panel with expertise in ILD (J.M.O., R.V., and I.N.) evaluated clinical data among participants who received an ICD-9 code–based ILD diagnosis from the four nontertiary medical centers. The determination of an adjudicated ILD diagnosis was performed in a blinded fashion among all participants who had clinical information available for review as previously described (21, 22). Participants were included if they had available documentation of index chest CT imaging within the electronic medical records and a multidisciplinary or adjudicated diagnosis of chronic ILD on the basis of clinical, pulmonary function, radiologic, and/or histopathologic evaluation.
Among participants meeting inclusion criteria, pertinent demographic, clinical, pulmonary function, and laboratory data were abstracted from the medical records of the initial ILD clinic evaluation. Participants were stratified based on presence or absence of honeycombing as documented on the chest CT imaging report, confirmed by imaging reread in a large subset of participants to determine the internal consistency of our findings. Independent assessment of chest CT imaging for rereads was performed by two dedicated thoracic radiologists (S.M. and J.H.C.) who had 33 and 12 years of thoracic imaging experience and were blinded to all other clinical data. Both thoracic radiologists jointly assessed each HRCT scan for consensus agreement on the presence or absence of honeycombing defined as clustered cystic airspaces, typically of comparable diameters ∼3 to 10 mm that are usually subpleural with well-defined walls (5, 12). HRCT scans were defined as noncontrast scans with volumetric acquisition of submillimeter collimation images with high pitch and short rotation times reconstructed as contiguous or overlapping thin-section CT images using a high-spatial-frequency iterative algorithm. Image acquisition consisted of inspiratory and expiratory supine scans with or without prone imaging. HRCT images were generated with lung kernel reconstructed pixels. A summary of the CT machines and reconstruction protocols used is outlined in Table E1. Vital status was determined from review of medical records and social security death index. Follow-up time was censored on July 31, 2016.
Statistical Analyses
In the main analyses, we conducted hypothesis testing using frequency counts of CT honeycombing as a categorical variable to determine prevalence within predefined categories of ILD subtypes. To examine the association of CT honeycombing and all-cause mortality, presence of CT honeycombing was treated as a binary variable, and duration of follow-up was treated as a continuous variable. Cox proportional hazard models were used for hazard ratio estimation.
We constructed a parsimonious Cox regression model with covariates on the basis of the study hypothesis. Covariate selection for inclusion in the model was based on potential confounding variables known to affect ILD disease severity and mortality (Figure E2), and missing covariates were infrequent in the study cohort (<10%). Univariate and multivariable Cox regression were used to examine associations between honeycombing on index chest CT scan and the hazard ratio (HR) for death. We assessed for effect modification through models stratified on distinct ILD subtypes and tertiary and nontertiary hospital centers. Models were adjusted for potential confounders selected a priori: hospital center and known prognostic determinants in ILD such as age, sex, immunosuppressive therapy, and physiologic indices of disease severity such as forced vital capacity (FVC) and diffusing capacity of the lung for carbon monoxide (DlCO). In the analyses of all multivariable outcome models, center was controlled for as a random effect. Sensitivity analyses were performed in which we used multiple imputation using chained equations to account for missing covariate values. Additional sensitivity analysis was performed in which the HRCT images reread by thoracic radiologists were jointly scored for the semiquantitative extent of honeycombing and its prognostic value assessed.
We calculated survival time as time from CT performance to death, lung transplantation, loss to follow-up, or end of study period. Survival time was censored on July 31, 2016 or when a participant was lost to follow-up. Survival curves are plotted using the Kaplan-Meier survival estimator. Estimates of the hazard functions were analyzed using the Nelson-Aalen cumulative hazard estimator (StataCorp 2017; R.15).
Results
Of 11,143 participants screened, 1,330 participants with MDD or independently adjudicated ILD across tertiary medical centers (n = 852, 64.1%) and nontertiary centers (n = 478, 35.9%) met inclusion criteria (mean age, 66.8 yr; 50% men) (Figure E1).
Prevalence of CT Honeycombing
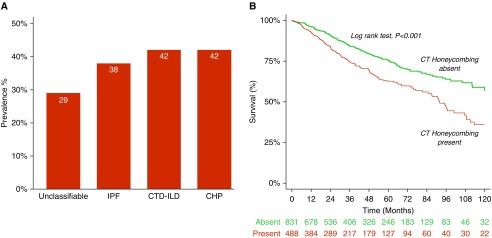
Among these, 492 (37.0%) participants with CT honeycombing were identified (IPF, n = 240, 48.8%; connective tissue disease–related ILD [CTD-ILD], n = 111, 22.6%; unclassifiable/other ILD, n = 81, 16.5%; and chronic hypersensitivity pneumonitis [CHP], n = 60, 12.2%, respectively) (Tables 1 and E2). The overall agreement between original documentation of CT honeycombing and imaging re-read in the large subset assessed by expert radiologists was 68% (kappa, 0.36; 95% confidence interval [CI], 0.26–0.46; P < 0.001; Table E3). Participants with CT honeycombing differed with regard to baseline demographics, clinical characteristics, and lung function (Tables 1 and E2). CT honeycombing was prevalent in the pooled ILD population and within distinct ILD subcategories (CHP, 42.0%; CTD-ILD, 41.9%; IPF, 37.6%; and unclassifiable/other ILDs, 28.6%, respectively; Table 1, Figure 1A). CT honeycombing was also prevalent in participants with ILD at the tertiary medical center (n = 335, 39.3%) and the nontertiary centers (n = 157, 32.9%), respectively.
Table 1.
Baseline characteristics of study participants stratified by computed tomography honeycombing
| Characteristics | CT Honeycombing Absent (n = 838) | CT Honeycombing Present (n = 492) |
|---|---|---|
| Age, yr | 66.1 (13.2) | 68.1 (11.8) |
| Male | 390 (46.5) | 270 (54.9) |
| Race/ethnicity | ||
| White | 636 (75.9) | 373 (75.8) |
| Black | 110 (13.1) | 65 (13.2) |
| Hispanic | 37 (4.4) | 27 (5.5) |
| Asian | 40 (4.8) | 20 (4.1) |
| Ever smoker | 329 (39.7) | 245 (49.8) |
| Body mass index, kg/m2 | 29.8 (6.9) | 28.2 (5.7) |
| Clinical features | ||
| Crackles | 519 (76.7) | 352 (84.0) |
| Clubbing | 74 (8.8) | 87 (17.7) |
| Lung function | ||
| FVC, % predicted | 68.9 (19.4) | 65.9 (18.8) |
| DlCO, % predicted | 52.3 (21.8) | 46.1 (19.6) |
| Oxygen therapy | 228 (27.2) | 172 (35.0) |
| Immunosuppressive therapy | 409 (50.0) | 259 (54.0) |
| CT fibrosis | ||
| Reticulation | 532 (63.5) | 328 (66.7) |
| Traction bronchiectasis | 157 (18.7) | 242 (49.2) |
| Antifibrotic therapy | 63 (7.5) | 38 (7.7) |
| Time from CT to ILD diagnosis, mo | 3.5 (23.1) | 2.6 (24.0) |
| ILD subcategory | ||
| Idiopathic pulmonary fibrosis | 399 (47.6) | 240 (48.8) |
| Connective tissue disease–associated ILD | 154 (18.4) | 111 (22.6) |
| Unclassifiable/others | 202 (24.1) | 81 (16.5) |
| Chronic hypersensitivity pneumonitis | 83 (9.9) | 60 (12.2) |
Definition of abbreviations: CT = computed tomography; DlCO = diffusing capacity of the lung for carbon monoxide; FVC = forced vital capacity; ILD = interstitial lung disease; SD = standard deviation.
Total sample size, N = 1,330. Categorical variables presented as n (%); continuous variables presented as mean (SD). Exception for participants: body mass index, n = 1,247; crackles, n = 1,096; clubbing, n = 1,329; FVC, n = 1,265; DlCO, n = 1,191; immunosuppressive therapy, n = 1,302; idiopathic pulmonary fibrosis, n = 639; connective tissue disease–associated ILD, n = 265; chronic hypersensitivity pneumonitis, n = 143; unclassifiable/other ILD, n = 283.
Figure 1.
(A) Computed tomography (CT) honeycombing is prevalent across diverse interstitial lung disease (ILD) subtypes; (B) Kaplan-Meier analysis demonstrates worsened 10-year survival in participants with ILD and CT honeycombing. CHP = chronic hypersensitivity pneumonitis; CTD-ILD = connective tissue disease–associated ILD; IPF = idiopathic pulmonary fibrosis.
Mortality in Participants with CT-Honeycombing
A total of 330 participants died over 4,831 person-years of follow-up. The mean survival time among participants with CT honeycombing was 107 months (95% CI, 92–122 mo) compared with 161 months (95% CI, 147 to 174 mo) in participants without CT honeycombing over the study period. Lung transplantation occurred in 3.9% (n = 19) of participants with CT honeycombing versus 2.7% (n = 23) of participants without CT honeycombing (Table 2).
Table 2.
Associations between computed tomography honeycombing and mortality
| Characteristics | CT Honeycombing Absent | CT Honeycombing Present | P Value |
|---|---|---|---|
| Participants | n = 838 | n = 492 | |
| Survival time, mo, mean (95% CI) | 161 (147–174) | 107 (92–122) | <0.001 |
| Number of deaths, n (%) | 170 (20.3) | 160 (32.5) | <0.001 |
| Crude mortality rate (events per 100 person-years) | 5.0 | 9.0 | <0.001 |
| Unadjusted hazard ratio* (95% CI) | — | 1.72 (1.38–2.14) | <0.001 |
| Adjusted hazard ratio*† (95% CI) | — | 1.62 (1.29–2.02) | <0.001 |
| Lung transplantation, n (%) | 23 (2.7) | 19 (3.9) | 0.26 |
Definition of abbreviations: CI = confidence interval; CT = computed tomography.
Computed using Cox proportional hazard models.
Adjusted for hospital center, sex, age, forced vital capacity, diffusing capacity of the lungs for carbon monoxide, interstitial lung disease subtype, and immunosuppressive therapy. Unadjusted model, n = 1,330; adjusted model, n = 1,232.
The crude mortality rate was higher in participants with CT honeycombing (9.0 deaths per 100 person-years) than in those without CT honeycombing (5.0 deaths per 100 person-years) (Table 2). Thirty-three percent (160 of 492) of those with CT honeycombing documented on the index chest CT imaging report died, compared with 20% (170 of 838) of those without documentation of CT honeycombing (P < 0.001; Table 2). Participants with CT honeycombing had an increased mortality rate (HR, 1.72; 95% CI, 1.38–2.14; P < 0.001) when compared with those without CT honeycombing, even after adjusting for hospital center, sex, age, FVC, DlCO, ILD subtype, and use of immunosuppressive therapy (HR, 1.62; 95% CI, 1.29–2.02) (Table 2). Sensitivity analyses performing multiple imputation using chained equations to account for missing covariate values demonstrated similar results (Table E4). Further adjustment for the presence of other forms of radiographic fibrosis (reticulation with or without traction bronchiectasis) did not significantly alter the statistical estimates of our analyses (Table E5).
Analysis of the subgroup with imaging reread (n = 361) showed similar results in participants with CT honeycombing (HR, 2.21; 95% CI, 1.51–3.23), even after covariate adjustment (HR, 1.44; 95% CI, 0.97–2.14) (Tables E6–E8). Additional sensitivity analysis assessing the prognostic value of semiquantitatively scoring HRCT images for extent of honeycombing showed similar results. The mortality rate increased by 40% for each 5% increase in the extent of CT honeycombing. Results remained consistent across tertiary (HR, 1.85; 95% CI, 1.43–2.38) and nontertiary hospitals (HR, 1.29; 95% CI, 0.90–1.86), even after covariate adjustment (HR, 1.49; 95% CI, 1.14–1.94; and HR, 1.44; 95% CI, 0.99–2.09, respectively), but this association in the nontertiary cohort was imprecise (Table E9).
Mortality in ILD Subtypes with CT Honeycombing
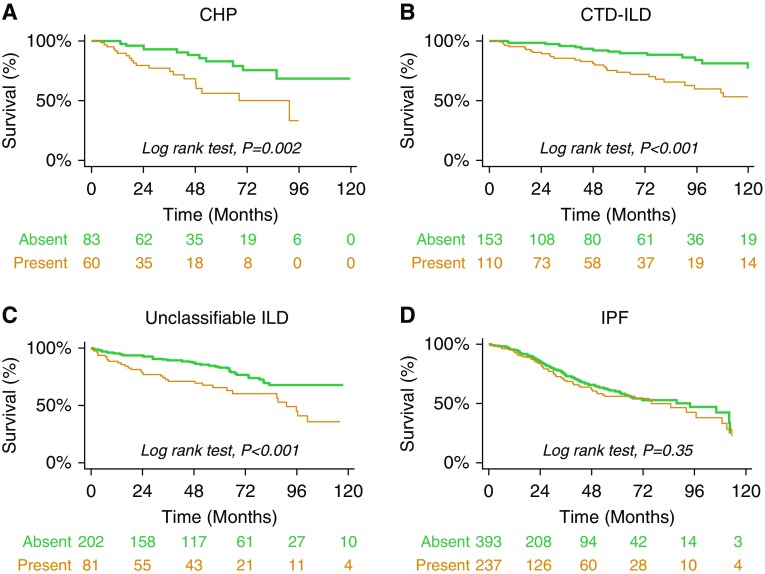
In unadjusted analyses, CT honeycombing was associated with a higher mortality rate within ILD subtypes: CHP (HR, 2.90; 95% CI, 1.58–5.30), CTD-ILD (HR, 3.04; 95% CI, 1.48–6.25), and unclassifiable ILD (HR, 2.32; 95% CI, 1.46–3.69) (Table 3, Figures 2A–2C).
Table 3.
Interstitial lung disease subtypes with computed tomography honeycombing
| ILD Subtype (N = 1,330) | Unadjusted Mortality Hazard |
Adjusted Mortality Hazard |
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| IPF (n = 639) | 1.16 (0.86–1.57) | 0.33 | 1.04 (0.76–1.43) | 0.81 |
| CHP (n = 143) | 2.90 (1.58–5.30) | 0.002 | 1.93 (0.91–4.13) | 0.09 |
| CTD-ILD (n = 265) | 3.04 (1.48–6.25) | 0.001 | 2.46 (1.34–4.53) | 0.004 |
| Unclassifiable* | 2.32 (1.46–3.69) | <0.001 | 2.22 (1.33–3.70) | 0.002 |
Definition of abbreviations: CHP = chronic hypersensitivity pneumonitis; CI = confidence interval; CTD-ILD = connective tissue disease–associated ILD; HR = hazard ratio; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis.
Eleven subjects (IPF n = 9 and CTD-ILD n = 2) were lost to follow-up immediately after their initial visit, and thus were censored at Time 0, the time of initial evaluation. Adjusted = adjusted for hospital center, sex, age, forced vital capacity, diffusing capacity of the lungs for carbon monoxide, and immunosuppressive therapy.
Subgroup includes unclassifiable ILD (n = 192), and other ILD (n = 91).
Figure 2.
Ten-year survival stratified by interstitial lung disease (ILD) subtype: (A) chronic hypersensitivity pneumonitis (CHP); (B) connective tissue disease–associated ILD (CTD-ILD); (C) Unclassifiable and other ILD; (D) idiopathic pulmonary fibrosis (IPF).
There findings were consistent after adjusting for hospital center, sex, age, FVC, DlCO, ILD subtype, and immunosuppressive therapy in those with CTD-ILD (HR, 2.46; 95% CI, 1.34–4.53) and unclassifiable ILD (HR, 2.22; 95% CI, 1.33–3.70), but for subjects with CHP this association was imprecise (HR, 1.93; 95% CI, 0.91–4.13) (Table 3).
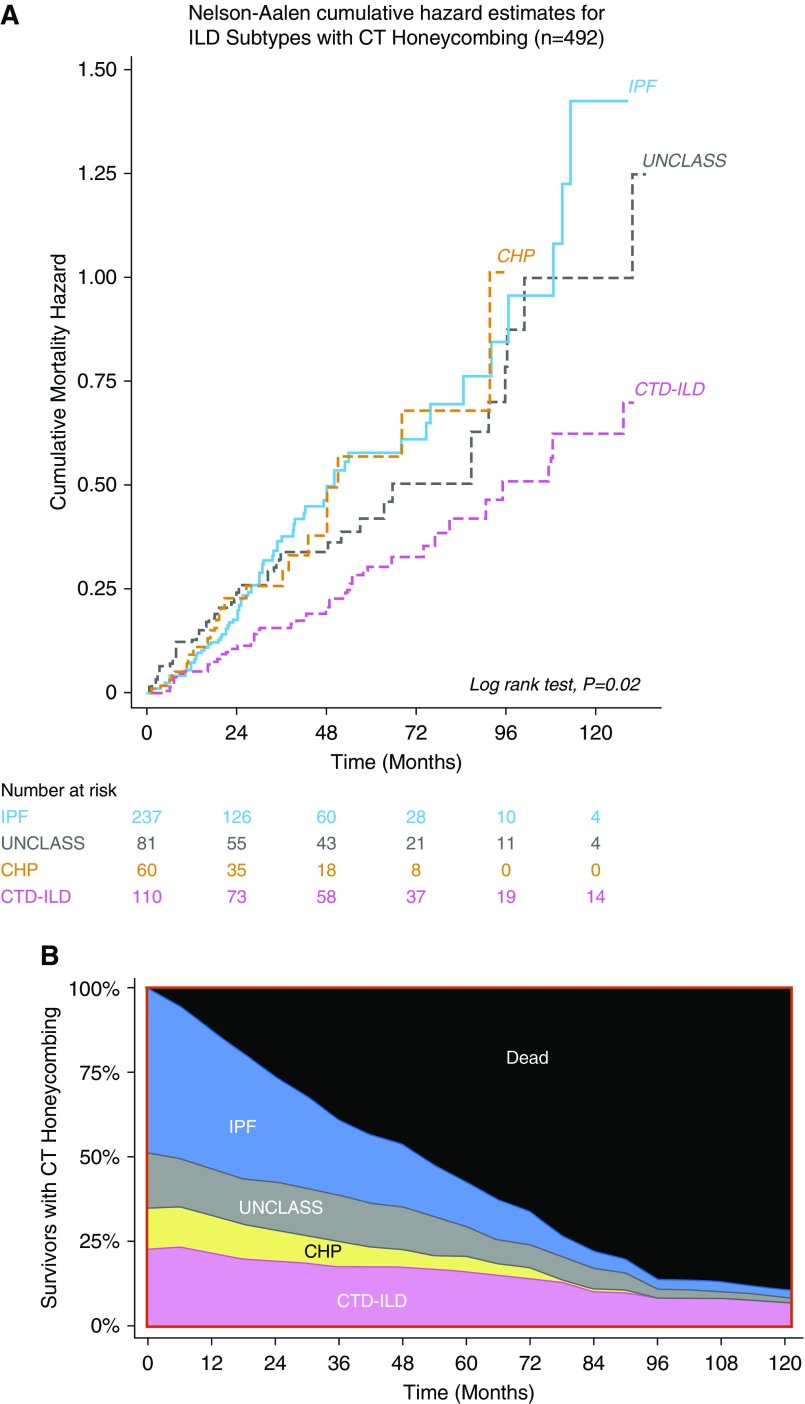
Among participants with IPF, there was no strong association between CT honeycombing and mortality in either unadjusted (HR, 1.16; 95% CI, 0.86–1.57) or adjusted (HR, 1.04; 95% CI, 0.76–1.43) models (Table 3, Figure 2D). Among those with CT honeycombing, cumulative mortality hazards were similar across ILD subtypes, except for CTD-ILD, which had a lower mortality hazard (Figures 3A and 3B).
Figure 3.
(A) Nelson-Aalen cumulative hazard estimates for interstitial lung disease (ILD) subtypes with computed tomography (CT) honeycombing. (B) Proportion of survivors for cohort with CT honeycombing (n = 492) stratified by ILD subtype; participants lost to follow-up were censored at time of loss to follow-up. CHP = chronic hypersensitivity pneumonitis; CTD-ILD = connective tissue disease–associated ILD; IPF = idiopathic pulmonary fibrosis; UNCLASS = unclassifiable and other ILD.
Discussion
We have shown that CT honeycombing is highly prevalent in diverse forms of ILD and that CT honeycombing is associated with an increased long-term mortality rate compared with those without honeycombing. Importantly, with the exception of CTD-ILD, CT honeycombing conferred mortality rates similar to IPF, suggesting that CT honeycombing denotes a progressive fibrotic ILD (PF-ILD) phenotype. It was not surprising that CT honeycombing was not associated with mortality in those with IPF, because IPF represents a PF-ILD phenotype. Our results support the need for a rigorous and systematic chest CT evaluation in all patients with ILD, as CT honeycombing has prognostic value and should be taken into consideration when counseling patients with ILD about their prognosis. CT honeycombing might also be used for clinical trial enrichment when selecting for those with a PF-ILD phenotype.
CT honeycombing may result from a progressive increase in the occurrence and size of subpleural cystic structures and parenchymal opacities gradually extending toward the center of the pulmonary lobule with eventual replacement of the entire lobule (27). This defining characteristic was highly prevalent across all major ILD subtypes in our study, thus underscoring the heterogeneity in disease severity within ILD subgroups (28). Our results are consistent with previously reported work describing CT honeycombing in ILD (12) and suggesting its prognostic value. Yunt and colleagues demonstrated that honeycombing might be found on the initial HRCT scan in up to 20% of subjects with biopsy-proven rheumatoid arthritis–associated ILD (RA-ILD) (16). Nurmi and colleagues quantitatively scored HRCTs from 60 patients with RA-ILD and found honeycombing in 53% of their cohort (18). Although honeycombing was not associated with worse survival in their study, they found that the extent of honeycombing correlated with respiratory hospitalizations (18). However, Jacob and colleagues showed that in RA-ILD, the presence of honeycombing conferred a worse prognosis (13).
Similarly, another study by Tateishi and colleagues assessed subjects with CHP and demonstrated a higher mortality risk among participants with honeycombing on HRCT (15). Among subjects with IPF, Salisbury and colleagues found that 35% had CT honeycombing with an increased risk of death or lung transplantation (HR, 2.04; 95% CI, 1.23–3.41) (3). They also showed that subjects with CHP or IPF have poor survival when CT honeycombing is present (29). Although CT honeycombing did not substantially increase mortality risk in IPF within our study, when all participants with honeycombing were assessed, those with IPF had the highest mortality. Although these prior studies assessed honeycombing in subjects with disparate ILD subtypes at academic medical centers, our study shows that across the ILD spectrum, CT honeycombing is prevalent and its identification is of prognostic value at both tertiary and community medical centers.
These intriguing results, therefore, provide strong evidence that CT honeycombing represents a PF-ILD phenotype regardless of underlying diagnosis. When present, this phenotype confers a radically different outcome. The importance of recognizing this distinct PF-ILD phenotype is justified by its clinical utility in the decision-making process. Realizing that subjects with this phenotype may inexorably progress to death provides a compelling justification for intervening more aggressively in the appropriate clinical context. Importantly, CT honeycombing identified at baseline evaluation would designate the subject as having the PF-ILD phenotype and obviate the need to wait for observed disease progression before considering appropriate interventions. Therefore, our findings underscore the utility of CT honeycombing as a practical and readily available biomarker for disease progression in ILD with immediate implications for routine clinical care (2, 4, 30). Identifying this biomarker in persons with fibrotic ILD would place further emphasis on the need for early referral to initiate approved therapies where indicated (31) and for consideration of enrollment in clinical trials and listing for lung transplantation (32).
Indeed, others have postulated the existence of a PF-ILD phenotype with IPF-like behavior, suggesting the need to explore “lumping” of IPF with other forms of progressive lung fibrosis (33–36). The distinct PF-ILD phenotype characterized by honeycombing lends credence to this idea and indicates that the high mortality frequently witnessed in ILD is not confined to patients with a UIP pattern. Rather, it suggests that common pathobiologic mechanisms linked to honeycombing may exist across diverse ILD subtypes. Beyond providing real-world prognostic data associated with honeycombing, our results have important potential implications in the design of future therapeutic trials. This phenotype provides a scientifically rational platform for studying subjects with similar longitudinal disease behavior and progression to death regardless of treatment. Importantly, designating subjects with CT honeycombing as PF-ILD could promote cohort enrichment and more robust sample sizes for ILD clinical trials.
Although the extent of honeycombing may be of additional prognostic value, we found that the dichotomous assessment of honeycombing as present on chest CT scans was associated with a similar mortality effect as systematic scoring of 5% honeycombing on chest CT scans. The prognostic impact of this binary categorization is reflected in the most recent American Thoracic Society guidelines that link CT honeycombing to a definitive UIP diagnosis, a pattern frequently present in IPF, the most devastating form of pulmonary fibrosis.
A fascinating finding in our study was the observation that although CT honeycombing was absent in a substantial proportion of participants with IPF, their mortality pattern did not differ from those with IPF who had CT honeycombing. This is of clinical relevance, as the UIP pattern that characterizes IPF confers a unique disease behavior typified by physiologic progression. Indeed, this is consistent with recent guidelines, which categorize participants who lack CT honeycombing but have a reticular pattern and peripheral traction bronchiectasis/bronchiolectasis as having a probable UIP pattern on CT scan (10, 37, 38). Histopathologic evaluation of surgical lung biopsy specimens obtained from these participants typically demonstrates a probable or definite UIP histopathologic pattern in the overwhelming majority (10, 37). As UIP is the hallmark radiologic pattern in IPF, subjects with histopathologic UIP on biopsy might be expected to have similar mortality patterns (38).
Our study has several limitations. First, as there is no universally standardized algorithm readily available for quantitatively scoring extent of honeycombing on CT scans, we used a qualitative assessment of honeycombing on the index chest CT scan obtained at baseline evaluation for ILD. This qualitative assessment of the presence or absence of CT honeycombing further maximizes its clinical utility as an objective metric in prognostication of outcomes at nontertiary medical centers without expertise in ILD. Second, the quality of the CT scans varied, and this was not always performed under “high-resolution” protocols; however, for temporal uniformity and to minimize lead-time bias, we elected to use the official documentation of the index chest CT imaging at ILD evaluation at the respective tertiary and nontertiary medical centers for all study participants. This may have decreased the study sensitivity, specificity, and positive predictive value for CT honeycombing among participants deemed to have ILD. However, we focused our analysis on the subset of participants with MDD or independently adjudicated ILD and confirmed presence of CT honeycombing by imaging reread in a large subset of participants (39).
Third, we excluded patients with sarcoidosis, as our study was designed to focus only on interstitial lung diseases, and the majority of patients with sarcoidosis have early-stage disease and lack evidence of parenchymal lung involvement. Fourth, this study was conducted retrospectively, limiting it to identification of association. Therefore, causality cannot be inferred; however, the consistency of the findings among all ILD subgroups suggests their merit.
Fifth, our assessment of the relationship between CT honeycombing and mortality may have been confounded by the extent and presence of various CT forms of fibrosis (reticulation, traction bronchiolectasis, traction bronchiectasis, or honeycombing), and paraseptal or centrilobular emphysema. Furthermore, chest CT reports used in this study occurred over a decade and at several medical centers or referring hospitals, with substantial heterogeneity in the variety of scanners, image quality, and scanning techniques, which could impact interobserver variability in assessment of the extent of honeycombing or other forms of fibrosis. However, these forms of fibrosis are believed to visually reflect increasing severity of parenchymal fibrosis, with CT honeycombing frequently regarded as the most severe. Therefore, to minimize the effect of these sources of heterogeneity, we focused our analyses on the dichotomous presence of CT honeycombing, given its ease of measurement and practical utility for the clinician.
In conclusion, CT honeycombing is prevalent in diverse forms of ILD and identifies a progressive fibrotic phenotype that is associated with increased mortality rates. Although CT honeycombing did not confer additional risk in IPF, other major ILD subtypes with this phenotype had higher mortality. This defining characteristic deserves further study to elucidate the common pathobiological mechanisms contributing to disease progression in subjects with CT honeycombing. Overall, the early recognition of the PF-ILD phenotype is of critical importance during the evaluation of persons with fibrotic lung disease, as this may improve the clinical decision-making process.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the support staff of the University of Chicago Respiratory Clinical Research Unit, the Interstitial Lung Disease Clinic (Nancy Trojan, Catherine Brown, and Spring Holland), and NorthShore University HealthSystem, Evanston, IL (Mohammad Imran Beig, Paul Chung, Kathleen M. Biblowitz, Daisy Zhu, Uday Mehta, Kiran Thakrar, and Naomi BenIsraelOlive), for their assistance with this study. Data from this study were provided by the Clinical Research Data Warehouse maintained by the Center for Research Informatics at University of Chicago. The Center for Research Informatics is funded by the Biological Sciences Division, the Institute for Translational Medicine/Clinical and Translational Science Awards (NIH UL1 TR000430) at the University of Chicago. The authors also thank the participants with ILD who made these research endeavors possible.
Footnotes
Supported by National Institutes of Health grants (NIH) K23HL138190 and NIH R01HL130796, and the Pulmonary Fibrosis Foundation.
Author Contributions: Conception and design: A.A., M.M.C., I.N., M.E.S., and J.H.C.; acquisition of data for the work: A.A., J.M.O., S.K.B., S.M., M.M.C., I.N., R.V., M.E.S., and J.H.C.; analysis and interpretation: A.A., M.M.C., I.N., M.E.S., and J.H.C.; drafting the manuscript for important intellectual content: A.A., J.M.O., S.K.B., S.M., M.M.C., I.N., R.V., M.E.S., and J.H.C.; critical revision for important intellectual content: all authors; final approval of the submitted manuscript and accountability for all aspects of the work: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378:1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salisbury ML, Tolle LB, Xia M, Murray S, Tayob N, Nambiar AM, et al. Possible UIP pattern on high-resolution computed tomography is associated with better survival than definite UIP in IPF patients. Respir Med. 2017;131:229–235. doi: 10.1016/j.rmed.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 5.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society; European Respiratory Society; Japanese Respiratory Society; Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 6.Lynch DA, Godwin JD, Safrin S, Starko KM, Hormel P, Brown KK, et al. Idiopathic Pulmonary Fibrosis Study Group. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med. 2005;172:488–493. doi: 10.1164/rccm.200412-1756OC. [DOI] [PubMed] [Google Scholar]
- 7.Sumikawa H, Johkoh T, Colby TV, Ichikado K, Suga M, Taniguchi H, et al. Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am J Respir Crit Care Med. 2008;177:433–439. doi: 10.1164/rccm.200611-1696OC. [DOI] [PubMed] [Google Scholar]
- 8.Watadani T, Sakai F, Johkoh T, Noma S, Akira M, Fujimoto K, et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology. 2013;266:936–944. doi: 10.1148/radiol.12112516. [DOI] [PubMed] [Google Scholar]
- 9.Leslie KO. Idiopathic pulmonary fibrosis may be a disease of recurrent, tractional injury to the periphery of the aging lung: a unifying hypothesis regarding etiology and pathogenesis. Arch Pathol Lab Med. 2012;136:591–600. doi: 10.5858/arpa.2011-0511-OA. [DOI] [PubMed] [Google Scholar]
- 10.Raghu G, Lynch D, Godwin JD, Webb R, Colby TV, Leslie KO, et al. Diagnosis of idiopathic pulmonary fibrosis with high-resolution CT in patients with little or no radiological evidence of honeycombing: secondary analysis of a randomised, controlled trial. Lancet Respir Med. 2014;2:277–284. doi: 10.1016/S2213-2600(14)70011-6. [DOI] [PubMed] [Google Scholar]
- 11.Morell F, Villar A, Montero MA, Muñoz X, Colby TV, Pipvath S, et al. Chronic hypersensitivity pneumonitis in patients diagnosed with idiopathic pulmonary fibrosis: a prospective case-cohort study. Lancet Respir Med. 2013;1:685–694. doi: 10.1016/S2213-2600(13)70191-7. [DOI] [PubMed] [Google Scholar]
- 12.Johkoh T, Sakai F, Noma S, Akira M, Fujimoto K, Watadani T, et al. Honeycombing on CT; its definition, pathologic correlation, and future direction of its diagnosis. Eur J Radiol. 2014;83:27–31. doi: 10.1016/j.ejrad.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Jacob J, Song JW, Yoon HY, Cross G, Barnett J, Woo WL, et al. Prevalence and effects of emphysema in never-smokers with rheumatoid arthritis interstitial lung disease. EBioMedicine. 2018;28:303–310. doi: 10.1016/j.ebiom.2018.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adegunsoye A, Oldham JM, Chung JH, Montner SM, Lee C, Witt LJ, et al. Phenotypic clusters predict outcomes in a longitudinal interstitial lung disease cohort. Chest. 2018;153:349–360. doi: 10.1016/j.chest.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tateishi T, Ohtani Y, Takemura T, Akashi T, Miyazaki Y, Inase N, et al. Serial high-resolution computed tomography findings of acute and chronic hypersensitivity pneumonitis induced by avian antigen. J Comput Assist Tomogr. 2011;35:272–279. doi: 10.1097/RCT.0b013e318209c5a6. [DOI] [PubMed] [Google Scholar]
- 16.Yunt ZX, Chung JH, Hobbs S, Fernandez-Perez ER, Olson AL, Huie TJ, et al. High resolution computed tomography pattern of usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease: relationship to survival. Respir Med. 2017;126:100–104. doi: 10.1016/j.rmed.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Chung JH, Zhan X, Cao M, Koelsch TL, Manjarres DCG, Brown KK, et al. Presence of air trapping and mosaic attenuation on chest computed tomography predicts survival in chronic hypersensitivity pneumonitis. Ann Am Thorac Soc. 2017;14:1533–1538. doi: 10.1513/AnnalsATS.201701-035OC. [DOI] [PubMed] [Google Scholar]
- 18.Nurmi HM, Kettunen HP, Suoranta SK, Purokivi MK, Karkkainen MS, Selander TA, et al. Several high-resolution computed tomography findings associate with survival and clinical features in rheumatoid arthritis-associated interstitial lung disease. Respir Med. 2018;134:24–30. doi: 10.1016/j.rmed.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Oldham JM, Adegunsoye A, Valenzi E, Lee C, Witt L, Chen L, et al. Characterisation of patients with interstitial pneumonia with autoimmune features. Eur Respir J. 2016;47:1767–1775. doi: 10.1183/13993003.01565-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adegunsoye AO, Chung JH, Bellam SK, Oldham J, Montner S, Churpek MM, et al CT honeycombing fibrosis is prevalent and associated with mortality across diverse interstitial lung diseases [abstract]. Am J Respir Crit Care Med 2018;197:A2536 [Google Scholar]
- 21.Adegunsoye A, Oldham JM, Bellam SK, Chung JH, Chung PA, Biblowitz KM, et al. African-American race and mortality in interstitial lung disease: a multicentre propensity-matched analysis. Eur Respir J. 2018;51:1800255. doi: 10.1183/13993003.00255-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adegunsoye A, Oldham JM, Bonham C, Hrusch C, Nolan P, Klejch W, et al. Prognosticating outcomes in interstitial lung disease by mediastinal lymph node assessment: an observational cohort study with independent validation Am J Respir Crit Care Med[online ahead of print] 14 Sep 2018. DOI: 10.1164/rccm.201804-0761OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. ATS/ERS Committee on Idiopathic Pulmonary Fibrosis. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001 Am J Respir Crit Care Med 2002165277–304.[Published erratum appears in Am J Respir Crit Care Med 166:426.] [DOI] [PubMed] [Google Scholar]
- 25.Fischer A, Antoniou KM, Brown KK, Cadranel J, Corte TJ, du Bois RM, et al. ERS/ATS Task Force on Undifferentiated Forms of CTD-ILD. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015;46:976–987. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 26.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. American Thoracic Society; European Respiratory Society; Japanese Respiratory Society; Latin American Thoracic Association. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis: an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 27.Mai C, Verleden SE, McDonough JE, Willems S, De Wever W, Coolen J, et al. Thin-section CT features of idiopathic pulmonary fibrosis correlated with micro-CT and histologic analysis. Radiology. 2017;283:252–263. doi: 10.1148/radiol.2016152362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guler SA, Ellison K, Algamdi M, Collard HR, Ryerson CJ.Heterogeneity in unclassifiable interstitial lung disease: a systematic review and meta-analysis Ann Am Thorac Soc 2018. 15:854–863 [DOI] [PubMed] [Google Scholar]
- 29.Salisbury ML, Gu T, Murray S, Gross BH, Chughtai A, Sayyouh M, et al. Hypersensitivity pneumonitis: radiologic phenotypes are associated with distinct survival time and pulmonary function trajectory Chest[online ahead of print] 19 Sep 2018. DOI: 10.1016/j.chest.2018.08.1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Podolanczuk AJ, Oelsner EC, Barr RG, Bernstein EJ, Hoffman EA, Easthausen IJ, et al. High-attenuation areas on chest computed tomography and clinical respiratory outcomes in community-dwelling adults. Am J Respir Crit Care Med. 2017;196:1434–1442. doi: 10.1164/rccm.201703-0555OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med. 2011;184:842–847. doi: 10.1164/rccm.201104-0668OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adegunsoye A, Strek ME, Garrity E, Guzy R, Bag R. Comprehensive care of the lung transplant patient. Chest. 2017;152:150–164. doi: 10.1016/j.chest.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells AU, Brown KK, Flaherty KR, Kolb M, Thannickal VJ IPF Consensus Working Group. What’s in a name? That which we call IPF, by any other name would act the same. Eur Respir J. 2018;51:1800692. doi: 10.1183/13993003.00692-2018. [DOI] [PubMed] [Google Scholar]
- 34.Ryerson CJ.Lumpers versus splitters: what to do with suspected idiopathic pulmonary fibrosis? Respirology[online ahead of print] 15 Nov 2018. DOI: 10.1111/resp.13442 [DOI] [PubMed] [Google Scholar]
- 35.Maher TM, Corte TJ, Fischer A, Kreuter M, Lederer DJ, Molina-Molina M, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: design of a double-blind, randomised, placebo-controlled phase II trial. BMJ Open Respir Res. 2018;5:e000289. doi: 10.1136/bmjresp-2018-000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flaherty KR, Brown KK, Wells AU, Clerisme-Beaty E, Collard HR, Cottin V, et al. Design of the PF-ILD trial: a double-blind, randomised, placebo-controlled phase III trial of nintedanib in patients with progressive fibrosing interstitial lung disease. BMJ Open Respir Res. 2017;4:e000212. doi: 10.1136/bmjresp-2017-000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung JH, Chawla A, Peljto AL, Cool CD, Groshong SD, Talbert JL, et al. CT scan findings of probable usual interstitial pneumonitis have a high predictive value for histologic usual interstitial pneumonitis. Chest. 2015;147:450–459. doi: 10.1378/chest.14-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018;6:138–153. doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 39.Chung JH, Oldham JM, Montner SM, Vij R, Adegunsoye A, Husain AN, et al. CT-pathologic correlation of major types of pulmonary fibrosis: insights for revisions to current guidelines. AJR Am J Roentgenol. 2018;210:1034–1041. doi: 10.2214/AJR.17.18947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.