Abstract
Background: Asthma and obesity are major public health problems, affecting hundreds of millions of people worldwide. Obesity is associated with increased asthma risk and severity, and lower asthma-related quality of life.
Objective: In this systematic review, we aimed to evaluate whether weight loss in subjects with obesity and asthma leads to improvement in asthma-related outcomes.
Data Sources and Extraction: We searched PubMed and Google Scholar for all studies in English published January 2000-December 2018. Studies were included if they were randomized, controlled clinical trials (RCTs) for overweight/obese children or adults with asthma, with sufficient data to assess outcomes and study quality. Non-randomized and non-controlled studies were excluded, as well as those in subjects without overweight/obesity, or with non-asthmatic controls.
Synthesis: We identified four RCTs involving children (total n = 246) and six involving adults (n = 502). All interventions were designed for weight loss and varied from dietary restrictions to multifactorial interventions with exercise training and cognitive behavioral therapy; the duration of intervention ranged from 8 weeks to 18 months. All RCTs reported successful improvements in weight or body mass index (−0.14 standard deviation scores to −15.9% BMI reduction in children, 1.8%–14.5% weight loss in adults). RCTs generally reported improvements in asthma-related quality of life and, to some degree, asthma control. RCTs involving adults also reported improvements in lung function (FEV1, FVC, TLC), while RCTs in children showed less consistent results.
Conclusions: These findings suggest that weight loss in subjects with obesity and asthma may improve asthma outcomes. However, there was wide variability in populations studied, baseline and post-intervention assessments, follow-up length, outcome definition and reporting, and statistical approaches, which hindered the ability to compare studies, perform a pooled analysis, or assess generalizability.
Primary Source of Funding: U.S. National Institutes of Health (NIH).
Keywords: obesity, asthma, childhood asthma, obese asthma, weight loss
Asthma affects more than 230 million people worldwide (1, 2). In the United States alone, asthma results each year in millions of missed days of school and work, almost 2 million emergency department visits, more than 400,000 hospitalizations, and 3,400 deaths (3). Total asthma costs reach ∼$82 billion per year in the United States (4) and ∼€72 billion in Europe (5, 6). Moreover, the number of people living with asthma has markedly increased over the past several decades (3). Although the causes for this increase are unclear, the reasons are likely multifactorial and include changes in microbial exposures in early life (the “hygiene hypothesis” [7]), environmental exposures (e.g., increased air pollution), and unhealthy lifestyle behaviors (e.g., obesity, sedentary lifestyle, and poor nutrition).
In parallel with the increasing prevalence of asthma, there has been a steady increase in obesity. Worldwide, more than 650 million adults and 380 million children are obese (8). In the United States alone, obesity affects ∼40% of adults and ∼20% of youth (9). Recent estimates predict that nearly 60% of today’s children will be obese by the age of 35 years (10). Obesity is associated with numerous health risks, including type 2 diabetes, hypertension, cardiovascular disease, and cancer (11). Likewise, obesity increases the risk of asthma and, at least in some individuals, is associated with worse asthma severity and control—a phenomenon increasingly recognized as the obese asthma phenotype (12, 13). Proposed mechanisms linking obesity and asthma include increased mechanical load on the lungs, increased airway or systemic inflammation, insulin resistance and alterations in glucose metabolism, or changes in the microbiome, among others (14, 15).
The impact of lifestyle interventions targeting weight loss and improved fitness in other chronic diseases has been well established: even modest weight loss can result in significant benefits to overall individual health, and weight management reduces the risk of obesity-associated conditions (16, 17). However, only recently have studies started to focus on the effect of weight loss on asthma in obesity, and previous reviews on the topic have only included a small number of noncontrolled studies and few, if any, randomized controlled clinical trials (RCTs) (18, 19). In this review, we assess the current RCT-based evidence on the effect of weight loss for children and adults with obesity and asthma.
Methods
A literature search was conducted using PubMed and Google Scholar for all studies from January 2000 to November 2018, using search terms (“weight loss”[MeSH Terms] OR (“weight”[All Fields] AND “loss”[All Fields]) OR “weight loss”[All Fields]) AND (“asthma”[MeSH Terms] OR “asthma”[All Fields]). In addition, relevant articles were cross-referenced for additional studies. Inclusion criteria were: 1) RCTs, 2) interventions designed for weight loss as reported by the original study authors and verified during this review, 3) involving overweight or obese children or adults with asthma, 4) sufficient data reported to assess outcomes and study quality, and 5) published in English. Exclusion criteria were: 1) nonrandomized or noncontrolled studies, 2) studies in subjects without asthma, 3) studies in subjects without overweight/obesity, or 4) not enough data reported to evaluate changes among overweight/obese subjects with asthma. Study quality, design, interventions, and results were evaluated independently by two researchers (W.O. and K.D.L.), with any discrepancies resolved by discussion and consensus or, if needed, by arbitration (E.F.). Both researchers also extracted and compared results from all studies with the aim of performing a meta-analysis if feasible.
We focused on four groups of outcomes: weight loss or improvement in body composition, changes in lung function, inflammatory biomarkers (systemic or related to the airways), and asthma-related quality of life (AR-QOL) and symptom control. The Cochrane Risk of Bias tool (20) was used to assess the quality of each study. Each criterion was assessed and categorized as having low risk, unclear risk, or high risk of bias. The protocol for this systematic review was prospectively registered in the International Prospective Register of Systematic Reviews, PROSPERO (CRD42018085045), and the review was conducted following the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (21).
Results
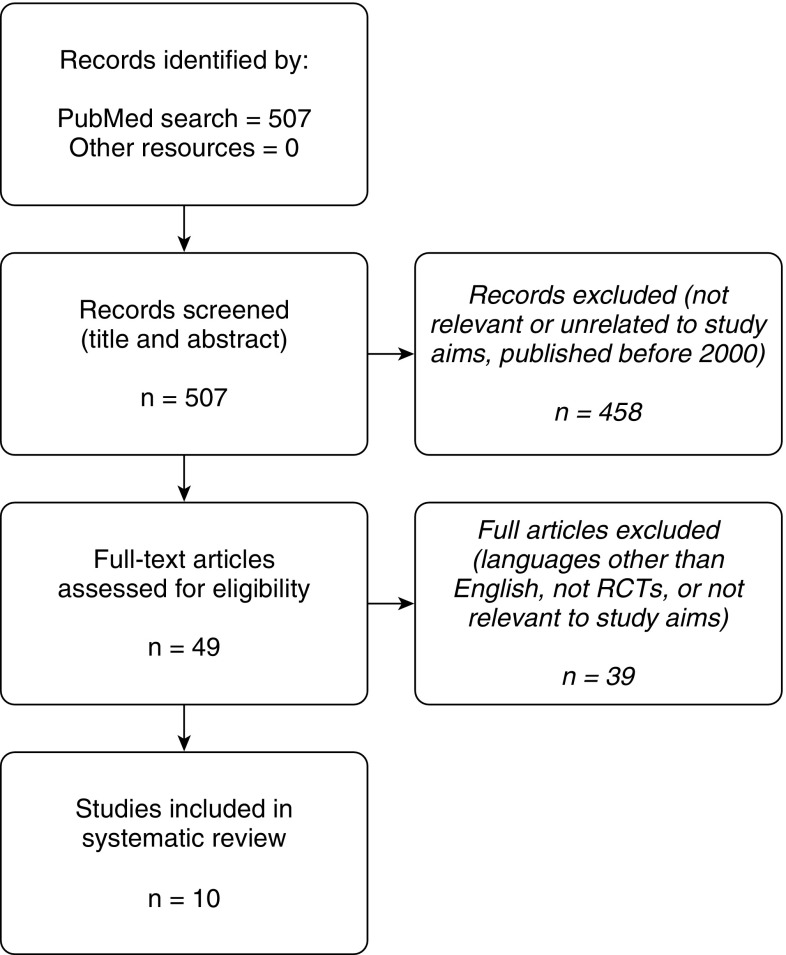
A diagram of the search process can be found in Figure 1. We found a total of 10 RCTs that qualified for inclusion in this review: 6 in adults and 4 in children/adolescents. A pooled analysis was not feasible because of the small number of RCTs and the wide differences in the interventions, the comparator groups, the outcomes reported, and/or the units used.
Figure 1.
Study selection overview: flow diagram of study selection. Adapted by permission from the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement (21). RCTs = randomized controlled clinical trials.
RCTs in Pediatric Populations
We found four RCTs evaluating weight loss in overweight/obese children with asthma (total n = 246; Table 1) (22–25). The interventions used varied markedly among the four studies: Abd El-Kader and colleagues (22) and Willeboordse and colleagues (25) used multifactorial interventions with exercise sessions and nutritional counseling; Willeboordse and colleagues (25) also incorporated cognitive behavioral therapy for study participants and their families. Luna-Pech and colleagues (24) and Jensen and colleagues (23) instead focused on nutritional interventions only, using normocaloric and restrictive diets, respectively. The interventions varied in duration from ∼8 to 10 weeks (Abd El-Kader and colleagues [22] and Jensen and colleagues [23]) to 28 weeks (Luna-Pech and colleagues [24]). Risk of bias ranged from low to high (Table 2). Of note, Willeboordse and colleagues included children with asthma as well as children at “high risk” of asthma because of family history (25).
Table 1.
Summary of studies in pediatric populations
| Study | Population | Intervention | Control | Timing | Outcome(s) | Result(s) |
|---|---|---|---|---|---|---|
| Willebordse et al., 2016 (25) NCT00998413 |
87 overweight or obese children (6–16 yr) with asthma or at high risk of developing asthma (family history) |
Multifactorial intervention, hospital outpatient setting: 18 lifestyle sessions (diet and CBT), 10 parental sessions, 8 individual sessions, and regular sport sessions |
Usual care; no weight loss intervention |
18 mo, with follow-up every 6 mo |
Primary: BMI-SDS, FEV1 (% predicted) | Weight: Intervention and control groups both showed reduced BMI-SDS (−0.14 and −0.12, respectively) at the end of the study, but no difference between groups (P = 0.65). |
| Other: FEV1, FVC, FEV1/FVC, ERV, TLC, c-ACT, PAQLQ, EIB, SABA use, ICS use, leptin, adiponectin, o2 peak |
PFTs: Significant between-group difference only in FVC (10% predicted). Significant within-group results (i.e., postintervention compared with baseline) included FEV1 (9.2%), ERV (12%), and TLC (4%). | |||||
| Inflam: No significant changes reported. | ||||||
| QOL: Significant within-group changes included (c)-ACT and PAQLQ (0.48 points) | ||||||
| Luna-Pech et al., 2014 (24) |
51 obese adolescents (12–16 yr) with asthma |
Supervised normocaloric diet. Follow-up visits to assess asthma, dietary recall, and make dietary adjustments with a certified nutritionist |
Free diet: follow-up visits to assess asthma and dietary recall; no dietary interventions |
28 wk, with follow-up visits every 2 wk |
Primary: PAQLQ |
Weight: Significant improvement within group and between group in BMI (−0.5 z-scores). Control group BMI reported as “practically unchanged” |
| Other: BMI z-score, dietary macronutrients, SABA use, daily ICS dose (budesonide). Lung function reported at baseline |
PFTs: Reported as no differences in FEV1, but postintervention lung function data not shown | |||||
| QOL and medications: Intervention group had significant improvement in PAQLQ scores. Between-group PAQLQ difference “greater than minimally significant difference.” The intervention group also showed a significant decrease in SABA use compared with control subjects. | ||||||
| Abd El-Kader et al., 2013 (22) |
80 obese adolescents (12–18 yr) with asthma |
Diet regimen and supervised exercise training in addition to usual medical treatment |
Usual medical treatment only |
8 wk, with exercise sessions 4 d per week |
BMI, leptin, adiponectin, TNF-α, IL-6, IL-8. PFTs reported only at baseline |
Weight: BMI significantly decreased in the intervention group (significant both within group and between group). |
| PFTs: No postintervention lung function data reported | ||||||
| Inflam: TNF-α, leptin, IL-6, and IL-8 decreased and adiponectin increased in postintervention group (significant both within group and between group). | ||||||
| QOL: No quality of life outcomes measured | ||||||
| Jensen et al., 2013 (23) ACTRN12610000955011 (A/NZ) |
28 obese children (8–17 yr) with asthma |
Dietary intervention: counseling sessions with a dietitian, targeted at a 500-kcal/d energy intake reduction |
Wait-list control: received intervention only after the 10-wk study period ended |
10 wk, with dietary sessions at Weeks 0, 1, 2, 4, 6, 8, and 10; and telephone contact the remaining weeks |
BMI z-score, percentage body fat, lean mass, glucose, insulin, lipid profiles, HOMA-IR, FEV1, FVC, FEV1/TLC, TLC, FRC, RV, RV/TLC, asthma control, methacholine challenge, FeNO, sputum cell counts, CRP, leptin, adiponectin, and IL-6 |
Weight: Significant between-group differences in weight loss, BMI z-score, and percent body fat |
| PFTs: Significant within-group changes in ERV, RV, and RV/TLC; these changes were higher magnitude compared with control subjects, but were not statistically significant. | ||||||
| Inflam: Significant between-group difference in CRP | ||||||
| QOL: Significant between-group difference in ACQ score |
Definition of abbreviations: ACQ = Asthma Control Questionnaire; ACT = Asthma Control Test; A/NZ = Australia/New Zealand; BMI = body mass index; c-ACT = childhood ACT; CBT = cognitive behavioral therapy; CRP = C-reactive protein; EIB = exercise-induced bronchospasm; ERV = expiratory residual volume; FeNO = fractional exhaled nitric oxide; FEV1 = forced expiratory volume in 1 second; FRC = functional residual capacity; FVC = forced vital capacity; HOMA-IR = homeostatic model assessment–insulin resistance; ICS = inhaled corticosteroid; IL = interleukin; inflam = inflammatory markers; PAQLQ = Pediatric Asthma Quality-of-Life Questionnaire; PFT = pulmonary function test; QOL = quality of life; RV = residual volume; SABA = short-acting β-agonist; SDS = standard deviation score; TLC = total lung capacity; TNF = tumor necrosis factor; o2 = oxygen consumption.
Results group by weight loss, PFTs, inflammatory markers, and QOL. Between-group differences refer to those between the intervention arm versus the control subjects. Within-group differences refer to those comparing postintervention to baseline within the intervention arm.
Table 2.
Risk of bias assessment summary
| Studies in Pediatric Populations | Studies in Adult Populations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Abd El-Kader et al. (22) | Jensen et al. (23) | Luna-Pech et al. (24) | Willeboordse et al. (25) | Stenius-Aarnalia et al. (39) | Scott et al. (34) | Dias-Júnior et al. (37) | Ma et al. (36) | Scott et al. (35) | Freitas et al. (38) | |
| Protocol found/registered | ☒ | ☑ | ☒ | ☑ | ☒ | ☐ | ☑ | ☑ | ☑ | ☑ |
| Random sequence generation (selection bias) | ☐ | ☑ | ☐ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ | ☑ |
| Allocation concealment (selection bias) | ☐ | ☑ | ☐ | ☐ | ☐ | ☑ | ☐ | ☑ | ☐ | ☑ |
| Blinding of participants and personnel (performance bias) | ☒ | ☒ | ☒ | ☒ | ☒ | ☒ | ☒ | ☒ | ☒ | ☑ |
| Blinding of outcome assessment (detection bias) | ☒ | ☒ | ☑ | ☒ | ☒ | ☒ | ☒ | ☑ | ☒ | ☑ |
| Incomplete outcome data (attrition bias) | ☐ | ☑ | ☑ | ☑ | ☐ | ☑ | ☑ | ☑ | ☐ | ☑ |
| Selective outcome reporting (reporting bias) | ☐ | ☑ | ☐ | ☐ | ☐ | ☐ | ☑ | ☐ | ☐ | ☑ |
| Other | ☐ | ☐ | ☑ | ☑ | ☑ | ☐ | ☑ | ☑ | ☐ | ☑ |
| Overall risk of bias | High | Low | Moderate | Moderate | Moderate | Moderate | Low | Low | Moderate | Low |
Assessment of quality of selected randomized controlled trials. Low risk of bias (check mark in squares), unclear risk of bias (open squares), and high risk of bias (x mark in squares). Adapted by permission from the Cochrane Bias Methods Group (https://methods.cochrane.org/bias/) (20).
Weight Loss
All four RCTs involving children reported significant reductions in body mass index (BMI) among participants in the intervention group compared with baseline: Jensen and colleagues (23) reported a −0.2 (95% confidence interval, −0.4 to −0.1) BMI z-score reduction, Abd El-Kader and colleagues (22) reported a −15.9% BMI reduction (no confidence interval reported), Luna-Pech and colleagues (24) reported a change in BMI z-score from 2.18 ± 0.3 at baseline to 1.66 ± 0.2 postintervention, and Willeboordse and colleagues (25) reported a BMI–standard deviation score reduction of −0.14 ± 0.29. All trials except Willeboordse and colleagues showed statistically significant differences in BMI reduction between the intervention and control group; Willeboordse and colleagues reported a significant BMI reduction in the control group (BMI–standard deviation score, −0.12 ± 0.34) in addition to the intervention group and noted that 29 of 44 control group participants sought professional weight loss help outside the study (25). Associations between the intervention type and the resulting weight loss could not be reasonably assessed because of the small numbers of trials. The importance of trial duration was also unclear: Luna-Pech and colleagues (24) reported that the most significant effects on BMI reduction were seen after 10 weeks and achieved statistical significance only after 20 weeks, whereas the other studies found significant results in less than 10 weeks.
Lung Function Changes
None of the pediatric studies reporting forced expiratory volume in 1 second (FEV1) or FEV1/forced vital capacity (FVC) found significant differences between groups (23–25). Willeboordse and colleagues reported a significant improvement in FEV1 (percent predicted) after 18 months in both intervention and control groups (90.5–99.7% and 89.3–95.1%, respectively) with no significant between-group differences (25). They also reported a significant between-group difference in FVC (percent predicted) improvement, with a mean increase of 10.1% in the intervention arm.
Expiratory reserve volume (ERV) is decreased in children with asthma and obesity (26), making it another potential disease severity marker. Jensen and colleagues (23) and Willeboordse and colleagues (25) reported within-group improvements in ERV in their intervention arms (i.e., compared with baseline), but these were not statistically different than the control subjects. Total lung capacity (TLC) improved in both arms in the study by Willeboordse and colleagues (25), with no between-group differences, whereas Jensen and colleagues (23) reported no changes in TLC in either arm. Jensen and colleagues further reported that residual volume (RV) and RV/TLC, which may be used to evaluate asthma-related airway obstruction and air trapping, significantly improved in the intervention group compared with baseline (mean improvement, −0.4 L for RV and −6.9% for RV/TLC), although again with no between-group differences (23).
Inflammatory Markers
Jensen and colleagues (23) reported that decreases in exhaled nitric oxide (FeNO, a marker of eosinophilic airway inflammation [27]) correlated with the reduction in BMI z-score (r = 0.46, P = 0.034) during their trial; they also noted a decrease in C-reactive protein (CRP) in their intervention group, with a significant between-group difference (P = 0.037). Abd El-Kader and colleagues (22) reported decreases in leptin and interleukin (IL)-6 and increases in adiponectin in the intervention group compared with control subjects, whereas Jensen and colleagues (23) and Willeboordse and colleagues (25) did not observe significant changes in any of these adipokines. Abd El-Kader and colleagues (22) also reported decreases in tumor necrosis factor-α and IL-8, both of which can be increased in asthma (28, 29) and in obesity (30, 31), in the intervention arm compared with control subjects.
AR-QOL and Symptom Control
Two studies reported on AR-QOL using the Pediatric Asthma Quality of Life Questionnaire, which measures AR-QOL for children on a seven-point scale, in which higher scores indicate better AR-QOL (32): Luna-Pech and colleagues (24) found a mean overall Pediatric Asthma Quality of Life Questionnaire increase of 0.8 points in the intervention arm that was significantly greater than in the control subjects, and Willeboordse and colleagues (25) reported a mean increase of 0.48 points in the intervention group (P < 0.05) that approached but did not reach statistical significance compared with their control subjects (P = 0.06). Jensen and colleagues (23) measured asthma control using the Asthma Control Questionnaire (ACQ), in which lower scores indicate better asthma control (33): they reported a significant improvement in the intervention arm compared with control subjects by a mean of −0.4 points, despite the fact that the intervention arm had a higher score at randomization.
RCTs in Adult Populations
We found six RCTs evaluating weight loss interventions in adults (total n = 502; Table 3) (34–39). As with the pediatric trials, interventions used varied widely and included diet, exercise, and combined approaches. All of the dietary interventions included caloric restrictions; Scott and colleagues (2013 [34] and 2015 [35]) used a meal replacement plan as part of their dietary intervention, and Dias-Júnior and colleagues (37) also included pharmaceutical weight loss with orlistat and sibutramine. Exercise interventions varied from weekly sessions to twice-weekly sessions with individual aerobic training goals. Programs ranged in duration from 8 weeks to 18 months. Most studies had a low to moderate risk of bias (Table 2).
Table 3.
Summary of studies in adult populations
| Study | Population | Intervention | Control | Timing | Outcome(s) | Result(s) |
|---|---|---|---|---|---|---|
| Freitas et al. 2017 (38) NCT02188940 |
55 obese patients (30–60 yr old) with moderate to severe asthma (50 of 51 included for analysis were women) |
Individual hypocaloric diet counseling sessions plus aerobic and resistance exercise |
Individual hypocaloric diet counseling sessions, plus sham exercise (stretching and breathing) |
3 mo (weekly counseling sessions, exercise sessions twice a week) |
Primary: ACQ score. Other: BMI, body composition, fitness and strength measures, FEV1, FVC, TLC, ERV, AQLQ, ACQ improvement, AQLQ improvement, FeNO, leptin, adiponectin, TNF-α, CRP, vitamin D, IL-4, IL-6, IL-10, other biomarkers |
Weight: Significant between-group differences in BMI and total fat mass improvements |
| PFTs: Significant between-group differences in ERV and FeNO | ||||||
| Inflam: Significant between-group differences in leptin, adiponectin, TNF-α, IL-4, IL-6, and IL-10 | ||||||
| QOL: Significant between-group differences in ACQ and AQLQ scores. ACQ improvement correlated with improvements in aerobic fitness, fat and lean mass, FVC, FeNO, IL-6, IL-10, and adiponectin | ||||||
| Ma et al. 2015 (36) NCT00901095 |
330 obese adults (18–70 yr old) with uncontrolled asthma |
Multistage intervention with increased physical activity and counseling focused on weight loss |
Standard care plus weight and asthma self-management tools |
12 mo; frequency of sessions varied by stage |
Primary: ACQ score. Other: Weight, BMI, waist circumference, physical activity, energy expenditure, FEV1, FVC, FEV1/FVC, ACT, mini AQLQ, medication use, exacerbations requiring corticosteroids, healthcare encounters |
Weight: Significant between-group differences in BMI and proportion of participants with weight loss ≥5%, 7%, and 10% of baseline |
| PFTs: No significant differences reported at 6 or 12 mo | ||||||
| QOL: No significant differences at 6 or 12 mo. Participants who lost >10% of baseline weight (including both groups) were more likely to show clinically significant improvements in ACQ (OR, 3.8; 95% CI, 1.72–8.31); those who lost 5–10% had a smaller effect (OR, 2.2; 95% CI, 1.08-4.46) | ||||||
| Scott et al. 2015 (35) ACTRN12611000235909 (A/NZ) |
38 overweight and obese adults (mean age, 40 yr) with asthma |
1) Dietary intervention with partial meal replacement, 2) exercise intervention with regular gym sessions, or 3) combined diet and exercise |
Not applicable; the three intervention arms were compared |
10 wk, with exercise sessions 3 times per week |
Weight, waist circumference, percentage body fat, eating behaviors, energy intake, food diary, steps/d, FEV1, FVC, FEV1/FVC, AQLQ, ACQ; as well as effect of sex on body composition, eating behaviors, intake, and physical activity. |
Weight: Subjects in the diet or combined arms lost more weight than those in the exercise-only group. |
| PFTs: Did not report pulmonary function tests postintervention. Lower baseline FEV1 was associated with greater weight loss. | ||||||
| QOL: Subjects with lower asthma-related QOL at baseline showed a greater weight loss. | ||||||
| Dias-Júnior et al. 2014 (37) NCT01049657 |
33 moderately obese adults (mean age, 43 yr) with severe uncontrolled asthma |
Weight loss program with low caloric intake, daily sibutramine (10 mg), and daily orlistat (max 120 mg/d) plus bimonthly asthma clinics |
Bimonthly asthma clinics |
6 mo |
Primary: ACQ. Other: BMI, ACT, SABA use, symptom-free days, ED visits, exacerbations, SGRQ, FEV1, FVC, FEV1/FVC, FEF25–75%, IC, TLC, ERV, RV, RV/TLC, Raw, Gaw, PD20, FeNO, sputum cell counts, serum biomarkers (IgE, CRP, leptin, eotaxin, TGF-β1) . |
Weight: Significant within-group and between-group differences in BMI in the intervention group |
| PFTs: Significant within-group change in FVC (liters, but not % predicted); also significant between-group difference in FVC (liters) for those with >10% weight loss. All other pulmonary function outcomes nonsignificant | ||||||
| Inflam: All changes in sputum and serum inflammatory markers were nonsignificant. | ||||||
| QOL: Within-group and between-group differences were significant for ACQ, ACT, and SGRQ scores. | ||||||
| Scott et al. 2013 (34) ACTRN12611000235909 (A/NZ) |
46 overweight and obese nonsmoking adults (mean age, 40 yr) with asthma |
1) Dietary intervention with partial meal replacement, 2) exercise intervention with regular gym exercise sessions, or 3) combined diet and exercise |
Not applicable; the three intervention arms were compared |
10 wk, with exercise sessions 3 times per week |
Weight, weight loss, waist circumference, fat and lean mass, total energy intake, dietary intake, physical activity, FEV1, FVC, FEV1/FVC, TLC, FRC, ERV, RV, airway hyperresponsiveness (hypertonic saline provocation test), ACQ, AQLQ, induced sputum cell counts, leptin, adiponectin, CRP, IL-6 |
Weight: Both the dietary (8.5%) and combined (8.3%) intervention groups achieved significant weight loss. |
| PFTs: Between-group differences in TLC were seen in the exercise (+2.2%) and combined (+1.2%) groups vs, the dietary group. ERV increased (21.4%) from baseline to postintervention in the dietary group. The proportion of subjects with AHR decreased in combined group (100% vs. 66.7%). When stratified by weight loss (rather than by intervention), FRC and ERV increased among those in the highest weight loss group. | ||||||
| Inflam: Within-group improvements: airway eosinophilia decreased from baseline in the exercise group only (−1.3%), leptin decreased in the dietary (−5.3 µg/L) and combined (−6.2 µg/L) groups, and IL-6 decreased in the combined group (−0.18 pg/mL). | ||||||
| QOL: ACQ scores improved in the dietary (−0.6 points) and combined (−0.5 points) arms compared with baseline; AQLQ scores improved in all groups compared with baseline. No between-group differences. | ||||||
| Stenius-Aarniala et al. 2000 (39) |
38 obese patients (mean age, 49 yr) with asthma |
12 weight reduction group sessions, low-energy diet preparation |
12 nonspecific group sessions |
14 wk, with an 8-wk “dieting period” in the intervention group, and follow-up at 1 year |
Weight, weight loss, PEF, FEV1, FVC, cough, dyspnea, medication use, exacerbations, oral steroid courses, SGRQ, serum and urine cortisol. |
Weight: Mean weight loss in the treatment group was 14.2 kg at 14 wk (end of program) and 11.1 kg at 1 yr; the control group lost 0.3 kg at 14 wk and gained 2.3 kg at 1 yr (no P values reported). |
| PFTs: Significant between-group improvements in FEV1 and FVC at all time points. At 1 yr, the mean changes in both FEV1 and FVC were 7.6% higher in the intervention group. No between-group differences in PEF. | ||||||
| QOL and medications: Dyspnea and rescue medication use decreased by the end of the intervention. Significant reduction in exacerbations between groups. At 1 yr, SGRQ symptom score was 12 points lower and total score 10 points lower in total score in the intervention group. |
Definition of abbreviations: ACQ = Asthma Control Questionnaire; ACT = Asthma Control Test; AHR = airway hyper-reactivity; A/NZ = Australia/New Zealand; AQLQ = Asthma Quality of Life Questionnaire; BMI = body mass index; CI = confidence interval; CRP = C-reactive protein; ED = emergency department; ERV = expiratory residual volume; FEF25–75% = forced expiratory flow at 25–75% of pulmonary volume; FeNO = fractional exhaled nitric oxide; FEV1 = forced expiratory volume in 1 second; FRC = functional residual capacity; FVC = forced vital capacity; Gaw = airway conductance; IC = inspiratory capacity; IgE = immunoglobulin E; IL = interleukin; inflam = inflammatory markers; OR = odds ratio; PD20 = provocative dose of methacholine causing a 20% fall in FEV1; PEF = peak expiratory flow; PFTs = pulmonary function tests; QOL = quality of life; Raw = airway resistance; RV = residual volume; SABA = short-acting β-agonist; SGRQ = St. George’s Respiratory Questionnaire; TGF = transforming growth factor; TLC = total lung capacity; TNF = tumor necrosis factor.
Results group by weight loss, PFTs, inflammatory markers, and QOL. Between-group differences refer to those between the intervention arm versus the control subjects. Within-group differences refer to those comparing postintervention to baseline within the intervention arm.
Weight Loss
All RCTs involving adults successfully achieved weight loss in their intervention groups, which was maintained even in the studies with longer follow-up periods: Stenius-Aarniala and colleagues (39) reported a weight reduction of 14.5% in the intervention group (vs. 0.3% in the control subjects) at the end of the 8-week program and an 11.3% reduction (vs. 2.2% gain) at the 1-year follow-up; likewise, Ma and colleagues (36) reported 5% and 4.1% weight loss at 6 and 12 months (vs. 1.3% and 2.1% in the control subjects; P < 0.01 for both). Scott and colleagues reported a greater weight loss in the dietary (8.5%) and combined (8.3%) groups than in the exercise-only group (1.8%), leading the authors to speculate that the effects were likely due to dietary restrictions rather than the exercise component (34). This is in contrast to the trial by Freitas and colleagues, which reported greater weight loss in the combined weight loss program (diet and exercise) than in the group with diet and sham activity (38).
The 2015 Scott and colleagues trial reported that men lost on average 3.6 kg weight and 3.3 cm waist circumference more than women (P = 0.087 and P = 0.022, respectively); they also reported sex differences in weight loss–associated behaviors, with reductions in emotional and uncontrolled eating associated with greater weight loss in women and more restrained eating associated with greater weight loss in men (35). No other studies analyzed weight loss differences between men and women. Of note, the trial by Freitas and colleagues included 50 women (and only one male participant), and they reported improvements in several body composition and fitness outcomes (38). Thus, the role of sex as an effect modifier in weight loss for obese asthma warrants further investigation.
Lung Function
Most adult RCTs reported at least one significant lung function outcome. Stenius-Aarniala and colleagues reported significant between-group changes in both FEV1 and FVC at all time points, with both FEV1 and FVC on average 7.6% higher at 1 year in the intervention group than in the control subjects (39). Freitas and colleagues reported improvements in FEV1, FVC, and ERV compared with baseline in the combined (weight loss plus exercise) group (38). Dias-Júnior and colleagues reported a significant improvement in FVC in liters (P = 0.006 between groups) but not FVC % predicted (37). On the other hand, the largest RCT to date (Ma and colleagues [36], n = 330) reported no significant changes in FEV1 or FVC, and none of the trials found intervention-related improvements in FEV1/FVC.
TLC was measured in two studies: Scott and colleagues’ 2013 trial (34) reported significant improvements in TLC among the exercise (+0.13 L) and combined (+0.06 L) groups compared with the diet-only group (P = 0.037), whereas Freitas and colleagues found no significant improvements (38). Similarly, Scott and colleagues (34) reported a change in ERV in the diet arm only (+0.28 L), whereas Freitas and colleagues (38) reported a significant difference in ERV between arms (group × time interaction P = 0.038).
Inflammation
In terms of airway inflammatory markers, Freitas and colleagues (38) reported decreased FeNO in the combined intervention group (−6.8 ppb, group × time P < 0.001), whereas Dias-Júnior and colleagues (37) found no significant changes. Scott and colleagues (34) reported a slight reduction in airway eosinophilia in the exercise group compared with baseline (−1.3%, P < 0.05), although it was not significantly different compared with the diet or combined groups (P = 0.76). In addition, they reported reductions in neutrophilic airway inflammation associated with gynoid adipose tissue reduction among women (P = 0.047) and with the reduction of dietary saturated fat in men (P = 0.041).
In addition, three of the six RCTs involving adults (34, 37, 38) reported serum inflammatory markers. Leptin significantly decreased in the combined arm (mean decrease, −1.8 mg/L) in the study by Freitas and colleagues (38), in both the dietary (−5.3 μg/L) and combined (−6.2 μg/L) arms in Scott and colleagues (34), and in both the intervention and control arms in Dias-Júnior and colleagues (but with no significant between-group differences) (37). Adiponectin levels increased in the combined arm in Freitas and colleagues (38) (1.1 mg/L, group × time interaction P = 0.027), whereas Scott and colleagues (34) reported no significant changes in any of their groups. IL-6 decreased significantly in the combined arm in Freitas and colleagues (38) (−0.49 pg/ml) and in the combined arm in Scott and colleagues (−0.18 pg/ml) (34). Freitas and colleagues also reported significant reductions and group × time interactions for serum IL-6, IL-10, tumor necrosis factor-α, and vitamin D (38). None of the three trials found significant changes in CRP.
Asthma-related Quality of Life and Symptom Control
Freitas and colleagues (38) reported a significant improvement in AQLQ scores in the combined group compared with the diet only (sham exercise) control subjects, whereas Scott and colleagues (34) reported improvements from baseline in all of their groups regardless of intervention. Similarly, Scott and colleagues reported that each 1-point improvement in AQLQ score corresponded to a 1.5% greater weight loss after adjusting for intervention type (35). In contrast, Ma and colleagues did not find a significant change in the mini-AQLQ (or any subscales) at 6 or 12 months (36). The St. George’s Respiratory Questionnaire (in which lower scores indicate better AR-QOL) (40) was reported in two trials: Dias-Júnior and colleagues (37) noted a significant between-group difference (65.12 ± 3.02 at baseline and 45.47 ± 4.42 postintervention vs. no improvement in the control subjects; P = 0.011), and Stenius-Aarniala and colleagues (39) reported the intervention group to be 10 points lower than control subjects at 1 year.
In terms of asthma control, Freitas and colleagues (38) reported that 69% of participants in the intervention group achieved a clinically significant improvement in ACQ (vs. 36% in the control subjects); Scott and colleagues (34) reported improvements from baseline in the diet and combined groups (−0.6 points and −0.5 points, respectively); and Dias-Júnior and colleagues (37) found significant between-group differences as well (baseline ACQ score 3.04 ± 0.25 and 1.64 ± 0.19 postintervention, vs. no improvement among control subjects). Of interest, Freitas and colleagues reported that ACQ reductions correlated with improvements in aerobic capacity, FVC, and inflammatory biomarkers (IL-6, FeNO, IL-10, and adiponectin) (38). On the other hand, Ma and colleagues reported no significant changes in ACQ from baseline or between groups (36). Only Dias-Júnior and colleagues assessed asthma control using the Asthma Control Test: they reported a significant improvement in the intervention group (mean Asthma Control Test, 12.3 ± 1.1 at baseline and 17.4 ± 1.1 at 6 mo) (37).
Discussion
Current evidence shows that weight loss interventions can be successful in reducing weight and improving fitness in overweight/obese children and adults with asthma. In fact, one could speculate that poorer asthma control could provide additional motivation for these individuals to lose weight, as has been reported for other diseases and conditions (41). Existing RCTs also generally tend to show improvements in obesity biomarkers, AR-QOL, and, to a certain extent, asthma symptom control and other subjective measures. However, it is less clear whether weight loss leads to improvements in lung function or asthma-related biomarkers, including markers of airway inflammation. In the pediatric trials, the large differences seen among studies could be due to differences in the populations studied: Abd El-Kader and colleagues (22) included the heaviest subjects with the lowest baseline FEV1 and FVC, whereas Luna-Pech and colleagues (24) and Jensen and colleagues (23) included participants with normal baseline lung function, which may have impacted the probability of improvement during the trial. In adults, several studies showed improvements in lung function and inflammatory biomarkers; however, the largest trial to date (Ma and colleagues [36]) found no significant changes in any of the outcomes they assessed. The fact that AR-QOL improved in the absence of objective improvements in lung function or other biomarkers makes it difficult to separate the general benefits of weight loss and improved fitness from any asthma-specific effects. Overall, however, there is significant lack of high-quality studies examining weight loss in a standardized manner in both adults and children with asthma. Thus, it is difficult to assess the generalizability of the existing studies, and further evidence is needed before clinical recommendations can be made.
Although this review focused on RCTs evaluating weight loss in obese children and adults with asthma, the role of exercise and improved fitness should also be studied. Independent of obesity, improvements in body composition and aerobic fitness are associated with decreased systemic inflammation (42), and exercise training in subjects with asthma may improve asthma control, quality of life, and airway inflammation (43). About half of the trials included in this review used exercise as part of their intervention, although duration and intensity varied widely. Only two studies evaluated aerobic fitness, and they reported significant improvements in their intervention groups (25, 38). Thus, improvements in aerobic fitness may have independent and complementary effects from changes in body weight.
Limitations of Existing RCTs
Likely owing to the difficulties in these types of interventions, most studies to date have included small sample sizes and thus may have been underpowered to detect small effects or effects with high variability. In at least one study, the small sample size also resulted in significantly different baseline ACQ scores, despite block randomization (23). There was wide variability in inclusion and exclusion criteria (e.g., patients with “stable asthma” vs. “uncontrolled asthma,” or patients at “high risk for asthma” but no formal diagnosis), baseline and postintervention measurements and outcomes, length of follow-up, and baseline population characteristics. One indeed expects trials to propose different interventions, but the broad variability in all other study characteristics leaves substantial room for confounding. Discrepancies between trials could be due to the intervention used but also due to length of follow-up; for example, weight may begin to increase again after an initial period of weight loss, and thus any differences could be short lived. Likewise, trials in which participants had normal baseline lung function may be less likely to find improvements than those in which participants had abnormal baseline lung function.
We strived to perform a meta-analysis of asthma-related outcomes and biomarkers where feasible, but we were unable to do so for several reasons, including the small number of studies, differences in outcomes and measurements reported, differing units used (e.g., liters vs. percent predicted), differences in reporting (e.g., change from baseline for each arm vs. differences between arms at each time point), and lack of objective data required to perform a pooled analysis (e.g., means or confidence intervals not reported). There were also substantial differences in statistical approach, with some trials reporting within-group differences (compared with baseline), others reporting between-group differences at the end of the study, and some reporting group × time interactions, which further hinders the ability to compare results among trials.
Conclusions and Recommendations
Overall, all of the RCTs involving children and adults were successful in improving weight or BMI. In general, RCTs reported improvements in AR-QOL and, to some degree, asthma control. Studies involving adults also reported improvements in lung function measures, whereas those involving children showed less consistent results. The existing studies have yielded important and useful information, but the heterogeneity makes it difficult to assess their generalizability and thus to make broad clinical recommendations at this time.
Both in pediatrics and adults, it will be critical that future studies report standardized outcomes in a uniform manner, so that interventions can be better compared and readers can assess whether results are generalizable or applicable to their own populations. Trials should adhere to recommended reporting guidelines, such as the CONSORT (CONsolidated Standards of Reporting Trials) statement (44) or others. Future studies should also investigate the possibility of a threshold effect, as some of the adult trials reported significant changes beyond a certain weight or BMI improvement level. In addition to subjective measures of asthma control and quality of life, studies should include objective outcomes, including lung function, systemic and airway inflammatory markers, and “hard” clinical outcomes such as moderate or severe exacerbations (45). Finally, it will be of critical importance for studies to describe their interventions in more detail, which will allow clinicians to better understand the interventions that patients might need as well as allow other researchers to reproduce their results in other populations.
As clinicians, we need to educate obese patients in general, and particularly those with asthma, about the importance of weight loss interventions (i.e., diet, exercise, behavioral therapy) to reduce weight and improve fitness overall. Although current evidence may not allow us to determine which types of interventions may be most successful, or whether they may lead to objective improvements in lung function and airway inflammation biomarkers, we can assure our patients that successful weight reduction can improve AR-QOL and possibly asthma control.
Supplementary Material
Footnotes
Supported by University of California at Irvine Clinical and Translational Science Award (CTSA) grant UL1-TR00141 (K.D.L.); National Institutes of Health grant HL125666 (E.F.); and an award from the Klosterfrau Foundation (E.F.). The funding agencies had no role in study design, data collection or interpretation, writing of the report, or in the decision to submit the manuscript for publication.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: national health interview survey, 2011. Vital Health Stat 10. 2012;(254):1–88. [PubMed] [Google Scholar]
- 2.World Health Organization. Asthma fact sheet. 2017 [Accessed 2018 Sep 2]. Available from: http://www.who.int/mediacentre/factsheets/fs307/en/.
- 3.Centers for Disease Control and Prevention. Asthma facts. 2013 [accessed 2018 Sep 2]. Available from: https://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdf.
- 4.Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15:348–356. doi: 10.1513/AnnalsATS.201703-259OC. [DOI] [PubMed] [Google Scholar]
- 5.Gibson GJ, Loddenkemper R, Sibille Y, Lundback B.The economic burden of lung disease.The European lung white book: respiratory health and disease in Europe. UK: Charlesworth Press; European Respiratory Society; 201316–27. [Google Scholar]
- 6.Gibson GJ, Loddenkemper R, Lundbäck B, Sibille Y. Respiratory health and disease in Europe: the new European Lung White Book. Eur Respir J. 2013;42:559–563. doi: 10.1183/09031936.00105513. [DOI] [PubMed] [Google Scholar]
- 7.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Obesity and overweight. 2018. [accessed 2018 Sep 2]. Available from: http://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight.
- 9.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief. 2017;(288):1–8. [PubMed] [Google Scholar]
- 10.Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med. 2017;377:2145–2153. doi: 10.1056/NEJMoa1703860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood LG. Metabolic dysregulation: driving the obese asthma phenotype in adolescents? Am J Respir Crit Care Med. 2015;191:121–122. doi: 10.1164/rccm.201412-2221ED. [DOI] [PubMed] [Google Scholar]
- 13.Lang JE, Hossain J, Dixon AE, Shade D, Wise RA, Peters SP, et al. American Lung Association-Asthma Clinical Research Centers. Does age impact the obese asthma phenotype? Longitudinal asthma control, airway function, and airflow perception among mild persistent asthmatics. Chest. 2011;140:1524–1533. doi: 10.1378/chest.11-0675. [DOI] [PubMed] [Google Scholar]
- 14.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141:1169–1179. doi: 10.1016/j.jaci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forno E, Celedón JC. The effect of obesity, weight gain, and weight loss on asthma inception and control. Curr Opin Allergy Clin Immunol. 2017;17:123–130. doi: 10.1097/ACI.0000000000000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults--the evidence report. Obes Res. 1998;6:51S–209S. [PubMed] [Google Scholar]
- 17.Thomas JG, Bond DS, Phelan S, Hill JO, Wing RR. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med. 2014;46:17–23. doi: 10.1016/j.amepre.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Ulrik CS. Asthma and obesity: is weight reduction the key to achieve asthma control? Curr Opin Pulm Med. 2016;22:69–73. doi: 10.1097/MCP.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 19.Lv N, Xiao L, Ma J.Weight management interventions in adult and pediatric asthma populations: a systematic review J Pulm Respir Med 20155pii: 1000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abd El-Kader MS, Al-Jiffri O, Ashmawy EM. Impact of weight loss on markers of systemic inflammation in obese Saudi children with asthma. Afr Health Sci. 2013;13:682–688. doi: 10.4314/ahs.v13i3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen ME, Gibson PG, Collins CE, Hilton JM, Wood LG. Diet-induced weight loss in obese children with asthma: a randomized controlled trial. Clin Exp Allergy. 2013;43:775–784. doi: 10.1111/cea.12115. [DOI] [PubMed] [Google Scholar]
- 24.Luna-Pech JA, Torres-Mendoza BM, Luna-Pech JA, Garcia-Cobas CY, Navarrete-Navarro S, Elizalde-Lozano AM. Normocaloric diet improves asthma-related quality of life in obese pubertal adolescents. Int Arch Allergy Immunol. 2014;163:252–258. doi: 10.1159/000360398. [DOI] [PubMed] [Google Scholar]
- 25.Willeboordse M, van de Kant KDG, Tan FE, Mulkens S, Schellings J, Crijns Y, et al. A multifactorial weight reduction programme for children with overweight and asthma: a randomized controlled trial. PLoS One. 2016;11:e0157158. doi: 10.1371/journal.pone.0157158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen ME, Gibson PG, Collins CE, Wood LG. Airway and systemic inflammation in obese children with asthma. Eur Respir J. 2013;42:1012–1019. doi: 10.1183/09031936.00124912. [DOI] [PubMed] [Google Scholar]
- 27.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi IW, Sun-Kim, Kim YS, Ko HM, Im SY, Kim JH, et al. TNF-alpha induces the late-phase airway hyperresponsiveness and airway inflammation through cytosolic phospholipase A(2) activation. J Allergy Clin Immunol. 2005;116:537–543. doi: 10.1016/j.jaci.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 29.Nocker RE, Schoonbrood DF, van de Graaf EA, Hack CE, Lutter R, Jansen HM, et al. Interleukin-8 in airway inflammation in patients with asthma and chronic obstructive pulmonary disease. Int Arch Allergy Immunol. 1996;109:183–191. doi: 10.1159/000237218. [DOI] [PubMed] [Google Scholar]
- 30.Castro-Giner F, Kogevinas M, Imboden M, de Cid R, Jarvis D, Machler M, et al. Joint effect of obesity and TNFA variability on asthma: two international cohort studies. Eur Respir J. 2009;33:1003–1009. doi: 10.1183/09031936.00140608. [DOI] [PubMed] [Google Scholar]
- 31.Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes. 2006;30:1347–1355. doi: 10.1038/sj.ijo.0803259. [DOI] [PubMed] [Google Scholar]
- 32.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47:76–83. doi: 10.1136/thx.47.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juniper EF, Bousquet J, Abetz L, Bateman ED GOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, Callister R, et al. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clin Exp Allergy. 2013;43:36–49. doi: 10.1111/cea.12004. [DOI] [PubMed] [Google Scholar]
- 35.Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, Callister R, et al. Determinants of weight loss success utilizing a meal replacement plan and/or exercise, in overweight and obese adults with asthma. Respirology. 2015;20:243–250. doi: 10.1111/resp.12423. [DOI] [PubMed] [Google Scholar]
- 36.Ma J, Strub P, Xiao L, Lavori PW, Camargo CA, Jr, Wilson SR, et al. Behavioral weight loss and physical activity intervention in obese adults with asthma: a randomized trial. Ann Am Thorac Soc. 2015;12:1–11. doi: 10.1513/AnnalsATS.201406-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dias-Júnior SA, Reis M, de Carvalho-Pinto RM, Stelmach R, Halpern A, Cukier A. Effects of weight loss on asthma control in obese patients with severe asthma. Eur Respir J. 2014;43:1368–1377. doi: 10.1183/09031936.00053413. [DOI] [PubMed] [Google Scholar]
- 38.Freitas PD, Ferreira PG, Silva AG, Stelmach R, Carvalho-Pinto RM, Fernandes FL, et al. The role of exercise in a weight-loss program on clinical control in obese adults with asthma: a randomized controlled trial. Am J Respir Crit Care Med. 2017;195:32–42. doi: 10.1164/rccm.201603-0446OC. [DOI] [PubMed] [Google Scholar]
- 39.Stenius-Aarniala B, Poussa T, Kvarnström J, Grönlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ. 2000;320:827–832. doi: 10.1136/bmj.320.7238.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones PW, Quirk FH, Baveystock CM.The St George’s Respiratory Questionnaire Respir Med 19918525–31.[Discussion, pp. 33–27.] [DOI] [PubMed] [Google Scholar]
- 41.Gorin AA, Phelan S, Hill JO, Wing RR. Medical triggers are associated with better short- and long-term weight loss outcomes. Prev Med. 2004;39:612–616. doi: 10.1016/j.ypmed.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 42.Wedell-Neergaard AS, Eriksen L, Grønbæk M, Pedersen BK, Krogh-Madsen R, Tolstrup J. Low fitness is associated with abdominal adiposity and low-grade inflammation independent of BMI. PLoS One. 2018;13:e0190645. doi: 10.1371/journal.pone.0190645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eichenberger PA, Diener SN, Kofmehl R, Spengler CM. Effects of exercise training on airway hyperreactivity in asthma: a systematic review and meta-analysis. Sports Med. 2013;43:1157–1170. doi: 10.1007/s40279-013-0077-2. [DOI] [PubMed] [Google Scholar]
- 44.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.