Abstract
Background
Vascular endothelial growth factor (VEGF) plays a key role in angiogenesis in foetal life. Researchers have recently attempted to use anti‐VEGF agents for the treatment of retinopathy of prematurity (ROP), a vasoproliferative disorder. The safety and efficacy of these agents in preterm infants with ROP is currently uncertain.
Objectives
To evaluate the efficacy and safety of anti‐VEGF drugs when used either as monotherapy, that is without concomitant cryotherapy or laser therapy, or in combination with planned cryo/laser therapy in preterm infants with type 1 ROP (defined as zone I any stage with plus disease, zone I stage 3 with or without plus disease, or zone II stage 2 or 3 with plus disease).
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 11), MEDLINE (1966 to 11 December 2016), Embase (1980 to 11 December 2016), CINAHL (1982 to 11 December 2016), and conference proceedings.
Selection criteria
Randomised or quasi‐randomised controlled trials that evaluated the efficacy or safety of administration, or both, of anti‐VEGF agents compared with conventional therapy in preterm infants with ROP.
Data collection and analysis
We used standard Cochrane and Cochrane Neonatal methods for data collection and analysis. We used the GRADE approach to assess the quality of the evidence.
Main results
Six trials involving a total of 383 infants fulfilled the inclusion criteria. Five trials compared intravitreal bevacizumab (n = 4) or ranibizumab (n = 1) with conventional laser therapy (monotherapy), while the sixth study compared intravitreal pegaptanib plus conventional laser therapy with laser/cryotherapy (combination therapy).
When used as monotherapy, bevacizumab/ranibizumab did not reduce the risk of complete or partial retinal detachment (3 studies; 272 infants; risk ratio (RR) 1.04, 95% confidence interval (CI) 0.21 to 5.13; risk difference (RD) 0.00, 95% CI ‐0.04 to 0.04; very low‐quality evidence), mortality before discharge (2 studies; 229 infants; RR 1.50, 95% CI 0.26 to 8.75), corneal opacity requiring corneal transplant (1 study; 286 eyes; RR 0.34, 95% CI 0.01 to 8.26), or lens opacity requiring cataract removal (3 studies; 544 eyes; RR 0.15, 95% CI 0.01 to 2.79). The risk of recurrence of ROP requiring retreatment also did not differ between groups (2 studies; 193 infants; RR 0.88, 95% CI 0.47 to 1.63; RD ‐0.02, 95% CI ‐0.12 to 0.07; very low‐quality evidence). Subgroup analysis showed a significant reduction in the risk of recurrence in infants with zone I ROP (RR 0.15, 95% CI 0.04 to 0.62), but an increased risk of recurrence in infants with zone II ROP (RR 2.53, 95% CI 1.01 to 6.32). Pooled analysis of studies that reported eye‐level outcomes also revealed significant increase in the risk of recurrence of ROP in the eyes that received bevacizumab (RR 5.36, 95% CI 1.22 to 23.50; RD 0.10, 95% CI 0.03 to 0.17). Infants who received intravitreal bevacizumab had a significantly lower risk of refractive errors (very high myopia) at 30 months of age (1 study; 211 eyes; RR 0.06, 95% CI 0.02 to 0.20; RD ‐0.40, 95% CI ‐0.50 to ‐0.30; low‐quality evidence).
When used in combination with laser therapy, intravitreal pegaptanib was found to reduce the risk of retinal detachment when compared to laser/cryotherapy alone (152 eyes; RR 0.26, 95% CI 0.12 to 0.55; RD ‐0.29, 95% CI ‐0.42 to ‐0.16; low‐quality evidence). The incidence of recurrence of ROP by 55 weeks' postmenstrual age was also lower in the pegaptanib + laser therapy group (76 infants; RR 0.29, 95% CI 0.12 to 0.7; RD ‐0.35, 95% CI ‐0.55 to ‐0.16; low‐quality evidence). There was no difference in the risk of perioperative retinal haemorrhages between the two groups (152 eyes; RR 0.62, 95% CI 0.24 to 1.56; RD ‐0.05, 95% CI ‐0.16 to 0.05; very low‐quality evidence). However, the risk of delayed systemic adverse effects with any of the three anti‐VEGF drugs is not known.
Authors' conclusions
Implications for practice: Intravitreal bevacizumab/ranibizumab, when used as monotherapy, reduces the risk of refractive errors during childhood but does not reduce the risk of retinal detachment or recurrence of ROP in infants with type 1 ROP. While the intervention might reduce the risk of recurrence of ROP in infants with zone I ROP, it can potentially result in higher risk of recurrence requiring retreatment in those with zone II ROP. Intravitreal pegaptanib, when used in conjunction with laser therapy, reduces the risk of retinal detachment as well as the recurrence of ROP in infants with type 1 ROP. However, the quality of the evidence was very low to low for most outcomes due to risk of detection bias and other biases. The effects on other critical outcomes and, more importantly, the long‐term systemic adverse effects of the drugs are not known. Insufficient data precludes strong conclusions favouring routine use of intravitreal anti‐VEGF agents ‐ either as monotherapy or in conjunction with laser therapy ‐ in preterm infants with type 1 ROP.
Implications for research: Further studies are needed to evaluate the effect of anti‐VEGF agents on structural and functional outcomes in childhood and delayed systemic effects including adverse neurodevelopmental outcomes.
Plain language summary
Anti‐vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity
Background
Retinopathy of prematurity (ROP) is a vascular disorder of the immature retina that can result in impairment of vision and even blindness in preterm infants. It is treated primarily by ablation of the avascular retina, the removal of the part of the retina without any blood vessels by cryotherapy or laser therapy. Though these treatments result in a significant improvement in long‐term outcomes, the results are far from perfect. In addition, they cause permanent loss of the peripheral visual field. Recently, studies have been done to evaluate the use of anti‐VEGF agents to treat ROP. These agents inhibit the action of vascular endothelial growth factor (VEGF), a key regulator of new vessel formation in foetal life. Animal studies had shown significant reduction in the neovascular response following injection of anti‐VEGF antibodies into the vitreous cavity of the eyes ('intravitreal' therapy).
Study characteristics
We searched scientific databases in December 2016 for studies evaluating the efficacy and safety of intravitreal therapy with anti‐VEGF agents in preterm infants with ROP. We identified six randomised controlled trials involving 383 infants. Five trials compared intravitreal bevacizumab or ranibizumab with conventional laser therapy. One trial compared intravitreal pegaptanib plus laser therapy with laser/cryotherapy alone.
Key results
The results suggest that intravitreal anti‐VEGF agents reduce the risk of refractive errors (high myopia) during childhood but do not reduce the risk of retinal detachment or recurrence of ROP when used alone. Intravitreal pegaptanib used in conjunction with laser therapy reduces the risk of retinal detachment. The effects on other critical outcomes, including delayed side effects such as stroke, are not known. Further studies are needed to assess these outcomes.
Quality of the evidence
We graded the quality of the evidence as very low or low for most of the key outcomes.
Setting
Neonatal units in China, Czech Republic, Italy, Iran, Ireland, and USA.
Summary of findings
Summary of findings for the main comparison. Anti‐vascular endothelial growth factor therapy compared to conventional laser/cryotherapy in preterm infants with type 1 retinopathy of prematurity.
| Anti‐vascular endothelial growth factor (anti‐VEGF) therapy compared to conventional laser/cryotherapy in preterm infants with type 1 retinopathy of prematurity (ROP) | ||||||
| Patient or population: preterm infants with type 1 ROP Setting: neonatal units Intervention: anti‐VEGF therapy Comparison: conventional laser/cryotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with conventional laser/cryotherapy | Risk with anti‐VEGF therapy | |||||
| Structural outcome ‐ retinal detachment | Study population | RR 1.04 (0.21 to 5.13) | 272 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| 15 per 1000 | 16 per 1000 (3 to 77) | |||||
|
Structural outcome ‐ complete retinal detachment (unit of analysis: eyes) |
Study population | RR 0.33 (0.01 to 7.50) | 26 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 3 4 5 | ||
| 77 per 1000 | 25 per 1000 (1 to 577) | |||||
|
Refractive error ‐ very high myopia ‐ at 30 months of age (unit of analysis: eyes) |
Study population | RR 0.06 (0.02 to 0.20) | 211 (1 RCT) | ⊕⊕⊝⊝ LOW 1 4 | ||
| 416 per 1000 | 25 per 1000 (8 to 83) | |||||
| Mortality before discharge from primary hospital | Study population | RR 1.50 (0.26 to 8.75) | 229 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 3 5 | ||
| 18 per 1000 | 27 per 1000 (5 to 158) | |||||
| Mortality at 30 months of age | Study population | RR 0.86 (0.30 to 2.45) | 150 (1 RCT) | ⊕⊕⊝⊝ LOW 2 3 5 | ||
| 93 per 1000 | 80 per 1000 (28 to 229) | |||||
|
Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes) |
Study population | RR 0.34 (0.01 to 8.26) | 286 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 | ||
| 7 per 1000 | 2 per 1000 (0 to 57) | |||||
|
Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes) |
Study population | RR 0.15 (0.01 to 2.79) | 544 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 | ||
| 11 per 1000 | 2 per 1000 (0 to 31) | |||||
| Recurrence of ROP (up to 6 months of age) | Study population | RR 0.88 (0.47 to 1.63) | 193 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 6 | ||
| 204 per 1000 | 180 per 1000 (96 to 333) | |||||
|
Recurrence of ROP (unit of analysis: eyes) |
Study population | RR 5.36 (1.22 to 23.50) | 188 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 7 | ||
| 23 per 1000 | 123 per 1000 (28 to 540) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Outcome assessment not masked. 295% CI around the pooled estimate includes both 1) no effect and 2) appreciable benefit or appreciable harm. 3Number of events too small. 4Serious risk of bias in analysis (unit of analysis error) in one or more of the included studies. 5Outcome assessment not masked, but outcome is objective. 6Evidence of large heterogeneity (I2 = 86%). 7Unclear risk of selection bias (details of allocation concealment not provided in the individual studies).
Summary of findings 2. Anti‐vascular endothelial growth factor therapy combined with laser/cryotherapy compared to laser/cryotherapy in preterm infants with type 1 retinopathy of prematurity.
| Anti‐vascular endothelial growth factor (anti‐VEGF) therapy combined with laser/cryotherapy compared to laser/cryotherapy in preterm infants with type 1 retinopathy of prematurity (ROP) | ||||||
| Patient or population: preterm infants with type 1 ROP Settings: neonatal units Intervention: anti‐VEGF combined with laser/cryotherapy Comparison: laser/cryotherapy | ||||||
| Outcomes* | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with conventional laser/cryotherapy | Risk with anti‐VEGF therapy | |||||
|
Structural outcome ‐ retinal detachment (unit of analysis: eyes) |
Study population | RR 0.26 (0.12 to 0.55) | 152 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2,3 | ||
| 393 per 1000 | 102 per 1000 (47 to 216) | |||||
|
Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes) |
Study population | RR 0.62 (0.24 to 1.56) | 152 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2,3,4 | ||
| 143 per 1000 | 89 per 1000 (34 to 223) | |||||
| Recurrence of ROP by 55 weeks' postmenstrual age | Study population | RR 0.29 (0.12 to 0.7) | 76 (1 RCT) | ⊕⊕⊝⊝ LOW 1,3 | ||
| 500 per 1000 | 145 per 1000 (60 to 350) | |||||
| *Only the outcomes for which data are available are reported here. #The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Outcome assessment not masked. 2Serious risk of bias in analysis (unit of analysis error). 3Unclear risk of selection bias 495% CI around the pooled estimate includes both 1) no effect and 2) appreciable benefit or appreciable harm.
Background
Description of the condition
Retinopathy of prematurity (ROP) is one of the major avoidable causes of childhood blindness in high‐, middle‐, and low‐income countries (Gilbert 2008). Essentially a neovascularising disease of the retina, ROP occurs mostly in preterm very low birth weight (VLBW) infants. With improved survival of VLBW infants, the absolute number of children with visual impairment secondary to ROP has increased in recent years (Gilbert 2005; Mantagos 2009).
The incidence of ROP varies inversely with gestation and weight at birth. A multicentre study of infants born between 1986 and 1987 reported that 81.6% of infants weighing less than 1000 g developed ROP, while only 46.9% of those weighing 1000 g to 1250 g had ROP (Palmer 1997).
Other risk factors of ROP include exposure to varying oxygen concentrations, hypercapnia, anaemia, acidosis, chronic lung disease, and intraventricular haemorrhage (Ashton 1953; Smith 2003; Tasman 2006).
The predisposition of preterm infants to the development of ROP relates to their immature retinal vasculature. In humans, retinal vascularisation begins at about 12 weeks and is completed by 36 to 40 weeks of gestation. Normally, the blood vessels develop from the optic disc and then progress outwards towards the ora serrata. Infants born before this period will, therefore, have an immature retina with a peripheral avascular zone. Retinopathy of prematurity develops if there is a disruption in the new vessel formation (angiogenesis) in this zone. The disruption of angiogenesis has been found to occur in two sequential phases: a vaso‐obliterative phase followed by a vaso‐proliferative phase (Ashton 1954). In the vaso‐obliterative phase (phase 1), the normally high arterial oxygen saturation in the postnatal life coupled with hyperoxia secondary to oxygen supplementation leads to involution and loss of formed blood vessels. In the vaso‐proliferative phase (phase 2), the relatively hypoxic environment due to ischaemia caused by vessel loss coupled with the high metabolic demands of the avascular retina leads to upregulation of various angiogenic factors, resulting in abnormal neovascularisation. In most infants, the newly formed vessels regress without leaving any sequelae. However, in some infants the neovascularisation goes unchecked leading to retinal scarring, traction, and finally detachment.
The extent and severity of ROP are traditionally described in terms of location (zones; I to III), severity (stages; 1 to 5), extent (clock hours; 1 to 12), and vascular dilatation and tortuosity (plus disease) according to the International Classification of ROP definitions (Committee for Classification of ROP 1984). In addition to defining the progression of the disease, this classification also serves as a guide for surgical intervention. In 2005, the classification was revised to include aggressive posterior ROP (AP‐ROP), pre‐plus disease, and a practical way to estimate the extent of zone I with the indirect ophthalmoscope. Concomitantly, the recommendations for treatment were also revised based on the results of the Early Treatment for Retinopathy of Prematurity trial (ETROP Group 2003). The new recommendations place more emphasis on the presence of plus disease, rather than the number of clock hours, to decide upon the need for treatment (American Academy of Pediatrics 2006). Accordingly, treatment is initiated for the following retinal findings (type 1 ROP).
Zone I ROP: any stage with plus disease
Zone I ROP: stage 3 ‐ no plus disease
Zone II ROP: stage 2 or stage 3 with plus disease
The current treatment strategy for infants with type 1 ROP involves peripheral retinal ablation by either cryotherapy or laser therapy. Both techniques result in significant improvement in the structural and functional outcomes; a Cochrane Review reported significant reduction in the risk of early unfavourable retinal structure from 47.9% to 28.1% and unfavourable visual acuity in early childhood from 63% to 50.6% following peripheral retinal ablation (Andersen 1999). However, in a small but significant proportion of preterm infants, the disease progresses despite treatment. Also, visual fields are slightly smaller in eyes subjected to peripheral retinal ablation as compared to 'control' eyes (Andersen 1999). Moreover, the ablation techniques are cumbersome and require sedation, general anaesthesia, or both. This has led to a quest for simpler and more effective treatment strategies.
Description of the intervention
Vascular endothelial growth factor (VEGF) is a key regulator of angiogenesis in foetal life. In the normally developing retina, VEGF is released in response to the higher oxygen demand of the retinal tissue, which leads to the development of blood vessels from the optic nerve to the periphery. In preterm infants with disrupted angiogenesis, however, the expression and levels of VEGF differ markedly in the two different phases. While the levels are suppressed in the vaso‐obliterative phase, there is an overproduction/expression of VEGF, leading to abnormal vascular proliferation in the vaso‐proliferative phase. Hugely elevated levels of VEGF have been documented in the vitreous cavity of eyes with stage 4 ROP (Lashkari 2000).
The key role of VEGF in inducing retinal neovascularisation prompted researchers to explore the role of anti‐VEGF drugs in the management of ROP. Intravitreal injection of neutralising anti‐VEGF antibodies had demonstrated a significant reduction in the neovascular response in animal studies (Aiello 1995). Two anti‐VEGF drugs, namely pegaptanib sodium (a pegylated anti‐VEGF aptamer) and ranibizumab (an anti‐VEGF monoclonal antibody) had been approved by the United States Food and Drug Administration (FDA) for intraocular use in adults in neovascular and age‐related macular degeneration. The third inhibitor, bevacizumab (a humanised anti‐VEGF monoclonal antibody), is being used off‐label for intraocular injection in adults with similar results. Recently, a fourth drug ‐ Aflibercept ‐ has been approved for the treatment of wet macular degeneration in adults. The drug is a recombinant fusion protein comprising the VEGF binding portions from the extracellular domains of human VEGF receptors 1 and 2 and the Fc portion of human IgG1. Unfortunately, none of these drugs have been approved for intraocular use in children to date (Mantagos 2009). However, given the limitations of existing treatment strategies (see above), many investigators have evaluated the off‐label use of these agents in infants with ROP (Shah 2007; Kong 2008; Mintz‐Hittner 2008). These studies ‐ predominantly case series and retrospective studies ‐ used one or more of the following approaches to evaluate the efficacy of VEGF inhibitors:
monotherapy: using an anti‐VEGF drug instead of cryo/laser therapy;
combination therapy: using anti‐VEGF simultaneously with cryo/laser therapy;
rescue therapy: using anti‐VEGF in infants with progression of the disease despite adequate treatment and in the rare instances where the infant presents with advanced ROP (stage 4 or more).
While most of the studies demonstrated the efficacy of anti‐VEGF drugs in ROP, the safety of the drugs is yet to be established. Though no significant adverse events have been reported so far, concerns still remain regarding their potential local and systemic adverse effects. By inhibiting VEGF, a key factor in regulation of angiogenesis in the developing retina as well as the central nervous system, these drugs could result in significant local and systemic adverse effects. Indeed, there have been concerns regarding the risk of cerebrovascular accidents following intravitreal ranibizumab injections in adults with age‐related macular degeneration (Ueta 2009). Though bevacizumab, the most frequently tested drug in ROP, has a lower risk of systemic absorption following intravitreal injections, the distinct possibility of its systemic effects cannot be ruled out in preterm infants with immature, and often impaired, blood‐retinal barrier (Law 2010).
The risk of systemic absorption, though small, brings its own complexity in the methods of randomisation and analysis in trials involving a locally administered drug. For a drug with truly local action, one can randomise either the study participant or a local body part of the participant to intervention and control groups. In the former, infants would be randomised ‐ both eyes of the infants (if needed) would receive the intervention or control therapy as per the group allocation. In the latter, eyes of the infants would be randomised ‐ one eye would receive the intervention, while the other eye would receive 'control' therapy. However, if the drug is likely to have systemic effects (like most anti‐VEGF agents), randomising the body part is not an ideal method of randomisation.
Why it is important to do this review
Treatment of ROP, to date, is largely by cryotherapy or laser therapy. Although these treatments result in a significant improvement in long‐term visual outcomes, the results are far from perfect. Despite appropriate treatment, progression to tractional retinal detachment occurs in 10% to 15% of infants with high‐risk prethreshold disease (ETROP Group 2003). In addition, the ablation procedures invariably cause permanent loss of the peripheral visual field. Simple, effective, and less destructive treatment strategies would be preferable to these procedures.
The recent reports of success following bevacizumab use have prompted various investigators to conduct clinical studies on the efficacy of this intervention. However, many of these studies are not powered to detect any serious adverse events ‐ local or systemic ‐ in the enrolled infants. Also, most have not systematically documented the risk of recurrence of ROP at a later age or examined the duration of follow‐up required to detect recurrence following intravitreal therapy. Given the protracted course of the disease and the short half‐life of anti‐VEGF drugs, the potential risk of recurrence is high (Wong 2015).
An earlier systematic review on the use of bevacizumab for severe ROP that included only case reports/case series and retrospective studies found considerable variability in how bevacizumab is used for the treatment of ROP (Micieli 2009). It concluded that "further randomized control trials are warranted". The purpose of this review was to identify all available randomised controlled trials on intravitreal anti‐VEGF therapy and to systematically analyse their results.
Objectives
To evaluate the efficacy and safety of anti‐VEGF drugs when used either as monotherapy, that is without concomitant cryotherapy or laser therapy, or in combination with planned cryo/laser therapy in preterm infants with type 1 ROP (defined as zone I any stage with plus disease, zone I stage 3 with or without plus disease, or zone II stage 2 or 3 with plus disease).
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised or quasi‐randomised controlled trials that evaluated the efficacy or safety of administration, or both, of anti‐VEGF agents in human preterm infants for inclusion in this review. We included only those trials that used VEGF inhibitors either alone (i.e. monotherapy) or in combination with cryo/laser therapy (i.e. combination therapy). We excluded those studies that used these drugs when other treatments such as cryo/laser therapy or vitrectomy had failed ('rescue therapy').
Types of participants
We considered studies that enrolled preterm (< 37 weeks' gestation at birth) infants with type 1 ROP at enrolment for inclusion. Type 1 ROP was defined as zone I any stage with plus disease, zone I stage 3 ROP with or without plus disease, or zone II stage 2 or 3 ROP with plus disease (ETROP Group 2003). For the purpose of this review, we excluded those studies that enrolled infants with more advanced ROP, that is stage 4 or more, at the time of enrolment (irrespective of the treatment strategy employed).
Types of interventions
Objective 1
Intervention: administration of any anti‐VEGF agent by intravitreal route
Control: cryotherapy/laser therapy
Objective 2
Intervention: intravitreal administration of VEGF inhibitors within seven days (before or after) of planned laser or cryotherapy
Control: cryotherapy/laser therapy alone
Types of outcome measures
Primary outcomes
Functional outcome: blindness or severe visual impairment (acuity ≤ 20/200) at 6 months to 12 months of corrected age.
Structural outcome: progression to retinal detachment involving the macula.
Secondary outcomes
-
Functional outcome(s) at 6 months to 12 months of corrected age:
amblyopia;
nystagmus; and/or
refractive error in either eye.
-
Unfavourable structural outcomes, assessed at 6 months to 12 months of corrected age, and defined as:
retinal fold involving the macula;
retinal detachment involving zone I of the posterior pole; and/or
retrolental tissue or 'mass' obscuring the view of the posterior pole.
Childhood unfavourable visual acuity, assessed at four years to six years, and defined as absence of vision or Snellen visual acuity of 20/200 or worse.
-
Mortality measured as:
death before discharge from the primary hospital;
death before two years corrected age.
-
Adverse neurodevelopmental outcomes at 18 months to 24 months of corrected age:
cerebral palsy; and/or
moderate to severe developmental delay as assessed on performance in formal neurodevelopmental testing such as Bayley scale.
Local adverse effects such as conjunctival haemorrhage, vitreous haemorrhage, and endophthalmitis after the procedure.
Acute systemic effects such as apnoea requiring respiratory support and cardiorespiratory arrest during or immediately after the treatment procedure.
Delayed systemic effects such as cerebrovascular accidents (stroke) and myocardial dysfunction (based on echocardiographic parameters such as ejection fraction and fractional shortening) diagnosed in the first 24 months of life.
Parental satisfaction regarding the treatment procedure employed (in Likert or other such scales).
Recurrence of ROP requiring retreatment up to 6 months of age.
Search methods for identification of studies
Electronic searches
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register). We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (Issue 11, 2016), MEDLINE (1966 to 11 December 2016) via PubMed, Embase (1980 to 11 December 2016), and CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1982 to 11 December 2016) using the following search terms: (bevacizumab OR Avastin OR ranibizumab OR pegaptanib sodium OR anti‐angio* OR angiogenesis inhibitors), plus database‐specific limiters for randomised controlled trials and neonates (see Appendix 1 for the full search strategies for each database). We limited the searches to human studies. We did not apply any language restrictions. We searched clinical trials registries for ongoing or recently completed trials (ClinicalTrials.gov (clinicaltrials.gov), the World Health Organization International Clinical Trials Registry Platform (www.whoint/ictrp/search/en/), and the ISRCTN registry (www.isrctn.com/)).
Searching other resources
We searched for unpublished studies by handsearching the conference proceedings of the Society for Pediatric Research (2002 to 2013) and American Academy of Ophthalmology Annual Meeting (1999 to 2016). We also searched the reference lists of all studies identified by the above methods.
Data collection and analysis
Selection of studies
For this updated review, two review authors (MJS and JS) independently searched and identified eligible trials based on the following characteristics: study population (preterm infants with type 1 ROP), study intervention (administration of anti‐VEGF drugs with or without laser/cryotherapy), and study design (randomised controlled trials).
The review authors screened the titles and abstracts to identify potentially relevant citations. We retrieved and reviewed the full text of the article if we could not ascertain relevance by screening the title and the abstract. The review authors independently assessed the eligibility of the studies by filling out eligibility forms designed in accordance with the specified inclusion criteria. Any disagreements were resolved by discussion.
We contacted the study investigators for additional information or for clarification of the method of randomisation, participant characteristics, details of interventions, definitions of events, additional relevant outcomes, and losses to follow‐up, as necessary.
Data extraction and management
We performed data extraction using a data extraction form designed and pilot tested by the review authors. We extracted information regarding:
study setting (e.g. country and settings);
study intervention;
sample size;
length of follow‐up;
randomisation procedure;
risk of different biases (see Assessment of risk of bias in included studies);
outcomes as listed above.
For dichotomous outcomes, we extracted the total number of participants for each group and the number of participants experiencing an event. For continuous outcomes, we extracted mean, standard deviation (or data required to calculate this), and the total number of participants for each group.
Assessment of risk of bias in included studies
Two review authors (MJS and JS) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2011).
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
Any disagreements were resolved by discussion or by a third assessor. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We used the standard methods of the Cochrane Neonatal Review Group to synthesise the data. We expressed effects as risk ratio (RR), risk difference (RD), and 95% confidence intervals (CI) for categorical data, and mean difference (MD) and 95% CI for continuous data. For significant differences, we also calculated the number needed to treat for an additional beneficial outcome (NNTB) based on 1/RD. We used the fixed‐effect model for pooling the results of individual studies.
Unit of analysis issues
We anticipated that the units of randomisation and analysis in the included trials would be individual infants and not eyes (it is difficult to randomise eyes because intravitreal anti‐VEGF can be absorbed into the systemic circulation). However, had a given study randomised eyes and not infants, we intended to use the study data but refrained from pooling these data with data of studies that had randomised infants. We decided a priori to use the eye‐level data (and not infant‐level data) in these studies, that is incidence of outcomes in eyes randomised to anti‐VEGF versus incidence of outcomes in eyes randomised to the control group; consequently, we did not consider individual‐level outcomes such as mortality or long‐term neurodevelopment in these studies. We a priori assumed that the beneficial effect, if any, would be diluted in these studies, that is the effect size would be closer to the null effect, if systemic absorption of anti‐VEGF agents were to occur (because the eye randomised to control group would be exposed to both anti‐VEGF agents and 'control' treatment).
Had a given study randomised infants but provided the outcome data for eyes, we planned to contact the authors to obtain infant‐level data so as to avoid unit of analysis error; using eyes as the denominator without adjusting for non‐independence between the eyes can result in spuriously precise results, that is narrow confidence intervals similar to those seen in cluster randomised trials when the clusters are randomised but the outcomes are analysed at the individual level without adjusting for 'cluster' effect (Higgins 2011a). If we could not obtain that information, we used the data for eyes but mentioned up‐front that the analysis referred to eyes and not infants.
Dealing with missing data
At the outcome level, if the data were measured but not reported, we planned to request such data from the study authors. If there was a discrepancy in the number randomised and the number analysed in each treatment group, we calculated and reported the percentage lost to follow‐up in each group.
Assessment of heterogeneity
We intended to assess heterogeneity between trial results by inspecting the forest plots and quantifying the impact of heterogeneity in any meta‐analysis using a measure of the degree of inconsistency in the studies’ results (Deeks 2011).
We estimated the proportion of total statistical heterogeneity not explained by chance using the I2 statistic (Higgins 2003). I2 (calculated as I2 = 100% x (Q ‐ df )/Q; where Q is Cochran’s heterogeneity statistic and df is the degrees of freedom) lies between 0% and 100%.
Data synthesis
We entered quantitative data into Review Manager 5 and analysed the data using the standard methods of Cochrane and Cochrane Neonatal (Review Manager 2014). We used the Mantel‐Haenszel method for estimates of typical RR and RD. We analysed continuous outcomes using the inverse variance method.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes.
Structural outcome: retinal detachment
Structural outcome: complete retinal detachment
Refractive error: very high myopia at 30 months of age
Mortality before discharge
Mortality at 30 months of age
Local adverse effects: corneal opacity requiring corneal transplant
Local adverse effects: lens opacity requiring cataract removal
Local adverse effects: perioperative retinal haemorrhages
Recurrence of ROP
Two review authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality, but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used GRADEpro GDT to create a ‘Summary of findings’ table to report the quality of the evidence (GRADEpro GDT).
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
We used GRADEpro GDT to import data from Review Manager 5 to create the 'Summary of findings' tables (GRADEpro GDT; Review Manager 2014). These tables provide outcome‐specific information concerning the overall quality of evidence from studies included in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes we considered.
Subgroup analysis and investigation of heterogeneity
Considerations of clinical diversity included assessment of differences in the nature of the surgical intervention, type and extent of disease, and the number and route of administration of VEGF inhibitors. Accordingly, we planned to analyse the studies based on differences in the following pre‐planned subgroups.
Nature of retinal ablation procedure (cryotherapy versus laser therapy)
Location of ROP at enrolment (zone I versus zone II)
Severity of ROP at enrolment (aggressive posterior retinopathy of prematurity versus others)
Specific anti‐VEGF agent administered
Number of doses of anti‐VEGF drug (single versus multiple)
Birth weights of the enrolled infants (< 1250 g versus ≥ 1250 g)
Sensitivity analysis
We intended to conduct a sensitivity analysis to investigate the robustness of the results for the primary outcome by excluding trials at high risk of bias or with dropout rates of more than 10% (overall).
Results
Description of studies
See Characteristics of included studies
Results of the search
Upon updating the search, we retrieved a total of 71 unique records, of which 58 were excluded after we scanned the title or abstract, or both (Figure 1).
1.
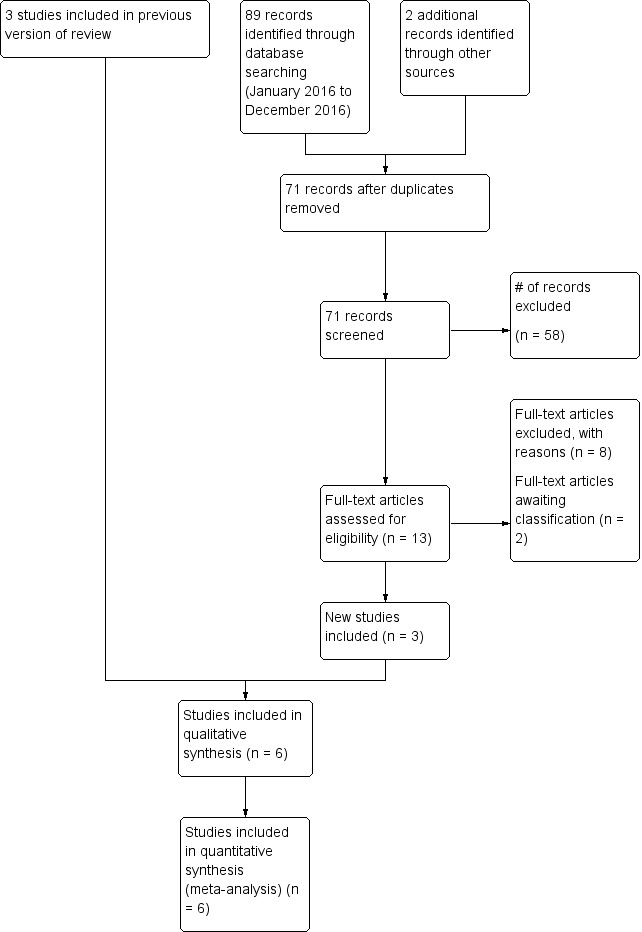
Study flow diagram: review update.
Six randomised trials fulfilled the eligibility criteria and were included in the review (BEAT‐ROP Trial 2011; Autrata 2012; Lepore 2014; Karkhaneh 2016; O'Keeffe 2016; Zhang 2016). Three articles remain in 'Studies awaiting classification'.
Included studies
Of the six included studies, four compared intravitreal bevacizumab monotherapy with conventional laser therapy (BEAT‐ROP Trial 2011; Lepore 2014; Karkhaneh 2016; O'Keeffe 2016); one compared ranibizumab monotherapy with laser therapy (Zhang 2016); and one study compared intravitreal pegaptanib plus conventional laser therapy with laser and cryotherapy (Autrata 2012). Four trials randomised the infants (BEAT‐ROP Trial 2011; Autrata 2012; Karkhaneh 2016; Zhang 2016), while the other two trials randomised the eyes of the infants to the two groups (Lepore 2014; O'Keeffe 2016). Two studies enrolled infants with only zone II ROP (Karkhaneh 2016; Zhang 2016), while one study included those with zone I ROP only (Lepore 2014); the remaining three studies enrolled infants with either zone I or zone II ROP (BEAT‐ROP Trial 2011; Autrata 2012; O'Keeffe 2016). Two trials allowed cross‐over treatment for infants with recurrence/reactivation of ROP (i.e. laser therapy for bevacizumab group and vice versa) (O'Keeffe 2016; Zhang 2016).
BEAT‐ROP Trial 2011 was a multicentre randomised trial conducted at 15 hospitals in the USA (BEAT‐ROP Trial 2011). It enrolled 150 preterm infants with zone I or zone II posterior stage 3+ ROP and randomly assigned them to receive intravitreal bevacizumab (0.625 mg in 0.025 mL of solution) or conventional laser therapy, bilaterally. The primary outcome was treatment failure, defined as the recurrence of neovascularisation in one or both eyes requiring retreatment, by 54 weeks’ postmenstrual age. RetCam (retinal imaging) photographs taken at different time points were evaluated to document recurrence of ROP. The study investigators reported the refractive outcomes at 30 months of age in a separate publication in 2014 by Geloneck (see BEAT‐ROP Trial 2011). Of the originally enrolled 150 infants, 131 infants underwent cycloplegic retinoscopic refraction to assess refractive outcomes at this age.
Autrata 2012 enrolled 76 preterm infants with zone I or zone II posterior stage 3+ ROP, admitted in a university hospital in the Czech Republic. Enrolled infants were randomly assigned to receive intravitreal pegaptanib (0.3 mg in 0.02 mL of solution) plus conventional diode laser therapy or laser therapy combined with cryotherapy, bilaterally. The primary outcome was treatment success, defined as absence of recurrence of stage 3+ ROP in one or both eyes by 55 weeks' postmenstrual age. RetCam photographs taken at different time points were evaluated to document recurrence of ROP. Infants who were randomised to the intervention group and had recurrence were given an additional intravitreal pegaptanib injection; those in the laser‐plus‐cryotherapy group did not receive pegaptanib for recurrence.
Karkhaneh 2016 enrolled 79 preterm infants (158 eyes) with zone II/stage 2 or 3 ROP and plus disease in a tertiary referral hospital in Tehran, Iran. Of them, 43 infants (86 eyes) were assigned to receive intravitreal bevacizumab injections (0.625 mg/0.025 mL) and 36 infants (72 eyes) were assigned to receive conventional indirect laser therapy. The primary outcome was defined as treatment failure, that is ROP persistence or recurrence by 90 weeks' postmenstrual age. Three experienced retina specialists (who were not involved in the initial treatment) performed follow‐up visits.
O'Keeffe 2016 conducted a prospective randomised study in 15 preterm infants with zone I or posterior zone II ROP admitted in a hospital in Dublin, Ireland. One eye of each infant was randomised to intravitreal bevacizumab, while the other eye was allocated to diode laser therapy. The investigators followed complications, regression/reactivation of ROP, visual outcome, refractive error, and systemic complications until five years of age.
Zhang 2016 conducted a randomised controlled trial of 50 infants with zone II treatment‐requiring ROP (i.e. stage 2 or 3 ROP with plus disease) admitted in the participating hospitals of 'Shenzhen Screening for ROP Cooperative Group', Shenzen, China. Infants were randomly assigned to receive intravitreal injection of ranibizumab monotherapy or laser therapy. Follow‐up interval was at least six months. Any eyes that developed recurrence of ROP underwent cross‐over retreatment.
Lepore 2014 conducted a single‐centre randomised trial and enrolled 13 infants with type 1 ROP in zone I in both eyes who required treatment according to Early Treatment for Retinopathy of Prematurity (ETROP) criteria (ETROP Group 2003). One eye of the enrolled infants was randomised to receive an intravitreal injection of 0.5 mg bevacizumab, while the fellow eye received conventional laser photoablation. The eye assigned to conventional laser peripheral ablation was treated first. The primary outcome was presence of retinal and choroidal abnormalities on fluorescein angiography at nine months of age. After treatment, binocular indirect ophthalmoscopy and RetCam imaging were performed every three days, and fluorescein angiography was performed every two weeks until discharge. Fluorescein angiography was done again at nine months of age under general anaesthesia.
Further details of the six studies are provided in the Characteristics of included studies table.
Excluded studies
We identified a large number of publications that were retrospective studies/case series of treatment with anti‐VEGF. For brevity, only the studies that provided data hitherto unreported in the randomised trials included in the review ‐ such as those comparing bevacizumab with ranibizumab, evaluating long‐term neurodevelopmental outcomes, etc. ‐ are mentioned here.
Alyamac 2016 reported a two‐centre retrospective study to compare the effects on the process of retinal vascularisation of intravitreal ranibizumab (IVR) and intravitreal bevacizumab (IVB) in the treatment of severe ROP. Forty‐four eyes of 22 participants in group 1 were applied 0.625 mg bevacizumab, and 46 eyes of 23 participants in group 2 were applied 0.25 mg ranibizumab. Retinal vascularisation was evaluated clinically.
Araz‐Ersan 2015 conducted a longitudinal follow‐up study of preterm infants who received 0.625 mg IVB therapy in addition to standard laser photocoagulation therapy. For comparison of the ophthalmological and neurological assessment outcomes of these infants, a control group was formed with 13 birth weight‐ and gestational age‐matched infants who were treated with laser therapy alone for type 1 ROP. The neurological status of the study group and the control group was examined systematically, and neurodevelopmental evaluation was assessed by the Bayley Scales of Infant Development (BSID‐III). The study included a total of 18 eyes of 13 infants.
Erol 2015 reported a retrospective evaluation of 36 eyes of 20 participants with type 1 ROP who received anti‐VEGF intravitreal injections between August 2011 and February 2013. Fifteen eyes of 8 participants received 0.25 mg ranibizumab (group 1), and 21 eyes of 12 participants received 0.625 mg bevacizumab (group 2). Eyes were examined by indirect ophthalmoscopy on the first day, third day, first week, and first month and as required after injections. Laser photocoagulation was performed in cases with progression of ROP.
Gunay 2016 conducted a retrospective interventional case series study including the data of 134 infants (264 eyes) who were treated with IVB, IVR, or laser photocoagulation for ROP. The data were collected from two major ROP treatment centres in Turkey without any randomisation or masking. Regression of ROP, recurrence profile, complications after each treatment modality, and indications for retreatment were evaluated. The main outcome measures included the total inactivation of ROP with anatomic and refractive outcomes at 1.5 years of adjusted age. There were 55 infants (41.1%) in the IVB group, 22 infants (16.4%) in the IVR group, and 57 infants (42.5%) in the laser photocoagulation group.
Han 2016 reported a case series addressing the clinical outcomes of IVB injection, with different dosing (0.25 mg/0.01 mL versus 0.625 mg/0.025 mL) in each eye of the same participant with ROP. Intravitreal bevacizumab was injected into 8 participants with stage 3+ in zone I or posterior zone II ROP (16 eyes). Different doses of bevacizumab (0.25 mg/0.01 mL and 0.625 mg/0.025 mL) were injected into the vitreous cavity of each eye.
Kabatas 2017 conducted a review of infants treated for ROP to evaluate the effectiveness of treatment modalities, major complications, and refractive errors in children who were treated with IVB, IVR, or laser photocoagulation for type 1 ROP. Preterm infants who underwent IVB monotherapy (group 1, 24 eyes of 12 infants), IVR monotherapy (group 2, 12 eyes of 6 infants), or laser photocoagulation (group 3, 72 eyes of 36 infants) for type 1 ROP and infants with spontaneously regressed ROP (group 4, 148 eyes of 74 infants) were included in the study. The study evaluated major complications, recurrence rate, recurrence time, total retinal vascularisation time, and refractive errors at 18 months of corrected age.
Lien 2016 conducted a retrospective observational case series at an institutional referral centre. The purpose of the study was to investigate the neurodevelopment of preterm infants after IVB for the treatment of ROP up to the two years of age. Infants with type 1 ROP were classified into three groups: laser only, IVB only, and a combination of IVB and laser treatment. Main outcome measures were neurodevelopmental outcomes of the infants after treatment assessed by BSID. Sixty‐one infants for whom the neurodevelopmental survey was finished were included.
Lin 2016 conducted a comparative, consecutive case study reporting on the axial length, refraction, and retinal vascularisation one year after ranibizumab or bevacizumab treatment for threshold ROP. Twenty‐five eyes of 13 participants with threshold ROP received one IVR treatment, and 15 eyes of eight participants received one IVB treatment.
Morin 2016 retrospectively reviewed data from the Canadian Neonatal Network and the Canadian Neonatal Follow‐Up Network databases on infants born at less than 29 weeks' gestation in 2010 to 2011 and treated for ROP. Neurodevelopmental outcome at 18 months was assessed by neurologic examination and the Bayley Scales of Infant and Toddler Development, Third Edition.
Wong 2015 reported a retrospective chart review on consecutive infants screened for ROP. Infants treated with peripheral retinal ablation, bevacizumab 0.625 mg/0.025 mL, or ranibizumab 0.25 mg/0.025 mL were specifically identified for review of their clinical outcomes. All treated infants had at least six months of follow‐up with the treating team and were examined until total regression of ROP. One hundred and forty‐two infants were screened over a two‐year period. Six infants with a mean gestational age of 23.48 weeks and mean birth weight of 620 g received anti‐VEGF agents. Ten eyes from the six infants received anti‐VEGF treatment.
Awaiting further assessment
The studies of Autrata 2012a, Kong 2015, and Moran 2014 are awaiting further assessment.
Risk of bias in included studies
Risk of bias assessments are detailed in the Characteristics of included studies table and are summarised in Figure 2.
2.
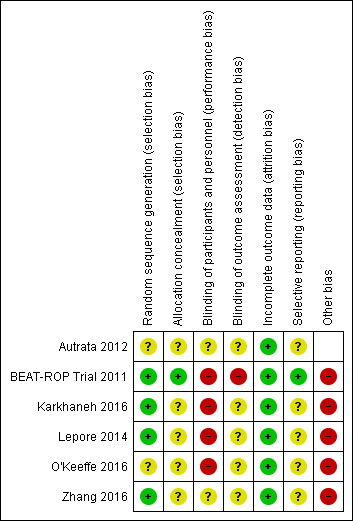
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only one trial reported an adequate allocation concealment method (BEAT‐ROP Trial 2011); details of allocation concealment were not reported in the remaining studies (Autrata 2012; Lepore 2014; Karkhaneh 2016; O'Keeffe 2016; Zhang 2016).
Blinding
Given the nature of the intervention, masking (blinding) of the intervention was not possible in any of the included studies.
BEAT‐ROP Trial 2011 formed a panel of six independent experts to examine the photographs taken at 54 weeks' postmenstrual age, and masked them to the treatment assignments by cropping the photographs to include only the optic disk and macula without laser marks. This enabled the study investigators to perform masked assessment of some of the secondary outcomes (e.g. macular dragging) but not the primary outcome of the study. Paediatric ophthalmologists who performed the cycloplegic retinoscopic refractions to assess the refractive errors at 30 months of age were not masked to the treatment assignments. In Karkhaneh 2016, all follow‐up visits were performed by three experienced retina specialists who were not involved in the initial treatment. However it remains unclear if the outcome assessors were truly masked to the groups, as experienced ophthalmologists could potentially identify the spots left by laser therapy in the control group.
It is not clear if the outcome assessors were masked to the group allocation in the other trials (Autrata 2012; Lepore 2014; O'Keeffe 2016; Zhang 2016).
Incomplete outcome data
Three trials reported no loss to follow‐up until 54 weeks' postmenstrual age (BEAT‐ROP Trial 2011; Autrata 2012; Karkhaneh 2016). Two trials reported no loss to follow‐up at six to nine months of age (Lepore 2014; Zhang 2016), while one trial reported no loss until five years of age (O'Keeffe 2016). In one trial (BEAT‐ROP Trial 2011), about 17% of eligible infants were lost to follow‐up at 30 months of age.
Selective reporting
BEAT‐ROP Trial 2011 reported all outcomes listed in the protocol (see NCT00622726 in BEAT‐ROP Trial 2011). We assessed the other trials as being at unclear risk of reporting bias. We could not refer to the study protocol of Autrata 2012, Karkhaneh 2016, O'Keeffe 2016, and Zhang 2016, and secondary outcomes were not provided in the study protocol of Lepore 2014.
Other potential sources of bias
Lepore 2014 and O'Keeffe 2016 randomised the eyes of enrolled infants. If there was significant systemic absorption of bevacizumab, the eye randomised to the control group would have been exposed to both the anti‐VEGF agent and control treatment, resulting in better outcomes in that eye.
We intended to assess the likelihood of potential publication bias using funnel plots, provided there were at least 8 to 10 trials (Sterne 2011).
Effects of interventions
Comparison 1: anti‐VEGF versus cryo/laser therapy ('monotherapy')
Four trials compared IVB with conventional laser therapy (BEAT‐ROP Trial 2011; Lepore 2014; Karkhaneh 2016; O'Keeffe 2016), while one trial compared IVR with laser therapy (Zhang 2016).
Primary outcomes
Functional outcome: blindness or severe visual impairment at 6 to 12 months of corrected age
None of the studies reported this outcome.
Structural outcome: progression to retinal detachment involving the macula (Outcomes 1.1 to 1.2)
Of the four trials that reported this outcome (BEAT‐ROP Trial 2011; Lepore 2014; Karkhaneh 2016; Zhang 2016), two reported no cases of retinal detachment in either of the two groups (Karkhaneh 2016; Zhang 2016). BEAT‐ROP Trial 2011 did not report any difference in the incidence of complete or partial retinal detachment between the two groups (risk ratio (RR) 1.04, 95% confidence interval (CI) 0.21 to 5.13; risk difference (RD) 0.00, 95% CI ‐0.06 to 0.07; Analysis 1.1). Only a small number of infants (two infants each in the two groups) had retinal detachment. We graded the quality of evidence as very low due to the small number of events and the potential risk of detection bias (Table 1). No separate data were available for the risk of retinal detachment involving only the macula.
1.1. Analysis.
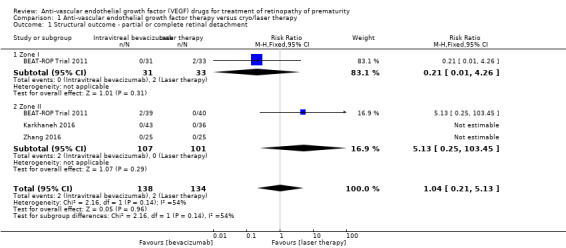
Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 1 Structural outcome ‐ partial or complete retinal detachment.
The fourth trial (Lepore 2014), which randomised eyes of the enrolled infants, reported progression to complete retinal detachment four weeks after treatment in one eye (8.5%) treated with conventional laser therapy; none of the eyes randomised to IVB had retinal detachment (RR 0.33, 95% CI 0.01 to 7.50; RD ‐0.08, 95% CI ‐0.27 to 0.11) (Analysis 1.2).
1.2. Analysis.
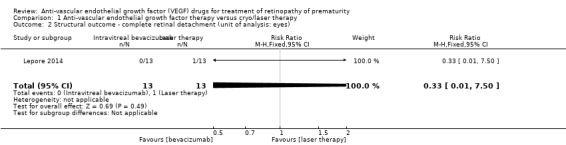
Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 2 Structural outcome ‐ complete retinal detachment (unit of analysis: eyes).
Secondary outcomes
Functional outcome(s): refractive error at or after 12 months of age (Outcomes 1.3 to 1.4)
One study reported the outcome using eyes as the denominator (BEAT‐ROP Trial 2011). The risk of very high myopia, defined as ‐8 dioptres (D) or more, at 30 months of age was significantly lower in the eyes of infants randomised to IVB (RR 0.06, 95% CI 0.02 to 0.20; RD ‐0.40, 95% CI ‐0.50 to ‐0.30) (Analysis 1.3). The magnitude of benefit was almost the same in both zone I and zone II posterior ROP. We graded the quality of evidence as low due to the unit of analysis error and risk of detection bias (Table 1). The mean spherical equivalent refractive error at 30 months of age was also significantly less in the eyes of infants who received IVB (mean difference (MD) 5.68 D, 95% CI 4.33 to 7.02) (BEAT‐ROP Trial 2011). The magnitude of difference was almost the same in both zone I (MD 6.93 D, 95% CI 4.26 to 9.60) and zone II posterior ROP (MD 5.25 D, 95% CI 3.69 to 6.81) (Analysis 1.4).
1.3. Analysis.
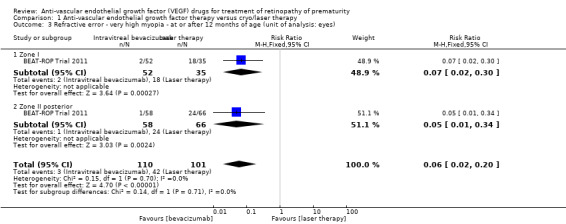
Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 3 Refractive error ‐ very high myopia ‐ at or after 12 months of age (unit of analysis: eyes).
1.4. Analysis.
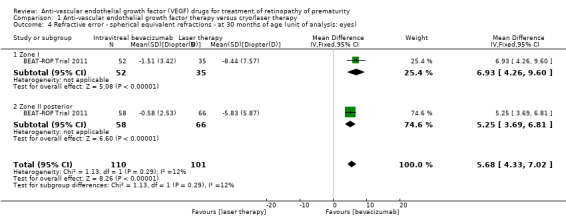
Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 4 Refractive error ‐ spherical equivalent refractions ‐ at 30 months of age (unit of analysis: eyes).
Another study reported no difference in refractive error between the bevacizumab‐treated and laser‐treated eyes at one‐year follow‐up (O'Keeffe 2016). However, the study allowed cross‐over retreatment (i.e. laser therapy for eyes randomised to bevacizumab and vice versa) in infants with recurrence of ROP, which makes it difficult to ascertain the true effect of the initial intervention. Moreover, the study did not provide relevant data to include the results in the pooled analysis.
Unfavourable structural outcomes, assessed at 6 to 12 months of corrected age
None of the studies reported this outcome.
Childhood unfavourable visual acuity, assessed at four to six years
One study reported significant myopic shift in the eyes treated with diode laser compared to the eyes that received IVB at five‐year follow‐up (O'Keeffe 2016). However, the study did not provide relevant data to estimate the risk ratio and its 95% confidence interval. Again, the study allowed cross‐over retreatment in infants with recurrent ROP.
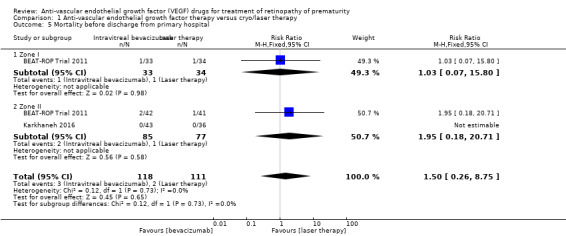
Mortality before discharge from the primary hospital and at 30 months of age (Outcomes 1.5 to 1.6)
BEAT‐ROP Trial 2011 reported no difference in the risk of mortality between the two groups, either before discharge from the primary hospital (RR 1.50, 95% CI 0.26 to 8.75; Analysis 1.5) or at a mean age of 30 months (RR 0.86, 95% CI 0.30 to 2.45; Analysis 1.6). However, the number of events was very small. Another study reported no deaths in either group until discharge from the hospital or until 90 weeks' postmenstrual age (Karkhaneh 2016).
1.5. Analysis.
Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 5 Mortality before discharge from primary hospital.
1.6. Analysis.
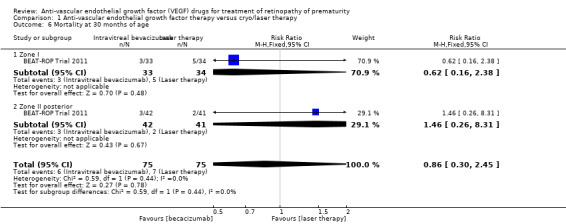
Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 6 Mortality at 30 months of age.
Adverse neurodevelopmental outcomes at 18 to 24 months of corrected age
None of the four studies that randomised infants to the two groups reported this outcome. One study that randomised eyes of the infants to the two groups reported no adverse changes attributable to bevacizumab therapy in magnetic resonance imaging (MRI) at one year of age (O'Keeffe 2016).
Local adverse effects (Outcomes 1.7 to 1.10)
Corneal opacity
One study reported this outcome using eyes as the denominator (BEAT‐ROP Trial 2011). There was no significant difference in the incidence of corneal opacity requiring corneal transplant between the two groups (RR 0.34, 95% CI 0.01 to 8.26) (Analysis 1.7).
1.7. Analysis.
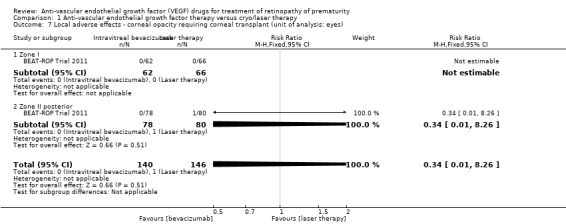
Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 7 Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes).
Lens opacity
Three studies reported this outcome using eyes as the denominator (BEAT‐ROP Trial 2011; Karkhaneh 2016; Zhang 2016). Of these, two studies did not report any case of cataract in either of the groups (Karkhaneh 2016; Zhang 2016). The third study did not find any significant difference in the incidence of lens opacity requiring cataract removal between the two groups (RR 0.15, 95% CI 0.01 to 2.79) (Analysis 1.8) (BEAT‐ROP Trial 2011).
1.8. Analysis.
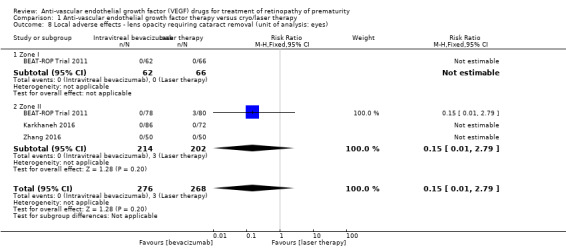
Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 8 Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes).
Endophthalmitis and vitreous haemorrhage
The two studies that reported this outcome did not find any case of endophthalmitis (Analysis 1.9) or vitreous haemorrhage (Analysis 1.10) in infants randomised to bevacizumab/ranibizumab or in those randomised to laser therapy (Karkhaneh 2016; Zhang 2016).
1.9. Analysis.
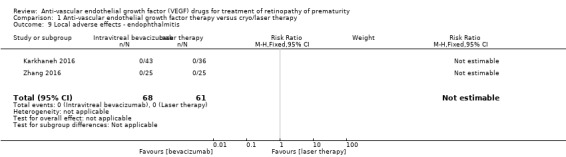
Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 9 Local adverse effects ‐ endophthalmitis.
1.10. Analysis.
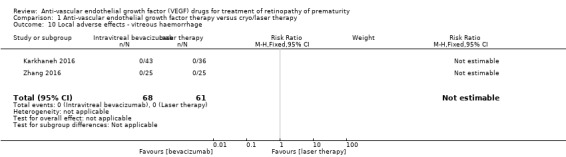
Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 10 Local adverse effects ‐ vitreous haemorrhage.
Choroidal ischaemia/rupture
None of the studies reported this outcome.
Acute systemic effects during or immediately after the treatment procedure
None of the studies reported this outcome.
Delayed systemic effects
One study that randomised eyes of the infants to the two groups reported no evidence of systemic adverse effects associated with bevacizumab at five‐year follow‐up (O'Keeffe 2016). None of the other studies reported this outcome.
Parental satisfaction regarding the treatment (in Likert or other such scales)
None of the studies reported this outcome.
Recurrence of ROP (Outcomes 1.11 to 1.12)
A total of four studies reported this outcome (BEAT‐ROP Trial 2011; Karkhaneh 2016; O'Keeffe 2016; Zhang 2016). Pooled analysis revealed no significant difference in the risk of recurrence of ROP requiring retreatment up to six months of age in infants randomised to IVB or IVR and those who received conventional laser therapy (RR 0.88, 95% CI 0.47 to 1.63; RD ‐0.02, 95% CI ‐0.12 to 0.07; 2 studies; fixed‐effect model) (Analysis 1.11). We graded the quality of evidence for this outcome as very low (Table 1). There was large heterogeneity (I2 = 86%), which was essentially due to the differences in the direction of effect between infants with zone I and those with zone II ROP (subgroup analysis); while there was a significant reduction in the risk of recurrence in infants with zone I ROP (RR 0.15, 95% CI 0.04 to 0.62), the risk of recurrence was significantly higher in infants with zone II ROP who received bevacizumab/ranibizumab monotherapy (RR 2.53, 95% CI 1.01 to 6.32) (Analysis 1.11).
1.11. Analysis.
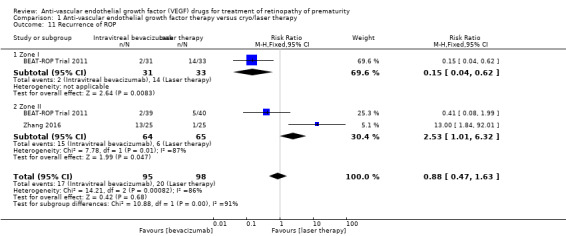
Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 11 Recurrence of ROP.
Pooled analysis of the other two studies, which reported eye‐level outcomes (not infant‐level outcomes), revealed significant increase in the risk of recurrence of ROP in the eyes that received bevacizumab therapy (RR 5.36, 95% CI 1.22 to 23.50) (Karkhaneh 2016; O'Keeffe 2016). Of these two studies, O'Keeffe 2016 enrolled infants with zone I or II ROP, while Karkhaneh 2016 enrolled infants with only zone II ROP (Analysis 1.12).
1.12. Analysis.
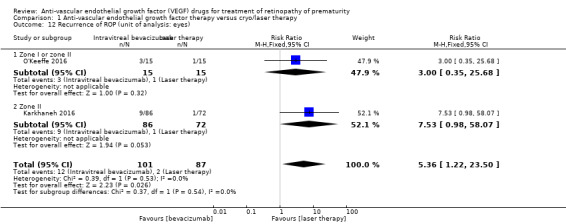
Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 12 Recurrence of ROP (unit of analysis: eyes).
Comparison 2: anti‐VEGF plus cryo/laser therapy ('combination therapy') versus cryo/laser therapy alone
Autrata 2012 compared intravitreal pegaptanib plus conventional laser therapy with laser and cryotherapy in preterm infants with stage 3+ ROP.
Primary outcomes
Functional outcome: blindness or severe visual impairment at 6 to 12 months of corrected age
The study did not report this outcome.
Structural outcome: progression to retinal detachment involving the macula (Outcome 2.1)
The study reported the outcome using eyes as the denominator. The risk of complete or partial retinal detachment was significantly lower in the eyes of infants randomised to intravitreal pegaptanib plus laser therapy (RR 0.26, 95% CI 0.12 to 0.55; RD ‐0.29, 95% CI ‐0.42 to ‐0.16; 1 study; 152 infants) (Analysis 2.1). We graded the quality of evidence as low due to the unit of analysis error and risk of detection bias (Table 2).
2.1. Analysis.
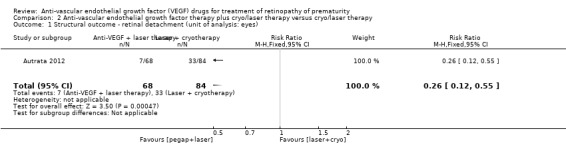
Comparison 2 Anti‐vascular endothelial growth factor therapy plus cryo/laser therapy versus cryo/laser therapy, Outcome 1 Structural outcome ‐ retinal detachment (unit of analysis: eyes).
No separate data were available for the risk of retinal detachment involving only the macula.
Secondary outcomes
Functional outcome(s): refractive error at 6 to 12 months of age or later
The study did not report this outcome.
Unfavourable structural outcomes at 6 to 12 months of age
The study did not report this outcome.
Childhood unfavourable visual acuity
The study did not report this outcome.
Mortality before discharge from the primary hospital and at 30 months of age
The study did not report this outcome.
Adverse neurodevelopmental outcomes at 18 to 24 months of corrected age
The study did not report this outcome.
Local adverse effects (Outcomes 2.2)
The study reported the outcome using eyes as the denominator. There was no significant difference in the risk of perioperative retinal haemorrhages after laser therapy between the two groups (RR 0.62, 95% CI 0.24 to 1.56; 1 study; 152 infants) (Analysis 2.2). The study did not report the risk of conjunctival haemorrhage or vitreous haemorrhage in the two groups.
2.2. Analysis.
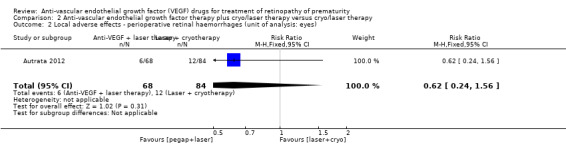
Comparison 2 Anti‐vascular endothelial growth factor therapy plus cryo/laser therapy versus cryo/laser therapy, Outcome 2 Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes).
The study reported that "no systemic or significant ocular complications of intravitreal pegaptanib injections, such as endophthalmitis or RD were found during the follow‐up period after treatment", but did not provide the corresponding data for the other group.
Acute systemic effects during or immediately after the treatment procedure
The study did not report this outcome (see 'Local adverse effects' above).
Delayed systemic effects
The study did not report this outcome.
Parental satisfaction regarding the treatment (in Likert or other such scales)
The study did not report this outcome.
Recurrence of ROP (Outcome 2.3)
Infants randomised to intravitreal pegaptanib plus laser therapy had a significantly lower risk of recurrence of ROP by 55 weeks' postmenstrual age compared to those randomised to laser therapy with cryotherapy (RR 0.29, 95% CI 0.12 to 0.70; RD ‐0.35, 95% CI ‐0.55 to ‐0.16; number needed to treat for an additional beneficial outcome 3, 95% CI 2 to 6; 1 study; 76 infants) (Analysis 2.3). We graded the quality of evidence for this outcome as low due to the risk of detection bias and unclear risk of selection bias (Table 2).
2.3. Analysis.
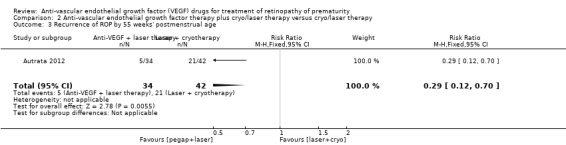
Comparison 2 Anti‐vascular endothelial growth factor therapy plus cryo/laser therapy versus cryo/laser therapy, Outcome 3 Recurrence of ROP by 55 weeks' postmenstrual age.
Discussion
Summary of main results
The systematic review included six randomised trials, of which five evaluated the effects of bevacizumab/ranibizumab monotherapy, while the sixth one examined the effects of intravitreal pegaptanib plus laser therapy. When used as monotherapy, IVB/IVR did not improve short‐term structural outcomes (partial or complete retinal detachment and recurrence of ROP) but significantly reduced the risk of refractive errors at 30 months of age. When used in conjunction with laser therapy, intravitreal pegaptanib reduced the risk of retinal detachment as well as recurrence of ROP in infants with stage 3+ ROP. We noted no significant difference in the incidence of local adverse events with any of the drugs. However, the quality of evidence was very low to low for most outcomes due to the risk of detection bias and other biases.
On subgroup analysis, we found the risk of recurrence of ROP requiring retreatment to be different in infants with zone I ROP and those with zone II ROP receiving IVB/IVR monotherapy: while the risk was reduced in infants with zone I ROP, we found it to be significantly higher in those with zone II ROP. However, the numbers were too small to draw any meaningful conclusion on this subgroup analysis.
Overall completeness and applicability of evidence
The updated evidence remains incomplete for three major reasons. The first reason is the limited number of studies included in the review. Despite the well‐established pathophysiological rationale for using anti‐VEGF agents and the short‐term benefits observed in numerous case reports, case series, and non‐randomised studies (Shah 2007; Kong 2008; Mintz‐Hittner 2008; Wu 2013; Nicoara 2016), only six randomised controlled trials enrolling 383 infants have been published so far. Consequently, the short‐term benefits observed with anti‐VEGF agents in the observational studies could be neither confirmed nor refuted with enough confidence in the current review. Secondly, the long‐term beneficial effects, if any, in terms of favourable structural and functional outcomes are not yet known. Though IVB monotherapy has been shown to reduce the risk of refractive errors (very high myopia) at 30 months of age, the effects of the intervention on other long‐term outcomes are largely unknown. Thirdly, the safety concerns of anti‐VEGF drugs have yet to be addressed. One trial reported no difference in the risk of mortality between intervention and control groups at a mean age of 30 months (BEAT‐ROP Trial 2011), but the number of events was very small. Another trial that randomised eyes of the infants to the two groups reported no evidence of abnormal MRI findings or systemic adverse effects attributable to bevacizumab therapy at one and five years of follow‐up, respectively (O'Keeffe 2016). Given the potential risk of systemic absorption and consequent adverse effects like cerebrovascular accidents following intravitreal anti‐VEGF therapy, the lack of evidence on safety outcomes is a major concern. A recently published study that used the data from the Canadian Neonatal Network demonstrated 3.1 times higher odds (95% CI 1.2 to 8.4) of severe neurodevelopmental disabilities in preterm infants born before 29 weeks' gestation and treated with bevacizumab, after adjusting for key confounders like gestation, gender, maternal education, Score for Neonatal Acute Physiology‐II (SNAP‐II) score, bronchopulmonary dysplasia, sepsis, and severe brain injury (Morin 2016). These findings further underscore the importance of evaluating long‐term safety outcomes of anti‐VEGF therapy .
The incomplete evidence indeed limits our ability to identify a simple, safe, and effective therapy for ROP. Unlike the current standard of treatment, laser therapy, anti‐VEGF administration is technically simple and does not require general anaesthesia or the services of a skilled retinal surgeon. This could be a great boon, particularly in settings with limited resources in low‐ and middle‐income countries. There is an urgent need to generate more evidence on the long‐term structural outcomes as well as the adverse effects following intravitreal therapy with anti‐VEGF agents before they can be considered for routine clinical use in infants with ROP. Future studies should also examine how these drugs affect the natural history of the disease, the focus being late recurrence that might warrant repeat doses of the drug, long‐term follow‐up, and the risk of local complications like infections following therapy, especially in resource‐restricted settings.
Quality of the evidence
We intended to include all primary and secondary outcomes of the review in the 'Summary of findings' tables. However, many of the outcomes were not reported in the included studies. We therefore reported only nine outcomes for the comparison of 'anti‐VEGF versus cryo/laser therapy' and three outcomes for the comparison of 'anti‐VEGF plus cryo/laser therapy versus cryo/laser therapy alone' in the 'Summary of findings' tables.
We graded the quality of evidence as very low to low for almost all outcomes (Table 1; Table 2). The risk of detection bias was high in all of the studies because the outcome assessors were not masked to the group allocation. The risk of other biases, including selection and reporting bias, was low in BEAT‐ROP Trial 2011, and unclear in the other studies (Autrata 2012; Lepore 2014; Karkhaneh 2016; O'Keeffe 2016; Zhang 2016).
Potential biases in the review process
Most outcomes of the review were not reported in the included studies. We are contacting the authors of the studies to collect additional information on these outcomes. Also, we did not perform a subgroup analysis based on the specific anti‐VEGF agent used because of the small number of studies (and the small number of infants enrolled in them).
Agreements and disagreements with other studies or reviews
An earlier systematic review, 'Off‐label use of bevacizumab for severe retinopathy of prematurity', was published in 2009 (Micieli 2009). It included nine articles, of which six were case reports, two were retrospective studies, and one was a prospective case series, and found considerable variability in how bevacizumab is used for the treatment of ROP, concluding that "further randomized control trials are warranted". Another systematic review, published in 2015, included 24 studies that evaluated anti‐VEGF therapy in 1457 eyes (Pertl 2015). Almost all the studies were observational except for one randomized and two case‐control studies. The review estimated a 6‐month risk of retreatment of 2.8% per eye, and a 6‐month risk of ocular complication without the need of retreatment of 1.6% per eye. Only isolated incidents of systemic complications were reported. The study concluded that "VEGF inhibitors seem to be associated with low recurrence rates and ocular complication rates".
The current review included six randomised trials, five on intravitreal bevacizumab/ranibizumab monotherapy and one on intravitreal pegaptanib combination therapy.
Authors' conclusions
Implications for practice.
Intravitreal bevacizumab/ranibizumab, when used as monotherapy, reduces the risk of refractive errors during childhood but does not reduce the risk of retinal detachment or recurrence of retinopathy of prematurity (ROP) in infants with type 1 ROP. While the intervention might reduce the risk of recurrence of ROP in infants with zone I ROP, it can potentially result in higher risk of recurrence requiring retreatment in those with zone II ROP. Intravitreal pegaptanib, when used in conjunction with laser therapy, reduces the risk of retinal detachment as well as the recurrence of ROP in infants with type 1 ROP. However, the quality of evidence was very low to low for most outcomes due to the risk of detection bias and other biases. The effects on other critical outcomes and, more importantly, the long‐term systemic adverse effects of the drugs are not known. The insufficient data precludes strong conclusions favouring routine use of intravitreal anti‐vascular endothelial growth factor (anti‐VEGF) agents, either as monotherapy or in conjunction with laser therapy, in preterm infants with type 1 ROP.
Implications for research.
Further randomised controlled trials are needed to evaluate the effect of anti‐VEGF agents, when used as monotherapy or as a part of combination therapy with laser/cryotherapy, on (i) structural and functional outcomes in childhood and (ii) delayed systemic adverse effects such as stroke, myocardial dysfunction, and adverse neurodevelopmental outcomes in preterm infants with severe ROP. The trials should ideally be large multicentre studies with adequate sample size to detect a clinically important difference in the risk of one or more of the delayed systemic adverse effects. The studies should also have adequate sample size to demonstrate benefit or harm in each of the two strata ‐ zone I and zone II ROP. An attempt should be made to ensure masking of caregivers and outcome assessors to the group allocation in these trials. Although there may be some apparently 'obvious' benefits to anti‐VEGF therapy (including simplicity of administration and cost), concerns regarding long‐term safety do not allow for more efficient trial designs such as 'non‐inferiority' studies. In addition to future trials, a registry of infants treated with any anti‐VEGF agent should be created to begin to follow the long‐term consequences of therapy in a more reliable fashion than isolated case reports.
Feedback
Comments from Zhou et al, 1 April 2016
Summary
"The authors mentioned that several safety concerns, such as cerebrovascular accidents following intravitreal anti‐VEGF therapy, would be examined. However, the impact of anti‐VEGF on choroid (the sole blood supply for the macula) is not fully discussed. A number of recent reports have demonstrated choroidal thinning in patients with a history of ROP (1‐3) as well as in a well‐established rodent model of ROP (4). Physiologically, VEGF plays a critical role for choroidal vascular development (5, 6). Mice lacking VEGF‐120 and VEGF‐164, major VEGF isoforms secreted by retinal pigment epithelium (RPE), experienced progressive choroidal degeneration (5). Meanwhile, a study on macaques showed that intravitreal bevacizumab (IVB) was able to reach choroid; and the drug was found to be concentrated in photoreceptors and choroidal endothelium (7). Lastly, serum VEGF levels have been found to be suppressed for two weeks after ROP patients received IVB treatment (8, 9), suggesting anti‐VEGF is able to penetrate retinal‐blood barrier. Indeed, adverse effects of IVB among ROP patients have been documented, including choroidal ruptures ten weeks after injection (10) and choroidal ischaemia in a neonate after a single, bilateral IVB injection (11). Since anti‐VEGF has a direct effect on vasculature, we feel that a thorough review about anti‐VEGF’s impact on choroid, such as linear choroidal filling pattern (mentioned in the review) and choroidal thickness (readily measured by optical coherent tomography), is warranted."
References 1. Anderson MF, Ramasamy B, Lythgoe DT, Clark D. Choroidal thickness in regressed retinopathy of prematurity. Eye (Lond) 2014;28(12):1461‐8. 2. Erol MK, Coban DT, Ozdemir O, Dogan B, Tunay ZO, Bulut M. Choroidal thickness in infants with retinopathy of prematurity. Retina 2015;36(6):1191‐8. 3. Park KA, Oh SY. Analysis of spectral‐domain optical coherence tomography in preterm children: retinal layer thickness and choroidal thickness profiles. Investigative Ophthalmology & Visual Science 2012;53(11):7201‐7. 4. Shao Z, Dorfman AL, Seshadri S, Djavari M, Kermorvant‐Duchemin E, Sennlaub F, et al. Choroidal involution is a key component of oxygen‐induced retinopathy. Investigative Ophthalmology & Visual Science 2011;52(9):6238‐48. 5. Saint‐Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA. An essential role for RPE‐derived soluble VEGF in the maintenance of the choriocapillaris. Proceedings of the National Academy of Sciences of the United States of America 2009;106(44):18751‐6. 6. Zhu M, Bai Y, Zheng L, Le YZ. Presence of RPE‐produced VEGF in a timely manner is critical to choroidal vascular development. Advances in Experimental Medicine and Biology 2012;723:299‐304. 7. Heiduschka P, Fietz H, Hofmeister S, Schultheiss S, Mack AF, Peters S, et al. Penetration of bevacizumab through the retina after intravitreal injection in the monkey. Investigative Ophthalmology & Visual Science 2007;48(6):2814‐23. 8. Wu WC, Lien R, Liao PJ, Wang NK, Chen YP, Chao AN, et al. Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmology 2015;133(4):391‐7. 9. Sato T, Wada K, Arahori H, Kuno N, Imoto K, Iwahashi‐Shima C, et al. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. American Journal of Ophthalmology 2012;153(2):327‐33.e1. 10. Atchaneeyasakul LO, Trinavarat A. Choroidal ruptures after adjuvant intravitreal injection of bevacizumab for aggressive posterior retinopathy of prematurity. Journal of Perinatology 2010;30(7):497‐9. 11. Chhablani J, Rani PK, Balakrishnan D, Jalali S. Unusual adverse choroidal reaction to intravitreal bevacizumab in aggressive posterior retinopathy of prematurity: the Indian Twin Cities ROP screening (ITCROPS) data base report number 7. Seminars Ophthalmology 2014;29(4):222‐5.
Reply
"We sincerely thank Zhou et al for their insightful comments on our review “Anti‐vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity”.
We do agree with them regarding the potential risk of choroidal thinning and ischaemia following anti‐VEGF therapy. Recent reports indicate that the choroidal thickness is inversely related to the severity of ROP.2 There is a risk that anti‐VEGF therapy might further aggravate this phenomenon. Our current protocol or review did not include these outcomes. We shall enlist the following two outcomes, namely:
median thickness of the choroid and
incidence of choroidal ischaemia/rupture
under ‘local adverse effects’ in the next update of our review." Review update publication is planned for 2017.
1. Erol MK, Coban DT, Ozdemir O, Dogan B, Tunay ZO, Bulut M. Choroidal thickness in infants with retinopathy of prematurity. Retina. 2015 Nov 18. [Epub ahead of print] PubMed PMID: 26583308.
Contributors
Tianwei Ellen Zhou, Department of Medicine, McGill University, Montréal, Québec, Canada
Sylvain Chemtob, Department of Pharmacology and Therapeutics, McGill University, Montréal, Québec, Canada
Mari Jeeva Sankar, Cochrane Neonatal Author
William McGuire, Cochrane Neonatal Feedback Editor
What's new
| Date | Event | Description |
|---|---|---|
| 1 December 2017 | New citation required and conclusions have changed | Three new randomised controlled trials included, resulting in a change of some of the conclusions. Many observational studies now included in the Discussion. |
| 1 December 2017 | New search has been performed | Search updated in December 2016 and new literature included in the updated review. |
History
Protocol first published: Issue 3, 2012 Review first published: Issue 2, 2016
| Date | Event | Description |
|---|---|---|
| 25 May 2016 | Amended | Review comment and author response added. Comments to be included in the next review update scheduled for 2017. |
Acknowledgements
The review authors (MJS and JS) acknowledge the contributions of the three authors (Dr Vishnu Bhat and Dr Renuka Srinivasan from JIPMER, Puducherry, India and Dr Manisha Mehta, New Delhi, India) of the earlier review (Sankar 2016).
Appendices
Appendix 1. Cochrane Neonatal standard search strategy
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial [ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) EMBASE: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial) CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) The Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. 'Risk of bias' tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies for which study protocols were published in advance, we compared prespecified outcomes versus outcomes reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; the study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Structural outcome ‐ partial or complete retinal detachment | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.21, 5.13] |
| 1.1 Zone I | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.26] |
| 1.2 Zone II | 3 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.13 [0.25, 103.45] |
| 2 Structural outcome ‐ complete retinal detachment (unit of analysis: eyes) | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.50] |
| 3 Refractive error ‐ very high myopia ‐ at or after 12 months of age (unit of analysis: eyes) | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.02, 0.20] |
| 3.1 Zone I | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.30] |
| 3.2 Zone II posterior | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.34] |
| 4 Refractive error ‐ spherical equivalent refractions ‐ at 30 months of age (unit of analysis: eyes) | 1 | 211 | Mean Difference (IV, Fixed, 95% CI) | 5.68 [4.33, 7.02] |
| 4.1 Zone I | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 6.93 [4.26, 9.60] |
| 4.2 Zone II posterior | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | 5.25 [3.69, 6.81] |
| 5 Mortality before discharge from primary hospital | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.26, 8.75] |
| 5.1 Zone I | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.80] |
| 5.2 Zone II | 2 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.18, 20.71] |
| 6 Mortality at 30 months of age | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.45] |
| 6.1 Zone I | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.16, 2.38] |
| 6.2 Zone II posterior | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.26, 8.31] |
| 7 Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes) | 1 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.26] |
| 7.1 Zone I | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Zone II posterior | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.26] |
| 8 Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes) | 3 | 544 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.79] |
| 8.1 Zone I | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Zone II | 3 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.79] |
| 9 Local adverse effects ‐ endophthalmitis | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Local adverse effects ‐ vitreous haemorrhage | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Recurrence of ROP | 2 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.47, 1.63] |
| 11.1 Zone I | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.62] |
| 11.2 Zone II | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.01, 6.32] |
| 12 Recurrence of ROP (unit of analysis: eyes) | 2 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.36 [1.22, 23.50] |
| 12.1 Zone I or zone II | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.35, 25.68] |
| 12.2 Zone II | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.53 [0.98, 58.07] |
Comparison 2. Anti‐vascular endothelial growth factor therapy plus cryo/laser therapy versus cryo/laser therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Structural outcome ‐ retinal detachment (unit of analysis: eyes) | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.12, 0.55] |
| 2 Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes) | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.24, 1.56] |
| 3 Recurrence of ROP by 55 weeks' postmenstrual age | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.70] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Autrata 2012.
| Methods | Randomised controlled trial | |
| Participants | Preterm infants with stage 3+ ROP in zone I or posterior zone II (n = 76); Single centre, university hospital, Brno, Czech Republic |
|
| Interventions | Intervention: intravitreal pegaptanib (0.3 mg in 0.02 mL of solution) combined with confluent laser therapy Control: conventional laser therapy |
|
| Outcomes | Primary: treatment success defined as absence of recurrence of stage 3+ ROP in 1 or both eyes by 55 weeks' postmenstrual age Secondary: time of regression and decrease of plus signs, development of peripheral retinal vessels after treatment, final structural/anatomic outcomes |
|
| Notes | We tried to contact the authors for additional information on methods and other outcomes (relevant to the review) but did not get any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study does not mention how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not clear if the random allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clear if the clinical team was masked to the intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear if the outcome assessors were masked to the group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not clear if all the outcomes were reported |
BEAT‐ROP Trial 2011.
| Methods | Randomised controlled trial | |
| Participants | Infants with birth weight 1500 g or less and gestational age of 30 weeks or less with stage 3+ ROP in zone I or zone II posterior in each eye (n = 150) Multicentre trial conducted at 15 hospitals in the USA |
|
| Interventions | Intervention: intravitreal bevacizumab monotherapy (0.625 mg in 0.025 mL of solution) Control: conventional laser therapy |
|
| Outcomes | Primary: treatment failure defined as recurrence of neovascularisation in one or both eyes and requiring retreatment by 54 weeks' postmenstrual age Secondary: structural outcomes of recurrence (macular dragging, retinal detachment), complications requiring intraocular surgery (cornea opacity requiring corneal transplant, lens opacity requiring cataract removal), mortality |
|
| Notes | We contacted the authors for additional information on other outcomes relevant to the review but did not get any response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Secure computer‐generated randomisation schedule stratified on the basis of zone by a study group member who did not participate in enrolment |
| Allocation concealment (selection bias) | Low risk | Treatment assignments were revealed to the investigators only after the eligibility for enrolment had been confirmed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was controlled but not masked owing to the marks made by the laser therapy. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | RetCam photographs to document recurrence by treating and confirming ophthalmologists without masking of treatment assignments, before deciding on additional treatment. Unmasked practicing paediatric ophthalmologists performed the cycloplegic retinoscopic refractions to assess the refractive errors at 30 months of age. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data of all infants who survived until 54 weeks' postmenstrual age were included in the analysis; about 17% of eligible infants were lost to follow‐up at 30 months of age (refractive outcomes). |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol were reported. |
| Other bias | High risk | Though the infants were randomised, results for some of the outcomes were provided for the individual eyes (unit of analysis error). |
Karkhaneh 2016.
| Methods | Randomised controlled trial | |
| Participants | Preterm infants (gestation less than 34 weeks) with birth weight less than 2000 g with zone II, stage 2 or 3 and plus disease were included (n = 79 infants; 158 eyes). Single centre, tertiary referral hospital, Tehran, Iran |
|
| Interventions | Intervention: intravitreal bevacizumab monotherapy (0.625 mg/0.025 mL) Control: conventional laser therapy |
|
| Outcomes | Primary: treatment failure defined as persistence (absence of regression of neovascularisation and plus disease 1 week after treatment) or recurrence (new extraretinal fibrovascular proliferation with the arrest of anterior progression of retinal vasculature) of ROP Secondary: need for surgery (scleral buckling or plana vitrectomy) and condition of retinal periphery by 54 weeks' postmenstrual age |
|
| Notes | Retreatment for those in the control group was additional laser ablation, while for those in the intervention group it was re‐injection of the drug (no cross‐over of treatment in the randomised groups). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐based randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Not clear if the random allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not masked. Quote: "Non‐blinded prospective clinical trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "all follow‐up visits were performed by three retina specialists ... surgeons performing the treatments were not among them"; still not clear if the outcome assessors were truly masked to the groups; experienced ophthalmologists could potentially identify the spots left by laser therapy in the control group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not clear if all the outcomes were reported |
| Other bias | High risk | Though the infants were randomised, results for some of the outcomes were provided for the individual eyes (unit of analysis error). |
Lepore 2014.
| Methods | Randomised controlled trial Randomised 1 eye of enrolled infants to conventional laser and the other eye to bevacizumab |
|
| Participants | Infants with type 1 ROP in zone I in both eyes requiring treatment, according to ETROP criteria (n = 13) | |
| Interventions | Intervention eye: intravitreal bevacizumab monotherapy (0.5 mg in 0.02 mL of balanced salt solution) Control eye: conventional laser therapy |
|
| Outcomes | Abnormalities on fluorescein angiography ‐ macular abnormalities (absence of foveolar avascular zone or hyperfluorescent lesion), capillary bed loss, linear choroidal filling pattern; complete retinal detachment (stage 5); mortality at 3 months of age | |
| Notes | The eye assigned to conventional laser peripheral ablation was treated first. The eye randomised to receive bevacizumab was then prepared, and 0.5 mg (0.02 mL) of bevacizumab was injected intravitreally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... randomly selected using a random number series" |
| Allocation concealment (selection bias) | Unclear risk | Not clear if the random allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible because 1 eye is randomised to intervention, while the other eye is randomised to control (laser) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear if the outcome assessors were masked to the group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not clear if all the outcomes were reported (secondary outcomes not available in the published protocol) |
| Other bias | High risk | Because eyes were randomised, the eye randomised to control group would have been exposed to both anti‐VEGF agents and control treatment, resulting in better outcomes if there was significant systemic absorption of bevacizumab. |
O'Keeffe 2016.
| Methods | Randomised trial Randomised 1 eye of enrolled infants to intravitreal bevacizumab and the other eye to conventional laser |
|
| Participants | Preterm infants (24 to 29 weeks' gestation) with zone I or posterior zone II ROP with plus disease (n = 15) Single centre (maternity/university hospital), Dublin, Ireland |
|
| Interventions | Intervention eye: intravitreal bevacizumab monotherapy (1.25 mg in 0.05 mL) Control eye: conventional laser therapy |
|
| Outcomes | Reactivation of ROP (return of plus disease and ridge formation anterior to the original site of pathology) and long‐term visual outcome/refractive error and systemic complications | |
| Notes | 3 eyes initially treated with bevacizumab that showed recurrence of ROP received laser therapy, while 1 eye in the laser group was treated with bevacizumab for recurrence (cross‐over of treatment). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear how random numbers were generated |
| Allocation concealment (selection bias) | Unclear risk | Not clear if the random allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible because 1 eye is randomised to intervention, while the other eye is randomised to control (laser) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear if the outcome assessors were masked to the group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not clear if all the outcomes were reported |
| Other bias | High risk | Because eyes were randomised, the eye randomised to the control group would have been exposed to both anti‐VEGF agents and control treatment, resulting in better outcomes if there was significant systemic absorption of bevacizumab. Eyes that developed recurrence of ROP underwent cross‐over retreatment; for some outcomes such as retinal detachment or lens opacity it is difficult to ascertain the effects of the original intervention. |
Zhang 2016.
| Methods | Randomised controlled trial | |
| Participants | Preterm infants with zone II stage 2 or 3 ROP with plus disease (n = 50 infants) Shenzen eye hospital as well as the other participating hospitals of 'Shenzhen Screening for ROP Cooperative Group', Shenzen, China |
|
| Interventions | Intervention: intravitreal ranibizumab monotherapy (0.3 mg in 0.03 mL) Control: conventional laser therapy Eyes that developed recurrence of ROP underwent cross‐over retreatment. |
|
| Outcomes | Regression of plus disease/resolution of neovascularisation, recurrence of ROP (recurrent plus disease, recurrent neovascularisation, or reformation of ridge despite treatment), and complications | |
| Notes | In cases of recurrence, cross‐over treatment was used (laser therapy for bevacizumab group and vice versa). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not clear if the random allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clear if the clinical team was masked to the intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear if the outcome assessors were masked to the group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not clear if all the outcomes were reported |
| Other bias | High risk | Because the eyes that developed recurrence of ROP underwent cross‐over retreatment, it is difficult to ascertain the effects of the original intervention on some outcomes such as retinal detachment or lens opacity. |
anti‐VEGF: anti‐vascular endothelial growth factor ETROP: Early Treatment for Retinopathy of Prematurity ROP: retinopathy of prematurity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alyamac 2016 | Retrospective study comparing the effects of IVR and IVB in the treatment of severe ROP |
| Araz‐Ersan 2015 | Longitudinal follow‐up study of preterm infants who received 0.625 mg IVB therapy in addition to standard laser photocoagulation therapy. For comparison , a control group was formed with 13 birth weight‐ and gestational age‐matched infants treated with laser therapy alone for type 1 ROP. The neurological status of the study group and the control group was examined systematically, and neurodevelopmental evaluation was assessed by the Bayley Scales of Infant Development (BSID‐III). At 2 years of age, no significant difference was found in terms of spherical or cylindrical refractive errors compared to the control group. Neurological examinations were abnormal in 2/13 infants in the control group and 3/13 infants in the study group. No significant difference was found in the mean cognitive, language, or motor BSID‐III test scores of the groups. |
| Chen 2015 | Case series involving 37 infants treated with intravitreal injections of either bevacizumab or ranibizumab as the primary treatment for type 1 ROP. There were no significant differences in mean refractive errors between the infants treated with intravitreal injections of bevacizumab or ranibizumab at the corrected age of 1 year. A significantly higher chance of high myopia was noted in the bevacizumab group (P = 0.03). |
| Erol 2015 | Retrospective study comparing the efficacy of intravitreal ranibizumab and bevacizumab treatment for type 1 ROP |
| Gunay 2016 | Retrospective study including the data of 134 infants (264 eyes) who were treated with IVB, IVR, and laser photocoagulation in Turkey Both IVB‐ and IVR‐treated infants had significantly better refractive outcomes in zone I ROP as compared to laser photocoagulation‐treated infants at 1.5 years of adjusted age. A higher rate of disease recurrence was associated with IVR. |
| Han 2016 | Study comparing different dosing (0.25 mg/0.01 mL versus 0.625 mg/0.025 mL) of IVB injection in 8 participants with stage 3+ in zone I or posterior zone II ROP (16 eyes). All eyes showed regression of plus sign after IVB injection within 1 week and revascularisation to the ora serrata at the time of final visit. There was no difference in the time of anatomical achievement between the eyes with different doses. |
| Kabatas 2017 | Evaluated the effectiveness of IVB, IVR, or laser photocoagulation for type 1 ROP. All 3 interventions could successfully treat ROP. Myopia was observed to be the main refractive error in all treatment groups. Vascularisation of the retina was completed later in the IVB group than in the IVR group. |
| Lien 2016 | Retrospective study to investigate the neurodevelopment of preterm infants up to the age of 2 years after intravitreal injections of bevacizumab for the treatment of ROP. Infants with type 1 ROP were classified into 3 groups: laser only, IVB only, and a combination of IVB and laser treatment. Main outcome measures were neurodevelopmental outcomes of the infants after treatment assessed by Bayley Scales for Infant Development. 61 infants who finished the neurodevelopmental survey were included. No detrimental effects on neurodevelopment were found in IVB group compared with the those who received laser treatment only. However, infants in the IVB + laser group had a higher incidence of significant mental (P = 0.028) and psychomotor (P = 0.002) impairment at 24 months than infants in the laser group. The odds ratio of having severe psychomotor defects in the IVB + laser group was 5.3 compared with the laser group (P = 0.041). |
| Lin 2016 | Comparative study reporting on the axial length, refraction, and retinal vascularisation 1 year after ranibizumab or bevacizumab treatment for threshold ROP. There were no significant differences in the axial length and refraction between children with threshold ROP who received intravitreal bevacizumab compared to those who received ranibizumab after 1 year of follow‐up. Ranibizumab treatment could achieve more complete retinal vascularisation than bevacizumab treatment, but the result was not significant. |
| Morin 2016 | Retrospective review of data from the Canadian Neonatal Network and the Canadian Neonatal Follow‐Up Network databases comparing neurodevelopment at 18 months' corrected age in preterm infants of < 29 weeks' gestation treated with bevacizumab versus laser ablation. Neurodevelopmental outcome was assessed by neurologic examination and the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley‐III). Of 125 treated infants, 27 received bevacizumab and 98 laser. The bevacizumab group, compared with laser, obtained a median Bayley‐III motor composite score of 81 (interquartile range 70 to 91) versus 88 (79 to 97), a language composite score of 79 (65 to 97) versus 89 (74 to 97), and a cognitive score of 90 (80 to 100) versus 90 (85 to 100). A difference was detected on the motor score only (P = 0.02). Odds of severe neurodevelopmental disabilities (Bayley‐III scores < 70, severe cerebral palsy, hearing aids, or bilateral blindness) was 3.1 times higher (95% confidence interval 1.2 to 8.4) in infants treated with bevacizumab versus laser after adjusting for gestational age, gender, maternal education, Score for Neonatal Acute Physiology‐II score, bronchopulmonary dysplasia, sepsis, and severe brain injury. The authors concluded that preterm infants treated with bevacizumab versus laser had higher odds of severe neurodevelopmental disabilities. |
| Wong 2015 | Retrospective chart review of infants treated with peripheral retinal ablation, bevacizumab 0.625 mg/0.025 mL, or ranibizumab 0.25 mg/0.025 mL. 10 eyes from the 6 infants received anti‐VEGF treatment. All 10 eyes demonstrated initial regression of ROP. However, ROP reactivation occurred in 5/6 (83%) eyes treated with ranibizumab, on average 5.9 weeks after treatment, whereas none of the 4 eyes treated with bevacizumab experienced reactivation (P < 0.05). 1 infant who received a unilateral injection of ranibizumab demonstrated bilateral regression of ROP. Although the shorter half‐life of ranibizumab makes it an attractive option, reactivation of ROP is possible. |
anti‐VEGF: anti‐vascular endothelial growth factor IVB: intravitreal bevacizumab IVR: intravitreal ranibizumab ROP: retinopathy of prematurity
Characteristics of studies awaiting assessment [ordered by study ID]
Autrata 2012a.
| Methods | Prospective comparative study |
| Participants | All infants diagnosed with stage 3+ ROP for zone I or zone II posterior (174 eyes of 87 premature infants) |
| Interventions | Intravitreal pegaptanib (0.3 mg) or bevacizumab (0.625 mg/0.025 mL of solution) with conventional diode laser photocoagulation (group A, 92 eyes of 46 infants) or laser therapy combined with cryotherapy (group B, 82 eyes of 41 infants), bilaterally |
| Outcomes | Major outcomes include treatment success/failure, time of regression and decrease of plus signs and development of peripheral retinal vessels after treatment, final structural anatomic outcomes |
| Notes | Not clear if (a) this is a randomised trial and (b) the infants enrolled in 2 of the 3 treatment groups were the same as the other study published by the same authors in the same year (Autrata 2012; included in the current review). We plan to contact the authors for clarification. |
Kong 2015.
| Methods | Randomised controlled trial |
| Participants | 24 infants with type 1 ROP |
| Interventions | 3 treatment groups: intravitreal injection of bevacizumab (IVB) at 0.625 mg per eye per dose, IVB at 0.25 mg per eye per dose, and laser. |
| Outcomes | Serum levels of bevacizumab, free VEGF, and IGF‐1 Blood samples were collected prior to treatment and on post‐treatment days 2, 14, 42, and 60. Weekly body weights were documented from birth until 60 days' post‐treatment. Serum bevacizumab was detected 2 days after the injection, peaked at 14 days, and persisted for up to 60 days with half‐life of 21 days. Area under the curve analysis showed that systemic exposure to bevacizumab was variable among the infants and was dose dependent. Serum free VEGF levels decreased in all 3 subgroups 2 days' post‐treatment, with more significant reductions found in both IVB‐treated groups, P = 0.0001. Serum IGF‐1 levels were lower in both IVB‐treated groups. |
| Notes | Only the serum levels of VEGF were reported in the study; we will contact the authors for data on relevant clinical outcomes, if any. |
Moran 2014.
| Methods | Prospective case–control study |
| Participants | 14 infants with symmetrical zone l or posterior zone ll stage 3+ ROP |
| Interventions | Intravitreal bevacizumab in 1 eye and laser therapy in the fellow eye |
| Outcomes | 4 of 14 eyes (28.6%) had recurrence of ROP, 3 eyes (21.4%) treated with bevacizumab and 1 eye (7.14%) with conventional laser therapy. There was a significant time delay to recurrence in the bevacizumab group compared with the laser group, with a mean age of 51 weeks' postmenstrual age at time of recurrence in bevacizumab‐treated eyes compared with 37 weeks' postmenstrual age in the laser‐treated eye. There was a rapid regression of ROP in all eyes injected with bevacizumab, as well as resolution of plus disease and flattening of the ridge by 48 hours' postinjection in all eyes. Further vascularisation was noted with complete regression taking up to 60 weeks in some eyes. |
| Notes | Reported as a letter to the editor. We will contact the authors regarding the confusion in study design (reported as "prospective case–control study", but it appears that the eyes of enrolled infants were randomised to either bevacizumab or laser therapy). |
IGF‐1: insulin‐like growth factor‐1 ROP: retinopathy of prematurity VEGF: vascular endothelial growth factor
Differences between protocol and review
We have listed the differences between the protocol and review below.
a. Secondary outcomes
Added: One additional outcome, recurrence of ROP requiring retreatment up to 6 months of age, that was not planned in the protocol.
b. Dealing with missing data
Deleted: “For dichotomous data, if drop‐outs exceed 10% for any trial, we will assign the worse outcomes to those who were lost to follow‐up and assess the impact in the study results in sensitivity analyses (Higgins 2011).”
c. Assessment of heterogeneity
Deleted: “If statistical heterogeneity is detected, we will explore the possible causes. We intend to use the fixed‐effect model if the I2 statistic is less than 60%; in the event that the I2 is more than 60%, we will use the random effects.”
d. Data synthesis
Deleted: “For ordinal outcomes (as in Likert scale for parental satisfaction), we will summarize the data using methods for continuous variables ‐ as a difference in means or standardized difference in means. Depending upon the heterogeneity, we plan to use either fixed‐effect or random effects models with inverse variance weighting for the meta‐analyses.”
e. Measures of treatment effect
Deleted: “We used the fixed‐effect model for pooling the results of individual studies.”
f. Unit of analysis issues
Added: “However, had a given study randomised eyes and not infants, we intended to use the study data but refrained from pooling these data with data of studies that had randomised infants. We decided a priori to use the eye‐level data (and not infant‐level data) in these studies, that is incidence of outcomes in eyes randomised to anti‐VEGF versus incidence of outcomes in eyes randomised to the control group; consequently, we did not consider individual‐level outcomes such as mortality or long‐term neurodevelopment in these studies. We a priori assumed that the beneficial effect, if any, would be diluted in these studies, that is the effect size would be closer to the null effect, if systemic absorption of anti‐VEGF agents were to occur (because the eye randomised to control group would be exposed to both anti‐VEGF agents and 'control' treatment).
Had a given study randomised infants but provided the outcome data for eyes, we planned to contact the authors to obtain infant‐level data so as to avoid unit of analysis error; using eyes as the denominator without adjusting for non‐independence between the eyes can result in spuriously precise results, that is narrow confidence intervals similar to those seen in cluster randomised trials when the clusters are randomised but the outcomes are analysed at the individual level without adjusting for 'cluster' effect (Higgins 2011). If we could not obtain that information, we used the data for eyes but mentioned up‐front that the analysis refers to eyes and not infants.”
Contributions of authors
MJS and JS updated the literature with the help of the Cochrane Neonatal Review Group Information Specialist. MJS and JS independently extracted data and assessed included studies for risk of bias. MJS conducted the data analysis and wrote the final draft with inputs from the remaining authors (JS and PC).
Sources of support
Internal sources
-
None, Other.
The authors did not receive any support from either external or internal resources
External sources
No sources of support supplied
Declarations of interest
Mari Jeeva Sankar: no conflict of interest
Jhuma Sankar: no conflict of interest
Parijat Chandra: no conflict of interest
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Autrata 2012 {published data only (unpublished sought but not used)}
- Autrata R, Krejčířová I, Šenková K, Holoušová M, Doležel Z, Borek I. Intravitreal pegaptanib combined with diode laser therapy for stage 3+ retinopathy of prematurity in zone I and posterior zone II. European Journal of Ophthalmology 2012;22(5):687‐94. [PUBMED: 22669848] [DOI] [PubMed] [Google Scholar]
BEAT‐ROP Trial 2011 {published data only (unpublished sought but not used)}
- Geloneck MM, Chuang AZ, Clark WL, Hunt MG, Norman AA, Packwood EA, et al. BEAT‐ROP Cooperative Group. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmology 2014;132(11):1327‐33. [PUBMED: 25103848] [DOI] [PubMed] [Google Scholar]
- Mintz‐Hittner HA, Kennedy KA, Chuang AZ, BEAT‐ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. New England Journal of Medicine 2011;364(7):603‐15. [PUBMED: 21323540] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00622726. Bevacizumab Eliminates the Angiogenic Threat for Retinopathy of Prematurity (BEAT‐ROP). clinicaltrials.gov/ct2/show/NCT00622726 25 February 2008.
Karkhaneh 2016 {published data only}
- Karkhaneh R, Khodabande A, Riazi‐Eafahani M, Roohipoor R, Ghassemi F, Imani M, et al. Efficacy of intravitreal bevacizumab for zone‐II retinopathy of prematurity. Acta Ophthalmologica 2016;94(6):e417‐20. [DOI: 10.1111/aos.13008; PUBMED: 27009449] [DOI] [PubMed] [Google Scholar]
Lepore 2014 {published data only}
- Lepore D, Quinn GE, Molle F, Baldascino A, Orazi L, Sammartino M, et al. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: report on fluorescein angiographic findings. Ophthalmology 2014;121(11):2212‐9. [PUBMED: 25001158] [DOI] [PubMed] [Google Scholar]
O'Keeffe 2016 {published data only}
- O'Keeffe N, Murphy J, O'Keefe M, Lanigan B. Bevacizumab compared with diode laser in stage 3 posterior retinopathy of prematurity: a 5 year follow up. Irish Medical Journal 2016;109(2):355. [PUBMED: 27685689] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang G, Yang M, Zeng J, Vakros G, Su K, Chen M, et al. Shenzhen Screening for Retinopathy of Prematurity Cooperative Group. Comparison of intravitreal injection of ranibuzumab versus laser therapy for zone II treatment‐requiring retinopathy of prematurity. Retina 2016 Aug 12 [Epub ahead of print]. [DOI: 10.1097/IAE.0000000000001241; PUBMED: 27529839] [DOI] [PMC free article] [PubMed]
References to studies excluded from this review
Alyamac 2016 {published data only}
- Alyamac Sukgen E, Comez A, Kocluk Y, Cevher S. The process of retinal vascularization after anti‐VEGF treatment in retinopathy of prematurity: a comparison study between ranibizumab and bevacizumab. Ophthalmologica. Journal International d'Ophtalmologie [International Journal of Ophthalmology] 2016;236(3):139‐47. [DOI: 10.1159/000449530; PUBMED: 27682852] [DOI] [PubMed] [Google Scholar]
Araz‐Ersan 2015 {published data only}
- Araz‐Ersan B, Kir N, Tuncer S, Aydinoglu‐Candan O, Yildiz‐Inec D, Akdogan B, et al. Preliminary anatomical and neurodevelopmental outcomes of intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. Current Eye Research 2015;40(6):585‐91. [DOI: 10.3109/02713683.2014.941070; PUBMED: 25025864] [DOI] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen SN, Lian I, Hwang YC, Chen YH, Chang YC, Lee KH, et al. Intravitreal anti‐vascular endothelial growth factor treatment for retinopathy of prematurity: comparison between ranibizumab and bevacizumab. Retina (Philadelphia, Pa.) 2015;35(4):667‐74. [PUBMED: 25462435] [DOI] [PubMed] [Google Scholar]
Erol 2015 {published data only}
- Erol MK, Coban DT, Sari ES, Bilgin AB, Dogan B, Ozdemir O, et al. Comparison of intravitreal ranibizumab and bevacizumab treatment for retinopathy of prematurity. Arquivos Brasileiros de Oftalmologia 2015;78(6):340‐3. [DOI: 10.5935/0004-2749.20150090; PUBMED: 26677033] [DOI] [PubMed] [Google Scholar]
Gunay 2016 {published data only}
- Gunay M, Sukgen EA, Celik G, Kocluk Y. Comparison of bevacizumab, ranibizumab, and laser photocoagulation in the treatment of retinopathy of prematurity in Turkey. Current Eye Research 2016;42(3):462‐9. [DOI: 10.1080/02713683.2016.1196709; PUBMED: 27420302] [DOI] [PubMed] [Google Scholar]
Han 2016 {published data only}
- Han J, Kim SE, Lee SC, Lee CS. Low dose versus conventional dose of intravitreal bevacizumab injection for retinopathy of prematurity: a case series with paired‐eye comparison. Acta Ophthalmologica 2016 Mar 24 [Epub ahead of print]. [DOI: 10.1111/aos.13004; PUBMED: 27011262] [DOI] [PubMed] [Google Scholar]
Kabatas 2017 {published data only}
- Kabatas EU, Kurtul BE, Altiaylik Ozer P, Kabatas N. Comparison of intravitreal bevacizumab, intravitreal ranibizumab and laser photocoagulation for treatment of type 1 retinopathy of prematurity in Turkish preterm children. Current Eye Research 2017 Jan 27 [Epub ahead of print]. [DOI: 10.1080/02713683.2016.1264607; PUBMED: 28128986] [DOI] [PubMed] [Google Scholar]
Lien 2016 {published data only}
- Lien R, Yu MH, Hsu KH, Liao PJ, Chen YP, Lai CC, et al. Neurodevelopmental outcomes in infants with retinopathy of prematurity and bevacizumab treatment. PLoS ONE 2016;11(1):e0148019. [DOI: 10.1371/journal.pone.0148019; PUBMED: 26815000] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2016 {published data only}
- Lin CJ, Tsai YY. Axial length, refraction, and retinal vascularization 1 year after ranibizumab or bevacizumab treatment for retinopathy of prematurity. Clinical Ophthalmology (Auckland, NZ) 2016;10:1323‐7. [DOI: 10.2147/OPTH.S110717; PUBMED: 27499611] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morin 2016 {published data only}
- Morin J, Luu TM, Superstein R, Ospina LH, Lefebvre F, Simard MN, et al. Canadian Neonatal Network and the Canadian Neonatal Follow‐Up Network Investigators. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics 2016;137(4):e20153218. [DOI: 10.1542/peds.2015-3218; PUBMED: 27244705] [DOI] [PubMed] [Google Scholar]
Wong 2015 {published data only}
- Wong RK, Hubschman S, Tsui I. Reactivation of retinopathy of prematurity after ranibizumab treatment. Retina (Philadelphia, Pa.) 2015;35(4):675‐80. [DOI: 10.1097/IAE.0000000000000578; PUBMED: 25768252] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Autrata 2012a {published data only}
- Autrata R, Senkova K, Holousova M, Krejcirova I, Dolezel Z, Borek I. Effects of intravitreal pegaptanib or bevacizumab and laser in treatment of threshold retinopathy of prematurity in zone I and posterior zone II ‐ four years results [Prinos intravitrealni aplikace anti‐VEGF preparatu v lecbe prahoveho stadia ROP 3+ v zone I‐II: vysledky ctyrlete studie]. Ceska a Slovenska Oftalmologie 2012;68(1):29‐36. [PubMed] [Google Scholar]
Kong 2015 {published data only}
- Kong L, Bhatt AR, Demny AB, Coats DK, Li A, Rahman EZ, et al. Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF‐1 in infants with retinopathy of prematurity. Investigative Ophthalmology & Visual Science 2015;56(2):956‐61. [DOI: 10.1167/iovs.14-15842; PUBMED: 25613938] [DOI] [PubMed] [Google Scholar]
Moran 2014 {published data only}
Additional references
Aiello 1995
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF‐receptor chimeric proteins. Proceedings of the National Academy of Sciences of the United States of America 1995;92(23):10457‐61. [PUBMED: 7479819] [DOI] [PMC free article] [PubMed] [Google Scholar]
American Academy of Pediatrics 2006
- Section on Ophthalmology American Academy of Pediatrics, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2006;117(2):572‐6. [DOI: 10.1542/peds.2005-2749; PUBMED: 16452383] [DOI] [PubMed] [Google Scholar]
Andersen 1999
- Andersen C, Phelps D. Peripheral retinal ablation for threshold retinopathy of prematurity in preterm infants. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001693] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashton 1953
- Ashton N, Ward B, Serpell G. Role of oxygen in the genesis of retrolental fibroplasia; a preliminary report. British Journal of Ophthalmology 1953;37(9):513‐20. [PUBMED: 13081949] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashton 1954
- Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. British Journal of Ophthalmology 1954;38(7):397‐432. [PUBMED: 13172417] [DOI] [PMC free article] [PubMed] [Google Scholar]
Committee for Classification of ROP 1984
- The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Archives of Ophthalmology 1984;102(8):1130‐4. [PUBMED: 6547831] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
ETROP Group 2003
- Early Treatment for Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the Early Treatment for Retinopathy Of Prematurity randomized trial. Archives of Ophthalmology 2003;121(12):1684‐94. [PUBMED: 14662586] [DOI] [PubMed] [Google Scholar]
Gilbert 2005
- Gilbert C, Fielder A, Gordillo L, Quinn G, Semiglia R, Visintin P, et al. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics 2005;115(5):e518‐25. [PUBMED: 15805336] [DOI] [PubMed] [Google Scholar]
Gilbert 2008
- Gilbert C, Muhit M. Twenty years of childhood blindness: what have we learnt?. Community Eye Health/International Centre for Eye Health 2008;21(67):46‐7. [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). Available at gradepro.org. GRADEpro GDT. Version (accessed 5 December 2017). Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org, 2015.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011a
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kong 2008
- Kong L, Mintz‐Hittner HA, Penland RL, Kretzer FL, Chevez‐Barrios P. Intravitreous bevacizumab as anti‐vascular endothelial growth factor therapy for retinopathy of prematurity: a morphologic study. Archives of Ophthalmology 2008;126(8):1161‐3. [PUBMED: 18695118] [DOI] [PubMed] [Google Scholar]
Lashkari 2000
- Lashkari K, Hirose T, Yazdany J, McMeel JW, Kazlauskas A, Rahimi N. Vascular endothelial growth factor and hepatocyte growth factor levels are differentially elevated in patients with advanced retinopathy of prematurity. American Journal of Pathology 2000;156(4):1337‐44. [PUBMED: 10751359] [DOI] [PMC free article] [PubMed] [Google Scholar]
Law 2010
- Law JC, Recchia FM, Morrison DG, Donahue SP, Estes RL. Intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. Journal of AAPOS 2010;14(1):6‐10. [PUBMED: 20227614] [DOI] [PubMed] [Google Scholar]
Mantagos 2009
- Mantagos IS, Vanderveen DK, Smith LE. Emerging treatments for retinopathy of prematurity. Seminars in Ophthalmology 2009;24(2):82‐6. [PUBMED: 19373691] [DOI] [PMC free article] [PubMed] [Google Scholar]
Micieli 2009
- Micieli JA, Surkont M, Smith AF. A systematic analysis of the off‐label use of bevacizumab for severe retinopathy of prematurity. American Journal of Ophthalmology 2009;148(4):536‐43.e2. [PUBMED: 19660736] [DOI] [PubMed] [Google Scholar]
Mintz‐Hittner 2008
- Mintz‐Hittner HA, Kuffel RR Jr. Intravitreal injection of bevacizumab (Avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina 2008;28(6):831‐8. [PUBMED: 18536599] [DOI] [PubMed] [Google Scholar]
Nicoara 2016
- Nicoară SD, Ștefănuţ AC, Nascutzy C, Zaharie GC, Toader LE, Drugan TC. Regression rates following the treatment of aggressive posterior retinopathy of prematurity with bevacizumab versus laser: 8‐year retrospective analysis. Medical Science Monitor 2016;22:1192‐209. [PUBMED: 27062023] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 1997
- Palmer EA. What have we learned about retinopathy of prematurity during the past ten years? Progress in retinopathy of prematurity. The International Symposium on Retinopathy of Prematurity; 1997; Taormina, Italy. Amsterdam/New York: Kugler Publications, 1997.
Pertl 2015
- Pertl L, Steinwender G, Mayer C, Hausberger S, Pöschl E‐M, Wackernagel W, et al. A systematic review and meta‐analysis on the safety of vascular endothelial growth factor (VEGF) inhibitors for the treatment of retinopathy of prematurity. PLoS ONE 2015;10(6):e0129383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed prior to 12 December 2017).
Shah 2007
- Shah PK, Narendran V, Tawansy KA, Raghuram A, Narendran K. Intravitreal bevacizumab (Avastin) for post laser anterior segment ischemia in aggressive posterior retinopathy of prematurity. Indian Journal of Ophthalmology 2007;55(1):75‐6. [PUBMED: 17189897] [DOI] [PubMed] [Google Scholar]
Smith 2003
- Smith LE. Pathogenesis of retinopathy of prematurity. Seminars in Neonatology 2003;8(6):469‐73. [PUBMED: 15001119] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tasman 2006
- Tasman W, Patz A, McNamara JA, Kaiser RS, Trese MT, Smith BT. Retinopathy of prematurity: the life of a lifetime disease. American Journal of Ophthalmology 2006;141(1):167‐74. [PUBMED: 16386993] [DOI] [PubMed] [Google Scholar]
Ueta 2009
Wu 2013
- Wu WC, Kuo HK, Yeh PT, Yang CM, Lai CC, Chen SN. An updated study of the use of bevacizumab in the treatment of patients with prethreshold retinopathy of prematurity in Taiwan. American Journal of Ophthalmology 2013;155(1):150‐8. [PUBMED: 22967867] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sankar 2016
- Sankar MJ, Sankar J, Mehta M, Bhat V, Srinivasan R. Anti‐vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD009734] [DOI] [PubMed] [Google Scholar]


