Abstract
Background
Children's exposure to other people's tobacco smoke (environmental tobacco smoke, or ETS) is associated with a range of adverse health outcomes for children. Parental smoking is a common source of children's exposure to ETS. Older children in child care or educational settings are also at risk of exposure to ETS. Preventing exposure to ETS during infancy and childhood has significant potential to improve children's health worldwide.
Objectives
To determine the effectiveness of interventions designed to reduce exposure of children to environmental tobacco smoke, or ETS.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialised Register and conducted additional searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, PsycINFO, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Education Resource Information Center (ERIC), and the Social Science Citation Index & Science Citation Index (Web of Knowledge). We conducted the most recent search in February 2017.
Selection criteria
We included controlled trials, with or without random allocation, that enrolled participants (parents and other family members, child care workers, and teachers) involved in the care and education of infants and young children (from birth to 12 years of age). All mechanisms for reducing children's ETS exposure were eligible, including smoking prevention, cessation, and control programmes. These include health promotion, social‐behavioural therapies, technology, education, and clinical interventions.
Data collection and analysis
Two review authors independently assessed studies and extracted data. Due to heterogeneity of methods and outcome measures, we did not pool results but instead synthesised study findings narratively.
Main results
Seventy‐eight studies met the inclusion criteria, and we assessed all evidence to be of low or very low quality based on GRADE assessment. We judged nine studies to be at low risk of bias, 35 to have unclear overall risk of bias, and 34 to have high risk of bias. Twenty‐one interventions targeted populations or community settings, 27 studies were conducted in the well‐child healthcare setting and 26 in the ill‐child healthcare setting. Two further studies conducted in paediatric clinics did not make clear whether visits were made to well‐ or ill‐children, and another included visits to both well‐ and ill‐children. Forty‐five studies were reported from North America, 22 from other high‐income countries, and 11 from low‐ or middle‐income countries. Only 26 of the 78 studies reported a beneficial intervention effect for reduction of child ETS exposure, 24 of which were statistically significant. Of these 24 studies, 13 used objective measures of children's ETS exposure. We were unable to pinpoint what made these programmes effective. Studies showing a significant effect used a range of interventions: nine used in‐person counselling or motivational interviewing; another study used telephone counselling, and one used a combination of in‐person and telephone counselling; three used multi‐component counselling‐based interventions; two used multi‐component education‐based interventions; one used a school‐based strategy; four used educational interventions, including one that used picture books; one used a smoking cessation intervention; one used a brief intervention; and another did not describe the intervention. Of the 52 studies that did not show a significant reduction in child ETS exposure, 19 used more intensive counselling approaches, including motivational interviewing, education, coaching, and smoking cessation brief advice. Other interventions consisted of brief advice or counselling (10 studies), feedback of a biological measure of children's ETS exposure (six studies), nicotine replacement therapy (two studies), feedback of maternal cotinine (one study), computerised risk assessment (one study), telephone smoking cessation support (two studies), educational home visits (eight studies), group sessions (one study), educational materials (three studies), and school‐based policy and health promotion (one study). Some studies employed more than one intervention. 35 of the 78 studies reported a reduction in ETS exposure for children, irrespective of assignment to intervention and comparison groups. One study did not aim to reduce children's tobacco smoke exposure but rather sought to reduce symptoms of asthma, and found a significant reduction in symptoms among the group exposed to motivational interviewing. We found little evidence of difference in effectiveness of interventions between the well infant, child respiratory illness, and other child illness settings as contexts for parental smoking cessation interventions.
Authors' conclusions
A minority of interventions have been shown to reduce children's exposure to environmental tobacco smoke and improve children's health, but the features that differentiate the effective interventions from those without clear evidence of effectiveness remain unclear. The evidence was judged to be of low or very low quality, as many of the trials are at a high risk of bias, are small and inadequately powered, with heterogeneous interventions and populations.
Plain language summary
Can interventions for parents and people caring for children reduce children's exposure to tobacco smoke?
Background
Children exposed to cigarette smoke (environmental tobacco smoke) are at greater risk of lung problems, infections, and serious complications including sudden infant death syndrome. Preventing exposure to cigarette smoke in infancy and childhood might significantly improve children's health worldwide. Parental smoking is a common source of cigarette exposure for children. Older children are also at risk of exposure to cigarette smoke in child care or educational settings.
Study characteristics
We searched six databases for relevant research. This is an update of a previously published review, and the date of the most recent search was February 2017. We found 78 studies on the effects of interventions aimed at family and carers with the goal of reducing children’s exposure to tobacco smoke. These studies included parents and other family members, child care workers, and teachers involved in the care and education of infants and young children (from birth to 12 years of age), and used a variety of interventions, including different kinds of counselling, brief advice, and educational materials.
Key results
Only 26 studies reported that an intervention was successful in reducing children’s exposure to tobacco smoke. These studies used a range of interventions. Nine studies used more intensive counselling methods or motivational interviewing, but in other studies, these types of interventions were not effective. Of the 52 studies that did not show a significant reduction in child tobacco smoke exposure, 19 used intensive counselling methods or motivational interviewing. One study successfully reduced children's asthma symptoms by using motivational interviewing. This review does not show whether any particular interventions reduced parental smoking and child smoke exposure more effectively than others.
Quality of evidence
The quality of evidence ranged from low to very low. Future studies should aim to provide evidence of higher quality by addressing study design problems, including more participants, and describing interventions in more detail.
Summary of findings
Background
Active smoking has been recognised as harmful to the smoker for over six decades, since the landmark Doll and Hill publication (Doll 1950), but it was not until 1974 that the medical literature first discussed parental smoking, exposure to environmental tobacco smoke (ETS), and the effects of ETS on children (Harlap 1974). Overwhelming evidence indicates that parental smoking is associated with a range of adverse health effects for children (NHMRC 1997). Perhaps its most obvious association is with increased risk, increased severity, and greater likelihood of admission to hospital of children with lower and upper respiratory tract disease (Strachan 1997; Strachan 1998, respectively). An increasing body of evidence describes an association between parental smoking and increased risk of serious bacterial infections such as meningitis among children (Iles 2001). In addition, Lam 2001 reported that ETS exposure increases health service use and costs, and Chiswell 2017 described associated poorer surgical outcomes.
Furthermore, parental smoking confers a significantly increased risk of sudden infant death syndrome (SIDS) (Golding 1997). This effect is present regardless of which parent is the smoker (Blair 1999), and it is the strongest modifiable risk factor for SIDS. In addition, research across several continents over the last two decades has found that children of smokers have an increased risk of uptake in adolescence, perhaps as a result of role modelling and/or increased access to cigarettes (Mays 2014). There is also an increased risk of respiratory symptoms persisting into adulthood among children exposed to ETS from their parents or carers, but who do not themselves take up smoking later in life (Pugmire 2014).
Parental smoking is a common but preventable source of infant and childhood morbidity. The World Health Organization (WHO) has identified the need to reduce parental smoking as a key element of action to encourage health and development in early childhood, particularly among those living in difficult social and economic circumstances (WHO 1999; WHO 2013). In some countries, strong relationships between socioeconomic status and environmental quality are evident (Moore 2012), and strategies to reduce smoking and improve child health outcomes must be underpinned by recognition of finite resources and the limited control that some individuals and families have over environmental and social situations.
Infants' and toddlers' exposure to smoking occurs primarily within the home environment, as this is where they spend most of their time. Older children may also be exposed to smoking in a variety of child care and educational settings in which they spend their time. As children increase their time spent in commercial and informal child care settings, the importance of child care workers' behaviours increases. Similarly, environments in which young children are exposed extend beyond the home and include shopping centres, meeting places, and other social environments.
Tobacco cessation strategies and interventions to reduce ETS have had mixed success, often providing small benefits on an individual level (Rosen 2014). Systematic reviews have previously demonstrated that individual counselling increases cessation rates (Lancaster 2017), and that simple advice from a physician may have a positive effect in triggering quit attempts (Stead 2013). In relation to children's exposure in utero and during the early years, smoking cessation interventions for pregnant women can be effective in reducing smoking (Coleman 2015; Chamberlain 2017). Although smoke‐free legislation in England has contributed to the 79% reduction in children’s ETS exposure since 1998 (Jarvis 2015), variability is ongoing, and children in families from lower socioeconomic status remain at greater risk of ETS exposure (Moore 2012). Globally, 80% of the world's smokers live in low‐ and middle‐income countries (WHO 2014), which have demonstrated less political will to enforce smoke‐free legislation (Pugmire 2017).
Objectives
To determine the effectiveness of interventions designed to reduce exposure of children to environmental tobacco smoke, or ETS.
Methods
Criteria for considering studies for this review
Types of studies
Controlled trials with or without random allocation.
Types of participants
People (parents and other family members, child care workers, and teachers) involved in the care and education of infants and young children (from birth to 12 years of age).
Types of interventions
We included all mechanisms for the reduction of children's ETS exposure, including smoking prevention, smoking cessation, and any other tobacco control programmes targeting the participants described above. These included health promotion, social‐behavioural therapy, technology, and educational and clinical interventions.
We included studies in which the primary aim was to reduce children's exposure to ETS (thereby preventing adverse health outcomes), but where secondary outcomes included reduction or cessation of familial/parental/carer smoking, or changes in infant and child health measures. We also included studies where the primary outcome was reduction or cessation of familial/parental/carer smoking, resulting in reduced exposure for children.
We excluded studies on uptake of smoking by minors.
We did not restrict inclusion based on who delivered the programmes. These could include researchers, general practitioners, midwives, paediatricians, community and hospital nurses, health promotion agencies, tobacco control and anti‐cancer organisations, or health departments.
Types of outcome measures
The primary outcome measures were children's exposure to tobacco smoke, child illness and health service utilisation, and the smoking behaviours of children's parents and carers. We included studies where the only outcome was parental or carer smoking status.
We used biological verification of exposure to or absorption of ETS as the 'gold standard', but we did not require this as an inclusion criterion. Where biological verification of exposure/absorption conflicted with the parental report of exposure, we regarded the biologically verified result as correct.
Outcomes for children
Exposure to ETS: biochemical measures of children's exposure to ETS based on air monitoring for levels of nicotine or other measures of ETS (including parent‐reported behaviour change, as described in the next section)
Absorption of ETS: biochemical measures of children's absorption of ETS through cotinine in urine, blood, saliva, or hair
Frequency of childhood illness events, respiratory problems (changes in lung function or symptom scores)
Use of health services: admission to hospital; frequency of use of general practitioners (GPs); frequency of medication use
Outcomes for parents and carers
Behaviour change in relation to children's exposure to ETS: We noted any reported bans or restrictions on smoking at home or in other environments or in designated smoking areas outside the home
Smoking behaviour, including cessation, reduction, or uptake, using biochemically validated measures of smoking behaviour (e.g. thiocyanates; cotinine levels in blood, urine, or saliva), or self‐report
Maternal postpartum smoking status
Costs and cost‐effectiveness associated with interventions and outcomes
We reported biochemical confirmation of parental self‐reported quit status or changes in behaviour such as moves to smoke outside, but we did not exclude studies without this measurement. Most studies did not use biochemical validation. However, there is conflicting evidence regarding the validity of self‐report of smoking status. Some trial authors suggest that self‐report is reasonably accurate in community settings (Dwyer 1986; Velicer 1992; Patrick 1994), whereas others suggest that parental self‐reports of smoke consumption and ETS are frequently underestimated (Jarvis 1987; Ford 1997; Matthews 1999). For example, in clinical situations where a clinician is the interviewer, social bias may influence the report towards the socially desired response.
Researchers and clinicians often prefer to use levels of nicotine or its breakdown products, by contrast, as a measure of real reductions in smoking or ETS. Cotinine is a metabolic breakdown product of nicotine with a half‐life of about one day (Haley 1983). Its half‐life is longer in non‐smokers such as infants and young children (Idle 1990). Smoke exposure can be detected by hair cotinine (Zahlsen 1994; Nafstad 1997; Al‐Delaimy 2002a; Al‐Delaimy 2002b), and absorption by urinary cotinine (Jarvis 1984; Bakoula 1995). Long‐term exposure is best estimated by hair cotinine, whereas urinary cotinine is more informative of short‐term exposure. Saliva cotinine approximates to blood cotinine concentrations, and collection is simple and non‐invasive.
Search methods for identification of studies
This is the fourth update of this review. Search methods for the previous searches are described in previously published versions of this review (Roseby 2002; Priest 2008; Baxi 2014).
Nia Wyn Roberts, Outreach Librarian, Bodleian Health Care Libraries, updated the search. We searched the Cochrane Central Register of Controlled Trials (Issue 2011) in the Cochrane Library, MEDLINE (OvidSP) (1948 to the present), Embase (OvidSP) (1974 to the present), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EbscoHOST) (1980 to the present), PsycINFO (OvidSP) (1967 to the present), and the Education Resource Information Center (ERIC) (ProQuest) (1966 to the present). In June 2011, we conducted a search for articles from 2007 to 2011. The Trial Search Co‐ordinator searched the CochraneTobacco Addiction Group Specialised Register. We conducted the most recent search in February 2017.
We obtained and reviewed reports of all references identified as possibly describing randomised controlled trials (RCTs) or controlled trials (CTs), and we checked the reference lists of all identified RCTs and CTs to identify potentially relevant citations. We made enquiries regarding other known published and unpublished studies so that we could include these results in our review.
We have presented search strategies for the key databases in Appendix 1 (MEDLINE); Appendix 2 (Embase); Appendix 3 (CINAHL); Appendix 4 (PsycINFO); Appendix 5 (ERIC); and Appendix 6 (the Cochrane Library).
Data collection and analysis
Two review authors (BB and MS) independently screened studies for inclusion using Covidence. Three review authors independently undertook assessment of quality and extraction of included study details and results. For this update, BB reviewed all studies; and MS, RB, and RR each reviewed one‐third of the studies and compared results. We created a data extraction spreadsheet in Microsoft Excel.
We extracted information on methods, participants, intervention and control conditions, and outcomes. We were particularly interested in aspects of intervention development that may have contributed to a stronger, more appropriate or sustained intervention. We extracted information on the theory underlying the intervention development and content, process indicators and descriptions of community consultation and/or participation in the planning and implementation of the intervention, incentives (if present), and concerns regarding intervention programmes. We also recorded any information about costs, either in terms of evaluations of cost‐effectiveness, or simply where costs were mentioned. Where possible, we examined outcomes by gender, age, and socioeconomic status.
We resolved differences between reviewers' screening and extraction results by discussion or by consultation with a third review author. Given the heterogeneity of study design and characteristics, we considered a quantitative estimate of effect to be inappropriate and therefore provided a narrative synthesis.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for all included studies, including those included in previous versions of this review. We categorised risk of bias as high, low, or unclear for randomisation, allocation concealment, incomplete data, blinding of outcome assessment, and other bias, in accordance with methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved differences by discussion.
Sequence generation (checking for possible selection bias)
We have described the methods used to generate the allocation sequence and have assessed these methods as having:
low risk of bias (any truly random process, e.g. random number table, computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); or
unclear risk of bias (insufficient information provided with which to judge).
Allocation concealment (checking for possible selection bias)
We have described the methods used to conceal the allocation sequence in sufficient detail to determine whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We have assessed these methods as having:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open allocation; unsealed or non‐opaque envelopes; alternation; date of birth); or
unclear risk of bias (insufficient information provided with which to judge).
Blinding (checking for possible detection bias)
We have described the methods reported, if any, to blind study participants and personnel from knowledge of which intervention a participant received. With educational interventions (such as those assessed in this review) it is often not possible to blind participants to group allocation, and hence we did not evaluate blinding based on performance bias but rather based solely on the potential to introduce detection bias. It is possible for outcome assessors to be blinded to group allocation and we have noted where there was partial blinding. We have assessed study methods as having high risk of bias, low risk of bias, or unclear risk.
When investigators objectively measured findings (e.g. biochemical validation, household air nicotine monitors), we assessed blinding as adequate to prevent detection bias.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, or protocol deviations)
Within each included study, we have described for each outcome or class of outcomes the completeness of data, including attrition and exclusions from analysis. We have noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups.
Other bias (e.g. selective reporting bias)
We have noted any other potential sources of bias that were not related to the four sources discussed above.
Overall risk of bias
We made explicit judgements about whether studies were at high, moderate, or low risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to the specific types of bias discussed above, we assessed the likely magnitude and direction of bias, and whether we considered it likely to impact study findings.
Results
Description of studies
We included 78 studies in this review, 21 of which were identified in the most recent update; see the search study flow diagram in Figure 1 (Abdullah 2015; Blaakman 2015; Borrelli 2016; Chen 2016; Collins 2015; Cooper 2014; Daly 2016; Eakin 2014; Hafkamp‐de 2014; Harutyunyan 2013; Joseph 2014; Kegler 2015; Nicholson 2015; Ortega 2015; Pollak 2015; Schuck 2014; Streja 2014; Ulbricht 2014; Walker 2015; Wang 2015; Yucel 2014). We have summarised the characteristics of included studies below, and have provided further detail in the Characteristics of included studies table.
1.
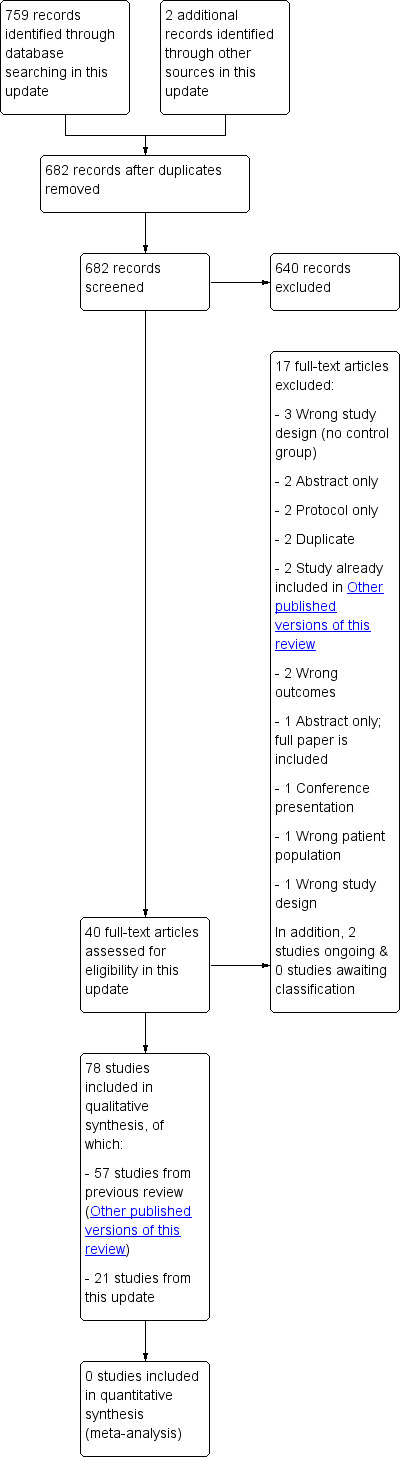
Study flow diagram.
We identified five additional studies for which outcome data are not yet available; we identified three of these in the previous update (Johnston 2010; Rosen 2011; Wagener 2012; Hutchinson 2013; Risica 2016). We have provided information about these ongoing studies in the Characteristics of ongoing studies table.
We have listed 35 studies as excluded. The most common reasons for exclusion were study design; participants not meeting inclusion criteria; outcomes not related to environmental tobacco smoke exposure; and lack of outcome data. Further information is available in the Characteristics of excluded studies table.
Intervention setting
One study evaluated outcomes for smoking mothers who called a telephone smoking cessation assistance counselling service (Davis 1992), and another recruited participants from callers to a 2‐1‐1 service (Kegler 2015). Seven studies introduced interventions in a school setting (Zhang 1993; Elder 1996; Ekerbicer 2007; Halterman 2011; Schuck 2014; Wang 2015; Chen 2016). Five further studies introduced interventions in other community settings (Conway 2004; Herbert 2011; Prokhorov 2013; Eakin 2014; Ulbricht 2014; see Characteristics of included studies for futher details).
Eight studies recruited from general healthcare settings (Harutyunyan 2013; Streja 2014; Yucel 2014; Abdullah 2015; Blaakman 2015; Collins 2015; Pollak 2015; Walker 2015; see Characteristics of included studies for futher details). Twenty‐five studies took place in well‐child healthcare settings, and recruited participants postnatally, at well‐child health visits or at infant immunisation clinics. Fourteen of these studies were peripartum, recruiting participants via maternity hospitals, from their records, or through midwives and general practitioners (Woodward 1987; Greenberg 1994; Severson 1997; Armstrong 2000; Van't Hof 2000; Emmons 2001; Ratner 2001; Pulley 2002; Schonberger 2005; Wiggins 2005; Culp 2007; French 2007; Hannover 2009; Cooper 2014). Chilmonczyk 1992, Vineis 1993, Eriksen 1996, Fossum 2004, Zakarian 2004, Abdullah 2005, Kallio 2006, Winickoff 2010, Baheiraei 2011, Hafkamp‐de 2014, Joseph 2014, and Daly 2016 used well‐child health check visits to a doctor or maternal child health nurse. Chellini 2013 recruited from hospital and public health facility waiting rooms, as well as from supermarkets.
Twenty‐six studies reported interventions conducted in an ill‐child healthcare setting. Fourteen of these identified families through their children's respiratory problems (Hughes 1991; McIntosh 1994; Wahlgren 1997; Irvine 1999; Wilson 2001; Hovell 2002; Krieger 2005; Ralston 2008; Borrelli 2010; Butz 2011; Halterman 2011 (recruited from school rather than healthcare setting); Wilson 2011; Stotts 2012; Borrelli 2016). Investigators conducted 10 studies in non‐respiratory ill‐child healthcare settings (Groner 2000; Hovell 2000; Wakefield 2002; Kimata 2004; Chan 2005; Chan 2006a; Hovell 2009; Phillips 2012; Tyc 2013; Nicholson 2015). Patel 2012 and Ralston 2013 targeted children presenting to the emergency department, approximately 40% of whom had a respiratory presenting complaint. Hovell 2000 and Hovell 2009 recruited mothers from a Special Supplemental Nutrition Program for Women, Infants, and Children, and looked at the effectiveness of counselling on smoking rates and children's ETS exposure among women of low income, high risk, and ethnically diverse backgrounds.
Two additional studies conducted in paediatric clinics did not specify whether they were conducted in the context of well‐child or ill‐child health visits (Curry 2003; Nuesslein 2006), and Yilmaz 2006 recruited children visiting paediatric clinics for treatment of primary conditions or for a well‐child visit.
Main target of intervention
Children's ETS exposure can be reduced by encouraging avoidance of children's exposure to cigarettes smoked, for example, by moving the child or the smoker to a different location, reducing the number of cigarettes smoked by the parent or carer, or having the smoker cease smoking altogether. The aims of studies identified by this review were heterogeneous. Here, we consider only smoking and ETS targets; we do not describe other intervention components, such as healthy eating (e.g. Elder 1996), asthma management (e.g. Hughes 1991), or household safety (e.g. Culp 2007).
Of the 78 included studies, 18 aimed solely for parental or carer smoking cessation or reduction (Vineis 1993; Zhang 1993; Severson 1997; Groner 2000; Emmons 2001; Wakefield 2002; Curry 2003; Kimata 2004; Chan 2005; Wiggins 2005; Kallio 2006; Nuesslein 2006; Ralston 2008; Borrelli 2010; Ralston 2013; Cooper 2014; Pollak 2015; Borrelli 2016). Twenty‐five studies aimed solely for reducing children's exposure to cigarettes smoked (Chilmonczyk 1992; Davis 1992; Elder 1996; Wahlgren 1997; Hovell 2000; Wilson 2001; Pulley 2002; Baheiraei 2011; Butz 2011; Herbert 2011; Wilson 2011; Stotts 2012; Chellini 2013; Prokhorov 2013; Tyc 2013; Harutyunyan 2013; Hafkamp‐de 2014; Schuck 2014; Streja 2014; Ulbricht 2014; Collins 2015; Kegler 2015; Nicholson 2015; Ortega 2015; Chen 2016), while 30 studies aimed for a combination of parental or carer cessation, reduction, or avoidance (Woodward 1987; Hughes 1991; Greenberg 1994; McIntosh 1994; Eriksen 1996; Irvine 1999; Armstrong 2000; Hovell 2000; Conway 2004; Fossum 2004; Zakarian 2004; Abdullah 2005; Krieger 2005; Schonberger 2005; Chan 2006a; Yilmaz 2006; Culp 2007; Ekerbicer 2007; Hovell 2009; Winickoff 2010; Halterman 2011; Patel 2012; Eakin 2014; Joseph 2014; Yucel 2014; Abdullah 2015; Blaakman 2015; Walker 2015; Wang 2015; Daly 2016). Five studies aimed to prevent reuptake of smoking postpartum (Van't Hof 2000; Ratner 2001; French 2007; Hannover 2009; Phillips 2012).
All studies aimed to achieve changes in behaviour in some way to reduce child ETS exposure. Eleven studies did not expressly include an educational or knowledge‐building component in their interventions but instead targeted change in attitudes and behaviours (Chilmonczyk 1992; Zhang 1993; Wahlgren 1997; Hovell 2000; Curry 2003; Zakarian 2004; Chan 2005; Nuesslein 2006; Cooper 2014; Abdullah 2015; Ortega 2015).
Location of studies
Most studies were reported from high‐income countries. Forty‐five studies were from North America, with 42 from the USA and three from Canada. Four studies were from Australia, and one was conducted in both Australia and New Zealand (Walker 2015). Three studies were from each of the UK, Germany, and the Netherlands. Two studies were from Italy (Vineis 1993; Chellini 2013). One study was reported from each of Finland (Kallio 2006), Japan (Kimata 2004), Sweden (Fossum 2004), Norway (Eriksen 1996), Taiwan (Chen 2016), and Spain (Ortega 2015). Fifteen of the studies conducted in high‐income countries specifically targeted disadvantaged, low‐income, and/or culturally diverse populations. Eleven studies were reported from low‐ or middle‐income countries, with six from China (Zhang 1993; Abdullah 2005; Chan 2005; Chan 2006a; Abdullah 2015; Wang 2015), three from Turkey (Yilmaz 2006; Ekerbicer 2007; Yucel 2014), and one from each of Iran (Baheiraei 2011) and Armenia (Harutyunyan 2013).
Participants
Twenty‐four studies targeted mothers only. Hovell 2009, Yucel 2014, and Pollak 2015 targeted mothers but invited partners or other family members to participate in counselling. One study targeted fathers by educating their non‐smoking wives (Chan 2006a). Thirty‐six studies targeted both parents. Zhang 1993 targeted fathers only; Borrelli 2010, Wilson 2011, Patel 2012, and Ralston 2013 targeted carers; Elder 1996 targeted teachers only; Wahlgren 1997, Butz 2011, and Stotts 2012 targeted families; and Krieger 2005, Halterman 2011, Harutyunyan 2013, Prokhorov 2013, and Kegler 2015 targeted households.
Age group
We stratified studies according to age groups of children: infants (younger than one year); preschoolers (up to age six); and school age (six to twelve years). Twenty‐three studies examined measures to reduce ETS exclusively for infants. Nineteen studies examined measures to reduce ETS for children up to and including preschool age, and 18 studies considered measures for children up to and including school age. One study followed pregnant women between 13 and 29 weeks' gestation for 12 months (Pollak 2015). Eight studies examined interventions to reduce ETS that included older age groups: Wahlgren 1997 included parents of children aged 6 to 17 years; Hovell 2002 and Borrelli 2016 included parents of children aged 3 to 17 years; Chan 2006a included parents of children from birth to 15 years; Yilmaz 2006 included mothers of children younger than 16 years of age; Streja 2014 included parents or guardians of children from 2 to 14 years of age; and Borrelli 2010, Chellini 2013, Prokhorov 2013, Tyc 2013,Kegler 2015, and Nicholson 2015 included children younger than 18 years of age. Five studies did not provide children's ages (Curry 2003; Chan 2005; Nuesslein 2006; Ralston 2008; Ralston 2013).
Theoretical framework
Forty‐five of the 78 studies expressly employed a theoretical framework in the design and/or development of the intervention. Fifteen studies used motivational interviewing (Emmons 2001; Curry 2003; Chan 2005; French 2007; Hannover 2009; Borrelli 2010; Baheiraei 2011; Halterman 2011; Phillips 2012; Stotts 2012; Ralston 2013; Eakin 2014; Blaakman 2015; Kegler 2015; Borrelli 2016). Seven used a social learning model (Greenberg 1994; Elder 1996; Conway 2004; Fossum 2004; Harutyunyan 2013; Ulbricht 2014; Blaakman 2015), and six used the stages of change component of Prochaska's transtheoretical model (Abdullah 2005; Krieger 2005; Ralston 2008; Winickoff 2010; Patel 2012; Ralston 2013). Chen 2016 combined transtheoretical and I‐change models, and Winickoff 2010 combined the transtheoretical stages of change model with social learning theory, the health beliefs model, cognitive‐behavioural theory, Wagner's chronic care model, and behavioural and systems theory. Several studies combined motivational interviewing with other frameworks, including stages of change (Ralston 2013; Wang 2015), Maori and Aboriginal holistic models of health (Walker 2015), the teachable moment (Borrelli 2016), cognitive‐behavioural therapy (Joseph 2014), cognitive‐behavioural skill building (Schuck 2014), and social cognitive theory. Kegler 2015 combined motivational interviewing with both the transtheoretical stages of change model and social cognitive theory, while Pollak 2015 combined motivational interviewing with both the teachable moment model and cognitive‐behavioural couples therapy.
McIntosh 1994 developed activities for the parent manual based on behaviour modification theory. Wahlgren 1997 tailored the programme to individual families and incorporated several behavioural modification techniques, including stimulus control, shaping, personal feedback, and contingency contracting. Groner 2000 employed the health belief model, and Wakefield 2002 used a harm minimisation approach that was based on previous research indicating that restrictions produced significantly lower urinary cotinine levels. Ratner 2001 utilised Marlatt's relapse model. Chan 2006a used Fishbein's theory of reasoned action and Ajzen's theory of planned behaviour in developing its educational intervention. Hovell 2009 used the behavioural ecological model in developing the counselling intervention. Herbert 2011 used a family‐centred assessment and intervention model to empower families to reduce cigarettes smoked in the home. Tyc 2013 and Nicholson 2015 used behavioural contracting, problem solving, and social reinforcement. Ortega 2015 used the 5 As (Ask, Advise, Assess, Assist, and Arrange) approach, and Streja 2014 employed the Health Behaviour Framework (previously the Adherence Model).
Acceptability of intervention to participants
Six studies appear to have involved consultation with potential participants as part of the development of the intervention (Hughes 1991; Davis 1992; Hovell 2000; Borrelli 2010; Streja 2014; Chen 2016). Davis 1992 employed focus groups with smokers and non‐smokers to understand their beliefs and attitudes towards smoking and cessation in order to develop improved self‐help materials. Borrelli 2010 conducted focus groups to better understand Latino culture and to modify the motivational interviewing technique accordingly.
Process indicators
Process indicators provide important information regarding the integrity of the way in which interventions were implemented. However, only 32 of the 78 studies described process indicators well (Hughes 1991; Chilmonczyk 1992; Davis 1992; Greenberg 1994; McIntosh 1994; Eriksen 1996; Severson 1997; Hovell 2000; Emmons 2001; Hovell 2002; Wakefield 2002; Fossum 2004; Zakarian 2004; Abdullah 2005; Wiggins 2005; Culp 2007; Hannover 2009; Hovell 2009; Borrelli 2010; Winickoff 2010; Stotts 2012; Tyc 2013; Cooper 2014; Eakin 2014; Hafkamp‐de 2014; Joseph 2014; Schuck 2014; Abdullah 2015; Blaakman 2015; Kegler 2015; Borrelli 2016; Daly 2016). More specifically, 11 studies reported that they maintained regular monitoring and support with those responsible for providing the intervention (Hughes 1991; Greenberg 1994; Emmons 2001; Culp 2007; Hannover 2009; Hovell 2009; Borrelli 2010; Eakin 2014; Hafkamp‐de 2014; Abdullah 2015; Daly 2016), and 19 reported that they evaluated the extent to which participants received, read, undertook, or adhered to the intervention as intended (Davis 1992; McIntosh 1994; Severson 1997; Hovell 2002; Wakefield 2002; Zakarian 2004; Abdullah 2005; Wiggins 2005; Culp 2007; Hovell 2009; Winickoff 2010; Stotts 2012; Cooper 2014; Joseph 2014; Schuck 2014; Abdullah 2015; Blaakman 2015; Kegler 2015; Borrelli 2016). Among those that commented on the monitoring of study implementation, one study recommended prompting providers over the course of the study to ensure appropriate implementation (Severson 1997). Another study reported the collection of qualitative data showing the opinions of nurses delivering the intervention (Fossum 2004).
Biological verification of children's exposure and absorption
Thirty studies used biological evidence of children's ETS absorption by measuring cotinine in urine or saliva, and 14 studies used environmental monitors of children's exposure to ETS. Eight of the 14 used passive sampling nicotine monitors as a primary study outcome. One study also measured particulate matter in the child's bedroom and living room (Butz 2011). The remaining studies used air nicotine monitors to promote or verify the accuracy of parent reporting of smoking behaviours. Wahlgren 1997 reported using air nicotine monitors in a room where greatest exposure to ETS was reported for two weeks before clinic visits to verify parent reports of cigarette consumption. Hovell 2000, Hovell 2002, Zakarian 2004, and Hovell 2009 used inactive air nicotine monitors placed in three rooms where children’s greatest ETS exposure was reported, to promote accurate self‐reporting of smoking behaviours by mothers. These studies also placed active air monitors for a selected proportion of the total sample: Hovell 2000 in a randomly selected half of the sample; both Hovell 2002 and Zakarian 2004 in 20% of the sample; and Hovell 2009 in a randomly selected 24% of the sample at six months. Zakarian 2004 reported randomly selecting these homes and placing monitors in the homes one week before data collection, while Hovell 2002 did not report how the 20% of homes were selected but reported that they were used only for baseline and post‐test measures. Cost was given as a reason for not using active air nicotine monitors across the whole sample. Eakin 2014 placed two monitors for seven days in the room where the child slept and in another room identified as a major activity room by the carer. Streja 2014 placed two monitors, each for one of two consecutive seven‐day periods in a major activity room. Kegler 2015 used passive air monitors after the three‐month visit for all participants reporting full or no bans, and for half of the participants reporting partial bans. However, investigators did not specify the location of the monitors. Borrelli 2016 placed two monitors for seven days at baseline and after call 5, they placed one in the room where the child spent the most time, and the child wore one.
Eleven interventions used feedback to parents of biological evidence of children's ETS absorption as a stimulus for parental behaviour change (Chilmonczyk 1992; McIntosh 1994; Wilson 2001; Wakefield 2002; Ekerbicer 2007; Wilson 2011; Harutyunyan 2013; Ulbricht 2014; Yucel 2014; Wang 2015; Daly 2016). Twenty‐three studies used biological validation of parental smoking cessation by measuring cotinine in urine, saliva, or serum (Woodward 1987; Irvine 1999; Hovell 2000; Hovell 2002; Fossum 2004; Zakarian 2004; Abdullah 2005; Kallio 2006; Nuesslein 2006; French 2007; Hovell 2009; Winickoff 2010; Phillips 2012; Tyc 2013; Cooper 2014), and/or expired carbon monoxide (Emmons 2001; Ratner 2001; Curry 2003; Abdullah 2005; Schonberger 2005; Borrelli 2010; Stotts 2012; Cooper 2014).
Length of follow‐up
For this review we determined length of follow‐up as extending from completion of the intervention to time of data collection. Length of follow‐up is important to determine, as it affects the extent to which sustainability and long‐term outcomes can be assessed. While short‐term reductions in children's ETS exposure have provided some benefit for children's health outcomes, the ultimate goal is long‐term and sustained change in order to maximise the positive impact on children's health and well‐being as they grow and develop. Twenty‐eight studies included in this review reported follow‐up of at least 12 months from the end of the intervention. Another 24 studies reported shorter follow‐up periods of between 6 and 12 months. Wahlgren 1997 debriefed participants at the six‐month follow‐up and reported ongoing follow‐up 8 and 18 months after that. Long‐term effectiveness was particularly difficult to assess in the remaining studies, specifically those with follow‐up periods of six months or less. McIntosh 1994 reported follow‐up periods that ranged between four and six months. Stotts 2012 reported a follow‐up period of six months from baseline, but it was unclear what the follow‐up was post intervention. The remaining studies (24) used a follow‐up time of less than six months.
Sample size
Thirty‐nine of the 78 studies mention conducting a power calculation in the design of their studies (Woodward 1987; Greenberg 1994; McIntosh 1994; Severson 1997; Wahlgren 1997; Irvine 1999; Armstrong 2000; Groner 2000; Hovell 2000; Emmons 2001; Wakefield 2002; Conway 2004; Krieger 2005; Schonberger 2005; Wiggins 2005; French 2007; Ralston 2008; Hannover 2009; Hovell 2009; Borrelli 2010; Baheiraei 2011; Butz 2011; Halterman 2011; Wilson 2011; Phillips 2012; Chellini 2013; Harutyunyan 2013; Prokhorov 2013; Ralston 2013; Cooper 2014; Ulbricht 2014; Abdullah 2015; Ortega 2015; Pollak 2015; Walker 2015; Wang 2015; Borrelli 2016; Chen 2016; Daly 2016). Of these, McIntosh 1994, Wahlgren 1997, Borrelli 2010, Harutyunyan 2013, Cooper 2014, Pollak 2015, and Daly 2016 explicitly mention that the statistical power of their study was limited by the small sample size. Although Streja 2014 did not present a power calculation, the authors did include a lack of statistical power as one of their limitations.
Risk of bias in included studies
To meet inclusion criteria for this review, studies had to be controlled trials. For this update, we assessed risk of bias for all of the included studies. We have summarised this assessment in Figure 2 and Figure 3.
2.
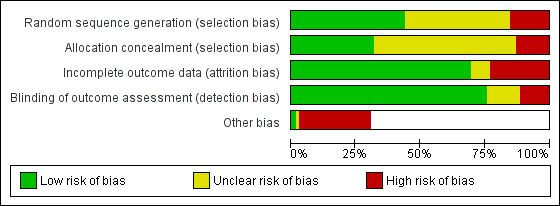
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
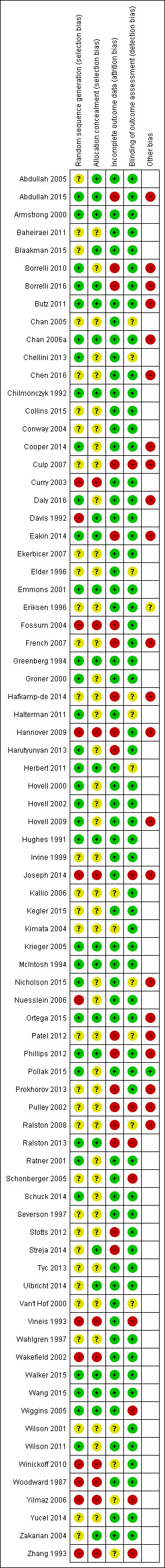
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Investigators rarely described the method of randomisation in sufficient detail to permit assessment of whether allocation was concealed at the time of trial entry. For example, it was common for studies to merely state that participants were randomised. Quasi‐randomisation was not uncommon even in large trials. Twelve and 32 studies, respectively, were at high and unclear risk of bias from poor randomisation and lack of randomisation. Ten and 43 studies, respectively, were at high and unclear risk of bias from allocation concealment, with many studies not describing allocation concealment.
Blinding (detection bias)
Very few trials had any blinding of participants or providers, largely due to pragmatic issues associated with administering an educational intervention. We have noted in the Characteristics of included studies tables where there was blinding of outcome assessors. We classified those trials without adequate blinding of outcome assessors or that used a subjective measure of outcome assessment as having high risk of bias. Nine and 10 studies, respectively, were at high and unclear risk of bias from blinding of outcome assessment.
Incomplete outcome data
Attrition from withdrawals and exclusions from trials were common, and often studies did not clearly specify the reasons for this. Attrition presents a potentially serious risk of bias in these studies. We have provided in the Characteristics of included studies table levels of attrition for each study, and information about any intention‐to‐treat analyses performed. Eighteen and six studies, respectively, were at high and unclear risk of bias due to incomplete outcome data.
Other potential sources of bias
We judged 22 studies to be at high risk of "other potential sources of bias". In 12 of these studies, this related to systematic differences in the characteristics of treatment groups (Pulley 2002; Culp 2007; French 2007; Ralston 2008; Hovell 2009; Butz 2011; Phillips 2012; Prokhorov 2013; Hafkamp‐de 2014; Abdullah 2015; Ortega 2015; Borrelli 2016). In four studies, this was due to potential exposure misclassification (Eakin 2014; Hafkamp‐de 2014; Joseph 2014; Daly 2016); in four this was due to a lack of intention‐to‐treat analysis (Pulley 2002; Hannover 2009; Patel 2012; Prokhorov 2013); in three this was due to the possibility of contamination between groups (Chan 2006a; Hafkamp‐de 2014; Abdullah 2015); in one it was due to a Hawthorne effect (Ortega 2015); and in another to the possibility of social desirability bias resulting from the interview format (Abdullah 2015).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Summary of findings: community‐based interventions for reducing children's exposure to environmental tobacco smoke.
| Community‐based interventions for reducing children's exposure to environmental tobacco smoke (ETS) | ||||
|
Patient or population: people who smoke and are involved in the care of young children (birth to 12 years of age) Settings: community Intervention: behavioural interventions Comparison: usual care or minimal intervention | ||||
| Intervention type and outcomes1 | Impact | No. of participants2 (studies) | Quality of the evidence (GRADE) | Comments |
| Multi‐component, counselling‐based interventions assessed with biochemical validation of ETS exposure and self‐report length of follow‐up: 3 to 12 months |
Of 7 studies in this group, 3 found that the intervention group was significantly more likely than the control group to implement full home smoking bans. One study found that the geometric mean hair nicotine level in the intervention group significantly decreased from 0.30 ng/mg to 0.23 ng/mg (P = 0.024), but not in the control group. Four studies found no significant differences in the change in cotinine levels between intervention and control groups. | 2880 (7 studies) | +‐‐‐ VERY LOW3 | |
| Multi‐comoponent, education‐based interventions assessed with biochemical validation of ETS exposure length of follow‐up: 6 months |
One study, with similar children’s urinary cotinine levels at baseline, found that cotinine levels were significantly lower (Z = ‐3.136; P = 0.002) in the intervention group (1.29 ng/mL) than in the control group (1.78 ng/mL) at 6 month follow‐up. The other study found no significant differences between intervention and control groups in child urine cotinine levels. | 307 (2 studies) | +‐‐‐ VERY LOW4 | |
| In‐person counselling (no additional components) assessed with biochemical validation of ETS exposure and self‐report length of follow‐up: 1 to 12 months |
Of the 6 studies in this group, 3 found significantly greater reductions in cotinine levels in the intervention compared with the control group. Two studies found that the intervention group was significantly more likely to implement home smoking bans. Two studies found no significant intervention impacts. | 1001 (6 studies) | +‐‐‐ VERY LOW5 | |
| Telephone counselling assessed with biochemical validation of ETS exposure length of follow‐up: 9 months |
One study found no significant difference in the proportion of children with low urinary cotinine levels (< 10 ng/mL) amongst parents receiving telephone counselling or a note regarding their child’s cotinine result. | 347 (1 study) | ++‐‐ LOW6 | |
| ETS: environmental tobacco smoke GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1 Not all studies reported length of follow‐up; length given based on those that reported.
2 Not all studies reported numbers of participants; number provided based on those that reported.
3 Downgraded two levels due to risk of bias: all studies at unclear or high risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous.
4 Downgraded one level due to risk of bias: one of two studies at high risk of bias. Downgraded two levels due to inconsistency: one study detected an effect and one did not; studies were clinically heterogeneous.
5 Downgraded two levels due to risk of bias: all studies at unclear or high risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous.
6 Downgraded one level due to risk of bias: one study at unclear risk of bias. Downgraded one level due to imprecision: only 186 participants with measured outcomes at nine‐month follow‐up.
Summary of findings 2. Summary of findings: interventions in the ill‐child setting for reducing children's exposure to environmental tobacco smoke.
| Interventions in the ill‐child setting for reducing children's exposure to environmental tobacco smoke (ETS) | ||||
|
Patient or population: people who smoke and are involved in the care of young children (birth to 12 years of age) Settings: healthcare ‐ ill‐child setting Intervention: behavioural interventions Comparison: usual care or minimal intervention | ||||
| Intervention type and outcomes1 | Impact | No. of participants2 (studies) | Quality of the evidence (GRADE) | Comments |
| Multi‐component, counselling‐based interventions assessed with biochemical validation of ETS exposure and self‐report length of follow‐up: 5 to 12 months |
Three studies found no significant differences between intervention and control groups. | 746 (3 studies) | +‐‐‐ VERY LOW3 | |
| Multi‐component, education‐based interventions assessed with biochemical validation of ETS exposure and self‐report length of follow‐up: 6 to 13 months |
One study reported significantly lower child's ETS exposure at home by any smoker at 12 months' follow‐up (52% vs 58%; P = 0.03). Six studies found no significant differences between intervention and control groups. | 2936 (7 studies) | +‐‐‐ VERY LOW4 | |
| In‐person counselling (no additional components) assessed with biochemical validation of ETS exposure, self‐report length of follow‐up: 3 to 18 months |
Eight studies appeared to show intervention benefits based on self‐reported ETS exposures but no significant differences between intervention and control groups in objective measures of exposure (e.g. cotinine). | 1835 (8 studies) | +‐‐‐ VERY LOW5 | |
| Telephone counselling | No studies examined telephone counselling delivered in the ill‐child setting and measured ETS exposure. | |||
| Brief interventions Assessed with presence of home and car smoking ban length of follow‐up: 24 weeks |
One study showed no significant differences between intervention and control groups in changed smoking policy: OR 2.0 (95% CI 0.166 to 24.069). | 100 (1 study) | +‐‐‐ VERY LOW6 | |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1 Not all studies reported length of follow‐up; length given based on those that reported.
2 Not all studies reported numbers of participants; number provided based on those that reported.
3 Downgraded one level due to risk of bias: two studies at unclear risk of bias. Downgraded one level due to imprecision. Downgraded one level due to indirectness: all studies were set in the USA and cannot be generalised to low income countries where smoking is more prevalent.
4 Downgraded two levels due to risk of bias: five of seven studies at high or unclear risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous.
5 Downgraded two levels due to risk of bias: all eight studies at high or unclear risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous.
6 Downgraded two levels due to risk of bias: only study was at high risk of bias. Downgraded one level due to imprecision: small study with a small number of events and wide confidence interval.
Summary of findings 3. Summary of findings: interventions in the well‐child setting for reducing children's exposure to environmental tobacco smoke.
| Interventions in the well‐child setting for reducing children's exposure to environmental tobacco smoke (ETS) | ||||
|
Patient or population: people who smoke and are involved in the care of young children (birth to 12 years of age) Settings: health care ‐ well‐child setting Intervention: behavioural interventions Comparison: usual care or minimal intervention | ||||
| Intervention type and outcomes1 | Impact | No. of participants2 (studies) | Quality of the evidence (GRADE) | Comments |
| Multi‐component, counselling‐based interventions assessed with biochemical validation of ETS exposure, self‐report length of follow‐up: 2 to 12 months |
One study found significant reduction in ETS exposure at home in the intervention group at age 6 years, but only on per‐protocol analysis (OR 0.71, 95% CI 0.59 to 0.87). One study found an increase in smoking bans in the home (19.3%) and in the car (7%) after 8 weeks' follow‐up in the intervention group, but not in the comparison group (2.5% increase in home ban and 0% change in car ban). One study found no significant difference between intervention and control groups in children’s urinary cotinine levels. | 8005 (3 studies) |
+‐‐‐ VERY LOW3 | |
| Multi‐component, education‐based interventions assessed with biochemical validation of ETS exposure, self‐report length of follow‐up: 2 to 12 months |
One study found that maternal self‐reported smoking at home around the infant was significantly less in the intervention group (8.6%) than in the control group (23.8%) (P < 0.05). Three studies found no evidence of effect of the intervention. | 1401 (4 studies) |
++‐‐ LOW4 | |
| In‐person counselling (no additional components) assessed with biochemical validation of ETS exposure, self‐report length of follow‐up: 3 to 90 months |
One study found significantly greater reductions in geometric mean urinary cotinine in the intervention group (decrease from 48.72 ng/mg to 28.68 ng/mg) compared to the control group (decrease from 40.43 to 36.32 ng/mg). In addition, the intervention group had a significantly greater increase in the proportion of households with smoking bans at home (15% to 33.3%) compared to the control group (11.5% to 19.5%). One study found a significantly beneficial reduction in kitchen and TV room air nicotine levels in the intervention group than in the control group (P < 0.05). One study found no difference in serum cotinine concentrations between the intervention and control groups. | 1483 (3 studies) |
++‐‐ LOW5 | |
| Telephone counselling assessed with self‐report length of follow‐up: 6 months |
One study found a greater proportion with partial home smoking bans in the intervention group (62.7%) than in the control group (56.4%), as well as a higher biochemically validated quit rate for the intervention group (10.6%) than for the control group (4.5%) at 6 months. | 952 (1 study) | ++‐‐ LOW6 | |
| Brief interventions assessed with self‐report length of follow‐up: not specified |
One study found no significant difference in home (OR 1.04, 95 CI 0.47 to 2.28) or car smoking bans (OR 1.47, 95 CI 0.69 to 3.11) between intervention and control groups. | 218 (1 study) | +‐‐‐ VERY LOW7 | |
| CI: confidence interval; OR: odds ratio GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1 Not all studies reported length of follow‐up; length given based on those that reported.
2 Not all studies reported numbers of participants; number provided based on those that reported.
3 Downgraded two levels due to risk of bias: all studies at unclear or high risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous.
4 Downgraded one level due to risk of bias: one study was at high risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous.
5 Downgraded one level due to risk of bias: two of three studies at unclear risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous.
6 Downgraded one level due to risk of bias: included study at unclear risk of bias. Downgraded one level due to indirectness: ETS exposure was measured indirectly as reported smoking restrictions in home.
7 Downgraded one level due to risk of bias: included study at unclear risk of bias. Downgraded one level due to indirectness: ETS exposure was measured indirectly as reported smoking restrictions in home and car. Downgraded one level due to imprecision: one study with a small number of participants and events.
We provide study results by outcome and by setting and child age below. We have discussed specific intervention types within individual outcomes, and more generally in the Discussion section. For further information, including effect sizes of interventions, see Analysis 1.1.
1.1. Analysis.
Comparison 1 Results, Outcome 1 Main outcomes.
| Main outcomes | |
|---|---|
| Study | |
| Abdullah 2005 | Counselling strategies based on the stages of change component of Prochaska's transtheoretical model. Results as N (%), intervention N = 444, control N = 459. Biochemically validated quit rate: Intervention 47 (10.6) Control 21 (4.5)
Had not quit but had reduced intake: Intervention 145 (32.6) Control 83 (18.1)
Stopped smoking for at least 24 hours: Intervention 145 (32.7) Control 136 (29.7)
Complete restriction: Intervention 113 (24.6) Control 151 (34.1)
Partial restriction: Intervention 278 (62.7) Control 259 (56.4) No measure of children's exposure or absorption via cotinine. |
| Abdullah 2015 | ETS exposure: 6 month follow‐up: 1) higher proportion of the intervention (62%) than the comparison (45%) group households adopted complete smoking restrictions at home (P = 0.022); 2) higher proportion of the intervention (38%) than the comparison (17%) group households did not smoke at home at all (P = 0.002); 3) total exposure from household members inside home in the past 7 days (measured by mean number of cigarettes smoked per week in front of the child by household members) was lower in the intervention (3.29) than the comparison (7.41) group (P = 0.021); 4) total exposure from all smokers indoors and outdoors in the past 7 days (measured by mean number of cigarettes smoked per week in front of the child) was significantly lower among children in the intervention (15.2) than the comparison (25.7) group (P = 0.005); 5) Comparison group: mean cotinine levels increased from baseline to 2 months and maximum at 6 months, with no statistically significant difference in time effects. Intervention group: mean cotinine levels increased at 2 months from baseline level but decreased again at 6 months, with statistically significant difference in time effects only from 2 to 6 months (P < 0.05); 6) No significant difference in allowing others to smoke around the child (P = 0.908). Air quality: At 6 month follow‐up: 1) mean number of cigarettes smoked daily was significantly lower in the intervention (11.02) than the comparison (13.6) group (P = 0.021); 2) significantly more participants in the intervention (48%) than the comparison (28%) group reduced the number of cigarettes smoked at home daily (P = 0.006) Child health: Perceived overall respiratory health of the child improved significantly in the intervention (35%) than the comparison (20%) group (P = 0.024). There were no significant differences in the reports respiratory symptoms of the child (P = 0.258). |
| Armstrong 2000 | Targeted disadvantaged mothers. Smoking in house around infant (maternal self report verified by researcher observation during home visit)
Intervention 8.6% v Control 23.8% (P < 0.05). included education about smoking near infants as a Sudden Infant Death Syndrome (SIDS) prevention strategy in a post‐natal nurse home visiting programme aimed to improve the quality of maternal‐child attachment, maternal health and child health parameters. At four months the intervention group had significantly more completed immunizations than the controls, although both groups had high immunization rates. At 12 months there was no statistically significant difference between the groups for immunization status. There was also no significant difference at four or 12 months for rates of utilisation of community services. |
| Baheiraei 2011 | Motivational Interviewing used. In 3 months geometric mean urinary cotinine: intervention decreased from 48.72 ng/mg to 28.68 ng/mg, control decreased from 40.43 to 36.32 ng/mg, differences between two groups statistically significant using one tailed t‐test. Greater decrease in total daily cigarette consumption in the presence of child in the intervention group than the control group (statistically significant with one tailed t‐test). Intervention median cigarettes at 3 month 0 (IQR 1 to 2.71), control 1 (IQR 0 to 3.21). Home smoking bans: intervention 15% to 33.3% (statistically significant increase), control 11.5% to 19.5% (not statistically significant increase), differences between two groups statistically significant using a one tailed t‐test. Car smoking bans in the intervention group increased from 4% to 8%, and didn't change in the control group. This was not a statistically significant difference. |
| Blaakman 2015 | ETS exposure: 5 months after discharge from NICY, caregivers in treatment group were sig more likely to report a home smoking ban than the comparison group (96% vs 84%; P = 0.03), and less likely to report routine infant contact with a smoker (40% vs 58%, P = 0.03). Differences in reported home bans (92% vs 83%, P = 0.14) and routine infant contact with smokers (44% vs 53%, P = 0.33) were no longer significantly different at study end (8 months after NICU discharge). No difference in car smoking bans or total smoking bans at any time. 8 months after NICU discharge, infants in intervention group had lower salivary cotinine and a greater decrease in salivary cotinine since baseline than infants in the comparison group. Air quality: Overall, very few caregivers quit smoking, which didn't differ between groups after intervention or at study end. Of the 29 total caregivers who reported smoking 5 months after NICU discharge, caregivers in the intervention group reported significantly higher confidence to quit than smoking caregivers in the comparison group at the 5‐month survey, but not at study end. No significant difference between groups in caregiver motivation to quit. Child health: No significant differences between groups in respiratory symptoms or use of health care services. |
| Borrelli 2010 | Latino families targeted. Used two interventions with different theoretical frameworks: one intervention used motivational interviewing, whilst the other intervention used the social cognitive theory. At 3 months 61.7% home monitors were returned and 98.8% were in good condition, whilst 60.9% child monitors returned and 100% in good condition. Household air nicotine significantly decreased from pretreatment to the 3 month follow‐up in the BAM condition, (baseline M = 1.07, SE 0.19, and 3‐month M = 0.28, SE 0.11, P = 0.01), whereas the decrease observed in the PAM condition was not statistically significant. Changes in secondhand smoke concentrations as assessed by the child monitors were not statistically significant. Continuous abstinence at 3 months 12.3% BAM group and 19.1% PAM group (OR 1.68, 95% CI 0.64 to 4.37). The child's level of functional morbidity due to asthma decreased significantly (P < 0.001) in both groups over time. Secondhand smoke exposure as measured by monitors directly on the child did not show a significant decrease in either group. |
| Borrelli 2016 | ETS exposure: SELF‐REPORTED: 1) PAM had significant reductions over time on one SHS exp variable, while HC had reductions on 4 of the 5 SHS exp variables, with a significant group x time interaction. 2) Enhanced PAM showed sig within‐group decreases in SHS exp over time on all 5 variables and HC showed sig within group decreases in SHS exp over time on 4 of the 5. Sig group x time interaction, such that enhanced PAM showed greater decreases in SHS exp over time versus HC for 3 of the 5 SHS exp variables; 3) Comparing PAM with enhanced PAM, no significant group x time interaction. OBJECTIVE: 1) No significant differences in levels of SHS exp at baseline; 2) At follow‐up, there were significant differences in detectable levels of SHS exp in the HOME monitors (PAM 92.1% vs HC 97.2%, P = 0.04), but NOT the CHILD monitors (PAM 91.4% vs HC 95.6%); 3) At follow up, no significant between‐group differences in detectable levels of SHS exp in either the home or child monitors, when comparing PAM with enhanced PAM. Air quality: 1) PAM more than 2x as likely to achieve 7‐day and 30‐day point‐prevalence abstinence than HC (statistically significant); 2) Enhanced PAM more than 2x as likely to achieve 7‐day PPA, 3x as likely to achieve 30‐day PPA than HCs, and 5x as likely to be continuously abstinent than HCs (statistically significant); 3) At 4‐months, enhanced PAm were more than 2x as likely to achieve 30‐day PPA versus PAM (significant). Child health: 1) At 6‐months, enhanced PAM had significantly lower child asthma hospitalisations than PAM; 2) At 2, 4 and 6 month follow‐up, enhanced PAM had sig lower missed school days due to asthma than PAM; 3) Odds of at least 1 day with asthma symptoms was sig lower in enhanced PAM than PAM at 6‐months; 3) No sig diff between groups in changes in asthma functional morbidity. |
| Butz 2011 | Low income households targeted. No statistically significant differences in urinary cotinine between baseline and follow up by group After combining the air cleaner groups, children assigned to those groups had a significant increase in symptom‐free days (SFDs) during the past 2 weeks (1.36 SFDs) compared with 0.24 SFDs for control group children from baseline to follow‐up No statistically significant differences In air nicotine at baseline and follow‐up by group Comparison of the combined air cleaner groups and the control group indicated that the combined air cleaner groups had significant mean differences in PM2.5 and PM2.5‐10 levels from baseline to follow‐up (mean differences for PM2.5: control, 3.5 [SD, 20.0]; combined air cleaner groups, ‐18.0 [SD, 33.2; P 0.001]; and for PM2.5‐10: control, 2.4 [SD, 20.8]; combined air cleaner groups, ‐9.6 [SD, 16.0; P = 0.009]) |
| Chan 2005 | Motivational Interviewing used. No statistically significant evidence of effect. Quit rate at 1 month post intervention: Intervention 7.5% [95%CI: 0 to 21] v 2.5% [95% CI: 0 to 7] control NS Reduced smoking consumption by half (self report): Intervention: 15% Control: 10% NS Reported quit attempts in last 30 days: Intervention 20% Control 7.5% NS Moved up the stage of readiness to quit: Intervention 17.5% Control 10% NS |
| Chan 2006a | Fishbein's theory of reasoned action and Ajzen's theory of planned behaviour used in the development of the educational intervention. Three most frequently reported actions taken by the mother to protect the child from passive smoking at home: opening the windows (N = 641, 43.9%), asking the father not to smoke near the child (N = 608, 41.6%), and moving the child away from the smoke (N = 482, 33%). Moved the children away when they were exposed to the fathers’ smoke at home at 3‐month follow up (78.4% vs. 71.1%; P = 0.01) NS at 6 and 12 months. Number of smokers (excluding the father) living with the child at 12 month follow up (11% vs 13% P = 0.049) Smokers who smoked at home (Excluding Child’s Father), at 12‐month follow up (92% vs 93% NS) Child’s ETS exposure at home by any smoker 3 months Intervention 37% vs Control 42% (P = 0.02) 6mths 51% vs 53% P = 0.48 12 mths 52% vs 58% P = 0.03 |
| Chellini 2013 | Post‐intervention smoke free homes were not significantly different between groups (increased in both): percentage increase in intervention group 12.7% and control group 11.1% (OR 1.04, 95 CI 0.47 to 2.28) . For cars: intervention group 18.2%, and control group 12.0% (OR 1.47 95 CI 0.69 to 3.11. Of the N = 131 smokers there was no significant difference in change of smoking habits. between intervention and control group (7% total stopped smoking, 5% stopped smoking indoors and n = 9 stopped smoking in the car). |
| Chen 2016 | ETS exposure: After intervention, the percentage of children with a urine cotinine concentration higher than 6ng/ml (indicating exposure) in the intervention group was significantly lower than that in the control group at both 8 weeks (P < 0.0001) and 6 months (P = 0.007). Air quality: Significantly less smoking in presence of children in intervention group at both 8 weeks and 6 months. Child health: N/A |
| Chilmonczyk 1992 | No evidence of effect. Intervention: 27/52 provided follow‐up urine. Control 29/51 provided follow‐up urine. Mean log urinary cotinine difference x100: Intervention group 2.05, control 2.17. P = 0.26 |
| Collins 2015 | ETS exposure: Associated with lower child urine cotinine compared with the control group. Air quality: Twenty (18.3%) of intervention group mothers and three (1.9%) of the control group mothers had bioverified quit status) P < 0.01). Child health: N/A |
| Conway 2004 | Participants (Latino families) for this study were recruited through advertising at community organisations and venues. Social learning model used. No significant effect. Hair nicotine (log ng/mg) 3mth Intervention 0.28, Control 0.32;12 mth Intervention 0.23, Control0.23 NS Hair cotinine (log ng/mg) 3mth Intervention 0.04, Control 0.04;12 mth Intervention 0.02, Control 0.04 NS Parent report reduction: % confirmed reducers 3mth Intervention 52%, Control 46%; 12mth Intervention 61%, Control 56% NS |
| Cooper 2014 | ETS exposure: N/A Air quality: After delivery, there were no statistically significant differences in cessation; self‐reported abstinence at 2 years was 2.9% in the NRT group and 1.7% in the placebo group. However, few participants reported using a full 8‐week course of NRT; 7.2% in NRT group and 2.8% in placebo group used their trial medications for over 1 month. Child health: At birth, significantly more Caesarian births occurred in the NRT group (20.7% vs 15.3%); at 2 years, significantly more infants in the NRT group (72.6% vs 65.5%) survived with 'no impairment'; 3) However, no sig difference between groups in infants' reported respiratory problems. |
| Culp 2007 | At 12 months the intervention group smokers smoked mean 2.1 fewer than control, which was not statistically significant: intervention 7.28 (s.d. 6.79), control 9.41 (s.d. 7.09) (t(147) = 1.82, P = 0.071). There were no significant differences between groups on number of hospital admissions or emergency room visits. At 12 months, intervention mothers were more likely to make use of health department clinics for well child care as compared to control group (chi square P =0.04) Knowledge of secondhand smoke exposure on child development: at 12 months significantly more intervention (N = 90, 58.1%) than control (N = 51, 47.7%) knew about SHS and impaired brain development, and significantly more intervention (N = 126, 80.6%) than control (N = 77, 72.0%) knew it takes longer to get well. No other significant differences with questions. |
| Curry 2003 | Ethnically diverse low income women targeted. Motivational Interviewing used. Abstinence rates: 3 mth Intervention 7.7% vs Control 3.4%; 12mth Intervention 13.5% vs Control 6.9% ‐ 12 mth difference statistically significant.
Serious attempt to quit at 12 months Adjusted OR 1.53 (95% CI 0.96 to 2.44)
Ever quit for 24h at 12 months Adjusted OR 0.94 (95% CI 0.59 to 1.5)
Prevalent abstinence 3 months Adjusted OR 2.40 (95% CI 0.85 to 7.8) 12 months Adjusted OR 2.77 (95% CI 1.24 to 6.60)
Sustained abstinence (abstinent at 3 and 12 months) Adjusted OR 1.83 (95% CI 0.29 to 14.30) Validation of smoking cessation by carbon monoxide expiration was completed by only a small subsample (13/156 in the intervention group and 5/147 in the control group). |
| Daly 2016 | ETS exposure: At 12 month follow‐up, 13% of all infants were reported to be exposed to SHS; however with urine cotinine validation, 17% overall were exposed. No significant time by group difference detected from baseline to follow‐up for either of the 2 treatment arms when compared with the control group. Air quality: At follow‐up, 47% of all parent/carers reported they were smokers. No significant time by group differences detected comparing either treatment arm with the control group. Child health: N/A |
| Davis 1992 | This study recruited participants through an advertising campaign that invited them to call a telephone smoking cessation assistance counselling service run by the National Cancer Institute in the USA. No evidence of difference between self‐help guides. Self‐reported quit attempts: Guide 1 121/198 (61%), Guide 2 122/204 (60%), Guide 3 147/229 (64%); Self‐reported abstinence for last week: Guide 1 28/198 (14%), Guide 2 24/204 (12%), Guide 3 27/229 (12%) P > 0.05 |
| Eakin 2014 | ETS exposure: Differences in salivary cotinine were not significant. However, among all families who reported a home smoking ban, salivary cotinine and air nicotine levels declined in both groups (P < 0.05). Air quality: Participants in the MI and education group had significantly lower air nicotine levels (0.29 vs 0.40 mg), 17% increase in prevalence of caregiver‐reported home smoking bans, and a 13% decrease in caregiver smokers compared with education‐alone group (all P values < 0.05). Child health: N/A |
| Ekerbicer 2007 | This study from Turkey recruited ETS exposed children from a primary school. Parents of identified children received telephone counselling or a note regarding their child's urinary cotinine result. At 9 months follow‐up: Group one 74/93 students had urinary cotinine levels < 10 ng/ml; group two 69/93 had urinary cotinine < 10 ng/ml. "The proportion of children with urinary cotinine values < 10ng/ml were statistically similar (P > 0.05) in both groups". |
| Elder 1996 | Social learning model used. No evidence of effect on tobacco‐free school policy after 3 years: Intervention 78% of 56 schools, Control 75% of 40 schools |
| Emmons 2001 | Motivational Interviewing used. Quit rates: Intervention 7.5%, Control 10.1%, P > 0.05 CPD: no effect Kitchen and TV room air nicotine measured by passive sampling diffusion monitors at 6 months (log transformed units): Intervention 3.7 & 3.1 fell to 2.6 & 2.3, Control 3.0 & 3.5 changed to 6.9 & 3.5. * P < 0.05, |
| Eriksen 1996 | No evidence of effect. Quit smoking: Intervention 7/222 (3%) vs Control 1/221 (0.5%); Stopped indoor smoking 4/222 vs 4/221; Any positive change 32/222 (14%) vs 34/221 (15%) |
| Fossum 2004 | Social learning model used. Self‐reported smoking (number of cigarettes) 1 month before childbirth: Intervention 13.1 vs Control 10.8 NS; 3 months after childbirth Intervention 12.8 vs Control 8.2 (significant); Past 24 hrs Intervention 11.8 vs Control 7.8 (significant). Salivary cotinine: Mean for Intervention reduced from 185 ng/ml to 165; mean for Control increased from 245 to 346 ng/ml. Weak correlation between mother's reported rate of smoking and cotinine levels for both control and intervention groups. |
| French 2007 | Six month follow‐up data Saliva cotinine verified non smoker: intervention (N = 26, 22%), control (N = 9, 10%) ‐ P < 0.025 Self‐reported non‐smoker: intervention (N = 40, 33%), control (N = 21, 22%) ‐ P < 0.10 |
| Greenberg 1994 | Social learning model used. Targeted ETS exposure in infants less than six months of age, and aimed to reduce the incidence of lower respiratory tract illness and the prevalence of respiratory symptoms. For infants of smoking mothers it demonstrated a lower prevalence of persistent symptoms in the intervention group (17.8%) compared with control group (30.9%; risk difference 13.1%; 95% CI: 1.0 to 27.0%). There was no difference in the incidence of illness. Parents report significant reduction in number of CPD: Intervention 12.5 CPD pre vs 7.7 CPD at 12month follow up, Control 12.3 CPD pre vs 13.3 at follow up P=0.01. Child urinary cotinine does not support this. Baseline mean urinary cotinine/ creatinine (nmol/mmol) Intervention 66 vs Control 51; at follow up Intervention 107 vs 98 Control. P = NS Prevalence of persistent lower respiratory symptoms Intervention 17.8%, Control 30.9% [difference 13.1%, 95% CI ‐1.0 to 27.0] |
| Groner 2000 | No evidence of effect. Self‐reported quit rates: Intervention Child Health Group 7/153, Mother's Health Group 4/164, Control 7/162. P = NS Self‐reported CPD reduced in all groups; Self‐reported not smoking indoors reduced: Intervention CHG 24, MHG 12, Control 13. P < 0.05 |
| Hafkamp‐de 2014 | ETS exposure: No significant difference in ETS exposure at home between intervention and control groups at age 6 years in the intention to treat analyses (OR 0.82, 95% CI: 0.66, 1.03); though this reached statistical significance in per‐protocol analysis with intervention group having less ETS exposure at age 6 years than the control group (OR 0.71, 95% CI: 0.59, 0.87). No effect modification by sociodemographic characteristics (data not shown). Air quality: N/A Child health: No significant differences between groups in asthma, wheezing frequency, airway inflammation (exhaled NO), or airway resistance (Rint). |
| Halterman 2011 | Motivational Interviewing used. Symptom‐free days/2 wk (difference) 0.96 (95% CI 0.39 to 1.52) Symptom nights/2 wk (difference) −0.63 (95% CI −1.09 to −0.18) Days with activity limitation/2 wk (difference) −0.44 (95% CI −0.87 to −0.02) Days with rescue medication use/2 wk (difference) −1.04 (95% CI −1.51 to −0.56) Days absent due to asthma/2 wk (difference) −0.22 (95% CI−0.36 to −0.07) ≥1 Visit for acute exacerbation of asthma (RR) 0.55 (95% CI 0.26 to 1.15) |
| Hannover 2009 | Motivational Interviewing used. At 24 months follow‐up Sustained abstinence: intervention (N = 36, 12%, 95% CI 8.8 to16.2), control (N = 39, 11%, 95% CI 8.4 to15.1), no statistically significant difference in proportions (0.7, 95% CI ‐4.2 to 5.8) Four week point prevalence: intervention (N = 72, 24% 95% CI 19.6 to29.2), control (N = 67, 19%, 95% CI 15.6 to23.9), no statically significant difference in proportions (4.7, 95 CI ‐1.7 to 11.1). |
| Harutyunyan 2013 | ETS exposure: Adjusting for baseline hair nicotine concentration, child's age and gender, the follow‐up geometric mean hair nicotine concentration in the intervention group was 17% lower than the control group (P = 0.239). The GM of hair nicotine in the intervention group significantly decreased from 0.30 ng/mg to 0.23 ng/mg (P = 0.024), but not in the control group. Adjusted odds of children's less than daily exposure to SHS at follow‐up was 1.87 times higher in the intervention group than in the control group (P = 0.077). Air quality: According to mothers, 4.5% intervention households and 5.4% control households completely banned indoor smoking at follow‐up. Also 4.5% smokers in the intervention group and 0.9% in the control group have reportedly stopped smoking at follow‐up. Child health: N/A |
| Herbert 2011 | Recruited families to participate in the study through five public health nursing offices, eight daycare centres, and kindergartens on Prince Edward Island. Used a family‐centred assessment and intervention model to empower families to reduce cigarettes smoked in the home. Those identified as having children exposed to ETS were then invited to participate in group counselling sessions. Intervention: decrease from median of 17 to 4.5 cigarettes/day and Control: decrease from 18.5 to 3.5 cigarettes/day. Both decreases statistically significant so did not detect a beneficial effect of the intervention. At 6 months follow‐up intervention participants smoked 0.65 (95% CI ‐5.68 to 6.98) more cigarettes per day compared to control participants |
| Hovell 2000 | Reduction in parent‐reported child exposure to cigarettes in the home and in total. At home reported exposure Intervention baseline 3.9 CPD, follow up 0.52 CPD vs Control 3.51 CPD baseline, 1.20 CPD follow up. The trend for parent‐reported total CPD exposure was similar.
Reports not supported by child urinary cotinine concentrations (ng/ml). Intervention baseline 10.93, follow up 10.47 vs Control baseline 9.43, follow up 17.47; 56% reduction (95% CI 48 to 63). Achieved a reduction in the number of parent‐reported cigarettes smoked in the presence of children per day at 12 months, following a three‐month intensive counselling intervention. There was, however, no change in cigarette smoke absorption as measured by children's urinary cotinine (ng/ml) for the intervention group over the 12 months (with measures collected at 3, 6 and 12 months). Cigarette smoke absorption for the control group increased from 9.4 ng/ml to 17.5 ng/ml over this time period, whereas there was almost no change in the intervention group (10.9 at baseline and 10.5 at 12 months). This increase in absorption observed for children in the control group appears to account for the apparent benefit of the intervention group. However the argument that this is solely due to reduced exposure in the home is uncertain, as the mothers in both the intervention and control groups reported falls in mothers' cigarettes smoked in the presence of the child from 3.9 to 0.5 (intervention) and 3.5 to 1.2 (control) cigarettes per day. In addition, they reported falls in total exposure to any source of cigarettes per day from 7.3 to 1.2 (intervention) and 7.2 to 2.8 (control). As the cotinine indicates a minimal fall for the intervention group and almost a doubling in urinary cotinine for the control group, either the cotinine measurement is unreliable or, more probably, that the parental report of cigarette exposure is not reliable. |
| Hovell 2002 | Latino families targeted. No significant effect. Decline in reported ETS exposure from (Intervention) 97% to 52% vs (Control) 93% to 69% at end of intervention (month 4). At follow up month 13, 9 months post‐intervention (Intervention) 52% to 45% and (Control) 69% to 54%. Average parent‐reported exposure levels declined over the follow‐up period from 0.57 to 0.47 CPD (Intervention) and 1.11 to 0.71 CPD (Control). These results show a difference of mean 0.34 CPD reduction in exposure by report. Biological verification of child exposure reveals a less successful outcome. Child cotinine levels fell in the intervention group immediately post‐intervention (month 4) 1.44 to 1.19 ng/mL, and rose in control group 1.17 to 1.35 ng/mL. Between end of intervention and follow up 9 months later levels fell 1.19 to 0.97 ng/mL (intervention) and 1.35 to 0.86 ng/mL (control). There was no significant difference in the mothers' rate of smoking cessation between groups. |
| Hovell 2009 | Low income households targeted. Behavioural ecological model used for development of the counselling intervention. Children's total SHSe showed a significant group by linear time interaction (P = 0.012) and a linear time effect (P < 0.001) from baseline to 6 months. Children's urinary cotinine showed no significant difference. Exposure from mothers in home (reported cigarettes/week) intervention 1.93 (95% CI 0.92 to3.48) control 6.16 (95% CI 3.61 to10.12); total reported exposure (cigarettes/week) intervention 5.15 (95% CI 2.71 to9.17) control 22.97 (95% CI 15.14 to34.58); mothers smoking reported cigarettes/week intervention 77.91 (95% CI 64.22 to91.60) control 92.88 (95% CI 80.59 to105.16); reported smoking by mothers indoors at home (cigarettes/week) intervention 3.94 (95% CI 2.06 to6.97) control 10.37 (95 CI 6.16 to17.06); reported smoking by all indoors at home (cigarettes/week) intervention 6.46 (95% CI 3.16 to12.40) control 19.18 (95% CI 11.15 to32.52). Children's urinary cotinine concentration and mother's reported smoking showed a significant group main effect, but did not show a significant difference in rates between intervention and control groups at 18 months. |
| Hughes 1991 | Intervention to reduce children's ETS exposure in a study of a comprehensive asthma education intervention. The outcome was improved asthma control but no change in exposure to ETS. No evidence of effect on homes with smoker: Intervention baseline 60% of 47 homes, follow up 52% vs Control baseline 57% of 48 homes, follow up 51% P = NS |
| Irvine 1999 | No evidence of effect. Mean decrease in child salivary cotinine (ng/ml): Intervention 0.70 vs Control 0.88. Difference= 0.19, 95% CI ‐0.86 to 0.48 Mean increase in mothers' salivary cotinine (ng/ml): Intervention 3.1 vs Control 1.8. Difference= 1.3, 95% CI ‐26.4 to 23.9 Self‐reported quit attempts: Intervention 101/213 vs Control 97/222, P = NS |
| Joseph 2014 | ETS exposure: Little change in household or car rules about smoking 8 weeks after index visit, but parents reported a high rate of total restriction at baseline. Air quality: 8 weeks after index visit, 11 of 38 (29%) parents in the intervention group reported 7‐day point‐prevalent abstinence. In contrast, only one parent in the comparison group reported abstinence from smoking (P = 0.001). There were fewer quit attempts and less readiness to quit in the comparison group. Child health: Not reported |
| Kallio 2006 | At child 8 years of age 10.1% (29/287) of mothers and 19.7% (43/218) fathers in the intervention group smoked regularly. The corresponding %s for the control group were 15.1% (45/298) mothers and 25.1% (60/239) fathers. Additionally 5.9% (17/287) of intervention group mothers and 8.3% (18/218) of intervention group fathers smoked occasionally compared with 5.7% (17/298) of control group mothers and 6.7% (16/239) of control group fathers (NS). |
| Kegler 2015 | ETS exposure: Significantly more intervention participants reported a full ban on smoking in the home than control participants at both 3 months (30.4% vs 14.9%, P < 0.001) and 6 months (40.0% vs 25.4%, P = 0.002) post‐baseline. The longitudinal intent‐to‐treat analysis showed that the difference in change was significant over time. When defining success more stringently by including only those reporting a full ban and no enforcement challenges, we found again that more intervention than control participants were successful in having and enforcing their smoke‐free home rule at 3 months (11.0% vs 5.6%; P = 0.03) and at 6 months post baseline (18.3% vs 8.7%; P = ).002). Air quality: Larger reduction in self‐reported exposure to SHS in the home among intervention participants at both follow‐up points, with a significantly larger decrease in the intervention group. In addition, significantly higher percentage of intervention participants (26.2% vs 18.0%) reported a full smoking ban in cars at 3 months (P = 0.02), although this difference was not observed 6 months post baseline. Smokers in the intervention group reported fewer cigarettes smoked per day at both follow‐up points, and the longitudinal analysis indicated that the intervention group had a significantly larger reduction over time. Although observed no difference in cessation rates between intervention and control groups, smokers in the intervention group had a higher number of quit attempts at the first follow‐up point, but not at 6 months post baseline. Also found that smokers in the intervention group had higher confidence in being able to quit at 3 months, but not at 6 months. The longitudinal intent‐to‐treat analysis, however, showed a significant difference in self‐efficacy to quit.. Child health: Not reported |
| Kimata 2004 | After 1 month urinary cotinine levels reduced 285±43 ngmL‐1 to 2.2±0.85 ngmL‐1 in AEDS cessation group, 257±31 ngmL‐1 to 1.8±52 ngmL‐1 in normal child cessation group and 274±42 ngmL‐1 vs 298±52 ngmL‐1 in control group of children with AEDS. AEDS children showed significant reduction in SCORAD index skin wheal (mm) from 9.9 baseline to 7.5; Control group 9.6 baseline to 9.3. Also significant changes in response to house dust mite & cat dander & lower neutrophil levels. |
| Krieger 2005 | Intervention guided by the transtheoretical stages of change model, as well as by social cognitive theory. Report that 20% of the sample quit smoking and that among smokers who did not go outside to smoke prior to intervention, a quarter did so after education, but data are not provided and it is unclear whether intervention outcomes were different between groups. Homes where smoking was reported as not allowed at baseline 80% (high intensity group) vs 76% (low intensity group) and at exit 77% (high) vs 80% (low) P = 0.33 NS. |
| McIntosh 1994 | Number of smokers who moved outside: Intervention 7/30, Control 4/30. Not statistically significant. Urinary cotinine concentrations of children of subjects reportedly smoking outside are above 10.0 in 4/6 (range 6.7 to 54) in Intervention children tested, and in 3/3 (range 12.2 to 21.5) control children tested. These levels suggest significant ETS exposure. |
| Nicholson 2015 | ETS exposure: At the end of the follow‐up phase, 45.4% of families reported a home ban (intervention: 47.2%; control: 43.6%) and 20.4% employed a full ban (intervention: 24.5%; control: 16.4%). Group assignment (intervention or control) was not a significant predictor of adopting a home ban. There was a marginal difference between intervention and control groups for the adoption of full bans (OR = 1.81, P = .060). Air quality: Not reported Child health: Not reported |
| Nuesslein 2006 | Calculated nicotine consumption Intervention: 12 micrograms to 4.65 micrograms vs Control: 12 micrograms to 7.5 micrograms NS Urinary cotinine levels Intervention 3520 ng/ml to 741 ng/ml vs Control 4572 ng/ml to 724 ng/ml P > 0.05 NS Across the entire sample (both intervention and control groups) there was an overall reduction in self‐reported smoking with average number of cigarettes smoked reduced from 17 to 10 per day and significant reduction in calculated nicotine consumption using self report data 12 micrograms to 5.5 micrograms (P < 0.05), urinary cotinine 4101 ng/ml to 741 ng/ml (P < 0.05). |
| Ortega 2015 | ETS exposure: TSP‐avoidance strategies improved more in the intervention group than in the control: 35.4% and 26.9% (P = 0.006) at home, and 62.2% and 53.1% in cars (P = 0.008). Logistic regression showed adjusted ORs for appropriate measures in the intervention group vs control group of 1.59 (95% CI 1.21 to 2.09) at home and 1.30 (95% CI 0.97 to 1.75) in cars. Air quality: Not reported Child health: Not reported |
| Patel 2012 | No significant differences between intervention compared to control groups in: Changed smoking policy: OR2.0 (95% CI 0.166 to 24.069) Reduced no. of cigarettes: OR 4.88 (95% CI 0.785 to 30.286) Quit smoking: OR 1.12 (95% CI 0.346 to 3.590) |
| Phillips 2012 | Where both saliva cotinine and self‐report were available, saliva cotinine was used. At eight weeks post‐partum, there was a significantly more smoke free mothers in the intervention (81%) compared with the control group (46%) ‐ P < 0.001. |
| Pollak 2015 | ETS exposure: Not reported Air quality: Found high rates of cessation but no arm differences in smoking rates at the end of pregnancy (0.31 vs. 0.30, materials only vs. counselling, respectively) and 12 months after randomisation (postpartum: 0.39 vs. 0.38). Found high quit rates among non daily smokers but no arm differences (0.43 vs. 0.46 in pregnancy and 0.52 vs. 0.48 postpartum). Among daily smokers, found lower quit rates with no arm differences but effects favouring the intervention arm (0.13 vs. 0.16 in pregnancy and 0.17 vs. 0.24 postpartum). Child health: Not reported |
| Prokhorov 2013 | Smoking status of smoker; 90% on baseline smokers in each group still using tobacco (N = 36 intervention, N = 35 control) Results for the environmental monitors: two monitors ‐ one in a "higher exposure" room than the other. In the high exposure room there was a significant main effect for time (P < 0.001) and time by condition effect (P < 0.05); for the intervention group the mean ambient nicotine level decreased from baseline at 12 months (1.14 μg/m3 to 0.20μg/m3, P < 0.01). There was a decrease in mean of control group but not significant (0.55 μg/m3 to 0.17μg/m3, P = .99), and a significant difference between average rate of change for intervention and control groups. In the low exposure there was a significant main effect for time but not time‐by‐condition and similar reductions in the intervention and control groups. Percentage of households banning smoking at 12 months: 73% of the intervention group and 56% of the control group. |
| Pulley 2002 | Follow‐up three weeks post‐intervention Cigarettes/day: intervention 16.17 (sd 9.10), control 11.33 (sd 4.69) ‐ P = 0.132 Mothers in the intervention group smoked more at enrolment compared with control group, an effect not present at the 2 week visit (baseline) but present again three weeks post intervention Respiratory illness: intervention N = 5 (42%), control N = 6 (66%) ‐ P = 0.666 |
| Ralston 2008 | Counselling strategies based on the stages of change component of Prochaska's transtheoretical model. N = 42, 33% (N = 14) lost to follow‐up. The quit rate: 14% intervention, 5% control group which did not reach statistical significance |
| Ralston 2013 | Differences between intervention and control groups were not significant (fisher's test): Self‐reported quit ‐ control 6/30 (20%, 95% CI 9 to 38%) and intervention 5/30 (17%, 95% CI 7 to 34%); any quit attempt during follow‐up ‐ control 11/30 (37%, 95 CI 22 to 55%) and intervention 16/30 (53%, 95% CI 36 to 70%); cut down ‐ control 11/30 (27%, 95% CI 22 to 55%) and intervention 15/30% (15%, 95 CI 33 to 67%); used quitline ‐ control 2/30 (7%, 95% CI 8 to 22%) and intervention 0/30 (0%, 95% CI 0 to 13%). |
| Ratner 2001 | 6 month Follow up: 36% abstinent, 26% occasional, 38% daily smoking. 76% homes smoke‐free. 12 month Follow up: 20% abstinent, 35% occasional, 46% daily. 76% homes smoke‐free No difference between groups. 6 month Follow up abstinence was 41% vs 30% (intervention vs control) but at 12 months abstinence was sustained in 21% vs 18.5% (intervention vs control) NS. Daily smoking at 6 months was 31% vs 45% (intervention vs control) but at 12 months was 41% vs 50% (intervention vs control). NS Abstinence reported as 38% vs 27% (treatment vs control) NS. |
| Schonberger 2005 | At 6 month Follow up Maternal post‐natal smoking Intervention 52% (14/27) vs. Control 28% (8/30) P = 0.04 Partner smoking Intervention 31% (14/44) vs Control 20% 9/45) NS Smoking by others Intervention 47% vs Control 50% NS |
| Schuck 2014 | ETS exposure: Not reported Air quality: Parents who received quitline counselling were more likely to report 7‐day point‐prevalence abstinence at 12‐month assessment (34.0 versus 18.0%, odds ratio (OR) = 2.35, confidence interval (CI) = 1.56–3.54) than those who received a standard self‐help brochure. Parents who received quitline counselling were more likely to use nicotine replacement therapy (P < 0.001) than those who received a standard self‐help brochure. Among parents who did not achieve abstinence, those who received quitline counselling smoked fewer cigarettes at 3‐month (P < 0.001) and 12‐month assessment (P < 0.001), were more likely to make a quit attempt (P < 0.001), to achieve 24 hours’ abstinence (P < 0.001) and to implement a complete home smoking ban (P < 0.01). Child health: Not reported |
| Severson 1997 | Cessation at 6 & 12 months: Intervention 25/1073 (2.3%), Control 10/802 (1.2%), P < 0.05*, 1‐tailed test
Cessation at 12 months: Intervention 59/1073 (5.5%), Control 38/802 (4.7%) NS. Only 35 of the 97 12‐month quitters had quit by six months, with more early quitters in the intervention group (25/59) compared with the control group (10/38). Relapse prevention at 6 & 12 months: Intervention 200/609 (33%). Control 109/417 (26%), P < 0.05*, 1‐tailed test Relapse prevention at 12 months: Intervention 261/609 (43%), Control 163/417 (39%) * when controlling for other variables this effect was lost. Significant benefits of intervention on CPD, readiness to quit, likelihood of making a quit attempt, attitude towards smoking, knowledge of ETS effects on children. |
| Stotts 2012 | Lower rates of total smoking bans in the usual care‐reduced measurement group (P < 0.012 for total ban, P < 0.01 for car) but not significantly different for home alone. 63.6% receiving motivational interviewing had a ban by 1 month post‐discharged compared to 20% of the usual care group. No significant differences in environmental nicotine monitors measurements |
| Streja 2014 | ETS exposure: No significant difference between intervention and control groups in child urine cotinine levels. Air quality: No significant difference between intervention and control groups in any of the measures. Child health: Not reported |
| Tyc 2013 | Group difference for average cigarettes smoked and child SHSe was not significantly different as the 12‐month follow‐up (P > 0.05). Child SHSe was significantly lower at 12 months from baseline for each group (P < 0.05). Children's urinary cotinine showed no significant difference, and did not change significantly over time in either group. |
| Ulbricht 2014 | ETS exposure: The child urine cotinine level difference between follow‐up and baseline was smaller in the control than in the intervention group, but the effect was not significant. Air quality: Not reported Child health: Not reported |
| Van't Hof 2000 | There was no statistically significant difference in the smoking relapse rate between women in the intervention (41%) and control (37%) groups. |
| Vineis 1993 | Smoking cessation for mothers: Intervention 12/74 vs Control 10/84, OR 1.4, 95% CI 0.6 to 3.5
Smoking cessation for fathers: Intervention 18/173 vs Control 26/244 OR 1.0 showed a trend towards smoking cessation for mothers classified as white collar workers in the intervention arm (5/33) versus the control arm (2/36) (Odds Ratio [OR] 3.0; 95% confidence intervals [CI] 0.6 to 16.0). No difference was detected for the other participants, comprising 80 blue collar mothers and a total of 411 men defined as white or blue collar workers. |
| Wahlgren 1997 | Intensive intervention was able to demonstrate a statistically significant but very small reduction in cigarette exposure from parents' cigarettes reported by parents without biological verification. Mean number of parent cigarettes smoked in presence of child fell in Intervention group: 5.8CPD baseline, 3.4CPD at clinic pre‐intervention to 1.2 CPD at 6 months following completion of intervention. In control group, parent reported exposure fell from 8.0 baseline, 5.7 pre‐intervention to 4.6 CPD at 6 month follow up. P for trend < 0.01. The effect size was small, however, and curiously, the largest fall in this measure occurred in the period after recruitment but before the intervention. After the intervention, parents reported a reduction of 1.1 cigarettes per day smoked in the presence of the children for the control group, and 2.2 cigarettes per day for the intervention group. There was no validation by measurement of children's exposure or absorption via cotinine, or validation of the parental reports, and the clinical significance of such a fall is unclear Environmental monitor (1 room with heaviest child exposure) measured air nicotine (mcg/ cubic metre). Intervention group baseline 1.7, follow up 1.9 vs Control baseline 2.3, follow up1.4. Measured child asthma symptoms but found no sustained difference between groups for this measure. |
| Wakefield 2002 | Home smoking ban: Intervention 41% at baseline, 49% at Follow up vs Control 40% at baseline, 42% at Follwo up. Relative increase in bans not significant; P = 0.40 Car smoking bans: Intervention baseline 33%, Follow up = 52%, Control baseline 37%, Follow up 48%, NS; Low rates of parental cessation, no difference between groups. Urinary cotinine measured for 209 children: Mean cotinine/ creatinine Intervention B = 22.8 nmol/mmol Follow up 21.0, Control baseline 25.7, Follow up 21.0, NS, P = 0.40 |
| Walker 2015 | ETS exposure: No significant difference between group in urine cotinine level change over time, self‐reported SHS exposure, smoking ban, smoking cessation. Air quality: No significant change in smoking prevalence and intensity was seen by group. Child health: No significant difference in infant cough, acute respiratory illness or rate of hospitalisations between treatment groups. |
| Wang 2015 | ETS exposure: Children's urinary cotinine was significantly lower (Z = ‐3.136; P = 0.002) in the intervention group (1.29 ng/mL) than the control group (1.78 ng/mL). After 6months, reported mean ETS exposure from caregivers decreased 40.6% from baseline among the intervention group and 3.4% among controls. Air quality: Caregiver's 7‐day quit rate was significantly higher (34.4% versus 0%) (p < 0.001; adjusted OR = 1.13; 95% CI: 1.02‐1.26) in the intervention group. Child health: Not reported |
| Wiggins 2005 | Mothers living in disadvantaged inner city areas targeted. No significant effect of either intervention. Support health visitor group vs control group, RR 0.86 (95% CI 0.86 to 1.19); Community support group RR 0.97 (95% CI 0.72 to 1.33). Reported no notable differences in child health outcomes for children receiving either post‐natal support intervention. |
| Wilson 2001 | Of 51 children with complete urinary cotinine: creatinine ratio (CCR) data. Log CCR (ng/mg) Intervention baseline 1.82, Follow up 1.27 vs Control baseline 2.34, Follow up 1.93, adjusted Diff ‐0.38, adjusted P = 0.26.
Proportion with >1 acute asthma visit/year: Intervention baseline 50, Follow up 29.6, Control baseline 37.2, Follow up 46.5, OR 0.32, P = 0.03
No significant differences in hospitalisation, prohibition of smoking in home, or smoking. examined the effect of an intervention targeting smoking behaviour change and asthma education on health care utilisation and asthma hospitalisations, and explored other measures of asthma control. It demonstrated a reduction in the prevalence of children making more than one acute care asthma visit in the year following the intervention. Given that there was no apparent benefit of the smoking‐related counselling on smoking‐related outcomes, it is likely that it was the asthma education that achieved the improvement in asthma morbidity, rather than the smoking behaviour programme. |
| Wilson 2011 | Mean urinary cotinine creatinine ratio (CCR) decreased in both groups (not shown data for 6 and 12 month follow‐up). The natural log of the urinary CCR decreased more in the intervention arm but it did not reach statistical significance (B coefficient ‐0.307 95% CI ‐0.633 to 0.018, P = 0.64) Decrease in asthma symptoms at follow‐up visits in both groups. The decrease in the intervention group did not reach statistical significance (B coefficient 0.035, 95% CI ‐0.208 to 0.277, P = 0.78) At 12 months 84.0% of the intervention group (N = 142) and 77.1% of the control group (N = 131) had home smoking bans (P = 0.11). |
| Winickoff 2010 | Prevalence of self‐reported 7 day abstinence 38% at baseline and 30% at follow up in the control group vs 31% at baseline and 30% at follow up in the intervention group (Effect size = 13% P = NS) Cotinine‐confirmed 7 day abstinence for baseline current smokers NS. For baseline current smokers 18% in the control and 64% in the intervention group reported making a 24hr quit attempt by follow up (P = 0.005). |
| Woodward 1987 | No evidence of effect. Mother self‐reported quitting: Intervention 6%, Control 2.2%, P = 0.25. Median infant urinary cotinine levels (mcg/litre): Intervention 11.0 (N = 48) vs Control 10.0 (N = 53), P = NS |
| Yilmaz 2006 | Quit smoking: Child intervention group 24.3%; Mother intervention group 13%; Control 0.8%. (χ2 = 29.5, P < 0.0001) Smoking location change: Child intervention: 73%, Mother intervention: 46.6%, Control 11.6% (χ2 = 90.1, P < 0.0001) Knowledge change (score on MCQ, possible score 0‐100): mean post‐intervention score in child intervention 63.51 (±7.35 ‐ not stated whether these ± is standard deviations, or 95% confidence intervals) mother intervention 57.69 (±10.46) control 56.68 (±7.67) (ANOVA showed that these scores differed) P < 0.0001 (Note: not an intention‐to‐treat analysis) |
| Yucel 2014 | ETS exposure: No significant difference between intensive and minimal intervention groups in change in child urine cotinine levels. Air quality: No significant difference in any outcome. Child health: Not reported |
| Zakarian 2004 | Low income ethnically diverse population. Both groups showed significant decline in reported exposure to mother's cigarette's/week (intervention group 18.89 at baseline to 5.41 at 12 months, control group 13.25 at baseline to 5.23 at 12 months) (P < 0.001). Total exposure to cigarettes/week (intervention group 53.2 at baseline to 21.99 at 12 months, control 54.48 at baseline to 18.22 at 12 months) (P < 0.001) however, no significant difference between groups. Children's urinary cotinine concentration did not show a significant change over time in either group ‐ No significant difference between groups. |
| Zhang 1993 | This was a study designed to increase public knowledge of the health consequences of cigarette smoking and to promote healthier attitudes among elementary school students in China, and encouraged these students to help their fathers to quit smoking. Schools in one district used a tobacco control curriculum, and the control group were students in another district. The other school‐based study was a cardiovascular health promotion programme that included an intervention designed to limit children's ETS exposure and negative role modelling from staff and visitors smoking at school (Elder 1996). Conducted in the USA, this study used a cluster‐randomized design with schools as the unit of allocation.Number (proportion) of smoking fathers: Intervention baseline 6843/9953 (68.8%) & follow up 60.7% vs Control baseline 6274/9580 (65.5%), follow up "approximately the same" [numbers are not stated] Proportion of fathers who quit smoking for at least 180 days: Intervention 800/9953 (11.7%), Control 14/6274 (0.2%) |
Tobacco smoke exposure outcomes
Of the 78 studies, 26 reported success in achieving reduced children's ETS exposure between intervention and control groups, 24 of which presented statistically significant findings (N = 33,811). Thirteen (N = 3640) used biochemical or environmental measures of children's ETS exposure (biological verification of cotinine in urine or saliva of the child, or use of environmental monitors) (Wahlgren 1997; Emmons 2001; Kimata 2004; Borrelli 2010; Baheiraei 2011; Harutyunyan 2013; Prokhorov 2013; Collins 2015; Kegler 2015; Ortega 2015; Wang 2015; Borrelli 2016; Chen 2016) and 11 (N = 30,171) did not use such measures (Zhang 1993; Armstrong 2000; Curry 2003; Abdullah 2005; Schonberger 2005; Yilmaz 2006; French 2007; Phillips 2012; Hafkamp‐de 2014; Abdullah 2015; Blaakman 2015). Of these, we judged 11 to be at high risk of bias, three at low risk of bias, and 10 at unclear risk of bias. We provide a brief summary of outcomes below, along with further details of available outcome measures in the section Analysis 1.1.
Of the 13 studies using biochemical or environmental measures of children's ETS exposure, five (N = 645) reported children's urinary cotinine measures (Kimata 2004; Baheiraei 2011; Collins 2015; Wang 2015; Chen 2016), two (N = 1351) reported children's hair nicotine measures (Harutyunyan 2013; Ortega 2015), and six (N = 1644) recorded household air nicotine assessed with monitors (Wahlgren 1997; Emmons 2001; Borrelli 2010; Prokhorov 2013; Kegler 2015; Borrelli 2016). Seven (N = 1580) of these 13 studies used in‐person counselling (Wahlgren 1997; Emmons 2001; Borrelli 2010; Baheiraei 2011; Collins 2015; Borrelli 2016; Chen 2016), two (N = 748) used complex interventions consisting of counselling plus additional components (Harutyunyan 2013; Kegler 2015), one (N = 65) used a complex intervention consisting of education plus additional components (Wang 2015), one (N = 1101) used a brief intervention (Ortega 2015), and one (N = 71) used "fotonovelas" and a comic book (Prokhorov 2013). In one study (N = 75) intervention methods are unclear as investigators do not describe how they encouraged participants to stop smoking, but do state that those in the intervention group "agreed to stop smoking" (Kimata 2004).
Eight studies reported success based on parents' reports of smoking cessation, with or without salivary cotinine verification, or reduction in smoking in the presence of children but without verification of children's ETS exposure. These studies employed a range of interventions including school‐based interventions (children writing letters to their fathers urging them to quit), intensive counselling, a home visiting programme, education and advice, and an intervention based on the Behavioural Action Model (BAM). Zhang 1993 (N = 19,533) used a school‐based intervention and reported the proportion of fathers who quit smoking for at least 180 days as 800/9953 (11.7%) for the intervention group, and as 14/6274 (0.2%) for the control group. At follow‐up, Armstrong 2000 (N = 181) reported smoking in the house around an infant (maternal self‐report) for the intervention group as 8.6% and for the control group as 23.8% when the intervention group received a home visiting programme. Curry 2003 (N = 303) reported smoking abstinence at 12 months as 13.5% in the intervention group, following a brief motivational message and telephone counselling, and as 6.9% in the control group. Abdullah 2005 (N = 952) used telephone counselling and reported a biochemically validated quit rate of 47/444 (10.6%) for the intervention group and 21/459 (4.5%) for the control group at six months. Schonberger 2005 (N = 476) reported that 52% (14/27) of postnatal mothers quit smoking in the intervention group, compared with 28% (8/30) in the control group, at six months' follow‐up when the intervention group received home visits. Yilmaz 2006 (N = 363) included two intervention groups that had discussions about effects of smoking on child or maternal health. Quit rates at follow‐up were as follows: child intervention group 24.3%; mother intervention group 13%; and control group 0.8%. French 2007 (N = 61) used motivational interviewing; and at six months' follow‐up, 26 (22%) participants in the intervention group and 9 (10%) in the control group were saliva cotinine‐verified non‐smokers. Phillips 2012 (N = 44) used motivational interviewing for both groups, and provided information about infant bonding to the intervention group. The study reported that at eight weeks postpartum, there were significantly more smoke‐free mothers in the intervention (81%) group compared with the control (46%) group.
Fifty‐two studies (N = 19,758) failed to detect an intervention effect on ETS outcomes (Woodward 1987; Hughes 1991; Chilmonczyk 1992; Davis 1992; Vineis 1993; Greenberg 1994; McIntosh 1994; Elder 1996; Eriksen 1996; Severson 1997; Irvine 1999; Groner 2000; Hovell 2000; Van't Hof 2000; Ratner 2001; Wilson 2001; Hovell 2002; Pulley 2002; Wakefield 2002; Conway 2004; Fossum 2004; Zakarian 2004; Chan 2005; Krieger 2005; Wiggins 2005; Chan 2006a; Kallio 2006; Nuesslein 2006; Culp 2007; Ekerbicer 2007; Ralston 2008; Hannover 2009; Hovell 2009; Winickoff 2010; Butz 2011; Halterman 2011; Herbert 2011; Wilson 2011; Stotts 2012; Chellini 2013; Patel 2012; Ralston 2013; Tyc 2013; Cooper 2014; Eakin 2014; Joseph 2014; Schuck 2014; Streja 2014; Yucel 2014; Pollak 2015; Walker 2015; Daly 2016). Three (N = 824) of these studies reported significant reduction in self‐reported parental smoking based on intensive counselling without a corresponding reduction in children’s urinary cotinine measurements (Hovell 2000; Hovell 2009; Schuck 2014). In Culp 2007 (N = 263), the intervention group received home visits, and whilst there was no significant reduction in smoking, the other outcome of relevance to our review was mothers' knowledge of the effects of smoking on child development. At 12 months, the intervention group answered two out of six questions better than the control group.
In all, 21 of these 52 studies (N = 6485) used biochemical measures of children's ETS exposure (child urinary, hair, or salivary cotinine levels) (Woodward 1987; Chilmonczyk 1992; Greenberg 1994; McIntosh 1994; Irvine 1999; Hovell 2000; Wilson 2001; Hovell 2002; Wakefield 2002; Conway 2004; Zakarian 2004; Kallio 2006; Ekerbicer 2007; Hovell 2009; Halterman 2011; Wilson 2011; Tyc 2013; Eakin 2014; Streja 2014;Yucel 2014; Walker 2015), while the rest used self‐reports of smoking behaviour, with or without salivary cotinine verification. Interventions used in these studies were varied; 29 studies (N = 8930) used complex interventions predominantly including counselling and/or education (Hughes 1991; Chilmonczyk 1992; Davis 1992; Vineis 1993; Greenberg 1994; McIntosh 1994; Eriksen 1996; Irvine 1999; Groner 2000; Wilson 2001; Hovell 2002; Pulley 2002; Wakefield 2002; Zakarian 2004; Krieger 2005; Chan 2006a; Ralston 2008; Winickoff 2010; Butz 2011; Wilson 2011; Ralston 2013; Tyc 2013; Eakin 2014; Joseph 2014; Schuck 2014; Streja 2014; Yucel 2014; Walker 2015; Daly 2016).
Thirty‐four of the 78 studies reported reduced children's ETS exposure among study participants regardless of assignment to intervention or control groups (Woodward 1987; Hughes 1991; Davis 1992; Vineis 1993; Elder 1996; Eriksen 1996; Severson 1997; Wahlgren 1997; Irvine 1999; Groner 2000; Ratner 2001; Wilson 2001; Hovell 2002; Wakefield 2002; Curry 2003; Fossum 2004; Abdullah 2005; Chan 2005; Krieger 2005; Chan 2006a; Kallio 2006; Nuesslein 2006; Ekerbicer 2007; Hovell 2009; Winickoff 2010; Halterman 2011; Herbert 2011; Wilson 2011; Chellini 2013; Prokhorov 2013; Ralston 2013; Tyc 2013; Eakin 2014; Nicholson 2015).
Household air quality
Eleven studies (N = 2636) reported household air nicotine measures (Wahlgren 1997; Emmons 2001; Hovell 2009; Borrelli 2010; Butz 2011; Stotts 2012; Prokhorov 2013; Eakin 2014; Streja 2014; Kegler 2015; Borrelli 2016). Of these studies, two did not use air nicotine measures to evaluate the impact of interventions; Hovell 2009 used air nicotine measures to validate reported exposures, while Kegler 2015 used air nicotine measures to validate home smoking bans. Of the remaining nine studies, five (N = 1385) found a statistically significant benefit of the intervention in reducing air nicotine levels (Emmons 2001; Borrelli 2010; Prokhorov 2013; Eakin 2014; Borrelli 2016).
Borrelli 2010 reported a significant decrease in nicotine concentrations as measured by home monitors in the Behaviour Action Model (BAM) group (intervention to increase self‐efficacy; baseline Mean = 1.07, standard error (SE) 0.19; three‐month Mean = 0.28, SE 0.11; P = 0.01) but not in the Precaution Adoption Model (PAM) (motivational interviewing) group at three‐month follow‐up. Borrelli 2016 used the PAM for two aims: first, to determine whether second‐hand smoke exposure (SHSe) feedback motivates cessation among parents of children with asthma versus parents of healthy children (HC) ‐ the study reported significant differences in levels of SHS exposure detected by home monitors (PAM 92.1% vs HC 97.2%; P = 0.04), but not by child monitors (PAM 91.4% vs HC 95.6%); second, to evaluate whether greater intervention intensity (enhanced‐precaution adoption model (PAM)) produces greater cessation than a previously tested intervention (PAM). However, data show no significant between‐group differences.
Emmons 2001 used motivational interviewing and telephone counselling and reported reduced household air nicotine measurements over time in the intervention groups (kitchen and TV room air nicotine at six months (log‐transformed units): intervention 3.7 and 3.1, falling to 2.6 and 2.3; Control 3.0 and 3.5, changing to 6.9 and 3.5; P < 0.05). As there was no change in the number of cigarettes smoked per day, nor in the cessation rate, the implication of the difference was that parents and carers had changed smoking location and had moved outside to smoke.
Eakin 2014 found that motivational interviewing and education resulted in significantly lower air nicotine levels compared to education alone (0.29 vs 0.40 mg) amongst carers of preschool children in a Head Start programme in the USA.
Prokhorov 2013 reported a significant decrease in nicotine concentrations for the intervention group, which received a comic book and "fotonovelas" for the "high‐exposure" room (1.14 μg/m³ to 0.20 μg/m³; P < 0.01) but not for the "low‐exposure" room, whilst the decrease noted in the control group was not significant.
Of the four studies (N = 603) that did not show a significant benefit, three used counselling, motivational interviewing, or a combination of air cleaners and health coaching in ill‐child settings (Wahlgren 1997; Butz 2011; Stotts 2012); while one used a combination of a video and a booklet with educational and risk reduction strategies, together with visual reminders, in a community setting (Streja 2014).
Child health outcomes
Sixteen studies (N = 12,520) assessed child health outcomes (Hughes 1991; Greenberg 1994; Armstrong 2000; Wilson 2001; Pulley 2002; Kimata 2004; Krieger 2005; Schonberger 2005; Wiggins 2005; Culp 2007; Borrelli 2010; Butz 2011; Halterman 2011; Wilson 2011; Hafkamp‐de 2014; Walker 2015), and five studies measured child health outcomes, although they were not regarded as a primary outcome variable (N = 2184; see Analysis 1.1) (Wahlgren 1997; Cooper 2014; Abdullah 2015; Blaakman 2015; Borrelli 2016). Of these, the child health outcome of interest in 10 studies was asthma related (symptom scores, quality of life, functional morbidity, symptom‐free days, and asthma‐related health services utilisation). In three studies, the health outcome of interest was respiratory illness, and another two reported health service utilisation alone ‐ community services in one, and hospital admissions and emergency visits in another. One study measured changes in neurotrophin levels but did not specify which neurotrophins were measured.
Nine studies found improvement in child health outcomes. Hughes 1991 (N = 95) embedded an intervention to reduce children’s ETS exposure in a study of a comprehensive asthma education intervention. Although asthma control was improved there was no change in exposure to ETS. Greenberg 1994 (N = 933) targeted ETS exposure in infants younger than six months of age and aimed to reduce the incidence of lower respiratory tract illness and the prevalence of respiratory symptoms. For infants of smoking mothers, the study demonstrated a lower prevalence of persistent symptoms in the intervention group (17.8%) compared with the control group (30.9%; risk difference 13.1%; 95% confidence interval (CI) 1.0% to 27.0%). There was no difference in the incidence of illness. Wilson 2001 (N = 87) examined the effects of an intervention targeting smoking behaviour change and asthma education on healthcare utilisation and asthma hospitalisations, and explored other measures of asthma control. The study demonstrated a reduction in the prevalence of children making more than one acute care asthma visit in the year following the intervention. Given that there was no apparent benefit of the smoking‐related counselling on smoking‐related outcomes, it is likely that asthma education, rather than the smoking behaviour programme, achieved improvement in asthma morbidity. Kimata 2004 (N = 75) found that cessation of smoking had no effect on skin wheal responses nor on plasma neurotrophins among normal children, but achieved a significant reduction in skin wheal response, responses to house dust mite, and cat dander, along with lower neutrophil levels for those with atopic eczema/dermatitis syndrome. Neurotrophins are a subset of growth factors with a range of functions throughout the body and include nerve growth factor and brain‐derived neurotrophic factor, as reported in Lackie 1999, which was the only study identified by this review to consider neurotrophin levels, and it does not specify which particular neurotrophins were measured. Krieger 2005 (N = 274) delivered a community home intervention to address conditions affecting childhood asthma and reported that the high‐intensity intervention group showed clinically significant improvement in paediatric carer asthma quality of life scores and a decline in urgent health service utilisation, but no significant difference in symptom‐free days, compared to the low‐intensity intervention group. However, they did not achieve a statistically significant intervention effect for carer reports of smoking in the home nor for reports of no smoking allowed in the home, so the child health intervention effect is probably due to other aspects of the intervention. Culp 2007 (N = 263) conducted home visits with the goal of promoting the health and development of first‐time mothers and infants and found no significant differences between groups in terms of numbers of hospital admissions or emergency room visits. At 12 months, intervention mothers were more likely to make use of health department clinics for well‐child care as compared to the control group (P = 0.04). Borrelli 2010 (N = 133) reported that the child’s level of functional morbidity due to asthma decreased significantly (P < .001) in both the BAM (intervention to increase self‐efficacy) and PAM (motivational interviewing) groups over time. Butz 2011 (N = 126) reported that after the two groups that used air cleaners were combined, children assigned to those groups showed a significant increase in symptom‐free days during the previous two weeks: 1.36 compared with 0.24 symptom‐free days for control group children from baseline to follow‐up. Halterman 2011 (N = 530) used motivational interviewing to counsel the primary carer and an additional smoker who spent the most time with the child and observed inhaler administration at school by a nurse. This study only measured child health outcomes and found a significant improvement in many asthma‐related outcome measures in the intervention compared to the control group. We have provided further details in the Analysis 1.1 table.
Seven studies (N = 9619) did not detect a significant intervention effect on child health outcomes (Wahlgren 1997; Armstrong 2000; Pulley 2002; Wiggins 2005; Wilson 2011; Hafkamp‐de 2014; Walker 2015). See Analysis 1.1 for further details. Of these seven studies, three used complex interventions consisting of counselling and additional components (Wilson 2011; Hafkamp‐de 2014; Walker 2015), two used complex interventions consisting of education and additional components (Armstrong 2000; Pulley 2002), one used in‐person counselling (Wahlgren 1997), and one used community support groups for mothers (Wiggins 2005).
Schonberger 2005 (N = 476) reported associations of exposure to passive smoking with parentally reported asthma symptoms without group allocation. Therefore it is not possible to determine an intervention effect on child health outcomes.
Results according to child age
A smaller proportion of studies of infants detected beneficial intervention effects compared with studies of older age groups. Four (N = 1187) of the 23 studies that examined measures to reduce ETS exclusively among infants detected a beneficial intervention effect (Abdullah 2005; French 2007; Baheiraei 2011; Phillips 2012). Eight (N = 10,576) of the nine studies examining measures to reduce ETS among children up to and including preschool age demonstrated a beneficial intervention effect (Emmons 2001; Schonberger 2005; Harutyunyan 2013; Hafkamp‐de 2014; Abdullah 2015; Collins 2015; Ortega 2015; Wang 2015). Ten (N = 22,078) of the 18 studies examining measures to reduce ETS among children up to and including school age and older demonstrated an intervention effect (Zhang 1993; Greenberg 1994; Wahlgren 1997; Kimata 2004; Krieger 2005; Yilmaz 2006; Borrelli 2010; Halterman 2011; Prokhorov 2013; Chen 2016).
Results according to setting
In the ill‐child respiratory setting, four (N = 1028) of 13 studies demonstrated a beneficial intervention effect (Wahlgren 1997; Krieger 2005; Borrelli 2010; Halterman 2011). Krieger 2005 and Halterman 2011 showed a significant effect on child health outcomes but not on tobacco smoke exposure outcomes. Three of these four studies used intensive counselling or motivational interviewing, whilst one used a community home intervention with elements of education and behaviour change. Of the nine studies that did not demonstrate an intervention effect, three used intensive counselling, one used motivational interviewing, one used a motivational health coach in addition to air cleaners, two used brief counselling methods, and two used home visits.
In the ill‐child non‐respiratory setting, two (N = 119) of nine studies showed a beneficial intervention effect (Kimata 2004; Phillips 2012). Kimata 2004 did not describe the intervention, and Phillips 2012 used motivational interviewing for both groups, with the intervention group also receiving information about infant bonding. Of the seven studies that did not demonstrate an intervention effect, three used brief counselling methods and four used more intensive counselling, including one study that used motivational interviewing, one that used a booklet, and one that used cotinine feedback.
In the clinical setting (not designated well‐child or ill‐child), one study (N = 303) out of two demonstrated a beneficial intervention effect (Curry 2003). This study used a brief motivational message and a motivational interview, along with follow‐up telephone counselling. Nuesslein 2006 (N = 40) did not find an intervention effect and used parental cotinine feedback.
In the clinical setting (both well‐child and ill‐child), Yilmaz 2006 (N = 3636) and Ortega 2015 (N = 1101) demonstrated a beneficial intervention effect. We included no other studies in this group.
In the well‐child clinical setting, seven (N = 9866) of the 27 studies demonstrated a beneficial intervention effect (Armstrong 2000; Emmons 2001; Abdullah 2005; Schonberger 2005; French 2007; Baheiraei 2011; Hafkamp‐de 2014). Three of these seven studies used motivational interviewing, two used home visiting interventions, one used telephone smoking cessation counselling, and one used a combination of counselling and education. Of the 20 studies that did not demonstrate an intervention effect, five used brief counselling methods; five used intensive counselling methods; four used home visits; one used cotinine feedback; one used telephone counselling; one used nicotine replacement therapy; one used an information kit and letter; one used a combination of counselling, education, and feedback on exposure level; and another used a combination of feedback on a computer risk assessment and nurse brief advice.
In the community setting, eight (N = 20,975) of 21 studies showed a beneficial intervention effect (Zhang 1993; Harutyunyan 2013; Prokhorov 2013; Abdullah 2015; Blaakman 2015; Kegler 2015; Wang 2015; Chen 2016). Four of these eight studies used counselling, one of which used motivational interviewing; two used a combination of counselling, education, and feedback on exposure level; one was a school‐based intervention; and one used a combination of telephone motivational interviewing and mailings. Of the 13 studies that did not demonstrate an intervention effect, two used telephone and two used in‐person counselling; four provided a combination of counselling and education, smoking cessation brief advice, or feedback on cotinine exposure level; two provided a combination of education with a video and visual reminders or culturally tailored couples‐based intervention with nicotine replacement therapy; one adopted a tobacco‐free school policy; and one used a support health visitor intervention consisting of monthly supportive listening home visits.
Biological validation of parents' self‐report
Of the 30 studies providing biological evidence of child ETS absorption, 16 (N = 4057) allowed an assessment of validation of parent‐reported change in exposure versus child ETS absorption (Greenberg 1994; McIntosh 1994; Hovell 2000; Wilson 2001; Hovell 2002; Wakefield 2002; Kimata 2004; Zakarian 2004; Kallio 2006; Hovell 2009; Baheiraei 2011; Tyc 2013; Streja 2014; Walker 2015; Wang 2015; Daly 2016). Of these studies, seven (N = 2116) did not show a discrepancy between reported exposure and an objective measure of absorption (Wilson 2001; Wakefield 2002; Kimata 2004; Kallio 2006; Streja 2014; Walker 2015; Wang 2015). Kallio 2006 (N = 1062) reported that parent serum cotinine values showed that parents reported smoking habits accurately but did not provide data. Of the studies using environmental monitors of child exposure to ETS, Wahlgren 1997 (N = 91) and Hovell 2009 (N = 150) allowed an assessment of validation of parent‐reported change in exposure versus objective measure. Wahlgren 1997 did not demonstrate a correlation between parental report and environmental monitoring, whilst Hovell 2009 reported a significant moderate correlation. For Hovell 2009, however, the results showed a significant reduction in child second‐hand smoke exposure associated with the intervention according to reports, but not according to child urinary cotinine. Tyc 2013 (N = 135) also noted a significant decrease in reported child second‐hand smoke exposure but not in child urinary cotinine in the intervention group. Borrelli 2010 (N = 133) noted that, according to monitors in the home, but not those on the child, there was a significantly greater reduction in exposure to children in the BAM (intervention to increase self‐efficacy) group, although quit rates in the PAM (motivational interviewing) group were higher. This was thought to have occurred as the result of a greater change in the number of cigarettes smoked in front of the child in the BAM group, rather than following use of monitors as a validation measure.
Cost data and cost‐effectiveness
Thirteen of the included studies made some reference to costs. However, this was generally limited to some statement of implementation costs. McIntosh 1994 (N = 92) mentioned the cost of the manual, and Severson 1997 (N = 1875) mentioned staff and intervention costs of the intervention per person. Conway 2004 (N = 143) and Wiggins 2005 (N = 731) also mentioned the costs of implementing the intervention but indicated that investigators did not conduct further analysis of cost‐effectiveness because of a lack of an intervention effect. Krieger 2005 (N = 274) reported reduced urgent healthcare costs during the two months before the exit interview among those receiving the intervention relative to those in the comparison group, but investigators did not provide an extensive cost‐benefit analysis. Cooper 2014 (N = 1050) reported total mean costs that were approximately £91 higher in the nicotine replacement therapy group and indicated that the incremental cost‐effectiveness ratio (ICER) associated with nicotine replacement therapy use was £4926 per additional quitter (95% CI ‐£114128 to £126747).
Discussion
Of the 78 included studies, a minority (26 studies) detected an effect in favour of the intervention, 24 of which reported statistically significant findings. Although the proportion of studies targeting the population or community level has increased since review authors conducted the previous update (Baxi 2014), most studies that detected an effect (15) were performed in clinical settings (eight well‐child; five ill‐child; two well‐ and ill‐child), with eight successful interventions delivered in community settings and one in an unspecified setting. The intervention most frequently used in 16 of the 24 successful studies was counselling, two instances of which were provided in combination with education and feedback on measures of exposure. Seven of the eight studies in community settings used counselling successfully ‐ five of the eight studies in well‐child clinical settings, and three of the five studies in ill‐child clinical settings.
However, counselling was also used in 29 of the 52 studies showing no effect of the intervention; most of which delivered the intervention in clinical settings (11 well‐child; 9 ill‐child), with nine delivering the intervention in community settings.
Our findings suggest that strategies that are effective in the adult healthcare setting may not be generalisable to the paediatric setting. Brief advice for adult smokers when they attend clinical services for their health has a positive effect in triggering quit attempts (Stead 2013). Trials of interventions for parents attending clinical paediatric or child health services did not detect this effect. However, this finding might suggest that either a different sort of brief intervention should be employed, or that this context should not be used for brief advice. Also, studies may have been underpowered to detect a small effect. Examination of the dynamics of the doctor‐child‐parent relationship may assist the development of brief strategies with a greater likelihood of success in this clinical setting. Given the unknowns about the doctor‐child‐parent interaction, interventions provided in this setting may potentially cause harm. One study reported a trend for mothers in the intervention group to smoke more than mothers in the control group after receiving the intervention (Irvine 1999). Several studies used only one‐tailed t‐tests to look for statistical significance. When an intervention may cause harm, even if the hypothesis is unidirectional, investigators should always employ two‐tailed tests of significance. Hovell 2009 undertook a regression analysis to examine factors associated with the longest participant smoking quit attempts following counselling. The odds favouring the longest quit attempt were significantly increased when participants had made a 24‐hour quit attempt in the year prior to baseline, had tried a greater number of methods to quit in the past, and had reduced permissiveness of home smoking. Researchers did not find significant associations between longer quit attempts and level of education, heaviness of smoking or the smoking status of a partner.
There are relatively high rates of smoking cessation in pregnancy, both spontaneously and with clinical interventions (Chamberlain 2017; Coleman 2015). With high postnatal relapse rates reported among women who have quit during pregnancy (Lelong 2001), prevention of relapse for this group is an obvious means of preventing environmental tobacco smoke (ETS) exposure for their children. Ratner 2001 and Van't Hof 2000 identified risk factors for relapse. Risk factors identified by Ratner 2001 included having a partner who smoked and smoking a greater number of sticks per day before quitting; data show that prolonged breast feeding and higher scores on a scale measuring mental health were protective. Van't Hof 2000 found that a lower level of confidence in maintaining cessation, a lower level of encouragement by family and friends to maintain cessation, and greater numbers of family and friends who smoked were all associated with significantly higher odds of postpartum relapse. Further work in this area will make an important contribution.
Many of the studies identified for this review demonstrated reduced child exposure to ETS among participants, regardless of assignment to intervention or control groups, which suggests that studies may be describing the natural history of smoking among parents. Parents may reduce their own smoking or their children's exposure over time, possibly as a result of social pressures. Indeed the prevalent social trend in many developed countries over the past decade has been increased community concern about exposing non‐smokers to ETS (although arguably more so among non‐smokers than among active smokers). This is especially true for adults in the workplace and in public spaces such as bars and restaurants, particularly in North America, Australia, and some countries within the EU, where total smoking bans for these settings are increasingly legislated. Campaigns and community concerns about children's exposure to ETS at home and in cars have also increased. It is possible that these studies have recorded parents responding to this social trend by limiting their children's exposure in the home. This being the case, studies need to aim not just for a reduction in children's ETS exposure, but for a greater than background reduction in ETS exposure. For a study to produce a significant effect, the impact of interventions must be greater than the rate of decline in comparison groups. It may be true that as most studies used comparison groups rather than control groups (i.e. no cessation or avoidance advice and no information), the comparison interventions may have been more effective than anticipated. As studies have generally involved comparison groups receiving a limited intervention rather than strict control groups, this is certainly possible. Moreover, measurement of tobacco smoke exposure outcomes alone may produce an intervention effect and thus may be an important component of any intervention.
We judged the inconclusive evidence presented in this review to be of low or very low quality, despite the fact that this review includes 78 studies (Table 1; Table 2; Table 3). Limitations include risk of bias, heterogeneity among study interventions and populations, and small sample sizes with low statistical power. Continuing to perform studies without adequate sample size, quality, or comparable interventions and populations will not allow for any conclusions to be reached regarding the clinical effects or cost‐effectiveness of interventions. Moreover, additional low‐quality studies may be an unethical use of resources and participants’ time.
Limitations of methods employed
The heterogeneity of study designs and characteristics rendered quantitative analysis inappropriate for this review. However, there is currently no best approach in narrative, rather than quantitative, syntheses of published studies. As we have included 78 studies, it would not be feasible to list results of each in the main text. Therefore, we have highlighted key results in our narrative summary and have recorded further results data in Analysis 1.1. However, we are aware that in some places, this means that studies with statistically significant results have been described in greater detail in the text than those that did not detect an effect. We have attempted to mitigate any impact of this by explicitly describing studies that tested similar interventions but did not detect an effect.
An additional limitation is that, of the 20 studies that used objective measures of children's ETS exposure or absorption, only four showed no discrepancy between parental reports of children's exposure and the biological measures. As most studies did not use objective measures, this calls into question the validity of self‐reported data provided in this review.
As noted above, many of the included studies had small sample sizes, and fewer than half (N = 28) reported a power calculation. For studies that did not detect an effect, this makes it difficult to establish whether the intervention was genuinely not effective, or if a result was not detected because the sample size was too small.
Included studies reported varying lengths of follow‐up. We used the longest reported follow‐up for the results. However, some studies reported short lengths of follow‐up, with 20 studies reporting follow‐up of less than six months. It is difficult to determine the sustainability and long‐term effectiveness of interventions when study follow‐up is short. Indeed, of the studies reporting longer follow‐up, some did show an initial difference between intervention and control groups that was not sustained at the final follow‐up period.
Finally, given that the burden of ETS is shifting more and more towards low‐ and middle‐income countries, and that in high‐income countries the burden is disproportionately falling on disadvantaged households, findings of the studies included in this review may not be generalisable, as these trials were conducted mainly in high‐income countries.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence to support one strategy over another to reduce the prevalence or level of children's environmental tobacco smoke exposure.
There is no clear evidence of difference in levels of success between different settings, including well‐child, ill‐child and community contexts.
There is limited support for the delivery of more intensive counselling interventions to parent(s).
Implications for research.
Given the potential for bias in parental reports of children's environmental tobacco smoke (ETS) exposure, future studies should use biochemical verification of children's exposure to or absorption of ETS.
Studies with larger sample sizes are needed to adequately explore the effects of family and carer interventions in reducing children's exposure to ETS.
Studies should be designed and powered with consideration of the reduction in children's ETS exposure that occurs in comparison groups and in the wider community.
Studies should minimise risk of bias, whilst providing detailed descriptions of methods used during randomisation and allocation concealment.
Researchers should provide detailed descriptions of interventions to aid reproducibility.
More studies are required to assess the impact of identical interventions to ascertain quantitative effect estimates.
Study reports must mention costs.
Further underpowered and/or low‐quality studies are unlikely to enhance understanding in this field.
What's new
| Date | Event | Description |
|---|---|---|
| 1 January 2018 | New search has been performed | Review update: Added 21 new studies, date of last search February 2017 |
| 1 January 2018 | New citation required but conclusions have not changed | Review update with changes to review authors |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 26 March 2014 | Amended | Changed date for 'Assessed as up‐to‐date' |
| 26 March 2014 | Amended | Changed contact to Ruchi Baxi |
| 18 December 2013 | New citation required but conclusions have not changed | Added review authors |
| 18 December 2013 | New search has been performed | Updated review; added 21 studies; date of last search September 2013 |
| 22 June 2011 | Amended | Converted additional table to appendix to correct pdf format |
| 8 August 2008 | New search has been performed | Updated review |
| 3 July 2008 | New citation required but conclusions have not changed | Added review authors |
Acknowledgements
Jamie Hartmann‐Boyce from the Cochrane Tobacco Addiction Group for assessing risk of bias for previously included studies. Study investigators Susan Blake, Sophia Chan, Ayman El‐Mohandes, Michele Kiely, Thurman Allen Merritt, Anne Turner‐Henson, and Jonathan Winickoff for providing information about their studies to the review author team. Behrooz Behbod for writing the review as part of his role as a Specialty Registrar in Public Health Medicine.
We gratefully acknowledge funding support for the original review ‐ Roseby 2002 ‐ provided by the Australian National Health & Medical Research Council (Trainee Research Scholarship (RR)), Murdoch Children's Research Institute, and VicHealth (Public Health Research Fellowship (EW)); and for the first update ‐ Priest 2008 ‐ provided by the Cochrane Public Health Group and the McCaughey Centre.
Thank you to all authors of previous versions of this review. Rona Campbell was involved in the development of the original review and the first update, extracted data from papers for previous versions, and edited both the original review and the first update. Grace Ferguson‐Thorne extracted data and assisted in editing the first review update. Ruchi Baxi and Mohit Sharma co‐ordinated the previous update, extracted data, and cowrote the previous update (Baxi 2014).
Appendices
Appendix 1. MEDLINE (Ovid SP) search strategy
| Searched February 2017 | |
| 1 | exp Smoking/ |
| 2 | Tobacco Smoke Pollution/ |
| 3 | 1 or 2 |
| 4 | Smoking Cessation/ |
| 5 | Environmental Medicine/ |
| 6 | exp Environmental Pollution/ |
| 7 | Public Health/ |
| 8 | Health Education/ |
| 9 | Health Promotion/ |
| 10 | Psychotherapy/ |
| 11 | 4 or 5 or 6 or 7 or 8 or 9 or 10 |
| 12 | exp Family/ |
| 13 | Child Day Care Centers/ or Child Care/ |
| 14 | Schools, Nursery/ |
| 15 | (child* or carer* or caregiver* or parent* or brother* or sister* or sibling* or nanny or nannies).ti,ab. |
| 16 | 12 or 13 or 14 or 15 |
| 17 | 3 and 11 and 16 |
| 18 | limit 17 to ("newborn infant (birth to 1 month)" or "infant (1 to 23 months)" or "preschool child (2 to 5 years)" or "child (6 to 12 years)") |
| 19randomisedd controlled trial.pt. | |
| 20 | controlled clinical trial.pt. |
| 21 | randomized.ab. |
| 22 | placebo.ab. |
| 23 | drug therapy.fs. |
| 24 | randomly.ab. |
| 25 | trial.ab. |
| 26 | groups.ab. |
| 27 | 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 |
| 28 | Research Design/ |
| 29 | Follow‐Up Studies/ |
| 30 | exp evaluation studies/ |
| 31 | Prospective Studies/ |
| 32 | Retrospective Studies/ |
| 33 | Comparative Study/ |
| 34 | Cross‐Sectional Studies/ |
| 35 | 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 |
| 36 | 18 and 35 |
| 37 | limit 36 to yr="2007 ‐Current" |
| 38 | (2011* or 2012*).yr,dp,ed. |
| 39 | 37 and 38 |
Appendix 2. Embase (Ovid SP) search strategy
| Searched February 2017 | |
| 1 | *smoking/ |
| 2 | *smoking cessation/ |
| 3 | *environmental health/ |
| 4 | *pollution/ |
| 5 | *public health/ |
| 6 | *health education/ |
| 7 | *psychotherapy/ |
| 8 | 2 or 3 or 4 or 5 or 6 or 7 |
| 9 | *family/ |
| 10 | *schools/ |
| 11 | *school/ |
| 12 | *nursery/ |
| 13 | *nurseries/ |
| 14 | *day care/ |
| 15 | *child care/ |
| 16 | *house/ |
| 17 | *home/ |
| 18 | (carer* or caregiver* or parent* or famil* or brother* or sister* or sibling* or nanny or nannies).ti,ab. |
| 19 | 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 |
| 20 | child/ |
| 21 | newborn/ |
| 22 | 20 or 21 |
| 23 | 1 and 8 and 19 and 22 |
| 24randomisedd controlled trial/ | |
| 25randomisationn/ | |
| 26 | controlled study/ |
| 27 | evidence based medicine/ |
| 28 | clinical trial/ |
| 29 | (clin* adj5 trial?).ti,ab. |
| 30 | ((singl* or doubl* or trebl* or tripl*) adj5 (blind* or mask*)).ti,ab. |
| 31 | placebos/ |
| 32 | placebo*.ti,ab. |
| 33 | methodology/ |
| 34 | comparative study/ |
| 35 | "evaluation and follow up"/ |
| 36 | prospective study/ |
| 37 | (control* or prospective* or volunteer?).ti,ab. |
| 38 | 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 |
| 39 | 23 and 38 |
| 40 | limit 39 to yr="2007 ‐Current" |
| 41 | (2011* or 2012*).yr,dp,em. |
| 42 | 40 and 41 |
Appendix 3. CINAHL (EbscoHOST) search strategy
| Searched February 2017 | |
| S31 | S15 and S29 Limiters ‐ Published Date from: 20110101‐20121231; Age Groups: Infant, Newborn: birth‐1 month, Infant: 1‐23 months, Child, Preschool: 2‐5 years, Child: 6‐12 years |
| S30 | S15 and S29 Limiters ‐ Published Date from: 20070101‐20111231; Age Groups: Infant, Newborn: birth‐1 month, Infant: 1‐23 months, Child, Preschool: 2‐5 years, Child: 6‐12 years |
| S29 | S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 |
| 28 | TI ( control* pr prospectiv* or volunteer* ) or AB ( control* or prospectiv* or volunteer* ) |
| S27 | (MH "Evaluation Research") |
| S26 | (MH "Comparative Studies") |
| S25 | (MH "Study Design") OR (MH "Cross Sectional Studies") OR (MH "Prospective Studies+") |
| S24 | TI random* or AB random* |
| S23 | TI placebo* or AB placebo* |
| S22 | (MH "Placebos") |
| S21 | TI tripl* n5 blind* or AB tripl* n5 blind* or TI tripl* n5 mask* or AB tripl* n5 mask* or TI trebl* n5 blind* or AB trebl* n5 blind* or TI trebl* n5 mask* or AB trebl* n5 mask* |
| S20 | TI doubl* n5 blind* or AB doubl* n5 blind* or TI doubl* n5 mask* or AB doubl* n5 mask* |
| S19 | TI singl* n5 blind* or AB singl* n5 blind* or TI singl* n5 mask* or AB singl* n5 mask* |
| S18 | TI clin* n5 trial* or AB clin* n5 trial* |
| S17 | (MH "Random Assignment") |
| S16 | (MH "Clinical Trials+") |
| S15 | S1 and S9 and S14 |
| S14 | S10 or S11 or S12 or S13 |
| S13 | TI ( child* or carer* or caregiver* or parent* or famil* or brother* or sister* or sibling* or nanny or nannies ) or AB ( child* or carer* or caregiver* or parent* or famil* or brother* or sister* or sibling* or nanny or nannies ) or MW ( child* or carer* or caregiver* or parent* or famil* or brother* or sister* or sibling* or nanny or nannies ) |
| S12 | (MH "Child Care+") |
| S11 | (MH "Schools, Nursery") |
| S10 | (MH "Family+") |
| S9 | S2 or S3 or S4 or S5 or S6 or S7 or S8 |
| S8 | (MH "Psychotherapy") |
| S7 | (MH "Health Promotion") |
| S6 | (MH "Health Education") |
| S5 | (MH "Public Health") |
| S4 | (MH "Environmental Pollution+") |
| S3 | (MH "Medicine, Environmental") |
| S2 | (MH "Smoking Cessation") |
| S1 | (MH "Smoking+") |
Appendix 4. PsycINFO search strategy
| Searched February 2017 | |
| 1 | exp tobacco smoking/ |
| 2 | Smoking Cessation/ |
| 3 | Environmental Medicine/ |
| 4 | exp pollution/ |
| 5 | Public Health/ |
| 6 | Health Education/ |
| 7 | Health Promotion/ |
| 8 | Psychotherapy/ |
| 9 | 2 or 4 or 5 or 6 or 7 or 8 |
| 10 | exp Family/ |
| 11 | exp health education/ |
| 12 | day care centers/ or child day care/ |
| 13 | Child Care/ |
| 14 | (child* or carer* or caregiver* or parent* or famil* or brother* or sister* or sibling* or nanny or nannies).ti,ab. |
| 15 | 10 or 11 or 12 or 13 or 14 |
| 16 | 1 and 9 and 15 |
| 17 | limit 16 to 100 childhood <birth to age 12 yrs> |
| 18 | limit 17 to yr="2007 ‐Current" |
| 19 | (2011* or 2012*).yr,dp. |
| 20 | 18 and 19 |
Appendix 5. ERIC (ProQuest) search strategy
| Searched February 2017 |
| su(smoking) AND (ab("smoking cessation") OR ti("smoking cessation")) AND (su(Pollution) OR su(Environmental influences) OR su(Public health) OR su(health education) OR su(health promotion) OR su(psychotherapy)) AND ((SU(family sociological unit) OR SU(parents) OR SU(child care) OR SU(Nursery schools)) OR pub(child* OR carer* OR caregiver* OR parent* OR brother OR sister* OR sibling* OR nanny OR nannies OR family*) OR ab(child* OR carer* OR caregiver* OR parent* OR brother OR sister* OR sibling* OR nanny OR nannies OR family*)) |
Appendix 6. Cochrane Library (Wiley) search strategy
| Searched February 2017 |
| #1 MeSH descriptor Smoking explode all trees #2 MeSH descriptor Tobacco Smoke Pollution explode all trees #3 (#1 OR #2) #4 MeSH descriptor Smoking Cessation explode all trees #5 MeSH descriptor Environmental Medicine explode all trees #6 MeSH descriptor Environmental Pollution explode all trees #7 MeSH descriptor Public Health, this term only #8 MeSH descriptor Health Education, this term only #9 MeSH descriptor Health Promotion, this term only #10 MeSH descriptor Psychotherapy, this term only #11 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10) #12 MeSH descriptor Family explode all trees #13 MeSH descriptor Schools, Nursery explode all trees #14 MeSH descriptor Child Care, this term only #15 MeSH descriptor Child Day Care Centers explode all trees #16 (child* or carer* or caregiver* or parent* or famil* or brother* or sister* or sibling* or nanny or nannies):ti,ab,kw #17 (#12 OR #13 OR #14 OR #15 OR #16) #18 (#1 AND #11 AND #17), from 2007 to 2011 #19 (#1 AND #11 AND #17), from 2011 to 2012 |
Data and analyses
Comparison 1. Results.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Main outcomes | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdullah 2005.
| Methods | Country: Hong Kong, China Setting: community (maternal and child health centres) Type: RCT | |
| Participants | 952 parents from a birth cohort who were listed as smokers in the '1997 Birth Cohort Study' of the Department of Community Medicine, University of Hong Kong | |
| Interventions | Intervention: 20 to 30 minutes of telephone counselling with information based on individual needs; no NRT information given unless asked, and even then, information given was kept minimal. Stage‐based printed self‐help materials (based on baseline) provided just once. Control: Recieved stage‐based printed self‐help material only. | |
| Outcomes | At 6 months: • Parental quitting: self‐reported 7‐day prevalence quit rate, self‐reported 24‐hour point prevalence quit rate, self‐reported continuous abstinence rate, biochemically validated (CO or urine cotinine or both) quit rate, reported implementation of total or partial smoking ban at home | |
| Type of intervention | Well‐child (child health check) | |
| Notes | Retention: 837/952 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; method not described |
| Allocation concealment (selection bias) | Low risk | Numbered sealed opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up 11% intervention/4% control. Included as continuing smokers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Independent interviewer...was unaware of subjects' group allocation... All respondents who reported they were not smoking during the preceding 7 days were invited to attend the research centre for biochemical validation." |
Abdullah 2015.
| Methods | Country: Shanghai, China Setting: community (households) Type: RCT |
|
| Participants | 318 households with smoking parents or caregivers who had children aged 5 years or younger at home | |
| Interventions |
Intervention: • Counselling, conceptualised on the basis of the protection motivation theory developed by Rogers 1975 • Smoking hygiene intervention (SHI) with brief advice to quit SHI: • Keeping child away from household members' and other people's smoke • Avoiding smoking in the car or in closed areas near the child • Not taking the child into smoky environments • Enforcing a strict no‐smoking policy at home and in the car Control: • Placebo intervention included counselling on child development issues • No SHI or second‐hand smoke (SHS) exposure reduction or quit smoking counselling provided by the study counsellor. When queries on smoking or SHS were raised by participants, they were given the hotline number of the Shanghai CDC's smoking cessation clinic. |
|
| Outcomes |
Child exposure: Primary outcomes at 6 months: • Participant‐reported improvement in smoking hygiene in the household (smoking restriction by household members at home) • Reduced exposure of child to SHS inside the home measured by mean number of cigarettes per week • Reduction in children's urine cotinine concentrations Secondary outcomes: • Total SHS exposure to child from all smokers inside and outside the home • Household members smoking cigarettes around the child • Smoking behaviour of household members (reducing the mean number of cigarettes smoked daily, making a quit smoking attempt for at least 24 hours, and quitting smoking) Child illness: Respiratory illness incidence among children as reported by key household members Target behaviour change: Secondary outcome at 6 months: • Smoking behaviour of household members (reducing mean number of cigarettes smoked daily, making a quit smoking attempt for at least 24 hours, and quitting smoking). Verified by CO measure |
|
| Type of intervention | Community‐based | |
| Notes | Conflict of interest: none declared Source of funding: Flight Attendant Medical Research Institute (FAMRI), USA, grant 072233_CIA; and American Academy of Pediatrics, Julius B. Richmond Center of Excellence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers were computer‐generated by the project manager (not counsellors) before participant recruitment. |
| Allocation concealment (selection bias) | Low risk | Counsellor opened a serially numbered, opaque, and sealed envelope to reveal the random assignment of each smoker to intervention or control group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large dropout rate; more than 40% of the households in each group were not available. This was the result of many households relocating to a new residential area, farther from the original study area. Analysis does not appear to be intention‐to‐treat. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded but objective measure (cotinine) |
| Other bias | High risk |
In addition to dropout rate: • Small possibility of cross‐contamination between intervention and comparison groups • Dosing (i.e. contact duration and frequency) of the intervention was not equal for the intervention and comparison groups • Social desirability bias due to interview format |
Armstrong 2000.
| Methods | Country: Australia Setting: community (child health nurse home visits) Type: RCT | |
| Participants | 181 women recruited from a postnatal ward who had given birth to a single live infant, identified as 'at risk' (1 or more of identified physical domestic violence, identified childhood abuse by either parent, sole parenthood, or ambivalence to pregnancy; as well as 3 or more of maternal age < 18 years, unstable housing, financial stress, poor maternal education, low family income, social isolation, history of mental health disorder, drug or alcohol abuse, and domestic violence other than physical abuse) | |
| Interventions |
Intervention: • Home‐based intervention focused on establishing trust with families, enhancing parenting self‐esteem and confidence, providing guidance for child development including crying and sleep behaviour, promoting preventive child health care and facilitating access to child health centres • Weekly home nurse visits for first 6 weeks, fortnightly for 3 months, then monthly until 6 months postpartum Control: • Usual care |
|
| Outcomes |
At 4 months: • Health outcomes only reported at 12 months • Maternal self‐report of smoking behaviour and observations by research assistants of smoking behaviour in the home • Child health questionnaire |
|
| Type of intervention | Well‐child (peripartum) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random number table was computer generated." |
| Allocation concealment (selection bias) | Low risk | The random number table was "used by a clerical officer not involved in determining eligibility to determine intervention status". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of retention at 12 months in both arms (76% intervention, 77% control) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Data were collected in the home by a researcher who was naive to the intervention status of the participants and was not involved in providing healthcare to the participants." |
Baheiraei 2011.
| Methods | Country: Iran Setting: recruited from health centres, intervention face‐to‐face/on phone RCT |
|
| Participants | 130 families with healthy infants younger than 12 months | |
| Interventions |
Intervention: • Counselling (motivational interviewing) of mothers and fathers Control: • Usual care (health visits for checking infant's growth and developmental milestones) • Parents given a pamphlet and sticker depicting a smoke‐free home |
|
| Outcomes | Infant urinary cotinine at baseline and at 3 months Change in parental smoking Home and car smoking bans |
|
| Type of intervention | Well‐child (child health check) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/65 lost to follow up in control group and 5/65 in intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The statistical analyst and outcome assessors were blinded to the group assignment, the control group was uninformed of the counselling processes. |
Blaakman 2015.
| Methods | Country: USA Setting: community (home) Type: RCT |
|
| Participants | 165 caregivers and their infants born at ≤ 32 weeks' gestational age, within 6 weeks of discharge from the NICU | |
| Interventions |
Intervention: • Counselling provided by 1 of 2 research nurses trained in motivational interviewing and actively supervised by an expert in the field • Sessions included smoking cessation or relapse prevention counselling for willing caregivers who were current or former smokers, while second‐hand smoke exposure control efforts were explored and reinforced for all • Motivational interviewing technique used: elicit‐provide‐elicit • Trialists also offered information on resources (e.g. smokers quit line, pharmacotherapy) Control: • Brief asthma education at baseline only |
|
| Outcomes |
Child exposure: • Postintervention infant exposure to second‐hand smoke assessed via caregiver‐reported data from survey that occurred closest to completion of the intervention (5‐month survey) • Salivary cotinine samples obtained at study end (8 months after NICU discharge) used as an objective measure of infant SHS exposure Child illness: • Respiratory symptoms assessed by asking caregivers to quantify in the past 2 weeks number of days with wheeze/cough, number of nights awakened because of wheeze/cough, number of days having taken rescue medication, and number of symptom‐free days Child health service utilisation: • Asked caregiver about numbers of visits to primary care provider and emergency department, and hospitalisations for wheezing or breathing problems since the prior survey Target behaviour change: • Smoking ban in home/car, caregiver confidence, and motivation to quit smoking |
|
| Type of intervention | Community‐based | |
| Notes | Conflict of interest: none declared Source of funding: grant from the Halcyon Hill Foundation (Halterman, PI), which had no involvement in submission of this manuscript for publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not specified |
| Allocation concealment (selection bias) | Low risk | Concealed envelope system, stratified by caregiver‐reported routine infant SHS exposure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12.7% of participants dropped out (18.1% in the treatment group vs 7.3% in comparison group) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments were completed by study team members blinded to the infants' randomisation category. Objective measure also used (cotinine) |
Borrelli 2010.
| Methods | Country: USA Setting: recruited from various sites including hospital in‐patient settings and clinics, Latino cultural events. Intervention involved counselling visits and phone calls. Type: RCT |
|
| Participants | Latino caregivers who smoked and had a child with asthma | |
| Interventions |
Group 1: Behavioral action model (BAM). This was modelled on clinical guidelines for smoking cessation. The model focused on increasing the smoker’s self‐efficacy to quit by teaching problem solving and coping skills. Group 2: Precaution adoption model (PAM). This model used feedback on the caregiver’s carbon monoxide level and the child’s second‐hand smoke exposure, using motivational interviewing techniques. Eight weeks of transdermal nicotine patches were available free of charge if participants were ready to quit. |
|
| Outcomes | Passive nicotine monitors at baseline and at 3 months after completion of treatment Level of functional morbidity due to asthma Smoking cessation by caregiver; self‐report and expired air CO concentration (continuous abstinence, 7‐day point prevalence abstinence) |
|
| Type of intervention | Child with health problems (respiratory disorders) | |
| Notes | Attrition 37/133 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition 37/133 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report assessments administered by research assistants blinded to the treatment condition |
| Other bias | High risk | Selection bias. Some participants were enrolled from other studies, so it may be difficult to elicit study‐specific effects. Inconsistencies in presentation of data: BAM group (n = 68) had results for n = 49 at the end of the study, and not all were accounted for. Similarly in the PAM group, n = 65 and completed n = 49 at end of treatment, and not all were accounted for. Outcomes presented for 'acculturation' and 'asthma morbidity', but no details on how these were assessed. |
Borrelli 2016.
| Methods | Country: USA Setting: community (home and telephone) Type: RCT |
|
| Participants | 560 smoking primary caregivers (parents) of both children with asthma and healthy children | |
| Interventions | • Precaution adoption model intervention (PAM; motivational interviewing to deliver feedback on child's second‐hand smoke (SHS) exposure and smokers' carbon monoxide levels and cessation induction strategies). • Home visit (for aim 1/teachable moment): Parents of children with asthma received NIH guideline‐based asthma education, while parents of healthy children received child wellness counselling. All participants received identical smoking cessation counselling via motivational interviewing. Verbal and graphical feedback was provided regarding smoking level, carbon monoxide level, how quitting could reduce disease risk and symptoms, the child's SHS exposure, risk of smoking on the child's SHS exposure, and how risks could be reduced by quitting smoking or reducing SHS exposure. • Telephone counselling (for aim 2/intervention intensity): Both PAM and enhanced PAM received six 15‐ to 20‐minute calls regarding asthma symptoms and management for 4 months after the home visits. Enhanced PAM also received smoking cessation and a second round of SHS exposure feedback. |
|
| Outcomes | Child exposure: 2 passive nicotine monitors (dosimetry) placed for 1 week during each of the 2 measurement periods (baseline and after call 5) ‐ 1 in the room where the child spends the most time and 1 worn by the child. Parent‐reported SHS exposure assessed by structured interview Child illness: asthma morbidity (numbers of asthma‐related hospitalisations, school days missed due to asthma, days with asthma symptoms, and Asthma Functional Morbidity Scale scores) Child health service utilisation: asthma‐related hospitalisations Target behaviour change: proportion of participants who quit; verified by expired air carbon monoxide testing at all follow‐up intervals |
|
| Type of intervention | Child with health problems (respiratory disorders) | |
| Notes | Conflict of interest: none declared Source of funding: NIH grant R01 HL062165‐06 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation (form of adaptive biased‐coin randomisation) |
| Allocation concealment (selection bias) | Low risk | Allocation sequence could not be accessed by staff. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although no significant difference was seen in the counselling call completion rate, this rate was only 55% by 12‐month follow‐up. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective measure used ‐ air nicotine |
| Other bias | High risk | • At baseline, comparison group (healthy children) was significantly different from the 2 intervention groups (PAM and enhanced PAM) with respect to child and parent age, cigarettes smoked per day, years smoked, nicotine dependence, and % household smoking ban. Note that randomisation only occurred for the 2 intervention arms. • Potential detection bias in that the half‐life of carbon monoxide is 4 to 6 hours, and so 7‐ and 30‐day point prevalence abstinence cannot be verified beyond that time frame. |
Butz 2011.
| Methods | Country: USA Settings: hospital and home RCT (3 arms) |
|
| Participants | Inner city families with a child aged 6 to 12 years with asthma, residing with a smoker | |
| Interventions | Health coach/air clear group: two air cleaners and four 30‐ to 45‐minute nurse health coach home visits, and a behavioural intervention to reduce child second‐hand smoke exposure Air cleaner group: two air cleaners and 4 asthma education sessions Control group: asthma education during 4 nurse home visits |
|
| Outcomes |
Six‐month follow‐up from baseline: • Child urinary cotinine at baseline and at 6‐month follow‐up • Asthma symptom‐free days • Acute asthma healthcare events • Change in air quality • Caregiver smoking frequency and location |
|
| Type of intervention | Child with health problems (respiratory disorders) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in 1:1:1 ratio with random block sizes; randomisation performed by study co‐ordinator using the function in the database |
| Allocation concealment (selection bias) | Low risk | All study staff, including all investigators, were blinded to subsequent group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 91.3% followed up |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study staff, including all investigators, were blinded to subsequent group assignment. |
| Other bias | High risk | Children randomised to the control group had caregivers who smoked significantly more at baseline and follow‐up than those in either intervention group. |
Chan 2005.
| Methods | Country: Hong Kong, China Setting: hospital (paediatric wards/outpatient settings) Type: RCT | |
| Participants | 80 parents of sick children presenting to a clinic or admitted to a children’s ward of a major Hong Kong hospital | |
| Interventions | Intervention: individualised motivational intervention for 30 minutes with nurse counsellor; appropriate stage‐matched intervention used to "increase motivation and lower resistance to quit"; telephone reminder 1 week after the intervention Control: healthy diet counselling for their sick children as a placebo intervention | |
| Outcomes | One‐month follow‐up: • Parent report of daily cigarette consumption in past 30 days | |
| Type of intervention | Child with health problems (ill‐child health care) | |
| Notes | Retention: 77/80 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized controlled trial"; no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Randomised after completion of questionnaire; no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low loss to follow‐up: 77 (of 80) participants followed‐up successfully |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "At 1 month, trained interviewers who were blinded to the group assignment delivered telephone follow‐up calls to both groups to evaluate the primary and secondary outcomes using a standardized questionnaire." Self‐reported outcome only; bias possible |
Chan 2006a.
| Methods | Country: Hong Kong, China Setting: hospital (paediatric wards and outpatient departments) RCT | |
| Participants | 1483 mothers of sick children admitted to the ward or attending the outpatient department from all participating trial centres, November 1997 to September 1998 | |
| Interventions | Intervention: Mothers received information from nurses including standardised health advice, booklet about preventing child exposure to passive smoking, booklet to give to fathers on quitting smoking, a no smoking sign to place in the home to remind the father not to smoke, and a telephone reminder 1 week later. Control: normal care by nurses | |
| Outcomes | 3‐, 6‐, and 12‐month follow‐up: • Mother self‐reports actions taken to reduce child passive smoke exposure. | |
| Type of intervention | Child with health problems (ill‐child health care) | |
| Notes | Retention: 1273/1483 (86%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random numbers were generated by the investigator using the computer and assigned to intervention (even) and control (odd) groups." |
| Allocation concealment (selection bias) | Low risk | "Nurses then randomized the subjects into the intervention or control group by opening a sealed envelope with serial numbers." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low loss to follow‐up, ITT analysis used, similar percentage lost in both groups: 86% intervention and 85% control retention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report only; differential misreport possible, but no difference found between groups, so unlikely |
| Other bias | High risk | Contamination of the control group possible: open ward setting "...the mothers in the control group could have by chance read the health education booklet from the mothers in the intervention group... furthermore, the nurses' health education could be easily overheard." |
Chellini 2013.
| Methods | Country: Italy Setting: well‐child, in the community RCT | |
| Participants | 218 women 30 to 49 years of age with children | |
| Interventions | Brief counselling and 3 gifts. Both groups received self‐help booklet. | |
| Outcomes | Reported smoking restrictions in home and car Change in smoking status reported |
|
| Type of intervention | Well‐child (child health check) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 of 218 lost to follow‐up and ITT analysis performed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed for observer; objective measure not used |
Chen 2016.
| Methods | Country: Taiwan Setting: community (schools) Type: RCT |
|
| Participants | 75 parent and child dyads in 6 elementary schools (grades 3 to 6); school was the unit of assignment | |
| Interventions |
Intervention: Parent‐child dyads received an interactive programme comprising 3 weekly group sessions and 1 individual telephone counselling session 4 weeks after group sessions. Control: Written materials related to tobacco information were received by mail 4 times during the same time period instead of the intervention sessions. |
|
| Outcomes | Child exposure: urine cotinine as well as parent and child reports of children's exposure to parental smoking Target knowledge change: Aims of intervention were to instil knowledge regarding the mechanism of the harmful effect of ETS, to correct people's perceptions of the smoking patterns that lead to ETS exposure at home, to introduce strategies for reducing ETS, and to assist parent‐child dyads in formulating strategies for maintaining a smoke‐free home. |
|
| Type of intervention | Community‐based | |
| Notes | Conflict of interest: none declared Source of funding: National Science Council of Taiwan (NSC97‐2314‐B‐038‐043‐MY3) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21% dropout rate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Single‐blind; objective measure (cotinine) |
| Other bias | High risk | Selection bias: differences in participation rates between intervention and control groups. Non‐simultaneous collection of self‐reported data and urine cotinine levels during post‐test 2 may have caused inconsistency in the data. |
Chilmonczyk 1992.
| Methods | Country: USA Setting: well baby check RCT | |
| Participants | 103 mothers smoking ≥ 10 cigarettes/d with infants presenting to a well baby check | |
| Interventions | Urine was collected from all infants and analysed for cotinine. Intervention: A report of the infant's urinary cotinine level along with a personalised letter to the mother to be signed was returned to the child's doctor. The letter outlined ways to reduce child ETS exposure (identify location of smoking, wash hands after smoking, ensure day care home is smoke‐free, ask friends to avoid smoking in the presence of the infant when visiting) but did not discuss cessation. The physician called the mother by telephone to further explain the results. Control: usual care | |
| Outcomes | At 2 months, all participants were contacted to obtain a second urine sample from the infant for analysis. | |
| Type of intervention | Well‐child (child health check) | |
| Notes | Retention: 56/103 (54%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned by computer on an individual basis to intervention or control groups" |
| Allocation concealment (selection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High loss to follow up ‐ 43% control and 48% intervention; "however, it is unlikely that exclusion bias would mask a true impact of the intervention. Characteristics of those who complied were similar to those of the noncompliers... even with the reduced participation... the data were adequate to indicate that the response to the intervention was poor" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes biochemically verified |
Collins 2015.
| Methods | Country: USA Setting: community (home and telephone) Type: RCT |
|
| Participants | 300 underserved smoking mothers of tobacco‐exposed infants and preschoolers | |
| Interventions |
Intervention: Behavioural counselling included 2 in‐home and 7 telephone sessions within 16 weeks. Home sessions aimed to offer skills training and modelled support for tobacco smoke exposure reduction efforts. Mothers also received 4 sections of written self‐help materials mailed at 2‐week intervals to supplement counselling content. Control: Participants mailed a single binder of written materials within a week of enrolment. Content was identical to the intervention group's 4 separate mailings. During telephone confirmation of receipt, staff provided a 5‐ to 10‐minute programme overview of the binder with brief advice and encouraged mothers to share materials with the family. |
|
| Outcomes | Child exposure: maternal report and child urine cotinine Target behavioural change: biological (maternal saliva cotinine) to verify self‐reported smoking status, reported cigarettes smoked per day, reported tobacco smoke exposure, reported presence of other smokers in home, and total smoking ban in home |
|
| Type of intervention | Community‐based | |
| Notes | Conflict of interest: none declared Source of funding: National Cancer Institute at the NIH (CA105183 and CA93756) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation via small blocks of random length, stratified by child race, gender, and recruitment site. Method not specified |
| Allocation concealment (selection bias) | Unclear risk | After baseline completion, the intervention manager obtained group assignment via a secured Internet interface. Unclear whether this was concealed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Control group: 3% of allocated did not initiate control intervention, and a further 17% were lost to follow‐up Intervention group: 11% of allocated did not initiate treatment, and a further 19% were lost to follow‐up |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Single‐blind and objective measure (cotinine) |
Conway 2004.
| Methods | Country: USA Setting: community RCT | |
| Participants | 143 Latino parents of children aged 1 to 9 who reported smoking at least 6 cigarettes a week | |
| Interventions | Intervention: 6 home and telephone sessions over a 4‐month period delivered by lay trained bicultural and bilingual Latina community health workers. Focused on problem solving aimed at lowering target child's exposure to ETS in the household. Intervention methods included contracting, shaping, positive reinforcement, problem solving, and social support to assist families in achieving their ETS goals. Control: survey completion only | |
| Outcomes | 3‐Month and 12‐month follow‐up: • Child hair nicotine and cotinine • Parent report of child's past month exposure from all sources in the household over previous 30 days as measured by numbers of cigarettes • Confirmed reduction based on both parents' reports and children's hair biomarkers | |
| Type of intervention | Community‐based | |
| Notes | Retention: 127/143 (89%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized"; no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 81% provided data at all assessments, "and analyses showed attrition introduced no significant biases". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
Cooper 2014.
| Methods | Country: UK Setting: hospital (antenatal clinic) Type: RCT |
|
| Participants | 1051 smoking 12‐ to 24‐week pregnant women who currently smoke 5 or more cigarettes per day and who smoked at least 10 cigarettes per day before pregnancy | |
| Interventions |
Intervention: biochemically validated smoking cessation with transdermal nicotine patches (15 mg per 16 hours) for 4 weeks, followed by another 4 weeks if abstinent Control: visually identical placebo |
|
| Outcomes | Child exposure: maternal self‐reported prolonged and total abstinence from smoking validated by exhaled CO and/or salivary cotinine Child illness: birth outcomes, infant impairment, infant respiratory symptoms up to age 2 Target behavioural change: smoking cessation |
|
| Type of intervention | Well‐child (antenatal health check) | |
| Notes | Conflict of interest: NM reports personal fees from Novartis and personal fees from Elsevier, outside of the submitted work; TC reports personal fees from Pierre Fabre Laboratories, France, outside the submitted work. Source of funding: HTA programme project number 06/07/016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Internet‐based randomisation that was stratified by recruiting site |
| Allocation concealment (selection bias) | Unclear risk | All pharmacists, research staff, and trial participants blinded to treatment allocations, but unclear about allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | By 2‐year follow‐up, 14% in NRT group and 15% in control group dropped out. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind and objective measure |
| Other bias | High risk | • Smoking data were not sought from all participants at predetermined time points, but were obtained opportunistically at multiple, different times between 8 and 54 months after childbirth, rendering smoking behaviour data difficult to interpret. • Smoking outcomes at 2 years were self‐reported, which may lead to bias; furthermore, these outcomes were not assessed in about 40% of participants. |
Culp 2007.
| Methods | Country: USA Setting: home Quasi‐experimental controlled study |
|
| Participants | Pregnant women in rural counties (first‐time mothers) with follow‐up until the child was 12 months old | |
| Interventions |
Intervention: home visits with the goal of promoting the health and development of first‐time mothers and infants (The Community‐Based Family Resource and Support (CBFRS) Program). The programme had 3 main foci: maternal health, child health and safety, and family functioning and parenting. Child's exposure to ETS was 1 part of this intervention. Control: received standard health department services that did not include home visits |
|
| Outcomes | Mother's reported number of cigarettes smoked per day at baseline, and when infant was aged 6 and 12 months Numbers of hospital admissions and emergency room visits, and visiting health department clinics for well‐child care Knowledge: Mother asked 6 questions (a set) about the effect of smoking on her child's growth and development |
|
| Type of intervention | Well‐child (peripartum) | |
| Notes | Part of a wider intervention federally funded programme, which also included several interventions unrelated to ETS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not applicable |
| Allocation concealment (selection bias) | Unclear risk | Not applicable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout from analysis rate was fairly low (26%), but dropout rate was higher in the control group (dropout 49/205 intervention group, 43/150 in control group). Characteristics of dropouts as a whole are described. No intention‐to‐treat analysis was carried out. Under these circumstances, attrition bias is certainly possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were assessed at interview by research staff, who were independent of the intervention staff. However, outcome assessors could very likely have been aware of which groups participants were in, as this was decided geographically, and blinding is not mentioned. The paper found a positive intervention effect. |
| Other bias | High risk | Not an RCT, so very open to selection bias ‐ significant difference in number of years of education between groups. Not much baseline questionnaire info provided, so unclear whether e.g. knowledge re smoking differed from the start between the 2 groups |
Curry 2003.
| Methods | Country: USA Setting: paediatric clinics serving ethnically diverse population of low‐income families RCT | |
| Participants | 303 self‐identified women smokers whose children received care at participating clinics | |
| Interventions | Intervention: During clinic visit, women received brief motivational message from the child’s clinician, a guide to quitting smoking, and a 10‐minute interview with a nurse or study interventionist. Women also received as many as 3 outreach telephone counselling calls from the clinic nurse or interventionist in the 3 months following the visit. Control: usual care | |
| Outcomes | 3‐Month and 12‐month follow‐up: • Maternal self‐reported 7‐day abstinence • Maternal CO testing | |
| Type of intervention | Mixed/not stated | |
| Notes | Retention: 81% at 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants "determined their randomization group by choosing a Ping‐Pong ball out of a brown paper bag. The bag contained several Ping‐Pong balls that were either white or yellow, and the color of the selected ball indicated their study group". |
| Allocation concealment (selection bias) | High risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19% lost at final follow‐up; counted as smokers. Similar numbers lost to follow‐up in both groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used in subset: "We determined the comparability of compliance with testing between the intervention and control groups and then examined the effect on self‐reported rates of abstinence of adjusting outcomes by the percentage of abstainers who tested above the cut‐off point." |
Daly 2016.
| Methods | Country: Australia Setting: community well‐child health clinic Type: RCT |
|
| Participants | 1424 parents of children aged 0 to 4 years attending well‐child health checks | |
| Interventions |
Interventions: Arm 1: • Computer‐delivered care ‐ tailored on‐screen information and a printed self‐help report regarding the risks of infant SHS exposure, how to reduce exposure risk, advice on quitting smoking, and contact details of the free quit line • Child health nurse‐delivered care ‐ During the subsequent clinic consultation, nurses provided a brief intervention focussing on risk reduction for the infant and offering NRT to parents/carers who were smokers. Contact details of the quit line were again provided, and nurses discussed the importance of complete home smoking bans, providing advice to address any barriers to their implementation. Arm 2: • Same as above, plus infant urine cotinine measured and results shared with parent, child health nurse, and their GP. A guide to preventing infant SHS exposure and strategies for quitting smoking were also included. Control: • Usual care from child health nurses |
|
| Outcomes |
Child exposure: Primary outcome: Parent/carer reported infant exposure to SHS, defined as a person smoking in the infant's presence in the past 3 days. At 12‐month follow‐up, if parent/caregiver reported the infant as NOT exposed, this was validated with urine cotinine test. Secondary outcomes: parent/caregiver smoking status and household smoking ban status of the home Target behavioural change: proportion who quit and proportion with complete household smoking ban |
|
| Type of intervention | Well‐child (child health check) | |
| Notes | Conflict of interest: unclear Source of funding: Financial Markets Foundation for Children, Community Health and Anti Tuberculosis Association, Centre for Health Research & Psycho‐oncology (CHeRP and infrastructure support from the Hunter Medical Research Institute) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clinics were randomly assigned to 1 of 2 treatment arms or to a control arm via random number function in SAS statistical software. |
| Allocation concealment (selection bias) | Unclear risk | Services not blind to study allocation but unclear about allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between 11% and 15% lost to follow‐up or declined to participate at 12‐month follow‐up |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective measure (cotinine) |
| Other bias | High risk | • Variability in quality and consistency of advice given to parents/caregivers to access NRT may bias the effect estimate towards the null. • Exposure misclassification; non‐smoking parents/caregivers had partners who smoked and this was not measured. Furthermore, self‐reported SHS exposure was not validated at baseline assessment. • Not blinded, meaning prone to detection and performance bias |
Davis 1992.
| Methods | Country: USA Setting: telephone smoking cessation helpline RCT. Randomised by day of the week, but counsellors blinded to the guide being used | |
| Participants | 630 smoking mothers with children younger than 6 years of age calling the helpline | |
| Interventions | Callers to a telephone smoking cessation assistance service were randomised to receive 1 of 3 self‐help guides. One was specifically written for the target audience, another was received from the American Lung Association, and 1 was developed by the National Cancer Institute. Callers to the line received individual stage‐based counselling and were sent the guide by mail. | |
| Outcomes | Six months later, the participant was called and was interviewed for 10 minutes about the use of the guide, opinion of the guide, quit attempts and strategies to quit, and current smoking. | |
| Type of intervention | Community‐based | |
| Notes | Retention: 630/873 (72%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised: "Guides were assigned randomly to those in the target audience based on a preassigned list randomized by the day of the week." |
| Allocation concealment (selection bias) | Low risk | "CIS counsellors were blinded regarding which self‐help guides subjects would receive." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 28% lost to follow‐up; "completion rates were similar for subjects in the three guide groups" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Follow‐up interviews were conducted by trained interviewers who were blinded regarding subject assignment.... Surrogate interviews were conducted to verify the smoking status of those who reported that they had quit smoking..." |
Eakin 2014.
| Methods | Country: USA Setting: community (Head Start programme) Type: RCT |
|
| Participants | 330 caregivers (parent or legal guardian) of children aged 6 months to 6 years who reported 1 or more smokers living in the home and who spoke English | |
| Interventions |
Intervention: motivational interviewing (MI) and education MI: over 3 months, offered caregivers 4 telephone counselling sessions (15 to 30 minutes in length each) plus 1 booster 15‐minute session after 3‐month assessment, for a total of 5 sessions Education: included EPA Smoke Free Home educational activities and materials as part of the Head Start programme, including staff training workshops about risks of and strategies for reducing SHS exposure, and expert facilitation of Head Start educational activities Control: education alone |
|
| Outcomes | Child exposure: air nicotine, salivary cotinine, caregiver‐reported home smoking ban, and smoking cessation Target behavioural change: smoking cessation and home smoking ban |
|
| Type of intervention | Community‐based | |
| Notes | Conflict of interest: unclear Source of funding: National Heart Lung Blood Institute grant HL092901 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation scheme of groups of 10 to ensure equal group sizes. Use of random number generator |
| Allocation concealment (selection bias) | Low risk | Randomisation assignments were placed into sealed envelopes, which were opened after families completed baseline surveys. Research assistants who completed assessments were not masked to the intervention condition. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 73% and 66% of the intervention group completed 6‐ and 12‐month assessments, compared with 85% of the education‐alone group completing both assessments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded but objective measure (cotinine) |
| Other bias | High risk | Misclassification bias; caregiver smoking status was not verified biochemically |
Ekerbicer 2007.
| Methods | Country: Turkey Setting: school with intervention including telephone calls RCT |
|
| Participants | Parents of school children exposed to ETS aged 9 to 11 years attending a private primary school | |
| Interventions |
Group 1: • Parents interviewed by a psychologist trained in smoking addiction Group 2: • Parents informed of child's urinary cotinine result through a letter |
|
| Outcomes | Child urinary cotinine concentrations at 9 months from baseline | |
| Type of intervention | Community‐based | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly assigned", but method was not described. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full follow‐up |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biological measure used |
Elder 1996.
| Methods | Country: USA Setting: schools RCT. Cluster randomisation by school | |
| Participants | 96 elementary schools in 4 states | |
| Interventions | Trial of school‐based cardiovascular health promotion, including an intervention designed to limit child ETS exposure Intervention: consisted of promoting adoption of a formal tobacco‐free policy for the school and providing classroom‐ and home‐based programmes for students Control: Schools participated in the evaluation but received no recommendations for policy or for classroom‐ or home‐based interventions. Control schools were not restricted from taking up tobacco‐free policies. | |
| Outcomes | At 2 years: • School principals (or delegates) were surveyed with respect to their school's policy on tobacco and the degree to which the policy was observed. |
|
| Type of intervention | Community‐based | |
| Notes | Retention: 96/96; this is the CATCH study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Ten schools at each site were randomly assigned to the control condition and 7 schools each to a school‐based intervention (food service, physical education, classroom curricula) or the school‐based plus family intervention program"; no further information given |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% of third grade teachers and 67% of students attended Family Fun Nights; 100% of schools remained in the dietary assessment process. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
Emmons 2001.
| Methods | Country: USA Setting: family home Type: RCT | |
| Participants | 291 smoking parents (or grandparents) living with a child younger than 3 years old, recruited from hospital labour and delivery logs; community health centres and healthcare providers; self‐referral | |
| Interventions | Intervention: received a 30‐ to 45‐minute motivational interview at the parent's home with a trained health educator and 4 follow‐up telephone counselling calls (approximately 10 minutes each), aiming to reduce household ETS exposure and to increase the smoker's level of readiness for change. Feedback was provided on baseline household air nicotine, parent's CO level, and smoking‐related respiratory symptoms. Self‐help materials targeting ETS reduction and smoking cessation strategies were also provided. Control: self‐help materials only; cessation manual; ETS reduction tip sheet; resource guide | |
| Outcomes | ETS exposure measured by air monitors at baseline and at 6 months Quitting and CPD by parent | |
| Type of intervention | Well‐child (peripartum) | |
| Notes | Retention: 247/291 (85%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated randomization table was used." |
| Allocation concealment (selection bias) | Low risk | "Randomization information was kept from study staff until the baseline assessment was completed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used; similar rates of follow‐up in both groups: 123/141 control, 124/150 intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ETS exposure was measured by air monitors; results did not rely on self‐report. |
Eriksen 1996.
| Methods | Country: Norway Setting: health centres RCT | |
| Participants | 443 families with 1 or more smoking parent presenting with a child to a well baby check at 6 weeks or 2 or 4 years | |
| Interventions | Intervention: 5‐minute counselling from health visitor on harmful effects of parent smoking on children and how to prevent them (stop smoking indoors/in living rooms or quit completely). Three brochures distributed (harm of passive smoking, measures to prevent passive smoking, self‐help cessation manual) along with a list of smoking cessation courses Control: given no information unless participants asked for it, until after the period of study. Physicians were asked to withhold their usual advice. Self‐completed questionnaires were administered at the visit and 1 month later. | |
| Outcomes | Parent behaviour by self‐report at baseline and at 1 month | |
| Type of intervention | Well‐child (child health check) | |
| Notes | Retention 363/443 (82%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated"; method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis; exact numbers not provided: "The withdrawal was small and probably not intervention related because the proportion of drop‐outs was about the same in both groups" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report only, no validation used; however no evidence of effect, so differential misreport judged to be unlikely |
| Other bias | Unclear risk | "A "contamination" of information may have taken place from the intervention group to the control group because parents from the two groups may have talked together during the study period." |
Fossum 2004.
| Methods | Country: Sweden Setting: community, child health centres CT | |
| Participants | 41 mothers of newborn infants attending participating child health centres | |
| Interventions | Intervention: 'smoke‐free children' counselling provided by nurses Control: usual care | |
| Outcomes | 3 months: • Self‐reported smoking habits (number of cigarettes smoked) • Maternal cotinine levels | |
| Type of intervention | Well‐child (child health check) | |
| Notes | Retention: 100% for self‐report measures. Cotinine follow‐up measures: 85% intervention, 57% control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomisation used |
| Allocation concealment (selection bias) | High risk | No randomisation used, and further control centres recruited due to low participant recruitment at original control centres |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 100% retention for self‐report, but more participants refused to provide cotinine samples in control (57% provided cotinine) than intervention (85% provided sample) groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
French 2007.
| Methods | Country: USA Setting: recruited from the hospital postpartum unit. Intervention involved home visits and telephone calls by nurses. CT: intervention and control groups enrolled over different time periods |
|
| Participants | Postpartum women who had quit smoking during their pregnancy | |
| Interventions |
Intervention: motivational interviewing, one 15‐minute home visit and 2 subsequent phone calls for less than 15 minutes each Control: usual care, which involved a home visit by a nurse with no smoking intervention |
|
| Outcomes | Final data collection 6 months from baseline Maternal self‐reported smoking status and salivary cotinine level |
|
| Type of intervention | Well‐child (peripartum) | |
| Notes | 71/219 attrition at 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not applicable |
| Allocation concealment (selection bias) | Unclear risk | Women in intervention and control groups had separate consents. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Control group: 80% and 65% were available for data collection at 3 and 6 months, respectively Intervention group: 87% and 69% provided information at 3 and 6 months, respectively |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
| Other bias | High risk | Groups differed in marital status, depression scores, and previous quit attempts. Separate consent forms were used for women in control and intervention groups. |
Greenberg 1994.
| Methods | Country: USA Setting: recruited at maternity hospitals; intervention in family home RCT | |
| Participants | 933 mothers (141 who smoked) of newborn babies | |
| Interventions | Factorial design, 'full' vs 'reduced' data collection. Full group visited at home when infants approximately 3 weeks old and had 2‐weekly telephone questionnaire. Intervention: A study nurse visited homes 4 times for 45 minutes delivering a programme aimed at developing a mother's skills at maintaining a smoke‐free environment for her child: information re child ETS exposure, sources of ETS, and required the mother's participation. Written resources were left with the mother. Follow‐up visits were made 1, 3, and 5 months later. Control: The only contact was made for data collection. | |
| Outcomes | 'Full' subgroup was surveyed and urine was collected at baseline. Data were collected again in homes when infants were 7 and 12 months old. Data on lower respiratory symptoms were collected by telephone survey every 2 weeks, in full subgroup. | |
| Type of intervention | Well‐child (peripartum) | |
| Notes | Full data for 583/933 (62%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated list of random numbers" |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by "a member of the administrative staff who was not involved with the conduct of the study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of follow‐up in both groups (67% intervention, 75% control) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
Groner 2000.
| Methods | Country: USA Setting: hospital RCT | |
| Participants | 479 smoking mothers accompanying a child younger than 12 years to a hospital | |
| Interventions | Two intervention groups ('Child's Health Group' (CHG); 'Mother's Health Group' (MHG)) and a control group Intervention: received a brief (10‐ to 15‐minute) counselling session given by a trained nurse while waiting to see a doctor. Participants in the CHG were informed of the hazards of ETS for their child, but not for themselves; participants in the MHG were informed of the effects of smoking on their own health, but not on their child's health. They were given standard self‐help manuals and materials specific to their group allocation. Notably, even mothers in the CHG were not encouraged to change their smoking location. They received reminder postcards at 2 weeks and at 4 months post intervention encouraging them to quit. Control: received usual care with no additional advice about smoking | |
| Outcomes | Maternal smoking status; stage of change; CPD; smoking location; knowledge of ETS effects at 6 months Assessment by telephone at 1 and 6 months post intervention, blinded assessor, or mailed questionnaire | |
| Type of intervention | Child with health problems (ill‐child health care) | |
| Notes | Retention: 232/479 (48%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High loss to follow‐up (52% lost at 6 months), but "there were no significant differences between subjects who completed the 2 follow‐ups and other subjects in terms of... group assignment or any other baseline variable. Subjects lost to follow‐up were considered continuing smokers, using the “intent to treat” model of analysis" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report only, but no evidence of effect shown, so differential misreport judged to be unlikely |
Hafkamp‐de 2014.
| Methods | Country: The Netherlands Setting: community well‐child centres Type: RCT |
|
| Participants | 7775 parents of children aged 1 to 4 years | |
| Interventions |
Intervention: If the child had recent asthma‐like symptoms, well‐child professionals provided an information leaflet and advised the parent to see a GP if not on treatment. If the child had already been treated by a GP or a paediatrician, well‐child professionals could refer them to an asthma nurse if symptom‐free; they were advised to see their GP if they experienced symptoms. If exposed to ETS, health risks of ETS exposure were discussed as well as whether parents could be motivated and prepared to stop exposing their child (house rules), and parents were given an info leaflet about preventing child ETS exposure. Control: routine practice, addressing the presence of general health symptoms and ETS exposure (at least at age 18 months). However, no specific or systematic assessments of asthma‐like symptoms and ETS exposure were performed. |
|
| Outcomes | Child exposure: parent‐reported ETS exposure at home Child illness: parent‐reported physician‐diagnosed asthma (ever), current wheezing frequency and quality of life; also measured airway inflammation (exhaled NO, FeNO) and airway resistance (Rint) |
|
| Type of intervention | Well‐child (child health check) | |
| Notes | Conflict of interest: none declared Source of funding: Netherlands Organization for Health Research and Development (ZonMw: project no. 22000128). LD received funding by means of a European Respiratory Society/Marie Curie Joint Research Fellowship (MC 1226–2009) under grant agreement RESPIRE, PCOFUND‐GA‐2008‐229571. VWJ received additional grants from the Netherlands Organization for Health Research and Development (ZonMw ‐ VIDI). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 16 well‐child centres ranked on the basis of the socioeconomic status of their neighbourhood. Then centres in each subsequent couple were randomly assigned to intervention (n = 8) or control (n = 8) groups. Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Response rate at first (71%), second (76%), third (72%), fourth (73%), and sixth (68%) years of life |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Parents not aware of research condition. However, unclear whether researchers measuring outcomes were aware of treatment group |
| Other bias | High risk | • Owing to possible contamination of intervention and control groups if families moved to other neighbourhoods and visited other well‐child centres, analyses were compared as both intention‐to‐treat and per‐protocol. • Sensitivity analyses were performed with and without multiple imputation to handle missingness. • Variation was evident in the way the intervention was delivered, with well‐child professionals tending not to repeat interventions that had been delivered at previous visits. • Information bias and misclassification were due to parental reports. |
Halterman 2011.
| Methods | Country: USA Setting: school, with intervention at home RCT |
|
| Participants | Children aged 3 to 10 years with diagnosed asthma attending preschool or elementary school in the Rochester City School District and their families | |
| Interventions |
Intervention: motivational interviewing to counsel the primary caregiver about reducing smoke in the home and to provide brief smoking cessation counselling with the primary caregiver (if a smoker). Counselling of an additional household smoker who spends the most time with the child. Booster telephone calls at 1 and 3 months after counselling. Children received observed inhaler administered by a school nurse. Control: Participants were advised to contact their child's paediatrician regarding persistent asthma symptoms. |
|
| Outcomes |
Seven‐ to nine‐month follow‐up from baseline: • Child salivary cotinine • Asthma symptoms in peak winter season, November to February • Asthma symptom‐free days per 2 weeks • Asthma symptom‐free nights per 2 weeks • Days with activity limitation per 2 weeks • Days with rescue medication use per 2 weeks • Days absent due to asthma per 2 weeks • Acute office and emergency department visits, and hospitalisations, for an acute exacerbation of asthma |
|
| Type of intervention | Child with health problems (respiratory disorders) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used blocked randomisation, 1:1 ratio, with scheme created by the biostatistics centre, stratified by smoking exposure at home |
| Allocation concealment (selection bias) | Unclear risk | Method of randomisation mentioned, but not clear whether allocation was adequately concealed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 withdrawals from each arm (N = 140 for intervention and N = 145 for control) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Interviewers blinded but children's parents not blinded |
Hannover 2009.
| Methods | Country: Germany Setting: recruited from maternity wards, with intervention at home RCT |
|
| Participants | Mothers of neonates who smoked during pregnancy or quit shortly before pregnancy | |
| Interventions |
Intervention: Counselling session based on motivational interviewing and relapse prevention and 2 telephone booster sessions 4 and 12 weeks after counselling Both groups received information brochures for themselves and their partners. |
|
| Outcomes |
Twenty‐four‐month follow‐up from baseline: • Proportion of mothers who quit • Proportion of mothers who did not restart smoking |
|
| Type of intervention | Well‐child (peripartum) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocated women to intervention or control, alternating the order on screening forms |
| Allocation concealment (selection bias) | High risk | Whether allocation sequences would begin with treatment or control condition was decided ad hoc. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High number revoked participation after randomisation, and 25% were not followed up at 24 months. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The nature of our intervention made blinding impossible." But later says follow‐up assessment interviews were conducted by trained interviewers, who did not screen or counsel the women and were blind to the women's group membership. |
| Other bias | High risk | No ITT analysis |
Harutyunyan 2013.
| Methods | Country: Armenia Setting: community Type: RCT |
|
| Participants | 250 households with non‐smoking mothers and at least 1 child 2 to 6 years of age living with at least 1 daily smoker | |
| Interventions |
Intervention: in‐person counselling session with the non‐smoking mother and at least 1 daily smoker in each household, with distribution of a tailored educational brochure and demonstration of measurement of indoor PM2.5 (at second baseline visit); also included 2 follow‐up counselling telephone calls 1 and 2 months after the initial session. Intervention based on the motivational interviewing technique Control: brief educational leaflet on the hazards of SHS only |
|
| Outcomes | Child exposure: children's hair nicotine and self‐report (questionnaire) Target behaviour change: smoking restriction Target knowledge change: health risks of ETS exposure |
|
| Type of intervention | Community‐based | |
| Notes | Conflict of interest: none declared Source of funding: FAMRI (Flight Attendant Medical Research Institute) Center of Excellence in Translational Research at Johns Hopkins University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 250 recruited households were assigned random numbers from 1 to 250; households with odd numbers were included in the intervention group, and those with even numbers were included in the control group. |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 92% follow‐up, but only 56% provided hair samples for nicotine measurement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Single‐blind; participants were unaware of their assignment status, the study hypothesis, and details of intervention and control group procedures |
Herbert 2011.
| Methods | Country: Canada Setting: recruited from 5 public health nursing offices, 8 day care centres and kindergartens on Prince Edward Island. Intervention in the community RCT |
|
| Participants | Parents with children younger than 5 years of age exposed to ETS | |
| Interventions | Group sessions held once a week for 3 consecutive weeks, followed by weekly telephone calls for 3 additional weeks Both groups received a brochure on ETS. |
|
| Outcomes |
Six‐month follow‐up from baseline: • Parent report on the average number of cigarettes smoked in the home daily • Implementation of a total ban on smoking in the household |
|
| Type of intervention | Community‐based | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence with block sizes of 4 or 6 |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque, sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9/30 non‐attenders for intervention; ITT analysis done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Phone interviews conducted and participants asked how they found the programme, so interviewer could not be blind |
Hovell 2000.
| Methods | Country: USA Setting: individual counselling in person and by phone RCT | |
| Participants | 108 mothers smoking at least 2 CPD with child/ren < 4 years, using a supplemental nutrition programme | |
| Interventions | Intervention: Mothers given 7 individualised counselling sessions (3 in person, 4 by phone) designed to reduce child exposure to ETS. Mothers recorded their smoking and child's exposure and were given "no smoking" signs and stickers; at subsequent sessions, new objectives were set and positive feedback was given to mothers, when appropriate. Total duration: 3 months Control: usual care nutritional and brief advice about smoking and child ETS exposure | |
| Outcomes | Child urine cotinine, reported exposure, parental smoking Mothers were surveyed at 3, 6, and 12 months; urine was collected at baseline and at 6 and 12 months. | |
| Type of intervention | Child with health problems (ill‐child health care) | |
| Notes | Retention: 96/108 (89%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random numbers were used to stratify assignments by three ethnic groups." |
| Allocation concealment (selection bias) | Unclear risk | "After the baseline measures, assistants opened an envelope to reveal assignments." No further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analyses; more losses to follow‐up in intervention than control groups (42/53 intervention provided 12‐month urine sample, 52/55 control provided sample) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used "Measurement assistants were blind to group assignment. Control families were unaware of counselling procedures, and investigators were blind to results until all data were collected." |
Hovell 2002.
| Methods | Country: USA Setting: community Type: RCT | |
| Participants | 204 families with an asthmatic child from 3 to 17 years of age whose natural parent(s) were Latino or Hispanic, who lived with at least 1 smoker, and who reported exposure to at least 6 cigarettes the previous week | |
| Interventions | Intervention: asthma management education session delivered in the home, including generic advice to reduce child exposure to ETS. Follow‐up coaching consisting of 7 in‐home sessions of 30 to 45 minutes over 3 months plus follow‐up phone call Control: asthma management education session and follow‐up visits for measurement only | |
| Outcomes | At 4, 7, 10, and 13 months: • Parental report of child ETS exposure • Child's urinary cotinine • Air nicotine levels (20% of homes) • Parental saliva cotinine | |
| Type of intervention | Child with health problems (respiratory disorders) | |
| Notes | Retention: 188/204 (92%). 11 participants dropped out before randomisation; 5 dropped out before outcome measurement. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An Excel computer‐generated list of random 3‐digit numbers was constructed by clinic site." |
| Allocation concealment (selection bias) | Unclear risk | "Participants were assigned to the coaching condition and control condition based on numbers ending with even and odd digits." No further information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis conducted. Low dropout rate: 3 control families, 2 intervention families; "little or no sampling bias attributable to attrition" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used "Control families were unaware of coaching procedures and continued in the study for measurement purposes only. Interviewers were blind to group assignment and investigators were blind to results until all data were collected." |
Hovell 2009.
| Methods | Country: USA Setting: at home RCT |
|
| Participants | Mothers who smoke, with children younger than 4 years | |
| Interventions |
Intervention: 10 in‐person at‐home and 4 telephone counselling sessions over 6 months, with additional pre‐quit and post‐quit telephone sessions Control: referral to the free California Smoker's Helpline (usual care) |
|
| Outcomes |
Eighteen‐month follow‐up from baseline: • Children's urine cotinine concentration • Parents' smoking status ‐ self‐reported and confirmed with salivary cotinine • Air nicotine measured in randomly selected homes |
|
| Type of intervention | Child with health problems (ill‐child health care) | |
| Notes | Recruited from the Supplemental Nutrition Programme for Women, Infants, and Children | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number list was used to assign pairs of participants matched on child's gender, ethnicity and recruitment site" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18‐Month interview 64/74 control group and 66/76 intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Data collection research assistants were blind to group assignment, and control families were unaware of counselling procedures. Investigators were blind to results until all data were collected." |
| Other bias | High risk | However, "baseline children's urinary cotinine concentration was significantly higher among controls, indicating that randomization did not balance the groups with respect to cotinine". |
Hughes 1991.
| Methods | Country: Canada Setting: hospital and home, asthma management programme RCT | |
| Participants | 95 children admitted to hospital in the previous 5 years with asthma, along with their parents (not all smokers) | |
| Interventions | Intervention: cared for by a paediatric respiratory physician through the 12‐month study period. In addition, seen at clinic visits and visited at home by a nurse co‐ordinator who provided written information about asthma care and carried out an asthma educational session around lung and airway anatomy, asthma episodes, and treatment. Participant's home visited at least 3 times. Environmental exposures checklist drawn up; role of cigarette smoke discussed; parents discouraged from smoking in the home and encouraged to participate in a smoking cessation programme Control: participants managed by their usual primary care physicians and reviewed by the study physician at intervals | |
| Outcomes | At 12 months: • Exposure to ETS at home (Primary study outcomes were related to asthma management.) | |
| Type of intervention | Child with health problems (respiratory disorders) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A process of restricted randomization based on age and number of previous hospitalizations during the previous 5 years was carried out. Subjects were alternately assigned to study or control groups, with the initial assignment for each pair determined by a coin toss." |
| Allocation concealment (selection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout ‐ 3 lost from each group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Smoking status reliant on self‐report; however, no evidence of effect, so differential misreport judged to be unlikely |
Irvine 1999.
| Methods | Country: Scotland Setting: home RCT | |
| Participants | 501 smoking parents of children with asthma | |
| Interventions | Intervention: brief advice from a nurse visiting the family home; information about passive smoking and asthma, financial and health benefits of quitting; information on how to stop smoking; advised to move to a different room or outside the home if they did not intend to quit; advised not to allow visitors to the home to smoke. Given 2 leaflets at baseline ‐ 1 commercially available, and the other provided to reinforce the brief advice. Questionnaires were completed. Further leaflets were distributed by mail at 4 and 8 months after baseline along with a letter encouraging them to stop smoking. Control: Participants received the commercial leaflet at baseline but nothing else. | |
| Outcomes |
At 12 months: • Child's saliva cotinine • Mother's saliva cotinine • Self‐reported quit attempts |
|
| Type of intervention | Child with health problems (respiratory disorders) | |
| Notes | Retention: 435/501 (87%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized"; no further information given |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 86.8% provided samples at follow‐up; percentage lost similar in both groups and reasons provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical measures used |
Joseph 2014.
| Methods | Country: USA Setting: community (well‐child clinic) Type: observational, quasi‐experimental (historical control) |
|
| Participants | Parents who smoke who have children aged 12 and 24 months; 40 parent‐child couples for intervention group and 40 for control group | |
| Interventions |
Intervention: Children had serum cotinine measured with lead screening. Lab results were sent to providers and parents. The letter included an explanation that cotinine came from tobacco exposure, and that the normal value was zero. One week later, the tobacco counsellor proactively telephoned to explain the lab result, to describe potential sources of tobacco smoke exposure, including third‐hand smoke, and to convey what is known about the potential health effects of exposure for their child. Counsellor used motivational interviewing and cognitive‐behavioural therapy to engage the parent in a smoking cessation attempt. All parents were encouraged to institute a strict home and car no‐smoking policy, regardless of whether they wanted to stop smoking. If parent wanted to stop smoking, counsellors offered an 8‐session weekly telephone intervention based on an evidence‐based telephone smoking cessation protocol. While no prescription or over‐the‐counter medicine was offered, counsellors did describe them as options and facilitated access where requested. Control: historical group that received usual care |
|
| Outcomes | Child exposure: outcomes assessed 8 weeks after initial call, including receipt of tobacco treatment, quit attempts, 7‐day point prevalent abstinence, and current home and car smoking policies Target behaviour change: receipt of tobacco treatment, parent quit attempts, 7‐day point prevalence abstinence |
|
| Type of intervention | Well‐child (child health check) | |
| Notes | Conflict of interest: unclear Source of funding: National Cancer Institute (R21CA137014) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Allocation concealment not possible, as the study was not randomised |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 95% followed up |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Other bias | High risk | • Recall bias as data ascertained historically in the comparison group • Misclassification, as smoking status not biochemically validated in control group |
Kallio 2006.
| Methods | Country: Finland Setting: community, well baby clinics RCT | |
| Participants | 1062 families presenting at a well baby clinic in Turku with a child 5 months old | |
| Interventions | Component of larger prospective intervention trial aimed at decreasing exposure of children to known environmental cardiovascular risk factors Intervention: Parents received booklet about the adverse effects of smoking at age 5 years. Counselling from paediatrician and dietician consisted of discussion with parents about major cardiovascular risk factors including smoking. Appointment with paediatrician and dietician at 1‐ to 3‐monthly intervals until age 2 years, then 6 monthly Control: normal health education given to all Finnish families at well baby clinics and throughout the school system. Appointment with paediatrician and dietician at 4‐ to 6‐monthly intervals until age 2 years, then 6‐monthly until age 7, then yearly | |
| Outcomes | Follow‐up when child 8 years of age: • Parent report of smoking status and habits, reported child exposure to ETS in past 3 days • Parent serum cotinine | |
| Type of intervention | Well‐child (child health check) | |
| Notes | Retention: 625/1062 (59%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Random numbers"; further details not provided |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High but similar dropout rates in both groups overall (serum cotinine measured in 306/540 intervention and 319/522 control). However, attrition of smokers not quantified and attrition analysis not reported. Trial authors write: "It is possible that smokers have discontinued participation in STRIP more frequently than non‐smokers". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
Kegler 2015.
| Methods | Country: USA Setting: community (2‐1‐1 callers) Type: RCT |
|
| Participants | 498 2‐1‐1 callers who were either smokers living with at least 1 child or other non‐smoker, or non‐smokers living with a smoker, and smoking was allowed in the home. Callers to 2‐1‐1 are disproportionately low income, unemployed, and uninsured, and have received fewer years of education relative to the general population. | |
| Interventions |
Intervention: Smoke‐Free Homes intervention consisted of 3 mailings and 1 coaching call, based on a theme of "Some things are better outside", with content focused on 5 steps to create a smoke‐free home. The intervention was delivered over a 6‐week period at 2‐week intervals, first as a mailing, then as a coaching call, and finally as 2 additional mailings. The coaching call used motivational interviewing. Control: measures alone |
|
| Outcomes | Child exposure: home smoking ban (self‐report), validated with air nicotine levels Target behaviour change: smoking away from home |
|
| Type of intervention | Community‐based | |
| Notes | Conflict of interest: unclear Source of funding: National Cancer Institute's State and Community Tobacco Control Research Initiative (U01CA154282) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Simple' (not block) randomisation, but method not described in detail |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 83.1% completed 3‐month data collection, and 79.1% completed 6‐month data collection. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | University‐based research assistants blinded to study condition collected outcome data at 3 and 6 months post randomisation; objective measure was also used. |
Kimata 2004.
| Methods | Country: Japan Setting: hospital outpatient clinic RCT | |
| Participants | Fifty children with mild atopic eczema/dermatitis syndrome and 25 normal children whose parents smoked 10 to 15 CPD at home | |
| Interventions | Intervention: not clear: “Parents of the cessation of passive smoking group agreed to stop smoking” Control: usual care | |
| Outcomes | At 1 month: • Child urinary cotinine • Child skin wheal response • Child plasma neurotrophin levels | |
| Type of intervention | Child with health problems (ill‐child health care) | |
| Notes | Not provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly divided"; no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
Krieger 2005.
| Methods | Country: USA Setting: community Type: RCT | |
| Participants | 274 low‐income households including a child aged 4 to 12 years who had asthma recruited by media publicity, hospitals, and emergency departments | |
| Interventions | Intervention: high‐intensity intervention with community health workers providing in‐home environmental assessments, education, support for behaviour change (7 sessions), and a full set of resources Control: low‐intensity intervention group received a single visit and limited resources | |
| Outcomes | Parent self‐report Paediatric asthma caregiver quality of life Self‐reported asthma‐related urgent healthcare service use Participant report of presence of asthma triggers in the home, including smoking behaviour | |
| Type of intervention | Child with health problems (respiratory disorders) | |
| Notes | Retention: 110/138 (80%) in high‐intensity group and 104/136 (76%) in low‐intensity group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We randomly assigned participants to groups using a permuted block design with varying block size." |
| Allocation concealment (selection bias) | Low risk | "Sequence numbers and group allocation were concealed in sealed, opaque, numbered envelopes prepared centrally and provided sequentially to interviewers." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "We performed an intention‐to‐treat analysis by using the baseline value of the outcome variable of interest as the exit value for participants who did not complete the study, which yields a conservative estimate of intervention effect." Similar follow‐up rates in both groups (110/138 intervention, 104/136 control) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The nature of the intervention made it impossible to blind participants and staff to group assignment." However, combination of objective and subjective measures, and all participants received visit from counsellor, so differential misreport unlikely |
McIntosh 1994.
| Methods | Country: USA Setting: clinic RCT | |
| Participants | 92 smoking parents of children with asthma | |
| Interventions | Intervention: Child's physician delivered a standardised passive smoking message to parents, consisting of counselling about the effects of passive smoking and advice to quit or smoke outside. Parents were given a specifically designed pamphlet that reinforced this message. About 1 month later, parents received a personalised letter from the principal investigator, containing the results and an explanation of their child's urine cotinine test. Included was a self‐help manual aimed at encouraging smoking outside. Control: Parents received the physician's message and the pamphlet only. | |
| Outcomes | At 4 to 6 months: • Self‐reported location of smoking, attempts to quit • Child urine cotinine | |
| Type of intervention | Child with health problems (respiratory disorders) | |
| Notes | Retention: 72/92 (78%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Families were randomly assigned... at the time of enrolment using a coin toss method." |
| Allocation concealment (selection bias) | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Slightly higher dropout rate in control group than in intervention group (37/44 followed up in intervention, 35/48 followed up in control), ITT analysis not reported, but per‐protocol analysis more conservative in this instance, so judged to be at low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome |
Nicholson 2015.
| Methods | Country: USA Setting: hospital Type: RCT |
|
| Participants | 119 parents or guardians of children receiving treatment for cancer who lived with at least 1 adult smoker and were exposed to SHS in the home or car setting | |
| Interventions |
Intervention: multi‐component behavioural programme over 3 months; counselling consisted of 3 individual, face‐to‐face, biweekly 1‐hour sessions followed by 3 25‐minute telephone sessions for a total of 6 individual contacts with the counsellor. Parents also received letters from their child's physician at the start and at the end of the counselling phase to acknowledge their participation and progress. Control: standard care and equivalent follow‐up to intervention arm |
|
| Outcomes | Child exposure (and target behaviour change): full smoking ban ‐ defined as a household with smokers that prohibited all smoking in the home and in the car | |
| Type of intervention | Child with health problems (ill‐child health care) | |
| Notes | Conflict of interest: none declared Source of funding: Grants CA085406 and CA21765 from the National Cancer Institute and the American Lebanese Associated Charities |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified, blocked randomisation scheme with strata including child's age and race, as well as smoking status of the participating parent |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 91% follow‐up rate |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Other bias | High risk | Reporting bias: smoking bans self‐reported, not validated biochemically |
Nuesslein 2006.
| Methods | Country: Germany Setting: paediatric clinic RCT | |
| Participants | 40 mothers attending participating paediatric practice and self‐reporting smoked at least 10 CPD | |
| Interventions | All participants received a quit smoking information sheet and had urinary cotinine levels taken. Intervention: received results of their cotinine levels within 1 week Control: did not receive results of cotinine levels until data collection was complete | |
| Outcomes | At 6 weeks: • Maternal self‐report of tobacco consumption • Urinary cotinine levels | |
| Type of intervention | Mixed/not stated | |
| Notes | Nicotine consumption did not differ at baseline (median 12 μg for both). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised by participant numbers (odd or even) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 (of 40) missing at final follow‐up |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
Ortega 2015.
| Methods | Country: Spain Setting: community (primary paediatric care) Type: RCT (cluster) |
|
| Participants | 1101 smoking parents of babies younger than 18 months | |
| Interventions |
Intervention: brief intervention based on the '5 A's' approach, carried out during regular well baby visits at paediatric primary care team offices, lasting less than 10 minutes each time and with at least 3 occurrences: at baseline, at 3‐month follow‐up, and at 6‐month follow‐up Control: usual care |
|
| Outcomes | Child exposure: hair nicotine level and parents' reported measures to avoid baby's exposure to tobacco smoke pollution at home, in the car, and in other settings Target behaviour change: smoking away from child in home, in car, or in other setting |
|
| Type of intervention | Mixed (primary paediatric care includes both well‐ and ill‐child healthcare services) | |
| Notes | Conflict of interest: none declared Source of funding: Spain’s National Committee on Smoking Prevention (Comité Nacional de Prevención del Tabaquismo) and the Public Health Agency of the Catalan Government (Direcció General de Salut Pública, Generalitat de Catalunya) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using SPSS version 15.0, with primary care teams as the unit of randomisation |
| Allocation concealment (selection bias) | Low risk | Not specified, but allocation was randomised by a central computer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 83% follow‐up rate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded but biological measure (objective) |
| Other bias | High risk | • Groups were statistically significantly different at baseline. • Hawthorne effect/observer bias in control group |
Patel 2012.
| Methods | Country: USA Setting: hospital emergency department RCT | |
| Participants | Child aged < 36 months with a smoking caregiver presenting to the emergency department | |
| Interventions | Intervention group received brief education about third‐hand smoke; control group received "routine education" from the emergency physician | |
| Outcomes | Caregivers' change in smoking status or policies for smoking in the home or in the car | |
| Type of intervention | Child with health problems (ill‐child health care) | |
| Notes | N = 40; 65% loss to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 65% loss to follow‐up from a small sample |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided; objective measure not used |
| Other bias | High risk | Selection ‐ very small sample size, convenience sample; reporting of results unclear in terms of how numbers were derived and whether ITT analysis was performed |
Phillips 2012.
| Methods | Country: USA Setting: hospital RCT |
|
| Participants | Mothers who had previously smoked who had babies in the neonatal intensive care unit | |
| Interventions |
Intervention: given information about bonding with the infant Both groups given handouts regarding second‐hand smoke exposure; neonatologist used motivational interviewing to prevent reuptake of smoking by the mother |
|
| Outcomes |
Eight‐week follow‐up from baseline: • Re‐uptake of smoking by mother, measured by self‐report, carbon monoxide oximetry, and salivary cotinine |
|
| Type of intervention | Child with health problems (ill‐child health care) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random table |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Salivary cotinine levels on only 67% of mothers who completed the study (45% from control and 55% from intervention) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biological measure used |
| Other bias | High risk | Small numbers ‐ intervention N = 24 and control N = 30. More mothers in the intervention than in the control group had private insurance (P = 0.02). Trend for infants in the intervention group to have lower birth weight (P = 0.08) and longer stay (P = 0.08). Insurance was found to be significantly associated with Kaplan‐Meier, remaining smoke free, and investigators tried to control for this. |
Pollak 2015.
| Methods | Country: USA Setting: community (home and telephone) Type: RCT |
|
| Participants | 348 expectant Latino couples (mothers and their male partners who smoked) | |
| Interventions |
Intervention: culturally tailored couples‐based intervention plus written materials (self‐help smoking cessation guide) and free NRT Control: minimal intervention involving written materials plus NRT |
|
| Outcomes | Target behaviour change: smoking cessation, measured by 7‐day point‐prevalence abstinence and 30‐day point‐prevalence abstinence at baseline, at the end of pregnancy, and 12 months post randomisation. Also assessed continuous abstinence and validated data with salivary cotinine from men | |
| Type of intervention | Community‐based | |
| Notes | Conflict of interest: none declared Source of funding: Grant R01CA127307 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Preset randomisation list stratified on whether men were daily or non‐daily smokers and first time fathers or not |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 81% follow‐up rate by 12 months |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective biological measure (unclear whether blinding occurred) |
| Other bias | Low risk | Social desirability bias is more common in Latinos, but this does not vary between intervention and control groups. |
Prokhorov 2013.
| Methods | Country: USA Setting: home RCT | |
| Participants | Households with a child younger than 18 years of age and 2 adults, 1 of whom was a smoker | |
| Interventions | One culturally appropriate bilingual comic book for children and 2 fotonovelas for adults | |
| Outcomes | Reduced household smoking ‐ report and 2 nicotine air sampling monitors Self‐reported smoking status given (for the smoker) Increase in knowledge of health effects of SHS |
|
| Type of intervention | Well‐child (child health check) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 76 of 91 households completed 12 months of follow‐up; no ITT analysis stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Environmental nicotine monitors as outcome |
| Other bias | High risk | No ITT analysis. Air nicotine levels higher in intervention group but not significantly so |
Pulley 2002.
| Methods | Country: USA Setting: recruited from postpartum units, intervention involved home visits Quasi‐experimental RCT |
|
| Participants | Postpartum mothers who smoke and breastfeed infants | |
| Interventions |
Intervention: educational intervention regarding "smoking hygiene" to reduce ETS exposure of infant. Education was delivered by a nurse, and participants were given an educational pamphlet. Air purifiers were provided. Control: data collection only |
|
| Outcomes | Mothers completed a smoking habits questionnaire at baseline and at completion of the follow‐up period, 3 weeks later. Frequency of respiratory symptoms in the infant and hospitalisation were recorded at baseline and 3 weeks later. |
|
| Type of intervention | Well‐child (peripartum) | |
| Notes | 8/29 dropped out after enrolment. Follow‐up period was 3 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Eight dropped out (25%), 4 from each arm ‐ very high attrition ‐ left 12 in intervention group and 9 in control group. No ITT performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Data collector aware to which group participants were assigned |
| Other bias | High risk | Significant difference in numbers of cigarettes smoked during pregnancy between intervention (significantly higher) and control groups ‐ P = 0.26. No ITT analysis. Very small study |
Ralston 2008.
| Methods | Country: USA Setting: hospital RCT |
|
| Participants | Smoking caregivers of children hospitalised for respiratory illness | |
| Interventions |
Intervention: counselling according to current clinical practice guidelines (US Public Health Guidelines: "Treating Tobacco Use and Dependence"). This includes nicotine replacement therapy. Control: received a brief antismoking message and referral to the state's quit line |
|
| Outcomes |
Six‐month follow‐up post hospitalisation: • Self‐report of parental smoking cessation • Parental quit attempts • Proportion reporting they set a quit date |
|
| Type of intervention | Child with health problems (respiratory disorders) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition but those lost to follow‐up treated as smokers. Unclear from which arm data are missing |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Other bias | High risk | Very small study, so may produce spurious results ‐ only 20% of those eligible participated. Differences in baseline group measurement |
Ralston 2013.
| Methods | Country: USA Setting: hospital RCT |
|
| Participants | Tobacco smoking caregiver over 18 years of age with a hospitalised child | |
| Interventions | Intervention: brief intervention recommending tobacco cessation followed by referral to the state tobacco quit line and receipt of a smoking cessation brochure produced by the American Cancer Society. Both groups received an age‐appropriate injury prevention brochure. | |
| Outcomes |
Primary outcome: Self‐reported quit status (defined as self‐reported abstinence for at least 1 week) Secondary outcomes: Decrease in cigarettes smoked per day; increase in importance of quitting on a 1 to 10 scale; report of any contact with state quit line |
|
| Type of intervention | Child with health problems (ill‐child health care) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers computer generated |
| Allocation concealment (selection bias) | Low risk | Sequential sealed envelopes used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High level of loss to follow‐up (N = 19/60; 32%). However, ITT analysis was performed, and those lost to follow‐up were treated as ongoing smokers. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Telephone interviewers were not always blinded (but did have a script). |
Ratner 2001.
| Methods | Country: Canada Setting: community Type: RCT | |
| Participants | 251 mothers who had quit smoking during pregnancy | |
| Interventions | Intervention: Mothers received nurse‐delivered telephone support, relapse prevention training, and information resources. Control: usual care | |
| Outcomes | Self‐report of smoking status Biological verification with exhaled CO | |
| Type of intervention | Well‐child (peripartum) | |
| Notes | Retention: 238/251 (95%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Identification numbers randomly assigned to 2 groups, in blocks of 50, via a computer software package." |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of follow‐up in both groups at 12 months and 95% retention (238/251) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used at in‐person follow‐ups (89% of participants) "Only 1.4% of the self‐reports of abstinence were contradicted by CO readings of ≥ 10 ppm; these women were classified as smokers." |
Schonberger 2005.
| Methods | Country: Netherlands Setting: community RCT; cluster | |
| Participants | 476 children seen to be at high risk of asthma recruited during the prenatal period | |
| Interventions | Intervention: 3 home visits (2 prenatal and 1 postnatal) with recommendations to reduce 4 main environmental exposures of mite allergens, pet allergens, food allergens, and passive smoking prenatally and postnatally Control: usual care | |
| Outcomes | Parent report of child ETS exposure Maternal CO Child IgE Tidal airway resistance and lung function Allergen measures | |
| Type of intervention | Well‐child (peripartum) | |
| Notes | Retention: 443/476 (93%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prerandomisation; no further information provided |
| Allocation concealment (selection bias) | Unclear risk | "To prevent contamination... the prerandomisation was performed in clusters, taking into account the post (zip) code of the domicile of the recruited family in combination with the location of the general practice the family attended. Once a general practice was allocated, every family subsequently recruited in that practice was allocated automatically to the same group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 93% retention; similar number completed follow‐up in both groups (222/242 intervention, 221/234 control); attrition and ITT analyses performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report only: "reporting bias cannot be excluded as an explanation for the decrease in asthma‐like symptoms in the intervention group at age 2 yrs." |
Schuck 2014.
| Methods | Country: Netherlands (nationwide) Setting: community (telephone) Type: RCT |
|
| Participants | 512 smoking parents of primary school children aged 9 to 12 years | |
| Interventions |
Intervention: up to 7 counsellor‐initiated telephone calls during a period of 3 months. All participants also received 3 books entitled "Smoke‐Free Parents". Booklets were sent at 3 time points throughout the study (immediately after the first call, 2 weeks after the first call, and 6 weeks after the first call). Time points corresponded with contents of the booklets (deciding and preparing, initiating and maintaining abstinence, and preventing relapse). Control: self‐help brochure, together with information on use of NRT and pharmacotherapy |
|
| Outcomes | Child exposure: home smoking ban Target behavioural change: smoking cessation, measured by 7‐day point‐prevalence abstinence at 12‐month follow‐up, 7‐day point‐prevalence abstinence at 3‐month follow‐up, and prolonged abstinence (defined as report of 7‐day point‐prevalence abstinence at 3 and 12 months and report of cessation for at least 6 months at 12‐month follow‐up). Also measured use of and adherence to NRT and pharmacotherapy. Subsample of those reporting abstinence were biochemically validated using exhaled CO and salivary cotinine. |
|
| Type of intervention | Community‐based | |
| Notes | Conflict of interest: none declared Source of funding: ZonMW, the Netherlands Organization for Health Care Research and Development (grant number: 50‐50110‐96‐639) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence, in blocks of 10 to ensure equal group sizes, and stratified to ensure balance of key characteristics (gender, educational level, and cigarettes smoked per day) |
| Allocation concealment (selection bias) | Unclear risk | Independent researcher performed allocation of participants, but first trial author prepared mailings informing participants about the treatment they would receive. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 85.5% follow‐up in treatment group, and 91.8% follow‐up in the control group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation (blinding not specified) |
Severson 1997.
| Methods | Country: USA Setting: hospital and well baby clinics RCT; randomization by practice | |
| Participants | 2901 mothers of newborn babies who had smoked before pregnancy (1875 smokers, 1026 non‐smokers at enrolment) | |
| Interventions | In the first 1 to 3 days after birth in hospital, mothers received a packet containing a brochure and a letter from the paediatrician about the health effects of passive smoking, along with a no smoking sign. Intervention: Mothers received further materials and brief oral counselling from the paediatrician at well baby visits at age 2 weeks and 2, 4, and 6 months. Paediatricians received a 45‐minute training session. Control: received the hospital packet only | |
| Outcomes |
Primary outcome: Assessment at 6 and 12 months by mailed questionnaire: • Quit rates (sustained at 6 and 12 months, and point prevalence at 12 months) • CPD, readiness to quit, likelihood of quit attempt. Secondary outcomes: • Knowledge of and attitudes towards ETS |
|
| Type of intervention | Well‐child (peripartum) | |
| Notes | Retention: 2003/2901 (69%) One‐tailed t‐test employed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised by practice; method not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocating practices not described. All eligible patients enrolled, "because the survey information was anonymous, and because smoking counselling was considered to be standard medical practice, the study was exempted from the requirements for obtaining informed consent". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up (31% in each group) assumed to have relapsed; attrition analyses performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No biochemical validation but cluster‐randomised by practice; followed up anonymously via survey; differential misreport unlikely |
Stotts 2012.
| Methods | Country: USA Setting: Neonatal Intensive Care Unit, Hospital RCT (3 groups) | |
| Participants | Families with a smoker at home; infant in NICU at high respiratory risk | |
| Interventions | Motivational interviewing. There were three groups; motivational interviewing, usual care, and usual care‐reduced measurement. The motivational interviewing group had 2 hospital‐based sessions of approximately 40 minutes each, 2 personalised letters, and 2 phone feedback sessions targeting infant ETS reduction. Reduced measurement group refers to reducing follow‐up, as this is thought to affect the behaviour of the control group. | |
| Outcomes | Air nicotine monitors Infant end‐tidal carbon monoxide Self‐report measures of home and car smoking bans |
|
| Type of intervention | Child with health problems (respiratory disorders) | |
| Notes | In process of publication, information taken from a report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High degree of loss to follow‐up by 6 months (intervention 51/70 completed, usual care 21/34 completed, and usual care reduced measurement 28/40 completed) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Air nicotine monitors used |
Streja 2014.
| Methods | Country: USA Setting: community (home) Type: RCT |
|
| Participants | 242 parents or guardians of children aged 2 to 14 years with asthma from low‐income, predominantly ethnic minority families, living in households with at least 1 current smoker where smoking had occurred at home | |
| Interventions |
Intervention: tailored Spanish/English video addressing implications of SHS exposure for children with asthma, possible efficacy of household SHS exposure reductions on the child's health and frequency of asthma attacks, and strategies to reduce household SHS exposure. A companion Spanish/English workbook was also provided to reinforce messages in the DVD and to encourage discussion among participating and non‐participating household members. Brief counselling consisted of asking participants to use the DVD and workbook only. Booster elements included a refrigerator magnet, a mug, and "no smoking" signs to serve as reminders. Control: received standard brochures describing the importance of SHS exposure as an asthma trigger |
|
| Outcomes | Child exposure: self‐reported SHS exposure with two separate surveys of parents/guardians and children; urinary cotinine in children; passive air nicotine monitors in major activity rooms Child illness: child's asthma severity, asthma‐related quality of life Target behavioural change: reduced smoking in household (including smoking ban) |
|
| Type of intervention | Community‐based | |
| Notes | Conflict of interest: unclear Source of funding: National Institutes of Health grants HL53957 from the National Heart, Lung and Blood Institute, Division of Lung Diseases, and CA16042 from the National Cancer Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes with allocations opened after baseline data collection |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 76% follow‐up in intervention group and 70% in control group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear whether blinded, but objective air nicotine measure used |
Tyc 2013.
| Methods | Country: USA Setting: hospital RCT | |
| Participants | Parents or guardians of children receiving treatment for cancer who lived with at least 1 adult smoker and were exposed to SHS in the home or car setting | |
| Interventions | Counselling (multi‐component behavioural programme) delivered by trained counsellors over 3 months ‐ 3 individual, face‐to‐face biweekly 1‐hour sessions followed by three 25‐minute telephone sessions. Parents received literature about SHS‐related health risks for children and for stress management. Did not involve formal cessation counselling. Standard care group given brief advice about removing child from sources of exposure, and advised about adverse health problems | |
| Outcomes | Parent‐reported child SHS exposure Child urinary cotinine Parent‐reported smoking |
|
| Type of intervention | Child with health problems (ill‐child health care) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Specific method used to achieve randomisation (e.g. computer‐generated random numbers, coin‐toss) not described. Stratified, blocked randomisation scheme with strata of child’s age (≤ 5, 6 to 12, 13 to 17 years), race (White, non‐White), and smoking status of the participating parent (smoker, nonsmoker) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10/135 lost to follow‐up; ITT analysis |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Urinary cotinine as measure (objective) |
Ulbricht 2014.
| Methods | Country: Germany Setting: community (home and telephone) Type: RCT |
|
| Participants | 917 households with parents of children younger than 4 years of age, where at least 1 parent was a smoker | |
| Interventions |
Intervention: 15 to 30‐minute in‐person behavioural change counselling session, a computer‐generated feedback letter (including the child's urine cotinine level), and a 5‐ to 15‐minute phone counselling session Control: received the same leaflet as the intervention group about the adverse effects of ETS on children. A letter containing information about the child urine cotinine level at baseline and 12 months later was sent after the 12‐month follow‐up assessment. |
|
| Outcomes | Child exposure: child urine cotinine and self‐reported SHS exposure, smoking status, and home smoking ban Target behavioural change: home smoking ban |
|
| Type of intervention | Community‐based | |
| Notes | Conflict of interest: none declared Source of funding: German Cancer AID (Deutsche Krebshilfe, grant no. 107539) and DZHK (German Centre for Cardiovascular Research), partner site Greifswald, Germany (grant no. 81/Z540100152) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Screening team blinded to allocation and separate from intervention team |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 89.7% follow‐up in intervention group; 96.4% follow‐up in control group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective biological measure, although assessors of baseline and 12‐month follow up data were not blind to study group assignment |
Van't Hof 2000.
| Methods | Country: USA Setting: hospital and well baby visits RCT |
|
| Participants | Postpartum women with a history of smoking in the 30 days before pregnancy | |
| Interventions |
Intervention: Initial nurse delivered relapse prevention counselling for 15 to 30 minutes. At 2‐week and 2‐ and 4‐month well baby visits with the paediatric provider, women received reinforcement if they had not restarted smoking. If they had restarted smoking, they were given encouragement and a plan to try to quit again. Control: received no counselling and "standard care" from the paediatric provider |
|
| Outcomes | Follow‐up 6 months from baseline Proportion of mothers who maintain smoking cessation postpartum |
|
| Type of intervention | Well‐child (peripartum) | |
| Notes | Had salivary cotinine at baseline only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
Vineis 1993.
| Methods | Country: Italy Setting: immunisation clinic CT: non‐random assignment | |
| Participants | 1015 parents of newborn babies (all mothers including non‐smokers recruited) recruited when attending the clinic for the 3‐month vaccination of the infant | |
| Interventions | Intervention: counselled for 15 minutes by a nurse on the health effects of active smoking and ETS, and given 3 booklets ‐ 1 of which was about the health effects of ETS on children Control: did not receive counselling or booklets | |
| Outcomes |
At 2 and 4 years: • Self‐reported cessation |
|
| Type of intervention | Well‐child (child health check) | |
| Notes | Retention: 747/1015 (74%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Non‐randomized experimental design" |
| Allocation concealment (selection bias) | High risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar follow‐up rates in both groups (304/402 intervention, 443/616 control). Participants who had moved away were excluded from analysis. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report only; differential misreport possible |
Wahlgren 1997.
| Methods | Country: USA Setting: paediatric allergy medical clinics RCT | |
| Participants | 91 families with children with asthma | |
| Interventions | Intervention: Parent and child attended a series of intensive counselling sessions over 6 months designed to reduce child's exposure to parental smoking. Diaries were used in the 2 weeks preceding visits to record parental smoking, child's ETS exposure, child's peak flow readings, and child's symptoms. These data were used for tailored counselling. Control (monitoring): used the same monitoring methods but did not receive counselling Control (usual care): attended clinics at the same frequency but did not maintain records nor receive counselling | |
| Outcomes |
At 6 months from end of intervention:
• Parent self‐report of cigarettes smoked in presence of the child
• Air nicotine in room with heaviest child exposure measured by environmental monitor
2 years later: • After debriefing about the study, the 2 comparison groups achieved similar reductions in parent‐reported rates of child exposure, and the intervention parent‐reported child exposure rate was similarly maintained. |
|
| Type of intervention | Child with health problems (respiratory disorders) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized"; no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High rate of follow‐up at 12 months across all groups (28/31 intervention, 28/28 monitoring control, 26/32 usual care) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report validated by environmental monitor |
Wakefield 2002.
| Methods | Country: Australia Setting: recruited from paediatric outpatient clinics, intervention by mail and phone CT: alternation by week of attendance at clinic | |
| Participants | 292 smoking parents of children aged 1 to 11 with asthma | |
| Interventions | At baseline, urine analysed for cotinine:creatinine ratio Intervention: parents sent a letter signed by the study co‐ordinator to explain child's baseline cotinine:creatinine ratio, and to encourage banning smoking at home. Two booklets enclosed: 1 explained the effects of ETS on children and gave advice to parents on its restriction; the other concerned quitting. The index parent was contacted by telephone 1 week and 1 month later for advice and encouragement. Control: usual advice about smoking from doctors and nurses | |
| Outcomes |
At 6 months: • Smoking bans at home Secondary study outcomes: • Parent reports of bans on smoking in car • CPD • Child urinary cotinine • Parent‐reported cessation |
|
| Type of intervention | Child with health problems (ill‐child health care) | |
| Notes | Retention 264/292 (90.4%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Families were allocated by alternate week to either an intervention or control group." |
| Allocation concealment (selection bias) | High risk | No information was provided, but method of sequence generation makes allocation concealment highly unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates lost to follow‐up in both groups (10.5% intervention, 8.7% control) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Children's cotinine levels used to validate self‐report of smoking bans |
Walker 2015.
| Methods | Country: Australia and New Zealand Setting: community (home) Type: RCT |
|
| Participants | 293 mothers of infants between birth and 5 weeks of age, when mothers self‐identified as Maori or Australian Aboriginal/Torres Strait Islander and mothers were current smokers, or the infant lived in a household with at least 1 smoker | |
| Interventions |
Intervention: Mothers (and family members present) received usual care plus behavioural coaching about dangers of SHS exposure to children, commitment to smoking restrictions in the home/car, positive role modelling, and strategies for overcoming obstacles to making smoke‐free changes. Smokers also were offered brief advice or intensive counselling to quit and were offered free NRT and/or a quit line referral. Control: usual care, which included brief quit advice and the provision of smoking cessation treatment |
|
| Outcomes | Child exposure: child urine cotinine, self‐report smoking restrictions in home/car, self‐reported SHS exposure, and self‐reported smoking cessation Child illness: parent‐reported cough in child Child health service utilisation: rate of health provider presentations and/or hospitalisations for new primary episodes of acute respiratory illness in the first year of life Target behavioural change: smoking cessation, restriction, and home/car smoking ban |
|
| Type of intervention | Community‐based | |
| Notes |
Conflicts of interest: All authors declare that (1) no trial authors have received support from any companies for the submitted work; (2) CB has previously undertaken research on behalf of NicoNovum, but before the purchase of the company by RJ Reynolds. NW has provided consultancy to the manufacturers of smoking cessation medications, received honoraria for speaking at a research meeting, and received benefits in kind and travel support from a manufacturer of smoking cessation medications. MG has provided consultancy to the manufacturers of smoking cessation medications; (3) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) all trial authors have no non‐financial interests that may be relevant to the submitted work. NW, CB, MG, and VP have also undertaken 2 trials of very low nicotine content cigarettes, which were purchased from 2 different tobacco companies. The companies concerned had no role in development of the study design, data collection, data analysis, data interpretation, or writing of the trial publications. Source of funding: National Health and Medical Research Council of Australia (545203); the Health Research Council of NZ (09/626); Cure Kids NZ (3525); and the James Russell Lewis Trust, New Zealand (13787/15734) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in a 1:1 ratio to 1 of 2 arms by central computer using block randomisation stratified by country |
| Allocation concealment (selection bias) | Low risk | Not specified, but allocation was randomised by a central computer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 88% follow‐up in intervention group and 86% follow‐up in control group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective measure and single‐blind (research staff assessing the primary outcome were blinded to treatment allocation) |
Wang 2015.
| Methods | Country: China Setting: community (preschools) Type: RCT |
|
| Participants | 65 caregivers (and children, but this Cochrane Review focuses on caregivers only) | |
| Interventions |
Intervention: health education classes for children aimed at encouraging children to persuade their smoker caregivers to change their behaviours. Children were given a bookmark, a card, and a sign that said "no smoking" to act as reminders for their caregivers. Also children were given materials about quitting and ETS exposure to be shared with their caregivers. Smoking cessation and ETS exposure counselling for caregivers consisted of 1 lecture and 5 monthly in‐person counselling sessions at school over 6 months, together with educational materials and text messages. Child's urine cotinine level was fed back to caregivers. Control: Group underwent all assessments but did not receive counselling. |
|
| Outcomes | Child exposure: child urine cotinine, self‐reported ETS exposure of children by caregivers, caregivers' self‐reported smoking status Target behavioural change: smoking cessation and reduced smoking in home or in presence of child |
|
| Type of intervention | Community‐based | |
| Notes | Conflict of interest: none declared Source of funding: Postgraduate Research Fund of Central South University, China (Grant Number 2013zzts076) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Two‐stage simple random sampling (first stage: district drawn at random; second stage: preschool drawn at random); unit of randomisation was the individual family; computer‐generated randomisation table used |
| Allocation concealment (selection bias) | Low risk | Randomisation information was kept from the study counsellor until the baseline assessment was completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Lab staff blinded to intervention status; cotinine used as an objective measure |
Wiggins 2005.
| Methods | Country: UK Setting: community Type: RCT | |
| Participants | 731 mothers who lived in deprived London districts and met the inclusion criteria after answering an information leaflet | |
| Interventions | Intervention Group 1: Support Health Visitor intervention consisting of monthly supportive listening visits to the mother's home, beginning when the baby was 10 weeks old. The primary focus was on the mother rather than on her child, as well as on providing practical support and information. Intervention Group 2: Assignment to 1 of 8 community groups that offered service for mothers with children younger than 5 years of age in the study area Control: usual care | |
| Outcomes | Childhood injury, maternal depression, and smoking Uptake and cost of health services, household resources, maternal and child health, experiences of motherhood and infant feeding | |
| Type of intervention | Well‐child (peripartum) | |
| Notes | Retention: 601/731 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation sequence was computer generated and minimisation was used to provide a reasonable balance on three potential confounders..." |
| Allocation concealment (selection bias) | Low risk | "Recruiters provided a centrally based administrator with the participant's name and information on the minimisation factors. These data were entered into the computer program to determine the participant's allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of follow‐up at 12 months in all groups (82% control, 85% community group intervention, 80% support health visitor intervention). Intention‐to‐treat analyses were performed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report via postal questionnaire: "because of the nature of the interventions, it was not possible for either the trial participants or the researchers to be blinded to group allocation" |
Wilson 2001.
| Methods | Country: USA Setting: paediatric pulmonary service of a paediatric hospital RCT | |
| Participants | 87 parents of children 3 to 12 years of age with asthma who were ETS exposed. (At baseline, 61% of intervention group maternal caregivers smoked vs 42% of controls.) | |
| Interventions | All children examined at baseline by a paediatric pulmonary specialist, and their treatment was adjusted as appropriate. Intervention: Caregiver received 3 nurse‐led sessions over a 5‐week period, employing behaviour change strategies and basic asthma and ETS education, along with repeated feedback on the child's urinary cotinine level (measured each session). The child and other family members were sometimes involved. Control: Caregivers received basic asthma advice from a nurse, along with the statement that ETS is to be avoided. Mothers who requested the cotinine result were told whether or not cotinine had been detected. | |
| Outcomes |
At 12 months:
• Urinary cotinine • Acute asthma episodes Secondary study outcomes: • Hospitalisation • Prohibition of smoking in the home • CPD • Parent‐reported exposure of children and asthma control |
|
| Type of intervention | Child with health problems (respiratory disorders) | |
| Notes | Follow‐up cotinine data obtained in 51/87 (59%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization design with blocks of length four"; no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis conducted; "attrition rates on the cotinine data were equivalent in the intervention and control groups" (25/44 intervention, 26/43 control) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical measure used |
Wilson 2011.
| Methods | Country: USA Setting: participants identified from insurer database, counselling intervention delivered in the community RCT |
|
| Participants | Caregivers of children aged 3 to 12 years who have asthma and are exposed to second‐hand smoke | |
| Interventions | Three counselling visits, including cotinine feedback, and 3 follow‐up phone calls | |
| Outcomes |
Twelve‐month follow‐up from baseline: • Child urinary cotinine:creatinine ratio • Child asthma‐related use of healthcare resources (asthma visits and medication use) • Home smoking bans • Caregiver smoking status |
|
| Type of intervention | Child with health problems (respiratory disorders) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer algorithm used |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low loss to follow‐up |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study staff performing follow‐up blinded; asthma assessments blinded Biological measure used |
Winickoff 2010.
| Methods | Country: USA Setting: hospital and community Quasi‐experimental RCT | |
| Participants | 101 mothers and fathers of newborns recruited on the postnatal ward who were current smokers or recent quitters | |
| Interventions | Intervention: A 15‐minute counselling session in person, enrolment in a proactive state quit line, follow‐up faxes to health professionals with tailored treatment measures Control: usual care | |
| Outcomes | 3‐Month follow‐up during which participant enrolment in the state smoking quit line was assessed and self‐reported smoking status was taken with a salivary cotinine level as confirmation of a self‐reported 7‐day point‐prevalence cessation | |
| Type of intervention | Well‐child (child health check) | |
| Notes | Retention: 75% control and 69% intervention available for follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Participants were assigned to either the control or the intervention condition on the basis of the date the mother was admitted to the postpartum floor." |
| Allocation concealment (selection bias) | High risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No significant difference in follow‐up between groups (75% control and 69% intervention); intention‐to‐treat analysis performed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
Woodward 1987.
| Methods | Country: Australia Setting: maternity hospital CT: allocation by month of delivery | |
| Participants | 184 parents of newborn babies whose mothers smoked during pregnancy | |
| Interventions | Intervention: Mothers in the maternity hospital were given an information kit about the effects of ETS on children and ways to quit smoking, along with a letter from the director of the Neonatal Intensive Care Unit urging parents to avoid exposing children to ETS. The kit was given to women by a research worker, who explained the material and answered questions. Women were telephoned at 1 month and were asked about their progress and use of the kit, and were given further information if required. Control and follow‐up only: did not receive the above intervention | |
| Outcomes | At 3 months: • Infant urine cotinine levels • Maternal quitting, maternal cotinine | |
| Type of intervention | Well‐child (peripartum) | |
| Notes | Retention: 157/184 (85%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised; group assignment by month of admission |
| Allocation concealment (selection bias) | High risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar and high rates of follow‐up in both groups (54/61 intervention, 57/62 control) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biological validation used |
Yilmaz 2006.
| Methods | Country: Turkey Setting: hospital RCT | |
| Participants | 375 mothers with children attending well‐child clinic or with any primary complaint | |
| Interventions | Intervention 1: smoking cessation intervention aimed at child's health Intervention 2: smoking cessation intervention aimed at mother's health Control: no smoking cessation advice | |
| Outcomes | Maternal smoking status Smoking location change Postintervention knowledge change | |
| Type of intervention | Mixed/not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Each mother was assigned the number of the questionnaire she filled in... Then the mothers were randomly assigned by a nurse who doesn't know anything about the study and the groups to one of three groups by randomly picking numbers form the list of questionnaire/mother numbers." |
| Allocation concealment (selection bias) | High risk | See above; breaking of allocation concealment possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "12 (out of 375) families could not be contacted and were therefore excluded from the analysis." Unclear which groups those not reached came from. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemical validation used; differential misreport possible |
Yucel 2014.
| Methods | Country: Turkey Setting: community (home and telephone) Type: RCT |
|
| Participants | 80 mothers of children aged 1 to 5 years who smoked and/or whose spouses smoked | |
| Interventions |
Intensive intervention: consisting of 3 home visits, 2 telephone follow‐ups, and urine cotinine notification. Initial home visit provided brochures for whole family to read. Five behavioural change techniques were used: (1) providing information, (2) engaging in goal‐setting behaviour (not smoking in the home) and outcome (to reduce children's ETS exposure), (3) using follow‐up prompts, (4) educating to use prompts (i.e. "no smoking" warning signs in the home), and (5) providing environmental restructuring (i.e. removing ashtrays in the house). Control: minimal intervention comprising 2 home visits and urine cotinine notification |
|
| Outcomes | Child exposure: urine cotinine; home smoking ban; number of cigarettes smoked in home Target behavioural change: home smoking ban |
|
| Type of intervention | Community‐based | |
| Notes | Conflict of interest: none declared Source of funding: Ege University Scientific Research Projects Commission (Project No. 2009 Medicine 037) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified using SAS statistical programme. However, 12 mothers were substituted with other mothers due not wishing to participate, inability to collect child urine, or not meeting the participation criteria. Unclear if this was before or after randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 97.5% follow up |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective cotinine measure (blinding not specified) |
Zakarian 2004.
| Methods | Country: USA Setting: community RCT | |
| Participants | 150 smoking mothers with children aged 4 or younger | |
| Interventions | Principal investigator and project co‐ordinator met with medical directors from each clinic to plan investigation implementation, then regularly throughout the study to "enlist participation and ongoing support". Intervention: Seven behavioural counselling sessions (3 in‐person and 4 over the telephone) over 6 months. Mothers were assisted in developing plans to reshape their and other household members' smoking behaviours. Mothers were asked to use pictorial charts and to self‐monitor their smoking and exposure. If participants asked counsellor for help in quitting smoking, they were issued a "Quit Kit" from the American Cancer Society. Control: usual care and 3‐, 6‐, and 12‐month follow‐up measures | |
| Outcomes | Mother report of smoking status and child's exposure to ETS Child urinary cotinine concentrations Air nicotine monitors | |
| Type of intervention | Well‐child (child health check) | |
| Notes | Retention: 128/150 (85%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Assignment was stratified by child's age, ethnicity, gender, and clinic site. Random number lists were generated for each strata." |
| Allocation concealment (selection bias) | Low risk | "Within each group of four numbers corresponding to four participants in that strata, the first two even numbers were assigned to the experimental group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analyses: "mothers who were lost to follow‐up and not measured were counted as smokers" 68/74 control and 60/76 intervention reached at final follow‐up |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used "Research assistants who obtained measurements were blind to group assignment, and control families were unaware of counselling procedures." |
Zhang 1993.
| Methods | Country: China Setting: school CT; schools in 1 district received intervention, compared with schools in a second district | |
| Participants | 20,382 children in 44 primary schools 68.8% of intervention and 65.5% of control fathers smoked at baseline. | |
| Interventions | Intervention: A tobacco prevention curriculum comprising social and health consequences of tobacco use and training in refusal skills was introduced. Smoking control policies for schools were encouraged. Children in intervention schools wrote letters to their fathers to ask them to quit smoking and monitored their smoking behaviour. Control: usual curriculum | |
| Outcomes |
At 8 months: • Self‐report of smoking cessation by smoking fathers during interview with health educator |
|
| Type of intervention | Community‐based | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomisation reported |
| Allocation concealment (selection bias) | High risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on missing data reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report only; differential misreport possible |
5 As: Ask, Advise, Assess, Assist, Arrange. BAM: Behavioural Action Model. CBFRS: Community‐Based Family Resource and Support. CHG: Child's Health Group. CO: carbon monoxide. CPD: cigarettes per day. CT: controlled trial. EPA: Environmental Protection Agency. ETS: environmental tobacco smoke. FeNO: fractional exhaled nitric oxide. GP; general practitioner. IgE: immunoglobulin E. ITT: intention‐to‐treat. MHG: Mother's Health Group. MI: motivational interviewing. min: minute(s). NICU: neonatal intensive care unit. NIH: National Institutes of Health. NRT: nicotine replacement therapy. PAM: Precaution Adoption Model. RCT: randomised controlled trial. Rint: interrupter resistance measurement. SHI: smoking hygiene intervention. SHS: second‐hand smoke. SPSS: Statistical Package for the Social Sciences.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arborelius 2001 | Longitudinal study |
| Bacewicz 2015 | Wrong study design (no control group) |
| Badger 2003 | Conference abstract only. Trial authors contacted and no further study information provided. |
| Burmaz 2007 | Minimal data on smoking at either baseline or follow‐up, as smoking only a very small component of the intervention. |
| Campion 1994 | Outcomes assessed by 2 surveys carried out before and after the campaign. This study targeted pregnant women. |
| Carlsson 2013 | Wrong study design (no control group) |
| Chamberlain 2013 | Wrong study design |
| Cookson 2000 | Before‐and‐after study |
| Eakin 2013 | Abstract only; full paper included |
| Emmons 2000 | Quasi‐experimental historical comparison design. |
| Gadomski 2011 | Uncontrolled study; no outcome data for control; only 3 versions of the intervention |
| Halterman 2011a | Conference presentation only |
| Hovell 2011 | Intervention aimed at preteens themselves, not at families or carers |
| Huang 2013 | Wrong outcomes |
| Hutchinson 2014 | Abstract only |
| Kegler 2012 | Pre‐post study; not a controlled study |
| Klinnert 2007 | Does not include outcome data related to ETS. |
| Lepore 2013 | Study protocol only |
| Loke 2005 | Intervention targeted pregnant women and their non‐smoking spouses during the perinatal period only. |
| Manfredi 1999 | This study targeted predominantly women, some of whom were mothers. |
| Meltzer 1993 | Multiple‐baseline, quasi‐experimental design |
| Morgan 2004 | Does not include outcome data related to ETS. |
| Murray 1993 | Longitudinal study |
| Oien 2008 | Study objective to assess the impact of an intervention on parental smoking during pregnancy. |
| Okah 2003 | Secondary analysis of an RCT of bupropion for smoking cessation |
| Philips 1990 | Met main inclusion criteria, but the outcome measure was the report by kindergarten students of their intent to avoid cigarette smoke (by leaving the room themselves or asking an adult smoker to stop smoking). This outcome measure is believed by trial authors to be too unreliable for inclusion of this study. |
| Sockrider 2003 | No ETS results published; this was an ongoing study from 2003 in the previous version of this Cochrane review (Baxi 2014); email contact with trial authors, no response. |
| Spencer 2000 | Pilot study only. No further results available. |
| Stepans 2006 | Pilot study only |
| Stotts 2013b | Study protocol only; full paper included |
| Tingen 2016 | Abstract only |
| Turner‐Henson 2005 | Intervention not described |
| Walley 2015 | Wrong study design (no control group) |
| Williams 2016 | Wrong patient population |
| Wilson 1996 | Baseline results only |
| Wilson 2005 | This ongoing study from 2005 was included in the previous version of this Cochrane Review (Baxi 2014); email contact with trial authors; no response |
| Winickoff 2013 | Outcome data related to ETS not included, but data related to implementation of an intervention provided |
ETS: environmental tobacco smoke. RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by year of study]
Johnston 2010.
| Trial name or title | The study protocol for a randomised controlled trial of a family‐centred tobacco control programme about environmental tobacco smoke (ETS) to reduce respiratory illness in Indigenous infants |
| Methods | Parallel RCT |
| Participants | Indigenous women from Australia and New Zealand and their infants recruited from birth to 5 weeks age and followed‐up until 12 months of age, when the mother herself smokes or someone else in the household is a smoker |
| Interventions | Face‐to‐face home visits. Indigenous model of health promotion ‐ information provision, health education, behavioural coaching for women. For other smokers in the household ‐ smoking cessation advice, counselling, and treatment options |
| Outcomes | Infant medically attended acute respiratory illness Hospitalisations for infant acute respiratory illness Infant urinary cotinine Carer's self‐report of infant tobacco smoke exposure Carer's report of home and car smoking bans Carer's self‐report of smoking cessation Carer's self‐report of number of quit attempts Process indicators |
| Starting date | 2009 |
| Contact information | Vanessa Johnston |
| Notes | Dr. Johnston contacted on 28 June 2017, but no response. Study results not yet published |
Rosen 2011.
| Trial name or title | Project zero exposure |
| Methods | Three‐stage approach: Stage 1 is intervention development, stage 2 is intervention pilot, and stage 3 is a cluster RCT. |
| Participants | Parents who smoke with a child younger than 3 years of age |
| Interventions | Developing a theory‐based intervention based on social marketing ‐ try to convince to stop smoking, (or) stop smoking around the child. Will have group support sessions, feedback of biochemical result of child tobacco smoke exposure, project website, video simulation game, and study information given to the participant's physician |
| Outcomes | Child tobacco smoke exposure assessed by hair nicotine Parental report of child tobacco smoke exposure Adoption of voluntary home and car smoking bans Child respiratory symptoms Parental smoking cessation |
| Starting date | Unclear |
| Contact information | Dr. L. J. Rosen |
| Notes | Dr. Rosen contacted on 28 June 2017; study results not yet available |
Wagener 2012.
| Trial name or title | Novel methods to reduce children's secondhand smoke exposure I |
| Methods | RCT |
| Participants | Carers of children (3 to 11 years of age) who smoke and who are not interested in quitting |
| Interventions | Three arms: Participants receive electronic cigarettes, dissolvable tobacco lozenges, or dissolvable nicotine lozenges (Nicorette) for use instead of cigarettes when in the presence of their child(ren) for up to 8 weeks. |
| Outcomes | Primary outcome measures: • Change in child salivary cotinine [Time Frame: 2, 4, 8, and 12 weeks] • Child salivary cotinine measured to assess the level of second‐hand smoke exposure. We will measure the change throughout the study. Secondary outcome measures: • Change in parent and child lung function [Time Frame: 2, 4, 8, and 12 weeks]. We will collect both parent and child spirometry data and will compare changes. |
| Starting date | April 2012 |
| Contact information | Theodore L. Wagener; theodore‐wagener@ouhsc.edu |
| Notes | Contacted trial author; currently analysing data; no published manuscripts yet |
Hutchinson 2013.
| Trial name or title | PREPASE (PREvent PAssive Smoke Exposure) |
| Methods | RCT |
| Participants | Families with children (birth to 13 years of age) having an asthma predisposition who experience passive smoke exposure at home |
| Interventions | A motivational interviewing tailored programme including urinary cotinine feedback with 6 sessions; based on the principles of the reasoned action model |
| Outcomes | Primary outcome measure: • Percentage of families curtailing passive smoking exposure in children (parental report verified with urine cotinine concentrations of children) after 6 months Secondary outcome measures: • Household nicotine level • Child’s lung function, airway inflammation and oxidative stress, presence of wheezing and questionnaires on respiratory symptoms, and quality of life. |
| Starting date | Unclear |
| Contact information | On paper: contact via Sasha Hutchinson |
| Notes | Dr. Hutchinson contacted on 28 June 2017; no response |
Risica 2016.
| Trial name or title | Baby's Breath |
| Methods | RCT |
| Participants | Pregnant women (not more than 16 weeks pregnant; spoke English and at least 18 years old) who are smokers, spontaneous quitters (women who quit on their own without project materials) or smoke‐exposed, not pregnant with more than 1 baby, and have access to a working telephone and VCR/DVD player |
| Interventions | A series of 5 tailored videos and newsletters addressing issues of tobacco smoke avoidance, including smoking cessation, were compared with written materials containing no tobacco‐related content. |
| Outcomes | The primary outcome measure is foetal exposure to ETS during pregnancy and in the infant at 6 months of life (salivary cotinine and self‐report). Other impact measures included were psychosocial variables used to assess possible determinants of ETS. Self‐reported outcomes and most impact variables were assessed at 16 and 32 weeks of pregnancy, and at 3 and 6 months postpartum. |
| Starting date | Unclear |
| Contact information | Patricia Risica; Patricia_risica@brown.edu |
| Notes | Dr. Risica contacted on 28 June 2017; no response |
ETS: environmental tobacco smoke. RCT: randomised controlled trial.
Differences between protocol and review
We have removed some secondary outcomes from the methods section in the most recent version of this review, as recent versions or the current version did not address them. These include:
knowledge, attitudes, and beliefs of carers about effects of passive smoking or environmental tobacco smoke (ETS) on self or children;
participants' views of the intervention;
measures of anxiety, depression, guilt, stress/locus of control, health, and well‐being/health‐related quality of life; and
measures of family functioning.
Contributions of authors
BB co‐ordinated the current review update, extracted data, and wrote and edited the current review update.
MS co‐ordinated and co‐wrote the previous update, extracted data, and edited the current review update.
RB co‐ordinated and co‐writing the previous update and extracting data and editing the current review update.
RR co‐ordinated the original review, wrote the original review, extracted data for the original review and for review updates, and edited review updates.
PW co‐ordinated the current review update, developed the original review and previous updates, extracted data for the original review and for the first update, and edited the original review and review updates.
Sources of support
Internal sources
The McCaughey Centre, Melbourne School of Population Health, University of Melbourne, Australia.
External sources
National Health & Medical Research Council, Australia.
Murdoch Children's Research Institute, Australia.
VicHealth (Victorian Health Promotion Foundation), Australia.
Declarations of interest
BB has no known conflicts of interest.
MS has no known conflicts of interest.
RB has no known conflicts of interest.
RR is a respiratory paediatrician in public and private practice. Many patients he sees have exposure to tobacco smoke. However he does not consider this a conflict of interest.
PW has no known conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abdullah 2005 {published data only}
- Abdullah ASM, Lam TH, Mak YW, Loke AY. A randomized control trial of a smoking cessation intervention on parents of young children ‐ a preliminary report (POS2‐011). Society for Research on Nicotine and Tobacco 10th Annual Meeting, 2005 February 18‐21; Phoenix, AZ. 2005:65.
- Abdullah ASM, Mak YW, Loke AY, Lam TH. Smoking cessation intervention in parents of young children: a randomised controlled trial. Addiction 2005;100(11):1731‐40. [DOI] [PubMed] [Google Scholar]
Abdullah 2015 {published data only}
- Abdullah AS, Hua F, Khan H, Xia X, Bing Q, Tarang K, et al. Secondhand smoke exposure reduction intervention in Chinese households of young children: a randomized controlled trial. Academy of Pediatrics 2015;15(6):588‐98. [DOI] [PubMed] [Google Scholar]
Armstrong 2000 {published data only}
- Armstrong KL, Fraser JA, Dadds MR, Morris J. A randomized, controlled trial of nurse home visiting to vulnerable families with newborns. Journal of Paediatrics and Child Health 1999;35(3):237‐44. [DOI] [PubMed] [Google Scholar]
- Armstrong KL, Fraser JA, Dadds MR, Morris J. Promoting secure attachment, maternal mood and child health in a vulnerable population: a randomized controlled trial. Journal of Paediatrics and Child Health 2000;36(6):555‐62. [DOI] [PubMed] [Google Scholar]
- Fraser JA, Armstrong KL, Morris JP, Dadds MR. Home visiting intervention for vulnerable families with newborns: follow‐up results of a randomized controlled trial. Child Abuse and Neglect 2000;24(11):1399‐429. [DOI] [PubMed] [Google Scholar]
Baheiraei 2011 {published data only}
- Baheiraei A, Kharaghani R, Mohsenifar A, Kazemnejad A, Alikhani S, Milani HS, et al. Reduction of secondhand smoke exposure among healthy infants in Iran: randomized controlled trial. Nicotine & Tobacco Research 2011;13(9):840‐7. [DOI] [PubMed] [Google Scholar]
Blaakman 2015 {published data only}
- Blaakman SW, Borrelli B, Wiesenthal EN, Fagnano M, Tremblay PJ, Stevens TP, et al. Secondhand smoke exposure reduction after NICU discharge: results of a randomized trial. Academy of Pediatrics 2015;15(6):605‐12. [DOI] [PubMed] [Google Scholar]
Borrelli 2010 {published data only}
- Borrelli B, McQuaid EL, Novak SP, Hammond SK, Becker B. Motivating Latino caregivers of children with asthma to quit smoking: a randomized trial. Journal of Consulting & Clinical Psychology 2010;78(1):34‐43. [DOI] [PubMed] [Google Scholar]
Borrelli 2016 {published data only}
- Borrelli B, McQuaid EL, Tooley EM, Busch AM, Hammond S, Becker B, et al. Motivating parents of kids with asthma to quit smoking: the effect of the teachable moment and increasing intervention intensity using a longitudinal randomized trial design. Addiction 2016;111(9):1646‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butz 2011 {published data only}
- Butz AM, Matsui EC, Breysse P, Curtin‐Brosnan J, Eggleston P, Diette G, et al. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner‐city children with asthma and secondhand smoke exposure. [Erratum appears in Arch Pediatr Adolesc Med 2011;165(9):791]. Archives of Pediatrics & Adolescent Medicine 2011;165(8):741‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Kim JS. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner‐city children with asthma and secondhand smoke exposure. Pediatrics 2012;130(Suppl 1):S33‐4. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2005 {published data only}
- Chan SS, Lam TH, Salili F, Leung GM, Wong DC, Botelho RJ, et al. A randomized controlled trial of an individualized motivational intervention on smoking cessation for parents of sick children: a pilot study. Applied Nursing Research 2005;18(3):178‐81. [DOI] [PubMed] [Google Scholar]
Chan 2006a {published data only}
- Chan S, Lam TH. Preventing exposure to second‐hand smoke. Seminars in Oncology Nursing 2003;19(4):284‐90. [DOI] [PubMed] [Google Scholar]
- Chan S, Lam TH. Protecting sick children from exposure to passive smoking through mothers' actions: a randomized controlled trial of a nursing intervention. Journal of Advanced Nursing 2006;54(4):440‐9. [DOI] [PubMed] [Google Scholar]
- Chan SC, Lam TH, Tudor‐Smith C. Working together for better health in children: the effectiveness of a health education intervention provided by nurses. Tackling Tobacco. Cardiff: Hybu lechyd Cymru Health Promotion Wales, 1999:39‐46. [Google Scholar]
- Chan SS, Leung GML, Wong DCN, Lam TH. Helping Chinese fathers quit smoking through educating their non‐smoking spouses: a randomized controlled trial. American Journal of Health Promotion 2008;23:31‐4. [DOI] [PubMed] [Google Scholar]
- Chan SS, Wong DC, Lam TH. Will mothers of sick children help their husbands to stop smoking after receiving a brief intervention from nurses? Secondary analysis of a randomised controlled trial. BMC Pediatrics 2013;13:50. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chellini 2013 {published data only}
- Chellini E, Gorini G, Carreras G, Da noi non si fuma Study Group. The "Don't smoke in our home" randomized controlled trial to protect children from second‐hand smoke exposure at home. Tumori 2013;99(1):23‐9. [] [DOI] [PubMed] [Google Scholar]
Chen 2016 {published data only}
- Chen YT, Hsiao FH, Lee CM, Wang RH, Chen PL. Effects of a parent‐child interactive program for families on reducing the exposure of school‐aged children to household smoking. Nicotine & Tobacco Research 2016;18(3):330‐40. [DOI] [PubMed] [Google Scholar]
Chilmonczyk 1992 {published data only}
- Chilmonczyk BA, Palomaki GE, Knight GJ, Williams J, Haddow JE. An unsuccessful cotinine‐assisted intervention strategy to reduce environmental tobacco smoke exposure during infancy. American Journal of Diseases of Children 1992;146(3):357‐60. [DOI] [PubMed] [Google Scholar]
Collins 2015 {published data only}
- Collins BN, Nair US, Hovell MF, DiSantis KI, Jaffe K, Tolley NM, et al. Reducing underserved children's exposure to tobacco smoke: a randomized counseling trial with maternal smokers. American Journal of Preventive Medicine 2015;49(4):534‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conway 2004 {published data only}
- Conway TL, Woodruff SI, Edwards CC, Hovell MF, Klein J. Intervention to reduce environmental tobacco smoke exposure in Latino children: null effects on hair biomarkers and parent reports. Tobacco Control 2004;13(1):90‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2014 {published data only}
- Cooper S, Lewis S, Thornton JG, Marlow N, Watts K, Britton J, et al. The SNAP trial: a randomised placebo‐controlled trial of nicotine replacement therapy in pregnancy ‐ clinical effectiveness and safety until 2 years after delivery, with economic evaluation. Health Technology Assessment 2014;18(54):1‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Culp 2007 {published data only}
- Culp AM, Culp RE, Anderson JW, Carter S. Health and safety intervention with first‐time mothers. Health Education Research 2007;22(2):285‐94. [DOI] [PubMed] [Google Scholar]
Curry 2003 {published data only}
- Curry SJ, Ludman EJ, Graham E, Stout J, Grothaus L, Lozano P. Pediatric‐based smoking cessation intervention for low‐income women: a randomized trial. Archives of Pediatrics and Adolescent Medicine 2003;157(3):295‐302. [DOI] [PubMed] [Google Scholar]
Daly 2016 {published data only}
- Daly JB, Freund M, Burrows S, Considine R, Bowman JA, Wiggers JH. A cluster randomised controlled trial of a brief child health nurse intervention to reduce infant secondhand smoke exposure. Maternal and Child Health Journal 2017;21(1):108‐17. [DOI] [PubMed] [Google Scholar]
Davis 1992 {published data only}
- Davis SW, Cummings KM, Rimer BK, Sciandra R, Stone JC. The impact of tailored self‐help smoking cessation guides on young mothers. Health Education Quarterly 1992;19(4):495‐504. [DOI] [PubMed] [Google Scholar]
Eakin 2014 {published data only}
- Eakin MN, Rand CS, Borrelli B, Bilderback A, Hovell M, Riekert KA. Effectiveness of motivational interviewing to reduce Head Start children's secondhand smoke exposure: a randomized clinical trial. American Journal of Respiratory and Critical Care Medicine 2014;189(12):1530‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekerbicer 2007 {published data only}
- Ekerbicer HC, Celik M, Guler E, Davutoglu M, Kilinc M. Evaluating environmental tobacco smoke exposure in a group of Turkish primary school students and developing intervention methods for prevention. BMC Public Health 2007;7:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elder 1996 {published data only}
- Elder JP, Perry CL, Stone EJ, Johnson CC, Yang M, Edmundson EW, et al. Tobacco use measurement, prediction, and intervention in elementary schools in four states: the CATCH study. Preventive Medicine 1996;25(4):486‐94. [DOI] [PubMed] [Google Scholar]
Emmons 2001 {published data only}
- Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low‐income households with young children. Pediatrics 2001;108(1):18‐24. [DOI] [PubMed] [Google Scholar]
- Emmons KM, Wong M, Hammond SK, Velicer WF, Fava JL, Monroe AD, et al. Intervention and policy issues related to children's exposure to environmental tobacco smoke. Preventive Medicine 2001;32:321‐31. [DOI] [PubMed] [Google Scholar]
Eriksen 1996 {published data only}
- Eriksen W, Sorum K, Bruusgaard D. Effects of information on smoking behaviour in families with preschool children. Acta Paediatrica 1996;85(2):209‐12. [DOI] [PubMed] [Google Scholar]
Fossum 2004 {published data only}
- Fossum B, Arborelius E, Bremberg S. Evaluation of a counseling method for the prevention of child exposure to tobacco smoke: an example of client‐centered communication. Preventive Medicine 2004;38(3):295‐301. [DOI] [PubMed] [Google Scholar]
French 2007 {published data only}
- French GM, Groner JA, Wewers ME, Ahijevych K. Staying smoke free: an intervention to prevent postpartum relapse. Nicotine & Tobacco Research 2007;9(6):663‐70. [DOI] [PubMed] [Google Scholar]
Greenberg 1994 {published data only}
- Greenberg RA, Strecher VJ, Bauman KE, Boat BW, Fowler MG, Keyes LL, et al. Evaluation of a home‐based intervention program to reduce infant passive smoking and lower respiratory illness. Journal of Behavioral Medicine 1994;17(3):273‐90. [DOI] [PubMed] [Google Scholar]
- Margolis PA, Keyes LL, Greenberg RA, Bauman KE, LaVange LM. Urinary cotinine and parent history (questionnaire) as indicators of passive smoking and predictors of lower respiratory illness in infants. Pediatric Pulmonology 1997;23(6):417‐23. [DOI] [PubMed] [Google Scholar]
- Strecher VJ, Bauman KE, Boat B, Fowler MG, Greenberg R, Stedman H. The role of outcome and efficacy expectations in an intervention designed to reduce infants' exposure to environmental tobacco smoke. Health Education Research 1993;8:137‐43. [DOI] [PubMed] [Google Scholar]
Groner 2000 {published data only}
- Groner JA, Ahijevych K, Grossman LK, Rich LN. The impact of a brief intervention on maternal smoking behavior. Pediatrics 2000;105(1 Pt 3):267‐71. [PubMed] [Google Scholar]
Hafkamp‐de 2014 {published data only}
- Hafkamp‐de Groen E, Valk RJ, Mohangoo AD, Wouden JC, Duijts L, Jaddoe VW, et al. Evaluation of systematic assessment of asthma‐like symptoms and tobacco smoke exposure in early childhood by well‐child professionals: a randomised trial. PLoS One 2014;9(3):e90982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halterman 2011 {published data only}
- Blaakman S, Tremblay PJ, Halterman JS, Fagnano M, Borrelli B. Implementation of a community‐based secondhand smoke reduction intervention for caregivers of urban children with asthma: process evaluation, successes and challenges. Health Education Research 2013;28(1):141‐52. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halterman JS, Borrelli B, Conn KM, Tremblay P, Blaakman S. Motivation to quit smoking among parents of urban children with asthma. Patient Education and Counseling 2010;79(2):152‐5. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halterman JS, Borrelli B, Fisher S, Szilagyi P, Yoos L. Improving care for urban children with asthma: design and methods of the School‐Based Asthma Therapy (SBAT) trial. Journal of Asthma 2008;45(4):279‐86. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halterman JS, Szilagyi PG, Fisher SG, Fagnano M, Tremblay P, Conn KM, et al. Randomized controlled trial to improve care for urban children with asthma: results of the School‐Based Asthma Therapy trial. Archives of Pediatrics & Adolescent Medicine 2011;165(3):262‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hannover 2009 {published data only}
- Hannover W, Thyrian JR, Roske K, Grempler J, Rumpf HJ, John U, et al. Smoking cessation and relapse prevention for postpartum women: results from a randomized controlled trial at 6, 12, 18 and 24 months. Addictive Behaviors 2009;34(1):1‐8. [DOI] [PubMed] [Google Scholar]
- Roske K, Hannover W, Thyrian JR, John U, Hannich HJ. Smoking cessation counselling for pregnant and postpartum women among midwives, gynaecologists and paediatricians in Germany. International Journal of Environmental Research and Public Health 2009;6(1):96‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyrian JR, Hannover W, Grempler J, Roske K, John U, Hapke U. An intervention to support postpartum women to quit smoking or remain smoke‐free. Journal of Midwifery & Women's Health 2006;51(1):45‐50. [DOI] [PubMed] [Google Scholar]
Harutyunyan 2013 {published data only}
- Harutyunyan A, Movsisyan N, Petrosyan V, Petrosyan D, Stillman F. Reducing children's exposure to secondhand smoke at home: a randomized trial. Pediatrics 2013;132(6):1071‐80. [DOI] [PubMed] [Google Scholar]
Herbert 2011 {published data only}
- Herbert RJ, Gagnon AJ, O'Loughlin JL, Rennick JE. Testing an empowerment intervention to help parents make homes smoke‐free: a randomized controlled trial. Journal of Community Health 2011;36(4):650‐7. [] [DOI] [PubMed] [Google Scholar]
Hovell 2000 {published data only}
- Hovell MF, Zakarian JM, Matt GE, Hofstetter CR, Bernert JT, Pirkle J. Decreasing environmental tobacco smoke exposure among low income children: preliminary findings. Tobacco Control 2000;9 Suppl 3:70‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovell MF, Zakarian JM, Matt GE, Hofstetter CR, Bernert JT, Pirkle J. Effect of counselling mothers on their children's exposure to environmental tobacco smoke: randomised controlled trial. BMJ 2000;321(7257):337‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hovell 2002 {published data only}
- Hovell MF, Meltzer SB, Wahlgren DR, Matt GE, Hofstetter CR, Jones JA, et al. Asthma management and environmental tobacco smoke exposure reduction in Latino children: a controlled trial. Pediatrics 2002;110(5):946‐56. [DOI] [PubMed] [Google Scholar]
Hovell 2009 {published data only}
- Hovell MF, Zakarian JM, Matt GE, Liles S, Jones JA, Hofstetter CR, et al. Counseling to reduce children's secondhand smoke exposure and help parents quit smoking: a controlled trial. Nicotine & Tobacco Research 2009;11(12):1383‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liles S, Hovell MF, Matt GE, Zakarian JM, Jones JA. Parent quit attempts after counseling to reduce children's secondhand smoke exposure and promote cessation: main and moderating relationships. Nicotine & Tobacco Research 2009;11(12):1395‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 1991 {published data only}
- Hughes DM, McLoed M, Garner B, Goldbloom RB. Controlled trial of a home and ambulatory program for asthmatic children. Pediatrics 1991;87(1):54‐61. [PubMed] [Google Scholar]
Irvine 1999 {published data only}
- Irvine L, Crombie IK, Clark RA, Slane PW, Feyerabend C, Goodman KE, et al. Advising parents of asthmatic children on passive smoking: randomised controlled trial. BMJ 1999;318(7196):1456‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine L, Crombie IK, Clark RA, Slane PW, Goodman KE, Feyerabend C, et al. What determines levels of passive smoking in children with asthma?. Thorax 1997;52:766‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph 2014 {published data only}
- Joseph A, Murphy S, Thomas J, Okuyemi KS, Hatsukami D, Wang Q, et al. A pilot study of concurrent lead and cotinine screening for childhood tobacco smoke exposure: effect on parental smoking. American Journal of Health Promotion 2014;28(5):316‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kallio 2006 {published data only}
- Kallio K, Jokinen E, Hamalainen M, Kaitosaari T, Volanen I, Viikari J, et al. Impact of repeated lifestyle counselling in an atherosclerosis prevention trial on parental smoking and children's exposure to tobacco smoke. Acta Paediatrica 2006;95(3):283‐90. [DOI] [PubMed] [Google Scholar]
Kegler 2015 {published data only}
- Kegler MC, Bundy L, Haardorfer R, Escoffery C, Berg C, Yembra D, et al. A minimal intervention to promote smoke‐free homes among 2‐1‐1 callers: a randomized controlled trial. American Journal of Public Health 2015;105(3):530‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kimata 2004 {published data only}
- Kimata H. Cessation of passive smoking reduces allergic responses and plasma neurotrophin. European Journal of Clinical Investigation 2004;34(2):165‐6. [DOI] [PubMed] [Google Scholar]
Krieger 2005 {published data only}
- Krieger JK, Takaro TK, Allen C, Song L, Weaver M, Chai S, et al. The Seattle‐King County healthy homes project: implementation of a comprehensive approach to improving indoor environmental quality for low‐income children with asthma. Environmental Health Perspectives 2002;110 Suppl 2:311‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger JW, Song L, Takaro TK, Stout J. Asthma and the home environment of low‐income urban children: preliminary findings from the Seattle‐King County healthy homes project. Journal of Urban Health‐Bulletin of the New York Academy of Medicine 2000;77:50‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger JW, Takaro TK, Song L, Weaver M. The Seattle‐King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. American Journal of Public Health 2005;95(4):652‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
McIntosh 1994 {published data only}
- McIntosh NA, Clark NM, Howatt WF. Reducing tobacco smoke in the environment of the child with asthma: a cotinine‐assisted, minimal‐contact intervention. Journal of Asthma 1994;31(6):453‐62. [DOI] [PubMed] [Google Scholar]
Nicholson 2015 {published data only}
- Nicholson JS, McDermott MJ, Huang Q, Zhang H, Tyc VL. Full and home smoking ban adoption after a randomized controlled trial targeting secondhand smoke exposure reduction. Nicotine & Tobacco Research 2015;17(5):612‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuesslein 2006 {published data only}
- Nuesslein TG, Struwe A, Maiwald N, Rieger C, Stephan V. [Maternal tobacco consumption can be reduced by simple intervention of the paediatrician]. [German]. Klinische Padiatrie 2006;218(5):283‐6. [DOI] [PubMed] [Google Scholar]
Ortega 2015 {published data only}
- Ortega Cuelva G, Cabezas Pena C, Almeda Ortega J, Saez Zafra M, Ballve Moreno JL, Pascual Esteban JA, et al. Effectiveness of a brief primary care intervention to reduce passive smoking in babies: a cluster randomised clinical trial. Journal of Epidemiology and Community Health 2015;69(3):249‐60. [DOI] [PubMed] [Google Scholar]
Patel 2012 {published data only}
- Patel S, Hendry P, Kalynych C, Butterfield R, Lott M, Lukens‐Bull K. The impact of third‐hand smoke education in a pediatric emergency department on caregiver smoking policies and quit status: a pilot study. International Journal on Disability and Human Development 2012;11(4):335‐42. [] [Google Scholar]
- Patel S, Hendry P, Kalynych C, Butterfield R, Lott M, Lukens‐Bull K. The impact of thirdhand smoke education in a pediatric emergency department on caregiver smoking. International Journal on Disability and Human Development 2013:41‐51. [Google Scholar]
Phillips 2012 {published data only}
- Phillips RM, Merritt TA, Goldstein MR, Deming DD, Slater LE, Angeles DM. Prevention of postpartum smoking relapse in mothers of infants in the neonatal intensive care unit. Journal of Perinatology 2012;32(5):374‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pollak 2015 {published data only}
- Pollak KI, Lyna P, Bilheimer AK, Gordon KC, Peterson BL, Gao X, et al. Efficacy of a couple‐based randomized controlled trial to help Latino fathers quit smoking during pregnancy and postpartum: the Parejas trial. Cancer Epidemiology, Biomarkers & Prevention 2015;24(2):379‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prokhorov 2013 {published data only}
- Prokhorov AV, Hudmon KS, Marani SK, Bondy ML, Gatus LA, Spitz MR, et al. Eliminating second‐hand smoke from Mexican‐American households: outcomes from Project Clean Air‐Safe Air (CASA). Addictive Behaviors 2013;38(1):1485‐92. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pulley 2002 {published data only}
- Pulley KR, Flanders‐Stepans M. Smoking hygiene: an educational intervention to reduce respiratory symptoms in breastfeeding infants exposed to tobacco. Journal of Perinatal Education 2002;11(3):28‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ralston 2008 {published data only}
- Ralston S, Roohi M. A randomized, controlled trial of smoking cessation counseling provided during child hospitalization for respiratory illness. Pediatric Pulmonology 2008;43(6):561‐6. [DOI] [PubMed] [Google Scholar]
Ralston 2013 {published data only}
- Ralston S, Grohman C, Word D, Williams J. A randomized trial of a brief intervention to promote smoking cessation for parents during child hospitalization. Pediatric Pulmonology 2013;48:608‐13. [DOI] [PubMed] [Google Scholar]
Ratner 2001 {published data only}
- Johnson JL, Ratner PA, Bottorff JL, Hall W, Dahinten S. Preventing smoking relapse in postpartum women. Nursing Research 2000;49(1):44‐52. [DOI] [PubMed] [Google Scholar]
- Ratner PA, Johnson JL, Bottorff JL. Mothers' efforts to protect their infants from environmental tobacco smoke. Canadian Journal of Public Health‐Revue Canadienne De Sante Publique 2001;92(1):46‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner PA, Johnson JL, Bottorff JL. Smoking during pregnancy: how well are we doing in encouraging women to quit?. BC Medical Journal 1997;39(9):492‐5. [Google Scholar]
- Ratner PA, Johnson JL, Bottorff JL. Smoking relapse and early weaning among postpartum women: is there an association?. Birth 1999;26(2):76‐82. [DOI] [PubMed] [Google Scholar]
- Ratner PA, Johnson JL, Bottorff JL, Dahinten S, Hall W. Twelve‐month follow‐up of a smoking relapse prevention intervention for postpartum women. Addictive Behaviors 2000;25(1):81‐92. [DOI] [PubMed] [Google Scholar]
Schonberger 2005 {published data only}
- Kuiper S, Maas T, Schayck CP, Muris JW, Schonberger HJ, Dompeling E, et al. The primary prevention of asthma in children study: design of a multifaceted prevention program. Pediatric Allergy and Immunology 2005;16(4):321‐31. [DOI] [PubMed] [Google Scholar]
- Maas T, Dompeling E, Muris JW, Wesseling G, Knottnerus JA, Schayck OC. Prevention of asthma in genetically susceptible children: a multifaceted intervention trial focussed on feasibility in general practice. Pediatric Allergy and Immunology 2011;22(8):794‐802. [] [DOI] [PubMed] [Google Scholar]
- Maas T, Schonberger HJ, Dompeling ED, Muris JW, Knottnerus JA, Schayck CP. Effectiveness of the PREVASC‐intervention on reducing the allergen and environmental tobacco smoke exposure [abstract]. European Respiratory Society Annual Congress 2002; 14‐18 September 2002; Stockholm, Sweden. Abstract No. P3261.
- Schonberger HJ, Dompeling E, Knottnerus JA, Maas T, Muris JW, Weel C, et al. The PREVASC study: the clinical effect of a multifaceted educational intervention to prevent childhood asthma. European Respiratory Journal 2005;25(4):660‐70. [DOI] [PubMed] [Google Scholar]
- Schonberger HJ, Maas T, Dompeling E, Knottnerus JA, Weel C, Schayck CP. Compliance of asthmatic families with a primary prevention programme of asthma and effectiveness of measures to reduce inhalant allergens ‐ a randomized trial. Clinical and Experimental Allergy 2004;34(7):1024‐31. [DOI] [PubMed] [Google Scholar]
Schuck 2014 {published data only}
- Schuck K, Bricker JB, Otten R, Kleinjan M, Brandon TH, Engels RC. Effectiveness of proactive quitline counselling for smoking parents recruited through primary schools: results of a randomized controlled trial. Addiction 2014;109(5):830‐41. [DOI] [PubMed] [Google Scholar]
Severson 1997 {published data only}
- Severson HH, Andrews JA, Lichtenstein E, Wall M, Akers L. Reducing maternal smoking and relapse: long‐term evaluation of a pediatric intervention. Preventive Medicine 1997;26(1):120‐30. [DOI] [PubMed] [Google Scholar]
- Wall MA, Severson HH, Andrews JA, Lichtenstein E, Zoref L. Pediatric office‐based smoking intervention: impact on maternal smoking and relapse. Pediatrics 1995;96(4):622‐8. [PubMed] [Google Scholar]
Stotts 2012 {published data only}
- NCT00670280. Reducing environmental tobacco smoke in NICU infants' homes. http://clinicaltrials.gov/show/NCT00670280, 2008. [] [Google Scholar]
- Northrup T, Green C, Evans PW, Stotts AL. Predicting attrition in a secondhand smoke intervention study with families in a neonatal intensive care unit (POS3‐102). Society for Research on Nicotine and Tobacco 18th Annual Meeting, 13‐16 March 2012; Houston, TX. 2012:120. [] [Google Scholar]
- Stotts AL, Green C, Northrup TF, Dodrill CL, Evans P, Tyson J, et al. Feasibility and efficacy of an intervention to reduce secondhand smoke exposure among infants discharged from a neonatal intensive care unit. Journal of Perinatology 2013;33(10):811‐6. [DOI] [PubMed] [Google Scholar]
- Stotts AL, Northrup TF, Green C, Evans PW, Tyson J, Hovell MF. The Baby's Breath project: a pilot trial to reduce secondhand smoke exposure in high respiratory risk infants in the neonatal intensive care unit (POS1‐69). Society for Research on Nicotine and Tobacco 18th Annual Meeting, 13‐16 March 2012; Houston, TX 2012:60. [] [Google Scholar]
Streja 2014 {published data only}
- Streja L, Crespi CM, Bastani R, Wong GC, Jones CA, Bernert JT, et al. Can a minimal intervention reduce secondhand smoke exposure among children with asthma from low income minority families? Results of a randomized trial. Journal of Immigrant and Minority Health 2014;16(2):256‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tyc 2013 {published data only}
- Klosky JL, Tyc VL, Lawford J, Ashford J, Lensing S, Buscemi J. Predictors of non‐participation in a randomized intervention trial to reduce environmental tobacco smoke (ETS) exposure in pediatric cancer patients. Pediatric Blood & Cancer 2009;52(5):644‐9. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyc VL, Huang Q, Nicholson J, Schultz B, Hovell MF, Lensing S, et al. Intervention to reduce secondhand smoke exposure among children with cancer: a controlled trial. Psycho‐oncology 2013;22(5):1104‐11. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyc VL, Puleo E, Emmons K, Moor JS, Ford JS. Smoking restrictions among households of childhood and young adult cancer survivors: implications for tobacco control efforts. Journal of Adolescent and Young Adult Oncology 2013;2(1):17‐24. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ulbricht 2014 {published data only}
- Ulbricht S, Gross S, Meyer C, Hannover W, Nauck M, John U. Reducing tobacco smoke exposure in children aged below 4 years ‐ a randomized controlled trial. Preventive Medicine 2014;69:208‐13. [DOI] [PubMed] [Google Scholar]
Van't Hof 2000 {published data only}
- Van't Hof SM, Wall MA, Dowler DW, Stark MJ. Randomised controlled trial of a postpartum relapse prevention intervention. Tobacco Control 2000;9 Suppl 3:III64‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vineis 1993 {published data only}
- Vineis P, Ronco G, Ciccone G, Vernero E, Troia B, D'Incalci T, et al. Prevention of exposure of young children to parental tobacco smoke: effectiveness of an educational program. Tumori 1993;79(3):183‐6. [DOI] [PubMed] [Google Scholar]
Wahlgren 1997 {published data only}
- Hovell MF, Meltzer MPH, Zakarian JM, Wahlgren DR, Emerson JA, Hofstetter CR, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: a controlled trial. Chest 1994;106(2):440‐6. [DOI] [PubMed] [Google Scholar]
- Wahlgren DR, Hovell MF, Meltzer SB, Hofstetter CR, Zakarian JM. Reduction of environmental tobacco smoke exposure in asthmatic children. A 2‐year follow‐up. Chest 1997;111(1):81‐8. [DOI] [PubMed] [Google Scholar]
Wakefield 2002 {published data only}
- Wakefield M, Banham D, McCaul K, Martin J, Ruffin R, Badcock N, et al. Effect of feedback regarding urinary cotinine and brief tailored advice on home smoking restrictions among low‐income parents of children with asthma: a controlled trial. Preventive Medicine 2002;34(1):58‐65. [DOI] [PubMed] [Google Scholar]
Walker 2015 {published data only}
- Walker N, Johnston V, Glover M, Bullen C, Trenholme A, Chang A, et al. Effect of a family‐centered, secondhand smoke intervention to reduce respiratory illness in indigenous infants in Australia and New Zealand: a randomized controlled trial. Nicotine & Tobacco Research 2015;17(1):48‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2015 {published data only}
- Wang Y, Huang Z, Yang M, Wang F, Xiao S. Reducing environmental tobacco smoke exposure of preschool children: a randomized controlled trial of class‐based health education and smoking cessation counseling for caregivers. International Journal of Environmental Research and Public Health 2015;12(1):692‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiggins 2005 {published data only}
- Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al. Postnatal support for mothers living in disadvantaged inner city areas: a randomised controlled trial. Journal of Epidemiology and Community Health 2005; Vol. 59, issue 4:288‐95. [DOI] [PMC free article] [PubMed]
- Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al. The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner‐city areas. Health Technology Assessment 2004; Vol. 8, issue 32:1‐120. [DOI] [PubMed]
Wilson 2001 {published data only}
- Wilson SR, Yamada EG, Sudhakar R, Roberto L, Mannino D, Mejia CM, et al. A controlled trial of an environmental tobacco smoke reduction Intervention in low‐income children with asthma. Chest 2001;120(5):1709‐22. [DOI] [PubMed] [Google Scholar]
Wilson 2011 {published data only}
- Wilson SR, Farber HJ, Knowles SB, Lavori PW. A randomized trial of parental behavioral counseling and cotinine feedback for lowering environmental tobacco smoke exposure in children with asthma: results of the LET'S Manage Asthma trial. Chest 2011;139(3):581‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winickoff 2010 {published and unpublished data}
- Winickoff J, Healey E, Regan S, Park E, Cole C, Rigotti N. Addressing parental smoking during the postpartum hospitalization. Society for Research on Nicotine and Tobacco 13th Annual Meeting, 21‐24 February 2007;. Austin, TX.
- Winickoff J, Healey E, Regan S, Park E, Friebely J, Rigotti N. The NEWS trial: using the postpartum hospitalization to address parental smoking. Unpublished manuscript.
- Winickoff JP, Healey EA, Regan S, Park ER, Cole C, Friebely J, et al. Using the postpartum hospital stay to address mothers' and fathers' smoking: the NEWS study. Pediatrics 2010;125(3):518‐25. [DOI] [PubMed] [Google Scholar]
Woodward 1987 {published data only}
- Woodward A, Owen N, Grgurinovich N, Griffith F, Linke H. Trial of an intervention to reduce passive smoking in infancy. Pediatric Pulmonology 1987;3(3):173‐8. [DOI] [PubMed] [Google Scholar]
Yilmaz 2006 {published data only}
- Yilmaz G, Karacan C, Yoney A, Yilmaz T. Brief intervention on maternal smoking: a randomized controlled trial. Child: Care, Health and Development 2006;32(1):73‐9. [DOI] [PubMed] [Google Scholar]
Yucel 2014 {published data only}
- Yucel U, Ocek ZA, Ciceklioglu M. Evaluation of an intensive intervention programme to protect children aged 1‐5 years from environmental tobacco smoke exposure at home in Turkey. Health Education Research 2014;29(3):442‐55. [DOI] [PubMed] [Google Scholar]
Zakarian 2004 {published data only}
- Jones JA, Hovell MF, Zakarian JM, Liles ST, Yap BS. Healthy tots intervention to reduce children's passive smoke exposure and to help mothers quit smoking ‐ program endorsed by mothers. Society for Research on Nicotine and Tobacco 10th Annual Meeting, 18‐21 February 2004; Phoenix, AZ. 2004:65.
- Jones JA, Zakarian JM, Liles ST, Hovell MF. Behavioral counseling intervention for passive smoking and smoking cessation with low‐income families with young children (POS1‐32). Society for Research on Nicotine and Tobacco 12th Annual Meeting; 15‐18 February 2006; Orlando, FL. 2006:49.
- Zakarian JM, Hovell MF, Sandweiss RD, Hofstetter CR, Matt GE, Bernert JT, et al. Behavioral counseling for reducing children's ETS exposure: implementation in community clinics. Nicotine & Tobacco Research 2004;6(6):1061‐74. [DOI] [PubMed] [Google Scholar]
Zhang 1993 {published data only}
- Zhang D, Qiu X. School‐based tobacco‐use prevention ‐ People's Republic of China, May 1989‐January 1990. JAMA 1993;269:2972. [PubMed] [Google Scholar]
- Zhang D, Qiu X. School‐based tobacco‐use prevention ‐ People's Republic of China, May 1989‐January 1990. MMWR 1993;42(19):370‐1, 377. [PubMed] [Google Scholar]
References to studies excluded from this review
Arborelius 2001 {published data only}
- Arborelius E, Bremberg S. Child health‐centre‐based promotion of a tobacco‐free environment ‐ a Swedish case study. Health Promotion International 2001;16(3):245‐54. [DOI] [PubMed] [Google Scholar]
Bacewicz 2015 {published data only}
- Bacewicz A, Wang W, Ashouri J, Mallah MK. Children with chronic lung disease: facilitating smoking cessation for their caregivers. Journal of Community Health 2015;40(3):409‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Badger 2003 {published data only}
- Badger A, Goldsmith M, Cullen D. Effects of secondhand tobacco education in Head‐Start parents [Abstract]. Respiratory Care 2003;48(11):1124. [Google Scholar]
Burmaz 2007 {published data only}
- Burmaz T, Villani M, Cattaneo A, Milinco M, Romero SQ, Bernal R. Compliance to preventive interventions in infancy among immigrants: a randomised trial. Quaderni ACP 2007;14(2):50‐5. [Google Scholar]
Campion 1994 {published data only}
- Campion P, Owen L, McNeill A, McGuire C. Evaluation of a mass media campaign on smoking and pregnancy. Addiction 1994;89(10):1245‐54. [DOI] [PubMed] [Google Scholar]
Carlsson 2013 {published data only}
- Carlsson N, Johansson A, Abrahamsson A, Gare BA. How to minimize children's environmental tobacco smoke exposure: an intervention in a clinical setting in high risk areas. BMC Pediatrics 2013;13:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamberlain 2013 {published data only}
- Chamberlain C, O'Mara‐Eves A, Oliver S, Caird JR, Perlen SM, Eades SJ, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2013, (10):CD001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cookson 2000 {published data only}
- Cookson S, Heath A, Bertrand L. The HeartSmart Family Fun Pack: an evaluation of family‐based intervention for cardiovascular risk reduction in children. Canadian Journal of Public Health [Revue Canadienne de Sante Publique] 2000;91(4):256‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eakin 2013 {published data only}
- Eakin MN, Bilderback A, Borrelli B, Hovell M, Welkom J, Hilliard ME, et al. Effectiveness of motivational interviewing to reduce head start children's secondhand smoke exposure. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emmons 2000 {published data only}
- Emmons KM, Sorensen G, Klar N, Digianni L, Barclay G, Schmidt K, et al. Healthy baby second‐hand smoke study: project brief. Tobacco Control 2000;9(Suppl 3):iii58‐iii60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gadomski 2011 {published data only}
- Gadomski A, Adams L, Tallman N, Krupa N, Jenkins P. Effectiveness of a combined prenatal and postpartum smoking cessation program. Maternal & Child Health Journal 2011;15(2):188‐97. [DOI] [PubMed] [Google Scholar]
Halterman 2011a {published data only}
- Halterman JS, Fagnano M, Isensee C, Tremblay P, Blaakman S, Borrelli B. Preliminary results of an asthma education and secondhand smoke reduction program to prevent respiratory illness among premature infants. Conference presentation 2011. [Google Scholar]
Hovell 2011 {published data only}
- Hovell MF, Wahlgren DR, Liles S, Jones JA, Hughes SC, Matt GE, et al. Providing coaching and cotinine results to preteens to reduce their secondhand smoke exposure: a randomized trial. Chest 2011;140(3):681‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2013 {published data only}
- Huang CM, Wu HL, Huang SH, Chien LY, Guo JL. Transtheoretical model‐based passive smoking prevention programme among pregnant women and mothers of young children. European Journal of Public Health 2013;23(5):777‐82. [DOI] [PubMed] [Google Scholar]
Hutchinson 2014 {published data only}
- Hutchinson S, Breukelen G, Schayck O, Essers B, Muris J, Feron F, et al. A randomised trial to stop passive smoking in children with a high risk of asthma [Abstract]. European Respiratory Journal 2014;44(Suppl 58):1404. [Google Scholar]
Kegler 2012 {published data only}
- Kegler MC, Escoffery C, Bundy L, Berg CJ, Haardorfer R, Yembra D, et al. Pilot study results from a brief intervention to create smoke‐free homes. Journal of Environmental and Public Health 2012;2012:951426. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klinnert 2007 {published data only}
- Klinnert MD, Liu AH, Pearson MR, Ellison MC, Budhiraja N, Robinson JL. Short‐term impact of a randomized multifaceted intervention for wheezing infants in low‐income families. Archives of Pediatrics & Adolescent Medicine 2005;159(1):75‐82. [DOI] [PubMed] [Google Scholar]
- Klinnert MD, Liu AH, Pearson MR, Tong S, Strand M, Luckow A, et al. Outcome of a randomized multifaceted intervention with low‐income families of wheezing infants. Archives of Pediatrics & Adolescent Medicine 2007;161(8):783‐90. [DOI] [PubMed] [Google Scholar]
Lepore 2013 {published data only}
- Lepore SJ, Winickoff JP, Moughan B, Bryant‐Stephens TC, Taylor DR, Fleece D, et al. Kids Safe and Smokefree (KiSS): a randomized controlled trial of a multilevel intervention to reduce secondhand tobacco smoke exposure in children. BMC Public Health 2013;13:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loke 2005 {published data only}
- Loke AY, Lam TH. A randomized controlled trial of the simple advice given by obstetricians in Guangzhou, China, to non‐smoking pregnant women to help their husbands quit smoking. Patient Education & Counseling 2005; Vol. 59, issue 1:31‐7. [DOI] [PubMed]
- Loke AY, Lam TH, Betson CL, Pan SC, Li SY, Gao SJ, et al. A randomised controlled trial of health education intervention in pregnant women to help husbands quit smoking [abstract]. Nicotine & Tobacco Research 1999;1(2):196. [Google Scholar]
Manfredi 1999 {published data only}
- Manfredi C, Crittenden KS, Warnecke R, Engler J, Cho YI, Shaligram C. Evaluation of a motivational smoking cessation intervention for women in public health clinics. Preventive Medicine 1999;28:51‐60. [DOI] [PubMed] [Google Scholar]
Meltzer 1993 {published data only}
- Meltzer SB, Hovell MF, Meltzer EO, Atkins CJ, Peyster A. Reduction of secondary smoke exposure in asthmatic children: parent counseling. Journal of Asthma 1993;30(5):391‐400. [DOI] [PubMed] [Google Scholar]
Morgan 2004 {published data only}
- Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, Evans R, et al. Results of a home‐based environmental intervention among urban children with asthma. New England Journal of Medicine 2004;351(11):1068‐80. [DOI] [PubMed] [Google Scholar]
Murray 1993 {published data only}
- Murray AB, Morrison BJ. The decrease in severity of asthma in children of parents who smoke since the parents have been exposing them to less cigarette smoke. Journal of Allergy & Clinical Immunology 1993;91(1 Pt 1):102‐10. [DOI] [PubMed] [Google Scholar]
Oien 2008 {published data only}
- Oien T, Storro O, Jenssen JA, Johnsen R. The impact of a minimal smoking cessation intervention for pregnant women and their partners on perinatal smoking behaviour in primary health care: a real‐life controlled study. BMC Public Health 2008;8:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okah 2003 {published data only}
- Okah FA, Okuyemi KS, Harris KJ, McCarter KS, Catley D, Ahluwalia JS. Predicting adoption of home smoking restrictions by inner‐city African American smokers. Pediatric Research 2002;51(4):196A. [DOI] [PubMed] [Google Scholar]
- Okah FA, Okuyemi KS, McCarter KS, Harris KJ, Catley D, Kaur H, et al. Predicting adoption of home smoking restriction by inner‐city black smokers. Archives of Pediatrics & Adolescent Medicine 2003;157(12):1202‐5. [DOI] [PubMed] [Google Scholar]
Philips 1990 {published data only}
- Philips BU, Longoria JM, Parcel GS, Ebeling EW. Expectations of preschool children to protect themselves from cigarette smoke: results of a smoking prevention program for preschool children. Journal of Cancer Education 1990;5(1):27‐31. [DOI] [PubMed] [Google Scholar]
Sockrider 2003 {published data only}
- Sockrider MM, Suchanek Hudmon K, Addy R, Dolan‐Mullen P. An exploratory study of control of smoking in the home to reduce infant exposure to environmental tobacco smoke. Nicotine & Tobacco Research 2003;5(6):901‐10. [DOI] [PubMed] [Google Scholar]
Spencer 2000 {published data only}
- Spencer D. Pilot study for a randomised controlled trial to determine the effectiveness of targetting smoking cessation interventions at mothers of children with asthma. NRR 2000.
Stepans 2006 {published data only}
- Stepans MB, Wilhelm SL, Dolence K. Smoking hygiene: reducing infant exposure to tobacco. Biological Research for Nursing 2006;8(2):104‐14. [DOI] [PubMed] [Google Scholar]
Stotts 2013b {published data only}
- Stotts AL, Northrup TF, Schmitz JM, Green C, Tyson J, Velasquez MM, et al. Baby's Breath II protocol development and design: a secondhand smoke exposure prevention program targeting infants discharged from a neonatal intensive care unit. Contemporary Clinical Trials 2013;35(1):97‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tingen 2016 {published data only}
- Tingen MS, Andrews JO, Heath J, Williams LB, Schroeder C, Dainer P, et al. Tailored parental cessation delivered concurrently with tobacco prevention in children enrolled in urban and rural southern elementary schools. Prevention Research 2016;25(3 suppl):Abstract B62. [Google Scholar]
Turner‐Henson 2005 {published and unpublished data}
- Turner‐Henson A, Sathiakumar N, Siddappa YS, Kohler C, Grad R, Schoenberger YM. Reducing secondhand smoke among young children, maternal smoking practices and household restrictions [abstract]. American Thoracic Society 2005 International Conference; 20 May 2005;San Diego, CA:C13. [Google Scholar]
Walley 2015 {published data only}
- Walley SC, Chime C, Powell J, Walker K, Burczyk‐Brown J, Funkhouser E. A brief inpatient intervention using a short video to promote reduction of child tobacco smoke exposure. Hospital Pediatrics 2015;5(10):534‐41. [DOI] [PubMed] [Google Scholar]
Williams 2016 {published data only}
- Williams RS, Stollings JH, Bundy U, Haardorfer R, Kreuter MW, Mullen PD, et al. A minimal intervention to promote smoke‐free homes among 2‐1‐1 callers: North Carolina randomized effectiveness trial. PLoS One 2016;11(11):e0165086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 1996 {published data only}
- Wilson SR, Latini D, Starr NJ, Fish L, Loes LM, Page A, et al. Education of parents of infants and very young children with asthma ‐ a developmental evaluation of the Wee Wheezers program. Journal of Asthma 1996;33:239‐54. [DOI] [PubMed] [Google Scholar]
Wilson 2005 {published and unpublished data}
- Wilson SE, Kahn RS, Khoury J, Lanphear BP. Racial differences in exposure to environmental tobacco smoke among children. Environmental Health Perspectives 2005;113(3):362‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winickoff 2013 {published data only}
- Friebely J, Rigotti NA, Chang Y, Hall N, Weiley V, Dempsey J, et al. Parent smoker role conflict and planning to quit smoking: a cross‐sectional study. BMC Public Health 2013;13:164. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi‐Burza E, Regan S, Drehmer J, Ossip D, Rigotti N, Hipple B, et al. Parents smoking in their cars with children present. Pediatrics 2012;130(6):e1471‐8. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winickoff J, Friebely J, Healey E, Hipple B, Park E, Regan S, et al. Addressing parental smoking by changing pediatric office systems. Society for Research on Nicotine and Tobacco 13th Annual Meeting, 21‐24 February 2007; Austin, TX. 2007.
- Winickoff JP, Nabi‐Burza E, Chang Y, Finch S, Regan S, Wasserman R, et al. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics 2013;132(1):109‐17. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Hutchinson 2013 {published data only}
- Hutchinson SG, Mesters I, Breukelen G, Muris JW, Feron FJ, Hammond SK, et al. A motivational interviewing intervention to PREvent PAssive Smoke Exposure (PREPASE) in children with a high risk of asthma: design of a randomised controlled trial. BMC Public Health 2013;13:177. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2010 {published data only}
- Johnston V, Walker N, Thomas DP, Glover M, Chang AB, Bullen C, et al. The study protocol for a randomized controlled trial of a family‐centred tobacco control program about environmental tobacco smoke (ETS) to reduce respiratory illness in Indigenous infants. BMC Public Health 2010;10:114‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Risica 2016 {unpublished data only}
- Baby's Breath. Ongoing study Unclear.
Rosen 2011 {published data only}
- Rosen LJ, Guttman N, Hovell MF, Noach MB, Winickoff JP, Tchernokovski S, et al. Development, design, and conceptual issues of project zero exposure: a program to protect young children from tobacco smoke exposure. BMC Public Health 2011;11:508‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagener 2012 {unpublished data only}
- Novel methods to reduce children's secondhand smoke exposure I. Ongoing study April 2012.
Additional references
Al‐Delaimy 2002a
- Al‐Delaimy WK, Crane J, Woodward A. Is hair nicotine level a more accurate biomarker of environmental tobacco smoke exposure than urine cotinine?. Journal of Epidemiology and Community Health 2002;56:66‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Delaimy 2002b
- Al‐Delaimy WK. Hair as a biomarker for exposure to tobacco smoke. Tobacco Control 2002;11:176‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakoula 1995
- Bakoula CG, Kafritsa YJ, Kavadias GD, Lazopoulou DD, Theodoridou MC, Maravelias KP, et al. Objective passive‐smoking indicators and respiratory morbidity in young children. Lancet 1995;346(8970):280‐1. [DOI] [PubMed] [Google Scholar]
Blair 1999
- Blair PS, Fleming PJ, Smith IJ, Platt MW, Young J, Nadin P, et al. Babies sleeping with parents: case‐control study of factors influencing the risk of the sudden infant death syndrome. BMJ 1999;319:1457‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamberlain 2017
- Chamberlain C, O'Mara‐Eves A, Porter J, Coleman T, Perlen S, Thomas J, McKenzie JE. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.CD001055.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiswell 2017
- Chiswell C, Akram Y. Impact of environmental tobacco smoke exposure on anaesthetic and surgical outcomes in children: a systematic review and meta‐analysis. Archives of Disease in Childhood 2017;102:123‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coleman 2015
- Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi‐Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD010078.pub2] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. www.covidence.org. Melbourne, Australia: Veritas Health Innovation, Version accessed February 2017.
Doll 1950
- Doll R, Hill AB. Smoking and carcinoma of the lung: preliminary report. British Medical Journal 1950;2(4682):739‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwyer 1986
- Dwyer T, Pierce JP, Hannam CD, Burke N. Evaluation of the Sydney "Quit For Life" anti‐smoking campaign. Part 2. Changes in smoking prevalence. Medical Journal of Australia 1986;144:344‐7. [PubMed] [Google Scholar]
Ford 1997
- Ford RP, Tappin DM, Schluter PJ, Wild CJ. Smoking during pregnancy: how reliable are maternal self reports in New Zealand?. Journal of Epidemiology and Community Health 1997;51:246‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Golding 1997
- Golding J. Sudden infant death syndrome and parental smoking ‐ a literature review. Paediatric & Perinatal Epidemiology 1997;11:67‐77. [DOI] [PubMed] [Google Scholar]
Haley 1983
- Haley NJ, Axelrad CM, Tilton KA. Validation of self‐reported smoking behavior: biochemical analyses of cotinine and thiocyanate. American Journal of Public Health 1983;73:1204‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harlap 1974
- Harlap S, Davies AM. Infant admissions to hospital and maternal smoking. Lancet 1974;1(7857):529‐32. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Idle 1990
- Idle JR. Titrating exposure to tobacco smoke using cotinine ‐ a minefield of misunderstandings. Journal of Clinical Epidemiology 1990;43:313‐7. [DOI] [PubMed] [Google Scholar]
Iles 2001
- Iles K, Poplawski NK, Couper RT. Passive exposure to tobacco smoke and bacterial meningitis in children. Journal of Paediatrics & Child Health 2001;37:388‐91. [DOI] [PubMed] [Google Scholar]
Jarvis 1984
- Jarvis M, Tunstall‐Pedoe H, Feyerabend C, Vesey C, Salloojee Y. Biochemical markers of smoke absorption and self reported exposure to passive smoking. Journal of Epidemiology and Community Health 1984;38(4):335‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarvis 1987
- Jarvis MJ, Tunstall‐Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from non‐smokers. American Journal of Public Health 1987;77:1435‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarvis 2015
- Jarvis MJ, Feyerabend C. Recent trends in children’s exposure to second‐hand smoke in England: cotinine evidence from the Health Survey for England. Addiction 2015;110:1484–92. [DOI] [PubMed] [Google Scholar]
Lackie 1999
- Lackie J. The Dictionary of Cell and Molecular Biology. 3rd Edition. Burlington, USA: Academic Press, 1999. [Google Scholar]
Lam 2001
- Lam TH, Leung GM, Ho LM. The effect of environmental tobacco smoke on health services utilization in the first eighteen months of life. Pediatrics 2001;107(6):e91. [DOI] [PubMed] [Google Scholar]
Lancaster 2017
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD001292.pub3] [DOI] [PubMed] [Google Scholar]
Lelong 2001
- Lelong N, Kaminski M, Saurel‐Cubizolles MJ, Bouvier‐Colle MH. Postpartum return to smoking among usual smokers who quit during pregnancy. European Journal of Public Health 2001;11:334‐9. [DOI] [PubMed] [Google Scholar]
Matthews 1999
- Matthews F. Birth outcome predicted by cotinine level. Annals of Clinical Biochemistry 1999;36:468‐76. [DOI] [PubMed] [Google Scholar]
Mays 2014
- Mays D, Gilman SE, Rende R, Luta G, Tercyak KP, Niaura RS. Parental smoking exposure and adolescent smoking trajectories. Pediatrics 2014;133(6):983‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2012
- Moore GF, Currie D, Gilmore G, et al. Socioeconomic inequalities in childhood exposure to secondhand smoke before and after smoke‐free legislation in three UK countries. Journal of Public Health (Oxford) 2012;34:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nafstad 1997
- Nafstad P, Jaakkola JJK, Hagen JA, Zahlsen K, Magnus P. Hair nicotine concentrations in mothers and children in relation to parental smoking. Journal of Exposure Analysis and Environmental Epidemiology 1997;7:235‐9. [PubMed] [Google Scholar]
NHMRC 1997
- National Health and Medical Research Council. The Health Effects of Passive Smoking: A Scientific Information Paper. Canberra: Australian Government Publishing Service,. Canberra, 1997.
Patrick 1994
- Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self‐reported smoking: a review and meta‐analysis. American Journal of Public Health 1994;84(7):1086‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pugmire 2014
- Pugmire J, Vasquez MM, Zhou M, et al. Exposure to parental smoking in childhood is associated with persistence of respiratory symptoms into young adult life. Journal of Allergy and Clinical Immunology 2014;134:962–5.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pugmire 2017
- Pugmire J, Sweeting H, Moore L. Environmental tobacco smoke exposure among infants, children and young people: now is no time to relax. Archives of Disease in Childhood 2017;102(2):117‐8. [DOI] [PubMed] [Google Scholar]
Rogers 1975
- Rogers RW. A protection motivation theory of fear appeals and attitude change. Journal of Psychology 1975;91:93‐114. [DOI] [PubMed] [Google Scholar]
Rosen 2014
- Rosen LJ, Myers V, Hovell M, Zucker D, Noach MB. Meta‐analysis of parental protection of children from tobacco smoke exposure. Pediatrics 2014;133:698–714. [DOI] [PubMed] [Google Scholar]
Stead 2013
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann‐Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD000165.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strachan 1997
- Strachan D, Cook D. Health effects of passive smoking. 1. Parental smoking and lower respiratory illness in infancy and early childhood. Thorax 1997;52:905‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strachan 1998
- Strachan DP, Cook DG. Health effects of passive smoking. 4. Parental smoking, middle ear disease and adenotonsillectomy in children. Thorax 1998;53:50‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Velicer 1992
- Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychological Bulletin 1992;111(1):23‐41. [DOI] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization. International Consultation on Environmental Tobacco Smoke (ETS) and Child Health. Geneva, Switzerland: World Health Organization, January 1999. [Google Scholar]
WHO 2013
- World Health Organization. WHO Framework Convention on Tobacco Control, 2013. http://www.who.int/fctc/en/ (accessed 25 July 2017)..
WHO 2014
- World Health Organization. World Cancer Report 2014. www.who.int/cancer/publications/WRC_2014/en/. [ISBN 92‐832‐0429‐8]
Zahlsen 1994
- Zahlsen K, Nilsen OG. Nicotine in smokers and non‐smokers: sampling procedure and gas chromatographic/mass spectrometric analysis. Pharmacology and Toxicology 1994;75:143‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Baxi 2014
- Baxi R, Sharma M, Roseby R, Polnay A, Priest N, Waters E, et al. Family and carer smoking control programmes for reducing children's exposure to environmental tobacco smoke. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD001746.pub3] [DOI] [PubMed] [Google Scholar]
Priest 2008
- Priest N, Roseby R, Waters E, Polnay A, Campbell R, Spencer N, et al. Family and carer smoking control programmes for reducing children's exposure to environmental tobacco smoke. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD001746.pub2] [DOI] [PubMed] [Google Scholar]
Roseby 2002
- Roseby R, Waters E, Polnay A, Campbell R, Webster P, Spencer N. Family and carer smoking control programmes for reducing children's exposure to environmental tobacco smoke. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001746] [DOI] [PubMed] [Google Scholar]
Waters 2000
- Waters E, Campbell R, Webster P, Spencer N. Family and carer smoking control programmes for reducing children's exposure to environmental tobacco smoke. Cochrane Database of Systematic Reviews 2000. [DOI: 10.1002/14651858.CD001746] [DOI] [PubMed] [Google Scholar]


