Abstract
Background
The female genital tract is not exposed to seminal plasma during standard assisted reproductive technology (ART) cycles. However, it is thought that the inflammatory reaction triggered by seminal plasma may be beneficial by inducing maternal tolerance to paternal antigens expressed by the products of conception, and may increase the chance of successful implantation and live birth.
Objectives
To assess the effectiveness and safety of application of seminal plasma to the female genital tract prior to embryo transfer in ART cycles.
Search methods
We searched the following databases from inception to October 2017: Cochrane Gynaecology and Fertility Group Specialised Register of Controlled Trials, Cochrane Central Register of Studies Online (CRSO), MEDLINE, Embase, CINAHL and PsycINFO. We also searched trial registers for ongoing trials, including International Clinical Trials Registry Platform (ICTRP) Search Portal and ClinicalTrials.gov. Other sources searched were; Web of Knowledge, OpenGrey, LILACS, PubMed, Google Scholar, and the reference lists of relevant articles.
Selection criteria
We included randomised controlled trials (RCTs) conducted among women undergoing ART, comparing any procedure that would expose the female genital tract to seminal plasma during the period starting five days before embryo transfer and ending two days after it versus no seminal plasma application.
Data collection and analysis
Two review authors independently selected trials, assessed risk of bias, and extracted data. We pooled data to calculate relative risks (RRs) and 95% confidence intervals (CIs). We assessed statistical heterogeneity using the I2 statistic. We assessed the overall quality of the evidence for the main outcomes using GRADE methods. Our primary outcomes were live birth rate and miscarriage rate. Secondary outcomes were live birth/ongoing pregnancy rate, clinical pregnancy rate, multiple pregnancy rate, ectopic pregnancy rate, and the incidence of other adverse events.
Main results
We included 11 RCTs (3215 women). The quality of the evidence ranged from very low to low. The main limitations were risk of bias (associated with poor reporting of allocation concealment and other methods) and imprecision for the primary outcome of live birth rate.
Live birth rates: Seminal plasma application made little or no difference in live birth rates (RR 1.10, 95% CI 0.86 to 1.43; 948 participants; 3 studies; I2 = 0%). Low‐quality evidence suggested that if the live birth rate following standard ART was 19%, it would be between 16% and 27% with seminal plasma application.
Miscarriage rate: Seminal plasma application made little or no difference in miscarriage rates (RR 1.01, 95% CI 0.57 to 1.79; 1209 participants; 4 studies; I2 = 0%). Low‐quality evidence suggested that if the miscarriage rate following standard ART was 3.7%, the miscarriage rate following seminal plasma application would be between 2.1% and 6.6%.
Live birth or ongoing pregnancy rates: Seminal plasma application made little or no difference in live birth or ongoing pregnancy rates (RR 1.19, 95% CI 0.95 to 1.49; 1178 participants; 4 studies; I2 = 4%, low‐quality evidence). The evidence suggested that if the live birth or ongoing pregnancy rate following standard ART was 19.5%, it would be between 18.5% and 29% with seminal plasma application.
Clinical pregnancy rates: We are uncertain whether seminal plasma application increases clinical pregnancy rates (RR 1.15, 95% CI 1.01 to 1.31; 2768 participants; 10 studies; I2 = 0%). Very low‐quality evidence suggested that if the clinical pregnancy rate following standard ART was 22.0%, it would be between 22.2% and 28.8% with seminal plasma application. This finding should be regarded with caution, as a post hoc sensitivity analysis restricted to studies at overall low risk of bias did not find a significant difference between the groups (RR 1.06, 95% CI 0.81 to 1.39; 547 participants; 3 studies; I2 = 0%).
Multiple pregnancy rate: Seminal plasma application may make little or no difference to multiple pregnancy rates (RR 1.11, 95% CI 0.76 to 1.64; 1642 participants; 5 studies; I2 = 9%). Low‐quality evidence suggested that if the multiple pregnancy rate following standard ART was 7%, the multiple pregnancy rate following seminal plasma application would be between 5% and 11.4%.
Ectopic pregnancy: There was insufficient evidence to determine whether seminal plasma application influenced the risk of ectopic pregnancy (RR 1.59, 95% CI 0.20 to 12.78, 1521 participants; 5 studies; I2 = 0%) .
Infectious complications or other adverse events: No data were available on these outcomes
Authors' conclusions
In women undergoing ART, there was insufficient evidence to determine whether there was a difference between the seminal plasma and the standard ART group in rates of live birth (low‐quality evidence) or miscarriage (low‐quality evidence). There was low‐quality evidence suggesting little or no difference between the groups in rates of live birth or ongoing pregnancy (composite outcome). We found low‐quality evidence that seminal plasma application may be associated with more clinical pregnancies than standard ART. There was low‐quality evidence suggesting little or no difference between the groups in rates of multiple pregnancy. There was insufficient evidence to reach any conclusions about the risk of ectopic pregnancy, and no data were available on infectious complications or other adverse events.
We conclude that seminal plasma application is worth further investigation, focusing on live birth and miscarriage rates.
Plain language summary
Seminal fluid application to improve assisted reproduction outcomes
Review question
The main aim of this review was to assess whether application of seminal plasma to the female genital tract around the time of embryo transfer improves live birth rates in assisted reproductive technology (ART) cycles. Seminal plasma is the fluid part of the ejaculate, and the female genital tract consists of the vagina, the neck of the womb and the womb.
Background
In ART cycles, the egg and sperm are mixed outside the body to develop embryos. One or two of the embryos are replaced into the womb in a very small amount of artificial fluid. During this process, the woman's body does not come into contact with seminal plasma at all, unlike during normal intercourse where the male partner ejaculates in the vagina, exposing the latter to seminal fluid. It has been suggested that seminal plasma contains several molecules which can help the embryos to attach to the womb. The logical question is whether application of some seminal plasma to the vagina/neck of the womb or womb increases the chances of a live birth after ART.
Study characteristics
This Cochrane review included 11 randomised controlled trials, in which women were randomly allocated to receive seminal plasma or not. These trials included a total of 3215 women undergoing ART. The evidence is current to October 2017.
Key results
We found no clear evidence to suggest whether seminal plasma application influences rates of live birth or miscarriage in women undergoing ART. However, we found low‐quality evidence suggesting that seminal plasma application may possibly lead to more clinical pregnancies than standard ART. There was low‐quality evidence suggesting little or no difference between the groups in rates of multiple pregnancy. There was insufficient evidence to reach any conclusions about the risk of ectopic pregnancy (pregnancy in which the embryo attaches outside the womb), and no data were available on infectious complications or other adverse events.
We conclude that seminal plasma application is worth further investigation focusing on live birth and miscarriage rates.
Quality of evidence
The quality of evidence ranged from very low to low.The main limitations were risk of bias (associated with poor reporting of study methods) and lack of data for the primary outcome of live birth rate.
Summary of findings
Summary of findings for the main comparison. ART with seminal plasma compared to standard ART (IVF, ICSI, and frozen embryo transfer).
| ART with seminal plasma compared to standard ART (IVF, ICSI, and frozen embryo transfer) | ||||||
| Population: Women undergoing ART (IVF, ICSI, or frozen embryo transfer) Setting: Assisted reproduction clinic Intervention: Seminal plasma Comparison: Standard IVF | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| with standard IVF | with seminal plasma | |||||
| Live birth | 191 per 1,000 | 210 per 1,000 (164 to 273) | RR 1.10 (0.86 to 1.43) | 948 (3 RCTs) | ⊕⊕⊝⊝1,2 low | |
| Miscarriage | 38 per 1,000 | 38 per 1,000 (21 to 67) | RR 1.01 (0.57 to 1.79) | 1209 (4 RCTs) | ⊕⊕⊝⊝3,4 low | |
| Live birth or ongoing pregnancy | 195 per 1,000 | 232 per 1,000 (185 to 291) | RR 1.19 (0.95 to 1.49) | 1178 (4 RCTs) | ⊕⊕⊝⊝2,5 low | |
| Clinical pregnancy | 220 per 1,000 | 252 per 1,000 (222 to 288) | RR 1.15 (1.01 to 1.31) | 2768 (10 RCTs) | ⊕⊕⊝⊝3,6,7 very low | A post hoc sensitivity analysis excluding studies at overall high risk of bias negated the statistical significance of the finding (RR 1.06, 95% CI 0.81 to 1.39; participants = 547; studies = 3; I2 = 0%) |
| Multiple pregnancy | 70 per 1,000 | 77 per 1,000 (53 to 114) | RR 1.11 (0.76 to 1.64) | 1642 (5 RCTs) | ⊕⊕⊝⊝4,8 low | |
| Ectopic pregnancy | 1 per 1,000 | 2 per 1,000 (0 to 17) | RR 1.59 (0.20 to 12.78) | 1521 (5 RCTs) | ⊕⊕⊝⊝9,10 very low | |
| Adverse events, including infectious complications | No data available | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level for serious risk of bias: method of allocation concealment was unclear in all of the included trials. The outcomes are/may have been incompletely reported in two of the three trials.
2 Downgraded one level for serious imprecision: total number of events was small and the confidence interval was not narrow enough to exclude potential significant benefit.
3 Downgraded one level for serious risk of bias: method of allocation concealment was unclear in 2/4 trials. Selective reporting is a concern for one trial, incomplete data for another trial.
4 Downgraded one level for serious imprecision: total number of events was small and the confidence interval was not narrow enough to exclude potential significant harm or benefit.
5 Downgraded one level for serious risk of bias: method of allocation concealment is unclear. There could be incomplete reporting for some participants.
6 Downgraded one level for serious risk of bias: method of allocation concealment was unclear in most trials and allocation was not concealed in one. Blinding was not possible in the trials of vaginal application and was not done in some others. Incomplete reporting and other biases are also concerning for two trials. Downgraded a further level for serious risk of bias, as a post hoc sensitivity analysis excluding studies at overall high risk of bias negated the statistical significance of the finding (RR 1.06, 95% CI 0.81 to 1.39; participants = 547; studies = 3; I2 = 0%).
7 Downgraded one level for imprecision ‐ confidence interval was compatible with benefit in the intervention arm or with no clinically meaningful effect.
8 Downgraded one level for serious risk of bias: method of allocation concealment was unclear in 2/4 trials and allocation was not concealed in 1/4 trials. Incomplete data can be a concern for all trials.
9 Downgraded one level for serious risk of bias: method of allocation concealment was unclear.
10 Downgraded two levels for very serious imprecision: only 3 events and very wide confidence intervals.
Background
Description of the condition
Despite advances in both clinical and laboratory aspects of assisted reproductive technologies, live birth rates have plateaued for the last decade. Embryo implantation, a delicate process requiring harmony between the implantation competent embryo and receptive endometrium (inner lining of the uterus), may be the rate‐limiting step. Currently, 20% to 35% of chromosomally normal embryos fail to implant (Lee 2015). This suggests that other factors than just aneuploidy (having abnormal number of chromosomes) are possibly preventing implantation. These could include inadequate maternal immune tolerance for the products of conception, which also express paternal genes. The products of the paternal genes, i.e. paternal antigens (molecules that can stimulate the immune system), can be recognised as 'foreign' by the maternal immune system and they can trigger an immune response (Tafuri 1995).
The progression of pregnancy therefore partially depends on protection of the products of conception from a destructive maternal immune response. A particular component of the maternal immune system, named regulatory T cells, can suppress such an immune reaction against the products of conception despite its expression of paternal 'foreign' antigens (Robertson 2013). However, proper activation of regulatory T cells requires their exposure to paternal antigens in advance (Samy 2006). The presence of immune‐modulatory molecules such as transforming growth factor‐beta (TGF‐β), interleukin (IL)‐10, granulocyte‐macrophage colony stimulating factor (GM‐CSF) and IL‐4 are also required during the initial contact of paternal antigens and T cells (Sato 2003). In summary, maternal regulatory T cells need to be primed before implantation by paternal antigens which are common with the products of conception, in order to generate immune tolerance.
The ejaculate is comprised of spermatozoa and seminal plasma. Seminal plasma is a combination of the secretions of seminal vesicles, and prostate and bulbourethral glands (secretory glands of the male reproductive system). It is a rich source of paternal antigens, cytokines (small proteins that enable communication between different cells), prostaglandins (short lived molecules that effect close by cells) and growth factors, which regulate endometrial receptivity and could play a role in inducing maternal immune tolerance (Achache 2006; Robertson 2002; Robertson 2005; Simon 2000).
However, spermatozoa are isolated from seminal plasma before being used for oocyte (egg) fertilisation in assisted reproductive technology cycles. Embryos generated in vitro (in the laboratory) are transferred to the uterus in artificial transfer media. Hence, the female genital tract is not exposed to seminal plasma during an assisted reproductive technology cycle. Already established live births with assisted reproductive technology attest to the fact that such exposure is not an absolute requirement for successful implantation. However, the lack of it could be a contributing factor to limited embryo implantation rates. It is thought that the inflammatory reaction triggered by seminal plasma in the female genital tract can induce maternal tolerance to the paternal antigens expressed by the products of conception and increase its chances to successfully implant and lead to a live birth.
Description of the intervention
In assisted reproductive technology cycles the female genital tract can be brought into contact with seminal plasma in several ways including unprotected vaginal intercourse around the time of the embryo transfer, and seminal plasma application to the vagina, cervical canal or into the endometrial cavity prior to embryo transfer. The effect on endometrial receptivity and implantation process may vary depending on the route of application. Application of seminal plasma to female genital tract is a quite straight forward procedure which is usually painless. Although very rare, upper genital tract infection is a potential complication.
How the intervention might work
Previous studies suggest that paternal antigens and the cytokines present in seminal plasma can induce regulatory T cell generation and interact with endometrial cells to suppress the maternal immune response against the products of conception that expresses similar paternal antigens (Bromfield 2014; Robertson 2013). Moreover, seminal plasma is also shown to up‐regulate expression of angiogenic factors (substances that promote new blood vessel formation) by endometrial cells, which could also help vascularisation (formation of new small blood vessels) of the feto‐maternal unit (Chen 2014). Eventually, seminal plasma exposure prior to embryo transfer could be expected to increase embryo implantation and live birth rates.
Why it is important to do this review
Trials investigating the effect of seminal plasma exposure on clinical outcome of assisted reproductive technology cycles have been published with conflicting results. Assessment of available evidence in its totality and conducting relevant subgroup analysis can provide either a definitive conclusion or inform future research on the subject. .
Objectives
To assess the effectiveness and safety of application of seminal plasma to the female genital tract prior to embryo transfer in assisted reproductive technology cycles.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs). We excluded non‐randomised and quasi‐randomised studies (e.g. studies with evidence of inadequate sequence generation, such as alternate days, patient numbers).
Types of participants
Women undergoing fresh or frozen thawed embryo transfer.
Types of interventions
Any procedure that would expose the female genital tract to seminal plasma during the period starting five days before embryo transfer and ending two days after it, including the following:
unprotected vaginal intercourse;
application of seminal plasma to the vagina;
application of seminal plasma into the cervical canal;
application of seminal plasma into the cervical canal and to the vagina;
instillation of seminal plasma into the endometrial cavity;
intrauterine insemination with unprocessed semen.
Our comparator was assisted reproductive technology cycles without any seminal plasma application. There were no studies comparing different locations of seminal plasma application.
Types of outcome measures
Primary outcomes
1. Live birth rate per randomised woman, defined as delivery of a live fetus after 20 completed weeks of gestation.
2. Miscarriage rate per randomised woman, defined as pregnancy loss before 20 weeks of gestation.
Secondary outcomes
3. Live birth or ongoing pregnancy rate per randomised woman. An ongoing pregnancy was defined as one that progresses beyond the 12th gestational week.
4. Clinical pregnancy rate per randomised woman, defined as evidence of a gestational sac, confirmed by ultrasound.
Adverse events
5. Multiple pregnancy rate per randomised woman, defined as the presence of more than one gestational sac, confirmed by ultrasound.
6. Ectopic pregnancy rate per randomised woman, defined as the presence of a gestational sac outside the endometrial cavity, confirmed by ultrasound.
7. Incidence of infections per randomised woman, as evidenced by the presence of fever > 37°C or clinical findings e.g. tenderness, mucopurulent discharge, or as reported by trialists.
8. Any other reported adverse events.
Search methods for identification of studies
We searched for all published and unpublished RCTs of seminal plasma application during assisted reproductive technology cycles, without language restriction, and in consultation with the Gynaecology and Fertility Group Information Specialist.
Electronic searches
We searched the following electronic databases, trial registers, and websites from the date of inception until 16 October 2017:
Cochrane Gynaecology and Fertility Group Specialised Register of Controlled Trials (Procite platform) (Appendix 1);
Cochrane Central Register of Studies Online (CRSO) (Web Platform) (Appendix 2);
MEDLINE (Ovid platform) (Appendix 3);
Embase (Ovid platform) (Appendix 4);
PsycINFO (Ovid platform) (Appendix 5);
CINAHL (EBSCO platform) (Appendix 6).
We combined our MEDLINE search with the 'Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format' (Higgins 2011). We combined our Embase, PsycINFO and CINAHL search strategies with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (http://www.sign.ac.uk/methodology/filters.html).
We also searched the following electronic sources (Appendix 7):
Trial registers for ongoing and registered trials (e.g. clinicaltrials.gov (https://clinicaltrials.gov/)), World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (http://apps.who.int/trialsearch/));
Database of Abstracts of Reviews of Effects (DARE) (the Cochrane Library);
Web of Knowledge;
OpenGrey;
LILACS;
PubMed;
Google Scholar.
Searching other resources
We handsearched reference lists of articles retrieved by the search and contacted experts in the field to obtain additional data. We also searched relevant journals and conference abstracts that were not covered in the Gynaecology and Fertility Group Specialised Register, in liaison with the Group's Information Specialist.
Data collection and analysis
Selection of studies
After an initial screen of titles and abstracts retrieved by the search, conducted by two review authors (AS and AMAS), we retrieved the full texts of all potentially eligible studies. Two authors independently examined these full‐text articles for compliance with the inclusion criteria and selected trials eligible for inclusion (BA and AS). We contacted study investigators, as required, to clarify study eligibility. We resolved disagreement as to study eligibility by discussion or by consulting a third review author (AMAS). We documented the selection process with a Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) flow chart.
Data extraction and management
Two review authors independently extracted data from eligible studies using a data extraction form designed and pilot‐tested by the authors. We resolved disagreements by discussion or by consulting a third review author. Extracted data included trial characteristics and outcome data. Where trials had multiple publications, we collated the multiple reports of the same study so that each trial, rather than each report, was the unit of interest in the review, and we used a single study identification number for such studies with multiple references. We contacted study investigators for further data on methods and/or results, as required.
Assessment of risk of bias in included studies
Two review authors (BA and AS) independently assessed the risk of bias in included studies using the Cochrane's tool for assessing risk of bias (Higgins 2011b). We considered the following domains:
selection bias (random sequence generation and allocation concealment);
performance bias (blinding of participants and personnel);
detection bias (blinding of outcome assessors);
attrition bias (incomplete outcome data);
reporting bias (selective reporting);
other bias, including probable unprotected intercourse during the time interval of the suggested intervention.
We resolved disagreements by discussion or by consulting a third review author (AMAS). We described all judgments fully and presented the conclusions in a 'Risk of bias' table, which we incorporated into the interpretation of review findings by means of sensitivity analyses.
We searched for within‐trial selective reporting, such as trials failing to report obvious outcomes, or reporting them in insufficient detail to allow inclusion. We sought published protocols and compared the outcomes between the protocol and the final published trial report.
Other biases of concern included allowing unprotected intercourse during the time interval of the intervention, potentially contaminating the results.
Where identified trials failed to report the primary outcome of live birth, but did report interim outcomes such as ongoing pregnancy, we pooled these data with live birth data from other studies. In this case, we performed a sensitivity analysis to test the robustness of the results. This was presented as the livebirth or ongoing pregnancy rate outcome.
Measures of treatment effect
As all outcomes in this review were dichotomous, we used the numbers of women with events in the control and intervention groups of each study to calculate Mantel‐Haenszel risk ratios (RRs); with 95% confidence intervals (CIs) for all outcomes. We compared the magnitude and direction of effect reported by trials with how they were presented in the review, taking account of legitimate differences.
Unit of analysis issues
Our primary analysis was per woman randomised. We also performed per‐pregnancy analysis for multiple pregnancy, miscarriage, and ectopic pregnancy rates.
We counted multiple live births (e.g. twins or triplets) as one live birth event.
Dealing with missing data
We analysed data on an intention‐to‐treat basis as far as possible and we attempted to obtain missing data from the original trialists. Where these were unobtainable, we undertook imputation of individual values for the primary outcome only. We assumed that live births had not occurred in participants without a reported outcome. For other outcomes, we analysed only the available data and no imputations were undertaken.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included trials were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the measure of the I2. We considered an I2 value greater than 50% as substantial heterogeneity.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible trials and by being alert for duplication of data. Publication bias testing was done by visual assessment of funnel plots for analyses which included 10 or more trials.
Data synthesis
We pooled data from the primary trials using the Mantel‐Haenzel random‐effects model for the following comparisons.
seminal plasma application versus no seminal plasma application;
one location of seminal plasma application versus another location of seminal plasma application.
We stratified comparisons by seminal plasma application method:
studies in which seminal plasma was applied vaginally either by vaginal unprotected vaginal intercourse or application of processed seminal plasma into the vagina;
studies in which seminal plasma was inseminated into the uterine cervix;
studies in which seminal plasma was applied to both vagina and cervix;
studies in which seminal plasma was applied by intrauterine instillation.
In our forest plots, an increase in the odds of a particular outcome, which may be beneficial (e.g. live birth) or detrimental (e.g. adverse effects), were displayed graphically in the meta‐analyses to the right of the centre‐line and a decrease in the odds of an outcome to the left of the centre‐line.
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analyses to assess whether the effects of the intervention differed according to the following:
seminal plasma application method (as described above)
type of ART cycle (fresh or frozen)
We took statistical heterogeneity into account when interpreting the results.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding the eligibility and analysis. These analyses included:
only studies without overall high risk of bias. We defined overall high risk of bias as studies at unclear or high risk of bias in multiple domains.
using a fixed effects model.
alternative imputation strategies for missing information.
We also conducted a post hoc sensitivity analysis for the secondary outcome of clinical pregnancy.
Overall quality of the body of evidence: Summary of findings table
Two review authors (BA and AMAS) working independently prepared a 'Summary of findings' table using the GRADEpro (GRADEpro GDT 2015) software (http://www.guidelinedevelopment.org). We evaluated the overall quality of the body of evidence for the main review outcomes (live birth, miscarriage, live birth or ongoing pregnancy, clinical pregnancy, multiple pregnancy, ectopic pregnancy, and adverse events), for the main review comparison; i.e. assisted reproductive technology cycles with seminal plasma application compared to standard assisted reproductive technology cycles without seminal plasma application (IVF, ICSI, and frozen embryo transfer), using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). We justified and documented our judgments about evidence quality (high, moderate, low or very low) in the full review and incorporated these findings into the reporting of results for each outcome.
Results
Description of studies
Results of the search
The electronic searches resulted in 2044 potential citations, with an additional two citations added from handsearching. After duplicate removal and screening, we identified 11 trials that met the inclusion criteria (Figure 1).
1.
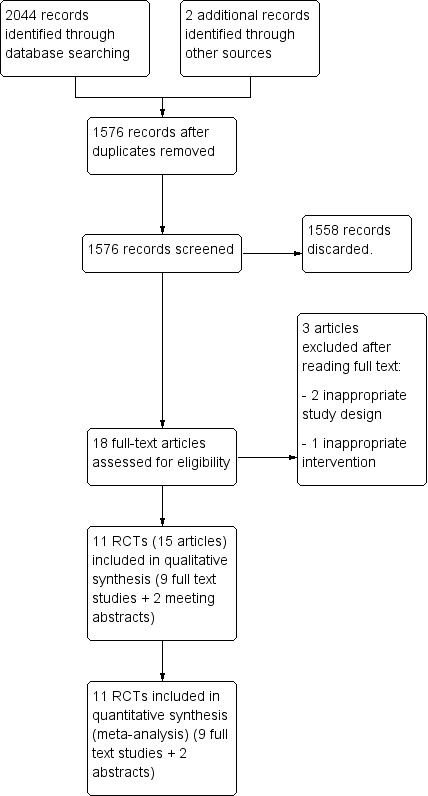
Study flow diagram.
Included studies
All 11 of the included studies identified from the electronic searches were conducted in 2015, 2016, and 2017. A summary of the methods, participants, interventions and outcomes are presented below. Further details are presented separately for each study in the Characteristics of included studies table.
Design
We included 11 studies with 3215 women randomised to treatment (Aflatoonian 2009; Bellinge 1986; Chicea 2013; Crawford 2015; Friedler 2013; Jafarabadi 2016; Karimian 2010; Mayer 2015; Tremellen 2000; Von Wolff 2009; Von Wolff 2013). Ten of the included studies were single centre, two‐arm parallel RCTs (Aflatoonian 2009; Bellinge 1986; Chicea 2013; Crawford 2015; Friedler 2013; Jafarabadi 2016; Karimian 2010; Mayer 2015; Von Wolff 2009; Von Wolff 2013). The remaining trial was a two centre RCT, in which couples undergoing fresh embryo transfers were recruited in one centre and frozen embryo transfers were recruited in the other centre (Tremellen 2000). Each group was randomised by itself and a similar number of participants were available in seminal plasma and control groups. None of the trials used a cross‐over design.
All trials included 100 or more women. The largest trials were Karimian 2010 (569 women) and Tremellen 2000 (478 women). Four trials included fewer than 200 women: Bellinge 1986 (113 women); Crawford 2015 (186 women); Mayer 2015 (100 women); and Von Wolff 2009 (133 women). The remaining five trials (Aflatoonian 2009; Chicea 2013; Friedler 2013; Jafarabadi 2016; Von Wolff 2013) included between 224 and 385 women.
The trials took place (or authors came from): Australia (Bellinge 1986); Australia and Spain (Tremellen 2000); Austria (Mayer 2015); England and Australia (Crawford 2015); Germany (Von Wolff 2009; Von Wolff 2013); Iran (Aflatoonian 2009; Jafarabadi 2016; Karimian 2010); Israel (Friedler 2013); and Romania (Chicea 2013).
Of the 11 studies, only three performed and adhered to an a priori sample size calculation (Friedler 2013; Mayer 2015; Tremellen 2000). However, Tremellen 2000 based the sample size calculation on the number of embryos transferred rather than the number of women. One study had an a priori sample size calculation but was terminated early for futility after a preplanned interim analysis (Von Wolff 2013). It was unclear whether the two studies that were published as abstracts were conducted according to a sample size calculation (Crawford 2015; Karimian 2010). The remaining five studies did not adhere to a sample size calculation (Aflatoonian 2009; Bellinge 1986; Chicea 2013; Jafarabadi 2016; Von Wolff 2009).
Participants
Inclusion Criteria
All 11 studies included women undergoing ART with a regular indication and with fresh ejaculate sperm.
One study required the couple to have at least five years of subfertility (Aflatoonian 2009).
Six studies imposed an age limit for the female partner; Chicea 2013 (< 38 years); Crawford 2015 (23 to 39 years); Friedler 2013 (< 40 years); Jafarabadi 2016 (< 40 years); Tremellen 2000 (18 to 40 years); Von Wolff 2009 (< 43 years).
Five studies mentioned a limit for number of prior ART cycles as an inclusion criterion; Chicea 2013 (< 4 prior ART cycles); Crawford 2015 (< 2 prior ART cycles); Friedler 2013 (at least one failed prior ART cycle); Jafarabadi 2016 (< 3 prior ART cycles); Mayer 2015 (< 2 prior ART cycles).
Only Tremellen 2000 included women undergoing frozen embryo transfers. This was a two centre study; one centre only recruited women undergoing fresh embryo transfers, and the other recruited only women undergoing frozen embryo transfers (Tremellen 2000). While the overall data from this trial were included in the main analyses, data from each centre were separately included in the 'fresh embryo transfer only' and 'frozen embryo transfer only' analyses.
Exclusion Criteria
Seven studies excluded couples in which the male partner had Hepatitis B, or C, HIV infection or leukocytospermia (Chicea 2013; Friedler 2013; Jafarabadi 2016; Mayer 2015; Tremellen 2000; Von Wolff 2009; Von Wolff 2013).
Only three studies excluded couples who did not have a minimum volume of seminal plasma; Mayer 2015 (0.5 ml); Von Wolff 2009 (0.5 ml); Von Wolff 2013 (0.3 ml).
Only two studies mentioned excluding women with uterine anomalies (Chicea 2013; Mayer 2015).
Four studies excluded couples based on embryology laboratory parameters. Friedler 2013 and Jafarabadi 2016 excluded couples who had no oocytes in a prior cycle. Mayer 2015 and Von Wolff 2009 excluded couples from their analyses if a couple had no embryos for transfer due to total fertilisation failure or pending ovarian hyperstimulation syndrome.
Interventions
Three studies required the couples to have unprotected vaginal intercourse around the time of embryo transfer; Aflatoonian 2009 (at least once during the 12 hours following embryo transfer); Karimian 2010 (only mentioned intercourse around the time of ART); Tremellen 2000 (for fresh embryo transfers, at least twice between 12 hours before oocyte pick up and 12 hours after embryo transfer; for frozen embryo transfers, at least once between four days before and two days after embryo transfer).
In only one study, untreated ejaculate was applied vaginally on the day of oocyte collection (Bellinge 1986). All other studies used seminal plasma (Chicea 2013; Crawford 2015; Friedler 2013; Jafarabadi 2016; Mayer 2015; Von Wolff 2009; Von Wolff 2013). Seminal plasma was applied to both cervix and vagina (Chicea 2013; Friedler 2013; Jafarabadi 2016; Mayer 2015; Von Wolff 2009; Von Wolff 2013) or only to the uterus (Crawford 2015; Von Wolff 2013).
Outcomes
Primary outcome
Only three studies reported live birth rate (Karimian 2010; Mayer 2015; Von Wolff 2013). Four studies reported miscarriage rates (Friedler 2013; Mayer 2015; Tremellen 2000; Von Wolff 2013). Definitions of these outcome measures were not clearly mentioned in the original publications.
Secondary outcomes
In addition to the three studies (Crawford 2015; Karimian 2010; Mayer 2015) reporting live birth rate, one study (Friedler 2013) reported ongoing pregnancy rate.
Clinical pregnancy was reported in 10 studies (Aflatoonian 2009; Bellinge 1986; Chicea 2013; Crawford 2015; Friedler 2013; Jafarabadi 2016; Mayer 2015; Tremellen 2000; Von Wolff 2009; Von Wolff 2013). All but one study defined clinical pregnancy with ultrasound visualisation of gestational sac and or fetal pole (Aflatoonian 2009; Bellinge 1986; Chicea 2013; Crawford 2015; Friedler 2013; Jafarabadi 2016; Tremellen 2000; Von Wolff 2009) and or fetal heart beat (Von Wolff 2013) between five and eight gestational weeks.
Five studies reported multiple pregnancy rates (Aflatoonian 2009; Bellinge 1986; Chicea 2013; Mayer 2015; Tremellen 2000). Likewise, five studies reported ectopic pregnancy rates (Aflatoonian 2009; Bellinge 1986; Mayer 2015; Tremellen 2000; Von Wolff 2013). The definitions of these outcome measures were not explicitly mentioned in the papers.
Excluded studies
Two studies (Fishel 1989; Lou 2014) were excluded since allocation was not by randomisation. Coulam 1995 was excluded because couples did not undergo ART but attempted spontaneous conception. Further details are presented in the Characteristics of excluded studies table.
Risk of bias in included studies
Of the 11 included trials, eight were at unclear or high risk of bias in multiple domains (Figure 2; Figure 3).
2.
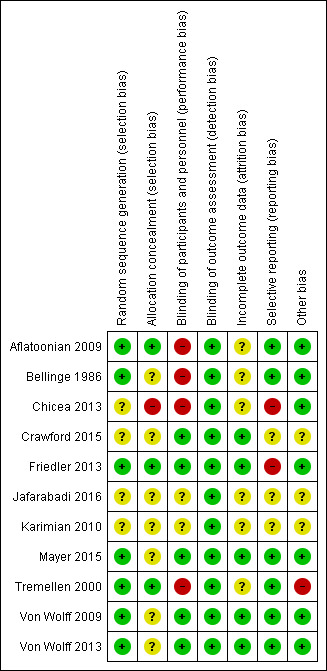
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
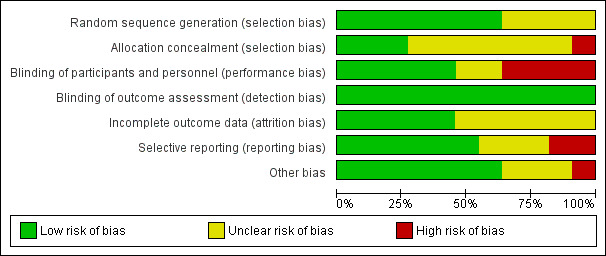
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Three studies did not mention how the randomisation sequence was generated (Chicea 2013; Crawford 2015; Karimian 2010). Two of these studies were published as an abstract (Crawford 2015; Karimian 2010). Jafarabadi 2016 mentioned use of permuted balanced block randomisation method but did not specify how this was done. Of the seven studies that were considered to be at low risk for sequence generation, one reported drawing from an equal number of paper slips from a bag (Aflatoonian 2009), three used random number tables or lists (Bellinge 1986; Von Wolff 2009; Von Wolff 2013) and three used computer‐generated randomisation sequences (Friedler 2013; Mayer 2015; Tremellen 2000).
Method of allocation concealment was clearly mentioned by three studies; Aflatoonian 2009 (drawing paper slips from a bag), Friedler 2013 (by an embryologist blinded to treating physician on the day of oocyte collection), and Tremellen 2000 (sealed envelopes). These three trials were judged to be at low risk of bias due to lack of allocation concealment. Seven studies did not mention the method used for allocation concealment and were thus judged to be at unclear risk (Bellinge 1986; Crawford 2015; Jafarabadi 2016; Karimian 2010; Mayer 2015; Von Wolff 2009; Von Wolff 2013). Chicea 2013 was considered to be at high risk of bias (randomisation sequence was not concealed from the investigators).
Blinding
Several trials (Crawford 2015; Friedler 2013; Mayer 2015; Von Wolff 2009; Von Wolff 2013) reported blinding the participants, personnel and outcome assessors, while the remaining trials were judged to be at unclear or high risk of bias (mainly due to issues surrounding performance bias).
Incomplete outcome data
Several trials (Aflatoonian 2009; Chicea 2013; Karimian 2010; Mayer 2015; Tremellen 2000) were at unclear risk of attrition bias. The remaining trials were at low risk.
Selective reporting
Several trials (Aflatoonian 2009; Chicea 2013; Mayer 2015; Tremellen 2000; Von Wolff 2009; Von Wolff 2013) were at low risk of selective outcome reporting bias. The remaining trials were at unclear or high risk.
Other potential sources of bias
Approximately half of the trials (Aflatoonian 2009; Chicea 2013; Crawford 2015; Friedler 2013; Karimian 2010; Tremellen 2000) were at unclear to high risk of bias due to other sources of bias. The remaining trials were at low risk.
Effects of interventions
See: Table 1
1. Seminal plasma versus no seminal plasma
Primary outcomes
1.1 Live birth
Seminal plasma application had little or no effect on live birth rates (RR 1.10, 95% CI 0.86 to 1.43; I2 = 0%; 3 trials; 948 participants, low‐quality evidence). Analysis 1.1, Figure 4
1.1. Analysis.
Comparison 1 Seminal plasma vs standard ART, Outcome 1 Live birth.
4.
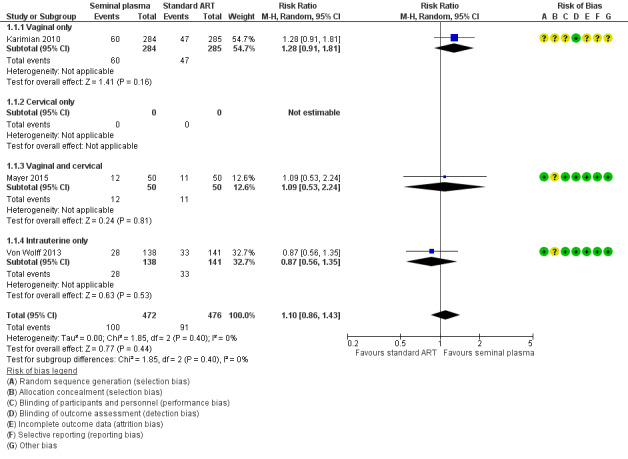
Forest plot of comparison: 1 Seminal plasma vs control, outcome: 1.1 Live birth.
Subgroup analyses
Seminal plasma application method
There was no evidence of a difference between the subgroups (test for subgroup differences: Chi² = 1.85, df = 2 (P = 0.40), I² = 0%). Analysis 1.1
Fresh versus frozen transfer
This subgroup comparison could not be conducted as none of the studies reporting this outcome used frozen embryos.
1.2 Miscarriage
Seminal plasma application had little or no effect on miscarriage rates (RR 1.01, 95% CI 0.57 to 1.79; I2 = 0%; 4 trials; 1209 participants, low‐quality evidence). Analysis 1.2, Figure 5
1.2. Analysis.
Comparison 1 Seminal plasma vs standard ART, Outcome 2 Miscarriage.
5.
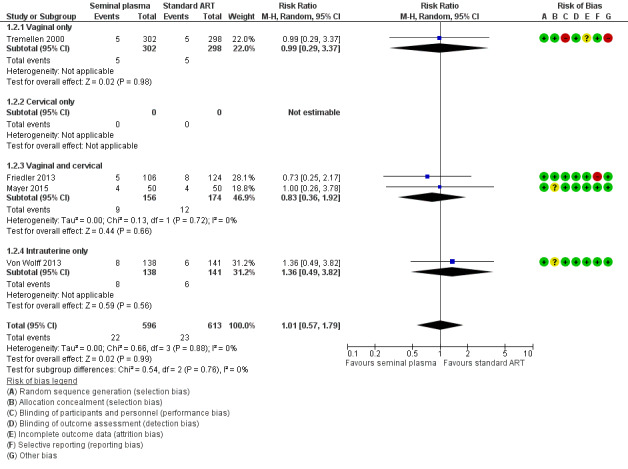
Forest plot of comparison: 1 Seminal plasma vs control, outcome: 1.2 Miscarriage.
Subgroup and sensitivity analyses
Seminal plasma application method
There was no evidence of a difference between the subgroups (test for subgroup differences: Chi² = 0.54, df = 2 (P = 0.76), I² = 0%). Analysis 1.2
Fresh versus frozen transfer
There was no evidence of a difference between the subgroups (test for subgroup differences: Chi² = 0.28, df = 1 (P = 0.60), I² = 0%). Analysis 3.2
3.2. Analysis.
Comparison 3 Grouped by fresh or frozen embryo transfer, Outcome 2 Miscarriage.
Per‐pregnancy analysis
There was insufficient evidence to determine whether there was a difference between the groups in rates of miscarriage per pregnancy (RR 0.92, 95% CI 0.54 to 1.57; 4 trials; 277 participants; I2 = 0%). Analysis 2.1
2.1. Analysis.
Comparison 2 Seminal plasma vs standard ART, per‐pregnancy analyses, Outcome 1 Miscarriage.
Secondary outcomes
1.3 Live birth or ongoing pregnancy (composite outcome)
Seminal plasma application had little or no effect on rates of live birth or ongoing pregnancy (RR 1.19, 95% CI 0.95 to 1.49; I2 = 4%; 4 trials; 1178 participants, low‐quality evidence). Analysis 1.3
1.3. Analysis.
Comparison 1 Seminal plasma vs standard ART, Outcome 3 Live birth or ongoing pregnancy.
Subgroup analyses
Seminal plasma application method
There was no evidence of a difference between the subgroups (test for subgroup differences: Chi² = 2.62, df = 2 (P = 0.27), I² = 23.6%). Analysis 1.3
Fresh versus frozen transfer
This subgroup comparison could not be conducted as none of the studies reporting this outcome used frozen embryos.
1.4 Clinical pregnancy
We are uncertain whether seminal plasma application improves clinical pregnancy rates (RR 1.15, 95% CI 1.01 to 1.31; 10 trials; 2768 participants; I2 = 0%, very low‐quality evidence). Analysis 1.4
1.4. Analysis.
Comparison 1 Seminal plasma vs standard ART, Outcome 4 Clinical pregnancy.
Subgroup analyses
Seminal plasma application method
There was no evidence of a difference between the subgroups (test for subgroup differences: Chi² = 2.43, df = 2 (P = 0.30), I² = 17.7%). Analysis 1.4
Fresh versus frozen transfer
There was no evidence of a difference between the subgroups (test for subgroup differences: Chi² = 0.21, df = 1 (P = 0.65), I² = 0%). Analysis 3.4
3.4. Analysis.
Comparison 3 Grouped by fresh or frozen embryo transfer, Outcome 4 Clinical pregnancy.
Post hoc sensitivity analysis
A post hoc sensitivity analysis restricted to studies at overall low risk of bias (Mayer 2015; Von Wolff 2009; Von Wolff 2013) did not find a significant difference between the groups (RR 1.06, 95% CI 0.81 to 1.39; 3 trials; 547 participants; I2 = 0%). Analysis 1.7Figure 6
1.7. Analysis.
Comparison 1 Seminal plasma vs standard ART, Outcome 7 Clinical pregnancy: Sensitivity analysis by RoB.
6.
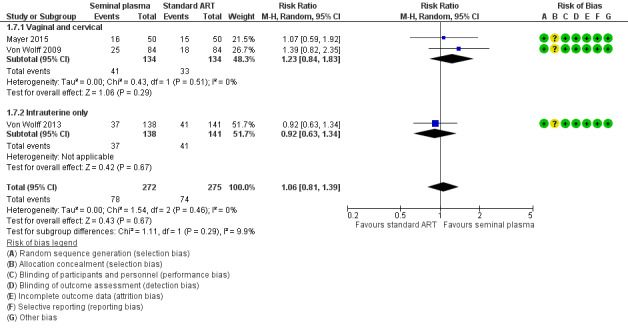
Forest plot of comparison: 1 Seminal plasma vs control, outcome: 1.7 Clinical pregnancy: Sensitivity analysis by RoB.
Safety outcomes
1.5 Multiple pregnancy
There was little or no effect of seminal plasma application on multiple pregnancy rates (RR 1.11, 95% CI 0.76 to 1.64; I2 = 9%; 5 trials; 1642 participants, low‐quality evidence). Analysis 1.5
1.5. Analysis.
Comparison 1 Seminal plasma vs standard ART, Outcome 5 Multiple pregnancy.
Subgroup and sensitivity analyses
Seminal plasma application method
There was no clear evidence of a difference between the subgroups (test for subgroup differences: Chi² = 2.66, df = 1 (P = 0.10), I² = 62.4%). Analysis 1.5
Fresh versus frozen transfer
There was no evidence of a difference between the subgroups (test for subgroup differences: Chi² = 1.52, df = 1 (P = 0.22), I² = 34.2%). Analysis 3.5
3.5. Analysis.
Comparison 3 Grouped by fresh or frozen embryo transfer, Outcome 5 Multiple pregnancy.
Per‐pregnancy analysis
There was no clear evidence of a difference between the groups in rates of multiple pregnancy per pregnancy (RR 0.93, 95% CI 0.69 to 1.24; 5 trials; 370 participants; I2 = 0%). Analysis 2.2
2.2. Analysis.
Comparison 2 Seminal plasma vs standard ART, per‐pregnancy analyses, Outcome 2 Multiple pregnancy.
1.6 Ectopic pregnancy
We are uncertain whether there was a difference between the groups (RR 1.59, 95% CI 0.20 to 12.78; I2 = 0%; 5 trials; 1521 participants; very low‐quality evidence). Analysis 1.6
1.6. Analysis.
Comparison 1 Seminal plasma vs standard ART, Outcome 6 Ectopic pregnancy.
Subgroup and sensitivity analyses
Seminal plasma application method
There was no evidence of a difference between the subgroups (test for subgroup differences: Chi² = 0.23, df = 1 (P = 0.63), I² = 0%). Analysis 1.6
Fresh versus frozen transfer
There was insufficient evidence to determine whether there was a difference between the groups in rates of ectopic pregnancy per pregnancy (RR 1.16, 95% CI 0.15 to 8.98; 277 participants; 5 trials; I2 = 0%). Analysis 3.6
3.6. Analysis.
Comparison 3 Grouped by fresh or frozen embryo transfer, Outcome 6 Ectopic pregnancy.
Per pregnancy analysis
There was insufficient evidence to determine whether there was a difference between the groups in rates of ectopic pregnancy per pregnancy ((RR 1.16, 95% CI 0.15 to 8.98; 277 participants; 5 trials;; I2 = 0%). Analysis 2.3
2.3. Analysis.
Comparison 2 Seminal plasma vs standard ART, per‐pregnancy analyses, Outcome 3 Ectopic pregnancy.
1.7 Infection
No extractable data were available.
1.8 Other adverse events
No extractable data were available.
Other sensitivity analyses
There was no or little heterogeneity and sensitivity analyses were not deemed necessary in this regard.
A sensitivity analysis restricted to studies without high risk of bias did not change the main findings for primary outcomes. However a post hoc sensitivity analysis for the outcome of clinical pregnancy negated the significant difference between the groups.
All the results remained unchanged when a fixed‐effect model was used.
The results were unchanged when the missing participants were assumed to have experienced the primary outcome measures.
Assessment of publication bias
Only two analyses included ten or more studies, and we have presented a funnel plot to assess publication bias for the outcome clinical pregnancy in the first comparison (Analysis 1.4); Analysis 3.4 included the same ten trials. The funnel plot did not suggest publication bias. Figure 7
7.
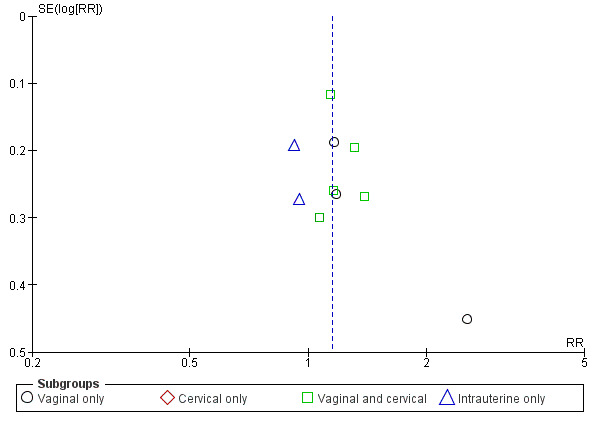
Funnel plot of comparison: 1 Seminal plasma vs Control, outcome: 1.4 Clinical pregnancy.
Discussion
This is the first Cochrane Review that aimed to determine whether exposure of the female genital tract to seminal plasma around the time of embryo transfer improves ART outcomes.
Summary of main results
Overall, there was insufficient evidence to determine whether seminal plasma exposure influenced rates of live birth or miscarriage or the composite outcome of livebirth or ongoing pregnancy. However, the clinical pregnancy rate was significantly increased. There was also insufficient evidence to determine whether seminal plasma exposure influenced rates of adverse events such as multiple pregnancy and ectopic pregnancy There was no information regarding infectious and other complications.
There was no clear evidence of any difference between the groups related to method of seminal plasma application or fresh versus frozen embryo transfers.
Overall completeness and applicability of evidence
We included 11 RCTs, totalling 3215 women. The sample sizes in the studies ranged between 100 and 569. Only three of the included trials, totalling 948 women, had data on the primary outcome measure, live birth rate. Four of the included trials, totalling 1209 women, had data on the other primary outcome measure, miscarriage. To be able to show a difference of 5% compared to a standard live birth rate of 19%, with 80% power, one would require at least 2114 couples. However, the number of RCTs reporting either live birth or ongoing pregnancy rate was 9, totalling 1178 women.
It is noteworthy that ten of the included RCTs, totalling 2768 women, reported clinical pregnancy rate. This is an adequate sample size to demonstrate as statistically significant a ≥ 5% increase over a 21.9% clinical pregnancy rate, at 5% alpha error rate with 80% power. Clinical pregnancy rate was significantly increased with seminal plasma exposure in the main analysis, with a number needed to treat value of 30 to achieve one additional clinical pregnancy with seminal plasma exposure. However, this finding was not robust to a post hoc sensitivity analysis by study risk of bias, so should be regarded very cautiously.
Unlike some other mammals (e.g. horses, pigs, or rodents), human beings do not ejaculate into the uterus. Only two of the included RCTs investigated intrauterine seminal plasma application (Crawford 2015; Von Wolff 2013) Despite the lack of statistical evidence of a subgroup difference, the point estimates observed in these two trials are on the opposite side of the unity line compared to the rest of the included trials. It is possible that intrauterine administration of a relatively large volume of seminal plasma could have affected the implantation process. A sensitivity analysis, excluding these two RCTs, showed similar live birth rates, but significantly increased the live birth/ongoing pregnancy rate (RR: 1.32, 95% CI 1.02, 1.70) and clinical pregnancy rate (RR: 1.21, 95% CI 1.04, 1.41). The absolute increase in clinical pregnancy rate in this sensitivity analysis was 4.7% with a number needed to treat value of 22 for one additional clinical pregnancy.
Per‐pregnancy comparisons for multiple pregnancy, miscarriage and ectopic pregnancy rates were similar to intention to treat analysis results, further strengthening our results.
The evidence is generally applicable to women undergoing fresh ART cycles with ejaculated sperm. There was only one trial reporting ART outcomes with seminal plasma exposure in frozen embryo transfers.
Quality of the evidence
The overall quality of the evidence was low for most outcomes.
The main limitations in the evidence were imprecision, and risk of bias associated with poor reporting of study methods. Seven RCTs did not report the method for allocation concealment.
None of the studies reported funding by pharmaceutical companies.
Potential biases in the review process
The review authors minimised the risk of bias by conducting a search that was systematic and thorough and by having two review authors independently perform the data extraction, 'risk of bias' assessment, and GRADE evaluation.
Agreements and disagreements with other studies or reviews
Our results are in agreement with those of a previous systematic review and meta‐analysis investigating the role of seminal plasma for improving ART outcomes (Crawford 2015b). The literature search in this study was conducted in December 2013 and the authors included seven of the 11 studies that are included in our review.
Authors' conclusions
Implications for practice.
In women undergoing ART, there was insufficient evidence to determine whether there was a difference between the seminal plasma and the standard ART group in rates of live birth (low‐quality evidence) or miscarriage (low‐quality evidence). There was low‐quality evidence suggesting little or no difference between the groups in rates of live birth or ongoing pregnancy (composite outcome). We found very low‐quality evidence that seminal plasma application may possibly be associated with more clinical pregnancies than standard ART. There was low‐quality evidence suggesting little or no difference between the groups in rates of multiple pregnancy. There was insufficient evidence to reach any conclusions about the risk of ectopic pregnancy, and no data were available on infectious complications or other adverse events.
We conclude that seminal plasma application is worth further investigation, focusing on live birth and miscarriage rates.
Implications for research.
We suggest more and adequately powered RCTs reporting live birth rates after seminal plasma application. The leading cause of implantation failure is embryo aneuploidy, therefore, ART cycles with only euploid embryo transfers would be the ideal setting to assess the effectiveness of seminal plasma application, particularly in women with recurrent implantation failure.
What's new
| Date | Event | Description |
|---|---|---|
| 9 March 2018 | Amended | Clarifications and corrections made to review text in order to meet Cochrane standards |
Acknowledgements
We thank the editorial office of the Cochrane Gynaecology and Fertility Group for their assistance.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group Specialised Register search strategy
Searched 16 October 2017
Procite platform
Keywords CONTAINS "ART" or "assisted reproduction" or "assisted reproduction techniques" or "IVF" or "ICSI" or "in vitro fertilisation" or "in‐vitro fertilisation techniques" or "in vitro fertilization" or "in vitro maturation" or "intracytoplasmic sperm injection" or "subfertility" or "Infertility" or "IUI" or "Intrauterine Insemination" or "*Embryo Transfer" or "ET" or Title CONTAINS "ART" or "assisted reproduction" or "assisted reproduction techniques" or "IVF" or "ICSI" or "in vitro fertilisation" or "in‐vitro fertilisation techniques" or "in vitro fertilization" or "in vitro maturation" or "intracytoplasmic sperm injection" or "subfertility" or "Infertility" or "IUI" or "Intrauterine Insemination" or "*Embryo Transfer" or "ET"
AND
Keywords CONTAINS "seminal fluid" or"*Semen" or "seminal plasma" or "sperm" or "ejaculated sperm" or "ejaculation" or "intercourse" or "coitus" or Title CONTAINS "seminal fluid" or"*Semen" or "seminal plasma" or "sperm" or "ejaculated sperm" or "ejaculation" or "intercourse" or "coitus"
AND
Keywords CONTAINS "Vaginal" or "vaginal application" or "vaginal preparation" or "intracervical" or "intracervical insemination" or "intrauterine" or "intrautero tuboperitoneal insemination" or "Intravaginal" or "cervical" or "cervix"or"insemination‐pericervical"or"insemination‐intrauterine"or"insemination, intracervical" or "insemination‐cervical cap" or "Endometrium" or Title CONTAINS "Vaginal" or "vaginal application" or "vaginal preparation" or "intracervical" or "intracervical insemination" or "intrauterine" or "intrautero tuboperitoneal insemination" or "Intravaginal" or "cervical" or "cervix"or"insemination‐pericervical"or"insemination‐intrauterine"or"insemination, intracervical" or "insemination‐cervical cap" or "Endometrium" (404 hits)
Appendix 2. CENTRAL Register of Studies Online (CRSO) search strategy
Searched 16 October 2017
Web platform
#1 MESH DESCRIPTOR Embryo Transfer EXPLODE ALL TREES 967
#2 MESH DESCRIPTOR Fertilization in Vitro EXPLODE ALL TREES 1861
#3 MESH DESCRIPTOR sperm injections, intracytoplasmic EXPLODE ALL TREES 481
#4 (embryo* transfer*):TI,AB,KY 2365
#5 (vitro fertili?ation):TI,AB,KY 2149
#6 (ivf or icsi):TI,AB,KY 4042
#7 (intracytoplasmic sperm injection*):TI,AB,KY 1315
#8 (blastocyst* adj2 transfer*):TI,AB,KY 253
#9 MESH DESCRIPTOR Reproductive Techniques, Assisted EXPLODE ALL TREES 2848
#10 MESH DESCRIPTOR Insemination, Artificial EXPLODE ALL TREES 345
#11 MESH DESCRIPTOR Ovulation Induction EXPLODE ALL TREES 1203
#12 (assisted reproduct*):TI,AB,KY 829
#13 (artificial insemination):TI,AB,KY 182
#14 iui:TI,AB,KY 550
#15 (intrauterine insemination):TI,AB,KY 707
#16 (ovulation induc*):TI,AB,KY 1915
#17 (ovary* adj2 stimulat*):TI,AB,KY 18
#18 superovulat*:TI,AB,KY 176
#19 (ovarian hyperstimulation):TI,AB,KY 950
#20 infertil*:TI,AB,KY 4472
#21 subfertil*:TI,AB,KY 598
#22 (ovary* adj2 induction):TI,AB,KY 137
#23 MESH DESCRIPTOR Oocyte Retrieval EXPLODE ALL TREES 147
#24 (Oocyte* adj2 Retrieval*):TI,AB,KY 818
#25 (Oocyte* adj2 pick up*):TI,AB,KY 51
#26 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 9185
#27 (seminal adj5 intravagina*):TI,AB,KY 1
#28 (seminal adj5 plasma*):TI,AB,KY 213
#29 (seminal adj5 vagina*):TI,AB,KY 4
#30 (seminal adj5 intracervi*):TI,AB,KY 0
#31 (seminal adj5 cervi*):TI,AB,KY 4
#32 (seminal adj5 instillation*):TI,AB,KY 0
#33 (seminal adj5 intrauter*):TI,AB,KY 0
#34 (seminal adj5 uter*):TI,AB,KY 2
#35 (seminal adj5 inseminat*):TI,AB,KY 5
#36 (seminal adj5 injection*):TI,AB,KY 1
#37 (seminal adj5 endometr*):TI,AB,KY 3
#38 (semen adj5 intravagina*):TI,AB,KY 0
#39 (semen adj5 vagina*):TI,AB,KY 15
#40 (semen adj5 intracervi*):TI,AB,KY 2
#41 (semen adj5 cervi*):TI,AB,KY 11
#42 (semen adj5 instillation*):TI,AB,KY 0
#43 (semen adj5 intrauter*):TI,AB,KY 12
#44 (semen adj5 uter*):TI,AB,KY 15
#45 (semen adj5 injection*):TI,AB,KY 40
#46 (semen adj5 endometr*):TI,AB,KY 1
#47 (semen adj5 intracervi*):TI,AB,KY 2
#48 (ejaculate* adj5 intracerv*):TI,AB,KY 1
#49 (ejaculate* adj5 vagina*):TI,AB,KY 1
#50 (ejaculate* adj5 intravagina*):TI,AB,KY 1
#51 (ejaculate* adj5 cervi*):TI,AB,KY 0
#52 (ejaculate* adj5 instillation*):TI,AB,KY 0
#53 (ejaculate* adj5 intrauter*):TI,AB,KY 1
#54 (ejaculate* adj5 uter*):TI,AB,KY 1
#55 (ejaculate* adj5 injection*):TI,AB,KY 1
#56 (ejaculate* adj5 endometr*):TI,AB,KY 0
#57 (intercourse adj7 embryo*):TI,AB,KY 5
#58 (coitus adj7 embryo*):TI,AB,KY 3
#59 (ejaculate* adj7 embryo*):TI,AB,KY 1
#60 MESH DESCRIPTOR coitus EXPLODE ALL TREES 311
#61 MESH DESCRIPTOR Ejaculation EXPLODE ALL TREES 198
#62 MESH DESCRIPTOR Spermatozoa EXPLODE ALL TREES 407
#63 #60 OR #61 OR #62 874
#64 #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 304
#65 #63 AND #64 57
#66 #64 OR #65 304
#67 #26 AND #66 192
Appendix 3. MEDLINE search strategy
Searched from 1946 to 16 October 2017
Ovid platform
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (40320) 2 embryo transfer$.tw. (10937) 3 vitro fertili?ation.tw. (22192) 4 ivf‐et.tw. (2465) 5 ivf.tw. (22151) 6 icsi.tw. (7338) 7 intracytoplasmic sperm injection$.tw. (6437) 8 (blastocyst adj2 transfer$).tw. (821) 9 exp reproductive techniques, assisted/ or exp insemination, artificial/ or exp ovulation induction/ (66566) 10 assisted reproduct$.tw. (12890) 11 artificial insemination.tw. (6205) 12 iui.tw. (1654) 13 intrauterine insemination$.tw. (2382) 14 ovulation induc$.tw. (4243) 15 (ovari$ adj2 stimulat$).tw. (6482) 16 superovulat$.tw. (3381) 17 ovarian hyperstimulation.tw. (4994) 18 COH.tw. (1559) 19 infertil$.tw. (57008) 20 subfertil$.tw. (4797) 21 (ovari$ adj2 induction).tw. (280) 22 exp Oocyte Retrieval/ (1384) 23 Oocyte Retrieval$.tw. (2666) 24 oocyte$ pick up$.tw. (204) 25 or/1‐24 (130075) 26 exp Semen/ (19619) 27 (seminal adj5 intravagina$).tw. (15) 28 (seminal adj5 plasma$).tw. (6706) 29 (seminal adj5 vagina$).tw. (173) 30 (seminal adj5 intracervi$).tw. (2) 31 (seminal adj5 cervi$).tw. (142) 32 (seminal adj5 instillation$).tw. (3) 33 (seminal adj5 intrauter$).tw. (19) 34 (seminal adj5 uter$).tw. (190) 35 (seminal adj5 inseminat$).tw. (63) 36 (seminal adj5 injection$).tw. (49) 37 (seminal adj5 endometr$).tw. (43) 38 (semen adj5 intravagina$).tw. (26) 39 (semen adj5 vagina$).tw. (503) 40 (semen adj5 intracervi$).tw. (14) 41 (semen adj5 cervi$).tw. (278) 42 (semen adj5 instillation$).tw. (2) 43 (semen adj5 intrauter$).tw. (167) 44 (semen adj5 uter$).tw. (147) 45 (semen adj5 injection$).tw. (118) 46 (semen adj5 endometr$).tw. (38) 47 (ejaculat$ adj5 intracervi$).tw. (3) 48 (ejaculat$ adj5 vagina$).tw. (182) 49 (ejaculat$ adj5 intravagina$).tw. (341) 50 (ejaculat$ adj5 cervi$).tw. (36) 51 (ejaculat$ adj5 instillation$).tw. (3) 52 (ejaculat$ adj5 intrauter$).tw. (17) 53 (ejaculat$ adj5 uter$).tw. (45) 54 (ejaculat$ adj5 injection$).tw. (133) 55 (ejaculat$ adj5 endometr$).tw. (1) 56 (ejaculat$ adj5 intravagina$).tw. (341) 57 (intercourse adj7 embryo$).tw. (7) 58 (coitus adj7 blastocyst$).tw. (9) 59 (coitus adj7 embryo$).tw. (63) 60 (ejaculat$ adj7 embryo$).tw. (76) 61 (ejaculat$ adj7 blastocyst$).tw. (18) 62 (seminal adj3 priming).tw. (7) 63 coitus/ or ejaculation/ (13611) 64 or/26‐63 (35255) 65 25 and 64 (8158) 66 randomized controlled trial.pt. (496904) 67 controlled clinical trial.pt. (99253) 68 randomized.ab. (433409) 69 randomised.ab. (87389) 70 placebo.tw. (208017) 71 clinical trials as topic.sh. (195527) 72 randomly.ab. (298737) 73 trial.ti. (195716) 74 (crossover or cross‐over or cross over).tw. (80801) 75 or/66‐74 (1269996) 76 exp animals/ not humans.sh. (4677556) 77 75 not 76 (1171818) 78 65 and 77 (416)
Appendix 4. Embase search strategy
Searched from 1980 to 16 October 2017
Ovid platform
1 (seminal adj5 intravagina$).tw. (23) 2 (seminal adj5 vagina$).tw. (173) 3 (seminal adj5 intracervi$).tw. (6) 4 (seminal adj5 cervi$).tw. (131) 5 (seminal adj5 instillation$).tw. (7) 6 (seminal adj5 intrauter$).tw. (22) 7 (seminal adj5 uter$).tw. (201) 8 (seminal adj5 inseminat$).tw. (59) 9 (seminal adj5 injection$).tw. (56) 10 (seminal adj5 endometr$).tw. (63) 11 (semen adj5 intravagina$).tw. (26) 12 (semen adj5 vagina$).tw. (502) 13 (semen adj5 intracervi$).tw. (14) 14 (semen adj5 cervi$).tw. (244) 15 (semen adj5 instillation$).tw. (0) 16 (semen adj5 intrauter$).tw. (176) 17 (semen adj5 uter$).tw. (146) 18 (semen adj5 injection$).tw. (136) 19 (semen adj5 endometr$).tw. (48) 20 seminal fluid.tw. (2481) 21 (seminal adj3 plasma$).tw. (6635) 22 exp seminal plasma/ (6375) 23 (ejaculat$ adj5 intracervi$).tw. (2) 24 (ejaculat$ adj5 vagina$).tw. (238) 25 (ejaculat$ adj5 intravagina$).tw. (496) 26 (ejaculat$ adj5 cervi$).tw. (35) 27 (ejaculat$ adj5 intrauter$).tw. (25) 28 (ejaculat$ adj5 uter$).tw. (48) 29 (ejaculat$ adj5 injection$).tw. (179) 30 (ejaculat$ adj5 endometr$).tw. (2) 31 (ejaculat$ adj5 intravagina$).tw. (496) 32 (intercourse adj7 embryo$).tw. (19) 33 (coitus adj7 blastocyst$).tw. (9) 34 (coitus adj7 embryo$).tw. (62) 35 (ejaculat$ adj7 embryo$).tw. (122) 36 (ejaculat$ adj7 blastocyst$).tw. (25) 37 (seminal adj3 priming).tw. (18) 38 coitus/ (5867) 39 ejaculation/ (8334) 40 or/1‐37 (13222) 41 38 or 39 (13953) 42 40 and 41 (1105) 43 40 or 42 (13222) 44 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (58626) 45 embryo$ transfer$.tw. (18006) 46 in vitro fertili?ation.tw. (26529) 47 icsi.tw. (13887) 48 intracytoplasmic sperm injection$.tw. (8367) 49 (blastocyst adj2 transfer$).tw. (1929) 50 ivf.tw. (34746) 51 exp infertility therapy/ or exp artificial insemination/ or exp intrauterine insemination/ or exp ovulation induction/ (86147) 52 assisted reproduct$.tw. (18955) 53 artificial insemination.tw. (5540) 54 iui.tw. (2796) 55 intrauterine insemination$.tw. (3297) 56 ovulation induc$.tw. (5203) 57 (ovari$ adj2 stimulat$).tw. (9655) 58 superovulat$.tw. (3517) 59 ovarian hyperstimulation.tw. (6740) 60 COH.tw. (2119) 61 infertil$.tw. (72808) 62 subfertil$.tw. (6020) 63 (ovari$ adj2 induction).tw. (333) 64 exp oocyte retrieval/ (5521) 65 Oocyte Retrieval$.tw. (4130) 66 oocyte$ pick up$.tw. (388) 67 or/44‐66 (167021) 68 43 and 67 (4341) 69 Clinical Trial/ (949969) 70 Randomized Controlled Trial/ (471914) 71 exp randomization/ (75860) 72 Single Blind Procedure/ (29732) 73 Double Blind Procedure/ (140776) 74 Crossover Procedure/ (53437) 75 Placebo/ (300796) 76 Randomi?ed controlled trial$.tw. (168408) 77 Rct.tw. (25850) 78 random allocation.tw. (1695) 79 randomly allocated.tw. (28434) 80 allocated randomly.tw. (2269) 81 (allocated adj2 random).tw. (785) 82 Single blind$.tw. (19880) 83 Double blind$.tw. (175965) 84 ((treble or triple) adj blind$).tw. (717) 85 placebo$.tw. (256628) 86 prospective study/ (405705) 87 or/69‐86 (1812459) 88 case study/ (50227) 89 case report.tw. (340144) 90 abstract report/ or letter/ (1013008) 91 or/88‐90 (1395186) 92 87 not 91 (1766305) 93 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5884177) 94 92 not 93 (1701104) 95 68 and 94 (418)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 16 October 2017
Ovid platform
1 exp reproductive technology/ (1654) 2 in vitro fertili?ation.tw. (672) 3 ivf‐et.tw. (17) 4 (ivf or et).tw. (123147) 5 icsi.tw. (67) 6 intracytoplasmic sperm injection$.tw. (50) 7 (blastocyst adj2 transfer$).tw. (4) 8 assisted reproduct$.tw. (819) 9 artificial insemination.tw. (243) 10 iui.tw. (31) 11 intrauterine insemination$.tw. (23) 12 ovulation induc$.tw. (27) 13 (ovari$ adj2 stimulat$).tw. (55) 14 ovarian hyperstimulation.tw. (11) 15 COH.tw. (97) 16 superovulat$.tw. (6) 17 infertil$.tw. (3145) 18 subfertil$.tw. (82) 19 (ovari$ adj2 induction).tw. (7) 20 or/1‐19 (127435) 21 exp Sperm/ (826) 22 semen.tw. (438) 23 seminal.tw. (4782) 24 or/21‐23 (5878) 25 20 and 24 (477) 26 random.tw. (51148) 27 control.tw. (395551) 28 double‐blind.tw. (21024) 29 clinical trials/ (10608) 30 placebo/ (4990) 31 exp Treatment/ (696683) 32 or/26‐31 (1080895) 33 25 and 32 (118)
Appendix 6. CINAHL search strategy
Searched 1961 to 16 October 2017
Ebsco platform
| # | Query | Results |
| S58 | S45 AND S57 | 53 |
| S57 | S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 | 1,168,494 |
| S56 | TX allocat* random* | 7,286 |
| S55 | (MH "Quantitative Studies") | 16,546 |
| S54 | (MH "Placebos") | 10,402 |
| S53 | TX placebo* | 47,633 |
| S52 | TX random* allocat* | 7,286 |
| S51 | (MH "Random Assignment") | 44,289 |
| S50 | TX randomi* control* trial* | 132,848 |
| S49 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 912,372 |
| S48 | TX clinic* n1 trial* | 212,242 |
| S47 | PT Clinical trial | 80,036 |
| S46 | (MH "Clinical Trials+") | 222,921 |
| S45 | S26 AND S44 | 242 |
| S44 | S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 | 1,023 |
| S43 | TX(seminal N3 priming) | 2 |
| S42 | TX(ejaculat* N7 embryo*) | 3 |
| S41 | TX (intercourse N7 embryo*) | 1 |
| S40 | TX semen N5 injection* | 3 |
| S39 | TX seminal plasma | 91 |
| S38 | TX(semen N5 inseminat*) | 20 |
| S37 | TX(semen N5 uter*) | 3 |
| S36 | TX (semen N5 intrauter*) | 9 |
| S35 | TX (semen N5 cervi*) | 15 |
| S34 | TX (semen N5 intravagina*) | 1 |
| S33 | TX (seminal N5 uter*) | 4 |
| S32 | TX (seminal N5 intrauter*) | 2 |
| S31 | TX (seminal N5 cervi*) | 6 |
| S30 | TX (seminal N5 vagina*) | 5 |
| S29 | TX (seminal N5 intravagina*) | 2 |
| S28 | (MM "Spermatozoa") | 656 |
| S27 | (MM "Semen") | 310 |
| S26 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 | 10,930 |
| S25 | TX intra‐uterine insemination | 15 |
| S24 | TX coitus | 2,005 |
| S23 | (MM "Coitus") | 880 |
| S22 | TX natural cycle* | 173 |
| S21 | TX expectant management | 638 |
| S20 | TX timed intercourse | 29 |
| S19 | TX oocyte* N2 pick up* | 11 |
| S18 | TX embryo transfer or TX oocyte* retrieval$ | 1,146 |
| S17 | TX ovarian hyperstimulation | 443 |
| S16 | TX superovulat* | 27 |
| S15 | TX ovulation induc* | 721 |
| S14 | TX intrauterine insemination | 214 |
| S13 | TX IUI | 143 |
| S12 | TX artificial insemination | 525 |
| S11 | TX assisted reproduct* | 1,940 |
| S10 | (MM "Insemination, Artificial") | 267 |
| S9 | (MM "Reproduction Techniques+") | 4,822 |
| S8 | TX intracytoplasmic sperm injection* | 376 |
| S7 | TX embryo* N3 transfer* | 1,115 |
| S6 | TX ovar* N3 hyperstimulat* | 447 |
| S5 | TX ovari* N3 stimulat* | 407 |
| S4 | TX IVF or TX ICSI | 2,112 |
| S3 | (MM "Fertilization in Vitro") | 1,763 |
| S2 | TX vitro fertilization | 3,806 |
| S1 | TX vitro fertilisation | 3,806 |
Appendix 7. Search strategy for ClinicalTrials.gov, WHO ICTRP Search Portal, DARE, Web of Knowledge, OpenGrey, LILACS, PubMed, Google Scholar
Web platforms
searched 16 October 2017
Combinations of words: "seminal plasma", "intercourse", ejaculate", "insemination", "IVF", "assisted reproduction", "embryo transfer"
Data and analyses
Comparison 1. Seminal plasma vs standard ART.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth | 3 | 948 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.86, 1.43] |
| 1.1 Vaginal only | 1 | 569 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.91, 1.81] |
| 1.2 Cervical only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Vaginal and cervical | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.53, 2.24] |
| 1.4 Intrauterine only | 1 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.56, 1.35] |
| 2 Miscarriage | 4 | 1209 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.57, 1.79] |
| 2.1 Vaginal only | 1 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.29, 3.37] |
| 2.2 Cervical only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Vaginal and cervical | 2 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.36, 1.92] |
| 2.4 Intrauterine only | 1 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.49, 3.82] |
| 3 Live birth or ongoing pregnancy | 4 | 1178 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.95, 1.49] |
| 3.1 Vaginal only | 1 | 569 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.91, 1.81] |
| 3.2 Cervical only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Vaginal and cervical | 2 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.93, 1.99] |
| 3.4 Intrauterine only | 1 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.56, 1.35] |
| 4 Clinical pregnancy | 10 | 2768 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.01, 1.31] |
| 4.1 Vaginal only | 3 | 1142 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.92, 1.85] |
| 4.2 Cervical only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Vaginal and cervical | 5 | 1161 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.01, 1.40] |
| 4.4 Intrauterine only | 2 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.68, 1.27] |
| 5 Multiple pregnancy | 5 | 1642 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.76, 1.64] |
| 5.1 Vaginal only | 3 | 1142 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.89, 2.69] |
| 5.2 Cervical only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Vaginal and cervical | 2 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.56, 1.33] |
| 5.4 Intrauterine only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Ectopic pregnancy | 5 | 1521 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.20, 12.78] |
| 6.1 Vaginal only | 3 | 1142 | Risk Ratio (M‐H, Random, 95% CI) | 2.85 [0.12, 68.83] |
| 6.2 Cervical only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Vaginal and cervical | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Intrauterine only | 1 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.06, 16.17] |
| 7 Clinical pregnancy: Sensitivity analysis by RoB | 3 | 547 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.81, 1.39] |
| 7.1 Vaginal and cervical | 2 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.84, 1.83] |
| 7.2 Intrauterine only | 1 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.63, 1.34] |
Comparison 2. Seminal plasma vs standard ART, per‐pregnancy analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Miscarriage | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.54, 1.57] |
| 1.1 Vaginal only | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.26, 2.73] |
| 1.2 Cervical only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Vaginal and cervical | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.32, 1.51] |
| 1.4 Intrauterine only | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.57, 3.86] |
| 2 Multiple pregnancy | 5 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.24] |
| 2.1 Vaginal only | 3 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.79, 1.99] |
| 2.2 Cervical only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Vaginal and cervical | 2 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.52, 1.11] |
| 2.4 Intrauterine only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Ectopic pregnancy | 5 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.15, 8.98] |
| 3.1 Vaginal only | 3 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.06, 26.80] |
| 3.2 Cervical only | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Vaginal and cervical | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Intrauterine only | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.07, 17.09] |
Comparison 3. Grouped by fresh or frozen embryo transfer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth | 3 | 948 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.86, 1.43] |
| 1.1 Fresh transfer | 3 | 948 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.86, 1.43] |
| 1.2 Frozen transfer | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Miscarriage | 4 | 1209 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.56, 1.79] |
| 2.1 Fresh transfer | 4 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.57, 1.95] |
| 2.2 Frozen transfer | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.11, 3.75] |
| 3 Live birth or ongoing pregnancy | 4 | 1178 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.95, 1.49] |
| 3.1 Fresh transfer | 4 | 1178 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.95, 1.49] |
| 3.2 Frozen transfer | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Clinical pregnancy | 10 | 2768 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.01, 1.31] |
| 4.1 Fresh transfer | 10 | 2568 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.02, 1.32] |
| 4.2 Frozen transfer | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.44, 2.11] |
| 5 Multiple pregnancy | 5 | 1642 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.75, 1.60] |
| 5.1 Fresh transfer | 5 | 1442 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.74, 1.46] |
| 5.2 Frozen transfer | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 6.73 [0.35, 128.59] |
| 6 Ectopic pregnancy | 5 | 1521 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.20, 12.78] |
| 6.1 Fresh transfer | 5 | 1321 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.20, 12.78] |
| 6.2 Frozen transfer | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
Comparison 3 Grouped by fresh or frozen embryo transfer, Outcome 1 Live birth.
3.3. Analysis.
Comparison 3 Grouped by fresh or frozen embryo transfer, Outcome 3 Live birth or ongoing pregnancy.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aflatoonian 2009.
| Methods | Parallel group study Number of women randomised: 390 (195 in the intervention group; 195 in the control group) Number of women analysed: 385 |
|
| Participants | Country of authors: Iran Inclusion criteria: couples undergoing assisted reproduction with at least 5 years of subfertility Exclusion criteria: none reported Mean age ± SD: intervention group: 29.4 ± 4.4 years; control group: 29.6 ± 4.9 years Mean duration of infertility ± SD: intervention group: 8.7 ± 3.8 years; control group: 9.0 ± 3.9 years Women with primary infertility: not reported Setting: assisted reproduction programme in Iran |
|
| Interventions | Intervention: vaginal intercourse at least once during the 12 hours following embryo transfer Control: abstinence during the entire ART cycle |
|
| Outcomes | Clinical pregnancy defined as the presence of a gestational sac or fetal cardiac activity three weeks after embryo transfer | |
| Notes | Pregnancy outcome was unknown for five women in the intervention arm. The corresponding author was contacted but was not able to provide missing data either for the five women with unknown primary outcome or other unreported outcome measures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Equal number of paper slips for each method put in a bag |
| Allocation concealment (selection bias) | Low risk | Drawing paper slips from the bag on the day of embryo transfer |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Couples in the control group could have had intercourse without informing the investigators and contaminated the results |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objectively assessed outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Pregnancy outcome missing for 5 women in the intervention group |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy rate was reported |
| Other bias | Low risk | No other cause of potential bias identified |
Bellinge 1986.
| Methods | Parallel group study Number of women randomised: 152 (78 in the intervention group; 74 in the control group) Number of women analysed: 113 |
|
| Participants | Country of authors: Australia Inclusion criteria: couples undergoing assisted reproduction with fresh ejaculate sperm Exclusion criteria: none reported Mean age (SD not stated): intervention group: 32.2 years; control group: 31.9 years Mean duration of infertility: not stated Women with primary infertility: not reported Setting: assisted reproduction programme in Western Australia |
|
| Interventions | Intervention: untreated fresh ejaculate was inseminated in the vagina at the time of oocyte fertilisation Control: women in both groups were instructed to abstain from vaginal intercourse 4 days before oocyte pick‐up |
|
| Outcomes | Clinical pregnancy defined as the presence of a gestational sac | |
| Notes | We were unable to contact the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table was used |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objectively measured outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10 to 30% of participants excluded after commencement, but similar proportions in both control and intervention groups. ITT analysis was presented |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy rate reported |
| Other bias | Low risk | No other cause of potential bias identified |
Chicea 2013.
| Methods | Parallel group study Number of women randomised: 400 (200 in the intervention group; 200 in the control group) Number of cancellations: 54 (36 in the intervention group due to severe leukocytospermia, severe ovarian hyperstimulation syndrome, lack of oocytes after follicular aspiration, fertilisation failure, lack of top quality embryos; 18 in control group due to severe ovarian hyperstimulation syndrome, lack of oocytes after follicular aspiration, lack of top quality embryos) Number of women analysed: 346 |
|
| Participants | Country of authors: Romania Inclusion criteria: women aged < 38 years, < 4 previous IVF attempts Exclusion criteria: Infection in the male partner or leukocytospermia, Hepatitis B, or C or HIV positivity in any partner, syphilis in any partner, uterine anomalies Transfer of top quality embryos was required for inclusion in the analysis Mean age (SD not reported): intervention group: 33.1 years; control group: 33.9 years Mean duration of infertility ± SD: intervention group: 8.7 ± 3.8 years; control group: 9.0 ± 3.9 years Women with primary infertility: not reported Setting: assisted reproduction programme in Romania |
|
| Interventions | Intervention: 0.5 ml of seminal plasma was injected 1 to 2 cm into the uterine cervix immediately after the oocyte pick‐up procedure. The rest of the available seminal plasma (0 to 1 ml) was deposited at the posterior vaginal fornix. Control: women in both groups were instructed to abstain from vaginal intercourse 3 days before oocyte pick‐up until the pregnancy test. |
|
| Outcomes | Clinical pregnancy defined as the presence of a gestational sac or fetal pole four weeks after oocyte pick‐up | |
| Notes | We tried to contact the corresponding author by email, twice, one month apart, but did not receive a response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | High risk | The randomisation sequence was not concealed from the investigators |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were randomised on the day of ovulation trigger, halfway through treatment. Although unlikely, it is not impossible that the rest of the treatment procedures could be affected by the knowledge of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objectively measured outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Even though the participants were randomised on the day of ovulation trigger, only those who had good quality embryos were included in the analyses. Embryo quality is a subjectively assessed parameter and selective inclusion is possible, especially in the absence of allocation concealment. |
| Selective reporting (reporting bias) | High risk | Only women undergoing embryo transfer, and in addition with good quality embryos were included |
| Other bias | Low risk | No other cause of potential bias identified |
Crawford 2015.
| Methods | Parallel group study Number of women randomised: 186 (91 in the intervention group; 95 in the control group) Number of cancellations: none reported Number of women analysed: 186 |
|
| Participants | Country of authors: United Kingdom and Australia Inclusion criteria: women aged 23 to 39 years, < 2 previous IVF attempts Exclusion criteria: not reported. Mean age: not reported Mean duration of infertility: not reported Women with primary infertility: not reported Setting: assisted reproduction programme in Homerton University Hospital |
|
| Interventions | Intervention: intrauterine injection of 0.5 ml seminal plasma immediately after the oocyte pick‐up procedure Control: intrauterine injection of 0.5 ml culture medium immediately after the oocyte pick‐up procedure |
|
| Outcomes | Clinical pregnancy rate | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both clinicians and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objectively measured outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Accounted for all randomised women |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess |
| Other bias | Unclear risk | Unable to assess |
Friedler 2013.
| Methods | Parallel group study Number of women randomised: 230 (106 in the intervention group; 124 in the control group) Number of cancellations: 10 (3 women in intervention group did not undergo an embryo transfer; 7 women in control group did not undergo an embryo transfer) Number of women analysed: 230 |
|
| Participants | Country of authors: Israel Inclusion criteria: women aged < 40 years, having at least 1 previous failed IVF attempt Exclusion criteria: prior cycle cancellation due to lack of oocytes, use of donor sperm, endometriosis, Hepatitis B, or C or HIV positivity or other infection in the male partner, leukocytospermia Mean age ± SD: intervention group: 31.2 ± 5.3 years; control group: 32 ± 5.7 years. Mean duration of infertility ± SD: intervention group: 2.5 ± 1.8 years; control group: 2.7 ± 1.7 years. Women with primary infertility: not reported Setting: assisted reproduction programme in Israel |
|
| Interventions | Intervention: intracervical injection of 0.5 ml seminal plasma was after the oocyte pick‐up procedure. The rest of the available seminal plasma was deposited at the posterior vaginal fornix. Control: intracervical injection of 0.5 ml culture medium was after the oocyte pick‐up procedure. The rest was deposited at the posterior vaginal fornix. Women in both groups were instructed to abstain from vaginal intercourse 2 days before oocyte pick ‐ up until 7 days after oocyte pick‐up. |
|
| Outcomes | Ongoing pregnancy defined as one that progressed beyond 22nd gestational week. Clinical pregnancy defined as the presence of gestational sac at 3 weeks gestational age. | |
| Notes | Live birth defined in the text but not reported. The corresponding author was contacted but live birth rates were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number sequence |
| Allocation concealment (selection bias) | Low risk | Allocation by an embryologist blinded to treating physician on the day of oocyte collection |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both physicians and patients were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objectively measured outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% attrition rate, which is similar between the study groups. Reasons for attrition given and intention to treat analysis was presented. |
| Selective reporting (reporting bias) | High risk | Live birth rate defined in the materials and methods, but not reported in the text. Multiple pregnancy rate was not reported. |
| Other bias | Low risk | No other cause of potential bias identified |
Jafarabadi 2016.
| Methods | Parallel group study Number of women randomised: 266 (133 in the intervention group; 133 in the control group) Number of cancellations: 3 women in the intervention group did not receive the intervention Number of women analysed: 263 |
|
| Participants | Country of authors: Iran Inclusion criteria: couples undergoing assisted reproduction with a female partner < 40 years of age Exclusion criteria: history of no oocytes in a former ART cycle, > 2 prior ART cycles, providing semen by any other means than ejaculation, active hepatitis B, or C or HIV in one partner, leukocytospermia Mean age ± SD: intervention group: 32.2 ± 6.0 years; control group: 31.1 ± 6.0 years. Mean duration of infertility ± SD: not reported. Women with primary infertility: not reported. Setting: assisted reproduction programme in Iran |
|
| Interventions | Intervention: intracervical injection of 0.5 ml seminal plasma was after the oocyte pick‐up procedure. The rest of the available seminal plasma was deposited at the posterior vaginal fornix. Control: no intervention |
|
| Outcomes | Clinical pregnancy defined as visualisation of embryo by ultrasound at sixth gestational week | |
| Notes | We tried to contact the authors by email, twice one month apart, however did not receive a response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Permuted block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Embryologist not blinded for allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objectively measured outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Three women in the intervention group were unaccounted for in the analyses |
| Selective reporting (reporting bias) | Unclear risk | Several outcome measures that should be available to the authors were not reported, e.g. multiple pregnancy rate, miscarriage rate |
| Other bias | Unclear risk | Poor reporting quality |
Karimian 2010.
| Methods | Parallel group study Number of women randomised: 569 (284 in intervention group; 285 in control group) Number of cancellations: not reported Number of women analysed: not reported |
|
| Participants | Country of authors: Iran Inclusion criteria: women undergoing ART Exclusion criteria: not reported Mean age: not reported Mean duration of infertility: not reported Women with primary infertility: not reported Setting: assisted reproduction programme in Tehran |
|
| Interventions | Intervention: intercourse around the time of ART Control: abstinence during ART cycle |
|
| Outcomes | Biocehmical pregnancy and live birth without gestational age at delivery | |
| Notes | Abstract. We were unable to contact the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objectively measured outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Unable to assess |
Mayer 2015.
| Methods | Parallel group study Number of women randomised: 100 (50 in the intervention group; 50 in the control group) Number of cancellations: 15 (6 women in the intervention group did not undergo an embryo transfer; 9 women in the control group did not undergo an embryo transfer) Number of women analysed: 87. Pregnancy data were available for all 100 and ITT analyses were possible for outcomes relevant to our review. |
|
| Participants | Country of authors: Austria Inclusion criteria: nulliparous women aged 18 to 41 years, nonsmoker, body mass index < 35 kg/m2, undergoing 1st or 2nd ART cycle Exclusion criteria: cancellation of embryo transfer due to total fertilisation failure or pending OHSS, Hepatitis B, or C or HIV infection or leukocytospermia in the male partner, seminal plasma volume < 0.5 ml, uterine or endometrial anomaly, indication for seminal plasma application Mean age ± SD: intervention group: 32.2 ± 4.48 years; control group: 31.1 ± 4.63 years Mean duration of infertility: not reported Women with primary infertility: not reported Setting: Landes Frauen und Kinderklinik Linz, Austria |
|
| Interventions | Intervention: 0.5 to 1.5 ml seminal plasma application into the cervical canal by an intrauterine insemination catheter right after oocyte pick‐up. The rest of SP was applied at the posterior fornix. Comparator: saline application in the same way. |
|
| Outcomes | Clinical pregnancy, live birth, multiple pregnancy, ectopic pregnancy, miscarriage. Definitions were not reported. | |
| Notes | The authors provided unpublished data on baseline characteristics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated block randomisation list prepared by an independent party |
| Allocation concealment (selection bias) | Unclear risk | The embryologist had the sequence, but whether the next allocation was concealed somehow is not mentioned at all. However, the patients were recruited by the clinician in advance. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinician and participant blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20% of women did not undergo an embryo transfer. Reasons for losses were reported and similar between the groups. ITT analysis done |
| Selective reporting (reporting bias) | Low risk | Live birth rate reported |
| Other bias | Low risk | None detected |
Tremellen 2000.
| Methods | Multicentre, parallel group study Randomisation stratified for centre, hence for fresh or frozen embryo transfer Number of women randomised: 600 (302 in the intervention group; 298 in the control group) Number of cancellations: 122 cycles (60 in the intervention group, 62 in the control group) which did not reach embryo transfer Number of cycles analysed: 478 |
|
| Participants | Country of authors: Spain and Australia Inclusion criteria: women aged 18 to 40 years, in a stable relationship, undergoing ART with fresh (in the Spanish centre) or frozen (in the Australian centre) embryo transfer Exclusion criteria: hepatitis B, or C or HIV positivity in the male partner Mean age ± SD: Spanish centre: intervention group: 33.3 ± 3.3 years, control group: 33.2 ± 3.3 years; Australian centre: intervention group: 33.8 ± 4.4 years, control group: 33.1 ± 4.4 years Mean duration of infertility ± SD: Spanish centre: intervention group: 4.7 ± 2.6 years, control group: 4.1 ± 2.8 years; Australian centre: intervention group: 5 ± 2.5 years, control group: 5.1 ± 2.8 years Women with primary infertility: not reported Setting: assisted reproduction programmes in Spain (Valencia and Murcia Clinics of the Instituto Valenciano de Infertilidad) and Australia (University of Adelaide Reproductive Medicine Unit) |
|
| Interventions | Interventions: fresh ART cycles in Spain: vaginal intercourse at least twice, 12 hours before oocyte pick up and 12 hours after embryo transfer Frozen embryo transfer cycles in Australia: vaginal intercourse at least once, during the period between four days before and two days after embryo transfer Comparator: abstinence during the same period. |
|
| Outcomes | Biochemical pregnancy, clinical pregnancy at 6 to 8 weeks of gestation, multiple pregnancy | |
| Notes | We contacted the corresponding author, who understandably did not have the missing data for this trial, which was published 18 years ago. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated block randomisation list stratified for centre |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes managed by 3rd party |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded, though they were asked about adherence to the protocol |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objectively measured outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 25% of cycles cancelled after randomisation. However, the proportion of cancelled cycles were similar between the groups and ITT analysis was done. |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy reported |
| Other bias | High risk | Significantly more embryos transferred in the control group |
Von Wolff 2009.
| Methods | Parallel group study Number of women randomised: 168 (84 in the intervention group; 84 in the control group) Number of cancellations: 31 cycles (14 in the intervention group, 17 in the control group) which did not reach embryo transfer Number of cycles analysed: 133 |
|
| Participants | Country of authors: Germany Inclusion criteria: women aged 18 to 42 years, in a stable relationship Exclusion criteria: cancellation of embryo transfer due to total fertilisation failure or pending OHSS, Hepatitis B, or C or HIV infection or leukocytospermia in the male partner, seminal plasma volume < 0.5 ml Mean age (SD not reported): intervention group: 34.4 years; control group: 34.1 years Mean duration of infertility: not reported Women with primary infertility: not reported Setting: ART Center of the Women's University Hospital, Heidelberg, Germany |
|
| Interventions | Intervention: 0.5 ml seminal plasma application into the cervical canal by an intrauterine insemination catheter right after oocyte pick‐up. The rest of SP was applied at the posterior fornix. Comparator: saline application in the same way. |
|
| Outcomes | Clinical pregnancy | |
| Notes | We tried to contact the authors by email, twice one month apart, however did not receive a response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified, block randomisation using lists |
| Allocation concealment (selection bias) | Unclear risk | Authors did not report that allocation concealment was used during the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical looking placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 to 30% attrition with reasons. Similar rate between the two groups |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy rate reported |
| Other bias | Low risk | No other cause of potential bias identified |
Von Wolff 2013.
| Methods | Parallel group study Number of women randomised: 279 (138 in the intervention group; 141 in the control group) Number of cancellations: 40 cycles (16 in the intervention group, 14 in the control group) which did not reach embryo transfer Number of cycles analysed: 279 |
|
| Participants | Country of authors: Germany Inclusion criteria: women undergoing IVF or ICSI Exclusion criteria: hepatitis B, or C or HIV infection or leukocytospermia in the male partner, seminal plasma volume < 0.3 ml Mean age (SD not reported): intervention group: 34.6 years; control group: 34.9 years Mean duration of infertility: not reported Women with primary infertility: not reported Setting: ART Center of the Women's University Hospital, Heidelberg, Germany |
|
| Interventions | Intervention: seminal plasma was collected 1 to 2 weeks before follicle aspiration and stored in sterile flasks. Following two rounds of centrifugation, 0.4 ml of supernatant was mixed with 1.6 ml sterile saline and stored at ‐20C. The solution was thawed 30 to 60 minutes before follicle aspiration and 1.5 ml was injected into the uterine cavity after follicle aspiration. Comparator: 1.5 ml sterile saline application after follicle aspiration |
|
| Outcomes | Clinical pregnancy, defined as an embryo with heart beat 4 to 5 weeks after follicle aspiration, live birth rate, ectopic pregnancy, miscarriage, and infection rates | |
| Notes | We tried to contact the authors by email, twice one month apart, however did not receive a response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified, block randomisation using lists |
| Allocation concealment (selection bias) | Unclear risk | Authors did not report that allocation concealment was used during the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical looking placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcome measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women were accounted for in the final analysis |
| Selective reporting (reporting bias) | Low risk | Live birth rate reported |
| Other bias | Low risk | None detected |
ART: assisted reproductive technology HIV: human immunodeficiency virus ICSI: intracytoplasmic sperm injection ITT: intention to treat IVF: in vitro fertilisation OHSS: ovarian hyperstimulation syndrome SD: standard deviation SP: seminal plasma
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Coulam 1995 | Couples who were trying spontaneous conception were recruited |
| Fishel 1989 | Quasi‐randomised design |
| Lou 2014 | Quasi‐randomised design |
Differences between protocol and review
The major change from the protocol was the formation of a new composite outcome measure of 'live birth/ongoing pregnancy rate'. We anticipated that few studies might report live birth and that this composite outcome might give us an extra and useful estimate of effectiveness.
We added a subgroup analysis comparing fresh versus frozen embryo transfer, as we considered this would be clinically useful.
We defined studies at (overall) high risk of bias for the purpose of the sensitivity analysis, as studies at unclear or high risk of bias in multiple domains.
Contributions of authors
Baris Ata conceived and wrote the protocol. He also drafted the text of review, extracted data from eligible trials, and conducted the analyses.
William Buckett provided a clinical perspective and reviewed the final text.
Ayse Seyhan provided a clinical perspective, screened literature search results, extracted data, and reviewed the final text.
Ahmed Abou‐Setta provided general advice on the protocol, screened the literature search results, adjudicated conflicting data extraction results, and reviewed the final text.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
WB, AS and AMAS have no interests to declare.
BA's institution has received fees for a consultancy, two lectures and a conference registration, all relating to contraception.
Edited (no change to conclusions)
References
References to studies included in this review
Aflatoonian 2009 {published data only (unpublished sought but not used)}
- Aflatoonian A, Ghandi S, Tabibnejad N. The effect of intercourse around embryo transfer on pregnancy rate in assisted reproductive technology cycles. International Journal of Fertility and Sterility 2009;2(4):169‐72. [Google Scholar]
Bellinge 1986 {published data only}
Chicea 2013 {published data only (unpublished sought but not used)}
- Chicea R, Ispasoiu F, Focsa M. Seminal plasma insemination during ovum‐pickup ‐ a method to increase pregnancy rate in IVF/ICSI procedure. A pilot randomized trial. Journal of Assisted Reproduction and Genetics 2013;30:569‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crawford 2015 {published data only (unpublished sought but not used)}
- Crawford G, Tovar G, Olivier F, Dilgil M, Gudi A, Shah A, et al. The effect of intrauterine injection of seminal plasma on IVF results a prospective double‐blind randomised placebo‐controlled trial. British Journal of Obstetrics and Gynaecology 2015;122(S1):379. [Google Scholar]
Friedler 2013 {published data only (unpublished sought but not used)}
- Friedler S, Ben‐Ami I, Gidoni Y, Strassburger D, Kasterstein E, Maslansky B, et al. Effect of seminal plasma application to the vaginal vault in in vitro fertilization or intracytoplasmic sperm injection treatment cycles ‐ a double‐blind, placebo‐controlled, randomized study. Journal of Assisted Reproduction and Genetics 2013;30:907‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jafarabadi 2016 {published data only (unpublished sought but not used)}
- Jafarabadi M, Sasani A, Ramezanzadeh F, Zandieh Z, Shariat M, Haghollahi F. Intracervical application of seminal plasma at the time of oocyte pickup during in vitro fertilization. Acta Medica Mediterranea 2016;32:2085‐90. [Google Scholar]
Karimian 2010 {published data only}
- Karimian L, Naghibi ZH, Yazdi PE, Moini A, Valojerdi MR, Akhondi MM, et al. The effect of intercourse on B HcG level and pregnancy outcome after IVF cycles. Reproductive Biomedicine Online 2010;20(Supplement 3):S65. [Google Scholar]
Mayer 2015 {published and unpublished data}
- Mayer RB, Ebner T, Yaman C, Hartl J, Sir A, Krain V, et al. Influence of intracervical and intravaginal seminal plasma on the endometrium in assisted reproduction: a double‐blind, placebo‐controlled, randomized study. Ultrasound in Obstetrics and Gynecology 2015;45:132‐8. [DOI] [PubMed] [Google Scholar]
- Mayer RB, Shebl O, Krain V, Hartl J, Oppelt P, Ebner T. The influence of intracervical and intravaginal application of seminal plasma on the endometrium and life birth rate: a prospective, double blind, placebo controlled, randomized study. Geburtshilfe und Frauenheilkunde 2013;73:P 08. [Google Scholar]
Tremellen 2000 {published data only}
Von Wolff 2009 {published data only}
- Germeyer A, Rösner S, Jauckus J, Strowitzki T, Wolff M. Intrauterine application of diluted seminal plasma in in vitro fertilization does not improve pregnancy rates ‐ a placebo controlled double blind randomized trial. Human Reproduction 2013;28(Supp 1):i215. [DOI] [PubMed] [Google Scholar]
- Wolff M, Rösner S, Thöne C, Pinheiro RM, Jauckus J, Bruckner T, et al. Intravaginal and intracervical application of seminal plasma in in vitro fertilization or intracytoplasmic sperm injection treatment cycles ‐ a double‐blind, placebo‐controlled, randomized pilot study. Fertility and Sterility 2009;91(1):167‐72. [DOI] [PubMed] [Google Scholar]
Von Wolff 2013 {published data only (unpublished sought but not used)}
- Germeyer A, Rosner S, Jauckus J, Strowitzki T, Wolff M. Intrauterine application of diluted seminal plasma in in vitro fertilization does not improve pregnancy rates ‐ a placebo controlled double blinded randomized trial. Human Reproduction 2013; Vol. 28, issue Supp 1:i215 (p240). [DOI] [PubMed]
- Wolff M, Rösner S, Germeyer A, Jauckus J, Griesinger G, Strowitzki T. Intrauterine instillation of diluted seminal plasma at oocyte pick‐up does not increase the IVF pregnancy rate: a double‐blind, placebo controlled, randomized study. Human Reproduction 2013;28(12):3247‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Coulam 1995 {published data only}
- Coulam CB, Stern J J. Effect of seminal plasma on implantation rates. Early Pregnancy 1995;1:33‐6. [PubMed] [Google Scholar]
Fishel 1989 {published data only}
- Fishel S, Webster J, Jackson P, Faratian B. Evaluation of high vaginal insemination at oocyte recovery in patients undergoing in vitro fertilization. Fertility and Sterility 1989;51(1):135‐8. [DOI] [PubMed] [Google Scholar]
Lou 2014 {published data only}
- Lou SG, Hagshafiha M, Yekta Z, Oshnouei S, Firoozi E, Pashapoor S, et al. Effects of intravaginal application of seminal plasma on embryo implantation and early abortion rate in patients undergoing intracytoplasmic sperm injection. Iranian Journal of Obstetrics, Gynecology and Infertility 2014;17(101):6‐12. [Google Scholar]
Additional references
Achache 2006
- Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Human Reproduction Update 2006;12(6):731‐46. [DOI] [PubMed] [Google Scholar]
Bromfield 2014
- Bromfield JJ. Seminal fluid and reproduction: much more than previously thought. Journal of Assisted Reproduction and Genetics 2014;31:627‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2014
- Chen JC, Johnson BA, Erikson DW, Piltonen TT, Barragan F, Chu S, et al. Seminal plasma induces global transcriptomic changes associated with cell migration, proliferation and viability in endometrial epithelial cells and stromal fibroblasts. Human Reproduction 2014;29:1255‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crawford 2015b
- Crawford G, Ray A, Gudi A, Shah S, Homburg R. The role of seminal plasma for improved outcomes during in vitro fertilization treatment: review of the literature and meta‐analysis. Human Reproduction Update 2015;21(2):275‐84. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 3 November 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Lee 2015
- Lee E, Illingworth P, Wilton L, Chambers GM. The clinical effectiveness of preimplantation genetic diagnosis for aneuploidy in all 24 chromosomes (PGD‐A): systematic review. Human Reproduction 2015;30(2):473‐83. [DOI] [PubMed] [Google Scholar]
Robertson 2002
- Robertson SA, Ingman WV, O'Leary S, Sharkey DJ, Tremellen KP. Transforming growth factor beta ‐ a mediator of immune deviation in seminal plasma. Journal of Reproductive Immunology 2002;57:109‐28. [DOI] [PubMed] [Google Scholar]
Robertson 2005
- Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell and Tissue Research 2005;322:43‐52. [DOI] [PubMed] [Google Scholar]
Robertson 2013
- Robertson SA, Prins JR, Sharkey DJ, Moldenhauer LM. Seminal fluid and the generation of regulatory T cells for embryo implantation. American Journal of Reproductive Immunology 2013;69:315‐30. [DOI] [PubMed] [Google Scholar]
Samy 2006
- Samy ET, Setiady YY, Ohno K, Pramoonjago P, Sharp C, Tung KS. The role of physiological self‐antigen in the acquisition and maintenance of regulatory T‐cell function. Immunological Reviews 2006;212:170‐84. [DOI] [PubMed] [Google Scholar]
Sato 2003
- Sato K, Yamashita N, Baba M, Matsuyama T. Modified myeloid dendritic cells act as regulatory dendritic cells to induce anergic and regulatory T cells. Blood 2003;101:3581‐9. [DOI] [PubMed] [Google Scholar]
Simon 2000
- Simon C, Martin JC, Pellicer A. Paracrine regulators of implantation. Best Practice & Research Clinical Obstetrics & Gynaecology 2000;14(5):815‐26. [DOI] [PubMed] [Google Scholar]
Tafuri 1995
- Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science 1995;270:630‐3. [DOI] [PubMed] [Google Scholar]


