Abstract
Background
Percutaneous exposure injuries from devices used for blood collection or for injections expose healthcare workers to the risk of blood borne infections such as hepatitis B and C, and human immunodeficiency virus (HIV). Safety features such as shields or retractable needles can possibly contribute to the prevention of these injuries and it is important to evaluate their effectiveness.
Objectives
To determine the benefits and harms of safety medical devices aiming to prevent percutaneous exposure injuries caused by needles in healthcare personnel versus no intervention or alternative interventions.
Search methods
We searched CENTRAL, MEDLINE, EMBASE, NHSEED, Science Citation Index Expanded, CINAHL, Nioshtic, CISdoc and PsycINFO (until 11 November 2016).
Selection criteria
We included randomised controlled trials (RCT), controlled before and after studies (CBA) and interrupted time‐series (ITS) designs of the effect of safety engineered medical devices on percutaneous exposure injuries in healthcare staff.
Data collection and analysis
Two of the authors independently assessed study eligibility and risk of bias and extracted data. We synthesized study results with a fixed‐effect or random‐effects model meta‐analysis where appropriate.
Main results
We included six RCTs with 1838 participants, two cluster‐RCTs with 795 participants and 73,454 patient days, five CBAs with approximately 22,000 participants and eleven ITS with an average of 13.8 data points. These studies evaluated safe modifications of blood collection systems, intravenous (IV) systems, injection systems, multiple devices, sharps containers and legislation on the implementation of safe devices. We estimated the needlestick injury (NSI) rate in the control groups to be about one to five NSIs per 1000 person‐years. There were only two studies from low‐ or middle‐income countries. The risk of bias was high in 20 of 24 studies.
Safe blood collection systems:
We found one RCT that found a safety engineered blood gas syringe having no considerable effect on NSIs (Relative Risk (RR) 0.2, 95% Confidence Interval (95% CI) 0.01 to 4.14, 550 patients, very low quality evidence). In one ITS study, safe blood collection systems decreased NSIs immediately after the introduction (effect size (ES) ‐6.9, 95% CI ‐9.5 to ‐4.2) but there was no further decrease over time (ES ‐1.2, 95% CI ‐2.5 to 0.1, very low quality evidence). Another ITS study evaluated an outdated recapping shield, which we did not consider further.
Safe Intravenous systems
There was very low quality evidence in two ITS studies that NSIs were reduced with the introduction of safe IV devices, whereas one RCT and one CBA study provided very low quality evidence of no effect. However, there was moderate quality evidence produced by four other RCT studies that these devices increased the number of blood splashes when the safety system had to be engaged actively (relative risk (RR) 1.6, 95% CI 1.08 to 2.36). In contrast there was low quality evidence produced by two RCTs of passive systems that showed no effect on blood splashes. Yet another RCT produced low quality evidence that a different safe active IV system also decreased the incidence of blood leakages.
Safe injection devices
There was very low quality evidence provided by one RCT and one CBA study showing that introduction of safe injection devices did not considerably change the NSI rate. One ITS study produced low quality evidence showing that the introduction of safe passive injection systems had no effect on NSI rate when compared to safe active injection systems.
Multiple safe devices
There was very low quality evidence from one CBA study and two ITS studies. According to the CBA study, the introduction of multiple safe devices resulted in a decrease in NSI,whereas the two ITS studies found no change.
Safety containers
One CBA study produced very low quality evidence showing that the introduction of safety containers decreased NSI. However, two ITS studies evaluating the same intervention found inconsistent results.
Legislation
There was low to moderate quality evidence in two ITS studies that introduction of legislation on the use of safety‐engineered devices reduced the rate of NSIs among healthcare workers. There was also low quality evidence which showed a decrease in the trend over time for NSI rates.
Twenty out of 24 studies had a high risk of bias and the lack of evidence of a beneficial effect could be due to both confounding and bias. This does not mean that these devices are not effective.
Authors' conclusions
For safe blood collection systems, we found very low quality evidence of inconsistent effects on NSIs. For safe passive intravenous systems, we found very low quality evidence of a decrease in NSI and a reduction in the incidence of blood leakage events but moderate quality evidence that active systems may increase exposure to blood. For safe injection needles, the introduction of multiple safety devices or the introduction of sharps containers the evidence was inconsistent or there was no clear evidence of a benefit. There was low to moderate quality evidence that introduction of legislation probably reduces NSI rates.
More high‐quality cluster‐randomised controlled studies that include cost‐effectiveness measures are needed, especially in countries where both NSIs and blood‐borne infections are highly prevalent.
Plain language summary
Devices with safety features for preventing percutaneous exposure injuries in healthcare staff
What is the aim of this review?
Healthcare workers use needles, syringes and other devices for collecting patients' bood and to inject drugs that are in liquid form. Sometimes healthcare workers come into contact with the sharp end of these devices by accident. Such instances are called needlestick injuries (NSI) and they may expose healthcare workers to the risk of serious infections such as hepatitis or human immunodeficiency virus (HIV). Safety features such as shields or retractable needles can help prevent these injuries. We searched in multiple databases for randomised (RCTs) and non‐randomised studies (NRS) that had evaluated these features.
Key messages
The evidence on safety devices preventing NSI is of low quality and inconsistent. The lack of a strong and consistent helpful effect could be due to bias. This does not mean that these devices are not effective. The risk of blood contamination may be greater.
More high‐quality experimental studies with groups of healthcare workers are needed to compare the effects and cost‐effectiveness of various types of safety devices on NSIs, especially in countries where both NSIs and blood‐borne infections are common.
What was studied in the review?
We included eight RCTs and 16 NRS. These studies evaluated the safety of blood collection systems, intravenous (IV) systems, injection systems, multiple devices, sharps containers and legislation. We estimated that one to five NSIs occur per 1000 workers every year without intervention. The risk of bias was high in 20 out of 24 studies.
What are the main results of the review?
For safe blood collection systems, one RCT found very low quality evidence showing no considerable effect and one NRS produced very low quality evidence showing a large reduction in NSI. Another NRS used an outdated cap shield.
For safe IV devices, there was very low‐quality evidence that NSIs decreased in two NRS but not in one RCT and one other NRS. However, four other RCT studies produced moderate quality evidence that the devices which had to be switched on increased the number of blood splashes. In two RCT studies where the safety feature automatically switched on produced low quality evidence showing no change in amount of blood splashes. Another RCT study found low quality evidence showing a decrease in the number of blood leakage events with these devices.
For safe injection devices, there was very low quality evidence that these reduced the NSI rate in one RCT and in one NRS. However, another NRS found low quality evidence no difference in NSI rate between active and passive safe injection devices.
For the introduction of several safety devices at once, there was very low quality evidence of inconsistent effects from three NRS. .One NRS showed a decrease in NSI rate but the other two studies showed no difference.
For the use of safety containers, there was very low quality evidence of inconsistent effects from three NRS. . One NRS showed a decrease in NSI but the other two studies showed inconsistent results.
For the introduction of legislation on safety‐engineered devices, there was low to moderate quality evidence produced by two NRS studies showing a reduction in NSIs.
How up‐to‐date is this review?
We searched for studies up until 11 November 2016.
Summary of findings
Summary of findings for the main comparison. (RCT) Safe blood collection systems compared to regular systems for preventing percutaneous exposure injuries caused by needles in healthcare personnel.
| Safe blood collection systems compared to regular systems for preventing percutaneous exposure injuries caused by needles in healthcare personnel (RCTs) | ||||||
| Patient or population: preventing percutaneous exposure injuries caused by needles in healthcare personnel (RCTs) Setting: emergency care department of hospital Intervention: Safe blood collection systems Comparison: regular systems | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with regular systems | Risk with Safe blood collection systems | |||||
| Needlestick injuries immediate follow up | Study population | RR 0.20 (0.01 to 4.15) | 550 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 7 per 1 000 | 1 per 1 000 (0 to 30) | |||||
| Blood splashes | Study population | RR 0.14 (0.02 to 1.15) | 550 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 4 | ||
| 25 per 1 000 | 4 per 1 000 (1 to 29) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by one level due to risk of bias (selection bias, performance bias and detection bias). 2 We downgraded the quality of evidence by two levels due to imprecision (wide confidence interval and very few events). 3 We downgraded the quality of evidence by one level due to indirectness (blood splashes were actually visible blood leakages). 4 We downgraded the quality of evidence by one level due to imprecision (confidence interval crosses 1).
Summary of findings 2. (ITS) Safe blood collection systems compared to regular systems for preventing percutaneous exposure injuries caused by needles in healthcare personnel.
| Safe blood collection systems compared to regular systems for preventing percutaneous exposure injuries caused by needles in healthcare personnel (ITS) | |||
| Patient or population: preventing percutaneous exposure injuries caused by needles in healthcare personnel (ITS) Setting: hospital Intervention: Safe blood collection systems Comparison: regular systems | |||
| Outcomes | Impact | № of participants (studies) | Quality of the evidence (GRADE) |
| Number of reported sharps injuries, level ‐ reported seperately for needle sheath and cap shield studies | Needle sheath study: effect size ‐6.88; confidence interval ‐9.53 to ‐4.23. Cap shield study: effect size ‐1.04; confidence interval ‐2.27 to 0.19. | (2 observational studies) | ⊕⊝⊝⊝ VERY LOW 1 2 3 |
| Number of reported sharps injuries, slope ‐ reported seperately for needle sheath and cap shield studies | Needle sheath study: effect size ‐1.19; confidence interval ‐2.50 to 0.12. Cap shield study: effect size ‐1.00; confidence interval ‐2.22 to ‐0.22. | (2 observational studies) | ⊕⊝⊝⊝ VERY LOW 2 3 |
| Interpretation of effect size: small (0‐0.2) medium (0.2‐0.5) large (0.6 and above), an effect size with negative sign implies decrease and positive sign implies increase of effect. | |||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 We downgraded the quality of evidence by two levels due to heterogeneity (I² = 93%). 2 We downgraded the quality of evidence by one level due to imprecision (wide confidence interval). 3 We downgraded the quality of evidence by one level due to risk of bias (incomplete data set in one study and use of SED in the intervention period varied in another).
Summary of findings 3. (RCT) Safe intravenous systems compared to regular systems for preventing percutaneous exposure injuries caused by needles in healthcare personnel.
| Safe intravenous systems compared to regular systems RCT for preventing percutaneous exposure injuries caused by needles in healthcare personnel | ||||||
| Patient or population: preventing percutaneous exposure injuries caused by needles in healthcare personnel Setting: hospital (general, medical, surgical and intensive care units) Intervention: Safe intravenous systems Comparison: regular systems RCT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with regular systems RCT | Risk with Safe intravenous systems | |||||
| Needlestick injuries | Study population | Rate ratio 0.62 (0.27 to 1.41) | (1 RCT, three arms) | ⊕⊝⊝⊝ VERY LOW 1 2 | Calculated based on 1000 patient days | |
| 0.71 per 1 000 | 0.44 per 1 000 (0.19 to 1.00) | |||||
| Incidences of blood contamination ‐ Active systems | Study population | RR 1.60 (1.08 to 2.36) | 961 (4 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | ||
| 92 per 1 000 | 148 per 1 000 (100 to 218) | |||||
| Incidences of blood contamination ‐ Passive systems | Study population | RR 0.94 (0.50 to 1.75) | 528 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | ||
| 79 per 1 000 | 74 per 1 000 (40 to 138) | |||||
| Incidence of blood leakage ‐ Active systems | Study population | RR 0.21 (0.11 to 0.37) | 147 (1 RCT) | ⊕⊕⊝⊝ LOW 5 | ||
| 684 per 1 000 | 144 per 1 000 (75 to 253) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by two levels due to risk of bias (serious attrition). 2 We downgraded the quality of evidence by one level due to imprecision (confidence interval includes 25% benefit and harm). 3 We downgraded the quality of evidence by one level due to risk of bias (studies with high risk of bias contribute most to summary estimate). 4 We downgraded the quality of evidence by one level due to imprecision (wide confidence interval). 5 We downgraded the quality of evidence by two levels due to risk of bias (no random sequence generation, allocation concealment or blinding).
Summary of findings 4. (CBA) Safe intravenous systems compared to regular systems for preventing percutaneous exposure injuries caused by needles in healthcare personnel.
| Safe intravenous systems compared to regular systems CBA for preventing percutaneous exposure injuries caused by needles in healthcare personnel | ||||||
| Patient or population: preventing percutaneous exposure injuries caused by needles in healthcare personnel Setting: hospital Intervention: Safe intravenous systems Comparison: regular systems CBA | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with regular systems CBA | Risk with Safe intravenous systems | |||||
| Number of needlestick injuries | Study population | Rate ratio 0.06 (0.00 to 1.09) | (1 observational study) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 36.36 per 1 000 | 2.18 per 1 000 (0.00 to 39.63) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by two levels due to risk of bias (no random sequence generation or allocation concealment). 2 We downgraded the quality of evidence by one level due to imprecision (wide confidence interval).
Summary of findings 5. (ITS) Safe intravenous systems compared to regular systems for preventing percutaneous exposure injuries caused by needles in healthcare personnel.
| Safe intravenous systems compared to regular systems ITS for preventing percutaneous exposure injuries caused by needles in healthcare personnel | |||
| Patient or population: preventing percutaneous exposure injuries caused by needles in healthcare personnel Setting: healthcare Intervention: Safe intravenous systems Comparison: regular systems ITS | |||
| Outcomes | Impact | № of participants (studies) | Quality of the evidence (GRADE) |
| Number of reported sharps injuries, level | Study 1: effect size ‐5.20; confidence interval ‐7.98 to ‐2.42. Study 2: effect size ‐1.78; confidence interval ‐3.09 to ‐0.47. | (2 observational studies) | ⊕⊝⊝⊝ VERY LOW 1 2 3 |
| Number of reported sharps injuries, slope | Study 1: Effect size ‐7.86; confidence interval ‐9.13 to ‐6.59. Study 2: Effect size 0.35; confidence interval ‐0.20 to 0.90. | (2 observational studies) | ⊕⊝⊝⊝ VERY LOW 1 3 4 |
| Interpretation of effect size: small (0‐0.2) medium (0.2‐0.5) large (0.6 and above), a effect size with negative sign implies decrease and positive sign implies increase of effect. | |||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 We downgraded the quality of evidence by one level due to risk of bias caused by lacking intervention fidelity (in the second study conventional devices were used during intervention period). 2 We downgraded the quality of evidence by one level due to heterogeneity (I² = 79%). 3 We downgraded the quality of evidence by one level due to imprecision (wide confidence interval). 4 We downgraded the quality of evidence by two levels due to heterogeneity (I² = 99%).
Summary of findings 6. (RCT) Safe injection systems compared to regular systems RCT for preventing percutaneous exposure injuries caused by needles in healthcare personnel.
| Safe injection systems compared to regular systems RCT for preventing percutaneous exposure injuries caused by needles in healthcare personnel | ||||||
| Patient or population: preventing percutaneous exposure injuries caused by needles in healthcare personnel Setting: hospital Intervention: Safe injection systems Comparison: regular systems RCT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with regular systems RCT | Risk with Safe injection systems | |||||
| Questionnaire reported Needlestick injuries 6 mo follow up | Study population | RR 0.42 (0.14 to 1.25) | 154 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 140 per 1 000 | 59 per 1 000 (20 to 174) | |||||
| Questionnaire reported Needlestick injuries 12 mo follow up | Study population | OR 0.20 (0.04 to 0.96) | 144 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 119 per 1 000 | 26 per 1 000 (5 to 115) | |||||
| Hospital reported Needlestick injuries 6 mo follow up | Study population | OR 1.20 (0.51 to 2.84) | 533 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 38 per 1 000 | 45 per 1 000 (20 to 100) | |||||
| Hospital reported Needlestick injuries 12 mo follow up | Study population | OR 0.72 (0.28 to 1.81) | 533 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 41 per 1 000 | 30 per 1 000 (12 to 72) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by two levels due to risk of bias (high attrition). 2 We downgraded the quality of evidence by one level due to imprecision (wide confidence interval).
Summary of findings 7. (CBA) Safe injection systems compared to regular systems for preventing percutaneous exposure injuries caused by needles in healthcare personnel.
| Safe injection systems compared to regular systems CBA for preventing percutaneous exposure injuries caused by needles in healthcare personnel | ||||||
| Patient or population: preventing percutaneous exposure injuries caused by needles in healthcare personnel Setting: dental clinic Intervention: Safe injection systems Comparison: regular systems CBA | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with regular systems CBA | Risk with Safe injection systems | |||||
| Needlestick injury rate | Study population | Rate ratio 0.34 (0.04 to 3.28) | (1 observational study) | ⊕⊝⊝⊝ VERY LOW 1 2 | Calculated based on 1000 person years | |
| 236 per 1 000 | 80.24 per 1 000 (9.44 to 774) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by two levels due to risk of bias (no random sequence generation or allocation concealment). 2 We downgraded the quality of evidence by two levels due to imprecision (wide confidence interval).
Summary of findings 8. (ITS) Safe passive injection systems compared to safe active injection systems for preventing percutaneous exposure injuries caused by needles in healthcare personnel.
| Safe passive injection systems compared to safe active injection systems ITS for preventing percutaneous exposure injuries caused by needles in healthcare personnel | |||
| Patient or population: preventing percutaneous exposure injuries caused by needles in healthcare personnel Setting: hospital Intervention: Safe passive injection systems Comparison: safe active injection systems ITS | |||
| Outcomes | Impact | № of participants (studies) | Quality of the evidence (GRADE) |
| Change in level of needlestick injuries | Effect size 0.23; confidence interval ‐1.89 to 2.35. | (1 observational study) | ⊕⊝⊝⊝ VERY LOW 1 |
| Change in slope of needlestick injuries | Effect size ‐0.74; confidence interval ‐1.66 to 0.18. | (1 observational study) | ⊕⊕⊝⊝ LOW 1 |
| Interpretation of effect size: small (0‐0.2) medium (0.2‐0.5) large (0.6 and above), a effect size with negative sign implies decrease and positive sign implies increase of effect. | |||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 We downgraded the quality of evidence by one level due to imprecision (wide confidence interval).
Summary of findings 9. (ITS) Multiple safe devices compared to regular devices for preventing percutaneous exposure injuries caused by needles in healthcare personnel.
| Multiple safe devices compared to regular devices ITS for preventing percutaneous exposure injuries caused by needles in healthcare personnel | |||
| Patient or population: preventing percutaneous exposure injuries caused by needles in healthcare personnel Setting: healthcare Intervention: Multiple safe devices Comparison: regular devices ITS | |||
| Outcomes | Impact | № of participants (studies) | Quality of the evidence (GRADE) |
| Number of reported sharps injuries, level | Study 1: effect size ‐1.04; confidence interval ‐2.20 to 0.12. Study 2: effect size 0.43; confidence interval ‐0.30 to 1.16. | (2 observational studies) | ⊕⊝⊝⊝ VERY LOW 1 2 3 |
| Number of reported sharps injuries, slope | Study 1: effect size ‐0.01; confidence interval ‐0.15 to 0.13. Study 2: effect size 0.56; confidence interval 0.23 to 0.89. | (2 observational studies) | ⊕⊝⊝⊝ VERY LOW 1 4 |
| Interpretation of effect size: small (0‐0.2) medium (0.2‐0.5) large (0.6 and above), a effect size with negative sign implies decrease and positive sign implies increase of effect. | |||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 We downgraded the quality of evidence by one level due to risk of bias (One study had a low risk of bias but the other study had a high risk as conventional devices were still available after the intervention began). 2 We downgraded the quality of evidence by one level due to heterogeneity (I² = 78%). 3 We downgraded the quality of evidence by one level due to imprecision (wide confidence interval). 4 We downgraded the quality of evidence by one level due to heterogeneity (I² = 90%).
Summary of findings 10. (CBA) Multiple safe devices compared to regular devices for preventing percutaneous exposure injuries caused by needles in healthcare personnel.
| Multiple safe devices compared to regular devices CBA for preventing percutaneous exposure injuries caused by needles in healthcare personnel | ||||||
| Patient or population: preventing percutaneous exposure injuries caused by needles in healthcare personnel Setting: hospital Intervention: Multiple safe devices Comparison: regular devices CBA | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with regular devices CBA | Risk with Multiple safe devices | |||||
| Needle stick injuries | Study population | Rate ratio 0.11 (0.01 to 0.81) | (1 observational study) | ⊕⊝⊝⊝ VERY LOW 1 2 | Calculated based on 1000 patient days | |
| 0.44 per 1 000 | 0.052 per 1 000 (0.004 to 0.35) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by two levels due to risk of bias (no random sequence generation or allocation concealment). 2 We downgraded the quality of evidence by one level due to imprecision (wide confidence interval).
Summary of findings 11. (ITS) Sharps containers compared to no containers for preventing percutaneous exposure injuries caused by needles in healthcare personnel.
| Sharps containers compared to no containers ITS for preventing percutaneous exposure injuries caused by needles in healthcare personnel | |||
| Patient or population: preventing percutaneous exposure injuries caused by needles in healthcare personnel Setting: hospital Intervention: Sharps containers Comparison: no containers ITS | |||
| Outcomes | Impact | № of participants (studies) | Quality of the evidence (GRADE) |
| Number of reported sharps injuries, level | Study 1: effect size 3.29; confidence interval 0.68 to 5.90. Study 2: effect size 1.35; confidence interval ‐1.75 to 4.45. | (2 observational studies) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| Number of reported sharps injuries, slope | Study 1: effect size 0.02; confidence interval ‐1.06 to 1.10. Study 2: effect size 2.55; confidence interval 1.20 to 3.90. | (2 observational studies) | ⊕⊝⊝⊝ VERY LOW 1 2 3 |
| Interpretation of effect size: small (0‐0.2) medium (0.2‐0.5) large (0.6 and above), a effect size with negative sign implies decrease and positive sign implies increase of effect. | |||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 We downgraded the quality of evidence by one level due to inconsistency (study 2 showed an increase in reporting). 2 We downgraded the quality of evidence by two levels due to imprecision (wide confidence interval). 3 We downgraded the quality of evidence by one level due to heterogeneity (I² = 88%).
Summary of findings 12. (CBA) Sharps containers compared to no containers for preventing percutaneous exposure injuries caused by needles in healthcare personnel.
| Sharps containers compared to no containers CBA for preventing percutaneous exposure injuries caused by needles in healthcare personnel | ||||||
| Patient or population: preventing percutaneous exposure injuries caused by needles in healthcare personnel Setting: hospital Intervention: Sharps containers Comparison: no containers CBA | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no containers CBA | Risk with Sharps containers | |||||
| Number of needlestick injuries | Study population | Rate ratio 0.88 (0.78 to 0.99) | (1 observational study) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 28.3 per 1 000 | 24.9 per 1 000 (22 to 28) | |||||
| Number of container related needlestick injuries | Study population | Rate ratio 0.22 (0.11 to 0.41) | (1 observational study) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 2.6 per 1 000 | 0.6 per 1 000 (0.28 to 1.06) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded the quality of evidence by two levels due to risk of bias (no random sequence generation or allocation concealment). 2 We downgraded the quality of evidence by one level due to imprecision (wide confidence interval).
Summary of findings 13. (ITS) Legislation compared to no legislation for preventing percutaneous exposure injuries caused by needles in healthcare personnel.
| Legislation compared to no legislation ITS for preventing percutaneous exposure injuries caused by needles in healthcare personnel | |||
| Patient or population: preventing percutaneous exposure injuries caused by needles in healthcare personnel Setting: healthcare Intervention: Legislation Comparison: no legislation ITS | |||
| Outcomes | Impact | № of participants (studies) | Quality of the evidence (GRADE) |
| NSI‐ change in level ‐ Interruption | Effect size ‐6.15; confidence interval ‐7.76 to ‐4.54. | (2 observational studies) | ⊕⊕⊕⊝ MODERATE 1 |
| NSI‐ change in level ‐ Gradual introduction | Effect size 0.80; confidence interval 0.41 to 1.19. | (1 observational study) | ⊕⊕⊝⊝ LOW 1 |
| NSI‐ Change in slope ‐ Interruption | Effect size ‐0.94; confidence interval ‐1.97 to 0.09 | (2 observational studies) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| NSI‐ Change in slope ‐ Gradual introduction | Effect size 0.50; confidence interval 0.36 to 0.64 | (1 observational study) | ⊕⊕⊝⊝ LOW 1 |
| Interpretation of effect size: small (0‐0.2) medium (0.2‐0.5) large (0.6 and above), a effect size with negative sign implies decrease and positive sign implies increase of effect. | |||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 We downgraded the quality of evidence by one level due to risk of bias (dataset did not represent the whole sample). 2 We downgraded the quality of evidence by one level due to imprecision (wide confidence interval).
Background
Healthcare workers (HCWs) are exposed to several occupational hazards, including biological agents. Percutaneous injury and occupational exposure to blood and body fluids increase the risk of exposure of HCWs to blood borne pathogens such as hepatitis B (HBV), hepatitis C (HCV) and human immunodeficiency virus (HIV). These infections can lead to chronic and fatal diseases. In the United States (US), the annual number of percutaneous injuries among hospital‐based HCWs was estimated to be 384,325 in 1997 to 1998 (Panlilio 2004). Percutaneous injury incidence rates have decreased since then. However, recently it was estimated that still 300,000 HCWs sustain percutaneous injuries annually in the US (Grimmond 2017). The World Health Organization (WHO) estimates that 16,000 HCV, 66,000 HBV and 1000 HIV infections may have occurred worldwide among HCWs in the year 2000 due to their occupational exposure to blood and body fluids (Pruss‐Ustun 2005). More recent information relating to recent global trends of percutaneous exposure injuries is not available. Nonetheless it is reasonable to assume that the trends are not considerably different from the US.
Description of the condition
A HCW's risk for acquiring infectious diseases at work is influenced by a variety of environmental and social factors. The population prevalence of specific diseases, percentage HBV vaccination coverage in the population, availability of medical supplies, adherence to standard precautions, accessibility and availability of post‐exposure prophylaxis, among others are important components influencing the risk of HCWs becoming infected by blood borne diseases. For HBV, the risk varies greatly based on the immunization coverage among health workers and the served population. For example, in 1990 the HBV infection rate among unvaccinated US healthcare personnel was three to five times greater than in the US general population (MacCannell 2010). This number decreased significantly due to the introduction of routine HBV immunization and comprehensive occupational health and safety policies. The prevalence of HBV among HCWs is now five times less than in the US general population (MacCannell 2010).
Occupational transmission of infectious diseases has a significant impact on the health of the workers and also on the healthcare system as a whole. The transmission of occupational blood borne infectious diseases leads to increased absenteeism and morbidity, and in some cases to higher mortality rates, among HCWs. These outcomes affect the delivery, provision, quality and safety of care. HCWs may suffer from psychological stress due to the risk of acquiring an infectious disease, which affects both their work and personal life (Fisman 2002; Sohn 2006). There is also the financial burden associated with occupational exposure to blood borne diseases, which includes costs related to blood tests, treatment, outpatient visits, and lost working hours (Jagger 1990; Leigh 2007).
Description of the intervention
Exposure to blood or body fluids is also called percutaneous exposure and happens most often when HCWs are injured with sharp needles or instruments, or when blood or body fluids are splashed on mucous membranes or wounds during medical interventions or accidents. These incidents are called percutaneous exposure incidents. The majority of these incidents are percutaneous injuries which include sharps injuries or needlestick injuries (NSIs). The actual causes of a NSI are multifactorial and include elements such as types of devices and procedures, lack of access to or availability of personal protective equipment for the HCWs, suboptimal use of personal protective equipment, lack of training and education on infection control and occupational health principles, improper management of needles, poor organisational climate, high workload and fatigue, working alternate shifts, high mental pressure and subjective perception of risk (Akduman 1999; Ansa 2002; Clarke 2002; Doebbeling 2003; Fisman 2007; Ilhan 2006; Ngatu 2011; Oh 2005; Orji 2002; Roberts 1999; Smith 2006; Smith 2006b; Wallis 2007). Most of these causes can be addressed by specific interventions.
Several epidemiological studies have demonstrated that some needlestick injuries are associated with specific actions and medical equipment, such as recapping and sharp devices respectively (De Carli 2003). The practice of recapping needles is a major factor contributing to needlestick injuries (Ngatu 2011) and specific devices have also been associated with an increased risk of percutaneous injuries. According to MacCannell 2010, needlestick injuries occurred more frequently with hollow‐bore needles compared to solid sharps (54% versus 40%). It is estimated that up to 25% of reported hollow‐bore needlestick injuries among nurses and physicians could have been prevented by the use of safer devices (MacCannell 2010). Almost two‐thirds of all reported injuries occurred with devices without safety features (MacCannell 2010).
Engineered medical devices such as retractable needles can reduce and eliminate the exposure to blood and body fluids. Even though sometime ago legislation has been introduced in the US and Europe that mandates that safety‐engineered devices should be used, there is no generally agreed definition of what constitutes a safety‐engineered device (OSHA 2001). Here, we define a safety‐engineered device as any medical device that purportedly protects against percutaneous injuries.
How the intervention might work
There are several possibilities to prevent infection from needlestick injuries. For hepatitis B, vaccination has been successful (Chen 2005). Vaccination is not yet possible for HCV or HIV (Mast 2004). Therefore, exposure elimination and reduction remain the main preventive strategies.
Many hospitals are now using safe medical devices as an intervention to reduce the risk of percutaneous injuries. These devices eliminate or encapsulate the needles. For example, needleless intravenous systems are defined as systems that administer medications through an intravenous access device without using needle connections. Some studies have noted a decrease in the risk of needlestick injuries following the introduction of safety medical devices such as a needle free system for intravenous therapy (Mendelson 1998), meanwhile other studies have found inconclusive findings for such systems (L'Ecuyer 1996 2wva).
Why it is important to do this review
There are several strategies available to abate percutaneous exposure injuries among HCWs workers, and these are widely used. Therefore, it is important to know if these preventive interventions are effective. Retrospective studies indicate that percutaneous exposure incidents would be reduced by more than 50% by behavioural interventions, either through education or adoption of new techniques (Bryce 1999; Castella 2003). The use of safety devices would probably also have a significant effect (Bryce 1999; Castella 2003; Jagger 1988; Waclawski 2004). There have been several reviews on the effectiveness of interventions (Hanrahan 1997; Hutin 2003; Rogers 2000; Trim 2004; Tuma 2006) but none have used the systematic Cochrane methodology. This review excluded studies where sharp suture needles were substituted with blunted ones as another Cochrane review (Parantainen 2011) has already addressed the effect of this intervention. Extra gloves or special types of gloves could theoretically be considered a device to prevent needlestick injuries while handling needles, but we excluded these studies because there is another Cochrane Review that shows that extra gloves are effective to prevent needlestick injuries (Mischke 2014).
Recently the WHO issued guidelines for the use of safety‐engineered devices in healthcare settings (WHO 2016). However, they based their recommendations on a judgment of moderate quality evidence which was different from the low quality evidence that we found in the 2014 version of this review.
Objectives
To determine the benefits and harms of safety medical devices aiming to prevent percutaneous exposure injuries caused by needles in healthcare personnel versus no intervention or alternative interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCT), cluster‐randomised trials (cluster‐RCT), interrupted time‐series (ITS) and controlled before and after studies (CBA) irrespective of language of publication, publication status, or blinding.
We expected that the availability of RCTs would be limited for this topic. Interventions for prevention are very different from clinical interventions. Many of these interventions are not implemented at the individual level. For example, new equipment is used by a group of workers or safety engineering controls are applied to the whole department simultaneously. This approach makes individual randomisation impossible. In principle, this can be partly overcome by randomisation at the department level as in a cluster‐RCT design. However, as the level of aggregation increases, the more difficult this is to perform due to the level of recruitment required. Therefore, we included the following non‐randomised study designs in our review: CBA studies with a concurrent control group, and ITS. CBA studies are also called prospective cohort studies. They are easier to perform, taking into account that the intervention is assigned at the group level, and still have reasonable validity.
ITS designs are often based on routinely collected administrative data from insurance or governmental sources, collected for injury outcomes. In many cases the data are collected independently from interventions and over long periods of time, offering reasonable validity. If there are at least three data points before and three data points after the intervention, we included these study designs as ITS (EPOC 2006). Both ITS with and without a control group were eligible for inclusion.
Types of participants
We included studies where participants were HCWs, including dentists, which means all persons that are professionally involved in providing health care to patients. The majority of study participants had to fulfil this criterion.
Types of interventions
Inclusion criteria
We included studies examining any medical devices that aim to prevent percutaneous exposure incidents and thus could reduce the risk of exposure to blood or bodily fluids.
We categorised the interventions based on the type of device in the following way.
‐ Safety engineered devices for blood collection.
‐ Safety engineered devices for Injecting fluids.
‐ Containers for collecting sharps.
Because these categories did not cover all studies that we found, we added two categories.
‐ The use of multiple safety devices in an intervention programme.
‐ Intravenous systems.
‐ The introduction of legislation
Exclusion criteria
We excluded studies where sharp suture needles were substituted with blunted ones. Another Cochrane review (Parantainen 2011) has addressed the effect of this intervention. We also excluded studies on devices that eliminate the use of suture needles or that encapsulate suture needles during surgery because the risk of a NSI is different with suture needles in surgery. Extra gloves or special types of gloves were also excluded because there is another Cochrane review on the effect of gloves to prevent needlestick injuries Mischke 2014.
Types of outcome measures
Primary outcomes
Our primary outcome measure was exposure of HCWs to potentially contaminated blood or bodily fluids. Exposure can be reported as self‐reported NSI, sharps injury, blood stains on the skin, or glove perforations. We considered all reports of such exposure as valid measures of the outcome, such as self‐reports, reports by the employer, or observations of blood stains.
Secondary outcomes
We considered ease of use of the devices (including user satisfaction) and information related to the cost of the intervention as secondary outcomes.
Search methods for identification of studies
Electronic searches
First, we generated search terms for percutaneous exposure incidents. We then combined these terms for percutaneous exposure incidents with the recommended search strings for randomised trials and for non‐randomised studies. We used the Robinson 2002 search strategy for randomised clinical trials and controlled clinical studies. For finding non‐randomised studies, we used the sensitive search strategy for occupational health intervention studies (Verbeek 2005).
We used the strategy to search CENTRAL, MEDLINE, EMBASE, NHSEED, Science Citation Index Expanded, CINAHL, OSH‐update, and PsycINFO from the earliest record to 1 November 2016. We also searched LILACS but only until 2012. We felt that the yield did not outweigh the efforts and decided to stop searching LILACS. In addition, we searched the databases of WHO, the UK National Health Service (NHS) and www.med.virginia.edu/epinet (Royle 2003).
We present the original search strategies for the databases listed above in Appendix 1.
In the first update of the original search that is common with Parantainen 2011, we used recap* and device* as additional search terms combined by OR and with the other terms as explained in Appendix 2.
We present the most recent updated search strategies for the databases listed above in Appendix 3.
Searching other resources
We screened the reference lists of all relevant studies for additional studies.
Data collection and analysis
Selection of studies
Using the inclusion and exclusion criteria, the authors (M‐CL, JV, VR, MP) worked individually and independently to screen the titles and abstracts of the references that were identified by the search strategy as potential studies. Pairs of authors went through the same references to increase the reliability of the results. We obtained the full texts of those references that appeared to meet the inclusion criteria. We did not blind ourselves regarding the trial author details because we felt that it would not increase validity. We solved disagreements between pairs by discussion. A pair consulted a third author if disagreement persisted.
Data extraction and management
Review authors worked in pairs (VR and JV, M‐CL and MP) but independently to extract the data onto a form. The form included the essential study characteristics about the participants, interventions, outcomes and results. We also noted any adverse events and the sponsorship of the study. Two pairs of authors (VR and JV, M‐CL and MP) independently assessed the risk of bias of the studies. The pairs used a consensus method if disagreements occurred. The pairs consulted a third author if disagreement persisted. Again, we did not mask trial names because we believed that it would not increase validity.
Assessment of risk of bias in included studies
For the assessment of risk of bias in RCTs we used the risk of bias tool in RevMan 2014. For CBA studies, we used two items additional to the Cochrane risk of bias tool from a validated instrument (Downs 1998): adjustment for baseline differences and similar timing of recruitment of intervention group.
For ITS studies we used the risk of bias criteria as presented by Ramsay 2003.
Overall judgement of risk of bias at study level
For RCT studies we judged a study to be at a low risk of bias if at least two of the following domains (random sequence generation, allocation concealment and blinding) had a low risk of bias and the remaining third domain had unclear risk of bias and none of the other domains (attrition bias, reporting bias, similar recruitment of groups, adjustment for baseline differences and other bias) had a high risk of bias.
For CBA and ITS studies, we judged a study to be at a low risk of bias if none of the domains were rated as high risk.
Measures of treatment effect
For RCTs and CBA studies with dichotomous outcomes, we used relative risks or risk ratios (RR) as the measure of the treatment effect. We did not use odds ratios because the incidence of most outcomes was higher than 10% and then odds ratios give an inflated impression of the relative risk.
In studies where needlestick injuries or glove perforations were reported more than once for an individual we used rates and rate ratios as the treatment effect. We calculated the log rate ratio and the standard error and used these data as the input for RevMan.
For ITS studies, we extracted and re‐analysed the data from the original papers according to the recommended methods for analysis of ITS designs for inclusion in systematic reviews (Ramsay 2003). These methods utilise a segmented time‐series regression analysis to estimate the effect of an intervention while taking into account secular time trends and any autocorrelation between individual observations. For each study, we fitted a first order autoregressive time‐series model to the data using a modification of the parameterization of Ramsay 2003. Details of the mode specification are as follows:
Y = ß0 + ß1 time + ß2 (time ‐ p) I (time > p) + ß3 I (time > p) + E, E ˜ N (0, s²).
For time = 1,...,T, where p is the time of the start of the intervention, I (time ≥ p) is a function which takes the value 1 if time is p or later and zero otherwise, and where the errors E are assumed to follow a first order autoregressive process (AR1) and the errors E are normally distributed with mean zero and variance s². The ß parameters have the following interpretation: ß1 is the pre‐intervention slope; ß2 is the difference between post‐ and pre‐intervention slopes; ß3 is the change in level at the beginning of the intervention period, meaning that it is the difference between the observed level at the first intervention time point and that predicted by the pre‐intervention time trend.
We used the change in slope and the change in level as two different measures of treatment effect for ITS studies.
Unit of analysis issues
For studies that employed a cluster‐randomised design but did not make an allowance for the design effect, we intended to calculate the design effect. If no intra‐cluster coefficients were reported, although they are needed to calculate the design effect, we would have assumed a fairly large intra‐cluster coefficient of 0.05 to enable the calculation of design effect. We intended to use the methods that are recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) for the calculations. However, the two studies that used a cluster‐randomised design either did not provide data on the size of the clusters (L'Ecuyer 1996 2wva) or had a loss to follow up of 50% (van der Molen 2011), which made the cluster calculations questionable. Therefore, we did not perform these calculations.
For studies with multiple study arms that belonged to the same comparison, we divided the number of events and participants in the control group equally over the study arms to prevent double counting of study participants in the meta‐analysis (Asai 2002 active; Asai 2002 passive).
Dealing with missing data
We contacted the authors for additional information if the data needed for meta‐analysis were missing (Hotaling 2009; Sossai 2010). If data were presented in figures only and the authors could not be reached, we extracted data from the figures presented in the article (Chambers 2015 hospitals; Chambers 2015 long‐term nursing care; Goldwater 1989; Goris 2015; Phillips 2013; Whitby 2008). If data such as standard deviations had been missing and they could be calculated from other data present in the article, such as P values, we would have done so according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), but there were no studies where this was necessary.
Assessment of heterogeneity
Clinical homogeneity among studies was defined based on the similarity of populations, interventions, and outcomes measured at the same follow‐up point. We regarded all healthcare professionals as sufficiently similar to assume a similar preventive effect from the use of similar devices. We categorised safe devices as indicated under types of interventions and assumed that different devices would lead to different effects. We added three extra categories: intravenous (IV) systems, the introduction of multiple safe devices at the same time and legislation that mandates the use of safe devices. We deemed the interventions contained within these categories to be conceptually similar and sufficiently homogeneous to be combined in a meta‐analysis.
We divided outcomes into a category of needlestick injuries and a category of blood or bodily fluid splashes. Thus, we had two different outcome measures: needlestick injuries and blood splashes. Even though the denominator of the NSI rates differed from patients to devices to workers we felt that they were sufficiently similar to be combined.
We did not combine various study designs as we assumed that there were large differences in risk of bias between the different study types. We have presented the results per comparison separately for each design type.
We assessed statistical heterogeneity by means of the I² statistic. We used the values of < 40%, between 30% and 60%, between 50% and 90%, and over 75% as indicating not important, moderate, substantial, and considerable heterogeneity respectively, as proposed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of reporting biases
We will assess for publication bias with a funnel plot in future updates of this review if more than five studies are available in a single comparison.
Data synthesis
We pooled studies that contained sufficient data and that we judged to be clinically and statistically homogeneous with RevMan 5 software (RevMan 2014).
When studies were statistically heterogeneous we used a random‐effects model or we refrained from meta‐analysis; otherwise we used a fixed‐effect model.
For ITS, we first standardised the data by dividing the outcome and standard error by the pre‐intervention standard deviation resulting in an effect size, as recommended by Ramsay 2001. Then, we entered the results into RevMan as the change in level and in slope as two different outcomes using the general inverse variance method.
Finally, we used the GRADE approach to assess the quality of the evidence per comparison and per outcome as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For comparisons that only included RCTs, we started at high quality evidence. Then, we reduced the quality of the evidence by one or more levels if there were one or more limitations in the following domains: risk of bias, consistency, directness of the evidence, precision of the pooled estimate, and the possibility of publication bias. When the comparison included non‐randomised studies we started at the low quality level and downgraded further if there were limitations, or we would have upgraded the quality if there were reasons to do so. We used the programme GRADEpro 2017 to generate summary of findings tables for the two most important outcomes for all comparisons but separated by design.
Subgroup analysis and investigation of heterogeneity
We intended to re‐analyse the results for studies with a high baseline or control group exposure rate, and for studies from low‐ and middle‐income countries, but this was not possible due to the few studies that we found per comparison and the lack of studies from low‐ and middle‐income countries.
Sensitivity analysis
We intended to re‐analyse the results including only studies with a low risk of bias in order to find out if risk of bias led to changes in the findings but this was only possible for one comparison as there weren't enough low risk of bias studies to do so.
Results
Description of studies
Results of the search
With the original search strategy described in Appendix 1 and after removal of duplicates we had a total of 11,239 references. Based on titles and abstracts, we selected 322 references for full‐text reading. Of these, we excluded those that did not fulfil our inclusion criteria. In cases where the article did not provide enough data we contacted the authors and asked them to send the missing information. If we did not receive sufficient information to judge if the study should be included, we excluded the study. This resulted in 84 full text articles on NSI prevention. Of these, 14 studies fulfilled the inclusion criteria for this review. We updated the search by adding the strategy described in Appendix 2 in January 2012. This resulted in 167 additional references from which we selected seven for full‐text reading. Of these full‐text studies, there were three additional studies that fulfilled our inclusion criteria. Another update of the whole search (Appendix 1 combined with Appendix 2) in January 2014 yielded another 292 references of which three could be potentially included but are awaiting classification. Six are pending more information from the authors (Perry 2012; Phillips 2010; Phillips 2011; Phillips 2012; Phillips 2012a; Uyen 2014) and one is pending translation from Italian (Ferrario 2012). In November 2016 we updated and reran the search strategy again and it yielded an additional 1194 references (Appendix 3) out of which we screened 60 for full‐text reading (see Figure 1). Out of these studies 7 studies fulfilled the inclusion criteria. Altogether, this process led to a total of 24 studies that fulfilled our inclusion criteria.
1.
Study flow diagram for 2017 update
Included studies
Interventions
We included a total of 24 studies, which contain three studies with two intervention arms (Asai 2002 active; Asai 2002 passive; Prunet 2008 active; Prunet 2008 passive; Chambers 2015 hospitals; Chambers 2015 long‐term nursing care) and one study with three intervention arms (L'Ecuyer 1996 2wva; L'Ecuyer 1996 mbc; L'Ecuyer 1996 pbc), corresponding to 29 different comparisons of safety medical devices that we named as different studies to increase transparency of the meta‐analyses. We elaborated on the details of the devices in Table 14. Based on the information in the articles, we checked on the Internet if the devices were still for sale and if they still resembled the original description given in the article. Even though we could not be sure that the devices currently sold were exactly similar to those in the articles, we are confident that the main safety features are still the same.
1. Content of the interventions.
| Study name | Device Commercial Names | Device Category | Safety Device type |
passive/ active |
For sale? |
| Asai 1999 active | Insyte AutoGuard intravenous cannula | Safe IV system (insertion) | button for actively retracting the needle | active | Yes |
| Asai 2002 active | Insyte Autoguard intravenous cannula | Safe IV system (insertion) | button for actively retracting the needle | active | Yes |
| Asai 2002 passive | Protective Acuvance | Safe IV system (insertion) | automated retraction of needle | passive | Yes |
| Azar‐Cavanagh 2007 | Unnamed intravenous catheter stylet | Safe IV system (insertion) | retractable protection shield | active? | ? |
| Baskin 2014 | BD Eclipse injector 3‐mL, BD preset syringe with BD Luer‐Lok tip 25G×1 | Blood collection | cannula protection shield is activated with one hand after puncture and clicks irreversibly over the cannula | active | Yes |
| Chambers 2015 hospitals | not reported | Multiple safe devices | safety engineered needles and needleless devices | passive or semi‐automatic | ? |
| Chambers 2015 long‐term nursing care | nor reported | Multiple safe devices | safety engineered needles and needleless devices | passive or semi‐automatic | ? |
| Cote 2003 | Angiocath Autoguard IV catheters | Safe IV system (insertion) | button for actively retracting the needle | active | Yes |
| Edmond 1988 | Winfield sharpsguard | Sharps container | bedside sharps container | n.a. | No |
| Gaballah 2010 | Unnamed safety dental syringes | Injection system | does not require re‐sheating or removal of the needle from its syringe | passive? | ? |
| Goldwater 1989 | Needle guard Biosafe New Zealand | Blood collection | shield on cap prevents injury while recapping | n.a. | No |
| Goris 2015 | Unnamed safety engineered passive retractable syringes | Injection system | automatically and instantly retracts the needle from the patient into the barrel of the syringe | passive | ? |
| Grimmond 2010 | Daniels sharpsmart | Sharps container | bedside sharps container | n.a. | Yes |
| L'Ecuyer 1996 2wva | 2‐way valve Safsite Braun medical | Safe IV system (insertion and needleless) | two valve system with plastic sharp that remains in the device | passive | Yes |
| L'Ecuyer 1996 mbc | Lifeshield metal blunt cannula | Safe IV system (needleless iv system) | metal blunt cannula | passive | Yes |
| L'Ecuyer 1996 pbc | Interlink PBC plastic cannula | Safe IV system (insertion and needleless) | plastic sharp covered by blunt plastic cannula | passive | Yes |
| Mendelson 1998 | 1‐valve Safsite Braun medical | Safe IV system (needleless) | valve of IV system incompatible with needle | passive | Yes |
| Phillips 2013 | safety engineered sharps | Multiple safe devices | not explained | ? | ? |
| Prunet 2008 active | Insyte Autoguard intravenous cannula | Safe IV system (insertion) | button for actively retracting the needle | active | Yes |
| Prunet 2008 passive | Introcan Safety IV system (Braun) | Safe IV system (insertion) | automatic shield on needle tip at withdrawing | passive | Yes |
| Reddy 2001 | 'safety syringes and needleless IV' | Multiple safe devices | not explained | ? | ? |
| Richard 2001 | 'sharps containers' | Sharps container | first in treatment rooms later bedside placements | ? | ? |
| Rogues 2004 | SafetyLock BD, resheathable winged steel needle | Blood collection | after pushing (two handed) needle retracts into sheath | active | Yes |
| Seiberlich 2016 | ViaValve safety I.V. catheter | Safe IV system (insertion) |
contains a valve that is designed to restrict blood flow back out of the catheter hub upon initial venipuncture | active | Yes |
| Sossai 2010 | Introcan safety IV system (Braun) | Safe IV system (insertion) | automatic shield on needle tip at withdrawing | passive | Yes |
| Valls 2007 | Eclipse BD; Saf‐T‐ E‐Z Set, BD; Surshield, Terumo; Preserts BD; Provent plus, Smiths; Genie BD; Surgilance Terumo; Blunt administration needles BD | Multiple systems | n.a. | active and passive | Yes |
| van der Molen 2011 | Eclipse BD | Injection system | after injection needle covered with shield | active | Yes |
| Whitby 2008 | VanishPoint; VanishPoint blood tube holders; BD Safety‐Lok; SmartSite needle‐free system; Smartsite Plus | Multiple systems | retractable syringes, needle‐free IV systems and safety winged butterfly needles. | passive | Yes |
| Zakrzweska 2001 | Safety Plus Septodont (Dental injections) | Injection system | Protective sheaths can be temporarily or definitely protect the needle | active | Yes |
The types of devices used in the various studies were:
safe blood collection devices (n = 3) (Baskin 2014; Goldwater 1989; Rogues 2004);
safe IV systems (n = 9) (Asai 1999 active; Asai 2002 active; Asai 2002 passive; Azar‐Cavanagh 2007; Cote 2003; L'Ecuyer 1996 2wva; L'Ecuyer 1996 mbc; L'Ecuyer 1996 pbc; Mendelson 1998; Prunet 2008 active; Prunet 2008 passive; Seiberlich 2016; Sossai 2010);
safe injection device (n = 4) (Gaballah 2012; Goris 2015; van der Molen 2011; Zakrzewska 2001);
multiple safety devices interventions (n = 5) (Chambers 2015 hospitals; Chambers 2015 long‐term nursing care; Phillips 2013; Reddy 2001; Valls 2007; Whitby 2008); and
safe needle disposal boxes (n = 3) (Edmond 1988; Grimmond 2010; Richard 2001).
Safety engineered devices can be divided into two broad categories, passive and active devices. Passive devices have a safety function that is automatically activated without the user's interference. This type of safety device is supposed to offer better protection because the human factor is excluded. Active devices require one‐ or two‐handed activation by a health worker after use.
Four studies used a similar type of safe active IV system (Autoguard IV) (Asai 1999 active; Asai 2002 active; Cote 2003; Prunet 2008 active). The safety mechanism of this device is activated by pushing a button which retracts the needle. Two studies evaluated a passive and an active system (Asai 2002 active; Asai 2002 passive; Prunet 2008 active; Prunet 2008 passive). In addition to the Autoguard IV, Asai 2002 passive and Prunet 2008 passive used a passive device. Asai 2002 passive used the Protective Acuvance, which consists of two needles (one inside the other) where the tip of the needle is automatically changed to a blunt needle upon withdrawing. Prunet 2008 passive used the Introcan safety, which automatically shields the needle tip upon withdrawing. The Introcan safety IV system was also used by Sossai 2010. Whereas Seiberlich 2016 used a safe active IV system (ViaValve), which consisted of a valve to prevent blood flow back out of the catheter hub on initial venipuncture.
A needleless system refers to a device that does not use needles for the collection of body fluids or administration of medication or fluid after initial IV access is established (Mendelson 1998). L'Ecuyer 1996 2wva; L'Ecuyer 1996 mbc; L'Ecuyer 1996 pbc used three needleless IV systems. One, the safety needleless IV tubing system (blunt metal cannula), was replaced after four months by a blunt plastic cannula due to dissatisfaction of employees with the device. Mendelson 1998 evaluated a needleless IV system which is incompatible with a needle. All other studies had employed either a combination of the needleless system and insertion or evaluated the effects of safe insertion only.
In the five studies involving multiple safety devices, one study included safety‐engineered needles and needleless devices that were either passive or semi‐automatic (Chambers 2015 hospitals; Chambers 2015 long‐term nursing care). The study by Phillips 2013 used safety‐engineered sharps. Reddy 2001 used safety syringes and needleless IV systems. Valls 2007 used safety vacuum phlebotomy systems, blood‐gas syringes with a needle sheath, lancets with retractable single‐use puncture sticks, safe IV catheters (passive and active), and safe injection devices. Whitby 2008 used multiple passive safety‐engineered devices including retractable syringes, needle‐free IV systems and safety winged butterfly needles.
In the studies on safe disposal boxes, Edmond 1988 evaluated a bedside needle disposal; Grimmond 2010 assessed a sharps container with enhanced safety features such as automatic lock‐out when full; and Richard 2001 introduced small containers in all patient areas combined with an educational program.
In studies focusing on safe blood collection, Rogues 2004 introduced two devices: re‐sheathable winged steel needles and Vacutainer blood‐collecting tubes with recapping sheaths. Goldwater 1989 used a shield on the needle cap to prevent the needle from injuring the worker. Baskin 2014 used a safety‐engineered blood gas syringe in which the cannula protection shield is activated with one hand after puncture and clicks irreversibly over the cannula.
Representing safe injection devices, Gaballah 2012 used safety dental syringes that did not require re‐sheating or removal of the needle from its syringe. Goris 2015 used passive subcutaneous retractable syringes that automatically and instantly retract the needle from the patient into the barrel of the syringe. van der Molen 2011 evaluated an injection needle with a safety feature shielding the needle after the injection, and Zakrzewska 2001 assessed one type of safety syringe for dentistry. The injection devices had an active safety mechanism that had to be activated by the workers.
A total of 17 studies reported introducing the safety devices together with training sessions (Azar‐Cavanagh 2007; Baskin 2014; Edmond 1988; Gaballah 2012; Goldwater 1989; Goris 2015; L'Ecuyer 1996 mbc; L'Ecuyer 1996 pbc; L'Ecuyer 1996 2wva; Mendelson 1998; Prunet 2008 active; Prunet 2008 passive; Richard 2001; Rogues 2004; Seiberlich 2016; Sossai 2010; Valls 2007; van der Molen 2011; Whitby 2008; Zakrzewska 2001). Goldwater 1989 briefly stated that staff completed an educational program. Two studies did not report on the integration of training or education as part of the study (Grimmond 2010; Reddy 2001).
Types of study designs
Study designs used to assess the effect of the intervention were:
six RCTs (Asai 1999 active; Asai 2002 active; Asai 2002 passive; Baskin 2014; Cote 2003; Prunet 2008 active; Prunet 2008 passive; Seiberlich 2016);
two cluster‐RCTs (L'Ecuyer 1996 2wva; L'Ecuyer 1996 mbc; L'Ecuyer 1996 pbc; van der Molen 2011);
five CBAs (Gaballah 2012; Grimmond 2010; Mendelson 1998; Valls 2007; Zakrzewska 2001); and
eleven ITS (Azar‐Cavanagh 2007; Chambers 2015 hospitals; Chambers 2015 long‐term nursing care; Edmond 1988; Goldwater 1989; Goris 2015; Phillips 2013; Reddy 2001; Richard 2001; Rogues 2004; Sossai 2010; Whitby 2008).
Participants
There were slight differences across studies in terms of selected participants for the study. In nine studies, researchers referred to the broad term of healthcare personnel or hospital workers as participants (Chambers 2015 hospitals; Chambers 2015 long‐term nursing care; Edmond 1988; Goris 2015; Grimmond 2010; Phillips 2013; Richard 2001; Rogues 2004; Sossai 2010; van der Molen 2011). Reddy 2001 included health personnel with the exception of physicians. Three studies included healthcare workers explicitly at risk of blood borne pathogen exposure from contaminated needles, referred to as house staff, physicians, medical students, nurses, nursing assistants, emergency medical technicians and environmental service workers (Azar‐Cavanagh 2007; Mendelson 1998; Whitby 2008). Three studies included nursing personnel only as participants (L'Ecuyer 1996 2wva; Seiberlich 2016; Valls 2007;). Two studies included anaesthesiologists (Cote 2003; Prunet 2008 active; Prunet 2008 passive). In two studies researchers and assistants were the persons handling the needles (Asai 1999 active; Asai 1999 active; Asai 2002 active). Dental clinic staff were the target group in one study (Zakrzewska 2001). One study included dental and nursing students (Gaballah 2012). One study included emergency department doctors (Baskin 2014). Another study included only laboratory staff (Goldwater 1989)
In one RCT the number of participants were 50 each in the intervention and control groups (Asai 1999 active; Asai 2002 active; Asai 2002 passive). In another RCT there were 254 and 251 participants in each of the intervention groups and 254 participants in the control group (Prunet 2008 active; Prunet 2008 passive). There were 119 participants in the control group and 211 in the intervention group in (Cote 2003) and 275 in each group in (Baskin 2014). In (Seiberlich 2016) there were 79 in the control group and 73 in the intervention group.
In the cluster‐RCTs, van der Molen 2011 reported on eight wards in each of the two intervention groups and the control group, representing approximately 265 workers in each of the these three groups during the initial phase. The authors adjusted for the cluster effect by means of a GEE‐analysis. L'Ecuyer 1996 2wva reported 19,436 patient‐days for the plastic two‐way valves, 3840 patient‐days for the metal blunt cannula (L'Ecuyer 1996 mbc) and 15,737 patient‐days for the plastic blunt needle (L'Ecuyer 1996 pbc). However, the study did not mention the number of wards that were randomised.
In the CBA studies, Grimmond 2010 recruited 14 hospitals in both the control and the intervention groups, approximating overall 19,880 full‐time equivalents (FTE) during the two‐year study period. Valls 2007 recruited seven wards for the intervention group and five wards for the control group from a hospital with 1000 workers. Zakrzewska 2001 had approximately 300 workers in both the intervention and control groups. Mendelson 1998 reported on eight medical units in both the intervention and control groups, corresponding to approximately 220 workers per group. Gaballah 2012 recruited three hospitals ‐ one for the control group and two for the intervention group. However, the authors did not report data relating to the number of participants.
In the ITS studies, Azar‐Cavanagh 2007 reported on 11,161 healthcare workers for the pre‐intervention period (18 months) and 12,851 healthcare workers for the post‐intervention period (18 months). Reddy 2001 reported on 3011 FTE for the pre‐intervention period (three years) and 3992 FTE for the post‐intervention period (three years). Rogues 2004 reported on 8500 FTE (2000 nurses) per year for the pre‐intervention period (four years) and post‐intervention period (three years). Edmond 1988 followed 278 nurses for the pre‐intervention period (eight months) but provided no information to determine if this number remained the same for the intervention period (four months). Richard 2001 did not report the number of participants in the one participating hospital during the seven‐year study period. Goldwater 1989 reported 127,000 venipunctures for the pre‐intervention period (six months), and 483,000 venipunctures with the device and 232,348 without the device during the intervention period (33 months). Sossai 2010 reported that the number of employees at the hospital fluctuated between 4447 and 4636 throughout the study period (two years pre‐intervention and three years post‐intervention). Chambers 2015 hospitals reported on an average of 325 000 FTE per year and included nine data points. Chambers 2015 long‐term nursing care also reported on an average of 325000 FTE per year and included nine data points. Goris 2015 reported on 857 895 employee productive hours for the pre‐intervention period and 237 202 employee productive hours for the post‐intervention period. Phillips 2013 reported on 184 years of cumulative data collected from 85 hospitals in the pre‐intervention period (six years) and 150 years of cumulative data collected from 85 hospitals in the post‐intervention period (five years). Whitby 2008 reported on 3053 FTE for the pre‐intervention period (12 months) and 6506 FTE for the post‐intervention period (24 months).
The average number of data points in the eleven ITS studies was 13.8 and ranged from six to 39.
Outcomes
Twenty‐one studies included self‐reported percutaneous injuries as their main outcome (Asai 1999 active; Asai 2002 active; Asai 2002 passive; Azar‐Cavanagh 2007; Chambers 2015 hospitals; Chambers 2015 long‐term nursing care; Cote 2003; Edmond 1988; Gaballah 2012; Goldwater 1989; Goris 2015; Grimmond 2010; L'Ecuyer 1996 2wva; L'Ecuyer 1996 mbc; L'Ecuyer 1996 pbc; Mendelson 1998; Phillips 2013; Reddy 2001; Richard 2001; Rogues 2004; Sossai 2010; Valls 2007; van der Molen 2011; Whitby 2008; Zakrzewska 2001). Seiberlich 2016 reported on incidence of blood leakage and blood exposure risk reduction. In two studies (Baskin 2014; Prunet 2008 active; Prunet 2008 passive) the main outcomes were both blood splashes and NSIs. In three studies researchers reported only blood splashes (Asai 1999 active; Asai 2002 passive; Cote 2003; Prunet 2008 active; Prunet 2008 passive). Three studies did not report NSIs as their main outcome as no injury was reported during the study (Asai 1999 active; Asai 2002 passive; Prunet 2008 active; Prunet 2008 passive). Cote 2003 reported that the study was underpowered to assess the difference in needlestick injuries between the groups.
The denominators for the self‐reported NSIs included: the number of procedures (Baskin 2014; Goldwater 1989; Rogues 2004), medical devices (Prunet 2008 active; Prunet 2008 passive; Sossai 2010), FTE (Chambers 2015 hospitals; Chambers 2015 long‐term nursing care; Grimmond 2010; Phillips 2013; Reddy 2001; Whitby 2008), health workers (Azar‐Cavanagh 2007; Edmond 1988; van der Molen 2011), patient‐days and productive hours worked (L'Ecuyer 1996 2wva; L'Ecuyer 1996 mbc; L'Ecuyer 1996 pbc), study weeks (Mendelson 1998), hours worked (Zakrzewska 2001), patients‐days and patients (Valls 2007), employee productive hours (Goris 2015). Richard 2001 reported the number of percutaneous injuries and the proportion of injuries due to improper disposal of sharps, which was defined by the authors as an NSI to worker assisting with a procedure, or NSI located on the non‐dominant hand while removing the needle. The denominators for the blood splashes were patients (Asai 1999 active; Asai 2002 active; Asai 2002 passive; Prunet 2008 active; Prunet 2008 passive) and number of procedures (Baskin 2014; Cote 2003). In one study the denominator for NSIs was not reported (Gaballah 2012).
Researchers reported the ease of use of the devices in six studies (Asai 1999 active; Asai 2002 active; Asai 2002 passive; Baskin 2014; Mendelson 1998; Prunet 2008 active; Prunet 2008 passive; Seiberlich 2016). Five studies included a cost analysis of the intervention (Goris 2015; Mendelson 1998; Valls 2007; Whitby 2008; Zakrzewska 2001).
To be able to estimate the absolute effect of an intervention it was important to know what the control group injury rate or the baseline rate was. The NSI rate varied from 5.0 percutaneous injuries (PIs) per 1000 person‐years for Azar‐Cavanagh 2007 to 1.03 per 1000 FTE‐years for Reddy 2001. Rogues 2004 reported a rate of 17.0 phlebotomy related PIs per 100,000 devices purchased. Sossai 2010 had a baseline rate of 9.67 per 100,000 catheters used per year. Goldwater 1989 reported a rate of about 49 per 100,000 venipuncture‐years.
Geographical location
The included studies originated from nine different countries. Nine studies were from the USA (Azar‐Cavanagh 2007; Cote 2003; Edmond 1988; Goris 2015; Grimmond 2010; L'Ecuyer 1996 2wva; L'Ecuyer 1996 mbc; L'Ecuyer 1996 pbc; Mendelson 1998; Phillips 2013; Reddy 2001), two from Japan (Asai 1999 active; Asai 2002 active; Asai 2002 passive), two from France (Prunet 2008 active; Prunet 2008 passive; Rogues 2004), two from Canada (Chambers 2015 hospitals; Chambers 2015 long‐term nursing care; Seiberlich 2016), two from the UK (Gaballah 2012; Zakrzewska 2001) and one each from New Zealand (Goldwater 1989), India (Richard 2001), Italy (Sossai 2010), Spain (Valls 2007), the Netherlands (van der Molen 2011), Turkey (Baskin 2014) and Australia (Whitby 2008).
Year of study
Of the 24 included studies, 19 had been published after the year 2000 (Asai 2002 active; Asai 2002 passive; Azar‐Cavanagh 2007; Baskin 2014; Chambers 2015 hospitals; Chambers 2015 long‐term nursing care; Cote 2003; Gaballah 2012; Goris 2015; Grimmond 2010; Phillips 2013; Prunet 2008 active; Prunet 2008 passive; Reddy 2001; Richard 2001; Rogues 2004; Seiberlich 2016; Sossai 2010; Valls 2007; van der Molen 2011; Whitby 2008; Zakrzewska 2001), whereas three studies had been published in the 1990s (Asai 1999 active; L'Ecuyer 1996 2wva; L'Ecuyer 1996 mbc; L'Ecuyer 1996 pbc; Mendelson 1998) and two studies in the 1980s (Edmond 1988; Goldwater 1989).
Excluded studies
The table Characteristics of excluded studies lists the reasons for exclusion of 44 studies.
Risk of bias in included studies
Risk of bias varied considerably across studies (Figure 2; Figure 3).
2.
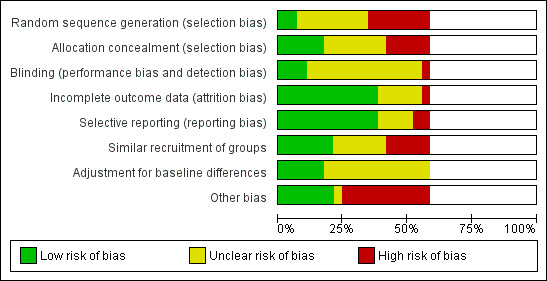
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Adequate sequence generation
We judged one of the six RCTs to have a low risk of bias for sequence generation because the researchers used a ballot box to randomise patients (Prunet 2008 active; Prunet 2008 passive). One RCT used randomisation by week (Cote 2003) and we judged it to have a high risk of bias due to the predictability of the randomisation. In one RCT (Seiberlich 2016) randomisation was done on a 1:1 basis by the participating clinicians and hence we judged it to have a high risk of bias. We judged three of the six RCTs to have an unclear risk of bias because the authors did not report specific information on the method used for randomisation (Asai 1999 active; Asai 2002 active; Asai 2002 passive; Baskin 2014).
Neither of the two cluster‐RCTs provided sufficient information about their randomisation process and therefore we judged them to have an unclear risk of bias (L'Ecuyer 1996 2wva; L'Ecuyer 1996 mbc; L'Ecuyer 1996 pbc; van der Molen 2011).
Allocation concealment
We judged three of the six RCTs to have a low risk of bias for allocation concealment because the researchers used sealed opaque envelopes or a single‐blinded envelope (Asai 2002 active; Asai 2002 passive; Baskin 2014; Prunet 2008 active; Prunet 2008 passive). We judged three RCTs and two cluster‐RCTs (Asai 1999 active; Cote 2003; L'Ecuyer 1996 2wva; L'Ecuyer 1996 mbc; L'Ecuyer 1996 pbc; Seiberlich 2016; van der Molen 2011) to have an unclear risk of bias because the authors reported no information about allocation concealment.
Blinding
Among the RCTs, Asai 1999 active and Asai 2002 passive reported that the presence or absence of blood on the tray was assessed by blinded researchers. We judged these two studies to have a low risk bias. Seiberlich 2016 reported it was not a double‐blind study which led to an inherent yet unavoidable clinician bias. Hence we judged this study to have a high risk of bias. Cote 2003; and Prunet 2008 active; Prunet 2008 passive also reported the presence or absence of blood spills but they did not report if the outcome assessors were blinded. Because of this we judged these two studies to have an unclear risk of bias. We judged the remaining 19 included studies to have an unclear risk of performance and detection bias as they provided no information on blinding.
One ITS study and another CBA study reported that healthcare workers were unaware of the study (Edmond 1988; Grimmond 2010). In these two studies it is unlikely that the staff changed their work practices or behaviours towards reporting NSIs due to the acknowledgment of the study. However, health workers would be aware of the change in the type of devices used. Consequently we judged these two studies to have an unclear risk of bias.
Incomplete outcome data
Among the six RCTs and two cluster‐RCTs, we judged six studies to have a low risk for incomplete outcome data because they reported all outcome data for all participants (Asai 1999 active; Asai 2002 active; Baskin 2014; Cote 2003; L'Ecuyer 1996 2wva; van der Molen 2011). Outcome information was unclear for the remaining two RCTs (Prunet 2008 active; Seiberlich 2016) and therefore we judged them to have an unclear risk of bias in this domain.
Among the five CBA studies, we judged three studies to have a low risk of bias because there was complete outcome data available (Grimmond 2010; Mendelson 1998; Zakrzewska 2001). The remaining two CBA studies reported outcome information unclearly and therefore we judged them to have an unclear risk of attrition bias (Gaballah 2012; Valls 2007).
Selective reporting
Among the six RCTs and two cluster‐RCTs, seven studies reported all outcomes as described in the method section and therefore we judged them to have a low risk of reporting bias (Asai 1999 active; Asai 2002 active; Asai 2002 passive; Baskin 2014; Cote 2003; Prunet 2008 active; Prunet 2008 passive; Seiberlich 2016; van der Molen 2011). We judged L'Ecuyer 1996 2wva to have an unclear risk of reporting bias as information that we expected based on the described methods appeared to be missing in the results section.
Among the five CBA studies, two studies reported all outcomes as described in the methods sections and therefore we judged them to have a low risk of reporting bias (Grimmond 2010; Mendelson 1998). We judged Valls 2007 to be at high risk of reporting bias because the authors did not fully report outcomes in the results section and they did not consistently report the denominator used for their analyses. We judged Gaballah 2012 to have a high risk of reporting bias because the type of syringe system causing NSIs among various departments was not mentioned in the results section. We judged Zakrzewska 2001 to have an unclear risk of reporting bias because the authors did not specifically mention their outcome measures in the methods section.
Similar recruitment of groups
Among the six RCTs and two cluster‐RCTs, we judged Baskin 2014; Prunet 2008 passive and van der Molen 2011 to have a low risk of recruitment bias. According to our judgment, four studies had an unclear risk of recruitment bias because they did not report information related to the recruitment of study groups (Asai 1999 active; Asai 2002 active; Cote 2003; Seiberlich 2016). We judged one study to be at high risk of recruitment bias due to a difference in the recruitment process for the intervention and control groups (L'Ecuyer 1996 2wva; L'Ecuyer 1996 mbc; L'Ecuyer 1996 pbc).
Among the five CBA studies, we judged two studies to have a low risk of recruitment bias (Grimmond 2010; Mendelson 1998). The study by Grimmond 2010 reported a small difference in staff full‐time equivalents (FTE) (< 1%) and the study by Mendelson 1998 was completed within a relatively short period of time (six months). We judged one study to have an unclear risk of recruitment bias due to the lack of information related to the recruitment of groups (Zakrzewska 2001). We judged two studies to be at high risk of recruitment bias because in one the researchers self‐assigned control and intervention hospital wards (Valls 2007) and in the other study the authors recruited control and intervention groups from different hospitals (Gaballah 2012).
Adjustment for baseline differences
For an RCT, any baseline difference should be due to chance if the randomisation process was appropriately completed. According to our judgment Asai 1999 active; Asai 2002 active; Asai 2002 passive; Cote 2003; L'Ecuyer 1996 2wva; L'Ecuyer 1996 mbc; L'Ecuyer 1996 pbc and Seiberlich 2016 had an unclear risk of bias due to baseline imbalance as they provided no information about the participants in the intervention and control groups. We judged Baskin 2014; Prunet 2008 active; Prunet 2008 passive and van der Molen 2011 to have a low risk of bias as they had adequately adjusted for baseline differences.
Among the five CBAs, we judged four studies to have an unclear risk of bias due to baseline imbalance as they reported no information regarding the adjustment for baseline difference (Gaballah 2012; Grimmond 2010; Mendelson 1998; Valls 2007). We judged Zakrzewska 2001 to have a low risk of bias in this domain because both groups were similar.
Risk of bias in ITS studies
See Table 15 for an overview of our judgment of all 11 included studies' risk of bias in all seven risk of bias domains relevant to the ITS design, and the consequent level of evidence provided. Among the 11 included ITS studies, five studies fulfilled the criterion that the intervention was independent of other changes (Azar‐Cavanagh 2007; Chambers 2015 hospitals; Chambers 2015 long‐term nursing care; Goris 2015; Phillips 2013; Rogues 2004). None of the studies reported a repeated measures analysis nor tested for trend, but this was overcome by our re‐analysis of the data. Six studies (Azar‐Cavanagh 2007; Edmond 1988; Goldwater 1989; Reddy 2001; Rogues 2004; Whitby 2008) used a data collection method which was sustained throughout the study and thus was unlikely to have affected the data collection. Three studies reported information to help determine if blind outcome assessment was used (Chambers 2015 hospitals; Chambers 2015 long‐term nursing care; Phillips 2013; Goris 2015). For the criterion of the completeness of the data set, five studies reported outcome data adequately (Azar‐Cavanagh 2007; Goldwater 1989; Goris 2015; Sossai 2010; Whitby 2008). We assessed the outcome measures of nine studies to be reliable because they used a consistent reporting system for NSI throughout the study period or they sourced data from a reliable source such as administrative data (Azar‐Cavanagh 2007; Chambers 2015 hospitals; Chambers 2015 long‐term nursing care; Edmond 1988; Goris 2015; Phillips 2013; Reddy 2001; Rogues 2004; Sossai 2010; Whitby 2008). One ITS study had an additional risk of bias due to participating health workers having access to conventional needles during the intervention period (Reddy 2001).
2. Risk of bias in ITS studies.
| Study | Intervention independent of other changes | Sufficient data points | Test for trend | Intervention did not affect data collection | Blinded outcome assessment | Complete data set | Reliable outcome measure | Total score |
| Goldwater 1989 | Not done (0) Comment: staff turnover during study period. Staff preference for the use of the intervention devices varied across study periods. |
Done (1) Comment: inlcusion of 3 data points before and after, the study was reanalysed using ARIMA model. |
Done (1) Comment: we reanalysed the study for trend. |
Done (1) Data collection seems to remained the same pre and post‐intervention. |
Not clear (0) Authors do not provide information on blinding. |
Done (1) | Not clear (0): Comment: no system for NSI seems to have been in placed during the study period. Uncertain about the consistency of the reporting during the study period. |
4 |
| Rogues 2004 | Done (1) Quote: "Conventional phlebotomy non‐safety devices were removed from all departments, and the new products were in place on implementation" Comment: only one device seems to have been introduced during intervention but authors do not specify if additional changes occurred during the study. |
Done (1) Comment: inlcusion of 3 data points before and after, the study was reanalysed using ARIMA model. |
Done (1) Comment: we reanalysed the study for trend. |
Done (1) Comment: the method of data collection remains the same throughout the study. It does not appears to be influenced by the intervention. |
Not clear (0) Comment No information is available on blinding. |
Not done (0) Comment: data not available for the estimated number of phlebotomies performed for 1993 and 1994. |
Done (1) Comment: hospital has a sharp injury surveillance system prior and after intervention. Althought not ideal as possibility of underreporting but appropriate for the study outcome. |
5 |
| Reddy 2001 | Not done (0) Quote: one of the confounder present throughout the post intervention phase was the availability of traditional needles devices. Comment: intervention occurs simultaneously with the availability of non‐safety device. |
Done (1) Comment: inlcusion of 3 data points before and after, the study was reanalysed using ARIMA model. |
Done (1) Comment: we reanalysed the study for trend. |
Done (1) Comment: the method of data collection remains the same throughout the study. It does not appears to be influenced by the intervention. |
Not clear (0) Comment: no information available on blinding |
Not done (0) Comment: physicians were excluded from analysis as no information on FTE. |
Done (1) Comment: hospital had a sharp injury surveillance system prior and after intervention. Althought no ideal as possibility of underreporting but appropriate for the study outcome. |
4 |
| Azar‐Cavanagh 2007 | Done (1) Comment: safety devices seem to have systematically replaced the conventional devices. Authors do not specify if additional changes occurred during the study. |
Done (1) Comment: inlcusion of 3 data points before and after, the study was reanalysed using ARIMA model. |
Done (1) Comment: we reanalysed the study for trend. |
Done (1) Comment: the method of data collection remains the same throughout the study. It does not appears to be influenced by the intervention. |
Not clear (0) Comment: authors do not specify if data analysts were blinded to the study. Healthcare workers could not have been blinded to the introduction of the new devices. |
Done (1) Comment: data is available for all health workers. |
Done (1) Coment: |
6 |
| Sosai 2010 | Not done (0) Comment: authors indicated that some conventional devices were still used during the intervention period despite study which aimed to replace all conventional devices by new safety devices. |
Done (1) comment: inlcusion of 3 data points before and after, the study was reanalysed using ARIMA model. |
Done (1) Comment: we reanalysed the study for trend. |
Not done (0) Quote: "after launching the sharps awareness campaign in 2003, # of injuries increased possibility because of sharps awareness campaign" Comment: intervention seems to have affected reporting of NSI. |
Not clear (0) Comment: information on blinding is not reported. |
Done (1) Comment: all hospital employees were included in the study. |
Done (1) Comment: used the incident reporting system throughout the study which appears to be adequate measure for NSI. |
4 |
| Edmond 1988 | Not clear (0) Comment: no information if additional changes were introduced during the same period at the hospital. |
Done (1) Comment: inlcusion of 3 data points before and after, the study was reanalysed using ARIMA model. |
Done (1) Comment: we reanalysed the study for trend. |
Done (1) Comment: intervention does not appears to have affected method of data collection. |
Not clear (0) Quote: "the subjects were unaware of the nature of the study". Comment: the reporting of the NSI was not likely to be affected by the staff knowing of the study. However, health workers would be aware of the change in the type of devices used. |
Not clear (0) Comment: information about the number of nurses for pre‐intervention but not for post‐intervention. For NSI, the number of staff per year is not available. |
Done (1) Comment: authors used employee health records for pre and post intervention. For NSI, this system appears reliable for the outcome of interest. |
4 |
| Richard 2001 | Not clear (0) Comment: no information if additional changes were introduced during the same period at the hospital. |
Done (1) Comment: inlcusion of 3 data points before and after, the study was reanalysed using ARIMA model. |
Done (1) Comment: we reanalysed the study for trend. |
Not done (0) Quote: the increase in total injuries reported in 1998 followed a better reporting stimulated by the second educational program. Comment: the reporting system started in 1993, it is possible that as more people became aware of the surveillance system, there was an increase in reporting. |
Not clear (0) Comment: No information is available on blinding |
Not clear (0) Comment: no information on the actual number of healthcare workers included during pre and post intervention. |
Not clear (0) Comment: it is unclear if the reporting system was used consistently throughout the years especially as it was launched during the early phase of the study. |
2 |
| Chambers 2015 hospitals | Done (1) Comment: safety devices seem to have replaced the conventional devices due to legislation. |
Done (1) Comment: inlcusion of 3 data points before and after, the study was reanalysed using ARIMA model. |
Done (1) Comment: we reanalysed the study for trend. |
Not clear (0) Comment: Increased attention to needle stick injury prevention during the period of regulatory change may have resulted in increased reporting. |
Done (1) Comment: data was obtained from an administrative source. |
Not done (0) Comment: the data set represented 63 percent of all needlestick injury claims. |
Done (1) Comment: authors used work place safety and insurance board data for compensation claims. For NSI, this system appears reliable for the outcome of interest. |
5 |
| Chambers 2015 long‐term nursing care | Done (1) Comment: safety devices seem to have replaced the conventional devices due to legislation. |
Done (1) Comment: inlcusion of 3 data points before and after, the study was reanalysed using ARIMA model. |
Done (1) Comment: we reanalysed the study for trend. |
Not clear (0) Comment: Increased attention to needle stick injury prevention during the period of regulatory change may have resulted in increased reporting. |
Done (1) Comment: data was obtained from an administrative source. |
Not done (0) Comment: the data set represented 63 percent of all needlestick injury claims. |
Done (1) Comment: authors used work place safety and insurance board data for compensation claims. For NSI, this system appears reliable for the outcome of interest. |
5 |
| Goris 2015 | Done (1) Quote: "The existing inventories of subcutaneous active safety‐engineered devices were removed and replaced with subcutaneous passive safety‐engineered devices" Comment: All conventional devices were replaced by safety‐engineered devices at the start of the intervention. |
Done (1) Comment: inlcusion of 3 data points before and after, the study was reanalysed using ARIMA model. |
Done (1) Comment: we reanalysed the study for trend. |
Not clear (0) Comment: the reporting might have increased after inrodcution of the passive safety‐engineered device due to heightened awareness. |
Done (1) Comment: data was obtained from an administrative source. |
Done (1) Comment: data for all the healthcare workers was provided in the form of employee productive hours in the pre and post intervention phase. |
Done(1) Comment: authors used BJC occupational health database records for pre and post intervention. This being administrative data appears to be reliable for the outcome of interest. |
6 |
| Phillips 2013 | Done (1) Comment: safety devices seem to have replaced the conventional devices due to legislation. |
Done (1) Comment: inlcusion of 3 data points before and after, the study was reanalysed using ARIMA model. |
Done (1) Comment: we reanalysed the study for trend. |
Not clear (0) | Done (1) Comment: data was obtained from an administrative source. |
Not done (0) Comment: Data set represented only 73% of the total sample. |
Done (1) Comment: Data was obtained from the US Exposure Prevention Information Network (EPINet) sharps injury surveillance database. This appears to be adequate measure for NSIs. |
5 |
| Whitby 2008 | Not clear (0) | Done (1) Comment: inlcusion of 3 data points before and after, the study was reanalysed using ARIMA model. |
Done (1) Comment: we reanalysed the study for trend. |
Done (1) Comment: the constant and unchanging rate of NSI with solid suture needles implies that reduction of NSI relates neither to the education program associated or increased reporting rates. |
Not done (0) Comment: health workers were aware of the change in the type of devices used. |
Done (1) Comment: data is available for all health workers. |
Done (1) Comment: used the same system of reporting of NSI in pre and post intervention period to the infectious diseases department which has been in place since 1996. |
5 |
Other potential sources of bias
In two RCTs (Asai 1999 active; Asai 2002 active; Asai 2002 passive) the authors reported that the industry supplied the medical safety devices, which could have potentially introduced bias. Therefore we judged these studies to have a high risk of bias. In one RCT (Seiberlich 2016) in addition to the study being funded by the manufacturer of the devices being evaluated a co‐author was an employee of the study sponsor. Consequently we judged the study to have a high risk of bias. In one study, health workers had access to conventional needles during the intervention period (L'Ecuyer 1996 2wva; L'Ecuyer 1996 mbc; L'Ecuyer 1996 pbc). Injuries during this period were attributed to the new devices even if they were caused by the conventional devices. Consequently we judged the study to have high risk of bias.
Among the five CBA studies, Zakrzewska 2001 reported that the industry supplied the medical safety devices, which could have potentially introduced bias. We judged this study to have a high risk of bias. In another study, the surveillance system for NSIs differed between the pre‐ and post‐intervention phases (Valls 2007). This difference may imply a high risk of bias because a more active case finding system was used during the intervention period. Finally, one study introduced another device parallel to the main intervention (Zakrzewska 2001).
The measurement of NSIs was a source of bias in all studies that used this outcome. NSIs can be based on self‐report or a proxy measure of glove perforations. However, none of the included studies used glove perforations as a measurement of NSIs. Like any occupational injury, the reporting of NSIs increases when workers are more aware of the problem, for example due to an awareness campaign. Any intervention has the same effect as an awareness campaign and will thus raise the number of reported injuries. This will probably lead to an underestimation of the true intervention effect.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13
1. Safe blood collection systems versus regular systems
RCT
Outcome: needlestick injuries (NSIs)
One RCT (Baskin 2014) randomised patients to two types of syringes and evaluated the effect of safety engineered blood gas syringes on NSI compared to a conventional heparinised syringe group in the physicians who drew the blood samples. Both intervention (n = 275) and control groups (n = 275) included patients who visited the emergency department. After an immediate follow up, there was a statistically non‐significant decrease in the NSI following the intervention (RR 0.20, 95% CI 0.01 to 4.15) (Analysis 1.1).
1.1. Analysis.
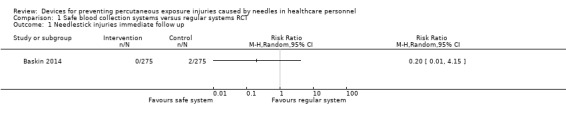
Comparison 1 Safe blood collection systems versus regular systems RCT, Outcome 1 Needlestick injuries immediate follow up.
Outcome: blood splashes
The same study (Baskin 2014) also examined contact with blood. There was a statistically non‐significant decrease in the incidence of blood splashes (RR 0.14, 95% CI 0.02 to 1.15) (Analysis 1.2).
1.2. Analysis.
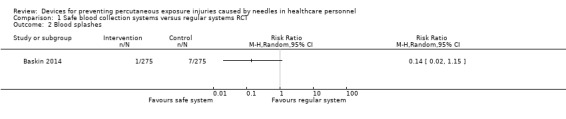
Comparison 1 Safe blood collection systems versus regular systems RCT, Outcome 2 Blood splashes.
ITS
Outcome: needlestick injuries (NSIs)
The two included ITS studies evaluated very different interventions. Therefore, we did not combine the studies in a meta‐analysis. One study evaluated a shield on the needle cap that should prevent the needle from injuring the worker when the cap is put back on the needle (Goldwater 1989). There was a non‐significant trend towards a decrease of injuries in this study (Analysis 2.1). The other used a needle sheath (Rogues 2004). In this study the level of injuries decreased substantially (effect size (ES) ‐6.88, 95% CI ‐9.53 to ‐4.23) but the trend over time showed a non‐significant decrease (Analysis 2.2).
2.1. Analysis.
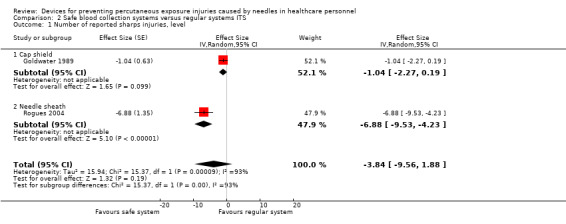
Comparison 2 Safe blood collection systems versus regular systems ITS, Outcome 1 Number of reported sharps injuries, level.
2.2. Analysis.

Comparison 2 Safe blood collection systems versus regular systems ITS, Outcome 2 Number of reported sharps injuries, slope.
2. Safe intravenous systems versus regular systems
RCT
Outcome: needlestick injuries (NSIs)
One trial evaluated the effect of three different safe IV systems to prevent NSI, which resulted in a non‐significant reduction of reported NSIs with a RR of 0.62 (95% CI 0.27 to 1.41) (Analysis 3.1) (L'Ecuyer 1996 mbc; L'Ecuyer 1996 2wva; L'Ecuyer 1996 pbc).
3.1. Analysis.
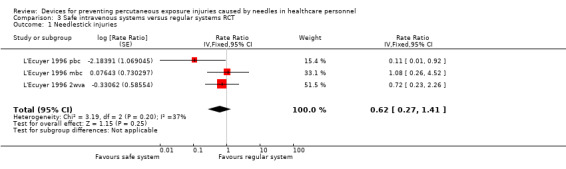
Comparison 3 Safe intravenous systems versus regular systems RCT, Outcome 1 Needlestick injuries.
Outcome: incidence of blood contamination
Seven trials with 1641 participants studied if safe IV systems resulted in a change in blood contamination compared to the usual systems. There was a statistically non‐significant increased risk of blood contamination with the safe systems with a RR of 1.38 (95% CI 1.00 to 1.92). Active systems, which had to be activated by health workers, displayed a statistically significant increase in blood splashes (RR 1.60, 95% CI 1.08 to 2.36). Passive systems, which don't have to be activated, displayed a similar incidence in blood splashes in both the intervention and control groups (RR 0.94, 95% CI 0.50 to 1.75) (Analysis 3.2).
3.2. Analysis.
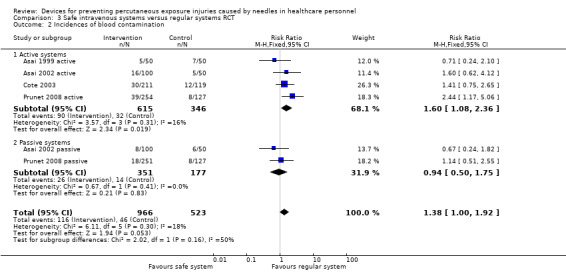
Comparison 3 Safe intravenous systems versus regular systems RCT, Outcome 2 Incidences of blood contamination.
Outcome: incidence of blood leakage
One RCT study (Seiberlich 2016) evaluated the effect of a passive safe IV system on the reduction of blood leakage events during insertion of the catheter, withdrawal of the needle and connection of the luer. The study showed a significant reduction in the incidence of blood leakage events with safe IV systems (RR 0.21, 95% CI 0.11 to 0.37) (Analysis 3.3).
3.3. Analysis.
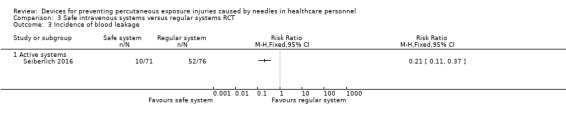
Comparison 3 Safe intravenous systems versus regular systems RCT, Outcome 3 Incidence of blood leakage.
CBA
Outcome: needlestick injuries (NSIs)
One CBA study (Mendelson 1998) evaluated the effect of safe IV systems to prevent NSI, which resulted in a non‐significant reduction of reported NSIs with a RR of 0.06 (95% CI 0.0 to 1.09) (Analysis 4.1).
4.1. Analysis.
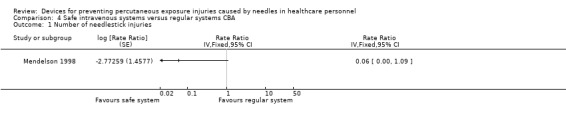
Comparison 4 Safe intravenous systems versus regular systems CBA, Outcome 1 Number of needlestick injuries.
ITS
Outcome: needlestick injuries (NSIs)
In two ITS studies (Azar‐Cavanagh 2007; Sossai 2010) the results were statistically very heterogenous (I² = 79% for level and I² = 99% for trend) and therefore we did not combine them in a meta‐analysis. The level in both studies decreased with a big effect size (Analysis 5.1). The trend over time decreased substantially in one study but not in the other (Analysis 5.2).
5.1. Analysis.
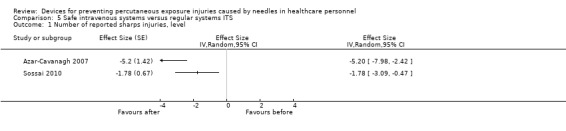
Comparison 5 Safe intravenous systems versus regular systems ITS, Outcome 1 Number of reported sharps injuries, level.
5.2. Analysis.
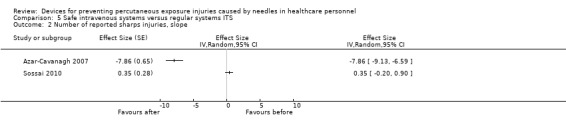
Comparison 5 Safe intravenous systems versus regular systems ITS, Outcome 2 Number of reported sharps injuries, slope.
3. Safe injection systems versus regular systems
RCT
Outcome: needlestick injuries (NSIs)
One RCT (van der Molen 2011) evaluated the effect of a workshop on NSI combined with the introduction of safety engineered injection needles in seven wards (n = 267) compared to a non‐intervention control group (eight wards, n = 266) and to a workshop on the prevention of NSIs only control group in eight wards (n = 263). NSIs were measured by questionnaires and by the hospital reporting system.
At six‐months follow‐up, there was a statistically non‐significant decrease in NSI based on the questionnaires (RR 0.49, 95% CI 0.16 to 1.56), but based on the hospital records there was a statistically non‐significant increase in NSI (RR 1.20, 95% CI 0.42 to 3.39) (Analysis 6.1; Analysis 6.2).
6.1. Analysis.

Comparison 6 Safe injection systems versus regular systems RCT, Outcome 1 Questionnaire reported Needlestick injuries 6 mo follow up.
6.2. Analysis.
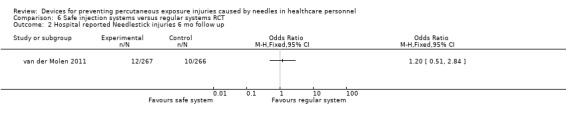
Comparison 6 Safe injection systems versus regular systems RCT, Outcome 2 Hospital reported Needlestick injuries 6 mo follow up.
At 12‐months follow‐up, based on the questionnaire results there was a statistically significant reduction of NSI with RR of 0.20 (95% CI 0.04 to 0.96), but based on the hospital recording system there was a statistically non‐significant reduction of NSI with RR 0.72 (95% CI 0.28 to 1.81) (Analysis 6.3; Analysis 6.4).
6.3. Analysis.
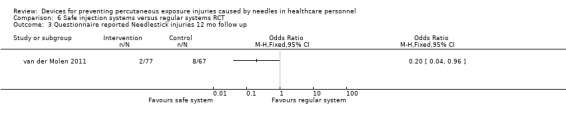
Comparison 6 Safe injection systems versus regular systems RCT, Outcome 3 Questionnaire reported Needlestick injuries 12 mo follow up.
6.4. Analysis.
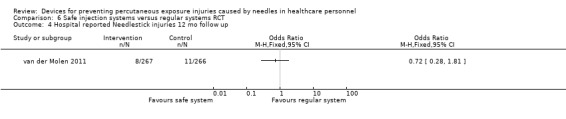
Comparison 6 Safe injection systems versus regular systems RCT, Outcome 4 Hospital reported Needlestick injuries 12 mo follow up.
CBA
In one study among dentists (Zakrzewska 2001) the risk of NSI was smaller with safe syringes compared to traditional ones but the difference was not significant (RR 0.34, 95% CI 0.04 to 3.28) (Analysis 7.1). Another study which was carried out among dental students (Gaballah 2012) evaluated the risk of NSI with safety dental syringes compared to conventional dental syringes. The authors did not report complete data regarding the type of syringe system causing NSIs for the departments in the intervention and control groups and therefore we did not analyse the results.
7.1. Analysis.
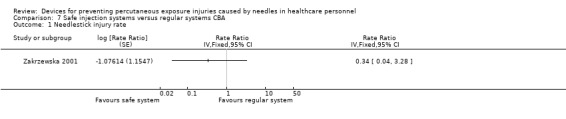
Comparison 7 Safe injection systems versus regular systems CBA, Outcome 1 Needlestick injury rate.
ITS
Outcome: needlestick injuries (NSIs) change in level
One study among healthcare workers (Goris 2015) evaluated the effect of a trial with passive safety‐engineered injection systems compared to active safety‐engineered injection systems on the incidence of NSI. There was no considerable effect on the level of NSI following the introduction of the intervention (ES 0.23, 95% CI ‐1.89 to 2.35) (Analysis 8.1).
8.1. Analysis.
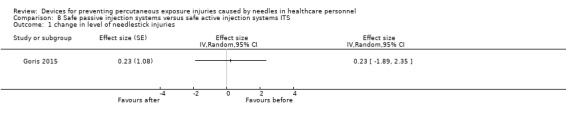
Comparison 8 Safe passive injection systems versus safe active injection systems ITS, Outcome 1 change in level of needlestick injuries.
Outcome: needlestick injuries (NSIs) change in slope
The same study showed a statistically non‐significant long term trend of a decrease in NSI (ES ‐0.74, 95% CI ‐1.66 to 0.18) (Analysis 8.2).
8.2. Analysis.
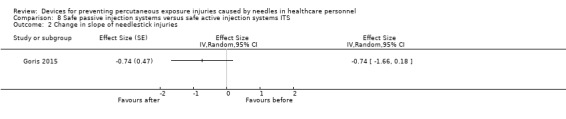
Comparison 8 Safe passive injection systems versus safe active injection systems ITS, Outcome 2 Change in slope of needlestick injuries.
4. Multiple safe devices versus regular devices
CBA
Outcome: needlestick injuries (NSIs)
One study that compared hospital level injury rates (Valls 2007) found a decrease in NSI in the hospitals that introduced safety devices compared to those that did not (RR 0.11, 95% CI 0.01 to 0.81) (Analysis 10.1).
10.1. Analysis.
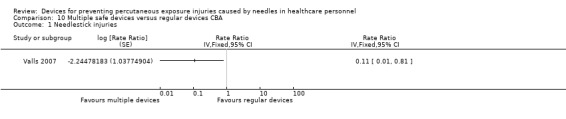
Comparison 10 Multiple safe devices versus regular devices CBA, Outcome 1 Needlestick injuries.
ITS
Outcome: needlestick injuries (NSIs) change in level
In one ITS study (Reddy 2001) there was a statistically non‐significant increase in the level of injuries following the introduction of the safety syringes and needleless IV system (ES 0.43, 95% CI ‐0.30 to 1.16) (Analysis 9.1). Another ITS study (Whitby 2008) showed a statistically non‐significant decrease in the level of NSI following the introduction of safety syringes, needless IV systems and safety‐engineered needles (ES ‐1.04, 95% CI ‐2.20 to 0.12) (Analysis 9.1).
9.1. Analysis.
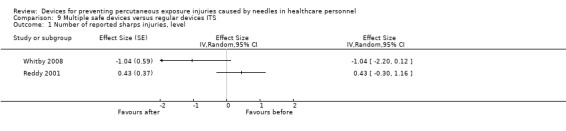
Comparison 9 Multiple safe devices versus regular devices ITS, Outcome 1 Number of reported sharps injuries, level.
Outcome: needlestick injuries (NSIs) change in slope
In the study by (Reddy 2001) the ES for the change in long‐term time trend showed an increase in the number of reported NSIs (ES 0.56, 95% CI 0.23 to 0.89) (Analysis 9.2). In the other ITS study (Whitby 2008) there was a statistically non‐significant decrease in the trend of reported NSI (ES ‐0.01, 95% CI ‐0.15 to 0.13) (Analysis 9.2).
9.2. Analysis.
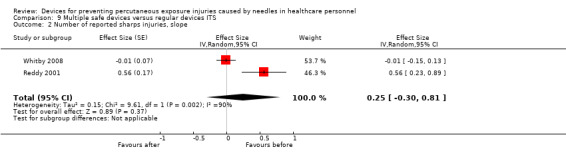
Comparison 9 Multiple safe devices versus regular devices ITS, Outcome 2 Number of reported sharps injuries, slope.
5. Sharps containers versus no containers
CBA
Outcome: needlestick injuries (NSIs)
In one CBA study (Grimmond 2010), the NSI rate decreased following the introduction of sharps containers compared to departments where these were not introduced with a RR of 0.88 (95% CI 0.78 to 0.99) (Analysis 12.1). This reduction was statistically significant when only container‐related NSIs were counted with a RR of 0.22 (95% CI 0.11 to 0.41) (Analysis 12.2).
12.1. Analysis.
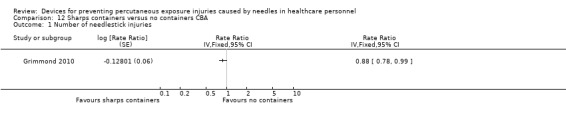
Comparison 12 Sharps containers versus no containers CBA, Outcome 1 Number of needlestick injuries.
12.2. Analysis.
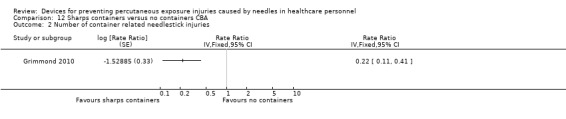
Comparison 12 Sharps containers versus no containers CBA, Outcome 2 Number of container related needlestick injuries.
ITS
Two ITS studies (Edmond 1988; Richard 2001) showed an increased level of NSI immediately after the introduction of sharps containers and a contradictory effect in the long‐term trend which prevented the synthesis of these studies in a meta‐analysis (Analysis 11.1; Analysis 11.2).
11.1. Analysis.

Comparison 11 Sharps containers versus no containers ITS, Outcome 1 Number of reported sharps injuries, level.
11.2. Analysis.

Comparison 11 Sharps containers versus no containers ITS, Outcome 2 Number of reported sharps injuries, slope.
6. Legislation versus no legislation
ITS
Outcome: needlestick injuries (NSIs) change in level
One ITS study had two intervention arms. One arm comprised of long‐term nursing care (Chambers 2015 long‐term nursing care) and the other comprised of hospitals (Chambers 2015 hospitals). According to the results the level of NSI decreased in long‐term nursing care after the introduction of legislation. However, the intervention arm comprising of hospitals showed an increase in the level of NSI. Another ITS study (Phillips 2013) also showed a decrease in the level of NSI following the introduction of legislation. Since these results were very heterogenous we did not combine them in a meta‐analysis (Analysis 13.1).
13.1. Analysis.
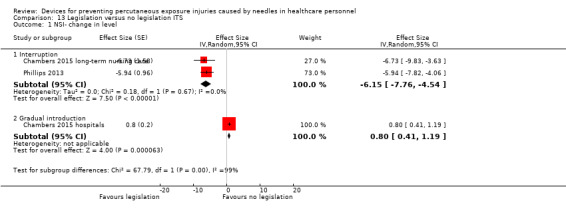
Comparison 13 Legislation versus no legislation ITS, Outcome 1 NSI‐ change in level.
Outcome: needlestick injuries (NSIs) change in slope
In one ITS study the NSI trend over time decreased in one of the intervention arms comprising of long‐term nursing care (Chambers 2015 long‐term nursing care) and increased in the other arm which included hospitals (Chambers 2015 hospitals). The other ITS study (Phillips 2013) showed a decrease in the long term trend of NSI (Analysis 13.2).
13.2. Analysis.
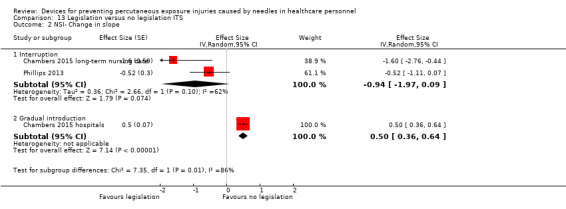
Comparison 13 Legislation versus no legislation ITS, Outcome 2 NSI‐ Change in slope.
Secondary outcomes
1. Cost
A total of five studies reported information regarding the cost of the intervention. Valls 2007 reported that the direct cost of the use of safety devices was an additional USD 19,417 (USD 0.75 per patient) for the emergency department and USD 16,336 (USD 0.56 per patient‐day) for the hospital wards compared to the pre‐intervention period. Zakrzewska 2001 reported that the price of the safety syringes was comparable to the non‐disposable syringes, approximately USD 0.33 per item. Mendelson 1998 reported that the estimated incremental hospital‐wide cost was USD 82,822 (in 1991) but the cost of injury prevented was USD 1593. Whitby 2008 reported that the overall increased cost for provision of safety‐engineered retractable syringes in the 800‐bed hopsital was USD 46,000 per annum, USD 14 for each at‐risk healthcare worker per year or USD 2 per occupied bed‐day per annum. Goris 2015 reported a net annual increase of USD 20,708.42 on conversion of ASED to PSED at the Barnes‐Jewish Hospital. The study also reported that the total cost avoidance of a conversion from ASED to PSED was USD 68,768.28.
2. Ease of use
Asai 1999 active reported no difference between the safety devices and the conventional devices in terms of ease of insertion. However, the authors reported statistically higher ease of handling for the safety device compared to the conventional one. Asai 1999 active, Asai 2002 active, and Asai 2002 passive reported that the Autoguard IV was significantly easier to insert and handle compared to the other safety device and the conventional catheter needle. Mendelson 1998 reported that 94% of the individuals who completed the survey (approximately 52% response rate) were comfortable using the safe IV system after five or less trials. Prunet 2008 active and Prunet 2008 passive reported that the Insyte Autoguard device was significantly more difficult to insert when compared to conventional devices and the passive devices. With both safety devices the needle was significantly more difficult to withdraw in comparison to the conventional catheter. Baskin 2014 reported that there was no significant difference between a conventional heparinised insulin syringe and safety‐engineered blood gas syringe in terms of ease of use. Seiberlich 2016 reported that the blood control PIVC and standard PIVC were similar in terms of ease of use.
Grading of the evidence
We graded the quality of the evidence per intervention‐outcome combination (Table 16). Because we based our conclusions upon results obtained with a range of study designs, we could not use the GRADEpro programme. We present our considerations in Table 16. For all but one combination we assessed the quality of the evidence as very low because of serious limitations in the study design and the inconsistency of the results. Starting with a low level of quality because of the non‐randomised studies included, the level goes down to very low quality. Only for the combination of safe IV systems and blood contamination, we assessed the quality of evidence as moderate because all included studies were RCTs and they did not have limitations in their design or in the other qualifiers.
3. Grading of the evidence.
| Comparison and outcome | Starting level | Risk of bias | Consistency | Directness | Precision | Publication bias | Quality of evidence |
| Safe versus traditional blood collection systems RCT ‐ all outcomes | high | 1 RCT high RoB | consistent | direct | wide CI | impossible to determine |
very low |
| Safe versus traditional blood collection systems ITS | low | 2 ITS high RoB | consistent | direct | wide CI | impossible to determine |
very low |
| Safe versus traditional IV systems RCT ‐ all outcomes | high | 5 RCT high RoB, 1 RCT low RoB | consistent | direct | wide CI | impossible to determine | very low |
| Safe versus traditional IV systems CBA | low | 1 CBA high RoB | consistent | direct | wide CI | impossible to determine | very low |
| Safe versus traditional IV systems ITS | low | 1 ITS low RoB, 1 ITS high RoB | consistent | direct | wide CI | impossible to determine | very low |
| Safe versus traditional injection systems RCT | high | 1 RCT high RoB | consistent | indirect; hospital | wide CI | impossible to determine | very low |
| Safe versus traditional injection systems CBA | low | 1 CBA high RoB | consistent | indirect; dentists | wdie CI | impossible to determine | very low |
| Safe pasive injection systems versus safe active injection systems ITS | low | 1 ITS low RoB | consistent | direct | wide CI | impossible to determine | very low |
| Multiple safe versus traditional devices ITS | low | 2 ITS high RoB | inconsistent | direct | wide CI | impossible to determine | very low |
| Multiple safe versus traditional devices CBA | low | 1 CBA high RoB | consistent | direct | wide CI | impossible to determine | very low |
| Sharps containers versus no containers ITS | low | 1 ITS low RoB, 1 ITS high RoB | inconsistent | direct | wide CI | impossible to determine | very low |
| Sharps containers versus no containers CBA ‐ all outcomes | low | 1 CBA high RoB | consistent | direct | wide CI | impossible to determine | very low |
| Legislation versus no legislation ITS | low | 2 ITS high RoB | consistent | direct | wide CI | impossible to determine | low |
Sensitivity analysis
We re‐analysed the results comparing safe IV systems for blood contamination leaving out the one study with a high risk of bias (Cote 2003), but that did not substantially change the results.
Publication bias
We did not have enough studies in any one comparison to assess the effect of publication bias with a funnel plot or a statistical test. However, because we also found small studies with negative results, we don't think that publication bias has played a significant role in the results of this review.
Subgroup analysis and exploration of heterogeneity
We intended to do a subgroup analysis based on the control group or baseline exposure rate. Since the exposures were measured in various ways and we had only a few studies in each comparison we refrained from doing so. In some comparisons, such as multiple safe devices and sharps containers, the results were inconsistent and we could not see any other reasons than the high risk of bias in the non‐randomised studies. We also intended to re‐analyse the results according to the origin of the study as one could expect low‐ and middle‐income countries to have a higher infectious disease prevalence (UNAIDS 2009). However, we included only two studies (Baskin 2014; Richard 2001) from low‐ or middle‐income countries (Turkey and India) that did not show a preventive effect from the introduction of safety‐engineered devices.
Discussion
Summary of main results
For safe blood collection systems, we found very low quality evidence of no considerable effect on NSIs in one underpowered RCT that introduced safe arterial blood gas collection systems. In one ITS study we found very low quality evidence of a large reduction in NSI following the use of a needle sheath on a winged steel needle. Another ITS study used cap shields that are outdated.
There was very low quality evidence in two ITS studies that NSIs were reduced with the introduction of safe IV devices. One RCT and one CBA study found no difference in NSIs. However, there was moderate quality evidence in four other RCTs that these devices increased the number of blood splashes where the safety system had to be engaged actively (relative risk (RR) 1.6, 95% CI 1.08 to 2.36) whereas two RCTs of passive systems produced low quality evidence that showed no effect on blood splashes. Yet another RCT produced low quality evidence that a different safe active IV system also decreased the incidence of blood leakages.
According to very low quality evidence from one RCT and one CBA study, the introduction of safe injection devices did not considerably change the NSI rate. One ITS study found low quality evidence of no effect on NSI rate following the introduction of safe passive injection systems compared to safe active injection systems.
According to very low quality evidence from one CBA study the introduction of multiple safety devices resulted in a decrease in NSIs (RR 0.1, 95% CI 0.01 to 0.81), whereas two ITS studies showed inconsistent results.
Similarly, the introduction of safety containers reduced NSIs in one CBA study but not in the two ITS studies (very low quality evidence).
Two ITS studies produced moderate quality evidence showing that the introduction of legislation on safety‐engineered devices reduced NSI rate. However, another ITS study reported in the same article that included hospitals the results showed the introduction of legislation having no effect on NSI rate. The reason for this could be that especially in this population safety‐engineered needles were available for early adoption already seven years prior to the legislation which invalidates the assumption that there is an interruption in the time‐series.
Overall completeness and applicability of evidence
The studies included in this review cover a time period from 1988 to 2016. With the exception of two studies, one from Turkey and the other from India, all the remaining studies were from high‐income countries. Studies covered a wide range of devices used for blood collection or injections. Some studies evaluated safety devices that are not in use anymore such as the standard needled IV system. This has been replaced by needleless IV systems. We included studies examining safety devices regardless of whether the devices were presently in use or not, as long as the studies evaluating them met our original inclusion criteria.
It is difficult to randomise complex interventions and therefore we also included non‐randomised studies. This provides the best avaliable evidence for these interventions. We felt that uncontrolled studies are at a too high risk of bias and therefore we did not include them. By including ITS studies we were able to detect both short‐term and long‐term effects on trends of injury rates.
Most studies could be named pragmatic trials because they were either carried out by the healthcare staff who were themselves at risk or they were based on routinely gathered data, such as NSI reports. This increased the applicability of the evidence but probably at the same time has decreased the quality of the studies. Most studies cover healthcare staff that are exposed to the risk of needlestick injuries, and as such the evidence is directly applicable to nurses, physicians and laboratory staff. Of the 24 included studies only two RCTs had researchers and assistants complete the procedures. Consequently their findings may not apply to the general population of healthcare workers. However, they completed the procedures in ordinary healthcare conditions and we assumed that they formed a part of the healthcare staff.
Among healthcare workers there is wide variation in skills, experience and working conditions that leads to a wide variation in NSI risk. For example, phlebotomists spend nearly all of their working hours drawing blood, and by repetition and practice will be more adept at this procedure than the average physician. At the same time their occupational exposure to needlestick injuries will also be higher than that of physicians due to the nature of their work. This variation can almost certainly lead to a difference in the rate of percutaneous exposure injuries. However, there was not enough variation in the included studies to assess this.
In the 2017 update of the review we found that there was low to moderate quality evidence that introduction of legislation on the use of safety‐engineered devices reduced the level of NSIs among healthcare workers.
Even though the number of studies increased from 17 to 24 in the 2017 update of this review, findings for various safety engineered devices remained largely unchanged from the original version of this review.
Quality of the evidence
We judged 20 of the 24 included studies to have a high risk of bias. The fact that we did find RCTs shows that rigorously controlled research methods can be used to evaluate the introduction of safety devices, especially in a cluster‐randomised design where hospital departments are randomised to the introduction of safety devices. Most of the often avoidable problems in study methodology like lack of randomisation (Table 16) might have been caused by the lack of involvement of professional research institutes.
With the exception of four studies, all included studies reported NSIs as their outcome. This outcome is problematic because these injuries are known to be under‐reported and are likely to increase with raised awareness, for example through an intervention study (Ratner 1994). This might explain the lack of effect in many studies, especially in the ITS studies. Nowadays, where the use of gloves with procedures that involve blood has increased, it would also be possible to use glove perforations as an outcome measure, which is less subject to reporting bias. Another problem with the NSI outcome is that the denominator varies across studies, with person‐years, employee productive hours, full time equivalents in some studies and 100,000 devices in others. We judged these all to be similar enough to be combined across studies because all these denominators reflect the hazard of needlestick injuries in a similar way, both in the intervention and the control group. There is most likely no single valid denominator for different purposes. It has been argued that for comparing hospitals the best denominator would be patient‐days, because of the accuracy and availability of the figures (Chen 2005).
Potential biases in the review process
We did not exclude studies published in languages other than English, but we found very few non‐English studies. Therefore, we are confident that there is no language bias in our review. We carried out all selection and data‐extraction processes in duplicate and involved a third assessor if we could not reach consensus easily.
The inclusion of non‐randomised studies further decreased the likelihood that we excluded important evidence. Because we analysed the non‐randomised studies separately, we believe that this has not introduced bias.
It was difficult to ascertain the validity of the outcome measures. Given the consistency of the results and the fact that the outcome was measured similarly in the intervention and control groups, we feel that this did not introduce bias. However, in some studies healthcare workers still had access to the conventional devices during the intervention period. Needlestick injuries caused by the conventional devices may have been misclassified as caused by safety devices, thus decreasing the effect of the intervention. The rate of needlestick injuries is a problematic outcome as attention to the problem has the potential to increase the rate of reporting thus nullifying the effect of the intervention. It could be that non‐significant results are due to this effect.
Agreements and disagreements with other studies or reviews
Several reviews have been published on prevention of percutaneous exposure injuries in the past years. Compared to earlier reviews (Hutin 2003; Rogers 2000), the number of studies has increased. Tuma 2006 reviewed the effect of safety engineered devices on percutaneous injuries, and reported that all 17 included studies reported a substantial decrease in injury rates. However, only five of these studies used a control group and the authors did not use meta‐analysis to combine results.
Harb 2015 reviewed the effect of safety‐engineered injection devices on the incidence of NSIs in healthcare delivery settings, and reported that there was moderate quality evidence that syringes with a sharps injury prevention feature reduced the incidence of needlestick injuries. The authors included uncontrolled before‐after studies which would normally be judged as having a high risk of bias. However, the authors arrived at the GRADE qualification moderate quality evidence for evidence based on uncontrolled before‐after studies. This is in disagreement with the GRADE guidance and our judgment of the quality of the available evidence.
Ballout 2016 reviewed the effect of safety‐engineered devices on the incidence of needlestick injuries during intravenous and phlebotomy procedures in healthcare settings. The authors included 21 NRS and one RCT and reported that there was moderate quality evidence that the use of safety‐engineered devices reduced the NSI rates of HCWs during phlebotomy and intravenous procedures. Here too the authors rated the evidence from uncontrolled before‐after studies as moderate quality which is in disagreement with the GRADE guidance and our judgment of the quality of the available evidence.
The HSE 2012 review states that there was low quality (SIGN level C) evidence that safety sharps devices lead to a reduction in sharps injuries and blood exposure for HCWs. However, even though the conclusion is more or less similar to our review, the HSE review included fewer studies and combined different types of interventions such as surgery needles and injection devices and the authors did not perform a meta‐analysis.
The review by Tarigan 2015 evaluated the effects of safety engineered devices combined with training and concluded that this intervention can substantially reduce the risk of NSIs. However, the authors included different study designs in one meta‐analysis and moreover analysed ITS studies as a simple before‐after comparison study which does not take into account trends over time.
Therefore we believe that the conclusions about the evidence put forth in this review are more realistic than in the other reviews mentioned above.
Authors' conclusions
Implications for practice.
We found very low quality evidence that safety features in blood collection systems and intravenous access systems has inconsistent effects on NSIs compared to systems without safety features. The extent of the effect and which features are best remain unclear.
Safety features on intravenous devices had inconsistent effects on NSIs and when they have to be actively switched on may increase the risk of blood exposure. Whereas devices that are automatically switched have no effect on the risk of blood contamination. Safe intravenous devices which have an active leakage control may decrease the incidence of blood leakages.
Studies found no difference in NSIs with the use of safe injection needles, the introduction of multiple safety devices or the introduction of sharps containers.
We found low to moderate quality evidence that the introduction of legislation probably reduces NSIs.
The lack of evidence of beneficial effects of the safety engineered devices could be due to bias in the included studies.
Implications for research.
The term safety medical devices or safety engineered devices, commonly used for devices that include built‐in safety features, could be misleading as it may lead users to believe that these devices are safer than conventional devices. However, to be able to call a particular device safety engineered there is no specific requirement to be proven effective in reducing needlestick injuries. Limitation of the name 'safe device' to devices that comply with minimum quality requirements would be helpful in practice. In the US, it has been estimated that there are over 300 sharps safety devices for injection and blood drawing, among other procedures which are in use nationwide (Jagger 2013).
Even though safety medical devices technically may reduce the risk of a NSI, the risk will not be eliminated completely. Comparisons of various types of safety engineered devices could show which device works best. Since there are considerable costs related to safety engineering, research is also needed on what are the most cost‐effective devices.
Studies that have a no‐intervention control group should consider integrating a pre‐intervention period in which an awareness campaign or training sessions, or both, are available to healthcare workers about needlestick injuries and reporting procedures. Without such a time period, an intervention may show no effect or an increase in needlestick injuries due to the increase in reporting but not in the actual number of needlestick injuries.
Since there are strict regulations on the use of safety‐engineered devices in practice, studies comparing safety‐engineered devices versus no safety devices are not feasible in Europe and North America. However, studies should focus on evaluating the most effective type of device. A large cluster‐randomised trial focused on NSI reporting in both the intervention and the control group would be the preferred research design. Because needlestick injuries are not very frequent, a large sample size is needed, with at least several large hospitals or groups of healthcare workers involved. There is also a need for similar trials in low‐ and middle‐income countries with a high prevalence of HIV or hepatitis C to evaluate low‐cost safety devices against the current use of conventional devices.
Surveillance systems for NSI could also contribute to the evidence base by collecting information on names of devices to identify more precisely which particular devices are associated with injuries.
More evaluation studies need to be carried out in countries that have newly adopted legislation regarding the use of safety‐engineered devices to prevent needlestick injuries.
What's new
| Date | Event | Description |
|---|---|---|
| 8 November 2017 | New citation required but conclusions have not changed | We updated the review with seven new studies which left the conclusions largely unchanged. |
| 11 August 2017 | New search has been performed | We updated the systematic searches and incorporated their results in the review. We also added a new comparison about the introduction of legislation. |
| 7 February 2012 | Amended | The original version of this protocol was published with the title: "Prevention of percutaneous injuries with risk of hepatitis B, hepatitis C, or other viral infections for health‐care workers". However, it turned out that the scope was far too wide and would result in an unmanageable number of studies for one review. Therefore the decision was taken to split the protocol into four new ones. The other three new titles are: "Blunt versus sharp suture needles for preventing percutaneous exposure incidents in surgical staff", "Education and training for preventing percutaneous exposure injuries in health care personnel" and "Extra gloves or special types of gloves versus a single pair of gloves for preventing percutaneous exposure injuries in healthcare personnel". |
Notes
The protocol for this review was first published as "Prevention of percutaneous injuries with risk of hepatitis B, hepatitis C, or other viral infections for healthcare workers" (Parantainen 2008). Our initial idea was to include all interventions used to prevent needlestick injuries. However, after the publication of the protocol it became apparent that very many studies would be eligible for inclusion. The decision was therefore made to split the protocol up into four new protocols. The resulting two published reviews and one protocol are titled: "Blunt versus sharp suture needles for preventing percutaneous exposure incidents in surgical staff" (Saarto 2011), "Education and training for preventing percutaneous exposure injuries in healthcare personnel" (Cheetham 2016) and "Gloves, extra gloves or special types of gloves for preventing percutaneous exposure injuries in healthcare personnel" (Mischke 2014).
The original protocol was hosted by Cochrane Hepato‐Biliary but due to the heavy involvement of Jos Verbeek and Cochrane Work the new titles were registered under their aegis.
Acknowledgements
We thank Annika Saarto (neé Parantainen) for her groundwork as the initial first author of this protocol. We thank Minna Anthoni and Ulla‐Maija Hellgren who participated in the writing of an early version of the first protocol. We extend our gratitude to Ms Leena Isotalo, the Trials Search Coordinator of the Cochrane Work Review Group, for designing the systematic search strategies. We would also like to thank Dimitrinka Nikolova and Christian Gluud from Cochrane Hepato‐Biliary for their comments on an early version of our protocol and Jani Ruotsalainen from Cochrane Work and Janet Wale from the Bone, Joint and Muscle Trauma for copy editing the text.
Appendices
Appendix 1. General search strategy, needle stick injury prevention interventions
| Database | Period of search | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | 1996 to 7 Oct 2010 | ('health care worker*' or 'health personnel' or 'HCWs' ) and ( 'virus disease*' or 'virus*' or 'viral infect*') |
| EMBASE | 1974 to 17 Sept 2010 |
#6 #5 AND [humans]/lim AND [embase]/lim #5 #3 AND #4 #4 [randomized controlled trial]/lim OR [controlled clinical trial]/lim OR random* OR 'double blind' OR 'single blind' OR (singl* OR doubl* OR trebl* OR tripl* AND (blind* OR mask*)) OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'triple blind procedure'/exp OR placebo* OR 'controlled study'/exp OR 'cross sectional study'/exp OR 'crossover procedure'/exp OR 'latin square design'/exp OR 'follow up'/exp OR 'comparative study'/exp OR 'evaluation studies'/exp OR 'evaluation study' OR prospectiv* OR volunteer* #3 #1 AND #2 #2 'health care personnel'/exp OR 'health care personnel' OR 'health care worker'/exp OR 'health care worker' OR 'health care workers' OR 'health care facilities and services'/exp OR 'medical profession'/exp OR 'nursing as a profession'/exp OR ('virus transmission'/exp AND 'patient'/exp AND professional) #1 'needlestick injury'/exp OR needlestick* OR 'needle stick'/exp OR 'sharp injury' OR 'sharp injuries' OR 'sharp medical' OR 'sharp instrument' OR 'sharp needle' OR 'sharp needles' OR sharps OR 'percutaneous exposure' OR 'percutaneous injury' OR 'percutaneous injuries' OR 'percutaneous trauma' OR 'stick injury' OR 'stick injuries' OR 'stab wound'/exp OR 'face injury'/de OR 'eye injury'/de OR 'arm injury'/de OR 'hand injury'/de OR 'needle'/exp OR (splash* AND ('blood'/exp OR blood OR secretion* OR fluid* OR 'body fluid'/exp OR 'body fluids'/exp)) |
| Wiley InterScience Cochrane Library Databases: CENTRAL and NHSEED | 1993 to 7 Oct 2010 |
#3 #1 AND #2 #2 EXP Needlestick Injuries (MeSH) OR needlestick* OR "needle stick OR "needle sticks" OR "percutaneous exposure" OR "percutaneous exposures" OR "percutaneous injury" OR "percutaneous injuries2 OR "stick injury" OR "stick injuries" OR Wounds, Stab (MeSH) OR Wounds, Penetrating (MeSH) OR Facial injuries (MeSH) OR EXP Eye Injuries, Penetrating (MeSH) OR Forearm Injuries (MeSH) OR EXP Hand Injuries (MeSH) OR [splash* AND blood OR secretion* OR fluid* OR EXP Body Fluids (MeSH) OR EXP Bodily Secretions (MeSH)] #1 EXP Health Occupations (MeSH) OR EXP Health Personnel (MeSH) OR EXP Health Facilities (MeSH) OR "health care worker" OR "health care workers" OR Disease Transmission, patient‐to‐Professional (MeSH) |
| Science Citation Index Expanded | 1986 to 5 October 2010 |
#4 #1 AND #2 AND #3 #3 TS=(random* OR control* OR trial OR trials OR "single blind" OR "double blind" OR "triple blind" OR "latin square" OR placebo* OR comparative OR "follow up" OR prospectiv* OR "cross over" OR volunteer*) #2 TS=(needlestick* OR "needle stick" OR "needle sticks" OR "stick injury" OR "stick injuries" OR "wound stab" OR "stab wound" OR "penetrating wound" OR "penetrating wounds") OR TS=(sharp* AND ( injury OR injuries OR medical OR instrument*)) OR TS=(percutaneous AND (exposure OR exposures OR injury OR injuries)) OR TS=(injur* AND (facial OR eye OR eyes OR arm OR hand OR finger OR fingers)) OR TS=(splash* AND (blood OR secretion* OR fluid OR fluids)) OR TS="blood borne infection" #1 TS=("health care worker" OR "health care workers" OR "health occupations" OR "health personnel" OR physician* OR nurse* OR hospital* OR clinic OR clinics) |
| CINAHL | 1982 to 30 Sept 2010 |
#5 #3 AND #4 #4 "randomized controlled trial" or "clinical trials" or "clinical trial" or "random allocation" or "double blind". or "single blind" or ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) or "latin square" or placebo# or random* or "research design" or "comparative study" or "comparative studies" or "evaluation study" or "evaluation studies" or "follow up study" or "follow up studies" or "prospective study" or "prospective studies" or "cross over study" or "cross over studies" or control* or prospective* or volunteer or (MH "Clinical Trials+") or (MH "Nonrandomized Trials") or (MH "Crossover Design") #3 #1 AND #2 #2 TX "needlestick injury" or needlestick# or "needle stick" or "needle sticks" or "sharp injury" or "sharp injuries" or "sharp medical device" or "sharp medical devices" or "sharp instrument" or "sharp instruments" or "sharp needle" or "sharp needles" or "percutaneous exposure" or "percutaneous exposures" or "percutaneous injury" or "percutaneous injuries" or "stick injury" or "stick injuries" or "wounds, stab" or "wounds, penetrating" or "facial injuries" or "eye injuries, penetrating" or "arm injuries" or "forearm injuries" or "hand injuries" or "finger injuries" or (splash# and (blood or secretion# or fluid#)) or ("occupational exposure" and ("body fluid" or "body fluids" or blood)) #1 (MH "Health Occupations") OR health occupations or (MH "Health Personnel+") or (MH "Health Facilities+") OR health facilities or TX "health care worker" or TX "health care workers" or (MH "Personnel, Health Facility+") or (MH "Occupational Health Services+") or (MH "Occupational Hazards+") or (MH "Occupational Exposure") or TX "health care personnel" or (MH "Health Personnel+") or (MH "HIV Infections+") |
| OSH UPDATE (NIOSHTIC‐2 and CISDOC) | NIOSHTIC‐2: 1900 to 7 Oct 2010 CISDOC: 1987 to 7 Oct 2010 |
#15 #13 AND #14 #14 PY{2007} OR PY{2008} OR PY{2009} #13 #7 AND #12 #12 #8 OR #11 #11 #9 AND #10 #10 GW{blind* OR mask*} #9 GW{singl* OR doubl* OR tripl* OR trebl*} #8 GW{random* OR control* OR trial OR trials OR comparativ* OR evaluation* OR "latin square" OR placebo OR "follow up" OR prospectiv* OR "cross over" OR volunteer*} #7 #1 AND #6 #6 #2 OR #5 #5 #3 AND #4 #4 GW{splash*} #3 GW{blood OR fluid* OR secretion*} #2 GW{"sharp medical" OR "sharp instrument" OR "sharp instruments" OR needlestick* OR "needle stick" OR "needle sticks" OR "sharp injury" OR "sharp injuries" OR "stab wound" OR "stab wounds" OR "wound penetrating" OR "stick injury" OR "stick injuries" OR "percutaneous injury" OR "percutaneous injuries" OR "percutaneous exposure" OR "percutaneous exposures" OR "sharp needle" OR "sharp needles"} #1 GW{nurse OR nurses OR physician OR physicians OR hospital* OR "health occupation" OR "health occupations" OR "health personnel" OR "health care personnel" OR "health care worker" OR "health care workers" OR "health worker" OR "health workers"} |
| MEDLINE in PubMed | from 1950 to 17 Sept 2010 |
#5 Search #1 AND #2 AND (#3 OR #4) #4 Search effect*[tw] OR control[tw] OR controls*[tw] OR controla*[tw] OR controle*[tw] OR controli*[tw] OR controll*[tw] OR control'*[tw] OR evaluation*[tw] OR program*[tw] #3 ("Randomized Controlled Trial"[pt] OR "Controlled Clinical Trial"[pt] OR "Randomized Controlled Trials as Topic"[mh] OR "Random Allocation"[mh] OR "Double‐Blind Method"[mh] OR "Single‐Blind Method"[mh] OR "Clinical Trial"[pt] OR "Clinical Trials as Topic"[mh] OR "clinical trial"[tw] OR ((singl*[tw] OR doubl*[tw] OR trebl*[tw] OR tripl*[tw]) AND (mask*[tw] OR blind*[tw])) OR "latin square"[tw] OR Placebos[mh] OR placebo*[tw] OR random*[tw] OR "Research Design"[mh:noexp] OR "Comparative Study"[pt] OR "Evaluation Studies as Topic"[mh] OR "Follow‐up Studies"[mh] OR "Prospective Studies"[mh] OR "Cross‐over Studies"[mh] OR control[tw] OR controls*[tw] OR controla*[tw] OR controle*[tw] OR controli*[tw] OR controll*[tw] OR control'*[tw] OR prospectiv*[tw] OR volunteer*[tw]) NOT (Animals[mh] NOT Humans[mh) #2 "Needlestick injuries"[mh] OR needlestick*[tw] OR "needle stick"[tw] or "needle sticks"[tw] OR "sharp injury"[tw] OR "sharp injuries"[tw] OR sharps[tw] OR "sharp medical device"[tw] OR "sharp medical devices"[tw] OR "sharp instrument"[tw] OR "sharp instruments"[tw] OR "sharp medical instrument"[tw] OR "sharp medical instruments"[tw] OR "sharp needle"[tw] OR "sharp needles"[tw] OR "percutaneous exposure"[tw] OR "percutaneous exposures"[tw] OR "percutaneous injury"[tw] OR "percutaneous injuries"[tw] OR "stick injury"[tw] OR "stick injuries"[tw] OR "Wounds, Stab"[mh:noexp] OR "Wounds, Penetrating"[mh:noexp] OR "Facial injuries"[mh:noexp] OR "Eye Injuries, Penetrating"[mh] OR "Arm Injuries"[mh:noexp] OR "Forearm Injuries"[mh:noexp] OR "Hand Injuries"[mh] OR (splash* AND (blood[tw] or secretion*[tw] OR fluid*[tw] OR "Body Fluids"[mh])) #1 "Health Occupations"[mh] OR "Health Personnel"[mh] OR "Health Facilities"[mh] OR "health care worker"[tw] OR "health care workers"[tw] OR "Infectious Disease Transmission, Patient‐to‐Professional"[mh] |
| PsycINFO (OvidSP) | 1967 to 6 Oct 2010 |
#5 limit 4 to all journals #4 #1 AND #2 AND #3 #3 random* OR control* OR trial OR trials OR comparativ* OR evaluation* OR ((singl* OR doubl* OR tripl* OR trebl*) AND (blind* OR mask*)) OR "latin square" OR placebo* OR "follow up" OR prospectiv* OR "cross over" OR volunteer* #2 (splash* AND (blood OR secretion* OR fluid OR fluids)) OR ("eye injuries" AND penetrating) OR (wound* AND (stab OR penetrating)) OR "percutaneous exposure" OR "percutaneous exposures" OR "percutaneous injury" OR "percutaneous injuries" OR "stick injury" OR "stick injuries" OR "sharp injury" OR "sharp injuries" OR "sharp medical" OR "sharp instrument" OR "sharp instruments" OR "sharp needle" OR "sharp needles" OR needlestick* OR "needle stick" OR "needle sticks" #1 (nursing or nurse or nurses or physician or physicians or "health care personnel" or "health personnel" or "health care worker" or "health care workers" or "Clinicians*" or "Dentist*" or "Health‐Personnel" or "Medical Personnel" or "Military‐Medical‐Personnel" or "Nurses*" or "Physician*" or "Psychiatric‐Hospital‐Staff*" or "medical students" or "hospitals" or "occupational exposure" or "occupational exposures").mp. [mp=title, abstract, heading word, table of contents, key concepts] |
| LILACS | "Health Occupations" or "Health Personnel" OR "Health Facilities" OR "health care worker" OR "health care workers" OR "Disease Transmission, Patient‐to‐Professional" OR "INJURIES" or "WOUNDS AND INJURIES/PC" or "accidents, OCCUPATIONAL" or "injuries, poisonings, and OCCUPATIONAL diseases" or "OCCUPATIONAL exposure" or "OCCUPATIONAL health policy" or "OCCUPATIONAL risks" OR "INJURIES" or "WOUNDS AND INJURIES/PC" or "accidents, OCCUPATIONAL" or "injuries, poisonings, and OCCUPATIONAL diseases" or "OCCUPATIONAL exposure" or "OCCUPATIONAL health policy" or "OCCUPATIONAL risks" [Descritor de assunto] and "CLINICAL TRIAL" OR "CLINICAL TRIAL, PHASE I" OR "CLINICAL TRIAL, PHASE II" OR "CLINICAL TRIAL, PHASE III" OR "CLINICAL TRIAL, PHASE IV" OR "COMPARATIVE STUDY" OR "CONTROLLED CLINICAL TRIAL" OR "EVALUATION STUDIES" OR "META‐ANALYSIS" OR "MULTICENTER STUDY" OR "RANDOMIZED CONTROLLED TRIAL" OR "REVIEW" [Tipo de publicação] and not "ANIMALS" or "HUMANS" [Palavras] |
Appendix 2. Updated search strategy, recapping prevention
We added the following search words to the general search strategy to restrict the interventions to prevention of recapping interventions:
(recap* OR device*)
Appendix 3. Updated search strategy (2016)
| Database |
Period of search |
Search strategy |
| EMBASE | 1 Jan 2014 to 1 November 2016 | #6 #5 AND [humans]/lim AND [embase]/lim #5 #1 AND #2 AND #3 AND #4 #4 [randomized controlled trial]/lim OR [controlled clinical trial]/lim OR random* OR 'double blind' OR 'single blind' OR (singl* OR doubl* OR trebl* OR tripl* NEAR1 (blind* OR mask*)) OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'triple blind procedure'/exp OR placebo* OR 'controlled study'/exp OR 'cross sectional study'/exp OR 'crossover procedure'/exp OR 'latin square design'/exp OR 'follow up'/exp OR 'comparative study'/exp OR 'evaluation studies'/exp OR 'evaluation study' OR prospectiv* OR cohort OR (comparative NEAR/1 study) OR ‘time series’/exp #3 'health care personnel'/exp OR 'health care personnel' OR 'health care worker'/exp OR 'health care worker’ OR ‘health workers’ OR ‘health professional’ OR ‘health care professionals’ OR ‘medical care professionals’ OR dentist* OR anesth* OR anaesth* OR phlebotomist OR surgeon* OR physician* OR doctor* OR nurse* OR 'health care workers' OR 'medical profession'/exp OR 'nursing as a profession'/exp OR hospital/exp OR 'health care facilities and services’/exp #2 (sharp NEAR/2 (instrument* OR needle*)) OR recap* OR ((medical OR safe) NEAR/1 device*) OR 'safety engineered' OR syringe* OR (iv NEAR/1 system*) OR (sharps NEAR/1 container*) #1 'needle stick injury'/exp OR (needle NEAR/1 stick*) OR 'sharps injury' OR 'sharps injuries' OR 'percutaneous exposure' OR 'percutaneous injury' OR 'percutaneous injuries' OR 'percutaneous trauma' OR (splash* NEAR/1 (blood OR secretion* OR fluid* OR 'body fluid' OR 'body fluids')) |
| Science Citation Index Expanded | 1 Jan 2014 to 1 November 2016 | #5 #1 AND #2 AND #3 AND #4 #4 TS=(random* OR control* OR trial OR trials OR "single blind" OR "double blind" OR "triple blind" OR "latin square" OR placebo* OR comparative OR "follow up" OR prospectiv* OR "cross over" OR volunteer*) #3 TS=(“sharps” OR “sharp medical device” OR “sharp medical devices” OR “sharp instrument” OR “sharp instruments” OR “sharp needle” OR “sharp needles” OR syringe* OR “IV‐system*” OR “sharps container*” OR “safety engineered” OR recap* OR device*) #2 TS=(needlestick* OR "needle stick" OR "needle sticks" OR "stick injury" OR "stick injuries" OR "wound stab" OR "stab wound" OR "penetrating wound" OR "penetrating wounds") OR TS=(sharp* AND ( injury OR injuries OR medical)) OR TS=(percutaneous AND (exposure OR exposures OR injury OR injuries)) OR TS=(injur* AND (facial OR eye OR eyes OR arm OR hand OR finger OR fingers)) OR TS=(splash* AND (blood OR secretion* OR fluid OR fluids)) OR TS="blood borne infection” #1 TS=("health care worker" OR "health care workers" OR "health occupations" OR "health personnel" OR physician* OR nurse* OR hospital* OR clinic OR clinics OR “healthcare worker” OR “health worker” OR “health professional” OR “health professionals” OR “healthcare professional” OR “health care professional” OR “medical care personnel” OR dentist* OR doctor* OR anesth* OR anaesth* OR phlebotomist* OR surgeon* OR veterinarian*) |
| PsycINFO (OvidSP) | 1 Jan 2014 to 1 November 2016 | #6 limit #5 to all journals #5 #1 AND #2 AND #3 AND #4 #4 random* OR control* OR trial OR trials OR comparativ* OR evaluation* OR ((singl* OR doubl* OR tripl* OR trebl*) AND (blind* OR mask*)) OR "latin square" OR placebo* OR "follow up" OR prospectiv* OR "cross over" OR volunteer* #3 (“sharps" OR "sharp medical device” OR "sharp medical devices" OR "sharp instrument" OR "sharp instruments" OR "sharp medical instrument" OR "sharp medical instruments” OR “needlestick*” OR “needle stick” OR “needle sticks” OR "sharp needle" OR "sharp needles" OR "syringe*" OR "IV‐system*" OR "sharps container*" OR "safety engineered” OR “recap*" OR “device*”) #2 (splash* AND (blood OR secretion* OR fluid OR fluids)) OR ("eye injuries" AND penetrating) OR (wound* AND (stab OR penetrating)) OR "percutaneous exposure" OR "percutaneous exposures" OR "percutaneous injury" OR "percutaneous injuries" OR "stick injury" OR "stick injuries" OR "sharp injury" OR "sharp injuries" needlestick* OR "needle stick" OR "needle sticks” #1 (nursing or nurse or nurses or physician or physicians or "health care personnel" or "health personnel" or "health care worker" or "health care workers" or "Clinicians*" or "Dentist*” or “anesth*” or “anaesth*” or “phlebotomist” or “surgeon” or “veterinarian" or "Health‐Personnel" or "Medical Personnel" or "Military‐Medical‐Personnel" or "Nurses*" or "Physician*" or "Psychiatric‐Hospital‐Staff*" or "medical students" or "hospitals" or "occupational exposure" or "occupational exposures").mp. [mp=title, abstract, heading word, table of contents, key concepts] |
| Wiley InterScience Cochrane Library Databases: CENTRAL and NHSEED | 1 Jan 2014 to 1 November 2016 | #4 #1 AND #2 AND #3 #3 EXP Health Occupations (MeSH) OR EXP Health Personnel (MeSH) OR EXP Health Facilities (MeSH) OR "health care worker" OR "health care workers" OR Disease Transmission, patient‐to‐Professional (MeSH) OR “health workers” Or “health worker” OR “health professional” OR “health professionals” OR “healthcare professional” OR “healthcare professionals” OR “health care professional” OR “health care professionals” OR “medical care personnel” OR dentist* OR surgeon* OR physician* OR doctor* OR anesth* OR anaesth* OR phlebotomist* OR nurse* OR veterinarian* #2 EXP Needlestick Injuries (MeSH) OR needlestick* OR “needle stick” OR “needle sticks” OR “percutaneous exposure” OR “percutaneous exposures” OR “percutaneous injury” OR “percutaneous injuries” OR “stick injury” OR “stick injuries” OR Wounds, Stab (MeSH) OR Wounds, Penetrating (MeSH) OR Facial injuries (MeSH) OR EXP Eye injuries, penetrating (MeSH) OR Forearm Injuries (MeSH) OR EXP Hand Injuries (MeSH) OR splash* AND blood OR secretion* OR fluid* OR EXP Body Fluids (MeSH) OR EXP Bodily Secretions (MeSH) #1 sharps OR "sharp medical device” OR “sharp medical devices” OR “sharp instrument” OR “sharp instruments” OR “sharp medical instrument” OR “sharp medical instruments” OR “sharp needle” OR “sharp needles” OR syringe* OR IV‐system* OR “sharps container*” OR "safety engineered” OR “recap*” OR “device" |
| CINAHL | 1 Jan 2014 to 1 November 2016 | S5 S1 AND S2 AND S3 AND S4 S4 "randomized controlled trial" or "clinical trials" or "clinical trial" or "random allocation" or "double blind". or "single blind" or ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) or "latin square" or placebo# or random* or "research design" or "comparative study" or "comparative studies" or "evaluation study" or "evaluation studies" or "follow up study" or "follow up studies" or "prospective study" or "prospective studies" or "cross over study" or "cross over studies" or control* or prospective* or volunteer or (MH "Clinical Trials+") or (MH "Nonrandomized Trials") or (MH "Crossover Design”) S3 TX "sharp medical device" or "sharp medical devices" or "sharp instrument" or "sharp instruments" or "sharp needle" or "sharp needles" or "sharps" or "sharp medical instrument" or "sharp medical instruments" or “syringe*" or "IV‐system*" or "sharps container*" or "safety engineered" or “recap*" or “device*” S2 TX "needlestick injury" or needlestick# or "needle stick" or "needle sticks" or "sharp injury" or "sharp injuries” or "percutaneous exposure" or "percutaneous exposures" or "percutaneous injury" or "percutaneous injuries" or "stick injury" or "stick injuries" or "wounds, stab" or "wounds, penetrating" or "facial injuries" or "eye injuries, penetrating" or "arm injuries" or "forearm injuries" or "hand injuries" or "finger injuries" or (splash# and (blood or secretion# or fluid#)) or ("occupational exposure" and ("body fluid" or "body fluids" or blood) S1 (MH "Health Occupations") OR health occupations or (MH "Health Personnel+") or (MH "Health Facilities+") OR health facilities or TX "health care worker" or TX "health care workers" or (MH "Personnel, Health Facility+") or (MH "Occupational Health Services+") or (MH "Occupational Hazards+") or (MH "Occupational Exposure") or TX "health care personnel" or (MH "Health Personnel+") or (MH "HIV Infections+") or TX "medical care professionals" or (MH "Infectious Disease Transmission, Patient‐to‐Professional+") or TX “dentist" or TX “anesth*" or TX “anaesth*" or TX “phlebotomist" or TX “surgeon*" or TX “physician*" or TX “doctor*" or TX “nurse*" or TX “veterinarian*” |
| OSH UPDATE | 1 Jan 2014 to 1 November 2016 |
#3 needlestick* OR "needle stick" OR "needle sticks" OR "sharps injury" OR "sharps injuries" OR "percutaneous injury" OR "percutaneous injuries" OR "percutaneous exposure" OR "percutaneous exposures" OR “blood splash” OR “blood splashes” #2 "sharp instrument" OR "sharp instruments" OR "sharp needle" OR "sharp needles" OR recap* OR “safe device” OR “safety engineered” OR “sharps containers” OR “IV system” OR device* #1 'health care personnel' OR 'health care worker’ OR ‘health care workers’ OR ‘health professional’ OR ‘health care professionals’ OR dentist* OR anesth* OR anaesth* OR phlebotomist OR surgeon* OR physician* OR doctor* OR nurse* OR hospital |
| MEDLINE in PubMed | 1 Jan 2014 to 1 November 2016 | #6 (#1 AND #2 AND #3 AND (#4 OR #5)) #5 (effect*[tw] OR control[tw] OR controls*[tw] OR controla*[tw] OR controle*[tw] OR controli*[tw] OR controll*[tw] OR control'*[tw] OR evaluation*[tw] OR program*[tw]) #4 (("Randomized Controlled Trial"[pt] OR "Controlled Clinical Trial"[pt] OR "Randomized Controlled Trials as Topic"[mh] OR "Random Allocation"[mh] OR "Double‐Blind Method"[mh] OR "Single‐Blind Method"[mh] OR "Clinical Trial"[pt] OR "Clinical Trials as Topic"[mh] OR "clinical trial"[tw] OR ((singl*[tw] OR doubl*[tw] OR trebl*[tw] OR tripl*[tw]) AND (mask*[tw] OR blind*[tw])) OR "latin square"[tw] OR Placebos[mh] OR placebo*[tw] OR random*[tw] OR "Research Design"[mh:noexp] OR "Comparative Study"[pt] OR "Evaluation Studies as Topic"[mh] OR "Follow‐up Studies"[mh] OR "Prospective Studies"[mh] OR "Cross‐over Studies"[mh] OR control[tw] OR controls*[tw] OR controla*[tw] OR controle*[tw] OR controli*[tw] OR controll*[tw] OR control'*[tw] OR prospectiv*[tw] OR volunteer*[tw]) NOT (Animals[mh] NOT Humans[mh)) #3 ("sharps"[tw] OR "sharp medical device"[tw] OR "sharp medical devices"[tw] OR "sharp instrument"[tw] OR "sharp instruments"[tw] OR "sharp medical instrument"[tw] OR "sharp medical instruments"[tw] OR "sharp needle"[tw] OR "sharp needles"[tw] OR "syringe*"[tw] OR "IV‐system*"[tw] OR "sharps container*"[tw] OR "safety engineered"[tw] OR “recap*"[tw] OR “device*"[tw]) #2 ("Needlestick injuries"[mh] OR needlestick*[tw] OR "needle stick"[tw] or "needle sticks"[tw] OR "sharp injury"[tw] OR "sharp injuries"[tw] OR "percutaneous exposure"[tw] OR "percutaneous exposures"[tw] OR "percutaneous injury"[tw] OR "percutaneous injuries"[tw] OR "stick injury"[tw] OR "stick injuries"[tw] OR "Wounds, Stab"[mh:noexp] OR "Wounds, Penetrating"[mh:noexp] OR "Facial injuries"[mh:noexp] OR "Eye Injuries, Penetrating"[mh] OR "Arm Injuries"[mh:noexp] OR "Forearm Injuries"[mh:noexp] OR "Hand Injuries"[mh] OR (splash* AND (blood[tw] or secretion*[tw] OR fluid*[tw] OR "Body Fluids"[mh]))) #1 (("Health Personnel"[Majr] OR "health personnel"[tiab] OR "health care personnel"[tw] OR "healthcare personnel"[tw] OR "health care worker"[tw] OR "health care workers"[tw] OR "healthcare worker"[tw] OR "healthcare workers"[tw] OR "health worker"[tw] OR "health workers"[tw] OR "health professional"[tw] OR "health professionals"[tw] OR "health care professional"[tw] OR "health care professionals"[tw] OR "healthcare professional"[tw] OR "healthcare professionals"[tw] OR "medical care personnel"[tw] OR "Health Occupations"[mh] OR "Health Personnel"[mh] OR "Health Facilities”[mh] OR "Infectious Disease Transmission, Patient‐to‐Professional"[mh] OR "dentist*"[tw] OR “anesth*”[tw] OR “anaesth*”[tw] OR “phlebotomist*”[tw] OR “surgeon*”[tw] OR “physician*”[tw] OR "doctor*"[tw] OR "nurse*"[tw] OR “veterinarian*"[tw])) |
Data and analyses
Comparison 1. Safe blood collection systems versus regular systems RCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Needlestick injuries immediate follow up | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Blood splashes | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Safe blood collection systems versus regular systems ITS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of reported sharps injuries, level | 2 | Effect Size (Random, 95% CI) | ‐3.84 [‐9.56, 1.88] | |
| 1.1 Cap shield | 1 | Effect Size (Random, 95% CI) | ‐1.04 [‐2.27, 0.19] | |
| 1.2 Needle sheath | 1 | Effect Size (Random, 95% CI) | ‐6.88 [‐9.53, ‐4.23] | |
| 2 Number of reported sharps injuries, slope | 2 | Effect Size (Fixed, 95% CI) | Totals not selected | |
| 2.1 Cap shield | 1 | Effect Size (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Needle sheath | 1 | Effect Size (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Safe intravenous systems versus regular systems RCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Needlestick injuries | 3 | Rate Ratio (Fixed, 95% CI) | 0.62 [0.27, 1.41] | |
| 2 Incidences of blood contamination | 6 | 1489 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.00, 1.92] |
| 2.1 Active systems | 4 | 961 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.08, 2.36] |
| 2.2 Passive systems | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.50, 1.75] |
| 3 Incidence of blood leakage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Active systems | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Safe intravenous systems versus regular systems CBA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of needlestick injuries | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected |
Comparison 5. Safe intravenous systems versus regular systems ITS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of reported sharps injuries, level | 2 | Effect Size (Random, 95% CI) | Totals not selected | |
| 2 Number of reported sharps injuries, slope | 2 | Effect Size (Random, 95% CI) | Totals not selected |
Comparison 6. Safe injection systems versus regular systems RCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Questionnaire reported Needlestick injuries 6 mo follow up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Hospital reported Needlestick injuries 6 mo follow up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Questionnaire reported Needlestick injuries 12 mo follow up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Hospital reported Needlestick injuries 12 mo follow up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Safe injection systems versus regular systems CBA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Needlestick injury rate | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected |
Comparison 8. Safe passive injection systems versus safe active injection systems ITS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 change in level of needlestick injuries | 1 | Effect size (Random, 95% CI) | Totals not selected | |
| 2 Change in slope of needlestick injuries | 1 | Effect Size (Random, 95% CI) | Totals not selected |
Comparison 9. Multiple safe devices versus regular devices ITS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of reported sharps injuries, level | 2 | Effect Size (Random, 95% CI) | Totals not selected | |
| 2 Number of reported sharps injuries, slope | 2 | Effect Size (Random, 95% CI) | 0.25 [‐0.30, 0.81] |
Comparison 10. Multiple safe devices versus regular devices CBA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Needlestick injuries | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected |
Comparison 11. Sharps containers versus no containers ITS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of reported sharps injuries, level | 2 | Effect Size (Random, 95% CI) | 2.49 [0.49, 4.48] | |
| 2 Number of reported sharps injuries, slope | 2 | Effect Size (Random, 95% CI) | Totals not selected |
Comparison 12. Sharps containers versus no containers CBA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of needlestick injuries | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Number of container related needlestick injuries | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected |
Comparison 13. Legislation versus no legislation ITS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NSI‐ change in level | 3 | Effect Size (Random, 95% CI) | Subtotals only | |
| 1.1 Interruption | 2 | Effect Size (Random, 95% CI) | ‐6.15 [‐7.76, ‐4.54] | |
| 1.2 Gradual introduction | 1 | Effect Size (Random, 95% CI) | 0.80 [0.41, 1.19] | |
| 2 NSI‐ Change in slope | 3 | Effect Size (Random, 95% CI) | Subtotals only | |
| 2.1 Interruption | 2 | Effect Size (Random, 95% CI) | ‐0.94 [‐1.97, 0.09] | |
| 2.2 Gradual introduction | 1 | Effect Size (Random, 95% CI) | 0.5 [0.36, 0.64] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asai 1999 active.
| Methods | Study design: Randomised Controlled Trial. Object of randomisation: patients | |
| Participants | Japan. Researchers and their assistants performing intravenous infusion on patients scheduled for elective surgery. Number studied: 100 patients. Intervention group n = 50. Control group n = 50. | |
| Interventions | Use of Insyte AutoGuard intravenous cannula where the needle can be retracted into a safety barrel by actively pushing a button. The control group used conventional Insyte intravenous cannula. | |
| Outcomes | (1) Number of needlestick injuries per total number of procedures; (2) blood contamination from either the inserted cannula or needle on researcher, assistant, patient or equipment; (3) blood stains on the collection tray. Measurement: (1) self‐reporting of needlestick injuries; (2) number of incidents of blood contamination by visual assessment; (3) number of blood stains with a maximum score of 10 if there were more than 10 stains. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the patient was allocated to one of the two groups by blocked randomisation (blocks of 10). " No additional information is available on the blocked randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Information about allocation concealment is not available in the article. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The presence or absence of blood on the tray was assessed by a blinded researcher" Healthcare workers could not have been blinded as they were using the devices but it is unlikely that this introduces bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Authors reported the outcomes mentioned in the method section. Information is available for the two groups for the number of attempts at insertion, ease of insertion, ease of handling needle, blood contamination, and needlestick injuries. |
| Similar recruitment of groups | Unclear risk | Patient characteristics were similar in terms of sex, age, weight and height. No information available on the characteristics of the researchers and assistants such as years of experience, professions, difference between the intervention and control groups in terms of staff. |
| Adjustment for baseline differences | Unclear risk | No information related to adjustment for baseline differences is reported. |
| Other bias | High risk | "We thank Japan Becton for supplying the Insyte and Autoguard cannulae." The involvement of a medical devices manufacturing company may have potentially introduced information bias. |
Asai 2002 active.
| Methods | Study design: Randomised Controlled Trial with two intervention arms and one control arm. Object of randomisation: patients. | |
| Participants | Japan. Researchers and assistants performing intravenous (n = 150) and intra‐arterial cannulations (n = 150) in elective surgery. Number studied: 300 patients. Intervention group one n = 100 (Insyte Autoguard cannula with a button for actively retracting the needle. Control group n = 100 (divided over the two intervention arms). | |
| Interventions | Arm one: Use of safeguarded needles (Insyte Autoguard) in intravenous cannulations. The control group used conventional Insyte catheter needles. | |
| Outcomes | Needlestick injuries (none detected), median number of blood contamination from inserted catheter or needles on staff, patients, equipment or tray. | |
| Notes | We combined the results of the intravenous and intra‐arterial cannulation when the same devices were used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "In each part of the study, patients were randomly allocated intro three groups. Block randomisation (in blocks of 15) was used for the allocation. No additional information available on randomisation process. |
| Allocation concealment (selection bias) | Low risk | "cards indicating allocations were placed in a serially numbered, sealed opaque envelope?" Adequate allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The presence or absence of blood on a tray was assessed by a researcher who was blinded to the allocation" Healthcare workers could not have been blinded as they were using the devices but bias seems unlikely here. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Authors reported the outcomes mentioned in the method section: information is available for the three groups for the ease of insertion, information on the backflow, ease of handling needle, blood contamination, needlestick injuries and problems at insertion. |
| Similar recruitment of groups | Unclear risk | Patients characteristics were similar in terms of the age, weight and height. There were differences between groups for sex. No information available on the characteristics of the researchers and assistants such as years of experience, professions, difference between the intervention and control groups in terms of the staff. |
| Adjustment for baseline differences | Unclear risk | No information related to adjustment for baseline differences is reported. |
| Other bias | High risk | "We thank Japan Becton for supplying Insyte and Insyte Autoguards and Johnson & Johnson Medical for supplying protective acuvance needles." The involvement of a medical devices manufacturing company may have potentially introduced information bias. |
Asai 2002 passive.
| Methods | Study design: Randomised Controlled Trial with two intervention arms and one control arm. Object of randomisation: patients. | |
| Participants | Japan. Researchers and assistants performing intravenous (n = 150) and intra‐arterial cannulations (n = 150) in elective surgery. Number studied: 300 patients. Intervention group two n = 100 (Protective Acuvance) cannula with a passive mechanism that retracts the needle, Control group n = 100 (divided over the two intervention arms). | |
| Interventions | Arm two: Use of safeguarded needles (Protective Acuvance) in intravenous and intra‐arterial cannulations. The control group used conventional Insyte catheter needles. | |
| Outcomes | Needlestick injuries (none detected), median number of blood contamination from inserted catheter or needles on staff, patients, equipment or tray, and median number of blood stains on tray. | |
| Notes | We combined the results of the intravenous and intra‐arterial cannulation when the same devices were used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "In each part of the study, patients were randomly allocated intro three groups. Block randomisation (in block of 15) was used for the allocation and cards indicating allocations we placed in a serially numbered, sealed opaque envelope". |
| Allocation concealment (selection bias) | Low risk | "cards indicating allocations were placed in a serially numbered, sealed opaque envelope" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The presence or absence of blood on a tray was assessed by a researcher who was blinded to the allocation" Healthcare workers could not been blinded as they were using the devices but bias is unlikely here. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Authors reported on outcomes mentioned in the method section:information is available for the three groups for the ease of insertion, information on the backflow, ease of handling needle, blood contamination, needlestick injuries and problem at insertion. |
| Similar recruitment of groups | Unclear risk | Patients characteristics were similar in terms of the age, weight and height. There were differences in between groups for sex No information available on the characteristics of the researchers and assistants such as years of experience, professions, difference between the intervention and control groups in terms of the staff. |
| Adjustment for baseline differences | Unclear risk | No information related to adjustment for baseline differences is reported. |
| Other bias | High risk | "We thank Japan Becton for supplying Insyte and Insyte Autoguards and Johnson & Johnson Medical fro supplying protective acuvance needles." The involvement of a medical devices manufacturing company may have potentially introduced information bias. |
Azar‐Cavanagh 2007.
| Methods | Study design: Interrupted Time‐Series Study | |
| Participants | USA. Healthcare workers handling needles and thus with potential exposure to blood borne pathogens. Number studied: 11,161 healthcare workers for the pre‐intervention period (18 months) and 12,851 healthcare workers for the post‐intervention period (18 months). |
|
| Interventions | Introduction of an intravenous catheter stylet with a safety engineered feature (a retractable protection shield). The mechanism has to be activated by the worker. Suture needles were not replaced by safety engineered needles and were thus used as control group. | |
| Outcomes | Number of percutaneous injuries per 1000 healthcare workers. | |
| Notes | Pre‐intervention rate (PI per 1000 health workers) IV catheter needle (2.5; 2.3, 2.5 for each six‐month period respectively). Total data points (n = 6). |
|
Baskin 2014.
| Methods | Study design: Randomised Controlled Trial. Object of randomisation: patients | |
| Participants | Turkey. Doctors who collected ABG samples from patients in the emergency care department. Number studied: 550 patients. Intervention group n = 275. Control group n = 275. | |
| Interventions | Use of safety‐engineered blood gas syringes which once in the artery filled automatically as a result of arterial pulse pressure. The control group used conventional heparinised syringes. | |
| Outcomes | (1) Number of needlestick injuries (2) Number of events of blood splashes (3) Number of attempts (4)The degree of difficulty of ABG extraction procedure according to physicians. | |
| Notes | Includes information about cost analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomization carried out was not mentioned. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. Data available includes all physicians who performed arterial blood gas extraction procedures (n = 27). |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes were reported accordingly. |
| Similar recruitment of groups | Low risk | The study included patients who visited the ED during the period of May 1, 2012 to June 30, 2012. |
| Adjustment for baseline differences | Low risk | There was no significant difference between groups in terms of age, weight, sex, height, wrist circumference and BMI. |
| Other bias | Low risk | The study appears to be free of other types of bias. |
Chambers 2015 hospitals.
| Methods | Study design: Interrupted Time‐Series Study | |
| Participants | Canada (Ontario). Healthcare workers registered with Work place Safety and Insurance Board (a workers' compensation claims organization). Number studied 16,364 in the period (2004‐2012). The study included two intervention arms, one comprising of long‐term nursing care and the other one comprising of hospitals. | |
| Interventions | Introduction of a legislation between, 2008‐2009 for the use of safety engineered needles which includes the use of needleless devices. Individual hospital had the discretion to choose the type of safety engineered needle either passive or semi‐automatic. In the pre‐intervention period there was no use of safety engineered needles. | |
| Outcomes | Rate of needlestick injuries per 10,000 full time equivalents as reported by healthcare workers to Work place Safety and Insurance board. | |
| Notes | Total number of data points long‐term nursing care (n = 9). Total number of data points hospitals (n = 9). |
|
Chambers 2015 long‐term nursing care.
| Methods | Study design: Interrupted Time‐Series Study | |
| Participants | Canada (Ontario). Healthcare workers registered with Work place Safety and Insurance Board (a workers' compensation claims organization). Number studied 16,364 in the period (2004‐2012). The study included two intervention arms, one comprising of long‐term nursing care and the other one comprising of hospitals. | |
| Interventions | Introduction of a legislation between, 2008‐2009 for the use of safety engineered needles which includes the use of needleless devices. Individual hospital had the discretion to choose the type of safety engineered needle either passive or semi‐automatic. In the pre‐intervention period there was no use of safety engineered needles. | |
| Outcomes | Rate of needlestick injuries per 10,000 full time equivalents as reported by healthcare workers to Work place Safety and Insurance board. | |
| Notes | Total number of data points long‐term nursing care (n = 9). Total number of data points hospitals (n = 9). |
|
Cote 2003.
| Methods | Study design: Randomised Controlled Trial. Object of randomisation: patients by calendar week | |
| Participants | USA. Staff of the operating theatre. Participation by attending anaesthesiologists was voluntary. Number randomised: 330 patients receiving IV catheter insertions. Intervention group n = 211. Control group n = 119. | |
| Interventions | The intervention group used Angiocath Autoguard IV catheters with retractable needles where retraction has to be activated with a button. The control group used traditional JELCO IV catheters. | |
| Outcomes | Number of spills and splatters of blood on linen, table, floor, skin or clothing per total number of procedures. Measurement: visual observations by the operating staff. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Assignment of catheter type was randomised by week" |
| Allocation concealment (selection bias) | Unclear risk | Researchers do not provide information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. Data available includes all participants (n = 330). |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes were reported accordingly. |
| Similar recruitment of groups | Unclear risk | The intervention and control groups were recruited from the same hospital. The study was completed over 20 days, 11 days for intervention and 9 days for the control. It is unclear if patients recruited to the study differed based on the week the person was selected to participate into the study. |
| Adjustment for baseline differences | Unclear risk | No information on the adjustment for baseline difference reported. |
| Other bias | Low risk | The study appears to be free of other types of bias. |
Edmond 1988.
| Methods | Study design: Interrupted Time‐Series Study | |
| Participants | USA. Registered nurses on medical and surgical wards, emergency department, intensive care unit and in the operating room performing tasks which require handling of needles. Number studied: 278 registered nurses with outcomes reported over 12 months. | |
| Interventions | Introduction of bedside needle disposal units. In the pre‐intervention period the disposal units were located in medication rooms and on medication carts. | |
| Outcomes | Number of reported needlestick per total number of healthcare personnel. Secondary outcome: recapping rate. | |
| Notes | Total number of data points (n = 12). | |
Gaballah 2012.
| Methods | Study design: Controlled Before and After Study | |
| Participants | UK (London). Bachelor of dental surgery students (3rd, 4th, 5th year) and dental nursing students from three hospitals in London. | |
| Interventions | Use of dental syringe that does not require re‐sheathing or removal of needle from the syringe. Control group used conventional metallic dental syringe. | |
| Outcomes | Outcome: incident reports of NSI sustained by dental students and nurse students over the period 1.2007 to 12.2008. The type of syringe system causing NSIs was not reported for the departments in the intervention and control groups. Unit: not specified. | |
| Notes | We contacted the authors but they did not respond. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not an RCT. |
| Allocation concealment (selection bias) | High risk | Not an RCT. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | High risk | Type of syringe system causing NSIs among various departments was not mentioned in the outcome. |
| Similar recruitment of groups | High risk | Same time period of recruitment but different groups recruited from different hospitals. |
| Adjustment for baseline differences | Unclear risk | No information regarding adjustment for baseline differences. |
| Other bias | Unclear risk | The study appears to be free of other types of bias. |
Goldwater 1989.
| Methods | Study design: Interrupted Time‐Series Study surrounding two interventions | |
| Participants | New Zealand. Laboratory staff performing venipunctures. Number studied: 644,000 venipunctures during a four‐year period. | |
| Interventions | 1. Adaption of Centers for Disease Control (CDC) guidelines on non‐recapping of needles. 2. Introduction of recapping injury prevention device Needle Guard and training on its use. In this review we only used the part on the introduction of the injury prevention device Needle Guard. The needle guard consists of a shield at the bottom of the protective cap that covers the needle. The shield should prevent a needle stick injury while the cap is placed beside the needle. Passive device because no worker intervention required. | |
| Outcomes | Number of needlestick injuries per total number of venipunctures performed. | |
| Notes | Not recapping prevention but prevention of PEI while recapping. During pre‐intervention, baseline rate estimated at 0.63 NSI per 1000 venipuncture‐years. Total number of data points (n = 39). |
|
Goris 2015.
| Methods | Study design: Interrupted Time‐Series Study | |
| Participants | USA (Missouri). Healthcare workers from four medical nursing divisions and one intensive care unit approximating overall 1,095,097 employee productive hours during the 30‐month pre‐trial and nine‐month trial period . Demographics and working experience of staff not reported. | |
| Interventions | 1. Introduction of passive safety engineered device for insulin and tuberculin injections 2. Extensive training and education during pre and post intervention periods. | |
| Outcomes | NSI rate per 100,000 employee productive hours. | |
| Notes | ||
Grimmond 2010.
| Methods | Study design: Controlled Before and After Study | |
| Participants | USA. Staff from non‐profit hospitals. Demographics and working experience of staff not reported. Number studied: 14 hospitals (control) and 14 hospitals (interventions). Approximating overall 19,880 FTE during the two‐year study period | |
| Interventions | 1. Engineered safety features of a sharps container | |
| Outcomes | Sharp injury (a) during procedure; b) after procedure but before disposal; c) container‐associated (CASI); d) inappropriate disposal. We used the total number and the container‐related injuries to calculate intervention effects. | |
| Notes | We calculated the RR of NSI after the introduction of containers and the SE. These were put into RevMan data tables. We did not adjust for baseline difference nor for a clustering effect. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not an RCT. |
| Allocation concealment (selection bias) | High risk | Not an RCT. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information on blinding. "Staff who suffered sharp injuries were not aware of the study at the time of their injury report". However, health workers would be aware of the change in the type of devices used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors reported that data on the outcome was obtained for the pre‐ and post‐intervention periods for the 14 participating hospitals. Authors do not include hospital‐level information. |
| Selective reporting (reporting bias) | Low risk | The pre‐specified outcomes are reported. |
| Similar recruitment of groups | Low risk | This study includes the same 14 hospitals for before and after intervention. There was minimum change in the number of FTE (0.6%) during the study period. |
| Adjustment for baseline differences | Unclear risk | Not reported in the analysis. |
| Other bias | Low risk | The study appears to be free of other types of bias. |
L'Ecuyer 1996 2wva.
| Methods | Study design: Cluster Randomised Controlled Trial. Object of randomisation: nursing divisions. Three‐armed trial with separate control groups | |
| Participants | USA. Nursing personnel from general, medical, surgical and intensive‐care units performing intravenous therapy. Number studied: 73,454 patient days (980,392 productive hours worked). Intervention three n = 19,436. Control n = 19,550. | |
| Interventions | Use of needleless intravenous device 2‐way valve. Passive system no need for activation. Control groups used standard IV needle systems. | |
| Outcomes | Reported needlestick injures per 1000 patient‐days and 1000 productive hours worked. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Four groups of nursing divisions were prospectively randomised to use one of the two safety devices" |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment is available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information available on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Selected nursing division were assigned to either the intervention (MBC then replaced by PBC, and 2‐way). The MBC was replaced after four months due to staff dissatisfaction. Authors reported all outcomes data for the intervention and control group. |
| Selective reporting (reporting bias) | Unclear risk | "Intravenenous‐therapy related injuries were categorized further as follows: low‐risk injuries involved needles without direct blood contact; intermediate risk injuries involved needles likely to have occult blood present and high risk injuries involved needles in direct contact with blood." However, there is no information available based on this categorization stipulated in the method section. |
| Similar recruitment of groups | High risk | The nursing divisions selected to participate to the study were from the same hospital. The recruitment time period of 2‐way device differed from the PBC. The PBC was selected to replace the MBC (after four months) due to staff dissatisfaction. |
| Adjustment for baseline differences | Unclear risk | The demographics of the workers (age, sex, years of experience) are not reported. The adjustment for baseline differences is not reported in the analysis. |
| Other bias | High risk | "Study participants generally have ready access to the traditional devices, which may contaminate the evaluation, so much attention must be focused on appropriate experimental device distributions and traditional device removal prior to study initiation." NSI reported in the study group may have been caused by the use of the traditional device. Based on the information available, it is not possible to separate NSI caused by the new devices or traditional ones. |
L'Ecuyer 1996 mbc.
| Methods | Study design: Cluster Randomised Controlled Trial. Object of randomisation: nursing divisions. Three‐armed trial with separate control groups | |
| Participants | USA. Nursing personnel from general, medical, surgical and intensive‐care units performing intravenous therapy. Number studied: 73,454 patient‐days (980,392 productive hours worked). Intervention two n = 3840. Control n = 2487 patient‐days. | |
| Interventions | Use of needleless intravenous device metal blunt cannula. Passive system no need for activation. Control groups used standard IV needle systems. | |
| Outcomes | Reported needlestick injures per 1000 patient‐days and 1000 productive hours worked. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Four groups of nursing divisions were prospectively randomised to use one of the two safety devices" |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment is available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information available on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Selected nursing division were assigned to either the intervention (MBC then replaced by PBC, and 2‐way). The MBC was replaced after four months due to staff dissatisfaction. Authors reported all outcomes data for the intervention and control group. |
| Selective reporting (reporting bias) | Unclear risk | "Intravenenous‐therapy related injuries were categorized further as follows: low‐risk injuries involved needles without direct blood contact; intermediate risk injuries involved needles likely to have occult blood present and high risk injuries involved needles in direct contact with blood." However, there is no information available based on this categorization stipulated in the method section. |
| Similar recruitment of groups | High risk | The nursing divisions selected to participate to the study were from the same hospital. The recruitment time period of 2‐way device differed from the PBC. The PBC was selected to replace the MBC (after four months) due to staff dissatisfaction. |
| Adjustment for baseline differences | Unclear risk | The demographics of the workers (age, sex, years of experience) are not reported. The adjustment for baseline differences is not reported in the analysis. |
| Other bias | High risk | "Study participants generally have ready access to the traditional devices, which may contaminate the evaluation, so much attention must be focused on appropriate experimental device distributions and traditional device removal prior to study initiation." NSI reported in the study group may have been caused by the use of the traditional device. Based on the information available, it is not possible to separate NSI caused by the new devices or traditional ones. |
L'Ecuyer 1996 pbc.
| Methods | Study design: Cluster Randomised Controlled Trial. Object of randomisation: Nursing divisions. Three‐armed trial with separate control groups | |
| Participants | USA. Nursing personnel from general, medical, surgical and intensive‐care units performing intravenous therapy. Number studied: 73,454 patient days (980,392 productive hours worked). Intervention one n = 15,737. Control n = 12,404. | |
| Interventions | Use of needleless intravenous device: plastic blunt cannula. Passive system no need for activation. Control groups used standard IV needle systems. | |
| Outcomes | Reported needlestick injures per 1000 patient‐days and 1000 productive hours worked. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Four groups of nursing divisions were prospectively randomised to use one of the two safety devices" |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment is available. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information available on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Selected nursing division were assigned to either the intervention (MBC then replaced by PBC, and 2‐way). The MBC was replaced after four months due to staff dissatisfaction. Authors reported all outcomes data for the intervention and control group. |
| Selective reporting (reporting bias) | Unclear risk | "Intravenenous‐therapy related injuries were categorized further as follows: low‐risk injuries involved needles without direct blood contact; intermediate risk injuries involved needles likely to have occult blood present and high risk injuries involved needles in direct contact with blood." However, there is no information available based on this categorization stipulated in the method section. |
| Similar recruitment of groups | High risk | The nursing divisions selected to participate to the study were from the same hospital. The recruitment time period of 2‐way device differed from the PBC. The PBC was selected to replace the MBC (after four months) due to staff dissatisfaction. |
| Adjustment for baseline differences | Unclear risk | The demographics of the workers (age, sex, years of experience) are not reported. The adjustment for baseline differences is not reported in the analysis. |
| Other bias | High risk | "Study participants generally have ready access to the traditional devices, which may contaminate the evaluation, so much attention must be focused on appropriate experimental device distributions and traditional device removal prior to study initiation." NSI reported in the study group may have been caused by the use of the traditional device. Based on the information available, it is not possible to separate NSI caused by the new devices or traditional ones. |
Mendelson 1998.
| Methods | Study design: Controlled Before‐After Study with Cross‐Over | |
| Participants | USA. Health care workers in sixteen nursing units excluding pediatrics, obstetrics‐gynaecology and intensive care, performing procedures which required the use of IV systems. We estimated that the number of workers in each groups was around 220. All IV insertions in the selected units during a period of six months. Eight units belonged to the intervention group and eight units to the control group, and the roles were switched in the middle of the study period. | |
| Interventions | Use of a needleless intermittent intravenous access system with a reflux valve. Control group used a conventional heparin lock. | |
| Outcomes | Number of reported percutaneous injuries per study week. Secondary outcomes: Local complications at insertion site, bacteraemia of patients, device‐related complications, staff satisfaction and cost analysis. | |
| Notes | Study includes information about costs; We calculated the RR (SE) for needlestick injuries of the intervention and the control group based on our estimates of the number of persons and the number of needlestick injuries reported by the authors. We added 0.5 to fill empty cells. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomisation. |
| Allocation concealment (selection bias) | High risk | No randomisation. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information about blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors indicated that study was completed in 16 medical and surgical units. The outcome data appears to be reported for the 16 units. No outcome data at the unit level. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are reported and correspond to the ones mentioned in the method section. |
| Similar recruitment of groups | Low risk | The intervention and control groups were from the same hospital. There is no information about the FTE change during study period. The study was completed within a short period of time (25 weeks), staff difference between before and after intervention is unlikely to be different. |
| Adjustment for baseline differences | Unclear risk | Authors specified that the wards for the control and intervention were similar in terms of staff‐to‐patient ratio and the type of illness of the patients. The units were different in terms of speciality for the control and intervention group. No information is available to compare the control and intervention groups for the number of staff, working experience, age and sex. Adjustment for baseline differences is not reported in the analysis. |
| Other bias | High risk | The outcome, NSI, is reported by study weeks. There is no information about number of FTE or number of devices used. Although the staff‐to‐patient ratios were similar, we do not know if the number or type of procedures were similar in both groups. |
Phillips 2013.
| Methods | Study design: Interrupted Time‐Series Study | |
| Participants | USA. Hospitals that used Exposure Prevention Information Network (a multi hospital sharps injury database). A total of 85 hospitals were selected of which 30 were removed. Numbers studied: during the pre‐NPSA period (1995‐2000) data representing to 13,377 per‐cutaneous injuries and for the post‐NPSA period (2001‐2005) a total of 5,379 per‐cutaneous injuries. | |
| Interventions | Introduction of a legislation on November 6, 2000 and as mandated, OSHA revised the standard in 2001 which required the provision of safety‐engineered sharps, evaluation of devices, maintenance of sharps injury logs and annual review of the facility's exposure control plan. | |
| Outcomes | Percutaneous injury rates per 100 FTEs. | |
| Notes | Total number of data points (n = 11). | |
Prunet 2008 active.
| Methods | Study design: Randomised Controlled Trial. Object of randomisation: procedures. Two intervention arms and one control arm | |
| Participants | France. Anaesthetist physicians and anaesthetist nurses in the operating room and emergency performing IV infusion. Number studied: 759 procedures. Intervention group two n = 254. Control group n = 254 (divided over the two arms). | |
| Interventions | Arm 2: use of active safety catheter (Insyte Autoguard). Control group used the Vialon traditional non‐safety catheter. We divided the control group over the two intervention arms. | |
| Outcomes | 1. Number of cases in which the patient's blood stained the operator's skin, gloves, mask, or any other clothing; 2. Number of cases in which the patient's blood stained the stretcher or floor. Secondary outcome: Ease of use and sense of protection. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "the type of venous catheter to use was determined randomly in a three ball ballot box." |
| Allocation concealment (selection bias) | Low risk | "The choice of the catheter was randomised by using a single blinded envelope method" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information on blinding available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information reported about the number of excluded patients. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported in introduction correspond to the ones mentioned in the method section. |
| Similar recruitment of groups | Low risk | Study uses randomisation. |
| Adjustment for baseline differences | Low risk | Adequate randomisation, no additional adjustment needed in the analysis. |
| Other bias | Low risk | The study appears to be free of other types of bias. |
Prunet 2008 passive.
| Methods | Study design: Randomised Controlled Trial. Object of randomisation: procedures. Two intervention arms and one control arm | |
| Participants | France. Anaesthetist physicians and anaesthetist nurses in the operating room and emergency performing IV infusion. Number studied: 759 procedures. Intervention group one n = 251, Control group n = 254 (divided over the two arms). | |
| Interventions | Arm 1: use of passive safety catheter (Introcan Safety). Intervention 2: use of active safety catheter (Insyte Autoguard). Control group used the Vialon non‐safety catheter. We divided the control group over the two intervention arms. | |
| Outcomes | 1. Number of cases in which the patient's blood stained the operator's skin, gloves, mask, or any other clothing; 2. Number of cases in which the patient's blood stained the stretcher or floor. Secondary outcome: Ease of use and sense of protection. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "the type of venous catheter to use was determined randomly in a three ball ballot box." |
| Allocation concealment (selection bias) | Low risk | "The choice of the catheter was randomised by using a single blinded envelope method" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information on blinding available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information reported about the number of excluded patients. |
| Selective reporting (reporting bias) | Low risk | "If the operator considered the patient's vein unsuitable for placing an 18 G catheter, the patient was excluded from the protocol" |
| Similar recruitment of groups | Low risk | Not reported but adequate randomisation to the control or intervention group. |
| Adjustment for baseline differences | Low risk | Adequate randomisation, no additional adjustment needed in the analysis. |
| Other bias | Low risk | The study appears to be free of other types of bias. |
Reddy 2001.
| Methods | Study design: Interrupted Time‐Series Study | |
| Participants | USA. Healthcare workers with direct patient contact, excluding physicians, or ancillary workers who may have been in areas where medical procedures had taken place during a six‐year period. Number studied: 3011 FTE for the pre‐intervention period (three years) and 3992 FTE for the post‐intervention period (three years). |
|
| Interventions | Implementation of safety syringes and needleless intravenous systems. It was unclear if these were active or passive. Co‐intervention: Educational in services attended by some or all healthcare workers. | |
| Outcomes | Reported needlestick injuries per 100 full time employees. | |
| Notes | Baseline incidence rate by 100 FTE per year Year Incidence rate 1994 10.6% 1995 10.3% 1996 6.4% Total number of data points (n = 6) |
|
Richard 2001.
| Methods | Study design: Interrupted Time‐Series Study | |
| Participants | India. Hospital healthcare workers during a seven‐year period. Number studied: Not reported. | |
| Interventions | 1. Introduction of sharps containers; 2. Education on blood borne pathogens and the importance of safe sharps disposal. | |
| Outcomes | Number of reported needlestick injuries due to improper disposal per total number of reported needlestick injuries. | |
| Notes | Total number of data points (n = 7). | |
Rogues 2004.
| Methods | Study design: Interrupted Time‐Series | |
| Participants | France. 3600 bed university hospital, sharp injuries reported on an annual of 8500 FTE (2900 nurses). Number of phlebotomist nurses, not reported. |
|
| Interventions | 1. re‐sheathable winged steel needles and Vacutainer blood collecting tube and 2. vacutainer blood collecting tubes with recapping sheaths. Each product required the healthcare worker to activate the safety feature immediately after phlebotomy. We regarded both devices as one intervention. The two safety mechanisms required two‐handed activation and were thus active. Pre‐intervention period (four years) and post‐intervention period (three years) |
|
| Outcomes | Phlebotomy‐related PIs (vacuum‐tube + winged steel needle) per 100 devices purchased. | |
| Notes | Baseline rate: Number of phlebotomy PI reported for first two years but no denominator available. For third year of baseline, rate was 18.8 phlebotomy PI related per 100,000 purchased devices. Total number of data points (n = 7). |
|
Seiberlich 2016.
| Methods | Study design: Randomised Controlled Trial. Object of randomisation: patients | |
| Participants | Canada (Alberta). Clinicians who carried out PIVC insertions in emergency department patients. Number studied: 150 patients. Number of study insertions: 152. Intervention group n = 73. Control group n = 79. | |
| Interventions | Use of blood control catheter (via valve safety IV catheter) which was an active safety device that includes a valve that is designed to restrict blood flow back out of the catheter hub upon initial venipuncture. It also contains a window within the introducer needle for easy confirmation of vessel entry. Control group used the straight hub version of standard device which also has to be actively switched on (ProtectIV safety IV catheter). | |
| Outcomes | (1) Number of blood leakage events (2) Number of blood exposure risk reduction events (we could not understand what the authors meant by this outcome measure and we decided to exclude this outcome measure. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Insertions were randomised 1:1 by participating clinicians. |
| Allocation concealment (selection bias) | Unclear risk | Researchers do not provide information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not a blinded study, the fact that the study could not be carried out as a double blind investigation lent some inherent, albeit unavoidable, clinician bias to the results. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Low risk | Authors reported the outcomes mentioned in the method section: information is available for clinical acceptability, incidence of blood leakage, risk of blood exposure, need for digital compression, insertion success and clinical usability. |
| Similar recruitment of groups | Unclear risk | Incomplete information on recruitment of groups. |
| Adjustment for baseline differences | Unclear risk | No information related to adjustment for baseline differences is reported. |
| Other bias | High risk | Clinicians were able to contribute to the endpoint multiple times, number of insertions performed by clinicians varied from nurse to nurse. This study was funded by Smiths Medical, the manufacturer of both the blood control and standard PIVCs that were evaluvated. The co‐author, Laura Seiberlich, is an employee of the study sponsor. |
Sossai 2010.
| Methods | Study design: Interrupted Time‐Series | |
| Participants | Healthcare workers from a hospital in Italy. The overall number of employees varied from 4447 and 4636 individuals from 2003 to 2007. | |
| Interventions | Sharps awareness program and passively activated Introcan safety IV catheter system. This has a self‐activating safety clip that automatically shields the needle’s sharp bevel during retraction of the needle after cannula insertion. With regard to design and handling, this safety catheter is identical to the conventional catheter. | |
| Outcomes | NSI with catheters and sharps. | |
| Notes | Total number of data points (n = 7) | |
Valls 2007.
| Methods | Study design: Controlled Before‐After Study | |
| Participants | Spain 350 bed general hospital. 1000 workers, seven wards assigned to intervention and five wards assigned as a control group. | |
| Interventions | 1. Educational session which included a three‐hour presentation and two hours of hands‐on training. 2. Safety devices which included blood‐culture collection tubes with a needle sheath, blood‐gas syringes with needle sheath, lancets with retractable single use puncture sticks, safety devices catheter and blunt needles. It was unclear if these devices were active or passive. Vacuum phlebotomy systems without needle sheaths were used prior the beginning of the study. | |
| Outcomes | Number of percutaneous injuries per 100,000 patient‐days. With the exception of the emergency department, NSI injuries per 100,000 patients. | |
| Notes | Information available on the cost of safety engineered devices. We used the rate ratios as reported by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised. |
| Allocation concealment (selection bias) | High risk | Not randomised. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information is provided about blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The intervention includes several wards. For the baseline, authors reported NSI rate for the different wards. This level of information is not available for the intervention as authors grouped the different medical wards into one category. |
| Selective reporting (reporting bias) | High risk | Figure 1: only absolute number is reported, no information available on the denominator for the study period. |
| Similar recruitment of groups | High risk | Researchers selected the wards for the intervention group, potentially introducing selection bias. The study was completed at the hospital at different times. Authors do not specify if the staff FTE and characteristics remain similar before and during intervention. |
| Adjustment for baseline differences | Unclear risk | The demographics of the workers (age, sex, years of experience) are not reported. Adjustment for baseline differences is not reported in the analysis. |
| Other bias | High risk | "injury reporting was voluntary during the pre intervention and intervention periods. However, the nurses in charge of the study carried out active surveillance reporting of injuries during the intervention period." This might have increased the number of cases reported. |
van der Molen 2011.
| Methods | Study design: Cluster‐RCT | |
| Participants | Netherlands. Workers of voluntarily participating hospital wards (academic hospital). Demographics and working experience of staff included. Number studied: 796 participants. Intervention one (safety device + workshop) = 267 participants (seven wards), intervention two (workshop only) = 263 (eight wards), control group = 266 (eight wards) | |
| Interventions | 1. (NW): one‐hour PowerPoint workshop about NSIs, introduction/demonstration by supplier of new device, plus replacement of existing injection needles on the ward with injection needle with safety device. The safety device had to be activated by the workers. 2. (W) only received workshop, no new needle device) |
|
| Outcomes | Self‐reported number of NSIs within six‐month period and official hospital database registered NSIs. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on randomisation process. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information available on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A. Questionnaire‐based NSI 1. Baseline: Workshop + device group: Data missing on 99/267 (37%) Workshop group: Data missing on 102/263 (39%) Control group: Data missing on 100/266 (38%) 2. At six months: Workshop + device group: Data missing on 197/267 (74%) Workshop group: Data missing on 179/263 (68%) Control group: Data missing on 180/266 (68%) 3. 12 months: Workshop + device group: Data missing on 187/267 (70%) Workshop group: Data missing on 160/263 (60%) Control group: Data missing on 192/266 (74%) B. Hospital registry NSI No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section reported. |
| Similar recruitment of groups | Low risk | Participants were randomised within the same hospital. |
| Adjustment for baseline differences | Low risk | There is difference among the groups in regards to sex and working experience. These differences may have influenced the results. For example, there are 17% apprentice nurse in the intervention group compared to 7% in the control group. "the differences in individual and job characteristics between the intervention groups and the control group at baseline were examined using generalized estimated equations (GEE) correcting for wards." |
| Other bias | Low risk | The study appears to be free of other types of bias. |
Whitby 2008.
| Methods | Study design: Interrupted Time‐Series Study | |
| Participants | Australia (Brisbane). All occupational groups with clinical exposure within the hospital whose FTE were avaliable (medical, nursing, allied health and housekeeping) in the period 2000‐2006. | |
| Interventions | 1. Introduction of safety engineered retractable syringes and needle‐free IV systems 2. Extensive education program at the commencement of the intervention in 2005. | |
| Outcomes | Reported needlestick injuries per 10,000 FTEs. | |
| Notes | Information available on the cost of safety engineered devices. Total number of data points (n = 36). |
|
Zakrzewska 2001.
| Methods | Study design: Controlled Before‐After Study | |
| Participants | UK. Staff of a dental clinic dealing exclusively with patients with blood‐borne viruses during a five‐year period. Number studied: approximately 600 workers. Intervention group n = approximately 300. Control group n = approximately 300. | |
| Interventions | Introduction of a safety syringe and training on its use by the manufacturer. The safety device had to be activated by the worker. Control group continued using non‐disposable metal syringes after having received education on safety issues. Co‐interventions: Testing of safety devices, ensuring adequate supplies and means of disposal, involvement of key partners, protocol for the changeover. | |
| Outcomes | Number of reported sharps injuries per 1000,000 hours worked; number of sharps injuries related to syringes per total number of sharps injuries. | |
| Notes | Includes information about cost. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not an RCT. |
| Allocation concealment (selection bias) | High risk | Not an RCT. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | In the method section, authors do not pre‐specify their outcome measures concretely. |
| Similar recruitment of groups | Unclear risk | The number of students and qualified staff remains constant throughout the pre‐intervention period and during intervention over the five‐year study period. It is unclear if pre‐ and post‐intervention group are composed of students with similar years of experience. For the concurrent control group, researchers provided limited information. It is unclear if the individuals in this group performed similar tasks as the pre‐ and post‐intervention group. Authors just indicated that a busy surgical unit was used as the control. |
| Adjustment for baseline differences | Low risk | Authors reported the participant's profession and working experience. The intervention and control groups appear comparable in terms of working experience. No information to enable comparing the control and intervention unit to assess homogeneity of the two groups. |
| Other bias | High risk | 1. "In view of the increased bulk of the safety syringes new waste disposal bins had to be ordered and distributed round the clinics." This co‐intervention may have affected the number of NSI but it is not possible to determine. 2. Possible conflict of interest: "We are indebted to Septodont for their supplies, training and help." |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beynon 2015 | The study was an ITS design but had insufficient data points. |
| Bowden 1993 | The study design did not match our inclusion criteria (not an intervention study). |
| Buswell 2014 | The study design did not match our inclusion criteria (not an intervention study), The study group did not match our inclusion criteria (livestock workers). |
| Carvalho 2016 | The study was an ITS design but had insufficient data points. |
| Chaillol 2010 | The study design did not match our inclusion criteria (surveillance data). |
| Chakravarthy 2014 | The study was an ITS design but had insufficient data points. |
| Cleveland 2007 | The study design did not match our inclusion criteria (surveillance data). |
| Cullen 2006 | The study design did not match our inclusion criteria (surveillance study follow up by expert analysis stating which NSI could have been prevented). |
| Di Bari 2015 | The study design did not match our inclusion criteria (assesment study). |
| Floret 2015 | The study design did not match our inclusion criteria (surveillance data). |
| Ford 2011 | The main outcome of the study does not include NSI. “The aim of the evaluation was to assess the range of sharp safety hypodermic needle devices available in the UK, in terms of device performance and user acceptability. The evaluation was not designed to assess reductions in needlestick injury rates.” |
| Fukuda 2016 | The study design was a CBA but the before data was missing. |
| Goossens 2011 | The study design did not match our inclusion criteria (no comparison group). |
| Gramling 2013 | The study design did not match our inclusion criteria (descriptive study). |
| Grimmond 2014 | The study design did not match our inclusion criteria (not an intervention study). |
| Guerlain 2010 | The study design did not match our inclusion criteria (no comparison group). |
| Hotaling 2009 | The study was an ITS design but had insufficient data points. |
| Iinuma 2005 | The study design did not match our inclusion criteria (surveillance data). |
| Jagger 2010 | The study was an ITS design but had insufficient data points. |
| Kanamori 2016 | The study was an ITS design but had insufficient data points. |
| Kim 2015 | The study design did not match our inclusion criteria (compliance study). |
| Lamontagne 2007 | The study design did not match our inclusion criteria (surveillance data). |
| Laramie 2011 | The study design did not match our inclusion criteria (surveillance data). |
| Lauer 2014 | The study was an ITS design but had insufficient data points. |
| Lipscomb 2010 | The study design did not match our inclusion criteria (descriptive study). |
| Lu 2015 | The study was an ITS design but had insufficient data points. |
| Markkanen 2015 | The study design did not match our inclusion criteria (qualitative study). |
| Massachusetts 2011 | The study design did not match our inclusion criteria (surveillance study). |
| McAllister 2014 | The main outcome of the study does not include NSI (study evaluvated patient safety). |
| Menezes 2014 | The study was an ITS design but had insufficient data points. |
| Montella 2014 | The study design did not match our inclusion criteria. |
| Neo 2016 | The study design did not match our inclusion criteria (not about safety‐engineered devices). |
| Perry 2012a | The study was an ITS design but had insufficient data points. |
| Pigman 1993 | The study was not a field study. |
| Rajkumari 2015 | The study intervention does not match our inclusion criteria (the paper describes effectiveness of interactive classes). |
| Roff 2014 | The paper describes spatter contamintaion by active SED but it is not a controlled study. |
| Shimatani 2011 | The study design did not match our inclusion criteria (CBA but no comparison group). |
| Sibbitt 2011 | The study design did not match our inclusion criteria (no comparison group). |
| Skolnick 1993 | The study was an ITS design but had insufficient data points. |
| Smith 2013 | The main outcome of the study does not include NSI. |
| Sossai 2016 | The study design did not match our inclusion criteria. |
| Steuten 2010 | The study design did not match our inclusion criteria (literature review ‐ not original research). |
| Tosini 2010 | The study design did not match our inclusion criteria (surveillance data). |
| Unahalekhaka 2015 | The study design did not match our inclusion criteria (descriptive study). |
Characteristics of studies awaiting assessment [ordered by study ID]
Ferrario 2012.
| Methods | Time‐series |
| Participants | Healthcare workers |
| Interventions | Devices ? |
| Outcomes | Needlestick injuries ? |
| Notes |
Perry 2012.
| Methods | Time‐series |
| Participants | Healthcare workers |
| Interventions | Regulations |
| Outcomes | Sharps injuries |
| Notes |
Phillips 2010.
| Methods | Time‐series |
| Participants | Healthcare workers |
| Interventions | Legislation |
| Outcomes | Needlestick injuries |
| Notes |
Phillips 2011.
| Methods | Time‐series |
| Participants | Healthcare workers |
| Interventions | Legislation |
| Outcomes | Needlestick injuries |
| Notes |
Phillips 2012.
| Methods | Time‐series |
| Participants | Healthcare workers |
| Interventions | Legislation |
| Outcomes | Needlestick injuries |
| Notes |
Phillips 2012a.
| Methods | Time‐series |
| Participants | Hospital workers |
| Interventions | Legislation |
| Outcomes | Needlestick injuries |
| Notes |
Uyen 2014.
| Methods | Time‐series |
| Participants | Healthcare workers |
| Interventions | Legislation |
| Outcomes | Needlestick injuries |
| Notes |
Differences between protocol and review
The protocol stated that the interventions would be categorized based on the type of device: 1) safety engineered devices for blood collection; 2) safety engineered devices for Injecting fluids; and 3) containers for collecting sharps. During the review process, we added three more categories, intravenous systems, multiple safety devices and legislation as two studies included more than one type of device as part of their intervention.
Contributions of authors
Conceiving and designing the review: JV, ML and VR.
Co‐ordinating the review: JV and VR.
Data extraction: JV, MC, MP and VR.
Data analyses: JV, MC and VR.
Data interpretation: JV, MC and VR.
Writing of the review: MC, JV and VR.
Sources of support
Internal sources
-
Finnish Institute of Occupational Health, Finland.
Provided salary and office facilities and resources for Jos Verbeek
-
Pan American Health Organization, USA.
Provided salaries and office facilities and resources as well as support to attend Cochrane training sessions for Manisha Pahwa and Marie‐Claude Lavoie
External sources
No sources of support supplied
Declarations of interest
Viraj Reddy: None known.
Marie‐Claude Lavoie: None known.
Jos Verbeek: None known.
Manisha Pahwa: None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Asai 1999 active {published data only}
- Asai T, Matsumoto S, Matsumoto H, Yamamoto K, Shingu K. Prevention of needlestick injury efficacy of a safeguarded intravenous cannula. Anaesthesia 1999;54(3):258‐61. [DOI] [PubMed] [Google Scholar]
Asai 2002 active {published data only}
- Asai T, Hidaka I, Kawashima A, Miki T, Inada K, Kawachi S. Efficacy of catheter needles with safeguard mechanisms. Anaesthesia 2002;57(6):572‐7. [DOI] [PubMed] [Google Scholar]
Asai 2002 passive {published data only}
- Asai T, Hidaka I, Kawashima A, Miki T, Inada K, Kawachi S. Efficacy of catheter needles with safeguard mechanisms. Anaesthesia 2002;57(6):572‐7. [DOI] [PubMed] [Google Scholar]
Azar‐Cavanagh 2007 {published data only}
- Azar‐Cavanagh M, Burdt P, Green‐McKenzie J. Effect of the introduction of an engineered sharps injury prevention device on the percutaneous injury rate in healthcare workers. Infection Control and Hospital Epidemiology 2007;28(2):165‐70. [DOI] [PubMed] [Google Scholar]
Baskin 2014 {published data only}
- Baskin SB, Oray NC, Yanturali S, Bayram B. The comparison of heparinized insulin syringes and safety‐engineered blood gas syringes used in arterial blood gas sampling in the ED setting (randomized controlled study). American Journal of Emergency Medicine 2014;32(5):432‐7. [DOI] [PubMed] [Google Scholar]
Chambers 2015 hospitals {published data only}
- Chambers A, Mustard CA, Etches J. Trends in needlestick injury incidence following regulatory change in Ontario, Canada (2004‐2012): an observational study. BMC Health Services Research 2015;15:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chambers 2015 long‐term nursing care {published data only}
- Chambers A, Mustard CA, Etches J. Trends in needlestick injury incidence following regulatory change in Ontario, Canada (2004‐2012): an observational study. BMC Health Services Research 2015;15:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cote 2003 {published data only}
- Cote CJ, Roth AG, Wheeler M, ter Rahe C, Rae BR, Dsida RM, et al. Traditional versus new needle retractable i.v. catheters in children: are they really safer, and whom are they protecting?. Anesthesia and Analgesia 2003;96(2):387‐91, table of contents. [DOI] [PubMed] [Google Scholar]
Edmond 1988 {published data only}
- Edmond M, Khakoo R, McTaggart B, Solomon R. Effect of bedside needle disposal units on needle recapping frequency and needlestick injury. Infection Control and Hospital Epidemiology 1988;9(3):114‐6. [DOI] [PubMed] [Google Scholar]
Gaballah 2012 {published data only}
- Gaballah K, Warbuton D, Sihmbly K, Renton T. Needle stick injuries among dental students: risk factors and recommendations for prevention. Libyan Journal of Medicine 2012;7:na. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldwater 1989 {published data only}
- Goldwater PN, Law R, Nixon AD, Officer JA, Cleland JF. Impact of a recapping device on venepuncture‐related needlestick injury. Infection Control and Hospital Epidemiology 1989;10(1):21‐5. [DOI] [PubMed] [Google Scholar]
Goris 2015 {published data only}
- Goris AL, Gemeinhart N, Babcock HM. Reducing needlestick injuries from active safety devices:a passive safety engineered conversion. American Journal of Infection Control 2015;43:S9‐S10. [Google Scholar]
Grimmond 2010 {published data only}
- Grimmond T, Bylund S, Anglea C, Beeke L, Callahan A, Christiansen E, et al. Sharps injury reduction using a sharps container with enhanced engineering: A 28 hospital non‐randomized intervention and cohort study. American Journal of Infection Control 2010;38(10):799‐805. [DOI] [PubMed] [Google Scholar]
L'Ecuyer 1996 2wva {published data only}
- L'Ecuyer PB, Schwab EO, lademarco E, Barr N, Aton EA, Fraser VJ. Randomized prospective study of the impact of three needleless intravenous systems on needlestick injury rates. Infection Control and Hospital Epidemiology 1996;17:803‐8. [DOI] [PubMed] [Google Scholar]
L'Ecuyer 1996 mbc {published data only}
- L'Ecuyer PB, Schwab EO, Iademarco E, Barr N, Aton EA, Fraser VJ. Randomized prospective study of the impact of three needleless intravenous systems on needlestick injury rates. Infection Control and Hospital Epidemiology 1996;17:803‐8. [DOI] [PubMed] [Google Scholar]
L'Ecuyer 1996 pbc {published data only}
- L'Ecuyer PB, Schwab EO, Iademarco E, Barr N, Aton EA, Fraser VJ. Randomized prospective study of the impact of three needleless intravenous systems on needlestick injury rates. Infection Control and Hospital Epidemiology 1996;17:803‐8. [DOI] [PubMed] [Google Scholar]
Mendelson 1998 {published data only}
- Mendelson MH, Short LJ, Schechter CB, Meyers BR, Rodriguez M, Cohen S, et al. Study of a needleless intermittent intravenous‐access system for peripheral infusions: analysis of staff, patient, and institutional outcomes. Infection Control and Hospital Epidemiology 1998;19(6):401‐6. [DOI] [PubMed] [Google Scholar]
Phillips 2013 {published data only}
- Phillips EK, Conaway M, Parker G, Perry J, Jagger J. Issues in understanding the impact of the Needlestick Safety and Prevention Act on hospital sharps injuries. Infection Control and Hospital Epidemiology 2013;34(9):935‐939. [DOI] [PubMed] [Google Scholar]
Prunet 2008 active {published data only}
- Prunet B, Meaudre E, Montcriol A, Asencio Y, Bordes J, Lacroix G, et al. A prospective randomized trial of two safety peripheral intravenous catheters. Anesthesia and Analgesia 2008;107(1):155. [DOI] [PubMed] [Google Scholar]
Prunet 2008 passive {published data only}
- Prunet B, Meaudre E, Montcriol A, Asencio Y, Bordes J, Lacroix G, et al. A prospective randomized trial of two safety peripheral intravenous catheters. Anesthesia and Analgesia 2008;107(1):155. [DOI] [PubMed] [Google Scholar]
Reddy 2001 {published data only}
- Reddy SG, Emery RJ. Assessing the effect of long‐term availability of engineering controls on needlestick injuries among health care workers: a 3‐year preimplementation and postimplementation comparison. American Journal of Infection Control 2001;29(6):425‐7. [DOI] [PubMed] [Google Scholar]
Richard 2001 {published data only}
- Richard VS, Kenneth J, Ramaprabha P, Kirupakaran H, Chandy GM. Impact of introduction of sharps containers and of education programmes on the pattern of needle stick injuries in a tertiary care centre in India. Journal of Hospital Infection 2001;47(2):163‐5. [DOI] [PubMed] [Google Scholar]
Rogues 2004 {published data only}
- Rogues AM, Verdun‐Esquer C, Buisson‐Valles I, Laville MF, Lashéras A, Sarrat A, et al. Impact of safety devices for preventing percutaneous injuries related to phlebotomy procedures in health care workers. American Journal of Infection Control 2004;32(8):441‐4. [DOI] [PubMed] [Google Scholar]
Seiberlich 2016 {published data only}
- Seiberlich LE, Keay V, Kallos S, Junghans T, Lang E, McRae AD. Clinical performance of a new blood control peripheral intravenous catheter: A prospective, randomized, controlled study. International Emergency Nursing 2016;25:59‐64. [DOI] [PubMed] [Google Scholar]
Sossai 2010 {published data only}
- Sossai D, Puro V, Chiappatoli L, Dagnino G, Odone B, Polimeri A, et al. Using an intravenous catheter system to prevent needlestick injury. Nursing Standard (Royal College of Nursing (Great Britain): 1987) 2010;24(29):42‐6. [DOI] [PubMed] [Google Scholar]
Valls 2007 {published data only}
- Valls V, Lozano MS, Yanez R, Martinez MJ, Pascual F, Lloret J, et al. Use of safety devices and the prevention of percutaneous injuries among healthcare workers. Infection Control and Hospital Epidemiology 2007;28(12):1352‐60. [DOI] [PubMed] [Google Scholar]
van der Molen 2011 {published data only}
- Molen HF, Zwinderman KAH, Sluiter JK, Frings‐Dresen MHW. Better effect of the use of a needle safety device in combination with an interactive workshop to prevent needle stick injuries. Safety Science 2011;49:1180‐6. [Google Scholar]
Whitby 2008 {published data only}
- Whitby M, McLaws ML, Slater K. Needlestick injuries in a major teaching hospital: the worthwhile effect of hospital‐wide replacement of conventional hollow‐bore needles. American Journal of Infection Control 2008;36(3):180‐6. [DOI] [PubMed] [Google Scholar]
Zakrzewska 2001 {published data only}
- Zakrzewska JM, Greenwood I, Jackson J. Cross‐infection control: Introducing safety syringes into a UK dental school ‐ a controlled study. British Dental Journal 2001;190(2):88‐92. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beynon 2015 {published data only}
- Beynon A. A quality improvement initiative to reduce needlestick injuries. Nursing Standard 2015;29(22):37‐42. [DOI] [PubMed] [Google Scholar]
Bowden 1993 {published data only}
- Bowden FJ, Pollett B, Birrell F, Dax EM. Occupational exposure to the human immunodeficiency virus and other blood‐borne pathogens. A six‐year prospective study. Medical journal of Australia 1993;158(12):810‐2. [DOI] [PubMed] [Google Scholar]
Buswell 2014 {published data only}
- Buswell ML, Hourigan M, Nault A, Bender J. Needlestick injuries in livestock workers and prevention programs. 2013 Agricultural Safety Summit. Journal of Agromedicine 2014;19(2):206‐7. [Google Scholar]
Carvalho 2016 {published data only}
- Carvalho PCF, Reis RK, Pereira FMV, Toffano SEM. Injury rates from peripheral catheters with or without safety devices in a Brazilian public hospital. American Journal of Infection Control 2016;44(7):853‐4. [DOI] [PubMed] [Google Scholar]
Chaillol 2010 {published data only}
- Chaillol I, Ecochard R, Denis MA, Iwaz J, Khoueiry P, Bergeret A. Fast and specific detection of moderate long‐term changes in occupational blood exposures. Occupational and Environmental Medicine 2010;67(11):785‐91. [DOI] [PubMed] [Google Scholar]
Chakravarthy 2014 {published data only}
- Chakravarthy M, Singh S, Arora A, Sengupta S, Munshi N, Rangaswamy S, et al. Epidemiology of sharp injuries ‐ Prospective EPINet data from five tertiary care hospitals in India ‐ Data for 144 cumulated months, 1.5 million inpatient days. Clinical Epidemiology and Global Health 2014;2(3):121‐6. [Google Scholar]
Cleveland 2007 {published data only}
- Cleveland JL, Barker LK, Cuny EJ, Panlilio AL. Preventing percutaneous injuries among dental health care personnel. Journal of the American Dental Association 2007;138(2):169‐78; quiz 247‐8. [DOI] [PubMed] [Google Scholar]
Cullen 2006 {published data only}
- Cullen BL, Genasi F, Symington I, Bagg J, McCreaddie M, Taylor A, et al. Potential for reported needlestick injury prevention among healthcare workers through safety device usage and improvement of guideline adherence: expert panel assessment. The Journal of Hospital Infection 2006;63(4):445‐51. [DOI] [PubMed] [Google Scholar]
Di Bari 2015 {published data only}
- Bari V, Carli G, Puro V. [Prevention of accidental needle sticks before the Directive 2010/32/EU in a sample of Italian hospitals]. La Medicina del Lavoro 2015;106(3):186‐205. [PubMed] [Google Scholar]
Floret 2015 {published data only}
- Floret N, Ali‐Brandmeyer O, L'Hériteau F, Bervas C, Barquins‐Guichard S, Pelissier G, et al. Sharp decrease of reported occupational blood and body fluid exposures in French hospitals, 2003‐2012: Results of the French National Network Survey, AES‐RAISIN. Infection Control and Hospital Epidemiology 2015;36(8):963‐8. [DOI] [PubMed] [Google Scholar]
Ford 2011 {published data only}
- Ford J, Phillips P. An evaluation of sharp safety blood evacuation devices. Nursing Standard (Royal College of Nursing (Great Britain): 1987) 2011;25(43):41‐7. [DOI] [PubMed] [Google Scholar]
Fukuda 2016 {published data only}
- Fukuda H, Yamanaka N. Reducing needlestick injuries through safety‐engineered devices: results of a Japanese multi‐centre study. J Hosp Infect 2016;92(2):147‐53. [DOI] [PubMed] [Google Scholar]
Goossens 2011 {published data only}
- Goossens GA, Moons P, Jerome M, Stas M. Prospective clinical evaluation of the Polyperf(R) Safe, a safety Huber needle, in cancer patients. The Journal of Vascular Access 2011;12(3):200‐6. [DOI] [PubMed] [Google Scholar]
Gramling 2013 {published data only}
- Gramling JJ, Nachreiner N. Implementing a sharps injury reduction program at a charity hospital in India. Workplace Health and Safety 2013;61(8):339‐45. [DOI] [PubMed] [Google Scholar]
Grimmond 2014 {published data only}
- Grimmond T. Frequency of use and activation of safety‐engineered sharps devices: A sharps container audit in five Australian capital cities. Healthcare Infection 2014;19(3):95‐100. [Google Scholar]
Guerlain 2010 {published data only}
- Guerlain S, Wang L, Hugine A. Intelliject's novel epinephrine autoinjector: sharps injury prevention validation and comparable analysis with EpiPen and Twinject. Annals of Allergy, Asthma & Immunology 2010;105(6):480‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hotaling 2009 {published data only}
- Hotaling M. A retractable winged steel (butterfly) needle performance improvement project. Joint Commission Journal on Quality and Patient Safety / Joint Commission Resources 2009;35(2):100‐5, 61. [DOI] [PubMed] [Google Scholar]
Iinuma 2005 {published data only}
- Iinuma Y, Igawa J, Takeshita M, Hashimoto Y, Fujihara N, Saito T, et al. Passive safety devices are more effective at reducing needlestick injuries. The Journal of Hospital Infection 2005;61(4):360‐1. [DOI] [PubMed] [Google Scholar]
Jagger 2010 {published data only}
- Jagger J, Berguer R, Phillips E K, Parker G, Gomaa A E. Increase in sharps injuries in surgical settings versus nonsurgical settings after passage of national needlestick legislation. JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS 2010;210:(4):496‐502. [DOI] [PubMed] [Google Scholar]
Kanamori 2016 {published data only}
- Kanamori H, Weber DJ, DiBiase LM, Pitman KL, Consoli SA, Hill J, et al. Impact of safety‐engineered devices on the Incidence of occupational blood and body fluid exposures among healthcare personnel in an academic facility, 2000‐2014. Infection Control and Hospital Epidemiology 2016;37(5):497‐504. [DOI] [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kim YG, Jeong IS, Park SM. Sharps injury prevention guidance among health care professionals: A comparison between self‐reported and observed compliance. American Journal of Infection Control 2015;43(9):977‐82. [DOI] [PubMed] [Google Scholar]
Lamontagne 2007 {published data only}
- Lamontagne F, Abiteboul D, Lolom I, Pellissier G, Tarantola A, Descamps JM, et al. Role of safety‐engineered devices in preventing needlestick injuries in 32 French hospitals. Infection Control and Hospital Epidemiology 2007;28(1):18‐23. [DOI] [PubMed] [Google Scholar]
Laramie 2011 {published data only}
- Laramie AK, Pun VC, Fang SC, Kriebel D, Davis L. Sharps injuries among employees of acute care hospitals in Massachusetts, 2002‐2007. Infection Control and Hospital Epidemiology 2011;32(6):538‐44. [DOI] [PubMed] [Google Scholar]
Lauer 2014 {published data only}
- Lauer A‐C, Reddemann A, Meier‐Wronski C‐P, Bias H, G̦decke K, Arendt M, et al. Needlestick and sharps injuries among medical undergraduate students. American Journal of Infection Control 2014;42(3):235‐9. [DOI] [PubMed] [Google Scholar]
Lipscomb 2010 {published data only}
- Lipscomb J, Geiger Brown J, Johnson J, McPhaul K, Trinkoff A, Storr C. Blood exposure and primary prevention in the home care workplace. NIOSH report. Cincinatti: National Institute of Occupational Safety and Health, 2010.
Lu 2015 {published data only}
- Lu Y, Senthilselvan A, Joffe A M, Beach J. Effectiveness of safety‐engineered devices in reducing sharp object injuries. Occupational medicine (Oxford, England) 2015;65(1):39‐44. [DOI] [PubMed] [Google Scholar]
Markkanen 2015 {published data only}
- Markkanen P, Galligan C, Laramie A, Fisher J, Sama S, Quinn M. Understanding sharps injuries in home healthcare: the Safe Home Care qualitative methods study to identify pathways for injury prevention. BMC Public Health 2015;na:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Massachusetts 2011 {published data only}
- Massachusetts Department of Public Health. Sharps injuries among hospital workers in Massachusetts. Massachusetts Department of Public Health: Occupational Health Surveillance Program 2011.
McAllister 2014 {published data only}
- McAllister L, Anderson J, Werth K, Cho I, Copeland K, Cam Bouveret N, et al. Needle‐free jet injection for administration of influenza vaccine: A randomised non‐inferiority trial. The Lancet 2014;384(9944):674‐81. [DOI] [PubMed] [Google Scholar]
Menezes 2014 {published data only}
- Menezes JA, Bandeira CS, Quintana M, Lima E Silva JC, Calvet GA, Brasil P. Impact of a single safety‐engineered device on the occurrence of percutaneous injuries in a general hospital in Brazil. American Journal of Infection Control 2014;42(2):174‐7. [DOI] [PubMed] [Google Scholar]
Montella 2014 {published data only}
- Montella E, Schiavone D, Apicella L, Silverio P, Gaudiosi M, Ambrosone E, et al. Cost‐benefit evaluation of a preventive intervention on the biological risk in health: the accidental puncture during the administration of insulin in the University Hospital "Federico II" of Naples. Annali di Igiene: Medicina Preventiva e di Comunità 2014;26(3):272‐8. [DOI] [PubMed] [Google Scholar]
Neo 2016 {published data only}
- Neo SHS, Khemlani MH, Sim LK, Seah AST. Winged metal needles versus plastic winged and nonwinged cannulae for subcutaneous infusions in palliative care: A quality improvement project to enhance patient care and medical staff safety in a Singaporean hospital. Journal of Palliative Medicine 2016;19(3):318‐22. [DOI] [PubMed] [Google Scholar]
Perry 2012a {published data only}
- Perry J, Jagger J, Parker G, Phillips E K, Gomaa A. Disposal of sharps medical waste in the United States: impact of recommendations and regulations, 1987‐2007. American Journal of Infection Control 2012;40(4):354‐8. [DOI] [PubMed] [Google Scholar]
Pigman 1993 {published data only}
- Pigman EC, Karch DB, Scott JL. Splatter during jet irrigation cleansing of a wound model: a comparison of three inexpensive devices. Annals of Emergency Medicine 1993;22(10):1563‐7. [DOI] [PubMed] [Google Scholar]
Rajkumari 2015 {published data only}
- Rajkumari N, Mathur P, Gunjiyal J, Misra MC. Effectiveness of Intensive Interactive Classes and Hands on Practice to Increase Awareness about Sharps Injuries and Splashes among Health Care Workers. Journal of Clinical and Diagnostic Research 2015;9(7):Dc17‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roff 2014 {published data only}
- Roff M, Basu S, Adisesh A. Do active safety‐needle devices cause spatter contamination?. Journal of Hospital Infection 2014;86(3):221‐3. [DOI] [PubMed] [Google Scholar]
Shimatani 2011 {published data only}
- Shimatani M, Matsui Y, Yano K. Comparison of the needlstick injuries due to active and passive design safety intravenous catheters. American Journal of Infection Control 2011;39(5):E79. [Google Scholar]
Sibbitt 2011 {published data only}
- Sibbitt WL Jr, Band PA, Kettwich LG, Sibbitt CR, Sibbitt LJ, Bankhurst AD. Safety syringes and anti‐needlestick devices in orthopaedic surgery. The Journal of Bone and Joint Surgery. American volume 2011;93(17):1641‐9. [DOI] [PubMed] [Google Scholar]
Skolnick 1993 {published data only}
- Skolnick R, LaRocca J, Barba D, Paicius L. Evaluation and implementation of a needleless intravenous system: making needlesticks a needless problem. American Journal of Infection Control 1993;21(1):39‐41. [DOI] [PubMed] [Google Scholar]
Smith 2013 {published data only}
- Smith SL, Shames A, Jeannerot F. Usability and validation of a new device for the administration of somatostatin analog therapy: An open‐label, randomized study using a simulated placebo gel. Endocrine Reviews 2013;34(suppl 4):na. [Google Scholar]
Sossai 2016 {published data only}
- Sossai D, Guardo M, Foscoli R, Pezzi R, Polimeni A, Ruzza L, et al. Efficacy of safety catheter devices in the prevention of occupational needlestick injuries: Applied research in the Liguria Region (Italy). Journal of Preventive Medicine and Hygiene 2016;57(2):E110‐4. [PMC free article] [PubMed] [Google Scholar]
Steuten 2010 {published data only}
- Steuten L, Buxton M. Economic evaluation of healthcare safety: which attributes of safety do healthcare professionals consider most important in resource allocation decisions?. Quality and Safety in Health Care 2010;19(5):e6. [DOI] [PubMed] [Google Scholar]
Tosini 2010 {published data only}
- Tosini W, Ciotti C, Goyer F, Lolom I, L'Heriteau F, Abiteboul D, et al. Needlestick injury rates according to different types of safety‐engineered devices: results of a French multicenter study. Infection Control and Hospital Epidemiology 2010;31(4):402‐7. [DOI] [PubMed] [Google Scholar]
Unahalekhaka 2015 {published data only}
- Unahalekhaka A, Lueang‐a‐papong S. Prevention of needlestick and sharp injuries among hospitals in Thailand. 42nd annual conference abstracts, APIC 2015, Nashville, TN June 2015. American Journal of Infection Control 2015;43:S44‐5. [Google Scholar]
References to studies awaiting assessment
Ferrario 2012 {published data only}
- Ferrario MM, Landone S, Biasi M, Tagliasacchi R, Riva R, Veronesi G, et al. Time trends of incidence rates of work accident with blood contamination in a North Italian teaching hospital [Analisi dei trend temporali (2004‐11) dei tassi di infortunio biologico in un ospedale universitario del nord Italia. Quali evidenze di efficacia dei sistemi di prelievo a vuoto?]. Giornale Italiano di Medicina del Lavoro ed Ergonomia 2012;34(3 suppl 1):275‐7. [PubMed] [Google Scholar]
Perry 2012 {published data only}
- Perry J, Jagger J, Parker G, Philips EK, Gomaa A. Disposal of sharps medical waste in the United States: impact of recommendations and regulations, 1987‐2007. American Journal of Infection Control 2012;40(4):354‐8. [DOI] [PubMed] [Google Scholar]
Phillips 2010 {published data only}
- Phillips EK. Healthcare worker injury risk and the impact of the Needlestick Safety and Prevention Act. 10th Anniversary of the Needlestick Safety and Prevention Act: Mapping Progress, Charting a Future Path, Charlottesville, VA, November 5‐6, 2010. Charlotsville, VA: University of Virginia School of Medicine, 2010 Nov; :1‐14. 2010.
Phillips 2011 {published data only}
- Phillips EK, Conaway M, Parker G, Perry J, Jagger J. Changes in sharps injuries among healthcare workers: the effect of HR 5178 (National Needlestick Safety and Prevention Act). NOIRS 2011‐Abstracts of the national occupational injury research symposium, october 18‐20, 2011, Morgantown, West Virginia. National Institute for Occupational Safety and Health, Oct; 130. 2011.
Phillips 2012 {published data only}
- Phillips EK, Conaway MR, Jagger JC. Percutaneous injuries before and after the needlestick safety and prevention act. New England Journal of Medicine 2012;366(7):670‐1. [DOI] [PubMed] [Google Scholar]
Phillips 2012a {published data only}
- Phillips E K. Impact of Needlestick Safety & Prevention Act (HR5178) on hospital worker injury. NIOSH report K01‐OH‐009140 2012:1‐33.
Uyen 2014 {published data only}
- Uyen Vu. The effective implementation of health and safety regulations: lessons learned from an iwh study on safety‐engineered needles. OOHNA Journal 2014;33(2):24‐6. [Google Scholar]
Additional references
Akduman 1999
- Akduman D, Kim LE, Parks RL, L'Ecuyer PB, Mutha S, Jeffe DB, et al. Use of personal protective equipment and operating room behaviors in four surgical subspecialties: personal protective equipment and behaviors in surgery. Infection Control and Hospital Epidemiology 1999;20(2):110‐4. [DOI] [PubMed] [Google Scholar]
Ansa 2002
- Ansa VO, Udoma EJ, Umoh MS, Anah MU. Occupational risk of infection by human immunodeficiency and hepatitis B viruses among health workers in south‐eastern Nigeria. East African Medical Journal 2002;79(5):254‐6. [DOI] [PubMed] [Google Scholar]
Ballout 2016
- Ballout RA, Diab B, Harb AC, Tarabay R, Khamassi S, Akl EA. Use of safety‐engineered devices by healthcare workers for intravenous and/or phlebotomy procedures in healthcare settings: a systematic review and meta‐analysis. BMC Health Services Research 2016;16:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bryce 1999
- Bryce EA, Ford J, Chase L, Taylor C, Scharf S. Sharps injuries: defining prevention priorities. American Journal of Infection Control 1999;27(5):447‐52. [DOI] [PubMed] [Google Scholar]
Castella 2003
- Castella A, Vallino A, Argentero PA, Zotti CM. Preventability of percutaneous injuries in healthcare workers: a year‐long survey in Italy. Journal of Hospital Infection 2003;55(4):290‐4. [DOI] [PubMed] [Google Scholar]
Cheetham 2016
- Cheetham S, Thompson SC, Liira J, Afilaka OA, Liira H. Education and training for preventing sharps injuries and splash exposures in healthcare workers. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD012060] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2005
- Chen W, Gluud C. Vaccines for preventing hepatitis B in healthcare workers. Cochrane Database of Systematic Reviews 2005, Issue Issue 4. [DOI: 10.1002/14651858.CD000100.pub3] [DOI] [PubMed] [Google Scholar]
Clarke 2002
- Clarke SP, Rockett JL, Sloane DM, Aiken LH. Organizational climate, staffing, and safety equipment as predictors of needlestick injuries and near‐misses in hospital nurses. American Journal of Infection Control 2002;30(4):207‐16. [DOI] [PubMed] [Google Scholar]
De Carli 2003
- Carli G, Puro V, Ippolito G, Studio Italiano Rischio Occupazionale da HIV Group. Risk of hepatitis C virus transmission following percutaneous exposure in healthcare workers. Infection 2003;31 Suppl 2:22‐7. [PubMed] [Google Scholar]
Doebbeling 2003
- Doebbeling BN, Vaughn TE, McCoy KD, Beekmann SE, Woolson RF, Ferguson KJ, et al. Percutaneous injury, blood exposure, and adherence to standard precautions: are hospital‐based health care providers still at risk?. Clinical Infectious Diseases 2003;37(8):1006‐13. [DOI] [PubMed] [Google Scholar]
Downs 1998
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. Journal of Epidemiology and Community Health 1998;52(6):377‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2006
- The Cochrane Effective Practice and Organisation of Care Group (EPOC). Including interrupted time‐series (ITS) designs in a EPOC review. http://www.epoc.uottawa.ca/inttime.pdf (accessed 5 September 2006).
Fisman 2002
- Fisman DN, Mittleman AM, Sorock GS, Harris AD. Willingness to pay to avoid sharps‐related injuries: a study in injured health care workers. American Journal of Infection Control 2002;30(5):283‐7. [DOI] [PubMed] [Google Scholar]
Fisman 2007
- Fisman DN, Harris AD, Rubin M, Sorock GS, Mittleman AM. Fatigue increases the risk of injury from sharp devices in medical trainees: results from a case‐crossover study. Infection Control and Hospital Epidemiology 2007;28(1):10‐7. [DOI] [PubMed] [Google Scholar]
GRADEpro 2017 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. GRADE working group, 2017.
Grimmond 2017
- Grimmond T, Good L. Exposure Survey of Trends in Occupational Practice (EXPO‐S.T.O.P.) 2015: A national survey of sharps injuries and mucocutaneous blood exposures among health care workers in US hospitals. American Journal of Infection Control 2017;S0196‐6553(17):30770‐8. [DOI] [PubMed] [Google Scholar]
Hanrahan 1997
- Hanrahan A, Reutter L. A critical review of the literature on sharps injuries: epidemiology, management of exposures and prevention. Journal of Advanced Nursing 1997;25(1):144‐54. [DOI] [PubMed] [Google Scholar]
Harb 2015
- Harb AC, Tarabay R, Diab B, Ballout RA, Khamassi S, Akl EA. Safety engineered injection devices for intramuscular, subcutaneous and intradermal injections in healthcare delivery settings: a systematic review and meta‐analysis. BMC nursing 2015;14:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org..
HSE 2012
- Beswick A, Robinson E, Evans G, Codling A. An evaluation of the efficacy of safer sharps devices. http://www.hse.gov.uk/research/rrpdf/rr914.pdf. HSE, (accessed May 20th, 2017).
Hutin 2003
- Hutin Y, Hauri A, Chiarello L, Catlin M, Stilwell B, Ghebrehiwet T, et al. Best infection control practices for intradermal, subcutaneous, and intramuscular needle injections. Bulletin of the World Health Organization 2003;81(7):491‐500. [PMC free article] [PubMed] [Google Scholar]
Ilhan 2006
- Ilhan MN, Durukan E, Aras E, Turkcuoglu S, Aygun R. Long working hours increase the risk of sharp and needlestick injury in nurses: the need for new policy implication. Journal of Advanced Nursing 2006;56(5):563‐8. [DOI] [PubMed] [Google Scholar]
Jagger 1988
- Jagger J, Hunt EH, Brand‐Elnaggar J, Pearson RD. Rates of needle‐stick injury caused by various devices in a university hospital. The New England Journal of Medicine 1988;319(5):284‐8. [DOI] [PubMed] [Google Scholar]
Jagger 1990
- Jagger J, Hunt EH, Pearson RD. Estimated cost of needlestick injuries for six major needled devices. Infection Control and Hospital Epidemiology 1990;11(11):584‐8. [DOI] [PubMed] [Google Scholar]
Jagger 2013
- Jagger J, Perry J. Safety‐engineered devices in 2012: the critical role of healthcare workers in device selection. Infection Control and Hospital Epidemiology 2013;34(6):615‐8. [DOI] [PubMed] [Google Scholar]
Leigh 2007
- Leigh JP, Gillen M, Franks P, Sutherland S, Nguyen HH, Steenland K, et al. Costs of needlestick injuries and subsequent hepatitis and HIV infection. Current Medical Research and Opinion 2007;23(9):2093‐105. [DOI] [PubMed] [Google Scholar]
MacCannell 2010
- MacCannell T, Laramie AK, Gomaa A, Perz JF. Occupational exposure of health care personnel to hepatitis B and hepatitis C: prevention and surveillance strategies. Clinics in Liver Disease 2010;14(1):23‐36, vii. [DOI] [PubMed] [Google Scholar]
Mast 2004
- Mast E, Mahoney F, Kane M, Margolis H. Hepatitis B vaccines. In: Plotkin OW editor(s). Vaccines. Philadelphia: Saunders, 2004:299‐337. [Google Scholar]
Mischke 2014
- Mischke C, Verbeek JH, Saarto A, Lavoie MC, Pahwa M, Ijaz S. Gloves, extra gloves or special types of gloves for preventing percutaneous exposure injuries in healthcare personnel. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD009573.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ngatu 2011
- Ngatu NR, Phillips EK, Wembonyama OS, Hirota R, Kaunge NJ, Mbutshu LH, et al. Practice of universal precautions and risk of occupational blood‐borne viral infection among Congolese health care workers. American Journal of Infection Control 2012;40(1):68‐70. [DOI] [PubMed] [Google Scholar]
Oh 2005
- Oh HS, Yi SE, Choe KW. Epidemiological characteristics of occupational blood exposures of healthcare workers in a university hospital in South Korea for 10 years. Journal of Hospital Infection 2005;60(3):269‐75. [DOI] [PubMed] [Google Scholar]
Orji 2002
- Orji EO, Fasubaa OB, Onwudiegwu U, Dare FO, Ogunniyi SO. Occupational health hazards among health care workers in an obstetrics and gynaecology unit of a Nigerian teaching hospital. Journal of Obstetrics and Gynaecology 2002;22(1):75‐8. [DOI] [PubMed] [Google Scholar]
OSHA 2001
- Occupational Safety and Health Administration (OSHA). Frequently asked questions needlestick safety and prevention act. https://www.osha.gov/needlesticks/needlefaq.html (accessed 8 August 2017).
Panlilio 2004
- Panlilio AL, Orelien JG, Srivastava PU, Jagger J, Cohn RD, Cardo DM, et al. Estimate of the annual number of percutaneous injuries among hospital‐based healthcare workers in the United States, 1997‐1998. Infection Control and Hospital Epidemiology 2004;25(7):556‐62. [DOI] [PubMed] [Google Scholar]
Parantainen 2011
- Parantainen A, Verbeek J, Lavoie MC, Pahwa M. Blunt versus sharp suture needles for preventing percutaneous exposure incidents in surgical staff. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009170.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pruss‐Ustun 2005
- Pruss‐Ustun A, Rapiti E, Hutin Y. Estimation of the global burden of disease attributable to contaminated sharps injuries among healthcare workers. American Journal of Industrial Medicine 2005;48(6):482‐90. [DOI] [PubMed] [Google Scholar]
Ramsay 2001
- Ramsay C. Robust methods for analysis of interrupted time‐series designs for inclusion in systematic reviews. Proceedings of the ninth Annual Cochrane Colloquium; 2001; Lyon. 2001.
Ramsay 2003
- Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted time‐series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. International Journal of Technology Assessment in Health Care 2003;19(4):613‐23. [DOI] [PubMed] [Google Scholar]
Ratner 1994
- Ratner SL, Norman SA, Berlin JA. Percuteous injuries on the frontline: a survey of house staff and nurses. American Journal of Preventive Medicine 1994;10:372‐7. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 1999
- Roberts RB, Lincoff AS, Frankel RE. Occupational exposure to HIV in an urban university hospital setting. Brazilian Journal of Infectious Diseases 1999;3(2):50‐62. [PubMed] [Google Scholar]
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology 2002;31(1):150‐3. [DOI] [PubMed] [Google Scholar]
Rogers 2000
- Rogers B, Goodno L. Evaluation of interventions to prevent needlestick injuries in health care occupations. American Journal of Preventive Medicine 2000;18(4 Suppl):90‐8. [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Saarto 2011
- Saarto A, Verbeek JH, Lavoie MC, Pahwa M. Blunt versus sharp suture needles for preventing percutaneous exposure incidents in surgical staff. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009170.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2006
- Smith DR, Choe MA, Jeong JS, Jeon MY, Chae YR, An GJ. Epidemiology of needlestick and sharps injuries among professional Korean nurses. Journal of Professional Nursing 2006;22(6):359‐66. [DOI] [PubMed] [Google Scholar]
Smith 2006b
- Smith DR, Mihashi M, Adachi Y, Nakashima Y, Ishitake T. Epidemiology of needlestick and sharps injuries among nurses in a Japanese teaching hospital. Journal of Hospital Infection 2006;64(1):44‐9. [DOI] [PubMed] [Google Scholar]
Sohn 2006
- Sohn JW, Kim BG, Kim SH, Han C. Mental health of healthcare workers who experience needlestick and sharps injuries. Journal of Occupational Health 2006;48(6):474‐9. [DOI] [PubMed] [Google Scholar]
Tarigan 2015
- Tarigan LH, Cifuentes M, Quinn M, Kriebel D. Prevention of needle‐stick injuries in healthcare facilities: a meta‐analysis. Infection Control and Hospital Epidemiology 2015;36(7):823‐829. [DOI] [PubMed] [Google Scholar]
Trim 2004
- Trim JC. A review of needle‐protective devices to prevent sharps injuries. British Journal of Nursing (Mark Allen Publishing) 2004;13(3):144, 146‐53. [PUBMED: 14997076] [DOI] [PubMed] [Google Scholar]
Tuma 2006
- Tuma S, Sepkowitz KA. Efficacy of safety‐engineered device implementation in the prevention of percutaneous injuries: a review of published studies. Clinical Infectious Diseases 2006;42(8):1159‐70. [DOI] [PubMed] [Google Scholar]
UNAIDS 2009
- UNAIDS. AIDS epidemic update: November 2009. http://www.unaids.org/en/media/unaids/contentassets/dataimport/pub/report/2009/jc1700_epi_update_2009_en.pdf. UNAIDS, (accessed Dec 15th, 2011).
Verbeek 2005
- Verbeek J, Salmi J, Pasternack I, Jauhiainen M, Laamanen I, Schaafsma F, et al. A search strategy for occupational health intervention studies. Journal of Occupational and Environmental Medicine 2005;62(10):682‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waclawski 2004
- Waclawski ER. Evaluation of potential reduction in blood and body fluid exposures by use of alternative instruments. Occupational Medicine 2004;54(8):567‐9. [DOI] [PubMed] [Google Scholar]
Wallis 2007
- Wallis GC, Kim WY, Chaudhary BR, Henderson JJ. Perceptions of orthopaedic surgeons regarding hepatitis C viral transmission: a questionnaire survey. Annals of the Royal College of Surgeons of England 2007;89(3):276‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2016
- WHO. WHO guideline on the use of safety‐engineered syringes for intramuscular, intradermal and subcutaneous injections in health care settings. http://apps.who.int/iris/bitstream/10665/250144/1/9789241549820‐eng.pdf. WHO, (accessed May 10th, 2017). [PubMed]
References to other published versions of this review
Lavoie 2012
- Lavoie, Marie‐Claude, Verbeek, Jos H, Parantainen, Annika, et al. Devices for preventing percutaneous exposure injuries caused by needles in health care personnel. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009740] [DOI] [Google Scholar]
Lavoie 2014
- Lavoie, Marie‐Claude, Verbeek, Jos H, Pahwa, Manisha. Devices for preventing percutaneous exposure injuries caused by needles in healthcare personnel. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD009740.pub2] [DOI] [PubMed] [Google Scholar]


