Abstract
Background
Preterm birth is a major health problem and contributes to more than 50% of the overall perinatal mortality. Preterm birth has multiple risk factors including cervical incompetence and multiple pregnancy. Different management strategies have been tried to prevent preterm birth, including cervical cerclage. Cervical cerclage is an invasive technique that needs anaesthesia and may be associated with complications. Moreover, there is still controversy regarding the efficacy and the group of patients that could benefit from this operation. Cervical pessary has been tried as a simple, non‐invasive alternative that might replace the above invasive cervical stitch operation to prevent preterm birth.
Objectives
To evaluate the efficacy of cervical pessary for the prevention of preterm birth in women with risk factors for cervical incompetence.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (1 September 2012), Current Controlled Trials and the Australian New Zealand Clinical Trials Registry (1 September 2012).
Selection criteria
We selected all published and unpublished randomised clinical trials comparing the use of cervical pessary with cervical cerclage or expectant management for prevention of preterm birth. We did not include quasi‐randomised trials. Cluster‐randomised or cross‐over trials were not eligible for inclusion.
Data collection and analysis
Two review authors independently assessed trials for inclusion.
Main results
The review included one randomised controlled trial. The study included 385 pregnant women with a short cervix of 25 mm or less who were between 18 to 22 weeks of pregnancy. The use of cervical pessary (192 women) was associated with a statistically significantly decrease in the incidence of spontaneous preterm birth less than 37 weeks' gestation compared with expectant management (22% versus 59 %; respectively, risk ratio (RR) 0.36, 95% confidence interval (CI) 0.27 to 0.49). Spontaneous preterm birth before 34 weeks was statistically significantly reduced in the pessary group (6% and 27% respectively, RR 0.24; 95% CI 0.13 to 0.43). Mean gestational age at delivery was 37.7 + 2 weeks in the pessary group and 34.9 + 4 weeks in the expectant group. Women in the pessary group used less tocolytics (RR 0.63; 95% CI 0.50 to 0.81) and corticosteroids (RR 0.66; 95% CI 0.54 to 0.81) than the expectant group. Vaginal discharge was more common in the pessary group (RR 2.18; 95% CI 1.87 to 2.54). Among the pessary group, 27 women needed pessary repositioning without removal and there was one case of pessary removal. Ninety‐five per cent of women in the pessary group would recommend this intervention to other people. Neonatal paediatric care admission was reduced in the pessary group in comparison to the expectant group (RR 0.17; 95% CI 0.07 to 0.42).
Authors' conclusions
The review included only one well‐designed randomised clinical trial that showed beneficial effect of cervical pessary in reducing preterm birth in women with a short cervix. There is a need for more trials in different settings (developed and developing countries), and with different risk factors including multiple pregnancy.
Plain language summary
Using a cervical pessary to prevent preterm birth
Giving birth before term contributes to more than half of the deaths of newborn babies. Weakness of the cervix (the neck of the womb) and multiple pregnancy are common risk factors. Different management techniques have been tried including tightening the cervix with a stitch (cervical cerclage) to prevent its premature opening. Although it is a simple operation, cervical cerclage is invasive requiring anaesthesia and can have bleeding complications and cause infection and pregnancy loss. There is also controversy regarding the efficacy of cervical cerclage and the women who benefit most from this operation. Closing the cervix with a silicone ring (cervical pessary) that is removed at around 37 weeks is a simple, less invasive procedure that does not require anaesthesia and might replace the cervical stitch operation. To date, data obtained from one well‐designed randomised clinical trial suggest that inserting a cervical pessary is superior to expectant management in the prevention of preterm birth in 385 women between 18 and 22 weeks of pregnancy. Neonatal paediatric care admission was reduced in the pessary group in comparison to the expectant group. These women had a singleton pregnancy and high risk of preterm birth because of the short length of the neck of the womb (cervix). Among the pessary group, 27 women needed pessary repositioning without removal and there was one case of pessary removal. Results of both the randomised trial and non‐randomised trials show that pessary users complained of increased vaginal discharge. More studies are needed in different settings, with singleton and multiple pregnancies where the weakness of the cervix is from other causes, to confirm the results of the single trial included in this review. Some studies are ongoing.
Background
Description of the condition
Preterm delivery is a major health problem; it complicates about 6% to 10% of pregnancies (Lumley 2003). Spontaneous preterm delivery represents a major cause of prenatal deaths (28.7%) (Ngoc 2006). It has also been demonstrated that preterm delivery contributes to about half of the overall perinatal mortality (AIHW 2005). Premature neonates represent a large economic burden; each day in the standard neonatal intensive care can cost approximately 1000 US$ (Rogowski 1999). In developed countries,10% of expenses for treating diseases in children result from preterm delivery (Lewitt 1995).
Cervical incompetence is one of the common causes of preterm birth; however, its firm diagnosis is far from being standardised. Diagnosis is often based retrospectively on history and exclusion of other causes of preterm delivery. Typical historical risk factors include: having two or more second‐trimester pregnancy losses, especially if there is a history of losing each pregnancy at an earlier gestational age; having preterm premature rupture of membranes prior to 32 weeks' gestation; a history of cervical trauma caused by cone biopsy, forced dilatation, or intrapartum cervical lacerations; or congenital uterine anomalies (Lo 2009). Clinical examination during pregnancy revealing short cervix, dilated cervix, protruding membranes or cervical tear(s) are suggestive of cervical incompetence. Ultrasound examination during pregnancy showing short cervical length (less than 25 mm at 20 weeks' gestation) (Owen 2004) or funnelling of the cervix during the second or early third trimester of pregnancy (Ayers 1988) have been suggested to be signs of cervical incompetence.
Multiple pregnancy is a another strong risk factor for preterm birth. About one in 60 pregnancies is a twin pregnancy, and about 30% of the preterm born children admitted in a neonatal care are from twin pregnancies (Lumley 2003). Prevention of preterm birth is therefore a major goal of obstetric care of multiple pregnancy. However, strategies to prevent preterm birth in these patients have been largely unsuccessful.
Different management strategies have been tried for prevention of preterm birth due to cervical incompetence, including trials to tighten the cervix (cervical cerclage) to prevent its premature opening (Anthony 1997; Gibb 1995; McDonald 1957; Shirodkar 1955). In spite of being a simple operation, it is an invasive technique that requires anaesthesia, and has its complications including haemorrhage, infection and even pregnancy loss (Grant 1989). Moreover, cervical cerclage is not always very effective in preventing preterm birth. A systematic review by Bachmann 2003 reported that elective cerclage has a significant effect in preventing spontaneous preterm birth before 34 weeks' gestation. The number needed to be treated to prevent one additional preterm birth before 34 weeks was 24 women (95% confidence interval (CI) 10 to 61). However, it has no significant effect on preventing preterm birth between 34 and 37 weeks of pregnancy. Another systematic review concluded that the use of a cervical stitch should not be offered to women at low or medium risk of mid‐trimester loss, regardless of cervical length by ultrasound. Cervical cerclage was associated with mild pyrexia, increased use of tocolytic therapy and hospital admissions, but no serious morbidity (Drakeley 2003). A third systematic review evaluated the role of cervical cerclage for a shortened cervix, and concluded that the available evidence does not support cerclage for a sonographically detected short cervix (Belej‐Rak 2003). On the other hand, a meta‐analysis was carried out of trials of women with singleton gestations and second‐trimester transvaginal sonographic cervical length (CL) less than 25 mm randomised to cerclage or no cerclage. The degree of CL shortening was correlated to the efficacy of cerclage in preventing preterm birth.There was a significant reduction in preterm birth before 35 weeks in the cerclage group compared with no the cerclage group in 208 singleton gestations with both a previous preterm birth and CL less than 25 mm (risk ratio (RR), 0.61; 95% CI, 0.40‐0.92). In these women, preterm birth before 37 weeks was significantly reduced with cerclage for CL less than or equal to 5.9 mm, less than or equal to 15.9 mm, 16 to 24.9 mm and less than 25 mm. None of the analyses for 344 women without a previous preterm birth was significant (Berghella 2010). The same researchers reported, in another meta‐analysis, that in twins, cerclage was associated with a significantly higher incidence of preterm birth (Berghella 2005).
Description of the intervention
Cervical pessary has been tried for management of cervical incompetence since the 1950s (Cross 1959). However, its use for this purpose has passed through waves of enthusiasm and loss of favour (Acharya 2006; Antczak‐Judycka 2003; Arabin 2003; Quaas 1990). Most of the studies have used the Arabin pessary which is a flexible, ring‐like silicone pessary available in different sizes with the outer diameter varying between 65 mm and 70 mm, the inner diameter between 32 mm and 35 mm, and the height of the curvature between 21 mm and 25 mm (Figure 1). It has been designed to be inserted with its curvature upwards so that the larger diameter is supported by the pelvic floor. The smaller inner diameter is supposed to encompass the cervix (Arabin 2003) Figure 1.
1.
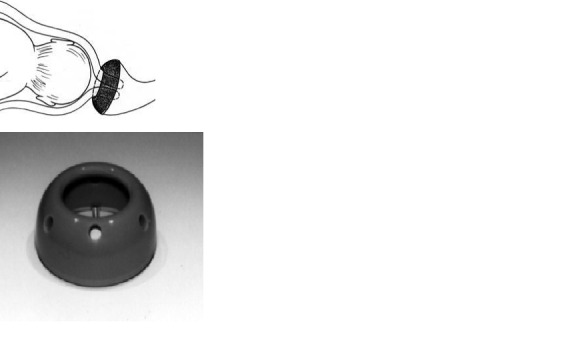
Cervical pessary in place. Images reproduced with the kind permission of Dr. Arabin GmbH & Co. KG
How the intervention might work
The mechanism by which pessaries can help women with an incompetent cervix is not known. In 1961, Vitsky suggested that the incompetent cervix is aligned centrally, with no support except the non‐resistant vagina (Vitsky 1961). A lever pessary, however, would change the inclination of the cervical canal, directing it more posteriorly. In doing so, the weight of the pregnancy would be more on the anterior lower segment (Arabin 2003). Another postulated mechanism is that the pessary might support the immunological barrier between the chorioamnion‐extraovular space and the vaginal microbiological flora as cerclage has been postulated to do (Goya 2012).
Why it is important to do this review
Cervical pessary is relatively non‐invasive, it is operator‐independent, easy to use, it does not require anaesthesia, it can be used in an outpatient clinic setting, and it is easily removed when necessary (Acharya 2006; Grzonka 2004; Newcomer 2000; Quaas 1990; Von Forster 1986). Oster et al conducted a non‐randomised trial in the USA in 1966 that involved 35 pregnant women. They used Hodge pessaries and reported 83% living children (Oster 1966). Dahl and Barz reported on 115 patients thought to have an incompetent cervix (Dahl 1979). They used a Mayer‐Ring pessary (glass ring and pushed around the cervix). Eighty per cent of women treated by pessaries gave birth to neonates more than 2500 gm. More recently, Quaas et al (Quaas 1990) reported on 107 patients using an Arabin‐cerclage pessary. The pessary was used instead of surgical cerclage prophylactically in 58 patients, in 44 cases therapeutically, and in five patients it was used instead of emergency cerclage. In 92% of the patients, the pregnancy was maintained until 36 weeks of gestation, when the Arabin‐cerclage pessary was removed. There were no infectious complications reported.
Other non‐randomised trials have shown that treating women with a short cervix with cervical pessary succeeded in prolonging the pregnancy compared to expectant management. The mean gestational age at delivery was 38 weeks (36 + 6 – 41) in the pessary group and 33 weeks (26 + 4 – 38) in the control group (P = 0.02) (Arabin 2003). In another comparative non‐randomised trial, cervical pessary was as effective as cervical cerclage in delaying the onset of labour. The primary outcome measure was prolongation of pregnancy (mean 13.4 weeks and 12.1 weeks for cerclage and pessary respectively) (P = 0.06) (Antczak‐Judycka 2003). However, the use of cervical pessary has not been assessed in a systematic way in singleton/multiple pregnancies.
Objectives
To evaluate the efficacy of cervical pessary for the prevention of preterm birth in women with risk factors for cervical incompetence.
Methods
Criteria for considering studies for this review
Types of studies
We included one randomised clinical trial that compared the use of cervical pessary with cervical cerclage or expectant management or other interventions for prevention of preterm birth. We did not include any quasi‐randomised trials (for example, randomisation by date of birth or day of admission). Cluster‐randomised or cross‐over trials were not eligible for inclusion.
Types of participants
Pregnant women with singleton/multiple viable fetus/fetuses in the second trimester of pregnancy and with risk factors for cervical incompetence. These include:
history of two or more second‐trimester pregnancy losses (excluding those resulting from induced preterm labour or abruption);
history of losing each pregnancy at an earlier gestational age;
preterm premature rupture of membranes prior to 32 weeks' gestation;
short cervical length (less than 25 mm at 20 weeks' gestation);
history of cervical trauma caused by cone biopsy, forced dilatation, intrapartum cervical lacerations;
history of painless cervical dilatation of up to 4 to 6 cm;
congenital uterine anomalies;
vaginal ultrasound evidence of cervical incompetence, including shortening (cervical length less than 25 mm at 20 weeks) and funnelling of the cervix during the second or early third trimester of pregnancy.
Types of interventions
To avoid duplication of comparisons in various reviews of interventions for preventing preterm birth, we planned to compare the intervention of interest (cervical pessary) with the following interventions.
Cervical pessary versus placebo/no treatment (singleton pregnancy).
Cervical pessary versus placebo/no treatment (multiple pregnancy).
Cervical pessary versus bedrest (singleton pregnancy).
Cervical pessary versus bedrest (multiple pregnancy).
Cervical pessary versus cervical cerclage(singleton pregnancy).
Cervical pessary versus cervical cerclage (multiple pregnancy).
Cervical pessary versus medical treatment (singleton pregnancy).
Cervical pessary versus medical treatment (multiple pregnancy).
Types of outcome measures
Primary
Delivery at less than 37 weeks' gestation.
Secondary
Maternal
Delivery at less than 34 weeks' gestation.
Delivery at less than 32 weeks' gestation.
Mean gestational age at time of delivery.
Maternal hospital admission.
Maternal medications (e.g. antibiotics, tocolytics).
Side effects of the intervention including expulsion of the pessary.
Patient’s satisfaction.
Additional costs over that of routine antenatal care.
Fetal
Neonatal paediatric care unit admission.
Perinatal death.
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (1 September 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
In addition, we searched Current Controlled Trials and the Australian New Zealand Clinical Trials Registry (September 2012), using the terms given in Appendix 1.
We did not apply any language restrictions.
Data collection and analysis
For this update, we used the following methods when assessing the reports identified by the updated search.
Selection of studies
Two review authors (O Shaaban and H Abdel‐Aleem) independently assessed for inclusion the studies resulting from the search. We resolved any disagreement through discussion with the third author (M Abdel‐Aleem).
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third author. We entered data into Review Manager software (RevMan 2011) and checked for accuracy. When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreement was resolved by discussion or by involving the third author.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We planned to assess blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We planned to assess blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups; or less than 20% losses to follow‐up);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary relative risk with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
Cluster‐randomised trials were not eligible for inclusion.
Cross‐over trials
Cross‐over trials were not eligible for inclusion.
Dealing with missing data
For the included study, we noted levels of attrition. In future updates if more studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
In future updates if more studies are included, we will assess statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We will regard heterogeneity as substantial if an I² is greater than 30% and either a T² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if 10 or more studies contribute data to meta‐analysis for any particular outcome, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess possible asymmetry visually, and if asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data for this update. In future updates, we will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analysis.
1. Women with a short cervix (25 mm or less) versus women with a cervix greater than 25 mm.
The following primary outcome will be used in subgroup analysis.
Delivery at less than 37 weeks' gestation.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2011). We will report the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
In future updates, we will carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates (greater than 20%), or both, with poor‐quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
The search retrieved 11 reports of eight trials. We included one randomised controlled trial conducted by Goya et al (Goya 2012) as it met our eligibility criteria. We excluded two other trials (Gmoser 1991; Von Forster 1986). We identified seven ongoing studies (Carreras 2008; Carreras 2011; Driggers 2011; Goya 2011; Hegeman 2009; Nicolaides 2008; Nizard 2007). For more information, seeCharacteristics of ongoing studies.
Included studies
We included one study that tested the efficacy of cervical pessary compared with expectant management in women with a short cervix of 25 mm or less (Goya 2012). In this trial, 385 pregnant women with a short cervix were assigned to the pessary (N = 192) and expectant management groups (N = 193), and 190 were analysed in each group. Analysis was by intention‐to‐treat.
Excluded studies
We excluded two studies (Gmoser 1991; Von Forster 1986). The Von Forster 1986 study was from Germany and was excluded because of unclear inclusion and exclusion criteria and the use of quasi‐randomisation (by the initial of the woman's surname). The Gmoser 1991 trial was from Austria and was excluded because of inadequate reporting on the methods in relation to randomisation, and inclusion and exclusion criteria. For more information, seeCharacteristics of excluded studies.
Risk of bias in included studies
The included study Goya 2012 is of moderate to high quality as double blinding was not possible.
Allocation
Both random sequence generation and allocation concealment were assessed as being at low risk of bias. Randomisation was performed using a computer‐generated random number table and allocation was done using central telephone randomisation.
Blinding
The study was open label and therefore at high risk of bias for blinding of participants, personnel and outcome assessors.
Incomplete outcome data
Loss to follow‐up was in the region of between 1% and 2% and therefore at low risk of attrition bias.
Selective reporting
The authors adhered to the study protocol and reported results for all specified outcomes and therefore was considered at low risk of reporting bias.
Other potential sources of bias
Baseline study characteristics are homogenous and no other sources of bias were apparent. The study was assessed as being at low risk of bias for this domain.
Effects of interventions
This review update includes one randomised controlled trial by Goya et al (Goya 2012).
Cervical pessary versus expectant management (singleton pregnancy)
Primary outcomes
The study included 385 pregnant women with a short cervix of 25 mm or less between 18 to 22 weeks of pregnancy. The use of cervical pessary (192 women) was associated with a statistically significantly decrease in the incidence of spontaneous preterm birth less than 37 weeks' gestation compared with expectant management (22% versus 59%; respectively, risk ratio (RR) 0.36; 95% confidence interval (CI) 0.27 to 0.49), Analysis 1.1.
1.1. Analysis.
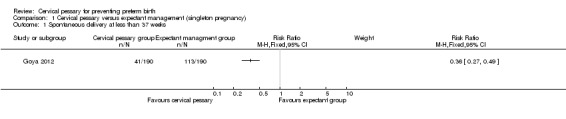
Comparison 1 Cervical pessary versus expectant management (singleton pregnancy), Outcome 1 Spontaneous delivery at less than 37 weeks.
Secondary outcomes
Spontaneous preterm birth before 34 weeks was statistically significantly reduced in the pessary group (RR 0.24; 95% CI 0.13 to 0.43), Analysis 1.2. Mean gestational age at delivery was 37.7 + 2 weeks in the pessary group and 34.9 + 4 weeks in the expectant group (mean difference (MD) 2.80 weeks; 95% CI 2.16 to 3.44), Analysis 1.3. Women in the pessary group used less tocolytics and corticosteroids than the expectant group (RR 0.63; 95% CI 0.50 to 0.81 and RR 0.66; 95% CI 0.54, 0.81 respectively), Analysis 1.4. Vaginal discharge was more common in the pessary group (RR 2.18; 95% CI 1.87 to 2.54), Analysis 1.5. Among the pessary group, 27 women needed pessary repositioning without removal and there was one case of pessary withdrawal. Ninety‐five per cent of women in the pessary group recommended this intervention to other people. Neonatal paediatric care admission was reduced in the pessary group in comparison to the expectant group (RR 0.17; 95% CI 0.07 to 0.42), Analysis 1.6.
1.2. Analysis.
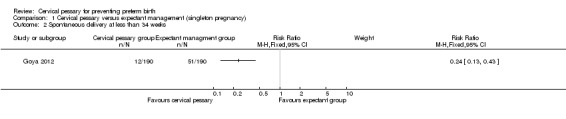
Comparison 1 Cervical pessary versus expectant management (singleton pregnancy), Outcome 2 Spontaneous delivery at less than 34 weeks.
1.3. Analysis.
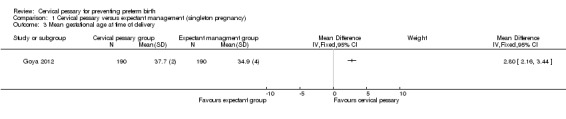
Comparison 1 Cervical pessary versus expectant management (singleton pregnancy), Outcome 3 Mean gestational age at time of delivery.
1.4. Analysis.
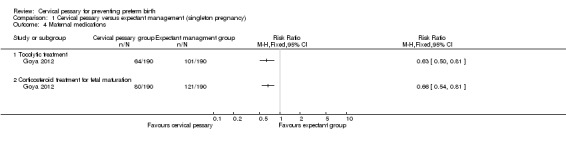
Comparison 1 Cervical pessary versus expectant management (singleton pregnancy), Outcome 4 Maternal medications.
1.5. Analysis.
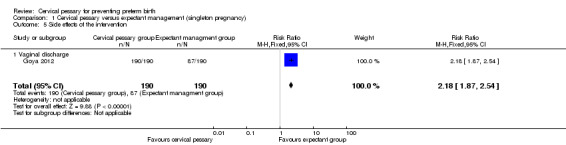
Comparison 1 Cervical pessary versus expectant management (singleton pregnancy), Outcome 5 Side effects of the intervention.
1.6. Analysis.
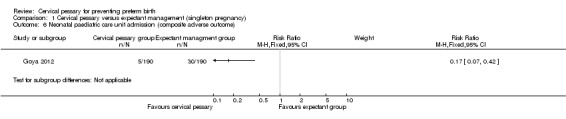
Comparison 1 Cervical pessary versus expectant management (singleton pregnancy), Outcome 6 Neonatal paediatric care unit admission (composite adverse outcome).
Discussion
Preterm birth has its major health (Lumley 2003; Ngoc 2006) and economic (Lewitt 1995; Rogowski 1999) burdens in developed and developing countries. Cervical incompetence and multiple pregnancy are blamed for a high percentage of preterm deliveries. Cervical cerclage has been administered for decades, as the only available option to prevent preterm birth in women with risk factors for cervical incompetency (Anthony 1997; Gibb 1995; Grant 1989; McDonald 1957). Falilure to identify the group of women who definitely get benefit from cervical cerclage, leads to using this invasive procedure unnecessarily in many occasions. Systematic reviews have failed to show a definite benefit from cervical cerclage in prevention of preterm birth or improving neonatal mortality for women with historical (Bachmann 2003) or ultrasonographically‐imaged short cervix as a risk factor (Belej‐Rak 2003). Berghella 2011 in a more recent meta‐analysis, concluded that in women with a previous spontaneous preterm birth, singleton gestation, and cervical length less than 25 mm, cerclage significantly prevents preterm birth and composite perinatal mortality and morbidity.
Cervical pessary, as an inexpensive and less invasive option to cervical stitch, may represent special importance to health services in low‐resource countries (Arabin 2003). Using a pessary instead of performing a cerclage operation can decrease hospital stays and costs. If a cervical pessary proves beneficial, this will definitely decrease the burden of premature delivery and care that is given to premature and extremely premature babies. Cervical pessaries have been used for prevention of preterm birth in several non‐randomised trials and shown to be effective in many of them. (Arabin 2003; Oster 1966; Quaas 1990; Seyffarth 1978; Vitsky 1968).
We assessed three randomised trials for inclusion in the current review (Gmoser 1991; Goya 2012; Von Forster 1986) Two studies were excluded. Gmoser 1991 found that cervical pessary was as effective as cerclage in the management of cervical incompetence. Pessary treatment was better at prolonging pregnancy and increasing the weight of the baby at birth, compared with no intervention (Gmoser 1991). In the second study (Von Forster 1986) both methods succeeded in prolonging pregnancy at least until 37 weeks in approximately 80% of cases (Von Forster 1986).
The only included study by Goya et al (Goya 2012) is a well‐designed multicentre trial involved 385 pregnant women. The study included women with singleton pregnancy and at high risk of preterm birth as evident by short cervix (less than 25 mm) between 18 to 22 weeks' gestation. Cervical pessary significantly decreased the incidence of spontaneous preterm birth before 37 and 34 weeks. The mean gestational age at delivery was statistically significantly higher in the pessary group in comparison to expectant group. Neonatal complications were significantly less in the pessary group. However, the incidence of preterm birth before 37 weeks and 34 weeks in the control group is high (59 % and 27%, respectively). These figures are higher than reported by the World Health Organization for the worldwide incidence of preterm birth (˜ 6.2 ‐11.8 %) with the lowest figure in Europe (6.2%)( Beck 2010). This could compromise the generalisability of the findings of this trial.
Few complications have been reported from pessary use during pregnancy. Increased vaginal discharge was complained by all pessary users in Goya 2012. Two studies have looked at changes in vaginal flora during pregnancy with pessary use. One study (Havlik 1986) compared the change in vaginal flora of 50 women wearing Mayer pessaries with 50 controls. They found that after two weeks, there were no differences in the change of flora between users and non‐users. Another study (Jorde 1983) also reported that 5.5% of women (in a cohort of 200) using pessaries had pathogenic organisms in the vagina during pregnancy, compared with 2% of controls. About half of the pessary users complained of increased vaginal discharge after the use of a cervical pessary (Arabin 2003). So, this could reflect foreign body irritation rather than infection.
Summary of main results
The review included only one randomised clinical trial of moderate to high quality. Double blinding is not possible in such type of studies (Goya 2012). The trial showed beneficial effect of pessary in reducing preterm birth in women with singleton pregnancy and a short cervix.
We also identified other ongoing randomised controlled trials using cervical pessary in pregnant women with a short cervix to prevent preterm birth in singleton and multiple pregnancy. We will assess these ongoing studies for inclusion in the next update of our review if data are available.
Authors' conclusions
Implications for practice.
There is evidence from one randomised controlled trial that using cervical pessary is superior than expectant management in prevention of preterm birth in women with a singleton pregnancy and a short cervix. Evidence for its beneficial effect in other settings and for other groups of patients is not yet documented.
Implications for research.
There is a need for more well‐designed randomised controlled trials to confirm the beneficial effect of cervical pessary in reducing preterm birth in women with a short cervix in different settings and in women with other risk factors for preterm birth including multiple pregnancy.
What's new
| Date | Event | Description |
|---|---|---|
| 15 February 2018 | Amended | Updated the Published notes to explain that this review will no longer be updated in it's current form. The review will be split into two new reviews following new protocols. |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 9, 2010
| Date | Event | Description |
|---|---|---|
| 26 September 2012 | New citation required and conclusions have changed | One new trial included. Previous published version did not include any trials. |
| 1 September 2012 | New search has been performed | Scope of the review has been widened to include use of cervical pessary in multiple pregnancy. Search updated. Methods updated. |
Notes
This Cochrane Review will no longer be updated in it's current form. The review will be split into two separate Cochrane reviews (one for singleton pregnancies/multiple pregnancies). The two new reviews will be prepared following new protocols.
Acknowledgements
Thanks to Emma Wood for help with translating Gmoser 1991.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Searching trials registry websites
We searched Current controlled trials and Australian and New Zealand Clinical Trials Registry (1 September 2012), using the terms pessary or pessaries.
Data and analyses
Comparison 1. Cervical pessary versus expectant management (singleton pregnancy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Spontaneous delivery at less than 37 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Spontaneous delivery at less than 34 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Mean gestational age at time of delivery | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Maternal medications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Tocolytic treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Corticosteroid treatment for fetal maturation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Side effects of the intervention | 1 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.87, 2.54] |
| 5.1 Vaginal discharge | 1 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.87, 2.54] |
| 6 Neonatal paediatric care unit admission (composite adverse outcome) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Perinatal death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
1.7. Analysis.
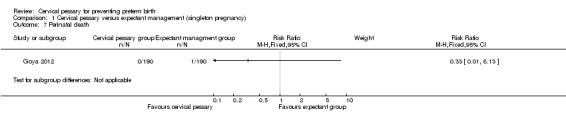
Comparison 1 Cervical pessary versus expectant management (singleton pregnancy), Outcome 7 Perinatal death.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Goya 2012.
| Methods | Open‐label, randomised controlled trial. | |
| Participants | Women with cervical length < 25 mm between 18‐22 weeks' gestation. | |
| Interventions | Arabin cervical pessary versus expectant management. | |
| Outcomes |
Primary outcome: delivery before 34 weeks' gestation. Secondary outcomes: spontaneous delivery before 37 weeks; gestational age at delivery (weeks); tocolytic treatment; corticosteroid treatment for fetal maturation; chorioamnionitis; pregnancy bleeding; premature preterm rupture of membranes; caesarean delivery; Side‐effects: (vaginal discharge, pessary repositioning without removal , pessary withdrawal); Perinatal outcome: (fetal death, neonatal death, birthweight less than 1500 g, birthweight less than 2500 g); Adverse outcomes: (necrotising enterocolitis; intraventricular haemorrhage; respiratory distress syndrome; retinopathy; treatment for sepsis; composite adverse outcomes). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random table. |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up rate is between 1% and 2%. |
| Selective reporting (reporting bias) | Low risk | The authors adhered to the study protocol. |
| Other bias | Low risk | Baseline study characteristics are homogenous. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gmoser 1991 |
This study was carried out by Gmoser and colleague, conducted in Austria and published in 1991 (Gmoser 1991). The investigators compared length of gestation and the weight at birth, between women treated with cervical pessary versus those with no intervention. The study included 300 cases. They found that the length of gestation and birthweight were higher in the pessary group compared with no intervention (39 versus 36 weeks and 2950 versus 2400 g, respectively). They did not reach a solid conclusion from their study (Gmoser 1991). |
| Von Forster 1986 |
The study was carried out by Von Forster and colleagues in Germany. It was a prospective randomised trial conducted between 1982 and 1983. In this study, patients were randomised into 3 groups based on the initial letter of their surname (quasi‐randomised). Patients in Group 1 were admitted as in‐patients and received cerclage (n = 112). Those in Group 2 (n = 130) were fitted with a pessary as outpatients. A third group was simply ordered to rest in bed. The type of pessary used was not mentioned. The therapies were used in patients for prophylactic as well as therapeutic reasons, although these terms are not defined in the article. However, at the analysis stage the investigators only analysed the intervention groups because all patients in the rest only group did need treatment. The results reported that the 2 groups were equal in the length of pregnancy (mean 37 to 38 weeks), birthweight (mean 3000 g), Apgar scores, and fetal survival. They investigators concluded that cerclage and pessary were equally effective in the management of cervical incompetence (Von Forster 1986). |
Characteristics of ongoing studies [ordered by study ID]
Carreras 2008.
| Trial name or title | Prevention of Preterm Birth Using Cervical Pessary in Pregnant Women After Threatened Preterm Labor (PECEP‐RETARD). |
| Methods | Multicentre, randomised, open‐label trial. |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Placement of cervical pessary (Arabin Pessary) between 23 weeks and 37 weeks. |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | June 2008. |
| Contact information | Elena Carreras 934893072 ecarrera@vhebron.net |
| Notes | NCT01242384. |
Carreras 2011.
| Trial name or title | Arabin Cervical Pessary for Prevention of Preterm Birth in Cases of Twin‐to‐twin Transfusion Syndrome Treated by Fetoscopic Selective Laser Coagulation: The PECEP Laser Trial. |
| Methods | Multicentre, randomised controlled trial. |
| Participants | Inclusion criteria
Exclusion criteria
Silicone allergy. |
| Interventions |
No Intervention: usual management of monochorionic pregnancy without the pessary placement. Experimental: pessary. The pessary will be inserted 24 hours after fetal surgery in the exploration room. The pessary will be removed at 37 weeks of gestation, or before if any unexpected event occurs. |
| Outcomes |
Primary outcome
Requirement of hospitalisation due to preterm contractions that need medical treatment to try to stop them before 33 + 6 weeks. |
| Starting date | April 12 2011. |
| Contact information | Carlota Rodo, MD 0034‐934893072 carlotarodo@gmail.com Elena Carreras, PhD 0034‐934893072 ecarreras@vhebron.net |
| Notes | ClinicalTrials.gov Identifier: NCT01334489. |
Driggers 2011.
| Trial name or title | Preventing Preterm Birth With a Pessary (PrePPy). |
| Methods | Multicentre, randomised controlled trial. |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Cervical pessary or no intervention. |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | January 2011. |
| Contact information | Rita W. Driggers, MD rita.w.driggers@medstar.net |
| Notes | NCT01380158. |
Goya 2011.
| Trial name or title | Prevention of Preterm Birth Using Cervical Pessary in Pregnant Women With Short Cervix in Twins (PECEP‐TWINS). |
| Methods | Randomised, open‐label, placebo‐controlled trial. |
| Participants | Inclusion criteria
Exclusion criteria
Placenta previa. |
| Interventions |
Cervical pessary (Arabin Cervical Pessary) since 23 weeks until 37 weeks: experimental. |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | September 28 2010. |
| Contact information | Hospital Vall d'Herbron Barcelona, Spain, 08036 Contact: Maria M Goya 934893185 mariagoya@mac.com |
| Notes | NCT01242410. |
Hegeman 2009.
| Trial name or title | Pessaries in multiple pregnancy as a prevention of preterm birth: the Pro Twin Trial. |
| Methods | Multicentre, randomised, open‐label trial. |
| Participants | Women with multiple pregnancy between 12 and 19 weeks of gestation. They will also include women with monochorionic pregnancies as well as women with triplet pregnancy or women with previous preterm birth. |
| Interventions | Cervical pessary (Arabin pessary) or no intervention. |
| Outcomes |
Primary outcome measures The composite morbidity rate of children in the 2 groups. Secondary outcomes
|
| Starting date | 1 September 2009. |
| Contact information | Prof. B.W.J. Mol e mail: b.w.mol@amc.nl |
| Notes | NTR1858. |
Nicolaides 2008.
| Trial name or title | Randomised study of pessary vs standard management in women with increased chance of premature birth. |
| Methods | Multicentre, randomised, open‐label trial. |
| Participants | Women with singleton pregnancies and with a cervical length of 25 mm or less. Women with twin pregnancies. |
| Interventions | Vaginal pessary (CE0482, MED/CERT ISO 9003 / EN 46003) versus standard management. |
| Outcomes |
Primary outcome
Seconday outcomes
|
| Starting date | . |
| Contact information | Prof, Kypros Nicolaides Tel: +442032999000 ext.: 8256 kypros@fetalmedicine.com |
| Notes | ISRCTN01096902. |
Nizard 2007.
| Trial name or title | Evaluation of pessaries in twin pregnancies with a short cervix (25 mm) between 20‐28 WG. |
| Methods | Randomised, open‐label, multicentre study. |
| Participants | |
| Interventions | Device: silicon ring positioned in the vagina, around the cervix versus control. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | June 2007. |
| Contact information | Jacky Nizard Hopital POISSY‐ST GERMAIN EN LAYE Poissy 78300 Status: Recruiting Contact: Jacky NIZARD, CCA Tel: +33(0) 1 39 27 40 50 jnizard@gmail.com |
| Notes | NCT00502190. |
IVH: intraventricular haemorrhage RDS: respiratory distress syndrome SROM: spontaneous rupture of membranes TTTS: twin‐to‐twin transfusion vs: versus WG: weeks gestation
Differences between protocol and review
We have updated the methods section in accordance with current guidelines. We searched the databases for registering clinical trials, to identify ongoing trials.
Contributions of authors
Hany Abdel‐Aleem is the guarantor of the review. He is responsible for conceiving the review; designing and co‐ordinating the review. Omar M Shaaban wrote the first draft of the review, assessed the trials retrieved from the search and shared in writing the final version of the review. Mahmoud Abdel‐Aleem participated in reviewing the updated review.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Goya 2012 {published data only}
- Goya M, Pratcorona L, Merced C, Rodo C, Valle L, Romero A, et al. Cervical pessary in pregnant women with a short cervix (PECEP): an open‐label randomised controlled trial.[Erratum appears in Lancet. 2012 May 12;379(9828):1790]. Lancet 2012;379(9828):1800‐6. [DOI] [PubMed] [Google Scholar]
- Moratonas EC. Prevention of preterm birth using cervical pessary in pregnant women with short cervix (PECEP). http://clinicaltrials.gov/ct2/show/NCT00706264 (accessed 3 February 2010) 2007.
References to studies excluded from this review
Gmoser 1991 {published data only}
- Gmoser G, Girardi F, Mayer HO, Hermann J, Haas J. The support pessary‐‐a therapeutic possibility in premature opening of the uterine cervix. Gynakologische Rundschau 1991;31 Suppl:117‐9. [PubMed] [Google Scholar]
Von Forster 1986 {published data only}
- Forster F, During R, Schwarzlos G. Treatment of cervix incompetence ‐ cerclage versus pessary?. Zentralblatt fur Gynakologie 1986;108:230‐7. [PubMed] [Google Scholar]
References to ongoing studies
Carreras 2008 {published data only}
- Carreras E. Prevention of preterm birth using cervical pessary in pregnant women after threatened preterm labor. http://clinicaltrials.gov/ct2/show/record/NCT01242384 (accessed 25 May 2012) 2008.
Carreras 2011 {published data only}
- Carreras E. Arabin cervical pessary for prevention of preterm birth in cases of twin‐to‐twin transfusion syndrome treated by fetoscopic selective laser coagulation: the PECEP laser trial. http://clinicaltrials.gov/ct2/show/record/NCT01334489 (accessed 5 June 2012) 2011. [DOI] [PMC free article] [PubMed]
Driggers 2011 {published data only}
- Driggers RW. Preventing preterm birth with a pessary (PrePPy). http://clinicaltrials.gov/ct2/show/record/NCT01380158 (accessed 5 June 2012) 2011.
Goya 2011 {published data only}
- Goya MM. Prevention of preterm birth using cervical pessary in pregnant women with short cervix in twins (PECEP‐TWINS). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 15 February 2011) 2011.
Hegeman 2009 {published data only}
- Hegeman MA, Bekedam DJ, Bloemenkamp KW, Kwee A, Papatsonis DN, vander Post JA, et al. Pessaries in multiple pregnancy as a prevention of preterm birth: the ProTwin Trial. BMC Pregnancy and Childbirth 2009;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicolaides 2008 {published data only}
- Nicolaides K. Randomized study of pessary vs standard management in women with increased chance of premature birth. http://www.controlled‐trials.com/ISRCTN01096902 (accessed 2010).
Nizard 2007 {published data only}
- Nizard J. Evaluation of pessaries in twin pregnancies with a short cervix (25 mm) between 20‐28 WG. http://www.controlled‐trials.com/mrct/trial/407673/pessary (accessed 15 May 2010).
Additional references
Acharya 2006
- Acharya G, Eschler B, Grønberg M, Hentemann M, Ottersen T, Maltau JM. Noninvasive cerclage for the management of cervical incompetence: a prospective study. Archives of Gynecology and Obstetrics 2006;273(5):283‐7. [DOI] [PubMed] [Google Scholar]
AIHW 2005
- Laws PJ, Sullivan EA. Australia's mothers and babies 2003: perinatal statistics series number 16. Sydney: AIHW National Perinatal Statistics Unit, 2005. [Google Scholar]
Antczak‐Judycka 2003
- Antczak‐Judycka A, Sawicki W, Spiewankiewicz B, Cendrowski K, Stelmachów J. Comparison of cerclage and cerclage pessary in the treatment of pregnant women with incompetent cervix and threatened preterm delivery. Ginekologia Polska 2003;74(10):1029‐36. [PubMed] [Google Scholar]
Anthony 1997
- Anthony GS, Walker RG, Cameron AD, Price JL, Walker JJ, Calder AA, et al. Trans‐abdominal cervico‐isthmic cerclage in the management of cervical incompetence. European Journal of Obstetrics & Gynecology and Reproductive Biology 1997;72:127‐30. [DOI] [PubMed] [Google Scholar]
Arabin 2003
- Arabin B, Halbesma JR, Vork F, Hübener M, Van‐Eyck J. Is treatment with vaginal pessaries an option in patients with a sonographically detected short cervix. Journal of Perinatal Medicine 2003;31:122‐33. [DOI] [PubMed] [Google Scholar]
Ayers 1988
- Ayers JW, DeGrood RM, Compton AA, Barclay M, Ansbacher R. Sonographic evaluation of cervical length in pregnancy: diagnosis and management of preterm clinical effacement in patients at risk for premature delivery. Obstetrics & Gynecology 1988;71:939‐44. [PubMed] [Google Scholar]
Bachmann 2003
- Bachmann LM, Coomarasamy A, Honest H, Khan KS. Elective cervical cerclage for prevention of preterm birth: a systematic review. Acta Obstetricia et Gynecologica Scandinavica 2003;82:398‐404. [DOI] [PubMed] [Google Scholar]
Beck 2010
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization 2010;88:31‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Belej‐Rak 2003
- Belej‐Rak T, Okun N, Windrim R, Ross S, Hannah ME. Effectiveness of cervical cerclage for a sonographically shortened cervix: a systematic review and meta‐analysis. American Journal of Obstetrics and Gynecology 2003;189:1679‐87. [DOI] [PubMed] [Google Scholar]
Berghella 2005
- Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: meta‐analysis of trials using individual patient‐level data. Obstetrics and Gynecology 2005;106:181‐9. [DOI] [PubMed] [Google Scholar]
Berghella 2010
- Berghella V, Keeler SM, To MS, Althuisius SM, Rust OA. Effectiveness of cerclage according to severity of cervical length shortening: a meta‐analysis. Ultrasound in Obstetrics and Gynecology 2010;35(4):468‐73. [DOI] [PubMed] [Google Scholar]
Berghella 2011
- Berghella V, Rafael TJ, Szychowski JM, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: a meta‐analysis. Obstetrics and Gynecology 2011;117:663‐71. [DOI] [PubMed] [Google Scholar]
Cross 1959
- Cross RG. Treatment of habitual abortion due to cervical incompetence. Lancet 1959;2:127. [Google Scholar]
Dahl 1979
- Dahl J, Barz MS. Prevention of premature labor by means of supporting pessaries (1st experiences). Zeitschrift für Ärztliche Fortbildung 1979;73:1010‐1. [PubMed] [Google Scholar]
Drakeley 2003
- Drakeley AJ, Roberts D, Alfirevic Z. Cervical stitch (cerclage) for preventing pregnancy loss in women. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003253] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibb 1995
- Gibb DMF, Salaria DA. Transabdominal cervico‐isthmic cerclage in the management of recurrent second trimester miscarriage and pre‐term delivery. British Journal of Obstetrics and Gynaecology 1995;102:802‐6. [DOI] [PubMed] [Google Scholar]
Grant 1989
- Grant A. Cervical cerclage to prolong pregnancy. In: Chalmers I, Enkin M, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. Oxford University Press, 1989. [Google Scholar]
Grzonka 2004
- Grzonka DT, Kazmierczak W, Cholewa D, Radzioch J. Herbich cervical pessary‐‐method of therapy for cervical incompetence and prophylaxis of prematurity. Wiadomosci Lekarskie 2004;57 Suppl 1:105‐7. [PubMed] [Google Scholar]
Havlik 1986
- Havlík I, Stasek K, Franek B, Havlíková S. Vaginal flora during supportive therapy using a pessary in pregnancy. Ceskoslovenská Gynekologie 1986;51:258‐9. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jorde 1983
- Jorde A, Kästli K, Hamann B, Pockrandt H. Changes in the vaginal flora caused by supporting pessary treatment in pregnancy. Zentralblatt fur Gynakologie 1983;105(13):855‐7. [PubMed] [Google Scholar]
Lewitt 1995
- Lewitt EM, Baker LS, Corman H, Shiono PH. The direct cost of low birthweight. The Future of Children. Los Altos, CA: David and Lucile Packard Foundation, 1995:35‐56. [PubMed] [Google Scholar]
Lo 2009
- Lo C. The incompetent cervix. O&G Magazine 2009;11(2):30‐2. [Google Scholar]
Lumley 2003
- Lumley J. Defining the problem: the epidemiology of preterm birth. BJOG: an international journal of obstetrics and gynaecology 2003;110 Suppl 20:3‐7. [PubMed] [Google Scholar]
McDonald 1957
- McDonald IA. Suture of the cervix for inevitable miscarriage. Journal of Obstetrics and Gynaecology of the British Commonwealth 1957;64:346‐53. [DOI] [PubMed] [Google Scholar]
Newcomer 2000
- Newcomer J. Pessaries for the treatment of incompetent cervix and premature delivery. Obstetrical and Gynecological Survey 2000;55(7):443‐8. [DOI] [PubMed] [Google Scholar]
Ngoc 2006
- Ngoc NT, Merialdi M, Abdel‐Aleem H, Carroli G, Purwar M, Zavaleta N, et al. Causes of stillbirths and early neonatal deaths: data from 7993 pregnancies in six developing countries. Bulletin of the World Health Organization 2006;84:699‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oster 1966
- Oster S, Javert CT. Treatment of the incompetent cervix with Hodge pessary. Obstetrics & Gynecology 1966;28:206‐8. [DOI] [PubMed] [Google Scholar]
Owen 2004
- Owen J, Yost N, Berghella V, MacPherson C, Swain M, Dildy GA 3rd, et al. Can shortened midtrimester cervical length predict very early spontaneous preterm birth?. American Journal of Obstetrics and Gynecology 2004;191:298‐303. [DOI] [PubMed] [Google Scholar]
Quaas 1990
- Quaas L, Hillemanns HG, du Bois A, Schillinger H. The Arabin cerclage pessary‐‐an alternative to surgical cerclage. Geburtshilfe und Frauenheilkunde 1990;50(6):429‐33. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rogowski 1999
- Rogowski, J. Measuring the cost of neonatal and perinatal care. Pediatrics 1999;103 (1 Suppl E):329‐35. [PubMed] [Google Scholar]
Seyffarth 1978
- Seyffarth K. Noninvasive cerclage using support pessaries for prevention and therapy of premature birth. Zentralblatt fur Gynakologie 1978;100:1566‐70. [PubMed] [Google Scholar]
Shirodkar 1955
- Shirodkar VN. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic 1955;52:299‐300. [Google Scholar]
Vitsky 1961
- Vitsky M. Simple treatment of the incompetent cervical os. American Journal of Obstetrics and Gynecology 1961;81:1194‐7. [DOI] [PubMed] [Google Scholar]
Vitsky 1968
- Vitsky M. Pessary treatment of the incompetent cervical os. Obstetrics and Gynecology 1968;31:732‐3. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Abdel‐Aleem 2009
- Abdel‐Aleem H, Shaaban OM, Abdel‐Aleem MA. Cervical pessary for preventing preterm birth. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007873] [DOI] [Google Scholar]
Abdel‐Aleem 2010
- Abdel‐Aleem H, Shaaban OM, Abdel‐Aleem MA. Cervical pessary for preventing preterm birth. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007873.pub2] [DOI] [PubMed] [Google Scholar]


