Abstract
Background
Diagnoses of endometrial cancer are increasing secondary to the rising prevalence of obesity. Obesity plays an important role in promoting the development of endometrial cancer, by inducing a state of unopposed oestrogen excess, insulin resistance and inflammation. It also affects treatment, increasing the risk of surgical complications and the complexity of radiotherapy planning, and may additionally impact on subsequent survival. Weight‐loss interventions have been associated with improvements in breast and colorectal cancer‐specific survival as well as a reduction in the risk of cardiovascular disease, a frequent cause of death in endometrial cancer survivors.
Objectives
To determine the impact of weight‐loss interventions, in addition to standard management of endometrial cancer, on overall survival and the frequency of adverse events.
Secondary objectives include an assessment of weight‐loss interventions on endometrial cancer‐specific survival, weight loss achieved, cardiovascular event frequency and quality of life both overall and stratified according to patient body mass index (BMI), where possible.
Search methods
This review searched Cochrane Central Register of Controlled Trials, MEDLINE, Embase and reference lists of articles, trial registries, and international gynaecological oncology conference abstracts from inception to January 2018.
Selection criteria
Randomised controlled trials (RCTs) of interventions to facilitate weight loss in overweight or obese women undergoing treatment for, or previously treated for, endometrial cancer were selected.
Data collection and analysis
Two review authors independently selected studies, assessed trial quality, and extracted data with disagreements resolved by a third review author. Study authors were contacted to obtain missing data, including details of any adverse events.
Main results
We included three RCTs in the review, randomising a total of 161 overweight and obese women with endometrial cancer. All studies compared combined behavioural and lifestyle interventions to facilitate weight loss through dietary modification and increased physical activity. The included RCTs were of low or very low quality, due to high risk of bias by failing to blind participants, personnel and outcome assessors, and significant loss to follow‐up (attrition rate up to 29%).
Combined behaviour and lifestyle interventions were not associated with improved overall survival (risk ratio (RR mortality), 0.23 95% confidence interval (CI) 0.01 to 4.55, P = 0.34, one RCT, 37 participants; very low‐certainty evidence) compared with usual care at 24 months. There was no evidence that such interventions were associated with improvements in cancer‐specific survival or cardiovascular event frequency as no cancer‐related deaths, myocardial infarctions or strokes were reported in the included studies. None of the included RCTs reported data for the outcome of recurrence‐free survival. Combined behaviour and lifestyle interventions were not associated with significant weight loss at either six months (mean difference (MD) ‐1.88 kg, 95% CI ‐5.98 to 2.21 kg, P = 0.37, three RCTs, 131 participants, I2= 0%; low‐certainty evidenc e)or 12 months (MD ‐8.98 kg, 95% CI ‐19.88 to 1.92 kg, P = 0.11, two RCTs, 91 participants, I2= 0%; very low‐certainty evidence) when compared with usual care. Combined behaviour and lifestyle interventions were not associated with increased quality of life, when measured using either the SF‐12 Physical Health questionnaire or FACT‐G at six months (FACT‐G MD 2.51, 95% CI ‐5.61 to 10.64, P = 0.54, two RCTs, 95 participants, I2= 83%; very low‐certainty evidence), or by FACT‐G alone at 12 months (MD 2.77, 95% CI ‐0.65 to 6.20, P = 0.11, two RCTs, 89 participants, I2= 0%; very low‐certainty evidence) when compared with usual care. No serious adverse events, for example hospitalisation or deaths, were reported in included trials. Lifestyle and behavioural interventions were associated with a higher risk of musculoskeletal symptoms (RR 19.03, 95% CI 1.17, 310.52, P = 0.04, two RCTs, 91 participants; low‐certainty evidence).
Authors' conclusions
There is currently insufficient high‐quality evidence to determine the effect of combined lifestyle and behavioural interventions on survival, quality of life, or significant weight loss in women with a history of endometrial cancer compared to those receiving usual care. The limited evidence suggests that there is little or no serious or life‐threatening adverse effects due to these interventions, although musculoskeletal problems were increased, presumably due to increased activity levels. Our conclusion is based on low‐ and very low‐quality evidence from a small number of trials and very few patients. We therefore have very little confidence in the evidence: the true effect of weight‐loss interventions in obese women with endometrial cancer is currently not known.
Further methodologically‐rigorous, adequately‐powered RCTs are required with follow‐up of 5 to 10 years duration. These should focus on the effects of varying dietary modification regimens, pharmacological treatments associated with weight loss and bariatric surgery on survival, quality of life, weight loss and adverse events.
Plain language summary
Weight‐loss interventions in endometrial cancer survivors
Background Endometrial or womb cancer is a common cancer in women and the number of cases is rising. This is due, in part, to increasing levels of obesity, which is a major risk factor for the disease. Whilst survival following endometrial cancer is generally excellent if diagnosed early, affected women are more likely to die early due to an increased risk of heart attacks and strokes and to have poorer quality of life. This review assessed the evidence for weight‐loss interventions in overweight and obese endometrial cancer survivors to determine whether they were of benefit compared with usual care.
Study characteristics We included three randomised controlled trials in which women were allocated at random to receive one of several interventions (treatments) and which involved 161 obese participants. The trials were conducted in the USA and the UK. All compared lifestyle advice (diet and exercise) plus self‐help techniques (to encourage adherence to the advice) with usual care. The evidence is current to January 2018.
Key results We found no benefit for endometrial cancer survivors from receiving lifestyle advice in terms of survival, cardiovascular events or quality of life, though such interventions were not associated with significant or serious harms to participants. They did, however, report higher rates of musculoskeletal symptoms, presumably due to increases in physical activity. Whilst some women lost weight with these interventions, others did not, meaning that overall there was little or no benefit.
Quality of the evidence The quality of included studies was, however, low or very low and all were small in terms of the number of participants and not designed to specifically look at the effect of their intervention on survival. Additional high‐quality studies are required in this field and currently there are five ongoing trials.
Summary of findings
Summary of findings for the main comparison. Lifestyle intervention versus usual care compared to placebo for weight reduction in obesity to improve survival in women with endometrial cancer.
| Lifestyle intervention versus usual care compared to placebo for weight reduction in obesity to improve survival in women with endometrial cancer | ||||||
| Patient or population: weight reduction in obesity to improve survival in women with endometrial cancer Setting: university hospitals in the USA Intervention: Lifestyle intervention versus. usual care Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Lifestyle intervention versus. usual care | |||||
| Overall survival (24 months) (Number of deaths from any cause) |
100 per 1000 | 23 per 1000 (1 to 455) | RR 0.23 (0.01 to 4.55) | 37 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 4 | Risk ratio for mortality calculated |
| Adverse events‐musculoskeletal (Number of musculoskeletal adverse events reported) |
0 per 1000 | 0 per 1000 (0 to 0) | RR 19.03 (1.17 to 310.52) | 91 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 5 6 7 | Unable to calculate assumed and corresponding risk as no events in control groups |
| Recurrence‐free survival (24 months) (Number of cases of disease recurrence or death) |
See comment | See comment | ‐ | ‐ | ‐ | No RCTs reported this outcome |
| Cancer‐specific survival (24 months) (Number of cancer‐related deaths) |
See comment | See comment | not estimable | 37 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 8 | Unable to calculate risk ratio for mortality as no cancer related deaths reported in either arm of the study |
| Weight loss (12 months) (Change in weight from baseline in kg; positive values = weight gain, negative values = weight lost) |
The mean weight loss (12 months) was + 1.5kg12 | MD 8.98 lower (19.88 lower to 1.92 higher) | ‐ | 91 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 9 10 | |
| Cardiovascular and metabolic event frequency (12 months) (Number of strokes, myocardial infarctions and hospitalisations for heart failure) |
See comment | See comment | ‐ | 93 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 8 9 | Unable to perform meta‐analysis as no events recorded in any study |
| Quality of life FACT‐G (12 months) (Change in QOL on FACT‐G questionnaire from baseline; positive values = improved QOL, negative values = worsening QOL) |
The mean quality of life FACT‐G (12 months) ranged from 0 to + 2 units13 | MD 2.77 units higher (0.65 lower to 6.20 higher) | ‐ | 89 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 9 11 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Although participants, personnel and outcome assessors were not blinded to treatment group allocation this is unlikely to affect this specific outcome measure
2 Downgraded by one point as included study at high risk of attrition bias due to incomplete outcome reporting
3 Downgraded by one point due to indirect results (included study contained two patients who, in addition to receiving the intervention, underwent gastric bypass during follow‐up and were included in the final analysis)
4 Downgraded by one point due to imprecision as low event number in included study and wide confidence intervals
5 Downgraded by one point as two of the included studies were at high risk of attrition bias due to incomplete outcome reporting
6 Downgraded by one point due to imprecision as no events in control arms of included studies and wide confidence intervals
7 One of the included studies contained two patients who, in addition to receiving the intervention, had undergone gastric bypass during follow‐up. This was not felt to impact on the number of adverse musculoskeletal events experienced and, therefore, the study was not downgraded for this reason.
8 Downgraded by one point due to imprecision as no events in any study
9 Downgraded by one point due to indirect results (one of the included studies contained one patient, who by this time, had undergone a gastric bypass in addition to receiving the specific study intervention and was included in the final analysis)
10 Downgraded by one point due to imprecision as wide confidence intervals in all included studies, which cross the line of unity
11 Downgraded by one point due to high risk of performance and detection bias in all included studies. Participants, personnel and outcome assessors were unblinded to treatment group allocation, which may have affected the subjective results
12The assumed (control) risk is the median weight change from baseline among the control groups in the included studies
13The assumed (control) risk is the range of scores for change in QOL from baseline at 12 months in the control groups from the included studies, presented in preference to the median change score due to significant variation
Background
Description of the condition
Endometrial cancer is a cancer of the lining of the womb and is the fourth most common cancer in women in the developed world (Cancer Research UK 2014a). Each year, 9000 new cases of endometrial cancer are diagnosed in the UK, and 60,000 in the USA (Cancer Research UK 2014a; NCI 2016). The incidence of the disease has doubled in the last 20 years, and this trajectory is expected to continue. Endometrial cancer has a generally good prognosis if diagnosed early, with eight out of 10 women still alive at five years after diagnosis (Cancer Research UK 2014b). With more women than ever surviving initial treatment for endometrial cancer, interventions aimed at reducing the risk of disease recurrence and optimising general health in the long term (at least 5 to 10 years following diagnosis) are required.
Endometrial cancer has a strong link with obesity and it is this relationship that is thought to underpin the rising number of cases (Renehan 2008). As the percentage of the female population who are obese has increased, so has the number of diagnoses of endometrial cancer. Three biological mechanisms, or themes, have been proposed to explain this association: unopposed oestrogen, insulin resistance, and the presence of an inflammatory milieu (tumour environment).
Oestrogen is a potent stimulator of endometrial cell proliferation or turnover, an effect that is normally counteracted by progesterone during the menstrual cycle. Unopposed oestrogen occurs in two different scenarios; if progesterone levels are low because of absent ovulation (anovulation), such as in polycystic ovary syndrome, or if oestrogen levels exceed progesterone levels. This occurs in obese postmenopausal women, when the ovaries no longer produce progesterone, but testosterone, secreted by the ovaries and adrenal glands, is converted into oestrogen by excess fat (adipose) tissue. Unopposed oestrogen is associated with an increased risk of endometrial cancer. It increases the rate of turnover of endometrial cells and thus the chance of acquiring alterations (mutations) within key genes associated with cancer development. Epidemiological studies have confirmed an increased risk of endometrial cancer in women with high oestrogen levels (Dossus 2013).
Insulin is also able to stimulate endometrial cell proliferation, activating many of the pathways shown to be critical to endometrial cancer development. Obese women have higher insulin levels than their normal‐weight counterparts; excess fat tissue reduces the responsiveness of the body to the effects of insulin, so levels increase to compensate. Elevated serum insulin levels have been shown to be present in women with endometrial cancer, compared with those without the disease (Dossus 2013).
Thirdly, fat tissue produces inflammatory and carcinogenic (cancer promoting) proteins, hence obese women have elevated levels compared with normal‐weight women. Any, or all of these proteins, may be responsible for the increase in endometrial cancer rates seen in this population (Dossus 2013).
Obesity plays an important role in promoting the development of endometrial cancer, and potentially affects treatment and subsequent survival. The mainstay of treatment for endometrial cancer is surgery to remove the uterus (womb), cervix, fallopian tubes and ovaries. This may be followed by radiotherapy, chemotherapy or both in some women. Obese women often have other health problems, including diabetes and sleep apnoea, which can adversely affect their medical fitness to undergo an operation, and increase the risk of complications associated with surgery and radiotherapy. This may lead to compromises in treatment (Papadia 2006). There is debate in the literature as to whether being overweight or obese has a negative impact on survival. Results from two large cohort studies, in which groups of women with endometrial cancer were followed up, have suggested that obese women, with a body mass index (BMI) of 30 or more, are twice as likely to die during this period as women of a healthy weight. This increases to a six‐fold elevation in risk if their BMI is over 40 (Calle 2003; Reeves 2007). However, these studies did not take into account differences in the cancer grade (how abnormal the cells appeared), stage (how far the disease had spread), or the type of treatment received.
When women with endometrial cancer received standardised treatment in the context of a randomised controlled trial (RCT), researchers were able to demonstrate that BMI had no impact on the risk of recurrence or overall survival. This was despite a high proportion of obese women having poorer general health (Crosbie 2012). The extra deaths observed in obese women with endometrial cancer may well be unrelated to their cancer. Women with early stage disease are twice as likely to die from cardiovascular disease, for example heart attacks and strokes, as they are to die from their endometrial cancer (Ward 2012). Excessive weight gain following diagnosis, and indeed, significant weight loss, may be more important than body mass per se. Data from observational studies demonstrate that large weight gains have a detrimental effect on survival, even after adjustment for other factors that influence prognosis, such as cancer grade and stage (El‐Safadi 2012; Matsuo 2016). Therefore, measures taken to reduce body weight following treatment for endometrial cancer may be beneficial in improving survival, either by reducing the risk of death from endometrial cancer, or by lowering the chance of dying from other causes, in particular cardiovascular disease.
Description of the intervention
This review focused on interventions designed to promote weight loss as their primary goal, and includes non‐pharmacological, pharmacological, and surgical interventions. These may be used alone, or in combination. Non‐pharmacological or 'lifestyle' interventions are those aimed at reducing nutrient intake and increasing physical activity, through diet and exercise, and may be used alongside psychological interventions such as stress management, stimulus control, and problem solving (addressing barriers to adhering to diet and exercise regimens) to induce permanent changes in behaviour. Pharmacological interventions include drugs that act to either reduce fat absorption, the most widely used of which is orlistat, or suppress appetite. Bariatric surgery encompasses procedures designed to limit food intake (e.g. gastric banding), cause malabsorption (e.g. intestinal bypass), or both (e.g. gastric bypass; Figuls 2013).
How the intervention might work
Weight‐loss interventions may improve survival by influencing any, or all of the pathways described above that link obesity and endometrial cancer, and have already been shown to be beneficial for survivors of other obesity‐related cancers, including breast and colorectal cancer (Morey 2009; Rock 2015; Stolley 2009). Like endometrial cancer, breast cancer also appears to be hormonally driven, and weight‐loss interventions that have been associated with a loss of 5% or more body weight have been shown to reduce total and free oestradiol (a type of oestrogen) levels in women following treatment for this cancer type, which may reduce the risk of disease recurrence (Rock 2013). Similarly, weight‐loss interventions have already been shown to lower levels of both insulin and adiponectin (a marker of insulin resistance), and improve insulin sensitivity in women following treatment for breast cancer (Rock 2013; Swisher 2015). They have also been associated with a reduction in the expression of inflammatory and cancer‐promoting proteins, and this may explain why they reduce the risk of disease recurrence (Irwin 2015).
In addition to potential improvements in cancer‐specific outcomes, weight‐loss interventions may also improve overall survival by reducing the risk of cardiovascular disease. This shares many of the same risk factors with endometrial cancer, including obesity and high blood pressure, both of which were improved when individuals with breast and colorectal cancer underwent intentional weight loss following treatment (Rock 2015). A previous Cochrane review concluded that physical activity may have a positive effect on quality of life in multiple different cancers, with reductions in anxiety, fatigue, sleep disturbance, and improved emotional well‐being. These results should be interpreted cautiously, as included studies were at risk of considerable bias (Mishra 2012). In particular, there was a high risk of performance bias (significant differences between groups beyond simply which intervention they received), as due to the nature of the intervention (i.e. exercise), it was not possible to conceal the treatment allocation from the participants and researcher. A proportion of the included studies were also assessed to be at high risk of selectively reporting only some of the outcomes (reporting bias), failing to be transparent in their allocation of participants to treatment groups (allocation bias), and not managing incomplete outcome data appropriately (attrition bias). The differences in exercise regimens tested meant it was difficult to combine the results to give an overall conclusion.
Why it is important to do this review
The impact of obesity on women's health has recently been highlighted in a number of high‐profile publications, including the UK Chief Medical Officer's report in December 2015 (Department of Health 2015), and the publication of the British Journal of Obstetrics and Gynaecology's themed issue, Obesity and Reproductive Health, in January 2016 (Crosbie 2016). The impact of lifestyle changes, including weight loss, on outcomes following treatment for endometrial cancer was also identified as one of the top 10 research priorities in endometrial cancer in the recent James Lind and Womb Cancer Alliance Priority Setting Partnership (Wan 2016). Therefore, this review is timely in its aim to establish the availability of evidence about the effects of weight‐loss interventions on survival and quality of life following treatment for endometrial cancer. There have been no previous Cochrane reviews of this topic, and such information will set the scene for high‐quality research to assess the feasibility, effectiveness, and cost‐effectiveness of such interventions.
Objectives
To determine the impact of weight‐loss interventions, in addition to standard management of endometrial cancer, on overall survival and the frequency of adverse events.
Secondary objectives include an assessment of weight‐loss interventions on endometrial cancer‐specific survival, weight loss achieved, cardiovascular event frequency and quality of life, both overall and stratified according to body mass index (BMI) and tumour characteristics, where possible.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), which are considered the highest level of evidence in clinical trials, to maximise the quality of included studies. We included studies reported as full text, those published as abstract only, and unpublished data, to ensure all relevant trials were incorporated.
Types of participants
We included trials that enrolled women of all ages, who were either overweight (BMI more than or equal to 25 kg/m²) or obese (BMI more than or equal to 30 kg/m²), and who were currently undergoing, or had been previously treated for endometrial cancer, of any grade, stage, or histological subtype. Trials were included regardless of primary treatment modality, i.e. surgery, radiotherapy, hormonal treatment, or a combination. When studies of participants with mixed BMI were identified but subgroup data were not provided, we contacted the study authors to request the subgroup data for overweight and obese participants only. If authors were unable or unwilling to provide these data, the study was not included in the meta‐analysis.
Types of interventions
We included studies reporting on interventions designed to promote weight loss as one of their primary stated goals, in any healthcare setting, including community‐based studies. These could include:
lifestyle interventions, including dietary and physical activity regimens;
behavioural strategies to improve adherence to treatment, which may include self‐monitoring of eating habits and physical activity, stress management, or stimulus control (eliminating environmental cues associated with undesired eating);
pharmacological interventions (such as, but not limited to, appetite suppressants, drugs that cause fat malabsorption or serotonin receptor antagonists (drugs that affect appetite) of any dose, route of delivery, or duration);
surgical interventions (including gastric band, sleeve (surgical removal of part of the stomach), or bypass procedure).
Any of these interventions were compared with any other intervention, usual care, or placebo.
Types of outcome measures
Primary and secondary outcome measures were described in terms of the effect of the weight‐loss intervention on survival, weight loss, cardiovascular events or quality of life, important measures that help determine whether these interventions should be included in routine clinical practice. Inclusion of these outcomes in the study design were not determinants of the eligibility of the trial for this review.
Primary outcomes
Overall survival; determined as the time from randomisation until death from any cause
Frequency of adverse events, of any nature
Secondary outcomes
Recurrence‐free survival; length of time from randomisation to recurrence of the disease or death
Cancer‐specific survival; length of time from randomisation to death from endometrial cancer
Weight loss; amount of weight lost between randomisation and end of study
Cardiovascular and metabolic event frequency; specifically the number of strokes, myocardial infarctions, and hospitalisations for heart failure
Quality of Life as measured on any validated scale
Search methods for identification of studies
We imposed no language restrictions on our searches. Where necessary, we translated the reports.
Electronic searches
We searched the following electronic databases from inception to January 2018:
Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library, 2017, Issue 12, Appendix 1);
MEDLINE Ovid SP (1946 to January week 2 2018, Appendix 2);
Embase Ovid SP (1980 to 2018 week 4, Appendix 3).
Searching other resources
We handsearched the citation lists of included studies and previous systematic reviews and contacted experts in the field to identify further reports of trials. Where additional information was required, we contacted the principal investigator of the trial.
Unpublished and grey literature
:We searched the following for ongoing clinical trials.
International Standard Randomised Controlled Trial Number (ISRCTN) ‐ metaRegister of Controlled Trials (www.isrctn.com/)
Physicians Data Query (www.cancer.gov/publications/pdqwww.nci.nih.gov)
PsycINFO
Handsearching
We also handsearched the reports of conferences in the following sources.
Gynecologic Oncology (Annual Meeting of the American Society of Gynecologic Oncologist)
International Journal of Gynecological Cancer (Annual Meeting of the International Gynecologic Cancer Society)
British Journal of Cancer
NCRI Cancer Conference
Annual Meeting of European Society of Medical Oncology (ESMO)
Annual Meeting of the American Society of Clinical Oncology (ASCO)
We searched for other conference abstracts and proceedings using ZETOC and WorldCat Dissertations.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database (EndNote) and removed duplicates.Two review authors (SK and NR) independently examined the remaining references. We excluded studies that clearly did not meet the inclusion criteria, and obtained full‐text copies of potentially relevant references. Two review authors (SK and NR) independently assessed the eligibility of the retrieved reports and publications. We resolved any disagreement through discussion, or if required, we consulted a third person (MM). We identified and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of included studies table (Liberati 2009).
Data extraction and management
Two review authors (SK and NR) independently extracted study characteristics and outcome data from included studies onto a pre‐piloted data collection form. We noted in the Characteristics of included studies table if outcome data were not reported in a usable format. We resolved disagreements by consensus or by involving a third person (MM). One review author (SK) transferred data into the Review Manager file (RevMan 2014). We double‐checked that data were entered correctly, by comparing the data in the RevMan file with the study reports. A second review author (MM) spot‐checked study characteristics for accuracy against the trial report. In the case where an included study had more than one report, we collated the available data to ensure maximal information yield and gave priority to the publication with the longest follow‐up associated with our review's primary and secondary outcomes.
We extracted the following data.
Author, year of publication, and journal citation (including language)
Country
Setting
Inclusion and exclusion criteria
Study design, methodology
Study population (total number enrolled; baseline patient characteristics: age, co‐morbidities (e.g. diabetes, cardiovascular disease); European Cooperative Oncology Group (ECOG) performance status; BMI; type of endometrial cancer; grade and stage of disease; timing of intervention in relation to treatment of endometrial cancer (i.e. before or after definitive treatment, nature of primary endometrial cancer treatment (e.g. surgery, radiotherapy, hormonal)).
Intervention details (type of intervention; dose, route of administration; duration of treatment; additional information as appropriate)
Comparison (nature of intervention; dose, route of administration; duration of treatment; additional information as appropriate)
Risk of bias in study (see below)
Duration of follow‐up
Outcomes: For each outcome, we extracted the outcome definition and unit of measurement (if relevant). For adjusted estimates, we recorded variables adjusted for in the analyses.
Results: We extracted the number of participants allocated to each intervention group, the total number analysed for each outcome, and the missing participants.
Notes: Funding for trial, and notable conflicts of interest of trial authors.
We extracted the results as follows.
For time‐to‐event data (survival and disease progression), we extracted the log of the hazard ratio [log (HR)] and its standard error from trial reports. If these were not reported, we attempted to estimate the log (HR) and its standard error using the methods of Parmar 1998. If this were not possible for survival data, they were treated as dichotomous outcomes and the risk ratio was estimated.
For dichotomous outcomes (e.g. adverse events, cardiovascular events or deaths), if it were not possible to calculate a hazard ratio, we estimated a risk ratio; we extracted the number of patients in each treatment arm who experienced the outcome of interest and the number of patients assessed at endpoint.
For continuous outcomes (e.g. quality of life measures, weight loss), we extracted the mean and standard deviation of the outcome of interest and the number of patients assessed in each treatment arm at specific time points and used this to estimate the mean difference and its standard deviation.
If reported, we extracted both unadjusted and adjusted statistics.
Where possible, we extracted data relevant to an intention‐to‐treat analysis, in which case participants were analysed in the groups to which they were assigned.
We noted the time points at which outcomes were collected and reported.
Assessment of risk of bias in included studies
We assessed and reported on the methodological risk of bias of included studies in accordance with the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011a), which recommends the explicit reporting of the following individual elements for RCTs.
Selection bias: random sequence generation and allocation concealment
Performance bias: blinding of participants and personnel (patients and treatment providers)
Detection bias: blinding of outcome assessment
Attrition bias: incomplete outcome data
Reporting bias: selective reporting of outcomes
Two review authors (SK and NR) independently applied the 'Risk of bias' criteria; we resolved differences by discussion, or by appealing to a third review author (MM). We checked clinical trial registries for a priori primary and secondary outcome measures to assess the risk of selective reporting. We judged each item as being at high, low, or unclear risk of bias, as set out in the criteria provided by Higgins 2011b and Higgins 2011a. We provided a quote from the study report and a statement to justify the judgement for each criteria. We summarised results in both a graph and a narrative summary. When interpreting treatment effects and meta‐analyses, we took into account the risk of bias for the studies that contributed to that outcome. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
Measures of treatment effect
We used the following measures of the effect of treatment.
For time‐to‐event data, we used the hazard ratio (HR), if possible. Where this was not the case, the data were treated as a dichotomous outcome and the risk ratio (RR) was estimated using the Mantel‐Haenszel method.
For dichotomous outcomes, we analysed data based on the number of events and the number of people assessed in the intervention and comparison groups. We used these to calculate the RR and 95% confidence interval (CI) using the Mantel‐Haenszel method.
For continuous outcomes, we analysed data based on the mean, standard deviation (SD), and number of people assessed for both the intervention and comparison groups, to calculate mean difference (MD) between treatment arms with a 95% CI. If the MD was reported without individual group data, we used this to report the study results. If more than one study measured the same outcome using different tools, we planned to calculate the standardised mean difference (SMD) and 95% CI using the inverse variance method in RevMan 2014 .
We undertook meta‐analyses only where this was meaningful, i.e. if the treatments, participants, and the underlying clinical question were similar enough for pooling to be appropriate. We described skewed data reported as medians and interquartile ranges. Where multiple trial arms were reported in a single trial, we included only the relevant arms and divided the 'shared' comparison group equally between the number of treatment groups, to avoid 'double‐counting'.
Unit of analysis issues
The unit of analysis was the participant. If any trials had multiple treatment groups, we combined similar intervention arms and control arms together in order to create single pair‐wise comparisons.
Dealing with missing data
We attempted to contact study authors to obtain missing data (participant, outcome, or summary data). Where possible, we conducted analysis of participant data on an intention‐to‐treat basis; otherwise, we analysed data as reported. We reported on the levels of loss to follow‐up, and assessed this as a source of potential bias.
We did not impute missing outcome data.
Assessment of heterogeneity
Where we considered studies similar enough (based on participants, intervention, comparison, settings and outcome measures) to pool the data using meta‐analysis, we assessed the degree of heterogeneity by visually inspecting forest plots, by estimating the percentage of heterogeneity (I² statistic ) between trials that cannot be ascribed to sampling variation (Higgins 2003), by formally testing the significance of the heterogeneity (Chi² statistic; Deeks 2001), and if possible, by conducting subgroup analyses. We used these I² statistic levels as a rough guide to assess heterogeneity as:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
We evaluated the value of the I² statistic alongside the magnitude and direction of effects, and the P value for the Chi² test (Higgins 2011).
If there was evidence of substantial clinical, methodological, or statistical heterogeneity across included studies, we did not report pooled results from the meta‐analysis, but instead used a narrative approach to data synthesis. In this event, we investigated and reported the possible clinical or methodological reasons for this.
Assessment of reporting biases
We aimed to minimise reporting bias by systematically searching for all eligible studies, including unpublished data and ongoing clinical trials, and by not including any language restrictions. Updates of this review will deal with any time lag bias.
Had we included 10 or more studies that investigated a particular outcome, we planned to examine funnel plots that correspond to the meta‐analysis of the outcome to assess the potential for small‐study effects, such as publication bias. We planned to visually assess funnel plot asymmetry; if asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it.
Data synthesis
If sufficient, clinically similar studies (in terms of participants, intervention, comparison, settings and outcome measures) were available to ensure meaningful conclusions, we pooled their results in meta‐analyses using the random‐effects model in RevMan. Given the number of possible interventions that could have been included in the incorporated studies, we only planned to perform the following meaningful comparisons.
Lifestyle interventions in addition to usual care versus usual care
Behavioural interventions in addition to usual care versus usual care
Pharmacological interventions in addition to usual care versus usual care
Surgical interventions in addition to usual care versus usual care
Lifestyle interventions versus behavioural interventions
Lifestyle interventions verus pharmacological interventions
Lifestyle interventions versus surgical interventions
Behavioural interventions versus pharmacological interventions
Behavioural interventions versus surgical interventions
Pharmacological intervention versus surgical interventions.
The specific method for pooling data depended upon the nature of the outcome measure. If we were unable to pool the data statistically using meta‐analysis, we conducted a narrative synthesis of results. We presented the major outcomes and results, organised by intervention categories, according to the major types or aims of the identified interventions.
'Summary of findings' table
We assessed and reported the quality of the evidence for each outcome, using the GRADE approach and these domains: study limitations (suggesting a high likelihood of bias), inconsistency (unexplained heterogeneity), imprecision (wide confidence intervals), indirectness of evidence, and publication bias. We created a 'Summary of findings' table, using GRADEpro GDT software (GRADEpro GDT), and two review authors (SK and NR) independently assessed the quality of the evidence, using Chapter 12.2 of the Cochrane Handbook of Systematic Reviews of Interventions as a guide (Schünemann 2011). We used a checklist to maximise consistent GRADE decisions, and the GRADE Working Group quality of evidence definitions (Meader 2014). We downgraded the evidence from high quality by one level for serious limitations (or by two for very serious limitations) for each outcome, and outlined our rationale in the footnotes.
High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
We included the following outcomes in the 'Summary of findings' table.
Overall survival
Adverse events
Recurrence‐free survival
Cancer‐specific survival
Weight loss
Cardiovascular and metabolic event frequency
Quality of life
If meta‐analyses had not been possible, we planned to present results in a narrative 'Summary of findings' table format, such as that used in the Cochrane review Chan 2011.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses for the following factors, where possible.
BMI
Histological type, stage, and grade of endometrial cancer
Sensitivity analysis
If adequate data were available, we planned to perform a sensitivity analysis comparing studies with high and unclear risk of bias and low risk of bias for attrition and outcome reporting, and allocation concealment (the latter is relevant only to pharmacological interventions).
Results
Description of studies
Results of the search
The electronic search retrieved 873 records. Thirty references were potentially eligible and were retrieved as full‐text articles. Three studies (five references) met the inclusion criteria and five studies were ongoing.
Please see study tables:Characteristics of included studies; Characteristics of excluded studies and Characteristics of ongoing studies and the PRISMA flow chart (Figure 1).
1.
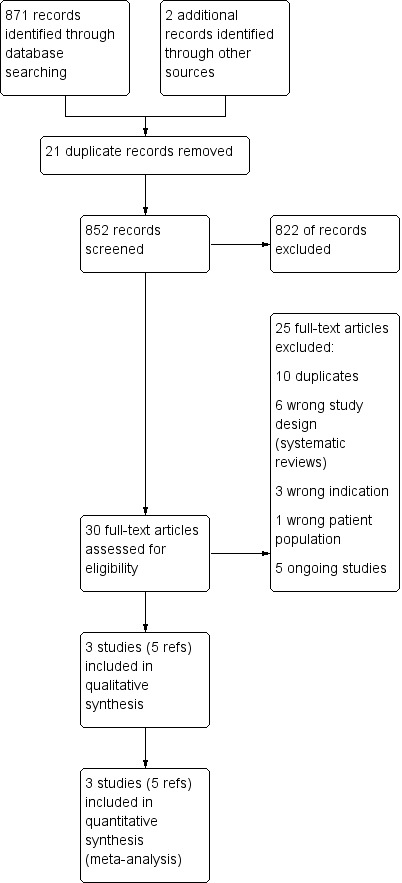
Study flow diagram.
Included studies
Study design and setting
Three randomised controlled trials (RCTs) were included in the review. Two RCTs were conducted in a single centre (McCarroll 2014; von Gruenigen 2009) and one was a multi‐centre trial (Allison 2016). All trials were conducted in university hospitals in the USA (Allison 2016; McCarroll 2014; von Gruenigen 2009).
Participants
Included trials randomised 161 overweight and obese female participants previously treated for endometrial cancer and with good performance status (0‐2, a way of quantifying the general well‐being and physical activity levels of cancer patients) (Allison 2016; McCarroll 2014; von Gruenigen 2009). The mean age of participants ranged from 54 years (von Gruenigen 2009) to 62 years (Allison 2016). Two RCTs included only patients with stage I or II disease (McCarroll 2014; von Gruenigen 2009). The other RCT did not provide details of the stage of disease of participants (Allison 2016). All patients underwent surgery as the primary treatment of their endometrial cancer (Allison 2016;McCarroll 2014; von Gruenigen 2009). In addition, one RCT included participants who had also received adjuvant brachytherapy, radiotherapy, or chemotherapy (McCarroll 2014). One RCT specifically excluded patients who had received, or were due to receive adjuvant treatment (Allison 2016), whilst the other trial did not provide details of radio‐ and chemotherapy exposure (von Gruenigen 2009).
Interventions
All studies compared combined behavioural and lifestyle interventions to facilitate weight loss through dietary modification and increased physical activity, with usual care. Two RCTs utilised a two‐arm design, comparing one intervention with usual care (McCarroll 2014; von Gruenigen 2009). One RCT had a three‐arm design, comparing two types of lifestyle interventions with usual care (Allison 2016). Counselling was provided either on an individual basis by telephone or text (Allison 2016) or a combination of face‐to‐face group and individual sessions (McCarroll 2014; von Gruenigen 2009).
Primary outcome
Overall survival
3/3 RCTs reported overall survival, defined as the number of deaths occurring during follow‐up (Allison 2016; McCarroll 2014; von Gruenigen 2009).
Adverse events
2/3 RCTs reported adverse events, defined as any undesirable symptom or sign occurring after the study had commenced, even if not thought to be directly related to the intervention (McCarroll 2014; von Gruenigen 2009). These were reported as two separate categories; mild to moderate adverse reactions and life‐threatening adverse reactions.
Secondary outcome
Recurrence‐free survival
No trials reported recurrence‐free survival
Cancer‐specific survival
3/3 RCTs reported cancer‐specific survival, defined as the number of deaths secondary to endometrial cancer occurring during follow‐up (Allison 2016; McCarroll 2014; von Gruenigen 2009).
Weight loss
3/3 RCTs reported change in weight from baseline, measured in kilograms (Allison 2016; McCarroll 2014; von Gruenigen 2009).
Cardiovascular and metabolic event frequency
3/3 RCTs reported cardiovascular events, defined as the number of myocardial infarctions, strokes, and hospitalisations for heart failure occurring during follow‐up (Allison 2016; McCarroll 2014; von Gruenigen 2009).
Quality of life
3/3 RCTs reported change in quality of life score from baseline (Allison 2016; McCarroll 2014; von Gruenigen 2009).
Quality of life was measured by four different instruments.
1/3 RCTs used SF‐12 Physical Health questionnaire (Allison 2016).
2/3 RCTs used Functional Assessment of Cancer Therapy‐General (FACT‐G) (McCarroll 2014; von Gruenigen 2009).
We contacted the principal investigator of each of the included RCTs for unpublished data where it was felt to be important to the results of the review. Full and detailed responses were obtained from the study authors (Table 2).
1. Authors' responses to additional information request.
| Study | Principle Investigator contacted | Additional information requested | Answers provided |
| Allison 2016 | Kelly Allison | Randomisation process Blinding process How was the study analysed? Exclusion criteria How was missing data dealt with? Baseline characteristics Duration of study intervention Was a power calculation performed? Results‐overall survival, adverse events, recurrence‐free survival, cancer‐specific survival, weight loss from baseline, cardiovascular and metabolic event frequency, change in quality of life from baseline Funding source Conflicts of interest |
"The coordinating center used a computer generated algorithm to produce the randomization envelopes for each clinical site, with the general parameters of randomizing 1:1:1 across the three conditions. The envelopes are then chosen sequentially as each participant was enrolled." "There was no blinding. The outcome assessments were conducted by study coordinators and trained medical personnel (for blood draws, DEXA). The coordinators knew which condition the participants were in, but other medical personnel were not informed." "Given we only had pre‐post assessment data and our main analyses used paired t‐tests and correlations, we were unable to do intention‐to treat analyses." "Exclusion criteria included: age less than 18, current or recent participation in a weight loss program or use of weight loss medications; uncontrolled serious medical or psychiatric condition(s) that would affect the patient’s ability to participate in the interventional study; invasive malignancy other than EC or non‐melanoma skin cancer which required active treatment currently or within the last 5 years, or current pregnancy." "Given the pre‐post assessment design, were excluded participants for variables that were not completed." See Characteristics of included studies. Data on co‐morbidities, performance status and type of endometrial cancer were not provided. " 6 months" "No ‐ From the grant: The purpose will be to provide estimates for the size of an intervention effect achievable by the experimental intervention in order to power and justify a grant application for a full‐scale trial of a weight loss program in women with endometrial cancer. With a sample size of 30 participants per group, the true difference in mean weight loss between the groups can be estimated with a 95% confidence interval size of ±0.50σ, where σ is the population standard deviation of weight loss, assumed in this calculation to be the same in each of the two intervention groups and the control group. We will assess the comparability of variance across the groups and do exploratory analyses of possibly variance‐stabilizing transformations. Because this is a pilot study to derive parameters to design an appropriately‐powered study, hypothesis testing is not a primary goal of the statistical analysis of the data, although p‐values will be calculated." See Data and analyses. No data provided on adverse events, recurrence‐free and cancer‐specific survival "Cross‐TREC study funded by NCI U54‐CA155850 – University of Pennsylvania; U54 CA155626 – Harvard University; U54 CA155496CC – Washington University; U01 CA116850 – Fred Hutchinson Cancer Research Center." None declared |
| McCarroll 2014 | Michele McCarroll | Single‐ or multi‐centre study? Reasons for non‐attendance at follow‐up visits Methods of group allocation concealment Prospectively published protocol? Results‐overall survival, adverse events, recurrence‐free survival, cancer‐specific survival, weight loss from baseline, cardiovascular and metabolic event frequency, change in quality of life from baseline |
Single centre None provided "Physician counseling was standardized. Clinical guidelines for professionals on the identification, evaluation, and treatment of overweight and obesity in adults, according to the NIH should include dietary therapy, behavior therapy, and an increase in physical activity. They recommend that the clinician and the patient devise goals and a treatment strategy for weight loss with periodic weight checks. A guideline for physicians consisting of a laminated 3 x 5 card was given to all treating physicians as a reminder of patient teaching points. Due to the interventions performed by the study team (dietitian, Physical therapist, psychologist, etc.), they were able to know who was in each group." "No" See Data and analyses |
| von Gruenigen 2009 | Michele McCarrroll | Single‐ or multi‐centre study? Reasons for non‐attendance at follow‐up visits Methods of group allocation concealment Prospectively published protocol? Results‐overall survival, adverse events, recurrence‐free survival, cancer‐specific survival, weight loss from baseline, cardiovascular and metabolic event frequency, change in quality of life from baseline |
Single centre None provided "Physician counselling was standardized. Clinical guidelines for professionals on the identification, evaluation, and treatment of overweight and obesity in adults, according to the NIH should include dietary therapy, behavior therapy, and an increase in physical activity. They recommend that the clinician and the patient devise goals and a treatment strategy for weight loss with periodic weight checks. A guideline for physicians consisting of a laminated 3 x 5 card was given to all treating physicians as a reminder of patient teaching points. Due to the interventions performed by the study team (dietitian, Physical therapist, psychologist, etc.), they were able to know who was in each group." No See Data and analyses |
Excluded studies
Ten full‐text articles were excluded from the review for the following reasons.
6/10 full‐text articles were systematic reviews (Babatunde 2016; Fasching 2009; Gil 2007; Koutoukidis 2015; Lin 2016; Smits 2015).
1/10 RCTs included a different patient population, enrolling patients with breast and colon cancer and only one patient with endometrial cancer (Beck 2015).
3/10 RCTs were for the wrong indication. One incorporated a physical activity‐based intervention for the treatment of cancer‐related fatigue rather than weight loss (Donnelly 2011), the primary aim of another was to study the effect of a diet and physical activity intervention on quality of life (Koutoukidis 2017) and another assessed the feasibility and effectiveness of physical activity and changes in self‐efficacy, outcome expectation and self‐regulation (Rossi 2016).
Risk of bias in included studies
Please refer to Characteristics of included studies; Figure 2; Figure 3
2.
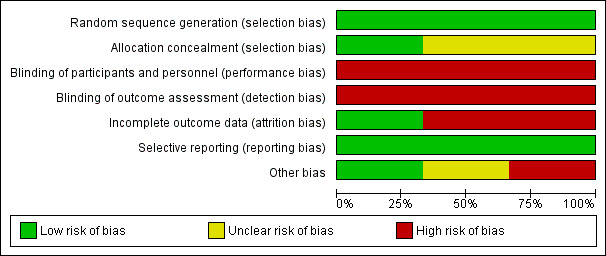
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
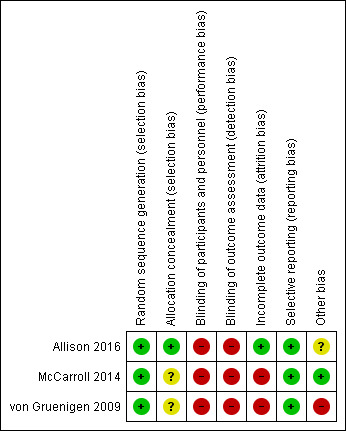
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All RCTs were at low risk of selection bias related to random sequence generation (Allison 2016; McCarroll 2014; von Gruenigen 2009). One RCT used computer‐generated randomisation (Allison 2016). The other two RCTs used block randomisation methods, stratifying patients according to baseline BMI (McCarroll 2014; von Gruenigen 2009).
One RCT was at low risk of selection bias related to allocation concealment as they used appropriate methods of sequentially numbered envelopes (Allison 2016). Two RCTs were at unclear risk of bias for allocation concealment as they did not describe the methods used (McCarroll 2014; von Gruenigen 2009).
Blinding
All RCTs were at high risk of performance bias related to blinding of participants and personnel (Allison 2016; McCarroll 2014; von Gruenigen 2009). Due to the nature of the intervention (either group or individual counselling sessions regarding weight loss and physical activity or usual care involving no additional counselling or generic health advice only), it was not possible to blind participants and the research team to group allocation.
It would, however, be possible to blind outcome assessors for all primary and secondary outcomes, thereby reducing the risk of detection bias. All RCTs were at high risk of detection bias as they used unblinded members of the research team to measure all outcomes (Allison 2016; McCarroll 2014; von Gruenigen 2009).
We considered that blinding was unlikely to affect the findings for the primary outcomes of overall survival and adverse events, nor the secondary outcomes of recurrence‐free and cancer‐specific survival, weight loss and cardiovascular event frequency, but that it may affect quality of life assessments.
Incomplete outcome data
One RCT was considered at low risk for attrition bias as they had no withdrawals from the study and no missing data (Allison 2016). The other two RCTs were considered to be at high risk for attrition bias as they had a participant withdrawal and missing data rate more than 10% (McCarroll 2014; von Gruenigen 2009). McCarroll 2014 had a withdrawal rate of 16/75 (21.3%) and von Gruenigen 2009 had a withdrawal rate of 7/45 (15.6%) and missing data for an additional 2/22 (9.1%) of participants in the control arm.
Selective reporting
None of the three RCTs published their protocols prospectively but all were registered prior to commencement of recruitment on clinicaltrials.gov and reported all of their prespecified outcomes (Allison 2016; McCarroll 2014; von Gruenigen 2009). These were, therefore, deemed at low risk of reporting bias.
Other potential sources of bias
No studies reported significant differences in baseline characteristics between their intervention and control groups. Only 30/41 (73.2%) of participants in one RCT had completed their outcome assessments at the time of correspondence with the study authors for this review (Allison 2016). Additional data will be available for future updates of the review. An additional source of bias was identified in one RCT where two participants in the intervention arm underwent gastric bypass during follow‐up and continued to be included in the final analysis (von Gruenigen 2009).
There were insufficient studies investigating each outcome to construct a funnel plot to assess for publication bias.
Effects of interventions
See: Table 1
See: Table 1
1. Lifestyle intervention compared with usual care
All three RCTs compared combined lifestyle and behavioural interventions with usual care (Allison 2016; McCarroll 2014; von Gruenigen 2009).
Primary outcomes
1. Overall survival (six, 12 and 24 months)
Insufficient data were available to calculate the effect of combined lifestyle and behavioural interventions on overall survival using the hazard ratio. Instead, mortality was treated as a dichotomous outcome and the risk ratio (RR) determined.
There was no evidence that a combined lifestyle and behavioural intervention, incorporating dietary and physical activity advice with self‐monitoring and stimulus control techniques, was associated with an improvement in overall survival at six months as no deaths were observed in the intervention or usual care groups of the two studies that reported this outcome (Analysis 1.1) (Allison 2016; McCarroll 2014). A risk ratio could not, therefore, be calculated and a meta‐analysis could not be performed. Neither sensitivity nor subgroup analyses were possible.
1.1. Analysis.
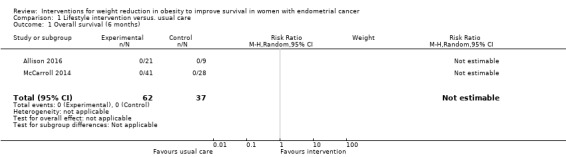
Comparison 1 Lifestyle intervention versus. usual care, Outcome 1 Overall survival (6 months).
There was no evidence that lifestyle and behavioural interventions were associated with an improvement in overall survival at 12 months as no deaths were observed in either the intervention or usual care groups of the one study that reported this outcome (Analysis 1.2) (McCarroll 2014). A risk ratio could not, therefore, be calculated. Sensitivity and subgroup analyses were not possible.
1.2. Analysis.
Comparison 1 Lifestyle intervention versus. usual care, Outcome 2 Overall survival (12 months).
Lifestyle and behavioural interventions were not associated with an improvement in overall survival at 24 months (RR (mortality) 0.23, 95% confidence interval (CI) 0.01 to 4.55, P = 0.34, one RCT, 37 participants, very low‐certainty evidence) (Analysis 1.3; Figure 4) (von Gruenigen 2009). Two deaths occurred in the control arm. Sensitivity and subgroup analyses were not possible.
1.3. Analysis.
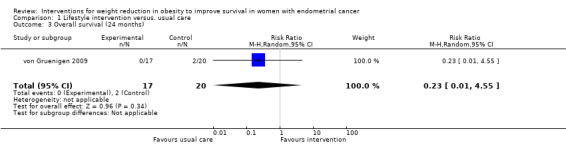
Comparison 1 Lifestyle intervention versus. usual care, Outcome 3 Overall survival (24 months).
4.

Forest plot of comparison: 1 Lifestyle intervention versus. usual care, outcome: 1.3 Overall survival (24 months).
2. Adverse events
Mild to moderate adverse events
One RCT reported no mild to moderate adverse events related to the study intervention (von Gruenigen 2009).
One RCT (McCarroll 2014) reported 13 musculoskeletal events in 10 participants in the intervention group, including knee and leg pain and muscle weakness, which were felt to be possibly related to the study intervention. Participants receiving combined lifestyle and behavioural interventions had a higher risk of musculoskeletal events than those receiving usual care (RR 19.03, 95% CI 1.17 to 310.52, P = 0.04, two RCTs , 91 participants, low‐certainty evidence) (Analysis 1.4; Figure 5) (McCarroll 2014; von Gruenigen 2009).
1.4. Analysis.
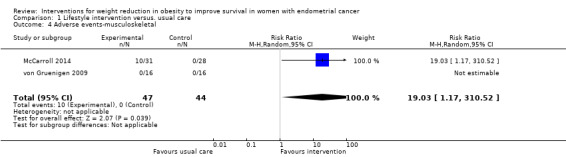
Comparison 1 Lifestyle intervention versus. usual care, Outcome 4 Adverse events‐musculoskeletal.
5.
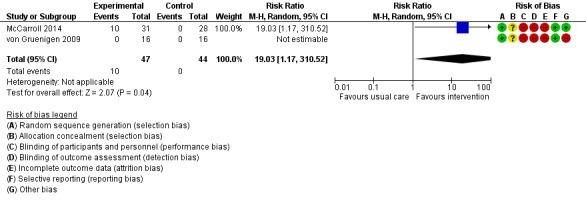
Forest plot of comparison: 1 Lifestyle intervention versus. usual care, outcome: 1.4 Adverse events‐musculoskeletal.
Two participants in the study by McCarroll 2014 also reported episodes of diarrhoea, which were felt to be possibly related to the study intervention. Lifestyle and behavioural interventions were not associated with an increased risk of diarrhoea (RR 4.53, 95% CI 0.23 to 90.51, P = 0.32, two RCTs, 91 participants, low‐certainty evidence) (Analysis 1.14) (McCarroll 2014; von Gruenigen 2009).
1.14. Analysis.
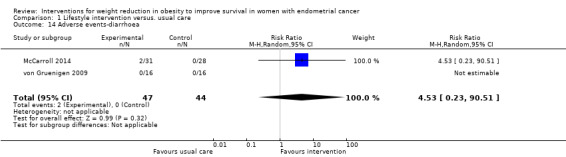
Comparison 1 Lifestyle intervention versus. usual care, Outcome 14 Adverse events‐diarrhoea.
Life‐threatening adverse events
No life‐threatening adverse events related to the study intervention were reported in any of the RCTs.
Secondary outcomes
1. Recurrence‐free survival
No RCTs reported this outcome.
2. Cancer‐specific survival (six, 12 and 24 months)
There was no evidence that combined lifestyle and behavioural interventions were associated with an improvement in cancer‐specific survival at six months as no deaths were reported in either the intervention or usual care groups for the two studies that reported this outcome (Analysis 1.5) (Allison 2016; McCarroll 2014). A risk ratio could not, therefore, be calculated and a meta‐analysis could not be performed. No sensitivity or subgroup analyses were possible.
1.5. Analysis.
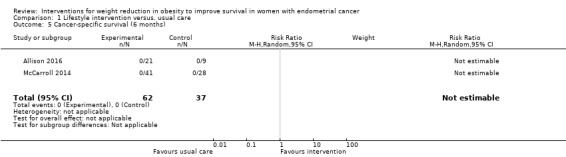
Comparison 1 Lifestyle intervention versus. usual care, Outcome 5 Cancer‐specific survival (6 months).
There was no evidence that combined lifestyle and behavioural interventions were associated with an improvement in cancer‐specific survival at 12 months as no deaths were reported in either group in the one study reporting this outcome (Analysis 1.6), (McCarroll 2014). A risk ratio could not, therefore, be calculated. No sensitivity or subgroup analyses were possible.
1.6. Analysis.
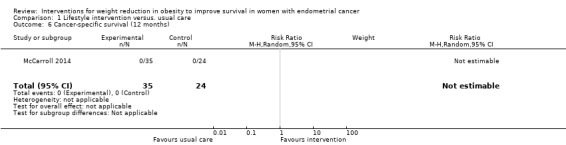
Comparison 1 Lifestyle intervention versus. usual care, Outcome 6 Cancer‐specific survival (12 months).
There was no evidence that combined lifestyle and behavioural interventions were associated with an improvement in cancer‐specific survival at 24 months as no cancer‐specific deaths were reported (Analysis 1.7) (von Gruenigen 2009). A risk ratio could not, therefore, be calculated. No sensitivity or subgroup analyses were possible.
1.7. Analysis.
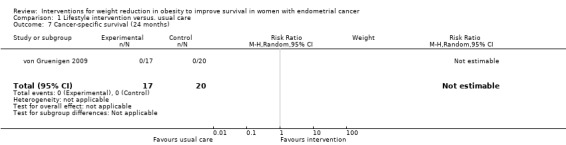
Comparison 1 Lifestyle intervention versus. usual care, Outcome 7 Cancer‐specific survival (24 months).
3. Weight loss (six, 12 and 24 months)
Combined lifestyle and behavioural intervention was not associated with weight loss at six months compared to usual care (mean difference (MD) ‐1.88 kg, 95% CI ‐5.98 to 2.21, P = 0.37, three RCTs , 131 participants, I2= 0%, low‐certainty evidence) (Analysis 1.8) (Allison 2016; McCarroll 2014; von Gruenigen 2009). Subgroup analysis according to baseline BMI was performed and did not affect the result (Analysis 1.9) (McCarroll 2014; von Gruenigen 2009). Insufficient data were available to perform a subgroup analyses according to histological type, stage and grade of endometrial cancer. No sensitivity analyses were possible.
1.8. Analysis.
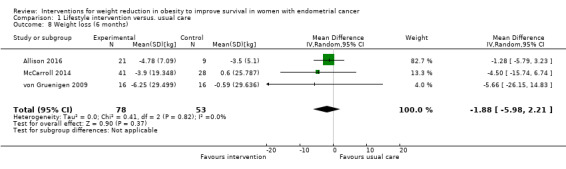
Comparison 1 Lifestyle intervention versus. usual care, Outcome 8 Weight loss (6 months).
1.9. Analysis.
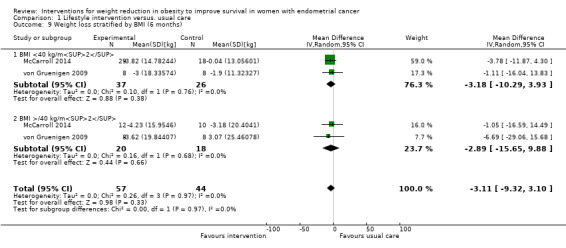
Comparison 1 Lifestyle intervention versus. usual care, Outcome 9 Weight loss stratified by BMI (6 months).
Lifestyle and behavioural intervention was not associated with weight loss at 12 months compared to usual care (MD ‐8.98 kg, 95% CI ‐19.88 to 1.92, P = 0.11, two RCTs , 91 participants, I2= 0%, very low‐certainty evidence) (Analysis 1.10). Although some individuals lost a lot of weight, most of the participants lost none or very little, which is why this result was not statistically significant. Subgroup analysis demonstrated no effect of baseline BMI on weight loss following the intervention (Analysis 1.11) (McCarroll 2014; von Gruenigen 2009). No sensitivity analysis was possible.
1.10. Analysis.
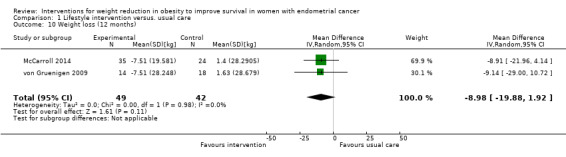
Comparison 1 Lifestyle intervention versus. usual care, Outcome 10 Weight loss (12 months).
1.11. Analysis.
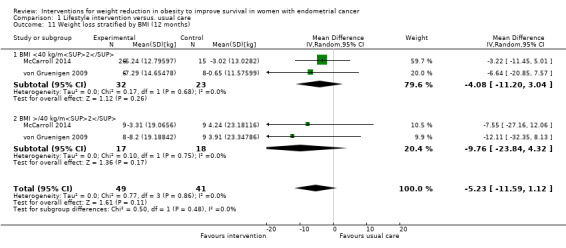
Comparison 1 Lifestyle intervention versus. usual care, Outcome 11 Weight loss stratified by BMI (12 months).
Overall, a lifestyle and behavioural intervention was not associated with weight loss at 24 months compared with usual care (MD ‐18.26 kg, 95% CI ‐38.73 to 2.21, P = 0.08, one RCT, 25 participants, very low‐certainty evidence) (Analysis 1.12) (von Gruenigen 2009). Subgroup analysis demonstrated significant differences in amount of weight lost according to baseline BMI (Chi2 10.10, df 1, P = 0.001) (Analysis 1.13). Participants with a BMI < 40 kg/m2 did not achieve greater weight loss following the intervention compared with those receiving usual care at 24 months (MD 2.12 kg, 95% CI ‐20.82 to 25.06, P = 0.86, one RCT, 13 participants, very low‐certainty evidence) (von Gruenigen 2009). Participants with a BMI greater than or equal to 40 kg/m2 who received the intervention, however, did achieve greater weight loss at 24 months than those receiving usual care (MD ‐54.58 kg, 95% CI ‐80.97 to ‐28.19, P < 0.0001,one RCT, 12 participants, very low‐certainty evidence) (Analysis 1.13; Figure 6). These results were influenced by the inclusion of two participants with a BMI over 40 kg/m2 who underwent bariatric surgery during follow‐up and lost a large amount of weight as a consequence. No sensitivity analysis was possible.
1.12. Analysis.
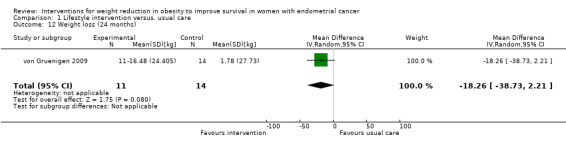
Comparison 1 Lifestyle intervention versus. usual care, Outcome 12 Weight loss (24 months).
1.13. Analysis.
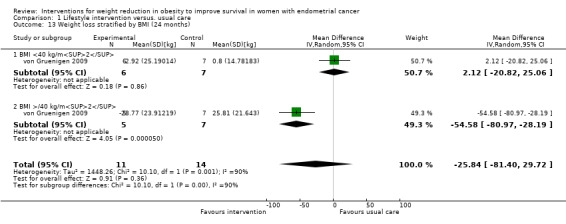
Comparison 1 Lifestyle intervention versus. usual care, Outcome 13 Weight loss stratified by BMI (24 months).
6.
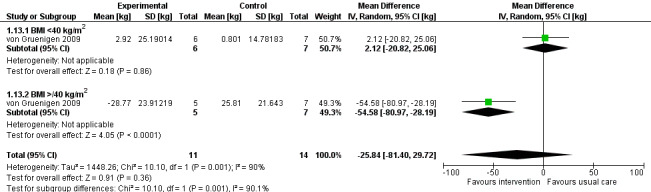
Forest plot of comparison: 1 Lifestyle intervention versus. usual care, outcome: 1.13 Weight loss stratified by BMI (24 months) [kg].
4. Cardiovascular and metabolic event frequency (six and 12 months)
No cardiovascular or metabolic events were reported at six and 12 months.(Analysis 1.15; Analysis 1.16)
1.15. Analysis.
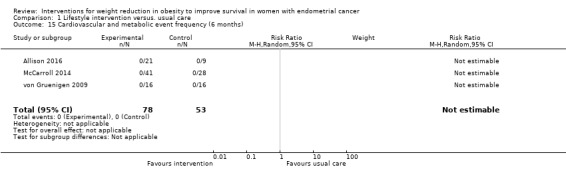
Comparison 1 Lifestyle intervention versus. usual care, Outcome 15 Cardiovascular and metabolic event frequency (6 months).
1.16. Analysis.

Comparison 1 Lifestyle intervention versus. usual care, Outcome 16 Cardiovascular and metabolic event frequency (12 months).
5. Quality of life (six and 12 months)
Six months
SF‐12 Physical Health questionnaire
Combined lifestyle and behavioural intervention was not associated with improvement in quality of life at six months compared with usual care when measured using the SF‐12 Physical Health questionnaire (MD ‐2.29, 95% CI ‐7.34 to 2.76, P = 0.37, one RCT, 30 participants, moderate‐certainty evidence) (Analysis 1.17) (Allison 2016).
1.17. Analysis.

Comparison 1 Lifestyle intervention versus. usual care, Outcome 17 Quality of life‐SF12 Physical Health component (6 months).
FACT‐G
Combined lifestyle and behavioural intervention was not associated with improvement in quality of life at six months compared with usual care when measured using the FACT‐G questionnaire (MD 2.51, 95% CI ‐5.61 to 10.64, P = 0.54, two RCTs (McCarroll 2014; von Gruenigen 2009), 95 participants, I2= 83%, very low‐certainty evidence) (Analysis 1.18). Baseline BMI did not impact on quality of life response to the intervention in a subgroup analysis (Analysis 1.19) (McCarroll 2014; von Gruenigen 2009). A sensitivity analysis was not possible.
1.18. Analysis.
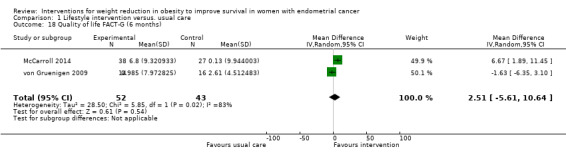
Comparison 1 Lifestyle intervention versus. usual care, Outcome 18 Quality of life FACT‐G (6 months).
1.19. Analysis.
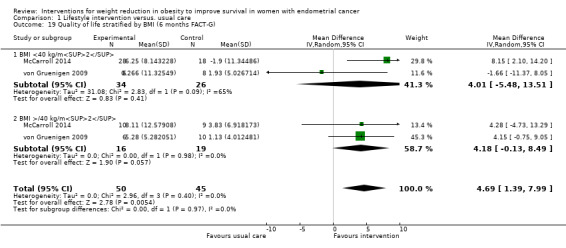
Comparison 1 Lifestyle intervention versus. usual care, Outcome 19 Quality of life stratified by BMI (6 months FACT‐G).
Twelve months
The effect of lifestyle and behavioural intervention on quality of life was measured at 12 months by two RCTs, both of which used the FACT‐G questionnaire (McCarroll 2014; von Gruenigen 2009). Lifestyle and behavioural intervention was not associated with improvement in quality of life at 12 months (MD 2.77, 95% CI ‐0.65 to 6.20, P = 0.11, 89 participants, I2=0%, very low‐certainty evidence) (Analysis 1.20) (McCarroll 2014; von Gruenigen 2009). The QoL response to the intervention did not differ according to baseline BMI in a subgroup analysis (Analysis 1.21). A sensitivity analysis was not possible.
1.20. Analysis.

Comparison 1 Lifestyle intervention versus. usual care, Outcome 20 Quality of life FACT‐G (12 months).
1.21. Analysis.

Comparison 1 Lifestyle intervention versus. usual care, Outcome 21 Quality of life stratified by BMI (12 months FACT‐G).
Discussion
Summary of main results
The limited evidence suggests that combined lifestyle and behavioural interventions had no effect on overall survival. There was no evidence that combined lifestyle and behavioural interventions affected cancer‐specific or recurrence‐free survival or reduced the number of cardiovascular and metabolic events in endometrial cancer survivors over a 12 month follow‐up period as either no events were recorded in the studies or the outcome was not reported. Dietary and physical activity advice, in combination with behavioural strategies to improve compliance, are not associated with significant weight loss or improvement in quality of life for women with a history of endometrial cancer over a similar follow‐up period, when compared with those receiving usual care. Body mass index (BMI) at baseline did not affect these results. These results should be viewed with caution, however, as only three randomised controlled trials (RCTs) met the eligibility criteria for inclusion in this review, all of which were small in size and meant that no events were recorded for many of these outcomes. At 24 months, super‐obese participants (BMI greater than or equal to 40 kg/m2) in one RCT (von Gruenigen 2009) lost significantly more weight than those receiving usual care. However, there were biases in the design of this study, namely the inclusion of participants who underwent gastric bypass surgery during follow‐up. Despite a lack of benefit with regards to the outcomes included in this review, lifestyle and behavioural interventions to induce weight loss in endometrial cancer survivors were associated with a significant risk of musculoskeletal side effects, though the low event numbers make relative risk estimates unreliable and none of the adverse events recorded were considered serious or life‐threatening.
The 'Summary of Findings' table summarises the main outcomes (Table 1).
Overall completeness and applicability of evidence
The evidence for each of the outcomes was limited as only three studies met the inclusion criteria and each had enrolled small numbers of participants. Two of the included studies were undertaken by the same study authors recruiting from the same hospital and pool of endometrial cancer survivors and were carried out as a pilot study (von Gruenigen 2009), followed by a definitive RCT using similar methodology (McCarroll 2014). This is likely to impact on the applicability of their findings to other populations.
All of the included studies were at high risk for performance bias, as due to the nature of the interventions, they were unable to blind participants and personnel to treatment group allocation. The RCTs were also at high risk for detection bias due to the use of unblinded outcome assessors. Whilst this is unlikely to have affected objective outcomes, such as weight loss and survival, it may have impacted on more subjective outcomes, such as quality of life. The use of independent, blind outcome assessors in future studies would remove this potential source of bias.
Two different questionnaires were used to measure quality of life in the three studies included in this review. The results presented in the 'Summary of findings' table are based on use of the FACT‐G (Functional Assessment of Cancer Therapy‐General) questionnaire as this was used by two studies and, hence, pools the individuals results from the greatest number of participants. These findings were considered, however, to be based on very low‐certainty evidence due to the risk of bias in the included studies. The study using the SF‐12 Physical Health Component questionnaire, whilst providing evidence of greater certainty, was based on a small number of participants and considered different aspects of quality of life, preventing pooling in the meta‐analysis. The overall findings of all three studies were, however, similar, with no significant improvement in quality of life found at six months following weight‐loss interventions. In order to improve the quality of evidence and to allow future meta‐analyses of the effect of weight‐loss interventions on quality of life to be conducted, it would be advisable for all studies going forward to use a common quality of life assessment tool.
While the study authors were able to provide additional data on the outcome measures included in this review, overall and cancer‐specific survival and cardiovascular and metabolic event frequency were not specific outcomes of these studies. This explains the paucity of data provided, which were insufficient to allow the calculation of hazard ratios for these outcomes. The short duration of the intervention (six months) and limited follow‐up time of the included RCTs, which was between six and 24 months, explains why so few deaths and cardiovascular and metabolic events were recorded by the study authors. Any conclusions with regards the effect of lifestyle and behavioural interventions on survival should, therefore, be made with caution. For weight‐loss interventions to be shown to impact on survival for women with a history of endometrial cancer, the duration of both the intervention and follow‐up period will need to be considerably longer (five to 10 years).
The only studies that met the inclusion criteria for this review had focused solely on lifestyle and behavioural strategies. There were no studies of pharmacological or surgical interventions, which are likely to be more effective than diet and physical activity advice in achieving significant sustained weight loss and hence impacting on the outcomes measured in this review (Bray 2016). Randomised controlled trials comparing these interventions with placebo/usual care are, therefore, required.
There were limited data available about the baseline characteristics of participants in the included studies, in particular with regards to their baseline BMI and histological type, stage and grade of endometrial cancer, which restricted the number of subgroup analyses that could be conducted. This information is vital to investigate whether all endometrial cancer survivors derive a similar benefit from weight‐loss interventions or whether efforts should be targeted at specific subpopulations, such as those with the greatest BMI. Adequately powered studies including participants with both early and late stage, endometrioid and non‐endometrioid endometrial cancer are required to explore these issues further.
Quality of the evidence
There were only three RCTs that met the inclusion criteria for the review, meaning that a meta‐analysis could rarely be performed. The small number of studies also meant that assessment of the heterogeneity between studies is unlikely to be reliable, particularly with regard to dichotomous outcomes. Ideally, the calculation of confidence intervals for I2 and sensitivity analyses would have been performed, but neither were possible in RevMan.
Using the GRADE method of assessment, the certainty of the evidence for all outcomes was either low or very low, meaning that our confidence in the effect estimate was limited or very limited and that the true effect may, or is likely to, be substantially different from the estimate of effect. The reasons for downgrading certainty of the evidence included serious and very serious risk of bias in the primary studies (for example, unblinded participants, study personnel and outcome assessors, significant, unexplained, loss of participants to follow‐up), imprecision due to small‐study sizes and the risk of introducing an indirect comparison. The latter applied particularly to the study with the longest follow‐up period of 24 months (von Gruenigen 2009), which was the only one to show an effect of lifestyle and behavioural interventions on weight loss. The fact that this was only observed at 24 months and not at six or 12 months, despite the intervention being limited to six months duration, is noteworthy, especially as the study was not originally planned to follow participants beyond 12 months and that, by this point, of the 25 participants remaining, two had undergone gastric bypass and continued to be included in the final analysis.
Potential biases in the review process
The search strategy was overseen by the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer group to reduce the risk of introducing bias into the review process. No limitations with regards to language or date of publication were applied and deliberate efforts were made to search for ongoing clinical trials. Additional unpublished data were gained through correspondence with study authors and were included in the review. Decisions regarding the eligibility of studies for inclusion, 'Risk of bias' assessment, data collection and grading of evidence were performed by two review authors independently, with disagreements settled by a third review author. The main bias relates to the small number of included studies, all of which had only limited participant numbers and were of low or very low methodological quality, which meant that it was frequently not possible to conduct a meta‐analysis and prevented the drawing of firm conclusions regarding the clinical effectiveness of the intervention. It also meant that it was not possible to assess for publication bias. No conflicts of interest were identified for any of the study authors.
Agreements and disagreements with other studies or reviews
Despite increasing awareness of the need to improve survival and quality of life in women with a history of endometrial cancer, there is little published literature evaluating weight‐loss interventions in this regard. Of the four systematic reviews previously conducted, three have included at least some of the data from three of the studies incorporated here (Allison 2016; McCarroll 2014; von Gruenigen 2009), though do not appear to have had the same access to unpublished data as this review's authors. Chlebowski 2016 described the results of the SUCCEED trial (McCarroll 2014) and preliminary findings from the study by Allison 2016 on weight loss and quality of life, but did not attempt a meta‐analysis. Where a meta‐analysis has been performed, the results have been similar to those reported here. Lin 2016 focused on the effect of interventions to increase physical activity, but noted that only one study used an exercise intervention alone without combining it with some form of lifestyle/dietary modification. They found no benefit of these interventions on health‐related quality of life (standardised mean difference (SMD) 0.05, 95% CI ‐0.28 to 0.37, P = 0.78), though there were significant improvements in BMI compared with those receiving usual care. The authors included studies conducted in survivors of all gynaecological malignancies and did not attempt to evaluate the effects of physical activity in specific cancer subtypes. There was also substantial methodological heterogeneity between RCTs, which had widely differing physical activity regimens, ranging from residential rehabilitation courses comprising physical activity education to pelvic floor exercises, which was not investigated further in their analysis. When the eligibility criteria for included studies was extended to non randomised trials the results were again similar, with no improvement seen in quality of life at three and six months (Smits 2015). A fourth systematic review included only epidemiological studies, two single‐arm intervention studies and five cross‐sectional studies of physical activity, and concluded that increased exercise could contribute to better quality of life in endometrial cancer survivors (Babatunde 2016). They did not, however, conduct a meta‐analysis and had undertaken only a limited search of the literature.
No other individual RCT or review to date has evaluated the role of weight‐loss interventions in improving survival for women with endometrial cancer. The only evidence available showed a trend towards increased mortality with greater levels of television viewing, as a surrogate marker of inactivity, in women recruited into the NIH‐AARP Diet and Health Study and who had developed endometrial cancer during long‐term follow‐up, though this result was not significant (Arem 2016). There was no association between self‐reported activity levels following diagnosis and overall survival and unfortunately the study was underpowered to look specifically at cardiovascular and cancer‐related deaths. An adequately powered RCT incorporating survival outcomes is, therefore, required to address this question.
Authors' conclusions
Implications for practice.
There is limited evidence available regarding the efficacy of weight‐loss interventions in improving survival and reducing cardiovascular and metabolic event frequency in endometrial cancer survivors. There is very low‐certainty evidence that combined lifestyle and behaviour interventions are not associated with significant weight loss at 12 months and that there is no improvement in quality of life compared to those receiving usual care. The small number and size of the included randomised controlled trials (RCTs) in this review mean that any effect size estimates should be viewed with caution, however. Whilst demonstration of a significant benefit from receiving diet and physical activity education has not been possible, the low‐certainty evidence available suggests that it may not be associated with significant or serious adverse events, apart from an increase in musculoskeletal symptoms, and could easily be incorporated into routine follow‐up reviews at low cost.
Implications for research.
Further trials are required to specifically address the effects of weight‐loss interventions on overall, cancer‐specific and recurrence‐free survival and to compare different dietary modification regimens, including intermittent fasting versus continuous low‐calorie diets, pharmacological treatments associated with weight loss, such as orlistat and metformin, and bariatric surgery, all of which may be more effective in achieving and sustaining significant weight loss and hence impacting upon these outcomes. Bariatric surgery, in particular, has already been shown to result in greater weight loss than non‐surgical weight management, which is maintained in the longer term, and leads to the resolution of diabetes, reducing overall and cardiovascular‐caused mortality as well as improving some aspects of quality of life in non‐cancer patients (Arterburn 2015; Colquitt 2014). It would be anticipated that women treated for endometrial cancer would derive similar benefits from undergoing weight‐loss surgery, though whether they would also notice improvements in cancer‐caused mortality is currently unknown. Any future trials in this area should be of high methodological quality, adequately powered and with at least five years of follow‐up to allow time for the impact of these interventions on survival to be determined. Larger trials would also allow the relative benefit of weight‐loss interventions on specific subgroups of endometrial cancer survivors, such as the super‐obese and those diagnosed with early and late stage disease, to be evaluated.
Of the five ongoing RCTs that could not be included in this version of the review, four will not address any of these issues as they involve randomisation to different lifestyle and/or behavioural interventions or usual care and do not include survival in their outcome measures (Bantum 2015; Basen‐Engquist 2016; Nock 2011; Yeh 2015). The exception to this is the RCT Hawkes 2014, in which participants with early stage endometrioid endometrial cancer are being randomised to a levonorgesterol‐intrauterine device alone or in combination with either metformin or a subscription to a weight‐loss programme at a ratio of 3:3:5. Whilst the follow‐up period is only of six months duration, the primary outcome of the study is absence of endometrial cancer or atypical endometrial hyperplasia at this time point. This will allow the effect of metformin on short‐term recurrence‐free survival to be evaluated.
Acknowledgements
We would like to thank Jo Morrison (Co‐ordinating Editor) for clinical and editorial advice, Jo Platt (Information Specialist) for designing the search strategy and Gail Quinn, Clare Jess, and Tracey Harrison (Managing and Assistant Managing Editors), for their contribution to the editorial process.
Dr Emma Crosbie was awarded funding via the Cochrane Review Support Programme to expedite the completion of this review which is a priority topic area.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health. We would like to thank the referees for many helpful suggestions and comments, some of these include Rebecca Beeken, Evangelos Kontopantelis and Katharine Tylko‐Hill.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Uterine Neoplasms] explode all trees #2 ((uterus or uterine or endometri* or womb or corpus uteri) near5 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan*)) #3 #1 or #2 #4 MeSH descriptor: [Body Mass Index] this term only #5 BMI #6 MeSH descriptor: [Obesity] explode all trees #7 MeSH descriptor: [Body Weight] explode all trees #8 MeSH descriptor: [Adiposity] this term only #9 obese or obesity or overweight or weight or adiposity or excess body fat #10 4 or 5 or 6 or 7 or 8 or 9 #11 #3 and #10
Appendix 2. MEDLINE Ovid search strategy
1. exp Uterine Neoplasms/ 2. ((uterus or uterine or endometri* or womb or corpus uteri) adj5 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan*)).mp. 3. 1 or 2 4. body mass index/ 5. BMI.mp. 6. exp obesity/ 7. exp body weight/ 8. Adiposity/ 9. (obese or obesity or overweight or weight or adiposity or excess body fat).mp. 10. 4 or 5 or 6 or 7 or 8 or 9 11. randomized controlled trial.pt. 12. controlled clinical trial.pt. 13. randomized.ab. 14. placebo.ab. 15. clinical trials as topic.sh. 16. randomly.ab. 17. trial.ti. 18. 11 or 12 or 13 or 14 or 15 or 16 or 17 19. 3 and 10 and 18
Appendix 3. Embase search strategy
1. exp uterus cancer/ 2. ((uterus or uterine or endometri* or womb or corpus uteri) adj5 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or malignan*)).mp. 3. 1 or 2 4. body mass/ 5. BMI.mp. 6. exp obesity/ 7. exp body weight/ 8. (obese or obesity or overweight or weight or adiposity or excess body fat).mp. 9. 4 or 5 or 6 or 7 or 8 10. crossover procedure/ 11. double‐blind procedure/ 12. randomized controlled trial/ 13. single‐blind procedure/ 14. random*.mp. 15. factorial*.mp. 16. (crossover* or cross over* or cross‐over*).mp. 17. placebo*.mp. 18. (double* adj blind*).mp. 19. (singl* adj blind*).mp. 20. assign*.mp. 21. allocat*.mp. 22. volunteer*.mp. 23. 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 24. 3 and 9 and 23
Data and analyses
Comparison 1. Lifestyle intervention versus. usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival (6 months) | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Overall survival (12 months) | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Overall survival (24 months) | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.55] |
| 4 Adverse events‐musculoskeletal | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 19.03 [1.17, 310.52] |
| 5 Cancer‐specific survival (6 months) | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cancer‐specific survival (12 months) | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Cancer‐specific survival (24 months) | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Weight loss (6 months) | 3 | 131 | Mean Difference (IV, Random, 95% CI) | ‐1.88 [‐5.98, 2.21] |
| 9 Weight loss stratified by BMI (6 months) | 2 | 101 | Mean Difference (IV, Random, 95% CI) | ‐3.11 [‐9.32, 3.10] |
| 9.1 BMI <40 kg/m2 | 2 | 63 | Mean Difference (IV, Random, 95% CI) | ‐3.18 [‐10.29, 3.93] |
| 9.2 BMI >/40 kg/m2 | 2 | 38 | Mean Difference (IV, Random, 95% CI) | ‐2.89 [‐15.65, 9.88] |
| 10 Weight loss (12 months) | 2 | 91 | Mean Difference (IV, Random, 95% CI) | ‐8.98 [‐19.88, 1.92] |
| 11 Weight loss stratified by BMI (12 months) | 2 | 90 | Mean Difference (IV, Random, 95% CI) | ‐5.23 [‐11.59, 1.12] |
| 11.1 BMI <40 kg/m2 | 2 | 55 | Mean Difference (IV, Random, 95% CI) | ‐4.08 [‐11.20, 3.04] |
| 11.2 BMI >/40 kg/m2 | 2 | 35 | Mean Difference (IV, Random, 95% CI) | ‐9.76 [‐23.84, 4.32] |
| 12 Weight loss (24 months) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐18.26 [‐38.73, 2.21] |
| 13 Weight loss stratified by BMI (24 months) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐25.84 [‐81.40, 29.72] |
| 13.1 BMI <40 kg/m2 | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 2.12 [‐20.82, 25.06] |
| 13.2 BMI >/40 kg/m2 | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐54.58 [‐80.97, ‐28.19] |
| 14 Adverse events‐diarrhoea | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 4.53 [0.23, 90.51] |
| 15 Cardiovascular and metabolic event frequency (6 months) | 3 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Cardiovascular and metabolic event frequency (12 months) | 2 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Quality of life‐SF12 Physical Health component (6 months) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐2.29 [‐7.34, 2.76] |
| 18 Quality of life FACT‐G (6 months) | 2 | 95 | Mean Difference (IV, Random, 95% CI) | 2.51 [‐5.61, 10.64] |
| 19 Quality of life stratified by BMI (6 months FACT‐G) | 2 | 95 | Mean Difference (IV, Random, 95% CI) | 4.69 [1.39, 7.99] |
| 19.1 BMI <40 kg/m2 | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 4.01 [‐5.48, 13.51] |
| 19.2 BMI >/40 kg/m2 | 2 | 35 | Mean Difference (IV, Random, 95% CI) | 4.18 [‐0.13, 8.49] |
| 20 Quality of life FACT‐G (12 months) | 2 | 89 | Mean Difference (IV, Random, 95% CI) | 2.77 [‐0.65, 6.20] |
| 21 Quality of life stratified by BMI (12 months FACT‐G) | 2 | 89 | Mean Difference (IV, Random, 95% CI) | 2.83 [0.15, 5.50] |
| 21.1 BMI <40k g/m2 | 2 | 56 | Mean Difference (IV, Random, 95% CI) | 2.90 [‐0.40, 6.20] |
| 21.2 BMI >/40 kg/m2 | 2 | 33 | Mean Difference (IV, Random, 95% CI) | 2.68 [‐1.90, 7.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allison 2016.
| Methods |
Design Comment: Parallel design, 3‐arm, open‐label randomised controlled trial Quote: "..randomised, controlled study..." Setting Comment: Multicentre study in USA. Quote: "...multi‐site, pilot feasibility study..." Follow‐up Duration: 6 months Quote: "...6 month follow‐up..." |
|
| Participants |
Number of participants enrolled 41 women were randomised; 13 into Arm A, 13 into Arm B and 15 into Arm C. Six‐month follow‐up data were only available for 30 women at the time of undertaking this review (11 Arm A, 10 Arm B, 9 Arm C) Inclusion criteria Women aged 18 years or older Biopsy proven endometrial cancer of any histological type BMI greater than or equal to 30 kg/m2 ECOG PS 0‐1 No concurrent or planned chemo‐radiation Access to wireless Internet and/or smartphone Life expectancy > 1 year Exclusion criteria Significant medical condition that would affect compliance with protocol or ability to participate, e.g. uncontrolled hypertension, symptomatic cardiac disease Current participation in another weight‐loss programme or taking weight‐loss medication Another invasive malignancy in last five years (excluding non‐melanoma skin cancer) Autoimmune or immunosuppressive condition Currenty taking immunosuppressant medication Currently pregnant Baseline participant characteristics The mean age of participants was 62.2 years (SD 8.7 years), with a mean BMI of 39.1 kg/m2 (range 30 kg/m2 to 67 kg/m2). Details of co‐morbid conditions not collected by study authors. Participants had both type I and type II endometrial cancer, though the grade and stage of their malignancy was not provided. All had ECOG PS 0‐1 and had undergone surgical treatment of their endometrial cancer. Baseline characteristics of participants according to group allocation were not provided. |
|
| Interventions |
Arm A Comment: Telemedicine arm. Telephone‐based weight‐loss counselling undertaken by trained interventionists with guided digital measurements of weight, lean and fat mass. Counselling and weight‐loss measurements occurred at least weekly for the six months duration of the intervention. Quote: "The telemedicine arm included a Wifi scale that recorded at least weekly weights of participants. The scale automatically graphs the weights on a password protected website which permitted counsellors to have immediate feedback during weekly 15‐20 minute counselling sessions teach standard weight‐loss skills, including self‐monitoring, problem‐solving, enlisting social support, and overcoming negative thoughts according to a standard curriculum." Arm B Comment: Text4Diet Group. Participants received 3‐5 Short Message Service (SMS) text messages each day for the six‐month intervention period. The text message provided tips and reminders to encourage healthy eating and weight loss. Participants also received a digital scale to track and report weight and were prompted to do so once a week by text message. Quote: "The texting arm receives personalized text messages daily, following different monthly themes, e.g. Do not go to a party hungry. Eat a healthy snack before or bring a healthy dish with you to share. You will be more likely to stick to your goals! Since you have been meal planning do you find that you eat out less often? Y or N‐remember the restaurant website is a great way to help you plan a healthy meal to order. Different styles included encouraging statements, yes/no questions or multiple choice questions." All participants in Arms A and B recorded dietary intake and restricted calories to 1200 kcal/day to 1500 kcal/day. They were given an exercise goal of 50 minutes/week to 175 minutes/week of moderate, aerobic physical activity, e.g. brisk walking. Arm C Comment: Enhanced Usual Care Group. Participants provided with handouts based on American Cancer Society guidelines on healthy eating and exercise and did not receive any additional input from the research team. Quote:"...printed information from American Cancer Society guidelines on healthy eating and exercise...encourage weight loss through dietary monitoring and a walking program...these efforts were not reinforced or monitored by study staff..." |
|
| Outcomes |
Primary outcomes Overall survival: No deaths were reported in any arm for the duration of the study. Adverse events: Not reported Second outcomes Recurence‐free survival: Not reported Cancer‐specific survival: No cancer‐specific deaths were reported in any arm for the duration of the study Weight loss: Change in weight from baseline at 6 months reported Cardiovascular and metabolic event frequency: No events reported in any arm QoL: Change in QoL from baseline at 6 months reported using SF‐12 Physical Health component change score Quote: "Change in quality of life from baseline... SF‐12 Physical Health component change score" Power Comment: No power calculation performed. Aim of study was to provide estimates of the effect size of the intervention in order to power a full‐scale trial Quote: "The purpose will be to provide estimates for the size of an intervention effect achievable by the experimental intervention in order to power and justify a grant application for a full‐scale trial of a weight loss program in women with endometrial cancer. With a sample size of 30 participants per group, the true difference in mean weight loss between the groups can be estimated with a 95% confidence interval size of ±0.50σ, where σ is the population standard deviation of weight loss, assumed in this calculation to be the same in each of the two intervention groups and the control group. We will assess the comparability of variance across the groups and do exploratory analyses of possibly variance‐stabilizing transformations. Because this is a pilot study to derive parameters to design an appropriately‐powered study, hypothesis testing is not a primary goal of the statistical analysis of the data, although P‐values will be calculated." |
|
| Notes | Study not yet published. Information obtained through correspondence with research team. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer‐generated algorithm used at co‐ordinating centre to produce randomisation envelopes for each site. Quote: "The coordinating center used a computer generated algorithm to produce the randomization envelopes for each clinical site, with the general parameters of randomizing 1:1:1 across the three conditions." |
| Allocation concealment (selection bias) | Low risk | Comment: Next envelope chosen for each enrolled participant Quote: " The envelopes are then chosen sequentially as each participant was enrolled." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants and personnel were unblinded Quote: "There was no blinding." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Outcome assessments performed by unblinded study co‐ordinators Quote: "The outcome assessments were conducted by study coordinators and trained medical personnel (for blood draws, DEXA). The coordinators knew which condition the participants were in, but other medical personnel were not informed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Follow‐up Entered the study: 13 into Arm A , 13 into Arm B and 15 into Arm C Withdrew from study: 0 in Arm A, 0 in Arm B, 0 in Arm C Completed the study (at the time of correspondence): 11 in Arm A, 10 in Arm B, 9 in Arm C No missing data reported. Intention‐to‐treat analysis Comment: Not performed Quote: "Given we only had pre‐post assessment data and our main analyses used paired t‐tests and correlations, we were unable to do intention‐to treat analyses." |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol not published but trial registered prospectively on clinicaltrials.gov and all prespecified outcomes reported |
| Other bias | Unclear risk |
Source of funding Comment: Source of funding described Quote: "Cross‐TREC study funded by NCI U54‐CA155850 – University of Pennsylvania; U54 CA155626 – Harvard University; U54 CA155496CC – Washington University; U01 CA116850 – Fred Hutchinson Cancer Research Center." Ethical approval Comment: Ethical approval obtained Conflicts of interest Comment: No conflicts of interest reported Other sources The study failed to enrol 30 participants into each group within their allotted time. The reasons for this were not provided. Four centres open to recruitment although only the UniversitIes of Washington and Pennsylvania enrolled patients into the study. Only 30 participants had completed 6 months of follow‐up at the time of correspondence with the study's chief investigator. Further results will be available for inclusion when the review is updated. |
McCarroll 2014.
| Methods |
Design Comment: Parallel design, two‐arm, randomised controlled trial Quote: "...two‐group randomised trial...Patients were randomised to either: 1) a lifestyle intervention (SUCCEED) group that received nutrition, exercise, and behavioral modification counselling and 2) a usual care (UC) group..." Setting Comment: Single‐centre study in Ohio, USA Quote: "The Case Comprehensive Cancer Center (affiliates University Hospitals Case Medical Center and Cleveland Clinic) tumor registry was used to identify potential subjects. A letter was sent to potential patients describing the study and women were invited to attend an informational session." Follow‐up Comment: 12 months Quote: "Outcome measures were assessed in both groups at baseline, 3, 6, and 12 months." |
|
| Participants |
Number of participants enrolled 75 participants enrolled; 41 in the intervention arm and 34 in the control arm Inclusion criteria Histologically‐confirmed endometrial cancer diagnosed within last three years Stage I or II Undergone surgical treatment of endometrial cancer in the form of total abdominal hysterectomy and bilateral salpingo‐oophorectomy +/‐ lymphadenectomy No evidence of disease at time of enrolment Performance status 0‐2 BMI greater than or equal to 25 kg/m2 Medical clearance from primary care physician and approval to contact patient by treating gynae‐oncologist Exclusion criteria Unable to read consent form Severe depression, dementia or cognitive deficit Unavailable for longitudinal follow‐up assessment Pre‐existing medical conditions that prevent participation in unsupervised walking Participation in weight‐loss or exercise programme in preceding six months Baseline participant characteristics There were no significant differences in the baseline characteristics of participants between the two groups. The mean age of participants in the intervention arm was 57 years (SD 8.6 years) compared with 58.9 years (SD 10.9 years) in the control arm. Overall, the mean BMI was 36.5 kg/m2; 36.4 kg/m2 (SD 5.5) in the intervention arm and 36.5 kg/m2 (SD 9.6) in the control arm. The co‐morbidities hypertension and diabetes were present in 31.7% and 17.1% of participants in the intervention arm compared with 35.3% and 26.5% of participants in the control arm, respectively. All participants had a performance status of 0‐2. All participants underwent surgical treatment of their endometrial cancer, on average, 20.7 months earlier. In addition, 39.0% of participants in the intervention arm and 35.3% of participants in the control arm had undergone adjuvant radiotherapy. Details of grade, stage and histological type of endometrial cancer were not provided. |
|
| Interventions |
Intervention arm Comment: Sixteen group sessions focusing on diet and physical activity over six months and an additional three face‐to‐face counselling visits at 3, 6 and 12 months. Feedback and support were provided by a registered dietitian after the end of the group sessions by phone, email and newsletters. Quote: "Sixteen group sessions were conducted (10 weekly followed by 6 bi‐weekly) in the SUCCEED group. Physician face‐to‐face counselling visits occurred at 3, 6 and 12 months. Group topics included PA, nutrition and improving diet quality and behavior modification designed to increase women's self‐efficacy. Sessions were 60 min in length with 8–10 women per group. The RD weighed participants in private at the beginning of each session and weekly food/activity records were reviewed. After 6 months when the group sessions ended, additional feedback and support was provided by the RD via newsletters, telephone and email. Newsletter topics included holiday recipes, reinforcement of goals for increasing calcium, decreasing sodium, and ways to increase PA. The intervention followed a stepwise, phased approach using strategies outlined by social cognitive theory, indicating that the optimal intervention for a major behavior change should focus on establishing short‐term goals, enabling the person to build self‐efficacy." Control arm Comment: Received information brochure only. Participants also attended physician counselling sessions at 3, 6 and 12 months, but these visits did not include any lifestyle advice related to weight loss, physical activity or nutrition. Quote: "Patients randomized to the (control) group received an informational brochure (“Healthy Eating & Physical Activity Across Your Lifespan, Better Health and You”). |
|
| Outcomes |
Primary outcomes Overall survival: No deaths reported for the duration of the study period (12 months) Adverse events: Reported adverse events in both intervention and control arms Quote:"(Adverse events were reported) as required by the IRB...The true adverse events were all in the intervention group" Second outcomes Recurence‐free survival: Not reported Cancer‐specific survival: No deaths reported for the duration of the study period (12 months) Weight loss: Weight change from baseline at 3, 6 and 12 months reported Cardiovascular and metabolic event frequency: No events reported QoL: Change in QoL from baseline measured at 3, 6 and 12 months using FACT‐G questionnaire Quote: "Quality of life (QoL) was measured by the Functional Assessment of Cancer Therapy—General (FACT‐G)... FACT‐G was measured at baseline, 3, 6, and 12 months post‐baseline." Power Comment: A power calculation was performed and sufficient detail was provided to allow it to be replicated Quote: "Approximately 37 patients per group were needed to provide 80% power to detect a difference between groups in mean weight change from baseline to 12 months of 4.0 kg or greater (alpha= 0.05, two‐sided, SD= 6.0; effect size= 0.67) and to assess changes in PA with a similar effect size. Effect sizes of 0.5 are considered medium and 0.8 or greater large." |
|
| Notes | This is the definitive RCT following on from the pilot study also included in this review (von Gruenigen 2009) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Block randomisation performed according to baseline BMI Quote: "Randomization was stratified using block sizes of 6 or 8 by baseline BMI (25.0–39.9 versus > 40)" |
| Allocation concealment (selection bias) | Unclear risk | Comment:No details of allocation concealment provided by study authors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Blinding of participants and personnel not possible due to nature of intervention. Principle investigator involved in delivery of intervention so aware of randomisation. Quote: "Due to the interventions performed by the study team (dietitian, Physical therapist, psychologist, etc.), they were able to know who was in each group." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Principle investigator performed outcome assessments and was unblinded to treatment group allocation. This is unlikely to affect weight measurements but may impact upon quality of life assessments |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Follow‐up Entered into the study: 41 in intervention arm and 34 in control arm Withdrew from study: 6 in intervention arm and 10 in control arm Completed study: 35 in intervention arm and 24 in control arm Reasons for withdrawal from study not provided by authors. The study was underpowered at 12 months to detect a weight loss of 4.0 kg or greater in the intervention arm. Quote: "Attrition in the trial overall was 21.3%. Six (14.6%) patients in the LI group versus 10 (29.4%) in UC did not complete the twelve‐month assessments, P = 0.159. Thirty‐one (75.6%) participants in the (intervention arm) attended 14 or more of the 16 sessions; mean adherence was 84.1%. Intention‐to‐treat analysis Comment: Analyses were conducted according to an intention‐to‐treat protocol, however, only 85.4% of participants in the intervention arm and 70.6% of participants in the control arm attended for the 12 month assessments. Missing data were imputed by multiple imputation. Quote: "Analyses were done according to intention‐to‐treat principles. Missing data were examined and imputed by multiple imputation" |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol not published but trial registered prospectively on clinicaltrials.gov and all prespecified outcomes reported |
| Other bias | Low risk |
Source of funding Comment: Source of funding described Quote:"This research was supported by the American Cancer Society." Ethical approval Comment: Ethical approval was obtained Quote: "Institutional review board approval was granted..." Conflicts of interest Comment: No significant conflicts of interest noted |
von Gruenigen 2009.
| Methods |
Design Comment: Parallel design, two‐arm, randomised controlled trial Quote: "...prospective, two‐group randomized controlled trial..." Setting Comment: Single‐centre study in Ohio, USA Quote: "...women included in the cancer registry at the Ireland Cancer Center diagnosed from 2001–2004..." Follow‐up Comment: 24 months |
|
| Participants |
Number of participants enrolled 45 participants enrolled; 23 into the intervention arm and 22 into the control arm Inclusion criteria Histologically‐confirmed endometrial cancer Stage I or II Undergone surgical treatment of endometrial cancer in the form of total abdominal hysterectomy and bilateral salpingo‐oophorectomy +/‐ lymphadenectomy No evidence of disease at time of enrolment Performance status 0‐2 BMI > 25 kg/m2 Exclusion criteria Clear cell or papillary serous histology Baseline participant characteristics There were no significant differences in the baseline characteristics of participants between the two groups. The mean age of participants in the intervention arm was 54 years (SEM 2.0 years) compared with 55.5 years (SEM 1.6 years) in the control arm. Overall, the mean BMI was 42.3 kg/m2; 43.5 kg/m2 (SEM 2.1) in the intervention arm and 41.1 kg/m2 (SEM 2.2) in the control arm. The co‐morbidities hypertension, diabetes and metabolic syndrome were present in 65.2%, 17.4% and 26.1% of participants in the intervention arm compared with 36.4%, 27.3% and 27.3% of participants in the control arm, respectively. All participants had a performance status of 0‐2. All participants underwent surgical treatment of their endometrial cancer, on average, 2 years earlier. Details of adjuvant treatment and grade, stage and histological type of endometrial cancer were not provided. |
|
| Interventions |
Intervention arm Comment: Consisted of group sessions based on other nutrition and exercise goals and delivered by a registered dietitian, principle investigator and psychologist for 6 months. Participants were encouraged to gradually increase walking or other aerobic activity to 5 days per week for 45 minutes or more per session. Reinforcement of the content of group sessions was provided on an individual basis by the principle investigator at 3, 6 and 12 months. Quote: "...Group session topics included: weight loss readiness and goal‐setting, physical activity, portion sizes and food intake per mypyramid.gov, emotional eating/negative thinking, behavior modification, grocery shopping and reading food labels, relapse prevention, eating out and in social situations, and stress management...(The groups) met weekly for 6 weeks, bi‐weekly for 1 month, and monthly for 3 months. Participants were contacted by the RD (MBK) by phone or newsletter every week that the group did not meet. Phone calls were structured in content and included reinforcement and discussion regarding the previous week's topic. Participants were also given feedback on individual progress towards physical activity and nutrition goals...Pedometers were provided to and used by the LI group for patient feedback...Study participants in both groups saw the PI at 3, 6 and 12 months. Both groups received counselling regarding overall health concerns and LI participants received specific reinforcement of group session topics. " Control arm Comment: Received usual care and were provided with a generic booklet on improving health. Individual meetings were held with the principle investigator at 3, 6 and 12 months, however, these consisted of counselling regarding overall health concerns rather than a discussion about weight loss and physical activity. Quote: "the (control arm) received only an informational brochure after randomization ("Better Health and You," Weight Control Information Network, June 2004)...(control arm) participants did not receive any advice related to weight loss, physical activity or nutrition at these visits..." |
|
| Outcomes |
Primary outcomes Overall survival: Deaths reported for the duration of the study period (24 months) but insufficient data available to determine hazard ratio. Quote: "Within 24‐months, 2 patients deceased: n=1 to brain aneurysm and n=1 to kidney cancer." Adverse events: No reported adverse events in either intervention and control arms Quote:"(Adverse events reported) as required by the IRB. No adverse events due to study procedures occurred Second outcomes Recurence‐free survival: Not reported Cancer‐specific survival: Deaths reported for the duration of the study period (24 months) but insufficient data available to determine hazard ratio Quote: "During the study period...2 patients deceased: n=1 to brain aneurysm and n=1 to kidney cancer. Both deaths were in the control arm..." Weight loss: Weight change from baseline at 3, 6, 9, 12 and 24 months reported Cardiovascular and metabolic event frequency: No events reported up to 24 months follow‐up QoL: Change in QoL from baseline at 3, 6, 9 and months reported using FACT‐G questionnaire Quote: "QoL and self‐efficacy were assessed at baseline and at 3, 6, and 12 months... QoL was measured by the Functional Assessment of Cancer Therapy‐General (FACT‐G), a valid and reliable questionnaire evaluating physical, functional, family‐social, and emotional well‐being domains. A fatigue subscale (‐F) and an endometrial symptom subscale (‐En) were also used..." Power Comment: A power calculation was performed and sufficient detail was provided to allow it to be replicated Quote: "Approximately 25 patients per group were needed to provide 80% power to detect a difference between groups in mean weight change from baseline to 12 months of 5 kg (11 lb) or greater, representing approximately 5% for an obese female (alpha = 0.05, two‐sided, SD = 5.0). Five percent weight change is considered clinically relevant and a recommended goal for weight loss over 6 months." |
|
| Notes | The follow‐up was described as being of 12 months duration in the publication, however, when contacted the authors were able to provide data for weight change up to 24 months This was the pilot study preceding the definitive trial, which is also included in this review (McCarroll 2014) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: stratified block randomisation based on BMI employed Quote: "After enrolment, participants were randomly assigned (to intervention or control arm)... Randomization was stratified according to patient BMI (25–39.9 versus N40 kg/m2 ) using a stratified blocked randomization scheme." |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details of allocation concealment provided by study authors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Blinding of participants and personnel not possible due to nature of intervention. Principle investigator involved in delivery of intervention so aware of randomisation. Quote: "Due to the interventions performed by the study team (dietitian, Physical therapist, psychologist, etc.), they were able to know who was in each group." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Principle investigator performed outcome assessments and was unblinded to treatment group allocation. This is unlikely to affect weight measurements but may impact upon quality of life assessments |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Follow‐up Entered into the study: 23 in intervention arm, 22 in control arm Withdrew from study: 5 in intervention arm, 2 in control arm Completed study: 17 in intervention arm, 20 in control arm (though data from assessment at 12 months missing for 2 participants in the control arm) Two withdrawals in the intervention arm were due to issues with work, the reason for the other three withdrawals in this group were not stipulated. The two withdrawals from the control arm occurred prior to the first assessment at 3 months and the reasons were not stipulated. Quote: "Attrition in the trial overall was 16% [2 patients (10%) in the UC group versus 5 (22%) in the LI group; P = 0.242], therefore 84% completed follow‐up assessments. Specifically, 78% of patients [LI: 17/23 (74%), UC: (18/22) (82%)] completed the 12‐month assessment time point and there was no difference between groups (P = 0.523)" Intention‐to‐treat analysis Comment: Analyses were conducted according to an intention‐to‐treat protocol. There was, however, significant missing data; 19% of weight values and 15% to 19% of QoL data were missing. Missing data were imputed using three different techniques; last and next average (average of last and next known values), previous row mean method and last observation carried forward. All produced similar findings and so only the results obtained using the first approach were included in the journal publication. Quote: "Imputation was done for 19% (35/ 180) of weight values, 10 patients (LI: 6 and UC: 4) had weight values imputed for the final weight. These patients opted to not complete the assessment and values were imputed based on the most recent physician visit, if they had one or were imputed...Imputation was done on between 15–19% of values for the various QoL and eating behavior measures." |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol not published but trial registered prospectively on clinicaltrials.gov and all prespecified outcomes reported. |
| Other bias | High risk |
Source of funding Comment: Source of funding described Quote:"This research was supported by a grant from the Lance Armstrong Foundation " Ethical approval Comment: Ethical approval was obtained Quote: "Institutional review board approval was obtained ..." Conflicts of interest Comment: No significant conflicts of interest noted Other sources Study failed to recruit sufficient numbers to meet a priori total in time frame. One patient in the intervention arm underwent gastric bypass at 9 months after the start of the intervention and another between 12 and 24 months. Both were included in the final analysis. |
BMI: body mass index; ECOG: European Cooperative Oncology Group; FACT‐G: Functional Assessment of Cancer Therapy‐General; QoL: quality of life; RCT: randomised controlled trial; SD: standard deviation; SEM: standard error of the mean
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Babatunde 2016 | Comment: Systematic review rather than randomised controlled trial |
| Beck 2015 | Comment: Wrong patient population Quote: "Obese (Mean BMI = 35.8) female patients (Mean age 58.41) with breast (n = 15), colon (n = 1), and endometrial cancers (n = 1) were recruited and randomly assigned to receive exercise and nutrition intervention without (POWER, n = 10) or with an additional mindfulness component (MORE POWER, n = 7)" |
| Donnelly 2011 | Comment: Wrong indication Quote: "To determine the feasibility and effectiveness of a physical activity (PA) behavioural change intervention in managing cancer‐related fatigue (CRF) among gynaecological cancer survivors during and post anti‐cancer treatments" |
| Fasching 2009 | Comment: Systematic review rather than randomised controlled trial |
| Gil 2007 | Comment: Systematic review rather than randomised controlled trial |
| Koutoukidis 2015 | Comment: Systematic review rather than randomised controlled trial |
| Koutoukidis 2017 | Comment: Wrong indication Quote: "Aim....(to determine if) Shape‐up following cancer treatment programme is more effective than usual care in improving the health‐related quality of life of endometrial cancer survivors" |
| Lin 2016 | Comment: Systematic review rather than randomised controlled trial |
| Rossi 2016 | Comment: Wrong indication Quote: "...aims of this study were to 1) assess the feasibility of a 12‐week physical activity intervention for obese socioculturally diverse endometrial cancer survivors in Bronx, NY; 2) determine the probable effectiveness of the intervention on physical activity, waist circumference, physical function and quality of life; and 3) evaluate changes in self‐efficacy, outcome expectations, social support, and self‐regulation during the 12‐week physical activity intervention." |
| Smits 2015 | Comment: Systematic review rather than randomised controlled trial |
BMI: body mass index
Characteristics of ongoing studies [ordered by study ID]
Bantum 2015.
| Trial name or title | Hula‐based exercise program in Increasing physical activity in breast, cervical, endometrial, or ovarian cancer survivors |
| Methods | Parallel‐design, open‐label, randomised trial |
| Participants | Women aged 21 years and older, living in Oahu, Hawaii, diagnosed with primary breast, cervical, endometrial or ovarian cancer (stage I‐III), completed initial regional and systemic breast cancer treatment at least 2 months earlier, physically capable of doing hula‐based physical activity, not currently undergoing chemo‐ or radiation therapy |
| Interventions | Arm I: Hula‐based exercise programme consisting of warm‐up, conditioning and cool‐down over 60 minutes, twice a week and a home‐based hula practice for 10‐15 minutes, three times per week for six months Arm II: The same hula‐based exercise programme beginning six months after study enrolment |
| Outcomes | Primary outcome measure: feasibility of programme (compliance, satisfaction) Secondary outcome measures: biomarkers (sex hormones, cytokines, inflammatory markers e.g. CRP, leptin, IGF‐1, IGFBP‐3), DNA methylation patterns, self‐reported physical activity, quality of life, depression, affective states, social constrains and cognitive functioning |
| Starting date | September 2013 |
| Contact information | Erin Bantum University of Hawaii Cancer Center, USA |
| Notes | Clinical trials.gov identifier: NCT02351479 |
Basen‐Engquist 2016.
| Trial name or title | Feasibility of the NEXT steps weight loss intervention +/‐ resistance training for endometrial cancer survivors: Effect on lean mass & biomarkers |
| Methods | Parallel‐design, unblinded, three‐arm, randomised controlled trial |
| Participants | Women aged 18 and over diagnosed with stage I and II endometrial cancer in the preceding three years and at least six months following treatment, with a BMI between 30 kg/m2 to 45 kg/m2, with access to a computer or smartphone and Wifi for syncing Fitbit devices and willing to travel to MD Andersen, Texas, USA |
| Interventions | NEXT Steps‐Aerobic Exercise and Resistance Training (NS‐ART). Participants entered into exercise plan focused on physical activity and resistance training of six months duration. Physical activity guidelines workbook distributed along with activity monitor. Participants receive phone calls and text messages for support in reaching diet and exercise goals. Participants also given resistance bands and exercise handouts. NEXT Steps‐Aerobic Exercise (NS‐AE). Partipants placed into an exercise plan of six months duration focused on physical activity only. Participants receive phone calls and text messages for support in reaching diet and exercise goals. Standard Care Control Group (CG). Participants receive standard care consisting of phone calls asking about their health and self‐help materials for six months. |
| Outcomes | Primary outcome measure: feasibility of interventions (consent, retention, adherence and satisfaction rates) Second outcome measure: change in lean body mass (weight and measured by dual‐energy x‐ray absorptiometry) |
| Starting date | October 2016 |
| Contact information | Karen Basen‐Engquist, MD Andersen Cancer Center, USA |
| Notes | Clinical Trials.gov identifier: NCT02774759 |
Hawkes 2014.
| Trial name or title | Improving treatment for obese women with early stage cancer of the uterus: rationale and design of the levonorgestrel intrauterine device +/‐ metformin +/‐ weight loss in endometrial cancer (feMME) trial |
| Methods | Parallel design, open‐label, three‐arm, randomised trial with patients randomised in a 3:3:5 ratio to the interventions |
| Participants | Grade 1 endometrioid, endometrial cancer, apparent stage I disease on CT and MRI scan, no lymphovascular space invasion on endometrial curettings, no or minimal myometrial invasion on MRI scan and a normal (less than or equal to 30 U/mL) CA‐125 level. |
| Interventions | Levonorgesterol‐IntraUterine Device only. Standard, Austrialian Therapeutic Goods Administration approved device to be inserted into the uterine cavity and left for six months Levonorgesterol‐IntraUterine Device plus Metformin at a dose of 1000 mg daily, given orally with meals for six months Levonorgesterol‐IntraUterine Device plus weight‐loss intervention. Participants will be provided with a voucher for a comprehensive subscription to a weight‐loss program (Weight Watchers®) and are encouraged to attend the face‐to‐face group meetings and to use the online tools and social networking opportunities for six months. |
| Outcomes | Primary outcome measure: Absence of invasive endometrial cancer or atypical endometrial hyperplasia at six months on dilatation and curettage Secondary outcome measures: change in weight and physical activity, quality of life, anxiety and depression symptomatology, health service usage, pelvic floor distress, diet, serum and tissue predictive biomarkers |
| Starting date | October 2012 |
| Contact information | Andreas Obermair Queensland Centre for Gynaecological Cancer, Australia |
| Notes | Clinical trials.gov identifier:NCT01686126 |
Nock 2011.
| Trial name or title | Assisted exercise in obese endometrial cancer patients |
| Methods | Parallel‐design, open‐label, randomised trial |
| Participants | Adult women with histologically‐confirmed grade 1‐2 , stage I endometrial cancer diagnosed in last four years, have not received adjuvant chemotherapy and completed treatment at least three months earlier, successfully completed a cardiopulmonary stress test, medical clearance by treating team to participate in exercise programme, BMI greater than or equal to 30 kg/m2 |
| Interventions | 'Assisted Rate' Exercise Intervention: cycling on stationary, recumbent exercise bike with motor assistance to maintain pedaling rate 35% greater than their voluntary rate. Participants will complete 45 to 60 minute sessions three times per week for eight weeks 'Voluntary Rate' Exercise Intervention: cycling on stationary, recumbent exercise bike at preferred pedaling rate for 45 to 60 minutes, three times per week for eight weeks |
| Outcomes | Primary outcome measures: changes in body weight, fitness, bi‐manual dexterity, exercise motivation and self‐reported eating behaviour Secondary outcome measures: changes in food behaviour in response to high‐ and low‐calorie visual stimuli under fed and starved conditions, genetic (e.g. dopamine receptor and transporter) and serum biomarkers (e.g. leptin) |
| Starting date | September 2011 |
| Contact information | Nora Nock Case Comprehensive Cancer Center, Cleveland, USA |
| Notes | Clinical Trials.gov identifier: NCT01870947 |
Yeh 2015.
| Trial name or title | Survivorship Promotion In Reducing IGF‐1 Trial (SPIRIT) |
| Methods | Parallel‐design, single‐blinded, three‐arm, randomised controlled trial |
| Participants | Women and men aged 18 years and older, with a prior diagnosis of a solid malignancy (including endometrial cancer), who have completed surgical, chemo‐ or radiation therapy at least three months previously and with an anticipated treatment‐free lifespan of more than 12 months, BMI greater than or equal to 25 kg/m2 and less than 400 lbs, with internet and phone access and willingness to change diet, physical activity and weight. |
| Interventions | Active Comparator: Self‐Directed. Meeting with trial team at beginning of study and provision of written information about weight management Experimental: Coach‐Directed Behavioral Weight Loss.Remote Lifestyle Coaching Intervention‐behaviour based telephonic coaching with web‐based support to promote healthy lifestyle and weight loss. The goal of the intervention is to achieve at least 5% weight loss in the first six months and to maintain these improvements through month 12 by meeting dietary and exercise goals Experimental: Metformin up to 2000 mg per day. Dosing can be flexible, depending on tolerance, and given 2‐3 times per day orally with meals for 12 months |
| Outcomes | Primary outcome measures: IGF‐1 levels, IGF‐1:IGFBP3 ratio at 6 months Secondary outcome measures: IGF‐1 levels, IGF‐1:IGFBP3 ratio at 12 months Other outcome measures: change in weight, BMI, dietary intake, physical activity, glucose, insulin, HbA1C, IL‐8, CRP levels and side effects in experimental arms |
| Starting date | May 2015 |
| Contact information | Jessica Yeh, John Hopkins, Maryland, USA |
| Notes | Clinical Trials.gov identifier: NCT02431676 |
BMI: body mass index; CA‐125: cancer antigen 125; CRP: C‐reactive protein; CT: computed tomography; iGF‐1: insulin growth factor; IGFBP‐3: insulin‐like growth factor binding protein‐3; IL‐8: interleukin‐8; MRI: magnetic resonance imaging
Differences between protocol and review
For the outcomes of overall and cancer‐specific survival insufficient data were available from published reports or correspondence with study authors to allow the calculation of hazard ratios. Instead, survival was treated as a dichotomous outcome and the risk ratio for survival was calculated in its place. Depending upon the assembled research, the study authors had planned to organise the data by population and, within the data categories, to explore the main comparisons of the review. Due to the small number of studies and participants included in the review this was not possible.
Contributions of authors
All review authors contributed to the study conception and design. Aquisition of data was undertaken by Sarah Kitson, Neil Ryan and Michelle MacKintosh Analysis and interpretation were undertaken by Sarah Kitson, Neil Ryan, James Duffy, Richard Edmondson and Emma Crosbie. Drafting of the manuscript was performed by Sarah Kitson, James Duffy, and Emma Crosbie and was reviewed by all authors. The review update will be undertaken by Emma Crosbie.
Sources of support
Internal sources
-
Cochrane Review Support Programme, UK.
Dr Emma Crosbie has been awarded funding via the Cochrane Review Support Programme to expedite the completion of this review which is a priority topic area.
External sources
-
National Institute for Health Research Clinician Scientist Fellowship, UK.
Dr Emma Crosbie and Dr Sarah Kitson are funded through an National Institute for Health Research (NIHR) Clinician Scientist award (NIHR‐CS‐012‐009). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
-
MRC, UK.
Dr Neil Ryan is an Medical Research Council Doctoral Research Fellow (MR/M018431/1)
Declarations of interest
Sarah Kitson: None known. Neil Ryan: None known. Michelle Mackintosh: None known. Richard Edmondson: None known. James Duffy: None known. Emma Crosbie: None known.
New
References
References to studies included in this review
Allison 2016 {published and unpublished data}
- Allison K, Haggerty A, Hagemann A, Horowitz N, Thornquist M. Lifestyle beyond cancer: Obesity and weight loss in endometrial cancer survivors: a randomized, multisite trial. TREC (Transdisciplinary Research on Energetics and Cancer) Scientific Grantee’s Meeting, National Cancer Institute. March 2016.
McCarroll 2014 {published and unpublished data}
- McCaroll ML, Armbuster S, Frasure HE, Gothard MD, Gil KM, Kavanagh MB, et al. Self‐efficacy, quality of life and weight loss in overweight/obese endometrial cancer survivors (SUCCEED): A randomized controlled trial. Gynecologic Oncology 2014;132:397‐402. [DOI] [PubMed] [Google Scholar]
- Gruenigen V, Frasure H, Kavanagh MB, Janata J, Waggoner S, Rose P, et al. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): a randomized controlled trial. Gynecologic Oncology 2012;125(3):699‐704. [DOI] [PubMed] [Google Scholar]
von Gruenigen 2009 {published and unpublished data}
- Gruenigen VE, Courneya KS, Gibbons HE, Kavanagh MB, Waggoner SE, Lerner E. Feasibility and effectiveness of a lifestyle intervention program in obese endometrial cancer patients: a randomized trial. Gyencologic Oncology 2008;109(1):19‐26. [DOI] [PubMed] [Google Scholar]
- Gruenigen VE, Gibbons HE, Kavanagh MB, Janata JW, Lerner E, Courneya KS. A randomized trial of a lifestyle intervention in obese endometrial cancer survivors: quality of life outcomes and mediators of behavior change. Health and Quality of Life Outcomes 2009;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Babatunde 2016 {published data only}
- Babatunde OA, Adams SA, Orekoya O, Basen‐Engquist K, Steck SE. Effect of physical activity on quality of life as perceived by endometrial cancer survivors: A systematic review. International Journal of Gynecological Cancer 2016;26(9):1727‐40. [DOI] [PubMed] [Google Scholar]
Beck 2015 {published data only}
- Beck AC, Garland EL, Hansen PA, Walker D, Reimers C, Thomas EA, et al. Integrated mindfulness‐oriented recovery enhancement (MORE) and physical health intervention for cancer survivors with obesity: Preliminary results from a pilot randomized controlled trial. Journal of Clinical Oncology 2015;33(29 SUPPL. 1):var. pagings. [Google Scholar]
Donnelly 2011 {published data only}
Fasching 2009 {published data only}
- Fasching PA, Hubner J, Kleeberg UR. Physical exercise and sport for preventing and treating cancers. Onkologe 2009;15(7):696‐701. [Google Scholar]
Gil 2007 {published data only}
- Gil KM, Gruenigen VE. Physical activity and gynecologic cancer survivorship. Recent Results in Cancer Research 2011;186:305‐15. [DOI] [PubMed] [Google Scholar]
Koutoukidis 2015 {published data only}
- Koutoukidis DA, Knobf MT, Lanceley A. Obesity, diet, physical activity, and health‐related quality of life in endometrial cancer survivors. Nutrition Reviews 2015;73(6):399‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koutoukidis 2017 {published and unpublished data}
- Koutoukidis DA, Beeken RJ, Manchanda R, Michalopoulou M, Burnell M, Knobf MT, et al. Recruitment, adherence, and retention of endometrial cancer survivors in a behavioural lifestyle programme: the Diet and Exercise in Uterine Cancer Survivors (DEUS) parallel randomised pilot trial. BMJ Open 2017;7(10):epub. [PUBMED: 10.1136/bmjopen‐2017‐018015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2016 {published data only}
- Lin K‐Y, Frawley HC, Denehy L, Feil D, Granger CL. Exercise interventions for patients with gynaecological cancer: a systematic review and meta‐analysis. Physiotherapy 2016;102(4):309‐19. [DOI] [PubMed] [Google Scholar]
Rossi 2016 {published data only}
- Rossi A, Garber CE, Ortiz M, Shankar V, Goldberg GL, Nevadunsky NS. Feasibility of a physical activity intervention for obese, socioculturally diverse endometrial cancer survivors. Gynecologic Oncology 2016;142(2):304‐10. [DOI] [PubMed] [Google Scholar]
Smits 2015 {published data only}
- Smits A, Lopes A, Das N, Bekkers R, Massuger L, Galaal K. The effect of lifestyle interventions on the quality of life of gynaecological cancer survivors A systematic review and meta‐analysis. Gynecologic Oncology 2015;139(3):546‐52. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Bantum 2015 {published data only (unpublished sought but not used)}
- Bantum E. Hula‐based exercise program in Increasing physical activity in breast, cervical, endometrial, or ovarian cancer survivors. National Institute for Health 2015.
Basen‐Engquist 2016 {published data only (unpublished sought but not used)}
- Basen‐Engquist K. Feasibility of the NEXT steps weight loss intervention +/‐ resistance training for endometrial cancer survivors: Effect on lean mass & biomarkers. Clinical Trials.gov 2017.
Hawkes 2014 {published data only (unpublished sought but not used)}
- Hawkes AL, Quinn M, Gebski V, Armes J, Brennan D, Janda M, et al. Improving treatment for obese women with early stage cancer of the uterus: rationale and design of the levonorgestrel intrauterine device +/‐ metformin +/‐ weight loss in endometrial cancer (feMME) trial. Comptemporary Clinical Trials 2014;39(1):14‐21. [DOI] [PubMed] [Google Scholar]
Nock 2011 {published data only (unpublished sought but not used)}
- Nock N. Assisted exercise in obese endometrial cancer patients. Clinical Trials.gov 2011.
Yeh 2015 {published data only (unpublished sought but not used)}
- Yeh J. Survivorship promotion In reducing IGF‐1 Trial (SPIRIT). Clinical Trials.gov 2015.
Additional references
Arem 2016
- Arem H, Pfeiffer RM, Moore SC, Brinton LA, Matthews CE. Body mass index, physical activity, and television time in relation to mortality risk among endometrial cancer survivors in the NIH‐AARP Diet and Health Study cohort. Cancer Causes Control 2016;27(11):1403‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arterburn 2015
- Arterburn DE, Olsen MK, Smith VA, Livingston EH, Scoyoc L, Yancy WS Jr, et al. Association between bariatric surgery and long‐term survival. JAMA 2015;313(1):62‐70. [DOI] [PubMed] [Google Scholar]
Bray 2016
- Bray GA, Fruhbeck G, Ryan DH, Wilding JPH. Management of obesity. Lancet 2016;387(10031):1947‐56. [DOI] [PubMed] [Google Scholar]
Calle 2003
- Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. New England Journal of Medicine 2003;348(17):1625‐38. [DOI] [PubMed] [Google Scholar]
Cancer Research UK 2014a
- Cancer Research UK. Uterine cancer incidence statistics. www.cancerresearchuk.org/health‐professional/cancer‐statistics/statistics‐by‐cancer‐type/uterine‐cancer/incidence; (accessed 7 December 2016).
Cancer Research UK 2014b
- Cancer Research UK. Uterne cancer mortality statistics. www.cancerresearchuk.org/health‐professional/cancer‐statistics/statistics‐by‐cancer‐type/uterine‐cancer/survival; (accessed 7 December 2106).
Chan 2011
- Chan RJ, Webster J, Marquart L. Information interventions for orienting patients and their carers to cancer care facilities. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008273.pub2] [DOI] [PubMed] [Google Scholar]
Chlebowski 2016
- Chlebowski RT, Reeves MM. Weight loss randomized intervention trials in female cancer survivors. Journal of Clinical Oncology 2016;34(35):4238‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colquitt 2014
- Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD003641.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crosbie 2012
- Crosbie EJ, Roberts C, Qian W, Swart AM, Kitchener HC, Renehan AG. Body mass index does not influence post‐treatment survival in early stage endometrial cancer: results from the MRC ASTEC trial. European Journal of Cancer 2012;48(6):853‐64. [DOI] [PubMed] [Google Scholar]
Crosbie 2016
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [DOI: 10.1002/9780470693926.ch15] [DOI] [Google Scholar]
Department of Health 2015
- Department of Health. Chief Medical Officer annual report 2014: women’s health. www.gov.uk/government/publications/chief‐medical‐officer‐annual‐report‐2014‐womens‐health (accessed 7 December 2016).
Dossus 2013
- Dossus L, Lukanova A, Rinaldi S, Allen N, Cust AE, Becker S, et al. Hormonal, metabolic, and inflammatory profiles and endometrial cancer risk within the EPIC cohort ‐ a factor analysis. American Journal of Epidemiology 2013;177(8):787‐99. [DOI] [PubMed] [Google Scholar]
El‐Safadi 2012
- El‐Safadi S, Sauerbier A, Hackethal A, Munstedt K. Body weight changes after the diagnosis of endometrial cancer and their influences on disease‐related prognosis. Archives of Gynecology and Obstetrics 2012;285(6):1725‐9. [PUBMED: 10.1007/s00404‐012‐2224‐7] [DOI] [PubMed] [Google Scholar]
Figuls 2013
- Roqué i Figuls M, Martínez García L, Martinez‐Zapata MJ, Pacheco R, Mauricio D, Bonfill Cosp X. Interventions for treating overweight or obesity in adults: an overview of systematic reviews. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD010665] [DOI] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 7 December 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Irwin 2015
- Irwin ML, Fabian C, McTiernan A. Risk reduction from weight management and physical activity interventions. Advances in Experimental Medicine and Biology. Vol. 862, Springer, 2015:193‐212. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuo 2016
- Matsuo K, Moeini A, Cahoon SS, Machida H, Ciccone MA, Grubbs BH, et al. Weight change pattern and survival outcome of women with endometrial cancer. Annals of Surgical Oncology 2016 [Epub ahead of print];9:5237‐9. [DOI: 10.1245/s10434-016-5237-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishra 2012
- Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, et al. Exercise interventions on health‐related quality of life for people with cancer during active treatment. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD008465] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morey 2009
- Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, et al. Effects of home‐based diet and exercise on functional outcomes among older, overweight long‐term cancer survivors: RENEW: a randomized controlled trial. JAMA 2009;301(18):1883‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCI 2016
- National Cancer Institute. Cancer stat facts: endometrial cancer. seer.cancer.gov/statfacts/html/corp.html (accessed 7 December 2016).
Papadia 2006
- Papadia A, Ragni N, Salom EM. The impact of obesity on surgery in gynecological oncology: a review. International Journal of Gynecological Cancer 2006;16:944‐52. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Reeves 2007
- Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 2007;335(7630):1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Renehan 2008
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body‐mass index and incidence of cancer: a systematic review and meta‐analysis of prospective observational studies. Lancet 2008;371(9612):569‐78. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rock 2013
- Rock CL, Pande C, Flatt SW, Ying C, Pakiz B, Parker BA, et al. Favorable changes in serum estrogens and other biologic factors after weight loss in breast cancer survivors who are overweight or obese. Clinical Breast Cancer 2013;13(3):188‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rock 2015
- Rock CL, Flatt SW, Byers TE, Colditz GA, Demark‐Wahnefried W, Ganz PA, et al. Results of the exercise and nutrition to enhance recovery and good health for you (ENERGY) trial: a behavioral weight loss intervention in overweight or obese breast cancer survivors. Journal of Clinical Oncology 2015;33(28):3169‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glaziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration. 2011. Available from handbook.cochrane.org. [available from http://handbook.cochrane.org/]
Stolley 2009
- Stolley MR, Sharp LK, Oh A, Schiffer L. A weight loss intervention for African American breast cancer survivors, 2006. Preventing Chronic Disease 2009;6(1):A22. [PMC free article] [PubMed] [Google Scholar]
Swisher 2015
- Swisher AK, Abraham J, Bonner D, Gilleland D, Hobbs G, Kurian S, et al. Exercise and dietary advice intervention for survivors of triple‐negative breast cancer: effects on body fat, physical function, quality of life, and adipokine profile. Support Care Cancer 2015;23(10):2995‐3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2016
- Wan YL, Beverley‐Stevenson R, Carlisle D, Clarke S, Edmondson RJ, Glover S, et al. Working together to shape the endometrial cancer research agenda: The top ten unanswered research questions. Gynecologic Oncology 2016;143(2):287‐93. [DOI] [PubMed] [Google Scholar]
Ward 2012
- Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecological Oncology 2012;126(2):176‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kitson 2017
- Kitson S, Duffy JMN, Ryan N, MacKintosh ML, Crosbie EJ. Interventions for weight reduction in obesity to improve survival in women with endometrial cancer. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD012513] [DOI] [PMC free article] [PubMed] [Google Scholar]


