Abstract
Background
People with dementia who are being cared for in long‐term care settings are often not engaged in meaningful activities. Offering them activities which are tailored to their individual interests and preferences might improve their quality of life and reduce challenging behaviour.
Objectives
∙ To assess the effects of personally tailored activities on psychosocial outcomes for people with dementia living in long‐term care facilities.
∙ To describe the components of the interventions.
∙ To describe conditions which enhance the effectiveness of personally tailored activities in this setting.
Search methods
We searched ALOIS, the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register, on 16 June 2017 using the terms: personally tailored OR individualized OR individualised OR individual OR person‐centred OR meaningful OR personhood OR involvement OR engagement OR engaging OR identity. We also performed additional searches in MEDLINE (Ovid SP), Embase (Ovid SP), PsycINFO (Ovid SP), CINAHL (EBSCOhost), Web of Science (ISI Web of Science), ClinicalTrials.gov, and the World Health Organization (WHO) ICTRP, to ensure that the search for the review was as up to date and as comprehensive as possible.
Selection criteria
We included randomised controlled trials and controlled clinical trials offering personally tailored activities. All interventions included an assessment of the participants' present or past preferences for, or interests in, particular activities as a basis for an individual activity plan. Control groups received either usual care or an active control intervention.
Data collection and analysis
Two authors independently checked the articles for inclusion, extracted data and assessed the methodological quality of included studies. For all studies, we assessed the risk of selection bias, performance bias, attrition bias and detection bias. In case of missing information, we contacted the study authors.
Main results
We included eight studies with 957 participants. The mean age of participants in the studies ranged from 78 to 88 years and in seven studies the mean MMSE score was 12 or lower. Seven studies were randomised controlled trials (three individually randomised, parallel group studies, one individually randomised cross‐over study and three cluster‐randomised trials) and one study was a non‐randomised clinical trial. Five studies included a control group receiving usual care, two studies an active control intervention (activities which were not personally tailored) and one study included both an active control and usual care. Personally tailored activities were mainly delivered directly to the participants; in one study the nursing staff were trained to deliver the activities. The selection of activities was based on different theoretical models but the activities did not vary substantially.
We found low‐quality evidence indicating that personally tailored activities may slightly improve challenging behaviour (standardised mean difference (SMD) −0.21, 95% confidence interval (CI) −0.49 to 0.08; I² = 50%; 6 studies; 439 participants). We also found low‐quality evidence from one study that was not included in the meta‐analysis, indicating that personally tailored activities may make little or no difference to general restlessness, aggression, uncooperative behaviour, very negative and negative verbal behaviour (180 participants). There was very little evidence related to our other primary outcome of quality of life, which was assessed in only one study. From this study, we found that quality of life rated by proxies was slightly worse in the group receiving personally tailored activities (moderate‐quality evidence, mean difference (MD) −1.93, 95% CI −3.63 to −0.23; 139 participants). Self‐rated quality of life was only available for a small number of participants, and there was little or no difference between personally tailored activities and usual care on this outcome (low‐quality evidence, MD 0.26, 95% CI −3.04 to 3.56; 42 participants). We found low‐quality evidence that personally tailored activities may make little or no difference to negative affect (SMD −0.02, 95% CI −0.19 to 0.14; I² = 0%; 6 studies; 589 participants). We found very low quality evidence and are therefore very uncertain whether personally tailored activities have any effect on positive affect (SMD 0.88, 95% CI 0.43 to 1.32; I² = 80%; 6 studies; 498 participants); or mood (SMD −0.02, 95% CI −0.27 to 0.23; I² = 0%; 3 studies; 247 participants). We were not able to undertake a meta‐analysis for engagement and the sleep‐related outcomes. We found very low quality evidence and are therefore very uncertain whether personally tailored activities improve engagement or sleep‐related outcomes (176 and 139 participants, respectively). Two studies that investigated the duration of the effects of personally tailored activities indicated that the intervention effects persisted only during the delivery of the activities. Two studies reported information about adverse effects and no adverse effects were observed.
Authors' conclusions
Offering personally tailored activities to people with dementia in long‐term care may slightly improve challenging behaviour. Evidence from one study suggested that it was probably associated with a slight reduction in the quality of life rated by proxies, but may have little or no effect on self‐rated quality of life. We acknowledge concerns about the validity of proxy ratings of quality of life in severe dementia. Personally tailored activities may have little or no effect on negative affect and we are uncertain whether they improve positive affect or mood. There was no evidence that interventions were more likely to be effective if based on one specific theoretical model rather than another. Our findings leave us unable to make recommendations about specific activities or the frequency and duration of delivery. Further research should focus on methods for selecting appropriate and meaningful activities for people in different stages of dementia.
Plain language summary
Personally tailored activities for people with dementia in long‐term care
Background
People with dementia living in nursing or residential homes often have too little to do. Activities which are available may not be meaningful to them. If a person with dementia has the chance to take part in activities which match his or her personal interests and preferences, this may lead to a better quality of life, may reduce challenging behaviour such as restlessness or aggression, and may have other positive effects.
Purpose of this review
We wanted to investigate the effects of offering people with dementia who were living in care homes activities tailored to their personal interests.
Studies included in the review
In June 2017 we searched for trials that had offered some participants an activity programme based on their individual interests (an intervention group) and had compared them with other participants who were not offered these activities (a control group).
We found eight studies including 957 people with dementia living in care homes. Seven of the studies were randomised controlled trials (RCTs), meaning that it was decided at random whether participants were in the intervention group or the control group. One study was not randomised, which puts it at higher risk of biased results. The number of participants included in the studies ranged from 25 to 180. They all had moderate or severe dementia and almost all had some kind of challenging behaviour when the study started. The studies lasted from 10 days to nine months. In all the studies, the people in the intervention groups got an individual activity plan. Most of the activities took place in special sessions run by trained staff, but in one study, the nursing staff were trained to provide the activities during the daily care routine. The activities actually offered in the different studies did not vary a lot, but the number of activity sessions per week and the duration of the sessions did vary. In five studies, the control group got only the usual care delivered in care homes; in three studies, the control group got different activities that were not personally tailored; one study had both types of control group.
The quality of the trials and how well they were reported varied, and this affected our confidence in the results.
Key findings
Offering personally tailored activities to people with dementia living in care homes may slightly improve challenging behaviour when compared with usual care, although we did not find evidence that it was any better than offering activities which were not personally tailored. In one study, staff members reported that people in the group receiving personally tailored activities had a slightly worse quality of life than the control group. Personally tailored activities may have little or no effect on the negative emotions expressed by the participants. Because the quality of some of the evidence was very low, we could not draw any conclusions about effects on the participants' positive emotions, mood, engagement (being involved in what is happening around them) or quality of sleep. Only two studies mentioned looking for harmful effects; none were reported. None of the studies measured effects on the amount of medication participants were given, or effects on carers.
Conclusions
We concluded that offering activity sessions to people with moderate or severe dementia living in care homes may help to manage challenging behaviour. However, we did not find any evidence to support the idea that activities were more effective if they were tailored to people's individual interests. More research of better quality is needed before we can be certain about the effects of personally tailored activities.
Summary of findings
Summary of findings for the main comparison. Personally tailored activities compared to usual care or unspecific activities for people with dementia.
| Personally tailored activities compared to usual care or unspecific activities for people with dementia | ||||||
| Patient or population: people with dementia Setting: Long‐term care facilities Intervention: Personally tailored activities Comparison: usual care or unspecific activities | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care or unspecific activities | Risk with Personally tailored activities | |||||
| Quality of life (self‐rating by the participants; assessed with Quality of Life in Alzheimer’s Disease scale; higher scores indicate a higher quality of life); follow‐up: 28 weeks | The mean quality of life was 33.00 (6.20) | MD 0.26 higher (3.04 lower to 3.56 higher) | ‐ | 42 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | Mean difference adjusted for baseline/demographic characteristics; clinical relevance (by study authors): 3 point difference; only about one‐third of the participants completed the self‐assessment. |
| Quality of life (proxy‐rating; assessed with Quality of Life in Alzheimer’s Disease scale; higher scores indicate a higher quality of life); follow‐up: 28 weeks | The mean quality of life was 31.35 (4.68) | MD 1.93 lower (3.63 lower to 0.23 lower) | ‐ | 139 (1 RCT) | ⊕⊕⊕⊝ MODERATE 2 | Proxy‐rating, mean difference adjusted for baseline/demographic characteristics; clinical relevance (by study authors): 3 point difference. |
| Challenging behaviour (assessed with different scales, higher scores indicate more challenging behaviour; follow‐up: range 10 days to 9 months | ‐ | SMD 0.21 SD lower (0.49 lower to 0.08 higher) | ‐ | 439 (6 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | |
| Adverse events; follow up: range 10 days to 4 weeks | Only 2 studies assessed adverse effects, but in both studies no adverse effects were reported. | ‐ | (2 RCTs) | ‐ | ||
| Positive affect (assessed with different scales, higher scores indicate a greater display of positive affect); follow‐up: range 10 days to 9 months | ‐ | SMD 0.88 SD higher (0.43 higher to 1.32 higher) | ‐ | 455 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 5 | |
| Negative affect (assessed with different scales, higher scores indicate a greater display of negative affect); follow‐up: range 10 days to 9 months | ‐ | SMD 0.02 SD lower (0.19 lower to 0.14 higher) | ‐ | 589 (6 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | |
| Mood (assessed with different scales, lower scores indicate improved mood); follow‐up: range 4 weeks to 9 months | ‐ | SMD 0.02 SD lower (0.27 lower to 0.23 higher) | ‐ | 247 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 6 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded 2 levels due to imprecision (wide confidence interval, crossing the borders of clinical relevance defined by the study authors in both directions)
2 Downgraded 1 level due to imprecision (wide confidence interval, crossing the border of clinical relevance defined by the study authors in 1 direction)
3 Downgraded one level due to study limitations: high risk of bias due to lack of adequate randomisation and allocation concealment, blinding of outcome assessors and recruitment bias in some included studies
4 Downgraded one level due to imprecision (wide confidence interval, crossing the border of small effects (SMD) in one direction)
5 Downgraded one level due to inconsistency: substantial heterogeneity
6 Downgraded two levels due to imprecision (wide confidence interval, crossing the border of small effects (SMD) in both directions)
Background
Description of the condition
Dementia is a syndrome of progressive cognitive and functional decline, threatening the affected person’s capacities to perform activities and to communicate. Approximately six million people in Europe are affected by dementia and the absolute number is expected to rise (Prince 2013; Wittchen 2011). In long‐term care facilities, the estimated prevalence of dementia ranges between 40% and 80% (Bernstein 2007; Nygaard 2003).
People with dementia living in long‐term care facilities often spend their time not engaged in meaningful activities or uninvolved with other people (Cohen‐Mansfield 2009a; Edvardsson 2014; Hill 2010; Kolanowski 2006). However, people with dementia wish to be involved in activities which meet their interests and are perceived as meaningful (Murphy 2007; Phinney 2007; Vernooij‐Dassen 2007).
To be engaged in activities experienced as meaningful might increase quality of life in residents with dementia (Cooney 2009; Edvardsson 2014; Murphy 2007; Zimmerman 2005). However, activities offered in nursing homes tend to be passive, e.g. watching television and listening to music, and are often not perceived as meaningful by people with dementia (Harmer 2008), or are addressed to residents with better cognitive and functional status (Buettner 2003; Edvardsson 2014). Hence, a lower cognitive function in people with dementia is associated with fewer social interactions and less participation in activities (Chen 2000; Dobbs 2005; Edvardsson 2014). Understimulation might magnify challenging behaviour, e.g. apathy, boredom, depression, loneliness and agitation (Cohen‐Mansfield 1992; Cohen‐Mansfield 2011; Cohen‐Mansfield 2012c). To be meaningful for a specific person with dementia, activities have to be individualised based on the person's interests, since the judgment of whether an activity is meaningful differs both between different people with dementia and between people with dementia and nurses (Harmer 2008).
Offering personally tailored activities to people with dementia primarily aims to improve psychosocial outcomes, e.g. challenging behaviour or quality of life, rather than to increase cognitive function or to improve particular skills. Since a remarkable sense of self‐identity can persist until late stages of dementia (Cohen‐Mansfield 2006; Hubbard 2002; Mills 1997), the engagement in personally tailored activities could be beneficial for people in all stages of dementia.
Description of the intervention
Interventions offering personally tailored activities for people with dementia living in long‐term care facilities are likely to be complex interventions, comprising different types of activities and different ways of delivering the intervention (Craig 2008). We focus on interventions aimed at improving psychosocial outcomes (e.g. challenging behaviours or quality of life in people with dementia) rather than on interventions exclusively aimed at improving particular skills (e.g. basic activities of daily living, or cognitive function).
All interventions have to include an assessment of interests or preferences of the participants. Interventions can be based on specific models or concepts, e.g. the principles of Montessori or the concept of person‐centred care. The choice of activities offered should be based on the assessment of personal interests or preferences. Activities offered within the interventions include instrumental activities of daily living (e.g. housework, preparing a meal), arts and crafts (e.g. painting, singing), work‐related tasks (e.g. gardening), and recreational activities (e.g. games). The interventions can be delivered in groups or individually; duration and frequency of the sessions can differ. Providers of the interventions we expected to find include different professionals or a multidisciplinary team.
How the intervention might work
Being involved in personally tailored activities may evoke positive emotions like interest and reduce challenging behaviour (Cohen‐Mansfield 2007; Cohen‐Mansfield 2009b; Harmer 2008; Phinney 2007). Also, participating in such activities can increase feelings of engagement which can reduce feelings of boredom and loneliness (Cohen‐Mansfield 2009a), and increase quality of life (Hoe 2009; Murphy 2007; Zimmerman 2005). Further expected benefits cover the evocation of autobiographical events (Guétin 2009), the preservation of a person's identity, and increasing their occupation and maintaining their relationships (Harmer 2008). These positive effects may reduce the use of psychotropic medication in people with dementia and may also result in benefits for the caregiver (e.g. increased sense of competence, decreased burden of care).
Why it is important to do this review
There is an increasing need of effective non‐pharmacological interventions to improve psychosocial outcomes in people with dementia in clinical practice (Ballard 2013; O'Neil 2011). In several dementia guidelines, the use of non‐pharmacological interventions is recommended as a primary approach for behavioural and psychological symptoms (BPSD) (Azermai 2012; Ngo 2015; Vasse 2012). Interventions offering personally tailored activities could be a promising approach due to their potential effects on challenging behaviours, quality of life and the level of engagement of people with dementia. Several studies evaluated complex interventions offering personally tailored activities to people with dementia in long‐term care facilities (Cohen‐Mansfield 2007; Kolanowski 2011). These interventions are of complex nature due to different underlying theoretical models, the composition of components, the types of activities offered, and intensity and duration of delivery.
To assess the effects of complex interventions, a description of the interventions' components is required to ensure comparability and reduce heterogeneity (Shepperd 2009). Since the effectiveness of complex interventions is also influenced by implementation fidelity, this information should be incorporated, e.g. adherence, exposure, quality of delivery, participants’ responsiveness and adherence (Shepperd 2009).
Currently, no systematic review is available describing the characteristics of these interventions and summarising their effects on people with dementia. We intended this review to expand the knowledge on non‐pharmacological treatments aiming to improve psychosocial outcomes and quality of life of people with dementia living in long‐term care facilities. We also hoped the results would be helpful for decision making about the implementation of evaluated programmes offering personally tailored activities as well as for the development of new interventions.
Objectives
To assess the effects of personally tailored activities on psychosocial outcomes for people with dementia living in long‐term care facilities.
To describe the components of the interventions.
To describe conditions which enhance the effectiveness of personally tailored activities in this setting.
Methods
Criteria for considering studies for this review
Types of studies
In this review, we included individual or cluster‐randomised controlled trials, controlled clinical trials and controlled before‐after studies.
Types of participants
People with dementia living in long‐term care facilities, irrespective of the stage of dementia.
Types of interventions
All the interventions aimed to improve psychosocial outcomes by offering personally tailored activities to people with dementia in long‐term care. The aims of the interventions did not necessarily include the improvement of a particular skill. The interventions had to comprise two elements.
Assessment of the participants' present or former preferences for particular activities or interests. We accepted both unstructured assessments, e.g. asking for the interests of the person with dementia, or the use of validated tools, e.g. the self‐identity questionnaire (Cohen‐Mansfield 2010) or the NEO‐FFI (Kolanowski 2005). This assessment had to be performed primarily with the person with dementia; however, relatives or health professionals could also be informants, e.g. in later stages of dementia.
An activity plan tailored to the individual participant's present or former preferences. We accepted activities of various kinds: instrumental activities of daily living (e.g. housework, preparing a meal); arts and crafts (e.g. painting, singing); work‐related tasks (e.g. gardening); and recreational activities (e.g. games). The intervention could be delivered by different professionals, e.g. nurses, occupational therapists, social workers or psychologists. The intervention could be delivered either to a group or to individual participants.
We excluded interventions which offered (1) only one specific type of activity (e.g. music or reminiscence), (2) specific care approaches (e.g. person‐centred care) which included the delivery of activities, (3) multi‐component interventions comprising drug treatment and the delivery of activities, and (4) interventions exclusively aimed at improving cognitive function or other particular skills (e.g. communication, basic activities of daily living).
Comparison: other types of psychosocial interventions, placebo interventions (e.g. non‐specific personal attention), usual or optimised usual care.
Types of outcome measures
Primary outcomes
Challenging behaviour, assessed by e.g. the Cohen‐Mansfield Agitation Inventory (CMAI).
Quality of life, assessed by e.g. Dementia Care Mapping, EuroQol (EQ‐5D).
Secondary outcomes
Affect (i.e. expression of emotion), assessed by e.g. Observed Emotion Rating Scale.
Level of engagement, assessed by e.g. Observational Measurement of Engagement Assessment, Index of Social Engagement.
Mood, assessed by e.g. Dementia Mood Picture Test.
Other dementia‐related symptoms such as sleep disturbances, hallucinations or delusions, assessed by e.g. Neuropsychiatric Inventory (NPI).
Use of psychotropic medication.
Effect on the caregivers, e.g. caregivers' distress (assessed by e.g. Neuropsychiatric Inventory Caregiver Distress Scale (NPI‐D)), sense of competence (assessed by e.g. Sense of Competence Questionnaire (SCQ)), quality of life, health status (assessed by e.g. General Health Questionnaire (GHQ‐12)).
Adverse effects of the interventions employed (e.g. injuries).
Cost.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois) — the Cochrane Dementia and Cognitive Improvement Group's Specialized Register — on 16 June 2017. The search terms used were: personally tailored OR individualized OR individualised OR individual OR person‐centred OR meaningful OR personhood OR involvement OR engagement OR engaging OR identity.
ALOIS is maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in healthy individuals. The studies are identified from:
monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and LILACS;
monthly searches of a number of trial registers: ISRCTN; UMIN (Japan's Trial Register); the WHO portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials; and the Netherlands National Trials Register, plus others);
quarterly search of the Cochrane Library’s Central Register of Controlled Trials (CENTRAL);
six‐monthly searches of grey literature source: ISI Web of Science Conference Proceedings.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘Methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group.
We also performed additional searches in many of the sources listed above to ensure that the search for the review was as up to date and as comprehensive as possible. The search strategies we used can be seen in Appendix 1.
Searching other resources
We screened reference lists and citations of all potentially eligible publications for additional trials and for additional data (e.g. interventions development, process‐related data).
Data collection and analysis
Selection of studies
Two reviewers (RM, AR) independently assessed all titles and abstracts obtained from the search for inclusion according to the inclusion criteria. We resolved disagreements by discussion or, if necessary, we referred to a third reviewer (GM).
Data extraction and management
Two reviewers (RM, AR) extracted data independently from all included publications using a standardised form. We checked results for accuracy and, in case of disagreement, called in a third reviewer (GM) to reach consensus.
For each study we extracted the following data: study design, characteristics of participants, baseline data, length of follow‐up, outcome measures, study results, and adverse effects. For each intervention we extracted the following information: method of assessing the individual preferences, types of activities offered, duration and frequency of the intervention's components, information of the implementation fidelity. Additionally, we collected information on the intervention's development (i.e. underlying theoretical considerations, components and delivery) and process‐related data. For cluster‐randomised trials, we also extracted estimates of the intra‐cluster correlation coefficient (ICCC) if possible. If necessary, we contacted study authors to obtain missing information.
Assessment of risk of bias in included studies
We followed the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed risk of bias in each study for the following criteria: selection bias, performance bias, attrition bias, detection bias, and additional design‐related criteria for cluster‐randomised and non‐randomised trials. Two authors (RM, AR) independently assessed methodological quality of studies in order to identify any potential sources of systematic bias. In case of unclear or missing information, we contacted the corresponding author of the trial. We assessed the quality of evidence using the criteria proposed by the GRADE working group (Guyatt 2011).
Measures of treatment effect
For challenging behaviour and affect (including mood), we used the standardised mean difference (SMD), which is the absolute mean difference divided by the standard deviation (SD), since the included studies used different rating scales (see also Unit of analysis issues). We used the post‐intervention means of each scale's total score or subscore (for affect). For continuous data that were not included in a meta‐analysis, we calculated the mean difference (MD). If it was not feasible for us to calculate the MD, e.g. in case of substantial baseline imbalances, we presented the study results in narrative form, e.g. as mean values and standard deviation).
None of the trials included in this review reported dichotomous data of interest to this review.
Unit of analysis issues
For all studies, we investigated whether individuals or groups (clusters) were randomised.
For cluster‐randomised trials, we extracted information about the intracluster correlation coefficient (ICC) if available. Only one of the included cluster‐randomised trials reported the ICC with values ranging from 0 to 0.3 (Wenborn 2013). We used the ICC values of the corresponding outcomes (0.19 for challenging behaviour and 0.09 for affect) from this study to incorporate the cluster effect in the studies without information on the ICC — Cohen‐Mansfield 2007 and Cohen‐Mansfield 2012a — by re‐calculating the effective sample size using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The number of included study participants and clusters in all three studies are comparable.
For cross‐over trials, we checked the risk of a carry‐over effect. There was no evidence for the occurrence of a carry‐over effect in the one included cross‐over study (van der Ploeg 2013); after the intervention sessions the values of most outcomes returned to the level assessed before the activities were offered. We used data from the complete study period for both conditions in our analysis since no results for the first period were available. We cannot be sure to have avoided a unit‐of‐analysis bias; however, this bias is conservative, being expected to lead to an under‐estimate of the intervention effect (Higgins 2011).
One study included four study groups (three different intervention groups and one control group) (Kolanowski 2011). We excluded two intervention groups from the analysis since they did not meet our inclusion criteria (see Description of studies) and we included only two groups (one intervention and the control group) in the analysis.
Dealing with missing data
For all included studies, numbers of participants lost to follow‐up, with reasons, were extracted and presented in Characteristics of included studies. Where information was missing, the study authors were contacted and asked for additional information.
Assessment of heterogeneity
We examined studies for clinical diversity in terms of characteristics of the interventions, participants, and outcomes. We combined data in meta‐analyses only if we considered the studies to be sufficiently clinically homogeneous. To test for statistical heterogeneity, we used the Chi² and I² statistics.
Assessment of reporting biases
In order to minimise the risk of publication bias we performed a comprehensive search, including multiple databases, snowballing techniques and searching trials registers to identify unpublished or ongoing trials. We did not investigate publication bias by means of a graphical funnel plot analysis since we included only a small number of studies. To detect cases of selective reporting in the included studies, we checked trial register information if available.
Data synthesis
We performed meta‐analyses for challenging behaviour, for positive and negative affect and for mood. In all cases, we used a random‐effects model as planned in the protocol due to the clinical diversity of the interventions or statistical heterogeneity (I² > 50%). In one study, different types of (positive and negative) affect were reported (Van Haitsma 2015). To include this study in the meta‐analysis, we combined the corresponding outcomes for positive and negative affect by calculating a combined score. To calculate the variance of the combined means, we assumed a positive correlation of 0.5 between the individual outcomes of each category. In the meta‐analysis for mood, the scales used in two studies differed in the direction of the scale (Orsulic‐Jeras 2000; Wenborn 2013). We re‐calculated the data of this study using the methods from the Cochrane Handbook for Systematic Reviews of Interventions (we multiplied the mean values by −1 as described in chapter 9.2.3.2) (Higgins 2011).
We did not perform meta‐analysis for any other outcomes and present the results in a narrative form.
Subgroup analysis and investigation of heterogeneity
We conducted the pre‐planned subgroup analyses for studies with and without an active control group. Since we included one study in both subgroup analyses (this study — Van Haitsma 2015 — compared the intervention group with both an active and a usual care control), we split the intervention group using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 16.5.4) (Higgins 2011). Where applicable, we also explored possible causes of heterogeneity by conducting pre‐planned analyses excluding studies with non‐overlapping confidence intervals (CI).
Sensitivity analysis
We performed a sensitivity analysis to explore the effects of including the study, for which we calculated the combined outcome for positive and negative affect (see Data synthesis).
Summary of findings
We used the GRADE approach to assess the quality of evidence for the most important outcomes. We assessed the quality of the evidence by judging study limitations, consistency of effect, imprecision, indirectness, and publication bias (Guyatt 2011). To determine imprecision, we defined the borders for minimal important difference as defined by study authors; e.g. in case of quality of life (Wenborn 2013), and for the analyses using the SMDs, we used an effect size of 0.2, which is described as a small effect for SMD in the Cochrane Handbook for Systematic Reviews of Interventions (chapter 12.6.2) (Higgins 2011). We rated quality of evidence as high, moderate, low or very low (Guyatt 2011). We created 'Table 1' for the outcomes 'challenging behaviour', 'positive affect' and 'negative affect' with GRADEpro GDT.
Results
Description of studies
Results of the search
The search retrieved a total of 19,357 citations (Figure 1). The Information Specialists of the Cochrane Dementia and Cognitive Improvement Group carried out an initial assessment; then two authors independently screened titles and abstracts of 919 records for potential eligibility. Thirty‐three publications were screened in full text and eight studies met all inclusion criteria (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Kolanowski 2011; Orsulic‐Jeras 2000; Richards 2005; van der Ploeg 2013; Van Haitsma 2015; Wenborn 2013).
1.
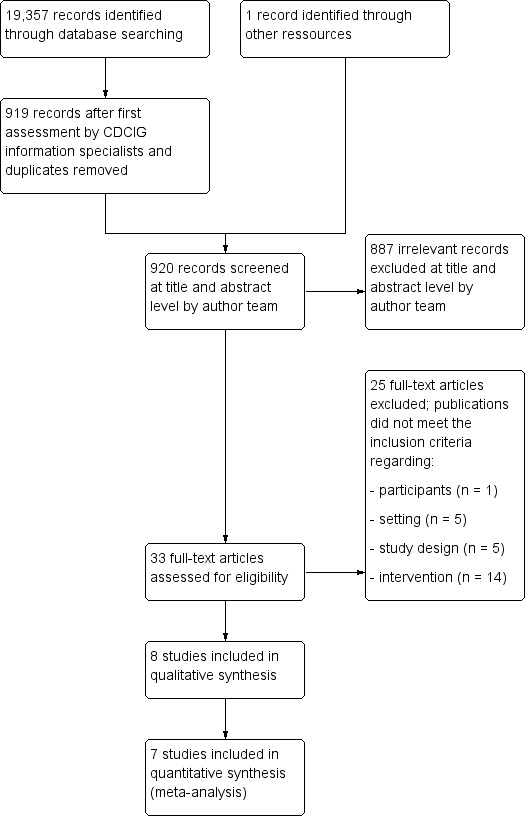
Study flow diagram.
Included studies
Seven included studies were randomised controlled trials (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Kolanowski 2011; Richards 2005; van der Ploeg 2013; Van Haitsma 2015; Wenborn 2013); and one study was a non‐randomised clinical trial (Orsulic‐Jeras 2000). Three of the RCTs used cluster‐randomisation (the units of allocation were nursing homes or nursing home wards) (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Wenborn 2013); three allocated individual participants (Kolanowski 2011; Richards 2005; Van Haitsma 2015); and one used a cross‐over design (van der Ploeg 2013). In the study by Wenborn 2013, matched pairs were randomised.
Setting and Participants
Six studies were conducted in the USA (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Kolanowski 2011; Orsulic‐Jeras 2000; Richards 2005; Van Haitsma 2015); one in Australia (van der Ploeg 2013); and one in the UK (Wenborn 2013). Six studies recruited participants from several nursing homes (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Kolanowski 2011; Richards 2005; van der Ploeg 2013; Wenborn 2013), the number of facilities ranging from 4 to 16. Two studies recruited participants from one facility; one study included participants from one large non‐profit nursing home (Van Haitsma 2015); and one study recruited people with dementia from a special care unit (Orsulic‐Jeras 2000).
A total of 1080 participants were recruited and 957 participants completed the studies. The number of participants completing the studies ranged from 25 (Orsulic‐Jeras 2000) to 180 (Van Haitsma 2015).
The mean age of participants ranged from 78 to 88 years. In six studies most participants were female (63% to 92%); in one study the proportion of women was 48% (Richards 2005). In seven studies the mean MMSE score was lower than 12 (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Orsulic‐Jeras 2000; Richards 2005; van der Ploeg 2013; Van Haitsma 2015; Wenborn 2013); and in one study the score ranged from 12 to 15 (Kolanowski 2011).
In three studies, challenging behaviour at baseline was an inclusion criterion for participants (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Kolanowski 2011); and in one study, physical agitation at baseline was an inclusion criterion (van der Ploeg 2013). In three studies without these inclusion criteria, all participants showed some form of challenging behaviour or agitation (Orsulic‐Jeras 2000; Van Haitsma 2015; Wenborn 2013). One study offered no information on challenging behaviour (Richards 2005) (see Characteristics of included studies).
Description of the interventions
In seven of the interventions, personally tailored activities were offered directly to the participants (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Kolanowski 2011; Orsulic‐Jeras 2000; Richards 2005; van der Ploeg 2013). In one study, members of the nursing staff were trained to deliver the personally tailored activities to the study participants (Wenborn 2013).
In this section, we describe the included interventions using categories relevant for complex interventions (Hoffmann 2014; Möhler 2015).
Theoretical basis and components of the interventions
Choice of activities in the included studies was based on different theoretical models. The theoretical basis guided the selection of activities which could be offered to the participants, and the methods by which the interventions were individually tailored, i.e. how the activities were chosen for the individual participants.
The interventions in Cohen‐Mansfield 2007 and Cohen‐Mansfield 2012a were based on the Treatment Routes for Exploring Agitation (TREA) framework. Kolanowski 2011 used the Need‐Driven Dementia‐Compromised Behavior (NDB) model and tested three different treatment conditions. The principles of Montessori were used in two studies (Orsulic‐Jeras 2000; van der Ploeg 2013). The interventions by Richards 2005 and Wenborn 2013 were not based on a specific theoretical framework; however, in both studies the choice of activities followed predefined principles.
The Treatment Routes for Exploring Agitation (TREA) framework
The TREA framework provides a systematic approach for individualizing non‐pharmacological interventions to unmet needs of people with dementia and agitation (Cohen‐Mansfield 2000). The TREA framework assumes that different types of agitated behaviours have different aetiologies. To create an individual intervention, the aetiology of the agitated behaviour must be identified. Individual interventions have to be developed based on the remaining abilities of the individual, his/her deficits, e.g. in sensory perception, cognition, and mobility, and personal preferences, e.g. past work, hobbies, important relationships, and sense of identity. With the TREA framework, individual needs and preferences of people with dementia exhibiting agitated behaviours could be assessed by using information from formal or informal caregivers (e.g. nursing staff or family members, respectively), or by observing the person's behaviour and environment. The TREA framework "can be viewed as a decision tree that guides caregivers through the necessary steps for exploring and identifying underlying unmet needs that contribute to agitated behaviours" (Cohen‐Mansfield 2007).
In the studies by Cohen‐Mansfield 2007 and Cohen‐Mansfield 2012a, the TREA decision tree protocol was used to identify all agitated behaviours exhibited by the individual participants and the possible reasons for these behaviours. For each participant, a 4‐hour peak period of agitation was identified at baseline. The intervention was individualised and administered to each participant based on this peak period. Information on the needs and preferences of the participant was identified by providing his or her relatives with a questionnaire to complete, including items concerning the participant's medical history, self‐identity, and social functioning. Based on this assessment, corresponding activities were offered (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a).
Examples of activities offered are: individualised music, family videotapes and pictures, illustrated magazines and large print books, board games and puzzles, plush toys, sorting cards with pictures and words, stress balls, baby dolls, electronic massagers, pain treatment, outdoor trips to the garden of the nursing home, perfume, and Play‐Doh (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a).
Need‐Driven Dementia‐Compromised Behavior (NDB) model
The NDB model defines behavioural symptoms as an indicator showing unmet needs of people with dementia (Algase 1996). Two aspects are described as potential reasons for behavioural symptoms: background risk factors (neuropathology, cognitive deficits, physical function, and premorbid personality); and proximal precipitating factors (qualities of the physical and social environment, and physiological and psychological need states) (Algase 1996). In this model, personally tailored activities can be seen as proximal factors that meet individual needs, since they aim at enriching the physical and social environment by matching the individual's background factors (Kolanowski 2005).
In the studies by Kolanowski 2011, the activities offered based on the NDB model were individually tailored to the participants' cognitive and physical functional level and to their style of interest. Style of interest was defined by the participants' personality traits of extraversion (preferred amount of social stimulation) and openness (individual tolerance for the unfamiliar). Kolanowski 2011 assessed style of interest by the use of the form F from the Revised NEO Personality Inventory (NEO‐PI‐R, Costa 1992). For choosing the activities, both the participants' style of interest and the cognitive and physical functional level was relevant. Kolanowski 2011 tested three treatment conditions based on this framework: (1) activities matched to the participants' (cognitive and physical) functional level, but opposite to their identified style of interest; (2) activities matched to the participants' style of interest, but not their functional level; (3) activities matched to both the participants' functional level and style of interest. Examples of activities offered are: games, puzzles, music (listening or making music), crafts (e.g. making a birdhouse), pet visits, sewing cards, cooking, painting (Kolanowski 2011). In this review, we considered only the activities matched both to the participants' functional level and style of interest to be personally tailored activities.
Principles of Montessori
The principles of Maria Montessori were developed to guide child education. They put emphasis on task breakdown, guided repetition, progression in difficulty from simple to complex, and the careful matching of demands to levels of competence. These principles were adapted to be used with people with dementia. Activities offered to people with dementia "are designed to tap procedural memory which is better preserved than verbal memory while minimising language demands and providing external cues to compensate for cognitive deficits" (van der Ploeg 2010).
In the study by van der Ploeg 2013 a maximum of 10 activities were selected based on discussion with families about participants' former interests and hobbies. Orsulic‐Jeras 2000 used the Myers Menorah Park/Montessori Assessment System (MMP/MAS) to individualise the activities. MMP/MAS is a Montessori‐based instrument and provides information on participants' areas of interest.
Examples of activities offered by Orsulic‐Jeras 2000 are: individual Montessori activities (with materials usually taken from the everyday environment e.g. utensils, bowls, flowers, baskets); group Montessori‐based activities (memory bingo); and a structured reading and discussion group. van der Ploeg 2013 offered activities like sorting cards or making puzzles from familiar photographs.
Individualized social activity intervention (ISAI)
The intervention by Richards 2005 was based on a conceptual framework which postulates (based on the two‐process model of sleep) that individualised activities can improve the homeostatic sleep drive and strengthen circadian processes; and that this may lead to improved nighttime sleep and decreased daytime sleep (Richards 2005).
The activities were preselected to match various interests as well as cognitive and functional abilities. About 100 different activities were identified by two therapeutic recreation specialists with more than 20 years of collective experience working with nursing home residents with dementia. A list was created comprising the following information for all activities: brief directions for use, which functional limitations preclude their use, and which previous interests of participants are associated with each activity. The activities were also grouped into activities which were appropriate for everyone, and those which were appropriate for participants with mild (MMSE > 15), moderate (MMSE 5 to 15), and severe (MMSE < 5) dementia. The activities offered were selected according to four characteristics of each participant: interests (work and leisure history), cognition and functional status (mobility, hearing, vision, fine motor skills), and napping patterns (time of unscheduled naps). This information was assessed by means of interviews with families, nursing staff, and participants, observation of participants' behaviour, chart review, and by using an Actigraph (for napping patterns).
Examples of the activities offered were listening to music, petting a toy cat, tossing a ball, writing a letter, playing checkers, making a wreath, preparing and serving a snack (Richards 2005).
Occupational therapy programme
Wenborn 2013 offered an occupational therapy programme. The intervention was developed by the primary author, an occupational therapist with experience in working with older people with dementia.
The intervention consists of two components.
An assessment of the care home's physical environment, including recommendations on how it could be adapted and enhanced to enable the residents to engage in activities.
An education programme for nursing staff aimed to enhance knowledge, attitudes and skills, based on the principles of experiential learning. The educational component comprised five two‐hour education sessions covering these topics: identify the residents' interests and abilities; choose and offer activities; review and record the outcomes. The care home manager joined the last session to agree an activity action plan for continued implementation of the programme. To ensure the use of the skills and tools in clinical practice, work‐based learning tasks with two residents were conducted between the educational sessions and one‐to‐one coaching sessions with the primary investigator were used. The activities were personalised to each resident by the use of the Pool Activity Level Checklist (Wenborn 2008).
Individualized Positive Psychosocial Intervention
The study by Van Haitsma 2015 was based on two theoretical models: the Self‐Determination Theory (Deci 2000); and Broaden‐and‐Build Theory (Fredrickson 2001). The Self‐Determination Theory proposes that all people have innate needs for autonomy and competence, which must be fulfilled for psychological well‐being; and the Broaden‐and‐Build Theory focusses on the critical role of positive emotions to improve the person's well‐being. The study is described as being based on the work of Kolanowski 2011, but it is not described how this study contributed to the design of the intervention or the study.
The Individualized Positive Psychosocial Intervention (IPPI) offered five basic types of activities reflective of the most common preferences.
Physical exercises (outdoor walk, work with clay).
Music (singing or listening to a favourite artist).
Reminiscence (reviewing family photos, writing letters).
Activities of daily living (manicures, preparing a snack).
Sensory stimulation (e.g. hand massage with lotion, smelling fresh flowers).
From each group, two or more specific activity options were offered (a total of 30 activity options). The activities were selected by researchers and clinicians for each resident based on the Preferences for Everyday Living Inventory‐Nursing Home (PELI‐NH; Van Haitsma 2000). The information was taken directly from the participant or from a family member, activity therapist, or other direct care staff.
Feasibility/pilot test
Richards 2005 tested their intervention in a pilot study (Richards 2001); the studies by Kolanowski 2011 and Cohen‐Mansfield 2012a used previous studies as a pilot‐test for their interventions (Kolanowski 2005; Cohen‐Mansfield 2007); and the intervention by Orsulic‐Jeras 2000 was based on experiences from an earlier project.
No information on a feasibility or pilot‐test was provided by Cohen‐Mansfield 2007, van der Ploeg 2013, Van Haitsma 2015, and Wenborn 2013.
Delivery of the intervention
In most studies, the interventions were delivered directly to the study participants (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Kolanowski 2011; Orsulic‐Jeras 2000; Richards 2005; van der Ploeg 2013; Van Haitsma 2015). In the study by Richards 2005, activities were delivered individually; however, when the same activity was selected for more than one participant at the same time, the activity was offered in groups of up to three participants. The intervention by Orsulic‐Jeras 2000 comprised both individual and group activities (see above: 'Theoretical basis and components of the interventions ‒ Principles of Montessori'). In the study by Wenborn 2013, members of the nursing staff were trained to select, plan and deliver the activities within daily care.
In all studies, trained staff delivered the interventions. Training was guided by written manuals or guidelines in three studies (van der Ploeg 2013; Van Haitsma 2015; Wenborn 2013); and a treatment fidelity plan in one study (Kolanowski 2011). The number and frequency of sessions delivered as well as the follow‐up period differed between studies. An overview is displayed in Table 1 (see also Characteristics of included studies).
Table 1 ‒Delivery of the intervention
| Reference | Delivered by | Frequency and duration of the sessions | Duration of follow‐up |
| Cohen‐Mansfield 2007 | Research assistant | Daily; up to 4 h per day (peak period of agitation) | 10 consecutive days |
| Cohen‐Mansfield 2012a | Research assistant | Daily; up to 4 h per day (peak period of agitation) | 10 consecutive days |
| Kolanowski 2011 | Research assistant | 5 days per week; up to 20 minutes twice per day (morning and afternoon) | 4 weeks (3‐week interventions period + 1‐week post‐intervention period) |
| Orsulic‐Jeras 2000 | Trained volunteer, nursing assistant or activities therapist | At least twice a week; individual activities 10 to 30 min, group activities 25 to 45 min, QAR 30 min to 1 h | 9 months |
| Richards 2005 | Nursing assistant | Daily; several sessions 15 to 30 min (max 1 to 2 h per day), between 9:00 a.m. and 5:00 p.m. | 21 consecutive days |
| van der Ploeg 2013 | Activity facilitators (psychologists or higher degree psychology students, received regular personal supervision throughout the study) | Twice a week; 30 min sessions (at times when participants' target behaviour was most frequent) | 4 weeks (2 weeks per condition) |
| Van Haitsma 2015 | Certified nursing assistants | 3 days per week; 10 min per session (not during mealtimes or shift change) | 3 weeks |
| Wenborn 2013 | Primary investigator | Not reported; five 2 h educational sessions for nursing staff | 28 weeks (16 weeks' intervention period + 12 weeks' post intervention period) |
Despite all studies basing the selection of the activities on an assessment of the participants' present or former preferences, no information was presented in any study about the number of participants who were able to express their individual interests or preferences. Also, no study reported information about the proportion of participants for whom preferences and interests were assessed through the primary caregiver or family members.
The degree of delivery of the interventions was assessed in three studies (Kolanowski 2011; Van Haitsma 2015; Wenborn 2013); and Cohen‐Mansfield 2012a assessed barriers to the intervention's implementation (Cohen‐Mansfield 2012b).
Kolanowski 2011 used a treatment fidelity plan to ensure the introduction of the intervention as planned. Also, the research assistants paid attention to potential confounding factors (e.g. pain, thirst, poor environmental conditions). Treatment fidelity was checked for 10% of the intervention sessions. Re‐training took place if the intervention was not implemented according to the protocol. Only one deviation from the protocol occurred.
Van Haitsma 2015 assessed implementation fidelity during randomly selected sessions. A member of the research team observed compliance with study procedures in both the intervention and active control group. Overall, adherence to protocol was 68%, with higher rates in the intervention group (73%) compared to the active control condition (60%).
In the study by Wenborn 2013, the number of staff attending each session was recorded and feedback regarding the work‐based learning activities was collected from nursing staff and residents. A mean staff attendance of 73% was recorded for the education sessions (range 63 to 86) and a mean uptake of 81% for the individual coaching sessions (range 49 to 100). Reasons for non‐attendance at the sessions included: being off duty (22%); annual leave (20%); on duty but not available (14%); sick leave (12%); study leave (11%); staff personal commitment (11%); and left the care home (9%). No information on the amount of activities delivered to the residents by the nursing staff was collected.
In the study by Cohen‐Mansfield 2012a, in approximately 22% of the sessions some participants were unwilling to participate in the activities offered, and 84% of the participants were unwilling to participate in at least one of the sessions (Cohen‐Mansfield 2012b).
Characteristics of the control conditions
An active control condition was used in three studies. Kolanowski 2011 offered a control group with activities that were functionally challenging and opposed to the participant's style of interest (based on the NDM model). van der Ploeg 2013 used non‐personalised one‐to‐one interactions aimed at engaging the participants in social interaction, e.g. general conversations or conversation based on newspaper stories and pictures. Van Haitsma 2015 offered standardised one‐to‐one social interaction activities (e.g. discussing a magazine).
In six studies, the control condition was usual care. (The study by Van Haitsma 2015 offered both an active control group and a control group with usual care). The nursing staff in the centres allocated to the control condition in Cohen‐Mansfield 2007 and Cohen‐Mansfield 2012a received a presentation on the different forms of agitation, their aetiologies and possible non‐pharmacological interventions. In the study by Orsulic‐Jeras 2000, the control group received the usual activities of the centre (individual, small group, and large group activities, including bingo, storytelling, trivia, exercise, modified sporting activities, watching movies, discussion groups, musical programmes, sensory stimulation, activities based on the participants' interests and hobbies, delivered by an activities therapist or nursing assistants). The participants in the control groups in the studies by Richards 2005, Van Haitsma 2015 and Wenborn 2013 received usual care of the nursing home, but no information on the type and amount of activities offered was published.
Outcomes and data collection methods
Challenging behaviour
In the studies by Cohen‐Mansfield 2007 and Cohen‐Mansfield 2012a, challenging behaviour was assessed with the Agitation Behavior Mapping Instrument (ABMI, Cohen‐Mansfield 1989a). ABMI is a 19‐item instrument to rate agitation in nursing homes by direct observation (a higher score indicates more agitation).
Kolanowski 2011 and Orsulic‐Jeras 2000 used the Cohen‐Mansfield Agitation Inventory (CMAI, Cohen‐Mansfield 1989b) to assess challenging behaviour. The CMAI is a proxy‐rating instrument used by nurses to assess agitation and comprises four subscales (physically non‐aggressive behaviours, physically aggressive behaviours, verbally non‐aggressive behaviours, and verbally aggressive behaviours; range 0 to 29; a higher score indicates greater agitation). Kolanowski 2011 also used the Passivity in Dementia Scale (PDS), a proxy‐rating instrument with 53 items (range −16 to 40, a higher score indicates less passivity; Colling 2000).
van der Ploeg 2013 selected one specific behaviour for each participant based on the nurses’ rating in a two‐week period before baseline assessment by the CMAI. For each participant, it was rated by direct observation whether this specific behaviour occurred within 30 minutes in 1‐minute intervals. The observation resulted in an individual behaviour score for each participant ranging from 0 to 30 points per session. The outcome score (mean and SD) was calculated using the observations from all sessions (n = 1.056 observations from all study participants). A higher score indicates a more frequent behaviour.
Van Haitsma 2015 assessed different categories of verbal and nonverbal behaviour by direct observation. Within a 10‐minute "behaviour stream", the onset and cessation of specific behaviours were recorded. Verbal behaviour was categorised as very negative (swearing, screaming, mocking), negative (incoherent, repetitious statements, muttering), positive (coherent conversation, responding to questions), very positive (complimenting, joking) or no verbal behaviour. Nonverbal behaviour was categorised as: psychosocial task (manipulates or gestures toward an object, engages in conversation), restlessness (pacing, fidgeting, disrobing), null behaviour (stares with fixed gaze, eyes unfocused), eyes closed (sits or lies with eyes closed), aggression (hitting, kicking, pushing, scratching, spitting), uncooperative (pulling away, saying “no”, turning head or body away), and positive touch (appropriate touching, hugging, kissing, hand holding). Higher scores indicated a higher frequency of the behaviour.
Wenborn 2013 used the Challenging Behaviour Scale (CBS, Moniz‐Cook 2001) to assess the incidence, frequency and severity of challenging behaviour. The CBS is a 25‐item proxy‐rating instrument used by nurses (higher scores indicate more challenging behaviour).
Quality of life
Quality of life was assessed in only one study — Wenborn 2013 — by the use of the Quality of Life in Alzheimer’s Disease (QOL‐AD) scale (self‐ and caregiver‐rating) (Logsdon 1999). Higher scores indicate a higher quality of life.
Affect
Cohen‐Mansfield 2007 and Cohen‐Mansfield 2012a used Lawton’s Modified Behavior Stream (LMBS, Lawton 1996), covering the following modes of affect: pleasure, interest, anger, anxiety, and sadness. A higher score indicates greater display of the affect.
Kolanowski 2011, Orsulic‐Jeras 2000 and van der Ploeg 2013 used the Philadelphia Geriatric Center Affect Rating Scale (ARS, Lawton 1996), covering the following modes of affect: pleasure, anger, anxiety, sadness, interest, and contentment. A higher score indicates greater display of the affect. In the study by Kolanowski 2011, anger and sadness were not used due to the inability to obtain adequate reliability for their measure. In two studies, results were categorised as positive or negative affect (Cohen‐Mansfield 2012a; van der Ploeg 2013); van der Ploeg 2013 used also the category neutral affect. van der Ploeg 2013 calculated outcome scores (mean and SD) based on the observations from all sessions (n = 1.056 observations from all study participants).
Van Haitsma 2015 assessed the duration of different types of affect by direct observation within a 10‐minute "behaviour stream". Positive affect included pleasure (smiling, laughing, singing, nodding) and alertness (eyes following object, intent fixation on object or person, visual scanning, eye contact maintained) and negative affect included sadness (crying, tears, moan, sigh, mouth turned down at corners), anger (clenched teeth, grimace, pursed lips, eyes narrowed), and anxiety (furrowed brow, motoric restlessness, repeated or agitated motion, hand wringing, leg jiggling). A higher score indicates more frequent occurrence of the specific type of affect.
Wenborn 2013 assessed anxiety by the use of the Rating Anxiety in Dementia scale (RAID, Shankar 1999), with scores of 11 or above indicating clinical anxiety.
Engagement
Three studies measured engagement. Kolanowski 2011 assessed time on task (minutes/seconds; range 0 to 20 minutes), and intensity of participation (ranging from 0 ("dozing") to 3 ("actively engaged"), based on Kovach 1998); Orsulic‐Jeras 2000 used the Myers Research Institute Engagement Scale (MRI‐ES, Judge 2000) (range 0 to 600, higher scores indicates more engagement); and van der Ploeg 2013 used the Menorah Park Engagement Scale (MPES) (range 0 to 30, higher values indicates more engagement) (Skrajner 2007).
Both scales assessed four types of engagement: constructive engagement (e.g. actively handling objects or talking); passive engagement (e.g. watching or listening); self‐engagement (e.g. fiddling with clothes); and non‐engagement (e.g. a blank stare). van der Ploeg 2013 combined non‐ and self‐engagement into the category "negative engagement"; and calculated outcome scores (mean and SD) based on the observations from all sessions (n = 1.056 observations from all study participants).
Mood
Kolanowski 2011 assessed mood by use of the Dementia Mood Picture Test (range 0 to 12, higher score indicates more positive mood; Tappen 1995). Orsulic‐Jeras 2000 and Wenborn 2013 assessed depression by the use of the Cornell Scale for Depression (CSD, Alexopoulos 1988). A score of 8 or above indicates depression.
Other outcomes
Richards 2005 assessed the daytime minutes slept, nighttime minutes to sleep onset, minutes slept, minutes awake, sleep efficiency, and the day/night sleep ratio using an Actigraph (motion‐sensing device), as well as the costs of implementing the intervention.
Duration of the effects
Two studies aimed to assess the duration of the intervention effects. Kolanowski 2011 assessed the intervention effect one week after the intervention period was completed; and van der Ploeg 2013 additionally assessed all outcomes after each session.
Excluded studies
Studies were excluded because the intervention or the study design did not meet our inclusion criteria. See Characteristics of excluded studies for the reasons for exclusion of the studies screened in full text.
Risk of bias in included studies
We contacted authors of all studies and asked for additional information on methodological details which were not reported in the publications (we sent one reminder to all non‐responding authors). Five authors responded to our request (A. Kolanowski, J. Cohen‐Mansfield, S. Orsulic‐Jeras, E. van der Ploeg, K. Van Haitsma) and four authors offered additional information; one author did not, for personal reasons.
The methodological quality of the included studies varied. We judged two studies to have no domains in which the risk of bias was high (Kolanowski 2011; Wenborn 2013). We judged all the other studies to be at high risk of bias in at least one domain (see Characteristics of included studies; Figure 2; Figure 3 and Appendix 2; Appendix 3; Appendix 4).
2.
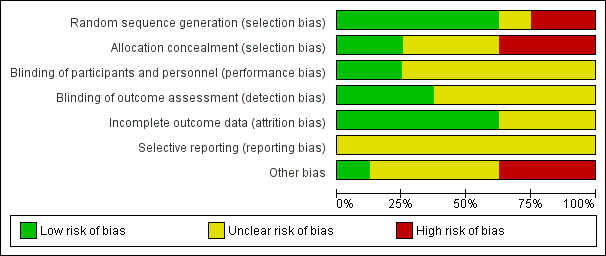
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
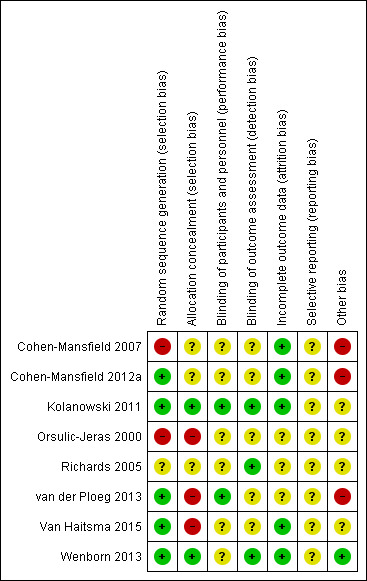
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The randomisation sequence was adequately generated in five studies (Cohen‐Mansfield 2012a; Kolanowski 2011; van der Ploeg 2013; Van Haitsma 2015; Wenborn 2013). Van Haitsma 2015 used a two‐step randomisation procedure. In the first step the included nursing home units were allocated to deliver one of the two active treatments (intervention or active control); and in the second step, the eligible residents in each ward were allocated to the active treatment or usual care (eligible participants were identified before allocation).
No information on the method of sequence generation was available in two studies (Cohen‐Mansfield 2007; Richards 2005). We considered the risk of bias in this domain to be unclear for Richards 2005. In the study by Cohen‐Mansfield 2007 two clusters were not assigned randomly due to preferences of the facility managers so we judged the risk of bias in this domain to be high.
Group allocation was adequately concealed in two studies (Kolanowski 2011; Wenborn 2013). In three studies no information on the allocation concealment was available (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Richards 2005) (risk of bias judged to be unclear); and in two studies allocation was not concealed (van der Ploeg 2013; Van Haitsma 2015) (risk of bias judged to be high). In two cluster‐randomised studies, the participants were identified after the allocation of clusters (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a).
In the study by Orsulic‐Jeras 2000 group allocation was not performed at random. Participants were allocated to the groups using matching based on the MMSE score, Myers Menorah Park/Montessori Assessment System (MMP/MAS) and the reading subtest of the Wide Range Achievement Test (WRAT3). We considered this study to be at high risk of selection bias.
Blinding
Blinding of participants and personnel was adequate in two studies (Kolanowski 2011; van der Ploeg 2013); both studies offered a form of active treatment to all participants. In six studies, blinding of participants and personnel was not possible (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Orsulic‐Jeras 2000; Richards 2005; Van Haitsma 2015; Wenborn 2013). We considered it to be unclear whether this introduced a bias.
Outcome assessors were blinded to group allocation in three studies (Kolanowski 2011; Richards 2005; Wenborn 2013). In five studies blinding of outcome assessors was not possible, because data were collected by proxies, such as unblinded nursing staff. Two studies attempted to assess the impact of the lack of blinding on the study results. Ten and 25 intervention sessions were videotaped and the outcomes were assessed by a blinded rater (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a). There was a high agreement between the blinded and unblinded rater. Generally, we considered it to be unclear whether the lack of blinding led to a bias.
Incomplete outcome data
In six studies attrition rates were low and reasons for attrition were documented (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Kolanowski 2011; Richards 2005; Van Haitsma 2015; Wenborn 2013). In the study by van der Ploeg 2013 the attrition rate was more than twice as high as anticipated (anticipated attrition rate 10%; actual attrition rate 23% (13/57)). In the study by Orsulic‐Jeras 2000, only 25 of 44 participants completed the study, but the group allocation of the participants lost to follow‐up was not reported. We considered the risk of attrition bias for these studies to be unclear, since the reasons for attrition were available and there was no evidence that attrition was due to the intervention.
Selective reporting
Four studies were registered, but all retrospectively (Cohen‐Mansfield 2012a; Kolanowski 2011; van der Ploeg 2013; Wenborn 2013); and a protocol was published for one study (van der Ploeg 2013). The primary outcome was defined in four studies (Cohen‐Mansfield 2007, Cohen‐Mansfield 2012a, van der Ploeg 2013; Wenborn 2013). Based on this information, results for all outcomes were reported as planned. We considered the risk of selective reporting bias in all studies to be unclear due to the retrospective or absent registration.
Other potential sources of bias
We considered there to be a high risk of bias in three studies (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; van der Ploeg 2013). There was a high risk of unit‐of‐analysis bias in Cohen‐Mansfield 2007 and Cohen‐Mansfield 2012a, since neither study considered the cluster effect in their analyses. The study by van der Ploeg 2013 was at high risk of a unit‐of‐analysis bias since no paired data were available.
We considered the risk of other bias to be unclear in four studies since they did not define a primary outcome and did not include an adequate adjustment for multiple testing (Kolanowski 2011; Orsulic‐Jeras 2000; Richards 2005; Van Haitsma 2015).
Effects of interventions
See: Table 1
Primary outcomes
Challenging behaviour
We performed a meta‐analysis for challenging behaviour, including six studies (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Kolanowski 2011; Orsulic‐Jeras 2000; van der Ploeg 2013; Wenborn 2013). One study assessing behaviour was not included in the meta‐analysis because the assessed types of behaviours were not comparable with the behavioural outcomes used in the other studies (Van Haitsma 2015).
We used the standardised mean difference (SMD), calculated from mean values assessed during or directly after the intervention period or session. For two studies, the number of participants was re‐calculated to incorporate the cluster effect, using an estimate of the intra‐cluster correlation coefficient (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a ‒ see Unit of analysis issues). We used a random‐effects model since there was clinical diversity and evidence for moderate heterogeneity (I² = 50%). Higher scores indicate more challenging behaviour.
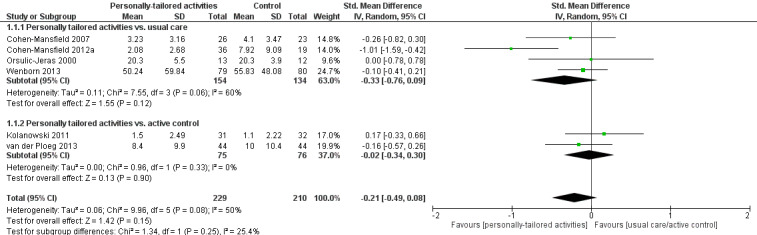
For challenging behaviour, we found low‐quality evidence (downgraded for risk of bias and imprecision) that personally tailored activities may slightly reduce challenging behaviour (SMD −0.21, 95% CI −0.49 to 0.08; I² = 50%; random‐effects model; 6 studies; 439 participants; Analysis 1.1; Figure 4). Compared with studies only including a usual care control group, personally tailored activities may slightly reduce challenging behaviour (SMD −0.33, 95% CI −0.76 to 0.09; 288 participants; I² = 60%; random‐effects model; 4 studies; 288 participants; Figure 4), but personally tailored activities may have little or no effect on challenging behaviour compared with studies including active control groups (SMD −0.02, 95% CI −0.34 to 0.30; I² = 0%; random‐effects model; 2 studies; 151 participants; Figure 4). However; there is no statistically significant difference between the results of the usual care active control subgroups (test for subgroup differences P = 0.25, I² = 25.4%). To further explore the potential reasons for heterogeneity, an analysis was performed excluding one study (Cohen‐Mansfield 2012a), which appeared to be an outlier. After excluding this study, the I² was reduced to 0% and the effect size was reduced to little or no effect (low‐quality evidence, SMD −0.08, 95% CI −0.28 to 0.12; 5 studies; 384 participants; Analysis 1.2). We could not explain the heterogeneity based on the characteristics of this study, e.g. population, intervention or outcome measures.
1.1. Analysis.
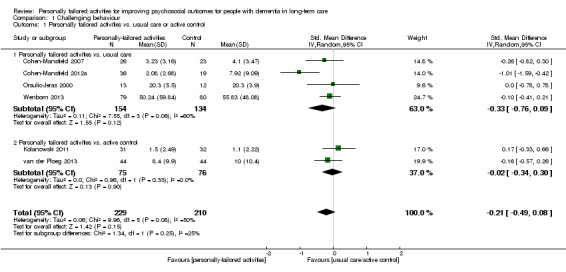
Comparison 1 Challenging behaviour, Outcome 1 Personally tailored activities vs. usual care or active control.
4.
Forest plot of comparison: 1 Challenging behaviour, outcome: 1.1 Personally tailored activities vs. usual care or active control.
1.2. Analysis.
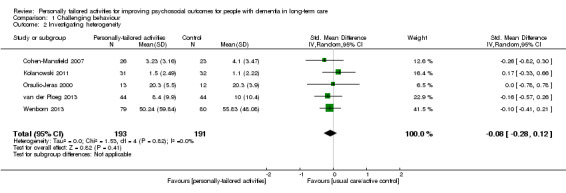
Comparison 1 Challenging behaviour, Outcome 2 Investigating heterogeneity.
In the study by Van Haitsma 2015, the outcomes of general restlessness, aggression, uncooperative behaviour, very negative and negative verbal behaviour seemed to best represent challenging behaviour. Higher scores indicate a higher frequency of the behaviour. We found low‐quality evidence (downgraded for risk of bias and imprecision) that personally tailored activities may slightly improve general restlessness compared to usual care (MD −16.97, 95% CI −18.80 to −15.14; 137 participants) but may make little or no difference compared to the active control group (MD 1.22, 95% CI −1.14 to 3.58; 87 participants). Aggression and uncooperative behaviours were rarely observed in all groups; we found low‐quality evidence (downgraded for risk of bias and imprecision) that personally tailored activities may have little or no effect on aggression and uncooperative behaviours (aggression: personally tailored activities vs usual care MD 0.06, 95% CI 0.05 to 0.07; 137 participants; personally tailored activities vs active control MD −0.06, 95% CI −0.07 to −0.04; 87 participants. Uncooperative behaviour: personally tailored activities vs usual care MD 0.01, 95% CI −0.00 to 0.02; 137 participants; personally tailored activities vs active control MD −0.13, 95% CI −0.15 to −0.12; 87 participants). We also found low‐quality evidence (downgraded for risk of bias and imprecision) that personally tailored activities may slightly increase very negative verbal behaviour compared to usual care (MD 7.75, 95% CI 5.51 to 9.99; 137 participants) but may reduce very negative verbal behaviour compared to the active control group (MD −29.33, 95% CI −32.22 to −26.44; 87 participants). For negative verbal behaviours, we found low‐quality evidence (downgraded for risk of bias and imprecision) that personally tailored activities may slightly increase negative verbal behaviour compared to usual care (MD 21.68, 95% CI 17.66 to 25.70; 137 participants) and may make little or no difference to negative verbal behaviour compared to the active control group (MD 3.07, 95% CI −2.13 to 8.27; 87 participants).
Quality of life
Only one study investigated the effects of personally tailored activities on quality of life (Wenborn 2013). Quality of life was assessed by the study personnel (proxy‐rating) and by a small group of participants who were able to complete the assessment (self‐rating, n = 42 out of n = 139). Clinical relevance was defined by the study authors as three points on the scale used (higher scores indicates better quality of life). For proxy‐rated quality of life, there was moderate‐quality evidence (downgraded one level for imprecision) that personally tailored activities were associated with a slight reduction in quality of life compared to usual care (MD −1.93, 95% CI −3.63 to −0.23; adjusting for baseline and demographic characteristics; 139 participants). For self‐rated quality of life, there was low‐quality evidence (downgraded two levels for imprecision) indicating little or no difference between personally tailored activities and usual care (MD 0.26, 95% CI −3.04 to 3.56; adjusting for baseline and demographic characteristics; 42 participants).
Secondary outcomes
Affect
We performed meta‐analyses for positive and negative affect (including six studies in each analysis) and mood (including three studies). For positive affect, we used the results from four studies assessing pleasure (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Kolanowski 2011; Orsulic‐Jeras 2000), from one study assessing a combination of pleasure and contentment (van der Ploeg 2013), and for one study we calculated a combination of pleasure and alertness (Van Haitsma 2015; see Data synthesis).
For negative affect, we used the following study data: negative affect calculated from anger, anxiety, and sadness (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a), negative affect calculated from anger, sadness, and anxiety/fear (van der Ploeg 2013), anxiety or fear (Kolanowski 2011), and anxiety (Wenborn 2013). From Van Haitsma 2015, we calculated negative affect from sadness, anger, and anxiety (see Data synthesis).
For mood we combined data on mood from one study (Kolanowski 2011, data were corrected for the differing direction of the scale (see Data synthesis)); and data on depression from two studies (Orsulic‐Jeras 2000; Wenborn 2013). We used the standardised mean difference (SMD), calculated from mean values assessed during or directly after the intervention period or session. For two studies, the number of participants was re‐calculated to incorporate the cluster effect, using an estimate of the intra‐cluster correlation coefficient (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a ‒ see Unit of analysis issues). We used a random‐effects model since there was clinical diversity and substantial heterogeneity (I² = 84% for positive affect). Higher scores indicate more positive and negative affect, and better mood.
Positive affect
For positive affect we found very low quality evidence (downgraded for risk of bias, inconsistency and imprecision) and we are therefore very uncertain whether personally tailored activities improve positive affect (SMD 0.88, 95% CI 0.43 to 1.32; I² = 80%; 6 studies; 498 participants; Analysis 2.1; Figure 5). We are also uncertain whether personally tailored activities improve positive affect compared to studies with active control groups (SMD 0.36, 95% CI 0.09 to 0.63; I² = 0%; 3 studies; 216 participants; Figure 5) or compared to studies with usual care control groups (SMD 1.30, 95% CI 0.77 to 1.84; I² = 69%; 4 studies; 282 participants; Figure 5) (one study contributed to both subgroup analyses). A sensitivity analysis excluding the study for which we calculated the combined effect showed an effect similar to the main analysis (SMD 0.76, 95% CI 0.38 to 1.13; I² = 58%; 5 studies; 318 participants; Analysis 2.2).
2.1. Analysis.
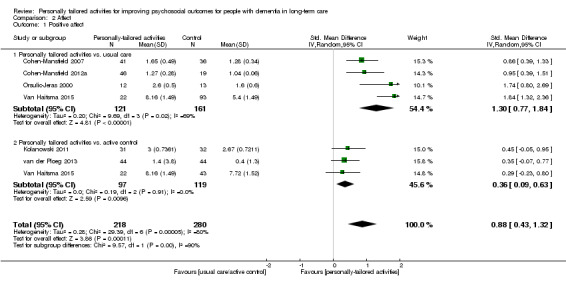
Comparison 2 Affect, Outcome 1 Positive affect.
5.
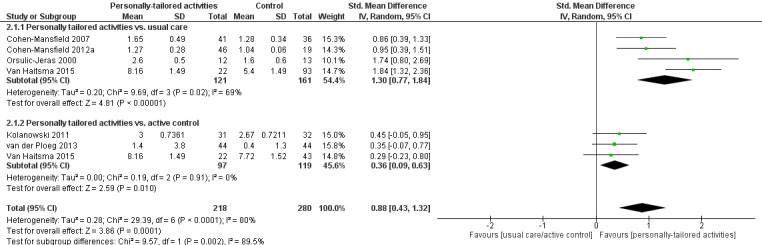
Forest plot of comparison: 2 Affect, outcome: 2.1 Positive affect.
2.2. Analysis.
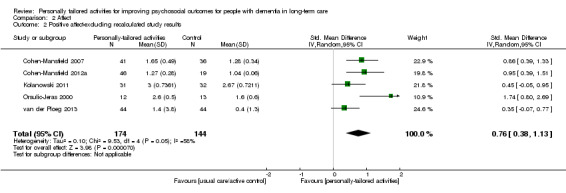
Comparison 2 Affect, Outcome 2 Positive affect‐excluding recalculated study results.
Negative affect
We found low‐quality evidence (downgraded for risk of bias and imprecision) that personally tailored activities may make little or no difference to negative affect (SMD 0.01, 95% CI −0.15 to 0.18; I² = 0%; 6 studies; 589 participants; Analysis 2.3; Figure 6). The subgroup analyses for the different types of control groups showed similar results (personally tailored activities vs. usual care: SMD 0.01, 95% CI −0.19 to 0.22; I² = 0%; 4 studies; 416 participants; personally tailored activities vs. active control group: SMD −0.09, 95% CI −0.36 to 0.18; I² = 0%; 3 studies; 216 participants; Analysis 2.3; Figure 6). The sensitivity analysis excluding the study for which we calculated the combined effect also showed similar results (SMD −0.03, 95% CI −0.22 to 0.16; I² = 0%; 5 studies; 452 participants; Analysis 2.4).
2.3. Analysis.
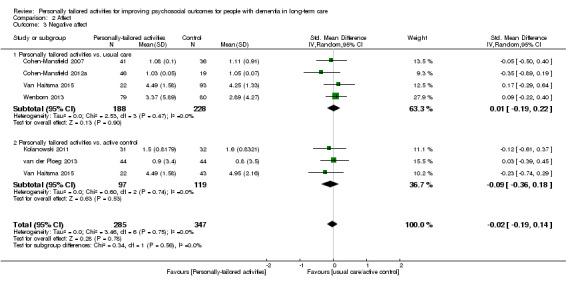
Comparison 2 Affect, Outcome 3 Negative affect.
6.
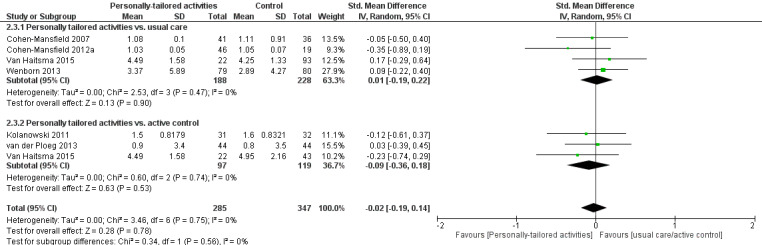
Forest plot of comparison: 2 Affect, outcome: 2.3 Negative affect.
2.4. Analysis.
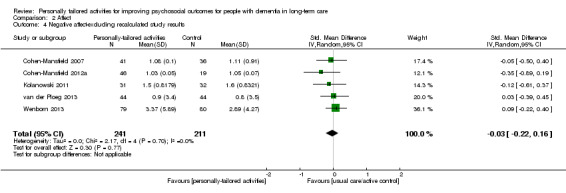
Comparison 2 Affect, Outcome 4 Negative affect‐excluding recalculated study results.
Mood
We found very low quality evidence (downgraded one level for risk of bias and two levels for imprecision) and we are therefore very uncertain whether personally tailored activities improve mood (SMD −0.02, 95% CI −0.27 to 0.23; I² = 0%; 3 studies; 247 participants; Analysis 2.5; Figure 7).
2.5. Analysis.
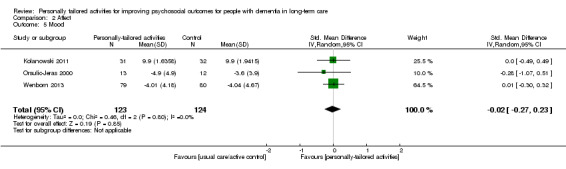
Comparison 2 Affect, Outcome 5 Mood.
7.
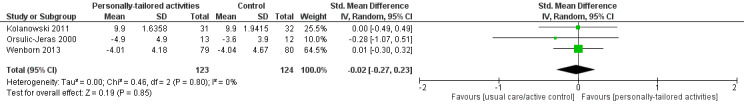
Forest plot of comparison: 2 Affect, outcome: 2.5 Mood.
Level of engagement
Three studies reported results on participants' engagement but assessed different types of engagement. The results were not pooled due to the diversity of the outcome measures. We calculated mean differences for the results of two studies (Kolanowski 2011; van der Ploeg 2013), but not for the study by Orsulic‐Jeras 2000 due to pronounced baseline imbalances. Higher scores indicate more engagement.
Kolanowski 2011 assessed the intensity of participation. We found low‐quality evidence (downgraded for indirectness and imprecision) that personally tailored activities may make little or no difference to the intensity of participation (MD 0.30, 95% CI 0.16 to 0.44; 63 participants).
For constructive engagement we found very low quality evidence (downgraded for risk of bias, indirectness and imprecision) and we are therefore very uncertain whether personally tailored activities improve constructive engagement (personally tailored activities vs. active control: MD 6.90, 95% CI 3.07 to 10.73; 88 participants; van der Ploeg 2013). In the study by Orsulic‐Jeras 2000, constructive engagement decreased in both groups (intervention group from 172 ± 171 at baseline to 96 ± 64 after six months; control group from 94 ± 79 at baseline to 49 ± 54 after six months; 25 participants).
For passive engagement, we found very low quality evidence (downgraded for risk of bias, indirectness and imprecision) and we are therefore very uncertain whether personally tailored activities improve passive engagement (personally tailored activities vs. active control: MD −1.60, 95% CI −4.99 to 1.79; 88 participants; van der Ploeg 2013). In the study by Orsulic‐Jeras 2000 passive engagement decreased in the intervention group and was nearly unchanged in the control group (intervention group baseline 207 ± 132, after six months 91 ± 66; control group baseline 354 ± 158, after six months 345 ± 188; 25 participants).
For negative engagement, we found very low quality evidence (downgraded for risk of bias, indirectness and imprecision) from one study and we are therefore very uncertain whether personally tailored activities improve negative engagement (MD personally tailored activities vs. active control −5.50, 95% CI −9.58 to −1.42; 88 participants) (van der Ploeg 2013).
Two studies investigated engagement after the sessions ended and all types of engagement returned to the baseline level (Kolanowski 2011; van der Ploeg 2013).
Sleep disturbances
For the sleep‐related outcomes, we found very low quality evidence (downgraded for risk of bias, indirectness and imprecision) from one study (Richards 2005). We are therefore very uncertain whether personally tailored activities improve the amount of daytime sleep (minutes slept: MD −39.16, 95% CI −62.06 to −16.26; 139 participants) or the amount of nighttime sleep (MD 28.81, 95% CI −22.65 to 80.27; 139 participants; there were baseline differences between the groups — minutes slept at baseline: intervention group 368.95 ± 158.13; control group 331.37 ± 135.20). We are also uncertain whether personally tailored activities improve the time awake during the night (no MD calculated due to pronounced baseline imbalances, minutes awake: intervention group baseline 266.19 ± 142.02, follow‐up 252.14 ± 138.57; control group baseline 310.44 ± 129.63, follow‐up 304.20 ± 151.31).
Duration of the effects
Two studies investigated the duration of intervention effects. In both studies, the values of most outcomes (challenging behaviour, positive and negative affect, engagement, and mood) returned to the baseline level (in the study by Kolanowski 2011, one week after the intervention period was completed; and in the study by van der Ploeg 2013, 30 minutes after the intervention sessions).
No information on the duration of the effects were available from the other studies (Cohen‐Mansfield 2007; Cohen‐Mansfield 2012a; Orsulic‐Jeras 2000; Richards 2005; Van Haitsma 2015; Wenborn 2013).
Psychotropic medication
No study offered information on the use of psychotropic medication.
Effects on caregivers
No study offered information on the effects of the interventions on caregivers.
Adverse effects
Only two studies reported any information on adverse effects. No adverse effects were observed in either study (Cohen‐Mansfield 2012a; Kolanowski 2011).
Costs
Only the study by Richards 2005 assessed costs related to staff training, delivery of activities and administration of the intervention. Training costs comprised USD 1200 for teaching the project nursing assistants to conduct the ISAI and training the registered nurses in the use of the outcome assessment. Costs for delivery of the activities were about USD 765 and included costs of commercial activities and perishable supplies. The mean cost per activity was estimated at USD 5. Administration costs were about USD 28 (one hour to complete the assessment).
Process evaluation
Four studies offered information about the implementation process or barriers to the implementation of the intervention (Cohen‐Mansfield 2012a; Kolanowski 2011; Van Haitsma 2015; Wenborn 2013).
Three studies offered information on the dose of the intervention received by the participants. Kolanowski 2011 calculated the dose received as the product of time on task and intensity of participation per day. The total dose of intervention per participant ranged widely, but the mean dose did not differ significantly between groups. Wenborn 2013 reported information on staff attendance at the training sessions: the participating nurses (n = 52) attended an average of 73% of the education sessions (range: 63 to 86) and 81% of the individual coaching sessions (range 49 to 100). No information was reported regarding the amount of activities offered to the residents in the intervention group. In the study by Van Haitsma 2015, each participant received on average seven intervention sessions (range 5 to 9).
In the study by Cohen‐Mansfield 2012a several barriers to implementation were identified. Participants were partly unwilling, unresponsive (e.g. due to the severity of dementia) or unavailable (e.g. asleep or eating) and did not participate in the offered activities. The participants were more engaged in activities related to food/drink and one‐to‐one socializing activities and less engaged with puzzles, board games, art and craft activities (Cohen‐Mansfield 2012b).
Discussion
Summary of main results
We included eight studies evaluating interventions offering personally tailored activity for people with dementia living in long‐term care facilities. The activities were offered directly to the people with dementia in seven studies and in one study the nursing staff were trained to deliver the activities. The interventions varied in terms of the theoretical basis, the way that personal interests of the participants were assessed, the frequency and duration of the activity sessions and the length of follow‐up; however, the activities themselves were comparable across studies.
Offering personally tailored activities to people with dementia in long‐term care may slightly improve challenging behaviour. In subgroup analyses, this effect was present when personally tailored activities were compared with usual care, but not when they were compared with an active control intervention. We also found that offering personally tailored activities probably slightly reduced proxy‐rated quality of life, but quality of life was only investigated in one study and the validity of proxy‐rating (in this case, by study personnel) of quality of life in severe dementia has been questioned. Personally tailored activities may also have little or no effect on negative affect and, due to the very low quality of the evidence, we are uncertain whether personally tailored activities improve positive affect, mood, engagement or sleep‐related outcomes. We found a relatively large effect size for positive affect, but in studies including an active control group there was only a small effect. Due to these differences and the very low quality of evidence we have very little confidence in this result. Adverse events related to personally tailored activities were assessed in only two studies and neither reported any adverse events. In summary, our results suggests that offering structured activities to people with dementia might have positive effects on some psychosocial outcomes, particularly challenging behaviour, when compared to usual care, but we found no evidence of additional effects of tailoring the activities to the person's preferences or interests.
Two studies investigated the duration of the intervention effects and in both studies the majority of the outcomes returned to the baseline level after the delivery of the activities ended (in one study 30 minutes after the activities were offered and in one study one week after the intervention period ended) (Kolanowski 2011; van der Ploeg 2013). In these studies, the positive effects of the activities offered persisted only during the time the activities were delivered. However, such results were not available for six studies and therefore it is not possible to draw clear conclusions.
Overall completeness and applicability of evidence
The number of studies contributing to the different outcomes of interest in this review was small. We included from three to six studies in the meta‐analyses. Only one study investigated quality of life. Due to the clinical heterogeneity of the interventions (e.g. the different theoretical basis, duration and frequency of the activity sessions) and the methodological limitations of the included studies, the results of this review must be interpreted with caution. Additional high‐quality studies are needed.
Almost all of the participants in the included studies had severe dementia. The results may not be applicable to residents of long‐term care facilities whose dementia is less severe.
Quality of the evidence
We evaluated the quality of evidence following the GRADE approach (Guyatt 2011). We judged the quality of evidence to be very low to moderate due to several methodological limitations in the studies, inconsistency between studies and imprecision of the results. The risk of bias varied in the included studies. Allocation concealment was adequate in only two out of seven randomised trials and one study was not randomised. The outcome assessors were blinded to group allocation in only three studies.
We also found moderate to substantial heterogeneity in the meta‐analyses on challenging behaviour and affect. For challenging behaviour, this heterogeneity was reduced by excluding one study (Cohen‐Mansfield 2012a); however, we could not explain this heterogeneity from characteristics of this study. For positive affect we could not identify the source of heterogeneity.
Generally, investigating the effects of personally tailored activities for people with dementia presents several methodological challenges. One challenge is the theoretical basis for preselecting the activities that could be offered to the participants and the process of choosing the activities for an individual person with dementia. The theoretical basis for selecting the activities differed between the studies. Two models, the Need‐Driven Dementia‐Compromised Behavior (NDB) model (Algase 1996) and Treatment Routes for Exploring Agitation (TREA) framework (Cohen‐Mansfield 2000), assume that challenging behaviour is a symptom of unmet needs in people with dementia. Both models postulate that by targeting the identified unmet needs the specific challenging behaviour could be modified. The principles of Montessori lay emphasis on offering activities which best fit the level of competence of people with dementia. The principles focus on task breakdown, guided repetition and progression in difficulty from simple to complex (van der Ploeg 2010). The Individualized Positive Psychosocial Intervention, employed by Van Haitsma 2015, did not focus on specific needs of people with dementia but more general assumptions about a person's needs for autonomy and competence and the importance of positive emotions to improve a person's well‐being. Irrespective of the different theoretical models, the activities offered were very similar. Based on the results of this review, there is no evidence that interventions were more likely to be effective if based on one theoretical model rather than another.
The methods for assessing the participants' interests in order to tailor the activities also differed between studies. In all studies data were collected directly from the participant, their relatives or the primary caregiver. No information was available in any study about the number of participants who were able to express their individual interests or preferences. The majority of participants had severe dementia (MMSE lower than 12), indicating a substantial risk of memory and ability loss. There is evidence that both family members and caregivers might have differing perceptions about the meaningfulness of activities compared to people with dementia themselves. People with dementia judge activities as personally meaningful if the activities are connected with self (which represents the personal interests and the individual motivation to take part in a specific activity), with others, and with the environment (Han 2016). Since the included studies did not investigate whether the relatives or primary caregivers were able to give valid information on the participants' interests and preferences or whether the former interests and preferences changed over time or with the progression of the cognitive impairment, it remains unclear whether the activities offered were judged as meaningful by the study participants. The active control activities might also be seen as meaningful from the perspective of the study participants, especially the one‐to‐one interactions offered as active control in two studies (van der Ploeg 2013; Van Haitsma 2015), which are likely to meet the need for connectedness of people with dementia (Han 2016). All studies included in this review hypothesised that personally tailored activities are more likely to be meaningful than activities which are not personally tailored, but this aspect was not investigated in the studies.
Care dependency and cognitive impairment are also expected to have a restricting influence on the selection of activities, e.g. sports or crafts might be difficult to offer in a nursing home and reading a book or a newspaper might be difficult due to poor sight. One study reported challenges in implementing several activities due to unwillingness or unresponsiveness of participants with severe cognitive impairment. Simple activities, e.g. one‐to‐one socialising activities, were implemented more easily than more complex activities, e.g. puzzles, board games or craft activities (Cohen‐Mansfield 2012b).
Another challenge is the characteristics of 'usual care'. There is evidence that people living in nursing homes have only few contacts with others and that activities offered to them are often not perceived as meaningful (Edvardsson 2014; Harmer 2008; Hill 2010). The usual care offered to the control groups was not well described in several studies and the amount of activity available to the control group may have varied substantially between studies.
It was difficult to distinguish clearly between some of the outcomes addressed in this review, i.e. challenging behaviour, engagement and affect. Van Haitsma 2015 categorised several types of behaviour differently from other studies, e.g. "staring with a fixed gaze" was categorised as non‐verbal behaviour in this study while a "blank stare" was categorised as engagement in the studies by Orsulic‐Jeras 2000 and van der Ploeg 2013. We did not include all behaviours assessed by Van Haitsma 2015 but selected behaviours which were most comparable with the concept of challenging behaviour assessed in the other studies. The different instruments used to assess challenging behaviour also warrant consideration. One group of instruments rated the outcome based on direct observation of the participants and another group used proxy rating by the nursing staff. There is some evidence that proxy‐rating instruments assessing quality of life are less valid than instruments based on direct observation, since there might be a stronger influence of personal factors of the proxy‐raters, e.g. personal attitudes (Arons 2013; Gomez‐Gallego 2015; Moyle 2012). For instruments assessing challenging behaviour some studies found that the reliability of instruments was moderate to good (Cohen‐Mansfield 2004; van der Linde 2014). In one study (van der Ploeg 2013), one single behaviour was investigated for each participant compared to the wide range of behaviours assessed by the rating scales used in other studies. Irrespective of these differences and uncertainties, the results of the different studies were quite similar, with the exception of one study (Cohen‐Mansfield 2012a). Therefore, pooling the results of the different instruments seemed to be feasible, with the caveats mentioned above.
Potential biases in the review process
We have made efforts in the review process to reduce the risk of bias. Publication bias is unlikely to have affected the results because we conducted an intensive literature search, covering database search (including electronic databases and trial registers, guided by the CDCIG) as well as snowballing techniques for all included studies. However, due to the small number of studies we were not able to investigate the risk for publication bias using formal statistical methods.
Two reviewers independently conducted study selection, quality appraisal, and data extraction. We also contacted all study authors for missing information.
Agreements and disagreements with other studies or reviews
Two systematic reviews investigating the effects of activities offered for people with dementia in nursing homes have been published in recent years. Both reviews used a broader approach investigating a wide range of non‐pharmacological interventions on psychosocial outcomes (Testad 2014; Travers 2016). Testad 2014 included, among others, interventions offering "pleasant activities with or without social interaction" but the inclusion criteria for both the interventions and the setting differed slightly from this review (e.g. cross‐over trials were excluded and different types of long‐term care settings were included). The review by Travers 2016 investigated, among others, the effects of individualised recreational activities. There was large agreement regarding the main results between this review and the reviews by Testad 2014 and Travers 2016, but in this review several studies were excluded based on the more specific inclusion and exclusion criteria.
Both reviews described positive effects on pleasure and interest and, in contrast to our analysis, an improvement of agitation. However, Testad 2014 performed no meta‐analysis and the narrative synthesis showed small intervention effects. Travers 2016 performed some meta‐analyses, including only two studies, and found no or small effects with wide CIs. Neither review rated the quality of evidence using the GRADE approach (Guyatt 2011); and therefore no information about the certainty of the evidence was reported.
Authors' conclusions
Implications for practice.
Offering personally tailored activities to people with dementia in long‐term care may be considered as an intervention for challenging behaviour. Offering any activities to people with dementia seems to be necessary from an ethical perspective. However, we can present no recommendations on the method for selecting activities, the types of activity, or the duration and frequency of activities based on the results of this review.
Implications for research.
The results indicate that further studies should be conducted to explore the potential benefits of personally tailored activities for improving positive affect and reducing challenging behaviour in people with dementia living in long‐term care facilities.
The development and evaluation of programmes offering personally tailored activities should adhere to the methodological recommendations for complex interventions (Craig 2008). When developing new interventions, more emphasis should be laid on potentially active components of such interventions. The theoretical basis on which the activities are chosen seems less important and it was not assessed whether the participants judged the offered activities as meaningful. The concept of 'meaningfulness' — how it could be assessed and how activities could be selected based on the results of such an assessment — needs to be investigated in more detail. Research on this topic seems to be feasible with people in earlier stages of dementia but more challenging in later stages of dementia. Assessing the interests and preferences of people with dementia and tailoring the activities to these interests, preferences and competencies (i.e. stage of dementia and the care dependency of the participants) also needs further investigation. In the context of active components, the effect of direct interaction alone (without offering specific activities) compared to direct interaction while offering specific (meaningful) activities has to be addressed. The role of direct interaction might also differ within the course of dementia, e.g. the activities might be more important in early stages of dementia. Because of these challenges, comprehensive feasibility and pilot studies should be performed prior to evaluation studies (Craig 2008).
Evaluation studies should be planned adhering to current methodological standards, e.g. a randomised and concealed allocation; adequate blinding (at least the participants (this is possible if an active control group is offered) and outcome assessors); recruitment of sufficiently large study samples; and adequate statistical methods, e.g. randomised clusters rather than individuals (Higgins 2011). In future studies we recommend comparing a personally tailored group with an active control group, offering direct interaction with participants or activities suitable for people with dementia; or with two control groups — an active control group and a 'usual care' control group. Studies including three groups are time‐ and personnel‐consuming; however, they can add valuable evidence to improve both research and clinical practice. Furthermore, a process evaluation should be an integral part of the evaluation study, assessing the degree of the intervention's implementation, information about the need to tailor the intervention to a specific study context (e.g. study centres) and barriers to, and facilitators of, the intervention's implementation (Grant 2013; Moore 2015).
To ensure comprehensive reporting covering the complete research process (development, piloting and evaluation), the corresponding reporting statements should be used, e.g. CReDECI 2 for complex interventions (Möhler 2015), the TiDieR criteria for the description of the interventions (Hoffmann 2014), and CONSORT or the corresponding extension, e.g. for randomised pilot and feasibility trials (Eldridge 2016), or cluster‐RCTs (Campbell 2012).
Acknowledgements
We thank Cochrane and the external peer reviewers for their helpful comments; and the group's information specalist for her support. We also acknowledge the statistical support by Gerta Rücker (Institute for Medical Biometry and Statistics, Medical Center ‒ University of Freiburg, Faculty of Medicine, University of Freiburg, Germany).
Appendices
Appendix 1. Searches May 2012, April 2013, March 2014, January 2015, January 2016, July 2017
|
Source |
Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) [most recent search date: 16 June 2017] |
#1 "personally tailored" OR individualized OR individualised OR individual OR person‐centred OR meaningful OR personhood #2 involvement OR engagement OR engaging OR identity #3 #1 OR #2 |
May 2012: 149 April 2013: 0 March 2014: 6 January 2015: 1 January 2016: 2 October 2016: 2 June 2017: 3 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1946 to present (Ovid SP) [most recent search date: 16 June 2017] |
1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*.mp. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (creutzfeldt or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. or/1‐19 21. activity.ti,ab. 22. activities.ti,ab. 23. psychosocial.ti,ab. 24. non‐pharmacological.ti,ab. 25. individually‐tailor*.ti,ab. 26. personally‐tailor*.ti,ab. 27. (individual or individuals or individually‐cent*).ti,ab. 28. meaning*.ti,ab. 29. involvement.ti,ab. 30. (engagement or engaging).ti,ab. 31. occupational*.ti,ab. 32. personhood.ti,ab. 33. person‐centred.ti,ab. 34. identity.ti,ab. 35. or/21‐34 36. 20 and 35 37. long‐term care.ti,ab. 38. "care home*".ti,ab. 39. "residential care".ti,ab. 40. "nursing home*".ti,ab. 41. "residential facilit*".ti,ab. 42. Residential Facilities/ 43. Nursing Homes/ 44. "old people* home*".ti,ab. 45. or/37‐44 46. 36 and 45 |
May 2012: 1656 April 2013: 205 March 2014: 177 January 2015: 182 January 2016: 185 October 2016: 367 June 2017: 358 |
| 3. Embase 1974 to 2011 December 29 (Ovid SP); then 1974 to 2013 week 12; then 1974 to 2014 week 11; then 1974 to 2016 October 14 [most recent search date: 16 June 2017] |
1. exp dementia/ 2. Lewy body/ 3. delirium/ 4. Wernicke encephalopathy/ 5. cognitive defect/ 6. dement*.mp. 7. alzheimer*.mp. 8. (lewy* adj2 bod*).mp. 9. deliri*.mp. 10. (chronic adj2 cerebrovascular).mp. 11. ("organic brain disease" or "organic brain syndrome").mp. 12. "supranuclear palsy".mp. 13. ("normal pressure hydrocephalus" and "shunt*").mp. 14. "benign senescent forgetfulness".mp. 15. (cerebr* adj2 deteriorat*).mp. 16. (cerebral* adj2 insufficient*).mp. 17. (pick* adj2 disease).mp. 18. (creutzfeldt or jcd or cjd).mp. 19. huntington*.mp. 20. binswanger*.mp. 21. korsako*.mp. 22. CADASIL.mp. 23. or/1‐22 24. activity.ti,ab. 25. activities.ti,ab. 26. psychosocial.ti,ab. 27. non‐pharmacological.ti,ab. 28. individually‐tailor*.ti,ab. 29. personally‐tailor*.ti,ab. 30. (individual or individuals or individually‐cent*).ti,ab. 31. meaning*.ti,ab. 32. involvement.ti,ab. 33. (engagement or engaging).ti,ab. 34. occupational*.ti,ab. 35. personhood.ti,ab. 36. person‐centred.ti,ab. 37. identity.ti,ab. 38. or/24‐37 39. 23 and 38 40. long‐term care.ti,ab. 41. "care home*".ti,ab. 42. "residential care".ti,ab. 43. "nursing home*".ti,ab. 44. "residential facilit*".ti,ab. 45. residential home/ 46. nursing home/ 47. "old people* home*".ti,ab. 48. or/40‐47 49. 39 and 48 50. 39 and 48 |
May 2012:2400 April 2013: 382 March 2014: 452 January 2015: 492 January 2016: 463 October 2016: 777 June 2017: 691 |
| 4. PsycINFO 1806 to May week 1 2012 (Ovid SP); then March week 4 2013 (Ovid SP); then 1806 to March week 1 (Ovid SP); then 1806 to October week 2 (Ovid SP) [most recent search date: 16 June 2017] |
1. exp Dementia/ 2. exp Delirium/ 3. exp Huntingtons Disease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. or/1‐23 25. activity.ti,ab. 26. activities.ti,ab. 27. psychosocial.ti,ab. 28. non‐pharmacological.ti,ab. 29. individually‐tailor*.ti,ab. 30. personally‐tailor*.ti,ab. 31. (individual or individuals or individually‐cent*).ti,ab. 32. meaning*.ti,ab. 33. involvement.ti,ab. 34. (engagement or engaging).ti,ab. 35. occupational*.ti,ab. 36. personhood.ti,ab. 37. person‐centred.ti,ab. 38. identity.ti,ab. 39. or/25‐38 40. 24 and 39 41. long‐term care.ti,ab. 42. "care home*".ti,ab. 43. "residential care".ti,ab. 44. "nursing home*".ti,ab. 45. "residential facilit*".ti,ab. 46. exp Nursing Homes/ or exp Residential Care Institutions/ 47. "old people* home*".ti,ab. 48. institutionali?ed.ti,ab. 49. or/41‐48 50. 40 and 49 |
May 2012: 1633 April 2012: 191 March 2014: 202 January 2015: 207 January 2016: 228 October 2016: 356 June 2017: 268 |
| 5. CINAHL (EBSCOhost) [most recent search date: 16 June 2017] |
S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 AB activity S21 AB activities S22 TX psychosocial S23 TX non‐pharmacological S24 TX individually‐tailor* S25 TX personally‐tailor* S26 AB individual OR individuals OR individually‐cent* S27 AB meaningful S28 AB involvement S29 TX engagement or engaging S30 TX occupational* S31 TX personhood S32 TX person‐centred S33 TX identity S34 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 S35 TX "long‐term care" S36 TX "care home*" S37 TX "residential care" S38 TX "nursing home*" S39 TX "residential facilit*" S40 (MH "Residential Facilities") S41 (MH "Nursing Homes") S42 TX "old people* home*" S43 TX institutional S44 TX institutionalised OR institutionalized S45 S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 S46 S19 and S34 and S45 |
May 2012: 2367 April 2013: 275 March 2014: 221 January 2015: 158 January 2016: 121 October 2016: 245 June 2017: 274 |
| 6. Web of Science and conference proceedings [most recent search date: 16 June 2017] |
Topic=(dementia OR alzheimer* OR lewy OR CJD OR JCD OR creutzfeldt OR binswanger OR korsako*) AND Topic=(activity OR activities OR psychosocial OR non‐pharmacological OR "individually tailor*" OR "personally tailor*" OR individual OR meaningful* OR occupaional OR personhood OR "person cent*" OR identity) AND Topic=("long term care" OR "longterm care" OR "residential care" OR "nursing home*" OR "residential facilit*" OR "old people* home*" OR institutionalised OR institutionalized) Timespan=All Years. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH. Lemmatization=On |
May 2012: 2153 April 2013: 311 March 2014: 104 January 2015: 216 January 2016: 391 October 2016: 773 June 2017: 766 |
| 7. LILACS (BIREME) [most recent search date: 16 June 2017] |
dementia OR demencia OR demência OR alzheimer OR alzheimers OR alzheimer’s [Words] and "personally tailored" OR "pessoalmente adaptado" OR "personal a medida" OR individual OR individualised OR individualized OR individualmente OR individualmente OR activity OR activites OR atividades OR "las actividades" OR occupational [Words] | May 2012: 313 April 2013: 21 March 2014: 0 January 2015: 0 January 2016: 3 October 2016: 52 June 2017: 67 |
| 8. CENTRAL (in The Cochrane Library) (Issue 4 2012 and Issue 2 2013) [most recent search date: 16 June 2017] |
#1 MeSH descriptor Dementia explode all trees #2 MeSH descriptor Delirium, this term only #3 MeSH descriptor Wernicke Encephalopathy, this term only #4 MeSH descriptor Delirium, Dementia, Amnestic, Cognitive Disorders, this term only #5 dement* #6 alzheimer* #7 "lewy* bod*" #8 deliri* #9 "chronic cerebrovascular" #10 "organic brain disease" or "organic brain syndrome" #11 "normal pressure hydrocephalus" and "shunt*" #12 "benign senescent forgetfulness" #13 "cerebr* deteriorat*" #14 "cerebral* insufficient*" #15 "pick* disease" #16 creutzfeldt or jcd or cjd #17 huntington* #18 binswanger* #19 korsako* #20 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) #21 activity #22 activities #23 psychosocial #24 non‐pharmacological #25 individually‐tailor* #26 personally‐tailor* #27 individual OR individuals OR individually‐cent* #28 meaning* #29 involvement #30 engagement or engaging #31 occupational* #32 personhood #33 person‐centred #34 identity #35 (#22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34) #36 "long‐term care" OT "longterm care" OR "long term care" #37 "care home*" #38 "residential care" #39 "nursing home*" #40 "residential facilit*" #41 MeSH descriptor Residential Facilities explode all trees #42 MeSH descriptor Nursing Homes explode all trees #43 "old people* home*" #44 institutionalised OR institutionalized #45 (#36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44) #46 (#20 AND #35 AND #45) |
May 2012: 280 April 2013: 3 March 2014: 10 January 2015: 18 January 2016: 13 October 2016: 50 June 2017: 17 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) [most recent search date: 16 June 2017] |
(personally tailored OR individual OR person‐centred OR meaningful OR personhood) | Interventional Studies | dementia OR VCI OR vascular dementia OR VaD OR vascular cognitive impairment OR cadasil OR multi‐infarct OR binswanger | Senior | May 2012: 271 April 2013: 47 March 2014: 88 January 2015: 14 January 2016: 13 October 2016: 58 June 2017: 94 |
| 10. ICTRP Search Portal (apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [most recent search date: 16 June 2017] |
personally tailored OR individual OR person‐centred OR meaningful OR personhood) | Interventional Studies | dementia OR VCI OR vascular dementia OR VaD OR vascular cognitive impairment OR cadasil OR multi‐infarct OR binswanger | May 2012: 127 April 2013: 12 March 2014: 13 January 2015: 8 January 2016: 16 October 2016: 4 June 2017: 11 |
| TOTAL before de‐duplication | May 2012: 11349 April 2012: 1455 March 2014: 1273 January 2015: 1296 January 2016: 1435 October 2016: 2682 June 2017: 2549 |
|
| TOTAL after de‐duplication and first assessment by CDCIG Information Specialists | May 2012: 532 April 2013: 50 March 2014: 52 January 2015: 54 January 2016: 61 October 2016: 105 June 2017: 180 |
|
Appendix 2. Appendix 2 ‐ Complete results of the risk of bias assessment (parallel group RCTs – individually and cluster randomised)
| Item | Cohen‐Mansfield 2007 | Cohen‐Mansfield 2012a | Kolanowski 2011 | Richards 2005 | Van Haitsma 2015 | Wenborn 2013 |
| Allocation sequence adequately generated | No | Yes | Yes | Unclear | Yes* | Yes* |
| Allocation adequately concealed | Unclear | Unclear | Yes | Unclear | No* | Yes* |
| Participants identified before randomisation | Unclear | Unclear | Yes | Yes | Yes | Yes |
| If no: no evidence for biased selection of participants | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Inclusion/exclusion criteria for participants clearly defined | Yes | Yes | Yes | Yes | Yes | Yes |
| Inclusion/exclusion criteria for clusters clearly defined | No | No | n. a. | n. a. | n. a. | Yes* |
| Adequate sample size calculation | No | Unclear | Yes | Yes | No | Yes |
| Absence of relevant differences between groups after randomisation | Yes | Yes | Unclear | Unclear | Yes | Yes |
| Loss to follow‐up less 5% of participants | Yes | Yes | Yes | No | No | No |
| Were incomplete data adequately explained | Yes | Yes | Yes | Yes | Yes | Yes |
| All groups treated equally, except of intervention or control | Yes | Yes | Yes | Yes | Yes | Yes |
| Primary outcome clearly stated? | Yes | Yes | No | No | No | Yes* |
| Participants blinded to group allocation | Unclear | Yes | Yes | Unclear | Yes* | Unclear |
| Personnel blinded to group allocation | No | No | Yes | Unclear | No | Yes |
| Outcome assessors blinded to group allocation | No | No | Yes | No | No | Yes |
| Data collection started immediately after randomisation | Unclear | Unclear | Yes* | Unclear | Varied* | Unclear |
| Intention to treat analysis | Unclear | Yes | Yes | Yes | Yes* | Yes |
| Complete reporting of outcome (as scheduled) | Yes | Yes | Yes | Yes | Yes | Yes |
| Methods of analysis adequate for cluster‐randomised trials | No | No | n. a. | n. a. | n. a. | Yes |
| Conflicts of interest mentioned | No | Yes | Yes | Yes | No | Yes |
| * Items marked with an asterisk have been answered by the study authors after personal request | ||||||
Appendix 3. Appendix 3 ‐ Complete results of the risk of bias assessment (cross‐over RCTs)
| Item | van der Ploeg 2013 |
| Cross‐over design appropriate | Yes |
| Absence of bias from a carry‐over effect | Yes |
| Allocation sequence adequately generated | Yes |
| Allocation adequately concealed | No* |
| Order of treatments randomised? | Yes |
| Participants identified before randomisation | Yes |
| If no: no evidence for biased selection of participants | ‐ |
| Inclusion/exclusion criteria for participants clearly defined | Yes |
| Adequate sample size calculation | Yes |
| Absence of relevant differences between groups after randomisation | Unclear |
| Loss to follow‐up less 5% of participants | No |
| Were incomplete data adequately explained | Yes |
| Primary outcome clearly stated? | Yes |
| Participants blinded to group allocation | Yes |
| Personnel blinded to group allocation | No |
| Outcome assessors blinded to group allocation | No |
| Data collection started immediately after randomisation | Unclear |
| Intention to treat analysis | Yes* |
| Complete reporting of outcome (as scheduled) | Yes |
| Conflicts of interest mentioned | Yes |
| * Items marked with an asterisk have been answered by the study authors after personal request | |
Appendix 4. Appendix 4 ‐ Complete results of the risk of bias assessment (non‐randomised trials)
| Item | Orsulic‐Jeras 2000 |
| Inclusion/exclusion criteria for participants clearly defined | No* |
| Reporting of all relevant characteristics of the participants | Yes |
| Absence of relevant differences between groups after randomisation | Yes* |
| Adequate sample size calculation | No* |
| Loss to follow‐up less 5% of participants | No |
| Were incomplete data adequately explained | Yes |
| All groups treated equally, except of intervention or control | Yes |
| Primary outcome clearly stated? | No |
| Participants blinded to group allocation | No |
| Personnel blinded to group allocation | No |
| Outcome assessors blinded to group allocation | No |
| Intention to treat analysis | Yes* |
| Complete reporting of outcome (as scheduled) | Yes |
| Conflicts of interest mentioned | No |
| * Items marked with an asterisk have been answered by the study authors after personal request | |
Data and analyses
Comparison 1. Challenging behaviour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Personally tailored activities vs. usual care or active control | 6 | 439 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.49, 0.08] |
| 1.1 Personally tailored activities vs. usual care | 4 | 288 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.76, 0.09] |
| 1.2 Personally tailored activities vs. active control | 2 | 151 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.34, 0.30] |
| 2 Investigating heterogeneity | 5 | 384 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.28, 0.12] |
| 3 Behaviours van Haitsma 2015 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 General restlessness | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Aggression | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Uncooperative | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Very negative verbal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Negative verbal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.3. Analysis.
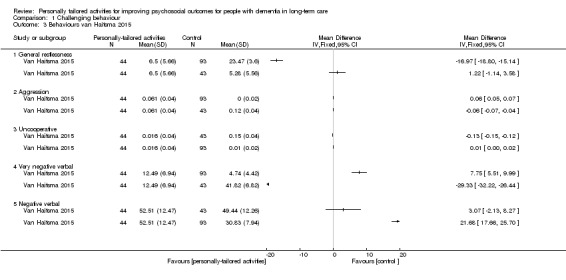
Comparison 1 Challenging behaviour, Outcome 3 Behaviours van Haitsma 2015.
Comparison 2. Affect.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Positive affect | 6 | 498 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [0.43, 1.32] |
| 1.1 Personally tailored activities vs. usual care | 4 | 282 | Std. Mean Difference (IV, Random, 95% CI) | 1.30 [0.77, 1.84] |
| 1.2 Personally tailored activities vs. active control | 3 | 216 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.09, 0.63] |
| 2 Positive affect‐excluding recalculated study results | 5 | 318 | Std. Mean Difference (IV, Random, 95% CI) | 0.76 [0.38, 1.13] |
| 3 Negative affect | 6 | 632 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.19, 0.14] |
| 3.1 Personally tailored activities vs. usual care | 4 | 416 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.19, 0.22] |
| 3.2 Personally tailored activities vs. active control | 3 | 216 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.36, 0.18] |
| 4 Negative affect‐excluding recalculated study results | 5 | 452 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.22, 0.16] |
| 5 Mood | 3 | 247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.27, 0.23] |
Comparison 3. Engagement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intensity of participation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Constructive engagement | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Passive engagement | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Negative engagement | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.

Comparison 3 Engagement, Outcome 1 Intensity of participation.
3.2. Analysis.
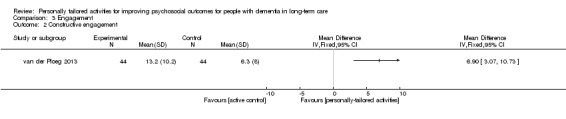
Comparison 3 Engagement, Outcome 2 Constructive engagement.
3.3. Analysis.

Comparison 3 Engagement, Outcome 3 Passive engagement.
3.4. Analysis.
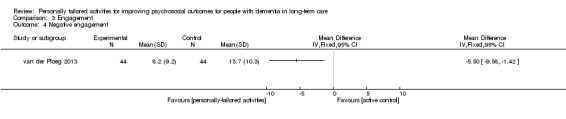
Comparison 3 Engagement, Outcome 4 Negative engagement.
Comparison 4. Sleep‐related outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Daytime minutes slept | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Nighttime minutes slept | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.

Comparison 4 Sleep‐related outcomes, Outcome 1 Daytime minutes slept.
4.2. Analysis.
Comparison 4 Sleep‐related outcomes, Outcome 2 Nighttime minutes slept.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cohen‐Mansfield 2007.
| Methods | Cluster‐randomised controlled trial Duration of follow‐up: 10 consecutive days |
|
| Participants | Country: USA Participants were recruited from 12 clusters in 11 suburban nursing home facilities (6 clusters per group) Inclusion criteria: all residents of the participating clusters with a diagnosis of dementia, who lived in the facility for more than 3 weeks and exhibited agitation several times per day Exclusion criteria: residents with a diagnosis of bipolar disorder or schizophrenia and residents who manifested aggressive behaviours Number of participants completing the study: n = 167 (intervention group n = 89, control group n = 78) Age (mean ± SD), years: intervention group 88.0 ± 6.4, control group 85.0 ± 8.6 Gender, female: intervention group 84%, control group 76% Cognitive status, MMSE (mean ± SD): intervention group 7.26 ± 6.0, control group 6.88 ± 6.5 Care dependency, ADL performance (from Minimum Data Set (MDS), 0 (independent) to 4 (total dependence)) (mean ± SD): intervention group 2.49 ± 1.01, control group 2.42 ± 1.03 |
|
| Interventions | Intervention: activity programme based on the Treatment Routes for Exploring Agitation (TREA) framework Control: presentation for nursing staff describing the syndromes of agitation, their aetiologies, and possible non‐pharmacologic interventions |
|
| Outcomes | Primary: agitation (ABMI) Secondary: affect (pleasure, interest, anger, anxiety, sadness) (LMBS) |
|
| Funding | National Institutes of Health; USA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "To limit contamination of the interventions’ effectiveness, buildings were assigned either control or intervention status (rather than having both within each building). We were unable at times to assign buildings randomly to either intervention or control groups because the administrators of two facilities insisted on making the decision as a condition of participation. Other facilities without such stipulations were randomly assigned to the treatment or control group while balancing the number of facilities in each group." No method of sequence generation was reported. |
| Allocation concealment (selection bias) | Unclear risk | No methods for allocation concealment was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information about blinding of personnel and participants reported, but blinding seems not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "...nonblindness of the observations". No further information reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants lost to follow‐up: intervention group: "1 excluded due to illness during intervention", control group: "2 excluded due to hospitalisation after baseline assessment". |
| Selective reporting (reporting bias) | Unclear risk | No trial registration and no study protocol is available. |
| Other bias | High risk | Cluster effect was not incorporated in the analysis (unit‐of‐analysis bias). |
Cohen‐Mansfield 2012a.
| Methods | Cluster‐randomised controlled trial; registration number: NCT00820859 Duration of follow‐up: 10 consecutive days Conducted between June 2006 and December 2011 |
|
| Participants | Country: USA Participants were recruited from 11 nursing homes (n = 6 intervention group, n = 5 control group), in Rockville, Silver Spring, Takoma Park, Chevy Chase, and Gaithersburg, Maryland, USA. Inclusion criteria: all residents of the participating clusters with a diagnosis of dementia, at age ≥ 60 years, who lived in the facility for more than 3 weeks and exhibited agitation several times per day Exclusion criteria: residents with a diagnosis of bipolar disorder or schizophrenia, an MMSE score ≥ 25, manifested aggressive behaviours, or took part in earlier studies testing a TREA intervention Number of participants completing the study: n = 125 (intervention group n = 89, control group n = 36) Age (mean ± SD) years: intervention group 85.9 ± 8.62, control group 85.3 ± 9.62 Gender, female: intervention group 73%, control group 77.8% Cognitive status, MMSE (mean ± SD): intervention group 7.62 ± 6.33, control group 9.38 ± 6.76 Care dependency, ADL performance (from Minimum Data Set (MDS), 0 (independent) to 4 (total dependence)) (mean ± SD): intervention group 2.72 ± 0.84, control group 2.75 ± 0.98 |
|
| Interventions | Intervention: activity programme based on the Treatment Routes for Exploring Agitation (TREA) framework Control: presentation for nursing staff describing the syndromes of agitation, their aetiologies, and possible non‐pharmacologic interventions |
|
| Outcomes | Primary: agitation (ABMI) Secondary: affect (pleasure, interest, anger, anxiety, sadness) (LMBS) |
|
| Funding | National Institutes of Health; USA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization to intervention or placebo control protocols was performed using random numbers via a ratio of 1.5: 1, with the intent of having more intervention than control participants in order to investigate process issues." |
| Allocation concealment (selection bias) | Unclear risk | No methods for allocation concealment were reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The research assistants who gathered initial baseline data were blind to the group allocation of residents; of course, once treatment started, research assistants were no longer blinded to group assignment." "Study participants were blinded as to their group assignment"; comment: since the control group did not receive an active control intervention, blinding of participants seems not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Research assistants could not be blinded once interventions began." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Residents not included after cluster randomisation: "Placebo (n = 36), bipolar disorder or schizophrenia diagnosis (n = 3), not agitated (n = 25), age < 60 years (n = 3), death(n = 2), participated in previous TREA study (n = 2), gave consent but could not be included before the data collection phase ended (n = 1). Intervention (n = 62), bipolar disorder or schizophrenia diagnosis (n = 6), not agitated (n = 29), age < 60 years (n = 3), MMSE > 25 (n = 2), no diagnosis of dementia (n = 1), death (n = 13), discharged (n = 6), life expectancy < 3 months (n = 1), gave consent but could not be included before the data collection phase ended (n = 1)" "Did not receive placebo as allocated (n = 4, lost to death), did not receive intervention as allocated (n = 4, lost to death)" |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported, but the study was registered retrospectively and no study protocol is available. |
| Other bias | High risk | Cluster effect was not incorporated in the analysis (unit‐of‐analysis bias). |
Kolanowski 2011.
| Methods | Randomised controlled trial; registration number: NCT00388544 Duration of follow‐up: 4 weeks (3‐week interventions period + 1‐week post‐intervention period) Conducted between August 2005 and November 2008 |
|
| Participants | Country: USA Participants were recruited from 9 community‐based nursing homes in Pennsylvania. Inclusion criteria: diagnosis of dementia, a willing informant who knows the participant well and who can provide past personality and other data, a stable dose of any psychoactive drug from pre‐baseline through final observation, and the presence of agitation or passivity Exclusion criteria: residents with delirium or an unstable medical condition, Parkinson's disease, Huntington's disease, seizure disorder, stroke, alcoholism, drug abuse, head trauma with loss of consciousness, psychiatric illness preceding the onset of memory loss, severe vision or hearing impairment; received a new psychoactive medication in a 30‐day period before baseline Number of participants completing the study: n = 128 (intervention group 1 n = 32, intervention group 2 n = 33, intervention group 3 n = 31, control group n = 32) Age (mean ± SD) years: intervention group 1 85.3 ± 6.1, intervention group 2 87.2 ± 5.9, intervention group 3 86 ± 7.1, control group 85.9 ± 4.9 Gender, female: intervention group 1 75%, intervention group 2 75.8%, intervention group 3 74.2%, control group 81.2% Cognitive status, MMSE (mean ± SD): intervention group 1 15.1 ± 4.2, intervention group 2 15.8 ± 4.9, intervention group 3 12.7 ± 3.3, control group 13.2 ± 4.6 Care dependency: not reported |
|
| Interventions | Activity programmes based on the Need‐Driven Dementia‐Compromised Behavior model. Intervention group A: activities matched to participants' cognitive and physical functional level and opposite to their identified style of interest Intervention group B: activities matched to participants' style of interest and challenging for their functional level Intervention group C: activities matched to both participants' functional level and style of interest Control group: activities opposite to participants' style of interest and challenging for their functional level |
|
| Outcomes | Agitation (CMAI, PDS), engagement, affect (ARS), mood (Dementia Mood Picture Test) | |
| Funding | 1 author was supported by National Institutes of Health and 1 author received royalties from the NEO‐PI‐R and the NEOFFI and was supported in part by National Institutes of Health; USA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The statistician determined participants’ group assignment using a random number generator with random block sizes to ensure equal assignment across the four groups at the completion of the study and approximately equal assignments throughout the study to control for unknown temporal effects." |
| Allocation concealment (selection bias) | Low risk | "Group assignment was concealed until after all screening data were collected. The project director obtained the assignment from a secure central location after verifying that the participant qualified for the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Because all participants received some type of activity, it was possible to blind the interventionists, data collectors, video raters, nursing home staff, and participants." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Because all participants received some type of activity, it was possible to blind the interventionists, data collectors, video raters, nursing home staff, and participants." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After randomisation, in both groups included in this review 1 participant was lost to follow‐up but no participants were excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported, but the study was registered retrospectively and no study protocol is available. |
| Other bias | Unclear risk | No primary outcome defined (multiple testing). |
Orsulic‐Jeras 2000.
| Methods | Non‐randomised trial Duration of follow‐up: 9 months |
|
| Participants | Country: USA Participants were recruited from 1 dementia special care unit of the Menorah Park Center for Senior Living, an Orthodox Jewish facility with over 350 long‐term care beds. No inclusion or exclusion criteria reported Number of participants completing the study: n = 25 (intervention group n = 13, control group n = 12). Age (mean ± SD) years: 88 ± 6 Gender, female: 92% Cognitive status, MMSE (mean ± SD): 11 ± 6 Care dependency: not reported |
|
| Interventions | Intervention: Montessori‐based activities (group or individual activities) Control: usual care (regular activities) |
|
| Outcomes | Agitation (CMAI), depression (CSD) (9 months' follow‐up); engagement (MRI‐ES), affect (ARS) (6 months' follow‐up) | |
| Funding | Not mentioned | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised trial; "Thirteen residents were assigned to the treatment condition and 12 to the control condition. Participants were matched across groups according to their scores on the MMSE, along with their performances on the Myers Menorah Park/Montessori Assessment System (MMP/MAS) and the reading subtest of the Wide Range Achievement Test (WRAT3)." |
| Allocation concealment (selection bias) | High risk | Not applicable (not an RCT) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Individual activity sessions "were run by either a trained volunteer, a research assistant, or the activities therapist on the unit." The 2 types of group activities were run "one day by a volunteer and one day by the activities therapist on the unit" and "led by either a trained volunteer or by the activities therapist on the unit" respectively. Comment: the control group received no additional activities and so it seems not possible to blind the personnel or the participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Research staff interviewed nursing assistants on the special care unit at pretest and at final posttest for approximately 20 minutes for all measures"; comment: nursing assistants were not blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Forty‐four residents from the dementia SCU were initially recruited. During the course of this 9‐month study, 19 participants dropped out of the study, either because of death (n = 3), transfer to another unit within the facility (n = 12), or excessive absence (n = 4). Thus, 25 participants (23 women and 2 men) completed the study." No information about the group allocation for the participants lost to follow‐up were reported. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration and no study protocol is available. |
| Other bias | Unclear risk | No primary outcome defined (multiple testing). |
Richards 2005.
| Methods | Randomised controlled trial Duration of follow‐up: 21 consecutive days |
|
| Participants | Country: USA Participants were recruited from 1 Department of Veterans Affairs nursing home and 6 for‐profit community nursing homes in the central southeastern United States. Inclusion criteria: age ≥ 55, baseline 85% sleep efficiency and at least 30 minutes of daytime sleep (Actigraph), living at the facility for at least 1 month, MMSE score ≤ 24 Number of participants completing the study: n = 139 (intervention group n = 71, control group n = 68) Age (mean ± SD) years: 79 ± 8.4 Gender, female: 48.2% Cognitive status, MMSE (mean ± SD): 8.7 ± 7.1 Care dependency: not reported |
|
| Interventions | Intervention: individualised activity‐programme Control: usual care (including any scheduled activities that the nursing home provided). |
|
| Outcomes | 24‐hour sleep/wake patterns (Actigraph), costs (training, activities, administration) | |
| Funding | Veterans Health Administration, National Institute of Nursing Research, National Institutes of Health/National Center for Research Resources to the General Clinical Research Center of the University of Arkansas for Medical Sciences; USA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Then participants were randomly assigned to one of two groups: ISAI or usual‐care control". No further information reported. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinded to group allocation. "The project nursing assistants implemented the ISAI and recorded the type, time, and duration of the activities. (...) As part of the ISAI, the project nursing assistants checked on the participants every hour, observed them for napping, wakened them if they were asleep, and provided ISAI. (...) Participants in this [control] group received usual care". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The Actigraph (Ambulatory Monitoring, Ardsley, NY), a motion‐sensing device that uses an algorithm to differentiate sleep from wake based on motor activity, measured sleep/wake pattern variables." Comment: no information about blinding reported, but the risk of bias was judged as low since only objective outcome were assessed via Actigraph. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Of the 147 remaining participants, seven were hospitalized, and one returned home." Comment: no information about the allocated groups of the participants lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration and no study protocol is available. |
| Other bias | Unclear risk | No primary outcome defined (multiple testing). |
van der Ploeg 2013.
| Methods | Crossover trial, registration number: ACTRN12609000564257 Duration of follow‐up: 4 weeks (2 weeks per condition, no washout period) Conducted between July 2009 and September 2011 |
|
| Participants | Country: Australia Participants were recruited from 9 residential facilities in metropolitan Melbourne, Australia. Inclusion criteria: diagnosis of dementia, a physically agitated behaviour that occurred at least several times a day outside nursing interventions, confirmation by nurses, visiting physician, and/or psychiatrist that the behaviour was not due to untreated pain, physical illness, major depression, or psychosis, residence in a specialist dementia unit or psychogeriatric nursing home for at least 3 months Exclusion criteria: psychotropic medications which were likely to be changed over the study period (medical and nursing staff were asked not to alter psychotropic medications during the study period if possible), an acutely life‐threatening physical illness or a behaviour presenting a potential hazard to the researchers Number of participants completing the study: n = 44 (group 1 (intervention first) n = 15, group 2 (control first) n = 29) Age (mean ± SD) years: 78.1 ± 9.8 Gender, female: 68.2% Cognitive status, MMSE (mean ± SD): 6 ± 8 Care dependency: not reported |
|
| Interventions | Intervention: personalised Montessori‐based activities Control: unspecific activities (active control) |
|
| Outcomes | Primary: 1 physically agitated behaviour specific for each participant (based on the CMAI) Secondary: affect (pleasure, contentment, interest, neutral affect, anger, sadness, anxiety/fear) (ARS), engagement (MPES) |
|
| Funding | Dementia Collaborative Research Centre (DCRC), Mason Foundation, National Health and Medical Research Council; Australia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The primary investigator generated the random allocation sequence using Excel Random Number Generator." |
| Allocation concealment (selection bias) | High risk | Group allocation was not concealed (unpublished information from the study author). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants and facility staff were blinded to the hypotheses of the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Because of the nature of the activities, it was not possible to blind observers to the Montessori or the control conditions but they were trained to record behavior, affect, and engagement styles consistently across sessions and their inter‐rater reliability was excellent." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Allocated to Montessori intervention first lost to follow‐up (n = 6): deceased (n = 3), refused intervention (n = 3); Allocated to Montessori intervention second lost to follow‐up (n = 7): deceased (n = 1), refused intervention (n = 1), moved to other facility (n = 1), too busy to schedule sessions (n = 4)." Since the attrition rate was more than twice as high as planned, risk of bias was rated as unclear. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported, but the study was registered retrospectively and the study protocol was published after first participants were recruited. |
| Other bias | High risk | No paired data were available (risk of a unit‐of‐analysis bias) and we used the (unpaired) data of the complete study period. |
Van Haitsma 2015.
| Methods | Randomised controlled trial Duration of follow‐up: 3 weeks |
|
| Participants | Country: USA Participants were recruited from 8 units of a large nonprofit nursing home in Pennsylvania. Inclusion criteria: all willing residents living in the nursing unit at baseline Exclusion criteria: residents living in the nursing unit for less than 1 month, actively psychotic residents or residents receiving end‐of‐life care Number of participants completed the study: n = 180 (intervention group n = 44, AC n = 43, control group n = 93) Age (mean (range)) years: 88.7 (64 to 105); (mean ± SD) intervention group 87.66 ± 8.37, AC 88.71 ± 6.13, control group 89.21 ± 6.87 Gender, female: 82.2% Cognitive status, MMSE (mean ± SD): intervention group 7.4 ± 7.13, AC 10.35 ± 7.95, control group 9.02 ± 7.64 Care dependency, MDS ADL (mean ± SD): intervention group 25.05 ± 12.52, AC 27.41 ± 10.49, control group 25.99 ± 11.18 |
|
| Interventions | Intervention: Individualized Positive Psychosocial Intervention (IPPI) Active control: standardised 1‐to‐1 activities Control: usual care |
|
| Outcomes | Behaviour (verbal and nonverbal), affect (positive affect: pleasure, alertness; negative affect: sadness, anger, anxiety) | |
| Funding | Alzheimer’s Association Tacrine Fund and the Pennsylvania Department of Health | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing sealed ward‐numbers (unpublished information). |
| Allocation concealment (selection bias) | High risk | No information reported, group allocation was not concealed (unpublished information from the study author). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Having each unit provide only one of the two experimental conditions mitigated the possibility of cross‐contamination because staff members were blinded to the condition of their unit." Comment: on each unit, 1 group of residents received 1 type of activity programme (intervention or active control) and another group of participants received usual care. Nursing staff was aware whether a participant received an activity programme or usual care; therefore blinding of personnel refers only to the type of activity programme (intervention or active control). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information reported; however, outcomes were assessed before, during and after the intervention session and therefore a blinding of the group allocation (activities vs. usual care) seems not possible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants lost to follow‐up: n = 15; intervention group: n = 5 (n = 3 refused, n = 1 died, n = 1 hospitalised), active control group: n = 6 (n = 1 refused, n = 0 died, n = 5 hospitalised), usual care group: n = 4 (n = 2 refused, n = 1 died, n = 1 hospitalised). |
| Selective reporting (reporting bias) | Unclear risk | No trial registration and no study protocol is available. |
| Other bias | Unclear risk | No primary outcome defined (multiple testing). |
Wenborn 2013.
| Methods | Cluster‐randomised controlled trial, registration number: ISRCTN67952488 Duration of follow‐up: total of 28 weeks (16 weeks delivery of the intervention, 12 weeks' post‐intervention follow‐up) |
|
| Participants | Country: UK (London) Number of participants completed the study: n = 159 (intervention group n = 79, control group n = 80) Number of clusters: 16 nursing homes (8 clusters per group) Age (mean ± SD) years: intervention group 84.2 ± 7.6, control group 84.2 ± 7.6 Gender, female: intervention group 63.5%, control group 70.8% Cognitive status, MMSE (mean ± SD): intervention group 5.8 ± 5.1, control group 5.5 ± 4.6 Care dependency, CAPE‐BRS (mean ± SD): intervention group 20.2 ± 4.3, control group 19.4 ± 4.6 Criteria for matching of clusters: provider (i.e. private company or statutory service or voluntary organisation), number of beds, registration category. For each participating organisation it was guaranteed to receive the intervention in at least 1 home Inclusion criteria for clusters: sufficient staff available to attend the intervention programme (minimum of 3 per home), sufficient residents eligible for inclusion (double the number of staff designated to participate in the intervention) Inclusion criteria: all residents ≥ 60 years, who had lived in the care home for at least 2 months and intending to stay, met the DSM‐IV criteria for dementia (American Psychiatric Association 1994) and had a MMSE score (Folstein 1975) less than 25 Exclusion criteria: residents with other serious physical or mental health problems |
|
| Interventions | Intervention: staff training designed to enable care home staff to provide personalised activities Control: usual care |
|
| Outcomes | Primary: Quality of Life (QOL‐AD, self‐ and caregiver‐rating) Secondary: challenging behaviour (CBS), depression (CSD), anxiety (RAID), number and type of medication |
|
| Funding | North East London Mental Health NHS Trust ‒ Occupational Therapy service; UK | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Care homes were recruited as matched pairs (matched according to provider: statutory, private or voluntary organisation and size). In each pair, 1 care home was allocated to the interventions group and the other to the control group using a computer random number generator (published and unpublished information). |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by a remote randomisation service (unpublished information). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information reported. The nursing staff was trained to deliver the intervention; therefore blinding seems not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed by the [primary investigator (before randomisation)] "at baseline and by blinded assessors at follow‐up." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants lost to follow‐up: n = 51; intervention group n = 25 (n = 17 died, n = 7 admitted to hospital, n = 1 moved), control group n = 26 (n = 23 died, n = 1 admitted to hospital, n = 2 moved). |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported, but the study was registered retrospectively and no study protocol is available. |
| Other bias | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beck 2002 | Intervention (no personally tailored activities) |
| Cohen‐Mansfield 2006 | Setting (participants recruited from both long‐term care facilities and day centres, no data on the different settings available) |
| DiNapoli 2016 | Setting (geriatric psychiatry) |
| Farina 2006 | Setting (day care centre) |
| Farina 2009 | Setting (day care centre) |
| Gerber 1991 | Setting (psychiatric hospital) |
| Hong 2011 | Intervention (multisensory stimulation) |
| Hopman‐Rock 1999 | Intervention (no personally tailored activities) |
| Hsu 2015 | Intervention (no personally tailored activities) |
| Kolanowski 2005 | Design (no randomised controlled trial) |
| Kovach 2004 | Intervention (re‐organisation of activities) |
| Lin 2009 | Intervention (acupressure and activities) |
| Luttenberger 2012 | Intervention (no personally tailored activities) |
| Mansbach 2017 | Intervention (no personally tailored activities) |
| Meeks 2015 | Population (not people with dementia) |
| Morley 2014 | Design (editorial) |
| Mowrey 2013 | Design (no control group, retrospective study) |
| Patel 2016 | Design (no control group) |
| Pieper 2016 | Intervention (no personally tailored activities) |
| Politis 2004 | Intervention (activity‐based cognitive stimulation) |
| Rapp 2013 | Intervention (non‐pharmacological and pharmacological components) |
| Sackley 2009 | Intervention (no personally tailored activities) |
| Schneider 2003 | Design (no control group) |
| Sung 2010 | Intervention (only listening to music as an activity) |
| Sánchez 2016 | Intervention (no personally tailored activities) |
| Treusch 2015 | Intervention (no personally tailored activities) |
| Vink 2014 | Intervention (no personally tailored activities) |
| Wilks 2017 | Design (no control group) |
Contributions of authors
RM and GM initially planned the study. RM, AR, HR and GM wrote the study protocol. RM, AR and HR selected studies, conducted the critical appraisal and extracted data. RM, AR and GM interpreted the study data. RM contacted the study authors and wrote the drafts of the review. All authors contributed to all drafts of the review.
Sources of support
Internal sources
Cochrane Germany, Medical Center ‐ University of Freiburg, Germany.
External sources
-
Ministry of Education and Research, Germany.
(Grant number 01 KG 1022)
-
NIHR, UK.
This review was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
None known.
New
References
References to studies included in this review
Cohen‐Mansfield 2007 {published data only}
- Cohen‐Mansfield J, Libin A, Marx MS. Nonpharmacological treatment of agitation: a controlled trial of systematic individualized intervention. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2007;62(8):908‐16. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 2012a {published data only}
- Cohen‐Mansfield J, Thein K, Marx MS, Dakheel‐Ali M, Freedman L. Efficacy of nonpharmacologic interventions for agitation in advanced dementia: a randomized, placebo‐controlled trial. Journal of Clinical Psychiatry 2012;73(9):1255‐61. [DOI] [PubMed] [Google Scholar]
Kolanowski 2011 {published and unpublished data}
- Kolanowski A, Litaker M, Buettner L, Moeller J, Costa PT Jr. A Randomized Clinical Trial of Theory‐Based Activities for the Behavioral Symptoms of Dementia in Nursing Home Residents. Journal of the American Geriatrics Society 2011;59(6):1032‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orsulic‐Jeras 2000 {published data only}
- Orsulic‐Jeras S, Schneider NM, Camp CJ. Special Feature: Montessori‐Based Activities for Long‐Term Care Residents with Dementia. Topics in Geriatric Rehabilitation 2000;16(1):78‐91. [Google Scholar]
Richards 2005 {published data only}
- Richards KC, Beck C, O'Sullivan PS, Shue VM. Effect of Individualized Social Activity on Sleep in Nursing HomeResidents with Dementia. Journal of the American Geriatrics Society 2005;53(9):1510‐7. [DOI] [PubMed] [Google Scholar]
van der Ploeg 2013 {published and unpublished data}
- Ploeg ES, Eppingstall B, Camp CJ, Runci SJ, Taffe J, O'Connor DW. A randomized crossover trial to study the effect of personalized, one‐to‐one interaction using Montessori‐based activities on agitation, affect, and engagement in nursing home residents with Dementia. International Psychogeriatrics 2013;25(4):565‐75. [DOI] [PubMed] [Google Scholar]
Van Haitsma 2015 {published data only}
- Haitsma KS, Curyto K, Abbott KM, Towsley GL, Spector A, Kleban M. A randomized controlled trial for an individualized positive psychosocial intervention for the affective and behavioral symptoms of dementia in nursing home residents. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2015;70(1):35‐45. [DOI] [PubMed] [Google Scholar]
Wenborn 2013 {published data only}
- Wenborn J, Challis D, Head J, Miranda‐Castillo C, Popham C, Thakur R, et al. Providing activity for people with dementia in care homes: A cluster randomised controlled trial. International Journal of Geriatric Psychiatry 2013;28(12):1296‐304. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beck 2002 {published data only}
- Beck CK, Vogelpohl TS, Rasin JH, Uriri JT, O'Sullivan P, Walls R, et al. Effects of behavioral interventions on disruptive behavior and affect in demented nursing home residents. Nursing Research 2002;51(4):219‐28. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 2006 {published data only}
- Cohen‐Mansfield J, Parpura‐Gill A, Golander H. Utilization of self‐identity roles for designing interventions for persons with dementia. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2006;61(4):P202‐12. [DOI] [PubMed] [Google Scholar]
DiNapoli 2016 {published data only}
- DiNapoli EA, Scogin F, Bryant AN, Sebastian S, Mundy MJ. Effect of individualized social activities on quality of life among older adults with mild to moderate cognitive impairment in a geriatric psychiatry facility. Aging & Mental Health 2016;20(3):262‐70. [DOI] [PubMed] [Google Scholar]
Farina 2006 {published data only}
- Farina E, Mantovani F, Fioravanti R, Pignatti R, Chiavari L, Imbornone E, et al. Evaluating two group programmes of cognitive training in mild‐to‐moderate AD: is there any difference between a 'global' stimulation and a 'cognitive‐specific' one?. Aging and Mental Health 2006;10(3):211‐8. [DOI] [PubMed] [Google Scholar]
Farina 2009 {published data only}
- Farina E, Villanelli F. Conducting an Intervention Program Mediated by Recreational Activities and Socialization in Groups for Clients with Alzheimer's Disease. In: Söderback I editor(s). International Handbook of Occupational Therapy Interventions. Springer, 2009:423‐9. [Google Scholar]
Gerber 1991 {published data only}
- Gerber GJ, Prince PN, Snider HG, Atchison K, Dubois L, Kilgour JA. Group activity and cognitive improvement among patients with Alzheimer's disease. Hospital and Community Psychiatry 1991;42(8):843‐5. [DOI] [PubMed] [Google Scholar]
Hong 2011 {published data only}
- Hong GR. [Effects of multisensory stimulation using familiarity: persons with dementia in long‐term care facility in Korea]. Journal of Korean Academy of Nursing 2011;41(4):528‐38. [DOI] [PubMed] [Google Scholar]
Hopman‐Rock 1999 {published data only}
- Hopman‐Rock M, Staats PG, Tak EC, Dröes RM. The effects of a psychomotor activation programme for use in groups of cognitively impaired people in homes for the elderly. International Journal of Geriatric Psychiatry 1999;14(8):633‐42. [DOI] [PubMed] [Google Scholar]
Hsu 2015 {published data only}
- Hsu MH, Flowerdew R, Parker M, Fachner J, Odell‐Miller H. Individual music therapy for managing neuropsychiatric symptoms for people with dementia and their carers: a cluster randomised controlled feasibility study. BMC Geriatrics 2015; Vol. 15:84. [DOI] [PMC free article] [PubMed]
Kolanowski 2005 {published and unpublished data}
- Kolanowski AM, Litaker M, Buettner L. Efficacy of theory‐based activities for behavioral symptoms of dementia. Nursing Research 2005;54(4):219‐28. [DOI] [PubMed] [Google Scholar]
Kovach 2004 {published data only}
- Kovach CR, Taneli Y, Dohearty P, Schlidt AM, Cashin S, Silva‐Smith AL. Effect of the BACE intervention on agitation of people with dementia. Gerontologist 2004;44(6):797‐806. [DOI] [PubMed] [Google Scholar]
Lin 2009 {published data only}
- Lin LC, Yang MH, Kao CC, Wu SC, Tang SH, Lin JG. Using acupressure and Montessori‐based activities to decrease agitation for residents with dementia: a cross‐over trial. Journal of the American Geriatrics Society 2009;57(6):1022‐9. [DOI] [PubMed] [Google Scholar]
Luttenberger 2012 {published data only}
- Luttenberger K, Donath C, Uter W, Graessel E. Effects of multimodal nondrug therapy on dementia symptoms and need for care in nursing home residents with degenerative dementia: a randomized‐controlled study with 6‐month follow‐up. Journal of the American Geriatrics Society 2012;60(5):830‐40. [DOI] [PubMed] [Google Scholar]
Mansbach 2017 {published data only}
- Mansbach WE, Mace RA, Clark KM, Firth IM. Meaningful activity for long‐term care residents with dementia: a comparison of activities and raters. Gerontologist 2017;57(1):461‐8. [DOI] [PubMed] [Google Scholar]
Meeks 2015 {published data only}
- Meeks S, Haitsma K, Schoenbachler B, Looney SW. BE‐ACTI V for depression in nursing homes: primary outcomes of a randomized clinical trial. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2015;70(1):13‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morley 2014 {published data only}
- Morley JE, Philpot CD, Gill D, Berg‐Weger M. Meaningful activities in the nursing home. Journal of the American Medical Directors Association 2014;15(2):79‐81. [DOI] [PubMed] [Google Scholar]
Mowrey 2013 {published data only}
- Mowrey C, Parikh PJ, Bharwani G, Bharwani M. Application of behavior‐based ergonomics therapies to improve quality of life and reduce medication usage for Alzheimer's/dementia residents. American Journal of Alzheimer's Disease and Other Dementias 2013;28(1):35‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2016 {published data only}
- Patel P, Porter T, Smith J, Mirk A. Montessori based activities in veterans with severe dementia: engagement, affect, and behavior. Journal of the American Geriatrics Society 2016;64(Supplement S1):S66‐7. [Google Scholar]
Pieper 2016 {published data only}
- Pieper MJ, Francke AL, Steen JT, Scherder EJ, Twisk JW, Kovach CR, Achterberg WP. Effects of a stepwise multidisciplinary intervention for challenging behavior in advanced dementia: a cluster randomized controlled trial. Journal of the American Geriatrics Society 2016;64(2):261‐9. [DOI] [PubMed] [Google Scholar]
Politis 2004 {published data only}
- Politis AM, Vozzella S, Mayer LS, Onyike CU, Baker AS, Lyketsos CG. A randomized, controlled, clinical trial of activity therapy for apathy in patients with dementia residing in long‐term care. International Journal of Geriatric Psychiatry 2004;19(11):1087‐94. [DOI] [PubMed] [Google Scholar]
Rapp 2013 {published data only}
- Rapp MA, Mell T, Majic T, Treusch Y, Nordheim J, Niemann‐Mirmehdi M, et al. Agitation in nursing home residents with dementia (VIDEANT Trial): effects of a cluster‐randomized, controlled, guideline implementation trial. Journal of the American Medical Directors Association 2013;14(9):690‐5. [DOI] [PubMed] [Google Scholar]
Sackley 2009 {published data only}
- Sackley CM, Berg ME, Lett K, Patel S, Hollands K, Wright CC, et al. Effects of a physiotherapy and occupational therapy intervention on mobility and activity in care home residents: A cluster randomised controlled trial. BMJ (Online) 2009;339(7722):670‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sánchez 2016 {published data only}
- Sánchez A, Marante‐Moar MP, Sarabia C, Labra C, Lorenzo T, Maseda A, et al. Multisensory stimulation as an intervention strategy for elderly patients with severe dementia: a pilot randomized controlled trial. American Journal of Alzheimer's Disease and Other Dementias 2016;31(4):341‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 2003 {published data only}
- Schneider NM, Camp CJ. Use of Montessori‐based activities by visitors of nursing home residents with dementia. Clinical Gerontologist 2003;26(1‐2):71‐84. [Google Scholar]
Sung 2010 {published data only}
- Sung HC, Chang AM, Lee WL. A preferred music listening intervention to reduce anxiety in older adults with dementia in nursing homes. Journal of Clinical Nursing 2010;19(7‐8):1056‐64. [DOI] [PubMed] [Google Scholar]
Treusch 2015 {published data only}
- Treusch Y, Majic T, Page J, Gutzmann H, Heinz A, Rapp MA. Apathy in nursing home residents with dementia: results from a cluster‐randomized controlled trial. European Psychiatry 2015;30(2):251‐7. [DOI] [PubMed] [Google Scholar]
Vink 2014 {published data only}
- Vink Annemieke C, Zuidersma Marij, Boersma Froukje, Jonge Peter, Zuidema Sytse U, Slaets Joris P. Effect of music therapy versus recreational activities on neuropsychiatric symptoms in elderly adults with dementia: an exploratory randomized controlled trial. Journal of the American Geriatrics Society 2014;62(2):392‐3. [DOI] [PubMed] [Google Scholar]
Wilks 2017 {published data only}
Additional references
Alexopoulos 1988
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biological Psychiatry 1988;23(3):271‐84. [DOI] [PubMed] [Google Scholar]
Algase 1996
- Algase DL, Beck C, Kolanowski A, Whall A, Berent SK, Richards K, et al. Need‐driven dementia‐compromised behavior: An alternative view of disruptive behavior. American Journal of Alzheimer's Disease and Other Dementias 1996;11(6):10–9. [Google Scholar]
American Psychiatric Association 1994
- American Psychiatric Association. Diagnosis and Statistical Manual of MentalDisorders (4th edition). Washington (DC): American Psychiatric Association, 1994. [Google Scholar]
Arons 2013
- Arons AM, Krabbe PF, Schölzel‐Dorenbos CJ, Wilt GJ, Rikkert MG. Quality of life in dementia: a study on proxy bias. BMC Medical Research Methodology 2013;13:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azermai 2012
- Azermai M, Petrovic M, Elseviers MM, Bourgeois J, Bortel LM, Vander Stichele RH. Systematic appraisal of dementia guidelines for the management of behavioural and psychological symptoms. Ageing Research Reviews 2012;11(1):78‐86. [DOI] [PubMed] [Google Scholar]
Ballard 2013
- Ballard C, Corbett A. Agitation and aggression in people with Alzheimer's disease. Current Opinion in Psychiatry 2013;26(3):252‐9. [DOI] [PubMed] [Google Scholar]
Bernstein 2007
- Bernstein AB, Remsburg RE. Estimated prevalence of people with cognitive impairment: results from nationally representative community and institutional studies. Gerontologist 2007;47(3):350‐4. [DOI] [PubMed] [Google Scholar]
Buettner 2003
- Buettner LL, Fitzsimmons S. Activity calendars for older adults with dementia: what you see is not what you get. American Journal of Alzheimer's Disease and other Dementias 2003;18(4):215‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2012
- Campbell MK, Piaggio G, Elbourne DR, Altman DG, CONSORT Group. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345:e5661. [DOI] [PubMed] [Google Scholar]
Chen 2000
- Chen YL, Ryden MB, Feldt K, Savik K. The relationship between social interaction and characteristics of aggressive, cognitively impaired nursing home residents. American Journal of Alzheimer's Disease 2000;15(1):10‐7. [Google Scholar]
Cohen‐Mansfield 1989a
- Cohen‐Mansfield J, Werner P, Marx MS. An observational study of agitation in agitated nursing home residents. International Psychogeriatrics / IPA 1989;1(2):153‐65. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 1989b
- Cohen‐Mansfield J, Marx M, Rosenthal S. A description of agitation in a nursing home. Journal of Gerontology 1989;44(3):M77–M84. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 1992
- Cohen‐Mansfield J, Marx MS, Werner P. Observational data on time use and behavior problems in the nursing home. Journal of Applied Gerontology 1992;11(1):111‐21. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 2000
- Cohen‐Mansfield J. Theoretical frameworks for behavioral problems in dementia. Alzheimer's Care Today 2000;1(4):8‐21. [Google Scholar]
Cohen‐Mansfield 2004
- Cohen‐Mansfield J, Libin A. Assessment of agitation in elderly patients with dementia: correlations between informant rating and direct observation. International Journal of Geriatric Psychiatry 2004;19(9):881‐91. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 2009a
- Cohen‐Mansfield J, Thein K, Dakheel‐Ali M, Marx MS. Engagement in persons with dementia: The concept and its measurement. American Journal of Geriatric Psychiatry 2009;17(4):299‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen‐Mansfield 2009b
- Cohen‐Mansfield J, Marx MS, Regier NG, Dakheel‐Ali M. The impact of personal characteristics on engagement in nursing home residents with dementia. International Journal of Geriatric Psychiatry 2009;24(7):755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen‐Mansfield 2010
- Cohen‐Mansfield J, Marx MS, Thein K, Dakheel‐Ali M. The impact of past and present preferences on stimulus engagement in nursing home residents with dementia. Aging & Mental Health 2010;14(1):67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen‐Mansfield 2011
- Cohen‐Mansfield J, Marx MS, Freedman LS, Murad H, Regier NG, Thein K, et al. The comprehensive process model of engagement. American Journal of Geriatric Psychiatry 2011;19(10):859‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen‐Mansfield 2012b
- Cohen‐Mansfield J, Thein K, Marx MS, Dakheel‐Ali M. What are the barriers to performing nonpharmacological interventions for behavioral symptoms in the nursing home?. Journal of the American Medical Directors Association 2012;13(4):400‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen‐Mansfield 2012c
- Cohen‐Mansfield J, Thein K, Marx MS, Dakheel‐Ali M, Murad H, Freedman LS. The relationships of environment and personal characteristics to agitated behaviors in nursing home residents with dementia. Journal of Clinical Psychiatry 2012;73(3):392‐9.. [DOI] [PubMed] [Google Scholar]
Colling 2000
- Colling KB. A taxonomy of passive behaviors in people with Alzheimer's disease. Journal of Nursing Scholarship 2000;32(3):239‐44. [DOI] [PubMed] [Google Scholar]
Cooney 2009
- Cooney A, Murphy K, O'Shea E. Resident perspectives of the determinants of quality of life in residential care in Ireland. Journal of Advanced Nursing 2009;65(5):1029‐38. [DOI] [PubMed] [Google Scholar]
Costa 1992
- Costa P, McCrae R. Revised NEO Personality Inventory and NEO Five‐Factor Inventory: Professional Manual. Odessa: Psychological Assessment Resources, 1992. [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deci 2000
- Deci EL, Ryan RM. The "what" and "why" of goal pursuits: Human needs and the self‐determination of behavior. Psychological Inquiry 2000;11(4):227‐68. [Google Scholar]
Dobbs 2005
- Dobbs D, Munn J, Zimmerman S, Boustani M, Williams CS, Sloane PD, et al. Characteristics associated with lower activity involvement in long‐term care residents with dementia. Gerontologist 2005;1(Suppl 1):81‐6. [DOI] [PubMed] [Google Scholar]
Edvardsson 2014
- Edvardsson David, Petersson Lisa, Sjogren Karin, Lindkvist Marie, Sandman Per‐Olof. Everyday activities for people with dementia in residential aged care: Associations with person‐centredness and quality of life. International Journal of Older People Nursing 2014;9(4):269‐76. [DOI] [PubMed] [Google Scholar]
Eldridge 2016
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot and Feasibility Studies 2016;2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. Mini Mental State: a practical guide for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
Fredrickson 2001
- Fredrickson BL. The role of positive emotions in positive psychology. The broaden‐and‐build theory of positive emotions. American Psychologist 2001;56(3):218‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomez‐Gallego 2015
- Gomez‐Gallego M, Gomez‐Garcia J, Ato‐Lozano E. Addressing the bias problem in the assessment of the quality of life of patients with dementia: determinants of the accuracy and precision of the proxy ratings. Journal of Nutrition, Health & Aging 2015;19(3):365‐72. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 08 February 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Grant 2013
- Grant A, Treweek S, Dreischulte T, Foy R, Guthrie B. Process evaluations for cluster‐randomised trials of complex interventions: a proposed framework for design and reporting. Trials 2013;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Guétin 2009
- Guétin S, Portet F, Picot MC, Pommié C, Messaoudi M, Djabelkir L, et al. Effect of music therapy on anxiety and depression in patients with Alzheimer's type dementia: randomised, controlled study. Dementia and Geriatric Cognitive Disorders 2009;28(1):36‐46. [DOI] [PubMed] [Google Scholar]
Han 2016
- Han A, Radel J, McDowd JM, Sabata D. Perspectives of People with Dementia About Meaningful Activities: A Synthesis. American Journal of Alzheimer's Disease and Other Dementias 2016;31(2):115‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harmer 2008
- Harmer BJ, Orrell M. What is meaningful activity for people with dementia living in care homes? A comparison of the views of older people with dementia, staff and family carers. Aging & Mental Health 2008;12(5):548‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hill 2010
- Hill NL, Kolanowski A, Kürüm E. Agreeableness and activity engagement in nursing home residents with dementia. Journal of Gerontological Nursing 2010;36(9):45‐52. [DOI] [PubMed] [Google Scholar]
Hoe 2009
- Hoe J, Hancock G, Livingston G, Woods B, Challis D, Orrell M. Changes in the quality of life of people with dementia living in care homes. Alzheimer Disease and Associated Disorders 2009;23(3):285‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Hubbard 2002
- Hubbard G, Cook A, Tester S, Downs M. Beyond words older people with dementia using and interpreting nonverbal behaviour. Journal of Aging Studies 2002;16(2):155‐67. [Google Scholar]
Judge 2000
- Judge KS, Camp CJ, Orsulic‐Jeras S. Use of Montessori‐based activities for clients with dementia in adult daycare: effects on engagement. American Journal of Alzheimer's Disease and Other Dementias 2000;15(1):42‐6. [Google Scholar]
Kolanowski 2006
- Kolanowski A, Litaker M. Social interaction, premorbid personality, and agitation in nursing home residents with dementia. Archives of Psychiatric Nursing 2006;20(1):12‐20. [DOI] [PubMed] [Google Scholar]
Kovach 1998
- Kovach CR, Magliocco JS. Late‐stage dementia and participation in therapeutic activities. Applied Nursing Research 1998;11(4):167‐73. [DOI] [PubMed] [Google Scholar]
Lawton 1996
- Lawton MP, Haitsma K, Klapper J. Observed affect in nursing home residents with Alzheimer's disease. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 1996;51(1):P3‐14. [DOI] [PubMed] [Google Scholar]
Logsdon 1999
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer’s disease: patient and caregiver reports. Journal of Mental Health and Aging 1999;5(1):21‐32. [Google Scholar]
Mills 1997
- Mills MA. Narrative identity and dementia: A study of emotion and narrative in older people with dementia. Ageing and Society 1997;17(6):673‐98. [Google Scholar]
Moniz‐Cook 2001
- Moniz‐Cook E, Woods R, Gardiner E, Silver M, Agar S. The Challenging Behaviour Scale (CBS): development of a scale for staff caring for older people in residential and nursing homes. British Journal of Clinical Psychology 2001;40(Pt 3):309‐22.. [DOI] [PubMed] [Google Scholar]
Moore 2015
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350:h1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moyle 2012
- Moyle W, Murfield JE, Griffiths SG, Venturato L. Assessing quality of life of older people with dementia: a comparison of quantitative self‐report and proxy accounts. Journal of Advanced Nursing 2012;68(10):2237‐46. [DOI] [PubMed] [Google Scholar]
Murphy 2007
- Murphy K, Shea EO, Cooney A. Quality of life for older people living in long‐stay settings in Ireland. Journal of Clinical Nursing 2007;16(11):2167‐77. [DOI] [PubMed] [Google Scholar]
Möhler 2015
- Möhler R, Köpke S, Meyer G. Criteria for Reporting the Development and Evaluation of Complex Interventions in healthcare: revised guideline (CReDECI 2). Trials 2015;16(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ngo 2015
- Ngo J, Holroyd‐Leduc JM. Systematic review of recent dementia practice guidelines. Age and Ageing 2015;44(1):25‐33. [DOI] [PubMed] [Google Scholar]
Nygaard 2003
- Nygaard HA, Ruths S. Missing the diagnosis: senile dementia in patients admitted to nursing homes. Scandinavian Journal of Primary Health Care 2003;21(3):148‐52. [DOI] [PubMed] [Google Scholar]
O'Neil 2011
- O'Neil ME, Freeman M, Christensen V, Telerant R, Addleman A, Kansagara D, Department of Veterans Affairs: Washington (DC). A systematic evidence review of non‐pharmacological interventions for behavioral symptoms of dementia. www.hsrd.research.va.gov/publications/esp/Dementia‐Nonpharm.pdf (accessed 08 February 2018). [PubMed]
Phinney 2007
- Phinney A, Chaudhury H, O'Connor DL. Doing as much as I can do: the meaning of activity for people with dementia. Aging & Mental Health 2007;11(4):384‐93. [DOI] [PubMed] [Google Scholar]
Prince 2013
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer's & Dementia 2013;9(1):63‐75.e2. [DOI] [PubMed] [Google Scholar]
Richards 2001
- Richards KC, Sullivan SC, Phillips RL, Beck CK, Overton‐McCoy AL. The effect of individualized activities on the sleep of nursing home residents who are cognitively impaired: a pilot study. Journal of Gerontological Nursing 2001;27(9):30‐7. [DOI] [PubMed] [Google Scholar]
Shankar 1999
- Shankar KK, Walker M, Frost D, Orrell M. The development of a valid and reliable scale for rating anxiety in dementia (RAID). Aging & Mental Health 1999;3(1):39‐49. [Google Scholar]
Shepperd 2009
- Shepperd S, Lewin S, Straus S, Clarke M, Eccles MP, Fitzpatrick R, et al. Can we systematically review studies that evaluate complex interventions?. PLoS Medicine 2009;6(8):e1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skrajner 2007
- Skrajner MJ, Camp CJ. Resident‐Assisted Montessori Programming (RAMP): use of a small group reading activity run by persons with dementia in adult day health care and long‐term care settings. American Journal of Alzheimer's Disease and Other Dementias 2007;22(1):27‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tappen 1995
- Tappen RM, Barry C. Assessment of affect in advanced Alzheimer's disease: the Dementia Mood Picture Test. Journal of Gerontological Nursing 1995;21(3):44‐6. [DOI] [PubMed] [Google Scholar]
Testad 2014
- Testad I, Corbett A, Aarsland D, Lexow KO, Fossey J, Woods B, et al. The value of personalized psychosocial interventions to address behavioral and psychological symptoms in people with dementia living in care home settings: a systematic review. International Psychogeriatrics / IPA 2014;26(7):1083‐98. [DOI] [PubMed] [Google Scholar]
Travers 2016
- Travers C, Brooks D, Hines S, O'Reilly M, McMaster M, He W, et al. Effectiveness of meaningful occupation interventions for people living with dementia in residential aged care: a systematic review. JBI Database of Systematic Reviews and Implementation Reports 2016;14(12):163‐225. [DOI] [PubMed] [Google Scholar]
van der Linde 2014
- Linde RM, Stephan BC, Dening T, Brayne C. Instruments to measure behavioural and psychological symptoms of dementia. International Journal of Methods in Psychiatric Research 2014;23(1):69‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Ploeg 2010
- Ploeg ES, O'Connor DW. Evaluation of personalised, one‐to‐one interaction using Montessori‐type activities as a treatment of challenging behaviours in people with dementia: the study protocol of a crossover trial. BMC Geriatrics 2010;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Haitsma 2000
- Haitsma K. The Assessment and integration of preferences into care practices for persons with dementia residing in the nursing home. In: Rubinstein R, Moss M, Kleban M editor(s). The many dimensions of aging. New York: Springer, 2000. [Google Scholar]
Vasse 2012
- Vasse E, Vernooij‐Dassen M, Cantegreil I, Franco M, Dorenlot P, Woods B, et al. Guidelines for psychosocial interventions in dementia care: a European survey and comparison. International Journal of Geriatric Psychiatry 2012;27(1):40‐8. [DOI] [PubMed] [Google Scholar]
Vernooij‐Dassen 2007
- Vernooij‐Dassen M. Meaningful activities for people with dementia. Aging & Mental Health 2007;11(4):359‐60. [DOI] [PubMed] [Google Scholar]
Wenborn 2008
- Wenborn J, Challis D, Pool J, Burgess J, Elliott N, Orrell M. Assessing the validity and reliability of the Pool Activity Level (PAL) Checklist for use with older people with dementia. Aging & Mental Health 2008;12(2):202‐11. [DOI] [PubMed] [Google Scholar]
Wittchen 2011
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. European Neuropsychopharmacology 2011;21(9):655‐79. [DOI] [PubMed] [Google Scholar]
Zimmerman 2005
- Zimmerman S, Sloane PD, Williams CS, Reed PS, Preisser JS, Eckert JK, et al. Dementia care and quality of life in assisted living and nursing homes. Gerontologist 2005;1(Suppl 1):133‐46. [DOI] [PubMed] [Google Scholar]


