Abstract
Background
The consequences of influenza in children and adults are mainly absenteeism from school and work. However, the risk of complications is greatest in children and people over 65 years of age. This is an update of a review published in 2011. Future updates of this review will be made only when new trials or vaccines become available. Observational data included in previous versions of the review have been retained for historical reasons but have not been updated because of their lack of influence on the review conclusions.
Objectives
To assess the effects (efficacy, effectiveness, and harm) of vaccines against influenza in healthy children.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2016, Issue 12), which includes the Cochrane Acute Respiratory Infections Group Specialised Register, MEDLINE (1966 to 31 December 2016), Embase (1974 to 31 December 2016), WHO International Clinical Trials Registry Platform (ICTRP; 1 July 2017), and ClinicalTrials.gov (1 July 2017).
Selection criteria
Randomised controlled trials comparing influenza vaccines with placebo or no intervention in naturally occurring influenza in healthy children under 16 years. Previous versions of this review included 19 cohort and 11 case‐control studies. We are no longer updating the searches for these study designs but have retained the observational studies for historical purposes.
Data collection and analysis
Review authors independently assessed risk of bias and extracted data. We used GRADE to rate the certainty of evidence for the key outcomes of influenza, influenza‐like illness (ILI), complications (hospitalisation, ear infection), and adverse events. Due to variation in control group risks for influenza and ILI, absolute effects are reported as the median control group risk, and numbers needed to vaccinate (NNVs) are reported accordingly. For other outcomes aggregate control group risks are used.
Main results
We included 41 clinical trials (> 200,000 children). Most of the studies were conducted in children over the age of two and compared live attenuated or inactivated vaccines with placebo or no vaccine. Studies were conducted over single influenza seasons in the USA, Western Europe, Russia, and Bangladesh between 1984 and 2013. Restricting analyses to studies at low risk of bias showed that influenza and otitis media were the only outcomes where the impact of bias was negligible. Variability in study design and reporting impeded meta‐analysis of harms outcomes.
Live attenuated vaccines
Compared with placebo or do nothing, live attenuated influenza vaccines probably reduce the risk of influenza infection in children aged 3 to 16 years from 18% to 4% (risk ratio (RR) 0.22, 95% confidence interval (CI) 0.11 to 0.41; 7718 children; moderate‐certainty evidence), and they may reduce ILI by a smaller degree, from 17% to 12% (RR 0.69, 95% CI 0.60 to 0.80; 124,606 children; low‐certainty evidence). Seven children would need to be vaccinated to prevent one case of influenza, and 20 children would need to be vaccinated to prevent one child experiencing an ILI. Acute otitis media is probably similar following vaccine or placebo during seasonal influenza, but this result comes from a single study with particularly high rates of acute otitis media (RR 0.98, 95% CI 0.95 to 1.01; moderate‐certainty evidence). There was insufficient information available to determine the effect of vaccines on school absenteeism due to very low‐certainty evidence from one study. Vaccinating children may lead to fewer parents taking time off work, although the CI includes no effect (RR 0.69, 95% CI 0.46 to 1.03; low‐certainty evidence). Data on the most serious consequences of influenza complications leading to hospitalisation were not available. Data from four studies measuring fever following vaccination varied considerably, from 0.16% to 15% in children who had live vaccines, while in the placebo groups the proportions ranged from 0.71% to 22% (very low‐certainty evidence). Data on nausea were not reported.
Inactivated vaccines
Compared with placebo or no vaccination, inactivated vaccines reduce the risk of influenza in children aged 2 to 16 years from 30% to 11% (RR 0.36, 95% CI 0.28 to 0.48; 1628 children; high‐certainty evidence), and they probably reduce ILI from 28% to 20% (RR 0.72, 95% CI 0.65 to 0.79; 19,044 children; moderate‐certainty evidence). Five children would need to be vaccinated to prevent one case of influenza, and 12 children would need to be vaccinated to avoid one case of ILI. The risk of otitis media is probably similar between vaccinated children and unvaccinated children (31% versus 27%), although the CI does not exclude a meaningful increase in otitis media following vaccination (RR 1.15, 95% CI 0.95 to 1.40; 884 participants; moderate‐certainty evidence). There was insufficient information available to determine the effect of vaccines on school absenteeism due to very low‐certainty evidence from one study. We identified no data on parental working time lost, hospitalisation, fever, or nausea.
We found limited evidence on secondary cases, requirement for treatment of lower respiratory tract disease, and drug prescriptions. One brand of monovalent pandemic vaccine was associated with a sudden loss of muscle tone triggered by the experience of an intense emotion (cataplexy) and a sleep disorder (narcolepsy) in children. Evidence of serious harms (such as febrile fits) was sparse.
Authors' conclusions
In children aged between 3 and 16 years, live influenza vaccines probably reduce influenza (moderate‐certainty evidence) and may reduce ILI (low‐certainty evidence) over a single influenza season. In this population inactivated vaccines also reduce influenza (high‐certainty evidence) and may reduce ILI (low‐certainty evidence). For both vaccine types, the absolute reduction in influenza and ILI varied considerably across the study populations, making it difficult to predict how these findings translate to different settings. We found very few randomised controlled trials in children under two years of age. Adverse event data were not well described in the available studies. Standardised approaches to the definition, ascertainment, and reporting of adverse events are needed. Identification of all global cases of potential harms is beyond the scope of this review.
Plain language summary
Vaccines for preventing influenza in healthy children
Review aim
The aim of this Cochrane Review, first published in 2007, was to summarise research on immunising healthy children up to the age of 16 with influenza vaccines during influenza seasons. We used randomised trials comparing either one of two types of vaccines with dummy vaccines or nothing. One type of vaccine is based on live but weakened influenza viruses (live attenuated influenza vaccines) and is given via the nose. The other is prepared by killing the influenza viruses with a chemical (inactivated virus) and is given by injection through the skin. We analysed the number of children with confirmed influenza and those who had influenza‐like illness (ILI) (headache, high temperature, cough, and muscle pain) and harms from vaccination. Future updates of this review will be made only when new trials or vaccines become available. Data from 33 observational studies included in previous versions of the review have been retained for historical reasons but have not been updated due to their lack of influence on the review conclusions.
Key messages
Live attenuated and inactivated vaccines can reduce the proportion of children who have influenza and ILI. Variation in the results of studies means that we are uncertain about the effects of these vaccines across different seasons.
What was studied in this review?
Over 200 viruses cause ILI and produce the same symptoms (fever, headache, aches, pains, cough, and runny nose) as influenza. Doctors cannot distinguish between them without laboratory tests because both last for days and rarely cause serious illness or death.
The types of virus contained in the vaccines are usually those that are expected to circulate in the following influenza seasons, according to recommendations of the World Health Organization (seasonal vaccine). Pandemic vaccine contains only the virus strain that is responsible for the pandemic (e.g. the type A H1N1 for the 2009 to 2010 pandemic).
Main results
We found 41 randomised studies. Most studies included children older than two years of age and were conducted in the USA, Western Europe, Russia, and Bangladesh.
Compared with placebo or do nothing, live attenuated vaccines probably reduced the proportion of children who had confirmed influenza from 18% to 4% (moderate‐certainty evidence), and may reduce ILI from 17% to 12% (low‐certainty evidence). Seven children would need to be vaccinated for one child to avoid influenza, and 20 children would need to prevent one child from experiencing an ILI. We found data from one study that showed similar risk of ear infection in the two groups. There was insufficient information available to assess school absence and parents needing to take time off work. We found no data on hospitalisation, and harms were not consistently reported.
Compared with placebo or no vaccination, inactivated vaccines reduce the risk of influenza from 30% to 11% (high‐certainty evidence), and they probably reduce ILI from 28% to 20% (moderate‐certainty evidence). Five children would need to be vaccinated for one child to avoid influenza, and 12 children would need to be vaccinated to prevent one case of ILI. The risk of otitis media is probably similar between vaccinated children and unvaccinated children (31% versus 27%, moderate‐certainty evidence). There was insufficient information available to assess school absenteeism due to very low‐certainty evidence from one study. We identified no data on parental working time lost, hospitalisation, fever, or nausea.
One brand of monovalent pandemic vaccine was associated with a sudden loss of muscle tone triggered by the experience of an intense emotion (cataplexy) and a sleep disorder (narcolepsy) in children.
Only a few studies were well designed and conducted, and the impact of studies at high risk of bias varied across the outcomes evaluated. Influenza and otitis media were the only outcomes where our confidence in the results was not affected by bias.
How up to date is this review?
The evidence is current to 31 December 2016.
Summary of findings
Background
Description of the condition
Viral respiratory disease imposes a heavy burden on society. The majority of viral respiratory disease (influenza‐like illness (ILI)) is caused by many different agents that are not clinically distinguishable from one another. A variable proportion of ILI (7% to 15% on average) is caused by influenza viruses and is known as influenza (Jefferson 2005b).
Influenza is an acute respiratory infection caused by a virus of the Orthomyxoviridae family. Three serotypes are known (A, B, and C). Influenza causes an acute febrile illness with myalgia, headache, and cough. Although the median duration of the acute illness is three days, cough and malaise can persist for weeks. Complications of influenza include otitis media, pneumonia, secondary bacterial pneumonia, exacerbations of chronic respiratory disease, and bronchiolitis in children. Additionally, influenza can cause a range of non‐respiratory complications including febrile convulsions, Reye's syndrome, and myocarditis (Treanor 2016; Wiselka 1994). Efforts to prevent or minimise the impact of seasonal influenza in the second part of the 20th century centred on the use of vaccines. Due to the yearly changes in viral antigenic configuration and the lack of carry‐over protection from year to year, a new vaccination campaign needs to be organised annually, with a huge scientific and logistic effort to ensure production and delivery of the vaccines.
Description of the intervention
There are four types of influenza vaccines currently available worldwide.
Whole‐virion inactivated vaccines, which consist of complete viruses that have been 'killed' or inactivated, so that they are not infectious but retain their strain‐specific antigenic properties.
Subunit inactivated vaccines, which are made of influenza surface antigens (H and N) only.
Split‐virion inactivated vaccines in which the viral structure is broken up by a disrupting agent. These vaccines contain both surface and internal antigens.
Live attenuated, cold‐adapted vaccines in which the live virus in the vaccine can only multiply in the cooler nasal passages and which are administered intranasally.
Periodic antigenic drifts and shifts pose problems for vaccine production and procurement. New vaccines closely matching the antigenic configuration of circulating strains must be produced and procured for the beginning of each new influenza 'season'. To achieve this, the World Health Organization (WHO) has established a worldwide surveillance system allowing early identification and isolation of viral strains circulating in the different parts of the world.
How the intervention might work
Vaccines work by simulating an infection and stimulating the body to produce antibodies against the threat and activate other defence mechanisms.
Efforts to prevent the spread of influenza have been shown to be unsuccessful due to the infectiousness of the condition, and public health interventions rely on vaccination to mitigate the worst consequences of the disease (death and hospitalisation).
Most high‐income countries have vaccination programmes covering the elderly and so‐called at‐risk groups (e.g. people with pre‐existing conditions likely to be made worse by influenza infection). However, for the influenza season 2004 to 2005, the American Academy of Pediatrics and the US Centers for Disease Control and Prevention (CDC) recommended that immunisation of healthy children between the ages of 6 and 23 months be instituted as a public health measure (AAPCID 2004). This was later extended to cover children aged 6 to 59 months (i.e. 6 months to 5 years) (CDC 2007), and to healthy household contacts (including children) and caregivers of children aged under 5 years (ACIP 2016; CDC 2007). In February 2004, the Canadian National Advisory Committee on Immunization followed the US authorities in recommending immunisation for the 6‐to‐23‐months age group (Orr 2004).
Finland is the only European country to have introduced routine vaccination of children aged six months to three years (from the beginning of the 2007 to 2008 influenza season). Other countries have also recommended childhood vaccination but have not included it in the routine childhood programmes. Slovenia and Latvia recommended vaccination of children aged six months to two years. Slovakia, Estonia, and Austria recommended it for children and adolescents aged six months to 18 years (Mereckiene 2010).
The European Medicines Agency (EMA) has made recent changes to the registration of seasonal, pre‐pandemic, and pandemic influenza vaccines (EMA 2014; Wijnans 2016).
The changes were introduced in 2014, triggered by the realisation that antibody responses are not sufficient predictors of field protection, as our reviews have consistently shown over the years. Most of the data for influenza vaccines included in our reviews are from registered vaccines, and yet the field protection afforded is modest or negligible. In addition, the methods of standardisation of antibody titres were lacking. The new rules for adults and elderly require demonstration of non‐inferiority of antibody response (immunogenicity) by a candidate seasonal influenza vaccine compared to an established one. In addition, whenever a demonstration of clinical efficacy is necessary, the EMA encourages the minimisation of the use of placebo and encourages the use of active controls (such a non‐influenza vaccine) with ILI (and relevant polymerase chain reaction results) as a primary endpoint. Clinical effectiveness should be tested by carrying out (preferably prospective) cohort studies or nested so‐called test‐negative case‐control studies following the European Centre for Disease Prevention and Control protocol (ECDC 2009).
Harms surveillance is now required with a follow‐up of at least six months’ duration and in the general elderly population a database of at least 3000 people exposed to the vaccine. Enhanced vaccine vigilance data should be collected as soon as possible at the beginning of the vaccination campaign each year.
Why it is important to do this review
The main arguments for immunising young children and those attending school include (Izurieta 2000; Neuzil 2000; Principi 2004; Reichert 2001):
reduction of the number of people with influenza;
reduction in the number of admissions to hospital;
reduction in mortality of the elderly in families with children;
reduction in illness in healthcare workers; and
reduction in the number of antibiotic prescriptions and reduction in absenteeism of children from school and their parents or carers or household contacts from work.
Rational decision‐making about the prevention of influenza is complicated by the absence of reliable forecasts, uncertainty about the effects of the vaccine in different age groups, and the vaccines efficacy versus effectiveness issue. Cochrane Reviews on the effects of the use of vaccines to prevent influenza in other age and risk groups show a striking difference between the vaccine efficacy (reduction in number of laboratory‐confirmed cases of influenza) and vaccine effectiveness against ILI (reduction in symptomatic cases), which can include illness caused by influenza viruses that is not laboratory confirmed or illness caused by other viruses, such as respiratory syncytial virus. To allow a reasoned choice between alternative prevention strategies, accurate assessment of both the efficacy and effectiveness of influenza vaccines is essential. The aim of this review was to identify, assess, and compare studies of vaccine efficacy and vaccine effectiveness in healthy children under 16 years of age, and to review the safety of vaccines in children up to 16 years of age.
Due to the unique production cycle of influenza vaccines (they are tested using surrogate outcomes ‐ antibody stimulation ‐ ahead of each influenza 'season'), past performance is probably the only reliable way to predict future performance. An accurate assessment of the effects (efficacy, effectiveness, and safety profile) of influenza vaccines is essential to allow rational choice between alternative strategies. This review, with its two companion reviews (Demicheli 2010; Jefferson 2012a), are long‐running reviews. They are among the most consistently accessed in the whole Cochrane Database of Systematic Reviews, confirming the importance of the topic and interest in it. Periodic updates, some stretching back almost two decades, have allowed us to include an increasing number of studies on the effects of influenza vaccines and monitor their impact on our reviews (Table 3).
1. Synthesis of results and conclusions from the previous versions of the present review.
| Review version (searches date) | Number of included trials (RCT/CCT) | Number of included observational studies | Estimates of effect (RCTs/CCTs only) | Conclusions from the Abstract |
| Version 1 (15 November 2005) |
38 | 13 (12 cohorts and 1 case‐control study) |
Influenza‐like illness LAIV = 33% (95% CI 28% to 38%) TIV = 36% (95% CI 24% to 46%) Influenza LAIV = 79% (95% CI 48% to 92%) TIV = 59% (95% CI 41% to 71%) |
Influenza vaccines are efficacious in children older than two years, but little evidence is available for children under two. There was a marked difference between vaccine efficacy and effectiveness. That no safety comparisons could be carried out emphasises the need for standardisation of methods and presentation of vaccine safety data in future studies. It was surprising to find only one study of inactivated vaccine in children under two years, given recent recommendations to vaccinate healthy children from six months old in the USA and Canada. If immunisation in children is to be recommended as public health policy, large‐scale studies assessing important outcomes and directly comparing vaccine types are urgently required. |
| Version 2 (20 February 2008) |
40 | 21 (18 cohorts and 3 case‐control studies) |
Influenza‐like illness LAIV = 33% (95% CI 28% to 38%) TIV = 36% (95% CI 24% to 46%) Influenza LAIV = 82% (95% CI 71% to 89%) TIV = 59% (95% CI 41% to 71%) |
Influenza vaccines are efficacious in children older than two, but little evidence is available for children under two. There was a marked difference between vaccine efficacy and effectiveness. No safety comparisons could be carried out, emphasising the need for standardisation of methods and presentation of vaccine safety data in future studies. It was surprising to find only one study of inactivated vaccine in children under two years, given current recommendations to vaccinate healthy children from six months old in the USA and Canada. If immunisation in children is to be recommended as a public health policy, large‐scale studies assessing important outcomes and directly comparing vaccine types are urgently required. |
| Version 3 (9 July 2012) |
43 | 33 (21 cohorts and 12 case‐control studies) |
Influenza‐like illness LAIV = 33% (95% CI 28% to 38%) TIV = 36% (95% CI 24% to 46%) Influenza LAIV = 80% (95% CI 68% to 87%) TIV = 59% (95% CI 41% to 71%) |
Influenza vaccines are efficacious in preventing cases of influenza in children older than two years of age, but little evidence is available for children younger than two years of age. There was a difference between vaccine efficacy and effectiveness, partly due to differing data sets, settings, and viral circulation patterns. No safety comparisons could be carried out, emphasising the need for standardisation of methods and presentation of vaccine safety data in future studies. In specific cases, influenza vaccines were associated with serious harms such as narcolepsy and febrile convulsions. It was surprising to find only one study of inactivated vaccine in children under two years, given current recommendations to vaccinate healthy children from six months of age in the USA, Canada, parts of Europe, and Australia. If immunisation in children is to be recommended as a public health policy, large‐scale studies assessing important outcomes and directly comparing vaccine types are urgently required. The degree of scrutiny needed to identify all global cases of potential harms is beyond the resources of this review. |
CCT: comparative controlled trial LAIV: live attenuated influenza vaccine RCT: randomised controlled trial TIV: trivalent inactivated influenza vaccine
The reviews are not methodologically homogeneous, as their methods reflect the history and development of Cochrane Reviews. In particular the inclusion of observational studies which was initially favoured for the assessment of harms, has been a source of discussion. In this review randomised evidence represents 55% of studies considered. To enhance the relevance of the review to decision‐makers, in the Jefferson 2005b update, we included comparative non‐randomised studies reporting evidence of serious or rare harms (or both).
Historically observational studies have been of poor methodological quality, often reporting conflicting or paradoxical results, preventing the drawing of firm conclusions. However, inclusion of particular study types and increasing size of the data sets has not led to a change in the conclusion of the reviews, while leading to a greatly increased workload. This is the main reason why the authors, the review group and the Cochrane editors have decided to stabilise all three reviews, that is not to carry out routine updates of the observational data set and update the randomised data set if certain conditions are fulfilled in the future.
For the same reason we have retained the observational content of this review and its companions as historical evidence of the life cycle of the reviews.
We plan to update the randomised evidence in this review if any or all of the following conditions are fulfilled in the future.
a trial assessing the clinical effects of the evolution of current technologies becomes available;
a new type of vaccine is developed; or
a new credible causal paradigm for influenza is put forward.
For an overview of the three reviews, see the covering editorial at https://community.cochrane.org/news/why‐have‐three‐long‐running‐cochrane‐reviews‐influenza‐vaccines‐been‐stabilised.
Objectives
To assess the effects (efficacy, effectiveness, and harm) of vaccines against influenza in healthy children.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs), quasi‐RCTs, comparative controlled trials (CCTs), and cohort and case‐control studies. See Appendix 1 for study design definitions. We decided to include evidence from comparative non‐randomised studies to enhance the relevance of the review. The searches for cohort and case‐control studies are no longer being updated but the observational studies have been retained for historical purposes.
Types of participants
Healthy children under 16 years of age in any geographical location. All participants were classified as healthy unless otherwise stated. We excluded studies that documented the inclusion of participants with chronic illnesses/conditions or immunodeficiency.
Types of interventions
Vaccination with any influenza vaccine given independently, in any dose, preparation, or time schedule (intervention), compared with placebo, or with no intervention (control).
We also considered newer, or as yet unlicensed types of vaccines (e.g. live attenuated and DNA vaccines).
Types of outcome measures
Primary outcomes
Primary outcome measures for treatment efficacy and effectiveness
Influenza: symptoms of influenza accompanied by a positive laboratory diagnosis (measure of vaccine efficacy).
Influenza‐like illness (ILI): symptoms of influenza only (measure of vaccine effectiveness).
Otitis media.
Lower respiratory tract diseases.
-
Cases admitted to hospital:
hospitalisation due to otitis media
Deaths of study participants (either from influenza or other causes).
Primary outcome measures for adverse events
All types of systemic and severe adverse events.
Secondary outcomes
Secondary outcome measures for treatment efficacy and effectiveness
-
Direct or indirect indicator of disease impact:
working days lost for influenza;
school absenteeism for influenza;
drug prescriptions; and
outpatients attendances.
Secondary outcome measures for adverse events
All types of local adverse events.
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 12, searched 31 December 2016 via the Cochrane Library), which contains the Cochrane Acute Respiratory Infections Group Specialised Register, MEDLINE (PubMed) (1966 to 31 December 2016), Embase (Elsevier) (1974 to 31 December 2016), World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en, 1 July 2017), and ClinicalTrials.gov (www.clinicaltrials.gov, 1 July 2017).
We used the search strategy in Appendix 2 to search MEDLINE. We adapted the search terms to search CENTRAL (Appendix 3), Embase (Appendix 4), WHO ICTRP (Appendix 5), and ClinicalTrials.gov (Appendix 6).
We did not impose any language or publication restrictions.
See Appendix 7 for details of previous searches.
Searching other resources
To identify additional published and unpublished studies, we searched the Vaccine Adverse Event Reporting System website (vaers.hhs.gov/index.html). For past versions, we contacted vaccine manufacturers and first or corresponding authors of relevant studies to identify further published or unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (TOJ, AR) independently excluded all studies not fulfilling the inclusion criteria of initially identified and retrieved articles. In the case of disagreement, VD carried out arbitration.
Data extraction and management
Four review authors (AR, TOJ, CDP, EF) performed data extraction using a data extraction form (Appendix 8). We checked the data and entered it into Review Manager 5 software (RevMan 2014). We extracted data on the following:
methodological quality of studies;
study design (Appendix 1);
description of setting;
characteristics of participants;
description of vaccines (content and antigenic match);
description of outcomes;
publication status;
date of study; and
location of study.
One review author (CDP) carried out statistical analyses.
Assessment of risk of bias in included studies
Experimental studies (trials)
The review authors independently assessed the methodological quality of the included studies using the criteria in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In the case of disagreement in assigning quality criteria amongst the review authors (TOJ, EF, CDP, AR), VD carried out arbitration.
We assessed risk of bias of the studies according to the following key domains (Higgins 2011).
Generation of the allocation sequence
Low risk of bias: e.g. use of a table of random numbers or computer‐generated random numbers.
High risk of bias: e.g. use of alternation, date of birth, day of the week, or case record number.
Unclear risk of bias: if insufficient information was provided.
Allocation concealment
Low risk of bias: e.g. numbered or coded identical containers administered sequentially, on‐site computer system that can only be accessed after entering the characteristics of an enrolled participant, or serially numbered, opaque, sealed envelopes were used, or sealed envelopes that were not sequentially numbered were used.
High risk of bias: e.g. use of an open table of random numbers.
Unclear risk of bias: if insufficient information was provided.
Blinding
Low risk of bias: if adequate double‐blinding, e.g. placebo vaccine, or single‐blinding, i.e. blinded outcome assessment, was used.
High risk of bias: if no blinding was used.
Unclear risk of bias: if insufficient information was provided.
Incomplete outcome data
Number of losses to follow‐up.
Low risk of bias: no missing data, or the proportion of missing data compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
High risk of bias: the proportion of missing data compared with observed event risk was enough to induce clinically relevant bias in the intervention effect estimate.
Unclear risk of bias: if insufficient information was provided.
Non‐experimental studies
We assessed the quality of non‐randomised studies in relation to the presence of potential confounders, which could make interpretation of the results difficult. We evaluated the quality of case‐control (prospective and retrospective) and cohort studies using the appropriate Newcastle‐Ottawa Scales (Appendix 9).
Using quality at the analysis stage as a means of interpretation of the results, we assigned 'Risk of bias' categories as (Higgins 2011):
low risk of bias: plausible bias unlikely to seriously alter the results;
unclear risk of bias: plausible bias that raises some doubt about the results; and
high risk of bias: plausible bias that seriously weakens confidence in the results.
Measures of treatment effect
We used the risk ratio (RR) and its 95% confidence interval (CI) as the summary measure. We calculated vaccine efficacy (or effectiveness) as VE = 1 ‐ RR expressed as a percentage, for cohort and RCT/CCT studies. For case‐control studies we adopted the odds ratio (OR) with 95% CIs.
To enhance relevance to everyday practice, we also expressed the summary measure of the most reliable and significant comparisons (those from RCTs with influenza cases as an outcome by age group) as a risk difference (RD). This is a measure of absolute efficacy of the vaccines that incorporates significant information such as the incidence in the control arm and allows the calculation of its reciprocal, the number needed to treat for an additional beneficial outcome (in this case, vaccinate or NNV). Number needed to vaccinate expresses the number of children needed to be vaccinated to prevent one case of influenza. Evidence was insufficient to calculate meaningful RDs for rarer outcomes (such as hospitalisations or pneumonia), or the evidence was of poor quality (as in the case of cohorts).
The NNV expresses the number of children needed to be vaccinated to prevent one case of influenza. The NNV can be computed as 1/RD. Since meta‐analysis estimates from RD are affected by spurious heterogeneity, we preferred to compute the NNV as CER*RR, where CER (control event rate) is the proportion of events in the control group. Given substantial variation in the control group rates across the studies for influenza and ILI outcomes, we have based NNVs on low, median, and high control group rates. This is a departure from the approach used in the previous version of this review (see Differences between protocol and review).
Unit of analysis issues
For cluster‐randomised trials we did not compute effective sample size as described by Higgins 2011 because the information supplied by papers was insufficient to compute intracluster correlation coefficient (ICC), and additional information was not available. However, for studies with a known cluster design (aa Alexandrova 1986; aa Rudenko 1993a; aa Rudenko 1993b), we conducted sensitivity analyses with effective sample size calculated from an assumed ICC of 0.004 (see Sensitivity analysis) (Loeb 2010). Information about ICC was unavailable for aa Clover 1991 and aa Gruber 1990. Nevertheless, even if we suppose for these studies an ICC different from zero, we have to take into account that average cluster size is small and that we expect a design effect close to 1. Because of the small sample size of each arm, we can suppose that the reduction to effective sample size was negligible.
We summarised evidence from non‐randomised studies (cohort and case‐control) in our review according to Higgins 2011.
Dealing with missing data
Our analysis relied on existing data. Whenever possible, we used the intention‐to‐treat (ITT) population.
Assessment of heterogeneity
We calculated the I2 statistic for each pooled estimate to assess the impact on statistical heterogeneity. The I2 statistic may be interpreted as the proportion of total variation among effect estimates that is due to heterogeneity rather than sampling error, and it is intrinsically independent of the number of studies. When the I2 is less than 30% there is little concern about statistical heterogeneity (Higgins 2011). We used random‐effects models throughout to take into account the between‐study variance in our findings (Higgins 2011). Variance is to be expected in influenza vaccine trials as there are unpredictable systematic differences between trials regarding the circulating strains, degree of antigenic matching of the vaccine, type of vaccine, and the levels of immunity presented by different populations in different settings. Not all studies reported sufficient details to enable a full analysis of the sources of heterogeneity, but we were able to take into account age group and number of doses.
Assessment of reporting biases
Due to the limited number of studies in each comparison, assessment of publication bias was not applicable.
The overall quality of retrieved studies was poor and was affected by poor reporting or limited descriptions of the design of the studies.
A detailed description is provided in the Quality of the evidence section of the review.
Data synthesis
We carried out data synthesis separately for live and inactivated vaccines. We grouped studies for analysis according to study design: trials, cohort studies, and case‐controlled studies.
Between‐trial variability is to be expected in influenza vaccine studies as there are unpredictable differences between effect estimates. We incorporated heterogeneity into the pooled estimates using the DerSimonian‐Laird random‐effects model.
We used RRs of events for the comparisons of vaccine with placebo/control groups for RCTs and cohort studies and ORs for the single case‐controlled study.
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: influenza, ILI, absence from school, parental leave from work, acute otitis media, hospitalisation, and nausea. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contributed data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT 2014). We justified all decisions to downgrade the quality of studies using footnotes, and made comments to aid readers’ understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analyses by age group for trials and cohort and case‐control studies as follows: under 2 years (from 0 to 23 months); under 6 years (from 24 months to 6 years), and over 6 years of age. We selected the under‐2‐years‐of‐age group, as the CDC recommends vaccination for healthy children aged 6 to 23 months (CDC 2011; Fiore 2011; Harper 2004). The under 6 years and over 6 years categories reflected the most frequent stratification in primary studies. One comparison (Analysis 8), which includes rare outcomes, included both vaccine types (live and inactivated). However, we only considered the subgroup analyses.
Sensitivity analysis
We performed three sensitivity analyses:
excluding studies translated from Russian (Table 4);
excluding studies with high risk of bias (Table 5);
estimating effective sample sizes for cluster‐randomised studies contributing data to influenza and ILI outcomes where adjustment could be made for the effect of clustering.
2. Sensitivity analysis.
| Comparison | Vaccine type | Study type | Outcome | Age group | Without Russian studies | Data sets | All studies | Data sets |
| Risk ratio (random) (95% CI) | Risk ratio (random) (95% CI) | |||||||
| 01.01 | Live | RCTs | Influenza | </= 2 years | ‐ | ‐ | ‐ | ‐ |
| </= 6 years | 0.15 (0.10 to 0.23) | 5 | 0.15 (0.10 to 0.23) | 5 | ||||
| > 6 years | 0.47 (0.23 to 0.97) | 1 | 0.47 (0.23 to 0.97) | 1 | ||||
| Total | 0.18 (0.11 to 0.29) | 6 | 0.18 (0.11 to 0.29) | 6 | ||||
| 01.02 | Live | RCTs | ILI | </= 2 years | ‐ | ‐ | ‐ | ‐ |
| </= 6 years | 0.54 (0.12 to 2.42)* | 1 | 0.67 (0.57 to 0.77) | 5 | ||||
| > 6 years | 0.12 (0.01 to 2.11)* | 1 | 0.67 (0.60 to 0.74) | 8 | ||||
| Total | 0.39 (0.10 to 1.48)* | 2 | 0.67 (0.62 to 0.72) | 13 | ||||
| 02.01 | Inactivated | RCTs | Influenza | </= 2 years | 0.55 (0.18 to 1.69) | 2 | 0.55 (0.18 to 1.69) | 2 |
| </= 6 years | 0.61 (0.34 to 1.08) | 2 | 0.61 (0.34 to 1.08) | 2 | ||||
| > 6 years | 0.31 (0.22 to 0.45) | 3 | 0.31 (0.22 to 0.45) | 3 | ||||
| Total | 0.41 (0.29 to 0.59) | 7 | 0.41 (0.29 to 0.59) | 7 | ||||
| 02.02 | Inactivated | RCTs | ILI | </= 2 years | ‐ | ‐ | ‐ | ‐ |
| </= 6 years | 0.39 (0.21 to 0.69) | 3 | 0.39 (0.21 to 0.69) | 3 | ||||
| > 6 years | 0.24 (0.08 to 0.70)+ | 2 | 0.72 (0.66 to 0.78) | 4 | ||||
| Total | 0.34 (0.24 to 0.50)+ | 5 | 0.64 (0.54 to 0.76) | 7 | ||||
| 03.01 | Live | Cohort studies | Influenza | </= 2 years | ‐ | ‐ | ‐ | ‐ |
| </= 6 years | ‐ | ‐ | ‐ | ‐ | ||||
| > 6 years | ‐ | ‐ | 0.56 (0.35 to 0.91) | 1 | ||||
| Total | No studies | ‐ | 0.56 (0.35 to 0.91) | 1 | ||||
| 03.02 | Live | Cohort studies | ILI | </= 2 years | ‐ | ‐ | ‐ | ‐ |
| </= 6 years | ‐ | ‐ | ‐ | ‐ | ||||
| > 6 years | 0.63 (0.57 to 0.69) | 1 | 0.63 (0.57 to 0.69) | 2 | ||||
| Total | 0.63 (0.57 to 0.69) | 1 | 0.63 (0.57 to 0.69) | 2 | ||||
| 04.01 | Inactivated | Cohort studies | Influenza | </= 2 years | 0.63 (0.27 to 1.47) | 3 | 0.63 (0.27 to 1.47) | 3 |
| </= 6 years | 0.34 (0.13 to 0.89) | 1 | 0.34 (0.13 to 0.89) | 1 | ||||
| > 6 years | 0.20 (0.10 to 0.39)* | 1 | 0.36 (0.12 to 1.11) | 2 | ||||
| Total | 0.36 (0.19 to 0.66) | 5 | 0.42 (0.25 to 0.73) | 6 | ||||
| 04.02 | Inactivated | Cohort studies | ILI | </= 2 years | ‐ | ‐ | ‐ | ‐ |
| </= 6 years | 0.40 (0.13 to 1.20) | 3 | 0.81 (0.65 to 1.01) | 4 | ||||
| > 6 years | 0.10 (0.05 to 0.21)+ | 1 | 0.44 (0.29 to 0.68) | 7 | ||||
| Total | 0.26 (0.07 to 0.92)+ | 4 | 0.55 (0.42 to 0.70) | 11 |
CI: confidence interval ILI: influenza‐like illness RCTs: randomised controlled trials *: significance change +: possible decision‐making significant change
3. 'Risk of bias' sensitivity analysis.
| Comparison | Effect measure | All data sets | Excluding studies at high risk of bias | VE absolute change | Change effect measure direction | Change in statistical significance | |||||||||
| Number of data sets | Effect estimate | LL 95% CI | UL 95% CI | Statistical significance | Number of data sets | Effect estimate* | LL 95% CI | UL 95% CI | Statistical significance | ||||||
| Analysis 1.1 | Influenza | Risk ratio | 8 | 0.22 | 0.11 | 0.41 | Significant | 5 | 0.25 | 0.12 | 0.26 | Significant | ‐4% | Unchanged | Unchanged |
| Analysis 1.2 | Influenza‐like illness | Risk ratio | 8 | 0.69 | 0.60 | 0.80 | Significant | 1 | 0.48 | 0.44 | 0.52 | Significant | 15% | Unchanged | Unchanged |
| Analysis 2.1 | Influenza | Risk ratio | 5 | 0.36 | 0.28 | 0.48 | Significant | 3 | 0.40 | 0.23 | 0.68 | Significant | ‐5% | Unchanged | Unchanged |
| Analysis 2.2 | Influenza‐like illness | Risk ratio | 7 | 0.64 | 0.54 | 0.76 | Significant | 0 | NA | NA | NA | NA | NA | NA | NA |
| Analysis 4.3 | Otitis media | Risk ratio | 1 | 0.48 | 0.22 | 1.03 | Not significant | 0 | 0 | 0 | 0 | ‐ | ‐ | Unchanged | Changed |
| Analysis 5.1 | Influenza | Risk ratio | 7 | 0.19 | 0.11 | 0.32 | Significant | 5 | 0.21 | 0.09 | 0.48 | Significant | ‐2% | Unchanged | Unchanged |
| Analysis 5.2 | Influenza‐like illness | Risk ratio | 8 | 0.69 | 0.6 | 0.80 | Significant | 3 | 0.53 | 0.39 | 0.74 | Significant | 16% | Unchanged | Unchanged |
| Analysis 5.3 | Otitis media (all episodes) | Risk ratio | 2 | 0.98 | 0.95 | 1.01 | Not significant | 1 | 0.98 | 0.95 | 1.01 | Not significant | 0% | Unchanged | Unchanged |
| Analysis 5.4 | Working days lost (number of events, parents) | Risk ratio | 2 | 0.69 | 0.46 | 1.03 | Not significant | 2 | 0.69 | 0.46 | 1.03 | Not significant | 0% | Unchanged | Unchanged |
| Analysis 5.5 | Drug prescriptions (number of events) | Risk ratio | 1 | 0.99 | 0.87 | 1.12 | Not significant | 1 | 0.99 | 0.87 | 1.12 | Not significant | 0% | Unchanged | Unchanged |
| Analysis 5.6 | Outpatients attendance for pneumonia and influenza | Risk ratio | 2 | 0.76 | 0.59 | 0.98 | Significant | 2 | 0.76 | 0.59 | 0.98 | Significant | 0% | Unchanged | Unchanged |
| Analysis 6.1 | Influenza | Risk ratio | 5 | 0.36 | 0.28 | 0.48 | Significant | 5 | 0.36 | 0.28 | 0.48 | Significant | 0% | Unchanged | Unchanged |
| Analysis 6.2 | Influenza‐like illness | Risk ratio | 4 | 0.72 | 0.65 | 0.79 | Significant | 2 | 0.35 | 0.15 | 0.81 | Significant | 37% | Unchanged | Unchanged |
| Analysis 7.1 | Influenza vs ILI (crude data) | Odds ratio | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Unchanged | Unchanged |
| Analysis 7.2.1 | Children aged below 23 months ‐ fully vaccinated | Odds ratio | 7 | 0.60 | 0.39 | 0.94 | Significant | 4 | 0.46 | 0.29 | 0.73 | Significant | 14% | Unchanged | Unchanged |
| Analysis 7.2.2 | Children aged 24 to 59 months ‐ fully vaccinated | Odds ratio | 4 | 0.40 | 0.22 | 0.70 | Significant | 4 | 0.4 | 0.22 | 0.70 | Significant | 0% | Unchanged | Unchanged |
| Analysis 7.2.3 | Children aged 6 to 59 months ‐ fully vaccinated | Odds ratio | 5 | 0.45 | 0.32 | 0.62 | Significant | 5 | 0.45 | 0.32 | 0.62 | Significant | 0% | Unchanged | Unchanged |
| Analysis 7.2.4 | Children aged below 14 years old ‐ fully vaccinated | Odds ratio | 1 | 0.23 | 0.06 | 0.84 | Significant | 1 | 0.23 | 0.06 | 0.84 | Significant | 0% | Unchanged | Unchanged |
| Analysis 7.3 | Influenza‐like illness vs no symptoms | Odds ratio | 2 | 0.49 | 0.28 | 0.86 | Significant | 0 | 0 | 0 | 0 | ‐ | ‐ | Unchanged | Changed |
| Analysis 8.1 | Influenza | Risk ratio | 11 | 0.27 | 0.18 | 0.42 | Significant | 11 | 0.27 | 0.18 | 0.42 | Significant | 0% | Unchanged | Unchanged |
| Analysis 8.2 | Influenza‐like illness | Risk ratio | 12 | 0.69 | 0.62 | 0.77 | Significant | 5 | 0.50 | 0.38 | 0.67 | Significant | 19% | Unchanged | Unchanged |
| Analysis 8.3 | Secondary cases | Risk ratio | 1 | 1.68 | 0.56 | 4.99 | Not significant | 1 | 1.68 | 0.56 | 4.99 | Not significant | 0% | Unchanged | Unchanged |
| Analysis 8.4 | School absenteeism | Risk ratio | 2 | 0.49 | 0.26 | 0.92 | Significant | 2 | 0.49 | 0.26 | 0.92 | Significant | 0% | Unchanged | Unchanged |
| Analysis 8.5 | Lower respiratory tract disease | Risk ratio | 3 | 0.2 | 0.03 | 1.54 | Not significant | 2 | 0.52 | 0.08 | 3.37 | Not significant | ‐32% | Unchanged | Unchanged |
| Analysis 8.6 | Acute otitis media | Risk ratio | 7 | 1.00 | 0.79 | 1.26 | Not significant | 5 | 1.03 | 0.91 | 1.17 | Not significant | ‐3% | Changed | Unchanged |
| Analysis 8.7 | Hospitalisation due to acute otitis media | Risk ratio | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Unchanged | Unchanged |
| Analysis 8.7.1 | Inactivated vaccine (2 doses) | Risk ratio | 2 | 1.41 | 0.62 | 3.24 | Not significant | 2 | 1.41 | 0.62 | 3.24 | Not significant | 0% | Unchanged | Unchanged |
| Analysis 8.8 | Consequences of acute otitis media | Mean difference | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Unchanged | Unchanged |
| Analysis 8.8.1 | Inactivated vaccine, 2 doses (the arm of the trial) visits (the outcome measure) | Mean difference | 2 | ‐0.02 | ‐0.27 | 0.23 | Not significant | 2 | ‐0.02 | ‐0.27 | 0.23 | Not significant | ‐ | Unchanged | Unchanged |
| Analysis 8.8.2 | Inactivated vaccine (2 doses; courses of antibiotics | Mean difference | 2 | 0.13 | ‐0.36 | 0.63 | Not significant | 2 | 0.13 | ‐0.36 | 0.63 | Not significant | ‐ | Unchanged | Unchanged |
| Analysis 8.9 | Outpatients attendance for pneumonia and influenza | Risk ratio | 2 | 0.76 | 0.59 | 0.98 | Significant | 1 | 0.85 | 0.75 | 0.96 | Significant | ‐9% | Unchanged | Unchanged |
| Analysis 8.10 | Working days lost (number of events, parents of children 6 to 36 months of age) | Risk ratio | 2 | 0.69 | 0.46 | 1.03 | Not significant | 1 | 0.83 | 0.71 | 0.98 | Significant | ‐14% | Unchanged | Changed |
| Analysis 8.11 | Drug prescriptions (number of events, 6 to 36 months of age) | Risk ratio | 1 | 0.99 | 0.87 | 1.12 | Not significant | 1 | 0.99 | 0.87 | 1.12 | Not significant | 0% | Unchanged | Unchanged |
CI: confidence interval ILI: influenza‐like illness LL: lower limit NA: not applicable RR: risk ratio UL: upper limit VE: vaccine efficacy/effectiveness VE absolute change = (1 ‐ RR*) ‐ (1 ‐ RR) *: effect estimate excluding data sets at high risk of bias
Results
Description of studies
Results of the search
Past searches identified 8340 records, corresponding to 8814 citations (after removal of duplicates). We screened 8814 records and excluded 8620 records based on the title and abstract. We retrieved the full text of 195 papers and excluded 107 papers (reasons are summarised in the Excluded studies section and the Characteristics of excluded studies tables). We finally included 77 papers. Eleven papers are in Studies awaiting classification, as some data are not presented in the papers (Figure 1).
1.
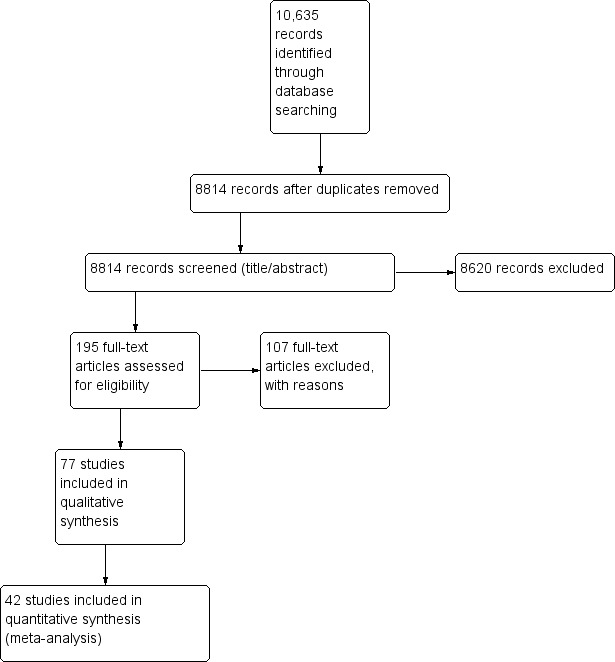
Study flow diagram.
We updated the searches in December 2016, identifying 2295 records (after removal of duplicates). After screening of title and abstracts, we retrieved the full text of seven citations for evaluation. We excluded five new trials (see Characteristics of excluded studies) and included two new trials. The updated version of the review includes 41 clinical trials with over 200,000 observations.
Trial register searches identified 18 completed trials with one or more corresponding publications, reporting methods, and study design. All 18 trials had been identified and dealt with appropriately in our searches of journal publication databases.
In this 2016 updated review we included only new RCTs. We are no longer updating searches for observational comparative studies, which were included previously.
Included studies
We have coded each trial on the basis of study design and type of data contributed to the review as follows.
The first letter before the study represents study design: 'a' denotes RCT, 'b' denotes case‐control study, and 'c' denotes cohort study. The second letter indicates the contribution to the evidence in the efficacy/effectiveness data set (letter a) or harms (letter b). So, for example, a case‐control study contributing safety or harms data is coded as bb, and a trial contributing efficacy/effectiveness data is coded as aa.
This review consists of 41 RCTs/CCTs (48 data sets), 12 case‐control studies (16 data sets), and 21 cohort studies (25 data sets). Eight trials (aa Grigor'eva 2002; aa Rudenko 1996b; ab Desheva 2002; ab Grigor'eva 1994; ab Rudenko 1991; ab Slepushkin 1974; ab Slepushkin 1991; ab Vasil'eva 1988a), eight cohort studies (aa Rudenko 1988; ab Vasil'eva 1988b; ca Burtseva 1991; ca Chumakov 1987; ca El'shina 2000; ca Slobodniuk 2002a; ca Vasil'eva 1982; cb Slepushkin 1994), and one CCT were translated from the Russian (ab Aksenov 1971). We sent six requests to trial authors for further data (split by age); two trial authors provided the requested data. In this 2016 update, we included two new trials (aa Brooks 2016; aa Cowling 2012). The aa Cowling 2012 trial is included in Table 6 because its design and reporting prevented us from including its results in our meta‐analysis.
4. Efficacy and effectiveness data from intra‐epidemic and non‐typical studies.
| Study reference | Exclusion reason | RCT/cohort | Vaccine | Age group | Outcome | n treatment | N treatment | n control | N control |
| Nicholls 2004 | Cohort from community not representative of local population | Cohort | Inactive, trivalent | 0 to 2 years | ILI | 11 | 18 | 3 | 5 |
| Nicholls 2004 | Cohort from community not representative of local population | Cohort | Inactive, trivalent | 3 to 4 years | ILI | 10 | 16 | 0 | 0 |
| Nicholls 2004 | Cohort from community not representative of local population | Cohort | Inactive, trivalent | 5 to 14 years | ILI | 39 | 91 | 0 | 3 |
| Slepushkin 1974 | Intra‐epidemic study of orally administered vaccine as emergency prophylaxis | RCT | Live (oral) H2N2+B | 1 to 3 years | Influenza or ARI >= 10 days after vaccination | 187 | 508 | 271 | 492 |
| Ritzwoller 2005 | Intra‐epidemic study | Cohort | Inactive, trivalent | 6 to 23 months | ILI | 65 | 1129 | 124 | 1615 |
| Aksenov 1971 | Intra‐epidemic study | Cohort | Live, H2N2+B, 3 doses 5 days apart | 4 to 7 years | Morbidity due to influenza and ARI | 107 | 760 | 164 | 594 |
| Aksenov 1971 | Intra‐epidemic study | Cohort | Live, H2N2+B, 3 doses 8 to 10 days apart | 4 to 7 years | Morbidity due to influenza and ARI | 81 | 728 | 193 | 674 |
| Aksenov 1971 | Intra‐epidemic study | Cohort | Live, H2N2+B, 3 doses 5 days apart | 7 to 15 years | Morbidity due to influenza and ARI | 143 | 1358 | 114 | 776 |
| Cowling 2012 | Intra‐epidemic study | RCT | TIV or saline placebo during the 2009–10 pandemic | 6 to 17 years | Morbidity due to influenza | Unclear | 479 | Unclear | 317 |
ARI: acute respiratory infection ILI: influenza‐like illness RCT: randomised controlled trial TIV: trivalent influenza vaccine
Included studies are classified below on the basis of the evidence provided. We included studies listed under 'Effectiveness and safety' and 'Effectiveness only' in our quantitative analysis. We included studies listed under 'Effectiveness and safety' and 'Safety only' in our qualitative analysis of vaccine safety only (see Adverse events section).
Effectiveness and safety
-
RCTs (16 studies/22 data sets):
RCT‐cluster randomisation:aa Alexandrova 1986; aa Clover 1991; aa Gruber 1990; aa Rudenko 1993a; aa Rudenko 1993b; aa Rudenko 1996a; aa Rudenko 1996b.
RCT‐individual randomisation: aa Belshe 1998; aa Belshe 2000a; aa Beutner 1979a; aa Beutner 1979b; aa Bracco Neto 2009a; aa Bracco Neto 2009b; aa Brooks 2016; aa Colombo 2001; aa Grigor'eva 2002; aa Khan 1996; aa Rudenko 1988; aa Tam 2007a; aa Tam 2007b; aa Vesikari 2006a; aa Vesikari 2006b.
Prospective cohort (5 studies/5 data sets): ca Burtseva 1991; ca El'shina 2000; ca Kawai 2003; ca King 2006; ca Vasil'eva 1982.
Effectiveness only
RCT (2 studies/3 data sets): aa Hoberman 2003a; aa Hoberman 2003b; aa Principi 2003.
Prospective cohort (9 studies/11 data sets): ca Chumakov 1987; ca Fujieda 2006; ca Jianping 1999; ca Maeda 2002; ca Maeda 2004a; ca Maeda 2004b; ca Maeda 2004c; ca Ozgur 2006; ca Salleras 2006; ca Wiggs‐Stayner 2006; ca Yin 2011.
Pandemic prospective cohort (1 study/1 data set): ca Ortqvist 2011.
Retrospective cohort (2 studies/4 data sets): ca Allison 2006; ca Slobodniuk 2002a; ca Slobodniuk 2002b; ca Slobodniuk 2002c.
Case‐control (7 studies/11 data sets): ba Anonymous 2005; ba Cochran 2010a; ba Cochran 2010b; ba Cochran 2010c; ba Eisenberg 2008a; ba Eisenberg 2008b; ba Hirota 1992; ba Kelly 2011; ba Kissling 2011; ba Staat 2011a; ba Staat 2011b.
Pandemic case‐control (4 studies/4 data sets): ba Gilca 2011; ba Mahmud 2011; ba Valenciano 2011; ba Van Buynder 2010.
Safety only
RCT (21 studies/21 data sets): ab Belshe 1992; ab Desheva 2002; ab Grigor'eva 1994; ab Gruber 1996; ab Gruber 1997; ab Gutman 1977; ab King 1998; ab Levine 1977; ab Mallory 2010; ab Obrosova‐Serova 1990; ab Plennevaux 2011; ab Rudenko 1991; ab Slepushkin 1988; ab Slepushkin 1991; ab Steinhoff 1990; ab Steinhoff 1991; ab Swierkosz 1994; ab Vasil'eva 1988a; ab Vasil'eva 1988b; ab Wright 1976a; ab Zangwill 2001.
Prospective cohort (1 study/1 data set): cb Slepushkin 1994.
Pandemic prospective cohort (1 study/1 data set): cb MPA 2011.
Case‐control (1 study/1 data set): bb Goodman 2006.
Interepidemic studies
RCT (2 studies/2 data sets): aa Cowling 2012; ab Slepushkin 1974.
CCT (1 study/1 data set): ab Aksenov 1971.
Retrospective cohort (2 studies/2 data sets): cb Nicholls 2004; cb Ritzwoller 2005.
Readers are reminded that one study may provide multiple data sets (e.g. aa Bracco Neto 2009a; aa Bracco Neto 2009b).
Excluded studies
We excluded 107 studies mainly because they were non‐comparative; had not been carried out in healthy children; had assessed the impact of vaccinating children to prevent influenza in the elderly; or presented only serological outcome or data published in studies already included in this review (See Characteristics of excluded studies tables).
Risk of bias in included studies
Randomised controlled trials/comparative controlled trials
We included 18 trials (corresponding to 25 data sets) in the vaccine efficacy or effectiveness analyses. We classified six RCTs (nine data sets) as having low risk of bias (aa Alexandrova 1986; aa Belshe 1998; (aa Beutner 1979a; aa Beutner 1979b); aa Brooks 2016; (aa Hoberman 2003a; aa Hoberman 2003b); (aa Tam 2007a; aa Tam 2007b)). Two RCTs reported data from two influenza seasons. In both cases we classified the first season as at low risk of bias (two data sets) (aa Bracco Neto 2009a; aa Vesikari 2006a), whereas we classified the second season for both studies (two data sets) as at high risk of bias (aa Bracco Neto 2009b; aa Vesikari 2006b). We assessed five RCTs (five data sets) as at unclear risk of bias for one or more key domains, there being plausible bias raising some doubts about the results (aa Clover 1991; aa Grigor'eva 2002; aa Gruber 1990; aa Khan 1996; aa Principi 2003). Finally, we considered five RCTs (seven data sets) to be at high risk of bias (aa Belshe 2000a; aa Colombo 2001; aa Rudenko 1988; (aa Rudenko 1993a; aa Rudenko 1993b); (aa Rudenko 1996a; aa Rudenko 1996b)). Overall, out of 25 data sets providing evidence of efficacy and effectiveness, 44% (11/25) were at low risk of bias; 20% (5/25) had an unclear risk of bias; and 36% (9/25) were at high risk of bias (Figure 2).
2.
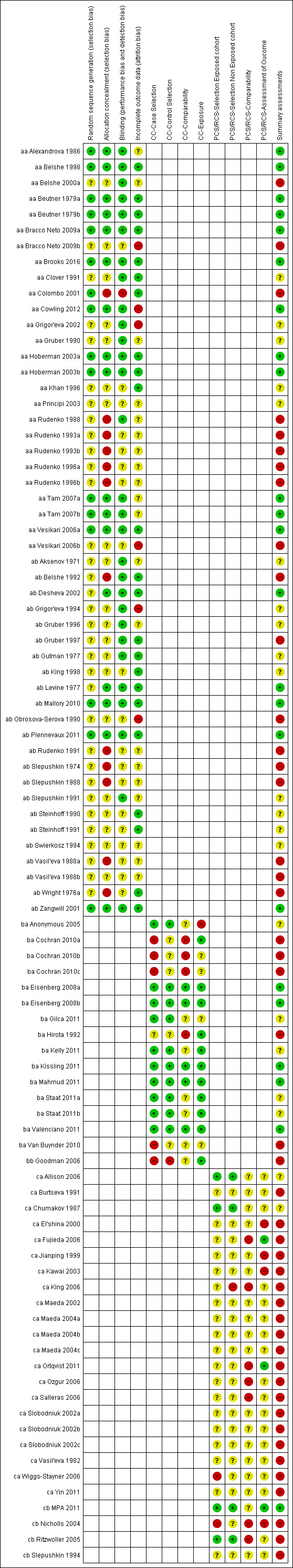
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We included 23 RCTs/CCTs (corresponding to 23 data sets) reporting vaccine safety outcome only. We assessed five RCTs as at low risk of bias (ab Desheva 2002; ab Levine 1977; ab Mallory 2010; ab Plennevaux 2011; ab Zangwill 2001). Nine RCTs (corresponding to nine data sets) had an unclear risk of bias (ab Aksenov 1971; ab Grigor'eva 1994; ab Gruber 1996; ab Gutman 1977; ab King 1998; ab Slepushkin 1991; ab Steinhoff 1990; ab Steinhoff 1991; ab Swierkosz 1994). Finally, we assessed nine RCTs (nine data sets) as at high risk of bias (ab Belshe 1992; ab Gruber 1997; ab Obrosova‐Serova 1990; ab Rudenko 1991; ab Slepushkin 1974; ab Slepushkin 1988; (ab Vasil'eva 1988a; ab Vasil'eva 1988b); ab Wright 1976a). Overall, out of 23 data sets providing evidence of vaccine safety only, 22% (5/23 ) were at low risk of bias; 39% (9/23) had an unclear risk of bias; and 39% (9/23) were at high risk of bias (Figure 2).
Case‐control studies
We included 11 case‐control studies (corresponding to 15 data sets) in vaccine efficacy analyses. Four studies (five data sets) were at low risk of bias ((ba Eisenberg 2008a; ba Eisenberg 2008b); ba Kissling 2011; ba Mahmud 2011; ba Valenciano 2011). Four studies (five data sets) had an unclear risk of bias for one or more key domains (ba Anonymous 2005; ba Gilca 2011; ba Kelly 2011; (ba Staat 2011a; ba Staat 2011b)). Three studies (five data sets) were at high risk of bias ((ba Cochran 2010a; ba Cochran 2010b; ba Cochran 2010c); ba Hirota 1992; ba Van Buynder 2010). Overall, out of 15 data sets providing evidence of vaccine efficacy, 33.3% (5/15) were at low risk of bias; 33.3% (5/15) had an unclear risk of bias; and 33.3% (5/15) were at high risk of bias (Figure 2). We assessed the only case‐control study with a safety outcome, bb Goodman 2006, as at high risk of bias.
Cohort studies
We included 17 cohort studies (corresponding to 21 data sets) in vaccine efficacy or effectiveness analyses. Two studies (two data sets) had an unclear risk of bias (ca Allison 2006; ca Chumakov 1987). Fifteen studies (19 data sets) were at high risk of bias (ca Burtseva 1991; ca El'shina 2000; ca Fujieda 2006; ca Jianping 1999; ca Kawai 2003; ca King 2006; ca Maeda 2002; (ca Maeda 2004a; ca Maeda 2004b; ca Maeda 2004c); ca Ortqvist 2011; ca Ozgur 2006; ca Salleras 2006; (ca Slobodniuk 2002a; ca Slobodniuk 2002b; ca Slobodniuk 2002c); ca Vasil'eva 1982; ca Wiggs‐Stayner 2006; ca Yin 2011). Overall, out of 21 data sets, 9.5% (2/21) had an unclear risk of bias, and 90.5% (19/21) were at high risk of bias (Figure 2).
Of the four cohort studies (corresponding to four data sets) included in vaccine safety only, only one (one data set) was at low risk of bias (cb MPA 2011), and three studies (three data sets) were at high risk of bias (cb Nicholls 2004; cb Ritzwoller 2005; cb Slepushkin 1994).
Allocation
Of the 19 included RCTs (26 data sets), nine studies (12 data sets) reported adequate allocation concealment. We assessed allocation concealment as satisfactory only for the first season of the two‐season trials by aa Belshe 1998, aa Bracco Neto 2009a, and aa Vesikari 2006a.
Blinding
We considered the blinding to be satisfactory in 29 data sets out of 49 (59%). Nineteen data sets showed unclear blinding, and one was not blinded.
Incomplete outcome data
Few studies reported information on influenza circulation in the surrounding community, making interpretation of the results and assessment of their generalisability difficult.
Selective reporting
There is evidence of sizeable reporting bias of all types in influenza vaccines studies in general (Jefferson 2009), in the publication of 2009 H1N1 pandemic vaccines studies (Ioannidis 2011), and in the harms in children (Jefferson 2005a). Other studies failed to report sufficient data for inclusion in meta‐analysis (aa Cowling 2012).
Other potential sources of bias
Twenty‐five studies reported that written consent had been obtained from the parents of study participants (aa Belshe 1998; aa Belshe 2000a; aa Beutner 1979a; aa Clover 1991; aa Colombo 2001; aa Gruber 1990; aa Hoberman 2003a; aa Khan 1996; aa Rudenko 1993a; aa Rudenko 1996a; ab Belshe 1992; ab Gruber 1996; ab Gruber 1997; ab Gutman 1977; ab King 1998; ab Levine 1977; ab Slepushkin 1988; ab Steinhoff 1990; ab Steinhoff 1991; ab Swierkosz 1994; ab Wright 1976a; ba Hirota 1992; ca Kawai 2003; ca Maeda 2002; ca Maeda 2004a); another two studies refer to parental permission being granted (ab Desheva 2002; ca El'shina 2000); and one study refers to voluntary participation (cb Slepushkin 1994). Seven studies reported that the trial had received approval from a local review body (aa Beutner 1979a; aa Clover 1991; aa Gruber 1990; aa Hoberman 2003a; aa Rudenko 1993a; ab Slepushkin 1991; cb Slepushkin 1994).
The main problem we encountered in interpreting studies included in the 2007 update was that of high risk of bias: all included studies were poorly reported and contained either contradictions between data in figures, tables, and text, or reported implausible events or showed evidence of reporting bias of one sort or another. The two placebo‐controlled trials of cold‐adapted influenza vaccine (CAIV) reported only partial safety data, with data missing for up to a third of participants. The reporting format of both trials (which had the same sponsors) was similar and so were the inconsistencies, which suggests either a pre‐set format from the same sponsor or the presence of one or more ghost authors, or both.
We encountered similar problems in the 2011 update, especially in cohort studies and a specific type of case‐control study.
Effects of interventions
Summary of findings for the main comparison. Live attenuated influenza vaccine compared to placebo or do nothing for preventing influenza in children.
| Influenza vaccine compared to placebo or do nothing for preventing influenza in children | ||||||
| Patient or population: healthy, community‐dwelling children younger than 16 years of age Setting: influenza seasons in the USA, Western Europe, Russia, and Bangladesh Intervention: live attenuated influenza vaccine Comparison: placebo or do nothing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or do nothing | Risk with influenza vaccine | |||||
| Influenza assessed by laboratory confirmation Follow‐up over 1 or 2 influenza seasons |
Low | RR 0.22 (0.11 to 0.41) | 7718 (7 RCTs) | ⊕⊕⊕⊝ MODERATE2 3 | ||
| 130 per 10001 | 29 per 1000 (14 to 53) | |||||
| Moderate | ||||||
| 180 per 1000 | 40 per 1000 (20 to 74) | |||||
| High | ||||||
| 480 per 1000 | 106 per 1000 (53 to 197) | |||||
| Influenza‐like illness assessed by subjective report Follow‐up over a single influenza season |
Low | RR 0.69 (0.60 to 0.80) | 124,606 (7 RCTs) | ⊕⊕⊝⊝ LOW4 5 | ||
| 121 per 10001 | 83 per 1000 (73 to 97) | |||||
| Moderate | ||||||
| 172 per 1000 | 119 per 1000 (103 to 138) | |||||
| High | ||||||
| 341 per 1000 | 235 per 1000 (205 to 273) | |||||
| Otitis media assessed by clinical confirmation (inspection of ear and symptoms) Follow‐up over a single influenza season |
915 per 1000 | 897 per 1000 (869 to 924) |
RR 0.98 (0.95 to 1.01) | 1784 (1 RCT) |
⊕⊕⊕⊝ MODERATE6 | |
| Number of children with time off from school during follow‐up Follow‐up over a single influenza season |
Study population | RR 0.51 (0.22 to 1.19) | 296 (1 RCT) | ⊕⊝⊝⊝ VERY LOW7 8 | ||
| 100 per 1000 | 51 per 1000 (22 to 119) | |||||
| Number of parents having to take off work during follow‐up Follow‐up over a single influenza season |
Study population | RR 0.69 (0.46 to 1.03) | 2874 (2 RCTs) | ⊕⊕⊝⊝ LOW9 1 0 | ||
| 262 per 1000 | 181 per 1000 (121 to 270) | |||||
| Hospitalisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Fever assessed as reported increase in temperature | Temperature rise as an outcome was presented in most of the RCTs, with large differences among trials. Considering only studies reporting raw data for this outcome, the proportion of vaccinated children with fever ranged from 0.16% to 15%, while the proportion of children in the placebo groups with fever ranged from 0.71% to 22%. | ‐ | 20,561 (4 RCTs) |
⊕⊝⊝⊝ VERY LOW11 12 | ||
| Nausea ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Control group risks stratified for low‐, moderate‐, and high‐risk groups due to wide variation in event rates across the studies. 2Sensitivity analysis by risk of bias did not change the size or direction of effect. 3Downgraded one level due to serious inconsistency. Although direction of effect across the studies is consistent, there is variation in the magnitude of effect (I2 94%). Statistical variation could not be explained by age or dose. The reasons for wide variation in control group risk could be due to variable viral circulation, but this is uncertain. 4Downgraded one level due to serious risk of bias. Analysis dominated by studies at high or unclear risk of bias for multiple domains. We considered only one study as at low risk of bias. 5Downgraded one level due to serious inconsistency. Direction and magnitude of effect varies between the studies, and there is a high level of statistical heterogeneity (96%). 6Downgraded one level due to serious indirectness. Single study following children over a single influenza season with particularly high rates of otitis media. It is uncertain whether a rate of 92% would be expected over a similar time frame in other settings. 7Downgraded one level due to serious risk of bias. Single study at unclear or high risk of bias for most domains. 8Downgraded two levels due to serious imprecision. The number of events is low, and the confidence interval for the absolute effect is consistent with a small increase and small reduction in school absenteeism. 9Downgraded one level due to serious risk of bias. Of two data sets used from the eligible study, we judged one to be at high risk of bias. 10Downgraded due to serious inconsistency. Study results are discordant in terms of size of reduction in parental working days lost. 11Downgraded due to very serious inconsistency. Proportion of children with fever differed substantially between studies. 12Downgraded due to serious risk of bias. Studies at high or unclear risk of bias across several domains.
Summary of findings 2. Inactivated influenza vaccine compared to placebo or do nothing for preventing influenza in children.
| Inactivated influenza vaccine compared to placebo or do nothing for preventing influenza in children | ||||||
| Patient or population: healthy, community‐dwelling children older than 2 years of age Setting: influenza seasons in the USA Intervention: inactivated influenza vaccine Comparison: placebo or do nothing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or do nothing | Risk with influenza vaccine | |||||
| Influenza assessed by laboratory confirmation Follow‐up over a single influenza season |
Low | RR 0.36 (0.28 to 0.48) | 1628 (5 RCTs) | ⊕⊕⊕⊕ HIGH1 | ||
| 3 per 10001 | 1 per 1000 (1 to 2) | |||||
| Moderate | ||||||
| 298 per 1000 | 107 per 1000 (83 to 143) | |||||
| High | ||||||
| 481 per 1000 | 173 per 1000 (135 to 231) | |||||
| Influenza‐like illness assessed by subjective report Follow‐up over a single influenza season |
Low | RR 0.72 (0.65 to 0.79) | 19,044 (4 RCTs) | ⊕⊕⊕⊝ MODERATE2 | ||
| 134 per 10001 | 96 per 1000 (87 to 106) | |||||
| Moderate | ||||||
| 282 per 1000 | 203 per 1000 (183 to 223) | |||||
| High | ||||||
| 328 per 1000 | 236 per 1000 (213 to 259) | |||||
| Otitis media assessed by clinical confirmation (inspection of ear and symptoms) Follow‐up over a single influenza season |
271 per 1000 | 312 per 1000 (257 to 379) |
RR 1.15 (0.95 to 1.4) |
884 (3 RCTs) |
⊕⊕⊕⊝ MODERATE3 | |
| Absence from school Follow‐up over a single influenza season |
92 per 1000 | 42 per 1000 (16 to 112) |
RR 0.46 (0.17 to 1.22) |
254 (1 RCT) |
⊕⊝⊝⊝ VERY LOW4 5 | |
| Number of parents having to take off work during follow‐up ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Hospitalisations ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Fever ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Nausea ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Control group risks stratified by low, moderate, and high due to wide variation in event rates. 2Downgraded one level due to serious risk of bias. Analysis based on studies at high or unclear risk of bias for multiple domains. 3Downgraded one level due to serious imprecision. Confidence interval includes meaningful increase in otitis media with vaccination. 4Downgraded one level due to serious risk of bias. Study contributing data had multiple domains at unclear risk of bias. 5Downgraded two levels due to very serious imprecision. Low number of events and wide confidence interval.
Quantitative data synthesis
We constructed the following eight comparisons for our meta‐analysis.
Four comparisons included evidence from RCTs subgrouped by dose: live vaccines (comparison 01 (Analysis 1.1, Analysis 1.2, Analysis 1.3, Analysis 1.5, Analysis 1.6, Analysis 1.7)) and inactivated vaccines (comparison 02 (Analysis 2.1, Analysis 2.2)). We subgrouped these same studies by age in comparison 03 (Analysis 3.1, Analysis 3.2) and comparison 04 (Analysis 4.1, Analysis 4.2).
One comparison was constructed for all outcomes for all vaccine types versus placebo: comparison 05 (Analysis 5.1, Analysis 5.2, Analysis 5.3, Analysis 5.4, Analysis 5.5, Analysis 5.6, Analysis 5.7, Analysis 5.8, Analysis 5.9, Analysis 5.10, Analysis 5.11).
Data from non‐randomised studies have been retained but not updated since 2011, as follows: case‐control studies in comparison 6 (Analysis 6.1, Analysis 6.2, Analysis 6.3); cohort studies for live attenuated vaccines in comparison 07 (Analysis 7.1, Analysis 7.2); and inactivated vaccines in comparison 08 (Analysis 8.1, Analysis 8.2, Analysis 8.3).
1.1. Analysis.
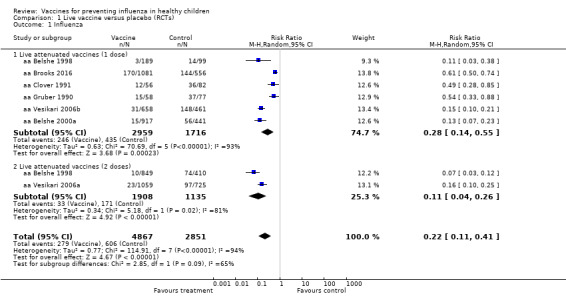
Comparison 1 Live vaccine versus placebo (RCTs), Outcome 1 Influenza.
1.2. Analysis.
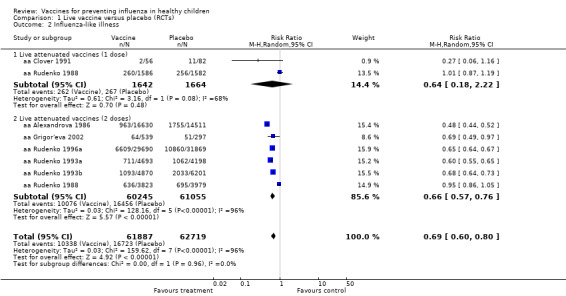
Comparison 1 Live vaccine versus placebo (RCTs), Outcome 2 Influenza‐like illness.
1.3. Analysis.
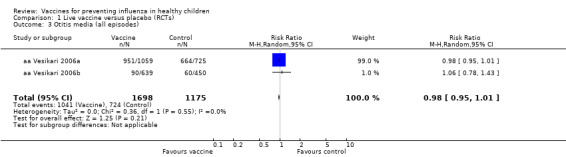
Comparison 1 Live vaccine versus placebo (RCTs), Outcome 3 Otitis media (all episodes).
1.5. Analysis.
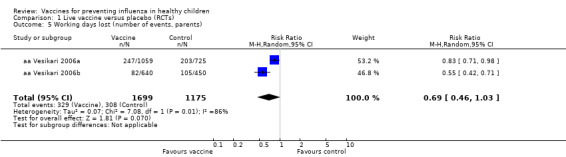
Comparison 1 Live vaccine versus placebo (RCTs), Outcome 5 Working days lost (number of events, parents).
1.6. Analysis.
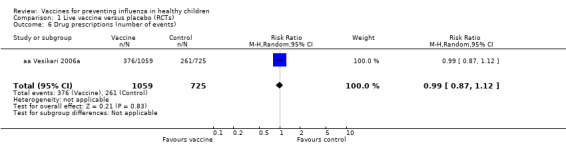
Comparison 1 Live vaccine versus placebo (RCTs), Outcome 6 Drug prescriptions (number of events).
1.7. Analysis.
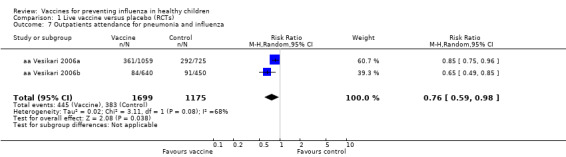
Comparison 1 Live vaccine versus placebo (RCTs), Outcome 7 Outpatients attendance for pneumonia and influenza.
2.1. Analysis.
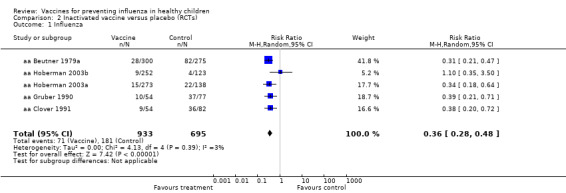
Comparison 2 Inactivated vaccine versus placebo (RCTs), Outcome 1 Influenza.
2.2. Analysis.
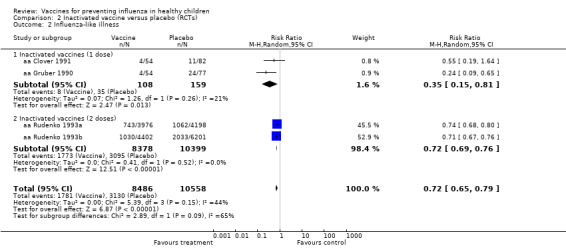
Comparison 2 Inactivated vaccine versus placebo (RCTs), Outcome 2 Influenza‐like illness.
3.1. Analysis.
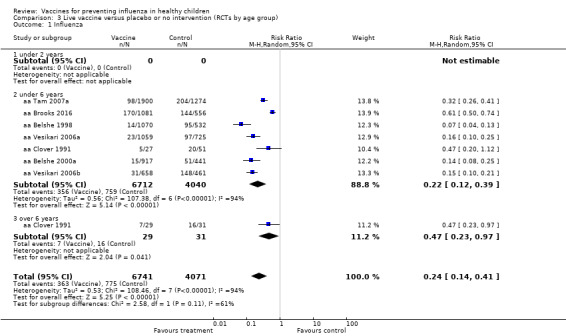
Comparison 3 Live vaccine versus placebo or no intervention (RCTs by age group), Outcome 1 Influenza.
3.2. Analysis.
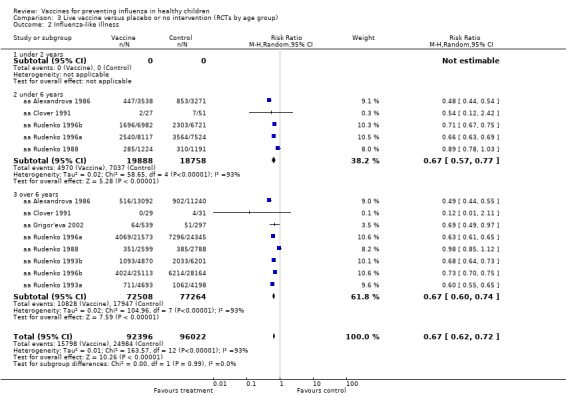
Comparison 3 Live vaccine versus placebo or no intervention (RCTs by age group), Outcome 2 Influenza‐like illness.
4.1. Analysis.
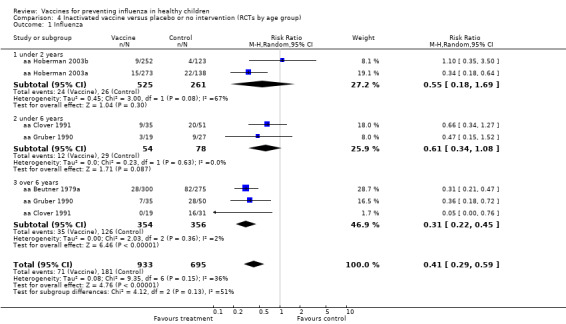
Comparison 4 Inactivated vaccine versus placebo or no intervention (RCTs by age group), Outcome 1 Influenza.
4.2. Analysis.
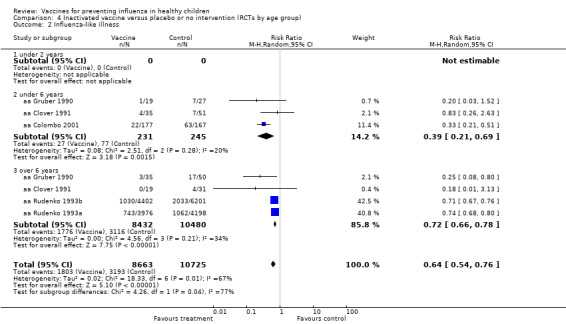
Comparison 4 Inactivated vaccine versus placebo or no intervention (RCTs by age group), Outcome 2 Influenza‐like illness.
5.1. Analysis.
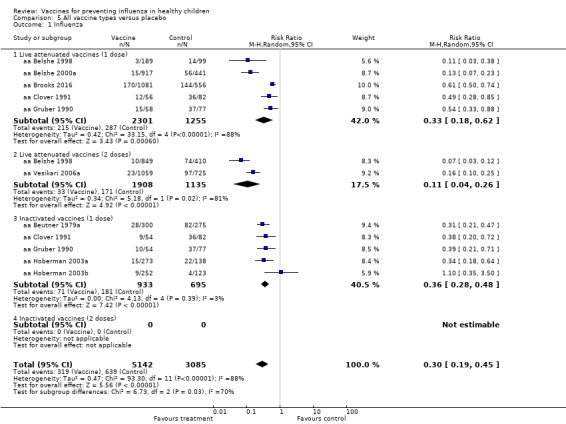
Comparison 5 All vaccine types versus placebo, Outcome 1 Influenza.
5.2. Analysis.
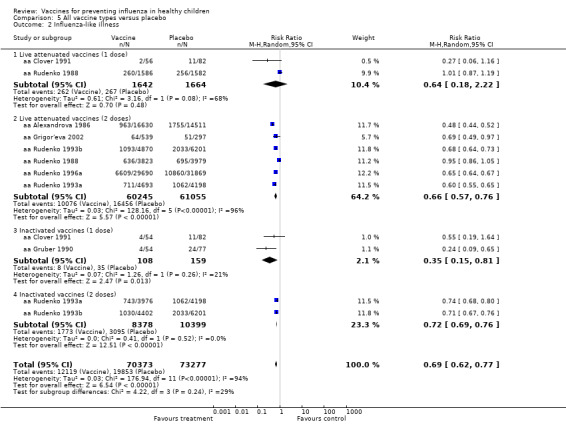
Comparison 5 All vaccine types versus placebo, Outcome 2 Influenza‐like illness.
5.3. Analysis.
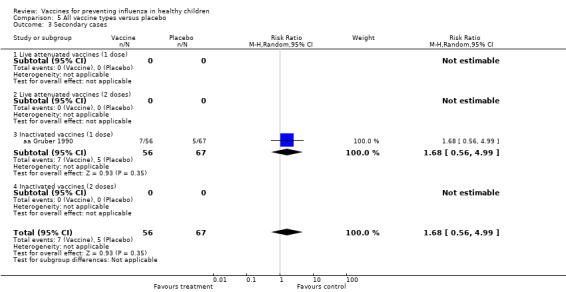
Comparison 5 All vaccine types versus placebo, Outcome 3 Secondary cases.
5.4. Analysis.
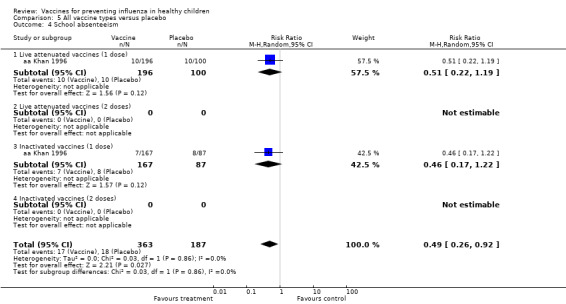
Comparison 5 All vaccine types versus placebo, Outcome 4 School absenteeism.
5.5. Analysis.
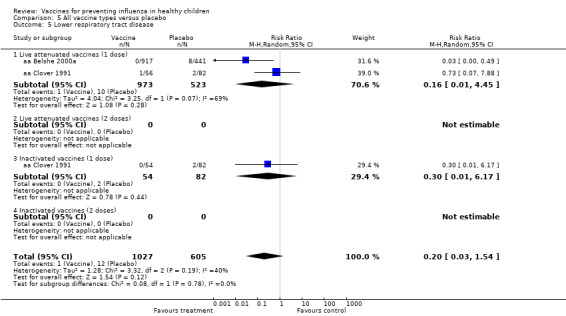
Comparison 5 All vaccine types versus placebo, Outcome 5 Lower respiratory tract disease.
5.6. Analysis.
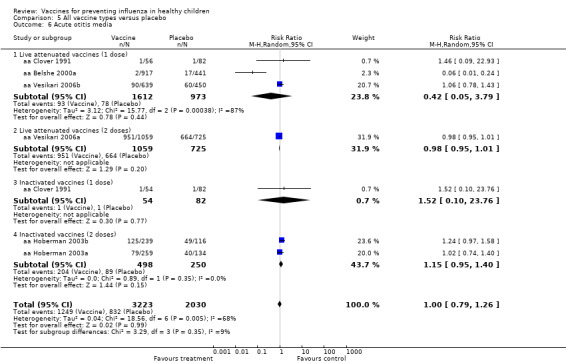
Comparison 5 All vaccine types versus placebo, Outcome 6 Acute otitis media.
5.7. Analysis.
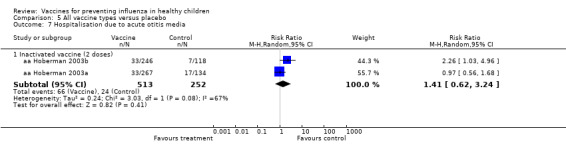
Comparison 5 All vaccine types versus placebo, Outcome 7 Hospitalisation due to acute otitis media.
5.8. Analysis.
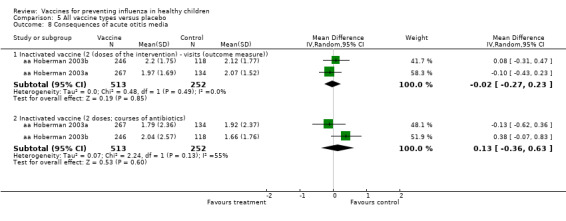
Comparison 5 All vaccine types versus placebo, Outcome 8 Consequences of acute otitis media.
5.9. Analysis.
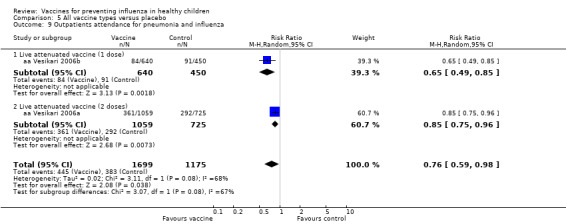
Comparison 5 All vaccine types versus placebo, Outcome 9 Outpatients attendance for pneumonia and influenza.
5.10. Analysis.
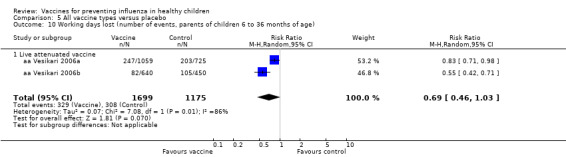
Comparison 5 All vaccine types versus placebo, Outcome 10 Working days lost (number of events, parents of children 6 to 36 months of age).
5.11. Analysis.
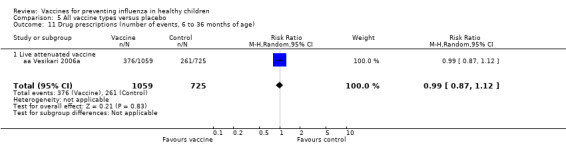
Comparison 5 All vaccine types versus placebo, Outcome 11 Drug prescriptions (number of events, 6 to 36 months of age).
6.1. Analysis.
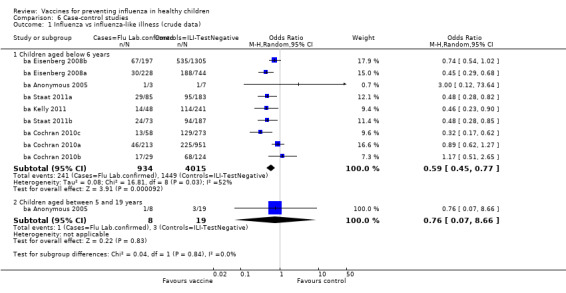
Comparison 6 Case‐control studies, Outcome 1 Influenza vs influenza‐like illness (crude data).
6.2. Analysis.
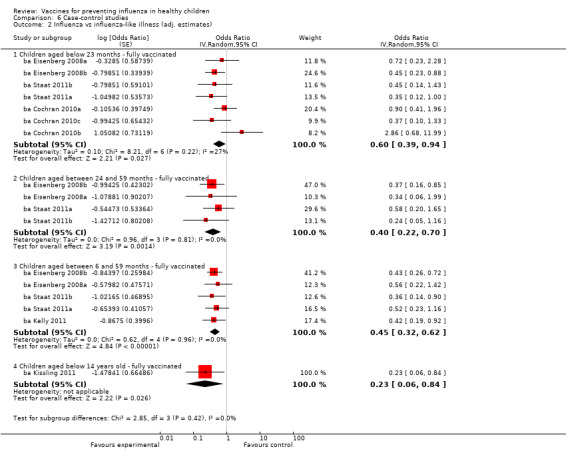
Comparison 6 Case‐control studies, Outcome 2 Influenza vs influenza‐like illness (adj. estimates).
6.3. Analysis.
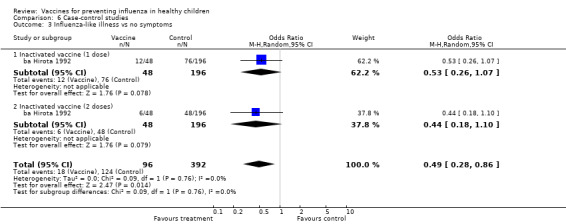
Comparison 6 Case‐control studies, Outcome 3 Influenza‐like illness vs no symptoms.
7.1. Analysis.
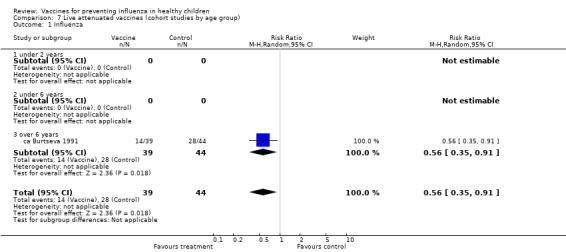
Comparison 7 Live attenuated vaccines (cohort studies by age group), Outcome 1 Influenza.
7.2. Analysis.
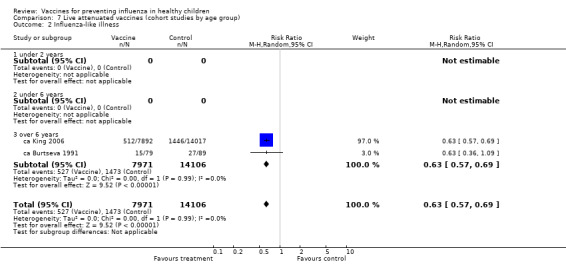
Comparison 7 Live attenuated vaccines (cohort studies by age group), Outcome 2 Influenza‐like illness.
8.1. Analysis.
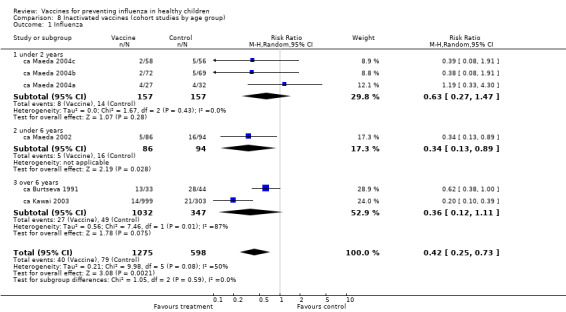
Comparison 8 Inactivated vaccines (cohort studies by age group), Outcome 1 Influenza.
8.2. Analysis.
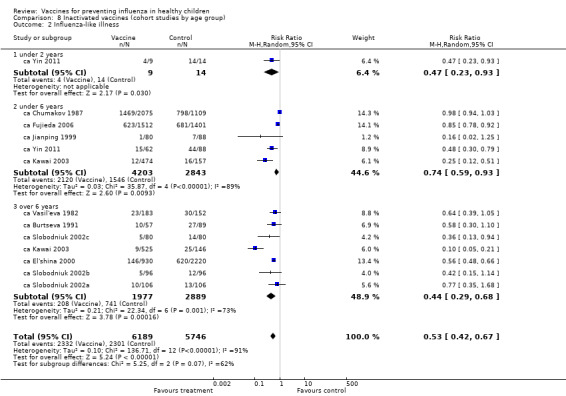
Comparison 8 Inactivated vaccines (cohort studies by age group), Outcome 2 Influenza‐like illness.
8.3. Analysis.
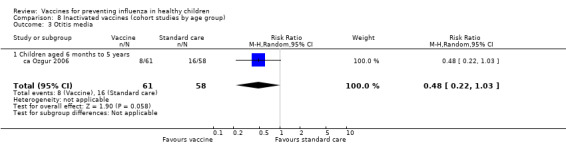
Comparison 8 Inactivated vaccines (cohort studies by age group), Outcome 3 Otitis media.
All comparators were placebo or do nothing, and comparisons 03,04,06, and 07 were stratified by available age groups, that is under two years, under six years, and over six years, and by type of outcome.
The comparisons with influenza as an outcome (Analysis 1.1 and Analysis 7.1 for live vaccines; Analysis 2.1 and Analysis 8.1 for inactivated vaccines) therefore summarise the evidence of vaccine efficacy. The comparisons with ILI as an outcome (Analysis 3.2 and Analysis 7.2 for live vaccines; Analysis 4.2 and Analysis 8.2 for inactivated vaccines) summarise vaccine effectiveness.
Comparison 05 (Analysis 5.3 to Analysis 5.8) (for placebo‐controlled trials) included data for rare outcomes (secondary cases, school absences, lower respiratory tract infection, acute otitis media and other consequences and socioeconomic impact). Due to scarcity of data (most outcomes were reported by one or two studies only), no age stratification was possible for these outcomes.
Comparisons showing vaccine efficacy (protection against influenza)
Comparison 01 (Analysis 1.1, evidence from RCTs) shows that live attenuated vaccines probably have 78% overall efficacy (risk ratio (RR) 0.22, 95% confidence interval (CI) 0.11 to 0.41; 7718 children; moderate‐certainty evidence). One study on 1632 children aged 15 to 71 months did report differences in incidence of influenza in 1‐year‐olds of 17% and 86% and for 2‐year‐olds of 24% and 96% for placebo and vaccination arms, respectively (aa Belshe 1998). We have presented these figures in the Discussion section, but in the absence of an age breakdown, could not include these data in the meta‐analysis. One study recruiting 3200 children aged between 6 to 36 months reported vaccine efficacy data that were consistent with those from our own analyses (73.5% in aa Bracco Neto 2009a).
Based on the median control group risk of 18% in children aged 3 to 16 years, live attenuated vaccination reduces the proportion of children with influenza to 4% (risk difference (RD) ‐14%, 95% CI ‐16% to ‐12%). At least seven children over the age of two need to be vaccinated to avoid one case of influenza (i.e. one set of symptoms with one infection). Risk differences for low and high control group risks yield numbers needed to vaccinate (NNVs) of 9 and 3, respectively. See Table 1.
Comparison 02 (Analysis 2.1, evidence from RCTs) shows that inactivated vaccines have a vaccine efficacy (VE) of 64% (RR 0.36, 95% CI 0.28 to 0.48; 1628 children; high‐certainty evidence). In one small study in children aged two or younger, there was not enough information about the vaccines to determine efficacy of vaccination (RR 0.55, 95% CI 0.18 to 1.69). The findings are consistent with results from other studies, but the 95% CI is wide and includes meaningful differences with either strategy.
Based on the median control group risk of 30% in children aged 2 to 16 years, inactivated vaccination reduces the proportion of children with influenza to 11% (RD ‐19%, 95% CI ‐21% to ‐15%). At least five children over the age of two need to be vaccinated to avoid one case of influenza (i.e. one set of symptoms with one infection). Risk differences for low and high control group risks yield NNVs of 50 and 3, respectively. See Table 2.
Comparison 07 (Analysis 7.1, evidence from cohort studies) shows that live attenuated vaccines have 44% VE (RR 0.56, 95% CI 0.35 to 0.91), although this observation is based on a single, small study in children aged over six years (ca Burtseva 1991).
Comparison 08 (Analysis 8.1, evidence from cohort studies) shows that inactivated vaccines have 64% VE (RR 0.36, 95% CI 0.12 to 1.11) in the over‐six‐years age group; 66% VE (RR 0.34, 95% CI 0.13 to 0.89) in children up to six years of age; and are no better than placebo in children aged below two years (RR 0.63, 95% CI 0.27 to 1.47).
Comparisons showing vaccines' effectiveness
Comparison 01 (Analysis 1.2, evidence from RCTs) shows that live attenuated vaccines may have 31% overall effectiveness (RR 0.69, 95% CI 0.6 to 0.80; 124,606 children; low‐certainty evidence), but we could find no evidence for children aged two years or younger.
Compared with the median control group risk of 17% in children aged 3 to 16 years, live attenuated vaccination reduces the proportion of children with ILI to 12% (RD ‐5%, 95% CI ‐7% to ‐4%). At least 20 children over the age of three need to be vaccinated to avoid one case of ILI (i.e. one set of symptoms). Risk differences for low and high control group risks yield NNVs of 25 and 10, respectively. See Table 1.
Comparison 02 (Analysis 2.2, evidence from RCTs) shows that inactivated vaccines probably have 28% overall effectiveness (RR 0.72, 95% CI 0.65 to 0.79; 19,044 children; moderate‐certainty evidence). We could find no evidence for children aged two years or younger.
Compared with the median control group risk of 28% in children aged 3 to 16 years, inactivated vaccination reduces the proportion of children with ILI to 20% (RD ‐8%, 95% CI ‐10% to ‐6%). At least 12 children over the age of three need to be vaccinated to avoid one case of ILI (i.e. one set of symptoms). Risk differences for low and high control group risks yield NNVs of 25 and 11, respectively. See Table 2.
Comparison 07 (Analysis 7.2, evidence from cohort studies) shows that live attenuated vaccines are 37% effective in the over‐five‐years age group (RR 0.63, 95% CI 0.57 to 0.69).
Comparison 08 (Analysis 8.2, evidence from cohort studies) shows that inactivated vaccines have overall 47% effectiveness (RR 0.53, 95% CI 0.42 to 0.67). Data from a very small single study report an effectiveness of 53% in children under the age of two (ca Yin 2011). Effectiveness in children aged up to six years (RR 0.74, 95% CI 0.59 to 0.93) is just statistically significant. However, this must be interpreted with caution because the sizeable decrease in RR since the 2005 version of our review is due to the inclusion of a large cohort study at high risk of bias (ca Fujieda 2006). Inactivated vaccines were more effective (VE = 56%) (RR 0.44, 95% CI 0.29 to 0.68) in children aged six years or older.
Evidence on rarer outcomes
There are uncertain effects of live attenuated vaccines on the number of children who were absent from school (RR 0.51, 0.22 to 1.19; Analysis 1.4) and the number of parents taking leave to care for their children (RR 0.69, 95% CI 0.46 to 1.03; 2874 children; 2 studies; Analysis 1.5), the evidence for both outcomes of low certainty.
1.4. Analysis.
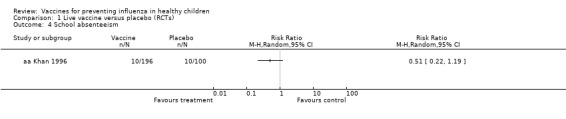
Comparison 1 Live vaccine versus placebo (RCTs), Outcome 4 School absenteeism.
Comparison 05 (Analysis 5.3 to Analysis 5.11) assessed evidence from RCTs on rare outcomes. Vaccines reduced school absence compared with placebo (RR 0.49, 95% CI 0.26 to 0.92) or standard care (RR 0.14, 95% CI 0.07 to 0.27). Both observations are based on single studies (aa Colombo 2001; aa Khan 1996). A third trial reported lower average school days missed by vaccinated children (mean difference ‐4.23, 95% CI ‐6.81 to ‐1.65) (aa Principi 2003). A trial showed a significant effect of trivalent, cold‐adapted influenza vaccine against outpatients' attendance for pneumonia and influenza (odds ratio (OR) 0.60, 95% CI 0.43 to 0.82) and parents' working days lost (OR 0.62, 95% CI 0.39 to 1.00) (aa Vesikari 2006a). The effects on all other outcomes (secondary cases, lower respiratory tract disease, drug prescriptions, acute otitis media and other consequences and socioeconomic impact) were not significantly different from those of placebo or standard care. According to one possible cohort study at high risk of bias (ca Ozgur 2006), inactivated vaccines do not reduce the risk of acute otitis media (although this may be due to the small denominator of 119). Virosomal vaccines (engineered to resemble the antigenic structure of the influenza virus) reduce antibiotic consumption (OR 0.77, 95% CI 0.61 to 0.98), school absenteeism (OR 0.42, 95% CI 0.34 to 0.51), and work absenteeism (OR 0.69, 95% CI 0.51 to 0.93). These observations must be interpreted with caution as they are based on a single cohort study at high risk of bias (ca Salleras 2006).
For completeness we have summarised the available evidence of efficacy and effectiveness from intra‐epidemic and non‐typical studies in Table 6.
In the case‐control study testing the effectiveness against ILI of an inactivated vaccine during an outbreak in 803 children aged 6 to 12 years (comparison 06, Analysis 6.3) (ba Hirota 1992), the vaccine was well matched antigenically to the circulating strain. Its administration was inversely associated with risk of severe ILI but not with mild ILI (no ORs are reported).
The case‐control study testing the effect against laboratory‐confirmed influenza of inactivated influenza vaccine in children aged below six years showed OR 0.59, 95% CI 0.45 to 0.77, 41% VE, 95% CI (23% to 55%) in comparison 06 (Analysis 6.1), performed with crude data.
In comparison 06 (Analysis 6.2), which was performed with adjusted estimates, the OR in children below 23 months was 0.60, 95% CI 0.39 to 0.94, 40% VE; in children between 24 and 59 months (OR 0.40, 95% CI 0.22 to 0.70), 60% VE; in children 6 to 59 months (OR 0.45, 95% CI 0.32 to 0.62), 55% VE; and in children below 14 years (OR 0.23, 95% CI 0.06 to 0.84), 77% VE.
Evidence on number of doses
There was no evidence of a subgroup effect between studies efficacy of one‐ and two‐dose schedules of live attenuated vaccines versus placebo. Results appear to favour the two‐dose schedule: 77% efficacy (RR 0.33, 95% CI 0.18 to 0.62) (aa Belshe 1998; aa Belshe 2000a; aa Brooks 2016; aa Clover 1991; aa Gruber 1990), compared with 89% efficacy (RR 0.11, 95% CI 0.04 to 0.26), although this estimate is based on two two‐dose studies only (aa Belshe 1998; aa Vesikari 2006a). All inactivated vaccine trials were conducted using a one‐dose schedule. The one‐dose virosomal vaccine was both efficacious and effective in children aged 3 to 14 years (RR 0.11, 95% CI 0.03 to 0.49) and (RR 0.26, 95% CI 0.17 to 0.60). However, these observations must be interpreted with caution, as they are based on a single cohort study at high risk of bias (ca Salleras 2006).
Sensitivity analysis
Sensitivity analysis based on assumed intracluster correlation coefficients to recalculate effective sample sizes in cluster studies where schools were the unit of allocation did not change the direction, size, or precision of effect for either influenza or ILI (see Analysis 1.8 and Analysis 2.5).
1.8. Analysis.
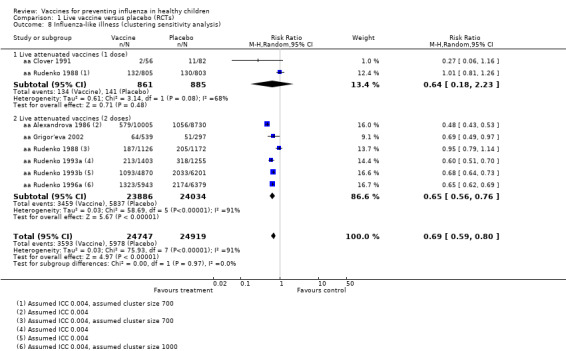
Comparison 1 Live vaccine versus placebo (RCTs), Outcome 8 Influenza‐like illness (clustering sensitivity analysis).
2.5. Analysis.
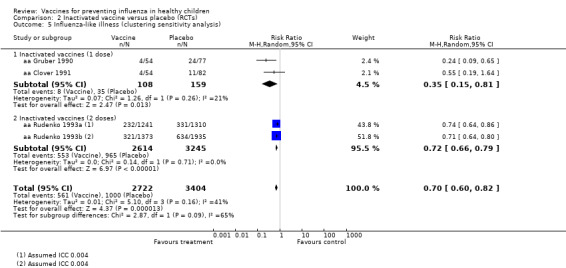
Comparison 2 Inactivated vaccine versus placebo (RCTs), Outcome 5 Influenza‐like illness (clustering sensitivity analysis).
Pooling all age data did not change our conclusions. Exclusion of evidence from Russian studies had the effect of making some of the comparisons not significant and depopulating single‐study comparisons, but did not materially affect our conclusions. However, we have no reason to believe that vaccines produced in Russia perform differently from vaccines produced elsewhere. The only study directly comparing the effectiveness of trivalent inactivated split‐virus vaccine (Wyeth‐Ayerst) with trivalent live attenuated, cold‐adapted influenza vaccine (Odessa Production Company for Biological Products; Odessa, Ukraine) with placebo on school absences failed to show any significant difference in performance (aa Khan 1996).
Table 4 shows the results of the stepwise sensitivity analysis excluding Russian/USSR studies. All comparisons except Analysis 3.1 and Analysis 3.2 (influenza and ILI in live vaccine trials) were sensitive to the exclusion of evidence from Russian/USSR studies. For comparison Analysis 3.2, exclusion of seven independent data sets made the effectiveness estimate non‐significant in children older than six years but enhanced the total effectiveness from 33% to 88%. For comparison Analysis 4.2, effectiveness estimates for children older than six years were not significantly affected but were increased from 28% to 76%. Comparisons Analysis 7.1 and Analysis 7.2 were depopulated by the removal of the one data set in each group. For comparison Analysis 8.1, the non‐significant 64% estimate for children older than six years became significant (80%), whereas for comparison Analysis 8.2, the estimates for children older than six years (56%) remained significant but increased in size.
Table 5 reports the results of the sensitivity analysis performed excluding studies (data sets) at high risk of bias. The results of Analysis 3.1 and Analysis 3.2 were sensitive to exclusion of the data sets at high risk of bias. However, these did not alter the results on vaccine efficacy or effectiveness described in the review. Analysis 4.1 does not include evidence from studies at high risk of bias, and its results are unchanged. Analysis 4.2 is sensitive to exclusion of evidence from studies at high risk of bias. Specifically, evidence of effectiveness in children under six years becomes not statistically significant with a VE reduction from 61% to 48%. In children over six years of age, exclusion of data sets at high risk of bias increased VE from 28% to 76%, and overall VE increased from 36% to 61%. Analysis 7.1, Analysis 7.2, Analysis 8.1, and Analysis 8.2 are depopulated because data for this comparison came from studies at high risk of bias only. Evidence from Analysis 1.1 to Analysis 6.2 is sensitive to exclusion of studies at high risk of bias, but this did not alter the conclusions. Evidence from Analysis 6.3 disappears. Evidence from Analysis 5.1 to Analysis 5.11 is sensitive to exclusion of studies at high risk of bias, but this did not alter the conclusions of the review.
Safety studies
Adverse events
In previous versions of the review we provided extensive documentation of the loss of evidence due to differing definitions and reporting formats of harms, chiefly local adverse events. For simplicity and to ease reading, we deleted the tables.
Randomised controlled trials
Twenty‐nine studies presented data on the safety of live influenza vaccines in children aged 2 months to 17 years old (aa Bracco Neto 2009a; ab Mallory 2010; Alexandrova 1986; Belshe 1992; Belshe 1998; Belshe 2000a; Beutner 1979a; Desheva 2002; Grigor'eva 1994; Grigor'eva 2002; Gruber 1990; Gruber 1996; Gruber 1997; Khan 1996; King 1998; Obrosova‐Serova 1990; Piedra 2002a; Rudenko 1988; Rudenko 1991; Rudenko 1993a; Rudenko 1996a; Slepushkin 1991; Slepushkin 1994; Steinhoff 1990; Swierkosz 1994; Tam 2007; Vesikari 2006a; Vesikari 2006b; Zangwill 2001).
Eight studies presented safety data for inactivated vaccines in children aged 6 months to 18 years old (ab Plennevaux 2011; Gruber 1990; Gutman 1977; Khan 1996; Levine 1977; Slepushkin 1991; Vasil'eva 1988a; Wright 1976a), and one paper, El'shina 2000, contained an RCT of short‐term safety data (≤ 5 days) and a cohort study of long‐term safety data (≤ 5 months).
Most of the RCTs presented temperature rise as an outcome, with large differences among trials. Considering only studies reporting raw data on this outcome, the proportion of vaccinated children with fever ranged from 0.16%, in Rudenko 1993a, to 15% (Belshe 1998), while in the placebo groups this proportion ranged from 0.71%, in Rudenko 1993a, to 22% (Gruber 1996).
Three studies reported raw data for nasal congestion (Belshe 1998; Belshe 2000a; Gruber 1996). Studies conducted by Belshe assessed safety of cold‐adapted trivalent influenza vaccine, while the study by Gruber and colleagues assessed live attenuated vaccine. The proportion of vaccinated children with nasal congestion ranged from 19%, in Belshe 2000a, to 78% (Gruber 1996), while in the control group this proportion ranged from 14%, in Belshe 2000a, to 68% (Gruber 1996).
Belshe 1992 reported data on upper respiratory tract infections: in the vaccinated arms the proportion of children affected ranged from 53% to 70%, while in the placebo group this outcome was reported in 47% of children.
aa Bracco Neto 2009a and aa Bracco Neto 2009b (one study run over two seasons) reported a significant difference in the rate of bronchitis between live attenuated influenza vaccine (LAIV) and saline placebo recipients (3.1% and 1.6%, respectively; P = 0.046), while the incidence of bronchospasm was also similar between groups (1.8% and 1.5%, respectively).
ab Mallory 2010 reported headache as the most common solicited symptom in children receiving H1N1 LAIV through day 8 after dose one, which was reported by 16.6% and 15.4% of H1N1 LAIV and placebo recipients respectively, rate difference 1.2% (95% CI –10.2% to 10.2).
Three RCTs included data on reactions to live vaccine within six weeks of inoculation (short‐term outcomes). Belshe 1998 included serious adverse events up to 42 days after vaccination. From the same trial, Piedra 2002a (see aa Belshe 1998) included the following outcomes between 11 and 42 days after vaccination: afebrile illness, analgesic/antipyretic use, antihistamine/decongestant/antitussive use, febrile illness, febrile otitis media, lower respiratory tract infection, oral antibiotics use, and otitis media. In the ab Plennevaux 2011 study, within 21 days since the last of the two injections a range of 42% to 55% of children in each age and vaccine group experienced unsolicited adverse events, considered by the investigator to not be vaccine related in most cases. ab Mallory 2010 reported adverse events during days 1 to 15 after doses one and two. Adverse events after dose one were reported in 18.1% and 16.9% of H1N1 LAIV and placebo recipients respectively, and in 13.7% and 14.3% after dose two. The most common adverse events in children after dose one were nausea (1.9% versus 3.1%), vomiting (2.7% versus 1.5%), and diarrhoea (1.5% versus 1.5%).
For longer‐term outcomes, Belshe 1998 included vaccine‐related serious adverse events within 102 days of inoculation. Three RCTs included safety outcome followed up for six months after inoculation. Desheva 2002 included three outcomes: allergies, infections (excluding influenza and acute respiratory infections), and other somatic illnesses. Rudenko 1988 included only morbidity (excluding influenza and acute respiratory infections). Rudenko 1996a evaluated 13 outcomes including allergies and five respiratory tract disease outcomes.
Seven RCTs reported data on short‐term outcomes following inoculation with inactivated vaccines. In particular, two RCTs reported data on erythema, swelling, and induration (Beutner 1979a; Wright 1976a); two other studies reported data on pain/tenderness (Beutner 1979a; Gruber 1990); and one study reported data on infiltration and hyperaemia (Vasil'eva 1988a).
One RCT presented safety outcomes data up to six months after inoculation of inactivated vaccine (15 outcomes) (Vasil'eva 1988a).
Observational studies
Three cohort studies presented safety data for inactivated vaccines in children aged 12 months to 18 years old (Slepushkin 1994; Vasil'eva 1982; Vasil'eva 1988b), and 1 paper, El'shina 2000, contained an RCT of short‐term safety data (≤ 5 days) and a cohort study of long‐term safety data (≤ 5 months).
One cohort study compared the reactogenicity and immunogenicity of live bivalent or trivalent vaccines and inactivated bivalent and trivalent vaccines in 1817 children in 3 cohorts between 1989 and 1991 (Slepushkin 1994). Reactions to the vaccines were studied for five days after vaccination. A temperature of 37.5 °C was considered a weak reaction, and from 37.6 to 38.5 °C a severe reaction. When a trivalent vaccine was administered subcutaneously to children aged 11 to 14 years in 1990, temperature reactions were recorded in 2.6% of participants, moderate local reactions in 3.2%, and severe local reaction in 0.7%. Consequently, the intramuscular route was used for the 7‐to‐10‐years age group where a lower frequency of reactions was recorded. In 1991, the inactive vaccine caused moderate temperature reactions (37.6 °C to 38.5 °C) in 1.3% of the participants and moderate local reactions (26 to 50 mm hyperaemia) in 4.4% of the participants.
Vasil'eva 1982 reported safety data of 335 children aged 7 to 15 years vaccinated with inactivated influenza vaccine. The children were monitored for reactions by daily physical examination for five days following inoculation. The outcomes of temperature, headache or malaise, sore throat, and local reactions (hyperaemia or cutaneous wheal) were recorded. Mild fever (37.0 °C to 37.5 °C) was observed in 20% to 25% of children aged 7 to 10 years and in 8% to 12% of children aged 11 to 15 years. Isolated cases of moderate and severe fever, that is above 37.6 °C, were recorded in all groups. There were no statistical differences in systemic reactions between vaccine and placebo, between age groups, or for method of administration. Local reactions were most frequent in children aged 11 to 15 years vaccinated with a syringe; 26.5% of children from this subgroup showed moderate reactions (26 to 50 mm hyperaemia).
El'shina 2000 reported long‐term safety data. The outcomes of cardiovascular illnesses, upper respiratory tract infection, illnesses of the stomach and intestines, skin diseases, allergies, and infectious illnesses were presented. There were no statistically significant differences between groups for the above safety outcomes. Incidences were rare, and there was no difference between vaccinated and unvaccinated groups.
Vasil'eva 1988b assessed the safety of multiple immunisations of an inactivated bivalent influenza A vaccine in 12,643 children aged 11 to 14 years. All children were followed up for 30 days after inoculation to determine the frequency of requests for urgent medical attention and of hospitalisation. The safety outcomes presented were increase in temperature, local reactions, and intoxication/catarrh in the nasopharynx. The frequency of weak temperature reactions (< 37.5 °C) varied from 6.6% to 37.9% in vaccinated groups and 2.9% to 29.0% in placebo groups. Moderate temperature reactions occurred in isolated cases; the maximum frequency was 1.9% in children vaccinated four times, who also showed the highest frequency of headaches and catarrh (11.1%). However, there were no statistically significant differences between vaccine and placebo groups. There was some increase in local reactions with an increase in number of inoculations (the percentage rising from 0.9% after one inoculation, 1.1% after three inoculations, and 1.9% after four inoculations), but these were not significantly different from responses in the placebo groups. No severe general or local reactions were observed in any child.
Serious adverse events
Three RCTs, aa Bracco Neto 2009a, ab Mallory 2010, and ab Plennevaux 2011, and one case‐control study, Goodman 2006, reported safety data on serious adverse events.
aa Bracco Neto 2009a reported in the first year of the study one or more serious adverse events in 5.0% of LAIV‐LAIV recipients, 3.8% of LAIV‐placebo recipients, 3.4% of excipient placebo recipients, and 4.1% of saline placebo recipients. During the second year of the study, 1.6% and 2.4% of LAIV and placebo recipients, respectively, reported one or more serious adverse event(s). Most of the events were respiratory. Twenty‐nine children experienced serious adverse events considered to be related to study product, the most frequent of which were pneumonia, bronchopneumonia, bronchiolitis, and bronchitis. Three deaths were reported: one was the result of Escherichia coli septicaemia diagnosed 18 days after receipt of the second dose of LAIV in year one, and 2 deaths were accidental. None of these cases was judged by the investigators to be related to the study product.
ab Plennevaux 2011 reported a maximum rate of 8% severe unsolicited events. In particular, for the age group 6 to 35 months, the proportion of children vaccinated with 7.5 µg haemagglutinin and 15 µg haemagglutinin vaccines who experienced severe adverse events was 5% and 6%, respectively, while no events occurred in the placebo group.
ab Mallory 2010 reported three serious adverse events in children during the study: hospitalisation for depression and osteomyelitis in vaccine recipients, and cellulitis in a placebo recipient; all were considered by the investigators to be unrelated to the study vaccine. One new onset chronic disease, attention deficit hyperactivity disorder, was reported in a placebo recipient.
The case‐control study assessing the safety of trivalent influenza vaccine (TIV) in 6‐ to 23‐months‐old children included in the 2007 update reported a series of outcomes identified by physicians combing the exposed population for possible outcomes of interest and then clustering the diagnosis by International Classification of Diseases (ICD) categories and then using Vaccine Safety Datalink (VSD) categories (Goodman 2006). This kind of data mining is not likely to clarify the safety profile of TIV.
The monovalent pandemic influenza vaccine Pandemrix (GSK) appears to be associated with the onset of narcolepsy and cataplexy in children. Current evidence does not support either a country‐specific spread or a lot‐related problem (cb MPA 2011; THL 2012).
Elsewere in the literature there were sparse reports of harms associated with particular brands of inactivated influenza vaccines. This was the case of the 2010 TIV by CSL Limited, which was used mainly in Australia. One child in every 110 aged below five who was vaccinated with the CSL vaccine had a febrile seizure. Australia consequently suspended its use. These episodes highlight the insufficient regulatory attention to potential harms from influenza vaccines in children, as the registration trials for the CSL vaccine had been carried out on 162 children aged up to three years (Collignon 2010).
This degree of scrutiny to identify all global cases of missed potential harms is beyond the resources of this review.
Discussion
Summary of main results
Our review shows that LAIVs have good relative efficacy (up to 80%), but lower relative effectiveness (around 33%) in children aged under 16 years and older than two years. Live attenuated influenza vaccines may be effective in controlling a school outbreak, although this observation is based on an old, poorly reported Russian study (ab Slepushkin 1974).
Inactivated vaccines have a lower relative efficacy (59%) than live attenuated vaccines. In children aged two years or younger, there is very limited evidence to determine their effects compared with placebo. This observation is based on a single small study (aa Hoberman 2003a). Under the age of six, TIV does not provide significant protection against influenza (has no significant efficacy). Below two years of age there is no evidence of effect of either LAIV or TIV.
Inactivated vaccines may reduce ILI by around 36% for children aged older than two years, but we could find no evidence for children aged two years or younger. Our conclusions on inactivated vaccines are based on almost 20,000 children from randomised studies.
Evidence from cohort studies (11,000 observations) yields higher estimates, suggesting that inactivated vaccines have higher (up to 64%) efficacy and effectiveness (56%) in the over‐six‐years age group; in children younger than two years of age, their efficacy is no better than that of a control arm, and there is evidence from a single study of 53% effectiveness. However, readers should bear in mind the very low quality of the cohort data sets.
The differences between efficacy and effectiveness of the vaccines are not surprising, as influenza vaccines are specifically targeted at influenza viruses and are not meant to prevent other causes of ILI.
We found little evidence for other outcomes. A trial at high risk of bias shows a significant effect of trivalent, cold‐adapted influenza vaccine against outpatients' attendance for pneumonia and influenza and parents' working days lost (aa Vesikari 2006a). Evidence for other outcomes (secondary cases, lower respiratory tract disease, drug prescriptions, acute otitis media and other consequences and socioeconomic impact) suggests no difference from placebo or standard care. However, these conclusions are based on single studies lacking statistical power, except for acute otitis media. Virosomal vaccines reduce antibiotic consumption and school and work absenteeism, but these observations are based on a single cohort study at high risk of bias (ca Salleras 2006).
Overall completeness and applicability of evidence
The studies included in our review assess the effects of vaccines over several decades. Our review has several potential limitations. We found insufficient data to allow us to draw firm conclusions on vaccination routes (intramuscular or intranasal) or one‐ or two‐dose schedules in inactivated vaccines. The evidence base for children younger than two years remains limited. We were unable to obtain dichotomous data from 1 study in 3200 children mainly younger than 2 years (mean age 20 months). aa Bracco Neto 2009a reports similar vaccine efficacy to our summary estimate. Most eligible studies report influenza and ILI outcomes, but the measurement and reporting of data for other key outcomes, notably otitis media, hospitalisation, and adverse effects, remain inconsistent or incomplete.
The small number of included studies in each comparison prevents a sufficiently powerful test to assess empirical evidence of publication bias. The only method to mitigate publication bias is to include published and (if retrievable) unpublished literature, regardless of language or country.
Decision‐makers looking to inform local or national policies with evidence summarised in this review should be aware of the wide variation in the NNVs we have calculated from the same risk ratios. Even where our results have shown consistent protective effects of vaccination, wide variation in control group risks point to additional sources of variation between settings that could explain why the absolute effects differ. This could be due to differences in between‐study follow‐up periods (the longer the follow‐up period, the more the potential for identification of cases with vaccine effectiveness dilution as viral circulation declines), differences in ILI case definitions (our sensitivity analysis failed to show significant differences in case definition specificity), differences in performance of different live vaccines (we have no reason to believe this is so), differences in case‐finding and in study quality, and differences in viral circulation levels. The included studies provided insufficient data to stratify for viral circulation or duration of follow‐up. Irrespective of the variation in absolute effects, our estimates point to high vaccine efficacy but lower effectiveness.
Quality of the evidence
We have made different downgrading decisions for influenza and ILI outcomes across the two vaccine types (Table 1; Table 2). We downgraded the evidence for live attenuated vaccine to moderate quality for influenza due to inconsistency and for ILI to low quality due to risk of bias and inconsistency. For ILI, the direction of effect was discordant across the studies. For influenza, we made the decision to downgrade for inconsistency despite the similar direction of effect across the studies. High between‐study variation of effect could not be explained by age or dose (Analysis 1.1; Analysis 2.1). The absence of an obvious relationship between control group risk and relative effect across the studies makes the variation in the absolute reduction a source of uncertainty. We downgraded the results for both absence from school and parental leave due to risk of bias. We further downgraded absence from school due to imprecision, and the discordant nature of the results for parental leave prompted us to downgrade further due to inconsistency. No data were available for hospitalisation or nausea. Variability in the reporting of fever prevented us from combining data. When taken together with risk of bias, we arrived at a rating of very low quality. The evidence for inactivated vaccines was high and moderate for influenza and ILI, respectively. The stability of the risk ratio for influenza gave us more confidence in its application to the variable control group risk across the studies. We considered bias a limitation only for ILI. Data for other outcomes were not available.
The general methodological quality of the included studies was poor. We found that description of vaccine content was variable, and no preservatives or excipients were reported. We could find no comment on the degree of matching between virus strains used in the studies, circulating strains, and composition of yearly World Health Organization (WHO) recommended vaccines. In healthy adults, antigenic composition is an important predictor of vaccine efficacy, as our Cochrane Review of influenza vaccines has shown (Jefferson 2010). The relative paucity of head‐to‐head comparisons of vaccines hinders meaningful comments on their relative performance and points to an absolute requirement for more direct‐comparison trials. Our 2005 decision to include non‐randomised evidence in the evaluation of efficacy/effectiveness resulted in the inclusion of a large number of studies of dubious quality. This is best observed in cohort studies. The majority of these are at high risk of bias, as are case‐control studies. We found several case‐control studies of similar design that claimed to be testing the effectiveness of inactivated influenza vaccines in a "real world setting" (e.g. ba Cochran 2010a; ba Eisenberg 2008a; ba Kelly 2011; ba Kissling 2011; ba Mahmud 2011; ba Staat 2011a; ba Valenciano 2011; ba Van Buynder 2010). The case test‐negative design is described in detail elsewhere (Foppa 2013; Valenciano 2012). Cases and controls were both selected on the basis of the presence of ILI symptoms. The discriminating variable between cases and controls was the positivity (for influenza) of a laboratory‐tested throat specimen. Consequently, this design does not test the effect of vaccine exposure on the onset of ILI. It tests the effect of the vaccines on microbiological specimens, which is of dubious public health significance.
The data included in a case test‐negative design are sometimes harvested from an ongoing networked surveillance cohort. In this case, the case test‐negative design is described as “nested” within the cohort. The source cohort can include community and hospitalised cases and controls, allowing flexibility (Kissling 2017). In Europe the surveillance programme has a formal structure and is known as Influenza ‐ Monitoring Vaccine Effectiveness (I‐MOVE). I‐MOVE is partly funded and co‐ordinated by the European Centre for Disease Prevention and Control.
Briefly, the study design that is similar to a case‐control design consists of selecting influenza cases (e.g. cases of ILI who have tested positive for influenza) and controls (cases of ILI who have tested negative) and calculating the relevant odds ratio (OR). Cases and controls are subsequently stratified by vaccination status. An estimate of vaccines' effectiveness (VE) is derived from the OR of influenza in vaccinated/unvaccinated participants using the standard formula VE = 1 ‐ OR%. The practice of using the OR estimate as an approximation of the risk ratio (RR) was first used in a 1980 study on pneumococcal vaccine (Broome 1980).
The design does not test field effectiveness, but the capacity of the vaccines to generate a negative polymerase chain reaction (PCR) result (what we would call laboratory efficacy). Both cases and controls are symptomatic, so any prevention is solely focused on the negativity of laboratory tests. In addition, no useful public health absolute measures of effect can be derived (such as absolute risk reduction and its reciprocal NNV) by such designs, as the background rates of infection and viral circulation are not part of the calculation of the estimates of effect. The mathematical method first used by Broome 1980 is correct if three key assumptions are met:
the risk of non‐influenza ILI is the same in vaccinated and non‐vaccinated individuals (a factor called “k” by Broome 1980);
the attack rate in the vaccinated population is low;
the circulating serotypes are similar to those in the selected population within the case‐control test‐negative design.
All assumptions are unlikely to be fulfilled at the same time, especially in multicentre/multi country surveillance cohorts with a non‐random sampling frame. For example, Cowling and colleagues reported an increased risk of non‐influenza respiratory virus infections associated with receipt of inactivated influenza vaccine. In addition, the OR will give falsely high VE if the attack rate in the vaccinated population is high (Cowling 2012; Orenstein 1985). Apart from these fundamental design problems, case test‐negative studies are also affected by poor reporting. (See also Perencevich 2013a; Perencevich 2013b).
Potential biases in the review process
We found a large data set showing variable quality evidence of vaccines' efficacy in children aged over three years.
As we had already observed in our Cochrane Review of influenza vaccines in healthy adults (Demicheli 2014), there is marked difference between the efficacy and effectiveness of the vaccines due to the large proportion of ILI ('the flu') caused by agents other than influenza viruses. This is an important point in the decision to vaccinate whole populations. In addition, we found limited evidence that vaccines reduce the burden of school absences. Decision‐makers' attention to the vaccination of very young children is not supported by the evidence summarised in our review. Although there is a growing body of evidence showing the impact of influenza on hospitalisations and deaths of children, at present we could find no convincing evidence that vaccines can reduce mortality, hospital admissions, serious complications, or community transmission of influenza.
We were surprised to find only one safety study of inactivated vaccine in children under 2 years, carried out nearly 30 years ago in 35 children (ab Wright 1976a). The lack of safety data for inactive vaccines in younger children is particularly surprising given that the inactive vaccine is now recommended for healthy children six months and older in the USA and Canada (AAPCID 2004; Harper 2004; Orr 2004). In contrast, while the live vaccine is only licenced for children aged 5 and older in the USA, we found 10 studies testing its safety in younger children. However, the manufacturers' refusal to release all safety outcome data from trials carried out in young children, together with obvious reporting bias and inconsistencies in the primary studies, does not bode well for a fair assessment of the safety of live attenuated vaccines.
We found a notable range and diversity of safety outcomes and definitions (or lack thereof) in the included studies, leading to a loss of data. This clearly demonstrates the difficulty of attempting to meta‐analyse safety data for a review when the data are not presented in a standardised format. The Brighton Collaboration, set up to facilitate the development, evaluation, and dissemination of high‐quality information about the safety of human vaccines, has produced guidelines on the recording and presentation of temperature and induration. The results of this search and review clearly show the need for the existence of such guidelines and their adoption by researchers worldwide.
Agreements and disagreements with other studies or reviews
The effects of influenza vaccination in those aged 16 or younger have recently been the topic of a number of other reviews. On the basis of their methods and the inclusion criteria adopted, we have classified the reviews into the following broad categories.
1) A first group of reviews that consider only studies with PCR‐confirmed or culture‐confirmed clinical cases as primary efficacy outcome (Ambrose 2012; Belshe 2010; Carter 2011; Osterholm 2012; Rhorer 2009). All of these reviews are focused on LAIV administration, but also include studies comparing LAIV with TIV.
Ambrose 2012 and Rhorer 2009 are based on the same evidence, including trials performed on populations aged between 6 months and 17 years. Rhorer 2009 includes one more placebo‐controlled trial than Ambrose 2012. In Ambrose 2012, the analysis is limited to the paediatric age group for which the vaccine is licenced (24 months or more at immunisation). Out of the nine included RCTs, six were placebo controlled (corresponding to nine data sets), whereas controls were immunised with TIV in the remaining three included trials (three data sets). Both reviews show a high relative efficacy of a two‐dose LAIV course against similar viral strains (about 80%) and a significantly lower efficacy of TIV.
The review by Osterholm 2012 includes evidence on both adult and paediatric populations. Included designs are either placebo‐controlled trials or case‐controlled studies. Considering only studies performed on paediatric populations, the placebo‐controlled RCTs are the same as those included in Rhorer 2009. A pooled meta‐analysis was carried out on the same data sets (apart from one study: aa Bracco Neto 2009a; aa Bracco Neto 2009b). Evidence from case‐control studies is also discussed (against seasonal or H1N1 pandemic influenza), even if it is not included in the analysis. Relative efficacy estimates of LAIV against PCR‐ or culture‐confirmed influenza in children aged six months to seven years was around 80%.
Carter 2011 reports results of studies on LAIV efficacy, reactogenicity, and immunogenicity in children and adult populations. Evidence of the vaccines’ efficacy in children (i.e. against influenza, as opposed to ILI) is the same as that included in Rhorer 2009 (with the exception of Forrest 2008). A meta‐analysis was not performed; only a descriptive review is presented. Estimates of LAIV efficacy in comparison with placebo or TIV are the same as that of the above mentioned studies. In contrast to the other reviews that included the study by Lum 2010 (Osterholm 2012; Rhorer 2009), Carter 2011 assessed the effect of measles, mumps, and rubella (MMR) vaccine co‐administration, concluding that this did not affect LAIV efficacy. The review also considers evidence on local and systemic reactions observed a few days after LAIV immunisation: runny nose, headache, and tiredness are likely to occur more frequently among LAIV than among placebo recipients, especially after the first dose. Live attenuated influenza vaccine is more reactogenic than TIV, and its administration is associated with an increased risk of wheezing within 42 days after vaccination in children younger than five years of age in comparison with TIV.
2) A second group of systematic reviews, Manzoli 2007 and Negri 2005, present several methodological analogies with our 2005 Cochrane Review; the exceptions are that only RCTs or CCTs are included; safety issues of the vaccines are not considered; and some included studies also had a vaccine control arm. The most recent review confirms that there are no significant differences between TIV and LAIV effects on the three assessed outcomes (influenza, ILI, and otitis media). A sensitivity analysis was performed excluding Russian studies (classified at a lower level of methodological quality) from a pooled analysis, which resulted in a higher estimate of effect against clinical disease (pooled estimates for LAIV and TIV). Rodrigo Pendas 2007 includes studies (seven RCTs) evaluating the efficacy of both LAIV and TIV in preventing ILI, confirmed influenza, otitis media, and other respiratory illnesses.
3) Only one review presented the effect of vaccination on the contacts of children (Jordan 2006). Results from this industry‐funded review including eight RCTs, three community studies, and three economic evaluations are discussed but a meta‐analysis is not performed. The authors conclude that child vaccination could produce significant health benefits and be cost‐saving to the community as a whole.
4) Two other reviews evaluated the immunogenicity of monovalent H1N1 pandemic influenza vaccines (in both adjuvanted and non‐adjuvanted formulations) (Manzoli 2011; Yin 2011). All tested split/subunit vaccines induced a satisfactory immunogenicity (over 70%) after only one dose in adolescents, while only non‐adjuvanted vaccines at high doses and oil‐in‐water adjuvanted vaccines showed acceptable results for children. Even if the rate of serious adverse events was low for all 2009 H1N1 vaccines (0.013% overall), the review does not allow a firm conclusion to be drawn for vaccine safety at the population level. Mild to moderate adverse reactions were more frequent for oil‐in‐water adjuvanted vaccines. Wijnans 2011 reviews several studies (clinical trials, case reports, results of surveillance) reporting safety data of monovalent H1N1 pandemic vaccine.
The key question of the relationship between a surrogate outcome (antibody production) and field outcomes (clinical illness) is left unaddressed, calling into question the rationale for applying results from the reviews in deciding vaccination policies.
5) One other review is based on evidence available in the Cochrane Library only (11 Cochrane Reviews; one other review/meta‐analysis; 14 RCTs; 3 CCTs) (Michiels 2011). The review provides a critical approach to the opportunity to administer inactivated influenza vaccine to children, adults, and the elderly, but also to individuals affected by comorbidity conditions (diabetes, chronic lung disease, cardiovascular disease, kidney or liver disease, and immune suppression). Inactivated influenza vaccines appear to be effective in healthy adults and children over six years but not in children younger than two years and institutionalised elderly. Inconsistent results are found in studies in children younger than six years, individuals with chronic obstructive airways disease, institutionalised elderly, elderly with comorbidity, and healthcare workers in elderly homes. Vaccination of children might be protective in non‐recipients of all ages living in the same community. The vaccination of pregnant women might be beneficial for their newborns.
Despite the great variations in methods, the reviews all reached similar conclusions to those of our 2005 Cochrane Review: TIV has few effects, and there is no evidence that it affects deaths, complications, or transmission of influenza. Live attenuated influenza vaccine performed a little better at the expense of safety.
Most reviews present estimates of vaccine efficacy derived from studies in which principal outcome measures are 'confirmed' cases of influenza, that is with laboratory confirmation of infection. The reviews express results in relative terms (RR) (i.e. regardless of the level of influenza viruses circulating in the study population). The relative efficacy measure represents the capacity of the vaccine to prevent cases specifically due to virus strains contained in the vaccine compared to the control. When used in vaccination campaigns, the same vaccines are unlikely to prevent the same proportion of cases simply because most ILI cases are attributable to other viruses. To assess the real benefit produced by a campaign, it would be preferable to also look at the impact of the vaccines on ILI and to present results using absolute measure such as RDs and their reciprocal, the NNV. These take into account the level of influenza viral circulation in the population.
Authors' conclusions
Implications for practice.
In children under the age of 16 years, live influenza vaccines probably reduce influenza (moderate‐certainty evidence) and may reduce influenza‐like illness (ILI) (low‐certainty evidence) over a single influenza season. In this population, inactivated vaccines also reduce influenza (high‐certainty evidence) and may reduce ILI (low‐certainty evidence). For both vaccine types, the size of effect when expressed in absolute terms varied considerably across the study populations. In addition to the variable certainty of the evidence, decision‐makers looking to inform local or national policies with evidence summarised in this review should be aware of the wide variation in absolute effects for protection against influenza and ILl. We did not find evidence that either vaccine reduces otitis media.
To date no studies have adequately measured or reported hospitalisation. Few randomised studies have measured school absenteeism and parental leave following vaccination, and effects on these outcomes are uncertain. We found few studies assessing vaccination during seasonal influenza on children younger than two years of age. Findings from single studies are consistent with estimates of vaccine efficacy from our own analyses, but do not currently provide clear evidence of equivalent effects. Adverse event data and effects on nausea are not well described in the available studies. Standardised approaches to the definition, ascertainment, and reporting of adverse events are needed. We found only one old study of the harms of inactivated vaccine in children younger than two years, despite current recommendations to vaccinate healthy children from six months of age. Identification of all global cases of potential harms is beyond the scope of this review.
Implications for research.
When a new vaccination or preventive technology becomes available, an adequately powered, publicly funded, high‐quality placebo‐controlled trial run over several seasons should be undertaken. New insights into the role of viruses and other agents in the genesis of influenza and ILI are also needed. This is especially important for the investigation of the non‐influenza causes of ILI, which could explain the findings of the study by Cowling 2012.
Further data on the safety of vaccines in children should also be collected or made available, particularly on inactivated vaccines in younger children. There is an immediate need to standardise safety outcome data according to Brighton Collaboration guidelines. Honest and full disclosure of all safety data to researchers is also a priority.
Feedback
Vaccines for preventing influenza in healthy children, 7 June 2012
Summary
The Cochrane article makes the claim that "Inactivated vaccines have a lower efficacy (65%) than live attenuated vaccines and in children aged two or less, they appear to have similar effects to placebo, although this observation is based on a single small study (Hoberman 2003a)." This conclusion regarding children under 2 years old seems to be erroneous, as the single study on which the result is based actually showed effectiveness in this age range against influenza infection in epidemic seasons. The reviewers may have been confused, as the paper by Hoberman et al does have lines like "Given that our study did not find a significant difference between vaccine and placebo," but this is regarding the primary objective of the study, which is (as is suggested by the study's title) to investigate the effectiveness of inactivated influenza vaccine in preventing Acute Otitis Media in young children. Note that while they may not have demonstrated a reduction in AOM, this does not mean that the vaccine was ineffective in preventing influenza infection. The first cohort was during an epidemic season, and showed, "efficacy rates against influenza in children aged 6 to 12 months, 13 to 18 months, and 19 to 24 months were 63%, 66%, and 69%, respectively." The second season failed to show an effect, but there were only 13 cases on influenza recorded for the second cohort, and influenza was infrequent, making it hard to come to any conclusion regarding effectiveness during that season. I hope that this may prompt a revision of the claim that the vaccine was ineffective in the children under 2 years old, as the evidence in fact showed a rather substantial protective effect during the cohort exposed to epidemic influenza, and the seroprotection levels recorded in the study suggest that it should have performed similarly in the second cohort, had their been sufficient circulating influenza to detect a difference. I agree with the conflict of interest statement below: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
Thank you. We have re‐examined the evidence commented on by the reader. As the reader remarks, the primary objective of the trial by Hoberman et al was to assess the effects of TIV on otitis media (OM) in under two year olds. The secondary objectives "were to evaluate the vaccine's safety, immunogenicity and efficacy against culture‐proven influenza.....as well as...on children's utilisation of selected health care and related resources." (pdf page 2, just before "Methods"). See also our descriptive table of included studies. Our comparison 2.1.1 shows the study's two influenza "seasons" (labelled as a and b). Overall the vaccine appears to have no effect. An equal lack of efficacy is seen against OM and resource utilisation. Wide yearly differences in virus circulation as remarked on by the reader and observed by Hoberman and colleagues are precisely the reason why influenza vaccines studies should be carried out over several seasons and reviews of several studies are the most meaningful public health way to estimate the effects of influenza vaccines. Hoberman A, Greenberg DP, Paradise JL, Rockette HE, Lave JR, Kearney DH, Colborn DK, Kurs‐Lasky M, Haralam MA, Byers CJ, Zoffel LM, Fabian IA, Bernard BS, Kerr JD. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 2003 Sep 24 ; 290 (12) : 1608 – 1616 .
Tom Jefferson and all co‐authors.
Contributors
Richard McAteer
Regulatory Project Officer Health Canada, Office of Regulatory Affairs Email Address: richard.mcateer@hc‐sc.gc.ca
What's new
| Date | Event | Description |
|---|---|---|
| 31 December 2016 | New search has been performed | For this update we included two new trials (aa Brooks 2016; aa Cowling 2012) and excluded five new trials (Ambrose 2014; Block 2011; Cakir 2012; Cowling 2014; Madhi 2014). |
| 31 December 2016 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 3 September 2014 | Amended | We amended the final sentence in paragraph three under 'Effects of interventions > Comparisons showing vaccine efficacy'. The value of number needed to vaccinate (NNV) reported in the text (NNV = 28) was incorrect; we have changed it to NNV = 2.8. |
| 9 July 2012 | Feedback has been incorporated | Feedback comment and reply added to the review. |
| 9 July 2012 | New citation required but conclusions have not changed | A new author joined the team to update this review. |
| 16 November 2011 | New search has been performed | For this 2011 update we included the following 15 new trials and data sets: (aa Bracco Neto 2009a; aa Bracco Neto 2009b); (ba Cochran 2010a; ba Cochran 2010b; ba Cochran 2010c); (ba Eisenberg 2008a; ba Eisenberg 2008b); ba Gilca 2011; ba Kelly 2011; ba Kissling 2011; ba Mahmud 2011; ab Mallory 2010; ca Ortqvist 2011; ab Plennevaux 2011; (ba Staat 2011a; ba Staat 2011b); ba Valenciano 2011; ba Van Buynder 2010; ca Yin 2011; cb MPA 2011. Readers are reminded that one study may provide multiple data sets (e.g. Bracco Neto 2009 a and b). We excluded 10 trials (Ambrose 2011; Belshe 2008; Fujieda 2008; Haba‐Rubio 2011; Jansen 2008; Kissling 2011a; McMahon 2008; Muhammad 2011; Stowe 2011; Wu 2010). |
| 20 February 2008 | New citation required and conclusions have changed | Substantive amendment |
| 20 February 2008 | New search has been performed | For the 2007 update, we re‐ran the searches and identified 1090 possible titles of interest. We retrieved 15 and excluded 5: Neuzil 2006, Hambidge 2006, and France 2004 because they were non‐comparative; Daubeney 1997 because it was not carried out in healthy children; and Gendon 2004 because it assessed the impact of vaccinating children to prevent influenza in the elderly. We included 10 studies. Two were placebo‐controlled trials of cold‐adapted live attenuated influenza vaccine (CAIV) (Tam 2007; Vesikari 2006); two were case‐control studies assessing respectively the efficacy and safety of trivalent influenza vaccine (TIV) (Anonymous 2005; Goodman 2006); three were prospective cohort studies assessing the effectiveness of respectively CAIV (Wiggs‐Stayner 2006), virosomal vaccine (Salleras 2006), and TIV vaccines (Fujieda 2006); and one was a retrospective cohort study assessing the effectiveness of an undescribed vaccine (Allison 2006). The remaining included studies were a prospective cohort study reporting effectiveness and safety of CAIV in school‐aged children (King 2006), and a prospective single‐blind cohort study assessing the effectiveness of TIV against otitis media (Ozgur 2006). Our conclusions remain unchanged. |
| 15 January 2008 | Amended | Converted to new review format |
Acknowledgements
The 2011 update was funded by Regione Piemonte, Italy and the UK National Institute for Health Research (NIHR) Cochrane Review Incentive Scheme. Oxford Childhood Infection Study, University of Oxford, UK (from MRC Programme Grant G0000340) funded the previous versions of the review.
The 2016 stabilisation and update was not funded.
Ruth Foxlee previously advised on search strategies and carried out duplicate searches. Vassily Vlassov and Frances Tilling carried out translations from Russian. Melanie Rudin provided logistical support. Gabriella Morandi retrieved papers. The review authors would like to thank Dr Kenneth Zangwill, ab Zangwill 2001, and Dr Jonathan Crofts, cb Nicholls 2004, for providing further data for use in the review.
Sue Smith, Anthony Harnden, Eliana Ferroni, and Nicholas J Matheson co‐authored previous versions of the review.
The review authors gratefully acknowledge the following people for commenting on previous drafts: Amy Zelmer, Leslie Goldstein, Mark Jones, George Swingler, Lamberto Manzoli, Márcia Alves Galvão, Robert Ware, and John Holden. For this 2016 update, we acknowledge help and comments received from Liz Dooley, Toby Lasserson, Theresa Wrangham, Colin Simpson, Robert Ware, and Jenny Doust. We thank Lisa Winer for copy‐editing this update.
Appendices
Appendix 1. Included study designs
A randomised controlled trial is any study on humans in which the individuals (or other experimental units) followed in the study were definitely or possibly assigned prospectively to one of two (or more) alternative forms of health care using random allocation.
A quasi‐randomised clinical trial is any study on humans in which the individuals (or other experimental units) followed in the study were definitely or possibly assigned prospectively to one of two (or more) alternative forms of health care using some quasi‐random method of allocation (such as alternation, date of birth, or case record number).
Appendix 2. MEDLINE (PubMed) search strategy
| No. | Query |
| #1 | "Influenza Vaccines"[MeSH] OR("Influenza,Human/complications"[MeSH]OR"Influenza,Human/epidemiology"[MeSH]OR"Influenza, Human/immunology"[MeSH] OR "Influenza, Human/mortality"[MeSH] OR "Influenza, Human/prevention and control"[MeSH] OR "Influenza, Human/transmission"[MeSH]) |
| #2 | ((influenza vaccin*[Text Word]) OR ((influenza [Text Word] OR flu[Text Word]) AND (vaccin*[Text Word] OR immuni*[Text Word] OR inocula*[Text Word] OR efficacy[Text Word] OR effectiveness[Text Word]))) |
| #3 | #1 OR #2 |
| #4 | (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) |
| #5 | ("cross over" OR "crossover" OR "Follow Up") OR ("Cross‐Over Studies"[MeSH] OR "Follow‐Up Studies"[MeSH] OR "Prospective Studies"[MeSH]) OR ("time series" OR "interrupted time series") OR (placebo* OR random* OR "double blind" OR "single blind" OR clinical trial* OR trial design) OR ("Case‐Control Studies"[MeSH] OR (cases[Title/Abstract] AND controls[Title/Abstract])) OR ("Cohort Studies"[MeSH] OR cohort*) OR ("Comparative Study"[Publication Type]) OR ("before after"[Title/Abstract] OR "before‐after"[Title/Abstract] OR "before/after"[Title/Abstract] OR "before and after"[Title/Abstract]) OR (volunteer*[Title/Abstract]) OR (control*[Text Word] AND evaluation[Text Word]) OR (longitudinal[Text Word]) OR (retrospective*[Text Word]) |
| #6 | #4 OR #5 |
| #7 | #3 AND #6 |
| #8 | #3 AND #6 Limits: All Child: 0‐18 years |
| #9 | child* OR preschool* OR school* OR young OR adolescent* OR infant* OR toddler* OR pediatric* OR paediatric* OR infant* |
| #10 | #7 AND #9 |
| #11 | #8 OR #10 |
Appendix 3. CENTRAL search strategy
| No. | ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐Query‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ |
| #1 | MeSH descriptor Influenza Vaccines explode all trees |
| #2 | MeSH descriptor Influenza, Human explode all trees with qualifiers: CO,EP,IM,MO,PC,TM |
| #3 | (influenza OR flu OR grippe) NEAR/5 (vaccin* OR immuni* OR inocul*) :ti,ab,kw |
| #4 | (#1 OR #2 OR #3) |
| #5 | (child* OR preschool* OR school* OR young OR adolescent* OR infant* OR toddler* OR pediatric* OR paediatric* OR infant*):ti,ab,kw |
| #6 | (#4 AND #5) |
| #7 | (#6)from 2007 to 2011 |
Appendix 4. Embase (Elsevier) search strategy
| No. | Query |
| #1 | 'influenza vaccine'/exp OR 'influenza vaccine' OR (influenza OR flu AND (vaccin* OR immuni* OR inoculat*)) OR 'influenza vaccine'/syn OR ('influenza'/exp AND 'vaccine'/exp) |
| #2 | 'case control study'/syn OR 'case control':de,ab,ti OR (cases:ab,ti AND controls:ab,ti) OR 'cohort analysis'/syn OR 'cohort study':de,ab,ti OR 'study cohort':de,ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti OR observational:ab,ti OR 'clinical trial':it OR 'randomized controlled trial':it OR 'drug therapy'/exp OR 'drug therapy':de OR randomized:ab,ti OR randomised:ab,ti OR placebo:ab,ti OR randomly:ab,ti OR trial:ab,ti OR groups:ab,ti |
| #3 | 'clinical trial':it OR 'randomized controlled trial':it OR 'randomized controlled trial'/exp OR 'randomisation'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'clinical trial'/exp OR 'clinical' NEAR/0 'trial' OR 'clinical trial' OR (singl* OR doubl* OR trebl* OR tripl* AND (mask* OR blind*)) OR 'placebo'/exp OR placebo* OR random* OR 'control group'/exp OR 'experimental design'/exp OR 'comparative study'/exp OR 'evaluation study' OR 'evaluation studies'/exp OR 'follow up'/exp OR 'prospective study'/exp OR control* OR prospectiv* OR volunteer* AND [humans]/lim |
| #4 | #2 OR #3 |
| #5 | #1 AND #4 |
| #6 | #5 AND ([newborn]/lim OR [infant]/lim OR [child]/lim OR [adolescent]/lim) |
| #7 | child*:de,ab,ti OR preschool*:de,ab,ti OR school*:de,ab,ti OR young:de,ab,ti OR adolescent*:de,ab,ti OR toddler*:de,ab,ti OR pediatric*:de,ab,ti OR paediatric*:de,ab,ti OR infant*:de,ab,ti |
| #8 | #5 AND #7 |
| #9 | #6 OR #8 |
Appendix 5. WHO ICTRP search strategies
vaccine* AND influenza
immuni* AND influenza
inocul* AND influenza
vaccine* AND flu
immuni* AND flu
inocul* AND flu
Appendix 6. ClinicalTrials.gov search strategies
(vaccine OR vaccines OR vaccinate OR vaccination OR vaccinated OR vaccinating OR immunise OR immunised OR immunising OR immunisation OR immunize OR immunized OR immunizing OR immunization) AND (influenza OR influenza OR flu)
(inoculate OR inoculated OR inoculating OR inoculation) AND (influenza OR influenza OR flu)
Appendix 7. Previous search strategy
For the 2007 version of the review we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, Issue 3); OLD MEDLINE (1950 to 1965); MEDLINE (1969 to September 2007); EMBASE (1974 to September 2007); Biological Abstracts (1969 to September 2007); and Science Citation Index (1974 to September 2007).
We used the following search terms to search MEDLINE and CENTRAL and adapted them for the other electronic databases. MEDLINE (OVID) 1 exp Influenza Vaccine 2 exp INFLUENZA/ 3 exp VACCINES/ 4 and/2‐3 5 ((influenza or flu) adj (vaccin$ or immuni$ or innoculat$)) 6 1 or 4 or 5 7 limit 6 to all child <0 to 18 years> 8 exp CHILD/ 9 (child or children or pediatric or paediatric) 10 or/8‐9 11 6 and 10 12 7 or 11 13 RANDOMIZED CONTROLLED TRIAL 14 CONTROLLED CLINICAL TRIAL 15 RANDOMIZED CONTROLLED TRIALS 16 RANDOM ALLOCATION 17 DOUBLE BLIND METHOD 18 SINGLE‐BLIND METHOD 19 or/13‐18 20 Animals/ 21 human 22 20 not 21 23 19 not 22 24 CLINICAL TRIAL 25 exp Clinical Trials/ 26 (clin$ adj25 trial$) 27 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)) 28 PLACEBOS 29 placebo$ 30 random$ 31 or/24‐30 32 31 not 22 33 exp Case‐Control Studies/ 34 case control stud$ 35 (case$ and control$) 36 exp Cohort Studies/ 37 cohort stud$ 38 exp Cross‐Over Studies/ 39 cross over stud$ 40 or/33‐39 41 40 not 22 42 23 or 32 or 41 43 12 and 42
We imposed no language or publication restrictions. The search of CENTRAL included any trial reports identified in the systematic handsearch of the journal, Vaccine. In order to identify additional published and unpublished studies we searched the Vaccine Adverse Event Reporting System Website (http://www.vaers.org). We contacted vaccine manufacturers and first or corresponding authors of relevant studies to identify further published or unpublished trials.
For the 2012 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 3; www.thecochranelibrary.com) (accessed on November 16th, 2011), which includes the Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to November 2011), and EMBASE (1974 to November 2011).We used the search strategy in Appendix 2 terms to search MEDLINE. We adapted the search terms to search CENTRAL (Appendix 3), and Embase (Appendix 4). We did not impose any language or publication restrictions. To identify additional published and unpublished studies we searched the Vaccine Adverse Event Reporting System Website (http://www.vaers.org). We contacted vaccine manufacturers and first or corresponding authors of relevant studies to identify further published or unpublished trials.
Appendix 8. Data extraction form
Part 1
Background information and description of study
Reviewer:
Study unique identifier:
Published: Y/N
Journal (if applicable):
Year of publication:
Period study conducted:
Abstract/Full paper:
Country or countries of study:
Number of studies included in this paper:
Funding source (delete non‐applicable items): Government, Pharmaceutical, Private, Unfunded, Unclear
Paper/abstract numbers of other studies with which these data are linked:
Reviewer's assessment of study design (delete non‐applicable items):
Study category ‐ study design Experimental studies ‐ RCT/CCT; historical controlled trial (HCT); cross‐over (X‐over) RCT Non‐randomised analytical studies (specifically designed to assess association) ‐ prospective/retrospective cohort; case control; X‐sectional Non‐randomised comparative studies (studies not specifically designed to assess association) ‐ case X‐over/time series; ecological study; indirect comparison (before and after) Non‐comparative studies ‐ EXCLUDE
Does the study present data distributed by age group/occupation/health status? (Yes/No)
Subgroup distribution Age group Y/N Occupation Y/N Health status Y/N Immunisation status/schedule Y/N Gender Y/N Risk group Y/N
Description of study
Methods
Participants
Interventions/Exposure
Outcomes
Notes
Part 2a
Methodological Quality Assessment RCT and CCT only
Randomisation: A = individual participants allocated to vaccine or control group B = groups of participants allocated to vaccine or control group
Generation of the allocation sequence: A = adequate, for example table of random numbers or computer generated random numbers B = inadequate, for example alternation, date of birth, day of the week or case record number C = not described
Allocation concealment: A = adequate, for example numbered or coded identical containers administered sequentially, on‐site computer system that can only be accessed after entering the characteristics of an enrolled participant or serially numbered, opaque, sealed envelopes B = possibly adequate, for example sealed envelopes that are not sequentially numbered or opaque C = inadequate, for example open table of random numbers D = not described
Blinding: A = adequate double‐blinding, for example placebo vaccine B = single‐blind, i.e. blinded outcome assessment C = no blinding
Follow up: Average duration of follow‐up and number of losses to follow‐up
Part 2b
Description of interventions and outcomes RCT and CCT only
Vaccines used
Vaccine and composition | Product and manufacturer | Schedule & dosage and status | Route of administration Arm 1 Arm 2 Arm 3 Arm 4 Placebo
Rule: index vaccine goes in the Arm 1 line, placebo in the last line
Status: primary, secondary or tertiary immunisation
Vaccine batch numbers
Details of participants
Enrolled | Missing | Reasons | Inclusion in analysis | Notes Active arm 1 Active arm 2 Active arm 3 Active arm 4 Controls
Outcomes List ‐ Efficacy and Effectiveness
Outcome | How defined | Description/Follow‐up/Notes
Outcomes List ‐ Safety
Outcome | How defined | Description/Follow‐up/Notes
Investigators to be contacted for more information? Yes/No
Contact details (principal investigator, fill in only if further contact is necessary):
Part 2c
Data Extraction and manipulation (to be used for dichotomous or continuous outcomes) RCT and CCT only
Comparison
Outcomes | n/N Index Arm | n/N Comparator
Outcomes | n/N Index Arm | n/N Comparator
Outcomes | n/N Index Arm | n/N Comparator
Outcomes | n/N Index Arm | n/N Comparator
Notes (for statistical use only)
Appendix 9. Methodological quality of non‐randomised studies
Newcastle‐Ottawa Quality Assessment Scale
Case‐control studies
Note: A study can be awarded a maximum of one star for each numbered item within the 'Selection' and 'Exposure' categories. A maximum of two stars can be given for 'Comparability'.
Selection
-
Is the case definition adequate?
yes, with independent validation*
yes, e.g. record linkage or based on self reports
no description
-
Representativeness of the cases
consecutive or obviously representative series of cases*
potential for selection biases or not stated
-
Selection of controls
community controls*
hospital controls
no description
-
Definition of controls
no history of disease (endpoint)*
no description of source
Comparability
-
Comparability of cases and controls on the basis of the design or analysis
study controls for _______________ (select the most important factor)*
study controls for any additional factor* (this criterion could be modified to indicate specific control for a second important factor)
Exposure
-
Ascertainment of exposure
secure record (e.g. surgical records)*
structured interview where blind to case/control status*
interview not blinded to case/control status
written self report or medical record only
no description
-
Same method of ascertainment for cases and controls
yes*
no
-
Non‐response rate
same rate for both groups*
non‐respondents described
rate different and no designation
Newcastle‐Ottawa Quality Assessment Scale
Cohort studies
Note: A study can be awarded a maximum of one star for each numbered item within the 'Selection' and 'Outcome' categories. A maximum of two stars can be given for 'Comparability'.
Selection
-
Representativeness of the exposed cohort
truly representative of the average _______________ (describe) in the community*
somewhat representative of the average ______________ in the community*
selected group of users, e.g. nurses, volunteers
no description of the derivation of the cohort
-
Selection of the non‐exposed cohort
drawn from the same community as the exposed cohort*
drawn from a different source
no description of the derivation of the non‐exposed cohort
-
Ascertainment of exposure
secure record (e.g. surgical records)*
structured interview*
written self report
no description
-
Demonstration that outcome of interest was not present at start of study
yes*
no
Comparability
-
Comparability of cohorts on the basis of the design or analysis
study controls for _____________ (select the most important factor)*
study controls for any additional factor* (this criterion could be modified to indicate specific control for a second important factor)
Outcome
-
Assessment of outcome
independent blind assessment*
record linkage*
self report
no description
-
Was follow‐up long enough for outcomes to occur?
yes (select an adequate follow‐up period for outcome of interest)*
no
-
Adequacy of follow‐up of cohorts
complete follow‐up ‐ all participants accounted for*
participants lost to follow‐up unlikely to introduce bias ‐ small number lost ‐ > ____ % (select an adequate %) follow‐up, or description provided of those lost*
follow‐up rate < ____% (select an adequate %) and no description of those lost
no statement
Data and analyses
Comparison 1. Live vaccine versus placebo (RCTs).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Influenza | 7 | 7718 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.11, 0.41] |
| 1.1 Live attenuated vaccines (1 dose) | 6 | 4675 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.14, 0.55] |
| 1.2 Live attenuated vaccines (2 doses) | 2 | 3043 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.04, 0.26] |
| 2 Influenza‐like illness | 7 | 124606 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.60, 0.80] |
| 2.1 Live attenuated vaccines (1 dose) | 2 | 3306 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.18, 2.22] |
| 2.2 Live attenuated vaccines (2 doses) | 6 | 121300 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.57, 0.76] |
| 3 Otitis media (all episodes) | 2 | 2873 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 4 School absenteeism | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Working days lost (number of events, parents) | 2 | 2874 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.46, 1.03] |
| 6 Drug prescriptions (number of events) | 1 | 1784 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| 7 Outpatients attendance for pneumonia and influenza | 2 | 2874 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.59, 0.98] |
| 8 Influenza‐like illness (clustering sensitivity analysis) | 7 | 49666 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.59, 0.80] |
| 8.1 Live attenuated vaccines (1 dose) | 2 | 1746 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.18, 2.23] |
| 8.2 Live attenuated vaccines (2 doses) | 6 | 47920 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.56, 0.76] |
Comparison 2. Inactivated vaccine versus placebo (RCTs).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Influenza | 5 | 1628 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.28, 0.48] |
| 2 Influenza‐like illness | 4 | 19044 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.65, 0.79] |
| 2.1 Inactivated vaccines (1 dose) | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.15, 0.81] |
| 2.2 Inactivated vaccines (2 doses) | 2 | 18777 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.69, 0.76] |
| 3 Acute otitis media | 3 | 884 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.95, 1.40] |
| 3.1 Inactivated vaccines (1 dose) | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.10, 23.76] |
| 3.2 Inactivated vaccines (2 doses) | 2 | 748 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.95, 1.40] |
| 4 School absenteeism | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Influenza‐like illness (clustering sensitivity analysis) | 4 | 6126 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.60, 0.82] |
| 5.1 Inactivated vaccines (1 dose) | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.15, 0.81] |
| 5.2 Inactivated vaccines (2 doses) | 2 | 5859 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.66, 0.79] |
2.3. Analysis.
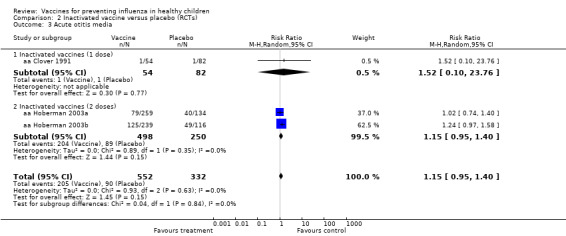
Comparison 2 Inactivated vaccine versus placebo (RCTs), Outcome 3 Acute otitis media.
2.4. Analysis.
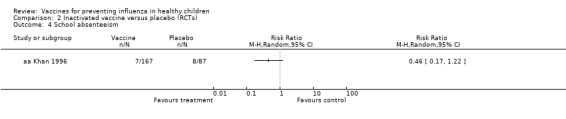
Comparison 2 Inactivated vaccine versus placebo (RCTs), Outcome 4 School absenteeism.
Comparison 3. Live vaccine versus placebo or no intervention (RCTs by age group).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Influenza | 7 | 10812 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.14, 0.41] |
| 1.1 under 2 years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 under 6 years | 7 | 10752 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.12, 0.39] |
| 1.3 over 6 years | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.23, 0.97] |
| 2 Influenza‐like illness | 8 | 188418 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.62, 0.72] |
| 2.1 under 2 years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 under 6 years | 5 | 38646 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.57, 0.77] |
| 2.3 over 6 years | 8 | 149772 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.60, 0.74] |
Comparison 4. Inactivated vaccine versus placebo or no intervention (RCTs by age group).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Influenza | 5 | 1628 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.29, 0.59] |
| 1.1 under 2 years | 2 | 786 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.18, 1.69] |
| 1.2 under 6 years | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.34, 1.08] |
| 1.3 over 6 years | 3 | 710 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.22, 0.45] |
| 2 Influenza‐like illness | 5 | 19388 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.54, 0.76] |
| 2.1 under 2 years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 under 6 years | 3 | 476 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.21, 0.69] |
| 2.3 over 6 years | 4 | 18912 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.66, 0.78] |
Comparison 5. All vaccine types versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Influenza | 9 | 8227 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.19, 0.45] |
| 1.1 Live attenuated vaccines (1 dose) | 5 | 3556 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.18, 0.62] |
| 1.2 Live attenuated vaccines (2 doses) | 2 | 3043 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.04, 0.26] |
| 1.3 Inactivated vaccines (1 dose) | 5 | 1628 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.28, 0.48] |
| 1.4 Inactivated vaccines (2 doses) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Influenza‐like illness | 8 | 143650 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.62, 0.77] |
| 2.1 Live attenuated vaccines (1 dose) | 2 | 3306 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.18, 2.22] |
| 2.2 Live attenuated vaccines (2 doses) | 6 | 121300 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.57, 0.76] |
| 2.3 Inactivated vaccines (1 dose) | 2 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.15, 0.81] |
| 2.4 Inactivated vaccines (2 doses) | 2 | 18777 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.69, 0.76] |
| 3 Secondary cases | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.56, 4.99] |
| 3.1 Live attenuated vaccines (1 dose) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Live attenuated vaccines (2 doses) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Inactivated vaccines (1 dose) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.56, 4.99] |
| 3.4 Inactivated vaccines (2 doses) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 School absenteeism | 1 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.26, 0.92] |
| 4.1 Live attenuated vaccines (1 dose) | 1 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.22, 1.19] |
| 4.2 Live attenuated vaccines (2 doses) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Inactivated vaccines (1 dose) | 1 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.17, 1.22] |
| 4.4 Inactivated vaccines (2 doses) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Lower respiratory tract disease | 2 | 1632 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.03, 1.54] |
| 5.1 Live attenuated vaccines (1 dose) | 2 | 1496 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.01, 4.45] |
| 5.2 Live attenuated vaccines (2 doses) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Inactivated vaccines (1 dose) | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 6.17] |
| 5.4 Inactivated vaccines (2 doses) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Acute otitis media | 6 | 5253 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.79, 1.26] |
| 6.1 Live attenuated vaccines (1 dose) | 3 | 2585 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.05, 3.79] |
| 6.2 Live attenuated vaccines (2 doses) | 1 | 1784 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 6.3 Inactivated vaccines (1 dose) | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.10, 23.76] |
| 6.4 Inactivated vaccines (2 doses) | 2 | 748 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.95, 1.40] |
| 7 Hospitalisation due to acute otitis media | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Inactivated vaccine (2 doses) | 2 | 765 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.62, 3.24] |
| 8 Consequences of acute otitis media | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Inactivated vaccine (2 (doses of the intervention) ‐ visits (outcome measure)) | 2 | 765 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.27, 0.23] |
| 8.2 Inactivated vaccine (2 doses; courses of antibiotics) | 2 | 765 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.36, 0.63] |
| 9 Outpatients attendance for pneumonia and influenza | 2 | 2874 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.59, 0.98] |
| 9.1 Live attenuated vaccine (1 dose) | 1 | 1090 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.49, 0.85] |
| 9.2 Live attenuated vaccine (2 doses) | 1 | 1784 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.75, 0.96] |
| 10 Working days lost (number of events, parents of children 6 to 36 months of age) | 2 | 2874 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.46, 1.03] |
| 10.1 Live attenuated vaccine | 2 | 2874 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.46, 1.03] |
| 11 Drug prescriptions (number of events, 6 to 36 months of age) | 1 | 1784 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| 11.1 Live attenuated vaccine | 1 | 1784 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
Comparison 6. Case‐control studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Influenza vs influenza‐like illness (crude data) | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Children aged below 6 years | 9 | 4949 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.45, 0.77] |
| 1.2 Children aged between 5 and 19 years | 1 | 27 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.07, 8.66] |
| 2 Influenza vs influenza‐like illness (adj. estimates) | 9 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Children aged below 23 months ‐ fully vaccinated | 7 | Odds Ratio (Random, 95% CI) | 0.60 [0.39, 0.94] | |
| 2.2 Children aged between 24 and 59 months ‐ fully vaccinated | 4 | Odds Ratio (Random, 95% CI) | 0.40 [0.22, 0.70] | |
| 2.3 Children aged between 6 and 59 months ‐ fully vaccinated | 5 | Odds Ratio (Random, 95% CI) | 0.45 [0.32, 0.62] | |
| 2.4 Children aged below 14 years old ‐ fully vaccinated | 1 | Odds Ratio (Random, 95% CI) | 0.23 [0.06, 0.84] | |
| 3 Influenza‐like illness vs no symptoms | 1 | 488 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.28, 0.86] |
| 3.1 Inactivated vaccine (1 dose) | 1 | 244 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.26, 1.07] |
| 3.2 Inactivated vaccine (2 doses) | 1 | 244 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.18, 1.10] |
Comparison 7. Live attenuated vaccines (cohort studies by age group).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Influenza | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.35, 0.91] |
| 1.1 under 2 years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 under 6 years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 over 6 years | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.35, 0.91] |
| 2 Influenza‐like illness | 2 | 22077 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.57, 0.69] |
| 2.1 under 2 years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 under 6 years | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 over 6 years | 2 | 22077 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.57, 0.69] |
Comparison 8. Inactivated vaccines (cohort studies by age group).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Influenza | 6 | 1873 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.25, 0.73] |
| 1.1 under 2 years | 3 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.27, 1.47] |
| 1.2 under 6 years | 1 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.13, 0.89] |
| 1.3 over 6 years | 2 | 1379 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.11] |
| 2 Influenza‐like illness | 11 | 11935 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.42, 0.67] |
| 2.1 under 2 years | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.23, 0.93] |
| 2.2 under 6 years | 5 | 7046 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.93] |
| 2.3 over 6 years | 7 | 4866 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.29, 0.68] |
| 3 Otitis media | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.22, 1.03] |
| 3.1 Children aged 6 months to 5 years | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.22, 1.03] |
Characteristics of studies
Characteristics of included studies [author‐defined order]
ab Aksenov 1971.
| Methods | Intrapandemic, placebo‐controlled CCT of live attenuated bivalent recombinant vaccine in schoolchildren in the Moscow area during the early part of 1969. Serological surveillance retrospectively showed that A2 Hong Kong caused most of the cases. | |
| Participants | Schoolchildren from 2 boarding schools aged 4 to 7 years and 8 to 15 years. There does not appear to be any attrition. | |
| Interventions | Live attenuated injected vaccine containing A2 and B type antigens, made in the central Moscow laboratories | |
| Outcomes | ILI, pneumonia, bronchitis, OM, tonsillitis, and duration and severity of influenza | |
| Funding Source | Government | |
| Notes | The authors conclude that vaccination did not prevent cases but shortened duration and severity of illness. Unfortunately, no standard deviations are reported for mean duration. The trial is reasonably reported but there is likely selection bias in serological testing. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pseudo‐random |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of losses to follow‐up is unknown. |
| Summary assessments | Unclear risk | Plausible bias that raises some doubt about the results |
aa Alexandrova 1986.
| Methods | C‐RCT possibly followed by 2 cohort studies | |
| Participants | Nearly 30,000 schoolchildren (aged 7 to 15) and preschool children (aged 3 to 6). The units sampled were schools and kindergartens. The samples were performed using random sampling numbers and stratified sampling in schools with different numbers of children. Initially reactogenicity of the vaccine was studied on a limited group of schoolchildren (190) and children between 3 and 6 years (267). After the low reactogenicity of the vaccine was assessed, vaccination of large groups of children was undertaken. The trial was extended to 45 schools (in 26 the bivaccine was administered, in 19 placebo) and to 142 community preschools (children from 76 preschools received vaccine, those from the other 66 received placebo). For each child a special form was completed in which data about immunisation and diseases were registered. No influenza was registered before the vaccination was carried out. | |
| Interventions | A reactogenicity study was carried out separately on a limited study population (those vaccinated in October 1982). This group consisted of 457 pupils and children who were divided into 2 groups. One group was given vaccine, the other received placebo. Cases of mild, moderate, or febrile reaction within 5 days of administration of vaccine or placebo were reported in consideration of the initial anti‐HA antibody level. These data were not considered because it is likely that the treatments were not randomly assigned. | |
| Outcomes | Incidence of influenza and acute respiratory disease during influenza epidemic 15 March to end of April 1983 Serological Antibody titres carried out on a non‐random section of the study population Effectiveness The prophylactic effectiveness of the bivaccine was estimated during an influenza epidemic caused by viruses A/Brazil/11/78 H1N1 and A/Bangkok/1/79 H3N2 (similar to the strains employed in the vaccine), which started in the middle of March 1983 and lasted for 6 weeks. The comparison of the influenza morbidity rates among vaccines and control groups of children were based on clinical diagnosis during the epidemic period. Safety
|
|
| Funding Source | Government | |
| Notes | 3 studies are reported in this paper. The first is a phase ll, 5‐day reactogenicity and safety trial carried out in 284 placebo recipients and 173 vaccine recipients. Although randomisation is claimed, it is unclear why the imbalance in numbers and due to unclear text we classified it as a C‐RCT. There appears to be an extension of the safety data to 14,228 placebo and 15,727 vaccine recipients. The second study (1 October 1982 to 14 March 1983) appears to be an extension of the first study, assessing effectiveness in 3538 bivalent vaccine recipients and 3271 placebo recipients. However, in the absence of influenza viral circulation, the vaccine appears to be highly effective against ILI, bronchitis, pneumonia, OM, and tonsillitis. A third study is the extension by 6 weeks (from 14 March 1983 of the second study) during the influenza epidemic. As the denominators are different in all 3 studies and the text is unclear, it is very difficult to classify study design. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomisation: random sampling numbers and stratified sampling were used |
| Allocation concealment (selection bias) | Low risk | Both vaccine and placebo batches were coded. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost to follow‐up is unknown. |
| Summary assessments | Low risk | Plausible bias unlikely to seriously alter the results. |
ca Allison 2006.
| Methods | 5‐practice retrospective cohort study taking place in Colorado during the 2003 to 2004 season. The study assessed the effectiveness of an undescribed vaccine in preventing ILI in healthy children aged 6 to 21 months. Participant data and immunisation status were identified from reimbursement registers and a web‐based immunisation register. Analysed data come from the period 1 November to 31 December 2003, when influenza A circulated in a prevalent fashion according to hospital isolates. Respiratory syncytial virus started circulating at the end of December, so the authors attempted to restrict analysis to the period of maximum influenza circulation. This, of course, does not mean that other pathogens may not have been co‐circulating. The results are presented for 2 peaks of ILI attendances, 1 corresponding with influenza A circulation and the other with RSV circulation ("influenza and RSV seasons"). | |
| Participants | 5193 healthy children aged 6 to 21 months. The 21‐month limit was chosen because of billing constraints. Participants were mostly white and privately insured. The authors classified participants as FV, PV, or UV, but as some UV became PVs and FVs as the season progressed, denominators are unstable. In addition, FV includes those who had 1 dose from the previous season, further increasing the confusion. At 1 March 2004 when the study ended there were 2087 FV, 1040 PV, and 2066 UV. | |
| Interventions | 1‐ and 2‐dose vaccinations versus do nothing. The vaccine must have been TIV, which is the only one registered in this age group in the USA. No mention is made of content or matching. | |
| Outcomes |
Serological N/A Effectiveness Physician's office attendance for: ILI or P&I as defined in ICD‐9. These were assessed only for first visits to the family physician. Safety N/A |
|
| Funding Source | Government | |
| Notes | The authors conclude that "a total of 28% of the patients had an ILI office visit and 5% had a pneumonia/influenza visit. HRs for FV versus UV were 0.31 (95% CI 0.3 to 0.4) for ILI and 0.13 (95% CI 0.1 to 0.2) for pneumonia/influenza, corresponding to a vaccine effectiveness (1 ‐ HR 100) of 69% for ILI and 87% for pneumonia/influenza. The corresponding HRs for PV versus UV were 1.0 (95% CI 0.9 to 1.2) and 1.1 (95% CI 0.8 to 1.5) Conclusions Although 2 doses of vaccine were 69% effective against ILI office visits and 87% effective against pneumonia/influenza office visits, 1 dose did not prevent office visits during the 2003 to 2004 influenza season." Summary estimates are presented as HR, and the authors used a Cox proportional hazards model, so no disaggregated numerators are available. As denominators are also moving, the study results are difficult to interpret. Data are reported by influenza (ILI and P&I) and RSV (ILI) seasons. Asymmetrical reporting? It is difficult to assign a design to this study, as the text is unclear on timings and buried in the text is the phrase "This study was conducted as part of a randomised controlled trial of registry‐based reminder recall in 5 private paediatric practices in Denver, Colorado from September 1, 2003 through February 29, 2004 (Kempe A, Daley MF, Barrow J, Allred N, Hester N, Beaty BL, et al). Implementation of universal influenza immunisation recommendations for healthy young children: results of a randomised, controlled trial with registry based recall. Pediatrics 2005;115:146‐54). There is also an implausible sharp division between influenza and RSV around New Year's Eve. High risk of bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Low risk | Selected group of users, secure records |
| PCS/RCS‐Selection Non Exposed cohort | Low risk | From the same community as the exposed cohort |
| PCS/RCS‐Comparability | Unclear risk | It is unclear whether the study took into account all possible confounders. |
| PCS/RCS‐Assessment of Oucome | Unclear risk | Physician's office attendance for: ILI or P&I as defined in ICD‐9. These were assessed only for first visits to the family physician. |
| Summary assessments | Unclear risk | No description of vaccine, content, or matching, no disaggregate numerators by event and arm, unstable denominators, low generalisability of results |
ba Anonymous 2005.
| Methods | Case‐control study based on the 45 British Columbia surveillance system sites in which during 2004 to 2005 sentinel physicians were encouraged to take more swabs. Cases were people who reported to sentinel physicians with acute onset respiratory illness with fever and cough and 1 or more of sore throat, arthralgia, myalgia, or prostration and had a positive specimen for influenza A. Controls were all other symptomatic reportees who tested negative. Once the specimens were taken, a questionnaire with details of the case was attached. The authors report that "there were 219 separate submissions of respiratory specimens by a known sentinel physician during the 2004 to 2005 surveillance period. Of these, only 32 (15%) had all questionnaire information completed on the original laboratory requisition; 187 required follow‐up interview with the submitting physician to complete missing information and 133 were completed. From the 165 patients with complete records, specimens were collected between 4 October, 2004 and 31 March, 2005 with the distribution of submissions mirroring the distribution of sentinel visits for ILI overall" | |
| Participants | 165 out of 219 participants had sufficient information as required by the study protocol. Of these, 134 were from the period of greatest circulation. 40 and 7 cases, respectively, had specimens positive for influenza A and B, and only 7 overall were aged 19 or below. The text appears to suggest that matching was partial. | |
| Interventions | Trivalent influenza vaccine (various suppliers) formulations were standardised to contain 15 µg each of A/H1N1/New Caledonia/ 20/99, A/H3N2/Wyoming/3/2003 (antigenically equivalent to A/H3N2/Fujian/411/2002), and B/Jiangsu/10/2003 strains. | |
| Outcomes |
Laboratory Specimens were swabs or nasal washouts on which PCR was used. |
|
| Funding Source | Government | |
| Notes | The authors conclude that "We found age‐adjusted point estimates for VE against medical consultation for laboratory‐confirmed influenza A during the mismatched 2004 to 2005 season to range as low as 40% and as high as 75%. VE varied with age, definition of immunisation status and whether analysis was restricted to presentation within 48 hours of ILI onset. Overall, our estimates suggest cross‐protection for the 2004 to 2005 season despite vaccine mismatch. Our VE estimates mostly reflect the protection conferred to young healthy adults; the sample included few elderly persons or those with underlying conditions. The higher than expected reports of facility outbreaks in 2004 to 2005 in BC may have reflected an even lower VE amongst the frail elderly. Because of small sample size, estimates are unstable with wide confidence intervals. The possibility of no protection cannot be ruled out". Attrition, small sample size, recall and performance bias. High risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | Low risk | Independent validation and representativeness series of cases |
| CC‐Control Selection | Low risk | Selected from the same population |
| CC‐Comparability | Unclear risk | Only sex and age adjustment |
| CC‐Exposure | High risk | No descriptions |
| Summary assessments | Unclear risk | Attrition, small sample size, recall and performance bias |
ab Belshe 1992.
| Methods | RCT of safety vaccine, double‐blind 0.5 mL of trivalent vaccine administered intranasally Children observed in own homes for 11 days by nursing staff Daily sampling ‐ nasopharyngeal swabbing for isolation of influenza virus Serum for antibody determination obtained on days 0 and 28 to 31. | |
| Participants | Healthy children age 6 months to 13 years | |
| Interventions | Live, trivalent vaccine, recombinant containing A/Kawasaki/9/86 (H1N1) CR125 + A/Korea/1/82 CR59 + B/Texas/1/84 CRB‐87 A/Kawasaki/9/86 and A/Korea/1/82 derived from cold‐adapted A/Ann/Arbor/6/60 parent virus. B/Texas/1/84 derived from cold‐adapted B/Ann Arbor/1/66 parent virus. | |
| Outcomes | Adverse reactions up to 11 days after vaccination Fever: rectal temperature > 38.3 °C (infants and young children); oral temperature > 37.8 °C (older children) Upper respiratory illness: rhinorrhoea on 2 consecutive days; lower respiratory illness; wheeze or pneumonia; OM Viral shedding (data not extracted) Serologic response to vaccine (data not extracted) | |
| Funding Source | Government | |
| Notes | Safety data presented separately for seronegative and seropositive responders, but were combined for extraction. Was significantly (P < 0.5) higher upper respiratory illness in seronegative individuals than in seropositive individuals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Summary assessments | High risk | Lack of allocation concealment; plausible bias that seriously weakens confidence in the results |
aa Belshe 1998.
| Methods | Multicentre, prospective, randomised, double‐blind, placebo‐controlled trial to assess efficacy and safety of a cold‐adapted influenza vaccine in single‐ and 2‐dose regimen versus placebo. Vaccine and placebo were randomly assigned sequential vaccination numbers. Randomisation sequence was incorporated in the preparation and labelling of materials. Each eligible child received the next available study number at a site, ensuring proper randomisation. Placebo was indistinguishable from the vaccine in appearance and smell. | |
| Participants | "Healthy children aged between 15 and 71 months at the time of their enrolment (August ’96). A total of 1314 children were enrolled in the 2‐dose group and 288 for the 1‐dose. No statistical differences in age, sex, race, daycare and household makeup were observed between vaccine and placebo groups Subjects scheduled to receive 2 doses of vaccine; received the first between August 21, 1996 and October 23, 1996; the second dose between October 15, 1996 and January 11, 1997. Subjects in the 1‐dose cohort were vaccinated between September 30, 1996 and December 5, 1996" | |
| Interventions | Cold‐adapted, trivalent influenza vaccine (supplied by Aviron; Mountain View, CA, USA). Vaccine reassortants contained the strains A/Texas/36/91‐like (H1N1), A/Wuhan/359/95‐like (H3N2), B/Harbin/7/94‐like in egg allantoic fluid with sucrose, phosphate, and glutamate. The mean dose of each attenuated strains was 106.7. These matched the antigens recommended for that year by the US Food and Drug Administration (1996 to 1997). Placebo consisted only of egg allantoic fluid with sucrose, phosphate, and glutamate. Both were administered intranasally through a spray applicator (0.25 mL of placebo or vaccine per nostril). In the 1‐dose group 189 participants were vaccinated and 89 received placebo; in the 2‐dose group 881 participants were randomised to receive vaccine and 433 to receive placebo. 42 participants in the 2‐dose group did not receive the second dose for the following reasons:
These 5 (and the 8 cases of influenza A) were included in the efficacy analysis considering the 2 groups together. |
|
| Outcomes |
Serological
Haemagglutination inhibiting antibody responses were evaluated after 1 or 2 doses of vaccine or placebo. Data for 136/849 (2‐dose recipients) vaccinated only reported ‐ likely selection bias. Effectiveness Influenza defined as any illness detected by active surveillance associated with positive culture for wild‐type influenza virus 28 days after the first dose and any time after the second dose during the influenza A H3N2 and B epidemic, which lasted up to April 1997. After the outbreak of influenza in the community (end November 1996), parents were contacted and reminded to notify if the child had symptoms suspected to be due to influenza: fever, runny nose, nasal congestion, sore throat, cough, headache, muscle aches, chills, vomiting, suspected or confirmed OM, decreased activity, irritability, wheezing, shortness of breath, and pulmonary congestion. Attempts were made to collect viral culture specimens within 4 days after the onset of any illnesses. Safety The parent or guardian of each child was given a digital thermometer and asked to record on a diary card temperature (fever was defined as an axillary temperature above 37.6 °C or oral temperature above 37.7 °C or rectal temperature above 38.1 °C) and occurrence of specific symptoms including decreasing activity, irritability, runny nose or nasal congestion, sore throat, cough, headache, muscle aches, chills, and vomiting, daily for 10 days after each vaccination. |
|
| Funding Source | Government/industry | |
| Notes | The authors conclude that live attenuated, cold‐adapted influenza vaccine is safe, immunogenic, and effective against influenza A and B in healthy children. Vaccine efficacy is equally high for older and younger children. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisations (block size of 6) |
| Allocation concealment (selection bias) | Low risk | "The randomisation sequence was incorporated in to the preparation and labelling of materials, and each eligible child received the next available study number at a site" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses to follow‐up |
| Summary assessments | Low risk | Plausible bias unlikely to seriously alter the results. |
aa Belshe 2000a.
| Methods | See aa Belshe 1998 | |
| Participants | 1358 healthy children who previously participated in year 1 of trial (aa Belshe 1998). Aged 26 to 85 months | |
| Interventions | Revaccination with live attenuated, cold‐adapted trivalent (H1N1, H2N3, and B) influenza vaccine, administered by nasal spray | |
| Outcomes |
|
|
| Funding Source | Government/industry | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Second year of study of aa Belshe 1998 not re‐randomised |
| Allocation concealment (selection bias) | Unclear risk | Second year of study of aa Belshe 1998, not sufficient description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At the start of the second study year (aa Belshe 1998) only 86% in the treatment arm and 83% in the placebo arm, from the first study year (aa Belshe 1998) were enrolled but insufficient information given to the end of this second study year |
| Summary assessments | High risk | Plausible bias that raises some doubt about the results |
aa Beutner 1979a.
| Methods | Randomised, placebo‐controlled trial to assess antibody response, efficacy, and safety of a neuraminidase‐specific influenza vaccine. Children were randomly divided into 3 groups to receive a single dose of 1 preparation (X – 41, X – 42, or placebo) under code. | |
| Participants | Study population consisted of 875 healthy children of both sexes aged 7 to 14 years who were recruited from the public school system after written informed consent for immunisation was obtained from their parents. | |
| Interventions |
Haemagglutinin titres were determined by the method of Horstaff and Tamm and were 1024 for X – 41 and 3072 for X – 42. X – 41 vaccine contains 2.3 fold greater neuraminidase activity than X – 42. All recruited children were intramuscularly inoculated with 1 0.5 mL dose of vaccine or placebo between September and November 1974. Serum samples were obtained before and at regular intervals after vaccination. |
|
| Outcomes |
Serological
Antibody titre rise Effectiveness "Influenza infection assessed during 2 epidemics. The first of these lasted between mid December 1974 and April 1975 and was due to the Port Chalmers (H3Ch N2 Ch) strain. An outbreak of Victoria strain was also observed in the population from January to March 1976. Serum samples were obtained before and at regular intervals after vaccination for determination of antibody response (1, 2, 6 months after vaccination). Clinical illnesses in the vaccinated were also evaluated by examination of all sick children within 24 hours during the subsequent outbreaks of natural influenza infection A minor outbreak of Victoria strain occurred in Buffalo from January to March 1976. Most of the immunised children were available for evaluation during this epidemic (220 in the X – 41 group, 200 in the X – 42 group, 185 in the placebo group)." Safety "Data on reactogenicity of influenza immunisation were obtained through telephone calls and questionnaire mailed to the parents of the vaccinees. All children reporting any reactions were immediately examined by a physician and evaluated for the degree of reactogenicity. Follow‐up for vaccine reactions was carried out for 1 to 4 weeks after vaccination. Data about local (pain‐tenderness, erythema, swelling, none) and systemic reactions (headache, nausea‐vomiting, soreness‐aching‐chills, none) are reported" |
|
| Funding Source | Government | |
| Notes | The authors conclude that both vaccines work as well as the bivalent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number |
| Allocation concealment (selection bias) | Low risk | Coded, identical‐looking compounds |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Summary assessments | Low risk | Plausible bias unlikely to seriously alter the results. |
aa Beutner 1979b.
| Methods | See aa Beutner 1979a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Funding Source | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number |
| Allocation concealment (selection bias) | Low risk | Coded, identical‐looking compounds |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Summary assessments | Low risk | Plausible bias unlikely to seriously alter the results. |
ca Burtseva 1991.
| Methods | Prospective cohort study of efficacy of live recombinant and inactivated influenza A (H3N2) vaccines versus placebo‐cold‐adapted recombinant live influenza vaccine A/47/F (H3N2) obtained by method described in other papers (Medvedeva et al, 1989. Vopr. Virusol.; 34: 564‐8 and Alexandrova et al. 1984. Infect. Immun.; 44: 734‐9).
|
|
| Participants | Children aged 8 to 15 years | |
| Interventions |
|
|
| Outcomes |
|
|
| Funding Source | Government | |
| Notes | The authors conclude that bivalent vaccine had better performance (they report protection indices), but the text has many contradictions, lacks clarity, and mentions exclusion of influenza B cases from the analysis. Children from "internat" roughly translates as state orphanage, could be ethical issues surrounding consent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | No description |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | No description |
| PCS/RCS‐Comparability | Unclear risk | No description |
| PCS/RCS‐Assessment of Oucome | Unclear risk | No description |
| Summary assessments | High risk | Plausible bias that raises some doubt about the results |
ca Chumakov 1987.
| Methods | Prospective cohort study, re‐analysis of data from Bashliaeva 1986, which did not take into account that influenza vaccine is not intended for prophylaxis of other ARIs, which make up about 70% of total and occur repeatedly in children aged 3 to 6 years
The authors claim that inoculations began late, when an epidemic situation has already arisen, and numbers of children attending nurseries had dropped by the time the second vaccination was administered (to a comparatively smaller number of children). The authors claim that antigenic activity was incorrectly analysed. |
|
| Participants | See Bashliaeva 1986 ‐ 2274 children were inoculated once with the 2 vaccine types, 876 were inoculated twice; 1321 and 573 children were inoculated with placebo, respectively. | |
| Interventions | See Bashliaeva 1986 ‐ 2 types of the vaccine were tested (15 and 16). The vaccines contained 3 strains (A/Brazil/11/78 (H1N1), A/Bangkok/1/79 (H3N2), and B/Singapore/222/79). The total amount of the B haemagglutinin varied: 31.9 μg (type 15) and 29.2 μg (type 16). The vaccines also contained ovalbumin (type 15 was 0.125 μg/mL; type 16 was 0.06 μg/mL). Sterile, apyrogenic, physiological solution was used for placebo. Vaccines or placebo were administered subcutaneously: 2 doses of 0.5 mL, with an interval of 28 to 30 days. | |
| Outcomes |
Effectiveness: Cases of ARI and influenza
Influenza and ILI. 2 statements assess the impact of influenza: "With the aim of serologically analysing the clinical diagnoses of influenza and acute respiratory illnesses from the children who fell ill during the period of observation, 470 coupled samples of serum were taken (I –in the first days of illness, II‐ 18 to 20 days later)" and "In order to analyse the aetiology of the spread of the virus, 380 children were observed who had contracted influenza or acute respiratory illnesses, both those who had received the vaccine and those who had received placebo. The division of viruses of influenza was determined from swabs taken from the nose and throat area, implanted onto chicken embryos and the subsequent identification of that which had been isolated" Serology There are 2 apparently contradictory statements concerning serology and partly safety assessment: "The reactogenicity and antigenic activity of the vaccine were studied by observing the 305 vaccinated children and the 237 children who had received the placebo in 15 schools. They were assessed according to a series of well known indices, characterising the frequency and intensiveness of the local and general reactions to the vaccination" and "in order to study the antigenic activity of ‘Grippovac SE‐AZH’, 320 samples of serum were taken from the inoculated children before vaccination, 280 samples were taken 21 days after the first injection and 170 samples were taken 21 days after the second injection". The reasons for this apparent attrition are unclear. Safety See above. Other harms data (headaches, etc.) are reported as 1‐liner with no data. |
|
| Funding Source | Government | |
| Notes | The authors report that there was a significant difference in the level of response in immunity in the recipients of type 15 (45.8%) and type 16 (76%) towards the serotype A (H1N1), which was probably due to vaccine antigen concentration. They concluded that "the preparation showed insignificant reactogenicity and moderate antigenic potency. The trial established that at the period of the epidemic rise of influenza B morbidity the vaccine showed, according to the data of the clinical diagnosis of influenza, insignificant effectiveness, its index of effectiveness (IE) being 1.08; according to the data of the serological diagnosis of influenza, only the A (H1N1) component of the vaccine was found to have IE equal to 1.58". This was a very difficult text to follow with many inconsistencies. Allocation and blinding are not described, denominators are not clear. See also criticism by Chumakov and colleagues in Chumakov 1987. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Low risk | Somewhat representative, secure record |
| PCS/RCS‐Selection Non Exposed cohort | Low risk | Drawn from the same community |
| PCS/RCS‐Comparability | Unclear risk | Only by age |
| PCS/RCS‐Assessment of Oucome | Unclear risk | No descriptions |
| Summary assessments | Unclear risk | Plausible bias that raises some doubt about the results |
aa Clover 1991.
| Methods | Multicentre, cluster‐randomised, placebo‐controlled clinical trial in which the efficacy of bivalent cold‐adapted and trivalent inactivated influenza vaccines were compared. 70% of the study population had already been immunised in the previous aa Gruber 1990, whose participants were enrolled at the same centres and which was carried out during the previous year. Design and methods of enrolment are similar to those adopted in that study. | |
| Participants | 103 families were enrolled from Houston Family Study, Baylor Family Practice Clinic (Houston), and Family Medicine Clinic (University of Oklahoma). They were randomly assigned to receive placebo (40%) or 1 of the 2 vaccines (each 30%). About 70% of the families were enrolled and randomised the previous year and received the same preparation. The entire study population consisted of 166 adults and 225 children. 98 families with 157 adults and 192 children aged almost 3 years and 20 children younger than 3 years completed the study. | |
| Interventions | Bivalent cold recombinant influenza A vaccine containing 107 TCID50 of CR – 90 (A/Bethesda/1/85 H3N2) and 10 7 TCID 50 of CR – 98 (A/Texas/1/85 H1N1) in 0.5 mL. 1 dose intranasally administered.
|
|
| Outcomes |
Serological
Children receiving vaccine or placebo were brought in 3 to 4 weeks after vaccination to obtain a second blood specimen to determine antibody responses to vaccine antigens. However, paired sera were taken from 112 children, with no explanation as to why. Effectiveness
When ongoing community surveillance at the Influenza Research Center indicated that influenza virus was spreading in the community (influenza A/Taiwan/86), weekly telephone contacts to families were made to evaluate respiratory illnesses. Home or clinic visits were scheduled for physical examination and collection of nasal washes or swab specimens for viral isolation. An illness was attributed to influenza A infection if influenza virus was isolated during the illness or, for a person with a postseason antibody rise only, if no other virus was detected in the illness specimen and if the illness occurred within 10 days of an isolate in household contact or during the period of most intense influenza activity in the community. Illnesses were characterised by review of records, which included date of onset, symptoms, physical signs diagnosis of each contact. Safety N/A |
|
| Funding Source | Unclear | |
| Notes | The authors conclude that TIV provided better protection against detectable infection in older children (P > 0.1 TIV versus placebo) than CR vaccine, which instead was more protective in younger children (however, based on a denominator of 27, 35, and 51 CR, TIV, and placebo recipients). There were no statistical differences in infection rates for family contacts of children receiving TI or CR or placebo. Analysis seems to have been done at individual level, whereas randomisation was at cluster level. The authors report that the vaccines were ineffective in preventing transmission. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses to follow‐up, unlikely to be related to true outcome |
| Summary assessments | Unclear risk | Plausible bias that raises some doubt about the results |
aa Colombo 2001.
| Methods | Randomised open trial to assess the efficacy of a trivalent subvirion vaccine | |
| Participants | Healthy children from the area of Sassari (North Sardinia). All were aged 1 to 6 years, and none had ever been immunised against influenza. Children with hypersensitivity reactions to eggs were excluded. Of the 398 children meeting the inclusion criteria, 344 enrolled. 177 were randomly assigned to receive trivalent subvirion vaccine and 167 to the control group (no treatment). | |
| Interventions |
|
|
| Outcomes |
Serological
Paired sera for 17 children, to test seroconversion and not diagnose influenza Effectiveness
"Follow‐up was carried out between December 1, 1995 and April 30, 1996. No participants were lost during this time. All children who developed influenza‐like symptoms were seen by the paediatrician. A clinical examination was conducted and repeated at the end of the illness with the aim to collect information regarding the duration of clinical symptoms and daycare absenteeism (also for the family members). Influenza‐like illness was defined as rectal temperature above 38.5°C and cough or sore throat lasting at least 72 hours" Safety
|
|
| Funding Source | Government | |
| Notes | The authors conclude that killed influenza vaccine is safe and effective in preschool children. Data about the rate of infection in parents were reported, but the number of parents involved is not stated. Only 85.5% of the children in the control group and 89.2% in the vaccinated group were in a day‐care centre. Quality of randomisation is suspect (different prevalence on passive smoking in the arms), lack of serological diagnosis despite 17 sera taken for seroconversion, no mention of circulating viruses in the season. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | High risk | No description |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Summary assessments | High risk | Plausible bias that seriously weakens confidence in the results |
ab Desheva 2002.
| Methods | RCT of adult variant (single‐dose) of live influenza vaccine in children aged 3 to 6 years. 2 groups of children were formed to receive vaccine, 1 group to receive placebo. Paediatricians from clinics serving nurseries selected children for immunisation. Parental consent was obtained for each child. Medical examination of children was carried out each day for 5 days after inoculation: body temperature measured, local and general reactions recorded.
|
|
| Participants | Children aged 3 to 6 years from nursery schools in the St Petersburg, Russia area | |
| Interventions | Trivalent, live influenza vaccine contained WHO recommended strains for 1999 to 2000: A/17/Peking/95/25 (H1N1), A/17/Sydney/97/76 (H3N2), and B/60/St‐Petersburg/95/20. Vaccine or placebo (allantoic fluid from chicken embryos) was administered once intranasally using RDZH‐M4 sprayer (0.25 mL per nostril). The difference between children and adult vaccines is the number of times passed at lower temperature and in the number of mutations of the base attenuated donor strains A(H1N1) and A(H3N2). | |
| Outcomes |
Serological
Paired serum samples were taken from subgroup prior to inoculation and 21 days after and analysed for haemagglutinin inhibition. Effectiveness ILI, bronchitis infections, somatic illness, and allergic pathologies (the last 2 are difficult to understand and have not been extracted) Safety Fever (in different temperature breakdowns), headache, and catarrhal symptoms |
|
| Funding Source | Government | |
| Notes | The authors conclude that the vaccine is safe and effective. We do not think the data support this conclusion, as for example the vaccine does not protect against bronchitis. No viral circulation in community is described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Low risk | Coded preparations |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Summary assessments | Low risk | Plausible bias unlikely to seriously alter the results. |
ca El'shina 2000.
| Methods | Report of a 2‐phase pilot RCT carried out in 1997 to 1998 among Moscow schoolchildren to assess safety of live attenuated trivalent vaccine (Grippol). The comparator was standard care. As usually happens in reports from Russia, there is a third study nested in the text. The study of cohort design was school based and assessed effectiveness against ILI. Data on general morbidity (excluding influenza and ARI) collected over entire observation period to determine possible side effects. Efficacy evaluated by comparing morbidity due to influenza and ARI using coefficient of efficacy. | |
| Participants | In the first study, 2 groups (aged 14 to 17 years) were formed by randomisation. Both groups had 30 participants. In the second study, 40 children aged 6 to 14 were again randomised to Grippol or standard care. The cohort study was carried out in 3 schools located near each other with a relatively similar level of morbidity and a comparable number of pupils. 1 school with a total number of 1835 students was assigned to the intervention group, and 2 schools with a total number of 1315 students were assigned to the control group. However, in the school assigned to the intervention group, "930 individuals were inoculated in the pre‐epidemical season. The remaining 905 pupils were also practically entirely healthy at the time of the inoculations but remained UV due to temporary medical exclusions. They acted as the so called ‘internal’ control group" | |
| Interventions | "The influenza tri‐valent polymer‐subunit ‘Grippol’ vaccine was created in the State Scientific Centre (the Institute of Immunology, the Ministry of Health for the Russian Federation) (7, 10). The preparation belongs to a new generation of vaccines. It is a sterile preparation, based on highly pure surface proteins of the influenza viruses A and B – hemagglutinins and neuraminidases. They are protective antigens (6). It is also based on synthetic high‐molecular immuno‐stimulator polyoxidonium, which has an adjuvant activity (10). ‘Grippol’ differs from other subunit influenza vaccines in the world because of its antigenic load, which is reduced by 3 times because of the inclusion of an immuno‐stimulator. The inoculation dose of the ‘Grippol’ vaccine contains 5 μg of hemagglutinin of each strain of the influenza virus and 500 μg of polyoxidonium". No mention is made of matching or content. | |
| Outcomes |
"From December to April, monthly collections and analysis of data for the morbidity of influenza and acute respiratory illnesses were organised in the working and control groups. Moreover, in order to correct the clinical diagnoses, the selective serological decoding of cases of illness diagnosed as influenza and acute respiratory illnesses was carried out". Table 3 reports ILI for the 930 in the intervention cohort and their 905 controls out of a total of 1835 and 1315 schoolchildren, respectively. This also includes "serological confirmation in 60.4% of cases". |
|
| Funding Source | Government | |
| Notes | The authors conclude that Grippol is safe and effective and recommend immunisation of children. The extensive contradictions between text and figures, unexplained selective serological testing and vaccination put this study at high risk of bias. Figure for serologically confirmed is 60.4% of calculated per 1000 figure for number with influenza and ARI. Serological confirmation is therefore an estimate, not an absolute figure, and it may not be appropriate to include in meta‐analysis of serologically confirmed influenza. Tables show period of seasonal rise from July 1997 to April 1998, which is likely a mistake. Text refers to period from December 1997 to April 1998. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Insufficient description |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Insufficient description |
| PCS/RCS‐Comparability | Unclear risk | Insufficient description |
| PCS/RCS‐Assessment of Oucome | High risk | No description |
| Summary assessments | High risk | Plausible bias that seriously weakens confidence in the results |
ca Fujieda 2006.
| Methods | Prospective cohort study carried out in 54 clinics around Japan during the 2002 to 2003 season. The study assessed the effectiveness of TIV against ILI. Baseline questionnaires were filled in at enrolment, and then an "attack" questionnaire in which every week for 17 weeks parents recorded children's body temperature in 3 steps of 1 °C. The authors report ILI surveillance Japan‐wide with peak isolates of A and B viruses in January to February. The authors describe an analysis stratified by age and other potential confounders (which are reported in Table 1). Systematic differences in age, birth and current body weight, number of siblings, family members, number and space in rooms, etc. are significantly different between hemicohorts. | |
| Participants | 2913 children (1512 vaccinees and 1401 non‐vaccinees) under 6 years of age (52% males). Allocation was on an alternation basis according to the provision of parental informed consent, and the following child whose parents did not give consent was allocated to the control arm. Attrition is not mentioned. Data by age group and location are reported but not extracted. | |
| Interventions | TIV (A/New Caledonia/20/99(H1N1), A/Panama/2007/99(H3N2), and B/Shandong/7/97) or no vaccination in 1 or 2 shots according to age. Producer not described. Matching not reported. | |
| Outcomes |
Serological
N/A Effectiveness ILI: acute febrile illness occurring during the highest epidemic period in each study area (but it is ILI, not influenza as claimed by the authors). Fever reported as below 38 °C, between 38 °C and 39 °C, and 39 °C or more (but no description of how temperature was taken by parents or whether follow‐up was complete). Safety N/A |
|
| Funding Source | Government | |
| Notes | The authors conclude that the adjusted OR and its 95% CI were calculated by the proportional odds model using logistic regression with 3‐level outcome variables (< 38.0/38.0 to 38.9/> or = 39.0 °C). A significantly decreased OR of vaccination was observed (OR 0.76, 95% CI 0.66 to 0.88), corresponding to a vaccine effectiveness (1 ‐ OR) of 24% (95% CI 12% to 34%). When the analysis was confined to those aged > or = 2 years, a more pronounced OR of 0.67 (95% CI 0.56 to 0.79) was obtained with a vaccine effectiveness of 33% (21% to 44%). On the other hand, no significant vaccine effectiveness was detected among very young children: the ORs were 1.84 (95% CI 0.81 to 4.19) for those < 1 year of age and 0.99 (95% CI 0.72 to 1.36) for those 1.0 to 1.9 years of age, and 1.07 (95% CI 0.80 to 1.44) when these 2 age groups were combined. Thus, among very young children vaccine effectiveness could not be demonstrated. Lack of description of matching, unacceptable ILI definition (fever only), recall bias, measurement bias, unknown attrition, systematic differences between hemicohorts, etc. put this study at high risk of bias. Of note, in the Results the range of percentage of A and B isolates in each study area is reported as a proportion of samples submitted during the height of the epidemic by sentinel physicians from symptomatic cases: 3% to 61%. In other words, if data from this non‐random sampling are generalisable, up to 97% of ILIs were not due to influenza. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Volunteer |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Volunteer |
| PCS/RCS‐Comparability | High risk | Several differences between groups at baseline |
| PCS/RCS‐Assessment of Oucome | Low risk | Secure record |
| Summary assessments | High risk | Plausible bias that seriously weakens confidence in the results |
bb Goodman 2006.
| Methods | Industry‐funded case‐control study conducted among healthy children of both sexes who were part of an HMO (or group practice?), HPMG, in Minneapolis, USA. The study was conducted to assess the safety of split TIV in small children after the 2002 decision by ACIP to extend the immunisation to this age group, and study data span 2 "seasons": 2002 to 2003 and 2003 to 2004. There is no declaration of conflicts of interest of the authors. Cases Healthy children aged 6 to 23 for 1 or more days during the TIV administration period enrolled in HPMG for 1 day or more during the study period and had 1 or more diagnostic code for an HPMG clinic during the study period Controls Children with same eligibility criteria matched by birth date and gender |
|
| Participants | 13,383 children of which 3697 received vaccination | |
| Interventions | TIV or no vaccination. Ascertainment of exposure was carried out through HPMG registry but no description of content or lot is given, although the authors report that this information is available. For the effectiveness, 1‐liner with no description of community viral circulation is reported. The authors report that they carried out multivariate modelling to allow for the effects of co‐administration of other vaccines. | |
| Outcomes |
Effectiveness Influenza 1‐liner ‐ no case definition given, although it appears to be based on ICD‐9, which means ILI Safety The following outcomes were identified either by physicians combing the exposed population for possible outcomes of interest and then clustering the diagnosis by ICD categories and then using VSD categories.
|
|
| Funding Source | Industry | |
| Notes | The authors conclude that "We found no statistically significantly elevated hazard ratios for the first TIV dose. An elevated risk of pharyngitis was found for children receiving a second TIV dose. No elevated risk of seizure was found. CONCLUSION: These results, for a population of healthy children, showed no medically significant adverse events related to TIV among children 6 to 23 months of age". Definitions of cases and controls are not reported and were reconstructed by the extractor. More worrying is the fact that the text clearly states that the authors identified the cases by looking at outcomes AND exposure, almost certainly introducing bias in the evaluation and not carrying out blinded assessment of exposure. Numerators and denominators are not reported by case and control status but only HR by first or second TIV injection. Population was selected, and there are very few data to compare cases and controls. 1‐liner by‐the‐ by effectiveness assessment of vaccine. Multivariate modelling use is unclear. How can you adjust for the effects of many concurrent vaccines if you do not have a non‐exposed window and the safety outcomes are highly unspecific (e.g. urticaria)? High risk of bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | High risk | No description |
| CC‐Control Selection | High risk | Insufficient description |
| CC‐Comparability | Unclear risk | No description |
| CC‐Exposure | Low risk | Secure record |
| Summary assessments | High risk | |
ab Grigor'eva 1994.
| Methods | Placebo‐controlled randomised trial of safety and effectiveness of live vaccine carried out in Havana, Cuba (with the collaboration of scientists from the former USSR) during the 1991 to 1992 season. The unit of allocation in schools was 1 child. The trial had 5 arms: 1 ‐ inoculated with A(H1N1) vaccine; 2 ‐ inoculated with A(H3N2) vaccine; 3 ‐ inoculated with B vaccine; 4 ‐ inoculated with trivalent vaccine A(H1N1)+A(H3N2)+B; and 5 ‐ placebo. Morbidity studied during period 1 December to 31 December 1991. The period of epidemic was defined according to serological data and epidemiological curves. Calculation of morbidity based on clinical diagnosis of influenza and ARI. | |
| Participants | 3663 children aged 5 to 14 years | |
| Interventions | Live influenza vaccines, industrially produced series: A (H1N1), strain A/47/T (epidemical virus A/Taiwan/1/86, attenuated donor A/Leningrad/134/47/57); A (H3N2), strain A/47/6/2 (epidemical virus A/Zakarpatye/354/89, attenuated donor A/Leningrad/134/47/57); and B strain B/60/32 (epidemical virus B/USSR/3/87, attenuated donor B/USSR/60/69) | |
| Outcomes |
Serological
Immogenicity ‐ seroconversion ‐ assessed on a sample basis (rule for sample selection not reported)
Recombination analysis of genetic stability Effectiveness Morbidity due to influenza and acute respiratory viral infections according to a variety of symptoms and signs (essentially ILI). Only effectiveness of the 2‐dose schedule was analysed. Background viral circulation was also assessed, as well as data from seroconversions. Safety The following outcomes were recorded: temperature, general ill‐health, dysphonia, reddening of the throat, nasal bleeding, conjunctivitis, cough. Safety was assessed on the basis of sampling (rule for sample selection not reported). Clinical examinations were carried out for 4 days after each vaccination to record temperature, examination of integuments, nasopharynx, and eye mucous and any complaints. |
|
| Funding Source | Government | |
| Notes | The authors conclude that live attenuated "polyvalent" vaccines are effective but no more than monovalent. Poor reporting (no description of blinding, placebo content and aspect, attrition, etc.) and likely selection bias of safety and immunological samples. However, there is a fairly detailed description of background viral circulation in Havana during January to December 1991 and an attempt at putting the results into this context. The authors show that there was no significant difference in morbidity between mono and polyvalent vaccine arms (49.7% in placebo arm versus 32.04% in arm 1 versus 28.29% in arm 2 versus 31.52% in arm 4 ‐ the trivalent vaccine). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No description |
| Summary assessments | Unclear risk | Plausible bias that raises some doubt about the results |
aa Grigor'eva 2002.
| Methods | Placebo‐controlled randomised trial carried out in 2 schools in the Lomonosovskii area and 2 schools in the Gatchinskii area, both in the Leningrad region of Russia. 6 arms were formed using a random selection method: 2 groups were inoculated with the live influenza vaccine I; 2 groups were inoculated with the live influenza vaccine II; and there was 1 placebo group for each vaccine. The unit of selection was 1 individual. The vaccine and placebo were administered as coded preparations. The influenza epidemic of the 1999 to 2000 season was caused by the influenza virus type A/Sydney/5/97 (H3N2). | |
| Participants | 2486 healthy children aged between 7 to 14 years during the 1999 to 2000 season | |
| Interventions | Child and adult variants of the live influenza vaccine (live influenza vaccine I and live influenza vaccine II, respectively). The vaccines were produced by the Irkutsk Federal State Unitary Company for the production of Immuno‐Biological preparations. The strains that formed both vaccines were identified and prepared on a base of the current epidemical influenza viruses A/Peking/262/95 (HINI), A/Sydney/5/97 (H3N2), and B/St‐Petersburg/95/20. The biological activity of each strain was not less than 10 6.5 EID50/0.2 mL for the influenza viruses type A and 10 6.0 EID50/0.2 mL for the influenza type B. The vaccine and placebo (allantoid fluid) were administered intranasally, using the RDZH‐M4 sprayer 0.25 mL in each nostril. The live influenza vaccine I was administered twice with an interval of 21 days, and the live influenza vaccine II was administered once. | |
| Outcomes |
Effectiveness
Influenza: "In order to carry out the serological correction of the clinical diagnosis, we tested 58 pairs of serum samples from those school children who had contracted influenza and acute respiratory illnesses in the inoculated and control groups. In 22 individuals, the diagnosis of influenza was confirmed serologically. Out of the 22, 18 (81.8%) individuals were from the control groups, 3 (13.6%) individuals had been inoculated twice with the live influenza vaccine I, and 1 (4.6%) individual had been inoculated with the live influenza vaccine II (for both the live influenza vaccine I and the live influenza vaccine II, P < 0.001)." This sentence does not make it clear whether there were only 58 children who reported sick or how the sample was chosen and why a separate group of children had to be recruited to test serological responses. Safety ARIs and allergic reactions. Harms' follow‐up was 7 days. |
|
| Funding Source | Government | |
| Notes | The authors conclude that "during the period of the maximum rise of morbidity, the coefficient of efficacy for those inoculated twice with the live influenza vaccine I was 48.8%. For those inoculated with the live influenza vaccine II, the figure was 44.6% (P < 0.05)." However, for influenza it was 83%. "Thus, both vaccines were highly effective. Moreover, the figures of efficacy for both preparations rose significantly after the serological correction of diagnoses". Possibly biased subset of influenza cases in follow‐up. Means of selection of them and of children to assess antibody responses not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No description |
| Summary assessments | Unclear risk | Plausible bias that raises some doubt about the results |
aa Gruber 1990.
| Methods | Multicentre, randomised, placebo‐controlled clinical trial to determine effectiveness and safety of cold bivalent cold recombinant (CR) and trivalent inactivated (TIV) influenza vaccines. Randomisation and allocation procedures were not described. | |
| Participants | "One hundred ninety one (191) healthy children aged 3 to 18 years from 92 families recruited from HFS, Oklahoma Family Practice Center (Oklahoma City), Baylor College of Medicine Family Practice Clinic (Houston, Texas) were enrolled. Recruited families were independently randomised at each participating institution to form 1 of three immunisation groups: 30% were assigned to each vaccine group and 40% to the placebo group. Placebo recipients were randomly assigned to receive intranasal buffered saline or intramuscular sterile saline. No significant differences were noted in socioeconomic status, average size of the family, age distribution of the vaccine recipients. Thirty families were assigned to the TIV group (54 children), 25 to the CR group (58 children) and 37 to the placebo (77). UV family contacts were also followed up during the epidemic of B/Ann Arbor/86 (TIV = 56; CR = 47; placebo = 72)" | |
| Interventions |
|
|
| Outcomes |
Serological
Antibody titres Effectiveness
"When ongoing community surveillance at the Influenza Research Center (Baylor College of Medicine) indicated that influenza virus was present in the community, weekly telephone contacts to families were initiated to evaluate all respiratory illnesses. Home or clinic visits were scheduled for physical examination and collection of nasal washes and throat swab specimens for virus isolation. Children and their families were followed up during the influenza B/Ann Arbor/86 epidemic (winter 85 – 86). An illness was attributed to influenza B infection if an isolate was obtained during the illness or, in a person with a postseason antibody rise only, if the illness occurred within 10 days of an isolate in household contact or during the period of most intensive viral activity in the community" Safety Families were contacted by telephone to record local, systemic, respiratory symptoms occurring within 2 weeks after vaccination. |
|
| Funding Source | Government | |
| Notes | The authors conclude that TIV is highly effective but that serological responses to CR vaccine depended on previous exposure and immunological memory.
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient description |
| Summary assessments | Unclear risk | Randomisation and allocation are not described in detail, so the success of randomisation is unclear. |
ab Gruber 1996.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled clinical trial to assess the efficacy and safety of live attenuated, cold‐adapted influenza vaccine in children aged 6 to 18 months. Vaccine was administered either as monovalent or bivalent preparation in a randomised, double‐blind manner (author contact is needed for description). | |
| Participants | Children aged 6 to 18 months who were enrolled at vaccination units: Baylor College of Medicine, St. Louis University, University of Rochester, Vanderbilt University, University of Maryland. 182 children were vaccinated, all were born after the last influenza A epidemic and had little opportunity for H3N2 exposure. | |
| Interventions |
Vaccines were prepared by Wyeth‐Ayerst (Philadelphia). Vaccine and placebo were administered as nose drops as 0.5 mL dose in the autumn of 1991. |
|
| Outcomes |
Serological
Haemagglutination antibody inhibition and ELISA were determined against H1N1 and H3N2. Effectiveness Children were monitored during the winter of 1991–92 to evaluate the protection against influenza A H3N2 (A/Beijing/89) epidemic. Once influenza was detected by community surveillance, all children were followed closely by weekly phone calls. A home visit was done if a child had symptoms of respiratory illness or any household contacts had fever > 37.8 °C and upper respiratory symptoms. In these cases a nasal wash for viral culture was obtained. Respiratory illnesses were classified as febrile or afebrile. The person performing the examination remained blinded to the treatment group. Otitis media was coded separately. A total of 128 illnesses among 181 children were identified. More than 50% of children with respiratory illnesses had viruses other than influenza. Influenza A/Beijing/89 was isolated from 23 children with respiratory illnesses. Safety During the 10 days after vaccination, parents and guardians recorded the child’s temperature twice a day (morning and evening) and symptoms including cough, rhinorrhoea, diarrhoea once a day (evening). Fever was considered any temperature > 37.8 °C. For the other symptoms were considered at least 3 stools in 24 hours. Parents had to contact the study site if a child had more than 1 symptom on a given day or had fever > 37.8 °C. These were clinically evaluated. Diary information was unavailable for 2 children. |
|
| Funding Source | Government/industry | |
| Notes | The authors conclude that live attenuated vaccines are significantly more effective than inactivated vaccines. Data about epidemic strain isolation in the 4 arms were pooled based on whether children received an H3N2‐containing vaccine or not. It is not possible to go back to the isolation in the single 4 arms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description |
| Summary assessments | Unclear risk | Plausible bias that raises some doubt about the results |
ab Gruber 1997.
| Methods | RCT, double‐blind, multicentre to assess reactogenicity and safety of a cold‐adapted bivalent influenza vaccine containing the strains A/Kawasaki/9/86 (H1N1) virus and ca A/Beijing/352/89 (H3N2) | |
| Participants | 1126 children aged 2 to 36 months enrolled from 13 participating institutes in autumn 1993. Children were excluded if they had received any vaccine within 3 weeks before vaccination with influenza or placebo. | |
| Interventions | Enrolled children were randomised to receive 1 0.5 mL dose of cold‐adapted bivalent flu vaccine containing 104, 106, or 107 TCDI50 ca A/Kawasaki/9/86 (H1N1) virus and ca A/Beijing/352/89 (H3N2) virus per 0.5 mL dose or placebo consisting of egg allantoic fluid. Vaccines and placebo were intranasally administered. |
|
| Outcomes |
Serological
Haemagglutination antibody inhibition titre against A/Kawasaki/9/86 and A/Beijing/352/89 were determined. Serum specimens were collected before vaccination and 35 days after by finger stick or venipuncture. Effectiveness Not assessed Safety Parent kept a diary card for 7 days after immunisation. Temperature (recorded axillary, rectal or orally) and other symptoms were reported. Fever was considered as temperature 38.6 °C rectal; 38.1 °C orally or 37.5 °C axillary. |
|
| Funding Source | Government/industry | |
| Notes | The authors conclude that CR vaccine is well tolerated and immunogenic but less so in very young children. The number of children in each study arm is not clearly reported. Data from the table of respiratory symptoms (table 2 of this paper) do not agree with those reported in table 1 (fever). A total of 1126 children were enrolled, but they resulted in 1249 from table 1 (and 1123 from table 2). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses to follow‐up, unlikely to be related to true outcome |
| Summary assessments | High risk | Follow‐up very short (7 days after each dose). Major denominator discrepancies between text and tables |
ab Gutman 1977.
| Methods | Placebo‐controlled clinical trial to assess safety and reactogenicity of monovalent A/New Jersey/8/76 administered as whole virus or split‐product (disrupted virion) vaccine in 4 different preparations from licensed manufacturers | |
| Participants | Children aged 3 to 10 years appeared at the Lincoln Community Health Center (LCHC, Durham, NC, USA) between 24 May and 28 May 1976, whose physicians allowed participation to the trial. Children were divided into 2 age groups (3 to 6 years and 6 to 10 years) and assigned to the preparation by continuous rotation of the vial numbers. | |
| Interventions | All vaccines were prepared from virus strain A/New Jersey/76 (Hsw1N1). Employed preparations were:
|
|
| Outcomes |
Serological
3 weeks after vaccination, a serum sample was taken to determine the antibody titre HAI to A/Victoria/3/75, A/swine/1976/31; A/Mayo Clinic/103/74, and A/ New Jersey/76 viruses. Children with titre above 1:20 to A/New Jersey were offered additional vaccination with MN 100 vaccine. Effectiveness N/A Safety After immunisation children were observed at the LCHC for 20 minutes. Mothers were provided with 2 thermometers to record temperatures 6 and 9 hours later. Both were returned on the next day to be read by investigators. On the day after, children returned to be examined for adverse reactions or fever. Mothers recorded on a sheet adverse reactions (pain at the injection site, malaise, myalgia, headache, fever, nausea, and tenderness, redness, induration). Sheets were completed the day after immunisation at the LCHC. A physician was available during the study when parents recognised or suspected an adverse reaction. |
|
| Funding Source | Government | |
| Notes | The authors conclude that reactogenicity of both types of vaccines were similar. It is not clear if assignation to the vaccine or placebo group was made separately for the 2 age groups. Safety data are expressed considering only the vaccine group type (i.e. split or whole virus) and not each arm that was effectively randomised. The placebo arm is reported in an aggregate fashion with no age breakdown, making vaccine comparison impossible. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not used |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Summary assessments | Unclear risk | It is not clear if assignation to the vaccine or placebo group was made separately for the 2 age groups. |
ba Hirota 1992.
| Methods | Case‐control study to asses correlation between ILIs and influenza immunisation status in schoolchildren aged between 6 and 12 years during an epidemic | |
| Participants | 814 children from 1 of the 9 elementary schools of Kasuga City (Fukoka Prefecture, Japan). Children were aged 6 to 12 years. | |
| Interventions | Immunisation with commercial inactivated flu vaccine prepared with the strains A/Yamagata/120/86 (H1N1), A/Fukoka/C29/85 (H3N2), B/Nagasaki/1/87. Each mL of vaccine contained 200 CCA units of each strain. Vaccine was subcutaneously administered in 2 doses of 0.3 mL. Vaccination was carried out after consensus from parents was obtained: the first dose was administered on 25 October and the second on 16 November 1988. 496 children (60.9%) were not immunised; 187 (23.0%) received 2 doses of vaccine; and 131 (16.1%) received 1 dose of vaccine. From data recorded by the Surveillance System for Tuberculosis and Infectious Diseases, an influenza epidemic lasted in Fukoka between 30 October and 1 April (with a sharp peak between 25 December and 11 February), which was caused mainly by the strains A/H1N1 (95%), A/H3N2 (3%), and B (2%). Percentages refer to 1575 isolates from all Japan. | |
| Outcomes |
Serological
N/A Effectiveness
Cases were defined as:
Controls defined as:
Questionnaires were returned from the parents of 803 children. MILI and SILI groups were composed of 48 and 80 children, respectively. Control group NS consisted of 196 children. Safety N/A |
|
| Funding Source | Unclear | |
| Notes | The authors conclude that vaccination was effective against SILI but not MILI ‐ case definition omits ARI onsets during the first 2 weeks of epidemic peak and those after the period (enhances it for the conservative determination for the risk factor). Immunisation data for MILI were not shown. Criteria for selection of case and controls (i.e. absenteeism and medical consultation) might have introduced selection bias. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | Unclear risk | Based on self report |
| CC‐Control Selection | Unclear risk | Not independent from case selection |
| CC‐Comparability | High risk | No description |
| CC‐Exposure | Low risk | Secure record |
| Summary assessments | High risk | Vaccination was voluntary, but its basis was not described. |
aa Hoberman 2003a.
| Methods | RCT to assess the effectiveness of inactivated influenza vaccine against OM and influenza. 2 groups in 2 following years were randomised before beginning of the respiratory season (1 December to 31 March of each year) to receive 2 doses of vaccine or placebo. | |
| Participants | Children aged 6 to 24 months enrolled at Children Hospital of Pittsburgh. In the first study year, 417 children were enrolled and randomised between 4 October and 30 November 1999 to receive 2 doses of vaccine or placebo. In the second study year, 376 children were randomised between 5 September and 8 December 2000. | |
| Interventions |
First study year:
versus
In both years, 2 doses were administered 4 weeks apart. Of the 417 initial participants, 278 were randomised to receive vaccine and 139 to placebo. 5 children in the vaccine group and 1 in the placebo group were discarded because of failure to meet eligibility criteria. The first dose was administered to 273 (vaccine) and 138 (placebo) children. The second dose was administered to 267 and 134 participants, respectively. Second study year:
versus
1 child from the placebo group was excluded for failure to reach eligibility. 252 children were administered vaccine, 123 placebo. The second dose was administered to 246 and 118 children, respectively. |
|
| Outcomes |
Serological
Effectiveness First study year: biweekly visit carried out after the second dose of vaccine up to 31 March 2000 (4 months); monthly visits up to 15 November 2000. Second study year: biweekly visits from after second dose was administered (December 2000) up to 31 March 2001 (4 months). Parents were instructed to contact staff for cases of upper respiratory tract infection or otitis. In these cases an interim visit was conducted.
In the first study year, 25 cases occurred during the epidemic and a further 12 in the following 25 weeks of surveillance. In the second study year, the corresponding values were 11 and 2 (16 weeks' surveillance). Safety
|
|
| Funding Source | Industry | |
| Notes | The authors conclude that the vaccine was well tolerated but had no effect on OM, resource consumption, or any of the other indicators. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number, computer‐generated list, block randomisation (block of 9) |
| Allocation concealment (selection bias) | Low risk | "randomisation lists were kept in locked files not accessible to blinded personnel" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. |
| Summary assessments | Low risk | Plausible bias unlikely to seriously alter the results. |
aa Hoberman 2003b.
| Methods | See aa Hoberman 2003a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Funding Source | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number, computer‐generated list, block randomisation (block of 9) |
| Allocation concealment (selection bias) | Low risk | "Randomisation lists were kept in locked files not accessible to blinded personnel" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. |
| Summary assessments | Low risk | |
ca Jianping 1999.
| Methods | Cohort study carried out on people from the Chinese People's Liberation Army (PLA) between December 1996 and May 1997 | |
| Participants | "One hundred and sixty‐eight children aged 3 to 6 years from the PLA in areas not considered at risk and who had not had influenza recently (adult and elderly data not extracted). Vaccinated groups consisted of 80 children aged between 3 and 6 years, 363 adults between 18 and 59 and 235 elderly over 60 years Controls were not immunised. Correspondent groups consisted respectively of 88 (children), 372 (adults) and 218 (elderly) people" | |
| Interventions | Inactivated influenza vaccine Vaxigrip (Pasteur Mérieux Connaught, France). Children up to 3 years were immunised with 2 doses of 0.25 mL administered 1 month apart. A single dose of 0.5 mL was administered to children over 3 years and adults. | |
| Outcomes |
Serological
N/A Effectiveness All participants were observed from 21 days to 6 months after vaccination. They were asked to report the following symptoms: fever over 38.5 °C, headache, myalgia or arthralgia, cough, sore throat, and coryza. Cases of fever due to other causes were excluded.
Safety Not assessed. Only serious adverse reactions that occurred during the study are reported. |
|
| Funding Source | Government | |
| Notes | "The examined vaccine was strongly protective in populations of different ages" The difference between outcomes is unclear. Gender was not considered in the reporting, and it seems strange that children are enrolled in the PLA. May have lost a lot in translation. Very confusing outcome definition and overlap. We have a problem believing that the vaccine protected from the common cold. Viral circulation was not discussed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Insufficient description |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Insufficient description |
| PCS/RCS‐Comparability | Unclear risk | Insufficient description |
| PCS/RCS‐Assessment of Oucome | High risk | Self reported |
| Summary assessments | High risk | Plausible bias that seriously weakens confidence in the results |
ca Kawai 2003.
| Methods | Prospective cohort study carried out during 2001 to 2002 in 38 practices in Japan (staffed by participating members of the Japanese Physicians Association). Doctors enrolled consenting vaccinated participants on an Internet‐based register from 1 October to 31 December 2001. Unvaccinated participants were selected by the researchers from the same clinic and matched by age and sex. By 31 May 2002, researchers had added data on symptoms of ILI and AE experienced by the participants. Information was elicited on the basis of self reported questionnaires, emails, or phone calls. | |
| Participants | Children aged 0 to 15 years (older children participated, but from 16 years are not separable from 16 to 64 years' age group), adults, and elderly up to the age of 104. A total of 8841 participants took part in the cohort study. | |
| Interventions | Inactivated influenza vaccine containing A/New Caledonia/20/99 + A/Panama/2007/99 + B/Johannesburg/5/99 once or twice. History of previous year's exposure was also elicited. A sliding scale of doses was used for age groups. Results are presented by 1, 2, or no immunisations. | |
| Outcomes |
Serological
Rapid kit testing was carried out in 75 of the 124 participants with ILI symptoms; 64 of these were positive (A viruses recovered from 3 participants). Paired sera were positive in 5 of the 6 participants in whom they were taken. Effectiveness ILI (sudden onset, temperature over 38 °C, sore throat and fatigue). Influenza was defined as ILI plus rapid test diagnosis, or serum antibody increase or viral isolation. Safety Data for 96 participants are reported for the vaccinated arm but not for participants in the UV arm. |
|
| Funding Source | Institutional | |
| Notes | The authors conclude that the vaccines were 67.6% and 84.5% effective, respectively against ILI (1 or 2 immunisations), and 54% and 79.8% effective against influenza (1 or 2 immunisations). No protection against ILI was conferred by immunisation the previous season. Despite an extensive baseline description of the 3 arms, the study has so many problems that the results are difficult to interpret: selection of participants, practices and controls, lack of specification of viral circulation and matching, non‐random serological testing, loss of safety data. Particularly non‐random kit testing makes a nonsense of the conclusions of the study. It is very strange that 64/8841 had influenza and yet had 84% efficacy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Volunteer, non‐information on number of doses |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Volunteer |
| PCS/RCS‐Comparability | Unclear risk | Matching by clinic age, sex |
| PCS/RCS‐Assessment of Oucome | High risk | No information on follow‐up length, self reported |
| Summary assessments | High risk | Despite an extensive baseline description of the 3 arms, the study has so many problems that the results are difficult to interpret: selection of participants, practices and controls, lack of specification of viral circulation and matching. |
aa Khan 1996.
| Methods | Single‐blind, placebo‐controlled randomised trial to compare the efficacy of trivalent cold‐adapted and trivalent inactivated split‐virus influenza vaccine. During the period 1 January to 2 February 1992, there was a local epidemic of A/H3N2 (not better defined). | |
| Participants | Children aged 9 to 12 years from 2 schools of Vologda (USSR). Children were excluded if they had an acute illness, oral herpetic lesions, temperature > 37.0 °C on the day of inoculation, or a history of egg allergy or severe reaction to previous influenza vaccination. A total of 555 children were enrolled between 21 October and 1 November 1991. 245 were enrolled from the school 1 and 310 from school 2. | |
| Interventions | After a physical examination, children were randomly assigned to receive vaccine or placebo, using the route of administration previously chosen by parents or guardians. For this purpose a blocked randomisation scheme was used with a vaccine to placebo ratio of 2:1. Vaccines
The vaccine groups do not differ significantly by age, sex, school, grade attended, or seronegativity for the 3 strains. Blood specimens were collected by fingerstick on the day of inoculation and again 28 days and 5 months after inoculation. |
|
| Outcomes |
Serological
3 sera samples were taken from about half the children over the 5‐month period. Effectiveness Schoolchildren absent for medical reasons were examined by a physician who was not affiliated with the study, and re‐examined before they returned to school. A letter stating the medical condition causing their absence was filled out. These data were recorded onto the child’s school medical card and covered the period 10 November 1991 to 17 March 1992; they were transcribed from the medical card at the time of serum collection 5 months after vaccination. Absenteeism due to ILI was defined as the first school absence with physician’s diagnosis of either acute respiratory disease or influenza. The epidemic lasted from 1 January to 2 February 1992. (Specific diagnosis of influenza refers to an acute respiratory illness occurring during the official influenza season and is a clinical diagnosis, moreover the employed criteria were not uniform, and these outcomes were not used). Vaccine efficacy was also estimated using 4‐fold serum antibody increase to A H3N2 (circulating virus). Safety Children enrolled during the first week were monitored daily for 4 days after inoculation. Those enrolled during the second week were monitored on the day after inoculation. Children with reaction after inoculation were monitored by paediatricians who were unaware of the child’s vaccine group until the symptoms resolved. Data on low‐grade axillary fever and other local reactions were reported. Some harms are reported with insufficient information for extraction (coryza and sore throat). |
|
| Funding Source | Government | |
| Notes | The authors conclude that there is no significant difference between live attenuated and inactivated vaccine in preventing school absence due to ILI, but both are significantly more effective than placebo. The authors report ILI and assume it to be influenza because of the background rate. The text is also contradictory because half the participants are supposed to have had serology carried out on a non‐random basis, but the middle line of Table 2 (reporting more than 4‐fold titre rise) appears to indicate that school absentees had titres done and lumps absences with titre rises under "both" with a calculation of vaccine efficacy. The 2 placebos are not reported separately, so it is impossible to assess safety apart from what is in the text on page 173 right‐hand column. Denominators do not match between tables and text, and the only mention of attrition is the statement that medical cards for 5 of the 555 participants were not received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Single |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses to follow‐up |
| Summary assessments | Unclear risk | Possible confounding by indication |
ab King 1998.
| Methods | Randomised, placebo‐controlled, multicentre trial | |
| Participants | Children aged 18 to 71 months in good health. 238 were enrolled altogether at Baylor College of Medicine Houston, Cincinnati Children Hospital, Saint Louis University, and University of Maryland at Baltimore in 3 steps. 118 were enrolled from 1 ambulatory clinic in the northern area of Santiago (Chile). | |
| Interventions | Cold‐adapted trivalent flu vaccine containing the strains A/Johannesburg/33/94 (H3N2), B/Panama/45/90, and A/Texas/36/91 (H1N1) in different titre (104, 105, 106, or 107 TCID50 of each strain) versus placebo. Vaccine and placebo (allantoic fluid) were assigned in double‐blind manner using a randomisation table provided by the manufacturer (Avion). Enrolment took place in 3 steps:
In the USA the randomisation was designed so that 50% of the children received vaccine or placebo as drops and the remaining 50% by spray. |
|
| Outcomes |
Serological
Antibody titres Effectiveness N/A Safety Temperature was recorded each evening within 10 days after vaccination on a diary card. Other daily recorded symptoms were: cough, wheezing, rhinorrhoea, sore throat, or irritability. Children were examined by clinicians if an axillary, oral, or rectal temperature > 38 °C was observed. |
|
| Funding Source | Government/industry | |
| Notes | The authors conclude that the vaccine was safe and immunogenic in 2 of the 3 strains. Small denominator | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Summary assessments | Unclear risk | Insufficient information about study design |
ca King 2006.
| Methods | Prospective cohort study carried out at 24 public elementary schools in Maryland, Texas, and Minnesota and 4 (kindergarten to elementary) in Washington during 2004 to 2005. The study aimed at assessing the effect of a school‐based vaccination programme on the households of children attenders. The schools were divided into 11 clusters, 7 of which had random selection of the intervention school and the other 4 of which were selected in a non‐random way. The remaining schools were controls. Clusters were matched by geographic, ethnic, and social class variables. There was a peak circulation period of influenza around the end of January 2005. Other household members could also have been vaccinated. After the peak week, all households who had children in study schools received an anonymised questionnaire. The text also refers to a post hoc analysis of vaccinated and non‐vaccinated children regardless of school. This appears to be a second study and also appears to imply that some of the "control school children" (as well as the household members) were vaccinated. | |
| Participants | 5840 pupils in intervention schools and 9451 in control schools, mainly whites in both arms | |
| Interventions | Live attenuated vaccine (MedImmune) intranasally (not better defined) to all children aged 5 or more or do nothing. Content of the vaccine was that of the 2004 to 2005 season. The paper describes main circulating virus (A/California/7/2004 H3N2) as drifted from the strain in the vaccine (not described). | |
| Outcomes |
Effectiveness
ILI, school absenteeism, serious harms at 42 days after vaccination Safety Reported in an appendix |
|
| Funding Source | Industry | |
| Notes | The authors conclude that "Most outcomes related to influenza‐like illness were significantly lower in intervention‐school households than in control‐school households. (ClinicalTrials.gov number, NCT00192218.)". There are several descriptions of the 2005 peak influenza period, but there is no information on vaccine content, although matching must have been at least incomplete as the text described a drifted circulating variant. There is no clear description of age of children or households, of vaccines, of very major discrepancies in denominators of the possible impact of bias of schools who refused to be controls and refused originally proposed placebos. How did this study achieve a trial registration number? It must be an aborted trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Insufficient description |
| PCS/RCS‐Selection Non Exposed cohort | High risk | No description |
| PCS/RCS‐Comparability | High risk | Insufficient information |
| PCS/RCS‐Assessment of Oucome | Unclear risk | Self report |
| Summary assessments | High risk | There is no clear description of age of children or households, of vaccines, of very major discrepancies in denominators of the possible impact of bias of schools who refused to be controls and refused originally proposed placebos. |
ab Levine 1977.
| Methods | Double‐blind, placebo‐controlled phase 1 randomised trial carried out in the summer of 1976 in Baltimore, USA. The aim was to compare reactogenicity and safety of various concentrations of whole‐virion vaccines with split products of various manufacturers. | |
| Participants | 158 Maryland children aged 3 to 5 years. 103 children took part in the 1‐dose evaluation of split products, 47 took part in the 1‐dose evaluation of whole‐virion products, and 28 took part in the 2‐dose evaluation of whole‐virion products. | |
| Interventions | 50, 100, and 200 CCA units of split vaccines (Parke Davis or Wyeth) or 50 or 100 CCA units of whole‐virion vaccines (MSD or Merrell) or placebo. All vaccines were monovalent containing A/New Jersey/8/76 (H1N1). All were administered as single doses, except for a follow‐up of second doses only for whole‐virion vaccines. Discontinuation of the use of split vaccines was due to disappointing antibody responses. | |
| Outcomes |
Serological
Paired sera for antibody titres Effectiveness N/A Safety Fever, nausea, and malaise and a reactogenicity score with definitions described in the Lerman 1977 study |
|
| Funding Source | Government | |
| Notes | The authors conclude that both vaccines were generally well tolerated, with whole‐virion products causing low‐grade pyrexia and split products being virtually non‐immunogenic in 1‐dose schedules. A well‐described study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description |
| Allocation concealment (selection bias) | Low risk | "preparations of vaccines and placebo in coded vials were supplied by the Bureau of Biologics" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Summary assessments | Low risk | A well‐described study |
ca Maeda 2002.
| Methods | Prospective open cohort study assessing the effects of TIV on children. The study took place in Japan between November 1999 and April 2000. | |
| Participants | 86 healthy recipients of TIV and 94 aged‐matched controls aged 5 to 83 months. Controls were randomly selected from hospital medical records of healthy infants. Age and sex of participants are described in Table 1. There is no mention of attrition, and age and gender of participants appear evenly matched. | |
| Interventions | TIV containing 200 CCA/mL of A/Beijing/262/95(H1N1), 350 CCA/mL of A/Sydney/5/97(H3N2), and 300 CCA/mL of B/Shandong/7/97. 2 injections were given subcutaneously 14 days apart. Dosage was on sliding scale per age: children < 1 year got 0.1 mL, those aged 1 to 6 years got 0.2 mL, and those > 6 years received 0.3 mL. The comparator was do nothing, as placebo administration was not possible "for ethical reasons". | |
| Outcomes |
Serological
Immunoassay (rapid test, Directigen Flu‐A; Becton Dickinson, USA), capable of detecting only influenza A Effectiveness Influenza A. Swabs were taken from children reporting to the hospital as instructed with a temperature > 37.8 °C. Follow‐up was from January to April 2000. Safety N/A |
|
| Funding Source | Unclear | |
| Notes | The authors conclude that inactivated influenza vaccine reduces the incidence of influenza A virus infection in children aged 2 to 6 years but not in children 6 to 24 months old (as 4 out of 5 infected vaccinees belonged to this group). Selection bias may be at play, as the enrolment procedure is not described, and the study controls only for age and sex. In addition, controls were selected on the basis of medical records, which may mean that the controls had had a recent medical contact (although none of them had been vaccinated in the previous 12 months). Viral circulation and vaccine matching are not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Insufficient description |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Matched infants in good health |
| PCS/RCS‐Comparability | Unclear risk | Matched infants |
| PCS/RCS‐Assessment of Oucome | Unclear risk | Laboratory |
| Summary assessments | High risk | Selection bias may be at play, as the enrolment procedure is not described, and the study controls only for age and sex. In addition, controls were selected on the basis of medical records, which may mean that the controls had had a recent medical contact. |
ca Maeda 2004a.
| Methods | Prospective open cohort study of inactivated TIV over 3 seasons in Japan. Placebo was not used for ethical reasons. Children came to hospital if they developed febrile illness within 48 hours of inoculation. The follow‐up period was from January to April of each year. | |
| Participants | 175 children were given vaccine every November or December of 1999, 2000, or 2001. For the control group, 171 aged‐matched children in good health who had not received influenza vaccine within 1 year of enrolment were randomly assigned from medical records of hospitals. | |
| Interventions | Inactivated vaccines for the 3 seasons:
|
|
| Outcomes |
Serological
Influenza A virus infection determined using Becton Dickinson Directigen Flu‐A antigen test performed according to direction of manufacturer. Test utilises enzyme‐conjugated monoclonal antibodies. Effectiveness Influenza A infection. If temperature > 38 °C throat swab taken and tested for influenza A. Safety N/A |
|
| Funding Source | Government | |
| Notes | The authors conclude that in small children younger than 24 months the vaccine is not protective. The authors report that there were no complications and no hospitalisations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Insufficient description |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Matched infants in good health |
| PCS/RCS‐Comparability | Unclear risk | Matched infants |
| PCS/RCS‐Assessment of Oucome | Unclear risk | Laboratory |
| Summary assessments | High risk | Selection bias may be at play as the enrolment procedure is not described. |
ca Maeda 2004b.
| Methods | See Maeda 2004a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Funding Source | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Insufficient description |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Matched infants in good health |
| PCS/RCS‐Comparability | Unclear risk | Matched infants |
| PCS/RCS‐Assessment of Oucome | Unclear risk | Laboratory |
| Summary assessments | High risk | Selection bias may be at play as the enrolment procedure is not described. |
ca Maeda 2004c.
| Methods | See Maeda 2004a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Funding Source | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Insufficient description |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Matched infants in good health |
| PCS/RCS‐Comparability | Unclear risk | Matched infants |
| PCS/RCS‐Assessment of Oucome | Unclear risk | Laboratory |
| Summary assessments | High risk | Selection bias may be at play as the enrolment procedure is not described. |
cb Nicholls 2004.
| Methods | Retrospective cohort study of an outbreak of influenza A(H3N2) between 10 March and 5 April 2002 in a semi‐closed, highly vaccinated religious community in the UK. 90% of members of the community had been vaccinated before 7 November 2001. Data collected by self completion questionnaire, response rate was 92% (350/380). | |
| Participants | 350 residents of religious community including 133 children aged 0 to 14 years | |
| Interventions | Inactivated trivalent influenza vaccine containing A/Moscow/10/99‐like (H3N2), A/New Caledonia/20/99‐like (H1N1), and B/Sichuan/379/99‐like. The study reports a comparison of efficacy of the vaccine in members vaccinated in the USA with those vaccinated in the UK, in effect testing the hypothesis of possible lower efficacy of the UK‐administered vaccine. | |
| Outcomes |
Serological
Throat swabs from 39 case volunteers, 10 non‐cases, and 5 of undefined status. Paired sera from 9 members and single sera from 2 were drawn. 27 throat swabs were positive for H3N2/Panama/2007/99‐like, which is well matched to vaccine content. Effectiveness A case was defined as self reported fever or chills accompanied by 1 or more of cough, sore throat, headache. Outcomes were evaluated by questionnaires distributed on 2 April 2002. Safety N/A |
|
| Funding Source | Unclear | |
| Notes | The authors conclude that the vaccine was not effective in preventing the outbreak, despite being well matched to the circulating virus (risk of developing ILI symptoms was not significantly different between vaccinated and UV: OR 1.14, 95% CI 0.41 to 3.14). VE was ‐5% in those vaccinated in the UK and 77% (53.2% to 88.4%) for those vaccinated elsewhere, mainly in the USA. The study reflects its mostly retrospective nature. The outbreak peaked on 20 March, 5 days before the arrival of the investigators. We do not understand why there is no matching of ILI cases with positive influenza diagnosis by vaccine exposure. Why report effectiveness when they could report efficacy? | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | High risk | Selected cohort |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Same community |
| PCS/RCS‐Comparability | High risk | Insufficient information |
| PCS/RCS‐Assessment of Oucome | High risk | Self report |
| Summary assessments | High risk | Plausible bias that seriously weakens confidence in the results |
ab Obrosova‐Serova 1990.
| Methods | Randomised, single‐blind, placebo‐controlled trial to assess reactogenicity in children of live attenuated cold‐adapted influenza B vaccine | |
| Participants | "The study was conducted in a children's nursery and in a children's boarding school. 109 children and 87 children 3 to 15 years old received respectively vaccine or placebo" | |
| Interventions | Enrolled participants were randomised to receive at least 1 dose or 2 doses of live attenuated cold‐adapted influenza B vaccine derived by reassortment between wild‐type B/Ann Arbor/1/86 and cold‐adapted B/Leningrad/14/55 viruses. First dose of vaccine or placebo was administered at day 0 and second dose after 3 weeks. 0.5 mL vaccine or placebo was administered intranasally by aerosol spray. Placebo consisted of distilled water. At the time of the study no evidence of circulation of influenza B viruses in Moscow was reported to the laboratory responsible for surveillance in the region. | |
| Outcomes |
Serological
HI titre against LEN‐B/14/5/1 reassortant virus. Sera were collected by finger stick before the first and second inoculations and 3 weeks later. Effectiveness N/A Safety Adverse reactions were defined as fever (axillary temperature > 37.5 °C) and upper respiratory symptoms (coryza or pharyngitis, or both) observed for 4 days after each inoculation. |
|
| Funding Source | Government | |
| Notes | The authors conclude that the vaccine was immunogenic in younger children but less so in older children. There was lot of unexplained attrition between the first and second inoculations. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Single‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was lot of unexplained attrition between the first and second inoculations. |
| Summary assessments | High risk | There was lot of unexplained attrition between the first and second inoculations. |
ca Ozgur 2006.
| Methods | Single‐blind prospective study carried out during the 2003 to 2004 season in children from 8 day‐care nurseries around Ankara, Turkey. The study aim was to assess the effectiveness of TIV in preventing AOM and OME. Randomisation is not mentioned, comparator is do nothing, and denominators are uneven. The single‐blind design refers to the ear, nose, and throat (ENT) tympanomtrist. The influenza period was defined as 15 December 2003 to 31 January 2004 on the basis of influenza and RSV isolates in the community. 3 other influenza periods are also described. | |
| Participants | 135 healthy children aged 6 to 60 months in day care. 16 children were excluded from the study (3 because of tympanostomy tubes, 11 because they could not complete the minimum of 3 follow‐up visits, and 3 due to failure to have the second vaccination). The authors report their analysis for 119 children (61 vaccinated and 58 UV, mean age 43 months). 22 children were aged less than 2 years. The arms were similar for breastfeeding, gender, dummy use, history of frequent URTIs, antibiotic use, allergy, asthma, previous OM, and passive smoking. | |
| Interventions | TIV containing A/Moscow/10/99 (H3N2), A/New Caledonia/20/99 (H1N1), or B/Hong Kong/330/2001 in 2 doses (Fluarix or Vaxigrip). No mention is made of the circulating strains, although the content of the vaccine was WHO recommended. | |
| Outcomes | Effectiveness OM diagnosed at tympanometry and otoscopy by a blinded ENT surgeon: normal ear (no abnormality and type A and C1 curves on tympanometry), AOM (hyperaemia, opacity, bulging or immobility of the TM together with any of the following: fever, earache, irritability, and vomiting), OME (retraction, opacity, bulging or immobility of the TM without clinical signs and with C2 or B tympanometry curve), OM (any episode of either AOM or OME) | |
| Funding Source | Unclear | |
| Notes | The authors conclude that "The frequencies of AOM, OME and total otitis media episodes in vaccinated children were 2.3%, 22.8% and 25.2%, respectively and these frequencies were 5.2%, 31.1% and 36.3% in the UV group. The difference was statistically significant (P < 0.01). This difference was especially prominent in the influenza season (P < 0.05). Influenza vaccine is effective in reducing AOM and OME episodes in 6‐ to 60‐month‐old day care children, especially during influenza season". The message is mixed, as the authors point out that the relatively low effectiveness of TIV makes mass vaccination to prevent an OM (a syndrome) impractical. Report was not very detailed, likely to be a cohort or CCT. Confusingly reported outcome data in Table 2. Numerators were extracted from the text. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Insufficient description |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Insufficient description |
| PCS/RCS‐Comparability | High risk | Possibly confounding by indication |
| PCS/RCS‐Assessment of Oucome | Unclear risk | Secure record |
| Summary assessments | High risk | Possibly confounding by indication |
aa Principi 2003.
| Methods | Randomised controlled open trial assessing the socioeconomic impact of virosomal vaccine compared to no intervention. The trial is reported very briefly within a wider descriptive paper reporting incidence of influenza in a prospective cohort of 3771 children aged around 3.5 years reporting to emergency room or family paediatricians with ILI symptoms. The cohort has been excluded because of lack of exposure to vaccines and the selected nature of participants. | |
| Participants | 303 children; mean age 3.2 years (range 6 months to 5 years) | |
| Interventions | Virosomal intramuscular vaccine (Inflexal, Berna, no further details given) or no intervention | |
| Outcomes |
Serological
N/A Effectiveness URTI, febrile URTI, LRTI, drug px, and days off school. Not otherwise defined, reported presumably as means and standard deviation. Safety N/A |
|
| Funding Source | Unclear | |
| Notes | The authors conclude that the findings support the wider use of influenza vaccine in healthy children of all ages to reduce the socioeconomic burden of influenza in the community. Brief reporting, randomisation, vaccine, circulation matching, and outcomes are not described. Confidence intervals are not reported, tables do not specify means and standard deviation, the recommendations on "children of all ages" is at odds with the lack of breakdown of age groups. No funding source is reported. Published in supplement sponsored by? THE STUDY IS LINKED TO ESPOSITO 2006, WHICH PRESENTS THE SAME DATA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Possibly no losses |
| Summary assessments | Unclear risk | Insufficient information to assess study design |
cb Ritzwoller 2005.
| Methods |
|
|
| Participants | Children aged 6 to 23 months | |
| Interventions | Vaccine not specified (see 2003 included strains below). 2003 to 2004 season will include A/New Caledonia/20/99‐like (H1N1), A/Moscow/10/99‐like (H3N2), and B/Hong Kong/330/2001‐like viruses. For the A/Moscow/10/99‐like (H3N2) virus, US manufacturers will use the antigenically equivalent A/Panama/2007/99 (H3N2) virus, and for the B/Hong Kong/330/2001‐like virus, they will use either B/Hong Kong/330/01 or the antigenically equivalent virus B/Hong Kong/1434/02. | |
| Outcomes |
|
|
| Funding Source | Government/industry | |
| Notes | Circulating strain of A (H3N2) Data collected during peak of influenza activity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Low risk | Selected group, secure record |
| PCS/RCS‐Selection Non Exposed cohort | Low risk | Same methods of the exposed cohort |
| PCS/RCS‐Comparability | High risk | Insufficient description |
| PCS/RCS‐Assessment of Oucome | Unclear risk | Record linkage |
| Summary assessments | High risk | Some doubt arises from the real comparability of the cohorts. |
aa Rudenko 1988.
| Methods | Apparently cluster‐randomised controlled trial of schoolchildren in the Kalinigrad, Russia in 1984‐85. The text seems to suggest that children were randomised by class. The children underwent daily clinical examination for 7 working days after inoculation: recorded temperature, complaints, inspection of skin, mucous from eyes, and condition of nasopharynx. Morbidity due to influenza and acute respiratory illness recorded during epidemic period (28 January to 3 March 1985). Antigenic activity determined by inhibition of haemagglutinin by "standard methods". Haematological and biochemical tests and analysis of urine carried out to evaluate safety of vaccine; samples taken before vaccination, 3 days after, and 1 month after each dose of vaccine. Haematological tests included full blood analysis, thrombocyte count, and lymphocyte index. Biochemical test included determination of C‐reactive protein, protein fraction, neuraminic acid, transaminase alanine‐aminotransferase, and urea. Antigenic activity carried out on subgroup of 240 children. Samples taken from 22 children who received vaccine and 18 who received placebo for re‐isolation of vaccine. Genetic stability of vaccine evaluated from swabs taken from nasopharynx after 1, 2, 3, 7, and 8 days. 3 criteria used: retention of antigenic specificity, temperature sensitive‐phenotype, localisation of temperature sensitive‐mutations in individual genes of re‐isolates. Statistical analysis of morbidity carried out using EVM employing the criteria of the "reliability of parameter differences of the binomial distribution". Influenza epidemic from 28 January to 3 March 1985, peak from 11 February to 17 February 1985. Epidemic caused by A(H3N2) (i.e. vaccine did not match circulating strain). | |
| Participants |
|
|
| Interventions | Live influenza A(H1N1) vaccine given intranasally, 2 doses 28 to 30 days apart administered using Smirnov apparatus. An influenza epidemic took place from 28 January to 3 March 1985, peaking from 11 February to 17 February 1985. The epidemic was caused by A(H3N2) (i.e. vaccine did not match circulating strain). | |
| Outcomes |
Serological
Antigenic activity was determined by HAI, haematological tests included full blood analysis, and biochemical tests were also performed. 3 serum samples were taken from 240 children to test seroconversion. The basis for the sampling is not described. Effectiveness
Safety Reactogenicity was studied in a sample of 596 children after the first dose and in 164 children after the second dose. It is unclear on what basis the children in the samples were selected. The only outcome reported by arm was fever of various degrees, but no definition is given. |
|
| Funding Source | Unclear | |
| Notes | The authors conclude that the vaccine did not affect morbidity because of mismatch between vaccine and circulating viruses. The vaccine also proved to be stable and not very reactogenic. No description of the vaccine content and unclear randomisation and attrition/sampling make interpretation of the results very difficult. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description; cluster‐randomised trial |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description |
| Summary assessments | High risk | No description of the vaccine content and unclear randomisation and attrition/sampling make interpretation of the results very difficult. |
ab Rudenko 1991.
| Methods |
|
|
| Participants | 1009 children age 3 to 14 years | |
| Interventions | Influenza virus B ‐ B/14/5/1 (recombinant) Commercial influenza A vaccine ‐ A/Taiwan/1/87 (H1N1) | |
| Outcomes |
|
|
| Funding Source | Unclear | |
| Notes | The text refers to 4 randomised arms with a total denominator of 1009 (this is not a mistranslation, as we have checked the original in Arab numerals). Table 2 reports data on 321 children. No mention is made of the missing children. We believe the data are uninterpretable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention is made of the missing children. |
| Summary assessments | High risk | Data are uninterpretable. |
aa Rudenko 1993a.
| Methods | 2‐year single‐blind, placebo‐controlled cluster‐RCT to assess the efficacy of both live cold‐adapted and inactivated influenza vaccine | |
| Participants | Children aged 7 to 14 years from 34 schools of Novgorod (USSR). School lists were randomly assigned as whole to 1 of the vaccine or placebo preparations. The assignment procedure was structured so that different regions of the city would be represented in each immunisation group. The assignment remained the same throughout the study, but new schools were introduced in the second year. In the first year a total of 30 schools participated in the study, of which 10 were in the live attenuated group, 9 in the inactivated group, and 11 in the placebo group. In the second year of the study, the numbers were respectively 14, 9, and 11. 6 of these schools comprised students who had not participated in the previous year, and 1 each of the inactivated vaccine and placebo schools had dropped out. Children aged 7 to 10 in the inactivated group received a more highly purified preparation than those aged 11 to 14. Placebo groups were also divided into 2 subgroups: 1 half was administered placebo intranasally, the other half intramuscularly. In the second year only intranasal placebo was administered. | |
| Interventions |
|
|
| Outcomes |
Serological
Paired sera were taken from approximately 100 children during the period preceding the immunisation campaign to test seroconversion. Effectiveness "Starting mid‐October the nurse in each participating school began to monitor illnesses recorded as acute respiratory disease on medical certificate (required by Russian Schools after an absence). A series of specific respiratory diagnoses was used. Any illness with diagnosis termed as 'respiratory illness' or 'influenza' was considered a case. Investigation by the polyclinic was conduct if any certificate was provided after an absence from school. When acute respiratory disease increased, virologic surveillance was started to identify influenza viruses To avoid the lack of independence associated with counting multiple illnesses separately, the presence of 1 or more respiratory illnesses in the epidemic period was counted as 1 outcome, whereas the absence of respiratory illnesses during this period was the other outcome. A child receiving vaccine or placebo was included for analysis only if he or she received the full schedule of doses. The 1989 – 90 outbreak of influenza in Novgorod was exclusively A H3N2. The first isolate was made on 15.1.1990 and isolation continued through 22.2.1990. The period used to determine frequency of influenza associated illnesses was 1.1. – 4.3.1990. 12,837 children received full immunisation in the first year. In the school year 1990 – 1991 the influenza outbreak was caused by both types A (A/Taiwan//86 H1N1)and B (B/Yagamata/16/88 or B/Victoria/11/87 like) strains. For the efficacy analysis was considered for the period 14.1 – 24.3.1991 (11 weeks)" Safety Reactogenicity was assessed 4 days postinoculation in approximately 100 children during the period preceding the immunisation campaign to test seroconversion. Fever During the first year of the study, 1 child out of 162 in the live vaccine group had low‐grade fever (< 38.5 °C). Any case of fever was observed in the controls and inactivated vaccine group, but how many children constituted these 2 subgroups was not reported. In the second year low‐grade fever was observed in 2 of 323 attenuated vaccine recipients, 2 of 278 placebo recipients, and 5 of 271 inactivated vaccine group (age 7 to 10). 8 of the 435 children aged 11 to 14 years (inactivated vaccine, second study year) also had low‐grade fever. 3 children in this group also had fever > 38.5 °C. Induration In the second study year, 3 of 271 children who received inactivated vaccine (group of 7‐ to 10‐year‐olds) developed induration, as did 17 of 435 in the group aged 11 to 14. These data were not extracted, as it is unclear how the children were selected. |
|
| Funding Source | Unclear | |
| Notes | The authors conclude that cold‐adapted live vaccine was more protective than TIV and possibly reduced transmission. Randomisation units were schools, and results were presented both at cluster (which is right) and individual (which is wrong) levels. How this affects the results is impossible to say, as no cluster coefficients are reported. The second‐year study had no intramuscular placebo. This unblinding could have had some effect if different schools were in communication. Data from the pilot reactogenicity cohort (?) study were not extracted, as provenance and allocation of children is not clear. Second‐season inactivated vaccine has no placebo arm, and data have not been extracted. No separate reporting of spray and subcutaneous placebo for first year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description |
| Summary assessments | High risk | Insufficient information |
aa Rudenko 1993b.
| Methods | See Rudenko 1993a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Funding Source | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description |
| Summary assessments | High risk | |
aa Rudenko 1996a.
| Methods | Cluster‐RCT(s) to determine the efficacy and safety of cold‐adapted flu vaccines prepared with different virus strains. The study was carried out in 4 sites (USSR (Kalinigrad, St Petersburg), Kazakhstan (Alma Ata), and Cuba (Havana). However no results are reported from St Petersburg. Neither randomisation nor allocation concealment is mentioned. | |
| Participants | Children aged between 3 and 14 years enrolled from schools and kindergartens in St Petersburg, Kalinigrad, Alma Ata, and Havana. About 131,930 children were involved in the study. | |
| Interventions | Children were randomly divided into groups to receive either live cold‐adapted influenza vaccine or placebo (2 doses of 0.5 mL, administered 21 to 28 days apart).
|
|
| Outcomes |
Serological
"Paired sera tested for seroconversion in subgroups of children and nasal swabs were taken from 22 vaccinated and 18 placebo recipient children to assess spread of vaccination strains (nil result). Haematological and biochemical full blood analysis and urine analysis were carried out on 20 children belonging to each group before vaccination, 3 days after the first dose, 1 month after the first dose, 3 days after the second dose and 1 month after the second dose)
IGE determination and lymphocyte functional action assessments were also carried out." Effectiveness A nurse in each participating school or kindergarten recorded details of acute respiratory diseases on (from) medical certificates starting in October of each year. A series of specific diagnoses were used. When acute respiratory diseases increased, virological surveillance (blood and nasal swabs) was started to identify influenza viruses. Effectiveness data are reported only for the trials conducted in Alma Ata (1986‐87 and 1988‐89) and Havana (1990 and 1991). The first epidemic season in Alma Ata was due to the strain A/Taiwan/1/86 (H1N1) and lasted between 17 November and 21 December. Considering that the epidemic began earlier than expected, it is possible that at this time not all study participants had received the second dose of vaccine or placebo, respectively. In the second study year (1988‐89), the epidemic was caused by the strains A/Taiwan/1/86 and B/Victoria/1/87 and lasted from 26 March 1989 for 9 weeks. In Havana clinical cases of influenza and acute respiratory diseases were registered from 1 December 1990 to 31 December 1991. Efficacy data from Kalinigrad are not reported. Influenza‐like illness is the only reported effectiveness outcome. Safety Table 5 reports a long list of common non‐ILI ailments that appear to be related to safety for 2 years. These are labelled infectious and somatic diseases up to 6 months after vaccination, but are not tied to any specific vaccine or study centre. Similarly, Table 3 reports the incidence of febrile reactions by degree of fever and by age for 3 years without relation to years or vaccine composition. Children were examined for 7 days after vaccination by paediatricians for AEs. Temperature was registered. Data for children who were immunised for 3 successive years are reported but have not been extracted, as it is unclear which year, which vaccine, and most of all how to reconcile massive differences in denominators (e.g. for year 1, data for a total of 262 children only are reported). |
|
| Funding Source | Unclear | |
| Notes | The authors conclude that "the CA (cold‐adapted) vaccines are effective against influenza B and against influenza in general". Febrile reactions and somatic and infectious diseases: to what group or groups do the children belong? It is not possible to take back these data with the vaccination plan in Table 1.
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description |
| Summary assessments | High risk | Insufficient information to assess study design |
aa Rudenko 1996b.
| Methods |
|
|
| Participants | Children aged 3 to 14 years | |
| Interventions | Live recombinant vaccine made from 2 mono vaccines containing A/47/25/1 (H1N1) and A/47/F (H3N2) | |
| Outcomes |
|
|
| Funding Source | Unclear | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description |
| Summary assessments | High risk | Insufficient information to assess study design |
ca Salleras 2006.
| Methods | Prospective cohort study carried out between 1 November 2004 and 31 March 2005 in 11 paediatric clinics in Barcelona, Spain. The study assessed the effectiveness of virosomal vaccine against ILI and its economic consequences. | |
| Participants | 966 vaccinated children and 985 non‐vaccinated controls attending respectively 5 and 6 clinics. The unit of selection was clinic enrolment. Children were aged 3 to 14, and age breakdown by exposure, sex, and by 2‐year groupings is reported. Systematic differences are reported (significantly smaller families and younger children in the non‐vaccinated cohort). No attrition is mentioned. | |
| Interventions | 1 dose of virosomal influenza vaccine (Inflexal, Berna). Content is not described. | |
| Outcomes |
Serological
Pharyngeal and nasal swabs sent to laboratory for culture. Follow‐up was by parents' questionnaire. Follow‐up unclear, no mention of how many children were followed up and whether there was attrition in reporting with symptoms. Effectiveness
Safety N/A |
|
| Funding Source | Industry | |
| Notes | The authors conclude that "Adjusted vaccination effectiveness was 58.6% (95% CI 49.2 to 66.3) in preventing acute febrile respiratory illnesses, 75.1% (95% CI 61.0 to 84.1) in preventing cases of influenza‐like illnesses and 88.4% (95% CI 49.2 to 97.3) in preventing laboratory‐confirmed cases of influenza A. The adjusted vaccination effectiveness in reducing antibiotic use (18.6%, 95% CI ‐4.2 to 3.64), absence from school (57.8%, 95% CI 47.9 to 65.9) and work‐loss of parents (33.3%, 95% CI 8.9 to 51.2) in children affected by an acute febrile respiratory illness was somewhat lower. Vaccination of children aged 3 to 14 years in paediatric practices with 1 dose of virosomal subunit inactivated influenza vaccine has the potential to considerably reduce the health and social burdens caused by influenza‐related illnesses". Systematic differences ("adjusted with logistic regression") between hemicohorts, lack of description of vaccine content, matching and influenza circulation make the conclusions unreliable. Why use PCR? Was the quantity of viral genome so tiny to need amplification? | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Selected group |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Same methods but different population |
| PCS/RCS‐Comparability | High risk | Clearly different populations, no adjustments |
| PCS/RCS‐Assessment of Oucome | Unclear risk | ILI self reported |
| Summary assessments | High risk | Some doubt arises from real comparability of the cohort |
ab Slepushkin 1974.
| Methods | Placebo‐ and do nothing‐controlled emergency randomised trial of live attenuated oral influenza vaccine carried out during the 1970‐71 season in Smolensk, USSR. During January 1971, at the beginning of an epidemic of influenza in the town, oral vaccination was carried out as an emergency on organised groups of children of nursery school age (1 to 3 years), and it appears that this study, carried out only in 2 arms, is the one for which we have data reported in the tables. The vaccine was given 2 to 3 times with an interval of 10 to 15 days. There appears to be another study included in the report to assess the effectiveness of the vaccine(s) in inducing interferon (data not extracted). | |
| Participants | The children in each establishment (children's nurseries, nursery groups in larger schools) were selected on a medical basis, and their temperature was measured. Although the text states that "Three equal groups of healthy children were formed at random", the tables report 571 and 552 children in the vaccine and UV groups, respectively. It could be that the 3‐arm trial is different from the trial undertaken in January 1971, but the text is very confusing. There may even be a fourth study, with again 3 arms. | |
| Interventions | For the vaccination, 2 types of the oral influenza vaccine were used, which were analysed at the Moscow Institute of Virological Preparations. The vaccine was composed of the strains of the influenza virus A2/Istra 10/96 and B/Liks 59, the infectious titre 10 exp.5.5 (the "two types" are not further discussed or reported). The single dose of the emergency prophylaxis vaccine for children was 1 mL for children aged 1 to 3 years, 2 mL for children aged 3 to 7 years, and 3 mL for children aged 8 to 16 years. | |
| Outcomes |
Serological
"In order to determine antibodies, blood serum was taken from those who had been inoculated, before vaccination and between 21 to 30 days after its completion. The blood serum was tested in a reaction of the inhibition of the hemagglutination with 1% red corpuscle from chickens and four units of hemagglutinins of the virus when the antigen was put into contact with the antibodies for two hours" Effectiveness Follow‐up was 45 days. The children in the first group received the live influenza vaccine, and the second group received the medium no. 199, applied in the capacity of placebo. The third group were those who were not inoculated. Records were maintained for each child containing the date of inoculation, the type of vaccine, and also information about reactions to the vaccine. This included the results of the contraction of acute respiratory illnesses, starting from 10 days after the completion of the inoculations. Study 1
Study 2
Safety "The reactogenicity of the vaccine was determined by measuring daily the temperature in certain groups of those who had been inoculated" |
|
| Funding Source | Unclear | |
| Notes | The authors conclude:
The text is so confusing that only the data from the tables have been extracted. However, we are not sure of their relationship with the text. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Summary assessments | High risk | Insufficient information to assess study design |
ab Slepushkin 1988.
| Methods | Randomised, single‐blinded, placebo‐controlled study conducted in a boarding school in Moscow in September to December 1984 | |
| Participants | 107 healthy children 8 to 11 years old without a history of current illness were examined and judged eligible for study | |
| Interventions | Attenuated influenza vaccine prepared by recombination of the cold‐adapted strain A/Leningrad/134/47/57 (H2N2) with A/Leningrad/322/79 (H1N1). Before use, lyophilised vaccine was diluted 1:2 with distilled water and administered intranasally by means of a Smirnov aerosol generator. Distilled water only was administered as placebo. 2 doses of 0.5 mL were administered 28 days apart. Vaccine titre was 102 EID50 for the first dose and 107 for the second. Children were randomly divided to receive vaccine or placebo. 58 children received the first dose of vaccine and 49 placebo. Of the 58 vaccinated children, 43 received second dose of vaccine, and 39 of 49 received second dose of placebo. | |
| Outcomes |
Serological
Haemagglutination inhibition test against A/Brasil/11/78 and enzyme immunoassay Effectiveness N/A Safety "All children were observed for 5 days after each vaccination Axillary temperature was measured once each day and children were interviewed about the presence of eventual symptoms and visited at home in case of absence from the school" |
|
| Funding Source | Government | |
| Notes | The authors conclude that despite the first dose being weakly immunogenic, the second dose response was much better, and the vaccine proved safe. Poorly conducted study: de facto unblinded, with unexplained attrition. Physical aspect of placebo and vaccine in coded vials differed, resulting in inadequate blinding. There is a strange subanalysis of respiratory symptoms classified as harms by arm after the first vaccination dose. The authors carried out nasal swabs in 10 children and found that 1 had tonsillitis and 5 had adenovirus rhinitis. Although the breakdown of these by arm is not reported, as this is an RCT, what surely matters is the difference in event between arms, even for harms. This leads us to suspect that the authors did not trust their own random allocation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unexplained losses to follow‐up |
| Summary assessments | High risk | Poorly conducted study: de facto unblinded, with unexplained attrition |
ab Slepushkin 1991.
| Methods | Randomised, placebo‐controlled trial carried out in the 1987‐88 season in Leningrad, former USSR on schoolchildren aged 8 to 15 years to test live cold‐adapted vaccine, with inactivated vaccine with intranasal and intramuscular placebo (data by placebo not presented split). There was an influenza A (H3N2) and B mixed epidemic reported in Slepushkin 1993, but the vaccines did not contain any B antigen. Influenza A peaked in mid‐January to mid‐February, whereas circulation of influenza B was constant. | |
| Participants | 241 healthy boarding school children aged 8 to 15 years (97, 56, 88 for cold‐adapted, bivalent vaccine, and placebo at first dose and 95 and 78 for cold‐adapted and placebo). The attrition between first and second dose of both active arm and placebo is not explained. | |
| Interventions | Intranasal live cold‐adapted A/47/F derived from A/Philippines/2/82‐like (H3N2) and A/Leningrad/134/47/57 (H2N2) or intramuscular normal saline placebo or bivalent vaccine (containing A/Philippines/2/82‐like (H3N2) and A/Chile/1/83/ (H1N1)) or intranasal allantoic fluid placebo. Intramuscular applications took place only once, whereas internasal took place twice approximately 4 weeks apart. | |
| Outcomes |
Serological Paired sera and "micro neutralisation test". Convalescent sera only on those children who reported with ILI symptoms to the school nurse. Effectiveness N/A in Slepushkin 1991, effectiveness was reported in Slepushkin 1993 for school 1: those children reporting with ILI (systemic illness or rhinitis or pharyngitis) symptoms had convalescent sera taken. Also reported are data from another school in the trial with asymptomatic cases (i.e. no symptoms but antibody rises). This is strange as the asymptomatics are all occurring in 1 school, and the explanation is in the text: data on clinical illness were not collected. DATA NOT EXTRACTED. Safety Temperature (37.1 °C to 37.5 °C), local reactions, headache, sore throat, cough, head cold |
|
| Funding Source | Government | |
| Notes | The authors conclude that "The inactivated vaccine was found to be superior to the live one in its capacity to stimulate humoral immunity studied by HI, EIA and micro‐neutralisation tests. In 69.7% of the children given the inactivated vaccine, seroconversion to the vaccine strain was detected by 2 or three methods of antibody titration used." Randomisation and attrition are not explained. Briefly reported study but clear text. The authors checked harm data against seroconversion, to ensure that for example temperature was not associated with seroconversion, that is with infection. Unfortunately, no effectiveness data are reported. Follow‐up not described. Problem with data collection and surveillance in school 2. In the 1993 paper the authors report efficacy as 13% (P = 0.82) for 2 doses of cold‐adapted and 73% (P = 0.08) for 1 dose of bivalent vaccine. This relates to school 1. They also report an efficacy estimate for school 2, but this is likely to be highly unreliable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Summary assessments | Unclear risk | Randomisation and attrition are not explained. |
cb Slepushkin 1994.
| Methods | Cohort study to compare reactogenicity and immunogenicity in children vaccinated with live vaccine, inactivated vaccine, or placebo, carried out over 3 years in Novogorod, former USSR. No mention of randomisation is made, and the study was classified as a cohort. Allocation was on a school basis. A subgroup was inoculated each year of study prior to mass inoculations to determine reactogenicity and immunogenicity. Reactogenicity and immunogenicity results were analysed using "generally accepted methods" (Slepushkin and colleagues 1991, Ibid, 5: 372‐4). | |
| Participants | Children aged 7 to 14 years | |
| Interventions |
No placebo arm is reported in the third year, which is strange as there is a placebo arm reported for immunogenicity in Table 2. For the second year there is also a mysterious second inactivated vaccine that appears in the results tables. Data not extracted. To obtain live recombinant vaccine, cold‐adapted strains A/Leningrad/134/47/57 (H2N2) and B/USSR/60/69 were used as attenuation donors. |
|
| Outcomes |
Serological
Seroconversion (not extracted) Effectiveness N/A Safety Temperature reactions and local hyperaemia and infiltration after vaccination |
|
| Funding Source | Government | |
| Notes | The authors do not draw clear conclusions, and it is difficult to understand what the purpose of the study was. Badly reported; no clear overall denominator, and safety data are reported for limited groups of participants with no clear sampling rule. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | No description |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | No description |
| PCS/RCS‐Comparability | Unclear risk | No description |
| PCS/RCS‐Assessment of Oucome | Unclear risk | No description |
| Summary assessments | High risk | Insufficient information |
ca Slobodniuk 2002a.
| Methods | "Cohort study of inactivated trivalent influenza vaccines compared with no treatment over 3 years. An additional aim of the study was to assess the impact on the immune system of vaccinating children for 3 years in a row. Children were immunised during three epidemics in 1998, 1999 and 2000 and controls were students from parallel classes, who received no intervention. The efficacy of the vaccines was determined from total morbidity rate for influenza and ARIs during outbreak periods 25/01/99 to 14/03/99; 10/01/00 to 21/02/00 and 21/01/01 to 23/02/01 in a boarding school in Yekaterinburg, Russia" | |
| Participants | 564 pupils of the boarding school aged 8 to 14 years | |
| Interventions |
|
|
| Outcomes |
Serological
Immune response was evaluated before and 30 days after vaccine was administered. Tests were carried out by serological status (i.e. in seropositive and seronegative children) in 70 children in year 1, 109 in year 2, and 73 paired sera in year 3. Effectiveness Number of children with influenza or ARI during outbreak period each year Safety N/A |
|
| Funding Source | Unclear | |
| Notes | The authors conclude that the vaccines offered increased protection with each new season, in effect having an additive effect. The first season the efficacy of Fluarix was low in the epidemic period (1.3?); the second inoculation achieved 2‐fold protection compared to the control group. The final year Grippol reduced morbidity by 2.8 times. According to the authors, a fourth injection could be unnecessary. The study is very difficult to interpret, there is no information on participants, community, matching, viral circulation disparity between paired sera and enrollees, etc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Not described |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Not described |
| PCS/RCS‐Comparability | Unclear risk | Not described |
| PCS/RCS‐Assessment of Oucome | Unclear risk | Not described |
| Summary assessments | High risk | Insufficient information to assess study design |
ca Slobodniuk 2002b.
| Methods | See Slobodniuk 2002a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Funding Source | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Not described |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Not described |
| PCS/RCS‐Comparability | Unclear risk | Not described |
| PCS/RCS‐Assessment of Oucome | Unclear risk | Not described |
| Summary assessments | High risk | Insufficient information to assess study design |
ca Slobodniuk 2002c.
| Methods | See Slobodniuk 2002a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Funding Source | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Not described |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Not described |
| PCS/RCS‐Comparability | Unclear risk | Not described |
| PCS/RCS‐Assessment of Oucome | Unclear risk | Not described |
| Summary assessments | High risk | Insufficient information to assess study design |
ab Steinhoff 1990.
| Methods | Randomised, double‐blind, placebo‐controlled trials of intranasal avian‐human and cold‐adapted vaccines. Conducted separately in a step‐wise, dose‐escalating fashion | |
| Participants | 63 seronegative (HAI no more than 1:8 to H3N2) children aged 6 to 48 months | |
| Interventions |
Both vaccines diluted in L‐15 medium (Whitaker Bioproducts; Walkersville, MD, USA). Placebo was L‐15 medium. |
|
| Outcomes |
Serological
Paired sera, duration of viral nasal shedding, production of mucosal antibodies Effectiveness N/A Safety
|
|
| Funding Source | Government | |
| Notes | The authors conclude that the vaccines are safe and induce immunity, protecting children from challenge with homologous virus.
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Summary assessments | Unclear risk | Insufficient information to assess study design |
ab Steinhoff 1991.
| Methods | RCT to compare characteristics of 2 live reassortant vaccines: cold‐adapted (ca) and avian‐human (ah) Vaccines were manufactured by isolating wild‐type A/Kawasaki/9/86 (H1N1) in tissue culture and 4 times passage in tissue culture and once in eggs. These were crossed with donor strains to produce reassortant vaccines. Each vaccine was diluted in L‐15 medium (Whitaker Bioproducts) to achieve desired number of infectious units. Vaccines were evaluated in 1987 and 1988 during periods when no influenza viruses were circulating. Vaccines initially tested in young adults (data not extracted) before continuing with children's study. | |
| Participants | 122 children aged 6 to 24 months seronegative to A/Kawasaki/86 (H1N1) were randomised to receive a first dose of either ah (40 children), ca (39), or placebo (43). | |
| Interventions |
Vaccines were administered in dose‐escalating fashion; after each dose was shown to be safe, 10‐fold higher dose administered until dose of 106 TCID50 was reached. Each child received 1 0.5 mL dose (0.25 mL per nostril). Children were observed for 1 to 2 hours daily for 3 days before inoculation; 7 to 9 days after each dose was shown to be safe, 10‐fold higher dose was administered until dose of 106 TCID50 was reached. |
|
| Outcomes |
Serological
Effectiveness N/A Safety
Children were observed for 1 to 2 hours daily for 3 days before inoculation and 7 to 9 days after. |
|
| Funding Source | Government | |
| Notes | The authors conclude that the ca A/Ann Arbor/6/60 donor virus reliably confers attenuation characteristics to a variety of H1N1 and H3N2 influenza A viruses. No description of randomisation, allocation, attrition, or placebo. Data on adults were not extracted. Data by TCID not extracted separately. Data on ILI with or without infection were extracted, as these are responses to viral challenge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Summary assessments | Unclear risk | Insufficient information to assess study design |
ab Swierkosz 1994.
| Methods | Randomised, double‐blinded, placebo‐controlled trial to assess safety of adding a third dose of a live attenuated, cold‐recombinant, trivalent influenza vaccine | |
| Participants | 22 healthy infants and children aged 2 to 22 months were recruited. 17 were seronegative to all 3 haemagglutinin types, while 2 were seronegative to H3 and B and 2 were seronegative to H1 and B. | |
| Interventions | Children were randomised to receive 3 doses of 0.5 mL vaccine or placebo intranasally in a double‐blinded fashion. 17 children received vaccine and 5 received placebo. Vaccine was administered at day 0, day 60, and day 120. Vaccine contained 3 strains: A/Kawasaki/9/86 (H1N1), A/Los Angeles/2/87 (H3N2), and B/Yamagata/16/88. The vaccine lots contained 108.0, 108.0, and 107.6 TCID50/mL H1N1, H3N2, and B. 106 TCID50 of each strain was present in 0.5 mL of trivalent vaccine. | |
| Outcomes |
Serological
"HAI titres against H1, H3, B and all types (H1, H3 and B) after first dose at day 0, second dose at day 60 and third dose at day 120
ELISA response to H1, H3, B and to all types (H1, H3 and B) after dose first dose at day 0, after second dose at day 60 and third dose at day 120" Effectiveness N/A Safety Adverse reactions were defined as fever (rectal temperature > 38.3 °C, or > 37.2 °C axillary); cough (2 or more episodes during examination on 2 consecutive days); otitis media (red immovable ear drum diagnosed by pneumotoscopy); and lower respiratory tract infection as indicated by wheezing (sustained musical sound during expiration) or pneumonia (a new alveolar consolidation seen radiographically). Clinical observations were recorded daily for 11 days. |
|
| Funding Source | Government | |
| Notes | The authors conclude that trivalent, cold‐adapted intranasal influenza vaccine is safe and immunogenic when administered in a 3‐dose regimen. A tiny schedule‐ranging trial. Only 4 children were aged less than 6 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Summary assessments | Unclear risk | Insufficient information to assess study design |
aa Tam 2007a.
| Methods | Multicentre (8 centres in Southeast Asia: China, Hong Kong, India, Malaysia, the Philippines, Singapore, Taiwan, and Thailand) RCT carried out over 3 seasons (enrolment and follow‐up carried out between 30 September 2000 and 31 May 2003) to assess efficacy, immunogenicity, and safety of live recombinant vaccine in small children. The randomisation schedule for each year was generated by Wyeth.
|
|
| Participants | Starting from 30 September 2000, 3174 children aged 12 to 36 months were enrolled and allocated either to CAIV (1900) or to placebo (1274). Each year the children were re‐randomised to either placebo or vaccine at a ratio of 2:3.
Mean age at first vaccination is reported as 23.5 (SD 7.4) months, which is strange because if the enrollees are always the same, most of them should have been out of age by the second season.
Figure 1 is not fully explained in the text. It shows 4 groups at year 2 with differing sequences of allocation to CAIV‐T and placebo. The initial trial description is that of a cross‐over, but that is not fully explained in the text as well as the third year of the study, which is not addressed in the text. |
|
| Interventions |
Both CAIV‐T and placebo were supplied in identically packaged sprayers; study participants, their parents or guardians, and the clinical personnel were blinded. Vaccine content was planned to be antigenically representative of the WHO recommendations for the Northern Hemisphere for each year, "However, in year 1, because of industry‐wide technical problems in the production of the A/H3N2/Moscow/10/99‐like virus, A/H3N2/Panama/2007/99 vaccine virus, the recommended strain was replaced with A/H3N2/Sydney/05/97.25 This decision was based on the antigenic similarity of the hemagglutinin (HA) antigens, a WHO report indicating that A/H3N2/Sydney/05/97‐like viruses were circulating before the 2000 to 2001 season, 26 and previous clinical trials with the frozen formulation of LAIV that had demonstrated efficacy against mismatched influenza A/H3N2 virus. In year 2, because of delays in manufacture, the recommended B vaccine component, B/Victoria/504/2000 (B/Sichuan/379/99‐like), was replaced with B/Yamanashi/166/98. Therefore, the B component of the second‐year vaccine formulation was not antigenically representative of the B/Victoria/504/2000 (B/Sichuan/379/99‐like) virus recommended by the WHO for the upcoming influenza season" In summary, the vaccines in both years were not well matched. |
|
| Outcomes |
Serological
Paired sera were taken from 111 children at 5 sites. However "the same participants did not necessarily participate in the cohort in both years". Blood samples were obtained before and after the second vaccination in year 1 and before and after vaccination in year 2. In summary, it is unclear what the relationship of these participants is with the rest of the study population. Nasal swabs were taken from symptomatic ILI cases. Effectiveness The primary efficacy endpoint was the first episode of culture‐confirmed influenza illness caused by a subtype antigenically similar to that in the vaccine after receipt of the second dose of study vaccine or placebo during year 1 in the PP population. Secondary efficacy endpoints included the first episode of culture‐confirmed influenza illness caused by any influenza virus subtype after receipt of the second dose of study vaccine or placebo during year 1 and the first episode of culture‐confirmed influenza caused by subtypes. It is unclear whether follow‐up included all children with ILI symptoms. The text reports that follow‐up was carried out by phone and clinic visits. Safety Parent or legal guardians recorded daily symptom information for 11 consecutive days including the day of administration. Adverse events were defined as any clinically significant event, including but not limited to:
Serious adverse events including hospitalisations were monitored from enrolment until the end of the study. Fever, runny nose, decreased activity or appetite, and use of increased fever medications. Other reported outcomes were bronchospasm (7 CAIV‐T, 3 placebo), bronchitis (3 CAIV‐T, 2 placebo), and rhinitis (3 CAIV‐T, 0 placebo) in year 1. In year 2, a child was hospitalised with pneumonia 6 days after receiving CAIV‐T. There was 1 dropout (20‐month‐old female developed fever that persisted for 3 days) after receiving the first dose of CAIV‐T in year 1. There were 2 deaths unrelated to vaccine. Perusal of reported safety denominators in Table 6 shows the usual discrepancies in trials of these CAIV‐T vaccines ‐ denominators that are reported as ranges with the usual (see Vesikari) caption "†n represents the number of participants with known values". According to Table 6, 1345 children received CAIV‐T in season 2, but according to Figure 1 the total should be 1757. There is no mention of the fate of the other children. |
|
| Funding Source | Industry | |
| Notes | The authors conclude that "In year 1, efficacy of CAIV‐T compared with placebo was 72.9% [95% confidence interval (CI): 62.8 to 80.5%] against antigenically similar influenza subtypes and 70.1% (95% CI: 60.9 to 77.3%) against any strain. In year 2, revaccination with CAIV‐T demonstrated significant efficacy against antigenically similar (84.3%; 95% CI: 70.1 to 92.4%) and any (64.2%; 95% CI: 54.2 to 77.3%) influenza strains. In year 1, fever, runny nose/nasal congestion, decreased activity and appetite and use of fever medication were more frequent with CAIV‐T after dose 1. Runny nose/nasal congestion after dose 2 (year 1) and dose 3 (year 2) and use of fever medication after dose 3 (year 2) were the only other events reported significantly more frequently in CAIV‐T recipients. CAIV‐T was well tolerated and effective in preventing culture‐confirmed influenza illness over multiple and complex influenza seasons in young children in Asia. Randomisation and allocation concealment are described very well, but inconsistencies in the text (a vanished season), unclear denominators, and a real possibility of biased follow‐up and reporting bias of safety outcomes put this study at high risk of bias. Safety remains a concern in these studies, with bronchospasm a possible AE". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number |
| Allocation concealment (selection bias) | Low risk | "At enrolment, each subject was assigned the next sequential subject number and received study product of the treatment code assigned to that subject number according to a preprinted randomisation allocation list" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Possibility of biased follow‐up and reporting bias |
| Summary assessments | Low risk | Plausible bias unlikely to seriously alter the results. |
ca Vasil'eva 1982.
| Methods | Comparative cohort study of a monovalent injected vaccine in children aged 7 to 15 years in Leningrad, former USSR. The setting, season, and viral circulation are not described. | |
| Participants | 335 children of unknown provenance | |
| Interventions | Monovalent inactivated vaccine containing A/Texas/1/77 (H3N2) (Leningrad Louis Pasteur laboratories) subcutaneous or by needleless injector or placebo. Placebo is not described. | |
| Outcomes |
Serological
Paired sera taken in an undescribed fashion. There were antibody rises to other influenza A viruses and PIV 1 in the placebo arm. Effectiveness Influenza‐like illness described in the translation as "influenza and URTI". Breakdown by age groups and type of injection is not reported. Safety Temperature, induration, headache, malaise, sore throat. Daily physical examinations for 5 days. |
|
| Funding Source | Unclear | |
| Notes | The authors conclude that the vaccine (incidence in the arms was 1.8 and 9.9, respectively) was effective, immunogenic, and safe. Very brief report. There is no description of randomisation, allocation, or attrition. The authors briefly described evidence of A/Khabarovsk/77, A/Texas/77, and PIV 1 circulation in the placebo arm, which could account for some of the febrile episodes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Not described |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Not described |
| PCS/RCS‐Comparability | Unclear risk | Not described |
| PCS/RCS‐Assessment of Oucome | Unclear risk | Not described |
| Summary assessments | High risk | Insufficient information to assess study design |
ab Vasil'eva 1988a.
| Methods | RCT assessing reactogenicity and immunogenicity of bivalent vaccine "RCT of inactivated influenza vaccine; large‐scale study of the effect of multiple immunisations on immunity. Children were randomised in groups for safety evaluation. Children were randomised (in sub‐group) as individuals for immunogenicity evaluation. Vaccination was carried out once, twice, 3 times, 3 times with interval of 2 years, 4 times but sub‐groups only were evaluated for 5 days after inoculation; measuring temperature, local reactions and subjective complaints Data on long‐term consequences, somatic and infectious disease (excluding influenza and ARI) and allergies were collected from all participants over a 6 month period after inoculation. Sub‐groups were monitored for any admissions to hospital during 30 days following immunisation" | |
| Participants | 12,643 children aged 11 to 14 years from Rostov‐on‐Don in the former USSR recruited during the period October 1984 to May 1986 | |
| Interventions | Bivalent inactivated, chromatographic influenza vaccine A/Philippines/82 (H3N2) and A/Kiev/59/79 (H1N1) | |
| Outcomes |
Serological
Immunological tests (with determination of concentration of IgA, IgE, and IgM) were carried out on a subgroup. "Allergising effect" of vaccine was determined by measuring IgE by radio‐immunological method and antibodies towards chicken embryos in haemagglutination neutralisation reaction. Effectiveness N/A Safety
|
|
| Funding Source | Unclear | |
| Notes | The authors conclude that multiple immunisations with bivalent vaccine do not have an immunity‐suppressing effect. Unclear rationale for subgroup sampling and sketchy description of methods. Much may have been lost in translation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description |
| Summary assessments | High risk | Unclear rationale for subgroup sampling and sketchy description of methods |
ab Vasil'eva 1988b.
| Methods | Randomised, placebo‐controlled trial carried out during 1983 to 1984 in the area of Rostov‐on‐Don in the former USSR. The study was conducted to assess efficacy, effectiveness, safety and immunogenicity of 2 types of bivalent vaccine versus placebo. These were administered by injection and needleless injector, although the data are presented by what the translator calls "chromatographic", "centrifugal", and "adsorptive" types of vaccines, whereas elsewhere they are reported as whole virion vs split. Randomisation is described only as that older children ("adolescents") were drawn individually into the randomisation sequence, whereas children aged 11 to 14 were selected on the basis of their class. It is unclear whether this means cluster randomisation, although denominators are roughly on a 3:1 basis. There was a B virus epidemic in January 1984, and then a H1N1 epidemic reported in Rostov‐on‐Don. | |
| Participants | 13,355 children aged 11 to 14 and "teenagers" observed, 9962 of whom took part in the vaccine evaluation (explanation not given). 6596 children were randomised to vaccines and 3393 to placebo. However, there are several inconsistencies in the text (see outcomes). The participants were recruited from schools, professional technical establishments, and technical colleges in Rostov‐on‐Don, Taganrog, and Novocherkassk. | |
| Interventions | Bivalent vaccine whole virion or split ("formed from the influenza virus strains A/Leningrad/385/80 (H3N2) and A/Kiev/79 (HINI): chromatographic, centrifugal and adsorbitive(?) chemical influenza vaccines") or placebo ("sterile apirogenic solution of sodium chloride, using a syringe or intravenous injector (as for the vaccine) in volumes of 0.2 ml to 0.5 ml") | |
| Outcomes |
Serological
Paired sera taken from 198 children who developed ILI symptoms during the season to confirm an influenza diagnosis. "Antigenic activity" (presumably immunogenicity) was tested on 655 children with paired sera taken 1 month apart. Effectiveness "Considering the mixed nature of the 1984 influenza epidemic and the fact that the tested preparations did not contain component B, it is interesting to analyse the rate of illness in children in the second half of the epidemic. At this time, the intensive circulation of the influenza virus type A (HINI) amongst children was confirmed by serological methods. A subsequent analysis showed that according to data from clinical diagnostics, 14.4% of children aged 11 to 14 years inoculated with the chromatographic preparation contracted influenza and acute respiratory illnesses in February to March 1984. For those inoculated with the centrifugal preparation the figure was 13.0% and for those who received placebo the figure was 12.6%. According to data from the serological correction of diagnoses, influenza A (HINI) was confirmed in 18.2% of those inoculated with the chromatographic preparation, 24.2% of those inoculated with the centrifugal preparation and 37.9% of children in the control groups. Figures for the corrected rate of illnesses were 2.6 and 3.1, as opposed to 4.8 in the control group. The indices of efficacy were 1.9 and 1.6 respectively. The differences in the figures given are statistically reliable (P < 0.001 and 0.01)". Safety "Reactogenicity was assessed on a sample of 866 school children aged 11 to 14 years. Paediatricians carried out a daily clinical examination of the children for 5 days after immunisation. This included the compulsory measuring temperatures, noting complaints of general reactions (feeling unwell, headaches, disturbed sleep etc.) and local reactions (reddening of skin, development of infiltrates, presence of illness at place of preparations’ administration" The basis for the sampling is unclear, and it is not at all clear whether this is a random sample (DATA NOT EXTRACTED). Earlier in the report, the text reports "When the groups were formed, with the aim of evaluating the preparations’ reactogenic properties and antigenic activity, the units of selection were individuals" ??? Data for the 866 children include several measures of induration and fever (Table 1). Elsewhere the text reports: "In order to evaluate the safety of the inactivated influenza vaccine, a comparative analysis was carried out of requests for emergency medical attention amongst those children who were inoculated and those who received placebo, for the 30 days after immunisation. The total figures for such requests amongst children aged 11 to 14 years and teenagers were 0.1% to 0.3% and 0.7% in the analogous group of children who had received placebo. The frequency of hospitalisation for inoculated children and those who had received placebo also did not reliably differ and did not exceed 0.04% to 0.06%". The outcomes reported in this analysis (Table 3) are very unusual (allergies, bronchitis, neuralgia, carbuncles, stomach ulcers, etc.), and there are gross imbalances and inconsistencies in the denominators of the arms (centrifugal 6625, adsorptive 491, chromatographic 4655, placebo 3493 = 15264). |
|
| Funding Source | Unclear | |
| Notes | The authors conclude the following. "The safety, low reactogenicity and high antigenic activity of the Soviet whole‐virion inactivated influenza vaccine has been established, when administered once subcutaneously in a dose of 7.0 μg of haemagglutinin to school children aged 11 to 14 years and to teenagers In view of the discovery of the residual reactogenicity of the adsorbitive(?) influenza chemical vaccine, it is recommended that further work should be carried out on the preparation, aiming to ensure the possibility of an intravenous method of administration The clear prophylactic efficacy of the whole‐virion vaccine during the mixed epidemic period of influenza B+A (HINI) was noted: the indices of efficacy, from the calculation of the serological correction of clinical diagnoses, were 1.6 and 1.9 The safety, high inoculation activity and prophylactic efficacy allow the inactivated influenza whole‐virion vaccines to be recommended to be introduced as part of the practical prevention of health of children aged 11 years and older" We are not happy about the large number of inconsistencies in the text and non‐random (or at least unexplained) sampling carried out. Terrible reporting leading to loss of data. We have tried extracting data for influenza from the effectiveness text assuming a denominator of 6596 for all vaccinees and 3393 for placebo, converting percentages from the text as follows for influenza A (H1N1) 18.2% of those inoculated with the chromatographic preparation (4655, i.e. 847), 24.2% of those inoculated with the centrifugal preparation (6625), and 37.9% (i.e. 1603) of children in the control groups (3393, not 3493 as it says in Table 3, i.e. 1286). The summed numerators exceeded the denominator reported. However, these numerators do not match even remotely the 198 paired sera taken for influenza diagnosis. Too many inconsistencies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Summary assessments | High risk | Insufficient information to assess study design |
aa Vesikari 2006a.
| Methods | Double‐blind RCT assessing efficacy and safety of CAIV‐T in children. Multicentre trial conducted in Belgium, Finland, the UK, Israel, Spain during the period 2 October 2000 to 31 May 2002. Follow‐up for each year lasted until 31 May and was a composite of phone calls, home and visit clinics. Coding as well as randomisation carried out centrally and assigned by a blind investigator on the basis of a pre‐printed randomisation schedule. Both ITT and PP populations were defined. Analyses were carried out only for outcomes occurring in periods of viral circulation in the different centre areas. | |
| Participants | 1616 healthy children aged 6 to 35 months attending day care (at least 12 hours weekly) in 1 of the centres who continued to be healthy during year 2 were included in the primary analysis (951 vaccine and 665 placebo recipients). 1784 children were originally randomised on 3:2 basis. There was considerable attrition between the year 1 ITT population (1059 in the active arm and 725 in the placebo arm) and the year 2 PP population (640 and 450, respectively), with 65 dropouts in the placebo arm and 132 in the intervention arm (calculated from the flow diagram of population, which does not add up). Table 1 reports 174 of the 1616 PP population as aged 6 to 12 months, 598 aged 12 to 23 months, and 844 aged 24 months or more. | |
| Interventions | CAIV‐T (Wyeth) containing A/New Caledonia/20/99 (H1N1), A/Sydney/05/97 (H3N2), and B/Yamanashi/166/98 in year 1 and A/New Caledonia/20/99 (H1N1), A/Panama/2007/99 (H3N2), and B/Victoria/504/2000 or sterile physiological solution placebo. For technical reasons, antigens in year 1 were similar to those recommended, and in year 2 they were those recommend by WHO. Dose was 0.2 mL in each nostril twice in year 1 (approximately 35 days apart) and once in year 2. Spray applicators were preloaded centrally and all identical. In year 1 the match was good, in year 2 the match was not so good because of drifted variants and the appearance of 2 different strains of influenza B vaccine. | |
| Outcomes |
Serological
Children with fever (rectal 38 °C or more and oral 37.5 °C or more), wheezing shortness of breath, pulmonary congestion, pneumonia, or ear infection got a nasal swab and those with 2 or more of the following: runny nose, nasal congestion, sore throat, cough, muscle aches, chills, irritability, decreased activity, or vomiting Effectiveness Influenza caused by subtypes antigenically similar to those contained in the vaccine (primary endpoint) and by those drifted from the recommended ones (secondary endpoint)
Safety Parents/guardians kept diary card to record axillary or rectal temperature, runny nose or nasal congestion, sore throat, cough, vomiting, activity level, appetite, irritability, headache, chills, muscle pain, and antipyretic medication use, unscheduled physician contacts for 11 consecutive days from vaccination and throughout the study any unscheduled event that required healthcare contact or study termination. Fevers were classified as mild, moderate, or severe (equal to or more than 37.5 °C, 38.6 °C, and 40 °C axillary respectively or 38 °C, 39.1 °C, and 40 °C rectally). Adverse events are reported in a mixture of table and text format. We have extracted the AEs for up to 11 days postvaccination, but the text reports no significant difference between those occurring within 11 days of vaccination and those occurring throughout the surveillance period. These are classed as possible, probable, or definitely caused by vaccination, but the definition of the association is unclear: "Lower respiratory tract illnesses reported as serious AEs from receipt of the first dose of study medication through the end of the first influenza surveillance period were also similar between treatment groups (pneumonia: 11 CAIV‐T recipients and 9 placebo recipients; bronchitis: 3 CAIV‐T recipients and 1 placebo recipient; bronchospasm: 2 CAIV‐T recipients and 2 placebo recipients; bronchiolitis: 1 CAIV‐T recipient and 2 placebo recipients) In participants 6 to 12 months of age, lower respiratory tract infections reported as serious AEs were pneumonia (2 CAIV‐T recipients and 1 placebo recipient), bronchitis (2 CAIV‐T recipients and 0 placebo recipients) and bronchospasm (1 CAIV‐T recipient and 0 placebo recipients). Serious AEs judged to be possibly, probably, or definitely related to study vaccination were reported for 9 CAIV‐T recipients (pneumonia and AOM, 2 recipients; bronchopneumonia, 2 recipients; pneumonia, 1 recipient; bronchiolitis, 1 recipient; bronchitis and AOM, 1 recipient; idiopathic thrombocytopenic purpura, 1 recipient; and fever, acute respiratory tract infection, dehydration and AOM, 1 recipient) and 5 placebo recipients (1 each for pneumonia and constipation; cough, wheeze and lung consolidation; pneumonia; idiopathic thrombocytopenic purpura; and hypersensitivity, erythema and periorbital edema). There were no statistically significant differences in serious AEs between treatment groups during the second influenza surveillance period. Six lower respiratory tract illnesses were reported, all among CAIV‐T recipients (5 cases of pneumonia and 1 of bronchospasm). 2 cases of pneumonia were judged to be possibly, probably, or definitely related to study vaccination. A total of 4 participants (2 CAIV‐T recipients and 2 placebo recipients) were withdrawn from the study because of AEs. No deaths occurred during the study period" |
|
| Funding Source | Industry | |
| Notes | The authors conclude that "cold‐adapted influenza vaccine‐trivalent was well tolerated and effective in preventing culture‐confirmed influenza illness in children as young as 6 months of age who attended day care". Formally this is a very well‐reported study following CONSORT guidelines. There are however numerous discrepancies in the text. Vaccine was not available until the end of November in year 2, and it is unclear what effect this had (immunisation was completed on 21 December, in the case of Israel this was after the beginning of viral circulation). In addition, the centres went from 70 in year 1 to 62 in year 2 for unexplained reasons. A major unexplained problem is seen in Table 7 (harm events reporting). 2 figures are shown for the 6 columns (vaccine and placebo by dose by year of the trial) representing "the number of subjects with known values" and then presumably the randomised denominator (which does not fit with either ITT or PP numbers). The figures show runny nose as significantly higher in dose 1 year 1 recipients, which could explain the high attrition between dose 1 year 1 and single dose year 2 (from 1021 to 631). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The proportion of missing outcomes compared with observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate. |
| Summary assessments | Low risk | Plausible bias unlikely to seriously alter the results. |
aa Vesikari 2006b.
| Methods | 2001 to 2002 season data from Vesikari 2006 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Funding Source | Industry | |
| Notes | This second year could be biased due to attrition. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient description |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The proportion of missing outcomes compared with observed event risk is enough to induce clinically relevant bias in intervention effect estimate. |
| Summary assessments | High risk | Some doubts arise from attrition bias. |
ca Wiggs‐Stayner 2006.
| Methods | Government‐funded, nurse‐led prospective cohort study carried out in the US state of Indiana. The study was conducted in 4 "entitlement 1" schools, which appear to have been populated by children of a lower socioeconomic class (80% to 90% were in receipt of free school lunches), evenly split between whites and blacks (Table 1 reports detailed ethnic background by school). With a range of students of 264 to 392. Attendance rates were 93.9% to 95.3%. | |
| Participants | In school 1, 277 children aged from 5 years and a number of adults (teachers) up to the age of 49. The criteria for selection were lack of contraindications, lack of self reported ongoing ILI, and parental consent. 51 were "medically excluded", and 143 finally had consent for and received the vaccine. In school 2, the figures were 273 "eligibles", 50 and 134. Overall coverage was 57%. We make the denominators 741 children in non‐vaccinated schools; out of 550 children in schools 1 and 2, 276 were vaccinated and 274 were not eligible for one reason or another. |
|
| Interventions | Cold‐adapted recombinant spray vaccine (FluMist) in 2 intranasal doses or no vaccination. Content, degree of matching, or surrounding community viral circulation is not described. | |
| Outcomes |
Effectiveness Days enrolled, days present, and days absent during the study period (which is not reported) |
|
| Funding Source | Government | |
| Notes | The authors conclude that "the 2 schools receiving FluMist increased their attendance rates from 95.3% and 93.9% to 96.1% and 95.8%. Previously, the comparison schools each had a 94.6% attendance rate; 1 fell to 94.4% and the other rose very slightly to 94.7%. The differences in self‐ or parent‐reported influenza absences were not significant. However, the difference in days absent between individual vaccinated and non‐vaccinated schools was statistically significant" Appalling reporting: no season, vaccine content, or viral circulation, no outcome definition, no incidence of ILI or definition of respiratory illness, selection bias, unclear conclusions, and mixture of 2 designs (before‐and‐after comparisons mixed with prospective cohort). High risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | High risk | No description |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Draw from different source |
| PCS/RCS‐Comparability | Unclear risk | No description |
| PCS/RCS‐Assessment of Oucome | Unclear risk | Not described |
| Summary assessments | High risk | No outcome definition, no incidence of ILI or definition of respiratory illness, selection bias, unclear conclusions, and mixture of 2 designs |
ab Wright 1976a.
| Methods | 2 studies are reported in the paper:
|
|
| Participants | 33 preschool children aged 3 to 6 were enrolled in Wright 1976 1. 35 children enrolled in the Pediatric Vaccine Clinic at Vanderbilt Hospital (Nashville, Tennessee) aged between 12 and 28 months | |
| Interventions | Children randomly received a single dose of 0.25 mL of monovalent inactivated flu vaccine B/Hong Kong/5/72 (zonally purified, Eli Lilly and Company) containing at least 250 CCA units per dose or saline control at the time of a routine clinic visit. Wright 1976 1 was conducted on preschool children. All children from 1 classroom received 1 dose of vaccine; 8 children from another classroom consisting of 12 children received vaccine, whereas the remaining 4 were given saline solution in double‐blind manner. 3 of these 4 controls received 1 dose of vaccine 6 weeks later. | |
| Outcomes |
Serological
Haemagglutinin inhibition antibody test against 4 units of Flu/B/HK/8/73 antigen Effectiveness N/A Safety Parents of the children completed a questionnaire to record local and systemic reactions such as the temperature at 20:00 on the day of vaccination. Parents were unaware if the children received immunisation. |
|
| Funding Source | Industry | |
| Notes | Parents of the children completed a questionnaire to record local and systemic reactions such as the temperature at 20:00 on the day of vaccination. Parents were unaware if the children received immunisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Single |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Summary assessments | High risk | Insufficient information to assess study design |
ab Zangwill 2001.
| Methods | Randomised, placebo‐controlled trial to assess safety and reactogenicity of 4 different lots of cold‐adapted influenza vaccine. The aim of the study was to test replicability of lots vs placebo versus a different concentration. | |
| Participants | Healthy children aged 12 to 36 months from the Kaiser Permanente paediatric clinic population. Children could be enrolled only in absence of the following conditions: hypersensitivity to eggs, presence of underlying chronic illnesses for which influenza vaccine was recommended, immunodeficiency diseases, acute febrile illnesses within 7 days or upper respiratory illnesses within 3 days of vaccination, prior receipt of inactivated flu vaccine or CAIV‐T, administration of an investigational drug within 1 month of vaccination in this study, administration of any live virus vaccine within 1 month of vaccination in this study, administration of any inactivated vaccine within 2 weeks of vaccination in the study, history of wheezing or bronchodilator medication use within 2 weeks before vaccination, receipt of any blood product within 3 months before vaccination, administration of nasal medication during the first 10 days after vaccination, no telephone in the household. 500 children were enrolled. | |
| Interventions | Children were randomised into 5 groups to receive 1 of the following preparations.
Each preparation was given as intranasal spray administered in 2 doses of 0.5 mL (0.25 mL per nostril) about 60 days apart. 500 children were enrolled; 474 children received 2 doses of vaccine or placebo. |
|
| Outcomes |
Serological
Paired sera for antibody response assessment Effectiveness N/A Safety After vaccination, children were observed for at least 15 minutes and families were provided with digital thermometer and diary cards to record temperature and occurrence of symptoms listed in the card (lethargy, irritability, runny nose/nasal congestion, sore throat, cough, headache, muscle aches, chills, vomiting) for 10 days. Other symptoms or medications taken were also reported. |
|
| Funding Source | Industry | |
| Notes | The authors concluded that all lots of vaccines were safe and immunogenic. The number of children in each arm was not stated in the paper but was obtained through contact with author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Coded |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Summary assessments | Low risk | Plausible bias unlikely to seriously alter the results. |
aa Bracco Neto 2009a.
| Methods | Placebo‐controlled (year 1 = 2001) Multicentre study conducted during the 2001 and 2002 influenza seasons at 35 sites in South Africa, Brazil, and Argentina (Southern Hemisphere) |
|
| Participants | 3200 children 6 to 36 months of age in good health were enrolled. Exclusion criteria in year 1 included any serious chronic disease, immunosuppression or presence of an immunocompromised household member, receipt of any commercial or investigational influenza vaccine before enrolment, a documented history of hypersensitivity to any component of LAIV or placebo. | |
| Interventions | LAIV versus 2 placebos: excipient or saline placebo. Saline placebo (Salplacebo) consisted of physiologic saline; excipient placebo (Eccplacebo) was the vaccine excipient alone (sucrose‐phosphate‐glutamate buffer, arginine, acid hydrolysed porcine gelatin, and normal allantoic fluid), in the same concentration as in LAIV. There were 4 arms in year 1: LAIV 2 doses, Eccplacebo, Salplacebo, and LAIV 1 dose plus Salplacebo 1 dose each. Vaccine content and degree of vaccine matching were unclear. | |
| Outcomes |
Laboratory Culture “standard techniques by laboratories in Argentina, Brazil and South Africa” Effectiveness Cultured‐confirmed influenza illness and all episodes of AOM and any LRTI, hospitalisation Safety Reactogenicity events and AEs |
|
| Funding Source | Industry | |
| Notes | The authors conclude “that a single dose of LAIV provided clinically significant protection against influenza in young children previously UV against influenza and 2 doses provided persistent protection through a second season without revaccination. These benefits, together with the vaccine’s safety profile in children 2 years of age and older, provide support for increased use of LAIV in children < 2 years of age. LAIV was well tolerated; no significant differences in solicited reactogenicity events were seen between treatment groups. LAIV was not associated with an increased rate of AEs through day 11 postvaccination. When AEs were assessed through day 28 postvaccination in year 2, the rate of bronchitis was significantly increased in LAIV recipients, although rates of bronchospasm and any respiratory AEs were similar between groups. Additionally, no differences in solicited reactogenicity events or other AEs were seen after either saline or excipient placebo. This suggests that the excipients in LAIV, which include egg protein and acid‐hydrolyzed gelatin, do not contribute to reactogenicity in vaccine recipients”. The description of trial methods and results is unclear. The rationale for the use of 2 placebos is unclear. An allocation mistake was made in year 2 of the study with a swap of a group from active to placebo and vice versa. It is unclear whether blinding was maintained throughout or not, but attrition appears to have gone up to 58% (Figure 1 is very difficult to interpret). In addition, numerators are not reported, and there is no mention of attempts at standardisation of laboratory procedures across 2 continents and 3 countries. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random lists |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation scheme |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses to follow‐up |
| Summary assessments | Low risk | Plausible bias unlikely to seriously alter the results. |
ba Cochran 2010a.
| Methods | Case‐control study to assess influenza vaccine effectiveness among children aged 6 to 23 months within the Northern California Kaiser Permanente Medical Care Program who tested positive for influenza during the years 2003 to 2006 | |
| Participants | Description of cases: children aged 6 to 23 months whose families were enrolled in Kaiser Permanente Northern California membership who tested positive for influenza during the years 2003 to 2006 Description of controls: participants without a positive influenza test were matched to each of these cases based on birth month/year and zip code | |
| Interventions | 1 and 2 doses of the trivalent inactivated vaccine against laboratory‐confirmed influenza | |
| Outcomes | ||
| Funding Source | Government | |
| Notes | The authors conclude that “during the 2005–2006 influenza season, when predominant circulating virus strains and vaccine strains were well‐matched, vaccination was 76% (95% CI: 37% to 91%) effective against laboratory‐confirmed infection. There was no statistically significant effect of vaccination, however, for the 2003–2004 or 2004–2005 seasons. Our results highlight the need for further study of influenza vaccine effectiveness in this age group” A very strangely reported study with Results before Methods (pages are numbered consecutively, though). Unclear case selection process and no mention of blind exposure assessment. No data were available on symptom status of cases or controls. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | High risk | Not clearly described |
| CC‐Control Selection | Unclear risk | Apparently same population |
| CC‐Comparability | High risk | Insufficient description |
| CC‐Exposure | Low risk | Secure record |
| Summary assessments | High risk | Lack of information about study design and matching method |
ba Eisenberg 2008a.
| Methods | A prospective, population‐based case‐control study of hospitalisations attributable to laboratory‐confirmed influenza was performed in counties that encompass Nashville, Tennessee; Rochester, New York; and Cincinnati, Ohio, during the 2003–04 and 2004–05 influenza seasons. Each site conducted surveillance at sufficient hospitals to capture 95% of hospitalisations attributable to ARI or fever among children residing in the respective county. Study nurses enrolled children within 48 hours after admission to surveillance hospitals Sunday through Thursday in the 2003–04 influenza season and 7 days per week during the 2004–05 season. | |
| Participants | Description of cases: Eligible children were county residents, 5 years of age or younger, with an admission diagnosis of ARI or fever with laboratory‐confirmed influenza. Description of controls: Children resident in the same county of cases who tested negative for influenza were control participants. | |
| Interventions | Unclear. Matching is described as suboptimal. | |
| Outcomes | ||
| Funding Source | Government | |
| Notes | The authors conclude that “even in an influenza season (2004–2005) with a suboptimal vaccine match, more than 1 half of these visits could be prevented with recommended influenza vaccination Partial vaccination did not seem to be effective These results offer additional evidence in support of recommendations for vaccinating children against influenza and they highlight the importance of children receiving the recommended number of influenza vaccinations” No mention of blind exposure assessment is made and effects of vaccine on laboratory outcomes supposedly assesses effectiveness. Broken‐down data not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | Low risk | Independent validation |
| CC‐Control Selection | Low risk | Same population |
| CC‐Comparability | Low risk | Possibly adequate |
| CC‐Exposure | Low risk | Secure record |
| Summary assessments | Low risk | Plausible bias unlikely to seriously alter the results. |
ba Gilca 2011.
| Methods | Matched case‐control study performed to assess the effectiveness of a single paediatric dose of AS03‐adjuvanted vaccine (Pandemrix, GSK) against hospitalisation in children aged 6 months to 9 years during the fall 2009 vaccination campaign in Quebec, Canada | |
| Participants | Description of cases: children hospitalised for PCR‐confirmed pH1N1 infection (“Quebec residents aged 6 months to 9 years hospitalised with laboratory‐confirmed pH1N1 infection”) Description of controls: non‐hospitalised children, matched by age and region of residence. For each case, 15 potential controls were selected at random from the health insurance registry. |
|
| Interventions | A single paediatric dose of AS03‐adjuvanted pH1N1 vaccine vs no intervention | |
| Outcomes | ||
| Funding Source | Government | |
| Notes | The authors conclude that a single paediatric dose of the AS03‐adjuvanted pH1N1 vaccine given to children aged 6 months to 9 years is highly protective against hospitalisation, beginning as early as 10 days after immunisation. The study is summarily reported. It is unclear whether blinded assessment of exposure status was carried out. In addition, it is unclear whether the children were hospitalised because of influenza or whether influenza was a chance finding and hospitalisation occurred for other reasons. This is a very important aspect in pandemic H1N1 infection, where most deaths were recorded for multiple pathologies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | Low risk | Independent validation |
| CC‐Control Selection | Low risk | Community control |
| CC‐Comparability | Unclear risk | Drawn from insurance registry |
| CC‐Exposure | Unclear risk | Self reported |
| Summary assessments | Unclear risk | Plausible bias that raises some doubts about the results |
ba Kelly 2011.
| Methods | The Western Australian Influenza Vaccine Effectiveness (WAIVE) study evaluated the protective effect of inactivated influenza vaccination in children aged 6 to 59 months, by means of a prospective case‐control study conducted in general practices and a hospital emergency department. Eligible participants were tested for influenza and a range of other common respiratory viruses. Influenza vaccine effectiveness (VE) against laboratory‐confirmed influenza was estimated with cases defined as children with an ILI who tested positive and controls as those with an ILI who tested negative for influenza virus. |
|
| Participants | Participants were children aged 6 to 59 months presenting with an ILI and from whom swabs had been taken for laboratory testing. ILI definition used in this study was “documented fever with oral (or aural) temperature 38°C (or axillary temperature 37.5°C), with at least 1 acute respiratory symptom or sign. Children were recruited if they had met the case definition for an ILI within the previous 72 hours”. All emergency department participants were recruited from the Emergency Department of Princess Margaret Hospital for Children, the only paediatric tertiary hospital in Western Australia. Children were also recruited from general practices in metropolitan Perth and Kalgoorlie. Those testing positive for influenza viruses were identified as cases, while those testing negative for influenza viruses were identified as controls. Cases and controls were recruited when they presented with an ILI, but their case or control status was not known at the time. |
|
| Interventions | Informed consent was obtained, parents were provided with a questionnaire to complete, which included demographic data, influenza vaccinations received in 2008 and previous years, and any underlying chronic illnesses. Vaccine status was validated for 87% of all participants with the vaccine provider of the child. Children were defined as FV if they had received 2 age‐appropriate doses of vaccine at least 21 days apart and more than 14 days before ILI onset in 2008. Children were also defined as FV if they had received at least 2 previous doses of influenza vaccine in any year and 1 dose of the age‐appropriate vaccine in 2008. Children who received no vaccine in 2008 were counted as UV, and all other children were defined as partially vaccinated. |
|
| Outcomes |
Laboratory “All samples were then tested by real‐time PCR directed to specific targets in the matrix genes of influenza A and B and the H1 and H3 genes of influenza A.13,14 Samples were also cultured for influenza viruses using centrifuge‐enhanced inoculation of Madin‐Darby canine kidney cells and those which were culture positive were referred to the World Health Organization Collaborating Centre for Reference and Research on Influenza in Melbourne, where detailed antigenic characterisation was performed. In addition to influenza viruses, the swabs were tested by PCR for the presence of rhinoviruses, respiratory syncytial viruses, parainfluenza virus types 1, 2 and 3, human metapneumoviruses and enteroviruses. Viral culture for adenoviruses was also performed using diploid lung fibroblast cells and monitoring for cytopathic effect” |
|
| Funding Source | Government | |
| Notes | The authors conclude that "A total of 75 children were enrolled from general practices and 214 through the emergency department, with 12 (27%) and 36 (17%), respectively, having laboratory‐confirmed influenza. Using all the influenza negative controls, the adjusted VE was 58% (95% confidence interval, 9–81). When controls were limited to those with another virus present, the adjusted VE was 68% (95% confidence interval, 26–86). VE estimates were higher when controls included only those children with another respiratory virus detected". A well‐reported and well‐conducted study; the only concern is about the role of confounding variables selected to adjust estimates. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | Low risk | Independent validation |
| CC‐Control Selection | Low risk | Drawn from the same population ‐ hospital control |
| CC‐Comparability | Unclear risk | Adjustment by confounders |
| CC‐Exposure | Low risk | Secure record ‐ interview |
| Summary assessments | Unclear risk | Plausible bias that raises some doubts about the results |
ba Kissling 2011.
| Methods | Third season of I‐MOVE (Influenza Monitoring Vaccine Effectiveness in Europe), a multicentre case‐control study based on sentinel practitioner surveillance networks in 8 European Union (EU) member states to estimate 2010/11 influenza vaccine effectiveness (VE) against medically attended ILI laboratory‐confirmed as influenza. The 8 study sites were settings in France, Hungary, Ireland, Italy, Poland, Portugal, Romania, and Spain. In 6 study sites, primary care practitioners belonging to the national influenza sentinel networks were invited to participate in the study. In Portugal and Italy, practitioners other than those participating in the national influenza sentinel networks were also invited to participate. The study population consisted of non‐institutionalised patients consulting a participating practitioner for ILI or ARI (France only) who had a nasal or throat swab taken less than 8 days after symptom onset and with no contraindication for influenza vaccination. In Hungary, the study population was restricted to those 18 years of age or older. The start of the study period in each of the study sites was defined as more than 14 days after the start of the 2010/11 influenza vaccination campaign. Practitioners in Ireland, Poland, Portugal, Spain, and France swabbed all ILI/ARI patients aged 65 and over; in Hungary they swabbed all ILI patients 60 and over; and in Italy they systematically swabbed 1 ILI/ARI patient aged 65 and over per week. In all study sites, practitioners systematically sampled ILI/ARI patients to swab among the other age groups, apart from Romania, where practitioners swabbed all ILI patients in all age groups. In all study sites, practitioners interviewed the ILI patients using country‐specific questionnaires. The common variables collected in the 8 study sites included ILI signs and symptoms, age, sex, pregnancy, presence of chronic conditions, severity of the chronic disease measured as the number of hospitalisations for the chronic disease in the previous 12 months, smoking history (none, past, current smoker), number of practitioner visits in the previous 12 months, 2009‐10 pandemic vaccination status, seasonal influenza vaccination in the 2009‐10 and the 2010‐11 season. ILI patients were excluded if they presented ILI symptoms before the week of onset of the first recruited influenza case. For each study site, ILI patients were excluded if presenting either after the onset week of the last recruited influenza case or after the onset week of the case prior to 2 consecutive weeks of no positive case recruited. To estimate VE against A(H1N1)2009 and against influenza B virus, the exclusion criteria were based on the week of onset of the first and last A(H1N1)2009 and influenza B case, respectively. |
|
| Participants | A case was defined as a patient with signs and symptoms adhering to the EU ILI case definition (sudden onset of symptoms and at least 1 of the following 4 systemic symptoms: fever or feverishness, malaise, headache, myalgia, and at least 1 of the following 3 respiratory symptoms: cough, sore throat, shortness of breath), who was swabbed and tested positive for influenza using real‐time polymerase chain reaction (qRT‐PCR) or culture. Controls were EU ILI patients who were swabbed and tested negative for influenza. |
|
| Interventions | An individual was considered vaccinated if he/she received at least 1 dose of the 2010‐11 seasonal vaccine more than 14 days before the date of onset of ILI symptoms. | |
| Outcomes |
Laboratory Those who were swabbed and tested positive for influenza using qRT‐PCR or culture. Swabs were tested for influenza at the respective countries’ National Influenza Reference Laboratory (in Spain, the laboratories of the regional sentinel networks integrated in the Spanish Influenza Sentinel Surveillance System). In each country, all or a subset of influenza isolates were antigenically characterised. Laboratory viral detection, typing, subtyping, and variant analysis performed in each of the National Reference Laboratories are described elsewhere (European Centre for Disease Prevention and Control (ECDC) (2010) European Influenza Surveillance Network (EISN). Table 2: Characteristics of the virological surveillance systems participating in EISN, Available from: www.ecdc.europa.eu/en/activities/surveillance/EISN/laboratory_network/ ages/laboratory_network.aspx. Accessed October 2011). |
|
| Funding Source | Government | |
| Notes | In conclusion, the I‐MOVE multicentre case‐control study provided summary influenza VE estimates across Europe and showed a moderate VE against medically attended ILI laboratory‐confirmed influenza in a season of good match between the circulating influenza strains and the strains included in the 2010‐11 trivalent vaccine. Next season further study sites may be included in the pooled analysis, and current study sites will focus on increasing sample size through recruitment of more GPs in order to obtain more precise estimates, to carry out an adjusted 2‐stage pooled analysis, and to obtain age‐specific estimates by influenza type among the target group for vaccination. Even if the trivalent inactivated influenza vaccines may only provide moderate protection against medically attended ILI laboratory confirmed as influenza, they remain, until more efficient vaccines are available, the most effective measure to prevent influenza infection and its consequences. Well‐conducted and well‐reported case‐control study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | Low risk | Independent validation |
| CC‐Control Selection | Low risk | Drawn from the same population |
| CC‐Comparability | Low risk | Study controls for age group, sex, presence of chronic conditions, at least 1 hospitalisation in the previous 12 months for chronic disease, smoking history, number of practitioner visits in the previous 12 months. |
| CC‐Exposure | Low risk | Secure record |
| Summary assessments | Low risk | Possible underestimation |
ba Mahmud 2011.
| Methods | Pandemic vaccines: population‐based case‐control study assessing the effectiveness of the pandemic H1N1 and seasonal TIV used during the 2009 mass vaccination campaign in Manitoba (Canada) in preventing laboratory‐confirmed H1N1 infections. Study uses data from Cadham Provincial Laboratory (CPL) and the Manitoba Immunization Monitoring System (MIMS). All Manitoba residents ≥ 6 months of age who had a respiratory specimen tested at CPL for H1N1 were included in the study. | |
| Participants | Any adult or child ≥ 6 months of age who normally resides in Manitoba and who had a respiratory sample submitted to CPL for influenza testing during the study period was eligible for inclusion in the study. The study was conducted from 2 November 2009 (1 week after the start of mass vaccination in Manitoba) to 10 February 2010. Description of cases Cases were individuals who tested positive for pandemic H1N1 influenza A by reverse transcriptase‐PCR (RT‐PCR). RT‐PCR assay developed by the National Microbiology Laboratory. Description of controls Controls were individuals who tested negative for both influenza A and B. Information on receipt of TIV or H1N1 vaccine was obtained by record linkage with MIMS, the population‐based, province‐wide immunisation registry. The date of specimen collection was considered the "index date". Exclusion criteria 12 individuals were excluded because they tested positive for influenza A but not for H1N1. |
|
| Interventions | For all cases and controls, information on the receipt of the pandemic H1N1, TIV, and the polyvalent pneumococcal polysaccharide (PPV23) vaccines during or before the 2009/10 season was obtained from MIMS, the population‐based, province‐wide registry recording virtually all immunisations administered to Manitoba residents since 1988. Estimates of the completeness and accuracy of the recorded vaccination information are high. Vaccinated individuals were classified into 3 groups depending on whether vaccination had occurred 1 to 6, 7 to 13, or ≥ 14 days before the index date. | |
| Outcomes | Laboratory‐confirmed influenza | |
| Funding Source | Government | |
| Notes | The authors conclude that "Overall, we found that the adjuvanted H1N1 vaccine was 86% effective in preventing laboratory‐confirmed H1N1 infections when received ≥ 14 days before testing, although effectiveness seemed lower among persons aged ≥ 50 years (51%) and among those with immunocompromising conditions (67%). We demonstrated that the adjuvanted H1N1 vaccine used during Manitoba’s mass vaccination campaign was highly effective against laboratory‐confirmed H1N1 infections, especially among children and younger adults. Despite logistical and communication challenges to vaccine delivery during the campaign, vaccine effectiveness appears comparable to that observed for influenza vaccines during non‐pandemic seasons in years with good antigenic match. This study demonstrates the utility of laboratory information systems and administrative databases for evaluating the effectiveness of influenza vaccines". A well‐conducted and well‐reported case‐control study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | Low risk | Record linkage |
| CC‐Control Selection | Low risk | Drawn from the same population |
| CC‐Comparability | Low risk | Adjustment by confounding factors |
| CC‐Exposure | Low risk | Secure record |
| Summary assessments | Low risk | Plausible bias unlikely to seriously alter the results. |
ab Mallory 2010.
| Methods | Randomised, placebo‐controlled trial carried out on children aged between 2 and 17 years in order to assess safety, tolerability, and immunogenicity of a monovalent intranasal 2009 A/H1N1 LAIV (MedImmune) | |
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions | H1N1 LAIV (2009 formulation) by MedImmune was derived by genetic reassortment of the haemagglutinin and neuraminidase genes from the wild‐type A/California/7/2009 virus and the remaining 6 gene segments from an attenuated master donor virus. The resulting 6:2 reassortant vaccine virus is grown in chicken eggs employing the same manufacturing process used to produce MedImmune’s seasonal trivalent LAIV.
Eligible participants were randomly assigned using an interactive voice response system in a 4:1 ratio to receive 2 doses of live monovalent H1N1 LAIV or placebo by intranasal spray 28 days apart. Randomisation was stratified by age (2 to 8 years and 9 to 17 years). Initially 326 children were enrolled and 261 (133 between 2 and 8 years and 128 between 9 and 17 years) were allocated to vaccine group, whereas 65 (29 between 2 and 8 years and 36 between 9 and 17 years) were allocated to control placebo. Children were further randomised (1:1) to provide a blood sample on either day 15 or day 29 after their first vaccination. A final immunogenicity blood sample was collected on day 57, approximately 28 days after the second vaccination. After conclusion of the blinded portion of the study, children randomised to receive placebo in the studies were offered optional H1N1 vaccination after collection of their day 57 blood sample. |
|
| Outcomes |
Laboratory Serum antibody titres were measured at baseline and on day 15 or 29 after dose 1 and on day 57 (28 days after dose 2) using a standardised HAI assay against antigenically matched influenza A/H1N1 6:2 virus reassortants. Safety A) The primary safety analysis compared the rates of fever (defined as a temperature of at least 38.3 °C) during days 1 to 8 after dose 1. B) Additional safety endpoints (from day 1 through day 8 and from day 1 through day 15 after each vaccination) included the following.
Memory aid worksheets were provided to record solicited symptoms, AEs, and concomitant medication use for 14 days after dosing. C) Serious adverse events and new‐onset chronic diseases were collected through 180 days after the final dose. Children who experienced a febrile illness within 7 days after dose 1 were instructed to return to the study site for evaluation. |
|
| Funding Source | Industry (MedImmune) | |
| Notes | The authors conclude that “This study demonstrates that 2 doses of 2009 H1N1 LAIV are safe in healthy children. Overall, the frequency of solicited symptoms and AEs were similar between H1N1 LAIV and placebo recipients, and most were mild to moderate in severity”. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random list |
| Allocation concealment (selection bias) | Low risk | Centralised |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses to follow‐up |
| Summary assessments | Low risk | |
ca Ortqvist 2011.
| Methods | Prospective cohort study carried out on Stockholm County inhabitants aged at least 6 months between week 44 and 52 in order to assess effectiveness of pandemic monovalent flu vaccine H1N1 (Pandemrix, GSK) in preventing laboratory‐confirmed H1N1 flu cases. Estimates were calculated by linking data from different databases: Sminet (for laboratory‐confirmed H1N1 flu cases), Vaccinera (on which data of vaccinated participants has been reported), Common Health‐Care Registers for Stockholm County Council (GVR, for detection of hospital admission cases due to influenza H1N1), Statistic Sweden (for demographical data). | |
| Participants | Inhabitants of Stockholm County (2,019,183, out of which 449,971 were aged under 19 years) | |
| Interventions |
Immunisation campaign started in week 42. Data about vaccination are recorded in the Vaccinera database, where date of vaccination, batch number of the vaccine, the person’s unique identification number, medical risk group of vaccinated are reported. A flu case was considered vaccinated if diagnosis/hospital admittance occurred more than 14 days after administration of the first vaccine dose. 25 cases of confirmed H1N1 flu cases were observed between weeks 44 and 52 among participants who had received 1 or 2 vaccine doses at least 14 days before diagnosis or hospitalisation. Out of them, 11 (10) were aged between 6 months and 12 years. |
|
| Outcomes | Laboratory Not assessed Effectiveness Cases of laboratory‐confirmed H1N1 flu cases notified to the Institute for Infectious Diseases Control and available in the Sminet database occurred between week 42 and 52 of 2009. Incidence rate ratios for a given week were calculated comparing the rate of people who developed influenza > 14 days after being vaccinated out of the cumulated number of people who had been vaccinated up until 2 weeks before with the rate of people with an influenza diagnosis out of all non‐vaccinated people, excluding people who had had a previous influenza diagnosis. Safety Not assessed | |
| Funding Source | Government: funding was provided by the County Council of Stockholm and by the Department of Communicable Diseases Control and Prevention, Stockholm County. Authors declare that they have no conflicts of interest. |
|
| Notes | Authors attempted to identify possible risk factors associated with vaccine failure in the study population, analysing incidence of several chronic conditions in cases (total cases of vaccine failures, n = 25) and in vaccinated controls (matched for age and vaccination date) using a case‐control design. For both chronic renal or hepatic disease and immunocompromised condition, a significant association was found (whole populations). As authors self note in the Discussion, “the sampling for the sampling for an influenza diagnosis was not made systematically but in routine medical care”. The authors conclude that “monovalent AS03‐adjuvanted influenza A(H1N1)v vaccine was very effective in preventing the pandemic influenza in Stockholm County”. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Voluntary vaccinee |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Refuse the vaccination |
| PCS/RCS‐Comparability | High risk | Insufficient description |
| PCS/RCS‐Assessment of Oucome | Low risk | Secure record |
| Summary assessments | High risk | Insufficient description as to how exposed and not exposed were selected ‐ possible bias by indication |
ab Plennevaux 2011.
| Methods | Randomised, placebo‐controlled trial assessing reactogenicity and immunogenicity of a split‐virion monovalent vaccine administered in children aged between 6 months and 9 years | |
| Participants | A total of 474 children were enrolled in the study; 229 of them were aged 6 to 35 months, 245 between 3 and 9 years. Exclusion criteria Known or suspected influenza infection since March 2009; any vaccination in the previous 4 weeks or planned within 6 weeks following the first trial vaccination; hypersensitivity to any vaccine component or life‐threatening reaction to a vaccine containing the same substances; known or suspected immunodeficiency; recent history (< 6 months) of immunosuppressive therapy or long‐term systemic corticosteroid therapy; known HIV, hepatitis B or C infection; receipt of blood or blood‐derived products in the previous 3 months; and febrile or acute illness on the day of enrolment |
|
| Interventions | Used vaccine was an inactivated split‐virion preparation of the New York Medical College (NYMC) X‐179A reassortant of the A/California/07/2009 (H1N1) strain and the PR8/8/34 strain, distributed by the US Centers for Disease Control and Prevention (CDC). Seed virus was propagated in embryonated chicken eggs, inactivated and split according to the process used to produce a seasonal influenza vaccine licensed in the US for people aged > 6 months (Fluzone, Sanofi Pasteur, Swiftwater, PA, USA). 2 different antigenic concentrations were tested: 7.5 mcg or 15 mcg HA per dose. Vaccine was supplied as single‐dose vials without preservative for 6‐ to 35‐month‐olds and multidose vials containing 0.01% thiomersal preservative for 3‐ to 9‐year‐olds. Children were randomly assigned to 1 of 3 study groups (7.5 mcg HA, 15 mcg HA, placebo) using randomisation lists with stratification by age group (6 to 35 months and 3 to 9 years). 2 doses were administered 21 days apart. |
|
| Outcomes | HI antibody titration against the vaccine strain using the standard HI assay with turkey erythrocytes had been performed on serum samples collected at baseline and 21 days after each inoculation. Immunogenicity data were summarised using geometric mean titre, geometric mean titre ratio, seroprotection rate (defined as % of participants with titres ≥ 1: 40), seroconversion rate (defined as % of participants with a prevaccination titre < 1:10 and a postvaccination titre ≥ 1:40, or with a prevaccination titre ≥ 1:10 and ≥ 4‐fold increase after vaccination).
|
|
| Funding Source | Industry | |
| Notes | The authors conclude that the safety and reactogenicity of the pandemic (H1N1) 2009 vaccine, at either dose, were acceptable and similar to placebo after both the first and second vaccinations. The safety results observed were similar to those seen historically with seasonal inactivated trivalent influenza vaccines. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses to follow‐up |
| Summary assessments | Low risk | |
ba Staat 2011a.
| Methods | Case‐control study assessing the efficacy of the trivalent inactivated influenza vaccine against laboratory‐confirmed influenza for the 2005–06 and 2006–07 influenza seasons. Vaccination rates among children 6 to 59 months of age with ARI or fever and laboratory‐confirmed influenza were compared with influenza test‐negative controls who also had a medically attended ARI. The design is based on active surveillance system in which the influenza vaccination status of children with laboratory‐confirmed influenza was compared with that of laboratory‐confirmed influenza‐negative matched controls. | |
| Participants |
Inpatient Children were enrolled 5 days a week after admission to surveillance hospitals. Eligible children were county residents younger than 5 years of age who were admitted with signs or symptoms of ARI. Children were excluded if they had fever and neutropenia associated with chemotherapy, were hospitalised in the prior 4 days, transferred from another surveillance hospital, or were newborns never discharged from the hospital. Outpatient settings Prospective surveillance of county children presenting with ARI to selected clinics and EDs was conducted during the 2 seasons. Study personnel enrolled children in the clinics and the EDs on specified surveillance days using similar inclusion and exclusion criteria to inpatient enrolment. Children were enrolled 1 or 2 days per week in 1 to 4 paediatric clinics per county and were enrolled 3 or 4 days per week in the EDs. Description of cases and controls Children whose specimens tested positive for influenza were eligible to be cases, and those who tested negative were eligible to be controls. To ensure that all children included in this study were eligible for vaccination based on current recommendations, the following parameters were used. Since the minimum age to receive a primary influenza vaccination is 24 weeks (168 days), followed by a second vaccination a minimum of 24 days later (192 days), and the child is considered protected 2 weeks following the final dose (206 days), 206 days was used as the lower age limit for this study. The upper age limit was 59 months at the onset of symptoms. The onset of the child’s symptoms must have occurred during influenza season for each geographic site. The start of the influenza season was defined as the occurrence of 1 or more positive influenza specimens in 2 consecutive weeks through local research or hospital laboratories at each site. The end of the influenza season was defined as the absence of 1 or more positive specimen(s) of influenza in 2 consecutive weeks. Control children were matched to case children by disease onset date (plus or minus 7 days), clinical setting (inpatient, ED, or clinic), geographic site (Nashville, Cincinnati, Rochester), and age (6 to 23 months, 24 to 59 months). The number of matched controls per case varied from 1 to 4 (1 control (28%), 2 controls (15%), 3 controls (12%), and 4 controls (34%)). For 18 children, 8 from the ED and 10 from outpatient practices, only 1 control that matched 2 cases was available, so both cases were matched to the same control. |
|
| Interventions |
Exposure Influenza vaccination status at the time of the ARI visit was determined through a telephone call or fax to the child’s primary care practice and subsequent extraction of influenza vaccination data from the child’s primary care medical record and/or the state immunisation registry, if available. Children were classified as FV if vaccinated according to ACIP guidelines, which included either 2 doses in the current season administered ≥ 24 days apart, or at least 1 vaccine dose in a previous influenza season and 1 dose in the current season, administered ≥ 14 days before ARI onset. Children were classified as being partially vaccinated if they received only 1 of the 2 recommended doses in the current season, ≥ 14 days before ARI onset or 2 vaccinations in the current season with the second dose administered within 14 days of ARI onset or < 24 days after the first dose. Children were classified as UV if they received no influenza vaccine doses during the study season or received the first of 2 recommended doses within 14 days before ARI onset during the study season. |
|
| Outcomes |
Laboratory Nasal and throat swabs obtained from each enrolled child were tested for influenza at each site’s research laboratory with standardisation of assays across sites using reverse transcription‐polymerase chain reaction assays, as described previously. A subset of children had viral cultures done. A specimen was defined as being influenza‐positive if viral culture or duplicate PCR assays were positive for influenza A or B. No children had a positive culture for influenza and a negative PCR, while 9 children with a negative culture had a positive PCR for influenza. |
|
| Funding Source | Government | |
| Notes | The authors conclude that "Each year, young children experience high rates of hospitalizations, ED visits and outpatient visits due to influenza. We found that full vaccination with the trivalent inactivated vaccine prevented nearly 60% of medically attended influenza visits across 2 influenza seasons for individual and combined age groups of children. An estimated 5% to 10% of children have an influenza‐related ARI visit each year and the visit often results in an antibiotic prescription [1,2,27]. This study and others’ suggest that widespread influenza vaccination of children will have a major impact on health care utilization. Our study supports recommendations from the CDC to vaccinate young children against influenza disease and highlights the importance of full vaccination, since partial vaccination showed no significant VE". Well‐reported and well‐conducted study; the only concern is about real effect of the matching procedure adopted by the authors. We are uncertain as to whether it assures good comparability between cases and control. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | Low risk | Independent validation |
| CC‐Control Selection | Low risk | Drawn from the same population |
| CC‐Comparability | Unclear risk | Matched |
| CC‐Exposure | Low risk | Secure record and interview |
| Summary assessments | Unclear risk | Plausible bias that raises some doubts about the results |
ba Valenciano 2011.
| Methods | A multicentre case‐control study based on sentinel practitioner surveillance networks from 7 European countries was undertaken to estimate the effectiveness of 2009–10 pandemic and seasonal influenza vaccines against medically attended ILI laboratory confirmed as pandemic influenza A (H1N1) (pH1N1). The study was conducted within the context of the existing European Influenza Surveillance Network (EISN). At the 7 study sites, EISN sentinel primary care practitioners were invited to participate in the study. In Portugal and Italy, practitioners other than those participating in EISN were also invited to participate. The study population consisted of patients consulting a participating practitioner for ILI (6 sites) or ARI (France) and having a nasal or throat swab taken within an interval of less than 8 days after symptom onset. In Hungary, the study population was restricted to patients aged more than 17 years. In Italy, the study population was restricted to patients who belonged to the groups for which the pandemic vaccine was recommended. In 5 of the 7 study sites, practitioners used a systematic random sample to select the patients to swab. In Ireland, each participating practice was asked to take a nasal or throat swab from 5 patients presenting with ILI each week. In France, each practitioner had an age group assigned and swabbed the first ARI patient of the week in the allocated age group. |
|
| Participants |
Exclusion criteria Individuals who tested positive for influenza A but had a non‐typeable strain, those testing positive for other strains of influenza A or for influenza B, and those with missing information on laboratory results were excluded. Description of cases A case of pandemic influenza A (H1N1) 2009 (pH1N1 case) was an ILI patient (defined according to the EU case definition as sudden onset of symptoms and at least 1 of the following 4 systemic symptoms: fever or feverishness, malaise, headache, myalgia, and at least 1 of the following 3 respiratory symptoms: cough, sore throat, shortness of breath) who was swabbed and tested positive for the pH1N1 using real‐time PCR or culture. Swabs were tested for influenza at the respective countries’ National Influenza Reference Laboratory. In France, Italy, and Spain, tests were also conducted in other laboratories participating in the National Influenza Sentinel Surveillance System. Description of controls Controls were ILI patients who were swabbed and tested negative for any influenza virus. |
|
| Interventions | Exposure For pandemic and seasonal influenza vaccine, individuals were considered vaccinated if they had received a dose of the vaccine more than 14 days before the date of onset of ILI symptoms and UV if they had received no vaccine or the vaccine was given less than 15 days before the onset of ILI symptoms. Vaccination status was ascertained using the practitioners’ medical records or during the patient interview. Each of the 7 study teams entered and validated data. Validation of the vaccination status and of other variables was attempted by contacting the practitioner or by checking existing vaccination registries in the case of missing information. | |
| Outcomes | pH1N1 using real‐time PCR or culture | |
| Funding Source | Government | |
| Notes | The authors conclude that the results suggest good protection of the pandemic monovalent vaccine against medically attended pH1N1 and no effect of the 2009–10 seasonal influenza vaccine. "However, the late availability of the pandemic vaccine and subsequent limited coverage with this vaccine hampered our ability to study vaccine benefits during the outbreak period." Future studies should include estimation of the effectiveness of the new trivalent vaccine in the upcoming 2010–11 season, when vaccination will occur before the influenza season starts. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | Low risk | Independent validation |
| CC‐Control Selection | Low risk | Drawn from the same population |
| CC‐Comparability | Low risk | Adjustment by confounders |
| CC‐Exposure | Low risk | Interview |
| Summary assessments | Low risk | Possible underestimation of vaccine efficacy |
ba Van Buynder 2010.
| Methods | Pandemic vaccines; case–control study on vaccine efficacy Carried out on children under 10 years of age with ILI who were tested for H1N1 infection at the central provincial laboratory. Laboratory‐confirmed influenza was the primary outcome and vaccination status the primary exposure to assess VE. |
|
| Participants | All children throughout New Brunswick, 6 months to 9 years of age, who were tested for H1N1 were selected for inclusion. Children were classified as cases if the respiratory sample was H1N1 positive. Children were classified as a control if the test was negative and the child met a clinical case definition of ILI (the presence of fever and at least 1 respiratory symptom or sign). Information on age, sex, hospitalisation, indigenous status, prematurity, immunosuppression, coexisting medical conditions, previous seasonal vaccination, and recent pandemic vaccination was collected by direct telephone interview. The diagnosis of an ILI was confirmed using a simple questionnaire. The interviews were conducted by staff from CDCB. |
|
| Interventions | Vaccination status and date of vaccination was determined through access to New Brunswick’s universal pandemic vaccination registration programme. This programme recorded the personal details of every person vaccinated in New Brunswick, including the date of administration. Children were classified as vaccinated if the child had received a dose of the H1N1 vaccine at least 14 days before the onset of symptoms and as "not vaccinated" if the child had received no vaccination or had received the first dose < 14 days before the onset of symptoms. No child in the study was 14 days' postreceipt of a second vaccine dose. | |
| Outcomes | ||
| Funding Source | Government | |
| Notes | The authors conclude that “A single 0.25 ml dose of the GSK adjuvanted vaccine (Arepanrix) protects children against laboratory‐confirmed pandemic influenza potentially avoiding any increased reactogenicity associated with second doses. Adjuvanted vaccines offer hope for improved seasonal vaccines in the future”. This is a poorly reported study in which selection criteria for cases are not clearly described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | High risk | Insufficient description |
| CC‐Control Selection | Unclear risk | Possibly drawn from the same population |
| CC‐Comparability | Unclear risk | Adjustment by confounding factors |
| CC‐Exposure | Unclear risk | Structured interview |
| Summary assessments | High risk | Plausible bias that seriously weakens confidence in the results |
ca Yin 2011.
| Methods | Prospective cohort study carried out on children aged 6 to 59 months from 2 day‐care centres (DCC) and 2 preschool centres (PSC). The study assessed effectiveness of trivalent inactivated seasonal vaccine in preventing ILI cases. | |
| Participants | Children from 2 day‐care centres (DCC1, n = 62 and DCC2, n = 73; age range 6 to 59 months) and 2 preschool centres (PSC1, n = 52 and PSC2, n = 52; age range 24 to 59 months) in Sydney | |
| Interventions | Administered vaccine was Vaxigrip Junior (Sanofi Pasteur, Lyon, France) prepared with the strain recommended for the 2007 season in the Southern Hemisphere:
Children were immunised with 2 doses of 0.5 mL (0.25 mL if aged less than 36 months) 1 month apart intramuscularly administered. Children from DCC1 and PSC1 were immunised, whereas children from DCC2 and PSC2 acted as control group and did not receive any treatment. Immunisation was performed between 11 July 2007 and 19 September 2007. |
|
| Outcomes | Laboratory Study nurses trained parents to collect nasal swabs by means of the Virocult system. Samples were sent by post to the Queensland Paediatric Infectious Diseases Laboratory, where the presence of the following viruses has been investigated: human rhinoviruses, influenza A, influenza B, RSV, adenoviruses, human metapneumovirus, parainfluenza viruses I, II, and III, bocavirus, hPyV‐WU, hPyV‐KI, and human coronaviruses OC43, 229E, NL6332, and HKU1.33. Effectiveness ILI: defined as illness with fever > 37.8 °C and at least 1 respiratory symptom (cough, blocked nose or runny nose). Parents assessed cases after education for ILI surveillance. This was begun 2 weeks after the second dose among vaccinated children and from 26 August 2007 onwards among controls, and was continued up to 21 October 2007. Households were also invited to monitor ILI symptoms by mail or phone call between 30 July and 21 October 2007. Safety Not assessed | |
| Funding Source | Government | |
| Notes |
The authors conclude that “No evidence was found for influenza VE but point estimates were all in the direction of protection”. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Unclear risk | Not clearly described |
| PCS/RCS‐Selection Non Exposed cohort | Unclear risk | Not clearly described |
| PCS/RCS‐Comparability | Unclear risk | Not clearly described |
| PCS/RCS‐Assessment of Oucome | Unclear risk | Self report |
| Summary assessments | High risk | Plausible bias that seriously weakens confidence in the results |
cb MPA 2011.
| Methods | Person‐time cohort study, based on a case inventory of narcolepsy cases observed in the 6 Swedish counties between 2009 and 2010 in order to assess its possible association with exposure to pandemic flu monovalent vaccine (Pandemrix) in children and adolescents, conducted by Medical Product Agency (MPA) | |
| Participants | Cases of narcolepsy with cataplexy were identified from departments of neurology, paediatrics, paediatric neurology, or paediatric psychiatry of hospitals, sleep laboratories, and laboratories of clinical physiology performing multiple sleep latency tests (MSLT), MPA database on spontaneous adverse drug reaction reports on narcolepsy. Medical records were collected for cases that had been diagnosed or were under review during 2009 through 2010. 2 external clinical experts in neurology/sleep disorders were commissioned by the MPA to review the medical records of all the collected cases and to classify (independently of each other) the diagnosis according to the American Academy of Sleep Medicine criteria for narcolepsy with cataplexy (see Safety) and to assess the onset of the narcolepsy disease through dating of the first symptom of narcolepsy. In cases of discrepancy, a third review was performed by an external expert in paediatric neurology. In the preliminary study, only cases occurring in Stockholm, Västra Götaland, Östergötland, and Skåne counties in participants aged below 19 were considered, whereas the whole Swedish “under 19” age class was included in the whole study. |
|
| Interventions |
Incident exposed cases occurring during the pandemic period (after 1 October 2009) were defined as vaccinated if the date of vaccination was before the date of the first symptom of narcolepsy (at least 1 vaccine dose). Cases were classified as non‐exposed when there was no exposure to vaccination or when vaccination had occurred after the onset of symptoms or during the same month as onset of symptoms. |
|
| Outcomes |
Laboratory Not assessed Effectiveness Not assessed Safety Incidence of narcolepsy with cataplexy was compared between vaccinated and non‐vaccinated participants. Diagnosis in medical records was reviewed by 2 neurologists according to the American Academy of Sleep Medicine diagnostic criteria for narcolepsy with cataplexy:
Altogether, 87 cases of narcolepsy with cataplexy were confirmed after review of the 135 cases initially identified. Of these, 69 were vaccinated before the onset of the first symptom; 7 were not vaccinated or had symptoms onset before vaccination; and a further 6 were also not vaccinated and thus had onset of first symptoms before 1 January 2009 and were therefore excluded from the study. A further 5 cases were classified under “unknown vaccination status” because they had vaccination during the same month of onset. |
|
| Funding Source | Government | |
| Notes | In the preliminary registry‐based study, carried out on the population of four counties (Stockholm, Västra Götaland, Östergötland, and Skåne) within which vaccination register and healthcare data were accessible and available, all participants registered in the respective county on 1 October 2009 without a known diagnosis of narcolepsy were followed until 31 December 2010, date of narcolepsy diagnosis, death, or migration from the county, whichever came first. In the cohort of vaccinated participants, the follow‐up time was defined as exposure from the date of vaccination until the end of follow‐up. Vaccinated participants contributed with exposure time in the UV cohort from 1 October to the date of vaccination. The incidence rates in the vaccinated and UV cohorts, respectively, were calculated as the number of persons diagnosed with an incident registration for narcolepsy in the health databases, divided by the person years at risk. The relative risk, vaccinated versus UV cohorts, was calculated as the corresponding ratio of incidence rates. Exact CIs for relative risk were calculated through exact CIs for binomial proportions. Since there is no nationwide vaccination register, it was not possible to calculate the risk time directly for the total vaccinated and non‐vaccinated cohorts in all of Sweden. However, risk time was extrapolated from the registry‐based study using data from 4 counties/regions of Sweden. It is not clear how the 5 cases with symptom onset and vaccination within the same month have been considered. They had initially been classified as not vaccinated (see page 5, lines 4 to 5 from the top, i.e. 9 vaccinated cases vs 7 + 5 non‐vaccinated cases) and simply excluded from the main analysis (69 vaccinated cases vs 7 non‐vaccinated cases), then the authors consider, erroneously, these 5 as part of the 7 unexposed in the “sensitivity analysis” (see page 6, third paragraph from the top and page 7, fourth paragraph from the bottom). It would be useful to complete the sensitivity analysis considering the 5 with uncertain vaccine exposure among vaccinated first and among not vaccinated. The authors conclude that “These new results provide strengthened evidence that vaccination with Pandemrix during the pandemic period was associated with an increase in the risk for narcolepsy with cataplexy in children/adolescents 19 years and younger. Further research is urgently needed to explain the possible causative mechanisms”. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| PCS/RCS‐Selection Exposed cohort | Low risk | Secure record |
| PCS/RCS‐Selection Non Exposed cohort | Low risk | Secure record |
| PCS/RCS‐Comparability | Unclear risk | Retrospective study |
| PCS/RCS‐Assessment of Oucome | Low risk | Secure record |
| Summary assessments | Low risk | |
aa Bracco Neto 2009b.
| Methods | See Bracco Neto 2009a (Year 2 = 2002) | |
| Participants | See Bracco Neto 2009a | |
| Interventions | See Bracco Neto 2000a | |
| Outcomes | See Bracco Neto 2009a | |
| Funding Source | See Bracco Neto 2009a | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Second year of the same randomisation procedure |
| Allocation concealment (selection bias) | Unclear risk | No descriptions, second year of the same study design |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No descriptions, second year of the same study design |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Year of the same study design |
| Summary assessments | High risk | Plausible bias that raises some doubt about the results |
ba Eisenberg 2008b.
| Methods | See Eisenberg 2008a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Funding Source | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | Low risk | Independent validation |
| CC‐Control Selection | Low risk | Same population |
| CC‐Comparability | Low risk | Adjustment by confounders |
| CC‐Exposure | Low risk | Secure record |
| Summary assessments | Low risk | Plausible bias unlikely to seriously alter the results |
aa Tam 2007b.
| Methods | see Tam 2007a (year 2) | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Funding Source | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number |
| Allocation concealment (selection bias) | Low risk | "At enrolment, each subject was assigned the next sequential subject number and received study product of the treatment code assigned to that subject number according to a preprinted randomisation allocation list" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Possible biased follow‐up and reporting bias |
| Summary assessments | Low risk | Plausible bias unlikely to seriously alter the results. |
ba Cochran 2010b.
| Methods | see ba Cochran 2010a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Funding Source | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | High risk | Not clearly described |
| CC‐Control Selection | Unclear risk | Apparently same population |
| CC‐Comparability | High risk | Insufficient description |
| CC‐Exposure | Unclear risk | Secure record |
| Summary assessments | High risk | Lack of information about study design and matching method |
ba Cochran 2010c.
| Methods | see ba Cochran 2010a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Funding Source | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | High risk | |
| CC‐Control Selection | Unclear risk | Apparently same population |
| CC‐Comparability | High risk | Insufficient description |
| CC‐Exposure | Unclear risk | Secure record |
| Summary assessments | High risk | Lack of information about study design and matching method |
ba Staat 2011b.
| Methods | See Staat 2011a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Funding Source | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| CC‐Case Selection | Low risk | Independent validation |
| CC‐Control Selection | Low risk | Drawn from the same population |
| CC‐Comparability | Unclear risk | Matched |
| CC‐Exposure | Low risk | Secure record and interview |
| Summary assessments | Unclear risk | Plausible bias that raises some doubt about the results |
aa Brooks 2016.
| Methods | Randomised, placebo‐controlled trial | |
| Participants | Healthy children aged 2 to 4 years | |
| Interventions | Trivalent live attenuated influenza vaccine containing antigens the 2 A strains and 1 B strain recommended by WHO in 2012 the Northern Hemisphere. 0.5 mL nasal spray administration. Placebo was vaccine vehicle without virus component (not better described). Same route of admnistration. Vaccine batch numbers: vaccine: 167E200; placebo: E9001PCB | |
| Outcomes | Symptomatic influenza cases laboratory confirmed by reverse transcription PCR. Mild, moderate, or severe adverse events recorded for 7 days after vaccination. | |
| Funding Source | Gates Foundation | |
| Notes | Immunisation took place between 27 February and 9 April 2013; influenza viruses circulated within study area between February and November 2013. "The per‐protocol analysis set included all children who met the inclusion criteria, were randomised, and received one dose of study vaccine or placebo, and who remained in the study area for at least 8 days after vaccination." This corresponds to the ITT population. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The random allocation sequence was computer generated by PATH staff not involved with the trial, using a ratio for LAIV and placebo of 2:1 and block sizes of three.” |
| Allocation concealment (selection bias) | Low risk | The sequence was delivered to the Serum Institute of India, Pune, India, where it was used to label the vaccine and placebo syringes, which were identical in appearance except for the allocation numbers. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Vaccine and placebo syringes were identical in appearance except for the allocation numbers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 93 out of 1174 vaccinated (7.9%) and 31 out of 587 placebo recipients (5.3%) were excluded or lost from efficacy/effectiveness follow‐up. |
| Summary assessments | Low risk | |
aa Cowling 2012.
| Methods | Cluster‐randomised, placebo‐controlled trial conducted in Hong Kong between 2009 and 2010 | |
| Participants | 796 children aged 6 to 17 in households | |
| Interventions | TIV (0.5 mL of Vaxigrip; Sanofi Pasteur) or placebo (0.5 mL of saline solution) intramuscularly. The 2009–10 TIV used included the strains A/Brisbane/59/2007(H1N1)‐like, A/Brisbane/10/ 2007(H3N2)‐like, and B/Brisbane/60/2008‐like. | |
| Outcomes | Influenza cases laboratory confirmed by viral culture and/or real‐time reverse transcription PCR or 4 fold in antibody titre. Mild, moderate, or severe adverse events recorded by parents for 4 days after vaccination. | |
| Funding Source | Hong Kong Government | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Groups of children allocated to vaccine or control group |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Numbered, identical‐looking syringes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Failure to report numerator numbers |
| Summary assessments | Low risk | |
ACIP: Advisory Committee on Immunization Practices AE: adverse event AOM: acute otitis media ARI: acute respiratory infection CAIV‐T: cold‐adapted influenza vaccine, trivalent CCA: chick cell‐agglutinating CCT: comparative controlled trial CI: confidence interval C‐RCT: cluster‐randomised controlled trial ED: emergency department EID: egg infective dose ELISA: enzyme‐linked immunosorbent assay EU: European Union EVM: Effective Vaccine Management FV: fully vaccinated HA: haemagglutinin HAI: haemagglutination antibody inhibition HI: haemagglutination‐inhibition HIPAA: Health Insurance Portability and Accountability Act of 1996 HMO: health maintenance organisation HPMG: HealthPartners Medical Group HR: hazard ratio ICD: International Classification of Diseases IgA: immunoglobulin A IgE: immunoglobulin E IgM: immunoglobulin M ILI: influenza‐like illness ITT: intention‐to‐treat IU: international unit LAIV: live attenuated influenza vaccine LRTI: lower respiratory tract infection N/A: not applicable NA: neuraminidase OM: otitis media OME: otitis media with effusion OR: odds ratio PCR: polymerase chain reaction P&I: pneumonia & influenza PIV: parainfluenza virus PV: partially vaccinated RCT: randomised controlled trial REM: rapid eye movement RSV: respiratory syncytial virus RT‐PCR: reverse transcription polymerase chain reaction SAE: serious adverse event SD: standard deviation TCID: tissue culture infective dose TIV: trivalent influenza vaccine TM: tympanic membrane URTI: upper respiratory tract infection UV: unvaccinated VE: vaccine efficacy/effectiveness VSD: Vaccine Safety Datalink WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ambrose 2011 | Pooled analysis of safety data from 20 RCTs of LAIV |
| Ambrose 2014 | Review of evidence of effects of LAIV different disease severity. No primary data fitting inclusion criteria reported. |
| Anderson 1992 | Only serological outcomes presented. |
| Anonymous 2003 | Editorial only |
| Beare 1968 | Study participants were adults. |
| Belshe 2000b | Only serological outcomes presented. |
| Belshe 2000c | Only aggregated outcomes presented, duplicate publication of Belshe 1998 and 2000. |
| Belshe 2008 | Data from studies already included in review |
| Bergen 2004 | Outcomes only presented if statistically significantly increased or decreased risk in vaccinated group. Outcomes were presented by age group and setting. Authors declined to grant access to data from settings and age groups where outcomes were not significantly different between treatment and control. |
| Betts 1977 | Study participants were university students aged 18 to 25. |
| Beutner 1976 | Same study as Beutner 1979 (included) |
| Bichurina 1982 | No denominators presented. |
| Block 2011 | Non‐inferiority study |
| Boyce 1999 | No clinical outcomes for efficacy and safety |
| Boyce 2000 | Study population aged 18 to 40 |
| Boyer 1977 | Only serological outcomes were presented. |
| Cakir 2012 | Study design inconsistent (randomised case control study) |
| Chow 1979 | Serological study on part of study population of Beutner 1979 |
| Clements 1995 | Hepatitis B vaccine as control |
| Coles 1992 | Study population consisted of elderly and staff from nursing home. |
| Cowling 2014 | Incidence data derived from an RCT published elsewhere |
| Daubeney 1997 | High‐risk children |
| Donatelli 1998 | No control (split vaccine versus trivalent subunit‐type) |
| Eddy 1970 | Participants were healthy adult males. |
| Edwards 1994 | Placebo arm present only in the first study year, for which neither efficacy nor safety data are available. Age group is 1 to 65 years, and no data are presented for children only. |
| El'shina 1998 | Age group 18 to 23 |
| Feldman 1985 | Only serological outcomes presented. |
| Foy 1981 | No control |
| France 2004 | Case cross‐over |
| Fujieda 2008 | Same data of Fujieda 2006 (included) |
| Gaglani 2004 | Ecological study |
| Gendon 2004 | Study addresses the question of whether vaccinating children interrupts transmission to elderly. Study should be included in the elderly review. |
| Glezen 2001 | Comment only (on Hurwitz 2000a) |
| Groothuis 1994 | Study participants were children with chronic pulmonary diseases; no control. |
| Groothuis 1998 | Trial of respiratory syncytial virus vaccine |
| Gross 1977a | Only serological outcomes |
| Gross 1977b | No placebo control |
| Gross 1982 | All recipients had cystic fibrosis. |
| Gruber 1993 | Follow‐up times for safety outcomes variable within groups. Total follow‐up time not stated in Methods, refers to other papers for methodology. |
| Haba‐Rubio 2011 | Case report of cases of narcolepsy |
| Halperin 2002 | Study participants had chronic cardiac or pulmonary disorders. |
| Hambidge 2006 | Case cross‐over study |
| Hatch 1956 | No control |
| Heikkinen 2003 | Survey carried out on children younger than 13 years to determine the attack of flu virus in those having fever or respiratory infections |
| Hoskins 1973 | No placebo control; excluded because an influenza B vaccine was used as control |
| Hoskins 1979 | No control |
| Howell 1964a | Adult population |
| Howell 1964b | Adult population |
| Hrabar 1977 | Likely that more than 25% of the study participants are older than 25 years (mean 15.8; range 14.0 to 17.9); efficacy outcomes only serological |
| Hurwitz 2000a | Hepatitis A vaccine as control |
| Hurwitz 2000b | Hepatitis A vaccine as control |
| Jansen 2008 | Head‐to‐head: TIV+PCV7 vs TIV+PLA vs HBV+PLA |
| Jovanovic 1979 | Non‐experimental design |
| Jurgenssen 1978 | No placebo control |
| Just 1978 | No placebo control |
| Karron 1995 | Influenza vaccine administered with routine immunisation. |
| Kaufman 2000 | Telephone survey to estimate the compliance rate with influenza vaccination |
| King 2001 | Study included HIV‐infected groups and uninfected groups; uninfected groups excluded because trial was a cross‐over design; safety data for 1st, 2nd, and 3rd doses were pooled so could not be used (some placebo recipients would have received vaccine 4 to 5 weeks previously, and participants would be included in N for placebo and vaccine). |
| Kissling 2011a | Data already presented in Kissling 2011. |
| Kramarz 2001 | Study participants are children with asthma. |
| Kuno‐Sakai 1994 | Study participants are aged 16 to 17 years. No control |
| La Montagne 1983 | No original data presented. |
| Lauteria 1974 | Study population aged 18 to 24 years. |
| Lerman 1977 | Only serological data presented. |
| Lina 2000 | No control |
| Longini 2000 | Comment on Belshe 1998 and 2000 only |
| Luce 2001 | Cost‐effectiveness analysis based on the results of Belshe 1998 and 2000 |
| Luthardt 1979 | No placebo control |
| Madhi 2014 | Results reported in a study already included in the influenza vaccines in healthy adults review. |
| Marchisio 2002 | Study participants are children with recurrent otitis media. |
| Martin Moreno 1998 | Review |
| Maynard 1968 | No placebo control |
| McMahon 2008 | Non‐comparative study |
| Mendelman 2001 | Review |
| Monto 1970 | Participants vaccinated just before or during epidemic. Vaccine effectiveness expressed as O‐E. No numerator or denominator data reported. |
| Monto 1977 | Review |
| Morio 1994 | Only cumulative data from 3 years were reported to evaluate effectiveness. |
| Morris 1976 | Study participants are college students aged 18 to 29. |
| Muhammad 2011 | Non‐comparative study |
| Neuzil 2001 | Re‐analysis of Edwards 1994 (in which placebo arm was present only in the first study year, neither efficacy nor safety data are available) |
| Neuzil 2006 | Non‐comparative study |
| Nolan 2003 | No control (2 different commercial preparations of the same vaccine were compared) |
| Ogra 1977 | Same study as Beutner 1979 |
| Piedra 1991 | 3 studies in 1; 2 already included, the third is of uncertain provenance |
| Piedra 1993 | Safety data are given cumulatively on 3‐year study. |
| Piedra 2002b | All the data in this paper are presented in either Piedra 2002 or King 1998 (both included). |
| Quach 2003 | Analysis of factors associated with hospitalisation |
| Rimmelzwaan 2000 | Participants aged 18 to 55 years |
| Ruben 1973 | No placebo control |
| Schaad 2000 | Study population consists of children and adolescents with cystic fibrosis. |
| Scheifele 1990 | Non‐comparative studies |
| Schiff 1975 | Safety outcomes combined for first and second doses of vaccine |
| Slepushkin 1993 | Participants received vaccine or placebo depending on their age. |
| Stowe 2011 | Case series or case cross‐over |
| Sugaya 1994 | Study participants are children with moderate to severe asthma. |
| Sumaya 1977 | Only serological data are presented. |
| Van Hoecke 1996 | No control |
| Vasil'eva 1986 | No denominators presented. |
| Vasil'eva 1987 | Denominators for vaccinated and placebo groups were combined in results tables. |
| Wahlberg 2003 | Trial of Hib vaccine |
| Welty 1977a | Safety outcomes only with no placebo control |
| Welty 1977b | Safety outcomes only with no placebo control |
| Wesselius‐de 1972 | Only serological efficacy outcomes presented. |
| Wright 1976b | Data duplicated in Wright 1976a. |
| Wright 1985 | Only immune responses and viral shedding outcomes presented. |
| Wu 2010 | Efficacy cohort with inadequate follow‐up length |
| Zhilova 1986 | Study population aged 18 to 23 |
HBV: hepatitis B vaccine HBV+PLA: human hepatitis B virus + placebo Hib: Haemophilus influenzae b LAIV: live attenuated influenza vaccine O‐E: observed‐expected PCV: pneumococcal conjugate vaccine PLA: placebo RCT: randomised controlled trial TIV: trivalent influenza vaccine TIV+PCV7: trivalent influenza vaccine + pneumococcal vaccine, heptavalent TIV+PLA: trivalent influenza vaccine + placebo
Differences between protocol and review
Prior to starting the 2016 update of this review, we decided that evidence from observational studies should no longer be included, given its inherent biases and our intention to focus the main synthesis, 'Summary of findings' tables, conclusions, and summary versions on randomised evidence. For historical purposes we have retained data from non‐randomised evidence in the review.
In previous versions of this review we computed the reciprocal of the pooled risk difference as the basis for calculating numbers needed to vaccinate (NNV). Given the variation in control group risks, we decided to revise this approach to better illustrate how effect sizes vary across the diversity of event rates observed. We have calculated NNVs for low, median, and high event rates from the control groups.
Contributions of authors
Tom Jefferson (TOJ) cowrote the Background, Methods, Results, and Discussion (efficacy and effectiveness) sections of the review and interpreted the data.
Alessandro Rivetti (AR) conducted searches, co‐ordinated retrieval of papers, identified papers for inclusion, and extracted and checked data.
Carlo Di Pietrantonj (CDP) constructed comparisons for meta‐analysis, checked data, identified papers for inclusion, analysed data, wrote statistical methods, cowrote the Results section, and interpreted the data.
Vittorio Demicheli (VD) cowrote the Background and Methods sections, assessed papers for inclusion, arbitrated on quality assessment, constructed comparisons for meta‐analysis, and performed a critical review of the existing review.
All authors contributed to and approved the revised text.
Sources of support
Internal sources
-
REGIONE PIEMONTE ASL 20 ALESSANDRIA, Italy.
for the 2007 update, now ASL AL for the 2012 update
MRC Programme Grant G0000340, UK.
External sources
No sources of support supplied
Declarations of interest
Tom Jefferson (TJ) was a recipient of a UK National Institute for Health Research grant for a Cochrane Review of neuraminidase inhibitors for influenza. In addition, TJ receives royalties from his books published by Il Pensiero Scientifico Editore, Rome and Blackwells. TJ is occasionally interviewed by market research companies about phase I or II pharmaceutical products. In 2011‐13, TJ acted as an expert witness in litigation related to the antiviral oseltamivir, in two litigation cases on potential vaccine‐related damage, and in a labour case on influenza vaccines in healthcare workers in Canada. He has acted as a consultant for Roche (1997‐99), GSK (2001‐2), Sanofi‐Synthelabo (2003), and IMS Health (2013). In 2014 he was retained as a scientific adviser to a legal team acting on oseltamivir. TJ has a potential financial conflict of interest in the drug oseltamivir. In 2014‐16, TJ was a member of three advisory boards for Boerhinger Ingelheim. He is holder of a Cochrane Methods Innovations Fund grant to develop guidance on the use of regulatory data in Cochrane Reviews. TJ was a member of an independent data monitoring committee for a Sanofi Pasteur clinical trial on an influenza vaccine. Between 1994 and 2013, TJ was the co‐ordinator of the Cochrane Vaccines Field. TJ is a cosignatory of the Nordic Cochrane Centre Complaint to the European Medicines Agency (EMA) over maladministration at the EMA in relation to the investigation of alleged harms of human papillomavirus vaccines and consequent complaints to the European Ombudsman.
Alessandro Rivetti: none known.
Carlo Di Pietrantonj: none known.
Vittorio Demicheli: none known.
Eliana Ferroni: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
aa Alexandrova 1986 {published data only}
- Alexandrova GI, Budilovsky GN, Koval TA, Polezhaev FI, Garmashova LM, Ghendon YuZ, et al. Study of live recombinant cold‐adapted influenza bivalent vaccine of type A for use in children: an epidemiological control trial. Vaccine 1986;4(2):114‐8. [DOI] [PubMed] [Google Scholar]
aa Belshe 1998 {published data only}
- Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, et al. The efficacy of live attenuated, cold‐adapted, trivalent, intranasal influenza virus vaccine in children. New England Journal of Medicine 1998;338(20):1405‐12. [DOI] [PubMed] [Google Scholar]
- Piedra PA, Yan L, Kotloff K, Zangwill K, Bernstein DI, King J, et al. Safety of the trivalent, cold‐adapted influenza vaccine in preschool‐aged children. Pediatrics 2002;110(4):662‐72. [DOI] [PubMed] [Google Scholar]
aa Belshe 2000a {published data only}
- Belshe RB, Gruber WC, Mendelman PM, Cho I, Reisinger K, Block SL, et al. Efficacy of vaccination with live attenuated, cold‐adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. Journal of Pediatrics 2000;136(2):168‐75. [DOI] [PubMed] [Google Scholar]
- Piedra PA, Yan L, Kotloff K, Zangwill K, Bernstein DI, King J, et al. Safety of the trivalent, cold‐adapted influenza vaccine in preschool‐aged children. Pediatrics 2002;110(4):662‐72. [DOI] [PubMed] [Google Scholar]
aa Beutner 1979a {published data only}
- Beutner KR, Chow T, Rubi E, Strussenberg J, Clement J, Ogra PL. Evaluation of a neuraminidase‐specific influenza A virus vaccine in children: antibody responses and effects on two successive outbreaks of natural infection. Journal of Infectious Diseases 1979;140(6):844‐50. [DOI] [PubMed] [Google Scholar]
aa Beutner 1979b {published data only}
- Beutner KR, Chow T, Rubi E, Strussenberg J, Clement J, Ogra PL. Evaluation of a neuraminidase‐specific influenza A virus vaccine in children: antibody responses and effects on two successive outbreaks of natural infection. Journal of Infectious Diseases 1979;140(6):844‐50. [DOI] [PubMed] [Google Scholar]
aa Bracco Neto 2009a {published data only}
- Bracco Neto H, Farhat CK, Tregnaghi MW, Madhi SA, Razmpour A, Palladino G, et al. Efficacy and safety of 1 and 2 doses of live attenuated influenza vaccine in vaccine‐naive children. Pediatric Infectious Disease Journal 2009;28(5):365‐71. [DOI] [PubMed] [Google Scholar]
aa Bracco Neto 2009b {published data only}
- Bracco Neto H, Farhat CK, Tregnaghi MW, Madhi SA, Razmpour A, Palladino G, et al. Efficacy and safety of 1 and 2 doses of live attenuated influenza vaccine in vaccine‐naive children. Pediatric Infectious Disease Journal 2009;28(5):365‐71. [DOI] [PubMed] [Google Scholar]
aa Brooks 2016 {published data only}
- Brooks WA, Zaman K, Lewis KD, Ortiz JR, Goswami D, Feser J, et al. Efficacy of a Russian‐backbone live attenuated influenza vaccine among young children in Bangladesh: a randomised, double‐blind, placebo‐controlled trial. Lancet Global Health 2016;4(12):e946‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
aa Clover 1991 {published data only}
- Clover RD, Crawford S, Glezen WP, Taber LH, Matson CC, Couch RB. Comparison of heterotypic protection against influenza A/Taiwan/86 (H1N1) by attenuated and inactivated vaccines to A/Chile/83‐like viruses. Journal of Infectious Diseases 1991;163(2):300‐4. [DOI] [PubMed] [Google Scholar]
aa Colombo 2001 {published data only}
- Colombo C, Argiolas L, Vecchia C, Negri E, Meloni G, Meloni T. Influenza vaccine in healthy preschool children. Revue d'Epidémiologie et de Santé Publique 2001;49(2):157‐62. [PubMed] [Google Scholar]
aa Cowling 2012 {published data only}
- Cowling BJ, Ng S, Ma ES, Fang VJ, So HC, Wai W, et al. Protective efficacy against pandemic influenza of seasonal influenza vaccination in children in Hong Kong: a randomized controlled trial. Clinical Infectious Diseases 2012;55(5):695‐702. [DOI] [PubMed] [Google Scholar]
aa Grigor'eva 2002 {published data only}
- Grigor'eva EP, Desheva I, Donina SA, Naikhin AN, Rekstin AR, Barantseva IB, et al. The comparative characteristics of the safety, immunogenic activity and prophylactic potency of the adult and children types of live influenza vaccine in schoolchildren aged 7‐14 years. Voprosy Virusologii 2002;47(4):24‐7. [PubMed] [Google Scholar]
aa Gruber 1990 {published data only}
- Gruber WC, Taber LH, Glezen WP, Clover RD, Abell TD, Demmler RW, et al. Live attenuated and inactivated influenza vaccine in school‐age children. American Journal of Diseases in Children 1990;144(5):595‐600. [DOI] [PubMed] [Google Scholar]
aa Hoberman 2003a {published data only}
- Hoberman A, Greenberg DP, Paradise JL, Rockette HE, Lave JR, Kearney DH, et al. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 2003;290(12):1608‐16. [DOI] [PubMed] [Google Scholar]
aa Hoberman 2003b {published data only}
- Hoberman A, Greenberg DP, Paradise JL, Rockette HE, Lave JR, Kearney DH, et al. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 2003;290(12):1608‐16. [DOI] [PubMed] [Google Scholar]
aa Khan 1996 {published data only}
- Khan AS, Polezhaev F, Vasiljeva R, Drinevsky V, Buffington J, Gary H, et al. Comparison of US inactivated split‐virus and Russian live attenuated, cold‐adapted trivalent influenza vaccines in Russian schoolchildren. Journal of Infectious Diseases 1996;173(2):453‐6. [DOI] [PubMed] [Google Scholar]
aa Principi 2003 {published data only}
- Principi N, Esposito S, Marchisio P, Gasparini R, Crovari P. Socioeconomic impact of influenza on healthy children and their families. Pediatric Infectious Disease Journal 2003;22(Suppl 10):207‐10. [DOI] [PubMed] [Google Scholar]
aa Rudenko 1988 {published data only}
- Rudenko LG, Grigor'eva EP, Drinevskii VP, Koval' TA, Doroshenko EM. Results of a study of a live intranasal influenza vaccine in the immunization of children 3 to 15 years old. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1988;5:41‐6. [PubMed] [Google Scholar]
aa Rudenko 1993a {published data only}
- Rudenko LG, Slepushkin AN, Monto AS, Kendal AP, Grigorieva EP, Burtseva EP, et al. Efficacy of live attenuated and inactivated influenza vaccines in schoolchildren and their unvaccinated contacts in Novgorod, Russia. Journal of Infectious Diseases 1993;168(4):881‐7. [DOI] [PubMed] [Google Scholar]
aa Rudenko 1993b {published data only}
- Rudenko LG, Slepushkin AN, Monto AS, Kendal AP, Grigorieva EP, Burtseva EP, et al. Efficacy of live attenuated and inactivated influenza vaccines in schoolchildren and their unvaccinated contacts in Novgorod, Russia. Journal of Infectious Diseases 1993;168(4):881‐7. [DOI] [PubMed] [Google Scholar]
aa Rudenko 1996a {published data only}
- Rudenko LG, Lonskaya NI, Klimov AI, Vasilieva RI, Ramirez A. Clinical and epidemiological evaluation of a live, cold‐adapted influenza vaccine for 3‐14‐year‐olds. Bulletin of the World Health Organization 1996;74(1):77‐84. [PMC free article] [PubMed] [Google Scholar]
aa Rudenko 1996b {published data only}
- Rudenko LG, Vasil'eva RI, Ismagulov AT, Karagodina VI, Slepushkin AN, Doroshenko EM, et al. Prophylactic effectiveness of a live recombinant influenza type A vaccine in immunizing children aged 3‐14 years. Voprosy Virusologii 1996;41:37‐9. [PubMed] [Google Scholar]
aa Tam 2007a {published data only}
- Tam JS, Capeding MR, Lum LC, Chotpitayasunondh T, Jiang Z, Huang LM, et al. Efficacy and safety of a live attenuated, cold‐adapted influenza vaccine, trivalent against culture‐confirmed influenza in young children in Asia. Pediatric Infectious Disease Journal 2007;26(7):619‐28. [DOI] [PubMed] [Google Scholar]
aa Tam 2007b {published data only}
- Tam JS, Capeding MR, Lum LC, Chotpitayasunondh T, Jiang Z, Huang LM, et al. Efficacy and safety of a live attenuated, cold‐adapted influenza vaccine, trivalent against culture‐confirmed influenza in young children in Asia. Pediatric Infectious Disease Journal 2007;26(7):619‐28. [DOI] [PubMed] [Google Scholar]
aa Vesikari 2006a {published data only}
- Vesikari T, Fleming DM, Aristegui JF, Vertruyen A, Ashkenazi S, Rappaport R, et al. Safety, efficacy, and effectiveness of cold‐adapted influenza vaccine‐trivalent against community‐acquired, culture‐confirmed influenza in young children attending day care. Pediatrics 2006;118:2298‐312. [DOI] [PubMed] [Google Scholar]
aa Vesikari 2006b {published data only}
- Vesikari T, Fleming DM, Aristegui JF, Vertruyen A, Ashkenazi S, Rappaport R, et al. Safety, efficacy, and effectiveness of cold‐adapted influenza vaccine‐trivalent against community‐acquired, culture‐confirmed influenza in young children attending day care. Pediatrics 2006;118:2298‐312. [DOI] [PubMed] [Google Scholar]
ab Aksenov 1971 {published data only}
- Aksenov VA, Selidovkin DA, Sorokina LV, Burnos AS, Sorokina AG. The effectiveness of influenza prevention with a special variation of live influenza vaccine for children in the 1969 epidemic. Voprosy Virusologii 1971;16:81‐6. [PubMed] [Google Scholar]
ab Belshe 1992 {published data only}
- Belshe RB, Swierkosz EM, Anderson EL, Newman FK, Nugent SL, Maassab HF. Immunization of infants and young children with live attenuated trivalent cold‐recombinant influenza A H1N1, H3N2, and B vaccine. Journal of Infectious Diseases 1992;165(4):727‐32. [DOI] [PubMed] [Google Scholar]
ab Desheva 2002 {published data only}
- Desheva I, Danini GV, Grigor'eva EP, Donina SA, Kiseleva IV, Rekstin AR, et al. The investigation of the safety, genetic stability and immunogenicity of live influenza vaccine for adults in vaccination of 3‐6 year old children. Voprosy Virusologii 2002;47(4):21‐4. [PubMed] [Google Scholar]
ab Grigor'eva 1994 {published data only}
- Grigor'eva EP, Rekstin AR, Rudenko LG, Ramirez A, Barro M, Lisovskaia KV, et al. The immunogenic properties and prophylactic efficacy of a live polyvalent influenza vaccine in children 5 to 14 years old. Voprosy Virusologii 1994;39(1):26‐9. [PubMed] [Google Scholar]
ab Gruber 1996 {published data only}
- Gruber WC, Belshe RB, King JC, Treanor JJ, Piedra PA, Wright PF, et al. Evaluation of live attenuated influenza vaccines in children 6‐18 months of age: safety, immunogenicity, and efficacy. National Institute of Allergy and Infectious Diseases, Vaccine and Treatment Evaluation Program and the Wyeth‐Ayerst ca Influenza Vaccine Investigators Group. Journal of Infectious Diseases 1996;173(6):1313‐9. [DOI] [PubMed] [Google Scholar]
ab Gruber 1997 {published data only}
- Gruber WC, Darden PM, Still JG, Lohr J, Reed G, Wright PF. Evaluation of bivalent live attenuated influenza A vaccines in children 2 months to 3 years of age: safety, immunogenicity and dose‐response. Vaccine 1997;15(12‐3):1379‐84. [DOI] [PubMed] [Google Scholar]
ab Gutman 1977 {published data only}
- Gutman LT, Wilfert CM, Idriss ZH, Schmidt E, Andrews S, Katz SL. Single‐dose trials of monovalent A/New Jersey/76 (Hsw1N1) influenza virus vaccine in children in Durham, North Carolina. Journal of Infectious Diseases 1977;136(Suppl):575‐8. [DOI] [PubMed] [Google Scholar]
ab King 1998 {published data only}
- King JC Jr, Lagos R, Bernstein DI, Piedra PA, Kotloff K, Bryant M, et al. Safety and immunogenicity of low and high doses of trivalent live cold‐adapted influenza vaccine administered intranasally as drops or spray to healthy children. Journal of Infectious Diseases 1998;17(5):1394‐7. [DOI] [PubMed] [Google Scholar]
ab Levine 1977 {published data only}
- Levine MM, Hughes TP, Simon P, O'Donnell S, Grauel S, Levine SG. Monovalent inactivated A/New Jersey/8/76 (Hsw1N1) vaccine in healthy children aged three to five years. Journal of Infectious Diseases 1977;136(Suppl):571‐4. [DOI] [PubMed] [Google Scholar]
ab Mallory 2010 {published data only}
- Mallory RM, Malkin E, Ambrose CS, Bellamy T, Shi L, Yi T, et al. Safety and immunogenicity following administration of a live, attenuated monovalent 2009 H1N1 influenza vaccine to children and adults in two randomized controlled trials. PLoS ONE 2010;5(10):e13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
ab Obrosova‐Serova 1990 {published data only}
- Obrosova‐Serova NP, Slepushkin AN, Kendal AP, Harmon MW, Burtseva EI, Bebesheva NI, et al. Evaluation in children of cold‐adapted influenza B live attenuated intranasal vaccine prepared by reassortment between wild‐type B/Ann Arbor/1/86 and cold‐adapted B/Leningrad/14/55 viruses. Vaccine 1990;8(1):57‐60. [DOI] [PubMed] [Google Scholar]
ab Plennevaux 2011 {published data only}
- Plennevaux E, Blatter M, Cornish MJ, Go K, Kirby D, Wali M, et al. Influenza A (H1N1) 2009 two‐dose immunization of US children: an observer‐blinded, randomized, placebo‐controlled trial. Vaccine 2011;29(8):1569‐75. [DOI] [PubMed] [Google Scholar]
ab Rudenko 1991 {published data only}
- Rudenko LG, Ramirez A, Barro M, Gushchina MI, Armanda RE, Ermachenko TA, et al. The inoculation properties of live recombinant influenza vaccine types A and B used separately and jointly in children 3 to 14. Voprosy Virusologii 1991;36(6):472‐4. [PubMed] [Google Scholar]
ab Slepushkin 1974 {published data only}
- Slepushkin AN, Dukova VS, Kalegaeva VA, Kagan AN, Temriuk EE. Results of studying the effectiveness of a live influenza vaccine for peroral use on preschool and schoolchildren. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1974;12:24‐9. [PubMed] [Google Scholar]
ab Slepushkin 1988 {published data only}
- Slepushkin AN, Patriarca PA, Obrosova‐Serova NP, Harmon MW, Kupryashina LN, Cheshik SG, et al. Class‐specific antibody responses in school children vaccinated with an A/Brazil/11/78 (H1N1)‐like recombinant influenza virus prepared from the A/Leningrad/134/57 paediatric cold‐adapted donor strain. Vaccine 1988;6(1):25‐8. [DOI] [PubMed] [Google Scholar]
ab Slepushkin 1991 {published data only}
- Slepushkin AN, Obrosova‐Serova NP, Burtseva EI, Govorkova EA, Rudenko LG, Vartanian RV, et al. A comparative study of the inoculation properties of live recombinant and inactivated influenza vaccines made from strain A/Philippines/2/82 (H3N2) in 8‐ to 15‐year‐old children. Voprosy Virusologii 1991;36(5):372‐4. [PubMed] [Google Scholar]
ab Steinhoff 1990 {published data only}
- Steinhoff MC, Halsey NA, Wilson MH, Burns BA, Samorodin RK, Fries LF, et al. Comparison of live attenuated cold‐adapted and avian‐human influenza A/Bethesda/85 (H3N2) reassortant virus vaccines in infants and children. Journal of Infectious Diseases 1990;162(2):394‐401. [DOI] [PubMed] [Google Scholar]
ab Steinhoff 1991 {published data only}
- Steinhoff MC, Halsey NA, Fries LF, Wilson MH, King J, Burns BA, et al. The A/Mallard/6750/78 avian‐human, but not the A/Ann Arbor/6/60 cold‐adapted, influenza A/Kawasaki/86 (H1N1) reassortant virus vaccine retains partial virulence for infants and children. Journal of Infectious Diseases 1991;163(5):1023‐8. [DOI] [PubMed] [Google Scholar]
ab Swierkosz 1994 {published data only}
- Swierkosz EM, Newman FK, Anderson EL, Nugent SL, Mills GB, Belshe RB. Multidose, live attenuated, cold‐recombinant, trivalent influenza vaccine in infants and young children. Journal of Infectious Diseases 1994;169(5):1121‐4. [DOI] [PubMed] [Google Scholar]
ab Vasil'eva 1988a {published data only}
- Vasil'eva RI, Merkur'eva LA, Latsenko VG, Vasil'eva AM, Shvager MM. Characteristics of the clinical and immunologic safety of inactivated influenza vaccines in children undergoing multiple immunizations. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1988;11:65‐9. [PubMed] [Google Scholar]
ab Vasil'eva 1988b {published data only}
- Vasil'eva RI, Smirnova LA, Rudenko LG, Drinevskii VP, Tsaritsina IM. The results of state trials of inactivated influenza vaccines for children. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1988;3:49‐54. [PubMed] [Google Scholar]
ab Wright 1976a {published data only}
- Wright PF, Sell SH, Thompson J, Karzon DT. Clinical reactions and serologic response following inactivated monovalent influenza type B vaccine in young children and infants. Journal of Pediatrics 1976;88(1):31‐5. [DOI] [PubMed] [Google Scholar]
ab Zangwill 2001 {published and unpublished data}
- Zangwill KM, Droge J, Mendelman P, Marcy SM, Partridge S, Chiu CY, et al. Prospective, randomized, placebo‐controlled evaluation of the safety and immunogenicity of three lots of intranasal trivalent influenza vaccine among young children. Pediatic Infectious Disease Journal 2001;20(8):740‐6. [DOI] [PubMed] [Google Scholar]
ba Anonymous 2005 {published data only}
- Effectiveness of vaccine against medical consultation due to laboratory‐confirmed influenza: results from a sentinel physician pilot project in British Columbia, 2004‐2005. Canada Communicable Disease Report 2005;31(18):181‐91. [PubMed] [Google Scholar]
ba Cochran 2010a {published data only}
- Cochran LW, Black S, Klein NP, Dekker CL, Lewis E, Reingold AL. Vaccine effectiveness against laboratory‐confirmed influenza in infants: a matched case control study. Human Vaccines 2010;6(9):729‐35. [DOI] [PubMed] [Google Scholar]
ba Cochran 2010b {published data only}
- Cochran LW, Black S, Klein NP, Dekker CL, Lewis E, Reingold AL. Vaccine effectiveness against laboratory‐confirmed influenza in infants: a matched case control study. Human Vaccines 2010;23(6):729‐35. [DOI] [PubMed] [Google Scholar]
ba Cochran 2010c {published data only}
- Cochran LW, Black S, Klein NP, Dekker CL, Lewis E, Reingold AL. Vaccine effectiveness against laboratory‐confirmed influenza in infants: a matched case control study. Human Vaccines 2010;23(6):729‐35. [DOI] [PubMed] [Google Scholar]
ba Eisenberg 2008a {published data only}
- Eisenberg KW, Szilagyi PG, Fairbrother G, Griffin MR, Staat M, Shone LP, et al. Vaccine effectiveness against laboratory‐confirmed influenza in children 6 to 59 months of age during the 2003‐2004 and 2004‐2005 influenza seasons. Pediatrics 2008;122(5):911‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
ba Eisenberg 2008b {published data only}
- Eisenberg KW, Szilagyi PG, Fairbrother G, Griffin MR, Staat M, Shone LP, et al. Vaccine effectiveness against laboratory‐confirmed influenza in children 6 to 59 months of age during the 2003‐2004 and 2004‐2005 influenza seasons. Pediatrics 2008;122(5):911‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
ba Gilca 2011 {published data only}
- Gilca R, Deceuninck G, Serres G, Boulianne N, Sauvageau C, Quach C, et al. Effectiveness of pandemic H1N1 vaccine against influenza‐related hospitalization in children. Pediatrics 2011;128(5):e1084‐91. [DOI] [PubMed] [Google Scholar]
ba Hirota 1992 {published data only}
- Hirota Y, Takeshita S, Ide S, Kataoka K, Ohkubo A, Fukuyoshi S, et al. Various factors associated with the manifestation of influenza‐like illness. International Journal of Epidemiology 1992;21(3):574‐82. [DOI] [PubMed] [Google Scholar]
ba Kelly 2011 {published data only}
- Kelly H, Jacoby P, Dixon GA, Carcione D, Williams S, Moore HC, et al. Vaccine effectiveness against laboratory‐confirmed influenza in healthy young children: a case‐control study. Pediatric Infectious Disease Journal 2011;30(2):107‐11. [DOI] [PubMed] [Google Scholar]
ba Kissling 2011 {published data only}
- Kissling E, Valenciano M, Cohen JM, Oroszi B, Barret AS, Rizzo C, et al. I‐MOVE multi‐centre case control study 2010‐11: overall and stratified estimates of influenza vaccine effectiveness in Europe. Eurosurveillance 2011;6(11):19818. [DOI] [PMC free article] [PubMed] [Google Scholar]
ba Mahmud 2011 {published data only}
- Mahmud S, Hammond G, Elliott L, Hilderman T, Kurbis C, Caetano P, et al. Effectiveness of the pandemic H1N1 influenza vaccines against laboratory‐confirmed H1N1 infections: population‐based case‐control study. Vaccine 2011;29(45):7975‐81. [DOI] [PubMed] [Google Scholar]
ba Staat 2011a {published data only}
- Staat MA, Griffin MR, Donauer S, Edwards KM, Szilagyi PG, Weinberg GA, et al. Vaccine effectiveness for laboratory‐confirmed influenza in children 6‐59 months of age, 2005‐2007. Vaccine 2011;29(48):9005‐11. [DOI] [PubMed] [Google Scholar]
ba Staat 2011b {published data only}
- Staat MA, Griffin MR, Donauer S, Edwards KM, Szilagyi PG, Weinberg GA, et al. Vaccine effectiveness for laboratory‐confirmed influenza in children 6‐59 months of age, 2005‐2007. Vaccine 2011;29(48):9005‐11. [DOI] [PubMed] [Google Scholar]
ba Valenciano 2011 {published data only}
- Valenciano M, Kissling E, Cohen JM, Oroszi B, Barret AS, Rizzo C, et al. Estimates of pandemic influenza vaccine effectiveness in Europe, 2009‐2010: results of Influenza Monitoring Vaccine Effectiveness in Europe (I‐MOVE) multicentre case‐control study. PLoS Medicine 2011;8(1):e1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
ba Van Buynder 2010 {published data only}
- Buynder PG, Dhaliwal JK, Buynder JL, Couturier C, Minville‐Leblanc M, Garceau R, et al. Protective effect of single‐dose adjuvanted pandemic influenza vaccine in children. Influenza and Other Respiratory Viruses 2010;4(4):171‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
bb Goodman 2006 {published data only}
- Goodman MJ, Nordin JD, Harper P, Defor T, Zhou X. The safety of trivalent influenza vaccine among healthy children 6 to 24 months of age. Pediatrics 2006;117(5):821‐6. [DOI] [PubMed] [Google Scholar]
ca Allison 2006 {published data only}
- Allison MA, Daley MF, Crane LA, Barrow J, Beaty BL, Allred N, et al. Influenza vaccine effectiveness in healthy 6‐ to 21‐month‐old children during the 2003‐2004 season. Journal of Pediatrics 2006;149(6):755‐62. [DOI] [PubMed] [Google Scholar]
ca Burtseva 1991 {published data only}
- Burtseva EI, Obrosova‐Serova NP, Govorkova EA, Rudenko LG, Vartanian RV, Beliaev AL, et al. A comparative study of the protective properties of live recombinant and inactivated influenza vaccines made from strain A/Philippines/2/82 (H3N2) in 8‐ to 15‐year‐old children. Voprosy Virusologii 1991;36(5):375‐7. [PubMed] [Google Scholar]
ca Chumakov 1987 {published data only}
- Bashliaeva ZA, Sumarokov AA, Nefedova LA, Iaroshevskaia II, Ozeretskovskaia NA. Basic results of a committee trial of the new vaccine Grippovac SE‐AZh. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1986;2:49‐54. [PubMed] [Google Scholar]
- Chumakov MP, Boiko VM, Malyshkina LP, Mel'nikova SK, Rodin VI. Results of coded trials of the activity of the trivalent subunit influenza vaccine Grippovak in Moscow kindergartens in December 1983 through the 1st quarter of 1984. Voprosy Virusologii 1987;32(2):175‐83. [PubMed] [Google Scholar]
ca El'shina 2000 {published data only}
- El'shina GA, Gorbunov MA, Bektimirov TA, Lonskaia NI, Pavlova LI, Nikul'shin AA, et al. The evaluation of the reactogenicity, harmlessness and prophylactic efficacy of Grippol trivalent polymer‐subunit influenza vaccine administered to schoolchildren. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2000;2:50‐4. [PubMed] [Google Scholar]
ca Fujieda 2006 {published data only}
- Fujieda M, Maeda A, Kondo K, Kaji M, Hirota Y. Inactivated influenza vaccine effectiveness in children under 6 years of age during the 2002‐2003 season. Vaccine 2006;24(7):957‐63. [DOI] [PubMed] [Google Scholar]
ca Jianping 1999 {published data only}
- Jianping H, Xin F, Changshun L, Bo Z, Linxiu G, Wei X, et al. Assessment of effectiveness of Vaxigrip. Vaccine 1999;17(Suppl 1):57‐8. [DOI] [PubMed] [Google Scholar]
ca Kawai 2003 {published data only}
- Kawai N, Ikematsu H, Iwaki N, Satoh I, Kawashima T, Tsuchimoto T, et al. A prospective, Internet‐based study of the effectiveness and safety of influenza vaccination in the 2001‐2002 influenza season. Vaccine 2003;21(31):4507‐13. [DOI] [PubMed] [Google Scholar]
ca King 2006 {published data only}
- King JC Jr, Stoddard JJ, Gaglani MJ, Moore KA, Magder L, McClure E, et al. Effectiveness of school‐based influenza vaccination. New England Journal of Medicine 2006;355:2523‐32. [DOI] [PubMed] [Google Scholar]
ca Maeda 2002 {published data only}
- Maeda T, Shintani Y, Miyamoto H, Kawagoe H, Nakano K, Nishiyama A, et al. Prophylactic effect of inactivated influenza vaccine on young children. Pediatrics International 2002;44(1):43‐6. [DOI] [PubMed] [Google Scholar]
ca Maeda 2004a {published data only}
- Maeda T, Shintani Y, Nakano K, Terashima K, Yamada Y. Failure of inactivated influenza A vaccine to protect healthy children aged 6‐24 months. Pediatrics International 2004;46(2):122‐5. [DOI] [PubMed] [Google Scholar]
ca Maeda 2004b {published data only}
- Maeda T, Shintani Y, Nakano K, Terashima K, Yamada Y. Failure of inactivated influenza A vaccine to protect healthy children aged 6‐24 months. Pediatrics International 2004;46(2):122‐5. [DOI] [PubMed] [Google Scholar]
ca Maeda 2004c {published data only}
- Maeda T, Shintani Y, Nakano K, Terashima K, Yamada Y. Failure of inactivated influenza A vaccine to protect healthy children aged 6‐24 months. Pediatrics International 2004;46(2):122‐5. [DOI] [PubMed] [Google Scholar]
ca Ortqvist 2011 {published data only}
- Ortqvist A, Berggren I, Insulander M, Jong B, Svenungsson B. Effectiveness of an adjuvanted monovalent vaccine against the 2009 pandemic strain of influenza A (H1N1)v, in Stockholm County, Sweden. Clinical Infectious Diseases 2011;52(10):1203‐11. [DOI] [PubMed] [Google Scholar]
ca Ozgur 2006 {published data only}
- Ozgur SK, Beyazova U, Kemaloglu YK, Maral I, Sahin F, Camurdan AD, et al. Effectiveness of inactivated influenza vaccine for prevention of otitis media in children. Pediatric Infectious Disease Journal 2006;25(5):401‐4. [DOI] [PubMed] [Google Scholar]
ca Salleras 2006 {published data only}
- Salleras L, Dominguez A, Pumarola T, Prat A, Marcos MA, Garrido P, et al. Effectiveness of virosomal subunit influenza vaccine in preventing influenza‐related illnesses and its social and economic consequences in children aged 3‐14 years: a prospective cohort study. Vaccine 2006;24(44‐6):6638‐42. [DOI] [PubMed] [Google Scholar]
ca Slobodniuk 2002a {published data only}
- Slobodniuk AV, Romanenko VV, Utnitskaia OS, Motus TM, Pereverzev AV. Influence of multiplicity of immunizations of children with inactivated influenza vaccine on immune response and the effectiveness of protection. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2002;4:36‐9. [PubMed] [Google Scholar]
ca Slobodniuk 2002b {published data only}
- Slobodniuk AV, Romanenko VV, Utnitskaia OS, Motus TM, Pereverzev AV. Influence of multiplicity of immunizations of children with inactivated influenza vaccine on immune response and the effectiveness of protection. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2002;4:36‐9. [PubMed] [Google Scholar]
ca Slobodniuk 2002c {published data only}
- Slobodniuk AV, Romanenko VV, Utnitskaia OS, Motus TM, Pereverzev AV. Influence of multiplicity of immunizations of children with inactivated influenza vaccine on immune response and the effectiveness of protection. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2002;4:36‐9. [PubMed] [Google Scholar]
ca Vasil'eva 1982 {published data only}
- Vasil'eva RI, Osidak LV, Bichurina MA, Mukhina LP, Chudina LV. Evaluation of the safety, reactogenicity and antigenic activity of inactivated chromatographic influenza vaccine in school children. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1982;4:96‐8. [PubMed] [Google Scholar]
ca Wiggs‐Stayner 2006 {published data only}
- Wiggs‐Stayner KS, Purdy TR, Go GN, McLaughlin NC, Tryzynka PS, Sines JR, et al. The impact of mass school immunization on school attendance. Journal of School of Nursing 2006;22(4):219‐22. [DOI] [PubMed] [Google Scholar]
ca Yin 2011 {published data only}
- Yin JK, Lahra MM, Iskander M, Lambert SB, Heron L, Nissen MD, et al. Pilot study of influenza vaccine effectiveness in urban Australian children attending childcare. Journal of Paediatrics and Child Health 2011;47(12):857‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
cb MPA 2011 {unpublished data only}
- MPA. Occurrence of narcolepsy with cataplexy among children and adolescents in relation to the H1N1 pandemic and Pandemrix vaccinations – results of a case inventory study by the MPA in Sweden during 2009–2010. www.lakemedelsverket.se/upload/nyheter/2011/Fallinventeringsrapport pandermrix 110630.pdf (accessed 10 January 2012).
cb Nicholls 2004 {published and unpublished data}
- Nicholls S, Carroll K, Crofts J, Ben‐Eliezer E, Paul J, Zambon M, et al. Outbreak of influenza A (H3N2) in a highly‐vaccinated religious community: a retrospective cohort study. Communicable Disease and Public Health 2004;7:272‐7. [PubMed] [Google Scholar]
cb Ritzwoller 2005 {published data only}
- Ritzwoller DP, Bridges CB, Shetterly S, Yamasaki K, Kolczak M, France EK. Effectiveness of the 2003‐2004 influenza vaccine among children 6 months to 8 years of age, with 1 vs 2 doses. Pediatrics 2005;116(1):153‐9. [DOI] [PubMed] [Google Scholar]
cb Slepushkin 1994 {published data only}
- Slepushkin AN, Rudenko LG, Kendal AP, Monto AS, Beliaev AL, Burtseva EI, et al. A comparative study of live and inactivated influenza vaccines: the organization of the observation and the results of a study of their reactogenicity and immunogenicity. Voprosy Virusologii 1994;39:129‐31. [PubMed] [Google Scholar]
References to studies excluded from this review
Ambrose 2011 {published data only}
- Ambrose CS, Yi T, Falloon J. An integrated, multi study analysis of the safety of Ann Arbor strain live attenuated influenza vaccine in children aged 2‐17 years. Influenza and Other Respiratory Viruses 2011;5(6):389‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ambrose 2014 {published data only}
- Ambrose CS, Wu X, Caspard H, Belshe RB. Efficacy of live attenuated influenza vaccine against influenza illness in children as a function of illness severity. Vaccine 2014;32(43):5546‐8. [DOI] [PubMed] [Google Scholar]
Anderson 1992 {published data only}
- Anderson EL, Newman FK, Maassab HF, Belshe RB. Evaluation of a cold‐adapted influenza B/Texas/84 reassortant virus (CRB‐87) vaccine in young children. Journal of Clinical Microbiology 1992;30(9):2230‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 2003 {published data only}
- FluMist: an intranasal live influenza vaccine. Medical Letter on Drugs and Therapeutics 2003;45(1163):65‐6. [PubMed] [Google Scholar]
Beare 1968 {published data only}
- Beare AS, Hobson D, Reed SE, Tyrrell DA. A comparison of live and killed influenza‐virus vaccines. Report to the Medical Research Council's Committee on Influenza and other Respiratory Virus Vaccines. Lancet 1968;2(7565):418‐22. [DOI] [PubMed] [Google Scholar]
Belshe 2000b {published data only}
- Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, Reisinger K, et al. Correlates of immune protection induced by live, attenuated, cold‐adapted, trivalent, intranasal influenza virus vaccine. Journal of Infectious Diseases 2000;181(3):1133‐7. [DOI] [PubMed] [Google Scholar]
Belshe 2000c {published data only}
- Belshe RB, Gruber WC. Prevention of otitis media in children with live attenuated influenza vaccine given intranasally. Pediatric Infectious Disease Journal 2000;19(Suppl 5):66‐71. [DOI] [PubMed] [Google Scholar]
Belshe 2008 {published data only}
- Belshe RB, Ambrose CS, Yi T. Safety and efficacy of live attenuated influenza vaccine in children 2‐7 years of age. Vaccine 2008;26(Suppl 4):D10‐6. [DOI] [PubMed] [Google Scholar]
Bergen 2004 {published data only}
- Bergen R, Black S, Shinefield H, Lewis E, Ray P, Hansen J, et al. Safety of cold‐adapted live attenuated influenza vaccine in a large cohort of children and adolescents. Pediatric Infectious Disease Journal 2004;23(2):138‐44. [DOI] [PubMed] [Google Scholar]
Betts 1977 {published data only}
- Betts RF, Douglas RG Jr, Roth FK, Little JW 3rd. Efficacy of live attenuated influenza A/Scotland/74 (H3N2) virus vaccine against challenge with influenza A/Victoria/3/75 (H3N2) virus. Journal of Infectious Diseases 1977;136:746‐53. [DOI] [PubMed] [Google Scholar]
Beutner 1976 {published data only}
- Beutner KR, Rizzone C, DeMello S, Ogra PL. Clinical evaluation of neuraminidase monospecific (HEqN2) recombinant influenza vaccine in children. Developments in Biological Standardization 1976;33:162‐70. [PubMed] [Google Scholar]
Bichurina 1982 {published data only}
- Bichurina MA, Polianskaia NI, Zhebrun AB, Rozaeva NR, Mukhina LP. Preliminary results of a trial of a highly purified inactivated chromatographic influenza vaccine for children. Trudy Instituta Imeni Pastera 1982;58:14‐6. [PubMed] [Google Scholar]
Block 2011 {published data only}
- Block SL, Yi T, Sheldon E, Dubovsky F, Falloon J. A randomized, double‐blind noninferiority study of quadrivalent live attenuated influenza vaccine in adults. Vaccine 2011;29(50):9391‐7. [DOI] [PubMed] [Google Scholar]
Boyce 1999 {published data only}
- Boyce TG, Gruber WC, Coleman‐Dockery SD, Sannella EC, Reed GW, Wolff M, et al. Mucosal immune response to trivalent live attenuated intranasal influenza vaccine in children. Vaccine 1999;18(1‐2):82‐8. [DOI] [PubMed] [Google Scholar]
Boyce 2000 {published data only}
- Boyce TG, Hsu HH, Sannella EC, Coleman‐Dockery SD, Baylis E, Zhu Y. Safety and immunogenicity of adjuvanted and unadjuvanted subunit influenza vaccines administered intranasally to healthy adults. Vaccine 2000;19(2‐3):217‐26. [DOI] [PubMed] [Google Scholar]
Boyer 1977 {published data only}
- Boyer KM, Cherry JD, Welliver RC, Deseda‐Tous J, Zahradnik JM, Dudley JP, et al. Clinical trials with inactivated monovalent (A/New Jersey/76) and bivalent (A/New Jersey/76‐A/Victoria/75) influenza vaccines in Los Angeles children. Journal of Infectious Diseases 1977;136(Suppl):661‐4. [DOI] [PubMed] [Google Scholar]
Cakir 2012 {published data only}
- Çakir BC, Beyazova U, Kemaloğlu YK, Özkan S, Gündüz B, Özdek A. Effectiveness of pandemic influenza A/H1N1 vaccine for prevention of otitis media in children. European Journal of Pediatrics 2012;171(11):1667‐71. [DOI] [PubMed] [Google Scholar]
Chow 1979 {published data only}
- Chow TC, Beutner KR, Ogra PL. Cell‐mediated immune responses to the hemagglutinin and neuraminidase antigens of influenza A virus after immunization in humans. Infection and Immunity 1979;25(1):103‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clements 1995 {published data only}
- Clements DA, Langdon L, Bland C, Walter E. Influenza A vaccine decreases the incidence of otitis media in 6‐ to 30‐month‐old children in day care. Archives of Pediatric and Adolescent Medicine 1995;149(10):1113‐7. [DOI] [PubMed] [Google Scholar]
Coles 1992 {published data only}
- Coles FB, Balzano GJ, Morse DL. An outbreak of influenza A (H3N2) in a well immunized nursing home population. Journal of the American Geriatrics Society 1992;40(6):589‐92. [DOI] [PubMed] [Google Scholar]
Cowling 2014 {published data only}
- Cowling BJ, Perera RA, Fang VJ, Chan KH, Wai W, So HC, et al. Incidence of influenza virus infections in children in Hong Kong in a 3‐year randomized placebo‐controlled vaccine study, 2009‐2012. Clinical Infectious Diseases 2014;59(4):517‐24. [DOI] [PubMed] [Google Scholar]
Daubeney 1997 {published data only}
- Daubeney P, Taylor CJ, McGaw J, Brown EM, Ghosal S, Keeton BR, et al. Immunogenicity and tolerability of a trivalent influenza subunit vaccine (Influvac) in high‐risk children aged 6 months to 4 years. British Journal of Clinical Practice 1997;51(2):87‐90. [PubMed] [Google Scholar]
Donatelli 1998 {published data only}
- Donatelli I, Zannolli R, Fuiano L, Biasio LR. Influenza vaccine in immunogenically naive healthy infants. European Journal of Pediatrics 1998;157(11):949‐50. [DOI] [PubMed] [Google Scholar]
Eddy 1970 {published data only}
- Eddy TS, Davies NA. The effect of vaccine on a closed epidemic of Hong Kong influenza. South African Medical Journal 1970;44(8):214‐6. [PubMed] [Google Scholar]
Edwards 1994 {published data only}
- Edwards KM, Dupont WD, Westrich MK, Plummer WD Jr, Palmer PS, Wright PF. A randomized controlled trial of cold‐adapted and inactivated vaccines for the prevention of influenza A disease. Journal of Infectious Diseases 1994;169(1):68‐76. [DOI] [PubMed] [Google Scholar]
El'shina 1998 {published data only}
- El'shina GA, Gorbunov MA, Shervarli VI, Lonskaia NI, Pavlova LI, Khaitov RM, et al. Evaluation of the effectiveness of influenza trivalent polymer subunit vaccine "Grippol". Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1998;3:40‐3. [PubMed] [Google Scholar]
Feldman 1985 {published data only}
- Feldman S, Wright PF, Webster RG, Roberson PK, Mahoney J, Thompson J, et al. Use of influenza A virus vaccines in seronegative children: live cold‐adapted versus inactivated whole virus. Journal of Infectious Diseases 1985;152(6):1212‐8. [DOI] [PubMed] [Google Scholar]
Foy 1981 {published data only}
- Foy HM, Cooney MK, Allan ID, Frost F, Blumhagen JM, Fox JP. Influenza B virus vaccines in children and adults: adverse reactions, immune response, and observations in the field. Journal of Infectious Diseases 1981;143(5):700‐6. [DOI] [PubMed] [Google Scholar]
France 2004 {published data only}
- France EK, Glanz JM, Xu S, Davis RL, Black SB, Shinefield HR, et al. Safety of the trivalent inactivated influenza vaccine among children: a population‐based study. Archives of Pediatric and Adolescent Medicine 2004;158:1031‐6. [DOI] [PubMed] [Google Scholar]
Fujieda 2008 {published data only}
- Fujieda M, Maeda A, Kondo K, Fukushima W, Ohfuji S, Kaji M, et al. Influenza vaccine effectiveness and confounding factors among young children. Vaccine 2008;26(50):6481‐5. [DOI] [PubMed] [Google Scholar]
Gaglani 2004 {published data only}
- Gaglani MJ, Piedra PA, Herschler GB, Griffith ME, Kozinetz CA, Riggs MW, et al. Direct and total effectiveness of the intranasal, live‐attenuated, trivalent cold‐adapted influenza virus vaccine against the 2000‐2001 influenza A(H1N1) and B epidemic in healthy children. Archives of Pediatric and Adolescent Medicine 2004;158(1):65‐73. [DOI] [PubMed] [Google Scholar]
Gendon 2004 {published data only}
- Gendon IuZ, Kaira AN, El'shina GA, Akhmadulina RR. Total immunization of children against influenza decreases morbidity in a number of diseases among elderly persons during influenza epidemic. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2004;5:62‐7. [PubMed] [Google Scholar]
Glezen 2001 {published data only}
- Glezen WP. Effectiveness of influenza vaccination of day care children in reducing influenza‐related morbidity among household contacts. Journal of Pediatrics 2001;138:782. [DOI] [PubMed] [Google Scholar]
Groothuis 1994 {published data only}
- Groothuis JR, Lehr MV, Levin MJ. Safety and immunogenicity of a purified haemagglutinin antigen in very young high‐risk children. Vaccine 1994;12(2):139‐41. [DOI] [PubMed] [Google Scholar]
Groothuis 1998 {published data only}
- Groothuis JR, King SJ, Hogerman DA, Paradiso PR, Simoes EA. Safety and immunogenicity of a purified F protein respiratory syncytial virus (PFP‐2) vaccine in seropositive children with bronchopulmonary dysplasia. Journal of Infectious Diseases 1998;177(2):467‐9. [DOI] [PubMed] [Google Scholar]
Gross 1977a {published data only}
- Gross PA. Reactogenicity and immunogenicity of bivalent influenza vaccine in one‐ and two‐dose trials in children: a summary. Journal of Infectious Diseases 1977;136(Suppl):616‐25. [DOI] [PubMed] [Google Scholar]
Gross 1977b {published data only}
- Gross PA, Ennis FA, Gaerlan PF, Denson LJ, Denning CR, Schiffman D. A controlled double‐blind comparison of reactogenicity, immunogenicity, and protective efficacy of whole‐virus and split‐product influenza vaccines in children. Journal of Infectious Diseases 1977;136(5):623‐32. [DOI] [PubMed] [Google Scholar]
Gross 1982 {published data only}
- Gross PA, Quinnan GV, Gaerlan PF, Denning CR, Davis A, Lazicki M, et al. Potential for single high‐dose influenza immunization in unprimed children. Pediatrics 1982;70(6):982‐6. [PubMed] [Google Scholar]
Gruber 1993 {published data only}
- Gruber WC, Kirschner K, Tollefson S, Thompson J, Reed G, Edwards KM, et al. Comparison of monovalent and trivalent live attenuated influenza vaccines in young children. Journal of Infectious Diseases 1993;168(1):53‐60. [DOI] [PubMed] [Google Scholar]
Haba‐Rubio 2011 {published data only}
- Haba‐Rubio J, Rossetti AO, Tafti M, Heinzer R. Narcolepsy with cataplexy associated with H1N1 vaccination [Narcolepsie avec cataplexie a pres vaccination anti‐H1N1]. Revista de Neurologia 2011;167(8‐9):563‐6. [DOI] [PubMed] [Google Scholar]
Halperin 2002 {published data only}
- Halperin SA, Smith B, Mabrouk T, Germain M, Trepanier P, Hassell T, et al. Safety and immunogenicity of a trivalent, inactivated, mammalian cell culture‐derived influenza vaccine in healthy adults, seniors, and children. Vaccine 2002;20(7‐8):1240‐7. [DOI] [PubMed] [Google Scholar]
Hambidge 2006 {published data only}
- Hambidge SJ, Glanz JM, France EK. Safety of inactivated influenza vaccine in children 6 to 23 months old. JAMA 2006;296:1990‐7. [DOI] [PubMed] [Google Scholar]
Hatch 1956 {published data only}
- Hatch LA, Hawkins GF, McDonald JC. Influenza vaccination in a residential boys' school; report to the medical research council committee on clinical trials of influenza vaccine. British Medical Journal 1956;12(5003):1200‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heikkinen 2003 {published data only}
- Heikkinen T, Ziegler T, Peltola V, Lehtinen P, Toikka P, Lintu M, et al. Incidence of influenza in Finnish children. Pediatic Infectious Disease Journal 2003;22(Suppl 10):204‐6. [DOI] [PubMed] [Google Scholar]
Hoskins 1973 {published data only}
- Hoskins TW, Davies JR, Allchin A, Miller CL, Pollock TM. Controlled trial of inactivated influenza vaccine containing the a‐Hong Kong strain during an outbreak of influenza due to the a‐England‐42‐72 strain. Lancet 1973;2(7821):116‐20. [DOI] [PubMed] [Google Scholar]
Hoskins 1979 {published data only}
- Hoskins TW, Davies JR, Smith AJ, Miller CL, Allchin A. Assessment of inactivated influenza‐A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet 1979;1(8106):33‐5. [DOI] [PubMed] [Google Scholar]
Howell 1964a {published data only}
- Howell RW, MacKenzie AB. A comparative trial of oil‐adjuvant and aqueous polyvalent influenza vaccines. British Journal of Industrial Medicine 1964;21:265‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howell 1964b {published data only}
- Howell RW, Stott AN. A trial of oil‐adjuvant influenza vaccine in a non‐epidemic season. British Journal of Industrial Medicine 1964;21:259‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hrabar 1977 {published data only}
- Hrabar A, Vodopija I, Andre FE, Mitchell JR, Maassab HF, Hennessy AV, et al. A placebo‐controlled dose‐response study of the reactogenicity and immunogenicity of a cold‐adapted recombinant A/Victoria/3/75 (H3N2) live influenza virus candidate vaccine in healthy volunteers. Developments in Biological Standardization 1977;39:53‐60. [PubMed] [Google Scholar]
Hurwitz 2000a {published data only}
- Hurwitz ES, Haber M, Chang A, Shope T, Teo S, Ginsberg M, et al. Effectiveness of influenza vaccination of day care children in reducing influenza‐related morbidity among household contacts. JAMA 2000;284(13):1677‐82. [DOI] [PubMed] [Google Scholar]
Hurwitz 2000b {published data only}
- Hurwitz ES, Haber M, Chang A, Shope T, Teo ST, Giesick JS, et al. Studies of the 1996‐1997 inactivated influenza vaccine among children attending day care: immunologic response, protection against infection, and clinical effectiveness. Journal of Infectious Diseases 2000;182:1218‐21. [DOI] [PubMed] [Google Scholar]
Jansen 2008 {published data only}
- Jansen AG, Sanders EA, Hoes AW, Loon AM, Hak E. Effects of influenza plus pneumococcal conjugate vaccination versus influenza vaccination alone in preventing respiratory tract infections in children: a randomized, double‐blind, placebo‐controlled trial. Journal of Pediatrics 2008;153(6):764‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jovanovic 1979 {published data only}
- Jovanovic D, Georges‐Delvaux AM, Delem A. Protective efficacy of live influenza vaccines. Its assessment in field and experimental conditions. Developments in Biological Standardization 1979;43:241‐5. [PubMed] [Google Scholar]
Jurgenssen 1978 {published data only}
- Jurgenssen O, Moritz A, Liehl E, Bachmayer H. Vaccination of infants and schoolchildren with an influenza subunit vaccine (author's translation). Infection 1978;6(5):221‐7. [DOI] [PubMed] [Google Scholar]
Just 1978 {published data only}
- Just M, Burgin‐Wolff A, Berger‐Hernandez R, Bachlin A, Ritzel G, Moritz AJ. A/New Jersey/76 influenza vaccine trial in seronegative schoolchildren: comparison of a subunit vaccine with a whole‐virus vaccine. Medical Microbiology and Immunology 1978;164(4):277‐84. [DOI] [PubMed] [Google Scholar]
Karron 1995 {published data only}
- Karron RA, Steinhoff MC, Subbarao EK, Wilson MH, Macleod K, Clements ML, et al. Safety and immunogenicity of a cold‐adapted influenza A (H1N1) reassortant virus vaccine administered to infants less than six months of age. Pediatric Infectious Disease Journal 1995;14(1):10‐6. [DOI] [PubMed] [Google Scholar]
Kaufman 2000 {published data only}
- Kaufman Z, Cohen‐Manheim I, Green MS. Compliance with influenza vaccination in Israel in two successive winters, 1998/1999 and 1999/2000. Israel Medical Association Journal 2000;2(10):742‐5. [PubMed] [Google Scholar]
King 2001 {published data only}
- King JC Jr, Fast PE, Zangwill KM, Weinberg GA, Wolff M, Yan L, et al. Safety, vaccine virus shedding and immunogenicity of trivalent, cold‐adapted, live attenuated influenza vaccine administered to human immunodeficiency virus‐infected and noninfected children. Pediatric Infectious Disease Journal 2001;20(12):1124‐31. [DOI] [PubMed] [Google Scholar]
Kissling 2011a {published data only}
- Kissling E, Valenciano M. Early estimates of seasonal influenza vaccine effectiveness in Europe, 2010/11: I‐MOVE, a multicentre case‐control study. Eurosurveillance 2011;16:11. [DOI] [PubMed] [Google Scholar]
Kramarz 2001 {published data only}
- Kramarz P, Destefano F, Gargiullo PM, Chen RT, Lieu TA, Davis RL, et al. Does influenza vaccination prevent asthma exacerbations in children?. Journal of Pediatrics 2001;138(3):306‐10. [DOI] [PubMed] [Google Scholar]
Kuno‐Sakai 1994 {published data only}
- Kuno‐Sakai H, Kimura M, Ohta K, Shimojima R, Oh Y, Fukumi H. Developments in mucosal influenza virus vaccines. Vaccine 1994;12(14):1303‐10. [DOI] [PubMed] [Google Scholar]
La Montagne 1983 {published data only}
- Montagne JR, Noble GR, Quinnan GV, Curlin GT, Blackwelder WC, Smith JI, et al. Summary of clinical trials of inactivated influenza vaccine ‐ 1978. Reviews of Infectious Diseases 1983;5(4):723‐36. [DOI] [PubMed] [Google Scholar]
Lauteria 1974 {published data only}
- Lauteria SF, Kantzler GB, High PC, Lee JD, Waldman RH. An attenuated influenza virus vaccine: reactogenicity, transmissibility, immunogenicity, and protective efficacy. Journal of Infectious Diseases 1974;130(4):380‐3. [DOI] [PubMed] [Google Scholar]
Lerman 1977 {published data only}
- Lerman SJ. Reactivity and immunogenicity of monovalent A/New Jersey/76 influenza virus vaccines in children. Journal of Infectious Diseases 1977;136(Suppl):563‐70. [DOI] [PubMed] [Google Scholar]
Lina 2000 {published data only}
- Lina B, Fletcher MA, Valette M, Saliou P, Aymard M. A TritonX‐100‐split virion influenza vaccine is safe and fulfills the Committee for Proprietary Medicinal Products (CPMP) recommendations for the European Community for immunogenicity, in children, adults and the elderly. Biologicals 2000;28(2):95‐103. [DOI] [PubMed] [Google Scholar]
Longini 2000 {published data only}
- Longini IM, Halloran ME, Nizam A, Wolff M, Mendelman PM, Fast PE, et al. Estimation of the efficacy of live, attenuated influenza vaccine from a two‐year, multi‐center vaccine trial: implications for influenza epidemic control. Vaccine 2000;18(18):1902‐9. [DOI] [PubMed] [Google Scholar]
Luce 2001 {published data only}
- Luce BR, Zangwill KM, Palmer CS, Mendelman PM, Yan L, Wolff MC, et al. Cost‐effectiveness analysis of an intranasal influenza vaccine for the prevention of influenza in healthy children. Pediatrics 2001;108(2):e24. [DOI] [PubMed] [Google Scholar]
Luthardt 1979 {published data only}
- Luthardt T, Lutz O, Kindt H. Immunisation against influenza with a new subunit vaccine tested on children at risk (author's translation). Deutsche Medizinische Wochenschrift 1979;10(2):56‐60. [DOI] [PubMed] [Google Scholar]
Madhi 2014 {published data only}
- Madhi S, Cutland C, Jones S, Hugo A, Treurnicht FK, Kuwanda L, et al. Randomized, placebo‐controlled trial on safety and efficacy of inactivated influenza vaccination of pregnant women in preventing illness in their infants. International Journal of Infectious Diseases 2014;21:32. [Google Scholar]
Marchisio 2002 {published data only}
- Marchisio P, Cavagna R, Maspes B, Gironi S, Esposito S, Lambertini L, et al. Efficacy of intranasal virosomal influenza vaccine in the prevention of recurrent acute otitis media in children. Clinical Infectious Diseases 2002;35(2):168‐74. [DOI] [PubMed] [Google Scholar]
Martin Moreno 1998 {published data only}
- Martín Moreno V, Molina Cabrerizo MR, Sotillo Rincón MJ, Gómez Gómez C, Alvarez Gómez J. Adverse effects associated with influenza vaccine in pediatrics. Revista Española de Salud Pública 1998;72:319‐29. [PubMed] [Google Scholar]
Maynard 1968 {published data only}
- Maynard JE, Dull HB, Hanson ML, Feltz ET, Berger R, Hammes L. Evaluation of monovalent and polyvalent influenza vaccines during an epidemic of type A2 and B influenza. American Journal of Epidemiology 1968;87(1):148‐57. [DOI] [PubMed] [Google Scholar]
McMahon 2008 {published data only}
- McMahon AW, Iskander JK, Haber P, Braun MM, Ball R. Inactivated influenza vaccine (IIV) in children < 2 years of age: examination of selected adverse events reported to the Vaccine Adverse Event Reporting System (VAERS) after thimerosal‐free or thimerosal‐containing vaccine. Vaccine 2008;26(3):427‐9. [DOI] [PubMed] [Google Scholar]
Mendelman 2001 {published data only}
- Mendelman PM, Cordova J, Cho I. Safety, efficacy and effectiveness of the influenza virus vaccine, trivalent, types A and B, live, cold‐adapted (CAIV‐T) in healthy children and healthy adults. Vaccine 2001;19(17‐9):2221‐6. [DOI] [PubMed] [Google Scholar]
Monto 1970 {published data only}
- Monto AS, Davenport FM, Napier JA, Francis T Jr. Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. Journal of Infectious Diseases 1970;122(1):16‐25. [DOI] [PubMed] [Google Scholar]
Monto 1977 {published data only}
- Monto AS, Maassab HF. Use of influenza vaccine in non‐high risk populations. Developments in Biological Standardization 1977;39:329‐35. [PubMed] [Google Scholar]
Morio 1994 {published data only}
- Morio S, Okamoto N, Kawamoto A, Suyama A, Okamoto M, Nakayama H. Three year follow‐up study of national influenza vaccination practices in Japan. Journal of Epidemiology and Community Health 1994;48(1):46‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 1976 {published data only}
- Morris CA, Freestone DS, Stealey VM. Natural challenge of subjects vaccinated with WRL 105 strain live influenza vaccine in a residential community. Developments in Biological Standardization 1976;33:197‐201. [PubMed] [Google Scholar]
Muhammad 2011 {published data only}
- Muhammad RD, Haber P, Broder KR, Leroy Z, Ball R, Braun MM, et al. Adverse events following trivalent inactivated influenza vaccination in children: analysis of the Vaccine Adverse Event Reporting System. Pediatric Infectious Disease Journal 2011;30(1):e1‐8. [DOI] [PubMed] [Google Scholar]
Neuzil 2001 {published data only}
- Neuzil KM, Dupont WD, Wright PF, Edwards KM. Efficacy of inactivated and cold‐adapted vaccines against influenza A infection, 1985 to 1990: the pediatric experience. Pediatric Infectious Disease Journal 2001;20(8):733‐40. [DOI] [PubMed] [Google Scholar]
Neuzil 2006 {published data only}
- Neuzil KM, Jackson LA, Nelson J, Klimov A, Cox N, Bridges CB, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine‐naive 5‐8‐year‐old children. Journal of Infectious Diseases 2006;194:1032‐9. [DOI] [PubMed] [Google Scholar]
Nolan 2003 {published data only}
- Nolan T, Lee MS, Cordova JM, Cho I, Walker RE, August MJ, et al. Safety and immunogenicity of a live‐attenuated influenza vaccine blended and filled at two manufacturing facilities. Vaccine 2003;21(11‐2):1224‐31. [DOI] [PubMed] [Google Scholar]
Ogra 1977 {published data only}
- Ogra PL, Chow T, Beutner KR, Rubi E, Strussenberg J, DeMello S, et al. Clinical and immunologic evaluation of neuraminidase‐specific influenza A virus vaccine in humans. Journal of Infectious Diseases 1977;135(4):499‐506. [DOI] [PubMed] [Google Scholar]
Piedra 1991 {published data only}
- Piedra PA, Glezen PW. Influenza in children: epidemiology, immunity, and vaccines. Seminars in Pediatric Infectious Diseases 1991;2(2):140‐6. [Google Scholar]
Piedra 1993 {published data only}
- Piedra PA, Glezen WP, Mbawuike I, Gruber WC, Baxter BD, Boland FJ, et al. Studies on reactogenicity and immunogenicity of attenuated bivalent cold recombinant influenza type A (CRA) and inactivated trivalent influenza virus (TI) vaccines in infants and young children. Vaccine 1993;11(7):718‐24. [DOI] [PubMed] [Google Scholar]
Piedra 2002b {published data only}
- Piedra PA. Safety of the trivalent, cold‐adapted influenza vaccine (CAIV‐T) in children. Seminars in Pediatric Infectious Diseases 2002;13(2):90‐6. [DOI] [PubMed] [Google Scholar]
Quach 2003 {published data only}
- Quach C, Piche‐Walker L, Platt R, Moore D. Risk factors associated with severe influenza infections in childhood: implication for vaccine strategy. Pediatrics 2003;112(3 Pt 1):e197‐201. [DOI] [PubMed] [Google Scholar]
Rimmelzwaan 2000 {published data only}
- Rimmelzwaan GF, Nieuwkoop N, Brandenburg A, Sutter G, Beyer WE, Maher D, et al. A randomized, double blind study in young healthy adults comparing cell mediated and humoral immune responses induced by influenza ISCOM vaccines and conventional vaccines. Vaccine 2000;19(9‐10):1180‐7. [DOI] [PubMed] [Google Scholar]
Ruben 1973 {published data only}
- Ruben FL, Akers LW, Stanley ED, Jackson GG. Protection with split and whole virus vaccines against influenza. Archives of Internal Medicine 1973;132(4):568‐71. [PubMed] [Google Scholar]
Schaad 2000 {published data only}
- Schaad UB, Buhlmann U, Burger R, Ruedeberg A, Wilder‐Smith A, Rutishauser M, et al. Comparison of immunogenicity and safety of a virosome influenza vaccine with those of a subunit influenza vaccine in pediatric patients with cystic fibrosis. Antimicrobial Agents and Chemotherapy 2000;44(5):1163‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scheifele 1990 {published data only}
- Scheifele DW, Bjornson G, Johnston J. Evaluation of adverse events after influenza vaccination in hospital personnel. Canadian Medical Association Journal 1990;142(2):127‐30. [PMC free article] [PubMed] [Google Scholar]
Schiff 1975 {published data only}
- Schiff GM, Linnemann CC Jr, Shea L, Lange B, Rotte T. Evaluation of a live, attenuated recombinant influenza vaccine in high school children. Infection and Immunity 1975;11(4):754‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slepushkin 1993 {published data only}
- Slepushkin AN, Obrosova‐Serova NP, Burtseva EI, Rudenko LG, Govorkova EA, Vartanyan RV, et al. Comparison of live attenuated and inactivated influenza vaccines in schoolchildren in Russia: I. Safety and efficacy in two Moscow schools, 1987/88. Vaccine 1993;11(3):323‐8. [DOI] [PubMed] [Google Scholar]
Stowe 2011 {published data only}
- Stowe J, Andrews N, Bryan P, Seabroke S, Miller E. Risk of convulsions in children after monovalent H1N1 (2009) and trivalent influenza vaccines: a database study. Vaccine 2011;29(51):9467‐72. [DOI] [PubMed] [Google Scholar]
Sugaya 1994 {published data only}
- Sugaya N, Nerome K, Ishida M, Matsumoto M, Mitamura K, Nirasawa M. Efficacy of inactivated vaccine in preventing antigenically drifted influenza type A and well‐matched type B. JAMA 1994;272(14):1122‐6. [PubMed] [Google Scholar]
Sumaya 1977 {published data only}
- Sumaya CV, Brunell PA. A clinical trial with monovalent influenza A/New Jersey/76 virus vaccine in preschool and school‐age children. Journal of Infectious Diseases 1977;Suppl 136:597‐600. [DOI] [PubMed] [Google Scholar]
Van Hoecke 1996 {published data only}
- Hoecke C, Raue W, Kunzel W, Engelmann H. Immunogenicity and safety of influenza vaccination in 3‐ to 6‐year‐old children with a two dose immunisation schedule. European Journal of Pediatrics 1996;155(4):346‐7. [DOI] [PubMed] [Google Scholar]
Vasil'eva 1986 {published data only}
- Vasil'eva RI, Liamtseva GA, Riazantseva TG, Oleinikova EV, Sosunov AV. Assessment of the prophylactic effectiveness of an inactivated influenza vaccine by immunizing schoolchildren in the springtime. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1986;3:10‐4. [PubMed] [Google Scholar]
Vasil'eva 1987 {published data only}
- Vasil'eva RI, Lonskaia NI, Nefedova LA, Iaroshevskaia II, Bichurina MA. Results of a study of new preparations of inactivated whole‐virion influenza vaccines for the protection of children in the USSR. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1987;3:38‐42. [PubMed] [Google Scholar]
Wahlberg 2003 {published data only}
- Wahlberg J, Fredriksson J, Vaarala O, Ludvigsson J. Vaccinations may induce diabetes‐related autoantibodies in one‐year‐old children. Annals of the New York Academy of Sciences 2003;1005:404‐8. [DOI] [PubMed] [Google Scholar]
Welty 1977a {published data only}
- Welty PB Jr, Epstein B, O'Brien J, Brackett RG, Brandon FB, Shillis JL. Reactions and serologic responses after administration of inactivated monovalent influenza A/swine virus vaccines. II. Immunization of children with influenza A/New Jersey/X‐53 virus vaccines. Journal of Infectious Diseases 1977;136(Suppl):609‐11. [DOI] [PubMed] [Google Scholar]
Welty 1977b {published data only}
- Welty PB Jr, Epstein B, O'Brien J, Brackett RG, Brandon FB, Shillis JL. Reactions and serologic responses after administration of inactivated monovalent influenza A/swine virus vaccines. I. Immunization of children and adults with influenza A/Shope virus vaccines. Journal of Infectious Diseases 1977;136(Suppl):604‐8. [DOI] [PubMed] [Google Scholar]
Wesselius‐de 1972 {published data only}
- Wesselius‐de Casparis A, Masurel N, Kerrebijn KF. Field trial with human and equine influenza vaccines in children: protection and antibody titres. Bulletin of the World Health Organization 1972;46(2):151‐7. [PMC free article] [PubMed] [Google Scholar]
Wright 1976b {published data only}
- Wright PF, Dolin R, Montagne JR. From the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, the Center for Disease Control, and the Bureau of Biologics of the Food and Drug Administration. Summary of clinical trials of influenza vaccines‐II. Journal of Infectious Diseases 1976;134:633‐8. [DOI] [PubMed] [Google Scholar]
Wright 1985 {published data only}
- Wright PF, Bhargava M, Johnson PR, Thompson J, Karzon DT. Simultaneous administration of live, attenuated influenza A vaccines representing different serotypes. Vaccine 1985;3(3):305‐8. [DOI] [PubMed] [Google Scholar]
Wu 2010 {published data only}
- Wu J, Xu F, Lu L, Lu M, Miao L, Gao T, et al. Safety and effectiveness of a 2009 H1N1 vaccine in Beijing. New England Journal of Medicine 2010;363(25):2416‐23. [DOI] [PubMed] [Google Scholar]
Zhilova 1986 {published data only}
- Zhilova GP, Ignat'eva GS, Orlov VA, Malikova EV, Maksakova VL. Results of a study of the effectiveness of simultaneous immunization against influenza with live and inactivated vaccines (1980‐1983). Voprosy Virusologii 1986;31(1):40‐4. [PubMed] [Google Scholar]
Additional references
AAPCID 2004
- American Academy of Pediatrics Committee on Infectious Diseases. Recommendations for influenza immunization of children. Pediatrics 2004;113:1441‐7. [DOI] [PubMed] [Google Scholar]
ACIP 2016
- Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and control of seasonal influenza with vaccines. Morbidity and Mortality Weekly Report 2016;65(5):1‐54. [DOI] [PubMed] [Google Scholar]
Ambrose 2012
- Ambrose CS, Wu X, Knuf M, Wutzler P. The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: a meta‐analysis of 8 randomized controlled studies. Vaccine 2012;30(5):886‐92. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Belshe 2010
- Belshe RB, Toback SL, Yi T, Ambrose CS. Efficacy of live attenuated influenza vaccine in children 6 months to 17 years of age. Influenza and Other Respiratory Viruses 2010;4(3):141‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Broome 1980
- Broome C, Facklam R, Fraser D. Pneumococcal disease after pneumococcal vaccination ‐ an alternative method to estimate the efficacy of pneumococcal vaccine. New England Journal of Medicine 1980;303(10):549‐52. [DOI] [PubMed] [Google Scholar]
Carter 2011
- Carter NJ, Curran MP. Live attenuated influenza vaccine (FluMist; Fluenz): a review of its use in the prevention of seasonal influenza in children and adults. Drugs 2011;71(12):1591‐622. [DOI] [PubMed] [Google Scholar]
CDC 2007
- Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, et al. Centers for Disease Control and Prevention (CDC). Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. Morbidity and Mortality Weekly Report 2007;56(RR‐6):1‐54. [PubMed] [Google Scholar]
CDC 2011
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. Morbidity and Mortality Weekly Report 2011;60(33):1128‐32. [PubMed] [Google Scholar]
Collignon 2010
- Collignon P, Doshi P, Jefferson T. Ramifications of adverse events in children in Australia (letter to the Editor). BMJ 2010;340:c2994. [DOI] [PubMed] [Google Scholar]
Cowling 2012
- Cowling B, Fang V, Nishiura H, Chan K‐H, Ng S, Ip DKM, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clinical Infectious Diseases 2012;54(12):1778‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demicheli 2010
- Demicheli V, Jefferson T, Pietrantonj C, Al‐Ansary LA, Ferroni E, Thorning S, et al. Vaccines for preventing influenza in the elderly. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD004876.pub3] [DOI] [PubMed] [Google Scholar]
Demicheli 2014
- Demicheli V, Jefferson T, Al‐Ansary LA, Ferroni E, Rivetti A, Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD001269.pub5] [DOI] [PubMed] [Google Scholar]
ECDC 2009
- European Centre for Disease Prevention and Control. Protocol for case‐control studies to measure pandemic and seasonal influenza vaccine effectiveness in the European Union and European Economic Area Member States ‐ Technical Document. ecdc.europa.eu/en/publications/Publications/0907_TED_Influenza_AH1N1_Measuring_Influenza_Vaccine_Effectiveness_Protocol_Case_Control_Studies.pdf (accessed prior to 23 January 2018).
EMA 2014
- European Medicines Agency. EMA/CHMP/VWP/457259/2014. Committee for Medicinal Products for Human Use. Guideline on influenza vaccines. Non‐clinical and clinical module. www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500211324 (accessed prior to 23 January 2018).
Fiore 2011
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. Morbidity and Mortality Weekly Report 2010;59(RR‐8):1‐62. [PubMed] [Google Scholar]
Foppa 2013
- Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test‐negative design for studies of the effectiveness of influenza vaccine. Vaccine 2013;31(30):3104‐9. [DOI] [PubMed] [Google Scholar]
Forrest 2008
- Forrest BD, Pride MW, Dunning AJ, Capeding MR, Chotpitayasunondh T, Tam JS, et al. Correlation of cellular immune responses with protection against culture‐confirmed influenza virus in young children. Clinical and Vaccine Immunology 2008;15(7):1042‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Harper 2004
- Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB, Centers for Disease Control and Prevention (CDC). Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report 2004;53(RR‐6):1‐40. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ioannidis 2011
- Ioannidis JPA, Manzoli L, Vito C, D’Addario M, Villari P. Publication delay of randomized trials on 2009 Influenza A (H1N1) vaccination. PLoS ONE 2011;6(12):e28346. [DOI: 10.1371/journal.pone.0028346] [DOI] [PMC free article] [PubMed] [Google Scholar]
Izurieta 2000
- Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. New England Journal of Medicine 2000;342:232‐9. [DOI] [PubMed] [Google Scholar]
Jefferson 2005a
- Jefferson T, Smith S, Demicheli V, Harnden A, Rivetti A. Safety of influenza vaccines in children. Lancet 2005;366(9488):803‐4. [DOI] [PubMed] [Google Scholar]
Jefferson 2009
- Jefferson T, Pietrantonj C, Debalini MG, Rivetti A, Demicheli V. Study quality, concordance, take home message, funding and impact: their relationship in influenza vaccines studies. BMJ 2009;338:b354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferson 2010
- Jefferson T, Pietrantonj C, Rivetti A, Bawazeer GA, Al‐Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD001269.pub4] [DOI] [PubMed] [Google Scholar]
Jefferson 2012a
- Jefferson T, Rivetti A, Pietrantonj C, Demicheli V, Ferroni E. Vaccines for preventing influenza in healthy children. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD004879.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jordan 2006
- Jordan R, Connock M, Albon E, Fry‐Smith A, Olowokure B, Hawker J, et al. Universal vaccination of children against influenza: are there indirect benefits to the community? A systematic review of the evidence. Vaccine 2006;24(8):1047‐62. [DOI] [PubMed] [Google Scholar]
Kissling 2017
- Kissling E, Rondy M, I‐MOVE/I‐MOVE+ study team. Early 2016/17 vaccine effectiveness estimates against influenza A(H3N2): I‐MOVE multicentre case control studies at primary care and hospital levels in Europe. Eurosurveillance 2017;22(7):30464. [DOI: 10.2807/1560-7917.ES.2017.22.7.30464] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loeb 2010
- Loeb M, Russell ML, Moss L, Fonseca K, Fox J, Earn DJD, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: a randomized trial. JAMA 2010;303(10):943–50. [DOI] [PubMed] [Google Scholar]
Lum 2010
- Lum LC, Borja‐Tabora CF, Breiman RF, Vesikari T, Sablan BP, Chay OM, et al. Influenza vaccine concurrently administered with a combination measles, mumps, and rubella vaccine to young children. Vaccine 2010;28(6):1566‐74. [DOI] [PubMed] [Google Scholar]
Manzoli 2007
- Manzoli L, Schioppa F, Boccia A, Villari P. The efficacy of influenza vaccine for healthy children: a meta‐analysis evaluating potential sources of variation in efficacy estimates including study quality. Pediatric Infectious Disease Journal 2007;26(2):97‐106. [DOI] [PubMed] [Google Scholar]
Manzoli 2011
- Manzoli L, Vito C, Salanti G, D'Addario M, Villari P, Ioannidis JPA. Meta‐analysis of the immunogenicity and tolerability of pandemic influenza A 2009 (H1N1) vaccines. PLoS ONE 2011;6(9):e24384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mereckiene 2010
- Mereckiene J, Cotter S, D’Ancona F, Giambi C, Nicoll A, Levy‐Bruhl D, et al. Differences in national influenza vaccination policies across the European Union, Norway and Iceland 2008‐2009. Eurosurveillance 2010;15(44):1‐10. [DOI] [PubMed] [Google Scholar]
Michiels 2011
- Michiels B, Govaerts F, Remmen R, Vermeire E, Coenen S. A systematic review of the evidence on the effectiveness and risks of inactivated influenza vaccines in different target groups. Vaccine 2011;29(49):9159‐70. [DOI] [PubMed] [Google Scholar]
Negri 2005
- Negri E, Colombo C, Giordano L, Groth N, Apolone G, Vecchia C. Influenza vaccine in healthy children: a meta‐analysis. Vaccine 2005;23(22):2851‐61. [DOI] [PubMed] [Google Scholar]
Neuzil 2000
- Neuzil KM, Mellen BG, Wright PF, Mitchel EF Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. New England Journal of Medicine 2000;342:225‐31. [DOI] [PubMed] [Google Scholar]
Orenstein 1985
- Orenstein W, Bernier R, Dondero T, Hinman AR, Marks JS, Bart KJ, et al. Field evaluation of vaccine efficacy. Bulletin of the World Health Organization 1985;63(6):1055‐68. [PMC free article] [PubMed] [Google Scholar]
Orr 2004
- Orr P. Statement on influenza vaccination for the 2004‐2005 season. Canada Communicable Disease Report 2004;30:1‐32. [PubMed] [Google Scholar]
Osterholm 2012
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta‐analysis. Lancet 2012;12(1):36‐44. [DOI] [PubMed] [Google Scholar]
Perencevich 2013a
- Perencevich EN. Pondering vexing issues in infection prevention and control. haicontroversies.blogspot.com.au/2015/09/there‐is‐no‐such‐thing‐as‐patient‐safety.html#!/ (accessed 22 May 2017).
Perencevich 2013b
- Perencevich EN. How should we calculate influenza vaccine effectiveness? ACP Internist Blog Archive. blog.acpinternist.org/2013/02/how‐should‐we‐calculate‐influenza_7.html (accessed 17 May 2017).
Principi 2004
- Principi N, Esposito S. Are we ready for universal influenza vaccination in paediatrics?. Lancet 2004;4:75‐83. [DOI] [PubMed] [Google Scholar]
Reichert 2001
- Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. New England Journal of Medicine 2001;344:889‐96. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhorer 2009
- Rhorer J, Ambrose CS, Dickinson S, Hamilton H, Oleka NA, Malinoski FJ, et al. Efficacy of live attenuated influenza vaccine in children: a meta‐analysis of nine randomized clinical trials. Vaccine 2009;27(7):1101‐10. [DOI] [PubMed] [Google Scholar]
Rodrigo Pendas 2007
- Rodrigo Pendas JA, Alemany Vilches L, Moraga Llop FA. Systematic reviews of the efficacy and effectiveness of influenza vaccination in infants, children and teenagers [Revisiones sistematicas de la eficacia o efectividad de las vacunas antigripales en el lactante, el nino y el adolescente sano]. Vacunas 2007;8(3):130‐42. [Google Scholar]
THL 2012
- National Institute for Health and Welfare (THL). National Narcolepsy Task Force Interim Report. www.thl.fi/thl‐client/pdfs/dce182fb‐651e‐48a1‐b018‐3f774d6d1875 (accessed 2 January 2012).
Treanor 2016
- Treanor JJ. Influenza vaccination. New England Journal of Medicine 2016;375(13):1261‐8. [DOI: 10.1056/NEJMcp1512870] [DOI] [PubMed] [Google Scholar]
Valenciano 2012
- Valenciano M, Ciancio BC, I‐MOVE study team. I‐MOVE: a European network to measure the effectiveness of influenza vaccine. Eurosurveillance 2012;17(39):20281. [DOI] [PubMed] [Google Scholar]
Wijnans 2011
- Wijnans L, Bie S, Dieleman J, Bonhoeffer J, Sturkenboom M. Safety of pandemic H1N1 vaccines in children and adolescents. Vaccine 2011;29(43):7559‐71. [DOI] [PubMed] [Google Scholar]
Wijnans 2016
- Wijnans L, Voordouw B. A review of the changes to the licensing of influenza vaccines in Europe. Influenza and Other Respiratory Viruses 2016;10(1):2‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiselka 1994
- Wiselka M. Influenza: diagnosis, management and prophylaxis. BMJ 1994;308(6940):1341‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yin 2011
- Yin JK, Khandaker G, Rashid H, Heron L, Ridda I, Booy R. Immunogenicity and safety of pandemic influenza A (H1N1) 2009 vaccine: systematic review and meta‐analysis. Influenza and Other Respiratory Viruses 2011;5(5):299‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Jefferson 2005b
- Jefferson T, Smith S, Demicheli V, Harnden A, Rivetti A, Pietrantonj C. Assessment of the efficacy and effectiveness of influenza vaccines in healthy children: systematic review. Lancet 2005;365(9461):773‐80. [DOI] [PubMed] [Google Scholar]
Jefferson 2008
- Jefferson T, Rivetti A, Harnden AR, Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD004879.pub3] [DOI] [PubMed] [Google Scholar]
Jefferson 2012b
- Jefferson T, Rivetti A, Pietrantonj C, Demicheli V, Ferroni E. Vaccines for preventing influenza in healthy children. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD004879.pub4] [DOI] [PubMed] [Google Scholar]
Smith 2004
- Smith S, Demicheli V, Jefferson T Harnden A, Matheson N, Pietrantonj C. Vaccines for preventing influenza in healthy children. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004879] [DOI] [PubMed] [Google Scholar]
Smith 2006
- Smith S, Demicheli V, Pietrantonj C, Harnden AR, Jefferson T, Matheson NJ, et al. Vaccines for preventing influenza in healthy children. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004879.pub2] [DOI] [PubMed] [Google Scholar]


