Abstract
Background
Rates of major depression among people living with HIV (PLWH) are substantially higher than those seen in the general population and this may adversely affect antiretroviral treatment outcomes. Several unique clinical and psychosocial factors may contribute to the development and persistence of depression in PLWH. Given these influences, it is unclear if antidepressant therapy is as effective for PLWH as the general population.
Objectives
To assess the efficacy of antidepressant therapy for treatment of depression in PLWH.
Search methods
We searched The Cochrane Common Mental Disorders Group's specialised register (CCMD‐CTR), the Cochrane Library, PubMed, Embase and ran a cited reference search on the Web of Science for reports of all included studies. We conducted additional searches of the international trial registers including; ClinicalTrials.gov, World Health Organization Trials Portal (ICTRP), and the HIV and AIDS ‐ Clinical trials register. We searched grey literature and reference lists to identify additional studies and contacted authors to obtain missing data. We applied no restrictions on date, language or publication status to the searches, which included studies conducted between 1 January 1980 and 18 April 2017.
Selection criteria
We included randomized controlled trials of antidepressant drug therapy compared to placebo or another antidepressant drug class. Participants eligible for inclusion had to be aged 18 years and older, from any setting, and have both HIV and depression. Depression was defined according to Diagnostic and Statistical Manual of Mental Disorders or International Statistical Classification of Diseases criteria.
Data collection and analysis
Two review authors independently applied the inclusion criteria and extracted data. We presented categorical outcomes as risk ratios (RR) with 95% confidence intervals (CIs). Continuous outcomes were presented mean (MD) or standardized mean differences (SMD) with standard deviations (SD). We assessed quality of evidence using the GRADE approach.
Main results
We included 10 studies with 709 participants in this review. Of the 10 studies, eight were conducted in high income countries (USA and Italy), seven were conducted prior to 2000 and seven had predominantly men. Seven studies assessed antidepressants versus placebo, two compared different antidepressant classes and one had three arms comparing two antidepressant classes with placebo.
Antidepressant therapy may result in a greater improvement in depression compared to placebo. There was a moderate improvement in depression when assessed with the Hamilton Depression Rating Scale (HAM‐D) score as a continuous outcome (SMD 0.59, 95% CI 0.21 to 0.96; participants = 357; studies = 6; I2 = 62%, low quality evidence). However, there was no evidence of improvement when this was assessed with HAM‐D score as a dichotomized outcome (RR 1.10, 95% CI 0.89 to 1.35; participants = 434; studies = 5; I2 = 0%, low quality evidence) or Clinical Global Impression of Improvement (CGI‐I) score (RR 1.28, 95% CI 0.93 to 1.77; participants = 346; studies = 4; I2 = 29%, low quality evidence). There was little to no difference in the proportion of study dropouts between study arms (RR 1.28, 95% CI 0.91 to 1.80; participants = 306; studies = 4; I2 = 0%, moderate quality evidence).
The methods of reporting adverse events varied substantially between studies, this resulted in very low quality evidence contributing to a pooled estimate (RR 0.88, 95% CI 0.64 to 1.21; participants = 167; studies = 2; I2 = 34%; very low quality evidence). Based on this, we were unable to determine if there was a difference in the proportion of participants experiencing adverse events in the antidepressant versus placebo arms. However, sexual dysfunction was reported commonly in people receiving selective serotonin reuptake inhibitors (SSRIs). People receiving tricyclic antidepressants (TCAs) frequently reported anticholinergic adverse effects such as dry mouth and constipation. There were no reported grade 3 or 4 adverse events in any study group.
There was no evidence of a difference in follow‐up CD4 count at study termination (MD ‐6.31 cells/mm3, 95% CI ‐72.76 to 60.14; participants = 176; studies = 3; I2 = 0%; low quality evidence). Only one study evaluated quality of life score (MD 3.60, 95% CI ‐0.38 to 7.58; participants = 87; studies = 1; very low quality evidence), due to the poor quality evidence we could not draw conclusions for this outcome.
There were few studies comparing different antidepressant classes. We are uncertain if SSRIs differ from TCAs with regard to improvement in depression as evaluated by HAM‐D score (MD ‐3.20, 95% CI ‐10.87 to 4.47; participants = 14; studies = 1; very low quality evidence). There was some evidence that mirtazapine resulted in a greater improvement in depression compared to an SSRI (MD 9.00, 95% CI 3.61 to 14.39; participants = 70; studies = 1; low quality evidence); however, this finding was not consistent for all measures of improvement in depression for this comparison.
No studies reported on virological suppression or any other HIV specific outcomes.
The studies included in this review had an overall unclear or high risk of bias due to under‐reporting of study methods, high risk of attrition bias and inadequate sequence generation methods. Heterogeneity between studies and the limited number of participants, and events lead to downgrading of the quality of the evidence for several outcomes.
Authors' conclusions
This review demonstrates that antidepressant therapy may be more beneficial than placebo for the treatment of depression in PLWH. The low quality of the evidence contributing to this assessment and the lack of studies representing PLWH from generalized epidemics in low‐ to middle‐income countries make the relevance of these finding in today's context limited. Future studies that evaluate the effectiveness of antidepressant therapy should be designed and conducted rigorously. Such studies should incorporate evaluation of stepped care models and health system strengthening interventions in the study design. In addition, outcomes related to HIV care and antiretroviral therapy should be reported.
Plain language summary
Antidepressant drugs for treatment of depression in people living with HIV
Why is this review important?
Depression is very common among people living with HIV. There are many unique issues which influence the development and possibly the recovery from depression in this group. We are therefore uncertain whether the antidepressant drugs which are usually used to treat depression in people without HIV will be as effective in PLWH.
Who will be interested in this review?
PLWH, general practitioners, HIV clinicians and professionals working in mental health services.
What questions does this review aim to answer?
‐ Are antidepressant medicines more effective than using a placebo (pretend treatment) for treatment of depression in PLWH?
‐ Do more people stop attending services (dropout) if they are receiving antidepressant medicines compared to placebos?
‐ Are there any serious side effects to antidepressant medicines which specifically affect PLWH?
‐ Which type of antidepressant medicine is most effective for depressed PLWH?
‐ Does treating depression with antidepressants in PLWH improve antiretroviral treatment outcomes among people also receiving HIV treatment?
Which studies were included in the review?
We searched several databases to find randomized controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) which compared antidepressant therapy to placebo or other antidepressant drugs for treatment of depression in PLWH. Studies had to have been conducted between 1 January 1980 and 18 April 2017 to be included in the review. Ten studies with 709 participants were included.
What does the evidence from the review tell us?
Most studies were conducted more than a decade ago, in the USA, in predominantly men. We found that antidepressant therapy may improve depressive symptoms when compared to a placebo tablet. There was no clear evidence of a difference in the number of people who dropped out of care when comparing people who received antidepressants with people who received a placebo. We cannot be certain if one type of antidepressant works better than another. Side effects were very common among all study participants. Although there were no clear conclusions on which side effects were most common or if side effects occurred more frequently in people taking antidepressants compared to a placebo, participants receiving antidepressants called selective serotonin reuptake inhibitors did report sexual problems frequently. People receiving medicines called tricyclic antidepressants reported constipation and dry mouth frequently. No studies reported on how antidepressant therapy affected the effectiveness of antiretroviral therapy. The evidence used to generate several of the results was assessed as low or very low quality.
What should happen next?
The review authors recommend that new studies on treatment of depression should be conducted in countries and population groups where HIV is most common. These studies should evaluate what causes depression in these populations and how to best to incorporate antidepressant therapy with other strategies for the management of PLWH and depression.
Summary of findings
Background
Description of the condition
HIV
Infection with HIV results in a multisystemic chronic illness. After initial infection, there is a period of latency before clinical symptoms emerge. The clinical effects of HIV are primarily due to the breakdown of the immune system, with a reduction in the number of infection‐fighting cells such as natural killer (NK) cells (Huang 1990) and T lymphocytes (Cohen 2001). A person is considered to have developed AIDS when he or she has CD4+ counts of less than 200 cells/mm3 or an AIDS‐defining illness (CDC 2008). Neurological and neuropsychiatric effects are seen throughout the course of the illness, but tend to worsen as the disease progresses (Grant 2008). In 2015, there were 36.7 million people living with HIV (PLWH) worldwide and the disease accounted for 1.1 million deaths in 2012 (UNAIDS 2016). The introduction of antiretroviral therapy (ART) has significantly improved life expectancy and quality of life among PLWH. The number of people receiving ART has increased markedly since the mid‐2000s and reached 17 million in 2015 (UNAIDS 2016).
Prevalence of depression in HIV‐positive people
Major depressive disorder may occur in as much as 42% of PLWH (Nanni 2015). Studies conducted in North America and Europe reported rates of major depression among PLWH as at least twice that of the general population (Dew 1997; Bing 2001; Ciesla 2001). One meta‐analysis of studies conducted in low‐ and middle‐income countries (LMIC) showed a wide range (0% to 63%) in the reported prevalence of depression (Collins 2006). This variation in prevalence may be due to the wide range of methods utilized in these studies. Of note, most studies were conducted before ART was widely available. More recent studies conducted among HIV‐positive people receiving ART in Uganda and Cameroon found a lifetime prevalence of a major depressive episode of 21% to 25% (Nakimuli‐Mpungu 2011; Gaynes 2012). It has been suggested that HIV‐positive women have higher rates of depression compared to HIV‐positive men, however, no consistent association has been demonstrated across studies (Cruess 2003; Nakimuli‐Mpungu 2011; Gaynes 2012).
Relationship between HIV and the development of depression
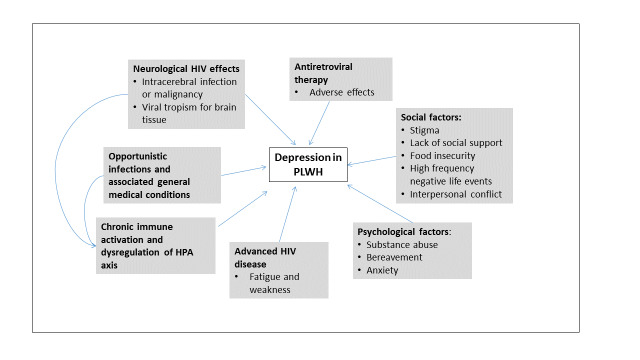
Many influences may contribute to the development of depressive illness in PLWH (Figure 1). Depression in HIV may be a consequence of a primary (or pre‐existing) depressive disorder and it may follow the effects of the virus on the brain, other infections or tumours, antiretroviral drugs and other medical treatments. Several of these factors may coexist within a person.
1.
Conceptual framework of factors influencing depression in people living with HIV
Psychosocial factors
Unique psychosocial issues such as stigmatization, lack of social support, substance abuse, bereavement and anxiety may contribute to the development of depression in this group (Nott 1999; Lichtenstein 2002; Morrison 2002; Akena 2012). In LMICs, financial stress, food insecurity, living in a rural setting, high frequency of negative life events and interpersonal conflict in PLWH have also been associated with an increased risk of depression (Kinyanda 2011; Unnikrishnan 2012; Gibbs 2016).
Neurological involvement
Neurological involvement is found in 60% of HIV‐positive people (Ghafouri 2006), and neurocognitive impairment has been found to be more common among HIV‐positive people compared to the general population (Nakasujja 2010). The virus predominantly affects the subcortical areas and fronto‐striatal circuits (McArthur 2005). Disruption of these circuits may result in depressive symptoms. Neuroimaging studies also reveal marked atrophy of the basal ganglia suggesting possible effects on dopaminergic pathways in HIV‐positive people(Foley 2008). Opportunistic infections of the central nervous system may also lead to depression. These secondary mood disorders become more likely as the disease progresses.
Chronic immune activation and multisystem involvement
Recurrent physical and psychological stressors contribute to chronic activation of immune cells via disruption of the hypothalamic‐pituitary‐adrenal (HPA) axis. This in turn leads to increased levels of inflammatory cytokines in the central nervous system and consequently a higher risk of developing depression among HIV‐positive people (Del Guerra 2013). In the absence of opportunistic infections, the main neuropsychiatric manifestations of HIV include impaired short‐term memory, reduced concentration, slowness of movement and gait, and depression (Ghafouri 2006). Systemic manifestations of HIV disease may also cause or precipitate depression. Hypogonadism is common in advanced HIV infection and may be associated with depressive symptoms (Fernandez 1991).
Antiretroviral therapy
There are several case reports that suggest an association between ART and the development or aggravation of depression. Efavirenz has been most commonly implicated with reports of depression, insomnia, disorientation, psychosis and vivid dreams after initiation of treatment (Cespedes 2006; Cavalcante 2010; Gaida 2016). The neurochemical pathways associated with efavirenz are not clearly understood. It has been postulated that efavirenz may have a direct effect on the serotonergic system as well as some indirect effects related to increased inflammatory cytokines and inhibition of creatine kinase in brain tissue (Cavalcante 2010).
Sequelae of depression in HIV‐positive people
Untreated depression in PLWH has been associated with a more rapid progression to AIDS (Elliott 2002). Lima and colleagues reported a shorter survival among HIV‐positive depressed people accessing ART (Lima 2007). Reducing depressive symptoms in HIV‐positive people may have a positive impact on health‐related quality of life and work status (Elliott 2002; Wagner 2014).
Depressive symptoms, especially in the presence of severe stress, have been related to declines in several lymphocyte subsets (e.g. CD16+ and CD56+ NK cells, and CD8+ cytotoxic‐suppressor cells) (Leserman 1997). Disturbances of HPA axis function increases adrenocorticotrophin‐releasing hormone and cortisol, and has been associated with stress and depression in humans. Such dysregulation can negatively impact the immune response. There is some evidence to suggest that a change in cortisol levels is positively related to stress and depression in HIV‐positive people (Gorman 1991; Schuster 2012).
Depression has been associated with poorer adherence to ART. Uthman 2014 pooled data from 111 studies globally and found that the likelihood of achieving good adherence was 42% lower among people with depressive symptoms compared to people without. This suggests that the compliant use of antidepressant therapy and improvement in depression should be associated with improved ART adherence and virological suppression (DiMatteo 2000; Ammassari 2004; Horberg 2008; Springer 2012). Pence 2015 evaluated an intervention where people were randomized to usual care for depression or measurement‐based‐care (including antidepressant treatment algorithms and depression case managers). This study showed no difference in any HIV outcomes (including ART adherence) between study arms. However, this study was compromised by high rates of loss to follow‐up and incomplete recruitment.
Description of the intervention
Treatment of depression in adults includes the use of medication or psychological therapies, or both. Drug treatment is reserved for people with: 1. moderate or severe depression (APA 1994), or 2. people who have mild or subthreshold depression (APA 1994) with a history of moderate/severe depression or persistent symptoms for a long period (two years) or a poor response to psychological therapies (NICE 2009). Among people with moderate to severe depression, drug therapy should ideally be offered in combination with a high intensity psychological intervention such as cognitive behavioural therapy or interpersonal therapy (NICE 2009).
Drugs that have been classified as antidepressants include reuptake inhibitors and modulators of noradrenaline or serotonin (or both), receptor blockers or enzyme inhibitors. Drug classes include selective serotonin reuptake inhibitors (SSRIs); serotonin‐noradrenaline reuptake inhibitors (SNRIs); serotonin modulators, tricyclics (TCAs) and tetracyclics antidepressants; monoamine oxidase inhibitors (MAOIs); and atypical agents such as bupropion, mirtazapine and agomelatine.
Most antidepressants have similar efficacy and the choice of antidepressant is influenced by previous antidepressant history, patient preference, adverse‐effect profile, safety in overdose, cost and interaction with other medications or physical conditions (NICE 2015). However, SSRIs are the favoured first choice antidepressant because of their good risk‐benefit profile. Adverse effects of SSRIs include; agitation, gastrointestinal symptoms, insomnia and sexual dysfunction. Paroxetine is the least well tolerated SSRI and is associated with high rates of treatment discontinuation (NICE 2009). There are many potential drug‐drug interactions which may occur with SSRIs as they inhibit cytochrome P450 enzymes in the liver. However, the clinical significance of many of these potential drug interactions are unclear. Fluoxetine, fluvoxamine and paroxetine have the highest risk of drug interactions as compared to other SSRIs.
TCAs commonly result in anticholinergic, antihistaminic and cardiac adverse effects and can be fatal in overdose. Slow titration to an optimum dose is required to prevent severe adverse effects and discontinuation. Marked sedation and postural hypotension are common reasons for discontinuation.
Adverse effects that occur with SNRIs include nausea, dizziness and diaphoresis. Among SNRIs, duloxetine has the greatest potential for drug interactions. Venlafaxine has been associated with a discontinuation syndrome if abruptly stopped and may also be lethal in overdose. Dosulepin can be fatal in overdose.
Serotonin modulators may also cause drug interactions due to inhibition of cytochrome P450 enzymes. These agents are highly sedating and are commonly used to treat depression with insomnia. They require slow titration to optimum effective dose. Additional adverse effects include dry mouth, nausea and dizziness.
MAOIs are not first‐line antidepressant treatment as they have several dietary restrictions, drug interactions and adverse effects (dry mouth, gastrointestinal upset, urinary hesitancy, headache, and myoclonic jerks and commonly dizziness due to hypotension). However, they do play a role in the treatment of atypical depression and treatment‐resistant depression.
Atypical agents such as bupropion, mirtazapine and agomelatine are mostly reserved for treatment of people who do not respond to, or have marked adverse effects to, more conventional antidepressants such as SSRIs. Bupropion can lower the seizure threshold and cannot be used in combination with other sedatives. Overdose of bupropion can be fatal. Agomelatine has significant interactions with other drugs metabolized by the liver and can be hepatotoxic; other adverse effects are relatively infrequent. Mirtazapine is commonly associated with dizziness, dry mouth and sedation and can also be fatal in overdose.
Antidepressant therapy has a gradual onset in effect and there should be evidence of an improvement in depressive symptoms within two to four weeks after the initiation of treatment. If no effect has been established by this stage, compliance should be reviewed and dosage adjustments or alternative/adjunctive treatment should be considered.
There are several potential pharmacological interactions between antiretroviral agents and antidepressant medications, the clinical significance of these remains an area of research. Non‐nuclueoside reverse transcriptase inhibitors and protease inhibitors boosted with ritonavir may induce or inhibit cytochrome P450 enzymes (or both) which play a role in the metabolism of several antidepressant agents (Siccardi 2013). Although no dosage adjustment has been recommended by the US Food and Drug Administration (FDA), the prolonged half‐life of fluoxetine when combined with ritonavir has been associated with serotonin syndrome (DeSilva 2001). Citalopram, which has a better pharmacokinetic profile when combined with ritonavir, is considered a better alternative to fluoxetine if protease inhibitors are used (Siccardi 2013). In many settings where HIV is prevalent, fluoxetine is preferred due to greater availability, affordability, longer half‐life and the activating properties of the drug. The US FDA recommends a reduction in the dosage of trazodone when used in combination with lopinavir‐ritonavir (US FDA 2013a), and reports that efavirenz is associated with reduced efficacy of sertraline (US FDA 2013b).
How the intervention might work
Antidepressant medications exert their effects at the level of neurotransmitters. However, the exact mechanisms by which antidepressants alleviate the symptoms of depression remain poorly understood. Improvement in depression using antidepressants could mitigate the negative effects of depression and provide substantial health and economic benefits in PLWH.
Why it is important to do this review
Although there is clear evidence of the effectiveness of antidepressant therapy in the general population, the unique clinical and psychosocial factors that influence the development and persistence of depression in PLWH warrants a separate evaluation of antidepressant therapy in this subgroup.
Himelhoch 2005 conducted a systematic review and meta‐analysis of antidepressant therapy in PLWH which showed antidepressant therapy to be effective in this group. However, this study was conducted in the mid‐2000s, prior to the widespread use of highly active ART and the expansion of ART provision in LMICs. The improved survival and changes in the patient population make the findings from this review less relevant in today's context.
Objectives
To assess the efficacy of antidepressant therapy for treatment of depression in PLWH.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCT), including cluster and cross‐over trials in which participants with HIV infection were randomly allocated to antidepressant therapy for depression versus placebo or alternative antidepressant treatment class.
Types of participants
Inclusion criteria
Participants of either sex aged 18 years and older, of any ethnicity and in any setting, and who had both depression and HIV. Participants could be receiving ART.
Participants were required to meet accepted diagnostic criteria for a major depressive disorder, persistent depressive disorder (dysthymia), adjustment disorder with depressed mood or minor (subthreshold) depression during the study period. This must have been defined in accordance with the Diagnostic and Statistical Manual of Mental Disorders (DSM); editions III (APA 1980), III‐R (APA 1987), IV (APA 1994), IV‐TR (APA 2000), and V (APA 2013) or the International Statistical Classification of Diseases, Injuries and Causes of Death (ICD) version 10 (WHO 1992).
The inclusion criteria for this review were expanded subsequent to publication of the protocol (Differences between protocol and review).
Exclusion criteria
Trials of antidepressants used for other indications (such as pain relief or insomnia) or other psychiatric disorders unless the participants were explicitly diagnosed and treated for depression.
Types of interventions
Experimental intervention
We considered studies including antidepressant medications in the following classes:
tricyclic/tetracyclic antidepressants (TCAs) (e.g. amitriptyline, imipramine, clomipramine, amoxapine, desipramine, doxepin, maprotiline, nortriptyline, protriptyline, trimipramine, dothiepin, lofepramine);
selective serotonin reuptake inhibitors (SSRIs) (e.g. citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline);
selective serotonin‐noradrenaline reuptake inhibitors (SNRIs) (e.g. duloxetine, venlafaxine, milnacipran, desvenlafaxine);
serotonin modulators (e.g. trazodone, nefazodone, vilazodone);
monoamine oxidase inhibitors (MAOIs) (e.g. isocarboxazid, phenelzine, selegiline, tranylcypromine);
atypical agents (e.g. bupropion (noradrenergic and dopaminergic reuptake inhibitor), mirtazapine (noradrenergic and specific serotoninergic antidepressant), reboxetine (noradrenergic reuptake inhibitor), agomelatine, mianserin, maprotiline). Non‐conventional herbal products (e.g. St John's wort) will not be included in this review due to unknown interactions between herbal products and ART.
There was no minimum dose requirement for inclusion.
We included studies where another adjunctive therapy (e.g. psychological therapy) was provided equally in all study arms.
Comparator interventions
Drugs in the above classes.
Placebo.
Changes made to the types of included interventions after protocol publication are detailed in the Differences between protocol and review section.
Types of outcome measures
Primary outcomes
Improvement in depression: (dichotomous: yes/no; or continuous) measured by rating scales such as the Beck Depression Inventory (Beck 1961), Patient Health Questionnaire (Spitzer 1999), Hamilton Depression Rating Scale (HAM‐D; Hamilton 1980), Montgomery‐Åsberg Depression Rating Scale (MADRS; Montgomery 1979), the Center for Epidemiologic Studies Depression Scale (CESD‐R; Eaton 2004) or Clinical Global Impression of Improvement (CGI‐I; Guy 1976).
Study dropouts: rate/proportion after initiation of study intervention.
Secondary outcomes
Adverse effects: we reported both serious and mild/moderate adverse effects as classified in the Adverse Event Toxicity Scale. Using this scale, grade 1 and 2 denote mild to moderate symptoms, grade 3 denotes serious symptoms and grade 4 denotes life‐threatening events requiring significant clinical intervention (DAIDS 2009).
Improvement in virological or immunological or clinical antiretroviral treatment outcomes (or a combination of these): as determined by the clinical trial.
Quality of life: as measured by a quality of life measurement instrument specified in the study, which may have evaluated: employment, health, leisure, living situation and relationship or other domains and presented these as a scale (Connell 2014). Examples of such scales included, but were not limited to, the Quality of Life Depression Scale (QLDS) and Quality of Life and Satisfaction Questionnaire (QLESQ) (Endicott 1993; Tuynman‐Qua 1997).
Health clinic attendance and hospitalizations: frequency of events.
Employment status: proportion employed.
Deaths, including suicide: proportion died.
For explanation of rating scales see Appendix 1.
Timing of outcome assessment
When studies reported different time points for outcome assessment, the primary outcomes were assessed after a minimum of six weeks and up to a maximum of 12 weeks of antidepressant therapy or placebo administration. The time point closest to eight weeks was given preference.
Secondary outcomes were assessed up to 12 months after the intervention was initiated. We planned to divide these outcome assessment periods into three time frames: zero to three months, more than three to six months and more than six to 12 months. The latest time point within each period was given preference. The zero‐ to three‐month time point was chosen for the 'Summary of findings' tables for these outcomes.
For adverse effects, outcomes reported up 12 weeks after treatment initiation were considered. The time point closest to eight weeks was given preference for reporting in the 'Summary of findings' tables.
Hierarchy of outcome measures
Where the HAM‐D was used to assess the outcome, this was the preferred outcome measure.
Outcome measures were selected according to the hierarchy below:
HAM‐D score;
HAM‐D dichotomous measure;
Montgomery‐Åsberg Depression Rating Scale (MADRS) score;
Center for Epidemiologic Studies Depression Scale (CESD‐R) score;
Clinical Global Impression of improvement (CGI‐I) score.
Changes to study outcomes after protocol publication are detailed in the Differences between protocol and review section.
Search methods for identification of studies
Specialised Register of the Cochrane Common Mental Disorders Group
The Cochrane Common Mental Disorders Group has a specialized register of RCTs, the CCMD‐CTR. This register contains over 39,000 reference records (reports of RCTs) for anxiety disorders, depression, bipolar disorder, eating disorders, self‐harm and other mental disorders within the scope of this Group. The CCMD‐CTR is a partially studies‐based register with more than 50% of reference records tagged to about 12,500 individually PICO (Patient/Problem, Intervention, Comparison, Outcome) coded study records. Reports of trials for inclusion in the register are collated from (weekly) generic searches of MEDLINE (from 1950), Embase (from 1974) and PsycINFO (from 1967), quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review‐specific searches of additional databases. Reports of trials are also sourced from international trial registries, drug companies, handsearching of key journals, conference proceedings, and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies (used to identify RCTs) can be found on the Group's website with an example of the core MEDLINE search displayed in Appendix 2.
Electronic searches
The Cochrane Group's Information Specialist searched the CCMD‐CTR‐Studies Register (to 6 June 2015) using the following terms: Condition = depress* and Comorbidity = HIV. Study records were manually screened for pharmacotherapy trials. The information specialist ran an additional search of the CCMD‐CTR‐References Register at this time using a more sensitive set of terms to find additional untagged, uncoded references (Appendix 3).
Routine databases
We conducted complementary searches on 15 June 2015, 6 June 2016 and again on 18 April 2017 on the following databases:
the Cochrane Library (CENTRAL, CDSR, HTA, DARE) for RCTs and systematic reviews;
PubMed (current year) to retrieve records not yet indexed on Ovid MEDLINE (e.g. Epubs ahead of print);
A cited reference search on the Web of Science (WoS) for reports of included studies;
Embase (Ovid)
Detailed search strategies for these databases can be found in Appendix 4.
International trial registries
We searched the following registries for unpublished or ongoing studies (from 1 Jan 1980 till 18 April 2017):
ClinicalTrials.gov (www.clinicaltrials.gov);
World Health Organization (WHO) International Clinical Trials Portal (ICTRP) (apps.who.int/trialsearch/);
HIV and AIDS ‐ clinical trials (www.nhs.uk/Conditions/HIV/Pages/clinical‐trial.aspx).
Searching other resources
Grey literature
We searched the following grey literature sources (from 1 Jan 2014 till 18 April 2017):
International AIDS Society Online Resource Library (library.iasociety.org/GlobalSearch.aspx);
RAND's Publication database (www.rand.org/search.html).
Reference lists
We checked the reference lists of all included studies and relevant systematic reviews to identify additional studies missed from the original electronic searches (e.g. unpublished or in‐press citations).
Correspondence
We contacted trialists and subject experts for information on unpublished or ongoing studies or to request additional trial data.
Changes to the search strategy after protocol publication are detailed in the Differences between protocol and review section.
Data collection and analysis
Selection of studies
Two review authors (IEW and DA) independently evaluated the studies identified by keyword searches, by reading abstracts to see if they met the inclusion criteria. We obtained the full articles for those studies that were potentially eligible and further decided on study eligibility with the aid of a study eligibility form. Studies were scrutinized to eliminate duplication of publication. All disagreements were resolved by a third review author (JJ). For the final search conducted in 2017, Marcel Kitenge at the Centre for Evidence Based Health Care (see Acknowledgements) and IEW conducted abstract screening.
Data extraction and management
For included studies, one review author (IEW) entered data using a data collection tool (excel spreadsheet) and a second reviewer (DA) checked the entries.
Main planned comparisons
Comparison 1: antidepressant versus placebo. This was stratified by drug class as stipulated in Types of interventions above. We conducted a pooled analysis with effect estimate if the studies were appropriate for a meta‐analysis.
Comparison 2: antidepressant versus other antidepressant. This was stratified by drug class as stipulated in Types of interventions above. We conducted a pooled analysis with effect estimate if the studies were appropriate for meta‐analysis.
Assessment of risk of bias in included studies
Two review authors (IEW and DA) examined the components of each included study for risk of bias using a standardized form. This included detailed information on sequence generation, allocation concealment, blinding (participants, personnel and outcome assessor), incomplete outcome data, selective outcome reporting and other sources of bias. We assessed the methodological components of the studies and classified these as adequate (low risk of bias), inadequate (high risk of bias) or unclear as explained in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2009). The likely magnitude and direction of biases and their likely impact on the findings was also assessed and reported. Where our judgement was uncertain, we attempted to contact the study authors.
Measures of treatment effect
Dichotomous data
We reported outcome measures for dichotomous data (e.g. less than 50% reduction in HAM‐D score) as risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
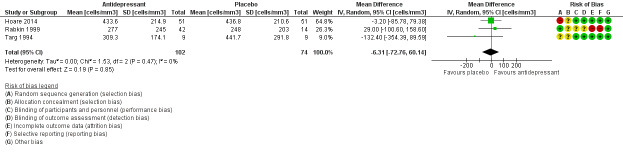
We reported continuous data (e.g. change in depression symptom scale) using the mean difference (MD) scores and standard deviations (SD). If mean change score was reported or calculated but no SD was available for this measure (Mauri 1994), we imputed the SD using the SD from the follow‐up score or we used the available P values and the calculator available in the Revman RevMan 2014 software to determine the SD (Targ 1994). If MD scores and SDs where not available for most of the studies in the analyses, we used mean follow‐up scores (Analysis 2.1). As studies used different versions of the HAM‐D score to measure the primary outcome, we calculated the standardized mean difference (SMD) and presented this as the effect estimate. We assumed an SMD of 0.2 to represent a small effect, 0.5 a medium effect and 0.8 a large effect according to Cohen 1988.
2.1. Analysis.
Comparison 2 Selective serotonin reuptake inhibitors (SSRI) versus tricyclic antidepressants (TCA), Outcome 1 Improvement in depression: HAM‐D score: continuous (follow‐up score).
Accessing primary data
Where possible, we contacted authors for their primary data if all outcomes were not reported in the published study. Hoare 2014 provided their analysis data set. From these data set, we extracted results on CGI‐I score, dichotomized HAM‐D score and calculated the SDs for mean HAM‐D change scores using Stata version 14.
Unit of analysis issues
There were no cluster‐randomized trials or cross‐over trial for analysis in this review. For studies with more than one intervention group compared to placebo, the results and number of participants from the placebo arm were divided. Each intervention group was then compared to the halved placebo group (Differences between protocol and review).
Dealing with missing data
Where possible, we contacted original investigators to request missing data. We analysed data as intention to treat for all categorical data. We conducted an available‐case analysis for continuous data (Differences between protocol and review).
Assessment of heterogeneity
As we anticipated heterogeneity between studies, we chose to use random‐effects models to generate pooled effects. We assessed statistical heterogeneity using the Chi2 test for heterogeneity (P < 0.1), and quantified it using the I2 statistic (Deeks 2011). We intended to explore statistical heterogeneity with subgroup analyses.
Heterogeneity was interpreted as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
There were not enough studies to assess publication bias using a funnel plot.
Data synthesis
The analysis was performed using the latest version of Review Manager 5 (RevMan 2014). When trials were considered clinically and methodologically suitable, we conducted meta‐analyses using a random‐effects model. For outcome measures for dichotomous data (e.g. relief or not of depression), we reported RR with 95% CIs. For continuous data (e.g. change in depression symptom scale), we used MD and SDs. If different psychometric scales were used between trials, we calculated the SMD. We used mean change scores were possible (Analysis 1.1); if these were not available, we used mean follow‐up scores in the data synthesis (Analysis 2.1). We presented a narrative synthesis for outcomes where data could not be pooled.
1.1. Analysis.
Comparison 1 Antidepressant versus placebo, Outcome 1 Improvement in depression: HAM‐D score: continuous (mean change).
Subgroup analysis and investigation of heterogeneity
Planned subgroup analyses included:
studies where participants were receiving ART versus no ART;
studies where a large proportion of participants were defined as having clinical or immunological AIDS (according to the international criteria used during the study period) as compared to studies where most participants were not categorized as having AIDS.
Most studies included both people who received ART and people who did not, making it difficult to distinguish between these subgroups for analysis 1. In addition, the type of ART received varied substantially over the time when the studies were conducted making the studies less comparable. Therefore, we only conducted subgroup analysis 2.
Sensitivity analysis
We conducted a sensitivity analysis of studies with a low risk of attrition, detection and performance bias.
'Summary of findings' table
We used GRADEpro version 3.6 to create 'Summary of findings' and evidence profile tables. The GRADEpro software was developed as part of a larger initiative led by the GRADE Working Group. GRADE offers a system for rating quality of evidence in systematic reviews and guidelines and grading strength of recommendations in guidelines (Guyatt 2011). Use of GRADEpro within a Cochrane systematic review facilitates the process of presenting and grading evidence transparently (ims.cochrane.org/revman/other‐resources/gradepro/about‐gradepro). In determining the quality of evidence for each outcome, we integrated both the efficacy results and the assessment of the risk of bias into a final assessment of the level of evidence and provide full details of the decision in footnotes.
Outcomes reported in the 'Summary of finding' tables included:
improvement in depression: HAM‐D score (continuous); at time point closest to eight weeks (range six to 12 weeks);
improvement in depression: HAM‐D score (dichotomized); at time point closest to eight weeks (range six to 12 weeks);
improvement in depression: CGI‐I score (score of 1 or 2); at time point closest to eight weeks (range six to 12 weeks);
study dropouts; at the end of the study period (range six to 12 weeks);
adverse effects reported during the entire study period (proportion of participants experiencing events);
follow‐up CD4 count (at the end of the study period, six to 12 weeks);
quality of life score (at the end of the study period, six to 12 weeks).
Results
Description of studies
Results of the search
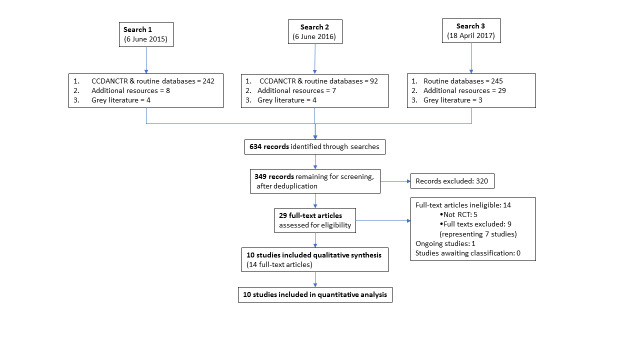
We conducted searches up to 18 April 2017 (first search 6 June 2015). The results of all searches are presented in a PRISMA flow diagram (Figure 2). Details of the excluded and ongoing studies are presented in the Characteristics of excluded studies and Characteristics of ongoing studies tables.
2.
PRISMA diagram
From the first search, we retrieved 58 records from the Cochrane Common Mental Disorders Group's specialised trials register and an additional 184 records from other databases (86 references from PubMed, 97 from CENTRAL/CRSO and one from DARE). Searches of international trial registries yielded three further records from ClinicalTrials.gov, one from the WHO ICTRP and four from the HIV/AIDS ‐ Clinical Trial register. Grey literature searches of International AIDS Society Online Resource Library and RAND's Publication database yielded an additional four records.
A second search conducted on 6 June 2016 identified a further 103 records (92 from CCMD‐CTR and other databases, seven from additional resources and four from grey literature).
A third search conducted on 18 April 2017 was during a period when the CCMD‐CTR was no longer up to date and therefore this search did not include outputs from this database. This search yielded an additional 277 records (245 from routine databases, 29 from additional resources and three from grey literature).
These searches in combination identified 634 records. After deduplication, we screened the titles and abstracts of 349 records and excluded 320 as obviously ineligible. We assessed 29 full‐text articles for eligibility. Fourteen articles representing 10 studies were eligible for inclusion. We excluded 14 articles with reasons (see Characteristics of excluded studies table). We found one ongoing study (see Characteristics of ongoing studies table).
Included studies
The review included 10 studies. The details of the individual studies are in the Characteristics of included studies tables. A summary of the main characteristics of all studies is presented in Table 4. Details of scales used in the included studies are presented in Appendix 1.
1. Summary of included studies.
| Author/ year | Country |
Randomized (n) |
Men (n; %) |
Baseline HAM‐D (score; SD)a |
CD4 count or HIV stage (mean; cells/mm3; SD)a |
ART (%)d |
Antidepressant | Placebo/comparison antidepressant | Adjunctive psychotherapy | Duration (weeks) | Dropouts (n; %) |
| Mauri 1994 | Italy | 26 | 19 (73%) | Fluv: 30 (1.3) P: 30 (6.9) |
9 had AIDS; 11 died within 1 year | 76% | Fluvoxamine | Placebo | No | 8 | NR |
| Rabkin 1994 | USA | 97 | 92 (95%) | I: 18 (4.1) P: 16 (4.1) |
301 (202) 341 (258)b |
53‐66%b | Imipramine | Placebo | No | 6 | 17 (18%) |
| Targ 1994 | USA | 20 | 20 (100%) | F: 21 (5.3) P: 20 (4.0) |
330 (145) 495 (176) |
100% | Fluoxetine | Placebo | Yes | 12 | 2 (10%) |
| Elliott 1998 | USA | 75 | 70 (93%) | 24.3 (5.7) | 368 (307) | 25% | Paroxetine | Imipramine/placebo | No | 12 | 41 (58%) |
| Zisook 1998 | USA | 47 | 47 (100%) | F: 20.2 (NR) P: 20 (NR) |
NR | 80% | Fluoxetine | Placebo | Yes | 7 | 10 (21%) |
| Rabkin 1999 | USA | 120 | 117 (98%) | F: 20 (4.7) P: 19 (5.1) |
295 (287) | 47% | Fluoxetine | Placebo | No | 8 | 33 (28%) |
| Schwartz 1999 | USA | 14 | 0 (0%) | F: 21 (6.0) D: 22 (10.8) |
F: 167 (unk) D: 191 (unk) |
Unk | Fluoxetine | Desipramine | No | 6 | 2 (14%) |
| Rabkin 2004 | USA | 85 | 85 (100%) |
F: 18 (4.5) P: 17 (3.3) |
F: 361 (237) P: 550 (359) |
F: 72% P: 74% |
Fluoxetine | Placebo | No | 8 | 25 (29.4%) |
| Patel 2013 | India | 70 | 30 (43%) | E: 36 (6) M: 38 (7) |
Unk | 100% | Escitalopram | Mirtazapine | No | 8 | 8 (11%) |
| Hoare 2014 | South Africa | 105 | 15 (14%) | E: 20 (5.5) P: 21 (5.2) |
E: 426 P: 350 c |
Unk | Escitalopram | Placebo | No | 6 | 3 (3%) |
ART: antiretroviral therapy; D: desipramine; E: escitalopram; F: fluoxetine; Fluv: fluvoxamine; HAM‐D: Hamilton Depression Rating Scale; I: imipramine; M: mirtazapine; n: number of participants; NR: not reported; P: placebo; SD: standard deviation; unk: unknown.
aPresented according to what was available in the publications.
bResults presented for 'completers' and 'non‐completers' of the study protocol.
cMedian values.
dReceiving at least one antiretroviral drug.
Design
All studies were RCTs. There were no cross‐over or cluster randomized trials eligible for inclusion.
Sample sizes
Rabkin 1999 had the largest sample size of the studies included in this review (120 participants). Hoare 2014 randomized 105 participants, Elliott 1998 randomized 75 participants and Rabkin 2004 randomized 85 participants to antidepressant or placebo. Patel 2013 had 70 participants and Zisook 1998 had 47 participants. Three studies had very small sample sizes with Mauri 1994 randomizing 26 participants, Targ 1994 had 20 participants and Schwartz 1999 had 14 participants. The total number of participants randomized in this review was 562.
Setting
Most studies took place in the USA (seven), the remaining three studies were conducted in South Africa, India and Italy.
Participants
Demographic characteristics
All participants were HIV‐positive and aged 18 years or older. In three studies, the participants were predominantly male (93% in Elliott 1998, 73% in Mauri 1994 and 95% in Rabkin 1994). All participants were men in Zisook 1998, Rabkin 2004, and Targ 1994. In Schwartz 1999, all participants were women. In Patel 2013, 57% were women and in Hoare 2014, 86% were women. One study did not report the gender of participants (Rabkin 1999). The mean or median age of participants reported in these studies ranged between 34 and 41 years.
Socioeconomic status
Socioeconomic details were similarly reported in some studies from the USA. Employment status and education varied between studies; in Elliott 1998, 29% were employed, in Rabkin 1994, 31% were receiving disability benefits or unemployed, in Rabkin 1999, 36% were receiving disability benefits, Schwartz 1999 reported 83% in the fluoxetine group and 88% in the desipramine group had "less than college education." Participants in Zisook 1998 had 13.4 and 13.5 total years of education in the two study arms and those in Targ 1994 had an average of 15.5 years of education. The participants in Elliott 1998 (75%), and Schwartz 1999 (88% or 100% depending on study arm) were predominantly single.
Diagnostic inclusion criteria
All participants were aged over 18 years and were HIV positive. Due to the different time periods when the studies were conducted, two different versions of DSM criteria were used; five studies used DSM‐III‐R (Mauri 1994; Rabkin 1994; Elliott 1998; Zisook 1998; Schwartz 1999), four studies used DSM‐IV (Rabkin 1999; Rabkin 2004; Patel 2013; Hoare 2014). In addition, Elliott 1998 required a HAM‐D score of 18 or more, Patel 2013 required a HAM‐D score of greater than 13 and MADRS score of greater than 19, Rabkin 1994 included people with dysthymia and required a HAM‐D score of 14 or greater, Rabkin 2004 and Rabkin 1999 also included people with dysthymia. Rabkin 2004 also included people with DSM‐IV classification of subthreshold depression. Schwartz 1999 required a HAM‐D score of greater than 14 and additionally a minimum of a 2‐point score on the depressed mood HAM‐D item. Targ 1994 did not specify DSM criteria but stated that "they met criteria for major depression or adjustment disorder with depressed mood" and had a score 16 or more on the HAM‐D scale. Mauri 1994 included people categorized as having adjustment disorder with depressed mood.
Diagnostic exclusion criteria
Most studies excluded people with alcoholism or substance abuse or dependence (or both alcohol and substance abuse problems) (Rabkin 1994; Targ 1994; Elliott 1998; Zisook 1998; Rabkin 1999; Schwartz 1999; Rabkin 2004; Hoare 2014). Six studies excluded people with evidence of dementia or cognitive impairment; Hoare 2014 defined this as a HIV Dementia Scale (HDS) score of less than 10 and Mini‐Mental State Examination (MMSE) score of less than 23, Zisook 1998 had stricter criteria and excluded people with an MMSE score of 27 or less, and Rabkin 1994 used a modified‐MMSE where a score of 50 resulted in more extensive neuropsychological testing and evaluation of eligibility. The remaining studies did not specify what method they used to evaluate cognitive function (Elliott 1998; Rabkin 1999; Rabkin 2004). Five studies used high suicide risk as an exclusion criterion (Rabkin 1994; Elliott 1998; Zisook 1998; Rabkin 1999; Rabkin 2004). Zisook 1998 evaluated suicidality as a score of 0 or 1 on item 3 of the HAM‐D. All studies except Mauri 1994 excluded people with underlying psychotic disorders or bipolar mood disorder. Mauri 1994 did not report any exclusion criteria. None of the included studies used failure of previous antidepressant regimens as an exclusion criterion.
Severity of depression at baseline
All studies reported the mean baseline HAM‐D score and SDs. In two studies, baseline HAM‐D scores indicated severe depression (score greater than 24); Mauri 1994 reported a baseline HAM‐D score of 30.37 (SD 1.31) and Patel 2013 reported a baseline score of 36 (SD 6) in the escitalopram group and 38 (SD 7) in the mirtazapine group. Six studies reported baseline HAM‐D scores of between 18 and 24 indicating moderate depression among participants (Targ 1994; Elliott 1998; Zisook 1998; Rabkin 1999; Schwartz 1999; Hoare 2014). Participants in Rabkin 1994 had the lowest baseline HAM‐D score of 17.5 (SD 4.1) indicating mild to moderate baseline level of depression in this study. Rabkin 2004 also recruited participants with low baseline levels of depression where the HAM‐D score was 18.2 (SD 4.5) in the fluoxetine arm and 16.8 (SD 3.3) in the placebo arm.
HIV illness severity
Several studies had participants with mild to moderate clinical HIV disease and immunosuppression. Baseline CD4 T‐cell count were reported as: 368 cell/mm3 (Elliott 1998), 425.5 cells/mm3 and 350 cells/mm3 between study arms (Hoare 2014), 301 cells/mm3 (Rabkin 1994), 330.2 cells/mm3 and 494.5 cells/mm3 between study arms (Targ 1994), 295 cells/mm3 (Rabkin 1999), and 455 cells/mm3 (Rabkin 2004). Zisook 1998 included only people with Centers for Disease Control and Prevention (CDC) (1993) category A or B HIV clinical disease. Elliott 1998 excluded people with any "severe concurrent HIV related illness" at initial screening. Patel 2013 excluded people with "abnormal lab results or serious disease" and restricted inclusion to people who had taken ART for six months or longer. Rabkin 1999 required participants to be "physically healthy" except for HIV‐related conditions for which they needed to be receiving treatment. They also excluded people with HIV wasting syndrome, diarrhoea or unstable health. Rabkin 1994 and Rabkin 2004 also specified that included participants had to be "medically stable." Participants included in the Schwartz 1999 and Mauri 1994 studies were more immunocompromised than in the remaining studies; Schwartz 1999 reported a lower mean CD4 T‐cell count of 167 cells/mm3 and 191 cells/mm3 among participants in the two arms, however, they excluded people with "serious concurrent HIV‐related physical illness" at screening; in the Mauri 1994 study, authors reported that 35% of participants had clinical AIDS at baseline with 42% of participants having died within one year of study completion.
Antiretroviral therapy
The use of ART was variable, different proportions of study participants received ART and among these, one or two antiretroviral medications was most common. In Elliott 1998, 25% were taking HIV‐related medications (e.g. dapsone) or an 'antiviral,' Mauri 1994 reported 73% were receiving zidovudine (AZT; also known as azidothymidine) monotherapy, 64% of participants in the Rabkin 1994 study were also reported to be receiving an 'antiviral' and Rabkin 1999 reported 47% of participants taking one or two antiretroviral medications. About 80% of people referred to the Zisook 1998 study were receiving at least one ART (most commonly AZT). Targ 1994 only included people receiving AZT. Patel 2013 included only people receiving highly active antiretroviral therapy (HAART), indicating that these people were likely receiving three antiretroviral medications. In Rabkin 2004, 72% to 74% of participants were receiving two or more antiretroviral agents. Hoare 2014 and Schwartz 1999 did not comment on the use of ART in their study population.
Interventions
Antidepressant versus placebo
Eight studies compared antidepressants with placebo. Duration of randomized assignments in these studies ranged between six and 12 weeks. Among these, six studies compared SSRIs (fluoxetine, fluvoxamine and escitalopram) with placebo (Mauri 1994; Targ 1994; Zisook 1998; Rabkin 1999; Rabkin 2004; Hoare 2014), one trial compared a TCA (imipramine) with placebo (Rabkin 1994), and one trial had three arms comparing an SSRI (paroxetine) with a TCA (desipramine) and a placebo (Elliott 1998).
Four trials compared fluoxetine versus placebo. In Rabkin 1999, the fluoxetine dose at initiation was 20 mg. If response was poor, the dose was increased every two weeks by 20 mg if this could be tolerated. The comparison arm received placebo. Similarly, Rabkin 2004 administered fluoxetine at 20 mg to 40 mg per dose. This study included a visually identical placebo tablet as well as a placebo injection (this was a three‐armed study with the third arm receiving placebo tablets and testosterone intramuscularly; see Characteristics of included studies table). In Zisook 1998, participants were initiated on fluoxetine 20 mg, which they received for three weeks; this could have been increased to 40 mg at week four and 60 mg at week five or the dose could be decreased. Targ 1994 similarly randomized participants to fluoxetine 20 mg or placebo (no dose adjustments were specified).
In Hoare 2014, participants were randomized to receive either escitalopram 10 mg or placebo. Prior to randomization, participants were given four to 10 days of single‐blind placebo to exclude early placebo responders and people with poor compliance. Mauri 1994 compared fluvoxamine 100 mg to 150 mg with placebo. In Elliott 1998, participants started with paroxetine 10 mg daily and were increased to 40 mg by week two. This was compared to imipramine (increased from 50 mg to 200 mg by week two) and placebo in a three‐armed study lasting 12 weeks. Rabkin 1994 was the only study that did not evaluate an SSRI. They compared imipramine (initiated at 50 mg and increased in 50 mg increments to 300 mg depending on clinical response) with a matching placebo.
Antidepressant versus other antidepressant
Two studies compared different antidepressant classes: escitalopram compared with mirtazapine (Patel 2013), and fluoxetine compared with desipramine (Schwartz 1999). Patel 2013 administered mirtazapine 15 mg daily (titrated up to 30 mg if improvement in HAM‐D/MADRS at four weeks was less than 20% and reduced by 5 mg daily if participants reported adverse effects) with escitalopram 10 mg also adjusted up to 20 mg based on response in scores or downtitrated by 7.5 mg if there were adverse effects. Schwartz 1999 compared desipramine 75 mg to 100 mg at night with fluoxetine 20 mg to 40 mg in the morning over a six‐week period.
Adjunctive psychotherapy
Two of the studies evaluating SSRIs versus placebo also provided adjunctive psychotherapy in both arms of the study. In Zisook 1998, participants in both arms were assigned to a concomitant supportive and educative psychotherapy group. A male licensed clinical social worker and female predoctoral level psychology graduate student who were blind to study drug assignment conducted groups. The group emphasized education about HIV, depression, mutual support, sharing, coping strategies and utilizing community resources. Participants were required to attend the group for the full seven‐week study duration. Targ 1994 provided adjunctive psychotherapy in both the fluoxetine and placebo group. Structured group therapy was provided including: relaxation techniques training, problem‐solving skills training, didactic presentations and open discussions. Three psychotherapy groups of six to eight participants were run by fourth year psychiatry residents. The groups were standardized through weekly supervision of the residents.
Outcomes
Primary outcome assessment
There were two primary outcomes evaluated in this review; improvement in depression and study dropout rate (see Appendix 1 for definitions of scoring systems used).
Improvement in depression
Improvement in depression was evaluated by several measures; however, a comparison of change in HAM‐D score as a continuous variable between intervention and comparison groups was the preferred primary outcome measure in this review. Of the 10 studies, four reported improvement in depression between groups using the 21‐item HAM‐D score (Rabkin 1994; Elliott 1998; Rabkin 1999; Rabkin 2004). The remaining studies used the 17‐item HAM‐D score. Eight studies reported HAM‐D score as a continuous measure (Mauri 1994; Rabkin 1994; Targ 1994; Zisook 1998; Rabkin 1999; Schwartz 1999; Patel 2013; Hoare 2014). Seven studies reported a 50% reduction in HAM‐D score at study completion as a measure of remission/response (Elliott 1998; Zisook 1998; Rabkin 1999; Schwartz 1999; Rabkin 2004; Patel 2013; Hoare 2014). Six studies reported the CGI‐I score as an outcome measure (Elliott 1998; Zisook 1998; Rabkin 1999; Schwartz 1999; Patel 2013; Hoare 2014). All studies categorized responders as those with a CGI‐I score of one or two. MADRS score was less commonly used to report outcomes; only two studies reported this and both studies presented it as a continuous outcome (Patel 2013; Hoare 2014).
Study dropouts
Nine studies reported study dropouts at the end of the study period (Rabkin 1994; Targ 1994; Elliott 1998; Zisook 1998; Rabkin 1999; Schwartz 1999; Rabkin 2004; Patel 2013; Hoare 2014).
Secondary outcome assessments
Adverse effects
Nine studies reported adverse effects (Mauri 1994; Rabkin 1994; Elliott 1998; Zisook 1998; Rabkin 1999; Schwartz 1999; Rabkin 2004; Patel 2013; Hoare 2014). Targ 1994 only reported "few side‐effects." Four studies used the Systematic Assessment for Treatment Emergent Effects (SAFTEE) tool (Rabkin 1994; Elliott 1998; Rabkin 1999; Rabkin 2004), and one study used the Dosage Record and Treatment of Symptoms Scale (DOTES) tool to monitor treatment emergent adverse effects (Mauri 1994). The studies performed safety assessments at one to four weekly intervals.
Improved antiretroviral therapy response
There were no studies specifically evaluating virological suppression on ART. However, three studies reported changes in immunological status over the study period by reporting the mean CD4 count for participants at baseline and at study termination (Targ 1994; Rabkin 1999; Hoare 2014).
Quality of life
Two studies reported quality of life measures (Elliott 1998; Rabkin 1999). Both studies used the QLESQ score to measure quality of life at baseline and at study termination. Rabkin 2004 evaluated QLESQ scores but did not present these results.
Other secondary outcomes
None of the studies reported the remaining three proposed secondary outcomes: frequency of health clinic attendance and hospitalizations; employment status; and deaths, including suicide.
Excluded studies
We excluded 14 articles after reviewing full‐texts. Among these, five had the incorrect study design (not RCTs). The remaining nine articles represented seven studies. Two had a different population and intervention groups: participants were HIV uninfected and the studies evaluated interventions to reduce HIV risk behaviour (Stein 2005; NCT00285584). Two further studies had interventions and comparisons which made them ineligible; in Tsai 2013, the intervention was directly observed antidepressant treatment compared to standard of care and the same antidepressant was given to both arms of the study, Brown 2016 randomized adolescents to cognitive behavioural therapy and a medication management algorithm compared to treatment as usual. In Chibanda 2014, the comparison group received a psychotherapeutic intervention and no antidepressant or placebo. In Markowitz 1998 the inclusion criteria did not include DSM or ICD diagnostic criteria for diagnosis of a depressive illness but rather HAM‐D rating and clinical judgement.
Ongoing studies
We found one ongoing study (NCT02620150; see Characteristics of ongoing studies table).
Studies awaiting classification
There were no studies awaiting classification.
Risk of bias in included studies
For details of the risk of bias judgements for each study, see Characteristics of included studies table. A graphical representation of the overall risk of bias in included studies is presented in Figure 3 and Figure 4.
3.
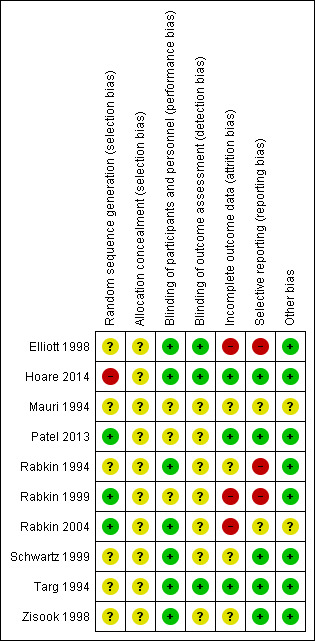
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
4.
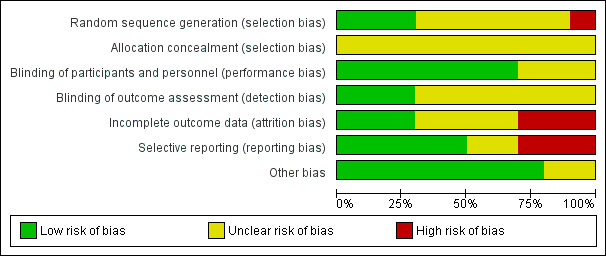
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
We used the Cochrane 'Risk of bias' assessment tool to evaluate each study with regard to the risk of bias in: random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective reporting and other sources of bias.
Allocation
Random sequence generation
Few included studies specified the method of random sequence generation. Hoare 2014 used a semi‐random sample of consecutively screened people, which put this study at high risk of bias for the domain. There was a low risk of bias in three studies: Patel 2013 used a computer‐generated list of random numbers and Rabkin 1999 utilized computer‐generated block randomization in a ratio of 2:1 fluoxetine to placebo. Rabkin 2004 also used computer‐generated block randomization. It was unclear if there was any bias in this domain for the remaining studies as they did not report sequence generation methods.
Allocation concealment
It was also unclear what bias may have existed in the included studies with regard to allocation concealment as none of the included studies commented on this aspect of study design and conduct.
Blinding
Blinding of participants and personnel
Seven studies were at low risk of bias with regard to blinding of participants (these were placebo‐controlled studies). None of the studies reported whether personnel were blinded. Author communication with Hoare 2014 confirmed blinding of personnel in addition to participants.
Three studies were at unclear risk of performance bias. It was unclear if participants or personnel were blinded in Mauri 1994. The authors stipulated that the study was conducted under 'double blind' conditions but provided no further details. Patel 2013 was an unblinded study and was at unclear risk of bias as the study compared two known effective antidepressants as opposed to an antidepressant versus placebo. Rabkin 1999 did not report on blinding of participants or personnel.
Blinding of outcome assessment
Three studies reported blinding of outcome assessment. Targ 1994 and Hoare 2014 (author communication) reported that outcome assessors were blinded. Elliott 1998 similarly reported that outcome assessments were made by staff who were blind to study drug assignment.
Overall, blinding of participants, personnel and outcome assessors was poorly reported in these studies leaving uncertainty with regard to the amount of bias that may have occurred in this domain.
Incomplete outcome data
Three studies had a high risk of attrition bias. In Elliott 1998, 25% of participants had dropped out at four weeks and by 12 weeks 58% had dropped out. The reasons for dropout were unevenly distributed between the three groups with more adverse effects in the imipramine group compared to the placebo and paroxetine groups, although the numbers in these subgroups were small. Similarly in Rabkin 1999, the high dropout rate of 27.5% could have biased the results; there were systematic differences between dropouts and completers as dropouts had milder depressive symptoms at baseline. Most dropouts in this study (73%) were in the fluoxetine arm. Rabkin 2004 had a similarly high proportion of dropouts of 29% in the placebo and fluoxetine arms.
It was unclear if there were any dropouts in the Mauri 1994 study as the results only showed statistical analysis with no total numbers and no comments with regard to this in the paper. In Schwartz 1999, five participants were excluded after screening due to substance abuse not previously detected; it was uncertain whether these were excluded after randomization or whether they were equally distributed in both groups. A further two participants dropped out during the study from the desipramine group. As the number of participants in the study was small (14), it was possible that these dropouts could have biased the results. In Zisook 1998, 21% of people randomized did not complete the study. There did not seem to be systematic differences between those who dropped out in either group. However, due to the similarly small number of participants in each group, these dropouts could have biased the results. In Rabkin 1994, 18% of people randomized dropped out. There did not seem to be any systematic differences between these groups at baseline and the reasons for dropout were comparable between groups, we assessed this study at unclear risk of attrition bias.
Three studies reported low dropout rates (Hoare 2014 2.9%; Patel 2013 11%; Targ 1994 10%). These studies were assessed at low risk of bias.
In Elliott 1998, the last observation from four weeks of treatment was carried forward for a portion of the study population. It is possible that an antidepressant effect would not have been detectable after this short duration of treatment. If such participants were unevenly distributed between the study arms this could have biased the results towards no effect in one of the study arms.
Selective reporting
Most studies were conducted more than a decade ago (pre‐2007) and few protocols were available for assessment. Therefore, selective reporting was difficult to assess fully.
In Rabkin 1999, the CGI‐I score was the only result reported as intention‐to‐treat, all other results were only reported for people who completed the study period. This suggests some bias in the way these results were presented.
Elliott 1998 presented the HAM‐D score as a dichotomous categorical outcome (greater than 50% reduction in HAM‐D score from baseline). There was no report of mean baseline scores in the treatment arms and follow‐up HAM‐D scores were only presented in figures without SDs or any representation of the uncertainty around the estimates. This study also described using the BSI assessment tool in their methodology and the findings from this were not presented in the results. A supplementary report from this study presented quality of life data for responders and non‐responders as opposed to the randomized treatment arms. This suggests reporting bias in this study.
It was unclear in Rabkin 1994 if there was any reporting bias as results of CGI‐I scores were reported as a composite with HAM‐D score as 'responders' (CGI rating of 1 or 2 and decline in HAM‐D by 50% and HAM‐D score of less than 8 at week six) or 'non‐responders.' Mean change in HAM‐D score was not presented. Rabkin 2004 described the measurement of BSI and QLESQ scores but did not report these results.
We assessed several studies as having a low risk of reporting bias. Patel 2013 reported results of several commonly used scales including MADRS, HAM‐D and CGI with both statistically 'significant' and 'non‐significant' findings. In Hoare 2014, Hospital Anxiety and Depression Scale (HADS) was listed in the secondary outcomes measures in the study methodology but not reported in results; however, this is unlikely to have an impact on the overall study outcome as this was not a key measure of depression and all other commonly used scales such as MADRS, HAM‐D and CGI were fully reported, and non‐significant results were also reported. We did not detect any evidence of reporting bias in the remaining studies.
Other potential sources of bias
For Mauri 1994 the authors provided very limited information on the study methods, so for this study it is possible that there may have been bias which we could not detect.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Antidepressant compared to placebo for depression in adults with HIV infection.
| Antidepressant compared to placebo for depression in adults with HIV infection | ||||||
| Patient or population: adults living with HIV and depression Setting: global Intervention: antidepressant therapy: SSRI or TCA Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with antidepressant | |||||
| Improvement in depression at 6‐12 weeks: HAM‐D score (continuous; standardized mean difference) | ‐ | The mean change in the antidepressant group was 0.59 standard deviations higher (0.21 higher to 0.96 higher) | ‐ | 357 (6 RCTs)1 | ⊕⊕⊝⊝ Low2,3 | There was a clinically relevant improvement in depression; however, the quality of evidence contributing to this outcome was low and the result should be interpreted with caution. |
| Improvement in depression: HAM‐D score at 6‐12 weeks (dichotomized) | 449 per 1000 | 494 per 1000 (400 to 607) | RR 1.10 (0.89 to 1.35) | 434 (5 RCTs) | ⊕⊕⊝⊝ Low4 | There was no evidence of an improvement in depression according to this measure. The quality of evidence contributing to this outcome was low and the result should be interpreted with caution. |
| Improvement in depression at 6‐12 weeks: CGI‐I (score of 1 or 2) | 412 per 1000 | 527 per 1000 (383 to 729) | RR 1.28 (0.93 to 1.77) | 346 (4 RCTs) | ⊕⊕⊝⊝ Low4 | There was no evidence of an improvement in depression according to this measure. The quality of the evidence contributing to this outcome was low and the result should be interpreted with caution. |
| Study dropouts at 6‐12 weeks | 237 per 1000 | 303 per 1000 (216 to 427) | RR 1.28 (0.91 to 1.80) | 306 (4 RCTs) | ⊕⊕⊕⊝ Moderate5 | Moderate quality evidence suggested that there was no difference in the number who dropout between antidepressant and placebo. |
| Adverse effects during antidepressant treatment period (0‐12 weeks) | 575 per 1000 | 506 per 1000 (368 to 696) | RR 0.88 (0.64 to 1.21) | 167 (2 RCTs) | ⊕⊝⊝⊝ Very low2,6 | We cannot be sure of a difference in the occurrence of adverse effects when antidepressants are compared to placebo as the evidence contributing to this outcome was very low quality. |
| Follow‐up CD4 count at 6‐18 weeks | The mean follow‐up CD4 count was 401.6 cells/mm3 | The mean follow‐up CD4 count was 6.31 cells/mm3 lower (72.76 lower to 60.14 higher) | ‐ | 176 (3 RCTs) | ⊕⊕⊝⊝ Low7,8 | There was no evidence of a change in CD4 count in participants receiving antidepressants vs placebo. The short follow‐up period in these studies made this outcome less clinically relevant. |
| Quality of life score at 8 weeks | The mean quality of life score was 6.4 | The mean quality of life score was 3.6 points higher (0.38 lower to 7.58 higher) | ‐ | 87 (1 RCT) | ⊕⊝⊝⊝ Very low9,10 | We cannot be sure what the effects of antidepressants were compared to placebo as the quality of evidence contributing to this outcome was very low. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CGI‐I: Clinical Global Impression of Improvement; CI: confidence interval; HAM‐D: Hamilton Depression Rating Scale; RCT: randomized controlled trial; RR: risk ratio; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Included five RCTs comparing SSRI versus placebo and one RCT comparing TCA versus placebo.
2Risk of bias: downgraded once for high or unclear risk of bias related to sequence generation and allocation concealment and attrition.
3Inconsistency: downgraded once as there was moderate statistical heterogeneity (heterogeneity: Tau2 = 0.10; Chi2 = 11.67, df = 5 (P = 0.04); I2 = 57%) and marked clinical heterogeneity between studies.
4Risk of bias: downgraded twice for high risk of bias in sequence generation in one study and very high risk of attrition bias (greater than 50% dropouts in Elliott 1998), in addition no studies reported on allocation concealment.
5Risk of bias: downgraded once due to unclear risk of bias related to sequence generation and lack of allocation concealment in all studies.
6Imprecision: downgraded twice due to limited participants, number of studies and wide confidence intervals that include appreciable benefit and harm.
7Risk of bias: downgraded once due to high risk of bias in sequence generation and attrition bias.
8Imprecision: downgraded once due to few events and wide confidence intervals.
9Risk of bias: downgraded once due to high risk of attrition bias in the included study.
10Imprecision: downgraded twice due to limited number of participants and studies and wide confidence intervals.
Summary of findings 2. Selective serotonin reuptake inhibitors (SSRI) compared to tricyclic antidepressant (TCA) for depression in adults with HIV infection.
| SSRI compared to TCA for depression in adults with HIV infection | ||||||
| Patient or population: adults living with HIV and depression Setting: global Intervention: SSRI Comparison: TCA | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with TCA | Risk with SSRI | |||||
| Improvement in depression: HAM‐D score at 6‐12 weeks (continuous) | The mean change in HAM‐D score was 12 | The mean change was 3.2 points lower (10.87 lower to 4.47 higher) | ‐ | 14 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | It was uncertain whether there was any difference in HAM‐D score between TCAs and SSRIs as quality of evidence contributing to this outcome was very low. |
| Improvement in depression at 6‐12 weeks: HAM‐D score (dichotomized) | 516 per 1000 | 459 per 1000 (279 to 754) | RR 0.89 (0.54 to 1.46) | 64 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2 | It was uncertain whether there was any difference in dichotomized HAM‐D score between TCAs and SSRIs as quality of evidence was very low. |
| Improvement in depression at 6‐12 weeks: CGI‐I (score of 1 or 2) | 355 per 1000 | 440 per 1000 (241 to 795) | RR 1.24 (0.68 to 2.24) | 64 (2 RCTs) | ⊕⊝⊝⊝ Very low1,2 | It was uncertain whether there was any difference in CGI‐I score between TCAs and SSRIs as quality of evidence contributing to this outcome was very low. |
| Study dropouts at 6‐12 weeks | 548 per 1000 | 444 per 1000 (280 to 713) | RR 0.81 (0.51 to 1.30) | 64 (2 RCTs) | ⊕⊝⊝⊝ Very low2,3 | It was uncertain whether there was any difference in study dropouts between TCAs and SSRIs as quality of evidence contributing to this outcome was very low. |
| Adverse effects during antidepressant treatment period (0‐12 weeks) | 833 per 1000 | 875 per 1000 (558 to 1000) | RR 1.05 (0.67 to 1.64) | 14 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | It was uncertain whether there was any difference in adverse effects between TCAs and SSRIs as quality of evidence contributing to this outcome was very low. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CGI‐I: Clinical Global Impression of Improvement; CI: confidence interval; HAM‐D: Hamilton Depression Rating Scale; RCT: randomized controlled trial; RR: risk ratio; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Risk of bias: downgraded once for uncertain risk of bias related to sequence generation, allocation concealment and attrition.
2Imprecision: downgraded twice due to very wide confidence intervals around effect estimate, limited number of participants and events.
3Risk of bias: downgraded once for uncertain risk of bias related to sequence generation and allocation concealment.
Summary of findings 3. Selective serotonin reuptake inhibitor (SSRI) compared to atypical agent for depression in adults with HIV infection.
| SSRI compared to atypical agent for depression in adults with HIV infection | ||||||
| Patient or population: adults living with HIV and depression Setting: global Intervention: SSRI Comparison: atypical agent (mirtazapine) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with mirtazapine | Risk with SSRI | |||||
| Improvement in depression at 6‐12 weeks: HAM‐D score (continuous) | The mean change in HAM‐D score was 13 | The mean change was 9 points higher (3.61 higher to 14.39 higher) | ‐ | 70 (1 RCT) | ⊕⊕⊝⊝ Low1 | There was low quality evidence that follow‐up HAM‐D scores may be lower among participants using mirtazapine compared to an SSRI. |
| Improvement in depression at 6‐12 weeks: HAM‐D score (dichotomized) | 914 per 1000 | 859 per 1000 (722 to 1000) | RR 0.94 (0.79 to 1.11) | 70 (1 RCT) | ⊕⊕⊝⊝ Low1 | There was low quality evidence of no difference in dichotomized HAM‐D score when mirtazapine was compared to an SSRI. |
| Improvement in depression at 6‐12 weeks: CGI‐I (score of 1 or 2) | 657 per 1000 | 513 per 1000 (342 to 769) | RR 0.78 (0.52 to 1.17) | 70 (1 RCT) | ⊕⊕⊝⊝ Low1 | There was low quality evidence suggesting that there was no difference in improvement in depression between arms based on CGI‐I score. |
| Study dropouts at 6‐12 weeks | 86 per 1000 | 143 per 1000 (37 to 553) | RR 1.67 (0.43 to 6.45) | 70 (1 RCT) | ⊕⊕⊝⊝ Low1 | There was low quality evidence which suggested that there was no difference in the number of study dropouts between arms. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CGI‐I: Clinical Global Impression of Improvement; CI: confidence interval; HAM‐D: Hamilton Depression Rating Scale; RCT: randomized controlled trial; RR: risk ratio; SSRI: selective serotonin reuptake inhibitor. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Imprecision: downgraded twice due to very wide confidence intervals around effect estimate, limited number of participants and events.
Comparison 1: antidepressant versus placebo
Seven studies including 575 participants contributed data to the comparison of antidepressant versus placebo. See Table 1.
Primary outcomes
1.1 Improvement in depression: HAM‐D score continuous
Six studies including 357 participants contributed to this outcome. There was a greater reduction in HAM‐D score among participants receiving antidepressant compared to placebo (SMD 0.59, 95% CI 0.21 to 0.96) (Analysis 1.1; Figure 5). The SMD of 0.59 can be interpreted as a medium effect size. The quality of this evidence was low. There was moderate statistical heterogeneity (Tau2 = 0.13; Chi2 = 13.24, degrees of freedom (df) = 5; P = 0.02; I2 = 62%) and marked clinical heterogeneity between the studies contributing to this outcome.
5.
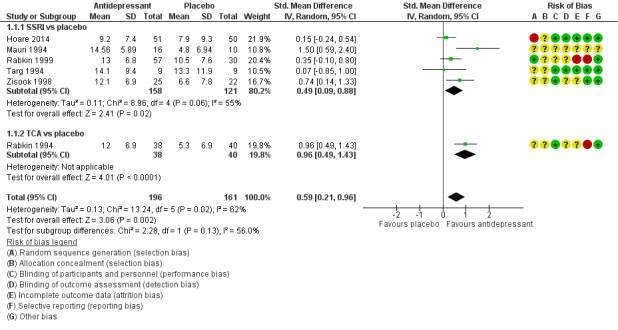
Forest plot and risk of bias assessment: Selective serotonin reuptake inhibitors (SSRI) versus placebo, outcome: 1.1 HAM‐D score.
1.2 Improvement in depression: HAM‐D score dichotomized
Five trials with 434 participants contributed to this outcome. There was no evidence of a difference in dichotomized change in HAM‐D score among participants receiving SSRIs compared to placebo (RR 1.10, 95% CI 0.89 to 1.35; I2 = 0%) (Analysis 1.2; Figure 6). The quality of this evidence was low.
1.2. Analysis.
Comparison 1 Antidepressant versus placebo, Outcome 2 Improvement in depression: HAM‐D score: dichotomized (> 50% reduction).
6.
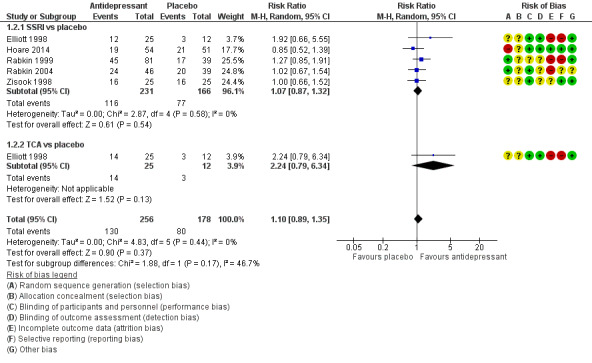
Forest plot and risk of bias assessment: Selective serotonin reuptake inhibitors (SSRI) versus placebo, outcome: 1.2 Dichotomized HAM‐D score (greater than 50% reduction).
1.3 Improvement in depression: CGI‐I score
Four trials with 346 participants contributed to this assessment. There was a slightly greater improvement in CGI‐I score (dichotomized as a score of 1 or 2 versus a higher score) among participants receiving SSRIs compared to participants receiving placebo (RR 1.28, 95% CI 0.93 to 1.77; I2 = 29%) (Analysis 1.3; Figure 7). The quality of the evidence contributing to this outcome was low.
1.3. Analysis.
Comparison 1 Antidepressant versus placebo, Outcome 3 Improvement in depression: CGI‐I (score of 1 or 2).
7.
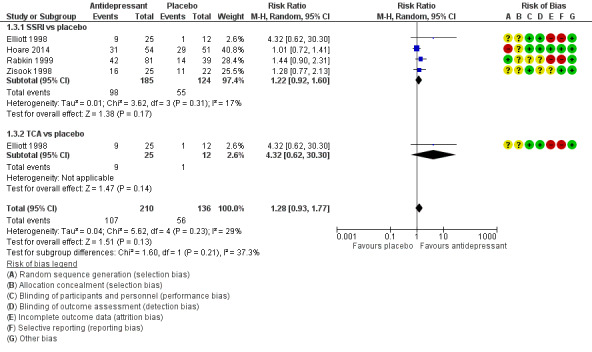
Forest plot and risk of bias assessment: Antidepressant versus placebo, outcome: 1.3 CGI‐I (score = 1 or 2).
1.4 Study dropouts
There was a wide range in the proportion of participants who dropped out in these studies (2.9% to 58%). The most common reasons cited for dropout were: adverse effects, clinical deterioration, substance abuse and loss to follow‐up. The risk of attrition bias related to dropouts is discussed elsewhere (see Incomplete outcome data (attrition bias)).
Five studies reported on proportion of dropouts by treatment arm. The pooled estimates from these studies show no evidence of a difference in the proportion of dropouts at study termination by treatment arm (RR 1.28, 95% CI 0.91 to 1.80; participants = 306; I2 = 0%) (Analysis 1.4; Figure 8). The quality of the evidence was moderate.
1.4. Analysis.
Comparison 1 Antidepressant versus placebo, Outcome 4 Study dropouts.
8.
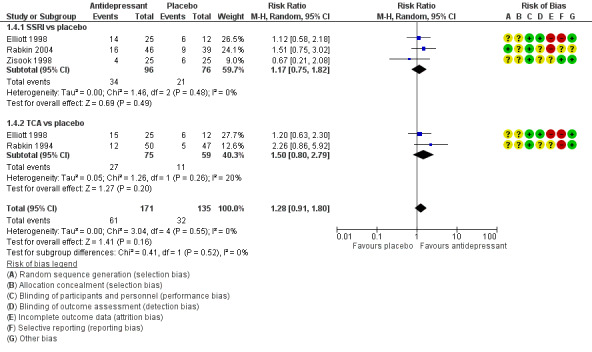
Forest plot and risk of bias assessment: Selective serotonin reuptake inhibitors (SSRI) versus placebo, outcome: 1.4 Study dropouts.
Secondary outcomes
1.5 Adverse effects
All seven studies comparing SSRI to placebo reported on adverse effects. The amount of detail provided varied considerably. Only two studies provided information on the proportion of participants experiencing any adverse effects in the placebo compared to antidepressant treatment arm. The pooled estimate from these two studies showed no difference in reported adverse effects between those receiving antidepressant or placebo (RR 0.88, 95% CI 0.64 to 1.21; participants = 167; studies = 2; I2 = 34%) (Analysis 1.5; Figure 9). The quality of evidence was very low.
1.5. Analysis.
Comparison 1 Antidepressant versus placebo, Outcome 5 Adverse effects.
9.
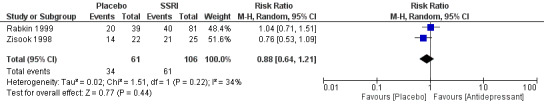
Forest plot of comparison: Antidepressant versus placebo, outcome: 1.5 Adverse effects.
Adverse effects reported for studies comparing SSRIs with placebo are presented in Table 5. Although most adverse effects occurred to some degree in both groups, features of sexual dysfunction seemed to be reported more commonly in the SSRI group. Two studies provided minimal details with regard to the occurrence of adverse effects (Mauri 1994; Targ 1994).
2. Reported adverse effects: selective serotonin reuptake inhibitors versus placebo.
| Study ID | Reported in all groups | Reported only in SSRI group | Reported only in placebo group | Dropouts due to adverse effects |
| Zisook 1998 | Nausea, headaches, diarrhoea, dry mouth, loss of appetite, agitation, fatigue, influenza‐like symptoms | Decreased libido, somnolence | Insomnia | 1/22 dropped out in placebo group due to agitation |
| Elliott 1998 | General sexual dysfunction, skin rash, sedation, nausea, poor memory/concentration, heart palpitations, fatigue, dry mouth, diarrhoea, dizziness, blurred vision, constipation, anxiety | Erectile dysfunction | Headache | 5/25 in paroxetine group; 6/25 in placebo group |
| Rabkin 2004 | Sleepiness, overstimulation, insomnia, nervousness, nausea, dry mouth, appetite loss, loose bowels, poor memory, agitation, diarrhoea, irritability, tension, easy to anger | Decreased ejaculate, headache, weight loss | Dizziness, anger | 6/30 in fluoxetine group |
| Rabkin 1999 | Upset stomach and diarrhoea, overstimulation, nervousness, sleepiness, loss of appetite, weight loss, dry mouth, sexual dysfunction | Headache | ‐ | 6/81 in fluoxetine group |
| Hoare 2014 | ‐ | Nausea, vomiting | ‐ | None |
| Mauri 1994 | ‐ | ‐ | ‐ | None |
| Targ 1994 | ‐ | ‐ | ‐ | None |
SSRI: selective serotonin reuptake inhibitor.
Among studies comparing TCAs with placebo, Elliott 1998 reported dry mouth, dizziness/postural hypotension and palpitations frequently in participants using imipramine, and in Rabkin 1994, eight participants in the imipramine group (16%) and three in the placebo group (6%) dropped out due to adverse effects. Participants receiving imipramine complained of anticholinergic adverse effects including drowsiness, headache, cognitive problems and dizziness. One participant developed a tremor. The most common adverse effect reported by participants receiving imipramine were constipation (47%), dry mouth (59%), tremors (34%), sexual dysfunction (31%) and sweating (53%).
1.6 Virological/immunological recovery
None of the included studies evaluated virological suppression.
Three trials with 176 participants reported change in mean CD4 count at follow‐up. The meta‐analysis of these studies showed no difference between SSRI and placebo in terms of follow‐up CD4 count (MD ‐6.31 cells/mm3, 95% CI ‐72.76 to 60.14 cells/mm3; I2 = 0%) (Analysis 1.6; Figure 10). The quality of the evidence was low.
1.6. Analysis.
Comparison 1 Antidepressant versus placebo, Outcome 6 Follow‐up CD4 count.
10.
Forest plot of comparison: Selective serotonin reuptake inhibitors (SSRI) versus placebo, outcome: 1.5 Follow‐up CD4 count.
1.7 Quality of life
Only one study reported on quality of life measures. Rabkin 1999 used the QLESQ score to evaluate changes in quality of life at baseline and study termination at eight weeks. There was no association between treatment arm and change in QLESQ score (MD 3.60, 95% CI ‐0.38 to 7.58; I2 = 0%) (Analysis 1.7; Figure 11). The quality of the evidence was very low.
1.7. Analysis.
Comparison 1 Antidepressant versus placebo, Outcome 7 Quality of life.
11.
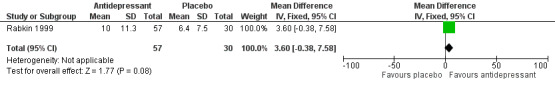
Forest plot of comparison: Antidepressant versus placebo, outcome: 1.7 Quality of life score.
Elliott 1998 compared changes in QLESQ score and Social Adjustment Scale‐Self Report (SAS) among 'responders' (reduction in HAM‐D score of greater than 50%) and 'non‐responders' after 12 weeks in the study. This study found that improvement in depression was associated with improvement of quality of life measures among all who completed the study. However, the authors did not report the differences found in the placebo versus antidepressant arms.
1.8 Frequency of health clinic attendance and hospitalizations
There were no data comparing antidepressant versus placebo for frequency of health clinic attendance and hospitalizations.
1.9 Employment status
There were no data comparing antidepressant versus placebo for employment status.
1.10 Deaths, including suicide
There were no data comparing antidepressant versus placebo for deaths, including suicide.
1.11 Placebo response rate
Among the five studies that reported a dichotomized HAM‐D score, the mean placebo response rate was 39% and ranged between 23% and 51% (Table 6).
3. Placebo response rate.
| Study | Placebo groupa | Respondersb |
Placebo response rate (%) |
| Hoare 2014 | 50 | 21 | 42 |
| Rabkin 2004 | 39 | 20 | 51 |
| Rabkin 1999 | 39 | 17 | 44 |
| Zisook 1998 | 22 | 5 | 23 |
| Elliott 1998 | 25 | 6 | 24 |
| Overall | 175 | 114 | 39 |
aNumber of participants randomized to placebo.
bNumber who had a reduction in Hamilton Depression Rating Scale score of more than 50% at the end of the study period.
Comparison 2: selective serotonin reuptake inhibitors versus tricyclic antidepressants
Two studies including 78 participants contributed data to the comparison of SSRIs versus TCAs (Elliott 1998; Schwartz 1999). See Table 2.
Primary outcomes
2.1 Improvement in depression: HAM‐D score: continuous
One study with 14 participants contributed to this analysis (Schwartz 1999). There was no evidence of a difference in improvement of HAM‐D score between the two study arms (MD ‐3.20, 95% CI ‐10.87 to 4.47) (Analysis 2.1; Figure 12). The quality of the evidence was very low.
12.
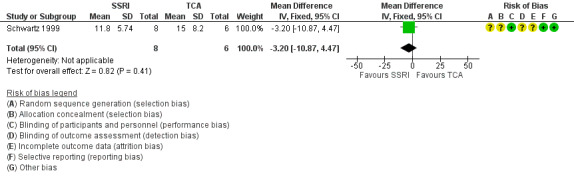
Forest plot of comparison: Selective serotonin reuptake inhibitors (SSRI) versus tricyclic antidepressants (TCA), outcome: 2.1 HAM‐D score.
2.2 Improvement in depression: HAM‐D score: dichotomized
Two studies with 64 participants contributed to this analysis. There was no evidence of a difference in improvement in dichotomized HAM‐D score between the two study arms (RR 0.89, 95% CI 0.54 to 1.46; I2 = 0%) (Analysis 2.2; Figure 13). The quality if the evidence was very low.
2.2. Analysis.
Comparison 2 Selective serotonin reuptake inhibitors (SSRI) versus tricyclic antidepressants (TCA), Outcome 2 Improvement in depression: HAM‐D score: dichotomized (> 50% reduction).
13.
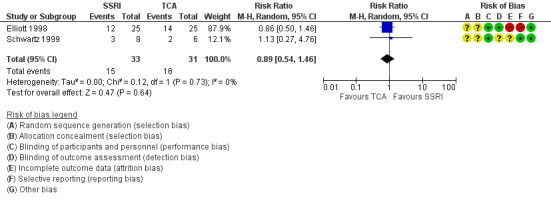
Forest plot of comparison: Selective serotonin reuptake inhibitors (SSRI) versus tricyclic antidepressants (TCA), outcome: 2.2 Dichotomized HAM‐D score (greater than 50% reduction).
2.3 Improvement in depression: CGI score
Two studies with 64 participants contributed to this outcome. There was no evidence of any difference in CGI‐I score between the two study arms (RR 1.24, 95% CI 0.68 to 2.24; I2 = 0%) (Analysis 2.3; Figure 14). The quality of the evidence was very low.
2.3. Analysis.
Comparison 2 Selective serotonin reuptake inhibitors (SSRI) versus tricyclic antidepressants (TCA), Outcome 3 Improvement in depression: CGI‐I (score of 1 or 2).
14.
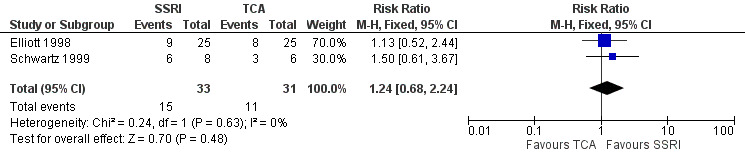
Forest plot of comparison: Selective serotonin reuptake inhibitors (SSRI) versus tricyclic antidepressants (TCA), outcome: 2.3 CGI‐I (score of 1 or 2).
2.4 Study dropouts
Two studies with 64 participants contributed to this outcome. There was no evidence of a difference in the proportion of dropouts in the comparison of SSRI with TCA (RR 0.81, 95% CI 0.51 to 1.30; I2 = 38%) (Analysis 2.4; Figure 15). The quality of the evidence was very low.
2.4. Analysis.
Comparison 2 Selective serotonin reuptake inhibitors (SSRI) versus tricyclic antidepressants (TCA), Outcome 4 Study dropouts.
15.
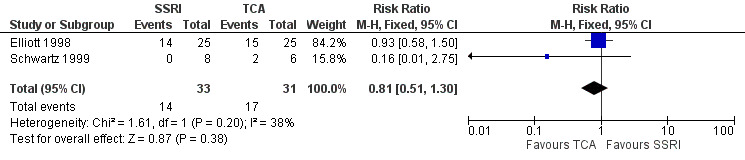
Forest plot of comparison: Selective serotonin reuptake inhibitors (SSRI) versus tricyclic antidepressants (TCA), outcome: 2.4 Study dropouts.
Secondary outcomes
2.5 Adverse effects
One study with 14 participants contributed to this outcome (Schwartz 1999). There was no difference in adverse effects (RR 1.05, 95% CI 0.67 to 1.64) (Analysis 2.5). In this study, one participant in the desipramine group discontinued therapy due to adverse effects, the remaining participants had mild‐moderate adverse effects. The quality of the evidence was very low.
2.5. Analysis.
Comparison 2 Selective serotonin reuptake inhibitors (SSRI) versus tricyclic antidepressants (TCA), Outcome 5 Adverse effects.
2.6 Virological/immunological recovery
There were no data comparing SSRIs versus TCAs for virological/immunological recovery.
2.7 Quality of life
There were no data comparing SSRIs versus TCAs for quality of life.
2.8 Health clinic attendance and hospitalizations
There were no data comparing SSRIs versus TCAs for frequency of health clinic attendance and hospitalizations.
2.9 Employment status
There were no data comparing SSRIs versus TCAs for employment status.
2.10 Deaths, including suicide
There were no data comparing SSRIs versus TCAs for deaths, including suicide.
Comparison 3: selective serotonin reuptake inhibitors versus mirtazapine
One study including 70 participants contributed data to the comparison of SSRIs versus mirtazapine (Patel 2013). See Table 3.
Primary outcomes
3.1 Improvement in depression: HAM‐D score: continuous
Mirtazapine had a better effect on depression score compared to fluoxetine (MD 9.00, 95% CI 3.61 to 14.39) (Analysis 3.1; Figure 16). The quality of the evidence was low.
3.1. Analysis.
Comparison 3 Selective serotonin reuptake inhibitors (SSRI) versus mirtazapine, Outcome 1 Improvement in depression: HAM‐D score: continuous (follow‐up score).
16.
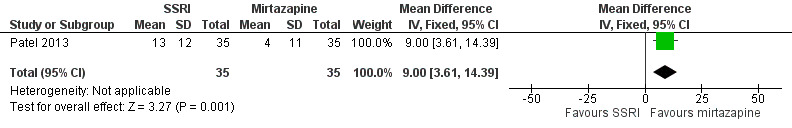
Forest plot of comparison: 3 Selective serotonin reuptake inhibitors (SSRI) versus mirtazapine, outcome: 3.1 Improvement in depression: HAM‐D score: continuous (follow‐up score).
3.2 Improvement in depression: HAM‐D score: dichotomized
There was no evidence of a difference in dichotomized depression score when mirtazapine was compared to escitalopram (RR 0.94, 95% CI 0.79 to 1.11) (Analysis 3.2). The quality of the evidence was low.
3.2. Analysis.
Comparison 3 Selective serotonin reuptake inhibitors (SSRI) versus mirtazapine, Outcome 2 Improvement in depression: HAM‐D score: dichotomized (> 50% reduction).
3.3 Improvement in depression: CGI score
There was no difference in follow‐up CGI‐I scores between SSRI and mirtazapine (RR 0.78, 95% CI 0.52 to 1.17) (Analysis 3.3). The quality of the evidence was low.
3.3. Analysis.
Comparison 3 Selective serotonin reuptake inhibitors (SSRI) versus mirtazapine, Outcome 3 Improvement in depression: CGI‐I (score of 1 or 2).
3.4 Study dropouts
There was no evidence of a difference in the proportion of dropouts in the comparison of SSRI with TCA (RR 1.67, 95% CI 0.43 to 6.45) (Analysis 3.4). The quality of the evidence was low.
3.4. Analysis.
Comparison 3 Selective serotonin reuptake inhibitors (SSRI) versus mirtazapine, Outcome 4 Study dropouts.
Secondary outcomes
3.5 Adverse effects
In Patel 2013, the most common adverse effect was nausea and vomiting (escitalopram 57%; mirtazapine 46%). Other common adverse effects were memory problems, dry mouth, constipation and dizziness. The total number of effects reported were 110 in the escitalopram group and 85 in the mirtazapine group. There were no serious adverse effects reported.
3.6 Virological/immunological recovery
There were no data comparing SSRIs versus mirtazapine for virological/immunological recovery.
3.7 Quality of life
There were no data comparing SSRIs versus mirtazapine for quality of life.
3.8 Health clinic attendance and hospitalizations
There were no data comparing SSRIs versus mirtazapine for frequency of health clinic attendance and hospitalizations.
3.9 Employment status
There were no data comparing SSRIs versus mirtazapine for employment status.
3.10 Deaths, including suicide
There were no data comparing SSRIs versus mirtazapine for deaths, including suicide.
Subgroup analyses
We conducted a subgroup analysis for the primary comparison of antidepressant versus placebo to investigate heterogeneity. This analysis evaluated studies where participants were diagnosed with clinical or immunological AIDS compared to studies where participants had more preserved immune function and were not characterized as having AIDS by the criteria at the time (Analysis 4.1; Figure 17). Only one study consisted primarily of people diagnosed with AIDS (SMD 1.50, 95% CI 0.59 to 2.40; participants = 26) (Mauri 1994). When the analysis of this study was separated from the remaining studies, the pooled HAM‐D score continued to show larger change scores among participants who received antidepressants compared to placebo and moderate heterogeneity persisted in the analysis (SMD 0.48, 95% CI 0.14 to 0.82; participants = 331; studies = 5; I2 = 53%).
4.1. Analysis.
Comparison 4 Subgroup analysis: HIV disease severity, Outcome 1 Improvement in depression: HAM‐D score: continuous (mean change).
17.
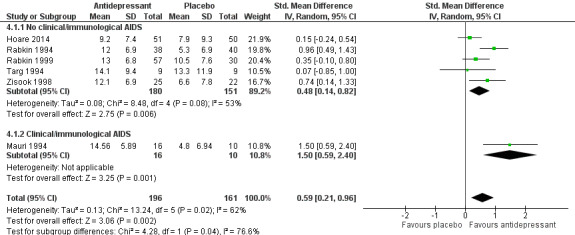
Forest plot of comparison: 4 Subgroup analysis: HIV disease severity, outcome: 4.1 Improvement in depression: HAM‐D score: continuous (mean change).
Sensitivity analyses
We conducted a sensitivity analysis for the primary outcome of improvement in HAM‐D score in studies evaluating antidepressant versus placebo (Analysis 5.1; Figure 18). All studies with a high risk or unclear risk of attrition, detection or performance bias were removed to determine if this contributed to the marked heterogeneity in the analysis of the primary outcome. This resulted in three studies being removed and only two studies remaining in the analysis. There was minimal heterogeneity when pooling the estimates from these studies. These two studies provided low quality evidence that there was no difference in the change in HAM‐D score between the SSRI and placebo arms (SMD 0.23, 95% CI ‐0.14 to 0.60; participants = 117; studies = 2; I2 = 1%). Although there was low risk of bias in these domains for these studies, there remained other methodological limitations and there were few participants contributing to the pooled estimates, which limits the interpretation of these results.
5.1. Analysis.
Comparison 5 Sensitivity analysis: low risk attrition, detection and performance bias, Outcome 1 Improvement in depression: HAM‐D score: continuous (mean change).
18.
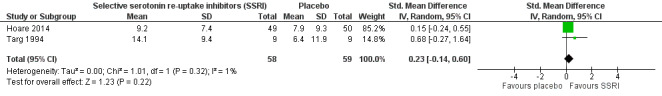
Forest plot of comparison: 5 Sensitivity analysis: low risk attrition, detection and performance bias, outcome: 5.1 Improvement in depression: HAM‐D score: continuous (mean change).
Reporting bias
There were too few studies for creation of a funnel plot. There did not seem to be evidence of reporting bias as studies with and without substantial treatment effects were identified in the published literature.
GRADE
We used GRADE to indicate the level of confidence we had in the results. For most outcomes, quality of evidence was graded as low.
Methodological quality: the methodological quality of the studies included in the review was poor overall. Few studies reported on the method of random sequence generation, allocation concealment or outcome assessment. Several studies had a high or unclear risk of attrition bias. Interpretations of the outcomes from all the comparisons were substantially hampered by the high or unclear risk of bias in the contributing studies.
Consistency: there was moderate statistical heterogeneity and marked clinical heterogeneity between studies in the meta‐analysis of SSRIs versus placebo (HAM‐D score). The variability in gender, baseline level of depression, severity of HIV illness and time periods when the studies were conducted in addition to the statistical heterogeneity resulted in the downgrading of this GRADE domain.
Indirectness: no studies were downgraded for indirectness
Imprecision: several outcomes had only one study with a limited number of participants and few events. This resulted in wide CIs and few events for the analyses comparing different antidepressant groups and the comparison of TCAs with placebo.
Publication bias: we did not detect any publication bias although a formal assessment of publication bias was not performed due to the limited number of included studies.
Discussion
Summary of main results
The search identified 10 studies evaluating antidepressants with placebo or different antidepressant classes for inclusion in this review. Six compared an SSRI with placebo, one study compared a TCA with placebo and one study had three arms evaluating TCAs and SSRIs compared to placebo (Table 1). Two studies compared different antidepressant classes (Table 2). Two studies provided adjunctive psychotherapy and eight of the 10 studies were conducted in the USA (Table 4). Most of the outcomes evaluated in these analyses were graded as having low quality evidence.
Primary outcomes
Improvement in depression
Overall, we found that antidepressants may improve depression compared to placebo. The quality of the evidence contributing to the assessment of this outcome was graded as low for all outcome measures. Data pooled from the six studies comparing mean change in HAM‐D scores showed a greater reduction in HAM‐D score among participants receiving antidepressant compared to placebo. This finding was evident for participants receiving SSRIs and participants receiving TCAs, although there was only one study with few participants contributing to the TCA subgroup. The quality of the evidence was low due to the overall high risk of bias and heterogeneity between studies.
The results of the meta‐analysis of the five studies that reported a dichotomized HAM‐D score found no benefit of antidepressants compared to placebo. This outcome had low quality evidence due to the very high risk of bias for several domains.
Pooled data from the four studies reporting CGI‐I scores showed a marginal statistical benefit of antidepressant over placebo. The evidence was low quality owing to a similarly high overall risk of bias in these studies.
There was a high placebo response rate among several of the studies where placebo response rate could be determined.
We were uncertain if there was any difference in effectiveness when SSRIs were compared to TCAs as the quality of the evidence in these analyses was very low. The data from the two studies comparing an SSRI (fluoxetine or paroxetine) with a TCA (imipramine or desipramine) found no statistical benefit of SSRIs over TCAs for all measures of this outcome (mean HAM‐D score, dichotomized HAM‐D score or CGI‐I score). The quality of the evidence was very low due to methodological limitations of the studies and small number of participants and events.
The use of escitalopram may result in a greater reduction in HAM‐D score compared to mirtazapine. However, there was little or no difference in improvement in depression symptoms as assessed by CGI‐I score or dichotomized HAM‐D score for this comparison. The quality of the evidence was low due to the small number of participants.
Study dropouts
The proportion of study dropouts varied markedly between studies. The individual studies did not consistently report the proportion of dropouts between study arms. A meta‐analysis including the four studies that reported this showed that antidepressant therapy may make little to no difference to the proportion of dropouts when compared to placebo (moderate quality evidence). We are uncertain if the proportion of dropouts differed between participants receiving an SSRI compared with a TCA as the quality of the evidence was very low. There was no discernible difference in the proportion of participants who dropped out between those receiving an SSRI compared to mirtazapine. The quality of the evidence was low.
Secondary outcomes
Adverse effects
There was marked variation in reporting of adverse effects and results from only two studies could be pooled to evaluate this outcome. Due to the very low quality evidence, we were uncertain whether there was a difference between the frequency of adverse effects in participants receiving antidepressants compared to placebo. However, adverse effects were reported frequently in both groups and features of sexual dysfunction were commonly reported in those receiving SSRIs. Anticholinergic adverse effects, including dry mouth and constipation, were reported frequently among participants receiving TCAs. No studies reported any serious adverse effects.
Immunological recovery
Antidepressants may make little or no difference to CD4 count when compared to placebo in the short‐term. Three studies contributed low quality evidence to this outcome.
Quality of life
One study compared quality of life measures between participants receiving antidepressant versus placebo and found no association between quality of life measures and assigned treatment arm. The low number of participants contributing to this outcome led to the evidence for this measure being graded as very low quality.
Studies did not report on any other secondary outcomes for assessment in this review.
Overall completeness and applicability of evidence
We identified few studies evaluating the use of antidepressants for depression in PLWH for inclusion in this review. Most studies were conducted in the USA, prior to 2004 and evaluated predominantly men. Only two studies were conducted in LMICs in the era of triple ART. There have been substantial changes in HIV survival and treatment of HIV and depression since most of these trials were conducted making the relevance of these findings in today's context limited. The pooled estimates from the main comparison of improvement in depression in the antidepressant versus placebo groups must be interpreted with caution due to the low quality evidence that contributed to these outcomes. For these comparisons, we combined the results for people with different baseline levels of depression, from varied population groups and different time periods. We were limited as to how many additional subgroups analyses could be performed due to the small number of participants and trials. As a result, these findings may not be generalizable to current populations of PLWH.
However, the studies did include people who had previously received or failed antidepressant therapy and this makes the findings more externally valid for people with depression, many of whom are likely to have received antidepressants in the past.
The meta‐analysis results were not consistent for different measures of improvement in depression. Although there was some improvement in HAM‐D score as a continuous measure, there was no strong evidence for improvement in CGI‐I score or dichotomized HAM‐D score. The modest and inconsistent changes in follow‐up depression scores using antidepressants in these studies may be the result of the methodological limitations of the included studies but may also be the result of the high placebo response rate seen in several trials. High placebo response rates in clinical trials evaluating antidepressants are well described averaging 31% and ranging from 12.5% to 51.8% (Walsh 2002; Rutherford 2013). The reasons for this variability in placebo response remains uncertain, but a low level of clinical severity of depression has been identified as a possible contributing factor (Stein 2006). Most participants included in these meta‐analyses had mild‐moderate levels of depression as evidenced by their mean baseline HAM‐D scores and several studies included participants with dysthymia or subthreshold depression. Current guidelines for the use of antidepressants do not recommend the initial use of antidepressants for the treatment of subthreshold or mild depressive illness; rather it is recommended that low‐intensity psychosocial interventions be attempted first (NICE 2009; WHO 2015).
A subgroup analysis stratified by severity of clinical HIV disease did not fully explain the heterogeneity that was present in the analysis for the primary outcome of improvement in depression.
Reported dropout rates were relatively high in several of the included studies. These rates were similar to what has been reported in antidepressant clinical trials conducted in general populations during this period. One systematic review by Machado 2006 determined that dropout rates in antidepressant clinical trials may vary from a mean of 28% in people receiving SSRIs to 35.7% in people receiving TCAs. This is similar to the dropout rates seen in several of the antidepressant trials included in this review.
The included studies reported no grade 3 or 4 adverse effects; however, there was a high frequency of adverse effects in all treatment arms. Results from the pooled results of two studies suggested that there was no difference in the frequency of adverse effects between participants receiving placebo or antidepressant. It would appear that features of sexual dysfunction were more common in participants receiving SSRIs, even though the data in this review were insufficient to draw firm conclusions on this, previous literature suggests that in general populations 25% to 73% of people receiving SSRIs may report some level of sexual dysfunction (Higgins 2010). Participants using antidepressants frequently reported anticholinergic adverse effects such as dry mouth and constipation.
None of the included studies reported the effects of antidepressants and improvement in depression on HIV parameters such as ART virological suppression. Although some studies assessed the difference in CD4 count at baseline and at treatment termination, changes in CD4 measurement in a two‐ to three‐month period offers little information, as CD4 values fluctuate substantially and are better assessed over long periods.
There was little to no evidence on additional measures such as quality of life, hospitalizations and suicidality in the included studies.
Despite the limitations of the data assessed in this review, it did appear that antidepressant therapy may improve depression in PLWH. This is supported by international recommendations for the use of antidepressants for moderate to severe depressive illness in the general population (NICE 2009; WHO 2015). The introduction of the WHO Mental Health Gap Action Programme highlights the need for future research in this field to focus on broader questions in order to be relevant in today's context (WHO 2015). This includes primary studies evaluating how antidepressant therapy may work as a component of a comprehensive package of care in PLWH, particularly in LMICs. This will allow for evaluation of the effectiveness of antidepressant therapy in combination with interventions aimed at the improvement of mental health services in these settings.
Quality of the evidence
The quality of evidence contributing to several outcomes in this study were graded as low or very low making it difficult to draw firm conclusions from the results. The main factor contributing to the poor quality evidence was the high or unclear risk of bias for several aspects of the risk of bias assessment. For most studies, methods were inadequately reported and several had high losses to follow‐up, which further hampered interpretation of the results. The limited number of participants and events in several analyses led to further downgrading for imprecision. The inclusion of participants with adjustment disorder, subthreshold depression and dysthymia in several studies led to marked clinical heterogeneity between studies and further contributed to downgrading of the evidence for several outcomes.
Potential biases in the review process
Selection bias was minimized by conducting an extensive search using a wide range of search terms and databases. Two review authors independently assessed the search outputs and evaluated eligibility. In addition, we evaluated reference lists of included papers and systematic reviews. There were updated searches in July 2016 and April 2017 to ensure that the most recent evidence was included in this review. As a result, we do not suspect that inadequate searches may have biased these results.
Several changes were made to the study protocol after publication and after the review process had begun. These changes were approved by the Cochrane Common Mental Disorders Group prior to implementation and continuation of the review process. The changes were made to strengthen the methods and meaningfulness of the review. These and any additional changes to the protocol are detailed in the Differences between protocol and review section.
Agreements and disagreements with other studies or reviews
Himelhoch 2005 published a systematic review and meta‐analysis that determined that antidepressants were "efficacious in treating depression" among PLWH with depression. Although several studies that contributed to our review were included in the 2005 review, the main difference in our results was the overall assessment of unclear or high risk of bias for several studies. This influenced our evaluation of evidence quality and contributed to our overall conclusions which incorporated evidence quality. A further difference between the Himelhoch 2005 paper and our review was our exclusion of one study by Markowitz 1998. This study compared imipramine combined with supportive psychotherapy to psychotherapy alone and found that the combined arm had no greater improvement in HAM‐D score compared to the supportive psychotherapy alone for participants who completed the study. The exclusion of this study from our review is unlikely to have a substantial impact on the overall conclusions made. Our review and the GRADE quality assessment highlights the benefit of evaluating statistical results within the framework of evidence quality.
Women were under‐represented in this review and we found that few studies were conducted in LMICs. Systematic reviews of the use of psychological therapies for the treatment of depression in PLWH have also highlighted that women a poorly represented in study populations (Honagodu 2013; Illa 2014) and that few high quality studies have been conducted in LMICs (Chibanda 2015).
Authors' conclusions
Implications for practice.
Overall, we found that antidepressants may improve depression compared to placebo, but we have little confidence in this result due to the low quality of the evidence.
Implications for research.
This review highlights that there is a lack of high quality research on the use of antidepressant therapy in people living with HIV. Future studies in this area should consider including participants from low‐ to middle‐income countries where HIV burden is greatest and sample both male and female participants. Adverse effects should be reported comprehensively, and research should consider collecting data on HIV‐specific outcomes, such as adherence, virological suppression and retention in HIV care. Rigorous research methods should be applied to allow for meaningful conclusions to be made from study findings.
What's new
| Date | Event | Description |
|---|---|---|
| 9 February 2018 | Amended | Acknowledgement corrected |
History
Protocol first published: Issue 5, 2010 Review first published: Issue 1, 2018
| Date | Event | Description |
|---|---|---|
| 31 July 2015 | Amended | Edits of protocol with new team |
| 27 June 2014 | New citation required and major changes | New team |
Acknowledgements
We thank:
the Cochrane HIV/AIDS Mentoring Programme, Cochrane South Africa, South Africa Medical Research Council;
the African Centre for Systematic Reviews and Knowledge Translation, Makerere University College of Health Sciences;
Ian Lewis at the Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa who drafted the first version of the protocol in 2008;
Marcel Kitenge at the Centre for Evidence Based Health Care, Division of Epidemiology and Biostatistics, Department of Global Health, University of Stellenbosch, Cape Town, South Africa who assisted with abstract screening for the third search.
Appendices
Appendix 1. Rating scales used in included studies
HADS: Hospital Anxiety and Depression Scale: self‐report measure of depressive and anxiety symptoms severity in medically ill populations.
HAM‐D: Hamilton Depression Rating Scale: an observer‐rated measure of depression severity. The original 17‐item HAM‐D version can score from 0 to 54. A score of 0 to 6 = no depression, 7 to 17 = mild depression, 18 to 24 = moderate depression and 25 or greater indicates severe depression. A total score of 7 or less after treatment is often used as an indicator of remission and a decrease of 50% or more from baseline indicates a clinically significant change. This scoring has been revised several times and further versions include a 7‐item, 21‐item, 24‐item and 29‐item version.
CGI: Clinical Global Impression Scale of Severity (CGI‐S) and Improvement (CGI‐I) is a clinician‐rated global assessment of disease severity (CGI‐S) and improvement (CGI‐I). The CGI‐I scale measures a change in symptom severity and is rated from 1 to 7, where 1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change, 5 = minimally worse, 6 = much worse and 7 = very much worse. The CGI‐S scale is a measure of disease severity and ranges from 1 to 7, where 1 = not at all ill, 2 = borderline mentally ill, 3 = mildly ill, 4 = moderately ill, 5 = markedly ill, 6 = severely ill and 7 = extremely ill.
MADRS: Montgomery‐Åsberg Depression Rating Scale: measures depression severity on a scale of 0 to 60. A score of 0 to 6 = no depression, 7 to 19 = mild depression; 20 to 34 = moderate depression and 35 or greater is indicative of severe depression.
Brief Symptom Inventory: 53‐item self‐report (patient‐reported) scale drawn from the longer SCL‐90 version. It gives an overview of a person's symptoms and their intensity at a particular time point. It is scored on a severity scale, where 0 = not at all and 4 = extremely. A global severity index is calculated. There are 9 subscales. Scores represent the mean item score. Higher scores signify greater distress.
Beck Hopelessness Scale: 20‐item self‐report inventory that measures three major aspects of hopelessness: feelings about the future, loss of motivation and expectations. The questionnaire consists of 20 true/false questions examining the respondents attitude for the past week. A score of 0 to 3 = minimal, 4 to 8 = mild, 9 to 14 = moderate and 15 to 20 = severe.
Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ): patient‐reported quality of life questionnaire designed to assess the degree of enjoyment and satisfaction experienced during the past week. It assesses 14 domains including: physical health activities, feelings, work, household duties, school/coursework, leisure time activities, social relations and general activities. Each is rated on a 5‐point scale with a higher score indicating greater enjoyment and satisfaction.
SAFTEE: Systematic Assessment for Treatment Emergent Effects: tool collects data on adverse effects onset, duration, pattern, severity (minimal, mild, moderate or severe), relationship to drug and action taken.
DOTES: 41‐item scale for recording dosage and symptoms associated with medication, it records symptom intensity, relationship with drug, action taken, type of symptom, daily dosage and global judgements by clinician.
HDS: HIV Dementia Scale: tool used to detect global cognitive function with a subcortical pattern. The tool tests 5 domains; memory, attention, psychomotor speed, memory/recall and construction. A score of less than 10 out of 16 suggests possible HIV dementia.
MMSE: Mini‐Mental State Examination: validated screening tool for evaluating cognitive impairment. The tool has 6 subsections which test skills at orientation, registration, attention and calculation, recall, language and copying. The test scores out of 30. A score of 24 to 30 suggests no cognitive impairment, 18 to 23 mild cognitive impairment and 0 to 17 severe cognitive impairment. The Modified MMSE is 57 item version of the MMSE that includes more extensive assessment of language and construction items as well as digit span.
Appendix 2. Core search strategy, CCMD‐CTR
CCMD's core search strategy used to inform the Group's specialised register: Ovid MEDLINE A weekly search alert based on condition + RCT filter only (all years to 6‐June‐2015) 1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/
2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf.
3. [RCT filter]: (controlled clinical trial.pt. or randomized controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomized controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
4. (1 and 2 and 3)
Records were screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs were tagged to the appropriate study record. Similar weekly search alerts were also conducted on Ovid EMBASE and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
N.B. With the relocation of the Cochrane Common Mental Disorders Group in 2015 and change of staff at the editorial unit, this register is currently out‐of‐date.
Appendix 3. Review search strategies
| Cochrane Common Mental Disorders Group's Specialised Register. CCMD‐CTR‐References Register (all years to 6‐June‐2015). | |
| #1. (HIV or ("human immun*" near virus) or ("acquired immun*" near syndrom*) or "aids virus*"):ti,ab,kw,ky,emt,mh,mc | |
| #2. (antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or “monoamine oxidase inhibit*” or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) NEAR (uptake or reuptake or re‐uptake or "re uptake")) or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic*):ti,ab,kw,ky,emt,mh,mc | |
| #3.(Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or (Buproprion or Amfebutamone) or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Demexiptiline or Deprenyl or (Desipramine* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233):ti,ab,kw,ky,emt,mh,mc | |
| #4. (Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or “St John*”) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or (“Lu AA21004” or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin*):ti,ab,kw,ky,emt,mh,mc | |
| #5. (Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone):ti,ab,kw,ky,emt,mh,mc | |
| #6. (amylobarbiton* or caffeine or *amphetamine or methylphenidat* or phenmetrazin* or amoxetin* or dexamfetamin* or cocaine or phenylpropanolamin* or pemolin* or ephedrin* or modafinil or methylen* or psychostimulant*):ti,ab,kw,ky,emt,mh,mc | |
| #7. #1 and (#2 or #3 or #4 or #5 or #6) [Key to field codes: ti:title; ab:abstract; kw:keywords: ky:additional keywords; emt:EMTREE headings; mh:MeSH headings; mc:MeSH checkwords] |
Appendix 4. Additional database search strategies
Pubmed History
| Search | Query | Items found |
| #22 | Search (((randomi* or placebo or randomly or "controlled trial" or "single blind*" or "double blind*" or allocated) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND (((((((((((((amylobarbiton* or caffeine or *amphetamine or methylphenidat* or phenmetrazin* or amoxetin* or dexamfetamin* or cocaine or phenylpropanolamin* or pemolin* or ephedrin* or modafinil or methylen* or psychostimulant*))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR ((opipramol OR oxaflozane OR paroxetine OR phenelzine OR pheniprazine OR pipofezine OR pirlindole OR pivagabine OR pizotyline OR propizepine OR Protriptylin* OR quinupramine OR reboxetine OR rolipram OR scopolamine OR selegiline OR sertraline OR setiptiline OR setiptiline OR thozalinone OR Tianeptin* OR toloxatone OR Tranylcypromin* OR trazodone OR trimipramine OR venlafaxine OR viloxazine OR vilazodone OR viqualine OR zalospirone) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (((Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone)) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR ((Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or “St John*”) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or (“Lu AA21004” or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin*)) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR ((agomelatine OR alaproclate OR amoxapine OR amineptine OR Amitriptylin* OR amitriptylinoxide OR atomoxetine OR befloxatone OR benactyzine OR binospirone OR brofaromine OR (bupropion OR amfebutamone) OR butriptyline OR caroxazone OR cianopramine OR cilobamine OR cimoxatone OR citalopram OR (Chlorimipramin* OR Clomipramin* OR Chlomipramin* OR clomipramine) OR clorgyline OR clovoxamine OR (CX157 OR tarima) OR demexiptiline OR deprenyl OR (Desipramine* OR pertofrane) OR desvenlafaxine OR dibenzepin OR diclofensine OR Dimetacrin* OR dosulepin OR dothiepin OR doxepin OR duloxetine OR desvenlafaxine OR DVS‐233) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (((antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or “monoamine oxidase inhibit*” or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) NEAR (uptake or reuptake or re‐uptake or "re uptake")) or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic*)) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND ((((((("HIV"[Mesh] AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (((HIV or ("human immun*" AND virus) or ("acquired immun*" AND syndrom*) or "aids virus*") AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (AIDS AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] )) Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 41 |
| #21 | Search randomi* or placebo or randomly or "controlled trial" or "single blind*" or "double blind*" or allocated Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 68428 |
| #19 | Search (((((((((((amylobarbiton* or caffeine or *amphetamine or methylphenidat* or phenmetrazin* or amoxetin* or dexamfetamin* or cocaine or phenylpropanolamin* or pemolin* or ephedrin* or modafinil or methylen* or psychostimulant*))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR ((opipramol OR oxaflozane OR paroxetine OR phenelzine OR pheniprazine OR pipofezine OR pirlindole OR pivagabine OR pizotyline OR propizepine OR Protriptylin* OR quinupramine OR reboxetine OR rolipram OR scopolamine OR selegiline OR sertraline OR setiptiline OR setiptiline OR thozalinone OR Tianeptin* OR toloxatone OR Tranylcypromin* OR trazodone OR trimipramine OR venlafaxine OR viloxazine OR vilazodone OR viqualine OR zalospirone) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (((Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone)) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR ((Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or “St John*”) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or (“Lu AA21004” or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin*)) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR ((agomelatine OR alaproclate OR amoxapine OR amineptine OR Amitriptylin* OR amitriptylinoxide OR atomoxetine OR befloxatone OR benactyzine OR binospirone OR brofaromine OR (bupropion OR amfebutamone) OR butriptyline OR caroxazone OR cianopramine OR cilobamine OR cimoxatone OR citalopram OR (Chlorimipramin* OR Clomipramin* OR Chlomipramin* OR clomipramine) OR clorgyline OR clovoxamine OR (CX157 OR tarima) OR demexiptiline OR deprenyl OR (Desipramine* OR pertofrane) OR desvenlafaxine OR dibenzepin OR diclofensine OR Dimetacrin* OR dosulepin OR dothiepin OR doxepin OR duloxetine OR desvenlafaxine OR DVS‐233) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (((antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or “monoamine oxidase inhibit*” or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) NEAR (uptake or reuptake or re‐uptake or "re uptake")) or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic*)) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND ((((((("HIV"[Mesh] AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (((HIV or ("human immun*" AND virus) or ("acquired immun*" AND syndrom*) or "aids virus*") AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (AIDS AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] )) Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 545 |
| #20 | Search (((((((((((amylobarbiton* or caffeine or *amphetamine or methylphenidat* or phenmetrazin* or amoxetin* or dexamfetamin* or cocaine or phenylpropanolamin* or pemolin* or ephedrin* or modafinil or methylen* or psychostimulant*))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR ((opipramol OR oxaflozane OR paroxetine OR phenelzine OR pheniprazine OR pipofezine OR pirlindole OR pivagabine OR pizotyline OR propizepine OR Protriptylin* OR quinupramine OR reboxetine OR rolipram OR scopolamine OR selegiline OR sertraline OR setiptiline OR setiptiline OR thozalinone OR Tianeptin* OR toloxatone OR Tranylcypromin* OR trazodone OR trimipramine OR venlafaxine OR viloxazine OR vilazodone OR viqualine OR zalospirone) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (((Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone)) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR ((Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or “St John*”) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or (“Lu AA21004” or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin*)) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR ((agomelatine OR alaproclate OR amoxapine OR amineptine OR Amitriptylin* OR amitriptylinoxide OR atomoxetine OR befloxatone OR benactyzine OR binospirone OR brofaromine OR (bupropion OR amfebutamone) OR butriptyline OR caroxazone OR cianopramine OR cilobamine OR cimoxatone OR citalopram OR (Chlorimipramin* OR Clomipramin* OR Chlomipramin* OR clomipramine) OR clorgyline OR clovoxamine OR (CX157 OR tarima) OR demexiptiline OR deprenyl OR (Desipramine* OR pertofrane) OR desvenlafaxine OR dibenzepin OR diclofensine OR Dimetacrin* OR dosulepin OR dothiepin OR doxepin OR duloxetine OR desvenlafaxine OR DVS‐233) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (((antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or “monoamine oxidase inhibit*” or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) NEAR (uptake or reuptake or re‐uptake or "re uptake")) or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic*)) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND ((((((("HIV"[Mesh] AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (((HIV or ("human immun*" AND virus) or ("acquired immun*" AND syndrom*) or "aids virus*") AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (AIDS AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] )) Sort by: PublicationDate Filters: Clinical Trial; Publication date from 2016/04/01 | 6 |
| #18 | Search ((((((((amylobarbiton* or caffeine or *amphetamine or methylphenidat* or phenmetrazin* or amoxetin* or dexamfetamin* or cocaine or phenylpropanolamin* or pemolin* or ephedrin* or modafinil or methylen* or psychostimulant*))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR ((opipramol OR oxaflozane OR paroxetine OR phenelzine OR pheniprazine OR pipofezine OR pirlindole OR pivagabine OR pizotyline OR propizepine OR Protriptylin* OR quinupramine OR reboxetine OR rolipram OR scopolamine OR selegiline OR sertraline OR setiptiline OR setiptiline OR thozalinone OR Tianeptin* OR toloxatone OR Tranylcypromin* OR trazodone OR trimipramine OR venlafaxine OR viloxazine OR vilazodone OR viqualine OR zalospirone) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (((Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone)) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR ((Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or “St John*”) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or (“Lu AA21004” or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin*)) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR ((agomelatine OR alaproclate OR amoxapine OR amineptine OR Amitriptylin* OR amitriptylinoxide OR atomoxetine OR befloxatone OR benactyzine OR binospirone OR brofaromine OR (bupropion OR amfebutamone) OR butriptyline OR caroxazone OR cianopramine OR cilobamine OR cimoxatone OR citalopram OR (Chlorimipramin* OR Clomipramin* OR Chlomipramin* OR clomipramine) OR clorgyline OR clovoxamine OR (CX157 OR tarima) OR demexiptiline OR deprenyl OR (Desipramine* OR pertofrane) OR desvenlafaxine OR dibenzepin OR diclofensine OR Dimetacrin* OR dosulepin OR dothiepin OR doxepin OR duloxetine OR desvenlafaxine OR DVS‐233) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (((antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or “monoamine oxidase inhibit*” or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) NEAR (uptake or reuptake or re‐uptake or "re uptake")) or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic*)) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] )) Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 33850 |
| #17 | Search (amylobarbiton* or caffeine or *amphetamine or methylphenidat* or phenmetrazin* or amoxetin* or dexamfetamin* or cocaine or phenylpropanolamin* or pemolin* or ephedrin* or modafinil or methylen* or psychostimulant*)) Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 5839 |
| #15 | Search (Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone) Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 1003 |
| #14 | Search Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or “St John*”) or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or (“Lu AA21004” or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin*) Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 18813 |
| #12 | Search Agomelatine or Alaproclate or Amoxapine or Amineptine or Amitriptylin* or Amitriptylinoxide or Atomoxetine or Befloxatone or Benactyzine or Binospirone or Brofaromine or (Buproprion or Amfebutamone) or Butriptyline or Caroxazone or Cianopramine or Cilobamine or Cimoxatone or Citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or Clomipramine) or Clorgyline or Clovoxamine or (CX157 or Tyrima) or Demexiptiline or Deprenyl or (Desipramine* or Pertofrane) or Desvenlafaxine or Dibenzepin or Diclofensine or Dimetacrin* or Dosulepin or Dothiepin or Doxepin or Duloxetine or Desvenlafaxine or DVS‐233 Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 1268 |
| #13 | Search agomelatine OR alaproclate OR amoxapine OR amineptine OR Amitriptylin* OR amitriptylinoxide OR atomoxetine OR befloxatone OR benactyzine OR binospirone OR brofaromine OR (bupropion OR amfebutamone) OR butriptyline OR caroxazone OR cianopramine OR cilobamine OR cimoxatone OR citalopram OR (Chlorimipramin* OR Clomipramin* OR Chlomipramin* OR clomipramine) OR clorgyline OR clovoxamine OR (CX157 OR tarima) OR demexiptiline OR deprenyl OR (Desipramine* OR pertofrane) OR desvenlafaxine OR dibenzepin OR diclofensine OR Dimetacrin* OR dosulepin OR dothiepin OR doxepin OR duloxetine OR desvenlafaxine OR DVS‐233 Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 1283 |
| #11 | Search (antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or “monoamine oxidase inhibit*” or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) NEAR (uptake or reuptake or re‐uptake or "re uptake")) or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic*) Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 9477 |
| #10 | Search ((((("HIV"[Mesh] AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (((HIV or ("human immun*" AND virus) or ("acquired immun*" AND syndrom*) or "aids virus*") AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (AIDS AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] )) Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 21537 |
| #9 | Search AIDS Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 10151 |
| #8 | Search (("HIV"[Mesh] AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] ))) OR (((HIV or ("human immun*" AND virus) or ("acquired immun*" AND syndrom*) or "aids virus*") AND ( "2016/04/01"[PDat] : "3000/12/31"[PDat] )) Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 18542 |
| #7 | Search "Acquired Immunodeficiency Syndrome"[Mesh] Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 146 |
| #5 | Search "HIV"[Mesh] Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 651 |
| #2 | Search (HIV or ("human immun*" AND virus) or ("acquired immun*" AND syndrom*) or "aids virus*" Sort by: PublicationDate Filters: Publication date from 2016/04/01 | 18542 |
| #1 | Search (HIV or ("human immun*" AND virus) or ("acquired immun*" AND syndrom*) or "aids virus*" Sort by: PublicationDate | 370871 |
| #0 | pubmed clipboard | 42 |
Search Name: Cochrane Library
Date Run: 18/04/17 12:01:46.371
Description:
ID Search Hits
#1 HIV or ("human immun*" and virus) or ("acquired immun*" and syndrom*) or "aids virus*":ti,ab,kw (Word variations have been searched) 16869
#2 MeSH descriptor: [HIV] explode all trees 2942
#3 MeSH descriptor: [HIV Infections] explode all trees 9362
#4 #1 or #2 or #3 Publication Year from 2016 to 2017 1799
#5 antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or "monoamine oxidase inhibit*" or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re‐uptake or "re uptake")) 20290
#6 agomelatine or alaproclate or amoxapine or amineptine or Amitriptylin* or amitriptylinoxide or atomoxetine or befloxatone or benactyzine or binospirone or brofaromine or (bupropion or amfebutamone) or butriptyline or caroxazone or cianopramine or cilobamine or cimoxatone or citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or clomipramine) or clorgyline or clovoxamine or (CX157 or tarima) or demexiptiline or deprenyl or (Desipramine* or pertofrane) or desvenlafaxine or dibenzepin or diclofensine or Dimetacrin* or dosulepin or dothiepin or doxepin or duloxetine or desvenlafaxine or DVS‐233 9275
#7 Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or "St John*") or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or ("Lu AA21004" or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* 10600
#8 Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone 8580
#9 amylobarbiton* or caffeine or *amphetamine or methylphenidat* or phenmetrazin* or amoxetin* or dexamfetamin* or cocaine or phenylpropanolamin* or pemolin* or ephedrin* or modafinil or methylen* or psychostimulant* 12996
#10 #5 or #6 or #7 or #8 or #9 43444
#11 #4 and #10 57
Database: Embase <1996 to 2017 April 18>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 (HIV or ("human immun*" adj1 virus) or ("acquired immun*" adj1 syndrom*) or "aids virus*:").mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading] (361442)
2 hiv infection.mp. or Human immunodeficiency virus infection/ (213639)
3 aids.mp. or acquired immune deficiency syndrome/ (143154)
4 1 or 2 or 3 (407342)
5 limit 4 to yr="2016 ‐Current" (26749)
6 (antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or "monoamine oxidase inhibit*" or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re‐uptake or "re uptake"))).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading] (185788)
7 (agomelatine or alaproclate or amoxapine or amineptine or Amitriptylin* or amitriptylinoxide or atomoxetine or befloxatone or benactyzine or binospirone or brofaromine or (bupropion or amfebutamone) or butriptyline or caroxazone or cianopramine or cilobamine or cimoxatone or citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or clomipramine) or clorgyline or clovoxamine or (CX157 or tarima) or demexiptiline or deprenyl or (Desipramine* or pertofrane) or desvenlafaxine or dibenzepin or diclofensine or Dimetacrin* or dosulepin or dothiepin or doxepin or duloxetine or desvenlafaxine or DVS‐233).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading] (73887)
8 (Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or "St John*") or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or ("Lu AA21004" or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading] (80046)
9 (Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading] (73128)
10 (amylobarbiton* or caffeine).mp. or *amphetamine/ or methylphenidat*.mp. or phenmetrazin*.mp. or amoxetin*.mp. or dexamfetamin*.mp. or cocaine.mp. or phenylpropanolamin*.mp. or pemolin*.mp. or ephedrin*.mp. or modafinil.mp. or methylen*.mp. or psychostimulant*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading] (164444)
11 6 or 7 or 8 or 9 or 10 (416153)
12 5 and 11 (587)
13 (randomized or randomised or placebo or double‐blind* or single‐blind*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading] (992922)
14 randomized controlled trial/ or controlled clinical trial/ (599309)
15 13 or 14 (1115195)
16 12 and 15 (83)
Database: Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to 18 April 2017>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 (HIV or ("human immun*" adj1 virus) or ("acquired immun*" adj1 syndrom*) or "aids virus*:").mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] (368246)
2 hiv infection.mp. or Human immunodeficiency virus infection/ (60785)
3 aids.mp. or acquired immune deficiency syndrome/ (204131)
4 1 or 2 or 3 (422617)
5 limit 4 to yr="2016 ‐Current" (25301)
6 (antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or "monoamine oxidase inhibit*" or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re‐uptake or "re uptake"))).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] (156007)
7 (agomelatine or alaproclate or amoxapine or amineptine or Amitriptylin* or amitriptylinoxide or atomoxetine or befloxatone or benactyzine or binospirone or brofaromine or (bupropion or amfebutamone) or butriptyline or caroxazone or cianopramine or cilobamine or cimoxatone or citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or clomipramine) or clorgyline or clovoxamine or (CX157 or tarima) or demexiptiline or deprenyl or (Desipramine* or pertofrane) or desvenlafaxine or dibenzepin or diclofensine or Dimetacrin* or dosulepin or dothiepin or doxepin or duloxetine or desvenlafaxine or DVS‐233).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] (37750)
8 (Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or "St John*") or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or ("Lu AA21004" or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] (46013)
9 (Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] (36650)
10 (amylobarbiton* or caffeine).mp. or *amphetamine/ or methylphenidat*.mp. or phenmetrazin*.mp. or amoxetin*.mp. or dexamfetamin*.mp. or cocaine.mp. or phenylpropanolamin*.mp. or pemolin*.mp. or ephedrin*.mp. or modafinil.mp. or methylen*.mp. or psychostimulant*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] (148677)
11 6 or 7 or 8 or 9 or 10 (340031)
12 5 and 11 (303)
13 (randomized or randomised or placebo or double‐blind* or single‐blind*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] (836821)
14 randomized controlled trial/ or controlled clinical trial/ (549025)
15 13 or 14 (901589)
16 12 and 15 (34)
| # | Query | Limiters/Expanders | Last Run Via | Results |
| S13 | S11 AND S12 | Search modes ‐ Boolean/Phrase | Interface ‐ EBSCOhost Research Databases Search Screen ‐ Advanced Search Database ‐ PsycINFO | 20 |
| S12 | randomized control trial OR controlled trial OR ( randomized or randomised or placebo or double‐blind* or single‐blind* ) | Search modes ‐ Boolean/Phrase | Interface ‐ EBSCOhost Research Databases Search Screen ‐ Advanced Search Database ‐ PsycINFO | 101,493 |
| S11 | S4 AND S10 | Search modes ‐ Boolean/Phrase | Interface ‐ EBSCOhost Research Databases Search Screen ‐ Advanced Search Database ‐ PsycINFO | 160 |
| S10 | S5 OR S6 OR S7 OR S8 OR S9 | Search modes ‐ Boolean/Phrase | Interface ‐ EBSCOhost Research Databases Search Screen ‐ Advanced Search Database ‐ PsycINFO | 123,010 |
| S9 | amylobarbiton* or caffeine or *amphetamine or methylphenidat* or phenmetrazin* or amoxetin* or dexamfetamin* or cocaine or phenylpropanolamin* or pemolin* or ephedrin* or modafinil or methylen* or psychostimulant* | Search modes ‐ Boolean/Phrase | Interface ‐ EBSCOhost Research Databases Search Screen ‐ Advanced Search Database ‐ PsycINFO | 44,354 |
| S8 | Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone | Search modes ‐ Boolean/Phrase | Interface ‐ EBSCOhost Research Databases Search Screen ‐ Advanced Search Database ‐ PsycINFO | 13,851 |
| S7 | Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or "St John*") or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or ("Lu AA21004" or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin* | Search modes ‐ Boolean/Phrase | Interface ‐ EBSCOhost Research Databases Search Screen ‐ Advanced Search Database ‐ PsycINFO | 25,871 |
| S6 | agomelatine or alaproclate or amoxapine or amineptine or Amitriptylin* or amitriptylinoxide or atomoxetine or befloxatone or benactyzine or binospirone or brofaromine or (bupropion or amfebutamone) or butriptyline or caroxazone or cianopramine or cilobamine or cimoxatone or citalopram or (Chlorimipramin* or Clomipramin* or Chlomipramin* or clomipramine) or clorgyline or clovoxamine or (CX157 or tarima) or demexiptiline or deprenyl or (Desipramine* or pertofrane) or desvenlafaxine or dibenzepin or diclofensine or Dimetacrin* or dosulepin or dothiepin or doxepin or duloxetine or desvenlafaxine or DVS‐233 | Search modes ‐ Boolean/Phrase | Interface ‐ EBSCOhost Research Databases Search Screen ‐ Advanced Search Database ‐ PsycINFO | 14,764 |
| S5 | (antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or "monoamine oxidase inhibit*" or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re‐uptake or "re uptake")) | Search modes ‐ Boolean/Phrase | Interface ‐ EBSCOhost Research Databases Search Screen ‐ Advanced Search Database ‐ PsycINFO | 65,024 |
| S4 | S2 OR S3 | Search modes ‐ Boolean/Phrase | Interface ‐ EBSCOhost Research Databases Search Screen ‐ Advanced Search Database ‐ PsycINFO | 3,154 |
| S3 | MA hiv OR MA acquired immunodeficiency syndrome | Limiters ‐ Publication Year: 2016‐2017 Search modes ‐ Boolean/Phrase | Interface ‐ EBSCOhost Research Databases Search Screen ‐ Advanced Search Database ‐ PsycINFO | 100 |
| S2 | ( HIV or ("acquired immun*" adj1 syndrom*) or ) OR aids virus* OR ("human immun*" adj1 virus) | Limiters ‐ Publication Year: 2016‐2017 Search modes ‐ Boolean/Phrase | Interface ‐ EBSCOhost Research Databases Search Screen ‐ Advanced Search Database ‐ PsycINFO | 3,154 |
| S1 | ( HIV or ("acquired immun*" adj1 syndrom*) or ) OR aids virus* OR ("human immun*" adj1 virus) | Search modes ‐ Boolean/Phrase | Interface ‐ EBSCOhost Research Databases Search Screen ‐ Advanced Search Database ‐ PsycINFO | 48,169 |
| Web of science | 1 Jan 2016 ‐ 18 April 2017 | |
| # 7 | 17 | #6 AND #5 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=2016‐2017 |
| # 6 | 111,577 |
TOPIC: (randomized or randomised or randomly or placebo or double‐blind* or single‐blind* or allocated) ORTOPIC: (ra) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=2016‐2017 |
| # 5 | 121 | #4 AND #3 Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=2016‐2017 |
| # 4 | 24,481 |
TOPIC: (antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or "monoamine oxidase inhibit*" or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re‐uptake or "re uptake"))) ORTOPIC: (antidepress* or anti‐depress* or "anti depress*" or MAOI* or RIMA* or "monoamine oxidase inhibit*" or SSRI* or SNRI* or NARI* or SARI* or NDRI* or TCA* or tricyclic* or tetracyclic* or ((serotonin or norepinephrine or noradrenaline or neurotransmitter* or dopamin*) and (uptake or reuptake or re‐uptake or "re uptake"))) ORTOPIC: (Escitalopram or Etoperidone or Femoxetine or Fluotracen or Fluoxetine or Fluvoxamine or (Hyperforin or Hypericum or "St John*") or Imipramin* or Iprindole or Iproniazid* or Ipsapirone or Isocarboxazid* or Levomilnacipran or Lofepramine* or ("Lu AA21004" or Vortioxetine) or "Lu AA24530" or (LY2216684 or Edivoxetine) or Maprotiline or Melitracen or Metapramine or Mianserin or Milnacipran or Minaprine or Mirtazapine or Moclobemide or Nefazodone or Nialamide or Nitroxazepine or Nomifensine or Norfenfluramine or Nortriptylin* or Noxiptilin*) ORTOPIC: (Opipramol or Oxaflozane or Paroxetine or Phenelzine or Pheniprazine or Pipofezine or Pirlindole or Pivagabine or Pizotyline or Propizepine or Protriptylin* or Quinupramine or Reboxetine or Rolipram or Scopolamine or Selegiline or Sertraline or Setiptiline or Teciptiline or Thozalinone or Tianeptin* or Toloxatone or Tranylcypromin* or Trazodone or Trimipramine or Venlafaxine or Viloxazine or Vilazodone or Viqualine or Zalospirone) ORTOPIC: (amylobarbiton* or caffeine or *amphetamine or methylphenidat* or phenmetrazin* or amoxetin* or dexamfetamin* or cocaine or phenylpropanolamin* or pemolin* or ephedrin* or modafinil or methylen* or psychostimulant*) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=2016‐2017 |
| # 3 | 9,198 |
TOPIC: (hiv or aids or "acquired immunodeficiency syndrome ") Refined by: WEB OF SCIENCE CATEGORIES: ( INFECTIOUS DISEASES OR IMMUNOLOGY OR PUBLIC ENVIRONMENTAL OCCUPATIONAL HEALTH ) Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=2016‐2017 |
| # 2 | 45,835 |
TOPIC: (hiv or aids or "acquired immunodeficiency syndrome ") Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=2016‐2017 |
| # 1 | 687,820 |
TOPIC: (hiv or aids or "acquired immunodeficiency syndrome ") Indexes=SCI‐EXPANDED, SSCI, CPCI‐S, CPCI‐SSH Timespan=All years |
Data and analyses
Comparison 1. Antidepressant versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in depression: HAM‐D score: continuous (mean change) | 6 | 357 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.21, 0.96] |
| 1.1 SSRI vs placebo | 5 | 279 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [0.09, 0.88] |
| 1.2 TCA vs placebo | 1 | 78 | Std. Mean Difference (IV, Random, 95% CI) | 0.96 [0.49, 1.43] |
| 2 Improvement in depression: HAM‐D score: dichotomized (> 50% reduction) | 5 | 434 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.89, 1.35] |
| 2.1 SSRI vs placebo | 5 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.87, 1.32] |
| 2.2 TCA vs placebo | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 2.24 [0.79, 6.34] |
| 3 Improvement in depression: CGI‐I (score of 1 or 2) | 4 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.93, 1.77] |
| 3.1 SSRI vs placebo | 4 | 309 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.92, 1.60] |
| 3.2 TCA vs placebo | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 4.32 [0.62, 30.30] |
| 4 Study dropouts | 4 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.91, 1.80] |
| 4.1 SSRI vs placebo | 3 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.75, 1.82] |
| 4.2 TCA vs placebo | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.80, 2.79] |
| 5 Adverse effects | 2 | 167 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.21] |
| 6 Follow‐up CD4 count | 3 | 176 | Mean Difference (IV, Random, 95% CI) | ‐6.31 [‐72.76, 60.14] |
| 7 Quality of life | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [‐0.38, 7.58] |
Comparison 2. Selective serotonin reuptake inhibitors (SSRI) versus tricyclic antidepressants (TCA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in depression: HAM‐D score: continuous (follow‐up score) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐10.87, 4.47] |
| 2 Improvement in depression: HAM‐D score: dichotomized (> 50% reduction) | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.54, 1.46] |
| 3 Improvement in depression: CGI‐I (score of 1 or 2) | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.68, 2.24] |
| 4 Study dropouts | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.51, 1.30] |
| 5 Adverse effects | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.67, 1.64] |
Comparison 3. Selective serotonin reuptake inhibitors (SSRI) versus mirtazapine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in depression: HAM‐D score: continuous (follow‐up score) | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [3.61, 14.39] |
| 2 Improvement in depression: HAM‐D score: dichotomized (> 50% reduction) | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.11] |
| 3 Improvement in depression: CGI‐I (score of 1 or 2) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.52, 1.17] |
| 4 Study dropouts | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.43, 6.45] |
Comparison 4. Subgroup analysis: HIV disease severity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in depression: HAM‐D score: continuous (mean change) | 6 | 357 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.21, 0.96] |
| 1.1 No clinical/immunological AIDS | 5 | 331 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.14, 0.82] |
| 1.2 Clinical/immunological AIDS | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | 1.50 [0.59, 2.40] |
Comparison 5. Sensitivity analysis: low risk attrition, detection and performance bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in depression: HAM‐D score: continuous (mean change) | 2 | 117 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.14, 0.60] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Elliott 1998.
| Methods | Randomized controlled trial. Randomization method: not specified. Power: no power calculation reported. Analysis: not ITT analysis. |
|
| Participants |
Country: USA. Setting: outpatient psychiatric and medical clinics in Seattle, USA. Recruitment: responses to advertisements at outpatient clinics. Inclusion criteria: PLWH with a DSM‐III‐R diagnosis of major depression according to SCID interview and HAM‐D score ≥ 18. Exclusion criteria: alcohol or substance abuse in previous month confirmed by urine drug testing, organic brain syndrome, dementia, severe concurrent HIV‐related physical illness, < 12 years of education, high suicide risk or a history of bipolar disorder, traumatic head injury or psychosis. Failure of previous antidepressant regimens was not an exclusion criterion in this study. Number randomized: 75. Number dropped out: 19 (25%) dropped out by 4 weeks, 41 (58%) dropped out by 12 weeks. Age: not reported. Gender: 70 (93%) men. Baseline HAM‐D score (mean): 24.33 (SD 5.66). CD4 T‐cell count (mean): 368 cells/mm3 (SD 307). ART: 19 (25%). Ethnicity: 56 (75%) white race. Socioeconomic details: 56(75%) 'single,' 22 (29%) employed. |
|
| Interventions |
Experimental arm: paroxetine started at 10 mg daily, increased to 20 mg by week 1 and then 40 mg by week 2 if tolerated. Comparison arm 1: imipramine started at 50 mg daily, steadily increased to 100 mg by week 1 and 200 mg by week 2 if tolerated. Comparison arm 2: placebo. Duration: 12 weeks. |
|
| Outcomes | All outcome assessments were carried out at 2, 4, 6, 8 and 12 weeks. Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Date of study: not reported. Funding: not reported. Declarations of conflict of interest by authors: not reported. Other: USD10 payment per visit, only offered after screening. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants were randomly assigned to blind treatment." Uncertain if personal were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All ratings were made by one of two authors who were blinded to study drug assignment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25% had dropped out at 4 weeks and by 12 weeks 58% had dropped out. 8‐week analysis was conducted using LOCF for participants who had dropped between 4 and 8 weeks. The reasons for dropout were unevenly distributed between the 3 groups; however, numbers were too small to make meaningful conclusions about which direction this might bias the results. 4 weeks of antidepressant therapy may not be adequate to evaluate true antidepressant effect. Analysis was conducted with LOCF from 4 weeks for a portion of study population. This would likely bias towards no effect of antidepressants. |
| Selective reporting (reporting bias) | High risk | HAM‐D score was dichotomized with no mean (SD) reported. BSI results not reported but not a major outcome of interest so unlikely to bias study outcome. Authors report quality of life outcome measures for 'responders' and 'non‐responders,' but these results were not given for the individual treatment arms (placebo vs antidepressant). |
| Other bias | Low risk | None noted. |
Hoare 2014.
| Methods | Randomized controlled trial. Randomization method: not specified. Power: sample size (56 participants) calculated to detect a 3‐point difference in MADRS scores at 6 weeks at 5% significance with 20% dropout rate. Analysis: no ITT analysis due to low dropout rate. |
|
| Participants |
Country: South Africa. Setting: primary healthcare clinics in urban setting referred people to tertiary academic centre where study took place Recruitment: semi‐random convenience sample of consecutively screened people attending primary care ART clinics were referred to an academic hospital to participate in the study. Inclusion criteria: PLWH, aged 18‐65 years, DSM‐IV diagnosis of major depressive episode without psychosis. Exclusion: current/last 6 months DSM‐IV diagnosis of alcohol or substance abuse/dependence; lifetime history of bipolar disorder/schizophrenia, other psychotic disorder, dementia; HDS < 10 and MMSE < 23; positive urine for prohibitive substances or medications such as benzodiazepines. Failure of previous antidepressant regimens was not an exclusion criterion in this study. Number randomized: 105. Number dropped out: 3. Age (median): escitalopram: 34 years; placebo: 34 years. Gender: 15 (14%) men. Baseline HAM‐D score (mean): escitalopram: 20 (SD 5.5); placebo: 21 (SD 5.2) Number on ART: unknown. Baseline CD4 T‐cell count (median): escitalopram: 425.5 cells/mm3; placebo: 350 cells/mm3 Ethnicity: no details. Socioeconomic details: no details. |
|
| Interventions | After a median of 7 days (range: 4 to 10 days) of single‐blind placebo in both arms. Experimental arm: escitalopram 10 mg. Comparison arm: matching placebo. Duration: 6 weeks. |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Date of study: 2004. Funding sources: Lundbeck South Africa subsidiary of H. Lundbeck A/S, an international, Danish research‐based pharmaceutical company. Declaration of conflict of interest by authors: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Semi‐random, convenience sample of consecutively screened participants. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The subjects were randomised to treatment of 10mg per day of either escitalopram or matching placebo for the full 6 weeks of the study." Study personnel and participants were blinded according to author communication. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded (communication with author). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout rate: "The drop‐out rate was 2.9%. Reasons for loss to follow‐up were unrelated to side effects and all three participants withdrew within the first 2 weeks of the first study visit." Unknown which treatment group these participants belonged to, but unlikely to have a large impact on study results. |
| Selective reporting (reporting bias) | Low risk | HADS was not reported in results but was included in the secondary outcomes measures in the study methodology. This was unlikely to have an impact on the overall study outcome as this was not a key measure of depression and all other commonly used scales such as MADRS, HAM‐D and CGI are reported. Protocol not reviewed. |
| Other bias | Low risk | None noted. |
Mauri 1994.
| Methods | Randomization controlled trial. Randomization method: not specified. Power: calculation not reported. Analysis: unknown if any dropouts, ITT analysis not stipulated. |
|
| Participants |
Country: Italy. Setting: unknown. Recruitment: unknown. Inclusion criteria: PLWH, DSM‐III‐R diagnosis of adjustment disorders with depressed mood. Exclusion: unknown. Number randomized: 26. Number dropped out: not reported. Age (mean): 35 years. Gender: 19 (73%) men. Baseline HAM‐D score (mean): Fluvoxamine: 30 (SD: 1.3); Placebo: 30(SD: 6.9) Number on ART: 19 on AZT. Baseline CD4 T‐cell count: unknown. HIV clinical staging: 9 people with AIDS (11 people died within 1 year of the trial ending). Ethnicity: no details. Socioeconomic details: no details. |
|
| Interventions |
Experimental arm: fluvoxamine 100‐150 mg 3 times daily. Comparison arm: placebo, unknown frequency. Duration: 8 weeks. |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Date of study: unknown. Funding: not reported. Declaration of conflict of interest by authors: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stimulated how blinding was achieved; however, reported that study was conducted "under double blind conditions." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reported dropouts; however, unclear if all participants were included in the 8‐week assessment of outcome as no total numbers reported only statistical analysis results. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for review but both "significant" (HAM‐D) and "non‐significant" (BPRS) results were reported. |
| Other bias | Unclear risk | Possible that there were several methodological issues that may have biased the results in this study as very little information was provided. |
Patel 2013.
| Methods | Randomized controlled trial. Randomization method: computer generated list of random numbers. Power: no power calculation reported. Analysis: ITT with LOCF. |
|
| Participants |
Country: India. Setting: people with HIV in an "outpatient department with symptoms of depression." Recruitment: uncertain recruitment method. Inclusion criteria: PLWH and on ART for ≥ 6 months, aged ≥ 18 years, meeting DSM‐IV criteria for major depression; HAM‐D score > 13; MADRS score > 19. Exclusion criteria: pregnant or nursing women; hypersensitivity to TCAs or SSRIs, previous use of mirtazapine or escitalopram, history of consumption of any psychotropic medication in past 4 weeks, history of seizures, bipolar disorder or other primary psychiatric diagnosis or abnormal laboratory results or serious disease. Failure of previous antidepressant regimens was not an exclusion criterion in this study. Number randomized: 70. Number dropped out: 8 dropped out by 8 weeks (11.4%). Age (mean): escitalopram: 37.9 years; mirtazapine: 36.8 years. Gender: 30 (43%) men. Baseline HAM‐D score (mean): escitalopram: 36 (SD 6); mirtazapine: 38 (SD 7). CD4 T‐cell count: unknown. ART: 100% receiving HAART. Ethnicity: unknown. Socioeconomic details: unknown. |
|
| Interventions |
Experimental arm: mirtazapine 15 mg once daily (titrated up to 30 mg daily); titrated up from initial dose if improvement < 20% in HAM‐D and MADRS at 4 weeks, downtitrated to 5 mg daily if any adverse effects reported. Comparison arm: escitalopram 10 mg once daily (titrated up to 20 mg daily); titrated up from initial dose if improvement < 20% in HAM‐D and MADRS at 4 weeks, and downtitrated to 7.5 mg daily if any adverse effects reported. Duration: 8 weeks. |
|
| Outcomes | All outcome assessments were carried out at baseline and 2 weekly assessments for 8 weeks. Primary outcomes:
Secondary outcome:
|
|
| Notes |
Date of study: not reported Funding: not reported Declaration of conflict of interest by authors: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unblinded study but comparison was between 2 known effective antidepressants and not placebo, therefore may not have biased the study outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unblinded study but comparison was between 2 known effective antidepressants and not placebo, therefore may not have biased the study outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 (11%) dropouts all lost to follow‐up (5 in escitalopram group; 3 in mirtazapine group). |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported. Protocol not assessed. |
| Other bias | Low risk | None noted. |
Rabkin 1994.
| Methods | Randomized controlled trial. Randomization method: not stated. Power: sample size calculation not stipulated. Analysis: no ITT analysis conducted. |
|
| Participants |
Country: USA. Setting: not stipulated. Recruitment: method unknown. Inclusion criteria: aged 18‐65 years; PLWH, DSM‐III‐R major depression, single or recurrent, with or without dysthymia, minimum HAM‐D score ≥ 14; medically stable; white cell count > 2000 cells/mm3, platelet count > 60,000 cells/mm3, haematocrit > 30%. Exclusion criteria: substantial suicide risk, previous treatment with imipramine ≥ 150 mg during current illness episode; substance abuse in past year, schizophrenia, schizoaffective disorder, bipolar mood disorder, dementia as assessed by modified‐MMSE, imipramine contraindicated. Failure of previous antidepressant regimens was not an exclusion criterion in this study. Number randomized: 97. Number dropped out: 17 (18%). Age (mean): study completers: 38 years; study dropouts: 37 years. Gender: 92 (95%) men. Baseline HAM‐D score (mean): 17.5 (SD 4.1). Number on ART: study completers: 53 (66%); study dropouts: 9 (53%). CD4 T‐cell count (mean): study completers: 301 cells/mm3 (SD 202); study dropouts: 341 cells/mm3 (SD 258). Ethnicity: white: 81, Hispanic: 8; black: 8. Socioeconomic details: full‐time employment: 55; part‐time employment: 11; disability grants: 14; unemployed: 16. |
|
| Interventions |
Experimental arm: imipramine initiated at 50 mg and increased in 50 mg increments to 200 mg at 4 weeks and up to 300 mg after week 4 if poor clinical response. Dispensed weekly. Comparison arm: matching placebo. Duration: randomized assignment continued for 6 weeks. After 6 weeks, blind was broken for treatment non‐responders. All were followed up for up to 26 weeks. |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Date of study: enrolment commenced 1989. Funding: NIMH grant MH‐45652; imipramine and matching placebo supplied by Ciba‐Geigy corporation. Declaration of conflict of interest by authors: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded: "...patients were randomly assigned to imipramine or matching placebo..." "After 6 weeks the blind was broken for nonresponders, while responders where maintained double‐blind for an additional 6 weeks." Unknown whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 18% dropouts, evenly distributed between study arms. Baseline characteristics were similar between dropouts and study completers. A greater number of participants on imipramine dropped out due to adverse effects (n = 7) as compared to those receiving placebo (n = 4), this is unlikely to have affected the assessment of the primary outcome. |
| Selective reporting (reporting bias) | High risk | CGI scores not tabulated with other rating scale results although stipulated as an outcome in the methodology it was only reported as a composite with HAM‐D as 'responder' or 'non‐responder.' |
| Other bias | Low risk | None noted. |
Rabkin 1999.
| Methods | Randomized controlled trial. Randomization method: computer‐generated block randomization; 2:1 for fluoxetine:placebo. Power: not reported. Analysis: ITT analysis reported for some outcomes but not all (CGI only). |
|
| Participants |
Country: USA. Setting: unknown. Recruitment: not specified. Inclusion criteria: known HIV seropositive for ≥ 2 months; physically healthy except HIV‐related conditions; aged 18‐70 years; people with AIDS defining condition had to be receiving treatment with primary care provider; DSM‐IV diagnosis of major depression or dysthymia (or both). Exclusion criteria: psychosis, bipolar disorder, past 6 months substance abuse, panic disorder, current risk of suicide, cognitive impairment, other antidepressant within last 2 weeks, psychotherapy within last 4 weeks, HIV wasting syndrome, significant diarrhoea, unstable health. Failure of previous antidepressant regimens was not an exclusion criterion in this study. Number enrolled: 120. Number dropped out: 33. Age (mean): 39 years. Baseline HAM‐D score (mean): Fluoxetine: 20 (SD 4.7) Placebo: 19 (SD 5.1) CD4 T‐cell count (mean): 295 cells/mm3 (SD 287). ART: 47%. Gender: not reported. Ethnicity: African American (20%), Latino (15%), white (65%). Socioeconomic details: 36% receiving disability benefits. |
|
| Interventions |
Experimental arm: fluoxetine 20 mg for 8 weeks, increased every two weeks by 20 mg if response poor. Comparison arm: placebo. Duration: participants maintained randomized assignment for 8 weeks, then treatment responders continued treatment and follow‐up to week 26. |
|
| Outcomes | All outcomes measured at: baseline, 4 weeks and 8 weeks (and 26 weeks for treatment responders). Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Date of study: 1993. Funding: not reported. Declaration of conflict of interest by authors: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned through computer‐generated blocks of six in a 2:1 ratio to fluoxetine or placebo." |
| Allocation concealment (selection bias) | Unclear risk | Block randomization may lead to lack of allocation concealment as the next assignment may be predictable. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate (27.5%). There were systematic differences between dropouts and completers, dropouts had milder depressive symptoms at baseline. Of 33 dropouts, 24 were on fluoxetine and 9 were on placebo. Difficult to draw conclusions about the impact of this on results. |
| Selective reporting (reporting bias) | High risk | Some reported outcomes were ITT, others were not. This suggests possible selection of reported results. |
| Other bias | Low risk | None noted. |
Rabkin 2004.
| Methods | Randomized controlled trial. Randomization method: computer‐generated list of numbers in blocks of 6. Power: not reported. Analysis: last CGI score brought forward for dropouts for CGI analysis. |
|
| Participants |
Country: USA. Setting: Unknown Recruitment: notices in HIV community publications, physician referral and personal recommendation. Inclusion criteria: men; aged ≥ 18 years; living with HIV; negative prostate specific antigen and digital rectal examination; primary healthcare provider consent; DSM‐IV diagnosis of major depression, subthreshold depression, dysthymia, or a combination of these. Exclusion criteria: current or recent substance use disorder, psychotic symptoms or history of psychosis, significant suicide risk, significant cognitive impairment likely to interfere with study procedures, antidepressant use in past 2‐5 weeks, psychotherapy started in past month, unstable medical condition, symptomatic benign prostatic hyperplasia, current or anticipated change of antiretroviral regimen in next 4 weeks, use of anabolic steroids in past 3 months, unprotected intercourse with partners of unknown or negative HIV serostatus in past 3 months. Failure of previous antidepressant regimens was not an exclusion criterion in this study. Number randomized: 85. Number dropped out: 25 (29.4%). Age (mean): 40‐41 years. Baseline HAM‐D score (mean): fluoxetine: 18.2 (SD 4.5); placebo: 16.8 (SD 3.3). CD4 T‐cell count (mean): fluoxetine: 361 cells/mm3 (SD 237); placebo: 550 cells/mm3 (SD 359). ART (≥ 2 ART medications): fluoxetine: 33 (72%); placebo: 29 (74%). Gender: 100% men. Socioeconomic details: not described. |
|
| Interventions |
Experimental arm 1: fluoxetine 20‐40 mg and placebo IM. Experimental arm 2: placebo tablets and testosterone IM. (Results for this arm not included in this review.) Comparison arm: placebo tablet and placebo IM. Duration: 8 weeks. |
|
| Outcomes | All outcomes were measured 2 weekly. Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Date of study: 1998‐2001. Funding: grant MH R01 MH52037 ‐ NIH Mental Health; Lilly pharmaceutical company provided fluoxetine and placebo; Pharmacia & Upjohn provided testosterone and placebo vials. Declaration of conflict of interest: None Additional information: 3‐armed study with an additional arm receiving placebo tablets and testosterone IM. We did not report results for this arm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated list of numbers in blocks of 6." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded participants with placebo; unknown if personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16/46 (35%) participants dropped out in fluoxetine group; 9/39(23%) participants dropped out in placebo group. High dropout rate. |
| Selective reporting (reporting bias) | Unclear risk | No protocol for evaluation. Described use of QLESQ and BSI but do not report the results. |
| Other bias | Unclear risk | None noted. |
Schwartz 1999.
| Methods | Randomized controlled trial. Randomization method: not specified. Power: no power calculation reported, but authors stated that study was underpowered to do analysis. Analysis: ITT with LOCF (but participants with substance abuse relapse during study were not included in ITT). |
|
| Participants |
Country: USA. Setting: Infectious Disease Program of Grady Health System, a public sector, multidisciplinary medical clinic affiliated with Emory University, serving HIV‐positive people in a metropolitan area. Recruitment: women were referred to the study by medical providers, mental health clinicians and by self‐referral in response to advertisements. Inclusion criteria: aged 18‐70 years with an HIV‐seropositive diagnosis; diagnosis of unipolar major depression according to DSM‐III‐R criteria verified by a modified version of the SCID for DSM‐III‐R; score ≥ 14 of the first 17 items on the 21‐item HAM‐D, with a minimum score of 2 on the "depressed mood" item. Exclusion criteria: presence of other Axis I or Axis II psychiatric diagnoses; substance abuse disorders in 6 months prior to study entry; use of other psychotropic drugs; serious concurrent HIV‐related physical illness; demonstrated placebo response (≥ 20% improvement in the HAM‐D total score between screening and baseline assessment; use of medications known to cause or complicate the treatment of depression; and monoamine oxidase inhibitor use within 14 days of study entry. Failure of previous antidepressant regimens was not an exclusion criterion in this study. Number randomized: 14. Number dropped out: 2 after randomization. Age (mean): fluoxetine: 34.5 years; desipramine: 37.2 years. Baseline HAM‐D score (mean): fluoxetine: 20.88 (SD 6.01); desipramine: 22 (SD 10.82). CD4 T‐cell count (mean): fluoxetine: 167 cells/mm3; desipramine: 191 cells/mm3. ART: not reported. Gender: 100% women. Ethnicity: predominantly African American (fluoxetine: 63%; desipramine: 100%). Socioeconomic details: unemployment: fluoxetine: 88%; desipramine: 83%; 'single:' fluoxetine: 88%; desipramine: 100%; "less than college education": fluoxetine: 88%; desipramine: 83%. |
|
| Interventions |
Experimental arm: fluoxetine 20 mg titrated to optimum dose over 28 days to a maximum 40 mg (morning dosage). Comparison arm: desipramine 75 mg titrated to optimum dose over 28 days to a maximum of 100 mg (evening dosage). Duration: 6 weeks. |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Date of study: not reported. Funding: not reported. Declaration on interest by authors: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded, unclear whether personnel where blinded. "Subjects were then randomly assigned to fluoxetine or desipramine treatment in a double‐blind study design;" "Study drugs were indistinguishable." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 participants were excluded after screening due to substance abuse not previously detected; uncertain whether these were excluded after randomization or whether they were equally distributed across groups Further 2 participants dropped out during the study from the desipramine group. This could have biased the results in either direction depending on which group the 5 excluded participants belonged to and if there were any other systematic differences between these and remaining participants. |
| Selective reporting (reporting bias) | Low risk | None noted. |
| Other bias | Low risk | None noted. |
Targ 1994.
| Methods | Randomized controlled trial. Randomization method: not specified. Power: not specified. Analysis: not ITT. |
|
| Participants |
Country: USA. Setting: unknown. Recruitment: from local community with newspaper advertisements. Inclusion criteria: asymptomatic PLWH receiving AZT, meeting criteria (not specified) for major depression or adjustment disorder with depressed mood and score of ≥ 16 on HAM‐D. Failure of previous antidepressant regimens was not an exclusion criterion in this study. Exclusion criteria: none reported. Number randomized: 20. Number dropped out: 2. Age (mean): 33 years (range 26‐49 years). Gender: 100% men. Baseline HAM‐D score (mean): fluoxetine: 20.8 (SD 5.3); placebo: 19.7 (SD 4.0). CD4 T‐cell count (mean): fluoxetine: 330.2 cells/mm3 (SD 144.7); placebo: 494.5 cell/mm3 (SD 175.8). ART: all participants receiving AZT. Ethnicity: 84% white; 16% Latino. Socioeconomic details: mean 15.5 years of education. |
|
| Interventions |
Experimental arm: fluoxetine 20 mg daily. Comparison arm: placebo daily. Adjunctive treatment: structured group therapy including: relaxation techniques training; problem‐solving skills training; didactic presentations; open discussion. 3 psychotherapy groups of 6‐8 participants run by 4th year psychiatry residents. Standardized through weekly supervision. Duration: 12 weeks. |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Date of study: unknown. Funding: none reported. Declaration on interest by authors: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded to treatment assignment through use of placebo. Unknown if remaining personnel blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 (10%) participants lost to follow‐up, 1 in each group; unlikely to have a significant impact on results. |
| Selective reporting (reporting bias) | Low risk | No evidence. Protocol not assessed. |
| Other bias | Low risk | None noted. |
Zisook 1998.
| Methods | Randomized controlled trial. Randomization method: not specified. Power: no power calculation reported. Analysis: ITT with LOCF. |
|
| Participants |
Country: USA. Setting: HIV‐positive men in a cohort being followed up at the University of California, San Diego HNRC and outpatient psychiatric services. Recruitment: directly referred from HNRC or from the UC San Diego outpatient psychiatric services. Inclusion criteria: PLWH and meeting CDC (1986) class II, III or IV.C.2 criteria; major depressive episode of moderate to severe intensity (SCID for DSM‐III‐R and DSM‐III‐R criteria) with symptoms for ≥ 4 weeks. Exclusion criteria: acutely ill; taking psychotropic medications; current alcohol abuse or other drug abuse; cognitive impairment as measured by a score of > 27 on MMSE; suicidality as measured by a score of 0 or 1 on item 3 of HAM‐D, psychosis or bipolar mood disorder. Failure of previous antidepressant regimens was not an exclusion criterion in this study. Number randomized: 47. Number dropped out: 10 dropped out by 7 weeks (21%). Age (mean): fluoxetine: 36.2 years; placebo: 34.9 years. Baseline HAM‐D score (mean): 20.2 (SD unknown). CD4 T‐cell count: not reported. Baseline clinical staging: CDC Category A or B HIV disease (1993 Classification system). ART: 80% of those referred to the study were on ≥ 1 antiretroviral agent. Gender: 100% men. Ethnicity: unknown. Socioeconomic details: years of education: fluoxetine: 13.4 years; placebo: 13.5 years. |
|
| Interventions |
Experimental arm: fluoxetine 20 mg for 3 weeks, increased to 40 mg in week 4 or 60 mg in week 5 if required, dose could be decreased. Intervention arm: identical placebo. Adjunctive therapy: all participants assigned to a concomitant supportive and educative psychotherapy group. Groups were conducted by male licensed clinical social worker and female predoctoral level psychology graduate student. "The group emphasized education about HIV, depression, mutual support, sharing, coping strategies and utilizing community resources." Duration: 7 weeks. |
|
| Outcomes | All outcome assessments were carried out at baseline and weekly assessments for 7 weeks. Primary outcomes:
Secondary outcome:
|
|
| Notes |
Date of study: not reported. Funding: NIH grant MH45294. Fluoxetine and placebo provided by Eli Lilly Company. Declaration of conflict of interest by authors: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and therapists were blinded, unknown if other study personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4/25 participants in fluoxetine group and 6/22 in placebo group did not complete the study. Overall, 21% of participants randomized did not complete study. There did not seem to be systematic differences between participants who dropped out in either group. |
| Selective reporting (reporting bias) | Low risk | None noted. |
| Other bias | Low risk | None noted. |
ART: antiretroviral therapy; AZT: zidovudine (also known as azidothymidine); BDI: Beck Depression Inventory; BPRS: Brief Psychiatric Rating Scale; BSI: Brief Symptom Inventory; CD4: cluster of differentiation 4; CD8: cluster of differentiation 8; CDC: Centers for Disease Control and Prevention; CGI: Clinical Global Impression; CGI‐I: Clinical Global Impression of Improvement; CGI‐S: Clinical Global Impression of Severity; DOTES: Dosage Record and Treatment of Symptoms Scale; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, third edition revised; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, fourth edition; HAART: highly active antiretroviral therapy; HADS: Hospital Anxiety and Depression Scale; HAM‐D: Hamilton Depression Rating Scale; HDS: Hasegawa's Dementia Scale; HNRC: HIV Neurobehavioural Research Center; IM: intramuscular; ITT: intention to treat; LOCF: last observation carried forward; MADRS: Montgomery‐Åsberg Depression Rating Scale; MMSE: Mini‐Mental State Examination; n: number of participants; NIMH: National Institute of Mental Health; PLWH: people living with HIV; QLESQ: Quality of Life Enjoyment and Satisfaction Questionnaire; SAFTEE: Systematic Assessment for Treatment Emergent Effects; SAS: Social Adjustment Scale‐Self Report; SCID: Structured Clinical Interview for DSM; SD: standard deviation; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant.
For explanation of rating scales see Appendix 1.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brown 2016 | Compared cognitive behavioural therapy plus medication treatment algorithm with treatment as usual. |
| Chibanda 2014 | Compared antidepressant therapy with psychological intervention. |
| Markowitz 1998 | Inclusion criteria did not include DSM or ICD diagnostic criteria for current depressive episode, rather HAM‐D score and clinical judgement. |
| NCT00285584 | Study population not primarily PLWH. Outcome was reduction in HIV risk behaviour. |
| Pence 2015 | Evaluated effect of a treatment care model and not antidepressant effect. |
| Stein 2005 | Study population not HIV infected. Outcome was reduction in HIV risk behaviour. |
| Tsai 2013 | Intervention was directly observed antidepressant treatment compared to standard of care (same antidepressant given in both groups). |
DSM: Diagnostic and Statistical Manual of Mental Disorders; HAM‐D: Hamilton Depression Rating Scale; ICD: International Statistical Classification of Diseases; PLWH: people living with HIV.
Characteristics of ongoing studies [ordered by study ID]
NCT02620150.
| Trial name or title | SSRI Effects on Depression and Immunity in HIV/AIDS. |
| Methods | Randomized controlled trial of escitalopram or placebo with background computerized cognitive behavioural therapy provided to both treatment arms. |
| Participants | Adults aged ≥ 18 years with HIV infection, on ART and SCID diagnosis of major depression. |
| Interventions | Escitalopram or placebo with background computerized cognitive behavioural therapy in both treatment arms. |
| Outcomes | Change in: natural killer cell activity, intracellular interferon gamma, plasma interleukin 6 and plasma C‐reactive protein. |
| Starting date | January 2016. |
| Contact information | Dwight L Evans, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA, 19104. |
| Notes | ClinicalTrials.gov identifier: NCT02620150. |
ART: antiretroviral therapy; SCID: Structured Clinical Interview for DSM (Diagnostic and Statistical Manual of Mental Disorders).
Differences between protocol and review
1. Background
A Description of the intervention and a section detailing How the intervention might work was added to the background.
2. Objectives
Secondary Objectives were removed in linke with the lastest requirements for Cochrane reviews.
3. Methods
We expanded our inclusion criteria to include studies where other DSM or ICD depressive diagnoses such as dysthymia and subthreshold depression were included due to the limited number of studies available for evaluation (Types of participants).
We removed psychological therapies from the comparison group when describing the types of interventions to be evaluated. The author team felt that the main rationale for this review was to determine if the neurological effects of HIV infection and if the drug interactions which occur between antiretroviral and antidepressant agents would lead to differential responses to antidepressants drugs in people living with HIV. Therefore, we focused on specific antidepressant drugs and drug classes (Types of interventions).
We removed psychostimulant therapies from the intervention and comparison group when describing the types of interventions to be evaluated. There is no evidence to suggest that psychostimulants are an effective treatment for depression and are not recommended in treatment of depression for people living with or without HIV (Types of interventions).
Atypical agents such as bupropion, mirtazapine, reboxetine, agomelatine, mianserin and maprotiline were included as these are recognized treatments for depression and should be included in a comprehensive review of antidepressant drugs (Types of interventions).
Studies where another adjunctive therapy (such as psychological therapy) is provided equally in both groups were added to the types of included studies. Providing both antidepressants and psychological therapies is common practice and if provided equally between both groups should not have an effect on the study results (Types of interventions).
Study dropout rate was changed to a primary outcome instead of secondary outcome to reflect potential harm as well as benefit in the Primary outcomes.
Mild/moderate adverse effects were added as Secondary outcomes as these were considered to be relevant to consumers.
Sections describing in detail the timing of outcome assessments and hierarchy of outcome measures were added to expand on what specific data points would be used from the selected studies (Types of outcome measures).
The Search methods for identification of studies was adapted to the methods used by the Cochrane Common Mental Disorders (CCMD) Group. This review was initially managed by the HIV review group; however, this group was disbanded and this review was adopted by the CCMD Group. Our protocol and search methods were adapted to methods used routinely by the CCMD Group.
Multiple imputation was not used to deal with missing data as this was not feasible. We did an intention‐to‐treat analysis where possible if data were missing (Dealing with missing data).
Where there were multiple treatment arms, we did not combine treatment arms, rather we chose to divide the placebo group between treatment arms (Unit of analysis issues). This occurred specifically for the study Elliott 1998, where there were three treatment arms; an SSRI arm, a TCA arm and a placebo arm. To avoid counting the same participants twice in the antidepressant versus placebo subgroup analysis, we split the number of participants in the placebo group between the two treatment arms.
There was no data evaluating time to resolution of symptoms and, therefore, evaluation of survival analyses was removed from the 'Methods.'
3. Data and analysis
A section was added on 'main planned comparisons' to state a‐priori the plan for analysis of the data (Data extraction and management).
More explicit details were added to the Assessment of heterogeneity section to facilitate the analysis process.
The number of sensitivity analyses were changed from four to two as it was deemed that the only relevant sensitivity analysis would be the evaluation of studies with a high or low risk of bias. GRADE was considered to reflect quality of evidence as opposed to risk of bias and this was, therefore, removed from sensitivity analyses. It was considered that performing a meta‐analysis of small versus large studies would not add any further information and this comparison was dropped (Sensitivity analysis).
Placebo response rate was added to the results to be comprehensive in the description of the included studies (Table 6).
Contributions of authors
Study concept and design: all review authors.
Drafting and revision of the manuscript: all review authors.
Sources of support
Internal sources
Department of Psychiatry and Mental Health, University of Cape Town, South Africa.
External sources
-
Cochrane HIV/AIDS Mentoring Programme, South Africa.
Based at the South African Cochrane Centre, Medical Research Council, and funded by the Cochrane HIV/AIDS Review Group
Declarations of interest
IEW: is supported by the Effective Health Care Research Consortium. This Consortium is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy. She was also supported by a fellowship offered by the Effective Health Care Research Consortium. This Consortium is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy. In 2009 she was further supported by South African Tuberculosis AIDS training, Fogarty international NIH grant (1U2RTW007370).
NS: none.
DA: none.
DJS: between 2011 and 2014, Dr Stein received research grants or consultancy honoraria (or both) from AMBRF, Biocodex, Cipla, Lundbeck, National Responsible Gambling Foundation, Novartis, Servier, and Sun. Prior to 2011, Dr Stein has received research grants or consultancy honoraria (or both) from Abbott, AMBRF, Astrazeneca, Biocodex, Eli‐Lilly, GlaxoSmithKline, Jazz Pharmaceuticals, Johnson & Johnson, Lundbeck, National Responsible Gambling Foundation, Novartis, Orion, Pfizer, Pharmacia, Roche, Servier, Solvay, Sumitomo, Sun, Takeda, Tikvah, and Wyeth. None of this past support influences Dr Stein's current work on this Cochrane Review.
EAO: none.
JJ: none.
Edited (no change to conclusions)
References
References to studies included in this review
Elliott 1998 {published data only}
- Elliot AJ, Russo J, Roy‐Byrne PP. The effect of changes in depression on health related quality of life (HRQoL) in HIV infection. General Hospital Psychiatry 2002;24:43‐7. [DOI] [PubMed] [Google Scholar]
- Elliot AJ, Uldhall KK, Bergam K, Russo J, Claypoole K, Roy‐Byrne PP. Randomized, placebo‐controlled trial of paroxetine versus imipramine in depressed HIV‐positive outpatients. American Journal of Psychiatry 1998;155:367‐72. [DOI] [PubMed] [Google Scholar]
Hoare 2014 {published and unpublished data}
- Hoare J, Carey P, Joska JA, Carrara H, Sorsdahl K, Stein DJ. Escitalopram treatment of depression in human immunodeficiency virus/acquired immunodeficiency syndrome: a randomized, double‐blind, placebo‐controlled study. Journal of nervous and mental disease. Journal of Nervous and Mental Disease 2014;202:133‐7. [DOI] [PubMed] [Google Scholar]
Mauri 1994 {published data only}
- Mauri MC, Ferrara A, Fabiano L, Ricci C, Invernizzi G. A double blind study on fluvoxamine vs. placebo in depressed HIV positive patients: short‐term and perspective results. Integrative Psychiatry 1994;10:199‐201. [Google Scholar]
Patel 2013 {published data only}
- Patel S, Kukreja S, Atram U, De SA, Shah N, Yadav S, et al. Escitalopram and mirtazapine for the treatment of depression in HIV patients: a randomized controlled open label trial. ASEAN Journal of Psychiatry 2013;14:31‐9. [Google Scholar]
Rabkin 1994 {published data only}
- Rabkin JG, Rabkin R, Harrison W, Wagner G. Effect of imipramine on mood and enumerative measures of immune status in depressed patients with HIV illness. American Journal of Psychiatry 1994;151:516‐23. [DOI] [PubMed] [Google Scholar]
Rabkin 1999 {published data only}
- Rabkin JG, Wagner GJ, Rabkin R. Fluoxetine treatment for depression in patients with HIV and Aids: a randomized, placebo‐controlled trial. American Journal of Psychiatry 1999;156:101‐7. [DOI] [PubMed] [Google Scholar]
- Wagner GJ, Maguen S, Rabkin JG. Ethnic differences in response to fluoxetine in a controlled trial with depressed HIV‐positive patients. Psychiatric Services (Washington, D.C.) 1998;49:239‐40. [DOI] [PubMed] [Google Scholar]
Rabkin 2004 {published data only}
- Rabkin JG, Wagner GJ, McElhiney MC, Rabkin R, Lin SH. Testosterone versus fluoxetine for depression and fatigue in HIV/AIDS. A placebo‐controlled trial. Clinical Psychopharmacology 2004;24(4):379‐85. [DOI] [PubMed] [Google Scholar]
Schwartz 1999 {published data only}
- Schwartz J. Double‐blind comparison of fluoxetine and desipramine in the treatment of depressed women with advanced HIV disease: a pilot study. Depression and Anxiety 1999;9(2):70‐4. [DOI] [PubMed] [Google Scholar]
Targ 1994 {published data only}
- Targ EF, Karasic DH, Diefenbach PN, Anderson DA, Bystritsky A, Fawzy FI. Structured group therapy and fluoxetine to treat depression in HIV‐positive persons. Psychosomatics 1994;35:132‐7. [DOI] [PubMed] [Google Scholar]
Zisook 1998 {published data only}
- Zisook S, Perekin J, Goggin KJ, Sledge P, Atkinson JH, Grant I. Treatment of major depression in HIV‐seropositive men. HIV Neurobehavioral Research Center Group. Journal of Clinical Psychiatry 1998;59:217‐24. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Brown 2016 {published data only}
- Brown LK, Kennard BD, Emslie GJ, Mayes TL, Whiteley LB, Bethel J, et al. Effective treatment of depressive disorders in medical clinics for adolescents and young adults living with HIV: a controlled trial. Journal of Acquired Immune Deficiency Syndromes 2016;71:38‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chibanda 2014 {published data only}
- Chibanda D, Shetty AK, Tshimanga M, Woelk G, Stranix‐Chibanda L, Rusakaniko S. Group problem‐solving therapy for postnatal depression among HIV‐positive and HIV‐negative mothers in Zimbabwe. Journal of the International Association of Providers of AIDS Care 2014;13(4):335‐41. [DOI] [PubMed] [Google Scholar]
Markowitz 1998 {published data only}
- Markowitz JC, Kocsis JH, Fishman B, Spielman LA, Jacobsberg LB, Frances AJ, et al. Treatment of depressive symptoms in human immunodeficiency virus‐positive patients. Archives of General Psychiatry 1998;55:452‐7. [DOI] [PubMed] [Google Scholar]
NCT00285584 {published data only}
- Marmor. Drug abuse, depression and responses to HIV counseling. clinicaltrials.gov/show/NCT00285584 Date first received: 2 February 2006.
Pence 2015 {published data only}
- Pence BW, Gaynes BN, Adams JL, Thielman NM, Heine AD, Mugavero MJ, et al. The effect of antidepressant treatment on HIV and depression outcomes: the SLAM DUNC randomized trial. AIDS 2015;19(15):1975‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2005 {published data only}
- NCT00228007. Antidepressant medication for reducing HIV risk behavior in depressed intravenous drug users. clinicaltrials.gov/show/NCT00228007 Date first registered: 28 September 2005.
- Stein MD, Anderson BJ, Solomon DA, Herman DS, Ramsey SE, Brown RA, et al. Reductions in HIV risk behaviors among depressed drug injectors. American Journal of Drug and Alcohol Abuse 2005;31:417‐32. [DOI] [PubMed] [Google Scholar]
Tsai 2013 {published data only}
- Grelotti DJ, Hammer GP, Dilley JW, Karasic DH, Sorensen JL, Bangsberg DR, et al. Does substance use compromise depression treatment? A randomized trial of homeless HIV+ persons. 21st Conference on Retroviruses and Opportunistic Infections, CROI; 2014 Mar 3‐6; Boston (MA) 2014.
- Tsai AC, Karasic DH, Hammer GP, Charlebois ED, Ragland K, Moss AR, et al. Directly observed antidepressant medication treatment and HIV outcomes among homeless and marginally housed HIV‐positive adults: a randomized controlled trial. American Journal of Public Health 2013;103:308‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT02620150 {unpublished data only}
- NCT02620150. SSRI effects on depression and immunity in HIV/AIDS. clinicaltrials.gov/show/NCT02620150 Date fist received: 2 December 2015. [NCT02620150]
Additional references
Akena 2012
- Akena D, Musisi S, Joska J, Stein DJ. The association between AIDS related stigma and major depressive disorder among HIV‐positive individuals in Uganda. PloS One 2012;7(11):e48671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ammassari 2004
- Ammassari A, Antinori A, Aloisi MS, Trotta MP, Murri R, Bartoli L, et al. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV‐infected persons. Psychosomatics 2004;45(5):394‐402. [DOI] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Arlington (VA): American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Arlington (VA): American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Arlington (VA): American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Arlington (VA): American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Arlington (VA): American Psychiatric Association, 2013. [Google Scholar]
Beck 1961
- Beck AT, Ward C, Mendelson M. "Beck Depression Inventory (BDI)". Archives of General Psychiatry 1961;4(6):561‐71. [DOI] [PubMed] [Google Scholar]
Bing 2001
- Bing EG, Burnam A, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human Immunodeficiency virus‐Infected adults in the United States. Archives of General Psychiatry 2001;58(8):721‐8. [DOI] [PubMed] [Google Scholar]
Cavalcante 2010
- Cavalcante GIT. Implications of efavirenz for neuropsychiatry: a review. International Journal of Neuroscience 2010;120:739‐45. [DOI] [PubMed] [Google Scholar]
CDC 2008
- Schneider E, Whitmore S, Glynn MK, Dominguez K, Mitsch A, McKenna MT, et al. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years ‐ United States, 2008. MMWR. Recommendations and Reports : Morbidity and Mortality Weekly Report 2008; Vol. 5, issue 57(RR‐10):1‐12. [PubMed]
Cespedes 2006
- Cespedes MS, Aberg JA. Neuropsychiatric complications of antiretroviral therapy. Drug Safety 2006;29(10):865‐74. [DOI] [PubMed] [Google Scholar]
Chibanda 2015
- Chibanda D, Cowan FM, Healy JL, Abas M, Lund C. Psychological interventions for common mental disorders for people living with HIV in low‐ and middle‐income countries: systematic review. Tropical Medicine and International Health 2015;20(7):830‐9. [DOI] [PubMed] [Google Scholar]
Ciesla 2001
- Ciesla JA, Roberts JE. Meta‐analysis of the relationship between HIV infection and risk for depressive disorders. American Journal of Psychiatry 2001;158(5):725‐30. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Erlbaum, 1988. [Google Scholar]
Cohen 2001
- Cohen DE, Walker BD. Human immunodeficiency virus pathogenesis and prospects for immune control in patients with established infection. Clinical Infectious Diseases 2001;32(12):1756‐68. [DOI] [PubMed] [Google Scholar]
Collins 2006
- Collins PY, Holman AR, Freeman MC, Patel V. What is the relevance of mental health to HIV/AIDS care and treatment programs in developing countries? A systematic review. AIDS 2006;20:1571‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Connell 2014
- Connell J, O'Cathian AO, Brazier J. Measuring quality of life in mental health: are we asking the right questions?. Social Science and Medicine 2014;120:12‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cruess 2003
- Cruess DG, Evans DL, Repetto MJ, Gettes D, Douglas SD, Petitto JM. Prevalence, diagnosis, and pharmacological treatment of mood disorders in HIV disease. Biological Psychiatry 2003;54(3):307‐16. [DOI] [PubMed] [Google Scholar]
DAIDS 2009
- National Institute of Allergy and Infectious Diseases. Division of AIDS table for grading the severity of adult and pediatric adverse events version 1.0, December 2004; clarification August 2009. rsc.tech‐res.com/docs/default‐source/safety/table_for_grading_severity_of_adult_pediatric_adverse_events.pdf (accessed 30 October 2017).
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Del Guerra 2013
- Guerra FB, Fonseca JLI, Figueiredo VM, Ziff EB, Castelon Konkiewitz E. Human immunodeficiency virus‐associated depression: contributions of immuno‐inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. Journal of Neurovirology 2013;19:314‐27. [DOI] [PubMed] [Google Scholar]
DeSilva 2001
- DeSilva KE, Flore DB, Marston BJ, Rimland D. Serotonin syndrome in HIV‐infected individuals receiving antiretroviral therapy and fluoxetine. AIDS 2001;15:1281‐5. [DOI] [PubMed] [Google Scholar]
Dew 1997
- Dew MA, Becker JT, Sanchez J, Caldarraro R, Lopez OL, Wess J, et al. Prevalence and predictors of depressive, anxiety and substance use disorders in HIV‐infected and uninfected men: a longitudinal evaluation. Psychological Medicine 1997;27:395‐409. [DOI] [PubMed] [Google Scholar]
DiMatteo 2000
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta‐analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine 2000;160(14):2101‐7. [DOI] [PubMed] [Google Scholar]
Eaton 2004
- Eaton WW, Muntaner C, Smith C, Tien A, Ybarra M. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD‐R). The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. 3rd Edition. Mahwah (NJ): Lawrence Erlbaum, 2004:363‐77. [Google Scholar]
Elliott 2002
- Elliott AJ, Russo J, Roy‐Byrne PP. The effect of changes in depression on health related quality of life (HRQoL) in HIV infection. General Hospital Psychiatry 2002;24(1):43‐7. [DOI] [PubMed] [Google Scholar]
Endicott 1993
- Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacology Bulletin 1993;29(2):321‐6. [PubMed] [Google Scholar]
Fernandez 1991
- Fernandez F, Levy JK. Psychopharmacotherapy of psychiatric syndromes in asymptomatic and symptomatic HIV infection. Psychiatric Medicine 1991;9(3):377‐94. [PubMed] [Google Scholar]
Foley 2008
- Foley J, Ettenhofer M, Wright M, Hinkin CH. Emerging issues in the neuropsychology of HIV infection. Current HIV/AIDS Reports 2008;5(4):204‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaida 2016
- Gaida R, Truter I, Grobler C, Kotze T, Godman B. A review of trials investigating efavirenz‐induced neuropsychiatric side effects and the implications. Expert Review of Anti‐infective Therapy 2016;14(4):377‐88. [DOI] [PubMed] [Google Scholar]
Gaynes 2012
- Gaynes BN, Pence BW, Atashili J, O'Donnel J, Kats D, Ndumbe PM. Prevalence and predictors of major depression in HIV‐infected patients on antiretroviral therapy in Bamenda, a semi‐urban center in Cameroon. PloS One 2012;7:e41699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghafouri 2006
- Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV‐1 associated dementia: symptoms and causes. Retrovirology 2006;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibbs 2016
- Gibbs A, Govender K, Jewkes R. An exploratory analysis of factors associated with depression in a vulnerable group of young people living in informal settlements in South Africa. Global Public Health Aug 2016 [Epub ahead of print]:1‐17. [DOI: 10.1080/17441692.2016.1214281] [DOI] [PubMed] [Google Scholar]
Gorman 1991
- Gorman JM, Kertzner R, Cooper T, Goetz RR, Lagomasino I, Novacenko H, et al. Glucocorticoid level and neuropsychiatric symptoms in homosexual men with HIV infection. American Journal of Psychiatry 1991;148(1):41‐5. [DOI] [PubMed] [Google Scholar]
Grant 2008
- Grant I. Neurocognitive disturbances in HIV. International Review of Psychiatry 2008;20(1):33‐47. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised. Rockville (MD): US Department of Health, Education, and Welfare, 1976. [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Hamilton 1980
- Hamilton M. Rating depressive patients. Journal of Clinical Psychiatry 1980;41:21‐4. [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions. 5.0.0. Chichester (UK): John Wiley & Sons, 2009. [Google Scholar]
Higgins 2010
- Higgins A, Nash M, Lynch AM. Antidepressant‐associated sexual dysfunction: impact, effects and treatment. Drug, Healthcare and Patient Safety 2010;2:141‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Himelhoch 2005
- Himelhoch S, Medoff DR. Efficacy of antidepressant medication among HIV positive individuals with depression: a systematic review and meta‐analysis. AIDS Patient Care and STDs 2005;19(12):813‐22. [DOI] [PubMed] [Google Scholar]
Honagodu 2013
- Honagodu AR, Kirshna M, Sundarachar R, Lepping P. Group psychotherapies for depression in persons with HIV: a systematic review. Indian Journal of Psychiatry 2013;55(4):323‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horberg 2008
- Horberg MA, Silverberg MJ, Hurley LB, Towner WJ, Klein DB, Bersoff‐Matcha S, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV‐infected patients. Journal of Acquired Immune Deficiency Syndromes 2008;47:384‐90. [DOI] [PubMed] [Google Scholar]
Huang 1990
- Huang XL, Cai Q, Rinaldo CR Jr. Natural killer cell responses in homosexual men with early HIV infection. Journal of Acquired Immune Deficiency Syndrome 1990;3:669‐76. [PubMed] [Google Scholar]
Illa 2014
- Illa L, Echenique M, Bustamante‐Avellaneda V, Sanchez‐Martinez M. Review of recent behavioural interventions targeting older adults living with HIV/AIDS. Current HIV/AIDS Reports 2014;11:413‐42. [DOI] [PubMed] [Google Scholar]
Kinyanda 2011
- Kinyanda E, Hoskins S, Nakku J, Nawaz S, Patel V. Prevalence and risk factors of major depressive disorder in HIV/AIDS as seen in semi‐urban Entebbe district, Uganda. BioMed Central Psychiatry 2011;11:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leserman 1997
- Leserman J, Petitto JM, Perkins DO, Folds JD, Golden RN, Evans DL. Severe stress, depressive symptoms, and changes in lymphocyte subsets in human immunodeficiency virus‐infected men. A 2‐year follow‐up study. Archives of General Psychiatry 1997;54(3):279‐85. [DOI] [PubMed] [Google Scholar]
Lichtenstein 2002
- Lichtenstein B, Laska MK, Clair JM. Chronic sorrow in the HIV‐positive patient: issues of race, gender, and social support. AIDS Patient Care and STDs 2002;16(1):27‐38. [DOI] [PubMed] [Google Scholar]
Lima 2007
- Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV‐infected individuals first initiating HAART. AIDS 2007;21(9):1175‐83. [DOI] [PubMed] [Google Scholar]
Machado 2006
- Machado M, Iskedjian M, Ruiz I, Einarson TR. Remission, dropouts, and adverse drug reaction rates in major depressive disorder: a meta‐analysis of head‐to‐head trials. Current Medical Research and Opinion 2006;22(9):1825‐37. [DOI] [PubMed] [Google Scholar]
McArthur 2005
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurology 2005;4(9):543‐55. [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134(4):382‐9. [DOI] [PubMed] [Google Scholar]
Morrison 2002
- Morrison MF, Petitto JM, Have T, Gettes DR, Chiappini MS, Weber AL, et al. Depressive and anxiety disorders in women with HIV infection. American Journal of Psychiatry 2002;159(5):789‐96. [DOI] [PubMed] [Google Scholar]
Nakasujja 2010
- Nakasujja N, Skolasky RL, Musisi S, Allebeck P, Robertson K, Ronald A, et al. Depression symptoms and cognitive function among individuals with advanced HIV infection initiating HAART in Uganda. BioMed Central Psychiatry 2010;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakimuli‐Mpungu 2011
- Nakimuli‐Mpungu E, Musisi S, Katabira E, Nachega J, Bass J. Prevalence and factors associated with depressive disorders in an HIV positive rural patient population in southern Uganda. Journal of Affective Disorders 2011;135(1‐3):160‐7. [DOI] [PubMed] [Google Scholar]
Nanni 2015
- Nanni MG, Caruso R, Mitchel AJ, Meggiolaro E, Grassi L. Depression in HIV infected patients: a review. Current Psychiatry Reports 2015;17(530):7‐11. [DOI] [PubMed] [Google Scholar]
Nelson 2009
- Nelson JC, Papkostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta‐analysis of placebo‐controlled randomized trials. American Journal of Psychiatry 2009;166(9):980. [DOI] [PubMed] [Google Scholar]
NICE 2009
- National Institute for Health and Clinical Excellence. Depression in adults: the treatment and management of depression in adults. 2009 (updated 2016). www.nice.org.uk/guidance/CG90 (accessed prior to 30 October 2017).
NICE 2015
- National Institute for Health and Care Excellence. First‐choice antidepressant use in adults with depression or generalised anxiety disorder, 2015. www.nice.org.uk/advice/ktt8/chapter/options‐for‐local‐implementation (accessed prior to 30 October 2017).
Nott 1999
- Nott KH, Vedhara K. Nature and consequences of stressful life events in homosexual HIV‐positive men: a review. AIDS Care 1999;11(2):235‐43. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rutherford 2013
- Rutherford BR. A model of placebo response in antidepressant clinical trials. American Journal of Psychiatry 2013;170(7):723‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schuster 2012
- Schuster R, Bornovalova M, Hunt E. The influence of depression on the progression of HIV: direct and indirect effects. Behaviour Modification 2012;36(2):123‐45. [DOI] [PubMed] [Google Scholar]
Siccardi 2013
- Siccardi M, Marzolini C, Seden K, Almond L, Kirov A, Khoo S, et al. Prediction of drug‐drug interactions between various antidepressants and efavirenz or boosted protease inhibitors using a physiologically based pharmacokinetic modelling approach. Clinical Pharmacokinetics 2013;52:583‐92. [DOI] [PubMed] [Google Scholar]
Spitzer 1999
- Spitzer R, Kroenke K, Williams J. Validation and utility of a self‐report Version of PRIME‐MD: the PHQ Primary Care Study. Journal of the American Medical Association 1999;282:1737‐44. [DOI] [PubMed] [Google Scholar]
Springer 2012
- Springer SA, Dushaj A, Azar MM. The impact of DSM‐IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. AIDS Behaviour 2012;16:2119‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2006
- Stein DJ, Baldwin DS, Dolberg OT, Despiegel N, Bandelow B. Which factors predict placebo response in anxiety disorders and major depression? An analysis of placebo‐controlled studies of escitalopram. Journal of Clinical Psychiatry 2006;67(11):1741‐6. [DOI] [PubMed] [Google Scholar]
Tuynman‐Qua 1997
- Tuynman‐Qua H, Jonghe F, McKenna SP. Quality of Life in Depression Scale (QLDS). Development, reliability, validity, responsiveness and application. European Psychiatry 1997;12(4):199‐202. [DOI] [PubMed] [Google Scholar]
UNAIDS 2016
- UNAIDS. Fact sheet 2016. www.unaids.org/en/resources/fact‐sheet (accessed prior to 30 October 2017).
Unnikrishnan 2012
- Unnikrishnan B, Jagannath V, Ramapuram JT, Achappa B, Madi D. Study of depression and its associated factors among women living with HIV/AIDS in coastal south India. International Scholarly Research Network AIDS 2012;2012(ID 684972):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
US FDA 2013b
- United States Food, Drug Administration. Prescribing information SUSTIVA. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020972s043,021360s031lbl.pdf (accessed prior to 30 October 2017).
US FDA 2013a
- United States Food, Drug Administration. Labeling Information KALETRA. www.accessdata.fda.gov/drugsatfda_docs/label/2013/021226s038lbl.pdf (accessed prior to 30 October 2017).
Uthman 2014
- Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low‐, middle‐ and high‐income countries: a systematic review and meta‐analysis. Current HIV/AIDS Reports 2014;11(3):291‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2014
- Wagner GJ, Ghosh‐Dastidar B, Slaughter M, Akena D, Nakasujja N, Okello E, et al. The role of depression in work‐related outcomes of HIV treatment in Uganda. International Journal of Behavioural Medicine 2014;21(6):946‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2002
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression. Variable, substantial, and growing. JAMA 2002;287(14):1840‐7. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD‐10). Geneva (Switzerland): World Health Organization, 1992. [Google Scholar]
WHO 2015
- World Health Organization. WHO mhGap guideline update, 2015. apps.who.int/iris/bitstream/10665/204132/1/9789241549417_eng.pdf?ua=1 (accessed prior to 30 October 2017).