Abstract
Background
Femoro‐popliteal bypass is implemented to save limbs that might otherwise require amputation, in patients with ischaemic rest pain or tissue loss; and to improve walking distance in patients with severe life‐limiting claudication. Contemporary practice involves grafts using autologous vein, polytetrafluoroethylene (PTFE) or Dacron as a bypass conduit. This is the second update of a Cochrane review first published in 1999 and last updated in 2010.
Objectives
To assess the effects of bypass graft type in the treatment of stenosis or occlusion of the femoro‐popliteal arterial segment, for above‐ and below‐knee femoro‐popliteal bypass grafts.
Search methods
For this update, the Cochrane Vascular Information Specialist searched the Vascular Specialised Register (13 March 2017) and CENTRAL (2017, Issue 2). Trial registries were also searched.
Selection criteria
We included randomised trials comparing at least two different types of femoro‐popliteal grafts for arterial reconstruction in patients with femoro‐popliteal ischaemia. Randomised controlled trials comparing bypass grafting to angioplasty or to other interventions were not included.
Data collection and analysis
Both review authors (GKA and CPT) independently screened studies, extracted data, assessed trials for risk of bias and graded the quality of the evidence using GRADE criteria.
Main results
We included nineteen randomised controlled trials, with a total of 3123 patients (2547 above‐knee, 576 below‐knee bypass surgery). In total, nine graft types were compared (autologous vein, polytetrafluoroethylene (PTFE) with and without vein cuff, human umbilical vein (HUV), polyurethane (PUR), Dacron and heparin bonded Dacron (HBD); FUSION BIOLINE and Dacron with external support). Studies differed in which graft types they compared and follow‐up ranged from six months to 10 years.
Above‐knee bypass
For above‐knee bypass, there was moderate‐quality evidence that autologous vein grafts improve primary patency compared to prosthetic grafts by 60 months (Peto odds ratio (OR) 0.47, 95% confidence interval (CI) 0.28 to 0.80; 3 studies, 269 limbs; P = 0.005). We found low‐quality evidence to suggest that this benefit translated to improved secondary patency by 60 months (Peto OR 0.41, 95% CI 0.22 to 0.74; 2 studies, 176 limbs; P = 0.003).
We found no clear difference between Dacron and PTFE graft types for primary patency by 60 months (Peto OR 1.67, 95% CI 0.96 to 2.90; 2 studies, 247 limbs; low‐quality evidence). We found low‐quality evidence that Dacron grafts improved secondary patency over PTFE by 24 months (Peto OR 1.54, 95% CI 1.04 to 2.28; 2 studies, 528 limbs; P = 0.03), an effect which continued to 60 months in the single trial reporting this timepoint (Peto OR 2.43, 95% CI 1.31 to 4.53; 167 limbs; P = 0.005).
Externally supported prosthetic grafts had inferior primary patency at 24 months when compared to unsupported prosthetic grafts (Peto OR 2.08, 95% CI 1.29 to 3.35; 2 studies, 270 limbs; P = 0.003). Secondary patency was similarly affected in the single trial reporting this outcome (Peto OR 2.25, 95% CI 1.24 to 4.07; 236 limbs; P = 0.008). No data were available for 60 months follow‐up.
HUV showed benefits in primary patency over PTFE at 24 months (Peto OR 4.80, 95% CI 1.76 to 13.06; 82 limbs; P = 0.002). This benefit was still seen at 60 months (Peto OR 3.75, 95% CI 1.46 to 9.62; 69 limbs; P = 0.006), but this was only compared in one trial. Results were similar for secondary patency at 24 months (Peto OR 4.01, 95% CI 1.44 to 11.17; 93 limbs) and at 60 months (Peto OR 3.87, 95% CI 1.65 to 9.05; 93 limbs).
We found HBD to be superior to PTFE for primary patency at 60 months for above‐knee bypass, but these results were based on a single trial (Peto OR 0.38, 95% CI 0.20 to 0.72; 146 limbs; very low‐quality evidence). There was no difference in primary patency between HBD and HUV for above‐knee bypass in the one small study which reported this outcome.
We found only one small trial studying PUR and it showed very poor primary and secondary patency rates which were inferior to Dacron at all time points.
Below‐knee bypass
For bypass below the knee, we found no graft type to be superior to any other in terms of primary patency, though one trial showed improved secondary patency of HUV over PTFE at all time points to 24 months (Peto OR 3.40, 95% CI 1.45 to 7.97; 88 limbs; P = 0.005).
One study compared PTFE alone to PTFE with vein cuff; very low‐quality evidence indicates no effect to either primary or secondary patency at 24 months (Peto OR 1.08, 95% CI 0.58 to 2.01; 182 limbs; 2 studies; P = 0.80 and Peto OR 1.22, 95% CI 0.67 to 2.23; 181 limbs; 2 studies; P = 0.51 respectively)
Limited data were available for limb survival, and those studies reporting on this outcome showed no clear difference between graft types for this outcome. Antiplatelet and anticoagulant protocols varied extensively between trials, and in some cases within trials.
The overall quality of the evidence ranged from very low to moderate. Issues which affected the quality of the evidence included differences in the design of the trials, and differences in the types of grafts they compared. These differences meant we were often only able to combine and analyse small numbers of participants and this resulted in uncertainty over the true effects of the graft type used.
Authors' conclusions
There was moderate‐quality evidence of improved long‐term (60 months) primary patency for autologous vein grafts when compared to prosthetic materials for above‐knee bypasses. In the long term (two to five years) there was low‐quality evidence that Dacron confers a small secondary patency benefit over PTFE for above‐knee bypass. Only very low‐quality data exist on below‐knee bypasses, so we are uncertain which graft type is best. Further randomised data are needed to ascertain whether this information translates into an improvement in limb survival.
Plain language summary
Choice of bypass graft material for lower‐limb arterial bypasses
Background
A person with severely diseased arteries in one or both legs can experience pain on walking (intermittent claudication), pain at rest, or death of tissues in the leg. When the main thigh artery has a long blockage, the best option is to insert a bypass to carry the blood from an artery with good blood flow to the affected artery below the blockage. Bypass is intended to improve walking, or to save limbs that might otherwise require amputation. The different types of material available to create the bypass include the person's own vein (autologous vein), human umbilical vein, and the prosthetic materials polytetrafluoroethylene (PTFE) or Dacron, alone or with the blood thinning agent heparin bonded to the inside of the graft. Bypass grafts extending to below the knee are not as effective at remaining patent (open) with good blood flow as those above the knee. The aim of this review was to determine the most effective type of material to use for above‐knee and below‐knee bypass grafts.
Study characteristics and key results
We identified 19 randomised controlled trials that included a total of 3123 people. Of these people, 2547 were given above‐knee bypass grafts and 576 were given bypass grafts below the knee. The evidence in our review is current until 13 March 2017. From our analysis, we found that grafts made from a person's own vein had a better primary patency (blood flow) rate than the prosthetic materials PTFE or Dacron for above‐knee bypass grafts. Meanwhile, Dacron (and possibly also human umbilical vein) achieved better blood flow (patency) than PTFE. We also found that Dacron with supporting rings around it (designed to prevent external compression) showed worse patency than non‐supported Dacron when used in grafts above the knee.
Adding a 'cuff' of vein did not improve the patency of PTFE for grafts extending to below the knee. The included trials provided few results on how long people's limbs survived following the bypass procedure. There was not much consistency between the trials (and sometimes within the trials) with regards to people taking additional medications such as antiplatelets or anticoagulants, and this might have affected the results.
Quality of the evidence
The overall quality of the evidence ranged from very low to moderate. Issues which affected the quality of the evidence included differences in the design of the trials, and differences in the types of grafts they compared. These differences meant we were often only able to combine and analyse small numbers of participants and this resulted in uncertainty over the true effects of the graft type used.
Summary of findings
Background
Description of the condition
Femoro‐popliteal bypass grafting for lower limb ischaemia is one of the most common procedures undertaken by vascular surgeons. Since its inception in the 1940s the procedure has evolved significantly in terms of technical intricacy, graft type, anticoagulant medication use and patient selection. Various graft types have been used, including: autologous vein (in situ or reversed), human umbilical vein (HUV), synthetic polymers, polytetrafluoroethylene (PTFE) and Dacron; and more recently heparin‐bonded synthetic polymers.
During femoro‐popliteal bypass grafting, the proximal anastomosis is taken from the common, superficial or profunda femoris artery and the distal anastomosis may be to the popliteal artery either above or below the knee (referred to as above‐ and below‐knee grafts).
Description of the intervention
Controversy still exists over the most appropriate type of graft to use in bypass surgery. It is generally accepted that autologous vein should be used wherever possible, but there are surgeons who believe that using vein is a more demanding and time‐consuming operation that involves a longer duration of anaesthesia in relatively frail patients. When vein is unavailable there are widespread differences in the material used. This is due, in part, to a lack of relevant randomised evidence. Early trials did not separate above‐ and below‐knee grafts, were underpowered, had inadequate randomisation and the patient populations were less relevant to modern practice. As new materials became available they were implemented as standard practice for many surgeons, but with a lack of high‐quality supporting evidence. Even fairly recent meta‐analyses have relied heavily on non‐randomised, retrospective data (Pereira 2006).
How the intervention might work
Arterial bypass grafting works by routing arterial blood around blocked or narrow sections of artery using an alternative conduit. This conduit may either be a section of the patient's own vein (reversed or with the valves cut and disrupted); or an alternative biological conduit such as human umbilical vein; or an artificial material.
Why it is important to do this review
Outcomes from infrainguinal bypass grafting continue to be poor; at a median follow‐up of five years, the landmark randomised trial comparing bypass surgery to angioplasty in severe limb ischaemia reported overall survival of less than 50% (Bradbury 2010). There are economic and patient advantages to successful bypass grafting (Luther 1997; Perler 1995). When this is considered in the context of the controversy surrounding choice of graft material and differences in surgical practice, it is vital to make decisions based on the best evidence currently available.
Objectives
To assess the effects of bypass graft type in the treatment of stenosis or occlusion of the femoro‐popliteal arterial segment, for above‐ and below‐knee femoro‐popliteal bypass grafts.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing at least two different graft types. All graft types were eligible for inclusion.
Types of participants
We included patients with femoro‐popliteal ischaemia requiring arterial reconstruction. These were mainly patients with critical claudication, rest pain or tissue loss (Rutherford category 3 to 6 Consensus Document), but could also include some stable claudicants (Rutherford grade 1 to 2) in earlier trials. Trials in which a clear distinction was not made between patients receiving grafts to the popliteal artery and to the tibial arteries were excluded. For trials analysing above‐ and below‐knee procedures together, trialists were contacted for data and excluded if the results were inseparable.
Types of interventions
We included studies comparing two or more graft materials. Randomised controlled trials comparing bypass grafting to angioplasty or to other interventions were not included.
Types of outcome measures
Primary outcomes
Primary patency, defined as continuous patency of the graft without need for further intervention (including primary assisted patency if performed during the primary procedure)
Secondary outcomes
Secondary patency, defined as continuous patency of the graft, with or without further procedures such as angioplasty or surgical patching to prevent occlusion
Limb survival or limb salvage
We assessed these outcomes at three months, six months, one year, two years, three years and five years after surgery.
Search methods for identification of studies
We placed no restrictions on language.
Electronic searches
For this update, the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials:
the Cochrane Vascular Specialised Register (13 March 2017);
the Cochrane Central Register of Controlled Trials (CENTRAL (2017, Issue 2)) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, EMBASE Ovid, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library (www.cochranelibrary.com).
The CIS also searched the following trial registries for details of ongoing and unpublished studies (13 March 2017); See Appendix 2 for details.
ClinicalTrials.gov (www.clinicaltrials.gov)
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch)
ISRCTN Register (www.isrctn.com/)
Searching other resources
We searched the reference lists of relevant articles identified through the electronic searches to identify further trials.
Data collection and analysis
Selection of studies
For this update, both review authors (GKA and CPT) independently selected trials for inclusion in the review. The section 'Criteria for considering studies for this review' details the inclusion criteria used for the selection process.
Data extraction and management
Data were independently extracted by GKA then cross checked by CPT. The following information was extracted on each trial.
Trial methods: method of randomisation, method of allocation.
Participants: country of origin, age, sex distribution, severity of disease as measured by the ankle brachial index (ABI) and the European Consensus definition of critical ischaemia (Consensus Document), presence of diabetes, inclusion and exclusion criteria.
Interventions: type of graft, level of anastomosis, use of aspirin or anticoagulants, smoking habit after surgery, attendance at a graft surveillance programme.
Outcomes: primary and secondary patency, limb survival.
Assessment of risk of bias in included studies
For this update, both review authors independently assessed the risk of bias in the included studies according to the guidelines given in the Cochrane Handbook for Systematic Reviews of Interventions, (Higgins 2011). We assessed the new studies included in the updated review and we re‐assessed the studies already included from the previous versions of the review.
We assessed the following domains as low risk of bias, unclear risk of bias, or high risk of bias:
sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
These assessments are reported for each individual study in the Characteristics of included studies tables.
Measures of treatment effect
We presented the results from the dichotomous outcomes (primary or secondary patency; limb salvage) as odds ratios (ORs) with 95% confidence intervals (CIs).
Unit of analysis issues
The unit of analysis was the limb. Some participants in some trials were enrolled more than once, as each lower limb was allowed to be entered into some of the trials independently. This created a unit of analysis issue when considering survival with intact limb, but it was felt that effects on both primary patency (our primary outcome) and secondary patency would be small, so these trials were not excluded. None of the included studies allowed previous bypass in the affected limb. Survival data were only considered where it was clear that participants could not be enrolled in the same trial more than once.
Dealing with missing data
Where data were missing we attempted to determine the reasons for this. If data were missing due to participants being lost to follow up or because participants were not followed up to a certain time point prior to publication (censoring) and reasons were clearly described, we assumed the data were missing at random.
Assessment of heterogeneity
Heterogeneity was assessed visually (for methodological or clinical heterogeneity) by inspecting the forest plots and statistically by using Review Manager 5 software (Higgins 2003). We obtained P values comparing the test statistic with a Chi2 distribution. The Chi2 statistic describes the percentage of total variation across studies due to heterogeneity rather than by chance. A value of 0% indicates no observed heterogeneity and larger values show increasing heterogeneity.
Assessment of reporting biases
We planned to assess reporting bias by presenting funnel plots if more than 10 studies were included in the analysis. We also searched trial registries to look for unreported studies.
Data synthesis
We analysed and presented data into groups according to whether the distal anastomosis was above or below the knee.
We only undertook meta‐analysis when we felt there was no significant methodological heterogeneity, and statistical heterogeneity was not revealed by either calculation of I2 or performing Chi2 tests. The effect estimate was calculated using Peto ORs with 95% CIs. Peto ORs were used as it was anticipated that intervention effects would mainly be small, and that most trials would have similar numbers in experimental and control groups. We used fixed‐effect methods as there was no significant heterogeneity detected. All analyses were based on endpoint data from the individual clinical trials, which all quoted intention‐to‐treat results. The data were synthesised by comparing group results. Individual patient data from different trials were not amalgamated.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis according to graft type.
Sensitivity analysis
We performed sensitivity analysis to consider whether excluding studies with higher risk of bias led to significant changes in the results.
Summary of findings
We created 'Summary of findings' tables using GRADEpro software (GRADEpro GDT 2015). The study population consisted of patients with femoro‐popliteal ischaemia requiring arterial reconstruction, and we created tables for the comparisons of 'Autologous vein compared to other graft types for above‐knee femoro‐popliteal bypass surgery' (Table 1); 'PTFE compared to Dacron for above‐knee femoro‐popliteal bypass surgery' (Table 2); 'Externally supported Dacron compared to unsupported Dacron for above‐knee femoro‐popliteal bypass surgery' (Table 3) and 'PTFE compared to PTFE with vein cuff for below‐knee femoro‐popliteal bypass surgery' (Table 4). The most important and clinically relevant outcomes (both desirable and undesirable) that were thought to be essential for decision‐making were the outcomes primary patency (at 24 and 60 months follow‐up), secondary patency (at 60 months follow‐up) and limb salvage (at 24 months follow‐up). Assumed control intervention risks were calculated by the mean number of events in the control groups of the selected studies for each outcome. We used the system developed by the Grades of Recommendation, Assessment, Development and Evaluation working group (GRADE working group) for grading the quality of evidence as high, moderate, low or very low, based on within‐study risk of bias, inconsistency, directness of evidence, imprecision, and publication bias (GRADE 2004; GRADEpro GDT 2015).
Summary of findings for the main comparison. Autologous vein compared to other graft types for above‐knee femoro‐popliteal bypass surgery.
| Autologous vein compared to other graft types for above‐knee femoro‐popliteal bypass surgery | ||||||
| Patient or population: people with peripheral vascular disease requiring above‐knee femoro‐popliteal bypass surgery Setting: hospital Intervention: autologous vein Comparison: other graft types | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of limbs (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with other graft types | Risk with autologous vein | |||||
| Primary patency (24 months) |
Study population | OR 0.59 (0.37 to 0.94) | 422 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | 92 fewer autologous vein grafts per 1000 (10 to 152 grafts per 1000) lose primary patency by 24 months compared to other grafts studied | |
| 275 per 1000 | 183 per 1000 (123 to 263) | |||||
| Primary patency (60 months) |
Study population | OR 0.47 (0.28 to 0.80) | 269 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | 172 fewer autologous vein grafts per 1000 (54 to 264 grafts per 1000) lose primary patency by 60 months compared to other grafts studied | |
| 451 per 1000 | 279 per 1000 (187 to 397) | |||||
| Secondary patency (60 months) |
Study population | OR 0.41 (0.22 to 0.74) | 176 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | 213 fewer autologous vein grafts per 1000 (75 to 330 grafts per 1000) lose secondary patency by 60 months compared to other grafts studied | |
| 526 per 1000 | 313 per 1000 (196 to 451) | |||||
| Limb salvage | ‐ | ‐ | ‐ | ‐ | ‐ | No studies of these graft types reported on this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded due to serious risk of bias resulting from lack of blinding and poor randomisation techniques 2 Downgraded due to imprecision because results based on small trials with few participants and events 3 Downgraded due to risk of bias resulting from lack of blinding and poor randomisation techniques. We did not downgrade further for imprecision because the effect was large and highly consistent between studies
Summary of findings 2. PTFE compared to Dacron for above‐knee femoro‐popliteal bypass surgery.
| PTFE compared to Dacron for above‐knee femoro‐popliteal bypass surgery | ||||||
| Patient or population: people with peripheral vascular disease requiring above‐knee femoro‐popliteal bypass surgery Setting: hospital Intervention: PTFE Comparison: Dacron | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of limbs (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with Dacron | Risk with PTFE | |||||
| Primary patency (24 months) |
Study population | OR 1.23 (0.92 to 1.65) | 764 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Our confidence in the effect is limited and this may differ substantially from the estimate of the effect | |
| 404 per 1000 | 454 per 1000 (384 to 528) | |||||
| Primary patency (60 months) |
Study population | OR 1.67 (0.96 to 2.90) | 247 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Our confidence in the effect is limited and this may differ substantially from the estimate of the effect | |
| 606 per 1000 | 720 per 1000 (597 to 817) | |||||
| Secondary patency (24 months) |
Study population | OR 1.54 (1.04 to 2.28) | 528 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | 81 more PTFE grafts per 1000 (7 to 168 per 1000) suffer from failed secondary patency by 24 months compared to Dacron | |
| 212 per 1000 | 293 per 1000 (219 to 380) | |||||
| Limb salvage (24 months) |
Study population | OR 0.82 (0.27 to 2.48) | 322 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | Our confidence in the effect is limited and this may differ substantially from the estimate of the effect | |
| 44 per 1000 | 37 per 1000 (12 to 103) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio;PTFE: polytetrafluoroethylene | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded because of serious risk of bias due to lack of blinding and poor randomisation techniques 2 Downgraded due to imprecision because of the low number of participants and events
Summary of findings 3. Externally supported graft compared to unsupported graft for above‐knee femoro‐popliteal bypass surgery.
| Externally supported graft compared to unsupported graft for above‐knee femoro‐popliteal bypass surgery | ||||||
| Patient or population: people with peripheral vascular disease requiring above‐knee femoro‐popliteal bypass surgery Setting: hospital Intervention: externally supported graft Comparison: unsupported graft | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of limbs (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with unsupported graft | Risk with externally supported graft | |||||
| Primary patency (24 months) |
Study population | OR 2.08 (1.29 to 3.35) | 270 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | 180 fewer unsupported prosthetic grafts per 1000 (61 to 293 grafts per 1000) lose primary patency by 24 months compared to externally supported prosthetic grafts | |
| 376 per 1000 | 556 per 1000 (437 to 669) | |||||
| Primary patency (60 months) |
‐ | ‐ | ‐ | ‐ | ‐ | No studies comparing supported and unsupported Dacron reported on primary patency at 60 months |
| Secondary patency (24 months) |
Study population | OR 2.25 (1.24 to 4.07) | 236 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | 143 fewer unsupported Dacron grafts per 1000 (32 to 281 grafts per 1,000) lose secondary patency by 24 months compared to externally supported Dacron grafts | |
| 165 per 1000 | 308 per 1000 (197 to 446) | |||||
| Limb salvage | ‐ | ‐ | ‐ | ‐ | ‐ | No studies of these graft types reported on this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded because of serious risk of bias due to lack of blinding and poor randomisation techniques 2 Downgraded due to imprecision because of the low number of participants and events
Summary of findings 4. PTFE compared to PTFE with vein cuff for below‐knee femoro‐popliteal bypass surgery.
| PTFE compared to PTFE with vein cuff for below‐knee femoro‐popliteal bypass surgery | ||||||
| Patient or population: people with peripheral vascular disease requiring below‐knee femoro‐popliteal bypass surgery Setting: hospital Intervention: PTFE Comparison: PTFE with vein cuff | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of limbs (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with PTFE with vein cuff | Risk with PTFE | |||||
| Primary patency (24 months) |
Study population | OR 1.08 (0.58 to 2.01) | 182 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Findings from two small trials were inconsistent so our confidence in the effect is limited and this may differ substantially from the estimate of the effect | |
| 626 per 1000 | 644 per 1000 (493 to 771) | |||||
| Primary patency (60 months) |
‐ | ‐ | ‐ | ‐ | ‐ | No studies comparing PTFE with and without a vein cuff for below‐knee bypass reported on primary patency at 60 months |
| Secondary patency (24 months) |
Study population | OR 1.22 (0.67 to 2.23) | 181 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | Findings from two small trials were inconsistent so our confidence in the effect is limited and this may differ substantially from the estimate of the effect | |
| 557 per 1000 | 605 per 1000 (457 to 737) | |||||
| Limb salvage (24 months) |
Study population | OR 1.34 (0.72 to 2.49) | 196 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | Our confidence in the effect is limited and this may differ substantially from the estimate of the effect | |
| 266 per 1000 | 327 per 1000 (207 to 474) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded due to serious risk of bias resulting from lack of blinding and poor randomisation techniques 2 Downgraded due to significant heterogeneity in studies 3 Downgraded due to imprecision because of the low number of participants and events
Results
Description of studies
Results of the search
See Figure 1.
1.
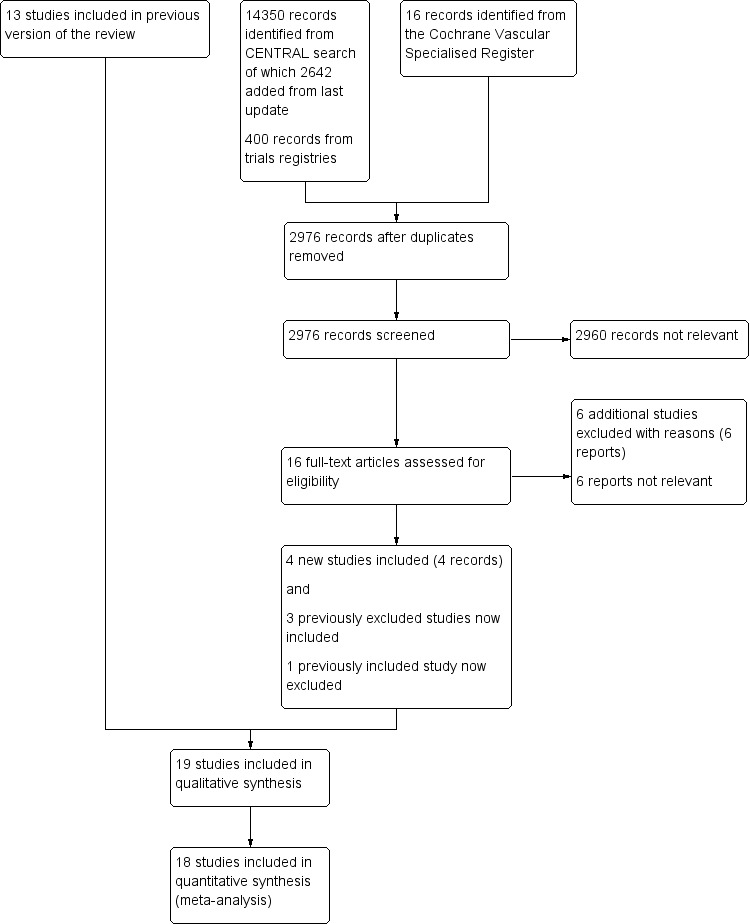
Study flow diagram.
Included studies
For summarised details of the included studies, see Characteristics of included studies.
We included seven additional studies in this review update (Davidovic 2010; Gloor 1996; Gupta 1991; Lumsden 2015; SCAMICOS 2010; Solakovic 2008; Vriens 2013), making a total of 19 randomised controlled trials which met the criteria for inclusion (Aalders 1992; Abbot 1997; Ballotta 2003; Davidovic 2010; Devine 2004; Eickhoff 1987; Gloor 1996; Gupta 1991; Jensen 2007; Klinkert 2003; Lumsden 2015; Post 2001; SCAMICOS 2010; Scharn 2008; Solakovic 2008; Stonebridge 1997; Tofigh 2007; van Det 2009; Vriens 2013). We had excluded three of the studies from the previous version of this review due to unclear randomisation methods (Gloor 1996; Gupta 1991; Solakovic 2008), but we were able to include them in this version due to the use of Cochrane's 'Risk of bias' tool. Follow‐up was reported to six months (Lumsden 2015), one year (Davidovic 2010; Gloor 1996), two years (Jensen 2007; Post 2001; Scharn 2008; Tofigh 2007; Vriens 2013), three years (Gupta 1991; SCAMICOS 2010), four years (Eickhoff 1987), five years (Aalders 1992; Abbot 1997; Ballotta 2003; Devine 2004; Klinkert 2003; Solakovic 2008; Stonebridge 1997) and 10 years (van Det 2009). There were a total of 3123 patients (2547 above‐knee, 576 below‐knee), with bypasses being performed on 3238 limbs (2662 above‐knee, 576 below‐knee). Nine types of graft were compared: autologous vein; polytetrafluoroethylene (PTFE) with and without vein cuff and with or without external support; human umbilical vein (HUV); Dacron and heparin bonded Dacron (HBD); FUSION BIOLINE and Dacron with external support).
Above‐knee bypass
Two trials compared autologous vein and PTFE grafts above the knee (Ballotta 2003; Klinkert 2003). In Ballotta 2003, 102 limbs (51 patients) with bilateral disabling claudication were randomised to receive reversed saphenous vein or PTFE. Klinkert 2003 also compared reversed saphenous vein with PTFE, in 151 limbs. Anticoagulation protocols and medication checks varied between these trials; see Characteristics of included studies for details.
In Tofigh 2007 autologous vein was compared with a polyester graft, while Solakovic 2008 compared autologous vein with a prosthetic graft, which was allowed to be either PTFE of Dacron. These have been considered separately for analysis from those trials where the prosthetic material was more clearly specified.
One trial compared PTFE with HUV in 93 limbs (Aalders 1992). Five trials compared PTFE with Dacron (Abbot 1997; Davidovic 2010; Jensen 2007; Post 2001; van Det 2009). We did not use Davidovic 2010 the quantitative analysis due to concerns over risk of bias in outcome data (see Characteristics of included studies). The trial with the largest number of limbs was Jensen 2007, in which 205 PTFE grafts were compared with 208 Dacron grafts. Unfortunately, anticoagulant and follow‐up protocols varied between departments in this study. In van Det 2009, 114 limbs were randomised to PTFE and 114 limbs to Dacron; the trialists used warfarin with a consistent protocol for anticoagulation, and they continued follow‐up for 10 years. One trial compared PTFE with the FUSION BIOLINE graft (Lumsden 2015), which is a two‐layer graft, the inner layer being heparin‐bonded expanded PTFE (ePTFE) which is glued to an outer knitted polyester textile. Above the knee, 88 limbs were randomised to FUSION BIOLINE graft, whilst 86 received standard ePTFE. Gupta 1991 considered PTFE with or without ringed support; 29 limbs received ringed grafts and 30 limbs received unringed grafts above the knee.
One trial looked at fluoropolymer‐coated Dacron graft with or without external support (Vriens 2013), with 134 limbs assigned to externally supported graft and 119 treated with unsupported graft.
One trial compared PTFE with PTFE and vein cuff in above‐knee bypass (Stonebridge 1997). The study included 74 limbs with PTFE and 76 with PTFE and vein cuff. The numbers of continuing smokers and of participants on antiplatelet and anticoagulant therapy were not given. Peri‐operative complications were not stated.
One study compared HBD with HUV (Scharn 2008) and one trial compared HBD with PTFE (Devine 2004). The anticoagulant protocol was not stated in the latter (Devine 2004).
One study compared polyurethane (PUR) with Dacron (Gloor 1996). Both primary and secondary patency rates were poor for the PUR grafts and the trial was stopped early due to safety concerns after only 20 limbs had been randomised.
Below‐knee bypass
There were far less data available for below‐knee bypass, with 651 procedures analysed. No studies compared autologous vein with PTFE, HUV or other graft types. One trial compared PTFE with Dacron (Post 2001), however there were low numbers of participants in each group (26 in the PTFE group, 27 in the Dacron group). Two trials (Stonebridge 1997; SCAMICOS 2010) compared PTFE with PTFE and vein cuff. One study (Lumsden 2015) compared standard ePTFE with the FUSION BIOLINE graft, though numbers of below‐knee popliteal procedures were low in each group (14 in the FUSION BIOLINE group, 14 in the PTFE group). Gupta 1991 included 63 below‐knee bypasses, and compared PTFE with or without ringed support in 29 and 34 limbs respectively.
One study (Eickhoff 1987) compared PTFE with HUV. This trial also separately analysed patency rates in claudicants and those with good distal runoff, and found those patients to have a patency advantage. The study authors did not state the anticoagulants used. Devine 2004 gave separate below‐knee data.
There were no statistically significant differences in the major cofounders of sex, age, smoking, dyslipidaemia (abnormal concentrations of lipids or lipoproteins in the blood), diabetes or hypertension reported between groups in any of the above‐ or below‐knee trials.
Excluded studies
For this update, we excluded six additional studies (Lindholt 2011; Linni 2015; Lundgren 2013; Midy 2016; NCT00617279; NCT00845585); we also excluded a study which had been included in previous versions of the review (Watelet 1997). We excluded three studies because above‐ and below‐the‐knee data could not be separated for analyses (Lindholt 2011, Linni 2015; Watelet 1997) . We excluded Lundgren 2013 because it included a mixture of femoro‐popliteal and femoro‐tibial bypass patients, and results for the subset of patients treated with femoro‐popliteal bypass were not presented separately. We excluded one study (Midy 2016) as it failed to recruit even 30% of the planned number of patients, and more than 25% of those recruited had no follow‐up. We excluded NCT00617279 and NCT00845585 for similar reasons; the former trial was terminated by the sponsor due to slow recruitment and no results were ever presented, whereas the latter trial was terminated before a single patient was recruited. Full reasons for trials being excluded can be found in the Characteristics of excluded studies table.
Ongoing studies
We identified two ongoing studies as being relevant to this review and these may be included in future updates (NCT00205790; NCT00147979). See Characteristics of ongoing studies.
Risk of bias in included studies
2.
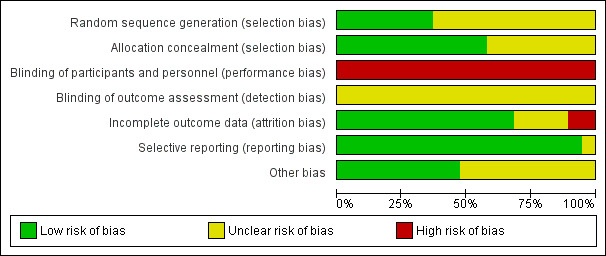
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
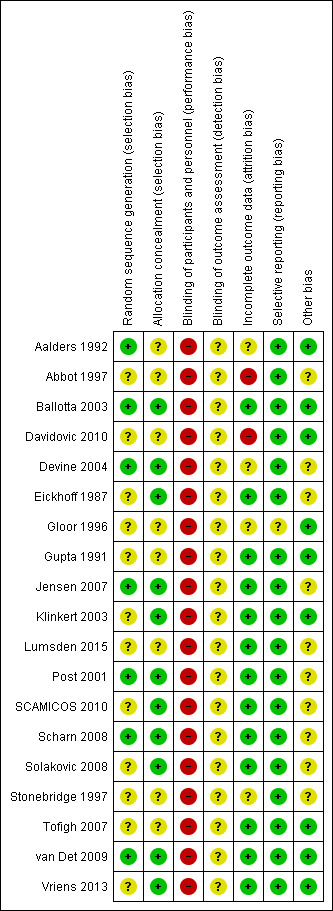
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Overall, the risk of bias was significant, principally due to a lack of blinding. There were issues to do with attrition and it was unclear whether there might have been issues of selection bias in some studies.
Allocation
Random sequence generation
Seven studies were at low risk of bias as their sequence generation was adequate (Aalders 1992; Ballotta 2003; Devine 2004; Jensen 2007; Post 2001; Scharn 2008; van Det 2009). We judged the remaining 12 studies to have unclear risk of bias as they failed to describe the method of randomisation, or used a non‐standard technique (Abbot 1997; Davidovic 2010; Eickhoff 1987; Gloor 1996; Gupta 1991; Klinkert 2003; Lumsden 2015; SCAMICOS 2010; Scharn 2008; Solakovic 2008; Stonebridge 1997; Tofigh 2007; Vriens 2013).
Allocation concealment
Eleven studies had adequate allocation concealment (Ballotta 2003; Devine 2004; Eickhoff 1987; Jensen 2007; Klinkert 2003; Post 2001; SCAMICOS 2010; Scharn 2008; Solakovic 2008; van Det 2009; Vriens 2013). The remaining eight were at unclear risk of bias as allocation concealment was not clearly discussed (Aalders 1992; Abbot 1997; Davidovic 2010; Gloor 1996; Gupta 1991; Lumsden 2015; Stonebridge 1997; Tofigh 2007).
Blinding
Blinding for graft insertion is impossible in surgical trials of this nature. Outcome assessment may be blinded, however this was not the case in any of the included studies and we are unsure what effect this may have had on the outcomes in question. For this reason all included studies were judged to be at high risk of performance bias and at unclear risk of detection bias.
Incomplete outcome data
We judged one study (Abbot 1997) to be at high risk of attrition bias as 13 participants were lost following randomisation and results were reported without specifically stating what happened to these participants. Davidovic 2010 failed to present numbers at risk at different time points and secondary patency was presented as worse than primary patency, which is impossible. Due to these issues we judged this study to be at high risk of bias and did not include it in meta‐analysis. We assessed Gloor 1996 as having unclear risk of bias as they failed to include a CONSORT flow diagram and there was no mention of patients excluded prior to randomisation or after randomisation. All the remaining studies were at low risk of bias, since any losses were minimal or described clearly.
Selective reporting
One study (Gloor 1996) failed to present details of complications occurring within the first 30 days which did not lead to reintervention, though this was a stated secondary outcome. As this is a patient population with significant comorbidity, it is likely that there were some undisclosed complications, so we judged the study to be at unclear risk of reporting bias. There were no concerns over selective reporting in any of the other included studies.
Other potential sources of bias
Three trials had antiplatelet and anticoagulant protocols which obviously varied within the trial: Post 2001 used heparin, warfarin or antiplatelet agents (specific agent not stated); Scharn 2008 used aspirin or coumarin derivatives; and Jensen 2007 used different anticoagulation protocols in each centre. One study (Lumsden 2015) left decisions about heparin, protamine and topical haemostatics to the operating surgeon, but specified that postoperative aspirin therapy was compulsory in all participants. Five trials did not state their anticoagulation protocol (Abbot 1997; Devine 2004; Eickhoff 1987; SCAMICOS 2010; Stonebridge 1997). One study (Solakovic 2008) gave a clear protocol of anticoagulants in the perioperative period and antiplatelet agents following discharge, but gave no details of compliance checks. We considered all these studies to have unclear risk of other sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Above‐knee bypass
Autologous vein compared to other graft types
Four studies compared autologous veins to other grafts prosthetic materials (Ballotta 2003; Klinkert 2003; Solakovic 2008; Tofigh 2007).
Primary patency
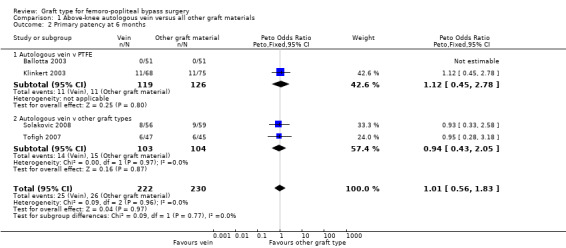
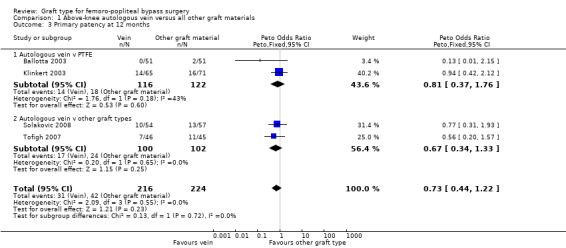
We were able to include four trials comparing autologous vein to prosthetic materials in a meta‐analysis (Ballotta 2003; Klinkert 2003; Solakovic 2008; Tofigh 2007). We found no clear difference between the groups in primary patency at 3, 6 or 12 months. See Analysis 1.1; Analysis 1.2; Analysis 1.3 respectively. Although individual trials failed to show clear benefit, once results of the four trials were combined a long‐term benefit for autologous vein was observed at 24 months (Peto odds ratio (OR) 0.59, 95% confidence interval (CI) 0.37 to 0.94; 422 limbs; 4 studies; P = 0.03; low‐quality evidence; Analysis 1.4). This was reflected in the continued benefit in primary patency for autologous vein over prosthetic grafts by five years (Peto OR 0.47, 95% CI 0.28 to 0.80; 269 limbs; 3 studies; P = 0.005; moderate‐quality evidence; Analysis 1.5). The comparison with polytetrafluoroethylen (PTFE) contributed the majority of weight to this result (weight 63.6%, OR 0.48, 95% CI 0.25 to 0.95).
1.1. Analysis.
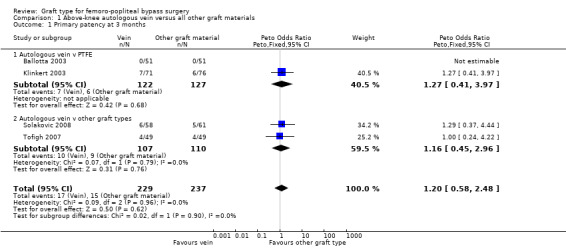
Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 1 Primary patency at 3 months.
1.2. Analysis.
Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 2 Primary patency at 6 months.
1.3. Analysis.
Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 3 Primary patency at 12 months.
1.4. Analysis.
Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 4 Primary patency at 24 months.
1.5. Analysis.
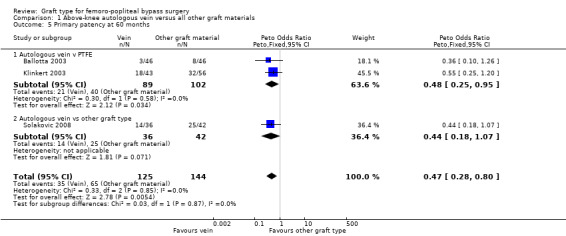
Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 5 Primary patency at 60 months.
Secondary patency
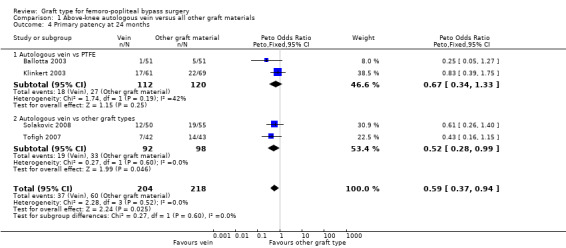
Three studies comparing autologous vein to prosthetic materials reported on this outcome and were pooled in a meta‐analysis (Klinkert 2003; Solakovic 2008; Tofigh 2007). No improvement in secondary patency was found at 3, 6, 12 or 24 months. See Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9 respectively. A benefit was seen at five years (Peto OR 0.41, 95% CI 0.22 to 0.74; 176 limbs; 2 studies; P = 0.003; low‐quality evidence; Analysis 1.10). However Ballotta 2003 and Tofigh 2007 were not included in analysis at this timepoint, reducing the power of the comparison. There was no evidence of significant statistical heterogeneity between these trials.
1.6. Analysis.
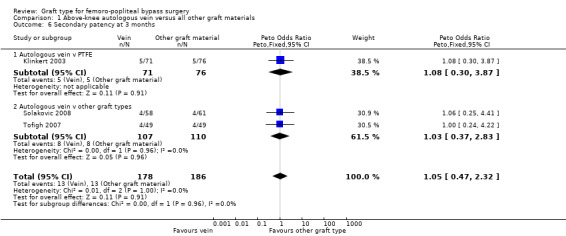
Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 6 Secondary patency at 3 months.
1.7. Analysis.
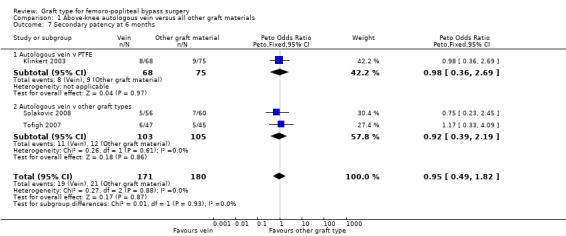
Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 7 Secondary patency at 6 months.
1.8. Analysis.
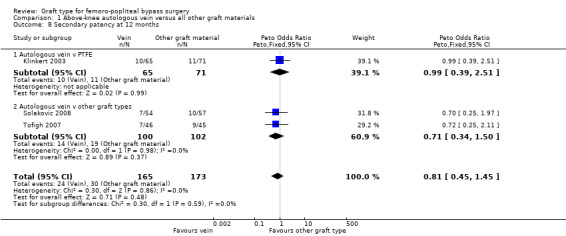
Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 8 Secondary patency at 12 months.
1.9. Analysis.
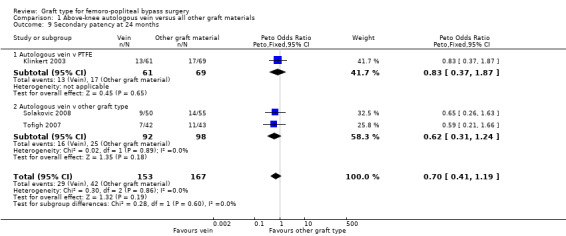
Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 9 Secondary patency at 24 months.
1.10. Analysis.
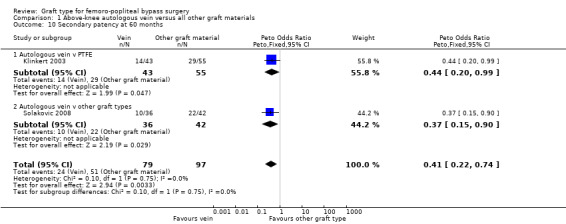
Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 10 Secondary patency at 60 months.
Limb survival or limb salvage
No data available
Polytetrafluoroethylen (PTFE) compared to other graft types
Eight studies compared PTFE to other grafts (Aalders 1992; Abbot 1997; Davidovic 2010; Jensen 2007; Lumsden 2015; Post 2001; Stonebridge 1997; van Det 2009).
Primary patency
Of the five studies comparing PTFE with Dacron (Abbot 1997; Davidovic 2010; Jensen 2007; Post 2001; van Det 2009), four were considered suitable for meta‐analysis (Abbot 1997; Jensen 2007; Post 2001; van Det 2009). We did not include Davidovic 2010 because of concerns about risk of bias (see Incomplete outcome data (attrition bias)). All four studies reported at 12 and 24 months; the remaining timepoints had data available from one or two studies. Three studies (Jensen 2007; van Det 2009; Post 2001) showed a non‐significant trend towards a greater benefit with Dacron and Abbot 1997 showed a non‐significant trend in favour of PTFE. Abbot 1997 was the weakest trial in terms of potential bias; see Figure 3 and the table Characteristics of included studies.
Once combined, we found no significant difference in primary patency between PTFE and Dacron at any time point. Removing the one trial with significant bias issues (Abbot 1997) did not change this result, except at 60 months, where data from one study (van Det 2009) suggested that Dacron grafts may potentially have a small benefit in primary patency at this time point (OR 1.87; 95% CI 1.01 to 3.43; Analysis 2.5).
2.5. Analysis.
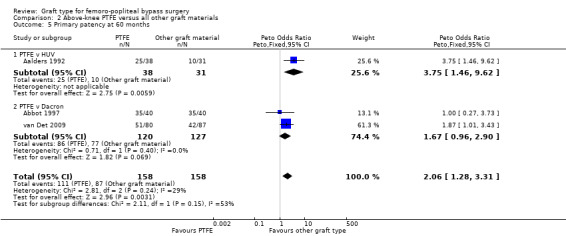
Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 5 Primary patency at 60 months.
One study (Aalders 1992) compared PTFE with human umbilical vein (HUV). No difference in primary patency was seen at three or six months (Analysis 2.1 and Analysis 2.2 respectively). Our analysis suggests a benefit in primary patency for HUV by 12 months (Peto OR 3.17, 95% CI 1.04 to 9.64; P = 0.04; 83 limbs; 1 study), which continued to 24 months (Peto OR 4.80, 95% CI 1.76 to 13.06; 82 limbs; 1 study; P = 0.002 (Analysis 2.4)). This benefit was still evident at five years (Peto OR 3.75, 95% CI 1.46 to 9.62; 69 limbs; 1 study; P = 0.006), Analysis 2.5), but the results are limited because of small numbers of participants.
2.1. Analysis.
Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 1 Primary patency at 3 months.
2.2. Analysis.
Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 2 Primary patency at 6 months.
2.4. Analysis.
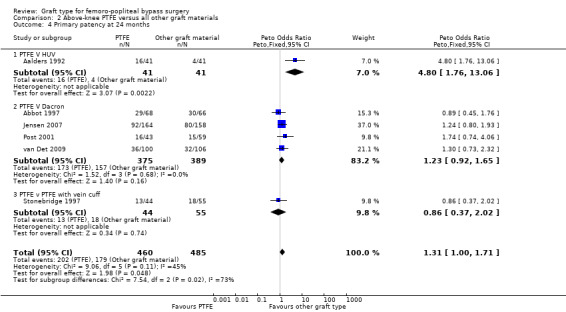
Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 4 Primary patency at 24 months.
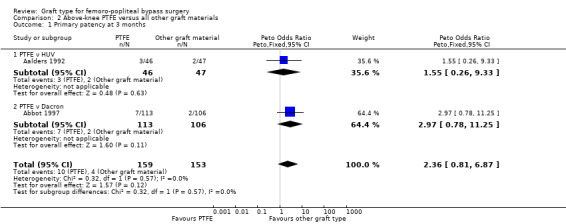
In Stonebridge 1997, there was no significant difference between PTFE and PTFE with vein cuff used above the knee for the outcome primary patency at any time point (Analysis 2.3; Analysis 2.4).
2.3. Analysis.
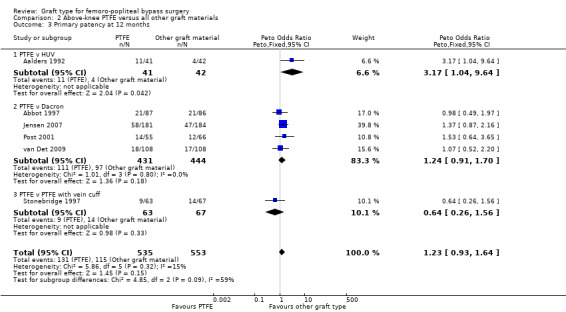
Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 3 Primary patency at 12 months.
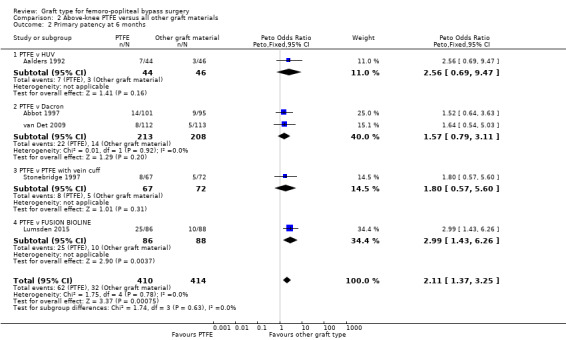
One study (Lumsden 2015) compared a new graft material, FUSION BIOLINE, which is composed of an inner heparin bonded PTFE layer glued to an outer knitted polyester layer. This study found a significant improvement in primary patency at six months for above‐knee bypass done with FUSION BIOLINE, when compared with a standard PTFE graft (Peto OR 2.99, 95% CI 1.43 to 6.26; 174 limbs; 1 study; P = 0.004; Analysis 2.2) . Results reported at other time points were only presented for both above‐ and below‐knee grafts combined, and failed to show a significant difference at either 90 days or 12 months, though the results at six months were also significant in the combined analysis.
Secondary patency
There was no clear difference in secondary patency between PTFE and Dacron at 6 months (Peto OR 1.01, 95% CI 0.25 to 4.13; 225 limbs; 1 study) or 12 months (Peto OR 1.19, 95% CI 0.76 to 1.86; 581 limbs; 2 studies). See Analysis 2.7 and Analysis 2.8. A benefit from the use of Dacron grafts was seen at 24 months (Peto OR 1.54, 95% CI 1.04 to 2.28; 528 limbs; 2 studies; P = 0.03) and 60 months (Peto OR 2.43, 95% CI 1.31 to 4.53; 167 limbs; 1 study; P = 0.005). See Analysis 2.9 and Analysis 2.10.
2.7. Analysis.
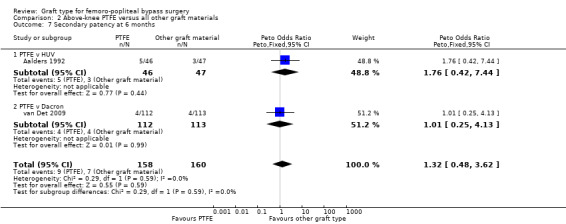
Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 7 Secondary patency at 6 months.
2.8. Analysis.
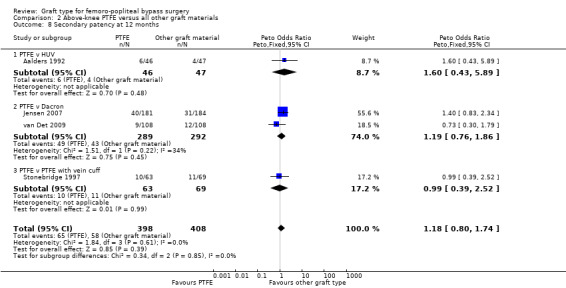
Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 8 Secondary patency at 12 months.
2.9. Analysis.
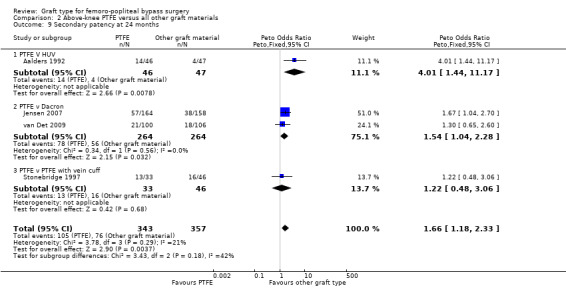
Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 9 Secondary patency at 24 months.
2.10. Analysis.
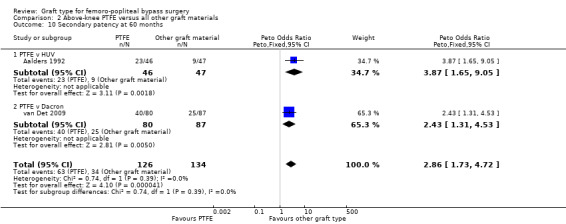
Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 10 Secondary patency at 60 months.
In Stonebridge 1997, there was no significant difference between PTFE and PTFE with vein cuff used above the knee for the outcome secondary patency at any time point (Analysis 2.8; Analysis 2.9).
One study (Aalders 1992) compared PTFE with human umbilical vein (HUV). No clear difference in secondary patency was seen at three, six and 12 months (Analysis 2.6; Analysis 2.7 and Analysis 2.8 respectively). Our analysis suggests a benefit in secondary patency for HUV by 24 months (Peto OR 4.01, 95% CI 1.44 to 11.17; 93 limbs; 1 study; P = 0.008), which continued to 60 months (Peto OR 3.87, 95% CI 1.65 to 9.05; 93 limbs; 1 study; P = 0.002) (Analysis 2.10).
2.6. Analysis.
Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 6 Secondary patency at 3 months.
Limb survival or limb salvage
Only two studies reported detailed limb salvage rates for above‐knee femoro‐popliteal bypass (Jensen 2007; Stonebridge 1997). Jensen 2007 compared PTFE with Dacron and Stonebridge 1997 compared PTFE with PTFE and vein cuff. Neither found differences in limb salvage rates between graft types at one month or 24 months (Analysis 2.11; Analysis 2.12).
2.11. Analysis.
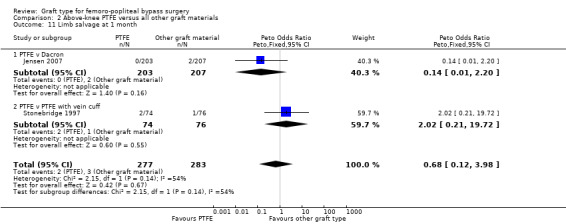
Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 11 Limb salvage at 1 month.
2.12. Analysis.
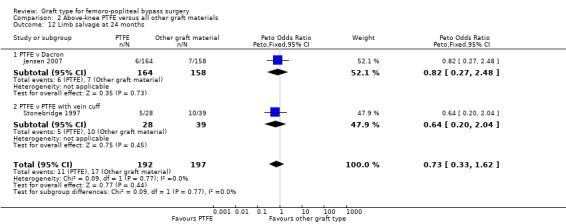
Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 12 Limb salvage at 24 months.
Heparin bonded Dacron (HBD) versus other grafts
Two studies compared heparin bonded Dacron grafts with other grafts (Devine 2004; Scharn 2008). Devine 2004 compared heparin bonded Dacron to PTFE and Scharn 2008 compared HBD to HUV.
Primary patency
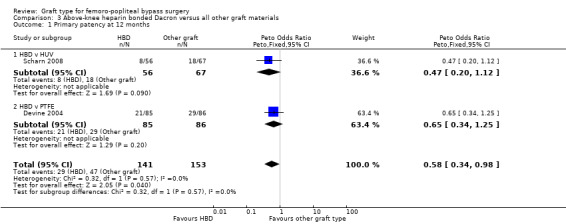
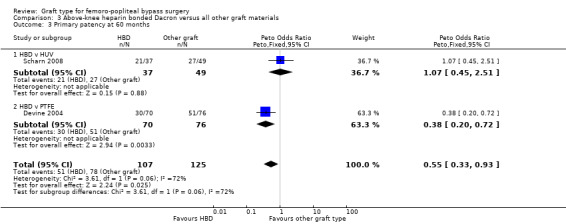
In Devine 2004, no difference in patency was detected at 12 or 24 months, though by 60 months, HBD showed improved patency compared to PTFE (Peto OR 0.38, 95% CI 0.20 to 0.72; 146 limbs; 1 study; P = 0.003). In Scharn 2008 there was no improvement in primary patency at any time interval when HBD was compared to HUV.
The combined overall primary patency for HBD compared to HUV/PTFE was improved at 12 months (Peto OR 0.58, 95% CI 0.34 to 0.98; 294 limbs; 2 studies); 24 months (Peto OR 0.62, 95% CI 0.38 to 1.02; 282 limbs; 2 studies); and 60 months (Peto OR 0.55, 95% CI 0.33 to 0.93; 232 limbs; 2 studies). See Analysis 3.1 to Analysis 3.3.
3.1. Analysis.
Comparison 3 Above‐knee heparin bonded Dacron versus all other graft materials, Outcome 1 Primary patency at 12 months.
3.3. Analysis.
Comparison 3 Above‐knee heparin bonded Dacron versus all other graft materials, Outcome 3 Primary patency at 60 months.
Secondary patency
No data available
Limb survival or limb salvage
No data available
Externally‐supported Dacron or PTFE grafts compared to other grafts
One trial examined whether adding external support to Dacron might improve outcomes in above‐knee femoro‐popliteal bypass (Vriens 2013), while another considered the same question for PTFE grafts (Gupta 1991).
Primary patency
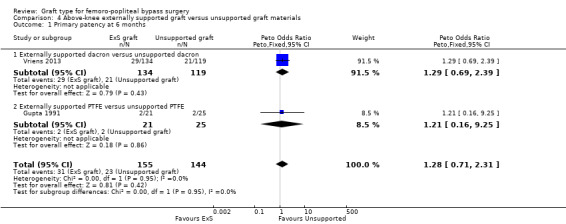
Although short‐term primary patency rates were comparable (Analysis 4.1; Analysis 4.2), by 24 months the externally supported Dacron grafts showed worse primary patency when compared to their unsupported counterparts (Peto OR 2.09, 95% CI 1.26 to 3.46; 240 limbs; 1 study; P = 0.004; Analysis 4.3).
4.1. Analysis.
Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 1 Primary patency at 6 months.
4.2. Analysis.
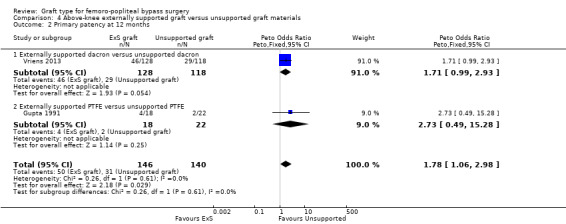
Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 2 Primary patency at 12 months.
4.3. Analysis.
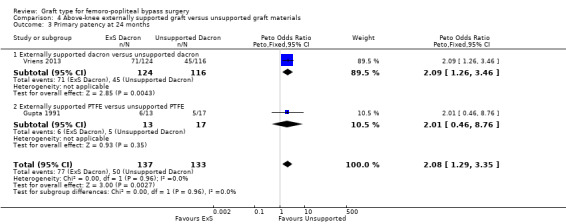
Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 3 Primary patency at 24 months.
Results from Gupta 1991 showed similar primary patency for PTFE grafts with and without ringed support at 6, 12 and 24 months (Analysis 4.1; Analysis 4.2; Analysis 4.3).
Secondary patency
Although short‐term secondary patency rates were comparable, by 24 months the externally supported Dacron grafts showed worse secondary patency when compared to their unsupported counterparts (Peto OR 2.25, 95% CI 1.24 to 4.07; 236 limbs; 1 study; P = 0.008; Analysis 4.6).
4.6. Analysis.
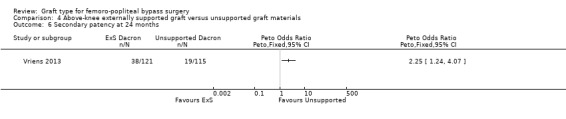
Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 6 Secondary patency at 24 months.
Limb survival or limb salvage
No data available
Polyurethane (PUR) graft compared to other grafts
One trial examined a new PUR graft type (Gloor 1996).
Primary patency
Primary patency was worse for the PUR grafts at all time points and the trial was stopped due to safety concerns after only 20 limbs had been randomised. See Analysis 5.1; Analysis 5.2; Analysis 5.3.
5.1. Analysis.
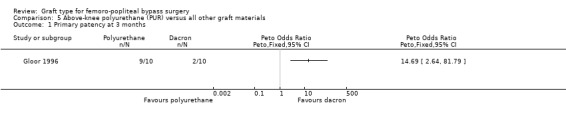
Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 1 Primary patency at 3 months.
5.2. Analysis.
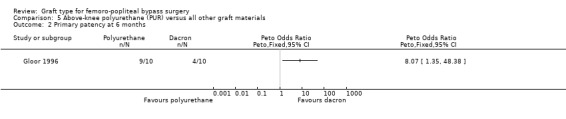
Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 2 Primary patency at 6 months.
5.3. Analysis.
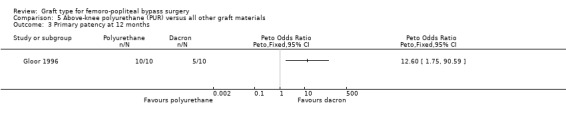
Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 3 Primary patency at 12 months.
Secondary patency
Secondary patency was worse for the PUR grafts at all time points and the trial was stopped due to safety concerns after only 20 limbs had been randomised. See Analysis 5.4; Analysis 5.5; Analysis 5.6.
5.4. Analysis.
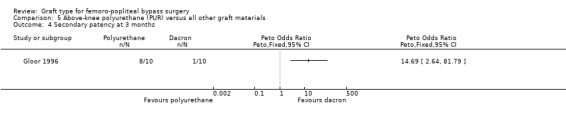
Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 4 Secondary patency at 3 months.
5.5. Analysis.
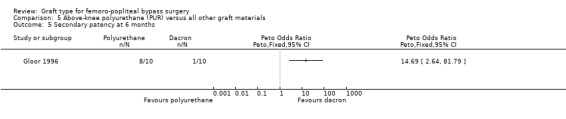
Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 5 Secondary patency at 6 months.
5.6. Analysis.
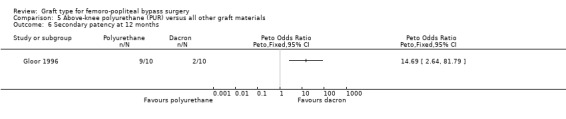
Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 6 Secondary patency at 12 months.
Limb survival or limb salvage
No data available
Below‐knee bypass
PTFE compared to other graft types
Six studies reported on primary or secondary patency, or both, but analysis was limited by different graft comparisons and reporting at different timepoints (Eickhoff 1987; Gupta 1991; Lumsden 2015; Post 2001; SCAMICOS 2010; Stonebridge 1997).
Primary patency
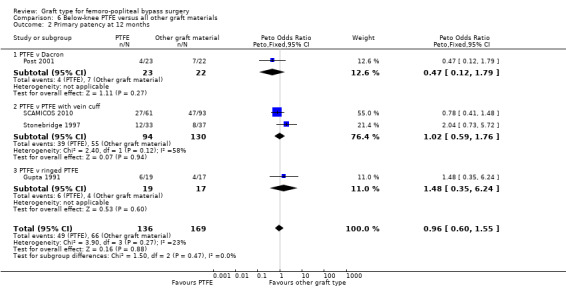
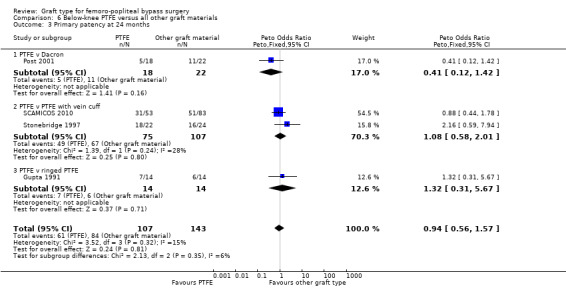
There was no clear difference in primary patency for PTFE compared to Dacron at 12 months (Peto OR 0.47, 95% CI 0.12 to 1.79; P = 0.27; 45 limbs; 1 study; Analysis 6.2) and 24 months (Peto OR 0.41, 95% CI 0.12 to 1.42; 40 limbs; 1 study; P = 0.16; Analysis 6.3), however the analysis only included one trial (Post 2001).
6.2. Analysis.
Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 2 Primary patency at 12 months.
6.3. Analysis.
Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 3 Primary patency at 24 months.
The two trials comparing PTFE with a vein cuff to PTFE alone in below‐knee femoro‐popliteal bypass were heterogeneous: Stonebridge 1997 suggested a benefit with the addition of a vein cuff, whilst SCAMICOS 2010 favoured no cuff. Pooling the data showed no difference in primary patency at six, 12 and 24 months (24 months: Peto OR 1.08, 95% CI 0.58 to 2.01; 182 limbs; 2 studies; Analysis 6.3). Allocation concealment and random sequence generation were not clearly described in Stonebridge 1997, so results may be attributable to selection bias in that trial.
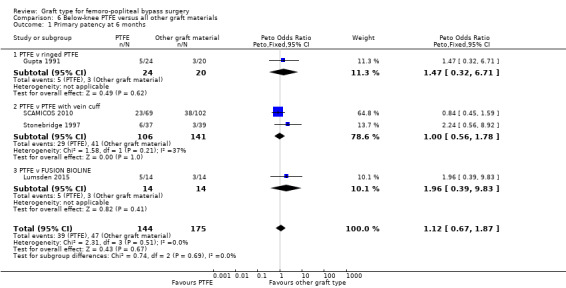
One study (Gupta 1991) considered whether ringed support was of benefit in PTFE grafts below the knee. We found no difference of effect at any time point (Analysis 6.2).
A small number of patients in the FUSION BIOLINE trial had below‐knee bypass (Lumsden 2015). We found no significant difference in primary patency between FUSION BIOLINE and PTFE in this case (Analysis 6.1).
6.1. Analysis.
Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 1 Primary patency at 6 months.
Secondary patency
One trial provided results on below‐the‐knee secondary patency for PTFE versus HUV (Eickhoff 1987).This trial showed improved patency rates for HUV grafts at all time intervals from three months to 24 months. See Analysis 6.5 to Analysis 6.8 (24 months: Peto OR 3.40; 95% CI 1.45 to 7.97, P = 0.005; 88 limbs; 1 study).
6.5. Analysis.
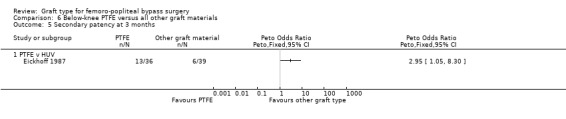
Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 5 Secondary patency at 3 months.
6.8. Analysis.
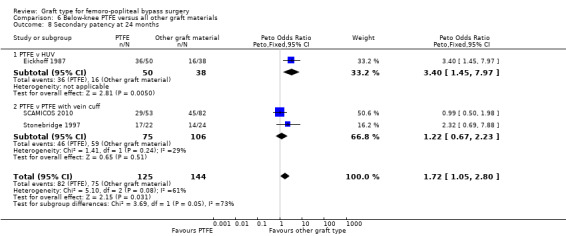
Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 8 Secondary patency at 24 months.
The two trials comparing PTFE with a vein cuff to PTFE alone in below‐knee femoro‐popliteal bypass were heterogeneous (SCAMICOS 2010; Stonebridge 1997). Pooling the data showed no difference in secondary patency at 12 and 24 months (24 months: (Peto OR 1.22, 95% CI 0.67 to 2.23; 181 limbs; 2 studies; Analysis 6.8). Allocation concealment and random sequence generation were not clearly described in Stonebridge 1997, so results may be attributable to selection bias in that trial.
Limb survival or limb salvage
Limited information was available on limb survival for below‐knee femoro‐popliteal bypass. Only Stonebridge 1997 and SCAMICOS 2010 reported this outcome, for PTFE versus PTFE with vein cuff. They found no clear difference at 12 months (Peto OR 1.35, 95% CI 0.72 to 2.55; 225 limbs; 2 studies) or 24 months (Peto OR 1.34, 95% CI 0.72 to 2.49; 196 limbs; 2 studies; Analysis 6.10 and Analysis 6.11).
6.10. Analysis.
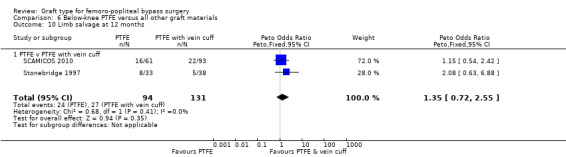
Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 10 Limb salvage at 12 months.
6.11. Analysis.
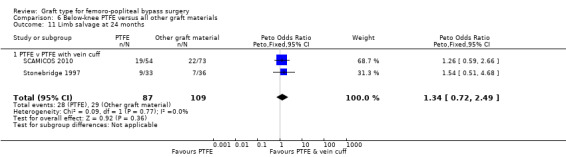
Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 11 Limb salvage at 24 months.
Heparin bonded Dacron versus all other graft materials
Primary patency
Only Devine 2004 compared HBD grafts with other grafts. No clear differences in primary patency were observed between HBD and PTFE below the knee at any time interval in this study (Devine 2004; Analysis 7.1; Analysis 7.2; Analysis 7.3; Analysis 7.4;Analysis 7.5).
7.1. Analysis.
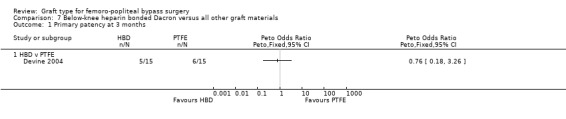
Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 1 Primary patency at 3 months.
7.2. Analysis.
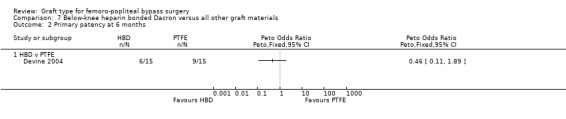
Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 2 Primary patency at 6 months.
7.3. Analysis.
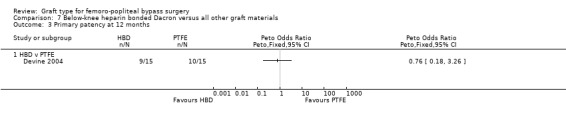
Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 3 Primary patency at 12 months.
7.4. Analysis.
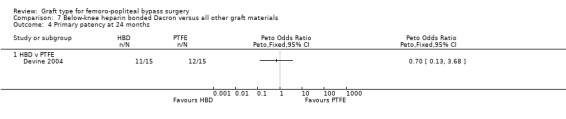
Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 4 Primary patency at 24 months.
7.5. Analysis.
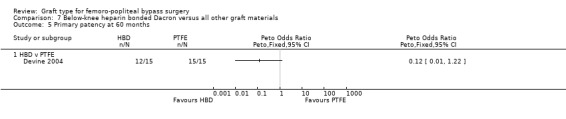
Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 5 Primary patency at 60 months.
Secondary patency
No data available
Limb survival or limb salvage
No data available
Discussion
Summary of main results
Our major findings were that autologous vein grafts have long‐term patency benefits over prosthetic grafts in above‐knee femoro‐popliteal bypass (moderate‐quality evidence). In the long term (greater than two years), we found that Dacron may confer a slight benefit in secondary patency over polytetrafluoroethylene (PTFE) for above‐knee bypasses (low‐quality evidence). There was no significant improvement in primary and secondary patency for below‐knee PTFE bypasses when a vein cuff was included. Limited evidence was available on below‐knee procedures for all graft types. There was also limited evidence on limb survival for both above‐ and below‐knee bypass surgery.
Overall completeness and applicability of evidence
While there have been many randomised controlled trials conducted for lower limb bypass surgery, the overall quality of these was poor and meant that we had to exclude 24 trials (see Characteristics of excluded studies). Some of the main reasons we excluded trials were because they failed to randomise patients, they did not report the data for above‐ and below‐knee procedures separately, or because they had severe methodological flaws which led to significant bias within the trial.
We only found low numbers of trials for some analyses, especially for below‐knee bypass, which is partly indicative of the numbers of new graft types being introduced and partly indicative of the reduced numbers of lower‐limb bypass procedures now performed. Inclusion criteria for randomised controlled trials produce the potential problem of reducing the applicability of the results to the overall patient population. This was especially a problem in older trials, which included stable or long‐distance claudicants, who are generally not offered surgery in contemporary practice. A subcomponent of any trial including such patients will therefore not be applicable to the overall patient population, but should have a minimal effect on the overall results as these trials have smaller numbers than the more recent included trials. The included trials are largely reflective of modern surgical practice in the UK and are therefore relevant.
Data on limb salvage and survival with limb intact were generally not included for analysis in trials. In the future this should be included as it is an important outcome, both for the patient and from a health economics point of view (Luther 1997; Perler 1995), and may therefore influence practice significantly. Quality‐of‐life data would also be useful in influencing treatment strategy (Nolan 2007). This information might augment the applicability of bypass surgery in general, as evidence is still lacking when comparing infrainguinal bypass with other treatments for lower limb ischaemia (Fowkes 2008).
Human umbilical vein (HUV) has primary patency results comparable with other non‐vein graft types, and may show an improvement in primary and secondary patency compared to polytetrafluoroethylene (PTFE) below the knee. However, in one trial up to 30% of HUV grafts showed graft dilation and aneurysm formation (Aalders 1992). This, in combination with other data at the time, has led to the diminished popularity of HUV in recent years. More recent reviews did not find these factors to be a significant issue (Dardik 2002) and the patency data from this meta‐analysis infer that HUV may be a suitable alternative to synthetic materials when no autologous vein is available.
Heparin bonded Dacron is showing promising early results in randomised trials (Devine 2004). Heparin bonded PTFE is also being widely utilised in contemporary practice. While there are case series data implying that this is an effective material, we could not include data from randomised trials in this review because the results are either awaited (see table Characteristics of ongoing studies), unavailable due to the trial being terminated early (NCT00617279), or reported in a way that does not separate above‐ and below‐knee results (Lindholt 2011).
A single small trial examined the use of polyurethane (PUR) grafts (Gloor 1996). The trial was stopped early due to astonishingly poor primary and secondary patency rates in the limbs treated with the new graft material, so this material cannot be recommended.
Several specific problems could not be assessed in this analysis. Firstly, infection of synthetic bypasses has disastrous consequences for the patient (Siracuse 2013), whereas infection of venous bypasses tends not to, and is easier to treat (Reifsnyder 1992). Occlusion of synthetic bypasses appears to lead to limb loss more frequently than venous (Jackson 2000), which is why it is so important that future trials measure limb survival. A second limitation of this review is the lack of information on antiplatelet and anticoagulant protocols in the included studies; this may have produced bias in the results and their interpretation. Finally, the majority of included studies were not stratified according to graft length, inflow site quality or inflow procedures, or patency of runoff vessels. While the randomisation of participants should have achieved balance with respect to these factors, the small numbers of participants could potentially have led to imbalance between treatment arms, in turn leading to biased results.
Quality of the evidence
While there were low numbers of trials for some comparisons, these trials are mainly of reasonable methodological quality with acceptable allocation concealment techniques, though often simple sealed envelopes were used and little—if any—effort appeared to have been made to blind participants, practitioners or outcome assessors (Figure 2; Figure 3). As a result, we assessed the majority of the evidence contributing to above‐knee bypass comparisons as low quality, which rose to moderate quality for one outcome. We assessed the quality of the evidence on below‐knee bypass comparisons as very low‐quality. Further details are included in Table 1, Table 2, Table 3 and Table 4.
All trials included a Kaplan‐Meier analysis, and most supplemented this with numbers‐at‐risk and life table analyses. The numbers of participants at each stage of the trial were usually clear. However, antiplatelet protocols were generally lacking. There is clear evidence for antiplatelet therapy in cardiovascular stenting (NICE 2003), which may be applicable to lower‐limb arterial stents (Twine 2009). While the evidence is less clear for lower‐limb bypass grafts (Brown 2008; Dorffler‐Melly 2003); clear protocols should be set in future trials to avoid the potential bias caused by individual preferences by surgeons or centres for particular antiplatelets or anticoagulants. Choice of anticoagulant for lower‐limb bypass grafts requires good‐quality randomised controlled trials to determine efficacy.
Potential biases in the review process
Although we are confident that a thorough search was carried out for all relevant studies, we were unable to separate data from trials from patients of below‐ and above‐the‐knee bypasses in all cases. It has been clear for some time that below‐knee bypass grafts have significantly inferior patency rates to above‐knee grafts (Cranley 1982; McCollum 1991). Most trials since the early 1990s have therefore separated the two types of bypass for reporting results, to avoid bias. This led to the division of above‐ and below‐knee procedures in this review. Three trials which were included in previous editions of the review have been excluded in this update or previous updates (or both) as the above‐ and below‐knee data were inseparable (McCollum 1991; Moody 1992; Watelet 1997). More recent trials with combined above‐ and below‐knee procedures had other severe methodological flaws which, in combination, led us to exclude them (Robinson 1999; Robinson 2003). In addition, we excluded two more recent trials either because of combined above‐ and below‐knee numbers (Lindholt 2011), or combined below‐knee and distal bypass numbers (Lundgren 2013). See the table Characteristics of excluded studies.
Agreements and disagreements with other studies or reviews
There are several recent meta‐analyses of graft type for femoro‐popliteal bypass grafts (Albers 2005; Pereira 2006; Roll 2008; Rychlik 2014a). In Albers 2005, alternative autologous vein (defined as any autologous venous conduit other than a single section of great saphenous vein) was compared with PTFE, HUV and cryopreserved vein. Randomised controlled trials and cohort controlled trials were considered for inclusion. The authors included retrospective data and combined above‐ and below‐knee bypasses. Thirty‐two articles with 2618 patients from studies conducted between 1982 and 2004 were included. Pooled estimate analysis was performed in which the authors found no difference in primary patency between autologous vein and PTFE, but reported a significant improvement in secondary patency and foot preservation for alternative autologous veins. While not directly comparable with our analysis, these data provide more evidence for autologous vein over prosthetic grafts.
In Pereira 2006, above‐knee autologous vein, PTFE and below‐knee autologous vein were compared. Randomised controlled trials and cohort trials were considered for inclusion. Forty‐nine retrospective articles and 24 prospective articles from 1986 to 2004 were included. As well as including retrospective data, the authors included several studies which we excluded from our analysis because of inadequate randomisation. Pooled estimate analysis was performed, in which the authors found a significant improvement in primary patency for above‐knee autologous vein when compared with PTFE. Secondary patency was lower for all graft types and showed no significant difference. Therefore, Pereira 2006 also broadly agrees with the findings of this analysis that autologous vein performs better than PTFE above the knee. The authors' findings should, however, be interpreted with caution due to the nature of the data included.
One meta‐analysis (Roll 2008), compared Dacron with PTFE and found no difference between the graft types. The authors included bypasses other than femoro‐popliteal (axillo‐bifemoral, aorto‐bifemoral, etc.) but had strict inclusion criteria and therefore included good‐quality trials. Our analysis is in broad agreement with the findings of Roll 2008 in terms of primary patency, though we did find an improvement in secondary patency at 24 months and five years, the latter as a result of data from the van Det study (van Det 2009), published after Roll (Roll 2008). Therefore, the findings of our analysis are broadly in agreement with Roll 2008. For this reason, the long‐term secondary patency benefit towards Dacon is tentative, as discussed throughout the text.
One meta‐analysis (Rychlik 2014a) compared Dacron with PTFE above the knee.It had similar exclusion criteria to our review and found results from five studies which are included in our analysis, in addition to one study which we excluded from our meta‐analysis due to its methodological flaws (Davidovic 2010). They chose to include the results of Devine 2004, which compared heparin bonded Dacron with PTFE, alongside the four studies comparing standard Dacron with PTFE (Abbot 1997; Jensen 2007; Post 2001; van Det 2009). Their conclusions were similar to our results in this context: that Dacron has superior patency to PTFE at 2 and 5 years follow‐up.
A previous meta‐analysis (Twine 2012) has shown benefit for PTFE with vein cuff for below‐knee bypass. This analysis included non‐randomised studies, and based on the results seen in our analysis, the benefit shown in Twine 2012 may be because of selection bias in the non‐randomised data. It is unlikely that another RCT of cuffed bypass will be performed, and most surgeons will perform a cuffed anastomosis for synthetic bypass distal to the knee. Registry data is becoming increasingly prevalent in vascular surgery and may help to answer this question more definitively in the future.
Authors' conclusions
Implications for practice.
We found moderate‐quality evidence that autologous vein grafts improve long‐term (60 months) primary patency over prosthetic graft materials for femoro‐popliteal bypass above the knee. There was low‐quality evidence that Dacron grafts had improved long‐term (two to five years) secondary patency compared to polytetrafluoroethylene (PTFE) above the knee. External reinforcement of Dacron grafts had inferior primary patency above the knee. Human umbilical cord (HUV) and heparin bonded Dacron (HBD) may also have superior patency to PTFE, but the results are from only one trial in each case. There was no evidence to support any one synthetic material for bypasses below the knee. Further randomised data are needed to ascertain whether this information translates into an improvement in limb survival.
Implications for research.
Randomised trials of synthetic materials versus autologous vein and other prosthetic materials are ongoing (NCT00205790; NCT00147979). While data on new graft types are invaluable, further randomised data are needed on 'established' materials used for femoro‐popliteal bypasses. This especially includes the use of vein cuffs with different prosthetic materials below the knee. Randomised trials of HBD versus Dacron would also be useful, as would randomised data comparing 'alternative' autologous vein (for example profunda femoris, arm vein and 'inadequate' saphenous vein) with prosthetic materials.
Future trials need to include data on limb survival, quality of life and costs, as well as patency rates, to ascertain whether the improvements in patency found in this analysis translate into improvements in these important outcomes. It would also be helpful if infection rates could be reported in future trials, though the low event rates seen in observational studies of graft infection would suggest that studies looking at this issue might need to be very large.
While vein cuffs or pre‐cuffed grafts are widely utilised below the knee, this practice is based on case‐series data. This would be a useful topic to study in future trials, since vein is not always available and the results of randomised studies of this technique are conflicting.
The effects of antiplatelets or anticoagulants on graft patency also need to be investigated further in the context of randomised controlled trials. This would facilitate graft‐type trial medication protocols and remove a major potential source of bias from future studies.
What's new
| Date | Event | Description |
|---|---|---|
| 13 March 2017 | New citation required but conclusions have not changed | Search updated. Seven new studies included, six new studies excluded and two new ongoing studies identified. Text updated to reflect recent Cochrane standards. All included studies assessed for risk of bias using Cochrane's 'Risk of bias' tool. 'Summary of findings' table added. No change to conclusions. |
| 13 March 2017 | New search has been performed | Search updated and seven new studies included, six new studies excluded and two new ongoing studies identified. |
History
Review first published: Issue 2, 1999
| Date | Event | Description |
|---|---|---|
| 10 March 2010 | New citation required and conclusions have changed | Review updated by new authors. Eight additional trials included and four trials which were included in the previous version of the review excluded. |
| 1 September 2008 | Amended | Converted to new review format. |
Acknowledgements
We are grateful to Drs Cathryn Broderick, Marlene Stewart and Karen Welch for their invaluable help and assistance in the preparation of this review.
Appendices
Appendix 1. CENTRAL search strategy
| Search run on Mon Mar 13 2017 | ||
| #1 | MESH DESCRIPTOR Arteriosclerosis | 869 |
| #2 | MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES | 0 |
| #3 | MESH DESCRIPTOR Arteriosclerosis Obliterans | 72 |
| #4 | MESH DESCRIPTOR Atherosclerosis | 641 |
| #5 | MESH DESCRIPTOR Arterial Occlusive Diseases | 734 |
| #6 | MESH DESCRIPTOR Intermittent Claudication | 723 |
| #7 | MESH DESCRIPTOR Ischemia | 801 |
| #8 | MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES | 2229 |
| #9 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY | 9491 |
| #10 | ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 8366 |
| #11 | (peripheral near3 dis*):TI,AB,KY | 3525 |
| #12 | (claudic* or IC):TI,AB,KY | 3219 |
| #13 | (isch* or CLI):TI,AB,KY | 24757 |
| #14 | arteriopathic:TI,AB,KY | 7 |
| #15 | dysvascular*:TI,AB,KY | 11 |
| #16 | (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 99 |
| #17 | (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 157 |
| #18 | ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 81 |
| #19 | MESH DESCRIPTOR Leg EXPLODE ALL TREES WITH QUALIFIERS BS | 1113 |
| #20 | MESH DESCRIPTOR Popliteal Artery | 280 |
| #21 | MESH DESCRIPTOR Femoral Artery | 826 |
| #22 | (femor* or popliteal or fempop* or poplite* or infrapopliteal or femdist* or infrainquinal or infra‐inquinal) :TI,AB,KY | 9481 |
| #23 | ((above or below) near2 knee) | 486 |
| #24 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 or #23 | 52728 |
| #25 | MESH DESCRIPTOR Blood Vessel Prosthesis EXPLODE ALL TREES | 411 |
| #26 | MESH DESCRIPTOR Blood Vessel Prosthesis Implantation EXPLODE ALL TREES | 405 |
| #27 | (bypass or surgery or construct* or reconstruct* or re‐construct* or re‐vasculari* or revasculari* or graft* ):TI,AB,KY | 139976 |
| #28 | #25 or #26 or #27 | 140022 |
| #29 | #24 and #28 | 14350 |
Appendix 2. Trials registries searches
ClinicalTrials.gov
354 studies found for: Peripheral Vascular Diseases OR Arterial Occlusive Diseases OR Ischemia | bypass AND (graft OR Polytetrafluoroethylene OR dacron OR polyester OR dacron OR umbilical OR PTFE)
World Health Organization International Clinical Trials Registry Platform
44 records for 36 trials found
bypass AND (graft OR Polytetrafluoroethylene OR dacron OR polyester OR dacron OR umbilical OR PTFE) in title
AND
Peripheral Vascular Diseases OR Arterial Occlusive Diseases OR Ischemia in Condition
ISRCTN Register
2 results for
(Peripheral Vascular Diseases OR Arterial Occlusive Diseases OR Ischemia) AND bypass AND (graft OR Polytetrafluoroethylene OR dacron OR umbilical OR PTFE)
Data and analyses
Comparison 1. Above‐knee autologous vein versus all other graft materials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary patency at 3 months | 4 | 466 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.58, 2.48] |
| 1.1 Autologous vein v PTFE | 2 | 249 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.41, 3.97] |
| 1.2 Autologous vein v other graft types | 2 | 217 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.45, 2.96] |
| 2 Primary patency at 6 months | 4 | 452 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.56, 1.83] |
| 2.1 Autologous vein v PTFE | 2 | 245 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.45, 2.78] |
| 2.2 Autologous vein v other graft types | 2 | 207 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.43, 2.05] |
| 3 Primary patency at 12 months | 4 | 440 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.44, 1.22] |
| 3.1 Autologous vein v PTFE | 2 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.37, 1.76] |
| 3.2 Autologous vein v other graft types | 2 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.34, 1.33] |
| 4 Primary patency at 24 months | 4 | 422 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.37, 0.94] |
| 4.1 Autologous vein vs PTFE | 2 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.34, 1.33] |
| 4.2 Autologous vein vs other graft types | 2 | 190 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.28, 0.99] |
| 5 Primary patency at 60 months | 3 | 269 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.28, 0.80] |
| 5.1 Autologous vein v PTFE | 2 | 191 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.25, 0.95] |
| 5.2 Autologous vein vs other graft type | 1 | 78 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.18, 1.07] |
| 6 Secondary patency at 3 months | 3 | 364 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.47, 2.32] |
| 6.1 Autologous vein v PTFE | 1 | 147 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.30, 3.87] |
| 6.2 Autologous vein v other graft types | 2 | 217 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.37, 2.83] |
| 7 Secondary patency at 6 months | 3 | 351 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.49, 1.82] |
| 7.1 Autologous vein v PTFE | 1 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.36, 2.69] |
| 7.2 Autologous vein v other graft types | 2 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.39, 2.19] |
| 8 Secondary patency at 12 months | 3 | 338 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.45, 1.45] |
| 8.1 Autologous vein v PTFE | 1 | 136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.39, 2.51] |
| 8.2 Autologous vein v other graft types | 2 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.34, 1.50] |
| 9 Secondary patency at 24 months | 3 | 320 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.41, 1.19] |
| 9.1 Autologous vein v PTFE | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.37, 1.87] |
| 9.2 Autologous vein v other graft type | 2 | 190 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.31, 1.24] |
| 10 Secondary patency at 60 months | 2 | 176 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.22, 0.74] |
| 10.1 Autologous vein v PTFE | 1 | 98 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.20, 0.99] |
| 10.2 Autologous vein v other graft types | 1 | 78 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.37 [0.15, 0.90] |
Comparison 2. Above‐knee PTFE versus all other graft materials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary patency at 3 months | 2 | 312 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.36 [0.81, 6.87] |
| 1.1 PTFE v HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.55 [0.26, 9.33] |
| 1.2 PTFE v Dacron | 1 | 219 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.97 [0.78, 11.25] |
| 2 Primary patency at 6 months | 5 | 824 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.11 [1.37, 3.25] |
| 2.1 PTFE v HUV | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.56 [0.69, 9.47] |
| 2.2 PTFE v Dacron | 2 | 421 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [0.79, 3.11] |
| 2.3 PTFE v PTFE with vein cuff | 1 | 139 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.80 [0.57, 5.60] |
| 2.4 PTFE v FUSION BIOLINE | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.99 [1.43, 6.26] |
| 3 Primary patency at 12 months | 6 | 1088 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.93, 1.64] |
| 3.1 PTFE v HUV | 1 | 83 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.17 [1.04, 9.64] |
| 3.2 PTFE v Dacron | 4 | 875 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.91, 1.70] |
| 3.3 PTFE v PTFE with vein cuff | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.26, 1.56] |
| 4 Primary patency at 24 months | 6 | 945 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [1.00, 1.71] |
| 4.1 PTFE V HUV | 1 | 82 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.80 [1.76, 13.06] |
| 4.2 PTFE V Dacron | 4 | 764 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.92, 1.65] |
| 4.3 PTFE v PTFE with vein cuff | 1 | 99 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.37, 2.02] |
| 5 Primary patency at 60 months | 3 | 316 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.06 [1.28, 3.31] |
| 5.1 PTFE v HUV | 1 | 69 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.75 [1.46, 9.62] |
| 5.2 PTFE v Dacron | 2 | 247 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.67 [0.96, 2.90] |
| 6 Secondary patency at 3 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 6.1 PTFE v HUV | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Secondary patency at 6 months | 2 | 318 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.48, 3.62] |
| 7.1 PTFE v HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.76 [0.42, 7.44] |
| 7.2 PTFE v Dacron | 1 | 225 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.25, 4.13] |
| 8 Secondary patency at 12 months | 4 | 806 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.80, 1.74] |
| 8.1 PTFE v HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.60 [0.43, 5.89] |
| 8.2 PTFE v Dacron | 2 | 581 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.76, 1.86] |
| 8.3 PTFE v PTFE with vein cuff | 1 | 132 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.39, 2.52] |
| 9 Secondary patency at 24 months | 4 | 700 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.66 [1.18, 2.33] |
| 9.1 PTFE V HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.01 [1.44, 11.17] |
| 9.2 PTFE v Dacron | 2 | 528 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.54 [1.04, 2.28] |
| 9.3 PTFE v PTFE with vein cuff | 1 | 79 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.48, 3.06] |
| 10 Secondary patency at 60 months | 2 | 260 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.86 [1.73, 4.72] |
| 10.1 PTFE v HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.87 [1.65, 9.05] |
| 10.2 PTFE v Dacron | 1 | 167 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.43 [1.31, 4.53] |
| 11 Limb salvage at 1 month | 2 | 560 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.12, 3.98] |
| 11.1 PTFE v Dacron | 1 | 410 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 2.20] |
| 11.2 PTFE v PTFE with vein cuff | 1 | 150 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.02 [0.21, 19.72] |
| 12 Limb salvage at 24 months | 2 | 389 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.33, 1.62] |
| 12.1 PTFE v Dacron | 1 | 322 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.27, 2.48] |
| 12.2 PTFE v PTFE with vein cuff | 1 | 67 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.20, 2.04] |
Comparison 3. Above‐knee heparin bonded Dacron versus all other graft materials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary patency at 12 months | 2 | 294 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.34, 0.98] |
| 1.1 HBD v HUV | 1 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.20, 1.12] |
| 1.2 HBD v PTFE | 1 | 171 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.34, 1.25] |
| 2 Primary patency at 24 months | 2 | 282 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.38, 1.02] |
| 2.1 HBD v HUV | 1 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.26, 1.33] |
| 2.2 HBD v PTFE | 1 | 165 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.34, 1.19] |
| 3 Primary patency at 60 months | 2 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.33, 0.93] |
| 3.1 HBD v HUV | 1 | 86 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.45, 2.51] |
| 3.2 HBD v PTFE | 1 | 146 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.20, 0.72] |
3.2. Analysis.
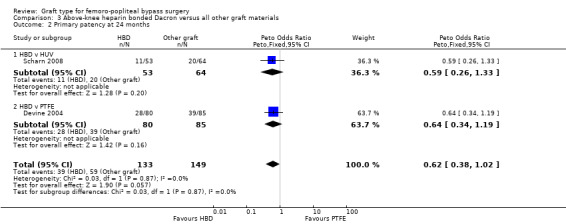
Comparison 3 Above‐knee heparin bonded Dacron versus all other graft materials, Outcome 2 Primary patency at 24 months.
Comparison 4. Above‐knee externally supported graft versus unsupported graft materials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary patency at 6 months | 2 | 299 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.71, 2.31] |
| 1.1 Externally supported dacron versus unsupported dacron | 1 | 253 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.29 [0.69, 2.39] |
| 1.2 Externally supported PTFE versus unsupported PTFE | 1 | 46 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.16, 9.25] |
| 2 Primary patency at 12 months | 2 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.78 [1.06, 2.98] |
| 2.1 Externally supported dacron versus unsupported dacron | 1 | 246 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.71 [0.99, 2.93] |
| 2.2 Externally supported PTFE versus unsupported PTFE | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.73 [0.49, 15.28] |
| 3 Primary patency at 24 months | 2 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.08 [1.29, 3.35] |
| 3.1 Externally supported dacron versus unsupported dacron | 1 | 240 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.09 [1.26, 3.46] |
| 3.2 Externally supported PTFE versus unsupported PTFE | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.01 [0.46, 8.76] |
| 4 Secondary patency at 6 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Secondary patency at 12 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 6 Secondary patency at 24 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
4.4. Analysis.
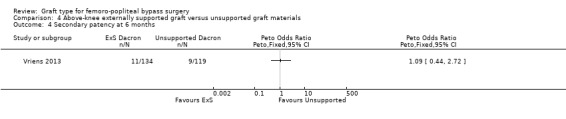
Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 4 Secondary patency at 6 months.
4.5. Analysis.
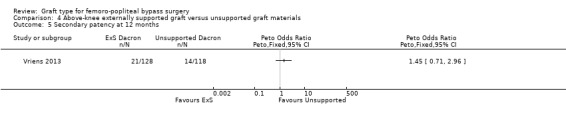
Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 5 Secondary patency at 12 months.
Comparison 5. Above‐knee polyurethane (PUR) versus all other graft materials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary patency at 3 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Primary patency at 6 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Primary patency at 12 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Secondary patency at 3 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Secondary patency at 6 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 6 Secondary patency at 12 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
Comparison 6. Below‐knee PTFE versus all other graft materials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary patency at 6 months | 4 | 319 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.67, 1.87] |
| 1.1 PTFE v ringed PTFE | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.32, 6.71] |
| 1.2 PTFE v PTFE with vein cuff | 2 | 247 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.56, 1.78] |
| 1.3 PTFE v FUSION BIOLINE | 1 | 28 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.96 [0.39, 9.83] |
| 2 Primary patency at 12 months | 4 | 305 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.60, 1.55] |
| 2.1 PTFE v Dacron | 1 | 45 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.12, 1.79] |
| 2.2 PTFE v PTFE with vein cuff | 2 | 224 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.59, 1.76] |
| 2.3 PTFE v ringed PTFE | 1 | 36 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.35, 6.24] |
| 3 Primary patency at 24 months | 4 | 250 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.56, 1.57] |
| 3.1 PTFE v Dacron | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.12, 1.42] |
| 3.2 PTFE v PTFE with vein cuff | 2 | 182 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.58, 2.01] |
| 3.3 PTFE v ringed PTFE | 1 | 28 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.31, 5.67] |
| 4 Primary patency at 36 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4.1 PTFE v PTFE with vein cuff | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Secondary patency at 3 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5.1 PTFE v HUV | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Secondary patency at 6 months | 2 | 242 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.69, 2.13] |
| 6.1 PTFE v HUV | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.01 [1.12, 8.07] |
| 6.2 PTFE v PTFE with vein cuff | 1 | 171 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.40, 1.56] |
| 7 Secondary patency at 12 months | 3 | 325 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.94, 2.34] |
| 7.1 PTFE v HUV | 1 | 101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.46 [1.10, 5.49] |
| 7.2 PTFE v PTFE with vein cuff | 2 | 224 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.66, 2.03] |
| 8 Secondary patency at 24 months | 3 | 269 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [1.05, 2.80] |
| 8.1 PTFE v HUV | 1 | 88 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.40 [1.45, 7.97] |
| 8.2 PTFE v PTFE with vein cuff | 2 | 181 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.67, 2.23] |
| 9 Secondary patency at 36 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 9.1 PTFE v PTFE with vein cuff | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Limb salvage at 12 months | 2 | 225 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.72, 2.55] |
| 10.1 PTFE v PTFE with vein cuff | 2 | 225 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.72, 2.55] |
| 11 Limb salvage at 24 months | 2 | 196 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.72, 2.49] |
| 11.1 PTFE v PTFE with vein cuff | 2 | 196 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.72, 2.49] |
6.4. Analysis.
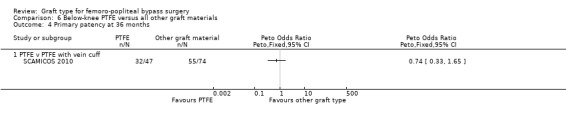
Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 4 Primary patency at 36 months.
6.6. Analysis.
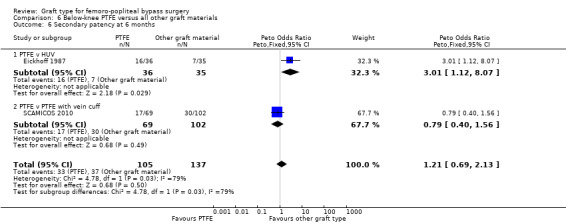
Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 6 Secondary patency at 6 months.
6.7. Analysis.
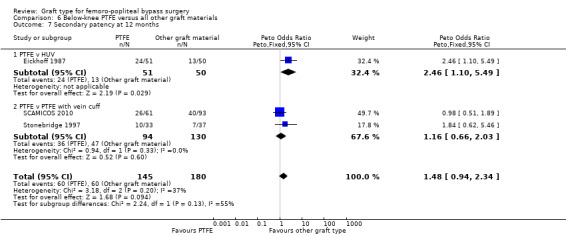
Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 7 Secondary patency at 12 months.
6.9. Analysis.
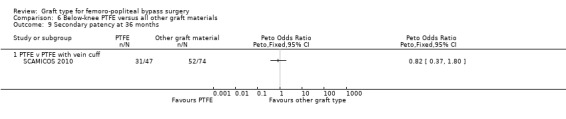
Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 9 Secondary patency at 36 months.
Comparison 7. Below‐knee heparin bonded Dacron versus all other graft materials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary patency at 3 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 1.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Primary patency at 6 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Primary patency at 12 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Primary patency at 24 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Primary patency at 60 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aalders 1992.
| Methods | Site: Femoral to AK popliteal Study design: Single‐centre RCT Method of randomisation: sealed envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: none |
|
| Participants | Country: Holland No. of participants: 85 patients(93 limbs; 46 PTFE, 47 HUV) Age: 64 yrs Sex: 67 male, 18 female DM 16, critical 17 Inclusion criteria: AK femoro‐popliteal graft for IC (or limb salvage if vein unavailable) Exclusion criteria: those with previous femoro‐popliteal graft |
|
| Interventions | 6 mm PTFE versus 6 mm HUV | |
| Outcomes | Primary patency, secondary patency, complications | |
| Notes | All had post‐op anticoagulants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random permuted blocks" |
| Allocation concealment (selection bias) | Unclear risk | Not specifically stated. Probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some patients lost to follow‐up early on, but clear life table data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other obvious bias |
Abbot 1997.
| Methods | Site: Femoral to AK popliteal Study design: Multicentre RCT Method of randomisation: central randomisation, but exact method unclear Blinding: unblinded, intention to treat Exclusions post randomisation: not discussed Losses to follow up: high rate of losses to follow‐up (37 within first 12 months of follow‐up) |
|
| Participants | Country: USA Setting: multicentre No. of participants: 231 patients (240 limbs; 122 PTFE, 118 Dacron) Age: mean 67.1 yrs Sex: 145 male, 95 female Inclusion criteria: angiographically demonstrated superficial femoral artery occlusion with reconstitution of a popliteal segment above the knee Exclusion criteria: earlier infrainguinal vascular procedures Unclear whether patients had IC or critical ischaemia |
|
| Interventions | PTFE versus Dacron (diameter at discretion of operating surgeon) | |
| Outcomes | Primary patency, secondary patency, peri‐operative complications | |
| Notes | 13 patients randomised but not described. Unclear how many patients had post‐op aspirin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised centrally after eligibility was determined by the operating surgeon and informed consent obtained." |
| Allocation concealment (selection bias) | Unclear risk | Not specifically stated. Probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 37 patients randomised lost by 12 months |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | Anticoagulation protocol not stated |
Ballotta 2003.
| Methods | Site: Femoral to AK popliteal Study design: RCT Method of randomisation: concealed randomisation using computer generated randomisation envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: none |
|
| Participants | Country: Italy Setting: hospital No. of participants: 51 (102 limbs; 51 PTFE, 51 reversed vein) Age (mean): 62 yrs Sex: 33 males, 18 females Inclusion criteria: severe claudication, SFA occlusion with one to three runoff vessels Exclusion criteria: untreated inflow disease of ipsilateral pelvic arteries (more than 50% stenosis or occlusion); previous bypass procedure or stent in target SFA; multiple lesions exceeding 10 cm; acute critical limb ischaemia; an untreated ipsilateral iliac artery stenosis; known intolerance to study medications or contrast agents |
|
| Interventions | 8 mm PTFE and reversed vein graft Oral warfarin from one day pre‐op and continued for 6 months; 325 mg aspirin afterwards |
|
| Outcomes | Primary assisted patency as remedial surgery for late bypass stenosis was not considered a primary failure 5‐year data |
|
| Notes | Compliance with medication not checked | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Concealed randomisation using computer generated randomisation envelopes." |
| Allocation concealment (selection bias) | Low risk | Envelopes sealed as above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients lost to long term follow up (mean 59 months) |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other obvious bias |
Davidovic 2010.
| Methods | Site: Femoral to AK popliteal Study design: RCT Method of randomisation: not described Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: not specified |
|
| Participants | Country: Serbia Setting: hospital No. of participants: 85 (43 ePTFE, 42 Dacron) Age (mean): 65.5 yrs Sex: 71 males, 14 females Inclusion criteria: severe claudication or critical ischaemia, "considered suitable for surgical revascularization using above‐knee prosthetic bypass graft" Exclusion criteria: previous procedures on aorto‐iliac or ipsilateral femoro‐politeal arterial segments |
|
| Interventions | 8 mm FlowNit Biosel (Dacron) or 8mm FlowLine BioPore (ePTFE) bypass graft from femoral to above‐knee popliteal artery. All patients given 4 days' antibiotic prophylaxis with a second generation cephalosporine and started on acetylsalicylic acid immediately after surgery | |
| Outcomes | Primary: primary patency, early complications (mortality, bleeding and infection), early limb salvage Secondary: secondary patency, mid‐term complications (mortality, false anastomotic aneurysms and infection), mid‐term limb salvage |
|
| Notes | Clear antibiotic and antiplatelet protocols | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Numbers at risk not presented with survival curves, secondary patency presented as worse than primary patency, which is impossible |
| Selective reporting (reporting bias) | Low risk | All outcomes presented, but numbers at risk at different time points not given so impossible to discern significance of different rates |
| Other bias | Low risk | Clear antiplatelet and antibiotic protocols |
Devine 2004.
| Methods | Site: Femoral to AK and BK popliteal Study design: RCT Method of randomisation: concealed randomisation using computer generated randomisation envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: none |
|
| Participants | Country: UK Setting: hospital No. of participants: 209 (AK: 88 PTFE, 91 HBD; BK: 15 PTFE, 15 HBD) Age (mean): 63 yrs Sex: 142 males, 67 females Inclusion criteria: severe claudication, SFA occlusion with one to three runoff vessels Exclusion criteria: emergency surgery for trauma, acute thrombosis, embolism, or popliteal artery thrombosis Symptoms not sufficiently severe to disrupt lifestyle or ABI > 0.8 at rest (unless aneurysm), the diagnosis or treatment for malignancy within 12 months including all cases with residual malignancy being followed up or observed, hospital inpatient treatment for cardiac failure in the previous 6 months, where adequate follow‐up would be impossible to arrange because the patient lived or was moving to an area where independent follow up could not be arranged |
|
| Interventions | HBD or PTFE (diameter at discretion of operating surgeon) Anticoagulation not stated |
|
| Outcomes | Primary patency | |
| Notes | Anticoagulation not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization, stratified for AK or BK and by surgeon, was performed for eligible patients, using a dedicated computer program." |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed randomization envelopes (1 for AK, 1 for BK) were delivered to the vascular surgeon before surgery." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No losses, but numbers at risk not given for below knee outcomes so attrition not clear for this outcome |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | Anticoagulation protocol not stated |
Eickhoff 1987.
| Methods | Site: Femoral to BK popliteal Study design: multicentre RCT Method of randomisation: sealed envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: none |
|
| Participants | Country: Scandinavia Setting: hospital No. of participants: 105 (55 PTFE, 50 HUV) Age: 68 yrs Sex: 60 male, 45 female Inclusion criteria: DM 12, critical ischaemia 80. BK fem‐pop for short distance IC or critical ischaemia, if no vein or CABG intended Exclusion criteria: short life expectancy, previous graft, Buerger's, coagulopathy |
|
| Interventions | PTFE versus HUV (diameter at discretion of operating surgeon) | |
| Outcomes | Secondary patency | |
| Notes | Post‐op anti‐thrombotic/coagulant therapy unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear as to how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses, clear life table data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | Anticoagulation protocol not stated |
Gloor 1996.
| Methods | Site: Ilio or femoral to AK popliteal Study design: single‐centre RCT Method of randomisation: not explicitly stated Blinding: stated to be single‐blind Exclusions post randomisation: not stated Losses to follow up: none Protocol violations: none stated |
|
| Participants | Country: France Setting: hospital No. of participants: 18 (20 limbs; 10 PUR graft, 10 Dacron) Age (mean): PUR group: 70.7 years; Dacron: 70.5 years Sex: Overall 13 men, 7 women; PUR group: 6 men, 4 women; Dacron group: 7 men, 3 women Inclusion criteria: peripheral arterial occlusion of lower limb graded Fontaine stage IIb‐IV requiring AK synthetic ilio‐ or femoro‐popliteal bypass Exclusion criteria: obesity, emergency surgery, critical threat to limb |
|
| Interventions | Iliac or Femoral to AK popliteal bypass graft with either 6 mm PUR or 6 mm Dacron | |
| Outcomes | Primary and secondary patency, complications in first 30 days, reintervention rate | |
| Notes | Clear anticoagulation/antiplatelet protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Timing of randomisation not declared |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial, though participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No PRISMA flow chart, no mention of patients excluded prior to randomisation or after randomisation |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary patency as well as reinterventions reported, but no complications in first 30 days which did not lead to reintervention mentioned |
| Other bias | Low risk | Clear anticoagulation and antiplatelet protocol |
Gupta 1991.
| Methods | Site: Femoral to AK or BK popliteal Study design: single‐centre RCT Method of randomisation: selecting a random card from an unsorted deck of cards marked with the choice of graft material Blinding: unblinded, no documented crossover so as treated/intention to treat analysis not discussed Exclusions post randomisation: none Losses to follow up: none Protocol violations: none |
|
| Participants | Country: USA Setting: hospital No. of participants: 122 (59 AK of whom 29 ringed, 63 BK of whom 29 ringed) Age (mean): 71 yrs Sex: split not specified Inclusion criteria: patients without an available ipsilateral ASV long enough to serve as femoro‐popliteal bypass on the basis of a history of prior removal, duplex ultrasonography, saphenous venography or operative findings requiring an AK or BK femoro‐popliteal bypass. Patients whose life expectancy was judged to be less than 3 years were also included whether or not an ipsilateral ASV was available Patients with Rutherford category 1 to 5 ischaemia were eligible, though all but 4 patients had rest pain or tissue loss Exclusion criteria: patients with extensive necrosis requiring sequential grafts to distal arteries, patients requiring bypass for reasons other than arteriosclerotic occlusive disease |
|
| Interventions | 6 mm ringed or unringed PTFE | |
| Outcomes | Primary patency, secondary patency, limb salvage (secondary patency and limb salvage not presented separately for above and below‐knee grafts so not included) | |
| Notes | Clear anticoagulation and antiplatelet protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by selection of "a random card from an unsorted deck of cards marked with the choice of graft material" |
| Allocation concealment (selection bias) | Unclear risk | Timing of randomisation not declared |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses, clear life table data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | Clear anticoagulation and antiplatelet protocol |
Jensen 2007.
| Methods | Site: Femoral to AK popliteal (POPUP study) Study design: RCT Method of randomisation: randomisation envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: 13 (8 Dacron, 5 PTFE) Losses to follow up: 51 (12%) |
|
| Participants | Country: Scandinavia Setting: hospital (13 departments) No. of participants: 426 (413 for analysis due to exclusions; 205 PTFE, 208 Dacron) Age (mean): 66 yrs Sex: 152 males, 261 females Inclusion criteria: "chronic lower limb ischaemia" Exclusion criteria: less than 18, pregnant, could not obtain informed consent |
|
| Interventions | 6 mm PTFE and 6 mm Dacron graft Anticoagulation as per individual centre protocol |
|
| Outcomes | Primary patency, secondary patency and limb survival | |
| Notes | No common anticoagulation pathway. Multiple, different surgeons | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Grafts were contained in envelopes, however the randomisation procedure is unclear. Probably done as other papers from this unit clearly use random sequences (Eiberg 2006; Vogt 2007) |
| Allocation concealment (selection bias) | Low risk | Quote: "Immediately before surgery, the graft material was selected by a pre‐processed sealed envelope. Randomisation was stratified for each centre." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses, clear life table data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | Anticoagulation as per individual centre protocol and therefore inconsistent |
Klinkert 2003.
| Methods | Site: Femoral to AK popliteal Study design: RCT Method of randomisation: concealed randomisation using computer generated randomisation envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: 11 (7%) |
|
| Participants | Country: the Netherlands Setting: hospital No. of participants: 136 (151 limbs; 75 Saphenous vein, 76 PTFE) Age (median): 69 yrs Gender: 88 males, 48 females Inclusion criteria: severe claudication, rest pain, tissue loss Exclusion criteria: patients with earlier bypass or previously removed long saphenous vein |
|
| Interventions | 6 mm PTFE and reversed vein graft Oral warfarin from one day pre‐op continued for 6 months. 38 mg aspirin afterwards |
|
| Outcomes | Primary and secondary patency 5‐year follow up |
|
| Notes | No compliance checks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. No specific description |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization took place with closed envelope allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 patients lost to long term follow up, clearly described |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | Oral warfarin from one day pre‐op continued for 6 months. 38mg aspirin afterwards |
Lumsden 2015.
| Methods | Site: Femoral to AK or BK popliteal Study design: multicentre RCT Method of randomisation: not stated Blinding: unblinded, as treated analysis Exclusions post randomisation: 3 (1.4%) Losses to follow up: 4 (1.9%) Protocol violations: 1 (treatment with a non test graft) |
|
| Participants | Country: 18 centres in the USA and 7 in Europe Setting: hospital No. of participants: 209 (105 FUSION BIOLINE, 101 standard ePTFE, 2 no graft implanted, 1 non test graft implanted so latter 3 excluded) Age (median): 62 yrs in standard ePTFE group, 67 in FUSION BIOLINE group Sex: 145 males, 58 females; 2 excluded Inclusion criteria: patients requiring an AK or BK femoro‐popliteal bypass with the proximal anastomosis at the level of the distal external iliac, common femoral, profunda femoral, or proximal superficial femoral artery. The study protocol specified that a prosthetic femoro‐popliteal bypass must be medically necessary, but did not, per se, exclude those without an adequate autogenous conduit. Patients with Rutherford category 1 to 5 ischaemia were eligible, with symptoms of claudication, rest pain, or with superficial ulceration in the target lower extremity Exclusion criteria: acute arterial occlusion requiring urgent intervention; prior open surgical bypass in the target extremity; angioplasty or stenting at the site of a planned anastomosis within the previous 30 days; serum creatinine > 2.5 mg/dL; recent (< 6 weeks) MI or stroke; coagulation or bleeding disorders; receiving warfarin therapy where oral anticoagulation could not be withheld |
|
| Interventions | FUSION BIOLINE heparin coated vascular graft or standard ePTFE graft (diameter at discretion of operating surgeon) | |
| Outcomes | Primary endpoints: efficacy: primary graft patency at 6 months as assessed by duplex ultrasound imaging and ABI. Safety: the composite of MALE and POD. MALE included major amputation, major graft reintervention with placement of a new graft or an interposition graft, open or percutaneous graft thrombectomy, pharmacologic thrombolysis, or graft excision. POD was defined as those that occurred within 30 days of the index procedure or any remedial procedure performed at the same anatomic site. Secondary endpoints: efficacy: primary assisted patency, secondary patency, and bleeding at the suture hole as judged subjectively by the operating surgeon and objectively by recording the time between restoration of flow into the graft and the absence of detectable bleeding from the suture holes | |
| Notes | No consistent anticoagulation protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation sequence generation technique |
| Allocation concealment (selection bias) | Unclear risk | Timing and method of randomisation allocation not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 4 patients had missing data at 6‐month follow‐up |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | No consistent anticoagulation protocol |
Post 2001.
| Methods | Site: Femoral to AK and BK popliteal Study design: RCT Method of randomisation: concealed randomisation using computer generated randomisation envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: 3 (1%) Losses to follow up: 6 (2%) |
|
| Participants | Country: Germany Setting: hospital No. of participants: 203 (194 limbs analysed. AK: 65 PTFE, 76 Dacron, BK: 26 PTFE, 27 Dacron) Age (median): 66 yrs Sex: 155 males, 48 females Inclusion criteria: severe claudication, rest pain, tissue loss Exclusion criteria: infection, emergency surgery for acute ischaemia, distal anastomosis below anterior tibial origin, concomitant disease not expected to live past 3 years, contraindication to anticoagulants |
|
| Interventions | PTFE and Dacron (diameter at discretion of operating surgeon) Post‐op warfarin, heparin or antiplatelet agents |
|
| Outcomes | Primary patency 3‐year follow up |
|
| Notes | No consistent anticoagulation protocol. No compliance checks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The order of Secondary end‐points assignment had been generated by random digits from a statistical software package (SAS)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised to either treatment arm intraoperatively by sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses, clear life table data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | No consistent anticoagulation protocol |
SCAMICOS 2010.
| Methods | Site: BK popliteal and distal (the latter not included in this review) Study design: multicentre RCT Method of randomisation: concealed randomisation using sealed envelopes in blocks of 16 per centre Blinding: unblinded, intention to treat Exclusions post randomisation: 3 (1%) Losses to follow up: 0 (0%) Protocol violations: 3 (1 ‐ suitable vein available, 1 ‐ distal reconstruction below popliteal artery, 1 ‐ crossover from non‐collar to collar group) |
|
| Participants | Country: 29 centres in Sweden and 3 in Denmark Setting: hospital No. of participants: 202 (87 PTFE, 115 PTFE with vein collar) Age (median): 79 yrs in PTFE group, 76 yrs in PTFE with collar group Gender: 77 males, 122 females; 3 excluded Inclusion criteria: rest pain, tissue loss Exclusion criteria: no suitable distal anastomotic target, distal anastomosis AK or below anterior tibial origin for BK popliteal group, or below‐ankle for distal group |
|
| Interventions | Gore or Impra PTFE graft with or without distal vein cuff, diameter not specified (diameter at discretion of operating surgeon) | |
| Outcomes | Primary patency; secondary patency; amputation; death | |
| Notes | No consistent anticoagulation protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation sequence generation technique |
| Allocation concealment (selection bias) | Low risk | Envelope selected at random after confirmation of suitable target vessel |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3 patients had missing follow‐up data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | No consistent anticoagulation protocol |
Scharn 2008.
| Methods | Site: AK Study design: RCT Method of randomisation: controlled by the BOA‐trial agency using a dedicated computer program Blinding: unblinded, intention to treat Exclusions post randomisation: 8 (6%) Losses to follow up: 13 (9%) |
|
| Participants | Country: the Netherlands Setting: hospital No. of participants: 137 (137 limbs with 8 excluded; 59 HBD, 70 HUV) Age (median): 65 yrs Sex: 87 males, 50 females Inclusion criteria: severe claudication, rest pain, tissue loss Exclusion criteria: patients younger than 30 or older than 90 yrs of age; patients with an ABI higher than 0.8 at rest, emergency surgery for trauma, acute thrombosis or embolism of the popliteal artery, the diagnosis or treatment for malignancy within 12 months, hospital in‐patient treatment for cardiac failure in the previous 6 months, the absence of the possibility for adequate follow up or contraindications for anticoagulant drug therapy |
|
| Interventions | Heparin bonded Dacron and HUV (diameter at discretion of operating surgeon) Aspirin 80 mg daily or coumarin derivates (Sintrom) |
|
| Outcomes | Primary patency. 5‐year follow‐up | |
| Notes | No consistent anticoagulation protocol. No compliance checks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was controlled by the BOA‐trial agency using a dedicated computer program." |
| Allocation concealment (selection bias) | Low risk | Not specifically stated but assumed done as BOA‐trial agency involved |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses, clear life table data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | No consistent anticoagulation protocol |
Solakovic 2008.
| Methods | Site: AK popliteal Study design: single‐centre RCT Method of randomisation: concealed randomisation using sealed envelopes following intraoperative assessment of artery and vein Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: 9 (7%) Protocol violations: none |
|
| Participants | Country: 1 centre in Bosnia Setting: hospital No. of participants: 109 patients, 121 limbs (12 patients had a second bypass in the contralateral limb during the study period). There were 60 reversed LSV bypasses and 61 prosthetic bypasses (PTFE or Dacron, material not further specified) Age (median): 70 yrs in reversed LSV group, 68 in prosthetic group Sex: 70 males, 51 females Inclusion criteria: rest pain, tissue loss, 'disabling claudication' Exclusion criteria: previous revascularisation in treated leg, LSV not available or suitable, CFA or AK popliteal not suitable site for anastomosis |
|
| Interventions | Reversed LSV or 6 mm prosthetic bypass from CFA to above‐knee popliteal artery | |
| Outcomes | Primary patency, secondary patency | |
| Notes | All patients received prophylactic clexane at a dose of 0.5 ml/kg while in hospital and then 150 mg/day aspirin after discharge. Compliance with this protocol was not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation sequence generation technique |
| Allocation concealment (selection bias) | Low risk | Envelope selected at random after confirmation of suitable target vessel and suitable vein |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 7% of patients lost to follow‐up over 5 years |
| Selective reporting (reporting bias) | Low risk | Stated outcomes reported |
| Other bias | Unclear risk | Consistent anticoagulation protocol but no compliance checks reported |
Stonebridge 1997.
| Methods | Site: Femoral to AK or BK popliteal Study design: multicentre RCT Method of randomisation: central randomisation centre assessment of artery and vein Blinding: unblinded, intention to treat Exclusions post randomisation: not specified Losses to follow up: not stated Protocol violations: none declared |
|
| Participants | Country: UK Setting: multicentre No. of participants: 246 Inclusion criteria: femoro‐popliteal graft to AK (76 cuff, 74 no cuff) or BK (48 cuff, 47 no cuff) popliteal Exclusion criteria: trauma |
|
| Interventions | 6 mm PTFE with and without a vein cuff | |
| Outcomes | Primary patency, secondary patency, limb salvage | |
| Notes | No consistent anticoagulation protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation technique |
| Allocation concealment (selection bias) | Unclear risk | No clear description |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rates not clearly presented |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | No consistent anticoagulation protocol |
Tofigh 2007.
| Methods | Site: AK Study design: RCT Method of randomisation: unclear Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: 6 (6%) |
|
| Participants | Country: France Setting: hospital No. of participants: 85 (103 limbs; 51 reversed vein, 52 polyester) Age (median): 69 yrs Sex: 49 males, 36 females Inclusion criteria: severe claudication, rest pain, tissue loss Exclusion criteria: patients with earlier bypass or un‐useable LSV |
|
| Interventions | 6 mm collagen‐impregnated woven polyester prosthesis and reversed vein graft Oral warfarin from one day pre‐op continued for 6 months. 38 mg aspirin afterwards |
|
| Outcomes | Primary and secondary patency 5‐year follow‐up |
|
| Notes | No medication compliance checks. Unclear randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation technique |
| Allocation concealment (selection bias) | Unclear risk | No clear description |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses, clear data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No obvious other source of bias |
van Det 2009.
| Methods | Site: AK Study design: RCT Method of randomisation: sealed envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: 4 (%) |
|
| Participants | Country: France Setting: hospital No. of participants: 228 (228 limbs; 114 Dacron, 114 PTFE) Age (median): 66 yrs Sex: 147 males, 81 females Inclusion criteria: severe claudication, rest pain, tissue loss Exclusion criteria: patients with earlier bypass contraindication to long term anticoagulant therapy, life expectancy less than 1 year |
|
| Interventions | 6 mm PTFE or 6 mm Dacron. Warfarin post‐op (all patients) | |
| Outcomes | Primary, primary assisted and secondary patency 10‐year follow‐up |
|
| Notes | Good anticoagulation protocol. Clear numbers of patients throughout (flow chart) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer program used for sequence generation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses, clear life table data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | Good anticoagulation protocol. Clear numbers of patients throughout (flow chart) |
Vriens 2013.
| Methods | Site: Femoral to AK popliteal Study design: multicentre RCT Method of randomisation: concealed randomisation using sealed envelopes in blocks of 4 per centre Blinding: unblinded, as treated analysis Exclusions post randomisation: 1 (0.4%) Losses to follow up: 4 (1.5%) Protocol violations: 1 (1 ‐ crossover from allocated group) |
|
| Participants | Country: 6 centres in the Netherlands Setting: hospital No. of participants: 266 (136 externally supported polyester, 129 non‐externally supported polyester, 1 not treated according to protocol so excluded) Age (median): 65 yrs in externally supported group, 67 in non externally supported group Sex: 199 males, 66 females; 1 excluded Inclusion criteria: all patients requiring AK femoro‐popliteal bypass for disabling claudication, rest pain, tissue loss in the absence of a suitable venous conduit Exclusion criteria: no suitable distal anastomotic target, distal anastomosis not above knee, previous ipsilateral femoro‐popliteal procedures, contra‐indication for the use of acetyl salicylic acid or anticoagulants, patients receiving chemo‐ or radiotherapy, malignancy diagnosed or treated within 12 months, known allergy to iodine or contrast medium, and impaired renal function |
|
| Interventions | Fluoropassiv 6 mm knitted polyester, either externally supported thin‐wall fluoropolymer coated or 6 mm externally unsupported thin wall | |
| Outcomes | Primary endpoints: primary patency at 1 and 2 years post‐op. Secondary endpoints: mortality, primary assisted and secondary patency | |
| Notes | Clear anticoagulation protocol. Clear numbers of patients throughout (flow chart) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation sequence generation technique |
| Allocation concealment (selection bias) | Low risk | Envelope selected at random after confirmation of suitable target vessel |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 4 patients (1.5%) were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | Good anticoagulation protocol. Clear numbers of patients throughout (flow chart) |
ABI: ankle brachial index AK: above knee ASV: autologous saphenous vein BK: below knee CABG: coronary bypass graft CFA: common femoral artery DM: diabetes mellitus HBD: heparin bonded Dacron HUV: human umbilical vein IC: intermittent claudication LSV: long saphenous vein MALE: major adverse limb events MI: myocardial infarction POD: peri‐procedural death post‐op: post‐operative/operatively pt: patient PTFE: polytetrafluoroethylene PUR: polyurethane RCT: randomised controlled trial SFA: superficial femoral artery yrs: years
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bennion 1985 | Results presented include non‐randomised patients. Randomisation technique unclear. Distal grafts included, not intention to treat |
| Chikiar 2003 | Retrospective, non‐randomised study (not an RCT or CCT): retrospective study where data were collected from patient records |
| Erasmi 1996 | The trial was performed in patients having femoro‐popliteal bypass both above and below the knee. Outcomes for the above‐ and below‐knee subgroups were not reported so it was not possible to include the trial |
| Hamann 1998 | Randomisation technique unclear, above‐knee, below‐knee and distal bypasses inseparable (English title states above‐knee but methods talk about below‐knee bypass) |
| Hobson 1980 | Case series, not randomised trial data |
| Johnson 2000 | Inadequate randomisation process. Quote: "the choice between a PTFE and HUV bypass graft was randomized in the operating room, initially to favour saphenous vein." The data were presented as vein versus HUV versus PTFE and was inseparable for analysis |
| Kreienberg 2002 | Bypass to any below‐knee artery, not just popliteal. Randomisation technique unclear |
| Kumar 1995 | Unclear randomisation process. Results never fully published in paper form, only as two abstracts. Data presented as vein versus PTFE versus Dacron and were inseparable for analysis |
| Lindholt 2011 | The trial was performed in patients having femoro‐popliteal bypass both above and below the knee. Outcomes for the above‐ and below‐knee subgroups were not reported so it was not possible to include the trial |
| Linni 2015 | The trial was performed in patients having femoro‐popliteal and more distal bypass. Outcomes for the subgroups of patients with distal anastomosis the above‐knee popliteal or below‐knee popliteal artery were not reported so the study could not be included |
| Lundgren 2013 | The trial was performed in both patients having femoro‐popliteal bypass below the knee and patients having femoro‐distal bypass. Outcomes for the subgroup having femoro‐popliteal bypass alone were not reported |
| McCollum 1991 | Unable to separate above‐ and below‐knee data |
| Midy 2016 | Trial failed to recruit 30% of planned patients, and lost 26% of these to follow up. Results only presented at 5 years follow‐up using an unusual system to impute missing data |
| Moody 1992 | Unable to separate above‐ and below‐knee data |
| Motta 1989 | Above‐knee, below‐knee and distal bypasses inseparable; unclear randomisation |
| NCT00617279 | Trial terminated by sponsor due to slow recruitment. No results available |
| NCT00845585 | Trial withdrawn prior to enrolment of any patients |
| Robinson 1999 | Unable to separate above‐ and below‐knee data. A proportion of both above‐ and below‐knee anastomoses included endarterectomies and or vein cuffs which the study authors concede produced a significant difference in patency without giving detailed subgroup analysis. Unclear randomisation |
| Robinson 2003 | Unable to separate above‐ and below‐knee data. Below‐knee anastomotic site described as 'distal' in some cases without detailed anatomical description. A proportion of both above‐ and below‐knee anastomoses included endarterectomies and or vein cuffs which the study authors concede produced a significant difference in patency without giving detailed subgroup analysis. Unclear randomisation |
| Schulman 1987 | Patients received both above‐ and below‐knee bypass grafts but results presented together. Poor randomisation (month of birth) |
| Tilanus 1985 | Unable to separate above‐ and below‐knee data. Unclear randomisation technique |
| Veith 1986 | Unable to separate above‐ and below‐knee data. Inadequate randomisation (hospital number, card pulling, random number generator) |
| Watelet 1997 | The trial was performed in patients having femoro‐popliteal bypass both above and below the knee. Outcomes for the above‐ and below‐knee subgroups were not reported so it was not possible to include the trial |
| Zilla 1994 | Unable to separate above‐ and below‐knee data, not intention to treat. Inadequate randomisation (random number generator, concealment not stated) |
CCT: clinically controlled trial HUV: human umbilical vein PTFE: polytetrafluoroethylene RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT00147979.
| Trial name or title | Multicentric, Prospective, Randomized, Comparing Trial Between Bypass of the Femoropoplitea by PTFE and Heparin Bounded PTFE |
| Methods | Randomised controlled trial |
| Participants | 18 years and older, peripheral vascular disease requiring above‐ or below‐knee femoro‐popliteal bypass |
| Interventions | PTFE versus PTFE with bonded heparin |
| Outcomes | Primary outcome measures: primary patency after 2 years Secondary outcome measures: secondary patency; limb salvage; mortality; re‐intervention |
| Starting date | April 2004 |
| Contact information | Frank Vermassen, MD, PhD, University Hospital, Ghent |
| Notes | A preliminary survival curve was presented at the Charing Cross Symposium in 2009. No useable data could be gleaned from this and no official abstract was published. The lead author was contacted for results but did not reply. The study is reported as completed on ClinicalTrials.gov but has not been published. ClinicalTrials.gov identifier: NCT00147979 |
NCT00205790.
| Trial name or title | GORE‐TEX PROPATEN Vascular Graft Study |
| Methods | Single‐blind randomised controlled trial |
| Participants | 21 years and older, peripheral vascular disease requiring above‐knee femoro‐popliteal bypass |
| Interventions | GORE‐TEX PROPATEN vascular grafts versus thin walled GORE‐TEX Stretch vascular grafts |
| Outcomes | Primary outcome measures: primary patency at 12 months; major device complication rates at 12 months Secondary outcome measures: technical failures; secondary patency |
| Starting date | February 2003. Trial completed recruitment in 2007 but still has not published results |
| Contact information | Enrico Ascher, MD Maimonides Hospital, Brooklyn NY |
| Notes | Sponsored by WL Gore & Associates ClinicalTrials.gov identifier: NCT00205790 |
PTFE: polytetrafluoroethylene
Differences between protocol and review
For this update, the risk of bias in all included studies was assessed using Cochrane's 'Risk of bias' tool and a 'Summary of findings' table has been added.
We reworded the objective so to adhere better to the Cochane guidelines.
We amended the 'types of studies' to include all possible graft types.
We provided definitions of the outcomes primary and secondary patency.
We analysed and presented data into groups according to whether the distal anastomosis was above or below the knee.
Contributions of authors
GA: identified relevant trials, assessed quality for all included trials, extracted data and updated the text of review.
CT: identified relevant trials, assessed quality, extracted data, wrote text of previous version of review, and reviewed updated text.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The editorial base of Cochrane Vascular is supported by the Chief Scientist Office.
Declarations of interest
GA: has declared that he previously held a National Institute for Health Research Academic clinical fellowship (2011‐2014) and that he received funds for a grant from Heath and Care Research Wales regarding research for patient and public benefit (grant number 1198); there are no known conflicts of interest with this review.
CT: has declared that he received money from Cook Medical for travel/accommodation/meeting expenses unrelated to this review and that he received funds for a grant from Heath and Care Research Wales regarding research for patient and public benefit (grant number 1198); there are no known conflicts of interest with this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aalders 1992 {published and unpublished data}
- Aalders GJ, Vroonhoven TJMV. Polytetrafluoroethylene versus human umbilical vein in above‐knee femoro‐popliteal bypass: Six‐year results of a randomized clinical trial. Journal of Vascular Surgery 1992;16:816‐24. [PubMed] [Google Scholar]
Abbot 1997 {published data only}
- Abbot WM, Green RM, Matsumoto T, Wheeler JR, Miller N, Veith FJ, et al. Prosthetic above‐knee femoro‐popliteal bypass grafting: results of a multicenter randomized prospective trial. Journal of Vascular Surgery 1997;25:19‐28. [DOI] [PubMed] [Google Scholar]
- Green RM, Abbott WM, Matsumoto T, Wheeler JR, Millem N, Veith FJ, et al. Prosthetic above‐knee femoro‐popliteal bypass grafting: Five‐year results of a randomized trial. Journal of Vascular Surgery 2000;31(3):417‐25. [PubMed] [Google Scholar]
Ballotta 2003 {published data only}
- Ballotta E, Renon L, Toffano M, Giau G. Prospective randomized study on bilateral above‐knee femoro‐popliteal revascularization: Polytetrafluoroethylene graft versus reversed saphenous vein. Journal of Vascular Surgery 2003;38(5):1051‐5. [PUBMED: 14603216] [DOI] [PubMed] [Google Scholar]
Davidovic 2010 {published data only}
- Davidovic L, Jakovljevic N, Radak D, Dragas M, Ilic N, Koncar I, et al. Dacron or ePTFE graft for above‐knee femoropopliteal bypass reconstruction. A bi‐centre randomised study. Vasa 2010;39:77‐84. [DOI] [PubMed] [Google Scholar]
Devine 2004 {published data only}
- Devine C, McCollum C. Heparin‐bonded Dacron or polytetrafluorethylene for femoro‐popliteal bypass: Five‐year results of a prospective randomized multicenter clinical trial. Journal of Vascular Surgery 2004;40(5):924‐31. [DOI] [PubMed] [Google Scholar]
- Devine CM, McCollum CN. Heparin‐bonded Dacron or polytetrafluoroethylene for femoro‐popliteal bypass grafting: a multicenter trial. Journal of Vascular Surgery 2001;33(3):533‐9. [DOI] [PubMed] [Google Scholar]
Eickhoff 1987 {published data only}
- Eickhoff JH, Broome A, Ericsson BF, Hansen HJB, Kordt K F, Mouritzen C, et al. Four years' results of a prospective, randomized clinical trial comparing polytetrafluoroethylene and modified human umbilical vein for below‐knee femoro‐popliteal bypass. Journal of Vascular Surgery 1987;6:506‐11. [DOI] [PubMed] [Google Scholar]
- Eickhoff JH, Hansen HJB, Bromme A, Ericsson BF, Kordt KF, Mouritzen C, et al. A randomized clinical trial of PTFE versus human umbilical vein for femoro‐popliteal bypass surgery. Preliminary results. British Journal of Surgery 1983;70:85‐8. [DOI] [PubMed] [Google Scholar]
Gloor 1996 {published data only}
- Gloor B, Wehrli E, Rotzer A, Brunner D, Wilms C, Largiader J. Polyurethane small artery substitutes for femoro‐popliteal above knee bypass. Swiss Surgery 1996;1 Suppl:13‐8. [PubMed] [Google Scholar]
Gupta 1991 {published data only}
- Gupta SK, Veith FJ, Kram HB, Wengerter KR. Prospective randomized comparison of ringed and nonringed polytetrafluoroethylene femoro‐popliteal bypass grafts: A preliminary report. Journal of Vascular Surgery 1991;13:162‐72. [PubMed] [Google Scholar]
Jensen 2007 {published data only}
- Jensen LP, Lepantalo M, Fossdal JE, Roder OC, Jensen BS, Madsen MS, et al. Dacron or PTFE for above‐knee femoro‐popliteal bypass. a multicenter randomised study. European Journal of Vascular and Endovascular Surgery 2007;34(1):44‐9. [PUBMED: 17400486] [DOI] [PubMed] [Google Scholar]
Klinkert 2003 {published data only}
- Burger DH, Kappetein AP, Bockel JH, Breslau PJ. A prospective randomized trial comparing vein with polytetrafluoroethylene in above‐knee femoro‐popliteal bypass grafting. Journal of Vascular Surgery 2000;32(2):278‐83. [PUBMED: 10917987] [DOI] [PubMed] [Google Scholar]
- Klinkert P, Schepers A, Burger DH, Bockel JH, Breslau PJ. Vein versus polytetrafluoroethylene in above‐knee femoro‐popliteal bypass grafting: five‐year results of a randomized controlled trial. Journal of Vascular Surgery 2003;37(1):149‐55. [PUBMED: 12514593] [DOI] [PubMed] [Google Scholar]
Lumsden 2015 {published data only}
- Lumsden AB, Morrissey NJ, Comparison of safety and primary patency between the FUSION BIOLINE heparin‐coated vascular graft and EXXCEL Soft ePTFE (FINEST) trial Co‐investigators. Randomized controlled trial comparing the safety and efficacy between the FUSION BIOLINE heparin‐coated vascular graft and the standard expanded polytetrafluoroethylene graft for femoropopliteal bypass. Journal of Vascular Surgery 2015;61:703‐12. [DOI] [PubMed] [Google Scholar]
Post 2001 {published data only}
- Post S, Kraus T, Muller‐Reinartz U, Weiss C, Kortmann H, Quentmeier A, et al. Dacron vs polytetrafluoroethylene grafts for femoro‐popliteal bypass: a prospective randomised multicentre trial. European Journal of Endovascular Surgery 2001;22:226‐31. [DOI] [PubMed] [Google Scholar]
SCAMICOS 2010 {published data only}
- SCAMICOS. PTFE bypass to below‐knee arteries: distal vein collar or not? A prospective randomised multicentre study. European Journal of Vascular and Endovascular Surgery 2010;39:747‐54. [DOI] [PubMed] [Google Scholar]
Scharn 2008 {published data only}
- Scharn DM, Dirven M, Barendregt WB, Boll AP, Roelofs D, Vliet JA. Human umbilical vein versus heparin‐bonded polyester for femoro‐popliteal bypass: 5‐year results of a prospective randomized multicentre trial. European Journal of Vascular and Endovascular Surgery 2008;35(1):61‐7. [DOI] [PubMed] [Google Scholar]
Solakovic 2008 {published data only}
- Solakovic E, Totic D, Solakovic S. Femoro‐popliteal bypass above knee with saphenous vein vs synthetic graft. Bosnian Journal of Basic Medical Sciences 2008;8:367‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stonebridge 1997 {published data only}
- Griffiths GD, Nagy J, Black D, Stonebridge PA. Randomized clinical trial of distal anastomotic interposition vein cuff in infrainguinal polytetrafluoroethylene bypass grafting. British Journal of Surgery 2004;91(5):560‐2. [PUBMED: 15122605] [DOI] [PubMed] [Google Scholar]
- Stonebridge PA, Prescott RJ, Ruckley CV. Randomized trial comparing infrainguinal polytetrafluoroethylene bypass grafting with and without vein interposition cuff at the distal anastamosis. Journal of Vascular Surgery 1997;26:543‐50. [DOI] [PubMed] [Google Scholar]
Tofigh 2007 {published data only}
- Tofigh AM, Warnier De Wailly G, Rhissassi B. Comparing vein with collagen impregnated woven polyester prosthesis in above‐knee femoro‐popliteal bypass grafting. International Journal of Surgery 2007;5(2):109‐13. [PUBMED: 17448975] [DOI] [PubMed] [Google Scholar]
van Det 2009 {published data only}
- Det RJ, Vriens BHR, Palen J, Geelkerken RH. Dacron or ePTFE for Femoro‐popliteal above knee bypass grafting: Short‐ and Long‐term results of a multicentre randomised trial. European Journal of Vascular and Endovascular Surgery 2009;37:457‐63. [DOI] [PubMed] [Google Scholar]
Vriens 2013 {published data only}
- Vriens BHR, Det RJ, Meerwaldt R, PJ, Gerrits DG, Zeebregts CJ, Geelkerken RH. Superior two‐year results of externally unsupported polyester compared to supported grafts in above‐knee bypass grafting: A multicenter randomised trial. European Journal of Vascular and Endovascular Surgery 2013;45(3):275‐81. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bennion 1985 {published data only}
- Bennion RS, Williams RA, Stabile BE, Fox MA, Owens ML, Wilson SE. Patency of autogenous saphenous vein versus polytetrafluoroethylene grafts in femoro‐popliteal bypass for advanced ischaemia of the extremity. Surgery, Gynecology & Obstetrics 1985;160:239‐42. [PubMed] [Google Scholar]
Chikiar 2003 {published data only}
- Chikiar DS, Grandjean M, Abelleyra A. Femoropoliteal bypass grafting for arterial occlusive disease. Patency and complications. Randomised retrospective study. Prensa Medica Argentina 2003;90(4):338‐44. [Google Scholar]
Erasmi 1996 {published data only}
- Erasmi H, Walter M, Schmitz‐Rixen T, Kristen F. Preliminary results of a prospective randomised study regarding femoro‐popliteal bypass material. Zentralblatt für Chirurgie 1996;121:228‐33. [PubMed] [Google Scholar]
Hamann 1998 {published data only}
- Hamann H, Krawczynski H, Mayer W, Wack HO. Above knee femoropopliteal vein bypasses versus vascular prostheses. Gefasschirurgie 1998;3:14‐9. [Google Scholar]
Hobson 1980 {published data only}
- Hobson RW, O'Donnell JA, Jamil Z, Mehta K. Below knee bypass for limb salvage. Archives of Surgery 1980;115:833‐7. [DOI] [PubMed] [Google Scholar]
Johnson 2000 {published data only}
- Johnson WC, Lee KK. A comparative evaluation of polytetrafluoroethylene, umbilical vein, and saphenous vein bypass grafts for femoral‐popliteal above‐knee revascularization:a prospective randomized Department of Veterans Affairs cooperative study. Journal of Vascular Surgery 2000;32(2):268‐77. [DOI] [PubMed] [Google Scholar]
- Johnson WC, Lee KK, VA Cooperative Study Group 141. Comparative evaluation of PTFE, HUV and saphenous vein bypasses in fem‐pop AK vascular reconstructions. Journal of Vascular Surgery 1992;15:1070. [Google Scholar]
Kreienberg 2002 {published data only}
- Kreienberg PB, Darling RC 3rd, Chang BB, Champagne BJ, Paty PS, Roddy SP, et al. Early results of a prospective randomized trial of spliced vein versus polytetrafluoroethylene graft with a distal vein cuff for limb‐threatening ischemia. Journal of Vascular Surgery 2002;35(2):299‐306. [PUBMED: 11854728] [DOI] [PubMed] [Google Scholar]
Kumar 1995 {published data only}
- Kumar KP, Crinnion JN, Ashley S, Case WG, Gough MJ. Vein, PTFE or Dacron for above‐knee femoro‐popliteal bypass?. International Angiology 1995;14:200. [Google Scholar]
- Kumar KP, Homer‐Vanniasinkam SM, Gough MJ. Femoro‐popliteal bypass grafting: fact or fiction?. Cardiovascular Surgery 22nd World Congress. 1995:116.
Lindholt 2011 {published data only (unpublished sought but not used)}
- Lindholt JS, Gottschalksen B, Johannesen N, Dueholm D, Ravn H, Christensen ED, Viddal B, Florenes T, Pedersen G, Rasmussen M, Carstensen M, Grondal N, Fasting H. The Scandinavian propaten trial ‐ 1‐year patency of PTFE vascular prostheses with heparin‐bonded luminal surfaces compared to ordinary pure PTFE vascular prostheses ‐ a randomised clinical controlled multi‐centre trial. European Journal of Vascular and Endovascular Surgery 2011;41:668‐73. [DOI] [PubMed] [Google Scholar]
Linni 2015 {published data only}
- Linni K, Aspalter M, Butturini E, Dabernig W, Guggenbichler S, Hitzl W, Hölzenbein T. Arm veins versus contralateral greater saphenous veins for lower extremity bypass reconstruction: preliminary data of a randomised study. Annals of Vascular Surgery 2015;29(3):551‐9. [DOI] [PubMed] [Google Scholar]
Lundgren 2013 {published data only}
- Lundgren F, Swedish External Support Study. External support of a polytetrafluoroethylene graft improves patency for bypass to below‐knee arteries. Annals of Vascular Surgery 2013;27(8):1124‐33. [DOI] [PubMed] [Google Scholar]
McCollum 1991 {published data only}
- McCollum C, Kenchington G, Alexander C, Franks PJ, Greenhalgh RM. PTFE or HUV for femoro‐popliteal bypass: A multicentre trial. European Journal of Vascular Surgery 1991;5:435‐43. [DOI] [PubMed] [Google Scholar]
- McCollum CN, Alexander CE, Kenchington G, Franks PJ, Greenhalgh RM. PTFE or HUV for femoro‐popliteal bypass: a multi‐centre trial. Proceedings of the European Society for Vascular Surgery 4th Annual Meeting. Rome, Italy, 1990:44. [DOI] [PubMed]
Midy 2016 {published data only}
- Midy D, Papon X, Patra P, Hassen Kodja R, Feugier P, Plissonnier D, et al. Randomized study of noninferiority comparing prosthetic and autologous vein above‐knee femoropopliteal bypasses. Annals of Vascular Surgery 2016;31:99‐104. [DOI] [PubMed] [Google Scholar]
Moody 1992 {published data only}
- Harris PL, How TV, Jones DR. Prospectively randomized clinical trial to compare in situ and reversed saphenous vein grafts for femoro‐popliteal bypass. The British Journal of Surgery 1987;74:252‐5. [DOI] [PubMed] [Google Scholar]
- Moody AP, Edwards PR, Harris PL. In situ versus reversed femoro‐popliteal vein grafts: long‐term follow up of a prospective randomized trial. The British Journal of Surgery 1992;79(8):750‐2. [DOI] [PubMed] [Google Scholar]
Motta 1989 {published data only}
- Motta G, Ratto GB, Sacco A. Long term evaluation of human umbilical vein as small caliber arterial substitute. Vascular Surgery 1988;22(5):328‐34. [Google Scholar]
NCT00617279 {published data only}
- NCT00617279. GORE PROPATEN vascular graft vs. disadvantaged autologous vein graft (PRODIGY). clinicaltrials.gov/ct2/show/NCT00617279?term=NCT00617279&rank=1 (first received 18 December 2007).
NCT00845585 {unpublished data only}
- NCT00845585. Ovine graft (Omniflow II) versus PTFE in below knee arterial reconstruction. clinicaltrials.gov/ct2/show/NCT00845585?term=NCT00845585&rank=1 (first received 16 February 2009).
Robinson 1999 {published data only}
- Fletcher JP, Robinson BI. A prospective randomised comparison of PTFE and Dacron for femoro‐popliteal bypass. ANZ Journal of Surgery 1995;67 Suppl 1:A85. [Google Scholar]
- Robinson BI, Fletcher JP, Tomlinson P, Allen RD, Hazelton SJ, Richardson AJ, et al. A prospective randomized multicentre comparison of expanded polytetrafluoroethylene and gelatin‐sealed knitted Dacron grafts for femoro‐popliteal bypass. Cardiovascular Surgery (London, England) 1999;7(2):214‐8. [PUBMED: 10353674] [DOI] [PubMed] [Google Scholar]
Robinson 2003 {published data only}
- Robinson BI, Fletcher JP. Fluoropolymer coated Dacron or polytetrafluoroethylene for femoro‐popliteal bypass grafting: a multicentre trial. ANZ Journal of Surgery 2003;73(3):95‐9. [PUBMED: 12608965] [DOI] [PubMed] [Google Scholar]
Schulman 1987 {published data only}
- Schulman ML, Badhey MR, Yatco R. Superficial femoro‐popliteal veins and reversed saphenous veins as primary femoro‐popliteal bypass grafts: A randomized comparative study. Journal of Vascular Surgery 1987;6:1‐10. [DOI] [PubMed] [Google Scholar]
- Schulman ML, Badhey MR, Yatco R, Pillari G. An 11‐year experience with deep leg veins as femoro‐popliteal bypass grafts. Archives of Surgery 1986;121:1010‐5. [DOI] [PubMed] [Google Scholar]
Tilanus 1985 {published data only}
- Tilanus HW, Obertrop H, Urk H. Saphenous vein or PTFE for femoro‐popliteal bypass. A prospective randomized trial. Annals of Surgery 1985;202:780‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veith 1986 {published and unpublished data}
- Bergan JJ, Veith FJ, Bernhard VM, Yao JST, Flinn WR, Gupta SK, et al. Randomization of autogenous vein and polytetrafluoroethylene grafts in femoro‐distal construction. Surgery 1982;92:921‐30. [PubMed] [Google Scholar]
- Veith FJ, Gupta SK, Ascer E, White‐Flores S, Samson RH, Scher LA, et al. Six‐year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial constructions. Journal of Vascular Surgery 1986;3:104‐14. [DOI] [PubMed] [Google Scholar]
Watelet 1997 {published and unpublished data}
- Watelet J, Cheysson E, Poels D, Menard J‐F, Papion H, Saour N, et al. In situ versus reversed saphenous vein for femoro‐popliteal bypass: a prospective randomized study of 100 cases. Annals of Vascular Surgery 1986;1:441‐52. [DOI] [PubMed] [Google Scholar]
- Watelet J, Soury P, Menard J‐F, Plissonnier D, Peillon C, Lestrat J‐P, et al. Femoropopliteal bypass: in situ or reversed vein grafts? Ten‐year results of a randomized prospective study. Annals of Vascular Surgery 1997;11:510‐9. [DOI] [PubMed] [Google Scholar]
Zilla 1994 {published data only}
- Zilla P, Deutsch M, Meinhart J, Puschmann R, Eberl T, Minar E, et al. Clinical in vitro endothelialization of femoro‐popliteal bypass grafts: an actuarial follow‐up over three years. Journal of Vascular Surgery 1994;19:540‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00147979 {published data only}
- NCT00147979. Multicentric, Prospective, Randomized, Comparing Trial Between Bypass of the Femoropoplitea by PTFE and Heparin Bounded PTFE. clinicaltrials.gov/ct2/show/NCT00147979?term=NCT00147979&rank=1 (first received 6 September 2005).
NCT00205790 {published data only}
- NCT00205790. GORE‐TEX PROPATEN Vascular Graft Study. clinicaltrials.gov/ct2/show/NCT00205790?term=NCT00205790&rank=1 (first received 12 September 2005).
Additional references
Albers 2005
- Albers A, Romiti M, Brochado‐Neto FC, Pereira CAB. Meta‐analysis of alternative autologous vein bypass grafts to infra popliteal arteries. Journal of Vascular Surgery 2005;42:449‐55. [DOI] [PubMed] [Google Scholar]
Bradbury 2010
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: An intention‐to‐treat analysis of amputation‐free and overall survival in patients randomized to a bypass surgery‐first or a balloon angioplasty‐first revascularization strategy. Journal of Vascular Surgery 2010;51:5S‐17S. [DOI] [PubMed] [Google Scholar]
Brown 2008
- Brown J, Lethaby A, Maxwell H, Wawrzyniak AJ, Prins MH. Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD000535.pub2] [DOI] [PubMed] [Google Scholar]
Consensus Document
- Anonymous. Second European Consensus Document on chronic critical leg ischemia. Circulation 1991;84 Suppl(4):1‐26. [PubMed] [Google Scholar]
Cranley 1982
- Cranley JJ, Hafner CD. Revascularisation of the femoro‐popliteal arteries using saphenous vein, polytetrafluoroethylene and umbilical vein grafts. Archives of Surgery 1982;117:1543‐50. [DOI] [PubMed] [Google Scholar]
Dardik 2002
- Dardik H, Wengerter K, Qin F, Pangilinan A, Silvestri F, Wolodiger F, et al. Comparative decades of experience with glutaraldehyde‐tanned human umbilical cord vein graft for lower limb revascularization: an analysis of 1275 cases. Journal of Vascular Surgery 2002;35(1):64‐71. [PUBMED: 11802134] [PubMed] [Google Scholar]
Dorffler‐Melly 2003
- Dorffler‐Melly J, Buller HR, Koopman MM, Prins MH. Antithrombotic agents for preventing thrombosis after infrainguinal arterial bypass surgery. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000536] [DOI] [PubMed] [Google Scholar]
Eiberg 2006
- Eiberg JP, Roder O, Stahl‐Madsen M, Eldrup N, Qvarfordt P, Laursen A, et al. Fluoropolymer‐coated dacron versus PTFE grafts for femorofemoral crossover bypass: randomised trial. European Journal of Vascular and Endovascular Surgery 2006;32(4):431‐8. [PUBMED: 16807001] [DOI] [PubMed] [Google Scholar]
Fowkes 2008
- Fowkes F, Leng GC. Bypass surgery for chronic lower limb ischaemia. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD002000.pub2] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 30 March 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Jackson 2000
- Jackson MR, Belott TP, Dickason T, Kaiser WJ, Modrall JG, Valentine RJ, et al. The consequences of a failed femoropopliteal bypass grafting: comparison of saphenous vein and PTFE grafts. Journal of Vascular Surgery 2000;32:498‐504. [DOI] [PubMed] [Google Scholar]
Luther 1997
- Luther M. Surgical treatment for chronic critical leg ischaemia: a 5 year follow‐up of socioeconomic outcome. European Journal of Vascular and Endovascular Surgery 1997;13(5):452‐9. [PUBMED: 9166267] [DOI] [PubMed] [Google Scholar]
NICE 2003
- The National Institute for Clinical Excellence. Coronary artery stents: rapid systematic review and economic evaluation. Liverpool: Liverpool reviews and implementation group, 2003. https://www.nice.org.uk/guidance/ta71/documents/assessment‐report‐coronary‐artery‐stents‐rapid‐systematic‐review‐and‐economic‐evaluation‐4. [Google Scholar]
Nolan 2007
- Nolan B, Finlayson S, Tosteson A, Powell R, Cronenwett J. The treatment of disabling intermittent claudication in patients with superficial femoral artery occlusive disease‐‐decision analysis. Journal of Vascular Surgery 2007;45(6):1179‐84. [PUBMED: 17543682] [DOI] [PubMed] [Google Scholar]
Pereira 2006
- Pereira CE, Albers M, Romiti M, Brochado‐Neto FC, Pereira CA. Meta‐analysis of femoro‐popliteal bypass grafts for lower extremity arterial insufficiency. Journal of Vascular Surgery 2006;44(3):510‐7. [PUBMED: 16950427] [DOI] [PubMed] [Google Scholar]
Perler 1995
- Perler BA. Cost‐efficacy issues in the treatment of peripheral vascular disease: primary amputation or revascularization for limb‐threatening ischemia. Journal of Vascular and Interventional Radiology: JVIR 1995;6 Suppl(6 Pt 2):111‐5. [PUBMED: 8770853] [DOI] [PubMed] [Google Scholar]
Reifsnyder 1992
- Reifsnyder T, Bandyk D, Seabrook G, Kinney E, Towne JB. Wound complications of the in situ saphenous vein bypass technique. Journal of Vascular Surgery 1992;15:843‐8. [DOI] [PubMed] [Google Scholar]
Roll 2008
- Roll S, Muller‐Nordhorn J, Keil T, Scholz H, Eidt D, Greiner W, et al. Dacron vs. PTFE as bypass materials in peripheral vascular surgery‐‐systematic review and meta‐analysis. BMC Surgery 2008;8:22. [PUBMED: 19099583] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rychlik 2014a
- Rychlik IJ, Davey P, Murphy J, O'Donnell ME. A meta‐analysis to compare Dacron versus polytetrafluroethylene grafts for above‐knee femoropopliteal artery bypass. Journal of Vascular Surgery 2014;60:506‐15. [DOI] [PubMed] [Google Scholar]
Siracuse 2013
- Siracuse JJ, Nandivada P, Giles KA, Hamdan AD, Wyers AC, Chaikov EL, et al. Prosthetic graft infections involving the femoral artery. Journal of Vascular Surgery 2013;57:700‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Twine 2009
- Twine CP, Coulston J, Shandall A, McLain AD. Angioplasty versus stenting for superficial femoral artery lesions. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006767.pub2] [DOI] [PubMed] [Google Scholar]
Twine 2012
- Twine CP, Williams IM, Fligelstone LJ. Systematic review and meta‐analysis of vein cuffs for below‐knee synthetic bypass. British Journal of Surgery 2012;99:1195‐202. [DOI] [PubMed] [Google Scholar]
Vogt 2007
- Vogt KC, Uhlyarik M, Schroeder TV. Moist wound healing compared with standard care of treatment of primary closed vascular surgical wounds: a prospective randomized controlled study. Wound Repair and Regeneration 2007;15(5):624‐7. [PUBMED: 17971007] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Mamode 1999
- Mamode N, Scott RN. Graft type for femoro‐popliteal bypass surgery. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD001487] [DOI] [PubMed] [Google Scholar]
Twine 2010
- Twine CP, McLain AD. Graft type for femoro‐popliteal bypass surgery. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD001487.pub2] [DOI] [PubMed] [Google Scholar]


