Abstract
Background
The routine prophylactic administration of an uterotonic agent is an integral part of active management of the third stage of labour, helping to prevent postpartum haemorrhage (PPH). The two most widely used uterotonic agents are: ergometrine‐oxytocin (Syntometrine®) (a combination of oxytocin 5 international units (iu) and ergometrine 0.5 mg) and oxytocin (Syntocinon®).
Objectives
To compare the effects of ergometrine‐oxytocin with oxytocin in reducing the risk of PPH (blood loss of at least 500 ml) and other maternal and neonatal outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 April 2007).
Selection criteria
Randomised trials comparing ergometrine‐oxytocin use with oxytocin use in women having the third stage of labour managed actively.
Data collection and analysis
We independently assessed trial eligibility and quality and extracted data. We contacted study authors for additional information.
Main results
Six trials were included (9332 women). Compared with oxytocin, ergometrine‐oxytocin was associated with a small reduction in the risk of PPH using the definition of PPH of blood loss of at least 500 ml (odds ratio 0.82, 95% confidence interval 0.71 to 0.95). This advantage was found for both a dose of 5 iu oxytocin and a dose of 10 iu oxytocin, but was greater for the lower dose. There was no difference detected between the groups using either 5 or 10 iu for the stricter definition of PPH of blood loss at least 1000 ml. Adverse effects of vomiting, nausea and hypertension were more likely to be associated with the use of ergometrine‐oxytocin. When heterogeneity between trials was taken into account there were no statistically significant differences found for the other maternal or neonatal outcomes.
Authors' conclusions
The use of ergometrine‐oxytocin as part of the routine active management of the third stage of labour appears to be associated with a small but statistically significant reduction in the risk of PPH when compared to oxytocin for blood loss of 500 ml or more. No statistically significant difference was observed between the groups for blood loss of 1000 ml or more. A statistically significant difference was observed in the presence of maternal side‐effects, including elevation of diastolic blood pressure, vomiting and nausea, associated with ergometrine‐oxytocin use compared to oxytocin use. Thus, the advantage of a reduction in the risk of PPH, between 500 and 1000 ml blood loss, needs to be weighed against the adverse side‐effects associated with the use of ergometrine‐oxytocin.
Plain language summary
Prophylactic ergometrine‐oxytocin versus oxytocin for the third stage of labour
Ergometrine‐oxytocin (Syntometrine®) is more effective than oxytocin (Syntocinon®) in reducing blood loss during the delivery of the placenta, but has more side‐effects.
Active management of the third stage of labour, when delivery of the placenta occurs, involves the clinician giving a drug as the baby's shoulder is born, clamping the umbilical cord immediately after birth and putting traction on the cord to speed delivery. This process is widely used to reduce the risk of excessive blood loss. The review of six trials (9332 women) found ergometrine‐oxytocin appears to be associated with less blood loss than oxytocin when a 'moderate' blood loss definition is taken rather than a 'severe' blood loss definition. However, ergometrine‐oxytocin was associated with more side‐effects of vomiting, nausea and high blood pressure.
Background
The best estimates of global mortality for mothers in childbirth are reported as between 500,000 and 600,000 annually (UNICEF 1996; WHO 1990; WHO 2002). More than 99% of maternal deaths occur in low‐income countries (WHO 2002), where other factors may contribute to death in the presence of severe postpartum haemorrhage (PPH). Many more women survive and suffer serious illness as a result, not only from the effects of acute anaemia but also from the interventions that a severe haemorrhage may necessitate (e.g. general anaesthesia, manual removal of the placenta, blood transfusion).
Despite the numerous risk strategies put in place in birth settings around the world, PPH remains a major contributor to maternal death in both high‐ and low‐resource countries and there has not been a major or sustained reduction in the numbers of PPH reported in most settings over the past decade regardless of the management strategy used or the risk status of the woman (McDonald 2007). The majority of women who experience a PPH are those who have been assessed as being at low risk for complications in the third stage and who have spontaneous vaginal births (McDonald 2007).
Most maternal deaths result from complications of the third stage of labour, and, in particular, from PPH. The third stage of labour is that period of time from birth of the infant until delivery of the placenta. The most widely accepted definition of PPH is that given by the Word Health Organization: blood loss of at least 500 millilitres (ml) from the genital tract during the first 24 hours postpartum (WHO 1990; WHO 2000). More stringent definitions of 600 ml (Beischer 1986) and 1000 ml (Burchell 1980) have been suggested although the assessment of blood loss is often significantly underestimated and is based on a clinical estimation of blood loss (Kwast 1991; WHO 1998). The 500 ml limit could be viewed as a warning sign while acknowledging that blood loss up to 1000 ml in healthy women may still be considered physiological, not necessitating treatment other than uterotonic drugs (agents that stimulate the uterus to contract). In low‐income countries, where the prevalence of severe anaemia is high, a 500 ml blood loss can be life threatening for many women (WHO 1996). However, even in high‐income countries, the risk of PPH should not be underestimated for any birth (McDonald 2003a). Any blood loss that facilitates symptoms of shock (breathlessness, hypotension, tachycardia, collapse) should be treated accordingly.
Reducing the likelihood of PPH by routine active management of the third stage of labour could play an important part in reducing maternal morbidity and mortality (Elbourne 1988). Active management of the third stage of labour involves the clinician intervening in the process through three interrelated processes; the administration of a prophylactic uterotonic drug; cord clamping and cutting; and controlled traction on the umbilical cord. The aim of administering a uterotonic drug as a precautionary measure is to reduce the risk of PPH. This injection is usually given to the mother at the same time as the infant's shoulders are born. In contrast to active management, expectant management is a non‐interventionist approach, which involves withholding the administration of the uterotonic drug, waiting for signs of placental separation and allowing the placenta to deliver spontaneously or aided by gravity, maternal effort or nipple stimulation.
The review of active versus expectant management of the third stage (seePrendiville 2000) reveals convincing evidence that active management is associated with a significant reduction in the risk of PPH, both in low‐risk women and in a total population. This review included the results of several large randomised controlled trials (Begley 1990; Khan 1997; Prendiville 1988; Rogers 1998; Thilaganathan 1993) and suggested that active management of the third stage of labour as a routine preventative measure is associated with a two to threefold reduction in the risk of PPH.
The routine prophylactic administration of an uterotonic agent is an integral part of the active management of labour. There are several different types of uterotonic drugs that may be given and the relative advantages and disadvantages of these different drugs are the subject of separate reviews (seeCotter 2001 (oxytocin); Gülmezoglu 2004 (prostaglandins and misoprostol); and Prendiville 2000 (active versus expectant management). For information on the cord clamping component of active management seeMcDonald 2003b (term infants) and Rabe 2004 (preterm infants). This review focuses on comparing the two most widely used uterotonic agents: ergometrine‐oxytocin (Syntometrine®) (a combination of oxytocin 5 international units (iu) and ergometrine 0.5 mg) and oxytocin (Syntocinon®) alone. Ergometrine‐oxytocin is known to increase the risk of hypertension whereas the use of oxytocin alone, used prophylactically in the recommended doses in the third stage of labour, is free of serious side‐effects. In a meta‐analytical overview in 1988, Elbourne et al (Elbourne 1988) attempted to determine which prophylactic uterotonic was associated with the least risk of PPH. They concluded that ergometrine‐oxytocin appeared to be the most effective, but the quality of the available evidence was not satisfactory.
Only one of the previous trials suggested there may be a dose‐related effect when using oxytocin. Dumoulin 1981 commenced his study using 5 iu and changed to 10 iu half way through the trial due to the high PPH rate encountered using 5 iu. Two other trials (Mitchell 1993; Nieminen 1963) compared 5 iu oxytocin (given intramuscularly) with ergometrine‐oxytocin (given intramuscularly). Four other trials (Choy 2002; Khan 1995; McDonald 1993; Yuen 1995) compared 10 iu oxytocin with ergometrine‐oxytocin. Therefore, the analyses have been divided into overall results and sub‐analysed for dose effect. We felt this was important as one of the reasons given by clinicians for preferring ergometrine‐oxytocin is the convenience of it being a one‐shot intramuscular injection, whereas oxytocin is commonly administered as an intravenous and intramuscular 'package' (i.e. 5 iu intravenously and 5 iu intramuscularly).
Objectives
To compare the effects of routine prophylactic administration of ergometrine‐oxytocin with administration of 5 international units (iu) and 10 iu oxytocin as part of the active management of the third stage of labour in respect of risk reduction for postpartum haemorrhage and other pre‐specified maternal and neonatal outcomes.
Methods
Criteria for considering studies for this review
Types of studies
All acceptably randomised trials identified in which prophylactic ergometrine‐oxytocin was compared with oxytocin, either 5 international units (iu) or 10 iu, as part of the prophylactic active management of the third stage of labour. For further details of these studies, please see table of 'Characteristics of included studies'.
Types of participants
The women recruited to the trials included in this review were in labour expecting to have a vaginal birth. In one trial (Khan 1995) the participants had only spontaneous vaginal births.
Types of interventions
All women in the studies included in this review had the third stage of labour managed actively whereby an uterotonic drug was administered, the umbilical cord was clamped and cut, and the placenta delivered by controlled cord traction. In all of these trials the uterotonic drug was under examination and use of ergometrine‐oxytocin was being compared with use of oxytocin.
Types of outcome measures
The outcome measures chosen in this review were based on those factors which were likely to be seen as clinically relevant in terms of an outcome changing clinical practice and what factors would be most likely to advantage or disadvantage the mother's and infant's recovery from childbirth.
The primary outcome measure in this review and in each of the trials included in this review was postpartum haemorrhage (PPH) (blood loss of at least 500 ml). This is a universally accepted index of blood loss which is the most important complication of the third stage of labour. The diagnosis was made clinically in each of these studies and this assessment is subject to significant subjective error. Because of this, each of the included studies also reported additional indices of blood loss, for example the need for blood transfusion, haemoglobin, need for therapeutic uterotonic therapy, length of the third stage and need for manual removal of the placenta. More severe blood loss was also recorded.
For this review, the following maternal and neonatal outcomes were assessed:
Maternal outcomes
'Moderate' PPH (clinically estimated blood loss of at least 500 ml);
'severe' PPH (blood loss of at least 1000 ml);
manual removal of the placenta;
blood transfusion;
elevation of diastolic blood pressure;
vomiting;
nausea;
use of therapeutic uterotonics;
third stage of labour lasting more than 30 minutes;
third stage of labour lasting more than 60 minutes.
Neonatal outcomes
Apgar score equal to or less than six at five minutes;
jaundice;
not breastfed at discharge;
admission to neonatal intensive care unit.
The definitions of these outcomes are discussed in the results section.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (30 April 2007).
The Cochrane Pregnancy and Childbirth Group's Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
monthly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness search of a further 36 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the 'Search strategies for identification of studies' section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are given a code (or codes) depending on the topic. The codes are linked to review topics. The Trials Search Co‐ordinator searches the register for each review using these codes rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
Each member of the review group assessed the methodological quality of each trial independently and we entered this information only when consensus had been attained. We contacted individual investigators if we required clarification before deciding if a trial met the inclusion criteria.
We performed subgroup analysis on the basis of the dosage of oxytocin administered when given on its own (5 international units (iu) or 10 iu).
Results are presented as odds ratios for dichotomous data, with 95% confidence intervals, using a fixed‐effect model. Where significant heterogeneity was found between trials, a random‐effects model was used as well.
Results
Description of studies
See table of 'Characteristics of included studies' for more detail, such as routes of administration of drugs, types of participants, method, interventions, and outcomes assessed.
The trials included in this review were conducted in a variety of countries (Australia, Finland, Hong Kong, UK and United Arab Emirates), in maternity units that practiced active management and used prophylactic uterotonics for management of the third stage of labour.
The women recruited to the trials included in this review were those who were expected to have a vaginal birth and fitted the eligibility criteria for receiving either drug. The Khan 1995 trial excluded women who had anything other than a spontaneous vaginal birth.
Risk of bias in included studies
Insufficient information was available for one of the trials (Nieminen 1963) to judge its methodological quality. Three of the more recently conducted trials (Khan 1995; McDonald 1993; Mitchell 1993) used a method of allocation involving independently pre‐coded drug ampoules; hence minimising the potential for bias at trial entry. Yuen 1995 used an independent staff member to draw up the drug and then hand it to a member of staff attending the birth. Choy 2002 had a second midwife, who was not otherwise involved in the study, prepare and administer the drug. This type of trial is quite difficult to blind completely as the different short‐term side‐effects can be dramatically different (e.g. vomiting).
Two studies (Khan 1995; Yuen 1995) withdrew women from the study post‐randomisation and excluded them from the subsequent analyses. The numbers were small in both cases and would probably not have had a major effect on final outcomes.
Effects of interventions
Six trials were analysed in this review, with a total of 9332 women. The exact number of trials and participants varied for each outcome, with some comparisons only having one trial available and 461 women. This limits the conclusions that can be drawn for some comparisons, as will be discussed below.
Postpartum haemorrhage (PPH)
The recorded rates for PPH varied considerably between trials, ranging from 1% to 17%. This is consistent with the published literature related to visual estimation of blood loss, which was the main method for measurement of blood loss used in the trials included in this review. The high rates reported in the McDonald 1993 trial were thought by the authors to be more a reflection of careful recording of blood loss, due to heightened awareness during the trial, rather than a higher background risk. Also, in this trial, a secondary analysis based on women perceived to be at high or low risk for PPH was conducted and we found no difference between the groups (data not shown in the review).
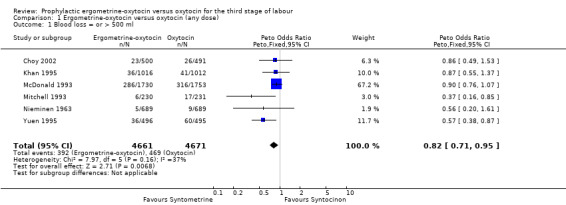
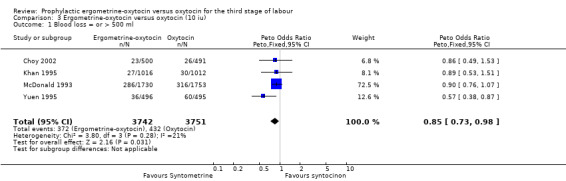
Primary outcome: PPH of at least 500 ml (any dose of oxytocin: six trials, 9332 women; 5 international units (iu) oxytocin: two trials, 1839 women; 10 iu oxytocin: four trials, 7493 women)
The primary outcome of moderate PPH was defined by the studies assessed in this review as blood loss of at least 500 ml, except for the Mitchell 1993 trial, which reported blood loss greater than 500 ml, and the Nieminen 1963 trial, for which only blood loss greater than 510 ml could be analysed. The summary odds ratio (OR) for all six trials, regardless of dosage of oxytocin, was OR 0.82 (95% confidence interval (CI) 0.71 to 0.95); where oxytocin 5 iu was used, it was OR 0.43 (95% CI 0.23 to 0.83) and where oxytocin 10 iu was used, it was OR 0.85 (95% CI 0.73 to 0.98). Thus, regardless of the dosage of oxytocin used, the use of ergometrine‐oxytocin compared to the use of oxytocin was associated with a significantly lower PPH rate when this is defined as blood loss of at least 500 ml. There was no significant heterogeneity observed regardless of dosage of oxytocin suggesting that the results can be combined.
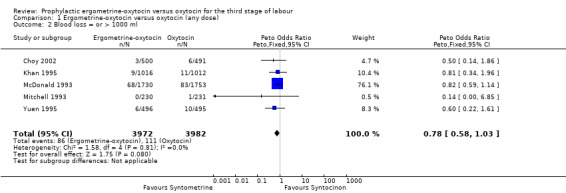
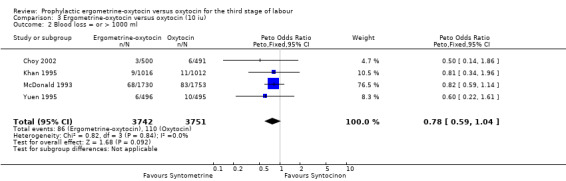
PPH of at least 1000 ml (any dose of oxytocin: five trials, 7954 women; 5 iu oxytocin: one trial, 461 women; 10 iu oxytocin: four trials, 7493 women)
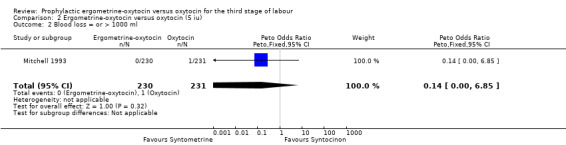
For the stricter definition of postpartum blood loss, three of the five trials defined severe PPH as at least 1000 ml, whilst the Khan 1995 and Mitchell 1993 trials defined it as blood loss more than 1000 ml. There was no statistically significant difference seen in the summary table for this severe PPH rate, regardless of dosage used (all trials: OR 0.78, 95% CI 0.58 to 1.03; 5 iu oxytocin: OR 0.14, 95% CI 0.00 to 6.85; 10 iu oxytocin: OR 0.78, 95% CI 0.59 to 1.04). Again no significant heterogeneity was observed.
Other maternal outcomes
Use of therapeutic uterotonics (any dose of oxytocin and 10 iu oxytocin: three trials, 5465 women; 5 iu oxytocin: no data available)
The use of ergometrine‐oxytocin compared with the use of oxytocin was associated with a reduced need for subsequent use of therapeutic uterotonics. The summary OR for these trials was OR 0.83 (95% CI 0.72 to 0.96). All three trials used 10 iu oxytocin. Significant heterogeneity was observed between the trials (X2 = 9.21, df = 2, P = 0.01). Re‐analysis of the data using a random‐effects model produced a non‐significant difference between the two drugs (summary OR 0.87, 95% CI 0.58 to 1.32). The observed heterogeneity is likely to be due to there being a non‐significant trend towards a greater need for therapeutic uterotonics associated with ergometrine‐oxytocin in the Choy 2002 trial (OR 1.46, 95% CI 0.94 to 2.26), whereas there were significantly reduced needs for such uterotonics in the Yuen 1995 trial (OR 0.60, 95% CI 0.40 to 0.88) and in the McDonald 1993 trial (OR 0.82, 95% CI 0.69 to 0.97).
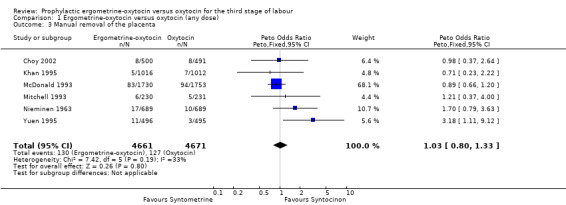
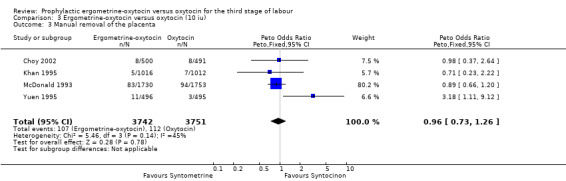
Manual removal of the placenta (any dose of oxytocin: six trials 9332 women; 5 iu oxytocin: two trials, 1839 women; 10 iu oxytocin: four trials, 7493 women)
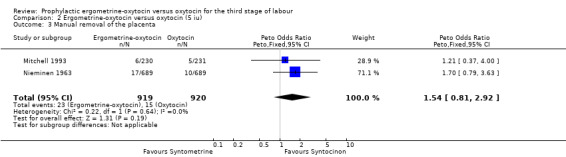
Generally, manual removal of the placenta is the surgical procedure undertaken when a placenta has been retained for longer than 30 to 60 minutes or is not able to be delivered spontaneously or by controlled cord traction. Very occasionally this may be due to snapping of the umbilical cord. For two of the six trials where data were available about manual removal of the placenta, this outcome was taken as the data for retained placenta: Khan 1995; Nieminen 1963. The summary OR shows no significant difference between groups for this outcome (any dose: OR 1.03, 95% CI 0.80 to 1.33; 5 iu oxytocin: OR 1.54, 95% CI 0.81 to 2.92; 10 iu oxytocin: OR 0.96, 95% CI 0.73 to 1.26). No significant heterogeneity was observed.
Length of the third stage (third stage greater than 30 minutes ‐ any dose of oxytocin: five trials, 7304 women; 5 iu oxytocin: two trials, 1839 women; 10 iu oxytocin: three trials, 5465 women; third stage greater than 60 minutes ‐ any dose of oxytocin: two trials, 4861 women; 5 iu oxytocin: one trial, 1378 women; 10 iu oxytocin: one trial, 3483 women)
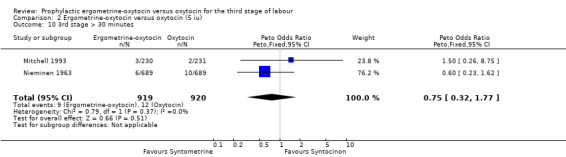
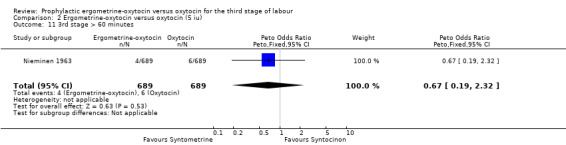
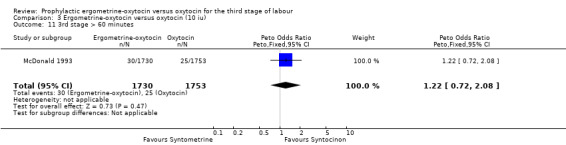
There were two durations of the third stage of labour analysed in this review: a third stage of labour greater than 30 minutes and a third stage of labour greater than 60 minutes. There was no significant difference between the ergometrine‐oxytocin and oxytocin groups for either duration of the third stage, nor for either dosage of oxytocin (third stage greater than 30 minutes ‐ any dose: OR 1.07, 95% CI 0.78 to 1.48; oxytocin 5 iu: OR 0.75, 95% CI 0.32 to 1.77; oxytocin 10 iu: OR 1.14, 95% CI 0.81 to 1.60; third stage greater than 60 minutes ‐ any dose: OR 1.11, 95% CI 0.68 to 1.81; oxytocin 5 iu: OR 0.67, 95% CI 0.19 to 2.32; oxytocin 10 iu: OR 1.22, 95% CI 0.72 to 2.08). No significant heterogeneity was observed for these outcomes.
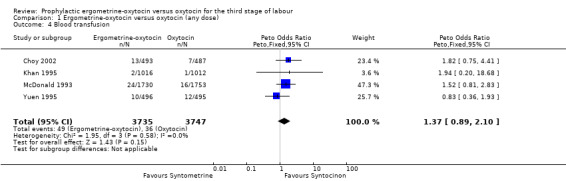
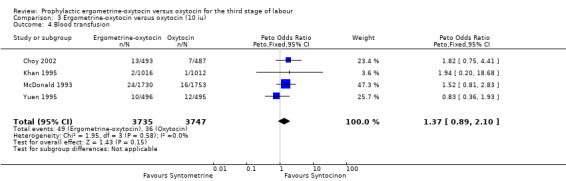
Blood transfusion (any dose of oxytocin and 10 iu oxytocin: four trials, 7482 women; 5 iu oxytocin: no data available)
No significant difference was found in the necessity for blood transfusion in the four trials that reported this outcome: Choy 2002; Khan 1995; McDonald 1993; Yuen 1995. All of these trials used 10 iu oxytocin. The summary OR was 1.37 (95% CI 0.89 to 2.10). No significant heterogeneity was observed.
Maternal side‐effects
Elevation of diastolic blood pressure (any dose of oxytocin and 10 iu oxytocin: four trials, 7486 women; 5 iu oxytocin: no data available)
From four trials, all using 10 iu oxytocin, a measure of elevation of diastolic blood pressure could be obtained. For Choy 2002, the measure of hypertension (blood pressure of at least 140/90 mmHg after birth) was used. For Khan 1995 the measure used was the number of participants who had a mean rise over three consecutive readings (15 minutes, 30 minutes and 60 minutes after injection of uterotonic at birth of the anterior shoulder of the baby) in diastolic blood pressure of 20 mmHg or over. It was diastolic blood pressure greater than 100 mmHg "in labour ward" for the McDonald 1993 trial and greater than 90 mmHg "immediately after delivery" for the Yuen 1995 trial. Use of ergometrine‐oxytocin was associated with a greater elevation in diastolic blood pressure than was use of oxytocin (OR 2.40, 95% CI 1.58 to 3.64). There was significant heterogeneity between trials (X2 = 7.96, df = 3, P = 0.047). When the data were re‐analysed using a random‐effects model, there was still a significantly higher rise in diastolic blood pressure associated with ergometrine‐oxytocin use (OR 2.81, 95% CI 1.17 to 6.73). The heterogeneity may be in part due to the Choy 2002 trial finding no significant difference between groups in terms of elevation of diastolic blood pressure, whereas in the other three trials, a greater elevation in diastolic blood pressure was associated with use of ergometrine‐oxytocin compared to oxytocin. The variations in definitions of elevation of diastolic blood pressure may have also contributed to the heterogeneity observed.
Vomiting and nausea (vomiting and nausea considered as separate outcomes ‐ any dose of oxytocin and 10 iu oxytocin: three trials, 5458 women; 5 iu oxytocin: no data available; vomiting and nausea considered as a combined outcome ‐ any dose of oxytocin and 10 iu oxytocin: four trials, 7486 women; 5 iu oxytocin: no data available)
Information about the incidence of nausea and vomiting among women was available from four trials, all using oxytocin 10 iu, with this information mostly being derived from direct complaints by participants of nausea and reports from the midwives that vomiting occurred. In the Choy 2002 trial the measure of nausea was obtained by asking participants to mark on a visual analogue scale the degree of nausea they were experiencing. The number of women indicating a score of five or more was analysed. In the Khan 1995 trial the data reported are for nausea and vomiting combined; no separate figures are given for the different side‐effects. Because of this, nausea and vomiting were assessed in this review both as separate outcomes, without the Khan 1995 trial, and as a combined outcome, with the Khan 1995 trial.
Regardless of whether vomiting and nausea were considered as separate outcomes or combined into one outcome, a greater incidence of these side‐effects was associated with ergometrine‐oxytocin use compared with oxytocin use (vomiting: OR 4.92, 95% CI 4.03 to 6.00; nausea: OR 4.07, 95% CI 3.43 to 4.84; vomiting and nausea combined: OR 5.71, 95% CI 4.97 to 6.57). However, there was significant heterogeneity observed for the combined vomiting and nausea outcome (X2 = 19.11, df = 3, P = 0.0003). When this outcome was re‐analysed with a random‐effects model, there was still significantly more nausea and vomiting observed in the same three trials, but with a smaller effect and greater confidence intervals (OR 3.40, 95% CI 1.33 to 8.67).
The heterogeneity observed is likely to be due to the inclusion of the Khan 1995 trial in the comparison of the two drugs for the combined outcome. It may be inappropriate to combine vomiting and nausea into two outcomes as was done in the Khan 1995 trial because, although related, they are different side‐effects. Another possibility for the heterogeneity is that there appeared to be a greater incidence of side‐effects associated with ergometrine‐oxytocin use in the McDonald 1993 trial compared to the three other trials.
Neonatal outcomes (Apgar score equal to or less than six at five minutes and jaundice ‐ any dose of oxytocin and 10 iu oxytocin: two trials, 5468 women; 5 iu oxytocin: no data available; not breastfed at discharge and admission to neonatal intensive care unit ‐ any dose of oxytocin and 10 iu oxytocin: one trial, 3440 women; 5 iu oxytocin: no data available)
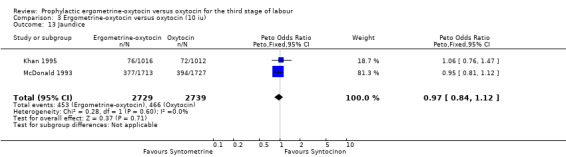
There were four neonatal outcomes analysed, but only two trials measured these neonatal outcomes (Khan 1995; McDonald 1993) both of these using oxytocin 10 iu. An Apgar score of less than or equal to six at five minutes was analysed for the Khan 1995 trial and an Apgar score of less than six at five minutes analysed for the McDonald 1993 trial. These two trials also measured jaundice; Khan 1995 defining this as bilirubin greater than 428 µmol/l and McDonald 1993 defining it as jaundice sufficiently elevated to require phototherapy. Only the McDonald 1993 trial measured the outcomes of the infant not having been breastfed at the time of discharge and admission to the neonatal intensive care unit. There were no significant differences between the ergometrine‐oxytocin and oxytocin groups for any of these neonatal outcomes (Apgar score: OR 1.00, 95% CI 0.67 to 1.50; jaundice: OR 0.97, 95% CI 0.84 to 1.12; not breastfed at discharge: OR 1.10, 95% CI 0.90 to 1.33; admission to neonatal intensive care unit: OR 1.04, 95% CI 0.88 to 1.24). No significant heterogeneity was observed for any of the neonatal outcomes.
Discussion
The results of this review show a statistically significant reduction in the risk of postpartum haemorrhage (PPH) (at least 500 ml) for women receiving ergometrine‐oxytocin when compared to oxytocin. In the studies undertaken where 5 international units (iu) of oxytocin were used, ergometrine‐oxytocin was shown to be of greater benefit in reducing the risk of PPH. In those studies where 10 iu of oxytocin was compared with ergometrine‐oxytocin, the benefit of using ergometrine‐oxytocin was reduced, although still marginally better in the group of women who experienced a blood loss of 500 ml or more.
There was no statistically significant difference seen between the two drugs when there was a more clinically significant (that is, likely to cause the clinician to initiate other interventions such as further uterotonic or surgical intervention) level of blood loss of at least 1000 ml.
There was also a statistically significant reduction in subsequent use of therapeutic uterotonics when ergometrine‐oxytocin was used compared to when oxytocin was used. However, when the heterogeneity observed between the trials was taken into account, by using a random‐effects model for the meta‐analysis, this overall advantage associated with ergometrine‐oxytocin use was no longer statistically significant.
Although there were advantages associated with ergometrine‐oxytocin use, these need to be weighed against the higher incidence of side‐effects observed when ergometrine‐oxytocin was used compared to when oxytocin was used. There were major differences recorded for unpleasant side‐effects of nausea, vomiting and elevation of blood pressure in previously normotensive women.
The summary odds ratio showed a significantly greater elevation in diastolic blood pressure associated with use of ergometrine‐oxytocin, compared with oxytocin use. This statistically significant difference was found both with a fixed‐effect model and a random‐effects model.
Ergometrine‐oxytocin use was also associated with more nausea and vomiting than oxytocin use, when the summary odds ratio was considered, and this was the case both when the side‐effects were considered separately and when they were combined.
There were no statistically significant differences found between groups for any of the other maternal outcomes analysed, nor for any of the neonatal outcomes.
The choice of the most appropriate uterotonic drug will be dependent on how much importance the individual clinician, or maternity unit, places on: (a) the lower category of blood loss (at least 500 ml); (b) the higher category of blood loss (at least 1000 ml); (c) maternal morbidity associated with side‐effects of vomiting and hypertension; (d) the setting in which maternity care is being undertaken.
This choice is important in the context of low‐income countries where the feasibility of introducing an active management policy is being explored. While ergometrine‐oxytocin may offer a slight advantage in terms of lower blood loss, the potential for harm also exists in areas where pre‐eclampsia and eclampsia are already high and availability of trained health professionals and medical facilities are in poor supply. Finally, the common side‐effect of vomiting associated with ergometrine‐oxytocin will carry weight with many clinicians.
Another factor that must not be considered in isolation from third stage management and choice of uterotonic is the management of the first and second stages of labour and what, if any, influence interventions such as induction of labour; epidural analgesia; and tolerance of longer second stage, where epidural is in situ, contribute to the risk of PPH.
Consistency across the trials was adequate for the outcomes measured though some, particularly those using 5 iu of oxytocin, studied fewer outcomes. Only two trials could be included in this review that used an oxytocin dose of 5 iu (Mitchell 1993; Nieminen 1963). The additional step in using a random‐effects model of analysis, where significant heterogeneity was observed between studies, led to an improved ability to interpret the data more accurately and to gain more precise estimates. The existence of heterogeneity between studies may suggest that there were one or more differences in the nature of the different studies.
The Yuen 1995 and Choy 2002 trials showed no significant difference in reporting of some or all of the side‐effects. Further information was sought from Yuen (Yuen 2002) since, in the trials in which he was one of the investigators (Choy 2002; Yuen 1995), the results differed greatly from the other large trials in regard to outcome for side‐effects. In almost all previous studies the side‐effects of nausea, vomiting and elevated blood pressure have been well documented as being considerably higher in women receiving ergometrine‐oxytocin. Yuen was unable to highlight any specific differences between the trials in terms of clinical management. It was, however, suggested that race may have been a factor for consideration. Participants recruited for the Choy 2002 and Yuen 1995 trials were in hospitals in Hong Kong whereas in the Khan 1995 and McDonald 1993 trials, in which a higher incidence of complaints of side‐effects was associated with ergometrine‐oxytocin use, participants were recruited in hospitals in the United Arab Emirates and Australia.
Authors' conclusions
Implications for practice.
The evidence from this review suggests a small risk reduction when ergometrine‐oxytocin is used, for blood loss exceeding 500 ml but not exceeding 1000 ml. There are significant side‐effects of vomiting and hypertension associated with the use of ergometrine‐oxytocin. The implications for practice in this situation of competing risks must depend on the different weights placed upon these outcomes.
During pregnancy the amount of blood circulating around a woman's body increases to up to 50% more than in the non‐pregnant woman. This enables the body to meet extra demands associated with an enlarging uterus and to provide extra blood flow to nourish the placenta (Murray 2003). In well‐nourished women it may be argued that once the infant is born, the loss of a fair proportion of the extra blood volume, now superfluous to circulatory requirements, would present no particular long‐term risk of increased morbidity. However, what has been shown in previous trials of third stage management, where women at low risk for complications have been the major participants (active versus expectant review Prendiville 2000), is the unpredictable nature of haemorrhage and the difficulty in reliably identifying women who are at risk of experiencing excessive blood loss.
In circumstances where the higher blood loss value of 1000 ml or more is considered a more likely point at which medical or surgical intervention in clinical management is likely to be initiated, and in view of the lack of evidence of important clinical benefits and clearly an increase in side‐effects with the use of ergometrine‐oxytocin, oxytocin would be considered as a first‐line prophylactic agent, at least in women at low risk of complications.
There also needs to be an acknowledgement of the difficulty of obtaining an accurate measurement of blood loss. It is widely accepted that estimation of blood loss provides a 'best guess' estimate at the time a judgement for further action is required. However, research evidence also suggests that clinicians are more likely to underestimate than overestimate and the reality of the practice domain is such that it is reasonable to rely on clinical expertise that is then followed up with an appropriate and objective diagnostic and risk assessment.
Implications for research.
None of the trials included in this review addressed any long‐term consequences that may be associated with PPH or women's preferences related to uterotonic choice. It would be of interest to include this aspect of care in future research on trials of uterotonic choice.
There was a dose‐related effect associated with the administration of oxytocin and risk reduction for PPH. Future studies may wish to consider exploring the effect of increasing the dose to 15 international units or 20 international units.
There has also been a lack of research relating to the third stage of labour management to what has occurred in the first and second stages of labour. All the trials included in the current review were undertaken over a decade ago. It may be timely to revisit trials of oxytocic choice in light of the different demographics of women attending for pregnancy care (e.g. older women having their first baby) and to assess possible effects of current strategies for management in labour on rates of PPH.
What's new
| Date | Event | Description |
|---|---|---|
| 5 February 2018 | Amended | Updated Published notes to clarify that this review will no longer be updated in it's current form. The review is now out‐of‐date and has been relinquished by the review authors. A new review team will prepare a new Cochrane review on this topic following a new protocol. |
History
Protocol first published: Issue 2, 1997 Review first published: Issue 2, 1997
| Date | Event | Description |
|---|---|---|
| 13 February 2009 | Amended | Contact details updated. |
| 12 February 2008 | Amended | Converted to new review format. |
| 30 April 2007 | New search has been performed | New studies sought but none found |
| 25 September 2003 | New citation required and conclusions have changed | This review is an update of the previously published 'Prophylactic syntometrine versus oxytocin for delivery of the placenta' review, which was last amended in February 1999. We have changed the title and have added three new studies to this update; one as an included study and two as excluded studies. Five new outcomes have been assessed. Every section of the original version has been modified, definitions of outcomes have been clarified and the format of the results section has been amended to provide more detail about the studies and discussion of heterogeneity. The contact author is the same but two new reviewers have worked on the review with her. The conclusions remain essentially the same. |
Notes
This review has become out of‐date and will no longer be updated by the existing review team. A new team will prepare a new Cochrane review on this topic, following a new protocol.
Acknowledgements
We wish to acknowledge Walter Prendiville and Diana Elbourne for all their work on the previous versions of this review and several other third stage of labour reviews.
Data and analyses
Comparison 1. Ergometrine‐oxytocin versus oxytocin (any dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss = or > 500 ml | 6 | 9332 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.71, 0.95] |
| 2 Blood loss = or > 1000 ml | 5 | 7954 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.58, 1.03] |
| 3 Manual removal of the placenta | 6 | 9332 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.80, 1.33] |
| 4 Blood transfusion | 4 | 7482 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.89, 2.10] |
| 5 Elevation diastolic blood pressure | 4 | 7486 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.40 [1.58, 3.64] |
| 6 Vomiting | 3 | 5458 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.92 [4.03, 6.00] |
| 7 Nausea | 3 | 5458 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.07 [3.43, 4.84] |
| 8 Vomiting + nausea combined | 4 | 7486 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.71 [4.97, 6.57] |
| 9 Therapeutic oxytocics | 3 | 5465 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.72, 0.96] |
| 10 3rd stage > 30 minutes | 5 | 7304 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.78, 1.48] |
| 11 3rd stage > 60 minutes | 2 | 4861 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.68, 1.81] |
| 12 Apgar score = or < 6 at 5 minutes | 2 | 5468 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.67, 1.50] |
| 13 Jaundice | 2 | 5468 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.84, 1.12] |
| 14 Not breastfed at discharge | 1 | 3440 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.90, 1.33] |
| 15 Admission to neonatal intensive care unit | 1 | 3440 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.88, 1.24] |
1.1. Analysis.
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 1 Blood loss = or > 500 ml.
1.2. Analysis.
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 2 Blood loss = or > 1000 ml.
1.3. Analysis.
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 3 Manual removal of the placenta.
1.4. Analysis.
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 4 Blood transfusion.
1.5. Analysis.
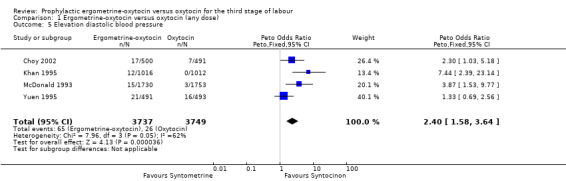
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 5 Elevation diastolic blood pressure.
1.6. Analysis.
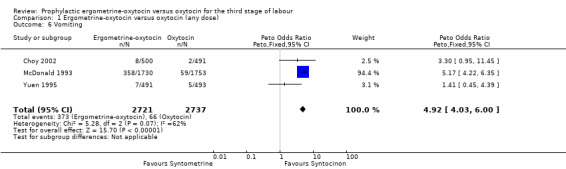
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 6 Vomiting.
1.7. Analysis.
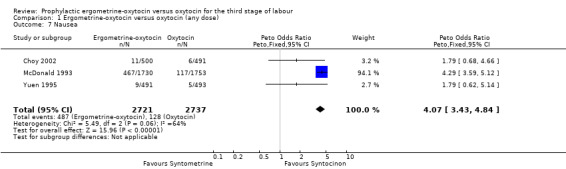
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 7 Nausea.
1.8. Analysis.
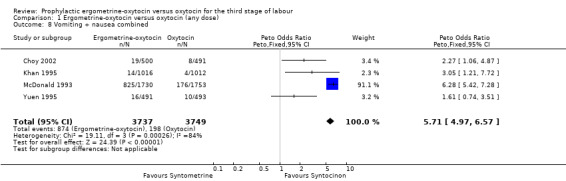
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 8 Vomiting + nausea combined.
1.9. Analysis.
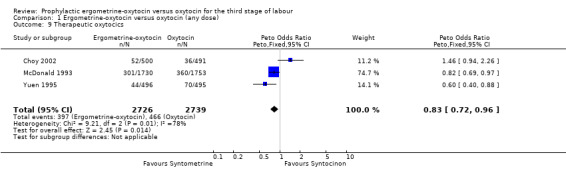
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 9 Therapeutic oxytocics.
1.10. Analysis.
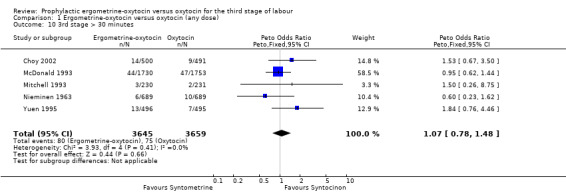
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 10 3rd stage > 30 minutes.
1.11. Analysis.
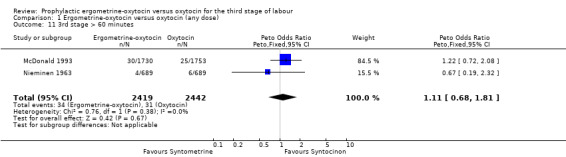
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 11 3rd stage > 60 minutes.
1.12. Analysis.
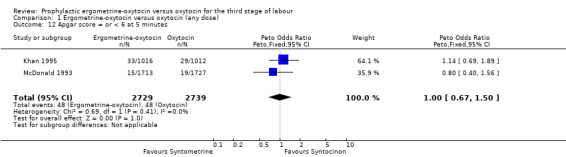
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 12 Apgar score = or < 6 at 5 minutes.
1.13. Analysis.
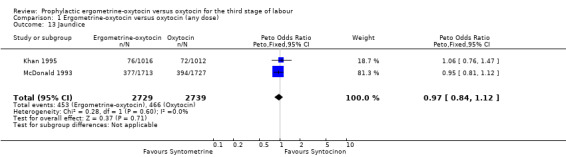
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 13 Jaundice.
1.14. Analysis.
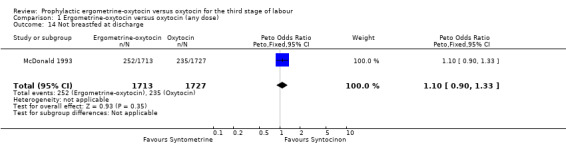
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 14 Not breastfed at discharge.
1.15. Analysis.
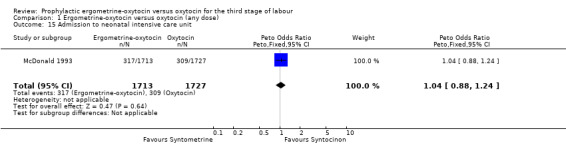
Comparison 1 Ergometrine‐oxytocin versus oxytocin (any dose), Outcome 15 Admission to neonatal intensive care unit.
Comparison 2. Ergometrine‐oxytocin versus oxytocin (5 iu).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss = or > 500 ml | 2 | 1839 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.43 [0.23, 0.83] |
| 2 Blood loss = or > 1000 ml | 1 | 461 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.85] |
| 3 Manual removal of the placenta | 2 | 1839 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.54 [0.81, 2.92] |
| 4 Blood transfusion | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Elevation of diastolic blood pressure | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Vomiting | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Nausea | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Vomiting + nausea combined | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Therapeutic oxytocics | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 3rd stage > 30 minutes | 2 | 1839 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.32, 1.77] |
| 11 3rd stage > 60 minutes | 1 | 1378 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.19, 2.32] |
| 12 Apgar score = or < 6 at 5 minutes | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Jaundice | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Not breastfed at discharge | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Admission to neonatal intensive care unit | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
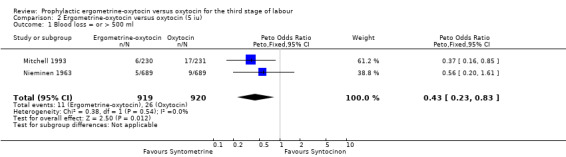
Comparison 2 Ergometrine‐oxytocin versus oxytocin (5 iu), Outcome 1 Blood loss = or > 500 ml.
2.2. Analysis.
Comparison 2 Ergometrine‐oxytocin versus oxytocin (5 iu), Outcome 2 Blood loss = or > 1000 ml.
2.3. Analysis.
Comparison 2 Ergometrine‐oxytocin versus oxytocin (5 iu), Outcome 3 Manual removal of the placenta.
2.10. Analysis.
Comparison 2 Ergometrine‐oxytocin versus oxytocin (5 iu), Outcome 10 3rd stage > 30 minutes.
2.11. Analysis.
Comparison 2 Ergometrine‐oxytocin versus oxytocin (5 iu), Outcome 11 3rd stage > 60 minutes.
Comparison 3. Ergometrine‐oxytocin versus oxytocin (10 iu).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss = or > 500 ml | 4 | 7493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.73, 0.98] |
| 2 Blood loss = or > 1000 ml | 4 | 7493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.59, 1.04] |
| 3 Manual removal of the placenta | 4 | 7493 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.73, 1.26] |
| 4 Blood transfusion | 4 | 7482 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.89, 2.10] |
| 5 Elevation of diastolic blood pressure | 4 | 7486 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.40 [1.58, 3.64] |
| 6 Vomiting | 3 | 5458 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.92 [4.03, 6.00] |
| 7 Nausea | 3 | 5458 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.07 [3.43, 4.84] |
| 8 Vomiting + nausea combined | 4 | 7486 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.71 [4.97, 6.57] |
| 9 Therapeutic oxytocics | 3 | 5465 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.72, 0.96] |
| 10 3rd stage > 30 minutes | 3 | 5465 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.81, 1.60] |
| 11 3rd stage > 60 minutes | 1 | 3483 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.72, 2.08] |
| 12 Apgar = or < 6 at 5 minutes | 2 | 5468 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.67, 1.50] |
| 13 Jaundice | 2 | 5468 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.84, 1.12] |
| 14 Not breastfed at discharge | 1 | 3440 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.90, 1.33] |
| 15 Admission to neonatal intensive care unit | 1 | 3440 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.88, 1.24] |
3.1. Analysis.
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 1 Blood loss = or > 500 ml.
3.2. Analysis.
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 2 Blood loss = or > 1000 ml.
3.3. Analysis.
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 3 Manual removal of the placenta.
3.4. Analysis.
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 4 Blood transfusion.
3.5. Analysis.
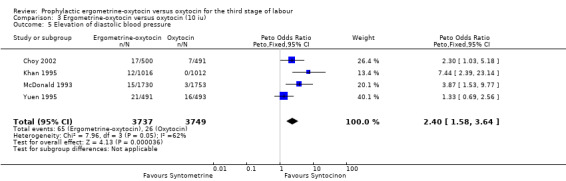
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 5 Elevation of diastolic blood pressure.
3.6. Analysis.
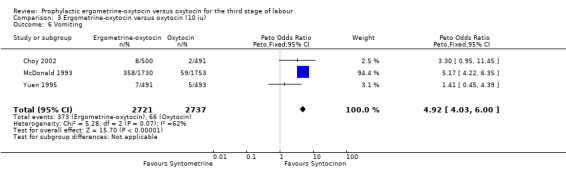
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 6 Vomiting.
3.7. Analysis.
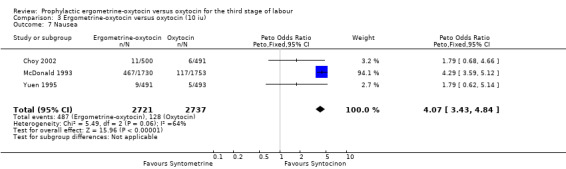
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 7 Nausea.
3.8. Analysis.
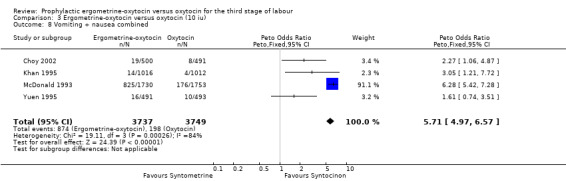
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 8 Vomiting + nausea combined.
3.9. Analysis.
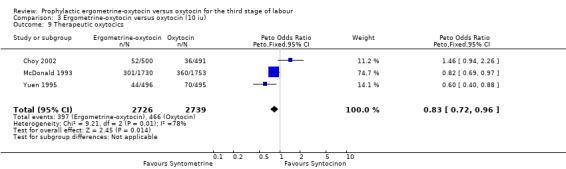
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 9 Therapeutic oxytocics.
3.10. Analysis.
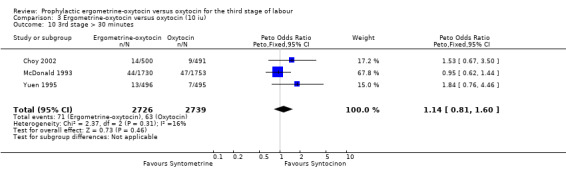
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 10 3rd stage > 30 minutes.
3.11. Analysis.
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 11 3rd stage > 60 minutes.
3.12. Analysis.
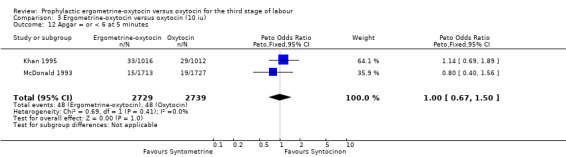
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 12 Apgar = or < 6 at 5 minutes.
3.13. Analysis.
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 13 Jaundice.
3.14. Analysis.
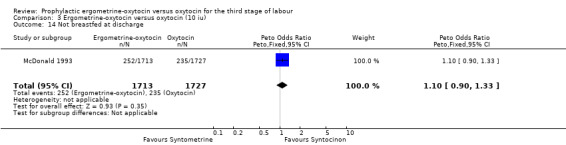
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 14 Not breastfed at discharge.
3.15. Analysis.
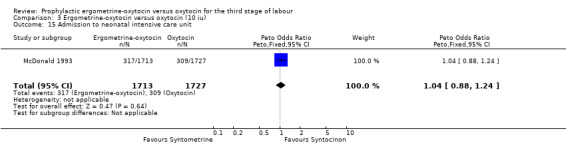
Comparison 3 Ergometrine‐oxytocin versus oxytocin (10 iu), Outcome 15 Admission to neonatal intensive care unit.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Choy 2002.
| Methods | Randomisation: double‐blind randomised controlled trial. Random allocation, when vaginal birth was imminent, from a sealed consecutively‐numbered opaque envelope, each containing a computer‐generated random number. The preparation and administration of the medication was carried out by a second midwife who was not otherwise involved in the management of the patient. The medical attendant who delivered the baby was not informed of the type of uterotonics used. Sample size calculation: sample size estimation was based on the incidence of PPH (> 500 ml) in the participating hospital (where IM ergometrine‐oxytocin is the routine uterotonic drug) being 4% and the incidence of PPH in the Soriano 1996 study (where intravenous oxytocin was used) being 9.7%. A total sample size of 980 participants was estimated as being necessary to detect such a difference with a power of 90% and a Type 1 error of 0.05. | |
| Participants | Inclusions: 991 women having a singleton pregnancy and vaginal birth at a university teaching hospital in Hong Kong. This included women who received oxytocin infusion in the first stage of labour, with this infusion stopped at the end of the second stage of labour. Exclusions: the presence of medical conditions that precluded the use of ergometrine, (such as pre‐eclampsia and cardiac disease) and conditions that require prophylactic oxytocin infusion after birth (such as grand multiparity ‐ parity = or > 4), presence of uterine fibroids. | |
| Interventions | Allocation to receiving either 1 ml of oxytocin (10 units of oxytocin) intravenously (n = 491) or 1 ml of ergometrine‐oxytocin (5 units of oxytocin and 0.5 mg ergometrine) IM (n = 500). | |
| Outcomes | Blood loss during birth, PPH = or > 500 ml, PPH = or > 1000 ml, need for repeated uterotonics, haemoglobin level before and 24 hours after birth, duration of 3rd stage, need for manual removal of placenta, side‐effects including hypertension, nausea, vomiting, headache and chest pain. | |
| Notes | The injection was given at birth of the anterior shoulder. The placenta was delivered by early clamping of the cord and controlled cord traction. An additional dose of ergometrine‐oxytocin was given if the uterus was not well contracted after birth of the placenta or if there was excessive vaginal bleeding as assessed by the attendant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Khan 1995.
| Methods | Randomisation: double‐blind randomised controlled trial. On admission to labour ward, those women eligible to enter the trial were assigned an opaque, sealed envelope that carried the allocation instructions. Immediately prior to birth, the envelope was opened by the attending midwife and the appropriate numbered vial (with pharmacy coded contents) was kept ready for injection in the third stage of labour. Sample size calculation: no sample size calculation was included. | |
| Participants | Inclusions: 2040 women meeting the strict inclusion criteria and who were expected to give birth vaginally were recruited (from a hospital in the United Arab Emirates. Exclusions: operative delivery (forceps, ventouse, caesarean section), antenatal BP 160/100 mmHg, need for antihypertensive drugs in pregnancy, GA, epidural or diazepam during labour, multiple pregnancy, antenatal anaemia 9 g/dl or less and cardiac disease. | |
| Interventions | Administration of either IM ergometrine‐oxytocin 1 ml (n = 1023) or IM oxytocin 10 iu (n = 1017) with the birth of the anterior shoulder of the baby. | |
| Outcomes | PPH: blood loss equal to or greater than 500 ml, retained placenta, nausea, vomiting, elevation of BP, headache. | |
| Notes | 12 women were removed from the trial after randomisation. Further information sought from the authors indicates that the analysis was based on treatment received rather than intention to treat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
McDonald 1993.
| Methods | Randomisation: double‐blind randomised controlled trial. The trial ampoules were coded by Sandoz, using simple randomisation, with no blocking or prognostic stratification. When the attending midwife deemed a vaginal birth to be imminent, the next available numbered trial ampoule was administered. Sample size calculation: it was estimated that if ergometrine‐oxytocin reduced the risk of PPH to 5% from a 'best guess estimate' of 7.5% for oxytocin alone, this would be sufficient to influence the choice of oxytocic in clinical practice. A sample size of at least 3100 women would be required to have an 80% chance of detecting such a difference at the 5% level of statistical significance. | |
| Participants | Inclusions: 3497 women (from 2 metropolitan teaching hospitals in Australia), in whom a vaginal birth was anticipated during the trial period. Exclusions: planned caesarean section, general anaesthetic given for operative delivery other than caesarean section, antepartum hypertension; maternal refusal; maternal distress; advanced stage in labour; language barrier; fetal abnormality or death in utero; and medical disease. | |
| Interventions | IM ergometrine‐oxytocin 1 ml (n = 1730) or IM oxytocin 10 iu (n = 1753) at time of birth of the anterior shoulder of the baby. | |
| Outcomes | PPH equal to or greater than 500 ml and 1000 ml, manual removal of the placenta, blood transfusion, nausea, vomiting, elevation of blood pressure. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Mitchell 1993.
| Methods | Randomisation: a double‐blind randomised controlled trial. Sandoz Products prepared the trial ampoules, giving them unique numbers and the code was not broken until after completion of the study. Simple randomisation was used, with no blocking or stratification. During the second stage of labour, when it was clear a birth would be vaginal, women who had consented to participate were allocated the next available ampoule. Sample size calculation: No prior power calculations were carried out. The study was aimed at recruiting as many women as possible during a 6‐month period during 1984. | |
| Participants | Inclusions: 461 women giving birth at the Hope Hospital in Salford, UK over the study period were recruited. Exclusions: women for whom a caesarean section was planned, or who had significant hypertension or cardiac disease. | |
| Interventions | Random allocation to either IM ergometrine‐oxytocin 1 ml (n = 230) or IM oxytocin 5 iu (n = 231) at the time of birth of the anterior shoulder of the baby. | |
| Outcomes | Postpartum blood loss, the length of the third stage of labour and manual removal of placenta. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Nieminen 1963.
| Methods | Randomisation: women were divided into groups, each group containing 285 primiparous and 404 multiparous women. No further description regarding randomisation process was given. Sample size calculation: no information. | |
| Participants | Inclusions: 1378 (689 in each group) women confined at the 2 clinics of Obstetrics and Gynaecology, Helsinki University Hospital. Exclusions: no information. | |
| Interventions | The study had 3 groups with different drugs administered: oxytocin, Methergin (a product similar to ergometrine) and OCM 505 ® (a product similar to ergometrine‐oxytocin, containing 0.5 mg Methergin and 5 iu oxytocin). For the purpose of this review, the data for the oxytocin group (administered 10 iu of oxytocin IM) and OCM 505 ® (administration of 1 ml OCM 505 ® IM) groups were analysed, with the OCM 505 ® group labelled ergometrine‐oxytocin in the data tables for ease of comparison and because it is an equivalent product. | |
| Outcomes | Duration of the third stage, PPH during the third stage and complete or partial retention of the placenta. | |
| Notes | The drugs were given as soon as the anterior shoulder was delivered or immediately afterwards. "Active treatment of expressing the placenta as soon as possible during the first contraction of the third stage." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Yuen 1995.
| Methods | Randomisation: a randomised double blind prospective study. Random allocation by computer‐generated random numbers contained in a consecutively‐labelled sealed envelope. When a women entered the study, a nursing officer not involved in the management of the management of the woman drew up the indicated medication and handed this to the woman's attendants. Sample size calculation: sample size estimation was based on preliminary study which found PPH incidence to be 6% in those given ergometrine‐oxytocin and 12% in those given oxytocin. 1000 women randomised to the study would be sufficient to confirm this observation with a power of 90% at the 5% level of significance. | |
| Participants | Inclusions: 991 women having a singleton pregnancy and vaginal birth at a university teaching hospital in Hong Kong. This included women who received oxytocin infusion in the first stage of labour, with this infusion stopped at the end of the second stage of labour. Exclusions: the presence of medical conditions that precluded the use of ergometrine, (such as pre‐eclampsia and cardiac disease) and conditions that require prophylactic oxytocin infusion after birth (such as grand multiparity ‐ parity = or > 4), presence of uterine fibroids. | |
| Interventions | Administration of IM ergometrine‐oxytocin 1 ml (n = 496) or IM oxytocin 10 iu (n = 495) at time of birth of the anterior shoulder of the baby. | |
| Outcomes | Blood loss equal to or greater than 500 ml and 1000 ml, delayed haemorrhage, maternal blood pressure retained placenta, manual removal of the placenta, nausea, vomiting, headache antenatal and postpartum haemoglobin. | |
| Notes | The study was not analysed on an 'intention‐to‐treat' basis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
BP: blood pressure GA: gestational age IM: intramuscular IU: international units PPH: postpartum haemorrhage
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abu‐Omar 2001 | Not random allocation: the 4 treatment protocols were used based on a temporal manner, with each given exclusively over a 3‐month period. |
| Docherty 1981 | Data not presented in a form that was suitable for incorporation in this review. We contacted the authors for additional information. |
| Dumoulin 1981 | This trial was originally included, but has been excluded after advice from the statistician who was part of the editorial team for this review. This study has questionable large discrepancies in sample sizes for the groups of women administered ergometrine‐oxytocin or 5 iu or 10 iu of oxytocin. It is not possible at this stage to determine the correct sample sizes for each group. This review may be included in a future revision if the correct sample sizes can be determined. |
| Soriano 1996 | Not random allocation: the 4 treatment protocols were assigned based on a temporal manner, with each given exclusively over a 10‐week period. |
| Symes 1984 | This study reported a single outcome only (serum prolactin). |
| Vaughan 1974 | Reported for a single outcome only (central venous pressure). |
Contributions of authors
Jo Abbott drafted the revised review with clinical input and assistance from Susan McDonald. Shane Higgins read and commented on the review. The original review (last updated February 1999) was written by Susan McDonald, Walter Prendiville and Diana Elbourne.
Declarations of interest
One of the review authors (SJ McDonald) is an author on one of the included studies within the review. A second review author (JM Abbott) assessed this study for inclusion and extracted the data.
Edited (no change to conclusions)
References
References to studies included in this review
Choy 2002 {published data only}
- Choy CMY, Lau WC, Tam WH, Yuen PM. A randomised controlled trial of intramuscular syntometrine and intravenous oxytocin in the management of the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2002;109:173‐7. [DOI] [PubMed] [Google Scholar]
Khan 1995 {published data only}
- Khan G, Susheela JI, Chan T, Wani S, Hughes A, Stirrat G. Abu Dhabi third stage trial: oxytocin versus syntometrine in the active management of the third stage of labour. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:212. [DOI] [PubMed]
- Khan GQ, John IS, Chan T, Wani S, Hughes AO, Stirrat GM. Abu Dhabi third stage trial: oxytocin versus Syntometrine in the active management of the third stage of labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 1995;58:147‐51. [DOI] [PubMed] [Google Scholar]
McDonald 1993 {published data only}
- McDonald S. The Perth third stage oxytocic trial. Proceedings of National Conference on Research in Midwifery; 1992; Reading, UK. 1992.
- McDonald S, Prendiville WJ. A randomized controlled trial of Syntocinon vs Syntometrine as part of the active management of the third stage of labour. Journal of Perinatal Medicine 1992;20(1):97. [Google Scholar]
- McDonald S, Prendiville WJ, Blair E. A randomised controlled trial of Syntocinon vs Syntometrine as part of the active management of the third stage of labour. Proceedings of 26th British Congress of Obstetrics and Gynaecology; 1992 July 7‐10; Manchester, UK. 1992.
- McDonald SJ. Management in the third stage of labour [dissertation]. Western Australia: University of Western Australia, 1996. [Google Scholar]
- McDonald SJ, Prendiville W, Blair E. Randomised controlled trial of oxytocin alone versus oxytocin and ergometrine in active management of the third stage of labour. BMJ 1993;307:1167‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 1993 {published data only}
- Mitchell GG, Elbourne DR. The Salford third stage trial: oxytocin plus ergometrine versus oxytocin alone in the active management of the third stage of labour. Online Journal of Current Clinical Trials 1993;2:Doc 83. [PubMed] [Google Scholar]
Nieminen 1963 {published data only}
- Nieminen U, Jarvinen PA. A comparative study of different medical treatments of the third stage of labour. Annales Chirurgiae et Gynaecologiae Fenniae 1963;53:424‐9. [PubMed] [Google Scholar]
Yuen 1995 {published data only}
- Yuen PM. Personal communication August 15, 2002.
- Yuen PM, Chan NST, Yim SF, Chang AMZ. A randomised double blind comparison of syntometrine and syntocinon in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1995;102:377‐80. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abu‐Omar 2001 {published data only}
- Abu‐Omar AA. Prevention of postpartum hemorrhage, safety and efficacy. Saudi Medical Journal 2001;22(12):1118‐21. [PubMed] [Google Scholar]
Docherty 1981 {published data only}
- Docherty PW, Hooper M. Choice of an oxytocic agent for routine use at delivery. Journal of Obstetrics and Gynaecology 1981;2:60. [Google Scholar]
Dumoulin 1981 {published data only}
- Dumoulin JG. A reappraisal of the use of ergometrine. Journal of Obstetrics and Gynaecology 1981;1:178‐81. [Google Scholar]
Soriano 1996 {published data only}
- Soriano D, Dulitzki M, Schiff E, Barkai G, Mashiach S, Seidman, DS. A prospective cohort study of oxytocin plus ergometrine with oxytocin alone for prevention of postpartum haemorrhage. British Journal of Obstetrics and Gynaecology 1996;103:1068‐73. [DOI] [PubMed] [Google Scholar]
Symes 1984 {published data only}
- Symes JB. A study on the effect of ergometrine on serum prolactin levels following delivery. Journal of Obstetrics and Gynaecology 1984;5:36‐8. [Google Scholar]
Vaughan 1974 {published data only}
- Vaughan Williams C, Johnson A, Ledward R. A comparison of central venous pressure changes in the third stage of labour following oxytocic drugs and diazepam. Journal of Obstetrics and Gynaecology of the British Commonwealth 1974;81:596‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Begley 1990
- Begley CM. Comparison of "active" and "physiological" management of the third stage of labour. Midwifery 1990;6:3‐17. [DOI] [PubMed] [Google Scholar]
Beischer 1986
- Beischer NA, Mackay EV. Obstetrics and the newborn. Eastbourne: Bailliere Tindall, 1986. [Google Scholar]
Burchell 1980
- Burchell RC. Postpartum haemorrhage. In: Quilligan ES editor(s). Current therapy in obstetrics and gynecology. Philadelphia: WB Saunders, 1980. [Google Scholar]
Cotter 2001
- Cotter A, Ness A, Tolosa J. Prophylactic oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [Art. No.: CD001808. DOI: 10.1002/14651858.CD001808] [DOI] [PubMed] [Google Scholar]
Elbourne 1988
- Elbourne D, Prendiville W, Chalmers I. Choice of oxytocic preparation for the routine use in the management of the third stage of labour: an overview of evidence from controlled trials. British Journal of Obstetrics and Gynaecology 1988;295:17‐30. [DOI] [PubMed] [Google Scholar]
Gülmezoglu 2004
- Gülmezoglu AM, Forna F, Villar J, Hofmeyr GJ. Prostaglandins for prevention of postpartum haemorrhage. Cochrane Database of Systematic Reviews 2004, Issue 1. [Art. No.: CD000494. DOI: 10.1002/14651858.CD000494.pub2] [DOI] [PubMed] [Google Scholar]
Khan 1997
- Khan GQ, John IS, Wani S, Doherty T, Sibari BM. Controlled cord traction versus minimal intervention techniques in delivery of the placenta: a randomized controlled trial. American Journal of Obstetrics and Gynecology 1997;177:770‐4. [DOI] [PubMed] [Google Scholar]
Kwast 1991
- Kwast BE. Postpartum haemorrhage: its contribution to maternal mortality. Midwifery 1991;7:64‐70. [DOI] [PubMed] [Google Scholar]
McDonald 2003a
- McDonald S. Physiology and management of the third stage of labour. In: Fraser D, Cooper M editor(s). Myles textbook for midwives. 14th Edition. Edinburgh: Churchill Livingstone, 2003. [Google Scholar]
McDonald 2003b
- McDonald SJ, Abbott JM. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database of Systematic Reviews 2003, Issue 1. [Art. No.: CD004074. DOI: 10.1002/14651858.CD004074] [DOI] [PubMed] [Google Scholar]
McDonald 2007
- McDonald S. Management of the third stage of labour. Journal of Midwifery & Women's Health 2007;52:254‐61. [DOI] [PubMed] [Google Scholar]
Murray 2003
- Murray I. Physiology and management of the third stage of labour. In: Fraser D, Cooper M editor(s). Myles textbook for midwives. 14th Edition. Edinburgh: Churchill Livingstone, 2003. [Google Scholar]
Prendiville 1988
- Prendiville W, Harding JE, Elbourne DR, Stirrat GM. The Bristol Third Stage Trial: "active" vs "physiological" management of the third stage of labour. BMJ 1988;297:1295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prendiville 2000
- Prendiville WJ, Elbourne D, McDonald S. Active versus expectant management in the third stage of labour. Cochrane Database of Systematic Reviews 2000, Issue 3. [Art. No.: CD000007. DOI: 10.1002/14651858.CD000007] [DOI] [PubMed] [Google Scholar]
Rabe 2004
- Rabe H, Reynolds G, Diaz‐Rossello J. Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database of Systematic Reviews 2004, Issue 4. [Art. No.: CD003248. DOI: 10.1002/14651858.CD003248.pub2] [DOI] [PubMed] [Google Scholar]
Rogers 1998
- Rogers J, Wood J, McCandlish R, Ayers S, Truesdale A, Elbourne D. Active versus expectant management of third stage of labour: the Hinchingbrook randomised controlled trial. Lancet 1998;351:593‐9. [DOI] [PubMed] [Google Scholar]
Thilaganathan 1993
- Thilaganathan B, Cutner A, Latimer J, Beard R. Management of the third stage of labour in women at low risk of postpartum haemorrhage. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;48:19‐22. [DOI] [PubMed] [Google Scholar]
UNICEF 1996
- Adamson P. A failure of imagination. Progress of Nations. UNICEF, 1996:2‐9. [Google Scholar]
WHO 1990
- World Health Organization. The prevention and management of postpartum haemorrhage. Report of Technical Working Group. Geneva: World Health Organisation, 1990:Report no.: WHO/MCH/90.7. [Google Scholar]
WHO 1996
- World Health Organization. Care in normal birth. Geneva: WHO, 1996. [Google Scholar]
WHO 1998
- World Health Organization. Postpartum care of the mother and newborn: a practical guide. Geneva: WHO, 1998. [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. Managing complications in pregnancy and childbirth: a guide for midwives and doctors. Geneva: WHO, 2000. [Google Scholar]
WHO 2002
- World Health Organization. Making pregnancy safer (MPR). http://www.who.int/reproductive‐health/mps/index.htm (accessed 24 October 2002).
Yuen 2002
- Yuen PM. Personal communication August 15, 2002.
References to other published versions of this review
CDSR 2003
- McDonald S, Prendiville WJ, Elbourne D. Prophylactic syntometrine versus oxytocin for delivery of the placenta. Cochrane Database of Systematic Reviews 2003, Issue 4. [Art. No.: CD000201. DOI: 10.1002/14651858.CD000201] [DOI] [PubMed] [Google Scholar]


