Abstract
The study examined the effects of in-utero exposure to maternal depression and Superstorm Sandy, a hurricane that hit metropolitan New York in 2012, on infant temperament at 6 months. Temperament was assessed using the Infant Behavior Questionnaire-Revised. Maternal depression was measured by the Edinburgh Postnatal Depression Scale. The main effects and the interaction of maternal depression and Sandy exposure on infant temperament were examined using Multivariable General Linear Model. Results show that prenatal maternal depression was associated with lower emotion-regulation and greater distress. Stratification and interaction analyses suggested that the adverse effects of prenatal maternal depression on problematic temperament were amplified by in-utero Sandy exposure. The study underscores the importance of providing prenatal screening and treatment for maternal depression during pregnancy, while simultaneously identifying high-risk families who may have suffered from disaster-related traumas in order to provide necessary services. As the frequency of natural disasters may increase owing to climate change, it is important to understand the consequences of in-utero stress on child development and to formulate plans for early identification.
Keywords: Superstorm Sandy, depression during pregnancy, traumatic stress, infant temperament, prospective study
A growing body of research shows that prenatal maternal stress and depression can have a dramatic impact on the developmental trajectory of offspring and potentially through adulthood and into future generations (Wadhwa, Sandman, & Garite, 2001). Maternal stress triggers a strong physiologic response that causes the release of cortisol and other stress-related neurotransmitters. The release of these neurotransmitters can have a profound and often harmful impact on the growing fetus, and has been linked to subsequent stress reactivity and psychopathology (Sandman, Wadhwa, Chicz-DeMet, Porto, & Garite, 1999).
One of the functions of the placenta is to filter and protect the fetus from exposure to stress-related programming effects attributable to these neurotransmitters. It regulates such exposure through proteins such as 11β-Hydroxysteroid dehydrogenase 2 (HSD11B2), which inactivates cortisol, and monoamine oxidase A (MAOA), which breaks down dopamine, norepinephrine, and serotonin. Mothers with mood disorders express lower levels of 11B-HSD2 in the placenta, with the concomitant risk of fetal exposure to a greater level of cortisol (Conradt, et al 2013). The placenta’s serotonin signaling pathway (5-HT) also plays a critical role in healthy fetal neural/brain development, and modulates several domains including cognition, attention, emotion, learning, and stress reactivity in the brain (Bonnin et al, 2011; Oberlander, 2012) and hypothalamus-pituitary-adrenal (HPA) system development (Brummelte, et al.2017). The placenta’s 5-HT is regulated by its level of MAOA; recent research has shown that prenatal maternal depression is linked to lower MAOA expression in the placenta, and hence lower 5-HT (Blakeley et al., 2013).
In an examination of the role of prenatal stress on offspring’s well-being, Glover (2011) suggested that variations in the responsiveness of HPA-axis plays an adaptive role in maximizing the organism’s survival. If prenatal maternal stress is censored, in-utero programming readies the fetus for survival in a similarly stressful postnatal environment. It is conceivable that the 5-HT and HPA systems may synergistically or independently affect offspring development. A mismatch between prenatal maternal stress, detected in-utero, and an anticipated postnatal environment would inadvertently lead to suboptimal consequences. Hence, prenatal maternal depression, plus the stress from a natural disaster, could result in greater negative consequences for the offspring.
Several examinations show the adverse influence of prenatal maternal depression on suboptimal neurodevelopment in childhood. It is linked to greater 4-month-old infant negative reactivity (Davis et al., 2004), more difficult temperament at 6-months (Austin et al., 2005), greater negative affectivity at 4- and 6-months (Blair et al., 2011; Zhang et al., 2017), and high cry reactivity at 2-years-old (Werner et al., 2007). Furthermore, elevated temperamental domains of activity and emotional reactivity have been linked to depression, anxiety, attention deficit hyperactivity disorder (ADHD), and conduct disorder later in childhood (Musser et al., 2013; Pagliaccio et al., 2014; de Pauw & Mervielde, 2010). These studies provide substantial evidence that evaluating temperament dimensions in early childhood may be beneficial to identify high-risk children for suboptimal neurobehavioral development and developmental psychopathology.
Capitalizing on the strength of the quasi-experiment with the in-utero Superstorm Sandy (i.e., Sandy), our primary aim is to examine what dimensions of infant temperament were associated with prenatal maternal depression and Sandy exposure. Our exploratory aim is to evaluate whether the magnitude of effects of prenatal maternal depression on temperament differed by Sandy exposure. We hypothesized that offspring exposed to maternal depression in-utero would have fearful attributes, a less happy disposition, and lower emotional regulation overall. We further explore whether there would be a differential impact of prenatal maternal depression on infant outcomes as a function of Sandy exposure, giving an overall magnified detrimental effect of prenatal maternal depression on infant fearfulness, emotional regulation, and sadness.
METHOD
Participants
The study utilized data from 408 mother-child dyads as a part of a longitudinal study of mothers exposed and unexposed to Sandy during pregnancy. Mothers were recruited from prenatal obstetric and gynecological clinics at Icahn School of Medicine at Mount Sinai (ISMMS) and New York Presbyterian/Queens (NYP/Q). The clinics draw patients from East Harlem and the South Bronx, New York City, and Flushing and Long Island, Queens, where most residents are low-income ethnic minorities. Criteria for exclusion included HIV infection, maternal psychosis, maternal age <15 years, and fetal life-threatening medical complications. Demographic information was obtained through self-administered questionnaires during the second trimester. Participants received reimbursement for participation during and 6-month post-pregnancy. A detailed description of the study can be found elsewhere (Finik & Nomura, 2017).
Of the 408 mothers, 310 (76%) provided information on their offspring at 6-month post-pregnancy. Between those provided information (n=310) and those who did not (n=98), there was no difference in major demographic variables, including marital status (p=.10), maternal education (p=.16), race (p=.12), maternal age (p=.49), paternal age (p=.43), parity (p=.36), prenatal smoking (p=.61), and child’s gender (p=.11).
The study was approved by the Institutional Review Boards at ISMMS, NYP/Q, and Queens College (CUNY).
Measures
Prenatal Maternal Depression:
Depression symptomatology - during antenatal and postpartum periods - was measured by the Edinburgh Postnatal Depression Scale (Murray & Carothers, 1990), a well-utilized self-report inventory that measures prenatal and postnatal depression. Mothers were asked how they had been feeling in the past 7 days, using a four-point Likert scale. Some questions were reverse coded and the sum score constituted the “maternal depression” scale. When the binary depression scale was used in the final interaction analysis, a score of 12 was used as a clinical cut-off point. The inventory is well-validated in different languages and has Cronbach’s α ranging from .79 to .86 with sensitivity (79%) and specificity (85%) (Kheirabadi et al., 2012). α in the current study was .84 for prenatal depression and .86 for post-partum depression.
Sandy exposure (during pregnancy) status:
Mothers pregnant during Sandy were given a value of 1. Mothers with offspring born prior to Sandy were given a value of 0.
Infant Temperament:
It was assessed at 6-months using the 91-item short-form of the Infant Behavior Questionnaire-Revised (IBQ-R) (Gartstein & Rothbart, 2003). The IBQ-R is a widely used, reliable, and validated measure of temperament (Gartstein & Rothbart, 2003). Mothers reported the relative frequency of specified infant reactions in the past week, using a seven-point Likert scale.
The items cover 14 sub-domains (activity, distress to limitations, fear, duration of orienting, smiling and laughter, high-pleasure seeking, low-pleasure seeking, soothability, falling reactivity, cuddliness, perceptual sensitivity, sadness, approach, and vocal reactivity). Cronbach’s α, for 14 sub-domains were at acceptable levels ranging from 0.70 to 0.87: activity level (α=.80), distress to limitations (α=.74), fear (α=.83), duration of orienting (α=.86), smiling and laughter (α=.84), high pleasure seeking (α=.87), low pleasure seeking (α=.75), soothability (α=.76), and falling reactivity (α=.73), cuddliness (α=.70), perceptual sensitivity (α=.86), sadness (α=.75), approach (α=.73), and vocal reactivity (α=.81).
Potential confounders:
Maternal education, marital status, race, parity, smoking, and child’s sex were prioritized. The information was obtained at the initial face-to-face evaluation with a social worker at the clinic. Maternal age at birth was calculated from mother’s and offspring’s date of birth. Additionally, severity of traumatic exposure due to Sandy, measured by Traumatic Exposure Instrument (Norris et al., 1990) and maternal 6-month postpartum depression were controlled for in the final model.
Statistical analysis
First, descriptive statistics were conducted to evaluate the correlation, mean, and standard deviation (SD) among the 14 sub-scales in the IBQ-R. As these sub-scales are inherently correlated to a certain degree, we used a multivariable analysis, the General Linear Model (GLM), which adjusts for correlations among dependent variables. Prior to the analysis, each sub-scale was evaluated for normality. If the assumption of univariate normal distribution was violated, log-transformation was applied to achieve normality. In the first, unadjusted GLM, we tested for the main effects of prenatal maternal depression and Sandy exposure without confounders and interactions (Model 1), followed by the same model with confounders, including sex of the child, maternal age, parity, education, marital status, race, and prenatal smoking (Model 2). Last, maternal postpartum depression and severity of trauma exposure due to Sandy were added (Model 3). To examine any moderation effect of in-utero Sandy exposure on the associations between prenatal maternal depression on the 14 offspring temperament dimensions, Model 3 was stratified by status of Sandy exposure. After this, the effect of prenatal maternal depression on the 14 temperamental dimensions was examined in each stratum. This was followed by a formal test of interactions between prenatal maternal depression and Sandy exposure on infant temperament. An improved Bonferroni procedure for multiple tests of significance (Simes, 1986) was applied when related dependent variables were analyzed together.
RESULTS
Demographic characteristics of participants
Table 1 shows the demographic characteristics. The mean age of mothers and fathers was 27.25 and 30.82 respectively. Mothers had on average three prior pregnancies. Most participants were from minority groups including Hispanic (53.2%), Black (24.5%), Asian (8.1%) and mixed-race (4.2%). Most mothers were single (56.5%), and 41.6% were either married or in a common-law relationship. Educational attainment was diverse; some had only primary education, some had graduate or professional degrees. A little over 64% were not pregnant during Sandy (i.e., pregnant prior to Sandy). Approximately 47% of children were girls and 53% boys.
Table 1:
Demographic characteristics of the sample population
| Total sample (N=310) | |
|---|---|
| Mean (SD) | |
| Parental characteristics | |
| Parents’ age | |
| Mother’s age | 27.25 (5.97) |
| Father’s age | 30.82 (10.68) |
| Parity | 3.30 (2.25) |
| Race | N (%) |
| White | 31 (10.0) |
| Black | 76 (24.5) |
| Hispanic | 165 (53.2) |
| Asian | 25 (8.1) |
| Others | 13 (4.2) |
| Marital status | N (%) |
| Married | 108 (34.8) |
| Common Law | 21 (6.8) |
| Single | 175 (56.5) |
| Widowed | 1 (0.3) |
| Divorced/separated | 5 (1.6) |
| Education level | N (%) |
| Primary School | 7 (2.3) |
| Some high school | 56 (18.1) |
| High school of GED | 65 (21.0) |
| Some college | 79 (25.5) |
| Associate degree (AA)a | 39 (12.6) |
| Bachelors degree (BA/BS)b | 38 (12.3) |
| Graduate/professional | 26 (8.4) |
| Superstorm Sandy exposure timing | N (%) |
| Pre-Sandy | 200 (64.5) |
| 3rd trimester | 15 (4.8) |
| 2nd trimester | 20 (6.5) |
| 1st trimester | 75 (24.2) |
| Child Gender | N (%) |
| Girls | 147 (47.4) |
| Boys | 163 (52.6) |
Associate degree is a 2-year undergraduate college education
Bachelors degree is a 4-year undergraduate college education.
BA denotes bachelor of arts and BS denotes bachelor of sciences.
Psychometrics of the dimensions of temperament
Table 2 shows the correlations among the 14 temperament sub-scales with means and SDs. Each has a theoretical range of 7.
Table 2.
Correlation table of the 14 temperament scales
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Mean (SD) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Activity level | 1.000 | 4.13 (1.13) | |||||||||||||
| 2. Distress | .323** | 1.000 | 3.82 (1.24) | ||||||||||||
| 3. Fearfulness | .178** | .364** | 1.000 | 3.23 (1.42) | |||||||||||
| 4. Attention duration | .232** | .045 | .144* | 1.000 | 4.70 (1.40) | ||||||||||
| 5. Smiling and laughter | .249** | .016 | .020 | .465** | 1.000 | 5.45 (1.25) | |||||||||
| 6. High pleasure seeking | .213** | .123* | .015 | .382** | .608** | 1.000 | 6.16 (1.04) | ||||||||
| 7. Low pleasure seeking | .067 | −.118* | .037 | .440** | .453** | .440** | 1.000 | 5.69 (0.93) | |||||||
| 8. Soothability | −.031 | −.211** | −.201** | .079 | .243** | .229** | .229** | 1.000 | 5.40 (0.87) | ||||||
| 9. Falling reactivity | −.089 | −.365** | −.136* | .108 | .244** | .301** | .310 | .414** | 1.000 | 5.06 (1.07) | |||||
| 10. Cuddliness | −.339** | −.340** | −.316** | −.033 | .033 | .047 | .192** | .456** | .252** | 1.000 | 5.74 (0.92) | ||||
| 11. Perceptual sensitivity | .255** | .125* | .174** | .369** | .357** | .410** | .415** | .143* | .150* | −.076 | 1.000 | 4.54 (1.60) | |||
| 12. Sadness | .223** | .612** | .390** | .111 | −.078 | −.041 | .035 | −.270** | −.352** | −.422** | .138* | 1.000 | 3.44 (1.20) | ||
| 13. Approach | .313** | .236** | .258** | .487** | .497** | .544** | .390** | .147* | .203** | −.130* | .491** | .184** | 1.000 | 5.42 (1.17) | |
| 14. Vocal reactivity | .317** | .121* | .043 | .377** | .714** | .593** | .388** | .222** | .231** | .031 | .433** | .028 | .466** | 1.000 | 5.37(1.12) |
NB:
.01 < p <.05;
p<.01
The effects of prenatal maternal depression on temperament at 6 months
Table 3 presents the effect of prenatal maternal depression on infant temperament. Model 1 shows the effect of prenatal maternal depression in the unadjusted model. Model 2 shows the effect in the adjusted model, with confounders for statistical adjustment, including sex of the infant, maternal age, race, marital status, parity, educational attainment, and prenatal smoking. Model 3 shows the effect of the final adjusted model, that includes all the confounders in Model 2 and additional maternal postnatal depression and severity of Sandy trauma.
Table 3.
The effects of maternal depression symptomatology during pregnancy on child temperament at 6 months of age
| Infant temperament | Unadjusted Model (Model l)a | Adjusted Model (Model 2)b | Adjusted Model (Model 3)c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Activity level | .015 | (.012) | .20 | .012 | (.012) | .32 | .009 | (.014) | .53 |
| Distress | .025 | (.012) | .04 | .027 | (.013) | .04 | .011 | (.015) | .47 |
| Fear | .026 | (.015) | .08 | .031 | (.015 | .04 | .008 | (.018) | .63 |
| Duration of attention | −.002 | (.015) | .88 | −.001 | (.016) | .95 | −.011 | (.018) | .55 |
| Smiling and laughter | −.043 | (.013) | .001 | −.046 | (.013) | .0004 | −.047 | (.015) | .002 |
| High pleasure seeking | −.020 | (.010) | .05 | −.024 | (.011) | .03 | −.020 | (.012) | .08 |
| Low pleasure seeking | −.017 | (.010) | .09 | −.024 | (.010) | .02 | −.019 | (.012) | .10 |
| Soothability | −.030 | (.010) | .004 | −.032 | (.011) | .004 | −.022 | (.011) | .05 |
| Falling (recovery from distress) | −.031 | (.011) | .005 | −.033 | (.012) | .006 | −.043 | (.015) | .03 |
| Cuddliness | −.034 | (.010) | .001 | −.037 | (010) | .0003 | −.029 | (013) | .03 |
| Perception | −.006 | (.017) | .74 | −.012 | (.018) | .49 | −.032 | (.020) | .11 |
| Sadness | .050 | (.012) | .00004 | .052 | (.012) | .00003 | .033 | (.014) | .02 |
| Approach | −.005 | (.012) | .65 | −.009 | (.013) | .53 | −.018 | (.015) | .21 |
| Vocal reactivity | −.016 | (.011) | .16 | −.022 | (.012) | .07 | −.011 | (.014) | .42 |
NB:
Model 1 is an unadjusted model.
In Model 2, sex of the infant, mother’s race, age, marital status, educational attainment, and smoking during pregnancy are statistically adjusted for.
In Model 3, perceived trauma due to Superstorm Sandy, and maternal postnatal depression at 6 months postpartum are added as additional covariates. Analysis is based on multivariable GLM with the 14 subscales of the temperament examined simultaneously. Maternal antenatal depression (Depression) was measured by the Edinburgh Postnatal Depression Scale (Murray & Carothers, 1990).
The two dimensions of temperament, including distress and fear, were no longer significant in Model 3. The remaining five dimensions, including smiling and laughter (p=.002), soothability (p=.05), falling reactivity (p=.03), cuddliness (p=.03), and sadness (p=.02), were significantly influenced by prenatal maternal depression.
Moderating effect of in-utero Sandy exposure on the associations between prenatal maternal depression and infant temperament
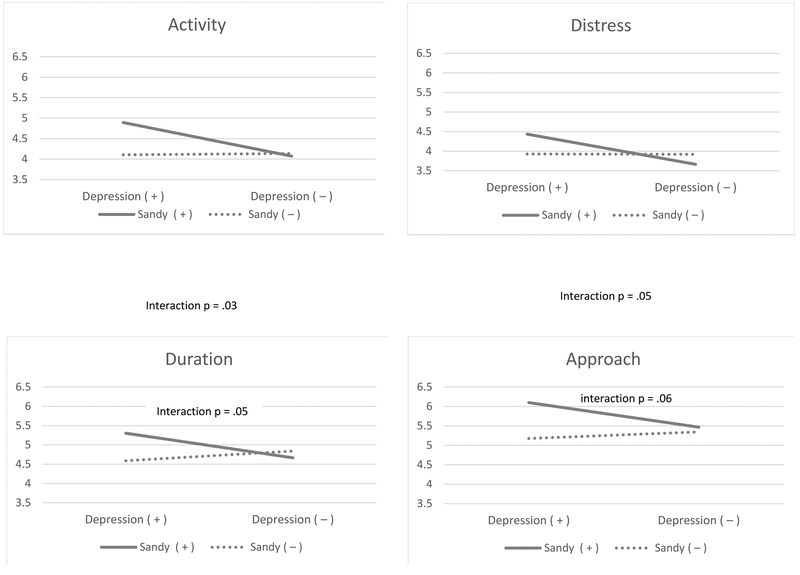
The 2nd and 3rd columns of Table 4 show the results from the stratified analysis on Sandy exposure. Among infants exposed to Sandy, prenatal maternal depression was associated with greater activity (p=.02), greater distress (p=.03), greater sadness (p=.05), and greater approach (p=.05). Among infants unexposed, prenatal maternal depression had a significant association with lower cuddliness (p=.02). Notably, among those exposed to Sandy, cuddliness was lower regardless of prenatal maternal depression status. Only among children exposed to Sandy, prenatal maternal depression was associated with increased activity, distress, duration of attention, and approach. Figure 1 shows the significant or marginally significant interactions between prenatal maternal depression and Sandy exposure on infant temperament. The interaction was significant for activity (p=.03), distress (p=.05) and approach (p=.05) and marginally significant for duration of attention (p=.06). The effect on other dimensions were not notable, including fear (p=.68), high-pleasure seeking (p=.58), low-pleasure seeking (p=.91), smile and laughter (p=.98), cuddliness (p=.49), perceptual sensitivity (p=.84), sadness (p=.52) and vocal reactivity (p=.90).
Table 4.
The effect maternal depression during pregnancy on child temperament at 6 months as a function of Superstorm Sandy exposure in-utero
| Superstorm Sandy Exposed in-utero | Superstorm Sandy Not exposed in-utero | |||||
|---|---|---|---|---|---|---|
| Activity | 4.06 (.17) | 4.87 (.29) | .02 | 4.15 (.11) | 4.10 (.21) | .82 |
| Distress | 3.65 (.18) | 4.50 (.32) | .03 | 3.79 (.12) | 3.78 (.22) | .80 |
| Fear | 3.26 (.19) | 3.91 (.34) | .11 | 2.81 (.13) | 3.25 (.24) | .20 |
| Duration of attention | 4.66 (.20) | 5.24 (.35) | .13 | 4.86 (.13) | 4.58 (.25) | .57 |
| Smiling and laughter | 5.48 (.17) | 5.21 (.22) | .93 | 5.63 (.11) | 5.34 (.22) | .23 |
| High pleasure seeking | 6.26 (.14) | 6.35 (.24) | .34 | 6.17 (.09) | 6.06 (.17) | .59 |
| Low pleasure seeking | 5.50 (.13) | 5.62 (.23) | .31 | 5.75 (.08) | 5.82 (.16) | .44 |
| Soothability | 5.07 (.13) | 5.07 (.23) | .99 | 5.68 (.09) | 5.42 (.16) | .24 |
| Falling (recovery from distress) | 4.92 (.16) | 4.60 (.28) | .37 | 5.27 (.13) | 4.96 (.19) | .26 |
| Cuddliness | 5.63 (.12) | 5.46 (.21) | .81 | 6.01 (.08) | 5.68 (.15) | .02 |
| Perceptual sensitivity | 4.72 (.23) | 4.71 (.41) | .75 | 4.51 (.15) | 4.45 (.29) | .58 |
| Sadness | 3.16 (.17) | 3.74 (.21) | .05 | 3.21 (.11) | 3.74 (.21) | .09 |
| Approach | 5.46 (.17) | 6.03 (.30) | .05 | 5.37 (.11) | 5.16 (.21) | .74 |
| Vocal reactivity | 5.27 (.15) | 5.61 (.26) | .11 | 5.40 (.10) | 5.68 (.19) | .22 |
NB: Values in the second and third column are based on stratified analysis on Sandy exposure status using Model 3 where sex of the infants, maternal education attainment, age, marital status, race, smoking status during pregnancy, perceived trauma due to Superstorm Sandy, and the level of depression at 6 months postpartum (when the assessment of temperament was done) were statistically controlled for.
Based on the Edinburgh Postnatal Depression Scale (Murray & Carothers, 1990), scores of 12 or more were considered to be depressed; scores of 11 or less, non-depressed.
Figure 1.
The mean score of the temperament scales among infants of mothers with and without depression during pregnancy by exposure to Superstorm Sandy in utero
DISCUSSION
Our findings are consistent with previous research (Sandman et al., 1999; Wadhwa et al., 2001) showing prenatal maternal depression directly influences infant temperament in early childhood. Our findings are important in that they extend our knowledge by providing evidence that prenatal maternal depression may have a differential impact on child temperament depending on the status of in-utero exposure to Sandy.
The study has two main findings: 1) prenatal maternal depression is associated with infant temperament related to emotion dysregulation and distressed emotion, including greater distress, greater fear, lower smiling and laughter, lower high- and low-pleasure seeking, lower soothability, slower falling reactivity, lower cuddliness, and greater sadness; and 2) there were interaction effects between prenatal maternal depression and Sandy exposure where prenatal maternal depression was associated with greater levels in activity, distress, approach, and shorter duration of attention only when they were also exposed to Sandy in-utero.
Stress is an intangible concept so it is difficult to synthesize results among studies with different designs and measures of stress, such as daily hassles (Buitelaar, Huizink, Mulder, de Medina, & Visser, 2003; Huizink, Robles de Medina, Mulder, Visser, & Buitelaar, 2003), stressful life-events (Hoffman & Hatch, 2000), maternal depression and anxiety (Glover, et al., 2015; Van den Bergh & Marcoen, 2004), and laboratory-induced stress through Stroop tests and mental arithmetic (De Weerth, Wied, Jansen, & Buitelaar, 2007; Schneider & Coe, 1993; Ward, Moore, Steptoe, Cockington, Robinson, & Phillips, 2004). Furthermore, measures of prenatal psychosocial stress, assessed by “daily hassles” or “stressful life-events” from everyday life, are generally broad and frequently lack negative valence.
To our knowledge, this is the first study to evaluate the relationship between prenatal depression plus a natural disaster (Sandy) that mothers experienced during pregnancy on infant temperament. Prior studies have shown that maternal prenatal depression is associated with infant temperament, but little is known about the additional effect of prenatal stress aggravated by a natural disaster on infant temperament. There are very few prospective investigations of the effects of prenatal stress that include maternal experiences of disaster, including natural disasters, man-made large-scale accidents, and wars/terrorism. These include the 1998 Canadian ice storm (Laplante, Brunet, Schmitz, Ciampi, & King, 2008), Hurricane Katrina (Oni, Harville, Xiong, & Buekens, 2012), the Three Mile Island nuclear plant explosion (Hoffman & Hatch, 2000), the Iowa floods (Ping et al., 2015), the Chernobyl nuclear plant disaster (Huizink, Dick, Sihvola, Pulkkinen, Rose & Kaprio., 2007), war (Meijer, 1985), and terrorism (Brand, 2006). Natural disasters are often unpredictable, intense, and short-lived but studies allow for simultaneous investigation of the effects of maternal mood during pregnancy and the additional stress caused by the disaster. In this study, we leveraged Sandy, which allowed us to evaluate the joint roles of prenatal maternal depression and external stress brought on by this unexpected and intense natural disaster.
In this study, we found a decreased level of smiling, soothability, falling reactivity, and cuddliness, as well as increased distress, fear, high- and low-pleasure seeking, and sadness among infants of depressed mothers during pregnancy, compared to infants of non-depressed mothers. Of those, smiling, soothability, falling reactivity, cuddliness, and sadness remained significant after controlling for maternal postpartum depression and severity of Sandy trauma. These findings suggest that there may be a biological consequence of prenatal maternal depression and resultant activated HPA-axis in offspring. Previously, our studies demonstrated the influence of prenatal maternal stress on placental MAOA gene expressions (Pehme, et al., in-press), and placental gene expression of HSD11B2 moderating the association between prenatal maternal depression and negative affectivity among 6-months-old children (Zhang et al., 2017). It is possible that through the epigenetic mechanism, compromised expressions caused by prenatal maternal stress led to excessive cortisol passed on to the fetus via umbilical-cord blood. The prenatal alterations in HPA tone and 5-HT, the serotonin signaling pathway, in the fetus may increase susceptibility to issues related to inhibited temperament, poorer emotional regulation, shyness, and fearfulness in infancy (Räikkönen et al., 2015).
Additionally, the study extended the existing literature by providing initial evidence that exposure to a natural disaster (i.e., Sandy) can aggravate the effect of existing problems during pregnancy, such as maternal depression, on infant outcomes. The allostatic load hypothesis proposed by McEwen posits that mothers who experience the stress of a disaster incur excessive wear and tear (i.e., allostatic load) on the body and brain (McEwen, 2003). It is conceivable that there was a synergistically elevated impact on the effect of depression among mothers exposed to Sandy in our cohort. Taken together, our findings on the differential impact by Sandy exposure on associations between prenatal maternal depression and some areas of temperament related to emotion regulation provide clues to explain how HPA-axis and 5-HT regulation in the fetus is aggravated and leads to susceptibility in temperament dysregulation. Given the recent increase in the frequency and intensity of natural disasters as a result of climate change (Trenberth, Fasullo, & Shepherd, 2015), greater understanding of how in-utero exposure to additional stress and the resultant negative impact on optimal child development is essential.
Prior studies clearly demonstrate that in-utero stress is associated with child neurodevelopmental outcomes and developmental psychopathology in mid/late childhood (Lupien et al., 2009; Glover, 2011), and among studies in early childhood, many examined “difficult” child temperament as the outcome (Jansen et al., 1995; Koniak-Griffin and VVerzemnieks, 1994; Oberklaid et al., 1993; Prior et al., 1992; Clerk-Stewart, et al., 2000; Austin et al., 2005). However, the notion of “difficult temperament” has often been subjectively interpreted across different studies. We followed psychobiological approach used in the IBQ-R (Putnam et al., 2014), which was created in part as a response to the problematic nature of the “difficult temperament” construct.
This study has several strengths. First, the occurrence of a natural disaster gave us an objective index of additional stress when testing the effect of prenatal maternal depression on infant outcomes. While our index of additional external stress is relatively simple, it provided an objective measure, especially considering that its effect is indiscriminant of SES, race, educational attainment, maternal psychopathology, or genetic makeup and therefore constituted an unparalleled opportunity to conduct a randomized controlled study of stress in a human population. We also included the severity of trauma exposure variable as a potential confounder in the final model, recognizing that not all cases had the same level of traumatic exposure. Second, the longitudinal design allowed us to follow the participants from pregnancy to the offspring’s first 6 months. We collected information on infant temperament concurrently as participants reached 6 months to minimize recall bias. As the first 6–12 months are an important developmental period, when rapid auditory and visual development takes place, the data are crucial in predicting childhood outcomes (Peterson, 2010). Maternal depression data was also prospectively ascertained. Finally, we used multivariable GLM to test all 14 sub-scales of temperament simultaneously. As they were inherently correlated to some degree, we could estimate the effect of the individual sub-scales simultaneously while adjusting for inter-correlation, thus minimizing Type I error.
This study also has limitations. First, infant temperament was based on maternal report. Maternal mood may introduce a potential bias (Mohler, Parzer, Brunner, Wiebel, & Resch, 2006; Najman, Williams, Nikles, Spence, Bor, O’Callaghan, & Andersen, 2000). To help counter this, maternal depression at 6-month postpartum, when temperament was assessed, was statistically controlled for in the final model. Although the IBQ-R is well-validated and mothers may be the best informants of temperament with infants, we acknowledge that more objective measures of infants’ temperament would have strengthened the findings, as would adjustment for other maternal psychopathology and measures of mother-child bond. Incorporating a biological measure of reactivity in offspring could have validated our findings in behavioral/phenotypic outcomes in reactivity (Ping et al. 2015). Second, temperament at 6 months was chosen as our outcome because it was the earliest available postnatal assessment point in our study, although the IBQ-R can be used to infants as early as 3 months old. We acknowledge that the 6-month assessment period being the earliest and only one measurement point in this paper as study limitation. Third, although we included mothers’ race, maternal age, prenatal smoking status, parity, education, marital status, and child’s sex in our analyses, our sample size restricted us from considering a wider range of potential confounders. Fourth, the statistical design of our study does not allow for analysis of the impact of the exact timing of exposure to Sandy in relation to the trimester of exposure. Future work should include these variables, as they may be related to child neurobehavioral outcomes. Lastly, the current study lacks biological markers of HPA-axis functioning such as cortisol levels, known to be associated with stressful life-events and psychopathology (Rubinow, Post, Savard, & Gold, 1984). A biological measure of maternal stress responsiveness during pregnancy might have broadened our understanding of the underlying biological pathway.
Despite these limitations, the study clearly demonstrates that prenatal maternal depression has important consequences for infant temperament. Furthermore, the negative impact of prenatal maternal depression appeared to be magnified among those exposed to Sandy in-utero. It is important for health care professionals to identify maternal depression and potential stressful life-events during pregnancy especially when there has been exposure to an external, grave, trauma as the first steps toward tailored interventions.
Financial support:
This work was supported by the Queens College, CUNY Research Enhancement Grant, and NIMH grants K01 MH080062, ARRA supplement K01 MH080062S, and R01 MH102729. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: None
IRB approvals:
The study was approved by the Institutional Review Boards at Icahn School of Medicine at Mount Sinai, New York Presbyterian at Queens, and Queens College (CUNY).
REFERENCES
- Austin MP, Hadzi-Pavlovic D, Leader L, Saint K, & Parker G (2005). Maternal trait anxiety, depression, and life event stress in pregnancy: relationships with infant temperament. Early Human Development, 81, 183–190 [DOI] [PubMed] [Google Scholar]
- Blair M, Glynn LM, Sandman C, & PoddidDavis E (2011). Prenatal maternal anxiety and early childhood temperament. Stress, 1, 1–8. doi. 10.3109/10253890.2011.594121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley PM, Capron LE, Jensen AB, O’Donnell KJ, & Glover V (2013). Maternal prenatal symptoms of depression and down regulation of placental monoamine oxidase A expression. Journal of Psychosomatic Research, 75(4), 341–345 [DOI] [PubMed] [Google Scholar]
- Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC & Levitt P (2011). A transient placental source of serotonin for the fetal forebrain. Nature, 472(7343), 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand SR (2006). The Effect of Maternal PTSD Following in Utero Trauma Exposure on Behavior and Temperament in the 9-Month-Old Infant. Annals of the New York Academy of Sciences, 1071(1), 454–458. doi: 10.1196/annals.1364.041 [DOI] [PubMed] [Google Scholar]
- Brummelte S, Mc Glanaghy E, Bonnin A, & Oberlander TF (2017). Developmental changes in serotonin signaling: implications for early brain function, behavior and adaptation. Neurosciences, 342, 212–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitelaar JK, Huizink AC, Mulder EJ, de Medina PGR, & Visser GHA (2003). Prenatal stress and cognitive development and temperament in infants. Neurobiology of Aging, 24, S53–S60. 10.1016/S0197-4580(03)00050-2 [DOI] [PubMed] [Google Scholar]
- Clarke-Stewart KA, Fitzpatrick MJ, Allhusen VD, & Goldberg WA (2000). Measuring difficult temperament the easy way. Journal of Developmental and Behavioral Pediatrics, 21(3), 207–220. [PubMed] [Google Scholar]
- Conradt E, Lester BM, Appleton AA, Armstrong DA, & Marsit CJ (2013). The roles of DNA methylation of NR3C1 and 11β-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics, 8(12), 1321–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, & Sandman CA (2004). Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy, 6(3), 319–331 [Google Scholar]
- De Pauw SS, & Mervielde I (2010). Temperament, personality and developmental psychopathology: A review based on the conceptual dimensions underlying childhood traits. Child Psychiatry & Human Development, 41(3), 313–329 [DOI] [PubMed] [Google Scholar]
- De Weerth C, Wied GD, Jansen LM, & Buitelaar JK (2007). Cardiovascular and cortisol responses to a psychological stressor during pregnancy. Acta Obstetrics Gynecological Scandinavia, 86(10), 1181–1192. doi: 10.1080/00016340701547442 [DOI] [PubMed] [Google Scholar]
- Finik J, & Nomura Y (2017). Cohort profile: Stress in Pregnancy (SIP) Study. International Journal of Epidemiology, 46(5), 1388–1388. doi.org/ 10.1093/ije/dyw264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, & Rothbart MK (2003). Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior and Development, 26(1), 64–86. doi: 10.1016/s0163-6383(02)00169-8 [DOI] [Google Scholar]
- Glover V (2011). The Effects of Prenatal Stress on Child Behavioural and Cognitive Outcomes Start at the Beginning. Stress and Pregnancy. 1–4. [Google Scholar]
- Glover V, O’Donnell K, O’Connor TG, Ramchandani P, & Capron L (2015). Prenatal anxiety and depression, fetal programming and placental function. Psychoneuroendocrinology, 61, 3–4. doi: 10.1016/j.psyneuen.2015.07.395 [DOI] [Google Scholar]
- Hoffman C, & Hatch MC (2000). Depressive symptomatology during pregnancy: evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychology, 19(6), 535–543 [PubMed] [Google Scholar]
- Huizink AC, Dick DM, Sihvola E, Pulkkinen L, Rose RJ, & Kaprio J (2007). Chernobyl exposure as stressor during pregnancy and behaviour in adolescent offspring. Acta Psychiatrica Scandinavica, 116(6), 438–446. doi: 10.1111/j.1600-0447.2007.01050.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Robles de M, Pascale GM, Eduard JH, Visser GHA, & Buitelaar JK (2003). Stress during pregnancy is associated with developmental outcome in infancy. Journal of Child Psychology & Psychiatry, 44(6), 810–818. doi: 10.1111/1469-7610.00166 [DOI] [PubMed] [Google Scholar]
- Jansen RE, Fitzgerald HE, Ham HP, & Zucker RA (1995). Pathways into risk: temperament and behavior problems in three- to five-year-old sons of alcoholics. Alcoholism: Clinical and Experimental Research, 19(2), 501–509. [DOI] [PubMed] [Google Scholar]
- Kheirabadi GR, Maracy MR, Akbaripour S, & Masaeli N (2012). Psychometric properties and diagnostic accuracy of the edinburgh postnatal depression scale in a sample of Iranian women. Iran J Med Sci, 37(1), 32–38 [PMC free article] [PubMed] [Google Scholar]
- Koniak-Griffin D & Verzemnieks I (1994). Relationship Between Patterns of Infant Temperament, Child Behavior Ratings, and Interactions During Toddlerhood. Journal of Child and Adolescent Psychiatric Nursing, 7(4), 26–37 [DOI] [PubMed] [Google Scholar]
- Laplante DP, Brunet A, Schmitz N, Ciampi A, & King S (2008). Project Ice Storm: Prenatal Maternal Stress Affects Cognitive and Linguistic Functioning in 51/2-Year-Old Children. Journal of the American Academy of Child & Adolescent Psychiatry, 47(9), 1063–1072. doi: 10.1097/chi.0b013e31817eec80 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C Effects of stress throughout the lifespan on the brain, behaiour, and cognition. Focus on Stress. 2009, 10, 434–445 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2003). Mood disorders and allostatic load. Biological Psychiatry, 54(3), 200–207. doi: 10.1016/s0006-3223(03)00177-x [DOI] [PubMed] [Google Scholar]
- Meijer A (1985). Child psychiatric sequelae of maternal war stress. Acta Psychiatrica Scandinavica, 72(6), 505–511. doi: 10.1111/j.1600-0447.1985.tb02647.x [DOI] [PubMed] [Google Scholar]
- Mohler E, Parzer P, Brunner R, Wiebel A, & Resch F (2006). Emotional stress in pregnancy predicts human infant reactivity. Early Human Development, 82(11), 731–737. doi: 10.1016/j.earlhumdev.2006.02.010 [DOI] [PubMed] [Google Scholar]
- Murray L, & Carothers AD (1990). The validation of the Edinburgh Post-natal Depression Scale on a community sample. The British Journal of Psychiatry, 157(2), 288–290. doi: 10.1192/bjp.157.2.288 [DOI] [PubMed] [Google Scholar]
- Musser ED, Galloway-Long HS, Frick PJ, & Nigg (2013). Emotion regulation and heterogeneity in attention-deficit/hyperactivity disorder. Journal of American Academy of Child & Adolescent Psychiatry, 52(2), 163–171.e2. doi: 10.1016/j.jaac.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najman JM, Williams GM, Nikles J, Spence S, Bor W, O’Callaghan M, & Andersen MJ (2000). Mothers’ mental illness and child behavior problems: cause-effect association or observation bias? Journal of American Academy of Child & Adolescent Psychiatry, 39(5), 592–602. doi: 10.1097/00004583-200005000-00013 [DOI] [PubMed] [Google Scholar]
- Norris FH, Kaniasty KZ, & Scheer DA (1990). Use of mental health services among victims of crime: frequency, correlates, and subsequent recovery. Journal of Consulting and Clinical Psychology; 58(5), 538–547 [DOI] [PubMed] [Google Scholar]
- Oberklaid F, Sanson A, Pedlow R, & Prior M (1993). Predicting preschool behavior problems from temperament and other variables in infancy. Pediatrics, 91(1), 113–120. [PubMed] [Google Scholar]
- Oberlander TF (2012). Fetal serotonin signaling: setting pathways for early childhood development and behavior. Journal of Adolescent Health, 51(2), S9–S16 [DOI] [PubMed] [Google Scholar]
- Oni O, Harville EW, Xiong X, & Buekens P (2012). Impact of coping styles on post-traumatic stress disorder and depressive symptoms among pregnant women exposed to Hurricane Katrina. American Journal of Disaster Medicine, 7(3), 199–209. doi: 10.5055/ajdm.2012.0095 [DOI] [PubMed] [Google Scholar]
- Pagliaccio D, Luby JL, Luking KR, Belden AC, & Barch DM (2014). Brain-behavior relationships in the experience and regulation of negative emotion in healthy children: implications for risk for childhood depression. Developmental Psychopathology, (4 Pt 2),1289–303. doi: 10.1017/S0954579414001035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehme MP, Zhang W, Finik J, Pritchett A, Buthmann J, Dana K, Hao K, & Nomura Y (in-press). Placental MAOA expression mediates prenatal stress effects on temperament in 12-month-olds. Infants & Child Development [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson EA (2010). Early Childhood Development: Building blocks for life. Greater twin cities united way research & planning (July). [Google Scholar]
- Ping EY, Laplante DP, Elgbeili G, Hillerer KM, Brunet A, O’Hara MW, & King S (2015). Prenatal maternal stress predicts stress reactivity at 21/2 years of age: The Iowa Flood Study. Psychoendocrinology, 56, 62–78. DOI: 10.1016/j.psyneuen.2015.02.015 [DOI] [PubMed] [Google Scholar]
- Prior M, Smart D, Sanson A, Pedlow R, & Oberklaid F (1992). Transient versus stable behavior problems in a normative sample: infancy to school age. Journal of Pediatric Psychology, 17(4), 423–443 [DOI] [PubMed] [Google Scholar]
- Putnam SP, Helbig AL, Gartstein MA, Rothbart MK, Leerkes E. Development and assessment of short and very short forms of the infant behavior questionnaire-revised. Journal of Personality Assessment. 2014;96(4):445–58. doi: 10.1080/00223891.2013.841171. [DOI] [PubMed] [Google Scholar]
- Räikkönen K, Pesonen AK, O’Reilly JR, Tuovinen S, Lahti M, Kajantie E, & Reynolds RM (2015). Maternal depressive symptoms during pregnancy, placental expression of genes regulating glucocorticoid and serotonin function and infant regulatory behaviors. Psychological Medicine, 45, 3217–3226. 10.1017/S003329171500121X [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Derryberry D, & Posner MI (1994). A psychobiological approach to the development of temperament In Bates JE & Wachs TD (Eds). Temperament: Individual differences at the interface of biology & behavior. Chapter 4 (pp. 83–114) American Psychological Association, Washington D.C., USA [Google Scholar]
- Rubinow DR, Post RM, Savard R, & Gold PW (1984). Cortisol hypersecretion and cognitive impairment in depression. Archives of General Psychiatry, 41(3), 279–283. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, & Garite TJ (1999). Maternal corticotropin-releasing hormone and habituation in the human fetus. Developmental Psychobiology, 34(3), 163–173. doi: 10.1002/(sici)1098–2302(199904)34:3<163::aid-dev1>3.0.co;2–9 [DOI] [PubMed] [Google Scholar]
- Schneider ML, & Coe CL (1993). Repeated social stress during pregnancy impairs neuromotor development of the primate infant. Journal of Developmental & Behavioral Pediatrics, 14(2), 81–87 [PubMed] [Google Scholar]
- Simes RJ (1986). An improved Bonferroni procedure for multiple tests of significance. Biometrika, 73(3), 751–754. doi: 10.1093/biomet/73.3.751 [DOI] [Google Scholar]
- Trenberth KE, Fasullo JT, & Shepherd TG (2015). Attribution of climate extreme events. Nature Climate Change, 5(8), 725–730. doi: 10.1038/nclimate2657 [DOI] [Google Scholar]
- Van den Bergh BR, & Marcoen A (2004). High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Development, 75(4), 1085–1097 [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, & Garite TJ (2001). The neurobiology of stress in human pregnancy: implications for prematurity and development of the fetal central nervous system Progress in Brain Research (pp. 131–142): Elsevier BV; [DOI] [PubMed] [Google Scholar]
- Ward AMV, Moore VM, Steptoe A, Cockington RA, Robinson JS, & Phillips DIW (2004). Size at birth and cardiovascular responses to psychological stressors. Journal of Hypertension, 22(12), 2295–2301. DOI: 0.1097/00004872-200412000-00011 [DOI] [PubMed] [Google Scholar]
- Werner EA, Myers MM, Fifer WP, Cheng B, Fang Y, Allen R, & Monk C (2007). Prenatal predictors of infant temperament. Developmental Psychobiology, 49, 474–484, [DOI] [PubMed] [Google Scholar]
- Zhang W, Finik J, Dana K, Glover V, Ham J, & Nomura Y (2018). Prenatal Depression and Infant Temperament: The Moderating Role of Placental Gene Expression. Infancy. 23(2), 211–231. doi: 10.1111/infa.12215 [DOI] [PMC free article] [PubMed] [Google Scholar]