Abstract
Cholesterol esterase-like (CE) activity from saliva and esterase from cariogenic bacteria hydrolyze ester linkages of dental methacrylate resins. Collagenolytic, matrix metalloproteinase-like (MMP) activities from dentin and bacteria degrade collagen in demineralized tooth dentin. Human neutrophils in the oral cavity contain factors that are hypothesized to have CE and MMP activities that could contribute to the degradation of methacrylate resins and dentinal collagen.
Objectives:
To measure the CE and MMP activities from human neutrophils and their ability to degrade dental methacrylate resin composite and dentinal collagen.
Neutrophils’ CE and MMP activities were measured using nitrophenyl-esters or fluorimetric MMP substrates, respectively. Neutrophils’ degradation of resin composite and dentinal collagen was quantified by measuring release of a universal 2,2-Bis[4-(2-hydroxy-3-methacryloxypropoxy)phenyl]propane (bisGMA)-derived resin composite degradation byproduct, bishydroxy-propoxy-phenyl-propane (BisHPPP), or a collagen degradation by-product, hydroxyproline, respectively using ultra performance liquid chromatography/mass spectrometry.
Neutrophils’ CE activity increased the release of bisHPPP from bisGMA monomer compared to control after 24 and 48 hours (p<0.05). Neutrophils degraded polymerized resin composite and produced higher amounts of bisHPPP than buffer after 48 hours of incubation (p<0.05). Neutrophils show generic MMP, gelatinase, MMP-2 and MMP-9, and collagenase, MMP-1 and MMP-8 activities that were stable or increased over the first 24 hours (p<0.05). Neutrophils degraded demineralized dentin more than buffer-only groups, indicated by higher amounts of hydroxyproline (p<0.05).
The ability of neutrophils to degrade both dental resin composite and tooth dentin, suggest neutrophil’s potential role in root caries, and in recurrent carries by accelerating the degradation of resin-dentin interfaces, and compromising the longevity of the restoration.
Keywords: Matrix metalloproteinases, esterase, biodegradation, dental caries, dental restoration failure
Graphical Abstract
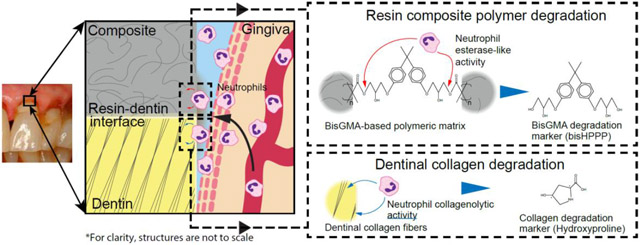
1. Introduction
Methacrylate resin composite are the most commonly used restoration in dentistry [1]; most dental resin composite restorations placed are replacements for failing restorations due to secondary caries [2]. The universal ingredient of methacrylate-based resin composite and adhesives, 2,2-Bis[4-(2-hydroxy-3-methacryloxypropoxy)phenyl]propane (bisGMA), is susceptible to degradation by salivary and bacterial esterases, CE-like activity yielding bishydroxy-propoxy-phenyl-propane (bisHPPP) [3-8]. Over time, resin composite and adhesives at the resin-dentin interface degrade, allowing for penetration of oral fluids and interfacial bacterial biofilm proliferation and further enlargement of the gap between the restoration and tooth [5, 9-11], leading to recurrent decay and early failure of the restoration [12].
The state of tooth dentin at the hybrid layer, the layer of dentinal collagen fibrils and tubules impregnated with resin adhesive that forms the critical restoration-tooth margins, is important for the longevity of resin composite restorations [13]. Tooth dentin can be degraded by metalloproteinases (MMPs) found in human saliva and dentin, and protease, MMP-like activities from bacteria, potentially contributing to recurrent and root caries [14-16]. A major portion of oral MMPs are believed to originate from the gingival crevicular fluid (GCF); however, the source for these activities have yet to be determined [17].
Neutrophils are part of the innate immune system and are constantly entering the oral cavity. The primary region of entry for neutrophils is the gingival sulcus [18], in direct contact with the tooth, restoration, restoration-tooth margins and pathogenic bacteria. Neutrophils contain high proteolytic and catabolic activities [19] and can cause tissue damage [20]. However, the role of human neutrophils in degradation of resin and dentin and the pathogenesis of dental caries has yet to be addressed.
Based on the above introduction, it is hypothesized that neutrophils release degradative moieties, including esterase-like and/or proteolytic activities that degrade methacrylate resin restorations and demineralized tooth dentin. This could potentially compromise the restoration-tooth interface, lead to recurrent caries formation and progression, and premature restoration failure. The aims of the current study were to measure the esterase, cholesterol esterase-like (CE) and protease, MMP-like activities from human neutrophils and their degradative effect toward resin monomer, resin composites and tooth dentin collagen.
2. Materials and methods
2.1. Isolation of human neutrophils
Neutrophils were freshly isolated from healthy human donors’ peripheral blood (University of Toronto Ethics Review Boards, Protocol # 29410) by gradient separation using 1-step polymorph (Accurate Chemical & Scientific Corp, Westbury, NY, USA) [21, 22]. Samples were centrifuged at 527xG, in room temperature for 35 minutes. The lower of the two bands containing neutrophils was collected and washed with Hanks balanced salt solution containing calcium and magnesium (Sigma-Aldrich, St. Louis, MO, USA) (HBSS+/+). Neutrophils were further washed and incubated with red blood cell lysis buffer, then resuspended in Hanks’ Balanced Salt solution (Sigma-Aldrich, St. Louis, MO, USA) (HBSS+/+), and counted with a hemocytometer (Z2 Coulter Counter, Beckman Coulter, Brea, CA). Cells were verified with diff-quick staining (Siemens, Deerfield, IL, USA), and cell viability was assessed with trypan blue exclusion tests. Cells were then diluted in HBSS+/+ containing 100 units/mL of penicillin and 100 μg/mL streptomycin and stored at −4°C, and used within 2 hours.
2.2. Measurement of esterase-like, CE-like activity from neutrophils
Neutrophils were incubated in HBSS+/+ at 37°C. Esterase-like, CE-like activity was measured immediately (0 hour) or after 2, 24 and 48 hours by mixing 1 mL of 15,000 neutrophils/mL in HBSS+/+ or HBSS+/+ alone (control) with 0.5 mL p-nitrophenyl-butyrate (p-NPB) (Sigma, St. Louis, MO, USA). Change in absorbance over time, corresponding to the production of nitrophenol from the nitrophenyl substrate was recorded at a wavelength of 401 nm using a spectrophotometer, (Cytation 3, BioTek, VT, USA), as previously described [4, 9-11, 23-27]. Data were normalized to the buffer control (HBSS+/+) without neutrophils. One CE activity unit was defined as release of 1 nmol of nitrophenol per minute [4, 6, 25, 26].
2.3. Degradation of resin monomers and cured resin composites by neutrophils
Solutions of 10 μM bisGMA monomer [6] in HBSS buffer were prepared with and without 106/mL neutrophils and incubated at 37°C. Standardized cylindrical samples (4×4 mm height by diameter) of cured resin composite (Z250, 3M™, St. Paul, MN, USA) were prepared, incubated at 60°C in a vacuum oven to remove volatile unreacted monomers as previously described [3], and were incubated with 1 mL of media with and without 106/mL neutrophils at 37°C. Viability of the neutrophils was measured for up to 96 hours. CE-like activity was measured at 0, 2, 24 and 48 hours and monomer degradation were measured at 0, 24 and 48 hours. Degradation of cured resin composite was assessed after 48 and 96 hours, and media with or without fresh neutrophils were replaced at each time point. Removed media were mixed with equal amounts of methanol to denature enzymes and halt the degradation reaction [5, 10]. Samples were filter centrifuged (AmiconUltra centrifugal filters, Millipore Corporation, Bedford, MA) at 18000xG, 4°C, for 20 minutes (Hermle Z400K, Wehingen, Germany). BisHPPP in sample solutions were quantified and confirmed using ultra performance liquid chromatography and mass spectrometry (UPLC-MS) (Waters Aquity, Waters Corporation, Milford, MA) as previously described [7].
2.4. Measurement of MMP activities from neutrophils
MMP-1, MMP-2, MMP-8, MMP-9 and generic MMP-like activities of neutrophils were determined immediately (0 hours), and after 2 and 24 hours post-harvesting using specific fluorimetric substrate kits (AnaSpec, San Jose, CA) [15]. At each time point, 2500 neutrophils or HBSS+/+ alone (control) were mixed with 1:100 diluted MMP substrates in assay buffer. Kinetic MMP activity fluorescence (excitation/emission=490nm/520nm) was measured using a spectrophotometer (Cytation3, BioTek, VT, USA) at 37°C [15]. Relative fluorescence units (RFU)/min were normalized to the buffer only benchmark and converted to MMP activity units using a standard curve. One MMP unit was defined as the release of 1 μmol of 5-FAM-Pro-Leu-OH substrate per minute at 37°C [15].
2.5. Degradation of dentinal collagen by neutrophils
Human dentin was obtained from extracted, non-carious human molars (University of Toronto Ethics Review Board, Protocol #28214), was cut into 2×2×4 mm slabs, completely demineralized with 10% phosphoric acid for 18 hours, neutralized with 10% sodium ascorbate, washed 3X with HBSS+/+ and then incubated with 2×106 neutrophils in HBSS+/+, HBSS+/+ alone (negative control), or collagenolytic enzyme (Collagenase Type I from Clostridium histolyticum, Gibco®, Sigma-Aldrich, Oakville, ON, Canada) at 125 U/mL (positive control) (24 hours, 37°C) [15]. The supernatant was filter-centrifuged (AmiconUltra centrifugal filters, Millipore Corporation, Bedford, MA) at 18000xG, 4°C, for 20 minutes (Hermle Z400K, Wehingen, Germany) and analyzed for free hydroxyproline by UPLC-UV at 254 nm at the Advanced Protein Technology Center, Hospital for Sick Children, Toronto, Ontario. Additionally dentin samples were processed for scanning electron microscopy (JEOL, USA, model 6610LV) [15].
2.6. Statistical Analysis
Differences between amounts of bisHPPP release from bisGMA monomers and resin composite, CE-like activity over time, generic and specific MMP activities at each time point, each MMP activity at different time points, and hydroxyproline release were analysed using ANOVA in conjunction with a post-hoc Tukey’s honestly significant difference (HSD) test (p<0.05). Each experiment was repeated independently for a minimum of three times.
3. Results:
3.1. Neutrophil Viability, Esterase, CE-like activity
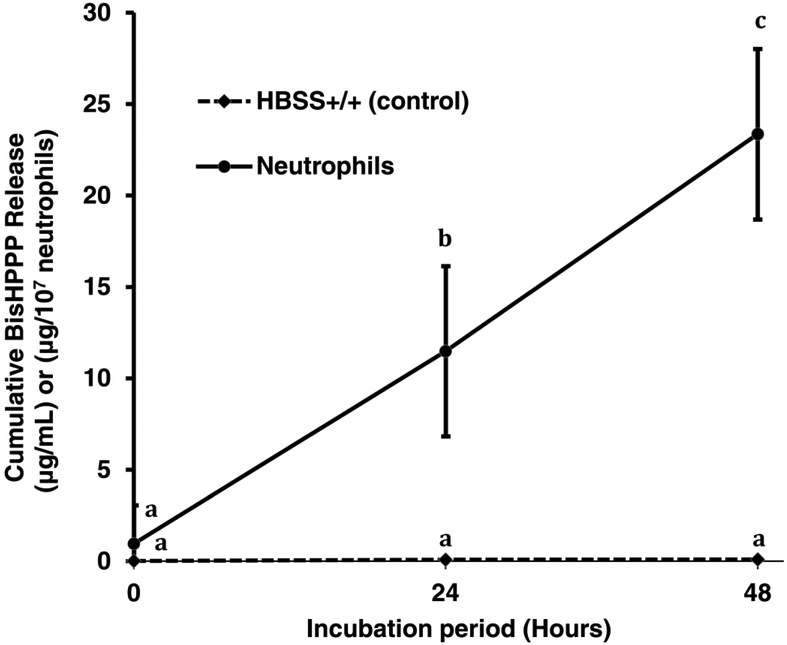
Viability of the neutrophils after 0, 24, 48, and 72 hours of incubation in HBSS+/+ (37 °C) was 97±4%, 66±18% and 18±8%, respectively (mean±SD, N=4). No viable neutrophils were found after 96 hours. Neutrophils showed CE-like activity (15.1±3.1 units/107 neutrophils), which remained stable for 24 hours (13.89±1.89 units/107 neutrophils), (p>0.05) and was slightly reduced after 48 hours of incubation (10.54±1.67 units/107 neutrophils) (p<0.05) (Fig. 1).
Fig. 1.
Esterase-like, CE-like activity of human neutrophils measured using p-NPB as a substrate (37 °C, in HBSS+/+). There were no significant differences in CE-like activity from neutrophils between time points (One-way ANOVA and Tukey’s HSD, p < 0.05). Data were normalized to 107 cells using HBSS+/+ w/o cells as baseline and shown as mean ± SD, n = 5.
3.2. Degradation of resin monomers and cured composites by neutrophils
Higher amounts of bisHPPP were produced from bisGMA monomer by neutrophils compared to media only after 24 (11.49±1.05 μg/107 neutrophils vs. 0.08±0.06 μg/mL) and 48 hours (23.36±1.19 μg/107 neutrophils vs. 0.09±0.02 μg/mL) (p<0.05) (Fig. 2). Degradation of cured resin composite was also accelerated by neutrophils compared to media only control after 48 (0.196±0.040 μg/cm2/107 neutrophils vs. 0.043±0.008 μg/cm2) and 96 hours (0.204±0.045 μg/cm2/107 neutrophils vs. 0.043±0.008 μg/cm2) (p<0.05) (Fig. 3).
Fig. 2.
Cumulative bisHPPP release after incubation (37 °C) of bisGMA monomer in HBSS+/+ alone (control) or with human neutrophils in HBSS+/+. Different letters indicate significant difference between groups (One-way ANOVA and Tukey’s HSD, p < 0.05). Data were normalized to 107 cells and surface area of the specimens and shown as mean ± SD, n = 6.
Fig. 3.
Cumulative bisHPPP release after incubation (37 °) of photocured resin composite in HBSS+/+ alone (control) or with human neutrophils in HBSS+/+. Different letters indicate significant difference between groups (One-way ANOVA and Tukey’s HSD, p < 0.05). Data were normalized to 107 neutrophils and surface area of the specimens and shown as mean ± SD, n = 3.
3.3. MMP-like activities from neutrophils
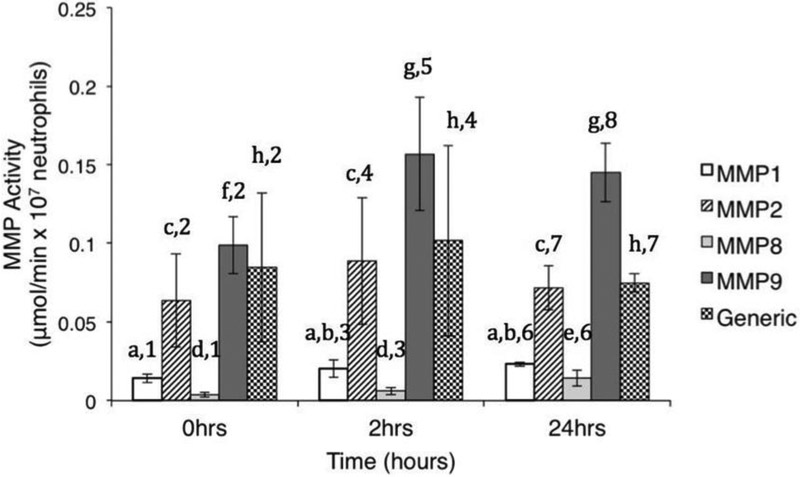
Human neutrophils had measurable specific and generic MMP-like activities throughout the incubation period (Fig. 4). MMP-1- (0.014±0.003 μmol/min per 107 neutrophils), MMP-2- (0.064±0.030 μmol/min per 107 neutrophils) and generic MMP-like (0.085±0.047 μmol/min per 107 neutrophils) activities did not change significantly over time (p>0.05). MMP-8-like activity had increased levels at 24 hours (0.14±0.005 μmol/min per 107 neutrophils) vs. initial (0.004±0.001 μmol/min per 107 neutrophils) and 2 hours (0.006±0.002 μmol/min per 107 neutrophils) levels (p<0.05). MMP-9-like activities increased activity at 2 (0.16±0.036 μmol/min per 107 neutrophils) and 24 (0.14±0.019 μmol/min per 107 neutrophils) hours vs. initial levels (0.099±0.018 μmol/min per 107 neutrophils) (p<0.05). MMP-2-, MMP-9- and generic MMP-like activities were significantly higher than MMP-1- and MMP-8-like activity throughout the incubation period; at 2 hours and 24 hours of incubation MMP-9-like activity was the highest, followed by MMP-2- and generic MMP-like activities (p<0.05) (Fig. 4).
Fig. 4.
MMP-like activities from human neutrophils (HBSS+/+, 37 °C). Different letters indicate significant differences between time points within each MMP type (One-way ANOVA and Tukey’s HSD, p < 0.05). Different numbers indicate significant difference between MMP activities at each time point (One-way ANOVA and Tukey’s HSD, p < 0.05). Data shown as mean ± SD (n = 6) and were normalized to 107 cells using HBSS+/+ w/o cells as baseline.
3.4. Degradation of dentinal collagen by neutrophils
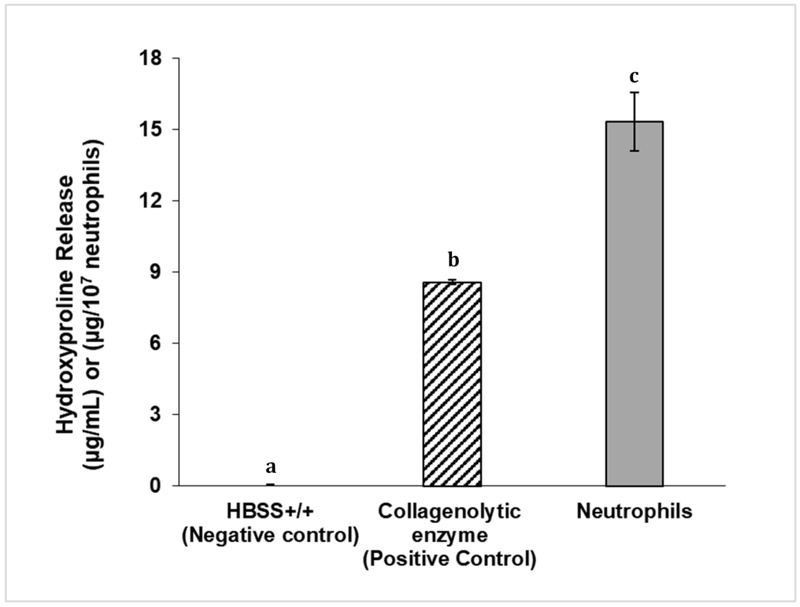
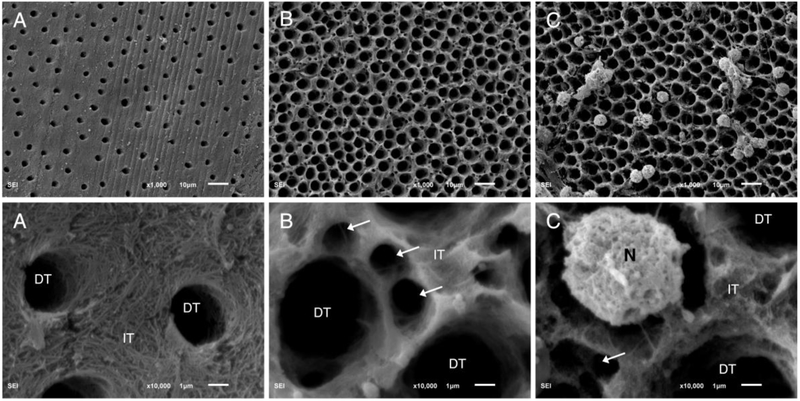
Incubation of demineralized dentin with neutrophils produced more hydroxyproline (15.32±1.2 μg/mL) compared to collagenolytic enzyme (positive control) (8.56±0.1 μg/mL) and buffer only (negative control) (0.03±0.04 μg/mL) (p<0.05) (Fig. 5). Fig. 6 depicts images of demineralized tooth dentin after exposure to media alone (negative control), collagenolytic enzyme (positive control) and neutrophils. SEM micrographs show loss of collagen fibril network, and degradation of peritubular and intertubular collagen after incubating specimens with neutrophils, similarly to the effect found by exposure to the collagenolytic enzyme (positive control). In contrast, media only (negative control) show intact collagen fibrils and was collagen rich at the intertubular dentin (Fig. 6).
Fig. 5.
Hydroxyproline release after 24 h of incubation (37 °C) with HBSS+/+ alone (negative control), collagenolytic enzyme (positive control), or neutrophils in HBSS +/+.Different letters indicate statistical significance (One-way ANOVA and Tukey’s HSD, p < 0.05). Data shown as mean ± SD, n = 3.
Fig. 6.
Representative scanning electron microscopy images at ×1000 (top row) and ×10,000 (bottom row) original magnification of demineralized tooth dentinal collagen and dentinal tubules (DT). A) Sample was incubated with HBSS+/+ alone for 24 h (37 °C), and has intact collagen fibrils and is collagen rich at the intertubular dentin (IT). B) Incubation of dentinal sample with collagenolytic enzyme (positive control) showing loss of collagen fibrils, and degradation of peritubular and intertubular collagen (arrows). C) Sample incubated with neutrophils (N) in HBSS +/+, showing loss of collagen fibrils and degradation of peritubular and intertubular collagen (arrow).
4. Discussion:
The current study is the first to characterize and quantify degradative activities from neutrophils toward methacrylate resin and demineralized dentin, the two main components of the restoration-tooth interface. Therefore, the hypothesis that neutrophils release degradative moieties, including esterase-like and/or proteolytic activities that degrade the methacrylate resin restoration and demineralized tooth dentin is accepted. These processes could potentially compromise the restoration-tooth interface, increase caries formation and progression, and premature restoration failure. As such, this study provides a significant finding to the oral health field by identifying a potential weakness in current restorative procedures and materials used to manage gingival proximal and cervical gingival or sub-gingival lesions and the pathogenesis of caries.
4.1. Esterase, CE-like activity from neutrophils
Nitrophenyl esters have been previously used to characterize esterases from human saliva and cariogenic dental bacteria with resin degrading properties [3, 4, 6-8]. In the current study, human neutrophils cleaved the nitrophenyl esters and showed CE-like activity in levels relevant to intraoral degradative conditions by saliva and bacteria [3, 4, 6, 24, 28]. The data in the current study demonstrate that human neutrophils are able to significantly hydrolyze bisGMA monomers for up to 48 hours post-harvesting, corroborating the finding of previous work that has shown that CE-like activities have a high affinity for hydrolyzing this monomer [4, 6]. It has been suggested that saliva contain a variety of enzymes responsible for CE-like activity and resin degradation [23, 27, 29-31]. The ability of human neutrophils to hydrolyze p-NPB and bisGMA suggest that they could be a source of some of the CE-like activity in saliva, contributing to the overall oral degradative activities towards bisGMA monomers and resin composites.
4.2. Biodegradation of resin composite by neutrophils
The resin composite used in the current study (Z250) has been previously used for biodegradation studies, thus providing a good database for comparison [3, 9-11, 28]. This bisGMA-containing material is commonly used as a representative for methacrylate resin based materials and when degraded produces the representative degradation product bisHPPP [31]. In the current study, degradation of cured resin composite was accelerated by neutrophils compared to media control as demonstrated by the increase in the release of bisHPPP. The reduction in the hydrolysis rate between the first and second 48 hours could be explained by the ability of neutrophils, as for human saliva and bacteria, to produce bisHPPP from unreacted and partially reacted bisGMA on the surface of the materials at a greater rate than the repeated bisGMA segments within the polymer [29]. Once the unreacted and partially reacted bisGMA molecules on and adjacent to the surface are consumed, the production of bisHPPP is lowered, yet still significant and higher than buffer alone. It then becomes dependent on the slow diffusion of bisGMA from within the bulk of the material and degradation of the polymer matrix that exposes deeper bisGMA molecules [32]. Under intra oral conditions, the masticatory activity will likely increase the mechanical degradation of the restoration and interface, accelerating the exposure of deeper parts of the matrix, allowing for increased degradation by neutrophils, bacteria and saliva to overall greater impact than their individual degradative activities.
In the study by Jaffer et al., [24] the degradative activity of human saliva (human salivary derived esterase, HSDE) toward the same commercial dental resin composite, measured by the production of bisHPPP was found to be similar to the amount of bisHPPP generated by the model enzyme CE as reported in previous studies [33, 34], when both measured activities were matched using nitrophenyl substrates. Similarly, in the current study, where CE-like measured activity from neutrophils toward the nitrophenyl substrate were at levels similar to those found for human saliva, levels of bisHPPP from the commercial resin composite were similar, confirming relevancy of this method to characterize degradative activity and actual degradation of resin composites, and relevancy of neutrophils degradation of the resin composites. Further research into CE-like activity from saliva, showed that longer incubation periods of up to 180 days, lead to a significant degradation of the resinous matrix, increase in cariogenic bacterial microleakage and a decrease in resin-dentin bond strength [5, 9–11]. Therefore, the comparable CE-like activity from neutrophils and their initial degradation of the resin composite to saliva and bacteria suggests a potential for neutrophils over time to negatively influence the resin-dentin interface and reduce interfacial bond strength. Studies for longer incubation period than done in the current investigation could provide a more definitive answer in this respect.
4.3. MMP activities from neutrophils
Fluorimetric MMP peptides have been previously used to measure and quantify MMP activities from dentin and oral bacteria as they have higher sensitivity than colorimetric assays [15, 17, 35]. In the current study, human neutrophils showed activity towards the MMP substrates, and the activities either remained constant or became elevated throughout the 48 hours replacement cycle, suggesting their potential ability to degrade the collagen type I in tooth dentin. Gelatinase MMP-2 and MMP-9 and generic MMP activities from neutrophils were measurably higher than collagenase MMP-1 and MMP-8 activities. MMP-1, MMP-8 and MMP-9 activities showed trends of increasing activity over time, while MMP-2 and generic MMP activities remained relatively steady over 24 hours of incubation. These findings corroborate previous reports identifying MMP-8 and MMP-9 in specific granules and MMP-9 in gelatinase granules of human neutrophils [36]. Additionally, the substrates used to measure MMP activities provide further characterization of protease activities and could aid the identification of proteolytic activities from neutrophils. These results provide evidence of active MMP properties from human neutrophils and means to better characterize the physiological mechanism of dentin degradation by these cells. Proteomic analyses would allow for identification of specific enzyme that are responsible for the above measured MMP-like activities [28]. Host derived MMPs play a role in the breakdown of dentinal collagen leading to caries [17]. Human MMP-9 has been isolated from caries infected dentin [37] and elevated levels of MMP-9 (“neutrophil gelatinase”) were found to correlate with caries lesion depth [38]. However, the above studies did not positively identify the source of these MMPs. Previous studies have looked at dentinal MMP enzymes and found that they cause only a slow gradual collagen degradation that require long incubation periods, on the order of ~250 days, to become measurable [39]. The result of the current study provide evidence that neutrophils, in concentrations similar to clinical situations, have collagenolytic, MMP-like activities in levels sufficient to degrade demineralized dentin within 24 hours of incubation. These results bring evidence to a novel contributor, neutrophils, to dentin degradation and suggest a potential involvement of neutrophils in caries progression and/or restoration failure.
4.4. Dentinal collagen degradation by neutrophils
Around 90% of the organic matrix in dentin is collagen, primarily type I collagen, which is rich in hydroxyproline [40]. The quantification of hydroxyproline release from dentinal collagen has been previously used for dentin degradation studies by bacteria [8]. The results of the current study show a significant increase in hydroxyproline release in the presence of neutrophils vs. media alone, suggesting the ability of the cells to degrade demineralized dentinal collagen. Neutrophils degradation of extracellular matrix has been previously shown, however, their ability to degrade demineralized dentin has never been previously demonstrated.
Scanning electron microscopy micrographs add qualitative evidence to the degradation of demineralized dentin by neutrophils. A clear increase in collagen destruction was visible when dentin was incubated with neutrophils compared to media only. Additionally, neutrophils incubated with demineralized dentin were found in the dentinal tubules, suggesting that they degrade their way through the dentin. There have been reports where neutrophils pursue bacteria into cracks in teeth [41]. The study by Kermanshahi et al., [9] showed a marginal gap of around 20 μm between resin composite and the dentin following aging in simulated human salivary esterases that allowed for interfacial bacterial biofilm formation. A gap like this is big enough also for neutrophils, with a size of 10-12 μm in diameter, to enter and follow the bacteria. Primary and secondary caries are located gingivally and wide voids could lead accumulation of bacteria and neutrophils, and consequential increase in lesion size through synergistic degradative activities from both bacteria and neutrophils, and restoration failure [42].
4.5. Neutrophils, restoration failure and caries
A concentration of 1-10 million neutrophils/mL was selected for the degradation studies as that number of neutrophils enters the oral cavity every minute in gingivitis patients with as many as 10-20 million neutrophils in patients with periodontitis diseases [18]. Based on viability data, neutrophils were replaced every 48 hours to maintain live functional cells and mimic intraoral conditions [43]. Isolated blood neutrophils were used as a model for oral neutrophils. Blood neutrophils are readily available and have well-defined isolation methods [21]. Due to invading microorganisms, oral neutrophils in healthy individuals are increased in activation compared to blood neutrophils, demonstrated by cell membrane markers CD11b, CD63, and CD66b [44]. Oral neutrophils are also able to respond to stimuli, like blood neutrophils [44]. Therefore, the results of the current study using resting blood neutrophils to assess degradative potential towards resin and dentin likely underestimate the effect of activated oral neutrophils in the clinical situation. The intra oral co-existence of cariogenic bacteria and neutrophils could result in exacerbated dentin degradation through complementary actions of acids from the bacteria that would expose demineralized dentinal structure (collagen), and degradative protease activity from neutrophils. Further, esterases from saliva [4], bacteria [3, 6, 28] and neutrophils may contribute synergistically to the degradation of resin and the restoration-tooth interface, thus potentially enhancing failure rate of resin composite restorations [12].
Neutrophils enter the mouth predominantly from the gingival sulcus [45]. Therefore, neutrophils are constantly populating areas adjacent to tooth dentin, root dentin or in the case of gingival proximal (Class II) and cervical (Class V) restorations, resin-dentin interfaces. Additionally, these areas are more prone to acid producing biofilms that demineralize the dentin [46]. In the confined space of the gingival sulcus, there is a relative increased concentration of neutrophils and associated degradative potential. Additionally, in the presence of oral biofilm, the neutrophils become activated to attack the bacteria and release their contents [47]. It is expected that neutrophils stimulated by the bacteria at the resin-dentin interfaces release their contents with a directionality and cause a great impact on the degradation of resin and/or dentin residing at the gingival margins. Degradation of this kind could be a contributor to primary root caries and recurrent caries, the latter being commonly found at the gingival margins of proximal (Class II) and cervical (Class V) restorations in healthy patients [42, 48, 49]. Studies investigating neutrophils degradation of the restoration-tooth could elucidate on the relationship between resin and dentin degradation and interfacial degradation. The degradative potential of neutrophils could also pose a threat to patients with periodontal disease. In individuals with periodontal disease there is an increased recruitment and concentration of neutrophils in the mouth [50]. There is also and increased activation of neutrophils (para and pro inflammatory) [51]. All of these factors suggest that there is a potential for increased neutrophil factors that degrade resin and dentin, providing a possible explanation for increased recurrent and root caries in patients with periodontal disease [46].
5. Conclusions
This study showed that neutrophils have CE-like and collagenolytic MMP-like activities, can degrade methacrylate resin monomer, cured resin composite and demineralized dentin, thus potentially affecting the resin-dentin interface. The results of this study provide a framework for future research, to explore the degradative capability of neutrophils and their potential role in primary and recurrent caries. This will allow for improved preventive measures and restoration quality and longevity, particularly pertaining to gingival proximal and cervical gingival or sub-gingival lesions. Future studies should look at the bond strength between resin and dentin when incubated with neutrophils [10, 11], and the degradative effects of neutrophils on the restoration-tooth interface [5, 9]. To better mimic pathogenic oral conditions, cariogenic and periodontal pathogens should be co-cultured with neutrophils so that the effect of the interactions between the two communities on their degradative activities could be assessed [6, 8, 15, 20, 28].
Statement of significance.
Neutrophils are part of the innate immune system and are constantly entering the oral cavity through the gingival sulcus, in direct contact with the tooth, restoration, restoration-tooth margins and pathogenic bacteria. The current study is the first to characterize and quantify degradative activities from neutrophils toward methacrylate resin and demineralized dentin, the two main components of the restoration-tooth interface, suggesting that this interface could be negatively influenced by neutrophils, potentially contributing to increase in caries formation and progression, and premature restoration failure. This study provides a significant finding to the biomaterials and oral health fields by identifying a potential weakness in current restorative procedures and materials used to manage gingival proximal and cervical gingival or sub-gingival carious lesions.
6.
Funding Sources
The authors declare no conflict of interest. This work was supported by the National Institutes of Health [R01DE021385-0]; the Canadian Institutes of Health Research [MOP 115113]; Canada Foundation for Innovation John R. Evans Leaders Fund (CFI_JELF) [project #35378], and Ministry of Research and Innovation (MRI), Ontario Research Fund (ORF) [ORF-35378].
Footnotes
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study. Data will be made available upon request.
Conflict of Interest Disclosure Statement
It is an independent study free of conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9 References:
- [1].Beazoglou T, Eklund S, Heffley D, Meiers J, Brown LJ, Bailit H, Economic impact of regulating the use of amalgam restorations, Public Health Rep 122(5) (2007) 657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Simecek JW, Diefenderfer KE, Cohen ME, An evaluation of replacement rates for posterior resin-based composite and amalgam restorations in U.S. Navy and marine corps recruits, J Am Dent Assoc 140(2) (2009) 200–9; quiz 249. [DOI] [PubMed] [Google Scholar]
- [3].Bourbia M, Ma D, Cvitkovitch DG, Santerre JP, Finer Y, Cariogenic bacteria degrade dental resin composites and adhesives, J Dent Res 92(11) (2013) 989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Finer Y, Santerre JP, Salivary esterase activity and its association with the biodegradation of dental composites, J Dent Res 83(1) (2004) 22–6. [DOI] [PubMed] [Google Scholar]
- [5].Huang B, Cvitkovitch DG, Santerre JP, Finer Y, Biodegradation of resin-dentin interfaces is dependent on the restorative material, mode of adhesion, esterase or MMP inhibition, Dent Mater 34(9) (2018) 1253–1262. [DOI] [PubMed] [Google Scholar]
- [6].Huang B, Siqueira WL, Cvitkovitch DG, Finer Y, Esterase from a cariogenic bacterium hydrolyzes dental resins, Acta Biomater 71 (2018) 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stewart CA, Hong JH, Hatton BD, Finer Y, Responsive antimicrobial dental adhesive based on drug-silica co-assembled particles, Acta Biomater 76 (2018) 283–294. [DOI] [PubMed] [Google Scholar]
- [8].Marashdeh MQ, Gitalis R, Levesque C, Finer Y, Enterococcus faecalis Hydrolyzes Dental Resin Composites and Adhesives, J Endod 44(4) (2018) 609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kermanshahi S, Santerre JP, Cvitkovitch DG, Finer Y, Biodegradation of resin-dentin interfaces increases bacterial microleakage, J Dent Res 89(9) (2010) 996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Serkies KB, Garcha R, Tam LE, De Souza GM, Finer Y, Matrix metalloproteinase inhibitor modulates esterase-catalyzed degradation of resin-dentin interfaces, Dent Mater 32(12) (2016) 1513–1523. [DOI] [PubMed] [Google Scholar]
- [11].Shokati B, Tam LE, Santerre JP, Finer Y, Effect of salivary esterase on the integrity and fracture toughness of the dentin-resin interface, J Biomed Mater Res B Appl Biomater 94(1) (2010) 230–7. [DOI] [PubMed] [Google Scholar]
- [12].Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, Eslick J, Camarda K, Katz JL, Adhesive/Dentin interface: the weak link in the composite restoration, Ann Biomed Eng 38(6) (2010) 1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jain A, Bahuguna R, Role of matrix metalloproteinases in dental caries, pulp and periapical inflammation: An overview, J Oral Biol Craniofac Res 5(3) (2015) 212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S, The role of matrix metalloproteinases (MMPs) in human caries, J Dent Res 85(1) (2006) 22–32. [DOI] [PubMed] [Google Scholar]
- [15].Marashdeh MQ, Gitalis R, Levesque C, Finer Y, Endodontic pathogens possess collagenolytic properties that degrade human dentine collagen matrix, Int Endod J Accepted (2018). [DOI] [PubMed] [Google Scholar]
- [16].Liu Y, Tjaderhane L, Breschi L, Mazzoni A, Li N, Mao J, Pashley DH, Tay FR, Limitations in bonding to dentin and experimental strategies to prevent bond degradation, J Dent Res 90(8) (2011) 953–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].van Strijp AJ, Jansen DC, DeGroot J, ten Cate JM, Everts V, Host-derived proteinases and degradation of dentine collagen in situ, Caries Res 37(1) (2003) 58–65. [DOI] [PubMed] [Google Scholar]
- [18].Bender JS, Thang H, Glogauer M, Novel rinse assay for the quantification of oral neutrophils and the monitoring of chronic periodontal disease, J Periodontal Res 41(3) (2006) 214–20. [DOI] [PubMed] [Google Scholar]
- [19].Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, Benarafa C, Roos D, Skokowa J, Hartl D, Neutrophils: Between host defence, immune modulation, and tissue injury, PLoS Pathog 11(3) (2015) e1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Borregaard N, Neutrophils, from marrow to microbes, Immunity 33(5) (2010) 657–70. [DOI] [PubMed] [Google Scholar]
- [21].Landzberg M, Doering H, Aboodi GM, Tenenbaum HC, Glogauer M, Quantifying oral inflammatory load: oral neutrophil counts in periodontal health and disease, J Periodontal Res 50(3) (2015) 330–6. [DOI] [PubMed] [Google Scholar]
- [22].Lakschevitz FS, Aboodi GM, Glogauer M, Oral neutrophils display a site-specific phenotype characterized by expression of T-cell receptors, J Periodontol 84(10) (2013)1493–503. [DOI] [PubMed] [Google Scholar]
- [23].Cai K, Delaviz Y, Banh M, Guo Y, Santerre JP, Biodegradation of composite resin with ester linkages: identifying human salivary enzyme activity with a potential role in the esterolytic process, Dent Mater 30(8) (2014) 848–60. [DOI] [PubMed] [Google Scholar]
- [24].Jaffer F, Finer Y, Santerre JP, Interactions between resin monomers and commercial composite resins with human saliva derived esterases, Biomaterials 23(7) (2002) 1707–19. [DOI] [PubMed] [Google Scholar]
- [25].Labow RS, Duguay DG, Santerre JP, The enzymatic hydrolysis of a synthetic biomembrane: a new substrate for cholesterol and carboxyl esterases, J Biomater Sci Polym Ed 6(2) (1994) 169–79. [DOI] [PubMed] [Google Scholar]
- [26].Labow RS, Meek E, Santerre JP, Synthesis of cholesterol esterase by monocyte-derived macrophages: a potential role in the biodegradation of poly(urethane)s, J Biomater Appl 13(3) (1999) 187–205. [DOI] [PubMed] [Google Scholar]
- [27].Lin BA, Jaffer F, Duff MD, Tang YW, Santerre JP, Identifying enzyme activities within human saliva which are relevant to dental resin composite biodegradation, Biomaterials 26(20) (2005) 4259–64. [DOI] [PubMed] [Google Scholar]
- [28].Huang B, Sadeghinejad L, Adebayo OIA, Ma D, Xiao Y, Siqueira WL, Cvitkovitch DG, Finer Y, Gene expression and protein synthesis of esterase from Streptococcus mutans are affected by biodegradation by-product from methacrylate resin composites and adhesives, Acta Biomater 81 (2018) 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Finer Y, Jaffer F, Santerre JP, Mutual influence of cholesterol esterase and pseudocholinesterase on the biodegradation of dental composites, Biomaterials 25(10) (2004) 1787–93. [DOI] [PubMed] [Google Scholar]
- [30].Delaviz Y, Finer Y, Santerre JP, Biodegradation of resin composites and adhesives by oral bacteria and saliva: a rationale for new material designs that consider the clinical environment and treatment challenges, Dent Mater 30(1) (2014) 16–32. [DOI] [PubMed] [Google Scholar]
- [31].MacAulay M, Tam LE, Santerre JP, Finer Y, In Vivo Biodegradation of bisGMA and Urethane-Modified bisGMA-Based Resin Composite Materials, JDR Clinical & Translational Research 2(4) (2017) 397–405. [DOI] [PubMed] [Google Scholar]
- [32].Finer Y, Santerre JP, Influence of silanated filler content on the biodegradation of bisGMA/TEGDMA dental composite resins, J Biomed Mater Res A 81(1) (2007) 75–84. [DOI] [PubMed] [Google Scholar]
- [33].Santerre JP, Shajii L, Leung BW, Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products, Crit Rev Oral Biol Med 12(2) (2001) 136–51. [DOI] [PubMed] [Google Scholar]
- [34].Santerre JP, Shajii L, Tsang H, Biodegradation of commercial dental composites by cholesterol esterase, J Dent Res 78(8) (1999) 1459–68. [DOI] [PubMed] [Google Scholar]
- [35].Roy A, Lushington GH, McGee J, Chaguturu R, Assay technologies for proteases Enzyme technologies., John Wiley & Sons, Inc; (2013) 1–53. [Google Scholar]
- [36].Borregaard N, Cowland JB, Granules of the human neutrophilic polymorphonuclear leukocyte, Blood 89(10) (1997) 3503–21. [PubMed] [Google Scholar]
- [37].Vidal CM, Tjaderhane L, Scaffa PM, Tersariol IL, Pashley D, Nader HB, Nascimento FD, Carrilho MR, Abundance of MMPs and cysteine cathepsins in caries-affected dentin, J Dent Res 93(3) (2014) 269–74. [DOI] [PubMed] [Google Scholar]
- [38].Ballal V, Rao S, Bagheri A, Bhat V, Attin T, Zehnder M, MMP-9 in Dentinal Fluid Correlates with Caries Lesion Depth, Caries Res 51(5) (2017) 460–465. [DOI] [PubMed] [Google Scholar]
- [39].Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S, Collagen degradation by host-derived enzymes during aging, J Dent Res 83(3) (2004) 216–21. [DOI] [PubMed] [Google Scholar]
- [40].Tjaderhane L, Buzalaf MA, Carrilho M, Chaussain C, Matrix metalloproteinases and other matrix proteinases in relation to cariology: the era of 'dentin degradomics', Caries Res 49(3) (2015) 193–208. [DOI] [PubMed] [Google Scholar]
- [41].Ricucci D, Siqueira JF Jr., Loghin S, Berman LH, The cracked tooth: histopathologic and histobacteriologic aspects, J Endod 41(3) (2015) 343–52. [DOI] [PubMed] [Google Scholar]
- [42].Mjor IA, Toffenetti F, Secondary caries: a literature review with case reports, Quintessence Int 31(3) (2000) 165–79. [PubMed] [Google Scholar]
- [43].Kolaczkowska E, Kubes P, Neutrophil recruitment and function in health and inflammation, Nat Rev Immunol 13(3) (2013) 159–75. [DOI] [PubMed] [Google Scholar]
- [44].Rijkschroeff P, Jansen ID, van der Weijden FA, Keijser BJ, Loos BG, Nicu EA, Oral polymorphonuclear neutrophil characteristics in relation to oral health: a cross-sectional, observational clinical study, Int J Oral Sci 8(3) (2016) 191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Delima AJ, Van Dyke TE, Origin and function of the cellular components in gingival crevice fluid, Periodontol 2000 31 (2003) 55–76. [DOI] [PubMed] [Google Scholar]
- [46].Gupta B, Marya C, Juneja V, Dahiya V, Root Caries: An Aging Problem, The Internet Journal of Dental Science 5(1) (2006). [Google Scholar]
- [47].Cortes-Vieyra R, Rosales C, Uribe-Querol E, Neutrophil Functions in Periodontal Homeostasis, J Immunol Res 2016 (2016) 1396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mjor IA, Clinical diagnosis of recurrent caries, J Am Dent Assoc 136(10) (2005) 1426–33. [DOI] [PubMed] [Google Scholar]
- [49].Mjor IA, Qvist V, Marginal failures of amalgam and composite restorations, J Dent 25(1) (1997) 25–30. [DOI] [PubMed] [Google Scholar]
- [50].Hajishengallis E, Hajishengallis G, Neutrophil homeostasis and periodontal health in children and adults, J Dent Res 93(3) (2014) 231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fine N, Hassanpour S, Borenstein A, Sima C, Oveisi M, Scholey J, Cherney D, Glogauer M, Distinct Oral Neutrophil Subsets Define Health and Periodontal Disease States, J Dent Res 95(8) (2016) 931–8. [DOI] [PubMed] [Google Scholar]