Abstract
Background
Cancer sufferers are amongst the most malnourished of all the patient groups. Studies have shown that ghrelin, a gut hormone can be a potential therapeutic agent for cachexia (wasting syndrome) associated with cancer. A variety of mechanisms of action of ghrelin in people with cancer cachexia have been proposed. However, safety and efficacy of ghrelin for cancer‐associated cachexia have not been systematically reviewed. The aim of this review was to assess whether ghrelin is associated with better food intake, body composition and survival than other options for adults with cancer cachexia.
Objectives
To assess the efficacy and safety of ghrelin in improving food intake, body composition and survival in people with cachexia associated with cancer.
Search methods
We searched CENTRAL, MEDLINE and Embase without language restrictions up to July 2017. We also searched for ongoing studies in trials registers, performed handsearching, checked bibliographic references of relevant articles and contacted authors and experts in the field to seek potentially relevant research. We applied no restrictions on language, date, or publication status.
Selection criteria
We included randomised controlled (parallel‐group or cross‐over) trials comparing ghrelin (any formulation or route of administration) with placebo or an active comparator in adults (aged 18 years and over) who met any of the international criteria for cancer cachexia.
Data collection and analysis
Two review authors independently assessed studies for eligibility. Two review authors then extracted data and assessed the risk of bias for individual studies using standard Cochrane methodology. For dichotomous variables, we planned to calculate risk ratio with 95% confidence intervals (CI) and for continuous data, we planned to calculate mean differences (MD) with 95% CI. We assessed the evidence using GRADE and created 'Summary of findings' tables.
Main results
We screened 926 individual references and identified three studies that satisfied the inclusion criteria. Fifty‐nine participants (37 men and 22 women) aged between 54 and 78 years were randomised initially, 47 participants completed the treatment. One study had a parallel design and two had a cross‐over design. The studies included people with a variety of cancers and also differed in the dosage, route of administration, frequency and duration of treatment.
One trial, which compared ghrelin with placebo, found that ghrelin improved food intake (very low‐quality evidence) and had no adverse events (very low‐quality evidence). Due to unavailability of data we were unable to report on comparisons for ghrelin versus no treatment or alternative experimental treatment modalities, or ghrelin in combination with other treatments or ghrelin analogues/ghrelin mimetics/ghrelin potentiators. Two studies compared a higher dose of ghrelin with a lower dose of ghrelin, however due to differences in study designs and great diversity in the treatment provided we did not pool the results. In both trials, food intake did not differ between participants on higher‐dose and lower‐dose ghrelin. None of the included studies assessed data on body weight. One study reported higher adverse events with a higher dose as compared to a lower dose of ghrelin.
All studies were at high risk of attrition bias and bias for size of the study. Risk of bias in other domains was unclear or low.
We rated the overall quality of the evidence for primary outcomes (food intake, body weight, adverse events) as very low. We downgraded the quality of the evidence due to lack of data, high or unclear risk of bias of the studies and small study size.
Authors' conclusions
There is insufficient evidence to be able to support or refute the use of ghrelin in people with cancer cachexia. Adequately powered randomised controlled trials focusing on evaluation of safety and efficacy of ghrelin in people with cancer cachexia is warranted.
Plain language summary
Ghrelin, a hunger hormone for management of cancer patients with loss of appetite and weight loss
Bottom line
There is no good evidence to support or reject the suggestion that ghrelin is useful in the management of cancer patients with loss of appetite and weight loss. There is insufficient evidence to recommend it for clinical practice.
Background
Sixty to eighty percent of cancer patients suffer from loss of appetite and weight loss, which in turn is associated with decreased life expectancy and quality of life. Ghrelin, a hunger hormone is secreted by the stomach and other organs of the body. Studies have shown that ghrelin can be used in treatment for loss of appetite and weight loss in cancer patients. However, the effectiveness and safety of ghrelin in such people have not been assessed. In this review we set out to examine all evidence on the effectiveness and safety of ghrelin in improving appetite and body weight in cancer patients with loss of appetite and weight loss.
Search date
The evidence is current to 20th July 2017.
Study characteristics
We found three studies that recruited a total of 59 cancer patients (37 men and 22 women) aged between 54 and 78 years. Forty‐seven cancer patients completed the treatment. Studies differed in study design and included people with a variety of cancers. Studies also differed in dosage, route of injection, frequency and duration of treatment. One study compared ghrelin with a placebo while two studies compared different doses of ghrelin (higher dose with lower dose). Outcomes of interest to cancer patients with loss of appetite and weight loss, such as improvement in food intake and improvement in body weight, were not adequately reported.
All three included studies were funded by government agencies. One study received an additional grant from a pharmaceutical company.
Key findings
We found insufficient evidence that using ghrelin demonstrated differences in food intake. We found no evidence that using ghrelin alone or in combination made any difference to body weight. We could not reach any conclusions about its side effects. The limited amount of information means that we could not draw any conclusions.
Quality of the evidence
We rated the quality of evidence from studies using four levels: high, moderate, low, or very low. High‐quality evidence means that we are very confident in the results. Very low‐quality evidence means that we are very uncertain about the results. The evidence in this review was of very low quality.
Summary of findings
Background
This review is partly based on suggested wording from Cochrane Pain, Palliative and Supportive Care (PaPaS).
Description of the condition
Cancer and its co‐morbidities like cancer cachexia (muscle wasting) have afflicted humans for centuries and still continue to be a major public health problem that profoundly affects more than 1.6 million people each year (National Cancer Institute 2015). People suffering from cancer are amongst the most malnourished of all the patient groups (Ryan 2016). It has been estimated that cachexia affects 60% to 80% of all advanced cancer patients (Baracos 2011) and more than 30% of patients die due to cachexia (von Haehling 2012). Cancer cachexia is commonly associated with poor quality of life (Fearon 2012; Loumaye 2017; Utech 2012).
Cancer cachexia is defined as "a multifactorial syndrome characterised by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment" (Fearon 2011). Cachexia syndrome can develop progressively, through stages of pre‐cachexia to cachexia to refractory cachexia (Fearon 2011). The incidence of cancer cachexia varies according to tumor type (Teunissen 2007; Tisdale 2009; Sun 2015). The prevalence of cachexia is highest in people with pancreatic cancer (88.9%), followed by gastric cancer (76.5% to 87%) and oesophageal cancer (52.9%) (Sun 2015; Tisdale 2009). The frequency of weight loss is lowest in patients with breast cancer, sarcomas, non‐Hodgkin's lymphoma and acute nonlymphocytic leukaemia (Teunissen 2007; Tisdale 2009). Although certain tumor types are more commonly associated with cachexia, even with the same tumor type there are variations in the extent to which people exhibit cachexia (Tisdale 2009). Cachexia can be a presenting symptom in a majority of people with advanced cancer, mainly those with hepatic, lung, or bone metastasis and primary cancers of the lung, cervix or head and neck (Mendes 2015). Cancer patients with muscle wasting are less able to tolerate chemotherapy, have poor treatment outcomes, and have shorter survival (Crawford 2015).
Extensive research has been carried out to understand the complex pathophysiology of cachexia associated with cancer (Bennani‐Baiti 2008; Mendes 2015; Ohnuma 2017; Penna 2016; Tisdale 2009). However, precise molecular mechanisms of cancer cachexia still remain poorly characterised (Loumaye 2017). The pathogenesis of cancer anorexia is multifactorial. It is suggested to be the result of tumour‐host interactions (Bennani‐Baiti 2008). Anorexia, anaemia, asthenia, inflammation, altered hormonal homeostasis, energy imbalance, perturbations in proinflammatory cytokines, impaired immunity and several cancer‐related metabolic changes (like negative protein balance and increased lipolysis) leading to significant weight loss have been attributed to the pathogenesis of cancer cachexia (Bennani‐Baiti 2008; Mendes 2015; Penna 2010; Stephens 2008). Therapies for cancer, such as chemotherapy, surgery and radiotherapy, also cause anorexia, muscle atrophy and weight loss (Chen 2015; Garcia 2005; Tisdale 2009). Deregulation of control of energy expenditure and hunger/satiety by the hypothalamus promotes cachexia in cancer patients (Mendes 2015). A discrepancy between anabolic and catabolic pathways mediated by chronic inflammation can cause muscle wasting in people with cancer cachexia (Madeddu 2015a). Depletion of adipose tissue as well as skeletal muscle mass with relative preservation of non‐muscle protein compartment can contribute to weight loss in cancer patients (Tisdale 2009). Studies suggest that the tumour cells secrete certain humoral factors that promote central and peripheral‐mediated cancer cachexia (Stewart 2006; Ohnuma 2017). Cachectic factors (like activin and proteolysis‐inducing factor) secreted by tumour cells decrease the synthesis and increase the breakdown of muscle proteins, and thereby induce sarcopenia (Stewart 2006). Excretion of cytokines and lipid‐mobilising factors may contribute to depletion of adipose tissue (Stewart 2006). Inflammation induced by host‐derived and tumor‐derived factors seems to be vital in the development of cancer cachexia (Loumaye 2017). Tumor cells secrete pro‐inflammatory factors that promote cachexia by signalling anorexia, wasting of muscles and atrophy of adipose tissue (Chen 2016; Johns 2013; Loumaye 2017). Release of inflammatory cytokines like TNF‐alpha (cachexin or cachectin), interferon gamma, interleukin‐6 and angiotensin II also have a role in cancer cachexia (Bennani‐Baiti 2008; Johns 2013; Ohnuma 2017; Tisdale 2009).
As cachexia progresses, wasting of skeletal muscles limits mobility and thereby leads to poor quality of life, which in turn pushes cancer patients towards isolation and depression (Stewart 2006; Watanabe 1996; Windsor 1988). Patients as well as family members, especially caregivers and healthcare professionals often suffer from depression as they try to palliate the symptoms (National Cancer Institute 2015; Reid 2012). Although cancer cachexia is associated with increased mortality and poor quality of life, treatment options for the condition are limited (Penna 2016). It has been proposed that multimodal strategies with anabolic focus ensuring sufficient energy and protein intake, pharmacological treatments, and non‐pharmacological therapies like physical training initiated early in the disease may provide benefit to people with cancer cachexia (Aapro 2014; Anderson 2017; Argilés 2017; Lucia 2012; Madeddu 2015a).
Description of the intervention
As cancer cachexia is associated with complex pathophysiological processes, pharmacologic treatment with potential orexigenic, anabolic and anti‐inflammatory effects should be targeted to counter this condition (Madeddu 2015b). Caloric supplementation or appetite stimulants like megestrol acetate, medroxyprogesterone acetate (MPA), cyproheptadine, marijuana, and corticosteroids such as dexamethasone, prednisolone and methylprednisolone have been used for enhancing appetite in people with cancer (Fearon 2011; Tisdale 2009). However, these interventions have limited efficacy. No definitive pharmacological treatment is available to address the relevant components of the cancer cachexia syndrome (Esposito 2015). Studies in animals and humans have shown that ghrelin, a gut hormone can be a potential therapeutic agent for treatment of cachexia associated with cancer (Chen 2015; Hatanaka 2015; Lundholm 2010; Neary 2004; Strasser 2008; Tsubouchi 2014).
Ghrelin, a 28‐amino acid peptide hormone, is an endogenous ligand for growth hormone secretagogue receptor (Khatib 2014f; Kojima 1999). This orexigenic gut hormone is primarily secreted by the endocrine X/A‐like cells of the stomach mucosa and also by intestinal mucosa, kidney, placenta, arcuate nucleus of the hypothalamus, pituitary gland, pancreatic islets, and other tissues (Gualillo 2001; Khatib 2015b; Kojima 1999; Korbonits 2001; Mori 2000; Van der Lely 2004; Volante 2002). It circulates in the blood stream under fasting conditions, indicating that it transmits the hunger signals from periphery to the central nervous system (Kojima 2008). Ghrelin plays an important role in regulation of release of growth hormone and energy homeostasis (Khatib 2014c; Kojima 1999; Kojima 2008). It has the potential to increase body weight and body composition through increased appetite, increased growth hormone (GH) secretion, prevention of muscle catabolism, promotion of gut motility, stimulation of gastric acid secretion, and regulation of metabolism (Chen 2012; Chen 2015; DeBoer 2007; Fujitsuka 2009; Fujitsuka 2011; Garcia 2005; Garcia 2013; Khatib 2014a; Khatib 2014d; Khatib 2014f; Khatib 2014e; Klok 2007; Lundholm 2010; Müller 2015; Neary 2004; Strasser 2008; Tsubouchi 2014; Wren 2001). These diverse actions of ghrelin raise the possibility of its clinical application for conditions like anorexia, cachexia, sarcopenia, gastrointestinal diseases, cardiovascular diseases, renal and pulmonary diseases, neurodegenerative disorders, inflammatory disorders and metabolic syndromes (Colldén 2017; Khatib 2014b; Khatib 2015a). It disturbs the vicious cycle of cachexia through its anabolic, orexigenic, and anti‐inflammatory effects (Müller 2015). Ghrelin regulates metabolism through activation of orexigenic neural circuits (Müller 2015). Studies have found that ghrelin is well‐tolerated and has no reported major adverse events (Akamizu 2010; Hiura 2012; Khatib 2015a; Neary 2004). Ghrelin agonists are being developed and tested for the treatment of anorexia/cachexia (Bai 2017; Currow 2017; Garcia 2015; Northrup 2013; Pietra 2014; Temel 2016; Zhang 2015).
Due to these and numerous other benefits of ghrelin, pharmacological targeting of the endogenous system of ghrelin is considered a promising and valuable approach for treatment of a variety of metabolic complications including cachexia associated with cancer (Colldén 2017; Hatanaka 2015; Ledderose 2011).
How the intervention might work
Although the mechanisms of action of ghrelin have not been fully elucidated, an increase in appetite (Cummings 2006; Wren 2001), decrease in energy expenditure (Garcia 2013; Murphy 1998), promotion of anabolic activity (Chen 2015), decrease in inflammation (Dixit 2004; Tsubouchi 2014), increase in growth hormone (Garcia 2009; Khatib 2014a; Khatib 2014d; Khatib 2014f), control of gastrointestinal motility (Fujino 2003), and effects in adipose tissue (Kos 2009) and skeletal muscle (Porporato 2013; Tsubouchi 2014) have been proposed.
Ghrelin and synthetic ghrelin receptor agonists cause weight gain by increasing food intake and by food intake‐independent mechanisms (Garcia 2007; Garcia 2013; Sugiyama 2012; Tschop 2000). Ghrelin is believed to be the only mammalian hormone that has been shown to increase appetite and food intake when delivered to humans (Neary 2004; Wren 2001). Administration of ghrelin increases appetite rapidly and briefly, principally by increasing appetitive feeding behaviours and number of meals (Cummings 2006; Faulconbridge 2003). Evidence implicates ghrelin in mealtime hunger and meal initiation (Cummings 2006). Circulating levels of ghrelin rise before meals and fall with feeding, attaining adequate concentrations to stimulate appetite and food intake (Cummings 2006). Pre‐prandial surges in ghrelin levels are possibly initiated by sympathetic nervous output while post‐prandial suppression is possibly mediated from post‐ingestive surges in insulin levels (Cummings 2001; Cummings 2006; Tschop 2000). As the growth hormone secretagogue receptors (GHS‐R) are expressed in vagal afferent neurons, the gastric vagus nerve may be involved in the effect of ghrelin on food intake and gastrointestinal motility (Date 2002). Ghrelin increases appetite through afferent vagal fibres to the caudal brainstem or directly to the hypothalamus (Suzuki 2010). Gastric ghrelin signalling via vagal afferents suppresses the activity of the sympathetic nerves and increases the discharge of both the gastric and the vagus efferent nerves (Fujitsuka 2009). Ghrelin has also been shown to promote faster motor activity by activating neuropeptide Y (NPY) neurons in the brain (Fujino 2003). Furthermore, ghrelin modulates taste sensation (Shin 2010) and acts on the stomach to enhance gastric acid secretion (Masuda 2000).
Ghrelin controls mediators involved in the cachectic process (Argilés 2013). Ghrelin promotes weight gain and lean body mass via anti‐inflammatory actions and effects involving orexigenic peptides (DeBoer 2007; Khatib 2014c; Khatib 2015b; Lin 2017). Ghrelin inhibits pro‐inflammatory cytokines such as IL‐1alpha, IL‐1beta, TNF‐alpha, which may cause anorexia (Guney 2007; Lin 2017). Animals treated with ghrelin exhibited decreased expression of IL‐1 receptor‐I transcript in the hypothalamus and brainstem and an increased expression of orexigenic peptides and NPY in the hypothalamus (DeBoer 2007; Tisdale 2009). Ghrelin inhibits the production and prevents the increase of pro‐inflammatory cytokines released by the tumour cells (Chen 2015; Dixit 2004; Tsubouchi 2014). Activation of the ghrelin receptor in the central nervous system releases growth hormone, which regulates insulin‐like growth factor‐1 (IGF‐1) (Khatib 2014a; Khatib 2014f; Velloso 2008). Growth hormone/IGF‐1 axis acts directly on bone, muscle and fat tissue, and also indirectly by producing anti‐cachectic cytokines and muscle‐restricted insulin‐like growth factor‐1 (mIGF‐1) (Fuoco 2015). Administration of ghrelin has been shown to prevent muscle atrophy by down‐regulating inflammation and activating protein kinase B (a protein kinase that plays a key role in apoptosis, cell proliferation, and cell migration), myogenin (a transcription factor involved in myogenesis and repair) and myoD (a protein that plays a major role in regulating muscle differentiation) (Chen 2015). Both acytylated and unacytylated ghrelin block skeletal muscle atrophy in a growth hormone‐independent manner (Porporato 2013). In‐vitro studies have demonstrated that ghrelin may regulate mesenchymal cell development by stimulating myogenesis (Zhang 2007). Cells expressing ghrelin have demonstrated a significant increase in the differentiation of premyocytes into myocytes (Zhang 2007). Short‐term administration of ghrelin at the start of cisplatin‐based chemotherapy has been shown to increase the efficiency of chemotherapy and also minimise adverse events associated with chemotherapy (Hiura 2012).
Ghrelin analogues can be viable treatment modalities for cancer‐associated cachexia. Anamorelin, a first‐in‐class, potent, orally‐active and highly‐specific ghrelin‐receptor agonist increases food intake, body weight, lean body mass and improves quality of life in people with cancer cachexia with good tolerability and no dose‐limiting toxicities (Bai 2017; Currow 2017; Garcia 2009; Garcia 2015; Northrup 2013; Pietra 2014; Temel 2016; Zhang 2015;). Oral administration of rikkunshito, a ghrelin enhancer, increases plasma acyl ghrelin levels in humans, mice, rats and dogs (Fujitsuka 2009; Fujitsuka 2011; Fujitsuka 2014; Takeda 2008, Shin 2010). Administration of RC‐1291, a ghrelin mimetic, has been shown to increase lean body mass in cachectic cancer patients (Garcia 2007).
Why it is important to do this review
Despite high prevalence of cancer cachexia, effective therapies are still limited and no definitive pharmacological treatment is available to address the relevant components of this syndrome (Esposito 2015). There is a strong need for more effective appetite‐stimulatory therapies for people with this condition. Several studies have demonstrated positive and encouraging effects of ghrelin or growth hormone secretagogues (GHS) in people with cancer cachexia (Lundholm 2010; Molfino 2014; Neary 2004; Strasser 2008). However, the safety and efficacy of ghrelin for cancer‐associated cachexia have not been systematically reviewed. There is a need to synthesise the evidence for patients, practitioners and policy makers to decide whether ghrelin can be incorporated in the management of cachexia associated with cancer and, if data permit, to explore the optimal drug programme for this group of patients. Therefore, systematic evaluation of the role of ghrelin in the treatment of cancer cachexia is warranted.
Objectives
To assess the efficacy and safety of ghrelin in improving food intake, body composition and survival in people with cachexia associated with cancer.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs) with open or blinded assessment of outcomes. We required full journal publication with the exception of extended abstracts of otherwise unpublished clinical trials. We excluded short abstracts (usually meeting reports), non‐randomised studies, studies of experimental pain, studies done on animal models, case reports, and clinical observational studies.
Types of participants
We included cachectic patients of 18 years and over with a histological or clinical diagnosis of cancer, or meeting any of the international criteria for cancer cachexia (Bozzetti 2009; Fearon 2006; Fearon 2011). We included both inpatients and outpatients with any type or stage of cancer irrespective of gender or race. We included patients in any healthcare setting (including hospice, hospital, oncology centre or community).
Types of interventions
We expected studies to vary in terms of form, dose, frequency, route and duration of treatment. We included studies in which ghrelin was administered in any form, at any dose, at any frequency, by any route and for any duration, administered for improving food intake, body composition and survival, compared to placebo or no treatment or any active comparator (such as appetisers, nutritional supplements, ghrelin analogues/ghrelin mimetics, ghrelin potentiators/enhancers, etc.). Studies examining ghrelin offered as a sole intervention or in combination with another intervention were eligible.
We had planned to undertake five comparisons. We added a sixth comparison group in this review: higher‐dose ghrelin versus lower‐dose ghrelin, as we found two studies addressing this. We thought it wiser to include this comparison rather than miss data on an intervention.
Ghrelin versus placebo
Ghrelin versus no treatment
Ghrelin versus alternative experimental treatment modality (like appetisers, nutritional supplements, etc.)
Ghrelin in combination with other treatments versus ghrelin treatment alone
Ghrelin treatment versus ghrelin analogues/ghrelin mimetics (anamorelin, ipamorelin, eganamorelin, hexarelin, MK‐677, etc.) or ghrelin potentiators/enhancers (rikkunshito)
Higher‐dose ghrelin versus lower‐dose ghrelin
Types of outcome measures
Primary outcomes
Change in food intake as difference between baseline and the end of treatment. We planned to express this outcome as a dichotomous variable (number of participants who experienced an increase in food intake) or a continuous variable (actual change in food intake).
Change in body weight as difference between baseline and at the end of treatment. We planned to express this outcome as a dichotomous variable (number of participants who experienced change in body weight) or a continuous variable (actual change in body weight).
Adverse events as the number of participants who suffered an event described as an adverse event by the authors of the studies.
Secondary outcomes
Change in survival measured as increase in survival in days. We planned to use hazard ratios for how many times more (or less) likely a participant was to suffer the event at a particular point in time if they received ghrelin rather than the control intervention.
Change in body composition (lean body mass, fat mass) as difference between baseline and the end of treatment. We planned to express this outcome as a dichotomous variable (number of participants who experienced change in body weight) or a continuous variable (actual change in body composition).
Plasma ghrelin levels as difference between baseline and the end of treatment. We planned to express this outcome as a dichotomous variable (number of participants who experienced increase in plasma ghrelin levels) or a continuous variable (actual change in plasma ghrelin levels).
Change in quality of life using any validated scale (CDC 2016).
Reporting of these outcome measures did not form part of the criteria for including studies in a review.
Search methods for identification of studies
Electronic searches
We searched the following databases without language restrictions.
The Cochrane Central Register of Controlled Trials (CENTRAL; 2017, issue 6) in the Cochrane Library
MEDLINE (via Ovid) searched 1947 to 20 July 2017
Embase (via Ovid) searched 1974 to 20 July 2017
We used Medical subject headings (MeSH) or equivalent and text‐word terms. We obtained full‐text translations of all relevant non‐English articles and tailored the searches to individual databases. The search strategies used can be found in Appendix 1.
Searching other resources
We searched the metaRegister of controlled trials (mRCT) (www.controlled-trials.com/mrct), National Cancer Institute (www.cancer.gov/clinicaltrials), clinicaltrials.gov (www.clinicaltrials.gov), and the WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) for ongoing trials in July 2017. We also searched ClinicalStudyResults.org (www.clinicalstudyresults.org) for clinical trials.
In addition, we checked the reference lists of reviews, retrieved articles for additional studies, and performed citation searches on key articles. We contacted study authors where necessary for additional information. We searched the British Association for Cancer Research (BACR), Society on Sarcopenia, Cachexia and Wasting Disorders (SCWD), the American Cancer Society. We contacted experts in the field for unpublished and ongoing trials to identify any additional literature related to the review.
Data collection and analysis
Selection of studies
Two review authors (MNK, SG) independently screened the articles retrieved from the searches using the Rayyan online screening tool (Elmagarmid 2014) and determined eligibility by reading the abstract of each study identified by the search. Review authors eliminated studies that clearly did not satisfy inclusion criteria, and obtained full copies of the remaining studies. Two review authors (ZQS, AG) screened full texts of these studies independently to select relevant studies. We contacted study authors by email, if missing information impaired the study selection, to clarify the necessary information. In the event of disagreement, a third author adjudicated (AS). We did not anonymise the studies in any way before assessment. We applied no language restrictions in the selection of studies. We included a PRISMA flow chart in the full review that shows the status of identified studies (Moher 2009) as recommended in Part 2, Section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We included studies in the review irrespective of whether measured outcome data are reported.
Data extraction and management
Two review authors (MNK, SG) independently extracted the data using a standard form and checked for agreement before entry into Review Manager 5 (RevMan 5) (RevMan 2014). We included information about all the primary outcomes. We collated multiple reports of the same study, so that each study rather than each report is the unit of interest in the review. We collected characteristics of the included studies in sufficient detail to populate a table of ‘Characteristics of included studies’.
Assessment of risk of bias in included studies
This section is taken from the PaPaS template for 'reviews'.
Two authors (MNK, PS) independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We completed a 'Risk of bias' table for each included study using the 'Risk of bias' tool in RevMan 5 (RevMan 2014).
We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table, computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes); unclear risk of bias (method not clearly stated).
Blinding of participants and personnel (checking for performance bias). We assessed the methods used to blind study participants and personnel about the receipt of the intervention. We assessed the methods as: low risk of bias (study states that it was blinded and describes the method used to achieve blinding); unclear risk of bias (study states that it was blinded but does not provide an adequate information of how it was done); high risk of bias (no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; or blinding of participants of the study and personnel was attempted, but it is likely that the blinding could have been broken).
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study states that it was blinded and describes the method used to achieve blinding, e.g. identical tablets, matched in appearance and smell); unclear risk of bias (study states that it was blinded but does not provide an adequate description of how blinding was achieved).
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (< 10% of participants did not complete the study and/or used ‘baseline observation carried forward’ analysis); unclear risk of bias (used 'last observation carried forward' analysis); high risk of bias (used 'completer' analysis).
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (≥ 200 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (< 50 participants per treatment arm).
Selective outcome reporting (checking for reporting bias). We assessed studies as being at low risk of bias (the study protocol is available; or the study protocol is not available but the study reported all expected and pre‐specified outcomes); high risk of bias (the study reported one or more outcomes of interest incompletely); unclear risk of bias (the study provides insufficient information to permit judgement of ‘low risk’ or ‘high risk’).
Other bias. We assessed the study as: low risk of bias (the study appears to be free of other sources of bias); unclear risk of bias (there may be a risk of bias, but there is insufficient information to judge whether risk of bias exists; or insufficient evidence that a problem under consideration will introduce bias); or high risk of bias (there is at least one important risk of bias).
Measures of treatment effect
We planned to record number of events and total number of participants in both the arms for dichotomous variables. For continuous variables we planned to record means with standard deviations and total number of participants in both the arms. We planned to summarise each study in our meta‐analysis using risk ratio (RR) or odds ratio (OR) with 95% confidence intervals (CI) for dichotomous variables and mean difference (MD) with 95% CI for continuous variables, then pool these effect estimates in a meta‐analysis. For continuous data we planned to use mean differences (MD) with 95% CI when the results were measured in the same way in different studies. We planned to use standardised mean differences (SMD) when the results obtained were conceptually the same but used different measurement scales. We planned to use a fixed‐effect model or random‐effects model to estimate the overall direction, size and consistency of an effect and obtain the change in standard deviation from CI, standard errors, t values, P values or F values (whichever was available) using the RevMan 5 calculator. If there was not enough information available to calculate the standard deviations for the changes, we planned to impute the values of the same. If the required data were not available, we planned to use a comparison of final measurements. We planned to evaluate the direction and size of the effect as well as looking at the consistency of the effect across the selected studies. We planned to consider clinically meaningful change taking into account the change in weight and appetite with a measurable entity and time span.
Unit of analysis issues
In parallel‐group RCTs, we considered an individual participant as the unit of analysis. When incorporating cross‐over trials into a meta‐analysis, we would have followed the approach suggested by Elbourne (Elbourne 2002). We would have incorporated these trials by taking measurements from experimental intervention periods and measurements from control intervention periods and analysing these as if the trial were a parallel group trial of intervention versus control. If carry‐over was thought to be a problem, we would have included only data from the first period. We would have included the effect estimate of cross‐over trials in meta‐analysis using the generic inverse‐variance method.
Dealing with missing data
If enough studies were available; we would have carried out an intention‐to‐treat analysis. We asked for further information from the authors or manufacturers when published data were missing, incomplete or inconsistent with RCT protocols. We contacted authors by email if studies did not report the outcome measures of interest, did not describe randomisation or intention‐to‐treat analysis or had missing data.
Assessment of heterogeneity
We planned to assess clinical heterogeneity by using the Chi2 test (P value < 0.10 for statistical significance) and use the I2 statistic to quantify heterogeneity. We would have regarded heterogeneity as considerable if I2 was more than 75%; substantial if it was between 50% and 90%; moderate if it was between 30% and 60% and mild if less than 40% (Deeks 2011). If we had identified statistical heterogeneity (I² greater than, or equal to 50%); we would have reported it and explored possible causes by prespecified subgroup analysis, and would have applied a random‐effects model.
Assessment of reporting biases
If there would have been 10 or more included studies; we planned to conduct a funnel plot test for asymmetry to assess for any evidence of reporting bias. Additionally; we had planned to explore the possible sources of asymmetry in a funnel plot.
Data synthesis
We planned to undertake a meta‐analysis only if participants, interventions, comparisons and outcomes were judged to be sufficiently similar to ensure an answer that is clinically meaningful and relevant. For analysis, we planned to use RevMan 2014, the statistical package provided by the Cochrane Collaboration.
If statistical heterogeneity (I² greater than, or equal to 50%) was detected, we would have identified the sources of the heterogeneity and would have performed subsequent meta‐analysis using a random‐effects model. When meta‐analysis seemed inappropriate, we did not pool the results of the included studies, but presented a qualitative description of these studies with supporting tables. We planned to perform a meta‐analysis using a fixed‐effect model if there were sufficient and homogeneous data with consistent or comparable outcomes.
‘Summary of findings' tables
We included 'Summary of findings' (SoF) tables as set out in the PaPaS author guide (AUREF 2012) and recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We have presented the SoF tables under the following comparisons for all of the primary outcomes:
Ghrelin versus placebo
Ghrelin versus an alternative experimental treatment modality
Ghrelin in combination with other treatments versus ghrelin treatment alone
Higher‐dose ghrelin versus lower‐dose ghrelin
Two review authors (ZQS, MNQ) assessed the overall quality of the evidence for each of the primary outcome using the GRADE system (GRADEpro GDT 2015) and presented the findings in the 'Summary of findings' tables. In particular, we included key information concerning the quality of evidence. We planned to include the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes.
We decreased the grade rating if there was:
A serious (‐1) or very serious (‐2) limitation to study quality;
Important inconsistency (‐1);
Some (‐1) or major (‐2) uncertainty about directness;
Imprecise or sparse data (‐1);
High probability of reporting bias (‐1).
We have justified all decisions to downgrade the quality of studies using footnotes.
Subgroup analysis and investigation of heterogeneity
We planned to undertake subgroup analyses for form, dose, duration and route of administration of ghrelin, and for different types of cancer.
Results
Description of studies
Results of the search
The electronic literature searches identified 1484 potential articles. Following the removal of duplicates, we independently examined 926 papers and after initial screening, we removed 892 papers. We then independently assessed the full text of 34 potentially relevant papers. We excluded 31 papers on the basis of different population (participants other than those with cancer cachexia: n = 5, animal models: n = 11), different intervention (intervention other than ghrelin: n = 14) and different study design (n = 1). Reasons for rejecting individual studies are detailed in the 'Characteristics of excluded studies' table. We identified three studies for potential inclusion (Lundholm 2010; Neary 2004; Strasser 2008) but none of the studies could be included in quantitative synthesis (meta‐analysis). We have presented a flow diagram detailing the selection of studies in Figure 1.
1.
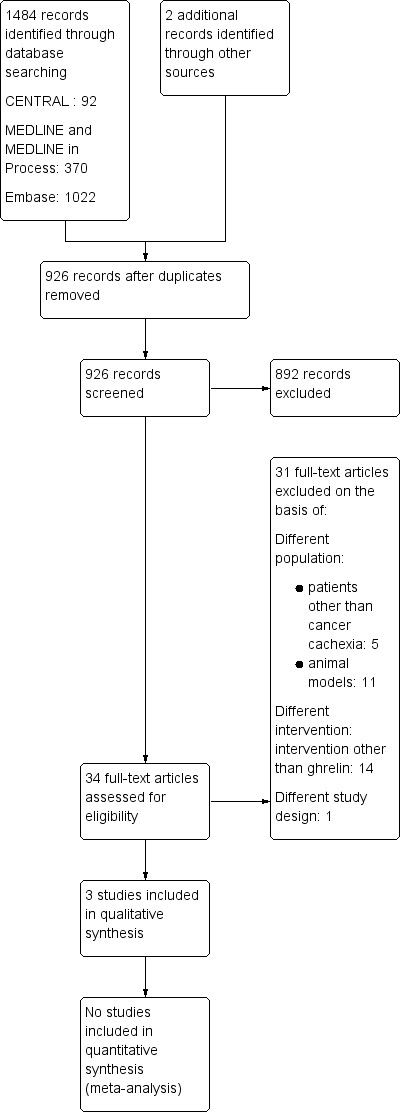
Study flow diagram
Included studies
In total, we identified three studies for inclusion (Lundholm 2010; Neary 2004; Strasser 2008). The included studies were conducted in Sweden (Lundholm 2010), the UK (Neary 2004) and Switzerland (Strasser 2008). All studies were randomised and double‐blind. Neary 2004 and Strasser 2008 were cross‐over clinical trials while Lundholm 2010 was a parallel‐group clinical trial. The cross‐over clinical trials did not report washout between treatment phases. All articles were written in English. Full details can be found in the Characteristics of included studies table.
Participants
The number of participants recruited were 31 in Lundholm 2010, seven in Neary 2004 and 21 in Strasser 2008. One of the seven participants failed to complete a food diary in Neary 2004. The number of participants who completed the study were 22 in Lundholm 2010 and 18 in Strasser 2008. All studies included people with cancer with weight loss and anorexia.
Lundholm 2010 randomised 18 men and 13 women suffering from progressive, weight‐losing gastrointestinal cancer with systemic spread into high‐dose ghrelin group (HDGG) and low‐dose ghrelin group (LDGG). The mean age of the participants in the two groups was 73 ± 2 years in HDGG and 76 ± 2 years in LDGG. The male:female ratio in the study for HDGG was 11 to 6 and for LDGG was 7 to 7. This study excluded participants aged under 40 years, people with diabetes (type I or II), people on steroid treatment, or any other treatment known or assumed to affect tumor host metabolism, as well as people receiving any nutritional support.
Neary 2004 randomised one man and six women with cancer with sustained anorexia and weight loss. Five participants had breast cancer, one participant had colon cancer and one malignant melanoma. All participants had metastasis. Two of the seven participants in Neary 2004 were on chemotherapy. The median age of participants was 54 years. The study excluded people who had undergone surgery or radiotherapy, and were on regular use of systemic steroids or progestins.
Strasser 2008 randomised 17 men and three women with far‐advanced, incurable cancer and involuntary loss of weight and appetite into two groups, a higher‐dose ghrelin group (HDGG) and a lower‐dose ghrelin group (LDGG). The participants had different cancer types including: pancreatic cancer (n = 4), mesothelioma (n = 2), prostate cancer (n = 3), colorectal cancer (n = 4), stomach/oesophageal cancer (n = 2), non‐small‐cell lung carcinoma (n = 3) , urogenital cancer (n = 1), and cholangiocarcinoma (n = 1). The median age of participants in the two groups was 66 years in LDGG and 70 in HDGG. This study excluded people receiving enteral or parenteral nutrition, who had significant cause of secondary anorexia and who required new systemic antineoplastic treatment for the study period of three weeks.
Intervention
One trial (Neary 2004) compared the effect of ghrelin with placebo while two trials (Lundholm 2010; Strasser 2008) studied the effect of two different doses (higher‐dose with lower‐dose) of ghrelin.
Lundholm 2010 randomised the participants into two groups: HDGG and LDGG. Participants randomised to HDGG received ~10 µg/kg daily and those randomised to LDGG received 0.5 µg/kg daily for eight weeks as once‐daily, subcutaneous injections, 30 to 45 minutes before the main daily meal. The amounts provided corresponded to 13 µg/kg daily (HDGG) or 0.7 µg/kg (LDGG) daily to account for individual body weight based on 56 doses for each group over eight weeks.
In Neary 2004 the participants were given two infusions at least three days apart (range: 3 to 21 days), one day for ghrelin and one day for saline. Participants on chemotherapy received the first infusion at least 17 days after their chemotherapy cycle and then the same number of days after their subsequent chemotherapy cycle. Ghrelin or saline infusion was administered over 90 minutes at the rate of 5 pmoL/kg/minute.
Strasser 2008 conducted a two‐week trial of ghrelin infusion, four to five days after baseline at one of two dose levels HDGG and LDGG, intravenous infusion over 60 minutes, once weekly on days one and eight and placebo on days four and 11 or vice versa, that is, three days apart. The LDGG received 10 pmoL/kg/minute (approximately 2 µg/kg). After observing treatment tolerance in the LDGG participants, the investigators administered the HDGG 40 pmoL/kg/minute (approximately 8 µg/kg). Normal saline was used as placebo.
Comparison
Neary 2004 compared ghrelin with placebo. Two trials (Lundholm 2010; Strasser 2008) compared higher‐dose of ghrelin with lower‐dose of ghrelin.
None of the trials compared ghrelin with no treatment or alternative experimental treatment modality (like appetisers, nutritional supplements, etc.) or ghrelin analogues/ghrelin mimetics (anamorelin, ipamorelin, eganamorelin, hexarelin, MK‐677, etc.) or ghrelin potentiators/enhancers (rikkunshito). None of the included trials compared ghrelin in combination with other treatments versus ghrelin treatment alone.
Outcomes
Primary outcomes
All the included trials assessed food intake and adverse events while none of the trials measured body weights. Lundholm 2010 reported the food intake at baseline and after eight weeks in two groups while Strasser 2008 reported the change in food intake.
Secondary outcomes
None of the included trials reported data on survival. Lundholm 2010 reported baseline and follow‐up (at eight weeks) data on body composition, plasma ghrelin levels and quality of life. Neary 2004 reported data only on plasma ghrelin levels at pre‐breakfast, at the start of infusion, and 60 minutes and 90 minutes after the start of infusion. Strasser 2008 did not assess data on any of our secondary outcomes.
In summary the three trials that met the inclusion criteria recruited 59 participants with cancer cachexia; of whom 47 were able to complete the study. All the trials were double‐blind with two trials of cross‐over design and one trial of parallel‐group design. The median age of participants across the trials ranged from 54 to 78 years. The ratio of men to women recruited in the included studies was 37 to 22. Each of the included participants suffered with various types of cancer (Neary 2004; Strasser 2008), except Lundholm 2010 that exclusively enrolled participants with gastrointestinal cancer. One trial (Neary 2004) compared the effect of ghrelin with placebo while two trials (Lundholm 2010; Strasser 2008) studied the effect of two different doses (higher dose with lower dose) of ghrelin. The doses and duration of ghrelin varied between the included studies. Lundholm 2010 administered ghrelin subcutaneously while Neary 2004 and Strasser 2008 administered ghrelin intravenously.
Excluded studies
We excluded Adachi 2010; Hiura 2012; Takata 2015; Takiguchi 2012; and Yamamoto 2010 because, although the participants suffered from cancer, they were not cachectic patients. One trial registered in a clinical trials registry (NCT00933361) was not an RCT and hence we excluded it.
Risk of bias in included studies
All studies were at high risk of attrition bias and bias for size of the study. Risk of bias in other domains was unclear or low (Figure 2; Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation
All studies were at low risk of selection bias for random sequence generation. Lundholm 2010 and Neary 2004 randomised the participants using a computerised algorithm and statistical package respectively. Strasser 2008 mentioned that a random allocation sequence was produced.
Allocation concealment
Lundholm 2010 stated that participants received ghrelin according to randomisation but the study authors did not adequately provide the method of allocation concealment and so we judged it as unclear risk. Neary 2004 concealed allocation through sealed containers and so we judged it as at low risk. In Strasser 2008 an independent investigator maintained randomisation procedure and opening of sealed envelopes disclosed treatment assignments and hence we judged this study as at low risk.
Blinding
All three included studies were double‐blind. Although Lundholm 2010 stated the study was 'double‐blind' the authors did not clearly describe the method of blinding. Lundholm 2010 did not report on blinding of the outcome assessor. Neary 2004 and Strasser 2008 blinded participants, investigators, and dieticians/clinicians to the type of infusion by producing identical bags containing indistinguishable liquids.
There is unclear risk in Lundholm 2010. Performance bias and detection bias appears unlikely to have occurred in Neary 2004 and Strasser 2008.
Incomplete outcome data
We judged all three studies at high risk of attrition bias because they had substantial attrition (> 10%).
In Neary 2004 one of the seven (14.28%) participants failed to complete a food diary. In Strasser 2008 three of 21 (14.28%) participants and in Lundholm 2010 nine of the 31 (29%) participants failed to complete the study.
Neary 2004 did not specify the reason for attrition. In Strasser 2008 two participants stopped treatment because of malignant bowel obstruction and one participant because of infection. Lundholm 2010 reported that interruption of treatment was either for personal reasons or because of progressive disease. None of the studies mentioned attrition due to the intervention.
Size of study
We judged all three studies (Lundholm 2010; Neary 2004; Strasser 2008) to be confounded by small size due to having fewer than 50 participants per treatment arm so at high risk of bias.
Selective reporting
The protocol of Neary 2004 was not available. Strasser 2008 and Lundholm 2010 had registered protocols (ISRCTN26185223; NCT00681486 respectively).
Neary 2004 did not describe the adjusted analyses, and did not present the first period results clearly in the cross‐over trial. Some outcomes, like food intake and appreciation of food were reported but with insufficient detail for the data to be included in analysis. This study provided insufficient information to permit judgement of 'low risk' or 'high risk' of bias.
Other potential sources of bias
We did not detect any other potential sources of bias in the included studies and hence all studies were at low risk.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. Ghrelin compared to placebo for cachexia associated with cancer.
| Ghrelin compared to placebo for cachexia associated with cancer | ||||||
|
Patient or population: people with cachexia associated with cancer Settings: hospital Intervention: ghrelin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with ghrelin | |||||
| Food intake | Not known | Not known | Not known | 7 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | The study reported, "31% increase in energy intake with ghrelin infusion" Too few data to be meaningful |
| Body weight | No data | No data | No data | No data | ⊕⊝⊝⊝ Very low3 | Not reported. No data available. No evidence to support or refute the use of ghrelin in people with cancer cachexia |
| Adverse events | Study population | Not estimable | 7 (1 RCT) | ⊕⊝⊝⊝ Very low1,4 | The study reported, "No side effects were observed" Too few data and number of events too small to be meaningful |
|
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded once for study limitations due to high risk of attrition bias and biases confounded by small size. 2Downgraded twice for imprecision due to sparse data and low participant numbers. 3Downgraded three times: in circumstances where there were no data reported for an outcome, we reported the level of evidence as very low with no evidence to support or refute the use of ghrelin in people with cancer cachexia. 4Downgraded twice for imprecision due to sparse data, low participant numbers and number of events too small to be meaningful.
Summary of findings 2. Ghrelin compared to alternative experimental treatment modality for cachexia associated with cancer.
| Ghrelin compared to alternative experimental treatment modality for cachexia associated with cancer | ||||||
|
Patient or population: people with cancer cachexia Settings: hospital Intervention: ghrelin Comparison: alternative experimental treatment modality (like appetisers, nutritional supplements, etc.) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with alternative experimental treatment modality | Risk with ghrelin | |||||
| Food intake | No data | No data | No data | No data | ⊕⊝⊝⊝ Very low1 | Not reported. No evidence to support or refute the use of ghrelin in people with cancer cachexia. |
| Body weight | No data | No data | No data | No data | ⊕⊝⊝⊝ Very low1 | Not reported. No evidence to support or refute the use of ghrelin in people with cancer cachexia. |
| Adverse events | No data | No data | No data | No data | ⊕⊝⊝⊝ Very low1 | Not reported. No evidence to support or refute the use of ghrelin in people with cancer cachexia. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1In circumstances where there were no data reported for an outcome, we report the level of evidence as 'very low' with no evidence to support or refute the use of ghrelin in people with cancer cachexia.
Summary of findings 3. Ghrelin in combination with other treatments compared to ghrelin treatment alone for cachexia associated with cancer.
| Ghrelin in combination with other treatments compared to ghrelin treatment alone for cachexia associated with cancer | ||||||
|
Patient or population: people with cancer cachexia Settings: hospital Intervention: ghrelin in combination with other treatments Comparison: ghrelin treatment alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with ghrelin treatment alone | Risk with ghrelin in combination with other treatments | |||||
| Food intake | No data | No data | No data | No data | ⊕⊝⊝⊝ Very low1 | Not reported. No evidence to support or refute the use of ghrelin in people with cancer cachexia. |
| Body weight | No data | No data | No data | No data | ⊕⊝⊝⊝ Very low1 | Not reported. No evidence to support or refute the use of ghrelin in people with cancer cachexia. |
| Adverse events | No data | No data | No data | No data | ⊕⊝⊝⊝ Very low1 | Not reported. No evidence to support or refute the use of ghrelin in people with cancer cachexia. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1In circumstances where there were no data reported for an outcome, we report the level of evidence as 'very low' with no evidence to support or refute the use of ghrelin in people with cancer cachexia.
Summary of findings 4. Higher‐dose ghrelin compared to lower‐dose ghrelin for cachexia associated with cancer.
| Higher‐dose ghrelin compared to lower‐dose ghrelin for cachexia associated with cancer | ||||||
|
Patient or population: people with cancer cachexia Settings: hospital Intervention: higher‐dose ghrelin Comparison: lower‐dose ghrelin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with lower‐dose ghrelin | Risk with higher‐dose ghrelin | |||||
| Food intake | See comment | See comment | N/A | 40 (2 RCTs) | ⊕⊝⊝⊝ Very low1 2 | Too few data to be meaningful Because of the differences in study design and diversity in the treatment provided we did not pool results in a meta‐analysis |
| Body weight | No data | No data | No data | No data | ⊕⊝⊝⊝ Very low3 | Not reported. No evidence to support or refute the use of ghrelin in people with cancer cachexia. |
| Adverse events | Study population | Not estimable | 40 (2 RCTs) | ⊕⊝⊝⊝ Very low1 2 | Too few data to be meaningful | |
| See comment | See comment | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval N/A: not applicable | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded once for study limitations due to high risk of attrition bias and biases confounded by small size. 2Downgraded twice for imprecision due to low participant numbers. 3In circumstances where there were no data reported for an outcome, we report the level of evidence as 'very low' with no evidence to support or refute the use of ghrelin in people with cancer cachexia.
Comparison 1. Ghrelin versus placebo
One trial (Neary 2004) with seven participants compared ghrelin with placebo (Table 5; Table 6).
1. Summary of results of individual studies: primary outcomes.
| Primary outcome | Lundholm 2010 | Neary 2004 | Strasser 2008 | |||
| Higher‐dose ghrelin group | Lower‐dose ghrelin group | Ghrelin group | Placebo group | Higher‐dose ghrelin group | Lower‐dose ghrelin group | |
| Food intake | Baseline: 32.5 ± 94 (SEM) kcal/kg/day At 8 weeks: 28.2 ± 3.8 (SEM) kcal/kg/day According to 4‐day schedule |
Baseline: 24.1 ± 3.0 (SEM) kcal/kg/day At 8 weeks: 25.5 ± 4.5 (SEM) kcal/kg/day According to 4‐day schedule |
Mean energy intake: 9270 kJ (95% CI 3249 to 15,290 kJ) As assessed by 24‐h food diary |
Mean energy intake: 6854 kJ (95% CI 3634 to 10,070 kJ) As assessed by 24‐h food diary |
Nutritrional intake at lunch compared to baseline Ghrelin: 251 kcal Placebo: 230 kcal Nutritrional intake at lunch and rest of the day compared to baseline Ghrelin: 244 kcal Placebo: 156 kcal Monitored daily |
Nutritrional intake at lunch compared to baseline Ghrelin: ‐105 kcal Placebo: ‐17 kcal Nutritrional intake at lunch and rest of the day compared to baseline Ghrelin: 145 kcal Placebo: 228 kcal Monitored daily |
| Body weight | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Adverse events | Nil | Nil | Nil | Nil | Ghrelin: 17 (increased bowel activity: 5; abdominal pain: 5; dry mouth: 3; worsening of pre‐existing neuropathy: 1; asthenia: 1; diarrhoea: 1; nausea: 1) Placebo: 6 (increased bowel activity: 3; dry mouth: 1; dizziness: 1; diarrhoea: 1) |
Ghrelin: 7 (increased bowel activity: 3; shortness of breath: 1; sweating: 2; vomiting: 1) Placebo: 12 (increased bowel activity: 5; shortness of breath: 1; nausea: 1; increased stool frequency: 1; apoplectiform deafness: 1; vomiting: 2; constipation: 1) |
SEM: standard error of mean
2. Summary of results of individual studies: secondary outcomes.
| Secondary Outcomes | Lundholm 2010 | Neary 2004 | Strasser 2008 | |||
| Higher‐dose ghrelin group | Lower‐dose ghrelin group | Ghrelin group | Placebo group | Higher‐dose ghrelin group | Lower‐dose ghrelin group | |
| Survival | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Body composition (lean body mass) | Baseline: 44.8 ± 2.9 (SEM) kg At 8 weeks: 47.8 ± 2.9 (SEM) kg Difference over time: 2.24 ± 0.71 (SEM) |
Baseline: 44.3 ± 2.2 (SEM) kg At 8 weeks: 45.1 ± 2.8 (SEM) kg Difference over time: 0.86 ± 1.18 (SEM) |
Not reported | Not reported | Not reported | Not reported |
| Mean difference 1.38 kg (95% CI ‐1.32 to 4.08) | ||||||
| Body composition (fat mass) | Baseline: 15.1 ± 1.9 (SEM) kg At 8 weeks: 13.5 ± 1.9 (SEM) kg Difference over time: ‐1.3 ± 0.7 (SEM) |
Baseline: 16.3 ± 3.0 (SEM) kg At 8 weeks: 12.6 ± 2.4 (SEM) kg Difference over time: ‐3.7 ± 0.8 (SEM) |
Not reported | Not reported | Not reported | Not reported |
| Mean difference 2.40 kg (95% CI 0.32 to 4.48 | ||||||
| Plasma ghrelin | Baseline: 563 ± 90 (SEM) ng/L At 8 weeks: 1229 ± 501 (SEM) ng/L |
Baseline: 3418 ± 2570 (SEM) ng/L At 8 weeks: 3817 ± 2997 (SEM) ng/L |
0 min (pre‐breakfast): 531 ± 83 (SEM) 90 min (at the start of infusion): 505 ± 90 (SEM) 150 min (60 min after the start of infusion): 1718 ± 169 (SEM) 180 min (90 min after the start of infusion): 1840 ± 221 (SEM) |
0 min (pre‐breakfast): 545 ± 58 (SEM) 90 min (at the start of infusion): 440 ± 59 (SEM) 150 min (60 min after the start of infusion): 509 ± 97 (SEM) 180 min (90 min after the start of infusion): 490 ± 63 (SEM) |
Not reported | Not reported |
| Baseline imbalances between the two groups | Mean difference 1209.00 pmoL/L (95% CI 827.08 to 1590.92) | |||||
| Quality of Life | HADS anxiety Baseline: 4.6 ± 1.07 (SEM) At 8 weeks: 5.4 ± 1.6 (SEM) |
HADS anxiety Baseline: 8.6 ± 1.6 (SEM) At 8 weeks: 8.8 ± 1.3 (SEM) |
Not reported | Not reported | Not reported | Not reported |
| Baseline imbalances between the two groups | ||||||
| HADS depression Baseline: 5.7 ± 0.9 (SEM) At 8 weeks: 6.8 ±1.2 (SEM) |
HADS depression Baseline: 7.6 ±1.4 (SEM) At 8 weeks: 9.3 ± 1.9 (SEM) |
Not reported | Not reported | Not reported | Not reported | |
| Mean difference ‐2.50 (95% CI ‐6.90 to 1.90) | ||||||
| SF‐36 PCS Baseline: 30 ± 3 (SEM) At 8 weeks: 27 ± 3 (SEM) |
SF‐36 PCS Baseline: 35 ± 3 (SEM) At 8 weeks: 30 ± 2 (SEM) |
Not reported | Not reported | Not reported | Not reported | |
| Mean difference ‐3.00 (95% CI ‐10.07 to 4.07) | ||||||
| SF‐36 MCS Baseline: 40 ± 3 (SEM) At 8 weeks: 41 ± 3 (SEM) |
SF‐36 MCS Baseline: 30 ± 2 (SEM) At 8 weeks: 34 ± 4 (SEM) |
Not reported | Not reported | Not reported | Not reported | |
| Baseline imbalances | ||||||
CI: confidence interval HADS: Hospital Anxiety and Depression Scale SEM: standard error of mean SF‐36 MCS: 36‐item Short Form Health Survey Mental Component Scale SF‐36 PCS: 36‐item Short Form Health Survey Physical Component Scale
We assessed the quality of evidence using GRADE for all primary outcomes in this comparison as very low. We downgraded the evidence for study limitations (due to high risk of attrition bias and biases confounded by small size) and imprecision or due to lack of data. See Table 1.
Primary outcomes
1. Food intake
Neary 2004 reported the effect on food intake after intravenous administration of a single dose of 5 pmoL/Kg/minute of ghrelin as assessed by 24‐hour food diary (Table 5). The energy intake at baseline differed amongst the participants. This trial reported a 31% increase in energy intake after administration of ghrelin. Over a day, mean energy intake after ghrelin administration was 9270 kJ (95% CI 3249 to 15,290) as compared to 6854 kJ (95% CI 3634 to 10,070) after saline administration (P = 0.09). The trial also found a 23% increase in perceived pleasantness of the meal (as assessed by visual analogue score) on the day of administration of ghrelin as compared to day of administration of saline. Appetite increased in all participants who received ghrelin.
We downgraded the quality of evidence using GRADE once for study limitations due to high risk of attrition bias and biases confounded by small size. Only seven participants participated in the study and the loss to follow‐up was more than 10%. The evidence was further downgraded twice for imprecision due to sparse data and low participant numbers. These data were insufficient to be meaningful.
2. Body weight
This study did not report the effect of ghrelin on body weight.
We reported the quality of evidence using GRADE for body weight as very low, downgraded three times due to a lack of data.
3. Adverse events
No adverse events were observed in Neary 2004 in either of the intervention groups (Table 5).
We rated the quality of evidence using GRADE for adverse events as very low, downgraded once for study limitations due to high risk of attrition bias and bias confounded by small size, and downgraded twice for imprecision due to sparse data and low event rate.
Secondary outcomes
1. Survival
This study did not report on survival.
2. Body composition
Body composition was not assessed in this study.
3. Plasma ghrelin levels
Neary 2004 assessed plasma ghrelin levels in seven participants before breakfast, at the start of infusion, 60 minutes after the start of infusion and 90 minutes after the start of infusion. They presented the data as mean with standard error of the mean (SEM) in pmoL/litre. We converted the SEM into SD with the help of the RevMan 5 calculator and found that after 60 minutes of infusion, plasma ghrelin was higher in the ghrelin group compared to the placebo group (MD 1209.00 pmoL/L, 95% CI 827.08 to 1590.92; one study; 7 participants). We could not analyse the change in plasma ghrelin levels or number of participants who experienced an increase in plasma ghrelin levels due to insufficient information (Table 6).
4. Quality of life
This study did not assess quality of life.
Comparison 2. Ghrelin versus no treatment
None of the trials compared ghrelin with no treatment
Comparison 3. Ghrelin versus alternative experimental treatment modality
None of the trials compared ghrelin with an alternative experimental treatment modality, like appetisers, nutritional supplements, etc. We judged the quality of the evidence using GRADE to be very low, downgraded three times due to a lack of data. See Table 2.
Comparison 4. Ghrelin in combination with other treatments versus ghrelin treatment alone
None of the trials compared ghrelin in combination with other treatments with ghrelin treatment alone.
We judged the quality of the evidence using GRADE to be very low, downgraded three times due to a lack of data. See Table 3
Comparison 5. Ghrelin treatment versus ghrelin analogues/ghrelin mimetics
None of the trials compared ghrelin with ghrelin analogues/ghrelin mimetics, like anamorelin, ipamorelin, eganamorelin, hexarelin, MK‐677, etc., or ghrelin potentiators/enhancers like rikkunshito.
Comparison 6. Higher dose of ghrelin versus lower dose of ghrelin
Two trials (Lundholm 2010; Strasser 2008) compared a higher dose of ghrelin with a lower dose of ghrelin. Neither of the studies reported data on body weight and survival. Because of the different study designs (parallel and cross‐over) and great diversity in the treatment provided (dosage, route of administration, frequency and duration of treatment) in the included trials; meta‐analysis seemed inappropriate and we did not pool the results. However, we have presented a narrative summary of each of these studies with supporting tables (Table 5; Table 6).
We assessed the quality of evidence using GRADE for all primary outcomes in this comparison as very low. The evidence was downgraded for study limitations (due to high risk of attrition bias and biases confounded by small size) and imprecision or due to lack of data. See Table 4.
Primary outcomes
1. Food intake
In Lundholm 2010 food intake (as assessed according to four‐day record) did not differ between 12 participants on higher‐dose and 10 participants on lower‐dose ghrelin over time. Appetite was higher in participants on higher‐dose ghrelin as compared with participants on lower‐dose ghrelin (Table 5).
In Strasser 2008, there were no differences in nutritional intake compared to baseline when participants received ghrelin or placebo. In 11 participants on higher‐dose ghrelin, nutritional intake at lunch compared to baseline was 251 kcal. In nine participants on lower‐dose ghrelin, nutritional intake at lunch compared to baseline was ‐105 kcal (Table 5).
The quality of evidence was downgraded once for study limitations due to high risk of attrition bias and biases confounded by small size. Forty participants participated in these two studies and each study had more than 10% loss to follow‐up. The evidence was further downgraded twice for imprecision due to sparse data and low participant numbers.
2. Body weight
Neither of the included studies (Lundholm 2010; Strasser 2008) reported the effect of ghrelin on body weight.
We reported the level of evidence for body weight as very low, downgraded three times due to a lack of data.
3. Adverse events
No objective adverse effects were reported with higher‐dose or lower‐dose ghrelin in Lundholm 2010 (Table 5).
Strasser 2008 reported 17 adverse events in 11 participants on upper‐dose and seven adverse events in nine participants on lower‐dose ghrelin. Adverse events observed with higher dose of ghrelin were increased bowel activity (n = 5), abdominal pain (n = 5), dry mouth (n = 3), worsening of pre‐existing neuropathy (n = 1), asthenia (n = 1), diarrhoea (n = 1) and nausea (n = 1). Adverse events observed with lower dose of ghrelin were increased bowel activity (n = 3), shortness of breath (n = 1), sweating (n = 2) and vomiting (n = 1) (Table 5). Although the study reported 13 serious adverse events, only one, transient apoplectiform deafness, was supposedly related to the treatment.
We rated the quality of evidence for adverse events as very low, downgraded once for study limitations due to high risk of attrition bias and biases confounded by small size, and downgraded twice for imprecision due to sparse data and low participant numbers.
Secondary outcomes
1. Survival
Neither of the included studies (Lundholm 2010; Strasser 2008) reported this outcome.
2. Body composition
Lean body mass
Lundholm 2010 reported that difference over time in lean body mass was more with higher‐dose ghrelin (2.24 ± 0.71 (SEM)) compared to lower‐dose ghrelin (0.86 ± 1.18 (SEM)) (Table 6).
Strasser 2008 did not measure lean body mass.
Fat mass
Lundholm 2010 reported that higher‐dose ghrelin reduced the loss of whole‐body fat. There was a total of 31 participants in this trial. Whole‐body fat loss was less pronounced in participants who were receiving higher‐dose ghrelin as compared with lower‐dose ghrelin (P < 0.04). The difference over time in fat mass was ‐1.3 ± 0.7 (SEM) with higher‐dose ghrelin and ‐3.7 ± 0.8 (SEM) with lower‐dose ghrelin (Table 6).
Strasser 2008 did not measure fat mass.
3. Plasma ghrelin levels
In Lundholm 2010, there were significant differences in the baseline plasma ghrelin values 563 ± 90 (SEM) ng/L in higher‐dose ghrelin group and 3418 ± 2570 (SEM) ng/L in lower‐dose ghrelin group. After eight weeks of treatment, plasma ghrelin increased in both the groups 1229 ± 501 (SEM) ng/L in higher‐dose ghrelin group and 3817 ± 2997 (SEM) ng/L in lower‐dose ghrelin group (Table 6).
Strasser 2008 did not measure plasma ghrelin levels.
4. Quality of life
Lundholm 2010 assessed health‐related quality of life using the 36‐item Short Form Health Survey (SF‐36) and the Hospital Anxiety and Depression Scale (HADS) in 31 participants. There were baseline imbalances in HADS anxiety score and SF‐36 Mental component scale between the two groups. Health‐related quality of life did not differ over time between participants in higher‐dose and lower‐dose ghrelin groups. However, participants receiving higher‐dose ghrelin had less anxiety and scored better for mental health at inclusion compared to participants receiving lower‐dose ghrelin (Table 6).
Strasser 2008 did not assess quality of life.
Discussion
Summary of main results
We included three studies reporting data from 47 participants (59 randomised initially), aged 54 to 78 years, comparing ghrelin with placebo, or higher‐dose ghrelin with lower‐dose ghrelin. None of the included studies assessed data on body weight and survival. One trial (Neary 2004), which compared ghrelin with placebo, found that ghrelin improved food intake and had no adverse events. Two studies (Lundholm 2010; Strasser 2008), which compared higher‐dose ghrelin with lower‐dose ghrelin, individually showed that participants on higher‐dose ghrelin had greater improvement in food intake, body composition, plasma ghrelin and quality of life. Reporting of adverse events provided no information. All the included studies were at high risk of attrition bias and other bias due to small size. Risk of bias in other domains were mostly low or unclear.
Due to differences in study designs (parallel and cross‐over) and diversity in the treatment provided (dosage, route of administration, frequency and duration of treatment), we could not undertake meta‐analysis. We are unable to make any judgement about the efficacy or safety of ghrelin for improving food intake, body composition and survival in people with cachexia associated with cancer. We found insufficient evidence that using ghrelin showed changes in food intake. We found no evidence that using ghrelin alone or in combination made any difference to body weight. No conclusions could be reached about adverse events. The limited amount of information means that we cannot draw any conclusions.
The evidence we found was very low quality and the true effect is very likely to be substantially different from that reported. The very low GRADE judgement was based on lack of data, methodological limitations of the included studies, and overall small study size (Table 1; Table 2; Table 3; Table 4).
Results of the studies available are insufficient to draw any conclusions regarding safety and efficacy of ghrelin in treating people with cancer cachexia. The evidence is insufficient to support or refute its use.
Overall completeness and applicability of evidence
This review highlights our lack of knowledge about the effectiveness of ghrelin for cancer cachexia. We found three studies that met our inclusion criteria. The three included studies randomised 59 participants and 47 participants completed the study. One cross‐over trial (Neary 2004) compared ghrelin with placebo while two trials, one cross‐over (Strasser 2008) and one parallel trial (Lundholm 2010) compared different doses of ghrelin (higher‐dose versus lower‐dose). We did not find studies that compared ghrelin versus no treatment or alternative experimental treatment modality or ghrelin analogues/ghrelin mimetics/ghrelin potentiators/enhancers; or in combination with other treatments. Outcomes of interest to people with cancer cachexia, such as improvement in food intake, body weight and survival were not adequately reported.
There is a small body of evidence that we were able to include in this review, which limits the conclusions we can draw. We found insufficient evidence that using ghrelin showed improvement in food intake. We found no evidence that using ghrelin alone or in combination made any difference to body weight. There is insufficient evidence to be able to conclude that ghrelin is effective and has no major adverse events in people with cancer cachexia.
Limited data from very few included studies with small sample sizes in this systematic review confirms the need for further large randomised controlled trials with long‐term follow‐up focusing on evaluation of efficacy of ghrelin as well as adverse events associated with use of ghrelin in people with cancer cachexia. We look forward to such trials being available for inclusion in future updates of this review.
Quality of the evidence
The quality of evidence for all our primary outcomes was very low, meaning we have little confidence in the effect estimate and the true effect may be substantially different from the estimate of the effect. In general, the assessment of the quality of the included studies was too limited by deficiencies in terms of quality of methodology and reporting of adequate data, and small study size to allow reasonable conclusions to be drawn. Sample sizes of all included studies were likely to be under‐powered to find a true effect, as they involved fewer than 50 participants.
The quality of evidence for ghrelin versus placebo across all our primary outcomes was very low, due to too few data and due to the fact that the number of events was too small to be meaningful (Table 1).
The quality of evidence for ghrelin versus no treatment or alternative experimental treatment modalities or ghrelin in combination with other treatments or ghrelin analogues/ghrelin mimetics/ghrelin potentiators across our primary outcomes was very low, due to no available data (Table 2; Table 3).
The quality of evidence for higher‐dose ghrelin versus lower‐dose ghrelin for all our primary outcomes was very low, due to high risk of attrition bias and biases confounded by small size, too few data and the number of events too small to be meaningful (Table 4).
This review does not provide a reliable indication of the likely effect. As a result, there is no evidence to support or refute the use of ghrelin in people with cancer cachexia.
Potential biases in the review process
We have estimated that the potential bias in the review process is low. The search was as comprehensive as possible. The evaluation of trials for inclusion was done in pairs. None of the authors of this review were involved in any of the included or excluded studies. Furthermore, none have any commercial or any other conflicts of interest regarding ghrelin. We are confident that we have included all relevant published studies. We have attempted to reduce bias in the review process by performing data extraction and assessing study quality independently. However, the possibility remains that we may have missed studies which have not been published.
We undertook the review in line with the recommendations of Cochrane as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b)
The authors of this review are from different disciplines with different focuses (e.g. public health, biostatistics, clinical medicine, nutrition). We consider this internal variety of expertise to be a strength of this review and made use of it by duplicating procedures during the entire review process when possible.
Agreements and disagreements with other studies or reviews
This is the first systematic review of ghrelin for the management of cachexia associated with cancer. Numerous studies have tried to summarise the applicability of ghrelin for cancer cachexia but have not have used a systematic approach (Argilés 2013; Garcia 2013; Molfino 2014).
We identified one non Cochrane systematic review (Sever 2016) involving human and animal in vivo studies to assess the safety and efficacy of ghrelin/ghrelin analogues on the risk, presence, or growth of tumours in people with cancer or animal models of cancer, irrespective of the presence or absence of cachexia. The authors could not undertake any meta‐analysis but suggested that the safety profile of ghrelin and ghrelin analogues may be favourable for the treatment of cachexia in people with cancer.
Authors' conclusions
Implications for practice.
For people with cancer cachexia
The amount and quality of evidence around the use of ghrelin for improving food intake and body weight in people with cancer cachexia is very low. This means that at present, treatment is based on clinical experience and advice from respected authorities. No judgement can be made about adverse events.
For clinicians
We are not able to state the effect of ghrelin for improving food intake and body weight in people with cancer cachexia due to very low‐quality evidence. The low number and small size of the included studies, and the very low quality of the evidence means that this result should be viewed with very little confidence. No judgement can be made about adverse events. The findings of our review do not provide evidence to support or refute supplementation with ghrelin in people with cancer cachexia. There is insufficient evidence to recommend it for clinical practice.
For policy makers
The small number of randomised controlled trials and the resulting paucity of information means that no conclusions concerning the use of ghrelin for improving food intake and body weight in people with cancer cachexia can be drawn. This review neither supports nor refutes the use of ghrelin for improving food intake and body weight in people with cancer cachexia. No judgement can be made about adverse events.
For funders of the intervention
We identified only a small number of studies with small numbers of participants and insufficient data for analysis. The lack of evidence highlighted in this review implies that there is a need to fund and support suitable research for the treatment of cancer cachexia.
We did not undertake cost‐effectiveness analysis.
Implications for research.
General implications
We found that the current evidence base is comprised of studies that contain small numbers of participants in which there was a significant dropout rate. The lack of robust evidence regarding efficacy of ghrelin found in this review confirms the need to design high‐quality and clinically relevant research on this topic. The challenge is to develop trials examining clinically meaningful, patient‐centred outcomes.
Design
Very few studies combined with small size, cross‐over design and consequent attrition, confirms the need for further adequately powered, methodologically sound, large, randomised controlled trials with long‐term follow‐up to ensure adequate data after the effect of attrition due to various causes. Studies with a cross‐over design often have significant attrition, therefore parallel‐group designs may be preferable. Improved quality in study designs and improvement in reporting of outcomes would add to the confidence in our estimates.
Measurement (endpoints)
There is a need for randomised controlled trials with long‐term follow‐up focusing on evaluation of safety and efficacy of ghrelin in people with cancer cachexia. Trials looking at key endpoints including food intake, body weight and adverse events are warranted. Several outcomes for different doses, routes and duration of ghrelin have been evaluated through single trials, and independent replication of these studies is desirable. Cost‐effectiveness analysis can also be taken up for evaluating improvements in this condition relative to the costs of other interventions.
Other
The study design of choice is the prospective randomised trial, but other pragmatic designs may be worth considering.
What's new
| Date | Event | Description |
|---|---|---|
| 24 August 2020 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 6, 2016 Review first published: Issue 2, 2018
| Date | Event | Description |
|---|---|---|
| 19 June 2018 | Amended | Minor amendment to the Plain Language Summary. |
Notes
Assessed for updating in 2020
In July 2020 we did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We acknowledge the support of Dr Prathap Tharyan, Director, South Asian Cochrane Network & Center, Prof. BV Moses Center for Evidence‐Informed Health Care and Health Policy, Christian Medical College, Vellore, India.
Cochrane Review Group funding acknowledgement: this project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pain, Palliative and Supportive Care (PaPaS). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health
We acknowledge the external peer referees Dr Ollie Minton, consultant and senior lecturer in palliative medicine, Roger Goucke, anaesthesia/palliative Care, Maria J Silveira, palliative care specialist and researcher, and Marília Seelaender, head of the Cancer Metabolism Group, University of São Paulo, for providing valuable input to the protocol. We also thank Joanne Abbott for developing the search strategy.
We acknowledge the external peer referees Marilia Seelaender, Dr Adrian Tookman, Bridget Candy and consumer referee Corrie Billedeau for providing valuable input to this review.
We acknowledge the support of Dr Vedprakash Mishra, Dr Tripti Shrivastava, Dr Mahjabeen Ahmed, Dr Deepak Saxena, Dr B Unnikrishnan and Mahafroz Khatib during the preparation of the review.
Appendices
Appendix 1. Search strategies
CENTRAL (the Cochrane Library)
#1 MESH DESCRIPTOR Ghrelin EXPLODE ALL TREES 541
#2 ((ghrelin or ppghrelin or (motilin adj2 peptide) or ghrl or obestatin or ppmtlrp or "appetite regulating hormone" or Anamorelin or Ipamorelin or Eganamorelin or Hexarelin or MK‐677 or Rikkunshito)):TI,AB,KY (1153)
#3 MESH DESCRIPTOR Cachexia EXPLODE ALL TREES (156)
#4 (Cachexia or cachexic):TI,AB,KY (512)
#5 ((weight or underweight or malnutrition or wasting)):TI,AB,KY (73015)
#6 MESH DESCRIPTOR Weight Loss (4714)
#7 #3 OR #4 OR #5 OR #6 (73180)
#8 #1 OR #2 (1153)
#9 MESH DESCRIPTOR Neoplasms EXPLODE ALL TREES (61680)
#10 ((cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choricarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*)):TI,AB,KY (152733)
#11 #9 OR #10 (155346)
#12 #7 AND #8 AND #11 (92)
MEDLINE OVID
1 Ghrelin/ (8958)
2 (ghrelin or ppghrelin or (motilin adj2 peptide) or ghrl or obestatin or ppmtlrp or "appetite regulating hormone" or Anamorelin or Ipamorelin or Eganamorelin or Hexarelin or MK‐677 or Rikkunshito).tw. (9407)
3 Cachexia/ (8248)
4 cachexia.tw. (7949)
5 cachexic.tw. (83)
6 (weight or underweight or malnutrition or wasting).tw. (994788)
7 Weight Loss/ (120212)
8 or/3‐7 (999660)
9 exp Neoplasms/ (2930112)
10 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choricarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).tw. (3708183)
11 or/9‐10 (3898945)
12 1 or 2 (9407)
13 8 and 11 and 12 (370)
Embase OVID
1 Ghrelin/(14303)
2 (ghrelin or ppghrelin or (motilinadj2 peptide) or ghrl or obestatin or ppmtlrp or "appetite regulating hormone"or Anamorelin or Ipamorelin or Eganamorelin or Hexarelin or MK‐677 or Rikkunshito).ab. (15252)
3 Cachexia/(14636)
4 cachexia.ab.(8135)
5 cachexic.tw.(129)
6 (weight or underweight or malnutrition or wasting).ab. (1318964)
7 Weight Reduction/(228902)
8 or/3‐7 (1326471)
9 exp Neoplasm/ (4134130)
10 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma* or choricarcinoma* or leukemia* or leukaemia* or metastat* or sarcoma* or teratoma*).ab. (5171585)
11 or/9‐10 (5481882)
12 1 or 2 (15222)
13 8 and 11 and 12 (1022)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lundholm 2010.
| Study characteristics | ||
| Methods |
Study design: single‐centric, randomised, double‐blinded clinical trial Duration/period of the study: participants recruited 2006‐2008 Withdrawals: 9 |
|
| Participants |
Participants: progressive, weight‐losing gastrointestinal cancer patients with systemic spread without ongoing specific tumor therapy Total participants recruited: 31 Number of participants allocated to each treatment group
Number of participants who completed the treatment
Mean age/age range: HDGG: 73 ± 2; LDGG: 76 ± 2 Gender distribution (male/female): HDGG: 11/6; LDGG: 7/7 Diagnostic criteria: weight loss > 5% and subjective appreciation of anorexia as a main cause behind health‐related problems Type of cancer: progressive gastrointestinal cancer with systemic spread Severity of cancer: all participants had metastatic cancer Inclusion criteria People with:
Exclusion criteria
|
|
| Interventions | Participants were randomly divided into two groups:
|
|
| Outcomes |
Outcomes related to the review
Outcomes unrelated to the review
All the outcomes were assessed at 4 weeks and 8 weeks. 31 participants were recruited (HDGG: 17; HDGG: 14). However, 22 participants (HDGG: 12; HDGG: 10) finished the intended treatment. All results were evaluated primarily on an ITT basis and then according to the protocol (HDGG: 12 participants; LDGG: 10 participants) |
|
| Notes |
Country: Sweden Funding: supported in parts by grants from the Swedish Cancer Society (2014), the Swedish Research Council (08712), Assar Gabrielsson Foundation (AB Volvo), Jubileumskliniken Foundation, IngaBritt and Arne Lundberg Research Foundation, Swedish and Gothenburg Medical Societies and the Medical Faculty, University of Gothenburg, Sahlgrenska University Hospital Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized by a computerized algorithm to high‐dose ghrelin (n = 17) or low‐dose ghrelin (n = 14) by stratification as described by Pocock and Simon" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients received ampoules that contained either 800 µg or 40 µg corresponding to high‐dose and low‐dose ghrelin, respectively, according to randomization." Method of allocation concealment not adequately reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "All injections were double blind" How blinding was done not adequately reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessor not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Twelve patients who were receiving high‐dose ghrelin and 10 patients who were receiving low‐dose ghrelin finished the intended 8 weeks of treatment. Interruption of treatment was either for personal (private) reasons (3 patients who were receiving high‐dose ghrelin and 2 patients who were receiving low‐dose ghrelin) or because of progressive disease (2 patients who were receiving high‐dose ghrelin and 2 patients who were receiving low‐dose ghrelin). The study had substantial attrition (> 10%). 9/31 (29%) participants failed to complete the study. 5 participants did not complete evaluations in HDGG (30%) and 4 in LDGG (28%) All outcomes were assessed initially with an ITT. However, outcome results were presented per protocol treatment for those participants who were able to complete 8 weeks of treatment. |
| Size of study | High risk | < 50 participants per treatment arm. We suspect bias confounded by small size |
| Selective reporting (reporting bias) | Low risk | Study protocol is available (NCT00681486) |
| Other bias | Low risk | No other potential sources of bias |
Neary 2004.
| Study characteristics | ||
| Methods |
Study design: single‐centric, randomised, crossover, double‐blinded clinical trial Duration/period of the study: participants recruited 10 September 2002‐6 January 2003 Withdrawals: Nil |
|
| Participants |
Participants: cancer patients established on chemotherapy with sustained anorexia and weight loss Total participants recruited: 7 Number of participants allocated to each treatment group
Mean age/age range: ghrelin: 75.4 ± 4.6; saline: 74.1 ± 8.7 Gender distribution (male/female): 1/6 Diagnostic criteria: Framingham criteria Type of cancer: breast cancer (n = 5), colon cancer (n = 1) and malignant melanoma (n = 1) Severity of cancer: all participants had metastatic cancer Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly divided into two groups:
Ghrelin group
Saline group
|
|
| Outcomes |
Outcomes related to the review
Outcomes unrelated to the review
|
|
| Notes |
Country: UK Study setting: oncology clinics at Charing Cross Hospital, London, UK Funding: this study was supported by Medical Research Council Program Grant G7811974 and Cancer Research (UK). Two authors are Wellcome Trust Clinical Research Training Fellows Comments
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study was randomized and double‐blinded. Subjects were randomized to receive ghrelin then saline (four patients) or saline then ghrelin (three patients) using the statistics package, SigmaStat version 2.0 (SPSS, Inc., Chicago, IL)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Vials of ghrelin and saline were indistinguishable visually; they were labeled by subject and infusion numbers and stored in a sealed container." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Subjects, investigators, and dieticians were blinded to the infusion order until study completion." Quote: "Vials of ghrelin and saline were indistinguishable visually; they were labeled by subject and infusion numbers and stored in a sealed container." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Subjects, investigators, and dieticians were blinded to the infusion order until study completion." Quote: "Vials of ghrelin and saline were indistinguishable visually; they were labeled by subject and infusion numbers and stored in a sealed container." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Except for one patient who failed to complete a food diary after one of her infusions; all the participants completed the study." The study had substantial attrition (> 10%). 1/7 (14.28%) participants failed to complete a food diary Reason for attrition not specified |
| Size of study | High risk | < 50 participants per treatment arm. We suspect bias confounded by small size |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not available but the study reported all the pre‐specified outcomes stated in objectives Study authors reported the mean energy intake after ghrelin/saline administration and did not report the baseline values of the same The study authors did not describe the adjusted analyses, and did not present the first period results clearly in the cross‐over trial. Study provides insufficient information to permit judgement of 'low risk' or 'high risk' of bias |
| Other bias | Low risk | No other potential sources of bias. |
Strasser 2008.
| Study characteristics | ||
| Methods |
Study design: single‐centric, randomised, double‐cross‐over, double‐blind clinical trial Duration/period of the study: not reported Withdrawals: 3 |
|
| Participants |
Participants: adults with advanced incurable cancer who had loss of appetite (≥ 3 VAS) and a weight loss of ≥ 2% within 2 months or ≥ 5% within 6 months before the study, not related to recent surgery Total participants recruited: 21 Number of participants allocated to each treatment group
Number of participants who completed the treatment
Median age (years): HDGG: 70 (45, 80); LDGG: 66 (45, 73) Gender distribution (male/female): HDGG: 8/1; LDGG: 9/2 Diagnostic criteria: not reported Type of cancer: pancreatic cancer (n = 4), mesothelioma (n = 2), prostate cancer (n = 3), colorectal cancer (n = 4), stomach/oesophageal cancer (n = 2), NSCLC (n = 3) , urogenital cancer (n = 1), cholangiocarcinoma (n = 1) Severity of cancer: advanced incurable cancer Inclusion criteria
Exclusion criteria
|
|
| Interventions | 20 participants were randomly divided into two groups:
Ghrelin intervention
Control Group
|
|
| Outcomes |
Outcomes related to the review
Outcomes unrelated to the review
|
|
| Notes |
Country: Switzerland Study setting: Kantonsspital in St Gallen, Switzerland Funding: research support was provided by OncoSuisse OCS – 01385 – 08 – 2003: scientific project grant; Eastern Switzerland Cancer Research Fund: scientific project support; Swiss Institute of Applied Cancer Research: pilot development grant; Gastrotech Pharm A/S, Denmark: unrestricted research support; and Amgen Switzerland: unrestricted grant. Protocol: this trial was registered at Current Controlled Trials: ISRCTN26185223. Received 13 August 2007; published online 8 January 2008 Values are reported in Mean ±SE |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised by independent personnel at the hospital pharmacy, where the random allocation sequence produced (switches after 1 to maximal 3 patients) was assigned and the sealed envelopes for each patient distributed." Quote: "In a randomised, double‐blind, placebo‐controlled, double‐cross‐ over trial" |
| Allocation concealment (selection bias) | Low risk | Quote: "an independent senior physician who had controlled the randomisation procedure, the master randomisation list, and the broken envelopes revealed the treatment assignments." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Less than 30 min before each infusion, the pharmacy produced identical bags containing indistinguishable liquids of 250 ml normal saline with or without ghrelin" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blinded clinicians rated the other adverse events as unrelated or probably unrelated to treatment" Quote: "Less than 30 min before each infusion, the pharmacy produced identical bags containing indistinguishable liquids of 250 ml normal saline with or without ghrelin" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "One patient having octreotide treatment was removed from analysis" Quote: "Two patients stopped study treatment early in the second week because of malignant bowel obstruction and blood‐culture positive infection, respectively." The study had substantial attrition (> 10%). Three of 21 (14.28%) participants failed to complete the study |
| Size of study | High risk | < 50 participants per treatment arm. We suspect bias confounded by small size |
| Selective reporting (reporting bias) | Low risk | Trial was registered at Current Controlled Trials: ISRCTN26185223 |
| Other bias | Low risk | No other potential sources of bias |
HADS: Hospital Anxiety and Depression Scale; HDGG: higher‐dose ghrelin group; HRQoL: health‐related quality of life; ITT: intention‐to‐treat; LDGG: lower‐dose ghrelin group; SEM: standard error of the mean; SF‐36: 36‐item Short Form Health Survey; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adachi 2010 | Different participants: included cancer patients without cachexia |
| Hiura 2012 | Different participants: included cancer patients without cachexia |
| NCT00933361 | Different study design: not an RCT. The study had only one arm with no comparison group |
| Takata 2015 | Different participants: included cancer patients without cachexia Quote from the study: "Most patients showed an advanced clinical stage, low body weight". However; no separate data available for cachectic patients. Also; the study seems to assess the effects of ghrelin after oesophagectomy rather than in people with cancer cachexia |
| Takiguchi 2012 | Different participants: included cancer patients without cachexia |
| Yamamoto 2010 | Different participants: patients with severe pre‐operative weight loss of > 10% over the past 3 months were ineligible for inclusion i.e. included people with cancer but without cachexia |
Differences between protocol and review
We updated the background section with recent references.
We had planned to undertake five comparisons. We added a sixth comparison group in this review: higher‐dose ghrelin versus lower‐dose ghrelin as we found two studies addressing this. We thought it wiser to include this comparison rather than miss data on an intervention.
We had intended to use changed scores for all our outcomes. However, for food intake, body composition, plasma ghrelin levels and quality of life (under comparison of ghrelin versus placebo), we presented the baseline and the end‐scores, as the data for changed scores were not available and could not be computed (Table 5; Table 6).
In the protocol, we had planned to include 'Summary of findings' tables for three comparisons: ghrelin versus placebo, ghrelin versus an alternative experimental treatment modality, and ghrelin in combination with other treatments versus ghrelin treatment alone. However, as per the availability of the data, we have included 'Summary of findings' tables for another comparison group: higher‐dose ghrelin versus lower‐dose ghrelin in this review.
Contributions of authors
MNK: led the design of the review as primary author, implemented search strategy with the help of Cochrane Pain, Palliative and Supportive Care's Information Specialist, screened the articles on the basis of title and abstract, extracted and analysed data, and led the write‐up.
AS: closely helped in design, resolved discrepancy in screening, aided in analysis and write‐up
RK: closely helped in design, provided statistical inputs for analysis, and assisted with the write‐up
AG: closely helped in design, screened full texts, provided statistical inputs for analysis, and assisted with the write‐up
SG: closely helped in design, screened the articles on the basis of title and abstract, extracted data, and assisted with the write‐up
PS: closely helped in design, assessed the risk of bias for each study, and assisted with the write‐up
ZQS: closely helped in design, screened full texts, provided statistical inputs for analysis, and assisted the write‐up
All authors are responsible for the full review, and updating the review in future.
Sources of support
Internal sources
Datta Meghe Institute of Medical Sciences, India
External sources
No sources of support supplied
Declarations of interest
MNK: none known AS: none known RK: none known AG: none known SG: none known. SG is a specialist physician and manages patients with cancer cachexia. PS: none known ZQS: none known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Lundholm 2010 {published data only}
- Lundholm K, Gunnebo L, Körner U, Iresjö B-M, Engström C, Hyltander A, et al. Effects by daily long term provision of ghrelin to unselected weight-losing cancer patients: a randomized double-blind study. Cancer 2010;116(8):2044-52. [DOI] [PubMed] [Google Scholar]
Neary 2004 {published data only}
- Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. The Journal of Clinical Endocrinology and Metabolism 2004;89(6):2832-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Strasser 2008 {published data only}
- Strasser F, Lutz T A, Maeder M T, Thuerlimann B, Bueche D, Tschöp M, et al. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: a randomised, placebo-controlled, double-blind, double-crossover study. British Journal of Cancer 2008;98(2):300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Adachi 2010 {published data only}
- Adachi S, Takiguchi S, Okada K, Yamamoto K, Yamasaki M, Miyata H, et al. Effects of ghrelin administration after total gastrectomy: a prospective, randomized, placebo-controlled phase II study. Gastroenterology 2010;138(4):1312-20. [DOI] [PubMed] [Google Scholar]
Hiura 2012 {published data only}
- Hiura Y, Takiguchi S, Yamamoto K, Takahashi T, Kurokawa Y, Yamasaki M, et al. Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients: a prospective, randomized, placebo-controlled phase 2 study. Cancer 2012;118(19):4785-94. [DOI] [PubMed] [Google Scholar]
NCT00933361 {published data only}
- NCT00933361. Individual dose-escalated bi-daily subcutaneously (sc) ghrelin in cancer cachexia: a Phase I/II Study. clinicaltrials.gov 2009. [NCT00933361]
Takata 2015 {published data only}
- Takata A, Takiguchi S, Miyazaki Y, Miyata H, Takahashi T, Kurokawa Y, et al. Randomized phase II study of the anti-inflammatory effect of ghrelin during the postoperative period of esophagectomy. Annals of Surgery 2015;262(2):230-6. [DOI] [PubMed] [Google Scholar]
Takiguchi 2012 {published data only}
- Takiguchi S, Hiura Y, Miyazaki Y, Takata A, Murakami K, Doki Y. Clinical trial of ghrelin synthesis administration for upper GI surgery. Methods in Enzymology 2012;514:409-31. [DOI] [PubMed] [Google Scholar]
Yamamoto 2010 {published data only}
- Yamamoto K, Takiguchi S, Miyata H, Adachi S, Hiura Y, Yamasaki M, et al. Randomized phase II study of clinical effects of ghrelin after esophagectomy with gastric tube reconstruction. Surgery 2010;148(1):31-8. [DOI] [PubMed] [Google Scholar]
Additional references
Aapro 2014
- Aapro M, Arends J, Bozzetti F, Fearon K, Grunberg SM, Herrstedt J, et al. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Annals of Oncology 2014;25(8):1492-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Akamizu 2010
- Akamizu T, Kangawa K. Ghrelin for cachexia. Journal of Cachexia, Sarcopenia and Muscle 2010;1(2):169-76. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2017
- Anderson LJ, Albrecht ED, Garcia JM. Update on management of cancer-related cachexia. Current Oncology Reports 2017;19(1):3. [DOI] [PubMed] [Google Scholar]
Argilés 2013
- Argilés JM, Stemmler B. The potential of ghrelin in the treatment of cancer cachexia. Expert Opinion on Biological Therapy 2013;13(1):67-76. [DOI] [PubMed] [Google Scholar]
Argilés 2017
- Argilés JM, Lopez-Soriano FJ, Stemmler B, Busquets S. Novel targeted therapies for cancer cachexia. The Biochemical Journal 2017;474(16):2663-78. [DOI: 10.1042/BCJ20170032] [PMID: 28751550 ] [DOI] [PubMed] [Google Scholar]
AUREF 2012
- Cochrane Pain, Palliative and Supportive Care Group. PaPaS Author and Referee Guidance. papas.cochrane.org/papas-documents (accessed on 16 August 2015).
Bai 2017
- Bai Y, Hu Y, Zhao Y, Yu X, Xu J, Hua Z, et al. Anamorelin for cancer anorexia-cachexia syndrome: a systematic review and meta-analysis. Supportive Care in Cancer 2017;25(5):1651-9. [DOI] [PubMed] [Google Scholar]
Baracos 2011
- Baracos VE. Pitfalls in defining and quantifying cachexia. Journal of Cachexia, Sarcopenia and Muscle 2011;2(2):71-3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennani‐Baiti 2008
- Bennani-Baiti N, Davis MP. Cytokines and cancer anorexia cachexia syndrome. American Journal of Hospice & Palliative Care 2008;25(5):407-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bozzetti 2009
- Bozzetti F, Mariani L. Defining and classifying cancer cachexia: a proposal by the SCRINIO Working Group. JPEN. Journal of Parenteral and Enteral Nutrition 2009;33(4):361-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
CDC 2016
- Centre for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion. Health-Related Quality of Life (HRQOL) | CDC. www.cdc.gov/hrqol/ May 2016.
Chen 2012
- Chen C-Y, Tsai C-Y. Ghrelin and motilin in the gastrointestinal system. Current Pharmaceutical Design 2012;18(31):4755-65. [DOI] [PubMed] [Google Scholar]
Chen 2015
- Chen JA, Splenser A, Guillory B, Luo J, Mendiratta M, Belinova B, et al. Ghrelin prevents tumour- and cisplatin-induced muscle wasting: characterization of multiple mechanisms involved. Journal of Cachexia, Sarcopenia and Muscle 2015;6(2):132-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2016
- Chen JL, Walton KL, Qian H, Colgan TD, Hagg A, Watt MJ, et al. Differential effects of IL6 and activin A in the development of cancer-associated cachexia. Cancer Research 2016;76(18):5372-82. [DOI] [PubMed] [Google Scholar]
Colldén 2017
- Colldén G, Tschöp MH, Müller TD. Therapeutic potential of targeting the ghrelin pathway. International Journal of Molecular Sciences 2017;18(4):798. [DOI: 10.3390/ijms18040798] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crawford 2015
- Crawford J. Cachexia. Journal of Thoracic Oncology 2015;2:S158. [Google Scholar]
Cummings 2001
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001;50(8):1714-9. [DOI] [PubMed] [Google Scholar]
Cummings 2006
- Cummings David E. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiology & Behavior 2006;89(1):71-84. [DOI] [PubMed] [Google Scholar]
Currow 2017
- Currow DC, Skipworth RJ. The emerging role of anamorelin hydrochloride in the management of patients with cancer anorexia-cachexia. Future Oncology 2017 (Accessed 28 Aug 2017);13(20):1767-83. [DOI: 10.2217/fon-2017-0141] [DOI] [PubMed] [Google Scholar]
Date 2002
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002;123(4):1120-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
DeBoer 2007
- DeBoer MD, Zhu X, Levasseur P, Meguid M, Suzuki S, Inui A, et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology 2007;148(6):3004-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Dixit 2004
- Dixit D, Schaffer M, Pyle S, Collins D, Sakthivel K, Palaniappan R, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. Journal of Clinical Investigation 2004;114(1):57-66. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Elmagarmid 2014
- Elmagarmid A, Fedorowicz Z, Hammady H, Ilyas I, Khabsa M, Ouzzani M. Rayyan: a systematic reviews web app for exploring and filtering searches for eligible studies for Cochrane Reviews [oral presentation]. In: Cochrane Colloquium. John Wiley & Sons, 2014.
Esposito 2015
- Esposito A, Criscitiello C, Gelao L, Pravettoni G, Locatelli M, Minchella I, et al. Mechanisms of anorexia-cachexia syndrome and rational for treatment with selective ghrelin receptor agonist. Cancer Treatment Reviews 2015;41(9):793-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Faulconbridge 2003
- Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes 2003;52(9):2260-5. [DOI] [PubMed] [Google Scholar]
Fearon 2006
- Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. The American Journal of Clinical Nutrition 2006;83(6):1345-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fearon 2011
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncology 2011;12(5):489-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fearon 2012
- Fearon KCH, Glass D, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metabolism 2012;16(2):153-66. [DOI] [PubMed] [Google Scholar]
Fujino 2003
- Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. The Journal of Physiology 2003;550(Pt 1):227-40. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fujitsuka 2009
- Fujitsuka N, Asakawa A, Hayashi M, Sameshima M, Amitani H, Kojima S, et al. Selective serotonin reuptake inhibitors modify physiological gastrointestinal motor activities via 5-HT2c receptor and acyl ghrelin. Biological Psychiatry 2009;65(9):748-59. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fujitsuka 2011
- Fujitsuka N, Asakawa A, Uezono Y, Minami K, Yamaguchi T, Niijima A, et al. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Translational Psychiatry 2011;1:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fujitsuka 2014
- Fujitsuka N, Uezono Y. Rikkunshito, a ghrelin potentiator, ameliorates anorexia-cachexia syndrome. Frontiers in Pharmacology 2014;5:271. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fuoco 2015
- Fuoco D, Kilgour RD, Vigano A. A hypothesis for a possible synergy between ghrelin and exercise in patients with cachexia: biochemical and physiological bases. Medical Hypotheses 2015;85(6):927-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garcia 2005
- Garcia JM, Garcia-Touza M, Hijazi RA, Taffet G, Epner D, Mann D, et al. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. Journal of Clinical Endocrinology and Metabolism 2005;90(5):2920-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garcia 2007
- Garcia J, Boccia R, Graham C, Kumor K, Polvino W. A phase II randomized, placebo-controlled, double-blind study of the efficacy and safety of RC-1291 (RC) for the treatment of cancer cachexia. Journal of Clinical Oncology 2007 (June 20 Supplement);25(18S):9133. [Google Scholar]
Garcia 2009
- Garcia JM, Polvino WJ. Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Hormone & IGF Research 2009;19(3):267-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garcia 2013
- Garcia JM, Friend J, Allen S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study. Supportive Care in Cancer 2013;21(1):129-37. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garcia 2015
- Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S, et al. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncology 2015;16(1):108-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), (accessed on September 2015). Available at gradepro.org.
Gualillo 2001
- Gualillo O, Caminos J, Blanco M, Garcìa-Caballero T, Kojima M, Kangawa K, et al. Ghrelin, a novel placental-derived hormone. Endocrinology 2001;142(2):788-94. [DOI] [PubMed] [Google Scholar]
Guney 2007
- Guney Y, Ozel TU, Hicsonmez A, Nalca AM, Kurtman C. Ghrelin may reduce radiation-induced mucositis and anorexia in head-neck cancer. Medical Hypotheses 2007;68(3):538-40. [DOI] [PubMed] [Google Scholar]
Hatanaka 2015
- Hatanaka M, Konishi M, Ishida J, Saito M, Springer J. Novel mechanism of ghrelin therapy for cachexia. Journal of Cachexia, Sarcopenia and Muscle 2015;6(4):393. [DOI: 10.1002/jcsm.12084] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
ISRCTN26185223
- ISRCTN26185223. Randomised phase I/II - study with ghrelin versus placebo for patients with cancer-related anorexia/cachexia. ISRCTN registry 2005 (accessed 28 July 2017). [DOI: 10.1186/ISRCTN26185223] [DOI]
Johns 2013
- Johns N, Stephens NA, Fearon KCH. Muscle wasting in cancer. The International Journal of Biochemistry & Cell Biology 2013;45(10):2215-29. [DOI] [PubMed] [Google Scholar]
Khatib 2014a
- Khatib MN, Gaidhane S, Gaidhane A, Syed ZQ. Role of ghrelin in regulation of growth hormone secretion by ghrelin-pituitary-GH axis. International Journal of Medical Science and Public Health 2014;3(4):425-9. [DOI: 10.5455/ijmsph.2014.250120141] [DOI] [Google Scholar]
Khatib 2014b
- Khatib MN, Simkhada P, Gode D. Cardioprotective effects of ghrelin in heart failure: from gut to heart. Heart Views 2014;15(3):74-6. [DOI: 10.4103/1995-705X.144792] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khatib 2014c
- Khatib MN, Khatib M, Gaidhane S, Gaidhane A, Quazi SZ. Ghrelin for regulating appetite and energy balance: a systematic review. National Journal of Physiology, Pharmacy and Pharmacology 2014;4(3):101-5. [DOI: 10.5455/njppp.2014.4.230420141] [DOI] [Google Scholar]
Khatib 2014d
- Khatib MN, Gode D, Simkhada P, Agho K, Gaidhane S, Saxena D, et al. Somatotropic and cardio-protective effects of ghrelin in experimental models of heart failure: a systematic review. Annals of Tropical Medicine and Public Health 2014;7(1):30-42. [DOI: 10.4103/1755-6783.145008] [DOI] [Google Scholar]
Khatib 2014e
- Khatib MN, Gaidhane S, Simkhada P, Gaidhane A, Quazi SZ. Cardiovascular effects of ghrelin in heart failure: a systematic review. International Journal of Medical Science and Public Health 2014;3(6):756-63. [DOI: 10.5455/ijmsph.2014.060420141] [DOI] [Google Scholar]
Khatib 2014f
- Khatib MN, Gaidhane S, Gaidhane AM, Khatib M, Simkhada P, Gode D, et al. Ghrelin: ghrelin as a regulatory peptide in growth hormone secretion. Journal of Clinical and Diagnostic Research 2014;8(8):MC13-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khatib 2015a
- Khatib MN, Shankar A, Kirubakaran R, Agho K, Simkhada P, Gaidhane S, et al. Effect of ghrelin on mortality and cardiovascular outcomes in experimental rat and mice models of heart failure: a systematic review and meta-analysis. PloS One 2015;10(5):e0126697. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khatib 2015b
- Khatib MN, Gaidhane S, Gaidhane AM, Simkhada P, Zahiruddin QS. Ghrelin-O-acyl-transferase (GOAT) as a novel metabolic regulatory enzyme. Journal of Clinical and Diagnostic Research 2015;9(2):LE01-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klok 2007
- Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obesity Reviews 2007;8(1):21-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kojima 1999
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402(6762):656-60. [DOI] [PubMed] [Google Scholar]
Kojima 2008
- Kojima M, Kangawa K. Structure and function of ghrelin. Results and Problems in Cell Differentiation 2008;46:89-115. [DOI] [PubMed] [Google Scholar]
Korbonits 2001
- Korbonits M, Bustin SA, Kojima M, Jordan S, Adams EF, Lowe DG, et al. The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. The Journal of Clinical Endocrinology and Metabolism 2001;86(2):881-7. [DOI] [PubMed] [Google Scholar]
Kos 2009
- Kos K, Harte AL, O'Hare PJ, Kumar S, McTernan PG. Ghrelin and the differential regulation of des-acyl (DSG) and oct-anoyl ghrelin (OTG) in human adipose tissue (AT). Clinical Endocrinology 2009;70(3):383-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ledderose 2011
- Ledderose C, Kreth S, Beiras-Fernandez A. Ghrelin, a novel peptide hormone in the regulation of energy balance and cardiovascular function. Recent Patents on Endocrine, Metabolic & Immune Drug Discovery 2011;5(1):1-6. [DOI] [PubMed] [Google Scholar]
Lin 2017
- Lin T-C, Hsiao M. Ghrelin and cancer progression. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 2017;1868(1):51-7. [DOI] [PubMed] [Google Scholar]
Loumaye 2017
- Loumaye A, Thissen J-P. Biomarkers of cancer cachexia. Clinical Biochemistry 2017;pii: S0009-9120(17):30427-7. [DOI: 10.1016/j.clinbiochem.2017.07.011. [Epub ahead of print]] [DOI] [PubMed] [Google Scholar]
Lucia 2012
- Lucia S, Esposito M, Rossi Fanelli F, Muscaritoli M. Cancer cachexia: from molecular mechanisms to patient's care. Critical Reviews in Oncogenesis 2012;17(3):315-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Madeddu 2015a
Madeddu 2015b
- Madeddu C, Mantovani G, Gramignano G, Maccio A. Advances in pharmacologic strategies for cancer cachexia. Expert Opinion on Pharmacotherapy 2015;16(14):2163-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Masuda 2000
- Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochemical and Biophysical Research Communications 2000;276(3):905-8. [DOI] [PubMed] [Google Scholar]
Mendes 2015
- Mendes MCS, Pimentel GD, Costa FO, Carvalheira JBC. Molecular and neuroendocrine mechanisms of cancer cachexia. Journal of Endocrinology 2015;226(3):R29-43. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Molfino 2014
- Molfino A, Formiconi A, Rossi Fanelli F, Muscaritoli M. Ghrelin: from discovery to cancer cachexia therapy. Current Opinion in Clinical Nutrition and Metabolic Care 2014;17(5):471-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mori 2000
- Mori K, Yoshimoto A, Takaya K, Hosoda K, Ariyasu H, Yahata K, et al. Kidney produces a novel acylated peptide, ghrelin. FEBS Letters 2000;486(3):213-6. [DOI] [PubMed] [Google Scholar]
Murphy 1998
- Murphy MG, Plunkett LM, Gertz BJ, He W, Wittreich J, Polvino WM, et al. MK-677, an orally active growth hormone secretagogue, reverses diet-induced catabolism. The Journal of Clinical Endocrinology and Metabolism 1998;83(2):320-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Müller 2015
- Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, et al. Ghrelin. Mol Metab 2015;4(6):437–60. [DOI: 10.1016/j.molmet.2015.03.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
National Cancer Institute 2015
- National Cancer Institute, NIH. Cancer Trends Progress Report. progressreport.cancer.gov March 2015 (accessed 26 July 2017).
NCT00681486
- NCT00681486. Ghrelin - a possible opportunity to improve appetite (phase 3). ClinicalTrials.gov 2008. [NCT00681486 ]
Northrup 2013
- Northrup R, Kuroda K, Duus EM, Barnes SR, Cheatham L, Wiley T, et al. Effect of ghrelin and anamorelin (ONO-7643), a selective ghrelin receptor agonist, on tumor growth in a lung cancer mouse xenograft model. Supportive Care in Cancer 2013;21(9):2409-15. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohnuma 2017
- Ohnuma T, Adigun R. Cancer, Anorexia and Cachexia. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2017. [PubMed] [Google Scholar]
Penna 2010
- Penna F, Costamagna D, Fanzani A, Bonelli G, Baccino FM, Costelli P. Muscle wasting and impaired myogenesis in tumor bearing mice are prevented by ERK inhibition. PloS one 2010;5(10):e13604. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Penna 2016
- Penna F, Busquets S, Argiles JM. Experimental cancer cachexia: evolving strategies for getting closer to the human scenario. Seminars in Cell and Developmental Biology 2016;54:20-7. [DOI: 10.1016/j.semcdb.2015.09.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pietra 2014
- Pietra C, Takeda Y, Tazawa-Ogata N, Minami M, Yuanfeng X, Duus EM, et al. Anamorelin HCl (ONO-7643), a novel ghrelin receptor agonist, for the treatment of cancer anorexia-cachexia syndrome: preclinical profile. Journal of Cachexia, Sarcopenia and Muscle 2014;5(4):329-37. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Porporato 2013
- Porporato PE, Filigheddu N, Reano S, Ferrara M, Angelino E, Gnocchi VF, et al. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. The Journal of Clinical Investigation 2013;123(2):611-22. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 2012
- Reid J, Mills M, Cantwell M, Cardwell CR, Murray LJ, Donnelly M. Thalidomide for managing cancer cachexia. Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No: CD008664. [DOI: 10.1002/14651858.CD008664.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ryan 2016
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sever 2016
- Sever S, White DL, Garcia JM. Is there an effect of ghrelin/ghrelin analogs on cancer? A systematic review. Endocrine-Related Cancer 2016;23(9):R393-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shin 2010
- Shin Y-K, Martin B, Kim W, White CM, Ji S, Sun Y, et al. Ghrelin is produced in taste cells and ghrelin receptor null mce show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS One 2010;5(9):e12729. [DOI: 10.1371/journal.pone.0012729] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stephens 2008
- Stephens NA, Skipworth RJ, Fearon KC. Cachexia, survival and the acute phase response. Current Opinion in Supportive and Palliative Care 2008;2(4):267-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stewart 2006
- Stewart GD, Skipworth RJ, Fearon KC. Cancer cachexia and fatigue. Clinical Medicine 2006;6(2):140-3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sugiyama 2012
- Sugiyama M, Yamaki A, Furuya M, Inomata N, Minamitake Y, Ohsuye K, et al. Ghrelin improves body weight loss and skeletal muscle catabolism associated with angiotensin II-induced cachexia in mice. Regulatory Peptides 2012;178(1-3):21-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sun 2015
- Sun L, Quan XQ, Yu S. An epidemiological survey of cachexia in advanced cancer patients and analysis on its diagnostic and treatment status. Nutrition and Cancer 2015;67(7):1056-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Suzuki 2010
- Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocrine Journal 2010;57(5):359-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Takeda 2008
- Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, Nagai K, et al. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology 2008;134(7):2004-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Temel 2016
- Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncology 2016;17(4):519-31. [DOI] [PubMed] [Google Scholar]
Teunissen 2007
- Teunissen SC, Wesker W, Kruitwagen C, De Haes HC, Voest EE, De Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. Journal of Pain and Symptom Management 2007;34(1):94-104. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tisdale 2009
- Tisdale MJ. Mechanisms of cancer cachexia. Physiological Reviews 2009;89(2):381-410. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tschop 2000
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000;407(6806):908-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tsubouchi 2014
- Tsubouchi H, Yanagi S, Miura A, Matsumoto N, Kangawa K, Nakazato M. Ghrelin relieves cancer cachexia associated with the development of lung adenocarcinoma in mice. European Journal of Pharmacology 2014;743:1-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Utech 2012
- Utech AE, Tadros EM, Hayes TG, Garcia JM. Predicting survival in cancer patients: the role of cachexia and hormonal, nutritional and inflammatory markers. Journal of Cachexia, Sarcopenia and Muscle 2012;3(4):245-51. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Lely 2004
- Van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocrine Reviews 2004;25(3):426-57. [DOI] [PubMed] [Google Scholar]
Velloso 2008
- Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. British Journal of Pharmacology 2008;154(3):557-68. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Volante 2002
- Volante M, Allìa E, Gugliotta P, Funaro A, Broglio F, Deghenghi R, et al. Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. The Journal of Clinical Endocrinology and Metabolism 2002;87(3):1300-8. [DOI] [PubMed] [Google Scholar]
von Haehling 2012
- Haehling S, Anker SD. Cachexia as major underestimated unmet medical need: facts and numbers. International Journal of Cardiology 2012;161(3):121-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Watanabe 1996
- Watanabe S, Bruera E. Anorexia and cachexia, asthenia, and lethargy. Hematology/Oncology Clinics of North America 1996;10(1):189-206. [PMID: ] [DOI] [PubMed] [Google Scholar]
Windsor 1988
- Windsor JA, Hill GL. Risk factors for postoperative pneumonia. The importance of protein depletion. Annals of Surgery 1988;208(2):209-14. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wren 2001
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. The Journal of Clinical Endocrinology and Metabolism 2001;86(12):5992. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhang 2007
- Zhang W, Zhao L, Mulholland MW. Ghrelin stimulates myocyte development. Cellular Physiology and Biochemistry 2007;20(5):659-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhang 2015
- Zhang H, Garcia JM. Anamorelin hydrochloride for the treatment of cancer-anorexia-cachexia in NSCLC. Expert Opinion on Pharmacotherapy 2015;16(8):1245-53. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


