Abstract
Skeletal muscle weakness occurs with aging and in females this is compounded by the loss of estrogen with ovarian failure. Estrogen deficiency mediates decrements in muscle strength from both inadequate preservation of skeletal muscle mass and decrements in the quality of the remaining skeletal muscle. Processes and components of skeletal muscle that are affected by estrogens are beginning to be identified. This review focuses on mechanisms that contribute to the loss of muscle force generation when estrogen is low in females, and conversely the maintenance of strength by estrogen. Evidence is accumulating that estrogen deficiency induces apoptosis in skeletal muscle contributing to loss of mass and thus strength. Estrogen sensitive processes that affect quality, i.e., force generating capacity of muscle, include myosin phosphorylation and satellite cell function. Further detailing these mechanisms and identifying additional mechanisms that underlie estrogenic effects on skeletal muscle is important foundation for the design of therapeutic strategies to minimize skeletal muscle pathologies, such as sarcopenia and dynapenia.
Keywords: Aging, Estrogen, Skeletal muscle, Strength
Introduction
Skeletal muscle weakness is an undesirable consequence of aging. Sarcopenia, the age-related loss of muscle mass and strength, has been identified as the crucial precursor to frailty, leading to disability, loss of independence [1], as well as hospitalization [2] for aged individuals. While decreased muscle mass contributes to sarcopenia, strength declines to a greater extent such that strength normalized for muscle size is reduced with age. Dynapenia is a relatively new term used to describe this age-associated loss of muscle strength that is independent of muscle atrophy [3]. The significance of dynapenia is exemplified by Visser and Schapp, who conclude that the loss of muscle strength consistently predicts falls, mortality and functional status, such as mobility in elderly individuals, while evidence for the relationship between low muscle mass and these outcomes is limited [4].
Skeletal muscle of females is dually affected by age due to the simultaneous loss of ovarian hormone production. In women, the production of estradiol and progesterone falls at menopause, occurring at 45–52 years of age [5, 6]. Declines in strength are accelerated at this age in women [7–13] and meta-analyses of nearly 10,000 post-menopausal women showed that those on hormone therapy (HT) had slightly greater muscle strength than those not on HT [14]. Such results point toward estrogen as being the key ovarian hormone affecting muscle strength in women. In this article, we will use the abbreviation HT to encompass both estrogen and combined estrogen-progesterone therapy in women as recommended by the 2017 North American Menopause Society position statement [15].
A systematic review and meta-analyses comparing force generation of muscle from ovarian hormone-deficient and–replete mice also indicate that muscle strength decrements occur when estrogen is deficient [14]. The most common method of inducing ovarian hormone deficiency in pre-clinical rodent research is ovariectomy, i.e., bilateral surgical removal of the ovaries, and this approach is often referred to as a model of menopause [16]. In rodents, estrous cycles become irregular and eventually cease with age. The age at which this occurs is variable across strains of mice and rats. For example, cessation of estrous cycles in C57BL6 mice was initially reported to occur between 13 and 16 months of age [17] and was more recently measured to occur between 17 and 20 months of age [18]. The term ovarian senescence is used to indicate when estrous cycles cease and this natural, aged-induced loss of ovarian hormone production is more similar to menopause in women than ovariectomy surgery (reviewed by [19]). Despite this more comparable physiology, the aged female rodent model is infrequently used to analyze how the loss of estrogen detrimentally affects muscle strength.
The focus of this review will be on the putative mechanisms that contribute to the loss of muscle force generation when estrogen is low in females, and conversely preserve strength when estrogen is present (Figure 1). Studies on menopausal women and estrogen-deficient and–replete rodents will be summarized as to how muscle strength is affected by estrogen, and lack thereof. The specific estrogenic mechanisms considered in the review will be limited to 1) regulation of skeletal muscle mass affected by estrogens and 2) processes in skeletal muscle contraction, which if perturbed by estrogen deficiency will diminish the generation of force.
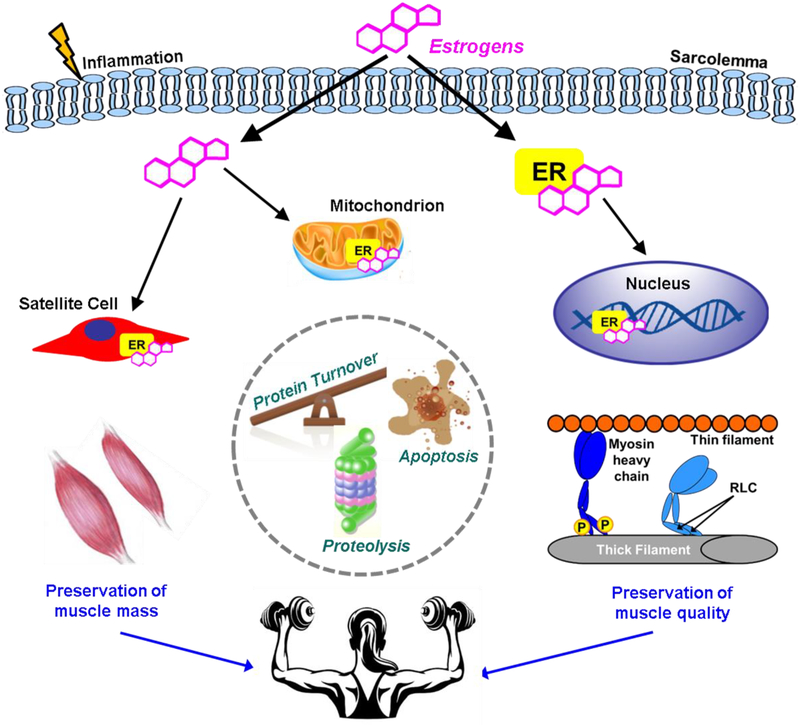
Figure 1.
Estrogens affect skeletal muscle strength in females by preserving muscle mass and quality of the contractile proteins. Estrogens influence the binding of myosin heavy chain to actin to generate force through phosphorylation of the regulatory light chain. Processes that are impacted by estrogens affecting muscle mass may include protein turnover, proteolysis, and apoptosis. Organelles that are sensitive to estrogens and contribute to maintenance of muscle mass and quality include nuclei and mitochondria. Inflammation and satellite cell function are also estrogen sensitive and can play a role in ultimately preserving muscle strength. ER = estrogen receptor; P = phosphorylation; RLC = regulatory light chain
1. Estrogen and skeletal muscle mass
While the anabolic effects of androgens on skeletal muscle in males are recognized to improve mass and thus strength (e.g., [20, 21]), by comparison there is much less known about effects of estrogens on skeletal muscle mass in females [22]. Current evidence indicates that estrogens have a role in maintaining muscle mass [23–25]. When estrogen is deficient, as occurs with advanced age in females, muscle atrophy ensues and contributes to muscle weakness. This section of the review will present evidence on cellular and molecular mechanisms by which estrogen may contribute to maintaining skeletal muscle mass and on the contrary, mechanisms underlying how estrogen deficiency may cause atrophy. While age-related weakness (dynapenia) can occur independent of atrophy, it is important to note that loss of muscle mass may also contribute to loss of strength.
Muscle protein turnover.
Dysregulation in muscle protein turnover during estrogen deficiency with the balance tipping away from protein synthesis and toward protein degradation would contribute to fiber atrophy and thus overall loss of muscle mass. The effects of estrogens on basal, resting skeletal muscle protein turnover have been studied in women under various conditions of estrogen manipulation (for example in [26]) and nominally in animal models.
Muscle protein synthesis. Relatively more studies have measured rates of muscle protein synthesis than degradation as related to estrogen status. Myofibrillar protein synthesis at rest in pre-menopausal women did not differ between those in follicular (~low estrogen) compared to luteal (relatively high estrogen) phase of their menstrual cycles [27]. A shortcoming of this type of study design is that progesterone fluctuates in addition to estradiol (the most bioactive estrogen in non-pregnant adult women) and therefore, delineating the impact of estrogen versus progesterone on muscle protein synthesis is not possible. Studies in which delivery of hormones is controlled circumvent this issue. Administering individual hormones to post-menopausal women showed that progesterone alone increased the basal rate of muscle protein synthesis but HT with estrogen alone had no significant effect [28]. Hansen and co-workers reported that myofibrillar rates of protein synthesis were lower in post-menopausal women on estrogen-only HT compared to post-menopausal women not on any HT [29]. In a separate study, those investigators reported that premenopausal women taking oral contraceptive containing ethinyl estradiol also had a lower rate of myofibrillar protein synthesis compared to young women not taking oral contraceptives [30].
Another approach to assess estrogenic impact on rates of muscle protein synthesis is to compare rates between pre- and post-menopausal women. Two studies by Smith and coworkers showed that basal rates of muscle protein synthesis were 20–30% greater in post-menopausal compared to young, pre-menopausal women [28, 31], indicating that estrogen deficiency may improve the rate of protein synthesis. Overall, the collective evidence points in the direction of estrogens suppressing the rate of muscle protein synthesis in women, which is counterintuitive to the concept that estrogens will blunt the loss of skeletal muscle mass with age.
Studies using various animal models do not clarify the impact of estrogen on muscle protein synthesis. Skeletal muscle of ovariectomized rats induced to grow by reloading the hindlimbs after unloading had low levels of p70s6k and Akt compared to sham-operated rats indicating that estrogen deficiency impaired protein synthesis [32]. On the contrary, female lambs treated with a synthetic estrogen had decreased rates of muscle protein synthesis, despite the treatment causing increased growth [33]. Toth and coworkers utilized a rat ovariectomy model with hormone treatments and measured muscle protein synthesis rates [34]. Ovariectomized rats that received a placebo treatment had rates of protein synthesis that approximately doubled that in sham-operated, control rats as well as in ovariectomized rats that received estradiol or progesterone. These results parallel the overall human results of estrogen deficiency with muscle protein turnover appearing to tip toward anabolism rather than catabolism, as rates of protein synthesis tend to be greater in the absence of estrogen.
Muscle protein degradation. Smith and coworkers deduced that because skeletal muscle of post-menopausal women is in an overall catabolic condition, the relatively high rate of protein synthesis in the basal, resting state must be offset by an even greater rate of protein degradation resulting in a net protein and muscle loss [31]. However, whole body protein breakdown did not differ between aged, post-menopausal women not on HT compared to young women with normal circulating estrogen [35], and myofibrillar protein breakdown at rest did not differ between premenopausal women who did and did not take estradiol-based HT [30]. Thus, results are insufficient to support the deduction that basal rates of muscle protein degradation are elevated with estrogen deficiency.
In animal studies, skeletal muscle protein degradation was not affected by estradiol treatment in growing steers [36]. Though, using bovine satellite cell cultures, Kamanga-Sollo and coworkers showed that estradiol treatment decreased the rate of protein degradation and increased the rate of protein synthesis lending support to the concept of estrogen being anabolic [37, 38].
Turnover summary.
Collectively, evidence that estrogen deficiency causes dysregulation in muscle protein turnover with the balance tipping away from protein synthesis and toward protein degradation and thus contributing to the loss of muscle mass is weak. Further research, particularly on the protein degradation side of protein turnover, is warranted. It is worth noting, that the evidence for estrogenic effects on protein synthesis and degradation presented above focus on resting basal rates. There is some evidence for reduced responsiveness of muscle protein synthesis to anabolic stimuli when estrogen is low [26].
Muscle protein ubiquitination and proteasome activity.
The ubiquitin-proteasome system contains intracellular machinery for degrading and turning over proteins, with ubiquitination having functions in addition to orchestrating proteolysis [39]. Protein ubiquitination involves three classes of enzymes acting synergistically; ubiquitin activating enzymes, ubiquitin conjugating enzymes and ubiquitin ligases. In addition, deubiquitinating enzymes, the ubiquitin-specific peptidases (USPs), are needed for recycling ubiquitin and for rescuing a protein from degradation. The ubiquitin-proteasome system is an important mediator of skeletal muscle protein homeostasis and regulation of muscle mass through crosstalk with anabolic and catabolic signaling pathways (reviewed by [40]). There has been little research to determine estrogenic impact on the function of the overall ubiquitin-proteasome system; what has been investigated has been directed mostly at ubiquitin ligases and peptidases.
The most prominent ubiquitin ligases in skeletal muscle are atrogin-1 (official name Fbxo32, also known as MAFbx) and MuRF1 (official name TRIM63, also known as MuRF2, IRF, RNF28), which were named as atrophy genes because their high expression leads to the loss of skeletal muscle mass whereas low expression generally leads to resistance against loss of muscle [41, 42]. The biology of ubiquitin ligases in skeletal muscle is complex as factors such as differences in muscle fiber types, acute versus gradual conditions inducing atrophy, and sex differences obscure the influence of these atrophy genes. For instance, an upregulation of atrogin-1 and MuRF1 genes was reported in atrophic muscles of aged male rats [43], while downregulation of atrogin-1 and MuRF1 was reported in muscles of aged female rats [44]. Such results of sex differences hint at a possible estrogenic effect, though the aged female rats were likely ovarian senescent and thus estrogen deficient. Rogers and co-workers more directly investigated estrogenic effects on ubiquitin ligases by comparing skeletal muscle from sham-operated and ovariectomized mice [45]. Muscle from ovariectomized mice had approximately 2-fold lower expression of atrogin-1 and MuRF1 compared to sham controls indicating that ubiquitin ligase-induced atrophy was suppressed when estrogen was lacking.
Svensson and coworkers performed a microarray study to investigate the effects of dihydrotestosterone, a non-aromatizable testosterone, and 17β-estradiol on muscle gene expression in gonadectomized male mice [46]. As expected, gonadectomy resulted in reduced muscle mass that was partially reversed by estradiol and completely reversed by dihydrotestosterone treatment. Dihydrotestosterone and estradiol regulated mainly a different set of genes with only 13 genes regulated by both sex hormones. Among those dually regulated was atrogin-1, which was upregulated in dihydrotestosterone- and estradiol-treated mice despite muscle mass being preserved. No other ubiquitin-proteasome system genes were identified in the study. Thus, the few reports on ubiquitin ligases in the rodent literature do not provide support to the concept that estrogens may help maintain muscle mass. Rather, many results are in opposition as estrogen deficiency was related to lower levels of atrophy genes and estrogen-replete muscle had relatively high levels of atrophy genes.
There is also a lack of consistent data in the human literature supporting the concept that estrogen exerts beneficial effects to muscle by suppressing atrophy genes. Dieli-Conwright and co-workers reported that postmenopausal women not on HT had lower muscle mass, higher baseline muscle expression of atrogin-1 and MuRF1, and smaller reductions in the expression of these atrophy genes in response to a single bout of resistance exercise compared to women on HT [47]. In contrast, Pöllänen and coworkers showed that muscle biopsies from non-HT, early postmenopausal women engaged in a one-year long, randomized HT trial had a higher number of differentially expressed ubiquitin-proteasome system genes, including atrogin-1, compared to biopsies from HT-using women [48]. HT users had upregulated atrogin-1 expression concomitant with a gain of muscle mass while non-users had reduced muscle mass over the year. Explanations for the seemingly contradicting results between these two studies may include age of the participants, type of HT, and duration of HT. In the latter study [48], women were relatively young (mean age 54 yr versus 59 yr in [47]) and were randomized to a specific HT containing both estradiol and a progesterone, whereas in the Dieli-Conwright study participants using either estrogen alone or estrogen plus progesterone HT were recruited. Furthermore, baseline atrogin-1 expression in the non-HT participants did not change over the one-year follow-up period in the Pöllänen study, while in the HT group there was 24% upregulation of atrogin-1. If the duration of HT were to continue for approximately 4 yr until study participants reached the same age range as in Dieli-Conwright study, it is unknown if atrogin-1 expression would eventually decline.
Minimal investigation into estrogenic effects on ubiquitin-specific peptidases in skeletal muscle of women has been conducted. In the microarray study by Pöllänen, differential expression of four ubiquitin-specific peptidases (usp1, usp2, usp15, usp50) as well as one ubiquitin conjugating enzyme (ube2g2) in muscle between non-HT and HT postmenopausal women were reported [48]. Estrogen deficiency was related to upregulated expression of usp1and usp15, and downregulation of usp2, usp50 and ube2g2, and real time RT-PCR validated these gene expression results, except for usp50. Any functional effects of estrogen on the deubiquitinating enzymes at the protein or activity level in muscle remain unknown.
A series of studies by Ogawa and co-workers investigated the role of estrogen in regulation of the ubiquitin-specific peptidase, USP19. Using a mouse immortalized skeletal myoblast cell line, C2C12, the group first showed that the level of USP19 protein was increased in a dose-dependent manner by estradiol [49]. Satellite cells isolated from mouse muscle and cultured in differentiation medium with and without estradiol also showed that protein and mRNA levels of USP19 were greater in the presence of estradiol. Consistent with the cellular results, USP19 protein was reduced after ovariectomy while a single intramuscular injection of estradiol restored the level back to the sham levels, which was shown to be mediated through estrogen receptor α [49]. The effect of USP19 was shown to be sex dependent with knockdown USP19 increasing muscle mass and fiber size in female but not male mouse soleus muscle [50]. Again, this was demonstrated to be mediated by estrogen receptor α as knockdown of the receptor caused a decrease in usp19 gene expression and an increase in soleus muscle mass in female but not male mice. A third study demonstrated that USP19 is an estrogen-sensitive factor in the negative regulation of muscle mass as estradiol treatment to ovariectomized mice resulted in higher soleus muscle USP19 gene and protein levels compared to those from untreated mice [51]. Collectively, the work by Ogawa and co-workers provides evidence that estrogen increases the expression of USP19 and contributes to muscle atrophy. Another group reported no difference in muscle mass between female mice ablated for USP19 and control littermates [39]. These results are discrepant from Ogawa and coworkers, perhaps due to differences in mouse strains or the specific muscles studied.
A last piece of evidence suggesting that the ubiquitin-proteasome system is involved in estrogen-mediated effects on skeletal muscle mass is that the system is important for the clearance of estrogen receptors, as these proteins carry conserved domains targeting them for ubiquitination-mediated degradation (reviewed by [52]). Because estrogens work primarily through estrogen receptors to mediate its effects, the turnover of estrogen receptors affects the transcriptional and signaling activity of estrogens.
To summarize, the evidence that estrogen influences the ubiquitin-proteasome system in regulating skeletal muscle mass is scant and conflicting. Genes for ubiquitin ligases, the so-called atrophy genes, tend to be expressed at higher levels in muscle from estrogen-replete rodents, while data from the human literature are inconsistent. Similarly, gene expression of various ubiquitin-specific peptidases are differentially up- or down-regulated in estrogen-deficient muscle of women. The ubiquitin-specific peptidase, USP19, is relatively high in soleus muscle of estrogen-replete mice, which indicates that estrogen may contribute to muscle atrophy. The ubiquitin-proteasome system is very complicated with the genes and proteins discussed here for associations with estrogen representing only a small fraction of the total genes and protein. Thus, results from current research are insufficient to conclude how estrogen modulates the ubiquitin-proteasome system in skeletal muscle to contribute to the regulation of mass and strength.
Skeletal muscle apoptosis.
Biological aging is associated with increased cellular apoptosis leading to tissue dysfunction such as muscle atrophy. Estrogen is known to protect against apoptosis in non-skeletal muscle tissues [53–56]. Moreover, it has long been suggested that women are more protected from cellular damage (e.g., oxidative stress) that results in apoptosis; however, protection is lost following the menopausal transition [57].
Sexually dimorphic action of heat shock proteins in part regulate anti-apoptotic signaling in tissues [58, 59], including skeletal muscle [58–60]. In normal conditions, heat shock proteins are in low abundance but adequate for facilitating protein folding and acting as chaperones for newly synthesized proteins to their site of action. The loss of heat shock proteins results in mislocalized proteins, aggregation, protein breakdown, and unwanted protein-protein interactions. Following a stress event in skeletal muscle (e.g., exercise or damage), the intracellular heat shock response is activated and is necessary for the repair process. However, the heat shock response is blunted with age and can result in a chronic stress response and incomplete recovery of skeletal muscle [61].
There is evidence that heat shock proteins are estrogen sensitive in skeletal muscle [62–64]. A body of literature by Boland and Vasconsuelo and coworkers using C2C12 cells demonstrates that estrogen treatment protects the cells against hydrogen peroxide-induced apoptosis through upregulation of HSP27 [64–70]. HSP27 binds caspase-3, preventing its cleavage and keeping the enzyme in its inactive state, which in turn modulates its downstream targets, Bcl-2, BAD, AKT, ERK, and MAPK to block apoptosis and promote survival of C2C12 cells [64–70]. The few rodent studies that have been conducted show that estrogen treatment to males or ovariectomized females attenuates exercise-induced HSP70 and HSP72 (official name HspA1A) response and has no effect on HSP27 in hindlimb muscles [63, 71–73]. Wang and coworkers further showed that loss of estrogen in female rats blunted basal protein levels of HSP70, HSP27, and HSP90 in skeletal muscle [74]. Thus, it is unclear how and which HSPs are regulated by estrogen; however, these studies provide impetus for future research investigating estrogen’s role in its protection against apoptosis through HSP modulation especially in the complex in vivo environment of skeletal muscle.
Only a few human studies to our knowledge have made measurements connecting estrogen and apoptosis in skeletal muscle. As such, even less is known whether HSPs are misregulated in skeletal muscle of post-menopausal women. Two studies utilized eccentric exercise protocols to induce muscle damage in young, adult men and women [75, 76]. Each study found men to have significantly higher basal levels of anti-apoptotic Bcl-2 in skeletal muscle than women, whereas only women had increased Bcl-2 in muscle due to the eccentric exercise. Kerksick and coworkers demonstrated greater change in cytoplasmic apoptotic activity in response to damaging exercise in skeletal muscle biopsies of men than in women [76]. These data, based on sex differences, suggest that estrogen may be protective against skeletal muscle apoptosis in women.
Further evidence supporting the regulation of apoptosis by estrogen comes from twin studies on postmenopausal women discordant for the use of HT. In a study by Kangas and coworkers, the expression of FasL, a ligand for cell death receptor Fas, and microRNAs miR-21 and miR-146a known to target FasL and Fas [77, 78], respectively, were studied and found to be less abundant in the serum samples of HT users compared to their non-using co-twins [79]. Additionally, miR-146a in skeletal muscle was lower in the HT using twins. While HSP’s were not measured, miR-146a has been implicated in regulating the immune response by targeting cytokine production, such as TNF-α and FasL [80]. These two cytokines are part of the TNF family of death receptors and clustering these ligands and receptors subsequently initiates signals for apoptosis [80]. Laakkonen et al also completed a functional muscle proteomic analysis on biopsies from the twins and predicted that the non-HT group had upregulated cell death and apoptotic pathways, as well as pathways related to the production of reactive oxygen species and necrosis [81]. The major cluster of differentially expressed proteins indicated that the predicted upregulation of cell death involves mitochondrial dysfunction occurring in women with low estrogen levels.
Mitochondrial dysfunction has dual effects; it induces apoptosis and can also lead to dysregulation of muscle energetic pathways, which in turn may contribute to muscle fiber atrophy and eventually to the loss of muscle mass. Transgenic manipulation of estrogen receptors has clearly demonstrated the importance of ERα-mediated estrogen signaling in metabolic health [82–84]. Such studies have started to delineate the mechanisms underlying the earlier observations pointing toward differences in signaling related to mitochondrial function at the transcriptome [85, 86] and proteome [81] levels in skeletal muscle of postmenopausal women. Muscle specific knockdown of ERα proved to have a critical role of estrogen signaling in mitochondrial functions [82, 84]. However, due to the complexities of increased fat and total body mass with estrogen receptor depletion [83, 87–90], it is difficult to differentiate if the loss in muscle mass is the result of apoptosis induced by mitochondrial dysfunction or as a side effect due to metabolic dysfunction in muscle.
Accumulating evidence indicates that estrogen protects skeletal muscle from apoptosis and thus defends against loss of muscle mass. However, the downstream, apoptotic signaling pathways that respond to estrogen have yet to be conclusively determined.
2. Processes and players in muscle contraction affected by estrogen
Strength loss due to estrogen deficiency in women and female rodents cannot be fully explained by loss of muscle mass because force generation that is normalized to muscle size (also known as specific force) declines as well. Theoretically, an impairment in any step in the process of muscle contraction, from propagation of an action potential at the motor end plate to the calcium-activated interaction of actin and myosin in the sarcomere, can cause diminished force generation. We are not aware of literature reporting estrogenic mechanisms in fiber excitation or excitation-contraction coupling of healthy muscle; rather the majority of investigations have focused on how estrogen affects sarcomeric proteins. Skeletal muscle incurs repetitive injury throughout life, requiring regeneration of the contractile machinery to regain strength. Here we will also review literature supporting the contention that regeneration of muscle is impaired when estrogen is lacking in females. The collective evidence presented indicates that dynapenia, due to aging or injury, is exacerbated by estrogen deficiency.
Myosin heavy chain.
Phillips and co-workers first speculated that ovarian hormones influence muscle strength by directly affecting crossbridges, i.e., contractile proteins [8]. They showed that women did not differ from men in specific force of adductor pollicis muscle at young ages but past the age of menopause were weaker than men; HT, however, preserved strength in postmenopausal women. Wattanapermpool and Reiser more directly implicated contractile proteins when they reported that permeabilized fibers from ovariectomized rats produced less specific force than did fibers from ovary-intact rates [91]. Contraction is initiated in permeabilized fibers by direct application of calcium, thus only processes downstream of calcium release from the sarcoplasmic reticulum are engaged. Such results indicate that ovarian hormones affect a process between calcium binding to troponin C and the interaction of myosin heavy chain binding to actin and generating force.
Since that time, evidence continues to support the contention that estrogen affects myosin. Placing a spin probe on the catalytic domain of myosin heavy chain in fibers from mice with and without their ovaries, and then measuring strong-binding of myosin to actin during contraction by electron paramagnetic resonance spectroscopy, directly demonstrated that the generation of force at the molecular level was low with estrogen deficiency [92]. A subsequent study showed that estradiol treatment to ovariectomized mice could prevent and restore loss of strong-binding of myosin to actin and force generation [93]. Active stiffness is an indirect estimate of myosin bound to actin during contraction and results using this measure are consistent with estrogen exerting a positive influence on myosin heavy chain function [93, 94]. Specific force of isolated mouse muscles in these studies were also greater in estrogen-replete compared to–deficient mice [92–95] and the estrogenic effects are mediated through estrogen receptors [96].
A study of permeabilized single muscle fibers from post-menopausal twins provides further convincing evidence that estrogen affects the function of myosin heavy chain. In this study by Qaisar and coworkers, specific force and myosin function, as determined by force per crossbridge, was greater in fibers from biopsies of sisters on HT compared to sisters not on HT [97].
Myosin heavy chain is an ATPase and recently a novel regulated state of this protein characterized by extremely slow ATP turnover during relaxation (i.e., not during contraction) was discovered and termed the super-relaxed state (SRX) [98]. Age-related changes in myosin SRX is specific to females [99] and is regulated at least in part by estradiol [100]. Downstream mechanisms underlying estrogenic effects on myosin function during relaxation are speculated to be related to phosphorylation of myosin’s regulatory light chain [99, 100]. Further studies are required to elucidate the extent to which the state of myosin during relaxation translates to modulation of force generation during contraction and thus strength in females.
Myosin regulatory light chain.
Myosin consists of two heavy chains and two pairs of light chains that are stabilized on the thick filament by myosin binding protein C. The regulatory light chain (RLC) acts as a lever arm on the α-helical neck region of the myosin heavy chain, transmitting the free energy of ATP hydrolysis down the neck domain to amplify movements of myosin and force production during contraction [101]. In skeletal muscle, RLC phosphorylation modulates myosin structure, force, and power output [102, 103] and can influence contractile strength during daily tasks [104]. Estrogen has been implicated in the regulation of RLC phosphorylation and contractility of cardiomyocytes [105]. In skeletal muscle, proteomic studies showed age-dependent reduction of RLC phosphorylation in human vastus lateralis muscle [106] and in rat fast-twitch muscle [107]. Muscle biopsies from young and older men and women also showed that phosphorylation of RLC was reduced in fibers of old, estrogen-deficient women but not old men, relative to young counterparts [108]. Such results implicate estrogenic impact on post-translational modification of myosin RLC.
Direct evidence that estrogen modulates phosphorylation of RLC in skeletal muscle comes from the work of Lai and coworkers [109]. Estradiol treatment increased RLC phosphorylation in C2C12 cells, as well as in skeletal muscles of ovariectomized mice [109, 110]. The estradiol-induced phosphorylation of RLC in female mice was related to force generation as measured by posttetanic potentiation of force [109]. Several other skeletal muscle proteins including myosin binding protein C, myosin essential light chain, troponin I, titin, and nebulin, affect contractility and/or myosin structure when phosphorylated, but whether or not those proteins are modulated by estrogen await additional studies.
The collective evidence supports the view that estrogen enhances muscle strength by affecting the function of myosin, specifically myosin through phosphorylation of the RLC. Future research to identify estrogenic effects on other sarcomeric proteins are warranted, as is determination of estrogen-sensitive kinases that phosphorylate sarcomeric proteins.
Impaired regeneration following injury.
The concept that repeated skeletal muscle injury followed by inadequate repair contributes to muscle weakness in the aged has been put forth [111–113]. Several lines of evidence indicate that estrogen affects the recovery of muscle following injury and thus, estrogen deficiency in females would theoretically further exacerbate muscle weakness.
The best indicator of recovery from injury is the capacity of skeletal muscle to produce force, that is, regain strength [114]. Ovarian hormones have been implicated in the recovery of strength in females because young, adult mice that are ovariectomized have an incomplete recovery of strength following contraction-induced injury [115] and estradiol treatment improves recovery of strength following a traumatic freeze injury [116]. Consistent but less direct evidence for a role of estrogen comes from two studies on aged mice by Rader and Faulkner. Results from those studies demonstrate that strength recovery after eccentric contractions is substantially worse in 25–29 month-old females [117], which are presumably ovarian senescent and thus estrogen deficient, compared to aged males [118].
Mechanistically, it has been hypothesized that regulation of muscle inflammation and satellite cell function are perturbed by the loss of estrogen and may contribute to impaired strength recovery. Although the effects of estrogen on the inflammatory response following injury has been reported in a fair number of studies on females, the results are conflicting. The effects of estrogen on muscle inflammation in studies on women are inconsistent with evidence that estrogen decreases [119], increases [120], or does not affect [121] inflammation following muscle injury. Using rodent models, studies show that estrogen treatment attenuates [122–125], enhances [120, 126–128], or has no effect [129, 130] on muscle inflammation. A recent study showed that a moderate, physiological dose of estradiol given to ovariectomized mice increased the inflammatory response via recruitment of neutrophils following muscle injury and also demonstrated that recovery of strength was enhanced compared to a placebo treatment [116]. It was further proposed that the serum level of estrogen is a critical component that needs to be considered in future research because supraphysiological levels of estrogen may elicit opposite inflammatory effects than physiological levels, contributing to inconsistencies in the literature, as serum estradiol levels are often not reliably measured in rodent studies.
The inflammatory response following skeletal muscle injury subsequently affects the myogenic response during regeneration (Reviewed by [131]). Studies from Tiidus and colleagues indicate that exercise-induced activation of satellite cells is less effective in the absence of estrogen [132, 133] and this response appears to be mediated by estrogen receptors [130, 134]. Similarly, cell culture studies have shown an estrogen dose-dependent increase in myogenic cell proliferation [135]. While, the data in humans is sparse, myogenic regulatory factor gene expression is acutely increased following eccentric exercise in post-menopausal women on HT compared to those not on HT [47].
Current evidence supports the premise that estrogen affects the regeneration of skeletal muscle by influencing the inflammatory and myogenic responses, although the underlying mechanisms are largely unknown and await future research. Studies with more controlled methods of estrogen treatment and reliable serum measurements, robust markers of inflammatory and satellite cell functions, and details of how those processes ultimately affect recovery of muscle strength are needed.
Conclusions
The loss of muscle strength in females due to estrogen deficiency results from both inadequate preservation of skeletal muscle mass and quality of the remaining skeletal muscle (Figure 1). Evidence that estrogen impacts muscle protein turnover and the ubiquitin-proteasome system is overall weak. There is stronger evidence that estrogen protects skeletal muscle against apoptosis via effects on HSPs and mitochondria. Thus, when estrogen is deficient it appears that apoptotic pathways contribute to the loss of muscle mass. The leading candidate causing dynapenia due to estrogen deficiency in females is myosin. Modifications to myosin heavy chain function through phosphorylation of myosin RLC have been demonstrated in both female rodent models and women. Also contributing to the loss of muscle strength in females is aberrant inflammatory and satellite cell responses when estrogen is deficient. Delving deeper into the mechanisms underlying estrogenic effects on skeletal muscle apoptosis, satellite cells, myosin, and overall signaling pathways will further inform scientists and clinicians designing therapeutic strategies to combat sarcopenia and dynapenia.
Highlights.
Estrogen deficiency results in loss of muscle mass through apoptotic mechanisms
Inconclusive evidence that loss of estrogen affects muscle protein turnover
Dynapenia due to estrogen deficiency in females is related to myosin dysfunction
Lack of estrogen impairs muscle regeneration ultimately impacting force generation
Acknowledgements
This work was supported by National Institutes of Health grants R01-AG031743 (DAL) and T32-DK091317 (BCC), and the Academy of Finland (309504; EKL). We thank Georgios Kararigas for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fielding RA, et al. , Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc, 2011. 12(4): p. 249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang XM, et al. , Sarcopenia as a predictor of hospitalization among older people: a systematic review and meta-analysis. Bmc Geriatrics, 2018. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark BC and Manini TM, What is dynapenia? Nutrition, 2012. 28(5): p. 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visser M and Schaap LA, Consequences of sarcopenia. Clinics in geriatric medicine, 2011. 27(3): p. 387–99. [DOI] [PubMed] [Google Scholar]
- 5.Thomas F, et al. , International variability of ages at menarche and menopause: Patterns and main determinants. Human Biology, 2001. 73(2): p. 271–290. [DOI] [PubMed] [Google Scholar]
- 6.Baber RJ, Panay N, and Fenton A, 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric, 2016. 19(2): p. 109–150. [DOI] [PubMed] [Google Scholar]
- 7.Meeuwsen IB, Samson MM, and Verhaar HJ, Evaluation of the applicability of HRT as a preservative of muscle strength in women. Maturitas, 2000. 36(1): p. 49–61. [DOI] [PubMed] [Google Scholar]
- 8.Phillips SK, et al. , Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clinical science, 1993. 84(1): p. 95–8. [DOI] [PubMed] [Google Scholar]
- 9.Samson MM, et al. , Relationships between physical performance measures, age, height and body weight in healthy adults. Age and ageing, 2000. 29(3): p. 235–42. [DOI] [PubMed] [Google Scholar]
- 10.Sowers M, et al. , Physical functioning and menopause states. Obstetrics and gynecology, 2007. 110(6): p. 1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng MH, et al. , Menopause and physical performance--a community-based cross-sectional study. Menopause, 2009. 16(5): p. 892–6. [DOI] [PubMed] [Google Scholar]
- 12.da Camara SM, et al. , Menopausal status and physical performance in middle aged women: a cross-sectional community-based study in Northeast Brazil. PLoS One, 2015. 10(3): p. e0119480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bondarev D, et al. , Physical performance in relation to menopause status and physical activity. 2018. Publish Ahead of Print. [DOI] [PubMed]
- 14.Greising SM, et al. , Hormone therapy and skeletal muscle strength: a meta-analysis. The journals of gerontology. Series A, Biological sciences and medical sciences, 2009. 64(10): p. 1071–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The 2017 hormone therapy position statement of The North American Menopause Society. Menopause, 2017. 24(7): p. 728–753. [DOI] [PubMed] [Google Scholar]
- 16.Koebele SV and Bimonte-Nelson HA, Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas, 2016. 87: p. 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson JF, et al. , A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod, 1982. 27(2): p. 327–39. [DOI] [PubMed] [Google Scholar]
- 18.Greising SM, et al. , Estradiol treatment, physical activity, and muscle function in ovarian-senescent mice. Exp Gerontol, 2011. 46(8): p. 685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brinton RD, Minireview: Translational Animal Models of Human Menopause: Challenges and Emerging Opportunities. Endocrinology, 2012. 153(8): p. 3571–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Spiegeleer A, et al. , Pharmacological Interventions to Improve Muscle Mass, Muscle Strength and Physical Performance in Older People: An Umbrella Review of Systematic Reviews and Meta-analyses. Drugs & aging, 2018. 35(8): p. 719–734. [DOI] [PubMed] [Google Scholar]
- 21.Ottenbacher KJ, et al. , Androgen treatment and muscle strength in elderly men: A meta-analysis. Journal of the American Geriatrics Society, 2006. 54(11): p. 1666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson LJ, Liu H, and Garcia JM, Sex Differences in Muscle Wasting. Advances in experimental medicine and biology, 2017. 1043: p. 153–197. [DOI] [PubMed] [Google Scholar]
- 23.Taaffe DR, et al. , Estrogen replacement, muscle composition, and physical function: The Health ABC Study. Medicine and science in sports and exercise, 2005. 37(10): p. 1741–7. [DOI] [PubMed] [Google Scholar]
- 24.Sipila S, et al. , Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond), 2001. 101(2): p. 147–57. [PubMed] [Google Scholar]
- 25.Ronkainen PH, et al. , Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: a study with monozygotic twin pairs. J Appl Physiol (1985), 2009. 107(1): p. 25–33. [DOI] [PubMed] [Google Scholar]
- 26.Hansen M, Female hormones: do they influence muscle and tendon protein metabolism? Proceedings of the Nutrition Society, 2018. 77(1): p. 32–41. [DOI] [PubMed] [Google Scholar]
- 27.Miller BF, et al. , No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism, 2006. 290(1): p. E163–E168. [DOI] [PubMed] [Google Scholar]
- 28.Smith GI, et al. , Testosterone and progesterone, but not estradiol, stimulate muscle protein synthesis in postmenopausal women. The Journal of clinical endocrinology and metabolism, 2014. 99(1): p. 256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen M, et al. , Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. The journals of gerontology. Series A, Biological sciences and medical sciences, 2012. 67(10): p. 1005–13. [DOI] [PubMed] [Google Scholar]
- 30.Hansen M, et al. , Effect of administration of oral contraceptives on the synthesis and breakdown of myofibrillar proteins in young women. Scandinavian journal of medicine & science in sports, 2011. 21(1): p. 62–72. [DOI] [PubMed] [Google Scholar]
- 31.Smith GI, et al. , Sexually dimorphic effect of aging on skeletal muscle protein synthesis. Biology of sex differences, 2012. 3(1): p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sitnick M, et al. , Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass. J Appl Physiol, 2006. 100(1): p. 286–93. [DOI] [PubMed] [Google Scholar]
- 33.Sinnettsmith PA, Dumelow NW, and Buttery PJ, Effects of Trenbolone Acetate and Zeranol on Protein-Metabolism in Male Castrate and Female Lambs. British Journal of Nutrition, 1983. 50(2): p. 225–234. [DOI] [PubMed] [Google Scholar]
- 34.Toth MJ, et al. , Effects of estradiol and progesterone on body composition, protein synthesis, and lipoprotein lipase in rats. American journal of physiology. Endocrinology and metabolism, 2001. 280(3): p. E496–501. [DOI] [PubMed] [Google Scholar]
- 35.Chevalier S, et al. , Protein anabolic responses to a fed steady state in healthy aging. The journals of gerontology. Series A, Biological sciences and medical sciences, 2011. 66(6): p. 681–8. [DOI] [PubMed] [Google Scholar]
- 36.Hayden JM, Bergen WG, and Merkel RA, Skeletal-Muscle Protein-Metabolism and Serum Growth-Hormone, Insulin, and Cortisol Concentrations in Growing Steers Implanted with Estradiol-17-Beta, Trenbolone Acetate, or Estradiol-17-Beta Plus Trenbolone Acetate. Journal of Animal Science, 1992. 70(7): p. 2109–2119. [DOI] [PubMed] [Google Scholar]
- 37.Kamanga-Sollo E, et al. , Role of G protein-coupled estrogen receptor-1 in estradiol 17 beta-induced alterations in protein synthesis and protein degradation rates in fused bovine satellite cell cultures. Domestic Animal Endocrinology, 2017. 58: p. 90–96. [DOI] [PubMed] [Google Scholar]
- 38.Kamanga-Sollo E, et al. , Effect of Estradiol-17beta on protein synthesis and degradation rates in fused bovine satellite cell cultures. Domestic animal endocrinology, 2010. 39(1): p. 54–62. [DOI] [PubMed] [Google Scholar]
- 39.Wing SS, Deubiquitinating enzymes in skeletal muscle atrophy-An essential role for USP19. The international journal of biochemistry & cell biology, 2016. 79: p. 462–468. [DOI] [PubMed] [Google Scholar]
- 40.Bilodeau PA, Coyne ES, and Wing SS, The ubiquitin proteasome system in atrophying skeletal muscle: roles and regulation. Am J Physiol Cell Physiol, 2016. 311(3): p. C392–403. [DOI] [PubMed] [Google Scholar]
- 41.Bodine SC, et al. , Identification of ubiquitin ligases required for skeletal muscle atrophy. Science, 2001. 294(5547): p. 1704–8. [DOI] [PubMed] [Google Scholar]
- 42.Gomes MD, et al. , Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A, 2001. 98(25): p. 14440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clavel S, et al. , Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat Tibialis Anterior muscle. Mechanisms of ageing and development, 2006. 127(10): p. 794–801. [DOI] [PubMed] [Google Scholar]
- 44.Edstrom E, et al. , Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. The journals of gerontology. Series A, Biological sciences and medical sciences, 2006. 61(7): p. 663–74. [DOI] [PubMed] [Google Scholar]
- 45.Rogers NH, et al. , Loss of ovarian function in mice results in abrogated skeletal muscle PPARdelta and FoxO1-mediated gene expression. Biochemical and biophysical research communications, 2010. 392(1): p. 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Svensson J, et al. , Stimulation of both estrogen and androgen receptors maintains skeletal muscle mass in gonadectomized male mice but mainly via different pathways. Journal of molecular endocrinology, 2010. 45(1): p. 45–57. [DOI] [PubMed] [Google Scholar]
- 47.Dieli-Conwright CM, et al. , Influence of hormone replacement therapy on eccentric exercise induced myogenic gene expression in postmenopausal women. J Appl Physiol (1985), 2009. 107(5): p. 1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pollanen E, et al. , Muscular transcriptome in postmenopausal women with or without hormone replacement. Rejuvenation Res, 2007. 10(4): p. 485–500. [DOI] [PubMed] [Google Scholar]
- 49.Ogawa M, et al. , 17beta-estradiol represses myogenic differentiation by increasing ubiquitin-specific peptidase 19 through estrogen receptor alpha. The Journal of biological chemistry, 2011. 286(48): p. 41455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogawa M, et al. , Female-specific regulation of skeletal muscle mass by USP19 in young mice. The Journal of endocrinology, 2015. 225(3): p. 135–45. [DOI] [PubMed] [Google Scholar]
- 51.Ogawa M, et al. , Daidzein down-regulates ubiquitin-specific protease 19 expression through estrogen receptor beta and increases skeletal muscle mass in young female mice. The Journal of nutritional biochemistry, 2017. 49: p. 63–70. [DOI] [PubMed] [Google Scholar]
- 52.Lee JH and Lee MJ, Emerging roles of the ubiquitin-proteasome system in the steroid receptor signaling. Arch Pharm Res, 2012. 35(3): p. 397–407. [DOI] [PubMed] [Google Scholar]
- 53.Guo L, et al. , MicroRNA-17–92a upregulation by estrogen leads to Bim targeting and inhibition of osteoblast apoptosis. J Cell Sci, 2013. 126(Pt 4): p. 978–88. [DOI] [PubMed] [Google Scholar]
- 54.Ruan Y, et al. , Retarding the senescence of human vascular endothelial cells induced by hydrogen peroxide: effects of 17beta-estradiol (E2) mediated mitochondria protection. Biogerontology, 2014. 15(4): p. 367–75. [DOI] [PubMed] [Google Scholar]
- 55.Xue B, et al. , Estrogen regulation of the brain renin-angiotensin system in protection against angiotensin II-induced sensitization of hypertension. Am J Physiol Heart Circ Physiol, 2014. 307(2): p. H191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou Y, et al. , Modulating expression of brain heat shock proteins by estrogen in ovariectomized mice model of aging. Exp Gerontol, 2010. 45(5): p. 323–30. [DOI] [PubMed] [Google Scholar]
- 57.Knowlton AA and Korzick DH, Estrogen and the female heart. Mol Cell Endocrinol, 2014. 389(1–2): p. 31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nickerson M, et al. , Sexual dimorphism of the intracellular heat shock protein 72 response. J Appl Physiol (1985), 2006. 101(2): p. 566–75. [DOI] [PubMed] [Google Scholar]
- 59.Stice JP and Knowlton AA, Estrogen, NFkappaB, and the heat shock response. Mol Med, 2008. 14(7–8): p. 517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romani WA and Russ DW, Acute effects of sex-specific sex hormones on heat shock proteins in fast muscle of male and female rats. Eur J Appl Physiol, 2013. 113(10): p. 2503–10. [DOI] [PubMed] [Google Scholar]
- 61.Kayani AC, Morton JP, and McArdle A, The exercise-induced stress response in skeletal muscle: failure during aging. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme, 2008. 33(5): p. 1033–41. [DOI] [PubMed] [Google Scholar]
- 62.Lee CE, McArdle A, and Griffiths RD, The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr, 2007. 26(5): p. 524–34. [DOI] [PubMed] [Google Scholar]
- 63.Paroo Z, Dipchand ES, and Noble EG, Estrogen attenuates postexercise HSP70 expression in skeletal muscle. Am J Physiol Cell Physiol, 2002. 282(2): p. C245–51. [DOI] [PubMed] [Google Scholar]
- 64.Vasconsuelo A, Milanesi L, and Boland R, Participation of HSP27 in the antiapoptotic action of 17beta-estradiol in skeletal muscle cells. Cell Stress Chaperones, 2010. 15(2): p. 183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boland R, et al. , 17beta-estradiol signaling in skeletal muscle cells and its relationship to apoptosis. Steroids, 2008. 73(9–10): p. 859–63. [DOI] [PubMed] [Google Scholar]
- 66.La Colla A, Boland R, and Vasconsuelo A, 17beta-Estradiol Abrogates Apoptosis Inhibiting PKCdelta, JNK, and p66Shc Activation in C2C12 Cells. J Cell Biochem, 2015. 116(7): p. 1454–65. [DOI] [PubMed] [Google Scholar]
- 67.La Colla A, Vasconsuelo A, and Boland R, Estradiol exerts antiapoptotic effects in skeletal myoblasts via mitochondrial PTP and MnSOD. J Endocrinol, 2013. 216(3): p. 331–41. [DOI] [PubMed] [Google Scholar]
- 68.La Colla A, et al. , 17-Estradiol Protects Skeletal Myoblasts From Apoptosis Through p53, Bcl-2, and FoxO Families. Journal of Cellular Biochemistry, 2017. 118(1): p. 104–115. [DOI] [PubMed] [Google Scholar]
- 69.Ronda AC, Vasconsuelo A, and Boland R, Extracellular-regulated kinase and p38 mitogen-activated protein kinases are involved in the antiapoptotic action of 17beta-estradiol in skeletal muscle cells. J Endocrinol, 2010. 206(2): p. 235–46. [DOI] [PubMed] [Google Scholar]
- 70.Ronda AC, Vasconsuelo A, and Boland R, 17beta-estradiol protects mitochondrial functions through extracellular-signal-regulated kinase in C2C12 muscle cells. Cell Physiol Biochem, 2013. 32(4): p. 1011–23. [DOI] [PubMed] [Google Scholar]
- 71.Paroo Z, Tiidus PM, and Noble EG, Estrogen attenuates HSP 72 expression in acutely exercised male rodents. Eur J Appl Physiol Occup Physiol, 1999. 80(3): p. 180–4. [DOI] [PubMed] [Google Scholar]
- 72.Bombardier E, et al. , The role of estrogen receptor-alpha in estrogen-mediated regulation of basal and exercise-induced Hsp70 and Hsp27 expression in rat soleus. Can J Physiol Pharmacol, 2013. 91(10): p. 823–29. [DOI] [PubMed] [Google Scholar]
- 73.Bombardier E, et al. , Effects of ovarian sex hormones and downhill running on fiber-type-specific HSP70 expression in rat soleus. J Appl Physiol (1985), 2009. 106(6): p. 2009–15. [DOI] [PubMed] [Google Scholar]
- 74.Wang H, et al. , Activation of GPR30 improves exercise capacity and skeletal muscle strength in senescent female Fischer344 x Brown Norway rats. Biochemical and biophysical research communications, 2016. 475(1): p. 81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stupka N, et al. , Gender differences in muscle inflammation after eccentric exercise. J Appl Physiol (1985), 2000. 89(6): p. 2325–32. [DOI] [PubMed] [Google Scholar]
- 76.Kerksick C, et al. , Gender-related differences in muscle injury, oxidative stress, and apoptosis. Med Sci Sports Exerc, 2008. 40(10): p. 1772–80. [DOI] [PubMed] [Google Scholar]
- 77.Sayed D, et al. , MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem, 2010. 285(26): p. 20281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suzuki Y, et al. , Diazoxide potentiates mesenchymal stem cell survival via NF-kappaB-dependent miR-146a expression by targeting Fas. Am J Physiol Heart Circ Physiol, 2010. 299(4): p. H1077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kangas R, et al. , Circulating miR-21, miR-146a and Fas ligand respond to postmenopausal estrogen-based hormone replacement therapy--a study with monozygotic twin pairs. Mech Ageing Dev, 2014. 143–144: p. 1–8. [DOI] [PubMed] [Google Scholar]
- 80.Ramaswamy M, et al. , Many checkpoints on the road to cell death: regulation of Fas-FasL interactions and Fas signaling in peripheral immune responses. Results Probl Cell Differ, 2009. 49: p. 17–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laakkonen EK, et al. , Estrogenic regulation of skeletal muscle proteome: a study of premenopausal women and postmenopausal MZ cotwins discordant for hormonal therapy. Aging Cell, 2017. 16(6): p. 1276–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ribas V, et al. , Skeletal muscle action of estrogen receptor alpha is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Science translational medicine, 2016. 8(334): p. 334ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ribas V, et al. , Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am J Physiol Endocrinol Metab, 2010. 298(2): p. E304–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Torres MJ, et al. , 17beta-Estradiol Directly Lowers Mitochondrial Membrane Microviscosity and Improves Bioenergetic Function in Skeletal Muscle. Cell metabolism, 2018. 27(1): p. 167–179 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ronkainen PH, et al. , Global gene expression profiles in skeletal muscle of monozygotic female twins discordant for hormone replacement therapy. Aging Cell, 2010. 9(6): p. 1098–110. [DOI] [PubMed] [Google Scholar]
- 86.Pollanen E, et al. , Power training and postmenopausal hormone therapy affect transcriptional control of specific co-regulated gene clusters in skeletal muscle. Age (Dordr), 2010. 32(3): p. 347–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Collins BC, et al. , Deletion of estrogen receptor alpha in skeletal muscle results in impaired contractility in female mice. J Appl Physiol (1985), 2018. 124(4): p. 980–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gorres BK, et al. , In vivo stimulation of oestrogen receptor alpha increases insulin-stimulated skeletal muscle glucose uptake. J Physiol, 2011. 589(Pt 8): p. 2041–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown M, et al. , Estrogen receptor-alpha and -beta and aromatase knockout effects on lower limb muscle mass and contractile function in female mice. Am J Physiol Endocrinol Metab, 2009. 296(4): p. E854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heine PA, et al. , Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A, 2000. 97(23): p. 12729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wattanapermpool J and Reiser PJ, Differential effects of ovariectomy on calcium activation of cardiac and soleus myofilaments. The American journal of physiology, 1999. 277(2): p. H467–73. [DOI] [PubMed] [Google Scholar]
- 92.Moran AL, Warren GL, and Lowe DA, Removal of ovarian hormones from mature mice detrimentally affects muscle contractile function and myosin structural distribution. J Appl Physiol, 2006. 100(2): p. 548–59. [DOI] [PubMed] [Google Scholar]
- 93.Moran AL, et al. , Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol, 2007. 102(4): p. 1387–93. [DOI] [PubMed] [Google Scholar]
- 94.Greising SM, et al. , Estradiol’s beneficial effect on murine muscle function is independent of muscle activity. J Appl Physiol, 2011. 110(1): p. 109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lowe DA, Baltgalvis KA, and Greising SM, Mechanisms Behind Estrogen’s Beneficial Effect on Muscle Strength in Females. Exercise and Sport Sciences Reviews, 2010. 38(2): p. 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Collins BC, et al. , Deletion of estrogen receptor alpha in skeletal muscle results in impaired contractility in female mice. Journal of applied physiology, 2018. 124(4): p. 980–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qaisar R, et al. , Hormone replacement therapy improves contractile function and myonuclear organization of single muscle fibres from postmenopausal monozygotic female twin pairs. The Journal of physiology, 2013. 591(9): p. 2333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stewart MA, et al. , Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc Natl Acad Sci U S A, 2010. 107(1): p. 430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Phung LA, et al. , Age affects myosin relaxation states in skeletal muscle fibers of female but not male mice. PloS one, 2018. 13(9): p. e0199062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Colson BA, et al. , The myosin super-relaxed state is disrupted by estradiol deficiency. Biochemical and biophysical research communications, 2015. 456(1): p. 151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rayment I, et al. , Structure of the actin-myosin complex and its implications for muscle contraction. Science, 1993. 261(5117): p. 58–65. [DOI] [PubMed] [Google Scholar]
- 102.Colson BA, et al. , Differential roles of regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J Physiol, 2010. 588(Pt 6): p. 981–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xeni J, et al. , Myosin light-chain phosphorylation and potentiation of dynamic function in mouse fast muscle. Pflugers Arch, 2011. 462(2): p. 349–58. [DOI] [PubMed] [Google Scholar]
- 104.Baudry S and Duchateau J, Postactivation potentiation in a human muscle: effect on the rate of torque development of tetanic and voluntary isometric contractions. J Appl Physiol (1985), 2007. 102(4): p. 1394–401. [DOI] [PubMed] [Google Scholar]
- 105.Kararigas G, et al. , Transcriptome characterization of estrogen-treated human myocardium identifies myosin regulatory light chain interacting protein as a sex-specific element influencing contractile function. Journal of the American College of Cardiology, 2012. 59(4): p. 410–7. [DOI] [PubMed] [Google Scholar]
- 106.Gelfi C, et al. , The human muscle proteome in aging. Journal of proteome research, 2006. 5(6): p. 1344–53. [DOI] [PubMed] [Google Scholar]
- 107.Gregorich ZR, et al. , Top-Down Targeted Proteomics Reveals Decrease in Myosin Regulatory Light-Chain Phosphorylation That Contributes to Sarcopenic Muscle Dysfunction. Journal of proteome research, 2016. 15(8): p. 2706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miller MS, et al. , Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. Journal of applied physiology, 2013. 115(7): p. 1004–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lai S, et al. , Estradiol modulates myosin regulatory light chain phosphorylation and contractility in skeletal muscle of female mice. Am J Physiol Endocrinol Metab, 2016. 310(9): p. E724–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Collins BC, et al. , Estrogen Regulates the Satellite Cell Compartment in Females. bioRxiv, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brooks SV and Faulkner JA, Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc, 1994. 26(4): p. 432–9. [PubMed] [Google Scholar]
- 112.Faulkner JA, Brooks SV, and Zerba E, Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism. J Gerontol A Biol Sci Med Sci, 1995. 50 Spec No: p. 124–9. [DOI] [PubMed] [Google Scholar]
- 113.Peake J, Della Gatta P, and Cameron-Smith D, Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am J Physiol Regul Integr Comp Physiol, 2010. 298(6): p. R1485–95. [DOI] [PubMed] [Google Scholar]
- 114.Warren GL, Lowe DA, and Armstrong RB, Measurement tools used in the study of eccentric contraction-induced injury. Sports Med, 1999. 27(1): p. 43–59. [DOI] [PubMed] [Google Scholar]
- 115.Kosir AM, et al. , Influence of Ovarian Hormones on Strength Loss in Healthy and Dystrophic Female Mice. Med Sci Sports Exerc, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Le G, et al. , A moderate oestradiol level enhances neutrophil number and activity in muscle after traumatic injury but strength recovery is accelerated. The Journal of physiology, 2018. 596(19): p. 4665–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rader EP and Faulkner JA, Effect of aging on the recovery following contraction-induced injury in muscles of female mice. J Appl Physiol, 2006. 101(3): p. 887–92. [DOI] [PubMed] [Google Scholar]
- 118.Rader EP and Faulkner JA, Recovery from contraction-induced injury is impaired in weight-bearing muscles of old male mice. Journal of applied physiology, 2006. 100(2): p. 656–61. [DOI] [PubMed] [Google Scholar]
- 119.Dieli-Conwright CM, et al. , Hormone therapy attenuates exercise-induced skeletal muscle damage in postmenopausal women. J Appl Physiol (1985), 2009. 107(3): p. 853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.MacIntyre DL, et al. , Different effects of strenuous eccentric exercise on the accumulation of neutrophils in muscle in women and men. European journal of applied physiology, 2000. 81(1–2): p. 47–53. [DOI] [PubMed] [Google Scholar]
- 121.Stupka N, et al. , Gender differences in muscle inflammation after eccentric exercise. Journal of applied physiology, 2000. 89(6): p. 2325–32. [DOI] [PubMed] [Google Scholar]
- 122.Iqbal S, et al. , Progesterone and estrogen influence postexercise leukocyte infiltration in overiectomized female rats. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme, 2008. 33(6): p. 1207–12. [DOI] [PubMed] [Google Scholar]
- 123.St Pierre Schneider B, Correia LA, and Cannon JG, Sex differences in leukocyte invasion in injured murine skeletal muscle. Research in nursing & health, 1999. 22(3): p. 243–50. [DOI] [PubMed] [Google Scholar]
- 124.Stupka N and Tiidus PM, Effects of ovariectomy and estrogen on ischemia reperfusion injury in hindlimbs of female rats. Journal of applied physiology, 2001. 91(4): p. 1828–35. [DOI] [PubMed] [Google Scholar]
- 125.Tiidus PM, et al. , Estrogen effect on post-exercise skeletal muscle neutrophil infiltration and calpain activity. Can J Physiol Pharmacol, 2001. 79(5): p. 400–6. [PubMed] [Google Scholar]
- 126.Fearing CM, et al. , Increased Adipocyte Area in Injured Muscle With Aging and Impaired Remodeling in Female Mice. The journals of gerontology. Series A, Biological sciences and medical sciences, 2016. 71(8): p. 992–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fulkerson ND, Nicholas J, and St Pierre Schneider B, Estrogen modulates 7/4 antigen distribution within eccentrically contracted injured skeletal muscle. Biotechnic & histochemistry: official publication of the Biological Stain Commission, 2015. 90(4): p. 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stupka N, et al. , Cellular adaptation to repeated eccentric exercise-induced muscle damage. Journal of applied physiology, 2001. 91(4): p. 1669–78. [DOI] [PubMed] [Google Scholar]
- 129.Schneider BS, Vigil SA, and Moonie S, Body weight and leukocyte infiltration after an acute exercise-related muscle injury in ovariectomized mice treated with estrogen and progesterone. Gen Comp Endocrinol, 2012. 176(2): p. 144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Velders M, et al. , Selective estrogen receptor-β activation stimulates skeletal muscle growth and regeneration. The FASEB Journal, 2012. 26(5): p. 1909–1920. [DOI] [PubMed] [Google Scholar]
- 131.Tidball JG and Villalta SA, Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol, 2010. 298(5): p. R1173–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Enns DL and Tiidus PM, Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol, 2008. 104(2): p. 347–53. [DOI] [PubMed] [Google Scholar]
- 133.Tiidus PM, Deller M, and Liu XL, Oestrogen influence on myogenic satellite cells following downhill running in male rats: a preliminary study. Acta Physiol Scand, 2005. 184(1): p. 67–72. [DOI] [PubMed] [Google Scholar]
- 134.Enns DL, Iqbal S, and Tiidus PM, Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiologica, 2008. 194(1): p. 81–93. [DOI] [PubMed] [Google Scholar]
- 135.Galluzzo P, et al. , 17β-Estradiol regulates the first steps of skeletal muscle cell differentiation via ER-α-mediated signals. American Journal of Physiology - Cell Physiology, 2009. 297(5): p. C1249–C1262. [DOI] [PubMed] [Google Scholar]