Abstract
Objective:
To investigate the effect of providing comprehensive personalized risk information on concern for chronic disease development.
Methods:
Unaffected first-degree relatives (FDRs) of rheumatoid arthritis (RA) patients (n = 238) were randomly allocated to: 1) disclosure of RA risk personalized to demographics, genetics, biomarkers, and behaviors using a web-based tool (PRE-RA arm, n = 78); 2) PRE-RA with interpretation by a health educator (PRE-RA Plus arm, n = 80); and 3) standard RA education (Comparison arm, n = 80). Concern for developing RA was assessed at baseline and immediately, 6 weeks, 6 months, and 12 months post-intervention.
Results:
FDRs randomized to PRE-RA arms were less concerned about developing RA than the Comparison arm at all post-intervention assessments (p < 0.05). Among those concerned about RA risk at baseline, the PRE-RA (OR = 4.7, 95%CI 1.5–14.4) and PRE-RA Plus (OR = 5.2, 95%CI 1.6–17.3) arms were more likely to have reassurance 6 months post-intervention than the Comparison arm.
Conclusion:
A comprehensive tool provided reassurance to those at risk for developing a chronic disease, with or without interpretation from a health educator, compared to standard education.
Practice implications:
Individuals may be more likely to be reassured using a personalized chronic disease risk disclosure tool than a standard non-personalized approach.
Keywords: Personalized medicine, Genetics, Concern, Rheumatoid arthritis, Prevention
1. Introduction
The pathogenesis and risk factors for chronic diseases are becoming better understood and disease risk tools tailored to these factors have become more popular. These tools seek to educate at-risk individuals about early signs and symptoms, encourage behavior changes to lower risk, and encouraging screening for individuals with early disease manifestations. Personalized risk tools aimed at the lay public have been developed for many cancers, cardiovascular disease, heart disease, stroke, osteoporosis, diabetes, chronic obstructive pulmonary disease, and Alzheimer’s disease, among others [1–6]. These tools are available in multiple formats including written or web-based materials, in-person or telephone counseling, and motivational interviewing sessions [1,2]. Some strategies present genetic information alone to communicate disease risk [3] while others focus on behavioral factors to help educate patients on modifiable risk [7]. However, presenting risk of a chronic disease without clear options to lower that risk may have unintended negative effects by inducing psychological distress, thus could result in more harm than benefit, particularly for those disclosed to have high risk [8]. Individuals at risk for a chronic disease with high levels of concern may be more likely to seek these tools. Therefore, the effect of novel personalized risk disclosure methods on psychological distress measures, such as concern for developing disease, is important to understand.
RA affects about 1% of the population, and is characterized by a chronic inflammatory arthritis that may result in disability as well as increased morbidity and mortality [9,10]. RA risk factors include demographics (female sex, increasing age), family history, genetics (HLA-DRB1), biomarkers (RA-related autoantibodies, cyclic citrullinated peptide [CCP] and rheumatoid factor [RF]), and lifestyle (smoking, low fish intake, obesity, and poor dental health).
Some prior research on risk education tools and anxiety suggests that distress may not be induced. Many chronic disease risk communication programs have demonstrated a more realistic estimate of chronic disease risk after initial overestimation [4,7] as well as lowered disease-related concern [11,12]. Many of these studies were performed using risk tools using only genetic factors so it is unclear whether more comprehensive chronic disease risk tools might have different effects on concern. We previously developed [13] a comprehensive chronic disease risk tool incorporating demographics, genetics, biomarkers, and lifestyle factors to estimate risk of rheumatoid arthritis (RA) and assessed effects on RA-related concern.
The Personalized Risk Estimator for RA (PRE-RA) Family Study (ClinicalTrials.gov identifier: NCT02046005) was a randomized controlled trial conducted among first-degree relatives (FDRs) of patients with RA, since they are familiar with RA and motivated to participate in prevention efforts. We randomized FDRs to receive RA risk to one of three strategies: 1) a web-based risk calculator called the PRE-RA tool, 2) the PRE-RA tool plus a session with a health educator using motivational interviewing concepts (with intent to improve RA risk-related health behaviors), or 3) a Comparison arm receiving standard information about RA risk factors and epidemiology. The PRE-RA tool was personalized to demographics, genetic, biomarker, and lifestyle risk factors. The main analysis of the PRE-RA Family Study found that those randomized to the PRE-RA tool were more likely to report increased motivation to improve at least one RA risk-related behavior (diet, exercise, dental hygiene and smoking) immediately and up to 6 months post intervention. Subjects randomized to PRE-RA arms also reported significantly more behavioral changes than the Comparison arm including increased fish intake, more frequent brushing and flossing, and higher levels of smoking cessation [14].
This secondary analysis aimed to investigate whether the PRERA tool affected levels of and decrease in concern for developing RA. We hypothesized that those who were randomized to the personalized PRE-RA tool would have decreased levels of disease-related concern compared to the Comparison arm.
2. Methods
2.1. Study population and sample
We recruited FDRs of patients with RA. Eligibility criteria was defined as age under 70 years, English-speaking, and no RA or other systemic rheumatic disease, as screened by the Connective Tissue Disease Screening Questionnaire [15] and examined by the study rheumatologist. The study was performed at Brigham and Women’s Hospital, a large tertiary medical center in Boston, Massachusetts. The Partners HealthCare institutional review board approved all aspects of the study.
2.2. Study design and interventions
We performed a randomized controlled trial to test the effects over one year of disclosing personalized RA risk compared to a standard, non-personalized strategy each with a 6-month booster education session. The protocol and prior results have previously been published [13,14,16]. At baseline, all participants completed demographic questionnaires and measures of self-perceived lifetime RA risk and RA risk-related concern and had blood specimens collected for genetic/biomarker testing. Participants were randomized to the Comparison arm or one of two active interventions in 1:1:1 ratio using permuted block randomization.
2.2.1. Comparison arm
Those randomized to the Comparison arm received a one-on-one interactive lecture with slides lasting about 20 min from a health educator consisting of standard, non-personalized RA education detailing epidemiology, risk factors, and signs/symptoms of disease with a summary handout. This arm was modeled on standard care, without the personalized results of genetics, autoantibody biomarker status, or lifestyle and participants could ask questions. Individuals randomized to this arm were provided the option to access the web-based PRE-RA tool after the conclusion of their participation in the study (12 months after intervention).
2.2.2. PRE-RA arm
Those randomized to the PRE-RA arm received the web-based, personalized RA risk educational tool. The PRE-RA web-based risk education tool was modified from YourDiseaseRisk (http://www.yourdiseaserisk.wustl.edu), a freely available website providing personalized risk estimation of 17 chronic diseases including 12 cancers, emphysema, cardiovascular disease, stroke, diabetes, and osteoporosis [6]. The details of the PRE-RA tool are described in detail elsewhere [13]. The PRE-RA tool calculated individualized estimates of RA risk by compiling demographic data, family history, lifestyle factors (diet, exercise, smoking, and dental hygiene), genetic information (presence or absence of risk polymorphisms in HLA-DRB1, the strongest genetic risk factor for RA) as well as RA-related autoantibody status (CCP and RF). Research assistants input genetic/biomarker results into the web-based tool prior to the study visit, but otherwise all data were reported by the participant and summarized by the tool in real time. Based on this information, individuals received a personalized RA risk result summary including both a relative and an absolute lifetime risk of developing RA. These risks were displayed in both a numeric and pictorial format for those with differing levels of numeric literacy. Since only lifestyle risk factors are modifiable, the risk results were interactive to emphasize RA risk-related behaviors; subjects could visualize how their RA risk might change if they adopted specific combinations of lifestyle changes. The risk education tool also contained links to websites with useful information on RA and tips on lifestyle changes for those interested in obtaining additional education. Subjects interacted with the PRE-RA tool without guidance from study staff, typically for about 15 min. Subjects also received printouts of the RA risk summary results and the same handout that those in the Comparison arm received.
2.2.3. PRE-RA Plus arm
Those randomized to the PRE-RA Plus arm received the web-based PRE-RA tool identical to those in the PRE-RA arm as well as a one-on-one session with a health educator trained in using motivational interviewing techniques. The health educator’s purpose was to facilitate the understanding of their personalized RA risk results and to identify modifiable lifestyle factors that could potentially lower risk. The health educator was trained by a behavioral scientist. The subject typically spent about 20 min for a face-to-face summary of the results and discussion on possible health behavior changes using motivational interviewing techniques with the health educator after the subject completed the PRE-RA tool. Subjects also received printouts of the RA risk summary results and the same handout that those in the Comparison arm received.
2.2.4. 6-month booster session
After completing questionnaires for the 6-month follow-up visit, all participants received a booster education session per the RA educational intervention they were originally assigned. The purpose of this session was to ensure that subjects randomized to PRE-RA and PRE-RA Plus had a second chance to review their RA risk results to refresh any information that may have been forgotten. The booster session also served as an attention control to ensure that subjects took time to review the broad spectrum of risk information.
2.3. Outcome
The primary outcome of this analysis was concern about RA risk, measured by questionnaires at baseline and immediately, 6 weeks, 6 months, and 12 months post-intervention. Concern was measured on a 5-point Likert scale: 0 (not at all concerned), 1 (a little concerned), 2 (somewhat concerned), 3 (quite concerned) and 4 (extremely concerned). Concern was also analyzed as both a continuous variable (based on the numerical values from the ordinal scale, ranging from 0 to 4) and a dichotomous variable (somewhat, quite, or extremely concerned vs. not at all or a little concerned).
2.4. Covariates
Covariates were assessed at baseline and included age, sex, race, education, relationship to affected relative with RA, and self-perceived lifetime RA risk. Self-perceived lifetime risk was assessed as a percentage by asking subjects “On a scale of 0–100%, what do you believe your chances are of developing RA sometime in your life?”. Risk tendency scores were calculated using the Risk Propensity Scale [17], a measure designed to assess tolerance or aversion to risk. A higher risk tendency score is interpreted as being more likely to take risks. Attitudes about contributors to RA risk were determined among subjects by asking them to rate the extent to which they believe lifestyle factors, autoantibodies, and genetics impact RA risk. Contemplation of RA risk was a composite measure defined as answering “yes” to at least three of five statements assessing aspects of RA risk contemplation: “I am worried about getting RA”, “I am curious about my risk for RA”, “I want to learn more about RA”, “I want to find out ways to lower my risk for RA”, and “I want to get blood tests for RA”.
2.5. Statistical analyses
We reported descriptive characteristics at baseline for randomized subjects stratified by study arm.
We first analyzed self-perceived lifetime RA risk at baseline as a continuous variable. Only among those in the PRE-RA and PRE-RA Plus arms, we used a paired-samples t-test to compare baseline self-perceived lifetime risk of RA to the lifetime RA risk calculated by the PRE-RA tool. We did not include subjects in the Comparison arm in this analysis because they did not complete the PRE-RA tool so did not have a calculated lifetime RA risk. We then derived the “overestimation” of RA risk by subtracting the calculated risk from the self-perceived risk. Among this same subset, we performed unadjusted and multivariable linear regression analyses to investigate whether baseline characteristics were associated with the outcome of overestimation of lifetime RA risk. We included all considered covariates in the multivariable model.
Our primary analysis compared RA risk-related concern in the PRE-RA and PRE-RA Plus arms to the Comparison arm at each post-intervention time-point (immediate, 6-week, 6-month, and 12-month) using the concern variable as a continuous value. We obtained p values using a linear regression model with continuous RA-risk related concern as the dependent variable and study arm as the independent variable (reference = Comparison arm), adjusting for concern at baseline. We analyzed each time point independently among those with data. There was relatively low (<10%) loss to follow-up and this was not differential by study arm, study flow diagram previously published [16]. To evaluate for the incremental effect of the health educator, we also compared the PRE-RA Plus arm to the PRE-RA arm.
In secondary analyses, we analyzed RA-related concern stratified by high risk vs. low risk scores calculated by the online risk education tool for the PRE-RA or PRE-RA Plus arms. As in our previous publications and based on prior literature risk stratifying for RA, the “high risk” group was defined as having ≥5% lifetime risk of RA and the “low risk” group <5% lifetime risk of RA, only among those randomized to the PRE-RA or PRE-RA Plus arms since they had calculated lifetime RA risk available [14]. We used linear regression to adjust for baseline concern and other covariates (age, sex, education, type of relative with RA, and perceived RA severity) to investigate how risk group was associated with changes in concern over each post-intervention time-point. As in the primary analysis, continuous RA risk-related concern was the outcome of the regression model and risk group (high risk, low risk, Comparison arm [reference]) was the independent variable adjusting for baseline RA-related concern. We also compared the high risk group to the low risk group.
We investigated whether a subset of subjects who were concerned at baseline were more or less likely to have a decrease in concern after the intervention. We defined “decreased concern” as any decrease in concern at the 6-month visit compared to the baseline visit among subjects who indicated “somewhat” or greater RA risk-related concern at baseline. We used logistic regression to calculate the odds ratio for decrease in concern about RA risk (i.e., reassurance) among individuals in the PRE-RA or PRERA Plus arm vs. Comparison arm at the 6-month post-randomization time point.
We considered a two-sided p value<0.05 as statistically significant. All analyses were performed using SAS v.9.4.
3. Results
3.1. Baseline characteristics
Baseline characteristics are displayed in Table 1, overall and by the 3 study arms. All baseline characteristics were balanced across study arms. A total of 238 subjects were randomized to either Comparison (n = 80), PRE-RA (n = 78), or PRE-RA Plus (n = 80) arms. Overall, the study sample was mostly female (77%), white (87%), and college educated or more (78%). At baseline, 56% of the overall study sample was at least “somewhat concerned’ about RA risk. Withdrawal and loss to follow-up were low, with 87% of subjects remaining in the study for the entire 12-month follow-up period [16].
Table 1.
Baseline characteristics of first-degrees relatives without rheumatoid arthritis in the PRE-RA Family Study (n = 238).
| All arms (n = 238) | Comparison arm (n = 80) | PRE-RA arm (n = 78) | PRE-RA Plus arm (n = 80) | |
|---|---|---|---|---|
| Sociodemographics and Lifestyle | ||||
| Mean age, years (SD) | 45.6 (14.5) | 43.4 (14.7) | 45.0 (14.9) | 48.3 (13.7) |
| Median age, years (IQR) | 46 (3357) | 43 (3055.5) | 47.5 (3156) | 50 (3959) |
| Female, n (%) | 182 (76.5) | 63 (78.8) | 62 (79.5) | 57 (71.3) |
| White, n (%) | 207 (87.0) | 69 (86.3) | 65 (83.3) | 73 (91.3) |
| College degree or more, n (%) | 186 (78.2) | 64 (80.0) | 59 (75.6) | 63 (78.8) |
| Type of relative affected with RA, n (%) | ||||
| Parent only | 155 (65.1) | 55 (68.8) | 53 (68.0) | 47 (58.8) |
| Sibling only | 38 (16.0) | 9 (11.3) | 13 (16.7) | 16 (20.0) |
| Offspring only | 24 (10.1) | 7 (8.8) | 9 (11.5) | 8 (10.0) |
| >1 relative with RA | 21 (8.8) | 9 (11.3) | 3 (3.9) | 9 (11.3) |
| Risk and Concern about RA | ||||
| Concern about RA risk, n (%) | ||||
| Not at all concerned | 25 (10.5) | 7 (8.8) | 6 (7.7) | 12 (15.0) |
| A little concerned | 81 (34.0) | 23 (28.8) | 30 (38.5) | 28 (35.0) |
| Somewhat concerned | 80 (33.6) | 32 (40.0) | 27 (34.6) | 21 (26.3) |
| Quite concerned | 44 (18.5) | 14 (17.5) | 12 (15.4) | 18 (22.5) |
| Extremely concerned | 8 (3.4) | 4 (5.0) | 3 (3.9) | 1 (1.3) |
| Concerned about RA risk*, n (%) | 132 (55.5) | 50 (62.5) | 42 (53.9) | 40 (50.0) |
| Mean concern about RA risk (SD) | 1.7 (1.0) | 1.8 (1.0) | 1.7 (1.0) | 1.6 (1.0) |
| Median concern about RA risk (IQR) | 2 (1,2) | 2 (1,2) | 2 (1,2) | 2 (1,2) |
| Mean self-perceived lifetime risk of RA, % (SD) | 41.3 (24.3) | 42.9 (25.1) | 38.9 (22.8) | 42.0 (25.0) |
| Median self-perceived lifetime risk of RA, % (IQR) | 45 (2550) | 50 (2060) | 30 (2550) | 50 (22.5,57.5) |
| Perceived RA severity of affected relative, n (%) | ||||
| Mild | 27 (11.3) | 11 (13.8) | 9 (11.5) | 7 (8.8) |
| Moderate | 137 (57.6) | 44 (55.0) | 50 (64.1) | 43 (53.8) |
| Severe | 59 (24.8) | 21 (26.3) | 12 (15.4) | 26 (32.5) |
| Unsure | 15 (6.3) | 4 (5.0) | 7 (9.0) | 4 (5.0) |
| Attitudes about Contributors to RA Risk | ||||
| Enrolled due to contemplating RA risk**, n (%) | 83 (34.9) | 24 (30.0) | 31 (39.7) | 28 (35.0) |
| Lifestyle factors are important for RA risk, n (%) | 52 (21.9) | 18 (22.5) | 19 (24.4) | 15 (18.8) |
| Autoantibodies are important for RA risk, n (%) | 131 (55.0) | 46 (57.5) | 43 (55.1) | 42 (52.5) |
| Genetics are important for RA risk, n (%) | 186 (78.2) | 61 (76.3) | 59 (75.6) | 66 (82.5) |
| Mean risk tendency score*** (SD) | 3.77 (1.1) | 3.85 (1.0) | 3.61 (1.1) | 3.84 (1.1) |
| Median risk tendency score*** (IQR) | 3.71 (3.00,4.43) | 3.86 (3.21,4.43) | 3.43 (2.71,4.43) | 3.71 (3.21,4.50) |
There were no missing data and all characteristics were balanced across the three study arms.
Concerned about RA risk was defined as somewhat, quite, or extremely concerned about RA risk.
Contemplation of RA risk was defined as answering “yes” to at least three of five statements assessing aspects of RA risk contemplation. Statements included: “I am worried about getting RA”, “I am curious about my risk for RA”, “I want to learn more about RA”, “I want to find out ways to lower my risk for RA”, and “I want to get blood tests for RA”.
A higher risk tendency score is interpreted as being more likely to take risks.
3.2. Self-perceived lifetime risk of RA analyses
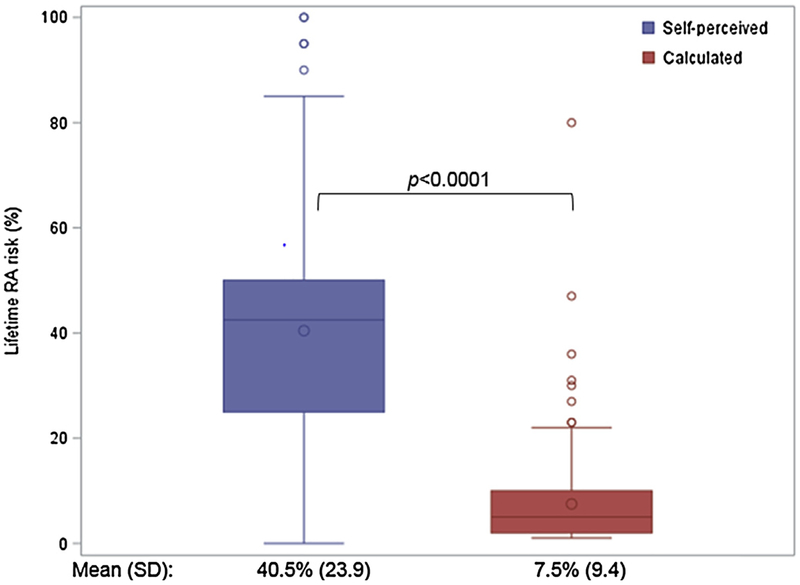
Fig. 1 shows the mean self-perceived lifetime risk of RA among the PRE-RA and PRE-RA plus arms at baseline and the calculated lifetime RA risk from the PRE-RA tool. FDRs overestimated their lifetime RA risk (mean: 40.5%, SD: 23.9%) compared to the calculated lifetime RA risk (mean: 7.5%, SD: 9.4; p < 0.0001). As displayed in Table 2, concern for developing RA (multivariable β = 15.6, 95%CI 7.2–24.1) and enrollment due to contemplating RA risk (β = 10.1, 95%CI 1.6–18.7) were the only baseline predictors significantly associated with overestimation of RA risk in the multivariable model.
Fig. 1.
Among subjects in the PRE-RA and PRE-RA Plus arms, self-perceived (blue) and calculated (red) lifetime RA risk (n = 158) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Table 2.
Unadjusted and multivariable associations for overestimation* of lifetime risk for RA (%) according to baseline characteristics among subjects in the PRE-RA and PRE-RA Plus arms (n = 158).
| Unadjusted | Multivariable** | |||||
|---|---|---|---|---|---|---|
| β | (95% CI) | p value | β | (95% CI) | p value | |
| Concerned about developing RA (vs. not)*** | 23.0 | (15.6,30.4) | <0.001 | 15.6 | (7.2,24.1) | <0.001 |
| Enrolled due to contemplating RA risk (vs. not)**** | 19.9 | (11.9,27.9) | <0.001 | 10.1 | (1.6,18.7) | 0.02 |
| Risk tendency score (continuous, per unit) | 3.1 | (−0.6,6.8) | 0.10 | 2.9 | (−0.5,6.3) | 0.09 |
| Genetics are important for RA risk (vs. not) | 14.8 | (4.8,24.8) | 0.004 | 8.1 | (−1.3,17.5) | 0.09 |
| Lifestyle factors are important for RA risk (vs. not) | 15.2 | (5.4,24.9) | 0.003 | 5.2 | (−4.4,14.7) | 0.29 |
| Autoantibodies are important for RA risk (vs. not) | 6.2 | (−2.1,14.4) | 0.14 | 1.1 | (−6.7,8.9) | 0.77 |
| College degree or more (vs. less education) | −1.7 | (−12.0,8.5) | 0.74 | −1.5 | (−11.4,8.3) | 0.76 |
| Female (vs. male) | 5.6 | (−3.1,14.4) | 0.20 | 1.5 | (−7.3,10.3) | 0.73 |
| Age (continuous, per year) | −0.2 | (−0.5,0.1) | 0.22 | −0.02 | (−0.3,0.2) | 0.86 |
CI, confidence interval; PRE-RA, Personalized Risk Estimator for Rheumatoid Arthritis; RA, rheumatoid arthritis.
Overestimation of lifetime RA risk was calculated by: self-perceived risk – calculated risk from the PRE-RA tool.
Adjusted for all variables listed in the table.
Those categorized as “concerned about RA risk” indicated somewhat, quite, or extremely concerned about RA risk on baseline survey.
Contemplation of RA risk was defined as answering “yes” to at least three of five statements assessing aspects of RA risk contemplation. Statements included: “I am worried about getting RA”, “I am curious about my risk for RA”, “I want to learn more about RA”, “I want to find out ways to lower my risk for RA”, and “I want to get blood tests for RA”.
3.3. RA risk-related concern over time
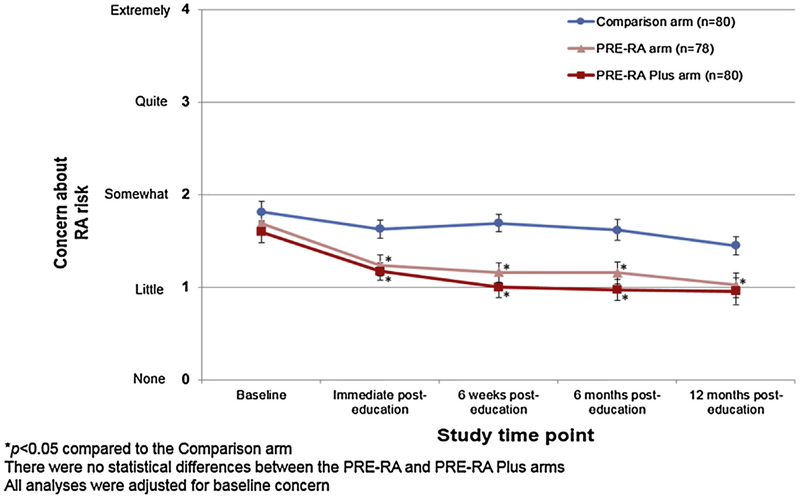
The primary analysis, displayed in Fig. 2, was the trend of RA risk-related concern over all study time points across the three study arms. At baseline prior to the intervention, all study arms had a similar mean RA risk-related concern, between somewhat and a little concerned. Immediately post-intervention, concern in both the PRE-RA (mean 1.2, SD 1.0) and PRE-RA Plus arms (mean 1.2, SD 0.8) was significantly decreased (p < 0.01) compared to the Comparison arm (mean 1.6, SD 0.9). This statistically significant decrease in concern persisted over the 12 months of follow-up in the PRE-RA arm. In the PRE-RA Plus arm, this decrease persisted until 12 months post-education. There were no significant differences between the PRE-RA and PRE-RA Plus arms for concern at any point.
Fig. 2.
Trajectory during follow-up of concern about developing RA by study arm (n = 238).
3.4. Secondary concern analyses
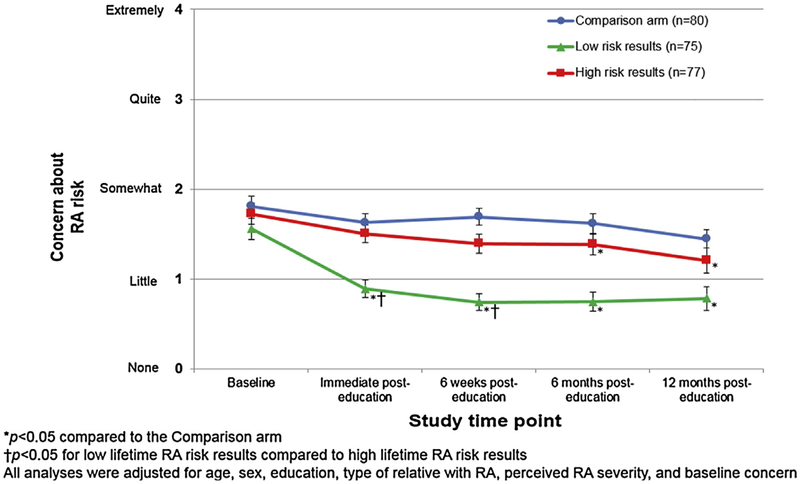
As displayed in Fig. 3, trends in RA risk-related concern were analyzed over time among subjects calculated by the web-based tool to be at high and low lifetime risk for RA vs. those randomized to the Comparison arm. Immediately post-intervention, those calculated to be at low lifetime risk of RA had a statistically significant decrease in concern compared to both the high risk group and Comparison arm. At 6 months and 12 months post-intervention, both the high risk and low risk groups had statistically significant lower levels of RA-related concern than the Comparison arm (p < 0.05). The high risk group did not demonstrate a higher level of concern than the Comparison arm, despite disclosure about their high risk status by the PRE-RA tool.
Fig. 3.
Trajectory during follow-up of concern about developing RA by Comparison arm or, among those in the PRE-RA or PRE-RA Plus arms, stratified by high (≥5%), or low (<5%) calculated lifetime RA risk results (n = 232).
Among subjects who indicated “somewhat” or more RA risk-related concern at baseline, those in the PRE-RA arm were found to have an OR of 4.7 (95%CI 1.5–14.4) for decrease in concern compared to the Comparison arm at 6 months post-intervention compared to baseline (Table 3). Subjects in the PRE-RA Plus arm had OR of 5.2 (95%CI, 1.6–17.3) for decrease in concern compared to the Comparison arm at 6 months post-intervention.
Table 3.
Odds ratios for decrease in concern about RA risk between baseline and 6 months* after RA educational intervention among subjects from all arms who were concerned at baseline (n = 132).
| Decreased concern about RA risk at 6 months / Total in stratum (n) | OR (95% CI) | p value | |
|---|---|---|---|
| By study arm | |||
| Comparison arm | 26/44 | 1.0 | Ref |
| PRE-RA arm | 34/39 | 4.7 (1.5,14.4) | 0.006 |
| PRE-RA Plus arm | 30/34 | 5.2 (1.6,17.3) | 0.007 |
CI, confidence interval; PRE-RA, OR, odds ratio; Personalized Risk Estimator for Rheumatoid Arthritis; RA, rheumatoid arthritis.
This subset analyzed subjects that were somewhat or more concerned about RA risk at baseline. The outcome was decrease in concern, defined as lower concern about RA risk at 6 months compared to baseline.
4. Discussion
This randomized controlled trial conducted among healthy FDRs of RA patients suggests that a personalized, web-based RA risk calculator lowered RA-related concern on a long-term basis post-intervention. Even subjects who were disclosed to be at high lifetime RA risk tended to have lower levels of concern than the Comparison arm, and demonstrated decrease in their RA risk-related concern. The addition of a health educator to the PRE-RA tool provided only a subtle incremental benefit in decreasing concern, suggesting that subjects were mostly reassured by using the web-based tool without much additional benefit of one-on-one interpretation. We found that subjects tended to overestimate their lifetime risk of RA by over five-fold compared to a calculated estimate personalized to their demographics, genetics, biomarkers, and lifestyle. RA risk-related concern was strongly associated with overestimation of lifetime RA risk, suggesting that having a relative with RA may influence risk perception and suggests that education may help individuals to better calibrate accurate disease risk. To our knowledge, our study is the first to investigate changes in concern for developing a chronic disease after risk disclosure using a comprehensive tool that includes genetic/biomarkers results and also personalized to demographics and behaviors.
Previous literature also suggests that many populations including FDRs, at-risk individuals, and healthy individuals may overestimate their self-perceived of chronic diseases [1,7,18–20]. FDRs in our study estimated their absolute lifetime risk of RA to be40.5%. Among those randomized to receive the PRE-RA tool, the mean calculated lifetime RA risk was 7.5%, consistent with literature estimates of RA risk in FDRs based on family history [21]. This result supports previous findings of perceived vulnerability and overestimation among relatives of affected individuals and could be a result of familiarity with the disease through experience with the affected family member and over-inflation of the genetic contribution to chronic disease risk [22,23]. Previous studies suggest that after presenting subjects with accurate risk of disease self-perceived risk drops to a more realistic level [1,20].
We found that individuals randomized to the PRE-RA arm had significantly decreased RA risk-related concern at all time points after intervention. Risk summary tools and risk counseling has been shown previously to decrease chronic risk-related concern [2,11,12,24]. Chronic disease risk-related concern has also been shown to decrease after risk-disclosure methods that involve a genetic counselor [2]. Prior studies have also stratified based on high or low risk groups for a chronic disease. For example, a prospective study investigating breast cancer risk found that regardless of risk group (high or low), women who attended genetic counseling were found to have less worry immediately afterward and this persisted for up to a year later [11]. Another study testing the effect of individual genetic counseling among a population of women at increased risk by having BRCA1 or BRCA2 mutations found a decrease in breast cancer worry [12]. In our study, even those disclosed to be at high risk for RA had lowered levels of concern during the follow-up period compared to subjects who did not receive personalized risk disclosure. These results suggest that a personalized medicine approach integrating a variety of risk factors for chronic disease can be successfully integrated with positive psychological effects without requiring interpretation from a health educator or counselor.
A major strength of this study was its design as a randomized controlled trial, and thus it was unlikely to be confounded by baseline factors. Another advantage of the study was the use of the PRE-RA tool, a comprehensive RA risk tool. The tool was modeled on YourDiseaseRisk (http://www.yourdiseaserisk.wustl.edu), which has been used successfully to estimate risk of many chronic diseases and cancers. We modified the tool to estimate RA risk by integrating information across many risk categories and to present risk estimates in a numeric, pictorial, and interactive way for ease of comprehension [13,25]. Next, our study had twelve months of follow-up data and little loss to follow-up, with 87% of subjects remaining in the study for the entire 12-month follow-up period [16]. Subjects disclosed to have low RA risk on the PRE-RA tool were still more likely to increase motivation than those in the Comparison arm [14]. This demonstrates that even though concern decreased, the intervention still had positive health benefits for this group. Finally, our study recruited FDRs of patients known to have RA, so there was no uncertainty as to whether subjects truly had a relative affected with RA.
Our study does have some limitations. First, the self-reported measure that we used to determine RA-related concern may not have accurately captured disease-related concern. However, a previous study showed that disclosure of genetics to those at risk for Alzheimer’s disease had minimal impact on a variety of validated psychological measures even among those who were disclosed to be at high risk, similar to our findings [26]. We did not measure self-perceived lifetime risk of RA after intervention, so were unable to study whether this changed during follow-up period and whether it may have mediated the disease risk-related concern. This study was a secondary analysis and therefore, findings should be considered hypothesis-generating as opposed to conclusive. Due to the nature of the intervention, we could not blind subjects and research staff to study arm, which may have resulted in performance or detection bias. While we modeled the content of our comparison arm after standard of care, we used a one-on-one presentation format to present these results to ensure the participant’s attention. Since the PRE-RA arm had no interaction with the health educator and still lowered RA risk-related concern, we find it unlikely that this explains our findings. However, alternate methods to communicate disease risk in the Comparison arm might have affected the results. Since RA is relatively uncommon even among FDRs, a majority of participants overestimated their risk. It is unclear if similar findings would occur for individuals at risk for more prevalent chronic diseases where overestimation of risk may not be as common as in our study. Finally, our study population was mostly female, white, and educated, so our findings may not be generalizable to other populations. This was an educated and motivated population, so less educated and unmotivated populations may be less likely to make lifestyle changes after the intervention. However, even more pronounced effects could occur in a population with more lifestyle risk factors and a larger knowledge deficit.
In conclusion, we demonstrated that a comprehensive chronic disease risk tool lowered RA risk-related concern in FDRs of RA patients. Subjects who received personalized RA risk were more likely to be reassured than those not receiving personalized RA risk, regardless of whether they were deemed to be high or low risk and interpretation from a health educator provided only subtle improvements beyond the web-based personalized risk disclosure tool. Future studies should further investigate the overall psychological and health behavior impact of personalized medicine strategies for chronic disease risk disclosure.
Acknowledgments
Funding/support
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) under award number P60 AR047782. Dr. Sparks is supported by NIAMS under award numbers K23 AR069688, L30 AR066953, P30 AR070253, and P30 AR072577. Dr. Karlson is supported by NIAMS under award numbers K24 AR052403, R01 AR049880, P30 AR070253, and P30 AR069625. Dr. Green is supported by U01 HG006500, U19 HD077671, R01 HG005092, U01 HG008685, and U41 HG006834. Dr. Deane is supported by UM1 AI110503. Dr. Iversen is supported by R01 AR059086, Pzifer, Norrebacka Eugenia Foundation, and the Swedish Rheumatism Foundation. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests statement
All authors report no competing interests.
References
- [1].Gurmankin AD, Domchek S, Stopfer J, Fels C, Armstrong K, Patients’ resistance to risk information in genetic counseling for BRCA1/2, Arch. Intern. Med 165 (5) (2005) 523–529. [DOI] [PubMed] [Google Scholar]
- [2].Helmes AW, Culver JO, Bowen DJ, Results of a randomized study of telephone versus in-person breast cancer risk counseling, Patient Educ. Couns 64 (1–3) (2006) 96–103. [DOI] [PubMed] [Google Scholar]
- [3].Chao S, Roberts JS, Marteau TM, Silliman R, Cupples LA, Green RC, Health behavior changes after genetic risk assessment for alzheimer disease: the REVEAL study, Alzheimer Dis. Assoc. Disord 22 (1) (2008) 94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Grant RW, O’Brien KE, Waxler JL, Vassy JL, Delahanty LM, Bissett LG, Green RC, Stember KG, Guiducci C, Park ER, Florez JC, Meigs JB, Personalized genetic risk counseling to motivate diabetes prevention: a randomized trial, Diabetes Care 36 (1) (2013) 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Emmons KM, Wong M, Puleo E, Weinstein N, Fletcher R, Colditz G, Tailored computer-based cancer risk communication: correcting colorectal cancer risk perception, J Health Commun 9 (2) (2004) 127–141. [DOI] [PubMed] [Google Scholar]
- [6].R.f. http://www.yourdiseaserisk.wustl.edu. (Accessed 4/6/2018 2018).
- [7].Weinstein ND, Atwood K, Puleo E, Fletcher R, Colditz G, Emmons K, Colon cancer: risk perceptions and risk communication, J. Health Commun 9 (1) (2004) 53–65. [DOI] [PubMed] [Google Scholar]
- [8].Stack RJ, Stoffer M, Englbrecht M, Mosor E, Falahee M, Simons G, Smolen J, Schett G, Buckley CD, Kumar K, Hansson M, Hueber A, Stamm T, Raza K, Perceptions of risk and predictive testing held by the first-degree relatives of patients with rheumatoid arthritis in England, Austria and Germany: a qualitative study, BMJ Open 6 (6) (2016)e010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Spector TD, Rheumatoid arthritis, Rheum. Dis. Clin. North Am 16 (3) (1990) 513–537. [PubMed] [Google Scholar]
- [10].Sparks JA, Chang SC, Liao KP, Lu B, Fine AR, Solomon DH, Costenbader KH, Karlson EW, Rheumatoid arthritis and mortality among women during 36 years of prospective follow-up: results from the nurses’ health study, Arthritis Care Res. (Hoboken) 68 (6) (2016) 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bish A, Sutton S, Jacobs C, Levene S, Ramirez A, Hodgson S, Changes in psychological distress after cancer genetic counselling: a comparison of affected and unaffected women, Br. J. Cancer 86 (1) (2002) 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bowen DJ, Burke W, Culver JO, Press N, Crystal S, Effects of counseling Ashkenazi Jewish women about breast cancer risk, Cultur. Divers. Ethnic Minor. Psychol 12 (1) (2006) 45–56. [DOI] [PubMed] [Google Scholar]
- [13].Sparks JA, Iversen MD, Miller Kroouze R, Mahmoud TG, Triedman NA, Kalia SS, Atkinson ML, Lu B, Deane KD, Costenbader KH, Green RC, Karlson EW, Personalized risk estimator for rheumatoid arthritis (PRE-RA) family study: rationale and design for a randomized controlled trial evaluating rheumatoid arthritis risk education to first-degree relatives, Contemp. Clin. Trials 39 (1) (2014) 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sparks JA, Iversen MD, Yu Z, Triedman NA, Prado MG, Miller Kroouze R, Kalia SS, Atkinson ML, Mody EA, Helfgott SM, Todd DJ, Dellaripa PF, Bermas BL, Costenbader KH, Deane KD, Lu B, Green RC, Karlson EW, Disclosure of personalized rheumatoid arthritis risk using genetics, biomarkers, and lifestyle factors to motivate health behavior improvements:a randomized controlled trial, Arthritis Care Res (Hoboken) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, Liang MH, A connective tissue disease screening questionnaire for population studies, Ann. Epidemiol 5 (4) (1995) 297–302. [DOI] [PubMed] [Google Scholar]
- [16].Prado MG, Iversen MD, Yu Z, Miller Kroouze R, Triedman NA, Kalia SS, Lu B, Green RC, Karlson EW, Sparks JA, Effectiveness of a web-based personalized rheumatoid arthritis risk tool with or without a health educator for knowledge of RA risk factors, Arthritis Care Res. (Hoboken) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Meertens RM, Lion R, Measuring an individual’s tendency to take risks: the risk propensity scale, J. Appl. Soc. Psychol 38 (6) (2008) 1506–1520. [Google Scholar]
- [18].Sivell S, Elwyn G, Gaff CL, Clarke AJ, Iredale R, Shaw C, Dundon J, Thornton H, Edwards A, How risk is perceived, constructed and interpreted by clients in clinical genetics, and the effects on decision making: systematic review, J. Genet. Couns 17 (1) (2008) 30–63. [DOI] [PubMed] [Google Scholar]
- [19].Shaheen NJ, Green B, Medapalli RK, Mitchell KL, Wei JT, Schmitz SM, West LM, Brown A, Noble M, Sultan S, Provenzale D, The perception of cancer risk in patients with prevalent Barrett’s esophagus enrolled in an endoscopic surveillance program, Gastroenterology 129 (2) (2005) 429–436. [DOI] [PubMed] [Google Scholar]
- [20].Linnenbringer E, Roberts JS, Hiraki S, Cupples LA, Green RC, I know what you told me, but this is what I think:” perceived risk of alzheimer disease among individuals who accurately recall their genetics-based risk estimate, Genet. Med 12 (4) (2010) 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Crowson CS, Matteson EL, Myasoedova E, Michet CJ, Ernste FC, Warrington KJ, Davis JM 3rd., Hunder GG, Therneau TM, Gabriel SE, The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases, Arthritis Rheum. 63 (3) (2011) 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jacobsen PB, Lamonde LA, Honour M, Kash K, Hudson PB, Pow-Sang J, Relation of family history of prostate cancer to perceived vulnerability and screening behavior, Psychooncology 13 (2) (2004) 80–85. [DOI] [PubMed] [Google Scholar]
- [23].Montgomery GH, Erblich J, DiLorenzo T, Bovbjerg DH, Family and friends with disease: their impact on perceived risk, Prev. Med 37 (3) (2003) 242–249. [DOI] [PubMed] [Google Scholar]
- [24].Bowen DJ, Powers D, Greenlee H, Effects of breast cancer risk counseling for sexual minority women, Health Care Women Int 27 (1) (2006) 59–74. [DOI] [PubMed] [Google Scholar]
- [25].Lautenbach DM, Christensen KD, Sparks JA, Green RC, Communicating genetic risk information for common disorders in the era of genomic medicine, Annu. Rev. Genomics Hum. Genet 14 (2013) 491–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ashida S, Koehly LM, Roberts JS, Chen CA, Hiraki S, Green RC, The role of disease perceptions and results sharing in psychological adaptation after genetic susceptibility testing: the REVEAL study, Eur. J. Hum. Genet 18 (12)(2010) 1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]