Abstract
Background:
Type-2 diabetes (T2D) increases the arrhythmias risk through incompletely elucidated mechanisms. Ventricular arrhythmias could be initiated by delayed afterdepolarizations (DADs) resulting from elevated spontaneous sarcoplasmic reticulum (SR) Ca2+ release (SR Ca2+ leak).
Objective:
To test the role of DADs and SR Ca2+ leak in triggering arrhythmias in T2D hearts.
Methods:
We compared rats with late-onset T2D that display pancreatic and cardiac phenotypes similar to those in humans with T2D (HIP rats) and their non-diabetic littermates (WT).
Results:
HIP rats showed higher propensity for premature ventricular complexes and ventricular tachyarrhythmias while HIP myocytes displayed more frequent DADs and had lower SR Ca2+ content compared to the WT. However, the threshold SR Ca2+ at which depolarizing transient inward currents (Itis) are generated was also significantly decreased in HIP myocytes and was below the actual SR Ca2+ load, which explains the increased DAD incidence despite reduced Ca2+ in SR. In agreement with these findings, Ca2+ spark frequency was augmented in myocytes from HIP vs. WT rats, which suggests activation of ryanodine receptors (RyR) in HIP hearts. Indeed, RyR phosphorylation (by CaMKII and PKA) and oxidation are enhanced in HIP hearts, while there is no RyR O-GlcNAcylation in either HIP or control hearts. CaMKII inhibition dissipated the difference in Ca2+ spark frequency between HIP and WT myocytes.
Conclusions:
The threshold SR Ca2+ for generating depolarizing Itis is lower in T2D due to RyR activation following hyperphosphorylation and oxidation, which favors the occurrence of DADs despite low SR Ca2+ loads.
Keywords: type-2 diabetes, transient inward current, afterdepolarizations, ryanodine receptors, CaMKII
Introduction
Type-2 diabetes (T2D) is generally associated with structural, contractile and electrical abnormalities of the heart, even in the absence of coronary artery disease or hypertension.1–3 Diabetic cardiomyopathy is characterized by diastolic and systolic dysfunction,4–6 hypertrophy,1,3,7 fibrosis3,4 and arrhythmias, including ventricular tachyarrhythmias and fibrillation.8–11 However, the mechanisms driving electrical remodeling of the heart in T2D are poorly understood.
Ventricular tachyarrhythmias may occur following a premature excitation of the ventricle caused by simultaneous delayed afterdepolarizations (DADs) in a large number of neighboring myocytes.12 At cellular level, DADs are produced by a transient inward current (Iti) generated by an increase in cytosolic Ca2+ due to spontaneous SR Ca2+ release during diastole (SR Ca2+ leak). SR leak is critically regulated by the amount of Ca2+ in the SR. Several studies reported lower SR Ca2+ content in hearts from various T2D animal models,13–17 which resulted in reduced amplitude of cytosolic Ca2+ transients. In agreement with impaired SR Ca2+ load, Pereira et al. found lower Ca2+ spark frequency in T2D db/db mice compared to their non-diabetic controls.15 However, other studies reported that SR Ca2+ leak is actually increased in myocytes from db/db mice16,17 Thus, it is unclear whether/how SR Ca2+ leak is altered in T2D hearts. Moreover, the db/db mouse model is not particularly translational because these mice are extremely obese and their pancreatic phenotype is different from that observed in humans with T2D as they lack deposits of amylin amyloid in the islets (because rodent amylin is not amyloidogenic)18 and the presence of β-cell mass depletion is strongly dependent on the genetic background.19 In addition, these prior studies focused entirely on Ca2+ handling and/or ryanodine receptor (RyR) function, without connecting them to the electrical activity of the myocyte.
Here we used the HIP rat model18 of late-onset T2D to test the role of DADs and SR Ca2+ leak in triggering ventricular arrhythmias in T2D hearts. These rats present the major manifestations of T2D in humans including insulin resistance, hyperglycemia, amylin amyloid deposition and loss of β-cell mass.18,20 Moreover, we have previously demonstrated that HIP rats develop cardiac hypertrophy,13,21 heart dysfunction13,21 and ventricular arrhythmias.22
Methods
Detailed methods are in the Online Supplement.
Experimental animals
All animal experiments conformed to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at University of Kentucky. The T2D HIP rats used here are obese Sprague-Dawley rats that express the human isoform of amylin, a pancreatic hormone co-secreted with insulin, specifically in the pancreatic β-cells.18 Secretion of amylin is increased in parallel with that of insulin in obesity and insulin resistance.23 Human, but not rodent, amylin is amyloidogenic. Therefore, hypersecretion of amylin in humans leads to amylin deposition in the pancreas, which is an active participant in the gradual decline in β-cell mass that results in late onset T2D.23 A similar mechanism occurs in HIP rats.18,20 Male HIP rats develop late-onset T2D (by 10–12 months of age) 18,21 and show cardiac hypertrophy and dysfunction.13,21 Only males were used here because HIP females develop T2D at more advanced aged (∼18 months). WT littermates served as non-diabetic controls. A total of N=32 HIP and N=32 WT rats were used in the study.
Standard surface ECGs were recorded on anaesthetized rats at baseline and for 20 min following injection of caffeine and dobutamine. Ventricular myocytes were isolated by Langendorff perfusion with collagenase.21
Experiments on isolated myocytes
DADs were recorded under current clamp for 10 s following pre-conditioning at 1Hz. Voltage-clamp was used to determine the threshold SR Ca2+ load required for generation of a depolarizing Iti as in Pogwizd et al.24 Iti occurrence was monitored for 30 sec at −80 mV, followed by emptying the SR with caffeine. SR Ca2+ load was calculated as the sum of the integrals of Iti and caffeine-induced current. The threshold SR Ca2+ load for Iti occurrence was determined as the average of the two lowest loads that gave an Iti and the two highest that did not.
SR Ca2+ leak was measured using the tetracaine method.25 Ca2+ sparks were recorded in linescan mode in myocytes pre-conditioned by pacing at 0.5 Hz.
Tissue analyses
Western blot experiments were performed on heart homogenates. Protein bands were visualized with a SynGene Imaging machine and band intensity was quantified using ImageJ software. RyR oxidation was assessed by measuring the amount of free thiol residues contained by RyRs using the monobrobimane (mBB) method.26 CaMKII activity was measured as a function of 32P-ATP incorporation into the synthetic substrate syntide-2, as previously described.22
Statistical analysis
Statistical differences between groups were determined using the student’s t-test or Fisher’s exact test, as appropriate. Data are presented as mean±standard error. *P<0.05, **P<0.01; ***P<0.001
Results
Increased incidence of arrhythmias and delayed afterdepolarizations in diabetic rats.
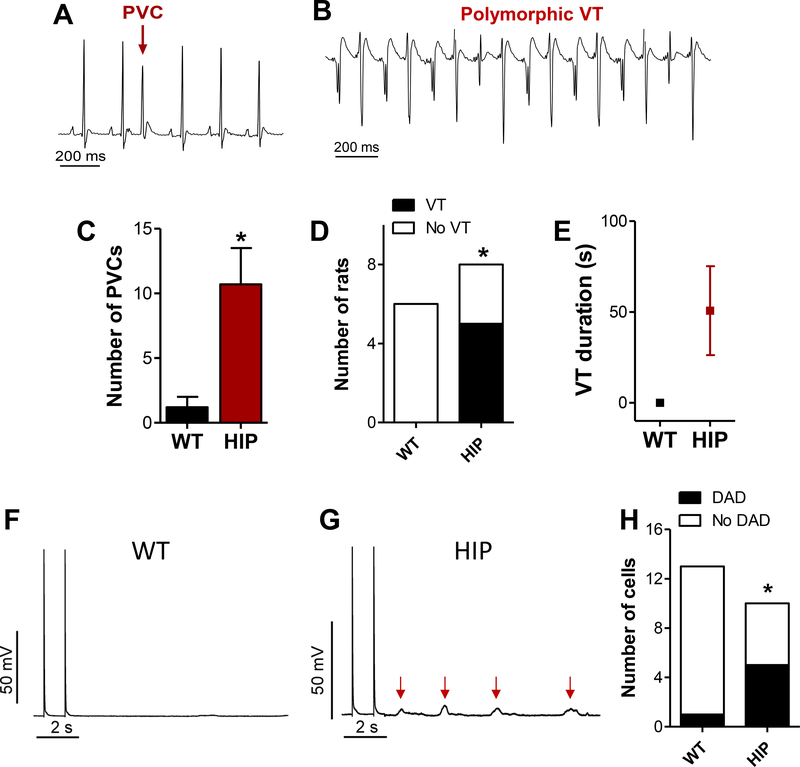
Similar to humans with T2D, diabetic HIP rats display abnormal electrical activity following a challenge with dobutamine and caffeine (Fig 1), including premature ventricular complexes (PVC; Fig 1A) and both polymorphic and monomorphic ventricular tachyarrhythmias (VT; Fig 1B). The frequency of PVCs was significantly higher in HIP vs. WT rats (Fig. 1C). The incidence (Fig. 1D) and average total duration (Fig. 1E) of VTs was also increased in HIP rats.
Figure 1. Abnormal electrical activity in diabetic HIP rats.
(A-B) Premature ventricular complex (PVC) and ventricular tachyarrhythmia (VT) recorded in HIP rats during a stress test with caffeine and dobutamine. (C) Mean number of PVCs recorded within 20 min following injection of caffeine and dobutamine in WT and HIP rats. (D) Number or rats showing VT within 20 min following injection of caffeine and dobutamine. (E) Average VT duration. (F-G) Measurement of DADs in WT (F) and HIP (G) myocytes. Current-clamped myocytes were pre-conditioned by pacing at 1Hz for 2 min. The occurrence of DADs was recorded for 10 s after pacing ended. Traces show the last two pacing-induced action potentials and the membrane potential during the following rest period. Red arrows point to DADs. (H) Frequency of DADs in myocytes from HIP and WT rats. N=13/10 myocytes/rats for WT and 15/10 for HIP. Statistical significance in panels D and H was assessed with Fisher’s exact test.
PVCs are generally triggered by DADs occurring simultaneously in a large number of neighboring myocytes and they may initiate VTs. Thus, we compared the frequency of DADs occurring in myocytes from WT and HIP rats during a 10 s period following pre-conditioning at 1Hz (Fig 1F-G). HIP myocytes showed higher propensity for generating DADs compared to WT cells (Fig 1H), which may explain the increased arrhythmogenicity in HIP rats. Most DADs were in the form of classical small depolarizations (Fig 1G). However, two HIP myocytes displayed large, action potential-like DADs, characterized by a slow initial depolarization (Online Figure I).
Lower threshold SR Ca2+ load for induction of transient inward currents and larger SR Ca2+ leak in myocytes from diabetic rats.
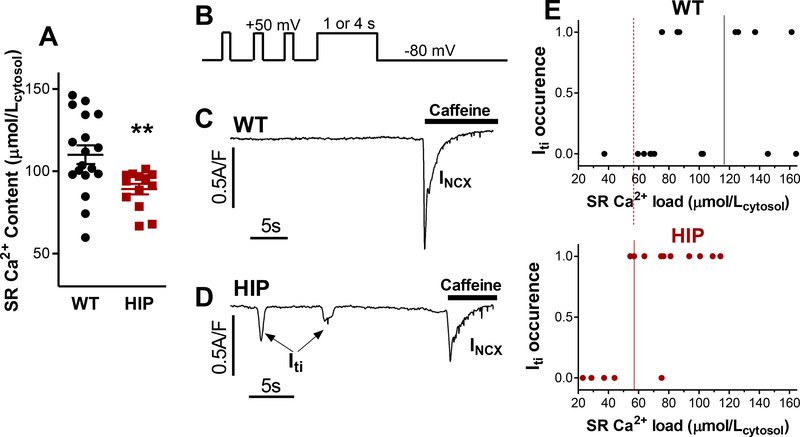
DADs are generated by Itis triggered by Ca2+ released from the SR. SR Ca2+ leak increases with higher Ca2+ levels in the SR. However, prior studies found lower SR Ca2+ content in T2D hearts.13–17 We found that this is also the case in HIP rats (89±3 μmol/Lcytosol vs. 110±6 μmol/Lcytosol in WT myocytes; Fig. 2A). The decreased SR Ca2+ load in HIP myocytes is likely the result of impaired SERCA activity, which is evidenced by the slower decline of twitch-induced Ca2+ transients (Online Figure II). To explain the higher DAD incidence despite reduced SR Ca2+ load in HIP myocytes, we measured the threshold SR Ca2+ at which depolarizing Itis are generated in myocytes from HIP vs. WT rats (Fig. 2B-E). In these experiments, the SR of voltage clamped-myocytes was pre-loaded with varying amounts of Ca2+ by depolarizing the myocyte to +50 mV for either 1 or 4 seconds (Fig 2B). Depolarization results in the reversal of the Na+/Ca2+ exchanger (NCX), which now transports Ca2+ into the cells. L-type Ca2+ channels will also open initially, and thus contribute to Ca2+ loading. Ca2+ is then taken from cytosol into the SR by SERCA. The occurrence of Iti was measured on repolarization to −80 mV. After 30 s, caffeine was applied to release all Ca2+ remaining in the SR; this Ca2+ is then extruded from the cell by NCX, generating a large inward current (INCX in Fig. 2C-D). Assuming that Iti is carried mainly by NCX,27 SR Ca2+ load was calculated from the sum of Iti and INCX integrals. Fig. 2C-D shows examples of such measurements in WT and HIP myocytes. These experiments indicate that the threshold SR Ca2+ content needed for generating Ca2+ waves that trigger Itis is greatly reduced in myocytes from HIP vs. WT rats (58±7 vs. 118±22 μmol/Lcytosol, P=0.04; Fig. 2E). The decreased threshold favors the occurrence of DADs at lower SR Ca2+ in HIP myocytes.
Figure 2. Reduced threshold SR Ca2+ for Iti occurrence in myocytes from HIP vs. WT rats.
(A) Steady-state SR Ca2+ content in myocytes from HIP and WT rats assessed from the amplitude of caffeine-induced Ca2+ transient. (B) Voltage protocol used for measuring the Iti threshold. Myocytes were conditioned by applying 100 ms depolarization pulses from −80 to +50 mV for 2 min (only last 3 are shown). Then, cells were depolarized to +50mV for 1 or 4 seconds, to drive Ca2+ into the cell via reverse-mode NCX, thus increasing the SR Ca2+ content. Iti was measured on repolarization to −80 mV (for 30 s). (C-D) Examples of current recordings following repolarization in a WT (C) and a HIP (D) myocyte. Caffeine was applied at the end to release all Ca2+ from the SR. The ensuing inward current is carried mainly by NCX (INCX). The sum of Iti and INCX integrals indicates the SR Ca2+ load. (E) Threshold SR Ca2+ load for Iti occurrence in HIP and WT myocytes.
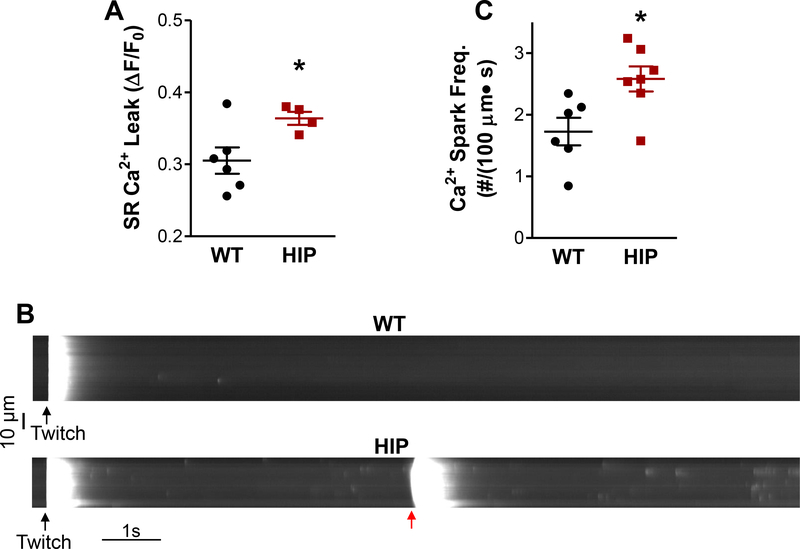
The data above suggest that SR Ca2+ leak is elevated in myocytes from HIP rats compared to the WT. We found that this is indeed the case (Fig. 3A). SR Ca2+ leak occurs as both Ca2+ sparks and smaller Ca2+ release events. Since occurrence of Ca2+ waves and consequent Itis is promoted by increased Ca2+ spark frequency, we recorded sparks in WT and HIP myocytes (Fig. 3B). Note the occurrence of a Ca2+ wave in the HIP cell (red arrow in Fig 3B). Ca2+ spark frequency was significantly higher in myocytes from diabetic HIP rats compared to non-diabetic WT littermates (2.6±0.2 vs. 1.7±0.2 sparks/100 μm·s; Fig. 3C). Ca2+ spark amplitude was lower in HIP rat myocytes (Online Figure IIIA), likely due to smaller SR Ca2+ content and thus reduced Ca2+ flux from the SR during a spark, while Ca2+ spark width and duration were similar in WT and HIP myocytes (Online Figure IIIB-C).
Figure 3. Higher SR Ca2+ leak and Ca2+ spark frequency in HIP rat myocytes.
(A) Total RyRmediated SR Ca2+ leak in WT and HIP myocytes. N=4 HIP and N=6 WT rats (3–8 myocytes/rat) (B) Representative line-scan recordings of spontaneous Ca2+ sparks in myocytes from HIP and WT rats preconditioned by steady-state pacing at 0.5 Hz (last stimulation-induced Ca2+ transient is indicated by black arrow). (C) Mean Ca2+ spark frequency in HIP and WT myocytes. N=7 HIP and N=6 WT rats (4–8 myocytes/rat). In (A) and (C), data were first averaged over cells from the same rat, then the mean SR leak/spark frequency per rat were averaged for each group.
Increased RyR phosphorylation and oxidation in diabetic rat hearts
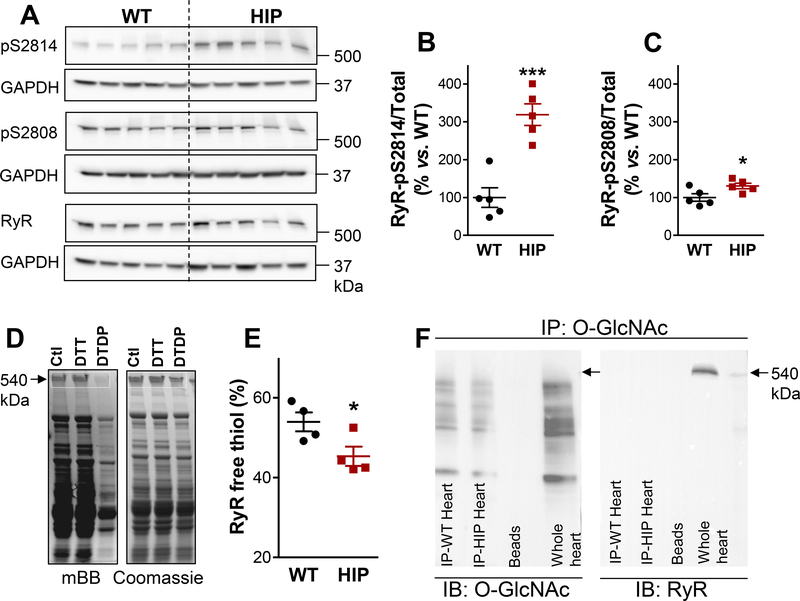
Higher SR Ca2+ leak at lower SR Ca2+ in HIP myocytes suggests that spontaneous RyR activity is enhanced compared to the WT. The total amount of RyRs is similar in hearts from HIP and WT rats (Online Figure IV). However, RyRs are activated by several post-translational modifications, including phosphorylation (by CAMKII at Ser2814 and PKA at Ser2808) and oxidation of thiol residues. We assessed the level of RyR phosphorylation using immunoblots and phospho-specific antibodies (Fig. 4A). While phosphorylation at both Ser2814 and Ser2808 sites was significantly enhanced in heart homogenates from HIP vs. WT rats (Fig. 4B-C), the effect was particularly strong at the CaMKII site (3fold increase vs. WT; Fig. 4B). Of note, phospholamban was also hyperphosphorylated by CaMKII in HIP hearts, whereas there were no significant differences in PKA-mediated phosphorylation between HIP and WT hearts (Online Figure V).
Figure 4. Ehanced RyR phosphorylation and oxidation in diabetic HIP rat hearts.
(A) Immunoblots on heart homogenates with antibodies that recognize RyR phosphorylated at the CaMKII (S2814) and PKA (S2808) sites and total RyR. GAPDH is the loading control. (B-C) Ratio of phosphorylated-to-total RyR for the S2814 (B) and S2808 (C) phosphorylation sites in HIP and WT hearts. N=5 hearts/group and immunoblots were repeated 4 times. (D) Measurement of free thiols contained in RyRs. Myocytes from each heart were divided in three groups, lysed and incubated under control conditions (Ctl), with the reducing agent dithiothreitol (DTT) or with the oxidizing agent 2,2’-dithiodipyridine (DTDP). Lysates were then incubated with mBB and loaded on two SDS-PAGE gels for measurements of mBB florescence and, respectively, protein content (by Coomassie Blue staining). (E) Percentage of RyR free thiols calculated from experiments as in panel D. Measurements were performed in duplicate on myocyte lysates from 4 WT and 4 HIP rats. (F) Measurement of RyR O-GlcNAcylation. O-GlcNAcylated proteins immunoprecipitated, separated by SDS-PAGE and probed with antibodies that recognize either OGlcNAc (left panel) or RyR (right panel). Whole heart homogenate was used as positive control for RyR (Whole heart column). Similar experiments were performed on homogenates from 3 WT and 3 HIP hearts.
To assess RyR oxidation, we measured the relative amount of free thiols contained in RyRs using the mBB method (Fig. 4D-E). For each heart, the free thiol level was calculated as the ratio between the mBB fluorescence of RyRs under control conditions and the maximum mBB signal obtained following incubation with the reducing agent DTT (after correcting for the background fluorescence obtained following incubation with the oxidizing agent DTDP). The percentage of free RyR thiols was lower in myocytes from HIP compared to WT rats (Fig. 4E), which denotes increased RyR oxidation in T2D hearts.
Addition of O-linked β-N-acetylglucosamine (O-GlcNAc) to Ser/Thr residues is generally enhanced in T2D and plays a key role in diabetic cardiomyopathy.28 Thus, we investigated whether RyRs are O-GlcNAcylated differently in WT and HIP hearts. For these measurements, heart homogenates containing Thiamet G (selective inhibitor of O-GlcNAcase, the enzyme that removes O-GlcNAc from proteins) were first immunoprecipitated with an anti-O-GlcNAc antibody, then separated by SDS-PAGE and probed with antibodies that recognize either O-GlcNAc or RyR (Fig. 4F). There was no O-GlcNAc protein band with the molecular weight corresponding to RyRs in hearts from either WT or HIP rats (Fig. 4F, left panel). Moreover, the RyR antibody produced no signal in O-GlcNAc immuneprecipates (Fig. 4F, right panel). In contrast, the RyR band was clearly seen in the whole heart homogenate (Fig. 4F, right panel). These results suggest that there is little RyR O-GlcNAcylation in hearts from both WT and HIP rats. Thus, increased RyR phosphorylation and oxidation, but not O-GlcNAc modification, contribute to RyR hyperactivity in diabetic HIP rat hearts.
Enhanced CaMKII activity and its role in SR Ca2+ leak in diabetic hearts
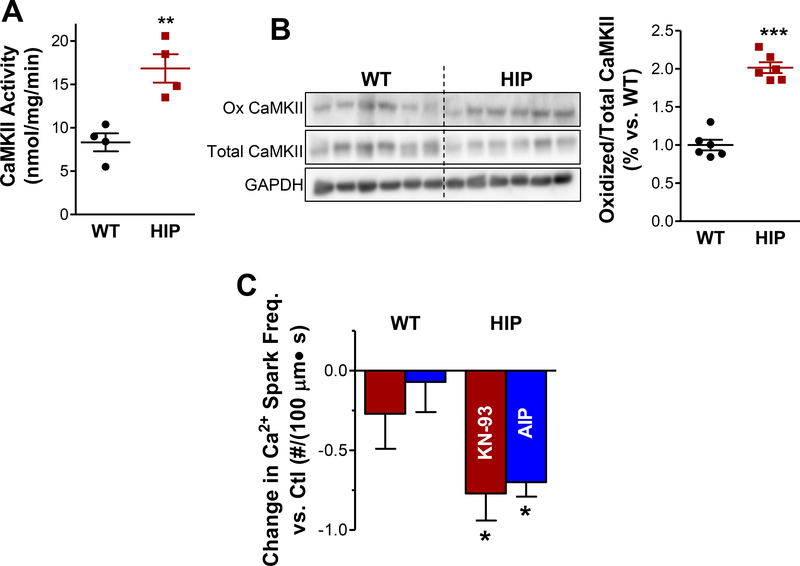
Experiments in Fig. 4 suggest that CaMKII-dependent RyR hyperphosphorylation plays an important role in augmenting the SR Ca2+ leak in myocytes from diabetic rats. In agreement with this result, we found higher CaMKII activity in hearts from diabetic HIP rats compared to WT (Fig. 5A). Ca2+/calmodulindependent CaMKII facilitates post-translational modifications of CaMKII, particularly autophosphorylation at the T287 site, oxidation at M281/M282 and O-GlcNAcylation at S280, that result in autonomous activation of the kinase.29 Increased CaMKII autophosphorylation and O-GlcNAcylation were previously reported in HIP rat hearts.22 We found that CaMKII oxidation is also significantly elevated in hearts from diabetic HIP rats vs. WT (Fig. 5B). Thus, multiple mechanisms contribute to CaMKII activation in diabetic hearts.
Figure 5. Increased CaMKII activity leads to higher Ca2+ spark frequency in HIP rat hearts.
(A) CaMKII activity in hearts from WT and HIP rats. N=4 hearts/group. (B) Representative immunoblots for oxidized and total CaMKII in HIP and WT hearts and the relative amount of oxidized CaMKII. N=6 hearts/group and immunoblots were repeated 5 times. (C) Change in Ca2+ spark frequency produced by CaMKII inhibitors KN-93 and myristoylated-AIP (both 1 μM). Data were collected from 4–6 HIP and 4–6 WT rats (4–8 myocytes/rat). Spark frequency was first averaged over the cells from the same rat and the mean spark frequency per rat was averaged for each group.
In agreement with a major role of CaMKII-mediated RyR hyperphosphorylation in enhancing SR Ca2+ leak, CaMKII inhibition with either KN-93 or the more specific myristoylated-AIP greatly reduced Ca2+ spark frequency in HIP rats while having little effect in the WT (Fig. 5C).
Discussion
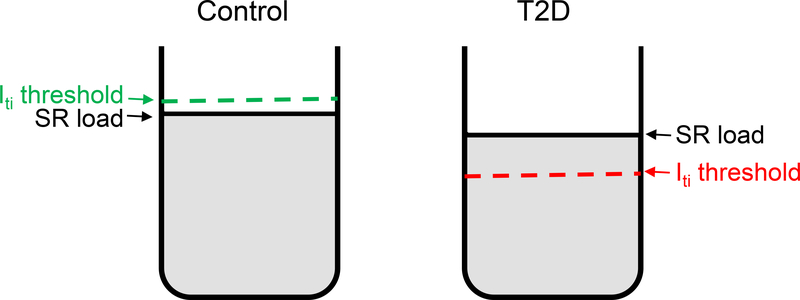
The mechanisms through which T2D increases the propensity for ventricular arrhythmias and sudden cardiac death are incompletely elucidated. Generally, diabetic hearts present structural alterations (fibrosis, etc) that predispose the heart to reentry arrhythmias.3,4 The contribution of triggered activity to the heightened arrhythmia risk is less clear. Using a rat model with late-onset T2D that largely recapitulates both the pancreatic18,20 and cardiac13,21,22 phenotypes present in humans with T2D we found that T2D significantly decreases both the amount of Ca2+ stored in the SR (Fig. 2A) and the threshold SR Ca2+ load at which depolarizing Itis occur (Fig. 2E). In myocytes from control rats the Iti threshold is slightly above the SR Ca2+ content (Fig 6), which makes the occurrence of Ca2+ waves and DADs unlikely. In contrast, in myocytes from HIP rats the Iti threshold is below the actual SR Ca2+ level (Fig 6), which favors the occurrence of DADs and premature beats. The lower Iti threshold in HIP myocytes is due to RyR activation following hyperphosphorylation (mainly by CaMKII, but also PKA) and oxidation. Several studies have linked the T2D-induced impairment in cardiac contraction and relaxation to alterations in myocyte Ca2+ handling.3,13–17 Smaller and slower electrically-triggered Ca2+ transients and decreased SR Ca2+ content have been reported in some T2D animal models and are caused mainly by SERCA downregulation.3,13–17 SERCA expression and function are also reduced in diabetic HIP rats (Despa et al.21 and Online Figure II). Phospholamban hyperphosphorylation (Online Figure V) partially offsets the effect of reduced expression on SERCA activity. Moreover, we reported previously30 that intracellular Na+ concentration is elevated in HIP vs. WT hearts, which reduces Ca2+ extrusion through NCX allowing SERCA to compete better for cytosolic Ca2+. Thus, phospholamban hyperphosphorylation and higher intracellular Na+ limit the decrease in SR Ca2+ load in diabetic hearts, keeping it above the threshold for generating Itis.
Figure 6. Proposed mechanism for the occurrence of DADs in myocytes from diabetic hearts.
In T2D, the threshold SR Ca2+ required for generating Itis and thus DADs decreases below the steady-state SR Ca2+ content.
Despite the lower SR Ca2+ load, SR Ca2+ leak is augmented in diabetic HIP rat hearts. This result is in agreement with some16,17 but not all15 prior studies in db/db mice, so far the only T2D model where SR Ca2+ leak was measured, as well as with a recent investigation in a model of pre-diabetes.31 Of note, the HIP rat is the only T2D animal model that reproduces the accumulation of amylin found in hearts from humans with T2D.13,21 We have previously found that amylin deposition directly perturbs myocyte Ca2+ cycling by increasing Ca2+ leak across the sarcolemma,21,32 which accelerates the remodeling of Ca2+ handling proteins.21 Thus, the T2D-induced alterations in myocyte Ca2+ homeostasis in HIP rats are likely to resemble closer those occurring in humans with T2D.
Besides SR Ca2+ content, RyR open probability is enhanced by posttranslational modifications, including phosphorylation and oxidation. CaMKII-dependent RyR hyperphosphorylation has been previously reported in pre-diabetic31 and T2D hearts.17 We found that it also occurs in HIP rats, where CaMKII-mediated RyR phosphorylation is increased 3-fold compared to non-diabetic hearts (Fig. 4) and CaMKII inhibition normalizes Ca2+ sparks (Fig. 5). In addition, RyR phosphorylation by PKA and RyR oxidation are also elevated in HIP hearts (Fig. 4) and thus contribute to the higher SR Ca2+ leak. We expected that RyRs will also be O-GlcNAcylated in HIP hearts, similar to CaMKII.22 However, we could not detect RyR O-GlcNAcylation in either HIP or WT hearts.
Oxidative stress is a hallmark of diabetic cardiomyopathy.3 Our data indicate that oxidative stress has a dual role in enhancing SR Ca2+ leak in diabetic hearts. On one hand, it directly activates RyRs through oxidation of RyR thiol residues (Fig. 4). On the other hand, it oxidizes CaMKII and thus contributes to its activation and consequent RyR hyperphosphorylation (Fig. 5).
Our results show that RyR activation facilitates the occurrence of Itis at reduced levels of Ca2+ in the SR. Lower SR Ca2+ threshold for triggering an Iti was previously shown to occur and increase the propensity for arrhythmias in a rabbit model of non-ischemic, arrhythmogenic heart failure.24,33 Thus, T2D and heart failure may share a similar cellular mechanism for ectopic events. Threshold SR Ca2+ for initiating spontaneous activity is also lowered by RyR mutations that cause catecholaminergic polymorphic ventricular tachyarrhythmias (CPVT).34 Interestingly however, β-adrenergic stimulation increases the threshold for Ca2+ waves in myocytes expressing both wild-type RyRs and RyRs with a CPVT mutation.35 This result implies that the combination of RyR CaMKII-mediated phosphorylation and oxidation is responsible for lowering the threshold SR Ca2+ load in T2D hearts and circumvents the opposing effect of PKA-mediated phosphorylation.
Clinical implications
Our findings suggest that increasing the threshold SR Ca2+ load for triggering Itis may be a useful therapeutic strategy for preventing DADs and arrhythmias in diabetic hearts. This could potentially be accomplished with dandrolene, a drug used for treating malignant hyperthermia caused by mutations in skeletal muscle RyR1. Indeed, dandrolene increased the wave initiation threshold in myocytes from failing rabbit hearts.33
Conclusions
T2D decreases the threshold SR Ca2+ load at which a transient inward current is generated by enhancing the SR Ca2+ leak through CaMKII-mediated RyR hyperphosphorylation and RyR oxidation. The lower threshold facilitates the occurrence of DADs despite a reduction in SR Ca2+ content and thus constitutes an important mechanism for ventricular arrhythmias in T2D.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institutes of Health [grants HL135000 and HL109501 to S.D. and HL118474 to F.D.]; the Health Research Council of New Zealand [grant 15/331 to J.R.E.].
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation 2000;101:2271–2276. [DOI] [PubMed] [Google Scholar]
- 2.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation 2002;105:1727–1733; [DOI] [PubMed] [Google Scholar]
- 3.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014;57:660671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 2016;12:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. J Am Med Assn 2005;294:334–341. [DOI] [PubMed] [Google Scholar]
- 6.Masoudi FA, Inzucchi SE. Diabetes mellitus and heart failure: epidemiology, mechanisms, and pharmacotherapy. Am J Cardiol 2007;99:113B–132B. [DOI] [PubMed] [Google Scholar]
- 7.Adeghate E, Singh J. Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart Fail Rev 2014;19:15–23. [DOI] [PubMed] [Google Scholar]
- 8.Balkau B, Jouven X, Ducimetière P, Eschwège E. Diabetes as a risk factor for sudden death. Lancet 1999;354:1968–1969. [DOI] [PubMed] [Google Scholar]
- 9.Chen-Scarabelli C, Scarabelli TM. Suboptimal glycemic control, independently of QT interval duration, is associated with increased risk of ventricular arrhythmias in a high-risk population. Pacing Clin Electrophysiol 2006;29:9–14. [DOI] [PubMed] [Google Scholar]
- 10.Movahed MR, Hashemzadeh M, Jamal M. Increased prevalence of ventricular fibrillation in patients with type 2 diabetes mellitus. Heart Vessels 2007;22:251–253. [DOI] [PubMed] [Google Scholar]
- 11.Koektuerk B, Aksoy M, Horlitz M, Bozdag-Turan I, Turan RG. Role of diabetes in heart rhythm disorders. World J Diabetes 2016;7:45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu MB, de Lange E, Garfinkel A, Weiss JN, Qu Z. Delayed afterdepolarizations generate both triggers and a vulnerable substrate promoting reentry in cardiac tissue. Heart Rhythm 2015;12:21152124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Despa S, Sharma S, Harris TR, Dong H, Li N, Chiamvimonvat N, Taegtmeyer H, Margulies KB, Hammock BD, Despa F. Cardioprotection by controlling hyperamylinemia in a “humanized” diabetic rat model. J Am Heart Assoc. 2014;3(4). pii: e001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guglielmino K, Jackson K, Harris TR, et al. Pharmacological inhibition of soluble epoxide hydrolase provides cardioprotection in hyperglycemic rats. Am J Physiol Heart Circ Physiol 2012;303:H853H862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira L, Matthes J, Schuster I, Valdivia HH, Herzig S, Richard S, Gómez AM. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes 2006;55:608–615. [DOI] [PubMed] [Google Scholar]
- 16.Belke DD, Swanson EA, Dillmann WH. Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes 2004;53:3201–3208. [DOI] [PubMed] [Google Scholar]
- 17.Stølen TO, Høydal MA, Kemi OJ, Catalucci D, Ceci M, Aasum E, Larsen T, Rolim N, Condorelli G, Smith GL, Wisløff U. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res 2009;105:527536. [DOI] [PubMed] [Google Scholar]
- 18.Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC. Diabetes due to a progressive defect in beta-cell mass in rats transgenic for human islet amyloid polypeptide (HIP Rat): a new model for type 2 diabetes. Diabetes 2004;53:1509–1516. [DOI] [PubMed] [Google Scholar]
- 19.Boquist L, Hellman B, Lernmark A, Täljedal IB. Influence of the mutation “diabetes” on insulin release and islet morphology in mice of different genetic backgrounds. J Cell Biol 1974;62:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matveyenko AV, Butler PC. β-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type-2 diabetes. Diabetes 2006;55:2106–2114. [DOI] [PubMed] [Google Scholar]
- 21.Despa S, Margulies KB, Chen L, Knowlton AA, Havel PJ, Taegtmeyer H, Bers DM, Despa F. Hyperamylinemia contributes to heart dysfunction in obesity and diabetes, a study in humans and rats. Circ Res 2012;110:598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature 2013;502:372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev 2011;91:795–826. [DOI] [PubMed] [Google Scholar]
- 24.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res 2001;88:1159–1167. [DOI] [PubMed] [Google Scholar]
- 25.Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res. 2002;91:594–600. [DOI] [PubMed] [Google Scholar]
- 26.Bovo E, Lipsius SL, Zima AV. Reactive oxygen species contribute to the development of arrhythmogenic Ca2⁺ waves during β-adrenergic receptor stimulation in rabbit cardiomyocytes. J Physiol 2012;590:3291–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bers DM. Excitation-contraction coupling and cardiac contractile force. Kluwer Academic; 2nd ed., 2001. [Google Scholar]
- 28.Banerjee PS, Lagerlöf O, Hart GW. Roles of O-GlcNAc in chronic diseases of aging. Mol Aspects Med 2016;51:1–15. [DOI] [PubMed] [Google Scholar]
- 29.Erickson JR. Mechanisms of CaMKII Activation in the Heart. Front Pharmacol 2014;5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert R, Srodulski S, Peng X, Margulies KB, Despa F, Despa S. Intracellular Na+ concentration ([Na+]i) is elevated in diabetic hearts due to enhanced Na+-glucose cotransport. J Am Heart Assoc 2015;4 pii:e002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sommese L, Valverde CA, Blanco P, Castro MC, Rueda OV, Kaetzel M, Dedman J, Anderson ME, Mattiazzi A, Palomeque J. Ryanodine receptor phosphorylation by CaMKII promotes spontaneous Ca2+ release events in a rodent model of early stage diabetes: The arrhythmogenic substrate. Int J Cardiol 2016;202:394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu M, Hoskins A, Verma N, Bers DM, Despa S, Despa F. Amylin and diabetic cardiomyopathy - amylin-induced sarcolemmal Ca2+ leak is independent of diabetic remodeling of myocardium. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maxwell JT, Domeier TL, Blatter LA. Dantrolene prevents arrhythmogenic Ca2+ release in heart failure. Am J Physiol Heart Circ Physiol 2012;302:H953–H963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res 2011;108:871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kashimura T, Briston SJ, Trafford AW, Napolitano C, Priori SG, Eisner DA, Venetucci LA. In the RyR2(R4496C) mouse model of CPVT, β-adrenergic stimulation induces Ca waves by increasing SR Ca content and not by decreasing the threshold for Ca waves. Circ Res 2010;107:1483–1489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.