Abstract
Background
The role of gefitinib for the treatment of advanced non‐small cell lung cancer (NSCLC) is evolving. We undertook a systematic review to evaluate the available evidence from all randomised trials.
Objectives
To determine the effectiveness and safety of gefitinib as first‐line, second‐line or maintenance treatment for advanced NSCLC.
Search methods
We performed searches in CENTRAL, MEDLINE and Embase from inception to 17 February 2017. We handsearched relevant conference proceedings, clinical trial registries and references lists of retrieved articles.
Selection criteria
We included trials assessing gefitinib, alone or in combination with other treatment, compared to placebo or other treatments in the first‐ or successive‐line treatment of patients with NSCLC, excluding compassionate use.
Data collection and analysis
We used the standard Cochrane methodology. Two authors independently assessed the search results to select those with sound methodological quality. We carried out all analyses on an intention‐to‐treat basis. We recorded the following outcome data: overall survival, progression‐free survival, toxicity, tumour response and quality of life. We also collected data for the following subgroups: Asian ethnicity and positive epidermal growth factor receptor (EGFR) mutation.
Main results
We included 35 eligible randomised controlled trials (RCTs), which examined 12,089 patients.
General population
Gefitinib did not statistically improve overall survival when compared with placebo or chemotherapy in either first‐ or second‐line settings. Second‐line gefitinib prolonged time to treatment failure (TTF) (hazard ratio (HR) 0.82, 95% confidence interval (CI) 0.75 to 0.90, P < 0.0001) when compared with placebo. Maintenance gefitinib improved progression‐free survival (HR 0.70, 95% CI 0.53 to 0.91, P = 0.007) after first‐line therapy.
Studies in patients of Asian ethnicity or that conducted subgroup analyses
Second‐line gefitinib prolonged overall survival over placebo (HR 0.66, 95% CI 0.48 to 0.91, P = 0.01). In the first‐line setting, progression‐free survival was improved with gefitinib over chemotherapy alone (HR 0.65, 95% CI 0.43 to 0.98, P = 0.04, moderate quality of evidence). Gefitinib given in combination with a chemotherapy regimen improved progression‐free survival versus either gefitinib alone or chemotherapy alone (HR 0.69, 95% CI 0.49 to 0.96, P = 0.03; HR 0.69, 95% CI 0.62 to 0.77, P < 0.00001, respectively). In the second‐line setting, progression‐free survival was superior in patients given gefitinib over placebo or chemotherapy (HR 0.69, 95% CI 0.52 to 0.91, P = 0.009; HR 0.71, 95% CI 0.57 to 0.88, P = 0.002; moderate quality of evidence, respectively). Combining gefitinib with chemotherapy in the second‐line setting was superior to gefitinib alone (HR 0.65, 95% CI 0.43 to 0.97, P = 0.04). As maintenance therapy, gefitinib improved progression‐free survival when compared with placebo (HR 0.42, 95% CI 0.33 to 0.54, P < 0.00001).
Patients with EGFR mutation‐positive tumours
Studies in patients with EGFR mutation‐positive tumours showed an improvement in progression‐free survival in favour of gefitinib over first‐line and second‐line chemotherapy (HR 0.47, 95% CI 0.36 to 0.61, P < 0.00001; HR 0.24, 95% CI 0.12 to 0.47, P < 0.0001, respectively). Gefitinib as maintenance therapy following chemotherapy improved overall and progression‐free survival (HR 0.39, 95% CI 0.15 to 0.98, P = 0.05; HR 0.17, 95% CI 0.07 to 0.41, P < 0.0001, respectively) in one phase III study when compared to placebo.
Toxicities from gefitinib included skin rash, diarrhoea and liver transaminase derangements. Toxicities from chemotherapy included anaemia, neutropenia and neurotoxicity.
In terms of quality of life, gefitinib improved Functional Assessment of Cancer Therapy‐Lung (FACT‐L) (standardised mean difference (SMD) 10.50, 95% CI 9.55 to 11.45, P < 0.000001), lung cancer subscale (SMD 3.63, 95% CI 3.08 to 4.19, P < 0.00001) and Trial Outcome Index (SMD 9.87, 95% CI 1.26 to 18.48, P < 0.00001) scores when compared with chemotherapy.
Authors' conclusions
This systematic review shows that gefitinib, when compared with standard first‐ or second‐line chemotherapy or maintenance therapy, probably has a beneficial effect on progression‐free survival and quality of life in selected patient populations, particularly those with tumours bearing sensitising EGFR mutations.
Patients with EGFR mutations lived longer when given maintenance gefitinib than those given placebo.
One study conducted subgroup analysis and showed that gefitinib improved overall survival over placebo in the second‐line setting in patients of Asian ethnicity. All other studies did not detect any benefit on overall survival. The data analysed in this review were very heterogenous. We were limited in the amount of data that could be pooled, largely due to variations in study design. The risk of bias in most studies was moderate, with some studies not adequately addressing potential selection, attrition and reporting bias. This heterogeneity may have an impact on the applicability of the results
Combining gefitinib with chemotherapy appears to be superior in improving progression‐free survival to either gefitinib or chemotherapy alone, however further data and phase III studies in these settings are required.
Gefitinib has a favourable toxicity profile when compared with current chemotherapy regimens. Although there is no improvement in overall survival, gefitinib compares favourably with cytotoxic chemotherapy in patients with EGFR mutations with a prolongation of progression‐free survival and a lesser side effect profile.
Plain language summary
A comparison of gefitinib with no therapy or chemotherapy for non‐small cell lung cancer
Review question
Do patients with non‐small cell lung cancer live longer if they are given gefitinib?
Background
Non‐small cell lung cancer (the most common type of lung cancer) is a leading cause of cancer death worldwide. People diagnosed with advanced lung cancer may be offered chemotherapy.
Some lung cancers have been found to have a gene mutation, which is an alteration in the chromosome sequence inside the cells. This mutation affects the epidermal growth factor receptor (EGFR), which is a switch on the surface of the cell leading to uncontrolled growth and spread. Gefitinib is a drug that targets cells with mutated EGFR, thus stopping their growth. Studies have found that this mutation is more commonly found in people who are non‐smokers, female, of Asian heritage and with adenocarcinoma (a type of lung cancer).
Study characteristics
We searched for relevant trials up to 17 February 2017. There were a total of 35 studies conducted between 2000 and 2017, evaluating 12,089 participants from multiple countries including North America, Europe and Asia.
Key results
This review showed that patients with advanced lung cancer do not live longer when treated with gefitinib when compared with no other treatment or chemotherapy. In people whose lung cancer has worsened after initial therapy, gefitinib may prolong the time before the cancer progresses further, but only in a selected group of patients of Asian ethnicity or with EGFR mutations. Combining gefitinib with chemotherapy probably increases the time to cancer progression over either gefitinib or chemotherapy alone. For EGFR‐mutation positive patients who are stable after chemotherapy, ongoing gefitinib has been shown to improve survival when compared to placebo.
Severe side effects, such as low red and white blood cell counts and nerve symptoms, occurred more frequently in patients given chemotherapy compared to those given gefitinib. Side effects caused by gefitinib included a skin rash, diarrhoea and liver dysfunction.
Quality of life may be improved in favour of gefitinib when compared with chemotherapy.
Quality of the evidence
When comparing gefitinib as a first‐ and second‐line treatment with chemotherapy, we downgraded the quality of the evidence to moderate for the outcomes overall survival and progression‐free survival because the results were not precise and they may not be applicable to all patients due to the inclusion of a population only over 70 years of age. However, the quality of the evidence when we compared toxicities from gefitinib with chemotherapy was high.
Summary of findings
Background
Description of the condition
Non‐small cell lung cancer (NSCLC) accounts for 14% of all cancer‐related deaths and is by far the leading cause of cancer death among both men and women. In the United States, it was predicted that about 234,030 new cases of NSCLC would be diagnosed, and 154,050 deaths would result from NSCLC in 2018 (ACS 2018). The survival rate for people diagnosed with NSCLC will vary according to the extent (stage) of the cancer. People with locally advanced NSCLC (stage III or more) have a five‐year survival rate of 5% to 36%, and survival estimates do vary according to stage at diagnosis (ACS 2018). Active treatment of NSCLC consists of surgery, radiotherapy and chemotherapy, given as single therapies or in combination. Although there have been major medical therapeutic advances in recent times, these have not been sufficient to significantly affect the high mortality and morbidity rates associated with lung cancer.
The pathogenesis of lung neoplasms is multifactorial, however most can be directly attributed to tobacco smoke exposure. NSCLC arising in smokers has a different spectrum of molecular abnormalities from those in non‐smokers, suggesting differences in aetiology, pathogenesis and possibly prognosis. Mutations of tumour suppressor genes such as p53 and retinoblastoma; stimulation of proto‐oncogenes such as K‐ras, c‐myc and c‐raf; and production of autocrine growth factors are some of the potential pathogenic mechanisms so far described in the development of lung cancer. Recent research has identified two oncogenic drivers, epidermal growth factor receptor (EGFR) mutation and EML4/ALK fusion, for which targeted therapies are available.
Description of the intervention
The epidermal growth factor receptor (EGFR) family of genes encodes a widely expressed transmembrane molecule that is frequently expressed in solid tumours. Overexpression of EGFR has been associated with the pathogenesis, proliferation, invasion and metastasis of various solid tumours, including NSCLC. EGFR is overexpressed in around 40% to 80% of documented cases of primary NSCLC and around 88% of advanced cases of NSCLC (Smith 2005).
Tyrosine kinase inhibitors (TKIs) bind to the intracellular domain of the tyrosine kinase and may inhibit EGFR downstream signalling. Inhibition of tyrosine kinase may, therefore, block EGFR‐mediated cancer cell propagation. TKIs may be classified as reversible or irreversible, and as selective against EGFR or active against other members of the receptor family. Somatic mutations in the region of EGFR that encodes the tyrosine kinase domain of the receptor (exons 18 through 21) have been identified in lung cancer. Such mutations occur more frequently in patients with NSCLC who have the adenocarcinoma sub‐type, women, Asian people and those who have never smoked (Kosaka 2004; Paez 2004). EGFR mutations are associated with both increased growth factor signalling and increased responsiveness to tyrosine kinase inhibitors (Mok 2011).
How the intervention might work
Gefitinib (Iressa, ZD 1839) is an orally active anilinoquinazoline that selectively and reversibly inhibits intracellular EGFR tyrosine kinase activity. Two large, randomised phase II clinical trials assessed the efficacy and safety of gefitinib monotherapy in patients with locally advanced or metastatic NSCLC who failed previous chemotherapy regimens (Fukuoka 2003 IDEAL I; Kris 2003 IDEAL II). Both showed no added benefit in terms of survival, time to progression or response rates compared with standard chemotherapy alone. However these monotherapy trials demonstrated a favourable safety profile. A phase III trial comparing gefitinib to placebo in advanced NSCLC patients who had received prior chemotherapy showed an improvement in progression‐free survival but no prolongation in overall survival (Thatcher 2005 ISEL). Since these early trials, a number of other randomised controlled trials (RCTs) have examined the effectiveness of gefitinib versus placebo or chemotherapy, or in combination with chemotherapy in the first‐ and second‐line settings. Several studies have also examined its role as maintenance therapy following treatment in patients with advanced NSCLC.
Why it is important to do this review
The precise clinical effectiveness of gefitinib in a range of clinical situations remains to be established. This review will bring together all the current evidence of effectiveness, in order to guide clinical management and the discussion of treatment risks and benefits in patients with NSCLC.
Objectives
To determine the effectiveness and safety of gefitinib as first‐line, second‐line or maintenance treatment for advanced NSCLC.
Methods
Criteria for considering studies for this review
Types of studies
We considered all published and unpublished randomised, controlled, phase II and phase III clinical trials of gefitinib as first‐ or second‐line or maintenance therapy in advanced NSCLC. We included any placebo‐controlled trials and trials using comparators. Trials with random allocation, double‐blinding and intention‐to‐treat analysis were preferred. We excluded cross‐over studies, studies that were quasi‐randomised and those that investigated the compassionate use of gefitinib.
Types of participants
Eligible trials included adult participants aged 18 years or older of either sex with histologically or cytologically confirmed NSCLC (stage IIIB/IV) not curable with surgery.
Types of interventions
We considered any administration of gefitinib for advanced NSCLC. This included the use of any dosage of gefitinib as first‐ or second‐line therapy or maintenance therapy:
Gefitinib at any dose compared with placebo or best supportive care.
Gefitinib at any dose compared with chemotherapeutic agents.
Gefitinib at a specific dose versus gefitinib at a different dose.
Gefitinib versus gefitinib combined with a chemotherapy regimen.
Gefitinib at any dose in combination with chemotherapeutic agents versus the same chemotherapy agents alone.
Gefitinib at any dose in combination with chemotherapeutic agents versus a different combination of chemotherapeutic agents.
Types of outcome measures
Primary outcomes
Overall survival (OS), assessed from date of randomisation to date of patient death (time to death).
-
Progression‐free survival (PFS):
Measured from the date of randomisation to the date of objective disease progression, based on Response Evaluation Criteria in Solid Tumours (RECIST), the revised version of the International Union Against Cancer/WHO criteria (Therasse 2000).
Time to treatment failure (TTF): measured from the date of randomisation to the date of study discontinuation (for any reason). This may be reported instead of PFS in some studies.
-
Toxicity (graded according to the National Cancer Institute Common Toxicity Criteria or the World Health Organization criteria (NCI CTCAE 2010).
However, we accepted whatever definitions had been used in the individual trials. A risk ratio (RR) significantly greater than 1 (RR > 1) is a positive response in favour of gefitinib.
Secondary outcomes
Median overall survival (OS) and progression‐free survival (PFS).
Survival rate at one year (1YSR).
-
Tumour response ‐ defined according to the RECIST criteria (Therasse 2000):
Complete response (CR) defined as the disappearance of all target lesions.
Partial response (PR) defined as at least a 30% decrease in the sum of the longest diameter of target lesions.
Overall response rate (ORR) taken as the sum of complete response (CR) rate and partial response (PR) rates.
Stable disease (SD) defined as neither sufficient shrinkage to qualify for partial response nor sufficient increase to qualify for progressive disease.
Disease control rate (DCR) defined as the sum of the ORR and SD rate. This represents all lesions that have either responded to the treatment or stabilised as a result of treatment.
Quality of life (QOL) and symptom response measured by the Functional Assessment of Cancer Therapy‐Lung (FACT‐L) quality of life instrument, the lung cancer subscale (LCS), the Trial Outcome Index (TOI) and the Pulmonary Symptom Index (PSI) (Cella 1995).
Search methods for identification of studies
Electronic searches
We electronically searched for eligible studies using:
The Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 2) (Appendix 1);
MEDLINE via PubMed (1966 to 17 February 2017) (Appendix 2);
Embase via OVID (1980 to Week 08, 2017) (Appendix 3).
We developed the search string for MEDLINE according to the Cochrane Highly Sensitive Search Strategy, sensitivity‐maximising version (2008 version) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.b of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Searching other resources
The authors (ES, IY) also screened reference lists of included and excluded studies, attempted to contact authors of relevant studies and examined registers of ongoing trials: ClinicalTrials.gov (ClinicalTrials.gov) and Current Controlled Trials (isrctn.com) to locate all significant published and unpublished data. We also reviewed conference proceedings of the American Society of Clinical Oncology, the European Cancer Conference, the European Society of Medical Oncology and the International Association for the Study of Lung Cancer, from January 1990 to February 2017. When two articles or more used the same data, we only used the most updated article, unless we found some additional information in that article.
Data collection and analysis
Selection of studies
We assessed the eligibility of retrieved articles from the title and abstract. Two investigators (ES, IY) reviewed potential trials for inclusion and extracted data from the published manuscripts. We resolved disagreements about relevance either by consensus or by referral to a third investigator (RWB). There was no blinding of the authors as to origin or conclusions of the articles for eligibility assessment, data extraction or 'Risk of bias' assessment. We sought data for all patients randomised in all eligible randomised trials. Two review authors (ES, IY) independently carried out data extraction using a specifically designed data extraction form. We recorded study details, including year of publication, numbers of people randomised and analysed per arm, age, sex, race/ethnicity of participants, staging and histological cell type, performance status and any previous treatment. We also recorded the dose and duration of gefitinib treatment, as well as the use of any chemotherapeutic agents. We double‐checked all data for consistency, plausibility and integrity of randomisation and follow‐up.
Data extraction and management
We extracted data from included studies using the guidelines set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
Two authors (ES, IY) independently assessed the risk of bias of included studies according to the areas and criteria proposed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered studies to be at low risk of bias when true randomisation occurred, when there was blinding of assessors to treatment received, when all patients were accounted for and included in the analysis on an 'intention‐to‐treat' basis and when all outcome measures were reported. We also considered studies that were terminated early to have a source of bias of interest.
The results of these judgements are presented in the 'Risk of bias' tables (Characteristics of included studies).
(1) Sequence generation (checking for possible selection bias)
For each included study, we assessed the method used to generate the allocation sequence in sufficient detail to allow an evaluation of whether it should produce comparable groups.
We assessed risk of bias as:
Low risk: any truly random process, e.g. random number table, computer random number generator.
High risk: any non‐random process, e.g. odd or even date of birth, hospital or clinic record number.
Unclear risk: insufficient information about sequence generation process to permit judgement of risk.
(2) Allocation concealment (checking for possible selection bias)
For each included study, we assessed the method used to conceal the allocation sequence to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment.
We assessed risk of bias as:
Low risk: e.g. central or telephone allocation, sequentially numbered drug containers of identical appearance.
High risk: e.g. open random allocation, unsealed or non‐opaque envelopes, alternation or rotation, date of birth.
Unclear risk: insufficient information to permit judgement of 'low risk' or 'high risk' or the study did not address this outcome.
(3) Blinding (checking for possible performance bias)
For each included study, we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received.
We assessed risk of bias as:
Low risk: blinding of participants and key study personnel was ensured and it was unlikely that the blinding could have been broken, or there was no blinding of outcome measurement, but outcome measurement is unlikely to be influenced by the lack of blinding.
High risk: no blinding or incomplete blinding and the outcome is likely to be influenced by lack of blinding.
Unclear risk: insufficient information to permit judgement of 'low risk' or 'high risk' or the study did not address this outcome.
(4) Incomplete outcome data (checking for attrition bias)
For each included study, we reported the completeness of data including attrition and exclusions, the numbers included in the analysis at each stage and the reasons for attrition or exclusion.
We assessed risk of bias as:
Low risk: e.g. if there were any missing outcome data, the reasons for missing outcome data were unlikely to be related to true outcome.
High risk: e.g. reasons for missing outcome data are likely to be related to true outcome.
Unclear risk: insufficient reporting of attrition/exclusions to permit judgement of 'low risk' or 'high risk' or the study did not address this outcome.
(5) Selective reporting (checking for whether the prespecified outcomes were met)
For each included study, we assessed if the study's protocol was available and that the study's prespecified (primary and secondary) outcomes had been reported in the prespecified way, utilising prespecified measurements and analysis methods.
We assessed risk of bias as:
Low risk: e.g. the study protocol was available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way, or if the protocol was not available, that the published report included all expected outcomes.
High risk: e.g. not all prespecified outcomes are reported, primary outcomes are reported using measurements or analysis methods that were not prespecified, the primary outcome reported was not prespecified, incomplete reporting of any outcomes, failure to include results for a key outcome that would be expected to have been reported.
Unclear risk: insufficient information available to permit a judgement of 'low risk' or 'high risk'.
(6) Other bias
For each included study, we assessed for bias due to problems are not covered elsewhere in the table.
We assess risk of bias as:
Low risk: e.g. study appears free of other bias.
High risk: e.g. there is at least one important risk of bias, such as a potential source of bias related to study design, or the study has been claimed to have been fraudulent.
Unclear risk: insufficient information or evidence that an identified problem will introduce bias.
Measures of treatment effect
Treatment effects are divided into quantitative data and patient‐reported outcomes. We analysed quantitative data such as survival and toxicity as dichotomous outcomes using the risk ratio (RR). We pooled time‐to‐event outcomes, such as hazard ratios (HR) for overall survival and progression‐free survival, provided that authors had analysed data using a Cox proportional hazards model. We summarised proportional outcomes, such as the proportion who survived, using a risk ratio (RR). We combined continuous outcomes with the inverse variance method. We combined quality of life outcomes if the same validated instrument was used, otherwise we utilised a descriptive approach. If data were combined, we presented the change from baseline as the standardised mean difference (SMD). All measures of effect included a 95% confidence interval (CI), P values and for pooled measures the I2 statistic value.
Assessment of heterogeneity
We performed tests for heterogeneity with Review Manager (RevMan 2014) using the I2 statistic and interpreting the I2 value using the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). An I2 value of greater than 75% is likely to represent considerable heterogeneity, a value of 50% to 90% is likely to represent substantial heterogeneity and a value of 30% to 60% represents moderate heterogeneity.
Data synthesis
We combined quantitative data using Review Manager 5.3 (RevMan 2014). We calculated hazard ratios (HR) for data presented as survival curves using logrank expected number of events and variance. We pooled hazard ratios across trials using a fixed‐effect model. We combined continuous data, where the mean, standard deviation (SD) and number of participants in each arm were available, generating a mean difference (MD) and 95% CI. We planned to use a fixed‐effect model in the meta‐analysis if heterogeneity was deemed to be small (an I2 value of less than 50%). We applied a random‐effects model to comparisons demonstrating significant heterogeneity (with an I2 value of greater than 50%).
GRADE and 'Summary of findings' tables
We employed the GRADE approach to interpret findings (Schünemann 2011). We used GRADEProGDT (GRADEpro GDT 2015) to import data from Review Manager (RevMan 2014) to create 'Summary of findings' tables for major comparisons in this review. These tables provide information concerning the overall quality of the evidence from the included studies, the magnitude of the effect of the interventions and the sum of available data on the primary outcome and selected secondary outcomes. We selected the most relevant comparison for presentation in the 'Summary of findings' tables and we selected the following outcomes that we considered important to clinical decision‐making for inclusion in these tables:
Overall survival.
Progression‐free survival.
Toxicity.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses for the outcomes of survival and tumour response. We categorised data from included studies into the following subgroups:
Asian population: if the study presented data specifically from patients who were of Asian ethnicity.
EGFR mutation positive: if the study presented data specifically from patients who were found to have EGFR activating mutations.
We undertook these subgroup analyses to determine whether there are differences between treatment groups depending on these biological and genomic factors.
Sensitivity analysis
Where applicable, we planned to perform a sensitivity analysis based on study quality, to assess the effect of this on the reported outcomes. We also applied a random‐effects model as part of our sensitivity analysis.
Results
Description of studies
Results of the search
The search strategy yielded 5703 studies or abstracts of which 127 studies were possibly eligible. Of these, we included 62 publications in this review, representing 35 primary studies and 27 publications that presented data from their respective primary studies. Fifty‐six were published in abstract form only and we found the remaining nine studies to be ineligible (Figure 1).
1.
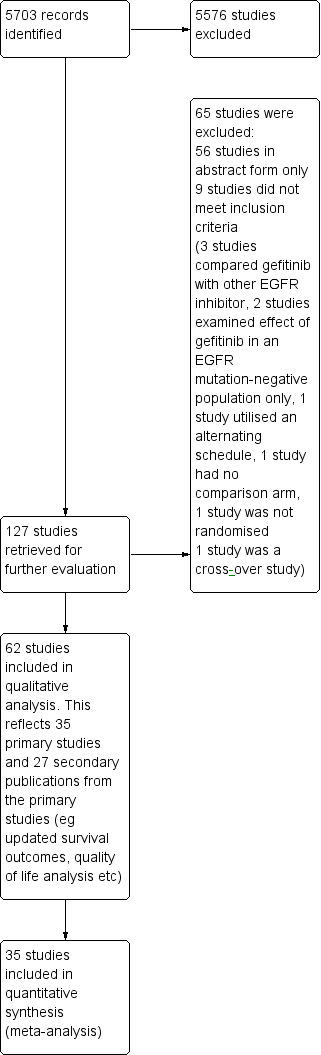
Study flow diagram for searches 1966‐2017.
(EGFR: epidermal growth factor receptor)
Included studies
We included a total of 35 separate primary studies in this review and these trials randomised a total of 12,089 patients. Seventeen of the eligible studies were multicentre, phase III trials (Gaafar 2011 EORTC08021; Giaccone 2004 INTACT I; Han 2012 First SIGNAL; Herbst 2004 INTACT II; Kelly 2008 SWOG S0023; Kim 2008 INTEREST; Lee 2010 ISTANA; Maemondo 2010 NEJ002; Maruyama 2008 V‐15‐32; Mitsudomi 2010 WJTOG3405; Mok 2009 IPASS; Soria 2015 IMPRESS; Sun 2012 KCSG‐LU08‐01; Takeda 2010 WJTOG0203; Thatcher 2005 ISEL; Yang 2014; Zhang 2012 INFORM). The remaining 18 were phase II studies (Ahn 2012; An 2016; Chen 2007; Chen 2011; Cheng 2016; Crino 2008 INVITE; Cufer 2006 SIGN; Dai 2013; Fukuoka 2003 IDEAL I; Goss 2009 INSTEP; Kim 2016; Kris 2003 IDEAL II; Li 2010; Lou 2014; Morere 2010 IFCT‐0301; Xu 2015; Xue 2015; Yu 2014). A summary of the 35 included primary studies is presented in Table 4. An additional 14 publications analysed data from their respective primary studies (Bell 2005; Boye 2016; Cella 2005; Chang 2006; Douillard 2010; Fukuoka 2011; Hirsch 2006; Herbst 2005; Inoue 2013; Oizumi 2012; Sekine 2009; Thongprasert 2011; Yamamoto 2010; Yang 2015). If we used data from these secondary studies, we did not duplicate with data from the respective primary studies and vice versa.
1. Included studies.
|
Author/Year (Study name) |
Journal | N | Comparison | Inclusion criteria | Phase | Asian | EGFR mutation | Line? |
| 1. Gefitinib versus placebo | ||||||||
| Goss 2009 (INSTEP) | JCO 27(13):2253‐2260 | 201 | Placebo | Poor PS | II | N | Subgroup | 1st line |
| Thatcher 2005 (ISEL) | Lancet 366:1527‐37 | 1692 | Placebo | — | III | Subset (Chang) | Subgroup (Hirsch) | 2nd line |
| Gaafar 2011 (EORTC08021) | Eur J Cancer (47):2331‐2340 | 173 | Placebo | Maintenance | III | N | N | Maintenance |
| Kelly 2008 (SWOGS0023) | JCO 26(15):2450‐2456 | 243 | Placebo | Consolidation | III | N | N | Maintenance |
| Zhang 2012 (INFORM) | Lancet Oncology 13:466‐475 | 296 | Placebo | Maintenance | III | Y | Subgroup | Maintenance |
| 2. Gefitinib versus chemotherapy | ||||||||
| Crino 2008 (INVITE) | JCO 26(26):4253‐4260 | 196 | Vinorelbine | Elderly patients | II | N | Subgroup | 1st line |
| Lou 2014 | Natl Med J China 94(30): 2337‐2341 | 51 | Carboplatin + paclitaxel | Asian | II | Y | N | 1st line |
| Morere 2010 (IFCT0301) | Lung Cancer 70:301‐307 | 85 | Docetaxel | Poor PS | II | N | N | 1st line |
| Han 2013 (First‐SIGNAL) | JCO 30(10): 1122‐1128 | 313 | Gemcitabine + cisplatin | — | III | Y | Planned Subgroup | 1st line |
| Mok 2009 (IPASS) | NEJM 361(10):947‐957 | 1217 | Carboplatin + paclitaxel | Asian, adenocarcinomas | III | Y | Subgroup | 1st line |
| Maemondo 2010 (NEJ002) | NEJM 362(25):2580‐2588 | 230 | Carboplatin + paclitaxel | Asian, EGFR mutation | III | Y | Y | 1st line |
| Mitsudomi 2010 (WJTOG3405) | Lancet Oncol 11:121‐128 | 177 | Cisplatin + docetaxel | Asian, EGFR mutation | III | Y | Y | 1st line |
| Yang 2014 | Eur J Cancer 50:2219‐2230 | 236 | Pemetrexed + cisplatin | Asian | III | Y | Subgroup | 1st line + maintenance |
| Cufer 2006 (SIGN) | Anti‐cancer Drugs 14:401‐409 | 141 | Docetaxel | Open‐label | II | N | N | 2nd line |
| Dai 2013 | Chin J Lung Cancer 16(8):405‐410 | 46 | Pemetrexed | Asian | II | Y | N | 2nd line |
| Kim 2016 | Cancer Res Treat 48(1):80‐87 | 95 | Pemetrexed | Asian | II | Y | N | 2nd/3rd line |
| Li 2010 | Chinese J Clin Onc 37:16‐18 | 98 | Docetaxel | Asian | II | Y | N | 2nd line |
| Kim 2008 (INTEREST) | Lancet 372:1809‐1818 | 1466 | Docetaxel | — | III | N | Subgroup (Doulliard) | 2nd line |
| Lee 2010 (ISTANA) | Clin Cancer Res 16(4):1307‐1314 | 161 | Docetaxel | Asian | III | Y | N | 2nd/3rd line |
| Maruyama 2008 (V‐15‐32) | JCO 26(26):4244‐4252 | 489 | Docetaxel | Asian | III | Y | Subgroup | 2nd/3rd line |
| Sun 2012 (KSCG‐LU08‐01) | Cancer 118:6234‐6242 | 141 | Pemetrexed | Adenocarcinoma, non‐smoker | III | Y | Subgroup | 2nd line |
| Ahn 2012 | Lung Cancer 77:346‐352 | 73 | Pemetrexed | Asian, never‐smokers | II | Y | N | Maintenance |
| Xu 2015 | Int J Clin Exp Med 8(4):6242‐6246 | 188 | Pemetrexed | Asian | II | Y | N | Maintenance |
| 3. Gefitinib 250 mg versus gefitinib 500 mg | ||||||||
| Fukuoka 2003 (IDEAL I) | JCO 21(12):2237‐2246 | 210 | G250 versus G500 | — | II | N | N | 2rd/3rd line |
| Kris 2003 (IDEAL II) | JAMA 290(16):2149‐2158 | 216 | G250 versus G500 | — | II | N | N | 3rd line |
| Xue 2015 | Int J Clin Exp Med 8(4):6242‐6246 | 188 | G250 versus G500 | Asian | II | Y | N | Maintenance |
| 4. Gefitinib versus gefitinib + chemotherapy | ||||||||
| An 2016 | Pathol Oncol Res 22:763‐768 | 90 | Gefitinib + Pemetrexed | Asian, EGFR mutation | II | Y | Y | 1st line |
| Cheng 2016 | JCO 34(27): 3258‐3266 | 191 | Gefitinib + Pemetrexed | Asian, EGFR mutation | II | Y | Y | 1st line |
| Chen 2007 | Cancer 109:1821‐8 | 48 | Gefitinib + Vinorelbine | Adenocarcinoma | II | N | Subgroup | 3rd line |
| Chen 2011 | J Thor Oncol 6:1110‐1116 | 115 | Gefitinib + Tegafur | Adenocarcinoma | II | Y | Subgroup | 2nd/3rd line |
| 5. Gefitinib + chemotherapy versus chemotherapy | ||||||||
| Giaccone 2004 (INTACT I) | JCO 22(5):777‐784 | 1093 | Gemcitabine + Cisplatin | — | III | N | N | 1st line |
| Herbst 2004 (INTACT II) |
JCO 22(5):785‐794 | 1037 | Carboplatin + paclitaxel | — | III | N | N | 1st line |
| Takeda 2010 (WTOG0203) | JCO 28(5):753‐760 | 604 | Platinum doublet | — | III | Y | N | 1st line |
| Yu 2014 | Cancer Biology & Therapy 15:832‐839 | 117 | Pemetrexed + platinum | Asian | II | Y | N | 1st line |
| Soria 2015 (IMPRESS) | Lancet Oncology 16:990‐98 | 265 | Pemetrexed + cisplatin | EGFR mutation positive | III | N | Y | 2nd line |
EGFR: epidermal growth factor receptor N: number of patients included PS: performance status
Journals:
Cancer Res Treat: Cancer Research and Treatment Chin J Lung Cancer: Chinese Journal of Lung Cancer Chinese J Clin Onc: Chinese Journal of Clinical Oncology Clin Cancer Res: Clinical Cancer Research Eur J Cancer: European Journal of Cancer Int J Clin Exp Med: International Journal of Clinical and Experimental Medicine J Thor Oncol: Journal of Thoracic Oncology JCO: Journal of Clinical Oncology Natl Med J China: National Medical Journal of China NMEJ: New England Journal of Medicine Pathol Oncol Res: Pathology and Oncology Research
The duration of gefitinib therapy varied between studies. Most studies continued therapy until there was disease progression, unacceptable toxicity or withdrawal. Two studies administered gefitinib for six or eight weeks (Chen 2007; Morere 2010 IFCT‐0301). The shortest reported median duration of treatment was 50 days (Goss 2009 INSTEP) and the longest 308 days (Maemondo 2010 NEJ002).
Please refer to the Characteristics of included studies for full details of included studies. Study characteristics have also been summarised in Table 4.
The various comparisons can be seen in the Data and analyses section.
1. Gefitinib at any dose compared with placebo or best supportive care for NSCLC
General population (Comparison 1)
Three phase III studies (Gaafar 2011 EORTC08021; Kelly 2008 SWOG S0023; Thatcher 2005 ISEL) and a single phase II study (Goss 2009 INSTEP) compared gefitinib with placebo. The ISEL (Thatcher 2005 ISEL), INSTEP (Goss 2009 INSTEP), EORTC 08021 (Gaafar 2011 EORTC08021) and SWOGS0023 (Kelly 2008 SWOG S0023) trials examined survival outcomes, objective response rates and toxicity in the general population. The INSTEP study randomised chemotherapy‐naive patients to 250 mg of gefitinib or placebo as first‐line therapy. The ISEL study studied its effects as second‐line therapy in advanced NSCLC. Detailed subgroup analysis was conducted in the ISEL population and subsequently published. These two studies are also presented below as subgroup analyses (Chang 2006; Hirsch 2006). Subgroups were assessed for evidence by subgroup interactions, thus ensuring that outcomes were indeed different. Pre‐planned subgroup analysis of patients of Asian ethnicity was presented in Chang 2006 and analysis of molecular predictors of outcome was presented in Hirsch 2006. The SWOGS0023 and EORTC08021 studies assessed the effect of gefitinib versus placebo as maintenance therapy after initial treatment. In the SWOG study, patients were included after receiving concurrent cisplatin/etoposide chemotherapy with thoracic radiation (45 Gy, 1.8 Gy per fraction). The EORTC08021 trial included patients not progressing after first‐line platinum doublet chemotherapy. We studied a total of 2605 patients in this group.
Asian population (Comparison 2)
The INFORM study assessed the use of gefitinib as maintenance therapy in an East Asian patient group (Zhang 2012 INFORM). These patients had achieved disease control after first‐line platinum‐based chemotherapy. Chang 2006 selected only ISEL patients who were of Asian ethnicity. This subgroup represented 20% of the original ISEL population, a total of 342 patients. We included a total of 638 patients in this group.
EGFR mutation positive population (Comparison 3)
Zhang 2012 INFORM performed planned subgroup analysis on EGFR mutation positive patients and 30 of 79 (38%) tissue tumour samples were positive for EGFR mutations. Hirsch 2006 analysed ISEL tumour biopsy samples to examine the relationships between biomarkers and clinical outcome after gefitinib administration. Two‐hundred and fifteen of 1692 patients (12.7%) in the ISEL trial were assessable for mutation detection. Of these, 26 (12.1%) patients were positive for EGFR mutations. Other biomarkers examined included EGFR gene copy number, EGFR and p‐Akt protein expression and KRAS and BRAF mutations. Data from these other biomarkers are beyond the scope of this review.
2. Gefitinib at any dose compared with other chemotherapeutic agents
We included 18 primary studies in this analysis (Ahn 2012; Crino 2008 INVITE; Cufer 2006 SIGN; Dai 2013; Han 2012 First SIGNAL; Kim 2008 INTEREST; Kim 2016; Lee 2010 ISTANA; Li 2010; Lou 2014; Maemondo 2010 NEJ002; Maruyama 2008 V‐15‐32; Mitsudomi 2010 WJTOG3405; Mok 2009 IPASS; Morere 2010 IFCT‐0301; Sun 2012 KCSG‐LU08‐01; Xu 2015; Yang 2014). Nine of these studies were multicentre, randomised, phase III trials.
These 18 primary studies randomised a total of 5400 patients.
General population (Comparison 4)
Four studies, SIGN (Cufer 2006 SIGN), INTEREST (Kim 2008 INTEREST), INVITE (Crino 2008 INVITE) and IFCT‐0301 (Morere 2010 IFCT‐0301), compared gefitinib with chemotherapy in 1888 patients and data from these are presented in Comparison 4. Two studies compared gefitinib with first‐line chemotherapy (Crino 2008 INVITE; Morere 2010 IFCT‐0301) and the other two studies compared it with second‐line chemotherapy (Cufer 2006 SIGN; Kim 2008 INTEREST). 'Iressa in NSCLC versus Vinorelbine Investigation in the Elderly' (INVITE) was a randomised, multicentre, phase II trial that compared gefitinib with vinorelbine as first‐line therapy in elderly patients (Crino 2008 INVITE). IFCT‐0301 compared gefitinib, gemcitabine and docetaxel in chemotherapy‐naive patients with a performance status of 2 or 3 (Morere 2010 IFCT‐0301). SIGN (Second‐line Indication of Gefitinib in NSCLC) was a phase II, randomised study comparing gefitinib with docetaxel as second‐line therapy (Cufer 2006 SIGN). INTEREST (Iressa NSCLC Trial Evaluating Response and Survival again Taxotere) was a phase III trial, which assessed the non‐inferiority of gefitinib to docetaxel as second‐line therapy (Kim 2008 INTEREST). Douillard 2010 performed a preplanned secondary analysis to investigate the relationship between biomarkers and clinical outcomes in the INTEREST population. We included a total of 1888 patients in this group.
Asian population (Comparison 5)
Fourteen studies selected Asian patients only (Ahn 2012; Dai 2013; Han 2012 First SIGNAL; Kim 2016; Lee 2010 ISTANA; Li 2010; Lou 2014; Maruyama 2008 V‐15‐32; Mok 2009 IPASS; Mitsudomi 2010 WJTOG3405; Maemondo 2010 NEJ002; Sun 2012 KCSG‐LU08‐01; Xu 2015; Yang 2014), of which all except six (Ahn 2012; Dai 2013; Kim 2016; Li 2010; Lou 2014; Xu 2015) were phase III studies. We included a total of 3512 patients in this group.
First‐line studies
Five phase III studies (Han 2012 First SIGNAL; Maemondo 2010 NEJ002; Mitsudomi 2010 WJTOG3405; Mok 2009 IPASS; Yang 2014) and one phase II study (Lou 2014) compared gefitinib with first‐line chemotherapy. IPASS compared gefitinib with carboplatin‐paclitaxel, but in Asian patients with adenocarcinoma who were light or never‐smokers (Mok 2009 IPASS). Maemondo 2010 NEJ002 randomised Asian chemotherapy‐naive patients with EGFR mutations to receive gefitinib or carboplatin‐paclitaxel. WJTOG3405 compared gefitinib with cisplatin plus docetaxel in Asian patients with EGFR mutations (Mitsudomi 2010 WJTOG3405). First‐SIGNAL compared first‐line gefitinib with gemcitabine plus cisplatin in Asian never‐smokers with lung adenocarcinoma (Han 2012 First SIGNAL). The phase III study by Yang 2014 compared first‐line pemetrexed and cisplatin followed by gefitinib maintenance therapy with gefitinib monotherapy alone in Asian non‐smoking patients. Patients were randomised at trial entry to either gefitinib or pemetrexed plus cisplatin chemotherapy. Patients in both arms then continued with maintenance gefitinib. Data were analysed in the intention‐to‐treat population and only data from the first phase of the study were included in this analysis. In the phase II study by Lou 2014, gefitinib was compared with carboplatin and paclitaxel in Asian patients who were either non‐smokers or light ex‐smokers.
We analysed a total of 2224 patients from the six studies in this group.
Second‐line studies
Three phase III studies (Lee 2010 ISTANA; Maruyama 2008 V‐15‐32; Sun 2012 KCSG‐LU08‐01) and three phase II studies (Dai 2013; Kim 2016; Li 2010) compared gefitinib with second‐line chemotherapy. ISTANA (Lee 2010 ISTANA), V‐15‐32 (Maruyama 2008 V‐15‐32) and the phase II study by Li 2010 included patients of Asian ethnicity but where mutation status was not always known, and compared gefitinib with docetaxel. KCSG‐LU08‐01 (Sun 2012 KCSG‐LU08‐01), Dai 2013 and Kim 2016 selected Asian patients with unknown EGFR status and compared gefitinib with second‐line pemetrexed. Secondary studies published by Sekine 2009 and Yamamoto 2010 conducted analyses on quality of life and disease control respectively in the V‐15‐32 trial.
We analysed a total of 1030 patients from the six studies in this group.
Maintenance studies
Two phase II studies compared the role of gefitinib as maintenance to chemotherapy. Ahn 2012 randomised Asian non‐smokers not progressing after first‐line pemetrexed‐cisplatin, to receive either gefitinib or pemetrexed ± cisplatin, in a two‐staged study design. Xu 2015 compared single‐agent pemetrexed with gefitinib in Asian patients not progressing after four to eight cycles of first‐line chemotherapy.
We analysed 258 patients in this group.
EGFR mutation positive population (Comparison 6)
Nine studies were included in this group, six of which were first‐line studies (Crino 2008 INVITE; Han 2012 First SIGNAL; Maemondo 2010 NEJ002; Mitsudomi 2010 WJTOG3405; Mok 2009 IPASS; Yang 2014) and three of which were second‐line studies (Kim 2008 INTEREST; Maruyama 2008 V‐15‐32; Sun 2012 KCSG‐LU08‐01).
We included a total of 879 patients in this group.
Two phase III studies selected patients of Asian ethnicity who were also positive for EGFR mutations and compared gefitinib with first‐line carboplatin and paclitaxel or cisplatin and docetaxel respectively (Maemondo 2010 NEJ002; Mitsudomi 2010 WJTOG3405). In contrast, the IPASS (Mok 2009 IPASS) and First‐SIGNAL (Han 2012 First SIGNAL) studies selected Asian patients with adenocarcinomas, and conducted planned subgroup analyses on the EGFR mutation positive patients. IPASS compared first‐line gefitinib with carboplatin and paclitaxel and First‐SIGNAL compared gefitinib with gemcitabine and cisplatin. Yang 2014 conducted a post‐hoc analysis of EGFR mutation positive patients and compared first‐line pemetrexed and cisplatin followed by gefitinib maintenance with gefitinib alone. The INVITE phase II study in elderly patients that compared first‐line gefitinib with vinorelbine also conducted analysis of EGFR mutation positive patients but this study did not include any data that could be pooled (Crino 2008 INVITE).
We analysed a total of 802 patients in this group.
A further three phase III studies compared second‐line gefitinib with chemotherapy and conducted subgroup analyses in the EGFR mutation positive patients (Kim 2008 INTEREST; Maruyama 2008 V‐15‐32; Sun 2012 KCSG‐LU08‐01). INTEREST and V‐15‐32 compared gefitinib with docetaxel and KCSG‐LU08‐01 compared gefitinib with pemetrexed in this second‐line setting. The INTEREST study also analysed other biomarkers, such as EGFR gene copy number, EGFR protein expression and KRAS mutations, in addition to EGFR mutations. One study did not publish data that could be pooled (Maruyama 2008 V‐15‐32) and thus we included a total of 77 patients in this group.
3. Gefitinib at a specific dose versus a different dose (Comparison 7)
Three phase II studies compared the effect of two different doses of gefitinib, 250 mg and 500 mg in 527 patients (Fukuoka 2003 IDEAL I; Kris 2003 IDEAL II; Xue 2015). IDEAL I (Fukuoka 2003 IDEAL I) and IDEAL II (Kris 2003 IDEAL II) were multicentre, randomised, double‐blind, phase II studies that evaluated two doses of gefitinib (250 mg/day and 500 mg/day) as second‐ or third‐line therapy.
We analysed 431 patients in this group.
The third study randomised 96 patients who were stable after one month of gefitinib (250 mg/day) to either 250 mg/day or 500 mg/day as maintenance therapy (Xue 2015).
4. Gefitinib versus gefitinib combined with a chemotherapy regimen (Comparison 8)
Four studies compared gefitinib alone or in combination with chemotherapy. Two recently published studies examined the addition of chemotherapy to gefitinib versus gefitinib alone in the first‐line setting. A small study by An 2016 recruited 90 East Asian patients with an EGFR mutation and randomised them to receive gefitinib or gefitinib plus pemetrexed (500 mg/m2). In this study, pemetrexed or placebo was administered via intravenous infusion on day 1 of a 21‐day cycle. Gefitinib 250 mg was administered on days 2 to 16. A multicentre, phase II study by Cheng 2016 also compared gefitinib with and without pemetrexed as first‐line therapy. This study recruited 191 East Asian patients from China, Japan, Korea and Taiwan with advanced non‐squamous NSCLC with an activating EGFR mutation. Patients either received gefitinib 250 mg per day or gefitinib plus pemetrexed (500 mg/m2) infusion on day 1 of a 21‐day cycle.
We included a total of 281 patients in this group.
Chen 2007 compared 250 mg of daily oral gefitinib with gefitinib plus vinorelbine (15 mg/m2) every two weeks in 48 patients of Asian ethnicity with stage IV adenocarcinoma who had failed at least two lines of chemotherapy. Chen 2011 compared gefitinib alone with the combination of gefitinib plus tegafur (100 mg)/uracil (224 mg) in 115 Taiwanese patients with stage IIIB or IV adenocarcinoma who had failed first‐line chemotherapy.
We included a total of 163 patients in this group.
5. Gefitinib at any dose in combination with other chemotherapeutic agents versus the same chemotherapy agents alone (Comparison 9)
Five studies examined survival outcomes, objective response rates and toxicity (Giaccone 2004 INTACT I; Herbst 2004 INTACT II; Soria 2015 IMPRESS; Takeda 2010 WJTOG0203; Yu 2014). Overall, we included a total of 3110 patients.
INTACT I (Giaccone 2004 INTACT I) and INTACT II (Herbst 2004 INTACT II) were large, multicentre trials that examined the effect of the addition of two different doses of gefitinib to a chemotherapy regimen with the chemotherapy alone in chemotherapy‐naive patients. INTACT I compared the effect of the addition of gefitinib to a chemotherapy regimen that included gemcitabine and cisplatin and INTACT II a paclitaxel and carboplatin regime. WJTOG0203 compared the addition of 250 mg of gefitinib to platinum‐doublet chemotherapy in chemotherapy‐naive Japanese patients (Takeda 2010 WJTOG0203). In this study, patients were randomised to receive platinum doublet chemotherapy (Arm A) or platinum‐doublet chemotherapy for three cycles followed by gefitinib until disease progression (Arm B). The phase II study by Yu 2014 examined the addition of gefitinib to a first‐line pemetrexed and cisplatin chemotherapy schedule in Asian patients who were non‐smokers or light ex‐smokers.
In this group, we included 2845 patients.
The IMPRESS study was a phase III, multicentre study conducted across Europe and the Asia‐Pacific region (Soria 2015 IMPRESS). This study selected patients with EGFR mutation positive advanced NSCLC who had failed first‐line therapy with gefitinib. This study compared second‐line gefitinib plus chemotherapy (cisplatin and pemetrexed) with placebo plus the same chemotherapy regimen (cisplatin and pemetrexed). Two hundred and sixty‐five patients were included in this trial.
6. Gefitinib at any dose in combination with other chemotherapeutic agents versus a different combination of chemotherapeutic agent (Comparison 10)
No studies compared gefitinib in combination with a chemotherapeutic regime with a different regime of agents.
Data for all endpoints were not available in all published reports. A summary of efficacy and survival data is presented in Table 5.
2. Efficacy and survival data.
| Study | ORR (%) | PFS (months) | OS (months) | ||||||
| 1. Gefitinib versus placebo | Gefitinib | Control | P | Gefitinib | Control | P | Gefitinib | Control | P |
| 1st line | |||||||||
| Goss 2009 | 6 | 1.0 | NS | 1.43 | 1.37 | NS | 3.7 | 2.8 | NS |
| 2nd line | |||||||||
| Thatcher 2005 ISEL | 37.5 | 48.3 | NS | 3 | 2.6 | 0.0006 | 5.6 | 5.1 | 0.087 |
| Maintenance therapy | |||||||||
| Kelly 2008 SWOGS0023 | ‐ | ‐ | ‐ | 8.3 | 11.7 | NS | 23 | 35 | 0.013 |
| Gaafar 2011 EORTC08021 | 12 | 1 | 0.004 | 4.1 | 2.9 | 0.0015 | 10.9 | 9.4 | NS |
| 2. Gefitinib versus placebo (Asian population) | Gefitinib | Control | P | Gefitinib | Control | P | Gefitinib | Control | P |
| Chang 2006 ISEL | 12.4 | 2.1 | 0.01 | 4.4 | 2.2 | 0.008 | 9.5 | 5.5 | 0.01 |
| Zhang 2012 INFORM | 24 | 1 | 0.0001 | 4.8 | 2.6 | < 0.0001 | 18.7 | 16.0 | NS |
| 3. Gefitinib versus placebo (EGFR mutation positive) | Gefitinib | Control | P | Gefitinib | Control | P | Gefitinib | Control | P |
| Zhang 2012 INFORM | ‐ | ‐ | ‐ | 16.6 | 2.8 | 0.0063 | 46.87 | 20.97 | 0.036 |
| Gefitinib vs chemotherapy | |||||||||
| 4. General population | Gefitinib | Chemo | P | Gefitinib | Chemo | P | Gefitinib | Chemo | P |
| versus 1st line chemotherapy | |||||||||
| Crino 2008 INVITE | 3.1 | 5.1 | ‐ | 2.7 | 2.9 | NS | 5.9 | 8 | NS |
| Morere 2010 IFCT0301 | ‐ | ‐ | ‐ | 1.9 | 2 | 0.078 | 2.2 | 3.5 | 0.088 |
| Morere 2010 IFCT0301 (Adenocarcinoma) | ‐ | ‐ | ‐ | 1.9 | 2.1 | 0.272 | 2.3 | 4.4 | NS |
| versus 2nd line chemotherapy | |||||||||
| Cufer 2006 SIGN | 13.2 | 13.7 | NS | 7.5 | 7.1 | NS | 3 | 3.4 | NS |
| Kim 2008 INTEREST | 9.1 | 7.6 | NS | 2.2 | 2.7 | NS | 7.6 | 8 | NS |
| Kim 2008 INTEREST | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 8.5 | 8.9 | NS |
| 5. Asian population | Gefitinib | Chemo | P | Gefitinib | Chemo | P | Gefitinib | Chemo | P |
| versus 1st line chemotherapy | |||||||||
| Lou 2014 | 36 | 42.3 | NS | 4.2 | 8.3 | NS | 14.4 | 15 | NS |
| Maemondo 2010 (EGFR mutation positive) | 73.7 | 30.7 | < 0.001 | 10.8 | 5.4 | < 0.001 | 30.5 | 23.6 | NS |
| Mitsudomi 2010 WJTOG (EGFR mutation positive) | 62.1 | 32.2 | < 0.0001 | 9.2 | 6.3 | < 0.0001 | ‐ | ‐ | ‐ |
| Mok 2009 IPASS | 43 | 32.2 | < 0.001 | 5.7 | 5.8 | NS | 18.6 | 17.3 | NS |
| Han 2012 First‐SIGNAL (adenocarcinoma) | 55.4 | 46 | NS | 5.8 | 6.4 | NS | 22.3 | 22.9 | NS |
| Yang 2014 (Asian) | 47.5 | 41.5 | NS | 9.63 | 8.38 | NS | 27.9 | 26.9 | NS |
| versus 2nd line chemotherapy | |||||||||
| Dai 2013 | 17.4 | 13 | NS | 4.4 | 3.1 | NS | ‐ | ‐ | ‐ |
| Kim 2016 | 8 | 13 | NS | 2 | 2 | NS | 8.5 | 8.5 | NS |
| Li 2010 | 22.4 | 18.8 | NS | ‐ | ‐ | ‐ | 7.1 | 6.9 | NS |
| Kim 2008 INTEREST (subgroup) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 10.4 | 12.2 | NS |
| Lee 2010 ISTANA | 28.1 | 7.6 | 0.0007 | 3.3 | 3.4 | NS | 14.1 | 12.2 | NS |
| Maruyama 2008 V‐15‐32 | 22.5 | 12.8 | 0.009 | 2 | 2 | NS | 11.5 | 14 | NS |
| Sun 2012 KCSG‐LU08‐01 (adenocarcinoma, subgroup) | 58.8 | 22.4 | < 0.001 | 9.0 | 3.0 | 0.0006 | 22.2 | 18.9 | NS |
| versus maintenance therapy | |||||||||
| Ahn 2012 (Asian) | 46 | 35 | NS | 9.95 | 6.83 | NS | ‐ | ‐ | ‐ |
| Xu 2015 (Asian) | 18.1 | 29.8 | NS | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| 6. EGFR mutation positive | Gefitinib | Chemo | P | Gefitinib | Chemo | P | Gefitinib | Chemo | P |
| versus 1st line chemotherapy | |||||||||
| Maemondo 2010 (EGFR mutation positive) | 73.7 | 30.7 | < 0.001 | 10.8 | 5.4 | < 0.001 | 30.5 | 23.6 | NS |
| Mitsudomi 2010 WJTOG (EGFR mutation positive) | 62.1 | 32.2 | < 0.0001 | 9.2 | 6.3 | < 0.0001 | ‐ | ‐ | ‐ |
| Mok 2009 IPASS (subgroup) | 71.2 | 47.3 | < 0.001 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Han 2012 First‐SIGNAL (subgroup) | 84.6 | 37.5 | 0.002 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Yang 2014 (subgroup) | 70.8 | 65.4 | NS | 16.62 | 12.91 | NS | 45.7 | 32.4 | 0.255 |
| versus 2nd line chemotherapy | |||||||||
| INTEREST Doulliard 2010 (subgroup) | 42.1 | 21.1 | 0.04 | 7 | 4.1 | 0.001 | 14.2 | 16.6 | NS |
| Maruyama 2008 (subgroup) | 67 | 46 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sun 2012 KCSG‐LU08‐01 (subgroup) | ‐ | ‐ | ‐ | 15.7 | 2.9 | 0.005 | ‐ | ‐ | ‐ |
| 7. Gefitinib 250 mg versus gefitinib 500 mg | 250 mg | 500 mg | P | 250 mg | 500 mg | P | 250 mg | 500 mg | P |
| 2nd+ line | |||||||||
| Fukuoka 2003 | 18.4 | 19 | NS | 2.7 | 2.8 | NS | 7.6 | 8 | NS |
| Kris 2004 | 12 | 9 | NS | ‐ | ‐ | ‐ | 7 | 6 | NS |
| Maintenance therapy | |||||||||
| Xue 2015 (Asian) | 12.5 | 12.5 | NS | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| 8. Gefitinib versus gefitinib + chemotherapy | Gefitinib | G + Chemo | P | Gefitinib | G + Chemo | P | Gefitinib | G + Chemo | P |
| 1st line | |||||||||
| An 2016 | 73.33 | 80 | NS | 14 | 18 | < 0.05 | 32 | 34 | NS |
| Cheng 2016 | 74 | 80 | NS | 10.9 | 15.8 | 0.014 | ‐ | ‐ | ‐ |
| 2nd+ line | |||||||||
| Chen 2007(Asian, adenocarcinoma) | 55.6 | 52.4 | NS | 7.1 | 12.8 | NS | 13.3 | 23.4 | NS |
| Chen 2011(Asian, adenocarcinoma) | 35 | 37 | NS | 5.3 | 8.3 | 0.04 | ‐ | ‐ | ‐ |
| Chen 2011 (EGFR mutation positive subgroup) | ‐ | ‐ | ‐ | 7.6 | 14.4 | 0.0061 | ‐ | ‐ | ‐ |
| 9. Gefitinib + chemotherapy versus chemotherapy | 250 mg + Chemo | Chemo | P | 250 mg + Chemo | Chemo | P | 250 mg + Chemo | Chemo | P |
| 1st line | |||||||||
| Giaccone 2004 | 51.2 | 47.2 | NS | 5.8 | 6 | NS | 9.9 | 10.9 | NS |
| Herbst 2004 | 30.4 | 28.7 | NS | 5.3 | 5 | NS | 9.8 | 9.9 | NS |
| Takeda 2010 (Asian) | 34.2 | 29.3 | NS | 4.3 | 4.6 | < 0.001 | 12.9 | 13.7 | NS |
| Yu 2014 (Asian) | 47.4 | 50 | NS | 7.9 | 7 | NS | 25.4 | 20.5 | NS |
| 2nd line | |||||||||
| Soria 2015 IMPRESS (EGFR mutation positive) | 32 | 34 | NS | 5.4 | 5.4 | NS | 14.8 | 17.2 | NS |
Chemo: chemotherapy G: gefitinib NS: non‐significant ORR: overall response rate OS: overall survival PFS: progression‐free survival
Risk of bias in included studies
We included trials that met our inclusion criteria. We checked all data extracted for accuracy and final database entries. We resolved any discrepancies through discussion. Overall, the risk of bias in the 35 included studies was moderate. The results of the 'Risk of bias' assessment are depicted graphically in Figure 2.
2.
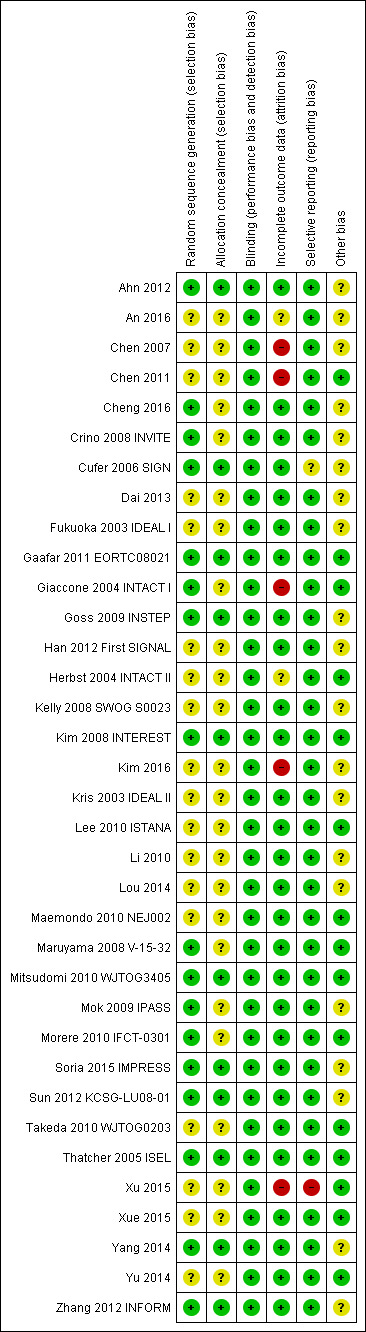
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Seventeen of the 35 included studies reported adequate sequence generation (Ahn 2012; Cheng 2016; Crino 2008 INVITE; Cufer 2006 SIGN; Gaafar 2011 EORTC08021; Giaccone 2004 INTACT I; Goss 2009 INSTEP; Kim 2008 INTEREST; Maruyama 2008 V‐15‐32; Mitsudomi 2010 WJTOG3405; Mok 2009 IPASS; Morere 2010 IFCT‐0301; Soria 2015 IMPRESS; Sun 2012 KCSG‐LU08‐01; Thatcher 2005 ISEL; Yang 2014; Zhang 2012 INFORM). The remaining 18 studies were all described as randomised, but none provided any further information and so we classified them as having an uncertain risk of bias (An 2016; Chen 2007; Chen 2011; Dai 2013; Fukuoka 2003 IDEAL I; Han 2012 First SIGNAL; Herbst 2004 INTACT II; Kelly 2008 SWOG S0023; Kim 2016; Kris 2003 IDEAL II; Lee 2010 ISTANA; Li 2010; Lou 2014; Maemondo 2010 NEJ002; Takeda 2010 WJTOG0203; Xu 2015; Xue 2015; Yu 2014).
Allocation concealment
Allocation concealment was adequate in 11 of the included studies (Ahn 2012; Cufer 2006 SIGN; Gaafar 2011 EORTC08021; Goss 2009 INSTEP; Kim 2008 INTEREST; Mitsudomi 2010 WJTOG3405; Soria 2015 IMPRESS; Sun 2012 KCSG‐LU08‐01; Thatcher 2005 ISEL; Yang 2014; Zhang 2012 INFORM). Most of these studies used a minimisation method or centralised allocation procedure. The remaining studies did not report whether allocation was concealed and so are possibly at risk of bias.
Blinding
Of the 35 included trials, we judged blinding to be adequate in all studies. Eight studies blinded participants and investigators using an identical placebo (Fukuoka 2003 IDEAL I; Gaafar 2011 EORTC08021; Giaccone 2004 INTACT I; Goss 2009 INSTEP; Soria 2015 IMPRESS; Thatcher 2005 ISEL; Yang 2014; Zhang 2012 INFORM). The remaining 27 studies were unblinded or open‐label (for example comparing gefitinib with intravenous chemotherapy), but we judged that this would not affect the measured outcomes.
Incomplete outcome data
The majority of studies adequately addressed incomplete outcome data. Of the 35 included trials, 28 had a low risk of bias from incomplete outcome data. Studies cited reasons such as death, disease progression and drug toxicity for dropouts. Five phase II studies did not address withdrawals or patients lost to follow‐up and thus are potentially at high risk of bias (Chen 2007; Chen 2011; Giaccone 2004 INTACT I; Kim 2016; Xu 2015). Two studies did not provide adequate outcome data and so are at a risk of bias from incomplete outcome data analysis (An 2016;Herbst 2004 INTACT II).
Selective reporting
We judged 33 of the 35 included studies as at low risk of reporting bias. One study reported an outcome (progression‐free survival) that was not pre‐specified (Cufer 2006 SIGN). We judged this as an unclear risk of bias. Another study did not report an outcome that was prespecified in the methods ("survival time"), with no reason provided for this in the paper (Xu 2015). We judged this as a high risk of bias
Other potential sources of bias
Three trials were stopped early (Kelly 2008 SWOG S0023; Maemondo 2010 NEJ002; Mitsudomi 2010 WJTOG3405), which may be another source of bias. The SWOGS0023 study was stopped because an unplanned interim analysis concluded that the alternate hypothesis of improved survival would not be met. The NEJ002 and WJTOG3405 studies were concluded early following the presentation of contemporary data showing a progression‐free survival benefit in EGFR mutated patients. These studies were then closed to accrual.
We judged the remaining studies as having an unclear risk of bias listed due to conflicts of interest, in particular pharmaceutical funding or significant affiliations, or because they did not adequately declare any conflicts of interest (Ahn 2012; An 2016; Chen 2007; Cheng 2016; Crino 2008 INVITE; Cufer 2006 SIGN; Dai 2013; Fukuoka 2003 IDEAL I; Goss 2009 INSTEP; Han 2012 First SIGNAL; Kelly 2008 SWOG S0023; Kim 2008 INTEREST; Kim 2016; Kris 2003 IDEAL II; Li 2010; Mok 2009 IPASS; Soria 2015 IMPRESS; Sun 2012 KCSG‐LU08‐01; Yang 2014; Zhang 2012 INFORM).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Gefitinib compared to chemotherapy for first‐line treatment of advanced NSCLC.
| Gefitinib compared to chemotherapy for first‐line treatment of advanced NSCLC | ||||||
| Patient or population: advanced NSCLC Settings: first‐line treatment Intervention: gefitinib Comparison: chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Chemotherapy | Gefitinib | |||||
| Overall survival (OS) | The mean OS ranged across control groups from 3.5 to 8 months | The mean OS in the intervention group ranged from 2.2 to 5.9 months | HR 0.98 (0.91 to 1.46) | 275 (2 RCTs) | ⊕⊕⊕⊝ MODERATE1 | OS similar in the Asian (HR 0.94, 0.82 to 1.06) and EGFR mutation positive subgroups (HR 0.97, 0.77 to 1.21) |
| Progression‐free survival (PFS) | The PFS ranged across control groups from 2 to 2.9 months | The mean PFS in the intervention group ranged from 1.9 to 2.7 months | HR 1.19 (0.86 to 1.65) | 275 (2 RCTs) | ⊕⊕⊕⊝ MODERATE1 | PFS improved with gefitinib in the Asian subgroup (HR 0.65, 0.43 to 0.98) and the EGFR mutation positive subgroup (HR 0.47, 0.36 to 0.61) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EGFR: epidermal growth factor receptor; HR: hazard ratio; NSCLC: non‐small cell lung cancer; OS: overall survival; PFS: progression‐free survival; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1We downgraded the quality of evidence by one level because of serious indirectness as one study included only elderly patients (> 70 years old).
Summary of findings 2. Gefitinib compared to chemotherapy for second‐line treatment of advanced NSCLC.
| Gefitinib compared to chemotherapy for second‐line treatment of advanced NSCLC | ||||||
| Patient or population: advanced NSCLC Settings: second‐line therapy Intervention: gefitinib Comparison: chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Chemotherapy | Gefitinib | |||||
| Overall survival (OS) | The mean OS ranged across control groups from 7.1 to 8 months | The mean OS in the intervention group ranged from 7.5 to 7.6 months | HR 1.02 (0.91 to 1.15) | 1607 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | OS similar in Asian patients (HR 0.94, 0.79 to 1.12) and EGFR mutation positive patients (HR 0.83, 0.41 to 1.66). |
| Progression‐free survival (PFS) | The mean PFS ranged across control groups from 2.7 to 3.4 months | The mean PFS in the intervention group ranged from 2.2 to 3 months | HR 1.04 (0.92 to 1.17) | 1607 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | PFS significantly improved in Asian patients (HR 0.71, 0.57 to 0.88) and in patients positive for EGFR mutation (HR 0.24, 0.12 to 0.47) (ranged from 2.7 to 4.1 months versus 4.5 to 7 months). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EGFR: epidermal growth factor receptor; HR: hazard ratio; NSCLC: non‐small cell lung cancer; OS: overall survival; PFS: progression‐free survival; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1We downgraded the quality of evidence by one level because of imprecision based on the wide confidence interval.
Summary of findings 3. Gefitinib compared to chemotherapy for advanced NSCLC ‐ toxicity.
| Gefitinib compared to chemotherapy for advanced NSCLC | |||||
| Patient or population: advanced NSCLC Settings: first‐line and second‐line therapy Intervention: gefitinib Comparison: chemotherapy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Chemotherapy | Gefitinib | ||||
| Skin rash | Study population | RR 2.40 (1.08 to 5.31) | 1858 (4 RCTs) | ⊕⊕⊕⊕ HIGH | |
| 9 per 1000 | 21 per 1000 (9 to 46) | ||||
| Constipation | Study population | RR 0.41 (0.17 to 0.97) | 1719 (3 studies) | ⊕⊕⊕⊕ HIGH | |
| 19 per 1000 | 8 per 1000 (3 to 18) | ||||
| Fatigue | Study population | RR 0.16 (0.03 to 0.88) | 275 (2 studies) | ⊕⊕⊕⊝ MODERATE1 | |
| 65 per 1000 | 10 per 1000 (2 to 57) | ||||
| Asthenia | Study population | RR 0.51 (0.35 to 0.75) | 1773 (3 studies) | ⊕⊕⊕⊕ HIGH | |
| 79 per 1000 | 40 per 1000 (28 to 60) | ||||
| Neurotoxicity | Study population | RR 0.07 (0.01 to 0.34) | 1529 (2 studies) | ⊕⊕⊕⊕ HIGH | |
| 29 per 1000 | 2 per 1000 (0 to 10) | ||||
| Neutropenia | Study population | RR 0.04 (0.02 to 0.06) | 1857 (4 studies) | ⊕⊕⊕⊕ HIGH | |
| 505 per 1000 | 20 per 1000 (10 to 30) | ||||
| Febrile neutropenia | Study population | RR 0.12 (0.06 to 0.23) | 1768 (3 studies) | ⊕⊕⊕⊕ HIGH | |
| 92 per 1000 | 11 per 1000 (6 to 21) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1We downgraded the quality of evidence by one level because of serious indirectness as one study included only elderly patients (> 70 years old).
See: Table 1 ('Gefitinib compared to chemotherapy for first‐line treatment of advanced NSCLC'); Table 2 ('Gefitinib compared to chemotherapy for second‐line treatment of advanced NSCLC'); Table 3 ('Gefitinib compared to chemotherapy for advanced NSCLC ‐ toxicity').
1. Gefitinib versus placebo or best supportive care
Survival
See Analysis 1.1; Analysis 1.2; Analysis 1.3.
1.1. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 1 HR Overall survival.
1.2. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 2 HR Progression‐free survival.
1.3. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 3 1‐year survival rate.
Four studies compared gefitinib with placebo in a general population (Gaafar 2011 EORTC08021; Goss 2009 INSTEP; Kelly 2008 SWOG S0023; Thatcher 2005 ISEL). The data presented examines the effect of gefitinib compared with placebo in the first‐line, second‐line and maintenance settings. Total pooling of data was not conducted for first‐ or second‐line therapy as only single studies were included. Pooling of data was only possible for maintenance treatment, as two studies were included (Gaafar 2011 EORTC08021; Kelly 2008 SWOG S0023). Gefitinib did not improve overall survival when compared with placebo, either when administered as first‐line (Goss 2009 INSTEP; hazard ratio (HR) 0.84, 95% confidence interval (CI) 0.62 to 1.14, P = 0.27), second‐line (Thatcher 2005 ISEL; HR 0.89, 95% CI 0.79 to 1.01, P = 0.06) or maintenance therapy (Gaafar 2011 EORTC08021; Kelly 2008 SWOG S0023; pooled HR 1.14, 95% CI 0.61 to 2.14, P = 0.69, I2 = 85%, random‐effects model).
One‐year survival rates were improved by administration of gefitinib versus placebo as second‐line therapy (risk ratio (RR) 1.28, 95% CI 1.05 to 1.57, P = 0.02), but not as maintenance therapy (RR 0.90, 95% CI 0.78 to 1.04, P = 0.15). Progression‐free survival was not improved when gefitinib was compared with placebo as first‐line therapy and median progression‐free survival was reported as 1.4 months in both groups (HR 0.82, 95% CI 0.60 to 1.12, P = 0.21). Time to treatment failure was improved in favour of gefitinib as second‐line therapy, with a HR of 0.82 (95% CI 0.75 to 0.90, P < 0.0001): median progression‐free survival was 3 months with gefitinib, 2.6 months with placebo. Maintenance use of gefitinib after first‐line treatment improved progression‐free survival (HR 0.70, 95% CI 0.53 to 0.91, P = 0.007, I2 = 32%).
Toxicity
See Analysis 1.4; Analysis 1.6.
1.4. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 4 Skin rash.
1.6. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 6 Diarrhoea.
We have pooled reported toxicity data from three studies in this comparison so as to examine the differences in toxicity between gefitinib and placebo or best supportive care (Gaafar 2011 EORTC08021; Goss 2009 INSTEP; Thatcher 2005 ISEL). Administration of gefitinib was significantly associated with Common Toxicity Criteria (CTC) grade 3 to 4 events such as skin rash (RR 7.92, 95% CI 1.46 to 43.03, P = 0.02, I2 = 0%) and diarrhoea (RR 2.48, 95% CI 1.15 to 5.35, P = 0.02, I2 = 0%). One study reported a statistically significant increase in alanine aminotransferase (ALT) with gefitinib (RR 9.11, 95% CI 1.18 to 70.32, P = 0.03). The risk of all other adverse events was either not estimable or not significantly different between the two groups.
Efficacy
See Analysis 1.22; Analysis 1.23.
1.22. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 22 Overall response rate.
1.23. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 23 Disease control rate.
Response was reported in only three of the four included studies (Gaafar 2011 EORTC08021; Goss 2009 INSTEP; Thatcher 2005 ISEL). We did not pool the data as the INSTEP study compared gefitinib with placebo as first‐line therapy, ISEL did so as second‐line therapy and the EORTC08021 trial as maintenance therapy. As first‐line therapy, gefitinib did not improve the overall response rate (RR 6.06, 95% CI 0.74 to 49.43, P = 0.09) or the disease control rate (RR 1.36, 95% CI 0.86 to 2.16, P = 0.19). This was reported as an overall response rate of 6% and 1% in the gefitinib and placebo groups, respectively, and the disease control rate was 31% and 23%, respectively. As second‐line therapy, the overall response rate was higher for gefitinib‐treated cases than for placebo (RR 6.42, 95% CI 2.82 to 14.64, P < 0.00001) and the disease control rate was also significantly higher for gefitinib (RR 1.24, 95% CI 1.06 to 1.44, P = 0.006). The overall response rate was 8% in the gefitinib group and 1% in the placebo group, and the disease control rate was 40% and 32%, respectively. Similarly, gefitinib improved the overall response rate and the disease control rate when used as maintenance therapy (RR 10.12, 95% CI 1.32 to 77.33, P = 0.03; RR 1.21, 95% CI 1.00 to 1.46, P = 0.05, respectively).
Quality of life and symptom improvement scores
Thatcher 2005 ISEL reported that the addition of gefitinib to "best supportive care" produced no significant changes in the quality of life subscale of the Functional Assessment of Cancer Therapy‐Lung (FACT‐L) questionnaire when compared with best supportive care alone. Gefitinib was associated with a statistically significant improvement in the symptom score (mean change from baseline ‐0.86 to ‐1.38; P = 0.019), but this did not meet predefined criteria. As described by Cella 2002, for changes in disease‐related symptoms to be classed as clinically relevant, the score must increase by two points. Goss 2009 INSTEP reported improvements in FACT‐L quality of life, FACT‐L Trial Outcome Index (TOI), lung cancer subscale (LCS) and Pulmonary Symptom Index (PSI) that were statistically non‐significant.
Subgroup analysis: Asian population
See Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4.
2.1. Analysis.
Comparison 2 Gefitinib versus placebo (Asian subgroup), Outcome 1 HR Overall survival.
2.2. Analysis.
Comparison 2 Gefitinib versus placebo (Asian subgroup), Outcome 2 HR Progression‐free survival.
2.3. Analysis.
Comparison 2 Gefitinib versus placebo (Asian subgroup), Outcome 3 1‐year survival rate.
2.4. Analysis.
Comparison 2 Gefitinib versus placebo (Asian subgroup), Outcome 4 Overall response rate.
The INFORM study prospectively recruited patients of East Asian ethnic origin without disease progression after first‐line chemotherapy (Zhang 2012 INFORM). Pre‐planned subgroup analysis in the ISEL trial found marked heterogeneity in survival between patient groups (Thatcher 2005 ISEL).
The ISEL study conducted a subgroup analysis in 342 patients of Asian ethnicity who were enrolled in the ISEL trial. Two hundred and thirty‐five patients received second‐line gefitinib and 107 received placebo. Pre‐planned analysis reported that gefitinib significantly improved overall survival (HR 0.66, 95% CI 0.48 to 0.91, P = 0.01), the one‐year survival rate (RR 1.75, 95% CI 1.20 to 2.55, P = 0.004) and progression‐free survival (HR 0.69, 95% CI 0.52 to 0.91, P = 0.009) compared to placebo. Median overall survival was 9.5 months for gefitinib compared with 5.5 months for placebo. Covariate analysis of demographic subgroups further demonstrated a survival advantage across multiple subgroups. Overall survival in this Asian subgroup of patients was also greater in never‐smokers (HR 0.37, 95% CI 0.21 to 0.64, P = 0.0004) compared with smokers (HR 0.85, 95% CI 0.58 to 1.25, P = 0.40); females (HR 0.46, 95% CI 0.26 to 0.79, P = 0.0045) compared with males (HR 0.80, 95% CI 0.54 to 1.18, P = 0.26); and patients with adenocarcinoma (HR 0.66, 95% CI 0.45 to 0.97, P = 0.04) compared with non‐adenocarcinoma (HR 0.86, 95% CI 0.50 to 1.47, P = 0.58). Objective response rates were higher in Asian patients treated with gefitinib compared with placebo (RR 6.03, 95% CI 1.46 to 24.91, P = 0.01).
The INFORM study showed that gefitinib in the maintenance setting did not improve overall survival (HR 0.88, 95% CI 0.68 to 1.14, P = 0.335). However, gefitinib improved progression‐free survival over placebo (HR 0.42, 95% CI 0.33 to 0.54, P < 0.00001), and median progression‐free survival was improved from 2.6 months to 4.8 months. The objective response rate was greater with gefitinib (RR 35.00, 95% CI 4.86 to 252.15, P = 0.0004). There was no difference in reported toxicities.
Quality of life improvement rates were higher in those administered gefitinib compared with placebo, as measured by FACT‐L (improvement rates 55% versus 24%, P < 0.001), TOI (51% versus 21%, P < 0.001) and LCS (50% versus 22%, P < 0.001) in the INFORM study (Zhang 2012 INFORM). Gefitinib also increased the time‐to‐worsening of quality of life when compared with placebo (FACT‐L: 2.8 months versus 1.4 months, P = 0.019; TOI: 3.5 months versus 1.4 months P = 0.006; LCS: 2.8 months versus 1.4 months P = 0.028). The relationship between the change in quality of life score and prognosis was also analysed in the INFORM study. Patients with an improvement in quality of life had significantly longer progression‐free survival and overall survival when compared with those that had a stable or worsened quality of life (FACT‐L: 9.4 months versus 2.8 months versus 2.7 months, P < 0.001 and 25.4 months versus 19.9 months versus 14.4 months, P = 0.003, respectively).
Subgroup analysis: biomarker
See Analysis 3.1; Analysis 3.2.
3.1. Analysis.
Comparison 3 Gefitinib versus placebo (biomarker subgroup), Outcome 1 HR Overall survival.
3.2. Analysis.
Comparison 3 Gefitinib versus placebo (biomarker subgroup), Outcome 2 HR Progression‐free survival.
Subgroup analysis of patients from the ISEL trial reported that the overall response rate was higher in patients with epidermal growth factor receptor (EGFR) mutations (37.5%; 6 of 16 patients) than those who were EGFR mutation negative (2.6%; 3 of 116 patients).
The INFORM study reported improved overall survival in 30 patients with EGFR mutations (HR 0.39, 95% CI 0.15 to 0.98, P = 0.036) with median overall survival improving from 20.97 months to 46.87 months when given gefitinib versus placebo. Whilst this subgroup only contained a very small number of patients, the study was able to show that gefitinib doubled the median overall survival. However, those with no detectable EGFR mutation or an unknown EGFR mutation status did not benefit from gefitinib maintenance therapy (HR 1.27, 95% CI 0.7 to 2.3, P = 0.431; HR 0.92, 95% CI 0.68 to 1.25, P = 0.603, respectively).
Progression‐free survival was also improved with gefitinib (HR 0.17, 95% CI 0.07 to 0.41, P < 0.0001) over placebo. Median progression‐free survival improved from 2.8 months to 16.6 months in this subgroup analysis of the INFORM trial.
2. Gefitinib versus chemotherapy
Survival
See Analysis 4.1; Analysis 4.2; Analysis 4.3.
4.1. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 1 HR Overall survival.
4.2. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 2 HR Progression‐free survival.
4.3. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 3 1‐year survival rate.
Gefitinib versus first‐line chemotherapy
As first‐line therapy, only one study reported hazard ratios for survival (Crino 2008 INVITE). Gefitinib did not prolong overall survival (HR 0.98, 95% CI 0.66 to 1.46, P = 0.92, moderate quality of evidence) or progression‐free survival (HR 1.19, 95% CI 0.86 to 1.65, P = 0.30, moderate quality of evidence) when compared with vinorelbine in this general population of patients aged at least 70 years. This study selected patients over the age of 70 years old, therefore this limits the applicability of the data to other patients and thus we downgraded the quality of evidence to moderate.
Two studies reported selected survival outcomes comparing gefitinib with first‐line chemotherapy (Crino 2008 INVITE; Morere 2010 IFCT‐0301). When we pooled data from these two studies there was no difference in one‐year survival rates between gefitinib and first‐line chemotherapy (RR 0.93, 95% CI 0.63 to 1.38, P = 0.73, I2 = 26%). Median overall survival ranged from 2.2 to 5.9 months and 3.5 to 8 months in the gefitinib and chemotherapy groups, respectively. Median progression‐free survival ranged from 1.9 to 2.7 months and 2.0 to 2.9 months in the gefitinib and chemotherapy groups, respectively.
Gefitinib versus second‐line chemotherapy
The SIGN and INTEREST studies compared gefitinib with docetaxel as second‐line therapy (Cufer 2006 SIGN; Kim 2008 INTEREST). Only Kim 2008 INTEREST reported survival outcomes and neither overall survival (HR 1.02, 95% CI 0.91 to 1.15, P = 0.74, moderate quality of evidence) nor progression‐free survival (HR 1.04, 95% CI 0.92 to 1.17, P = 0.51, moderate quality of evidence) were prolonged by gefitinib. Median overall survival ranged from 7.5 to 7.6 months and 7.1 to 8 months in the gefitinib and chemotherapy groups, respectively. There was no difference in the one‐year survival rate (RR 0.94, 95% CI 0.82 to 1.09, P = 0.44). Median progression‐free survival in the non‐selected population ranged from 2.2 to 3 months and 2.7 to 3.4 months in the gefitinib and chemotherapy groups, respectively.
Cufer 2006 SIGN randomised patients to either second‐line gefitinib or docetaxel, however the trial was not formally powered to detect any statistical differences for any endpoint. We judged this to be at risk of serious imprecision and thus downgraded it one level.
Toxicity
See Analysis 4.4; Analysis 4.5; Analysis 4.6; Analysis 4.7; Analysis 4.8; Analysis 4.9; Analysis 4.10; Analysis 4.11.
4.4. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 4 Skin rash.
4.5. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 5 Constipation.
4.6. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 6 Fatigue.
4.7. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 7 Asthenia.
4.8. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 8 Neurotoxicity.
4.9. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 9 Neutropenia.
4.10. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 10 Leukopenia.
4.11. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 11 Febrile neutropenia.
We combined data to compare the toxicity profile of gefitinib with chemotherapy for first‐ and second‐line therapy to assess the overall effect in both groups. Data from Cufer 2006 SIGN, Crino 2008 INVITE, Kim 2008 INTEREST and Morere 2010 IFCT‐0301 were included. Gefitinib was generally better tolerated than chemotherapy. Gefitinib was associated with an increased risk of skin rash when compared with chemotherapy (RR 2.40, 95% CI 1.08 to 5.31, P = 0.03, I2 = 4.7%, high quality of evidence). Gefitinib was associated with a decreased risk of constipation (RR 0.41, 95% CI 0.17 to 0.97, P = 0.04, I2 = 0%, high quality of evidence), fatigue (RR 0.16, 95% CI 0.03 to 0.88, P = 0.04, I2 = 8.2%, moderate quality of evidence), asthenia (RR 0.51, 95% CI 0.35 to 0.75, P = 0.0007, I2 = 0%, high quality of evidence), neurotoxicity (RR 0.07, 95% CI 0.01 to 0.34, P = 0.001, I2 = 0%, high quality of evidence), neutropenia (RR 0.04, 95% CI 0.02 to 0.06, P < 0.00001, I2 = 43.1%, high quality of evidence), leukopenia (RR 0.03, 95% CI 0.00 to 0.22, P = 0.0005, I2 = 0%, high quality of evidence) and febrile neutropenia (RR 0.12, 95% CI 0.06 to 0.23, P < 0.00001, I2 = 0%, high quality of evidence). There were no differences between groups for any other measured adverse side effects including pruritus, diarrhoea, vomiting, anorexia, stomatitis, arthralgia, peripheral oedema, respiratory tract infection, dyspnoea, cough, anaemia, thrombocytopenia, hypokalaemia or pyrexia.
We assessed most of the toxicity outcomes as high‐quality evidence. We downgraded one outcome, fatigue, to a moderate quality of evidence as the study by Crino 2008 INVITE enrolled only 190 patients who were older than 70 years old, thus there was a risk of serious indirectness.
Efficacy
See Analysis 4.26; Analysis 4.27.
4.26. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 26 Overall response rate.
4.27. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 27 Disease control rate.
Only one first‐line study presented data on disease control rates and there was no reported improvement when administering gefitinib versus vinorelbine (RR 0.82, 95% CI 0.61 to 1.10, P = 0.19) (Crino 2008 INVITE). Disease control rates were 43.3% and 53.5% for gefitinib and chemotherapy, respectively. Two second‐line studies reported efficacy data (Cufer 2006 SIGN; Kim 2008 INTEREST). Pooled data showed that there was no improvement in overall response rate when comparing gefitinib and docetaxel as second‐line therapy (RR 1.16, 95% CI 0.85 to 1.59, P = 0.35, I2 = 0%). Overall response rates were 9% to 13% for both the gefitinib and chemotherapy groups.
Quality of life and symptom improvement scores
See Analysis 4.28; Analysis 4.29; Analysis 4.30; Analysis 4.31.
4.28. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 28 FACT‐L QOL improvement rate.
4.29. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 29 LCS QOL improvement rate.
4.30. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 30 TOI QOL improvement rate.
4.31. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 31 PSI QOL improvement rate.
We pooled data from the INVITE (Crino 2008 INVITE) and INTEREST (Kim 2008 INTEREST) studies. Patients who received gefitinib reported statistically significant improvements in quality of life as assessed by scores on the FACT‐L (standardised mean difference (SMD) 10.50, 95% CI 9.55 to 11.45, P < 0.00001, I2 = 21%), LCS (SMD 3.63, 95% CI 3.08 to 4.19, P < 0.00001, I2 = 0%) and TOI (SMD 9.87, 95% CI 1.26 to 18.48, P = 0.02, I2 = 59%). One study also described an improvement in PSI scores (SMD 5.60, 95% CI 3.55 to 7.65, P < 0.00001) in patients who received gefitinib (Crino 2008 INVITE).
Subgroup analysis: Asian population
Survival
See Analysis 5.1; Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6; Analysis 5.7.
5.1. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 1 HR Overall survival = 1st line.
5.2. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 2 HR Overall survival = 2nd line.
5.3. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 3 HR Overall survival = Maintenance.
5.4. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 4 HR Progression‐free survival = 1st line.
5.5. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 5 HR Progression‐free survival = 2nd line.
5.6. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 6 HR Progression‐free survival = Maintenance.
5.7. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 7 1‐year survival rate.
Gefitinib versus first‐line chemotherapy
Five phase III studies compared gefitinib with first‐line platinum doublet chemotherapy (Han 2012 First SIGNAL; Maemondo 2010 NEJ002; Mitsudomi 2010 WJTOG3405; Mok 2009 IPASS; Yang 2014). The IPASS (Mok 2009 IPASS) and NEJ002 (Maemondo 2010 NEJ002) studies compared gefitinib with carboplatin‐paclitaxel. The WJTOG3405 study compared gefitinib with cisplatin‐docetaxel (Mitsudomi 2010 WJTOG3405). The First‐SIGNAL study compared gefitinib with gemcitabine‐cisplatin (Han 2012 First SIGNAL). The study by Yang 2014 compared gefitinib monotherapy with pemetrexed‐cisplatin followed by gefitinib maintenance.
Pooled analysis showed that gefitinib did not improve overall survival (HR 0.94, 95% CI 0.82 to 1.06, P = 0.31, I2 = 0%) or the one‐year survival rate (RR 1.03, 95% C 0.97 to 1.09, P = 0.33, I2 = 1%). One study reported median overall survival as 22 months in both groups. Progression‐free survival was higher in the gefitinib group than in the chemotherapy group (HR 0.65, 95% CI 0.43 to 0.98, P = 0.04, I2 = 93%). Median progression‐free survival ranged from 5.5 to 6.4 months with chemotherapy to 5.7 to 10.4 months with gefitinib. Please refer to Figure 3 for the pooled progression‐free survival data from first‐line studies that included Asian patients.
3.
Progression‐free survival: Gefitinib versus first‐line chemotherapy in an Asian population (Analysis 5.4).
Gefitinib versus second‐line chemotherapy
Two phase III studies compared gefitinib with second‐line docetaxel in patients of Asian ethnicity (Lee 2010 ISTANA; Maruyama 2008 V‐15‐32) and one phase III study compared gefitinib with pemetrexed (Sun 2012 KCSG‐LU08‐01). In pooled analysis of these three trials, there was no benefit on either overall survival or the one‐year survival rate for gefitinib over second‐line chemotherapy (HR 0.94, 95% CI 0.79 to 1.12, P = 0.50, I2 = 0%; RR 0.94, 95% CI 0.81 to 1.11, P = 0.48, I2 = 0%, respectively). Progression‐free survival was prolonged (HR 0.71, 95% CI 0.57 to 0.88, P = 0.002, I2 = 40%; see Figure 4) in favour of gefitinib. Median progression‐free survival was 2 to 6.8 months with second‐line chemotherapy, and 2 to 10 months with gefitinib in the second‐line setting.
4.
Progression‐free survival: Gefitinib versus second‐line chemotherapy in an Asian population (Analysis 5.5).
Gefitinib versus maintenance chemotherapy
Two phase II studies compared maintenance gefitinib with chemotherapy, however only one of them presented survival data (Ahn 2012). There was no difference in overall survival (HR 2.15, 95% CI 0.83 to 5.55, P = 0.11) or progression‐free survival (HR 0.53, 95% CI 0.27 to 1.04, P = 0.06) between the gefitinib and chemotherapy treatment arms. There was an improved one‐year survival rate (RR 0.79, 95% CI 0.65 to 0.98, P = 0.03) with maintenance gefitinib over chemotherapy.
Toxicity
See Analysis 5.8; Analysis 5.9; Analysis 5.10; Analysis 5.11; Analysis 5.12; Analysis 5.13; Analysis 5.14; Analysis 5.15; Analysis 5.16; Analysis 5.17; Analysis 5.18; Analysis 5.19; Analysis 5.20; Analysis 5.21; Analysis 5.22; Analysis 5.23.
5.8. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 8 Nausea.
5.9. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 9 Vomiting.
5.10. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 10 Anorexia.
5.11. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 11 Fatigue.
5.12. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 12 Arthralgia/myalgia.
5.13. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 13 Asthenia.
5.14. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 14 Neurotoxicity.
5.15. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 15 Neutropenia.
5.16. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 16 Anaemia.
5.17. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 17 Leukopenia.
5.18. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 18 Thrombocytopenia.
5.19. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 19 Febrile neutropenia.
5.20. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 20 Skin rash.
5.21. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 21 Diarrhoea.
5.22. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 22 Increased ALT.
5.23. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 23 Increased AST.
Gefitinib was generally well tolerated in this population. We pooled toxicity data from all studies. Compared to chemotherapy, the gefitinib group reported fewer adverse side effects such as nausea (RR 0.34, 95% CI 0.17 to 0.64, P = 0.001, I2 = 0%), vomiting (RR 0.19, 95% CI 0.05 to 0.77, P = 0.02, I2 = 56%, random‐effects model), anorexia (RR 0.36, 95% CI 0.27 to 0.49, P < 0.00001, I2 = 18%), fatigue (RR 0.32, 95% CI 0.22 to 0.46, P < 0.00001, I2 = 50%), arthralgia (RR 0.14, 95% CI 0.03 to 0.61, P = 0.009, I2 = 0%), asthenia (RR 0.22, 95% CI 0.08 to 0.58, P = 0.002, I2 = 13%), neurotoxicity (RR 0.07, 95% CI 0.02 to 0.24, P < 0.0001, I2 = 0%), neutropenia (RR 0.11, 95% CI 0.05 to 0.27, P < 0.00001, I2 = 82%, random‐effects model), anaemia (RR 0.18, 95% CI 0.12 to 0.29, P < 0.00001, I2 = 4%), leukopenia (RR 0.07, 95% CI 0.02 to 0.23, P < 0.00001, I2 = 77%, random‐effects model), thrombocytopaenia (RR 0.32, 95% CI 0.14 to 0.72, P = 0.006, I2 = 22%) and febrile neutropenia (RR 0.09, 95% CI 0.03 to 0.28, P < 0.0001, I2 = 0%). Other side effects were seen more frequently in the gefitinib group. Skin rash (RR 3.11, 95% CI 1.28 to 7.55, P = 0.01, I2 = 60%, random‐effects model), diarrhoea (RR 2.79, 95% CI 1.57 to 4.94, P = 0.0005, I2 = 0%), increased alanine aminotransferase (ALT) (RR 10.03, 95% CI 5.23 to 19.26, P < 0.00001, I2 = 37%) and increased aspartate transaminase (AST) (RR 7.73, 95% CI 2.78 to 21.46, P < 0.0001, I2 = 0%) were more frequent in gefitinib‐treated cases.
Efficacy
See Analysis 5.24; Analysis 5.25; Analysis 5.26.
5.24. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 24 Overall response rate.
5.25. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 25 Stable disease.
5.26. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 26 Disease control rate.
Objective response rates were higher in the gefitinib group when compared with first‐line chemotherapy (RR 1.43, 95% CI 1.13 to 1.82, P = 0.003, I2 = 76%, random‐effects model). The overall response rate ranged from 43% to 62.1% in the gefitinib group and 30.7% to 32.2% in the chemotherapy group. There was no effect on the disease control rate (RR 0.99, 95% CI 0.86 to 1.13, P = 0.86, I2 = 80%, random‐effects model): 73% to 94% and 78% to 81%, respectively.
The overall response rate was not significantly improved in the gefitinib group compared with second‐line chemotherapy (RR 1.43, 95% CI 0.92 to 2.22, P = 0.11, I2 = 46%). Two studies found that overall response rates were poor overall, but the gefitinib group performed better (23% to 28%) than the second‐line chemotherapy group (8% to 13%) (Lee 2010 ISTANA; Maruyama 2008 V‐15‐32). The disease control rate (RR 0.99, 95% CI 0.78 to 1.25, P = 0.92, I2 = 46%) was statistically similar for both groups (34% and 33%, respectively).
Pooled data from two maintenance studies found that gefitinib improved the stable disease rate and the disease control rate (RR 0.64, 95% CI 0.44 to 0.93, P = 0.02; RR 0.65, 95% CI 0.49 to 0.85, P = 0.002, respectively). There was no improvement in the overall response rate with maintenance gefitinib (RR 0.88, 95% CI 0.41 to 1.87, P = 0.06, I2 = 73%, random‐effects model).
Quality of life and symptom improvement scores
Three studies explored the impact of gefitinib versus chemotherapy on quality of life, but unfortunately the data could not be pooled (Lee 2010 ISTANA; Maruyama 2008 V‐15‐32; Mok 2009 IPASS). All three studies reported significantly improved quality of life in patients who received gefitinib as measured by the Trial Outcome Index (TOI). Mok 2009 IPASS and Maruyama 2008 V‐15‐32 stated that improvements as measured by FACT‐L were significant, but none recorded significant improvements on the lung cancer subscale (LCS).
Subgroup analysis: EGFR mutation positive population
Survival
See Analysis 6.1; Analysis 6.2; Analysis 6.3; Analysis 6.4.
6.1. Analysis.
Comparison 6 Gefitinib versus chemotherapy (EGFR mutation), Outcome 1 HR Overall survival = 1st line.
6.2. Analysis.
Comparison 6 Gefitinib versus chemotherapy (EGFR mutation), Outcome 2 HR Overall survival = 2nd line.
6.3. Analysis.
Comparison 6 Gefitinib versus chemotherapy (EGFR mutation), Outcome 3 HR Progression‐free survival = 1st line.
6.4. Analysis.
Comparison 6 Gefitinib versus chemotherapy (EGFR mutation), Outcome 4 HR Progression‐free survival = 2nd line.
Gefitinib versus first‐line chemotherapy
Five studies compared gefitinib with first‐line chemotherapy. Two of these studies selected patients with EGFR mutations (Maemondo 2010 NEJ002; Mitsudomi 2010 WJTOG3405), and the others selected patients based on clinical features and conducted subgroup analyses on patients positive for EGFR mutations (Han 2012 First SIGNAL; Mok 2009 IPASS; Yang 2014). We have separately analysed studies that selected EGFR mutants and those that selected patients based on clinical features then conducted subgroup analyses and progression‐free survival results are presented in Figure 5.
5.
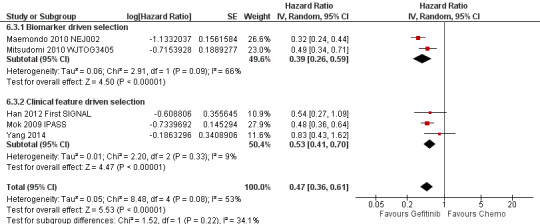
Progression‐free survival: Gefitinib versus first‐line chemotherapy in an EGFR mutation positive population (Analysis 6.3).
The two biomarker driven studies did not show any improvement in overall survival (HR 0.98, 95% CI 0.72 to 1.33, P = 0.90, I2 = 54%). Progression‐free survival was significantly increased with gefitinib compared with first‐line chemotherapy (HR 0.39, 95% CI 0.26 to 0.59, P < 0.00001, I2 = 66%, random‐effects model).
Three phase III studies conducted subgroup analyses in EGFR mutation positive patients. There was no improvement in overall survival (HR 0.95, 95% CI 0.68 to 1.33, P = 0.75, I2 = 20%). However, there was a statistically significant improvement in progression‐free survival (HR 0.53, 95% CI 0.41 to 0.70, P < 0.00001, I2 = 9%).
Pooled analysis of all first‐line studies that examined EGFR mutation positive patients showed that there was no difference in overall survival (HR 0.97, 95% CI 0.77 to 1.21, P = 0.76, I2 = 15%). However, pooled data from these five studies showed that gefitinib was able to prolong progression‐free survival when compared with first‐line chemotherapy (HR 0.47, 95% CI 0.36 to 0.61, P < 0.00001, I2 = 53%, random‐effects model), with median progression‐free survival improving from 5.5 to 6.3 months in the chemotherapy group to 9.2 to 10.4 months in the gefitinib group.
Gefitinib versus second‐line chemotherapy
When comparing gefitinib with second‐line chemotherapy, data were available from two studies (Kim 2008 INTEREST; Sun 2012 KCSG‐LU08‐01). This showed that gefitinib did not improve overall survival (HR 0.83, 95% CI 0.41 to 1.66, P = 0.60). There was a statistically significant improvement in progression‐free survival (HR 0.24, 95% CI 0.12 to 0.47, P < 0.0001, I2 = 0%) in EGFR mutation positive patients. Progression‐free survival for this analysis is presented in Figure 6.
6.
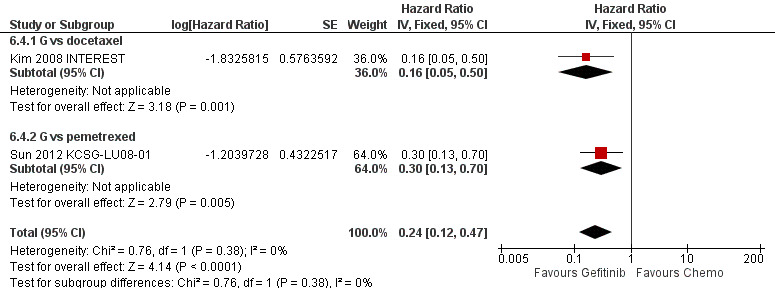
Progression‐free survival: Gefitinib versus second‐line chemotherapy in an EGFR mutation positive population (Analysis 6.4).
Efficacy
See Analysis 6.5; Analysis 6.6; Analysis 6.7.
6.5. Analysis.
Comparison 6 Gefitinib versus chemotherapy (EGFR mutation), Outcome 5 Overall response rate.
6.6. Analysis.
Comparison 6 Gefitinib versus chemotherapy (EGFR mutation), Outcome 6 Stable disease.
6.7. Analysis.
Comparison 6 Gefitinib versus chemotherapy (EGFR mutation), Outcome 7 Disease control rate.
Gefitinib versus first‐line chemotherapy
Pooled analysis comparing first‐line gefitinib with chemotherapy showed that the overall response rate was significantly improved in favour of gefitinib. The two studies that selected patients with EGFR mutations (Maemondo 2010 NEJ002; Mitsudomi 2010 WJTOG3405), as well as the phase III studies that conducted subgroup analyses on EGFR mutation positive patients found significant improvements in overall response rate (RR 2.23, 95% CI 1.75 to 2.85, P < 0.00001, I2 = 0% and RR 1.45, 95% CI 1.05 to 1.99, P = 0.02, I2 = 53%, random‐effects model, respectively). Pooled analysis of all studies showed that first‐line gefitinib improved the overall response rate over chemotherapy (RR 1.73, 95% CI 1.29 to 2.31, P = 0.002, I2 = 70%, random‐effects model) and overall response rates ranged from 62% to 76% in the gefitinib group, compared with 31% to 47% in the first‐line chemotherapy group. The stable disease rate was improved in favour of first‐line chemotherapy (RR 0.52, 95% CI 0.28 to 0.97, P = 0.04, I2 = 66%, random‐effects model) but there was no difference in the disease control rate (RR 1.06, 95% CI 0.91 to 1.22, P = 0.46, I2 = 82%, random‐effects model).
Gefitinib versus second‐line chemotherapy
Gefitinib as second‐line therapy did not result in a significant difference in overall response rate (RR 1.65, 95% CI 0.88 to 3.09, P = 0.12). Overall response rates were reported as 67% in the gefitinib group and 46% in the chemotherapy group.
3. Gefitinib at a specific dose versus gefitinib at a different dose
Survival
See Analysis 7.1.
7.1. Analysis.
Comparison 7 Gefitinib 250 mg versus gefitinib 500 mg, Outcome 1 1‐year survival rate.
Two multicentre, randomised, double‐blind, phase II studies evaluated differing doses of gefitinib (250 mg and 500 mg) in the second‐line setting (Fukuoka 2003 IDEAL I; Kris 2003 IDEAL II). There was no significant effect on one‐year survival (RR 0.83, 95% CI 0.61 to 1.11, P = 0.21, I2 = 0%). HRs were not available for meta‐analysis. Median overall survival ranged from 7 to 7.6 months in patients given 250 mg, and 6 to 8 months in those given 500 mg of gefitinib. Median progression‐free survival ranged from 2.7 to 7 months and from 2.8 to 6 months in patients given 250 mg and 500 mg, respectively.
One study examined the effect of a higher dose of gefitinib in patients that had been stable after one month of 250 mg/day dosing of gefitinib (Xue 2015). In this study, there was no difference in progression‐free or overall survival with a higher dose of gefitinib (500 mg/day versus 250 mg/day: median progression‐free survival 5.30 months versus 6.23 months, P = 0.167; median overall survival 13.70 months versus 18.87 months, P = 0.156).
Toxicity
See Analysis 7.2; Analysis 7.3; Analysis 7.4; Analysis 7.5; Analysis 7.6; Analysis 7.7; Analysis 7.8; Analysis 7.9.
7.2. Analysis.
Comparison 7 Gefitinib 250 mg versus gefitinib 500 mg, Outcome 2 Skin rash.
7.3. Analysis.
Comparison 7 Gefitinib 250 mg versus gefitinib 500 mg, Outcome 3 Acne.
7.4. Analysis.
Comparison 7 Gefitinib 250 mg versus gefitinib 500 mg, Outcome 4 Pruritus.
7.5. Analysis.
Comparison 7 Gefitinib 250 mg versus gefitinib 500 mg, Outcome 5 Diarrhoea.
7.6. Analysis.
Comparison 7 Gefitinib 250 mg versus gefitinib 500 mg, Outcome 6 Nausea.
7.7. Analysis.
Comparison 7 Gefitinib 250 mg versus gefitinib 500 mg, Outcome 7 Vomiting.
7.8. Analysis.
Comparison 7 Gefitinib 250 mg versus gefitinib 500 mg, Outcome 8 Anorexia.
7.9. Analysis.
Comparison 7 Gefitinib 250 mg versus gefitinib 500 mg, Outcome 9 Asthenia.
Data from all three studies were available for pooling (Fukuoka 2003 IDEAL I; Kris 2003 IDEAL II; Xue 2015). A gefitinib dose of 500 mg had a marginally worse toxicity profile when compared with the lower dose of 250 mg. This higher dose was associated with an increased rate of diarrhoea (RR 8.36, 95% CI 1.58 to 44.34, P = 0.01, I2 = 0%) and skin rash (RR 8.13, 95% CI 1.51 to 43.72, P = 0.01, I2 = 0%). Other reported side effects such as pruritus, acne, vomiting, anorexia, asthenia, neutropenia, leukopenia and dyspnoea were not significantly different between doses.
Efficacy
See Analysis 7.10; Analysis 7.11.
7.10. Analysis.
Comparison 7 Gefitinib 250 mg versus gefitinib 500 mg, Outcome 10 Overall response rate.
7.11. Analysis.
Comparison 7 Gefitinib 250 mg versus gefitinib 500 mg, Outcome 11 Partial response.
Pooled analysis of two studies found no significant difference in overall response rate (RR 0.92, 95% CI 0.58 to 1.46, P = 0.72, I2 = 0%) between doses (Fukuoka 2003 IDEAL I; Kris 2003 IDEAL II). Overall response rates in the 250mg arm were reported as 18% and 12% in the IDEAL I and IDEAL II trials respectively, compared with ORR rates of 19% and 9% respectively, in patients receiving 500mg of gefitinib. Complete and partial response rates were only reported individually in the IDEAL I paper, and were 10% and 18.1%, respectively.
A higher dose of gefitinib as maintenance treatment did not improve the overall response rate (12.5% versus 12.5%, P = 1) (Xue 2015).
Quality of life and symptom improvement scores
See Analysis 7.12; Analysis 7.13.
7.12. Analysis.
Comparison 7 Gefitinib 250 mg versus gefitinib 500 mg, Outcome 12 FACT‐L Symptom improvement rate.
7.13. Analysis.
Comparison 7 Gefitinib 250 mg versus gefitinib 500 mg, Outcome 13 TOI QOL improvement rate.
Two studies reported changes in quality of life and symptom improvement scores (Fukuoka 2003 IDEAL I; Kris 2003 IDEAL II).
Quality of life improvements were also measured using the Trial Outcome Index (TOI), a summary score of the physical and functional domains of FACT‐L and the lung cancer subscale (a validated subscale of the FACT‐L questionnaire). No statistically significant difference was found between 250 mg and 500 mg of gefitinib in the rate of change of the FACT‐L and TOI scales (SMD 3.70, 95% CI ‐7.28 to 14.69; P = 0.51, I2 = 0% and SMD 7.38, 95% CI ‐2.30 to 17.05; P = 0.14, I2 = 0%, respectively). Unfortunately, extractable data from the published papers were inconsistently reported and thus not all data were pooled for analysis.
Data from the IDEAL II study further correlated symptom improvement with objective response and survival. When given a dose of 250 mg of gefitinib, all patients who experienced a partial response also experienced symptom improvement. Patients with stable or progressive disease who experienced symptom improvement also had a longer median survival time compared to those in the same tumour progression category without symptom improvement.
Subgroup analysis
Both studies performed subgroup analyses.
Fukuoka 2003 IDEAL I found that the objective tumour response rate was higher for Japanese patients versus non‐Japanese patients (27.5% versus 10.4%; odds ratio (OR) 3.27; P = 0.0023). A planned subgroup multivariate analysis revealed seven factors that predicted response in Japanese patients: baseline lung cancer subscale, body mass index, performance status, prior radiotherapy, histology, prior immuno/hormonal therapy and gender. After accounting for all the baseline imbalances, the odds ratio indicated that Japanese patients had response rates 1.64 times that of non‐Japanese patients, but this was not considered statistically significant.
Kris 2003 IDEAL II reported observation of symptom improvement and radiographic responses in all patient subgroups. Multivariate analysis identified female gender to be predictive of both symptom improvement and radiographic responses.
Symptom improvement was rapid, with a median time to onset of less than two weeks: 10 days in the 250 mg group (95% CI 8 to 22 days) and 9 days (95% CI 9 to 16 days) in the 500 mg group.
It was also reported that patients receiving third‐, fourth‐ and fifth‐line and above therapy had similar rates of symptom improvement both for 250 mg and 500 mg doses of gefitinib. Post‐hoc analysis showed that RRs for symptom improvement for the subgroup of patients who had previously received a platinum and taxane were 24% at 250 mg and 28% at 500 mg and for patients who had previously received platinum and docetaxel, 24% and 26% for the 250 mg and 500 mg groups, respectively.
4. Gefitinib versus gefitinib plus chemotherapy
Survival
See Analysis 8.1; Analysis 8.2; Analysis 8.3.
8.1. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 1 HR Progression‐free survival.
8.2. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 2 1‐year survival rate.
8.3. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 3 1‐year progression‐free survival.
In the first‐line setting, two studies compared gefitinib alone with gefitinib plus pemetrexed (An 2016; Cheng 2016). One study reported no difference in median survival between the gefitinib and gefitinib plus chemotherapy group (32 months versus 34 months respectively) (An 2016). The other study did not present survival data (Cheng 2016). There was, however, a statistically significant improvement in progression‐free survival in favour of gefitinib plus chemotherapy over gefitinib alone (HR 0.69, 95% CI 0.49 to 0.96; P = 0.03; median progression‐free survival 12.6 months versus 18.3 months) (Cheng 2016).
In the second‐line or greater setting, median overall survival improved from 13.3 months (Chen 2007) and 18.3 months (Chen 2011) to 23.4 months and 23.6 months, respectively. This improvement was not statistically significant. Combining gefitinib with either vinorelbine or tegafur/uracil did not improve the one‐year survival rate (RR 1.15, 95% CI 0.92 to 1.43; P = 0.22; I2 = 43%). Gefitinib plus chemotherapy improved one‐year progression‐free survival (RR 2.29, 95% CI 1.38 to 3.80; P = 0.001). However, the HR for progression‐free survival was only presented in Chen 2011 (HR 0.65, 95% CI 0.43 to 0.97; P = 0.04: median progression‐free survival improved from 7.1 months (Chen 2007) and 5.3 months (Chen 2011) to 12.8 months and 8.3 months, respectively).
Toxicity
See Analysis 8.4; Analysis 8.5; Analysis 8.6; Analysis 8.7; Analysis 8.8; Analysis 8.9; Analysis 8.10; Analysis 8.11; Analysis 8.12; Analysis 8.13; Analysis 8.14; Analysis 8.15.
8.4. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 4 Skin rash.
8.5. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 5 Diarrhoea.
8.6. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 6 Constipation.
8.7. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 7 Fatigue.
8.8. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 8 Leukopenia.
8.9. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 9 Anaemia.
8.10. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 10 Thrombocytopenia.
8.11. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 11 Neutropenia.
8.12. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 12 Increased ALT.
8.13. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 13 Increased AST.
8.14. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 14 Vomiting.
8.15. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 15 Nausea.
We pooled toxicity data from three studies (An 2016; Chen 2007; Cheng 2016). Both regimens were well tolerated with no significant difference in rates of skin rash, diarrhoea, constipation, fatigue, blood counts, nausea or vomiting.
Pooled data from both first‐line studies did show that the addition of pemetrexed chemotherapy to gefitinib resulted in higher rates of raised ALT (RR 2.57, 95% CI 1.09 to 6.04; P = 0.03; I2 = 63%) but not AST (RR 1.47, 95% CI 0.56 to 3.88; P = 0.44; I2 = 0%).
Efficacy
See Analysis 8.16; Analysis 8.17; Analysis 8.18.
8.16. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 16 Overall response rate.
8.17. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 17 Partial response.
8.18. Analysis.
Comparison 8 Gefitinib versus gefitinib + chemotherapy, Outcome 18 Stable disease.
When comparing gefitinib alone to gefitinib plus chemotherapy as first‐line therapy, there was no improvement in overall response rate (RR 1.02, 95% CI 0.89 to 1.17; P = 0.73; I2 = 26%) or rate of stable disease (RR 0.67, 95% CI 0.39 to 1.16; P = 0.16; I2 = 0%).
In the second‐line setting, the addition of chemotherapy to gefitinib did not result in an improvement in either partial radiological response (RR 1.02, 95% CI 0.71 to 1.47; P = 0.92; I2 = 0%) or stable disease (RR 1.30, 95% CI 0.84 to 2.03; P = 0.24; I2 = 16%).
5. Gefitinib plus chemotherapy versus chemotherapy
Survival
See Analysis 9.1; Analysis 9.2; Analysis 9.3.
9.1. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 1 HR Overall survival.
9.2. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 2 HR Progression‐free survival.
9.3. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 3 1‐year survival rate.
Meta‐analysis of two phase II, first‐line trials examining 1411 patients showed that the addition of gefitinib (250 mg/day) to a chemotherapy regimen in chemotherapy‐naive patients did not change the one‐year survival rate (RR 0.95, 95% CI 0.84 to 1.08, P = 0.44, I2 = 0%) (Giaccone 2004 INTACT I; Herbst 2004 INTACT II).
Two trials compared the addition of first‐line gefitinib to chemotherapy with chemotherapy alone in Asian patients only (Takeda 2010 WJTOG0203; Yu 2014). There was no improvement in overall survival (HR 0.86, 95% CI 0.72 to 1.02, P = 0.08, I2 = 0%), however there was a statistically significant improvement in progression‐free survival (HR 0.69, 95% CI 0.62 to 0.77, P < 0.00001, I2 = 18%).
A single phase III trial recruited only EGFR mutation positive patients who had failed prior first‐line gefitinib, and the addition of gefitinib to chemotherapy did not improve progression‐free survival (HR 0.86, 95% CI 0.65 to 1.13, P = 0.28) (Soria 2015 IMPRESS). Overall survival appeared to be better in the chemotherapy alone group (HR 1.62, 95% CI 1.05 to 2.50, P = 0.03).
Toxicity
See Analysis 9.4; Analysis 9.5; Analysis 9.6.
9.4. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 4 Skin rash.
9.5. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 5 Acne.
9.6. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 6 Diarrhoea.
Pooled data from all five trials showed that the addition of gefitinib to a chemotherapeutic regimen resulted in increased rates of skin rash (RR 2.98, 95% CI 1.54 to 5.77, P = 0.001, I2 = 28%), acne (RR 4.95, 95% CI 1.09 to 22.51, P = 0.04, I2 = 0%) and diarrhoea (RR 2.04, 95% CI 1.17 to 3.58, P = 0.01, I2 = 17%). Other measured side effects such as pruritus, vomiting, nausea, anorexia, asthenia, dyspnoea, anaemia, neutropenia and leukopenia were not significantly increased.
Efficacy
See Analysis 9.16.
9.16. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 16 Overall response rate.
In the first‐line setting, the addition of gefitinib to chemotherapy did not effect the overall response rate in either the unselected population (RR 1.07, 95% CI 0.94 to 1.22, P = 0.28, I2 = 0%) or the Asian population (RR 1.14, 95% CI 0.93 to 1.40, P = 0.20, I2 = 0%). The overall response rate ranged from 30% to 51% in the gefitinib plus chemotherapy group and 29% to 50% in the chemotherapy group.
There was also no improvement in the overall response rate in the second‐line setting (RR 0.93, 95% CI 0.66 to 1.31, P = 0.66, I2 = 0%), and the overall response rate was 32% in the gefitinib plus chemotherapy group and 34% in the chemotherapy alone group.
Quality of life and symptom improvement scores
In the first‐line setting, the WJTOG0203 study reported a disease‐related symptoms assessment (Takeda 2010 WJTOG0203). Sequential administration of gefitinib was reported to provide better symptom control, however these differences were not statistically significant. The adjusted mean of initial summed scores of the lung cancer subscale were 20.3 for Arm A and 20.6 for Arm B. The adjusted lung cancer subscale scores at 12 and 18 weeks were 21 and 20.9 for Arm A and 21.8 and 21.2 for Arm B, respectively.
In the second‐line setting, the IMPRESS study reported that the improvements in quality of life were no different when gefitinib plus chemotherapy was compared to placebo plus chemotherapy as measured by the Trial Outcome Index (TOI) (29% versus 30.2%, respectively), FACT‐L (35.5% versus 38%, respectively) or lung cancer subscale (43.5% versus 42.6%, respectively) (Soria 2015 IMPRESS). There was also no difference in the time to worsening of health‐related quality of life as measured by the TOI, FACT‐L and lung cancer subscale.
These data could not be pooled for meta‐analysis.
Subgroup analysis
A planned exploratory subgroup analysis in Japanese patients of overall survival by histological group reported that patients with adenocarcinoma that were given sequential gefitinib had better outcomes than patients given chemotherapy alone (n = 467; progression‐free survival: HR 0.79, 95% CI 0.65 to 0.98, P = 0.03; overall survival: HR 0.60, 95% CI 0.50 to 0.73, P < 0.001) (Takeda 2010 WJTOG0203). There was no difference in overall survival or progression‐free survival in those with non‐adenocarcinoma (overall survival: HR 1.24, 95% CI 0.85 to 1.79, P = 0.25 and progression‐free survival: HR 1.14, 95% CI 0.80 to 1.62, P = 0.47). This study also reported that smokers also experienced improved survival with sequential gefitinib (HR 0.79, 95% CI 0.64 to 0.98), as opposed to non‐smokers (HR 0.94, 95% CI 0.66 to 1.33), however P values were not published.
Discussion
This meta‐analysis examined published data on the effectiveness and safety of gefitinib in patients with non‐small cell lung cancer (NSCLC). We performed an extensive search of electronic databases and carried out handsearching, and 35 randomised studies fulfilled the inclusion criteria. Some were phase II, open‐label design trials and limited pooling of data was possible due to methodological differences between studies.
Summary of main results
A total of 35 studies were included in this review.
Five studies compared gefitinib with placebo: one study in the first‐line, one study in the second‐line and three studies in the maintenance setting. Gefitinib did not improve survival in the first‐line setting in a general population of NSCLC patients. The ISEL study found that gefitinib as a second‐line therapy was able to reduce the risk of disease progression by 18%, and improve the objective response rate from 1% to 6% when compared to placebo (Thatcher 2005 ISEL). Three studies compared gefitinib with placebo in the maintenance setting. Gefitinib reduced the risk of disease progression by 31%.
In patients of Asian ethnicity, preplanned subgroup analysis in the ISEL study found that second‐line gefitinib improved overall and progression‐free survival by 34% and 31%, respectively (Chang 2006). The INFORM study compared gefitinib with placebo in the maintenance setting and selected patients of Asian ethnicity (Zhang 2012 INFORM). This study found that gefitinib prolonged progression‐free survival by 58% and the overall response rate improved from 1% to 24%. Quality of life analysis from the INFORM study also showed that improvement rates as measured by the Functional Assessment of Cancer Therapy‐Lung (FACT‐L), Trial Outcome Index (TOI) and lung cancer subscale were higher in the patients who were given gefitinib as maintenance therapy. These patients also experienced a longer time‐to‐worsening of quality of life scores.
In patients positive for an epidermal growth factor receptor (EGFR) mutation, subgroup analysis of the INFORM study showed an improvement in median overall survival from 21 months to 47 months and maintenance gefitinib reduced the risk of death by 61% (Zhang 2012 INFORM). Maintenance gefitinib also improved progression‐free survival from 2.8 months to 16.6 months.
Several phase II and III studies compared gefitinib with chemotherapy. Eighteen randomised studies have examined the effectiveness of gefitinib compared with recommended chemotherapy regimes. Meta‐analysis of four studies failed to demonstrate any benefit for survival or response rate in a general population (moderate quality of evidence). (Please refer to Table 1 and Table 2). Quality of life was significantly better for patients on gefitinib than for those having chemotherapy, and gefitinib was significantly less toxic and generally well tolerated when compared with chemotherapy (high quality of evidence), in keeping with results from other studies. Skin rash, diarrhoea and increased liver transaminases were more frequent in the gefitinib group, but other significant side effects such as neutropenia, anaemia, leukopenia and febrile neutropenia were less frequent. (Please refer to Table 3).
Fourteen trials included patients exclusively of Asian ethnicity, with some additionally selecting by EGFR mutation status or clinical criteria that are likely to have enriched EGFR mutations. Gefitinib improved the overall response rate by 43% and progression‐free survival by 35% when compared with first‐line chemotherapy, but this did not translate into an improvement in overall survival. Comparing gefitinib with second‐line chemotherapy found that progression‐free survival was improved by 29%, but there was no effect on overall survival or overall response rate. The effect of Asian ethnicity is complicated, and may be confounded by higher rates of EGFR mutation and the biologically plausible predictive biomarker characteristic of EGFR mutations. Two trials compared maintenance gefitinib with maintenance chemotherapy. There was no difference in either progression‐free survival or overall survival, but gefitinib was able to improve the one‐year survival rate by 21% and the disease control rate by 35%. Skin rash, diarrhoea and elevated liver transaminases were more common in those treated with gefitinib, however severe adverse side effects such as haematological derangements, neurotoxicity, nausea, anorexia, fatigue and arthralgia were much more common in the chemotherapy group.
Eight studies either selected patients with tumours expressing EGFR mutations for comparison or conducted subgroup analyses in these patients. Use of gefitinib in the first‐line setting improved progression‐free survival over platinum‐doublet chemotherapy. Studies that selected patients with EGFR mutations exclusively were able to show a 61% improvement in progression‐free survival over first‐line chemotherapy. Two studies recruited patients with clinical features likely to respond favourably to gefitinib, and showed a 51% improvement in progression‐free survival after subgroup analysis of EGFR mutation positive patients. Gefitinib also improved the overall response rate by 73% over first‐line chemotherapy. However, none were able to demonstrate an improvement in overall survival, arguably due to high rates of cross‐over. When comparing gefitinib with second‐line chemotherapy, a similar improvement in progression‐free survival of 76% was seen. There was no impact on overall survival or overall response rate.
Increasing the dose of gefitinib from 250 mg/day to 500 mg/day yielded no additional benefit in survival or response rate in three phase II trials. This increased dose, however, was associated with greater toxicity.
Two phase II studies compared pemetrexed plus gefitinib with gefitinib alone as first‐line treatment. Progression‐free survival was improved by 31% with a median improvement from 12.6 months to 18.3 months. There were, however, increased rates of raised ALT in this treatment arm. All other toxicities were similar. The two studies comparing gefitinib plus chemotherapy with gefitinib alone in the second‐line setting showed improved one‐year progression‐free survival when chemotherapy was added to gefitinib.
Five studies showed that the addition of gefitinib to a chemotherapy regimen compared to chemotherapy alone did not confer any survival benefit. In patients of Asian ethnicity, two studies showed that first‐line gefitinib plus chemotherapy improved progression‐free survival by 31% compared to chemotherapy alone. One phase III study compared gefitinib plus chemotherapy to chemotherapy alone and found that survival was improved in favour of chemotherapy alone. All patients in this study were EGFR mutation positive, but had failed prior first‐line therapy with gefitinib.
A summary of the efficacy results is presented in Table 5.
Overall completeness and applicability of evidence
Much of the data analysed in this review has predated the routine assessment of EGFR mutation status in NSCLC. This testing is now done routinely in many countries before starting treatment, and the status of EGFR mutation now guides the therapeutic options. Gefitinib has already been registered in occidental countries for treatment of NSCLC with EGFR activating mutations. The treatment options for patients with advanced NSCLC continue to evolve rapidly and although some of the data in this review may be considered historical, it still provides a foundation upon which ongoing studies examining the relationship between the effectiveness of gefitinib and the timing of its use with other treatment modalities can be built.
The inclusion criteria for selecting patients for these studies may have adversely affected their ability to provide statistically significant results. For example, some studies selected patients with highly refractory disease who may have been less likely to respond to any additional therapy. Some studies selected chemotherapy‐naive patients for inclusion (Giaccone 2004 INTACT I; Herbst 2004 INTACT II), whereas others included patients who had received at least one prior platinum‐containing chemotherapy regimen (Kris 2003 IDEAL II; Fukuoka 2003 IDEAL I). Thatcher 2005 ISEL selected patients who had recurrence or progressive disease during treatment or within 90 days of the last dose of chemotherapy.
In some studies, patients who progressed on a certain treatment were allowed the opportunity to switch to the comparison arm. This was reported in some studies (e.g. Mok 2009 IPASS) and in some data were censored accordingly (e.g. Mitsudomi 2010 WJTOG3405). The impact of this cross‐over is difficult to analyse and may contribute to a lack of survival benefit seen in these large phase III studies.
We analysed EGFR mutation positive patients in this review, finding that gefitinib improved progression‐free survival over first‐ and second‐line chemotherapy and over placebo in the maintenance setting. However, patients with EGFR wild type NSCLC were not formally included in this meta‐analysis. Studies such as Zhou 2014 CTONG 0806 were excluded from this meta‐analysis as they selected only EGFR wild type NSCLC. This study showed that second‐line pemetrexed chemotherapy was superior to gefitinib in terms of progression‐free survival but a trend towards improved overall survival was also seen. Thus, this highlights the importance of determining the EGFR mutation status in patients with advanced NSCLC, as this result will guide further management decisions.
Patients with progressive NSCLC who have failed to respond to first‐line chemotherapy have an extremely poor prognosis and often exhibit severe symptoms. One difficulty with meta‐analyses of quality of life data is that outcomes are not consistently reported in the published papers, limiting the pooling of data. Some studies reported changes in FACT‐L and the lung cancer subscale that reached the pre‐defined criteria for clinical significance (Cella 2005; Kris 2003 IDEAL II), whereas others failed to show any improvement (Fukuoka 2003 IDEAL I; Thatcher 2005 ISEL). Cella 2005 reported a correlation between symptom improvement, objective response and survival, and found that 30% of patients showed a quality of life improvement that was correlated with tumour response. Kris 2003 IDEAL II reported that symptoms improved in 96% of patients with partial radiographic responses. Pre‐planned subgroup analysis in Thatcher 2005 ISEL found that gefitinib was associated with a significant improvement in symptom score compared with placebo in never‐smokers and patients of Asian origin.
Quality of the evidence
The 'Risk of bias' tables have enabled a methodical and thorough assessment of the quality of evidence. We included a total of 35 randomised controlled trials (RCTs) randomising 12,089 patients in this review. (Please refer to Figure 2). Trials included in this meta‐analysis generally had a low risk of selection and attrition bias. Unfortunately, differences in reporting of outcomes, such as survival times, and a lack of survival curves, meant that only extractable data could be included in the analyses. The duration of gefitinib treatment and duration of follow‐up may also have affected outcomes in these RCTs. Despite these limitations, the included RCTs were generally consistent with their findings.
For studies that compared gefitinib with first‐line chemotherapy, we judged the quality of evidence as moderate. One study enrolled elderly patients (over 70 years old) and thus we downgraded the quality of evidence as this may be at serious risk of indirectness (Crino 2008 INVITE). When comparing gefitinib with second‐line chemotherapy, we also judged the quality of evidence as moderate as one study was not statistically powered to detect differences in any endpoint and was thus at serious risk of imprecision (Cufer 2006 SIGN). When considering toxicity outcomes, generally the quality of data was high, except for fatigue, which we judged as moderate quality of evidence. We downgraded this outcome one level, as we judged one study as having a serious risk of indirectness for enrolling only patients over the age of 70 years old (Crino 2008 INVITE).
Agreements and disagreements with other studies or reviews
Two published meta‐analyses also examined the effect of gefitinib in NSCLC. The first by Ibrahim 2010 reported on seven studies that included chemotherapy‐naive patients (Crino 2008 INVITE; Giaccone 2004 INTACT I; Goss 2009 INSTEP; Herbst 2004 INTACT II; Kelly 2008 SWOG S0023; Mok 2009 IPASS; Takeda 2010 WJTOG0203), analysing a total of 2545 and 1939 patients in the gefitinib and control arms. The same seven studies also fulfilled the inclusion criteria for this review; however our review included a further 17 studies that analysed the use of gefitinib as second‐ or third‐line and maintenance therapy. The authors were not able to show any benefit in objective response rate, progression‐free survival or overall survival in this general population. In a small subset of patients with EGFR mutations, gefitinib was shown to significantly improve the overall response rate (odds ratio (OR) 2.81, 95% CI 1.71 to 4.62, P < 0.0001). This benefit was not associated with a progression‐free or overall survival advantage in that group. Only three of the included seven studies reported on quality of life, showing a measurable and statistically significant improvement as measured by FACT‐L.
The second meta‐analysis by Jiang 2011 compared gefitinib with docetaxel as second‐line therapy. Four studies were included, all of which were also included in this review (Cufer 2006 SIGN; Kim 2008 INTEREST; Lee 2010 ISTANA; Maruyama 2008 V‐15‐32). A total of 2247 patients received either gefitinib or docetaxel as second‐line therapy. Similar results were also found in this meta‐analysis. There was an improved overall response rate with gefitinib compared with docetaxel (risk ratio (RR) 1.58, 95% CI 1.02 to 2.45, P = 0.04) and quality of life as measured with the FACT‐L and TOI questionnaires (RR 1.55, 95% CI 1.27 to 1.88, P < 0.001; RR 1.86, 95% 1.43 to 2.42, P < 0.001, respectively). There was no benefit in overall survival or progression‐free survival.
Both of these systematic reviews reported results similar to those of this meta‐analysis.
Patients with tumours bearing EGFR mutations derive benefit from gefitinib treatment. It has been shown that in patients of Asian ethnicity with tumours with EGFR mutations, progression‐free survival and overall response rate were significantly improved by the use of gefitinib as first‐line therapy; however there was no effect on overall survival, perhaps because of cross‐over between study interventions.
An interaction between ethnicity, EGFR mutation status and other clinical features is likely to confound a straightforward analysis of factors predictive of a gefitinib response. Patients of Asian descent, who are non‐smokers or with adenocarcinoma histology are also more likely to have tumours harbouring EGFR mutations.
There is increasing evidence to justify the use of molecular markers in clinical practice and the EGFR mutation status appears to be a significant predictor of benefit in terms of progression‐free survival and response to gefitinib. Other markers of EGFR status such as EGFR protein expression and EGFR gene copy number appear to be related to EGFR mutations, but interpretation criteria still need to be established. Further research into optimal sampling, mutation testing methods and the precise spectrum of predictive EGFR mutations is required.
Authors' conclusions
Implications for practice.
In patients of Asian ethnicity or who are epidermal growth factor receptor (EGFR) mutation positive, first‐line gefitinib significantly improves progression‐free survival and overall response rate but not overall survival when compared with chemotherapy.
Side effects such as skin rash, diarrhoea and increased alanine aminotransferase (ALT) and aspartate transaminase (AST) are more common with gefitinib. Side effects such as nausea, vomiting, anorexia, fatigue, arthralgia, asthenia, neurotoxicity, neutropenia, leukopenia, thrombocytopaenia and anaemia are more common with chemotherapy.
In patients of Asian ethnicity, first‐line gefitinib plus chemotherapy improves progression‐free survival over either gefitinib or chemotherapy alone.
In the second‐line setting, gefitinib is more effective than placebo, with improvements in the one‐year survival rate, progression‐free survival and overall response rate. There was no improvement in overall survival.
One study demonstrated an improvement in overall survival, time to treatment failure and overall response rate when comparing second‐line gefitinib to placebo in patients of Asian ethnicity. However, the prevalence of EGFR mutations in cancers from Asian patients means that caution needs to be exercised in interpreting these results.
Second‐line gefitinib plus chemotherapy is probably more effective in improving progression‐free survival than gefitinib alone.
One second‐line study selected EGFR mutation positive patients and showed that chemotherapy is more effective in improving survival than gefitinib plus chemotherapy in patients who have failed first‐line gefitinib.
Maintenance treatment with gefitinib was shown to be more effective in improving overall survival and progression‐free survival than placebo in patients with EGFR mutation positive tumours. In unselected patients or those of Asian ethnicity, gefitinib improves progression‐free survival but not overall survival or overall response rate over placebo.
Increasing the dose of gefitinib from 250 mg/day to 500 mg/day results in significantly more adverse side effects, without any impact on response rate, survival or reported quality of life.
Quality of life is higher in patients who receive gefitinib than those who either receive placebo or chemotherapy.
Implications for research.
Extended follow‐up of existing trials and the inclusion of other randomised trials will provide additional evidence on the use of gefitinib in advanced non‐small cell lung cancer.
Gaining a clearer understanding of why most, but not all, patients with tumours bearing EGFR mutations respond to gefitinib, as well as identifying new predictive factors, and the mechanisms and the management of drug resistance, are high‐priority research issues.
Acknowledgements
We would like to thank the Cochrane Lung Cancer Review Group, including Fédérico Capuzzo, Jean‐Paul Sculier, Noelle O’Rourke, Fergus Macbeth, Virginie Westeel, Corynne Marchal, Tom Haswell and Marta Roqué for expert advice and extensive support with this review.
We would also like to thank Vivian Sun, Senior Clinical Research Co‐ordinator (50% Melanoma), Adult Oncology Research Centre, Auckland Regional Cancer and Blood Service for her translation of the Dai 2013 and Li 2010 studies that were published in Mandarin.
Esther Sim was supported by an Australian Lung Foundation/Lung Cancer Consultative Group Cochrane Review Scholarship in 2007. Ian Yang was supported by an NHMRC Career Development Fellowship, and project grants from NHMRC, Cancer Council Queensland, The Prince Charles Hospital Foundation and Queensland Smart State grants. Rayleen Bowman was supported by project grants from NHMRC, Dust Diseases Board, Cancer Australia, Cancer Council Queensland and The Prince Charles Hospital Foundation. Kwun Fong was supported by an NHMRC Practitioner Fellowship, and project grants from NHMRC, Cancer Australia, Dust Diseases Board, Cancer Council Queensland, The Prince Charles Hospital Foundation and Queensland Smart State grants. Richard Wood‐Baker was supported by project grants from NHMRC and Royal Hobart Hospital Research Foundation.
Appendices
Appendix 1. CENTRAL search strategy (Cochrane Library 2017, Issue 2)
#1 MeSH descriptor: [Lung Neoplasms] explode all trees 5740
#2 MeSH descriptor: [Carcinoma, Non‐Small‐Cell Lung] explode all trees 2918
#3 (lung or pulmon*) and (neoplas* or cancer or carcinoma* or tumor or tumour) 17027
#4 #1 or #2 or #3 17106
#5 gefitinib or zd 1839 or zd1839 or iressa 490
#6 #4 and #5 360
Appendix 2. MEDLINE search strategy (PubMed; 17 February 2017)
#11 Add Search #9 AND #10 3906 00:07:34
#10 Add Search (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals [mh] NOT humans [mh]) 3415417 00:06:59
#9 Add Search #5 AND #8 5006 00:02:59
#8 Add Search #6 OR #7 11742 00:02:47
#7 Add Search gefitinib[tw] OR ZD1839[tw] OR ZD 1839[tw] OR Iressa[tw] 5828 00:02:34
#6 Add Search "Receptor, Epidermal Growth Factor/antagonists and inhibitors"[Mesh] 7886 00:01:43
#5 Add Search #1 OR #2 OR #3 OR #4 208140 00:01:13
#4 Add Search NSCLC[tiab] 28799 00:00:30
#3 Add Search Non Small Cell[tiab] 45118 00:00:14
#2 Add Search "Carcinoma, Non‐Small‐Cell Lung"[MeSH] 39657 23:59:42
#1 Add Search Lung Neoplasms[MeSH] 196596 23:59:14
Appendix 3. Embase search strategy (Ovid; 1980 to 2017 Week 08)
1 exp lung cancer/ (275,340)
2 exp lung non small cell cancer/ (102,369)
3 non small cell.ti,ab. (66,846)
4 NSCLC.ti,ab. (49,669)
5 1 or 2 or 3 or 4 (286,738)
6 exp GEFITINIB/ (19,445)
7 gefitinib.mp. (19,987)
8 (ZD1839 or ZD 1839 or iressa).mp. (4,876)
9 6 or 7 or 8 (20,033)
10 5 and 9 (11,699)
11 random:.tw. or placebo:.mp. or double‐blind:.mp. (699,255)
12 10 and 11 (1,437)
Data and analyses
Comparison 1. Gefitinib versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HR Overall survival | 4 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 G(250) vs P = 1st line | 1 | Hazard Ratio (Random, 95% CI) | 0.84 [0.62, 1.14] | |
| 1.2 G(250) vs P = 2nd line | 1 | Hazard Ratio (Random, 95% CI) | 0.89 [0.79, 1.01] | |
| 1.3 G(500) vs P = Maintenance | 2 | Hazard Ratio (Random, 95% CI) | 1.14 [0.61, 2.14] | |
| 2 HR Progression‐free survival | 4 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 G(250) vs P = 1st line | 1 | Hazard Ratio (Random, 95% CI) | 0.82 [0.60, 1.12] | |
| 2.2 G(250) vs P = 2nd line | 1 | Hazard Ratio (Random, 95% CI) | 0.82 [0.75, 0.90] | |
| 2.3 G(500) vs P = Maintenance | 2 | Hazard Ratio (Random, 95% CI) | 0.70 [0.53, 0.91] | |
| 3 1‐year survival rate | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 G(250) vs P = 2nd line | 1 | 1439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.05, 1.57] |
| 3.2 G(500) vs P = Maintenance | 1 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.78, 1.04] |
| 4 Skin rash | 3 | 2060 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.92 [1.46, 43.03] |
| 4.1 G(250) vs P = 1st line | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 G(250) vs P = 2nd line | 1 | 1688 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.98 [1.20, 67.13] |
| 4.3 G(250) vs P = Maintenance | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.06 [0.25, 103.82] |
| 5 Pruritus | 2 | 1889 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.22, 17.82] |
| 5.1 G(250) vs P = 1st line | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 G(250) vs P = 2nd line | 1 | 1688 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.22, 17.82] |
| 6 Diarrhoea | 3 | 2060 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.15, 5.35] |
| 6.1 G(250) vs P = 1st line | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.21, 4.89] |
| 6.2 G(250) vs P = 2nd line | 1 | 1688 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [1.21, 7.91] |
| 6.3 G(250) vs P = Maintenance | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.13, 73.47] |
| 7 Constipation | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 15.93] |
| 7.1 G(250) vs P = 1st line | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 15.93] |
| 8 Nausea | 2 | 1889 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.03, 12.44] |
| 8.1 G(250) vs P = 1st line | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.06] |
| 8.2 G(250) vs P = 2nd line | 1 | 1688 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [0.49, 10.36] |
| 9 Vomiting | 2 | 1859 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.83, 12.38] |
| 9.1 G(250) vs P = 2nd line | 1 | 1688 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.73, 14.33] |
| 9.2 G(250) vs P = Maintenance | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.13, 73.47] |
| 10 Anorexia | 3 | 2060 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.64, 2.33] |
| 10.1 G(250) vs P = 1st line | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.25, 103.87] |
| 10.2 G(250) vs P = 2nd line | 1 | 1688 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.59, 2.37] |
| 10.3 G(250) vs P = Maintenance | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.16] |
| 11 Fatigue | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 G(250) vs P = 1st line | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.27, 2.10] |
| 11.2 G(250) vs P = Maintenance | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.05 [0.46, 35.47] |
| 12 Asthenia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 G(250) vs P = 2nd line | 1 | 1688 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.66, 2.17] |
| 13 Respiratory tract infection | 2 | 1889 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.07, 3.83] |
| 13.1 G(250) vs P = 1st line | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.06] |
| 13.2 G(250) vs P = 2nd line | 1 | 1688 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.54, 1.84] |
| 14 Dyspnoea | 3 | 2060 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.59, 1.63] |
| 14.1 G(250) vs P = 1st line | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.71, 4.81] |
| 14.2 G(250) vs P = 2nd line | 1 | 1688 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.49, 1.42] |
| 14.3 G(250) vs P = Maintenance | 1 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.20, 2.31] |
| 15 Anaemia | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.07 [0.37, 135.12] |
| 15.1 G(250) vs P = 1st line | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.07 [0.37, 135.12] |
| 16 Abdominal pain | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.48] |
| 16.1 G(250) vs P = 1st line | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.48] |
| 17 Increased ALT | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.11 [1.18, 70.32] |
| 17.1 G(250) vs P = Maintenance | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.11 [1.18, 70.32] |
| 18 Increased AST | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.08 [0.89, 56.34] |
| 18.1 G(250) vs P = Maintenance | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.08 [0.89, 56.34] |
| 19 Neutropenia | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.13, 73.47] |
| 19.1 G(250) vs P = Maintenance | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.13, 73.47] |
| 20 Anaemia | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.15] |
| 20.1 G(250) vs P = Maintenance | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.15] |
| 21 Thrombocytopaenia | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.13, 73.47] |
| 21.1 G(250) vs P = Maintenance | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.13, 73.47] |
| 22 Overall response rate | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 G(250) vs P = 1st line | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.06 [0.74, 49.43] |
| 22.2 G(250) vs P= 2nd line | 1 | 1439 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.42 [2.82, 14.64] |
| 22.3 G(250) vs P = Maintenance | 1 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.12 [1.32, 77.33] |
| 23 Disease control rate | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 G(250) vs P = 1st line | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.86, 2.16] |
| 23.2 G(250) vs P = 2nd line | 1 | 1439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.06, 1.44] |
| 23.3 G(250) vs P = Maintenance | 1 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.00, 1.46] |
1.5. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 5 Pruritus.
1.7. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 7 Constipation.
1.8. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 8 Nausea.
1.9. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 9 Vomiting.
1.10. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 10 Anorexia.
1.11. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 11 Fatigue.
1.12. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 12 Asthenia.
1.13. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 13 Respiratory tract infection.
1.14. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 14 Dyspnoea.
1.15. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 15 Anaemia.
1.16. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 16 Abdominal pain.
1.17. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 17 Increased ALT.
1.18. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 18 Increased AST.
1.19. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 19 Neutropenia.
1.20. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 20 Anaemia.
1.21. Analysis.
Comparison 1 Gefitinib versus placebo, Outcome 21 Thrombocytopaenia.
Comparison 2. Gefitinib versus placebo (Asian subgroup).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HR Overall survival | 2 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 G(250) vs P = 2nd line | 1 | Hazard Ratio (Fixed, 95% CI) | 0.66 [0.48, 0.91] | |
| 1.2 G(250) vs P = Maintenance | 1 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.68, 1.14] | |
| 2 HR Progression‐free survival | 2 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 G(250) vs P = 2nd line | 1 | Hazard Ratio (Fixed, 95% CI) | 0.69 [0.52, 0.91] | |
| 2.2 G(250) vs P = Maintenance | 1 | Hazard Ratio (Fixed, 95% CI) | 0.42 [0.33, 0.54] | |
| 3 1‐year survival rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 G(250) vs P = 2nd line | 1 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.20, 2.55] |
| 4 Overall response rate | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 G(250) vs P = 2nd line | 1 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 6.03 [1.46, 24.91] |
| 4.2 G(250) vs P = Maintenance | 1 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 35.00 [4.86, 252.15] |
Comparison 3. Gefitinib versus placebo (biomarker subgroup).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HR Overall survival | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 G(250) vs P = Maintenance | 1 | Hazard Ratio (Fixed, 95% CI) | 0.39 [0.15, 0.98] | |
| 2 HR Progression‐free survival | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 G(250) vs P = Maintenance | 1 | Hazard Ratio (Fixed, 95% CI) | 0.17 [0.07, 0.41] |
Comparison 4. Gefitinib versus chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HR Overall survival | 2 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 G vs vinorelbine = 1st line | 1 | Hazard Ratio (Fixed, 95% CI) | 0.98 [0.66, 1.46] | |
| 1.2 G vs docetaxel = 2nd line | 1 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.91, 1.15] | |
| 2 HR Progression‐free survival | 2 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 G vs vinorelbine = 1st line | 1 | Hazard Ratio (Fixed, 95% CI) | 1.19 [0.86, 1.65] | |
| 2.2 G vs docetaxel = 2nd line | 1 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.92, 1.17] | |
| 3 1‐year survival rate | 3 | 1741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.08] |
| 3.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.69, 1.52] |
| 3.2 G vs docetaxel = 1st line | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 1.90] |
| 3.3 G vs docetaxel = 2nd line | 1 | 1466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.09] |
| 4 Skin rash | 4 | 1858 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.08, 5.31] |
| 4.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.11 [0.25, 104.94] |
| 4.2 G vs docetaxel = 1st line | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.05, 5.19] |
| 4.3 G vs docetaxel = 2nd line | 2 | 1583 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [1.11, 7.13] |
| 5 Constipation | 3 | 1719 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.17, 0.97] |
| 5.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.20] |
| 5.2 G vs docetaxel = 1st line | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.78] |
| 5.3 G vs docetaxel = 2nd line | 1 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.17, 1.18] |
| 6 Fatigue | 2 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.03, 0.88] |
| 6.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.18] |
| 6.2 G vs docetaxel = 1st line | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.05, 5.19] |
| 7 Asthenia | 3 | 1773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.35, 0.75] |
| 7.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.79] |
| 7.2 G vs docetaxel = 2nd line | 2 | 1583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.36, 0.78] |
| 8 Neurotoxicity | 2 | 1529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.34] |
| 8.1 G vs docetaxel = 1st line | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.56] |
| 8.2 G vs docetaxel = 2nd line | 1 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.43] |
| 9 Neutropenia | 4 | 1857 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.02, 0.06] |
| 9.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.43] |
| 9.2 G vs docetaxel = 1st line | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.63] |
| 9.3 G vs docetaxel = 2nd line | 2 | 1582 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.02, 0.06] |
| 10 Leukopenia | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.22] |
| 10.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.18] |
| 10.2 G vs docetaxel = 2nd line | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.32] |
| 11 Febrile neutropenia | 3 | 1768 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.06, 0.23] |
| 11.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.18] |
| 11.2 G vs docetaxel = 2nd line | 2 | 1578 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.06, 0.24] |
| 12 Pruritus | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.22 [0.26, 106.74] |
| 12.1 G vs docetaxel = 2nd line | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.22 [0.26, 106.74] |
| 13 Diarrhoea | 4 | 1858 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.48, 1.34] |
| 13.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.26, 3.96] |
| 13.2 G vs docetaxel = 1st line | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.62] |
| 13.3 G vs docetaxel = 2nd line | 2 | 1583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.43, 1.35] |
| 14 Vomiting | 2 | 1583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.19, 1.63] |
| 14.1 G vs docetaxel = 2nd line | 2 | 1583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.19, 1.63] |
| 15 Anorexia | 3 | 1719 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.61, 3.32] |
| 15.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.15, 7.10] |
| 15.2 G vs docetaxel = 1st line | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 G vs docetaxel = 2nd line | 1 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.60, 3.95] |
| 16 Stomatitis | 1 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.71] |
| 16.1 G vs docetaxel = 2nd line | 1 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.71] |
| 17 Arthralgia/myalgia | 2 | 1529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.19] |
| 17.1 G vs docetaxel = 1st line | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 G vs docetaxel = 2nd line | 1 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.19] |
| 18 Peripheral oedema | 2 | 1634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.61] |
| 18.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 G vs docetaxel = 2nd line | 1 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.61] |
| 19 Respiratory tract infection | 1 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.52, 1.57] |
| 19.1 G vs docetaxel = 2nd line | 1 | 1444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.52, 1.57] |
| 20 Dyspnoea | 3 | 1773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.16] |
| 20.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.24] |
| 20.2 G vs docetaxel = 2nd line | 2 | 1583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.59, 1.22] |
| 21 Cough | 2 | 1583 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.36, 3.84] |
| 21.1 G vs docetaxel = 2nd line | 2 | 1583 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.36, 3.84] |
| 22 Anaemia | 4 | 1853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.36, 1.36] |
| 22.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.25] |
| 22.2 G vs docetaxel = 1st line | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.62] |
| 22.3 G vs docetaxel = 2nd line | 2 | 1578 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.42, 1.75] |
| 23 Thrombocytopenia | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.35] |
| 23.1 G vs docetaxel = 1st line | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 G vs docetaxel = 2nd line | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.35] |
| 24 Hypokalaemia | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.06, 16.09] |
| 24.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.06, 16.09] |
| 25 Pyrexia | 3 | 1773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.14, 2.47] |
| 25.1 G vs vinorelbine = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.06, 16.09] |
| 25.2 G vs docetaxel = 2nd line | 2 | 1583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.67] |
| 26 Overall response rate | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 G vs docetaxel = 2nd line | 2 | 1607 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.85, 1.59] |
| 27 Disease control rate | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 G vs docetaxel = 1st line | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.10] |
| 27.2 G vs docetaxel = 2nd line | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.40] |
| 28 FACT‐L QOL improvement rate | 2 | 1656 | Mean Difference (IV, Fixed, 95% CI) | 10.50 [9.55, 11.45] |
| 28.1 G vs vinorelbine = 1st line | 1 | 190 | Mean Difference (IV, Fixed, 95% CI) | 13.4 [8.25, 18.55] |
| 28.2 G vs docetaxel = 2nd line | 1 | 1466 | Mean Difference (IV, Fixed, 95% CI) | 10.40 [9.43, 11.37] |
| 29 LCS QOL improvement rate | 2 | 1656 | Mean Difference (IV, Fixed, 95% CI) | 3.63 [3.08, 4.19] |
| 29.1 G vs vinorelbine = 1st line | 1 | 190 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [2.42, 5.18] |
| 29.2 G vs docetaxel = 2nd line | 1 | 1466 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [2.99, 4.21] |
| 30 TOI QOL improvement rate | 2 | 1656 | Mean Difference (IV, Random, 95% CI) | 9.87 [1.26, 18.48] |
| 30.1 G vs vinorelbine = 1st line | 1 | 190 | Mean Difference (IV, Random, 95% CI) | 16.60 [4.61, 28.59] |
| 30.2 G vs docetaxel = 2nd line | 1 | 1466 | Mean Difference (IV, Random, 95% CI) | 7.0 [5.97, 8.03] |
| 31 PSI QOL improvement rate | 1 | 190 | Mean Difference (IV, Fixed, 95% CI) | 5.60 [3.55, 7.65] |
| 31.1 G vs vinorelbine = 1st line | 1 | 190 | Mean Difference (IV, Fixed, 95% CI) | 5.60 [3.55, 7.65] |
4.12. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 12 Pruritus.
4.13. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 13 Diarrhoea.
4.14. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 14 Vomiting.
4.15. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 15 Anorexia.
4.16. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 16 Stomatitis.
4.17. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 17 Arthralgia/myalgia.
4.18. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 18 Peripheral oedema.
4.19. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 19 Respiratory tract infection.
4.20. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 20 Dyspnoea.
4.21. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 21 Cough.
4.22. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 22 Anaemia.
4.23. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 23 Thrombocytopenia.
4.24. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 24 Hypokalaemia.
4.25. Analysis.
Comparison 4 Gefitinib versus chemotherapy, Outcome 25 Pyrexia.
Comparison 5. Gefitinib versus chemotherapy (Asian subgroup).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HR Overall survival = 1st line | 4 | Hazard Ratio (Random, 95% CI) | 0.94 [0.82, 1.06] | |
| 1.1 G vs carboplatin + paclitaxel | 2 | Hazard Ratio (Random, 95% CI) | 1.09 [0.64, 1.84] | |
| 1.2 G vs gemcitabine + cisplatin | 1 | Hazard Ratio (Random, 95% CI) | 0.93 [0.72, 1.21] | |
| 1.3 G vs pemetrexed + cisplatin | 1 | Hazard Ratio (Random, 95% CI) | 0.94 [0.68, 1.30] | |
| 2 HR Overall survival = 2nd line | 3 | Hazard Ratio (Random, 95% CI) | 0.94 [0.79, 1.12] | |
| 2.1 G vs docetaxel | 2 | Hazard Ratio (Random, 95% CI) | 0.97 [0.80, 1.17] | |
| 2.2 G vs pemetrexed | 1 | Hazard Ratio (Random, 95% CI) | 0.80 [0.50, 1.28] | |
| 3 HR Overall survival = Maintenance | 1 | Hazard Ratio (Random, 95% CI) | 2.15 [0.83, 5.55] | |
| 3.1 G vs pemetrexed | 1 | Hazard Ratio (Random, 95% CI) | 2.15 [0.83, 5.55] | |
| 4 HR Progression‐free survival = 1st line | 5 | Hazard Ratio (Random, 95% CI) | 0.65 [0.43, 0.98] | |
| 4.1 G vs carboplatin + paclitaxel | 2 | Hazard Ratio (Random, 95% CI) | 0.48 [0.20, 1.15] | |
| 4.2 G vs cisplatin + docetaxel | 1 | Hazard Ratio (Random, 95% CI) | 0.49 [0.34, 0.71] | |
| 4.3 G vs gemcitabine + cisplatin | 1 | Hazard Ratio (Random, 95% CI) | 1.20 [0.95, 1.52] | |
| 4.4 G vs pemetrexed + cisplatin | 1 | Hazard Ratio (Random, 95% CI) | 0.85 [0.64, 1.14] | |
| 5 HR Progression‐free survival = 2nd line | 3 | Hazard Ratio (Random, 95% CI) | 0.71 [0.57, 0.88] | |
| 5.1 G vs docetaxel | 2 | Hazard Ratio (Random, 95% CI) | 0.78 [0.65, 0.94] | |
| 5.2 G vs pemetrexed | 1 | Hazard Ratio (Random, 95% CI) | 0.54 [0.37, 0.79] | |
| 6 HR Progression‐free survival = Maintenance | 1 | Hazard Ratio (Random, 95% CI) | 0.53 [0.27, 1.04] | |
| 6.1 G vs pemetrexed | 1 | Hazard Ratio (Random, 95% CI) | 0.53 [0.27, 1.04] | |
| 7 1‐year survival rate | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 1st line | 3 | 1754 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.97, 1.09] |
| 7.2 2nd line | 3 | 681 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.11] |
| 7.3 Maintenance | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.65, 0.98] |
| 8 Nausea | 10 | 2898 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.64] |
| 8.1 1st line | 4 | 1912 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.06, 0.54] |
| 8.2 2nd line | 5 | 916 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.22, 1.60] |
| 8.3 Maintenance | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.09, 2.98] |
| 9 Vomiting | 6 | 2447 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.05, 0.77] |
| 9.1 1st line | 3 | 1737 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.02, 0.29] |
| 9.2 2nd line | 2 | 640 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.30, 5.77] |
| 9.3 Maintenance | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.69] |
| 10 Anorexia | 10 | 2950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.27, 0.49] |
| 10.1 1st line | 4 | 1964 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.23, 0.45] |
| 10.2 2nd line | 5 | 916 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.27, 1.02] |
| 10.3 Maintenance | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.05, 12.20] |
| 11 Fatigue | 10 | 1960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.22, 0.46] |
| 11.1 1st line | 4 | 943 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.17, 0.40] |
| 11.2 2nd line | 4 | 759 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.06, 1.03] |
| 11.3 Maintenance | 2 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.41, 2.89] |
| 12 Arthralgia/myalgia | 4 | 2063 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.61] |
| 12.1 1st line | 2 | 1423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.61] |
| 12.2 2nd line | 2 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Asthenia | 4 | 1755 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.08, 0.58] |
| 13.1 1st line | 3 | 1598 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.61] |
| 13.2 2nd line | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 2.94] |
| 14 Neurotoxicity | 4 | 1797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.24] |
| 14.1 1st line | 2 | 1505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.24] |
| 14.2 2nd line | 2 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Neutropenia | 10 | 3061 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.05, 0.27] |
| 15.1 1st line | 5 | 2139 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.03, 0.07] |
| 15.2 2nd line | 3 | 664 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.08, 0.18] |
| 15.3 Maintenance | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.49, 2.96] |
| 16 Anaemia | 9 | 2538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.12, 0.29] |
| 16.1 1st line | 5 | 2139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.10, 0.26] |
| 16.2 2nd line | 2 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.02, 1.61] |
| 16.3 Maintenance | 2 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.24, 7.87] |
| 17 Leukopenia | 4 | 2086 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.02, 0.23] |
| 17.1 1st line | 3 | 1603 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.02, 0.08] |
| 17.2 2nd line | 1 | 483 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.09, 0.26] |
| 18 Thrombocytopenia | 7 | 1070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.14, 0.72] |
| 18.1 1st line | 2 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.04, 0.51] |
| 18.2 2nd line | 3 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.15] |
| 18.3 Maintenance | 2 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.63 [0.42, 31.44] |
| 19 Febrile neutropenia | 2 | 1679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.28] |
| 19.1 1st line | 1 | 1196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.43] |
| 19.2 2nd line | 1 | 483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.49] |
| 20 Skin rash | 10 | 3174 | Risk Ratio (M‐H, Random, 95% CI) | 3.11 [1.28, 7.55] |
| 20.1 1st line | 5 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 5.09 [2.21, 11.72] |
| 20.2 2nd line | 3 | 775 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [0.46, 13.95] |
| 20.3 Maintenance | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.24, 3.44] |
| 21 Diarrhoea | 10 | 3055 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.79 [1.57, 4.94] |
| 21.1 1st line | 5 | 2139 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [1.43, 5.27] |
| 21.2 2nd line | 5 | 916 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.88, 9.73] |
| 22 Increased ALT | 7 | 1542 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.03 [5.23, 19.26] |
| 22.1 1st line | 4 | 943 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.66 [5.13, 26.49] |
| 22.2 2nd line | 2 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.22 [3.18, 54.99] |
| 22.3 Maintenance | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.01, 6.33] |
| 23 Increased AST | 4 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.73 [2.78, 21.46] |
| 23.1 1st line | 3 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.73 [2.78, 21.46] |
| 23.2 2nd line | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Overall response rate | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.1 1st line | 6 | 2158 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.13, 1.82] |
| 24.2 2nd line | 6 | 921 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.92, 2.22] |
| 24.3 Maintenance | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.41, 1.87] |
| 25 Stable disease | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 25.1 1st line | 5 | 941 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.34, 0.64] |
| 25.2 2nd line | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.64, 1.82] |
| 25.3 Maintenance | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.44, 0.93] |
| 26 Disease control rate | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 26.1 1st line | 5 | 1848 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.13] |
| 26.2 2nd line | 3 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.78, 1.25] |
| 26.3 Maintenance | 1 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.49, 0.85] |
| 27 FACT‐L QOL improvement rate | 3 | 1670 | Mean Difference (IV, Fixed, 95% CI) | 9.50 [7.95, 11.05] |
| 27.1 1st line | 1 | 1151 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 2nd line | 2 | 519 | Mean Difference (IV, Fixed, 95% CI) | 9.50 [7.95, 11.05] |
| 28 LCS QOL improvement rate | 3 | 1748 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [1.53, 3.07] |
| 28.1 1st line | 1 | 1151 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 2nd line | 2 | 597 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [1.53, 3.07] |
| 29 TOI QOL improvement rate | 3 | 1670 | Mean Difference (IV, Fixed, 95% CI) | 11.8 [9.17, 14.43] |
| 29.1 1st line | 1 | 1151 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 2nd line | 2 | 519 | Mean Difference (IV, Fixed, 95% CI) | 11.8 [9.17, 14.43] |
5.27. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 27 FACT‐L QOL improvement rate.
5.28. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 28 LCS QOL improvement rate.
5.29. Analysis.
Comparison 5 Gefitinib versus chemotherapy (Asian subgroup), Outcome 29 TOI QOL improvement rate.
Comparison 6. Gefitinib versus chemotherapy (EGFR mutation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HR Overall survival = 1st line | 5 | Hazard Ratio (Fixed, 95% CI) | 0.97 [0.77, 1.21] | |
| 1.1 Biomarker driven selection | 2 | Hazard Ratio (Fixed, 95% CI) | 0.98 [0.72, 1.33] | |
| 1.2 Clinical feature driven selection | 3 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.68, 1.33] | |
| 2 HR Overall survival = 2nd line | 1 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.41, 1.66] | |
| 2.1 G vs docetaxel | 1 | Hazard Ratio (Fixed, 95% CI) | 0.83 [0.41, 1.66] | |
| 3 HR Progression‐free survival = 1st line | 5 | Hazard Ratio (Random, 95% CI) | 0.47 [0.36, 0.61] | |
| 3.1 Biomarker driven selection | 2 | Hazard Ratio (Random, 95% CI) | 0.39 [0.26, 0.59] | |
| 3.2 Clinical feature driven selection | 3 | Hazard Ratio (Random, 95% CI) | 0.53 [0.41, 0.70] | |
| 4 HR Progression‐free survival = 2nd line | 2 | Hazard Ratio (Fixed, 95% CI) | 0.24 [0.12, 0.47] | |
| 4.1 G vs docetaxel | 1 | Hazard Ratio (Fixed, 95% CI) | 0.16 [0.05, 0.50] | |
| 4.2 G vs pemetrexed | 1 | Hazard Ratio (Fixed, 95% CI) | 0.30 [0.13, 0.70] | |
| 5 Overall response rate | 7 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.34, 2.19] |
| 5.1 First‐line biomarker driven selection | 2 | 347 | Risk Ratio (M‐H, Random, 95% CI) | 2.23 [1.75, 2.85] |
| 5.2 First‐line, clinical feature driven selection | 3 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.05, 1.99] |
| 5.3 2nd line | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.88, 3.09] |
| 6 Stable disease | 3 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.28, 0.97] |
| 6.1 First‐line, biomarker driven selection | 2 | 347 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.22, 0.98] |
| 6.2 First‐line, clinical feature driven selection | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.26, 2.85] |
| 7 Disease control rate | 5 | 2001 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.93, 1.19] |
| 7.1 First‐line, biomarker driven selection | 2 | 347 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.05, 1.26] |
| 7.2 First‐line, clinical feature driven selection | 2 | 1267 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 7.3 Second‐line | 1 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.77, 1.36] |
Comparison 7. Gefitinib 250 mg versus gefitinib 500 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 1‐year survival rate | 2 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.61, 1.11] |
| 1.1 2nd line | 2 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.61, 1.11] |
| 2 Skin rash | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.13 [1.51, 43.72] |
| 2.1 2nd line | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.80 [0.85, 54.32] |
| 2.2 Maintenance | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.41 [0.61, 176.21] |
| 3 Acne | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.86 [0.24, 100.02] |
| 3.1 2nd line | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.86 [0.24, 100.02] |
| 4 Pruritus | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.12, 70.77] |
| 4.1 2nd line | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.12, 70.77] |
| 5 Diarrhoea | 3 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.36 [1.58, 44.34] |
| 5.1 2nd line | 3 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.36 [1.58, 44.34] |
| 6 Nausea | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 15.33] |
| 6.1 2nd line | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 15.33] |
| 7 Vomiting | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 2nd line | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Anorexia | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.12, 70.77] |
| 8.1 2nd line | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.12, 70.77] |
| 9 Asthenia | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.12, 70.77] |
| 9.1 2nd line | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.12, 70.77] |
| 10 Overall response rate | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 2nd line | 2 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.58, 1.46] |
| 10.2 Maintenance | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.35, 2.88] |
| 11 Partial response | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.34, 1.65] |
| 11.1 2nd line | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.34, 1.65] |
| 12 FACT‐L Symptom improvement rate | 2 | 356 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐7.28, 14.69] |
| 12.1 2nd line | 2 | 356 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐7.28, 14.69] |
| 13 TOI QOL improvement rate | 2 | 424 | Mean Difference (IV, Fixed, 95% CI) | 7.38 [‐2.30, 17.05] |
| 13.1 2nd line | 2 | 424 | Mean Difference (IV, Fixed, 95% CI) | 7.38 [‐2.30, 17.05] |
Comparison 8. Gefitinib versus gefitinib + chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HR Progression‐free survival | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 1st line | 1 | Hazard Ratio (Random, 95% CI) | 0.69 [0.49, 0.96] | |
| 1.2 2nd line | 1 | Hazard Ratio (Random, 95% CI) | 0.65 [0.43, 0.97] | |
| 2 1‐year survival rate | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.92, 1.43] |
| 2.1 2nd line | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.92, 1.43] |
| 3 1‐year progression‐free survival | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [1.38, 3.80] |
| 3.1 2nd line | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [1.38, 3.80] |
| 4 Skin rash | 3 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.39, 4.57] |
| 4.1 1st line | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.23, 4.51] |
| 4.2 2nd line | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.25, 26.47] |
| 5 Diarrhoea | 3 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.21, 6.34] |
| 5.1 1st line | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.21, 6.34] |
| 5.2 2nd line | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Constipation | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.02, 9.92] |
| 6.1 2nd line | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.02, 9.92] |
| 7 Fatigue | 3 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [0.60, 11.90] |
| 7.1 1st line | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [0.60, 11.90] |
| 7.2 2nd line | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Leukopenia | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.48, 4.70] |
| 8.1 1st line | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.36, 4.35] |
| 8.2 2nd line | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.82 [0.16, 89.24] |
| 9 Anaemia | 3 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.22 [0.66, 15.72] |
| 9.1 1st line | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.49, 19.15] |
| 9.2 2nd line | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.82 [0.16, 89.24] |
| 10 Thrombocytopenia | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 1st line | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 2nd line | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Neutropenia | 3 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.71, 3.02] |
| 11.1 1st line | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.65, 2.88] |
| 11.2 2nd line | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.82 [0.16, 89.24] |
| 12 Increased ALT | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [1.09, 6.04] |
| 12.1 1st line | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [1.09, 6.04] |
| 13 Increased AST | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.56, 3.88] |
| 13.1 1st line | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.56, 3.88] |
| 14 Vomiting | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.06, 37.74] |
| 14.1 1st line | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.06, 37.74] |
| 15 Nausea | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.06, 37.74] |
| 15.1 1st line | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.06, 37.74] |
| 16 Overall response rate | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.17] |
| 16.1 1st line | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.17] |
| 17 Partial response | 4 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88, 1.16] |
| 17.1 1st line | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88, 1.16] |
| 17.2 2nd line | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.71, 1.47] |
| 18 Stable disease | 4 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.69, 1.37] |
| 18.1 1st line | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.39, 1.16] |
| 18.2 2nd line | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.84, 2.03] |
Comparison 9. Gefitinib + chemotherapy versus chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HR Overall survival | 3 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 1st line [Asian] | 2 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.72, 1.02] | |
| 1.2 2nd line [EGFRm] | 1 | Hazard Ratio (Fixed, 95% CI) | 1.62 [1.05, 2.50] | |
| 2 HR Progression‐free survival | 3 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 1st line [Asian] | 2 | Hazard Ratio (Fixed, 95% CI) | 0.69 [0.62, 0.77] | |
| 2.2 2nd line [EGFRm] | 1 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.65, 1.13] | |
| 3 1‐year survival rate | 2 | 1411 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.08] |
| 3.1 1st line | 2 | 1411 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.08] |
| 4 Skin rash | 5 | 2379 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [1.54, 5.77] |
| 4.1 1st line | 2 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.23, 5.63] |
| 4.2 1st line [Asian] | 2 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.23 [1.08, 16.54] |
| 4.3 2nd line [EGFRm] | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Acne | 3 | 1664 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.95 [1.09, 22.51] |
| 5.1 1st line | 2 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.59 [0.99, 31.60] |
| 5.2 2nd line [EGFRm] | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.98] |
| 6 Diarrhoea | 5 | 2379 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [1.17, 3.58] |
| 6.1 1st line | 2 | 1400 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.17, 5.09] |
| 6.2 1st line [Asian] | 2 | 715 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.32, 2.92] |
| 6.3 2nd line [EGFRm] | 1 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.32, 28.47] |
| 7 Pruritus | 2 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.18, 21.89] |
| 7.1 1st line | 2 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.18, 21.89] |
| 8 Vomiting | 5 | 2379 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.81, 1.89] |
| 8.1 1st line | 2 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.53, 2.06] |
| 8.2 1st line [Asian] | 2 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.70, 2.32] |
| 8.3 2nd line [EGFRm] | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.51, 7.83] |
| 9 Nausea | 5 | 2379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.58, 1.17] |
| 9.1 1st line | 2 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.51, 2.18] |
| 9.2 1st line [Asian] | 2 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.14] |
| 9.3 2nd line [EGFRm] | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.26, 2.66] |
| 10 Anorexia | 5 | 2379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.20] |
| 10.1 1st line | 2 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.36, 10.76] |
| 10.2 1st line [Asian] | 2 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.53, 1.20] |
| 10.3 2nd line [EGFRm] | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.16] |
| 11 Asthenia | 3 | 1664 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.21, 2.99] |
| 11.1 1st line | 2 | 1400 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.10, 7.76] |
| 11.2 2nd line [EGFRm] | 1 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.09, 2.68] |
| 12 Dyspnoea | 2 | 947 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.25, 3.96] |
| 12.1 1st line | 1 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.18, 21.89] |
| 12.2 2nd line [EGFRm] | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.93] |
| 13 Anaemia | 3 | 979 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.53, 1.03] |
| 13.1 1st line [Asian] | 2 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.90] |
| 13.2 2nd line [EGFRm] | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.2 [0.79, 6.16] |
| 14 Neutropenia | 5 | 2379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.87, 1.08] |
| 14.1 1st line | 2 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.77, 1.80] |
| 14.2 1st line [Asian] | 2 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| 14.3 2nd line [EGFRm] | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.49, 3.35] |
| 15 Leukopenia | 4 | 2262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.89, 1.31] |
| 15.1 1st line | 2 | 1400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.61, 2.26] |
| 15.2 1st line [Asian] | 1 | 598 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.30] |
| 15.3 2nd line [EGFRm] | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.86] |
| 16 Overall response rate | 5 | 2314 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.97, 1.20] |
| 16.1 1st line | 2 | 1343 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.94, 1.22] |
| 16.2 1st line [Asian] | 2 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.93, 1.40] |
| 16.3 2nd line [EGFRm] | 1 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.31] |
9.7. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 7 Pruritus.
9.8. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 8 Vomiting.
9.9. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 9 Nausea.
9.10. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 10 Anorexia.
9.11. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 11 Asthenia.
9.12. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 12 Dyspnoea.
9.13. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 13 Anaemia.
9.14. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 14 Neutropenia.
9.15. Analysis.
Comparison 9 Gefitinib + chemotherapy versus chemotherapy, Outcome 15 Leukopenia.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahn 2012.
| Methods | Design: parallel‐group Randomisation: yes, method stated Blinding: open‐label Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 86 Number enrolled: 73 Number in treatment group: 39 Number in control group: 31 Number of withdrawals (treatment/control): TP1 14/31; TPII 25/24 Number completing trial (treatment/control): 0/0 Age range: (treatment/control) 35 to 73 years/29 to 76 years Sex: 15 M, 55 F Ethnicity: East Asian NSCLC diagnosis: histologic/cytologic diagnosis of NSCLC, stage IIIB to IV disease Inclusion criteria: stage IIIB to IV NSCLC with at least one measurable lesion, ECOG PS 0 or 1, EGFR mutation status unknown Exclusion criteria: received treatment for NSCLC other than palliative radiotherapy, smoker of more than 100 cigarettes in lifetime, life expectancy of < 12 weeks Baseline characteristics of treatment/control groups: comparable |
|
| Interventions |
TP1 All patients received first‐line chemotherapy: Pemetrexed 500 mg/m2 + cisplatin 75 mg/m2 Intravenously on day 1 of 3‐week cycle for 4 cycles TPII Received either: Gefitinib 250 mg/day OR Pemetrexed 500 mg/m2 with optional cisplatin 75 mg/m2 in first 2 cycles intravenously |
|
| Outcomes | Progression‐free survival Overall survival Tumour response – RECIST Duration of response ASEs – NCI‐CTC Haematology and biochemical parameters |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Stratified random assignment method, random allocation sequence generated by central computerised voice response unit" Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "random allocation sequence generated by central computerised voice response unit" Comment: this was judged as adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions and reasons for withdrawals presented in Figure 1. Missing outcome data balanced in numbers across interventional groups with similar reasons for missing data across groups. Data analysed using intention‐to‐treat analysis Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | Funded by Eli Lilly and Company. Authors have received honoraria from Eli Lilly and some authors are current employees or previous employees of Eli Lilly. Comment: this was judged as an unclear risk of bias |
An 2016.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: not stated |
|
| Participants | Setting: single‐centre study, hospital outpatient department Number eligible: 90 Number enrolled: 90 Number in treatment group: 45 Number in control group: 45 Number of withdrawals (treatment/control): not stated Number completing trial (treatment/control): not stated Age range: 57 to 83 years Sex: 50 M, 40 F Ethnicity: East Asian NSCLC diagnosis: histologic/cytologic diagnosis of non‐squamous NSCLC, stage IIIB to IV disease. Presence of EGFR sensitive mutation Inclusion criteria: at least one measurable lesion, an estimated life expectancy of at least 12 weeks, adequate major organ function Exclusion criteria: any of the following: myocardial infarction within the previous 3 months, uncontrolled angina pectoris or arrhythmia, brain metastasis, uncontrolled hypertension or diabetes, active infection, pulmonary fibrosis, pleural effusion or ascites requiring drainage, or cerebrovascular disease Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Pemetrexed 500 mg/m2 on day 1 PLUS gefitinib 250 mg on day 2 to 16 Cycles repeated every 3 weeks until disease progression Gefitinib 250 mg on day 2 to 16 PLUS placebo on day 1 Cycles repeated every 3 weeks until disease progression |
|
| Outcomes | Tumour response – RECIST ASEs – NCI‐CTC Progression‐free survival Overall survival |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly divided.." but no further information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All investigators and patients were masked to treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | There were no declarations of potential conflicts of interest or indication of funding or support Comment: there was insufficient information to permit a clear judgement of the risk of bias |
Chen 2007.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: nil Withdrawals: stated | |
| Participants | Setting: single‐centre study, hospital outpatient department Number eligible: 48 Number enrolled: 48 Number in treatment group: 21 Number in control group: 27 Number of withdrawals (treatment/control): 6/0 Number completing trial (treatment/control): 15/27 Age range: treatment 39 to 80; control 35 to 85 Sex: 25 M, 23 F Ethnicity: Ethnic Chinese NSCLC diagnosis: histologic or cytologic diagnosis of stage IV adenocarcinoma Inclusion criteria: failed previous chemotherapy with ?2 regimens (including taxanes and platinum‐based chemotherapy); clinically measurable disease; no previous radiotherapy directed at lesions; adequate bone marrow reserve with WBC count < 4000/mm3; platelets < 100,000/mm3; haemoglobin < 10 g/dL; life expectancy of > 2 months Exclusion criteria: inadequate liver function (total bilirubin > 1.5 times upper limit of normal and alanine and aspartate aminotransferase levels > 3 times upper limit of normal) or inadequate renal function with creatinine levels > 2 mg/dL Baseline characteristics of treatment/control groups: no difference between groups |
|
| Interventions | 250 mg gefitinib daily Vinorelbine (15 mg/m2) on day 1, 250 mg gefitinib daily on days 2 to 14 every 2 weeks | |
| Outcomes | Overall survival Time to progression, 1‐year progression‐free survival Tumour response ‐ RECIST ASEs ‐ NCI‐CTC EGFR FISH examination LCS of FACT‐L | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomized..." but no further information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information provided Comment: this was judged as a high risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | There were no declarations of potential conflicts of interest or indication of funding or support Comment: there was insufficient information to permit a clear judgement of the risk of bias |
Chen 2011.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: open‐label Withdrawals: stated Jadad score: 2 |
|
| Participants | Setting: single‐centre study, hospital outpatient department Number eligible: 115 Number enrolled: 115 Number in treatment group: 58 Number in control group: 57 Number of withdrawals (treatment/control): 0/0 Number completing trial (treatment/control): 58/57 Age range (treatment/control): 37 to 87 years/30 to 85 years Sex: 69 M, 45 F Ethnicity: Taiwanese NSCLC diagnosis: histological and cytological diagnosis of adenocarcinoma of the lung Inclusion criteria: stage IIIB or IV adenocarcinoma of the lung, age 18 years or older, failed previous chemotherapy, WHO PS of 0 to 3, clinically measurable disease, no previous radiotherapy directed at the measurable lesion(s), adequate bone marrow reserve with a white blood count > 4000/mm3 Exclusion criteria: previous treatment with 5FU‐related chemotherapeutic agent, interstitial lung disease, with inadequate liver function (total bilirubin > 1.5 times and alanine aminotransferase/aspartate transaminase > 3 times the upper limit normal) or inadequate renal function with creatinine > 2.0 mg/dl Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg/day Gefitinib (250 mg/day) + UFT (tegafur 100 mg + uracil 224 mg) |
|
| Outcomes | Progression‐free survival Overall survival Tumour response – RECIST ASEs – NCI‐CTC Haematology and biochemical parameters Quality of life (LCS) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no details provided Comment: there was insufficient detail reported about the method used to generate the allocation sequence to allow a clear assessment of whether it would produce comparable groups |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information provided Comment: this was judged as a high risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Low risk | Grants from National Science Council of the Republic of China and Taipei Veterans General Hospital |
Cheng 2016.
| Methods | Design: parallel‐group Randomisation: yes, method stated Blinding: open‐label Withdrawals: not stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 232 Number enrolled: 195 Number in treatment group: 129 Number in control group: 66 Number of withdrawals (treatment/control): 106/59 Number completing trial (treatment/control): 23/7 Age range: (treatment/control) 33 to 84 years/41 to 80 years Sex: 68 M, 123 F Ethnicity: East Asian NSCLC diagnosis: histologic/cytologic diagnosis of non‐squamous NSCLC, stage IV or recurrent disease. Presence of activating EGFR mutation Inclusion criteria: age ≥ 18 years, ECOG 0 or 1, measurable disease documented by CT or MRI as defined by RECIST criteria Exclusion criteria: prior systemic chemotherapy, immunotherapy or biologic therapy, including targeted therapy (e.g. EGFR‐TKI) for stage IV or recurrent NSCLC. Receipt of adjuvant or neoadjuvant treatment with pemetrexed or an EGFR‐TKI, thoracic radiation therapy within 28 days before enrolment or could not take folic acid, vitamin B12 and dexamethasone Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Pemetrexed 500 mg/m2 on day 1 PLUS gefitinib 250 mg daily Cycles repeated every 3 weeks Gefitinib 250 mg daily |
|
| Outcomes | Progression‐free survival Overall survival Time to progressive disease (TtPD) Tumour response – RECIST Duration of response ASEs – NCI‐CTC Quality of life Biomarker analysis |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "random assignment was conducted using a computer‐generated random sequence and an interactive voice‐response system." Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions and reasons for withdrawals presented in Figure 1. Missing outcomes balanced in numbers across intervention groups with similar reasons for missing data across groups. Data analysed using intention‐to‐treat analysis Comment: this was judged as low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported, except for overall survival Quote: "At time of PFS analysis, OS data were immature, and therefore, are not presented" Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | Authors report consultative roles within industry, and other potential financial conflicts of interest Comment: this was judged as an unclear risk of bias |
Crino 2008 INVITE.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: open‐label Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 205 Number enrolled: 196 Number in treatment group: 97 Number in control group: 99 Number of withdrawals (treatment/control): 20/38 Number completing trial (treatment/control): 77/61 Age range: treatment 70 to 89, control 70 to 86 Sex: M 148, F 48 Ethnicity: white 162, Asian 31, other 3 NSCLC diagnosis: histologically confirmed stage IIIB or stage IV NSCLC Inclusion criteria: age > 70 years, at least 1 measurable lesion according to RECIST criteria, histological biopsy and paraffin block from the original tumour or metastatic site, no prior chemotherapy, biologic or immunologic therapy, WHO performance status of 0 to 2, life expectancy of at least 12 weeks Exclusion criteria: newly diagnosed central nervous system metastases that had not yet been treated, any evidence of clinically active interstitial lung disease, other coexisting malignancies or malignancies discovered within the last 5 years other than basal cell carcinoma or cervical cancer in situ, prior treatment with EGFR inhibitors, treatment with an investigational drug within 30 days Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg/day Vinorelbine 30 mg/m2 infusion on days 1 and 8 of a 21‐day cycle |
|
| Outcomes | Overall survival Progression‐free survival (PFS) Tumour response ‐ RECIST ASEs ‐ NCI‐CTC Quality of life ‐ LCS/FACT‐L |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned in 1:1 manner" Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions and reasons for withdrawals presented in Figure 1. Missing outcomes balanced in numbers across intervention groups with similar reasons for missing data across groups. Data analysed using intention‐to‐treat analysis Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | Authors report consultative roles within industry and other potential financial conflicts of interest Comment: this was judged as an unclear risk of bias |
Cufer 2006 SIGN.
| Methods | Design: parallel‐group Randomisation: yes, method stated Blinding: open‐label Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 141 Number enrolled: 141 Number in treatment 1 group: 68 Number in treatment 2 group: 73 Number of withdrawals (treatment 1/treatment 2): 0/0 Number completing trial (treatment 1/treatment 2): 68/73 Age range: treatment 1 34 to 85 years; treatment 2 29 to 83 years Sex: 98 M, 43 F Ethnicity: 42.6% Caucasian; 44.0% Hispanic; 5.0% Oriental; 1.5% Black; 7.1% other NSCLC diagnosis: histologically or cytologically confirmed advanced (stage IIIb or IV) NSCLC that had progressed on or after 1 previous chemotherapy regimen. Also 1 or more measurable lesion according to RECIST Inclusion criteria: WHO PS 0 to 2; life expectancy > 12 weeks, age > 18 years, symptomatic (LCS score < 24), capable of understanding the FACT‐L questionnaire Exclusion criteria: previous taxane treatment, treatment with any chemotherapeutic within 30 days prior to study, radiotherapy within 3 weeks prior to study, known cerebral metastasis, any evidence of ongoing interstitial lung disease (ILD), coexisting malignancies, malignancies diagnosed within the last 5 years, with exception of basal cell carcinoma or cervical carcinoma in situ, any unresolved chronic toxicity above grade 2 NCI‐CTC from previous anti‐cancer therapy, laboratory values outside requested limits, psychiatric disorders that may affect completion of FACT‐L questionnaire Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Treatment 1: gefitinib 250 mg/day Treatment 2: docetaxel 75 mg/m2 IV every 3 weeks |
|
| Outcomes | LCS component of FACT‐L Tumour response ‐ RECIST Overall survival, progression‐free survival ASEs ‐ NCI‐CTC |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "sealed randomisation envelopes which were allocated sequentially to patients" Comment: this was judged as low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed randomisation envelopes which were allocated sequentially to patients" Comment: this was judged as low risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding. Comment: this was judged as low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcomes balanced in numbers across intervention groups with similar reasons for missing data across groups. 139/141 completed the trial. Comment: this was judged as low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes were reported. Progression‐free survival was not a prespecified outcome but included in results. Quote: "Progression‐free survival was not defined as a study variable in the protocol, but as tumour assessments were performed consistently for both treatment arms, it was also estimated." Comment: this was judged as an unclear risk of bias |
| Other bias | Unclear risk | There were no declarations of potential conflicts of interest or indication of funding or support Comment: there was insufficient information to permit a clear judgement of the risk of bias |
Dai 2013.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: open‐label Withdrawals: stated |
|
| Participants | Setting: single‐centre study, hospital outpatient department Number eligible: 46 Number enrolled: 46 Number in treatment group: 23 Number in control group: 23 Number of withdrawals (treatment/control): 0/0 Number completing trial (treatment/control): 23/23 Age range: (treatment/control): 41 to 74years/47 to 72 years Sex: 29 M, 17 F Ethnicity: East Asian NSCLC diagnosis: histologic or pathologically proven diagnosis of nonsquamous NSCLC, stage IIIB to IV disease Inclusion criteria: age 18 to 75 years, Received prior platinum‐based chemotherapy of 4 to 6 cycles and has had progressive disease,at least 1 target lesion, ECOG 0 to 2, adequate bone marrow and organ function Exclusion criteria: not stated Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg/day Pemetrexed 500 mg/m2 intravenously, every 4 weeks until disease progression or unacceptable toxicity |
|
| Outcomes | Tumour response – RECIST Progression‐free survival Overall survival Toxicity – CTCAE Quality of life – FACT‐L |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized" but random sequence generation not discussed Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data Comment: this was judged as low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as low risk of bias |
| Other bias | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
Fukuoka 2003 IDEAL I.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 210 Number enrolled: 209 Number in treatment group 1: 103 Number in treatment group 2: 106 Number of withdrawals (treatment 1/treatment 2): 1/0 Number completing trial (treatment 1/treatment 2): 103/105 Age range: treatment 1 28 to 85 years; treatment 2 37 to 78 years Sex: 148 M, 62 F Ethnicity: 50% Caucasian, 50% Japanese NSCLC diagnosis: histologic or cytologic confirmation of advanced or metastatic NSCLC; stage III or IV not curable with surgery or radiotherapy at study entry Inclusion criteria: recurrent or refractory disease following 1 or 2 previous chemotherapy regimens (at least 1 containing platinum); at least 1 bi‐dimensionally measurable or radiographically assessable lesion, age 18 or older, WHO PS 0 to 2, life expectancy of 12 weeks or longer Exclusion criteria: more than 2 previous chemotherapy regimens, systemic anticancer therapy within 21 days, radiotherapy within 14 days before start of treatment; unresolved chronic toxicity higher than in NCI‐CTC grade 2; ALT or AST levels greater than 2.5 times upper limit of reference range; serum creatinine levels greater than 1.5 times the upper limit of reference range; neutrophils less than 1.5 x 109/L or platelets less than 75 x 109/L Baseline characteristics of treatment/control groups: comparable except for sex. Some demographic imbalances between Japanese and non‐Japanese populations. |
|
| Interventions | Treatment 1: gefitinib 250 mg/day Treatment 2: gefitinib 500 mg/day | |
| Outcomes | Objective tumour response rate ‐ RECIST Disease control rate (response + stable disease) Progression‐free survival Overall survival FACT‐L questionnaire LCS of FACT‐L ASEs ‐ NCI‐CTC | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized" but no further information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind", "blinded treatment supplies" Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "1 patient excluded due to protocol violation", otherwise no missing data Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | Co‐authors are recipients of research grants and honoraria from Astra Zeneca Comment: this was judged as an unclear risk of bias |
Gaafar 2011 EORTC08021.
| Methods | Design: parallel‐group Randomisation: yes, method stated Blinding: double‐blind, double‐dummy Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 173 Number enrolled: 173 Number in treatment group: 86 Number in control group: 87 Number of withdrawals (treatment/control): 15/9 Number completing trial (treatment/control): 71/78 Age range: 28 to 80 years Sex: M 133, F 40 Ethnicity: not stated NSCLC diagnosis: histologically or cytologically confirmed stage IIIB or IV NSCLC (UICC 6th ed) Inclusion criteria: not amenable to local therapy, non‐progressing after prior platinum‐based chemotherapy (2 to 6 cycles) and without unacceptable toxicity. Age older than 18 years, WHO PS 2 or less, adequate renal, hepatic and haematological function. Patients with brain metastasis were eligible, provided asymptomatic after cranial irradiation. Exclusion criteria: previous EGFR therapy, symptomatic brain metastasis, other malignancies, pregnancy or breastfeeding and interstitial pulmonary disease. Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Treatment: gefitinib 250 mg daily Control: placebo |
|
| Outcomes | Overall survival Time to progression Tumour response – RECIST ASEs – NCI‐CTC Haematology and biochemical parameters |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "centralised double blind random assignment using minimisation technique" Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "centralised double blind random assignment using minimisation technique" Comment: this was judged as a low risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. Quote: "matched daily placebo tablet" Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions and reasons for withdrawals presented in Figure 1. Missing outcomes balanced in numbers across intervention groups with similar reasons for missing data across groups (24/173 lost to follow‐up/censored). Intention‐to‐treat analysis Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Low risk | Funding from EORTC, ILCP, National Cancer Institute, Fonda Cancer (FOCA) Belgium Comment: this was judged as a low risk of bias |
Giaccone 2004 INTACT I.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: not stated | |
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 1093 Number enrolled: 1093 Number in treatment 1 group: 365 Number in treatment 2 group: 365 Number in control group: 363 Number of withdrawals (treatment 1/treatment 2/control): 3/7/8 Number completing trial (treatment 1/treatment 2/control): 362/358/355 Age range: median age 60 years Sex: 805 M, 288 F Ethnicity: 998 white (90.4%) NSCLC diagnosis: histologically or cytologically confirmed NSCLC Inclusion criteria: NSCLC locally advanced stage II disease not curable with surgery or radiotherapy or stage IV disease; aged < 18 years; WHO PS 0 to 2 Exclusion criteria: previously received chemotherapy (prior radiotherapy or surgery allowed); hypersensitive to mannitol, corticosteroids, H2‐antagonists, antihistamines or agents formulated with polyoxyethylated castor oil; had received radiotherapy within the last 2 weeks; had unresolved toxicity from previous radiation therapy or incomplete healing from previous surgery; had pre‐existing motor or sensory neurotoxicity (NCI‐CTC < grade 2); showed evidence of severe or uncontrolled systemic disease; had recent conditions requiring medication or uncontrolled significant active infections; had absolute neutrophils count < 2000/mm3; WBC < 4000/mm3; platelets < 100000/mm3; serum bilirubin greater than 1.25 times normal upper limit; ALT or AST greater than 2.5 times normal upper limit; creatinine clearance < 60 mL/min; were pregnant or breastfeeding; other coexisting malignancies or malignancies diagnosed within the last 5 years with the exception of basal cell carcinoma or cervical cancer in situ; had mixed NSCLC plus small cell lung cancer Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Regime A: 3‐week cycle of IV gemcitabine 1250 mg/m2 for 30 min of day 1 and day 8; IV Cisplatin 80 mg/m2 after gemcitabine administration on day 1 only Treatment 1: gefitinib 250 mg/day + 6 cycles of regime A Treatment 2: gefitinib 500 mg/day + 6 cycles of regime A Control: placebo + 6 cycles of regime A |
|
| Outcomes | Overall survival Time to progression Tumour response ‐ RECIST ASE ‐ NCI‐CTC | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned to one of three groups.. further stratification by dynamic randomisation..." Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind manner" Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information provided Comment: this was judged as a high risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Low risk | Financial conflicts of interest declared Comment: this was judged as a low risk of bias |
Goss 2009 INSTEP.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: double‐blind, double‐dummy Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 220 Number enrolled: 201 Number in treatment group: 100 Number in control group: 101 Number of withdrawals (treatment/control): 26/19 Number completing trial (treatment/control): 100/101 Age range: treatment 43 to 89, control 42 to 90 Sex: M 122, F 79 Ethnicity: white 193 NSCLC diagnosis: histologically or cytologically confirmed locally advanced or metastatic NSCLC not amenable to curative surgery or radiotherapy Inclusion criteria: age > 18 years, chemotherapy‐naive, WHO performance of 2 or 3, measurable disease (RECIST), no prior EGFR inhibitor therapy Exclusion criteria: untreated, newly diagnosed metastases in the CNS; other coexisting malignancies or malignancies diagnosed within the last 5 years other than basal cell carcinoma or cervical cancer in situ; fewer than 4 weeks since completion of wide‐field radiotherapy or persistence of any radiotherapy‐related toxicity; unresolved chronic toxicity greater than National Cancer Institute Common Toxicity Criteria for Adverse Events grade 2 from previous anticancer therapy (except alopecia); evidence of clinically active interstitial lung disease; prior treatment with epidermal growth factor receptor inhibitors, biologic or immunological therapy; and treatment with an investigational drug within the prior 30 days. Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg/day plus best supportive care Placebo plus best supportive care |
|
| Outcomes | Overall survival Progression‐free survival (PFS) Tumour response ‐ RECIST ASEs ‐ NCI‐CTC Haematology and biochemical parameters Quality of life Pulmonary symptom improvement (PSI) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned 1:1 according to a randomisation scheme prepared by biostatics group, AstraZeneca" Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Low risk | Central allocation Comment: this was judged as a low risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind", "gefitinib and placebo tablets physically identical and presented in identical packaging" Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition presented in Figure 1. Missing outcomes balanced in numbers across intervention groups with similar reasons for missing data across groups. Intention‐to‐treat analysis performed Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | Co‐authors are recipients of research grants and honoraria from industry Comment: this was judged as an unclear risk of bias |
Han 2012 First SIGNAL.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: open‐label Withdrawals: stated Jadad score: 2 |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 316 Number enrolled: 313 Number in treatment group: 159 Number in control group: 154 Number of withdrawals (treatment/control): 0/4 Number completing trial (treatment/control): 159/150 Age range: (treatment/control): 32 to 74 years/19 to 74 years Sex: 35 M, 174 F Ethnicity: Asian NSCLC diagnosis: stage IIIB (ineligible for curative radiotherapy) or IV adenocarcinoma of the lung with measurable or non‐measurable disease Inclusion criteria: stage IIIB or IV adenocarcinoma of the lung. ECOG PS 0 to 2, adequate bone marrow, liver and renal function. Exclusion criteria: known severe hypersensitivity to gefitinib or any constituents of this product, any evidence of clinically active interstitial lung disease; severe or uncontrolled systemic disease; concomitant use of phenytoin, carbamazepine, rifampin, barbiturate or St John's Wort; non‐stable brain metastasis Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg/day Gemcitabine (1250 mg/m2) on days 1 and 8 plus cisplatin (75 mg/m2) on day 1. Cycles repeated every 3 weeks for up to a maximum of 9 cycles as tolerated. |
|
| Outcomes | Overall survival Progression‐free survival Tumour response – RECIST ASEs – NCI‐CTC Quality of life – EORTC Quality of Life Questionnaire C30 and LC13 |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no further details provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions and reasons for withdrawals presented in Figure 1. Reasons for missing data unlikely to be related to true outcome (4/313 withdrawn, but all from the chemotherapy arm). Intention‐to‐treat analysis performed Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | Co‐authors are recipients of research grants and honoraria from industry Comment: this was judged as an unclear risk of bias |
Herbst 2004 INTACT II.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: not stated | |
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 1037 Number enrolled: 1037 Number in treatment 1 group: 345 Number in treatment 2 group: 347 Number in control group: 345 Number of withdrawals (treatment 1/treatment 2/control): 3/5/4 Number completing trial (treatment 1/treatment 2/control): 342/342/341 Age range: treatment 1 median 62 years; treatment 2 median 61 years; control median 63 years Sex: 619 M, 418 F Ethnicity: treatment 1 88.5% white, 7.5% black, 4% other; treatment 2 90.4% white, 4.1% black, 5.5% other; control 91.9% white, 5.2% black, 2.9% other NSCLC diagnosis: histologically or cytologically diagnosed NSCLC; unresectable stage III or IV disease Inclusion criteria: no prior chemotherapy; age < 18 years; WHO PS 0 to 2 Exclusion criteria: presence of mixed NSCLC or small cell lung cancer; brain metastases that were newly diagnosed or had not been treated with surgery or radiation; previously treated CNS metastases or spinal cord compression in presence of clinically stable disease; less than 2 weeks since radiotherapy; unresolved toxicity from prior radiotherapy or incomplete healing from surgery; evidence of severe systemic disease; greater than trace protein or blood on repeat urinalysis; absolute neutrophils count < 2000/µL; WBC < 4000/µL; platelets < 100,000/µL; serum bilirubin greater than 1.25 times normal upper limit; ALT or AST greater than 2.5 times normal upper limit; serum creatinine greater 1.5 times normal upper limit; pregnancy; breastfeeding; hypersensitive to mannitol, corticosteroids, H2‐antagonists, antihistamines or agents formulated with polyoxyethylated castor oil Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Regime A: IV Paclitaxel 225 mg/m3 over 3 hours on day 1 of 3 week cycle immediately followed by IV carboplatin AUC of 6 mg/mL over 15 to 30 min on day 1 Treatment 1: gefitinib 250 mg/day + 6 cycles of regime A Treatment 2: gefitinib 500 mg/day + 6 cycles of regime A Control: placebo + 6 cycles of regime A |
|
| Outcomes | Overall survival Time to progression Tumour response ‐ RECIST ASEs ‐ NCI‐CTC Haematology and biochemical parameters | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized to receive..." Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind" with use of placebo tablets Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided Intention‐to‐treat analysis performed Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Low risk | Financial conflicts of interest declared Comment: this was judged as a low risk of bias |
Kelly 2008 SWOG S0023.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: not stated Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 243 Number enrolled: 243 Number in treatment group: 118 Number in control group: 125 Number of withdrawals (treatment/control): 0/0 Number completing trial (treatment/control): 118/125 Age range: treatment: 24 to 79 years, control 37 to 81 years Sex: M 153, F 90 Ethnicity: White 221, Black 18, Asian 2, other 2 NSCLC diagnosis: pathologically confirmed and inoperable stage IIIA or IIIB NSCLC Inclusion criteria: ECOG 0 or 1, measurable or non‐measurable disease, no prior systemic therapy, radiation therapy or complete surgical resection, adequate organ function, FEV1 of less then 2.0 L if also have a minimum FEV1 of 800 mL in contralateral lung Exclusion criteria: pleural or pericardial effusions, patients with multiple tumours within the lung Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | All patients received concurrent cisplatin and etoposide with thoracic radiation according to SWOG 9504 Treatment 1: gefitinib 250 mg/day Treatment 2: placebo |
|
| Outcomes | Overall survival Progression‐free survival (PFS) ASEs ‐ NCI‐CTC |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised" but no further information given Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions and attrition stated in text. Missing outcomes balanced in numbers across intervention groups with similar reasons for missing data across groups. 115/571 (20%) eligible patients dropped out before random assignment due to progressive disease and 27 (5%) dropped out as a result of death from cancer, treatment or other causes. Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | Co‐authors are recipients of research grants and honoraria from industry Quote: "Study closed early as unplanned interim analysis rejected alternative hypothesis of improved survival..." Comment: this was judged as an unclear risk of bias |
Kim 2008 INTEREST.
| Methods | Design: parallel‐group Randomisation: yes, method stated Blinding: open‐label Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 1607 Number enrolled: 1466 Number in treatment group: 733 Number in control group: 733 Number of withdrawals (treatment/control): 701/711 Number completing trial (treatment/control): 116/107 Age range: treatment 27 to 84 years, control 20 to 84 years Sex: M 954, F 512 Ethnicity: White 1090, Asian 323, Black 22, other 31 NSCLC diagnosis: histologically or cytologically confirmed locally advanced or metastatic NSCLC Inclusion criteria: 18 years or older, NSCLC that progressed or recurred after at least 1 previous platinum‐based chemotherapy regimen (up to 2 regimens allowed), WHO performance status 0 to 2, measurable or non‐measurable disease by Response Evaluation Criteria in Solid Tumours (RECIST), had no previous therapy with EGFR tyrosine kinase inhibitor, absolute neutrophil count < 1.5 x 109/L, adequate hepatic function Exclusion criteria: not stated Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Treatment: gefitinib (250 mg/day) Control: docetaxel (75 mg/m2 in a 1‐hour infusion every 3 weeks) with standard premedication |
|
| Outcomes | Overall survival Progression‐free survival (PFS) Tumour response ‐ RECIST ASEs ‐ NCI‐CTC FACT‐L, TOI, LCS EGFR gene copy number |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Use of a centralised registration and randomisation centre, contacted by telephone, to assign patients to a specific treatment group" Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Use of a centralised registration and randomisation centre, contacted by telephone, to assign patients to a specific treatment group" Comment: this was judged as a low risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions and attrition presented in Figure 1. Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups. Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Low risk | Co‐authors are recipients of research grants and honoraria from industry. Study was supported by Astra Zeneca, but principal investigators had unrestricted access to the study data and gave assurance for the accuracy and completeness of the reported analyses. Comment: this was judged as a low risk of bias |
Kim 2016.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: open‐label Withdrawals: stated |
|
| Participants | Setting: single‐centre study, hospital outpatient department Number eligible: 95 Number enrolled: 95 Number in treatment group: 48 Number in control group: 47 Number of withdrawals (treatment/control): not stated Number completing trial (treatment/control): 0/2 Age range: (treatment/control) 42 to 82 years/31 to 81 years Sex: 68 M, 27 F Ethnicity: East Asian NSCLC diagnosis: histologically or cytologically proven advanced (stage IIIB or IV) or recurrent NSCLC; disease progression after first‐line or second‐line chemotherapy; age ≥ 18 years; ECOG PS ≤ 2; at least one measurable lesion; adequate bone marrow (absolute neutrophil count ≥ 1500/mL and platelet count ≥ 100,000/mL), normal hepatic (bilirubin ≥ 1.5 ULN and hepatic transaminase ≤ 2.5 ULN) and renal (serum creatinine < 1.5 mg/dL) functions; and an estimated life expectancy of at least 3 months Patients with brain metastases were eligible if treated with radiotherapy and clinically stable. Exclusion criteria: patients with chronic diarrhoea of any grade, inflammatory bowel disease, uncontrolled comorbid illness or other malignancies Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Pemetrexed 500 mg/m2 intravenously on day 1 of 21‐day cycle Gefitinib 250 mg/day oral 1 cycle for 21 days Cycles to continue until disease progression, unacceptable toxicity or until patient declined further treatment |
|
| Outcomes | Progression‐free survival rate at 6 months Progression‐free survival Tumour response – RECIST ASEs – NCI‐CTC Overall survival |
|
| Notes | Study closed early due to poor accrual | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized.." but no further information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals not stated Comment: this was judged as a high risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | No specific funding was disclosed and authors made no disclosure of conflicts of interest Comment: this was judged as an unclear risk of bias |
Kris 2003 IDEAL II.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 261 Number enrolled: 221 Number in treatment 1 group: 106 Number in treatment 2 group: 115 Number of withdrawals (treatment 1/treatment 2): 4/1 Number completing trial (treatment 1/treatment 2): 102/114 Age range: treatment 1 34 to 84 years; treatment 2 30 to 80 years Sex: 128 M, 93 F Ethnicity: not stated NSCLC diagnosis: pathological diagnosis of NSCLC, stage IIIB or IV disease extent Inclusion criteria: treatment with 2 or more regimens containing cisplatin or carboplatin and docetaxel, given either concurrently or as separate regimens; disease progression or unacceptable toxicity with last chemotherapy regimen; symptomatic NSCLC as determined by score of 24 of 28 on LCS of FACT‐L; measurable or evaluable indicator lesions, WHO PS 0 to 2 Exclusion criteria: received chemotherapy or irradiation within 14 days; unresolved toxicity greater than grade 2 from prior chemotherapy; neutrophil count less than 1.5 x 109/L; platelet count less than 75 x 109/L; bilirubin level more than 1.25 times the upper limit of normal; creatinine clearance less than 30 mL/min Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Treatment 1: gefitinib 250 mg/day (1 x 250 mg tablet + 1 placebo tablet) Treatment 2: gefitinib 500 mg/day (2 x 250 mg tablets) | |
| Outcomes | FACT‐L ‐ Time to symptom improvement as measured by FACT‐L ‐ Duration of improvement as measured by FACT‐L Radiographic assessments (PR ‐ 50% decrease in lesion size) ‐ Duration of radiographic response ‐ Radiographic response rates ASEs ‐ NCI‐CTC Overall survival by dose, frequency, severity of ASE | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized.." but no further information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/261 "lost to follow‐up" Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | Research support received from Astra Zeneca Comment: this was judged as an unclear risk of bias |
Lee 2010 ISTANA.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: double‐blind Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 163 Number enrolled: 161 Number in treatment group: 82 Number in control group: 79 Number of withdrawals (treatment/control): 8/12 Number completing trial (treatment/control): 82/79 Age range: treatment 21 to 74 years, control 20 to 73 years Sex: M 100, F 61 Ethnicity: Korean NSCLC diagnosis: histologically or cytologically confirmed NSCLC with stage IIB or IV Inclusion criteria: patients with NSCLC who received only 1 previous platinum‐doublet chemotherapy regimen, and who were considered candidates for further chemotherapy. Age 18 years or older, WHO performance status of 0 to 2, progressive or recurrent disease following previous chemotherapy (adjuvant chemotherapy was allowed if full cytotoxic doses of platinum‐based doublet therapy was given in patients with early disease having progressed), measurable disease by RECIST, adequate bone marrow, renal and hepatic function Exclusion criteria: previous docetaxel or any other EGFR‐targeted treatment, any evidence of clinically active interstitial lung disease, newly diagnosed central nervous system metastases, or any unresolved chronic toxicity greater than NCI‐CTCAE grade 2 from previous anti‐cancer therapy Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg/day Docetaxel 75 mg/m2 as a 1‐hour intravenous infusion on day 1 every 3 weeks |
|
| Outcomes | Overall survival Progression‐free survival (PFS) Tumour response ‐ RECIST ASEs ‐ NCI‐CTC Quality of life ‐ LCS of FACT‐L, the Trial Outcome Index |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned .. after stratification..." but no further information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition presented in Figure 1. Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups. Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Low risk | Quote: "No potential conflicts of interest were disclosed." Comment: this was judged as a low risk of bias |
Li 2010.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: open‐label Withdrawals: stated |
|
| Participants | Setting: single‐centre study, hospital outpatient department Number eligible: 98 Number enrolled: 98 Number in treatment group: 50 Number in control group: 48 Number of withdrawals (treatment/control): 1/0 Number completing trial (treatment/control): 49/48 Age range: (treatment/control): 42 to 69 years Sex: 59 M, 39 F Ethnicity: East Asian NSCLC diagnosis: pathologically proven diagnosis of NSCLC, stage IIIB to IV disease Inclusion criteria: age ≥ 18 years, Karnofsky score of ≥ 70, life expectancy ≥ 3 months, Received at least 1 cycles of prior chemotherapy (Navelbine, Gemzar or cisplatin), Have at least 1 target lesion, adequate organ function, normal ECG Exclusion criteria: not stated Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg/day Docetaxel 75 mg/m2 intravenously, every 3 weeks for 2 to 4 cycles or until disease progression or unacceptable toxicity |
|
| Outcomes | Tumour response – RECIST Survival Toxicity – CTCAE Quality of life – WHO criteria |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized" but no further information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data Comment: this was judged as low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
Lou 2014.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: open‐label Withdrawals: stated |
|
| Participants | Setting: single‐centre study, hospital outpatient department Number eligible: 51 Number enrolled: 51 Number in treatment group: 25 Number in control group: 26 Number of withdrawals (treatment/control): 0/0 Number completing trial (treatment/control): 25/26 Age range: (treatment/control): 34 to 73years/36 to 76 years Sex: 9 M, 42 F Ethnicity: East Asian NSCLC diagnosis: histologic or pathologically proven diagnosis of NSCLC, stage IIIB to IV disease Inclusion criteria: age ≥ 18 years, non‐smoker (< 100 cigarettes consumed in lifetime) or former light smoker (< 10 pack‐year history), received no prior chemotherapy of biological/immunological anti‐cancer therapy Exclusion criteria: not stated Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg/day Paclitaxel 200 mg/m2 with carboplatin AUC5 intravenously for 6 cycles or until disease progression |
|
| Outcomes | Progression‐free survival Overall survival Tumour response – RECIST |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized" but no further information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
Maemondo 2010 NEJ002.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: not blinded Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 230 Number enrolled: 230 Number in treatment group: 115 Number in control group: 115 Number of withdrawals (treatment/control): 1/5 Number completing trial (treatment/control): 114/110 Age range: treatment: 43 to 75 years, control: 35 to 75 years Sex: 48 M, 145 F Ethnicity: not stated ‐ Japanese NSCLC diagnosis: advanced NSCLC Inclusion criteria: harbouring sensitive EGFR mutations, absence of resistant EGFR mutation T790M, no history of chemotherapy, age 75 or younger Exclusion criteria: presence of resistant EGFR mutation Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Treatment 1: gefitinib 250 mg/day Treatment 2: Paclitaxel (at least dose of 200 mg/m2 of body‐surface area, given intravenously over 3‐hour period) and carboplatin (at a dose equivalent to an area under the concentration‐time curve of 6, given intravenously over a 1‐hour period), both administered on the first day of every 3‐week cycle |
|
| Outcomes | Overall survival ‐ date of randomisation to date of death Progression‐free survival (PFS) Tumour response ‐ RECIST ASEs ‐ NCI‐CTC |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized" but no further information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions presented in Figure 1, attrition stated in text. Reasons for missing data unlikely to be related to true outcome. 224/230 patients included in PFS population, 227/230 patients included in safety population. Intention‐to‐treat analysis performed Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Low risk | Quote: "In the planned interim analysis of data, PFS was significantly longer in the gefitinib group than in standard‐chemotherapy group resulting in early termination of the study" Funded by Japan Society for Promotion and Science and Japanese Foundation for Multidisciplinary Treatment of Cancer and Tokyo Cooperative Oncology Group Comment: this was judged as an unclear risk of bias |
Maruyama 2008 V‐15‐32.
| Methods | Design: parallel‐group Randomisation: yes, method stated Blinding: open‐label Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 511 Number enrolled: 489 Number in treatment group: 245 Number in control group: 244 Number of withdrawals (treatment/control): 233/241 Number completing trial (treatment/control): 12/3 Age range: < 64 years = 275, > 65 years = 216 Sex: M 302, F 187 Ethnicity: Japanese NSCLC diagnosis: histologically or cytologically confirmed NSCLC (stage IIIB/IV) Inclusion criteria: age 20 years or older, pretreated locally advanced/metastatic (stage IIIB/IV) NSCLC, or recurrent NSCLC, NSCLC not amenable to curative surgery or radiotherapy or postoperative recurrent NSCLC, failure of prior treatment with 1 or 2 chemotherapy regimens (> 1 platinum based regimen), life expectancy of 3 months or greater, WHO PS score 0 to 2, measurable disease by RECIST, WBC count of 4.0 to 12.0 x 109 cells/L, neutrophil count < 2.0 x 109 cells/L, platelet count > 100 x 109 cells/L, serum bilirubin < 1.5 x 109 cells/L, ALT or AST < 2.5 x upper limit of reference range, serum creatinine < 1.5 mg/dL, arterial oxygen tension > 70 torr. Exclusion criteria: received last chemotherapy within 4 weeks before enrolment, received prior treatment with a docetaxel‐containing regimen or any anti‐EGFR therapy, an allergy or suspected allergy to gefitinib or docetaxel, other coexisting malignancies diagnosed within the last 5 years, with exceptions, any unresolved chronic toxicity greater than NCI‐CTC grade 2 from previous anticancer therapy, any evidence of severe or uncontrolled systemic disease, as judged by investigator, current status of pregnancy or breastfeeding, treatment with a non‐approved or investigational drug within 30 drugs before enrolment, intracerebral metastases, significant malabsorption syndrome, past history of or concurrent interstitial lung disease, idiopathic pulmonary fibrosis or pneumoconiosis, or radiation pneumonia or drug‐induced pneumonia, that required corticosteroids, fever with suspected infection or treatment with systemic corticosteroids for > 4 weeks Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg/day Docetaxel every 3 weeks as a 1‐hour intravenous infusion of 60 mg/m2 |
|
| Outcomes | Overall survival Progression‐free survival (PFS) Tumour response ‐ RECIST ASEs ‐ NCI‐CTC |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned by using stratification..." Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusions presented in Figure 1. Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups. 483/489 patients analysed for safety, 387/489 (79%) analysed for response (balanced between treatment arms) Intention‐to‐treat analysis performed Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Low risk | Co‐authors have received honoraria from industry Comment: this was judged as a low risk of bias |
Mitsudomi 2010 WJTOG3405.
| Methods | Design: parallel‐group Randomisation: yes, method stated Blinding: open‐label Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 177 Number enrolled: 177 Number in treatment group: 88 Number in control group: 89 Number of withdrawals (treatment/control): 50/30 Number completing trial (treatment/control): 30/59 Age range: treatment: 34 to 73 years, control: 41 to 75 years Sex: M 53, F 119 Ethnicity: Japanese NSCLC diagnosis: histologically or cytologically confirmed NSCLC (stage IIIB/IV) harbouring an activating mutation of EGFR gene (either exon 19 deletion or L858R in exon 21) Inclusion criteria: aged 75 or younger, WHO performance status 0 to 1, had measurable or non‐measurable disease according to RECIST, adequate organ function. Patients with postoperative recurrence, treated with adjuvant therapy other than cisplatin plus docetaxel, were included when interval between end of adjuvant chemotherapy and registration exceeded 6 months for platinum‐doublet therapy and more than 1 month for oral tegafur plus uracil therapy. Exclusion criteria: received previous drug therapy that had targeted the EGFR, a history of interstitial lung disease, severe drug allergy, active infection or other serious disease condition, symptomatic brain metastases, poorly controlled pleural effusion, pericardial effusion or ascites necessitating drainage, active double cancer, or severe hypersensitivity to drugs containing poly solvate 80, pregnancy or lactation. Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg/day Docetaxel 60 mg/m2, administered intravenously over a 1‐hour period, followed by cisplatin 80 mg/m2, administered intravenously over a 90‐min period with adequate hydration, in cycles of once every 21 days for 3 to 6 cycles |
|
| Outcomes | Progression‐free survival (PFS) Overall survival Tumour response ‐ RECIST Disease control rate ASEs ‐ NCI‐CTC Mutation‐type‐specific survival |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned in 1:1 ratio" Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were allocated at the WJOG data centre to each treatment group using a desktop computer programmed for the minimisation method." Comment: this was judged as a low risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions and attrition presented in Figure 1. Reasons for missing data unlikely to be related to true outcome. 5/177 withdrawn. Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported, except for overall survival. Quote: "data for overall survival were immature, with follow‐up still ongoing" Comment: this was judged as a low risk of bias |
| Other bias | Low risk | Trial designed and conducted independently of any pharmaceutical company Author conflicts of interest declared Trial closed early as results of contemporary studies showing improved PFS in EGFR mutation positive NSCLC. Further trial accrual was felt to be futile and unethical. Comment: this was judged as low risk of bias |
Mok 2009 IPASS.
| Methods | Design: parallel‐group Randomisation: yes, method stated Blinding: not blinded Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 1329 Number enrolled: 1217 Number in treatment group: 609 Number in control group: 608 Number of withdrawals (treatment/control): 12/28 Number completing trial (treatment/control): 597/580 Age range: treatment: 24 to 84 years, control: 25 to 84 years Sex: M 252, F 965 Ethnicity: Chinese 618, Japanese 233, other East Asian 363, other 3 NSCLC diagnosis: histologically or cytologically confirmed stage IIIB or IV NSCLC with histological features of adenocarcinoma Inclusion criteria: 18 years or older, non‐smoker or former light smokers (those who had stopped smoking at least 15 years previously and had a total of ?10 pack‐years of smoking), no previous chemotherapy or biologic or immunologic therapy, WHO PS 0 to 2, measurable disease according to RECIST criteria with at least 1 measurable lesion, not previously irradiated, adjuvant chemotherapy permitted if not platinum‐based and completed > 6 months previously, absolute neutrophil count > 2.0 x 109 and adequate hepatic function Exclusion criteria: not stated Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Treatment: gefitinib 250 mg/day Control: Paclitaxel (200 mg/m2 of body‐surface area, administered intravenously over a 3‐hour period on the first day of the cycle) followed immediately by carboplatin (at a dose calculated to produce an area under the curve of 5.0 to 6.0 per mL per min, administered intravenously over a period of 15 to 60 min) |
|
| Outcomes | Overall survival Progression‐free survival (PFS) Tumour response ‐ RECIST ASEs ‐ NCI‐CTC Quality of life ‐ FACT‐L, TOI, LCS score of FACT‐L |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was performed with the use of dynamic balancing..." Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions and attrition presented in Figure 1. Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups. 1159/1217 (95%) included in analysis Intention‐to‐treat analysis performed Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | Funding from the Chinese Lung Cancer Research Foundation. Co‐authors received consulting fees and grant support from industry. Comment: this was judged as an unclear risk of bias |
Morere 2010 IFCT‐0301.
| Methods | Design: parallel‐group Randomisation: yes, method stated Blinding: double‐blind Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 128 Number enrolled: 85 Number in treatment group: 43 Number in control group: 42 Number of withdrawals (treatment/control): 43/41 Number completing trial (treatment/control): 0/0 Age range: treatment 45 to 79 years, control 30 to 79 years Sex: M 71, F 14 Ethnicity: not stated NSCLC diagnosis: stage IIIb/IV NSCLC Inclusion criteria: age 18 to 80, NSCLC with measurable disease, ECOG PS 2 or 3, adequate organ function Exclusion criteria: prior chemotherapy, prior EGFR therapy or prior thoracic radiotherapy Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg daily Docetaxel 75 mg/m2 day 1 every 3 weeks |
|
| Outcomes | Overall survival Time to progression Tumour response ‐ RECIST ASEs ‐ NCI‐CTC |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random assignment was block stratified..." Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions and attrition presented in Figure 1. Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups. Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Low risk | Co‐authors have received honoraria from industry Comment: this was judged as a low risk of bias |
Soria 2015 IMPRESS.
| Methods | Design: parallel‐group Randomisation: yes, method stated Blinding: placebo‐controlled Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 287 Number enrolled: 265 Number in treatment group: 133 Number in control group: 132 Number of withdrawals (treatment/control): 1/0 Number completing trial (treatment/control): 23/18 Age range: (treatment/control) 33 to 79 years/35 to 79 years Sex: 94 M, 171 F Ethnicity: East Asian 78%; Spanish/French/German/Italian/Russia 22% NSCLC diagnosis: histologic/cytologic diagnosis of NSCLC, stage IIIB to IV disease, chemotherapy‐naive Inclusion criteria: age ≥ 18 years; chemotherapy‐naive advanced NSCLC and an activating EGFR mutation as confirmed by local testing, who had achieved a complete or partial response for longer than 4 months, or durable stable disease for at least 6 months on first‐line gefitinib and had subsequently developed radiological disease progression. Life expectancy of > 12 months, and a WHO PS of 0 or 1. Exclusion criteria: NSCLC of predominately squamous cell histology, a history of interstitial lung disease, any other coexisting malignancies diagnosed within the past 5 years (excluding basal cell carcinoma, cervical cancer in situ, or completely resected intramucosal gastric cancer) or treatment with another investigational drug 4 weeks of less before random allocation Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg daily PLUS cisplatin 75 mg/m2 and pemetrexed 500 mg/m2 on day 1 of cycle Placebo PLUS cisplatin 75 mg/m2 and pemetrexed 500 mg/m2 on day 1 of cycle |
|
| Outcomes | Progression‐free survival Tumour response – RECIST Overall survival ASEs – NCI‐CTC Health‐related quality of life – FACT‐L, LCS, TOI |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of "central block randomisation to allocate patients (1:1)..." Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Low risk | Patients were assigned a unique enrolment number using an interactive web response system Comment: this was judged as a low risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled with identical packaging Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals stated in Figure 1 Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | Authors have received honoraria, consultant and advisor fees from industry Study funded by Astra Zeneca, who co‐ordinated the trial, managed the database and undertook analyses Comment: this was judged as an unclear risk of bias |
Sun 2012 KCSG‐LU08‐01.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: open‐label Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 147 Number enrolled: 141 Number in treatment group: 71 Number in control group: 70 Number of withdrawals (treatment/control): 3/3 Number completing trial (treatment/control): 68/67 Age range: (treatment/control): 40 to 77 years/30 to 78 years Sex: 20 M, 115 F Ethnicity: Asian NSCLC diagnosis: histologically or cytologically confirmed pulmonary adenocarcinoma Inclusion criteria: histologically or cytologically confirmed pulmonary adenocarcinoma that progressed after just 1 previous platinum‐based chemotherapy regimen for advanced disease, never‐smoker, 18 years or older, ECOG PS 0 to 2, measurable or evaluable disease, adequate bone marrow, renal and hepatic function Exclusion criteria: prior EGFR TKI or pemetrexed treatment and symptomatic or uncontrolled brain metastases Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg/day Pemetrexed 500 mg/m2 on day 1 of a 21‐day cycle Cycles repeated until disease progression, unacceptable toxicity, or until patient or investigator requested therapy discontinuation |
|
| Outcomes | Tumour response – RECIST Overall survival Progression‐free survival ASEs – NCI‐CTC Haematology and biochemical parameters Quality of life – EORTC Quality of Life Questionnaire C30 (EORTC QLQ‐C30) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "consecutively assigned to either arm according to a predefined computer‐generated randomisation scheme developed by statisticians" Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "consecutively assigned to either arm according to a predefined computer‐generated randomisation scheme developed by statisticians" Comment: this was judged as a low risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions and attrition presented in Figure 1. Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups. 135/141 patients analysed for efficacy. Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | No specific funding was disclosed and authors made no disclosure of conflicts of interest Comment: this was judged as an unclear risk of bias |
Takeda 2010 WJTOG0203.
| Methods | Design: parallel‐group Randomisation: yes, method stated Blinding: double‐blind Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 604 Number enrolled: 603 Number in treatment group: 302 Number in control group: 301 Number of withdrawals (treatment/control): 4/4 Number completing trial (treatment/control): 298/297 Age range: treatment 25 to 74 years; control 35 to 74 years Sex: M 383, F 215 Ethnicity: Japanese NSCLC diagnosis: histologically or cytologically confirmed stage IIIB (with malignant pleural effusion or contralateral hilar lymph node metastases) or stage IV NSCLC Inclusion criteria: NSCLC who had not previously received any chemotherapy, patients who had recurrence after complete surgical resection were permitted, ECOG performance status 0 to 1, adequate organ function as indicated as WBC count > 4000/µL, absolute neutrophil count > 2000/µL, haemoglobin > 9.5 g/dL, AST/ALT < 2.5 times the upper limit of normal, total bilirubin < 1.5 mg/dL, serum creatinine < 1.2 mg/dL, PaO2 in arterial blood > 70 mmHg. Asymptomatic brain metastases were allowed provided they had been irradiated and were clinically and radiologically stable. Exclusion criteria: patients treated with either adjuvant or neoadjuvant chemotherapy. Radiologically or clinically apparent interstitial pneumonitis or pulmonary fibrosis. Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Arm A: platinum doublet chemotherapy ‐ up to 6 cycles Arm B: 3 cycles of chemotherapy followed by gefitinib 250g/day orally until disease progression |
|
| Outcomes | Overall survival Progression‐free survival (PFS) Tumour response ‐ RECIST Quality of life ‐ FACT‐L |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but no further information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions presented in Figure 1; withdrawals were stated in text. Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups. 595/604 included in analysis. Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Low risk | Co‐authors have received honoraria from industry Comment: this was judged as a low risk of bias |
Thatcher 2005 ISEL.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: double‐blind, double‐dummy Withdrawals: stated | |
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 1836 Number enrolled: 1692 Number in treatment group: 1126 Number in control group: 562 Number of withdrawals (treatment/control): 818/451 Number completing trial (treatment/control): 308/111 Age range: treatment 28 to 90 years, control 31 to 87 years Sex: 1139 M, 553 F Ethnicity: 1274 Caucasian; 342 Asian; 14 Black; 62 other NSCLC diagnosis: histologically or cytologically proven NSCLC Inclusion criteria: NSCLC not curable with surgery or radiotherapy; previously received 1 or 2 chemotherapy regimens; refractory to (recurrent or progressive disease within 90 days of chemotherapy) or intolerant of latest chemotherapy regimen; younger than 70 years; received at least 1 previous platinum‐based chemotherapy regimen; WHO PS 0 to 2; life expectancy of at least 8 weeks Exclusion criteria: presence of small cell lung cancer alone or with NSCLC; administration of last dose of single‐agent chemotherapy within the previous 21 days; untreated or clinically unstable newly diagnosed metastasis in central nervous system; less than 1 week since completion of previous radiotherapy or persistence of any radiotherapy‐related toxic effects; unresolved chronic toxic effects from previous anticancer therapy; known severe hypersensitivity to gefitinib or any tablet excipients; inability to swallow tablets; other coexisting malignant disease (apart from basal cell carcinoma); absolute neutrophils count less than 1.0 x 109/L; platelet count less than 100 x 109/L; serum bilirubin concentration more than 3 times upper limit of normal; AST or AST concentration more than 5x upper limit of normal; more than 2 previous chemotherapy regimens for NSCLC; previous treatment with an experimental agent of which the main mechanism of action is inhibition of epidermal growth receptor or its associated tyrosine kinase; concomitant use of phenytoin, carbamazepine, rifampicin, barbiturates, St John's wort; severe or uncontrolled systemic disease; clinically active interstitial lung disease (except uncomplicated lymphangitic carcinomatosis); pregnancy; breastfeeding Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg/day Placebo | |
| Outcomes | Overall survival Time to treatment failure Tumour progression ‐ RECIST FACT‐L LCS of FACT‐L ASEs ‐ NCI‐CTC | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation done by a minimisation method" Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "central registration and randomisation centre" Comment: this was judged as a low risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind", "physically identical tablets and packaging" Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "818/1126 in treatment group and 451/562 in placebo group discontinued". Missing outcomes balanced in numbers across intervention groups with similar reasons for missing data across groups. Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Low risk | Co‐authors have received honoraria from industry Comment: this was judged as a low risk of bias |
Xu 2015.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: open‐label Withdrawals: not stated |
|
| Participants | Setting: single‐centre study, hospital outpatient department Number eligible: 188 Number enrolled: 188 Number in treatment group: 94 Number in control group: 94 Number of withdrawals (treatment/control): not stated Number completing trial (treatment/control): not stated Age range: (treatment/control): 60 to 82 years Sex: 98 M, 90 F Ethnicity: East Asian NSCLC diagnosis: histologic/cytologic diagnosis of NSCLC, stage IIIB to IV disease Inclusion criteria: stage IIIB to IV NSCLC, inoperable due to medical reasons or rejecting surgery, or the patients accepting 4 to 8 cycles of first‐line chemotherapy and achieving complete remission, partial response or stability. KPS ≥ 60, no other disease interfering patients to complete the treatment; no brain metastases, with good compliance Exclusion criteria: not stated Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Pemetrexed 500 mg/m2 day 1 to 3 Gefitinib 250 mg/daily |
|
| Outcomes | Tumour response – RECIST ASEs – NCI‐CTC |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomized" Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals not stated Comment: this was judged as a high risk of bias |
| Selective reporting (reporting bias) | High risk | "survival time" was a prespecified outcome but not reported in methods; reason for this is unclear Comment: this was judged as a high risk of bias |
| Other bias | Low risk | Disclosed no conflicts of interest Comment: this was judged as a low risk of bias |
Xue 2015.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: open‐label Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 155 Number enrolled: 100 Number in treatment group: 48 Number in control group: 48 Number of withdrawals (treatment/control): 2/2 Number completing trial (treatment/control): 8/10 Age range: (treatment/control) 33 to 83 years/32 to 83 years Sex: 43 M, 53 F Ethnicity: East Asian NSCLC diagnosis: histologic/cytologic diagnosis of NSCLC, stage IIIB to IV disease Inclusion criteria: advanced, refractory or recurrent NSCLC after at least 1 previous regimen; achievement of stable disease after 1 month of gefitinib 250 mg daily therapy; measurable lesions by RECIST criteria; ECOG PS 0 to 2; satisfactory renal, haematological and cardiac function. Stable brain metastases were allowed. Exclusion criteria: previous EGFR TKI therapy, pregnancy, breastfeeding, or unable to take oral medications Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 500 mg/day Gefitinib 250 mg/day |
|
| Outcomes | Tumour response – RECIST Progression‐free survival Overall survival ASEs – NCI‐CTC |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomized " Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals stated with reasons such as "consent not given" provided Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Low risk | Study supported by Wu Jieping Medical Foundation Project grant and National funding programmes. One author has declared having received research support from industry. Comment: this was judged as a low risk of bias |
Yang 2014.
| Methods | Design: parallel‐group Randomisation: yes, method stated Blinding: open‐label Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 253 Number enrolled: 236 Number in treatment group: 118 Number in control group: 118 Number of withdrawals (treatment/control): 4/0 Number completing trial (treatment/control): 12/46 Age range: treatment 24 to 81 years; control 31 to 79 years Sex: 59 M, 177 F Ethnicity: East Asian NSCLC diagnosis: histologic/cytologic diagnosis of non‐squamous NSCLC, stage IIIB to IV disease Inclusion criteria: chemotherapy‐naive patients of East Asian ethnicity and unknown EGFR mutation status. Stage IIIB to IV non‐squamous NSCLC. Age ≥ 18 years, "light ex smokers" or "never smokers" measurable disease by RECIST version 1.0, ECOG PS 0 or 1 Exclusion criteria: known EGFR status before study entry, documented brain metastasis (previously treated stable brain metastases were allowed), clinically significant third space fluid collections, inability to interrupt aspirin or other non‐steroidal anti‐inflammatory agents (except aspirin at a dose of 1300 mg daily for a 5‐day period) and concomitant use of CYP3A4 inducers Baseline characteristics of treatment/control groups: comparable |
|
| Interventions |
PC/Gefitinib arm Pemetrexed (500 mg/m2) + cisplatin (75 mg/m2) on day 1 of 21‐day cycle. Maximum of 6 cycles. Then non‐progressing patients received gefitinib 250 mg daily as maintenance Gefitinib arm Gefitinib 250 mg daily as maintenance |
|
| Outcomes | Progression‐free survival Overall survival Tumour response – RECIST Time to progressive disease (TtPD) Duration of response (DoR) ASEs – NCI‐CTC Association between EGFR mutation status and clinical outcomes |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... randomisation was controlled by a centrally located computerised voice response unit using a computer‐generated random sequence and an interactive voice response system..." Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Low risk | External computer generated random sequence Comment: this was judged as a low risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals presented in Figure 1. 58 patients completed the study, with balanced numbers between both arms. Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | Authors have declared paid consultancies, honorarium and research funding from industry Comment: this was judged as an unclear risk of bias |
Yu 2014.
| Methods | Design: parallel‐group Randomisation: yes, method not stated Blinding: open‐label Withdrawals: stated |
|
| Participants | Setting: single‐centre study, hospital outpatient department Number eligible: 120 Number enrolled: 117 Number in treatment group: 58 Number in control group: 59 Number of withdrawals (treatment/control): 6/2 Number completing trial (treatment/control): 27/27 Age range: treatmnet 36 to 72 years;control 33 to 70 years Sex: 58 M, 59 F Ethnicity: East Asian NSCLC diagnosis: histologic/cytologic diagnosis of advanced or recurrent non‐squamous NSCLC, stage IIIB to IV disease Inclusion criteria: ≥ 18 years, stage IIIB to IV non‐squamous NSCLC ECOG PS 0 to 1; measurable disease according to RECIST, adequate haematological hepatic and renal functions, life expectancy of > 12 weeks Exclusion criteria: received previous systemic anticancer treatment or had severe drug allergy, or another serious disease or condition, uncontrolled brain metastases, uncontrolled pleural effusion and/or pericardial effusion, or second malignancy, pregnancy or lactation. Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | PC/G Pemetrexed 500 mg/m2 + either cisplatin (75 mg/m2) or carboplatin (AUC5) Intravenously on day 1 of a 3‐week cycle Gefitinib 250 mg orally on day 3 to 16 of a 21‐day cycle PC Pemetrexed 500 mg/m2 + either cisplatin (75 mg/m2) or carboplatin (AUC5) Intravenously on day 1 of a 3‐week cycle Continued until disease progression |
|
| Outcomes | Non‐progression rate (NPR) Tumour response – RECIST Progression‐free survival Overall survival ASEs – NCI‐CTC |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomized in 1:1 ratio" and stratified by smoking status, EGFR genotype Comment: this was judged as an unclear risk of bias |
| Allocation concealment (selection bias) | Unclear risk | No information provided Comment: there was insufficient information to permit a clear judgement of risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding but review authors judge that outcome is not likely to be influenced by lack of blinding Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals stated in text Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Low risk | Authors declared no competing conflicts of interest |
Zhang 2012 INFORM.
| Methods | Design: parallel‐group Randomisation: yes, method stated Blinding: double‐blind Withdrawals: stated |
|
| Participants | Setting: multicentre study, hospital outpatient department Number eligible: 298 Number enrolled: 296 Number in treatment group: 148 Number in control group: 148 Number of withdrawals (treatment/control): 81/95 Number completing trial (treatment/control): 67/53 Age range: treatment 31 to 79 years; control 20 to 75 years Sex: 175 M, 121 F Ethnicity: East Asian (Chinese) NSCLC diagnosis: histologically or cytologically confirmed stage IIIB or IV NSCLC Inclusion criteria: stage IIIB or IV NSCLC, 18 years or older, life expectancy of > 12 weeks, WHO PS 0 to 2, completed 4 cycles of first‐line platinum doublet chemotherapy without disease progression and acceptable toxic effects Exclusion criteria: patients with known EGFR status to avoid selection bias. Prior exposure to monoclonal antibodies or small molecule inhibitors against EGFR receptors (e.g. gefitinib, erlotinib, C225). Participation in another clinical study or received treatment with a non‐approved agent within 42 days before Day 1 of study treatment. Serum bilirubin > 3 x ULRR, Aspartate aminotransferase (AST/SGOT) or alanine aminotransferase (ALT/SGPT) ≥ 2.5 x ULN if no demonstrable liver metastases (or > 5 x in presence of liver metastases). Any unresolved chronic toxicity greater than common toxicity criteria (CTCAE) grade 2 from previous anticancer therapy excluding peripheral neuropathy. Patients with previously diagnosed and treated CNS metastases or spinal cord compression may be considered if they are clinically stable and have been discontinued from steroid therapy for at least 4 weeks prior to first dose of study medication. Any evidence of clinically active interstitial lung disease (patients with chronic, stable, radiographic changes who are asymptomatic need not be excluded). Pre‐existing idiopathic pulmonary fibrosis evidence by CT scan at baseline. Patients who have undergone complete tumour resection after responding to platinum‐based chemotherapy. As judged by the investigator, any evidence of severe or uncontrolled systemic disease (e.g. unstable or uncompensated respiratory, cardiac, hepatic or renal disease). Treatment with any systemic anticancer therapies other than the prescribed protocol chemotherapy regimen (refer to Inclusion criterion). Exception: palliative radiotherapy for symptom relief of lesions present at diagnosis will be allowed; however, palliative wide field radiotherapy to the lung must be completed at least 4 weeks before day 1 with no persistence of any radiotherapy‐related toxicity. Other co‐existing malignancies or malignancies diagnosed within the last 5 years with the exception of basal cell carcinoma or cervical cancer in situ. Pregnancy or breastfeeding (women of child bearing potential). Concomitant use of phenytoin, carbamazepine, rifampicin, barbiturates or St. John’s wort. Previous bone marrow transplant. Whole blood transfusion within 120 days of the date of genetic sample collection. Known biomarker status of one or more of the following: tumour EGFR gene copy number, tumour EGFR gene mutation status, tumour EGFR protein expression. Baseline characteristics of treatment/control groups: comparable |
|
| Interventions | Gefitinib 250 mg/day Placebo (oral) |
|
| Outcomes | Progression‐free survival Overall survival Time to progression Tumour response – RECIST ASEs – NCI‐CTC Haematology and biochemical parameters Quality of life (FACT‐L) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was done centrally by a third‐party randomisation centre that had no other role in the study", "Randomization was performed using dynamic balancing..." Comment: this was judged as a low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation was done centrally by a third‐party randomisation centre that had no other role in the study" Comment: this was judged as a low risk of bias |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "active and placebo drugs were identical in form and packaging to ensure blinding" Comment: this was judged as a low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions and attrition presented in Figure 1. Missing outcomes balanced in numbers across intervention groups with similar reasons for missing data across groups. All 296 patients were available for analysis. Comment: this was judged as a low risk of bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported Comment: this was judged as a low risk of bias |
| Other bias | Unclear risk | Funding for study from Astra Zeneca. Co‐authors have received research support from industry. Comment: this was judged as an unclear risk of bias |
ALT: alanine transaminase ASE: adverse side effects AST: aspartate transaminase AUC: area under curve CNS: central nervous system CT: computerised tomography CTCAE: Common Toxicity Criteria for Adverse Events ECG: electrocardiogram ECOG: Eastern Cooperative Oncology Group ECOG PS: ECOG Performance Status EGFR: epidermal growth factor receptor EORTC: European Organisation for Research and Treatment of Cancer F: female FACT‐L: Functional Assessment of Cancer Therapy‐Lung FEV1: forced expiratory volume in one second ILCP: Italian Lung Cancer Project IV: intravenous KPS: Karnofsky Performance Status LCS: lung cancer subscale M: male MRI: magnetic resonance imaging NCI‐CTC: National Cancer Institute Common Toxicity Criteria NSCLC: non‐small cell lung cancer PFS: progression‐free survival PR: partial response RECIST: Response Evaluation Criteria in Solid Tumours SGOT: serum glutamic‐oxaloacetic transaminase SGPT: serum glutamic‐pyruvic transaminase TKI: tyrosine kinase inhibitor TOI: Trial Outcome Index UFT: tegafur + uracil UICC: Union for International Cancer Control ULN: upper limit of normal ULRR: upper limit of the reference range WBC: white blood cell WHO: World Health Organization WHO PS: WHO Performance Status
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Choi 2015 | Recruited only EGFR‐mutation negative patients |
| Kim 2012 | Gefitinib versus other EGFR TKI |
| Lee 2009 | Open‐label, non‐randomised study |
| Manegold 2005 | No direct comparison arm |
| Natale 2009 | Cross‐over study |
| Shi 2013 ICOGEN | Gefitinib versus other EGFR TKI |
| Sugawara 2015 | Gefitinib + chemotherapy (sequential) versus gefitinib + chemotherapy (alternating) |
| Urata 2016 | Gefitinib versus other EGFR TKI |
| Zhou 2014 CTONG 0806 | Recruited only EGFR‐mutation negative patients |
EGFR: epidermal growth factor receptor TKI: tyrosine kinase inhibitor
Characteristics of ongoing studies [ordered by study ID]
Bhatnagar 2012.
| Trial name or title | Docetaxel versus gefitinib in patients with locally advanced or metastatic NSCLC pretreated with platinum‐based chemotherapy |
| Methods | Randomised |
| Participants | 30 patients with locally advanced or metastatic NSCLC previously treated with cisplatin‐based chemotherapy, who had progressive or recurrent disease and ECOG performance score 0 to 2 |
| Interventions | Gefitinib 250 mg/day versus docetaxel 75 mg/m2 every 3 weeks |
| Outcomes | Tumour response Adverse events |
| Starting date | Not known |
| Contact information | Not known |
| Notes | — |
Gaafar 2010.
| Trial name or title | A double‐blind, randomized, placebo‐controlled phase III intergroup study of gefitinib (G) in patients (pts) with advanced NSCLC, non‐progressing after first‐line platinum‐based chemotherapy (EORTC 08021‐ILCP 01/03) |
| Methods | Randomised |
| Participants | Advanced NSCLC |
| Interventions | Gefitinib 250 mg daily versus placebo |
| Outcomes | Overall survival Progression‐free survival Toxicity |
| Starting date | Not known |
| Contact information | Not known |
| Notes | — |
Hong 2010.
| Trial name or title | Randomized phase II study of pemetrexed versus gefitinib for patients with previously treated non‐small cell lung cancer |
| Methods | Randomised |
| Participants | Patients with histologically or cytologically confirmed advanced (stage IIIB or IV) or recurrent NSCLC were eligible if they were; age > 18 years, with measurable lesion, previously treated, an Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0 to 2, and with adequate organ function |
| Interventions | 500 mg/m2 of pemetrexed intravenously every 3 weeks with vitamin supplementation versus gefitinib 250 mg/day until disease progression, intolerable toxicity or withdrawal of consent |
| Outcomes | Tumour response PFS OS Toxicity |
| Starting date | Not known |
| Contact information | Not known |
| Notes | — |
Laurie 2000.
| Trial name or title | Pilot trial of ZD1839 (Iressa‐TM‐), an oral inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase, in combination with carboplatin (C) and paclitaxel (P) in previously untreated advanced non‐small cell lung cancer |
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Starting date | Not known |
| Contact information | Not known |
| Notes | — |
Lee 2013.
| Trial name or title | Randomized phase II study comparing paclitaxel/carboplatin intercalated with gefitinib to paclitaxel/carboplatin alone for chemotherapy‐naive non‐small cell lung cancer patients either with history of smoking or with wild‐type EGFR |
| Methods | Randomised |
| Participants | Chemotherapy‐naive advanced NSCLC patients with good ECOG PS of 0 or 1 |
| Interventions | PCG arm: P 175 mg/m2 and C AUC 5 intravenously on day 1 intercalated with G 250 mg orally on days 2 through 15 every 3 weeks for 4 cycles followed by G 250 mg orally until progressive disease PC arm: P 175 mg/m2 and C AUC 5 on day 1 every 3 weeks for 4 cycles only without maintenance therapy |
| Outcomes | Tumour response PFS OS Toxicity |
| Starting date | Not known |
| Contact information | Not known |
| Notes | — |
Liang 2010.
| Trial name or title | First‐line treatment (txt) with pemetrexed‐cisplatin (PC), followed sequentially by gefitinib (G) or pemetrexed, in Asian, never‐smoker (n/smkr) patients (pts) with advanced NSCLC: an open‐label, randomized phase II trial |
| Methods | Randomised |
| Participants | Advanced NSCLC Asian, chemotherapy‐naive, non‐smoker |
| Interventions | First‐line PC + TXT followed by gefitinib 250 mg daily versus placebo |
| Outcomes | Progression‐free survival Response rate Toxicities |
| Starting date | February 2007 |
| Contact information | Not known |
| Notes | — |
Nokihara 2006.
| Trial name or title | A randomized phase II study of sequential carboplatin/paclitaxel (CP) and gefitinib (G) in chemotherapy‐naive patients with advanced non‐small‐cell lung cancer (NSCLC): preliminary results |
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Starting date | Not known |
| Contact information | Not known |
| Notes | — |
Puri 2013.
| Trial name or title | A randomized phase 2 trial of pemetrexed (P) and gefitinib (G) versus G as first‐line treatment for patients with stage IV non‐squamous (NS) non‐small cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations |
| Methods | Randomised |
| Participants | Stage IV NS NSCLC, an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 1 and an activating EGFR mutation |
| Interventions | Gefitinib versus pemetrexed |
| Outcomes | PFS Time to progressive disease OS ORR DCR Adverse events |
| Starting date | Not known |
| Contact information | Not known |
| Notes | — |
AUC: Area under curve C: carboplatin DCR: disease control rate ECOG: Eastern Cooperative Oncology Group ECOG PS: ECOG Performance Status EGFR: epidermal growth factor receptor G: gefitinib NSCLC: non‐small cell lung cancer ORR: overall response rate OS: overall survival P: paclitaxel PC: pemetrexed‐cisplatin PFS: progression‐free survival TXT: first‐line treatment
Differences between protocol and review
The text of the protocol section of the review has been updated to included new subheadings.
We have added toxicity to the list of our primary outcomes.
We have included three 'Summary of findings' tables giving overall survival and progression‐free survival for gefitinib compared to chemotherapy for first‐line treatment of advanced NSCLC, gefitinib compared to chemotherapy for second‐line treatment of advanced NSCLC and the toxicity of gefitinib compared to chemotherapy for advanced NSCLC.
Contributions of authors
All authors contributed to the design and methodology of this review. Esther Sim assessed the trials for inclusion, extracted data on included trials, entered data to RevMan, undertook the analysis and wrote the review. Ian Yang independently assessed the trials for inclusion, independently extracted data from included trials, checked the analysis and contributed to writing the review. Rayleen Bowman, Kwun Fong and Richard Wood‐Baker critically commented on the review and provided advice for improving the review.
Sources of support
Internal sources
No sources of support supplied
External sources
The Lung Foundation (Australia)/Lung Cancer Consultative Group Cochrane Review Scholarship (ES), Australia.
Lung Foundation (Australia) Lung Consultative Group (IY, KF, RB), Australia.
National Health and Medical Research Council (IY, KF, RB, RWB), Australia.
Declarations of interest
Esther HA Sim (ES): none known
Ian A Yang (IY): none known
Rayleen V Bowman (RB) has received pharmaceutical company sponsored items, meals and travel expenses associated with attendance at scientific meetings including Australian Lung Cancer Conference and Thoracic Society of Australia and New Zealand. RB is a current member of the Lung Foundation (Australia)'s Lung Cancer Consultative Group, which receives financial sponsorship from a number of pharmaceutical companies.
Kwun M Fong (KF) was an investigator for a clinical trial of gefitinib for lung cancer (Astra Zeneca international trial) ‐ funding received by the Hospital funds the clinical trial and the employment of a trials nurse. KF's laboratory has also undertaken contract research for a clinical study looking at immunohistochemistry of certain proteins in lung cancer for Novartis ‐ funding received will go to the Project and employment of Research staff for the Project. KF was previously offered an honorarium from a pharmaceutical company for attending an one‐off Advisory Board Meeting; this was not accepted and asked to be given to a charity. KF has received occasional pens, pads and minor stationery from industry. KF has occasionally attended/spoken at meetings organised by pharmaceutical companies where meals/travel costs would be sponsored. KF has organised the Queensland Lung Cancer Interest Group Meeting (two to three meetings per year, a teleconference meeting, which is supported by Eli Lilly. KF has/is also involved in organising and attending professional meetings including those run by the Thoracic Society of Australia and New Zealand, Asia‐Pacific Society of Respirology, Australian Lung Cancer Conference, IASLC, where some sponsorship is usually provided by industry. KF is involved with the Lung Foundation (Australia)'s Lung Cancer Cooperative Group (not‐for‐profit, public benevolent institution) (http://www.lungnet.org/www.lungnet.org.au) and its activities, which includes promotion of Cochrane Reviews. The LFA receives support from pharmaceutical companies. KF was Chair of the Australian Lung Cancer trials Group, which receives some support funding from pharmaceutical companies.
Richard Wood‐Baker (RWB): none known
New
References
References to studies included in this review
Ahn 2012 {published data only}
- Ahn MJ, Yang JCH, Liang J, Kang JH, Xiu Q, Chen YM, et al. Randomized phase II trial of first‐line treatment with pemetrexed‐cisplatin, followed sequentially by gefitinib or pemetrexed, in East Asian, never‐smoker patients with advanced non‐small cell lung cancer. Lung Cancer 2012;77:346‐52. [DOI] [PubMed] [Google Scholar]
An 2016 {published data only}
- An C, Zhang J, Chu H, Gu C, Xiao F, Zhu F, et al. Study of gefitinib and pemetrexed as first‐line treatment in patients with advanced non‐small cell lung cancer harboring EGFR mutation. Pathology and Oncology Research 2016;22:763‐8. [DOI] [PubMed] [Google Scholar]
Chen 2007 {published data only}
- Chen YM, Liu JM, Chou TY, Perng RP, Tsai CM, Whang‐Peng J. Phase II randomized study of daily gefitinib treatment alone or with vinorelbine every 2 weeks in patients with adenocarcinoma of the lung who failed at least 2 regimens of chemotherapy. Cancer 2007;109(9):1821‐8. [DOI] [PubMed] [Google Scholar]
Chen 2011 {published data only}
- Chen YM, Fan WC, Tsai CM, Liu SH, Shih JF, Chou TY, et al. A phase II randomized trial of gefitinib alone or with Tegafur/uracil treatment in patients with pulmonary adenocarcinoma who had failed previous chemotherapy. Journal of Thoracic Oncology 2011;6:1110‐6. [DOI] [PubMed] [Google Scholar]
Cheng 2016 {published data only}
- Cheng Y, Murakami H, Yang PC, He J, Nakagawa K, Kang JH, et al. Randomized phase II trial of gefitinib with and without pemetrexed as first‐line therapy in patients with advanced nonsquamous non‐small‐cell lung cancer with activating epidermal growth factor receptor mutations. Journal of Clinical Oncology 2016;27(20):3258‐66. [DOI] [PubMed] [Google Scholar]
Crino 2008 INVITE {published data only}
- Crino L, Cappuzzo F, Zatloukal P, Reck M, Pesek M, Thompson JC, et al. Gefitinib versus vinorelbine in chemotherapy‐naive elderly patients with advanced non‐small‐cell lung cancer (INVITE): a randomized, phase II study. Journal of Clinical Oncology 2008;26(26):4253‐60. [DOI] [PubMed] [Google Scholar]
Cufer 2006 SIGN {published data only}
- Cufer T, Vrdoljak E, Gaafar R, Erensoy I, Pemberton K, SIGN study group. Phase II, open‐label, randomized study (SIGN) or single‐agent gefitinib (IRESSA) or docetaxel as second‐line therapy in patients with advanced (stage IIIb or IV) non‐small‐cell lung cancer. Anticancer Drugs 2006;17(4):401‐9. [DOI] [PubMed] [Google Scholar]
Dai 2013 {published data only}
- Dai H, Xu L, Xia C, Chen W. A randomized clinical study of gefitinib and pemetrexed as second line therapy for advanced non‐squamous non‐small cell lung cancer. Chinese Journal of Lung Cancer 2013;16:405‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fukuoka 2003 IDEAL I {published data only}
- Baelga J, Kris M, Yano S, Natale R, Giaccone G, Brahmer J, et al. Phase II trials (IDEAL 1 and IDEAL 2) of ZD1839 in advanced or metastatic non‐small cell lung cancer (NSCLC) patient. Annals of Oncology 2002;13(Suppl 5):131 Abs 481. [Google Scholar]
- Douillard JY, Giaccone G, Horai T, Noda K, Vansteenkiste JF, Takata I, et al. Improvement in disease‐related symptoms and quality of life in patients with advanced non‐small cell lung cancer (NSCLC) treated with ZD1839 ('Iressa') (IDEAL 1) [abstract]. 38th Annual Meeting of the American Society of Clinical Oncology; 18‐21 May 2002; Orlando, Florida, USA. 2002; Vol. 21 Pt 1:299a, Abs 1195.
- Douillard JY, Skarin A, Baselga J, Natale R, Giaccone G, Maddox AM, et al. Improvement in disease‐related symptoms and quality of life (QOL) for advanced non‐small cell lung cancer (NSCLC) patients treated with ZD1839 in IDEAL 1 and IDEAL 2. Annals of Oncology 2002;13 Suppl 5:131, Abs 480. [Google Scholar]
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi‐institutional randomized phase II trial of gefitinib for previously treated patients with advanced non‐small‐cell lung cancer. Journal of Clinical Oncology 2003;21(12):2237‐46. [DOI] [PubMed] [Google Scholar]
- Fukuoka M, Yano S, Giaccone G, et al. [Final results from a phase II trial of ZD1839 ('Iressa') for patients with advanced non‐small cell lung cancer (IDEAL 1) [abstract]]. 38th Annual Meeting of the American Society of Clinical Oncology; 18‐21 May 2002; Orlando, Florida, USA. 2002; Vol. 21 Pt 1:298a, Abs 1188.
- Manegold C, Gatzemeier U, Kaukel E. Results from a randomised, double blind phase II trial of ZD1839 (IRESSA) as 2nd/3rd‐line monotherapy in advanced non small cell lung cancer (NSCLC) (IDEAL 1). Journal of Cancer Research & Clinical Oncology 2002;128 Suppl 1:S45. [Google Scholar]
- Nishiwaki Y, Yano S, Tamura T, Nakagawa K, Kudoh S, Horai T, et al. Subset analysis of data in the Japanese patients with NSCLC from IDEAL I study on gefitinib. Gan to Kagaku Ryoho. Cancer & Chemotherapy 2004;31(4):567‐73. [PubMed] [Google Scholar]
- Vansteenkiste J, Natale R, Giaccone G, et al. Two randomised, double‐blind studies of ZD1839 in 425 patients with pretreated advanced non‐small‐call lung cancer (IDEAL 1 and IDEAL 2) [abstract]. European Respiratory Society Annual Congress. Stockholm, 2002:Abstract 2537.
Gaafar 2011 EORTC08021 {published data only}
- Gaafar R M, Surmont V, Scagliotti G, Klaveren R, Papamichael D, Welch J, et al. A double‐blind, randomized, placebo‐controlled phase III intergroup study of gefitinib (G) in patients (pts) with advanced NSCLC, non‐progressing after first‐line platinum‐based chemotherapy (EORTC 08021‐ILCP 01/03). 2010 Annual Meeting of the Americal Society of CLinical Oncology, ASCO, Chicago, IL United States. American Society of Clinical Oncology, 2010; Vol. 28.
- Gaafar RM, Surmont VF, Scagliotti GV, Klaveren RJ, Papamichael D, Welch JJ, et al. A double‐blind, randomised, placebo‐controlled phase III intergroup study of gefitinib in patients with advanced NSCLC, non‐progressing after first line platinum‐based chemotherapy (EORTC 08021/ILCP 01/03). European Journal of Cancer 2011;47:2331‐40. [DOI] [PubMed] [Google Scholar]
- Surmont VF, Gaafar RM, Scagliotti GV, Klaveren RJ, Papamichael D, Hasan B, et al. A double‐blind, randomized, placebo‐controlled phase III study of gefitinib (G) versus placebo (P) in patients (pts) with advanced NSCLC, non progressing after first‐line platinum‐based chemotherapy (EORTC 08021‐ ILCP). 35th ESMO Congress Milan Italy. Oxford University Press, 2010; Vol. 21:viii124.
Giaccone 2004 INTACT I {published data only}
- Bell DW, Lynch TJ, Maserlat SM, Harris PL, Okimoto FA, Brannigan BW, et al. Epidermal growth factor receptor mutations and gene amplification in non‐small‐cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. Journal of Clinical Oncology 2005;23(31):8081‐92. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non‐small cell lung cancer: a phase III trial ‐ INTACT I. Journal of Clinical Oncology 2004;22(5):777‐84. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Johnson DH, Manegold C, et al. A phase III clinical trial of ZD1839 ('Iressa') in combination with gemcitabine and cisplatin in chemotherapy‐naive patients with advanced non‐small cell lung cancer (INTACT 1). Annals of Oncology 2002;13 Suppl 5:2, Abstract 4. [Google Scholar]
Goss 2009 INSTEP {published data only}
- Goss G, Ferry D, Wierzibicki R, Laurie SA, Thompson J, Biesma B, et al. Randomized phase II study of gefitinib compared with placebo in chemotherapy‐naive patients with advanced non‐small‐cell lung cancer and poor performance status. Journal of Clinical Oncology 2009;27(13):2253‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2012 First SIGNAL {published data only}
- Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First‐SIGNAL: first‐line single‐agent Iressa versus gemcitabine and cisplatin trial in never‐smokers with adenocarcinoma of the lung. Journal of Clinical Oncology 2012;30:1122‐8. [DOI] [PubMed] [Google Scholar]
- Han JY, Yoon KA, Park JH, Lee YJ, Lee GK, Han JH, et al. DNA repair gene polymorphisms and benefit from gefitinib in never‐smokers with lung adenocarcinoma. Cancer 2011;117(14):3201‐8. [DOI] [PubMed] [Google Scholar]
Herbst 2004 INTACT II {published data only}
- Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non‐small cell lung cancer: a phase III trial ‐ INTACT 2. Journal of Clinical Oncology 2004;22(5):785‐94. [DOI] [PubMed] [Google Scholar]
- Johnson DH, Herbst R, Giaccone G, et al. ZD1839 ('Iressa') in combination with paclitaxel & carboplatin in chemotherapy‐naive patients with advanced non‐small cell lung cancer (NSCLC): results from a phase III clinical trial (INTACT 2). Annals of Oncology 2002;13 Suppl 5:127, Abstract 468. [Google Scholar]
Kelly 2008 SWOG S0023 {published data only}
- Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, Ung YC, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non‐small‐cell lung cancer: SWOG S0023. Journal of Clinical Oncology 2008;26(15):2450‐6. [SWOG S0023] [DOI] [PubMed] [Google Scholar]
- Kelly K, Gaspar LE, Chansky K, Albain KS, Crowley J, Gandara DR. Low incidence of pneumonitis on SWOG 0023: A preliminary analysis of an ongoing phase III trial of concurrent chemoradiotherapy followed by consolidation docetaxel and Iressa/placebo maintenance in patients with inoperable stage III non‐small cell lung cancer [abstract]. 41st Annual Meeting of the American Society of Clinical Oncology, Orlando, FL, 13‐17 May, 2005 (Abstract No. 7058). 2005; Vol. 23:634.
Kim 2008 INTEREST {published data only}
- Douillard JY, Shephard FA, Hirsh V, Mok T, Socinski MA, Gervais R, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non‐small‐cell lung cancer: from the randomized phase III INTEREST Trial. Journal of Clinical Oncology 2010;28(5):744‐52. [DOI] [PubMed] [Google Scholar]
- Horgan AM, Bradbury PA, Amir E, Ng R, Douillard JY, Kim ES, et al. An economic analysis of the INTEREST trial, a randomized trial of docetaxel versus gefitinib as second‐/third‐line therapy in advanced non‐small‐cell lung cancer. Annals of Oncology. United Kingdom: Oxford University Press (Great Clarendon Street, Oxford OX2 6DP, United Kingdom), 2011; Vol. 22:1805‐11. [DOI] [PubMed]
- Horgan AM, Shepherd FA, Bradbury PA, Ng R, Leighl NB. Preliminary cost‐consequence analysis of the INTEREST trial, a randomized trial of docetaxel versus gefitinib as 2nd line therapy in advanced non‐small cell lung cancer [abstract no. 8110]. Journal of Clinical Oncology: ASCO Annual Meeting Proceedings 2008;25(15 Suppl Pt 1):451. [Google Scholar]
- Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non‐small‐cell lung cancer (INTEREST): a randomized phase III trial. Lancet 2008;372:1809‐18. [DOI] [PubMed] [Google Scholar]
Kim 2016 {published data only}
- Kim YS, Cho EK, Woo HS, Hong J, Ahn HK, Park I, et al. Randomized phase II study of pemetrexed versus gefitinib in previously treated patients with advanced non‐small cell lung cancer. Cancer Research and Treatment 2016;48(1):80‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kris 2003 IDEAL II {published data only}
- Cella D, Herbst RS, Lynch TJ, Prager D, Belani CP, Schiller JH, et al. Clinically meaningful improvement in symptoms and quality of life for patients with non‐small cell lung cancer receiving gefitinib in a randomized controlled trial. Journal of Clinical Oncology 2005;23(13):2946‐54. [DOI] [PubMed] [Google Scholar]
- Kris MG, Natale RB, Herbst RS, et al. A phase II trial of ZD1839 ('Iressa') in advanced non‐small cell lung cancer (NSCLC) patients who had failed platinum‐ and docetaxel‐based regimens (IDEAL 2) [abstract]. 38th Annual Meeting of the American Society of Clinical Oncology; 18‐21 May 2002; Orlando, Florida, USA. 2002; Vol. 21 Pt 1:292a Abstract 1166.
- Kris MG, Natale RB, Herbst RS, Lynch TJ, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non‐small cell lung cancer: a randomized trial. JAMA 2003;290(16):2149‐58. [DOI] [PubMed] [Google Scholar]
- Natale RB, Skarin A, Maddox AM, et al. Improvement in symptoms and quality of life for advanced non‐small cell lung cancer patients receiving ZD1839 ('Iressa') in IDEAL 2 [abstract]. 38th Annual Meeting of the American Society of Clinical Oncology; 18‐21 May 2002; Orlando, Florida, USA. 2002; Vol. 21 Pt 1:292a, Abstract 1167.
Lee 2010 ISTANA {published data only}
- Lee D, Kim S, Park K, et al. A randomized open‐label study of gefitinib versus docetaxel in patients with advanced/metastatic non‐small cell lung cancer (NSCLC) who have previously received platinum‐based chemotherapy [abstract no. 8025]. Journal of Clinical Oncology: ASCO Annual Meeting Proceedings 2008;26(15 Suppl Pt I):430. [Google Scholar]
- Lee DH, Park K, Kim JH, Lee JS, Shin SW, Kang JH, et al. Randomized phase III trial of gefitinib versus docetaxel in non‐small‐cell lung cancer patients who have previously received platinum‐based chemotherapy. Clinical Cancer Research 2010;16(4):1307‐14. [ISTANA] [DOI] [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li H, Wang X, Hua F. Second‐line treatment with gefitinib or docetaxel for advanced non‐small cell lung cancer. Chinese Journal of Clinical Oncology 2010;37:16‐8. [Google Scholar]
Lou 2014 {published data only}
- Lou N, Yang J, Yan H, Qing Z, Liao R, Xu C, et al. Efficacies of gefitinib versus paclitaxel/carboplatin for patients with advanced pulmonary adenocarcinoma. National Medical Journal of China 2014;94(30):2337‐41. [PubMed] [Google Scholar]
Maemondo 2010 NEJ002 {published data only}
- Fukuhara T, Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, et al. Factors associated with a poor response to gefitinib in the NEJ002 study: smoking and the L858R mutation. Lung Cancer 2015;88:181‐6. [DOI] [PubMed] [Google Scholar]
- Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin‐paclitaxel for chemo‐naive non‐small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Annals of Oncology. United Kingdom: Oxford University Press (Great Clarendon Street, Oxford OX2 6DP, United Kingdom), 2013; Vol. 24:54‐9. [DOI] [PubMed]
- Inoue A, Kobayashi K, Maemondo M, et al. A randomized phase III study comparing gefitinib with carboplatin (CBDCA) plus paclitaxel (TXL) for the first‐line treatment of non‐small cell lung cancer (NSCLC) with sensitive EGFR mutations: NEJ002 study. European Journal of Cancer. 2009:Abstract 9LBA.
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. New England Journal of Medicine 2010;362(25):2380‐8. [DOI] [PubMed] [Google Scholar]
- Miyauchi E, Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, et al. Efficacy of chemotherapy after first‐line gefitinib therapy in EGFR mutation‐positive advanced non‐small cell lung cancer ‐ data from a randomized phase III study comparing gefitinib with carboplatin plus paclitaxel (NEJ002). Japanese Journal of Clinical Oncology 2015;45(7):670‐6. [DOI] [PubMed] [Google Scholar]
- Oizumi S, Kobayashi K, Inoue A, Maemondo M, Sugawara S, Yoshizawa H, et al. Quality of life with gefitinib in patients with EGFR‐mutated non‐small cell lung cancer: quality of life analysis of North East Japan study group 002 trial. Oncologist. United States: AlphaMed Press (318 Blackwell St. Suite 260, Durham NC 27701‐2884, United States), 2012; Vol. 17:863‐70. [DOI] [PMC free article] [PubMed]
- Yoshizawa H, Kobayashi K, Inoue A, Maemondo M, Sugawara S, Oizumi S, et al. QOL analysis from NEJ 002 study comparing gefitinib to chemotherapy for non‐small cell lung cancer with mutated EGFR. 35th ESMO Congress Milan Italy. Oxford University Press, 2010; Vol. 21:viii122.
Maruyama 2008 V‐15‐32 {published data only}
- Leki R, Kawahara M, Watanabe H, et al. The impact of response evaluation committee in a Phase III study (V‐15‐32) of gefitinib versus docetaxel in Japanese patients with non‐small cell lung cancer [Abstract No. 298P]. Annals of Oncology 2009;19 Suppl 8:109‐10. [Google Scholar]
- Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, et al. Phase III study, V‐15‐32, of gefitinib versus docetaxel in previously treated Japanese patients with non‐small‐cell lung cancer. Journal of Clinical Oncology 2008;26(26):4245‐52. [DOI] [PubMed] [Google Scholar]
- Sekine I, Ichinose Y, Nishiwaki Y, Yamamoto N, Tsuboi M, Nakagawa K, et al. Quality of life and disease‐related symptoms in previously treated Japanese patients with non‐small‐cell lung cancer: results of a randomized phase III study (V‐15‐32) of gefitinib versus docetaxel. Annals of Oncology 2009;20(9):1483‐8. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Nishiwaki Y, Negoro S, Jiang H, Itoh Y, Saijo N, et al. Disease control as a predictor of survival with gefitinib and docetaxel in a phase III study (V‐15‐32) in advanced non‐small‐cell lung cancer patients. Journal of Thoracic Oncology 2010;5(7):1042‐7. [DOI] [PubMed] [Google Scholar]
Mitsudomi 2010 WJTOG3405 {published data only}
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Seto T, et al. Updated overall survival results of WJTOG 3405, a randomized phase III trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first‐line treatment for patients with non‐small cell lung cancer harboring mutations of the epidermal growth factor receptor (EGFR). 2012 Annual Meeting of the American Society of Clinical Oncology, ASCO Chicago, IL United States. American Society of Clinical Oncology, 2012; Vol. 30.
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase III trial. Lancet 2010;11:121‐8. [DOI] [PubMed] [Google Scholar]
- Tsurutani J, Mitsudomi T, Mori S, Okamoto I, Nozaki K, Tada H, et al. A phase III, first‐line trial of gefitinib versus cisplatin plus docetaxel for patients with advanced or recurrent non‐small cell lung cancer (NSCLC) harboring activating mutation of the epidermal growth factor receptor (EGFR) gene: a preliminary results of WJTOG 3405 [abstract O‐9002]. European Journal of Cancer. 2009; Vol. 7 Suppl:505.
Mok 2009 IPASS {published data only}
- Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open‐label, first‐line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non ‐ small‐cell lung cancer in Asia (IPASS). Journal of Clinical Oncology 2011;29:2866‐74. [DOI] [PubMed] [Google Scholar]
- Mok T, Wu Y‐L, Thongprasert S, et al. Phase III, randomised, open‐label, first‐line study of gefitinib (G) vs carboplatin/paclitaxel (C/P) in clinically selected patients (PTS) with advanced non‐small cell lung cancer (NSCLC) (IPASS). Annals of Oncology 2008;19 Suppl 8:Viii1. [Google Scholar]
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. New England Journal of Medicine 2009;361(10):947‐57. [DOI] [PubMed] [Google Scholar]
- Ohe Y, Ichinose Y, Nishiwaki Y, et al. Phase III, randomized, open‐label, first‐line study of gefitinib (G) versus carboplatin/paclitaxel (C/P) in selected patients (pts) with advanced non‐small‐cell lung cancer (NSCLC) (IPASS): Evaluation of recruits in Japan. Journal of Clinical Oncology 2009;29(15 Suppl 1):8044. [Google Scholar]
- Thongprasert S, Duffield E, Saijo N, Wu YL, Yang JC, Chu DT, et al. Health‐related quality‐of‐life in a randomized phase III first‐line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS). Journal of Thoracic Oncology 2011;6:1872‐80. [DOI] [PubMed] [Google Scholar]
- Thongprasert S, Duffield E, Wu Y, et al. Quality of life (QOL) in a randomized phase III first‐line study of gefitinib (G) vs carboplatin/paclitaxel (CP) in clinically selected Asian patients (pts) with advanced NSCLC (IPASS). Journal of Thoracic Oncology 2010;5(5 Suppl 1):S80. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chu D, Han B, Liu X, Zhang L, Zhou C, et al. Phase III, randomized, open‐label, first‐line study in Asia of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non‐small‐cell lung cancer: evaluation of patients recruited from mainland China. Asia‐Pacific Journal of Clinical Oncology 2012;8:232‐43. [DOI] [PubMed] [Google Scholar]
- Wu Y, Fukuoka M, Mok T S K, Saijo N, Thongprasert S, Yang J C H, et al. Tumour response, skin rash and health‐related quality of life (HRQOL) ‐ Post‐HOC data from the IPASS study. European Multidisciplinary Cancer Congress Stockholm Sweden. Elsevier Ltd, 2011; Vol. 47:S633‐4.
- Wu Y, Mok T, Chu D, et al. Evaluation of clinically selected patients (pts) with advanced non‐small cell lung cancer (NSCLC) recruited in China in a phase III, randomized, open‐label, first‐line study in Asia of gefitinib (G) versus carboplatin/paclitaxel (C/P) (IPASS). Journal of Clinical Oncology. 2009; Vol. 27 (15 Suppl 1):8041.
- Wu YL, Fukuoka M, Mok TSK, Saijo N, Thongprasert S, Yang JCH, et al. Tumor response and health‐related quality of life in clinically selected patients from Asia with advanced non‐small‐cell lung cancer treated with first‐line gefitinib: post hoc analyses from the IPASS study. Lung Cancer 2013;81:280‐7. [DOI] [PubMed] [Google Scholar]
- Yang CH, Fukuoka M, Mok TS, Wu YL, Thongprasert S, Saijo N, et al. Final overall survival (OS) results from a phase III, randomised, open‐label, first‐line study of gefitinib (G) v carboplatin/paclitaxel (C/P) in clinically selected patients with advanced nonsmall cell lung cancer (NSCLC) in Asia (IPASS). 35th ESMO Congress Milan Italy. Oxford University Press, 2010; Vol. 21:viii1‐2.
- Yang J, Wu Y L, Saijo N, Thongprasert S, Chu D T, Chen Y M, et al. Efficacy outcomes in first‐line treatment of advanced NSCLC with gefitinib (G) vs carboplatin/paclitaxel (C/P) by epidermal growth factor receptor (EGFR) gene‐copy number score and by most common EGFR mutation subtypes ‐ Exploratory data from IPASS. 2011 European Multidisciplinary Cancer Congress Stockholm Sweden. Elsevier Ltd, 2011; Vol. 47:S633.
- Yang J, Wu Y, Chan V, Kurnianda J, Nakagawa K. Epidermal growth factor receptor mutation analysis in previously unanalyzed histology samples and cytology samples from the phase III Iressa Pan‐ASia Study (IPASS). Lung Cancer 2014;83:174‐81. [DOI] [PubMed] [Google Scholar]
- Yang JCH, Wu YL, Chan V, Kurnianda J, Nakagawa K, Saijo N, et al. Epidermal growth factor receptor mutation analysis in previously unanalyzed histology samples and cytology samples from the phase III Iressa Pan‐ASia Study (IPASS). Lung Cancer 2014;83:174‐81. [DOI] [PubMed] [Google Scholar]
Morere 2010 IFCT‐0301 {published data only}
- Guetz G, Landre T, Westeel V, Milleron B, Vaylet F, Urban T, et al. Similar survival rates with first‐line gefitinib, gemcitabine or docetaxel in a randomized phase II trial in elderly patients with advanced non‐small cell lung cancer and a poor performance status (IFCT‐0301). Journal of Geriatric Oncology 2015;6:233‐40. [DOI] [PubMed] [Google Scholar]
- Morere J, Westeel V, Morin F, et al. Randomized phase II trial of first‐line gefitinib,gemcitabine or docetaxel in performance status (PS) 2 or 3 non‐small‐cell lung cancer (NSCLC) patients (IFCT‐0301) [abstract no. 8086]. Journal of Clinical Oncology: ASCO Annual Meeting Proceedings 2008;26(15 Suppl Pt I):445. [Google Scholar]
- Morere JF, Brechot JM, Westeel V, Gounant V, Lebeau B, Vaylet F, et al. Randomized phase II trial of gefitinib or gemcitabine or docetaxel chemotherapy in patients with advanced non‐small‐cell lung cancer and a performance status of 2 or 3 (IFCT‐0301 study). Lung Cancer 2010;70(1):301‐7. [DOI] [PubMed] [Google Scholar]
Soria 2015 IMPRESS {published data only}
- Soria JC, Wu YL, Nakagawa K, Kim SW, Yang JJ, Ahn MJ, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR‐mutation‐positive non‐small‐cell lung cancer after progression on first‐line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncology 2015;16:990‐8. [DOI] [PubMed] [Google Scholar]
Sun 2012 KCSG‐LU08‐01 {published data only}
- Ahn M, Sun J, Ahn JS, Kim S, Min YJ, Yun HJ, et al. Randomized phase III trial of gefitinib or pemetrexed as second‐line treatment in patients with non‐small cell lung cancer previously treated with platinum‐based chemotherapy (KCSG‐LU08‐01). ASCO Annual Meeting 2011 Chicago, IL United States. American Society of Clinical Oncology, 2011; Vol. 29.
- Ahn MJ, Sun JM, Lee KH, Ahn JS, Kim SW, Min YJ, et al. Randomized phase III trial of gefitinib or pemetrexed as second‐line treatment in patients with non‐small cell lung cancer previously treated with platinum‐based chemotherapy (KCSG‐LU08‐01). 14th World Conference on Lung Cancer Amsterdam Netherlands. International Association for the Study of Lung Cancer, 2011; Vol. 6:S317.
- Sun JM, Lee KH, Kim SW, Lee DH, Min YJ, Yun HJ, et al. Gefitinib versus pemetrexed as second‐line treatment in patients with nonsmall cell lung cancer previously treated with platinum‐based chemotherapy (KCSG‐LU08‐01): an open‐label, phase 3 trial. Cancer 2012;118:6234‐42. [DOI] [PubMed] [Google Scholar]
Takeda 2010 WJTOG0203 {published data only}
- Okamoto I, Hida T, Kashii T, et al. Randomized phase III study of platinum‐doublet chemotherapy followed by gefitinib versus continued platinum‐doublet chemotherapy in patients (pts) with advanced non‐small cell lung cancer (NSCLC): Results of West Japan Thoracic Oncology Group Trial (WJTOG0203) [Abstract No. 2 33PD]. Annals of Oncology 2008;19 Suppl 8:91. [Google Scholar]
- Takeda K, Hida T, Sato T, Ando M, Seto T, Satouchi M, et al. Randomized phase III trial of platinum‐doublet chemotherapy followed by gefitinib comparted with continued platinum‐doublet chemotherapy in Japanese patients with advanced non‐small cell lung cancer: results of a West Japan Thoracic Oncology Group Trial (WJTOG0203). Journal of Clinical Oncology 2010;28(5):753‐60. [DOI] [PubMed] [Google Scholar]
Thatcher 2005 ISEL {published data only}
- Chang A, Parikh P, Thongprasert S, Tan EH, Perng RP, Ganzon D, et al. Gefitinib (IRESSA) in patients of Asian origin with refractory advanced non‐small cell lung cancer: subset analysis from the ISEL study. Journal of Thoracic Oncology 2006;1(8):847‐55. [PubMed] [Google Scholar]
- Hirsch FR, Dziadziuszko R, Thatcher N, Mann H, Watkins C, Parums DV, et al. Epidermal growth factor receptor immunohistochemistry: comparison of antibodies and cutoff points to predict benefit from gefitinib in a phase 3 placebo‐controlled study in advanced non small‐cell lung cancer. Cancer 2008;112(5):1114‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch FR, Varella‐Garcia M, Bunn Jr PA, Franklin WA, Dziadziuszko R, Thatcher N, et al. Molecular predictors of outcome with gefitinib in a phase III placebo‐controlled study in advanced non‐small cell lung cancer. Journal of Clinical Oncology 2006;24(31):5034‐42. [DOI] [PubMed] [Google Scholar]
- Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non‐small‐cell lung cancer: results from a randomised, placebo‐controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366(9496):1527‐37. [DOI] [PubMed] [Google Scholar]
Xu 2015 {published data only}
- Xu YH, Mei JS, Zhou J. Randomized study of gefitinib versus pemetrexed as maintenance treatment in patients with advanced glandular non‐small cell lung cancer. International Journal of Clinical and Experimental Medicine 2015;8(4):6242‐6. [PMC free article] [PubMed] [Google Scholar]
Xue 2015 {published data only}
- Xue C, Hong S, Li N, Feng W, Jia J, Peng J, et al. Randomized, multicenter study of gefitinib dose‐escalation in advanced non‐small‐cell lung cancer patients achieved stable disease after one‐month gefitinib treatment. Scientific Reports 2015;5:10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Xue C, Li N, Feng W, Jia J, Peng J, et al. Randomized, open‐label, multi‐center study of gefitinib dose‐escalation (500mg/d versus 250mg/d) in advanced NSCLC patient achieved stable disease (SD) after one‐month gefitinib treatment. 15th World Conference on Lung Cancer Sydney, NSW Australia. International Association for the Study of Lung Cancer, 2013; Vol. 8:S1185.
Yang 2014 {published data only}
- Boye M, Wang X, Srimuninnimit V, Kang JH, Tsai CM, Orlando M, et al. First‐line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian never‐smoker patients with locally advanced or metastatic nonsquamous non‐small cell lung cancer: quality of life results from a randomized phase III trial. Clinical Lung Cancer 2016;17(2):150‐60. [DOI] [PubMed] [Google Scholar]
- Kang JH, Ahn M, Kim D, Cho EK, Kim J, Shin SW, et al. Tolerability and outcomes of first‐line pemetrexed‐cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in Korean patients with advanced non‐squamous non‐small cell lung cancer: a post hoc descriptive subgroup analysis of a randomized, phase 3 trial. Cancer Research and Treatment 2015;48(2):458‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JC, Kang JH, Mok T, Ahn M, Srimuninnimit V, Lin C, et al. First‐line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian patients with locally advanced or metastatic non‐squamous non‐small cell lung cancer: a randomised, phase 3 trial. European Journal of Cancer 2014;50:2219‐30. [DOI] [PubMed] [Google Scholar]
- Yang JC, Park K, Mok TSK, Kang JH, Srimuninnimit V, Lin CC, et al. A randomized phase 3 study comparing first‐line pemetrexed plus cisplatin followed by gefitinib as maintenance with gefitinib monotherapy in east Asian patients with locally advanced or metastatic nonsquamous non‐small cell lung cancer (NSQNSCLC). 15th World Conference on Lung Cancer Sydney, NSW Australia. International Association for the Study of Lung Cancer, 2013; Vol. 8:S288.
- Yang JC, Srimuninnimit V, Ahn M, Lin C, Kim S, Tsai C, et al. A randomized phase 3 study comparing first‐line pemetrexed plus cisplatin followed by gefitinib maintenance (PC/G) with gefitinib monotherapy (G) in East Asian patients (pts) with locally advanced or metastatic nonsquamous non‐small cell lung cancer (nSqNSCLC): final survival results. Journal of Clinical Oncology 2015;15 Suppl:8041. [Google Scholar]
- Yang JC, Srimunninnimit V, Ahn M, Lin C, Kim S, Tsai C, et al. First‐line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in East Asian never‐smoker patients with locally advanced or metastatic nonsquamous non‐small cell lung cancer: final overall survival results from a randomized phase 3 study. Journal of Thoracic Oncology 2016;11(3):370‐9. [DOI] [PubMed] [Google Scholar]
Yu 2014 {published data only}
- Yu H, Zhang J, Wu X, Luo Z, Wang H, Sun S, et al. A phase II randomized trial evaluating gefitinib intercalated with pemetrexed/platinum chemotherapy or pemetrexed/platinum chemotherapy alone in unselected patients with advanced non‐squamous non‐small cell lung cancer. Cancer Biology and Therapy 2014;15(7):832‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2012 INFORM {published data only}
- Yang Y, Ma Y, Zhao Y, Fang W, Hong S, et al. QoL analyses from INFORM study, a phase III study of gefitinib versus placebo as maintenance therapy in advanced NSCLC. Scientific Reports 2015;5:11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ma S, Song X, Han B, Cheng Y, Huang C, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non‐small‐cell lung cancer (INFORM; C‐TONG 0804): a multicentre, double‐blind randomised phase 3 trial. Lancet Oncology 2012;13:466‐75. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma SL, Song XQ, Cheng Y, Huang C, Yang SJ, et al. Pre‐planned subgroup analyses from the phase III, randomised, placebo‐controlled, parallel‐group study of gefitinib (G) as maintenance therapy in patients (pts) with locally advanced or metastatic non‐small‐cell lung cancer (NSCLC) (INFORM; C‐TONG 0804). European Multidisciplinary Cancer Congress Stockholm Sweden. Elsevier Ltd, 2011; Vol. 47:S622‐3.
- Zhang L, Ma SL, Song XQ, Han BH, Cheng Y, Huang C, et al. Efficacy, tolerability and biomarker analyses from a phase III, randomized, placebo controlled, parallel group study of gefitinib as maintenance therapy in patients with locally advanced or metastatic non‐small‐cell lung cancer (NSCLC) in China (INFORM) (C‐TONG 0804). 14th World Conference on Lung Cancer Amsterdam Netherlands. International Association for the Study of Lung Cancer, 2011; Vol. 6:S558‐9.
- Zhang L, Shenglin M, Song X, Han B, Cheng Y, Huang C, et al. Efficacy, tolerability, and biomarker analyses from a phase III, randomized, placebo‐controlled, parallel group study of gefitinib as maintenance therapy in patients with locally advanced or metastatic non‐small cell lung cancer (NSCLC; INFORM; C‐TONG 0804). ASCO Annual Meeting 2011 Chicago, IL United States. American Society of Clinical Oncology, 2011; Vol. 29.
- Zhao H, Fan Y, Ma S, Song X, Han B, Cheng Y, et al. Final overall survival results from a phase III, randomized placebo‐controlled, parallel‐group study of gefitinib versus placebo as maintenance therapy in patients with advanced or metastatic non‐small‐cell lung cancer (INFORM; C‐TONG 0804). Journal of Thoracic Oncology 2015;10(4):655‐64. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Choi 2015 {published data only}
- Choi YJ, Lee DH, Choi CM, Lee JS, Lee SJ, Ahn J, et al. Randomized phase II study of paclitaxel/carboplatin intercalated with gefitinib compared to paclitaxel/carboplatin alone for chemotherapy‐naive non‐small cell lung cancer in a clinically selected population excluding patients with non‐smoking adenocarcinoma or mutated EGFR. BMC Cancer 2015;15:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim ST, Uhm JE, Lee J, Sun JM, Sohn I, Kim SW, et al. Randomized phase II study of gefitinib versus erlotinib in patients with advanced non‐small cell lung cancer who failed previous chemotherapy. Lung Cancer 2012;75:82‐8. [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee KH, Lee KY, Jeon YJ, et al. A phase IV, multicenter, non‐randomized, open‐labelled study to evaluate the efficacy of gefitinib (Iressa®) as a second‐line therapy in Korean patients with non‐small cell lung cancer (NSCLC). Respirology. 2009; Vol. 14 Suppl S3:A161.
Manegold 2005 {published data only}
- Manegold C, Gatzemeier U, Buchholz E, Smith RP, Fandi A. A pilot trial of gefitinib in combination with docetaxel in patients with locally advanced or metastatic non‐small‐cell lung cancer. Clinical Lung Cancer 2005;6(6):343‐9. [DOI] [PubMed] [Google Scholar]
Natale 2009 {published data only}
- Natale RB, Bodkin D, Govindan R, Sleckman BG, Rizvi NA, Capo A, et al. Vandetanib versus gefitinib in patients with advanced non‐small‐cell lung cancer: results from a two‐part, double‐blind, randomized phase II study. Journal of Clinical Oncology 2009;27(15):2523‐9. [DOI] [PubMed] [Google Scholar]
Shi 2013 ICOGEN {published data only}
- Shi Y, Zhang L, Liu X, Zhou C, Zhang L, Zhang S, et al. Icotinib versus gefitinib in previously treated advanced non‐small‐cell lung cancer (ICOGEN): a randomised, double‐blind phase 3 non‐inferiority trial. Lancet Oncology 2013;14:953‐61. [DOI] [PubMed] [Google Scholar]
- Sun Y, Shi Y, Zhang L, Liu X, Zhou C, Li Z, et al. Final overall survival and updated biomarker analysis results from the randomized phase III ICOGEN trial. 2012 Annual Meeting of the American Society of Clinical Oncology, ASCO Chicago, IL United States. American Society of Clinical Oncology, 2012; Vol. 30.
- Sun Y, Shi Y, Zhang L, Liu X, Zhou C, Wang D, et al. A randomized, doubleblind phase III study of icotinib versus gefitinib in patients with advanced non‐small cell lung cancer (NSCLC) previously treated with chemotherapy (ICOGEN). 14th World Conference on Lung Cancer Amsterdam Netherlands. International Association for the Study of Lung Cancer, 2011; Vol. 6:S317‐8.
Sugawara 2015 {published data only}
- Sugawara S, Oizumi S, Minato K, Harada T, Inoue A, Fujita Y, et al. Randomized phase II study of concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated non‐small cell lung cancer with sensitive EGFR mutations: NEJ005/TCOG0902. Annals of Oncology 2015;26:888‐94. [DOI] [PubMed] [Google Scholar]
Urata 2016 {published data only}
- Urata Y, Katakami N, Morita S, Kaji R, Yoshioka H, Seto T, et al. Randomized phase III study comparing gefitinib with erlotinib in patients with previously treated advanced lung adenocarcinoma: WJOG 5108L. Journal of Clinical Oncology 2016;34(27):3248‐57. [DOI] [PubMed] [Google Scholar]
Zhou 2014 CTONG 0806 {published data only}
- Yang J, Cheng Y, Zhao M, Zhou Q, Yan HH, Zhang L, et al. A phase II trial comparing pemetrexed with gefitinib as the second‐line treatment of nonsquamous NSCLC patients with wild‐type EGFR (CTONG0806). 2013 Annual Meeting of the American Society of Clinical Oncology, ASCO Chicago, IL United States. American Society of Clinical Oncology, 2013; Vol. 31.
- Zhou Q, Cheng Y, Yang JJ, Zhao MF, Zhang L, Zhang XC, et al. Pemetrexed versus gefitinib as second‐line treatment in advanced non‐squamous nonsmall‐cell lung cancer patients harbouring wild‐type EGFR (CTONG 0806): a multicenter randomized trial. Annals of Oncology 2014;25(12):2385‐91. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Cheng Y, Zhao MF, Yang JJ, Yan H H, Zhang L, et al. Final results of CTONG 0806: a phase II trial comparing pemetrexed with gefitinib as second‐line treatment of advanced non‐squamous NSCLC patients with wild‐type EGFR. 15th World Conference on Lung Cancer Sydney, NSW Australia. International Association for the Study of Lung Cancer, 2013; Vol. 8:S194‐5.
References to ongoing studies
Bhatnagar 2012 {published data only}
- Bhatnagar AR, Singh DP, Sharma R, Kumbhaj P. Docetaxel versus geftinib in patients with locally advanced or metastatic NSCLC pretreated with platinum‐based chemotherapy. 4th Australian Lung Cancer Conference, ALCC 2012 Adelaide, SA Australia. International Association for the Study of Lung Cancer, 2012; Vol. 7:S159.
Gaafar 2010 {published data only}
- Gaafar RM, Surmont V, Scagliotti G, Klaveren R, Papamichael G, Welch J, et al. A double‐blind, randomized, placebo‐controlled phase III intergroup study of gefitinib (G) in patients (pts) with advanced NSCLC, non‐progressing after first‐line platinum‐based chemotherapy (EORTC 08021‐ILCP 01/03). Journal of Clinical Oncology. 2010; Vol. 28 Suppl 15.
Hong 2010 {published data only}
- Hong J, Kyung SY, Lee SP, Park JW, Jung SH, Sym SJ, et al. Randomized phase II study of pemetrexed versus gefitinib for patients with previously treated non‐small cell lung cancer. 4th Asia Pacific Lung Cancer Conference, APLCC 2010 Seoul, South Korea. International Association for the Study of Lung Cancer, 2010; Vol. 5:S401.
Laurie 2000 {published data only}
- Laurie SA, Miller VA, Johnson D, Ng KK, Heelan RT, Pizzo BA, et al. Pilot trial of ZD1839 (Iressa‐TM‐), an oral inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase, in combination with carboplatin (C) and paclitaxel (P) in previously untreated advanced non‐small cell lung cancer. Lung Cancer. 2000; Vol. 29 Suppl 1:S71.
Lee 2013 {published data only}
- Lee DH, Kim JE, Choi YJ, Choi CM, Lee JS, Kim SW. Randomized phase II study comparing paclitaxel/carboplatin intercalated with gefitinib to paclitaxel/carboplatin alone for chemotherapy‐naive non‐small cell lung cancer patients either with history of smoking or with wild‐type EGFR. AACR‐NCI‐EORTC International Conference: Molecular Targets and Cancer Therapeutics 2013 Boston, MA United States. American Association for Cancer Research Inc, 2013; Vol. 12.
Liang 2010 {published data only}
- Liang J, Ahn M, Kang J, et al. First‐line treatment (txt) with pemetrexed‐cisplatin (PC), followed sequentially by gefitinib (G) or pemetrexed, in Asian, never‐smoker (n/smkr) patients (pts) with advanced NSCLC: An open‐label, randomized phase II trial. Journal of Clinical Oncology 2010;28(15 Suppl 1):Abstract 7591. [Google Scholar]
Nokihara 2006 {published data only}
- Nokihara H, Ohe Y, Kawaishi M, et al. A randomized phase II study of sequential carboplatin/paclitaxel (CP) and gefitinib (G) in chemotherapy‐naive patients with advanced non‐small‐cell lung cancer (NSCLC): Preliminary results. Journal of Clinical Oncology: ASCO Annual Meeting Proceedings 2006;24(18 Suppl):7096. [Google Scholar]
- Nokihara H, Ohe Y, Yamada K, et al. Randomized phase II study of sequential carboplatin/paclitaxel (CP) and gefitinib (G) in chemotherapy‐naive patients with advanced non‐small‐cell lung cancer (NSCLC): final results [abstract no. 8069]. Journal of Clinical Oncology: ASCO Annual Meeting Proceedings 2008;26(15 Suppl Pt I):441. [Google Scholar]
Puri 2013 {published data only}
- Puri T, Orlando M, Barraclough H, Enatsu S. A randomized phase 2 trial of pemetrexed (P) and gefitinib (G) versus g as first‐line treatment for patients with stage IV non‐squamous (NS) non‐small cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations. 15th World Conference on Lung Cancer Sydney, NSW Australia. International Association for the Study of Lung Cancer, 2013; Vol. 8:S1156‐7.
Additional references
ACS 2018
- American Cancer Society. Cancer facts and figures 2018. Available at: http://www.cancer.org/cancer/lungcancer‐non‐smallcell/ (accessed 11th Jan 2018).
Bell 2005
- Bell DW, Lynch TJ, Maserlat SM, Harris PL, Okimoto FA, Brannigan BW, et al. Epidermal growth factor receptor mutations and gene amplification in non‐small‐cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. Journal of Clinical Oncology 2005;23(31):8081‐92. [DOI] [PubMed] [Google Scholar]
Boye 2016
- Boye M, Wang X, Srimuninnimit V, Kang JH, Tsai CM, Orlando M, et al. First‐line pemetrexed plus cisplatin followed by gefitinib maintenance therapy versus gefitinib monotherapy in east Asian never‐smoker patients with locally advanced or metastatic nonsquamous non‐small cell lung cancer: quality of life results from a randomized phase III trial. Clinical Lung Cancer 2016;17(2):150‐60. [DOI] [PubMed] [Google Scholar]
Cella 1995
- Cella D, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy ‐ Lung (FACT‐L) quality of life instrument. Lung Cancer 1995;12:199‐220. [DOI] [PubMed] [Google Scholar]
Cella 2002
- Cella D, Eton DT, Fairclough DL, Bonomi P, Heyes AE, Silberman C, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy‐Lung (FACT‐L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. Journal of Clinical Epidemiology 2002;55:285‐95. [DOI] [PubMed] [Google Scholar]
Cella 2005
- Cella D, Herbst RS, Lynch TJ, Prager D, Belani CP, Schiller JH, et al. Clinically meaningful improvement in symptoms and quality of life for patients with non‐small cell lung cancer receiving gefitinib in a randomized controlled trial. Journal of Clinical Oncology 2005;23(13):2946‐54. [DOI] [PubMed] [Google Scholar]
Chang 2006
- Chang A, Parikh P, Thongprasert S, Tan EH, Perng RP, Ganzon D, et al. Gefitinib (IRESSA) in patients of Asian origin with refractory advanced non‐small‐cell lung cancer: Subset analysis from the ISEL study. Journal of Thoracic Oncology 2006;1(8):847‐55. [PubMed] [Google Scholar]
Douillard 2010
- Douillard JY, Shephard FA, Hirsh V, Mok T, Socinski MA, Gervais R, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non‐small‐cell lung cancer: from the randomized phase III INTEREST trial. Journal of Clinical Oncology 2010;28(5):744‐52. [DOI] [PubMed] [Google Scholar]
Fukuoka 2011
- Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open‐label, first‐line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non ‐ small‐cell lung cancer in Asia (IPASS). Journal of Clinical Oncology. United States: American Society of Clinical Oncology (330 John Carlyle Street, Suite 300, Alexandria VA 22314, United States), 2011; Vol. 29:2866‐74. [DOI] [PubMed]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Herbst 2005
- Herbst RS, Prager D, Hermann R, Fenrenbacher L, Johnson BE, Sandler A, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI‐774) combined with carboplatin and paclitaxel chemotherapy in advanced non‐small‐cell lung cancer. Journal of Clinical Oncology 2005;23(25):5892‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [www.cochrane‐handbook.org]
Hirsch 2006
- Hirsch FR, Varella‐Garcia M, Bunn Jr PA, Franklin WA, Dziadziuszko R, Thatcher N, et al. Molecular predictors of outcome with gefitinib in a phase III placebo‐controlled study in advanced non‐small cell lung cancer. Journal of Clinical Oncology 2006;24(31):5034‐42. [DOI] [PubMed] [Google Scholar]
Ibrahim 2010
- Ibrahim EM. Frontline gefitinib in advanced non‐small cell lung cancer: meta‐analysis of published randomized trials. Annals of Thoracic Medicine 2010;5(3):153‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inoue 2013
- Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin‐paclitaxel for chemo‐naive non‐small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Annals of Oncology 2013;24:54‐9. [DOI] [PubMed] [Google Scholar]
Jiang 2011
- Jiang J, Huang L, Liang X, Zhou X, Huang R, Chu Z, et al. Gefitinib versus docetaxel in previously treated advanced non‐small‐cell lung cancer: a meta‐analysis of randomized controlled trials. Acta Oncologica 2011;50(4):582‐8. [DOI] [PubMed] [Google Scholar]
Kosaka 2004
- Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Research 2004;64:8919‐23. [DOI] [PubMed] [Google Scholar]
Mok 2011
- Mok TSK. Personalized medicine in lung cancer: what we need to know. Nature Reviews Clinical Oncology 2011;8:661‐8. [DOI] [PubMed] [Google Scholar]
NCI CTCAE 2010
- National Cancer Institute. Cancer Therapy Evaluation Program. Common terminology criteria for adverse events. Common toxicity criteria, version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010‐06‐14_QuickReference_5x7.pdf (accessed 17 February 2017).
Oizumi 2012
- Oizumi S, Kobayashi K, Inoue A, Maemondo M, Sugawara S, Yoshizawa H, et al. Quality of life with gefitinib in patients with EGFR‐mutated non‐small cell lung cancer: quality of life analysis of north east Japan study group 002 trial. Oncologist. United States: AlphaMed Press (318 Blackwell St. Suite 260, Durham NC 27701‐2884, United States), 2012; Vol. 17:863‐70. [DOI] [PMC free article] [PubMed]
Paez 2004
- Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497‐500. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sekine 2009
- Sekine I, Ichinose Y, Nishiwaki Y, Yamamoto N, Tsuboi M, Nakagawa K, et al. Quality of life and disease‐related symptoms in previously treated Japanese patients with non‐small‐cell lung cancer: results of a randomized phase III study (V‐15‐32) of gefitinib versus docetaxel. Annals of Oncology 2009;20(9):1483‐8. [DOI] [PubMed] [Google Scholar]
Smith 2005
- Smith J. Erlotinib: small molecule targeted therapy in the treatment of non‐small‐cell lung cancer. Clinical Therapeutics 2005;27(10):1513‐34. [DOI] [PubMed] [Google Scholar]
Therasse 2000
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92(3):205‐16. [DOI] [PubMed] [Google Scholar]
Thongprasert 2011
- Thongprasert S, Duffield E, Saijo N, Wu YL, Yang JC, Chu DT, et al. Health‐related quality‐of‐life in a randomized phase III first‐line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS). Journal of Thoracic Oncology 2011;6:1872‐80. [DOI] [PubMed] [Google Scholar]
Yamamoto 2010
- Yamamoto N, Nishiwaki Y, Negoro S, Jiang H, Itoh Y, Saijo N, et al. Disease control as a predictor of survival with gefitinib and docetaxel in a phase III study (V‐15‐32) in advanced non‐small‐cell lung cancer patients. Journal of Thoracic Oncology 2010;5(7):1042‐7. [DOI] [PubMed] [Google Scholar]
Yang 2015
- Yang YP, Ma YX, Huang Y, Zhao YY, Fang WF, Hong SD, et al. QoL analyses from INFORM study, a phase III study of gefitinib versus placebo as maintenance therapy in advanced NSCLC. Scientific Reports 2015;5:11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Sim 2007
- Sim EH, Yang IA, Fong K, Wood‐Baker R, Bowman R. Gefitinib for advanced non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006847] [DOI] [PMC free article] [PubMed] [Google Scholar]


