Abstract
Antibiotic stewardship programs aim to optimize antimicrobial use to prevent the emergence of resistance species and protect patients from the side effects of unnecessary medication. The high incidence of systemic infection and associated mortality from these infections leads neonatal providers to frequently initiate antibiotic therapy and make empiric antibiotic courses one of the main contributors of antibiotic use in the neonatal units. Yet, premature infants are also at risk for acute life-threatening complications associated with antibiotic use such as necrotizing enterocolitis and for long-term morbidities such as asthma. In this review, we discuss specific aspects of antibiotic use in the very-low birth weight preterm infants, with a focus on empiric use, that provide opportunities for stewardship practice. We discuss strategies to risk-stratify antibiotic initiation for the risk of early-onset sepsis, optimize empiric therapy duration and antibiotic choice in late onset sepsis, standardize decisions for stopping empiric therapy. Lastly review the evolving role of biomarkers in antibiotic stewardship.
Keywords: very-low birth weight, prematurity, antibiotic stewardship
RISKS AND BENEFITS OF ANTIBIOTICS FOR PRETERM INFANTS
Antibiotics are the most commonly used drugs in the NICU1. More than 75% of very-low birth weight infants (birth weight <1500 grams, VLBW) and over 80% of extremely-low birth weight infants (birth weight <1000 grams, ELBW) receive antibiotics for the risk of early-onset sepsis (EOS, infection occurring < 72 hours of age)2,3. Two-thirds of VLBW infants cared for in the NICHD Neonatal Research Network (NRN) centers are evaluated at least once for late-onset sepsis (LOS, infection occurring ≥72 hours of age)4. Neonatal antibiotic use is as variable as it is prevalent. Among 22 centers participating in the NRN the proportion of preterm infants administered prolonged antibiotics in the first week after birth varied from 16–77% across sites, while the incidence of culture-confirmed EOS ranged from 0–7%5. Similarly, overall antibiotic use varied 40-fold in a study of 127 NICUs; variation that was unexplained by differences in rates of proven infection, necrotizing enterocolitis (NEC) surgical volume or mortality6. Such variation strongly suggests a contribution of antibiotic prescriber preferences beyond biological drivers of antibiotic usage.
Clinicians administer antibiotics to ensure the safety of preterm infants, but antibiotic administration is not without risk. The risk/benefit analysis that drives the common practice of administering antibiotics in the absence of culture-confirmed infection is based on two assumptions: (1) the risk associated with antibiotic use is predictable and manageable, and (2) antibiotic use, even in absence of known organism or antibiotic susceptibility data, has significant benefit. In recent years both these assumptions have been questioned.
The predictable risks of antibiotics exposure include issues such as acute drug toxicities, the need for intravenous access, and the costs and potential unintended consequences of escalated monitoring. An evolving understanding of the role of microbiome in human health has added new, potentially more pervasive, and currently unpredictable risks to neonatal antibiotic exposure. Newborn antibiotic exposure via maternal antibiotic administration just prior to delivery7,8 and neonatal administration after birth9, both alter the neonate’s microbiome composition well into infancy. Observational studies find an association of early life antibiotics in premature infants with increased risks of NEC, fungal infection and death2,10,11. Multiple preclinical studies provide insight into the mechanistic pathways by which altered microbiota can influence the host immune state and overall health12–14. Finally, the emergence of multi-drug resistant organisms remains an underappreciated but increasingly reported threat in neonatal units across the world15,16. The extent to which any individual infant will suffer from one or more of the adverse outcomes associated with antibiotic use is difficult to predict. However, it is clear that antibiotic use is associated with a cumulative risk that is neither entirely predictable nor immediately manageable.
The benefits of antibiotic administration are clear for infected infants, and infection is prevalent among preterm infants. Culture-confirmed EOS occurs in ~1% of VLBW infants, an incidence that is 20-fold higher than that in infants born with birth weight >2500 grams17. Culture-confirmed LOS is reported in ~20% of ELBW infants18. Additionally, 5% of overall mortality in the ELBW population is attributable to infection and NEC19. Antibiotic therapy is potentially life-saving. However, extrapolating the efficacy of antibiotics to infants without culture-confirmed infection or NEC generally requires three assumptions: (1) microbiological cultures lack sufficient sensitivity for detecting existing infection; (2) clinical exam and non-microbiological tests serve as reasonable surrogates for identifying infection; and (3) clinical decompensation observed on examination or the inflammatory state captured in the test, is not only bacterial in origin but also from a susceptible bacterium that will respond to the chosen antibiotic type and duration. No clinical trials have tested these assumptions. Indeed, most observational studies challenge these assumptions, finding increased mortality and morbidity in infants treated with prolonged antibiotics in the absence of culture-confirmed infection compared to infants with similar clinical characteristics managed without antibiotics2,10,11,20,21. One explanation could be residual confounding in these studies. However, additional concerns should be that the worse outcome is related to negative effects of antibiotics or due to inadequate treatment of the true cause of decompensation.
DRIVERS OF ANTIBIOTIC USE AND OPPORTUNITIES FOR ANTIBIOTIC STEWARDSHIP AMONG PREMATURE INFANTS
The increased understanding of risk and limited evidence for benefit of antibiotic therapy in the absence of culture-confirmed infectiondemands that neonatal providers examine current practice. Specific drivers of neonatal antibiotic use inform the opportunities for antibiotic stewardship. Cantey et al quantified indications for antibiotic use among all infants cared for at a single center over 14 months22. Most antibiotic initiation (79%) occurred within 72 hours after birth. Sixty-three percent of antibiotic use was for “rule-out sepsis,” targeting both EOS and LOS, which ended when cultures were reported as sterile. For the remaining 31%, ~7% was for the combined indication of culture confirmed infection (5%), NEC (2%), or cellulitis (<1%), and the rest was for presumed and culture-negative infection (CNI) or pneumonia. Patel et al14 reported on indications for antibiotic use for LOS in 4 neonatal centers. Empiric initiation again accounted for the majority of initiation (66%); and presumed, CNI treatment accounted for ~1/3 of the continued use. Empiric initiation and antibiotic use for CNI is driven by the non-specific nature of VLBW instability. Indications for LOS antibiotic initiation at a perinatal center and a referral center with surgical cases, included feeding intolerance (25–35%), increased need for respiratory support (15–25%), increased apnea and bradycardia (10–15%) and ill-appearance (5–10%), with few evaluation for specific indications such as cellulitis, seizures or recent surgery23.
The general goals of hospital-based antibiotic stewardship are described by the Centers for Disease Control and Prevention 24. These guidelines focus on the elements of initiating and discontinuing antibiotics, optimizing infection and biomarker testing, choosing empiric and definitive antibiotic therapy, and limiting duration of empiric therapy. Decreases in overall neonatal antibiotic utilization are reported in conjunction with multiple antimicrobial stewardship interventions25–27 and among term infants with use of multivariate risk models for EOS risk assessment28,29. Schulman et al recently reported a significant decrease in antibiotic use rate of 21.9% in ~130 NICUs across California from 2013 to 201630. They found a greater reduction in centers participating in study-defined antibiotic stewardship efforts compared with centers without such participation (28.7% vs. 16.2%) underscoring the importance of stewardship efforts. However, NICUs utilizing antimicrobial stewardship committees report less impact specifically among VLBW infants31,32. A possible reason for this is that the major contributor of antibiotic use in the VLBW population is the empiric ‘rule out’. A labile clinical status, high incidence of infection and an extended turn-around time for culture results limits the scope for safely reducing empiric initiation. While factors such as the infection rate maybe modifiable, clinical lability is unlikely to change. Thus a focus on the choice and duration of empiric therapy and risk-stratification to minimize empiric early initiation are likely to have the most immediate impact on VLBW antibiotic use. Here we will discuss aspects of empiric antibiotic use that provide opportunities for stewardship practice among VLBW preterm infants.
EMPIRIC INITIATION FOR SUSPECTED EARLY ONSET SEPSIS
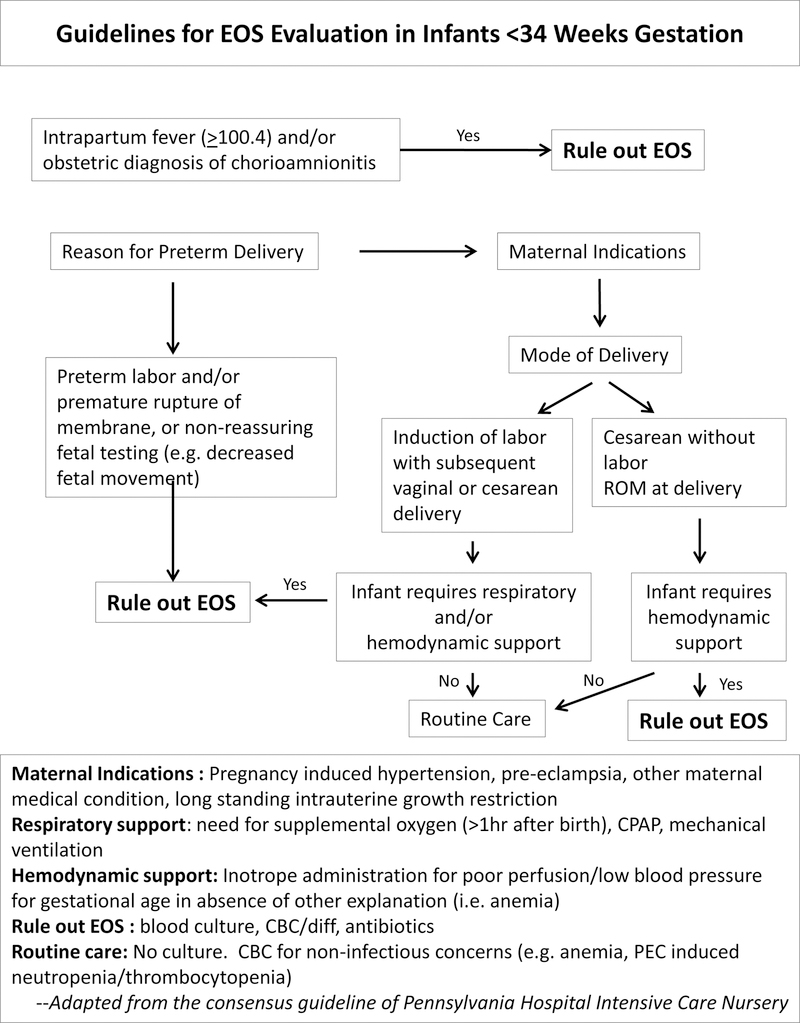
EOS pathogenesis predominantly involves colonization and invasion of the fetus or newborn by maternal genitourinary flora. A breach in the gravid uterine environment with onset of spontaneous or induced labor and/or membrane rupture provides opportunity for ascending infection. However, a substantial proportion of preterm infants are delivered for maternal indication by cesarean section (CS), without labor and with membrane rupture at delivery. We have reported the significantly different risk of EOS based on delivery characteristics. In a study of 15,433 infants born 22–28 weeks gestation, 5759 (37%) infants were delivered by CS, with rupture at delivery and without a diagnosis of chorioamnionitis5. The composite outcome of death at <12 hours or EOS was 64% lower in infants delivered with these criteria (2.6% vs 15.1%; aRR [95% CI] 0.36 [0.30–0.43]). Yet, among infants with low-risk delivery criteria, 66 infants were treated for presumed CNI for each case of confirmed EOS, compared with 19 infants per case in the comparison group (p<.001). In a 25-year single-center study, where detailed chart review for the presence of labor was feasible, only 3/109 cases of culture confirmed EOS were found among infants delivered by CS without labor or rupture33. As empiric antibiotic initiation for EOS is a major driver of NICU antibiotic use, restricting initiation in infants based on delivery characteristics can have a substantial impact on overall antibiotic use in preterm infants. A written policy and auditing may be necessary to establish practice, ensure compliance and determine balance measures. An example of such a policy is shown in Figure 1. Of note, the goal of the algorithm is to provide clarity for situations where antibiotic can be withheld, not to mandate management approach in all other situations which should continue to be informed by local practices.
Figure 1:
Guidelines for Evaluation of EOS in Infants Born <34 weeks
DURATION OF EMPIRIC ANTIBIOTIC THERAPY
Optimal duration for empiric therapy balances the provision of antibiotic therapy to infants whose blood culture will grow a pathogen with minimized antibiotic exposure in those where the blood culture will remain sterile. Traditional 48- and 72-hour “rule out” periods are based on data from Pichichero et al34, in which positive cultures were identified by daily examination of broth culture for visual turbidity and 12-hourly review of plate subcultures. Current blood culture techniques rely on production of CO2 or change in gas pressure for continuous detection of bacterial growth and are optimized to detect very low levels of bacteremia. Reports of modern blood culture performance rarely focus on the VLBW population (Table 1). We reviewed 84 cases of VLBW EOS detected in aerobic culture at a single center over 25 years and found a cut-off of 36 hours would have detected ~90% of the infections33. Had we stopped antibiotics at 36 hours, 4/8 cases detected after 36 hours would have had an ampicillin dose delayed by ≤3 hours, 2/8 by ≤9 hours and the remaining ≥10 hours. During this time period 5313 VLBW infants were admitted to the NICU and ~85% underwent empiric EOS therapy. We estimate that using a cut-off of 36 hours of incubation to discontinue antibiotics would have reduced ~4400 doses of ampicillin in uninfected preterm infants. Given that EOS risk is the primary indication for empiric antibiotic use among VLBW infants, as well as the prolonged half-life of antibiotics such as ampicillin and gentamicin in premature infants in the first week after birth35,36 limiting duration of empiric therapy could be a meaningful component of VLBW antimicrobial stewardship. Implementation of standard cut-off periods to rule out sepsis regardless of duration is best implemented by hard stops in order-sets26,37.
Table 1.
Blood culture time-to-positivity among neonates
| Reference | Study Cohort | Culture Timing† | Culture n* | Culture positive by hours of incubation (%) | |||
|---|---|---|---|---|---|---|---|
| 24 | >24–36 | >36–48 | >48–72 | ||||
| Pichiero et al, 197934 | 24–41 weeks | n/a | 105 | 67.6 | n/a | 96.2 | 98.1 |
| Mukhopadhyay et al, 201733 | Birth weight <1500 g | ≤72 hours of age | 84 | 74.4 | 90.2 | 97.6 | 100.0 |
| Garcia-Prats et al, 200038 | All gestations (including CONS) | ~84% at >72 hours of age | 248 | 81.5 | 92.7 | 94.8 | 97.2 |
| Garcia-Prats et al, 200038 | All gestations (excluding CONS) | n/a | 113 | 91.2 | 93.8 | 94.7 | 96.5 |
| Kumar et al, 200139 | All gestations | Majority >48 hours of age | 366 | 51.9 | 88.3 | 95.9 | 98.9 |
| Guerti et al, 201040 | All gestations | ~80% at >72 hours | 358 | 59.5 | n/a | 91.1 | 97.5 |
| Guerti et al, 201040 | All gestations (excluding CONS) | n/a | 144 | 86.8 | n/a | 93.8 | 95.8 |
| Biondi, 201441 | Infants in non-ICU setting | <90 days of age | 392 | 91 | 96 | 99 | 100 |
Footnotes:
Number of culture shown exclude contaminants (micrococcus, Bacillus sp., Corynebacterium sp.); yeast isolates; and cultures obtained after start of antimicrobials, except Biondi et al41, where contaminants could not be excluded in time to positivity results. n/a-not reported.
Timing shown for all positive cultures reported in the study including those classified as contaminants. ref 34 specifies subjects were neonates but does not clarify infant age at time of cultures. CONS-coagulase negative staphylococcus
EMPIRIC ANTIBIOTIC CHOICE
Ampicillin, gentamicin and vancomycin are among the top medications used in NICU’s across the U.S.1 The most common empiric antibiotic choice for EOS is ampicillin and gentamicin1. Recent national surveillance demonstrates ~70% VLBW EOS is due to GBS or E. coli, and >90% of these isolates were sensitive to one of these antibiotics42. There is less clarity in empiric antibiotic choice for LOS, which may provide an opportunity for VLBW stewardship. Stoll et al reported that 62% of 6215 VLBW infants were evaluated for risk of LOS at least once4. Vancomycin was prescribed to ~40% of the infants including 30% of infants without proven infection. The choice for vancomycin in LOS empiric therapy is driven by the predominance of coagulase-negative staphylococcus (CONS) as a cause of LOS and concern for methicillin-resistant Staphylococcus aureus (MRSA) infection. However, there are several arguments against use of vancomycin as first line empiric therapy for LOS. The discrepancy between the frequency of “rule-out LOS” and the actual incidence of LOS means a substantial proportion of uninfected infants are exposed to this medication which should be reserved for clinical situations in which its use is mandated by culture data43,44. Ototoxicity, renal failure and development of resistant organisms have all been reported with vancomycin use37,42. Among infants with CONS infection, the adjusted odds of mortality before hospital discharge when empiric vancomycin was administered immediately after blood culture was obtained (n= 2848) was similar to infants (n=1516) where vancomycin was initiated ≥1 day after culture (1.06, 95% CI 0.81– 1.39)45. Possible misclassification of CONS that are contaminants as pathogens and the non-fulminant nature of CONS sepsis provide further arguments for not basing empiric regimen on this organism. A concern for MRSA infection is a justified indication for vancomycin empiric therapy with reported mortality benefit46. However, the reported incidence of MRSA LOS among VLBW infants is significantly lower (~1%) compared to incidence of methicillin-sensitive Staphylococcus aureus (MSSA) sepsis (2.7%) with comparable mortality47. Beta-lactam medications such as oxacillin are more quickly bactericidal compared to vancomycin and may provide benefit as the empiric choice in cases of MSSA infection. MRSA screening in NICUs may provide an opportunity to restrict vancomycin empiric therapy to patients known to be colonized. Multiple studies have demonstrated the success of reducing unnecessary vancomycin use in neonates without increasing adverse events23,48. Thus a review of local pathogen distribution and screening policies may provide an opportunity to reduce empiric vancomycin use in VLBW infants.
STOPPING EMPIRIC ANTIBIOTIC THERAPY
Continuation of antibiotics in the absence of evidence for infection such as bacteremia or cellulitis contributes significantly to overall antibiotic use in the NICU49. There are no standard clinical algorithms to inform such decisions, likely adding to the wide center-specific variation in CNI treatment4. The potential of biomarkers to inform antibiotic decisions is discussed below. In Table 2, we summarize strategies to support decision making when considering CNI treatment.
Table 2.
Strategies to Optimize Use of Antibiotics for Culture-negative sepsis among VLBW Infants
| Sepsis Evaluation | |
| Blood culture | 1. Obtain minimum 1 mL blood per pediatric blood culture bottle 2. Consider 2 separate cultures to optimize identification of contaminant species after 72 hours of life |
| CSF culture | Strongly consider obtaining CSF culture prior to initiation of antibiotics for evaluations after 72 hours of life |
| Urine culture | 1. Strongly consider urine culture in older VLBW infants without central lines 2. Obtain culture by sterile catheter or supra-pubic aspirate to minimize isolation of contaminant species |
| Non-bacterial cultures | 1. Consider urine CMV testing for infants with BW <1000 grams fed mother’s own milk 2. Consider additional respiratory and gastrointestinal viral testing in site-specific appropriate seasons |
| Antibiotic Choice and Duration | |
| Conduct annual review of unit-specific culture isolates and antibiotic susceptibility data to inform empiric antibiotic choice | |
| Discontinue antibiotics when blood cultures are sterile by 36–48 hours incubation | |
| If decision is made to empirically administer antibiotics for presumed and culture-negative infection (CNI): 1. Use patient-specific colonizing data to choose least broad-spectrum antibiotic 2. Set local guidelines for antibiotic duration for presumed and CNI 3. Ensure that the rationale, risks and benefits of antibiotic administration for presumed and CNI are discussed with all members of the clinical care team and with the infant’s parent(s) or guardians 4. Involve the stewardship team in decision making | |
BIOMARKERS OF INFLAMMATION
The use of biomarkers, specifically acute phase reactants, to guide antibiotic therapy in the NICU has long been proposed among neonates50. An obvious drawback is that these biomarkers reflect systemic inflammation that can be caused by other conditions besides bacterial infection. The scope for biomarker-guided antibiotic use includes: determining initiation, determining continuation when an infection is suspected but not confirmed and determining duration in diseases with an unambiguous need for antibiotics such as bacteremic sepsis.
The sensitivity of biomarkers, even in combination, is not high enough to be the sole determinant in withholding antibiotic initiation in the extremely preterm population51–53. Biomarker-guided antibiotic initiation is likely to perform best in scenarios with a low prior probability of true infection, high antibiotic use rates and among populations with low risk of morbidity if therapy is delayed. Consequently, most studies of biomarker-guided initiation have been conducted in outpatient settings 54–56. In ICU, two trials in adult patients have studied antibiotic initiation based on procalcitonin (PCT)-guided algorithms. One study found no effect, and although the other found a significant reduction in antibiotic use57, a 53% non-compliance in the PCT arm raised concerns about generalizability of the study55,57,58. One trial in neonates found the addition of IL8 (≥70 pg/ml) to standard management (clinical signs and CRP >10 mg/L) among 1291 NICU admissions resulted in a reduction of antibiotic initiation for suspected EOS from 49.6% to 36.1% (p <0.001) without any ‘missed’ infections59. The study however was not focused on preterm infants and included only 13 cases of culture-confirmed infection. Multiple studies report significant reductions in antibiotic duration using biomarker-guided decision tools (particularly PCT) without changes in mortality or re-infection55. Most of these studies include patients with a known focus of infection such as community-acquired pneumonia and exclude immunosuppressed patients or patients with an unknown infection source55. In contrast, biomarkers have been used in neonates to determine duration of therapy for CNI with variable impact52. The NeoPINS trial randomized infants born ≥34 weeks gestation with suspected EOS to antibiotic therapy guided by PCT measurements60. Antibiotic duration was decreased by 9.9 hours (55.1 vs 65 hours, p <0.001) and length of stay by 3.5 hours (123 vs. 126.5 hours, P=0.002) in the PCT arm. The recommended antibiotic regimens of 5–7 days for culture-negative infants in the standard arm classified as intermediate risk is greater than that reported at other centers28 suggesting that the efficacy of this intervention will vary depending on local practice. The opposite effect, of significantly increased antibiotic use, has been reported with CRP use and argues against incorporation of using biomarkers to guide antibiotic durations in CNI without evidence for improved outcomes with such practice61,62.
A final use of biomarkers may be to determine length of therapy for conditions in which antibiotic use in mandated. Hemels et al, observed that most CONS bacteremia at their center resolved within 72 hours of initiation antibiotic initiation when the infants demonstrated clinical improvement, CRP levels decreased and central catheters were removed30. This group later reported an observational experience of treating infants meeting such criteria with 3 or 7 days of antibiotics, and found no difference in resolution of bacteremia or clinical outcome63. Cuoto et al conducted a pre-post study utilizing CRP measurements to determine length of antibiotic treatment among infants with culture-confirmed sepsis (77 vs 122 infants)64. A significant reduction (16 to 9 medians days) was observed in the post-implementation period when antibiotics were stopped if CRP declined to <12 mg/L64. Whether resolution of a host inflammatory marker consistently reflects resolution of tissue level bacteremia with negligible recurrent bacteremia requires larger studies.
Overall, the role of biomarkers in determining antibiotic prescription among preterm infants remains promising but unclear. Studies reporting antibiotic use reduction with biomarker-guided therapy have high baseline antibiotic use rates that will impact the anticipated effect size when translated to a different setting. While we await definitive studies perhaps the best first step would be to optimize blood culture collection technique and considering discontinuation of antibiotics in culture negative infants65.
CONCLUSION
Despite the challenges of implementing antibiotic stewardship in the premature infant, promising strategies that specifically target this population exist. Incorporating these strategies require a re-adjustment of our risk-benefit mind-set such that we acknowledge and account for the risk of unnecessary antibiotic use. Much of the focus in antibiotic use among premature infants has been to protect the child from infection – while undoubtedly a relevant focus, that protection should not happen at the risk of injury to the uninfected child. Achieving this balance requires standardization of care, measurement of antibiotic use, strong balance measures and continuous evaluation of practices and outcomes. An urgent need of the field lies in building the “equipoise” around the current approach to antibiotic use that will enable systematic examination of the risk and benefits.
What is known about this topic:
Empiric antibiotic use for “rule-outs” is a major contributor of overall antibiotic use in neonatal units. In the presence of high incidence of infection, labile clinical status and long turn-around time for cultures, finding strategies that allow safe antibiotic restriction can be challenging.
What this study adds:
This review focuses on aspects of empiric therapy commonly prescribed in the very low birth weight infant. We describe strategies that are likely to have the most immediate impact on optimizing antibiotic use in his population.
Acknowledgments
Funding Source: 1K23HD088753-01A1 NICHD; 1K08HL132053-01A1 NHLBI
Financial disclosure and conflict of interest: The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.Hsieh EM, Hornik CP, Clark RH, et al. Medication use in the neonatal intensive care unit. Am J Perinatol 2014;31:811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009;123:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flannery DD, Ross RK, Mukhopadhyay S, Tribble AC, Puopolo KM, Gerber JS. Temporal Trends and Center Variation in Early Antibiotic Use Among Premature Infants. JAMA Network Open 2018;1:e180164–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen N, Fanaroff AA, et al. Late–onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110:285–91. [DOI] [PubMed] [Google Scholar]
- 5.Puopolo KM, Mukhopadhyay S, Hansen NI, et al. Identification of Extremely Premature Infants at Low Risk for Early–Onset Sepsis. Pediatrics 2017;140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulman J, Dimand RJ, Lee HC, Duenas GV, Bennett MV, Gould JB. Neonatal intensive care unit antibiotic use. Pediatrics 2015;135:826–33. [DOI] [PubMed] [Google Scholar]
- 7.Azad MB, Moossavi S, Owora A, Sepehri S. Early–Life Antibiotic Exposure, Gut Microbiota Development, and Predisposition to Obesity. Nestle Nutr Inst Workshop Ser 2017;88:67–79. [DOI] [PubMed] [Google Scholar]
- 8.Corvaglia L, Tonti G, Martini S, et al. Influence of Intrapartum Antibiotic Prophylaxis for Group B Streptococcus on Gut Microbiota in the First Month of Life. J Pediatr Gastroenterol Nutr 2016;62:304–8. [DOI] [PubMed] [Google Scholar]
- 9.Fouhy F, Guinane CM, Hussey S, et al. High–throughput sequencing reveals the incomplete, shortterm recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob Agents Chemother 2012;56:5811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuppala VS, Meinzen–Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr 2011;159:720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ting JY, Synnes A, Roberts A, et al. Association Between Antibiotic Use and Neonatal Mortality and Morbidities in Very Low–Birth–Weight Infants Without Culture–Proven Sepsis or Necrotizing Enterocolitis. JAMA Pediatr 2016;170:1181–7. [DOI] [PubMed] [Google Scholar]
- 12.Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014;158:705–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshmukh HS, Liu Y, Menkiti OR, et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med 2014;20:524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012;336:489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carr D, Barnes EH, Gordon A, Isaacs D. Effect of antibiotic use on antimicrobial antibiotic resistance and late–onset neonatal infections over 25 years in an Australian tertiary neonatal unit. Arch Dis Child Fetal Neonatal Ed 2017;102:F244–F50. [DOI] [PubMed] [Google Scholar]
- 16.Investigators of the Delhi Neonatal Infection Study c. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: a cohort study. Lancet Glob Health 2016;4:e752–60. [DOI] [PubMed] [Google Scholar]
- 17.Stoll BJ, Hansen NI, Sanchez PJ, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics 2011;127:817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg RG, Kandefer S, Do BT, et al. Late–onset Sepsis in Extremely Premature Infants: 2000–2011. Pediatr Infect Dis J 2017;36:774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel RM, Kandefer S, Walsh MC, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med 2015;372:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaoutis TE, Prasad PA, Localio AR, et al. Risk factors and predictors for candidemia in pediatric intensive care unit patients: implications for prevention. Clin Infect Dis 2010;51:e38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ting JY, Synnes A, Roberts A, et al. Association of Antibiotic Utilization and Neurodevelopmental Outcomes among Extremely Low Gestational Age Neonates without Proven Sepsis or Necrotizing Enterocolitis. Am J Perinatol 2018;35:972–8. [DOI] [PubMed] [Google Scholar]
- 22.Cantey JB, Wozniak PS, Pruszynski JE, Sanchez PJ. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time–series study. Lancet Infect Dis 2016;16:1178–84. [DOI] [PubMed] [Google Scholar]
- 23.Chiu CH, Michelow IC, Cronin J, Ringer SA, Ferris TG, Puopolo KM. Effectiveness of a guideline to reduce vancomycin use in the neonatal intensive care unit. Pediatr Infect Dis J 2011;30:273–8. [DOI] [PubMed] [Google Scholar]
- 24.(HICPAC) TRotHICPAC. Healthcare Infection Control Practices Advisory Committee. Antibiotic Stewardship Statement for Antibiotic Guidelines 2016.
- 25.Patel SJ, Saiman L. Principles and strategies of antimicrobial stewardship in the neonatal intensive care unit. Semin Perinatol 2012;36:431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantey JB, Baird SD. Ending the Culture of Culture–Negative Sepsis in the Neonatal ICU. Pediatrics 2017;140. [DOI] [PubMed] [Google Scholar]
- 27.Schelonka RL, Scruggs S, Nichols K, Dimmitt RA, Carlo WA. Sustained reductions in neonatal nosocomial infection rates following a comprehensive infection control intervention. J Perinatol 2006;26:176–9. [DOI] [PubMed] [Google Scholar]
- 28.Dhudasia MB, Mukhopadhyay S, Puopolo KM. Implementation of the Sepsis Risk Calculator at an Academic Birth Hospital. Hosp Pediatr 2018;8:243–50. [DOI] [PubMed] [Google Scholar]
- 29.Kuzniewicz MW, Puopolo KM, Fischer A, et al. A Quantitative, Risk–Based Approach to the Management of Neonatal Early–Onset Sepsis. JAMA Pediatr 2017;171:365–71. [DOI] [PubMed] [Google Scholar]
- 30.Schulman J, Profit J, Lee HC, et al. Variations in Neonatal Antibiotic Use. Pediatrics 2018:e20180115. [DOI] [PMC free article] [PubMed]
- 31.Bizzarro MJ, Shabanova V, Baltimore RS, Dembry LM, Ehrenkranz RA, Gallagher PG. Neonatal sepsis 2004–2013: the rise and fall of coagulase–negative staphylococci. J Pediatr 2015;166:1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nzegwu NI, Rychalsky MR, Nallu LA, et al. Implementation of an Antimicrobial Stewardship Program in a Neonatal Intensive Care Unit. Infect Control Hosp Epidemiol 2017;38:1137–43. [DOI] [PubMed] [Google Scholar]
- 33.Mukhopadhyay S, Puopolo KM. Clinical and Microbiologic Characteristics of Early–onset Sepsis Among Very Low Birth Weight Infants: Opportunities for Antibiotic Stewardship. Pediatr Infect Dis J 2017;36:477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pichichero ME, Todd JK. Detection of neonatal bacteremia. J Pediatr 1979;94:958–60. [DOI] [PubMed] [Google Scholar]
- 35.Tremoulet A, Le J, Poindexter B, et al. Characterization of the population pharmacokinetics of ampicillin in neonates using an opportunistic study design. Antimicrob Agents Chemother 2014;58:3013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen EI, Sandstrom M, Honore PH, Ewald U, Friberg LE. Developmental pharmacokinetics of gentamicin in preterm and term neonates: population modelling of a prospective study. Clin Pharmacokinet 2009;48:253–63. [DOI] [PubMed] [Google Scholar]
- 37.McCarthy KN, Hawke A, Dempsey EM. Antimicrobial stewardship in the neonatal unit reduces antibiotic exposure. Acta Paediatr 2018. [DOI] [PubMed]
- 38.Garcia–Prats JA, Cooper TR, Schneider VF, Stager CE, Hansen TN. Rapid detection of microorganisms in blood cultures of newborn infants utilizing an automated blood culture system. Pediatrics 2000;105:523–7. [DOI] [PubMed] [Google Scholar]
- 39.Kumar Y, Qunibi M, Neal TJ, Yoxall CW. Time to positivity of neonatal blood cultures. Arch Dis Child Fetal Neonatal Ed 2001;85:F182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guerti K, Devos H, Ieven MM, Mahieu LM. Time to positivity of neonatal blood cultures: fast and furious? J Med Microbiol 2011;60:446–53. [DOI] [PubMed] [Google Scholar]
- 41.Biondi E, Evans R, Mischler M, et al. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics 2013;132:990–6. [DOI] [PubMed] [Google Scholar]
- 42.Schrag SJ, Farley MM, Petit S, et al. Epidemiology of Invasive Early–Onset Neonatal Sepsis, 2005 to 2014. Pediatrics 2016;138. [DOI] [PubMed]
- 43.Dellit TH, Owens RC, McGowan JE Jr., et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44:159–77. [DOI] [PubMed] [Google Scholar]
- 44.Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 1995;44:1–13. [PubMed] [Google Scholar]
- 45.Ericson JE, Thaden J, Cross HR, et al. No survival benefit with empirical vancomycin therapy for coagulase–negative staphylococcal bloodstream infections in infants. Pediatr Infect Dis J 2015;34:371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thaden JT, Ericson JE, Cross H, et al. Survival Benefit of Empirical Therapy for Staphylococcus aureus Bloodstream Infections in Infants. Pediatr Infect Dis J 2015;34:1175–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shane AL, Hansen NI, Stoll BJ, et al. Methicillin–resistant and susceptible Staphylococcus aureus bacteremia and meningitis in preterm infants. Pediatrics 2012:peds. 2011–0966. [DOI] [PMC free article] [PubMed]
- 48.Holzmann–Pazgal G, Khan AM, Northrup TF, Domonoske C, Eichenwald EC. Decreasing vancomycin utilization in a neonatal intensive care unit. Am J Infect Control 2015;43:1255–7. [DOI] [PubMed] [Google Scholar]
- 49.Cantey JB, Wozniak PS, Sanchez PJ. Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT study. Pediatr Infect Dis J 2015;34:267–72. [DOI] [PubMed] [Google Scholar]
- 50.Philip AG, Mills PC. Use of C–reactive protein in minimizing antibiotic exposure: experience with infants initially admitted to a well–baby nursery. Pediatrics 2000;106:E4. [DOI] [PubMed] [Google Scholar]
- 51.Benitz WE, Han MY, Madan A, Ramachandra P. Serial serum C–reactive protein levels in the diagnosis of neonatal infection. Pediatrics 1998;102:E41. [DOI] [PubMed] [Google Scholar]
- 52.Coggins SA, Wynn JL, Hill ML, et al. Use of a computerized C–reactive protein (CRP) based sepsis evaluation in very low birth weight (VLBW) infants: a five–year experience. PLoS One 2013;8:e78602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner M, Power S, Emmerson A. Gestational age and the C reactive protein response. Archives of Disease in Childhood–Fetal and Neonatal Edition 2004;89:F272–F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cals JW, Schot MJ, de Jong SA, Dinant G–J, Hopstaken RM. Point–of–care C–reactive protein testing and antibiotic prescribing for respiratory tract infections: a randomized controlled trial. The Annals of Family Medicine 2010;8:124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quenot JP, Luyt CE, Roche N, et al. Role of biomarkers in the management of antibiotic therapy: an expert panel review II: clinical use of biomarkers for initiation or discontinuation of antibiotic therapy. Ann Intensive Care 2013;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nora D, Salluh J, Martin–Loeches I, Povoa P. Biomarker–guided antibiotic therapy–strengths and limitations. Ann Transl Med 2017;5:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375:463–74. [DOI] [PubMed] [Google Scholar]
- 58.Layios N, Lambermont B. Procalcitonin for antibiotic treatment in intensive care unit patients. Curr Infect Dis Rep 2013;15:394–9. [DOI] [PubMed] [Google Scholar]
- 59.Franz AR, Bauer K, Schalk A, et al. Measurement of interleukin 8 in combination with C–reactive protein reduced unnecessary antibiotic therapy in newborn infants: a multicenter, randomized, controlled trial. Pediatrics 2004;114:1–8. [DOI] [PubMed] [Google Scholar]
- 60.Stocker M, van Herk W, El Helou S, et al. Procalcitonin–guided decision making for duration of antibiotic therapy in neonates with suspected early–onset sepsis: a multicentre, randomised controlled trial (NeoPIns). Lancet 2017;390:871–81. [DOI] [PubMed] [Google Scholar]
- 61.Mukherjee A, Davidson L, Anguvaa L, Duffy DA, Kennea N. NICE neonatal early onset sepsis guidance: greater consistency, but more investigations, and greater length of stay. Arch Dis Child Fetal Neonatal Ed 2015;100:F248–9. [DOI] [PubMed] [Google Scholar]
- 62.Kiser C, Nawab U, McKenna K, Aghai ZH. Role of guidelines on length of therapy in chorioamnionitis and neonatal sepsis. Pediatrics 2014;133:992–8. [DOI] [PubMed] [Google Scholar]
- 63.Hemels MA, van den Hoogen A, Verboon–Maciolek MA, Fleer A, Krediet TG. Shortening the antibiotic course for the treatment of neonatal coagulase–negative staphylococcal sepsis: fine with three days? Neonatology 2012;101:101–5. [DOI] [PubMed] [Google Scholar]
- 64.Couto RC, Barbosa JA, Pedrosa TM, Biscione FM. C–reactive protein–guided approach may shorten length of antimicrobial treatment of culture–proven late–onset sepsis: an intervention study. Braz J Infect Dis 2007;11:240–5. [DOI] [PubMed] [Google Scholar]
- 65.Mukhopadhyay S, Puopolo KM. Relevance of Neonatal Anaerobic Blood Cultures: New Information for an Old Question. J Pediatric Infect Dis Soc 2017. [DOI] [PubMed]