Abstract
Background:
We recently developed a novel ciprofloxacin-coated sinus stent capable of releasing antibiotics over a sustained period of time. Ivacaftor is a CFTR potentiator that has synergistic bactericidal activity with ciprofloxacin and also enhances sinus mucociliary clearance. The objective of this study was to optimize and evaluate the efficacy of a ciprofloxacin and ivacaftor releasing biodegradable sinus stent (CISS) in vitro.
Methods:
A CISS was created by coating ciprofloxacin/ivacaftor-embedded nanoparticles with an acrylate and ammonium methacrylate copolymer onto a biodegradable poly-L-lactic acid stent. In vitro evaluation of the CISS included: 1) assessment of drug stability in nanoparticles by zeta potential, and drug coating stability within the CISS using scanning electron microscopy (SEM); 2) determination of ciprofloxacin and ivacaftor release kinetics; and 3) assessment of anti-Pseudomonas aeruginosa biofilm formation by calculating relative optical density units (RODUs) compared to control stents at 590nm.
Results:
The presence of drugs and a uniform coating on the stent were confirmed by zeta potential and SEM. Sustained drug release was observed through 21 days without an initial burst release. Anti-biofilm formation was observed after placing the CISS for 3 days on to a preformed 1 day P. aeruginosa biofilm. The CISS significantly reduced biofilm mass compared to bare stents and controls (RODUs at OD590, CISS=0.31+/− 0.01, bare stent=0.78+/−0.12, control=1.0+/−0.00, p=0.001, n=3).
Conclusion:
The CISS maintains a uniform coating and sustained delivery of drugs providing a marked reduction in P. aeruginosa biofilm formation. Further studies evaluating the efficacy of CISS in a preclinical model are planned.
Keywords: Pseudomonas Aeruginosa, Ciprofloxacin, Ivacaftor, Sinus stent Biofilm, Chronic Rhinosinusitis, recalcitrant sinusitis
INTRODUCTION
Chronic rhinosinusitis (CRS) is a chronic inflammatory and infectious process of the sinus and nasal cavities and is a common chronic disease afflicting 14–16% of the adult population in the United States.1 Bacterial biofilms, aggregates of extracellular polysaccharides and bacteria that form a “sludge”, are likely a key modulator of the refractory nature of CRS and increase the tolerance of bacteria to antibiotics through numerous mechanisms.2 They have been found on the sinonasal mucosa of up to 54% of CRS sufferers, compared to 8% of control patients.3 Multiple studies have noted a higher prevalence of biofilms in patients who are undergoing revision sinus surgery.3,4 In particular, the presence of biofilm-forming Pseudomonas aeruginosa strains has been associated with poor resolution of symptoms and signs of CRS following endoscopic sinus surgery.5
As antibiotic treatments often involve a long course of therapy, sufficient antibiotic exposure is needed to ensure the eradication of the microorganism. To avoid systemic side effects, topical drug-eluting implants with prolonged mucosal contact time and sustained drug release may provide a suitable therapeutic option.6,7 This approach allows for a larger dose and more efficient delivery of the drug to penetrate into biofilms, and thus result in a potent therapeutic effect. We recently developed a ciprofloxacin eluting sinus stent that was shown to be effective in clearing P. aeruginosa biofilms both in-vitro and in-vivo using a rabbit sinusitis model.6 In treating multi-drug resistant bacterial pathogens, combining 2 or more distinct antibiotics represents a common strategy in treating bacterial infections with the aim to broaden the antimicrobial spectrum, generate synergistic effects, and counteract antibiotic resistance.8 In addition, agents that enhance the antimicrobial activity of currently available antibiotics may represent a valuable and cost-effective means for improving clinical efficacy.9
Ivacaftor is a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator that enhances Cl− secretion in airway epithelia, including the sinonasal mucosa.10–13 The drug was recently identified to have potentially beneficial off-target effects as a weak inhibitor of bacterial DNA gyrase and topoisomerase IV due to its potential chemical similarity with ciprofloxacin (fluoroquinolones).9,14 Simultaneous delivery of ciprofloxacin and ivacaftor may have synergistic bactericidal effects, while also increasing mucociliary clearance (MCC) by promoting Cl− transport.14,15 We have shown previously that ivacaftor and ciprofloxacin have synergistic bactericidal activity when used concurrently in vitro (in press).16 The impact of the 2 drugs on P. aeruginosa biofilms has yet to be evaluated. The objective of the current study was to optimize and evaluate the efficacy of a ciprofloxacin and ivacaftor releasing biodegradable sinus stent (CISS) in vitro.
METHODS
Materials
Ciprofloxacin HCl (99.5% purity) was purchased from GenHunter Corporation (Nashville, TN). Ivacaftor (VX-770) was obtained from Selleckchem (Houston, TX). Poly (D, L-lactide-co-glycolide) (PLGA) was purchased from PolySciTech (West Layfeyette, IN). All other chemicals and reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO)
Preparation of PLGA nanoparticles with ciprofloxacin and ivacaftor
Ciprofloxacin-loaded PLGA nanoparticles
An emulsion/solvent technique was used to create ciprofloxacin-loaded PLGA nanoparticles.17 First, 15 mg of ciprofloxacin was dissolved in 750 μl deionized water and subsequently mixed with 2.5% (w/v) of PLGA solution in dichloromethane (DCM). This solution was then placed in an iced water bath and ultrasonically agitated to prepare a homogenous water in oil emulsion (W/O). For each batch, 1ml of the resulting W/O emulsion was immediately poured into 25 ml of a 1% (w/v) polyvinyl alcohol solution (PVA, 98.0 ~ 98.8 % hydrolyzed, Mw = 31,000 – 50,000) and stirred vigorously for 2 min using a homogenizer. The suspended nanoparticles were then placed onto a stir plate to evaporate the organic solvent in a chemical fume hood overnight. The nanoparticles were then collected by centrifugation at 4000 rpm for 20 min at 4 °C, and resuspended in 10 ml of distilled water, following which large particulates and aggregates were removed through the use of a 40μm cell strainer.
Ivacaftor-loaded PLGA nanoparticles
Ivacaftor-containing nanoparticles were fabricated using a solvent evaporation method in a similar fashion to ciprofloxacin nanoparticles. An ivacaftor stock solution (25 mg/ml) was prepared in dimethyl sulfoxide (DMSO) and aliquoted into separate 300 μl ivacaftor solutions. To prepare a mixture of ivacaftor and PLGA in DCM, the 300 μl ivacaftor solution was dissolved in 10 ml of dichloromethane containing 250 mg of PLGA polymer. Subsequently, 1 ml of the mixture was slowly added into 25ml of 1% (w/v) PVA solution, and then homogenized at 32,000 rpm for 2 min to create ivacaftor-containing nanoparticles. After creating ivacaftor-loaded PLGA nanoparticles, the collection was made based on similar protocols of ciprofloxacin-loaded PLGA nanoparticles, described above.18
Characterization of the prepared PLGA nanoparticles with ciprofloxacin or ivacaftor
To examine the morphology of the fabricated PLGA nanoparticles, a field emission scanning electron microscope (SEM) was used (FE-SEM, Quanta FEG-650, USA). Prior to scanning, the PLGA nanoparticles were coated with an Au-Pd sputter to enhance surface conductivity while reducing charging artifacts. An accelerating voltage of 20 kV was used in most cases, and the SEM images were processed at the UAB SEM Laboratory. Zeta potentials (the electrostatic potential at the electrical double layer surrounding a nanoparticle in solution) were determined by using a Malvern Zetasizer Nano ZS apparatus (Malvern Instruments Ltd, Worcestershire, UK).
Fabrication of a ciprofloxacin and ivacaftor coated sinus stent for in vitro analysis
Model biodegradable poly-D/L-lactic acid (PLLA) stents (Biogeneral, Inc. San Diego CA) were utilized to create the CISS. Two separate layers of coating were generated: 1) inner layer – ciprofloxacin only and 2) outer layer – ciprofloxacin + ivacaftor. The inner layer (ciprofloxacin nanoparticles) was first coated in the model PLLA stents with a solution of Eudragit RS 100 polymer in acetone (25 % w/v). Eudragit RS 100 (a copolymer of ethyl acrylate, methyl methacrylate and a low content of methacrylic acid ester with quaternary ammonium groups) was used for time-controlled drug release by sustained release formulations.19 Ciprofloxacin nanoparticles were coated in the inner layer to prevent burst release of hydrophilic ciprofloxacin.20 In the outer layer, the stents were coated with both ciprofloxacin and ivacaftor nanoparticles suspended in the solution of Eudragit RS 100. Sixty μg of ciprofloxacin and 300 μg of ivacaftor were coated on the stent. Finally, the stents were dried under vacuum for 2 days at room temperature.
In vitro release profile of the CISS
To assess their in vitro release profiles, model CISS stents containing ciprofloxacin (60 μg) and ivacaftor (300 μg) were placed in 3 mL of sterilized phosphate buffered saline (PBS) and collected periodically for up to 21 days. For the assay of released ciprofloxacin concentration, a ciprofloxacin enzyme-linked immunosorbent assay (ELISA) kit (REAGEN™, Moorestown NJ) was used according to the manufacture’s protocol. The ivacaftor concentration was evaluated by measuring the absorbance at 230 nm using a microplate reader (Synergy HK, BIO-TEK Instruments, Winooski, VT).
Evaluation of anti-biofilm activity of CISS
Quantitative analysis by crystal violet staining
To assess the efficacy of the fabricated CISS against P. aeruginosa (PAO-1 strain) biofilms21, a crystal violet assay was used. Briefly, stents loaded with the drugs were placed in a 24-well tissue culture plate and inoculated with 100 μL of 100-fold diluted overnight culture grown at 30°C. Stents without loaded drugs served as negative controls. After 3 days, the attached biofilm was assessed as previously described. Three ml of 0.1% (w/v) crystal violet was used to stain the biofilms. Next, 900 μL of 30% acetic acid was used to dissolve the PAO-1 biofilms and release the conjugated crystal violet dye. Absorbance was then measured at 590nm to quantify the amount of crystal violet present.
Quanitative analysis by confocal laser scanning microscopy (CLSM)
To create pre-formed PAO-1 biofilms, PAO-1 was cultured for 24 hours on 14mm glass coverslips within a 35mm dish (MatTek, Ashland, MA). In the pre-formed PAO-1 biofilms, stents containing loaded drugs (ciprofloxacin and ivacftor) were then placed in a 24-well tissue culture plate and cultured for an additional three days. Stents without loaded drugs were also introduced to serve as a negative control. To visualize both viable and dead bacterial populations, biofilms were stained with SYTO9 and propidium iodide (PI) staining (BacLight™ Live/Dead Bacterial Viability Kit; Molecular Probes, Eugene, OR). The biofilms were imaged with CLSM (A1R, Nikon, Tokyo, Japan) and biofilm thickness quantified using Image J by referencing at least 4 different images per condition. The proportions of live and dead bacteria were also quantified using BioFilmAnalyzer v.1.0 (https://bitbucket.org/rogex/biofilmanalyzer/downloads/)22 by counting fluorescence specific pixels in digital fluorescent images. Five different images were selected for analysis per condition.
Statistical Analysis
All experiments were performed in triplicate. Statistical analysis was performed with GraphPad Prism 6.0 (La Jolla, Ca). For assessing the anti-biofilm activity of stents, a one-way ANOVA was performed with a post-hoc Dunnett’s multiple comparison test with significance set at p < 0.05. Normalized values for relative biofilm quantification were expressed as ± standard error of the mean.
RESULTS
Surface morphology and size of drug-loaded PLGA nanoparticles
To visualize the surface morphology and size distribution, nanoparticles loaded with ciprofloxacin or ivacaftor were imaged with SEM. Drug-loaded nanoparticles were spherical in shape (Figure 1). In terms of particle size, both nanoparticle configurations had similar size distributions (mean diameter of ciprofloxacin nanoparticle = 556.6 +/− 64.05 nm, mean diameter of ivacaftor nanoparticle = 553.8 +/− 72.93 nm, p > 0.05). The measurement of zeta potential was performed to confirm the surface charge property of the nanoparticles formulations, which identifies the presence of ciprofloxacin or ivacaftor within the PLGA nanoparticles. The zeta potential of the empty PLGA nanoparticles was −1.28 +/− 1.3 mV, a negative charge, indicating the lack of any drug present on the surface of the nanoparticles. In contrast, drug loaded nanoparticles produced a higher, positive charge, with ciprofloxacin-loaded particles exhibiting a zeta potential of 0.17 +/− 1.1 mV (p < 0.01), and ivacaftor-loaded particles exhibiting a zeta potential of 0.27 +/− 1.4 mV (p = 0.005). These results confirmed the presence of ciprofloxacin and ivacaftor on the PLGA nanoparticles.
Figure 1.
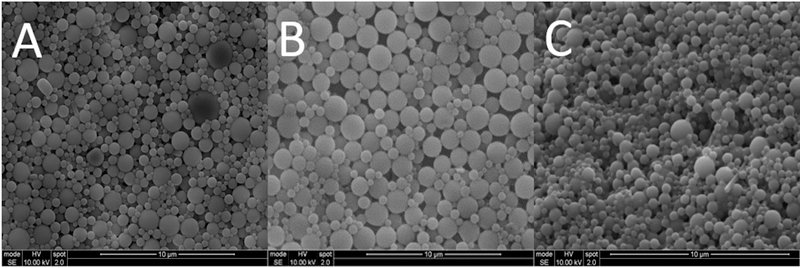
Scanning electron microscopy (SEM) images of the ciprofloxacin and ivacaftor loaded nanoparticles. The scale bar at lower right is 10 μms. A: Ciprofloxacin nanoparticles, B: Ivacaftor nanoparticles, C: Mixture of both Ciprofloxacin + Ivacaftor nanoparticles.
Structural morphology of the CISS
The surface morphology of the CISS was characterized using SEM (Figure 2). Both ciprofloxacin and ivacaftor loaded nanoparticles were embedded within an acrylate and ammonium methacrylate copolymer polymeric matrix coating on the surface of the PLLA stents. Figure 2A demonstrates the top-down view of the CISS. The presence of nanoparticles within the coating matrix produced a “bumpy”, mountainous appearance with size distribution approaching a similar size to nanoparticle images shown in Figure 1. Cross sectional images (Figure 2B) of the CISS demonstrated that 2 layers of the nanoparticles are present throughout the full thickness of the coating. Additionally, both inner and outer layers can be easily visualized with clear distinction. To understand the degradation of the CISS over time, cross-sectional SEM imaging was performed on stents used in the drug release profile experiment at 21 days (Figure 2C). There was a bulk loss of the outer layer over the course of the experiment, with thinning of both layers present on the stent surface and absence of the “bumpy” appearance. This confirms degradation of the coated layers with the nanoparticles released over time.
Figure 2.
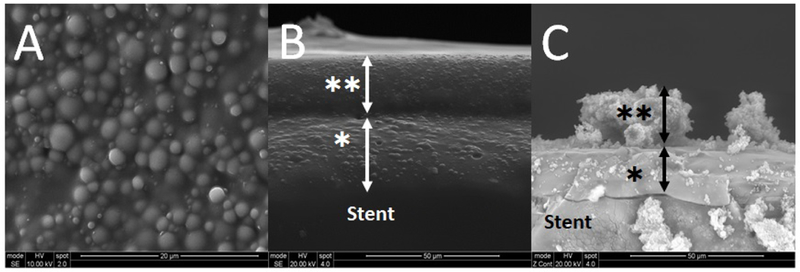
SEM images of the ciprofloxacin and ivacaftor releasing biodegradable sinus stent (CISS). The scale bar is located at lower right. * : Inner layer, **: Outer layer (arrow: thickness of each layer). A.Top-down view of the CISS surface. B. Cross sectional view of the dual layers of CISS (before use) C. Cross sectional view of the dual layers of CISS (21 days after in vitro release)
In vitro release profile of the CISS
To determine the specific release profile for the drug eluting stent, an in-vitro release profile assay was performed (Figure 3). A similar steady-state release was achieved with both drugs without an initial burst release. Approximately 50% of the coated ciprofloxacin and ivacaftor were released by 10 days (ciprofloxacin 49.1 +/− 9.4 %, ivacaftor 51.2 +/− 2.3 % at day 10) and 80% by 21days (ciprofloxacin 77.1 +/− 9.6 %, ivacaftor 82.2 +/− 5.3 % at day 21). Although ivacaftor was only present on the outer layer of the CISS stent, a sustained release profile over the course of the 21 days was identified, The hydrophobic nature of the ivacaftor is likely responsible for the consistent release.
Figure 3.
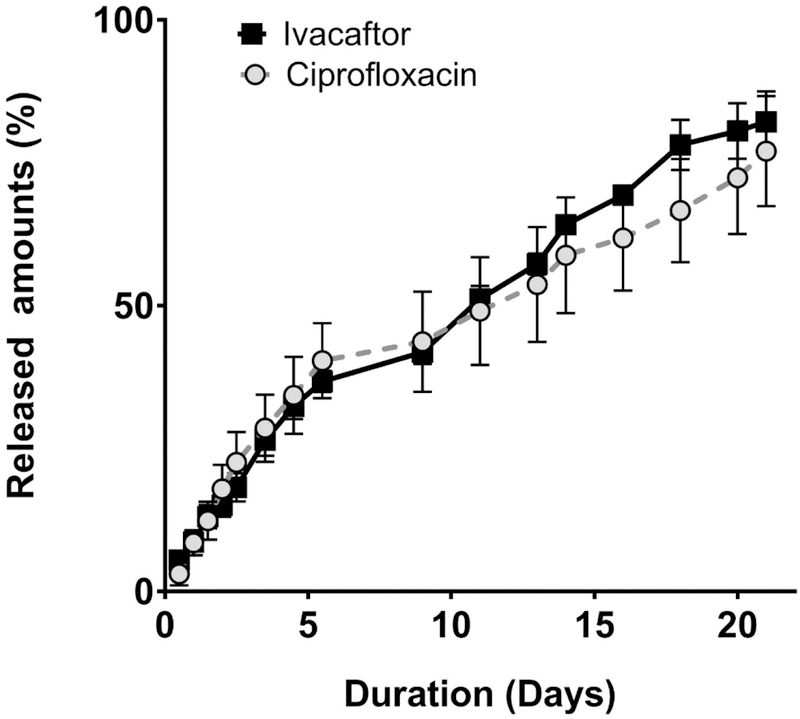
In vitro release profile of the CISS
Anti-biofilm activity of the CISS against P. aeruginosa biofilms
Crystal violet staining
To determine the anti-biofilm activity of CISS stents, a standard crystal biofilm assay was performed (Figure 6). In this assay, PAO-1 biofilms were grown for 24 hours and then subjected to 1 of the following 3 conditions for 72 hours: 1) CISS, 2) PLLA stent without drugs (bare stent), 3) control. The CISS significantly reduced biofilm mass compared to bare stents and controls (relative biofilm value compared to control at OD590, CISS = 0.31 +/− 0.01, bare stent = 0.78 +/− 0.12, control = 1.0 +/− 0.00, p = 0.001, n = 3).
Figure 6.
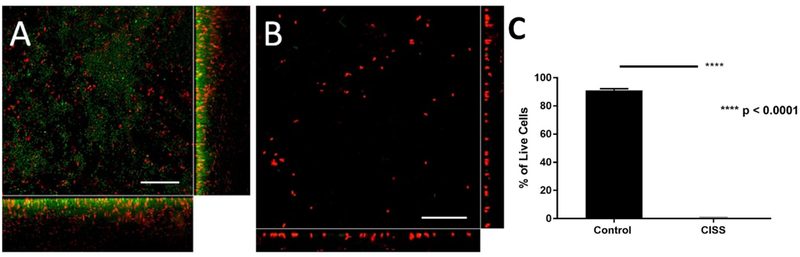
Efficacy (Inhibition of preformed PAO-1 biofilms) of CISS. Representative confocal laser scanning microscopy (CLSM) images of PAO-1 biofilms grown three days with or without placing CISS on preformed biofilms. CLSM orthogonal images of Z-stacks show a plane view (sqaure) looking down the biofilm and side views through the biofilm (right and below). Scale bar indicates 20 μm. A: PAO-1 biofilm without CISS B: PAO-1 biofilm with CISS C: C: % of Live Cells between Control and CISS
CLSM analysis
The bactericidal efficacy of CISS stents against P. aeruginosa biofilms was further assessed using live-dead staining. To evaluate whether CISS stents can prevent the formation of biofilms, bacteria were grown in the presence of CISS stents and biofilm height was measured on day 1. Biofilm thickness was significantly lower in the presence of CISS (11.66 +/− 2.8 μm) compared to without CISS (22.94 +/− 3.30 μm), indicating that CISS stents can inhibit the formation of PAO-1 biofilms (p < 0.01) (Figure 5). Biofilm formation was significantly decreased in the presence of CISS compared to controls without CISS (% of live cells; control without CISS = 88.9 +/− 2 %; CISS = 8.5 +/− 3.2 %; n = 4 per condition, p < 0.0001) (Figure 5C). To assess the capability of the stent to eradicate biofilms (efficacy), the CISS was placed on pre-formed 1-day old PAO-1 biofilms and grown for additional 3 days (Figure 6). There was a significant reduction in the PAO-1 biofilm height (4.16 +/− 1.87 μm) when compared to those from controls without exposure to the CISS (14.94 +/− 2.78 μm) (p < 0.001). Biofilm presence was significantly decreased in the presence of CISS on day 4 compared to controls without CISS (% of live cells; control without CISS = 91.1 +/− 1 %; CISS = 0.3 +/− 0.6 %; n = 4 per condition, p < 0.0001) (Figure 6C). Biofilms within the control group also had a mixture of both live and dead cells likely due to nutrient deprivation from over confluence of bacteria.
Figure 5.
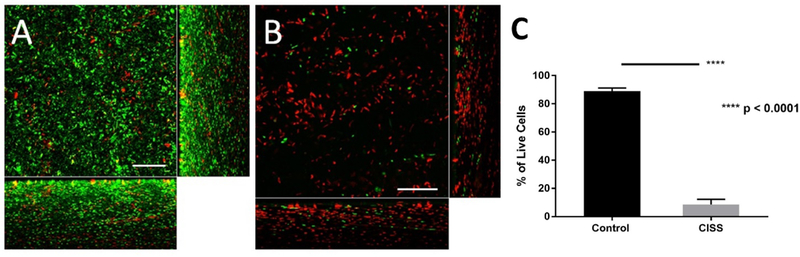
Effect of the CISS on biofilm formation. Representative confocal laser scanning microscopy (CLSM) images of PAO-1 biofilms with and without CISS after 24 hours. CLSM orthogonal images of Z-stacks show a plane view (square) looking down the biofilm and side views through the biofilm (right and below). Scale bar indicates 20 μm. A: PAO-1 biofilm without CISS B: PAO-1 biofilm with CISS C: % of Live Cells between Control and CISS
DISCUSSION
One of the most commonly encountered pathogens responsible for medically recalcitrant CRS is P. aeroginosa.23 In the current study, ciprofloxacin and ivacaftor embedded nanoparticle coated stents were characterized using SEM, zeta potential measurements and drug release kinetics. Bactericidal activity of the CISS on P. aeroginosa was also evaluated. A sustained release of ciprofloxacin and ivacaftor from the nanoparticle coated sinus stents over a 21-day time frame was observed and the CISS was able to eradicate P.aeruginosa PAO-1 biofilms in the in vitro assay.
Besides antimicrobial synergism described in our previous publication, the dual coating (hydrophilic and hydrophobic layers) has the additional advantage of having improved controlled release of the two drugs since the single coating of ciprofloxacin led to an initial burst release of the drug. The double layer coating (inner layer = ciprofloxacin, outer layer = ciprofloxacin + ivacaftor) was planned so that ivacaftor will be released during the initial insertion to enhance clearance of thick mucus by stimulating transepithelial Cl− secretion. Theoretically, this would enable more effective delivery of ciprofloxacin to the biofilm without additional interference from thick mucus. Simultaneously, ciprofloxacin was released at a more constant rate over a longer timeframe, providing long-term bactericidal activity against PAO-1 based biofilms. The ciprofloxacin concentrations coated in this model stent (60 μg) were calculated based on 1) the surface of area, 2) previous analysis and 3) coating technique.6 The surface area of this model stent (5mm in length) is approximately 40mm2, which is about 1/10 of the future in vivo CISS stent.6 The double coating in the CISS achieved a constant release of ciprofloxacin over time (week 1= 3.46 +/− 0.56 μg/day, week 2 = 1.58 +/− 0.30 μg/day, and week 3 = 1.52 ± 0.04 μg/day). Notably, the area under the concentration-time curve (AUC0–24) to the MIC ratio (concentration-dependent killing) (MIC of ciprofloxacin for P. aeruginosa PAO1 strain H103 = 0.06 μg/ml)24 was around 57.6 at week 1 (day 7) and around 25.3 at week 3 from 60 μg of ciprofloxacin coating. The CISS for in vivo studies will be coated with 0.6mg, which should provide concentrations 10 times higher than the in vitro stent and yield AUC0–24/MIC far in excess of the minimum goal (>100).25,26 By coating the hydrophilic drug in the inner layer and the hydrophobic ivacaftor with ciprofloxacin in the outer layer(double coating), the drug release is much higher than what was measured with the 2 mg coating in the single layer ciprofloxacin stent used previously.6,27
Regarding ivacaftor, the concentration was calculated based on our previous in vitro experiments.28 PAO-1 growth was significantly reduced in the presence of ivacaftor (8 or 16μg/ml) and ciprofloxacin. Therefore, our goal was to release the ivacaftor at least 8 μg/ml per day to achieve this synergism. In this study, the ivacaftor release rate was more linear (week 1 = 15.75 ± 0.80 μg/day, week 2 = 11.77 ± 0.24 μg/day, and week 3 = 7.70 ± 1.71 μg/day). As the rabbit’s sinus volume is approximately 2500 mm3 (2.5 ml),29 the total amount of ivacaftor coated in the CISS will be 1 mg (3 times higher than current concentrations) for future in vivo preclinical studies to achieve the targeted release rate (around 8 μg/ml/day). Interestingly, even though ivacaftor was only present on the outer coating of the CISS stent, there was consistent release over 21 days and is likely due to the hydrophobic nature of ivacaftor.
Many drugs used for treating sinusitis are administered as nasal sprays/irrigation or oral/intravenous formulations. CRS is a prolonged condition (> 12 weeks), so a drug delivery system providing prolonged mucosal contact time with local absorption and minimal depletion are the desired requirements, especially for eradicating biofilms.30 The addition of ivacaftor to the sinus stent incorporates several synergistic elements: 1) sustained release of ciprofloxacin and ivacaftor for bactericidal effects, and 2) ivacaftor release to enhance sinus mucociliary clearance. Controlled delivery of ciprofloxacin and ivacaftor using the sinus stent could clear P. aeruginosa infection more efficiently and represents an exciting treatment strategy for recalcitrant CRS.
There are several limitations to this study. Although the synergism of ciprofloxacin and ivacaftor has been described in our previous publication,16 we did not use the single drug stents as a control in this study. Thus, the synergism of the drugs noted in previous in vitro studies may not translate to improved antimicrobial activity when released from the stent and will need further evaluation. Only 1 strain of Pseudomonas was tested in this study. This bacteria is well known for its intrinsic resistance to a variety of antimicrobial agents and toxic compounds and there is significant variability among clinical isolates.31 Further studies are planned to test against different multi-drug resistant strains of Pseudomonas. Additionally, the drug release with the current stent will need modifications to improve concentrations on future iterations prior to planned animal studies.
CONCLUSION
The CISS is capable of delivering both ciprofloxacin and ivacaftor at sustained concentrations for up to 3 weeks and has significant bactericidal activity and biofilm reduction in in vitro assays. The innovative design using a double layered drug coating on the surface of the PLLA stent may provide therapeutic advantages over current treatment strategies for recalcitrant bacterial infections in CRS. Future studies assessing the CISS stents in preclinical animal models with active maxillary P. aeruginosa sinusitis are planned.
Figure 4.
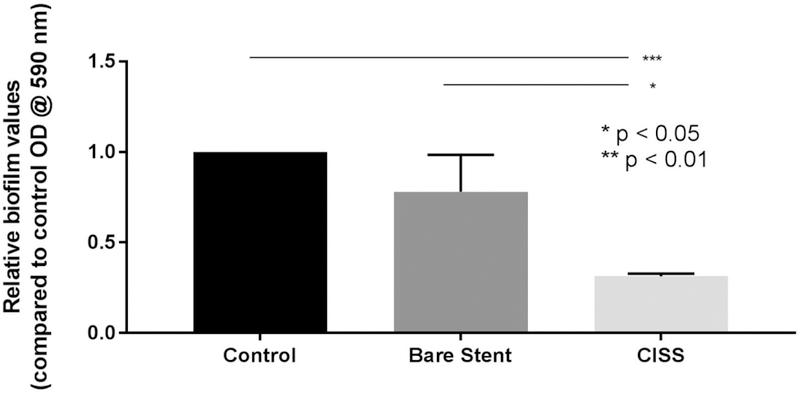
Effect of CISS on Pseudomonas aeruginosa PAO-1 biofilms: CISS significantly reduced biofilm mass. #: Statistical significance when compared to control (p < 0.0001) in reducing PAO-1 biofilm formation (n = 3, respectively).
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006–03) and National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482–05, CF Research Center Pilot Award) to B.A.W. and John W. Kirklin Research and Education Foundation Fellowship Award, UAB Faculty Development Research Award, American Rhinologic Society New Investigator Award, and Cystic Fibrosis Foundation Research Development Pilot grant (ROWE15R0) to D.Y.C.
Do-Yeon Cho, MD receives research grant support from Bionorica Inc.
Bradford A. Woodworth, M.D. is a consultant for Olympus and Cook Medical. He also receives grant support from Cook Medical and Bionorica Inc.
Footnotes
Manuscript was presented at the American Rhinologic Society Meetings, Atlanta, GA, Oct 5th, 2018.
References
- 1.Anand VK. Epidemiology and economic impact of rhinosinusitis. Ann Otol Rhinol Laryngol Suppl 2004; 193:3–5. [DOI] [PubMed] [Google Scholar]
- 2.Fastenberg JH, Hsueh WD, Mustafa A, Akbar NA, Abuzeid WM. Biofilms in chronic rhinosinusitis: Pathophysiology and therapeutic strategies. World J Otorhinolaryngol Head Neck Surg 2016; 2:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H-H, Liu X, Ni C et al. Bacterial biofilms in chronic rhinosinusitis and their relationship with inflammation severity. Auris Nasus Larynx 2012; 39:169–174. [DOI] [PubMed] [Google Scholar]
- 4.Psaltis AJ, Ha KR, Beule AG, Tan LW, Wormald PJ. Confocal scanning laser microscopy evidence of biofilms in patients with chronic rhinosinusitis. Laryngoscope 2007; 117:1302–1306. [DOI] [PubMed] [Google Scholar]
- 5.Bendouah Z, Barbeau J, Hamad WA, Desrosiers M. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol Head Neck Surg 2006; 134:991–996. [DOI] [PubMed] [Google Scholar]
- 6.Cho DY, Hoffman K, Skinner D et al. Tolerance and pharmacokinetics of a ciprofloxacin-coated sinus stent in a preclinical model. Int Forum Allergy Rhinol 2017; 7:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parikh A, Anand U, Ugwu MC, Feridooni T, Massoud E, Agu RU. Drug-eluting nasal implants: formulation, characterization, clinical applications and challenges. Pharmaceutics 2014; 6:249–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao W, Thamphiwatana S, Angsantikul P, Zhang L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2014; 6:532–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider EK, Azad MA, Han ML et al. An “Unlikely” Pair: The Antimicrobial Synergy of Polymyxin B in Combination with the Cystic Fibrosis Transmembrane Conductance Regulator Drugs KALYDECO and ORKAMBI. ACS Infect Dis 2016; 2:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaaban MR, Kejner A, Rowe SM, Woodworth BA. Cystic fibrosis chronic rhinosinusitis: a comprehensive review. Am J Rhinol Allergy 2013; 27:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Illing EA, Woodworth BA. Management of the upper airway in cystic fibrosis. Curr Opin Pulm Med 2014; 20:623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe SM, Liu B, Hill A et al. Optimizing nasal potential difference analysis for CFTR modulator development: assessment of ivacaftor in CF subjects with the G551D-CFTR mutation. PLoS One 2013; 8:e66955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Blount AC, McNicholas CM et al. Resveratrol enhances airway surface liquid depth in sinonasal epithelium by increasing cystic fibrosis transmembrane conductance regulator open probability. PLoS One 2013; 8:e81589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reznikov LR, Abou Alaiwa MH, Dohrn CL et al. Antibacterial properties of the CFTR potentiator ivacaftor. J Cyst Fibros 2014; 13:515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernarde C, Keravec M, Mounier J et al. Impact of the CFTR-potentiator ivacaftor on airway microbiota in cystic fibrosis patients carrying a G551D mutation. PLoS One 2015; 10:e0124124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho DYL, Dong Jin; Mackey Calvin; Skinner Daniel; Zhang Shaoyan; McCormick Justin; Woodworth Bradford A. Cystic Fibrosis Transmembrane Conductance Regulator Potentiator, Ivacaftor, Enhances Ciprofloxacin Activity Against Pseudomonas Aeruginosa American Journal of Rhinology and Allergy 2018(In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. J Biomater Sci Polym Ed 2006; 17:247–289. [DOI] [PubMed] [Google Scholar]
- 18.Gunday Tureli N, Torge A, Juntke J et al. Ciprofloxacin-loaded PLGA nanoparticles against cystic fibrosis P. aeruginosa lung infections. Eur J Pharm Biopharm 2017; 117:363–371. [DOI] [PubMed] [Google Scholar]
- 19.Li HS, Singh B, Park TE et al. Mannandecorated thiolated Eudragit microspheres for targeting antigen presenting cells via nasal vaccination. Eur J Pharm Sci 2015; 80:16–25. [DOI] [PubMed] [Google Scholar]
- 20.Garhwal R, Shady SF, Ellis EJ et al. Sustained ocular delivery of ciprofloxacin using nanospheres and conventional contact lens materials. Invest Ophthalmol Vis Sci 2012; 53:1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merritt JH, Kadouri DE, O’Toole GA. Growing and analyzing static biofilms. Curr Protoc Microbiol 2005; Chapter 1:Unit 1B 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogachev MI, Volkov VY, Markelov OA et al. Fast and simple tool for the quantification of biofilm-embedded cells sub-populations from fluorescent microscopic images. PLoS One 2018; 13:e0193267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meltzer EO, Hamilos DL, Hadley JA et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol 2004; 114:155–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brazas MD, Hancock RE. Ciprofloxacin induction of a susceptibility determinant in Pseudomonas aeruginosa. Antimicrob Agents Chemother 2005; 49:3222–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao P, Nie X, Zou M, Shi Y, Cheng G. Recent advances in materials for extended-release antibiotic delivery system. J Antibiot (Tokyo) 2011; 64:625–634. [DOI] [PubMed] [Google Scholar]
- 26.Haeseker M, Stolk L, Nieman F et al. The ciprofloxacin target AUC : MIC ratio is not reached in hospitalized patients with the recommended dosing regimens. Br J Clin Pharmacol 2013; 75:180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho DY, Lim DJ, Mackey C et al. Preclinical therapeutic efficacy of the ciprofloxacin-eluting sinus stent for Pseudomonas aeruginosa sinusitis. Int Forum Allergy Rhinol 2018; 8:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho DY. Ciprofloxacin Antimicrobial Activity against Pseudomonas Aeruginosa Is Enhanced by the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Potentiator, Ivacaftor Presented at: American Rhinologic Society Spring Meeting 2017 (COSM) [Google Scholar]
- 29.Xi J, Si XA, Kim J et al. Anatomical Details of the Rabbit Nasal Passages and Their Implications in Breathing, Air Conditioning, and Olfaction. Anat Rec (Hoboken) 2016; 299:853–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albu S Novel drug-delivery systems for patients with chronic rhinosinusitis. Drug Des Devel Ther 2012; 6:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolter DJ, Black JA, Lister PD, Hanson ND. Multiple genotypic changes in hypersusceptible strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients do not always correlate with the phenotype. J Antimicrob Chemother 2009; 64:294–300. [DOI] [PubMed] [Google Scholar]


