Abstract
Sleep and wakefulness control in the mammalian brain requires the coordination of various discrete interconnected neurons. According to the most conventional sleep model, wake-promoting neurons (WPNs) and sleep-promoting neurons (SPNs) compete for network dominance, creating a systematic “switch” that results in either the sleep or awake state. WPNs and SPNs are ubiquitous in the brainstem and diencephalon, areas that together contain less than 1% of the neurons in the human brain. Interestingly, many of these WPNs and SPNs co-express and co-release various types of the neurotransmitters that often have opposing modulatory effects on the network. Co-transmission is often beneficial to structures with limited numbers of neurons because it provides increasing computational capability and flexibility. Moreover, co-transmission allows subcortical structures to bi-directionally control postsynaptic neurons, thus helping to orchestrate several complex physiological functions such as sleep. Here, we present an in-depth review of co-transmission in hypothalamic WPNs and SPNs and discuss its functional significance in the sleep-wake network.
1. Introduction
Multiple brain regions interact to modulate sleep and wakefulness, and distinct neuronal populations synthesize different neurotransmitters that regulate sleep-wake dynamics in a hierarchical manner. Wakefulness is governed by wake-promoting neurons (WPNs), including noradrenergic neurons of the locus coeruleus (LC), serotonergic neurons of the raphe nuclei (RN), histaminergic neurons of the tuberomammillary nucleus (TMN), and orexin/hypocretin-producing neurons of the perifornical nuclei (PFN)/lateral hypothalamic area (LHA)1-8. Sleep-promoting neurons (SPNs) include melanin-concentrating hormone-producing (MCH) neurons in the diencephalon and GABAergic neurons in the ventrolateral preoptic nuclei (VLPO)/intermediate nuclei, median preoptic nuclei (MnPO), and brainstem parafacial zone9-13. A prominent hypothesis of a sleep-wake control model suggests that WPNs and SPNs compete for network dominance through mutual inhibition, creating a systematic “switch” that results in the sleep or awake state14-16. However, this model does not fully account for the complexity of the neurobiology behind sleep/wakefulness, and research into the identity and functional significance of co-transmitting neurons in the sleep-wake network is still a work in progress17.
Central nervous system (CNS) neurons can co-transmit different neurotransmitter types through distinct mechanisms, thereby allowing for versatile synaptic signaling18. Moreover, the neurophysiological effect of co-transmission is determined by differences in neurotransmitter concentrations at the presynaptic terminals and composition of postsynaptic receptors19, 20. Different signaling molecules may be packed in the same vesicle21, 22, which can affect their postsynaptic receptivity and functions. For example, one of the best-studied co-transmission systems is the co-release of glutamate by primary GABAergic/glycinergic neuron terminals in the medial nucleus of the trapezoid body (MNTB), part of the sound localization pathway during the development process. Co-released glutamate activates post-synaptic NMDA receptors and reorganizes the MNTB-lateral superior olive inhibitory circuitry23. Disrupting glutamate co-transmission during this critical period impairs the tonotopic refinement of the auditory brainstem24. Additionally, co-transmission facilitates various functions in the adult brain. For example, starburst amacrine cells (SACs) in the retina co-release GABA and acetylcholine (ACh) through separate vesicles in a Ca2+-dependent process21. This allows a small number of SACs to encode both motion and direction sensitivities using GABA and ACh signaling, respectively. Glutamate is co-released from dopaminergic neurons in the ventral tegmental area (VTA)25. Dopamine acts on a slow time scale by binding to G-protein-coupled receptors, whereas glutamate acts on a fast time scale when bound to ionotropic glutamate receptors and conveys temporally precise signals. Glutamate co-release is useful for accurate prediction-error signals, allowing reward to be encoded in the firing rates of dopaminergic neurons and mediating dopamine-dependent behaviors26, 27.
Co-transmission, a concept introduced decades ago, is increasingly being incorporated into models attempting to explain the sleep-wake circuit, thus rapidly replacing models that privileged the “one neuron = one neurotransmitter” hypothesis. Most WPNs and SPNs release more than one neurotransmitter type, and neurotransmitter co-release is vital in increasing the computational capabilities of this relatively short-numbered neuronal population which modulates the sleep-wake circuit. In this review, we discuss recent findings on co-transmission in hypothalamic WPNs and SPNs and examine its functional significance in the sleep-wake circuit.
2.1. Histamine and GABA of the Tuberomammillary Nuclei
The hypothalamic TMN contains almost all histaminergic neurons (about 64,000) found in the adult mammalian brain28, 29. Moreover, TMN neurons also synthesize GABA30 and have been shown to express galanin, enkephalins, thyrotropin release hormone, and substance-P in some species30, 31. Accumulating evidence suggests that the interaction of histamine and GABA transmission is relevant to the sleep-wake circuit.
Histamine in neurons is synthesized by decarboxylation of L-histidine by histidine decarboxylase (HDC), transported into vesicles by vesicular monoamine transporter-2 (VMAT2), and inactivated by histamine-N-methyltransferase and monoamine oxidase-B following release32, 33. Histaminergic neurons can innervate multiple brain regions such as the basal forebrain, LHA, and neocortex. Pharmacological studies in animals have shown that histamine is a critical wake-promoting neurotransmitter; however, the underlying mechanisms are unclear. Bilateral histamine injection into the TMN increases arousal and sleep latency in cats33. In contrast, irreversible HDC inhibitor (alpha-fluoromethylhistidine) administration reduces wakefulness and increases non-rapid eye movement (NREM) and rapid eye movement (REM) sleep in rats and cats34.
Previous studies have demonstrated that glutamate decarboxylase-67 (GAD67) and vesicular GABA transporter (VGAT), the enzymes needed for GABA synthesis and transmission, respectively, are expressed in the TMN31, 35. VMAT-positive neurons in TMN-explants also expressed GABA in in vitro cultures, indicating histamine and GABA co-localization36. The same study reported that GABA and VMAT2 were located in different granular deposits, suggesting that they are packed in distinct presynaptic vesicles. Although the hypothesis of separate packing needs confirmation by electron microscopy36, this finding indicates that histamine and GABA are capable of acting independently on the diverse populations of postsynaptic neurons. This is not surprising because the TMN projects to many parts of the CNS and co-regulates various brain functions, such as learning and memory, appetite, metabolism, and thermoregulation, besides promoting wakefulness37-40.
At the cellular level, co-transmitted GABA can potentially influence the overall postsynaptic excitability by acting on a different time course than that of histamine. The histamine-1 receptors (H1Rs) are coupled to G-protein (Gq/11) and phospholipase C (PLC), which activate secondary messengers, diacylglycerol (DAG) and triphosphoinositol (IP3). IP3 facilitates Ca2+ release from the endoplasmic reticulum (ER), leading to Ca2+-dependent depolarization through cation channels. Histamine-2 receptor (H2R) activation results in CREB transcription factor phosphorylation through Gs, adenylyl cyclase, and PKA cascade, leading to an enhanced excitatory response41. Compared with the slow-acting G-protein coupled receptors (GPCRs), synaptic ionotropic GABA-A receptors regulate neuronal inhibition on a millisecond time scale42. Therefore, if GABA binds synaptic receptors, it may produce inhibitory postsynaptic potentials (IPSPs) before the onset of H1R and H2R-mediated depolarization. Initially, IPSPs activate hyperpolarization-activated cation currents (Ih) and steadily depolarize the resting potential43. It is possible that the delayed H1R- and H2R-mediated inward currents enhance post-inhibitory rebound spiking and create a biphasic inhibition-excitation signal commonly found in other monoamine-GABA co-transmission cases44-46. During low TMN activity, this mechanism may attenuate spontaneous and subthreshold excitatory postsynaptic potentials (EPSPs) in receiving WPNs. Only when sufficient presynaptic TMN neurons are discharged may the rebound spiking generate a postsynaptic excitatory switch. We hypothesize that an increase in the signal-to-noise ratio is an essential mechanism governing accurate and rapid sleep-to-wake transitions while preventing uncommitted transitions.
Moreover, co-transmitted GABA acts as an inhibitory regulator for an overactive histaminergic system. Yu et al. demonstrated that mice with a Cre recombination VGAT knock-down (HDC-VGAT KD) as well as those with VGAT knock-out (TMN-ΔVGAT) displayed hyperactivity and an increased and sustained wakefulness at night (dark phase)35. Even after sleep deprivation, HDC-VGAT KD and TMN-ΔVGAT mice maintained the hyperactive states and their hours of sleep were less than controls. As noted by the same study, specific optogenetic activation of TMN-ΔVGAT/HDC-Channelrhodopsin in neocortex and caudate-putamen slices resulted in the absence of tonic inhibitory conductance usually generated by VGAT-dependent GABA release from the TMN onto extrasynaptic ionotropic GABA-A receptors. These findings corroborate the hypothesis that co-transmitted GABA plays a critical role in preventing histamine-induced overexcitement of post-synaptic neurons and promotes optimal wakefulness35. Conversely, in Williams, Chee et al., optogenetic stimulation of TMN neurons evoked histamine release in the ventrolateral TMN (vlTMN) and ventrolateral preoptic nucleus (VLPO) but evidence of GABA co-release was lacking 47. Thus, it is possible that TMN co-transmission is target-specific and a study incorporating a larger number of brain areas is sought to clarify this matter.
At other neuronal terminals, histamine and GABA may elicit synergistic, inhibitory actions on target neurons. In addition to activating metabotropic histamine receptors, recent in vitro electrophysiological studies have shown that histamine acts through positive allosteric sites on heteromultimeric GABA-A receptors to enhance GABA-dependent tonic inhibition48-51. In fact, while histamine is mainly known for eliciting an excitatory-modulatory response, TMN stimulation has been shown to also evoke fast IPSPs, with kinetics that resembles GABA-A receptors in supraoptic oxytocin neurons52, 53. Furthermore, iontophoretically administered histamine directly reduces neuronal firing in the anterior and intralaminar thalamic nuclei, perigeniculate nuclei (PGN), and other regions54, 55. Lee et al. hypothesize that increased histamine release during wakefulness can dampen a thalamic oscillation in sleep-wake transition by PGN neuron inhibition55. The inhibitory effects via enhanced Cl- conductance may be mediated by GABA-histamine binding on GABA-A receptor. Further research is needed to clarify how an interaction between the two neurotransmitters regulates circuit-specific and selective inhibition49, 56.
Recent identification of cortical GABAergic interneurons by Kilduff et al. demonstrated an increased Fos-immunoreactivity during recovery and spontaneous sleep57, 58. This led to the speculation that inhibitory effects of GABA-histamine on GABAergic interneurons that contain GABA-A receptors may underlie an indirect excitation of arousal centers. These interneurons are sleep-active and co-express nitric oxide (NO), neuropeptide Y(NPY), and somatostatin (SST)57, 58. The authors also speculated that a combination of co-transmitters (GABA, NO, NPY, and SST) contributes to synchronization of cortical EEG activity during sleep58. NO has been previously implicated in REM regulation59, although its role in interneurons remains unclear. Thus, the hypothesis that inhibitory TMN inputs during wakefulness modulate the activity of NO/GABA cortical interneurons needs testing.
In conclusion, the mechanisms underlying histamine modulation of wakefulness in the context of GABA co-transmission still remain elusive. Nevertheless, mounting evidence suggests that GABA is an active component of histaminergic neurotransmission (Figure 1A), and GABA and histamine co-transmission may promote bi-directional postsynaptic modulation of coincidental excitation, inhibitory regulation, and synergistic inhibition. These mechanisms may promote appropriate sleep-to-wake transitions and wakefulness while suppressing sleep-related neuronal activity.
Figure 1. Possible mechanisms governing sleep and wakefulness via co-transmission.
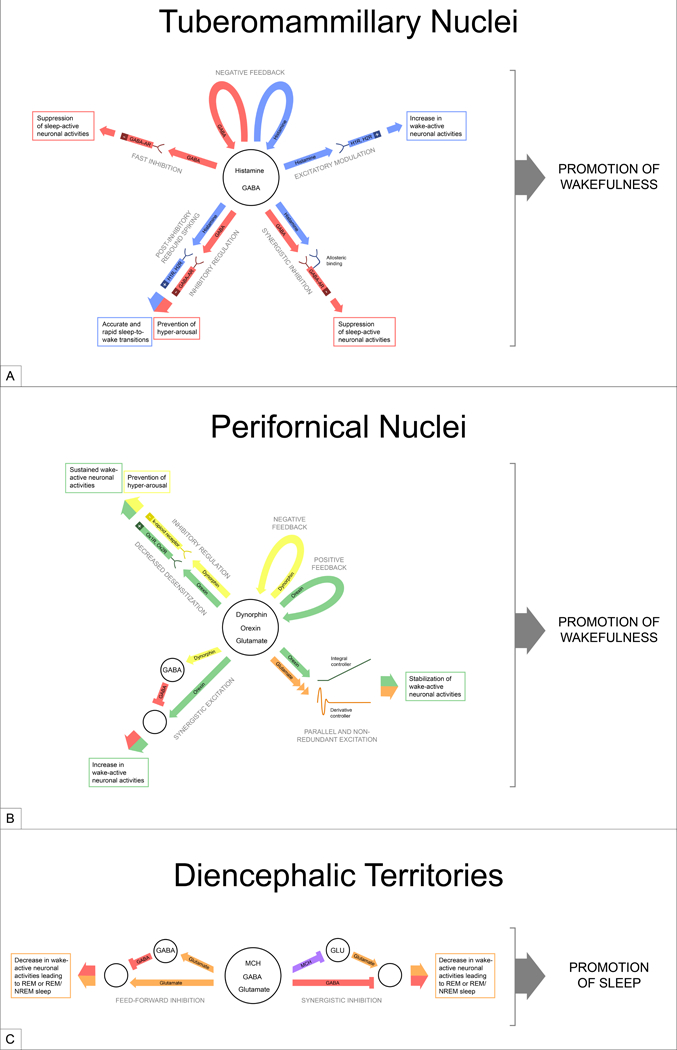
(a) While TMN neurons are mainly known as histaminergic, they also synthesize and release GABA, which may segregate into different presynaptic terminals than histamine and act independently on distinct postsynaptic neurons. GABA may also work in opposition or synergy with histamine when released. (b) In addition to orexin, PFN neurons release inhibitory neuropeptides, dynorphin, and the fast-neurotransmitter, glutamate. The interaction between these neurotransmitters may allow PFN neurons to promote optimal wakefulness. (c) MCH neurons produce many neurotransmitters, including GABA and glutamate. Although postsynaptic responses to fast-neurotransmitters are readily observable by photo-stimulation, this may not be the case for MCH and other co-neuropeptides. In all the three hypothalamic neuron populations (a-c), the common motif is that the neurons synthesize “opposing” neurotransmitters can modulate postsynaptic neurons bi-directionally. In small neuron populations, this may allow target-specific neuronal influence and flexibility to regulate sleep and wakefulness. This diagram depicts only a few possible mechanisms. Abbreviations: H1R, H2R: histamine receptor-1, histamine-receptor-2; GABA-AR: GABA-A receptor; Ox1R, Ox2R: orexin-1 receptor, orexin-2 receptor; MCH: melanin-concentrating hormone; GLU: glutamate
2.2. Dynorphin, Glutamate, and Orexin in the Lateral Hypothalamic Area
The orexinergic (hypocretinergic) neurons in the LHA promote and stabilize wakefulness by innervating WPNs of the basal forebrain, LC, TMN, and RN. Orexinergic neurons co-express dynorphin and/or glutamate. Co-release of orexin and dynorphin or orexin and glutamate may modulate activity of LHA neurons through distinct feedback mechanisms.
Orexinergic neurons (~70,000) play a critical role in sleep-wake homeostasis. Selective optogenetic activation of orexin neurons increases the probability of NREM or REM transition to wakefulness in mice60, and selective optogenetic inhibition of these cells induces NREM sleep in mice61, 62. Orexin or Orexin-receptor (OxRs) knock-out animal models mimic human narcolepsy63. Neuropeptides Orexin-A and B are synthesized by prepro-orexin cleavage and pre-synaptically packaged into large dense core vesicles. Orexin-A binds to both Ox1Rs and Ox2Rs, whereas Orexin-B binds mainly to Ox2Rs64. G-protein activation coupled with the receptors increases neuronal excitability by inhibiting G protein-coupled inward-rectifying K+ channels (GIRKs), inducing Ca2+ influx through voltage-dependent calcium channels (VDCCs) and influencing Na+/Ca2+ exchange64-66. Ox1Rs and OxR2s occur in distinct anatomical locations. For example, LC neurons and TMN neurons exclusively express Ox1Rs and Ox2Rs respectively, whereas RN neurons express both.
Interestingly, nearly all mice and rat orexinergic neurons contain dynorphin67. In the LHA, only orexinergic neurons co-expressed dynorphin67. Dynorphins are neuropeptides derived from prodynorphin and act as endogenous ligands for k-opioid receptors. Dynorphins show high affinity for other opioids (μ and δ) and non-opioid receptors (NMDARs)68, 69. Opioid receptors inhibit adenylyl cyclase by coupling to inhibitory G-proteins. They also stimulate GIRK channels and inhibit presynaptically expressed N-type Ca2+ channels, which increase K+ conductance and decrease Ca2+ conductance70. Thus, dynorphins are mainly inhibitory peptides. However, at sub-nanomolar dynorphin concentrations, opioid receptors may bind to stimulatory G-proteins and prolong action potential duration69, 71, making dynorphin a multi-functional neurotransmitter in the CNS. Electron micrograph (EM) images show orexin and dynorphin co-localization in large dense core vesicles, suggesting co-packaging and co-release on the same postsynaptic target72. Many orexin-innervated SPNs and WPNs contain dynorphin fibers and opioid receptors67. Thus, orexin and dynorphin likely coordinate closely to regulate diverse physiological functions such as arousal.
The significance of dynorphin and orexin co-release remains largely unknown, but both types of neurotransmitters have been shown previously to influence the sleep-wake circuit. In the LC, the selective k-opioid receptor agonist depresses the excitatory synaptic inputs into noradrenergic neurons in vitro73. A recent in vivo electrophysiological study supports these findings: dynorphin attenuated LC neuronal discharge through presynaptic inhibition in vivo74. In the RN, the k-opioid receptor agonist reduces EPSPs and decreases extracellular 5-HT75, 76. In the basal forebrain, dynorphin inhibits cholinergic neurons through pre- and postsynaptic mechanisms77. Thus, despite being co-released, dynorphin seems to have an opposing role to that of stimulatory orexin78. In the VTA, orexin and dynorphin tune dopaminergic output by simultaneously inhibiting and activating different neuronal subsets79. A study on reward circuitry in mice showed that when applied separately, orexin excited while dynorphin inhibited dopaminergic VTA neurons72. However, upon co-application, no net change in firing rate was observed, suggesting that the effects of these co-released neuropeptides compensated each other during saturating concentrations. At the behavioral level, orexin disruption using orexin-1 receptor antagonist (SB334867) blunted the reward effects of LHA electrical stimulation, eliminated cocaine-induced impulsivity, and reduced cocaine self-administration72. However, disruption of both orexin and dynorphin, using SB334867 and K-opioid receptor antagonist (norbinaltorphimine), reversed these effects, suggesting that the dynamic interactions between the two neuropeptides may regulate reward-driven behavior. While this study focused on reward circuitry, it has strong implications on the wake-circuit as well because (1) VTA is a wake-promoting center80, 81 and (2) other WPNs innervated by orexin-dynorphin fibers may be similarly regulated (Figure 1B).
What, then, is the purpose of co-releasing signals with opposing effects? Antagonistic neurotransmitter co-release may allow pre-synaptic neurons to balance excitation and inhibition of postsynaptic neurons. Thus, LHA neurons may control firing rates and prevent postsynaptic overexcitation during wakefulness. Similar to the effects in the VTA, dynorphin or orexin application in the basal forebrain produces opposite effects on cholinergic neurons77. When co-applied at a membrane potential of −40mV, dynorphin exerts a stronger effect than orexin, resulting in net neuronal inhibition. At a more hyperpolarized potential (−70mV), the opposite effect is observed. Thus, depending on the membrane potential, orexin and dynorphin co-release results in either net inhibition or excitation. In an overstimulated neuron, this mechanism may prevent excitotoxicity and signal saturation. Moreover, co-released dynorphin may act on presynaptic LHA neurons themselves to attenuate overexcitation. Orexinergic neurons form synapses and directly activate neighboring orexinergic neurons through Ox2Rs82, allowing signal amplification and maintenance of wakefulness. However, a positive feedback circuitry may be prone to instabilities (runaway excitation). Co-released dynorphin directly inhibits local orexinergic neurons through multiple mechanisms, including GIRK channel activation and calcium current reduction83. While orexin and glutamate (see below) promote positive feedback, dynorphin may lead to negative feedback and optimize LHA neuronal activity.
In other regions, dynorphin may act in concert with orexin to increase postsynaptic neuronal excitability. During sleep, TMN neurons are hypothesized to receive inhibitory inputs from GABA/galaninergic sleep-promoting neurons of the VLPO, which express Fos protein during sleep but not during wakefulness 84, 85. Dynorphin suppresses these inhibitory inputs and attenuates TMN neuron inhibition86. A similar mechanism is employed by NPY neurons in the hypothalamic arcuate nucleus. Orexin directly excites NPY neurons while dynorphin attenuates GABAergic inputs, resulting in enhanced excitation83. In this case, co-released dynorphin and orexin function synergistically.
Repeated pharmacological application of either dynorphin or orexin in mice brain slices in vitro resulted in desensitization of cholinergic neurons, whereas the same cholinergic neurons showed a sustained electrophysiological response when exposed to dynorphin and orexin simultaneously77. Melanin-concentrating hormone (MCH) neurons desensitize faster when exposed to dynorphin than orexin, and when dynorphin and orexin are repeatedly co-applied, orexinergic excitation remains83. In contrast to previous studies suggesting a collective role of dynorphin and orexin in modulating MCH neurons, a more recent study in mouse brain slices showed that optogenetic stimulation of orexinergic neurons inhibits most MCH neurons through a dynorphin-independent mechanism87. In this study, a k-opioid receptor antagonist (nor-binaltorphimine), intended to block the effect of dynorphin, did not cause changes in MCH cell membrane potential. Conversely, GABA-A receptor blocker (Gabazine) abolished the orexin-driven inhibition of MCH neurons87. The authors further speculated that orexinergic inhibition of MCH neurons is mediated by local GABAergic interneurons containing orexin receptors87. Therefore, the effect of co-transmission originating from LHA neurons varies and depends on the targeted neuron. Understanding the interactions of these neuropeptides in postsynaptic signaling may provide further insights on their downstream modulatory effects88, 89.
Along with dynorphin, over half of orexinergic neurons synthesize glutamate90, suggesting that orexinergic neurons may be heterogeneous91. However, the exact topographic organization and significance of orexinergic neurons remain unknown. Orexinergic neurons containing glutamate are directly involved in the wake circuit. For instance, orexinergic terminals in the LC contain vesicular transporter for glutamate (VGLUT2). They also contain synaptophysin, a protein found in small synaptic vesicles of glutamatergic terminals92. In the TMN, EM studies show orexin immunoreactivity in large dense-core vesicles; the same orexinergic terminals contain glutamate in synaptic vesicles93. This is consistent with the notion that small amino acid neurotransmitters and neuropeptides are differentially stored and regulated. Orexin and dynorphin are synthesized and processed in the rough ER and Golgi apparatus. Then, dense core vesicles, containing the neuropeptides, are transported to the release site, resulting in a relatively slow replenishment rate94. In contrast, glutamate can be refilled into small synaptic vesicles directly at the axonal bouton. Additionally, while dense-core vesicles are released extra-synaptically and act more diffusely (volume transmission), glutamate tends to congregate in the active zone near the synapse, providing a limited but fast synchronous excitation-release coupling94-96. The spatial and temporal differences (fast versus slow) of co-neurotransmitters may modulate various input-output operations on the downstream wake circuit.
Co-released orexin and glutamate may produce distinct excitatory spike outputs in postsynaptic neurons (Figure 1B). A recent study explored the relationship in histaminergic neurons using optogenetic manipulation of upstream orexinergic neurons97. Orexin-dependent excitation occurred only at high-frequency stimulation (20 Hz), whereas glutamate-dependent excitation occurred at all frequencies. In contrast to large dense core vesicles, small synaptic vesicles containing glutamate are docked near voltage-gated Ca2+-channels, requiring less calcium influx to be released98. In the same study, glutamate produced a brief, rapid excitation through AMPAR, whereas orexin induced a delayed but linearly-increasing excitation through Ox2Rs. These two outputs were temporarily and pharmacologically separable, suggesting a parallel and non-redundant co-transmission signaling at the postsynaptic level97. The authors further speculated that orexin may act as an integral controller because the orexin-dependent output and input are proportional. Meanwhile, glutamate acts as a derivative controller, which spikes only at stimulus onset, possibly detecting input changes. Together, these two components increase the computational capacity of the LHA-TMN circuit and may stabilize wakefulness. An interesting experiment would be to selectively knock out glutamate in orexinergic neurons in vivo and observe whether glutamate co-transmission loss affects wakefulness. Co-released glutamate and orexin act independently on histaminergic neurons. However, it is unclear whether these independent effects are observed in other postsynaptic and local orexinergic neurons; this should be investigated to shed light on mechanisms governing target-dependent computation. For instance, Apergis-Schoute et al. used optogenetics in mouse brain slices, showing that glutamate released from orexinergic neurons was not required for activating local GABAergic interneurons87. Lastly, investigating how dynorphin cross-communicates with glutamate at both the molecular and physiological level is essential in unraveling the wake circuit. For example, while dynorphin mainly binds to opioid receptors, it has also been implicated in blocking NMDA receptors, which may decrease glutamate-induced excitation and plasticity99.
2.3. Co-transmitters and Melanin-Concentrating Hormone in the Diencephalic Areas
MCH neurons are located mainly in the LHA, incerta-hypothalamic area (IHy), and zona incerta (ZI)100-103. The dorsomedial part of the TMN also contains non-histaminergic MCH neurons104. MCH neurons interact with neighboring orexinergic neurons to modulate physiological functions such as learning and memory, stress and anxiety, and energy homeostasis105-107. Additionally, MCH neurons promote sleep by innervating WPNs and REM-generating pons, although the mechanism of this process is elusive108-110. Some evidence suggests that the different signaling patterns of MCH neurons on sleep promotion could relate to the interplay of MCH, GABA and glutamate release from these neurons. Besides GABA and glutamate, MCH neurons co-express other neurotransmitters, including nesfatin, cocaine-amphetamine-regulated transcript (CART), neuropeptide-EI, and neuropeptide-GE101, 111-113. Further work is still necessary to pinpoint how each neurotransmitter type contributes to sleep regulation.
MCH binds two GPCRs: MCHR1s and MCHR2s. Studies using MCHR1-expressing, non-neuronal CHO cells show that MCH activates diverse intracellular signaling pathways by coupling to Gi, Go, and Gq proteins114 Studies in hypothalamic neuronal cultures suggest that MCH-MCHR1 predominantly plays an inhibitory role in the CNS. One such study showed that MCH inhibited VDCCs through the Gi/o-mediated pathway115. MCH also causes miniature excitatory postsynaptic currents (mEPSC) depression by modulating presynaptic glutamate release and postsynaptic glutamate receptors116. MCHR2 function remains largely unclear because although humans express both receptors, rodents express only MCHR1s117. However, some studies suggest that MCHR2s activate Gq protein118.
To date, it remains unclear whether MCH neurons promote only REM or also NREM sleep. Some studies have shown that optogenetic stimulation of MCH neurons increased NREM and REM sleep in mice119 and rats120. However, others show that it extended only REM duration121, 122. This may be because of a variation in MCH neuron clusters (zona incerta vs. perifornical/LHA/dorsomedial TMN) and channelrhodopsin expression rate. The first two studies119, 120 used chronic stimulation (1 min stimulation / 4 min of no stimulation cycles for 24 h). The latter studies121, 122 used acute stimulation.
Curiously, acute stimulation (stimulation at the onset of a stable NREM or REM sleep episode) extends REM sleep in both MCHR1-expressing and MCHR1-knock out mice, suggesting that other co-released neurotransmitters, and not MCH, modulate REM behavior121. As also noted, GABA co-release evoked bicuculline-sensitive inhibitory postsynaptic currents (IPSCs) in postsynaptic neurons in vitro. This is consistent with the findings that among the co-transmitters GABA, neuropeptide-EI, and MCH, a site-specific microinjection of GABA promotes the most rapid increase in REM sleep and shows no change in NREM sleep123. In contrast, increased NREM sleep seen in the experiments using chronic stimulation may derive from additional recruitment of MCH and other slow-acting inhibitory co-neuropeptides, such as neuropeptide-EI, neuropeptide-GE, nesfatin-1, and CART. As such, the intraventricular MCH peptide administration induces a dose-dependent increase in NREM and REM sleep124. Blocking MCH1 receptors with antagonists decreased the time spent in both types of sleep125. Similarly, a near-complete MCH neuron ablation by cell-specific diphtheria toxin-A expression decreased NREM sleep but showed no effect on REM sleep122. However, Vetrivelan et al. and Naganuma et al. demonstrated that selective activation of MCH neurons using chemogenetics causes an increase in REM sleep without altering NREM sleep in mice126, 127.
Given the contradicting nature of these above-mentioned results, further investigation is necessary to clarify whether different MCH neuronal subpopulations modulate particular sleep components. MCH subpopulations are shown to have distinct intrahypothalamic localization and projection patterns128. For example, MCH neurons bordering the cerebral peduncle are devoid of CART whereas MCH subpopulations near the zona incerta, the perifornical area, and the area between the ventromedial hypothalamus (VMH) and dorsomedial hypothalamus (DMH) co-express CART128.
At the circuit level, classical co-neurotransmitters may work in synergy with MCH peptides to enhance postsynaptic inhibition (Figure 1C). In fact, GABA or glutamate co-transmission may be necessary to inhibit postsynaptic neurons126, 129. GABA from MCH neurons evokes IPSPs121. Moreover, some MCH neurons reveal a glutamate-mediated feedforward inhibition of neurons in lateral septal nucleus (LS). Optogenetic stimulation of MCH neurons directly releases glutamate onto LS neurons and GABAergic interneurons/afferents129. By activating GABAergic interneurons, MCH neurons can robustly inhibit LS neurons. In both studies led by Jego and Chee, MCH peptides themselves failed to inhibit postsynaptic neurons121, 129. This is not too surprising because MCH peptides modulate presynaptic neurons to reduce transmission rather than to directly affect postsynaptic membrane potential or conductance116. These studies have highlighted the critical role of fast-acting co-transmitters in modulating the postsynaptic network.
The mechanism underlying the presynaptic release of co-neurotransmitters remains largely unknown. A recent study using single-cell qPCR analysis in mice demonstrated that MCH neurons and neighboring orexinergic neurons express mRNA for VGLUT2 (Slc17a6+)91, supporting previous findings showing that these two neuronal populations are functionally glutamatergic97, 129. Jego et al.121 suggested that MCH neurons also release GABA. Interestingly, the study from Mickelsen et al. with single cell analysis showed that although MCH neurons and about 50% of orexinergic neurons are capable of synthesizing GABA (Gad1+), they lack the machinery for vesicular GABA release (Slc32a1-)91. These latter results led to the speculation that GABA release, at least from MCH neurons, likely occurs through a non-synaptic or non-canonical release pathway91. Meanwhile, using double transgenic reporter mice, Blanco-Centurion et al. showed that there are four major populations of neurons in the hypothalamus: VGAT+, orexin+/VGLUT2+, orexin-/VGLUT2+, and MCH+ neurons130. These and other recent studies point to the complexity of neurotransmitter phenotypes in the LHA neuronal populations and the need to clarify the role of neurochemical heterogeneity in eliciting dynamic homeostatic changes. These complexities also provide some background for explaining the challenges in treating sleep dysfunction in the clinical practice.
3. Conclusion
In this review, we focus on the complexity and relevance of co-transmission in modulating the sleep-wake network. Although current methods are still subpar to investigate how neuronal populations control different behaviors in dynamic conditions (e.g., studies using animal models often focus on one behavioral paradigm at a time), the idea that WPNs and SPNs are unidirectional-signaling neurons has been repeatedly challenged. Furthermore, validation studies from animal models to humans remain an obstacle. Although molecular imaging technique improvement has allowed neurotransmitter circuit detection in vivo131, 132, spatial/temporal resolution is too low to discriminate most subcortical structures and to observe their activities. However, recent improvements in human tissue techniques are widening the analytical scope of post-mortem studies. Moreover, sleep assessments are more widely accessible and have been incorporated to aging cohorts, enabling sleep-focused clinicopathological studies.
Co-transmission in histaminergic, orexinergic, and MCH-producing neurons increases the computational capabilities of these small neuronal populations (less than 300,000 out of 88 billion neurons133) by promoting postsynaptic synergistic excitation or inhibition, regulating negative or positive feedback, and modulating temporal responses. Co-transmission with fast-acting neurotransmitters may be a key to fine tune sleep-wake transitions and homeostasis, following the circadian rhythms and environmental cues. Thus, it is imperative to acknowledge the heterogeneous chemical identities of these neurons and investigate the consequences of the co-neurotransmitter imbalance. Co-transmission increases neuronal flexibility and enables more specific neuron targeting. This review focused on the hypothalamus and sleep-wake regulation. However, co-transmission has also been described in other sleep-wake regulating regions, such as the LC (norepinephrine, dopamine, NPY, galanin) and the RN (serotonin, glutamate)134-139, and in regulating other physiological functions, such as appetite, addiction, mood, and metabolism. Understanding how these co-transmitters interact to drive sleep and wakefulness in animal models and human subjects has immense implications for the next generation of treatments for sleep dysfunction and may explain the differential vulnerability of similar neuronal populations to neurodegenerative conditions140.
Acknowledgments
We gratefully acknowledge support from the Tau Consortium/Rainwater Charitable Foundation and NIH grants: K24AG053435 (LTG), R56MH107042 (TCN). The Grants that support JCB came from the Fundação de Amparo à Pesquisa do Estado de São Paulo [São Paulo Research Foundation - FAPESP] grant #2016/02224-1 [JCB], the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [Agency for the Advancement of Higher Education - CAPES grant #848/15], and the Conselho Nacional de Desenvolvimento Científico e Tecnológico [National Council for Scientific and Technological Development - CNPq] with grant #426378/2016-4. JCB is an investigator with the CNPq. JYO is a Tau Consortium Fellow. We also thank Dulce Morales for histology and Evan T. Keum and Brian Hitchin for manuscript editing.
Footnotes
Conflict of interest:
Authors declare no competing financial interests in relation to the work described.
References
- 1. Ng MC. Orexin and Epilepsy: Potential Role of REM Sleep. Sleep 2017; 40(3). [DOI] [PubMed] [Google Scholar]
- 2. Kroeger D, Ferrari LL, Petit G, Mahoney CE, Fuller PM, Arrigoni E et al. Cholinergic, Glutamatergic, and GABAergic Neurons of the Pedunculopontine Tegmental Nucleus Have Distinct Effects on Sleep/Wake Behavior in Mice. J Neurosci 2017; 37(5): 1352–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacobson LH, Chen S, Mir S, Hoyer D. Orexin OX2 Receptor Antagonists as Sleep Aids. Curr Top Behav Neurosci 2017; 33: 105–136. [DOI] [PubMed] [Google Scholar]
- 4. Khanday MA, Somarajan BI, Mehta R, Mallick BN. Noradrenaline from Locus Coeruleus Neurons Acts on Pedunculo-Pontine Neurons to Prevent REM Sleep and Induces Its Loss-Associated Effects in Rats. eNeuro 2016; 3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gotter AL, Forman MS, Harrell CM, Stevens J, Svetnik V, Yee KL et al. Orexin 2 Receptor Antagonism is Sufficient to Promote NREM and REM Sleep from Mouse to Man. Sci Rep 2016; 6: 27147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takahashi K, Kayama Y, Lin JS, Sakai K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience 2010; 169(3): 1115–1126. [DOI] [PubMed] [Google Scholar]
- 7. Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci 2006; 26(40): 10292–10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao LZ, Zhang GL, Gao J, Zhang JX, Zhong MK, Zhang J. [Role of serotonergic neurons in dorsal raphe nuclei in regulation of sleep]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2003; 19(2): 175–178. [PubMed] [Google Scholar]
- 9. Anaclet C, Ferrari L, Arrigoni E, Bass CE, Saper CB, Lu J et al. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci 2014; 17(9): 1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung S, Weber F, Zhong P, Tan CL, Nguyen TN, Beier KT et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 2017; 545(7655): 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J et al. Identification of sleep-promoting neurons in vitro. Nature 2000; 404(6781): 992–995. [DOI] [PubMed] [Google Scholar]
- 12. Pelluru D, Konadhode R, Shiromani PJ. MCH neurons are the primary sleep-promoting group. Sleep 2013; 36(12): 1779–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alam MA, Kumar S, McGinty D, Alam MN, Szymusiak R. Neuronal activity in the preoptic hypothalamus during sleep deprivation and recovery sleep. J Neurophysiol 2014; 111(2): 287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu BS, Zee PC. Neurobiology of sleep. Clin Chest Med 2010; 31(2): 309–318. [DOI] [PubMed] [Google Scholar]
- 15. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 2001; 24(12): 726–731. [DOI] [PubMed] [Google Scholar]
- 16. Szymusiak R, McGinty D. Hypothalamic regulation of sleep and arousal. Ann N Y Acad Sci 2008; 1129: 275–286. [DOI] [PubMed] [Google Scholar]
- 17. Saper CB, Fuller PM. Wake-sleep circuitry: an overview. Curr Opin Neurobiol 2017; 44: 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hokfelt T Neuropeptides in perspective: the last ten years. Neuron 1991; 7(6): 867–879. [DOI] [PubMed] [Google Scholar]
- 19. Apostolides PF, Trussell LO. Rapid, activity-independent turnover of vesicular transmitter content at a mixed glycine/GABA synapse. J Neurosci 2013; 33(11): 4768–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tritsch NX, Granger AJ, Sabatini BL. Mechanisms and functions of GABA co-release. Nat Rev Neurosci 2016; 17(3): 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee S, Kim K, Zhou ZJ. Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron 2010; 68(6): 1159–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N et al. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron 2006; 50(4): 575–587. [DOI] [PubMed] [Google Scholar]
- 23. Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci 2005; 8(3): 332–338. [DOI] [PubMed] [Google Scholar]
- 24. Noh J, Seal RP, Garver JA, Edwards RH, Kandler K. Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat Neurosci 2010; 13(2): 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chuhma N, Choi WY, Mingote S, Rayport S. Dopamine neuron glutamate cotransmission: frequency-dependent modulation in the mesoventromedial projection. Neuroscience 2009; 164(3): 1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lapish CC, Seamans JK, Chandler LJ. Glutamate-dopamine cotransmission and reward processing in addiction. Alcohol Clin Exp Res 2006; 30(9): 1451–1465. [DOI] [PubMed] [Google Scholar]
- 27. Mingote S, Chuhma N, Kalmbach A, Thomsen GM, Wang Y, Mihali A et al. Dopamine neuron dependent behaviors mediated by glutamate cotransmission. Elife 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Panula P, Yang HY, Costa E. Histamine-containing neurons in the rat hypothalamus. Proc Natl Acad Sci U S A 1984; 81(8): 2572–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Airaksinen MS, Paetau A, Paljärvi L, Reinikainen K, Riekkinen P, Suomalainen R et al. Histamine neurons in human hypothalamus: anatomy in normal and Alzheimer diseased brains. Neuroscience 1991; 44: 465–481. [DOI] [PubMed] [Google Scholar]
- 30. Airaksinen MS, Alanen S, Szabat E, Visser TJ, Panula P. Multiple neurotransmitters in the tuberomammillary nucleus: comparison of rat, mouse, and guinea pig. J Comp Neurol 1992; 323(1): 103–116. [DOI] [PubMed] [Google Scholar]
- 31. Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev 2008; 88(3): 1183–1241. [DOI] [PubMed] [Google Scholar]
- 32. Merickel A, Edwards RH. Transport of histamine by vesicular monoamine transporter-2. Neuropharmacology 1995; 34(11): 1543–1547. [DOI] [PubMed] [Google Scholar]
- 33. Thakkar MM. Histamine in the regulation of wakefulness. Sleep Med Rev 2011; 15(1): 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Monti JM, D’Angelo L, Jantos H, Pazos S. Effects of a-fluoromethylhistidine on sleep and wakefulness in the rat. Short note. J Neural Transm 1988; 72(2): 141–145. [DOI] [PubMed] [Google Scholar]
- 35. Yu X, Ye Z, Houston CM, Zecharia AY, Ma Y, Zhang Z et al. Wakefulness Is Governed by GABA and Histamine Cotransmission. Neuron 2015; 87(1): 164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kukko-Lukjanov TK, Panula P. Subcellular distribution of histamine, GABA and galanin in tuberomamillary neurons in vitro. J Chem Neuroanat 2003; 25(4): 279–292. [DOI] [PubMed] [Google Scholar]
- 37. Kjaer A, Knigge U, Rouleau A, Garbarg M, Warberg J. Dehydration-induced release of vasopressin involves activation of hypothalamic histaminergic neurons. Endocrinology 1994; 135(2): 675–681. [DOI] [PubMed] [Google Scholar]
- 38. Ookuma K, Sakata T, Fukagawa K, Yoshimatsu H, Kurokawa M, Machidori H et al. Neuronal histamine in the hypothalamus suppresses food intake in rats. Brain Res 1993; 628(1–2): 235–242. [DOI] [PubMed] [Google Scholar]
- 39. Sakata T, Kurokawa M, Oohara A, Yoshimatsu H. A physiological role of brain histamine during energy deficiency. Brain Res Bull 1994; 35(2): 135–139. [DOI] [PubMed] [Google Scholar]
- 40. Lundius EG, Sanchez-Alavez M, Ghochani Y, Klaus J, Tabarean IV. Histamine influences body temperature by acting at H1 and H3 receptors on distinct populations of preoptic neurons. J Neurosci 2010; 30(12): 4369–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci 2003; 4(2): 121–130. [DOI] [PubMed] [Google Scholar]
- 42. Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J Biol Chem 2012; 287(48): 40224–40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kamondi A, Reiner PB. Hyperpolarization-activated inward current in histaminergic tuberomammillary neurons of the rat hypothalamus. J Neurophysiol 1991; 66(6): 1902–1911. [DOI] [PubMed] [Google Scholar]
- 44. Liu S, Plachez C, Shao Z, Puche A, Shipley MT. Olfactory bulb short axon cell release of GABA and dopamine produces a temporally biphasic inhibition-excitation response in external tufted cells. J Neurosci 2013; 33(7): 2916–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu J, Hablitz JJ. Cooperative activation of D1 and D2 dopamine receptors enhances a hyperpolarization-activated inward current in layer I interneurons. J Neurosci 2005; 25(27): 6322–6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu S, Shipley MT. Intrinsic conductances actively shape excitatory and inhibitory postsynaptic responses in olfactory bulb external tufted cells. J Neurosci 2008; 28(41): 10311–10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Williams RH, Chee MJ, Kroeger D, Ferrari LL, Maratos-Flier E, Scammell TE et al. Optogenetic-mediated release of histamine reveals distal and autoregulatory mechanisms for controlling arousal. J Neurosci 2014; 34(17): 6023–6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saras A, Gisselmann G, Vogt-Eisele AK, Erlkamp KS, Kletke O, Pusch H et al. Histamine action on vertebrate GABAA receptors: direct channel gating and potentiation of GABA responses. J Biol Chem 2008; 283(16): 10470–10475. [DOI] [PubMed] [Google Scholar]
- 49. Bianchi MT, Clark AG, Fisher JL. The wake-promoting transmitter histamine preferentially enhances alpha-4 subunit-containing GABAA receptors. Neuropharmacology 2011; 61(4): 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hoerbelt P, Ramerstorfer J, Ernst M, Sieghart W, Thomson JL, Hough LB et al. Mutagenesis and computational docking studies support the existence of a histamine binding site at the extracellular beta3+beta3- interface of homooligomeric beta3 GABAA receptors. Neuropharmacology 2016; 108: 252–263. [DOI] [PubMed] [Google Scholar]
- 51. Thiel U, Platt SJ, Wolf S, Hatt H, Gisselmann G. Identification of amino acids involved in histamine potentiation of GABA A receptors. Front Pharmacol 2015; 6: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hatton GI, Yang QZ. Ionotropic histamine receptors and H2 receptors modulate supraoptic oxytocin neuronal excitability and dye coupling. J Neurosci 2001; 21(9): 2974–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yang QZ, Hatton GI. Histamine mediates fast synaptic inhibition of rat supraoptic oxytocin neurons via chloride conductance activation. Neuroscience 1994; 61(4): 955–964. [DOI] [PubMed] [Google Scholar]
- 54. Sittig N, Davidowa H. Histamine reduces firing and bursting of anterior and intralaminar thalamic neurons and activates striatal cells in anesthetized rats. Behav Brain Res 2001; 124(2): 137–143. [DOI] [PubMed] [Google Scholar]
- 55. Lee KH, Broberger C, Kim U, McCormick DA. Histamine modulates thalamocortical activity by activating a chloride conductance in ferret perigeniculate neurons. Proc Natl Acad Sci U S A 2004; 101(17): 6716–6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fleck MW, Thomson JL, Hough LB. Histamine-gated ion channels in mammals? Biochem Pharmacol 2012; 83(9): 1127–1135. [DOI] [PubMed] [Google Scholar]
- 57. Gerashchenko D, Wisor JP, Burns D, Reh RK, Shiromani PJ, Sakurai T et al. Identification of a population of sleep-active cerebral cortex neurons. Proc Natl Acad Sci U S A 2008; 105(29): 10227–10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kilduff TS, Cauli B, Gerashchenko D. Activation of cortical interneurons during sleep: an anatomical link to homeostatic sleep regulation? Trends Neurosci 2011; 34(1): 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen L, Majde JA, Krueger JM. Spontaneous sleep in mice with targeted disruptions of neuronal or inducible nitric oxide synthase genes. Brain Res 2003; 973(2): 214–222. [DOI] [PubMed] [Google Scholar]
- 60. Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 2007; 450(7168): 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tsunematsu T, Kilduff TS, Boyden ES, Takahashi S, Tominaga M, Yamanaka A. Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J Neurosci 2011; 31(29): 10529–10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsunematsu T, Tabuchi S, Tanaka KF, Boyden ES, Tominaga M, Yamanaka A. Long-lasting silencing of orexin/hypocretin neurons using archaerhodopsin induces slow-wave sleep in mice. Behav Brain Res 2013; 255: 64–74. [DOI] [PubMed] [Google Scholar]
- 63. Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 1999; 98(4): 437–451. [DOI] [PubMed] [Google Scholar]
- 64. Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol 2011; 51: 243–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hoang QV, Bajic D, Yanagisawa M, Nakajima S, Nakajima Y. Effects of orexin (hypocretin) on GIRK channels. J Neurophysiol 2003; 90(2): 693–702. [DOI] [PubMed] [Google Scholar]
- 66. Burdakov D, Liss B, Ashcroft FM. Orexin excites GABAergic neurons of the arcuate nucleus by activating the sodium--calcium exchanger. J Neurosci 2003; 23(12): 4951–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT et al. Orexin (hypocretin) neurons contain dynorphin. J Neurosci 2001; 21(19): RC168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shukla VK, Lemaire S. Non-opioid effects of dynorphins: possible role of the NMDA receptor. Trends Pharmacol Sci 1994; 15(11): 420–424. [DOI] [PubMed] [Google Scholar]
- 69. Tejeda HA, Shippenberg TS, Henriksson R. The dynorphin/kappa-opioid receptor system and its role in psychiatric disorders. Cell Mol Life Sci 2012; 69(6): 857–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bruijnzeel AW. kappa-Opioid receptor signaling and brain reward function. Brain Res Rev 2009; 62(1): 127–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Crain SM, Shen KF. Modulatory effects of Gs-coupled excitatory opioid receptor functions on opioid analgesia, tolerance, and dependence. Neurochem Res 1996; 21(11): 1347–1351. [DOI] [PubMed] [Google Scholar]
- 72. Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S et al. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A 2014; 111(16): E1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McFadzean I, Lacey MG, Hill RG, Henderson G. Kappa opioid receptor activation depresses excitatory synaptic input to rat locus coeruleus neurons in vitro. Neuroscience 1987; 20(1): 231–239. [DOI] [PubMed] [Google Scholar]
- 74. Kreibich A, Reyes BA, Curtis AL, Ecke L, Chavkin C, Van Bockstaele EJ et al. Presynaptic inhibition of diverse afferents to the locus ceruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J Neurosci 2008; 28(25): 6516–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pinnock RD. Activation of kappa-opioid receptors depresses electrically evoked excitatory postsynaptic potentials on 5-HT-sensitive neurones in the rat dorsal raphe nucleus in vitro. Brain Res 1992; 583(1–2): 237–246. [DOI] [PubMed] [Google Scholar]
- 76. Tao R, Auerbach SB. Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther 2002; 303(2): 549–556. [DOI] [PubMed] [Google Scholar]
- 77. Ferrari LL, Agostinelli LJ, Krashes MJ, Lowell BB, Scammell TE, Arrigoni E. Dynorphin inhibits basal forebrain cholinergic neurons by pre- and postsynaptic mechanisms. J Physiol 2016; 594(4): 1069–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Matzeu A, Kallupi M, George O, Schweitzer P, Martin-Fardon R. Dynorphin Counteracts Orexin in the Paraventricular Nucleus of the Thalamus: Cellular and Behavioral Evidence. Neuropsychopharmacology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Baimel C, Lau BK, Qiao M, Borgland SL. Projection-Target-Defined Effects of Orexin and Dynorphin on VTA Dopamine Neurons. Cell Rep 2017; 18(6): 1346–1355. [DOI] [PubMed] [Google Scholar]
- 80. Sun HX, Wang DR, Ye CB, Hu ZZ, Wang CY, Huang ZL et al. Activation of the ventral tegmental area increased wakefulness in mice. Sleep Biol Rhythms 2017; 15(2): 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev 2007; 11(2): 113–133. [DOI] [PubMed] [Google Scholar]
- 82. Yamanaka A, Tabuchi S, Tsunematsu T, Fukazawa Y, Tominaga M. Orexin directly excites orexin neurons through orexin 2 receptor. J Neurosci 2010; 30(38): 12642–12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li Y, van den Pol AN. Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci 2006; 26(50): 13037–13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci 1998; 18(12): 4705–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science 1996; 271(5246): 216–219. [DOI] [PubMed] [Google Scholar]
- 86. Eriksson KS, Sergeeva OA, Selbach O, Haas HL. Orexin (hypocretin)/dynorphin neurons control GABAergic inputs to tuberomammillary neurons. Eur J Neurosci 2004; 19(5): 1278–1284. [DOI] [PubMed] [Google Scholar]
- 87. Apergis-Schoute J, Iordanidou P, Faure C, Jego S, Schone C, Aitta-Aho T et al. Optogenetic evidence for inhibitory signaling from orexin to MCH neurons via local microcircuits. J Neurosci 2015; 35(14): 5435–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Robinson JD, McDonald PH. The orexin 1 receptor modulates kappa opioid receptor function via a JNK-dependent mechanism. Cell Signal 2015; 27(7): 1449–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen J, Zhang R, Chen X, Wang C, Cai X, Liu H et al. Heterodimerization of human orexin receptor 1 and kappa opioid receptor promotes protein kinase A/cAMP-response element binding protein signaling via a Galphas-mediated mechanism. Cell Signal 2015; 27(7): 1426–1438. [DOI] [PubMed] [Google Scholar]
- 90. Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol 2003; 465(4): 593–603. [DOI] [PubMed] [Google Scholar]
- 91. Mickelsen LE, Kolling FWt, Chimileski BR, Fujita A, Norris C, Chen K et al. Neurochemical Heterogeneity Among Lateral Hypothalamic Hypocretin/Orexin and Melanin-Concentrating Hormone Neurons Identified Through Single-Cell Gene Expression Analysis . eNeuro 2017; 4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Henny P, Brischoux F, Mainville L, Stroh T, Jones BE. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience 2010; 169(3): 1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Torrealba F, Yanagisawa M, Saper CB. Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience 2003; 119(4): 1033–1044. [DOI] [PubMed] [Google Scholar]
- 94. van den Pol AN. Neuropeptide transmission in brain circuits. Neuron 2012; 76(1): 98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Baimel C, Borgland SL. Hypocretin/Orexin and Plastic Adaptations Associated with Drug Abuse. Curr Top Behav Neurosci 2017; 33: 283–304. [DOI] [PubMed] [Google Scholar]
- 96. Sudhof TC. The presynaptic active zone. Neuron 2012; 75(1): 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schone C, Apergis-Schoute J, Sakurai T, Adamantidis A, Burdakov D. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep 2014; 7(3): 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sudhof TC. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol 2012; 4(1): a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chen L, Gu Y, Huang LY. The mechanism of action for the block of NMDA receptor channels by the opioid peptide dynorphin. J Neurosci 1995; 15(6): 4602–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sita LV, Elias CF, Bittencourt JC. Connectivity pattern suggests that incerto-hypothalamic area belongs to the medial hypothalamic system. Neuroscience 2007; 148(4): 949–969. [DOI] [PubMed] [Google Scholar]
- 101. Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL et al. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol 1992; 319(2): 218–245. [DOI] [PubMed] [Google Scholar]
- 102. Bittencourt JC. Anatomical organization of the melanin-concentrating hormone peptide family in the mammalian brain. Gen Comp Endocrinol 2011; 172(2): 185–197. [DOI] [PubMed] [Google Scholar]
- 103. Diniz GB, Bittencourt JC. The Melanin-Concentrating Hormone as an Integrative Peptide Driving Motivated Behaviors. Front Syst Neurosci 2017; 11: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Casatti CA, Elias CF, Sita LV, Frigo L, Furlani VC, Bauer JA et al. Distribution of melanin-concentrating hormone neurons projecting to the medial mammillary nucleus. Neuroscience 2002; 115(3): 899–915. [DOI] [PubMed] [Google Scholar]
- 105. Adamantidis A, de Lecea L. A role for Melanin-Concentrating Hormone in learning and memory. Peptides 2009; 30(11): 2066–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Smith DG, Davis RJ, Rorick-Kehn L, Morin M, Witkin JM, McKinzie DL et al. Melanin-concentrating hormone-1 receptor modulates neuroendocrine, behavioral, and corticolimbic neurochemical stress responses in mice. Neuropsychopharmacology 2006; 31(6): 1135–1145. [DOI] [PubMed] [Google Scholar]
- 107. Glick M, Segal-Lieberman G, Cohen R, Kronfeld-Schor N. Chronic MCH infusion causes a decrease in energy expenditure and body temperature, and an increase in serum IGF-1 levels in mice. Endocrine 2009; 36(3): 479–485. [DOI] [PubMed] [Google Scholar]
- 108. Yoon YS, Lee HS. Projections from melanin-concentrating hormone (MCH) neurons to the dorsal raphe or the nuclear core of the locus coeruleus in the rat. Brain Res 2013; 1490: 72–82. [DOI] [PubMed] [Google Scholar]
- 109. Torterolo P, Sampogna S, Chase MH. MCHergic projections to the nucleus pontis oralis participate in the control of active (REM) sleep. Brain Res 2009; 1268: 76–87. [DOI] [PubMed] [Google Scholar]
- 110. Fraigne JJ, Peever JH. Melanin-concentrating hormone neurons promote and stabilize sleep. Sleep 2013; 36(12): 1767–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Whiddon BB, Palmiter RD. Ablation of neurons expressing melanin-concentrating hormone (MCH) in adult mice improves glucose tolerance independent of MCH signaling. J Neurosci 2013; 33(5): 2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fort P, Salvert D, Hanriot L, Jego S, Shimizu H, Hashimoto K et al. The satiety molecule nesfatin-1 is co-expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience 2008; 155(1): 174–181. [DOI] [PubMed] [Google Scholar]
- 113. Elias CF, Lee CE, Kelly JF, Ahima RS, Kuhar M, Saper CB et al. Characterization of CART neurons in the rat and human hypothalamus. J Comp Neurol 2001; 432(1): 1–19. [DOI] [PubMed] [Google Scholar]
- 114. Hawes BE, Kil E, Green B, O’Neill K, Fried S, Graziano MP. The melanin-concentrating hormone receptor couples to multiple G proteins to activate diverse intracellular signaling pathways. Endocrinology 2000; 141(12): 4524–4532. [DOI] [PubMed] [Google Scholar]
- 115. Gao XB, van den Pol AN. Melanin-concentrating hormone depresses L-, N-, and P/Q-type voltage-dependent calcium channels in rat lateral hypothalamic neurons. J Physiol 2002; 542(Pt 1): 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gao XB, van den Pol AN. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J Physiol 2001; 533(Pt 1): 237–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tan CP, Sano H, Iwaasa H, Pan J, Sailer AW, Hreniuk DL et al. Melanin-concentrating hormone receptor subtypes 1 and 2: species-specific gene expression. Genomics 2002; 79(6): 785–792. [DOI] [PubMed] [Google Scholar]
- 118. Sailer AW, Sano H, Zeng Z, McDonald TP, Pan J, Pong SS et al. Identification and characterization of a second melanin-concentrating hormone receptor, MCH-2R. Proc Natl Acad Sci U S A 2001; 98(13): 7564–7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Konadhode RR, Pelluru D, Blanco-Centurion C, Zayachkivsky A, Liu M, Uhde T et al. Optogenetic stimulation of MCH neurons increases sleep. J Neurosci 2013; 33(25): 10257–10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Blanco-Centurion C, Liu M, Konadhode RP, Zhang X, Pelluru D, van den Pol AN et al. Optogenetic activation of melanin-concentrating hormone neurons increases non-rapid eye movement and rapid eye movement sleep during the night in rats. Eur J Neurosci 2016; 44(10): 2846–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R et al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci 2013; 16(11): 1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tsunematsu T, Ueno T, Tabuchi S, Inutsuka A, Tanaka KF, Hasuwa H et al. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J Neurosci 2014; 34(20): 6896–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Fujimoto M, Fukuda S, Sakamoto H, Takata J, Sawamura S. Neuropeptide glutamic acid-isoleucine (NEI)-induced paradoxical sleep in rats. Peptides 2017; 87: 28–33. [DOI] [PubMed] [Google Scholar]
- 124. Verret L, Goutagny R, Fort P, Cagnon L, Salvert D, Leger L et al. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci 2003; 4: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ahnaou A, Drinkenburg WH, Bouwknecht JA, Alcazar J, Steckler T, Dautzenberg FM. Blocking melanin-concentrating hormone MCH1 receptor affects rat sleep-wake architecture. Eur J Pharmacol 2008; 579(1–3): 177–188. [DOI] [PubMed] [Google Scholar]
- 126. Vetrivelan R, Kong D, Ferrari LL, Arrigoni E, Madara JC, Bandaru SS et al. Melanin-concentrating hormone neurons specifically promote rapid eye movement sleep in mice. Neuroscience 2016; 336: 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Naganuma F, Bandaru SS, Absi G, Mahoney CE, Scammell TE, Vetrivelan R. Melanin-concentrating hormone neurons contribute to dysregulation of rapid eye movement sleep in narcolepsy. Neurobiol Dis 2018; 120: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Jancsik V, Bene R, Sotonyi P, Zachar G. Sub-cellular organization of the melanin-concentrating hormone neurons in the hypothalamus. Peptides 2017. [DOI] [PubMed] [Google Scholar]
- 129. Chee MJ, Arrigoni E, Maratos-Flier E. Melanin-concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. J Neurosci 2015; 35(8): 3644–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Blanco-Centurion C, Bendell E, Zou B, Sun Y, Shiromani PJ, Liu M. VGAT and VGLUT2 expression in MCH and orexin neurons in double transgenic reporter mice. IBRO Rep 2018; 4: 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tuominen L, Nummenmaa L, Keltikangas-Jarvinen L, Raitakari O, Hietala J. Mapping neurotransmitter networks with PET: an example on serotonin and opioid systems. Hum Brain Mapp 2014; 35(5): 1875–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Liu ZY, Liu FT, Zuo CT, Koprich JB, Wang J. Update on Molecular Imaging in Parkinson’s Disease. Neurosci Bull 2018; 34(2): 330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 2009; 513(5): 532–541. [DOI] [PubMed] [Google Scholar]
- 134. Schwarz LA, Luo L. Organization of the locus coeruleus-norepinephrine system. Curr Biol 2015; 25(21): R1051–R1056. [DOI] [PubMed] [Google Scholar]
- 135. Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 2014; 81(6): 1360–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sengupta A, Bocchio M, Bannerman DM, Sharp T, Capogna M. Control of Amygdala Circuits by 5-HT Neurons via 5-HT and Glutamate Cotransmission. J Neurosci 2017; 37(7): 1785–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Le Maitre E, Barde SS, Palkovits M, Diaz-Heijtz R, Hokfelt TG. Distinct features of neurotransmitter systems in the human brain with focus on the galanin system in locus coeruleus and dorsal raphe. Proc Natl Acad Sci U S A 2013; 110(6): E536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wagatsuma A, Okuyama T, Sun C, Smith LM, Abe K, Tonegawa S. Locus coeruleus input to hippocampal CA3 drives single-trial learning of a novel context. Proc Natl Acad Sci U S A 2018; 115(2): E310–E316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci U S A 2016; 113(51): 14835–14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Eser RA, Ehrenberg AJ, Petersen C, Dunlop S, Mejia MB, Suemoto CK et al. Selective Vulnerability of Brainstem Nuclei in Distinct Tauopathies: A Postmortem Study. J Neuropathol Exp Neurol 2018; 77(2): 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]


