Abstract
BACKGROUND
Postoperative arrhythmias after pediatric congenital heart disease (CHD) surgery are a known cause of morbidity and are associated with mortality. A comprehensive evaluation of early postoperative ventricular arrhythmias (VAs) after CHD surgery has not been reported.
OBJECTIVES
We sought to determine the incidence of in-hospital VAs after CHD surgery and assess the clinical relevance of this arrhythmia during the postoperative hospital course.
METHODS
Patients undergoing CHD surgery at our center from September 2007 through December 2016 were prospectively enrolled. Univariate and multivariate analysis was used to assess the association between postoperative VAs and in-hospital mortality, adjusting for postoperative extracorporeal membrane oxygenation and stage 1 single ventricle palliation operations.
RESULTS
A total of 2503 postoperative courses in 1835 patients were included. In all, 464 (18.5%) had VAs, of whom 135 (29.1%) received treatment. Monomorphic ventricular tachycardia was the most frequently treated ventricular arrhythmia (TVA; n=91 [62.3%]). TVAs were associated with increased postoperative extracorporeal membrane oxygenation (13.3% vs 5.5%; P < .001) and in-hospital mortality (14.9% vs 4.0%; P < .001). In multivariate analysis, TVA was an independent risk factor for in-hospital mortality (adjusted odds ratio 2.44; 95% confidence interval 1.21–4.92).
CONCLUSION
Early postoperative VAs after CHD surgery are more common than previously reported. Postoperative VAs are associated with increased in-hospital mortality, and the subgroup of TVAs is an independent risk factor for in-hospital mortality.
Keywords: Arrhythmia, Cardiac surgery, Congenital heart disease, Pediatric, Ventricular tachycardia
Introduction
Postoperative arrhythmias are common after surgery for congenital heart disease (CHD)1–4 and are associated with increased morbidity.5–8 Most neonates and children will experience an arrhythmia in the postoperative period after CHD surgery.5 Often these arrhythmias occur during a hemo-dynamically vulnerable period and are associated with prolonged mechanical ventilation, longer intensive care unit stay, longer hospital stay, and increased mortality.5–8 Studies of specific postoperative arrhythmias have focused on atrial9–13 and junctional14–18 tachycardias. Ventricular arrhythmias (VAs), particularly ventricular tachycardias (VTs), are less well studied.19 The reported incidence of early postoperative VT ranges from ≤2%2,6,20 to 13%,5,8 and the clinical significance of VAs in the postoperative period is not well understood. The objectives of the present study were to determine the incidence of postoperative in-hospital VAs using, to our knowledge, the largest cohort of patients with CHD prospectively followed for postoperative arrhythmias and to assess the clinical relevance of this arrhythmia during the early postoperative period.
Methods
Study population
All patients in the analysis were enrolled in an ongoing prospective observational study.21 Every patient undergoing CHD surgery at our center is approached for enrollment, thereby minimizing sampling bias. Each patient’s parents or guardians provided written informed consent, and patient assent was obtained as age appropriate. The Vanderbilt University Institutional Review Board approved the study. This analysis is limited to surgeries between September 2007 and December 2016. The volume of surgical cases was consistent over this period, with a total of 2637 CHD surgeries in the study. Patients were excluded if they were still hospitalized on December 31, 2016. Additional exclusion criteria included the following noncardiac primary operative procedures: vascular ring repair, thoracic duct ligation, sympathectomy, and pacemaker implantation or revision.
Data collection
Perioperative data collection included demographic characteristics, diagnoses, medical history, preoperative medications, history of arrhythmias, history of extracorporeal membrane oxygenation (ECMO) support after previous surgery, and number of cardiac surgeries performed during each hospitalization. Operative details included surgical procedure (primary and secondary procedures) and durations of bypass duration and cross-clamp duration. Operations were classified by Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery (STAT) scores.22 Laboratory results and continuous infusions administered at postoperative intensive care unit admission were recorded. Each patient underwent continuous telemetry monitoring (Philips Medical Systems, Bothell, WA) for the entirety of hospitalization.8 Telemetry data were reviewed daily by study personnel. When feasible, atrial electrograms using temporary epicardial wires (routinely placed during surgery at our center) were used to aid in discerning tachycardia mechanisms. All recorded arrhythmias were reviewed and confirmed by pediatric electrophysiologists. Each patient was monitored for the entirety of their postoperative course, to cover the time from surgical procedure until discharge from hospital, the next surgical procedure if performed before discharge, or death.
Arrhythmia definitions
Early postoperative arrhythmia was defined as an arrhythmia that occurred during hospitalization after cardiac surgery. Postoperative recurrences of arrhythmias that had occurred preoperatively were excluded. Arrhythmias occurring after discharge were not analyzed for this study. VAs included the following: VT–monomorphic (uniform QRS morphology), polymorphic (variable QRS morphology), or wide complex not otherwise specified; accelerated ventricular rhythm; and ventricular fibrillation. VT was defined as a wide complex tachycardia consistent with a ventricular origin, ≥3 beats in duration, and with rate >10% above the baseline rate.23 Single premature ventricular contractions, regardless of their frequency or burden were not included, nor were premature ventricular contraction pairs. Ventricular escape rhythms (slower than the baseline rhythm) were not included.
The onset and duration of each arrhythmia were noted. Treatment of an arrhythmia was defined and categorized as the following: antiarrhythmic medication, electrolyte repletion, other medical intervention (including dexmedetomidine, sodium bicarbonate, and/or changes to inotropic support), cardioversion, defibrillation, pace termination, temporary pacing, permanent pacing, cardiopulmonary resuscitation (CPR) requiring only compressions, CPR requiring both compressions and drugs, manipulation of a central line, cannulation to ECMO support, or implantable cardioverter-defibrillator placement. VAs that received at least one of these treatments were classified as treated ventricular arrhythmias (TVAs). The TVA group was further divided by treatment type into 3 subcategories, namely, complex, moderate, and simple treatment types. Complex treatment consisted of targeted and potentially invasive antiarrhythmic treatments including any of the following: cardioversion, defibrillation, pace termination, temporary pacing, permanent pacing, CPR (with or without drug), cannulation to ECMO, or implantable cardioverter-defibrillator. Moderate treatment consisted of initiating or titrating antiarrhythmic medications. Simple treatment consisted of supportive routine postoperative care including electrolyte repletion, administration of sodium bicarbonate, adjustment of dexmedetomidine or inotropic infusions, or manipulation of a central line.
Treatment decisions were made at the discretion of the clinical teams. Treatment serves as a surrogate marker for clinical severity of VAs, as our intentionally broad definition includes nonsustained arrhythmias that may not elicit clinical attention. Traditionally the institution adheres to the following goals to guide electrolyte replacement: maintaining a potassium level of 3.5–5.0 mEq/L; an ionized calcium level of 4.5–5.5 mg/dL; and a magnesium level of 1.8–2.2 mEq/L.
Power calculation and data analysis
Using a conservative estimate of postoperative VA incidence of 5.7% and the established in-hospital mortality rate of 3.9% after pediatric heart surgery,24 our sample size was adequately powered to demonstrate an increased in-hospital mortality rate of at least 10% in patients with postoperative VAs. The type 1 error probability associated with rejecting the null hypothesis that in-hospital mortality rates for patients with postoperative VAs was the same was .05 with a power of 80%. Power calculations were performed using Power and Sample Size Calculation software (version 3.1.2 Vanderbilt University, Nashville, TN).25
Continuous data are reported as medians with interquartile ranges (IQRs), and categorical data are reported as frequencies with percentages. The 95% confidence intervals (CIs) of in-hospital mortality rates were calculated by bootstrapping with 1000 intervals. Univariate analyses were performed using the Mann-Whitney U test for continuous variables and Fisher exact test for categorical variables. Multivariate logistic regression using in-hospital mortality as the outcome was completed to assess the clinical importance of TVAs. Other covariates, in addition to TVA, within this model were chosen a priori as known high morbidity clinical factors. These covariates included STAT score, history of ECMO, history of multiple cardiac surgeries within a single postoperative admission, lactate level, bypass duration, and cross-clamp duration. The model used a 1:10 rule of predictors in the model per outcome measured.26 This mortality analysis was completed using the subgroup of 1835 unique patients. We assessed model fit using the Hosmer-Lemeshow test. The TVA group was further divided into subcategories on the basis of type of therapy received: complex, moderate, and simple (as defined above); and association of each treatment category with mortality was assessed. Significance tests were 2-tailed, with a P value of <.05 considered significant. Subgroup analyses, including exclusions for a history of ECMO and stage 1 single ventricle palliation (S1-SVP), are described further in Supplemental Methods. Data were analyzed using SPSS for Windows version 24.0 (IBM Corporation, Armonk, NY) and Stata version 14 (StataCorp LP, College Station, TX).
Results
A total of 2503 surgeries in 1835 patients were included. Baseline characteristics and perioperative data are summarized in Table 1. As expected for a cohort of patients with postoperative CHD, patients were young (median age at surgery 186 days), with a slight male predominance (54.6% male), and 23.2% had a known chromosomal abnormality. Preoperative antiarrhythmic medication (atenolol, carvedilol, digoxin, esmolol, flecainide, lidocaine, metoprolol, propafenone, propranolol, or sotalol) was present in 14.1%. More than one-third of the cohort had a STAT score of ≥3. Compared to the case mix in the Society of Thoracic Surgeons Congenital Heart Surgery database, our cohort had relatively fewer low complexity STAT category 2 cases (28% vs 36%) and nearly double the most complex STAT category 5 cases (7.2% vs 3.9%) than did the average reporting center.27
Table 1.
Baseline demographic and perioperative characteristics of the study cohort*
| Characteristic | Value |
|---|---|
| Age (d) | 186 (61–1182) |
| Weight (kg) | 6.6 (4.0–13.6) |
| Sex: male | 1366 (54.6) |
| Chromosomal abnormality | 580 (23.2) |
| Most common primary diagnoses | |
| HLHS† | 321 (12.8) |
| Tetralogy of Fallot | 225 (9.0) |
| Single ventricle anatomy other than HLHS | 224 (8.9) |
| AVSD | 210 (8.4) |
| Ventricular septal defect | 193 (7.7) |
| Most common surgical procedures | |
| Ventricular septal defect closure | 202 (8.1) |
| Glenn procedure | 183 (7.3) |
| Repair of AVSD | 166 (6.6) |
| Total repair of tetralogy of Fallot | 159 (6.4) |
| Fontan procedure | 155 (6.2) |
| History of preoperative antiarrhythmic medication | 354 (14.1) |
| Cardiopulmonary bypass duration (min) | 104 (65–150) |
| Aortic cross-clamp duration (min) | 36 (3–64) |
| Lactate level (mmol/L) | 1.6 (1.1–2.8) |
| STAT score | |
| 1 | 764 (30.5) |
| 2 | 698 (27.9) |
| 3 | 332 (13.3) |
| 4 | 525 (21.0) |
| 5 | 181 (7.2) |
| Missing or unable to classify | 3 (0.1) |
| Cannulation to ECMO | 137 (5.5) |
| In-hospital mortality‡ | 74 (4.0) |
Values are presented as median (interquartile range) or as n (%).
AVSD = atrioventricular septal defect (includes complete and partial AVSDs); ECMO = extracorporeal membrane oxygenation; HLHS = hypoplastic left heart syndrome; STAT = Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery.
N = 2503 postoperative courses.
The HLHS group includes patients with Shone’s syndrome requiring single ventricle palliation.
In-hospital mortality was calculated using 1835 unique individuals.
At least 1 VA occurred in 464 unique postoperative courses for an incidence of 18.5% (Table 2). Nearly one-third of the 464 postoperative courses with VA (n=135) received treatment (Table 3). TVA occurred a median of 18.7 hours (IQR 4.6–126.9 hours) postoperatively. Monomorphic VT was the most common form of TVA (n=91 [62.3%]). The most frequent treatments of VA were electrolyte repletion (n=95 [65.1%]) and initiation or manipulation of antiarrhythmic medication (n=32 [21.9%]). Forty patents received >1 treatment modality type. Further details on the frequency of each type of TVA and treatment modalities can be found in Supplemental Tables 1 and 2.
Table 2.
Comparison of perioperative characteristics of patients with and without early postoperative VAs
| Characteristic | VA (n=464) | No VA (n=2039) | P |
|---|---|---|---|
| Age (d) | 174 (14–1821) | 190 (73–1116) | .43 |
| Weight (kg) | 6.2 (3.6–17.7) | 6.6 (4.2–13.2) | .86 |
| Sex: male | 256 (55.2) | 1110 (54.4) | .80 |
| Chromosomal abnormality | 98 (21.1) | 482 (23.6) | .27 |
| Most common primary diagnosis | |||
| Tetralogy of Fallot | 58 (12.5) | 167 (8.2) | .005 |
| HLHS* | 52 (11.2) | 269 (13.2) | .28 |
| Ventricular septal defect | 37 (8.0) | 156 (7.7) | .85 |
| Transposition of the great arteries | 33 (7.1) | 75 (3.7) | .002 |
| AVSD | 30 (6.5) | 180 (8.8) | .11 |
| Single ventricle anatomy other than HLHS | 30 (6.5) | 194 (9.5) | .04 |
| Most common primary surgical procedure | |||
| Tetralogy of Fallot (complete repair) | 40 (8.6) | 119 (5.8) | .03 |
| Ventricular septal defect closure | 38 (8.2) | 164 (8.0) | .93 |
| S1-SVP† operation | 41 (8.8) | 119 (5.8) | .02 |
| Repair of AvSD | 26 (5.6) | 140 (6.9) | .35 |
| Heart transplantation | 22 (4.7) | 33 (1.6) | <.001 |
| History of preoperative antiarrhythmic medication | 46 (9.9) | 308 (15.1) | .003 |
| Cardiopulmonary bypass duration (min) | 128 (84–174) | 99 (62–144) | <.001 |
| Aortic cross-clamp duration (min) | 49 (28–76) | 32 (0–60) | <.001 |
| Lactate level (mmol/L) | 2.0 (1.3-3.8) | 1.6 (1.1-2.7) | <.001 |
| STAT score | |||
| 1 | 148 (31.9) | 616 (30.2) | .50 |
| 2 | 100 (21.6) | 598 (29.3) | .001 |
| 3 | 57 (12.3) | 275 (13.4) | .54 |
| 4 | 114 (24.6) | 411 (20.2) | .04 |
| 5 | 43 (9.3) | 138 (6.8) | .07 |
| Unable to classify | 2 (0.43) | 1 (0.05) | .09 |
| Cannulation to ECMO | 35 (7.5) | 102 (5.0) | .04 |
| In-hospital mortality‡ | 30 (6.5) | 44 (3.2) | .002 |
Values are presented as median (interquartile range) or as n (%). P values were calculated using the Mann-Whitney U test for continuous variables and Fisher exact test for categorical variables. The most common diagnoses and surgical procedures listed are organized by frequency in the VA subgroup.
AVSD = atrioventricular septal defect (includes complete and partial AVSDs); ECMO = extracorporeal membrane oxygenation; HLHS = hypoplastic left heart syndrome; S1-SVP = stage 1 single ventricle palliation; STAT = Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery; VA = ventricular arrhythmia.
The HLHS group includes patients with Shone’s syndrome requiring single ventricle palliation.
Sl-SVP operation was defined as a primary surgery of the Norwood procedure, hybrid approach, or Damus-Kaye-Stansel procedure resulting in single ventricle palliation pathway operation.
In-hospital mortality rates were calculated using 1835 unique individuals (consisting of 454 with VA and 1381 without VA).
Table 3.
Comparison of perioperative characteristics of patients with treated vs untreated early postoperative VA
| Characteristic | Treated VA (n=135) | Untreated VA (n=329) | P |
|---|---|---|---|
| Age (d) | 182 (11–2832) | 170 (15–1550) | .65 |
| Weight (kg) | 6.4 (3.6–6.4) | 6.1 (3.7–15.6) | .80 |
| Sex: male | 74 (54.8) | 182 (55.3) | .92 |
| Chromosomal abnormality | 28 (20.7) | 70 (21.3) | 1.00 |
| Most common primary diagnosis | |||
| HLHS* | 22 (16.3) | 30 (9.1) | .03 |
| Tetralogy of Fallot | 14 (10.4) | 44 (13.4) | .44 |
| Single ventricle other than HLHS | 14 (10.4) | 16 (4.9) | .04 |
| Ventricular septal defect | 8 (5.9) | 29 (8.8) | .35 |
| AVSD | 8 (5.9) | 22 (6.7) | .84 |
| Most common primary surgical procedure | |||
| S1-SVP operation† | 18 (13.3) | 23 (7.0) | .046 |
| Ventricular septal defect repair | 10 (7.4) | 28 (8.5) | .85 |
| Heart transplantation | 10 (7.4) | 12 (3.6) | .10 |
| Pulmonary valve replacement | 9 (6.7) | 12 (3.6) | .22 |
| Repair of AVSD | 7 (5.2) | 19 (5.8) | 1.00 |
| History of preoperative antiarrhythmic medication | 17 (12.6) | 29 (8.8) | .23 |
| Cardiopulmonary bypass duration (min) | 148 (100–191) | 119 (80–165) | .001 |
| Aortic cross-clamp duration (min) | 53 (30–82) | 26 (47–75) | .21 |
| Lactate level (mmol/L) | 2.6 (1.5–5.2) | 1.6 (1.1–2.8) | <.001 |
| STAT score | |||
| 1 | 37 (27.4) | 111 (33.7) | .19 |
| 2 | 28 (20.7) | 72 (21.9) | .90 |
| 3 | 15 (11.1) | 42 (12.8) | .76 |
| 4 | 36 (26.7) | 78 (23.7) | .55 |
| 5 | 18 (13.3) | 25 (7.6) | .08 |
| Unable to classify | 1 (0.7) | 1 (0.3) | .50 |
| Cannulation to ECMO | 18 (13.3) | 17 (5.2) | .006 |
| In-hospital mortality‡ | 20 (14.9) | 10 (3.1) | <.001 |
Values are presented as median (interquartile range) or as n (%). P values were calculated using the Mann-Whitney U test for continuous variables and Fisher exact test for categorical variables. The most common diagnoses and surgical procedures listed are organized by frequency in the treated VA subgroup.
AVSD = atrioventricular septal defect (includes complete and partial AVSDs); ECMO = extracorporeal membrane oxygenation; HLHS = hypoplastic left heart syndrome; S1-SVP = stage 1 single ventricle palliation; STAT = Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery; VA = ventricular arrhythmia.
The HLHS group includes patients with Shone’s syndrome requiring single ventricle palliation.
S1-SVP operation was defined as a primary surgery of the Norwood procedure, hybrid approach, or Damus-Kaye-Stansel procedure resulting in single ventricle palliation pathway operation.
In-hospital mortality rates were calculated using 454 unique individuals (consisting of 134 with treated VA and 320 with untreated VA).
Patients with postoperative VA had higher rates of morbidity and in-hospital mortality than did patients without VA. The use of ECMO for patients with VA was 7.5% as compared with 5.0% in patients without VA (P = .04). The in-hospital mortality rate for patients with VA was also higher (6.5% vs 3.2%; P = .002). Subgroup analysis demonstrated that within the VA group, it was the patients with TVA who had the highest rate of in-hospital mortality (14.9% vs 3.1%; P < .001) (see Figure 1 and Supplemental Tables 3 and 4). The median time from TVA onset to death was 52.5 days (IQR 4.0–79.5 days).
Figure 1.
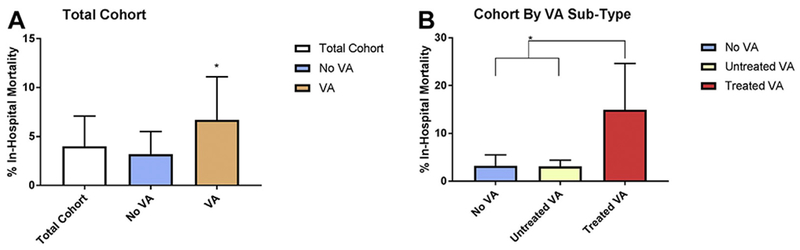
In-hospital mortality rate by each postoperative VA type. In-hospital mortality rates with 95% confidence intervals, which were calculated by boot-strapping with 1000 intervals, were as follows: total cohort, 4.0% (3.1%–4.9%); no VA, 3.2% (2.3%–4.1%); VA, 6.7% (4.4%–9.0%); untreated VA, 3.1% (1.3%–5.0%); treated VA, 14.9% (9.7%–20.9%). *Statistically significant P value. A: The rate of in-hospital mortality in the VA group is higher than that in the no VA group (Fisher exact test, P = .002). B: The rate of in-hospital mortality in the treated VA group is higher than that in the no VA and untreated VA groups (Fisher exact test, P < .001). In-hospital mortality was calculated using 1835 unique individuals for the total cohort, 1381 unique individuals with no VA, and 454 with VA, divided into the subgroups of 320 with untreated VA and 134 with treated VA. VA = ventricular arrhythmia.
In the multivariate logistic model, TVA was independently associated with in-hospital mortality (adjusted odds ratio [OR] 2.44; 95% CI 1.21–4.92). This model adjusted for clinical factors associated with morbidity, including bypass duration, cross-clamp duration, history of ECMO, postoperative admission lactate level, history of cardiac surgery during the current admission, and STAT score >3. The summary of each covariate’s association with in-hospital mortality is given in Figure 2 and Supplemental Table 5. TVA was independently associated with increased in-hospital mortality in both the ECMO-negative and S1-SVP-negative subgroups (Supplemental Results and Supplemental Tables 6 and 7).
Figure 2.
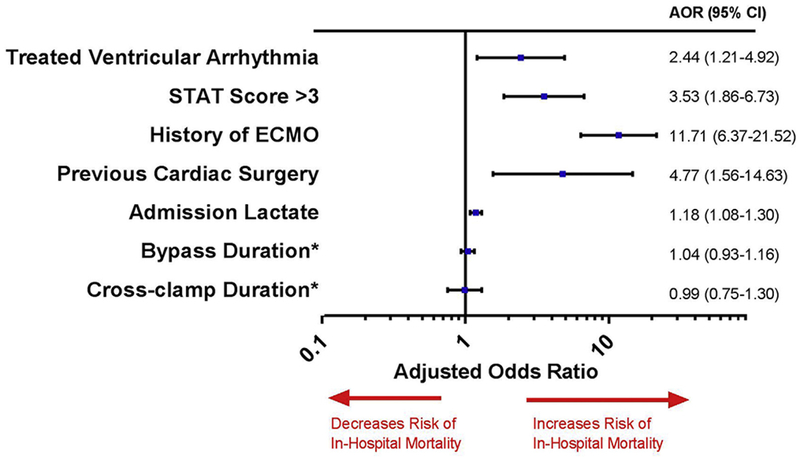
Multivariate analysis of in-hospital mortality in the total cohort (1835 unique patients with a total of 74 deaths). Previous cardiac surgery refers to a history of multiple cardiac surgeries within a single postoperative admission (Hosmer-Lemeshow test, P = .408). The full details of the multivariate analysis are given in Supplemental Table 3. *The cross-clamp and bypass durations are reported as 30-minute interval durations. AOR = adjusted odds ratio; CI = confidence interval; ECMO = extracorporeal membrane oxygenation; STAT = Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery.
To further analyze the relationship between TVA and in-hospital mortality, TVA was further divided into the subcategories of complex, moderate, and simple forms of treatment received for VA. In total, 134 individual patients (>135 postoperative courses) received treatment for VA. Both complex (OR 17.43; 95% CI 8.16–37.24) and moderate (OR 3.81; 95% CI 1.11–13.05) treatment measures are associated with a significant risk of in-hospital mortality when compared to patients without TVA (those without VA and with untreated VA) (Table 4).
Table 4.
Type of treatment patient received for VA and risk of in-hospital mortality
| Treatment category | n* | Mortality rate (%) | OR (95% CI)† |
|---|---|---|---|
| Complex | 33 | 36.4 | 17.43 (8.16–37.24) |
| Moderate | 27 | 11.1 | 3.81 (1.11–13.05) |
| Simple | 74 | 6.8 | 2.21 (0.86–5.70) |
CI = confidence interval; OR = odds ratio; VA = ventricular arrhythmia.
There were a total of 146 episodes of treated VAs that occurred in a total of 134 unique patients (during 135 postoperative courses).
Compared to the combined non-treated VA group (patients with no VA or untreated VA), which has a mortality rate of 3.2%.
Discussion
The major findings of this study are that early postoperative VAs occur after 18.5% of CHD surgeries and are clinically significant, as they are associated with >2-fold increased risk of in-hospital mortality (P = .002). TVAs are an independent risk factor for in-hospital mortality (adjusted OR 2.44; 95% CI 1.21–4.92) after adjustment for multiple clinical covariates. The reported in-hospital mortality rate after CHD surgery of 3.9%24 could potentially be improved if postoperative risk factors, such as VAs, were better understood.
In this single-center prospective investigation, we demonstrate that the incidence of postoperative VAs in patients with CHD is higher than previously reported. Previous publications reported that the onset of VT generally occurs decades after surgical repair,19 while our study shows a significant burden of VT occurring earlier in the postoperative hospital course. Previously reported incidence rates likely underestimated the true incidence of postoperative VA by not including patients with untreated VA’s, the lack of continuous monitoring through hospital discharge,5 and excluding hypoplastic left heart syndrome (HLHS) patients, a known high risk group, in their studied cohorts. In addition, we have used a novel approach to distinguish TVA from untreated VA in order to identify the subgroup of postoperative VA that is most associated with mortality. Treatment was used as a proxy of arrhythmia severity, and we do not suggest that treatment itself has a negative effect on survival. More likely, VAs that did not warrant treatment were brief and/or mild and we found that these were not associated with increased mortality. Although we do not assert that postoperative VA is the cause of death, with multivariate modeling we have shown TVA to be a notable risk factor for death. To our knowledge, this is the first study to demonstrate in the pediatric population with CHD that TVA is an independent risk factor for in-hospital mortality after cardiac surgery. Previous studies published on postoperative VAs analyzed smaller cohort sizes ranging from 189 to 494 patients with postoperative CHD.2,5,20 Our sample size of >2500 surgeries is >5-fold greater. With our notably increased sample size, our study was powered to detect a meaningful increased mortality rate.
Understanding that in the hours and minutes before death an unstable patient may experience a fatal arrhythmia, including types of VAs, we reviewed the timing of TVA events to discern whether TVA events occurred immediately before death. TVA occurred a median of 18.7 hours (IQR 4.6–126.9 hours) postoperatively. For those patients who experienced a TVA event and had an in-hospital mortality, the median time from TVA onset to death was 52.5 days (IQR 4.0–79.5 days). This implies that the TVA event was unlikely to be an aspect of a terminal event for the patient.
Our group has recently shown that postoperative VA after the Norwood procedure are correlated with late mortality in patients with HLHS.28 The present study further supports that this subgroup is highly susceptible to clinically significant VAs. In the TVA subgroup (Table 3), the most prevalent diagnosis is HLHS (16.3%) and S1-SVP operation is the most common surgery type (13.3%). In addition to HLHS, the clinical effect of TVA can be seen across multiple lesions and TVA remains an independent risk factor for in-hospital mortality after excluding S1-SVP operations.
Study limitations
The analysis was performed in a single-center cohort, and thus further studies at other centers would be necessary to validate these findings. In addition, the review of the “treated” status of TVA is retrospective, with an assumption that the VA was clinically significant in order to receive treatment. The treatment method was also at the discretion of the provider, and not driven by a study or clinical protocol. Overall, the previously discussed analysis supports that postoperative VA, specifically TVA, is associated with increased mortality, but it does not establish a causal link between the arrhythmia and death.
Conclusion
Postoperative VA is a clinically significant arrhythmia that occurs with higher frequency, morbidity, and mortality than previously reported. The subgroup of TVAs is an independent risk factor for postoperative in-hospital mortality. Further research into the specific factors, both clinical and genetic, that may cause VA in our postoperative patients with innate myocardial compromise after CHD surgery is necessary. Such work will allow more meaningful patient risk stratification and paves the way for the development of personalized and potentially prophylactic medical therapies for our patients.
Supplementary Material
Acknowledgments
This work was supported in part by grants T32HL105334, R01HD084461, and UL1TR000445 from the National Institutes of Health, 12CRP10560001 from the American Heart Association, and CSDA 2017075 from the Doris Duke Charitable Foundation. Dr Van Driest has received an honorarium from Merck as an invited speaker. The other authors have no relationships with industry to disclose.
Appendix
Supplementary data
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrthm.2018.11.032.
References
- 1.Rękawek J, Kansy A, Miszczak-Knecht M, et al. Risk factors for cardiac arrhythmias in children with congenital heart disease after surgical intervention in the early postoperative period. J Thorac Cardiovasc Surg 2007;133:900–904. [DOI] [PubMed] [Google Scholar]
- 2.Delaney JW, Moltedo JM, Dziura JD, Kopf GS, Snyder CS. Early postoperative arrhythmias after pediatric cardiac surgery. J Thorac Cardiovasc Surg 2006; 131:1296–1300. [DOI] [PubMed] [Google Scholar]
- 3.Valsangiacomo E, Schmid ER, Schupbach RW, Schmidlin D, Waldvogel K, Bauersfeld U. Early postoperative arrhythmias after cardiac operation in children. Ann Thorac Surg 2002;74:792–796. [DOI] [PubMed] [Google Scholar]
- 4.Houyel L, Vaksmann G, Fournier A, Davignon A. Ventricular arrhythmias after correction of ventricular septal defects: importance of surgical approach. J Am Coll Cardiol 1990;16:1224–1228. [DOI] [PubMed] [Google Scholar]
- 5.Grosse-Wortmann L, Kreitz S, Grabitz RG, et al. Prevalence of and risk factors for perioperative arrhythmias in neonates and children after cardiopulmonary bypass: continuous Holter monitoring before and for three days after surgery. J Cardiothorac Surg 2010;5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva JN, Van Hare G. Management of postoperative pediatric cardiac arrhythmias: current state of the art. Curr Treat Options Cardiovasc Med 2009;11:410–416. [DOI] [PubMed] [Google Scholar]
- 7.Peretto G, Durante A, Limite LR, Cianflone D. Postoperative arrhythmias after cardiac surgery: incidence, risk factors, and therapeutic management. Cardiol Res Pract 2014;2014:615987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith AH, Flack EC, Borgman KY, et al. A common angiotensin-converting enzyme polymorphism and preoperative angiotensin-converting enzyme inhibition modify risk of tachyarrhythmias after congenital heart surgery. Heart Rhythm 2014;11:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao SO, Boramanand NK, Burton DA, Perry JC. Atrial tachycardias in young adults and adolescents with congenital heart disease: conversion using single dose oral sotalol. Int J Cardiol 2009;136:253–257. [DOI] [PubMed] [Google Scholar]
- 10.Beaufort-Krol GC, Bink-Boelkens MT. Effectiveness of sotalol for atrial flutter in children after surgery for congenital heart disease. Am J Cardiol 1997;79:92–94. [DOI] [PubMed] [Google Scholar]
- 11.Maragnes P, Tipple M, Fournier A. Effectiveness of oral sotalol for treatment of pediatric arrhythmias. Am J Cardiol 1992;69:751–754. [DOI] [PubMed] [Google Scholar]
- 12.Clark BC, Berger JT, Berul CI, et al. Risk factors for development of ectopic atrial tachycardia in postoperative congenital heart disease. Pediatr Cardiol 2018;39:459–465. [DOI] [PubMed] [Google Scholar]
- 13.Rosales AM, Walsh EP, Wessel DL, Triedman JK. Postoperative ectopic atrial tachycardia in children with congenital heart disease. Am J Cardiol 2001; 88:1169–1172. [DOI] [PubMed] [Google Scholar]
- 14.Dodge-Khatami A, Miller OI, Anderson RH, et al. Surgical substrates of postoperative junctional ectopic tachycardia in congenital heart defects. J Thorac Cardiovasc Surg 2002;123:624–630. [DOI] [PubMed] [Google Scholar]
- 15.Borgman KY, Smith AH, Owen JP, Fish FA, Kannankeril PJ. A genetic contribution to risk for postoperative junctional ectopic tachycardia in children undergoing surgery for congenital heart disease. Heart Rhythm 2011;8:1900–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zampi JD, Hirsch JC, Gurney JG, et al. Junctional ectopic tachycardia after infant heart surgery: incidence and outcomes. Pediatr Cardiol 2012;33:1362–1369. [DOI] [PubMed] [Google Scholar]
- 17.Batra AS, Chun DS, Johnson TR, et al. A prospective analysis of the incidence and risk factors associated with junctional ectopic tachycardia following surgery for congenital heart disease. Pediatr Cardiol 2006;27:51–55. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman TM, Bush DM, Wernovsky G, et al. Postoperative junctional ectopic tachycardia in children: incidence, risk factors, and treatment. Ann Thorac Surg 2002;74:1607–1611. [DOI] [PubMed] [Google Scholar]
- 19.Snyder CS. Postoperative ventricular tachycardia in patients with congenital heart disease: diagnosis and management. Nat Clin Pract Cardiovasc Med 2008; 5:469–476. [DOI] [PubMed] [Google Scholar]
- 20.Pfammatter JP, Bachmann DC, Wagner BP, et al. Early postoperative arrhythmias after open-heart procedures in children with congenital heart disease. Pediatr Crit Care Med 2001;2:217–222. [DOI] [PubMed] [Google Scholar]
- 21.Murray LE, Smith AH, Flack EC, Crum K, Owen J, Kannankeril PJ Genotypic and phenotypic predictors of complete heart block and recovery of conduction after surgical repair of congenital heart disease. Heart Rhythm 2017; 14:402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs JP, Jacobs ML, Lacour-Gayet FG, et al. Stratification of complexity improves the utility and accuracy of outcomes analysis in a Multi-Institutional Congenital Heart Surgery Database: application of the Risk Adjustment in Congenital Heart Surgery (RACHS-1) and Aristotle Systems in the Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database. Pediatr Cardiol 2009;30:1117–1130. [DOI] [PubMed] [Google Scholar]
- 23.Crosson JE, Callans DJ, Bradley DJ, et al. PACES/HRS expert consensus statement on the evaluation and management of ventricular arrhythmias in the child with a structurally normal heart. Heart Rhythm 2014;11:e55–e78. [DOI] [PubMed] [Google Scholar]
- 24.Pasquali SK, Li JS, Burstein DS, et al. Association of center volume with mortality and complications in pediatric heart surgery. Pediatrics 2012; 129:e370–e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dupont WD, Plummer WD Jr. Power and sample size calculations: a review and computer program. Control Clin Trials 1990;11:116–128. [DOI] [PubMed] [Google Scholar]
- 26.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373–1379. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs JP, Mayer JE Jr, Pasquali SK, et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2018 Update on Outcomes and Quality. Ann Thorac Surg 2018;105:680–689. [DOI] [PubMed] [Google Scholar]
- 28.Hall EJ, Smith AH, Fish FA, et al. Association of shunt type with arrhythmias after Norwood procedure. Ann Thorac Surg 2018;105:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


