Abstract
Background
Tonic‐clonic convulsions and convulsive status epilepticus (currently defined as a tonic‐clonic convulsion lasting at least 30 minutes) are medical emergencies and require urgent and appropriate anticonvulsant treatment. International consensus is that an anticonvulsant drug should be administered for any tonic‐clonic convulsion that has been continuing for at least five minutes. Benzodiazepines (diazepam, lorazepam, midazolam) are traditionally regarded as first‐line drugs and phenobarbital, phenytoin and paraldehyde as second‐line drugs. This is an update of a Cochrane Review first published in 2002 and updated in 2008.
Objectives
To evaluate the effectiveness and safety of anticonvulsant drugs used to treat any acute tonic‐clonic convulsion of any duration, including established convulsive (tonic‐clonic) status epilepticus in children who present to a hospital or emergency medical department.
Search methods
For the latest update we searched the Cochrane Epilepsy Group's Specialised Register (23 May 2017), the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, 23 May 2017), MEDLINE (Ovid, 1946 to 23 May 2017), ClinicalTrials.gov (23 May 2017), and the WHO International Clinical Trials Registry Platform (ICTRP, 23 May 2017).
Selection criteria
Randomised and quasi‐randomised trials comparing any anticonvulsant drugs used for the treatment of an acute tonic‐clonic convulsion including convulsive status epilepticus in children.
Data collection and analysis
Two review authors independently assessed trials for inclusion and extracted data. We contacted study authors for additional information.
Main results
The review includes 18 randomised trials involving 2199 participants, and a range of drug treatment options, doses and routes of administration (rectal, buccal, nasal, intramuscular and intravenous). The studies vary by design, setting and population, both in terms of their ages and also in their clinical situation. We have made many comparisons of drugs and of routes of administration of drugs in this review; our key findings are as follows:
(1) This review provides only low‐ to very low‐quality evidence comparing buccal midazolam with rectal diazepam for the treatment of acute tonic‐clonic convulsions (risk ratio (RR) for seizure cessation 1.25, 95% confidence interval (CI) 1.13 to 1.38; 4 trials; 690 children). However, there is uncertainty about the effect and therefore insufficient evidence to support its use. There were no included studies which compare intranasal and buccal midazolam.
(2) Buccal and intranasal anticonvulsants were shown to lead to similar rates of seizure cessation as intravenous anticonvulsants, e.g. intranasal lorazepam appears to be as effective as intravenous lorazepam (RR 0.96, 95% CI 0.82 to 1.13; 1 trial; 141 children; high‐quality evidence) and intranasal midazolam was equivalent to intravenous diazepam (RR 0.98, 95% CI 0.91 to 1.06; 2 trials; 122 children; moderate‐quality evidence).
(3) Intramuscular midazolam also showed a similar rate of seizure cessation to intravenous diazepam (RR 0.97, 95% CI 0.87 to 1.09; 2 trials; 105 children; low‐quality evidence).
(4) For intravenous routes of administration, lorazepam appears to be as effective as diazepam in stopping acute tonic clonic convulsions: RR 1.04, 95% CI 0.94 to 1.16; 3 trials; 414 children; low‐quality evidence. Furthermore, we found no statistically significant or clinically important differences between intravenous midazolam and diazepam (RR for seizure cessation 1.08, 95% CI 0.97 to 1.21; 1 trial; 80 children; moderate‐quality evidence) or intravenous midazolam and lorazepam (RR for seizure cessation 0.98, 95% CI 0.91 to 1.04; 1 trial; 80 children; moderate‐quality evidence). In general, intravenously‐administered anticonvulsants led to more rapid seizure cessation but this was usually compromised by the time taken to establish intravenous access. (5) There is limited evidence from a single trial to suggest that intranasal lorazepam may be more effective than intramuscular paraldehyde in stopping acute tonic‐clonic convulsions (RR 1.22, 95% CI 0.99 to 1.52; 160 children; moderate‐quality evidence).
(6) Adverse side effects were observed and reported very infrequently in the included studies. Respiratory depression was the most common and most clinically relevant side effect and, where reported, the frequency of this adverse event was observed in 0% to up to 18% of children. None of the studies individually demonstrated any difference in the rates of respiratory depression between the different anticonvulsants or their different routes of administration; but when pooled, three studies (439 children) provided moderate‐quality evidence that lorazepam was significantly associated with fewer occurrences of respiratory depression than diazepam (RR 0.72, 95% CI 0.55 to 0.93).
Much of the evidence provided in this review is of mostly moderate to high quality. However, the quality of the evidence provided for some important outcomes is low to very low, particularly for comparisons of non‐intravenous routes of drug administration. Low‐ to very low‐quality evidence was provided where limited data and imprecise results were available for analysis, methodological inadequacies were present in some studies which may have introduced bias into the results, study settings were not applicable to wider clinical practice, and where inconsistency was present in some pooled analyses.
Authors' conclusions
We have not identified any new high‐quality evidence on the efficacy or safety of an anticonvulsant in stopping an acute tonic‐clonic convulsion that would inform clinical practice. There appears to be a very low risk of adverse events, specifically respiratory depression. Intravenous lorazepam and diazepam appear to be associated with similar rates of seizure cessation and respiratory depression. Although intravenous lorazepam and intravenous diazepam lead to more rapid seizure cessation, the time taken to obtain intravenous access may undermine this effect. In the absence of intravenous access, buccal midazolam or rectal diazepam are therefore acceptable first‐line anticonvulsants for the treatment of an acute tonic‐clonic convulsion that has lasted at least five minutes. There is no evidence provided by this review to support the use of intranasal midazolam or lorazepam as alternatives to buccal midazolam or rectal diazepam.
Plain language summary
Drug management for acute tonic‐clonic convulsions (fits), including convulsive status epilepticus in children
Review question
This review aimed to assess whether the use of different anticonvulsant drugs, given by different routes of administration, have an impact on how quickly an acute tonic‐clonic‐convulsion (fit) can be stopped. The review also investigated whether different anticonvulsant drugs were accompanied by less frequent or different serious side effects.
Background
Tonic‐clonic convulsions and convulsive status epilepticus are medical emergencies. In children, the first anticonvulsant drug is usually given in the Accident and Emergency (A&E) Department of a hospital. This drug may be administered in a number of ways, including into a vein (intravenously), into the mouth and between the cheeks (buccally), into the nostrils (intranasally) or into the rectum (rectally). The first‐choice drug should be effective, work rapidly and not be associated with any serious adverse effects. Research is important to try and find the most effective and the safest anticonvulsant drug in this clinical situation.
Study characteristics
We carried out a review of all available and relevant evidence on the effectiveness and safety of anticonvulsant drugs used in the first‐line treatment of tonic‐clonic convulsions in children who attended hospital A&E departments. This review examined data from 18 randomised controlled trials (RCTs); RCTs provide the most reliable evidence. They investigated the use of different anticonvulsant drugs and given by different routes.
Key Results
The review included 18 RCTs involving 2199 children, and investigated many different anticonvulsant drugs, doses of the drugs and routes of administration of the drugs. The studies also had some differences in their designs, their settings and the populations of children included, in terms of their ages and their clinical situation (such as how long their convulsion had been going on when they were recruited into the trial).
Analysis of two trials found no clear evidence of a different effect between intravenous lorazepam and intravenous diazepam in stopping a tonic‐clonic convulsion taken to an Emergency Department. There is uncertainty about whether buccal midazolam is more effective than rectal diazepam as the first management of a tonic‐clonic convulsion or convulsive status epilepticus when intravenous access is unavailable. There is no good evidence that the intranasal route is as effective as the intravenous route. Consequently there is no evidence that it can be used as an alternative route of administration.
Although medications such as midazolam, lorazepam and paraldehyde can reduce breathing rates, this is not a common complication and was not seen very often in the included studies. Rates of serious side effects of these medications are generally very low.
Quality of the evidence
Many of the trials used different drugs, different dosages and different routes of administration. This has to be taken into account when looking at the overall conclusion of this review. Most of the trials took place in large children’s hospitals or in large children’s departments in a general hospital. This means that the results found in this review are probably relevant for similar clinical situations throughout the world.
The quality of the evidence provided in this review ranged from very low to high. The quality of the evidence provided for some outcomes is low to very low, due to imprecise results where limited information was available for analysis. There were also variability and problems within the designs of some studies, which may have influenced the findings. The quality of evidence was lower in some study settings which were specific to the country in which they were conducted, so the results may not reflect clinical practice worldwide.
The evidence is current to May 2017.
Summary of findings
Summary of findings for the main comparison. Summary of findings ‐ Lorazepam compared with diazepam.
| Lorazepam compared with diazepam for children with acute tonic‐clonic seizures | ||||||
|
Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Lorazepam Comparison: Diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Diazepam | Lorazepam | |||||
|
Seizure cessation Follow‐up: up to 24 hours |
708 per 1000 | 765 per 1000 (694 to 850) |
RR 1.08 (0.98 to 1.20) |
439 (3 trials) | ⊕⊕⊝⊝ low1, 2 | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible Subgroup analysis showed a significant difference by route of intervention (intravenous: RR 1.04 (95% CI 0.94 to 1.16) compared to rectally RR: 2.86 (95% CI 1.47 to 5.55), test of subgroups P = 0.003) |
|
Time from drug administration to termination of seizures Follow‐up: up to 24 hours |
The mean time to cessation of seizures was 84.94 seconds in the diazepam group | The mean time to cessation of seizures was 6.18 faster (7.83 slower to 20.19 faster) in the lorazepam group | NA | 80 (1 trial) | ⊕⊕⊕⊝ moderate3 | Drugs were administered intravenously Another trial (where drugs were administered intravenously or rectally) reported similar mean times to seizure cessation. Standard deviations were not available so data could not be entered into analysis |
|
Incidence of respiratory depression Follow‐up: up to 24 hours |
356 per 1000 | 256 per 1000 (196 to 331) |
RR 0.72 (0.55 to 0.93) |
439 (3 trials) | ⊕⊕⊕⊝ moderate1 | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible There was no difference between the routes of intervention (test of subgroups, P = 0.86) |
|
Additional drugs required to terminate the seizure: additional dose of study drug Follow‐up: up to 24 hours |
305 per 1000 | 268 per 1000 (195 to 366) |
RR 0.88 (0.64 to 1.20) |
439 (3 trials) | ⊕⊕⊝⊝ low1, 2 | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible Subgroup analysis by route of intervention (intravenous: RR 0.97 (95% CI 0.71 to 1.33) compared to rectally RR: 0.11 (95% CI 0.01 to 1.56), test of subgroups P = 0.11). Two trials also reported whether additional (other) antiepileptic drugs were required to stop the seizure. There were no significant differences overall or by route of intervention |
|
Seizure recurrence within 24 hours Follow‐up: up to 24 hours |
266 per 1000 | 229 per 1000 (162 to 319) |
RR 0.86 (0.61 to 1.20) |
439 (3 trials) | ⊕⊕⊕⊝ moderate1 | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible There was no difference between the routes of intervention (test of subgroups, P = 0.27) |
|
Incidence of admissions to the ICU Follow‐up: up to 24 hours |
116 per 1000 | 17 per 1000 (2 to 114) |
RR 0.15 (0.02 to 0.98) |
86 (1 trial) | ⊕⊕⊝⊝ low1, 4 | In the included trial, drugs were administered intravenously or rectally if intravenous access was not possible There was no difference between the routes of intervention (test of subgroups P = 0.32). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ICU: Intensive Care Unit; NA: Not applicable; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded once due to risk of bias: one included study was quasi‐randomised, which may have led to selection bias and an intention‐to‐treat approach was not used in the study. 2Downgraded once due to inconsistency: a high proportion of heterogeneity was present in the analysis, probably due to differences in the route of administration and differences in definition of 'seizure cessation'. 3Downgraded once due to imprecision: wide confidence intervals around the effect size, 4Downgraded once due to imprecision: wide confidence intervals around the effect size (due to zero events in the intervention group).
Summary of findings 2. Summary of findings ‐ Intranasal lorazepam compared with intramuscular paraldehyde.
| Intranasal lorazepam compared with intramuscular paraldehyde for children with acute tonic‐clonic seizures | ||||||
|
Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intranasal lorazepam Comparison: Intramuscular paraldehyde | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intramuscular paraldehyde | Intranasal lorazepam | |||||
|
Seizure cessation: within 10 minutes Follow‐up: up to 24 hours |
613 per 1000 | 747 per 1000 (606 to 931) |
RR 1.22 (0.99 to 1.52) |
160 (1 study) |
⊕⊕⊕⊝ moderate1 | ‐ |
|
Time from drug administration to termination of seizures Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
|
Incidence of respiratory depression Follow‐up: up to 24 hours |
No difference was found between either treatment group in terms of clinically important cardiorespiratory events. | NA | 160 (1 study) |
⊕⊕⊝⊝ low1, 2 | ‐ | |
|
Additional drugs required to terminate the seizure: 2 or more additional anticonvulsants required Follow‐up: up to 24 hours |
263 per 1000 | 100 per 1000 (47 to 213) |
RR 0.38 (0.18 to 0.81) |
160 (1 study) |
⊕⊕⊝⊝ low1, 3 | ‐ |
|
Seizure recurrence within 24 hours Follow‐up: up to 24 hours |
138 per 1000 | 100 per 1000 (43 to 235) |
RR 0.73 (0.31 to 1.71) |
160 (1 study) |
⊕⊕⊝⊝ low1, 3 | ‐ |
|
Incidence of admissions to the ICU Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ICU: Intensive Care Unit; NA: Not applicable; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded once due to applicability: a high proportion of the children recruited had either cerebral malaria or meningitis. These comorbidities may have impacted upon the results. 2Downgraded once due to imprecision: no numerical data reported. 3Downgraded once due to imprecision: wide confidence intervals around the effect size (due to low event numbers in one or both treatment groups).
Summary of findings 3. Summary of findings ‐ Intravenous lorazepam compared with intravenous diazepam/intravenous phenytoin combination.
| Intravenous lorazepam compared with intravenous diazepam/intravenous phenytoin combination for children with acute tonic‐clonic seizures | ||||||
|
Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous lorazepam Comparison: Intravenous diazepam/intravenous phenytoin combination | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam/intravenous phenytoin combination | Intravenous lorazepam | |||||
|
Seizure cessation: within 10 minutes Follow‐up: up to 24 hours |
Seizures were stopped for all individuals in the Intravenous diazepam/intravenous phenytoin combination group | Seizures were stopped for all individuals in the Intravenous lorazepam group |
RR 1.00 (0.98 to 1.02) |
178 (1 trial) | ⊕⊕⊕⊝ moderate1 | ‐ |
|
Time from drug administration to stopping of seizures Follow‐up: up to 24 hours |
There was no significant difference in the median time to seizure cessation (20 seconds in each group). | NA | 178 (1 trial) | ⊕⊕⊕⊝ moderate2 | ‐ | |
|
Incidence of respiratory depression Follow‐up: up to 24 hours |
57 per 1000 |
44 per 1000 (13 to 160) |
RR 0.78 (0.22 to 2.82) |
178 (1 trial) | ⊕⊕⊕⊝ moderate3 | ‐ |
|
Additional drugs required to stop the seizure Follow‐up: up to 24 hours |
159 per 1000 |
67 per 1000 (27 to 165) |
RR 0.42 (0.17 to 1.04) |
178 (1 trial) | ⊕⊕⊕⊝ moderate3 | ‐ |
|
Seizure recurrence within 24 hours Follow‐up: up to 24 hours |
There were no seizure recurrences in either group. | NA | 178 (1 trial) | ⊕⊕⊕⊝ moderate4 | ‐ | |
|
Incidence of admissions to the ICU Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ICU: Intensive Care Unit; NA: Not applicable; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded once due toapplicability: Both treatment arms showed a 100% seizure cessation rate, which is higher than expected. Unclear whether this high success rate was due to a particular element of the trial design. 2Downgraded once due to imprecision: limited numerical data reported. 3Downgraded once due to imprecision: wide confidence intervals around the effect size (due to low event numbers in one or both treatment groups). 4Downgraded once due to applicability: the control intervention included a long‐acting anti‐convulsant (phenytoin) which may have influenced the seizure recurrence rate in the control group.
Summary of findings 4. Summary of findings ‐ Intravenous lorazepam compared with intranasal lorazepam.
| Intravenous lorazepam compared with intranasal lorazepam for children with acute tonic‐clonic seizures | ||||||
|
Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous lorazepam Comparison: Intranasal lorazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intranasal lorazepam | Intravenous lorazepam | |||||
|
Seizure cessation: within 10 minutes Follow‐up: up to 24 hours |
696 per 1000 | 744 per 1000 (536 to 1000) |
RR 1.07 (0.77 to 1.49) |
58 (1 trial) | ⊕⊕⊕⊝ moderate 1 | There was also no significant difference between treatments for seizure cessation at 1 hour: RR 0.70 (95% CI 0.43 to 1.17) |
|
Time from drug administration to stopping of seizures Follow‐up: up to 24 hours |
Median time to achieve seizure control from drug administration was 4 minutes in both groups. | NA | 58 (1 trial) | ⊕⊕⊕⊝ moderate2 | Time taken to achieve intravenous access ranged from 1 to 25 minutes with a median of 4 minutes across all participants in the trial. If this had been included in the response time for the intravenous lorazepam, the results would have been skewed significantly in favour of intranasal lorazepam | |
|
Incidence of respiratory depression Follow‐up: up to 24 hours |
One child required respiratory support | Two children required respiratory support | NA | 141 (1 trial, see comment) | ⊕⊕⊕⊝ moderate3 | Incidence of respiratory depression was not reported for the subgroup of participants with generalised tonic‐clonic seizures in the trial, therefore these results refer to all participants (including 83 participants without generalised tonic‐clonic seizures). |
|
Additional drugs required to stop the seizure Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
|
Seizure recurrence within 24 hours Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
|
Incidence of admissions to the ICU Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ICU: Intensive Care Unit; NA: Not applicable; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded once due to imprecision: imbalance in the number of participants randomised to each intervention with generalised tonic‐clonic seizures and overall direction of effect seems to change when measured at 10 minutes or at 1 hour
2Downgraded once due to imprecision: limited numerical data reported. 3Downgraded once due to imprecision: Low event numbers and outcome data not available for the subgroup participants with generalised tonic‐clonic seizures in the trial
Summary of findings 5. Summary of findings ‐ Buccal midazolam compared with rectal diazepam.
| Buccal midazolam compared with rectal diazepam for children with acute tonic‐clonic seizures | ||||||
|
Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Buccal midazolam Comparison: Rectal diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Rectal diazepam | Buccal midazolam | |||||
|
Seizure cessation: within 5 minutes to 1 hour Follow‐up: up to 24 hours |
584 per 1000 | 730 per 1000 (660 to 806) |
RR 1.25 (1.13 to 1.38) |
648 (4 trials) 690 seizure episodes |
⊕⊝⊝⊝ very low1, 2, 3 | The measurement time of seizure cessation was examined in a subgroup analysis 5 minutes: RR 1.22 (95% CI 1.07 to 1.40, P = 0.004); 10 minutes: RR 1.07 (95% CI 0.95 to 1.21, P = 0.26); 1 hour; RR 2.05 (95% CI 1.45 to 2.91, P < 0.001). There was a significant difference between the subgroups (P = 0.002) |
|
Time from drug administration to of seizures Follow‐up: up to 24 hours |
One trial found no difference between groups in the time from drug administration to seizure cessation One trial reported that both the median treatment initiation time and drug effect time were significantly shorter in the buccal midazolam group than the rectal diazepam group. |
NA | 141 (2 trials) |
⊕⊕⊝⊝ low1, 4 | No numerical data presented for either trial | |
|
Incidence of respiratory depression Follow‐up: up to 24 hours |
76 per 1000 |
67 per 1000 (46 to 94) |
RR 0.88 (0.61 to 1.25) |
648 (4 trials) 690 seizure episodes |
⊕⊕⊝⊝ low1, 3 | ‐ |
|
Additional drugs required to stop the seizure: intravenous lorazepam required Follow‐up: up to 24 hours |
573 per 1000 | 332 per 1000 (241 to 452) |
RR 0.58 (0.42 to 0.79) |
177 (1 trial) 219 seizure episodes |
⊕⊕⊝⊝ low3, 5 | A second trial reported that there was no difference between groups in the need for a second drug |
|
Seizure recurrence within 24 hours Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
|
Incidence of admissions to the ICU Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ICU: Intensive Care Unit; NA: Not applicable; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded once due to risk of bias: one included study was quasi‐randomised and one study did not conceal allocation. Both of these studies were at risk of selection bias. 2Downgraded once due to inconsistency: a high proportion of heterogeneity was present in analysis, probably due to differences in the measurement times of the outcome and potentially also the doses of the drugs across the studies and comorbidities of participants recruited. 3Downgraded once due to imprecision: Results are not available at the participant level so results reported for McIntyre 2005 are at the episode level. This is a limitation, as meta‐analysis assumes independence between measurements, and more than one treated seizure per participant would not be statistically independent. A result of ignoring this unit‐of‐analysis issue could be overoptimistic confidence intervals. 4Downgraded once due to imprecision: no numerical data reported. 5Downgraded once due to risk of bias: the included study was quasi‐randomised, did not conceal allocation and was at risk of selection bias.
Summary of findings 6. Summary of findings ‐ Buccal midazolam compared with intravenous diazepam.
| Buccal midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
|
Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Buccal midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Buccal midazolam | |||||
|
Seizure cessation Follow‐up: up to 24 hours |
933 per 1000 | 849 per 1000 (747 to 961) | RR 0.91 (0.80 to 1.03) | 120 (1 trial) |
⊕⊕⊕⊕ high | ‐ |
|
Time from drug administration to termination of seizures Follow‐up: up to 24 hours |
The mean time to cessation of seizures was 1.13 minutes in the intravenous diazepam group. | The mean time to cessation of seizures was 0.56 minutes higher in the buccal diazepam group (0.29 to 0.83 minutes higher). | NA | 120 (1 trial) |
⊕⊕⊕⊝ moderate1 | The mean time for initiation of treatment was significantly shorter in the buccal midazolam group (MD ‐1.09 minutes, 95% CI ‐1.31 to ‐0.87) and therefore the mean total time to controlling the seizures was significantly shorter in the buccal midazolam group compared to the intravenous diazepam group (MD ‐0.59, 95% CI ‐0.96 to ‐0.22) |
|
Incidence of respiratory depression Follow‐up: up to 24 hours |
There were no adverse events in either group | NA | 120 (1 trial) |
⊕⊕⊕⊕ high | ‐ | |
|
Additional drugs required to stop the seizure Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
|
Seizure recurrence within 24 hours Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
|
Incidence of admissions to the ICU Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ICU: Intensive Care Unit; MD: Mean difference; NA: Not applicable; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded once due to applicability: the route of intervention of the drug has been shown to influence the outcome.
Summary of findings 7. Summary of findings ‐ Intranasal midazolam compared with intravenous diazepam.
| Intranasal midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
|
Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intranasal midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Intranasal midazolam | |||||
|
Seizure cessation Follow‐up: up to 24 hours |
967 per 1000 | 948 per 1000 (880 to 1000) |
RR 0.98 (0.91 to 1.06) |
122 (2 trials) |
⊕⊕⊕⊝ moderate1 | ‐ |
|
Time from drug administration to stopping of seizures Follow‐up: up to 24 hours |
The mean time to cessation of seizures ranged from 2.5 to 2.94 minutes in the intravenous diazepam group. | The mean time to cessation of seizures was 0.62 minutes higher in the intranasal midazolam group (0.14 lower to 1.38 minutes higher). | NA | 122 (2 trials) |
⊕⊕⊕⊝ moderate2 | One trial reports that the time for initiation of treatment was significantly shorter in the intranasal midazolam group (MD ‐2.00 minutes, 95% CI ‐3.03 to ‐0.97). The other trial also reports that time for initiation of treatment was significantly shorter in the intranasal midazolam group but does not account for this in analysis |
|
Incidence of respiratory depression Follow‐up: up to 24 hours |
No adverse events including respiratory depression occurred in either group. | NA | 122 (2 trials) |
⊕⊕⊕⊕ high | ‐ | |
|
Additional drugs required to stop the seizure Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
|
Seizure recurrence within 24 hours Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
|
Incidence of admissions to the ICU Follow‐up: up to 24 hours |
There were no admissions to the ICU in either group | NA | 52 (1 trial) |
⊕⊕⊕⊕ high | ‐ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ICU: Intensive Care Unit; MD: Mean difference; NA: Not applicable; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded once due to risk of bias: one of the studies included in this comparison did not report this outcome. As this is an expected outcome, this may be selective reporting. Additionally, in one trial both treatment arms showed a 100% seizure cessation rate, which is higher than expected. Unclear whether this high success rate was due to a particular element of the trial design. 2Downgraded once due to applicability: the route of intervention of the drug has been shown to influence the outcome.
Summary of findings 8. Summary of findings ‐ Intranasal midazolam compared with rectal diazepam.
| Intranasal midazolam compared with rectal diazepam for children with acute tonic‐clonic seizures | ||||||
|
Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intranasal midazolam Comparison: Rectal diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Rectal diazepam | Intranasal midazolam | |||||
|
Seizure cessation: within 10 minutes Follow‐up: up to 24 hours |
591 per 1000 | 869 per 1000 (591 to 1000) |
RR 1.47 (1.00 to 2.16) |
45 (1 trial) | ⊕⊕⊝⊝ low1, 2 | ‐ |
|
Time from drug administration to termination of seizures Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
|
Incidence of respiratory depression Follow‐up: |
There was no significant difference between the two groups for of cardiorespiratory or adverse effects. | NA | 45 (1 trial) | ⊕⊕⊝⊝ low1, 3 | No numerical data reported | |
|
Additional drugs required to stop the seizure Follow‐up: up to 24 hours |
409 per 1000 |
131 per 1000 (41 to 421) |
RR 0.32 (0.10 to 1.03) |
45 (1 trial) | ⊕⊕⊝⊝ low1, 4 | ‐ |
|
Seizure recurrence within 24 hours Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
|
Incidence of admissions to the ICU Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ICU: Intensive Care Unit; NA: Not applicable; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded once due to risk of bias: one included study was quasi‐randomised, which may have led to selection bias. Additionally, the description of the seizure type and aetiology of the included children was unclear, so it is unclear if the population of this study is generalisable. 2Downgraded once due to imprecision: wide confidence intervals around the effect size (due to high event rates in both treatment groups). 3Downgraded once due to imprecision: no numerical data reported. 4Downgraded once due to imprecision: wide confidence intervals around the effect size (due to low event rates in both treatment groups).
Summary of findings 9. Summary of findings ‐ Intramuscular midazolam compared with intravenous diazepam.
| Intramuscular midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
|
Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intramsucular midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Intramsucular midazolam | |||||
|
Seizure cessation Follow‐up: up to 24 hours |
929 per 1000 | 901 per 1000 (808 to 1000) |
RR 0.97 (0.87 to 1.09) |
105 (2 trials) | ⊕⊕⊝⊝ low1,2 | ‐ |
|
Time from drug administration to stopping of seizures: total time to seizure cessation Follow‐up: up to 24 hours |
The mean total time to cessation of seizures was 2.68 minutes lower (3.94 to 1.42 minutes lower) in the intramuscular midazolam group compared to the intravenous diazepam group | NA | 105 (2 trials) | ⊕⊝⊝⊝ very low1, 2, 3 | One trial also showed that the initiation of treatment was significantly shorter in the intramuscular midazolam group (MD ‐4.50 minutes (‐6.68 to ‐2.32)) but there was no significant difference between treatments for the time to drug effect (MD 1.10 minutes (95% CI ‐0.91 to 3.11) | |
|
Incidence of respiratory depression Follow‐up: up to 24 hours |
There were no adverse events or complications in either trial | NA | 105 (2 trials) | ⊕⊕⊝⊝ low1, 2 | ‐ | |
|
Additional drugs required to terminate the seizure Follow‐up: up to 24 hours |
71 per 1000 | 96 per 1000 (25 to 366) |
RR 1.34 (0.35 to 5.13) |
105 (2 trials) | ⊕⊝⊝⊝ very low1, 2, 4 | ‐ |
|
Seizure recurrence within 24 hours: within one hour Follow‐up: up to 24 hours |
364 per 1000 | 309 per 1000 (98 to 983) |
RR 0.85 (0.27 to 2.62) |
24 (1 trial) |
⊕⊝⊝⊝ very low1, 2, 4 | There was also no significant difference between treatments at within 15 minutes (RR: 0.85 (95% CI 0.06,to12.01) |
|
Incidence of admissions to the ICU Follow‐up: up to 24 hours |
There were no admissions to the ICU | NA | 81 (1 trial) |
⊕⊕⊕⊝ moderate1 | ‐ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ICU: Intensive Care Unit; MD: Mean difference; NA: Not applicable; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded once due to risk of bias: in both included trials, methods of randomisation were unclear so the trials may be at risk of selection bias. 2Downgraded once due to applicability: one child was randomised twice in one trial and included in both groups. It was not possible to identify this child in analysis and results are not adjusted for the correlation between measurements from the same child. 3Downgraded once due to applicability: the route of intervention of the drug has been shown to influence the outcome. 4Downgraded once due to imprecision: wide confidence intervals around the effect size or pooled effect size (due to low event rates in both treatment groups).
Summary of findings 10. Summary of findings ‐ Intramuscular midazolam compared with rectal diazepam.
| Intramuscular midazolam compared with rectal diazepam for children with acute tonic‐clonic seizures | ||||||
|
Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intramuscular midazolam Comparison: Rectal diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Rectal diazepam | Intramuscular midazolam | |||||
|
Seizure cessation Follow‐up: up to 24 hours |
940 per 1000 | 959 per 1000 (874 to 1000) | RR 1.02 (0.93 to 1.12) | 100 (1 trial) |
⊕⊕⊕⊝ moderate1 | ‐ |
|
Time from drug administration to stopping of seizures Follow‐up: up to 24 hours |
There was a significant difference in time from administration to seizure cessation in favour of midazolam (median 66 seconds, diazepam, median 130 seconds, P < 0.001) | NA | 100 (1 trial) |
⊕⊕⊕⊝ moderate1 | It is noted that the speed of administration was similarly fast for both medications, so this seems to reflect a medication difference. | |
|
Incidence of respiratory depression Follow‐up: up to 24 hours |
No patients developed respiratory depression except for one patient who received an accidental double dose of intramuscular midazolam. | NA | 100 (1 trial) |
⊕⊕⊕⊝ moderate1 | ‐ | |
|
Additional drugs required to stop the seizure Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
|
Seizure recurrence within 24 hours Follow‐up: up to 24 hours |
Among those with seizures terminated, there were no recurrences at 24 hours | NA | 100 (1 trial) |
⊕⊕⊕⊝ moderate1 | ||
|
Incidence of admissions to the ICU Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ICU: Intensive Care Unit; NA: Not applicable; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded once due to risk of bias: the included study did not conceal allocation so is at risk of selection bias.
Summary of findings 11. Summary of findings ‐ Intravenous midazolam compared with intravenous diazepam.
| Intravenous midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
|
Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Intravenous midazolam | |||||
|
Seizure cessation Follow‐up: up to 24 hours |
900 per 1000 | 972 per 1000 (873 to 1000) |
RR 1.08 (0.97 to 1.21) |
80 (1 trial) |
⊕⊕⊕⊝ moderate1 | ‐ |
|
Time from drug administration to stopping of seizures Follow‐up: up to 24 hours |
The mean time to cessation of seizures was 84.94 seconds in the intravenous diazepam group. | The mean time to cessation of seizures was 7.68 seconds higher in the intravenous midazolam group (6.73 seconds lower to 22.09 seconds higher) . | NA | 80 (1 trial) |
⊕⊕⊕⊝ moderate2 | ‐ |
|
Incidence of respiratory depression Follow‐up: up to 24 hours |
25 per 1000 | 8 per 1000 (0 to 199) | RR 0.33 (0.01 to 7.95) | 80 (1 trial) |
⊕⊕⊕⊝ moderate3 | ‐ |
|
Additional drugs required to stop the seizure: additional dose of the trial drug required Follow‐up: up to 24 hours |
100 per 1000 | 25 per 1000 (3 to 214) |
RR 0.25 (0.03 to 2.14) |
80 (1 trial) |
⊕⊕⊕⊝ moderate3 | ‐ |
|
Seizure recurrence within 24 hours Follow‐up: up to 24 hours |
100 per 1000 | 50 per 1000 (10 to 258) | RR 0.50 (0.10 to 2.58) | 80 (1 trial) |
⊕⊕⊕⊝ moderate3 | ‐ |
|
Incidence of admissions to the ICU Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ICU: Intensive Care Unit; NA: Not applicable; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded once due to risk of bias: the definition of the 'seizure cessation' outcome is not an appropriate criterion for judging seizure cessation. This definition is likely to have impacted upon results. 2Downgraded once due to imprecision: wide confidence intervals around the effect size. 3Downgraded once due to imprecision: wide confidence intervals around the effect size (due to low event rates in both treatment groups).
Summary of findings 12. Summary of findings ‐ Intravenous midazolam compared with intravenous lorazepam.
| Intravenous midazolam compared with intravenous lorazepam for children with acute tonic‐clonic seizures | ||||||
|
Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous midazolam Comparison: Intravenous lorazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous lorazepam | Intravenous midazolam | |||||
|
Seizure cessation Follow‐up: up to 24 hours |
Seizures were terminated for all children in the Intravenous lorazepam group | Seizures were terminated for 39 out of 40 children in the intravenous midazolam group | RR 0.98 (0.91 to 1.04) | 80 (1 trial) |
⊕⊕⊕⊝ moderate1 | ‐ |
|
Time from drug administration to termination of seizures Follow‐up: up to 24 hours |
The mean time to cessation of seizures was 91.12 seconds in the intravenous lorazepam group. | The mean time to cessation of seizures was 1.50 seconds higher in the intravenous midazolam group (9.37 seconds lower to 12.37 seconds higher) . | NA | 80 (1 trial) |
⊕⊕⊕⊝ moderate2 | ‐ |
|
Incidence of respiratory depression Follow‐up: up to 24 hours |
There were no occurrences of respiratory depression in either group | NA | 80 (1 trial) |
⊕⊕⊕⊕ high | ‐ | |
|
Additional drugs required to terminate the seizure: additional dose of the trial drug required Follow‐up: up to 24 hours |
No children in the intravenous lorazepam group required an additional dose of the trial drug. | One child in the intravenous midazolam group required an additional dose of the trial drug. | RR 3.00 (0.13 to 71.51) | 80 (1 trial) |
⊕⊕⊕⊝ moderate3 | ‐ |
|
Seizure recurrence within 24 hours Follow‐up: up to 24 hours |
50 per 1000 | 50 per 1000 (8 to 338) | RR 1.00 (0.15 to 6.76) | 80 (1 trial) |
⊕⊕⊕⊝ moderate3 | ‐ |
|
Incidence of admissions to the ICU Follow‐up: NA |
Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; ICU: Intensive Care Unit; NA: Not applicable; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded once due to risk of bias: the definition of the 'seizure cessation' outcome is not an appropriate criterion for judging seizure cessation. This definition is likely to have impacted upon results. 2Downgraded once due to imprecision: wide confidence intervals around the effect size. 3Downgraded once due to imprecision: wide confidence intervals around the effect size (due to low event rates in both treatment groups).
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Issue 3, 2008; Appleton 2008).
Description of the condition
Convulsive status epilepticus (CSE) is a medical and neurological emergency and if under‐ or inappropriately treated may result in death or significant morbidity. Convulsive status epilepticus is defined as more than 30 minutes of either continuous seizure activity or two or more sequential seizures without full recovery of consciousness between seizures (Glauser 2016). The 30‐minute definition is based on the duration of convulsive status epilepticus that may lead to irreversible neuronal injury. Since most seizures are brief, and once a seizure lasts more than five minutes it is likely to be prolonged (Shinnar 2001), status treatment protocols are based on a five‐minute definition to minimise both the risk of seizures reaching 30 minutes and potential adverse outcomes associated with treating brief, self‐resolving tonic‐clonic convulsions. It is generally believed that the longer the episode of CSE, the more difficult it is to stop.
When the exact time of onset or duration of the convulsion is not known, any person presenting to the A&E department in an acute tonic‐clonic convulsion tends to be managed according to the definition of status epilepticus, with the primary objective of stopping the convulsion, irrespective of its duration. Most published national and international guidance, including from the National Institute of Health and Care Excellence (NICE) in the UK (NICE 2012), the International League Against Epilepsy (ILAE) (Trinka 2015) and the American Epilepsy Society (AES) (Glauser 2016), recommend treating a tonic‐clonic seizure after five minutes. This is because in over 90% of cases a tonic‐clonic seizure will end spontaneously within four minutes; it is assumed and likely that a seizure that has continued for more than four minutes will not stop spontaneously.
Description of the intervention
Twenty‐five years ago, the first drug used to treat an acute tonic‐clonic convulsion in children was usually administered in the A&E department (Garr 1999). However it is now more common that parents/carers of children with either prolonged or recurrent (serial) convulsions are prescribed ‘rescue’ medications, such as rectal diazepam or buccal/intranasal midazolam to administer at home (or even at school). An epidemiological study published in 2008 demonstrated that 61% of episodes of convulsive status epilepticus in children were treated with pre‐hospital emergency medication and predominantly rectal diazepam (Chin 2008). Over‐treatment may be as potentially damaging as under‐treatment by causing respiratory depression/arrest (with a risk of consequent cerebral hypoxia) or a potentially fatal cardiac arrhythmia.
How the intervention might work
Convulsive status epilepticus is a medical and neurological emergency that may result in death or significant morbidity. The intended aim of the intervention is to stop the acute tonic‐clonic seizure as rapidly as possible, without causing serious and potentially life‐threatening adverse side effects, and avoiding the need for a second‐line treatment.
Why it is important to do this review
This is an update of a Cochrane Review first published in 2002, and updated in 2008. Since the last update (Appleton 2008), there have been a number of newly‐published randomised controlled trials in children. These data contribute to the growing evidence base on the management of acute tonic‐clonic convulsions in children. We therefore consider it appropriate to update the review.
Objectives
To evaluate the effectiveness and safety of the anticonvulsant drugs used to treat any acute tonic‐clonic convulsion of any duration, including established convulsive (tonic‐clonic) status epilepticus in children presenting to a hospital or emergency medical department.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials of a parallel design, blinded or unblinded.
Cluster‐randomised and cross‐over trials are not suitable designs for the review, due to the nature of the condition and the treatment.
Types of participants
Children aged between one month and 16 years, presenting to an A&E department or to a hospital ward (direct from the community) in an acute tonic‐clonic convulsion and who received treatment with an anticonvulsant drug, irrespective of the duration of the presenting convulsion.
Children included those presenting with a first convulsion and those with an established diagnosis of epilepsy. Any and all causes of the convulsion (including convulsive status epilepticus) were included in the review. We included studies where 70% or more of the study population had generalised tonic‐clonic seizures (GTC) or secondarily generalised seizures, or where subgroup data for children with GTC were available.
Types of interventions
In children presenting with an acute tonic‐clonic seizure including status epilepticus, we included trials if they compared two or more treatments or two or more treatment protocols of the same anticonvulsant. We included studies comparing first‐line treatments only (i.e. the first treatment a child received at the hospital). Studies of second‐line treatments (e.g. the second treatment given at hospital after a first seizure treatment had failed) were not within the scope of this review. Specific drugs considered within this review included the benzodiazepines (diazepam, lorazepam and midazolam), phenytoin, and paraldehyde. Different routes of drug administration were also analysed where possible, including intravenous (IV), intranasal, buccal, rectal and intramuscular administration. We consider different routes of drug administration separately in analyses.
Types of outcome measures
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion.
3. Incidence of admissions to the intensive care unit (ICU).
Search methods for identification of studies
We ran searches for the original review in 2002 and again in 2003, 2005, 2007, 2010, 2011, 2012, 2013, 2014, and 2017. For this update we searched the following databases:
Cochrane Epilepsy Group Specialised Register (23 May 2017) using the search strategy outlined in Appendix 1;
The Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, 23 May 2017), using the search strategy outlined in Appendix 2;
MEDLINE (Ovid, 1946 to 23 May 2017) using the strategy outlined in Appendix 3;
ClinicalTrials.gov (23 May 2017) using the strategy outlined in Appendix 4;
WHO International Clinical Trials Registry Platform (ICTRP, 23 May 2017) using the strategy outlined in Appendix 5.
There were no language restrictions.
Data collection and analysis
Selection of studies
Two review authors (Amy McTague and Richard Appleton) independently assessed trials for inclusion. We first screened titles and abstracts, followed by full‐text reports of potentially eligible trials, resolving any disagreements by discussion.
Data extraction and management
All three review authors (Amy McTague, Richard Appleton and Tim Martland) independently extracted the outcome data specified above, as well as the following data. We resolved any disagreements by discussion.
Methodological/trial design
Method of randomisation.
Method of double‐blinding.
Whether any participants had been excluded from the reported analyses.
Participant/demographic information
Total number of participants allocated to each treatment group/audited in any protocol.
Age/sex.
Number and type of background anti‐epileptic drugs.
Whether any pre‐hospital emergency anticonvulsant treatment was given.
Duration of presenting tonic‐clonic seizure/episode of convulsive status.
Cause of acute tonic‐clonic seizure/episode of convulsive status.
Assessment of risk of bias in included studies
Two review authors (Amy McTague and Richard Appleton) independently assessed the risks of bias in the included studies, using the Cochrane 'Risk of bias' tool (Higgins 2011b). We judged whether each study was at high, low or unclear risk of bias in each of the following domains:
Random sequence generation;
Allocation concealment;
Blinding;
Incomplete outcome data;
Selective outcome reporting.
Other potential risks of bias.
We resolved any disagreements by discussion.
Measures of treatment effect
Dichotomous outcomes (e.g. number of children with convulsions stopped, number of children with specific adverse events, etc.) were expressed as risk ratios (RRs) with 95% confidence intervals (CIs). Continuous outcomes (e.g. time to stop the seizure/status episode) were expressed as mean differences (MDs) with 95% CIs.
Unit of analysis issues
We did not anticipate unit‐of‐analysis issues, as the unit of allocation and analysis must be the individual for all included trials, and cross‐over designs would not be suitable for this review, given the acute nature of the convulsions.
The participant was the preferred unit of analysis, but where results were reported in terms of 'episodes' (i.e. the same child being treated for multiple seizures in the same trial), where participant‐specific information could not be extracted we accepted episode‐level information. This is a limitation, as meta‐analysis assumes independence between measurements, and more than one treated seizure per child would not be statistically independent. A consequence of ignoring this unit‐of‐analysis issue could be over‐optimistic confidence intervals.
Where we included studies with multiple treatment arms, multiple treatment doses or different routes of administration, we considered each eligible treatment, dose or route of intervention in separate comparisons.
Dealing with missing data
The analyses conducted in this review aimed to take an 'intention‐to‐treat' approach where possible, i.e. including all randomised participants, analysed in the treatment group to which they were allocated, irrespective of which treatment they actually received.
Where data were missing, we attempted to contact the study authors for this information. If we could not acquire the missing data, we conducted a 'complete‐case' analysis and took account of the limitations of this approach when interpreting results.
Assessment of heterogeneity
We assessed clinical heterogeneity by reviewing the differences across trials in characteristics of recruited participants and treatment protocols. We also estimated heterogeneity statistically using a Chi2 test for heterogeneity and the I2 statistic. We interpreted the I2 statistic as follows (Higgins 2011a):
might not be important (I2 values 0% to 40%);
may represent moderate heterogeneity (I2 values 30% to 60%);
may represent substantial heterogeneity (I2 values 50% to 90%); and
considerable heterogeneity (I2 values 75% to 100%).
Assessment of reporting biases
To assess selective reporting bias, we compared the measurements and outcomes planned by the original iInvestigators during the trial with those reported within the published paper, by checking the trial protocols (when available) against the information in the final publication. Where protocols were not available, we compared the 'Methods' and the 'Results' sections of the published papers. We also used our knowledge of the clinical background to identify standard outcome measures usually taken, but not reported by the trial investigators.
If a sufficient number of trials (10 or more) had been included for any comparison, we would have investigated publication bias using a funnel plot.
Data synthesis
We analysed data using the fixed‐effect model in the first instance. Where we found substantial or considerable heterogeneity, we repeated the analysis with a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We assessed clinical and statistical heterogeneity using the methods outlined in Assessment of heterogeneity.
If appropriate, we considered different measurement times of the primary outcome (seizure cessation); i.e. if different trials reported this outcome at different time points or if any trials reported this outcome at multiple time points. In the former case, we also calculated a pooled summary of the measurement time subgroups and performed the Chi2 test for differences between subgroups. In the latter case, where a trial reported multiple time points, we reported subgroup results only and did not pool the results.
Sensitivity analysis
We planned a sensitivity analysis based on the methodological quality of the studies. However, given the small number of studies included in each comparison, we did not deem this sensitivity analysis to be appropriate, but we will consider a sensitivity analysis based on study quality for future updates of the review.
Summary of Findings and Quality of the Evidence (GRADE)
In a post hoc change in line with current Cochrane guidance, for the 2017 update we added a 'Summary of findings' table for each comparison presented in the review, reporting all of the primary and secondary outcomes.
We determined the quality of the evidence using the GRADE approach (GRADEPro 2004), downgrading evidence in the presence of a high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, or high probability of publication bias. We downgraded evidence by one level if we considered the limitation to be serious, and by two levels if very serious.
Results
Description of studies
Results of the search
The original Cochrane Review (2002) identified a single study (Appleton 1995).
The update in 2008 identified three further studies (Ahmad 2006; Lahat 2000; McIntyre 2005).
For this update, we have identified 14 further studies that meet the main inclusion criteria in addition to the single study in the original review and the three studies identified for the 2008 update. (Arya 2011; Ashrafi 2010; Baysun 2005; Chamberlain 1997; Chamberlain 2014; Fişgin 2002; Gathwala 2012; Javadzadeh 2012; Mahmoudian 2004; Momen 2015; Mpimbaza 2008; Shah 2005; Sreenath 2010; Talukdar 2009).
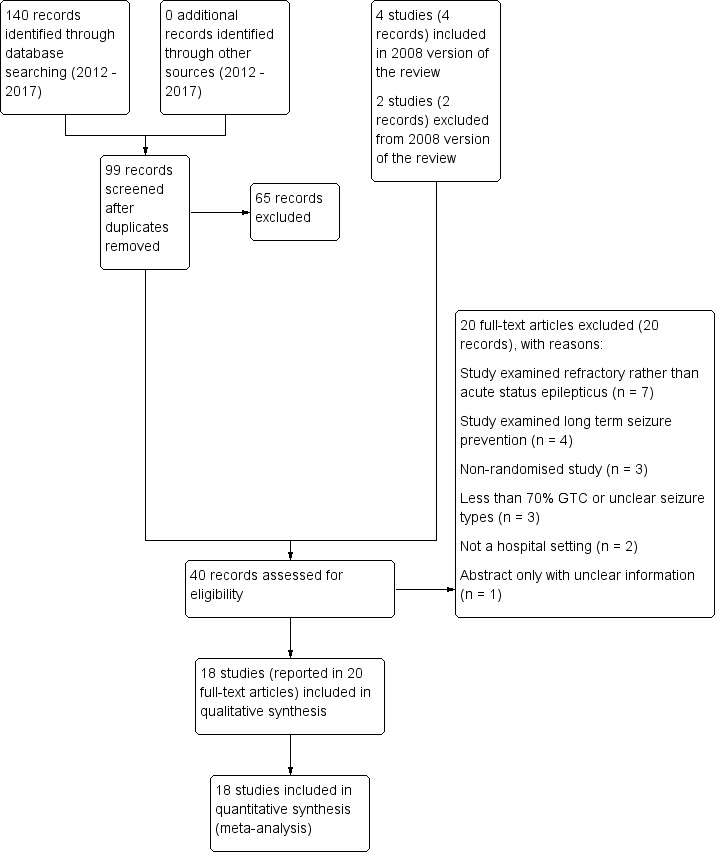
Full details of searches conducted before 2012 are unavailable. Figure 1 shows the study flow diagram for searches completed between 2012 and 2017, in addition to the studies already listed in the 2008 update of the review.
1.
Study flow diagram.
Searches conducted been 2012 and 2017 identified 140 records, including 41 duplicate records. We screened 99 records (title and abstract) for inclusion in the review and excluded 65 clearly irrelevant records. With the four studies included in previous versions of the review and two studies excluded from previous versions of the review, we assessed 40 full‐text articles or clinical trials registry entries. We excluded 20 studies (see Excluded studies) and included 18 studies (reported in 20 full‐text articles or clinical trials registry entries) in the review.
Included studies
We included 18 trials in this review (Ahmad 2006; Appleton 1995; Arya 2011; Ashrafi 2010; Baysun 2005; Chamberlain 1997; Chamberlain 2014; Fişgin 2002; Gathwala 2012; Javadzadeh 2012; Lahat 2000; Mahmoudian 2004; McIntyre 2005; Momen 2015; Mpimbaza 2008; Shah 2005; Sreenath 2010; Talukdar 2009). All were hospital‐based studies.
This section gives a brief description of the characteristics and participants of each included trial; see Characteristics of included studies for further details.
Ahmad 2006 was a 12‐month, open, randomised study comparing intranasal lorazepam (0.1 mg (100 micrograms)/kg) and intramuscular paraldehyde (0.2 mg (200 micrograms)/kg) as the first‐line treatment of children aged two months to 12 years, presenting to a paediatric emergency centre with a generalised convulsion continuing for at least five minutes. The study was carried out in Malawi, Africa. Intramuscular paraldehyde is commonly used as a first‐line treatment for acute tonic‐clonic seizures in sub‐Saharan Africa but is associated with injury around the injection site, sterile abscesses and is incompatible with plastics. Patient demographics were similar in each group. Because of the geographical location of this study most of the children had acute symptomatic seizures, mainly due to acute brain infection (cerebral malaria or bacterial meningitis in two‐thirds of each of the two study groups). Randomisation was allocated in advance by computer in blocks of 10; after identification and treatment of children with hypoglycaemic seizures, investigators opened an unmarked envelope which contained details of treatment allocation. Primary outcome was the clinical cessation of the seizure within 10 minutes of drug administration. Children with features of hepatic or hypertensive encephalopathy or organophosphate poisoning were excluded, as were children who had received an anticonvulsant agent within one hour of presentation. For children in whom clinical seizure activity continued after 10 minutes, investigators followed a locally‐agreed protocol. The study evaluated 160 children of both sexes.
Appleton 1995 was a one‐year open, quasi‐randomised study, comparing lorazepam and diazepam, with the drugs given either intravenously or rectally depending on ease of venous access. This study evaluated 102 children, aged between one month and 16 years, of both sexes, presenting with an acute tonic‐clonic convulsion including established convulsive status epilepticus to an A&E department of a large children's hospital. The study accepted all causes of the convulsion or status, including symptomatic and idiopathic. No child had evidence of acute head trauma, metabolic encephalopathy, bacterial meningitis or herpes simplex encephalitis as a cause of their presenting convulsion. No children were included with known pseudo‐tonic‐clonic convulsions or pseudo‐convulsive, absence or complex partial status. The demography of the two treatment groups was very similar (age; sex; numbers with pre‐existing epilepsy; numbers with a pre‐existing neurological disorder and duration of the presenting convulsion prior to treatment with the two study drugs). Cessation of the seizure was defined as the seizure or episode of status stopping within seven or eight minutes of administration of the first dose of the study anticonvulsant. If the presenting convulsion had not stopped by eight minutes, then a second dose of either lorazepam or diazepam would be given. If this seizure persisted, then an additional anticonvulsant would be given, based on the hospital's protocol for managing convulsive status epilepticus (Garr 1999).
Arya 2011 was a randomised controlled trial comparing intranasal and intravenous (IV) lorazepam for the treatment of convulsive status epilepticus in children. The trial took place in the emergency room of a hospital in New Delhi, India. Inclusion criteria were children aged six to 14 years who presented convulsing or who developed a seizure during the emergency room attendance. Exclusion criteria were receipt of any anti‐epileptic drug (AED) within one hour of enrolment, the presence of severe cardiovascular compromise, and the presence of cerebrospinal fluid (CSF) rhinorrhoea or upper respiratory infection severe enough to prevent intranasal administration. Fifty‐eight children (41%) had GTC, 77 (54%) had partial seizures and six were described as having "others/unclear". The groups were evenly matched for age, gender, seizure type and prior AED administration. The primary outcome measure was cessation of visible motor activity by 10 minutes. Secondary outcome measures were persistent cessation of seizure activity at one hour, time to IV access, time from drug administration to stopping of the seizure and development of hypotension/respiratory depression. Further seizures were treated with intravenous phenytoin.
Ashrafi 2010 was a randomised controlled trial conducted in two large hospitals in Tehran, Iran, comparing buccal midazolam and rectal diazepam for the control of acute convulsive seizures. Ninety‐eight children aged more than three months with an acute prolonged seizure lasting more than five minutes and those convulsing while attending the emergency rooms were enrolled, irrespective of the cause of the seizure. Patients who already had intravenous access or who were younger than three months were excluded. Most (84 patients or 86%) had GTC, with the remainder being myoclonic, focal clonic and focal tonic seizures. There was no significant difference between the two groups for age, sex or seizure type. Randomisation was by a random‐number table to either buccal midazolam (0.3 to 0.5 mg/kg) or rectal diazepam (0.5 mg/kg). The primary outcome measure was cessation of all motor activity in less than five minutes, without respiratory depression and without another seizure. Further seizures were treated with intravenous diazepam. The outcome measures were further defined as treatment initiation time and drug effect time. The authors also examined the convenience of drug use and parental acceptance of the drug/route of administration for each group.
Baysun 2005 was a prospective randomised study of all children attending the emergency room of a children's hospital in Turkey with a seizure, regardless of type, aetiology and whether the seizure was prolonged (this was assumed). No exclusion criteria were stated. Forty‐three children ranging in age from two months to 12 years were recruited and randomised to buccal midazolam (0.25 mg/kg) on even days of the month and rectal diazepam (0.5 mg/kg for under‐fives and 0.3 mg/kg for those aged six or more) on odd days of the month. The two groups did not differ significantly by sex, age, type of seizures or anti‐epileptic drug used. Ten children in the midazolam group and 10 in the diazepam group had GTC. The remaining participants presented with generalised tonic, simple partial and complex partial seizures. Outcome measures were cessation of convulsive seizure activity within 10 minutes, time to response, and need for a second drug. Those who did not respond within 10 minutes were given the alternative drug, i.e. midazolam given to those who had already received diazepam and vice versa.
Chamberlain 1997 was a prospective, open randomised study of the management of children aged 0 to 18 years presenting to the emergency department of two large hospitals in the USA, with motor seizures of at least 10 minutes' duration (all had tonic‐clonic or clonic seizures ‐ clarified in personal communication). The study compared intramuscular midazolam (0.2 mg/kg) with intravenous diazepam (0.3 mg/kg). Children who had established intravenous access or who had already received treatment for this seizure episode were excluded. Primary outcome measures were seizure cessation within five minutes of administration, seizure cessation between five and 10 minutes after administration (defined as delayed seizure control), and treatment failure (lack of cessation by 10 minutes). Those who had treatment failure were subsequently given intravenous diazepam or phenytoin. Other outcome measures included recurrence of seizures, defined as early recurrence if within 15 minutes, or recurrence if within 60 minutes. Twenty‐eight children were identified for enrolment, but three were excluded as their seizures did not persist beyond 10 minutes. One child who was randomised to diazepam was a protocol violation due to failure to establish intravenous access, necessitating treatment with intramuscular midazolam. Twenty‐three children with 24 seizure episodes were studied (one child had two episodes and appears in the study twice, once in each group). The demographics were similar between the two groups.
Chamberlain 2014 was a large multicentre randomised controlled trial conducted in the emergency departments of 11 North American hospitals, comparing intravenous lorazepam with intravenous diazepam for convulsive status epilepticus in children. Inclusion criteria were children aged three months to 18 years with generalised tonic‐clonic status epilepticus. This was defined as three or more seizures in the previous hour, two or more successive seizures with no recovery of consciousness with an ongoing seizure, or an ongoing seizure lasting at least five minutes. Children who had initial focal seizures rapidly evolving to bilaterally convulsive seizures were included. Patients with the following factors were excluded: known pregnancy, significant cardiac arrhythmia, urgent need for surgical intervention and anaesthesia, known contraindication to benzodiazepines or benzodiazepine use in the previous seven days (including pre‐hospital use by ambulance personnel). "Early terminators" were children removed from the study following administration of the study drug due to discovery of an exclusion factor or a refusal to participate by the family. The study assessed 11,630 patients for eligibility, of whom 11,320 were excluded, mainly because they were not having an acute seizure or did not meet the inclusion criteria. The study randomised 310 children to one of the two study drugs. Twenty‐two and 15 children were early terminators from each treatment arm respectively, and were excluded from the efficacy analysis. Further exclusions largely due to protocol deviations resulted in 102 and 107 children being available for per protocol analysis in each treatment arm. The participants in each treatment arm were well‐matched in demographics and seizure aetiology. Primary efficacy outcome measures were cessation of status epilepticus (defined as cessation of generalised convulsive activity with return of consciousness within the four‐hour observation period) within 10 minutes of the initial dose, and seizure freedom for 30 minutes. Secondary outcome measures included latency of drug response (time to cessation of convulsions), need for a dose of study medication, need for further anticonvulsants and sustained seizure freedom for 60 minutes and four hours. Primary safety outcomes were severe respiratory depression (needing assisted ventilation) within four hours of the study drug administration; secondary safety outcomes were aspiration pneumonia, any degree of respiratory depression, time required to return to baseline mental status, and degree of sedation or agitation as measured by the Riker Sedation‐Agitation scale.
Fişgin 2002 was a prospective, randomised, single‐centre study of children aged between one month and 13 years, presenting with an acute seizure to the emergency room of a children's hospital in Turkey. All children who were seizing on arrival were included, as it was presumed that their seizure had been ongoing for at least five minutes.No exclusion criteria are stated and the aetiology of the seizures is not given. Of 45 enrolled in the study, 28 children (14 per treatment group) had generalised tonic‐clonic seizures, the rest presenting with simple focal (10), secondarily generalised (4), tonic (1) and myoclonic (2) seizures. Both febrile and afebrile seizures were included, although only 10% of the participants had a febrile seizure, and were equally distributed between the two groups. Children were randomised on alternate days to rectal diazepam (0.3 mg/kg) or nasal midazolam (0.2 mg/kg). If the seizure continued beyond 10 minutes, the alternative drug was given, i.e. there was cross‐over between the two groups. Persistent convulsions (not clearly defined) were treated with intravenous midazolam by bolus, then infusion. Outcome measures were stopping of the seizure within 10 minutes, response time, and necessity for a second drug. There was no significant difference between the two groups for age or seizure type.
Gathwala 2012 was a randomised controlled trial undertaken in an Indian teaching hospital, comparing intravenous diazepam, midazolam and lorazepam for the treatment of acute convulsive seizures in children. Children aged six months to 14 years presenting with a convulsion to the emergency department were recruited. Children with liver or renal disease, cardiovascular abnormalities, head injury, diabetes mellitus or hypoglycaemia were excluded, as were those whose seizure had already stopped or where intravenous access could not be established. The study assessed 185 children for inclusion, of whom 65 were excluded; 55 did not meet the inclusion criteria, seven declined, and intravenous access could not be established in three. Participants were randomised into three treatment groups, which were evenly matched for demographics, mean duration of seizure, prolonged seizures, those presenting with first episode, and cause of seizure. The primary outcome measure was time to seizure cessation, defined as cessation of visible epileptic phenomena or return of purposeful response to external stimuli within 15 minutes of drug administration. The secondary outcomes were the effects of the drugs, i.e. vomiting, apnoea, somnolence, respiratory depression and requirement for mechanical ventilation. Other secondary outcomes were the number of participants with seizure recurrence, requirement for a second dose of medication, uncontrolled seizures, and the time to seizure recurrence.
Javadzadeh 2012 was a randomised unblinded study of 60 children aged between two months and 15 years, presenting to the emergency department with an acute seizure. Exclusion criteria were patients with prior IV access, previous anticonvulsant treatment, or concurrent respiratory tract infection. Participants were randomised to intranasal midazolam or intravenous diazepam, although all patients were cannulated on arrival. Outcome measures included time needed to control seizure, oxygen saturations, and heart rate pre‐ and post‐treatment.
Lahat 2000 was a 12‐month single‐centre randomised study comparing intranasal midazolam (0.2 mg/kg) and intravenous diazepam (0.3 mg/kg) in the treatment of prolonged febrile seizures (a seizure of at least 10 minutes duration) in children aged six months to five years. The study was carried out in a paediatric emergency department within a general hospital. Patient demographics were similar in both groups. Treatment was successful if the clinical features of the seizure stopped within five minutes. If the seizure stopped at between five and 10 minutes, this was identified as a delayed but successful treatment. Treatment failures (continued seizure activity after 10 minutes) received intravenous diazepam and then phenobarbital in accordance with local guidelines. Randomisation was allocated in advance by a random‐number table, with investigators receiving an opaque envelope with each allocation at the time of administration. Forty‐four children of both sexes were evaluated, with a total of 52 seizure episodes. Children who had received an anticonvulsant or had an intravenous line sited by paramedics prior to hospital attendance were excluded from the study.
Mahmoudian 2004 is a prospective randomised study of children aged two months to 15 years, presenting with an acute seizure to the paediatric emergency department of a general hospital in Iran over a two‐month period. Seventy children who presented with an acute seizure (length not specified) were randomised by an odd‐ and even‐number table to receive 0.2 mg/kg of intravenous diazepam or 0.2 mg/kg of intranasal midazolam. Fifty children presented with GTC, six with simple partial seizures, twelve with complex partial seizures and five with myoclonic seizures (note that multiple seizure types can occur in a single child). Outcome measures were time from treatment to cessation of seizure, with treatment considered successful if the seizure stopped within 10 minutes. Seizures that did not stop within 10 minutes were defined as treatment failures. Treatment failures in the midazolam group were given intravenous diazepam and those in the diazepam group were given intravenous phenobarbitone. Aetiologies of the seizures were reported, and were not evenly distributed between the groups; 14 of the midazolam group versus one of the diazepam group had febrile convulsions, and 10 of the diazepam group versus four in the midazolam group had central nervous system (CNS) infection.
McIntyre 2005 was a 40‐month, multicentre, randomised, controlled trial comparing buccal midazolam (approximately 0.5 mg/kg) with rectal diazepam (0.5 mg/kg) as the first‐line treatment of children aged six months to 15 years, presenting to a paediatric A&E department with active seizures.The primary outcome measure was clinical cessation of the seizure within 10 minutes of drug administration, without seizure recurrence within one hour and without respiratory depression. Children with partial seizures or non‐convulsive status epilepticus were excluded from the trial. Weekly blocks of treatment of either buccal midazolam or rectal diazepam were randomly selected in each of the four participating centres. Participant demographics were similar between groups. Locally‐agreed guidelines were followed in the event of continued seizure activity after the 10‐minute period. The study evaluated 219 seizure episodes in 177 children of both sexes. Separate results were reported both for total episodes and for first presenting episodes, to minimise potential bias of children with multiple entries. In contrast with the other studies included in the previous review, children were not excluded if they had received anticonvulsant agents prior to their attendance at the A&E department.
Momen 2015 was an unblinded randomised trial of 100 children aged one month to 16 years. Inclusion criteria were children older than one month who were convulsing on arrival. The length of the ongoing seizure was not taken into account, so children with relatively short‐lived seizure may have been included. Exclusion criteria were established IV access, prior administration of rectal or nasal benzodiazepines, lack of consent or serial seizures with no recovery of consciousness. In addition, a history of serious adverse reactions to either of the study medications was an exclusion criterion. Participants were randomised to intramuscular midazolam or rectal diazepam. The main outcome measure was seizure cessation without recurrence within 60 minutes. Respiratory rate and blood pressure were also monitored.
Mpimbaza 2008 was a single‐blinded, placebo‐controlled randomised clinical trial in a paediatric emergency unit in Kampala, Uganda. The inclusion criteria were children aged three months to 12 years who presented while convulsing or who experienced a seizure that lasted more than five minutes while in the unit, and who had no documented evidence of having received intravenous diazepam or phenobarbitone in the 24 hours before presentation. Children aged less than three months or more than 12 years, who had evidence of prior treatment or whose convulsion stopped prior to treatment, were excluded. The study recruited 330 participants (note that multiple seizure types can occur in a single participant): 269 (82%) had generalised tonic‐clonic seizures, 18 had tonic seizures, 61 had focal seizures and three had myoclonic seizures. Participants were randomised by a random‐number table to 0.5 mg/kg of rectal diazepam or buccal midazolam. A placebo which was identical in volume and similar in colour was simultaneously given with the study drug. The participants were well‐balanced by age, sex, and type of seizure between the two groups. The primary outcome measure was cessation of visible seizure activity within 10 minutes, without recurrence in the subsequent hour. If the convulsion lasted longer than 10 minutes or recurred within one hour, this was considered a treatment failure and the child was given intravenous diazepam. Secondary outcome measures were the proportion with cessation of convulsions within 10 minutes, the proportion with recurrence in the next hour and within 24 hours of initial control, and time to recurrence within these periods.
Shah 2005 was a prospective controlled quasi‐randomised study of the treatment of acute seizures in children in a tertiary general hospital in Mumbai, India, including children presenting to the emergency department and those who were already admitted to the ward or intensive care unit (ICU). The study enrolled 115 children with an acute seizure (definition unclear) over a one‐year period in a single centre. Those who had already had treatment for the seizure were excluded. Participants who already had intravenous access were treated with 0.2 mg/kg of intravenous diazepam. Those without were randomised to treatment with 0.2 mg/kg of intramuscular midazolam or to the establishment of intravenous access and treatment with intravenous diazepam. Sixty‐three children had generalised tonic‐clonic seizures, and were equally divided between the two treatment groups. Of the remaining participants, 47 had focal, four had tonic and one had clonic seizures. Outcome measures were mean time to cessation of seizures and the presence of adverse effects. Those who did not respond after five minutes were treated with other "anticonvulsants" (not specified).
Sreenath 2010 was a randomised controlled study of the management of convulsive status epilepticus (defined as continuous convulsive activity lasting for five minutes or more) in children presenting to a single centre in North India. Exclusion criteria were treatment with any anti‐epileptic medication in the preceding four weeks, acute head trauma, history of poisoning and jaundice, suspected renal failure, or diarrhoea presenting with seizures. The study randomised 178 children aged one to 12 years by computer‐generated table to receive lorazepam 0.1 mg/kg or diazepam 0.2 mg/kg. If intravenous access was not present, the drug was given rectally at the same dose. If the seizures recurred within an undefined time frame, a second dose of the same drug was given. The diazepam group were given a loading dose of 18 mg/kg of phenytoin after 15 to 30 minutes, regardless of whether the seizure had recurred. Sixty‐three per cent had generalised tonic‐clonic seizures, while the rest were tonic (10%), clonic (10%), myoclonic (0.5%), simple partial (2%), complex partial (10%), and partial with secondary generalisation (4%). The majority were therefore generalised convulsive or motor seizures. The primary outcome measure was cessation of seizure activity, with treatment considered successful if this occurred within 10 minutes of the first intervention and without recurrence over the next 18 hours. Secondary outcomes were time taken for initial (presenting) convulsion to stop after administration of the first drug, the number of doses of study drug required to treat the initial convulsion, the use of an additional anti‐epileptic drug, the total number of seizures occurring in the first 18 hours following administration of the study drug, the development of respiratory depression, the number of participants requiring transfer to ICU for mechanical ventilation, and the number of participants requiring cross‐over to an alternative regimen (i.e. from diazepam to lorazepam and vice versa).
Talukdar 2009 was a prospective randomised study of 120 children who attended the paediatric emergency department of a Delhi hospital with a seizure, the length of which was not defined. Those with myoclonic, absence and atonic seizures were excluded. The mean age of the participants was 3.2 years, with 73.3% under five years of age and 53.3% under one year. Seventy‐four per cent of the children presented with GTC, while the rest were complex partial or tonic seizures. The groups were not significantly different in age, sex, seizure type or underlying aetiology. Participants were allocated by a random‐number table to 0.2 mg/kg of buccal midazolam or 0.3 mg/kg of intravenous diazepam. The primary outcome measure was cessation of all motor activity within or by five minutes of administration of the drug. The drug response was further analysed as treatment initiation time (time from noting seizure to drug administration), drug effect time (time from drug administration to effect) and total controlling time, a combination of the previous two.
Excluded studies
We excluded 20 studies from the review for the following reasons (see Characteristics of excluded studies for further information):
The study evaluated refractory status epilepticus (i.e. children who have failed a first or second treatment for status epilepticus) rather than acute status epilepticus (Agarwal 2007; Arpita 2014; Mahmoudian 2006; Mehta 2007; Mittal 2014; Rosati 2016; Singhi 2002).
The study was not randomised or did not have a control group, or both (Kutlu 2003; Morton 2007; Qureshi 2002).
Fewer than 70% of included participants had GTCs (or it was unclear how many participants had GTCs) (Bhattacharyya 2006; Scott 1999; Tonekaboni 2012).
The study examined drug management for the long‐term prevention of recurring febrile seizures (Camfield 1980; Heckmatt 1976; Strengell 2009) or clusters of seizures (Cereghino 1998), rather than management of acute convulsions.
The study was not conducted in a hospital setting (Holsti 2010; Silbergleit 2012).
The study was published only as a conference abstract and we could not contact the authors for additional information to assess eligibility (McCormick 1999).
Risk of bias in included studies
The results of our 'Risk of bias' evaluations are summarised in Figure 2 and Figure 3.
2.
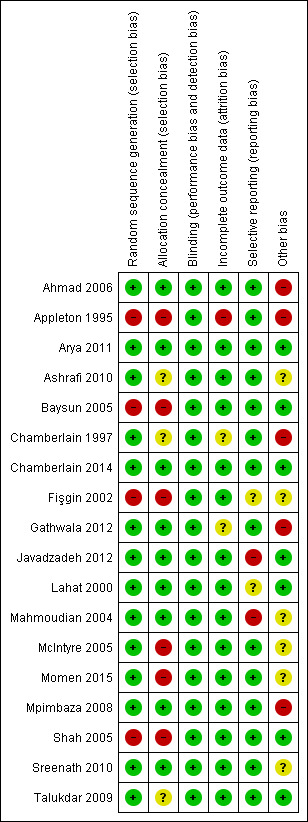
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
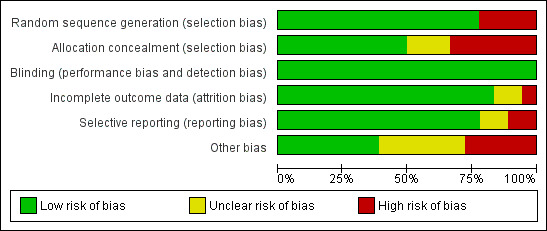
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Fourteen studies reported adequate methods for random sequence generation (e.g. computer‐generated randomisation, block randomisation, etc.) and we judged them to be at low risk of bias. Four studies (Appleton 1995; Baysun 2005; Fişgin 2002; Shah 2005) reported inadequate methods for random sequence generation and we judged these to be at high risk of bias. Three of these studies (Appleton 1995; Baysun 2005; Fişgin 2002) used an alternating ('odd' and 'even') day approach to randomisation and one study (Shah 2005) was partially randomised, including patients attending the emergency department and inpatients in the paediatric ward or intensive care unit. Those who already had intravenous access were selected to receive intravenous diazepam, and those without were further randomised to intramuscular midazolam or intravenous diazepam. For those who were randomised, it was unclear how randomisation was performed.
Regarding allocation concealment, nine studies described adequate methods of concealment such as centralised allocation or sealed opaque envelopes and we judged them to be at low risk of bias. Six studies (Appleton 1995; Baysun 2005; Fişgin 2002; McIntyre 2005; Momen 2015; Shah 2005) did not conceal allocation and we judged them to be at high risk of bias. The remaining three studies did not mention allocation concealment and we judged them to be at unclear risk of bias (Ashrafi 2010; Chamberlain 1997; Talukdar 2009).
Blinding
One study was double‐blinded (Chamberlain 2014) and one trial had single‐blinded participants (Mpimbaza 2008). The remaining sixteen studies were unblinded, and for many of them blinding would have been impractical, due to different routes of intervention. However, given the objective nature of the main outcomes of these studies (e.g. seizure cessation), it is unlikely that the lack of blinding would affect results, so we rated all studies at low risk of bias.
Incomplete outcome data
We judged 15 studies to be at low risk of bias, since all recruited participants were included and analysed on an intention‐to‐treat basis. We judged one study (Appleton 1995) to be at high risk of bias. In this study there were a relatively large number of protocol violators (16 of 102 children, or 16% of the total study population) and these violators were excluded from the analyses. The analysis was therefore not an intention‐to‐treat analysis.
We judged two studies (Chamberlain 1997; Gathwala 2012) as being at unclear risk of bias. In Chamberlain 1997, three children who were randomised to receive diazepam were subsequently excluded, as their seizures did not persist for 10 minutes. There was also a protocol violator who was randomised to receive intravenous diazepam but received intramuscular midazolam after 25 minutes, due to unsuccessful intravenous access. This participant was excluded from the analysis and obviously would have skewed the results significantly if he/she had been included. It may have been helpful to know the response time of this child once treatment was administered, as this is an important example of the disadvantages of the intravenous route. In Gathwala 2012, three children were excluded due to difficulties obtaining intravenous access, and this may have introduced a source of bias. It could be argued that the data cannot be considered to have been analysed on an intention‐to‐treat basis, as these participants were excluded from the analysis. However, given that all routes were intravenous the effect of this is likely to have been small.
Selective reporting
Fourteen studies reported all expected and prespecified outcomes and we judged them to be at low risk of bias. We rated two studies at high risk of bias; Javadzadeh 2012 did not report seizure cessation, which we would expect to be reported, and in Mahmoudian 2004 the authors did not report the time taken to insert intravenous cannulae in the intravenous diazepam group. This would have a significant effect on the time from arrival to seizure cessation. Other studies that compared intravenous with other routes have included this information.
We judged two studies to be at unclear risk of bias. Fişgin 2002 stated that information about previous convulsions and history of anti‐epileptic medication were collected according to the Methods but not reported in the Results section. It is unlikely that this information influenced outcomes, but we are unclear why the information was not reported. Lahat 2000 defined seizure cessation in the Methods section as "successful" if seizures stopped in less than five minutes, "successful but delayed" if seizures stopped after five to 10 minutes, and "failure" if seizures had not stopped after 10 minutes. However, results seem to be presented only in terms of treatment success and failure. It is unclear if this is selective reporting of results.
Other potential sources of bias
We identified additional high risks of bias in five studies. In two studies, a high proportion of the children recruited had either cerebral malaria or meningitis, which may have impacted upon the results (Ahmad 2006; Mpimbaza 2008). In Appleton 1995 there was a large discrepancy in the two routes of administration used in the study, probably due to clinician uncertainty about the use of rectal lorazepam. This discrepancy is likely to have impacted upon results. In Chamberlain 1997, one child was enrolled in the study twice, and is represented in both groups. Due to the small numbers of children included in the study, this double‐enrolment may have impacted on the results. In Gathwala 2012, the definition of the 'seizure cessation' outcome used is different from all the other included studies, and is not an appropriate criterion for judging seizure cessation. This definition is likely to have impacted upon results.
In four studies, it was unclear whether additional bias was present. In three studies (Ashrafi 2010; Mahmoudian 2004; Sreenath 2010), one or both treatment arms showed a 100% seizure cessation rate, which is higher than expected. However, it was unclear whether these unexpected results were due to a particular element of the trial design. In Fişgin 2002, the description of the seizure type and aetiology of participating children was unclear, so we cannot be sure that the population of this study is generalisable.
We found no other biases in the remaining nine studies (Arya 2011; Baysun 2005; Chamberlain 2014; Javadzadeh 2012; Lahat 2000; McIntyre 2005; Momen 2015; Shah 2005; Talukdar 2009).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12
In the 18 included trials, specific drugs (i.e. benzodiazepines (diazepam, lorazepam and midazolam), phenytoin, and paraldehyde) were compared to each other; different routes of drug administration (i.e. intravenous, intranasal, buccal, rectal and intramuscular) of the same drug or different drugs were compared.
Considering the different drugs and different routes of administration, this review makes 12 comparisons. The results of each comparison are also summarised in 'Summary of findings' tables:
Table 1: Lorazepam versus diazepam; Table 2: Intranasal lorazepam versus intramuscular paraldehyde; Table 3: Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination; Table 4: Intravenous lorazepam versus intranasal lorazepam; Table 5: Buccal midazolam versus rectal diazepam; Table 6: Buccal midazolam versus intravenous diazepam; Table 7: Intranasal midazolam versus intravenous diazepam; Table 8: Intranasal midazolam versus rectal diazepam; Table 9: Intramuscular midazolam versus intravenous diazepam; Table 10: Intramuscular midazolam versus rectal diazepam; Table 11: Intravenous midazolam versus intravenous diazepam; Table 12: Intravenous midazolam versus intravenous lorazepam.
Table 13 also shows the study‐specific event rates for the outcomes 'Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used,' 'Incidence of respiratory depression' and 'The need to use additional anti‐epileptic drugs to stop the presenting convulsion'.
1. Event rates for seizure cessation, respiratory depression and additional drugs required.
| Study | Drug | Seizure cessation | Respiratory Depression | Additional drugs required | ||||||
|
No. of Events |
No. of Children |
% |
No. of Events |
No. of Children |
% |
No. of Events |
No. of Children |
% | ||
| Ahmad 2006 | IN lorazepam | 60 | 80 | 75 | 0 | 80 | 0 | 8 | 80 | 10 |
| IM paraldehyde | 49 | 80 | 60 | 0 | 80 | 0 | 21 | 80 | 26 | |
| Appleton 1995 | IV lorazepam | 19 | 27 | 70 | 1 | 27 | 4 | 1 | 27 | 4 |
| Rectal lorazepam | 6 | 6 | 100 | 0 | 6 | 0 | 0 | 6 | 0 | |
| IV diazepam | 22 | 34 | 65 | 7 | 34 | 21 | 5 | 34 | 15 | |
| Rectal diazepam | 6 | 19 | 32 | 1 | 19 | 5 | 12 | 19 | 63 | |
| Arya 2011* | IN lorazepam | 16 | 23 | 70 | 1 | 71 | 1 | NR | 23 | NA |
| IV lorazepam | 26 | 35 | 74 | 2 | 70 | 3 | NR | 35 | NA | |
| Ashrafi 2010 | Buccal midazolam | 49 | 49 | 100 | 0 | 49 | 0 | 0 | 49 | 0 |
| Rectal diazepam | 40 | 49 | 82 | 0 | 49 | 0 | 9 | 49 | 18 | |
| Baysun 2005 | Buccal midazolam | 18 | 23 | 78 | 0 | 23 | 0 | 5 | 23 | 22 |
| Rectal diazepam | 17 | 20 | 85 | 1 | 20 | 5 | 3 | 20 | 15 | |
| Chamberlain 1997 | IM midazolam | 12 | 13 | 92 | 0 | 13 | 0 | 1 | 13 | 8 |
| IV diazepam | 10 | 11 | 91 | 0 | 11 | 0 | 1 | 11 | 9 | |
| Chamberlain 2014 | IV diazepam | 101 | 140 | 72 | 26 | 140 | 16 | 21 | 140 | 15 |
| IV lorazepam | 97 | 133 | 73 | 26 | 133 | 18 | 21 | 133 | 16 | |
| Fişgin 2002 | IN midazolam | 20 | 23 | 87 | 0 | 23 | 0 | 3 | 23 | 13 |
| Rectal diazepam | 13 | 22 | 60 | 0 | 22 | 0 | 9 | 22 | 40 | |
| Gathwala 2012 | IV diazepam | 36 | 40 | 90 | 1 | 40 | 3 | 4 | 40 | 10 |
| IV midazolam | 39 | 40 | 98 | 0 | 40 | 0 | 1 | 40 | 3 | |
| IV lorazepam | 40 | 40 | 100 | 0 | 40 | 0 | 0 | 40 | 0 | |
| Javadzadeh 2012 | IN midazolam | NR | 30 | NA | NR | 30 | NA | NR | 30 | NA |
| IV diazepam | NR | 30 | NA | NR | 30 | NA | NR | 30 | NA | |
| Lahat 2000 | IN midazolam | 23 | 26 | 88 | 0 | 26 | 0 | NR | 26 | NA |
| IV diazepam | 24 | 26 | 92 | 0 | 26 | 0 | NR | 26 | NA | |
| Mahmoudian 2004 | IN midazolam | 35 | 35 | 100 | 0 | 35 | 0 | 0 | 35 | 0 |
| IV diazepam | 35 | 35 | 100 | 0 | 35 | 0 | 0 | 35 | 0 | |
| McIntyre 2005 | Buccal midazolam | 61 | 109 | 56 | 5 | 109 | 5 | 36 | 109 | 33 |
| Rectal diazepam | 30 | 110 | 27 | 7 | 110 | 6 | 63 | 110 | 57 | |
| Momen 2015 | IM midazolam | 48 | 50 | 96 | 1 | 50 | 2 | NR | 50 | NA |
| Rectal diazepam | 47 | 50 | 94 | 0 | 50 | 0 | NR | 50 | NA | |
| Mpimbaza 2008 | Buccal midazolam | 125 | 165 | 76 | 2 | 165 | 1 | NR | 165 | NA |
| Rectal diazepam | 114 | 165 | 69 | 2 | 165 | 1 | NR | 165 | NA | |
| Shah 2005 | IM midazolam | 45 | 50 | 90 | 0 | 50 | 0 | 5 | 50 | 10 |
| IV diazepam | 29 | 31 | 90 | 0 | 31 | 0 | 2 | 31 | 6 | |
| Sreenath 2010 | IV lorazepam | 90 | 90 | 100 | 4 | 90 | 4 | 6 | 90 | 7 |
| IV diazepam with phenytoin |
88 | 88 | 100 | 5 | 88 | 6 | 14 | 88 | 16 | |
| Talukdar 2009 | Buccal midazolam | 51 | 60 | 85 | 0 | 60 | 0 | 9 | 60 | 15 |
| IV diazepam | 56 | 60 | 93 | 0 | 60 | 0 | 4 | 60 | 7 | |
Abbreviations: IM: Intramuscular; IN: Intranasal; IV: Intravenous; NR: Not reported; NA: Not available (percentages could not be calculated where event rate was NR)
*Occurences of respiratory depression were not reported for the subgroup of participants with generalised tonic‐clonic seizures in Arya 2011, therefore these results refer to all participants (including 83 participants without generalised tonic‐clonic seizures).
1. Lorazepam versus diazepam
Three trials recruiting 455 participants compared lorazepam to diazepam (Appleton 1995; Chamberlain 2014; Gathwala 2012). All participants in Chamberlain 2014 and Gathwala 2012 received drugs intravenously; in Appleton 1995, children received the drugs either intravenously or rectally (where intravenous access was not possible). As the route of administration in this trial was not randomised, we test the route of administration for lorazepam and diazepam by subgroup analyses rather than by separate comparisons.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used.
All three studies reported the number of children with their presenting seizure(s) stopped by the trial drug. There was no statistically significant difference between the treatments when administered intravenously; risk ratio (RR) 1.04, 95% confidence interval (CI) 0.94 to 1.16, P = 0.43, Analysis 1.1. There was no heterogeneity present in this analysis (I2 = 0%).
1.1. Analysis.
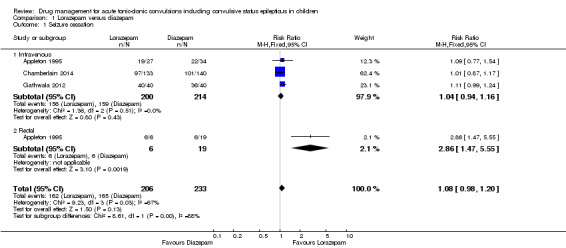
Comparison 1 Lorazepam versus diazepam, Outcome 1 Seizure cessation.
For the 25 participants in Appleton 1995 who received the treatments rectally, lorazepam was statistically significantly more effective for seizure cessation than diazepam; RR 2.86, 95% CI 1.47 to 5.55, P = 0.002, Analysis 1.1. We are cautious when interpreting this result, due to the unbalanced number of children receiving each drug rectally (six received lorazepam and 19 received diazepam).
Overall, for both routes of administration, there was no statistically significant difference between the treatments; RR 1.08, 95% CI 0.98 to 1.20, P = 0.13, low‐quality evidence, Analysis 1.1. We note that this analysis has substantial heterogeneity (I2 = 67%), which may be due to the differences in the results by the different routes of administration (test for subgroup differences: Chi2 = 8.61, df = 1, P = 0.003, I2 = 88.4%).
2. Time taken from administration of any drug in the hospital to stopping the convulsion.
Gathwala 2012 reported that there was no significant difference for time to seizure cessation, with a mean difference 6.18 seconds, 95% CI ‐7.83 to 20.19, P = 0.39, moderate‐quality evidence, Analysis 1.2.
1.2. Analysis.
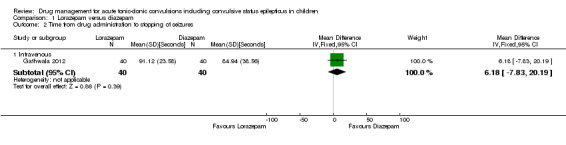
Comparison 1 Lorazepam versus diazepam, Outcome 2 Time from drug administration to stopping of seizures.
Appleton 1995 reports the mean and range of times for the presenting convulsion to stop is 29 seconds (range 25 to 60) for the intravenous lorazepam, 26 seconds (range 20 to 51) for the intravenous diazepam group, 37 seconds (range 31 to 48) for the rectal lorazepam group, and 38 seconds (range 35 to 49) for the rectal diazepam group. Standard deviations were not available, so we cannot enter data into analysis.
Chamberlain 2014 did not report on this outcome.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
All three studies reported the incidence of respiratory depression. When administered intravenously, there were significantly fewer occurrences of respiratory depression with lorazepam compared to diazepam; RR 0.71, 95% CI 0.55 to 0.92, P = 0.01, 414 children, Analysis 1.3. There was no heterogeneity present in this analysis (I2 = 0%).
1.3. Analysis.
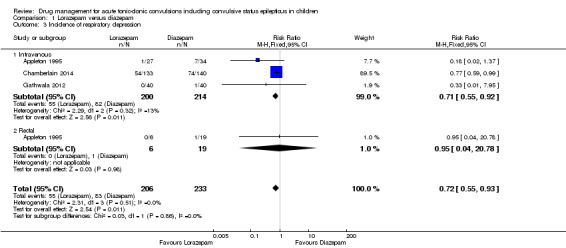
Comparison 1 Lorazepam versus diazepam, Outcome 3 Incidence of respiratory depression.
For the 25 participants in Appleton 1995 who received the treatments rectally, there was no significant difference between treatments; RR 0.95, 95% CI 0.04 to 20.78, P = 0.98, Analysis 1.3. However, as above we are cautious when interpreting this result, due to the unbalanced number of children receiving each drug rectally.
Overall for both routes of administration, there were significantly fewer occurrences of respiratory depression with lorazepam compared to diazepam; RR 0.72, 95% CI 0.55 to 0.93, P = 0.01, moderate‐quality evidence, Analysis 1.3. There was no heterogeneity present in this analysis (I2 = 0%) and no significant difference between the routes of administration (test for subgroup differences: Chi2 = 0.03, df = 1, P = 0.86, I2 = 0%).
Gathwala 2012 reported that there was a significant increase in somnolence between the diazepam and both the midazolam and lorazepam groups, but other adverse effects were evenly distributed. Chamberlain 2014 also reported that there was an increased incidence of sedation in the lorazepam group (absolute risk difference (ARD) 16.9%, 95% CI 6.1 to 27.7) and increased time taken to return to baseline mental status in the lorazepam group (hazard ratio (HR) 1.96, 95% CI 1.35 to 2.84, P < 0.001).
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion.
All three studies reported the number of children requiring an extra dose of trial medication to stop the presenting seizure. There was no statistically significant difference between the treatments when administered intravenously (RR 0.97, 95% CI 0.71 to 1.33, P = 0.86, 414 children, Analysis 1.4), rectally (RR 0.11, 95% CI 0.01 to 1.56, P = 0.10, 25 children, Analysis 1.4) or for both routes of administration (RR 0.88, 95% CI 0.64 to 1.20, P = 0.41, low‐quality evidence, Analysis 1.4). Some heterogeneity was present in the combined analysis (I2 = 50%), which is probably due to the differences in routes of administration, although the test for subgroup differences did not reach statistical significance (test for subgroup differences: Chi2 = 2.58, df = 1, P = 0.11, I2 = 61.3%).
1.4. Analysis.
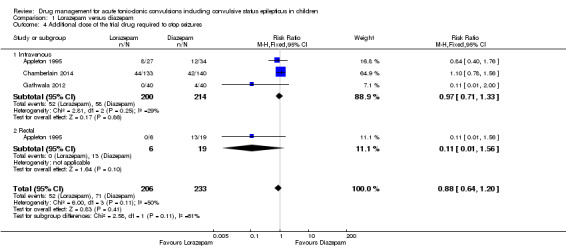
Comparison 1 Lorazepam versus diazepam, Outcome 4 Additional dose of the trial drug required to stop seizures.
Appleton 1995 and Chamberlain 2014 also reported the number of children requiring treatment with additional anti‐epileptic drugs to stop the presenting seizure. There was no statistically significant difference between the treatments when administered intravenously (RR 0.91, 95% CI 0.54 to 1.55, P = 0.73, 334 children, Analysis 1.5), rectally (RR 0.11, 95% CI 0.01 to 1.69, P = 0.11, 25 children, Analysis 1.5) or for both routes of administration (RR 0.75, 95% CI 0.45 to 1.24, P = 0.26, 359 children, Analysis 1.5). Some heterogeneity was present in the analysis combining both routes of administration (I2 = 54%), which is probably due to the differences in routes of administration, although the test for subgroup differences did not reach statistical significance (test for subgroup differences: Chi2 = 2.20, df = 1, P = 0.14, I2 = 54.5%).
1.5. Analysis.
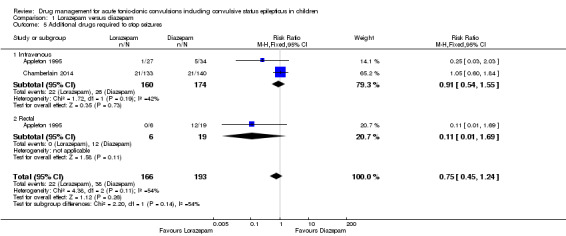
Comparison 1 Lorazepam versus diazepam, Outcome 5 Additional drugs required to stop seizures.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion.
All three trials reported the number of children with recurrences of seizures within 24 hours. There was no statistically significant difference between the treatments when administered intravenously (RR 0.91, 95% CI 0.65 to 1.27, P = 0.56, 414 children, Analysis 1.6), rectally (RR 0.19, 95% CI 0.01 to 2.92, P = 0.23, 25 children, Analysis 1.6) or for both routes of administration (RR 0.86, 95% CI 0.61 to 1.20, P = 0.36, moderate‐quality evidence, 439 children, Analysis 1.6). There was no heterogeneity present in the pooled analyses and no differences by route of administration were found (test for subgroup differences: Chi2 = 1.23, df = 1, P = 0.27, I2 = 19.0%).
1.6. Analysis.
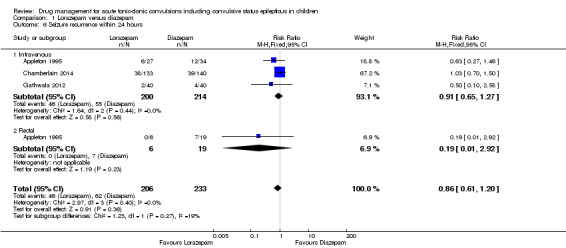
Comparison 1 Lorazepam versus diazepam, Outcome 6 Seizure recurrence within 24 hours.
3. Incidence of admissions to the intensive care unit (ICU)
Appleton 1995 reported the number of admissions to the ICU. There was no statistically significant difference between the intravenously (RR 0.07, 95% CI 0.00 to 1.22, P = 0.07, 61 children, Analysis 1.7), and rectally (RR 0.57, 95% CI 0.03 to 10.51, P = 0.71, 25 children, Analysis 1.7) separately. However, when combining both routes of administration, significantly more children who received diazepam were admitted to the ICU (10 compared to 0 who received lorazepam) (RR 0.15, 95% CI 0.02 to 0.98, P = 0.05, low‐quality evidence, 86 children, Analysis 1.7). There was very little heterogeneity present in the pooled analysis and no difference by route of administration (test for subgroup differences: Chi2 = 0.99, df = 1, P = 0.32, I2 = 0%).
1.7. Analysis.
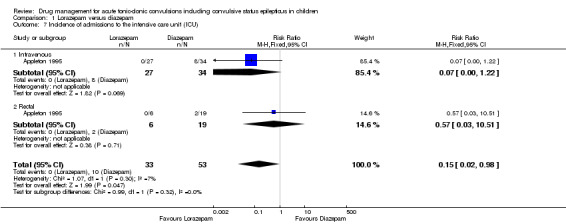
Comparison 1 Lorazepam versus diazepam, Outcome 7 Incidence of admissions to the intensive care unit (ICU).
2. Intranasal lorazepam versus intramuscular paraldehyde
One trial (Ahmad 2006), recruiting 160 participants, compared intranasal lorazepam to intramuscular paraldehyde.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus terminated with the drug(s) used
There was no statistically significant difference between the intranasal lorazepam and intramuscular paraldehyde groups for stopping the presenting seizure, with 60/80 (75%) in the intranasal lorazepam group compared to 49/80 (61%) in the intramuscular paraldehyde group: RR 1.22, 95% CI 0.99 to 1.52, P = 0.07, moderate‐quality evidence, Analysis 2.1.
2.1. Analysis.
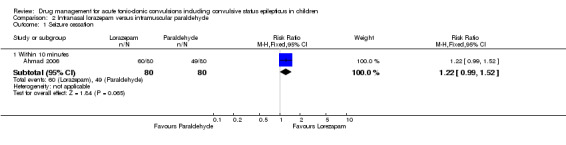
Comparison 2 Intranasal lorazepam versus intramuscular paraldehyde, Outcome 1 Seizure cessation.
2. Time taken from administration of any drug in the hospital to stopping the convulsion
This outcome was not reported in the trial.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
There was no difference between the treatment groups for clinically‐important cardiorespiratory events (low‐quality evidence).
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
Statistically significantly more children (8/80 (10%)) in the intranasal lorazepam group required two or more additional anticonvulsant doses to stop the seizures, compared to 21/80 children (26%) in the intramuscular paraldehyde group: RR 0.38, 95% CI 0.18 to 0.81, P = 0.01, low‐quality evidence, Analysis 2.2.
2.2. Analysis.
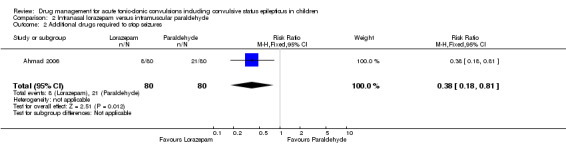
Comparison 2 Intranasal lorazepam versus intramuscular paraldehyde, Outcome 2 Additional drugs required to stop seizures.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
There was no statistically significant difference between the treatment groups for seizure recurrence within 24 hours: RR 0.73, 95% CI 0.31 to 1.71, P = 0.47, low‐quality evidence, Analysis 2.3.
2.3. Analysis.
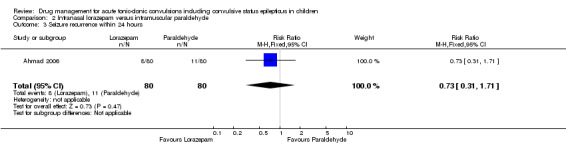
Comparison 2 Intranasal lorazepam versus intramuscular paraldehyde, Outcome 3 Seizure recurrence within 24 hours.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
3. Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination
One trial (Sreenath 2010), recruiting 178 participants, compared intravenous lorazepam to intravenous diazepam/intravenous phenytoin combination.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
There was no difference between intravenous lorazepam and intravenous diazepam‐phenytoin combination for seizure cessation within 10 minutes (100% in both groups: RR 1.00, 95% CI 0.98 to 1.02, P = 1.00, moderate‐quality evidence, Analysis 3.1.
3.1. Analysis.
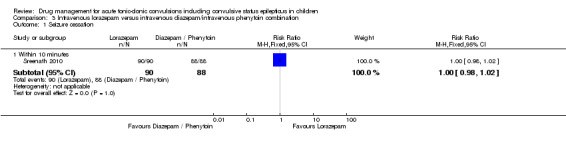
Comparison 3 Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination, Outcome 1 Seizure cessation.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
There was no statistically significant difference in the median time to seizure cessation (20 seconds in each group, moderate‐quality evidence).
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
Four participants in the intravenous lorazepam group experienced respiratory depression, compared to five in the intravenous diazepam‐phenytoin combination group. This difference was not statistically significant: RR 0.78, 95% CI 0.22 to 2.82, P = 0.71, moderate‐quality evidence, Analysis 3.2.
3.2. Analysis.
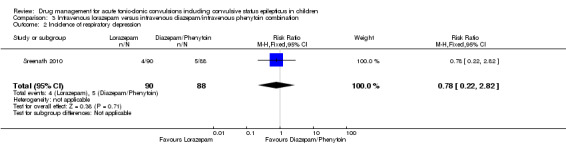
Comparison 3 Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination, Outcome 2 Incidence of respiratory depression.
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
No additional anti‐epileptic drugs were required, as seizures stopped in all children within 10 minutes (Analysis 3.1). However, only six participants in the intravenous lorazepam group required more than one dose of the trial drug to stop the seizures, compared to 14 in the intravenous diazepam‐phenytoin combination group. This difference was not statistically significant: RR 0.42, 95% CI 0.17 to 1.04, P = 0.06, moderate‐quality evidence, Analysis 3.3.
3.3. Analysis.
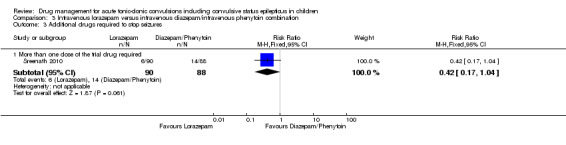
Comparison 3 Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination, Outcome 3 Additional drugs required to stop seizures.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
There were no seizure recurrences in either group (moderate‐quality evidence). The authors suggest that the lack of recurrences in the diazepam‐phenytoin group may have been due to the addition of phenytoin as a longer‐acting drug.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
4. Intravenous lorazepam versus intranasal lorazepam
One trial (Arya 2011), recruiting 141 participants, compared intravenous lorazepam to intranasal lorazepam. Results are presented for the subgroup of 58 participants with GTC.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
There were no statistically significant differences between intravenous and intranasal lorazepam for seizure cessation within 10 minutes: RR 1.07, 95% CI 0.77 to 1.49, P = 0.70, moderate‐quality evidence, or within one hour: RR 0.70, 95% CI 0.43 to 1.17, P = 0.17, Analysis 4.1.
4.1. Analysis.

Comparison 4 Intravenous lorazepam versus intranasal lorazepam, Outcome 1 Seizure cessation.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
Median time to achieve seizure control from drug administration was four minutes in both groups (moderate‐quality evidence).
The authors note that across all participants (including those without GTC), the time taken to achieve intravenous access ranged from one to 25 minutes, with a median of four minutes. If this had been included in the response time for the intravenous lorazepam, the results would have been skewed significantly in favour of intranasal lorazepam.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
Results were not available for the subgroup of participants with GTC. Across all participants (including those without GTC), one child from the intranasal group and two children from the intravenous group required respiratory support ( moderate‐quality evidence) No participant in either group demonstrated significant hypotension.
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
This outcome was not reported in the trial.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
This outcome was not reported in the trial.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
5. Buccal midazolam versus rectal diazepam
Four trials, recruiting 648 participants, compared buccal midazolam to rectal diazepam (Ashrafi 2010; Baysun 2005; McIntyre 2005; Mpimbaza 2008). One trial (177 participants; McIntyre 2005) reported on 219 seizure episodes; in other words, the same child was randomised and treated for multiple seizures in the same trial. Results are not available at the participant level so results reported for McIntyre 2005 are by episode. This is a limitation, as meta‐analysis assumes independence between measurements, and more than one treated seizure per child would not be statistically independent. A result of ignoring this unit‐of‐analysis issue could be overoptimistic confidence intervals.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
All four studies reported the number of children with their presenting seizure(s) stopped by the trial drug. Buccal midazolam was statistically significantly more effective than rectal diazepam for seizure cessation: RR 1.25, 95% CI 1.13 to 1.38, P < 0.001, very low‐quality evidence, Analysis 5.1.
5.1. Analysis.
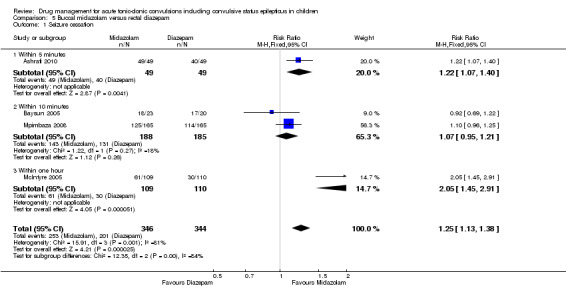
Comparison 5 Buccal midazolam versus rectal diazepam, Outcome 1 Seizure cessation.
However, there was considerable heterogeneity in the analysis (I2 = 81%). When we repeated the analysis with a random‐effects model, there was no statistically significant difference between the treatments: RR 1.23, 95% CI 0.98 to 1.54, P = 0.08. The heterogeneity may be due to the four trials measuring seizure cessation at different time points (Ashrafi 2010 five minutes; RR 1.22, 95% CI 1.07 to 1.40, P = 0.004; Baysun 2005 and Mpimbaza 2008 10 minutes; RR 1.07, 95% CI 0.95 to 1.21, P = 0.26; McIntyre 2005 one hour; RR 2.05, 95% CI 1.45 to 2.91, P < 0.001). We considered these different times in a subgroup analysis and found a significant difference between the subgroups (test for subgroup differences: Chi2 = 12.35, df = 2, P = 0.002, I2 = 83.8%, Analysis 5.1).
Seizure cessation data at one hour were also provided by the authors of Mpimbaza 2008, showing that buccal midazolam was significantly more effective than rectal diazepam: RR 1.42, 95% CI 1.06 to 1.90.
The doses of the drugs used in the studies were also different: Baysun 2005 used 0.25 mg/kg for buccal midazolam, whereas Mpimbaza 2008 and McIntyre 2005 used 0.5 mg/kg, and Ashrafi 2010 used 0.3 to 0.5 mg/kg. Furthermore, in Mpimbaza 2008 67.3% of the children had malaria and 13.7% had cerebral malaria; when only children without malaria were analysed, buccal midazolam was statistically significantly more effective than rectal diazepam for seizure cessation (RR 2.11, 95% CI 1.26 to 3.54; data provided by the author). Furthermore, for the subgroup of children with only GTC, 109/135 (80.7%) in the buccal midazolam group compared to 97/134 (72.4%) in the rectal diazepam group had seizure cessation; RR 1.12, 95% CI 0.98 to 1.27; data provided by the author.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
Baysun 2005 noted that there was no difference between groups in the time from drug administration to seizure cessation, and Ashrafi 2010 reported that both the median treatment initiation time and drug effect time were significantly shorter in the buccal midazolam group than in the rectal diazepam group (low‐quality evidence).
McIntyre 2005 and Mpimbaza 2008 did not report this outcome.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
All four studies reported on the incidence of respiratory depression. Across the four trials, 25/346 in the buccal midazolam groups and 26/344 in the rectal diazepam groups experienced respiratory depression, but this difference was not statistically significant; RR 0.88, 95% 0.61 to 1.25, P = 0.47, low‐quality evidence, Analysis 5.2.
5.2. Analysis.
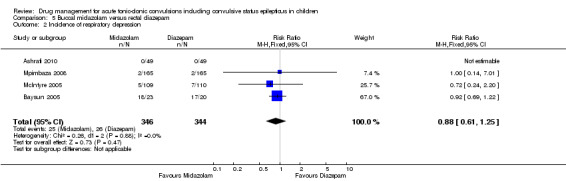
Comparison 5 Buccal midazolam versus rectal diazepam, Outcome 2 Incidence of respiratory depression.
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
McIntyre 2005 reported that fewer children in the buccal midazolam group required intravenous lorazepam to stop the seizure compared to the rectal diazepam group; RR 0.58, 95% CI 0.42 to 0.79, P < 0.001, low‐quality evidence, Analysis 5.3. Baysun 2005 also noted no difference in the need for a second drug.
5.3. Analysis.
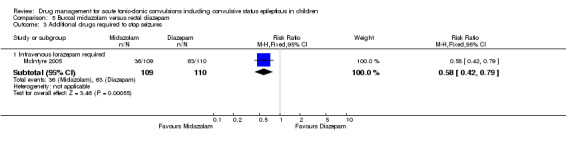
Comparison 5 Buccal midazolam versus rectal diazepam, Outcome 3 Additional drugs required to stop seizures.
Ashrafi 2010 and Mpimbaza 2008 did not report this outcome.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
None of the trials reported this outcome.
3. Incidence of admissions to the intensive care unit (ICU)
None of the trials reported this outcome.
6. Buccal midazolam versus intravenous diazepam
One trial (Talukdar 2009), recruiting 120 participants, compared buccal midazolam to intravenous diazepam.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used.
There was no statistically significant difference in seizure cessation rates between the groups treated with buccal midazolam or intravenous diazepam: RR 0.91, 95% CI 0.80 to 1.03, P = 0.15, high‐quality evidence, Analysis 6.1. Both treatments were effective, with 85% seizure cessation rate for buccal midazolam and 93% for intravenous diazepam.
6.1. Analysis.
Comparison 6 Buccal midazolam versus intravenous diazepam, Outcome 1 Seizure cessation within five minutes.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
The time to control of the seizure from drug administration (time for drug effect) was significantly shorter for intravenous diazepam compared to buccal midazolam (mean difference 0.56 minutes, 95% CI 0.29 to 0.83, P < 0.001, moderate‐quality evidence, Analysis 6.2). However the mean time for initiation of treatment was significantly shorter in the buccal midazolam group (mean difference ‐1.09 minutes, 95% CI ‐1.31 to ‐0.87, P < 0.001, Analysis 6.2), making the mean total time to controlling the seizures significantly shorter in the buccal midazolam group compared to the intravenous diazepam group (mean difference ‐0.59 minutes, 95% CI ‐0.96 to ‐0.22, P = 0.002, Analysis 6.2). The faster drug action of intravenous diazepam is therefore compromised by the need to gain intravenous access.
6.2. Analysis.
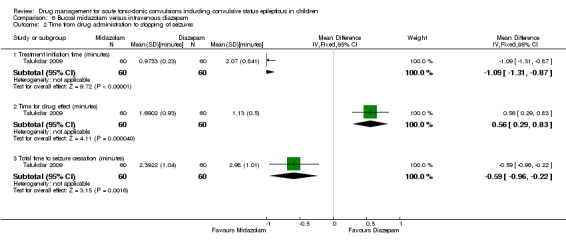
Comparison 6 Buccal midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
There were no significant adverse events in either group (high‐quality evidence).
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
This outcome was not reported in the trial.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
This outcome was not reported in the trial.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
7. Intranasal midazolam versus intravenous diazepam
Three trials, recruiting 174 participants, compared intranasal midazolam to intravenous diazepam (Javadzadeh 2012; Lahat 2000; Mahmoudian 2004).
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
Two of the trials reported the number of children with their presenting seizure(s) stopped by the trial drug (Lahat 2000; Mahmoudian 2004).
Most of the children in the two trials experienced seizure cessation, with no statistically significant difference between treatments; RR 0.98, 95% CI 0.91 to 1.06, P = 0.67, 122 children, moderate‐quality evidence, Analysis 7.1. There was no heterogeneity present in the analysis (I2 = 0%).
7.1. Analysis.

Comparison 7 Intranasal midazolam versus intravenous diazepam, Outcome 1 Seizure Cessation.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
Lahat 2000 reported that the mean time for initiation of treatment was significantly shorter in the intranasal midazolam group compared to the intravenous diazepam group (mean difference ‐2.00 minutes, 95% CI ‐3.03 to ‐0.97, P < 0.001, 52 children, Analysis 7.2). Mahmoudian 2004 also stated that the time from seizure onset to treatment was faster in the midazolam group due to cannula insertion in the diazepam group (numerical data not reported).
7.2. Analysis.
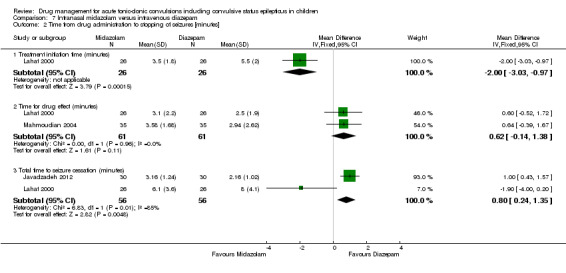
Comparison 7 Intranasal midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures [minutes].
There was no statistically significant difference between groups in two trials (Lahat 2000; Mahmoudian 2004) in the time to control of the seizure from drug administration (time for drug effect): mean difference 0.62 minutes, 95% CI ‐0.14 to 1.38, P = 0.11, 122 children, moderate‐quality evidence, Analysis 7.2.
Overall, the mean total time to controlling the seizures was significantly shorter in the intravenous diazepam group compared to the intranasal midazolam group in two trials (Javadzadeh 2012; Lahat 2000): mean difference 0.80, 95% CI 0.24 to 1.35, P = 0.005, 112 children, Analysis 7.2. There is considerable heterogeneity in this analysis (I2 = 85%), which probably originated from the calculation of the total time to controlling seizures in the Javadzadeh 2012 trial, which seems to adjust for the time taken to insert an intravenous line.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
Lahat 2000 and Mahmoudian 2004 stated that no adverse events, including respiratory depression, occurred in either group (high‐quality evidence).
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
None of the trials reported this outcome.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
None of the trials reported this outcome.
3. Incidence of admissions to the intensive care unit (ICU)
Mahmoudian 2004 stated that no children required admission to the ICU (high‐quality evidence).
8. Intranasal midazolam and rectal diazepam
One trial (Fişgin 2002), recruiting 45 participants, compared intranasal midazolam and rectal diazepam.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
Intranasal midazolam was significantly more effective than rectal diazepam in stopping seizures within 10 minutes; 20/23 children with stopped seizures in the intranasal midazolam group, compared to 13/22 in the rectal diazepam group: RR 1.47, 95% CI 1.00 to 2.16, P = 0.05, low‐quality evidence, Analysis 8.1.
8.1. Analysis.
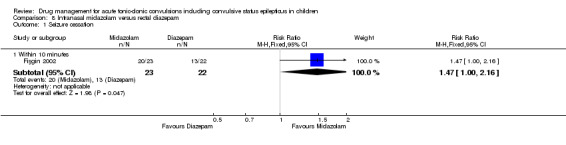
Comparison 8 Intranasal midazolam versus rectal diazepam, Outcome 1 Seizure cessation.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion.
This outcome was not reported in the trial.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
There was no significant difference between the two groups for of cardiorespiratory or adverse effects (low‐quality evidence).
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
The requirement for a second drug to treat the seizures was higher in the rectal diazepam group (9/22 children compared to 3/23 children in the intranasal midazolam group), but this was not statistically significant; RR 0.32, 95% CI 0.10 to 1.03, P = 0.06, low‐quality evidence, Analysis 8.2.
8.2. Analysis.
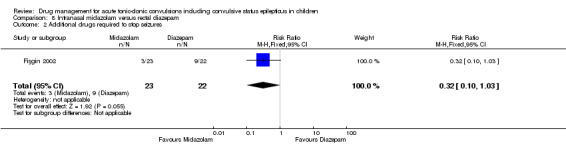
Comparison 8 Intranasal midazolam versus rectal diazepam, Outcome 2 Additional drugs required to stop seizures.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
Data were only collected for one hour after seizure onset, so there is no information about seizure recurrence over 24 hours in Fişgin 2002.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
9. Intramuscular midazolam versus intravenous diazepam
Two trials, recruiting 138 participants, compared intramuscular midazolam to intravenous diazepam (Chamberlain 1997; Shah 2005). Shah 2005 included some non‐randomised participants who received intravenous diazepam as they already had intravenous access; only randomised children are reported in this review. Chamberlain 1997 reported that one child was enrolled in the study twice, so is represented in both groups. It was not possible to identify this child in the reported results so we note that results presented in this section must be interpreted with caution, due to the representation of this child in both treatment groups.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
Both trials reported the number of children with their presenting seizure(s) stopped by the trial drug.There was no statistically significant difference between the treatments; RR 0.97, 95% CI 0.87 to 1.09, P = 0.66, low‐quality evidence, Analysis 9.1. There was no heterogeneity present in the analysis (I2 = 0%).
9.1. Analysis.
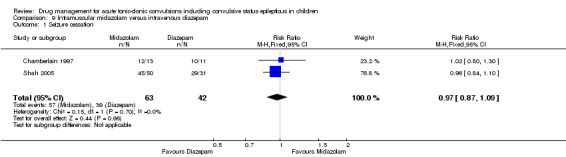
Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 1 Seizure cessation.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
Chamberlain 1997 reported that the time after arrival to initiating treatment was shorter in the intramuscular midazolam group compared to the intravenous diazepam group (mean difference ‐4.50 minutes, 95% CI ‐6.68 to ‐2.32, P < 0.001, 24 children, Analysis 9.2) and that this offsets the time to drug effect of the two treatments (mean difference 1.10 minutes, 95% CI ‐0.91 to 3.11, P = 0.28, Analysis 9.2), resulting in an overall shorter time to cessation of seizures in the intramuscular midazolam group.
9.2. Analysis.
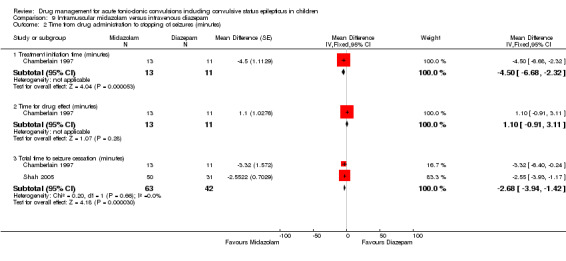
Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures (minutes).
This is demonstrated in both trials, with the mean total cessation time converted from seconds to minutes to allow meta‐analysis; mean difference ‐2.68 minutes, 95% CI ‐3.94 to ‐1.42, P < 0.001, 105 children, very low‐quality evidence, Analysis 9.2
Chamberlain 1997 also reported that delayed seizure control (between five and 10 minutes) occurred in four midazolam participants and one diazepam participant, but this did not reach statistical significance.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐ administered anticonvulsant
Chamberlain 1997 reported that there were no significant complications. Shah 2005 reported that there were no occurrences of hypotension or respiratory depression, but identified an important adverse effect in that seven (10.8%) of the children treated with intravenous diazepam developed thrombophlebitis, while none in the intramuscular midazolam group had complications.
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
Both trials reported the number of children requiring additional drugs to stop their presenting seizure(s).There was no statistically significant difference between the treatments; RR 1.34, 95% CI 0.35 to 5.13, P = 0.67, 105 children, very low‐quality evidence, Analysis 9.3. There was no heterogeneity in the analysis (I2 = 0%).
9.3. Analysis.
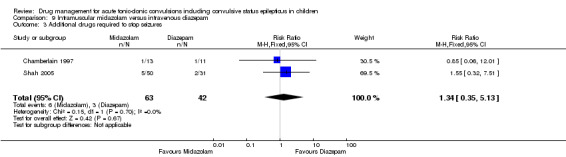
Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 3 Additional drugs required to stop seizures.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
Chamberlain 1997 reported that after initial seizure cessation four participants in each group had recurrent seizures requiring further medication, with one child from each group having a recurrence within the first 15 minutes. There was no statistically significant difference between groups at 15 minutes (RR 0.85, 95% CI 0.06 to 12.01, P = 0.90) or at one hour (RR 0.85, 95% CI 0.27 to 2.62, P = 0.77, very low‐quality evidence, Analysis 9.4). Shah 2005 did not report this outcome.
9.4. Analysis.
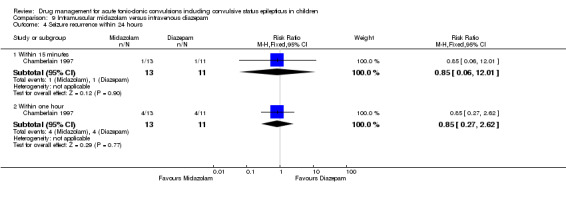
Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 4 Seizure recurrence within 24 hours.
3. Incidence of admissions to the intensive care unit (ICU)
Shah 2005 reported that there were no ICU admissions (moderate‐quality evidence). Chamberlain 1997 did not report this outcome.
10. Intramuscular midazolam versus rectal diazepam
One trial (Momen 2015), recruiting 100 participants, compared intramuscular midazolam to rectal diazepam.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
Presenting convulsions were stopped for most participants (48/50 in the intramuscular midazolam group and 47/50 in the rectal diazepam group) with no significant difference between the treatments: RR 1.02, 95% CI 0.93 to 1.12, P = 0.65, moderate‐quality evidence, Analysis 10.1.
10.1. Analysis.
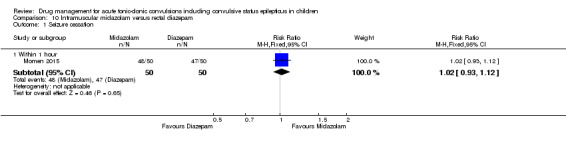
Comparison 10 Intramuscular midazolam versus rectal diazepam, Outcome 1 Seizure cessation.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
Time from administration of drug to seizure cessation was expressed in terms of medians in Momen 2015, so we cannot present data as a forest plot for this review and we report the results narratively.
There was a statistically significant difference in time from administration to seizure cessation in favour of midazolam: median 66 seconds; diazepam: median 130 seconds, P < 0.001 (moderate‐quality evidence). We note that the speed of administration was similar for both medications, so this seems to reflect a medication difference.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
No participants developed respiratory depression, except for one child who received an accidental double‐dose of midazolam (moderate‐quality evidence).
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
This outcome was not reported in the trial.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
Among those who achieved seizure cessation (see Analysis 10.1), there was no recurrence within 60 minutes (moderate‐quality evidence). No data are available for recurrence up to 24 hours.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
11. Intravenous midazolam versus intravenous diazepam
One trial (Gathwala 2012), recruiting 80 participants, compared intravenous midazolam to intravenous diazepam.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
The presenting seizure was stopped in most children, with no statistically significant difference between treatment groups: RR 1.08, 95% CI 0.97 to 1.21, P = 0.17, moderate‐quality evidence, Analysis 11.1.
11.1. Analysis.
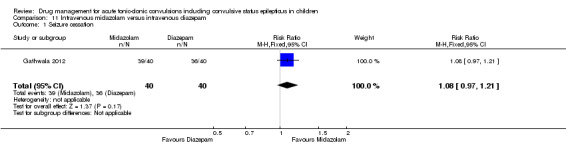
Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 1 Seizure cessation.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
There was no statistically significant difference between treatments in the time to cessation of seizures; mean difference 7.68 seconds, 95% CI ‐6.73 to 22.09, P = 0.30, moderate‐quality evidence, Analysis 11.2.
11.2. Analysis.
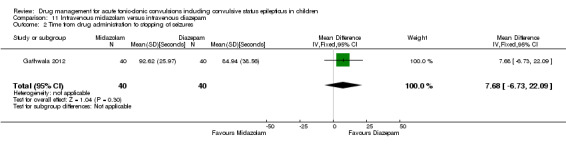
Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
One child in the intravenous diazepam group and no children in the intravenous midazolam group experienced respiratory depression; this difference was not statistically significant: RR 0.33, 95% CI 0.01 to 7.95, P = 0.50, moderate‐quality evidence, Analysis 11.3. Gathwala 2012 also reported that there was a significant increase in somnolence in the diazepam compared to the midazolam groups, but that other adverse effects were evenly distributed.
11.3. Analysis.

Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 3 Incidence of respiratory depression.
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
There was no statistically significant difference between treatments in the number of children requiring an additional dose of the trial drug to stop the seizure (one child in the midazolam group and four children in the diazepam group); RR 0.25, 95% CI 0.03 to 2.14, P = 0.21, moderate‐quality evidence, Analysis 11.4.
11.4. Analysis.
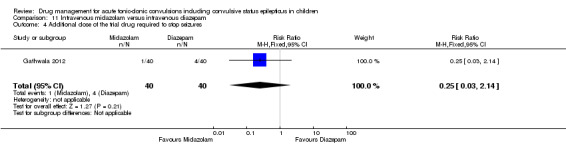
Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 4 Additional dose of the trial drug required to stop seizures.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
There was no statistically significant difference between treatment groups in the number of children with seizure recurrence within 24 hours (two children in the midazolam group and four children in the diazepam group); RR 0.50, 95% CI 0.10 to 2.58, P = 0.41, moderate‐quality evidence, Analysis 11.5.
11.5. Analysis.
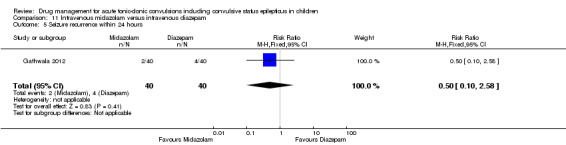
Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 5 Seizure recurrence within 24 hours.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
12. Intravenous midazolam versus intravenous lorazepam
One trial (Gathwala 2012), recruiting 80 participants, compared Intravenous midazolam to intravenous lorazepam.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
The presenting seizure was stopped in most children in the Gathwala 2012 trial; there was no statistically significant difference between treatment groups; RR 0.98, 95% CI 0.91 to 1.04, P = 0.48, moderate‐quality evidence, Analysis 12.1.
12.1. Analysis.
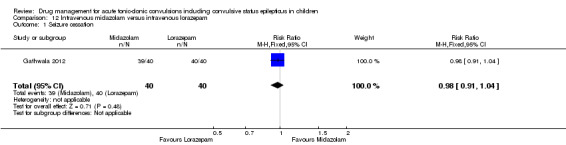
Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 1 Seizure cessation.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
There was no significant difference between treatment groups in the time to cessation of seizures; mean difference 1.50 seconds, 95% CI ‐9.37 to 12.37, P = 0.79, moderate‐quality evidence, Analysis 12.2.
12.2. Analysis.
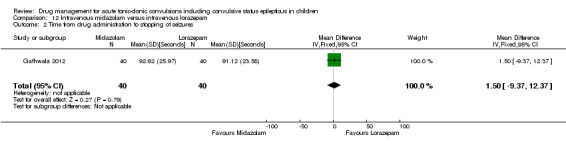
Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 2 Time from drug administration to stopping of seizures.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
There were no occurrences of respiratory depression in either group in the Gathwala 2012 trial. Gathwala 2012 also reported that other adverse effects were evenly distributed between the groups (high‐quality evidence).
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
There was no statistically significant difference between treatment groups in the number of children requiring an additional dose of the trial drug to stop the seizure (one child in the midazolam group and no children in the lorazepam group); RR 3.00, 95% CI 0.13 to 71.51, P = 0.50, moderate‐quality evidence, Analysis 12.3.
12.3. Analysis.
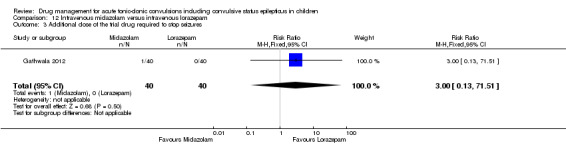
Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 3 Additional dose of the trial drug required to stop seizures.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
There was no statistically significant difference between treatment groups in the number of children with seizure recurrence within 24 hours (two children in each group); RR 1.00, 95% CI 0.15 to 6.76, P = 1.00, moderate‐quality evidence, Analysis 12.4.
12.4. Analysis.
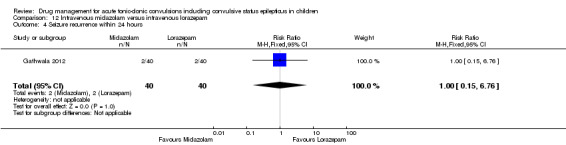
Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 4 Seizure recurrence within 24 hours.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
Discussion
Summary of main results
The 14 newly‐identified studies in this updated review include a range of drug treatment options (midazolam, diazepam, lorazepam, paraldehyde and phenytoin), treatment doses (for the same drug) and a range of routes of administration (rectal, buccal, nasal, intramuscular and intravenous). A number of the new studies have evaluated and emphasised the use of non‐intravenous routes. These have included intranasal, intramuscular and buccal routes. The role of the intramuscular route in children is uncertain, particularly in view of its relatively invasive nature as well as potentially serious complications, including trauma to the sciatic nerve.
The 18 studies included in this review vary by design, setting and populations, in their ages and also in their clinical situation. We have conducted many comparisons of drugs and of routes of administration of drugs in this review, with the quality of the evidence for each comparison varying from high to very low, depending on the homogeneity and quality of design of the studies contributing to the comparison.
This update has shown that for intravenous administration, lorazepam and diazepam seem to be associated with similar rates of seizure cessation (Appleton 1995; Chamberlain 2014; Gathwala 2012), but risks of bias in the included studies and heterogeneity of design may have confounded the results.
Two studies in this update have shown that buccal midazolam may be associated with a higher rate of seizure cessation than rectal diazepam (Ashrafi 2010; Mpimbaza 2008). However, we are very uncertain about the estimate of this effect. In part, this reflects the different range of doses of buccal midazolam used in these studies and the different characteristics of their participants. A single study also provides moderate‐ to high‐quality evidence that buccal midazolam may be associated with a higher rate of seizure cessation than intravenous diazepam (Talukdar 2009). However, as for all studies included within this review, with different routes of administration, time to cessation of seizures was influenced by the way it was delivered.
There are currently insufficient data to determine whether there are any significant or clinically important differences in efficacy or safety between the buccal and intranasal routes of administration of midazolam; this issue will only be resolved by at least one robust RCT that compares buccal to intranasal midazolam. The intranasal route of administration was used in five studies and was compared with rectal or intravenous routes (Ahmad 2006; Arya 2011; Fişgin 2002; Javadzadeh 2012; Mahmoudian 2004). Generally, the intranasal/buccal/intramuscular routes appear to show similar rates of the most common (and most clinically important) primary outcome, seizure cessation, compared to intravenous routes of administration. However, the rapid action of the intravenously‐administered drug is compromised by the time taken to achieve intravenous access. This was particularly demonstrated in three studies (Arya 2011; Shah 2005; Talukdar 2009). This is an important issue, particularly in infants but also in older children who are in shock with circulatory collapse, and where intravenous access is likely to be more difficult and therefore delay effective anticonvulsive treatment.
Adverse side effects were observed very infrequently in the included studies. Respiratory depression was the most common and most clinically relevant side effect. Where reported in the study, the frequency ranged from none (Ashrafi 2010; Chamberlain 1997; Fişgin 2002; Mahmoudian 2004; Shah 2005; Talukdar 2009), to 1% to 2% (Arya 2011), almost 6% (Sreenath 2010) and up to almost 18% (Chamberlain 2014). The latter study defined respiratory depression as ‘assisted ventilation’; the incidence of respiratory depression is considerably higher than in the other studies that reported this outcome. None of the studies individually demonstrated any difference in the rates of respiratory depression between the different anticonvulsants or their different routes of administration; but when pooled, three studies provided moderate‐quality evidence that lorazepam was significantly associated with fewer occurrences of respiratory depression than diazepam (RR 0.72, 95% CI 0.55 to 0.93).
Overall completeness and applicability of evidence
The evidence presented in previous versions of the review has supported previously‐published open, anecdotal data. Buccal midazolam has become established as the first‐line non‐intravenous drug, and intravenous lorazepam has become established as the first‐line intravenous drug in treating an acute tonic‐clonic convulsion (and established convulsive status epilepticus) in children. The evidence has contributed to the evidence base for the Status Epilepticus Working Group to revise the convulsive status epilepticus guideline which was first published in 2000 (Working Party 2000) and has been incorporated into the partially revised and updated National Institute for Health and Care Excellence (NICE) Clinical Guideline in Epilepsy (NICE 2012) and the Advanced Paediatric Life Support (APLS) guidelines (APLS 2016).
Most studies were undertaken in unselected populations of children presenting to the Emergency Department (ED) of a single centre, or a group of centres (between three and 11) based either in a general or a children’s hospital. Consequently, these data are likely to be generalisable and applicable to other children with acute tonic‐clonic convulsions in this clinical situation. However, there were two studies undertaken in a very specific population, of African children, in whom cerebral malaria was the cause of the convulsion in 49% to 67% (Ahmad 2006; Mpimbaza 2008 respectively). The treatment arms in Ahmad 2006 were somewhat unusual, comprising intranasal lorazepam and intramuscular paraldehyde; no other study used these treatments. The authors justified the use of paraldehyde on the basis of it's being the “first or second‐line anticonvulsant agent in much of sub‐Saharan Africa because of its favourable safety and efficacy profile”. Paraldehyde has been used as an anticonvulsant for over 50 years. It is currently used when other anticonvulsants, including benzodiazepines or phenytoin and phenobarbital, have failed to stop an acute tonic–clonic convulsion and is often effective (Rowland 2009). The rectal route is preferred, because of the risk of sterile abscesses and damage to the sciatic nerve with the intramuscular route. Rectal paraldehyde is included in the UK’s APLS algorithm (APLS 2016) for the management of status epilepticus.
Two early studies (Chamberlain 1997; Lahat 2000) used a seizure duration of 10 rather than five minutes as the time to institute emergency treatment; all other studies used five or "at least 5" minutes, which is standard international practice.
The age range of the children in the 18 studies varied between birth and under 18 years. Most assessed children aged two months to 12 or 15 years, although two assessed a much narrower age range from two months to approximately five years (Ahmad 2006; Lahat 2000). Most epidemiological studies have demonstrated that more than 80% of children who present with an acute tonic‐clonic convulsion, including convulsive status epilepticus, are under 10 years of age, and of these most will be under five years of age. In addition, most causes of convulsive status epilepticus in children under five will be febrile status or due to an acute symptomatic cause. Consequently, this might introduce some bias in those studies that assessed only young children.
Quality of the evidence
The extent of the evidence provided by this updated review, both in terms of the 14 new studies (making a total of 18 and comprising 2199 participants) and their scientific robustness, has strengthened its quality and its conclusions. We have consequently achieved some of the objectives of the review.
Much of the evidence provided in this review is of moderate to high quality. However, the quality of the evidence provided for some important outcomes is low to very low, particularly for comparisons of non‐intravenous routes of drug administration. We downgraded the quality of the evidence due to imprecise results where limited data were available for analysis or where confidence intervals of effect sizes were wide, making interpretation of results difficult. Quality of the evidence was also downgraded due to the methodological inadequacies of some studies which may have introduced bias into the results, to study settings which were not applicable to wider clinical practice, and to inconsistency in some pooled analyses.
The dose of lorazepam in all preparations (predominantly intravenous, but also rectal and intranasal) was the same in all six studies where it was a treatment arm (0.1 mg/kg). In contrast, the dose of midazolam (in predominantly buccal but also intranasal preparation) ranged from 0.2 to 0.5 mg/kg, and the dose of either rectal or intravenous diazepam varied from 0.2 to 0.5 mg/kg. The reasons for the wide range of doses are not clear. Previous studies had not suggested that respiratory depression was a significant problem with doses of buccal midazolam of 0.5 mg/kg (McIntyre 2005), and the methodology and findings of this large study would have helped to inform subsequent studies on an effective and safe dose.
One potential bias throughout all the studies is how the original trial authors defined cessation of the seizure or convulsion following the intervention. Observer variation and inconsistency is well recognised when deciding when a tonic‐clonic convulsion has stopped. A proposed definition by Appleton (personal opinion) is that a tonic‐clonic convulsion has stopped when there is “no visible sign of ongoing rhythmic clonic activity”. The included studies' definitions of seizure cessation ranged from no definition to “the practitioner’s clinical judgement” to “generalized convulsions have stopped”, “cessation of all visible convulsive activity”, “cessation of all visible motor seizure activity” or “cessation of all motor activity”. The use of ‘motor activity’ is arguably too vague, as the brief, asymmetric and asynchronous myoclonus that commonly follows a tonic‐clonic seizure may be misinterpreted as “ongoing motor activity”; this is likely to impact on the efficacy result and lead to bias between studies.
Potential biases in the review process
It is unlikely that the methods used in this updated review will have introduced any significant bias. We successfully addressed outstanding queries and resolved them in most cases by personal contact with the leading or corresponding authors of the included studies.
We identified all relevant new studies, as far as we could ascertain. The methodology of most of the new studies was more robust than those included in the first review. However, there was some variation in methodology and the reporting of results between these studies, as detailed earlier in the review. Two of the 18 studies reported 100% seizure cessation in both treatment arms (Mahmoudian 2004; Sreenath 2010), and in one treatment arm (Ashrafi 2010), which is unusual as the median seizure‐cessation rate was approximately 75% in all other studies. In addition, the dose of intravenous diazepam in the two studies that reported 100% seizure cessation in both was the lowest used throughout the included studies (0.2 mg/kg).
Agreements and disagreements with other studies or reviews
This review is in broad general agreement with the recently‐published Evidence‐based guideline on the treatment of convulsive status epilepticus in children and adults, published by the Guideline Committee of the American Epilepsy Society (Glauser 2016).
Our findings are also consistent with a recent meta‐analysis (McMullan 2010) of midazolam versus diazepam in children and young adults which included many of the studies in this review. They also concluded that non‐intravenous midazolam was as effective as intravenous diazepam and that buccal midazolam was superior to rectal diazepam.
The above studies would appear to have been subjected to a similar systematic review process.
Authors' conclusions
Implications for practice.
This updated review provides limited and low‐ or very‐low quality evidence regarding the use of buccal midazolam as the first‐line treatment for an acute tonic‐clonic convulsion and convulsive status epilepticus in children where intravenous access is not available. Limited new data, of moderate to low quality, shows no clear differences between intravenous lorazepam and intravenous diazepam as the first‐line intravenous drug in the management of acute tonic‐clonic convulsions in children. The review provides limited and low‐quality evidence that the intranasal route, using either lorazepam or midazolam, may be an effective alternative non‐intravenous route of administration to stop tonic‐clonic seizures. This is of particular importance in countries with a high incidence of central nervous system infectious diseases, where children often present late and in shock, making it difficult to obtain rapid intravenous access, and where intravenous cannulae and equipment are likely to be in limited supply.
Implications for research.
This review has identified a large number of new randomised clinical trials since 2007. Despite these new data, much was of low quality for important comparisons. Consequently, there is a clear need for additional paediatric randomised controlled trials of the treatment of acute tonic‐clonic convulsions and convulsive status epilepticus. Potential areas for research and specifically for randomised controlled trials include:
Efficacy of commonly‐used first‐line treatments such as lorazepam and midazolam, mode of delivery including data on optimal drug doses, and timing of interventions. The most appropriate randomised control trial would use a factorial design to compare drugs and modes of delivery efficiently.
Role and efficacy of pre‐hospital medications, usually benzodiazepines, administered by parents, carers or paramedical staff.
Efficacy and safety of second‐line treatments, including fosphenytoin, phenobarbital, phenytoin and sodium valproate.
The role of rectal paraldehyde.
The potential efficacy and safety of newer anticonvulsants, including intravenous levetiracetam and lacosamide.
The pre‐hospital treatment of acute tonic‐clonic convulsions is not within the remit of this review. However, it seems appropriate to comment on possible future research initiatives. Traditionally, rectal diazepam has been the preferred pre‐hospital rescue (emergency) medication, but this has now been replaced by buccal midazolam in routine clinical practice in the UK and the rest of Europe. This has been on the basis of midazolam’s perceived similar or slightly superior efficacy to diazepam, and its easier and more acceptable route of administration by carers, school nurses and teaching staff. More recently, pre‐hospital randomised controlled trials have examined the role of intramuscular midazolam administered by paramedics in acute tonic‐clonic convulsions, including status epilepticus. The Rapid Anticonvulsant Medication Prior to Arrival Trial (RAMPART) was a double‐blind randomised, non‐inferiority clinical trial of the efficacy of intramuscular midazolam versus intravenous lorazepam in the pre‐hospital treatment of status epilepticus by paramedics (Silbergleit 2013). A secondary analysis of the RAMPART study undertaken in children aged under 18 (Welch 2015) showed no statistically significant difference between the two treatment arms in achieving the study’s primary outcome, namely seizure cessation prior to arrival in the emergency department. Although intramuscular midazolam might become the preferred pre‐hospital, first‐line emergency medication by paramedic staff (as intravenous access may be difficult), this route is unlikely to be adopted by carers, school nurses and teachers who administer most pre‐hospital rescue medications. Nevertheless, it would be interesting to undertake an RCT of intramuscular midazolam and buccal midazolam amongst paramedics.
What's new
| Date | Event | Description |
|---|---|---|
| 23 May 2017 | New search has been performed | Searches updated 23 May 2017. |
| 23 May 2017 | New citation required and conclusions have changed | We have added 14 new studies in this update. The authors' conclusions have changed to suggest that buccal midazolam is more effective than rectal diazepam, with a very low risk of adverse events. Intravenous benzodiazepines lead to more rapid seizure cessation but time taken to establish IV access undermines this effect. The findings of the previous review (intravenous lorazepam is at least as effective as intravenous diazepam and is associated with fewer adverse events) are supported by this update. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 21 October 2008 | Amended | Search strategy amended to comply with RevMan 5 format. |
| 8 May 2008 | New search has been performed | We re‐ran our searches on 1st July 2007 and found three new studies (Ahmad 2006;Lahat 2000; McIntyre 2005) with 381 participants so there are now four included studies with a total of 483 participants ‐ all hospital based. |
| 7 May 2008 | New citation required and conclusions have changed | Substantive amendment |
| 7 May 2008 | Amended | Converted to new review format. |
Acknowledgements
Ms Anita Aindow, Pharmacy Department, Alder Hey Children's Hospital, Liverpool, UK.
Appendices
Appendix 1. Cochrane Epilepsy Group Specialised Register search strategy
#1 MeSH DESCRIPTOR Child Explode All
#2 MeSH DESCRIPTOR Infant Explode All
#3 paediatr* or pediatr* or child* or infant*
#4 #1 OR #2 OR #3
#5 emergency or emergencies or acute
#6 #4 AND #5
#7 >17/10/2013:CRSCREATED
#8 #6 AND #7
Appendix 2. CENTRAL via CRSO search strategy
#1 MESH DESCRIPTOR Epilepsy, Tonic‐Clonic EXPLODE ALL TREES
#2 (tonic ADJ2 clonic):TI,AB,KY
#3 MESH DESCRIPTOR Status Epilepticus EXPLODE ALL TREES
#4 (status ADJ2 epilepti*):TI,AB,KY
#5 MESH DESCRIPTOR Seizures EXPLODE ALL TREES
#6 (epilep* and (seizure* or convuls*)):TI,AB,KY
#7 #1 OR #2 OR #3 OR #4 OR #5 OR #6
#8 MESH DESCRIPTOR Pediatric Emergency Medicine EXPLODE ALL TREES
#9 MESH DESCRIPTOR Child EXPLODE ALL TREES
#10 MESH DESCRIPTOR Infant EXPLODE ALL TREES
#11 (paediatr* or pediatr* or child* or infant*):TI,AB,KY
#12 #9 OR #10 OR #11
#13 (emergency or emergencies or acute):TI,AB,KY
#14 #12 AND #13
#15 #8 OR #14
#16 #7 AND #15
#17 * NOT INMEDLINE AND 30/09/2013 TO 30/06/2017:DL
#18 #16 AND #17
Appendix 3. MEDLINE search strategy
This strategy is based on the Cochrane Highly Sensitive Search Strategy for identifying randomized trials (Lefebvre 2011).
1. exp Epilepsy, Tonic‐Clonic/
2. (tonic adj2 clonic).tw.
3. exp Status Epilepticus/
4. (status adj2 epilepti$).tw.
5. exp Seizures/
6. (epilep$ and (seizure$ or convuls$)).tw.
7. 1 or 2 or 3 or 4 or 5 or 6
8. exp Pediatric Emergency Medicine/
9. exp child/ or exp infant/
10. (paediatr$ or pediatr$ or child$ or infant$).tw.
11. 9 or 10
12. (emergency or emergencies or acute).tw.
13. 11 and 12
14. 8 or 13
15. 7 and 14
16. (randomized controlled trial or controlled clinical trial or pragmatic clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
17. clinical trials as topic.sh.
18. trial.ti.
19. exp Random Allocation/
20. exp Double‐Blind Method/
21. exp Single‐Blind Method/
22. exp Clinical Trial/
23. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
24. (clin$ adj2 (study or studies or trial?)).tw.
25. ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).tw.
26. (control$ adj2 (study or studies or trial?)).tw.
27. exp cross‐over studies/
28. (cross?over adj2 (analy$ or method or procedure or study or studies or trial?)).tw.
29. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28
30. (animals not human).sh.
31. 29 not 30
32. 15 and 31
33. remove duplicates from 32
34. limit 33 to ed=20131017‐20170523
35. 33 not (1$ or 2$).ed.
36. 35 and (2013$ or 2014$ or 2015$ or 2016$ or 2017$).dc.
37. 34 or 36
Appendix 4. ClinicalTrials.gov search strategy
acute OR emergency OR emergencies | tonic‐clonic convulsions OR status epilepticus | drug | Child
Appendix 5. ICTRP search strategy
Condition: tonic‐clonic convulsions OR status epilepticus
Intervention: drug
Clinical trials in children
Recruitment status: all
Data and analyses
Comparison 1. Lorazepam versus diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure cessation | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.98, 1.20] |
| 1.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.16] |
| 1.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [1.47, 5.55] |
| 2 Time from drug administration to stopping of seizures | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Intravenous | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 6.18 [‐7.83, 20.19] |
| 3 Incidence of respiratory depression | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.55, 0.93] |
| 3.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.55, 0.92] |
| 3.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.04, 20.78] |
| 4 Additional dose of the trial drug required to stop seizures | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.20] |
| 4.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.71, 1.33] |
| 4.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.56] |
| 5 Additional drugs required to stop seizures | 2 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.45, 1.24] |
| 5.1 Intravenous | 2 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.55] |
| 5.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.69] |
| 6 Seizure recurrence within 24 hours | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.20] |
| 6.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.65, 1.27] |
| 6.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 2.92] |
| 7 Incidence of admissions to the intensive care unit (ICU) | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 0.98] |
| 7.1 Intravenous | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.22] |
| 7.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.03, 10.51] |
Comparison 2. Intranasal lorazepam versus intramuscular paraldehyde.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 10 minutes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.99, 1.52] |
| 2 Additional drugs required to stop seizures | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.81] |
| 3 Seizure recurrence within 24 hours | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.31, 1.71] |
Comparison 3. Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 10 minutes | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.98, 1.02] |
| 2 Incidence of respiratory depression | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.22, 2.82] |
| 3 Additional drugs required to stop seizures | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 More than one dose of the trial drug required | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.04] |
Comparison 4. Intravenous lorazepam versus intranasal lorazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 10 minutes | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.77, 1.49] |
| 1.2 Within 1 hour | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.43, 1.17] |
Comparison 5. Buccal midazolam versus rectal diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure cessation | 4 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.13, 1.38] |
| 1.1 Within 5 minutes | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.07, 1.40] |
| 1.2 Within 10 minutes | 2 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.95, 1.21] |
| 1.3 Within one hour | 1 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.45, 2.91] |
| 2 Incidence of respiratory depression | 4 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.25] |
| 3 Additional drugs required to stop seizures | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Intravenous lorazepam required | 1 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.42, 0.79] |
Comparison 6. Buccal midazolam versus intravenous diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure cessation within five minutes | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 2 Time from drug administration to stopping of seizures | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Treatment initiation time (minutes) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐1.31, ‐0.87] |
| 2.2 Time for drug effect (minutes) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.29, 0.83] |
| 2.3 Total time to seizure cessation (minutes) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐0.96, ‐0.22] |
Comparison 7. Intranasal midazolam versus intravenous diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure Cessation | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| 2 Time from drug administration to stopping of seizures [minutes] | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Treatment initiation time (minutes) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐3.03, ‐0.97] |
| 2.2 Time for drug effect (minutes) | 2 | 122 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [‐0.14, 1.38] |
| 2.3 Total time to seizure cessation (minutes) | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.24, 1.35] |
Comparison 8. Intranasal midazolam versus rectal diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 10 minutes | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.00, 2.16] |
| 2 Additional drugs required to stop seizures | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.10, 1.03] |
Comparison 9. Intramuscular midazolam versus intravenous diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure cessation | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.87, 1.09] |
| 2 Time from drug administration to stopping of seizures (minutes) | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 Treatment initiation time (minutes) | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐4.5 [‐6.68, ‐2.32] |
| 2.2 Time for drug effect (minutes) | 1 | 24 | Mean Difference (Fixed, 95% CI) | 1.1 [‐0.91, 3.11] |
| 2.3 Total time to seizure cessation (minutes) | 2 | 105 | Mean Difference (Fixed, 95% CI) | ‐2.68 [‐3.94, ‐1.42] |
| 3 Additional drugs required to stop seizures | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.35, 5.13] |
| 4 Seizure recurrence within 24 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Within 15 minutes | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
| 4.2 Within one hour | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.27, 2.62] |
Comparison 10. Intramuscular midazolam versus rectal diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.12] |
Comparison 11. Intravenous midazolam versus intravenous diazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure cessation | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.97, 1.21] |
| 2 Time from drug administration to stopping of seizures | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 7.68 [‐6.73, 22.09] |
| 3 Incidence of respiratory depression | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 4 Additional dose of the trial drug required to stop seizures | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.14] |
| 5 Seizure recurrence within 24 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.58] |
Comparison 12. Intravenous midazolam versus intravenous lorazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Seizure cessation | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.04] |
| 2 Time from drug administration to stopping of seizures | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐9.37, 12.37] |
| 3 Additional dose of the trial drug required to stop seizures | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |
| 4 Seizure recurrence within 24 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.76] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmad 2006.
| Methods | Randomised controlled trial carried out over 12 months in Malawi | |
| Participants | 160 children of both sexes and aged 2 months to 12 years presenting to a paediatric emergency department in a generalised seizure. Exclusion criteria: features of hepatic or hypertensive encephalopathy or organophosphate poisoning, children who had received an anticonvulsant within 1 hour of presentation | |
| Interventions | Intranasal lorazepam versus intramuscular paraldehyde | |
| Outcomes | Seizure cessation Incidence of cardiorespiratory depression Need for further anti‐convulsant/s | |
| Notes | Study conducted in Africa with a high proportion of children with either cerebral malaria or meningitis. Consequently, not readily generalisable to western populations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Blocked randomisation was done in advance by a computer that randomly generated a table of numbers in batches of ten" Comment: adequate randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: " treatment allocations were sealed in unmarked identical envelopes. Investigators were masked to these allocations before the point of patient treatment. Quote: adequate concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study unblinded, but blinding would have been difficult due to the different routes of administration of the 2 study drugs. This is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the final analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | High risk | A high proportion of the children recruited had either cerebral malaria or meningitis. These comorbidities may have impacted upon the results |
Appleton 1995.
| Methods | Quasi‐randomised controlled trial (odd and even days randomisation of the 2 drugs) over a 12‐month study period | |
| Participants | 102 children of both sexes and aged < 16 years presenting to a single Accident and Emergency department in a tonic‐clonic convulsion including established convulsive status epilepticus. Participants treated included those with an established diagnosis of epilepsy, febrile convulsions and those presenting with a first convulsion. Exclusion criteria: known pseudo‐tonic‐clonic convulsions or pseudo‐convulsive, absence or complex partial status | |
| Interventions | Lorazepam versus diazepam: rectal and intravenous administration. Diazepam dose: 0.3 to 0.4 mg/kg and lorazepam dose: 0.05 to 0.1 mg/kg. These doses were used for both intravenous and rectal routes of administration | |
| Outcomes | Seizure cessation Seizure recurrence within 24 hours after the presenting seizure had been stopped Additional drugs needed to control the presenting seizure Adverse effects | |
| Notes | Numerous protocol violators in the study who were then excluded from analysis. The study population was small and there were substantial differences in the size of the 2 treatment groups (lorazepam 33 participants and diazepam 53 participants). There was an even larger discrepancy in the children who received the drug rectally; rectal lorazepam (6 children) versus rectal diazepam (19 children) This clearly suggests a higher violation rate for these children who should have received rectal lorazepam. This may have been due to clinician uncertainty about the use of rectal lorazepam, as this drug and route of administration are not used in routine clinical practice |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: " children were assigned..on an odd and even dates basis" Comment: this was done to avoid any delay incurred by another randomisation method. The randomisation method may have contributed to the unequal sizes of the groups |
| Allocation concealment (selection bias) | High risk | As described above, clinicians would be aware of the allocation by whether the day was odd or even |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study was unblinded, but this would have been impractical and is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were a relatively large number of protocol violators (16/102 children, or 16% of the total study population) and these violators were excluded from the analyses. The analysis was therefore not an intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in the Results section |
| Other bias | High risk | Large discrepancy in the 2 routes of administration used in the study, probably due to clinician uncertainty about the use of rectal lorazepam. This discrepancy is likely to have impacted upon results |
Arya 2011.
| Methods | Randomised controlled trial, not blinded | |
| Participants | 141 children aged 6 ‐ 14 years attending the emergency room of a hospital in New Delhi, India with a seizure, or those having a seizure during attendance
Exclusion criteria: known hypersensitivity to benzodiazepine, child having received any parenteral anti‐epileptic drug within 1 hour of enrolment, presence of severe cardiorespiratory compromise, presence of cerebrospinal fluid rhinorrhoea and upper respiratory tract infection sufficiently severe to preclude intranasal administration 58 out of 141 of the children (41%) had generalised tonic‐clonic seizures but primary outcome results are presented separately for the subgroup of generalised tonic‐clonic seizures |
|
| Interventions | Intranasal versus intravenous lorazepam | |
| Outcomes | Cessation of all visible motor activity by 10 minutes Persistent cessation of seizures by 1 hour Time to achieve IV access, time from drug administration to stopping of seizure Development of hypotension/respiratory depression | |
| Notes | Results are presented for the subgroup of 58 children with generalised tonic‐clonic seizures Inclusion criteria did not include duration of seizure, unlike most of the studies There was 1 protocol violation when intravenous access could not be obtained in 1 child who was randomised to intravenous lorazepam. This child was treated with intranasal lorazepam. However the results were analysed on an intention‐to‐treat basis and no participants were excluded from the analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:" randomisation was done using blocks of variable length" |
| Allocation concealment (selection bias) | Low risk | Quote: "Opaque sealed envelopes containing allocation of randomisation" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study was unblinded; this would have been difficult, due to the different routes of administration and is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All recruited participants were included in the analysis and analysed on an intention to treat basis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in the Results section |
| Other bias | Low risk | None identified |
Ashrafi 2010.
| Methods | Randomised controlled trial, not blinded and no placebo | |
| Participants | 98 children of both sexes and aged 3 months to 12 years attending the emergency department of two large paediatric hospitals in Tehran, Iran between April 2007 and April 2008. Children who already had intravenous access or who were younger than 3 months were excluded | |
| Interventions | Buccal midazolam versus rectal diazepam | |
| Outcomes | Cessation of all motor activity within 5 minutes, without respiratory depression and without seizure recurrence Treatment initiation time (time spent preparing the drug) and drug effect time (time from drug administration to seizure cessation) also recorded Parental satisfaction assessed | |
| Notes | Buccal midazolam associated with 100% seizure cessation rate, which is higher than expected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A random number table was used for randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess this |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study was unblinded; blinding would have been difficult due to the different routes used, but this is unlikely to have had a significant impact on the results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All recruited participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in the Results section |
| Other bias | Unclear risk | Buccal midazolam associated with 100% seizure cessation rate, which is higher than expected. Unclear whether this high success rate was due to a particular element of the trial design |
Baysun 2005.
| Methods | Prospective quasi‐randomised trial (odd and even days randomisation of the 2 drugs) in 1 centre | |
| Participants | 43 children of both sexes aged 2 months to 12 years who presented with a seizure to the emergency room, regardless of seizure type, aetiology or duration No exclusion criteria were stated | |
| Interventions | Buccal midazolam versus rectal diazepam | |
| Outcomes | Cessation of convulsive seizure activity within 10 minutes Time to seizure cessation Need for a second drug to control seizures Presence of adverse events | |
| Notes | Children who were seizing on arrival were included, on the assumption that the seizure was prolonged. This is different from most of the other studies, which require a period of seizure activity lasting 5 ‐ 10 minutes before inclusion and randomisation. However, this should not have introduced bias, as these children should have been equally distributed between the 2 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Diazepam was given on odd days of the month and midazolam on the even days" Comment: inadequate randomisation |
| Allocation concealment (selection bias) | High risk | See above; no concealment of allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study unblinded, but blinding would not have been possible, due to the different routes of administration of the 2 study drugs, so this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All recruited participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | Low risk | None identified |
Chamberlain 1997.
| Methods | Prospective randomised study in two centres | |
| Participants | 23 children of both sexes and aged birth to 18 years presenting to an emergency department with a motor seizure lasting at least 10 minutes Children who had established intravenous access or who had already received treatment for this seizure episode were excluded. | |
| Interventions | Intramuscular midazolam versus intravenous diazepam | |
| Outcomes | Seizure cessation within 5 minutes of drug administration Delayed seizure control defined as cessation of seizures 5 ‐ 10 minutes after drug administration Treatment failure, defined as lack of seizure cessation at 10 minutes Early recurrence, defined as return of seizures within 5 minutes Recurrence, defined as return of seizures within 60 minutes of drug administration Presence of respiratory depression | |
| Notes | 1 child was enrolled in the study twice, so is represented in both groups. It was not possible to identify this child in the reported results There was also a protocol violator who was randomised to receive intravenous diazepam but received intramuscular midazolam after 25 minutes, due to unsuccessful intravenous access. This participant was excluded from the analysis and would have skewed the results significantly if he/she had been included. It may have been helpful to know the response time of this child once treatment was administered, as this is an important example of the disadvantages of the intravenous route |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly selected by computer" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess this |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding would not have been possible, due to the different routes of administration of the 2 study drugs, but this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote "Three children were randomised to receive diazepam but were excluded because their seizures did not persist for 10 minutes." Comment: this is unlikely to have made a significant difference to the analysis Quote "One child was a protocol deviation and was excluded‐ was randomised to diazepam but received midazolam instead due to unsuccessful attempts at IV access" Comment: this child should have been included in the analysis for it to be considered an intention‐to‐treat analysis. However it would have skewed the results significantly, as midazolam was not given until after 25 minutes of attempting intravenous access |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | High risk | 1 child was enrolled in the study twice, so is represented in both groups. It was not possible to identify this child in the reported results. Due to the small numbers of children included in the study, this double‐enrolment may have impacted on the results |
Chamberlain 2014.
| Methods | Double‐blind multicentre randomised trial | |
| Participants | 273 patients aged 3 months up to 18 years presenting with convulsive status epilepticus | |
| Interventions | intravenous diazepam versus intravenous lorazepam | |
| Outcomes | Primary outcomes: Cessation of status epilepticus by 10 minutes without recurrence within 30 minutes Requirement for assisted ventilation Secondary outcomes: Rates of seizure recurrence Presence of sedation Times to cessation of status epilepticus Return to baseline mental status | |
| Notes | Consideration was given to sample size with an estimate of 120 participants per group for 80% power to detect a significant difference between treatments. After an interim analysis halfway through the study, this was increased to 131 participants per group, probably because there was less treatment effect difference than anticipated between the treatment arms. Analysis of data was transparent, with all participants who were randomised analysed on an intention‐to‐treat basis but with further per protocol analysis limited to those with no protocol violation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation (1:1) with stratification to 3 age groups was performed |
| Allocation concealment (selection bias) | Low risk | Measures taken to ensure allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who were randomised were analysed on an intention to treat basis. An additional per protocol analysis limited to those with <1 no protocol violation |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | Low risk | None identified |
Fişgin 2002.
| Methods | Prospective quasi‐randomised study (odd and even days randomisation of the 2 drugs) over 15 months | |
| Participants | 45 children of both sexes and aged 1 month to 13 years presenting to the emergency room with a seizure lasting at least 5 minutes No exclusion criteria stated | |
| Interventions | intranasal midazolam versus rectal diazepam | |
| Outcomes | Stopping of seizure within 10 minutes Time to cessation of seizure Efficacy of anticonvulsant effect Need for a second drug to control seizures Presence of complications | |
| Notes | Some methodology described unclear, particularly relating to seizure type and aetiology of included children. It is therefore unclear if the population of this study is generalisable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Diazepam was given on odd days of the month and midazolam on the even days" |
| Allocation concealment (selection bias) | High risk | See above; no concealment of allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study unblinded, but blinding would not have been possible, due to the different routes of administration of the 2 study drugs, but this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Quote: "information about previous convulsions and history of antiepileptic medication was obtained.." Comment: this information was not reported in the Results section but as this is not one of the primary outcome measures it is not likely to be significant |
| Other bias | Unclear risk | Unclear description of the seizure type and aetiology of included children, so it is unclear if the population of this study is generalisable |
Gathwala 2012.
| Methods | Randomised controlled trial, unblinded | |
| Participants | 120 children aged 6 months to 14 years, attending emergency room with an acute seizure | |
| Interventions | Intravenous diazepam versus midazolam versus lorazepam | |
| Outcomes | Time to seizure cessation Side effects of drugs: vomiting, apnoea, somnolence, respiratory depression and requirement for mechanical ventilation Number of participants with seizure recurrence, requiring a second dose of medication or with uncontrolled seizures Time to seizure recurrence | |
| Notes | Unclear exactly when participants were given second dose of drug (range 5 ‐ 20 minutes); the convention would be to wait 10 minutes.
Large number with prolonged seizures
The differences in underlying causes may affect applicability to western populations Seizure cessation is defined as "Cessation of visible epileptic phenomenon or return of purposeful response to external stimuli within 15 minutes of drug administration". This definition is different from all other included studies and latter part of this definition is not an appropriate criterion for judging seizure cessation, as most individuals following a tonic‐clonic seizure will have a post‐ictal phase in which they do not respond to external stimuli |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by shuffling of envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation by sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study was unblinded, but this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The 3 excluded participants where IV access was not possible were not included in the analysis. However, as all routes were intravenous this is unlikely to have introduced bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | High risk | The definition of the 'Seizure Cessation' outcome used is different from all other included studies and is not an appropriate criterion for judging seizure cessation. This definition is likely to have impacted upon results. |
Javadzadeh 2012.
| Methods | Randomised unblinded study | |
| Participants | 60 children aged 2 months to 15 years old presenting to emergency department with acute seizure episode | |
| Interventions | Intranasal midazolam versus intravenous diazepam | |
| Outcomes | Time needed to control seizure Oxygen saturation and heart rate before and after treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study was unblinded, but this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis and analysed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | High risk | Number of children with seizure cessation not reported; we would expect this outcome to be reported |
| Other bias | Low risk | None identified |
Lahat 2000.
| Methods | 12‐month randomised controlled trial | |
| Participants | 44 children of both sexes and aged 6 months to 5 years presenting to a paediatric emergency department with a febrile seizure Children with established intravenous lines or those who had received anticonvulsants before admission were excluded. | |
| Interventions | Intravenous diazepam versus intranasal midazolam | |
| Outcomes | Seizure cessation Time to seizure cessation Incidence of cardiorespiratory distress | |
| Notes | In addition this study evaluated a specific subgroup of children with prolonged convulsive febrile seizures. This is important, as the aetiology of seizures varies across the age ranges during childhood, thereby potentially affecting results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed in advance with a random number table by a hospital pharmacist not involved in the study" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "and treatment allocations were sealed in opaque envelopes. Investigators were blind to these allocations. Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study was unblinded‐;blinding would have been difficult, due to the different routes of administration. This is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were available for all participants enrolled in the study |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes were reported in the Results section. In the Methods section, seizure cessation was defined as 'successful' if seizures stopped in < 5 minutes, 'successful but delayed' if seizures stopped after 5 ‐ 10 minutes and 'failure' if seizures had not stopped after 10 minutes. However, results seem to be presented only in terms of treatment success and failure. It is unclear if this is selective reporting of results |
| Other bias | Low risk | None identified |
Mahmoudian 2004.
| Methods | Prospective randomised study in 1 centre | |
| Participants | 70 children of both sexes and aged 2 months to 15 years presenting with an acute seizure to the emergency department. Children who had received anticonvulsants before admission were excluded | |
| Interventions | intranasal midazolam versus intravenous diazepam | |
| Outcomes | Time from drug treatment to seizure cessation (Treatment successful if seizures stopped within 10 minutes) | |
| Notes | Both treatment arms showed a 100% seizure cessation rate, which is higher than expected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed in advance with an odd and even number table" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "treatment allocations were sealed in opaque envelopes" Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study unblinded, but blinding would not have been possible, due to the different routes of administration of the 2 study drugs, but this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis |
| Selective reporting (reporting bias) | High risk | Time taken to insert intravenous cannula in the intravenous diazepam group should have been included, as this would have a significant effect on the time from arrival to seizure cessation. Other studies comparing intravenous with other routes have included this information |
| Other bias | Unclear risk | Both treatment arms showed a 100% seizure cessation rate, which is higher than expected. Unclear whether this high success rate was due to a particular element of the trial design |
McIntyre 2005.
| Methods | Multicentre randomised controlled trial over 3 years 4 months. Randomisation of 2 drugs in weekly blocks | |
| Participants | 177 children of both sexes aged 6 months to 16 years presenting to a children's accident and emergency department with active generalised tonic‐clonic seizures including established convulsive status epilepticus. Children with partial seizures or non‐convulsive status epilepticus were excluded. |
|
| Interventions | Buccal midazolam versus rectal diazepam | |
| Outcomes | Seizure cessation without recurrence within 1 hour and without respiratory depression | |
| Notes | 219 convulsive episodes were recorded in the 177 children. Some results are reported only as the number of episodes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " weekly blocks of treatment were randomly selected for each of the four centres. The randomisation sequence was generated ...from a table of random numbers" Comment: probably done |
| Allocation concealment (selection bias) | High risk | Quote: "Allocation was not concealed from attending staff" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study unblinded, but blinding would not have been possible, due to the different routes of administration of the 2 study drugs. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "46 episodes were excluded". 46 episodes were screened for eligibility but did not meet criteria; all participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | Unclear risk | None identified |
Momen 2015.
| Methods | Unblinded randomised trial | |
| Participants | 100 children with convulsive status epilepticus aged 1 month to 16 years | |
| Interventions | Intramuscular midazolam versus rectal diazepam | |
| Outcomes | Seizure cessation after drug administration without recurrence within 60 minutes Respiratory rate and blood pressure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study was unblinded, but this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis and analysed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | Unclear risk | None identified |
Mpimbaza 2008.
| Methods | Placebo‐controlled single‐blinded randomised study in 1 centre | |
| Participants | 330 children of both sexes and aged 3 months to 12 years who presented while convulsing or experienced a seizure lasting > 5 minutes to an emergency department in Uganda. Note 67.3% of children had malaria and 13.7% had cerebral malaria Children aged less than 3 months or more than 12 years, who had evidence of prior treatment or whose convulsion stopped prior to treatment were excluded | |
| Interventions | Buccal midazolam versus rectal diazepam | |
| Outcomes | Cessation of visible seizure activity within 10 minutes, without recurrence in the subsequent hour Convulsion lasting > 10 minutes or recurring within 1 hour, defined as treatment failures Time to cessation of convulsions Seizure recurrence in first hour or within subsequent 24 hours, time to seizure recurrence Presence of respiratory depression | |
| Notes | Study conducted in Africa with a high proportion of children with either cerebral malaria or meningitis. Consequently, not readily generalisable to western populations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer was used to generate a list of sequential random treatment codes" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "each treatment code ... placed in a opaque envelope, sealed. Investigators were not aware of a patient's treatment allocation" Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote; "Study drugs and placebo were pre‐packaged by a pharmacist not involved with patient care. " Comment: probably done Quote: "Although the study team were not aware which treatment a patient received they were aware of the treatment code, therefore we considered this single‐blinded" Comment: blinding probably adequate as each participant received placebo and study drug |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data analysed on an intention to treat basis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | High risk | A high proportion of the children recruited had either cerebral malaria or meningitis. These co‐morbidities may have impacted upon the results. |
Shah 2005.
| Methods | Partly‐randomised prospective trial in a single centre over 1 year | |
| Participants | 115 children of both sexes aged 1 month to 12 years either presenting to the emergency department with acute convulsions or who developed acute seizures on the ward or PICU Those who had already had treatment for the seizure were excluded. | |
| Interventions | intramuscular midazolam versus intravenous diazepam | |
| Outcomes | Mean time from administration of drug to cessation of seizures Adverse events such as thrombophlebitis | |
| Notes | Not all participants were randomised; only those who were randomised are included in the results of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients who already had an intravenous access present were treated with intravenous diazepam.. patients without an intravenous access were randomised into 2 groups" Comment: randomisation is inadequate, as treatment determined by presence of IV access which may introduce bias (patients not randomised are not included in the review) Method of randomisation of those without an IV access is unclear |
| Allocation concealment (selection bias) | High risk | No information about whether allocation in those without an IV access was concealed. Allocation definitely not concealed in those with an intravenous access |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study unblinded, but blinding would not have been possible due to the different routes of administration of the 2 study drugs, but this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | Low risk | None identified |
Sreenath 2010.
| Methods | Randomised prospective trial in a single centre | |
| Participants | 178 children of both sexes aged 1 ‐ 12 years presenting with convulsive status epilepticus (continuous convulsive activity for 5 minutes or more). Exclusion criteria were treatment with any anti‐epileptic medication in preceding 4 weeks, acute head trauma, history of poisoning and jaundice, suspected renal failure or diarrhoea presenting with seizures | |
| Interventions | intravenous lorazepam versus intravenous diazepam‐phenytoin combination | |
| Outcomes | Cessation of seizure activity within 10 minutes and no recurrence over the subsequent 18 hours Time to seizure cessation Number of doses of study drug required to stop convulsions Use of additional anti‐epileptic drugs Total number of seizures in first 18 hours following administration of study drug Presence of respiratory depression Requirement for PICU transfer for mechanical ventilation Requirement to cross over to alternative regimen due to ongoing seizures | |
| Notes | One child received lorazepam despite being randomised to diazepam‐phenytoin. This led to a difference in the number of participants in each group The study protocol states that where access could not be obtained, rectal lorazepam or diazepam would be used instead. The number of participants receiving rectal drugs should have been included in the paper,‐but was clarified through personal communication with the author who informed us that all drugs were given intravenously Both treatment arms showed a 100% seizure cessation rate, which is higher than expected |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple randomisation was done using a computer generated random number table" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was by sealed envelope technique" Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study was unblinded but this is unlikely to have had a significant impact on the results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis. One received lorazepam despite being randomised to diazepam‐phenytoin, i.e. was a protocol violation. Data were analysed on intention‐to‐treat basis. This is unlikely to have had a significant impact on the overall findings of the study |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | Unclear risk | Both treatment arms showed a 100% seizure cessation rate, which is higher than expected. Unclear whether this high success rate was due to a particular element of the trial design |
Talukdar 2009.
| Methods | Prospective randomised trial in a single centre | |
| Participants | 120 children of both sexes aged 0 ‐ 12 years (mean age 3.2 years) presenting with an episode of convulsion, irrespective of cause and duration. Those patients with myoclonic, absence and atonic seizures were excluded | |
| Interventions | Buccal midazolam versus intravenous diazepam | |
| Outcomes | Cessation of all motor activity within or by 5 minutes of administration of the drug Treatment initiation time (time from noting seizure to drug administration), drug effect time (time from drug administration to effect) and total controlling time, a combination of the previous two | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was done using the random number table" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess this |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study unblinded, but blinding would not have been possible due to the different routes of administration of the 2 study drugs; this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | Low risk | None identified |
IV: intravenous PICU: paediatric intensive care unit RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agarwal 2007 | Only 38 of 100 participants in this study were under 16 years of age. In addition, this study examined the treatment of benzodiazepine‐refractory status epilepticus, whereas we are concerned with the treatment of children presenting with acute convulsive status epilepticus |
| Arpita 2014 | This study examined the management of refractory not acute status epilepticus |
| Bhattacharyya 2006 | Most seizures were simple partial seizures as opposed to generalised tonic‐clonic seizures. Study also included children with absence, myoclonic and atonic seizures |
| Camfield 1980 | The study examined drug management for the long‐term prevention of recurring febrile seizures, rather than management of acute convulsions |
| Cereghino 1998 | This study examined diazepam treatment for clusters of seizures rather than acute convulsions |
| Heckmatt 1976 | The study examined drug management for the long‐term prevention of recurring febrile seizures, rather than management of acute convulsions |
| Holsti 2010 | This study compared intranasal midazolam and rectal diazepam for the treatment of seizures at home, not in a hospital‐based setting, so did not meet our inclusion criteria |
| Kutlu 2003 | This was a study of the use of buccal midazolam for acute seizures in children, but without any comparison or placebo group |
| Mahmoudian 2006 | This study examined the treatment of children already treated with IV diazepam, phenytoin and phenobarbital and whose seizures had lasted at least 60 minutes. The comparison was between rectal sodium valproate and intravenous midazolam. We excluded this study as it was examining the treatment of refractory status epilepticus |
| McCormick 1999 | This study was a prospective comparison of intravenous midazolam and lorazepam in 27 paediatric patients. However this was only published in abstract form as conference proceedings, so there was insufficient information on which to base assessment of the trial. Attempts to contact the authors were unsuccessful |
| Mehta 2007 | This study included children with refractory status epilepticus who were initially treated with intravenous diazepam and 2 doses of intravenous phenytoin, then randomised to either IV SVA or diazepam infusion. We excluded this study as it was examining the management of refractory not acute status epilepticus |
| Mittal 2014 | This study examined the management of refractory not acute status epilepticus |
| Morton 2007 | This was a study of the use of intravenous valproate for acute seizures in children, but without any comparison or placebo group |
| Qureshi 2002 | This was excluded as it was a retrospective audit of practice, comparing two different time periods when different seizure protocols were used. It did not meet our inclusion criteria of being a randomised, quasi‐randomised or controlled study |
| Rosati 2016 | This study examined the management of refractory not acute status epilepticus |
| Scott 1999 | Quasi randomised study of rectal diazepam and buccal midazolam in treating 79 seizure episodes in 18 patients with severe and refractory epilepsy in a residential institution. The study does not make clear how many of the 11 paediatric patients had experienced a tonic‐clonic and not a complex partial or myoclonic seizure when treated with diazepam or midazolam. Only 11 of the 18 patients were aged 16 years or under |
| Silbergleit 2012 | This double‐blind, randomised study compared intramuscular midazolam with intravenous lorazepam for the pre‐hospital treatment of status epilepticus in children and adults. As the study did not take place in a hospital setting it did not meet our inclusion criteria. |
| Singhi 2002 | This study compared continuous midazolam or diazepam infusion in patients with refractory status epilepticus, defined as motor seizures uncontrolled after two doses of diazepam and a phenytoin infusion. We excluded this study as it concerned the management of refractory not acute status epilepticus |
| Strengell 2009 | The study examined drug management for the long‐term prevention of recurring febrile seizures, rather than management of acute convulsions. |
| Tonekaboni 2012 | Less than 70% of participants had generalised tonic‐clonic seizures. We contacted the authors to request subgroup data but these were not supplied |
Differences between protocol and review
The protocol was published in 2000 (Appleton 2000), and the review was last updated in 2008 (Appleton 2008). Therefore changes have been made to the format and content of the methods and the review from the protocol and from the last update to this version of the review, in line with current MECIR standards (MECIR 2012) and the Cochrane Style Manual.
The original protocol specified that we would include non‐randomised studies and adult studies (including adolescents between the ages of 12 and 16), as we anticipated that we would find few randomised paediatric studies. The number of published studies in this research field has greatly increased since publication of the protocol, so for this update we revised the inclusion criteria to cover only randomised paediatric studies. These criteria were changed before we ran the updated searches and before starting the review update.
Contributions of authors
Amy McTague undertook the literature review, performed the data extraction and wrote the review. Richard Appleton undertook the literature review, reviewed the included and excluded studies and commented on the draft review. Tim Martland commented on the draft review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
This review update was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Richard Appleton is the lead investigator of the study included in the original review and is a co‐author of another study included in this update. Tim Martland is a co‐author of one of the studies included in this review. Amy McTague has no known declarations of interests.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ahmad 2006 {published data only}
- Ahmad S, Ellis JC, Kamwendo H, Molyneux E. Efficacy and safety of intranasal lorazepam versus intramuscular paraldehyde for protracted convulsions in children: an open randomised trial. Lancet 2006;367(9522):1591‐7. [DOI] [PubMed] [Google Scholar]
- NCT00116064. Intranasal lorazepam versus intramuscular paraldehyde in paediatric convulsions. ClinicalTrials.gov/show/NCT00116064 (first received July 2004).
Appleton 1995 {published data only}
- Appleton RE, Sweeney A, Choonara I, Robson J, Molyneux E. Lorazepam versus diazepam in the acute treatment of epileptic seizures and status epilepticus. Developmental Medicine and Child Neurology 1995;37:682‐8. [DOI] [PubMed] [Google Scholar]
Arya 2011 {published data only}
- Arya R, Gulati S, Kabra M, Sahu J, Kalra V. Intranasal versus intravenous lorazepam for control of acute seizures in children: a randomized open‐label study. Epilepsia 2011;52(4):788‐93. [DOI] [PubMed] [Google Scholar]
Ashrafi 2010 {published data only}
- Ashrafi M, Khosroshahi N, Karimi P, Malamiri R, Bavarian B, Zarch A, et al. Efficacy and usability of buccal midazolam in controlling acute prolonged convulsive seizures in children. European Journal of Paediatric Neurology 2010;14(5):434‐8. [DOI] [PubMed] [Google Scholar]
Baysun 2005 {published data only}
- Baysun S, Aydin OF, Atmaca E, Gurer Y. A comparison of buccal midazolam and rectal diazepam for the acute treatment of seizures. Clinical Pediatrics 2005;44(9):771‐6. [DOI] [PubMed] [Google Scholar]
Chamberlain 1997 {published and unpublished data}
Chamberlain 2014 {published data only}
- Chamberlain JM, Okada P, Holsti M, Mahajan P, Brown KM, Vance C, et al. Lorazepam vs diazepam for pediatric status epilepticus: a randomized clinical trial. JAMA 2014;311(16):1652‐60. [DOI] [PubMed] [Google Scholar]
Fişgin 2002 {published data only}
- Fişgin T, Gurer Y, Teziç T, Senbil N, Zorlu P, Okuyaz C, et al. Effects of intranasal midazolam and rectal diazepam on acute convulsions in children: prospective randomized study. Journal of Child Neurology 2002;17(2):123‐6. [DOI] [PubMed] [Google Scholar]
Gathwala 2012 {published data only}
- Gathwala G, Goel M, Singh J, Mittal K. Intravenous diazepam, midazolam and lorazepam in acute seizure control. Indian Journal of Pediatrics 2012;79(3):327‐32. [DOI] [PubMed] [Google Scholar]
Javadzadeh 2012 {published data only}
- Javadzadeh M, Sheibani K, Hashemieh M, Saneifard H. Intranasal midazolam compared with intravenous diazepam in patients suffering from acute seizure: a randomized clinical trial. Iranian Journal of Pediatrics 2012;22(1):1‐8. [PMC free article] [PubMed] [Google Scholar]
Lahat 2000 {published data only}
- Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ 2000;321(7253):83‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahmoudian 2004 {published data only}
- Mahmoudian T, Mohammadi Zadeh M. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy and Behavior 2004;5:253‐5. [DOI] [PubMed] [Google Scholar]
McIntyre 2005 {published data only}
- Appleton RE, McIntyre JW, Choonara IA, Whitehouse WP, Robertson S, Norris E. Randomised controlled trial of buccal midazolam versus rectal diazepam for the emergency treatment of seizures in children. Epilepsia 2004;45(Suppl 7):186, Abstract no: B.02. [DOI] [PubMed] [Google Scholar]
- McIntyre J, Robertson S, Norris E, Appleton RE, Whitehouse WP, Phillips B, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet 2005;366(9481):205‐10. [DOI] [PubMed] [Google Scholar]
Momen 2015 {published data only}
- Momen AA, Azizi Malamiri R, Nikkhah A, Jafari M, Fayezi A, Riahi K, Maraghi E. Efficacy and safety of intramuscular midazolam versus rectal diazepam in controlling status epilepticus in children.. European Journal of Paediatric Neurology: EJPN 2015;19(2):149‐54. [DOI] [PubMed] [Google Scholar]
Mpimbaza 2008 {published data only}
- Mpimbaza A, Ndeezi G, Staedke S, Rosenthal PJ, Byarugaba J. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Ugandan children: a randomized clinical trial. Pediatrics 2008;121(1):e58‐64. [PUBMED: 18166545] [DOI] [PubMed] [Google Scholar]
Shah 2005 {published data only}
Sreenath 2010 {published and unpublished data}
- Sreenath TG, Gupta P, Sharma KK, Krishnamurthy S. Lorazepam versus diazepam‐phenytoin combination in the treatment of convulsive status epilepticus in children: a randomized controlled trial. European Journal of Paediatric Neurology 2010;14(2):162‐8. [DOI] [PubMed] [Google Scholar]
Talukdar 2009 {published data only}
- Talukdar B, Chakrabarty B. Efficacy of buccal midazolam compared to intravenous diazepam in controlling convulsions in children: a randomised controlled trial. Brain and Development 2009;31(10):744‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agarwal 2007 {published data only}
- Agarwal P, Kumar N, Chandra R, Gupta G, Antony AR, Garg N. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure 2007;16(6):527‐32. [DOI] [PubMed] [Google Scholar]
Arpita 2014 {published data only}
- Arpita A, Chandrakanta, Kumar R, Singh SN. Efficacy of intravenous valproate versus intravenous phenytoin in children with status epilepticus: a randomized controlled trial in tertiary care centre. Pediatric Critical Care Medicine 2014;15(4 Suppl 1):11, Abstract no: 31. [DOI: ] [Google Scholar]
Bhattacharyya 2006 {published data only}
- Bhattacharyya M, Kalra V, Gulati S. Intranasal midazolam vs rectal diazepam in acute childhood seizures. Pediatric Neurology 2006;34(5):355‐9. [DOI] [PubMed] [Google Scholar]
Camfield 1980 {published data only}
- Camfield PR, Camfield CS, Shapiro SH, Cummings C. The first febrile seizure‐‐antipyretic instruction plus either phenobarbital or placebo to prevent recurrence. Journal of Pediatrics 1980;97(1):16‐21. [DOI] [PubMed] [Google Scholar]
Cereghino 1998 {published data only}
- Cereghino JJ, Mitchell WG, Murphy J, Kriel RL, Rosenfeld WE, Trevathan E. Treating repetitive seizures with a rectal diazepam formulation: a randomized study. The North American Diastat Study Group. Neurology 1998;51(5):1274‐82. [DOI] [PubMed] [Google Scholar]
Heckmatt 1976 {published data only}
- Heckmatt JZ, Houston AB, Clow DJ, Strephenson JB, Dodd KL, Lealman GT, et al. Failure of phenobarbitone to prevent febrile convulsions. British Medical Journal 1976;1(6009):559‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holsti 2010 {published data only}
- Holsti M, Dudley N, Schunk J, Adelgais K, Greenberg R, Olsen C, et al. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in pediatric patients with epilepsy. Archives of Pediatrics and Adolescent Medicine 2010;164(8):747‐53. [DOI] [PubMed] [Google Scholar]
Kutlu 2003 {published data only}
- Kutlu NO, Dogrul M, Yakinci C, Soylu H. Buccal midazolam for treatment of prolonged seizures. Brain and Development 2003;25(4):275‐8. [DOI] [PubMed] [Google Scholar]
Mahmoudian 2006 {published data only}
- Mahmoudian T, Najafian M. Comparing the effects of intravenous midazolam with rectal sodium valproate in controlling of children with refractory status epilepticus. Journal of Research in Medical Science 2006;11(1):1‐5. [Google Scholar]
McCormick 1999 {published data only}
- McCormick EM, Lieh‐Lai M, Knazik S, Nigro M. A prospective comparison of midazolam and lorazepam in the initial treatment of status epilepticus in the pediatric patient. Epilepsia 1999; Vol. 40 Suppl 7:160.
Mehta 2007 {published data only}
- Mehta V, Singhi P, Singhi S. Intravenous sodium valproate versus diazepam for the control of refractory status epilepticus in children: a randomised controlled trial. Journal of Child Neurology 2007;22(10):1191‐7. [DOI] [PubMed] [Google Scholar]
Mittal 2014 {published data only}
- Mittal K, Gupta A. Efficacy & safety: Intravenous sodium valproate and phenytoin in status epilepticus. Critical Care Medicine 2014;42(12 Suppl 1):A1486‐A7. [DOI: 10.1097/01.ccm.0000458023.53658.51] [DOI] [Google Scholar]
Morton 2007 {published data only}
Qureshi 2002 {published data only}
- Qureshi A, Wassmer E, Davies P, Berry K, Whitehouse W. Comparative audit of intravenous lorazepam and diazepam in the emergency treatment of convulsive status epilepticus in children. Seizure 2002;11(3):141‐4. [DOI] [PubMed] [Google Scholar]
Rosati 2016 {published data only}
- Rosati A, Ilvento L, L'Erario M, Masi S, Biggeri A, Fabbro G, et al. Efficacy of ketamine in refractory convulsive status epilepticus in children: a protocol for a sequential design, multicentre, randomised, controlled, open‐label, non‐profit trial (KETASER01). BMJ Open 2016;6(6):e011565. [DOI: 10.1136/bmjopen-2016-011565.; PUBMED: 27311915] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 1999 {published data only}
- Scott RC, Besag FM, Neville BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet 1999;353(9153):623‐6. [DOI] [PubMed] [Google Scholar]
Silbergleit 2012 {published data only}
- Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. New England Journal of Medicine 2012;366(7):591‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singhi 2002 {published data only}
- Singhi S, Murthy A, Singhi P, Jayashree M. Continuous midazolam versus diazepam for refractory convulsive status epilepticus. Journal of Child Neurology 2002;17(2):106‐10. [DOI] [PubMed] [Google Scholar]
Strengell 2009 {published data only}
- Strengell T, Uhari M, Tarkka R, Uusimaa J, Alen R, Lautala P, et al. Antipyretic agents for preventing recurrences of febrile seizures: randomized controlled trial. Archives of Pediatrics & Adolescent Medicine 2009;163(9):799‐804. [DOI] [PubMed] [Google Scholar]
Tonekaboni 2012 {published data only (unpublished sought but not used)}
- Tonekaboni SH, Shamsabadi FM, Anvari SS, Mazrooei A, Ghofrani M. A comparison of buccal midazolam and intravenous diazepam for the acute treatment of seizures in children.. Iranian Journal of Pediatrics 2012;22.(3):303‐8. [PMC free article] [PubMed] [Google Scholar]
Additional references
APLS 2016
- Advanced Life Support Group. Advanced Paediatric Life Support (APLS): A Practical Approach to Emergencies. 6th Edition. New Jersey (USA): Wiley‐Blackwell Publishing, 2016. [Google Scholar]
Chin 2008
- Chin R, Neville B, Peckham C, Wade A, Bedford H, Scott R. Treatment of community‐onset, childhood convulsive status epilepticus: a prospective, population‐based study. Lancet. Neurology 2008;7(8):696‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garr 1999
- Garr RE, Appleton RE, Robson WJ, Molyneux EM. Children presenting with convulsions (including status epilepticus) to a paediatric accident and emergency department: an audit of a treatment protocol. Developmental Medicine and Child Neurology 1999;41(1):44‐7. [DOI] [PubMed] [Google Scholar]
Glauser 2016
- Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence‐based guideline: treatment of convulsive status epilepticus in children and adults: Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Currents 2016;16(1):48‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEPro 2004 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEPro Version 3.6 for Windows. GRADE Working Group, 2004.
Higgins 2011a
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
McMullan 2010
- McMullan J, Sasson C, Pancioli A, Silbergleit R. Midazolam versus diazepam for the treatment of status epilepticus in children and young adults: a meta‐analysis. Academic Emergency Medicine 2010;17(6):575‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
MECIR 2012
- Methodological Expectations of Cochrane Intervention Reviews (MECIR): Standards for the conduct and reporting of new Cochrane Intervention Reviews 2012. editorial‐unit.cochrane.org.
NICE 2012
- National Institute for Health and Care Excellence. Epilepsies: diagnosis and management. Available from www.nice.org.uk/guidance/cg137 Accessed 1st July 2017.
Rowland 2009
- Rowland AG, Gill AM, Stewart AB, Appleton RE, Al Kharusi A, Cramp C, et al. Review of the efficacy of rectal paraldehyde in the management of acute and prolonged tonic–clonic convulsions. Archives of Disease in Childhood 2009;94(9):720‐3. [DOI] [PubMed] [Google Scholar]
Shinnar 2001
- Shinnar S, Berg AT. How long do new‐onset seizures in children last?. Annals of Neurology 2001;49(5):659‐64. [PubMed] [Google Scholar]
Silbergleit 2013
- Silbergleit R, Lowenstein D, Durkalski V, Conwit R, NETT Investigators. Lessons from the RAMPART study‐‐and which is the best route of administration of benzodiazepines in status epilepticus.. Epilepsia. 2013;54(6):74‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trinka 2015
- Trinka E, Cock H, Hesdorffer D. A definition and classification of status epilepticus – Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015;56(10):1515‐23. [DOI] [PubMed] [Google Scholar]
Welch 2015
- Welch RD, Nicholas K, Durkalski‐Mauldin VL, Lowenstein DH, Conwit R, Mahajan PV, et al. Neurological Emergencies Treatment Trials (NETT) Network Investigators. Intramuscular midazolam versus intravenous lorazepam for the prehospital treatment of status epilepticus in the pediatric population.. Epilepsia 2015;56(2):254‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Working Party 2000
- Appleton R, Choonara I, Martland T, Phillips B, Scott R, Whitehouse W, The Status Epilepticus Working Party. The treatment of convulsive status epilepticus in children. Archives of Disease in Childhood 2000;83(5):415‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Appleton 2000
- Appleton RE, Martland T, Phillips B. Drug management for acute tonic‐clonic convulsions including convulsive status epilepticus in children (Protocol). Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD001905] [DOI] [PMC free article] [PubMed] [Google Scholar]
Appleton 2007
- Appleton R, Martland T, Phillips B. Drug management for acute tonic‐clonic convulsions including convulsive status epilepticus in children. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001905] [DOI] [PMC free article] [PubMed] [Google Scholar]
Appleton 2008
- Appleton R, Macleod S, Martland T. Drug management for acute tonic‐clonic convulsions including convulsive status epilepticus in children. Cochrane Database of Systematic Reviews 2008, Issue 7. [DOI: 10.1002/14651858.CD001905.pub2] [DOI] [PubMed] [Google Scholar]


