Abstract
Background
Available evidence has been inconclusive on whether pulmonary artery perfusion during cardiopulmonary bypass (CPB) is associated with decreased or increased mortality, pulmonary events, and serious adverse events (SAEs) after open heart surgery. To our knowledge, no previous systematic reviews have included meta‐analyses of these interventions.
Objectives
To assess the benefits and harms of single‐shot or continuous pulmonary artery perfusion with blood (oxygenated or deoxygenated) or a preservation solution compared with no perfusion during cardiopulmonary bypass (CPB) in terms of mortality, pulmonary events, serious adverse events (SAEs), and increased inflammatory markers for adult surgical patients.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Science Citation Index Expanded, and advanced Google for relevant studies. We handsearched retrieved study reports and scanned citations of included studies and relevant reviews to ensure that no relevant trials were missed. We searched for ongoing trials and unpublished trials in the World Health Organization International Clinical Trials Registry Platform (ICTRP) and at clinicaltrials.gov (4 July 2017). We contacted medicinal firms producing preservation solutions to retrieve additional studies conducted to examine relevant interventions.
Selection criteria
We included randomized controlled trials (RCTs) that compared pulmonary artery perfusion versus no perfusion during CPB in adult patients (≧ 18 years).
Data collection and analysis
Two independent review authors extracted data, conducted fixed‐effect and random‐effects meta‐analyses, and calculated risk ratios (RRs) or odds ratios (ORs) for dichotomous outcomes. For continuous data, we have presented mean differences (MDs) and 95% confidence intervals (CIs) as estimates of the intervention effect. To minimize the risk of systematic error, we assessed risk of bias of included trials. To reduce the risk of random errors caused by sparse data and repetitive updating of cumulative meta‐analyses, we applied Trial Sequential Analyses (TSAs). We used GRADE principles to assess the quality of evidence.
Main results
We included in this review four RCTs (210 participants) reporting relevant outcomes. Investigators randomly assigned participants to pulmonary artery perfusion with blood versus no perfusion during CPB. Only one trial included the pulmonary artery perfusion intervention with a preservation solution; therefore we did not perform meta‐analysis. Likewise, only one trial reported patient‐specific data for the outcome "pulmonary events"; therefore we have provided no results from meta‐analysis. Instead, review authors added two explorative secondary outcomes for this version of the review: the ratio of partial pressure of oxygen in arterial blood (PaO2) to fraction of inspired oxygen (FiO2); and intubation time. Last, review authors found no comparable data for the secondary outcome inflammatory markers.
The effect of pulmonary artery perfusion on all‐cause mortality was uncertain (Peto OR 1.78, 95% CI 0.43 to 7.40; TSA adjusted CI 0.01 to 493; 4 studies, 210 participants; GRADE: very low quality). Sensitivity analysis of one trial with overall low risk of bias (except for blinding of personnel during the surgical procedure) yielded no evidence of a difference for mortality (Peto OR 1.65, 95% CI 0.27 to 10.15; 1 study, 60 participants). The TSA calculated required information size was not reached and the futility boundaries did not cross; thus this analysis cannot refute a 100% increase in mortality.
The effect of pulmonary artery perfusion with blood on SAEs was likewise uncertain (RR 1.12, 95% CI 0.66 to 1.89; 3 studies, 180 participants; GRADE: very low quality). Data show an association between pulmonary artery perfusion with blood during CPB and a higher postoperative PaO2/FiO2 ratio (MD 27.80, 95% CI 5.67 to 49.93; 3 studies, 119 participants; TSA adjusted CI 5.67 to 49.93; GRADE: very low quality), although TSA could not confirm or refute a 10% increase in the PaO2/FiO2 ratio, as the required information size was not reached.
Authors' conclusions
The effects of pulmonary artery perfusion with blood during cardiopulmonary bypass (CPB) are uncertain owing to the small numbers of participants included in meta‐analyses. Risks of death and serious adverse events may be higher with pulmonary artery perfusion with blood during CPB, and robust evidence for any beneficial effects is lacking. Future randomized controlled trials (RCTs) should provide long‐term follow‐up and patient stratification by preoperative lung function and other documented risk factors for mortality. One study that is awaiting classification (epub abstract with preliminary results) may change the results of this review when full study details have been published.
Plain language summary
The effects of perfusing the pulmonary circuit during open heart surgery in adults
Review question
During open heart surgery, the heart‐lung machine temporarily takes over the function of the heart and lungs. During extracorporeal circulation (ECC), only the systemic circuit is perfused with oxygenated blood with no blood supply to the lungs. This systematic review assesses the beneficial and harmful effects of additional perfusion of the pulmonary circuit with blood or a preservation solution compared with no blood supply to the lungs during ECC in adults undergoing open heart surgery. We report numbers of deaths, serious adverse events, and pulmonary events (for this version of the review, mechanical ventilation and oxygenation after surgery).
Background
Pulmonary complications are often seen after open heart surgery with ECC when insufficient perfusion of the lungs leads to reduced tissue oxygenation. Previous trials have led to different conclusions on whether additional perfusion of the pulmonary circuit during ECC may decrease or increase risks of death, serious adverse events, and pulmonary events. This systematic review follows the Cochrane method for systematic reviews to access evidence from randomized controlled trials (RCTs).
We identified four RCTs (210 participants) reporting on risk of death and mechanical ventilation time. Three trials reported on serious adverse events and oxygenation after surgery. All trials were conducted without direct industry funding. The number of participants in each trial ranged from 30 to 89. The mean age of participants was 59 years (range 37 to 70 years), and 65% were women. Types of surgery included coronary artery bypass graft and valve replacement surgery. Only one trial included the intervention pulmonary perfusion with a preservation solution. Therefore, in this version of the review, we report only results of the intervention pulmonary perfusion with blood compared with no perfusion during ECC.
Key results
Pulmonary perfusion with blood during cardiopulmonary bypass was not associated with increased risk of death nor with decreased serious adverse events and mechanical ventilation time. Trial results do not prove that a higher oxygenation value after surgery was beneficial or harmful for pulmonary perfusion with blood during ECC.
Quality and quantity of the evidence
Only one of the included trials had low risk of bias (except for blinding of personnel during the surgical procedure). Trials randomly assigned 210 participants, and the number of participants required to detect or reject a 100% risk ratio reduction in deaths was not reached; therefore observed results are uncertain. Overall the quality of evidence is low.
Summary of findings
Background
Pulmonary dysfunction is a common complication of open heart surgery with cardiopulmonary bypass (CPB). The causes are multi‐factorial, but prevailing theories comprise lung ischaemia and activation of the inflammatory system mainly as a consequence of CPB. Lung ischaemia occurs as a result of insufficient blood supply to the pulmonary circuit during aortic cross‐clamping, leaving the lungs without ventilation or blood perfusion. During this time, the only oxygen supply to the lungs is received from the bronchial arteries, amounting to only 10% to 15% of the normal blood supply to the pulmonary circuit (Massoudy 2001). Activation of the inflammatory system occurs as a result of tissue ischaemia and continuous contact between circulating blood and artificial surfaces of the heart‐lung machine. This leads to a cellular and humoral immunity‐mediated inflammatory response together with endotoxin release from the splanchnic area (Apostolakis 2010; McNicol 1999). Activation of the inflammatory system is most evident after aortic cross‐clamp release, during which reperfusion of ischaemic lungs causes an interleukin‐6 (IL‐6)‐driven inflammatory response shown by an increased IL‐6 level in the right atrium compared with the pulmonary veins, indicating that ischaemic lungs could be the site of IL‐6 production (Massoudy 2001). Pulmonary inflammatory activation results in endothelial leakage seen histologically as alveolar septal thickening and decreased alveolar surface area, leading to abnormal gas exchange with compromised oxygenation (Schlensak 2002).
Pulmonary artery perfusion during CPB has been shown to improve postoperative oxygenation and lung compliance, to reduce the inflammatory response, and to positively influence haemodynamics (e.g. a higher cardiac index both during and after surgery) (Li 2010; Massoudy 2000; Santini 2011; Sievers 2002). For clinical outcomes with potential economic benefits, pulmonary artery perfusion has been shown to reduce intubation time and thereby intensive care unit (ICU) length of stay (ICU‐LOS) (Li 2010). To perfuse the lung circuit during CPB, one or two extra cannulations (pulmonal artery and vein) are needed. Adverse events most commonly associated with cannulation of blood vessels include bleeding, dissection, and tearing (Kirklin/Barratt‐Boyes 2012), although investigators have reported no complications related to the pulmonary artery perfusion procedure (Santini 2011).
Description of the condition
Reduced postoperative oxygenation is observed in all patients following open heart surgery. Severity varies from a short need for supplemental oxygen therapy or mechanical ventilation (< three hours) to fever, productive cough, pulmonary oedema, pneumonia, respiratory failure, and, in the most severe cases, acute respiratory distress syndrome (ARDS) with a mortality rate of approximately 50% (Apostolakis 2010; Myles 2005; Schlensak 2002) ‐ all prolonging ICU‐LOS. The severity of the postoperative pulmonary dysfunction and associated increased mortality are known to be related to patients' preoperative lung function (Adabag 2010; Ried 2010; Roques 1999). Patients with reduced lung function (both restrictive and obstructive pulmonary disease) therefore face a multitude of problems both during and after open heart surgery.
Description of the intervention
Pulmonary artery perfusion is administered during aortic cross‐clamping when natural lung perfusion pathways are clamped and ventilation is ceased. It is administered as a single shot or continuously until clamp release, at which time the natural perfusion of the lung circuit is re‐established. To perfuse the lung circuit during CPB, two extra cannulations are needed. Adverse events most commonly associated with cannulation of blood vessels are bleeding, dissection, and tearing (Kirklin/Barratt‐Boyes 2012), although investigators have reported no complications related to the specific pulmonary artery perfusion procedure performed (Santini 2011). For perfusion with autologous blood or a preservation solution, an extra inflow cannula is placed in the pulmonary artery along with an outflow cannula in the left atrium to ensure a bloodless operating field during surgery. With autologous blood, perfusion is administered continuously from an extra circuit in the heart‐lung machine (an arterial and venous pump) and the preservation solution is administered once during primary cardioplegia. In the control group, researchers performed standard CPB without simultaneous pulmonary artery perfusion (Buggeskov 2013).
How the intervention might work
Five clinical trials (three randomized and two prospective studies) have indicated preserved postoperative oxygenation by less pitch in the ratio of partial pressure of oxygen in arterial blood (PaO2) to fraction of inspired oxygen (FiO2) (PaO2/FiO2 ratio) or less fall in the oxygenation index (OI) (where OI = FiO2 × mean airway pressure (Mpaw)/PaO2) when compared with baseline measurements. Investigators have also reported a decrease in immunological mediators and a positive influence on haemodynamics during CPB as a result of pulmonary artery perfusion with blood (Massoudy 2000; Santini 2011; Sievers 2002) or with a preservation solution (Li 2010; Wei 2004).
Why it is important to do this review
Currently, no systematic review has compared pulmonary artery perfusion with no intervention for prevention of pulmonary dysfunction after open heart surgery. Therefore we aim to provide a systematic evaluation of the evidence for pulmonary artery perfusion with blood or with a preservation solution for primary prevention of pulmonary dysfunction after open heart surgery. In particular, this review will indicate which interventions ought to be investigated thoroughly in future larger randomized trials with low risk of bias. From an economic perspective, interventions to prevent postoperative pulmonary complications may help to reduce the financial burden on the healthcare system by reducing the associated need for longer hospitalization.
Objectives
To assess the benefits and harms of single‐shot or continuous pulmonary artery perfusion with blood (oxygenated or deoxygenated) or a preservation solution compared with no perfusion during cardiopulmonary bypass (CPB) in terms of mortality, pulmonary events, serious adverse events (SAEs), and increased inflammatory markers for adult surgical patients.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) including adults irrespective of blinding, language, sample size, or publication status. We excluded RCTs including children (< 18 years old).
We also excluded controlled clinical trials (CCTs) that used quasi‐randomization methods (day of the week, date of birth, medical record number, etc.) and other non‐randomized studies (including observational studies) in both adults and children as identified through the search for RCTs.
Types of participants
We included trials with adults of all ethnicities undergoing open heart surgery with CPB that compared the relevant intervention with no intervention.
Types of interventions
We considered trials for inclusion when investigators allocated at least one study group to receive pulmonary artery perfusion during CPB (irrespective of perfusate, dose, temperature, or pharmacological class of administered fluid or drug(s)) in accordance with a standard (within trial) protocol for lung perfusion during open heart surgery. This protocol included the following interventions (single or multiple per trial) separated into two comparisons.
Pulmonary artery perfusion with oxygenated or deoxygenated blood compared with no perfusion during CPB.
Pulmonary artery perfusion with a preservation solution compared with no perfusion during CPB.
Types of outcome measures
Primary outcomes
All‐cause mortality (at maximum follow‐up)
Pulmonary events: numbers of participants with reduced postoperative oxygenation values (specified as a decrease in the PaO2/FiO2 ratio, or an increase in the OI), prolonged mechanical ventilation (> three hours), pneumonia, and/or ARDS
Secondary outcomes
Numbers of participants with one or more SAEs during the index admission (e.g. patient‐ or clinician‐reported adverse effects, morbidity). The International Conference on Harmonization Expert Working Group (ICH) defines an adverse event as any untoward medical occurrence that does not have a causal relationship to a specific treatment and can include any unfavourable and unintended signs (e.g. abnormal laboratory findings), symptoms, or diseases temporarily associated with use of the respective medicinal product being assessed, regardless of whether the event is related to this medicinal product (ICH‐GCP 1997). The ICH describes an SAE as any adverse event that results in death; is life threatening; requires hospitalization or prolongation of existing hospitalization; or results in persistent or significant disability or incapacity; or any medical event that may jeopardize the patient or require an intervention to prevent it (ICH‐GCP 1997). As we already report mortality in the primary outcome, the secondary outcome "Number of patients with one or more SAEs" does not include death, although this differs from the ICH definition
Change in inflammatory markers in plasma or bronchoalveolar lavage fluid
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for relevant studies: Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 6), MEDLINE (from 1946 to July 2017), Embase (from 1974 to 2017, week 27), and Science Citation Index Expanded (Web of Science; from 1900 to 4 July 2017), using a combination of subject terms and text words.
These sites are reported to yield approximately 95% or more of relevant studies sought by the search (Royle 2003). We have provided search strategies in Appendix 1, together with time spans of all searches.
We identified duplicate publications by comparing publications of the same authors with respect to study populations, dates, locations, and follow‐up times.
We imposed no language restrictions.
Searching other resources
We handsearched the study reports retrieved and scanned the citations of included studies and relevant reviews to ensure that relevant trials were not missed. We searched for ongoing trials and unpublished trials in the World Health Organization International Clinical Trials Registry Platform (ICTRP) and at clinicaltrials.gov (4 July 2017). We contacted medicinal firms producing preservation solutions to retrieve additional studies with relevant interventions.
Data collection and analysis
Selection of studies
Two review authors (KBB and LG) independently reviewed titles and abstracts to select potentially relevant trials. We assessed the full texts of provisionally included trials to determine whether they met inclusion criteria. We resolved differences in opinion by discussion and if necessary consulted with a third review author (JW). We listed all excluded studies together with reasons for their exclusion.
Data extraction and management
We performed this systematic review in accordance with instructions provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We performed analyses using the Cochrane software Review Manager (RevMan) Version 5 (RevMan 5.3) and Trial Sequential Analysis software Version 0.9 downloaded from http://www.ctu.dk/tsa/. We have presented results by following the recommended approach detailed in the Cochrane Handbook for Systematic Reviews of Interventions.
Two review authors (KBB and LGR) independently extracted data as listed in the data extraction form (see Appendix 2) from identified RCTs (although this list was not yet exhaustive). In the case of a discrepancy, we consulted a third review author (JW). We contacted the authors of individual trials to request unclear or missing information.
Assessment of risk of bias in included studies
Trials with inadequate bias control increase the risk that beneficial intervention effects may be overestimated (Gluud 2006; Savović 2012). Therefore we (KBB and LG) independently assessed the risk of bias of included trials by following the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We did not mask the trial name. We contacted study authors to request unavailable information that we deemed necessary.
We assessed the domains listed below to determine the extent of systematic error and the risk of bias for each trial (Kjaergard 2001; Moher 1998; Schulz 1995; Wood 2008). We have presented this information in the Characteristics of included studies table.
Random sequence generation
Low risk of bias: Investigators achieved sequence generation by using computer random number generation or a random numbers table. Drawing lots, tossing a coin, shuffling cards, and throwing dice are adequate if performed by an independent adjudicator.
Unclear risk of bias: Investigators described the trial as randomized but did not specify the method of sequence generation.
High risk of bias: Investigators described a sequence generation method that was not, or may not be, random. We excluded quasi‐randomized studies that used dates, names, or admittance numbers to allocate participants for assessment of benefits but not for assessment of harms.
Allocation concealment
Low risk of bias: Investigators used a central and independent randomization unit ‐ opaque and sealed envelopes ‐ or similar methods and did not reveal intervention allocations in advance of or during enrolment.
Unclear risk of bias: Investigators described the trial as randomized but did not describe the method used to conceal the allocation when intervention allocation could have been revealed in advance of or during enrolment.
High risk of bias: Investigators who assigned participants knew the allocation sequence, or the study was quasi‐randomized. We excluded quasi‐randomized studies for assessment of benefits but not for assessment of harms.
Blinding of participants and personnel
Low risk of bias: The report describes that both participants and personnel were blinded (and describes the method of blinding) with knowledge of allocation adequately prevented during the trial.
Unclear risk of bias: The report does not describe whether the trial was blinded or describes the trial as blinded but does not describe the method of blinding and indicates that knowledge of allocation was possible during the trial.
High risk of bias: The report states that the trial was not blinded and that allocation was known during the trial.
Blinded outcome assessment
Low risk of bias: Investigators assessed all relevant outcomes while blinded and described the method of blinding with knowledge of allocation adequately prevented.
Unclear risk of bias: Investigators did not describe whether outcome assessment was blinded, or described outcome assessment as blinded but did not describe the method of blinding, with knowledge of allocation possible.
High risk of bias: Investigators described outcome assessment as blinded and indicated that outcome assessors were knowledgeable about allocation.
Incomplete outcome data
Low risk of bias: Underlying reasons for missingness were unlikely to make treatment effects depart from plausible values, or investigators used proper methods when handling missing data.
Uncertain risk of bias: Information was insufficient for assessment of whether the missing data mechanism, in combination with the method used to handle missing data, was likely to induce bias in the estimate of effect.
High risk of bias: The crude estimate of effects (e.g. complete case estimate) is clearly biased because of underlying reasons for missingness and/or because methods used to handle missing data were unsatisfactory.
Selective outcome reporting
Low risk of bias: Investigators reported predefined or clinically relevant and reasonably expected outcomes.
Unclear risk of bias: Investigators did not report, or did not fully report, all predefined or clinically relevant and reasonably expected outcomes, making it unclear whether data on these outcomes were recorded.
High risk of bias: Investigators did not report one or more clinically relevant and reasonably expected outcomes and/or likely recorded data for these outcomes.
Vested interest bias
Low risk of bias: Funding for the trial did not come from any parties that might have confounding interests, or any academic, professional, financial, or other benefits to the person responsible for the trial were independent of the direction or statistical significance of trial results.
Unclear risk of bias: The source of funding is not clear, or it is unclear whether the person responsible for the trial stands to benefit according to the direction or statistical significance of trial results.
Review authors assessing risk of bias also considered administration of inappropriate treatment to control groups. We have presented a risk of bias figure and a risk of bias summary in the main body of the review to show our findings. We assigned trials a degree of bias, for example, high, low, or unclear. Trials fell into the "low risk of bias" group if we judged the trial as having "low risk" in all domains except blinding of personnel during the surgical procedure. If we judged risk of bias as "unclear" or "high", the trial fell into the group of trials with "high risk of bias".
Measures of treatment effect
We performed the meta‐analysis and displayed results according to Cochrane's recommendations (Higgins 2011a). We grouped data on the basis of similarities of the interventions implemented. We presented dichotomous data (benefit or harm) as meta‐analysed odds ratios (ORs) or risk ratios (RRs), with 95% confidence intervals (CIs), P values, and Trial Sequential Analysis (TSA) adjusted 95% CIs, and displayed the findings in a forest plot based on a logarithmic scale. For rare event data, we used the Peto odds ratio (POR) to summarize intervention effects. For continuous data, we presented the mean difference (MD) and the 95% CI as estimates of the intervention effect (or the standardised mean difference (SMD) if diverse scales were used). When possible, we derived exact P values (instead of above or below 0.05) for all comparisons. We included the risk difference, if this was presented, to illustrate the above results in absolute terms (e.g. number of participants who developed pneumonia). We converted the OR or RR in the case of statistically significant intervention effects to provide a number needed to treat for an additional beneficial outcome (NNTB) or a number needed to treat for an additional harmful outcome (NNTH) estimate.
Only one trial reported results in accordance with the intention‐to‐treat (ITT) principle use in the meta‐analysis (Buggeskov 2016). We extracted per‐protocol data for the remaining trials for use in the meta‐analysis (Kiessling 2014; Liu 2005; Santini 2012).
Unit of analysis issues
Included trials may report multiple observations for the same outcome (e.g. PaO2/FiO2 ratio or OI index as a single measurement or as area under the curve (AUC) for continuous measurements preoperatively, perioperatively, and postoperatively). In this case, review authors may need to compute an effect measure that incorporates all time points for each individual participant (e.g. AUC).
Dealing with missing data
We contacted trial authors to request original data when data on outcomes of excluded participants were missing. We collected dropout rates together with reasons for dropping out as reported by trial authors. We documented this information in the Characteristics of included studies table.
Assessment of heterogeneity
We used the Chi2 test to confirm expected between‐study heterogeneity (Higgins 2002), and we used the I2 statistic (which incorporates the Chi2 statistic (Q)) to inform the degree of heterogeneity. We conducted subgroup analyses to investigate possible sources of heterogeneity (see Subgroup analysis and investigation of heterogeneity), and if sufficient numbers of trials were present, we carried out meta‐regression.
We seriously considered not doing the meta‐analysis if the I2 value for binary outcomes was larger than 75% owing to high risk of heterogeneity due to pooling of trial results.
We performed the meta‐analysis for continuous outcomes regardless of the I2 value.
Assessment of reporting biases
We planned to prepare a funnel plot, using RevMan to investigate the presence of bias, by plotting treatment effect against standard error if the review included the 10 RCTs required to generate a funnel plot. For future versions of the review when 10 or more RCTs report on the same outcome, we will prepare a funnel plot. We will use a linear regression approach to determine funnel plot asymmetry (Egger 1997).
Data synthesis
We applied the random‐effects model, except when Peto OR was calculated and a fixed‐effect model was appropriate (DerSimonian 1986). We used the Mantel‐Haenszel method for meta‐analysis of trial data (Mantel 1959). The Mantel‐Haenszel method assumes a fixed‐effect meta‐analysis and is used for dichotomous data. The Cochrane Handbook for Systematic Reviews of Interventions reports that the Mantel‐Haenszel method is best for combining trials with small sample sizes (Higgins 2011a).
Trial Sequential Analysis
Cumulative meta‐analyses are at risk of producing random errors as the result of sparse data and multiple testing of accumulating data (Brok 2009; Higgins 2011b; Pogue 1997; Thorlund 2009; Wetterslev 2008). Trial Sequential Analysis can be applied to assess this risk (http://ctu.dk/tsa; Thorlund 2011). Researchers can calculate the required information size (i.e. the number of participants needed for a meta‐analysis to detect or reject a certain intervention effect) to minimize random errors (Wetterslev 2008; Wetterslev 2009). The required information size takes into account the event proportion in the control group; the assumption of a plausible risk ratio (RR) reduction, or the RR reduction observed in included trials with low risk of bias; and assumed heterogeneity ‐ Turner 2013 ‐ or diversity of the meta‐analysis (Wetterslev 2008; Wetterslev 2009). Trial Sequential Analysis enables testing for significance each time a new trial is included in the meta‐analysis. On the basis of the required information size, researchers can construct trial sequential monitoring boundaries. This enables one to determine the statistical inference concerning cumulative meta‐analysis that has not yet reached the required information size (Wetterslev 2008).
Firm evidence is established if the trial sequential monitoring boundary is crossed before the required information size is reached, in which case further trials may turn out to be superfluous. In contrast, if the boundary is not surpassed, one may conclude that it is necessary to continue with further trials before a specific intervention effect can be detected or rejected. Firm evidence for lack of the postulated intervention effect can also be assessed via Trial Sequential Analysis. This occurs when the cumulative Z‐score crosses the trial sequential beta spending monitoring boundaries and enters the area of futility. We planned to perform Trial Sequential Analysis with an a priori anticipated risk ratio reduction (RRR) of 30% for mortality and SAEs, along with a sensitivity analysis based on the RRR suggested by trials with low risk of bias in all bias domains, or with lower risk of methodological bias (adequate sequence generation, adequate allocation concealment, and adequate blinding). For Trial Sequential Analysis of the effect on continuous outcomes (PaO2/FiO2 ratio or OI), we used an anticipated MD corresponding to a 10% reduction (PaO2/FiO2 ratio) or increase (OI) as the anticipated a priori intervention effect. For all Trial Sequential Analyses, we used a priori anticipated diversity of 25% and the diversity found in actual meta‐analyses for estimation of the diversity‐adjusted required information size in the meta‐analyses (Trial Sequential Analysis Manual; http://www.ctu.dk/tsa/).
Subgroup analysis and investigation of heterogeneity
When appropriate, we performed subgroup analyses to assess possible sources of heterogeneity. Specifically, if we found enough trials to justify subgroup analysis, we identified differences or similarities among the results of trials with common features. We planned to perform subgroup analyses to help answer specific questions about particular patient groups, or types of interventions.
We planned to investigate the following subgroups.
Type of intervention: single shot versus continuous pulmonary artery perfusion with oxygenated or deoxygenated blood.
Baseline lung function: trials including participants with reduced lung function (> 50% of participants with forced expiratory volume in one second (FEV1) < 80% of predicted) versus trials including participants with normal lung function.
Duration of follow‐up: trials with longer follow‐up than the median follow‐up of included trials versus trials with shorter follow‐up than the median follow‐up of included trials.
We declared a subgroup effect only when results of the test of interaction were statistically significant, with a P value less than 0.05 (Altman 2003).
Sensitivity analysis
We conducted analyses by using both models (fixed‐effect and random‐effects) to determine whether trial results showed a discrepancy. In case one or two trials dominated reported evidence, we synthesized data (when similarities existed) and emphasized results of the fixed‐effect model meta‐analysis (DeMets 1987).
We performed a sensitivity analysis by excluding trials with high risk of bias. We stratified analyses by low risk of bias and high or uncertain risk of bias. In future updates, when we identify sufficient studies, we plan to test for subgroup heterogeneity to see if a difference in intervention effect is evident between low and high or uncertain risk of bias trials.
Economic issues
Although we predicted that a full economic evaluation would not be feasible for this review, we discussed the cost and cost‐effectiveness of interventions and, if data permitted, preliminarily reflected on efficient use of resources with respect to the efficacy of interventions, for example, quality of life (as assessed by a validated questionnaire) and length of hospital stay.
'Summary of findings' table and GRADE
We planned to include all primary and secondary outcome measures in two 'Summary of findings' (SoF) tables (GRADE tables) ‐ one for each comparison (GRADEproGDT).
Continuous or single‐shot pulmonary artery perfusion with oxygenated or deoxygenated blood compared with no pulmonary perfusion during CPB.
Continuous or single‐shot pulmonary artery perfusion with a preservation solution compared with no pulmonary perfusion during CPB.
As only one trial included the intervention "pulmonary artery perfusion with a preservation solution" (Buggeskov 2016), we did not conduct a meta‐analysis and we produced only one SoF table (for the first comparison).
We used GRADE principles to assess the quality of the body of evidence associated with the following specific outcomes (Schünemann 2008).
All‐cause mortality (at maximum follow‐up) in all trials.
Pulmonary events (e.g. numbers of participants with reduced postoperative oxygenation values (specified as reduction in the OI or increase in the PaO2/FiO2 ratio), prolonged mechanical ventilation (> three hours), pneumonia, ARDS).
Numbers of participants with one or more SAEs (at maximum follow‐up) in all trials.
Last measured PaO2/FiO2 ratio (mmHg) reported in trials.
We constructed a 'Summary of findings' table using the GRADE approach to appraise the quality of a body of evidence on the basis of the extent to which one can be confident that an estimate of effect or association reflects the item being assessed (GRADEproGDT). The quality of a body of evidence reflects within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision (risk of random error) derived from Trial Sequential Analysis of effect estimates, and risk of publication bias.
Results
Description of studies
Results of the search
We identified a total of 336 references of possible interest by searching the Cochrane Central Register of Controlled Studies (CENTRAL), MEDLINE, Embase, Science Citation Index Expanded, and reference lists of identified studies. We identified eight additional trials by handsearching. We searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) for ongoing trials but found none. We found one epub abstract with preliminary results ahead of the full article (Pichugin 2016), listed under Studies awaiting classification.
We excluded 100 duplicates and 222 clearly irrelevant references upon reading the abstracts. Accordingly, we retrieved 14 references for further assessment. Of these, we excluded nine references (four CCTs in adults, four RCTs in children, and one RCT in adults without a control group) from the meta‐analyses and reported only data on harm. We listed reasons for exclusion in the Characteristics of excluded studies table.
In total, four RCTs described in five references fulfilled our inclusion criteria (Figure 2) (Buggeskov 2016; Kiessling 2014; Liu 2005; Santini 2012). These trials included a total of 270 participants, with 210 participants providing data for the outcomes analysed in this review.
2.
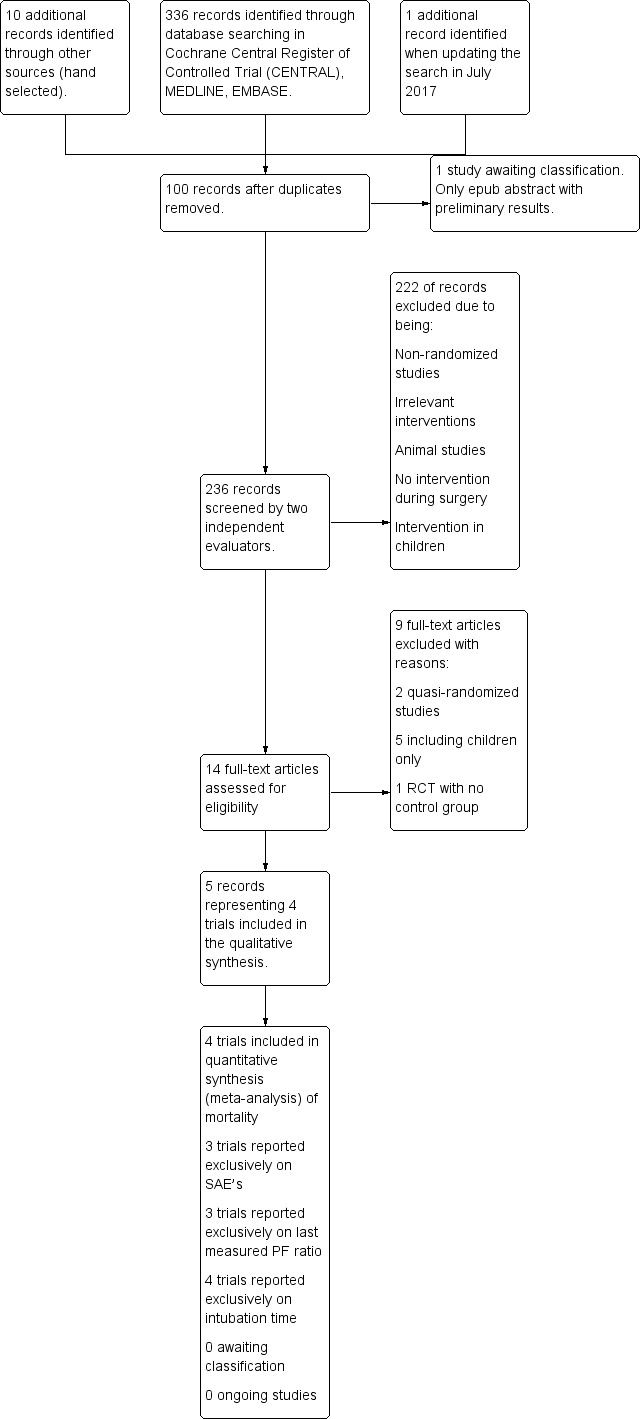
Study flow diagram.
We approached two corresponding or first study authors to request missing or unclear information (Kiessling 2014; Santini 2012). We received a reply from one of the two authors (Kiessling 2014). We contacted the second author on several occasions but received no reply (Santini 2012). One question not answered by this trial author was whether participants included in one paper were also included in a second paper (Santini 2012). The inclusion period for the two reported populations overlapped, and it was not specified in the later article whether participants were also included in the primary trial (Santini 2012). For this reason, we considered the two papers to describe a single trial with 64 participants (Santini 2012; primary reference), from which we primarily extracted data, except outcomes data for the PaO2/FiO2 ratio and intubation time, reported only in the 2011 publication (Santini 2011).
We present detailed descriptions in the Characteristics of included studies table and in the appendices. A summary overview follows.
Included studies
Trial characteristics
The four included RCTs, described in five references, included 210 participants and reported on mortality (210 participants), SAEs (180 participants), PaO2/FiO2 ratio (119 participants), and SAEs and intubation time (176 participants) (Buggeskov 2016; Kiessling 2014; Liu 2005; Santini 2012). Three of these trials (four references) used a two‐arm parallel‐group design (Kiessling 2014; Liu 2005; Santini 2012), and the remaining trial used a three‐arm parallel‐group design (Buggeskov 2016). All four RCTs were published from 2005 to 2016.
Participants
Investigators randomly assigned a total of 210 participants to pulmonary artery perfusion versus no perfusion during CPB in the four trials reporting on outcomes (Buggeskov 2016; Kiessling 2014; Liu 2005; Santini 2012). Numbers of participants in each trial were 64 (one trial, two references; Santini 2012), 56 (Kiessling 2014), 30 (Liu 2005), and 60 (Buggeskov 2016), respectively. The approximate weighted mean age of trial participants was 59 (range 37 to 70). The mean proportion of women was 65% (137 of 210). One trial included only young participants (mean age 37 ± 5.2) undergoing mitral valve replacement and in some cases concomitant tricuspid valvuloplasty owing to primary rheumatic fever (Liu 2005). The remaining three trials included participants undergoing standard coronary artery bypass graft (CABG) and/or aortic valve replacement (AVR), in some cases with concomitant surgery on the ascending aorta (Buggeskov 2016; Kiessling 2014; Santini 2012). Two trials included only participants with no history of pulmonary disease (Liu 2005; Santini 2012), and the remaining two trials included only participants with chronic obstructive pulmonary disease (COPD) (Buggeskov 2016; Kiessling 2014).
Funding
Two of the included trials were conducted without direct funding by industry (Liu 2005; Santini 2012), and one trial reported support received from a company producing equipment used during heart surgery and in the intensive care unit (Kiessling 2014). One trial, authored by two of the authors of this Cochrane review, was not directly funded by industry but was supported by two restricted grants ‐ one from a pharmaceutical company, and one from an independent pharmaceutical foundation (Buggeskov 2016; see Declarations of interest section).
Three of the included trials were conducted in high‐income countries in Europe (Buggeskov 2016; Kiessling 2014; Santini 2012), and one trial was conducted in an upper‐middle‐income country (Liu 2005).
Experimental interventions
Two trials performed continuous pulmonary artery perfusion with oxygenated blood during CPB (Liu 2005; Santini 2012), and one trial performed non‐pulsatile intermittent pulmonary artery perfusion (4 L of venous blood initially after primary cardioplegia and 3 L every 20 minutes during the CPB period) (Kiessling 2014). The last trial performed continuous non‐pulsatile pulmonary artery perfusion with oxygenated blood in one group, while performing single‐shot pulmonary artery perfusion with a hypothermic preservation solution in a second intervention group (Buggeskov 2016).
Comparator interventions
In all four trials, the control group received no pulmonary artery perfusion during CPB (Buggeskov 2016; Kiessling 2014; Liu 2005; Santini 2012).
Co‐interventions used in experimental and comparator intervention groups
Anaesthesia, perfusion, and surgical protocols for both experimental and comparator groups were comparable in the four trials (Buggeskov 2016; Kiessling 2014; Liu 2005; Santini 2012). Protocols showed small deviations with regards to perfusion techniques, with three trials performing hypothermic CPB (Kiessling 2014; Liu 2005; Santini 2012), and one trial performing normothermic CPB (Buggeskov 2016). In all four trials, CPB was non‐pulsatile and mechanical ventilation ceased during CPB.
Excluded studies
We excluded nine studies from the review and meta‐analysis; all reported data on harm (De Santo 2003a; De Santo 2003b; Li 2010; Sievers 2002; Suzuki 1997; Suzuki 2000; Suzuki 2001; Wei 2004; Yi 2006). Four studies were CCTs in adults (De Santo 2003a; De Santo 2003b; Sievers 2002; Suzuki 1997), four were RCTs including children (Li 2010; Suzuki 2000; Suzuki 2001; Wei 2004), and one was an RCT with no control group but two intervention groups ‐ both receiving pulmonary artery perfusion with blood (one group with Chinese medicine added) (Yi 2006).
We present a detailed description of the characteristics of the eight excluded studies in the section Characteristics of excluded studies.
Studies awaiting classification
One study awaits classification with only an epub abstract with preliminary results currently available ahead of the full article (Pichugin 2016).
Ongoing studies
We found no ongoing studies.
Risk of bias in included studies
Three trials had high or unclear risk of bias in one or more bias domains other than outcome reporting bias and blinding of personnel during the surgical procedure (Kiessling 2014; Liu 2005; Santini 2012). One trial had low risk of bias in all domains, except for blinding of personnel during the surgical procedure (Buggeskov 2016). See the risk of bias graph (Figure 1) and the risk of bias summary (Figure 3).
1.
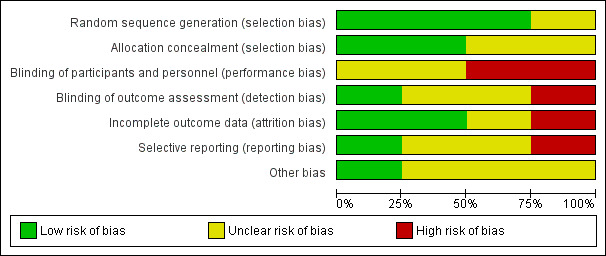
Risk of bias graph: a plot of review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
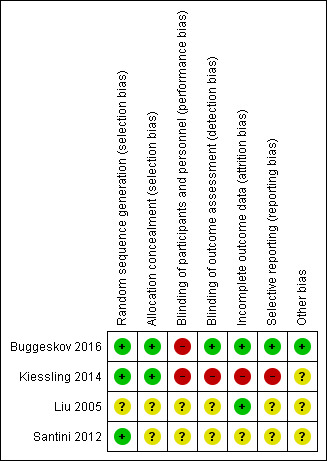
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
For the key outcomes mortality, pulmonary events, and SAEs, we considered only one trial to be at low risk of bias, except for blinding of personnel during the surgical procedure (Buggeskov 2016).
Allocation
Generation of the allocation sequence
Three trials reported the primary outcome mortality and described generation of the allocation sequence by block randomization or lottery (Buggeskov 2016; Kiessling 2014; Santini 2012).
One trial provided no description of allocation sequence generation (Liu 2005).
Allocation concealment
Three trials reported the primary outcome mortality and described allocation concealment performed by drawing pre‐prepared sealed envelopes containing group assignment or centrally computer‐generated block randomization(Buggeskov 2016; Kiessling 2014; Santini 2012).
One trial provided no description of allocation concealment (Liu 2005).
Blinding
Blinding of participants and personnel
Two trials reported the primary outcome mortality and described incomplete blinding of personnel or unclear blinding of participants (Kiessling 2014; Santini 2012). One trial reported the primary outcome of mortality and described blinding of personnel (except for blinding of personnel during the surgical procedure) and blinding of participants (Buggeskov 2016). Owing to the inherent problem with blinding of a surgical intervention, we did not expect any trials to describe blinding of the surgical team. One trial provided no description of blinding of participants and/or personnel (Liu 2005).
Blinding of outcome assessors
Two trials reporting the primary outcome mortality did not describe blinding of outcome assessors (Liu 2005; Santini 2012). One trial specifically described that outcome assessors were unblinded (Kiessling 2014), and one trial described blinding of both the statistician and those drawing conclusions (Buggeskov 2016).
Incomplete outcome data
Three trials reported the primary outcome mortality and provided numbers and reasons for dropouts and withdrawals from the intervention groups (Buggeskov 2016; Liu 2005; Kiessling 2014). One trial did not consistently report on dropouts nor on withdrawals (Santini 2012).
Selective reporting
All four trials reported mortality, pulmonary events in relation to postoperative oxygenation values, intubation time, and ICU‐LOS and SAEs (Buggeskov 2016; Kiessling 2014; Liu 2005; Santini 2012). One trial described inflammatory markers in both bronchoalveolar lavage fluid and plasma (Santini 2012), one reported inflammatory markers only in plasma (Kiessling 2014), and another reported pathological changes in lung tissue (Liu 2005).
Other potential sources of bias
One trial had unclear risk of industry bias, as a company selling equipment used for patients in the ICU supported the trial (Kiessling 2014). Two trials had uncertain risk of industry bias, as researchers did not describe financial support in the article (Liu 2005; Santini 2012). One trial was not directly supported by industry, but the first trial author had received two restricted grants ‐ one from a pharmaceutical company, and one from an independent pharmaceutical foundation. Neither the company nor the foundation had any influence on the protocol (Buggeskov 2013), and neither was involved in conduct of the trial, analysis of the data, or writing of the trial report; thus we classified this trial as having low risk of bias (Buggeskov 2016; see Declarations of interest).
Effects of interventions
See: Table 1
Summary of findings for the main comparison. Pulmonary artery perfusion with blood versus no perfusion during cardiopulmonary bypass for open heart surgery in adults.
| Pulmonary artery perfusion with blood versus no perfusion during cardiopulmonary bypass | ||||||
| Patient or population: adult surgical patients with the need for cardiopulmonary bypass‐dependent heart surgery Setting: hospitals in Europe and China Intervention: pulmonary artery perfusion with blood during cardiopulmonary bypass Comparison: no pulmonary artery perfusion during cardiopulmonary bypass | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk ‐ no pulmonary artery perfusion | Corresponding risk ‐ pulmonary artery perfusion with blood | |||||
| All‐cause mortality within longest follow‐up in all trials irrespective of overall risk of bias | Study population | Peto OR 1.78 (0.43 to 7.40) | 210 (4 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b | Results of sensitivity analysis restricted to studies at low risk of bias (1 study, 60 participants): Peto OR 1.65 (0.27 to 10.15) | |
| 28 per 1000 | 49 per 1000 (12 to 196) | |||||
| Number of participants with pulmonary events within longest follow‐up | No patient‐specific data | No patient‐specific data | No patient‐specific data | No patient‐specific data | All trials reported event‐specific and not patient‐specific pulmonary events. It was not possible to perform a meta‐analysis, as some participants may have had more than 1 event. | |
| Participants with ≥ 1SAE within longest follow‐up in all trials, irrespective of overall risk of bias | Study population | RR 1.12 (0.66 to 1.89) | 180 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWc,d | Results of sensitivity analysis restricted to studies at low risk of bias (1 study, 60 participants): RR 0.95 (0.74 to 1.20) | |
| 429 per 1000 | 514 per 1000 (347 to 767) | |||||
| Last measured PaO2/FiO2 ratio (mmHg) in all trials, irrespective of overall risk of bias | Last measured mean PaO2/FiO2 ratio in all trials, irrespective of overall risk of bias, was301.92 mmHg. | MD 27.8 mmHg higher (5.67 higher to 49.93 higher) | ‐ | 119 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWe,f,g | Results of sensitivity analysis restricted to studies at low risk of bias (1 study, 59 participants): MD 39.67 (16.33 to 95.67) |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; OR: odds ratio (Peto); PaO2/FiO2: ratio of partial pressure of alveolar oxygen to fraction of inspired oxygen; RCT: randomized controlled trial; RR: risk ratio; RRI; relative risk increase; SAE: serious adverse event; TSA: Trial Sequential Analysis; TSMBs: trial sequential monitoring boundaries. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for imprecision (wide CI) as TSA showed that the required information size of 1500 for an RRI of 100% (a priori planned 30%) has not been reached, and that no TSMBs have been crossed (Figure 1; Characteristics of included studies).
bDowngraded one level for risk of bias because three out of four trials had overall high risk of bias (Figure 1; Characteristics of included studies).
cDowngraded two levels for imprecision (wide CI) as TSA showed that the required information size of 504 for an RRI of 30% has not been reached, and that no TSMBs have been crossed (Figure 1; Characteristics of included studies).
dDowngraded one level for risk of bias because two out of three trials had overall high risk of bias (Figure 1; Characteristics of included studies).
eDowngraded one level for imprecision (wide CI).
fDowngraded one level for indirectness because the PaO2/FiO2 ratio is a surrogate outcome with questionable clinical relevance.
gDowngraded one level for risk of bias because two out of three trials had overall high risk of bias (Figure 1; Characteristics of included studies).
Intervention: Pulmonary artery perfusion with blood versus no pulmonary perfusion during cardiopulmonary bypass
See Table 1.
Primary outcomes
1.1 All‐cause mortality (at maximum follow‐up)
Four trials including 210 participants provided data for analyses on all‐cause mortality (Buggeskov 2016; Kiessling 2014; Liu 2005; Santini 2012). Investigators did not find that pulmonary artery perfusion with blood compared with no perfusion during CPB was associated with a difference in mortality (Peto OR 1.78, 95% CI 0.43 to 7.40; 4 studies, 210 participants; I2 = 0%; Analysis 1.1; GRADE: very low quality). We downgraded the quality of evidence to very low by two levels for imprecision (wide CI) and by one level for high risk of bias (Figure 1; Characteristics of included studies). In a sensitivity analysis of one trial with an overall low risk of bias (except for blinding of personnel during the surgical procedure) (Buggeskov 2016), we found no evidence of a difference for mortality (Peto OR 1.65, 95% CI 0.27 to 10.15; 1 study, 60 participants).
1.1. Analysis.
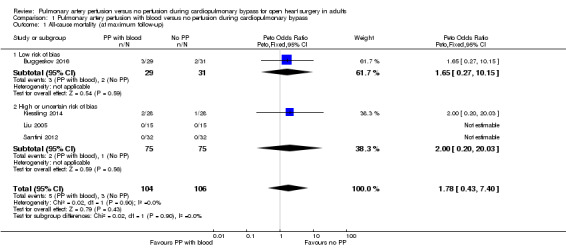
Comparison 1 Pulmonary artery perfusion with blood versus no perfusion during cardiopulmonary bypass, Outcome 1 All‐cause mortality (at maximum follow‐up).
Trial Sequential Analysis on all‐cause mortality (at maximum follow‐up)
Trial Sequential Analysis (Figure 4) in two of four trials reporting death showed that even with an anticipated relative risk increase (RRI) of 100% (a priori anticipated RRR was 30%), along with mortality in the control group of 3%, type I error of 5%, type II error of 20%, and diversity (D2) of 0%, the required information size was 1500 participants (Buggeskov 2016; Kiessling 2014; 116 participants). The anticipated RRI was changed from a priori 30% to 100%, as the actual information size was less than 3% to 4% of the required information size for a 30% RRR. The cumulative Z‐curve did not cross boundaries for benefit, harm, nor trial sequential monitoring boundaries for futility, indicating that, in the light of sparse date and repetitive testing, evidence was insufficient to refute even a 100% RRI or a 100% RRR for benefit or harm of pulmonary artery perfusion with blood compared with no perfusion during CPB (Imberger 2015; Mascha 2015; Terkawi 2016). The Trial Sequential Analysis adjusted CI for the intervention effect measured in the two trials reporting deaths was 0.01 to 493. The traditional 95% CI was 0.43 to 7.40.
4.
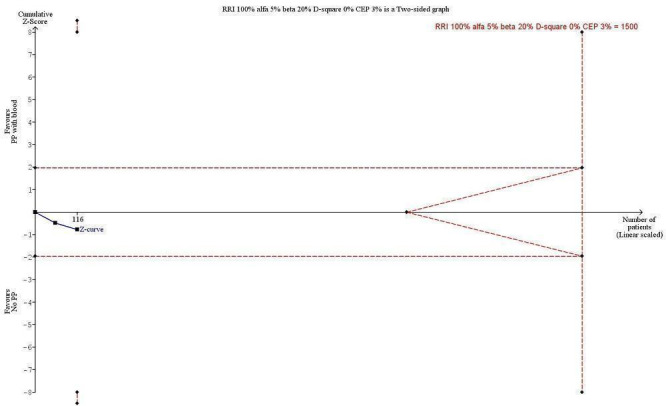
Trial Sequential Analysis (TSA) of the meta‐analysis of 2 trials reporting all‐cause mortality at the maximal length of follow‐up. The blue line is the cumulative Z‐curve, and the boundaries for benefit and harm are shown in the upper and lower panels as a line between two points. The futility boundaries are shown as an inner wedge to the right side as a triangle with the base adhering to the vertical line demonstrating the required information size. The control event proportion (CEP) is 3%, and we are addressing an alternative hypothesis that pulmonary artery perfusion with oxygenated blood may double mortality to 6% within maximal length of follow‐up, with maximal type I and II errors of 5% and 20%, respectively. The required information size based on actual diversity of trials of 0% is 1500 participants, and none of the TSA boundaries is crossed. The TSA adjusted confidence interval (CI) is 0.01 to 493. The traditional 95% CI is 0.43 to 6.91.
2.1 Pulmonary events (numbers of participants with reduced postoperative oxygenation values, prolonged mechanical ventilation, pneumonia, ARDS)
For the secondary primary outcome "pulmonary events", only one trial reported pneumonia (Buggeskov 2016), and no trials reported ARDS. We therefore made a pragmatic decision and added the PaO2/FiO2 ratio and intubation time as two new secondary explorative outcomes for this version of the review, representing surrogate outcomes for lung function. Investigators reported these as mean differences that were not patient specific, as was first intended for the outcome "numbers of patients with reduced postoperative oxygenation values, prolonged mechanical ventilation, pneumonia, ARDS". If it becomes possible, we will report in future versions of this review the numbers of participants with reduced postoperative oxygenation values, prolonged mechanical ventilation, pneumonia, and ARDS.
Secondary outcomes
3.1 Serious adverse events (SAEs) (at maximum follow‐up)
Three trials reported SAEs defined according to the rules in International Conference of Harmonization‐Good Clinical Practice (ICH‐GCP) (and Directive 2001) as “any event that led to death, was life‐threatening, required in‐patient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability, and any important medical event which jeopardised the patient or required intervention to prevent it” (Buggeskov 2016; Kiessling 2014; Santini 2012); we have listed these trials in Appendix 3. Trials, including those with high and low risk of bias, found that pulmonary artery perfusion with blood compared with no pulmonary artery perfusion during CPB was not associated with the proportion of SAEs in a random‐effects model (RR 1.12, 95% CI 0.66 to 1.89; 3 studies, 180 participants;I2 = 29%; Analysis 1.2; GRADE: very low quality). We downgraded the quality of evidence to very low by two levels for imprecision (wide CI) and by one level for high risk of bias (Figure 1; Characteristics of included studies). In a sensitivity analysis of one trial with an overall low risk of bias (except for blinding of personnel during the surgical procedure) (Buggeskov 2016), we found no evidence of a difference for proportions of SAEs (RR 0.95, 95% CI 0.74 to 1.20; 1 study, 60 participants).
1.2. Analysis.
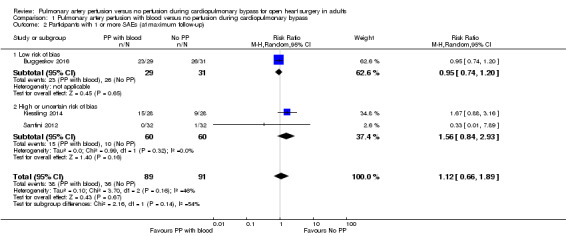
Comparison 1 Pulmonary artery perfusion with blood versus no perfusion during cardiopulmonary bypass, Outcome 2 Participants with 1 or more SAEs (at maximum follow‐up).
3.2 SAEs according to preoperative lung function (at maximum follow‐up)
One trial including 64 participants with a preoperative normal lung function found that pulmonary artery perfusion with blood compared with no pulmonary artery perfusion during CPB was not associated with proportions of SAEs (RR 0.33, 95% CI 0.01 to 7.89; 1 study, 64 participants; Analysis 1.3; GRADE: very low quality) (Santini 2012). Two trials including 116 participants with preoperatively diagnosed COPD found that pulmonary artery perfusion with blood compared with no pulmonary artery perfusion during CPB was not associated with proportions of SAEs in a random‐effects model (RR 1.18, 95% CI 0.63 to 2.23; 2 studies, 116 participants; I2 = 64%; Analysis 1.3; GRADE: very low quality) (Buggeskov 2016; Kiessling 2014). When testing for interaction, we found no subgroup differences (P = 0.44; I2= 0%).
1.3. Analysis.
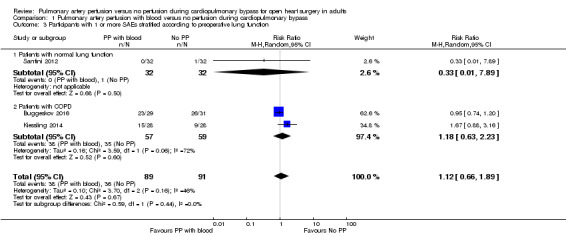
Comparison 1 Pulmonary artery perfusion with blood versus no perfusion during cardiopulmonary bypass, Outcome 3 Participants with 1 or more SAEs stratified according to preoperative lung function.
3.3 SAEs according to the follow‐up period for each trial
One trial (64 participants) reporting SAEs within the index admission found that pulmonary artery perfusion with blood compared with no pulmonary artery perfusion during CPB was not associated with proportions of SAEs (RR 0.33, 95% CI 0.01 to 7.89; 1 study, 64 participants; Analysis 1.4; GRADE: very low quality) (Santini 2012). Two trials (116 participants) reporting long‐term follow‐up with a minimum follow‐up of 30 days reported that pulmonary artery perfusion with blood compared with no pulmonary artery perfusion during CPB was not associated with proportions of SAEs within the longest follow‐up period in a random‐effects model (RR 1.18, 95% CI 0.63 to 2.23; 2 studies, 116 participants; I2 = 64%; Analysis 1.4; GRADE: very low quality) (Buggeskov 2016; Kiessling 2014). When testing for interaction, we found no subgroup differences (P = 0.44; I2 = 0%).
1.4. Analysis.
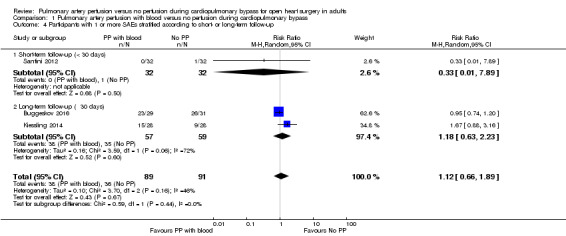
Comparison 1 Pulmonary artery perfusion with blood versus no perfusion during cardiopulmonary bypass, Outcome 4 Participants with 1 or more SAEs stratified according to short‐ or long‐term follow‐up.
Trial Sequential Analysis on SAEs (at maximum follow‐up)
Trial Sequential Analyses of the three trials reporting SAEs showed that with an anticipated RRI of 30%, SAEs in the control group of 40%, type I error of 5%, type II error of 20%, and diversity (D2) of 53%, the required information size was 1070 participants (Buggeskov 2016; Kiessling 2014; Santini 2012; 180 participants). The cumulative Z‐curve did not cross any boundaries for benefit and harm nor trial sequential monitoring boundaries for futility, indicating that evidence was insufficient to refute a 30% RRI or a 30% RRR for benefit or harm of pulmonary artery perfusion with blood, in the light of sparse data and repetitive testing (Imberger 2015; Mascha 2015; Terkawi 2016). The Trial Sequential Analysis adjusted confidence interval for the intervention effect measured in the three trials reporting SAEs was RR 1.18 with adjusted CI 0.37 to 3.52. The traditional 95% CI was 0.80 to 1.75.
4.1 Inflammatory markers
Two trials reported on inflammatory markers in plasma and bronchoalveolar lavage fluid (Kiessling 2014; Santini 2012). Both trials reported only two identical inflammatory markers (interleukin (IL)‐1 and tumour necrosis factor (TNF)‐alpha) measured in plasma but as repeated measurements at different time points and with different time intervals. Therefore, we could not perform a meta‐analysis on inflammatory markers in plasma or bronchoalveolar lavage fluid.
Santini 2012 reported no differences in plasma inflammatory markers between participants receiving pulmonary artery perfusion with blood compared with no perfusion during CPB. Investigators found a higher level of anti‐inflammatory markers and a lower level of pro‐inflammatory markers in bronchoalveolar lavage fluid from participants receiving pulmonary artery perfusion with blood compared with no perfusion during CPB (Santini 2012).
Kiessling 2014 reported an increase for all measured inflammatory markers after initiation of CPB but with no differences between groups.
5.1 Last measured PaO2/FiO2 ratio (mmHg)
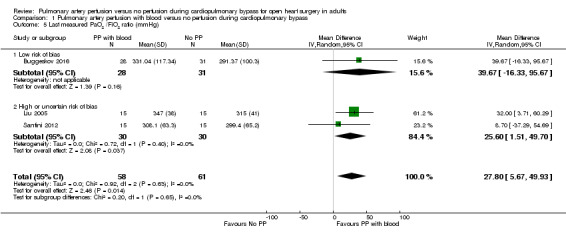
In all trials reporting on the last measured PaO2/FiO2 ratio (Buggeskov 2016; Liu 2005; Santini 2012), investigators found that pulmonary artery perfusion with blood compared with no perfusion during CPB was associated with a higher PaO2/FiO2 ratio in a random‐effects model (MD 27.80, 95% CI 5.67 to 49.93; 3 studies, 119 participants; I2 = 0%; Analysis 1.5; GRADE: very low quality). We downgraded the quality of evidence to very low by one level for imprecision (wide CI), by one level for risk of bias because two of three trials had overall high risk of bias (Figure 1; Characteristics of included studies), and by one level for indirectness because the PaO2/FiO2 ratio is a surrogate outcome with questionable clinical relevance. Sensitivity analysis of one trial with an overall low risk of bias (except for blinding of personnel during the surgical procedure) revealed no evidence of a difference in the last measured PaO2/FiO2 ratio (MD 39.67, 95% CI ‐16.33 to 95.67; 1 study, 59 participants) (Buggeskov 2016). These investigators reported a difference for the OI (which includes mean airway pressure in the calculation) but not for the PaO2/FiO2 ratio used in the current analysis, and remaining trials reported only the PaO2/FiO2 ratio (Liu 2005; Santini 2012). PaO2/FiO2 ratio data were available for only 28 of 29 participants receiving pulmonary artery perfusion with blood.
1.5. Analysis.
Comparison 1 Pulmonary artery perfusion with blood versus no perfusion during cardiopulmonary bypass, Outcome 5 Last measured PaO2 /FiO2 ratio (mmHg).
5.2 Last measured PaO2/FiO2 ratio (mmHg) according to lung function
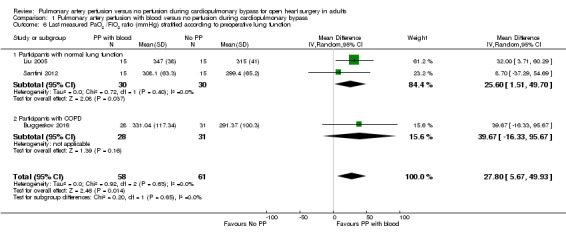
Trials including participants with a preoperative normal lung function found that pulmonary artery perfusion with blood compared with no perfusion during CPB was associated with a higher postoperative PaO2/FiO2 ratio in a random‐effects model (MD 25.60, 95% CI 1.51 to 49.70; 2 studies, 60 participants; I2 = 0%; Analysis 1.6; GRADE: very low quality) (Liu 2005; Santini 2012). One trial including participants with diagnosed COPD found that pulmonary artery perfusion with blood compared with no perfusion during CPB was not associated with a difference in the PaO2/FiO2 ratio (MD 39.67, 95% CI ‐16.33 to 95.67; 1 study, 59 participants; Analysis 1.6; GRADE: very low quality) (Buggeskov 2016). When testing for interaction, we found no subgroup differences (P = 0.65; I2 = 0%).
1.6. Analysis.
Comparison 1 Pulmonary artery perfusion with blood versus no perfusion during cardiopulmonary bypass, Outcome 6 Last measured PaO2 /FiO2 ratio (mmHg) stratified according to preoperative lung function.
Trial sequential analysis on the PaO2/FiO2 ratio (mmHg) measured at least six hours after surgery and anaesthesia
Trial Sequential Analysis (Figure 5) in three of four trials reporting a PaO2/FiO2 ratio showed that with an anticipated increase in PaO2/FiO2 ratio of 10%, along with PaO2/FiO2 mean difference of 29.1 mmHg in the control group, type I error of 5%, type II error of 20%, and diversity (D2) of 0%, the required information size was 310 participants (Buggeskov 2016; Liu 2005; Santini 2012; 119 participants). The cumulative Z‐curve did cross the traditional boundary for benefit (P < 0.05) favouring pulmonary artery perfusion with blood but did not cross the trial sequential monitoring boundaries for benefit, indicating that evidence was insufficient to confirm that a 10% increase in PaO2/FiO2 ratio for pulmonary artery perfusion with blood during CPB leads to a 10% higher PaO2/FiO2 ratio adjusted for sparse data and repetitive testing. The TSA adjusted CI for the intervention effect measured in the three trials reporting a PaO2/FiO2 ratio was ‐10.7 to 66.4. The traditional 95% CI was 5.67 to 49.93.
5.
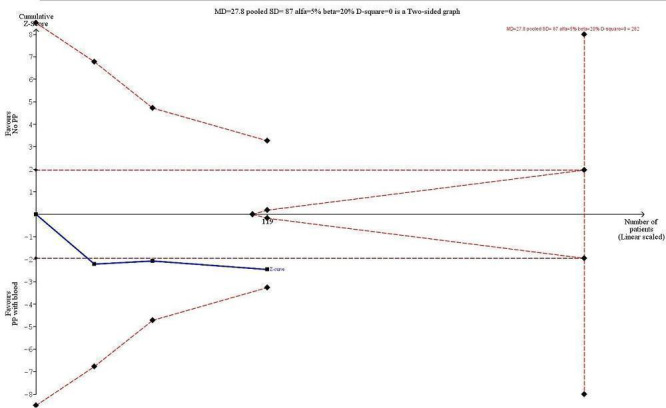
Trial Sequential Analysis (TSA) of the meta‐analysis of three trials reporting the ratio of partial pressure of alveolar oxygen to fraction of inspired oxygen (PaO2/FiO2) at least six hours after surgery and anaesthesia. The blue line is the cumulative Z‐curve, and the boundaries for benefit and harm are shown in the upper and lower panels as a line between two points. The futility boundaries are shown as an inner wedge to the right side as a triangle with the base adhering to the vertical line demonstrating the required information size. We are addressing an alternative hypothesis that pulmonary artery perfusion with oxygenated blood may increase the PaO2/FiO2 ratio within 10% (29.1 mmHg absolute increase) at six hours after surgery and maximal type I and II errors of 5% and 20%, respectively. The required information size based on actual diversity of trials of 0% is 282 participants, and none of the TSA boundaries is crossed. The TSA adjusted confidence interval (CI) is ‐10.7 to 66.4. The traditional 95% CI is 5.67 to 49.93.
6.1 Intubation time (hours) after primary surgery
Four trials provided data for analyses on Intubation time (Buggeskov 2016; Kiessling 2014; Liu 2005; Santini 2012). Data were not normally distributed; therefore we chose to report the median values as approximated means and to calculate the standard deviation (SD) from the interquartile ranges. For one trial (Santini 2012), the interquartile range was 0; we therefore imputed an SD of 0.01.
All trials found that pulmonary artery perfusion with blood compared with no perfusion during CPB was not associated with a difference for intubation time in a random‐effects model (MD ‐0.35, 95% CI ‐1.12 to 0.42; 4 studies, 176 participants; I2 = 23%) nor in a fixed‐effect model (MD ‐0.10, 95% CI ‐0.46 to 0.25; 4 studies, 176 participants; I2 = 23%; Analysis 1.7; GRADE: very low quality). We downgraded the quality of evidence to very low by one level for imprecision (wide CI), by one level for risk of bias because three of four trials had overall high risk of bias (Figure 1; Characteristics of included studies), and by one level for indirectness because intubation time is a surrogate outcome with questionable clinical relevance. A sensitivity analysis of one trial with an overall low risk of bias (except for blinding of personnel during the surgical procedure) revealed no evidence of a difference in intubation time after primary surgery (MD ‐1.00, 95% CI ‐2.24 to 0.24; 1 study, 60 participants) (Buggeskov 2016).
1.7. Analysis.
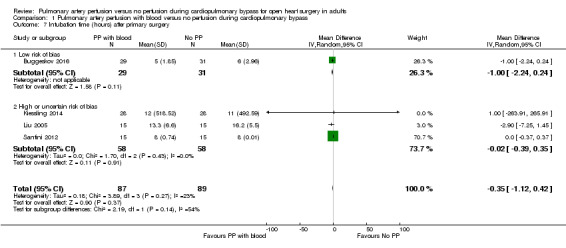
Comparison 1 Pulmonary artery perfusion with blood versus no perfusion during cardiopulmonary bypass, Outcome 7 Intubation time (hours) after primary surgery.
As converted skewed data from Santini 2012 resulted in a narrow SD, data weighted the overall analysis. A sensitivity analysis excluding data from Santini 2012 resulted in a tendency towards a shorter intubation time for participants receiving pulmonary artery perfusion with blood (MD ‐1.14, 95% CI ‐2.34 to 0.05; 3 studies, 146 participants; I2 = 0%; Analysis 1.7; GRADE: very low quality).
6.2 Intubation time (hours) after primary surgery according to lung function
Trials including participants with a preoperative normal lung function found that pulmonary artery perfusion with blood compared with no perfusion during CPB was not associated with a difference for intubation time in a random‐effects model (MD ‐0.61, 95% CI ‐2.92 to 1.71; 2 studies, 60 participants; I2 = 41%; Analysis 1.8; GRADE: very low quality) (Liu 2005; Santini 2012). Trials including participants with diagnosed COPD found that pulmonary artery perfusion with blood compared with no perfusion during CPB was not associated with a difference for intubation time in a random‐effects model (MD ‐1.00, 95% CI ‐2.24 to 0.24; 2 studies, 116 participants; I2 = 0%; Analysis 1.8; GRADE: very low quality) (Buggeskov 2016; Kiessling 2014). When testing for interaction, we found no subgroup differences (P = 0.77; I2 = 0%).
1.8. Analysis.
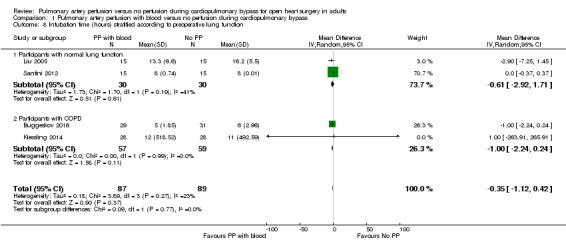
Comparison 1 Pulmonary artery perfusion with blood versus no perfusion during cardiopulmonary bypass, Outcome 8 Intubation time (hours) stratified according to preoperative lung function.
Trial Sequential Analysis on intubation time (hours) after surgery and anaesthesia
Trial Sequential Analysis (Figure 6) in the four trials reporting intubation time showed that with anticipated decrease in intubation time of 1.5 hours, mean difference in intubation time for the control group of 1.5 hours, type I error of 5%, type II error of 20%, and diversity (D2) of 78%, the required information size was 188 participants (Buggeskov 2016; Kiessling 2014; Liu 2005; Santini 2012; 176 participants). The cumulative Z‐curve crossed the trial sequential monitoring boundaries for futility, indicating that evidence was sufficient to refute a 10% decrease or increase in intubation time for benefit or harm of pulmonary artery perfusion with blood, in the light of sparse data and repetitive testing (Imberger 2015; Mascha 2015; Terkawi 2016). The TSA adjusted CI for the intervention effect measured in the four trials reporting intubation time was ‐1.28 to 0.46. The traditional 95% CI was ‐1.12 to 0.42.
6.
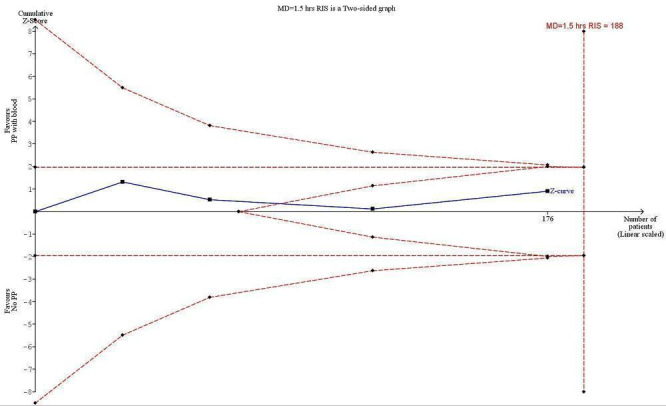
Trial Sequential Analysis (TSA) of the meta‐analysis of four trials reporting intubation time after surgery and anaesthesia. The blue line is the cumulative Z‐curve, and the boundaries for benefit and harm are shown in the upper and lower panels as a line between two points. The futility boundaries are shown as an inner wedge to the right side as a triangle with the base adhering to the vertical line demonstrating the required information size. We are addressing an alternative hypothesis that pulmonary artery perfusion with oxygenated blood may decrease intubation time within 1.5 hours after surgery with maximal type I and II errors of 5% and 20%, respectively. The required information based on actual diversity of trials of 78% is 188 participants, and none of the TSA boundaries is crossed. The TSA adjusted confidence interval (CI) is ‐1.28 to 0.46 hours, excluding a difference in intubation time of 1.5 hours or more. The traditional 95% CI is ‐1.12 to 0.42.
Intervention: Pulmonary artery perfusion with a preservation solution compared with no perfusion during CPB
Only one study reported results for the comparison pulmonary artery perfusion with a preservation solution versus no perfusion during CPB. Therefore it was not possible to perform a meta‐analysis. Buggeskov 2016 reported no differences in mortality (RR 1.60, 95% CI 0.29 to 8.92; 1 study, 60 participants; Analysis 2.1), SAEs (RR 1.07, 95% CI 0.88 to 1.30; 1 study, 60 participants; Analysis 2.2), nor last measured PaO2 /FiO2 ratio (MD ‐25.34, 95% CI ‐69.16 to ‐18.48; 1 study, 60 participants; Analysis 2.3) between participants receiving pulmonary artery perfusion with a preservation solution compared with no perfusion during CPB. Likewise, Buggeskov 2016 reported no differences in intubation times between participants receiving pulmonary artery perfusion with a preservation solution compared with no perfusion during CPB, using the van Elteren test, owing to non‐normal distribution (count‐data) of data. The Review Manager analysis in our present review, unduly based on the assumption of normal distribution of intubation times, resulted in detection of a difference in intubation time between the two groups (MD ‐2.00, 95% CI ‐3.50 to ‐0.50; 1 study, 60 participants; Analysis 2.4).
2.1. Analysis.
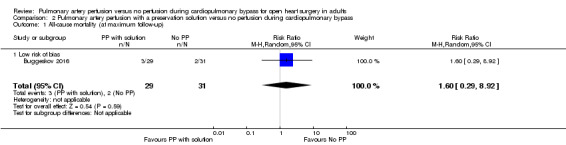
Comparison 2 Pulmonary artery perfusion with a preservation solution versus no perfusion during cardiopulmonary bypass, Outcome 1 All‐cause mortality (at maximum follow‐up).
2.2. Analysis.
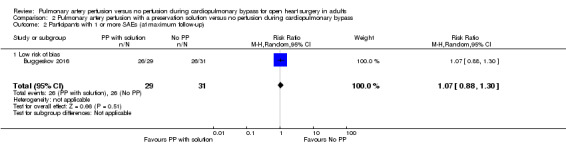
Comparison 2 Pulmonary artery perfusion with a preservation solution versus no perfusion during cardiopulmonary bypass, Outcome 2 Participants with 1 or more SAEs (at maximum follow‐up).
2.3. Analysis.
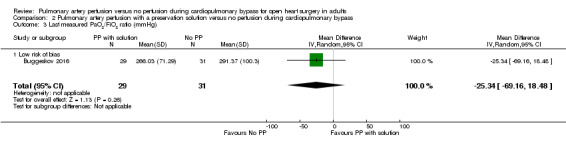
Comparison 2 Pulmonary artery perfusion with a preservation solution versus no perfusion during cardiopulmonary bypass, Outcome 3 Last measured PaO2/FiO2 ratio (mmHg).
2.4. Analysis.
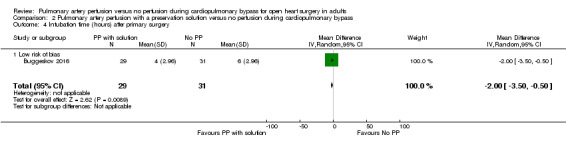
Comparison 2 Pulmonary artery perfusion with a preservation solution versus no perfusion during cardiopulmonary bypass, Outcome 4 Intubation time (hours) after primary surgery.
Discussion
Summary of main results
All trials reported that the effect of pulmonary artery perfusion with blood during cardiopulmonary bypass (CPB) on all‐cause mortality was uncertain (Peto odds ratio (OR) 1.78, 95% confidence interval (CI) 0.43 to 7.40; 4 studies, 210 participants; I2 = 0%; Analysis 1.1; GRADE: very low quality) (Buggeskov 2016; Kiessling 2014; Liu 2005; Santini 2012). Sensitivity analysis of one trial with an overall low risk of bias (except for blinding of personnel during the surgical procedure) yielded no evidence of a difference for mortality (Peto OR 1.65, 95% CI 0.27 to 10.15; 1 study, 60 participants) (Buggeskov 2016). Trial Sequential Analysis on mortality showed that the information size required to detect or reject a risk ratio increase (RRI) of 100% was not achieved; therefore we could not conclude whether an intervention effect was evident (Figure 4). When further data become available and are added to this review, the cumulative meta‐analysis may reveal an effect (risk ratio reduction (RRR) or RRI of 100% or as a priori planned 30%) of pulmonary artery perfusion with blood during CPB on mortality. Subgroup analyses on preoperative lung function and short‐ versus long‐term follow‐up showed no differences in intervention effects on all‐cause mortality (within maximum follow‐up).
All trials reporting serious adverse events (SAEs) indicated that the effect of pulmonary artery perfusion with blood on the proportion of SAEs was uncertain (risk ratio (RR) 1.12, 95% CI 0.66 to 1.89; 3 studies, 180 participants; I2 = 29%; Analysis 1.2; GRADE: very low quality) (Buggeskov 2016; Kiessling 2014; Santini 2012). Sensitivity analysis of one trial with an overall low risk of bias (except for blinding of personnel during the surgical procedure) yielded no evidence of a difference in SAEs (RR 0.95, 95% CI 0.74 to 1.20; 1 study, 60 participants) (Buggeskov 2016). Trial Sequential Analysis revealed that only 17% of the required information size (1070 participants) had been reached, and that neither conventional nor trial sequential monitoring boundaries for benefit, harm, and futility had been crossed. Subgroup analyses on preoperative lung function and short‐ versus long‐term follow‐up revealed no differences in intervention effects on proportions of SAEs.
All trials reporting a ratio of partial pressure of oxygen in arterial blood (PaO2) to fraction of inspired oxygen (FiO2) at least six hours after surgery found that pulmonary artery perfusion with blood during CPB was associated with a higher PaO2/FiO2 ratio (mean difference (MD) 27.80, 95% CI 5.67 to 49.93; 3 studies, 119 participants; I2 = 0%; P = 0.01; Analysis 1.5; GRADE: very low quality) (Buggeskov 2016; Liu 2005; Santini 2012). Sensitivity and subgroup analyses on risk of bias, preoperative lung function, and short‐ versus long‐term follow‐up demonstrated similar results. Despite the overall result of traditional meta‐analysis, Trial Sequential Analysis showed that only 39% of the information size required to detect or reject a 10% (absolute 29.1 mmHg) increase in PaO2/FiO2 ratio was reached, and that no Trial Sequential Analysis boundaries were crossed. Therefore we cannot confirm the presence of an intervention effect of pulmonary artery perfusion with blood compared with no perfusion during CPB seen as a 10% increase or decrease in the PaO2/FiO2 ratio (Figure 5).
All trials showed that the effect of pulmonary artery perfusion with blood on intubation time after primary surgery was uncertain (MD ‐0.35, 95% CI ‐1.12 to 0.42; 4 studies, 176 participants; I2 = 23%; Analysis 1.7; GRADE: very low quality) (Buggeskov 2016; Liu 2005; Kiessling 2014; Santini 2012). Sensitivity and subgroup analyses on risk of bias, preoperative lung function, and short‐ versus long‐term follow‐up yielded similar results. Trial Sequential Analysis showed that the information size required to detect or reject an increase in intubation time of 1.5 hours was not surpassed,although trial sequential monitoring boundaries for futility were surpassed, refuting even a 1.5‐hour reduction in intubation time (Figure 6).
We were not able to detect a 30% excess in all‐cause mortality of pulmonary artery perfusion with blood during CPB in all trials, nor in all trials with low risk of bias. Furthermore, we did not find consistent evidence that pulmonary artery perfusion with blood during CPB is associated with risk of SAEs and prolonged or shortened intubation time. We found an association between pulmonary artery perfusion with blood and a higher postoperative PaO2/FiO2 ratio, but we were not able to confirm a 10% increase, as the cumulative Z‐curve of the Trial Sequential Analysis did not surpass the boundary for benefit. An association between a higher postoperative PaO2 /FiO2 ratio in patients receiving pulmonary artery perfusion with blood during CPB and clinical relevant outcomes such as mortality and pulmonary and serious adverse events is therefore arguable. Especially in the light of current discussions on targeting oxygen treatment to avoid hypoxaemia but without applying hyperoxia, this can lead to vasoconstriction on normal vasculature and can consequently reduce blood flow to at‐risk tissues (Hafner 2015; Sjöberg 2013).
Only one study included the intervention pulmonary artery perfusion with a preservation solution (Buggeskov 2016). Therefore it was not possible to include these data in a meta‐analysis. Buggeskov 2016 found no differences in mortality, SAEs, nor last measured PaO2 /FiO2 ratio. Likewise, Buggeskov 2016 reported no differences in intubation times between patients receiving pulmonary artery perfusion with a preservation solution and those given no perfusion during CPB, although the different statistical approach applied in the current review resulted in detection of a difference in intubation time between the two groups.
Overall completeness and applicability of evidence
We included all eligible randomized controlled trials (RCTs) up to July 2017. Buggeskov 2016, Kiessling 2014, and Santini 2012 were conducted in high‐income countries, and Liu 2005 in an upper‐middle‐income country. Trials included both sexes (35% women, 65% men), and participants were generally patients undergoing cardiac valve replacement or coronary artery bypass graft (CABG). All participants came from primary intervention trials. We found low statistical heterogeneity in our analyses of mortality and last measured PaO2/FiO2 ratio.
Only one trial reported the effect of pulmonary artery perfusion with blood on pulmonary events; accordingly we could not meta‐analyse study data (Buggeskov 2016). This trial did not report an association between pulmonary artery perfusion with blood and pulmonary events. Two trials reported effects on inflammatory markers but at different time points using only two of the same markers (Kiessling 2014; Santini 2012). Therefore we could not meta‐analyse those data.
In general we found insufficient data to conduct meaningful subgroup analyses and Trial Sequential Analyses.
Quality of the evidence
A major drawback of meta‐analyses performed for this review is the small total number of participants combined with multiple outcomes, which may account for high risk of both false‐positive and false‐negative results due to type II and type I errors, respectively. In addition, we classified three of the four included trials as having high risk of bias (Kiessling 2014; Liu 2005; Santini 2012). Therefore we graded the overall quality of evidence for all outcomes as very low.
We found no effect of pulmonary artery perfusion with blood during CPB on mortality, proportions of SAEs, nor intubation. However, for the outcome intubation time, the cumulative Z‐curve did cross the trial sequential monitoring boundaries for futility in the Trial Sequential Analysis, indicating that evidence was sufficient to refute a 10% decrease or increase. We found a higher postoperative PaO2/FiO2 ratio for participants receiving pulmonary artery perfusion with blood, although the cumulative Z‐curve did not cross trial sequential monitoring boundaries for benefit or harm. Trial Sequential Analysis does not remove risks of bias ‐ detected or undetected. It is relevant to discuss how much evidence is required to detect benefit or harm of the intervention, as estimated beneficial or harmful effects can occur as the result of random errors. Therefore assessment of sufficient information is needed to demonstrate a reliable benefit or harm of an intervention. For this review, Trial Sequential Analyses were based on anticipation of a rather large, but possibly not realistic, clinically relevant intervention effect. To detect or reject an even smaller intervention effect, much larger information sizes are required (Imberger 2015; Mascha 2015; Terkawi 2016).
Potential biases in the review process
We repeatedly searched several databases and contacted authors of trials investigating pulmonary artery perfusion compared with no perfusion during CPB, but the fact that one study ‐ Pichugin 2016 ‐ has not yet been incorporated may be a source of potential bias (see Studies awaiting classification). We also contacted medical firms producing preservation solutions to ask about former and ongoing trials. We therefore assume that we have not overlooked important RCTs.
In general, only about every second trial is reported, so possible reporting biases cannot be fully excluded (Gluud 2008). On the other hand, we obtained more information than was previously published in any systematic reviews or meta‐analyses.
We selected all trials and checked all data in duplicate, and we reached a high level of agreement. We were not blinded to study authors and bias risks while conducting quality assessments and data extractions. Two authors of this review (KB and JW) have been involved in one of the included trials (Buggeskov 2016); however, review authors not involved in the trial (LGR and ECR) extracted data and assessed bias for this trial.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review on effects of pulmonary artery perfusion with blood during CPB for open heart surgery in adults. Overview articles with data from both animal and human studies have been published previously but included no meta‐analyses (Apostolakis 2010; Carvalho 2008; Ng 2002; Suzuki 2010).
One RCT including 14 consecutive infants with ventricular or atrioventricular septal defect and pulmonary hypertension reported no effects of pulmonary artery perfusion with blood during CPB on death nor on adverse events (Suzuki 2001). A larger methodologically identical RCT including 30 infants and an earlier controlled clinical trial (CCT) reported similar results (Suzuki 1997; Suzuki 2001). An RCT including 24 infants with congenital heart defects and pulmonary hypertension randomized to receive pulmonary artery perfusion with hypothermic histidine‐tryptophan‐ketoglutarate (HTK) solution compared with no perfusion during CPB reported no deaths and did not report SAEs (Li 2010).
Two CCTs assigned 42 adults with acute aortic dissection prospectively and alternately to receive pulmonary artery perfusion with hypothermic Celsior (single‐shot) compared with no perfusion during selective cerebral perfusion (De Santo 2003a; De Santo 2003b). Both articles reported no differences in deaths nor in SAEs between groups. Last, one CCT prospectively assigned 16 participants who underwent CABG or AVR to receive pulmonary artery perfusion with hypothermic autologous blood (single‐shot) compared with no perfusion during CPB (Sievers 2002); investigators reported no deaths in either group and did not report SAEs.
Overall, data show no differences in reported harms including death between excluded studies.
Authors' conclusions
Implications for practice.
The effects of pulmonary artery perfusion with blood during cardiopulmonary bypass (CPB) are uncertain owing to the small numbers of participants included in meta‐analyses. Risks of death and serious adverse events may be higher with pulmonary artery perfusion with blood during CPB, and robust evidence for any beneficial effects is lacking. Future randomized controlled trials (RCTs) should provide long‐term follow‐up and patient stratification by preoperative lung function and other documented risk factors for mortality. One study that is awaiting classification (epub abstract with preliminary results) may change the results of this review when the full study report is published.
Implications for research.
Before definitive conclusions can be drawn, additional RCTs must explore the effects of pulmonary artery perfusion with blood compared with no perfusion during CPB on mortality and serious adverse events (SAEs). Larger trials with low risk of bias in all bias domains must examine patient‐important outcomes such as all‐cause mortality, SAEs, and health‐related quality of life. A recent systematic review described the need for a core set of patient‐relevant clinical and quality of life‐related outcomes for inclusion in future cardiac surgery trials (Benstoem 2015). Future RCTs should provide long‐term follow‐up while including patients stratified by preoperative lung function and other documented risk factors for mortality.
Investigators should design future trials according to SPIRIT guidelines and should report results according to CONSORT guidelines (Chan 2013; www.consort‐statement.org). Future trials should also provide individual participant data for inclusion in meta‐analyses on longitudinal outcomes adjusted for relevant confounders. In future updates of this review, we will seek to conduct analyses of the effects of pulmonary artery perfusion during CPB on pulmonary events.
Acknowledgements
We would like to thank Sarah Klingenberg, Trials Search Co‐ordinator, for preliminary searches of electronic databases, and the Cochrane Hepato‐Biliary Group (CHBG) for their support. We would also like to thank Arash Afshari (Content Editor), Nathan Pace (Statistical Editor), and Peter Alston and Ben Gibbison (Peer Reviewers) for help and editorial advice provided during preparation of the protocol for this systematic review. We thank Arash Afshari (Content Editor); the ACE Editorial Screening Board (Bronagh Blackwood, Jane Cracknell, and Harald Herkner); Nathan Pace (Senior Statistical Editor and Co‐ed); Janne Vendt (Information Specialist); Cathal Walsh (Statistical Editor); R. Peter Alston, Hamdy Awad, and Ben Gibbison (Peer Reviewers); and Brian Stafford (Consumer Referee) for help and editorial advice provided during preparation of this systematic review.
Appendices
Appendix 1. Search strategies for identification of studies
| Database | Period of search | Search strategy |
| Cochrane Central Register of Controlled Trials (CENTRAL) |
Latest issue | #1 MeSH descriptor Thoracic Surgery explode all trees #2 MeSH descriptor Extracorporeal Circulation explode all trees #3 MeSH descriptor Heart‐Lung Machine explode all trees #4 ((cardiac OR ' heart') AND surgery) OR 'cardiopulmonary bypass' OR CPB OR (extra corporeal near circulation) OR ECC OR ((heart or lung) near machine) #5 MeSH descriptor Perfusion explode all trees #6 MeSH descriptor Pulmonary Circulation explode all trees #7 (pulmonary OR lung) AND (perfusion OR flow OR oxygenation) #8 (cannulation near pulmonary artery) OR pulmoplegia #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 'blood' OR custodiol OR histidine‐tryptophan‐ketoglutarate OR HTK #11 (#9 AND #10) |
| MEDLINE (OvidSP) | From 1946 | 1. exp Thoracic Surgery/ 2. exp Extracorporeal Circulation/ 3. exp Heart‐Lung Machine/ 4. (((cardiac or ' heart') and surgery) or 'cardiopulmonary bypass' or CPB or (extra corporeal adj3 circulation) or ECC or ((heart or lung) adj3 machine)).mp. 5. exp Perfusion/ 6. exp Pulmonary Circulation/ 7. ((pulmonary or lung) and (perfusion or flow or oxygenation)).mp. 8. ((cannulation adj3 pulmonary artery) or pulmoplegia).mp. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. ('blood' or custodiol or histidine‐tryptophan‐ketoglutarate or HTK).mp. 11. 9 and 10 12. (random* or blind* or placebo* or meta‐analys*).mp. 13. 11 and 12 |
| Embase (OvidSP) | From 1974 | 1. exp thorax surgery/ 2. exp extracorporeal circulation/ 3. exp heart lung machine/ 4. (((cardiac or ' heart') and surgery) or 'cardiopulmonary bypass' or CPB or (extra corporeal adj3 circulation) or ECC or ((heart or lung) adj3 machine)).mp. 5. exp perfusion/ 6. exp lung circulation/ 7. ((pulmonary or lung) and (perfusion or flow or oxygenation)).mp. 8. ((cannulation adj3 pulmonary artery) or pulmoplegia).mp. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. ('blood' or custodiol or histidine‐tryptophan‐ketoglutarate or HTK).mp. 11. 9 and 10 12. (random* or blind* or placebo* or meta‐analys*).mp. 13. 11 and 12 |
| Science Citation Index Expanded | From 1900 | # 1 TS=(((cardiac or ' heart') and surgery) or 'cardiopulmonary bypass' or CPB or 'extra corporeal circulation' or ECC or 'heart lung machine') # 2 TS=((pulmonary or lung) and (perfusion or flow or oxygenation)) # 3 TS=((cannulation SAME pulmonary artery) or pulmoplegia) # 4 #3 OR #2 OR #1 # 5 TS=('blood' or custodiol or histidine‐tryptophan‐ketoglutarate or HTK) # 6 #5 AND #4 # 7 TS=(random* or blind* or placebo* or meta‐analys*) # 8 #7 AND #6 |
| WHO ICTRP | From 2005 | #1 MeSH descriptor Thoracic Surgery #2 MeSH descriptor Extracorporeal Circulation #3 MeSH descriptor Heart‐Lung Machine #4 ((cardiac OR ' heart') AND surgery) OR 'cardiopulmonary bypass' OR CPB OR (extra corporeal near circulation) OR ECC OR ((heart or lung) near machine) #5 MeSH descriptor Perfusion #6 MeSH descriptor Pulmonary Circulation #7 (pulmonary OR lung) AND (perfusion OR flow OR oxygenation) #8 (cannulation near pulmonary artery) OR pulmoplegia #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 'blood' OR custodiol OR histidine‐tryptophan‐ketoglutarate OR HTK #11 (#9 AND #10) |
Appendix 2. Data extraction form
| TRIAL IDENTIFICATION | ||||
| Review | Pulmonary artery perfusion versus no perfusion during cardiopulmonary bypass for cardiac surgical |
YES | ||
| Title | ||||
| Authors | ||||
| Country | ||||
| Language | ||||
| Publication type | Lead trial | YES | Secondary publication to: | TRIAL NAME: |
| NO | ||||
| TRIAL | ||
| Clinical trial | Yes | No |
| Randomized | Yes | No |
| TRIAL INFORMATION | |||
| Date published | |||
| Time frame of trial period and duration of follow‐up period | |||
| Funding | |||
| Inclusion and exclusion criteria |
Inclusion criteria: |
Exclusion criteria: |
|
| Whether a calculation of sample size has been published | YES | NO | |
| Number of research participants | |||
| Number of included research participants | |||
| Whether a protocol has been published before launch of randomization | YES | NO | |
| Is the trial registered athttp://www.clinicaltrials.gov/? | YES | NO | |
| METHODS | ||||
|
Generation of allocation sequence |
Adequate (If randomizing is performed by computer or a “random number table”. If the randomizing is a random process, e.g. “heads or tails” or a throw of a dice; and the person performing the procedure in no other way is involved in the trial) |
Uncertain (If the procedure in respect of randomizing is not sufficiently described) |
Inadequate (If the trial uses, e.g. date of admission or alternation for allocating the participants. Such trials will be included only in the assessment of harms) |
|
|
Allocation concealment |
Adequate If the allocation sequence is concealed from the investigators, treatment providers, and participants, for example, by central randomization. And this procedure is described and documented |
Uncertain If the procedure to conceal allocation is not sufficiently described |
Inadequate If treatment providers/clinical principal investigators/study participants are able to predict the allocation sequence. Such trials will be included only in the assessment of harms |
|
| Intention‐to‐treat analysis | Adequate |
Uncertain |
Inadequate |
|
|
Blinding |
Adequate If the personnel who instruct or supply or assess the observer‐dependent questionnaire are blinded and this is described. Thus, personnel performing these procedures must not be otherwise involved in the trial |
Uncertain If the procedure of blinding is insufficiently described |
Inadequate If blinding is not performed or if the procedure cannot be classified as ‘adequate’ or ‘uncertain’ |
|
|
Dropouts |
Adequate If dropouts following randomizing can be described as being the same in the two intervention groups |
Uncertain If dropouts are not stated, or if the reasons why participants dropped out are unclear |
Inadequate If the pattern of dropouts can be described as different in the 2 intervention groups |
|
| Reporting of outcome measures |
Adequate If all outcome measures are stated in the results. And the hierarchy of the efficacy variables are documented in a protocol before launch of randomization |
Uncertain If the method of choosing outcome measures is inadequately described |
Inadequate If incongruence is evident between the original protocol and the outcome measures used in the results, or if not all of the outcome measures are stated |
|
|
Economic bias |
Adequate If the trial is not financed by an authority that might have an interest in a given result |
Uncertain If no description is provided of how the trial is financed |
Inadequate If the trial is financed by an authority that could have an interest in a specific result from the trial |
|
|
Academic bias sources |
Adequate If trialists do not have an academic/personal interest in a given result from the trial |
Uncertain If no description is provided of any academic interests that trialists might have |
Inadequate If trialists have a direct interest in a given result from the trial |
|
| Overall bias assessment |
LOW (adequate everything) |
HIGH | ||
| PARTICIPANTS: | ||
| 18 years or older | Yes | No |
| Under 18 years | Yes | No |
| Equal distribution of age | Yes | No |
| Equal distribution of gender | Yes | No |
| Heart surgery with CPB | Yes | No |
| Elective surgery | Yes | No |
| Urgent, emergency or salvage surgery | Yes | No |
| Aortic valve replacement | Yes | No |
| Mitral valve replacement | Yes | No |
| Coronary artery bypass surgery | Yes | No |
| Double procedure | Yes | No |
| Surgery for congenital heart defects | Yes | No |
| Pulmonary hypertension (PAMP > 25 mmHg) | Yes | No |
| Reduced lung function (FEV1 < 80 PP) | Yes | No |
| Preoperative normal renal function (P‐creatinine from 50‐90 µmol/L) | Yes | No |
| IDDM | Yes | No |
| Recent MI (within 3 months before surgery) | Yes | No |
| LV function good (LVEF > 50%) | Yes | No |
| LV function moderate (LVEF 31%‐50%) | Yes | No |
| LV function poor (LVEF 21%‐30%) | Yes | No |
| LV function very poor (LVEF ≤ 20%) | Yes | No |
| INTERVENTIONS AND SURGICAL DETAILS: | |||
| Assess whether the trial intervention should be classified as ‘adequately defined’ |
|||
| Pulmonary artery perfusion during CPB | Yes | No | |
| Pulmonary artery perfusion during aortic cross‐clamp | Yes | No | |
| Hypothermic CPB | Yes | No | |
| Normothermic CPB | Yes | No | |
| Single‐shot pulmonary artery perfusion with oxygenated blood | Yes | No | |
| Continuous pulmonary artery perfusion with oxygenated blood | Yes | No | |
| Single‐shot pulmonary artery perfusion with deoxygenated blood | Yes | No | |
| Continuous pulmonary artery perfusion with deoxygenated blood | Yes | No | |
| Single‐shot pulmonary artery perfusion with a solution used for perfusion and flushing of organs | Yes | No | |
| Continuous pulmonary artery perfusion with a solution used for perfusion and flushing of organs | Yes | No | |
| CO‐INTERVENTIONS | Yes | No | |
| Ventilation of the lungs during CPB | Yes | No | |
| Add‐on therapy to any kind of therapy described and delivered similarly to different intervention groups |
|||
| OUTCOMES FOR INTERVENTIONS AND CONTROL GROUPS | |||||||||||
|
Primary outcome specified in the trial |
Pulmonary artery perfusion |
Pulmoplegia |
Control |
||||||||
|
Primary outcome measures for intervention and control groups (all responses will be calculated based on total numbers of randomized participants) | |||||||||||
|
All‐cause mortality (at maximum follow‐up). |
Pulmonary artery perfusion (# of people) |
Pulmoplegia (# of people) |
Control (# of people) |
||||||||
| Pulmonary events (e.g. numbers of participants with reduced postoperative oxygenation values (specified as reduction in OI or increase in PaO2/FiO2 ratio), prolonged mechanical ventilation (> 3 hours), pneumonia, ARDS) | (# of people) | (# of people) | (# of people) | ||||||||
|
Secondary outcomes (all responses will be calculated based on total numbers of randomized participants) | |||||||||||
| Adverse events. Serious adverse events are defined as medical events that are life‐threatening; result in death, disability, or significant loss of function; or cause hospital admission or prolonged hospitalization or a hereditary anomaly or foetal injury. All other adverse events (i.e. events that have not necessarily had a causal relationship with the treatment, but that resulted in change in or cessation of treatment) will be considered non‐serious events. |
Pulmonary artery perfusion (# of people) |
Pulmoplegia (# of people) |
Control (# of people) |
||||||||
| PaO2/FiO2ratio (PaO2/FiO2= PaO2/FiO2) or OI index ( FiO2 ×Mpaw/PaO2) |
(MEAN and # of people) |
(SD) |
(MEAN and # of people) |
(SD) |
(MEAN and # of people) |
(SD) |
|||||
| Intubation time (hours) | (MEAN and # of people) |
(SD) |
(MEAN and # of people) |
(SD) |
(MEAN and # of people) |
(SD) |
|||||
| Time at the ICU (hours) | (MEAN and # of people) |
(SD) |
(MEAN and # of people) |
(SD) |
(MEAN and # of people) |
(SD) |
|||||
|
Reintubation |
(# of people) |
(# of people) |
(# of people) |
||||||||
|
Readmission to the ICU |
(# of people) |
(# of people) |
(# of people) |
||||||||
| Number of cases of pneumonia | (# of people) |
(# of people) |
(# of people) |
||||||||
| Effect of the intervention on markers of inflammation in plasma (write which inflammatory markers were measured) | (MEAN for each inflammatory marker) |
SD (for each inflammatory marker) |
(MEAN for each inflammatory marker) |
SD (for each inflammatory marker) |
(MEAN for each inflammatory marker) |
SD (for each inflammatory marker) |
|||||
| Effect of the intervention on markers of inflammation in bronchoalveolar lavage fluid (write which inflammatory markers were measured) | (MEAN for each inflammatory marker) |
SD (for each inflammatory marker) |
(MEAN for each inflammatory marker) |
SD (for each inflammatory marker) |
(MEAN for each inflammatory marker) |
SD (for each inflammatory marker) |
|||||
|
Days alive outside hospital within 90 days |
(MEAN and # of people) |
SD |
(MEAN and # of people) |
SD |
(MEAN and # of people) |
SD |
|||||
| Number of readmissions with a ventilation‐related problem or dyspnoea treated by the patient’s general practitioner | (MEAN and # of people) |
SD |
(MEAN and # of people) |
SD |
(MEAN and # of people) |
SD |
|||||
Appendix 3. Serious adverse events
| SAEs for PP with blood | SAEs for no PP | |
| Santini et al (2012) | 1 Reoperation | |
| Kiessling et al | 2 Deaths 3 Tracheotomies 3 Reoperations 2 Strokes 7 Respiratory failures |
1 Death 3 Reoperations 1 Stroke 5 Respiratory failures |
| Buggeskov et al | 3 Deaths 5 Pneumothoraces requiring drain 1 Pleural effusions requiring drainage 2 Major bleedings 3 Reoperations 9 Severe infections 1 Acute myocardial infarctions 2 Cases of cardiac arrest 4 Maligned arrhythmias 3 Cases requiring dialysis |
2 Deaths 7 Pneumothoraces requiring drain 7 Pleural effusion requiring drainage 2 Reoperations 10 Severe infections 1 Stroke 1 Acute myocardial infarction 1 Cardiac arrest 3 Maligned arrhythmias |
Data and analyses
Comparison 1. Pulmonary artery perfusion with blood versus no perfusion during cardiopulmonary bypass.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (at maximum follow‐up) | 4 | 210 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.78 [0.43, 7.40] |
| 1.1 Low risk of bias | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [0.27, 10.15] |
| 1.2 High or uncertain risk of bias | 3 | 150 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.00 [0.20, 20.03] |
| 2 Participants with 1 or more SAEs (at maximum follow‐up) | 3 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.66, 1.89] |
| 2.1 Low risk of bias | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.74, 1.20] |
| 2.2 High or uncertain risk of bias | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.84, 2.93] |
| 3 Participants with 1 or more SAEs stratified according to preoperative lung function | 3 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.66, 1.89] |
| 3.1 Patients with normal lung function | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.89] |
| 3.2 Patients with COPD | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.63, 2.23] |
| 4 Participants with 1 or more SAEs stratified according to short‐ or long‐term follow‐up | 3 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.66, 1.89] |
| 4.1 Short‐term follow‐up (< 30 days) | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.89] |
| 4.2 Long‐term follow‐up (≧ 30 days) | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.63, 2.23] |
| 5 Last measured PaO2 /FiO2 ratio (mmHg) | 3 | 119 | Mean Difference (IV, Random, 95% CI) | 27.80 [5.67, 49.93] |
| 5.1 Low risk of bias | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 39.67 [‐16.33, 95.67] |
| 5.2 High or uncertain risk of bias | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 25.60 [1.51, 49.70] |
| 6 Last measured PaO2 /FiO2 ratio (mmHg) stratified according to preoperative lung function | 3 | 119 | Mean Difference (IV, Random, 95% CI) | 27.80 [5.67, 49.93] |
| 6.1 Participants with normal lung function | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 25.60 [1.51, 49.70] |
| 6.2 Participants with COPD | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 39.67 [‐16.33, 95.67] |
| 7 Intubation time (hours) after primary surgery | 4 | 176 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.12, 0.42] |
| 7.1 Low risk of bias | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.24, 0.24] |
| 7.2 High or uncertain risk of bias | 3 | 116 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.39, 0.35] |
| 8 Intubation time (hours) stratified according to preoperative lung function | 4 | 176 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.12, 0.42] |
| 8.1 Participants with normal lung function | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐2.92, 1.71] |
| 8.2 Participants with COPD | 2 | 116 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐2.24, 0.24] |
Comparison 2. Pulmonary artery perfusion with a preservation solution versus no perfusion during cardiopulmonary bypass.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (at maximum follow‐up) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.29, 8.92] |
| 1.1 Low risk of bias | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.29, 8.92] |
| 2 Participants with 1 or more SAEs (at maximum follow‐up) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.88, 1.30] |
| 2.1 Low risk of bias | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.88, 1.30] |
| 3 Last measured PaO2/FiO2 ratio (mmHg) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐25.34 [‐69.16, 18.48] |
| 3.1 Low risk of bias | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐25.34 [‐69.16, 18.48] |
| 4 Intubation time (hours) after primary surgery | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐3.50, ‐0.50] |
| 4.1 Low risk of bias | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐3.50, ‐0.50] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Buggeskov 2016.
| Methods | Parallel‐group randomized controlled trial conducted from July 2012 to November 2013 in Denmark | |
| Participants | 90 participants > 18 years of age with COPD undergoing CABG and/or AVR | |
| Interventions | Continous pulmonary artery perfusion with normothermic oxygenated blood or single‐shot pulmonary artery perfusion with hypothermic HTK Solution compared with no pulmonary artery perfusion during cardiopulmonary bypass | |
| Outcomes | OI measured from surgery start until 24 hours after, mortality within 90 days, SAEs, intubation time, ICU, and in‐hospital length of stay | |
| Notes | Potential conflicts of interest due to pharmaceutical funding. First trial author received in January 2012 a restricted grant of 4 months' salary to write the protocol for The Pulmonary Protection Trial (200.000 DKR out of a total budget for The Pulmonary Protection Trial of 3.2 million DKR) from a pharmaceutical company distributing (but not producing) Custodiol HTK Solution (Buggeskov 2013). In addition, the first trial author was granted an independent scholarship from The Lundbeck Foundation, which is a not‐for‐profit independent foundation (see Declarations of interest). Lars Grønlykke and Emilie C. Risom extracted these data to avoid bias. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Performed centrally at a trial web page by block randomization |
| Allocation concealment (selection bias) | Low risk | Performed centrally at a trial web page by block randomization. Allocations were performed by a computer‐generated sequence blinded to investigators, treatment providers, and participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was performed for participants, care personnel at the ICU and surgical ward, the statistician, and those drawing conclusions. Investigators and personnel at the operating room were not blinded to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The statistician and those drawing conclusions were blinded to intervention group assignment during the analysis phase and during writing of the abstracts. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were included in the intension‐to‐treat analysis, and none were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | A prepublished protocol describing trial outcomes was found comparable with the article in terms of outcomes reported. |
| Other bias | Low risk | A prepublished statistical analysis plan was found comparable with the article in terms of outcomes reported. |
Kiessling 2014.
| Methods | Parallel‐group randomized controlled trial conducted from January 2011 to September 2012 in Germany | |
| Participants | 59 participants > 18 years old with COPD undergoing CABG and valve surgery | |
| Interventions | Single‐shot pulmonary artery perfusion with deoxygenated blood (every 20 minutes) during CPB vs no perfusion during CPB | |
| Outcomes | Alveolar‐arterial oxygen gradient (A‐aDo2), OI measured before and after surgery, mortality, SAEs, intubation time, ICU and in‐hospital lengths of stay, and serum cytokine | |
| Notes | Potential economic bias and conflicts of interests. The trial was funded by a pharmaceutical company. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized by the study co‐ordinator and by the clinical research institute. |
| Allocation concealment (selection bias) | Low risk | Randomization was performed by the study co‐ordinator and by the clinical research institute as block randomization based on an Excel sheet. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was performed unblinded for all parties. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The trial was performed unblinded for all parties. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Statistical analysis was based on per‐protocol as‐treated analysis. |
| Selective reporting (reporting bias) | High risk | No prelaunch randomization written protocol specified planned outcome measures. |
| Other bias | Unclear risk | Potential economic bias and conflicts of interests. The trial was funded by a pharmaceutical company. |
Liu 2005.
| Methods | Parallel‐group randomized controlled trial conducted from 2005 to 2006 in China | |
| Participants | 30 participants with rheumatic mitral stenosis who had undergone mitral valve replacement and in some cases also tricuspid valvuloplasty | |
| Interventions | Continuous pulmonary artery perfusion with oxygenated blood during CPB vs no perfusion during CPB | |
| Outcomes | CPB time, aortic cross‐clamp time, intubation time, ICU‐LOS, PaO2/FiO2 ratio measured before surgery and until 6 hours after surgery, mortality, pathological changes in lung tissue | |
| Notes | SAEs were not reported. Funding and potential conflicts of interest were not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. Just states "randomized" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants or personnel was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was performed. Dropouts were described. |
| Selective reporting (reporting bias) | Unclear risk | A prelaunch randomization protocol was not described. |
| Other bias | Unclear risk | Funding and potential conflicts of interest were not described. |
Santini 2012.
| Methods | Parallel‐group randomized controlled trial conducted from November 2008 to April 2011 in Italy | |
| Participants | 64 participants > 18 years of age undergoing elective, first‐time, isolated, low‐risk (logistic EuroSCORE (European System for Cardiac Operative Risk Evaluation) 5%), 3‐vessel CABG | |
| Interventions | Pulsatile continuous pulmonary artery perfusion with oxygenated blood during CPB vs no perfusion during CPB | |
| Outcomes | Bronchoalveolar lavage fluid cellular count and cytokines, serum cytokines, A‐aDO2, mortality, SAEs | |
| Notes | Data for the PaO2/FiO2 ratio (measured before surgery, 3 hours after surgery, and at extubation) and intubation time were drawn from the 2011 publication (Santini 2011), as they were not reported in the 2012 publication (Santini 2012). Santini 2011 was conducted from November 2008 to November 2011 in Italy. Funding and potential conflicts of interest were not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Prospectively randomized by lottery, with drawing of pre‐prepared sealed envelopes containing group assignment at hospital admission |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants or personnel was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Postrandomization dropouts were described, but intention‐to‐treat analysis or per‐protocol analysis was not described. |
| Selective reporting (reporting bias) | Unclear risk | A pre‐randomization protocol describing outcome measures was not provided. |
| Other bias | Unclear risk | Funding and potential conflicts of interest were not described. |
A‐aDo2: alveolar‐arterial oxygen gradient. AVR: aorta valve replacement. CABG: coronary artery bypass graft. COPD: chronic obstructive pulmonary disease. CPB: cardiopulmonary bypass. DKR: Danish krone. EuroSCORE: European System for Cardiac Operative Risk Evaluation. HTK: histidine‐tryptophan‐ketoglutarate. ICU: intensive care unit. LOS: length of stay. OI: oxygenation index. PaO2/FiO2: ratio of partial pressure of alveolar oxygen to fraction of inspired oxygen. SAE: serious adverse event.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| De Santo 2003a | CCT in adults |
| De Santo 2003b | CCT in adults |
| Li 2010 | RCT including infants with congenital heart disease |
| Sievers 2002 | CCT in adults |
| Suzuki 1997 | CCT including infants with ventricular septal defect |
| Suzuki 2000 | RCT including children with congenital heart defects and pulmonary hypertension |
| Suzuki 2001 | RCT including children with congenital heart defects |
| Wei 2004 | RCT including children with congestive heart failure |
| Yi 2006 | RCT in adults comparing pulmonary artery perfusion with blood vs pulmonary artery perfusion with blood with a Chinese medicine named Shenqi Fuzheng added. No control group without pulmonary artery perfusion |
CCT: controlled clinical trial. RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Pichugin 2016.
| Methods | Parallel‐group randomized controlled trial |
| Participants | 70 participants > 18 years of age with pulmonary hypertension undergoing valve surgery with CPB |
| Interventions | Pulmonary artery perfusion with oxygenated or non‐oxygenated blood and reduced volumes of lung ventilation during CPB compared with no perfusion and no ventilation during CPB |
| Outcomes | Alveolar‐arterial oxygen difference, oxygenation index, PaCO2, F‐shunt parameter, lung compliance during operation |
| Notes | Epub abstract with preliminary results available ahead of the full article |
CPB: cardiopulmonary bypass. PaCO2: partial pressure of carbon dioxide in arterial blood.
Differences between protocol and review
We made the following changes to the published protocol(Buggeskov 2014).
We changed the title from "Pulmonary perfusion versus no pulmonary perfusion during cardiopulmonary bypass for heart surgery" to the more explanatory title "Pulmonary artery perfusion versus no perfusion during cardiopulmonary bypass for open heart surgery".
Three trials performed continuous pulmonary artery perfusion during CPB (Buggeskov 2016; Liu 2005; Santini 2012), and one trial performed repetitive pulmonary artery perfusion every 20 minutes during CPB (Kiessling 2014). Owing to insufficient data, we did not perform the subgroup analysis "single shot versus continuous pulmonary artery perfusion".
We assigned overall low risk of bias to RCTs with low risk of bias in all domains, except for blinding of personnel in the operating room, owing to the inherent problem with blinding of a surgical intervention. Therefore results should still be interpreted in the light of risk of bias.
Our primary plan was to report results from trials with overall low risk of bias in the review text and in the 'Summary of findings' table (Table 1). Owing to sparse data, we have reported results from all trials in this version of the review, but in future updates with more RCTs, we will prioritize results from analyses of trials with overall low risk of bias.
We did not include the subgroup analysis of "All‐cause mortality" according to lung function and follow‐up period in this version of the review, given the small number of included studies and lack of statistical heterogeneity.
For the secondary primary outcome "pulmonary events", only one trial reported pneumonia (Buggeskov 2016), and no trials reported ARDS. For this version of the review, we report oxygenation values (the PaO2/FiO2 ratio) and intubation time only as continuous outcomes, not as patient‐specific pulmonary events, because data show only mean differences. Therefore, we could not calculate the secondary primary outcome "pulmonary events", and we made a pragmatic decision to add PaO2/FiO2 ratio and intubation time as two new secondary explorative outcomes for this version of the review.
For future versions of the review, we will if possible change the interval for "prolonged mechanical ventilation" from > three hours to > 12 hours, as it correlates better in severity with other pulmonary events such as pneumonia and ARDS. The same applies for OI as a dichotomous outcome, which in future versions of the review we will define as a pulmonary event when △OI is 20% higher 24 hours after surgery compared with baseline OI.
Regarding the secondary outcome "Number of patients with one or more SAEs", the ICH describes an SAE as that which results in death; is life threatening; requires hospitalization or prolongation of existing hospitalization; or leads to persistent or significant disability or incapacity; or any medical event that may jeopardize the patient or require an intervention to prevent it (ICH‐GCP 1997). As we already report mortality in the primary outcome, the secondary outcome "Number of patients with one or more SAEs" does not include death, although this differs from the ICH definition.
Regarding the secondary outcome "Increases in inflammatory markers in the plasma or bronchoalveolar lavage fluid", only one trial reported on inflammatory markers in bronchoalveolar lavage fluid (Santini 2012). We will refer to these markers under the section "Effects of interventions" but will not include them in the statistical analysis. For inflammatory markers, Kiessling 2014 and Santini 2012 reported plasma interleukin‐1 and tumour necrosis factor alpha as the only identical inflammatory markers but measured these at different time points. We will therefore refer to inflammatory markers in plasma only under the section "Effects of interventions" and will not include these in statistical analyses.
Contributions of authors
Contact author: Katrine Bredahl Buggeskov (KBB).
Conceiving the review: KBB.
Co‐ordinating the review: KBB, Jonas B Nielsen (JBN), Jørn Wetterslev (JW).
Undertaking manual searches: Sarah Klingenberg (CHBG), Karen Hovhannisyan (CARG).
Screening search results: KBB, Lars Grønlykke (LG).
Organizing retrieval of papers: KBB.
Screening retrieved papers against inclusion criteria: KBB, LG, Emilie C. Risom (ECR), Mao Ling Wei (MLW).
Appraising quality of papers: KBB, LG, ECR, MLW.
Abstracting data from papers: KBB, LG, ECR, MLW.
Writing to authors of papers for additional information: KBB.
Providing additional data about papers: KBB.
Obtaining and screening data on unpublished studies: KBB.
Managing data for the review: KBB, JW.
Entering data into Review Manager (RevMan 5.3): KBB.
Analysing RevMan statistical data: KBB, JW.
Performing other statistical analysis not using RevMan: KBB, JW.
Interpreting data: KBB, JW.
Making statistical inferences: KBB, JW.
Performing GRADE assessment: KBB, JW.
Writing the review: KBB, JW.
Securing funding for the review: KBB.
Performing previous work that was the foundation of the present review: KBB.
Serving as guarantor for the review (one review author): KBB.
Taking responsibility for reading and checking the review before submission: KBB, LG, ECR, MLW, JW.
Declarations of interest
Katrine B Buggeskov (KBB) participated in The Pulmonary Protection Trial (Buggeskov 2013; Buggeskov 2016). To avoid bias, Lars Grønlykke and Emilie C. Rison extracted data from this trial.
KBB was granted a scholarship in March 2012 (1.000.000 DKR out of a total budget for The Pulmonary Protection Trial of 3.2 million DKR) from The Lundbeck Foundation. This is an independent not‐for‐profit foundation established in 1954 by Grete Lundbeck, widow of the founder of the pharmaceutical company H. Lundbeck A/S. The independent scholarship was granted for conduction of The Pulmonary Protection Trial as a part of Dr. Buggeskov's PhD programme. The Lundbeck Foundation does not produce or distribute Custodiol HTK solution. In addition, in January 2012, KBB received from a pharmaceutical company (Nordmedica A/S, now Pharmanovia A/) a restricted grant of four months' salary to write the protocol for The Pulmonary Protection Trial (200.000 DKR out of a total budget for The Pulmonary Protection Trial of 3.2 million DKR) (Buggeskov 2013). Normedica (Pharmanovia) distributes, but does not produce, Custodiol HTK Solution. Pharmanovia did not support the conduct of the trial nor analyses and writing of the trial report, and had no influence whatsoever on design, analysis, or reporting of trial results.
The grant was not related to writing of the Cochrane review protocol nor the review, and was issued approximately one year before initiation of the application for the title of the review in March 2013 (Buggeskov 2014). The Cochrane review was first written in 2016 (four years after receipt of the restricted grant from Normedica A/S and the scholarship from the independent Lundbeck Foundation) because KBB was on maternity leave in 2015. For further information, see the Characteristics of included studies table.
Jonas B Nielsen: none declared.
Lars Grønlykke: none declared.
Emilie C. Risom: none declared.
Mao Ling Wei: none declared.
Jørn Wetterslev (JW) participated in The Pulmonary Protection Trial and co‐authored Buggeskov 2013 and Buggeskov 2016. Therefore Lars Grønlykke and Emilie C. Risom extracted data from this trial to avoid bias. JW is a member of the task force at the Copenhagen Trial Unit commissioned to develop theory and software for Trial Sequential Analysis, available as freeware at http://www.ctu.dk/tsa/.
New
References
References to studies included in this review
Buggeskov 2016 {published data only}
- Buggeskov KB, Sundskard MM, Jonassen T, Andersen LW, Secher NH, Ravn HB, et al. Pulmonary artery perfusion versus no pulmonary perfusion during cardiopulmonary bypass in patients with chronic obstructive pulmonary disease: a randomised clinical trial. BMJ Open Respiratory Research 2016;6(3):e000146. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiessling 2014 {published and unpublished data}
- Kiessling A‐H, Guo FW, Gökdemir Y, Thudt M, Reyher C, Scherer M, et al. The influence of selective pulmonary perfusion on the inflammatory response and clinical outcome of patients with chronic obstructive pulmonary disease undergoing cardiopulmonary bypass. Interactive Cardiovascular Thoracic Surgery 2014;18(6):732–9. [PUBMED: 24632426] [DOI] [PubMed] [Google Scholar]
Liu 2005 {published data only}
- Liu L, Hu J, Yin B, Yang Y, Zhang W, Yi D, et al. Lung protection of continuous pulmonary artery perfusion with oxygenated blood during cardiopulmonary bypass. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2005;30(4):413–6. [PUBMED: 16190386] [PubMed] [Google Scholar]
Santini 2012 {published data only}
- Santini F, Onorati F, Telesca M, Menon T, Mazzi P, Berton G, et al. Selective pulmonary pulsatile perfusion with oxygenated blood during cardiopulmonary bypass attenuates lung tissue inflammation but does not affect circulating cytokine levels. European Journal of Cardiothoracic Surgery 2012;42(6):942–50. [PUBMED: 22561658] [DOI] [PubMed] [Google Scholar]
- Santini F, Onorati F, Telesca M, Patelli F, Berton G, Franchi G, et al. Pulsatile pulmonary perfusion with oxygenated blood ameliorates pulmonary hemodynamic and respiratory indices in low‐risk coronary artery bypass patients. European Journal of Cardiothoracic Surgery 2011;40(4):794–803. [PUBMED: 21388821] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
De Santo 2003a {published data only}
- Santo LS, Romano G, Amarelli C, Della Corte A, Onorati F, Torella M, et al. Pilot study on prevention of lung injury during surgery for type A acute aortic dissection: no evident improvements with celsior flushing through the pulmonary artery. International Journal of Artificial Organs 2003;26(11):1032–8. [PUBMED: 14708832] [DOI] [PubMed] [Google Scholar]
De Santo 2003b {published data only}
- Santo LS, Romano G, Amarelli C, Onorati F, Torella M, Renzulli A, et al. Surgical repair of acute type A aortic dissection: continuous pulmonary perfusion during retrograde cerebral perfusion prevents lung injury in a pilot study. Journal of Thoracic and Cardiovascular Surgery 2003;126(3):826‐31. [PUBMED: 14502161] [DOI] [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li J‐A, Liu Y‐L, Liu J‐P, Li X‐F. Pulmonary artery perfusion with HTK solution prevents lung injury in infants after cardiopulmonary bypass. Chinese Medical Journal 2010;123(19):2645‐50. [PUBMED: 21034646] [PubMed] [Google Scholar]
Sievers 2002 {published data only}
- Sievers H‐H, Freund‐Kaas C, Eleftheriadis S, Fischer T, Kuppe H, Kraatz EG, et al. Lung protection during total cardiopulmonary bypass by isolated lung perfusion: preliminary results of a novel perfusion strategy. Annals of Thoracic Surgery 2002;74(4):1167‐72. [PUBMED: 12400763] [DOI] [PubMed] [Google Scholar]
Suzuki 1997 {published data only}
- Suzuki T, Fukuda T, Inoue Y, Aki A, Cho Y. Effectiveness of continuous pulmonary perfusion during total cardiopulmonary bypass to prevent lung reperfusion injury. Zasshi J Nihon Kyōbu Geka Gakkai 1997;45(1):31–6. [PUBMED: 9028120] [PubMed] [Google Scholar]
Suzuki 2000 {published data only}
- Suzuki T, Fukuda T, Ito T, Inoue Y, Cho Y, Kashima I. Continuous pulmonary perfusion during cardiopulmonary bypass prevents lung injury in infants. Annals of Thoracic Surgery 2000;69(2):602–6. [PUBMED: 10735706] [DOI] [PubMed] [Google Scholar]
Suzuki 2001 {published data only}
- Suzuki T, Ito T, Kashima I, Teruya K, Fukuda T. Continuous perfusion of pulmonary arteries during total cardiopulmonary bypass favorably affects levels of circulating adhesion molecules and lung function. Journal of Thoracic and Cardiovascular Surgery 2001;122(2):242‐8. [PUBMED: 11479496] [DOI] [PubMed] [Google Scholar]
Wei 2004 {published data only}
- Wei B, Liu Y, Wang Q, Yu C, Long C, Chang Y, et al. Lung perfusion with protective solution relieves lung injury in corrections of Tetralogy of Fallot. Annals of Thoracic Surgery 2004;77(3):918‐24. [PUBMED: 14992899] [DOI] [PubMed] [Google Scholar]
Yi 2006 {published data only}
- Yi S, Ran L, Gu XH. Effects of pulmonary arterial perfusion with shenqi fuzheng injection on lung injury during cardiopulmonary bypass. Chinese Journal of Journal Integrated Traditional and Western Medicine 2006;26(10):938–41. [PUBMED: 17121051] [PubMed] [Google Scholar]
References to studies awaiting assessment
Pichugin 2016 {published data only}
- Pichugin V, Domnin S, Kurapeev I, Bober V. Abstract PR045: Comparative Evaluation of Lungs Protection Techniques in Adults with Pulmonary Hypertension During Open Heart Surgery. Anesthesia & Analgesia 2016;123(3S_Suppl):70. [Google Scholar]
Additional references
Adabag 2010
- Adabag AS, Wassif HS, Rice K, Mithani S, Johnson D, Bonawitz‐Conlin J, et al. Preoperative pulmonary function and mortality after cardiac surgery. American Heart Journal 2010;159(4):691–7. [PUBMED: 20362731] [DOI] [PubMed] [Google Scholar]
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [PUBMED: 12543843] [DOI] [PMC free article] [PubMed] [Google Scholar]
Apostolakis 2010
- Apostolakis E, Filos KS, Koletsis E, Dougenis D. Lung dysfunction following cardiopulmonary bypass. Journal of Cardiac Surgery 2010;25(1):47–55. [PUBMED: 19549041] [DOI] [PubMed] [Google Scholar]
Benstoem 2015
- Benstoem C, Moza A, Autschbach R, Stoppe C, Goetzenich A. Evaluating outcomes used in cardiothoracic surgery interventional research: a systematic review of reviews to develop a core outcome set. PLOS One 2015;10(4):e0122204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] [DOI] [PubMed] [Google Scholar]
Buggeskov 2013
- Buggeskov KB, Wetterslev J, Secher NH, Andersen LW, Jonassen T, Steinbrüchel DA. Pulmonary perfusion with oxygenated blood or custodiol HTK solution during cardiac surgery for postoperative pulmonary function in COPD patients: a trial protocol for the randomized, clinical, parallel group, assessor and data analyst blinded Pulmonary Protection Trial. Trials 2013;14:30. [DOI: 10.1186/1745-6215-14-30; PUBMED: 23363494] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carvalho 2008
- Carvalho EM, Gabriel EA, Salerno TA. Pulmonary protection during cardiac surgery: systematic literature review. Asian Cardiovascular Thoracic Anaesthesia 2008;16(6):503–7. [PUBMED: 18984765] [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, GøtzschePC, Krlea‐JeriK, et al. SPIRIT 2013 statement:defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200–7. [DOI: 10.7326/0003-4819-158-3-201302050-00583; PUBMED: 23295957] [DOI] [PMC free article] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [PUBMED: 3616287] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
Directive 2001
- Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations, and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Official Journal of the European Union. Vol. Vol. L 121:34–44:Accessed 2012: http://ec.europa.eu/health/files/eudralex/vol‐1/dir_2001_83_cons/dir2001_83_cons_20081230_en.pdf. [PubMed]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gluud 2006
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology 2006;163(6):493‐501. [PUBMED: 16443796] [DOI] [PubMed] [Google Scholar]
Gluud 2008
- Gluud C, Hilden J, Heiberg P. 1897 methodological study on the statistical method as an aid in therapeutic trials. JLL Bulletin: commentaries on the history of treatment evaluation 2008. Accessed 23 March 2016: http://www.jameslindlibrary.org/articles/povl–heibergs‐1897‐methodological‐study‐on‐the‐statistical‐method‐as‐an‐aid‐in‐therapeutic‐trials/.
GRADEproGDT [Computer program]
- McMaster University (developed by Evidence Prime), GRADE Working Group. GRADEproGDT. Version accessed May 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), GRADE Working Group, 2015. Available from gradepro.org.
Hafner 2015
- Hafner S, Beloncle F, Koch A, Radermacher P, Asfar P. Dr. Jekyll or Mr. Hyde. Hyperoxia in intensive care, emergency, and peri‐operative medicine? A 2015 update. Annals of Intensive Care 2015;5(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JP, Whitehead A, Simmonds M. Sequential methods for random‐effects meta‐analysis. Statistics in Medicine 2011;30(9):903–21. [PUBMED: 21472757] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice. CFR & ICH Guidelines. 1997. USA: Barnett International/PAREXEL; Vol. 1:1963‐2043.
ICTRP
- ICTRP Search Portal. http://apps.who.int/trialsearch/ (accessed 19 May 2016).
Imberger 2015
- Imberger G, Gluud C, Boylan J, Wetterslev J. Systematic reviews of anesthesiologic interventions reported as statistically significant: problems with power, precision, and type 1 error protection. Anesthesia and Analgesia 2015;121(6):1611‐22. [PUBMED: 26579662] [DOI] [PubMed] [Google Scholar]
Kirklin/Barratt‐Boyes 2012
- Kouchoukos NT, Blackstone EH, Hanley FL, Kirklin JK. Kirklin/Barratt‐Boyes Cardiac Surgery. Kirklin/Barratt‐Boyes Cardiac Surgery. Fourth. St. Louis, MO: Elsevier Health Sciences, 2012. [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of International Medicine 2001;135(11):982‐9. [PUBMED: 11730399] [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719‐48. [PUBMED: 13655060] [PubMed] [Google Scholar]
Mascha 2015
- Mascha EJ. Alpha, beta, meta: guidelines for assessing power and type I error in meta‐analyses. Anesthesia and Analgesia 2015;121(6):1430‐3. [PUBMED: 26579648] [DOI] [PubMed] [Google Scholar]
Massoudy 2000
- Massoudy P, Zahler S, Tassani P, Becker BF, Richter JA, Pfauder M, et al. Reduction of pro‐inflammatory cytokine levels and cellular adhesion in CABG procedures with separated pulmonary and systemic extracorporeal circulation without an oxygenator. European Journal of Cardio‐Thoracic Surgery 2000;17(6):729–36. [PUBMED: 10856868] [DOI] [PubMed] [Google Scholar]
Massoudy 2001
- Massoudy P, Zahler S, Becker BF, Braun SL, Barankay A, Meisner H. Evidence for inflammatory responses of the lungs during coronary artery bypass grafting with cardiopulmonary bypass. Chest 2001;119(1):31–6. [PUBMED: 11157581] [DOI] [PubMed] [Google Scholar]
McNicol 1999
- McNicol L, Andersen LW, Liu G, Doolan L, Baek L. Markers of splanchnic perfusion and intestinal translocation of endotoxins during cardiopulmonary bypass: effects of dopamine and milrinone. Journal of Cardiothoracic and Vascular Anesthesia 1999;13(3):292–8. [PUBMED: 10392680] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [PUBMED: 9746022] [DOI] [PubMed] [Google Scholar]
Myles 2005
- Myles PS, McIlroy D. Fast‐track cardiac anesthesia: choice of anesthetic agents and techniques. Seminars in Cardiothoracic and Vascular Anesthesia 2005;9(1):5–16. [PUBMED: 15735840] [DOI] [PubMed] [Google Scholar]
Ng 2002
- Ng CSH, Wan S, Yim APC, Arifi AA. Pulmonary dysfunction after cardiac surgery. Chest 2002;121(4):1269–77. [PUBMED: 11948063] [DOI] [PubMed] [Google Scholar]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93. [PUBMED: 9408720] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ried 2010
- Ried M, Unger P, Puehler T, Haneya A, Schmidt C, Diez C. Mild‐to‐moderate COPD as a risk factor for increased 30‐day mortality in cardiac surgery. Journal of Thoracic and Cardiovascular Surgery 2010;58:387–91. [PUBMED: 20922620] [DOI] [PubMed] [Google Scholar]
Roques 1999
- Roques F, Nashef SA, Michel P, Gauducheau E, Vincentiis C de, Baudet E, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. European Journal of Cardio‐Thoracic Surgery 1999;15(6):816–22. [PUBMED: 10431864] [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [PUBMED: 15095765] [DOI] [PubMed] [Google Scholar]
Santini 2011
- Santini F, Onorati F, Telesca M, Patelli F, Berton G, Franchi G, et al. Pulsatile pulmonary perfusion with oxygenated blood ameliorates pulmonary hemodynamic and respiratory indices in low‐risk coronary artery bypass patients. European Journal of Cardio‐Thoracic Surgery 2011;40(4):794‐803. [PUBMED: 21388821] [DOI] [PubMed] [Google Scholar]
Savović 2012
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [PUBMED: 22945832] [DOI] [PubMed] [Google Scholar]
Schlensak 2002
- Schlensak C, Doenst T, Preusser S, Wunderlich M, Kleinschmidt M, Beyersdorf F. Cardiopulmonary bypass reduction of bronchial blood flow: a potential mechanism for lung injury in a neonatal pig model. Journal of Thoracic and Cardiovascular Surgery 2002;123(6):1199–205. [PUBMED: 12063469] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [PUBMED: 7823387] [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, et al. GRADE: assessing the quality of evidence for diagnostic recommendations. Evidence‐Based Medicine 2008;13(6):162‐3. [PUBMED: 19043023] [DOI] [PubMed] [Google Scholar]
Sjöberg 2013
- Sjöberg F, Singer M. The medical use of oxygen: a time for critical reappraisal. Journal of Internal Medicine 2013;274(6):505–28. [DOI] [PubMed] [Google Scholar]
Suzuki 2010
- Suzuki T. Additional lung‐protective perfusion techniques during cardiopulmonary bypass. Annals of Thorac Cardiovascular Surgery 2010;16(3):150–5. [PUBMED: 20930674] [PubMed] [Google Scholar]
Terkawi 2016
- Terkawi AS, Mavridis D, Flood P, Wetterslev J, Terkawi RS, Bin Abdulhak AA, et al. Does ondansetron modify sympathectomy due to subarachnoid anesthesia? Meta‐analysis, meta‐regression, and trial sequential analysis. Anesthesiology 2016;124(4):846‐69. [PUBMED: 26835645] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [PUBMED: 18824467] [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA). Available from http://ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 2 May 2012).
Turner 2013
- Turner RM, Bird SM, Higgins JP. The impact of study size on meta‐analyses: examination of underpowered studies in Cochrane reviews. PLOS One 2013;8(3):e59202. [PUBMED: 23544056] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [PUBMED: 20042080] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [PUBMED: 18316340] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Buggeskov 2014
- Buggeskov KB, Nielsen JB, Wetterslev J. Pulmonary perfusion versus no pulmonary perfusion during cardiopulmonary bypass for cardiac surgery. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD011098] [DOI] [PMC free article] [PubMed] [Google Scholar]


