Abstract
Background
Alcoholic hepatitis is a form of alcoholic liver disease, characterised by steatosis, necroinflammation, fibrosis, and potential complications to the liver disease. Typically, alcoholic hepatitis presents in people between 40 and 50 years of age. Alcoholic hepatitis can be resolved if people abstain from drinking, but the risk of death will depend on the severity of the liver damage and abstinence from alcohol. Glucocorticosteroids are used as anti‐inflammatory drugs for people with alcoholic hepatitis. Glucocorticosteroids have been studied extensively in randomised clinical trials in order to assess their benefits and harms. However, the results have been contradictory.
Objectives
To assess the benefits and harms of glucocorticosteroids in people with alcoholic hepatitis.
Search methods
We identified trials through electronic searches in Cochrane Hepato‐Biliary's (CHB) Controlled Trials Register, CENTRAL, MEDLINE, Embase, LILACS, and Science Citation Index Expanded. We looked for ongoing or unpublished trials in clinical trials registers and pharmaceutical company sources. We also scanned reference lists of the studies retrieved. The last search was 20 October 2016.
Selection criteria
Randomised clinical trials assessing glucocorticosteroids versus placebo or no intervention in people with alcoholic hepatitis, irrespective of year, language of publication, or format. We considered trials with adult participants diagnosed with alcoholic hepatitis, which could have been established through clinical or biochemical diagnostic criteria or both. We defined alcoholic hepatitis as mild (Maddrey's score less than 32) and severe (Maddrey's score 32 or more). We allowed co‐interventions in the trial groups, provided they were similar.
Data collection and analysis
We followed Cochrane and CHB methodology, performing the meta‐analyses using Review Manager 5 and Trial Sequential Analysis. We presented the results of dichotomous outcomes as risk ratios (RR) and those of the continuous outcomes as mean difference (MD). We applied both the fixed‐effect model and the random‐effects model meta‐analyses. Whenever there were significant discrepancies in the results, we reported the more conservative point estimate of the two. We considered a P value of 0.01 or less, two‐tailed, as statistically significant if the required information size was reached due to our three primary outcomes (all‐cause mortality, health‐related quality of life, and serious adverse events during treatment) and our post hoc decision to include analyses of mortality at more time points. We presented heterogeneity using the I² statistic. If trialists used intention‐to‐treat analysis to deal with missing data, we used these data in our primary analysis; otherwise, we used the available data. We assessed the bias risk of the trials using bias risk domains and the quality of the evidence using GRADE.
Main results
Sixteen trials fulfilled the inclusion criteria. All trials were at high risk of bias. Fifteen trials provided data for analysis (927 participants received glucocorticosteroids and 934 participants received placebo or no intervention). The glucocorticosteroids were administered orally or parenterally for a median of 28 days (range 3 days to 12 weeks). The participants were between 25 and 70 years old, had different stages of alcoholic liver disease, and 65% were men. The follow‐up of trial participants, when it was reported, was up to the moment of discharge from the hospital, until they died (a median of 63 days), or for at least a year. There was no evidence of effect of glucocorticosteroids on all‐cause mortality up to three months following randomisation neither with traditional meta‐analysis (random‐effects RR 0.90, 95% CI 0.70 to 1.15; participants = 1861; trials = 15; I² = 45% (moderate heterogeneity) nor with Trial Sequential Analysis. Meta‐analysis showed no evidence of effect on health‐related quality of life up to three months (MD ‐0.04 points; 95% CI ‐0.11 to 0.03; participants = 377; trial = 1; low‐quality evidence), measured with the European Quality of Life ‐ 5 Dimensions‐3 Levels (EQ‐ 5D‐3L) scale. There was no evidence of effect on the occurrence of serious adverse events during treatment, neither with traditional meta‐analysis (random‐effects RR 1.05, 95% CI 0.85 to 1.29; participants = 1861; trials = 15; I² = 36% (moderate heterogeneity), liver‐related mortality up to three months following randomisation (random‐effects RR 0.89, 95% CI 0.69 to 1.14; participants = 1861; trials = 15; I² = 46% (moderate heterogeneity), frequency of any complications up to three months following randomisation (random‐effects RR 1.04, 95% CI 0.86 to 1.27; participants = 1861; I² = 42% (moderate heterogeneity), and frequency of non‐serious adverse events up to three months' follow‐up after end of treatment (random‐effects RR 1.99, 95% CI 0.72 to 5.48; participants = 160; trials = 4; I² = 0% (no heterogeneity) nor with Trial Sequential Analysis. Nine of the trials were industry‐funded.
Authors' conclusions
We found no evidence of a difference between glucocorticosteroids and placebo or no intervention on all‐cause mortality, health‐related quality of life, and serious adverse events during treatment. The risk of bias was high and the quality of evidence was very low or low. Therefore, we are very uncertain about this effect estimate. Due to inadequate reporting, we cannot exclude increases in adverse events. As the confidence intervals were wide, we cannot rule out significant benefits and harms of glucocorticosteroids. Therefore, we need placebo‐controlled, randomised clinical trials, designed according to the SPIRIT guidelines and reported according to the CONSORT guidelines. Future trials ought to report depersonalised individual participant data, so that proper individual participant data meta‐analyses of the effects of glucocorticosteroids in subgroups can be conducted.
Glucocorticosteroids for people with alcoholic hepatitis
Review question To assess the benefits and harms of glucocorticosteroids administered in any route, dose, and duration versus placebo or no intervention in people with alcoholic hepatitis in terms of death, health‐related quality of life, and complications.
Background Excessive alcoholic consumption may damage the liver, causing alcoholic hepatitis. The first stage of liver damage in alcoholic hepatitis is usually reversible if people abstain from drinking, but the risk of the disease developing further and getting more complications increases with resumed drinking. A heavy drinker is considered a person who consumes more than 60 g to 80 g (for men) or more than 20 g (for women) alcohol per day. Only 10 to 35 people out of 100 heavy drinkers with evidence of excessive fat in the liver would most probably develop alcoholic hepatitis. With time, alcoholic hepatitis will cause liver fibrosis (scarring of the liver) or liver cirrhosis with complications (bleeding, infections, liver cancer, etc).
Glucocorticosteroids are considered to have anti‐inflammatory effects (relieving pain, oedema, fever). They are administered to people with alcoholic hepatitis in order to repair their liver injury. However, the benefits and harms of glucocorticosteroids are not well studied in randomised clinical trials, and therefore, it is uncertain if they should be used in clinical practice for people with alcoholic liver disease.
Search date
The date of the last search was 20 October 2016.
Study characteristics
Sixteen randomised clinical trials compared glucocorticosteroids with placebo or no intervention in people with alcoholic hepatitis. Fifteen trials provided data for analysis (927 participants received glucocorticosteroids and 934 participants received placebo or no intervention). Glucocorticosteroids were administered orally or as an injection for a median of 28 days (range 3 days to 12 weeks). The trial participants were between 25 and 70 years old (men: 65%) and had different stages of alcoholic liver disease. Trial participants were followed up to the moment of discharge from the hospital, or until they died (a median of 63 days), or for at least a year. Not all trials reported the follow‐up of participants. The trials were conducted in France, India, UK, and USA. Two trials administered pentoxifylline to both glucocorticosteroids and placebo intervention groups.
Funding Nine of the trials were industry‐funded.
Quality of evidence
The overall quality of evidence was very low, low, or moderate, and all the trials were at high risk of bias, which means that there is possibility of drawing wrong conclusions, exaggerating benefits or underestimating harms of glucocorticosteroids because of the way that the trials were conducted and analysed.
Key results
Glucocorticosteroids did not benefit clinical outcomes of importance to people with alcoholic liver disease, such as mortality, no matter the cause, and health‐related quality of life. In addition, glucocorticosteroids may increase the number of adverse events. We cannot exclude benefits and harms of glucocorticosteroids but researchers need to study further their effects in high‐quality, placebo‐controlled, randomised clinical trials. Such trials ought to be registered before they are launched and openly report depersonalised individual participant data so that individual participant data meta‐analysis can be conducted.
Summary of findings
Summary of findings for the main comparison.
Glucocorticosteroids for people with alcoholic hepatitis
| Glucocorticosteroids for people with alcoholic hepatitis | ||||||
|
Patient or population: participants with alcoholic hepatitis at high risk of mortality and morbidity Settings: hospitals and clinics Intervention: glucocorticosteroids Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | Glucocorticosteroids | |||||
| All‐cause mortality: 3 months following randomisation | 298 per 1000 | 278 per 1000 |
RR 0.90 (0.70 to 1.15) |
1861 (15 RCTs) |
⊕⊝⊝⊝1 very low | The Trial Sequential Analysis‐adjusted CI was 0.36 to 2.32 |
|
Health‐related quality of life: up to 3 months (measured withEuropean Quality of Life ‐ 5 Dimensions‐3 Levels (EQ‐ 5D‐3L) scale) |
The mean value is 0.592 | The mean value is 0.553 | MD ‐0.04; (‐0.11 to 0.03) | 377 (1 RCT) |
⊕⊕⊝⊝2 low |
We did not perform Trial Sequential Analysis |
| Serious adverse events during treatment | 361 per 1000 | 389 per 1000 |
RR 1.05 (0.85 to 1.29) |
1861 (15 RCTs) |
⊕⊕⊝⊝3 low | The Trial Sequential Analysis‐adjusted CI was 0.60 to 1.82 |
| Liver‐related mortality: up to 3 months following randomisation | 298 per 1000 | 277 per 1000 |
RR 0.89 (0.69 to 1.14) |
1861 (15 RCTs) |
⊕⊕⊝⊝4 low | The Trial Sequential Analysis‐adjusted CI was 0.32 to 2.45 |
| Any complication: up to 3 months following randomisation | 443 per 1000 | 474 per 1000 |
RR 1.04 (0.86 to 1.27) |
1861 (15 RCTs) |
⊕⊕⊝⊝5 low | The Trial Sequential Analysis‐adjusted CI was 0.67 to 1.63 |
| Number of participants with non‐serious adverse events: up to 3 months' follow‐up after end of treatment | 51 per 1000 | 120 per 1000 |
RR 1.99 (0.72 to 5.48) |
160 (4 RCTs) |
⊕⊝⊝⊝6 very low | The Trial Sequential Analysis‐adjusted CI was 0.01 to 249.60 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: this research provides a very good indication of the likely effect; the likelihood that the effect will be substantially different is low. Moderate quality: this research provides a good indication of the likely effect; the likelihood that the effect will be substantially different is moderate. Low quality: this research provides some indication of the likely effect; however, the likelihood that it will be substantially different is high. Very low quality: this research does not provide a reliable indication of the likely effect; the likelihood that the effect will be substantially different is very high. | ||||||
1Downgraded 3 levels: 1 level due to within‐study risk of bias (high overall risk of bias in all the trials); 1 level due to inconsistency of the data (there is wide variation in the effect estimates across studies; there is little overlap of confidence intervals associated with the effect estimates; presence of moderate heterogeneity: I² = 45%; heterogeneity could be explained with selection bias); 1 level due to imprecision of effect estimates (the trial sequential analysis showed that additional evidence is needed and that we have not yet reached the required information size). 2Downgraded 2 levels: 1 level due to within‐study risk of bias (high overall risk of bias in the trial); 1 level due to imprecision of effect estimates. 3Downgraded 2 levels: 1 level due to within‐study risk of bias (high overall risk of bias in all the trials); 1 level due to inconsistency of the data (there is wide variation in the effect estimates across studies; there is little overlap of confidence intervals associated with the effect estimates; presence of moderate heterogeneity: I² = 36%; heterogeneity could be explained with selection bias). 4Downgraded 2 levels: 1 level due to within‐study risk of bias (high overall risk of bias in all the trials); 1 level due to inconsistency of the data (there is wide variation in the effect estimates across studies; there is little overlap of confidence intervals associated with the effect estimates; presence of moderate heterogeneity: I² = 46%; heterogeneity could be explained with selection bias). 5Downgraded 2 levels: 1 level due to within‐study risk of bias (high overall risk of bias in all the trials); 1 level due to inconsistency of the data (there is wide variation in the effect estimates across studies; there is little overlap of confidence intervals associated with the effect estimates; presence of moderate heterogeneity: I² = 41%; heterogeneity could be explained with selection bias). 6Downgraded 4 levels: 1 level due to within‐study risk of bias (high overall risk of bias in all the trials); 1 level due to inconsistency of the data (there is little overlap of confidence intervals associated with the effect estimates);1 level due to imprecision of effect estimates (the Trial Sequential Analysis showed that additional evidence is needed and that we have not yet reached the required information size); 1 level due to publication bias (only 4 trials with a small number of participants reported on non‐serious adverse events).
Background
Description of the condition
The term 'alcoholic hepatitis' was used for the first time in a paper by Beckett and colleagues in 1961 (Beckett 1961), but clinical jaundice after excessive ethanol consumption was reported in the literature long before that (Gerber 1973). Most probably, these reports represented people with alcoholic hepatitis (Mendenhall 1984; Jensen 1994).
Alcoholic hepatitis is a serious form of alcoholic liver disease (injury of the liver due to excessive alcohol consumption) (WHO 2010).
The first stage of liver damage in alcoholic hepatitis is usually reversible if people abstain from drinking, but the risk of progression to fibrosis and cirrhosis increases with resumed drinking (Ellis 2012). The accumulation of fat in the hepatocytes causes disruption of the mitochondrial beta‐oxidation of fatty acids, accumulation of lipotoxic metabolites, and release of reactive oxygen species (Lieber 1999; Wu 1999; Petrasek 2013). Lipotoxic metabolites and reactive oxygen species lead to cell death and liver inflammation (Wu 1999; Petrasek 2013;WHO 2013). Alcoholic hepatitis is a histological form of alcoholic liver disease, characterised by steatosis (the earliest stage of alcoholic liver damage) and necroinflammation (EASL 2012b). Alcoholic hepatitis can be resolved if people abstain from drinking, but the risk of death will depend on the severity of the liver damage and drinking patterns. In 20% to 40% of persistent heavy drinkers (defined as alcohol consumption per day of more than 60 g to 80 g in men and more than 20 g in women), alcoholic hepatitis and other complications may develop (WHO 2013).
Severe alcoholic hepatitis may be characterised by clinically clear signs of jaundice, coagulopathy, liver decompensation with ascites, portal hypertension, variceal bleeding, hepatorenal syndrome, hepatic encephalopathy, systemic inflammatory response syndrome, or sepsis (Becker 1996; EASL 2012b). Typically, alcoholic hepatitis presents in people aged between 40 and 50 years. Among the risk factors of developing severe alcoholic hepatitis are being female, Hispanic ethnicity, various types of alcohol, binge drinking, poor nutrition, obesity, etc (WHO 2010). Several composite prognostic scores exist to distinguish people with poor prognosis from those who can become abstinent, instituting supportive care, until recovery is achieved. Some of these scores, designed to predict mortality, are Maddrey's discriminant function (Maddrey 1978), the model of end‐stage liver disease (MELD) score (Dunn 2005), the Glasgow alcoholic hepatitis score (Forrest 2005), and the age, bilirubin, international normalised ratio, creatinine (ABIC) score (Dominguez 2008).
The Maddrey Discriminant Function is the most often used score in severe alcoholic hepatitis to identify people in potential need of glucocorticosteroids (also known as glucocorticosteroids, corticosteroids, or steroids). The one‐month survival of people with alcoholic hepatitis and with Maddrey's score higher than 32 varied between 50% and 65% (Carithers 1989; Phillips 2006). The Lille Model (www.lillemodel.com) is the only validated model so far to assess glucocorticosteroid response and is highly predictive of death at six months (P value < 0.000001) in people with severe alcoholic hepatitis (Louvet 2007). A Lille Model score greater than 0.45, calculated after seven days of treatment with prednisolone, means failure to respond to treatment and predicts a six‐month mortality of about 75% (Lefkowitch 2005).
Description of the intervention
Glucocorticosteroids are used as anti‐inflammatory drugs. Glucocorticosteroid agents mimic the endogenous‐produced glucocorticoid (cortisol) (Rhen 2005). Glucocorticosteroids, primarily regulated by corticotropin, are considered to have anti‐inflammatory effects as well as metabolic and immunogenic effects in our body (Rhen 2005). It is agreed that the anti‐inflammatory effects of glucocorticosteroids are mediated primarily through repression of gene transcription (Schäcke 2002).
How the intervention might work
Glucocorticosteroids administered to people with alcoholic hepatitis repair the liver injury by decreasing the liver polymorphonuclear neutrophil (PMN) (effector cells) infiltrates and the level of pro‐inflammatory mediators such as tumour necrosis factor‐alpha (TNF‐alpha), intercellular adhesion molecule 1, and interleukin (IL)‐6 and IL‐8 in the liver tissue (Taïeb 2000; Spahr 2001). The benefits of corticosteroids ensue from short‐term vascular changes (Schäcke 2002). However, adverse events have still been poorly reported (Christensen 1995; Rambaldi 2008).
Why it is important to do this review
Over the years, a number of randomised clinical trials have studied the benefits and harms of corticosteroids for people with alcoholic hepatitis, in order to determine the best route of administration, dose, and duration. However, results have been contradictory. Some systematic reviews (Christensen 1995; Rambaldi 2008) and meta‐analyses of randomised clinical trials (Reynolds 1989; Imperiale 1990; Daures 1991; Christensen 1999; Mathurin 2011) have been published. The review authors explained their various conclusions regarding patient‐orientated outcomes as being due to differences in glucocorticosteroid regimens, trial quality, participants' characteristics, and clinical spectrum of the disease. Reynolds 1989 concluded that corticosteroid treatment could help only the most severely ill people with severe alcoholic hepatitis characterised by high levels of serum bilirubin, prolonged prothrombin times, and development of hepatic encephalopathy. Imperiale 1990 concluded that glucocorticosteroids reduced short‐term mortality in people with severe alcoholic hepatitis, provided that they also had hepatic encephalopathy but did not have severe gastrointestinal bleeding. Daures 1991 concluded that further randomised clinical trials were needed to confirm the benefits and harms of glucocorticosteroids, especially in people with severe alcoholic hepatitis. Christensen 1995,Christensen 1999, and Rambaldi 2006 could not find sufficient proof supporting the routine use of glucocorticosteroids in people with alcoholic hepatitis, including those with hepatic encephalopathy. Rambaldi 2008 concluded that glucocorticosteroids did not improve overall survival in people with alcoholic hepatitis. Based on the Trial Sequential Analysis of the subgroup of people with Maddrey's score of at least 32 or spontaneous hepatic encephalopathy, the required information size of 2420 people for the outcome mortality was far from reached, with only 249 participants randomised in the six randomised trials (Rambaldi 2008). Using the Lille model, Mathurin 2011 concluded that glucocorticosteroids significantly improved 28‐day survival in people with severe alcoholic hepatitis. The Mathurin 2011 meta‐analysis was based on individual data from five selected randomised clinical trials and is accordingly at risk of 'cherry picking'. This is why we decided to conduct this Cochrane systematic review in order to assess the efficacy of glucocorticosteroids in people with severe alcoholic hepatitis with or without complications.
Objectives
To assess the benefits and harms of glucocorticosteroids in people with alcoholic hepatitis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials in which glucocorticosteroids were assessed in people with alcoholic hepatitis, irrespective of year or language of publication or format.
We found no reports of quasi‐randomised or observational studies retrieved with our searches for randomised clinical trials, in order to extract data on harm.
Types of participants
We included adult participants with alcoholic hepatitis, diagnosed according to the diagnostic work‐up used in the individual randomised clinical trial. Alcoholic hepatitis could have been established through clinical or biochemical diagnostic criteria or both.
We considered alcoholic hepatitis as mild if a randomised participant had Maddrey's score less than 32 (Maddrey's score = 4.6 x (prothrombin time ‐ control time)(s) + serum bilirubin (mg per dL)) (Maddrey 1978). Usually, people with mild alcoholic hepatitis do not have concomitant gastrointestinal bleeding.
We considered alcoholic hepatitis as severe at any stage of the alcoholic liver disease with the presence of spontaneous hepatic encephalopathy; or Maddrey's score equal to or higher than 32. We also examined whether there was a difference in terms of initiation of treatment with glucocorticosteroids in trials using the Maddrey's score where severe alcoholic hepatitis was defined as equal to or higher than 32.
Included trial participants diagnosed with severe alcoholic hepatitis could also manifest with hepatic encephalopathy, gastro‐intestinal bleeding, cirrhosis (e.g. classified with Child‐Pugh score ‐ Child‐Pugh type C (Pugh 1973)), ascites, hepatorenal syndrome, hyponatraemia, or spontaneous bacterial peritonitis.
For studies not reporting the Maddrey's score, we used the classifications for mild and severe alcoholic hepatitis as provided by the trialists.
Types of interventions
Glucocorticosteroids administered in any route, dose, and duration versus placebo or no intervention.
We allowed co‐interventions in the trial groups, provided they were the same.
Types of outcome measures
Primary outcomes
All‐cause mortality: up to three months' follow‐up after randomisation (the primary time point for drawing our main conclusion); at the end of treatment (post hoc analysis); and one year following randomisation (post hoc analysis)
Health‐related quality of life as defined by the trial authors
Serious adverse events during treatment. We used the International Conference on Harmonisation (ICH) Guidelines for Good Clinical Practice's definition of a serious adverse event (ICH‐GCP 1997), that is, any untoward medical occurrence that results in death, is life threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, or is a congenital anomaly or birth defect. We considered all other adverse events as non‐serious (see below).
Secondary outcomes
Liver‐related mortality up to three months' follow‐up after randomisation
Participants with any complication up to three months' follow‐up after randomisation (i.e. ascites, hepatorenal syndrome, spontaneous bacterial peritonitis, gastrointestinal bleeding, hepatic encephalopathy, nonobstructive jaundice, systemic inflammatory response syndrome, sepsis, or hepatocellular carcinoma, or a combination of any of these)
Participants with non‐serious adverse events up to three months' follow‐up after randomisation
Exploratory analysis
Participants with an increase of liver enzymes as defined by the trialists
Participants with a decrease of prothrombin index as defined by the trialists
Participants with a decrease of serum albumin as defined by the trialists
Search methods for identification of studies
Electronic searches
We searched Cochrane Hepato‐Biliary's Controlled Trials Register (Gluud 2017; September 2016), Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8) in the Cochrane Library, MEDLINE Ovid (1946 to September 2016), Embase Ovid (1974 to September 2016), LILACS, and Science Citation Index Expanded (Web of Science; 1900 to September 2016) (Royle 2003). We applied no language or document‐type restrictions. Appendix 1 shows the search strategies with the time spans of the searches.
Searching other resources
We searched online trials registries such as ClinicalTrials.gov (clinicaltrials.gov), European Medicines Agency (EMA; www.ema.europa.eu), WHO International Clinical Trial Registry Platform www.who.int/ictrp), the Food and Drug Administration (FDA; www.fda.gov), and pharmaceutical company sources for ongoing or unpublished trials (last search 20 October 2016).
Data collection and analysis
We followed the available guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), and the Cochrane Hepato‐Biliary Module (Gluud 2017). We performed the analyses using Review Manager 5 (RevMan 2014) and Trial Sequential Analysis (Thorlund 2011; TSA 2011; Wetterslev 2017). We assessed the evidence according to Jakobsen and colleagues (Jakobsen 2014).
Selection of studies
We retrieved the publications that we considered as potentially eligible for inclusion, after reading their titles and abstracts. Three review authors (CP, DV, GC) independently reviewed the full‐text publications for eligibility. The review authors assessed each publication to determine if trial participants and the interventions administered met the inclusion criteria. We included abstracts if sufficient data were provided for analysis. We resolved disagreements by discussion or consulting any of the remaining review authors for arbitration.
Data extraction and management
Three review authors (CP, DV, GC) independently completed a data extraction form for all included trials. Authors extracted general information on the trial, such as publication title; place and year of publication; trial design; inclusion and exclusion criteria; preliminary sample size calculation reached or not; number of participants randomised in each trial and following treatment allocation; diagnostic work‐up; age (mean or median); sex or sex ratio; race; co‐infection; type, dose, and route of administration of glucocorticosteroids and their possible link with adverse events; concurrent medications used; length of trial; and length of follow‐up. CP, DV and GC also extracted data on malnutrition whenever it was clearly defined by the trial authors.
The review authors resolved disagreements by discussion or asking the advice of the review arbitrator, CG.
Assessment of risk of bias in included studies
Three review authors (CP, DV, and GC) independently assessed the risk of bias of each included trial according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), the Cochrane Hepato‐Biliary Module (Gluud 2017), and methodological studies (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Lundh 2017). We used the following definitions in the assessment of risk of bias.
Allocation sequence generation
Low risk of bias: the study performed sequence generation using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if an independent person not otherwise involved in the study performed them.
Unclear risk of bias: the study authors did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not random. We will only include such studies for assessment of harms.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. A central and independent randomisation unit controlled allocation. The investigators were unaware of the allocation sequence (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the study authors did not describe the method used to conceal the allocation so the intervention allocations may have been foreseen before, or during, enrolment.
High risk of bias: it is likely that the investigators who assigned the participants knew the allocation sequence. We will only include such studies for assessment of harms.
Blinding of participants and personnel
Low risk of bias: any of the following: no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; or blinding of participants and key study personnel ensured, and it is unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of ‘low risk’ or ‘high risk’; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
Blinded outcome assessment
Low risk of bias: any of the following: no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; or blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit judgement of ‘low risk’ or ‘high risk’; or the trial did not address this outcome.
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk: the trial reported the following pre‐defined outcomes: all‐cause mortality, serious adverse events, and liver‐related mortality. If the original trial protocol was available, the outcomes should be those called for in that protocol. If the trial protocol was obtained from a trials registry (e.g. www.clinicaltrials.gov), the outcomes sought should have been those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes will not be considered to be reliable.
Unclear risk: not all pre‐defined outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk: one or more pre‐defined outcomes were not reported.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support that may manipulate the trial design, conduct, or analyses of results of the trial.
Unclear risk of bias: the trial may or may not be free of for‐profit bias as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support.
Other bias
Low risk of bias: the trial appeared to be free of other factors that could put it at risk of bias.
Unclear risk of bias: the trial may or may not have been free of other factors that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias.
We judged each trial as having a low, uncertain, or high risk of bias based on the definitions described above. We included a bias risk assessment combining all domains and judged the trials to be at low risk of bias if none of the trial domains was assessed as being with high or unclear risk of bias. Moreover, we considered trials with one or more domains with unclear or high risk of bias as trials at high risks of bias.
Measures of treatment effect
Dichotomous outcomes
We used risk ratio (RR) with 95% confidence interval (CI) and Trial Sequential Analysis‐adjusted CI for dichotomous outcomes.
Continuous outcomes
We used mean difference (MD) with 95% CI and Trial Sequential Analysis‐adjusted CI for health‐related quality of life. We planned to use the standardised mean difference (SMD) with 95% CI if trials used different measures for health‐related quality of life.
Unit of analysis issues
Trial participants as randomised per intervention group. In case of multiple treatment groups, we considered only the trial group to which glucocorticosteroids were administered versus the group that received placebo or no intervention. If a trial consisted of more than two groups (either parallel or factorial design), we compared the participants from all the glucocorticosteroid groups versus all participants from the placebo group(s). Had we been able to include a cross‐over trial from which we could extract data for analyses, we would have used the data from the first treatment period of the cross‐over trial.
Dealing with missing data
If dichotomous or continuous data were missing in a published report, we, whenever possible, contacted the original investigators to request the missing data.
If trialists used intention‐to‐treat analysis to deal with missing data, we used these data in our primary analysis. Otherwise, we used the data that were available to us.
Dealing with missing data using sensitivity analysis
As some trials reported only per‐protocol analysis results, we included missing data by considering participants as treatment failures or treatment successes by imputing them according to the following two scenarios:
extreme case analysis favouring the experimental intervention ('best‐worse' case scenario): none of the participants who dropped out from the experimental trial group experienced the outcome, but all of the participants who dropped out from the control trial group experienced the outcome; including all randomised participants in the denominator.
extreme case analysis favouring the control ('worst‐best' case scenario): all participants who dropped out from the experimental trial group, but none from the control trial group experienced the outcome; including all randomised participants in the denominator.
For continuous outcomes, as in our case health‐related quality of life, we planned to perform a ‘best‐worst’ case scenario analysis assuming that all participants lost to follow‐up in the experimental group had an improved outcome (the group mean plus 1 standard deviation (SD)); and all those with missing outcomes in the control group had a worsened outcome (the group mean minus 1 SD) (Jakobsen 2014). We also planned to perform 'worst‐best' case scenario analysis assuming that all participants lost to follow‐up in the experimental group had a worsened outcome (the group mean minus 1 SD); and all those with missing outcomes in the control group had an improved outcome (the group mean plus 1 SD) (Jakobsen 2014).
We performed the two sensitivity scenario analyses only for our primary outcomes.
Assessment of heterogeneity
We addressed the presence of heterogeneity in both clinical and statistical ways.
To assess heterogeneity between the trials, we specifically examined the degree of heterogeneity observed in the results using the I² statistic (Higgins 2002). As thresholds for the interpretation of the I² statistic could be misleading, we used the following rough guide for interpretation of heterogeneity provided in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity*;
50% to 90%: may represent substantial heterogeneity*;
75% to 100%: considerable heterogeneity*.
*The importance of the observed value of the I² statistic depends on (i) the magnitude and direction of effects and (ii) the strength of evidence for heterogeneity (e.g. P value from the Chi² test, or a CI for I² statistic).
For the heterogeneity adjustment of the required information size in the Trial Sequential Analysis, we used diversity (D²) because the I² statistics used for this purpose underestimates the required information size (Wetterslev 2009).
Depending on the number of eligible trials, we planned to add covariates to a meta‐regression model to adjust for heterogeneity.
Assessment of reporting biases
We drew funnel plots to assess reporting biases from the individual trials by plotting the RR on a logarithmic scale against its standard error (Egger 1997; Sterne 2011).
For dichotomous outcomes, we tested asymmetry using the Harbord test in cases where Tau² was less than 0.1 (Harbord 2006), and we planned to use Rücker 2008 in cases where Tau² was more than 0.1. For continuous outcomes, we planned to use the regression asymmetry test (Egger 1997), and the adjusted rank correlation (Begg 1994).
Data synthesis
Meta‐analysis
We performed the meta‐analyses using Review Manager 5 (RevMan 2014) and according to the recommendations stated in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
We presented the results of dichotomous outcomes of individual trials as risk ratios (RR) with 95% CI and the results of the continuous outcomes as mean difference (MD) with 95% CI and Trial Sequential Analysis‐adjusted CI. We applied both the fixed‐effect model (DeMets 1987) and the random‐effects model (DerSimonian 1986) meta‐analyses. If there were statistically significant discrepancies in the results (e.g. one giving a significant intervention effect and the other no significant intervention effect), we reported the more conservative point estimate of the two (Jakobsen 2014). The more conservative point estimate is the estimate closest to zero effect. If the two point estimates were equal, we used the estimate with the widest CI as our main result of the two analyses. We considered a P value of 0.025 or less, two‐tailed, as statistically significant if the required information size was reached due to the three primary outcomes (Jakobsen 2014). Due to us expanding the number of analyses conducted, we post hoc made the alpha level even lower. We used the eight‐step procedure to assess if the thresholds for significance were crossed (Jakobsen 2014). We presented heterogeneity using the I2 statistic (Higgins 2002). We presented the results of the individual trials and meta‐analyses in the form of forest plots.
Where data were only available from one trial (in our case continuous data on health‐related quality of life), we used Student's t‐test (Student 1908). We planned to use Fisher's exact test for dichotomous data in a single trial (Fisher 1922).
Trial Sequential Analysis
We applied Trial Sequential Analysis for both dichotomous and continuous outcomes (Thorlund 2011; TSA 2011; Wetterslev 2017), as cumulative meta‐analyses are at risk of producing random errors due to sparse data and repetitive testing of the accumulating data (Wetterslev 2008; Wetterslev 2017). To control random errors, we calculated the diversity‐adjusted required information size (DARIS) (i.e. the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect) (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009, Wetterslev 2009; Thorlund 2010).
In our meta‐analysis, we based the DARIS for dichotomous outcomes on the event proportion in the control group; assumption of a plausible risk ratio reduction of 20% of the risk observed in the included trials; a risk of type I error of 1% due to more than three outcomes, as we decided to perform post hoc analyses on mortality at end of treatment and at one year following randomisation, a risk of type II error of 20%, and the diversity of the included trials in the meta‐analysis. For health‐related quality of life, we planned to: estimate DARIS using a minimal relevant difference of 10% of the mean response observed in the control group; the standard deviation; alpha of 1% (Jakobsen 2014); beta of 20%; and the diversity as estimated from the trials in the meta‐analysis (Wetterslev 2009). However, we did not conduct Trial Sequential Analysis because only one trial provided data on health‐related quality of life. We also calculated and reported the Trial Sequential Analysis‐adjusted CI (Thorlund 2011).
The underlying assumption of Trial Sequential Analysis is that testing for statistical significance may be performed each time a new trial is added to the meta‐analysis. We added the trials according to the year of publication, and, if more than one trial was published in a year, we added trials alphabetically according to the last name of the first author. On the basis of the DARIS, we constructed the trial sequential monitoring boundaries for benefit, harm, and futility (Wetterslev 2008; Thorlund 2011). These boundaries determine the statistical inference one may draw regarding the cumulative meta‐analysis that has not reached the DARIS; if the trial sequential monitoring boundary for benefit or harm is crossed before the DARIS is reached, firm evidence may be established and further trials may be superfluous. However, if the boundaries are not crossed, it is most probably necessary to continue doing trials in order to detect or reject a certain intervention effect. However, if the cumulative Z‐curve crosses the trial sequential monitoring boundaries for futility, no more trials may be needed.
A more detailed description of Trial Sequential Analysis can be found at www.ctu.dk/tsa/ (Thorlund 2011).
Subgroup analysis and investigation of heterogeneity
Whenever possible, we performed the following subgroup analyses for all‐cause mortality up to three months after randomisation.
Trials at low risk of bias compared to trials at high risk of bias.
Trials with people with mild alcoholic hepatitis compared to trials with severe alcoholic hepatitis, following Maddrey's score lower than 32 or equal to or higher than 32 or presence of hepatic encephalopathy; or as provided by the trialists.
Trials with glucocorticosteroid dose equal to or less than 40 mg compared to trials with glucocorticosteroid dose more than 40 mg.
Trials with people with severe alcoholic hepatitis without cirrhosis compared to trials with people with severe alcoholic hepatitis with cirrhosis. If cirrhosis is classified by Child‐Pugh score, then we may be able to perform additional subgroup analyses in order to adjust for the clinical spectrum of the disease.
Trials with people with severe alcoholic hepatitis without hepatorenal syndrome compared to trials with people with severe alcoholic hepatitis with hepatorenal syndrome.
Trials with people with severe alcoholic hepatitis without ascites compared to trials with people with severe alcoholic hepatitis with ascites.
We did not perform any additional subgroup analyses to those planned in advance.
Sensitivity analysis
We planned to undertake additional sensitivity analyses to those specified under Dealing with missing data should we have considered it necessary (e.g. trials published as full‐paper articles, abstracts, and unpublished trials).
Summary of findings' tables
We created 'Summary of findings' tables on all review outcomes using GRADEpro GDT 2015. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias, indirectness of the evidence (population, intervention, control, outcomes), unexplained inconsistency (heterogeneity) of results (including problems with subgroup analyses); imprecision of results (wide CIs as evaluated with our Trial Sequential Analyses) (Jakobsen 2014), and risk of publication bias (Balshem 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h; Guyatt 2013a; Guyatt 2013b; Guyatt 2013c; Mustafa 2013; Guyatt 2017).
We defined the levels of evidence as 'high', 'moderate', 'low', or 'very low'. These grades are defined as follows:
High quality: this research provides a very good indication of the likely effect; the likelihood that the effect will be substantially different is low.
Moderate quality: this research provides a good indication of the likely effect; the likelihood that the effect will be substantially different is moderate.
Low quality: this research provides some indication of the likely effect; however, the likelihood that it will be substantially different is high.
Very low quality: this research does not provide a reliable indication of the likely effect; the likelihood that the effect will be substantially different is very high.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We identified 932 potentially relevant records through the electronic searches (Figure 1). Of these, 37 records that referred to 16 randomised clinical trials fulfilled our inclusion criteria. We found two trials published in abstract form (Mendenhall 1977; Richardet 1993), and fourteen trials described in full paper articles (Helman 1971; Porter 1971; Campra 1973; Blitzer 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Theodossi 1982; Mendenhall 1984; Bories 1987; Carithers 1989; Ramond 1992; De 2014; Thursz 2015). Our searches retrieved no quasi‐randomised trials or observational studies. We identified no additional references by handsearching the reference lists of articles, retrieved through the computerised databases.
Figure 1.
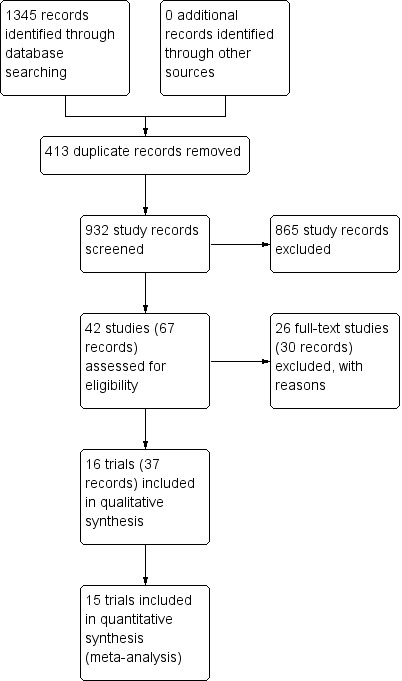
Study flow diagram
Included studies
Sixteen randomised clinical trials fulfilled our review protocol inclusion criteria (Helman 1971; Porter 1971; Campra 1973; Blitzer 1977; Mendenhall 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Theodossi 1982; Mendenhall 1984; Bories 1987; Carithers 1989; Ramond 1992; Richardet 1993; De 2014; Thursz 2015). Two were three‐armed trials (Mendenhall 1977; Mendenhall 1984), one trial was a randomised trial with a two‐by‐two factorial design (Thursz 2015), one trial was a cross‐over trial (Richardet 1993), and the remaining were parallel, two‐group design trials. There were 1884 participants randomised in all trials. Some participants from Mendenhall 1977 (pilot trial or feasibility trial) continued participation in Mendenhall 1984. The trials were conducted in France (n = 3), India (n = 1), UK (n = 2), and USA (n = 10) (Characteristics of included studies). All the trials reported the sex (65% of the participants were men) and age of the participants (range 25 years to 70 years). Four trials excluded women (Blitzer 1977; Mendenhall 1977; Mendenhall 1984; De 2014). Eleven trials reported to have included trial participants at different stages of alcoholic liver disease due to hepatitis, fibrosis, or cirrhosis (Helman 1971; Porter 1971; Campra 1973; Blitzer 1977; Maddrey 1978; Depew 1980; Theodossi 1982; Mendenhall 1984,Bories 1987; Ramond 1992; Thursz 2015). Most trials established diagnosis primarily through liver biopsy. One trial included only participants with liver cirrhosis in addition to alcoholic hepatitis (De 2014). The remaining trials did not provide information on the stage of disease. All the trials included participants with recent history of alcohol consumption, increase of serum bilirubin, liver enzymes, prolonged prothrombin time, and participants without previous treatment with glucocorticosteroids within the last three months before the start of the trial. Ten trials performed liver biopsy whenever possible (Helman 1971; Porter 1971; Campra 1973; Blitzer 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Bories 1987; Ramond 1992; Thursz 2015); however, it was an inclusion criterion in only one trial, performed at the admission and after treatment (Helman 1971).
Ten trials reported the period of trial enrolment (range of one year to five years, with the median of three years) (Campra 1973; Blitzer 1977; Mendenhall 1977; Maddrey 1978; Depew 1980; Mendenhall 1984; Bories 1987; Carithers 1989; De 2014; Thursz 2015). The earliest trial began participant recruitment in 1966 (Campra 1973), and the most recently published trial began recruitment in 2011 and completed it in 2014 (Thursz 2015).
Three trials followed participants up to one year Mendenhall 1984, De 2014 and Thursz 2015. The remaining trials followed their participants to the moment of discharge from the hospital or until death occurred, with a median duration of follow‐up of 63 days (range 28 to 120).
We could extract data for analysis from all 16 trials but one (Richardet 1993). We contacted Richardet in 2006, but we did not receive a reply. In the remaining 15 trials, 182 participants had mild alcoholic hepatitis and 1679 had severe alcoholic hepatitis. The analyses of the 15 trials accounted for 927 participants randomised to glucocorticosteroids, and 934 participants randomised to placebo or no intervention.
Experimental interventions
Glucocorticosteroids (prednisolone or 6‐methylprednisolone in equivalent dose of prednisolone) were administered orally or parenterally at different dose regimens and different durations. Twelve trials assessed oral glucocorticosteroids at a dose equal to or more than 40 mg prednisolone (Helman 1971; Mendenhall 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Mendenhall 1984; Bories 1987; Carithers 1989; Ramond 1992; Richardet 1993; De 2014; Thursz 2015), but three trials also allowed parenteral administration to participants who were not able to swallow (Shumaker 1978; Carithers 1989; Ramond 1992). Two trials assessed oral glucocorticosteroids at a dose less than 40 mg prednisolone (Campra 1973; Blitzer 1977), and in one trial the initial therapy was parenteral and then it was administered orally (Porter 1971). One trial used only parenteral (intravenous) glucocorticosteroids (Theodossi 1982).
The median duration of glucocorticosteroid administration was 28 days with a range of three days (Theodossi 1982) to 11 weeks (De 2014): one week (Richardet 1993), three weeks (Mendenhall 1977), four weeks (Ramond 1992; Thursz 2015), 26 days (Blitzer 1977), one month (Maddrey 1978; Mendenhall 1984; Bories 1987), five weeks (Shumaker 1978; Carithers 1989), six weeks (Helman 1971;Campra 1973; Depew 1980), 45 days (Porter 1971). The dose of prednisolone was tapered until it was stopped in Helman 1971; Porter 1971; Campra 1973; Blitzer 1977; Mendenhall 1977; Shumaker 1978; Depew 1980; Mendenhall 1984; Carithers 1989; and De 2014.
Control interventions
Twelve trials used identical placebos (Helman 1971; Porter 1971; Blitzer 1977; Mendenhall 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Mendenhall 1984; Carithers 1989; Ramond 1992; De 2014; Thursz 2015) and four trials used no intervention (Campra 1973; Theodossi 1982; Bories 1987; Richardet 1993).
Co‐interventions
Two trials administered pentoxifylline to both glucocorticosteroids and placebo intervention groups (De 2014; Thursz 2015). There seemed to be no interaction between the intervention effects of pentoxifylline and glucocorticosteroids (De 2014; Thursz 2015).
Outcomes
The Characteristics of included studies tables detail the outcomes reported in the individual trials. Five trials reported on outcomes with a follow‐up period up to three months after randomisation (Helman 1971; Mendenhall 1977; Bories 1987; De 2014; Thursz 2015). Twelve trials reported on outcomes at the end of treatment or at the moment of discharge from the hospital (Helman 1971; Porter 1971; Campra 1973; Blitzer 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Theodossi 1982; Bories 1987; Carithers 1989; Ramond 1992; Richardet 1993). Three trials exceeded the 12‐month follow‐up period (Mendenhall 1984; De 2014; Thursz 2015).
Only one trial reported health‐related quality of life, using the European quality of life‐5 dimensions (EQ‐5D) score registered to Eudra CT 2009‐ 013897‐42 and ISRCTN 88782125 and it was reported in all the groups at three months' follow‐up after randomisation, and at one year (Thursz 2015; see Notes in Characteristics of included studies).
None of the trials provided usable data for meta‐analyses of our exploratory outcomes.
For further details on trial characteristics, please see Characteristics of included studies.
Excluded studies
We excluded 26 trials from the final assessment with the reasons for their exclusion provided in Characteristics of excluded studies. Among the excluded trials are two trials that used a nutritional intervention in the control group (Lesesne 1978; Cabré 2000). Although nutritional intervention as an overall intervention does not seem to influence mortality or serious adverse events (Feinberg 2017), including the Cabré 2000 and Lesesne 1978 trials in our review would not have affected our results noticeably because these trials were small and had very few events.
Risk of bias in included studies
Allocation
Allocation sequence generation
Based on the information that we collected from the published reports and information from study authors, we assessed the allocation sequence generation as low risk of bias in eight trials (Porter 1971; Campra 1973; Blitzer 1977; Maddrey 1978; Carithers 1989; Ramond 1992; De 2014; Thursz 2015) and as unclear in the remaining trials (Helman 1971; Mendenhall 1977; Shumaker 1978; Depew 1980; Theodossi 1982; Mendenhall 1984; Bories 1987; Richardet 1993).
Allocation concealment
Based on the information that we collected from the published reports and information from study authors, we assessed the allocation concealment as low risk of bias in ten trials (Helman 1971; Porter 1971; Campra 1973; Blitzer 1977; Shumaker 1978; Theodossi 1982; Mendenhall 1984; Carithers 1989; Ramond 1992; Thursz 2015) and as unclear in the remaining trials (Mendenhall 1977; Maddrey 1978; Depew 1980; Bories 1987; Richardet 1993; De 2014).
Blinding
Three trials were at high risk of performance bias as they were open‐label trials, without blinding of participants or investigators (Campra 1973; Bories 1987; Theodossi 1982) and placebo was used in the Richardet 1993 trial, but there was no description of it and we judged the risk of bias as unclear. Twelve trials were blinded, using identical placebo (Helman 1971; Porter 1971; Blitzer 1977; Mendenhall 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Mendenhall 1984b: Carithers 1989; Ramond 1992; De 2014; Thursz 2015), and hence, at low risk of bias.
We assessed five trials at low risk of detection bias (Porter 1971; Shumaker 1978; Carithers 1989; De 2014; Thursz 2015), and the remaining eleven trials as unclear risk of detection bias (Helman 1971; Campra 1973; Blitzer 1977; Mendenhall 1977; Maddrey 1978; Depew 1980; Theodossi 1982; Mendenhall 1984; Bories 1987; Ramond 1992; Richardet 1993).
Incomplete outcome data
We classed four trials at high risk of attrition bias because they did not account for participants with missing outcomes (Porter 1971; Blitzer 1977; Theodossi 1982; Thursz 2015). Eleven trials were assessed as having low risk of attrition bias (Helman 1971; Campra 1973; Mendenhall 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Mendenhall 1984; Bories 1987; Carithers 1989; Ramond 1992; De 2014). We judged the risk of bias as unclear in Richardet 1993.
Selective reporting
All trials but Richardet 1993 reported pre‐defined outcomes in our review. We assessed the remaining 15 trials at low risk of reporting bias.
For‐profit bias
Based on the information that we collected from the published reports, we judged two trials to be at low risk of for‐profit bias (Porter 1971; Thursz 2015). We assessed the profit‐bias as unclear in the remaining trials (Helman 1971; Campra 1973; Blitzer 1977; Mendenhall 1977; Maddrey 1978; Shumaker 1978; Depew 1980; Theodossi 1982; Mendenhall 1984; Bories 1987; Carithers 1989; Ramond 1992; Richardet 1993; De 2014).
Other potential sources of bias
We did not identify other biases for any of the included trials.
We judged all trials as high risk‐of‐bias trials. Figure 2 and Figure 3 show our assessment of risk of bias of the published trial reports (Characteristics of included studies).
Figure 2.
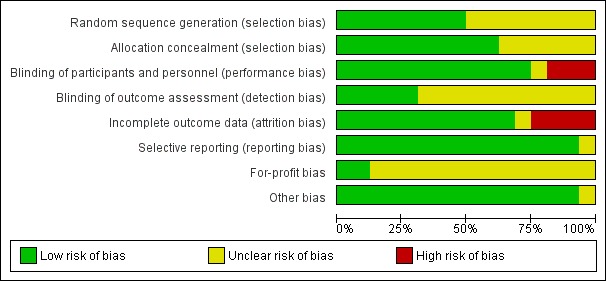
Risk of bias graph: review authors' judgements about each risk of bias domain presented as percentages across all included studies
Figure 3.
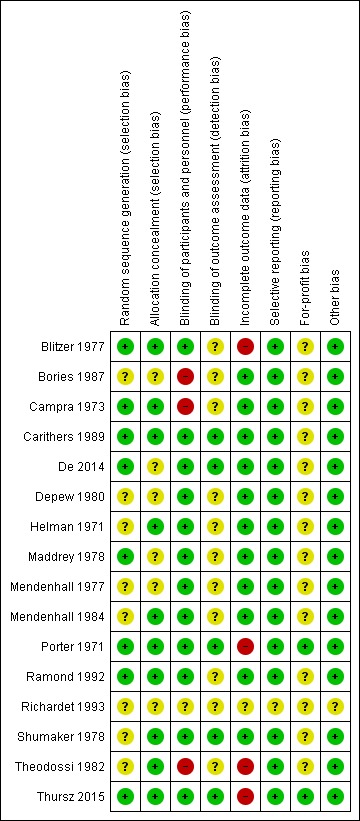
Risk of bias summary: review authors' judgements about each risk of bias domain for each included study
Effects of interventions
See: Table 1
Primary outcomes
All‐cause mortality
Up to three months following randomisation
In total, 258 of 927 (27.8%) participants in the group treated with glucocorticosteroids died versus 279 of 934 (29.9%) participants in the control group. There was no evidence of effect of glucocorticosteroids on all‐cause mortality (random‐effects RR 0.90, 95% CI 0.70 to 1.15; participants = 1861; trials = 15; I² = 45% (moderate heterogeneity; Analysis 1.1). We observed a similar result with the Trial Sequential Analysis showing that the cumulative Z‐curve did not cross the trial sequential monitoring boundaries for benefit or harm, nor enter the trial sequential monitoring area for futility in order to include an intervention effect of 20% risk ratio reduction (RRR) (Figure 4). The Trial Sequential analysis‐adjusted CI was 0.36 to 2.32. We rated the quality of the evidence as very low (Table 1).
Analysis 1.1.
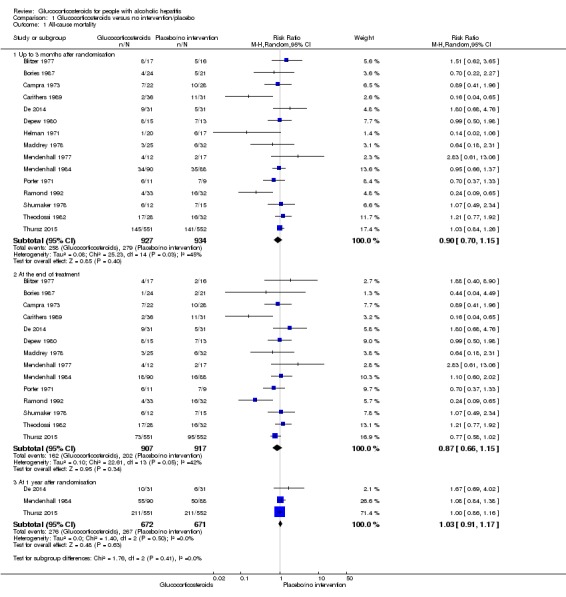
Comparison 1 Glucocorticosteroids versus no intervention/placebo, Outcome 1 All‐cause mortality.
Figure 4.
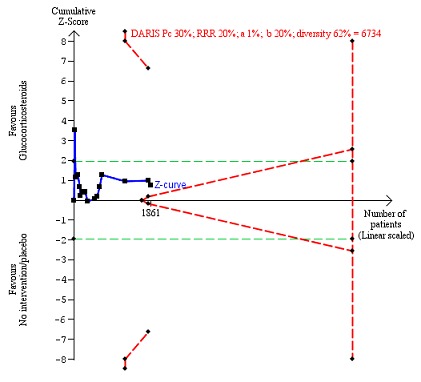
All‐cause mortality up to three months after randomisation. Fifteen trials provided data. The diversity‐adjusted required information size (DARIS) was calculated based on all‐cause mortality of 30% in the control group; risk ratio reduction in the glucocorticosteroid group of 20%; type I error of 1%; and type II error of 20% (80% power). Trial diversity was 62%. The required information size was 6734 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (red inward sloping lines) and did not enter the trial sequential monitoring area for futility (inner‐wedge with red outward sloping lines). The green dotted lines show the conventional boundaries of the naive alpha of 5% equal to Z‐scores of +1.96 and ‐1.96.
We constructed a funnel plot for publication bias, and using the Harbord 2006 test, we found no evidence of reporting bias (P = 0.31) (Figure 5).
Figure 5.
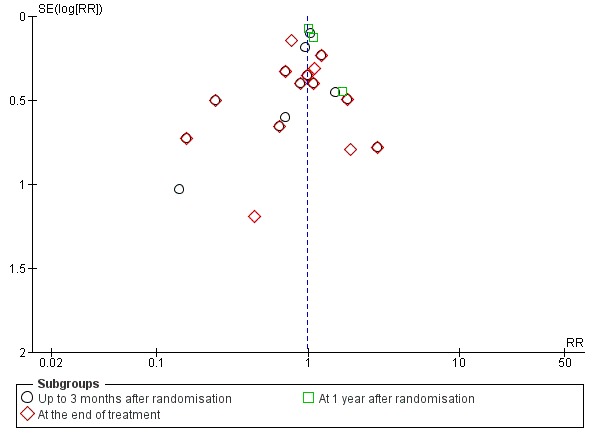
Funnel plot of comparison 1. Glucocorticosteroids versus no intervention/placebo, outcome 1.1 all‐cause mortality
'Best‐worst' case scenario analysis
The 'best‐worst' case scenario analysis on mortality up to three months after randomisation produced two different results. While there was no evidence of effect of glucocorticosteroids with the random‐effects model (RR 0.82, 95% CI 0.64 to 1.05; I² = 47%), there was evidence of beneficial effect with the fixed‐effect model (RR 0.74, 95% CI 0.65 to 0.84; participants = 1861; trials = 15; I² = 47%; Analysis 3.1). The heterogeneity in both analyses was moderate.
Analysis 3.1.
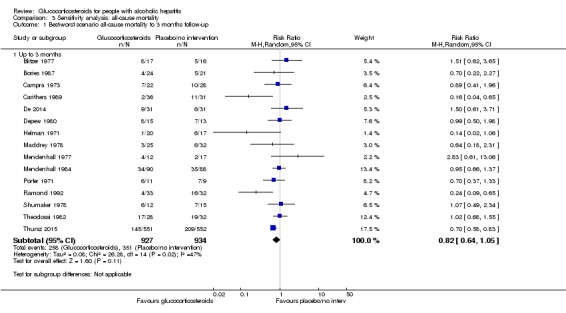
Comparison 3 Sensitivity analysis: all‐cause mortality, Outcome 1 Best‐worst scenario all‐cause mortality to 3 months follow‐up.
'Worst‐best' case scenario analysis
The 'worst‐best' case scenario analysis on mortality up to three months after randomisation produced two different results. While there was no evidence of effect of glucocorticosteroids with the random‐effects model (RR 0.97, 95% CI 0.73 to 1.29; I² = 62%), there was evidence of harmful effect with the fixed‐effect model (RR 1.21, 95% CI 1.06 to 1.37; I² = 62%); Analysis 3.2).
Analysis 3.2.
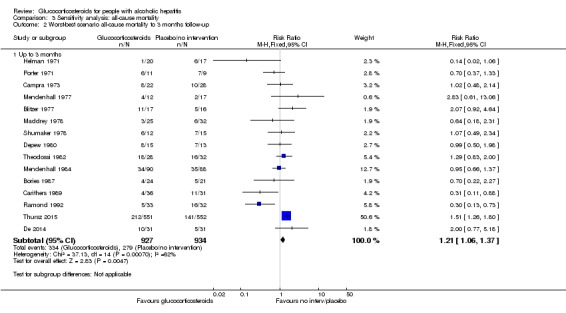
Comparison 3 Sensitivity analysis: all‐cause mortality, Outcome 2 Worst‐best scenario all‐cause mortality to 3 months follow‐up.
At the end of treatment (post hoc analysis)
The treatment lasted for a median of 28 days (range 3 days to 12 weeks). In total, 162 of 907 (17%) participants in the group treated with glucocorticosteroids died versus 202 of 917 (22%) participants in the control group. There was no evidence of effect of glucocorticosteroids on all‐cause mortality (random‐effects (RR 0.87, 95% CI 0.66 to 1.15; participants = 1824; trials = 14; I² = 42% (moderate heterogeneity); Analysis 1.1.1). We observed a similar result with the Trial Sequential Analysis showing that the cumulative Z‐curve did not cross the trial sequential monitoring boundaries for benefit or harm, and did not enter the trial sequential monitoring area for futility in order to exclude an intervention effect of 20% RRR (Figure 6). The Trial Sequential Analysis‐adjusted CI was CI 0.29 to 2.68. We rated the quality of the evidence as very low (Table 1).
Figure 6.
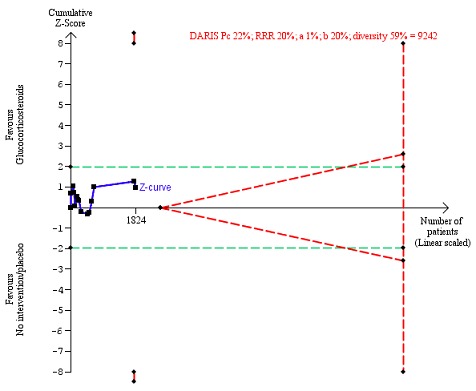
All‐cause mortality at the end of treatment (median 28 days (range 3 days to 12 weeks) (post hoc analysis). Fourteen trials provided data. The diversity‐adjusted required information size (DARIS) was calculated based on all‐cause mortality of 22% in the control group; risk ratio reduction in the glucocorticosteroid group of 20%; type I error of 1%; and type II error of 20% (80% power). Trial diversity was 59%. The required information size was 9242 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (red inward sloping lines) and did not enter the trial sequential monitoring area for futility (inner‐wedge with red outward sloping lines). The green dotted lines show the conventional boundaries of the naive alpha of 5% equal to Z‐scores of +1.96 and ‐1.96.
We constructed a funnel plot for publication bias, and using the Harbord 2006 test, we found no evidence of reporting bias (P = 0.84) (Figure 5).
At one year following randomisation (post hoc analysis)
Three of the included trials provided data on all‐cause mortality one year following randomisation (Mendenhall 1984; De 2014; Thursz 2015). In total, 274 of 668 (41%) participants in the group treated with glucocorticosteroids died versus 265 of 664 (40%) participants in the control group. There was no evidence of effect of glucocorticosteroids on all‐cause mortality (random‐effects RR 1.03, 95% CI 0.91 to 1.17; participants = 1343; trials = 3; I² = 0% (no heterogeneity among the trials); Analysis 1.1.3). We observed a similar result with the Trial Sequential Analysis showing that the cumulative Z‐curve entered the area of futility, which excludes an intervention effect of 20% RRR (Figure 7). The Trial Sequential analysis‐adjusted CI was CI 0.85 to 1.25. We rated the quality of the evidence as moderate (Table 1).
Figure 7.
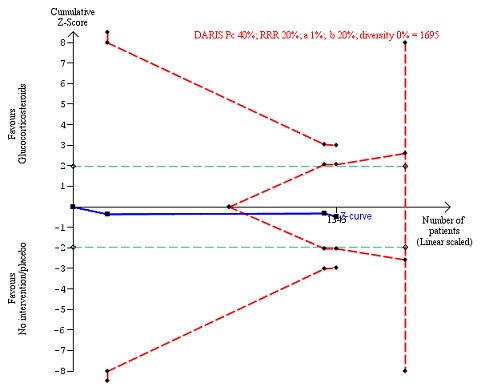
All‐cause mortality up to 1 year (post hoc analysis). Three trials provided data. The diversity‐adjusted required information size (DARIS) was calculated based on mortality in the control group of 40%; risk ratio reduction of 20% in the glucocorticosteroid group; type I error of 1%; and type II error of 20% (80% power). Trial diversity was 0%. The required information size was 1695 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (red inward sloping lines). The cumulative Z‐curve crossed the inner‐wedge futility line (red outward sloping lines). The green dotted lines show the conventional boundaries of the naive alpha of 5% equal to Z‐scores of +1.96 and ‐1.96.
Subgroup analysis and investigation of heterogeneity: all‐cause mortality up to three months after randomisation
Trials at low risk of bias compared to trials at high risk of bias
As all the trials were at high risk of bias, we could not perform subgroup analysis on risk of bias.
Trials with people with mild alcoholic hepatitis compared to trials with severe alcoholic hepatitis, following Maddrey's score lower than or equal to or higher than 32, or presence of hepatic encephalopathy; or as provided by the trialists
There was no significant difference (P = 0.75) between the subgroups (mild alcoholic hepatitis RR 1.02, 95% CI 0.58 to 1.80; participants = 182; trials = 4; I² = 0%; Analysis 2.1.1) and severe alcoholic hepatitis (RR 0.92, 95% CI 0.73 to 1.16; participants = 1679; trials = 14; I² = 37%; Analysis 2.1.2).
Analysis 2.1.
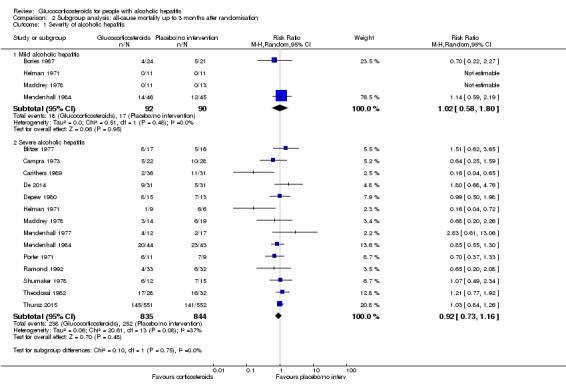
Comparison 2 Subgroup analysis: all‐cause mortality up to 3 months after randomisation, Outcome 1 Severity of alcoholic hepatitis.
Trials with glucocorticosteroid dose equal to or less than 40 mg compared to trials with glucocorticosteroid dose more than 40 mg
There was no significant difference (P = 0.22) between the subgroups of the trials with glucocorticosteroid dose less than or equal to 40 mg (RR 0.75, 95% CI 0.50 to 1.14; participants = 1547; trials = 10; I² = 58%; Analysis 2.2.1) and trials with glucocorticosteroid dose more than 40 mg (RR 1.02, 95% CI 0.79 to 1.30; participants = 314; trials = 5; I² = 0%; Analysis 2.2.2).
Analysis 2.2.
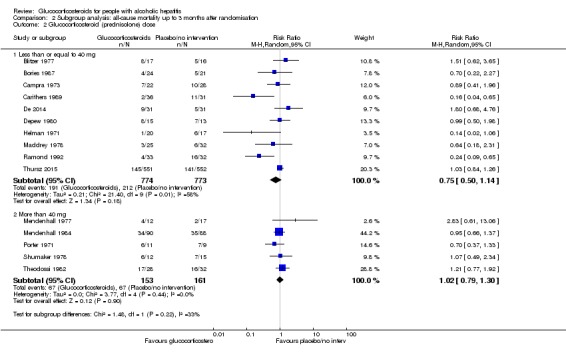
Comparison 2 Subgroup analysis: all‐cause mortality up to 3 months after randomisation, Outcome 2 Glucocorticosteroid (prednisolone) dose.
Trials with people with severe alcoholic hepatitis without cirrhosis compared to trials with people with severe alcoholic hepatitis with cirrhosis
There was no significant difference (P = 0.83) between the subgroups of the trials with severe alcoholic hepatitis without cirrhosis (RR 0.79, 95% CI 0.18 to 3.48; participants = 123; trials = 3; I² = 77%; Analysis 2.3.1) and trials with people with severe alcoholic hepatitis with cirrhosis (RR 0.92, 95% CI 0.74 to 1.16; participants = 1738; studies = 12; I2 = 35%; ; Analysis 2.3.2).
Analysis 2.3.
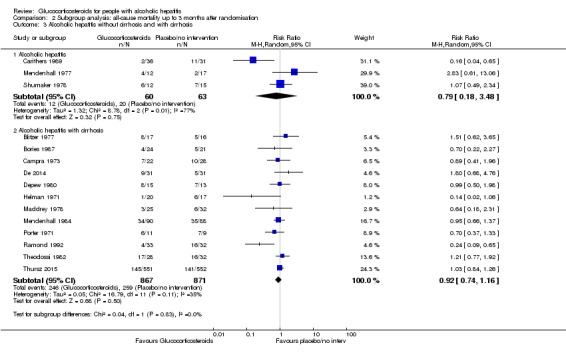
Comparison 2 Subgroup analysis: all‐cause mortality up to 3 months after randomisation, Outcome 3 Alcoholic hepatitis without cirrhosis and with cirrhosis.
As only two trials classified cirrhosis by Child‐Pugh score (Bories 1987; De 2014) and we did not know what classification system the remaining trials had used, we could not perform a subgroup analysis in order to adjust for the clinical spectrum of the disease.
Trials with people with severe alcoholic hepatitis without hepatorenal syndrome compared to trials with people with severe alcoholic hepatitis with hepatorenal syndrome
There was no significant difference (P = 0.64) between the subgroups of the trials with people with severe alcoholic hepatitis without hepatorenal syndrome (RR 1.00, 95% CI 0.85 to 1.17; participants = 1382; studies = 8; I2 = 0%; Analysis 2.4.1) compared to trials with people with severe alcoholic hepatitis with hepatorenal syndrome (RR 0.56, 95% CI 0.05 to 6.49; participants = 129; studies = 2; I2 = 88%; Analysis 2.4.2). The presence of hepatorenal syndrome was not clearly described in five trials (Blitzer 1977; Bories 1987; Mendenhall 1977; Mendenhall 1984; Ramond 1992).
Analysis 2.4.
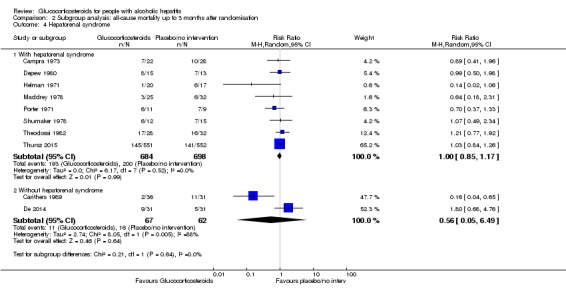
Comparison 2 Subgroup analysis: all‐cause mortality up to 3 months after randomisation, Outcome 4 Hepatorenal syndrome.
Trials with people with severe alcoholic hepatitis without ascites compared to trials with people with severe alcoholic hepatitis with ascites
As we did not have data on trials with participants not having ascites, we could analyse only the subgroup of trials including participants with ascites (RR 0.82, 95% CI 0.60 to 1.12; participants = 729; trials = 13; I² = 48%) (Analysis 2.5.1). In addition, the presence of ascites was not clearly described in two trials (Mendenhall 1977; Thursz 2015).
Analysis 2.5.
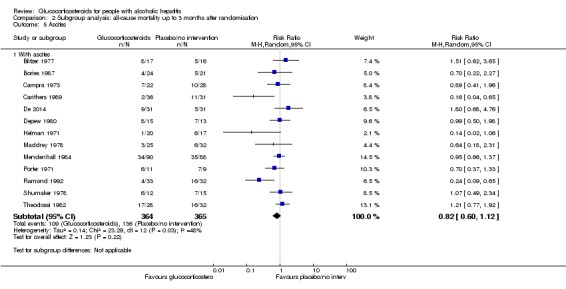
Comparison 2 Subgroup analysis: all‐cause mortality up to 3 months after randomisation, Outcome 5 Ascites.
Health‐related quality of life
Up to three months
Only one trial reported on quality of life at follow‐up period of up to three months, using responses to the European Quality of Life ‐ 5 Dimensions‐3 Levels (EQ‐5D‐3L) (Thursz 2015). We applied the Student's t‐test for the glucocorticosteroids versus the placebo group. We observed no difference between the two groups (MD ‐0.04 points; 95% CI ‐0.11 to 0.03; Analysis 1.2). We rated the quality of the evidence as low (Table 1). We did not perform Trial Sequential Analysis.
Analysis 1.2.
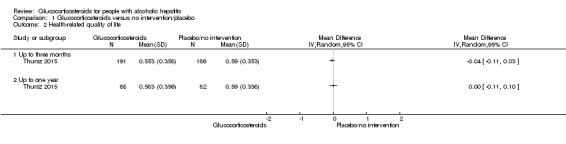
Comparison 1 Glucocorticosteroids versus no intervention/placebo, Outcome 2 Health‐related quality of life.
Up to one year
Only one trial reported on quality of life at follow‐up period of up to one year, using responses to the European Quality of Life ‐ 5 Dimensions‐3 Levels (EQ‐5D‐3L) (Thursz 2015). We applied the Student's t‐test for the glucocorticosteroids versus the placebo group. We observed no difference between the two groups (MD 0.00 points; 95% CI ‐0.11 to 0.10; Analysis 1.2). We rated the quality of the evidence as very low (Table 1). We did not perform Trial Sequential Analysis.
Serious adverse events during treatment
Fifteen trials reported number of participants with serious adverse events during treatment. In total, 361 of 927 (38%) participants in the group treated with glucocorticosteroids had serious adverse events during treatment versus 338 of 934 (36%) participants in the control group. There was no evidence of effect of glucocorticosteroids on the occurrence of serious adverse events (random‐effects RR 1.05, 95% CI 0.85 to 1.29; participants = 1861; trials = 15; I² = 36% (moderate heterogeneity); Analysis 1.3). We observed a similar result with the Trial Sequential Analysis showing that the cumulative Z‐curve entered the area of futility which excludes an intervention effect of 20% RRR (Figure 8). The Trial Sequential analysis‐adjusted CI was 0.60 to 1.82. We rated the quality of the evidence as low (Table 1).
Analysis 1.3.
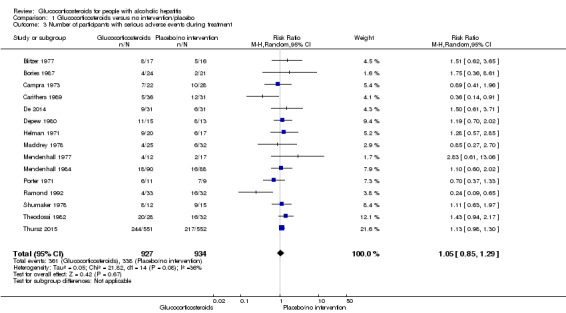
Comparison 1 Glucocorticosteroids versus no intervention/placebo, Outcome 3 Number of participants with serious adverse events during treatment.
Figure 8.
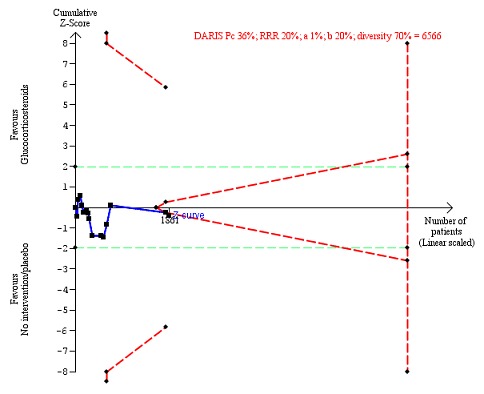
Serious adverse events during treatment. There are 15 trials providing data. The diversity‐adjusted required information size (DARIS) was calculated based on an incidence rate of serious adverse events in the control group of 36%; risk ratio reduction of 20% in the glucocorticosteroid group; type I error of 1%; and type II error of 20% (80% power). Trial diversity was 70%. The required information size was 6566 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (red inward sloping lines), but it entered the trial sequential monitoring area for futility (inner‐wedge futility line red outward sloping lines) indicating that sufficient information was provided. The green dotted lines show the conventional boundaries of the naive alpha of 5% equal to Z‐scores of +1.96 and ‐1.96.
Table 6 shows the number of participants with the most often occurring serious adverse events in 14 included trials; mortality is not included. Table 7 presents the most often occurring serious adverse events in Thursz 2015 because this trial did not specify the individual number of participants with a serious adverse event.
Table 1.
Number of participants with most often occurring serious adverse events during treatment
| Trial | Gastrointestinal haemorrhage | Hepatorenal syndrome (with or without hepatic failure) | Septicaemia | Hepatocellular carcinoma | ||||
| Prednisolone | Placebo | Prednisolone | Placebo | Prednisolone | Placebo | Prednisolone | Placebo | |
| Helman 1971 | 3 | |||||||
| Porter 1971 | 4 | 2 | ||||||
| Campra 1973 | 3 | 5 | 4 | |||||
| Blitzer 1977 | 3 | 2 | 2 | 2 fungal | 1 | |||
| Mendenhall 1977 | Not reported | |||||||
| Maddrey 1978 | 1 | 1 | 3 | 6 | ||||
| Shumaker 1978 | 3 | 3 | 2 | |||||
| Depew 1980 | 2 | 1 | 2 | 1 | ||||
| Theodossi 1982 | 11 | 6 | 7 | 6 | ||||
| Bories 1987 | 3 | 3 | 2 | |||||
| Carithers 1989 | 2 | 4 | 1 | |||||
| Mendenhall 1984 | 2 | |||||||
| Ramond 1992 | 1 | 2 | 1 | 1 | ||||
| De 2014 | 2 | 3 | 3 | 3 | 1 | |||
Table 2.
Most often occurring serious adverse events in Thursz trial. Number of events.
| Type of adverse event | Prednisolone group | Placebo group |
| Gastrointestinal haemorrhage plus variceal bleeding |
40 | 28 |
| Infections | 74 | 43 |
| ‐ lung | 38 | 17 |
| ‐ sepsis | 14 | 14 |
We constructed a funnel plot for publication bias, and using the Harbord 2006 test, we found no evidence of reporting bias (P = 0.63).
'Best‐worst' case scenario analysis
There was no evidence of effect of glucocorticosteroids on serious adverse events during treatment, with neither of the models (random‐effects model (RR 1.00, 95% CI 0.83 to 1.21; participants = 1861; studies = 15; I2 = 28%) (not important heterogeneity) and fixed‐effect model RR 0.99, 95% CI 0.89 to 1.11; participants = 1861; I² = 28% (not important heterogeneity); Analysis 4.1).
Analysis 4.1.
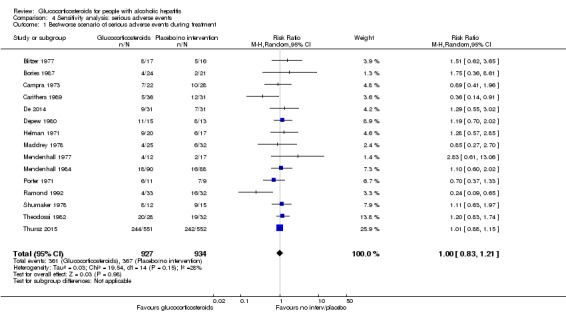
Comparison 4 Sensitivity analysis: serious adverse events, Outcome 1 Best‐worse scenario of serious adverse events during treatment.
'Worst‐best' case scenario analysis
While there was evidence of harmful effect of glucocorticosteroids with the fixed‐effect model (RR 1.18, 95% CI 1.05 to 1.31; participants = 1861; I² = 38%), there was no evidence of effect of glucocorticosteroids with the random‐effects model (RR 1.11, 95% CI 0.91 to 1.36; I² = 38%; Analysis 4.2).
Analysis 4.2.
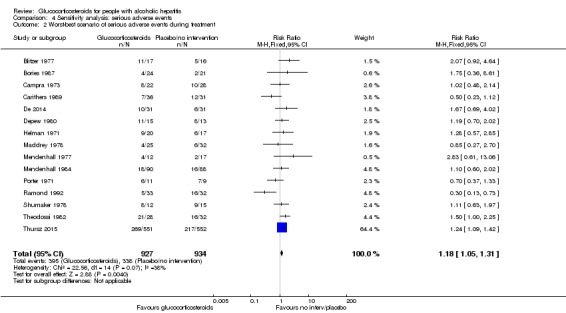
Comparison 4 Sensitivity analysis: serious adverse events, Outcome 2 Worst‐best scenario of serious adverse events during treatment.
Secondary outcomes
Liver‐related mortality up to three months following randomisation
In total, 257 of 927 (27.7%) participants in the group treated with glucocorticosteroids died versus 279 of 934 (29.9%) participants in the control group.
There was no evidence of effect of glucocorticosteroids on liver‐related mortality (random‐effects RR 0.89, 95% CI 0.69 to 1.14; participants = 1861; trials = 15; I² = 46% (moderate heterogeneity); Analysis 1.4). The heterogeneity among the trials was moderate. We observed a similar result with the Trial Sequential Analysis showing that the cumulative Z‐curve did not cross the trial sequential monitoring boundaries for benefit or harm, nor enter the trial sequential monitoring area for futility in order to include an intervention effect of 20% RRR (Figure 9). The Trial Sequential analysis‐adjusted CI was 0.32 to 2.45. We rated the quality of the evidence as low (Table 1).
Analysis 1.4.
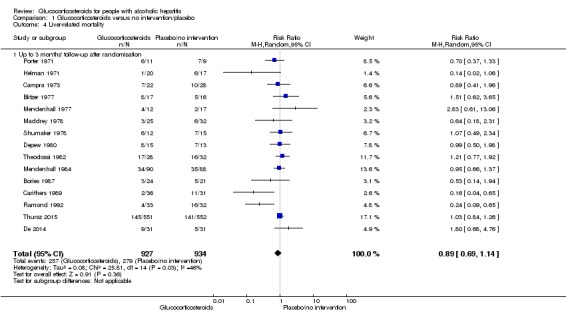
Comparison 1 Glucocorticosteroids versus no intervention/placebo, Outcome 4 Liver‐related mortality.
Figure 9.
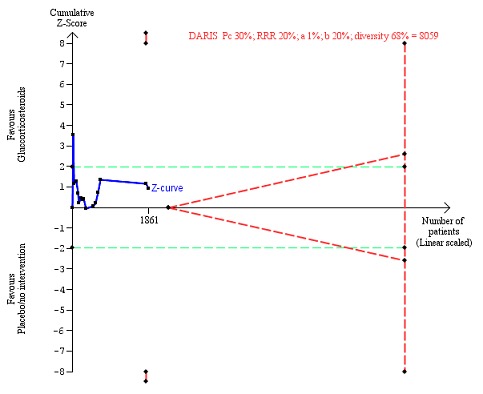
Liver‐related mortality up to three months after randomisation. Fifteen trials provided data. The diversity‐adjusted required information size (DARIS) was calculated based on liver‐ related mortality of 30% in the control group; risk ratio reduction in the glucocorticosteroid group of 20%; type I error of 1%; and type II error of 20% (80% power). Trial diversity was 68%. The required information size was 8059 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (red inward sloping lines) and did not enter the trial sequential monitoring area for futility (inner‐wedge with red outward sloping lines). The green dotted lines show the conventional boundaries of the naive alpha of 5% equal to Z‐scores of +1.96 and ‐1.96.
Number of participants with any complication up to three months' follow‐up after randomisation
In total, 440 of 927 (47%) participants in the group treated with glucocorticosteroids had one or more complications versus 414 of 934 (44%) participants in the control group. There was no evidence of effect of glucocorticosteroids on frequency of any complications (random‐effects RR 1.04, 95% CI 0.86 to 1.27; participants = 1861; I² = 42% (moderate heterogeneity); Analysis 1.5). We observed a similar result with the Trial Sequential Analysis showing that the cumulative Z‐curve did not cross the trial sequential monitoring boundaries for benefit or harm, nor enter the trial sequential monitoring area for futility in order to include an intervention effect of 20% RRR (Figure 10). The Trial Sequential analysis‐adjusted CI was 0.67 to 1.63. We rated the quality of the evidence as very low, mainly due to within‐study bias, inconsistency, and imprecision (Table 1).
Analysis 1.5.
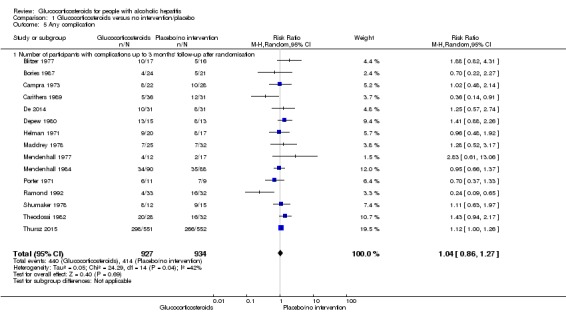
Comparison 1 Glucocorticosteroids versus no intervention/placebo, Outcome 5 Any complication.
Figure 10.
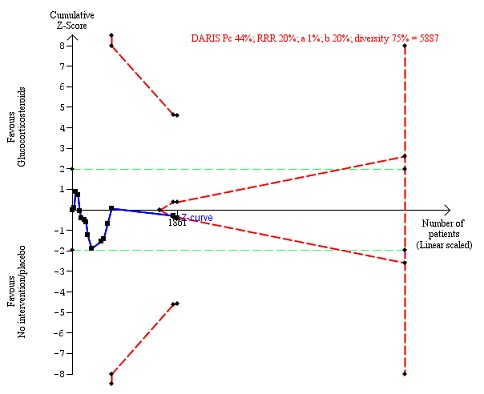
Any complications up to three months after randomisation. Fifteen trials provided data. The diversity‐adjusted required information size (DARIS) was calculated based on any complications of 44% in the control group; risk ratio reduction in the glucocorticosteroid group of 20%; type I error of 1%; and type II error of 20% (80% power). Trial diversity was 75%. The required information size was 5887 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (red inward sloping lines). The cumulative Z‐curve crossed the inner‐wedge futility line (red outward sloping lines). The green dotted lines show the conventional boundaries of the naive alpha of 5% equal to Z‐scores of +1.96 and ‐1.96.
Number of people with non‐serious adverse events up to three months following randomisation
Only four trials reported non‐serious adverse events such as Cushingoid symptoms, vertigo, and fungal lesions. There was no evidence of effect of glucocorticosteroids on frequency of non‐serious adverse events (random‐effects RR 1.99, 95% CI 0.72 to 5.48; participants = 160; trials = 4; I² = 0% (no heterogeneity); Analysis 1.6). We observed a similar result with the Trial Sequential Analysis showing that the cumulative Z‐curve did not cross the trial sequential monitoring boundaries for benefit or harm, nor enter the trial sequential monitoring area for futility in order to include an intervention effect of 50% RRR (Figure 11). The Trial Sequential Analysis‐adjusted CI was 0.01 to 249.60. We rated the quality of the evidence as very low (Table 1).
Analysis 1.6.
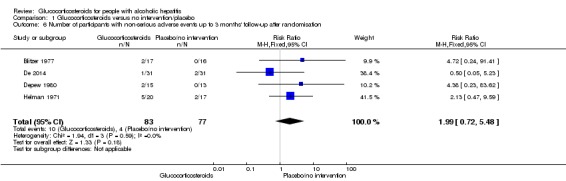
Comparison 1 Glucocorticosteroids versus no intervention/placebo, Outcome 6 Number of participants with non‐serious adverse events up to 3 months' follow‐up after randomisation.
Figure 11.
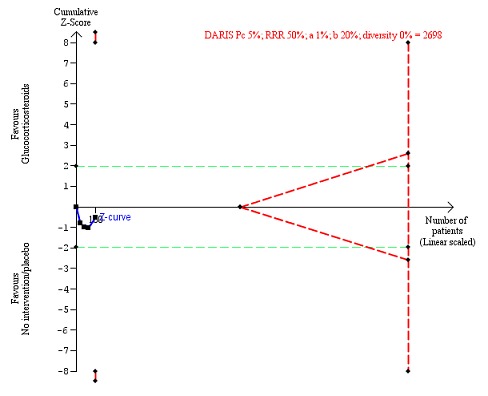
Non‐serious adverse events up to three months after randomisation. Four trials provided data. The diversity‐adjusted required information size (DARIS) was calculated based on non‐serious adverse events of 5% in the control group; risk ratio reduction in the glucocorticosteroid group of 50%; type I error of 1%; and type II error of 20% (80% power). Trial diversity was 0%. The required information size was 2698 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (red inward sloping lines) and did not enter the trial sequential monitoring area for futility (inner‐wedge with red outward sloping lines). The green dotted lines show the conventional boundaries of the naive alpha of 5% equal to Z‐scores of +1.96 and ‐1.96.
Exploratory outcomes at the end of treatment
No trial reported on number of participants with change of level of liver enzymes, prothrombin index, or serum albumin at the end of treatment. This is why we could not perform the planned exploratory analyses. Instead, post hoc, we decided to present in a tabular way the extracted information on level of liver enzymes reported in the trials by Campra 1973; Maddrey 1978; Theodossi 1982; and Carithers 1989 (Appendix 2); prothrombin index or international normalised ratio reported in the trials by Campra 1973; Maddrey 1978; Theodossi 1982; Carithers 1989; Ramond 1992 (Appendix 3); and level of serum albumin (and bilirubin ‐ post hoc again) reported in the trials by Campra 1973; Maddrey 1978; Depew 1980; Theodossi 1982; Carithers 1989; and Ramond 1992 ( Appendix 4; Appendix 5).
'Summary of findings' table
We have presented the key results on the outcomes mortality, health‐related quality of life, serious adverse events, liver‐related mortality, all complications, and non‐serious adverse events in Table 1. We assessed the evidence as being very low to low.
Discussion
Summary of main results
We included 16 randomised clinical trials comparing glucocorticosteroids versus placebo or no intervention in people with alcoholic hepatitis. Fifteen of the trials provided data for analyses. Our traditional meta‐analyses showed no beneficial or detrimental effects of glucocorticosteroids on any of our outcomes. In general, serious and non‐serious adverse events as well as complications were poorly reported or the information was unclear, and hence, these analyses may be subject to outcome reporting bias (Ioannidis 2009). Trial Sequential Analyses showed similar results. Based on methodological concerns, we classified the strength of the evidence as very low to moderate. As the trials were at high risk of bias, it is more likely that we are overestimating benefits and overlooking harms.
Overall completeness and applicability of evidence
The trial participants varied according to severity of alcoholic hepatitis and the trials were published between 1971 and 2014. However, only 1861 participants were included. During this time period, glucocorticosteroid interventions varied regarding dose and duration. The small number of trials and trial participants, except for the Thursz 2015 trial, the poor trial design and reporting, make the results of our review inconclusive. The high risk of bias of the trials undermines the precision of our meta‐analyses results.
We were not able to assess if ethnicity had any influence on our results, as data were either lacking or insufficient. The same applied for the nutritional status of the participants, as only one trial reported on it (Mendenhall 1984). Mathurin and coworkers have proposed that people with alcoholic hepatitis with Madderey's score of at least 32 should likely benefit from glucocorticosteroids (Mathurin 2011). However, we did not find a significant effect of glucocorticosteroids in this subgroup of trial participants.
This review is applicable in people with alcoholic hepatitis at different stages of the disease. Our meta‐analyses and Trial Sequential Analyses seem to provide no evidence of benefit of glucocorticosteroids on all‐cause mortality at one‐year follow‐up after randomisation. It is also unlikely that glucocorticosteroids may have a beneficial effect on mortality at end of treatment and three months following randomisation; however, due to mainly imprecision (the confidence interval crossed the clinical decision threshold between recommending and not recommending treatment and the required number of participants is far from reached), we cannot exclude the possibility of a short‐term beneficial or harmful effect. We cannot say if glucocorticosteroids may influence infection and gastrointestinal bleeding as we had no data for meta‐analysis. However, Thursz 2015 and colleagues' analysis shows an increase in the number of these complications in treated participants.
Quality of the evidence
The quality of the evidence reflects only the quality of the included trials so we cannot be certain of our conclusions. We judged the overall quality of evidence as very low to low for all outcomes except for all‐cause mortality at one year after randomisation, for which the quality of the evidence was moderate. All trials were at high risk of bias, mainly because the randomisation procedures were insufficiently reported. In addition to downgrading the trials for within‐study risk of bias, we also downgraded the trials for imprecision of effect estimates due to the number of participants included in the trials (all but one of the 14 trials had fewer than 400 participants), and for inconsistency of our results (there was wide variation in the effect estimates across the trials; there was little overlap of confidence intervals associated with the effect estimates; and we assessed heterogeneity of the data as moderate with I² of 36% to 46%, which could be explained with selection bias). We also found some evidence of publication bias or small study bias.
In spite of the quality of the evidence being mostly low to very low, we are pretty confident in our recommendations regarding implications for practice and for research. This ensues from our analysis results and is based on the knowledge that trials at high risk of bias overestimate benefits and underestimate harms. Therefore, we do not find supporting evidence for using glucocorticosteroids in clinical practice. There is definitely a need for more transparent reporting of individual participant data (NTAWG 2015; Garattini 2016).
Potential biases in the review process
The strengths of our review are that we have conducted our review following the recommendations of Cochrane Hepato‐Biliary and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Gluud 2017). We included only randomised clinical trials in our review. This creates a bias towards benefits as short‐term randomised trials often overlook harms. We attempted to minimise possible selection biases by using a comprehensive search strategy. We combined searches in electronic databases with extensive manual searches. In addition, we also searched conference proceedings and abstract books, irrespective of language. We think it is unlikely that we have missed any published trials, but we cannot exclude the possibility that we have missed unpublished trials. In fact, visual inspection of the funnel plots suggests publication bias or small trial bias on the outcomes, mortality at end of treatment and following three months after randomisation (Figure 5). We wrote to pharmaceutical companies as well as to regulatory authorities. We made extensive attempts to avoid risk of system and random errors. We assessed the evidence with GRADE approach.
Limitations of our review were the small number of trials and the small total number of participants. Having in mind that hepatitis C viral disease was discovered as late as 1989, we might have run the risk that the included trials initiated before 1989 did not include participants with only alcoholic hepatitis (Houghton 2009). Furthermore, our results are hampered by the quality of the included trials as well as imprecision and severe inconsistency. Even though all trials provided data on mortality, data on other serious adverse events and complications were rarely reported, which calls into question the reliability of the two latter analyses. Moreover, by including primarily randomised clinical trials we have focused on potential beneficial effects and overlooked the many known harms connected with the administration of glucocorticosteroids. Again, these flaws in our review make us suspect that benefits are overestimated and harms are underestimated.
When conducting our Trial Sequential Analyses, we used plausible parameters to calculate our required information sizes. However, we only used 80% power (beta = 20%). Had we used 90% power (beta = 10%) or less, which is relevant in meta‐analyses where one does not want to discharge a potentially relevant intervention, then we would have obtained larger required information sizes and wider Trial Sequential Analyses‐adjusted Confidence Intervals (Garattini 2016; Castellini 2017). Accordingly, the imprecision may be worse than signalled by our analyses.
Agreements and disagreements with other studies or reviews
The meta‐analysis by Christensen 1995 and colleagues found no effect of glucocorticosteroids versus placebo on mortality. This review included data from 13 trials with 659 participants randomised. Rambaldi 2008 and colleagues updated the meta‐analysis by Christensen 1995, adding two more trials with 62 participants randomised. Hence, Rambaldi 2008 and colleagues concluded that depending on the estimation of the information size, their review lacked another 1000 to 2000 participants randomised to glucocorticosteroids versus placebo in order to be able to either demonstrate or reject a clinically relevant 20% mortality reduction.
We found two new trials for inclusion in our review (De 2014; Thursz 2015). However, we excluded two of the trials from the Rambaldi and colleagues review (Rambaldi 2008) as they assessed glucocorticosteroids versus nutrition (Lesesne 1978; Cabré 2000). In addition, two trial reports turned out to be the same trial (Shumaker 1978; Galambos 1984), and thus, we counted them as one trial.
Our systematic review of pair‐wise comparison randomised clinical trials is in agreement with the recent meta‐analysis by Buzzetti 2017. In this network meta‐analysis, the authors found no significant effects of glucocorticosteroids on mortality at maximal follow‐up and up to 90 days of follow‐up.
Due to the inclusion of the two new trials, our review now includes 1861 participants. The Thursz 2015 trial included 1103 participants and found "a reduction in the 28‐day mortality in the prednisolone‐treated group on logistic regression model analysis, but there was not clear evidence of benefit, sustained beyond this point". Mathurin 2011 performed "analysis of individual data from five randomised clinical trials which showed that corticosteroids significantly improved 28‐day survival in patients with severe alcoholic hepatitis". In our present aggregate meta‐analysis, we cannot see an effect of glucocorticosteroids on mortality at 'end of treatment', which is quite close to 28 days.
Modern clinical guidelines recommend prescribing glucocorticosteroids: "Patients with severe disease (Maddrey's Discriminant Function (MDF) score of > 32, with or without hepatic encephalopathy) and lacking contraindications to steroid use should be considered for a four‐week course of prednisolone (40 mg/day for 28 days, typically followed by discontinuation or a 2‐week taper) (Class I, level A)" (AASLD 2010); and "First‐line therapy in patients with severe alcoholic hepatitis includes corticosteroids or, in case of ongoing sepsis, pentoxifylline (Recommendation B1) (see original publication for Figure 2)" (EASL 2012). In our present aggregate meta‐analysis, we cannot find a beneficial effect of glucocorticosteroids in people with severe alcoholic hepatitis.
Authors' conclusions
We found no evidence of a difference between glucocorticosteroids and placebo or no intervention on all‐cause mortality, health‐related quality of life, and serious adverse events during treatment. The risk of bias was high and the quality of evidence was very low or low. Therefore, we are very uncertain about this effect estimate. Due to inadequate reporting, we cannot exclude increases in adverse events. As the confidence intervals were wide, we cannot rule out significant benefits and harms of glucocorticosteroids.
As there could be some people with alcoholic hepatitis who could benefit from glucocorticosteroids, it could be of use for researchers to study further the effects of glucocorticosteroids in randomised clinical trials on short‐term all‐cause mortality. Additional evidence evaluating the effect on health‐related quality of life may also be needed. Future trials ought to be designed according to the SPIRIT guidelines (http://www.spirit‐statement.org/) and reported according to the CONSORT guidelines (www.consort‐statement.org). Future trials ought to report individual participant data, so that proper individual participant data meta‐analyses of the effects of glucocorticosteroids in subgroups can be conducted (NTAWG 2015; Garattini 2016).
Acknowledgements
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of Cochrane Hepato‐Biliary through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark. Disclaimer: The views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
Peer reviewers: Kurinchi S Gurusamy, UK; Michael Ronan Lucey, USA. Contact editor: Dario Conte, Italy. Sign‐off editor: Mirella Fraquelli, Italy.
Appendices
Appendix 1. Search strategies
| Database | Search performed | Search strategy |
| Cochrane Hepato‐Biliary Controlled Trials Register | September 2016 | (glucocortico* or steroid* or dexamethasone or prednis* or hydrocortisone or corticosteroid* or cortiso* or budesonide* or beclomethasone*) AND (alcohol* and (liver or hepati*)) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | 2016, Issue 8 | #1 MeSH descriptor: [Adrenal Cortex Hormones] explode all trees #2 (glucocortico* or steroid* or dexamethasone or prednis* or hydrocortisone or corticosteroid* or cortiso* or budesonide* or beclomethasone*) #3 #1 or #2 #4 MeSH descriptor: [Hepatitis, Alcoholic] explode all trees #5 (alcohol* and (liver or hepati*)) #6 #4 or #5 #7 #3 and #6 |
| MEDLINE Ovid | 1946 to September 2016 | 1. exp Adrenal Cortex Hormones/ 2. (glucocortico* or steroid* or dexamethasone or prednis* or hydrocortisone or corticosteroid* or cortiso* or budesonide* or beclomethasone*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 3. 1 or 2 4. exp Hepatitis, Alcoholic/ 5. (alcohol* and (liver or hepati*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 6. 4 or 5 7. 3 and 6 8. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 9. 7 and 8 |
| Embase Ovid | 1974 to September 2016 | 1. exp corticosteroid/ 2. (glucocortico* or steroid* or dexamethasone or prednis* or hydrocortisone or corticosteroid* or cortiso* or budesonide* or beclomethasone*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 3. 1 or 2 4. exp alcohol liver disease/ 5. (alcohol* and (liver or hepati*)).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 6. 4 or 5 7. 3 and 6 8. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 9. 7 and 8 |
| Science Citation Index Expanded (Web of Science) | 1900 to September 2016 | #5 217 #4 AND #3 #4 1,347,943 TS=(random* or blind* or placebo* or meta‐analys*) #3 1,060 #2 AND #1 #2 36,574 TS=(alcohol* and (liver or hepati*)) #1 425,242 TS=(glucocortico* or steroid* or dexamethasone or prednis* or hydrocortisone or corticosteroid* or cortiso* or budesonide* or beclomethasone*) |
Appendix 2. Level of liver enzymes (at the end of treatment)
| Glucocorticosteroids | Placebo/no intervention | |||||
| Mean | SD | Number of participants | Mean | SD | Number of participants | |
| Campra 1973 | 62.5 | 3.72 | 22 | 79.2 | 5.83 | 28 |
| Maddrey 1978 | 34.5 | 4 | 23 | 41.7 | 4.1 | 27 |
| Theodossi 1982 | 164.0 | ‐ | 28 | 118 | ‐ | 32 |
| Carithers 1989 | 74.9 | 4.19 | 36 | 119.9 | 7.79 | 31 |
| SD = standard deviation ‐ = SD data not reported | ||||||
Appendix 3. Prothrombin index (s)
| Glucocorticosteroids | Placebo/no intervention | |||||
| Mean | SD | Number of participants | Mean | SD | Number of participants | |
| Percentage of normal | ||||||
| Campra 1973 | 77.5 | 2.5 | 36 | 83.1 | 2 | 31 |
| Ramond 1992 | 48 | 0.5 | 32 | 45 | 0.5 | 29 |
| At the end of treatment | ||||||
| Maddrey 1978 | 14 | 0.5 | 23 | 15.5 | 0.9 | 27 |
| Theodossi 1982 | 13 | ‐ | 28 | 10 | ‐ | 32 |
| Carithers 1989 | 15.5 | 1.25 | 36 | 16.25 | 1 | 31 |
| SD = standard deviation ‐ = SD data not reported | ||||||
Appendix 4. Level of serum albumin (g/L)
| Glucocorticosteroids | Placebo/no intervention | |||||
| Mean | SD | Number of participants | Mean | SD | Number of participants | |
| Campra 1973 | 33.3 | 1.48 | 22 | 30.08 | 0.66 | 28 |
| Maddrey 1978 | 33 | 2 | 23 | 30 | 2 | 27 |
| Depew 1980 | 32 | 0.17 | 15 | 25.8 | 0.13 | 13 |
| Carithers 1989 | 27 | 0.35 | 36 | 29 | 0.4 | 31 |
| Ramond 1992 | 33 | 0.17 | 32 | 30 | 0.37 | 29 |
| SD = standard deviation | ||||||
Appendix 5. Level of bilirubin (μmol/L) (at the end of treatment)
| Glucocorticosteroids | Placebo/no intervention | |||||
| Mean | SD | Number of participants | Mean | SD | Number of participants | |
| Campra 1973 | 43.2 | 2.9 | 22 | 63.95 | 6.84 | 28 |
| Maddrey 1978 | 64.98 | 4.97 | 24 | 66.69 | 3.69 | 31 |
| Depew 1980 | 68.4 | 6.5 | 15 | 136.8 | 11.79 | 13 |
| Carithers 1989 | 125 | 8.12 | 36 | 190 | 17.9 | 31 |
| Ramond 1992 | 100 | 7.1 | 32 | 150 | 18.8 | 29 |
| SD = standard deviation | ||||||
Appendix 6. Age
| Glucocorticosteroids | Placebo/no intervention | |||||
| Mean | SD | Number of participants | Mean | SD | Number of participants | |
| Porter 1971 | 44.6 | 4.4 | 11 | 49.5 | 8.9 | 9 |
| Campra 1973 | 43.1 | 11.1 | 22 | 42.7 | 8.1 | 28 |
| Blitzer 1977 | 47.2 | ‐ | 17 | 48.4 | ‐ | 16 |
| Maddrey 1978 | 40 | 8.5 | 25 | 42.3 | 11.1 | 32 |
| Shumaker 1978 | 45.5 | ‐ | 15 | 44.5 | ‐ | 13 |
| Depew 1980 | 49.8 | 2.1 | 15 | 48.2 | 2.3 | 13 |
| Mendenhall 1984 | 51.5 | 8.2 | 90 | 50.4 | 9.2 | 88 |
| Bories 1987 | 41 | ‐ | 24 | 49 | ‐ | 21 |
| Carithers 1989 | 43.1 | 2 | 36 | 44.4 | 1.7 | 31 |
| Ramond 1992 | 48.1 | 1.3 | 33 | 48.2 | 1.6 | 32 |
| De 2014 | 42.7 | 0.4 | 31 | 41.3 | 7.8 | 31 |
| Thursz 2015 | 48.6 | 9.8 | 277 | 47.9 | 9.2 | 276 |
| Thursz 2015 | 49.3 | 10.6 | 274 | 48.8 | 10.3 | 276 |
| SD = standard deviation ‐ = SD data not reported | ||||||
Data and analyses
Comparison 1.
Glucocorticosteroids versus no intervention/placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Up to 3 months after randomisation | 15 | 1861 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.70, 1.15] |
| 1.2 At the end of treatment | 14 | 1824 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.66, 1.15] |
| 1.3 At 1 year after randomisation | 3 | 1343 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.91, 1.17] |
| 2 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Up to three months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Up to one year | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of participants with serious adverse events during treatment | 15 | 1861 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.85, 1.29] |
| 4 Liver‐related mortality | 15 | 1861 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.69, 1.14] |
| 4.1 Up to 3 months' follow‐up after randomisation | 15 | 1861 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.69, 1.14] |
| 5 Any complication | 15 | 1861 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.86, 1.27] |
| 5.1 Number of participants with complications up to 3 months' follow‐up after randomisation | 15 | 1861 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.86, 1.27] |
| 6 Number of participants with non‐serious adverse events up to 3 months' follow‐up after randomisation | 4 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.72, 5.48] |
Comparison 2.
Subgroup analysis: all‐cause mortality up to 3 months after randomisation
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severity of alcoholic hepatitis | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Mild alcoholic hepatitis | 4 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.58, 1.80] |
| 1.2 Severe alcoholic hepatitis | 14 | 1679 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.73, 1.16] |
| 2 Glucocorticosteroid (prednisolone) dose | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Less than or equal to 40 mg | 10 | 1547 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.50, 1.14] |
| 2.2 More than 40 mg | 5 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.79, 1.30] |
| 3 Alcoholic hepatitis without cirrhosis and with cirrhosis | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Alcoholic hepatitis | 3 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.18, 3.48] |
| 3.2 Alcoholic hepatitis with cirrhosis | 12 | 1738 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.74, 1.16] |
| 4 Hepatorenal syndrome | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 With hepatorenal syndrome | 8 | 1382 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.85, 1.17] |
| 4.2 Without hepatorenal syndrome | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.05, 6.49] |
| 5 Ascites | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 With ascites | 13 | 729 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.60, 1.12] |
Comparison 3.
Sensitivity analysis: all‐cause mortality
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Best‐worst scenario all‐cause mortality to 3 months follow‐up | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Up to 3 months | 15 | 1861 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.64, 1.05] |
| 2 Worst‐best scenario all‐cause mortality to 3 months follow‐up | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Up to 3 months | 15 | 1861 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.06, 1.37] |
Comparison 4.
Sensitivity analysis: serious adverse events
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Best‐worse scenario of serious adverse events during treatment | 15 | 1861 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.21] |
| 2 Worst‐best scenario of serious adverse events during treatment | 15 | 1861 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.05, 1.31] |
Differences between protocol and review
Review author team changed.
We removed the word 'alcohol' from the outcome "Alcohol liver‐related mortality up to three months follow‐up after end of treatment" as it was superfluous.
-
Outcomes
All‐cause mortality is now better defined. Duration of treatment varied across the trials and also mortality data for up to three‐months' follow‐up. This is why we have modified all‐cause mortality to all‐cause mortality at the end of treatment, up to three months' follow‐up after randomisation, and one year following randomisation. Thus, our primary time point has become "all‐cause mortality up to three months' follow‐up after randomisation".
Trials also reported data on liver‐related mortality, any complication, and non‐serious adverse events up to three months' follow‐up after randomisation. Thus, three months' follow‐up after randomisation has also become our primary time point for the latter outcomes. However, serious adverse events were reported mostly during the treatment period.
Regarding exploratory outcomes, we created tables, as we did not have sufficient data for analysis.
As we did not have trials with low risk of bias, we calculated the diversity‐adjusted required information size (DARIS) for our Trial Sequential Analysis using data from all included trials.
We calculated and reported the Trial Sequential Analysis‐adjusted CI as a supplement to the naive 95% CI.
We changed the risk of type I error from 2.5% (as originally planned based on the three primary outcomes) into type I error of 1%, as we performed Trial Sequential Analysis on all primary and secondary outcomes.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | A prospective, double‐blind randomised trial Country: USA, 1971‐1973 Analyses were not performed according to the intention‐to‐treat principle. Sample size calculation was not reported |
|
| Participants |
Demographic characteristics Age (mean): prednisolone/placebo group 47.2/48.4 Sex: male (all participants) "There were no significant differences between them with respect to mean age, sex, race, duration of hospitalization prior to entry into the study, frequency of histologically proved cirrhosis, or to the histologic severity of the alcoholic hepatitis." Inclusion criteria and degree of severity ".. patients with alcoholic hepatitis who met the following criteria after at least five days in the hospital were included in the study: (1) recent history of heavy alcohol consumption (more than one pint of whiskey per day or its alcoholic equivalent); (2) hepatomegaly based on physical examination (palpable more than 5 cm below the costal margin) and/or liver scan; (3) total serum bilirubin greater than 5 mg/100 mL; and (4) at least two of the following abnormalities: (a) serum glutamic oxaloacetic transaminase (SGOT) greater than 100 Reitman‐Frankel units per ml, (b) serum albumin concentration less than 3g/100 mL, or (c) prothrombin time more than 2 s greater than control value. Liver biopsies were performed whenever possible, but were not required for admission to the study." "14 biopsies proved alcoholic hepatitis." "Neither positive PPD skin tests nor active tuberculosis excluded patients from randomization. None of the former were encountered, and one of the latter continued to receive INH and PAS throughout the study. If serious life‐threatening infection was present, patients' entry into the protocol was postponed until it was eradicated. Patients with either a history of peptic ulcer, active peptic ulcer disease, or gastrointestinal bleeding were included." The study authors did not clearly describe the degree of severity of alcoholic hepatitis; however, the participants probably had moderate to severe alcoholic hepatitis, since they presented patients with alcoholic hepatitis who met the described criteria. Exclusion criteria "...who had been treated with adrenocorticosteroid in the six months prior to admission or who showed evidence of psychotic behavior precluding their cooperation during the investigation were excluded". Randomisation procedure "... Patients were assigned by random, sealed‐envelope technique to receive either placebo or steroid". Number of participants randomised n = 33 Prednisolone group: n = 17 Placebo group: n = 16 |
|
| Interventions |
Experimental group: oral prednisolone Dose: "10 mg q.i.d [four times a day], for 14 days, 5 mg qid [four times a day] for 4 days, 2.5 mg qid [four times a day] for 4 days, and 2.5 nag bid [four times a day] for 4 days" Control group: placebo tablets Dose: the same dosage schedule as the prednisolone group Additional interventions to the trial groups: "Patients were encouraged to eat the standard hospital 2600‐calorie diet and were offered supplements when caloric intake seemed inadequate. Low‐protein, low‐sodium, and other special diets were employed as the clinical situation dictated." Duration of treatment: 26 days Follow‐up after randomisation: 9 weeks |
|
| Outcomes | The trial outcomes were:
|
|
| Notes | Letter sent to authors in March 2000. No answer received. No further attempts were made as the trial was conducted between 1971‐1973. One participant received placebo treatment during the trial. At the end of the therapy, due to lack of improvement, the ward physician requested the code be broken. The participant received a 7‐day course of prednisolone. He died 17 days later; his death was included in the mortality data of the placebo group on an intention‐to‐treat basis. On the 26th day treatment period, three participants in the placebo group and one in the glucocorticosteroid group received the alternative medication on a double‐blind basis. "Both prednisolone and placebo tablets were kindly supplied by the Upjohn Co., Kalamazoo, Michigan." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned by random, sealed‐envelope technique to receive either placebo or steroid" |
| Allocation concealment (selection bias) | Low risk | "Patients were assigned by random, sealed‐envelope technique to receive either placebo or steroid" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Only the pharmacist was aware of the type of therapy which any individual patient was receiving." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "5 participants, who had each received less than 5 days of therapy, were subsequently excluded from analysis. Of these, three had left the hospital against medical advice or withdrew from the study, and in two participants experimental therapy had been stopped following gastrointestinal haemorrhage. One bled after 4 days of therapy from a gastric varix and the other from an unknown site after three days of treatment. On breaking the code at the end of the investigation, it was learned that all five participants had been in the steroid group....Furthemore, the addition of two deaths among the five excluded participants ......" 3/17 people in the prednisolone group and 0/16 people in the placebo group dropped out (9%) |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality, serious adverse events, and liver‐related mortality were reported |
| For‐profit bias | Unclear risk | Prednisolone and placebo tablets were supplied by the Upjohn Co., Kalamazoo, Michigan. |
| Other bias | Low risk | None suspected |
| Methods | Randomised controlled trial Country: France, 1979‐1982 Analyses were performed according to intention‐to‐treat method. Sample size calculation was not reported. |
|
| Participants |
Demographic characteristics Age: prednisolone group: average 41 years old (26 to 68); control group: average 49 years old (30 to 70) Sex: 16 men and 8 women in the treatment group, and 11 men and 10 women in the control group Inclusion criteria and degree of severity Not stated clearly, but the average level of bilirubin was ≥ 147 + 30.78 mmol/L Alcohol consumption: 155 g + 46 g per day in men; 140 g + 32 g per day in women Exclusion criteria 48 patients were excluded due to infections (45 patients), diabetes (2 patients), and tuberculosis (1 patient). Randomisation procedure Random number table Number of participants randomised n = 45 Prednisolone group: n = 24 Control group: n = 21 |
|
| Interventions |
Experimental group: oral prednisolone Dose: 40 mg/d Control group: no intervention Additional interventions to the trial groups: 1500 calories and 50 g of protein/d. Encephalopathy was treated with lactulose and neomycin. In case of infection, participants were treated with antibiotics. Duration of treatment: 1 month Duration of follow‐up: 3 months after randomisation |
|
| Outcomes | The trial outcomes were:
|
|
| Notes | Letter sent to authors in March 2000. No answer received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "By random number table" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality, serious adverse events, and liver‐related mortality were reported |
| For‐profit bias | Unclear risk | Not reported |
| Other bias | Low risk | None suspected |
| Methods | A prospective randomised control trial Country: USA, 1971 Analyses were not performed according to intention‐to‐treat method Sample size calculation was not reported |
|
| Participants |
Demographic characteristics Age: (mean + SD): prednisolone/control group 43.1 + 11.1/42.7 + 8.1 Sex: male (%): prednisolone/control group: 8% (40)/9% (35) Inclusion criteria and degree of severity ".....a clinical diagnosis of severe acute alcoholic liver disease, absence of contraindication to corticosteroids therapy, absence of a prior history of liver disease." Liver biopsy was not required for admission in the trial since some participants had prothrombin time < 50% of normal value. No clear definition of severity criteria used Exclusion criteria Participants with other known illness or illnesses Randomisation procedure "...by using previously prepared sealed envelopes, patients were randomly allocated to one of the two treatment groups" Number of participants randomised "50 patients entered the trial, but 5 were subsequently withdrawn when additional data favoured another diagnosis. In one case (group 2), jaundice proved to be caused by hepatitis B....the patient died .....2 of these patients were in group 2, one patient in group 1; all survived. The fifth patient was removed from the trial when peptic ulcer was diagnosed after 15 days of prednisolone therapy". Prednisolone group: n = 22 (20) Control group: n = 28 (25) Analyses of 45 participants: 20 prednisolone + 25 control |
|
| Interventions |
Experimental group: oral prednisone Dose: 0.5 mg/kg body weight daily, during 3 weeks; and then 0.25 mg/kg body weight, daily, for additional 3 weeks Control group: no intervention Additional interventions to the trial groups: vitamin supplements, folic acids; "high calorie, high protein diet was given if tolerated .......In patients with encephalopathy, protein intake was reduced to 20 or 40 g..... and neomycin 500 x 4 times daily was given. ...In case of bleeding , vomiting and extreme anorexia, 5% or 10% solutions of dextrose in water was administered." Duration of treatment: 6 weeks Duration of follow‐up: "hospital stay after randomisation ranged between 42 and 92 days, with a mean of 47 days, for group 1; and between 43 and 95 days, with a mean of 48 days, for group 2." |
|
| Outcomes | The trial outcomes were:
|
|
| Notes | Letter sent to authors in March 2000. AG Redeker answered in January 2001 (see the risk of bias table) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...using previously prepared sealed envelopes, patients were randomly allocated to one of the two treatment groups" |
| Allocation concealment (selection bias) | Low risk | Information obtained through personal communication with the authors in 2001 reads: "they were never in the possession of the investigators, but were kept by the department secretary who opened them upon request" However, the publication reads: "using previously prepared sealed envelopes, patients were randomly allocated to one of the two treatment groups" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "the trial was not double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | ".. all statistical analyses and interpretation were done under supervision of Dr. John Weiner of the Department of Biostatics, University of Southern California School of Medicine" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "50 patients entered the trial, but five were subsequently withdrawn when additional data favoured another diagnosis. In one case (group 2), jaundice proved to be caused by hepatitis B...the patient died .....2 of these patients were in group 2, one patient in group 1; all survived. The fifth patient was removed from the trial when peptic ulcer was diagnosed after 15 days of prednisolone therapy". 0/22 prednisolone group/control group 0/28 |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality, serious adverse events, and liver‐related mortality were reported |
| For‐profit bias | Unclear risk | Not reported |
| Other bias | Low risk | Not suspected |
| Methods | A randomised, multi central, double‐blind, placebo‐controlled, clinical trial Country: USA, 1979‐1984 Analyses were performed according to intention‐to‐treat method Sample size calculation was reported It was ".. calculated that 62 patients should be entered to have a 95% chance of detecting a difference in survival between the two groups" |
|
| Participants |
Demographic characteristics Age (mean) + SD: 43.1 + 2.0 in the treatment group; 44.4 + 1.7 in the placebo group Sex: (male (%)): 20 (57)/21 (68) Inclusion criteria and degree of severity "... a history of long‐standing alcoholism and clinical features of alcoholic hepatitis were evaluated by one of the principal investigators within 3 days of admission. All patients admitted to the study had either clinical evidence of spontaneous hepatic encephalopathy or a discriminant function value greater than 32 or both. Hepatic encephalopathy was assessed using standard clinical criteria. Spontaneous hepatic encephalopathy was considered to be present when correctable causes of encephalopathy had been excluded." "Each patient had to have a negative hepatitis B surface antigen within the first 3 days of hospitalisation and also have no history of previous viral hepatitis." Exclusion criteria "Patients with gastrointestinal haemorrhage requiring transfusions, diabetes requiring insulin administration, active infection requiring treatment, clinical and laboratory evidence of acute pancreatitis, history of recent head trauma, known prior heroin addiction, or preexisting chronic renal disease with a serum creatinine level greater than 175 mmol/L were excluded" Randomisation procedure ".. Random sequences for drug or placebo were submitted to the Upjohn Company (Kalamazoo, Michigan), which provided methylprednisolone (Medrol) in 4‐mg tablets and identical placebo tablets as well as intravenous preparations of methylprednisolone sodium succinate (SoluMedrol) and placebo. A random code was prepared for each of the four participating institutions such that within each group of 10 patients, 5 would receive methylprednisolone and 5 placebo. The random code sequence was kept by an independent source." Number of participants randomised n = 67 ("...67 patients ranging in age from 25 to 66 years were entered into the study over 4 years") Prednisolone group n = 36 (2 refused, 1 was excluded from analysis) Control group n = 31 |
|
| Interventions |
Experimental group: methylprednisolone Dose: .......given in a single dose of eight pills (4‐mg tablets) each morning for 28 days. 32 mg methylprednisolone (equivalent to 40 mg prednisolone). In patients unable to take oral medications, intravenous infusions of the study drugs were administered daily (methylprednisolone sodium succinate (SoluMedrol) or identical placebo". After 4 weeks, four tablets were administered daily for 1 week followed by two tablets daily for an additional week; then therapy was discontinued. Control group: placebo ‐ identical tablets Additional treatment: "All patients were offered a 3000‐caIorie diet. Protein (1 to 1.5 g/kg body weight) was provided for patients who had no evidence of hepatic encephalopathy. Protein restriction to 20 g/d or less and lactulose therapy were instituted in patients with signs of hepatic encephalopathy. Ascites was managed with sodium restriction or by the addition of spironolactone in patients who did not respond with diuresis within 5 days. Fluid intake was restricted in patients with hyponatraemia. B‐compIex multivitamins and folic acid (1 mg) were given each day. In patients who developed tremulousness or delirium tremens, Valium, or oxazepam was administered." Duration of treatment: 5 weeks; 28 days ‐ 32 mg. "After 4 weeks, four tablets were administered daily for 1 week followed by two tablets daily for an additional week; then therapy was discontinued". Duration of follow‐up after randomisation: at the moment of discharge |
|
| Outcomes | The trial outcomes were:
|
|
| Notes | Letter sent to authors in March 2000. No answer received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random code was prepared for each of the four participating institutions such that within each group of 10 patients, 5 would receive methylprednisolone and 5 placebo. ......". |
| Allocation concealment (selection bias) | Low risk | "the random code sequence was kept by an independent source" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "neither the principal investigators nor their associates were aware of which regimen patients received throughout the trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Data obtained at initial evaluation and follow‐up were recorded on standardized data collection forms that were submitted to the statistical coordinating center at the end of each study.... A study overview committee, chaired by Dr. Hyman Zimmerman, reviewed the ongoing results of the study on a yearly basis from reports generated by the statistical coordinating center, which had access to the randomisation codes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3% "One patient who received methylprednisolone was belatedly discovered to have had neither encephalopathy nor elevation of discriminant function sufficient to meet the entry criteria and was excluded from analysis. Of the remaining 66 patients, 64 remained in the hospital for the duration of the study. Two methylprednisolone recipients refused to continue in the study. The first patient signed out of the hospital after 10 days on the trial and was alive at the end of the study. The second patient was discharged at his insistence after 15 days on the trial and was given the study drug to take at home, but he never returned for follow‐up. His status at the end of the study was unknown. He was the only patient lost to follow‐up." 2/36 prednisolone group / placebo group 0/31 |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality, serious adverse events, and liver‐related mortality were reported. |
| For‐profit bias | Unclear risk | "random sequences for drug or placebo were submitted to the Upjohn Company (Kalamazoo, Michigan), which provided methylprednisolone (Medrol) in 4‐mg tablets and identical placebo tablets as well as intravenous preparations of methylprednisolone sodium succinate (Solu‐Medrol) and placebo." |
| Other bias | Low risk | Not suspected |
| Methods | A randomised controlled clinical trial Country: India (the Medical College and Hospital, Kolkata); January 2010 ‐ August 2012 The study protocol was approved by the institutional ethical committee. |
|
| Participants |
Demographic characteristics Age: mean (year) (PTX +prednisolone)/PTX 42.73 (0.43)/41.33 + 7.81 Sex: male Inclusion criteria and degree of severity "Patients who had a history of chronic alcohol intake of more than 50 g/day with clinical and biochemical features of severe alcoholic hepatitis (MDF score ≥ 32 and aspartate aminotransferase (AST): alanine aminotransferase (ALT) > 2:1 with absolute value of AST < 500 IU/L and ALT < 200 IU/L)" Model for end‑stage liver disease (MELD) score and Glasgow alcoholic hepatitis score (GAHS) and Child‑Pugh score were calculated for all participants who were included in the study. Exclusion criteria "..Patients with any other potential etiology of liver injury (acute or chronic viral hepatitis, autoimmune liver disease, Wilson’s disease) even in the background of chronic alcohol intake, positive for HIV antibodies or patients with a history of abstinence from alcohol in the last month, infection, sepsis or spontaneous bacterial peritonitis, acute pancreatitis, gastrointestinal bleeding, hepatorenal syndrome or any other severe associated disease such as uncontrolled diabetes mellitus, systemic hypertension, heart failure, pulmonary disease or malignancy at the time of inclusion or in the previous 3 months". Randomisation procedure "The recruited patients were then divided into 2 groups by a computer generated randomization table" Number of participants randomised n = 62 "... who fulfilled the inclusion and exclusion criteria and who gave informed written consent were randomized and divided into 2 groups: Group 1 (PTX only) had 31 patients Group 2 (prednisolone plus PTX) had 31 patients. One patient in Group 1 developed severe vertigo within 7 days after starting PTX and one patient in Group 2 withdrew voluntarily from the study, and hence they were excluded. Hence, a total of 60 patients, 30 in each group, were considered for the final analysis." Prednisolone group n = 31 (1 voluntary dropped out) Control group n = 31 (1 vertigo and withdrew) |
|
| Interventions |
Experimental group: "received prednisolone tablet (Wysolone, Wreath, Mumbai, India) at a dose of 40 mg once daily for 4 weeks and PTX tablets at a dose of 400 mg thrice daily for the duration for the first 4 weeks". Dose: 40 mg prednisolone Control group: "received PTX (Trental tablets, Sanofi Aventis, Mumbai, India) at a dose of 400 mg thrice daily orally and a placebo tablet in the place of prednisolone for the first 4 weeks." Dose: 40 mg placebo Duration of treatment: 12 weeks "After the initial 4 weeks, the study was opened and the patients allocated to different groups were revealed. Patients in Group 1 (PTX) who tolerated the drug well, continued to receive the medication at the same dose for the next 8 weeks and then stopped. After 4 weeks of initial therapy, the dose of prednisolone in Group 2 was tapered by 5 mg/week over a period of 7 weeks and then stopped and received PTX like Group 1 patients". (Thus, we can use only 3 months.) Duration of follow‐up: 12 months |
|
| Outcomes | The trial outcomes were:
|
|
| Notes | "One patient in Group 1 developed severe vertigo within 7 days after starting PTX, and one patient in Group 2 withdrew voluntarily from the study and hence they were excluded. A total of 60 patients, 30 in each group, were considered for the final analysis." Letter sent to SK Mandal 12.10.2016. No reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The recruited patients were then divided into 2 groups by a computer generated randomization table" |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The investigator, who allocated the patients to the groups, administered the drugs and collected the clinical and laboratory data, as well as statisticians were all blinded regarding the nature of the pharmacotherapy." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "as well as statisticians were all blinded regarding the nature of the pharmacotherapy." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1.6% "One patient in Group 1 developed severe vertigo within 7 days after starting PTX, and one patient in Group 2 withdrew voluntarily from the study and hence they were excluded." 1/31 prednisolone/1/31 placebo group |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality, serious adverse events, and liver‐related mortality are reported. |
| For‐profit bias | Unclear risk | "Prednisolone tablet (Wysolone, Wreath, Mumbai, India) and ptx (trental tablets, Sanofi Aventis, Mumbai, India" |
| Other bias | Low risk | Not suspected |
| Methods | A randomised, double‐blind, controlled clinical trial Country: USA, 1977‐1979 The study was approved by the Human Experimentation Committee of the John Wesley County Hospital. Analyses were performed according to intention‐to‐treat method. Sample size calculation was reported. |
|
| Participants |
Demographic characteristics: Age: (mean) + SD: 49.8 + 2.1 in the treatment group, 48.2 + 2.3 in the placebo group Sex: (male (%)): 10 (67)/6 (43), hepatic encephalopathy %: 100/100; ascites %: 87/92 Inclusion criteria and degree of severity ".... all patients were alcohol abusers from the lower socioeconomic strata with a clinical diagnosis of severe alcoholic hepatitis manifested by hepatomegaly, leukocytosis, and a serum‐bilirubin greater than 5 mg/dL, spontaneous hepatic encephalopathy occurring in the absence of gastrointestinal haemorrhage, sedation, diuretic usage, or major electrolyte disturbances." Exclusion criteria "Severe diabetes, active tuberculosis, and serious bacterial infection prevented participation in the trial" Liver biopsy was not required. Randomisation procedure Unclear. It is not described. However, it reads: "All patients fulfilling the criteria who gave informed consent were randomised into two treatment protocols" and "...to avoid introducing bias based on the presence of the hepatorenal syndrome, the randomisation procedure was stratified to distinguish those with a serum creatinine greater than 2.5 mg/dL." Number of participants randomised n = 28 Prednisolone group: n = 15 Control group: n = 13 |
|
| Interventions |
Prednisolone group: prednisolone Dose: 40 mg daily by mouth Control group: placebo Dose: 40 mg daily by mouth Duration of treatment: "....28 days followed by tapered withdrawal over the ensuing 14 days." Additional treatment: "none of the patients received prophylactic isoniazid. Supportive measures were attention to fluid and electrolyte balance, multiple vitamin supplementation, and parenteral glucose administration when food intake was poor. Encephalopathy was treated with catharsis, protein restriction, and oral neomycin." Duration of treatment: 6 weeks to 28 days followed by tapered withdrawal over the ensuing 14 days Duration of follow‐up: 6 weeks |
|
| Outcomes | The trial outcomes were:
|
|
| Notes | Letter sent to authors in March 2000. No answer received. No further attempts were made as the trial was conducted between 1977‐1979. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. "All patients fulfilling the criteria who gave informed consent were randomised into two treatment protocols" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Neither the principal investigators nor the physicians attending the patients were aware of the identity of the coded drugs. Provision was made for breaking the code if serious complications developed which could be related to steroid therapy" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals and dropouts |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality, serious adverse events, and liver‐related mortality were reported. |
| For‐profit bias | Unclear risk | Not reported |
| Other bias | Low risk | None suspected |
| Methods | Randomised controlled trial Country: USA Analyses were performed according to intention‐to‐treat method. Sample size calculation was not reported. |
|
| Participants |
Demographic characteristics: Age: (average) 47.8 years, with a range of 30 to 67 years Sex : male 12, female 25 Inclusion criteria and degree of severity ". .... the diagnosis of alcoholic hepatitis was confirmed in all patients by percutaneous needle biopsy....Seventy per cent of patients had anaemia on admission. The anaemia was attributed to folate deficiency, blood loss, alcoholism, and haemolysis." Exclusion criteria "Patients with clinical evidence of alcoholic hepatitis were excluded from the trial if a biopsy could not be obtained within the first week of hospitalisation, if gastrointestinal bleeding requiring transfusion occurred during this period, or if the purified protein derivative test was positive." The authors reported that participants were classified into three groups, according to clinical severity of their disease. "Group I were severely ill‐ manifesting precoma or coma, group 2 ‐were moderately ill,but no evidence of encephalopathy, group 3 ‐ asymptomatic ambulatory patients." Randomisation procedure "... patients were selected by a random, double‐blind technique....Drug treatment was randomly determined by the hospital pharmacist, without informing physicians, nurses, or patients until completion of the study" Number of participants randomised n = 37, divided in 3 groups according to the severity of the disease Prednisolone group: n = 20 Control group: n = 17 |
|
| Interventions |
Experimental group: prednisolone daily Dose: 40 mg Control group: daily lactose placebo Dose: 40 mg Additional intervention: "all patients were treated with bed rest, a high protein (100 g) and high calorie diet (3 000 Kcal) when tolerated and vitamin supplementation including folic acid. Sodium restriction was instituted and all patients with ascites and oedema were treated with diuretics." Duration of treatment: 6 weeks: 4 weeks and 2‐week period tapered Duration of follow‐up after randomisation: 4 months |
|
| Outcomes | The trial outcomes were
|
|
| Notes | "prednisolone and lactose placebo provided by Upjohn Co, Kalamazoo, Mich" Letter sent to authors in March 2000. No answer received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. It only reads: "patients were selected by a random, double‐blind technique...." |
| Allocation concealment (selection bias) | Low risk | "Drug treatment was randomly determined by the hospital pharmacist, without informing physicians, nurses, or patients until completion of the study " |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | ".....The treatment code was broken during the study in only one case because of a medical emergency" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None of the participants withdrew or dropped out |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality was reported |
| For‐profit bias | Unclear risk | "tablets 40 mg of prednisolone daily or lactose placebo (provided by Upjohn Co, Kalamazoo, Mich)" |
| Other bias | Low risk | None suspected |
| Methods | Randomised, double‐blind clinical trial with parallel‐group design (3 groups) Country: USA Analyses were not performed according to the intention‐to‐treat principle. Sample size calculation was not reported. |
|
| Participants |
Demographic characteristics Age: (mean + SD): 40 + 8.5 in the treatment group and 42.3 + 11.1 in the control group Sex: 12 men (50%) in the treatment group and 23 men (74%) in the control group Inclusion criteria and degree of severity People with a history of long‐standing and recent alcoholism were referred to the Liver Service (The Johns Hopkins Hospital). A percutaneous liver biopsy was performed unless precluded by coagulation abnormalities. Alcoholic hepatitis was defined histologically as an inflammatory hepatic disease with cell swelling and hydropic change, cell necrosis, and polymorphonuclear leukocytic infiltration. Exclusion criteria People with active gastro‐intestinal haemorrhage, pancreatitis, history of peptic ulcer, active infection, presence of hepatitis B infection, or history of previous viral hepatitis were excluded. Maddrey's discriminant‐function. All people had wedged hepatic venous pressure determination. Randomisation procedure "Patients were randomized for treatment within three groups based on apparent severity of disease. Random drug sequences were arranged within each group." Number of participants randomised n = 57 "Patients were randomised for treatment within three groups based on apparent severity of disease. Group A (moderately ill), serum bilirubin > 3 mg per dL; hepatomegaly; and clotting factors adequate to allow liver biopsy Group B (more severely ill), hyperbilirubinaemia and hepatomegaly as in A with additional presence of ascites and/or hepatic encephalopathy, but coagulation studies adequate for liver biopsy Group C (severely ill), hyperbilirubinaemia and hepatomegaly as in A and B, with or without ascites and/or hepatic encephalopathy, but coagulation abnormalities precluded liver biopsy." Prednisolone group: n = 25 Control group: n = 32 |
|
| Interventions |
Experimental group: Dose: 40 mg/d orally (eight tablets 5 mg each every morning) Control group: identical placebo tablets Additional interventions to the trial groups: all trial participants were offered a 3000 calorie diet. Protein (1 g to 1.5 g per kg) was provided for people with no evidence of hepatic encephalopathy. In people with encephalopathy, protein restriction to 20 g/d or less and lactulose therapy. Ascites was managed with sodium restriction alone or with the addition of spironolactone in those who did not respond with diuresis in 5 days. All participants initially received 100 mg of thiamine intramuscularly. B‐complex multivitamins and folic acid U mg) were given each day Duration of treatment: from 28 to 32 days Follow‐up: until discharge |
|
| Outcomes | The trial outcomes were"factors important in determining outcome in alcoholic hepatitis and to evaluate further the effects of corticosteroid therapy on early mortality and on the progression to cirrhosis, as reflected by histological changes and wedged hepatic venous pressure." Reported outcomes in the trial
|
|
| Notes | This study was supported by Research Grant AA00201 from the National Institute of Alcohol Abuse and Alcoholism of the National Institutes of Health, and by Grant RR‐35 from the Clinical Research Centers Program, United States Public Health Service. Prednisolone and placebo tablets were provided by the Division of Steroid Research, The Upjohn Company, Kalamazoo, Mich. Letter sent to authors in March 2000. No answer received. No further attempts were made. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised for treatment within three groups based on apparent severity of disease. Random drug sequences were arranged within each group" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Prednisolone (5 mg) or identical placebo tablets were given in a single dose of 8 pills each morning for 28 to 32 days. (Prednisolone (5 mg) and identical placebo tablets were provided by the Division of Steroid Research, The Upjohn Company, Kalamazoo, Mich.). The investigators were not aware of which regimen the patient was receiving until the completion of the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3.5% dropped out or were withdrawn, i.e. "Two additional patients were removed from the study after randomisation. One patient who was randomised to the placebo group bled from oesophageal varices before receiving the study drug. He subsequently stopped bleeding and survived. Another patient had an episode of upper gastrointestinal haemorrhage presumably from oesophageal varices after receiving prednisolone for 9 days and the drug was stopped." 1/25 prednisolone group and 1/32 placebo group |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality, serious adverse events, and liver‐related mortality were reported. |
| For‐profit bias | Unclear risk | Prednisolone and placebo tablets were provided by the Division of Steroid Research, The Upjohn Company, Kalamazoo, Mich. However, no further details were provided. |
| Other bias | Low risk | None suspected |
| Methods | A prospective, randomised clinical trial (three intervention groups) Country: USA Intention‐to‐treat analysis: not mentioned Sample size calculation was not reported. |
|
| Participants |
Demographic characteristics Age: not reported Sex: not reported; most probably men as they came from V.A. (Veteran Affairs) Medical Centers Inclusion criteria and degree of severity
Exclusion criteria Not described Randomisation procedure Not described, but mentioned that "regimens were chosen randomly and blinded so that neither physician nor patient was aware of the treatment modality" Number of participants randomised n = 46 29 (n = prednisolone and placebo) + 17 (n = oxandrolone) Prednisolone group: n = 12 (all severe alcoholic hepatitis) Control group: n = 17 (all severe alcoholic hepatitis) |
|
| Interventions | Experimental group: "prednisolone 60 mg/d x 5, then decreased over a 16 day period" Dose: 60 mg Placebo group: placebo Dose: unknown Additional treatment: supportive care Duration of treatment: 21 days Duration of follow‐up: at the moment of discharge |
|
| Outcomes | The trial outcomes were
|
|
| Notes | Letter sent to study authors in 2006. No answer received. Only published as an abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ".....regimens were chosen randomly" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "blinded so that neither physician nor patient was aware of the treatment modality" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "one additional mortality withdrew from the study on the 8 day" (not mentioned from what group out of 50 participants 17 participants were treated with oxandrolone |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality was reported |
| For‐profit bias | Unclear risk | Not reported |
| Other bias | Low risk | None suspected |
| Methods | A co‐operative, multicentre, randomised clinical trial (three intervention groups) Country: USA, 1980‐1983 Intention‐to‐treat analysis Sample size calculation was reported. |
|
| Participants |
Demographic characteristics Age: (mean 90) prednisolone group 51.5 + 8.2; (mean 88) placebo group 50.4 + 9.2 Sex: male Inclusion criteria and degree of severity Men hospitalised at 6 Veterans Administration Medical Centers in whom diagnosis of moderate or severe alcoholic hepatitis was based on conventional clinical and laboratory changes of this disease. "Histological confirmation was not required, so that severely ill patients were not excluded." Criteria to classify severity: "the degree of jaundice (bilirubin) and coagulopathy (prothrombin time)" Exclusion criteria
Randomisation procedure "...One hundred thirty‐two patients with moderate disease and 131 with severe disease were randomly assigned to one of three treatments: prednisolone, oxandrolone, or placebo...Treatment assignment were made by the Coordinating Center (Hines, Ill.)The random assignment of treatments was balanced within each hospital, as well as according to disease severity." Number of participants randomised 178 (n = prednisolone and placebo) + 85 (n = oxandrolone) Prednisolone group: n = 90 (moderate 46, severe 44) Control group: n = 88 (moderate 45, severe 43) |
|
| Interventions |
Experimental group: prednisolone Dose: "60 mg per day for four days; 40 mg per day for four days; 30 mg per day for four days; 20 mg per day for four days; 10 mg per day for seven days; 5 mg per day for seven final days" Control group: corresponding placebos Duration of treatment: 30 days When possible, participants were evaluated monthly at outpatient clinics. If alcoholic hepatitis recurred and required re‐hospitalisation, the person was reassigned to the same therapy for 30 days with his permission. Duration of follow‐up after randomisation: 1 year (350 days for prednisolone group ) |
|
| Outcomes | The trial outcomes were
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Treatment assignment was made by the Coordinating Center (Hines, Ill.). The random assignment of treatments was balanced within each hospital, as well as according to disease severity". |
| Allocation concealment (selection bias) | Low risk | "Treatment assignment were made by the Coordinating Center (Hines,Ill.)". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Medication was packed into unit dose kits at the Veterans Administration Center Pharmacy(Albuquerque, N.M.). The patient, physician and the local hospital pharmacy had no knowledge of the specific medication in use" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4.5% "Ten patients withdrew from the study before completing treatment (5 given placebo, 3 prednisolone). However, these patients were included in the outcome assessment" 3/ 90 prednisolone group/ 5/88 control group "324 days...37 patients were lost to follow‐up: 13 given placebo, 11 prednisolone" |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality, serious adverse events, and liver‐related mortality are reported. |
| For‐profit bias | Unclear risk | "Matching placebos were prepared for each of these medications by Upjohn Company and G.D. Searle and Company." |
| Other bias | Low risk | None suspected |
| Methods | A prospective, double‐blind, controlled pilot trial Country: USA Analyses were not performed according to the intention‐to‐treat method. Sample size calculation was not reported. |
|
| Participants |
Demographic characteristics steroid/placebo group Age (years) 44.6 + 4.4/49.5 + 8.9 (age range 27 to 61 years) Sex: male 13/7 (64%/67%) Inclusion criteria and degree of severity: "A history of recent heavy alcohol ingestion a serum bilirubin concentration of 5 mg per 100 mL or more clinical and laboratory deterioration over the first five hospital days, a striking lack of improvement in the patient's clinical and biochemical status over the same period, or rapid marked deterioration in less than 24 hours for admission to the study all three absolute criteria were required In addition, two or more major criteria or one major and four or more minor criteria had to be met. The major criteria were liver biopsy showing alcoholic hepatitis; hepatic encephalopathy (including asterixis); persistent or progressive azotaemia not explained by another process, and total bilirubin levels > 20 mg/100 mL. The minor criteria for inclusion were fever that was not obviously secondary to another process; white blood count > 12,000 not obviously secondary to another process; anorexia or nausea or vomiting; palpable hepatomegaly; palpable splenomegaly; oesophageal varices; spider angiomas, edema or ascites; palmar erythema and a prothrombin time prolonged three or more seconds over control. The Australia antigen was absent from the serum of all 16 patients in whom it was sought. Before the trial, a percutaneous needle biopsy of the liver was performed if the prothrombin time was not prolonged more than four seconds over control and there was no clinical bleeding tendency. Exclusion criteria active gastrointestinal bleeding, pancreatitis, radiological evidence of peptic‐ulcer disease, active or questionably active pulmonary tuberculosis and potentially life threatening bacterial infection. Randomisation procedure: "the case was randomised into one of the two treatment groups. Both the steroid (6‐ methyl prednisolone, or Medrol) and the placebo (lactose) were packaged and coded by number in both parenteral and oral forms (prepared and supplied through the courtesy of the Upjohn Co, Kalamazoo,Mich)and randomisation was achieved by a number drawn from a pool. Neither patients nor physicians knew which form of treatment was used until the study had been completed, when the code was broken." Number of participants randomised: n = 23 (20) "23 accepted to participate, but 3 died within 36 hours of the start of therapy, and were excluded from the analysis before the code was broken, and did not receive adequate medication. The final series consisted of 20 patients" Prednisolone group: n = 11 Placebo group: n = 9 |
|
| Interventions |
Prednisolone group: "6‐methyl‐prednisolone (or Medrol) 40 mg per day (equivalent to 50 mg prednisolone, or 200 mg hydrocortisone) in three divided doses, parenterally for the first 10 days. If clinical improvement occurred over this interval and if nausea and vomiting were absent the drug was administered orally, and the dose gradually tapered (decreased every second day by 4 mg for the 11th to the 18th days, by 2 mg for the 19th to 30th days and every third day by 2 mg for the 31st to 45th days). If there was no clinical improvement within 10 days. The initial parenteral dose of 40 mg daily was continued until improvement or death occurred." Dose: 50 mg prednisolone Placebo group: placebo(lactose). "...packaged and coded by number in both parenteral and oral forms (prepared and supplied through the courtesy of the Upjohn Co, Kalamazoo, Mich)" Additional treatment: "early in the study only the patients with a positive intermediate strength PPD test or a suspicious chest film were given isoniazid coverage , however, later in the study, all patients were so treated" "general supportive care required in hepatic decompensation. Special attention was given to fluid and electrolyte balance , prompt treatment of hepatic encephalopathy and repeated evaluation for infection... most patients had daily estimation of the caloric and protein intake by a hospital dietitian... who were unable to take oral nutrition received parenterally a minimum of 400 calories per day as glucose Duration of treatment and of follow‐up: 45 days after randomisation |
|
| Outcomes | The trial outcomes were:
|
|
| Notes | Country: USA Letter sent to study authors in March 2000. No answer received. "Twenty‐three patients were accepted for studying. However, three died within 36 hours of the start of the therapy and were excluded from analysis before the code was broken because they did not receive adequate medication." Supported in part by a gastroenterology‐research training grant (AM‐05099) from the National Institute of Artrithis and Metabolic Diseases (a portion of this work was conducted within the Clinical Research Center of the University of Washington, with support by a grant MO1 FR‐37 from the National Institutes of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... randomisation was achieved by a number drawn from a pool" |
| Allocation concealment (selection bias) | Low risk | "the case was randomised into one of the two treatment groups. Both the steroid (6‐ methyl prednisolone, or Medrol) and the placebo (lactose) were packaged and coded by number in both parenteral and oral forms (prepared and supplied through the courtesy of the Upjohn Co, Kalamazoo, Mich)..." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Neither patients nor physicians knew which form of treatment was used until the study had been completed, when the code was broken" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "At the conclusion of the study, all needle biopsy and post‐mortem liver specimens were coded and read in blind review by the same observer" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13% "Twenty three patients were accepted for study, three died within 36 hours of the start of therapy and were excluded from analysis before the code was broken because they didn't receive adequate medication. The final series thus consisted of 20 patients " |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality, serious adverse events, and liver‐related mortality are reported. |
| For‐profit bias | Low risk | "The drugs were provided by Upjohn Co., Kalamazoo, Mich." Supported in part by a gastroenterology‐research training grant (AM‐05099) from the National Institute of Artrithis and Metabolic Diseases (a portion of this work was conducted within the Clinical Research Center of the University of Washington, with support by a grant MO1 FR‐37 from the National Institutes of Health |
| Other bias | Low risk | None suspected. |
| Methods | A randomised, double‐blind trial Intention to‐treat analysis "The study was approved by the hospital ethics committees" |
|
| Participants |
Demographic characteristics: 124 alcoholic patients were admitted to 2 centres. Mean age + SD: 48.1 + 1.3; 48.2 + 1.6 Sex: (male): prednisolone group/placebo group 10/9 Randomisation procedure: "... a random code was prepared by computer for each participating center. There was a different code for men and women in each center, so that within each group of six patients (male or female), three patients received prednisolone and three patients placebo. Random sequence of drug or placebo were prepared by the pharmacists at each hospital" Inclusion criteria and degree of severity: "all the patients had biopsy proven alcoholic hepatitis (characterised by hyaline necrosis and infiltration of polymorphonuclear leucocytes) and spontaneous hepatic encephalopathy or a discriminant‐function value higher than 32 (or both)." "Eight patients died before inclusion in the trial." Exclusion criteria "gastrointestinal bleeding or bacterial infection were excluded from the trial unless they could be effectively treated within 48 hours" Presence of hepatitis B surface antigen, presence of HIV antibodies, refusal of liver biopsy, non‐alcoholic hepatitis at histology Number of participants randomised: n = 65 "65 patients were randomly assigned, but 4 were excluded‐ one patient assigned to receive prednisolone was found to have anguilluliasis and her treatment was stopped one day after her inclusion in the study. Three patients assigned to placebo were found not to have satisfied the inclusion criteria . These 4 patients were alive at the end of treatment" Prednisolone group: n = 33 (analysed 32) Placebo group: n = 32 (analysed 29) |
|
| Interventions |
Prednisolone group: "prednisolone (Solupred) in 20 mg tablets and identical placebo were provided by the pharmacists at each hospital" Dose: "prednisolone 40 mg (40 mg of prednisolone equivalent to 32 mg of methylprednisolone) was given in a single dose of two pills each morning for 28 days", if the participant was unable to take oral medication, "received intravenous infusions of prednisolone (Hydrocortancyl) or placebo prepared by the pharmacists and administered by the pharmacist and administered by the attending physician." Placebo group: "identical placebo was given in a single dose of two pills" Additional treatment: "All patients were provided with 3,000 Kcal containing one gram protein/kg. Patients with hepatic encephalopathy received lactulose therapy. Ascites was managed with sodium restriction or by the spironolactone to the treatment regimen" "B complex multivitamins, folic acid, and antiacids were given each day" Duration of treatment: 28 days Duration of follow‐up: 8 weeks |
|
| Outcomes | The trial outcomes were:
|
|
| Notes | Country: France. From March 1987‐June 1990 Letter sent to study authors in March 2000. No answer received The trial was stopped at the first interim analysis conducted after inclusion of 61 participants out of the planned 130 participants. The authors used an alpha error below 0.025. This is too high a value to prevent early stopping at a random high. "Drug therapy was interrupted by the attending physician if there was severe bacterial infection or gastrointestinal bleeding, or if a corticosteroid‐related complication was suspected... in patients with such complications the remaining tablets of the study drug were replaced with placebo tablets provided by the pharmacist (the only person who knew which regimen the patient had received first). The principal investigator and their associates were not aware of randomisation procedure or of the medication that the patients were receiving throughout the trial." "65 patients were randomly assigned, but 4 were excluded ‐ one patient assigned to receive prednisolone was found to have anguilluliasis and her treatment was stopped one day after her inclusion in the study. Three patients assigned to placebo were found not to have satisfied the inclusion criteria. These 4 patients were alive at the end of treatment." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a random code was prepared by computer for each participating centre... There was a different code prepared for men and women in each center, so that within each group of six patients (male and female), three patients received prednisolone and three received placebo" |
| Allocation concealment (selection bias) | Low risk | "a random code was prepared by computer for each participating centre. Random sequences of drug or placebo were prepared by the pharmacist at each hospital" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "a random code was prepared by computer for each participating centre. Random sequences of drug or placebo were prepared by the pharmacist at each hospital" "prednisolone (Solupred) in 20 mg tablets and identical placebo were provided by the pharmacists at each hospital" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1.5% 1 woman left the hospital and then "she was re hospitalised 56 days after enrolment and left again the following day. She was the only patient lost to follow up" 1/33 prednisolone group 0/32 placebo group |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality, serious adverse events, and liver‐related mortality were reported |
| For‐profit bias | Unclear risk | Prednisolone tablets and placebo were provided by Laboratoire Houdé (Paris) |
| Other bias | Low risk | Not suspected |
| Methods | A randomised clinical trial with a cross‐over design Intention to‐treat analysis: not mentioned Sample size calculation was not reported |
|
| Participants |
Demographic characteristics: no information. Inclusion criteria and degree of severity: non‐infected patients with histologically proven alcoholic hepatitis. All patients had severe hepatic failure (prothrombin time < 50%, or bilirubin > 5.6 mg/dL, or encephalopathy) Number of participants randomised: 23 Glucocorticosteroid group: 12 participants Placebo group: 11 participants |
|
| Interventions |
Prednisolone group: prednisolone 40 mg daily Control group: placebo 40 mg daily Duration of treatment: Prednisolone group: 1 week of treatment followed by one week of no treatment Control group: 1 week of no treatment followed by one week of treatment After that, both groups received glucocorticosteroids for three weeks. Duration of follow‐up: at discharge from hospital (three months) |
|
| Outcomes | The trial outcomes were:
|
|
| Notes | Country: France. Letter sent to study authors in 2006. No answer received. Only published as abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
| For‐profit bias | Unclear risk | Not reported |
| Other bias | Unclear risk | No information |
| Methods | A prospective, double‐blind, randomised clinical trial Sample size calculation was not reported. Intention‐to‐treat analysis: not mentioned, but presumably used |
|
| Participants |
Demographic characteristics: mean age + SD: 47/43 Sex: male were 25% in the glucocorticosteroid group and 44% in the placebo group Inclusion criteria and degree of severity: "history of recent heavy alcoholic ingestion, serum bilirubin greater than 5 mg %, hospitalisation for at least 5 days without improvement in liver tests; or rapid deterioration of the clinical condition during a 24‐hour period while under observation. In addition, a patient had to have a minimum of two "major criteria" or one "major" and two "minor" criteria to be placed in the study. The major criteria were a liver biopsy showing alcoholic hepatitis (with or without Mallory bodies), hepatic encephalopathy, azotaemia unexplained by another process (BUN [blood urea nitrogen] > 20 mg % or creatinine > 1.5 mg % ), hyperbilirubinaemia (> 20 mg%) and prothrombin time prolonged more than four seconds over control; and unresponsive to parenteral administration of vitamin K. The minor criteria included fever not obviously secondary to another process, WBC (white blood count) > 12,000, hepatomegaly (span greater than 14 cm), splenomegaly, or liver stigmas (spider telangiectasias, palmar erythema, ascites, edema, etc)" ...."a positive HBAg did not exclude them from the study if a percutaneous liver biopsy confirmed alcoholic hepatitis" Exclusion criteria: "Patients were not considered for the trial if their SGOT [serum glutamic oxaloacetic transaminase] was greater than 500....Patients were rejected from the study if they manifested active gastrointestinal bleeding, pancreatitis, X‐ray evidence of peptic ulcer disease, active or questionably active tuberculosis, active infection, or severe psychiatric disorder" Randomisation procedure: "The patient was then randomised into a predetermined code provided by the drug manufacturer. Immediately prior to randomisation, patients were stratified into two categories based on the presence or abstinence of criteria permitting liver biopsy" the purpose of this procedure was to provide comparable case material for both steroid and placebo control groups.in the absence of other contradictions, patients with prothrombin times less than four seconds prolonged were placed in the "Biopsy feasible" group (n = 10) whether or not they agreed to a biopsy. All other patients constituted the "Biopsy‐ Disallowed" group (n = 17)" Number of participants randomised: 27 Prednisolone group: n = 12 Control group: n = 15 |
|
| Interventions |
Prednisolone group: "80 mg of 6‐methylprednisolone or equivalent number of placebo tablets (or parenteral therapy of the same dosage intravenously if gastrointestinal function precluded oral intake) for four to seven days; the medication was then tapered on a flexible schedule with cessation of therapy planned for four weeks unless death or complications" Dose: 100 mg prednisolone Control group: placebo Additional interventions to the trial groups: "Both groups received comparable supportive care required in hepatic decompensation" All participants with positive tuberculin tests were treated with INH 300 mg daily, and 50 mg of pyridoxine daily. Duration of treatment: 5 weeks; participants were placed on treatment for 4‐7 days. Then the medication was tapered on flexible schedule with cessation of therapy planned for 4 weeks unless death or complication intervened. Duration of follow‐up: until the moment of discharge |
|
| Outcomes | The trial outcomes were:
|
|
| Notes | Country: USA Letter sent to study authors in March 2000. No answer received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "The patient was then randomised into a predetermined code provided by the drug manufacturer" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind: "80 mg of 6‐methylprednisolone or equivalent number of placebo tablets (or parenteral therapy of the same dosage intravenously if gastrointestinal function precluded oral intake" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Clinical evaluation was carried out by junior staff physicians blinded to treatment status of the patients." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3.7 % "a steroid treated patient voluntarily withdrew from the study after eight days but was retained for statistical purposes" 1/12 prednisolone group; 0/15 control group |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality, serious adverse events, and liver‐related mortality were reported. |
| For‐profit bias | Unclear risk | "The patient was then randomised into a predetermined code provided by the drug manufacturer. (Upjohn Co., Kalamazoo, MI, prepared and supplied the medication and placebo." |
| Other bias | Low risk | None suspected |
| Methods | A randomised controlled trial Analyses were not by intention‐to‐treat method. Sample size calculation was not reported. |
|
| Participants |
Demographic characteristics: Sex: men/women prednisolone group: 19/8, control group 12/16 Age: not mentioned Inclusion criteria and degree of severity: "patients had to satisfy the following criteria: ‐ a history of alcohol intake of about 80 g or more daily for at least five years, ‐ a serum bilirubin concentration greater than 80 pmol/l (normal up to 20 timol/L), ‐ a serum aspartate transaminase level at least twice the upper limit of normal (normal up to 40 IU/1), ‐ a prothrombin time prolonged by at least nine seconds." "Gastrointestinal bleeding, renal failure, and sepsis did not invalidate entry." Exclusion criteria: "Patients with hepatoma and those with other diseases such as recent myocardial infarction, an accompanying cerebrovascular accident including evidence of subdural hematoma, and active tuberculosis" Randomisation procedure "Patients .... referred from other hospitals because of the severity of their illness. Patients were allocated by random sealed envelope technique to a control or treatment group..." Number of patients: 60 (55). They were referred from other hospitals because of the severity of their illness (after excluded patients from the analyses because of doubts in initial diagnosis) Prednisolone group: n = 28 (27) Control group: n = 32 (28) |
|
| Interventions |
Prednisolone group: intravenous methylprednisolone 1 g daily for three days Dose: 1.25 g prednisolone Control group: no intervention Additional treatment: "Patients who were too ill to take the standard hospital diet received a minimum of 2000 calories as intravenous 20% glucose. Encephalopathy was treated with protein restriction (maximum of 20 g/day), lactulose (15 to 30 mL twice daily), and daily magnesium sulphate enemas" Duration of treatment: 3 days Duration of follow‐up: "there was a little difference between the groups in the mean length of stay in hospital (treatment group 24.2 days and control group 28.1 days)" |
|
| Outcomes | The trial outcomes were:
|
|
| Notes | Country: UK Letter sent to study authors in March 2000. No answer received "Of the 60 patients who satisfied the entry criteria, one in the treatment group and four in the control group were excluded from the final analysis because subsequent findings in four cases cast doubt on the initial diagnosis, and one patient was later found to have been given corticosteroids at the referring hospital. Thus there were 27 patients in the treatment and 28 in the control group." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "Patients were allocated by random sealed envelope technique to a control or treatment group,..." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded "Patients were allocated by random sealed envelope technique to a control or treatment group, the latter receiving intravenous methylprednisolone 1 g daily for three days." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8% "Of the 60 patients who satisfied the entry criteria, one in the treatment group and four in the control group were excluded from the final analysis because subsequent findings in four cases cast doubt on the initial diagnosis, and one patient was later found to have been given corticosteroids at the referring hospital. Thus there were 27 patients in the treatment and 28 in the control group." 1/28 prednisolone and 4/32 control group |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality, serious adverse events, and liver‐related mortality were reported. |
| For‐profit bias | Unclear risk | Not reported |
| Other bias | Low risk | None suspected |
| Methods | Multicentre, randomised trial with a 2‐by‐2 factorial design (09/MRE09/59) Country: UK (65 hospitals). January 2011‐February 2014 Analyses were performed according to intention‐to‐treat basis Sample size calculation was reported. |
|
| Participants |
Demographic characteristics Age: (mean) + SD: glucocorticosteroids group/control group 49.3 ± 10.6; 48.6 ± 9.8/47.9 ± 10.2; 48.8 ± 10.3 Sex (male (%)): glucocorticosteroids group/control group 359 (65,6%)/326 (59,8%) Hepatic encephalopathy (%): 152/143 (28%/26%) Inclusion criteria and degree of severity "all patients were alcohol abusers with a clinical diagnosis of severe alcoholic hepatitis manifested by hepatomegaly, leukocytosis, and a serum‐bilirubin greater than 5 mg/dL, spontaneous hepatic encephalopathy"
Exclusion criteria
Randomisation procedure "...A web‐based computer system (Tenalea, Forms‐Vision) was used to enrol eligible patients and randomly assign them to study groups. The randomization schedule was created with the use of Stata software, version 11 (StataCorp). Randomization was performed with a block size of four, with stratification according to geographic area and risk category. The high‐risk category consisted of patients who had an occurrence of gastrointestinal bleeding, renal impairment, or sepsis before randomisation. All other patients were assigned to the intermediate‐risk category." Number of participants randomised n = 1103 ("...1103 patients underwent randomisation (data from 1053 were available for the primary end‐point analysis..") Prednisolone group: 274 + 277 Control group: 276 + 276 |
|
| Interventions |
Experimental groups: "the second group receiving 40 mg of prednisolone daily and a pentoxifylline‐matched placebo (n = 277), the fourth group receiving 40 mg of prednisolone daily, and 400 mg of pentoxifylline three times daily (n = 274) Dose: 40 mg Control groups: "one group receiving a pentoxifylline matched placebo and a prednisolone matched placebo, placebo (n = 276). The third group receiving 400 mg of pentoxifylline three times daily and prednisolone matched placebo (n = 274)". Additional treatment: "Standard supportive care and nutritional support were given to each patient. The clinician responsible for each patient made the decision regarding other treatments, such as terlipressin for patients in whom hepatorenal failure was developing, acid suppression for prophylaxis against gastrointestinal haemorrhage, antibiotics, and vitamin supplementation. Patients with renal failure (defined as a creatinine level > 500 μmol per liter [> 5.7 mg per decilitre] or the requirement for renal‐replacement therapy), active gastrointestinal bleeding, or untreated sepsis, and patients requiring inotropic support with epinephrine or norepinephrine, were excluded unless the condition stabilized within the first 7 days after admission to the hospital." Duration of treatment: 28 days Duration of follow‐up: 1 year |
|
| Outcomes | The trial outcomes were
|
|
| Notes | The "European Quality of Life Five Dimension Five Level Scale (EQ‐5D‐5L): The EQ‐5D‐5L is a self‐report, multiple choice questionnaire that provides a simple descriptive profile and a single index value for health status. The EQ‐5D‐5L essentially consists of 2 pages – the EQ‐5D descriptive system (page 2) and the EQ visual analogue scale (EQ VAS) (page 3). The descriptive system comprises the following 5 dimensions: mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has 5 levels: no problems, slight problems, moderate problems, severe problems, and extreme problems. The EQ VAS records the respondent’s self‐rated health on a vertical, visual analogue scale. The EQ‐5D‐5L takes only a few minutes to complete. A summary index with a maximum score of 1 can be derived from these five dimensions by conversion with a table of scores. The maximum score of 1 indicates the best health state, by contrast with the scores of individual questions, where higher scores indicate more severe or frequent problems. In addition, there is a visual analogue scale (VAS) to indicate the general health status with 100 indicating the best health status." "The study was approved by the Multicenter Research Ethics Committee (reference number 09/MRE09/59), and clinical trial authorization was received from the Medicines and Healthcare Products Regulatory Agency (Funded by the National Institute for Health Research Health Technology Assessment program; STOPAH EudraCT number, 2009‐013897‐42, and Current Controlled Trials number, ISRCTN88782125.)" "The trial was conducted and reported with fidelity to the protocol, the Medicines for Human Use (Clinical Trials) Regulations 2004, as amended in 2006, the European Union Clinical Trials Directive (Directive 2001/20/EC) guidelines, the principles of the International Conference on Harmonisation Good Clinical Practice under the oversight of University Hospital Southampton NHS Foundation Trust, and the provisions of the Declaration of Helsinki. Letter sent to M. Thursz 12 October 2016. No reply received yet |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A web‐based computer system (Tenalea, Forms‐Vision) was used to enrol eligible patients and randomly assign them to study groups." |
| Allocation concealment (selection bias) | Low risk | "The randomization schedule was created with the use of Stata software, version 11 (StataCorp). Randomization was performed with a block size of four, with stratification according to geographic area and risk category. "Treatment allocation was blinded to site staff and the patient by providing each patient with a unique four‐digit patient pack number." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded "The treatment arm was also concealed to investigators and researchers. Only the study statisticians were unblinded and this was for analysis purposes only." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An independent data monitoring and ethics committee, whose members were aware of the group assignments, was convened to review the conduct of the trial and to analyze primary end‐point data, using prespecified stopping guidelines, after the recruitment of 200, 400, and 800 patients, to avoid continued recruitment in the event that a definitive result had been achieved. Data collected by site investigative teams were submitted to the clinical trials unit and analysed by study statisticians. The first author wrote the first draft of the manuscript, with substantial contributions from the coauthors. All the authors vouch for the accuracy and completeness of the data and analyses." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "At the time the trial was stopped, 33 patients who underwent randomization during the last 90 days of the trial could not be included in the 90‐day or 12‐month analyses. In addition, there were 159 patients who underwent randomization within 90 days to 12 months before the end of trial who could not be included in 12‐month analyses. The four groups were well matched with regard to their baseline characteristics, including laboratory values (See Table 1 in the published article). At 28 days, 16% of the patients had died, 1% had been lost to follow‐up, and 2% had withdrawn from the study. At 90 days, 29% of the patients (285 of 968 patients) had died, 5% had been lost to follow‐up, 3% had withdrawn, and 4% had not completed follow‐up owing to cessation of the study. At 1 year, 56% of the patients (421 of 747 patients) had died or undergone liver transplantation (the latter were 3 patients), 8% had been lost to follow‐up, 4% had withdrawn, and 20% had not completed follow‐up owing to cessation of the study due to limitations on funding." |
| Selective reporting (reporting bias) | Low risk | All‐cause mortality, serious adverse events, liver‐related mortality, and quality of life were reported. |
| For‐profit bias | Low risk | "Owing to limitations on funding, the trial was stopped after all enrolled patients had completed at least 28 days of follow‐up." "This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment programme. The NIHR Clinical Research Network provided research nurse support and the Imperial College Biomedical Research Centre also provided funding." |
| Other bias | Low risk | None suspected |
INH = isoniazid; PAS = para‐aminosalicylic acid; PPD = purified protein derivative; PTX = pentoxifylline
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvarez 2004 | An observational study (patient series). Thirteen participants with severe alcoholic hepatitis were treated with systemic glucocorticosteroids and total enteral nutrition. |
| Cabré 2000 | A randomised trial of glucocorticosteroids versus nutrition in people with alcoholic hepatitis. Participants received oral or intravenous prednisolone or enteral nutrition (2000 kcal/d of a chemically defined polymeric enteral diet enriched in branched‐chain amino acids) |
| Christensen 1981 | A quasi‐randomised clinical study |
| Copenhagen 1969 | A meta‐analysis |
| Daures 1991 | A meta‐analysis |
| Dhanda 2016 | A prospective single‐ centre cohort of patients with severe alcoholic hepatitis treated with steroids ‐ the incidence and significance of infection |
| Galambos 1984 | Reported in article through private contacts as part of Shumaker 1978 |
| Gill 1984 | The trial randomised 10 patients with severe alcoholic hepatitis to prednisolone, testosterone, and amino acid supplement versus no intervention. |
| Goldis 2000 | An observational study (patient series); the authors used a control group from the same centre. |
| Hozo 1996 | The trial randomised patients with alcoholic liver cirrhosis to glucocorticosteroids versus placebo. |
| Imperiale 1990 | A meta‐analysis |
| Lesesne 1978 | A randomised trial of glucocorticosteroids versus nutrition in patients with alcoholic hepatitis. Participants received glucocorticosteroids plus permission to eat as they wanted or a maximum of 600 kcal/d as intravenous glucose, while the control group received caloric supplements of at least 1600 kcal/d |
| Mal 1991 | Abstract about influence of corticosteroids on the level of serum TNF concentrations |
| Mendenhall 1993 | A chapter on alcoholic hepatitis in a book |
| Moreno 2014 | A multi central study with two groups of comparison of intensive enteral nutrition with complete nutrition, in both groups receiving prednisolone |
| Morris 2005 | An observational study (patient series) |
| Naganuma 2014 | The trial of granulocytapheresis and leukocytapheresis for the treatment of severe alcoholic hepatitis |
| Naveau 2004 | The trial randomised patients with alcoholic hepatitis to receive infliximab versus placebo. All participants received prednisone too |
| Phillips 2001 | The trial randomised participants to antioxidants versus glucocorticosteroids |
| Poynard 1991 | A meta‐analysis on alcoholic hepatitis |
| Reynolds 1989 | A narrative review on alcoholic hepatitis |
| Schlichting 1976 | A quasi‐randomised clinical study |
| Spahr 2002 | The trial randomised patients with alcoholic hepatitis to receive infliximab versus placebo. All participants received prednisone too |
| Stewart 2002 | The trial stratified participants by gender and glucocorticosteroid use, and then randomised participants to receive antioxidants versus placebo |
| Tygstrup 1979 | A meta‐analysis |
| Young‐Sun Lee | A review |
TNF: tumour necrosis factor
Contributions of authors
CP, DV, and GC: drafted the review. DN, ET, and CG: revised the review. CP and DV are the guarantors of the review. All authors approved the review.
Sources of support
Internal sources
The Cochrane Hepato‐Biliary Group Editorial Team Office, Denmark.
External sources
No sources of support supplied
Declarations of interest
CP: no financial, academic, or personal conflicts of interest. DV: no financial, academic, or personal conflicts of interest. GC: no financial, academic, or personal conflicts of interest. ET: no financial, academic, or personal conflicts of interest. DN: no financial, academic, or personal conflicts of interest. CG: no financial, academic, or personal conflicts of interest.
Notes
Cochrane Reviews can be expected to have a high percentage of overlap in the methods section because of standardised methods. In addition, overlap may be observed across two of our protocols as they share at least four common authors.
New
References
References to studies included in this review
- Blitzer BL, Mutchnick MG, Joshi PH, Phillips MM, Fessel JM, Conn HO. Adrenocorticosteroid therapy in alcoholic hepatitis. A prospective, double‐blind randomized study. Digestive Diseases 1977;22(6):477‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Blitzer BL, Mutchnick MG, Joshi PH, Phillips MM, Fessel JM, Conn HO. Adrenocorticosteroid therapy in alcoholic hepatitis. A prospective, double‐blind randomized study. Gastroenterology 1973;64:880. [DOI] [PubMed] [Google Scholar]
- Bories P, Guedj JY, Mirouze D, Yousfi A, Michel H. Prednisolone treatment of alcoholic hepatitis: forty‐five patients [Traitement de l'hepatite alcoolique aigue par la prednisolone. Quarante‐cinq malades]. La Presse Medicale 1987;16:769‐72. [MEDLINE: ] [PubMed] [Google Scholar]
- Campra JL, Hamlin EM Jr, Kirshbaum RJ, Olivier M, Redeker AG, Reynolds TB. Prednisone therapy of acute alcoholic hepatitis. Report of a controlled trial. Annals of Internal Medicine 1973;79(5):625‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Erratum: Methylprednisolone therapy in patients with severe alcoholic hepatitis: Randomized multicenter trial (American Journal of Gastroenterology, Vol. 85, No. 4 (473)). American Journal of Gastroenterology1990; Vol. 85, issue 6:776. [CRS: 6800131000045704; EMBASE: 1990185566]; Carithers RL Jr, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, et al. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Annals of Internal Medicine 1989;110:685‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Carithers RLJ, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, et al. Corticosteroid therapy of alcoholic hepatitis: how many studies will it take?. Hepatology (Baltimore, Md.) 1990;12(3):619‐20. [CENTRAL: 844269; CRS: 6800100000024896; CN‐00844269] [DOI] [PubMed] [Google Scholar]; Maddrey WC, Carithers R Jr, Herlong HF, Combes B, Diehl AM, Shaw E, et al. Prednisolone therapy in patients with severe alcoholic hepatitis: results of a multicenter trial. Hepatology (Baltimore, Md.) 1986;6(5):1202. [Google Scholar]
- De BK, Mandal SK, Sau D, Mani S, Chatterjee S, Mondal SS, et al. Pentoxifylline plus prednisolone versus pentoxifylline only for severe alcoholic hepatitis: a randomised controlled clinical trial. Annals of Medical and Health Sciences Research 2014;4(5):810‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew W, Boyer T, Omata M, Redeker A, Reynolds T. Double‐blind controlled trial of prednisolone therapy in patients with severe acute alcoholic hepatitis and spontaneous encephalopathy. Gastroenterology 1980;78(3):524‐9. [MEDLINE: ] [PubMed] [Google Scholar]; Depew WT, Boyer TD, Omata M, Redeker AG, Reynolds TB. Double‐blind controlled trial of corticosteroid‐therapy in severe alcoholic hepatitis with encephalopathy. Clinical Research 1978;26(2):A150. [CRS: 6800131000045795] [Google Scholar]
- Helman RA, Temko MH, Nye SW, Fallon HJ. Alcoholic hepatitis. Natural history and evaluation of prednisolone therapy. Annals of Internal Medicine 1971;74(3):311‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Maddrey WC, Boitnott JK, Bedine MS. Corticosteroid treatment of alcoholic liver disease: a controlled trial. Gastroenterology 1977;72(5 II):A‐148. [CRS: 6800131000045726; EMBASE: 0978117751] [Google Scholar]; Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978;75(2):193‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Mendenhall CL, Goldberg S. Risk factors and therapy in alcoholic hepatitis (AH). Gastroenterology 1977;72(5):1100. [Google Scholar]
- Mendenhall C, The Cooperative Study on Alcoholic Hepatitis. Survival after steroid treatment (T) for alcoholic hepatitis (AH). Hepatology (Baltimore, Md.) 1983;3(5):850. [CENTRAL: 221691; CRS: 6800100000005197; CN‐00221691] [Google Scholar]; Mendenhall CL, Anderson S, Garcia‐Pont P, Goldberg S, Kiernan T, Seeff LB, et al. Short‐term and long‐term survival in patients with alcoholic hepatitis treated with oxandrolone and prednisolone. New England Journal of Medicine 1984;311(23):1464‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Mendenhall CL, Roselle GA, Gartside P, Moritz T. Relationship of protein calorie malnutrition to alcoholic liver disease: a reexamination of data from two Veterans Administration Cooperative Studies. Alcoholism, Clinical and Experimental Research 1995;19(3):635‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Porter HP, Simon FR, Pope CE II, Volwiler W, Fenster LF. Corticosteroid therapy in severe alcoholic hepatitis. A double‐blind drug trial. New England Journal of Medicine 1971;284(24):1350‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mathurin P, Duchatelle V, Ramond MJ, Degott C, Bedossa P, Erlinger S, et al. Long term survival and prognostic factors in patients with severe biopsy‐proved alcoholic hepatitis (AH) treated by prednisolone: randomized trial, new cohort and simulation. Hepatology (Baltimore, Md.) 1994;20(4):319A. [Google Scholar]; Mathurin P, Duchatelle V, Ramond MJ, Degott C, Bedossa P, Erlinger S, et al. Survival and prognostic factors in patients with severe alcoholic hepatitis treated with prednisolone. Gastroenterology 1996;110(6):1847‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Ramond M‐J, Poynard T, Rueff B, Carey WD. Steroids in alcoholic hepatitis: another salvo of data. American Journal of Gastroenterology 1992;87(9):1219‐20. [CRS: 6800131000045543; EMBASE: 1992277170; CN‐00196579] [PubMed] [Google Scholar]; Ramond MJ, Poynard T, Rueff B, Horwitz RJ. Prednisolone for severe alcoholic hepatitis. Annals of Internal Medicine 1992;117(Suppl 2):36. [CRS: 6800131000045702; EMBASE: 1992360747] [Google Scholar]; Ramond MJ, Poynard T, Rueff B, Mathurin P, Theodore C, Chaput JC, et al. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. New England Journal of Medicine 1992;326(8):507‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Ramond MJ, Poynard T, Rueff B, Theodore C, Matthurin P, Chaput JC, et al. Prednisolone markedly improves short‐term survival of patients with severe biopsy‐proven alcoholic hepatitis A double blind randomized bicenter trial. Journal of Hepatology 1991;13(Suppl 2):S63. [CENTRAL: 222028; CRS: 6800100000005449; CN‐00222028]1667670 [Google Scholar]
- Mal F, Pham Huu T, Richardet JP, Roulot D, Labadie H, Pol S, et al. Corticosteroids (Cs) does not influence plasma tumor necrosis factor‐alpha (pTNF) and interleukin 6 (pIL6) concentrations in severe alcoholic hepatitis (AH): a cross over study. Hepatology (Baltimore, Md.) 1992;16:234A. [Google Scholar]; Richardet JP, Dehoux M, Mal F, Roulot D, Labadie H, Pol S, et al. Influence of corticosteroids (CS) on plasma cytokines concentrations in patients with severe alcoholic hepatitis (HA): results of a randomized study. Journal of Hepatology 1993;18:S75. [Google Scholar]
- Conn HO. Steroid treatment of alcoholic hepatitis. The yeas and the nays. Gastroenterology 1978;74(2 (Pt 1)):319‐22. [PUBMED: 620902] [PubMed] [Google Scholar]; Galambos JT. Alcoholic liver disease, new aspects of an old problem. Schweizerische Medizinische Wochenschrift. Journal Suisse de Medecine 1978;108(28):1050‐2. [CENTRAL: 221034; CRS: 6800100000004689; EMBASE: 0978366839; JC‐‐NLM: Journal ID:uei, 0404401; PUBMED: 78228296] [PubMed] [Google Scholar]; Shumaker JB, Resnick RH, Galambos JT, Makopour H, Iber FL. A controlled trial of 6‐methylprednisolone in acute alcoholic hepatitis. With a note on published results in encephalopathic patients. American Journal of Gastroenterology 1978;69(4):443‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Theodossi A, Eddleston AL, Williams R. Controlled trial of methylprednisolone therapy in severe acute alcoholic hepatitis. Gut 1982;23(1):75‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest E, Mellor J, Stanton L, Bowers M, Ryder P, Austin A, et al. Steroids or pentoxifylline for alcoholic hepatitis (STOPAH): study protocol for a randomised controlled trial. Trials 2013;14(1):262. [CRS: 6800131000045566; EMBASE: 2013572163; CN‐00917636] [DOI] [PMC free article] [PubMed] [Google Scholar]; Petts G, Lloyd K, Vergis N, Kudo H, Quaglia A, Forrest E, et al. The liver biopsy in alcoholic hepatitis: data from the steroids or pentoxifylline in alcoholic hepatitis (STOPAH) clinical trial. Gut 2015;64(Suppl 1):A262. [CRS: 6800131000059987; EMBASE: 72009796] [Google Scholar]; Petts G, Lloyd K, Vergis N, Kudo H, Quaglia A, Forrest E, et al. The liver biopsy in alcoholic hepatitis: data from the steroids or pentoxifylline in alcoholic hepatitis (STOPAH) clinical trial. Journal of Pathology 2015;237(Suppl 1):S23. [CRS: 6800131000075013; EMBASE: 72082064] [Google Scholar]; Petts G, Lloyd K, Vergis N, Kudo H, Quaglia A, Forrest E, et al. Utility of liver biopsy in alcoholic hepatitis: data from the steroids or pentoxyfilline in alcoholic hepatitis (STOPAH) clinical trial. Journal of Hepatology 2015;62(Suppl 2):S776‐7. [CRS: 6800131000059992; EMBASE: 71937847] [Google Scholar]; Thursz M, Forrest E, Roderick P, Day C, Austin A, O'Grady J, et al. The clinical effectiveness and cost‐effectiveness of steroids or pentoxifylline for alcoholic hepatitis (STOPAH): a 2 x 2 factorial randomised controlled trial. Health Technology Assessment 2015;19(102):1‐138. [CRS: 6800131000075049] [DOI] [PMC free article] [PubMed] [Google Scholar]; Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. New England Journal of Medicine 2015;372(17):1619‐28. [CRS: 6800131000075005; JC‐‐NLM: Journal ID:0255562, now; PUBMED: 25901427] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Alvarez MA, Cabre E, Lorenzo‐Zuniga V, Montoliu S, Planas R, Gassull MA. Combining steroids with enteral nutrition: a better therapeutic strategy for severe alcoholic hepatitis? Results of a pilot study. European Journal of Gastroenterology & Hepatology 2004;16:1375‐80. [DOI] [PubMed] [Google Scholar]
- Cabré E, Rodríguez‐Iglesias P, Caballería J, Quer JC, Sánchez‐Lombraña JL, Parés A, et al. Short‐ and long‐term outcome of severe alcohol‐induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology (Baltimore, Md.) 2000;32:36‐42. [DOI] [PubMed] [Google Scholar]; Cabré E, on behalf of the Spanish Group for the Study of Alcoholic Hepatitis. Treatment of severe alcoholic hepatitis with steroids or total enteral nutrition: interim results of a prospective, randomized, multicentric trial. Gastroenterology 1998;114:A868‐9. [Google Scholar]; Gassull M, Cabré E. Short and long‐term outcome in severe alcoholic hepatitis (SAH) treated with steroids or total enteral nutrition (TEN). A multicentric randomized controlled trial by the Spanish Group for the Study of Alcoholic Hepatitis. Journal of Parenteral and Enteral Nutrition 2000;24:S5. [Google Scholar]
- Christensen E, Fauerholdt L, Schlichting P, Juhl E, Poulsen H, Tygstrup N. Aspects of the natural history of gastrointestinal bleeding in cirrhosis and the effect of prednisone. Gastroenterology 1981;81(5):944‐52. [PubMed] [Google Scholar]
- Copenhagen Study Group for Liver Diseases. Effect of prednisone on the survival of patients with cirrhosis of the liver. A report from the Copenhagen Study Group for Liver Diseases. Lancet 1969;1(586):119‐21. [CENTRAL: 844264; CRS: 6800100000024872; PUBMED: 69076529] [PubMed] [Google Scholar]
- Daures J‐P, Peray P, Bories P, Blanc P, Yousfi A, Michel H, et al. Steroid therapy in acute alcoholic hepatitis: a pooled estimate based on published randomized controlled trials [Place de la corticotherapie dans le traitement des hépatites alcooliques aiguës. Résultants d'une méta‐analyse]. Gastroenterologie Clinique et Biologique 1991;51:223‐8. [PubMed] [Google Scholar]
- Dhanda A, Collins P. Infection is common in patients with severe alcoholic hepatitis treated with steroids but not associated with poor outcome. Journal of Hepatology 2016;64:S213–S424. [Google Scholar]
- Galambos JT, Riepe SP. Use of colchicine and steroids in the treatment of alcoholic liver disease. Recent Developments in Alcoholism 1984;2:181‐94. [CENTRAL: 34655; CRS: 6800100000000618; EMBASE: 6374780; JC‐‐NLM: Journal ID:rda, 8301996; PUBMED: 84222884] [DOI] [PubMed] [Google Scholar]
- Gill R, Zieve L, Logan G. Severe alcoholic hepatitis improved by combined treatment with prednisolone, testosterone and an amino acid supplement. Hepatology (Baltimore, Md.) 1984;4:2894. [Google Scholar]
- Goldis A, Matei R, Vernic C, Strain R. Treatment of acute alcoholic hepatitis with glucocorticosteroids‐prognostic factors. Steatohepatitis (NASH and ASH). Falk Symposium 121. Den Haag (Netherlands), 2000; Vol. 123:25. [Google Scholar]
- Hozo I, Mise S, Rumboldt Z, Bagatin J, Tonkic A. A controlled clinical trial of methylprednisolone in patients with the cholestatic form of alcoholic liver cirrhosis [Kontrolirano klinicko ispitivanje metilprednisolona u bolesnika sa kolestatskim oblikom alkoholne ciroze jetre]. Medicinski Arhiv 1996;50:81‐3. [PMID: 9601759] [PubMed] [Google Scholar]; Hozo I, Mise S, Rumboldt Z, Bagatin J, Tonkic A. Controlled clinical trial of methylprednisolone in patients with the cholestatic form of alcoholic liver cirrhosis. Gastroenterology International 1997;10:137‐9. [PubMed] [Google Scholar]
- Imperiale TF, McCullough AJ. Do corticosteroids reduce mortality from alcoholic hepatitis? A meta‐analysis of the randomized trials. Annals of Internal Medicine 1990;113(4):299‐307. [DOI] [PubMed] [Google Scholar]
- Lesesne HR, Bozymski EM, Fallon HJ. Treatment of alcoholic hepatitis with encephalopathy. Comparison of prednisolone with caloric supplements. Gastroenterology 1978;74(2 Pt 1):169‐73. [MEDLINE: ] [PubMed] [Google Scholar]
- Mal F, Huu TRP, Roulot D, Pol S, Labadie H, Ricardet JP, et al. In severe alcoholic hepatitis (AH) plasma tumor necrosis factor (pTNF) is inconstantly elevated and is not influenced by corticosteroid (Cs) therapy. Hepatology (Baltimore, Md.) 1991;14(4 (Pt 2)):232A. [CENTRAL: 221590; CRS: 6800100000005112; CN‐00221590] [Google Scholar]
- Mendenhall CL. Alcoholic hepatitis. In: Schiff L, Schiff ER editor(s). Diseases of the Liver. 7th Edition. Vol. 2, Philadelphia: JB Lippincott Co, 1993:856‐74. [Google Scholar]
- Moreno C, Deltenre P, Senterre C, Louvet A, Gustot T, Bastens B, et al. Intensive enteral nutrition is ineffective for patients with severe alcoholic hepatitis treated with corticosteroids. Gastroenterology 2016;150:903–10. [DOI] [PubMed] [Google Scholar]; Moreno C, Trepo E, Louvet A, Degre D, Bastens B, Hittelet A, et al. Impact of intensive enteral nutrition in association with corticosteroids in the treatment of severe alcoholic hepatitis: a multicenter randomized controlled trial. Hepatology (Baltimore, Md.) 2014;60(Suppl 1):269A‐70A. [CRS: 6800131000024411; EMBASE: 71638823; CN‐01023754] [Google Scholar]
- Morris JM, Forrest EH. Bilirubin response to corticosteroids in severe alcoholic hepatitis. European Journal of Gastroenterology & Hepatology 2005;17:759‐62. [PMID: 15947554] [DOI] [PubMed] [Google Scholar]
- Naganuma A, Hoshino T, Ogashiwa T, Hayashi E, Uehara S, Miyamae N, et al. Pilot study of granulocytapheresis and leukocytapheresis for the treatment of severe alcoholic hepatitis. Hepatology International 2014;8(1 Suppl 1):S8. [CRS: 6800131000045556; CN‐01011068] [Google Scholar]
- Naveau S, Chollet‐Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, et al. A double‐blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology (Baltimore, Md.) 2004;39:1390‐7. [PMID: 15122768] [DOI] [PubMed] [Google Scholar]
- Philips M, Curtis H, Portmann B, Donaldson N, Bomford A, O´Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis: a randomized trial. Hepatology (Baltimore, Md.) 2001;34:250A. [DOI] [PubMed] [Google Scholar]
- Poynard T, Ramond MJ, Rueff B, Mathurin P, Chaput JC, Benhamou JP. Corticosteroids reduce mortality from alcoholic hepatitis in patients without encephalopathy. A meta‐analysis of the randomized trials (RCTs) including French trials. Hepatology (Baltimore, Md.) 1991;14:234A. [Google Scholar]
- Reynolds TB, Benhamou JP, Blake J, Naccarato R, Orrego H. Treatment of acute alcoholic hepatitis. Gastroenterology International 1989;2(4):208‐16. [Google Scholar]
- Schlichting P, Juhl E, Poulsen H, Winkel P. Alcoholic hepatitis superimposed on cirrhosis. Clinical significance and effect of long‐term prednisone treatment. Scandinavian Journal of Gastroenterology 1976;11:305‐12. [MEDLINE: ] [PubMed] [Google Scholar]
- Spahr L, Rubbia‐Brandt L, Giostra E, Rougemont A‐L, Pugin J, Fischer M, et al. Combination of steroids with anti‐TNFa (INFLIXIMAB) or placebo in severe alcoholic hepatitis: early changes in ICAM‐1, histology and biochemical parameters. A pilot study. Journal of Hepatology 2002;37:448‐55. [PMID: 12217597] [DOI] [PubMed] [Google Scholar]
- Stewart S, Prince M, Bassendine M, Hudson M, James O, Jones D, et al. A trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. Journal of Hepatology 2002;36(Suppl 1):42. [DOI] [PubMed] [Google Scholar]
- Tygstrup N, Christensen E, Juhl E. Randomised clinical trials in hepatology [Randomisierte klinische therapiestudien in der hepatologie]. Internist 1979;20:565‐70. [PubMed] [Google Scholar]
- Lee Y‐S, Kim HJ, Kim JH, Yoo YJ, Kim TS, Kang SH, et al. Systematic review: steroid, pentoxifylline or combined therapy for acute alcoholic hepatitis. Hepatology International 2016;10(Suppl 1):424. 26758592 [Google Scholar]
Additional references
- O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology (Baltimore, Md.) 2010;51:307‐28. [DOI] [PubMed] [Google Scholar]
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
- Becker U, Deis A, Sorensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology (Baltimore, Md.) 1996;23:1025‐9. [DOI] [PubMed] [Google Scholar]
- Beckett AG, Livingstone AV, Hill KR. Acute alcoholic hepatitis. British Medical Journal 1961;2:1113‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PUBMED: 7786990] [PubMed] [Google Scholar]
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial Sequential Analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive‐‐Trial Sequential Analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
- Buzzetti E, Kalafateli M, Thorburn D, Davidson BR, Thiele M, Gluud LL, et al. Pharmacological interventions for alcoholic liver disease (alcohol‐related liver disease). Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD011646.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellini G, Nielsen E, Gluud, C. Comment on: “Cell therapy for heart disease: Trial Sequential Analyses of two Cochrane Reviews”. Clinical Pharmacology and Therapeutics 2017;102:21–4. [DOI] [PubMed] [Google Scholar]
- Christensen E, Gluud C. Glucocorticoids are ineffective in alcoholic hepatitis: a meta‐analysis adjusting for confounding variables [see comments]. Gut 1995;37(1):113‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen E, Gluud C. Glucocorticosteroids are not effective in alcoholic hepatitis. American Journal of Gastroenterology 1999;94(10)(3065):66. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Rincon D, Abraldes JG, Miquel R, Colmenero J, Bellot P, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. American Journal of Gastroenterology 2008;103:2747–56. [DOI] [PubMed] [Google Scholar]
- Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KVN, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology (Baltimore, Md.) 2005;41:353–8. [DOI] [PubMed] [Google Scholar]
- European Association for the Study of the Liver. EASL Clinical Practical Guidelines: management of alcoholic liver disease. Journal of Hepatology 2012;57:399–420. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. Journal of Hepatology 2012;56(5):1171‐80. [DOI] [PubMed] [Google Scholar]
- Feinberg J, Nielsen EE, Korang SK, Halberg Engell K, Nielsen MS, Zhang K, et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD011598.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. On the interpretation of X^2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society 1922;85(1):87‐94. [Google Scholar]
- Forrest EH, Evans CD, Stewart S, Phillips M, Oo YH, McAvoy NC, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005;54:1174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garattini S, Jakobsen JC, Wetterslev J, Bertelé V, Banzi R, Rath A, et al. Evidence‐based clinical practice: overview of threats to the validity of evidence and how to minimise them. European Journal of Internernal Medicine 2016;32:13‐21. [DOI] [PubMed] [Google Scholar]
- Gerber MA, Orr W, Denk H, Schaffner F, Popper H. Hepatocellular hyalin in cholestasis and cirrhosis: its diagnostic significance. Gastroenterology 1973;64(1):89‐98. [PubMed] [Google Scholar]
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2017, Issue 2. Art. No.: LIVER2017. [PubMed]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [PUBMED: 22542023] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables‐binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [PUBMED: 22609141] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles‐continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [PUBMED: 23116689] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Ebrahim S, Alonso‐Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;May 18:Epub ahead of print. [PUBMED: 28529188] [DOI] [PubMed] [Google Scholar]
- Harbord RM, Egger M, Sterne JAC. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Houghton M. Discovery of the hepatitis C virus. Liver International 2009;29(Suppl 1):82‐8. [DOI] [PubMed] [Google Scholar]
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
- Ioannidis JP. Adverse events in randomized trials: neglected, restricted, distorted, and silenced. Archives of Internal Medicine 2009;169(19):1737‐9. [DOI] [PubMed] [Google Scholar]
- Jakobsen J, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K, Gluud C. The Mallory body: morphological, clinical and experimental studies (Part 1 of a literature survey). Hepatology (Baltimore, Md.) 1994;20:1061‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
- Lefkowitch JH. Morphology of alcoholic liver disease. Clinics in Liver Disease 2005;9(1):37‐53. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Role of S‐adenosyl‐L‐methionine in the treatment of liver diseases. Journal of Hepatology 1999;30:1155‐9. [DOI] [PubMed] [Google Scholar]
- Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology (Baltimore, Md.) 2007;45(6):1348‐54. [DOI] [PubMed] [Google Scholar]
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathurin P, O'Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, et al. Corticosteroids improve short‐term survival in patients with severe alcoholic hepatitis: meta‐analysis of individual patient data. Gut 2011;60(2):255‐60. [DOI] [PubMed] [Google Scholar]
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42; quiz 742.e1‐5. [PUBMED: 23623694] [DOI] [PubMed] [Google Scholar]
- Nordic Trial Alliance Working Group on Transparency and Registration. Transparency and registration in clinical research in the Nordic countries (Report). nta.nordforsk.org/projects/nta_transparency_report.pdf 2015 (accessed 21 June 2017).
- Petrasek J, Iracheta‐Vellve A, Csak T, Satishchandran A, Kodys K, Kurt‐Jones EA, et al. STING‐IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proceedings of the National Academy of Sciences of the United States of America 2013;110(41):16544‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O’Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis – a randomised clinical trial. Journal of Hepatology 2006;44:784–90. [DOI] [PubMed] [Google Scholar]
- Pugh RNH, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. British Journal of Surgery 1973;60:646‐9. [DOI] [PubMed] [Google Scholar]
- Rambaldi A, Gluud C. Anabolic‐androgenic steroids for alcoholic liver disease. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003045.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis ‐‐ a Cochrane Hepato‐Biliary Group systematic review with meta‐analyses and trial sequential analyses of randomized clinical trials. Alimentary Pharmacology & Therapeutics 2008;27(12):1167‐78. [CRS: 6800131000005200; EMBASE: 2008243224; JC‐‐NLM: Journal ID:a5d, 8707234; PUBMED: 18363896] [DOI] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids‐‐new mechanisms for old drugs. New England Journal of Medicine 2005;353(16):1711‐23. [DOI] [PubMed] [Google Scholar]
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane Reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27:746‐63. [DOI] [PubMed] [Google Scholar]
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Chalmers I, Hayes, R, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment in controlled trials. JAMA 1995;273:408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacology & Therapeutics 2002;96(1):23‐43. [DOI] [PubMed] [Google Scholar]
- Spahr L, Rubbia‐Brandt L, Pugin J, Giostra E, Frossard JL, Borisch B, et al. Rapid changes in alcoholic hepatitis histology under steroids: correlation with soluble intercellular adhesion molecule‐1 in hepatic venous blood. Journal of Hepatology 2001;35(5):582‐9. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Student. The probable error of a mean. Biometrika 1908;6(1):1‐25. [Google Scholar]
- Taïeb J, Mathurin P, Elbim C, Cluzel P, Arce‐Vicioso M, Bernard B, et al. Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: effect of corticosteroids. Journal of Hepatology 2000;32(4):579‐86. [DOI] [PubMed] [Google Scholar]
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 21 June 2017).
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global strategy to reduce the harmful use of alcohol. www.who.int/substance_abuse/msbalcstragegy.pdf 2010 (accessed 21 June 2017).
- Sauced RS. Update on Background Paper 6.14. Harmful use of alcohol, alcohol use, disorders, and alcoholic liver diseases. www.who.int/medicines/areas/priority_medicines/BP6_14Alcohol.pdf (accessed 21 June 2017).
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Cederbaum AI. Ethanol‐induced apoptosis to stable HepG2 cell lines expressing human cytochrome P‐4502E1. Alcoholism: Clinical and Experimental Research 1999;23:67–76. [PubMed] [Google Scholar]
References to other published versions of this review
- Pavlov CS, Tsochatzis E, Casazza G, Nikolova D, Volcek E, Gluud C. Glucocorticosteroids for people with alcoholic hepatitis. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD001511.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saconato H, Gluud C, Christensen E, Atallah ÁN. Glucocorticosteroids for alcoholic hepatitis. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD001511] [DOI] [Google Scholar]


