Abstract
Background
Pigs/bovines share with humans some of the antigens present on cardiac valves. Two such antigens are: the major xenogenic Ag, “Gal” present in all pig/bovine very close to human B-antigen of ABO-blood-group system; the minor Ag, pig histo-blood-group AH-antigen identical to human AH-antigen and present by some animals. We hypothesize that these antigens may modify the immunogenicity of the bioprosthesis and also its longevity. ABO distribution may vary between patients with low (<6 years) and high (≥15 years) bioprostheses longevity.
Methods
Single-centre registry study (Paris, France) including all degenerative porcine bioprostheses (mostly Carpentier-Edwards 2nd/3rd generation heart valves) explanted between 1985 and 1998 and some bovine bioprostheses. For period 1998–2014, all porcine bioprostheses with longevity ≥13 years (follow-up ≥29 years). Important predictive factors for bioprosthesis longevity: number, site of implantation, age were collected. Blood group and other variables were entered into an ordinal logistic regression analysis model predicting valve longevity, categorized as low (<6 years), medium (6–14.9 years), and high (≥15 years).
Findings
Longevity and ABO-blood group were obtained for 483 explanted porcine bioprostheses. Mean longevity was 10.2 ± 3.9 years [0–28] and significantly higher for A-patients than others (P = 0.009). Using multivariate analysis, group A was a strong predictive factor of longevity (OR 2.09; P < 0.001). For the 64 explanted bovine bioprosthesis with low/medium longevity, the association, with A-group was even more significant.
Interpretation
Patients of A-group but not B have a higher longevity of their bioprostheses. Future graft-host phenotyping and matching may give rise to a new generation of long-lasting bioprosthesis for implantation in humans, especially for the younger population.
Fund
None.
Research in context
Evidence before this study
Cardiac valvular bioprosthesis are made from xenogenic tissue bovine or porcine chemically reticulated by glutaraldehyde. Persistence of immunogenicity and its real impact on bioprosthesis longevity after implantation is still under discussion. The only clinical demonstration of this putative effect is the nonspecific accelerated failure of the bioprosthesis in children. Recently, it was shown that the major carbohydrate xenoantigen alphaGal that is very structurally similar to Human B antigen was present on commercial bioprosthesis and elicited an early specific immune response after implantation. Another minor xenoantigen also carbohydrate has been shown to be also present in some pig or bovine tissue: the human A antigen. Thus patients may have a different reactivity against bioprosthesis based on their ABO blood group that may influence bioprosthesis longevity.
Added value of this study
We performed a single centre propective study evaluating a very large number of porcine bioprosthesis dysfunctional and needing surgical replacement for intrinsic structural degeneration for a period of time that exceed bioprosthesis classical longevity and evaluate classical risk factor for bioprosthesis degeneration in addition to ABO blood group. As anticipated, the distribution of ABO blood group changed between early failing bioprosthesis < 6 year and late failing ≥ 15 year bioprosthesis. Using multivariate analysis including all the classical risk factor of longevity, the group A was the most predictive factor and not B. This effect was also present for the need generation of bovine bioprosthesis.
Implications of all the available evidence
The longevity of bioprosthesis depends of patient ABO blood group with higher longevity in group A patient. Immunogencity against carbohydrates remaining on the bioprosthesis after reticulation maybe involve in this effect. Better adequation between patient ABO blood group and bioprosthesis carbohydrates especially regarding A antigen may give rise to bioprosthesis with lower immunogenicity and thus longevity.
1. Introduction
Approximately 200,000 patients worldwide undergo aortic valve replacement annually and 1/5 are aged between 40 and 60 years old [1]. The current trend is to use bioprostheses [2] to avoid anticoagulation [1]. The main disadvantage of bioprostheses is their limited durability, which is problematic in young patients [3]. Bioprosthesis failure is mostly due to calcification [3], possibly caused by exaggerated immune response [4] which has not been fully characterized yet [3] [5,6].
According to AHA Guidelines 2008, bioprostheses are recommended for: patients with a relatively short life expectancy; patients who are already 65 years old or more; implantation in the aortic rather than mitral position (higher success rate); patients without problems of atrial fibrillation; patients with no need for anticoagulation [7,8]. However, biological factors such as immunological reactivity are not taken into account.
The possible influence of immune reactivity on bioprosthesis longevity is not well established. Humans and pigs share several carbohydrate antigens that are expressed in the bioprostheses even after reticulation, which can trigger an immune response in the recipient. One of these antigens is the “α-Gal” [9], the major antigen of xenogenic rejection, which is expressed in the extracellular matrix [10,11], is present in all non-primate mammals such as pigs or bovines, and is still present after chemical reticulation [12]. The α-Gal are present in the commercially available bioprostheses [13] [14]. The α-Gal titer antibodies increase after bioprosthesis implantation [14] [15]. The α-Gal is structurally almost identical to the human group B-antigen [16,17] and, although the subject of some discussion [[18], [19], [20]], B-type patients may have a better tolerance towards this and related antigens [21] [19]. Bioprostheses obtained from Gal-Knockout pigs are possibly less immunogenic in New World primates [22].
Another possible immunogenic carbohydrate antigen is the Pig A-antigen of the pig histo-blood group O system (i.e. locus EAO on pig chromosome 6) corresponding to the orthologous site for the A transferase gene (OMIA 006089-9823) [23,24] and controlling A antigen expression in the tissue. Unlike the α-Gal, which is expressed by all pigs in all their tissues, expression of the A-antigen is restricted to A-type pigs [[25], [26], [27], [28]]. The porcine A-antigen [27,29] [24,30] [31], is identical to the human A-antigen of the ABO blood group [32] and is synthesized by the same enzyme (A-transferase) [23]. Unlike humans, pigs can also add the A-terminal antigen to other substances, but not necessarily the H-substance, and thus A+ pigs may have the A + H+ or A + H– phenotypes [25,28]. Furthermore, pig and human H-antigens are synthesized with a shared enzyme (Fucozyl transferase (FUT1) [29,31] [23].
The AH histo-blood group system also exists in most mammals and it is from bovine tissue [23] that most new generations of bioprotheses are made. The fucozyl transferase FUT1 is a common gene in mammals [31], including bovine (i.e. Bos Taurus) heart (FUT-1/NM_177499) [31,33], and is very similar to that observed in humans [31]. Bovine A-transferase has also been isolated and cloned (chr.11/BC126634) [27,34], and it too has a high level of homology to that found in humans.
In this study, we hypothesize that human ABO blood groups and pig AH systems share common antigens, A (human)/A (pig) and B (human)/α-Gal (pig), that might influence patients' individual immunological reactivity to bioprosthesis implantation, depending on the match between patient ABO phenotype and the AH phenotype. This in turn may influence the longevity of the bioprosthesis. We also sought to determine whether there was any possible effect from the presence of blood group A, given the early degeneration observed in new generations of bovine pericardial bioprosthesis.
2. Methods
2.1. Ethics statement
The ethics committee was not implicated due to the retrospective nature of the study. All patients gave consent for the use of their data for research at the University Hospital.
2.2. Study population
Single-centre study of patients requiring reoperation for degenerative bioprostheses at Broussais-Hospital/Georges Pompidou European Hospital in Paris, (Chairman Professor Alain Carpentier). Professor A. Carpentier has developed the concept of valvular heart bioprothesis [35] and several generations of porcine and bovine bioprostheses, and a large number of cases, have undergone initial evaluation studies at this centre [35,36].
Patients' characteristics before 1985 (1975–1980 [[37], [38], [39]] and 1980–1985 [40,41]) and the number of implanted bioprostheses in mitral or aortic position in the Broussais Hospital have already been reported in the literature [42]. Due to a mean longevity of heart valve bioprosthesis of 10 years, the period 1985–1997 corresponded to CE-2nd/3rd generations that were mostly implanted in the institution between 1975 and 1985. Unlike the 2nd-CE generation made from one pig, the 3rd-CE was manufactured using the cusps of two pigs in order to improve the hemodynamics [37] [42] [43],. New generations of CE-bioprostheses made of xenogenic bovine pericardium [44,45], have been implanted since 1980 [42] [40], and have almost totally replaced porcine bioprotheses since 1985. Chemical fixation by glutaraldehyde is still the main reticulating reagent. Patients' clinical conditions was not taken into account for the choice between a porcine or bovine bioprosthesis and after 1985 almost all patients did receive the new generation of bovine bioprostheses.
All patients reoperated for degenerative porcine bioprosthesis during the 13-year period from January 1985 to December1997 were eligible for the study and mainly comprised patients from our institution.
In addition, we included all patients reoperated on from January 1998 to January 2014 who had a porcine bioprosthesis with exceptional longevity (≥13 years). Since most porcine bioprostheses were implanted before 1985, we have a total follow-up period of 29 years for most of them.
Criteria for inclusion: degenerative porcine bioprosthesis that needs replacement during this period because of bioprosthetic valve dysfunction due to intrinsic structural valve deterioration (i.e. bioprosthesis degeneration (SVD)). Excluded are bioprosthetic replacements for another causes of valvular dysfunction such as nonstructural valve deterioration (i.e. any abnormality not intrinsic to the valve itself such as para-prosthetic regurgitation, malposition) [46], endocarditis or thrombosis. The cause of valve replacement and the type of intrinsic structural valve anomalies were prospectively specified by the surgeon at the time of bioprosthesis replacement, with observations on the presence of tears, fibrosis, calcification or pannus. A standardized classification of bioprosthesis valve degeneration in the aortic position has recently been proposed that excludes bioprosthesis thrombosis [[46], [47], [48]]. In a recent prospective study evaluating for 25 years Carpentier-Edwards bovine Bioprosthesis in aortic position, the causes for valvular replacement were: SVD (73%), endocarditis (15%), nonstructural dysfunction (11%), thrombosis (exceptional) [49].
Additional recent factors leading to accelerated bioprosthetic valve dysfunction, such as patient prosthesis mismatch or the small size of some prostheses, were not specifically investigated.
In the case of multiple bioprosthesis implants, only degenerative bioprostheses were considered.
Some cases of early failure or intermediate longevity in the new generation of bovine bioprostheses were explanted during this period (1985–1998) and analysed separately.
2.3. Study variables
The main outcome variable was the interval between valve implantation and explantation (longevity). The main risk factor of interest was the patient's blood type (ABO and rhesus). Other known classical risk factors for structural degeneneration of bioprostheses, and for which data were prospectively collected at replacement, were as follows: patient's age at the moment of implantation, sex, valve location and number of bioprostheses implanted initially. Some additional risk factors in bioprosthesis degeneration, especially in the aortic position, have also been reported recently and include factors that increase hemodynamic stress (larger body surface area, small prosthesis size, prosthesis-patient mismatch, left ventricle hypertrophy) and cardiovascular risk factors such as smoking, hypertension, metabolic syndrome, diabetes melitus, dyslipidemia [47,48]. Chronic dialyses and hyperparathyroidism have also been shown to be associated with early structural valve degeneration, although patients presenting these characteristics are rare in this study. None of these factors have been shown to be related to the ABO blood group of patients and could not therefore explain the different levels of bioprosthesis longevity between the different ABO blood groups.
2.4. Data collection
A prospective database for bioprosthetic heart valves has been developed since 1985. Thus data were collected prospectively and valve information was recorded in the operating room from 1985 to 1998. Information collected on other variables included: bioprosthetic valve degeneration and other factors resulting in bioprosthesis replacement (e.g. thrombosis, endocarditis, non-dysfunction valve); date of implantation; longevity; site of implantation; number, origin (bovine or porcine) and type of bioprosthesis. The patient's blood group information was obtained from the blood bank (2/3 of cases) and patients' records (1/3 of cases). If the information was not available in the blood bank, patients' medical records were consulted for those patients with the highest (≥13 years) and lowest longevity (≤7 years).
After 1998, all porcine bioprostheses with a longevity of ≥13 years were systematically sent to the laboratory for further analysis. This enabled us to go back to a patient's name and chart.
Since 1985 the new generations of bioprostheses made from bovine tissue have replaced porcine prostheses, so that for the year 2014 we have at least 29 years of follow-up for porcine bioprostheses.
2.5. Statistical analysis
We obtained frequency distributions for all study variables, for all replaced valves, and for all patients. For the main outcome variable, i.e. valve longevity, discrete categories were defined: the approximate lower and upper deciles were isolated (early and late failure), and the remainder was split into 3 classes resulting in the following 5 longevity categories (years): 0–5.9, 6–8.9, 9–11.9, 12–14.9 and 15–28. We cross-tabulated the 5-levels of longevity variable with ABO and rhesus blood types. This analysis suggested that the three middle categories were homogenous, so for simplicity we continued the analysis with a 3-level longevity variable (0–5.9, 6–14.9, 15–28). We cross-tabulated this variable with blood types and other valve and patient characteristics. We did not choose the class of longevity initially, but these classes of longevity appear to be clinically relevant.
Since we were not convinced that risk factors for low longevity (<6 years) would be similar (but opposite in their effect) to risk factors for high longevity (≥15 years), we conducted separate analyses comparing short versus medium longevity, and long versus medium longevity. Each of these analyses excluded the group at the opposite extreme of longevity. These analyses were conducted using logistic regression. Blood group and known predictors of longevity were included as predictors in the models.
Because these models turned out to be similar, especially for blood type, we obtained an ordinal logistic regression model, where the dependent variable was longevity based on 3 categories. The odds ratios obtained from this model corresponded to the odds ratio of being in a higher outcome category, averaged over the 2 transitions (medium vs low and high vs medium longevity).
The analyses were conducted using the SPSS-version18.
3. Results
3.1. Patient and valve characteristics
Between 1985 and 1998, 828 porcine bioprostheses were explanted from 641 patients. From 1998 to 2014, 32 additional porcine bioprostheses with longevity ≥13 years were removed. The longevity and blood groups were known in 426 patients (483 porcine bioprostheses) and constitute the study cohort. For these patients we do have all the variables.
Types of explanted porcine bioprostheses were as follows: CE-2nd 49.4%, 3rd 35.4%, 1rst 1.8%, Hancock-II™ 7.5%, Liotta™ 3.2%, others 2.7%. During the period 1987–1998, we also explanted 66 additional new generations of bovine bioprostheses (CE bovine pericardium) for degenerative reason, the majority of which (n = 64) exhibited short or intermediate longevities of <15 years.
Demographic data and porcine valve characteristics are shown in Table 1A. 22.2% of patients had more than one porcine bioprosthesis. The distributions according to age, sex, and blood group were similar for valves and patients. Mean age at implantation was 40.9 ± 14.0 years, the time elapsed before reoperation for degenerative porcine bioprosthesis was 10.2 years ± 3.9 [0–28] and the quartiles were 8/10/12 years. Most valves lasted between 6 and 15 years. About 10.6% failed after <6 years and another 12.2% failed after ≥15 years. In our group of reoperations for failing bioprosthesis, a significant number of young patients (36.6%) were ≤ 35 years old at implantation and only 10.8% were ≥ 60 years old. The prevalence of ABO in this small cohort was: (A: 38.2%, 95% CI +33.3 to +42.8; B: 14.2%, 95% CI +10.9 to +17.5; AB: 4.9%, 95% CI, +2.9 to +6.9; O: 42.7%, 95% CI +38.0 to 47.4) and Rhesus (−): 10.8%, 95% CI +7.9 to 13.7.
Table 1A.
Characteristics of 483 explanted bioprothesis in 426 patientsTable 1.
| Characteristic | Bioprosthesis n (%) | Patients n (%) |
|---|---|---|
| n | 483 | 426 |
| Male sex, n (%) | 257 (53.5) | 232 (54.4) |
| Age at first implantation, y, mean ± SD | 40.9 ± 14.0 | 40.9 ± 14.0 |
| [7–29] n (%) | 128 (26.5) | 107 (25.1) |
| [30–39] | 100 (20.7) | 89 (20.9) |
| [40–49] | 106 (21.9) | 93 (21.8) |
| [50–59] | 97 (20.1) | 88 (20.7) |
| [60–82] | 52 (10.8) | 49 (11.5) |
| Age at replacement, y, mean ± SD | 51.3 ± 14.4 | 51.2 ± 14.3 |
| [18–39] n (%) | 116 (24.0) | 95 (22.3) |
| [40–49] | 112 (23.2) | 98 (23.0) |
| [50–59] | 94 (19.4) | 84 (19.7) |
| [60–69] | 113 (23.4) | 105 (24.6) |
| [70–87] | 48 (10.0) | 44 (10.3) |
| Valve replacement | ||
| Mitral n (%) | 281 (58.2) | |
| Aortic | 190 (39.3) | |
| Tricuspid | 11 (2.3) | |
| Pulmonary | 1 (0.2) | |
| Number of valve replaced | ||
| n = 1 n (%) | 375 (77.8) | |
| n = 2 | 92 (19.1) | |
| n = 3 | 15 (3.1) | |
| Blood Type | ||
| A n (%) | 173 (35.8) | 164 (38.2) |
| B | 75 (15.5) | 61 (14.2) |
| AB | 27 (5.6) | 21 (4.9) |
| O | 208 (43.1) | 183 (42.7) |
| Rhesus | ||
| Positive n (%) | 430 (89.2) | 383 (89.5) |
| Bioprosth. Longevity, y, mean ± SD | 10.2 ± 3.9 | |
| [0–5.9] n (%) | 51 (10.6) | |
| [6–8.9] | 132 (27.3) | |
| [9–11.9] | 155 (32.1) | |
| [12–14.9] | 86 (17.8) | |
| [15–28] | 59 (12.2) |
3.2. Porcine valve longevity and blood type
The mean bioprosthetic valve longevity varied significantly between blood types and was highest for patients in group A (Table 1B). Mean longevity (years) was: for A group 10.7± 3.9, for B group 9.33 ± 4.3, for AB group 9.5± 3.2, and for O group 9.9 ± 3.6 (P = 0.008). This difference in longevity is in the range of what we observed when we considered the difference in mean longevity between the aortic position 10.6 ± 4.3 and the mitral position 10.0 ± 3.2 (P = 0.081), a difference that is known to be clinically relevant.
Table 1B.
Association between bioprosthesis longevity and patient ABO/Rhesus blood group.
| Variable | ABO Blood group |
P Value | ||||
|---|---|---|---|---|---|---|
| A | B | AB | O | |||
| Longevity, y | mean ± SD | 10.7 ± 3.9 | 9.93 ± 4.3 | 9.5 ± 3.2 | 9.9 ± 3.6 | 0.009 |
| [0–5.9] | n (%) | 9 (17.6) | 12 (23.5) | 4 (7.8) | 26 (51.0) | 0.097 |
| [6–8.9] | 49 (37.1) | 19 (14.4) | 7 (5.3) | 57 (43.2) | ||
| [9–11.9] | 57 (36.8) | 21 (13.5) | 9 (5.8) | 68 (43.9) | ||
| [12–14.9] | 28 (32.6) | 13 (15.1) | 7 (8.1) | 38 (44.2) | ||
| [15–28] |
30 (50.8) | 10 (16.9) | 0 (0.0) | 19 (32.2) | ||
| Variable | Rhesus blood group |
P Value | |
|---|---|---|---|
| Positive | Negative | ||
| Longevity, y mean ± /SD | 10.0 ± 3.8 | 11.0 ± 3.5 | 0.068 |
| [0–5.9] n (%) | 48 (94.1) | 3 (5.9) | 0.12 |
| [6–8.9] | 121 (91.7) | 11 (8.3) | |
| [9–11.9] | 139 (89.1) | 17 (10.9) | |
| [12–14.9] | 69 (81.2) | 16 (18.8) | |
| [15–28] | 54 (91.5) | 5 (8.5) | |
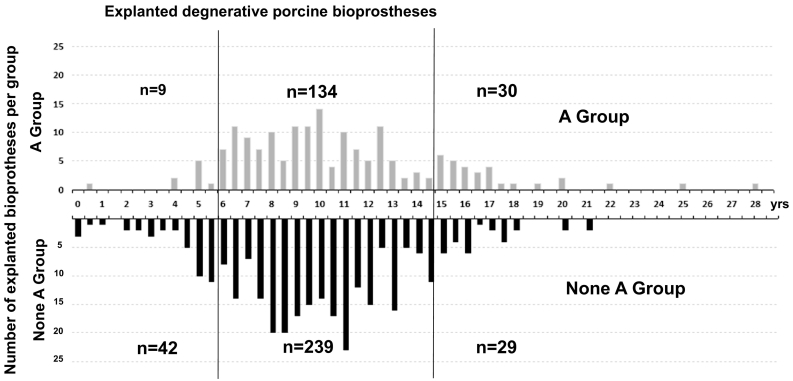
The prevalence of blood type A was low among early failures (<20%), higher, at around 30–40%, in the intermediate categories of valve longevity, and highest (>50%) for valves with longevity of 15 years or more (Table 1B and Fig. 1). There was no strong association between longevity and Rhesus positivity. Prevalence of ABO blood group in France or Caucasian populations is around 45% for Group A, 43% for Group O, 9% for Group B, 3% for Group AB, and 15% for Rhesus negative [50]. Thus the distribution for Group A matched the expected prevalence only for the intermediate longevity category, and was lower for the early group and higher for the group with high longevity.
Fig. 1.
Histogram of longevity of explanted porcine bioprostheses (n = 483) according to patient ABO blood [A Group (gray) or non-A Group (black)]. Each group is further divided into 3 categories of longevity: low <6 years, intermediate [6–14.9] or high ≥15 years (vertical lines); n = number of patients in each category. Horizontal axis: Longevity (in years); Vertical axis: number of cases per half year. P value is for subgroup effect. SD = Standard Deviation; y = year; n = number; Bioprosth = Bioprosthesis.
3.3. Characteristics associated with porcine valve longevity
Valve longevity was not associated with the sex of patients (Table 2). Older age at the time of implantation was associated with early failure, but the oldest patients were likely to die before they needed a valve replacement. For the 377 patients aged <60 years, there was a significant positive linear association between category of age at the time of implantation and bioprosthesis longevity.
Table 2.
Association between patient /valve characteristics and categories of bioprosthesis longevity.
| Characteristic | Bioprosthesis longevity, y |
P Value | ||
|---|---|---|---|---|
| [0–5.9] | [6–14.9] | [15–28] | ||
| Sex, n (%) | 0.60 | |||
| Women | 28 (12.4) | 171 (75.6) | 27 (12.0) | |
| Men | 24 (9.3) | 200 (77.8) | 33 (12.8) | |
| Age at first implantation, n (%) | 0.035 | |||
| [7–29] | 13 (10.1) | 103 (80.5) | 12 (9.4) | |
| [30–39] | 9 (8.9) | 80 (79.2) | 12 (11.9) | |
| [40–49] | 13 (11.9) | 81 (73.4) | 15 (14.7) | |
| [50–59] | 5 (5.4) | 72 (77.4) | 16 (17.2) | |
| [60–82] | 11 (21.1) | 37 (71.1) | 4 (7.8) | |
| Age at first implantation, n (%) | 0.003 | |||
| [7–59] | 39 (9.1) | 336 (78.5) | 53 (12.4) | |
| [60–82] | 12 (23.1) | 37 (71.2) | 3 (5.8) | |
| Blood type, n (%) | 0.008 | |||
| A | 9 (5.2) | 134 (77.5) | 30 (17.3) | |
| B | 12 (16.0) | 53 (70.7) | 10 (13.7) | |
| AB | 4 (14.8) | 23 (85.2) | 0 (0.0) | |
| O | 26 (12.5) | 163 (78.4) | 19 (9.1) | |
| Antigen A, n (%) | 0.008 | |||
| Positive (A or AB) | 13 (6.5) | 157 (78.5) | 30 (15.0) | |
| Negative (B or O) | 38 (13.4) | 216 (76.3) | 29 (10.2) | |
| Antigen B, n (%) | 0.14 | |||
| Positive (B or AB) | 16 (15.7) | 76 (74.5) | 10 (9.8) | |
| Negative (A or O) | 35 (9.2) | 297 (78.0) | 49 (12.9) | |
| Rhesus, n (%) | 0.73 | |||
| Positive | 48 (11.1) | 329 (76.3) | 54 (12.6) | |
| Negative | 3 (5.8) | 44 (84.6) | 5 (9.6) | |
| Valve replaced,n(%) | 0.46 | |||
| Mitral | 29 (10.3) | 226 (80.4) | 26 (9.3) | |
| Aortic | 22 (11.6) | 138 (72.6) | 30 (15.8) | |
| Tricuspid or pulmonary | 1 (8.3) | 10 (83.4) | 1 (8.3) | |
| Number of valves replaced, n (%) | 0.001 | |||
| 1 | 31 (8.2) | 292 (77.6) | 53 (14.2) | |
| 2 or 3 | 20 (18.7) | 82 (76.6) | 5 (4.7) | |
Blood group frequencies differed significantly according to the class of longevity. The A-blood type was rare among early failures and more common among late failures. The same trend was observed if we considered the presence of A-antigen. The B-antigen was not associated with valve longevity. Single-valve replacement was associated with high longevity. Aortic valves were somewhat more common among late failures.
3.4. Predictors of short porcine longevity
Older age at implantation was a significant predictor of long-term survival. A-blood group and single-valve replacement appeared to be protective against short-term valve durability (i.e., more common in the medium longevity group) but the site of implantation was not predictive (Table 3A). The B-group was more common both in the early failure group (OR: 1.42; P = 0.064) and the higher longevity group (OR: 1.62; P = 0.23), but these differences were not statistically significant. The multivariate model confirmed these risk factors. There was no evidence of confounding, in particular regarding blood type.
Table 3A.
Association between short valve longevity (<6 years) and valve characteristics.
| Characteristic | Univariate |
Multivariate |
||
|---|---|---|---|---|
| OR [95% CI] | P Value | OR [95% CI] | P Value | |
| Women (versus men) | 1.32 [0.74–2.38] | 0.35 | 1.20 [0.63–2.26] | 0.58 |
| Older age at first operation (≥60 y versus younger) |
2.79 [1.35–5.80] | 0.006 | 3.45 [1.57–7.57] | 0.002 |
| Blood type | 0.064 | – | ||
| A | 0.42 [0.19–0.93] | |||
| B | 1.42 [0.67–3.01] | |||
| AB | 1.09 [0.35–3.41] | |||
| O | 1.0 (reference) | |||
| Antigen A | 0.47 [0.24–0.91] | 0.026 | – | |
| Blood type A (versus other) | 0.38 [0.18–0.81] | 0.012 | 0.44 [0.20–0.95] | 0.037 |
| Rhesus positive (versus negative) | 2.15 [0.64–7.18] | 0.21 | 2.70 [0.79–9.23] | 0.11 |
| Aortic valve (versus other) | 1.30 [0.72–2.35] | 0.38 | 1.43 [0.75–2.72] | 0.28 |
| Single valve replaced | 0.44 [0.24–0.81] | 0.008 | 0.45 [0.23–0.93] | 0.016 |
3.5. Predictors of high porcine longevity
Significant predictors of greater longevity were the aortic valve location and single-valve replacement. A-blood type was also associated with high longevity (Table 3B).
Table 3B.
Associations between long valve longevity (≥15 years) and valve characteristics.
| Characteristic | Univariate |
Multivariate |
||
|---|---|---|---|---|
| OR [95% CI] | P Value | OR [95% CI] | P Value | |
| Women (versus men) | 0.93 [0.53–1.61] | 0.79 | 1.36 [0.61–2.60] | 0.35 |
| Younger age at first operation (<60 y versus older) | 1.96 [0.58–6.67] | 0.28 | 1.79 (0.52–6.25] | 0.36 |
| Blood type | 0.23 | |||
| A | 1.92 [1.04–3.56] | – | ||
| B | 1.62 [0.71–3.70] | |||
| AB | (not estimated)* | |||
| O | 1.0 (reference) | |||
| Antigen A | 1.42 [0.82–2.47] | 0.21 | – | |
| Blood type A (versus other) | 1.84 [1.06–3.21] | 0.030 | 1.81 [1.00–3.28] | 0.049 |
| Rhesus positive (versus negative) | 1.45 [0.55–3.82] | 0.45 | 1.41 [0.48–4.20] | 0.53 |
| Aortic valve (versus other) | 1.91 [1.08–3.38] | 0.026 | 2.20 [1.15–4.19] | 0.017 |
| Single valve replaced | 2.99 [1.16–7.72] | 0.025 | 2.62 [0.98–6.96] | 0.054 |
The B-antigen was also potentially associated with increased longevity. Using a multivariate logistic regression model, the effect was pronounced in aortic vs mitral position and single vs multiple bioprosthesis and also for the A-blood group (vs others).
3.6. Ordinal regression model for porcine bioprostheses
This analysis combines the 2 previous models into one, but forces the same effect for the transition from short to medium longevity and for the transition from medium to high longevity (Table 3C). In this analysis, older age at the time of implantation (OR: 3.05; P < 0.001), a single-valve replacement (OR: 2.56; P = 0.001), and A-blood type (vs. others) (OR: 2.09; P < 0.001) were all significantly associated with higher valve longevity but not the site of implantation (OR: 1.28; P = 0.31). The model was unchanged when patients of 60 years or more were excluded from the analysis.
Table 3C.
Associations between long valve longevity in 3 categories (<6 years, [6–14.9], ≥15 years) and valve characteristics
| Characteristic | Univariate |
Multivariate |
||
|---|---|---|---|---|
| OR [95% CI] | P Value | OR [95% CI] | P Value | |
| Women (versus men) | 0.82 [0.54–1.26] | 0.37 | 1.02 [0.64–1.61] | 0.95 |
| Younger age at first operation (<60 y versus older) | 2.82 [1.45–5.50] | <0.001 | 3.05 [1.52–6.09] | <0.001 |
| Blood type A (versus other) | 2.29 [1.45–3.62] | <0.001 | 2.09 [1.29–3.38] | <0.001 |
| Rhesus positive (versus negative) | 0.89 [0.45–1.56] | 0.74 | 0.75 [0.37–1.52] | 0.42 |
| Aortic valve (versus other) | 1.28 [0.82–1.99] | 0.27 | 1.28 [0.80–2.05] | 0.31 |
| Single valve replaced | 2.76 [1.63–4.67] | <0.001 | 2.56 [1.48–4.44] | 0.001 |
In a post-hoc analysis, we compared 2nd (n = 218) and 3rd generation (n = 156) CE valves, which are derived from one and two pigs respectively [2]. The prevalence of blood type A according to valve longevity (0–5; 6–14.9; 15–28 years) was 16.7%, 31.6% and 55.6% for the 2nd generation vs. 23.1%, 40.2% and 30.8% for the 3rd generation. In the ordinal logistic regression, the association between blood type A and longevity appeared to be stronger for CE 2nd (OR: 2.79; 95% CI, 1.15 to 6,77; P = 0.024) than for CE 3rd (OR: 1.45, 95% CI, 0.68 to 3.1; P = 0.33).
3.7. Distribution of ABO blood group among early and intermediate failing degenerative bovine or porcine bioprostheses
A new generation of bovine pericardium has been implanted at our institution since 1980 but most of the implanted bovine bioprostheses have been implanted since 1985 so that, for the period of observation, we had only bovine bioprostheses explanted with short or intermediate longevity available for separate analysis. The results are shown in Table 4A, Table 4B. Of the eleven early failing degenerative bovine bioprostheses, none of them was observed in a patient of the A group (P = 0.0005). The P value was even more significant than that observed for the porcine valve (P = 0.011).
Table 4A.
Characteristics of explanted bovine bioprosthesis with longevity < 15 years.
| n = 64 |
ABO Blood group |
P Value | |
|---|---|---|---|
| Longevity, y | Non A group, n (%) | A group, n (%) | |
| [0–5.9] | 11 (27.5) | 0 (0.0) | 0.005 |
| [6–14.9] | 29 (72.5) | 23 (100.0) | |
Table 4B.
Characteristics of explanted porcine bioprosthesis with longevity < 15 years.
|
n = 424 |
ABO Blood group |
P Value | |
|---|---|---|---|
| Longevity, y | Non A group, n (%) | A group, n (%) | |
| [0–5.9] | 42 (14.9) | 9 (6.3) | 0.011 |
| [6–14.9] | 239 (85.1) | 134 (93.7) | |
4. Discussion
In this study, we have demonstrated that the presence of the ABO blood group in a patient may influence the outcome of an implanted bioprosthesis. The effect of group A was independent of all other known predictive factors.
We hypothesize that, on average, A-patients may have a better compatibility with bioprosthesis, and are therefore less likely to experience early failure and thus more likely to experience bioprosthesis longevity of >15 years.
4.1. Human ABO blood group and xenogenic tissue carbohydrate antigens
The human A antigen has been shown to be present in some pig cardiac tissue [25,27,51] and also, less frequently, associated with H substances. As in the case of the human heart [32,52], and probably the bovine heart, the pig A antigen has been shown to be expressed in the same locations, such as: the endocardium and the endothelial cells of myocardium and mesothelial cells and capillaries on the surface of the cardiac epicardium [51]. The adherent cardiac pericardium is in continuity with, and has the same origin as, the free cardiac pericardium [53]. The pericardium is widely used for manufacturing the new generation of bovine bioprostheses that are used for surgical implantation but also for all the new generation of porcine and bovine pericardial bioprostheses used in percutaneous implantation (i.e. TAVI) [54].
The fucozyl transferase FUT1 involved in H core synthesis is a widespread gene in mammals with high homomlogy [31]. H substance [25,55] and FUT1 [27] are present in some pig cardiac tissue. Pig FUT1 is located on chromosome 6 gene U70883 [23,31].
A transferase expression in pig tissue is regulated by the genetic locus EAO on pig chromosome 6 corresponding to the orthologous site for the A transferase gene (OMIA 006089-9823) [23,24]. There are four genotypes for the “pig O histo-group system” coding for A histo group antigenicity: AA, AO, OO, and negative [24,[26], [27], [28]]. This locus coding is different from that for the A transferase gene coding for the A antigen expression on pig erythrocyte (i.e. locus EAA) and located on another chromosome (i.e. pig chr.1) [29,56].
The genetic determination of allele A frequency and genotype for the histo group was determined recently in a large series in commercial pigs of different strains as well as mini-pigs. In commercial pigs used for bioprotheses, the A allele frequency varies from 0.15 to 0.67 [57]. Currently, the bioprosthetic tissue phenotype is not checked during fabrication. The A-allele frequency depends on the breed and varies from 0.15 to 0.67 [[26], [27], [28], [29],58] [57]. According to Oriol et al., [51%] of pigs are A+ and [12%] A + H+ [25]. For A-patients, this advantage is only present if by chance a corresponding A-phenotype is present on the implanted bioprosthesis.
Multivariate analysis showed that the ABO group was not only a new additional risk factor, but also one of the most predictive. Moreover, we may also underestimate its positive influence for several reasons. First, we did not have information about the AH phenotype of the pig. Since the prevalence of A-allele in pig varies from 0.15 to 0.67 [29,58] [57], only a fraction of A-patients could randomly receive the corresponding A-bioprosthesis. This probability is further decreased by the fact that only a fraction of A-pigs express the A-antigen in the heart [28,32,51,55,59].
This reasoning if supported by our observation that for bioprostheses made from two pigs' valves, patient blood type A was less strongly associated with valve longevity. If the prevalence of A+ is 0.4 among pigs, the probability that two randomly selected pigs are A+ is only 0.16, and the likelihood of a match between an A+ valve and an A+ patient is similarly reduced.
In some bovines, as in some pigs, the human A/H-type2 antigen has been shown to be present in saliva, gut epithelial cells, the urinary tract and respiratory tract cells [60,61], and has a similar role to that of α-Gal [62] in the general control of virus infection, a mechanism that has been well preserved throughout the evolution of the species.
Several of the main carbohydrate antigens have been identified as being the major antigens of xenograft tissue recognition. These antigens are α-Gal, N-glycolylneuramic acid (Neu5Gc), and the Forshsmann antigen [20,63]. All these antigens, and especially α-Gal, have structural similarities with the B antigen but not the A antigen [64]. The α-Gal epitope is structurally related to the histo-blood group B type 2 since both share a terminal galactose in 1,3 linkage and the type 2 backbone structure (Galα4GlcNAC). They only structural difference is the fucose residue of the B antigen, which allows some reagents, such as certain anti-B mAbs or GS1-isolectin that recognise α-Gal, to cross-react. This cross-reactivity does not exist between A and B antigens mAbs. One of the main factors in xenograft rejection is the binding of antibodies on xenograft antigen and the activation of the complement by IgG mAbs or the destruction of cells by NK cells [65]. Although some cross reactivity may exist between α-Gal and B antigen, the reactivity to α-Gal is generally the same for the different ABO blood groups in most studies [18,20].
Recently, several approaches have been developed to reduce the immuno-reactivity of bioprostheses by controlling the expression of carbohydrate antigens, especially α-Gal. Two approaches have been used to reduce the expression of α-Gal. The first uses a specific enzyme, alpha Galatoctosidase, but this treatment has a negative impact on the physical characteristics of the pericardium [66]. In the second approach, valves can be prepared from genetically-modified pigs (α-galactozyl-transferase gene–knockout-pigs (GTKO)) that do not express Gal [[67], [68], [69]]. Tissues from GTKO-pigs are still immunogenic [70] [71] with increased reactivity against minor antigens derived from the same Gal-αframework [70] [17] [71,72], including the N-glycolylneuramic acid (Neu5Gc) [70]. Interestingly, the reactions against unrelated minor antigens such as A/H are also increased in GTKO-pigs [55,73].
As for α-Gal, the binding and functionality of human serum of different ABO blood groups has been evaluated recently against Neu5Gc, but no difference has been found between ABO blood group for the type of antibodies, the amount of binding, and functionality [19]. There is also no correlation between the reactivity against α-Gal or Neu5Gc [19]. Furthermore it has recently been shown that reactivity with regard to antibody binding could be further decreased by targeting glycan products of β4GalNT2 in the pericardium [74].
The immunogenicity of the bovine pericardium has been evaluated by affinity chromatography and the immunoproteomic approach to determine the reactivity of rat serum before and after subcutaneous implantation of pericardial patches [75]. 133 antigens have been identified, many of which were associated with the extracellular matrix [75]. This may limit the possibility of reducing tissue antigenicity just by cell removal [76,77].
The ABO system in other contexts, such as allotransplantation, can be defined by the presence of alloantibodies. In our study, A-patients with circulating anti-B antibodies or immune cells (potentially reactive with αGal) had a better compatibility with bioprosthesis, while B-patients with anti-A antibodies or immune cells had a lower valvular bioprosthesis survival rate. Cross reactivity of alloantibodies or immune cells, because of shared carbohydrate antigens between humans and animals, could explain part of the valve survival difference observed in this study. Moreover, apparent autoantibodies can be found in healthy donors [78]. There is some clinical evidence for a higher antigenicity of the A-antigen compared with the B-antigen in human diseases. Indeed, red blood cell transfusion requirements after major ABO-mismatched hematopoietic progenitor cell transplantation (HPCT) differed depending on the donor ABO blood group. This suggests that the ABO blood group antigens themselves are major determinants in the immunological process [79]. Higher antigenicity of the A-antigen can also be observed in ABO hemolytic disease of the newborn infants [80]. Thus, the increased immunogenicity of A antigen, unlike B-type antigens, is in line with these clinical observations.
4.2. Limitations of the present study
The main limitation was that the tissue antigens borne by the bioprosthesis were unknown, unlike the patient's blood group. Therefore, we could not directly demonstrate that biocompatibility was associated with long-term valve durability.
Another limitation was that we only had access to patients whose bioprostheses failed, thus requiring replacement, and not to the full cohort of patients who initially received a bioprosthesis. For this reason, we were not able to compute risks of failure or to construct Kaplan-Meier time-to-failure curves.
This probably also explains why we observed that older age at the time of implantation was associated with a shorter survival of the bioprosthesis. Older patients were more likely to die before their valve prosthesis failed, and therefore to be excluded from this analysis. Age was not associated with valve survival among patients younger than 60 years at the time of implantation while the order of magnitude and significance of other risk factors remained stable (results not shown).
Today, we can access >29 years of observations, since most of the porcine bioprostheses were implanted before 1985. This interval exceeds the mean longevity of bioprostheses in published prospective studies and in our cohort of patients followed prospectively after mitral implantation (12-year longevity of mitral bioprosthesis (patient survival [48%] [39], and valve failures [31%] [39], most of which were due to degeneration [39]). Thus, we can expect to have recovered all the bioprostheses that have failed, but to have “missed” bioprostheses of patients who have died before their bioprostheses required replacement.
In this study in agreement with literature we exclude dysfunctional valvular replacement for thrombosis. Bioprosthesis thrombosis leading to surgical valvular replacement as in our series is a very rare complication in prospective surgical studies with <0.54% (8/1463) for porcine bioprosthesis and an even lower rate for bovine bioprosthesis 0/3031 [81]. In 90% of the cases, bioprothesis thrombosis leading to surgical replacement occurs in the first 2 years [81]. In surgery, the incidence of bioprosthesis thrombosis leading to valvular replacement is >10 times lower than that observed for bioprosthesis degeneration [82,83]. After 25 years, >90% of the bioprostheses fail from structural degeneration if the patients are <60 years old at the time of implantation [49]. Early valvular thrombosis not needing surgical replacement has been reported in 10 to 15% of bioprostheses [84]. It is even possible that this may be related to the immunogenicity of the tissue that might trigger inflammation and subsequent fibrocalcic remodeling of valve leaflets leading to late structural bioprosthesis degeneration [48]. In the aortic position, it was shown that an increased risk of thrombosis of the mechanical valve in A group patients due to coagulation annomalies [85] was not able to explain the improved longevity of the bioprosthesis reported in this location.
Some studies have shown a possible association between longevity and the ABO blood group [86]. In Japan, the B antigen has been shown to be possibly associated with higher longevity [87]. On the other hand, the A antigen has been shown to be associated with higher cardiac mortality, including ischemic events [88,89]) and strokes. ‘A' patients have a higher level of circulating vWF and factor FVIII [90,91] and possibly higher circulating cholesterol levels [92]. The A antigen has also recently been found to be associated with an increased risk of mechanical cardiac valve thrombosis [85]. In patients with chronic heart failure, the ABO blood group appears to have no impact on patient outcome [93].
In A patients, there does not appear to be an overall higher mortality rate from cancer, but there is a specific association with some types of cancer, such as gastric or pancreatic cancers [89].
All these factors cannot explain the overall representation of A patients in the group with the best survival rates following bioprosthetic valve implantation. Censoring due to patient deaths should not bias the association between blood type and valve longevity in explaining the increased prevalence of A patients in the group with high longevity rates, given that the literature shows that A patients should have an increased mortality rate especially from cardiac problems.
Another limitation of the study is that we do not have data on all the parameters that have been recently identified as influencing bioprosthetic heart valve degeneration. Furthermore, most of these factors have been shown to exist for aortic sites but not for the mitral position. The meta-analysis of prospective study data reporting the rate of bioprosthesis degeneration have shown that the risk is very low (21.4% at 15 years and 51.5% at 20 years) [94]. Following 2758 patients for 20 years with Carpentier-Edwards bioprosthetic valves in the aortic position, Bourguinon T. et al. reported only 123 patients requiring reoperation after 20 years [49]. This has to be compared with the 426 patients of the present study. Large size populations as in our study will allow for an equal distribution of confounding factors. The low frequency of bioprosthetic valve degeneration could also make it more difficult to properly evaluate all the clinical parameters that have been reported as possible influences on bioprosthetic valve degeneration in prospective studies.
4.3. Clinical implications
Our results show that persistent immunological reactions might determine the future of an implanted bioprosthetic valve. Thus the design of bioprostheses that are more resistant to hosts' immune response systems would be a major improvement in valvular heart surgery [95].
For the first time, we have identified a patient-related immunological variable that may determine the outcome of bioprosthesis. Up to now the only variable that has been shown to be possibly related to immunological parameters is the accelerated bioprosthesis degeneration in younger age patients [48]. In addition, no targeted antigen has been identified.
Our data show that the patient's ABO blood type influences bioprosthesis longevity. Our hypothesis is that shared carbohydrate antigens between humans and animals may determine patient immune-response upon implantation and subsequent bioprosthesis longevity.
Nowadays, both bovine and porcine valves are being used. New generations of valves, including most stentless valves and some percutaneous valves, are being made from porcine or bovine tissues. However, to date, none of them is superior to any others and there are no specific clinical guidelines for a particular bioprosthesis [2].
Moreover, the issue of blood-type compatibility may be extended to other species such as bovines. Human A-transferase has also been isolated and cloned [27,34], in bovine species and enzymes that are involved in the synthesis of human H antigen are widely expressed in animals [33], including bovine heart [31,33].
In our study, when we performed a sub-analysis for patients under 60 years old at implantation, the patient's age category was no longer a risk factor in valve longevity. Using multivariate analysis, the only factor identified was multiple valve replacement (OR 2.56 P = 0.001 in the same range of values as blood type A (OR 2.09; P < 0.001)), which was far more important than the implantation site (aortic versus mitral) (OR 1.26; P = 0.31). This observation could be particularly relevant clinically in terms of bioprosthesis attribution. Since we are measuring this effect on the basis of a purely arbitrary adequate allocation between pig AH tissue and human A, we would probably be able to multiply it by a factor of 2 to 5 if we deliberately matched patient and prosthesis phenotypes.
5. Conclusions
In the near future, we propose checking the phenotypes of the animals used in bioprostheses, especially with regard to A and H antigens, and matching them accordingly with the patient's ABO blood group. This will give rise to a new generation of bioprostheses with less risk of early failure and hence better longevity.
Funding sources
None.
Acknowledgments
Acknowledgements
None.
Declaration of interests
None declared for any of the authors OS, NL, TP, PM, JG, AF, DS, YL, MR, AC.
Author contributions
Study design: Olivier Schussler; Litterature search: Olivier Schussler -David Smadja; Data Collection: Olivier Schussler-Nermine Lila-Anne Francois; Data Analysis: Olivier Schussler-Thomas Pernerger-Marc Ruel; Figures and Tables: Olivier Schussler; Data Interpretation: Olivier Schussler-Thomas Pernerger-David Smadja-Marc Ruel-Alain Carpentier; Writting: Olivier Schussler-Thomas Pernerger-Parmeeseeven Mootoosamy-Juan Grau-Yves Lecarpentier-Alain Carpentier + N. Lila, T. Pernerger, P. Mootoosamy and J. Grau contributed equally to this work.
Conflict of interest
None declared by any authors.
References
- 1.Chikwe J., Filsoufi F., Carpentier A.F. Prosthetic valve selection for middle-aged patients with aortic stenosis. Nat Rev Cardiol. 2010 Dec;7(12):711–719. doi: 10.1038/nrcardio.2010.164. [DOI] [PubMed] [Google Scholar]
- 2.Butany J., Fayet C., Ahluwalia M.S., Blit P., Ahn C., Munroe C. Biological replacement heart valves. Identification and evaluation. Cardiovasc Pathol. 2003 May-Jun;12(3):119–139. doi: 10.1016/s1054-8807(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui R.F., Abraham J.R., Butany J. Bioprosthetic heart valves: modes of failure. Histopathology. 2009 Aug;55(2):135–144. doi: 10.1111/j.1365-2559.2008.03190.x. [DOI] [PubMed] [Google Scholar]
- 4.Milano A., Guglielmi C., De Carlo M., Di Gregorio O., Borzoni G., Verunelli F. Valve-related complications in elderly patients with biological and mechanical aortic valves. Ann Thorac Surg. 1998 Dec;66(6 Suppl):S82–S87. doi: 10.1016/s0003-4975(98)01097-2. [DOI] [PubMed] [Google Scholar]
- 5.Badylak S.F., Gilbert T.W. Immune response to biologic scaffold materials. Semin Immunol. 2008 Apr;20(2):109–116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keane T.J., Badylak S.F. The host response to allogeneic and xenogeneic biological scaffold materials. J Tissue Eng Regen Med. 2014 Feb;18 doi: 10.1002/term.1874. [DOI] [PubMed] [Google Scholar]
- 7.Bonow R.O., Carabello B.A., Chatterjee K., De Leon A.C., Jr., Faxon D.P., Freed M.D. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll Cardiol. 2008;52(13) doi: 10.1016/j.jacc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 8.El Oakley R., Kleine P., Bach D.S. Choice of prosthetic heart valve in today's practice. Circulation. 2008 Jan 15;117(2):253–256. doi: 10.1161/CIRCULATIONAHA.107.736819. [DOI] [PubMed] [Google Scholar]
- 9.McMorrow I.M., Comrack C.A., Nazarey P.P., Sachs D.H., DerSimonian H. Relationship between ABO blood group and levels of Gal alpha,3Galactose-reactive human immunoglobulin G. Transplantation. 1997 Aug 15;64(3):546–549. doi: 10.1097/00007890-199708150-00032. [DOI] [PubMed] [Google Scholar]
- 10.Maruyama S., Cantu E., 3rd, Galili U., D'Agati V., Godman G., Stern D.M. Alpha-galactosyl epitopes on glycoproteins of porcine renal extracellular matrix. Kidney Int. 2000 Feb;57(2):655–663. doi: 10.1046/j.1523-1755.2000.00887.x. [DOI] [PubMed] [Google Scholar]
- 11.Daly K.A., Stewart-Akers A.M., Hara H., Ezzelarab M., Long C., Cordero K. Effect of the alphaGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model. Tissue Eng Part A. 2009 Dec;15(12):3877–3888. doi: 10.1089/ten.TEA.2009.0089. [DOI] [PubMed] [Google Scholar]
- 12.Naso F., Gandaglia A., Bottio T., Tarzia V., Nottle M.B., d'Apice A.J. First quantification of alpha-Gal epitope in current glutaraldehyde-fixed heart valve bioprostheses. Xenotransplantation. 2013 Jul-Aug;20(4):252–261. doi: 10.1111/xen.12044. [DOI] [PubMed] [Google Scholar]
- 13.Farivar R.S., Filsoufi F., Adams D.H. Mechanisms of Gal(alpha)1-3Gal(beta)1-4GlcNAc-R (alphaGal) expression on porcine valve endothelial cells. J Thorac Cardiovasc Surg. 2003 Feb;125(2):306–314. doi: 10.1067/mtc.2003.76. [DOI] [PubMed] [Google Scholar]
- 14.Konakci K.Z., Bohle B., Blumer R., Hoetzenecker W., Roth G., Moser B. Alpha-Gal on bioprostheses: xenograft immune response in cardiac surgery. Eur J Clin Invest. 2005 Jan;35(1):17–23. doi: 10.1111/j.1365-2362.2005.01441.x. [DOI] [PubMed] [Google Scholar]
- 15.Park C.S., Park S.S., Choi S.Y., Yoon S.H., Kim W.H., Kim Y.J. Anti alpha-gal immune response following porcine bioprosthesis implantation in children. J Heart Valve Dis. 2010 Jan;19(1):124–130. [PubMed] [Google Scholar]
- 16.Ezzelarab M., Ayares D., Cooper D.K. Carbohydrates in xenotransplantation. Immunol Cell Biol. 2005 Aug;83(4):396–404. doi: 10.1111/j.1440-1711.2005.01344.x. [DOI] [PubMed] [Google Scholar]
- 17.Milland J., Sandrin M.S. ABO blood group and related antigens, natural antibodies and transplantation. Tissue Antigens. 2006 Dec;68(6):459–466. doi: 10.1111/j.1399-0039.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- 18.Buonomano R., Tinguely C., Rieben R., Mohacsi P.J., Nydegger U.E. Quantitation and characterization of anti-Galalpha1-3Gal antibodies in sera of 200 healthy persons. Xenotransplantation. 1999 Aug;6(3):173–180. doi: 10.1034/j.1399-3089.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 19.Hurh S., Kang B., Choi I., Cho B., Lee E.M., Kim H. Human antibody reactivity against xenogeneic N-glycolylneuraminic acid and galactose-alpha-1,3-galactose antigen. Xenotransplantation. 2016 Jul;23(4):279–292. doi: 10.1111/xen.12239. [DOI] [PubMed] [Google Scholar]
- 20.Gao B., Long C., Lee W., Zhang Z., Gao X., Landsittel D. Anti-Neu5Gc and anti-non-Neu5Gc antibodies in healthy humans. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0180768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manji J.S., Rajotte R.V., Koshal A., Manji R.A. Human anti-A and anti-B antibodies interact with alphaGal antibody affecting xenograft survival. Xenotransplantation. 2004 Jul;11(4):376–377. doi: 10.1111/j.1399-3089.2004.00141.x. [DOI] [PubMed] [Google Scholar]
- 22.McGregor CG, Kogelberg H, Vlasin M, Byrne GW. Gal-knockout bioprostheses exhibit less immune stimulation compared to standard biological heart valves. J Heart Valve Dis. May;22(3):383–90. [PubMed]
- 23.Yamamoto F., Cid E., Yamamoto M., Saitou N., Bertranpetit J., Blancher A. An integrative evolution theory of histo-blood group ABO and related genes. Sci Rep. 2014;4:6601. doi: 10.1038/srep06601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto F., Yamamoto M. Molecular genetic basis of porcine histo-blood group AO system. Blood. 2001 May 15;97(10):3308–3310. doi: 10.1182/blood.v97.10.3308. [DOI] [PubMed] [Google Scholar]
- 25.Oriol R., Barthod F., Bergemer A.M., Ye Y., Koren E., Cooper D.K. Monomorphic and polymorphic carbohydrate antigens on pig tissues: implications for organ xenotransplantation in the pig-to-human model. Transpl Int. 1994;7(6):405–413. doi: 10.1007/BF00346034. [DOI] [PubMed] [Google Scholar]
- 26.Choi M.K., Le M.T., Cho H., Yum J., Kang M., Song H. Determination of complete sequence information of the human ABO blood group orthologous gene in pigs and breed difference in blood type frequencies. Gene. 2018 Jan 15;640:1–5. doi: 10.1016/j.gene.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen D.T., Choi H., Jo H., Kim J.H., Dirisala V.R., Lee K.T. Molecular characterization of the human ABO blood group orthologus system in pigs. Anim Genet. 2011 Jun;42(3):325–328. doi: 10.1111/j.1365-2052.2010.02152.x. [DOI] [PubMed] [Google Scholar]
- 28.Yeom S.C., Oh B.C., Cho S.Y., Park C.G., Lee B.C., Lee W.J. Investigation of blood typing method for Seoul National University miniature pig. Transplant Proc. 2009 Jun;41(5):1921–1926. doi: 10.1016/j.transproceed.2009.01.096. [DOI] [PubMed] [Google Scholar]
- 29.Smith D.M., Newhouse M., Naziruddin B., Kresie L. Blood groups and transfusions in pigs. Xenotransplantation. 2006 May;13(3):186–194. doi: 10.1111/j.1399-3089.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- 30.Meijerink E., Neuenschwander S., Dinter A., Yerle M., Stranzinger G., Vogeli P. Isolation of a porcine UDP-GalNAc transferase cDNA mapping to the region of the blood group EAA locus on pig chromosome 1. Anim Genet. 2001 Jun;32(3):132–138. doi: 10.1046/j.1365-2052.2001.00752.x. [DOI] [PubMed] [Google Scholar]
- 31.Abrantes J., Posada D., Guillon P., Esteves P.J., Le Pendu J. Widespread gene conversion of alpha-2-fucosyltransferase genes in mammals. J Mol Evol. 2009 Jul;69(1):22–31. doi: 10.1007/s00239-009-9239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gehrie E.A., Cates J.M., Nian H., Olson S.J., Young P.P. Blood Group A antigen expression on cardiac endothelium is highly individualized: possible implications for transplantation. Cardiovasc Pathol. 2013 Jul-Aug;22(4):251–256. doi: 10.1016/j.carpath.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barreaud J.P., Saunier K., Souchaire J., Delourme D., Oulmouden A., Oriol R. Three bovine alpha2-fucosyltransferase genes encode enzymes that preferentially transfer fucose on Galbeta1-3GalNAc acceptor substrates. Glycobiology. 2000 Jun;10(6):611–621. doi: 10.1093/glycob/10.6.611. [DOI] [PubMed] [Google Scholar]
- 34.Joziasse D.H., Shaper J.H., Van den Eijnden D.H., Van Tunen A.J., Shaper N.L. Bovine alpha 1-3-galactosyltransferase: isolation and characterization of a cDNA clone. Identification of homologous sequences in human genomic DNA. J Biol Chem. 1989 Aug 25;264(24):14290–14297. [PubMed] [Google Scholar]
- 35.Lifton R.P. Lasker Award to heart valve pioneers. Cell. 2007 Sep 21;130(6):971–974. doi: 10.1016/j.cell.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 36.Carpentier A. Lasker Clinical Research Award. The surprising rise of nonthrombogenic valvular surgery. Nat Med. 2007 Oct;13(10):1165–1168. doi: 10.1038/nm1645. [DOI] [PubMed] [Google Scholar]
- 37.Perier P., Bessou J.P., Swanson J.S., Bensasson D., Chachques J.C., Chauvaud S. Comparative evaluation of aortic valve replacement with Starr, Bjork, and porcine valve prostheses. Circulation. 1985 Sep;72(3 Pt 2):II140–5. [PubMed] [Google Scholar]
- 38.Perier P., Deloche A., Chauvaud S., Chachques J.C., Relland J., Fabiani J.N. A 10-year comparison of mitral valve replacement with Carpentier-Edwards and Hancock porcine bioprostheses. Ann Thorac Surg. 1989 Jul;48(1):54–59. doi: 10.1016/0003-4975(89)90176-8. [DOI] [PubMed] [Google Scholar]
- 39.Perier P., Deloche A., Chauvaud S., Fabiani J.N., Stephan Y., Dreyfus G. Clinical comparison of mitral valve replacement using porcine, Starr, and Bjork valves. J Card Surg. 1988 Sep;3(3 Suppl):359–368. doi: 10.1111/jocs.1988.3.3s.359. [DOI] [PubMed] [Google Scholar]
- 40.Pellerin M., Mihaileanu S., Couetil J.P., Relland J.Y., Deloche A., Fabiani J.N. Carpentier-Edwards pericardial bioprosthesis in aortic position: long-term follow-up 1980 to 1994. Ann Thorac Surg. 1995 Aug;60(2 Suppl) doi: 10.1016/0003-4975(95)00225-a. S292–5; discussion S5–6. [DOI] [PubMed] [Google Scholar]
- 41.Perier P., Mihaileanu S., Fabiani J.N., Deloche A., Chauvaud S., Jindani A. Long-term evaluation of the carpentier-Edwards pericardial valve in the aortic position. J Card Surg. 1991;6(suppl):589–594. doi: 10.1111/jocs.1991.6.4s.589. [DOI] [PubMed] [Google Scholar]
- 42.Relland J., Perier P., Lecointe B. The third generation Carpentier-Edwards bioprosthesis: early results. J Am Coll Cardiol. 1985 Nov;6(5):1149–1154. doi: 10.1016/s0735-1097(85)80323-5. [DOI] [PubMed] [Google Scholar]
- 43.Jamieson W.R., Janusz M.T., Burr L.H., Ling H., Miyagishima R.T., Germann E. Carpentier-Edwards supraannular porcine bioprosthesis: second-generation prosthesis in aortic valve replacement. Ann Thorac Surg. 2001 May;71(5 Suppl):S224–S227. doi: 10.1016/s0003-4975(01)02549-8. [DOI] [PubMed] [Google Scholar]
- 44.Gao G., Wu Y., Grunkemeier G.L., Furnary A.P., Starr A. Durability of pericardial versus porcine aortic valves. J Am Coll Cardiol. 2004 Jul 21;44(2):384–388. doi: 10.1016/j.jacc.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 45.McClure R.S., Narayanasamy N., Wiegerinck E., Lipsitz S., Maloney A., Byrne J.G. Late outcomes for aortic valve replacement with the Carpentier-Edwards pericardial bioprosthesis: up to 17-year follow-up in 1,000 patients. Ann Thorac Surg. 2010 May;89(5):1410–1416. doi: 10.1016/j.athoracsur.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 46.Dvir D., Bourguignon T., Otto C.M., Hahn R.T., Rosenhek R., Webb J.G. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018 Jan 23;137(4):388–399. doi: 10.1161/CIRCULATIONAHA.117.030729. [DOI] [PubMed] [Google Scholar]
- 47.Capodanno D., Petronio A.S., Prendergast B., Eltchaninoff H., Vahanian A., Modine T. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur J Cardiothorac Surg. 2017 Sep 1;52(3):408–417. doi: 10.1093/ejcts/ezx244. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Gabella T., Voisine P., Puri R., Pibarot P., Rodes-Cabau J. Aortic bioprosthetic valve durability: incidence, mechanisms, predictors, and management of surgical and transcatheter valve degeneration. J Am Coll Cardiol. 2017 Aug 22;70(8):1013–1028. doi: 10.1016/j.jacc.2017.07.715. [DOI] [PubMed] [Google Scholar]
- 49.Bourguignon T., Bouquiaux-Stablo A.L., Candolfi P., Mirza A., Loardi C., May M.A. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg. 2015 Mar;99(3):831–837. doi: 10.1016/j.athoracsur.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 50.Reid M.E., Lomas-Francis C., Olsson M. 3rd ed. Elsevier; 2012. The Blood group antigen facts. [Google Scholar]
- 51.Bravery C.A., Hunt B.J., Thorpe S.J., Yacoub M. Expression of ABH antigens in porcine heart tissue. Transplant Proc. 1992 Apr;24(2):445–446. [PubMed] [Google Scholar]
- 52.Thorpe S.J., Hunt B., Yacoub M. Expression of ABH blood group antigens in human heart tissue and its relevance to cardiac transplantation. Transplantation. 1991 Jun;51(6):1290–1295. doi: 10.1097/00007890-199106000-00027. [DOI] [PubMed] [Google Scholar]
- 53.Ariza L., Carmona R., Canete A., Cano E., Munoz-Chapuli R. Coelomic epithelium-derived cells in visceral morphogenesis. Dev Dyn. 2015 Mar;245(3):307–322. doi: 10.1002/dvdy.24373. [DOI] [PubMed] [Google Scholar]
- 54.Cribier A. The development of transcatheter aortic valve replacement (TAVR) Glob Cardiol Sci Pract. 2016 Dec 30;2016(4) doi: 10.21542/gcsp.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diswall M., Angstrom J., Karlsson H., Phelps C.J., Ayares D., Teneberg S. Structural characterization of alpha1,3-galactosyltransferase knockout pig heart and kidney glycolipids and their reactivity with human and baboon antibodies. Xenotransplantation. 2010 Jan-Feb;17(1):48–60. doi: 10.1111/j.1399-3089.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- 56.Andresen E. Blood group in pigs. Ann N Y Acad Sci. 1962;97:205–225. [PubMed] [Google Scholar]
- 57.Choi M.K., Le M.T., Cho H., Yum J., Kang M., Song H. Determination of complete sequence information of the human ABO blood group orthologous gene in pigs and breed difference in blood type frequencies. Gene. 2017 Jan 15;640:1–5. doi: 10.1016/j.gene.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 58.Andresen E. Blood groups in pigs. Ann N Y Acad Sci. 1962 May 3;97:205–225. [PubMed] [Google Scholar]
- 59.Feingold B., Wearden P.D., Morell V.O., Galvis D., Galambos C. Expression of A and B blood group antigens on cryopreserved homografts. Ann Thorac Surg. 2009 Jan;87(1):211–214. doi: 10.1016/j.athoracsur.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 60.Cho E.H., Soliman M., Alfajaro M.M., Kim J.Y., Seo J.Y., Park J.G. Bovine nebovirus interacts with a wide spectrum of histo-blood group antigens. J Virol. 2018 May;1:92(9). doi: 10.1128/JVI.02160-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nasir W., Frank M., Kunze A., Bally M., Parra F., Nyholm P.G. Histo-blood group antigen presentation is critical for binding of norovirus VLP to glycosphingolipids in model membranes. ACS Chem Biol. 2017 May 19;12(5):1288–1296. doi: 10.1021/acschembio.7b00152. [DOI] [PubMed] [Google Scholar]
- 62.Zakhour M., Ruvoen-Clouet N., Charpilienne A., Langpap B., Poncet D., Peters T. The alphaGal epitope of the histo-blood group antigen family is a ligand for bovine norovirus Newbury2 expected to prevent cross-species transmission. PLoS Pathog. 2009 Jul;5(7) doi: 10.1371/journal.ppat.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yeh P., Ezzelarab M., Bovin N., Hara H., Long C., Tomiyama K. Investigation of potential carbohydrate antigen targets for human and baboon antibodies. Xenotransplantation. 2010 May-Jun;17(3):197–206. doi: 10.1111/j.1399-3089.2010.00579.x. [DOI] [PubMed] [Google Scholar]
- 64.Milland J., Sandrin M.S. ABO blood group and related antigens, natural antibodies and transplantation. Tiisue Antigens. 2016;68(6):459–466. doi: 10.1111/j.1399-0039.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- 65.Puga Yung G., Schneider M.K., Seebach J.D. Immune responses to alpha1,3 galactosyltransferase knockout pigs. Curr Opin Organ Transplant. 2009 Apr;14(2):154–160. doi: 10.1097/MOT.0b013e328329250d. [DOI] [PubMed] [Google Scholar]
- 66.McGregor C., Byrne G., Rahmani B., Chisari E., Kyriakopoulou K., Burriesci G. Physical equivalency of wild type and galactose alpha 1,3 galactose free porcine pericardium; a new source material for bioprosthetic heart valves. Acta Biomater. 2016 Sep 1;41:204–209. doi: 10.1016/j.actbio.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuwaki K., Tseng Y.L., Dor F.J., Shimizu A., Houser S.L., Sanderson T.M. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005 Jan;11(1):29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 68.Lila N., McGregor C.G., Carpentier S., Rancic J., Byrne G.W., Carpentier A. Gal knockout pig pericardium: new source of material for heart valve bioprostheses. J Heart Lung Transplant. 2010 May;29(5):538–543. doi: 10.1016/j.healun.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 69.McGregor C.G., Carpentier A., Lila N., Logan J.S., Byrne G.W. Cardiac xenotransplantation technology provides materials for improved bioprosthetic heart valves. J Thorac Cardiovasc Surg. 2011 Jan;141(1):269–275. doi: 10.1016/j.jtcvs.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 70.Park J.Y., Park M.R., Bui H.T., Kwon D.N., Kang M.H., Oh M. alpha1,3-galactosyltransferase deficiency in germ-free miniature pigs increases N-glycolylneuraminic acids as the xenoantigenic determinant in pig-human xenotransplantation. Cell Reprogram. 2012 Aug;14(4):353–363. doi: 10.1089/cell.2011.0083. [DOI] [PubMed] [Google Scholar]
- 71.Burlak C., Bern M., Brito A.E., Isailovic D., Wang Z.Y., Estrada J.L. N-linked glycan profiling of GGTA1/CMAH knockout pigs identifies new potential carbohydrate xenoantigens. Xenotransplantation. 2013 Sep-Oct;20(5):277–291. doi: 10.1111/xen.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuriev E., Agostino M., Farrugia W., Christiansen D., Sandrin M.S., Ramsland P.A. Structural biology of carbohydrate xenoantigens. Expert Opin Biol Ther. 2009 Aug;9(8):1017–1029. doi: 10.1517/14712590903066703. [DOI] [PubMed] [Google Scholar]
- 73.Miyagawa S., Takeishi S., Yamamoto A., Ikeda K., Matsunari H., Yamada M. Survey of glycoantigens in cells from alpha1-3galactosyltransferase knockout pig using a lectin microarray. Xenotransplantation. 2010 Jan-Feb;17(1):61–70. doi: 10.1111/j.1399-3089.2009.00565.x. [DOI] [PubMed] [Google Scholar]
- 74.Zhang R., Wang Y., Chen L., Wang R., Li C., Li X. Reducing immunoreactivity of porcine bioprosthetic heart valves by genetically-deleting three major glycan antigens, GGTA1/beta4GalNT2/CMAH. Acta Biomater. 2018 May;72:196–205. doi: 10.1016/j.actbio.2018.03.055. [DOI] [PubMed] [Google Scholar]
- 75.Gates K.V., Dalgliesh A.J., Griffiths L.G. Antigenicity of bovine pericardium determined by a novel immunoproteomic approach. Sci Rep. 2017 May 26;7(1):2446. doi: 10.1038/s41598-017-02719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aamodt J.M., Grainger D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials. 2016 Apr;86:68–82. doi: 10.1016/j.biomaterials.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dalgliesh A.J., Parvizi M., Lopera-Higuita M., Shklover J., Griffiths L.G. Graft-specific immune tolerance is determined by residual antigenicity of xenogeneic extracellular matrix scaffolds. Acta Biomater. 2018 Oct 1;79:253–264. doi: 10.1016/j.actbio.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spalter S.H., Kaveri S.V., Bonnin E., Mani J.C., Cartron J.P., Kazatchkine M.D. Normal human serum contains natural antibodies reactive with autologous ABO blood group antigens. Blood. 1999 Jun 15;93(12):4418–4424. [PubMed] [Google Scholar]
- 79.Schetelig J., Breitschaft A., Kroger N., Zabelina T., Ebell W., Bornhauser M. After major ABO-mismatched allogeneic hematopoietic progenitor cell transplantation, erythroid engraftment occurs later in patients with donor blood group A than donor blood group B. Transfusion. 2005 May;45(5):779–787. doi: 10.1111/j.1537-2995.2005.04236.x. [DOI] [PubMed] [Google Scholar]
- 80.Ozolek J.A., Watchko J.F., Mimouni F. Prevalence and lack of clinical significance of blood group incompatibility in mothers with blood type A or B. J Pediatr. 1994 Jul;125(1):87–91. doi: 10.1016/s0022-3476(94)70131-8. [DOI] [PubMed] [Google Scholar]
- 81.Brown M.L., Park S.J., Sundt T.M., Schaff H.V. Early thrombosis risk in patients with biologic valves in the aortic position. J Thorac Cardiovasc Surg. 2012 Jul;144(1):108–111. doi: 10.1016/j.jtcvs.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 82.Huygens S.A., Mokhles M.M., Hanif M., Bekkers J.A., Bogers A.J., Rutten-van Molken M.P. Contemporary outcomes after surgical aortic valve replacement with bioprostheses and allografts: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2016 Oct;50(4):605–616. doi: 10.1093/ejcts/ezw101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Puvimanasinghe J.P., Steyerberg E.W., Takkenberg J.J., Eijkemans M.J., van Herwerden L.A., Bogers A.J. Prognosis after aortic valve replacement with a bioprosthesis: predictions based on meta-analysis and microsimulation. Circulation. 2001 Mar 20;103(11):1535–1541. doi: 10.1161/01.cir.103.11.1535. [DOI] [PubMed] [Google Scholar]
- 84.Puri R., Auffret V., Rodes-Cabau J. Bioprosthetic valve thrombosis. J Am Coll Cardiol. 2017 May 2;69(17):2193–2211. doi: 10.1016/j.jacc.2017.02.051. [DOI] [PubMed] [Google Scholar]
- 85.Astarcioglu M.A., Kalcik M., Yesin M., Gursoy M.O., Sen T., Karakoyun S. AB0 blood types: impact on development of prosthetic mechanical valve thrombosis. Anatol J Cardiol. 2016 Nov;16(11):820–823. doi: 10.14744/AnatolJCardiol.2016.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rizzo C., Caruso C., Vasto S. Possible role of ABO system in age-related diseases and longevity: a narrative review. Immun Ageing. 2014;11:16. doi: 10.1186/1742-4933-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shimizu K., Hirose N., Ebihara Y., Arai Y., Hamamatsu M., Nakazawa S. Blood type B might imply longevity. Exp Gerontol. 2004 Oct;39(10):1563–1565. doi: 10.1016/j.exger.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 88.Rumley A., Lowe G.D., Sweetnam P.M., Yarnell J.W., Ford R.P. Factor VIII, von Willebrand factor and the risk of major ischaemic heart disease in the Caerphilly Heart Study. Br J Haematol. 1999 Apr;105(1):110–116. [PubMed] [Google Scholar]
- 89.Etemadi A., Kamangar F., Islami F., Poustchi H., Pourshams A., Brennan P. Mortality and cancer in relation to ABO blood group phenotypes in the Golestan Cohort Study. BMC Med. 2015 Jan 15;13:8. doi: 10.1186/s12916-014-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bowen D.J. An influence of ABO blood group on the rate of proteolysis of von Willebrand factor by ADAMTS13. J Thromb Haemost. 2003 Jan;1(1):33–40. doi: 10.1046/j.1538-7836.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 91.Franchini M., Mannucci P.M. ABO blood group and thrombotic vascular disease. Thromb Haemost. 2014 Dec;112(6):1103–1109. doi: 10.1160/TH14-05-0457. [DOI] [PubMed] [Google Scholar]
- 92.Chen Y., Chen C., Ke X., Xiong L., Shi Y., Li J. Analysis of circulating cholesterol levels as a mediator of an association between ABO blood group and coronary heart disease. Circ Cardiovasc Genet. 2014 Feb;7(1):43–48. doi: 10.1161/CIRCGENETICS.113.000299. [DOI] [PubMed] [Google Scholar]
- 93.Gotsman I., Keren A., Zwas D.R., Lotan C., Admon D. Clinical impact of ABO and rhesus D blood type groups in patients with chronic heart failure. Am J Cardiol. 2018 Aug 1;122(3):413–419. doi: 10.1016/j.amjcard.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 94.Johnston D.R., Soltesz E.G., Vakil N., Rajeswaran J., Roselli E.E., Sabik J.F., 3rd Long-term durability of bioprosthetic aortic valves: implications from 12,569 implants. Ann Thorac Surg. 2015 Apr;99(4):1239–1247. doi: 10.1016/j.athoracsur.2014.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Manji R.A., Menkis A.H., Ekser B., Cooper D.K. Porcine bioprosthetic heart valves: The next generation. Am Heart J. 2012 Aug;164(2):177–185. doi: 10.1016/j.ahj.2012.05.011. [DOI] [PubMed] [Google Scholar]