Abstract
Background
Although temozolomide (TMZ) resistance is a significant clinical problem in glioblastoma (GBM), its underlying molecular mechanisms are poorly understood. In this study, we identified the role of exosomal microRNAs (miRNAs) from TMZ-resistant cells as important mediators of chemoresistance in GBM cells.
Methods
Exosomes were isolated from TMZ-resistant GBM cells and characterized via scanning electron microscopy (SEM). Expression levels of miR-1238 in GBM cell lines and their exosomes, clinical tissues, and sera were evaluated by RT-qPCR. In vitro and in vivo experiments were performed to elucidate the function of exosomal miR-1238 in TMZ resistance in GBM cells. Co-immunoprecipitation assays and western blot analysis were used to investigate the potential mechanisms of miR-1238/CAV1 that contribute to TMZ resistance.
Findings
MiR-1238 levels were higher in TMZ-resistant GBM cells and their exosomes than in sensitive cells. Higher levels of miR-1238 were found in the sera of GBM patients than in healthy people. The loss of miR-1238 may sensitize resistant GBM cells by directly targeting the CAV1/EGFR pathway. Furthermore, bioactive miR-1238 may be incorporated into the exosomes shed by TMZ-resistant cells and taken up by TMZ-sensitive cells, thus disseminating TMZ resistance.
Interpretation
Our findings establish that miR-1238 plays an important role in mediating the acquired chemoresistance of GBM and that exosomal miR-1238 may confer chemoresistance in the tumour microenvironment. These results suggest that circulating miR-1238 serves as a clinical biomarker and a promising therapeutic target for TMZ resistance in GBM.
Fund
This study was supported by the National Natural Science Foundation of China (No·81402056, 81472362, and 81772951) and the National High Technology Research and Development Program of China (863) (No·2012AA02A508).
Keywords: Glioblastoma, Temozolomide, Chemoresistance, miRNA, Exosomes
Abbreviations: GBM, glioblastoma; LGG, low grade glioma; HGG, high grade glioma; TMZ, temozolomide; CAV1, caveolin-1; miRNA, microRNA; SEM, scanning electron microscope; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labelling; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; Co-IP, Co-immunoprecipitation; sExo, exosomes from sensitive cells; rExo, exosomes from resistant cells; 1238-down rExo, the miR-1238 levels were downregulated in exosomes from resistant cells
Research in context.
Evidence before this study
GBM is one of the most aggressive tumours in adults. The current standard treatment for GBM consists of surgical resection, adjuvant radiation therapy, and chemotherapy. Temozolomide (TMZ) is employed as a first-line drug for GBM. However, drug resistance significantly limits the durability of the treatment response. Exosomes are newly discovered extracellular vesicles. Exosomes are involved in intercellular communication by transporting their intracellular cargos, such as proteins and RNAs. Recent research results indicate that the exosomal transfer of miRNAs plays an important role in glioma progression and chemoresistance. For example, exosomes from glioma-associated mesenchymal stem cells increase the tumorigenicity of glioma stem-like cells via transfer of miR-1587. Our previous study also showed that exosomal transfer of miR-151a enhances chemosensitivity to TMZ in drug-resistant GBM. Thus, we tend to figure out more available miRNAs in exosomes, which contribute to chemoresistance for GBM.
Added value of this study
In this study, we found that exosomal miR-1238 from TMZ resistant GBM cells confers acquired chemoresistance to TMZ sensitive GBM cells. In addition, the level of exosomal miR-1238 might serve as a biomarker for predicting treatment reaction of GBM patients. Furthermore, we investigated the mechanisms of exosomal miR-1238 inducing TMZ resistance. Results showed that exosomal miR-1238 activates EGFR pathway via directly targeting CAV1. In summary, this study demonstrated that exosomal miR-1238 plays an essential role in modulating chemoresistance in GBM cells.
Implications of all the available evidence
This study identified a novel exosomal miRNA that contributes to the advanced chemoresistance of GBM, and suggested that circulating miR-1238 serves as a clinical biomarker and a promising therapeutic target for TMZ resistance in GBM.
Alt-text: Unlabelled Box
1. Introduction
GBM is one of the most aggressive human cancers. Despite intensive therapeutic strategies including surgery, chemotherapy, and radiotherapy, the median survival remains <15 months [1,2]. TMZ has been used as a first-line chemotherapy; however, resistance to the drug significantly limits the prognosis associated with GBM patients [3,4]. Therefore, research on the molecular mechanisms of the malignant phenotype of GBM is necessary for prognostic and treatment strategies for this tumour.
Extracellular vesicles (EVs) shed by cells are membrane-enclosed nanospheres that serve as important mediators of intercellular communication [5,6]. Exosomes are generally the most abundant type of EVs, ranging from 40 to 100 nm in size. Their capacity as efficient carriers of RNAs, proteins, and other bioactive molecules make them important players during tumorigenesis and drug resistance [7,8]. Results from our previous study indicated that exosomes from GBM cells harbouring PTPRZ1-MET fusion mediated the aggressive character of GBM [9]. MicroRNAs are small endogenous noncoding RNAs that act as gene regulators at the transcriptional and posttranscriptional levels by binding to 3′-untranslated regions (UTRs) [10]. For example, miR-423-5p functioned as an oncogene in GBM tissues by suppressing ING-4 [11]. Recently, exosomal miRNAs have received substantial attention. MiR-21-rich exosomes derived from hypoxic oral squamous cell carcinoma (OSCC) can promote pro-metastatic behaviours [12]. Moreover, exosomal miR-29a binds to toll-like receptors in nearby tumour-associated macrophages and triggers a proinflammatory reaction in lung cancer [13]. However, whether exosomal miRNAs contribute to TMZ resistance in GBM tissues remains to be elucidated. Here, we identified a significant role of exosomal miR-1238 on TMZ resistance in GBM.
2. Materials and methods
2.1. Human tissue samples
Thirteen primary GBM specimens and thirteen recurrent samples were obtained from the First Affiliated Hospital of Nanjing Medical University (Nanjing, Jiangsu, China). The detailed characteristics of enrolled patients are provided in Supplementary Table 1. All tissue samples were collected during surgery, frozen immediately in liquid nitrogen, and stored for total RNA or protein extraction.
2.2. Ethics approval and consent to participate
This study was approved by the institutional review board and the ethics committee of Nanjing Medical University and written informed consent was obtained from all patients, in accordance with the guidelines established in the Declaration of Helsinki. All animal studies were approved by Jiangsu Animal Experimental for Medical and Pharmaceutical Research Center and were conducted in compliance with animal-use guidelines established in Nanjing China.
2.3. Cell culture
The human GBM cell line U251 was obtained from the Chinese Academy of Sciences Cell Bank (Shanghai, China) and primary human N3 GBM cells were obtained from Beijing Neurosurgical Institute, Capital Medical University (Beijing, China). Both cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) with high glucose and sodium pyruvate supplemented with 10% foetal bovine serum and antibiotics (100 units/mL penicillin and 100 units/mL streptomycin). The primary GBM cells (GBM1) were established from a newly diagnosed GBM patient (51-year-old female; frontal; MGMT: unmethylated; IDH1/2: wild-type). rGBM1 cells were established from a recurrent GBM patient (63-year-old male; frontal; MGMT: methylated; IDH1/2: wild-type). sGBM2 cells were established from a newly diagnosed GBM patient (45-year-old male; frontal; MGMT: unmethylated; IDH1/2: wild-type). rGBM2 cells were established from a recurrent GBM patient (67-year-old female; frontal; MGMT: methylated; IDH1/2: wild-type). GBM surgical specimens were collected and dissociated into single cells by placing them in Accutase® (purchased from Sigma-Aldrich, St. Louis, MO, USA) or an enzyme cocktail for 15–20 min at 37 °C. The primary GBM cells used for the experiments were cultured in DMEM supplemented with 10% foetal bovine serum and were replenished every three months from frozen stocks.
2.4. RNA extraction and quantitative reverse transcription PCR (RT-qPCR)
Total RNA was isolated from human GBM tissues or cultured cells using TRIzol reagent (Invitrogen/Thermo Fisher Scientific, Carlsbad, CA, USA) according to the standard protocol. For exosomal RNA extraction, 750 uL TRIzol-LS was used to crack 250 ul exosome suspension, then chloroform was used to separate the pyrolysis liquid phase and the upper liquid was added to isopropanol to precipitate RNA in the exosome suspension. A stem-loop-specific primer method was used to measure miR-1238 expression as described previously [14]. U6 expression was used as an endogenous control [15]. The cDNA was amplified by qRT-PCR using SYBR® Premix Ex Taq™ (Takara, Kusatsu, Japan) on a 7900HT system (Applied Biosystems/Thermo Fisher Scientific, Foster City, CA, USA). Fold changes were calculated by relative quantification (2-ΔΔCt).
2.5. Protein extraction and immunoblotting
Protein extraction and western blot analysis were performed as described previously [16]. Antibodies against CAV1 (Cell Signaling Technology, Danvers, MA, USA)), cleaved caspase-3 (Cell Signaling Technology), CD63 (Abcam, Cambridge, UK)), CD81 (Cell Signaling Technology), EGFR and phosphorylated EGFR (Cell Signaling Technology), PI3K and phosphorylated PI3K (Cell Signaling Technology), Akt and pAkt Ser473 (Cell Signaling Technology), mTOR and phosphorylated mTOR (Cell Signaling Technology), and β-actin (1:10000, AC-74; Sigma-Aldrich) were used.
2.6. Plasmid construction, lentiviral packaging, and stable cell line establishment
CAV1 and EGFR cDNA were purified by Genechem (Shanghai, China) and cloned into pcDNA3·1-FLAG and pcDNA3·1-Myc vectors to generate pcDNA3·1-FLAG-CAV1 and pcDNA3·1-Myc-EGFR plasmids. The transfection was performed according to the manufacturer's instructions for LipofectamineTM 2000 transfection reagent (Invitrogen/Thermo Fisher Scientific). A lentiviral packaging kit was purchased from Genechem (Shanghai, China). Lentivirus carrying miR-1238 inhibitor or miR-NC was packaged in human embryonic kidney 293 T cells and the virus was collected according to the manufacturer's instructions. Stable cell lines were established by infecting U251r, N3r, rGBM1, and rGBM2 cells with lentivirus, followed by puromycin selection.
2.7. Cell viability and colony formation assay
For the cell counting kit-8 (CCK-8) assay, stable transfected or exosome-treated U251r, N3r, rGBM1, and rGBM2 cells were seeded in 96-well plates and cultured for 24, 48, 72, and 96 h with TMZ treatment. After a 1 h incubation with CCK-8 at 37 °C, absorbance was detected (OD value) at a wavelength of 450 nm.
For the colony formation assay, cells were harvested 24 h after transfection and then seeded in 6-well plates and cultured for approximately 2 weeks with or without TMZ treatment until colony formation was observed. The colony formation rate was used to calculate the cell survival rate.
2.8. Apoptosis and flow cytometric analysis
The apoptosis assay was performed after transfection or exosomal treatment using the annexin V-fluorescein isothiocyanate Apoptosis Detection Kit I (BD Biosciences, San Jose, CA, USA) and assessed by fluorescence-activated cell sorting (FACS).
2.9. Terminal deoxynucleotidyl transferase dUTP nick end labelling analysis
After transfection or exosomal treatment of GBM cells, a terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) apoptosis detection kit (MilliporeSigma, Burlington, MA, USA) was used according to the manufacturer's instructions.
2.10. Luciferase reporter assay
The wild-type and mutated putative miR-1238 target in the CAV1 3′-UTR were cloned into a pGL3-control luciferase reporter plasmid (Invitrogen/Thermo Fisher Scientific). Firefly and Renilla luciferase signals were determined using a Dual-Luciferase® Assay Kit (Promega, Madison, WI, USA).
2.11. Immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH)
Immunohistochemistry to detect CAV1 and cleaved caspase-3 in GBM samples was performed as described previously [17].
The expression levels of miR-1238 in GBM samples were detected by FISH as previously described [18]. The mature human miR-1238 sequence was: 3′-CCCCGGUCGCCCUC-5′. We used (LNA)-based probes directed against the full length mature miRNA sequence. The 5′-FAM-labelled miR-1238 probe sequence was: 5′-GGGGCCAGCGGGAG-3′ purchased from BioSense (Guangzhou, China). The FISH procedure was performed according to the BioSense instructions.
2.12. Co-immunoprecipitation
Co-immunoprecipitation assays were performed using a Co-IP kit (Thermo Fisher Scientific, Waltham, MA, USA). Briefly, precleared cell lysates were incubated with anti-FLAG or anti-Myc antibody on a rotator overnight at 4 °C. Next, the mixtures were incubated with immobilized protein A/G beads (Thermo Fisher Scientific) on a rotator for 2 h at 4 °C. The beads were collected and washed five times with IP wash buffer. SDS loading buffer was then added to the beads and the samples were denatured at 95 °C for 8–10 min. Finally, the supernatants were collected and analysed through western blotting as described above.
2.13. Exosome isolation and characterization
Exosomes were isolated from GBM cell culture supernatants or the serum of GBM patients and healthy volunteers as previously described [19]. In brief, culture supernatants or the serum were collected and differentially centrifuged at 300g for 10 min, 1000 ×g for 20 min and 10,000 ×g for 30 min. Next, the supernatants were filtered using 0·22 μm filter units (Millex-GP; EMD Millipore, Darmstadt, Germany) and ultracentrifuged at 100,000 ×g for 3 h at 4 °C. After removing the supernatant, the pellets were resuspended in ice-cold PBS. Then the suspension was centrifuged at 100,000 ×g for another 3 h at 4 °C. Exosome pellets were resuspended in PBS and stored at −80 °C. The concentration of exosomes was measured via BCA methods. Exosomes were visualized by transmission electron microscopy and confirmed by the expression of CD63 and CD81, which are specific proteins of exosomes. The exosome samples were detected on a NanoSight NS300 particle size analyser (NTA; Malvern Panalytical, Malvern, UK) equipped with a 450 nm laser. After the exosome particles were irradiated by the laser beam, they were visualized by a microscope equipped with a camera and the video files of the Brownian motion of the exosomes were captured. The Einstein equation was used to calculate concentration and hydrodynamic diameter based on motion.
2.14. Xenograft studies and treatment experiments
Male nude mice (6-weeks-old) were purchased from Shanghai Experimental Animal Centre of the Chinese Academy of Sciences and the in vivo studies were performed as previously described [20]. To establish intracranial GBMs, 0·5 × 105 U251r and U251s cells stably expressing the luciferase reporter were stereotactically implanted. Before implantation, U251r cells were transfected with a lentivirus carrying miR-1238 inhibitor and U251s cells were treated with 50 μg of exosomes purified from the culture supernatants of U251r cells and cultured for 6 days in Exo-free medium. The mice were imaged for Fluc activity using bioluminescence imaging after an intraperitoneal injection of D-luciferin (10 μL/g). Tumours from mouse flanks and brains were fixed in 4% paraformaldehyde for 24 h followed by incubation in 30% sucrose for 48 h. Paraffin-embedded tissue sections were stained with haematoxylin–eosin (H&E). Three sections per tumour were analysed to quantify staining.
2.15. Statistical analysis
All experiments were performed three times and all values are presented as the mean ± standard deviation (SD). The correlations between miR-1238 expression and CAV1 levels in GBM tissues were analysed using the Spearman rank test. Statistical evaluation for data analysis was determined using the t-test. The differences were considered to be statistically significant at P < .05.
3. Results
3.1. MiR-1238 is highly expressed in TMZ-resistant GBM cells and tissues
In our previous study [21], we identified four miRNAs (miR-1280, miR-1238, miR-938, and miR-423-5p) overexpressing in TMZ chemoresistant tissues compared with TMZ chemosensitive tissues Meanwhile, the aberrant expression of these miRNAs confer a relatively poor prognosis. However, further research is necessary to clarify the role of these miRNAs in the development of GBM. Therefore, we selected miR-1238, one of the miRNAs exhibiting the highest expression levels in the TMZ chemoresistant subtype.
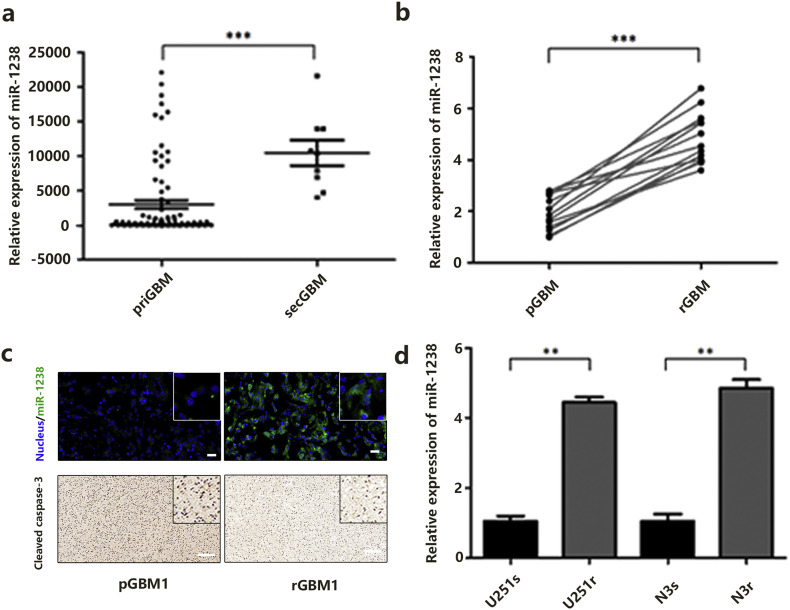
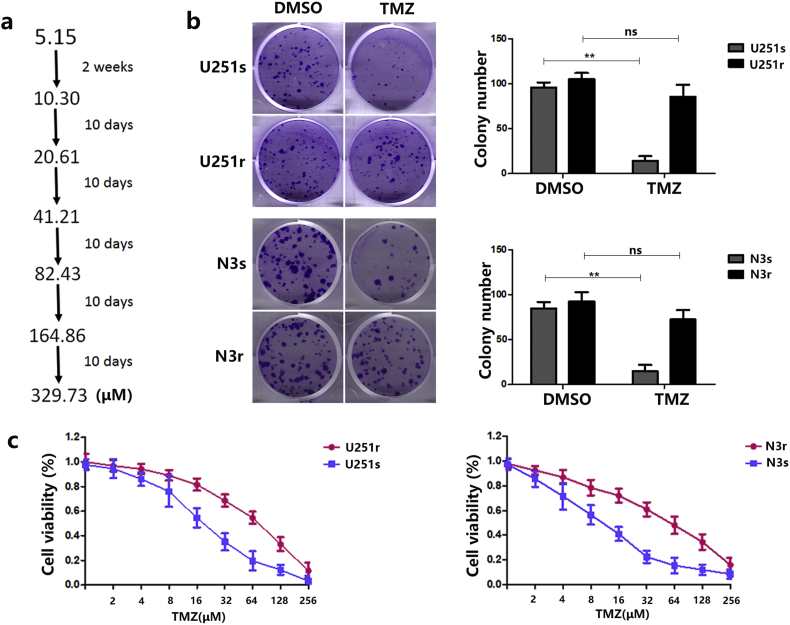
To fully assess the expression of miR-1238 in GBM, all miRNA profiles of 198 GBM samples were downloaded from the Chinese GBM Genome Atlas (CGGA) data portal (newly updated) and analysed. As shown in Fig. 1A, miR-1238 expression was significantly higher in SecGBM samples than in PriGBM samples. Then, using quantitative reverse transcription PCR (RT-qPCR) analysis, we confirmed that miR-1238 was dramatically upregulated in the recurrent GBM samples (n = 13) versus primary GBM tissues (n = 13) (Fig. 1B). In addition, using fluorescent in situ hybridization (FISH), we found that the recurrent tumours exhibited significantly higher miR-1238 staining intensity compared with primary tumours (Fig. 1C). Based on these results, we next sought to explore whether miR-1238 played an important role in TMZ resistance. We established two TMZ-resistant cell lines using U251 and N3 cells (U251r and N3r) by stepwise revulsion with TMZ over 6 months (Fig. S1A). To validate the chemoresistance of U251r and N3r, we performed cell colony formation assays and RF (resistance factor) to test the effect (Fig. S1B and C). The results showed that U251r and N3r exhibit about a 6- or 8-fold resistance to TMZ compared with U251s and N3s, respectively (Table 1:, Table 2:). RT-qPCR indicated that U251r and N3r express higher miR-1238 levels than the respective parental cell lines U251s and N3s (Fig. 1D).
Fig. 1.
MiR-1238 is highly expressed in TMZ-resistant GBM cells and tissues. A: The CGGA database was used to analyse the expression of miR-1238 in primary and recurrent GBM samples. B: RT-qPCR analysis of miR-1238 expression in thirteen primary and thirteen recurrent GBM samples. C: Upper: The expression levels of miR-1238 in primary GBM specimen (pGBM1) and recurrent GBM specimen (rGBM1) were assessed by FISH (scale bars = 50 μm). Lower: The expression levels of cleaved caspase-3 in pGBM1 and rGBM1 were assessed by IHC (scale bars = 50 μm). D: RT-qPCR analysis of miR-1238 expression in TMZ-sensitive U251s and N3s cells as well as TMZ-resistant cells U251r and N3r (normalized by U6 expression). In all experiments, data are mean ± SD of triplicate measurements. (Statistical analysis was performed by Student‘s t-test, *P < .05, **P < .01.)
Fig. S1.
A: Schematic of the establishment of TMZ-resistant cells. B: Plate colony formation assays were used to estimate the TMZ resistance of U251r and N3r cells. C: Relative cell viability of U251 s, U251r, N3 s, and N3r cells with TMZ treatment. In all experiments, bars represent mean ± SD for three replicates. (Statistical analysis was performed by Student‘s t-test, *P < .05, **P < .01.)
Table 1:
IC50 of U251s and U251r calculated after TMZ treatment
| Drug | Rf | p value | ||
|---|---|---|---|---|
| IC50a(mM) | ||||
| U251r | U251 s | |||
| TMZ | 14.26 ± 0.04 | 2.31 ± 0.04 | 6.17 | p < .05 |
Table 2:
IC50 of N3s and N3r calculated after TMZ treatment
| Drug | Rf | p value | ||
|---|---|---|---|---|
| IC50a(mM) | ||||
| N3r | N3 s | |||
| TMZ | 8.39 ± 0.09 | 1.04 ± 0.05 | 8.07 | p < .05 |
3.2. MiR-1238 levels in serum and GBM exosomes correlate with TMZ resistance
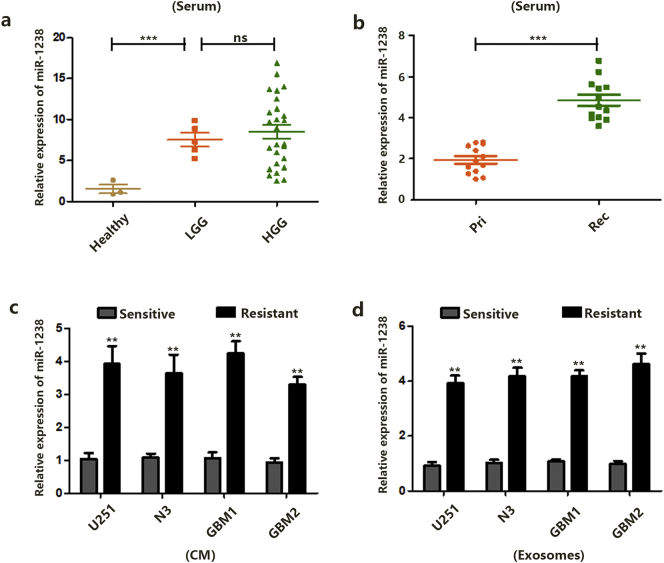
We next explored whether miR-1238 could exist in an extracellular milieu and act as a biomarker for GBM patients. As shown in Fig. 2A, the average expression levels of miR-1238 in serum from LGG and HGG patients were higher than in healthy volunteers. However, there was no significant difference between LGG and HGG samples. Furthermore, as shown in Fig. 2B, the average expression level of miR-1238 in serum from recurrent GBM patients (mentioned previously) was upregulated compared with primary GBM patient serum (Fig. 1B). These results indicated that the expression level of miR-1238 in the extracellular milieu may correlate with TMZ resistance in GBM patients. Next, RNAs in the culture medium (CM) and exosomes of TMZ-sensitive and resistant U251, N3, GBM1, and GBM2 cells were extracted. Compared with sensitive CM, miR-1238 levels were increased in resistant CM (Fig. 2C). A correlation was also observed between sensitive and resistant exosomes (Fig. 2D).
Fig. 2.
MiR-1238 levels in serum and GBM exosomes correlate with TMZ resistance. A: Relative expression of miR-1238 in serum samples was measured by RT-qPCR. B: Relative expression of miR-1238 in serum samples was measured by RT-qPCR. C: Relative expression of miR-1238 in culture medium (CM) of GBM cells was measured by RT-qPCR. D: Relative expression of miR-1238 in exosomes of GBM cells was measured by RT-qPCR. In all experiments, data are mean ± SD of triplicate measurements. (Statistical analysis was performed by Student‘s t-test, *P < .05, **P < .01.)
Together, these results indicate a possible role of miR-1238 in the regulation of TMZ resistance. In addition, circulating miR-1238 may provide a new biomarker for GBM patients.
3.3. Exosomal miR-1238 conferred TMZ resistance to GBM cells in vitro
Cell-secreted exosomes have been shown to act as mediators of cell-cell communication and their cargoes can be internalized by neighbouring cells [22,23]. Exosomal transfer of miRNAs between living cells can facilitate their targeted exchange [24]. Recently, certain studies have suggested that exosomes derived from resistant cancer cells can confer drug resistance to sensitive cells [25]. Moreover, components embedded in circulating exosomes may serve as easily accessible biomarkers for the evaluation of drug response in patients [26]. Thus, we investigated whether resistant GBM cells could confer TMZ resistance to sensitive cells via exosomal miRNAs.
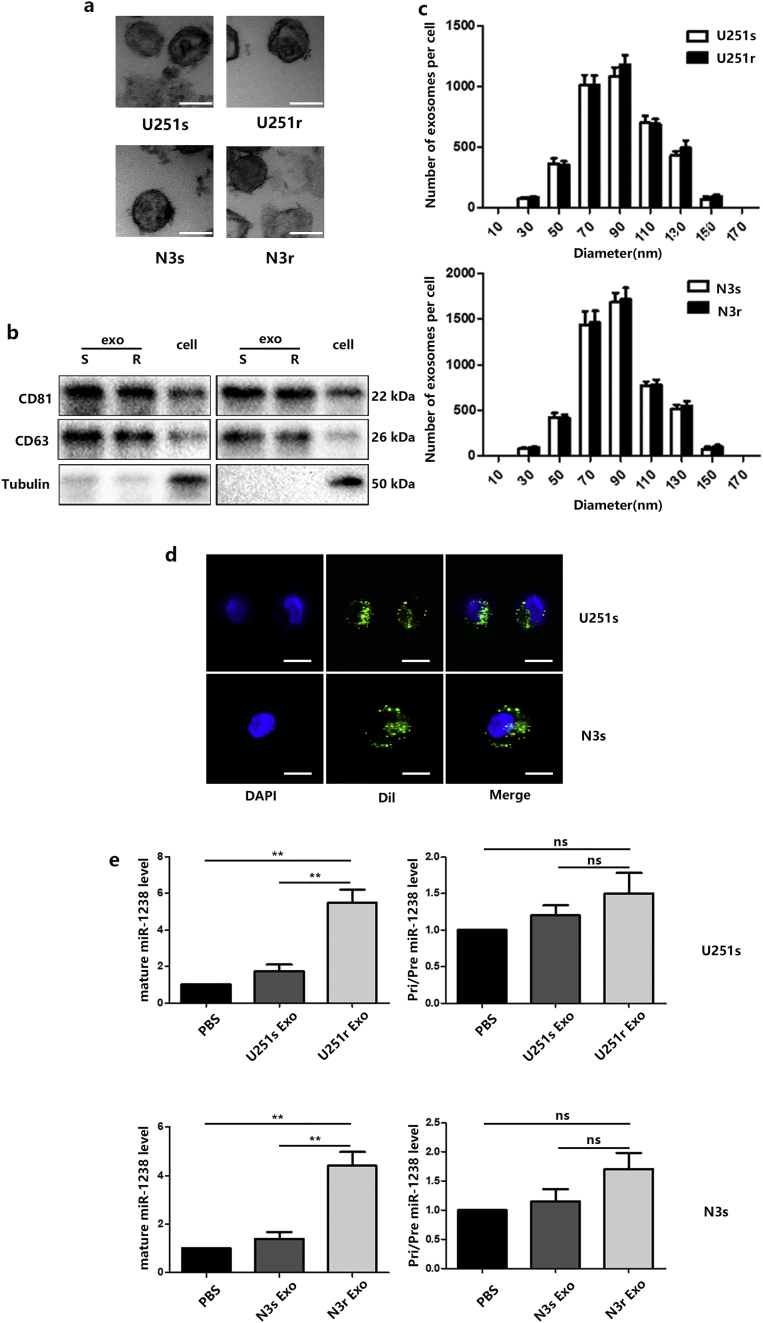
First, exosomes were isolated from U251r and N3r cells. Next, transmission electron microscopy (TEM), immunoblot (using CD63, CD81), and the NanoSight particle-tracking system were used to characterize and quantify (Fig. 3A, B, and C). The number of U251r and N3r exosomes were similar and they had similar morphology and size distributions. Then, exosomes derived from U251r and N3r cells were labelled with fluorescent Dil. Confocal microscopy captured the Dil-labelled exosomes that were internalized by U251s and N3s cells during a 2-hour incubation (Fig. 3D). An increase in cellular levels of mature miR-1238, but not pri/pre-miR-1238, was observed in recipient U251s and N3s treated with the exosomes isolated from resistant cell lines (Fig. 3E).
Fig. 3.
Exosomal transfer of miR-1238. A: Electron microscopy images of exosomes derived from TMZ-resistant and TMZ-sensitive cells (scale bars = 100 nm). B: Western blot analysis showing the presence of CD63 and CD81 in exosomes and cells. C: The NanoSight particle-tracking system was used to measure the size (diameters) of exosomes. D: Representative confocal microscopy image showing the internalization of Dil-labelled exosomes by TMZ-sensitive cells (scale bars = 10 μm). E: The level of mature and pri/pre miR-1238 in exosome-treated TMZ-sensitive cells controlled by PBS treatment. In all experiments, data are mean ± SD of triplicate measurements. (Statistical analysis was performed by Student‘s t-test, *P < .05, **P < .01.)
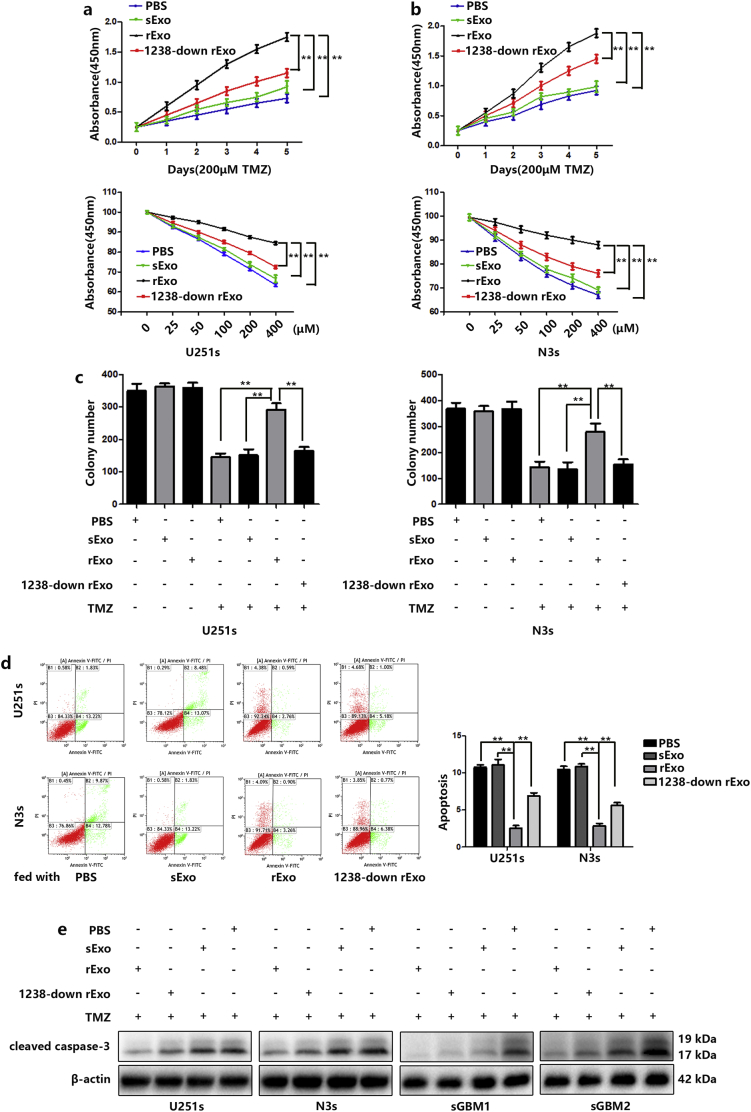
Next, we examined whether exosome-transferred miR-1238 could confer the resistant phenotype to recipient GBM cells. First, we generated 1238-down Exos (blockade of exosomal miR-1238) from miR-1238 down cells (Fig. S2A and B). CCK-8 and colony formation assays were performed, and the results indicated that resistant tumour-derived exosomes significantly increased the resistance of U251s and N3s to TMZ (Fig. 4A, B, and C). Exosomes derived from TMZ resistant cells also inhibited the apoptosis of TMZ sensitive cells (Fig. 4D). Similarly, the apoptosis-related protein cleaved caspase-3 was measured by western blotting and the results indicated that exosomes derived from TMZ resistant cells significantly decreased the protein level of cleaved caspase-3 in U251s and N3s cells under TMZ treatment (Fig. 4E). Moreover, decreased exosomal miR-1238 partially failed to increase cell resistance to TMZ.
Fig. S2.
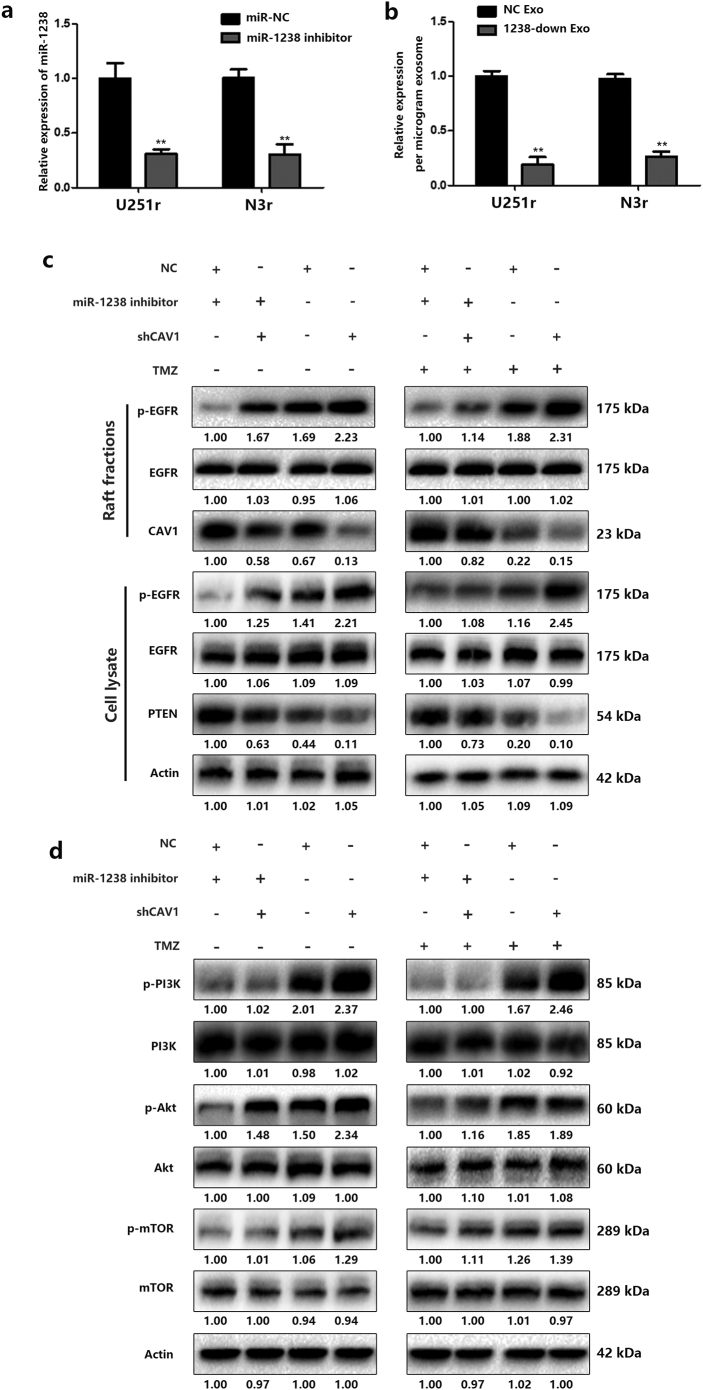
A: Relative expression of miR-1238 levels after transfection of miR-NC or miR-1238 inhibitor in U251r and N3r cells. B: Relative expression of miR-1238 levels of NC exosomes and 1238-down exosomes. C: Parallel experiments were performed to evaluate the modulation of the EGFR-PI3K-Akt-mTOR pathway by miR-1238/CAV1 signaling in N3r cells. In all experiments, bars represent mean ± SD for three replicates. (Statistical analysis was performed by Student‘s t-test, *P < .05, **P < .01.)
Fig. 4.
Exosomal miR-1238 conferred TMZ resistance to GBM cells in vitro. A and B: Relative cell viability of TMZ-sensitive cells, which were pre-treated with different exosomes (30 μg/mL) for 48 h under TMZ treatment for indicated times or indicated concentrations. C: Plate colony formation assays of the TMZ-sensitive cells after treatment with different exosomes (30 μg/mL) with or without TMZ treatment. D: FACS analysis was performed to assess the apoptotic rates of TMZ-sensitive cells fed with different exosomal treatments (30 μg/mL) with 200 μM TMZ for 48 h. E: Apoptotic protein cleaved caspase-3 in TMZ-sensitive cells after treatment with different exosomes (30 μg/mL) with 200 μM TMZ for 48 h. In all experiments, data are mean ± SD of triplicate measurements. (Statistical analysis was performed by Student‘s t-test, *P < .05, **P < .01.)
3.4. CAV1 is a direct target of miR-1238
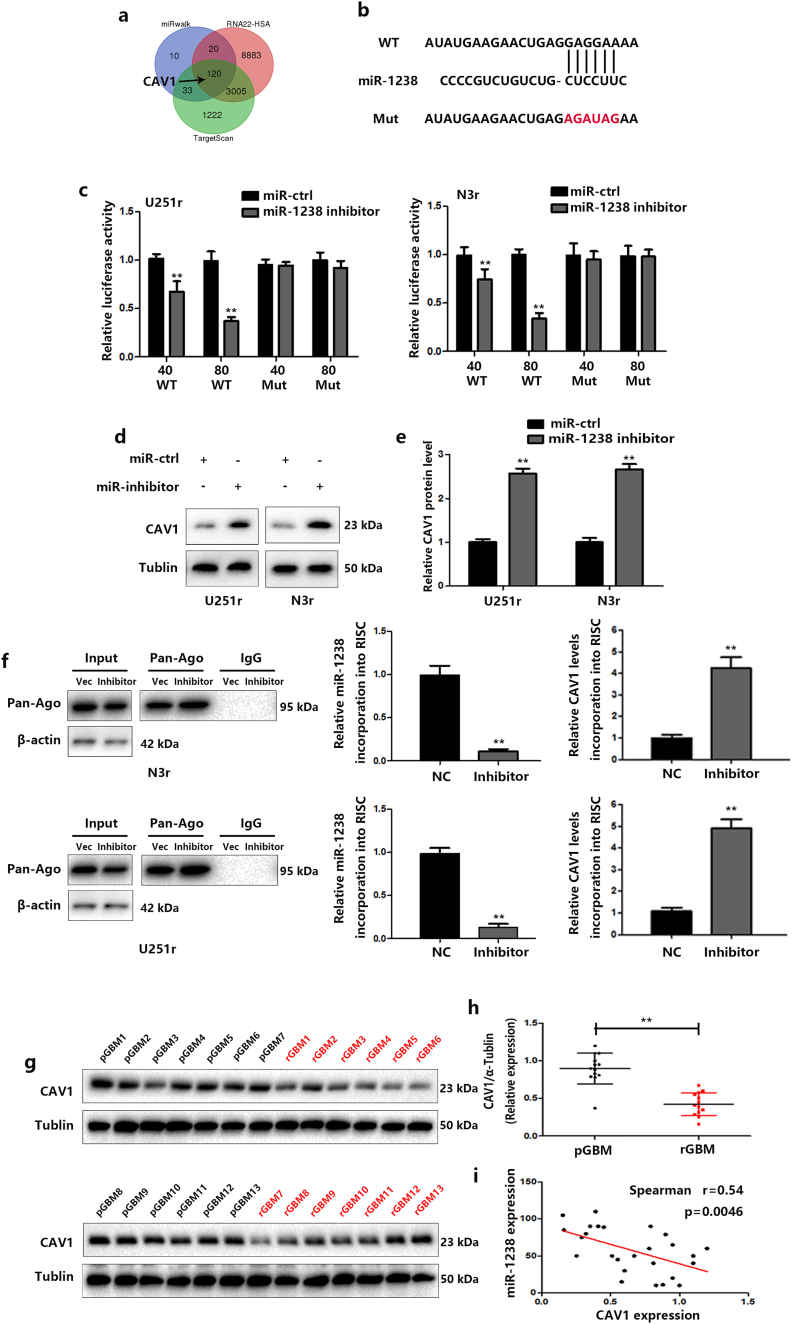
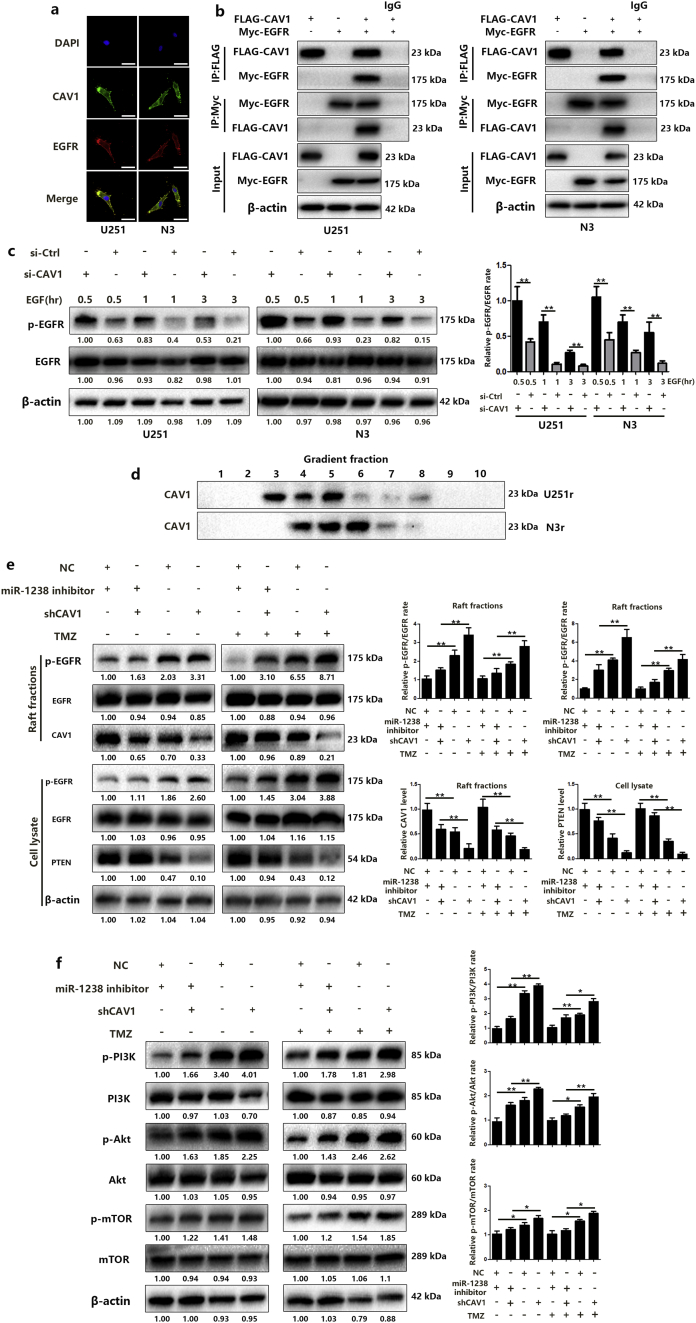
To identify the potential mechanism by which miR-1238 induces chemoresistance, we used TargetScan, miWalk, and RNA22 to predict potential target genes and caveolin-1 (CAV1) was found to be our target (Fig. 5A). CAV1 is the most studied member of the Caveolin family, acting either as a tumour suppressor or as an oncogene [27]. Previous studies indicated that CAV1 might act as a tumour suppressor in GBM cells [28,29]. To verify the functional significance of this finding, we mutated the predicted 3′-UTR of the binding sites of miR-1238 (Fig. 5B) and performed luciferase expression assays. The results indicated that wild-type miR-1238 binding sites of the 3′-UTR of CAV1 led to decreased luciferase activity compared with mutated binding sites (Fig. 5C). Lower expression levels of miR-1238 significantly increased CAV1 protein and mRNA levels in U251r and N3r cells (Fig. 5D and E). Meanwhile, exosomal transfer of miR-1238 also induced a significant decline in CAV1 level in recipient cells (Fig. S3). To further verify the role of CAV1 as a direct target of miR-1238, we used RNA-ChIP (chromatin immunoprecipitation) analysis to identify the mRNAs that were selectively abundant in the Ago/RISC complex after miR-1238 knockdown. As an internal positive control, miR-1238 incorporation into RISC was significantly decreased in miR1238-down cells (Fig. 5F). Next, we determined the correlation between CAV1 levels and miR-1238 expression levels in the 13 pairs of GBM samples mentioned previously. CAV1 expression in GBM samples were measured by western blot (Fig. 5G and H) and as shown in Fig. 5I, Spearman's correlation analysis demonstrated that CAV1 levels in GBM samples were inversely correlated with miR-1238 expression levels (Spearman's correlation r = −0.54). These data indicated that CAV1 is a direct target of miR-1238.
Fig. 5.
Identification of CAV1 as a direct target of miR-1238. A: Venn diagram displaying potential targets of miR-1238 by three prediction algorithms: TargetScan, miRWalk, and RNA22. B: Wild-type and mutant CAV1 3’-UTR reporter constructs. C: Luciferase/s'-UTR reporter assays were used to validate the directing of the CAV1 3’-UTR by miR-1238. D: Western blot analysis showed that CAV1 expression levels were lower in cells with miR-1238 inhibitor transfection. E: The statistic analysis of CAV1 protein level in cells with miR-1238 inhibitor transfection. F: Left: Immunoprecipitation of the RNA-induced silencing complex (Ago2-RISC) using the Ago2 antibody in TMZ-resistant cells transfected with miR-NC or miR-1238 inhibitor. IgG was employed as a negative control and β-actin was used as an internal control. Middle: RT-qPCR analysis was performed to measure miR-1238 levels incorporated into RISC. U6 RNA was used as an internal control. Right: RT-qPCR analysis was performed to measure the levels of CAV1 mRNA incorporated into RISC. GAPDH was used as an internal control. G and H: Western blot analysis was performed to estimate the levels of CAV1 in pGBM and the corresponding rGBM specimens (n = 13). The fold changes were normalized to α-tublin. I: Pearson's correlation analysis of the relative expression levels of miR-1238 and CAV1. In all experiments, bars represent mean ± SD from three replicates. (Statistical analysis was performed by Student‘s t-test, *P < .05, **P < .01.)
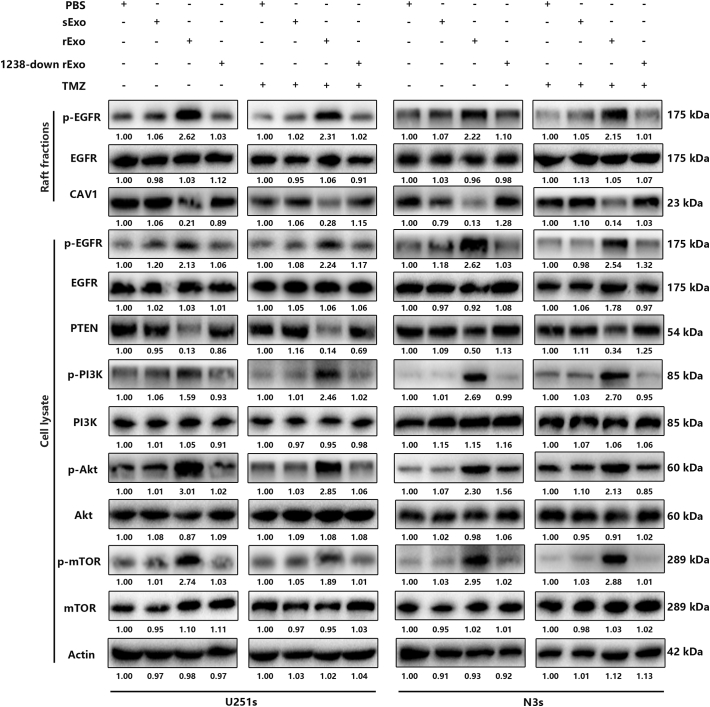
Fig. S3.
Relative expression of CAV1 and activation of the EGFR-PI3K-Akt-mTOR pathway in recipient cells after incubation with resistant exosomes.
Based on our preceding results, which support a critical role for exosomal miR-1238 in mediating TMZ resistance and previous findings that CAV1 could confer chemosensitivity in GBM cells [30]. Furthermore, it has been reported that TMZ can increase CAV1 expression in experimental GBM grown in vivo [31]. Therefore, we hypothesized that miR-1238 could confer resistance to TMZ by modulating CAV1.
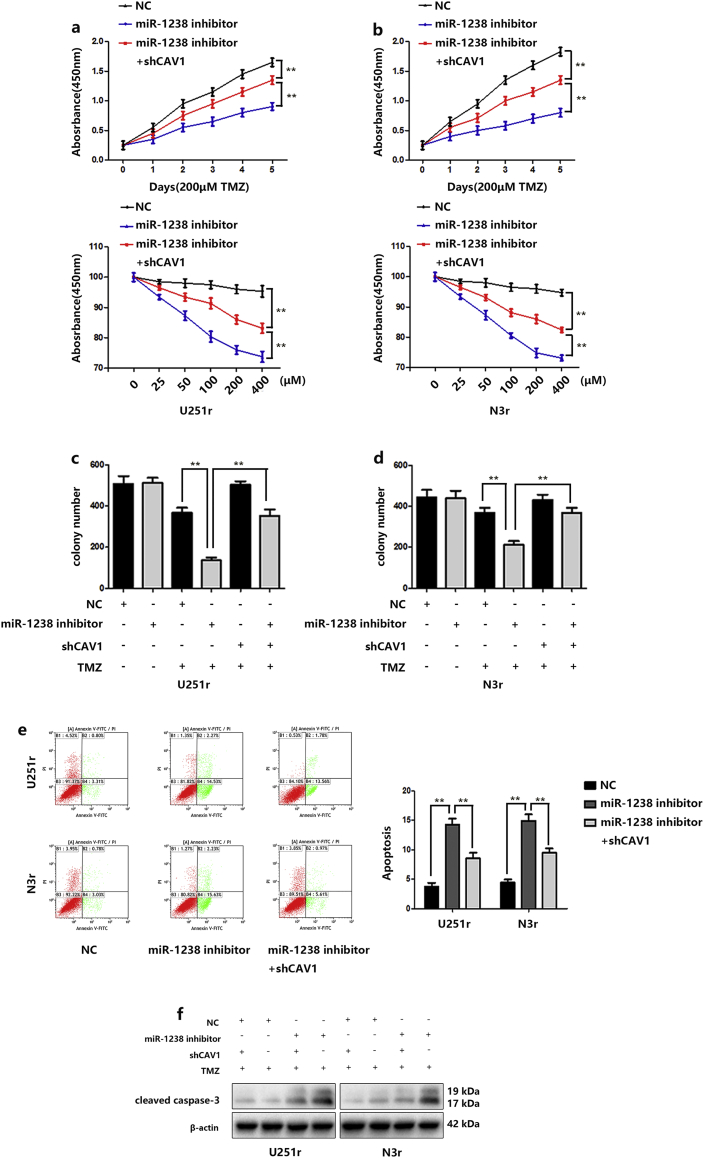
Indeed, reduced expression of miR-1238 sensitized U251r cells to TMZ treatment, as indicated by CCK-8 assays (Fig. 6A and B) and colony formation assays supported these results (Fig. 6C). Fluorescence-activated cell sorting (FACS) analysis was performed to detect cell apoptosis rates. The results showed that miR1238-downregulation induced more apoptotic cells compared with that of control cells in the presence of TMZ treatment (Fig. 6D). Then, the apoptosis-related protein cleaved caspase-3 was measured by western blotting and the results indicated that downregulation of miR-1238 significantly increased the protein level of cleaved caspase-3 in resistant cells (Fig. 6E). Further experiments on chemoresistance were conducted. As indicated in Fig. 6A–E, the increased chemosensitivity induced by the downregulation of miR-1238 was reversed by CAV1 downregulation. These results further stated that CAV1 is a direct target of miR-1238 and miR-1238/CAV1 can regulate TMZ resistance in GBM cells.
Fig. 6.
Verification of CAV1 as a direct target of miR-1238. A and B: Relative cell viability of the miR-NC or miR-1238 inhibitor-transfected TMZ-resistant cell lines (U251r and N3r) with or without sh-CAV1 transfection under 200 μM TMZ treatment for indicated times or indicated concentrations for 48 h. C and D: Plate colony formation assays of the miR-NC or miR-1238 inhibitor-transfected TMZ-resistant cell lines (U251r and N3r) with or without sh-CAV1 transfection under TMZ treatment or not. E: FACS analysis was performed to assess the apoptotic rates of TMZ-resistant cells transduced with miR-NC, miR-1238 inhibitor, or miR-1238 plus shCAV1 treatment with 200 μM TMZ for 48 h. F: Western blot analysis of the expression of the apoptotic protein caspase-3 in TMZ-resistant cells transduced with miR-NC, miR-NC plus shCAV1, miR-1238 inhibitor, or miR-1238 inhibitor plus shCAV1 upon treatment with 200 μM TMZ for 48 h. In all experiments, bars represent mean ± SD from three replicates. (Statistical analysis was performed by Student‘s t-test, *P < .05, **P < .01.)
3.5. MiR-1238/CAV1 signaling modulates TMZ resistance via EGFR pathway
Next, we wanted to determine the mechanism by which miR-1238/CAV1 contributes to TMZ resistance in GBM cells. CAV1 has been demonstrated to interact with EGFR and stabilize the receptor kinase in an inactive conformation thus inhibiting the autophosphorylation of EGFR [32]. Different studies have revealed an important role for CAV1 in EGFR-induced effects on different cells [33,34]. Therefore, we examined the effects of miR-1238/CAV1 on EGFR activation in TMZ resistant GBM cells. First, the colocalization of EGFR with CAV1 in U251r and N3r cells were examined by confocal microscopy (Fig. 7A). To further confirm the interaction of CAV1 with EGFR, co-immunoprecipitations were performed in U251 and N3 cells transfected with FLA-CAV1 and Myc-EGFR. After immunoprecipitating CAV1, we detected the associated EGFR and vice versa (Fig. 7B). Significant differences were observed in the abundance of phosphorylated EGFR (p-EGFR) among EGF-treated U251 and N3 cells with si-CAV1 or si-Ctrl plasmids transfected (Fig. 7C). Together, these results indicated that the activation of EGFR was suppressed when interacted with CAV1 and CAV1 is a negative regulator of EGFR activation. To measure the function of CAV1-EGFR interaction on TMZ resistance, lipid raft fractions (fractions 3–5) were collected and pooled (Fig. 7D). As shown in Fig. 7E and Fig. S2C, miR-1238 decrease in U251r and N3r cells induced a dramatic decrease in p-EGFR to EGFR levels and increased PTEN, a famous tumour suppressor that is mutated in a large number of GBMs and which can inhibit the development of tumours by antagonistic tyrosine kinase and other phosphorylase activity [35] levels with TMZ treatment. It is well-known that the activation of EGFR contributes to the activation of multiple downstream signal transduction pathways such as the PI3K-AKT-mTOR pathway, which has been verified to contribute to chemoresistance in different tumours, including GBM [36,37]. To illustrate the effect of CAV1 on the activation of the PI3K-AKT-mTOR pathway, the phosphorylation of EGFR, PI3K, AKT, and mTOR was analysed. The results showed that downregulation of miR-1238 suppressed the activation of the PI3K-Akt-mTOR signal transduction pathway, which could be rescued by CAV1 knockdown (Fig. 7F and Fig. S2D). In conclusion, down-regulation of CAV1 induced by increased miR-1238 activated the PI3K-AKT-mTOR pathway in TMZ resistant GBM cells. At the same time, we evaluated the activation of this pathway in TMZ-sensitive cells after incubation with resistant exosomes. The results suggest that exosomal transfer of miR-1238 from resistant cells to sensitive ones could confer chemoresistance via activating the EGFR-PI3K-AKT-mTOR pathway (Fig. S3).
Fig. 7.
MiR-1238/CAV1 signaling modulates TMZ resistance via EGFR pathway. A: Representative confocal images of CAV1 (green) and EGFR (red) colocalized in U251 and N3 cells (scale bars = 10 μm). B: Co-IP was performed using lysates from U251 and N3 cells transfected with FLAG-CAV1, Myc-EGFR, or vector control. Western blotting was performed with the indicated antibodies. C: U251 and N3 cells transfected with si-CAV1 or control were treated with EGF (50 ng/mL) for the times shown prior to harvest, lysis, and analysis by western blot using the antibodies indicated. D: Subcellular fractionation of U251r and N3r cells created 10 fractions and each was tested for the presence of CAV1 by western blotting. Fractions 3, 4, and 5 from U251r and 4, 5, and 6 from N3r were pooled and considered as caveolae membranes. E: Western blot analysis for phospho-EGFR, EGFR, CAV1, and PTEN in collected fractions or cell lysate of U251r cells. F: Western blot analysis was performed to estimate the activation of the PI3K-Akt-mTOR pathway in U251r cells transfected with miR-1238 inhibitor or shCAV1 with TMZ treatment or not. All results are representative of at least three independent experiments. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6. Exosomal miR-1238 conferred TMZ resistance to GBM cells in vivo
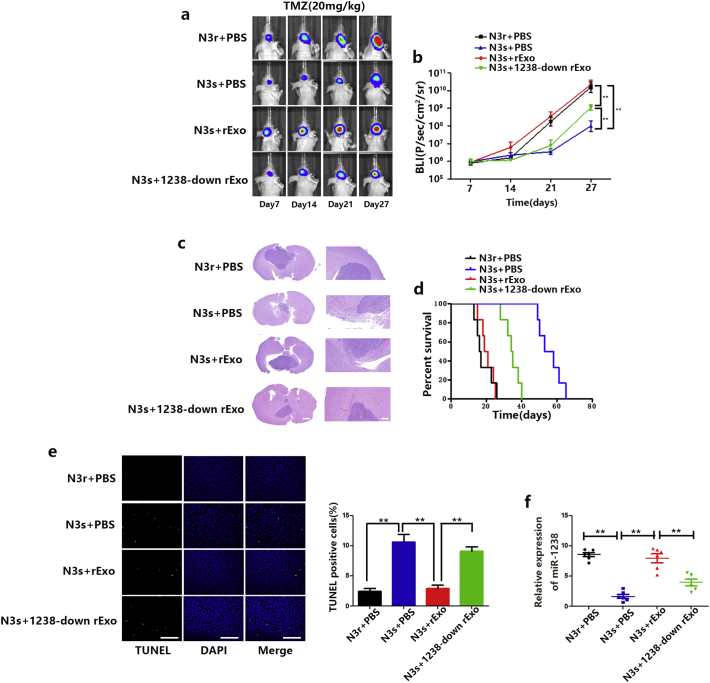
We next investigated whether exosomal miR-1238 regulated its target genes and TMZ resistance in vivo. Prior to orthotopic injection in mice, N3s/luciferase cells were pre-incubated with N3r exosomes for 6 days. Then, in the presence of TMZ, tumour volume in mice was analysed by bioluminescence imaging. Consistent with the in vitro experiments, resistant exosomes increased TMZ resistance in mice and decreased miR-1238 expression levels negated this effect (Fig. 8A–C). Survival analysis of the three groups also yielded statistically significant results (Fig. 8D). TUNEL staining was significantly increased in tumours pre-treated with resistant exosomes and downregulation of exosomal miR-1238 defeated the function of resistant exosomes (Fig. 8E). Moreover, we retrieved blood samples from all mice 27 days after tumour formation. RT-qPCR analysis of exosomal miR-1238 extracted from the sera of mice indicated that resistant mice have higher levels of miR-1238 (Fig. 8F). These results confirmed our hypothesis that exosomal miR-1238 in serum may provide a potential indicator of TMZ resistance.
Fig. 8.
Exosomal miR-1238 conferred TMZ resistance to GBM cells in vivo. A: Representative pseudocolour bioluminescence images of orthotopic tumours derived from N3 s cells fed with resistant exosomes or 1238-down exosomes after treatment with vehicle or TMZ on the days indicated (n = 10 per group). B: Bioluminescence was quantified in tumours from four groups. C: Representative H&E staining for tumour cytostructures. Scale bars = 100 mm (left panels) and 100 mm (bottom panels). D: Survival curves of mice harbouring intracranial xenografts. E: TUNEL staining of N3 s cells injected into mice treated with TMZ plus exosomal treatment (scale bars = 200 μm). F: Relative expression of miR-1238 in serum samples was measured by RT-qPCR. In all experiments, bars represent mean ± SD from three replicates. (Statistical analysis was performed by Student‘s t-test, *P < .05, **P < .01.)
4. Discussion
Previous studies have demonstrated that processes such as cell proliferation, apoptosis, survival, and metastasis are regulated by miRNAs functioning as either oncogenes or tumour suppressor genes [38,39]. And, targeting aberrantly expressed miRNAs in tumours is a promising therapeutic direction [40]. Our group has been devoted to the investigation of miRNAs for decades to find more effient therapeutic targets for treatment of GBM. For example, we firstly identified the role of miR-181a and miR-181b as tumour suppressors in human glioma cells [41]. In addition, we reported that the MIR155HG/miR-155 axis plays a critical role in GBM progression and NSC141562, an MIR155/miR-155 axis inhibitor, may provide a candidate anti-GBM drug [42]. In this study, we focused on miR-1238, which is a newly discovered miRNA that lacks adequate attention in the literature. Zhang HT et al. reported that miR-1238 functions as a tumour suppressor via inhibiting cell proliferation by targeting LHX2 in non-small cell lung cancer [43]. On the contrary, our previous results indicated that a higher level of miR-1238 is correlated with TMZ resistance in GBM patients. Our results also showed that downregulation of TMZ resistant cells significantly decreased the apoptosis rate with TMZ treatment, thus conferring chemoresistance to GBM cells. EGFR has been shown to play an important role in TMZ resistance [44,45]. EGFR kinase inhibitor erlotinib plus TMZ has been shown to improve the overall survival of glioblastoma patients compared to TMZ alone [46]. In our study, confocal microscopy and co-immunoprecipitation indicated that CAV1 and EGFR are mutually interactive. Loss of CAV1 binding induced the activation of the EGFR-PI3K-Akt-mTOR pathway, leading to enhanced anti-apoptosis of GBM cells under TMZ treatment. To the best of our knowledge, this is the first study of miR-1238 expression and biological function in GBM cells. The miR-1238/CAV1 axis may provide a potential target for future anti-tumour drugs.
Exosomes are a novel class of extracellular vesicles that have gained enormous attention lately as facilitators of the progression of various tumours [47]. Temozolomide resistance in glioblastoma stem cells can be reflected by extracellular vesicles [48]. In this study, we found that circulating exosomes from GBM patients contain higher levels of miR-1238 than healthy circulating exosomes. In addition, exosomal miR-1238 transferred to recipient cells induced the upregulation of miR-1238 levels in TMZ sensitive cells. This process may cause partial resistance in TMZ sensitive cells in vitro and in vivo via the intensive activation of the EGFR-PI3K-Akt-mTOR pathway. Thus we assume that exosomal miR-1238 in the serum of GBM patients could function as a reminder for doctors to adopt efficacious chemotherapeutic protocols. However, more clinical data in addition to the development of more convenient and rapid methods for the detection of circulating miRNAs are required.
5. Conclusion
In conclusion, our results demonstrate the importance of miR-1238/CAV1 signaling in acquired TMZ-resistance in GBM cells and that exosomes act as carriers of miR-1238. Moreover, circulating miR-1238 may be used as a clinical biomarker for TMZ response. Taken together, these findings reveal a novel molecular mechanism of TMZ resistance in GBM.
The following are the supplementary data related to this article.
Clinicopathological features of 26 GBM patients and treatment characteristic
Acknowledgments
Acknowledgments
We thank the other members of our laboratory.
Funding sources
This study was supported by the National Natural Science Foundation of China (No. 81402056, 81472362, and 81772951) and the National High Technology Research and Development Program of China (863) (No. 2012AA02A508). And the funders are not involved in study design, data collection, data analysis, interpretation, writing of the report.
Declaration of interests
There are no relevant conflicts of interest.
Author contributions
Jianxing Yin, Ailiang Zeng, and Zhuoran Zhang performed the experiments and analysed the data. Zhumei Shi, Wei Yan, and Yongping You provided clinical samples and approved the manuscript. All authors have read and approved the manuscript, and ensure that it is the case.
References
- 1.Stupp R., Hegi M.E., Mason W.P. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Kotliarova S., Fine H.A. SnapShot: glioblastoma multiforme. Cancer Cell. 2012;21(5):710–e1. doi: 10.1016/j.ccr.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 3.Lefranc F., Sadeghi N., Camby I., Metens T., Dewitte O., Kiss R. Present and potential future issues in glioblastoma treatment. Expert Rev Anticancer Ther. 2006;6(5):719–732. doi: 10.1586/14737140.6.5.719. [DOI] [PubMed] [Google Scholar]
- 4.Saito T., Sugiyama K., Takeshima Y. Prognostic implications of the subcellular localization of survivin in glioblastomas treated with radiotherapy plus concomitant and adjuvant temozolomide. J Neurosurg. 2017:1–6. doi: 10.3171/2016.11.JNS162326. [DOI] [PubMed] [Google Scholar]
- 5.Bei Y, Das S, Rodosthenous RS, et al. Extracellular vesicles in cardiovascular Theranostics. Theranostics 2017; 7(17): 4168–82. [DOI] [PMC free article] [PubMed]
- 6.Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azmi A.S., Bao B., Sarkar F.H. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32(3–4):623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng A.L., Yan W., Liu Y.W. Tumour exosomes from cells harbouring PTPRZ1-MET fusion contribute to a malignant phenotype and temozolomide chemoresistance in glioblastoma. Oncogene. 2017;36(38):5369–5381. doi: 10.1038/onc.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janga S.C., Vallabhaneni S. MicroRNAs as post-transcriptional machines and their interplay with cellular networks. Adv Exp Med Biol. 2011;722:59–74. doi: 10.1007/978-1-4614-0332-6_4. [DOI] [PubMed] [Google Scholar]
- 11.Li S., Zeng A., Hu Q., Yan W., Liu Y., You Y. miR-423-5p contributes to a malignant phenotype and temozolomide chemoresistance in glioblastomas. Neuro Oncol. 2017;19(1):55–65. doi: 10.1093/neuonc/now129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L., Li C., Wang S. Exosomes derived from hypoxic Oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a Prometastatic phenotype. Cancer Res. 2016;76(7):1770–1780. doi: 10.1158/0008-5472.CAN-15-1625. [DOI] [PubMed] [Google Scholar]
- 13.Fabbri M., Paone A., Calore F. MicroRNAs bind to toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109(31):E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X. A PCR-based platform for microRNA expression profiling studies. RNA. 2009;15(4):716–723. doi: 10.1261/rna.1460509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian X., Yu J., Yin Y. MicroRNA-143 inhibits tumor growth and angiogenesis and sensitizes chemosensitivity to oxaliplatin in colorectal cancers. Cell Cycle. 2013;12(9):1385–1394. doi: 10.4161/cc.24477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Z., Chen Q., Li C. MiR-124 governs glioma growth and angiogenesis and enhances chemosensitivity by targeting R-Ras and N-Ras. Neuro Oncol. 2014;16(10):1341–1353. doi: 10.1093/neuonc/nou084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y.Y., Sun G., Luo H. MiR-21 modulates hTERT through a STAT3-dependent manner on glioblastoma cell growth. CNS Neurosci Ther. 2012;18(9):722–728. doi: 10.1111/j.1755-5949.2012.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo H., Chen Z., Wang S. C-Myc-miR-29c-REV3L signalling pathway drives the acquisition of temozolomide resistance in glioblastoma. Brain. 2015;138:3654–3672. doi: 10.1093/brain/awv287. Pt 12. [DOI] [PubMed] [Google Scholar]
- 19.Witwer K.W., Buzas E.I., Bemis L.T. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han D., Wei W., Chen X. NF-kappaB/RelA-PKM2 mediates inhibition of glycolysis by fenofibrate in glioblastoma cells. Oncotarget. 2015;6(28):26119–26128. doi: 10.18632/oncotarget.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan W., Liu Y., Yang P., Wang Z., You Y., Jiang T. MicroRNA profiling of Chinese primary glioblastoma reveals a temozolomide-chemoresistant subtype. Oncotarget. 2015;6(13):11676–11682. doi: 10.18632/oncotarget.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melo S.A., Sugimoto H., O'Connell J.T. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26(5):707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peinado H., Aleckovic M., Lavotshkin S. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 25.Au Yeung C.L., Co N.N., Tsuruga T. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7 doi: 10.1038/ncomms11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 27.Quest A.F., Lobos-Gonzalez L., Nunez S. The caveolin-1 connection to cell death and survival. Curr Mol Med. 2013;13(2):266–281. doi: 10.2174/156652413804810745. [DOI] [PubMed] [Google Scholar]
- 28.Cosset E.C., Godet J., Entz-Werle N. Involvement of the TGFbeta pathway in the regulation of alpha5 beta1 integrins by caveolin-1 in human glioblastoma. Int J Cancer. 2012;131(3):601–611. doi: 10.1002/ijc.26415. [DOI] [PubMed] [Google Scholar]
- 29.Cameron P.L., Liu C., Smart D.K., Hantus S.T., Fick J.R., Cameron R.S. Caveolin-1 expression is maintained in rat and human astroglioma cell lines. Glia. 2002;37(3):275–290. doi: 10.1002/glia.10036. [DOI] [PubMed] [Google Scholar]
- 30.Quann K., Gonzales D.M., Mercier I. Caveolin-1 is a negative regulator of tumor growth in glioblastoma and modulates chemosensitivity to temozolomide. Cell Cycle. 2013;12(10):1510–1520. doi: 10.4161/cc.24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruyère C., Abeloos L., Lamoral-Theys D. Temozolomide modifies Caveolin-1 expression in experimental malignant Gliomas in vitro and in vivo. Translational Oncology. 2011;4(2):92–100. doi: 10.1593/tlo.10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Couet J., Sargiacomo M., Lisanti M.P. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997;272(48):30429–30438. doi: 10.1074/jbc.272.48.30429. [DOI] [PubMed] [Google Scholar]
- 33.Abulrob A., Giuseppin S., Andrade M.F., McDermid A., Moreno M., Stanimirovic D. Interactions of EGFR and caveolin-1 in human glioblastoma cells: evidence that tyrosine phosphorylation regulates EGFR association with caveolae. Oncogene. 2004;23(41):6967–6979. doi: 10.1038/sj.onc.1207911. [DOI] [PubMed] [Google Scholar]
- 34.Parat M.O., Riggins G.J. Caveolin-1, caveolae, and glioblastoma. Neuro Oncol. 2012;14(6):679–688. doi: 10.1093/neuonc/nos079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng H., Ying H., Yan H. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455(7216):1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slomovitz B.M., Coleman R.L. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res. 2012;18(21):5856–5864. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
- 37.Wu S.H., Bi J.F., Cloughesy T., Cavenee W.K., Mischel P.S. Emerging function of mTORC2 as a core regulator in glioblastoma: metabolic reprogramming and drug resistance. Cancer Biol Med. 2014;11(4):255–263. doi: 10.7497/j.issn.2095-3941.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wurdinger T., Tannous B.A., Saydam O. miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell. 2008;14(5):382–393. doi: 10.1016/j.ccr.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu J., Sun T., Wang H. MiR-215 is induced post-transcriptionally via HIF-Drosha complex and mediates Glioma-initiating cell adaptation to hypoxia by targeting KDM1B. Cancer Cell. 2016;29(1):49–60. doi: 10.1016/j.ccell.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu X., Wang Y., Yu T. Blocking MIR155HG/miR-155 axis inhibits mesenchymal transition in glioma. Neuro Oncol. 2017;19(9):1195–1205. doi: 10.1093/neuonc/nox017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi X., Zhan L., Xiao C. miR-1238 inhibits cell proliferation by targeting LHX2 in non-small cell lung cancer. Oncotarget. 2015;6(22):19043–19054. doi: 10.18632/oncotarget.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munoz J.L., Rodriguez-Cruz V., Greco S.J., Ramkissoon S.H., Ligon K.L., Rameshwar P. Temozolomide resistance in glioblastoma cells occurs partly through epidermal growth factor receptor-mediated induction of connexin 43. Cell Death Dis. 2014;5:e1145. doi: 10.1038/cddis.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeom S.Y., Nam D.H., Park C. RRAD promotes EGFR-mediated STAT3 activation and induces temozolomide resistance of malignant glioblastoma. Mol Cancer Ther. 2014;13(12):3049–3061. doi: 10.1158/1535-7163.MCT-14-0244. [DOI] [PubMed] [Google Scholar]
- 44.Prados M.D., Chang S.M., Butowski N. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27(4):579–584. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Becker A., Thakur B.K., Weiss J.M., Kim H.S., Peinado H., Lyden D. Extracellular vesicles in Cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30(6):836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garnier D., Meehan B., Kislinger T. Divergent evolution of temozolomide resistance in glioblastoma stem cells is reflected in extracellular vesicles and coupled with radiosensitization. Neuro Oncol. 2018;20(2):236–248. doi: 10.1093/neuonc/nox142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garofalo M., Croce C.M. microRNAs: master regulators as potential therapeutics in cancer. Annu Rev Pharmacol Toxicol. 2011;51:25–43. doi: 10.1146/annurev-pharmtox-010510-100517. [DOI] [PubMed] [Google Scholar]
- 48.Shi L., Cheng Z., Zhang J. Hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res. 2008;1236:185–193. doi: 10.1016/j.brainres.2008.07.085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinicopathological features of 26 GBM patients and treatment characteristic