Abstract Abstract
The branchial-attaching cymothoid genus, Elthusa Schioedte & Meinert, 1884 is a genus with a worldwide distribution of 36 species, including the three species described here. Elthusaraynaudii (Milne Edwards, 1840) is the only species that has been described from southern Africa. All South African material held at the National Museum of Natural History, Paris, France (MNHN) and the Iziko South African Museum, Cape Town (SAMC) identified as, or appearing to belong to, Elthusa was examined. Four species were identified, Elthusaraynaudii and three species that proved to be undescribed. Elthusaxenasp. n. can be distinguished by an evenly rounded pereonite 1 anterior margin, a roughly rectangular pleotelson, and narrowly rounded uropod apices that extend to more than half the length of the pleotelson. Elthusaacutinasasp. n. is identified by the produced and narrowly rounded cephalon anterior margin, acute uropods that are shorter than half the length of the pleotelson, and pereonite 1 anterior margin with medial projection. Elthusarotundasp. n. is characterised by the round body shape, broadly rounded uropod apices, and protrusions on the proximal and lateral margins of the merus and carpus of pereopod 7. A key to the South African Elthusa species is provided, together with a table summarising the hosts and localities of the 33 previously known species of Elthusa.
Keywords: Alexander Bay, Atlantic Ocean, Clinus superciliosus , Elthusa raynaudii , fish parasites, Indian Ocean, taxonomy
Introduction
Elthusa Schioedte & Meinert, 1884 is a branchial cavity-inhabiting cymothoid genus that was described as a monotypic genus for Elthusaemarginata (Bleeker, 1857). Elthusa was subsequently largely overlooked until Bruce (1990) provided a new diagnosis based on one of Bleeker’s (1857) syntypes and the Australian species of the genus. Most species of Elthusa were originally described and placed within the genus Livoneca before their revision and redescription by Bruce (1990).
Currently, there are 33 known and accepted Elthusa species (Öktener et al. 2018a). Elthusa is one of the more speciose genera within the family Cymothoidae Leach, 1818, however many species of Elthusa still need to be studied and redescribed due to their original descriptions being inadequate, lacking morphological detail and illustrations. The high morphological intraspecific variability that exists within this genus (Hadfield et al. 2017) has also contributed, in some cases, to misidentifications and confusion regarding the placement of species.
Most species of Elthusa inhabit the branchial cavities of their fish hosts (Smit et al. 2014), with the exception of two species. Elthusaneocytta (Avdeev, 1975) ovigerous females have been recorded from the buccal cavity of the spiky oreo, Neocyttusrhomboidalis Gilchrist, 1906 (see Stephenson 1987), and Elthusasplendida (Sadowsky & Moreira, 1981) has been described from the buccal cavity of the spiny dogfish Squaluscubensis Rivero, 1936 (see Sadowsky and Moreira 1981).
Elthusa is considered to be cosmopolitan, except for polar waters (Bruce 1990, Bruce et al. 2002, Rocha-Ramírez et al. 2005, Hadfield et al. 2017), and is predominantly recorded from the Indo-West Pacific (see Bruce 1990, Trilles and Justine 2006) with only occasional records of species from the Eastern Pacific (Brusca 1978, Espinosa-Pérez and Hendrickx 2001), the Atlantic (Kensley and Brusca 2001) and the Mediterranean (Trilles and Justine 2006, Öktener et al. 2018a). Elthusaraynaudii (Milne Edwards, 1840) is the only species of Elthusa that has been described from sub-Saharan Africa. The lack of species records is most likely due to the lack of studying cymothoid isopods from this region and is not a true representation of the biodiversity of this genus. This paper forms part of a detailed study on the cymothoids from sub-Saharan Africa and confirms this postulation with the identification of three new species from the region.
Materials and methods
Twenty-seven specimens of Elthusa were examined. Material loaned from the National Museum of Natural History, Paris, France (MNHN) and the Iziko South African Museum, Cape Town (SAMC) was included in the examination. These specimens were collected as early as 1840 (MNHN) and 1960 (SAMC). Non-museum material was collected during 1993 in the intertidal zone of Alexander Bay, as well as from deep-sea trawlers during January 1999 and April 2003 off the south coast (RV Africana), and during February 2010 off the west coast of South Africa (RV Dr Fridtjof Nansen).
Specimens were identified by illustrating all body parts and appendages using a Nikon SMZ1500 Stereo Microscope and a Nikon Eclipse80i Compound Microscope, both equipped with drawing tubes. The position of specimens and dissected parts were manipulated to obtain the most accurate direct and complete view in order to minimise errors in illustrated ratios of segments. Material loaned from national museums was not dissected. Species descriptions were made with the aid of the taxonomy software package DELTA (Descriptive Language for Taxonomy) (see Coleman et al. 2010), following a general Cymothoidae character data set originally developed by Hadfield et al. (2010) and recently updated for other genera (Hadfield et al. 2013, 2016b). Ratios and measurements for the descriptions were made using the maximum values at the middle of the specific measured segment, and all proportional measurements were rounded to one decimal place. Higher-level classification follows that of Brandt and Poore (2003). Host authorities are not included in the text or references; host nomenclature and distribution were sourced from FishBase (see Froese and Pauly 2018) and Catalog of Fishes (see Eschmeyer 2018).
Abbreviations:
DELTA Descriptive Language for Taxonomy
MNHN National Museum of Natural History, Paris, France
NWU North-West University, Potchefstroom Campus
OH other hosts
OL other localities
RV research vessel
SAMC Iziko South African Museum
Syn synonym
TH type host
TL total length
TLoc type locality
W width
Taxonomy
Suborder Cymothoida Wägele, 1989
Superfamily Cymothooidea Leach, 1814
Family Cymothoidae Leach, 1814
Genus. Elthusa
Schioedte & Meinert, 1884
Elthusa : Schioedte and Meinert 1884: 337; Bruce 1990: 254; Trilles and Randall 2011: 453; Hadfield et al. 2017: 3.
Type species.
Livonecaemarginata Bleeker, 1857; by monotypy (Schioedte and Meinert 1884). The original number of type specimens that were available to Bleeker (1857) is unknown. A single female syntype, examined by Bleeker (1857), is deposited at the Naturalis Biodiversity Center (previously the Rijksmuseum von Natuurlijke Historie), Leiden (RMNH.CRUS.I.66). Another type specimen from the latter museum has been lost. The specimen examined by Schioedte and Meinert (1884) is held at the Natural History Museum in Paris (MNHN241) (Trilles 1976).
Remarks.
Species from Elthusa can be distinguished from other genera by having a weakly vaulted dorsum with a wide pleon; antennulae that are shorter than, or subequal in length to antennae, bases not in contact; a cephalon posterior margin that is not trilobed; and lamellar pleopods. Other diagnostic characters include a slender maxilliped palp article 3, with setae present; as well as pereopods with relatively short dactyli (see Bruce 1990 for a revised diagnosis of the genus).
Trilles and Randall (2011) redescribed the type species for the genus, E.emarginata. This redescription provided a more detailed description and more accurate drawings of the species that had previously not been possible due to the fragility of the syntype. It also allows for a diagnosis and description of the genus based on the type material. However, Trilles and Randall (2011) designated one of the examined specimens [material deposited by Schioedte and Meinert (1884) into the Natural History Museum in Paris, MNHN No. 241] as the lectotype for the species. This does not follow the ICZN rules (Article 74.1) for lectotype designation as there is extant type material (RMNH.CRUS.I.66). Furthermore, no figures were provided of the designated lectotype material to ensure recognition of the specimen designated (ICZN Article 74.7.2). As such this lectotype designation is invalid and set aside (ICZN Article 74.2).
The original description by Bleeker (1857) did not specify any host species, genus or even family (“the skin of various species of fish”) and Trilles and Randall’s (2011) redescription is not supported by or based on specimens being from the same host species or genus. Trilles and Randall (2011) did not examine Bleeker’s remaining syntype, and comparison of the two accounts suggest that there are some differences between the Bleeker (1857) figures and those of Trilles and Randall (2011); most notably being the shape of the cephalon, which is truncate or subtruncate in the syntype but anteriorly concave in Trilles and Randall’s redescription; and the pleotelson in the syntype is broadly rounded (“semi-circular”) while distally narrowed in Trilles and Randall’s redescription. Trilles and Randall (2011) made no direct reference to Bleeker’s (1857) description and did not comment on any perceived character difference. These differences suggest that direct comparison to Bleeker’s syntype is needed to confirm conspecificity of the specimens identified by Trilles and Randall (2011) as E.emarginata.
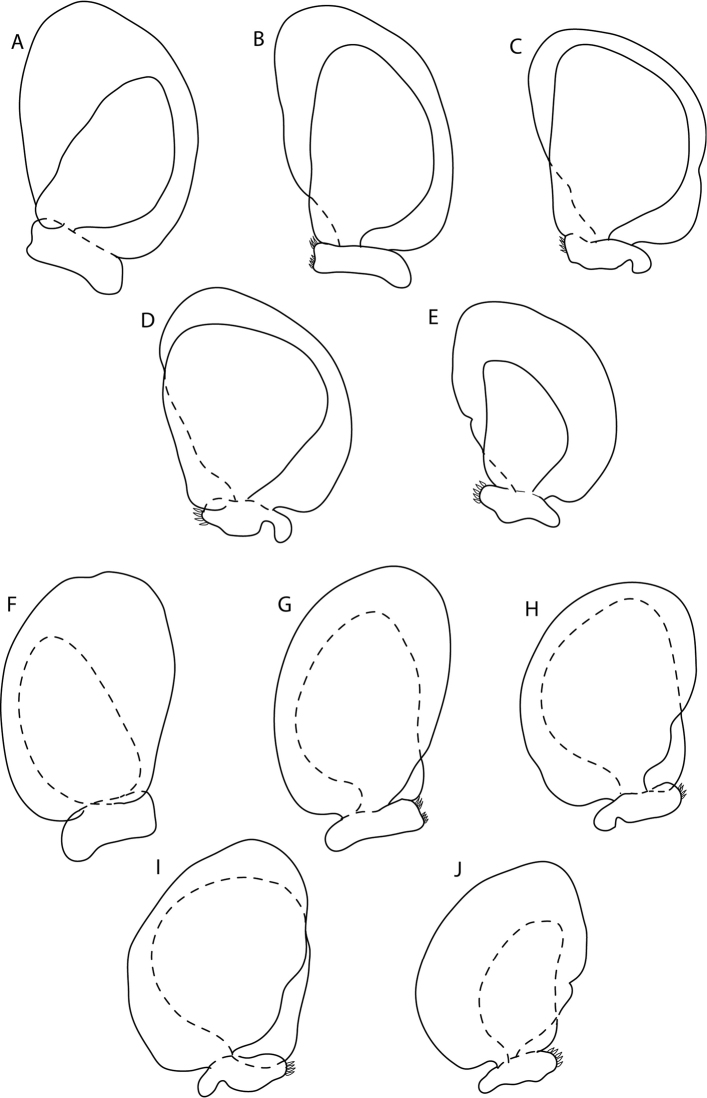
Key to the species of Elthusa from southern Africa
| 1 | Pleonite 5 lateral margins visible; uropods half the length of pleotelson or longer; pereonite 1 anterior margin without medial projections; pereonite 1 anterolateral margin extending to medial region of the eye | 2 |
| – | Pleonite 5 lateral margins largely concealed by pleonite 4; uropods short, less than half the length of pleotelson; pereonite 1 anterior margin medially pointed; pereonite 1 anterolateral margin extending to posterior margin of the eye | Elthusaacutinasa sp. n. |
| 2 | Cephalon with rounded anterior margin; uropod rami apices broadly rounded; pleotelson evenly rounded | 3 |
| – | Cephalon anterior margin narrowly rounded; uropod rami apices narrowly rounded; pleotelson sub-quadrate | Elthusaxena sp. n. |
| 3 | Pereon 1.2–1.4 times as long as wide; cephalon anterior margin blunt; pereopod 7 without bulbous protrusions; uropods more than half the length of pleotelson; pleonites subequal in length | Elthusa raynaudii |
| – | Pereon as long as wide; cephalon anterior margin concave; pereopod 7 merus and carpus with bulbous protrusions; uropods half the length of pleotelson; pleonite 5 longest | Elthusarotunda sp. n. |
Elthusa raynaudii
(Milne Edwards, 1840)
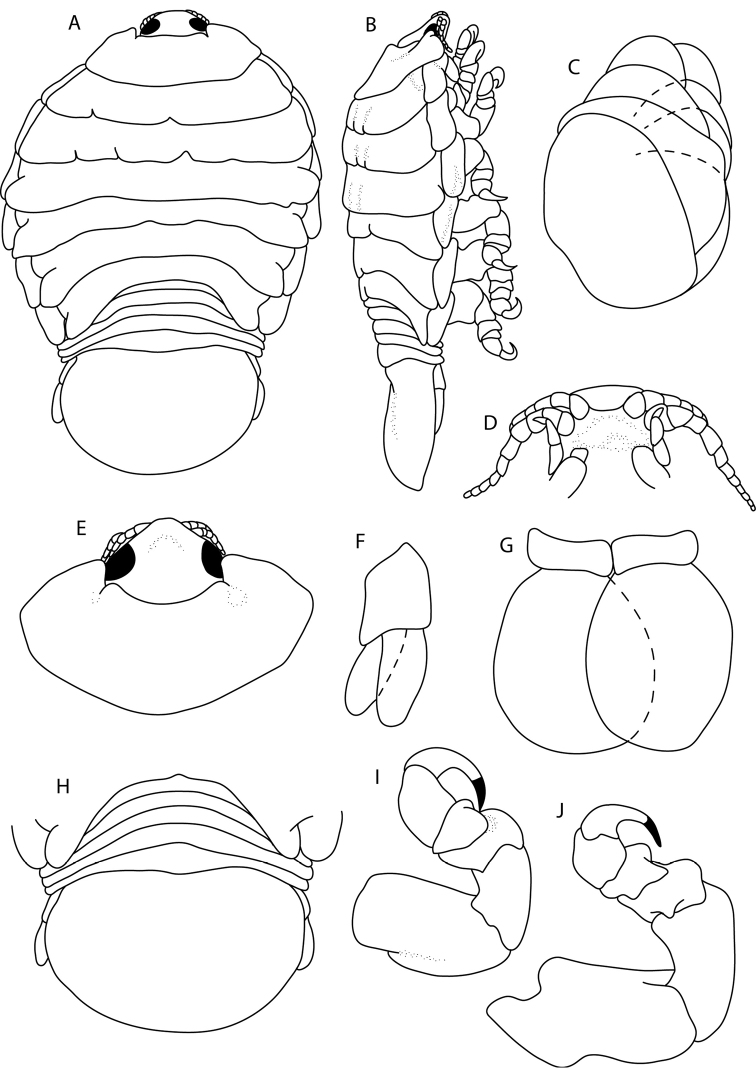
Figure 1.
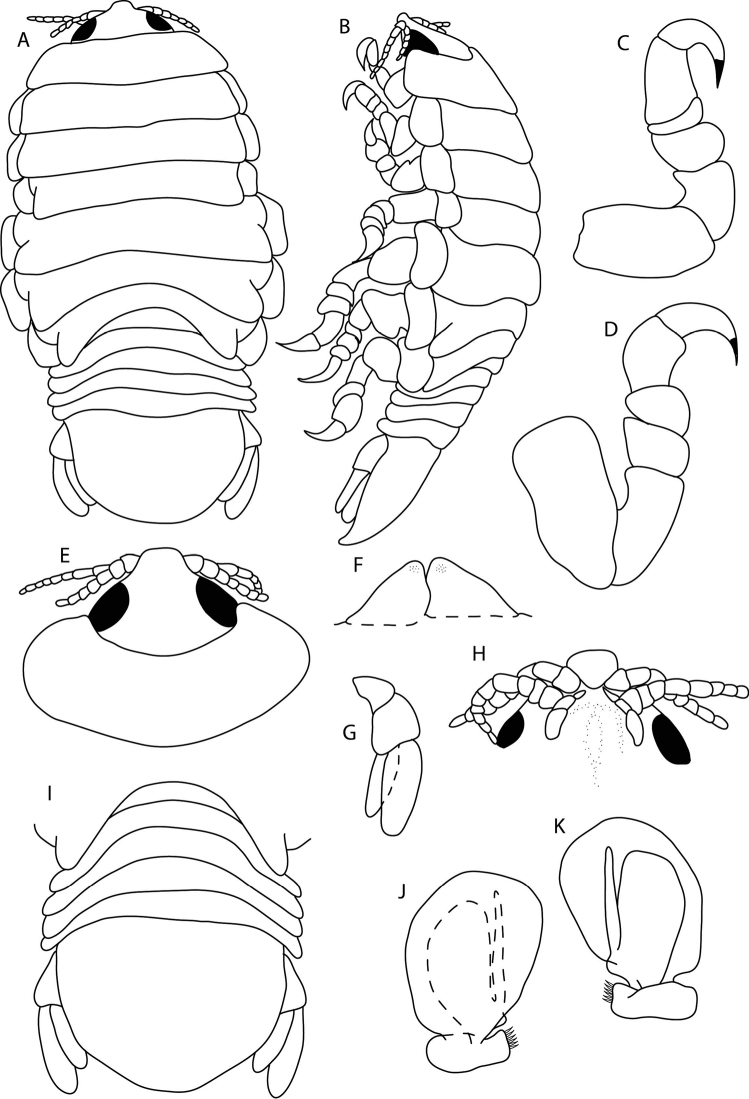
Elthusaraynaudii (Milne Edwards, 1840) ♀ (ovigerous, 20.0 mm TL, 12.0 mm W) (SAMC-A089957) from Dr Fridtjof Nansen research vessel A dorsal body B lateral body C oostegites D dorsal view of cephalon and pereonite 1 E uropod F ventral cephalon G pleopod 1 H dorsal view of pleon I pereopod 1 J pereopod 7.
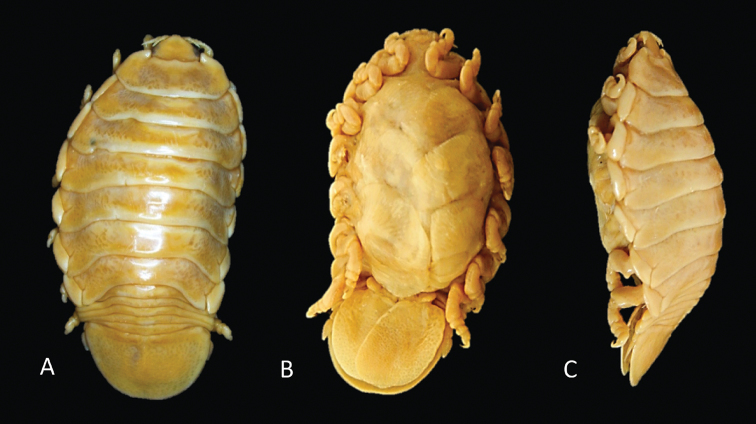
Figure 2.
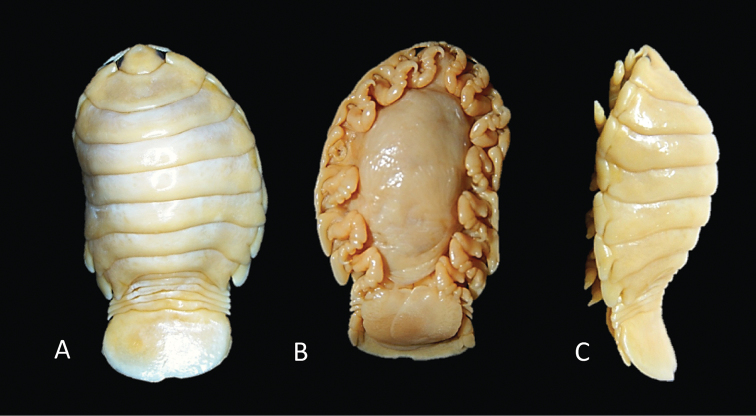
Photos of Elthusaraynaudii (Milne Edwards, 1840) ♀ (ovigerous, 26.0 mm TL, 15.0 mm W) (SAMC-A47881) from Dr Fridtjof Nansen research vessel A dorsal view B ventral view C lateral view.
Figure 3.
Photos of syntype material Livonecaraynaudii Milne Edwards, 1840 ♀ (ovigerous, 26.7 mm TL, 14.1 mm W) (MNHN–IU–2016–9885) A dorsal view B ventral view C lateral view.
Table 1.
Interspecific character states between Elthusaraynaudii (Milne Edwards, 1840), Elthusaxena sp. n., Elthusaacutinasa sp. n., and Elthusarotunda sp. n. from sub-Saharan African marine waters.
| Morphological feature | Elthusaraynaudii (Milne Edwards, 1840) | Elthusaxena sp. n. | Elthusaacutinasa sp. n. | Elthusarotunda sp. n. |
| Body shape | Ovoid | Elongate ovoid | Elongate ovoid | Round |
| Shape of cephalon and anterior margin | Sub-truncate, blunt anterior margin | Sub-triangular, bluntly pointed anterior margin | Sub-triangular, pointed anterior margin | Sub-triangular, blunt anterior margin |
| Pereonite 1 anterior margin | Straight | Medially indented | Medial projection | Concave |
| Coxae 7 posterior margin | Not extending past posterior margin of pereonite 7 | Not extending past posterior margin of pereonite 7 | Extending past posterior margin of pereonite 7 | Not extending past posterior margin of pereonite 7 |
| Pereopod 7 protrusions | Absent | Absent | Absent | Present on merus and carpus |
| Pleonite length | Pleonites 1–5 sub-equal | Pleonite 5 longest and indented | Pleonite 1 longest | Pleonite 5 longest, medially convex |
| Pleonite 1 width | Narrower than other pleonites | As long as other pleonites | As wide as pleotelson | Narrower than other pleonites |
| Pleonite 5 lateral margins | Visible | Visible | Largely concealed by pleonite 4 | Slightly concealed by pleonite 4 |
| Pleotelson shape | Evenly rounded | Roughly quadrate and curved upwards | Rounded | Broadly rounded |
| Pleopod 5 endopod | Slightly smaller than exopod | Smaller than exopod (not dissected) | Half the size of exopod | Smaller than exopod (not dissected) |
| Uropods | Broadly rounded, more than half the length of pleotelson | Apices narrowly rounded, more than half the length of pleotelson | Short, pointed, less than half the length of pleotelson | Broadly rounded, half the length of pleotelson |
Livoneca Raynaudii : Milne Edwards 1840: 262; Krauss 1843: 66; Bleeker 1857: 30; Schioedte and Meinert 1884: 367, pl. 12, figs 9–13; Thielemann 1910: 42; Hale 1926: 215–217, figs 10a–j.
Cymothoa Novae-Zealandia: White 1847: 110 (nomen nudum).
Lironeca novae-zealandia : Miers 1874: 228; 1876: 106, pl. III, fig. 2; 1881: 64, 67.
Lironeca laticauda : Miers 1877: 677, pl. 69, fig. 5; Ellis 1981: 124.
Livoneca Raynaudi .–Gerstaecker 1882: 259.
Livoneca Novae Zelandiae.–Gerstaecker 1882: 263.
Lironeca Stewarti: Filhol 1885: 450, pl. 4, fig. 6.
Lironeca neo-zelanica .–Thomson and Chilton 1886: 154.
Livoneca raynaudii .–Whitelegge 1902: 236; Chilton 1909: 606; 1911: 309; 1912: 135; Stebbing 1910: 125; Young 1926: 283; Hale 1926: 215, fig. 10; 1929: 261, figs 253, 259; 1940: 303; Barnard 1940: 491; 1955: 6; Hurley 1961: 268; Hewitt and Hine 1972: 108; Sivertsen and Holthuis 1980: 34; Beumer et al. 1982: 33.
Livoneca epimerias : Richardson 1909: 88, fig. 13; Kussakin 1979: 301, figs 69, 170.
Livoneca raynaudi .–Nierstrasz 1915: 97; 1931: 145; Barnard 1920: 358; Pillai 1954: 16.
Livoneca laticauda .–Nierstrasz 1931: 143.
Lironeca raynaudii .–Brian and Dartevelle 1949: 176; Avdeev 1975: 250; 1978: 281; Trilles 1976: 778, pl. 1, fig. 4; Poore 1981: 341.
Lironeca raynaudi .–Menzies 1962: 115, fig. 36A–B; Kensley 1978: 80, fig. 33B; Moreira and Sadowsky 1978: 111.
Lironeca magna : Mañé-Garzón 1979: 18, figs 1–5.
Elthusa raynaudii .–Bruce 1990: 263; Bruce et al. 2002: 177; Williams et al. 2010: 99–101.
Elthusa raynaudi .–Ghani 2003: 218.
Type material.
Type material held at the Museum national d’Histoire naturelle, Paris (syntypes MNHN-IU-2016-9885; MNHN-IU-2016-9884).
Type locality.
Cape of Good Hope, South Africa.
Type host.
Unknown.
Material examined
(all from South Africa). Syntype. SOUTH AFRICA • 1 ♀ (ovigerous, 26.7 mm TL, 14.1 mm W); south coast of South Africa, Cape of Good Hope; MNHN-IU-2016-9885. Other material. SOUTH AFRICA • 1 ♀ (ovigerous, 26.0 mm TL, 14.0 mm W); Indian Ocean, south coast of South Africa, RV Africana (fish sorting table); 34°38'S, 25°38'E; April 2003; coll. Nico J. Smit; dissected; in the collection of the authors at NWU • 1 ♀ (ovigerous, 26.0 mm TL, 15.0 mm W); Atlantic Ocean, RV Dr Fridtjof Nansen trawl (Station NAN401T062); January 2007; coll. L Atkinson; SAMC-A47881 • 1 ♀ (ovigerous, 20.0 mm TL, 12.0 mm W); Atlantic Ocean, RV Dr Fridtjof Nansen (fish sorting table); 32°17'S, 16°54'E; 269 m; February 2010; coll. KA Hadfield; dissected; SAMC-A089957.
Description
(ovigerous ♀). Figs 1–3. Body ovoid, slightly twisted to the left, 1.7 times as long as greatest width; dorsal surfaces smooth and polished in appearance, widest at pereonite 5, most narrow at pereonite 1; pereonite lateral margins mostly posteriorly ovate, medially indented. Cephalon 0.9 times longer than wide, visible in dorsal view, sub-truncate with blunt anterior margin. Frontal margin thickened, ventrally folded. Eyes oval with distinct margins; one eye 0.2 times width of cephalon, 0.4 times length of cephalon. Pereonite 1 smooth; anterior border medially straight, curved laterally; anterolateral angle narrowly rounded, extending to the medial region of eyes. Posterior margins of pereonites smooth, slightly curved laterally. Coxae 2–3 wide, with posteroventral angles rounded; coxae 4–7 with rounded point, not extending past pereonite posterior margin. Pereonites 2–5 subequal, becoming more progressively rounded posteriorly; pereonites 6 and 7 slightly narrower. Pleon 0.4 times as long as total body length, with pleonite 1 largely concealed by pereonite 7, slightly visible in dorsal view; pleonites posterior margin mostly concave. Pleonite 2 partially overlapped by pereonite 7. Pleonites 3–5 similar in form to pleonite 2; pleonites subequal in length, with posterolateral angles narrowly rounded, posterior margin straight. Pleotelson 0.6 times as long as anterior width, dorsal surface smooth; lateral margins weakly convex; posterior margin evenly rounded.
Antennula shorter than antenna, consisting of eight articles; antennula peduncle articles I and II distinct and articulated, extending to anterior of pereonite 1. Antenna consists of eleven articles, extending to middle of pereonite 1.
Pereopod 1 basis 1.6 times as long as greatest width; ischium 0.7 times as long as basis; merus proximal margin without bulbous protrusion; carpus with rounded proximal margin; propodus 1.4 times as long as wide; dactylus slender, 1.6 times as long as propodus, 2.9 times as long as basal width. All pereopods without robust or simple setae. Pereopod 7 basis with carina, 2.5 times as long as greatest width; ischium without protrusions, 0.5 times as long as basis; merus 0.7 times as long as wide, 0.4 times as long as ischium; carpus without bulbous protrusion, 0.7 times as long as wide, 0.3 times as long as ischium; propodus 0.8 times as long as wide, 0.3 times as long as ischium; dactylus slender, 2.3 times as long as propodus, 3.5 times as long as basal width.
Pleopods simple, exopod larger than endopod. Pleopod 1 exopod 1.3 times as long as wide, lateral margin weakly convex, distally narrowly rounded, mesial margin straight; peduncle 2.3 times as wide as long.
Uropod more than half the length of pleotelson; peduncle 0.5 times longer than rami, lateral margin without setae; rami not extending beyond pleotelson, apices broadly rounded. Endopod apically rounded, 2.7 times as long as greatest width, terminating without setae. Exopod extending to end of endopod, 2.2 times as long as greatest width, apically rounded, terminating without setae.
Variations. Intra-specific variations can cause difficulty in identification and should be taken into consideration. One of the more obvious variations is the overall body shape of examined individuals, as seen from the dorsal view. While the syntype (MNHN–IU–2016–9885) has weakly convex, symmetrical lateral margins, specimen SAMC-A089957 is not as symmetrical, with the right margin being strongly convex and that of the left margin, weakly convex. The latter specimen therefore appears to be less symmetrical. Bruce (1990) mentioned this occasional asymmetrical body shape as an observed variation, as a result of slightly twisted individuals. The body shape of the South African specimen (SAMC-A089957) accords to the shape of individuals illustrated and described by Bruce (1990). In addition, the widest part of this species may vary between pereonite 4 and pereonite 5. This variation may also cause individual body shapes to appear dissimilar. The anterior margin of the cephalon of the syntype (MNHN–IU–2016–9885) appears to be rounded rather than subtruncate. The posterior margin of pleonite 5 can be roughly straight (AM G2181 from Bruce 1990), have a slight medial point, or be weakly concave (Bruce 1990, present study). Although Bruce (1990) described the uropods as being short, most measure more than half the length of the pleotelson.
Size. Ovigerous females 20.0–26.7 mm TL, 14.0–15.0 mm W. Other material: ovigerous females 22.0–67.0 mm TL (average 30.83 mm TL) (Bruce 1990).
Distribution.
Records listed from west to east. North Pacific Ocean: Bering Sea (Kensley 1976). South America: Punta Quillaipe (Menzies 1962) and Chile (Nierstrasz 1931); Uruguay (Mañé-Garzón 1979). South Atlantic Ocean: Saint Helena and Tristan da Cunha (Sivertsen and Holthuis 1980). South Africa: Table Bay (Barnard 1920); Cape of Good Hope (Milne Edwards 1840); Durban (Barnard 1955). India: Travancore (Pillai 1954). Southern Indian Ocean: Amsterdam Island (Kensley 1976). Australia: southern and south-eastern Australia (Schioedte and Meinert 1884, Hale 1926, Bruce 1990, Whitelegge 1901). Japan: Yokohama (Schioedte and Meinert 1884). New Zealand (Filhol 1885, Chilton 1909, Nierstrasz 1915, Hurley 1961, Bruce 1990).
Hosts.
Elthusaraynaudii has been recorded from various fish hosts of multiple orders and families. These hosts are: Chelidonichthyskumu (Cuvier, 1829) (see Avdeev 1978); Chorisochismusdentex (Pallas, 1769) (see Barnard 1920); Cyttusaustralis (Richardson, 1843) (see Avdeev 1978, 1984, Bruce 1990); Cyttusnovaezelandiae (Arthur, 1885) (see Avdeev 1978, 1984); Cyttustraversi Hutton, 1872, previously Cyttoidopsmccullochi (Whitley, 1947) (see Avdeev 1984, Bruce 1990); Genypterusblacodes (Bloch and Schneider, 1801) (see Hewitt and Hine 1972); Gnathanacanthusgoetzeei Bleeker, 1855 (see Bruce 1990); Hyporhamphusintermedius (Cantor, 1842) (see Powell 1959, Stephenson 1969); Latrislineata (Forster, 1801) (see Kensley 1976); Meuscheniafreycineti (Quoy and Gaimard, 1824) (see Bruce 1990); Mustelusantarcticus Günther, 1870 (see Hewitt and Hine 1972); Nemadactylusmonodactylus (Carmichael, 1819), previously Acantholatrismonodactylus (Carmichael, 1819) (see Sivertsen and Holthuis 1980); Nematalosanasus (Bloch, 1795) (see Ghani 2003); Notacanthussexspinis Richardson, 1846 (see Avdeev 1978, 1984); Nototheniamicrolepidota Hutton, 1875, previously Nototheniacolbecki (see Chilton 1909, Hewitt and Hine 1972, Avdeev 1978, 1984); Notolabrustetricus (Richardson, 1840), previously Pseudolabrustetricus (see Bruce 1990); Paranototheniamagellanica (Forster, 1801), previously Nototheniamacrocephala (see Avdeev 1978); Ilishamelastoma (Bloch and Schneider, 1801) previously Pellonabrachysoma (see Pillai 1954); Pelotretisflavilatus Waite, 1911 (see Chilton 1911); Pseudophycisbachus (Forster, 1801), previously Physiculusbachus (see Hewitt and Hine 1972); Physiculus sp. (see Bruce 1990); Pseudophycisbarbata Günther, 1863, previously Physiculusbarbatus (Günther, 1863) (see Bruce 1990); Pseudolabrusmiles (Schneider and Forster, 1801) (see Poore 1981, Bruce 1990); Pseudophycisbachus (Forster, 1801) (see Chilton 1911, Bruce 1990); Rexeasolandri (Cuvier, 1832) (see Bruce 1990); Rhombosolea sp. (see Hewitt and Hine 1972); Sardinopssagax (Jenyns, 1842), previously Clupeaneopilchardus Steindachner, 1879 (see Chilton 1911); Scorpaenacardinalis Solander and Richardson, 1842 (see Poore 1981); Sebastescapensis (Gmelin, 1789), previously Sebastichthyscapensis (Gmelin, 1789) (see Sivertsen and Holthuis 1980); Stolephoruscommersonnii Lacepède, 1803 (see Pillai 1954); Thyrsitesatun (Euphrasen, 1791) (see Sivertsen and Holthuis 1980); Zenopsisnebulosa (Temminck and Schlegel, 1845), previously Zenopsisnebulosus (see Bruce 1990); Zeusfaber Linnaeus, 1758 (see Hale 1926, Avdeev 1984). Unidentified by scientific names: banded perch (Serranidae), flathead (Platycephalidae) (see Bruce 1990).
Remarks.
Elthusaraynaudii can be distinguished by the cephalon having a narrowly truncate rostrum; pereonite 1 with anterior margin straight; pleonites subequal in shape and width; and broadly rounded uropod apices that extend to more than half the length of the pleotelson.
Originally described in 1840, from the Cape of Good Hope in South Africa, from an unknown host, Elthusaraynaudii has been recorded numerous times from a wide range of localities within the Indo-Pacific region. It is the only species of Elthusa that has been described from sub-Saharan Africa. It has been recorded from an unknown host from the Cape of Good Hope (see Milne Edwards 1840); from the rocksucker, Chorisochismusdentex (Pallas, 1769) near Cape Town (Table Bay) (see Barnard 1920); from a wrasse in Durban (see Barnard 1955); as well as from the striped trumpeter, Latrislineata (Forster, 1801) (see Kensley 1976).
Elthusasigani Bruce, 1990, which is only known from its type locality in Queensland, Australia, seems to be most similar to E.raynaudii. Elthusasigani can be distinguished from E.raynaudii by having an evenly concave pereonite 1 anterior margin; a flat, straight cephalon anterior margin; and coxae 7 that extend past the posterior margin of pereonite 7. In addition, E.sigani is a much smaller species in overall body length range (9.0–13.0 mm), compared to E.raynaudii (20.0–26.7 mm).
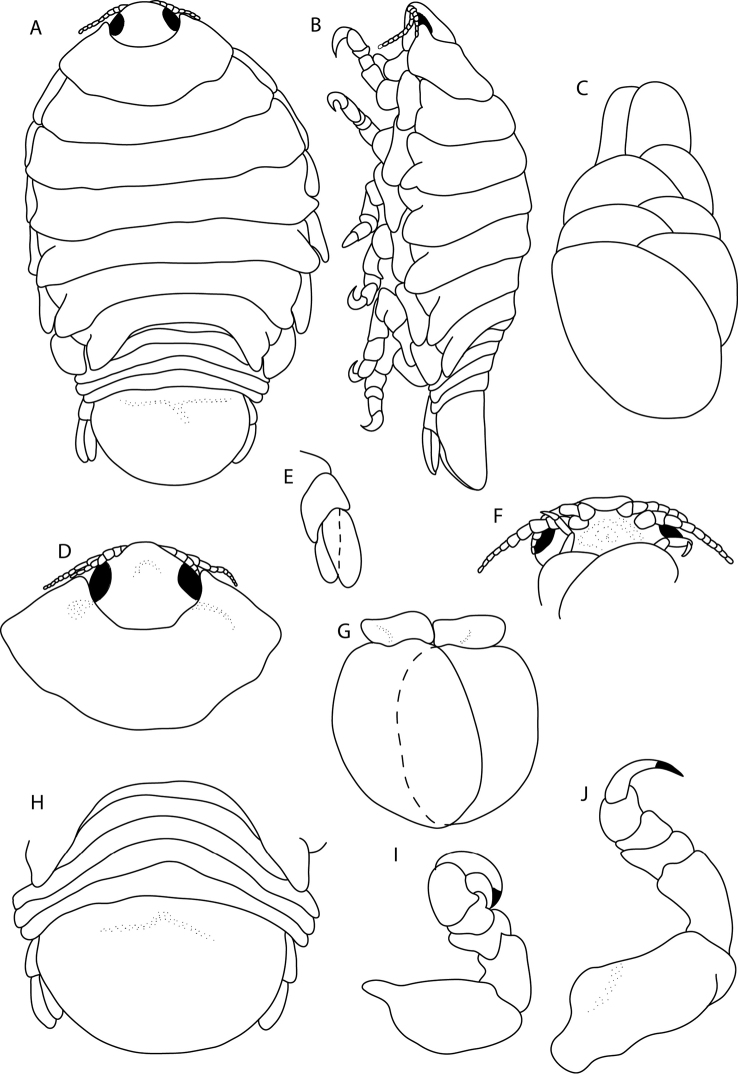
Elthusa xena sp. n.
http://zoobank.org/338A44A2-746F-4D9B-B890-5372D1E45B4C
Figures 4 , 5 , 6 , 7 , Table 1
Figure 4.
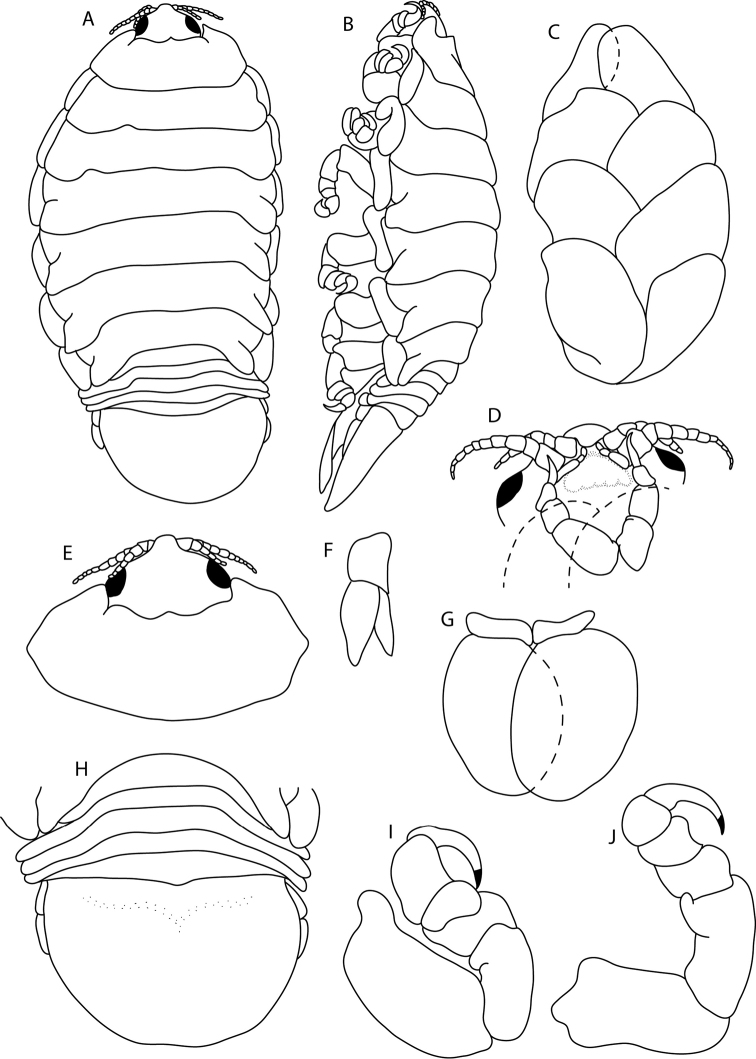
Elthusaxena sp. n. holotype ♀ (ovigerous, 34.0 mm TL, 17.0 mm W) (SAMC-A089958) from Alexander Bay, South Africa A dorsal body B lateral body C oostegites D dorsal view of cephalon and pereonite 1 E uropod F ventral cephalon G pleopod 1 H dorsal view of pleon I pereopod 1 J pereopod 7.
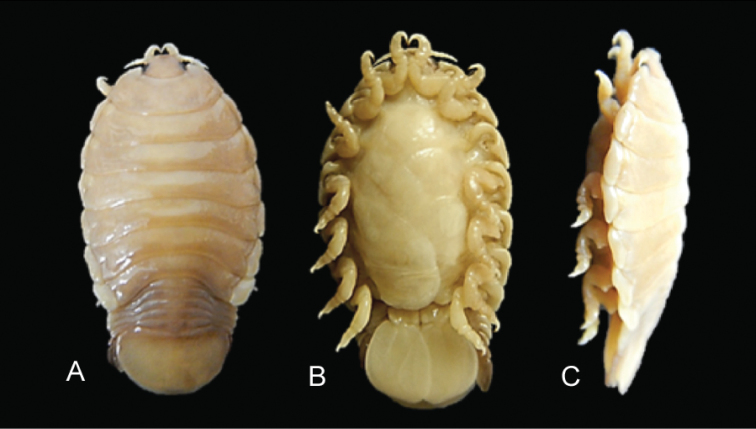
Figure 5.
Photos of Elthusaxena sp. n. holotype ♀ (ovigerous, 34.0 mm TL, 17.0 mm W) (SAMC-A089958) from Alexander Bay, South Africa A dorsal view B ventral view C lateral view.
Figure 6.
Elthusaxena sp. n. paratype ♂ (intermoult) (8 mm TL, 4 mm W) (SAMC-A089959) from Alexander Bay, South Africa A dorsal body B lateral body C pereopod 1 D pereopod 7 E dorsal view of cephalon F penes G uropod H ventral cephalon I dorsal view of pleon J ventral pleopod 2 K dorsal pleopod 2.
Figure 7.
Photos of Elthusaxena sp. n. paratype ♂ (intermoult) (8.0 mm TL, 4.0 mm W) (SAMC-A089959) from Alexander Bay, South Africa A dorsal view B ventral view.
Material examined.
Holotype. SOUTH AFRICA • 1 ♀ (ovigerous, 34.0 mm TL, 17.0 mm W); Alexander Bay, mouth of the Orange River; 28°38'S, 16°27'E; July 1993; coll. J Laubscher; from the super klipfish, Clinussuperciliosus (Linnaeus, 1758); SAMC-A089958.
Paratype. SOUTH AFRICA • 1 ♂ (intermoult, 8.0 mm TL, 4.0 mm W); same data as holotype; SAMC-A089959.
Description
(ovigerous ♀). Figs 4–5. Body slightly twisted to the left, elongated ovoid, twice as long as greatest width; dorsal surfaces smooth and polished in appearance, widest at pereonite 5, most narrow at pereonite 1, pereonite lateral margins mostly rounded, medially indented. Cephalon 0.8 times longer than wide, visible from dorsal view, sub-triangular with blunt anterior point. Frontal margin thickened, ventrally folded. Eyes oval with distinct margins; one eye 0.1 times width of cephalon, 0.3 times length of cephalon. Pereonite 1 smooth, anterior border slightly concave; anterolateral angle rounded, extending to the medial region of eyes. Posterior margins of pereonites smooth, slightly curved laterally. Coxae 2–3 narrow with posteroventral angles narrowly rounded; coxae 4–7 with rounded point, not extending past pereonite margin. Pereonites 2–5 subequal, pereonites 6 and 7 slightly narrower. Pleon 0.4 times as long as total body length, with pleonite 1 same width as other pleonites, lateral margins concealed by pereonite 7, slightly visible in dorsal view; pleonites posterior margin smooth, slightly curved laterally. Pleonite 2 partially overlapped by pereonite 7; posterolateral angles of pleonite 2 rounded. Pleonites 3–5 similar in form to pleonite 2; pleonite 5 longest, free, not overlapped by lateral margins of pleonite 4, with posterolateral angles narrowly rounded, posterior margin with 3 indentations. Pleotelson 0.6 times as long as anterior width, dorsal surface smooth; lateral margins convex; posterior margin evenly rounded.
Antennula shorter than antenna, consisting of eight articles; peduncle articles I and II distinct and articulated, extending to anterior of pereonite 1. Antenna consists of eleven articles, extending to past anterior margin of pereonite 1.
Pereopod 1 basis 1.8 times as long as greatest width; ischium 0.7 times as long as basis; merus proximal margin without bulbous protrusion; carpus with rounded proximal margin; propodus 1.8 times as long as wide; dactylus slender, 0.8 times as long as propodus, 2.3 times as long as basal width. Pereopods 2–3 similar to pereopod 1, all pereopods without robust or simple setae. Pereopod 7 basis with carina, 1.5 times as long as greatest width; ischium without protrusions, 0.9 times as long as basis; merus proximal margin with slight bulbous protrusion, 0.6 times as long as wide, 0.3 times as long as ischium; carpus with bulbous protrusion, 0.9 times as long as wide, 0.5 times as long as ischium; propodus as long as wide, 0.4 times as long as ischium; dactylus slender, 1.9 times as long as propodus, 3.1 times as long as basal width.
Pleopods simple, exopod larger than endopod. Pleopod 1 exopod 1.1 times as long as wide, lateral margin strongly convex, distally broadly rounded, mesial margin weakly convex; peduncle 2.8 times as wide as long.
Uropod more than half the length of pleotelson, peduncle 0.8 times longer than rami, peduncle lateral margin without setae; rami not extending beyond pleotelson, apices narrowly rounded. Endopod apically rounded, 2.5 times as long as greatest width, lateral margin weakly convex, mesial margin straight, terminating without setae. Exopod extending beyond end of endopod, twice as long as greatest width, apically rounded, lateral margin weakly convex, mesial margin straight, terminating without setae.
Description
(paratype intermoult ♂). Figs 6, 7. Male similar to female but smaller. Specimen mid-moult. Body rectangular, not twisted, twice as long as greatest width. Pereonite lateral margins mostly subparallel. Cephalon 0.7 times longer than wide. Frontal margin rounded to form blunt rostrum. Eyes oval with distinct margins; one eye 0.2 times width of cephalon; 0.5 times length of cephalon. Pereonite 1 smooth, anterior border concave, extending past base of cephalon. Posterior margins of pereonites smooth and straight, except pereonite 4 and 5. Coxae 2–3 wide, with posteroventral angles rounded; coxae 4–7 rounded. Pereonites 6 and 7 narrower, becoming more progressively rounded posteriorly. Pleon 0.3 times as long as total body length, with pleonite 1 largely concealed by pereonite 7, slightly visible in dorsal view; pleonites 1–3 posterior margin posteriorly concave, smooth and slightly curved laterally. Pleonite 5 overlapped by lateral margins of pleonite 4, with posterolateral angles narrowly rounded, posterior margin straight. Pleotelson 0.8 times as long as anterior width, lateral margins straight or weakly convex, posterior margin broadly truncate.
Antennula shorter than antenna, consisting of eight articles. Antenna consists of ten articles, extending to middle of pereonite 1.
Pereopod 1 basis twice as long as greatest width; ischium 0.6 times as long as basis; propodus 1.6 times as long as wide; dactylus 1.1 times as long as propodus, 3 times as long as basal width. Pereopod 7 twice as long as greatest width; ischium 0.7 times as long as basis; merus proximal margin without bulbous protrusion, 0.7 times as long as wide, 0.4 times as long as ischium; carpus without bulbous protrusion, 0.7 times as long as wide, 0.4 times as long as ischium; propodus 1.3 times as long as wide, 0.6 as long as ischium; dactylus slender, 1.4 times as long as propodus, 2.7 times as long as basal width.
Pleopod 1 exopod 1.2 times as long as wide, lateral margin weakly convex, distally broadly rounded, mesial margin straight; endopod 2.1 times as long as wide, lateral margin weakly convex, mesial margin straight, peduncle 2.2 times as wide as long. Pleopod 2 appendix masculina with parallel margins, 1.1 times as long as endopod, distally narrowly rounded.
Uropod same length or slightly longer than the pleotelson, peduncle 0.4 times longer than rami, rami extending slightly beyond pleotelson, apices narrowly rounded. Endopod apically slightly pointed, 3 times as long as greatest width. Exopod 2.6 times as long as greatest width.
Penes medially adjacent; penial process 0.7 times as long as basal width.
Etymology.
The epithet is constructed in a possessive form of a personal name. This species is named after Xena, the warrior princess, in reference to the strong nature of the female cymothoid isopod.
Size.
Ovigerous female (34.0 mm TL, 17.0 mm W), male (8.0 mm TL, 4.0 mm W).
Distribution.
Currently only known from the mouth of the Orange River, Alexander Bay, South Africa (Atlantic Ocean).
Hosts.
Clinussuperciliosus (Linnaeus, 1758). This is the first record of a klipfish (of the genus Clinus Cuvier, 1816), and of the intertidal super klipfish, Clinussupercilious, as a fish host of a species of Elthusa. This host belongs to the fish order Perciformes, and is endemic to the Southeast Atlantic Ocean, from northern Namibia to the Kei River of South Africa (Smith and Heemstra 1986).
Remarks.
Elthusaxena sp. n. female can be identified by the elongate, ovoid body shape; coxae 7 that do not extend past the posterior margin of pereonite 7; a bluntly pointed anterior margin of the cephalon; evenly rounded, slightly concave anterior margin of pereonite 1; uropod rami with apices narrowly rounded and more than half the length of pleotelson; pleonite 5 posterior margin with indentations; and the pleotelson is short, roughly quadrate, with margins that curl upward.
Two other Elthusa species have been recorded from related perciform fish hosts from the family Clinidae Swainson, 1839 (blennies). Elthusacalifornica (Schioedte & Meinert, 1884) was noted from the striped kelpfish Gibbonsiametzi Hubbs, 1927; and Elthusamenziesi (Brusca, 1981) from both the spotted kelpfish Gibbonsiaelegans (Cooper, 1864) and the crevice kelpfish Gibbonsiamontereyensis Hubbs, 1927. However, this is the first record of Elthusa collected from a Clinus sp.
Elthusaxena sp. n. can be distinguished from E.raynaudii by having a bluntly pointed cephalon anterior margin, compared to the narrowly truncate margin of E.raynaudii. Other differences include the shape of the pleotelson (which is quadrate, wide and short for E.xena sp. n., and evenly rounded for E.raynaudii); pleonite 1 is the same length as the other pleonites in Elthusaxena sp. n. but narrower in E.raynaudii; and the uropod apices of E.xena sp. n. are narrowly rounded compared to the broadly rounded apices of E.raynaudii uropods. See Table 1 for further morphological variation and comparisons.
Elthusa acutinasa sp. n.
http://zoobank.org/D5AFAEC4-F03D-400F-98A0-8D86631E495E
Figures 8 , 9 , 10 , 11 , Table 1
Figure 8.
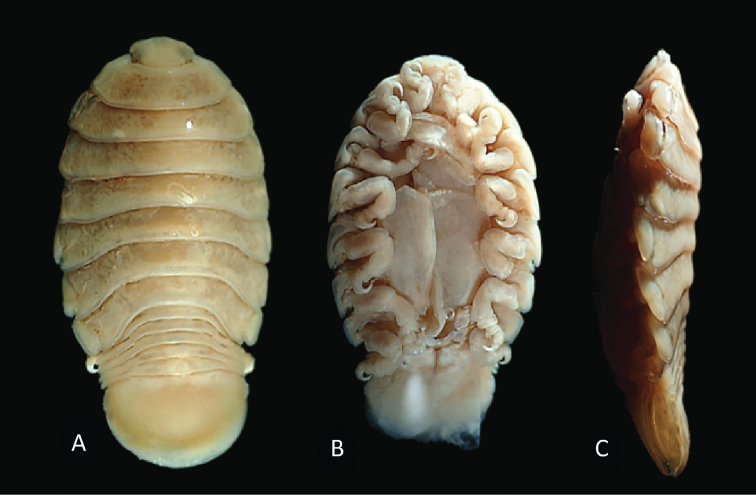
Elthusaacutinasa sp. n. holotype ♀ (ovigerous, 39.0 mm TL, 19.0 mm W) (SAMC-A089960) from Africana research vessel A dorsal body B lateral body C oostegites D ventral cephalon E dorsal view of cephalon and pereonite 1 F uropod G pleopod 1 H dorsal view of pleon I pereopod 1 J pereopod 7.
Figure 9.
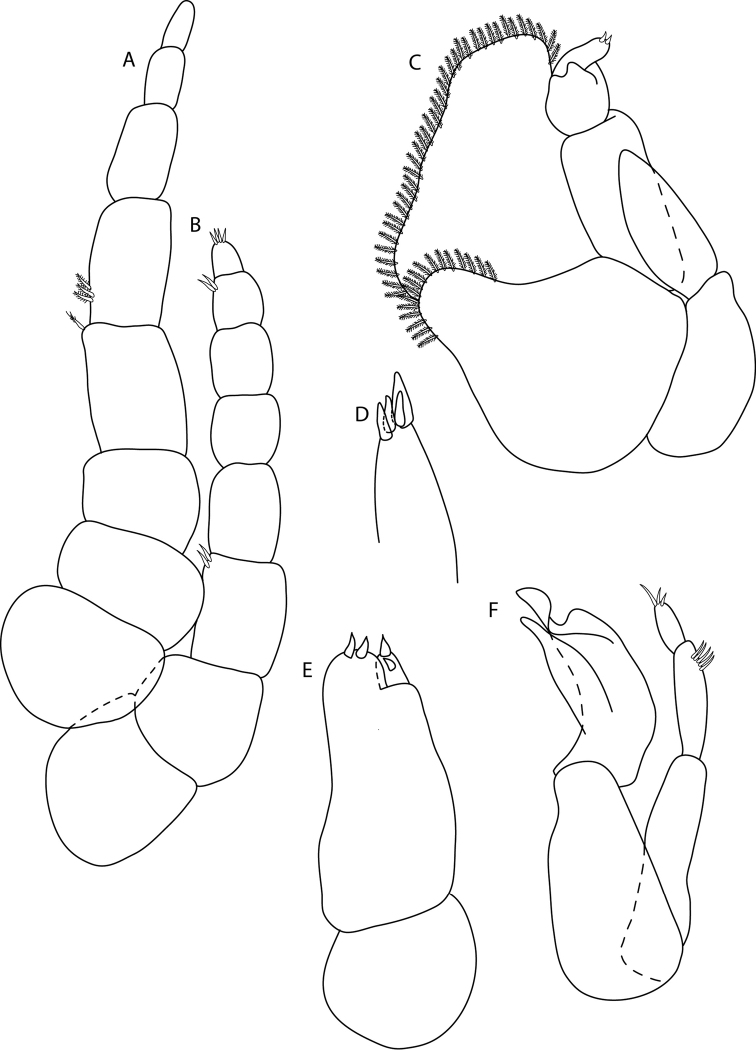
Elthusaacutinasa sp. n. paratype ♀ (ovigerous, 33.0 mm TL, 16.0 mm W) (SAMC-A089961) from Africana research vessel A antennula B antenna C maxilliped D tip of maxillula E maxilla F mandible.
Figure 10.
Elthusaacutinasa sp. n. paratype ♀ (ovigerous, 33.0 mm TL, 16.0 mm W) (SAMC-A089961) from Africana research vessel A–E dorsal view of pleopods 1–5 respectively F–J ventral view of pleopods 1–5 respectively.
Figure 11.
Photos of Elthusaacutinasa sp. n. holotype ♀ (ovigerous, 39.0 mm TL, 19.0 mm W) (SAMC-A089960) from Africana research vessel A dorsal view B ventral view C lateral view.
Material examined.
Holotype. SOUTH AFRICA • 1 ♀ (ovigerous, 39.0 mm TL, 19.0 mm W); Indian Ocean, south coast of South Africa, RV Africana (fish sorting table); 34°38'S, 25°38'E; April 2003; coll. Nico J Smit; SAMC-A089960.
Paratypes. SOUTH AFRICA • 3 ♀♀ (ovigerous, 28.0–30.0 mm TL, 15.0–17.0 mm W); same data as holotype; SAMC-A089961.
Other material. SOUTH AFRICA • 1 ♀ (ovigerous, 29.0 mm TL, 17.0 mm W); same data as holotype; dissected; in the collection of the authors at NWU • 4 ♀♀ (non-ovigerous, 19.0–24.0 mm TL, 10.0–14.0 mm W); same data as holotype; in the collection of the authors at NWU • 9 ♀♀ (three ovigerous, six non-ovigerous, 15.0–40.0 mm TL, 8.0–19.0 mm W); Indian Ocean, south coast of South Africa, RV Africana (fish sorting table); 30°29'S, 16°0'E; 213 m depth; January 1999; SAMC-A091307 • 1 ♀ (ovigerous, 40.0 mm TL, 19.0 mm W); same data as preceding; 30°25'S, 16°9'E; 259 m depth; SAMC-A091308 • 1 ♀ (ovigerous, 30.0 mm TL, 15.0 mm W); same data as preceding; 31°8'S, 15°20'E; 234 m depth; SAMC-A091309.
Description
(ovigerous ♀). Figs 8–11. Body slightly twisted to the right, elongated ovoid, 2.1 times as long as greatest width. Body dorsal surfaces smooth and polished in appearance, widest at pereonite 4, most narrow at pereonite 1, pereonite lateral margins mostly posteriorly ovate, medially indented. Cephalon 0.4 times longer than wide, visible from dorsal view, sub-triangular with narrowly rounded anterior point. Frontal margin thickened, ventrally folded. Eyes oval with distinct margins; one eye 0.2 times width of cephalon, 0.4 times length of cephalon. Pereonite 1 smooth, anterior border with medially produced point, with two indentations; anterolateral angle rounded, extending to posterior margin of eyes. Posterior margins of pereonites smooth and slightly curved laterally. Coxae 2–3 wide; with posteroventral angles rounded; 4–7 with rounded point. Coxae 7 extending slightly past pereonite posterior margin. Pereonites 2–5 subequal, becoming more progressively rounded posteriorly. Pleon 0.4 times as long as total body length, with pleonite 1 longest, lateral margins concealed by pereonite 7, visible in dorsal view; pleonites posterior margin smooth and slightly curved laterally. Pleonite 2 partially overlapped by pereonite 7; posterolateral angles of pleonite 2 rounded. Pleonites 3–5 similar in form to pleonite 2; pleonite 5 overlapped by lateral margins of pleonite 4, posterior margin straight, with slight medial point. Pleotelson 0.7 times as long as anterior width, dorsal surface smooth; lateral margins weakly convex; posterior margin rounded, with slight medial indent.
Antennula shorter than antenna, consisting of eight articles; antennula peduncle articles I and II distinct and articulated; article II 0.9 times as long as article 1; article III 1.4 times as long as wide, 0.5 times as long as combined lengths of articles I and II; antennula flagellum with five articles, extending to middle of eye, with tufts of setae on articles I–III and article VIII. Antenna consists of twelve articles. Antenna peduncle article III 1.3 times as long as article II; article IV 1.3 times as long as wide, 1.2 times as long as article III; article V 1.5 times as long as wide, 1.1 times as long as article IV. Antenna flagellum with six articles, terminal article terminating in 1–5 short simple setae, extending to past anterior margin of pereonite 1. Mandible palp article II with five distolateral setae, and article III with three simple setae. Maxillula simple with four terminal robust setae. Maxilla mesial lobe not fused to lateral lobe; lateral lobe without simple setae, two recurved robust setae; mesial lobe without simple setae, and two large recurved robust setae. Maxilliped consists of III articles, with lamellar oostegite lobe or second, smaller oostegite lobe on basal part of article, palp article II without simple setae, article III with three recurved robust setae. Oostegites margin covered in numerous plumose setae.
Pereopod 1 basis 1.9 times as long as greatest width; ischium 0.7 times as long as basis; merus proximal margin with slight bulbous protrusion; carpus with rounded proximal margin; propodus 1.1 times as long as wide; dactylus slender, 1.3 times as long as propodus, 3 times as long as basal width. Pereopod 3 similar to pereopod 2, all pereopods without robust or simple setae. Pereopod 7 basis 1.9 times as long as greatest width; ischium with slight bulbous protrusion on distal margin, 0.9 times as long as basis; merus proximal margin with slight bulbous protrusion, 0.6 times as long as wide, 0.3 times as long as ischium; carpus with bulbous protrusion, 0.7 times as long as wide, 0.3 times as long as ischium; propodus 1 times as long as wide, 0.3 times as long as ischium; dactylus slender, 1.9 times as long as propodus, 3.3 times as long as basal width.
Pleopods simple; exopod larger than endopod, with 4–7 simple setae on peduncle of pleopods 2–5. Pleopod 1 exopod 1.3 times as long as wide, lateral margin weakly convex, distally broadly rounded, mesial margin straight; peduncle 3 times as wide as long. Endopod 1.6 times as long as wide, lateral margin convex, distally narrowly rounded, mesial margin straight, peduncle 2.4 times as wide as long. Pleopods 2–5 similar to pleopod 1, mesial margins becoming more strongly produced, peduncle lobes absent.
Uropod less than half the length of the pleotelson, peduncle 0.7 times longer than rami, peduncle lateral margin without setae, marginal setae absent, apices narrowly rounded. Endopod apically slightly pointed, 3.4 times as long as greatest width, lateral margin weakly convex, mesial margin straight, terminating without setae. Exopod extending to end of endopod, 2.3 times as long as greatest width, apically rounded, lateral margin distally convex, mesial margin straight, terminating without setae.
Variations. Intra-specific variation was observed among the examined specimens of Elthusaacutinasa sp. n. The size of the medial point formed at the anterior margin of pereonite 1 may vary. Some specimens portrayed an obvious, sharp medial point, while others only had a weak medial projection of the anterior margin of pereonite 1. Variation in the length of the uropods are slight, but one specimen had uropod rami extending to half the length of the pleotelson, while all the others specimens’ uropods were remarkably short. The overlapping of pleonite 5 lateral margins by pleonite 4 was consistent, except with one of the other examined paratype females, where pleonite 5 lateral margins were slightly visible. Some variation was also noted in the width of pleonite 1.
Etymology.
The epithet is a noun in the genitive singular. The species name acutinasa was derived by the son of one of us (NJS) from a combination of the two Latin words acute and nasus. The word acute translates to a feature that is pointy or ends with a sharp point; while nasus translates to nose. The combined word, acutinasa, therefore means pointy nose, and appropriately describes one of the characters of this species, which is its pointed anterior margin of the rostrum.
Size.
Ovigerous females (28.0–40.0 mm TL, 15.0–19.0 mm W), non-ovigerous females (19.0–24.0 mm TL, 10.0–14.0 mm W).
Distribution.
Known from the Indian Ocean, off the south coast of South Africa.
Hosts.
Not known (type material was collected from the fish sorting table following a trawl and not from a specific fish species).
Remarks.
Elthusaacutinasa sp. n. can be identified by its elongate, ovoid body shape; pointed anterior margin of the cephalon; anterior margin of pereonite 1 with short medial point; short, apically pointed uropod rami, which extend to less than half of the length of the pleotelson; coxae 7 that extends past the posterior margin of pereonite 7; pleonite 5 lateral margins that are largely concealed by pleonite 4; pleonite 5 posterior margin with a slight medial point; pleonite 1 the longest of the pleonites; and pleopod 5 endopod approximately half the size of the exopod.
Several characters differentiate between E.acutinasa sp. n. from E.raynaudii (see Table 1). Elthusaacutinasa sp. n. has a prominent, pointed cephalon anterior margin with a medially pointed pereonite 1 anterior margin compared to the straight anterior margin of E.raynaudii cephalon and pereonite 1. Pleon differences include the longer pleotelson of E.acutinasa sp. n. with pleonite 1 widest and pleonite 5 lateral margins concealed by those of pleonite 4 (not seen in E.raynaudii). Elthusaacutinasa sp. n. also has short uropods that do not extend to the half of the pleotelson length, whereas those of E.raynaudii reach to, or extend past, the half of the pleotelson length.
Elthusaacutinasa sp. n. can also be distinguished from E.xena sp. n. by its short uropods and coxae 7 that extend past the posterior margin of pereonite 7. Further differences are found within pleon morphology, where E.acutinasa sp. n. pleonite 5 lateral margins are largely concealed by pleonite 4, whereas those of E.xena sp. n. are visible. Pleonite 1 in E.xena sp. n. is as wide as the other pleonites, whereas pleonite 1 in E.acutinasa sp. n. is narrower than the other pleonites. The pleotelson shape of E.acutinasa sp. n. is evenly rounded, compared to the roughly quadrate pleotelson of E.xena sp. n. (see Table 1).
Elthusa rotunda sp. n.
http://zoobank.org/138FBF0D-2E4B-4561-86C8-F209B78A33E0
Figure 12.
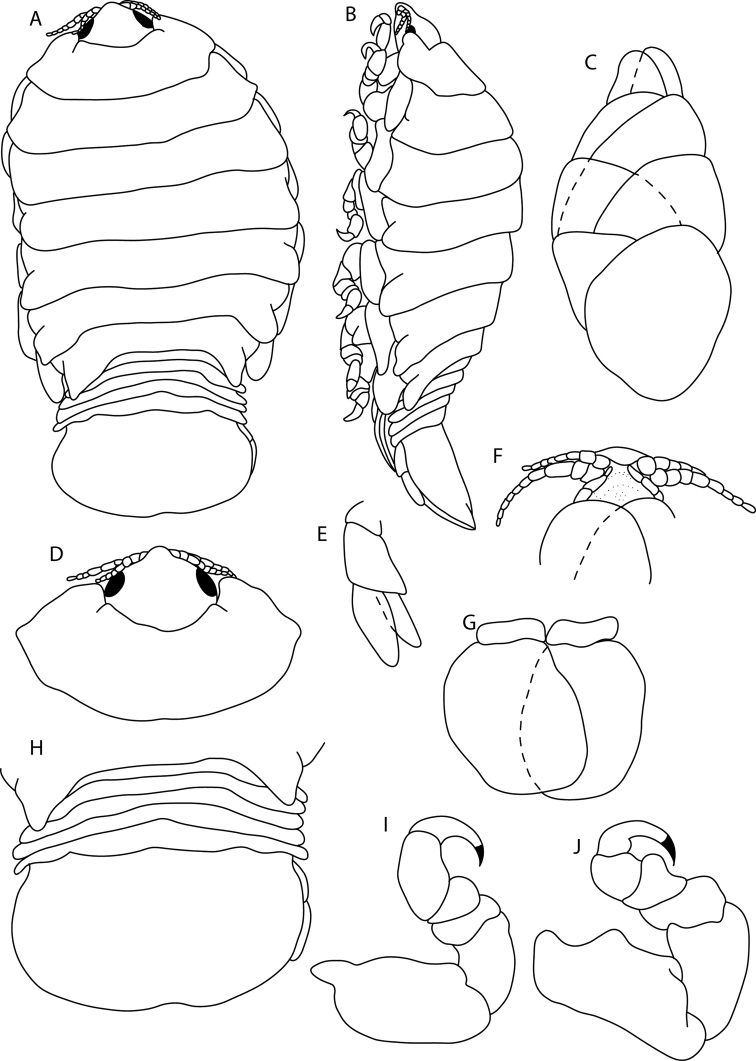
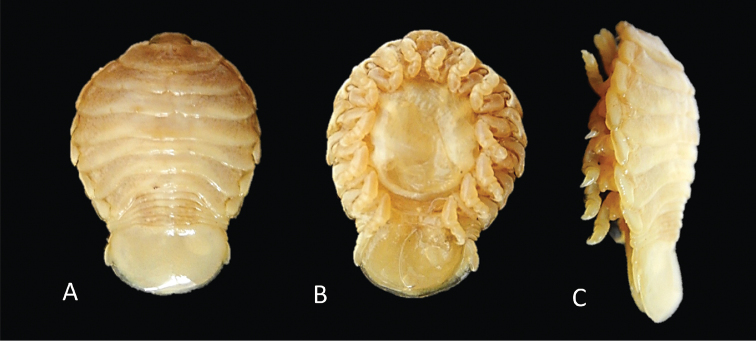
Elthusarotunda sp. n. holotype ♀ (ovigerous, 28 mm TL, 19 mm W) (SAMC-A11001) from Sea Point, South Africa A dorsal body B lateral body C oostegites D ventral cephalon E dorsal view of cephalon and pereonite 1 F uropod G pleopod 1 H dorsal view of pleon I pereopod 1 J pereopod 7.
Figure 13.
Photos of Elthusarotunda sp. n. holotype ♀ (ovigerous, 28 mm TL, 19 mm W) (SAMC-A11001) from Sea Point, South Africa A dorsal view B ventral view C lateral view.
Material examined.
Holotype. SOUTH AFRICA • 1 ♀ (ovigerous, 29.0 mm TL; 20.0 mm W); Cape Town, Sea Point; 33°55'S, 18°23'E; January 1960; coll. G Branch; SAMC A11001.
Description
(ovigerous ♀). Figs 12–13. Body round, not twisted, 1.4 times as long as greatest width; dorsal surfaces smooth and polished in appearance, widest at pereonite 4, most narrow at pereonite 1; pereonite lateral margins mostly posteriorly ovate, medially indented. Cephalon 0.4 times longer than wide, visible from dorsal view, sub-triangular with blunt anterior point. Frontal margin thickened, ventrally folded. Eyes oval with distinct margins; one eye 0.2 times width of cephalon; 0.5 times length of cephalon. Pereonite 1 smooth, anterior border evenly concave; anterolateral angles rounded, extending to the medial region of eyes. Posterior margins of pereonites smooth, slightly curved laterally, posterior margins of pereonites 2–3 uneven. Coxae 2–3 wide; with posteroventral angles rounded; coxae 4–7 with rounded point, not extending past pereonite posterior margin. Pereonites becoming more progressively rounded posteriorly; pereonite 5 most narrow. Pleon 0.4 times as long as total body length; pleonite 1 largely concealed by pereonite 7, slightly visible in dorsal view; pleonites posterior margin slightly concave, smooth, slightly curved laterally. Pleonite 2 lateral margins overlapped by pereonite 7. Pleonites 3–4 similar in form to pleonite 2; pleonite 5 longest, overlapped by lateral margins of pleonite 4, posterior margin medially convex. Pleotelson broadly rounded, 0.7 times as long as anterior width, dorsal surface smooth; lateral margins convex; posterior margin evenly rounded.
Antennula shorter than antenna, consisting of eight articles; peduncle articles I and II distinct and articulated; extending to middle of eye. Antenna consists of ten articles, extending to past anterior margin of pereonite 1.
Pereopod 1 basis 1.7 times as long as greatest width; ischium 0.7 times as long as basis; merus proximal margin without bulbous protrusion; propodus 1.4 times as long as wide; dactylus slender, 1.3 times as long as propodus, 2.9 times as long as basal width. All pereopods without robust or simple setae. Pereopod 7 basis with carina, 2.1 times as long as greatest width; ischium with slight bulbous protrusion, 0.8 times as long as basis; merus proximal margin with bulbous protrusion, 0.6 times as long as wide, 0.3 times as long as ischium; carpus with bulbous protrusion, 0.7 times as long as wide, 0.3 times as long as ischium; propodus 1.2 times as long as wide, 0.9 times as long as ischium; dactylus slender, 1.7 times as long as propodus, 2.5 times as long as basal width.
Pleopods simple, exopod larger than endopod. Pleopod 1 exopod 1.3 times as long as wide, lateral margin weakly convex, distally broadly rounded, mesial margin weakly convex; peduncle 2.5 times as wide as long.
Uropod half the length of pleotelson, peduncle 0.9 times longer than rami, peduncle lateral margin without setae; rami not extending beyond pleotelson, marginal setae absent, apices broadly rounded. Endopod apically rounded, 2.6 times as long as greatest width, lateral margin weakly convex, mesial margin weakly convex. Exopod extending to end of endopod, 2.2 times as long as greatest width, apically rounded, lateral margin weakly convex, mesial margin straight.
Size.
Ovigerous female (29.0 mm TL, 20.0 mm W).
Etymology.
The epithet is a noun in the nominative singular. It is named after its most distinct, defining character, which is the rounded shape of the body. The Latin word for round is rotundus.
Distribution.
Currently only known from Sea Point, Cape Town, South Africa.
Hosts.
Not known.
Remarks.
The diagnostic characters of E.rotunda sp. n. include its circular body shape; a sub-triangular cephalon with blunt anterior margin; pereopod 7 merus and carpus with protrusions on the proximal and lateral margins; pereonite 7 lateral margins that extend to pleonite 4; pleonite 5 longest and medially convex; a broadly rounded pleotelson posterior margin; and uropod rami that are sub-equal in length to the peduncle.
When comparing E.rotunda sp. n. to the rest of the identified Elthusa species, its closest resemblance is to that of E.raynaudii. This is especially in regards to the shape of the uropods, pleon, and cephalon anterior margin. It can be distinguished from E.raynaudii in having a more rounded body shape compared to the ovoid body shape of E.raynaudii; triangular cephalon as opposed to the narrowly truncate cephalon of E.raynaudii; the broadly rounded pereonite 1 anterolateral margins of E.rotunda sp. n. compared to the narrowly rounded to pointed anterolateral margins of E.raynaudii pereonite 1; as well as the uropod rami and peduncles that are subequal in length, as opposed to the longer rami of E.raynaudii (see Table 1).
Elthusarotunda sp. n. can be distinguished from E.xena sp. n. by the cephalon anterior margin which is more pointed in E.xena sp. n. and more rounded in E.rotunda sp. n.; broadly rounded uropod apices compared to the narrowly rounded ones from E.xena sp. n.; the shape of the pleotelson, which is broadly rounded for E.rotunda sp. n. and roughly quadrate for E.xena sp. n.; as well as the prominent presence of pereopod 7 protrusions on the merus and carpus of E.rotunda sp. n., that are less bulbous on E.xena sp. n.
The main differentiating characters between E.rotunda sp. n. and E.acutinasa sp. n. include the shape of the cephalon anterior margin (bluntly rounded versus produced point); and the uropod morphology, with E.rotunda sp. n. having broadly rounded, longer uropodal rami in comparison to the short, pointed uropodal rami of E.acutinasa sp. n. Elthusarotunda sp. n. pleonite 5 is the longest, whereas E.acutinasa sp. n. pleonite 1 is the longest; the presence of pereopod 7 protrusions on E.rotunda sp. n. is more prominent and bulbous that those of E.acutinasa sp. n. pereopod 7 (see Table 1).
Conclusions
From previous collections across South Africa, four Elthusa species were recognised. Elthusaraynaudii, the only known Elthusa species from South Africa, was identified along with three new species from this genus. These new species, E.xena sp. n., E.acutinasa sp. n., and E.rotunda sp. n., more than double the known records of Elthusa from this region. Descriptions were provided for the three new Elthusa species along with an identification key with diagnostic characters to distinguish between the sub-Saharan Elthusa species (Table 1). A summative table was provided with currently known information on all species from the genus Elthusa, including host and location records of each (Table 2).
Table 2.
Summary of the hosts, distribution, and attachment sites of all 33 species from the genus Elthusa Schioedte & Meinert, 1884, as well as the references for each record.
| Species | Distribution | Hosts | References |
|---|---|---|---|
| Elthusaalvaradoensis Rocha-Ramírez, Chávez-López & Bruce, 2005 | TLoc: Alvarado, Veracruz, Mexico. | TH: Synodusfoetens (Linnaeus, 1766) | Rocha-Ramírez et al. (2005) |
| Elthusaarnoglossi Trilles & Justine, 2006 | TLoc: Chesterfield Islands, New Caledonia. | TH: Arnoglossus sp. | Trilles and Justine (2006) |
| Elthusaatlantniroi (Kononenko, 1988) | TLoc: Bay of Biscay, northeast Atlantic Ocean | TH: Cepolamacrophthalma (Linnaeus, 1758) | Kononenko (1988) |
| Elthusacalifornica (Schioedte & Meinert, 1884) Syn: Livonecacalifornica Schioedte & Meinert, 1884 | TLoc: California, near San Francisco | TH: Holconoti sp. | Schioedte and Meinert (1884); Keys (1928); Hatch (1947); Olson (1972); Iverson (1974); Miller (1975); Waugh et al. (1989); Bennett (1993); Brusca (1981); Brusca et al. (2001); Gamble et al. (2013) |
| OL: Pacific coast from Alaska to Peru; Canada; USA; Mexico | OH: Species from the families Atherinidae; Aulorhynchidae; Clinidae; Clupeidae; Cottidae; Embiotocidae; Fundulidae; Gasterosteidae; Gobiidae; Hexagrammidae; Moronidae; Mugilidae; Pholidae; Osmeridae; Paralichthyidae; Pholidae; Pleuronectidae; Sebastidae | ||
| Elthusacaudata (Schioedte & Meinert, 1884) Syn: Livonecacaudata Schioedte & Meinert, 1884 | TLoc: Laponica islands, Japan | TH: Unknown | Schioedte and Meinert (1884); Avdeev (1978) |
| OL: New Zealand | Other hosts: Genypterusblacodes (Forster, 1801) | ||
| Elthusaemarginata (Bleeker, 1857) Syn: Livonecaemarginata Bleeker, 1857 | TLoc: Java, Indonesia | TH: Unknown | Bleeker (1857); Miers (1881); Schioedte and Meinert (1884); Nierstrasz (1915); Trilles and Randall (2011) |
| OL: East India; Malaysia; Indonesia | OH: Species from the family Mullidae | ||
| Elthusaepinepheli Trilles & Justine, 2010 | TLoc: Off Nouméa, New Caledonia | TH: Epinephelushowlandi (Günther, 1873) | Trilles and Justine (2010) |
| Elthusafoveolata (Hansen, 1897) Syn: Ironafoveolata Hansen, 1897 | TLoc: Sri Lanka | TH: Unknown | Hansen (1897) |
| Elthusafrontalis (Richardson, 1910) Syn: Livonecafrontalis Richardson, 1910 | TLoc: Sablayan, Philippines | TH: Balistes sp. | Richardson (1910) |
| Elthusamenziesi (Brusca, 1981) Syn: Lironecamenziesi Brusca, 1981 | TLoc: San Quintin Bays, Baja California, Mexico | TH: Clinocottusanalis (Girard, 1858) | Brusca (1981); Ruiz-Campos (1986); Wetzer et al. (1991); Espinosa-Pérez and Hendrickx (2001) |
| OL: Mexico and Western Baja California | OH: Species from the families of Atherinidae; Blenniidae; Clinidae; Cottidae; Gobiesocidae; Kyphosidae; Labrisomidae; Lessoniaceae | ||
| Elthusamethepia (Schioedte & Meinert, 1884) | TLoc: Rio de Janeiro, Brazil | TH: Achirus sp. | Schioedte and Meinert (1884) |
| Elthusamoritakii Saito & Yamauchi, 2016 | TLoc: Honshu and east China Sea coast of Kyushu, Japan | TH: Ereuniasgrallator Jordan & Snyder, 1901 | Saito and Yamauchi (2016) |
| Elthusamyripristae Bruce, 1990 | TLoc: Escape Reef, outer Barrier Reef, Australia | TH: Myripristisviolaceus Bleeker, 1851 | Bruce (1990) |
| Elthusananoides (Stebbing, 1905) Syn: Ironananoides Stebbing, 1905 | TLoc: Galle, Sri Lanka (old Ceylon) | TH: Unknown | Stebbing (1905); Monod (1923); Trilles (1976) |
| OL: Gulf of Suez, Red Sea | OH: Species from the families Holothuriidae; Leiognathidae; Molidae; Plotosidae; Scorpaenidae; Sparidae | ||
| Elthusaneocytta (Avdeev, 1975) Syn: Lironecaneocytta Avdeev, 1975 | TLoc: New Zealand | TH: Neocyttusrhomboidalis Gilchrist, 1906 | Avdeev (1975, 1984); Stephenson (1987); Bruce (1990) |
| OL: Tasmania and south-east New Zealand | OH: species from the families Cyttidae; Oreosomatidae; Scombridae; Zeidae | ||
| Elthusanierstraszi Hadfield, Bruce & Smit, 2016 Syn: Lironecaparva Nierstrasz, 1915. | TLoc: Kisar Island, Moluccas, Indonesia | TH: Ereuniasgrallator Jordan & Snyder, 1901 | Nierstrasz (1915); Avdeev (1984); Hadfield et al. (2016a) |
| Elthusaochotensis (Kussakin, 1979) Syn: Lironecaochotensis Kussakin, 1979 | TLoc: Sea of Ochosk (near the city of Ayan), western Pacific Ocean | TH: Unknown | Kussakin (1979) |
| Elthusaparabothi Trilles & Justine, 2004 | TLoc: New Caledonia, off Coëtlogon Bank | TH: Parabothuskiensis (Tanaka, 1918) | Trilles and Justine (2004) |
| Elthusaparva (Richardson, 1910) Syn: Ceratothoaparva (Richardson, 1910) | TLoc: Opol, Mindanao, Philippines | TH: Unknown | Richardson (1910); Hadfield et al. (2016b) |
| Elthusaphilippinensis (Richardson, 1910) Syn: Livonecaphilippinensis Richardson, 1910 | TLoc: Jolo Light, Philippines | TH: Unknown | Richardson (1910) |
| Elthusapoutassouiensis (Penso, 1939) Syn: Ceratothoapoutassouiensis (Penso, 1939) | TLoc: Babakale Port, Aegean Sea Coasts, Turkey | TH: Micromesistiuspoutassou (Risso, 1827) | Brian (1939); Penso (1939); Öktener et al. (2018b) |
| OL: Genova Gulf, Italy | |||
| Elthusapropinqua (Richardson, 1904) Syn: Livonecapropinqua Richardson, 1904 | TLoc: Port Heda, Japan | TH: Unknown | Richardson (1904, 1910); Barnard (1936); Bruce (1990) |
| OL: Arabian Sea; Laccadive Islands; India; Maldives; Myanmar; Japan; Philippines; Australia | OH: “chalinura”; “a macrurid”, “Macrurus”; Ventrifossacf.nigrodorsalis | ||
| Elthusaraynaudii (Milne Edwards, 1840) Syn: Livonecaraynaudii Milne Edwards, 1840 | TLoc: Cape of Good Hope, South Africa | TH: Unknown | See in text. |
| OL: See text | OH: See text | ||
| Elthusasacciger (Richardson, 1909) Syn: Livonecasacciger Richardson, 1909 | TLoc: Bungo Channel; Japan | TH: Synaphobranchus sp. | Avdeev (1984); Bruce (1990); Hata et al. (2017); Richardson (1909); Shiino (1951); Yamauchi (2009) |
| OL: North-western Pacific; Australia; Japan and Pacific coast | OH: Species from the families Synaphobranchidae; Sebastidae | ||
| Elthusasamariscii (Shiino, 1951) Syn: Lironecasamariscii Shiino, 1951 | TLoc: Japan | TH: Samariscusjaponicus Kamohara, 1936 | Shiino (1951); Biju Kumar and Bruce (1997) |
| OL: Kerala coast, India | Other hosts: Samariscristatus Gray, 1831 | ||
| Elthusasamoensis (Schioedte & Meinert, 1884) Syn: Livonecasamoensis Schioedte & Meinert, 1884 | TLoc: Samoa Islands (Samoenses islands) | TH: Unknown | Schioedte and Meinert (1884) |
| Elthusasigani Bruce, 1990 | TLoc: North Stradbroke Island, Moreton Bay, southeastern Queensland, Australia | TH: Siganusspinus (Linnaeus, 1758) | Bruce (1990) |
| Elthusasinuata (Koelbel, 1879) Syn: Livonecasinuata Koelbel, 1879 | TLoc: Mediterranean coast | TH: Cepolamacrophthalma (Linnaeus, 1758) | Koelbel (1879); Schioedte and Meinert (1884); Carus (1885); Gourret (1891); Gerstaecker (1901); Galati-Mosella (1920); Brian (1921); Monod (1924); Trilles (1968, 1977, 2008); Trilles and Raibaut (1973); Dollfus and Trilles (1976); Rokicki (1984, 1985); Trilles et al. (1989); Bello and Mariniello (1998); Trilles and Öktener (2004); Öktener et al. (2009, 2018a) |
| OL: North-West Africa; United Kingdom; Mediterranean; Adriatic Sea; Spain; France; Algeria; Tunisia; Italy; Yugoslavia; Montenegro; Turkey | OH: Species from the families Argentinidae; Bramidae; Cepolidae; Gobiidae; Loliginidae; Pleuronectidae; Rajidae; Sepiolidae; Sparidae; Trichiuridae | ||
| Elthusasplendida (Sadowsky & Moreira, 1981) Syn: Lironecasplendida Sadowsky & Moreira, 1981 | TLoc: South Western Atlantic Ocean | TH: Squaluscubensis Howell Rivero, 1936 | Sadowsky and Moreira (1981) |
| Elthusatropicalis (Menzies & Kruczynski, 1983) Syn: Lironecatropicalis Menzies & Kruczynski, 1983 | TLoc: off Egmont Key, Florida, USA | TH: Ogcocephalusparvus Longley & Hildebrand, 1940 | Menzies and Kruczynski (1983) |
| Elthusaturgidula (Hale, 1926) Syn: Livonecaturgidula Hale, 1926 | TLoc: Western Australia | TH: Unknown | Hale (1926); Bruce (1990) |
| OL: One Tree Island, Great Barrier Reef | OH: Species from the families Scaridae; Scaridae | ||
| Elthusavulgaris (Simpson, 1857) Syn: Livonecavulgaris Stimpson, 1857 | TLoc: San Francisco Bay; Tomales Bay; Monterey | TH: Unknown | Stimpson (1857); Richardson (1904); Turner et al. (1969); Hobson (1971); Brusca (1978, 1981); Bennett (1993); Espinosa-Pérez and Hendrickx (2001); Gamble et al. (2013) |
| OL: Pacific Ocean including the western coast of USA, Mexico and Colombia | OH: Species from the families Carangidae; Chaenopsidae; Cottidae; Cynoglossidae; Embiotocidae;Engraulidae; Gobiidae; Hexagrammidae; Moronidae; Paralichthyidae; Pleuronectidae; Scorpaenidae; Sebastidae; Serranidae; Synodontidae. Also “rock cod”, “flounder”, “lingcod” | ||
| Elthusawinstoni Hadfield, Tuttle & Smit, 2017 | TLoc: Hawaii | TH: Ctenochaetusstrigosus (Bennett, 1828); Acanthurusnigroris Valenciennes, 1835 | Hadfield et al. (2017) |
Supplementary Material
Acknowledgments
The project was funded through a Western Indian Ocean Marine Science Association (WIOMSA) Marine Research Grant for KA Hadfield. The financial assistance of the National Research Foundation (NRF) (NRF project IFR170210222411 Grant 109352, NJ Smit, PI) and the South African National Biodiversity Institute (SANBI) in conjunction with the Foundational Biodiversity Information programme (FBIP, S van der Wal) supported this research and is hereby acknowledged. Opinions expressed, and conclusions arrived at, are those of the authors and are not necessarily those of the NRF. This is contribution number 336 from the NWU–Water Research Group. Prof Liesl van As, Aquatic Parasitology Research Group, University of the Free State, is thanked for the donation of the specimen here described as Elthusaxena sp. n. We also thank the Captain and crew of the FRS Africana and Sharon du Plessis (Chief Scientist) for the opportunity to collect material during the April/May 2003 South Coast Hake Biomass Survey. We would also like to thank Albé Bosman, curator at the Iziko South African Museum and Lauré Corbari, curator at the National Museum of Natural History, Paris, for providing specimens and information on collections.
Citation
van der Wal S, Smit NJ, Hadfield KA (2019) Review of the fish parasitic genus Elthusa Schioedte & Meinert, 1884 (Crustacea, Isopoda, Cymothoidae) from South Africa, including the description of three new species. ZooKeys 841: 1–37. https://doi.org/10.3897/zookeys.841.32364
References
- Avdeev VV. (1975) Two representatives of parasitic isopods of the genus Lironeca (Cymothoidae) from the region of Australia and New Zealand. Parasitologia 3: 247–251. [PubMed] [Google Scholar]
- Avdeev VV. (1978) Notes on the distribution of the marine Cymothoidae (Isopoda, Crustacea) in the Australian–New Zealand region. Folia Parasitologica 25: 281–283. [Google Scholar]
- Avdeev VV. (1984) Localization of isopods of the family Cymothoidae in the gill and oral cavities of fishes. Parasitology 18: 23–29. [Google Scholar]
- Barnard KH. (1920) Contributions to the crustacean fauna of South Africa, 6: Further additions to the list of marine Isopoda. Annals of the South African Museum 17: 319–438. 10.5962/bhl.part.22318 [DOI] [Google Scholar]
- Barnard KH. (1936) Isopods collected by the RIMS “Investigator”. Records of the Indian Museum 38: 147–191. [Google Scholar]
- Barnard KH. (1940) Contributions to the crustacean fauna of South Africa, XII: Further additions to the Tanaidacea, Isopoda and Amphipoda, together with keys for the identification of the hitherto recorded marine and freshwater species. Annals of the South African Museum 33: 381–543. 10.5962/bhl.part.22318 [DOI] [Google Scholar]
- Barnard KH. (1955) Additions to the fauna-list of South African Crustacea and Pycnogonida. Annals of the South African Museum 43(1): 1–107. [Google Scholar]
- Bello G, Mariniello L. (1998) Occurrence of Livonecasinuata (isopoda, Cymothoidae) in the mantle cavity of Sepiolaligulata (Cephalopoda, Sepiolidae). Archive of Fishery and Marine Research 46: 37–42. [Google Scholar]
- Bennett T. (1993) Resource partitioning of two fish ectoparasites, Lironecavulgaris and Lironecacalifornica (Class Isopoda, Family Cymothoidae). MSc Thesis and Graduate Research, San Jose State University, San Jose.
- Beumer JP, Ashburner LD, Burbury ME, Jette E, Latham DJ. (1982) A checklist of the parasites of fishes from Australia and its adjacent Antarctic territories. Technical Communication no. 48. St Albans: Commonwealth Institute of Parasitology. Commonwealth Agricultural Bureaux, Farnham Royal, Slough, 107 pp 10.1017/s0022149x00028091 [DOI] [Google Scholar]
- Bleeker P. (1857) Recherches sur les Crustacés de L’Inde Archipelagique. II. Sur les Isopodes Cymothoadiens de L’Archipel Indien. Natuurkundige vereeniging in Nederlandsche-Indie, Batavia, Verhandelingen 2: 20–40. 10.5962/bhl.title.9908 [DOI] [Google Scholar]
- Brandt A, Poore GC. (2003) Higher classification of the flabelliferan and related Isopoda based on a reappraisal of relationships. Invertebrate Systematics 17: 893–923. 10.1071/IS02032 [DOI] [Google Scholar]
- Brian A. (1921) A proposito di un isopoda parassita detl’Atherinamochon cur. Monitore Zoologico Italiano 32: 20–24. [Google Scholar]
- Brian A. (1939) I parassiti del Nasello nel Mare Ligure (Clavellastellata (Kröyer) nuova pel Mediterraneo). Corriere della Pesca 13(9): 3–11. [Google Scholar]
- Brian A, Darteville E. (1949) Contribution a l’etude des isopodes marins et fluviatiles du Congo. Annals du Musee du Congo Beige, Série C. Zoologie 3, 1: 77–208.
- Bruce NL. (1990) The genera Catoessa, Elthusa, Enispa, Ichthyoxenus, Idusa, Livoneca and Norileca n. gen. (Isopoda, Cymothoidae), crustacean parasites of marine fishes, with descriptions of eastern Australian species. Records of the Australian Museum 42: 247–300. 10.3853/j.0067-1975.42.1990.118 [DOI] [Google Scholar]
- Bruce NL, Lew Ton HM, Poore GCB. (2002) Cymothoidae Leach, 1814. In: Poore GCB. (Ed.) Crustacea: Malacostraca: Syncarida and Peracarida: Isopoda, Tanaidacea, Mictacea, Thermosbaenacea, Spelaeogriphacea.CSIRO Publishing, Melbourne, 168–183. 10.1163/156854008x361012 [DOI]
- Brusca RC. (1978) Studies on the Cymothoid Fish Symbionts of the Eastern Pacific: (Crustacea: Isopoda: Cymothoidae); II, Systematics and Biology of Lironecavulgaris Stimpson 1857. Occasional papers of the Allan Hancock Foundation (NS) 2: 1–19. 10.1163/156854078x00718 [DOI] [Google Scholar]
- Brusca RC. (1981) A monograph on the IsopodaCymothoidae (Crustacea) of the eastern Pacific. Zoological Journal of the Linnaean Society 73: 117–199. 10.1111/j.1096-3642.1981.tb01592.x [DOI] [Google Scholar]
- Brusca RC, Coelho V, Taiti S. (2001) A guide to the coastal isopods of California. http://tolweb.org/notes/?note_id=3004
- Boyko CB, Bruce NL, Hadfield KA, Merrin KL, Ota Y, Poore GCB, Taiti S, Schotte M, Wilson GDF. (2008) World Marine, Freshwater and Terrestrial Isopod Crustaceans database – Elthusa Schioedte & Meinert, 1884. Register of Antarctic Species. http://ras.biodiversity.aq/aphia.php?p=taxdetails&id=118410 [on 2018-02-13]
- Carus JV. (1885) Coelenterata, Echinodermata, vermes, Arthropoda. Prodromus fauna Mediterranae, sive descriptio animalium Maris Mediterrenei incolarum quam comparata silva rerum quatenus innotuit odiectis locis et nominibus vulgaribus e orumque auctoribus in commodum Zoologorum. E. Schweizerbatsche, Stuttgart, 525 pp 10.5962/bhl.title.11523 [DOI] [Google Scholar]
- Chilton C. (1909) The Crustacea of the subantarctic Islands of New Zealand. Subantarctic Islands of New Zealand 26: 601–671. [Google Scholar]
- Chilton C. (1911) Scientific Results of the New Zealand Government Trawling Expedition, 1907. Crustacea. Records of the Canterbury Museum 1: 285–312. 10.5962/bhl.title.14022 [DOI] [Google Scholar]
- Chilton C. (1912) Miscellaneous notes on some New Zealand Crustacea. Transactions of the New Zealand Institute 44: 128–135. [Google Scholar]
- Coleman CO, Lowry JK, Macfarlane T. (2010) DELTA for Beginners: An introduction into the taxonomy software package DELTA. ZooKeys 45: 1–75. 10.3897/zookeys.45.263 [DOI] [Google Scholar]
- Dollfus RP, Trilles JP. (1976) A propos de la collection R.P. Dollfus, mise au point sur les cymothoadiens jusqu’à présent récoltés sur des téléostéens du Maroc et de l’Algérie. Bulletin du Muséum d’histoire naturelle 272: 821–830. 10.5962/bhl.title.46654 [DOI] [Google Scholar]
- Ellis I. (1981) Some type specimens of Isopoda (Flabellifera) in the British Museum (Natural History) and the isopods in the Linnaean collection. Bulletin of the British Museum of Natural History (Zoology) 40: 121–128. 10.5962/bhl.title.25418 [DOI] [Google Scholar]
- Eschmeyer WN. (2018) Catalog of Fishes: Genera, Species, References. http://research.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
- Espinosa-Pérez M, Hendrickx ME. (2001) Checklist of isopods (Crustacea: Peracarida: Isopoda) from the eastern tropical Pacific. Belgian Journal of Zoology 131: 43–56. [Google Scholar]
- Filhol H. (1885) Considerations relative it la faune des Crustaces de la Nouvelle Zelande. Bibliotheque de l’Ecole des Hautes it etudes, Section des Sciences naturelles 30: 1–60. 10.1093/ww/9780199540884.013.u212849 [DOI] [Google Scholar]
- Froese R, Pauly D. (2018) FishBase. World Wide Web electronic publication, version (05/2018). http://www.fishbase.org
- Galati-Mosella R. (1920) Sulla Livonecasinuata Koelbel, parassita di Cepolarubescens e di Atherinamocho. Monitore Zoologico Italiano 31: 1–10. [Google Scholar]
- Gamble M, Smith M, Chi Y. (2013) Cymothoid isopod parasitism of fishes in Campbell Cove, Bodega Bay, California, USA. Comparative Parasitology 80: 247–250. 10.1654/4540.1 [DOI] [Google Scholar]
- Gerstaecker A. (1882) Sechste Ordnung: Isopoda–Asseln. Bronn, HG, Klassen und Ordnungen des Thier-Reichs 5: 8–278. 10.5962/bhl.title.2054 [DOI] [Google Scholar]
- Gerstaecker A. (1901) Isopoda. In: Bronn HG (Ed.) Die Klassen und Ordnungen der Arthropoden wissenschaftlich dargestellt in Wort und Bild. Crustacea (Zweite Hälfte: Malacostraca), Fünfter Band. II Abtheilung, 278 pp 10.5962/bhl.title.9999 [DOI] [Google Scholar]
- Ghani N. (2003) Isopod parasites of marine fishes of Pakistan. Proceedings of Pakistan Congress of Zoology 23: 217–221. [Google Scholar]
- Gourret P. (1891) Les lemodipodes et les isopodes du Golfe deMarseille. Annales du Musée colonial de Marseille 4: 1–44. [Google Scholar]
- Hadfield KA, Bruce NL, Smit NJ. (2010) Redescription of the monotypic genus Cinusa Schioedte and Meinert, 1884 (Isopoda, Cymothoidae), a buccal-cavity isopod from South Africa. Zootaxa 2437: 51–68. [Google Scholar]
- Hadfield KA, Bruce NL, Smit NJ. (2013) Review of the fish-parasitic genus Cymothoa Fabricius, 1793 (Isopoda, Cymothoidae, Crustacea) from the southwestern Indian Ocean, including a new species from South Africa. Zootaxa 3640: 152–176. 10.11646/zootaxa.3640.2.2 [DOI] [PubMed] [Google Scholar]
- Hadfield KA, Bruce NL, Smit NJ. (2016a) Elthusanierstraszi nom. n., the replacement name for Elthusaparva (Nierstrasz, 1915), a junior secondary homonym of Elthusaparva (Richardson, 1910) (Isopoda, Cymothoidae). ZooKeys 619: 167–170. 10.3897/zookeys.619.10143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield KA, Bruce NL, Smit NJ. (2016b) Redescription of poorly known species of Ceratothoa Dana, 1852 (Crustacea, Isopoda, Cymothoidae), based on original type material. ZooKeys 592: 39–91. 10.3897/zookeys.592.8098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield KA, Tuttle LJ, Smit NJ. (2017) Elthusawinstoni sp. n. (Isopoda, Cymothoidae), a new fish parasitic isopod from Hawaii. ZooKeys 661: 125–135. 10.3897/zookeys.661.11251.figure1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale HM. (1926) Review of the Australian isopods of the cymothoid group. Part II. Transactions of the Royal Society of South Australia 50: 201–234. [Google Scholar]
- Hansen HJ. (1897) Reports on the dredging operations off the west coast of central America to the Galapagos, to the west coast of Mexico, and in the Gulf of California, in charge of Alexander Agassiz, carried on by the U.S. Fish Commission Steamer Albatross, during 1891, Lieut. Commander Z.L. Tanner, U.S.N., commanding. Bulletin of the Museum of Comparative Zoology at Harvard College 3: 95–129. 10.5962/bhl.part.27494 [DOI] [Google Scholar]
- Hata H, Sogabe A, Tada S, Nishimoto R, Nakano R, Kohya N, Takeshima H, Kawanishi R. (2017) Molecular phylogeny of obligate fish parasites of the family Cymothoidae (Isopoda, Crustacea): evolution of the attachment mode to host fish and the habitat shift from saline water to freshwater. Marine Biology 164(105): 1–15. 10.1007/s00227-017-3138-527980349 [DOI] [Google Scholar]
- Hatch MH. (1947) The Chelifera and Isopoda of Washington and adjacent regions. University of Washington Press 10: 155–274. [Google Scholar]
- Hewitt GC, Hine PM. (1972) Checklist of parasites of New Zealand fishes and of their hosts. New Zealand journal of marine and freshwater research 6: 69–114. 10.1080/00288330.1977.9515410 [DOI] [Google Scholar]
- Hobson ES. (1971) Cleaning symbiosis among California inshore fishes. Fishery Bulletin 69: 491–523. [Google Scholar]
- Hurley DE. (1961) A checklist and key to the Crustacean Isopoda of New Zealand and Subantarctic Is. Transactions of the Royal Society of New Zealand (Zoology) 1: 239–292. [Google Scholar]
- Iverson EW. (1974) Range extensions for some California marine lsopod Crustaceans. Bulletin of the Southern California Academy of Sciences 73: 164–169. [Google Scholar]
- Kensley B. (1976) Isopodan and Tanaidacean Crustacea from St. Paul and Amsterdam Islands, southern Indian Ocean. Annals of the South African Museum 69: 261–323. [Google Scholar]
- Kensley B. (1978) Guide to the marine isopods of southern Africa. The Rustica Press (Pty.) Ltd. , Trustees of the South African Museum, Wynberg, Cape Town, 173 pp. [Google Scholar]
- Kensley BF, Brusca RC. (2001) Isopod systematics and evolution. Crustacean Issues 13. A. A. Balkema, Rotterdam/Brookfield, 357 pp. [Google Scholar]
- Keys AB. (1928) Ectoparasites and vitality. The American Naturalist, New York, 63: 279–282. [Google Scholar]
- Koelbel C. (1879) Uber einige neue Cymothoiden. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Classe 78: 401–416. 10.5962/bhl.title.60847 [DOI] [Google Scholar]
- Kononenko AF. (1988) Lironecaatlantniroi sp. n (Isopoda, Cymothoidae), a parasitic isopoda from fishes of the north-atlantic. Parazitologiya 22: 425–428. [Google Scholar]
- Krauss F. (1843) Die Siidafrikanischen Crustaceen. Eine Zusammenstellung aller bekannten Malacostraca, Bemerkungen iiber deren Lebensweise und geographische Verbreitung, nebst Beschreibung und Abbildung mehrer neuen Arten, Stuttgart, 1–68. 10.5962/bhl.title.4825 [DOI]
- Kussakin OG. (1979) Marine and brackish isopods (Isopoda) of cold and temperate waters of the northern hemisphere, vol. 1. Suborder Flabellifera. Opredeliteli po faune SSSR, izdavaemye Zoologicheskim institutom Akademii nauk SSSR 122: 1–470. [Google Scholar]
- Mañé-Garzón F. (1979) Una nueve especie del genero Lironeca Leach, 1818 (IsopodaCymothoidae) de la costa oceanica del Uruguay. Revista de Biologia del Uruguay 12: 11–22. [Google Scholar]
- Menzies RJ. (1962) The zoogeography, ecology, and systematics of the Chilian marine isopods. The Lund University Chile Expedition, 1948–49, no. 42. Lunds Universitets Arskriffter, 2, Bund 57: 1–162.
- Miers EJ. (1874) Descriptions of some new species of Crustacea, chiefly from New Zealand. Annals and Magazine of Natural History series 4, 17: 218–229. 10.1080/00222937608681934 [DOI]
- Miers EJ. (1877) On a collection of Crustacea, Decapoda and Isopoda, chiefly from South America, with descriptions of new species. Proceedings of the Zoological Society (London) 43: 653–679. [Google Scholar]
- Miers EJ. (1881) Crustacea. In: Account of the Zoological Collection made during the Survey of H.M.S. Alert in the Straits of Magellan and on the Coast of Patagonia. Proceedings of the Zoological Society of London 21: 61–140. 10.1111/j.1096-3642.1881.tb01270.x [DOI] [Google Scholar]
- Milne Edwards H. (1840) Histoire Naturelle des Crustacés III, Comprenent l’anatomie, la physiologie et la classification de ces animaux, vol. 3. Librairie encyclopédique de Roret, Paris, 638 pp 10.5962/bhl.title.6234 [DOI] [Google Scholar]
- Monod T. (1924) Isopoda. In: Parasitologia Mauritanica. Matériaux pour la faune parasitologique en Mauritanie.Bulletin de ComitéÉtudes Historiques et Scientifiques de I’Afrique Occidentale Française 9: 67–84 (428–445). [Google Scholar]
- Moreira PS, Sadowsky V. (1978) An annotated bibliography of parasitic lsopoda (Crustacea) of Chondrichthyes. Boletim do Instituto Oceanográfico 27: 95–152. 10.1590/s0373-55241978000200005 [DOI] [Google Scholar]
- Nierstrasz HF. (1915) Die Isopoden-Sammlung im Naturhistorischen Reichsmuseum zu Leiden – 1. Cymothoidae. Zoologische Mededelingen (Leiden) 1: 71–108. [Google Scholar]
- Nierstrasz HF. (1931) Isopoda genuina. II. Flabellifera In: Weber M, De Beaufort LF (Eds) Die Isopoden der Siboga-Expedition Siboga Expeditie (Uitkomsten op Zoölogisch, Botanisch, Oceanographisch en Geologisch Gebied verzameld in de Oost-Indische 1899–1900 aan boord HM Siboga onder commando van Luitenant ter zee 1e kl GF Tydeman) EJ Brill, Leiden, 123–233. 10.5962/bhl.title.10641 [DOI]
- Öktener A, Trilles J-P, Alas A. (2018b) Elthusapoutassouiensis (Penso, 1939), comb. nov (Isopoda, Cymothoidae) for Meinertia (Ceratothoa) poutassouiensis, parasite of the blue whiting, Micromesistiuspoutassou. Bulletin of the European Association of Fish Pathologists, 38(1): 12–23. [Google Scholar]
- Öktener A, Trilles J-P, Alaş A, Solak K. (2009) Cymothoid (Crustacea, isopoda) records on marine fishes (Teleostei and Chondrichthyes) from Turkey. Bulletin European Association of Fish Pathologists 29: 51–57. [Google Scholar]
- Öktener A, Trilles J-P, Kocabaş E, İnceoğlu H, Alaş A. (2018a) Redescription of Elthusasinuata (Koelbel, 1879) comb. nov. (Isopoda, Cymothoidae) parasitizing the red bandfish in Turkey. Thalassas: An International Journal of Marine Sciences. 10.1007/s41208-018-0068-z [DOI]
- Olson AC. (1972) Argulusmelanostictus and other parasitic crustaceans on the California grunion, Leuresthestenuis (Osteichthyes: Atherinidae). The Journal of Parasitology 58: 1201–1204. 10.2307/3278165 [DOI] [PubMed] [Google Scholar]
- Penso G. (1939) Nuovo parassita e nuova parassitosi del “Gadus potassou”. Corriere della Pesca Anno 12: 1.
- Pillai NK. (1954) A preliminary note on the Tanaidacea and Isopoda of Travancore. Bulletin of the Central Research Institute, University of Travancore, Trivandrum 3: 1–22. [Google Scholar]
- Poore GC. (1981) Marine Isopoda of the Snares Islands, New Zealand–1. Gnathiidea, Valvifera, Anthuridea, and Flabellifera. New Zealand Journal of Zoology 8: 331–348. 10.1080/03014223.1981.10430613 [DOI] [Google Scholar]
- Powell AWB. (1959) Native Animals of New Zealand. Auckland Museum Handbook of Zoology. Unity Press, Auckland, 96 pp. [Google Scholar]
- Richardson H. (1904) Contributions to the natural history of the Isopoda. Proceedings of the United States National Museum 27: 1–89. 10.5479/si.00963801.27-1350.113 [DOI] [Google Scholar]
- Richardson H. (1909) Isopods collected in the northwest Pacific by the U.S. bureau of fisheries steamer “Albatross” in 1906. Proceedings of the United States National Museum 37: 75–129. 10.5479/si.00963801.37-1701.75 [DOI] [Google Scholar]
- Richardson H. (1910) Marine isopods collected in the Philippines by the US Fisheries steamer Albatross in 1907–1908. Department of Commerce and Labor (USA), Bureau of fisheries document. Bureau of Fisheries 736: 1–44. 10.5962/bhl.title.82673 [DOI] [Google Scholar]
- Rocha-Ramírez A, Chávez-López R, Bruce NL. (2005) Elthusaalvaradoensis n. sp. (Isopoda, Cymothoidae) from the gill chamber of the lizardfish, Synodusfoetens (Linnaeus, 1766). Crustaceana 78: 701–707. 10.1163/156854005774353430 [DOI] [Google Scholar]
- Rokicki J. (1984) Parasitic isopods of the NW African shelf in connection with their occurrence in fish. Zedzyty Naukowe. Rozporządzenie Monograms 50: 1–222. [Google Scholar]
- Rokicki J. (1985) Biology of adult isopoda (Crustacea) parasitizing fishes of north-West Africa shelf. Acta ichthyologica et piscatoria 15: 95–122. 10.3750/aip1985.15.1.06 [DOI] [Google Scholar]
- Ruiz-Campos G. (1986) New Hosts for Lironecamenziesi Brusca, 1981 (Crustacea, Isopoda, Cymothoidae) with some comments on preference for them. Ciencias Marinas 12: 99–104. 10.7773/cm.v12i2.505 [DOI] [Google Scholar]
- Sadowsky V, Moreira PS. (1981) Occurrence of Squaluscubensis Rivero, 1936, in the Western South Atlantic Ocean, and incidence of its parasitic isopod Lironecasplendida sp. n. Studies on Neotropical Fauna and Environment 16: 137–150. 10.1080/01650528109360588 [DOI] [Google Scholar]
- Saito N, Yamauchi T. (2016) A new species and new host records of the genus Elthusa (Crustacea: Isopoda: Cymothoidae) from Japan. Crustacean Research 45: 59–67. 10.18353/crustacea.45.0_59 [DOI] [Google Scholar]
- Schioedte JC, Meinert F. (1884) Symbolæ ad monographium Cymothoarum crustaceorum isopodum familiæ. IV. Cymothoidæ Trib. II. Cymothoinæ. Trib. III: Livonecinæ. Naturhistorisk tidsskrift, Kjøbenhavn 14: 221–454. 10.5962/bhl.title.10300 [DOI] [Google Scholar]
- Shiino SM. (1951) On the cymothoid Isopoda parasitic on Japanese fishes. Bulletin of the Japanese Society of Scientific Fisheries 16: 81–89. [Google Scholar]
- Sivertsen E, Holthuis LB. (1980) The marine isopod Crustacea of the Tristan da Cunha Archipelago. Gunneria 33: 1–128. [Google Scholar]
- Smit NJ, Bruce NL, Hadfield KA. (2014) Global diversity of fish parasitic isopod crustaceans of the family Cymothoidae. International Journal for Parasitology: Parasites and Wildlife 3: 188–197. 10.1016/j.ijppaw.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MM, Heemstra PC. (1986) Smith’s sea fishes. Springer, South Africa, 1047 pp. [Google Scholar]
- Stebbing TRR. (1905) Report on the Isopods collected by Professor Herdman at Ceylon, in 1902. In: Herdman WA. (Ed.) Report to the Government of Ceylon on the Pearl Oyster Fisheries in the Gulf of Manaar.Vol 4, Supplementary report 23. The Royal Society, London, 1–64. 10.5962/bhl.title.23477 [DOI]
- Stebbing TRR. (1910) General catalogue of South African Crustacea Part V of SA Crustacea, for the Marine Investigations in South Africa. Annals of the South African Museum 6: 281–599. 10.5962/bhl.part.15558 [DOI] [Google Scholar]
- Stephenson AB. (1969) Ironamelanosfiicta (Isopoda, Cymothoidae). A new record for New Zealand waters, with descriptions of male, female and larval states. Records of the Auckland Institute and Museum 6: 427–34. [Google Scholar]
- Stephenson AB. (1987) Additional notes on Lironecaneocyuus (Isopoda, Cymothoidae). Records of the Auckland Institute and Museum 24: 135–142. [Google Scholar]
- Stimpson W. (1857) The Crustacea and Echinodermata of the Pacific shores of North America. Boston Society of Natural History 6: 503–513. 10.5962/bhl.title.59693 [DOI] [Google Scholar]
- Thielemann M. (1910) Beitriige zur Naturgeschichte Ostasiens. Herausgegeben von F. Doflein. Band H, no. 9. Beitrage zu Kenntnis der isopodenfauna Ostasiens. Abhandlungen der Mathematisch-Naturwissenschaftlichen Klasse der K. Bayer. Akademia der Wissenschaften 2, 3: 1–109. 10.1002/ange.19390522011 [DOI]
- Thomson GM, Chilton C. (1886) Critical list of the CrustaceaMalacostraca of New Zealand. Transactions of the New Zealand Institute 18: 141–159. [Google Scholar]
- Trilles J-P. (1968) Recherches sur les Isopodes Cymothoidae des côtes Françaises. PhD Thesis, University of Montpellier, Montpellier.
- Trilles J-P. (1976) Les Cymothoidae (Isopoda, Flabellifera) des collections du Muséum National d’Histoire Naturelle de Paris. IV. Les Lironecinae Schioedte & Meinert, 1884. Bulletin du Muséum National d’Histoire Naturelle, 3e serie (Zoologie) 390: 773–800. 10.5962/bhl.title.58212 [DOI]
- Trilles J-P. (1977) Les Cymothoidae (Isopoda, Flabellifera; parasites de poissons) du Rijksmuseum van Natuurlijke Historie de Leiden Méditerranée et Atlantique nord-oriental. Zoologische Mededelingen 52: 7–17. [Google Scholar]
- Trilles J-P. (1994) Les Cymothoidae (Crustacea, Isopoda) du Monde. Prodrome pour une faune. Studia Marina 21–22: 1–288.
- Trilles J-P. (2008) Some marine isopods from the Senckenberg research institute (Frankfurt am main, Germany). Senckenbergiana Biologica 88: 21–28. [Google Scholar]
- Trilles J-P, Justine JL. (2004) A new cymothoid species, and three aegids (Crustacea, Isopoda), collected from deep-sea fish off New Caledonia. Zoosystema 26: 211–233. [Google Scholar]
- Trilles J-P, Justine JL. (2006) Elthusaarnoglossi sp. nov. (Crustacea: Isopoda: Cymothoidae), a branchial parasite of flatfishes (Bothidae) from the Chesterfield Islands, New Caledonia. Zootaxa 1338: 57–68. 10.2478/s11686-010-0020-8 [DOI] [Google Scholar]
- Trilles J-P, Justine JL. (2010) Elthusaepinepheli sp. nov. (Crustacea, Isopoda, Cymothoidae) a branchial parasite of the grouper Epinephelushowlandi (Serranidae, Epinephelinae) from off New Caledonia. Acta Parasitologica 55: 177–187. 10.2478/s11686-010-0020-8 [DOI] [Google Scholar]
- Trilles J-P, Radujkovic BM, Romestand B. (1989) Parasites de poisons marins du Montenegro: isopodes. In: Radujkovic BM, Raibaut A. (Eds) Faune des parasites des poissons marins des côtes du Montenegro (Adriatique sud).Acta Adriatica 30: 279–306.
- Trilles JP, Raibaut A. (1973) Sur les Cymothoidae (Isopoda, Flabellifera) parasites de poissons marins de Tunisie (2ème note). Bulletin du Muséum d’histoire naturelle 88: 273–281. [Google Scholar]
- Trilles J-P, Randall JE. (2011) Redescription of Elthusaemarginata (Bleeker, 1857) (Crustacea, Isopoda, Cymothoidae), type species of the genus Elthusa Schioedte & Meinert, 1884. Marine Biology Research 7: 453–465. 10.1080/17451000.2010.528770 [DOI] [Google Scholar]
- Trilles JP, Öktener A. (2004) Livonecasinuata (Crustacea; isopoda; Cymothoidae) on Loligovulgaris from Turkey, and unusual cymothoid associations. Diseases of Aquatic Organisms 61: 235–240. 10.3354/dao061235 [DOI] [PubMed] [Google Scholar]
- Turner CH, Ebert EE, Given RR. (1969) Man-made reef ecology. California Department of Fish and Game Bulletin 146: 1–221. [Google Scholar]
- Waugh DN, Bennett T, Dugon TL. (1989) The Incidence of the Cymothoid Isopod Lironecacalifornica on Fishes in Campbell Cove, Sonoma County, California. Bulletin of the Southern California Academy of Sciences 88: 33–39. [Google Scholar]
- Wetzer R, Kuck HG, Baez P, Brusca RC, Jurkevics LM. (1991) Catalog of the isopod Crustacea type collection of the Natural History Museum of Los Angeles County. Natural History Museum, Los Angeles, 69 pp 10.1130/abs/2016am-285330 [DOI] [Google Scholar]
- White A. (1847) List of the specimens of Crustacea in the collection of the British Museum. British Museum (Natural History), Department of Zoology, London, 143 pp 10.5962/bhl.title.1708 [DOI] [Google Scholar]
- Whitelegge T. (1901) Crustacea. Part II. ln: Scientific Results of the trawling Expedition of H.M.C.S. “Thelis” of the Coast of New South Wales in February and March, 1898. Australian Museum, Sydney 4: 203–246. 10.3853/j.0067-1967.4.1901.471 [DOI] [Google Scholar]
- Whitelegge T. (1902) Crustacea. Part II. Isopoda. Part 1. In: Scientific Results of the Trawling Expedition of HMCS Thetis, Vol. 1. Memoirs of the Australian Museum 4: 201–246. [Google Scholar]
- Williams EH, Bunkley-Williams L, Ebert DA. (2010) An accidental attachment of Elthusaraynaudii (Isopoda, Cymothoidae) in Etmopterus sp. (Squaliformes, Etmopteridae). Acta Parasitologica 55: 99–101. 10.2478/s11686-010-0006-6 [DOI] [Google Scholar]
- Yamauchi T. (2009) Deep-sea cymothoid isopods (Crustacea: Isopoda: Cymothoidae) of Pacific coast of northern Honshu, Japan. National Museum of Nature and Science Monographs 39: 467–481. [Google Scholar]
- Young MW. (1926) Marine biological notes no. 2. Fecundity of Livonecaraynaudii Milne-Edw. (synonym: Livonecanovae-zeelantjiae Miers). New Zealand Journal of Science and Technology 8: 282–286. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.