Abstract
Background
Cell division cycle 20 (CDC20) is frequently overexpressed in malignant tumours and involved in the differentiation process of hematopoietic stem cells. However, the role of CDC20 in prostate cancer stem-like cells (CSCs) remains poorly understood.
Methods
The expression of CDC20, CD44, β-catenin were examined in prostate cancer specimens by immunohistochemistry assay, the role of CDC20 on the stem-like properties of prostate CSCs was accessed by real-time quantitive PCR, spheroid formation, in vitro and in vivo limiting dilution assay.
Finding
CDC20 was associated with malignant progression of prostate cancer, the patients with both high expression CDC20 and CD44 or β-catenin were associated with more aggressive clinicopathological features and poor prognosis. CDC20 was usually enriched in CD44+ prostate CSCs. Knockdown of CDC20 could inhibit the expression of stemness-related genes, self-renewal ability, chemo-resistance, invasion capability and tumorigenicity of CD44+ prostate CSCs. Mechanistically, CDC20 promoted degradation of Axin1, the core member of β-catenin destruction complex, sequentially reduced the phosphorylation of β-catenin, promoting the latter into the nucleus, thereby enhancing the self-renewal capacity of CD44+ prostate CSCs.
Interpretation
Our results indicated that CDC20 maintains the self-renewal ability of CD44+ prostate CSCs by promoting nuclear translocation and trans-activation of β-catenin. In addition, CDC20 combined with CD44 or β-catenin can serve as an important indicator for prognosis of patients with prostate cancer.
Keywords: Prostate cancer, CDC20, Cancer stem like cell, β-catenin, Disease progression
Research in context.
Evidence before this study
Cancer stem-like cells (CSCs) have been implicated in drug resistance and disease progression of prostate cancer, which is the first most frequently diagnosed malignancies and the second leading cause of cancer-related deaths among men in developed countries. We previously reported that cell division cycle 20 (CDC20) was up-regulated in high Gleason score tissues of prostate cancer, and CDC20 exerts its carcinogenic function in many malignancies mainly during the metaphase to anaphase by disrupting key cell cycle regulators.
Added value of this study
Our study showed that CDC20 maintains the self-renewal ability of CD44+ prostate CSCs by promoting nuclear translocation and trans-activation of β-catenin. In addition, CDC20 combined with CD44 or β-catenin can serve as an important indicator for prognosis of patients with prostate cancer.
Implications of all the available evidence
Our findings shared new insights into the relationship between CDC20 and stem-like features of CSCs during tumorigenesis in prostate cancer, suggesting inhibition of CDC20 may prevent or slow prostate cancer progression by inactivating CSCs subset.
Alt-text: Unlabelled Box
1. Introduction
Prostate cancer is the first most frequently diagnosed malignancies and the second leading cause of cancer-related deaths among men in developed countries [1]. Although prostate cancer can often be cured at early stage [2], patients with metastasis have to go through androgen deprivation therapy (ADT) [3] and inevitably progress into a drug resistance stage called castration-resistant prostate cancer (CRPC), leading to increased cancer-related deaths [4]. For patients with metastatic CRPC, docetaxel-based chemotherapy is recommended as one of the first-line treatments by multiple guidelines [5,6]. However, a limited survival benefit has been proven due to the chemo-resistance and relapse of prostate cancer, the mechanism underlying the resistance to docetaxel for metastatic CRPC, therefore, remains obscure.
Increasing evidence has revealed that the critical role of cancer stem-like cells (CSCs) in chemo-resistance and relapse of prostate cancer [7,8]. The hypothesis of CSC suggests that tumours are maintained in the hierarchical tissues of cells, and that cancer stem cell-like cell subsets are essential for initiating and maintaining tumour growth due to their strong self-renewal capacity [9]. The presence of CSC was first identified in myeloid leukemia [10] and has recently been observed in various solid malignancies [11]. Concrete evidence indicated the importance of prostate CSCs in tumorigenesis and anticancer drug resistance [12]. Previous studies identified several cell surface antigens to characterize prostate CSCs, such as CD44 [[13], [14], [15], [16], [17]], CD133 [18] and OV6 [19]. Therefore, targeting prostate CSCs to make them sensitive to chemotherapy can be used as a novel therapeutic paradigm for metastatic CRPC patients.
In our previous study, we identified five hub genes associated with poorly differentiated prostate cancer, including cell division cycle 20 (CDC20) [20], a regulator of cell cycle checkpoints which activates adenomatous polyposis coli (APC) [21]. CDC20 is a key E3 ligase, a protein containing the WD40 repeat domain that binds to APC and recognises D-box or KEN box substrates to promote proteasomal degradation. APC/CDC20 exerts its carcinogenic function during the metaphase to anaphase by disrupting key cell cycle regulators [22]. Abnormal levels or dysfunction of CDC20 can abolish mitotic arrest, thereby promoting premature lateness by relaxing APC activation, leading to aneuploidy in daughter cells [23]. Interestingly, increased CDC20 expression has been reported to be associated with poor pathological features and poor prognosis in many human cancer types [[24], [25], [26]]. In addition, previous studies showed that CDC20 regulates hematopoiesis and leukemia in hematopoietic stem cells by promoting ubiquitination of MEIS1 and p21, and maintains the self-renewal potential of human glioblastoma by regulating SOX2 protein and promoting SOX2-dependent transcription [27]. However, the expression and role of CDC20 in prostate CSCs remains poorly understood.
In this study, the functional role of CDC20 in maintaining the stem like characteristics of prostate CSCs was assessed. Our founding indicated that CDC20 was preferentially enriched in prostate CSC and associated with poor prognosis in prostate cancer patients. Simultaneously, the interference of CDC20 resulted in the reduction of prostate CSCs by inhibiting their self-renewal, drug resistance, invasion capability and tumorigenicity. In addition, our results suggested that CDC20 exerts a key role in maintaining the stem-like properties of CD44+ CSCs in prostate cancer by promoting nuclear translocation and transactivation of β-catenin. Thus, our findings shared new insights into the relationship between CDC20 and stem-like features of CSC during tumorigenesis. Therefore, inhibition of CDC20 may be utilized to inhibit prostate cancer progression by inactivating CSC subset.
2. Materials and methods
2.1. Patients and specimens
We followed the reporting recommendations for tumour marker prognostic studies (REMARK) in this study [28]. Clinical data, tissue specimens and follow-up information were collected for 121 consecutive prostate cancer patients who were pathologically diagnosed and received prostatectomy between 2012 and 2013, and 8 prostate cancer patients who received prostate needle biopsy after docetaxel treatment. The clinical data of 121 prostate cancer patients included age at diagnosis, preoperative PSA (the maximum within 6 months before the operation), the postoperative pathological status including Gleason score (GS), pathological T stage, surgical margins (SM). Localised PCa refers to T1-T2, and locally advanced PCa (LAPC) refers to the patients with T stage ≥ T3 (Tumour extends through the prostatic capsule) according to the 2018th guideline of European Association of Urology. The time to biochemical recurrence (BCR) (cutoff: PSA = 0.2 ng/mL) and disease progression identified by MRI, CT or ECT were selected as the clinical endpoint of biochemical relapse free survival and disease-free survival, respectively. Hematoxylin and eosin (H&E)-stained sections of the prostate cancer specimens were re-evaluated by two experienced pathologists (Jun-hui Ge and Xi-quan Yang) to identify representative areas in double blind procedure. Tumour stage and GS were assessed according to the American Joint Committee on Cancer (AJCC) 2002 and the international society of urological pathology (ISUP) consensus conference 2014 [29]. The samples were obtained after writing informed consent from patients according to an established protocol approved by the Ethics Committee of Second Military Medical University. The clinical features are summarized in Supplementary Table S1.
2.2. Gene knockdown, RNA interference and plasmid transfection
Short hairpin RNA (shRNA) interference vector pLKO.1-GFP containing an U6 promoter upstream of the shRNA, lentivirus packaging vector pVSVG-I and pCMV-GAG-POL were obtained from Shanghai Integrated Biotech Solutions Co, Ltd., (Shanghai, China). Green fluorescent protein (GFP) was used as an internal control with an independent promoter. The C4–2B or DU145 cell line was cultured in 6-well plates, inoculated at a density of 5 × 104 cells/mL, and transfected with the shRNAs-expressing lentivirus (Lv-shCDC20 or Lv-sh-β-catenin) or control lentivirus at a multiplicity of infection (MOI) of 45. After 72 h transfection, they were observed and photographed under microscope. The sequences for shRNA were shown in Supplementary Table S2.
The siRNA (si-CDC20) were purchased from Shanghai Integrated Biotech Solutions Co, Ltd., (Shanghai, China), and siRNA transfection was carried out using RNA iMAX reagents (L3000015, Invitrogen, Shanghai, China) according to the manufacturer's protocols. The sequences for siRNA were presented in Supplementary Table S2.
Transfection of CDC20 pcDNA 3.1-WT or -MT plasmid was carried out using Lipofectamine 3000 reagents (L3000015, Invitrogen) according to the manufacturer's protocols, the sequence of CDC20 plasmids were shown in Supplementary Table S2.
2.3. Real-time polymerase chain reaction (PCR)
Total RNA was prepared from cultured cells from C4–2B or DU145 using RNAiso Plus (9109, Takara, Japan) according to the manufacturer's protocol. Reverse transcriptase PCR (RT-PCR) was performed using PrimeScript One Step RT reagent Kit (RR037B, Takara, Japan) in the presence of random primers. Amplification of the generated cDNA was carried out in SYBR Green Realtime PCR Master Mix (QPK201, Toyobo, Japan) with ABI PRISM 7300HT Sequence Detection System. Each measurement was performed in triplicate and the results were normalized by the expression of the β-actin gene. Fold change relative to mean value was determined by 2–△△Ct. The primer sequences were presented in Supplementary Table S2.
2.4. Animal experiments
All experimental animal procedures were approved by the Animal Care and Use Committee of the Second Military Medical University (Shanghai, China). Nude mice (male, 6-weeks old) were purchased from the Shanghai Laboratory Animal Centre (SLAC, China) and housed under specific pathogen-free (SPF) conditions.
For the in vivo limiting dilution assay, the sorted C4–2B CD44+ and DU145 CD44+ cells with or without Lv-shCDC20 stable transfection were serially diluted to the appropriate cell dose. Cells were injected and the number of tumours formed from each cell dose injected was scored. The frequency of cancer stem cells had been calculated using the ELDA software provided by the Walter and Eliza Hall Institute (http://bioinf.wehi.edu.au/software/elda/index.html).
2.5. Statistical analysis
Numerical data were expressed as the Mean ± S.D. Statistical differences between variables were analysed by chi-square test or Fisher's exact test for categorical/binary measures and ANOVA for continuous measures. Survival curve was plotted by the Kaplan-Meier method and compared using the log-rank analysis. Difference was considered significant at P < .05. All experiments for cell cultures were performed independently at least three times and in triplicate each time. Data analysis was performed by the GraphPad Prism 5 and SPSS 22.0.
Supplementary information of materials and methods are described in Supplementary materials and methods.
3. Results
3.1. Up-regulated CDC20 is associated with poor prognosis, and combination of CDC20 and CD44 expression is a significant prognostic factor in prostate cancer patients
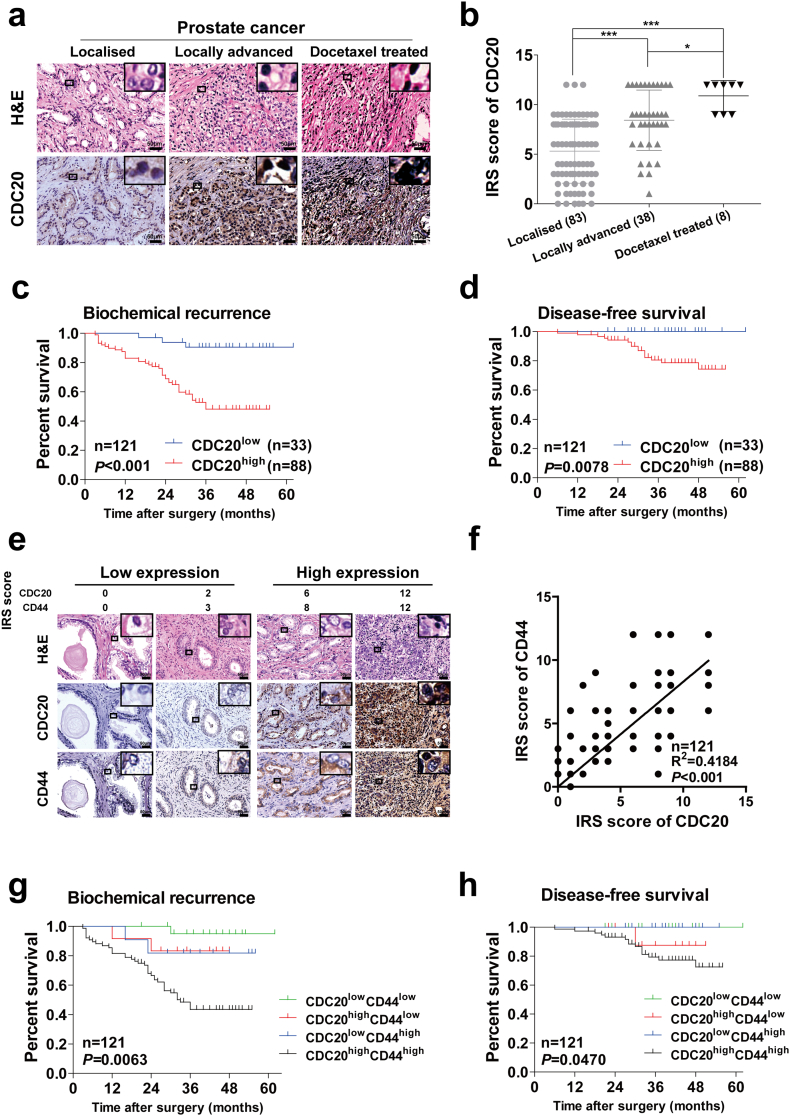
Previous study has indicated that CDC20 is up-regulated in prostate cancer tissues [30], and higher Gleason score tissues exert higher CDC20 expression level which was shown in our recent study [20]. To confirm the fundamental role of CDC20 in prostate cancer progression, firstly, we detected the CDC20 expression level in different stages of prostate cancer development. Samples from locally advanced prostate cancer (LAPC), docetaxel treated prostate cancer exhibited higher CDC20 expression than localised prostate cancers (Fig. 1a, b), Similarly, analysis of various types of prostate cancer cell lines demonstrated relatively higher expression of CDC20 in the more aggressive cell lines such as C4–2B and DU145 (Supplementary Fig. S1a). According to the expression level of CDC20, all 121 prostate cancer patients were divided into CDC20low and CDC20high groups, as shown in Supplementary Table S1, the patients with high expression of CDC20 showed more aggressive features in superior Gleason score, higher T-stage and positive surgical margin, they presented much worse biochemical recurrence (BCR) (P < .001) and disease-free survival (DFS) (P = .0078) through Kaplan-Meier analysis (Fig. 1c, d), which further indicated that CDC20 may be used as a predictor for poor survival of prostate cancer patients.
Fig. 1.
Up-regulated CDC20 in advanced prostate cancer tissues was associated with poor prognosis, and combination of CDC20 and CD44 expression is a significant prognostic factor in prostate cancer.
(a) Hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) staining for CDC20 in prostate cancer tissues ranked by different disease stages as localised (n = 83), locally advanced (n = 38), docetaxel treated (n = 8) were presented (scale bar = 50 μm). (b) Expression of CDC20 in different stages was evaluated by IRS score, which was listed in Materials and Methods (P value: Wilcoxon test), values represented as the mean ± SD. (c, d) Kaplan-Meier curves for biochemical recurrence (BCR) (c) and disease-free survival (DFS) (d) of prostate cancer patients were analysed according to CDC20 expression (P value: log rank test). (e) H&E staining and IHC staining of CDC20 and CD44 in prostate cancer tissues (scale bar = 50 μm); (f) Correlation analysis of CDC20 and CD44 expression in different prostate cancer tissues (Spearman r2 = 0.4184; P value <.001). (g, h) BCR (g) and DFS (h) of prostate cancer patients in different groups were compared according to CDC20 and CD44 expression using Kaplan-Meier curves (P value: log rank test). All p values were defined as: *p < .05, **p < .01 and ***p < .001.
Given that the crucial role of CSCs in recurrence and aggressive progression of prostate cancer [7], we further investigated the potential role of CDC20 in regulating prostate CSCs. Many studies showed that CD44 positive cells can be recognised as putative prostate CSC subpopulation in prostate cancer [[13], [14], [15], [16], [17]], we therefore assessed the relationship between CDC20 and CD44 expression in 121 prostate cancer patients using immunohistochemical (IHC) analysis, high levels of CDC20 indeed exhibited elevated CD44 staining in prostate cancer tissues (Fig. 1e, f), suggesting a positive correlation between CDC20 and CD44 expression. We next explored if co-expression of CDC20 and CD44 exerts aggressive biological behaviors of prostate cancer. All 121 patients were divided into four groups according to the expression level of CDC20 and CD44 in prostate cancer samples. As expected, the patients with CDC20high/CD44high were associated with more aggressive characteristics such as Gleason score, T-stage (Supplementary Table S3), and suffered worse BCR (P = .0063) (Fig. 1g) and inferior DFS (P = .047) (Fig. 1h), compared with other groups. Taken together, CDC20 may correlate with expansion of prostate CSCs, and co-expression of CDC20 and CD44 could serve as an effective predictor for the prognosis of prostate cancer.
3.2. CDC20 is predominantly enriched in prostate CSCs
Given that CDC20 expression is correlated with CD44 in prostate cancer tissues, whether it is required for maintenance of prostate cancer stem-like cells (CSCs) needed to be addressed. We first established three CSC enrichment model: spheroid cells, CD44+ subpopulation (Supplementary Fig. S1b) and chemo-resistant cell lines (Supplementary Fig. S1c). The expression level of CDC20 in these three CSCs enrichment model was determined. CDC20 expression level was higher in spheres from C4–2B and DU145 cells than in their corresponding adherent cells, which was similar as some representative stemness associated genes such as CD44, CD133, SOX2, OCT4 (Fig. 2a–c). Next, we examined the CDC20 expression in CD44 positive and negative cells from C4–2B and DU145, as anticipated, CDC20 expression level was higher in CD44 positive cells than that in negative cells, and so as some representative stemness associated genes (Fig. 2d–f). It is acknowledged that chemo-resistant cells are enriched in CSCs [31], we then detected the CDC20 expression in docetaxel (first-line chemotherapy for metastatic CRPC patients) resistant and control cells from C4–2B and DU145, CDC20 expression level was higher in docetaxel resistant cells than that in control cells, which is analogous to some representative stemness associated genes (Fig. 2g–i), and this founding was accordance to the previous studies [32,33]. In conclusion, CDC20 is predominantly enriched in prostate CSCs, suggesting a putative role of CDC20 in the maintenance of prostate CSCs.
Fig. 2.
CDC20 is predominantly enriched in prostate CSCs.
(a–c) The mRNA expression of a series of stemness related genes (CD44, CD133, SOX2, OCT4) was analysed by quantitative real time PCR (qRT-PCR) in adherent and spheroid prostate cancer cell lines C4–2B (a) and DU145 (b); CDC20 mRNA and protein expression were compared between adherent and spheroid cells using qRT-PCR (a, b) and western blot (c) (P value: Wilcoxon test). (d–f) The mRNA expression of a series of stemness related genes (CD44, CD133, SOX2, OCT4) was analysed by qRT-PCR in prostate cancer cell spheroids derived from sorted CD44− and CD44+ of C4–2B (d) and DU145 (e); CDC20 mRNA and protein expression were compared between CD44− and CD44+ cells using qRT-PCR (d, e) and western blot (f) (P value: Wilcoxon test). (g–i) The mRNA expression of a series of stemness related genes (CD44, CD133, SOX2, OCT4) was analysed by qRT-PCR in prostate cancer cell derived from control and docetaxel resistant C4–2B (g) and DU145 (h); CDC20 mRNA and protein expression were compared between control and docetaxel resistant cells (DTX-R) using qRT-PCR (g, h) and western blot (i) (P value: Wilcoxon test). The results were collected from three independent experiments and all data represent Mean ± SD. All p values were defined as: *p < .05, **p < .01 and ***p < .001.
3.3. CDC20 is required to maintain stem cell-like features of CD44+ prostate CSCs
To further investigate the functional role of CDC20 in regulating the stem-like properties of CD44+ prostate CSCs, we establish Lv-shCDC20-stable transfectants of prostate cells (C4–2B and DU145) via a lentivirus-based knockdown approach. In addition, a wide-type (WT) or mutant type (MT) with a modified codon of CDC20 escaping from shRNA in Lv-shCDC20-infected prostate cancer cell lines to test if up-regulation of CDC20 could rescue these inhibitory effects. The knockdown and rescue efficiency were verified by western blot (Supplementary Fig. S1d). As presented in Fig. 3a, b and Supplementary Fig. S1d, e, CD44+ cells magnetic sorted from Lv-shCDC20 infection C4–2B or DU145 cells exhibited down-regulated mRNA and protein levels of CDC20 and some crucial stemness-related genes (including CD44, SOX2, OCT4, MYC, KLF4). Considering that strengthened self-renewal capacity is one of the most crucial features of CSCs, the spheroid formation and in vitro limiting dilution assays were performed to investigate if CDC20 could affect the self-renewal ability of prostate CSCs. Consistently, both quantity and size of the primary and secondary spheroids of CD44+ prostate cancer cells were decreased due to the knockdown of CDC20 (Fig. 3c, d). Meanwhile, decreased expression of CDC20 led to a lower spheroid initiating frequency (SIF) which further supported that the silence of CDC20 impeded self-renewal ability of CSCs in vitro (Fig. 3e, f and Supplementary Table S4). Since drug resistance of CSCs is considered to be involved in chemotherapeutic failure and tumour progression [8], we next determined whether CDC20 could regulate chemoresistance of prostate CSCs. As shown in Fig. 3g, h, CD44+ CSCs sorted from Lv-shCDC20 infection C4–2B or DU145 cells exhibited more vulnerable to docetaxel treatment as compared to the controls. More importantly, down-regulation of CDC20 significantly inhibited the invasion and migration ability of CD44+ prostate CSCs as compared to control (Supplementary Fig. 1f, g), while no significantly difference was found in cell cycle distribution between the control and Lv-shCDC20 groups (Supplementary Fig. 1h).
Fig. 3.
CDC20 is required to maintain stem cell-like features of CD44+ prostate CSCs.
(a, b) The mRNA expression levels of multiple stemness-related genes in CDC20-silenced or re-expressed C4–2B CD44+ (a) or DU145 CD44+ (b) cells were evaluated by qRT-PCR. Quantified mRNA levels were normalized to β-actin and presented relative to the controls (P value: Wilcoxon test). (c, d) Relative expression of CDC20 and a series of stemness related genes (CD44, MYC, SOX2, OCT4, KLF4) was examined in primary and secondary passaged prostate cancer cell spheroids derived from CD44+ C4–2B (c) or DU145 (d) with CDC20 knockdown or re-expression (Scale bar = 75 μm), the spheroid number was counted (P value: Wilcoxon test). (e, f) The limiting dilution assay of spheroid formation was performed in control and Lv-shCDC20 C4–2B CD44+ (e) or DU145 CD44+ (f) cells with different gradient cell numbers as indicated. (g, h) The indicated CD44+ C4–2B (g) or DU145 (h) cells were treated with docetaxel of different gradient concentration and cell viability was measured by CCK-8 assay (P value: Wilcoxon test). (i) A total of 1000, 5000 or 10,000 Con or shCDC20 CD44+ C4–2B cells were subcutaneously injected into 6-week-old, male, nude mice (n = 6/group). The tumour xenografts derived from Con or shCDC20 CD44+ C4–2B cells and the tumour incidence (inc.) in two generations were shown. (j) H&E and IHC staining of CDC20 and CD44 were examined in second generation tumours derived from Con or shCDC20 CD44+ C4–2B cells (Scale bar = 50 μm). All representative results were collected from three independent experiments and all data were represented as Mean ± SD. All p values were defined as: *p < .05, **p < .01 and ***p < .001.
Previous studies reported that CD44+ prostate cancer cells exhibited superior tumour initiating rate in vivo [34]. To evaluate whether CDC20 could manipulate tumorigenicity in vivo of CD44+ CSCs subset, xenograft assay was performed by inoculation of several gradient CD44+ cells sorted from CD44-Lv-shCon or Lv-shCDC20 cells. As shown in Fig. 3i and Supplementary Table S5, 1 × 104 CD44+ Lv-shCon C4–2B cells efficiently initiated larger tumours in 66.7% (4/6) of mice, while the equal numbers of CD44+/Lv-shCDC20 C4–2B cells produced only 1 tumour at 7 weeks after injection among 6 injected nude mice. Consistently, only 1 × 103 CD44+/Lv-shCon cells could generate tumours in 1 of 6 injected mice, whereas 1 × 103 CD44+ cells with CDC20 knokdown failed to do so at 7 weeks after transplantation, and IHC assay showed a weakened expression of CDC20 and CD44 in 1 × 104 Lv-shCDC20 cell group (Fig. 3j), indicating that knockdown of CDC20 attenuated the CD44+ fractions-driven tumorigenicity in vivo. In conclusion, our data suggested CDC20 is required to maintain stem cell-like features of CD44+ prostate CSCs, and may take a part in promoting the tumorigenicity of CD44+ prostate CSCs during prostate cancer progression.
3.4. CDC20 activates β-catenin signaling via collapsing the destructive complex and promoting β-catenin nuclear translocation and transactivation
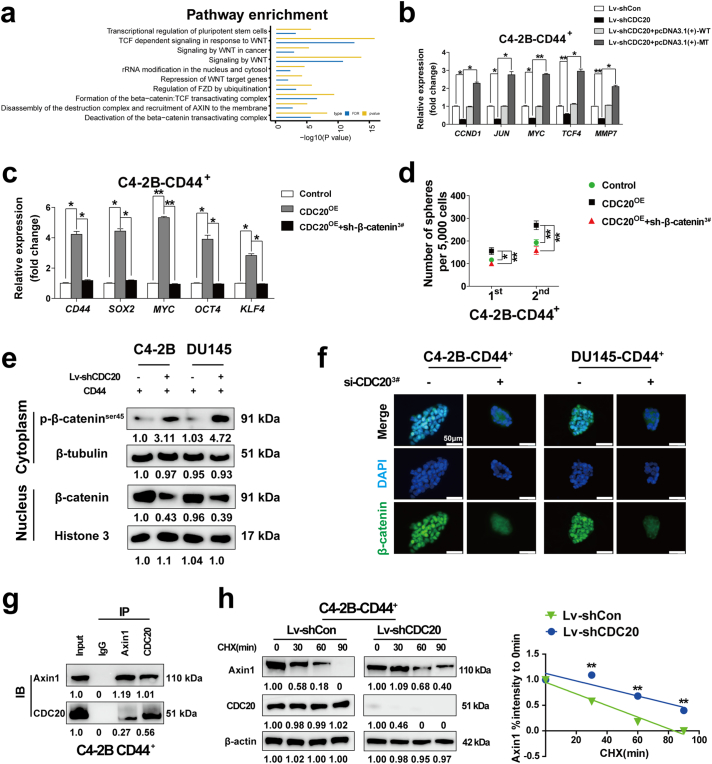
To examine the underlying mechanism by which CDC20 maintains the stem-like properties of CD44+ prostate CSCs, RNA-sequence was employed to detect differentially expressed genes in CDC20 knockdown CD44+ C4–2B cells compared to that in control cells, a total of 1600 differentially expressed genes were identified using a 2-fold cut-off with a P-value <.05 (Supplementary Fig. S2a and Supplementary Table S6). The first top 60 differentially up- and down-regulated genes were shown and we noticed that many stemness related genes or β-catenin/TCF4 target genes exhibited a decreased expression in Lv-shCDC20 CD44+ C4–2B cells in Supplementary Fig. S2b. Consistently, the pathway analysis showed that transcriptional regulation of pluripotent stem cells and β-catenin: TCF complex related pathway was differentially regulated in Lv-shCDC20 CD44+ C4–2B cells (Fig. 4a and Supplementary Table S7) as well as gene ontology (GO) analysis (Supplementary Fig. S2c and Supplementary Table S8). Interestingly, qRT-PCR assay revealed that a set of important β-catenin target genes such as c-Jun, c-Myc, cyclin D1, TCF4 and MMP7 were down-regulated in CDC20 knockdown CD44+ C4–2B cells, whereas overexpression of CDC20 could recue these inhibitory effectors in mRNA levels (Fig. 4b and Supplementary Fig. S2d). Next, we investigated whether β-catenin took a crucial part in the CDC20-regulated stem-like properties of CD44+ prostate CSCs. The expression of stem-related genes was higher in CD44+ prostate CSCs with CDC20 overexpression compared with the control CD44+ prostate CSCs by qRT-PCR, knockdown of β-catenin (Supplementary Fig. S2e) repressed this effect (Fig. 4c and Supplementary Fig. S2f). Meanwhile, spheroid formation assay indicated that CDC20 reinforced the self-renewal of CD44+ prostate CSCs, depletion of β-catenin abolished this effect of CDC20 (Fig. 4d and Supplementary Fig. S2g). These results suggested that β-catenin is indispensable for the CDC20-dependent stem-like properties of CD44+ prostate CSCs.
Fig. 4.
CDC20 activates β-catenin signaling via collapsing the destructive complex and promoting β-catenin nuclear translocation and transactivation.
(a) Signaling pathway analysis of differentially expressed genes in control relative to shCDC20 CD44+ C4–2B cells; FDR: false discovery rate. (b) The mRNA expression levels of a series of β-catenin/TCF targeted genes in CDC20-silenced or re-expressed C4–2B CD44+ cells were evaluated by qRT-PCR (P value: Wilcoxon test). (c) The qRT-PCR assay was performed to detect the mRNA expression of multiple stemness related genes in indicated groups (P value: Wilcoxon test). (d) The spheroid formation assay of CD44+ C4–2B of indicated groups was conducted and spheroid number was counted (P value: Wilcoxon test). (e) Western blotting assay of cytoplasmic and nuclear proteins were extracted from CD44+ C4–2B or DU145 cells with and without CDC20 knockdown. (f) Immunofluorescence staining of β-catenin was performed in CD44+ C4–2B or DU145 cells with and without CDC20 knockdown. (g) Interaction of Axin1 and CDC20 in CD44+ C4–2B cells was detected by western blot. (h) Western blot assay for Axin1 and CDC20 expression level was examined in CD44+ C4–2B cells with Con or CDC20 knockdown upon treatment of 50 μg/mL of cycloheximide (CHX) at various time points, and the percentage of intensity compared to 0 min was calculated. The results were come from three independent experiments and all data were represented as Mean ± SD. All p values were defined as: *p < .05, **p < .01 and ***p < .001.
Concrete evidence has proven that Wnt/β-catenin signaling was involved in carcinogenesis and stem-like feature manipulation of CSCs by activating β-catenin and its nucleus translocation [35]. However, the pool of β-catenin in the cytoplasm is usually withered accounting for degradation by the proteasome via phosphorylated at Ser45 [36]. Therefore, we assessed if CDC20 could maintain β-catenin and promote its nuclear localization. Western blot assay showed that the expression level of p-β-cateninSer45 was elevated in cytoplasm of CD44+ prostate CSCs after CDC20 knockdown, meanwhile the expression of β-catenin was down-regulated in nucleus (Fig. 4e–f). Thus, these results suggested that CDC20 expression is necessary for the stability of β-catenin in the cytosolic and its nuclear translocation.
Considering that the degradation of cytoplasmic β-catenin is manipulated by the “destructive complex” which comprised of Axin1, APC, and glycogen synthase kinase 3β (GSK-3β) [36], we examined the potential link between CDC20 and β-catenin or its destructive complex members. Western blot assay indicated that alternations of CDC20 in prostate cancer cells influenced β-catenin and Axin1 expression level but not APC and GSK-3β (Supplementary Fig. S2h,i). We next detected the direct interaction between CDC20 and β-catenin or Axin1, the co-immunoprecipitation assay suggested that CDC20 and Axin1 could bond to each other in CD44+ C4–2B (Fig. 4g) and CD44+ DU145 cells (Supplementary Fig. S2j), while there was no interaction between CDC20 and β-catenin (Supplementary Fig. S2k). Given that CDC20 is an E3 Ubiquitination ligase, we next explored whether knockdown CDC20 could impede the degradation of Axin1. As expected, western blot assay revealed that Axin1 was more stable with CDC20 knockdown under the treatment by cycloheximide (CHX), a protein synthesis inhibitor (Fig. 4h). Meanwhile, the enhanced maintenance of Axin1 was not detected in the presence of MG132 (a proteasome inhibitor) (Supplementary Fig. S2l) Thus, these results indicated that CDC20 could promote Axin1 degradation through a proteasomal-dependent pathway rather than by protein synthesis, although the concrete interaction mode between CDC20 and Axin1 needs to be further elucidated. Taken together, CDC20 directly binds with Axin1 and to some extent intensified Axin1 degradation activities, which may impede the destructive complex, resulting in enhancement of the stability of β-catenin and its translocation to nucleus.
3.5. Expression of CDC20 and β-catenin predicts malignant clinicopathological features and prognosis for prostate cancer patients
Considering the crucial role of CDC20 and β-catenin in the stem-like features of CD44+ prostate CSCs, we next explored the clinical correlation between CDC20 and β-catenin in prostate cancer specimens. IHC analysis was performed in prostate cancer specimens from 121 patients (Fig. 5a), positive correlation was identified between CDC20 and β-catenin expression (p < .001) (Fig. 5b). Next, we evaluated whether the combined expression of CDC20 and β-catenin could serve as a predicter for malignant clinicopathological features and prognosis for prostate cancer patients. According to their tumoral CDC20 and β-catenin expression, all 121 patients were divided into 4 groups (Supplementary Table S9), the concomitant high expression of CDC20 and β-catenin was associated with worst clinicopathological features and prognosis for prostate cancer patients (Supplementary Table S9, Fig. 5c, d). Thus, the concomitant over-expression of CDC20 and β-catenin can serve as an effective predictor for unsatisfactory prognosis of prostate cancer patients, further supporting the important role of CDC20 and β-catenin in the progression of prostate CSCs.
Fig. 5.
The expression of CDC20 and β-catenin predicts malignant clinicopathological features and prognosis for prostate cancer patients.
(a) H&E staining and IHC staining of CDC20 and β-catenin were shown in prostate cancer tissues (scale bar = 50 μm). (b) Correlation analysis of CDC20 and β-catenin expression was performed in different prostate cancer tissues (Spearman r2 = 0.5979; P value <.001). (c, d) BCR (c) and DFS (d) of prostate cancer patients in different groups were compared according to CDC20 and β-catenin expression using Kaplan-Meier curves (P value: log rank test). (e) The schematic diagram of underlying mechanisms was described in our study. All p values were defined as: *p < .05, **p < .01 and ***p < .001.
4. Discussion
Despite recent advances in new drugs for the treatment of metastatic prostate cancer, current treatments gain unsatisfied survival benefits due to acquired drug resistance and disease progression [3]. Recent studies have proven that CSCs exert a critical role in tumorigenesis and spread of prostate cancer [34]. Thus, targeted elimination of prostate CSCs may be an effective option to inhibit malignant biological behaviors of prostate cancer. For this purpose, the potential molecular mechanisms by which CDC20 manipulates prostate CSCs require to be elucidated. In this study, we reported that CDC20 is required for maintenance of CD44+ prostate CSCs via enhancing Axin1 degradation and promoting β-catenin translocation to nuclear and transactivation (Fig. 5e). To our best knowledge, it is the first report that there was a positive correlation between CDC20 and CD44 expression in clinical prostate cancer specimens. Likely, CDC20 is preferentially expressed in parts of spheroids or CD44+ or chemo-resistant prostate cancer cell lines. In addition, lentiviral-based methods of interfering with CDC20 significantly inhibited CSC self-renewal ability and inhibited CSCs-driven in vivo tumorigenicity. Our further studies have also found that Wnt/β-catenin signaling plays a critical role in the maintenance of prostate CSC. Mechanically, knockdown of CDC20 expression impairs nuclear translocation of β-catenin and impedes its transcriptional activity, thereby attenuating activation and expansion of prostate CSCs. These findings also indicated that CDC20 can be used as a therapeutic target to eradicate CSCs in prostate cancer management.
Recent studies have highlighted the critical role of CDC20 in hematopoietic stem cells and cancer stem cells. CDC20 regulates hematopoietic stem cell hematopoiesis and leukemia by promoting ubiquitination of MEIS1 and p21 [27]. It is preferentially expressed in the basal and superior layers of the epidermis rather than differentiated cells, and serves as an effective key regulator for adult stem cell fate [37]. CDC20 also can enhance the stability of SOX2 by directly interacting with SOX2, thereby conferring self-renewal and tumorigenicity in glioblastoma [38].
So far, high expression of cancer stem-like cell marker CD44 is associated with chemical and radiotherapy resistance in breast [39], colorectal [40], pancreatic [41] and prostate cancers [[13], [14], [15], [16], [17]]. It is also associated with aggressive proliferation and metastasis of cancers [42,43]. We assessed the expression level of CDC20 in spheroid, CD44+ or chemo-resistant cells. The results supported that CDC20 promotes the development of prostate cancer by regulating the prostate CSCs, targeting CDC20 in CD44+ prostate CSCs impeded their stemness and attenuated their ability to tumorigenesis in vivo.
To uncover the underlying mechanisms how CDC20 regulates prostate CSC stemness, RNA sequences were performed to screen the differentially regulated genes and corresponding pathways under knockdown of CDC20 in CD44+ C4–2B prostate cancer cells. Interestingly, the pathway of transcriptional regulation of pluripotent stem cells was significantly affected in the Lv-shCDC20 group, as well as a series of pathways consistently down-regulated, such as the β-catenin/TCF transactivation complex, the Hippo pathway, the Notch pathway and the ABC family, which were involved in the regulation of biological functions and characteristics of stem-like cells and drug resistance in previous study [44]. Among them, abnormal activation of the Wnt/β-catenin signaling pathway usually exerts a critical role in regulating prostate CSCs expansion during prostate cancer progression and androgen deprivation treatment resistance [45,46]. Our data found that down-regulation of CDC20 inhibits mRNA expression of c-Jun, c-Myc, MMP7, cyclin D1 and TCF4, which are the major downstream targets for β-catenin/TCF signaling, and the MMP7 expression was weaken may give a clue for illuminating its role on impeding the invasion ability of cancer stem-like cells. It is well known that β-catenin is always phosphorylated in cytoplasm by its “destructive complex” and sequentially degraded by the proteasome system [47]. Thus, disrupting the formation of this complex can result in the accumulation of stabilised β-catenin and its translocation into nucleus to transactive the downstream genes such as MMP7 and c-Myc. To elucidate how CDC20 affects the Wnt/β-catenin pathway, we found that CDC20-driven regulation on β-catenin signaling by promoting Axin1 degradation. Thus, the inactivation of destructive complexes stabilised β-catenin and enhanced its nuclear translocation and transactive ability in prostate CSCs. Previous study suggested that c-Myc, the key target effector of β-catenin pathway, is closely related to stem-like properties regulation, whereas depletion of c-Myc could reverse the tumourigenic potential of CSCs [48]. CDC20 is an important target gene for c-Myc [49], therefore, whether a positive feedback loop could be formed between CDC20 and c-Myc still needs to be further elucidated.
Based on our results, we believe that CDC20, through its regulation of prostate CSCs, contributes at least to some extent to the aggressiveness and chemo-resistance of prostate cancer, which was further supported by clinical studies showing that CDC20 in combination with CD44 or β-catenin provides a new prognostic indicator for prostate cancer patients. In conclusion, knockdown of CDC20 can be used for therapeutic benefit and represents an effective adjuvant anti-cancer treatment to eliminate CSCs during prostate cancer progression.
Funding sources and acknowledgement
National Natural Science Foundation of China (No. 81572525, 81772747, 81773251 and 81572863), the Shanghai Medical Guidance (Chinese and Western Medicine) Science and Technology Support Project (No. 18411960100).
Declaration of interests
The authors declare no conflict of interest in this study.
Author contributions
Qin Zhang, Hai Huang contributed to study concept, design and acquisition of data. Hai Huang and Qin Zhang contributed to drafting of the manuscript. Qin Zhang, Hai Huang, Jiang Li, Chunying Liu and Bin Sun contributed to acquisition of data. Lu Chen and Ao Liu contributed to analysis and interpretation of data. Lu Chen and Jiang Li contributed to statistical analysis. Changqing Su, Danfeng Xu and Yi Gao contributed to critical revision of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.03.032.
Contributor Information
Qin Zhang, Email: xiaoqin1989@gmail.com.
Hai Huang, Email: yellowsea201314@163.com.
Ao Liu, Email: liuao19951028@sjtu.edu.cn.
Jiang Li, Email: iamlijiang@hotmail.com.
Chunying Liu, Email: cyliu@sibcb.ac.cn.
Bin Sun, Email: sunbin05301984@aliyun.com.
Lu Chen, Email: cl12063@rjh.com.cn.
Yi Gao, Email: gy12065@rjh.com.cn.
Danfeng Xu, Email: xdf12036@rjh.com.cn.
Changqing Su, Email: suchangqing@gmail.com.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
Supplementary material 3
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. 2018-01-01. [DOI] [PubMed] [Google Scholar]
- 2.Kohli M., Tindall D.J. New developments in the medical management of prostate cancer. Mayo Clin. Proc. 2010;85(1):77–86. doi: 10.4065/mcp.2009.0442. 2010-01-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James N.D., Sydes M.R., Clarke N.W., Mason M.D., Dearnaley D.P., Spears M.R. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–1177. doi: 10.1016/S0140-6736(15)01037-5. 2016-03-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritch C.R., Cookson M.S. Advances in the management of castration resistant prostate cancer. BMJ. 2016;355:i4405. doi: 10.1136/bmj.i4405. 2016-10-17. [DOI] [PubMed] [Google Scholar]
- 5.Lowrance W.T., Murad M.H., Oh W.K., Jarrard D.F., Resnick M.J., Cookson M.S. Castration-resistant prostate Cancer: AUA guideline amendment 2018. J. Urol. 2018;200(6):1264–1272. doi: 10.1016/j.juro.2018.07.090. [DOI] [PubMed] [Google Scholar]
- 6.Cornford P., Bellmunt J., Bolla M., Briers E., De Santis M., Gross T. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 2017;71(4):630–642. doi: 10.1016/j.eururo.2016.08.002. 2017-04-01. [DOI] [PubMed] [Google Scholar]
- 7.Li C., Liu S., Yan R., Han N., Wong K.K., Li L. CD54-NOTCH1 axis controls tumor initiation and cancer stem cell functions in human prostate cancer. Theranostics. 2017;7(1):67–80. doi: 10.7150/thno.16752. 2017-01-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W., Meng Y., Liu N., Wen X.F., Yang T. Insights into chemoresistance of prostate cancer. Int. J. Biol. Sci. 2015;11(10):1160–1170. doi: 10.7150/ijbs.11439. 2015-01-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan C.T., Guzman M.L., Noble M. Cancer stem cells. N. Engl. J. Med. 2006;355(12):1253–1261. doi: 10.1056/NEJMra061808. 2006-09-21. [DOI] [PubMed] [Google Scholar]
- 10.Lapidot T., Sirard C., Vormoor J., Murdoch B., Hoang T., Caceres-Cortes J. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. 1994-02-17. [DOI] [PubMed] [Google Scholar]
- 11.Visvader J.E. Cells of origin in cancer. Nature. 2011;469(7330):314–322. doi: 10.1038/nature09781. 2011-01-20. [DOI] [PubMed] [Google Scholar]
- 12.Collins A.T., Berry P.A., Hyde C., Stower M.J., Maitland N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. 2005-12-01. [DOI] [PubMed] [Google Scholar]
- 13.Erdogan S., Turkekul K., Serttas R., Erdogan Z. The natural flavonoid apigenin sensitizes human CD44(+) prostate cancer stem cells to cisplatin therapy. Biomed. Pharmacother. 2017;88:210–217. doi: 10.1016/j.biopha.2017.01.056. 2017-04-01. [DOI] [PubMed] [Google Scholar]
- 14.Patrawala L., Calhoun T., Schneider-Broussard R., Li H., Bhatia B., Tang S. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25(12):1696–1708. doi: 10.1038/sj.onc.1209327. 2006-03-16. [DOI] [PubMed] [Google Scholar]
- 15.Saini S., Majid S., Shahryari V., Arora S., Yamamura S., Chang I. miRNA-708 control of CD44(+) prostate cancer-initiating cells. Cancer Res. 2012;72(14):3618–3630. doi: 10.1158/0008-5472.CAN-12-0540. 2012-07-15. [DOI] [PubMed] [Google Scholar]
- 16.Patrawala L., Calhoun-Davis T., Schneider-Broussard R., Tang D.G. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67(14):6796–6805. doi: 10.1158/0008-5472.CAN-07-0490. 2007-07-15. [DOI] [PubMed] [Google Scholar]
- 17.Liu C., Kelnar K., Liu B., Chen X., Calhoun-Davis T., Li H. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 2011;17(2):211–215. doi: 10.1038/nm.2284. 2011-02-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao C.P., Lin T.P., Li P.C., Geary L.A., Chen K., Vaikari V.P. Loss of MAOA in epithelia inhibits adenocarcinoma development, cell proliferation and cancer stem cells in prostate. Oncogene. 2018;37(38):5175–5190. doi: 10.1038/s41388-018-0325-x. 2018-09-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H., Wang C., Liu F., Li H.Z., Peng G., Gao X. Reciprocal network between cancer stem-like cells and macrophages facilitates the progression and androgen deprivation therapy resistance of prostate cancer. Clin. Cancer Res. 2018;24(18):4612–4626. doi: 10.1158/1078-0432.CCR-18-0461. 2018-09-15. [DOI] [PubMed] [Google Scholar]
- 20.Huang H., Zhang Q., Ye C., Lv J., Liu X., Chen L. Identification of prognostic markers of high grade prostate cancer through an integrated bioinformatics approach. J. Cancer Res. Clin. 2017;143(12):2571–2579. doi: 10.1007/s00432-017-2497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinstein J., Jacobsen F.W., Hsu-Chen J., Wu T., Baum L.G. A novel mammalian protein, p55CDC, present in dividing cells is associated with protein kinase activity and has homology to the Saccharomyces cerevisiae cell division cycle proteins Cdc20 and Cdc4. Mol Cell Biol. 1994;14(5):3350–3363. doi: 10.1128/mcb.14.5.3350. 1994-05-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol. Cell. 2007;27(1):3–16. doi: 10.1016/j.molcel.2007.06.009. 2007-07-06. [DOI] [PubMed] [Google Scholar]
- 23.Rajagopalan H., Lengauer C. Aneuploidy and cancer. Nature. 2004;432(7015):338–341. doi: 10.1038/nature03099. 2004-11-18. [DOI] [PubMed] [Google Scholar]
- 24.Moura I.M., Delgado M.L., Silva P.M., Lopes C.A., Do A.J., Monteiro L.S. High CDC20 expression is associated with poor prognosis in oral squamous cell carcinoma. J Oral Pathol Med. 2014;43(3):225–231. doi: 10.1111/jop.12115. 2014-03-01. [DOI] [PubMed] [Google Scholar]
- 25.Choi J.W., Kim Y., Lee J.H., Kim Y.S. High expression of spindle assembly checkpoint proteins CDC20 and MAD2 is associated with poor prognosis in urothelial bladder cancer. Virchows Arch. 2013;463(5):681–687. doi: 10.1007/s00428-013-1473-6. 2013-11-01. [DOI] [PubMed] [Google Scholar]
- 26.Chang D.Z., Ma Y., Ji B., Liu Y., Hwu P., Abbruzzese J.L. Increased CDC20 expression is associated with pancreatic ductal adenocarcinoma differentiation and progression. J. Hematol. Oncol. 2012;5:15. doi: 10.1186/1756-8722-5-15. 2012-04-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X., Zhang F., Zhang Y., Li X., Chen C., Zhou M. PPM1K regulates hematopoiesis and leukemogenesis through CDC20-mediated ubiquitination of MEIS1 and p21. Cell Rep. 2018;23(5):1461–1475. doi: 10.1016/j.celrep.2018.03.140. 2018-05-01. [DOI] [PubMed] [Google Scholar]
- 28.McShane L.M., Altman D.G., Sauerbrei W., Taube S.E., Gion M., Clark G.M. Reporting recommendations for tumor marker prognostic studies (Remark) J. Natl. Cancer Inst. 2005;97(16):1180–1184. doi: 10.1093/jnci/dji237. 2005-08-17. [DOI] [PubMed] [Google Scholar]
- 29.Epstein J.I., Egevad L., Amin M.B., Delahunt B., Srigley J.R., Humphrey P.A. The 2014 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 2016;40(2):244–252. doi: 10.1097/PAS.0000000000000530. 2016-02-01. [DOI] [PubMed] [Google Scholar]
- 30.Mao Y., Li K., Lu L., Si-tu J., Lu M., Gao X. Overexpression of Cdc20 in clinically localized prostate cancer: relation to high Gleason score and biochemical recurrence after laparoscopic radical prostatectomy. Cancer Biomark. 2016;16(3):351–358. doi: 10.3233/CBM-160573. [DOI] [PubMed] [Google Scholar]
- 31.Zhao J. Cancer stem cells and chemoresistance: the smartest survives the raid. Pharmacol. Ther. 2016;160:145–158. doi: 10.1016/j.pharmthera.2016.02.008. 2016-04-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu F., Lin Y., Cui P., Li H., Zhang L., Sun Z. Cdc20/p55 mediates the resistance to docetaxel in castration-resistant prostate cancer in a Bim-dependent manner. Cancer Chemoth. Pharm. 2018;81(6):999–1006. doi: 10.1007/s00280-018-3578-8. [DOI] [PubMed] [Google Scholar]
- 33.Li K., Mao Y., Lu L., Hu C., Wang D., Si-Tu J. Silencing of CDC20 suppresses metastatic castration-resistant prostate cancer growth and enhances chemosensitivity to docetaxel. Int. J. Oncol. 2016;49(4):1679–1685. doi: 10.3892/ijo.2016.3671. 2016-10-01. [DOI] [PubMed] [Google Scholar]
- 34.Yun E.J., Zhou J., Lin C.J., Hernandez E., Fazli L., Gleave M. Targeting cancer stem cells in castration-resistant prostate cancer. Clin Cancer Res. 2016;22(3):670–679. doi: 10.1158/1078-0432.CCR-15-0190. 2016-02-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C., Fu S., Wang M., Yu W., Cui Q., Wang H. Zinc finger protein X-linked promotes expansion of EpCAM+ cancer stem-like cells in hepatocellular carcinoma. Mol. Oncol. 2017;11(5):455–469. doi: 10.1002/1878-0261.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li V.S., Ng S.S., Boersema P.J., Low T.Y., Karthaus W.R., Gerlach J.P. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. 2012;149(6):1245–1256. doi: 10.1016/j.cell.2012.05.002. 2012-06-08. [DOI] [PubMed] [Google Scholar]
- 37.Quek L.S., Grasset N., Jasmen J.B., Robinson K.S., Bellanger S. Dual role of the anaphase promoting complex/cyclosome in regulating stemness and differentiation in human primary keratinocytes. J Invest Dermatol. 2018;138(8):1851–1861. doi: 10.1016/j.jid.2018.02.033. 2018-08-01. [DOI] [PubMed] [Google Scholar]
- 38.Mao D.D., Gujar A.D., Mahlokozera T., Chen I., Pan Y., Luo J. A CDC20-APC/SOX2 signaling axis regulates human glioblastoma stem-like cells. Cell Rep. 2015;11(11):1809–1821. doi: 10.1016/j.celrep.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips T.M., McBride W.H., Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J. Natl. Cancer Inst. 2006;98(24):1777–1785. doi: 10.1093/jnci/djj495. 2006-12-20. [DOI] [PubMed] [Google Scholar]
- 40.Hwang WL., Yang MH., Tsai ML., Lan HY., Su SH., Chang SC. SNAIL regulates interleukin-8 expression, stem cell-like activity, and tumorigenicity of human colorectal carcinoma cells. Gastroenterology. 2011;141:279–291. doi: 10.1053/j.gastro.2011.04.008. 2011-07-01; 291. [DOI] [PubMed] [Google Scholar]
- 41.Hamada S., Masamune A., Shimosegawa T. Pancreatic cancer stem cell. 2015;73(5):844–849. Nihon Rinsho. 2015-05-01. [PubMed] [Google Scholar]
- 42.Marhaba R., Zoller M. CD44 in cancer progression: adhesion, migration and growth regulation. J. Mol. Histol. 2004;35(3):211–231. doi: 10.1023/b:hijo.0000032354.94213.69. 2004-03-01. [DOI] [PubMed] [Google Scholar]
- 43.Negi L.M., Talegaonkar S., Jaggi M., Ahmad F.J., Iqbal Z., Khar R.K. Role of CD44 in tumour progression and strategies for targeting. J Drug Target. 2012;20(7):561–573. doi: 10.3109/1061186X.2012.702767. 2012-08-01. [DOI] [PubMed] [Google Scholar]
- 44.Batlle E., Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–1134. doi: 10.1038/nm.4409. 2017-10-06; [DOI] [PubMed] [Google Scholar]
- 45.Song X.L., Huang B., Zhou B.W., Wang C., Liao Z.W., Yu Y. miR-1301-3p promotes prostate cancer stem cell expansion by targeting SFRP1 and GSK3beta. Biomed. Pharmacother. 2018;vol. 99:369–374. doi: 10.1016/j.biopha.2018.01.086. 2018-03-01. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z., Cheng L., Li J., Farah E., Atallah N.M., Pascuzzi P.E. Inhibition of the Wnt/beta-catenin pathway overcomes resistance to enzalutamide in castration-resistant prostate cancer. Cancer Res. 2018;78(12):3147–3162. doi: 10.1158/0008-5472.CAN-17-3006. 2018-06-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clevers H., Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. 2012-06-08. [DOI] [PubMed] [Google Scholar]
- 48.Vyas A.R., Moura M.B., Hahm E.R., Singh K.B., Singh S.V. Sulforaphane inhibits c-myc-mediated prostate cancer stem-like traits. J. Cell Biochem. 2016;117(11):2482–2495. doi: 10.1002/jcb.25541. 2016-11-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blanco-Bose W.E., Murphy M.J., Ehninger A., Offner S., Dubey C., Huang W. C-Myc and its target FoxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology. 2008;48(4):1302–1311. doi: 10.1002/hep.22475. 2008-10-01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Supplementary material 3