Abstract
Background
Hypertensive patients exhibit decline in capillary density and endothelial progenitor cells (EPCs). However, whether capillary rarefaction in hypertension is associated with defect angiogenesis of EPCs remains unknown. We hypothesized that impaired mitochondrial function of late EPCs in hypertension is associated with the structural lack of capillary microcirculation via deficient CXCR4/JAK2/SIRT5 signaling.
Methods
We performed capillary microcirculation detection in hypertensive patients and healthy subjects. Angiogenic capacity and mitochondrial function of circulating EPCs were evaluated. The underlying mechanisms were further investigated by genetic inhibition and overexpression.
Findings
Capillary density of nail fold and eye fundus were significantly reduced in hypertensive patients, which was paralleled to decreased in vitro late EPC function and in vivo angiogenic capacity. Meanwhile the decline of EPC function in hypertension was accompanied by impaired mitochondrial ultrastructure, diminished mitochondrial membrane potential, reduced oxygen consumption, increased ROS generation and NADH level. Rotenone induced inhibition of oxygen consumption rate, excessive ROS generation and loss of MMP, which markedly decreased the in vitro functions of EPCs. Furthermore, SIRT5 expression of EPCs in hypertension was markedly reduced, which was correlated to mitochondrial dysfunction. CXCR4 gene transfer enhanced SIRT5 expression, improved mitochondrial functions and augmented angiogenic capacity of EPCs. The beneficial impacts of SIRT5 up-regulation on late EPC-mediated angiogenesis can be abrogated by blockade of CXCR4/JAK2/SIRT5 signaling pathway.
Interpretation
Mitochondrial dysfunction-mediated fall in angiogenic capacity due to deficient CXCR4/JAK2/SIRT5 signaling of late EPCs is probably responsible for the capillary rarefaction in hypertension. Our findings provide insight into the potential of EPC mitochondria as a novel target for the treatment of hypertension-related loss of microvascular density.
Funds
National Nature Science Foundation of China, 973Program, the Nature Science Foundation of Guangdong.
Keywords: Hypertension, EPCs, CXCR4, Mitochondria, SIRT5, Angiogenesis, Microcirculation
Research in context.
Evidence before this study
Hypertensive patients exhibit capillary rarefaction and impaired EPC functions. Late EPCs are essential for normal angiogenesis through endothelial tubulogenesis as an endogenous repair mechanism. Mitochondrial activity plays a pivotal role in the maintenance of normal cellular functions. However, seldom researches focus on the relationship between defective late EPCs-mediated angiogenesis and microvascular rarefaction in hypertension. Besides, no data have been provided for a potential position of mitochondrial dysfunction related to decreased angiogenic activity of late EPCs and its underlying molecular mechanisms in hypertension.
Added value of this study
Exploring the mechanisms of capillary rarefaction has important clinical implications in management of hypertension. These results contribute to further understanding of the occurrence and development of hypertension, providing insight into the potential of EPCs for the treatment of hypertension.
Implications of all the available evidence
In this study, we found that mitochondrial dysfunction of late EPCs from hypertensive patients is, at least in part, responsible for the decline in EPC-based angiogenic capacity. CXCR4 gene transfer contributes to the restoration of mitochondrial function which results in the improvement of EPC angiogenic capacity in patients with hypertension via regulating CXCR4/JAK2/SIRT5 signal pathway. The study provides the novel insight into the potential of late EPC mitochondria as a novel target for the treatment of hypertension related loss of microvascular density.
Alt-text: Unlabelled Box
1. Introduction
Hypertension is a major independent risk factor for the development of atherosclerotic vascular disease, including vascular abnormalities in the macro- and microvasculature [1,2]. Capillary rarefaction is the hallmark of hypertensive microvascular disorders [[3], [4], [5]]. Further understanding the mechanisms of capillary rarefaction has important clinical implications in management of hypertension. Our previous data and other studies showed that the impaired function of EPCs was observed in patients with hypertension [[6], [7], [8], [9]]. Endothelial progenitor cells (EPCs), which can be classified at least into early and late EPCs, is essential for normal angiogenesis through endothelial tubulogenesis as an endogenous repair mechanism [10,11]. Accumulating data showed that late EPCs possess the capacity to form de novo blood vessels, indicating that maintenance of normal late EPC function plays a key role in the homeostasis between angiogenesis and microvascular regression [12,13]. However, the relationship between defective late EPCs-mediated angiogenesis and capillary rarefaction in hypertension and the underlying molecular mechanisms remain largely unknown.
It has been demonstrated that mitochondrial activity plays a pivotal role in the maintenance of normal cellular functions [14,15]. Therefore, we assume that mitochondrial dysfunction might also be a key mediator of impaired EPC function under pathologic conditions including hypertension. However, no data have been provided for a potential position of mitochondrial dysfunction related to decreased angiogenic activity of late EPCs in hypertension. Moreover, the underlying molecular mechanisms of mitochondrial dysfunction-mediated decline in late EPCs angiogenic capacity remain unknown.
Silent mating type information regulation 2 homolog 5 (SIRT5), one of the 7 mammalian sirtuins family of nicotinamide adenine dinucleotide (NAD)-dependent enzymes involved in the dynamic regulation of cellular physiology, is located in mitochondria, which can regulate mitochondrial metabolic networks and eliminate reactive oxidative stress (ROS) [16]. Chemokine (C-X-C motif) receptor 4(CXCR4), as a receptor for stromal cell derived factor 1 (SDF-1), regulates many biological processes of cells [17]. Our previous studies demonstrate that diminished CXCR4 and its downstream signaling janus kinase 2 (JAK2) contribute to impaired function of EPCs from hypertensive patients [18]. Interestingly, recent studies reported that the crosstalk of CXCR4 and ROS is involved in the regulation of cellular function [19,20]. Also, one study showed that SDF-1/CXCR4 modulates mitochondrial respiration of immature blood cells by regulating mitochondrial oxidative phosphorylation, adenosine triphosphate production and mitochondrial content [21]. Accordingly, we supposed that CXCR4 and SIRT5 signaling play an important role in mitochondrial function of EPCs. However, there are no data available on mitochondrial function alterations and its relationship with CXCR4 signaling involved in angiogenic capacity of late EPCs from hypertensive patients. Furthermore, there are no prior studies on the impact of SIRT5 mediated mitochondrial dysfunction related to CXCR4 signaling in late EPCs from patients with hypertension.
Based on these uncertainties mentioned above, we hypothesized that mitochondrial dysfunction leads to the impaired angiogenic capacity of late EPCs from hypertensive patients and diminished CXCR4/JAK2/SIRT5 signaling is responsible for the mitochondrial dysfunction, which contributes to the fall in angiogenic function of late EPCs related to capillary rarefaction in hypertension. To address these assumptions, the present study was designed to investigate: 1.whether defect angiogenic function of EPCs is associated with capillary rarefaction in hypertension; 2.whether mitochondrial dysfunction due to deficient CXCR4/JAK2/SIRT5 signaling in late EPCs leads to the fall of angiogenic capacity in hypertensive patients.
2. Materials and methods
2.1. Study subjects
Thirty consecutive outpatients with newly diagnosed hypertension (BP ≥ 140/90 mmHg) and 30 age-matched healthy subjects were enrolled into the study after informed consent were obtained. Exclusion criteria included cardiovascular events, malignancy, active inflammatory disease and those with other cardiovascular risk factors or taking other medications. Blood pressure measurements were performed according to JNC-7 [22]. The baseline characteristics are shown in Table S1. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (Guangzhou, China).
2.2. Animal prepare
The 8-week-old male Wistar rats and Spontaneously Hypertensive rats (SHRs) were purchased from Vital River Experimental Animal Center (Beijing, China). The 8-week-old male BALB/c nude mice were purchased from SLAC Laboratory Animal Center (Shanghai, China). All animal experiments were complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and the Animal Care and Use Committees of Sun Yat-sen University.
2.3. Capillary density measurement
Nail fold capillaroscopy was performed by a video dermatoscope with 30× magnification (XS3000, JIANNENG Optical Instrument Lmt.com, An Hui, China). All patients had rest for at least 15 min before the procedure in the examination room in where the temperature was kept approximately 24 °C. With the software of the instrument, all fingers (but not the thumbs) except those affected by recent trauma of both hands were examined using a video capillaroscope and the images were captured, coded and stored using Videocap 8.14 software (DS-Medica, Milan, Italy). Capillary density in the resting state was counted during a 15-second period. During the 15-second period, visible continuously and intermittently perfused capillaries were counted. We applied venous congestion (VC), with the digital cuff (OMRON, HEM-7131, Japan) inflated to 60 mmHg for 2 min, to expose a maximal number of non-perfused capillaries. The images captured all of the nail-fold areas in which capillary visibility was good, and were scanned for the main parameters of nail fold capillary density (NCD).
2.4. Optical coherence tomography angiography dectetion
OCTA imaging was performed using the RTVue XR Avantispectral-domain OCT device with AngioVue software (version 2015.1.1.98; Optovue, Inc., Freemont, CA, USA) with an 840-nm light source, an A-scan rate of 70,000 scans/s and a bandwidth of 50 nm. Each volume contains 304 × 304 A-scans with two consecutive B-scans captured at each fixed position. Each volume scan is acquired in 3 s and consists of two orthogonal volumes that are used to minimize motion artifacts arising from microsaccades and fixation changes. The split-spectrum amplitude-decorrelation angiography (SSADA) method was performed to acquire the dynamic motion of red blood cells and establish a high-resolution 3D visualization of perfused retinal vasculature.
AngioVue characterizes vascular information at various user-defined retinal layers as a capillary density map and quantitatively as capillary density (%) as previously described [23]. Capillary density was automatically calculated as the proportion of measured area occupied by flowing blood vessels defined as pixels having decorrelation values acquired by the SSADA algorithm above the threshold level.
2.5. EPC culture
EPCs were isolated and cultured as previously described in detail [24,25]. After 4 days culture, non-adherent cells were removed by thoroughly washing with endothelial cell basal medium-2 (EBM-2) (Lonza, Swiss). Medium was changed daily for 7 days, and then every 3 days was changed. After 4 weeks' culture, late EPCs were defined as cells dually positive for 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine (DiI)-acetylated low density lipoprotein (acLDL) uptake (0.02 mg/ml; Invitrogen, Carlsbad, CA, USA) and BS-1 lectin binding (0.01 mg/ml; Sigma-Aldrich, St. Louis, MO, USA). Endothelial markers of cultured EPCs were also examined by flow cytometry analysis using CD31 (BD Pharmingen), von Willebrand factor (vWF) and kinase-insert domain receptor (KDR) (R&D Systems Inc) as previously described [26,27]. Based on the isolation and cultivation protocol, the adherent mononuclear cells were identified as late EPCs, and then were used for the following experiments.
2.6. Isolation of rat BM-derived EPCs
Wistar rats and SHRs in each group were sacrificed by cervical dislocation, and bone marrow was aspirated from bilateral femurs of rats aseptically. Using rat lymphocyte separation medium (TBD, China) and density gradient centrifugation, mononuclear cells (MNCs) were isolated from bone marrow. And then BM-isolated MNCs were resuspended in EGM-2 medium (Lonza, Swiss) containing 20% fetal bovine serum (Gibco, AU)., seeded into fibronectin (5μg/cm2) coated six-well plates at a density of 3 × 106/cm2 and maintained at a 37 °C incubator for 8 days. EGM-2 medium was replaced every two days. Non-adherent cells were removed and adherent cells were harvested by trypsinization for analysis.
2.7. Retinal detachments and immunofluorescence staining
Wistar rats and SHRs were used to prepare whole-mount retinas. Rats were sacrificed by cervical dislocation. Then, the eyeballs were taken out and fixed in 4% paraformaldehyde at 4 °C for 5–10 min. Then the ocular anterior segment (cornea and crystalline lens) was cut off; the retina was carefully ablated under dissection microscope (BX61, Olympus, Tokyo, Japan) and a petal shape was cut into the four edges of the retina for flattening. The sample was dropped in cold methanol at −20 °C for 20 min before proceeding with immunostaining. Subsequently, the retinas were washed 2 times (15 min each) in 0.01 mol/L PBS (pH 7.4). Then the sample was covered with 100ul of perm/block solution and shaked gently for 1 h. Then the perm/block solution was removed and retina was incubated with 100ul of IsolectinB4(Sigma-Aldrich, Merck) overnight at 4 °C. After washing 3 times in 0.01 mol/L PBS for 15 min each time, the samples were examined under a fluorescence microscope (BX61, Olympus).
2.8. EPC gene transfer
After 4-weeks culture, cells were transduced with the adenovirus serotype 5 (Ad5) encoding the human CXCR4 gene (Ad5/CXCR4), human SIRT5 gene (Ad5/SIRT5) and enhanced green fluorescent protein gene (Ad5/EGFP) (GeneChem company, Ltd., Shanghai) for 90 min in culture medium without serum. After transduction, cells were washed with PBS and incubated with cell basal medium for 48 h before subsequent experiments.
2.9. EPC migration in vitro
A total of 2 × 104 EPCs were resuspended in 250 μl EBM-2 and pipetted in the upper chamber of a modified Boyden chamber (Costar Transwell ® assay, 8 μm pore size, Corning, NY). The chamber was placed in a 24-well culture dish containing 500 μL EBM-2 supplemented with either PBS or 100 ng/ml SDF-1. After 24 h incubation at 37 °C, transmigrated cells were counted.
2.10. EPC adhesion in vitro
EPC adhesion assay was performed as described [24,25]. A monolayer of human umbilical vein endothelial cells (HUVECs) was prepared 48 h before the assay by plating 2 × 105 cells in each well of a 24-well plate. HUVECs were pretreated with or without 1 ng/ml tumor necrosis factor-α (TNF-α, Peprotech) for 12 h. Then 1 × 105 CM-DiI (CellTracker™ CM-DiI, Invitrogen)-labeled EPCs were added to each well and incubated for 3 h at 37 °C. Nonattached cells were gently removed with PBS, and adherent EPCs were fixed with 4% paraformaldehyde and counted.
2.11. EPC tube formation in vitro
A growth factor-reduced Matrigel (BD Biosciences, USA) was dissolved at 4 °C, added to 96-well plates at 60/well, and then incubated at 37 °C for 1 h. EPCs (2 × 104 cells/100 μL) were resuspended in EBM-2 serum-free medium, and loaded on the top of the Matrigel. After 2 h of incubation at 37 °C, EPC tube formation was assessed by a microscopy and each well was photographed at 400× magnification under a light microscope. An average of tubules was counted from 3 to 5 random fields.
2.12. Determination of ROS generation and mitochondrial membrane potential (MMP) disruption
ROS generation was detected with the carboxy derivative of fluorescein (carboxy-H2DCFDA, 1.0 mM, Beyotime Institute of Biotechnology, China) and MMP disruption was detected with JC-1 cationic dye (5 μg/ml, KeyGen Biotech, China), respectively. In brief, the treated EPCs were labeled with a specific fluorescent dye for 30 min at 37 °C. After labeling, cells were washed with PBS and resuspended in PBS at a concentration of 1 × 106 cells/ml before analysis via a FACSCanto™ flow cytometry (BD Biosciences).
2.13. Determination of intracellular nicotinamide adenine dinucleotide (NADH) levels
NADH level was determined with a Frex probe. The Frex probe was generously provided by Dr. Yi Yang (East China University of Science and Technology, China). Cells were seeded at 1 × 106 per well in 6-well culture plate and Frex probe was transfected into EPCs using lipo2000 (Invitrogen). Intracellular NADH levels were measured after calibration of Frex fluorescence in EPCs using flow cytometers (Beckman, USA).
2.14. Measurement of oxygen consumption
Oxygen consumption was quantified using oxygen sensitive probe MitoXpress® (Luxcel Biosciences). In brief, EPCs were grown in 96-well plates and were labeled with the oxygen sensitive probe. All wells were overlaid with mineral oil. Fluorescence of each well was then measured at 2 min intervals for a total of 200 min using a fluorescence plate reader (Corning, NY). Rate of oxygen consumption was determined from the slope of fluorescence vs.time for each sample using arbitrary units.
2.15. Mitochondrial ultrastructure assay
Transmission electron microscopy was used to observe mitochondrial ultrastructure of EPCs. EPCs were treated and then harvested by trypsinization, washed twice with PBS, fixed with a buffer containing 2.5% glutaraldehyde for 24 h and refixed in 1% osmium tetroxide for 30 min. Then, they were dehydrated in graded ethanol, washed with propylene oxide, embedded in Epong, and finally sectioned on a Reichert–Jung ultramicrotome at 90 nm thickness. Sections were stained with methylene buffer ArumeII before using a Philips electron microscope CM-120.
2.16. Real-time PCR assay for mRNA level of CXCR4 and SIRT genes
Total RNA was extracted with the High pure RNA isolation kit (Roche, Indianapolis, USA). The first strand cDNA was synthesized using PrimeScript® RT reagent Kit (Takara Biotechnology, Japan). The mRNA expression of CXCR4 was quantified using the 2−ΔΔCTanalytical method in triplicate using a StepOne Plus real-time PCR System (ABI, USA). The mRNA level of β-actin gene was measured in each sample as an internal normalization standard. The primer CXCR4 A (sense) is 5′-TCTTCCTGCCCACCATCTACTC-3′, and the primer CXCR4 B (anti-sense) is 5′-GTAGATGACATGGACTGCCTTGC-3′. Real-time PCR was performed in a 20 μL reaction mixture containing primers, FastStart Universal SYBR Green master (ROX) reagent (Roche Applied Science, Mannheim, Germany) and 2 μL cDNA sample.
2.17. Western blot analysis
Total EPC protein were extracted and quantified by cytoBuster TM protein extraction reagent (Beyotime Biotechnology, China) and bicinchoninic acid protein assay kit (I Thermo, USA) separately. Protein extracts were subjected to SDS-PAGE, transferred to polyvinylidene fluoride membranes (Roche, Indianapolis, IN, USA). The following antibodies were used: rabbit anti-CXCR4 antibody (1:500; ABCAM, USA), rabbit anti-actin antibody (1:2000; Cell Signaling Technology), rabbit anti-SIRT5 antibody rabbit (1:500; Santa Cruz, USA), rabbit anti-Phospho-JAK2 antibody rabbit (1:1000; Immunoway, USA) and rabbit anti-GADPH antibody (1:3000; Cell Signaling Technology). Proteins were visualized with HRP-conjugated anti-rabbit IgG (1:2000; Cell Signaling Technology), followed by use of the ECL chemiluminescence system (Thermo).
2.18. In vivo Angiogenic assay
Male BALB/c nude mice were used to allow injection of human EPCs. The animals were kept under controlled environmental conditions with constant laminar airflow, temperature of 20–23 °C, and humidity of 40–60%, and a 12/12-h light/dark cycle. Intraperitoneal anesthesia was administered using pentobarbital (80 mg/kg). The right femoral artery ligation and superficial femoral artery (SFA) excision was performed. Mice were positioned in dorsal recumbency with their hind limbs externally rotated. A skin incision was made over the femoral artery beginning at the inguinal ligament and continued caudally to the popliteal bifurcation. The femoral artery was isolated above the level of the profunda and epigastric arterial branches, doubly ligated using 7–0 Prolene suture, and transected. The SFA caudal to the major branch points was dissected, ligated, and excised in its entirety.
After 4 weeks culture, EPCs (1 × 105 cells) were resuspended in 100 μL of pre-warmed PBS (37 °C) and transplanted intramuscularly into three sites of ischemic gastrocnemius muscle. The same volume of PBS as a control was injected into placebo mice. Detection of hindlimb subcutaneous blood flow was performed using a Laser Doppler imager (Perimed Instruments, Sweden). Blood flow in both ischemic and nonischemic hindlimbs was measured at 0, 3, 7, 14 and 21 days after the injection. After 21 days, mice were sacrificed.
2.19. Statistical analysis
All results are expressed as mean value ± SEM. Statistical significance was evaluated by means of a Student's t-test or ANOVA. Pearson's test was performed to evaluate correlation between mitochondrial function and EPC function. P < .05 was considered statistically significant. All statistical analyses used SPSS statistical software (SPSS version 13.0).
3. Results
3.1. Baseline characteristics
As listed in Table S1, baseline characteristics were not significantly different between healthy subjects and hypertensive patients with the exception of systolic BP and diastolic BP.
3.2. Nail fold and retinal capillary density are reduced in hypertensive patients
To investigate the systematic capillary density status in hypertensive patients, we performed capillaroscopy measurement in the nail fold and optical coherence tomography angiography (OCTA) in the fundus. Compared with healthy subjects, NCD was markedly decreased in hypertensive patients both in the rest state and after 2-min venous congestion (VC) (Fig. S1a-b). Consistent with testing results of NCD, retinal capillary density (RCD) was significantly decreased in hypertensive patients (Fig. S2a-b). These findings indicated that the microcirculation was systemically impaired in hypertension.
3.3. Decreased in vitro function and in vivo angiogenic capacity of EPCs are related to capillary rarefaction in hypertension
EPCs are involved in angiogenesis and vasculogenesis, however the relationship between EPC function and microcirculation has not been explored in patients with hypertension. Here data showed that both the basal level of migration and adhesion onto TNF-α-pre-stimulated monolayer HUVECs were significantly lower in EPCs from hypertensive patients than those from healthy subjects (Fig. S3a-b and Fig. S3c-d). Tube formation function from hypertensive patients was also decreased (Fig. S3e-f). Besides, a significant positive correlation was found between NCD and in vitro migration function (Fig. 1a), as well as in vitro adhesion function (Fig. 1b) and tube formation function (Fig. 1c). Moreover, in vivo angiogenic capacity of EPCs from hypertensive patients was significantly reduced in comparison with EPCs from healthy subjects in 21 days after EPCs injected into hindlimb ischemia model mice after EPCs injected into hindlimb ischemia model mice (Fig. 1d). These data demonstrated that EPC angiogenic function was decreased in hypertension and had obviously positive correlation with NCD. To further clarify capillary rarefaction in hypertension, we detected the retinal capillary and EPC function in hypertensive animal models. Our data suggested that the number of retinal capillary (Fig. S4a-c) of SHRs and in vitro adhesion(Fig. S5a-b), migration(Fig. S5c-d), tube formation(Fig. S5e-f)function of bone marrow derived EPCs from SHRs were remarkably decreased compared with wistar rats. The results of animal experiments further confirmed that hypertension display capillary rarefaction and decreased EPC function.
Fig. 1.
The in vivo angiogenic capacity of EPCs from hypertensive patients are reduced in hind limb ischemia model mice, and the correlations between capillary density and in vitro functions of EPCs.
a-c, The positive association between NCD and EPCs function of migration (a, r2 = 0.144; P < .01, n = 50), adhesion (b, r2 = 0.100; P < .05, n = 50) and tube formation (c, r2 = 0.161; P < .01, n = 50). Pearson's test was performed to evaluate the correlation. d, Quantification analysis and representative photographs of blood flow in hindlimb ischemia in mice after injection of EPCs (1 × 105 cells) from hypertensive patients or healthy subjects (*P < .05 versus Day 0 group; n = 6 per group). Paired t-test was used to analyze statistical significance. Error bars denote standard deviation.
3.4. Mitochondrial dysfunction is present in EPCs from hypertensive patients
Then we detected EPC mitochondrial function of hypertensive patients to determine whether EPC mitochondrial dysfunction was related to cell dysfunction. Using the Frex probe in EPCs, the NADH level was significant increased by ~1.5-fold in hypertensive patients (Fig. 2a). ROS level was found obviously increased ~1.8-fold in hypertensive patients (Fig. 2b). As shown in Fig. 2c, the oxygen consumption rate also decreased in EPCs from hypertensive patients compared to healthy subjects. In addition, we found that MMP (Ψm) of EPCs was markedly decreased by ~3-fold in EPCs of hypertensive patients compared with healthy control (Fig. 2d). Moreover, swollen mitochondria and a more conspicuous loss of mitochondrial cristae were observed in EPCs from hypertensive patients (Fig. 2e). Therefore, these data suggested that EPCs from hypertensive patients had mitochondrial dysfunction and abnormal structure of mitochondria, which may play a central role in decreased EPC function and angiogenic capacity.
Fig. 2.
Mitochondrial dysfunction is present in EPCs of hypertensive patients.
a-c, Quantification analysis of the NADH level (a), ROS level (b) and oxygen consumption rate (c) of EPCs (*P < .05 versus healthy group). d, Quantification analysis and representative photographs of MMP (ΔΨm) of EPCs (*P < .05 versus healthy group). e, Representative photographs of electron microscopy of mitochondrial ultrastructure in EPCs (magnification: 12500). n = 10 per group. Paired t-test was performed to analyze statistical significance. Error bars denote standard deviation. Scale bars, 20um.
3.5. Mitochondrial dysfunction is responsible for the impaired function of EPCs from healthy subjects
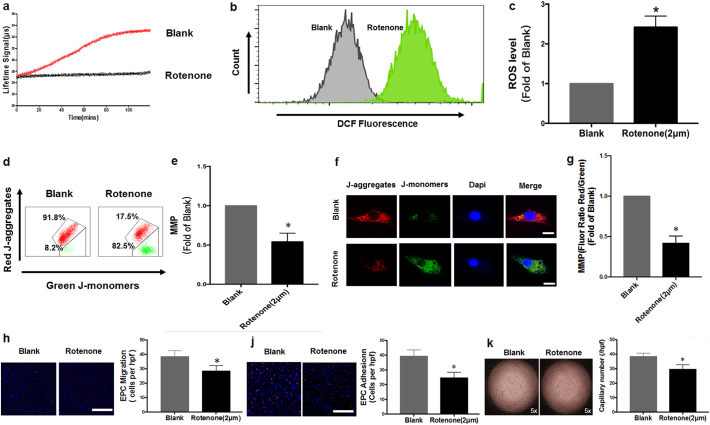
To further clarify whether mitochondrial dysfunction leads to impaired function of EPCs, we investigate EPCs function under the condition of mitochondrial respiration inhibition by Rotenone, a mitochondrial respiratory chain complex I inhibitor. As shown in Fig. 3a, oxygen consumption rate significantly decreased in EPCs pre-treated with rotenone. Rotenone induced excessive ROS generation (Fig. 3b–c) and loss of MMP (Fig. 3d–g). In addition, rotenone administration markedly impaired migration (Fig. 3h), adhesion (Fig. 3j) and tube formation capacity (Fig. 3k) of EPCs from healthy subjects. These data demonstrated that mitochondrial dysfunction contributed to decline in EPC function.
Fig. 3.
Mitochondrial dysfunction is Responsible for the impaired function of EPCs from healthy subjects. a-g, Quantification analysis and representative photographs of oxygen consumption rate (a), ROS level (b-c), MMP (ΔΨm) of EPCs (d-g) with rotenone administrated (*P < .05 versus blank group). h-k, Quantification analysis and representative photographs of migration (h),adhesion (j) and tube formation (k) function of EPCs (*P < .05 versus blank group). n = 6 per group. Paired t-test was performed to analyze statistical significance. Error bars denote standard deviation. Scale bars, 20um.
3.6. CXCR4 expression is essential for mitochondrial function and angiogenic capacity of EPCs from healthy subjects
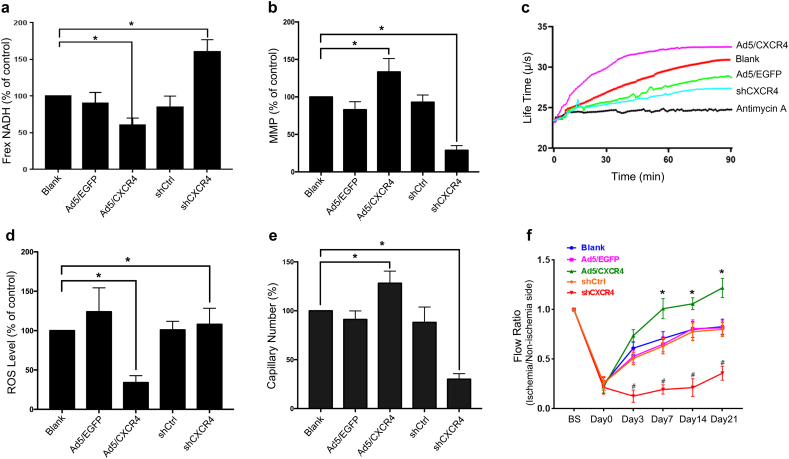
CXCR4 is a key modulator to regulate EPC functions and its level was decreased in EPCs from hypertensive patients (Fig. S6a-b), similar with our previous study [18]. To confirm the effect of CXCR4 on the regulation of mitochondrial function, we transduced EPCs from healthy subjects with Ad5 vector encoding CXCR4 cDNA and short hairpin CXCR4 (shCXCR4) (Fig. S6c). At 48 h after transduction, data showed that the MMP (Ψm) and oxygen consumption rate were obviously increased in Ad5/CXCR4-transduced EPCs from healthy subjects compared with Ad5/EGFP-transduced EPCs or non-transduced EPCs from healthy subjects (Fig. 4b-c), while ROS level and NADH level was reduced (Fig. 4a, d), which are consistent with its effect on tube formation function (Fig. 4e). Meanwhile, as opposed to Ad5/CXCR4-transduced EPCs, shCXCR4 can markedly reduce mitochondrial function and tube formation function (Fig. 4a-e). In addition, Ad5/CXCR4-transduced EPCs increased the flow ratio of hind limbs in contrast with non-transduced EPCs and Ad5/EGFP-transduced EPCs (Fig. 4F and Fig. S6d). Compared with Ad/CXCR4 EPCs, shCXCR4 was significantly able to inhibit in vivo angiogenic capacity of EPCs (Fig. 4f and Fig. S6d). Therefore, these data suggested that CXCR4 plays an important role in mitochondrial function and angiogenic capacity of EPCs from healthy subjects.
Fig. 4.
Effects of Ad5/CXCR4 gene transfer and shCXCR4 on mitochondrial function and angiogenic capacity of EPCs from healthy subjects.
a-d, Quantification analysis of the NADH level (a), MMP (b), oxygen consumption rate (c) and ROS level (d) of EPCs with or without CXCR4 gene transfection and shCXCR4 (*P < .05 versus blank group). e, Quantification analysis of tube formation capacity of EPCs. (*P < .05 versus blank group). f, Quantification analysis of blood flow in hindlimb ischemia in mice after injection of EPCs (1 × 105 cells) (*P < .05 versus Day0 group). n = 10 per group. A one-way analysis of variance (ANOVA) test was performed to determine the statistically significant differences among groups. Paired t-test was performed to analyze statistical significance. Error bars denote standard deviation.
3.7. CXCR4 gene transfer improves mitochondrial function and Angiogenic capacity of EPCs from hypertensive patients
We then investigated the effect of CXCR4 on regulating mitochondrial function in relation to the angiogenic capacity of EPCs from hypertensive patients. Ad5/CXCR4 gene transfer increased CXCR4 expression in EPCs (Fig. S7a). Our data showed that, paralleling with Ad5/CXCR4-transduced EPCs from healthy subjects, mitochondrial function (Fig. S7b-e), tube formation function (Fig. S7f) and angiogenic capacity (Fig. S7 g) were significantly enhanced in Ad5/CXCR4-transduced EPCs from hypertensive patients compared with non-transduced EPCs from hypertensive patients or Ad5/EGFP-transduced EPCs from hypertensive patients. These findings indicated that CXCR4 can improve reduced mitochondrial function and angiogenic capacity of EPCs from hypertensive patients.
3.8. SIRT5 expression induced by CXCR4 gene transfer is essential for the improvement of in vitro functions and in vivo angiogenic capacity of EPCs from healthy subjects
We tested the expression of sirtuin family (SIRT1- SIRT7), and our data showed that SIRT5 expression was significantly lower in EPCs from hypertensive patients (Fig. 5a-b). To further define the mechanistic link between CXCR4 and SIRT5, we then transduced EPCs from healthy subjects with Ad5/CXCR4 and shCXCR4. Our data showed that the level of SIRT5 protein was significantly higher in EPCs from Ad5/CXCR4-transduced EPCs from healthy subjects than non-transduced EPCs from healthy subjects or Ad5/EGFP-transduced EPCs from healthy subjects (Fig. S8a-b). In order to evaluate the effect of SIRT5 expression on in vitro and in vivo function, EPCs were also transduced with Ad5/SIRT5 and shSIRT5. Compared with Ad/EGFP-EPCs and shSIRT5-EPCs from healthy subjects, Ad5/SIRT5 was significantly able to increase in vitro function (Fig. 5c-e) and in vivo angiogenic capacity (Fig. 5f and Fig. S8c) of EPCs. Meanwhile, shSIRT5 can inhibit the Ad5/CXCR4-induced the increase of in vitro function (Fig. 5c-e) and in vivo angiogenic capacity (Fig. 5f and Fig. S8c) of EPCs from healthy subjects. Indeed, our data demonstrated that abnormality in CXCR4/SIRT5 signaling pathway is, at least in part, responsible for the fall in angiogenic capacity of EPCs.
Fig. 5.
Effects of SIRT5 regulated by CXCR4 on in vitro functions and in vivo angiogenic capacity of EPCs.
a, Quantitative analysis of SIRT1-SIRT7 mRNA level in EPCs (*P < .05 versus healthy group). b, Quantitative analysis and representative photographs of SIRT5 protein expression in EPCs (*P < .05 versus healthy group). c-e, Quantification analysis of migration (c), adhesion to HUVECs (d) and tube formation (e) of EPCs (*P < .05 versus blank group). f, Quantification analysis of blood flow in hindlimb ischemia in mice after injection of EPCs (1 × 105 cells) (*P < .05 versus Day0 group). n = 10 per group. A one-way analysis of variance (ANOVA) test was performed to determine the statistically significant differences among groups. Paired t-test was performed to analyze statistical significance. Error bars denote standard deviation.
3.9. CXCR4 upregulates SIRT5 in a JAK2-dependent manner
Our previous research demonstrated that decline of JAK2 phosphorylation is often correlated with decreased CXCR4, which contributes to the fall in EPC-mediated endothelial reparative function of hypertensive patients [18]. Indeed, our data showed that both of SIRT5 expression and JAK2 phosphorylation were significantly lower in EPCs from hypertensive patients than EPCs from healthy subjects (Fig. 6a). To explore whether JAK2 was involved in CXCR4-regulated SIRT5 expression in EPC-related to mitochondrial function and angiogenic capacity, we transduced EPCs from healthy subjects with Ad5/CXCR4 and shCXCR4. Data reported here showed that the level of p-JAK2 was significantly increased in EPCs from Ad5/CXCR4-transduced EPCs compared with shCXCR4-transduced EPCs (Fig. 6b), which was consistent with the change of SIRT5 expression. Then we treated EPCs with 10 μM JAK2 inhibitor AG490 for 15, 30, 45, 60 min. The data showed that AG490 can markedly inhibit SIRT5 expression in a time-dependent manner (Fig. 6c). Moreover, AG490 can markedly decrease the Ad5/CXCR4 up-regulated SIRT5 level (Fig. 6d). Therefore, our results demonstrated that CXCR4 regulated SIRT5 via JAK2 signaling in EPCs.
Fig. 6.
CXCR4 regulates SIRT5 expression via JAK2 signal pathway.
a, Quantification analysis and representative photographs of p-JAK2 and SIRT5 protein expression in EPCs (*P < .05 versus healthy group). b, Quantification analysis and representative photographs of p-JAK2 and SIRT5 protein expression in EPCs (*P < .05 versus healthy group). c, Quantification analysis and representative photographs of SIRT5 expression in EPCs pretreated with AG490 for 15, 30, 45, 60 min (*P < .05 versus blank group). d, Representative photographs and quantification analysis of SIRT5 expression in EPCs (*P < .05 versus blank group). n = 10 per group. A one-way analysis of variance (ANOVA) test was performed to determine the statistically significant differences among groups. Paired t-test was performed to analyze statistical significance. Error bars denote standard deviation.
3.10. CXCR4/JAK2/SIRT5 signaling is involved in regulating in vitro functions and in vivo angiogenic capacity of EPCs from hypertensive patients
In order to further prove CXCR4/JAK2/SIRT5 signal pathway contributing to decreased angiogenic capacity of EPCs from hypertensive patients, we then investigated the effect of up-regulation of CXCR4 and SIRT5 in relation to the capabilities of EPCs from hypertensive patients. Similar to the effects of Ad/CXCR4 and Ad/SIRT5 gene transfer into EPCs from healthy subjects, in vitro migration (Fig. 7a), in vitro adhesion function (Fig. 7b), tube formation function (Fig. 7c) and in vivo angiogenic capacity (Fig. 7d and Fig. S9) were significantly increased in Ad5/CXCR4-transduced and Ad5/SIRT5-transduced EPCs from hypertensive patients compared with non-pretreated and Ad5/EGFP-transduced EPCs. In addition, as shown in Fig. 7a-d, the inhibitory effects of shSIRT5 on Ad5/CXCR4-transduced EPCs from hypertensive patients were in accord with that of shSIRT5 on in vitro functions and in vivo angiogenic capacity of EPCs from healthy subjects.
Fig. 7.
CXCR4/SIRT5 signaling is involved in the reduced functions and angiogenic capacity of EPCs from hypertensive patients.
a-b, Quantification analysis of migration (a) and adhesion to HUVECs (b) of EPCs (*P < .05 versus blank group; *P < .05 Ad/CXCR4-EPCs versus Ad/CXCR4-EPCs+shSIRT5). c, Quantification analysis of tube formation capacity of EPCs (*P < .05). d, Quantification analysis of blood flow in hindlimb ischemia in mice after injection of EPCs (1 × 105 cells) (*P < .05 versus Day0 group). e, Proposed model. Mitochondrial dysfunction-mediated fall in angiogenic capacity due to deficient CXCR4/JAK2/SIRT5 signaling of late EPCs is probably responsible for the capillary rarefaction in hypertension. n = 10 per group. A one-way analysis of variance (ANOVA) test was performed to determine the statistically significant differences among groups. Paired t-test was performed to analyze statistical significance. Error bars denote standard deviation.
4. Discussion
The aim of this study was to determine whether mitochondrial dysfunction in late EPCs from hypertensive patients due to reduced CXCR4/JAK2/SIRT5 signaling results in the fall of EPC-related angiogenic capacity, which is paralleled to the development of capillary rarefaction in hypertension. Our present study clearly showed that the association of the reduced capillary density of nail fold with decreased late EPC angiogenic capacity in hypertensive patients. The decline in EPC activities of hypertensive patients was accompanied by impaired mitochondrial ultrastructure, diminished MMP, increased ROS generation and NADH level and reduced oxygen consumption. Rotenone, a mitochondrial respiratory chain complex I inhibitor, induced inhibition of oxygen consumption rate, excessive ROS generation and loss of MMP, which markedly decreased the in vitro functions of EPCs. Mitochondrial SIRT5 expression of EPCs from hypertensive patients was reduced, which is correlated to mitochondrial dysfunction. CXCR4 gene transfer facilitated SIRT5 expression, improved mitochondrial function and augmented angiogenic capacity of late EPCs. Furthermore, the beneficial impacts of SIRT5 up-regulation on EPC-mediated angiogenesis can be inhibited by JAK2-inhibitor AG490. Our present study demonstrates for the first time that mitochondrial dysfunction of late EPCs from hypertensive patients is, at least in part, responsible for the decline in EPC-based angiogenic capacity. CXCR4 gene transfer contributes to the restoration of mitochondrial function which results in the improvement of EPC angiogenic capacity in patients with hypertension via regulating CXCR4/JAK2/SIRT5 signal pathway.
It is widely accepted that capillary rarefaction occurs in patients with untreated essential hypertension and plays an important role in increasing peripheral vascular resistance, thereby resulting in elevated BP and aggravating BP-related target organ damage [3,4,28]. Indeed, our current results showed that both of untreated hypertensive patients and SHRs had significant reduction in capillary density, similar with previous studies [3,5]. Although microvascular abnormality in hypertension was convincingly illustrated, the cause of capillary rarefaction has not been completely elucidated.
It has been proved that hypertension is characterized by imbalance of pro-angiogenic and anti-angiogenic factors [29]. Previous studies demonstrated that anti-VEGF therapy related to angiogenic inhibition can lead to capillary rarefaction in nontumor tissues and thus result in elevated BP [30]. Moreover, animal experiments have confirmed that capillary rarefaction involves a suppressed angiogenesis in SHRs, and cytokines-induced angiogenesis can ameliorate long-lasting BP in SHRs and prevent the occurrence of hypertension in young prehypertensive SHRs [31,32]. The mechanisms underlying capillary rarefaction in hypertension is still unclear and probably multifactorial, involving hypoxia, reduced growth factors, increased ROS and inflammation. However, it is not yet clear whether circulating late EPC dysfunction is associated with capillary rarefaction in patients with hypertension. Data reported here for the first time found that an reduction in capillary density are correlated to decreased EPC angiogenic capacity in patients with essential hypertension, suggesting that fall in late EPC-mediated angiogenesis is associated with microvascular abnormality. However, the molecular mechanisms underlying the impaired late EPC-based angiogenesis in hypertension remain further to be elucidated.
Recent studies have demonstrated that mitochondrial function involves in modulation of stem and progenitor function under various physiologic and pathologic conditions [33]. Meanwhile, it is proposed that mitochondrial dysfunction may participate in the pathogenesis of hypertension [34]. However, the impact of mitochondrial dysfunction on EPC angiogenic capacity is completely unclear. Given the importance of mitochondrial function on cell function, thus, the deep insight into the role of mitochondrial dysfunction in the modulation of EPC angiogenic capacity may be of important clinical implication for our further understanding of the pathogenesis of EPC dysfunction in hypertension, thereby developing a novel potential therapeutic target. Thus, we put forward the notion that mitochondrial dysfunction contributes to diminished late EPC angiogenic capacity. Indeed, here we found that impaired mitochondrial ultrastructure, diminished MMP, increased ROS level and NADH level and reduced oxygen consumption occur in late EPCs from hypertensive patients compared with normal subjects. In parallel, in vitro migration, adhesion, tube formation function and in vivo angiogenic capacity were markedly decreased in EPCs from hypertensive patients. Moreover, as a mitochondrial respiratory chain complex I inhibitor, rotenone significantly induced inhibition of oxygen consumption rate, excessive ROS generation and loss of MMP. Meanwhile, rotenone markedly decreased the in vitro functions of EPCs. Our data reported here for the first time suggest that mitochondrial dysfunction is responsible for the decline in late EPC-based angiogenic capacity in patient with hypertension. In agreement, previous studies indicate that oxidative stress can modulate EPC bioactivities, such as mobilization, migration, and neovascularization, and that inhibition of NADPH oxidase has been shown to improve EPC functions [35]. Moreover, mitochondria produce ROS during oxidative phosphorylation, and prolonged oxidative stress can damage mitochondria [36]. However, further elucidation of the underlying mechanisms in the regulation of mitochondrial function of late EPCs should be still examined.
As it was mentioned before, CXCR4 plays an important role in regulating EPC function [18]. Accumulating evidences also showed that several factors including ROS modulate expression of CXCR4 in human cells [19,20]. However, whether CXCR4 can mediate mitochondrial function of late EPCs related to angiogenic capacity and its molecular pathway is still unclear in hypertension. Interestingly, our data showed that increased the expression of CXCR4 by Ad5/CXCR4 gene transfer can markedly reduce ROS level and NADH level, increase MMP and oxygen consumption. In parallel, this CXCR4 expression augmentation is consistent with increased in vitro EPC migration, adhesion, tube formation function as well as accelerated in vivo angiogenic capacity respectively, which can be blocked by shCXCR4. Furthermore, our data for the first time showed that the expression of SIRT5, involved in the CXCR4 downstream signaling, is also significantly reduced concomitant with decreased phosphorylation of JAK2. Besides, our data showed that both SIRT5 and CXCR4 gene transfer can respectively increase SIRT5 expression of EPCs from hypertensive patients. Similar with CXCR4 gene transfer, SIRT5 augmentation is consistent with increased in vitro EPC migration, adhesion, tube formation function as well as accelerated in vivo angiogenic capacity. Upregulation of late EPC functions by SIRT5 overexpression can be also attenuated by shSIRT5,and thus highlights the putative signaling pathways that involve JAK2 and SIRT5. Indeed, SIRT5 overexpression can be blocked by JAK2 inhibitor AG490 and shSIRT5 respectively. Therefore, our data demonstrates for the first time that mitochondrial dysfunction due to diminished CXCR4/JAK2/SIRT5 signaling leads to the decline in angiogenic capacity of late EPCs, which is paralleled with the capillary rarefaction in hypertension. Our current study provides the novel insight into the potential of late EPC mitochondria as a new target for the treatment of hypertension-related loss of microvascular density.
Our present study still had some limitations. Firstly, although we found that declined angiogenic capacity in late EPC is associated with capillary rarefaction in hypertensive patients, the causal relationship should be further investigated in our future work. Secondly, even though our current data supported that mitochondria may be a novel target in treating hypertension-related capillary rarefaction, the notion put forward here should be evidenced by further clinical investigations. Finally, our data showed that decline in CXCR4 signaling of late EPCs is involved in mitochondrial dysfunction and the impaired angiogenic capacity of EPCs. However, the exact mechanism responsible for diminished CXCR4 expression in hypertension remains to be further elucidated.
In conclusion, we identified mitochondrial dysfunction as a crucial regulator of late EPC function in hypertension. Our present study demonstrates for the first time that mitochondrial dysfunction-mediated the fall in angiogenic capacity due to deficient CXCR4/JAK2/SIRT5 signaling of late EPCs is, at least in part, related to the capillary rarefaction in hypertension, providing the novel insight into the potential of late EPC mitochondria as a novel target for the treatment of hypertension-related loss of microvascular density.
Conflicts of interest
None.
Sources of funding
This work was supported by the National Nature Science Foundation of China (31530023, 81500205, 81671379), 973 Program of China (2013CB531200), the Nature Science Foundation of Guangdong (2016A030310184, 2016A020215056) and Science and Technology Planning Project of Guangdong (2017A020215085).
Author contributions
BBY designed and performed mouse studies. BBY, HZ and XYZ performed endothelial progenitor cell studies. JWL designed and performed clinical studies. JH, HZ, XYZ, and BBY performed mitochondrial studies. HZ, XYZ and CS performed gene transfer experiments. BBY and JH performed Statistical Analysis. JT, GXZ and WHX designed and conceived of studies. BBY, HZ and YXZ wrote the manuscript. All authors provided feedback and edited the manuscript.
Acknowledgements
We thank Dr. Yi Yang, East China University of Science and Technology, for sharing reagent. The authors thank the patients and healthy volunteers involved in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.03.031.
Contributor Information
Gao-Xing Zhang, Email: zhang.g.x@medmail.com.cn.
Jun Tao, Email: taojungz123@163.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.Messerli F.H., Fischer U., Rimoldi S.F., Bangalore S. Hypertension control and cardiovascular disease. Lancet. 2017;389(10065):153. doi: 10.1016/S0140-6736(17)30017-X. 2017-01-14. [DOI] [PubMed] [Google Scholar]
- 2.Mills K.T., Bundy J.D., Kelly T.N., Reed J.E., Kearney P.M., Reynolds K. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. 2016-08-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noon J.P., Walker B.R., Webb D.J., Shore A.C., Holton D.W., Edwards H.V. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J. Clin. Invest. 1997;99(8):1873–1879. doi: 10.1172/JCI119354. 1997-04-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonios T.F., Singer D.R., Markandu N.D., Mortimer P.S., MacGregor G.A. Structural skin capillary rarefaction in essential hypertension. Hypertension. 1999;33(4):998–1001. doi: 10.1161/01.hyp.33.4.998. 1999-04-01. [DOI] [PubMed] [Google Scholar]
- 5.Serne E.H., Gans R.O., ter Maaten J.C., Tangelder G.J., Donker A.J., Stehouwer C.D. Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension. 2001;38(2):238–242. doi: 10.1161/01.hyp.38.2.238. 2001-08-01. [DOI] [PubMed] [Google Scholar]
- 6.Luo S., Xia W., Chen C., Robinson E.A., Tao J. Endothelial progenitor cells and hypertension: current concepts and future implications. Clin. Sci. (Lond.) 2016;130(22):2029–2042. doi: 10.1042/CS20160587. 2016-11-01. [DOI] [PubMed] [Google Scholar]
- 7.Chen L., Ding M.L., Wu F., He W., Li J., Zhang X.Y. Impaired endothelial repair capacity of early endothelial progenitor cells in hypertensive patients with primary hyperaldosteronemia: role of 5,6,7,8-tetrahydrobiopterin oxidation and endothelial nitric oxide synthase uncoupling. Hypertension. 2016;67(2):430–439. doi: 10.1161/HYPERTENSIONAHA.115.06597. 2016-02-01. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X.Y., Su C., Cao Z., Xu S.Y., Xia W.H., Xie W.L. CXCR7 upregulation is required for early endothelial progenitor cell-mediated endothelial repair in patients with hypertension. Hypertension. 2014;63(2):383–389. doi: 10.1161/HYPERTENSIONAHA.113.02273. 2014-02-01. [DOI] [PubMed] [Google Scholar]
- 9.Giannotti G., Doerries C., Mocharla P.S., Mueller M.F., Bahlmann F.H., Horvath T. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension. 2010;55(6):1389–1397. doi: 10.1161/HYPERTENSIONAHA.109.141614. 2010-06-01. [DOI] [PubMed] [Google Scholar]
- 10.Asahara T., Murohara T., Sullivan A., Silver M., van der Zee R., Li T. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. 1997-02-14. [DOI] [PubMed] [Google Scholar]
- 11.Yoon C.H., Hur J., Park K.W., Kim J.H., Lee C.S., Oh I.Y. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112(11):1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. 2005-09-13. [DOI] [PubMed] [Google Scholar]
- 12.Sieveking D.P., Buckle A., Celermajer D.S., Ng M.K. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J. Am. Coll. Cardiol. 2008;51(6):660–668. doi: 10.1016/j.jacc.2007.09.059. 2008-02-12. [DOI] [PubMed] [Google Scholar]
- 13.Kim S.W., Jin H.L., Kang S.M., Kim S., Yoo K.J., Jang Y. Therapeutic effects of late outgrowth endothelial progenitor cells or mesenchymal stem cells derived from human umbilical cord blood on infarct repair. Int. J. Cardiol. 2016;203:498–507. doi: 10.1016/j.ijcard.2015.10.110. 2016-01-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman J.R., Nunnari J. Mitochondrial form and function. Nature. 2014;505(7483):335–343. doi: 10.1038/nature12985. 2014-01-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunnari J., Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–1159. doi: 10.1016/j.cell.2012.02.035. 2012-03-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verdin E., Hirschey M.D., Finley L.W., Haigis M.C. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem. Sci. 2010;35(12):669–675. doi: 10.1016/j.tibs.2010.07.003. 2010-12-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo W., Chen J., Patel A., Zhang L., Chau V., Li Y. CXCR4/CXCL12 mediate autocrine cell- cycle progression in NF1-associated malignant peripheral nerve sheath tumors. Cell. 2013;152(5):1077–1090. doi: 10.1016/j.cell.2013.01.053. 2013-02-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X., Zhang G.X., Zhang X.Y., Xia W.H., Yang Z., Su C. Lacidipine improves endothelial repair capacity of endothelial progenitor cells from patients with essential hypertension. Int. J. Cardiol. 2013;168(4):3317–3326. doi: 10.1016/j.ijcard.2013.04.041. 2013-10-09. [DOI] [PubMed] [Google Scholar]
- 19.Dooley D., Vidal P., Hendrix S. Immunopharmacological intervention for successful neural stem cell therapy: new perspectives in CNS neurogenesis and repair. Pharmacol. Ther. 2014;141(1):21–31. doi: 10.1016/j.pharmthera.2013.08.001. 2014-01-01. [DOI] [PubMed] [Google Scholar]
- 20.Dar A., Schajnovitz A., Lapid K., Kalinkovich A., Itkin T., Ludin A. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 2011;25(8):1286–1296. doi: 10.1038/leu.2011.62. 2011-08-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messina-Graham S., Broxmeyer H. SDF-1/CXCL12 modulates mitochondrial respiration of immature blood cells in a bi-phasic manner. Blood Cells Mol. Dis. 2016;58:13–18. doi: 10.1016/j.bcmd.2016.01.008. 2016-05-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenfant C., Chobanian A.V., Jones D.W., Roccella E.J. Seventh report of the joint national committee on the prevention, detection, evaluation, and treatment of high blood pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41(6):1178–1179. doi: 10.1161/01.HYP.0000075790.33892.AE. 2003-06-01. [DOI] [PubMed] [Google Scholar]
- 23.Garrity S.T., Iafe N.A., Phasukkijwatana N., Chen X., Sarraf D. Quantitative analysis of three distinct retinal capillary plexuses in healthy eyes using optical coherence tomography angiography. Invest. Ophthalmol. Vis. Sci. 2017;58(12):5548–5555. doi: 10.1167/iovs.17-22036. 2017-10-01. [DOI] [PubMed] [Google Scholar]
- 24.Yoon C.H., Hur J., Park K.W., Kim J.H., Lee C.S., Oh I.Y. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112(11):1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. 2005-09-13. [DOI] [PubMed] [Google Scholar]
- 25.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science Support, Non-U.S. Gov't; Research Support, U.S. Gov't, P.H.S.]. 1997 1997-02-14;275(5302):964–7. [DOI] [PubMed]
- 26.Chen L., Ding M.L., Wu F., He W., Li J., Zhang X.Y. Impaired endothelial repair capacity of early endothelial progenitor cells in hypertensive patients with primary Hyperaldosteronemia: role of 5,6,7,8-tetrahydrobiopterin oxidation and endothelial nitric oxide synthase uncoupling. Hypertension. 2016;67(2):430–439. doi: 10.1161/HYPERTENSIONAHA.115.06597. 2016-02-01. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X.Y., Su C., Cao Z., Xu S.Y., Xia W.H., Xie W.L. CXCR7 upregulation is required for early endothelial progenitor cell-mediated endothelial repair in patients with hypertension. Hypertension. 2014;63(2):383–389. doi: 10.1161/HYPERTENSIONAHA.113.02273. 2014-02-01. [DOI] [PubMed] [Google Scholar]
- 28.Greene A.S., Tonellato P.J., Lui J., Lombard J.H., Cowley A.J. Microvascular rarefaction and tissue vascular resistance in hypertension. Am. J. Phys. 1989;256(1 Pt 2) doi: 10.1152/ajpheart.1989.256.1.H126. 1989-01-01. (H126–31) [DOI] [PubMed] [Google Scholar]
- 29.Sane D.C., Anton L., Brosnihan K.B. Angiogenic growth factors and hypertension. Angiogenesis. 2004;7(3):193–201. doi: 10.1007/s10456-004-2699-3. 2004-01-20. [DOI] [PubMed] [Google Scholar]
- 30.Belcik J.T., Qi Y., Kaufmann B.A., Xie A., Bullens S., Morgan T.K. Cardiovascular and systemic microvascular effects of anti-vascular endothelial growth factor therapy for cancer. J. Am. Coll. Cardiol. 2012;60(7) doi: 10.1016/j.jacc.2012.02.053. 2012-08-14. (618–25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rusai K., Jianxing C., Schneider R., Struijker-Boudier H., Lutz J., Heemann U. Renin inhibition mitigates anti-angiogenesis in spontaneously hypertensive rats. J. Hypertens. 2011;29(2):266–272. doi: 10.1097/HJH.0b013e328340aa72. 2011-02-01. [DOI] [PubMed] [Google Scholar]
- 32.Vilar J., Waeckel L., Bonnin P., Cochain C., Loinard C., Duriez M. Chronic hypoxia-induced angiogenesis normalizes blood pressure in spontaneously hypertensive rats. Circ. Res. 2008;103(7):761–769. doi: 10.1161/CIRCRESAHA.108.182758. 2008-09-26. [DOI] [PubMed] [Google Scholar]
- 33.Garrison B.S., Rossi D.J. Reactive oxygen species resulting from mitochondrial mutation diminishes stem and progenitor cell function. Cell Metab. 2012;15(1):2–3. doi: 10.1016/j.cmet.2011.12.008. 2012-01-04. [DOI] [PubMed] [Google Scholar]
- 34.Johnstone M. Mitochondrial calcium overload, a cause of essential hypertension. Lancet. 1977;1(8012) doi: 10.1016/s0140-6736(77)92085-2. 1977-03-19. (650–1) [DOI] [PubMed] [Google Scholar]
- 35.Chen D.D., Dong Y.G., Yuan H., Chen A.F. Endothelin 1 activation of endothelin a receptor/NADPH oxidase pathway and diminished antioxidants critically contribute to endothelial progenitor cell reduction and dysfunction in salt-sensitive hypertension. Hypertension. 2012;59(5):1037–1043. doi: 10.1161/HYPERTENSIONAHA.111.183368. 2012-05-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willems P.H., Rossignol R., Dieteren C.E., Murphy M.P., Koopman W.J. Redox homeostasis and mitochondrial dynamics. Cell Metab. 2015;22(2):207–218. doi: 10.1016/j.cmet.2015.06.006. 2015-08-04. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material