Abstract
The study of polymorphic immune genes in host populations is critical for understanding genetic variation in susceptibility to pathogens. Controlled infection experiments are necessary to separate variation in the probability of exposure from genetic variation in susceptibility to infection, but such experiments are rare for wild vertebrate reservoir hosts and their zoonotic pathogens. The bank vole (Myodes glareolus) is an important reservoir host of Borrelia afzelii, a tick-borne spirochete that causes Lyme disease. Bank vole populations are polymorphic for Toll-like receptor 2 (TLR2), an innate immune receptor that recognizes bacterial lipoproteins. To test whether the TLR2 polymorphism influences variation in the susceptibility to infection with B. afzelii, we challenged pathogen-free, lab-born individuals of known TLR2 genotype with B. afzelii-infected ticks. We measured the spirochete load in tissues of the bank voles. The susceptibility to infection with B. afzelii following an infected tick bite was very high (95%) and did not differ between TLR2 genotypes. The TLR2 polymorphism also had no effect on the spirochete abundance in the tissues of the bank voles. Under the laboratory conditions of our study, we did not find that the TLR2 polymorphism in bank voles influenced variation in the susceptibility to B. afzelii infection.
Subject terms: Ecological genetics, Immunogenetics
Introduction
The Toll-like receptors (TLRs) are an important family of pathogen recognition receptors (PRRs) that are found in all vertebrates1. These receptors of the innate immune system play a critical role in the recognition of pathogen-associated molecular patterns (PAMPs). PAMPs are highly conserved molecules that are essential for the pathogen’s existence such as the cell wall components of bacteria. To date, 12 to 15 different TLR genes have been discovered in humans and mice, and each TLR receptor recognizes a distinct set of PAMPs2. For example, TLR4 recognizes lipopolysaccharides (LPS), whereas TLR5 recognizes the bacterial flagellin protein2,3. The recognition of pathogens by the TLR receptors is a critical step in the activation of the adaptive immune system in the vertebrate host4. Recent reviews have described numerous polymorphisms in the different TLRs and their associations with susceptibility or resistance to different infectious diseases4,5.
The toll-like receptor 2 (TLR2) recognizes a wide variety of PAMPs including peptidoglycan, zymosan, and lipoproteins3. TLR2 can also dimerize with TLR1 and TLR6 to recognize additional PAMPs6. TLR2 plays an important role in recognizing the lipoproteins of the spirochete bacteria of the Borrelia burgdorferi sensu lato (sl) genospecies complex, which includes the causative agents of Lyme borreliosis in humans7,8. Functional studies have shown that TLR2-knockout mice have much higher loads of B. burgdorferi sensu stricto (ss) in their tissues than immunocompetent control mice7,9. Furthermore, population genetic studies have found associations between genetic polymorphisms at the TLR2 locus and resistance to B. burgdorferi sl pathogens in humans and wild rodents10–14.
Lyme borreliosis is the most common vector-borne disease in the northern hemisphere15. In Europe, Borrelia afzelii is the most important etiological agent of Lyme borreliosis and is transmitted by the hard tick Ixodes ricinus16,17. The main reservoir hosts of B. afzelii include small mammals such as mice (Apodemus species) and the bank vole (Myodes glareolus). Experimental infections have shown that B. afzelii establishes chronic infections in its rodent reservoir hosts that can last for months or even years18–20. Larval ticks acquire spirochetes after feeding on an infected reservoir host, as there is no vertical transmission21,22. Blood-engorged larvae subsequently moult into nymphs that infect the reservoir hosts the following year15. In areas with high tick densities, wild rodents are repeatedly bitten by nymphs and exposed to B. burgdorferi sl pathogens23,24. Long-term field studies in Sweden have shown that a quarter of all wild rodents were infected with B. afzelii25. Thus, B. afzelii is an important pathogen in wild rodent populations that could exert strong selection on the host immune system13,14,26.
The bank vole (M. glareolus) is an important wild reservoir host for B. afzelii and for immature Ixodes ticks17,23,27. Genetic studies on the TLR2 locus in this species found three different clusters of alleles: C1, C2, and C313,28. A field study in Sweden found that individuals with the C1C1, C1C2, and C2C2 genotype had the highest (48.6%), intermediate (30.6%), and lowest (17.9%) prevalence of B. afzelii13. Thus, the C1 and C2 alleles appear to confer susceptibility and resistance, respectively, to B. afzelii in bank voles. The C3 allele was rare in this population and its phenotype with respect to B. afzelii infection was not investigated. A recent study of the TLR2 polymorphism in bank vole populations across 19 countries in Europe found a strong positive correlation between the frequency of the C2 resistance allele and the case load of human Lyme borreliosis26. This study suggests that B. afzelii is driving the evolution of the C2 resistance allele in European bank vole populations26. Nevertheless, it remains to be conclusively demonstrated that the TLR2 polymorphism in bank voles causes variation in susceptibility to B. afzelii in this important reservoir host.
To date, all the work on the TLR2 polymorphism and B. afzelii infection in bank voles has been correlative in nature13,14,26. Experimental infections are needed to further confirm whether the TLR2 polymorphism in this species influences variation in susceptibility to infection with B. afzelii. The two aims of this study were to determine whether the TLR2 polymorphism in wild bank voles influences variation in the probability of infection with B. afzelii (hereafter referred to as host susceptibility) and variation in B. afzelii spirochete abundance in host tissues (hereafter referred to as host spirochete load). To test this hypothesis, we challenged Borrelia-free, lab-born bank voles of known TLR2 genotype with B. afzelii via tick bite. The TLR2 gene could influence the prevalence of B. afzelii by different mechanisms such as preventing systemic infection and/or spirochete clearance following infection. We expected that compared to the C1C1 genotype, the C2C2 genotype would be less likely to develop a systemic infection following infestation with B. afzelii-infected nymphs. We also expected that the C2C2 genotype would have a lower spirochete load in the tissues than the C1C1 genotype. The importance of the C3 allele was assessed for the first time in this study.
Materials and Methods
General experimental design
The present study consists of two separate infection experiments: the first experiment was conducted in the summer of 2015 using bank voles from a Swiss colony, whereas the second experiment was conducted in the summer of 2016 using bank voles from a Finnish colony. In these infection experiments, the Swiss and Finnish bank voles of known TLR2 genotype were challenged via tick bite with different isolates of B. afzelii, respectively. The Swiss bank vole colony contained the C1 susceptible allele and the C3 allele, for which the B. afzelii infection phenotype was unknown. The Finnish bank vole colony contained the C1 susceptible allele, the C2 resistance allele, and the C3 allele.
Ethics statement and animal experimentation permits
All the experiments were performed following the Swiss and Finnish legislation on animal experimentation. For the infection experiment conducted in Switzerland, the commission that is part of the ‘Service de la Consommation et des Affaires Vétérinaires (SCAV)’ of the canton of Vaud, Switzerland evaluated and approved the ethics of this part of the study. The SCAV of the canton of Neuchâtel, Switzerland issued the animal experimentation permit (NE2/2014). The infection experiment conducted in Finland was carried out under the permits of the Finnish Animal Experiment Board (ESAVI/3834/04.10.03/2011, ESAVI/7256/04.10.07/2014 and ESAVI/3457/04.10.07/2015).
Breeding of the bank voles in the laboratory
The Swiss and Finnish colonies of bank voles were established by trapping bank voles near Neuchâtel, Switzerland and Jyväskylä, Finland, respectively (section 1 in the electronic supplementary material (ESM)). TLR2 genotyping found that the Swiss bank voles carried the C1 and C3 alleles (n = 36 animals; frequencies were 42.2% and and 57.8%, respectively), but not the C2 putative resistance allele (section 2 in the ESM). For the Swiss infection experiment, male and female bank voles of known TLR2 genotype were crossed to maximize the number of homozygous C1C1 and C3C3 offspring. A sample of the Finnish colony (n = 48 animals) revealed that the frequencies of the TLR2 allele were as follows: 88.5% C1, 4.2% C2, and 7.3% C3. For the Finnish infection experiment, bank voles were bred to maximize the number of homozygous C1C1, C2C2 and C3C3 offspring. All lab-born offspring were genotyped with respect to their TLR2 locus. TLR2 genotyping of the bank voles is described in section 2 in the ESM.
Strains of B. afzelii
Our original intention was to challenge the Swiss and Finnish bank voles with ticks carrying a single Swiss strain (NE4049) and a single Finnish strain (Fin-Jyv-A3) of B. afzelii, respectively. We chose these two strains because our previous work had shown that they are competent at establishing infection in laboratory mice29–32. Due to a combination of experimental error and time constraints, we used nymphs that were co-infected with two strains of B. afzelii. The Swiss bank voles were challenged with nymphs co-infected with a local Swiss strain (NE4049) and a foreign Austrian strain (E61). The Finnish bank voles were challenged with nymphs that were co-infected with a local Finnish strain (Fin-Jyv-A3) and a foreign Swiss strain (NE4049). The origin and genetics of these B. afzelii strains is described in section 3 in the ESM. We used a strain-specific qPCR to confirm that the local strain of B. afzelii had established infection in the bank voles (section 3 in the ESM).
Infectious challenge of bank voles with B. afzelii-infected nymphal ticks
All animals were Borrelia-free, lab-born individuals and were in the adult stage (older than 2 months) at the time of the challenge with B. afzelii-infected nymphs. For the Swiss infection experiment, 50 animals were challenged with B. afzelii-infected nymphs and the TLR2 genotypes were as follows: 17 C1C1 (12 females, 5 males), 15 C1C3 (7 females, 8 males), and 18 C3C3 (8 females, 10 males). For the Finnish infection experiment, 50 animals were challenged and their TLR2 genotypes were as follows: 11 C1C1 (4 females, 7 males), 8 C1C2 (4 females, 4 males), 12 C2C2 (4 females, 8 males), 1 C2C3 (1 male), 8 C1C3 (4 females, 4 males), and 10 C3C3 (6 females, 4 males).
In the field, rodents are rarely infested with more than one I. ricinus nymph at a time13,23,24,33. To simulate the natural conditions while also ensuring a good probability of infectious challenge, each animal was infested with 3 randomly selected nymphs. The creation of the experimentally infected nymphs is described in section 3 in the ESM. The percentage of B. afzelii-infected nymphs was >90% for both the Swiss and Finnish infection experiments. The nymphs were placed in a neoprene capsule that had been glued to the shaved backs of the bank voles. During this procedure, animals were anesthetized with a mixture of xylazine, ketamine and PBS (1:2:9; 5 µl per gram of bank vole). The purpose of the capsule is to prevent the bank vole from grooming off and killing the attached nymphs. The animals were fitted with an Elizabethan collar to prevent them from removing the capsules. The capsules were checked on a daily basis over a period of 7 days, and detached engorged nymphs were collected from the capsule and frozen at −20 °C.
At 49 days post-infection, the animals were sacrificed using CO2 asphyxiation. Our previous work had shown that this is enough time for B. afzelii to disseminate to all of the organs20,29,30. For each animal, a tissue biopsy was taken from the ear and the dorsal skin from the site of the tick bite using a 2 mm forceps-type punch. The bladder and ankle joints were aseptically dissected29. These internal tissues are typically used to demonstrate that B. burgdorferi sl has disseminated and established a systemic infection9,34–38. To determine the infection status of the bank voles, these four tissue samples were tested for B. afzelii using qPCR (see below).
Borrelia afzelii infection status of the engorged nymphs and the bank voles
We extracted the DNA from engorged nymphs and bank vole tissue samples and used qPCR targeting the flagellin gene to estimate the spirochete load (for details, see section 3 in the ESM). For the bank vole tissue samples, we used the DNA concentration of the DNA extraction to standardize the spirochete load as the number of spirochetes per mg of host DNA (for details, see section 3 in the ESM).
Statistical analyses
General approach
The three response variables included the number of engorged nymphs collected from each bank vole, the infection status of the bank vole (infected, uninfected) and the spirochete load of B. afzelii in the bank vole tissues. These three response variables were analysed using generalized linear models (GLMs) with binomial errors, GLMs with binomial errors, and linear mixed effects models (LMEMs) with normal errors, respectively. The fixed effects included experiment (2 levels: Swiss, Finnish), sex (2 levels: female, male), organ (4 levels: bladder, ear, joint, dorsal skin), and TLR2 genotype. TLR2 genotype was coded in eight different ways that corresponded to different assumptions about the effects of the TLR2 alleles (Table 1).
Table 1.
The eight different ways in which TLR2 genotype was modelled are shown.
| Name | Type of variable | # of genotypes | Identity of genotypes | # of alleles | Identity of alleles |
|---|---|---|---|---|---|
| Geno1 | Categorical | 6 | C1C1, C2C2, C3C3, C1C2, C1C3, C2C3 | NA | NA |
| Geno2 | Categorical | 3 | C2C2, C2Cx, CxCx; (Cx = C1 = C3) | NA | NA |
| Geno3 | Categorical | 3 | C1C1, C1Cy, CyCy; (Cy = C2 = C3) | NA | NA |
| Geno4 | Categorical | 3 | C3C3, C2Cz, CzCz; (Cz = C1 = C2) | NA | NA |
| Geno5 | 1 Covariate | NA | NA | 2 | # of C2 alleles; (C1 = C3) |
| Geno6 | 1 Covariate | NA | NA | 2 | # of C1 alleles; (C2 = C3) |
| Geno7 | 1 Covariate | NA | NA | 2 | # of C3 alleles; (C1 = C2) |
| Geno8 | 3 Covariates | NA | NA | 3 | # of C1, C2, and C3 alleles |
TLR2 genotype was either modelled as a categorical factor where each TLR2 genotype was a different category or as a covariate that counted the number of TLR2 alleles. We reduced the number of genotypes or alleles by setting pairs of alleles or genotypes as equivalent. For example, for Geno2, we assumed that the C1 and C3 alleles are equivalent so that there are only 3 distinct genotypes: C2C2, C2Cx, CxCx, where Cx = C1 = C3. Combining similar genotypes (or alleles) increases the sample size for the remaining genotype categories and thereby increases the power of the statistical test.
Experiment and TLR2 could not be included in the same model because of partial or complete redundancy between these factors (i.e. the C2 allele only occurs in the Finnish experiment). We therefore used model selection based on the Akaike Information Criterion (AIC) to determine the best model; the advantage of this approach is that it allows us to compare non-nested models. We used the model weights to calculate the % support for each of the fixed effects. We used model averaging to obtain robust parameter estimates and 95% confidence intervals (95% CI) for the fixed effects. A fixed effect is significant when its model-averaged 95% CI does not overlap 0. We used R version 3.4.3 to analyse the data. The GLMs and LMEMs were run using the glm() function in the base package and the lmer() function in the lme4 package, respectively. Model selection and calculation of model-averaged parameter estimates were run using the model.sel() and the model.avg() functions in the MuMIn package.
Number of engorged nymphs per bank vole
Each bank vole was infested with 3 nymphs, and the number of engorged nymphs collected from each bank vole was therefore a binomial response variable that ranged from 0/3 to 3/3. To test whether the 100 bank voles were exposed to the same infectious challenge, we analysed two response variables: the number of engorged nymphs that were collected per bank vole and the number of engorged B. afzelii-infected nymphs that were collected per bank vole. These two response variables were modelled using GLMs with binomial errors. The fixed factors were experiment, sex, and the eight different ways to code TLR2 genotype. Each vole occurred only once in the analysis so it was not necessary to include bank vole ID as a random effect.
TLR2 genotype and infection status of the bank voles
Bank vole infection status is a binomial variable (uninfected, infected) that was modelled using GLMs with binomial errors. The fixed effects were number of B. afzelii-infected nymphs (0, 1, 2, 3), experiment, sex, and the eight different ways to code TLR2 genotype. As there was no variation in infection status among the four organs, we did not include this factor in the analysis. Each bank vole occurred only once in the analysis so it was not necessary to include bank vole ID as a random effect. The analysis was performed for all the bank voles (n = 100) and for the subset of bank voles that had been successfully challenged with B. afzelii-infected nymphs (n = 88).
B. afzelii spirochete load of the bank vole tissue samples
The statistical analysis of the B. afzelii spirochete load in the tissue samples was restricted to the subset of infected bank voles (n = 84). For each tissue sample, the geometric mean spirochete load (in 3 μl of DNA template) was calculated for the three replicate qPCR runs. The tissue spirochete loads were standardized to mg of DNA (by dividing by the DNA concentration). This variable was log10-transformed to normalize the residuals. The log10-transformed tissue spirochete loads were modelled using LMEMs with normal errors. The fixed factors were experiment, sex, organ, and the eight different ways to code TLR2 genotype. Organs sampled from the same bank vole are not independent and bank vole identity was therefore modelled as a random factor.
Results
The C1, C2, and C3 allele clusters encode different TLR2 proteins
The TLR2 gene sequences of the animals in the experimental infection formed three clearly separated clusters that coded for three different protein variants: cluster 1 (C1), cluster 2 (C2) and cluster 3 (C3); these three clusters were the same as the ones described by Tschirren et al.13. The C1 and C2 clusters, C1 and C3 clusters, and C2 and C3 clusters were separated by a genetic distance of 12, 15, and 15 nucleotide differences, respectively, which corresponded to a protein distance of 7, 7, and 4 amino acids, respectively (Fig. S1). A protein model of TLR2 had previously shown that the variants encoded by the C1 and C2 alleles would differ in at least one amino acid in the binding site that is believed to be critical for ligand binding13. We therefore expected that these three variants of the TLR2 protein would differ in their ability to recognize the lipoproteins of B. afzelii and therefore to protect the bank vole against infection via tick bite.
Definition of a successful infectious challenge
We collected at least 1 engorged B. afzelii-infected nymph from 83 of the 100 bank voles (Tables S01 and S02 in the ESM). There were 17 bank voles for which we did not recover any engorged B. afzelii-infected nymphs (Tables S01 and S02 in the ESM). Five of these 17 individuals became infected with B. afzelii proving that they had been exposed to an infected nymph (which we failed to recover). Hence, we have proof that 88 of the 100 bank voles were successfully challenged in this study (83 + 5 = 88). The 12 remaining individuals for which there was no proof of an infectious challenge (no infected engorged nymphs, bank voles are uninfected) all came from the Swiss experiment.
Number of engorged nymphs per bank vole
For the 50 Swiss bank voles, we collected 84 engorged nymphs (mean = 2.00, range = 0–3 engorged nymphs per bank vole), of which 52 were infected with B. afzelii (mean = 1.24, range = 0–3 engorged infected nymphs per bank vole). For the 50 Finnish bank voles, we collected 131 engorged nymphs (mean = 2.72, range = 2–3 engorged nymphs per bank vole), of which 96 were infected with B. afzelii (mean = 1.92, range = 0–3 engorged infected nymphs per bank vole).
For the model selection analysis of the number of engorged nymphs per bank vole, the top 3 models had 99.999% of the support; the remaining 26 models had <0.001% of the support (Table 2 and Table S07 in the ESM). The support for individual factors was as follows: experiment (99.999%), sex (32.4%), and TLR2 genotype (<0.001% for 24 models). The model-averaged parameter estimates found that significantly more engorged nymphs were collected per bank vole in the Finnish experiment (Table S08 in the ESM) compared to the Swiss experiment.
Table 2.
Model selection table is shown for the generalized linear models (with binomial errors) of the number of engorged nymphs per bank vole (nymphs.engorged).
| Model | Model structure | df | logLik | AICc | Delta | Weight (%) |
|---|---|---|---|---|---|---|
| model003 | nymphs.engorged~E | 2 | −109.353 | 222.831 | 0.000 | 67.575 |
| model002 | nymphs.engorged~E + S | 3 | −109.316 | 224.881 | 2.051 | 24.235 |
| model001 | nymphs.engorged~E + S + E:S | 4 | −109.315 | 227.051 | 4.221 | 8.189 |
| model010 | nymphs.engorged~geno2 | 3 | −121.250 | 248.751 | 25.920 | 0.000 |
| model022 | nymphs.engorged~geno5 | 2 | −122.638 | 249.399 | 26.569 | 0.000 |
The analysis was done on the entire data set of 100 bank voles. Of the 29 models, the top 5 models are shown; the top 3 models have 99.999% of the support. Table S09 in the ESM shows all 29 models. The fixed factors are experiment (E), sex (S), and TLR2 genotype. TLR2 genotype was modelled in 8 different ways (geno1, geno2, geno3, geno4, geno5, geno6, geno7, and geno8). For each model, the model structure, model degrees of freedom (df), log likelihood, corrected AIC value (AICc), difference in AICc from the top model (Delta), and the support (Weight) expressed as a percent are shown.
The results were similar for the number of engorged B. afzelii-infected nymphs and when the analyses were done on the subset of 88 bank voles that had been successfully challenged. These analyses show that the infectious challenge was the same for all TLR2 genotypes.
Infection status of the bank voles
A bank vole was defined as being infected if it tested positive for B. afzelii for at least one of four organs: (1) bladder, (2) ear, (3) joint, and (4) dorsal skin. There was no ambiguity about the infection status of the 100 bank voles: 84 tested positive for all four organs and 16 tested negative for all four organs. The susceptibility of a host to a pathogen is the probability of acquiring an infection following exposure. In our study, the susceptibility of bank voles to infection with B. afzelii was 95.5% (84 infected/ 88 total; 95% CI = 88.1–98.5%) and was therefore very high.
Infection was higher in the Finnish experiment (100.0% = 50/50) than the Swiss experiment (68.0% = 34/50). When the analysis was restricted to the subset of 88 bank voles that had been successfully challenged, infection in the Finnish experiment (100.0% = 50/50) and Swiss experiment (89.5% = 34/38) were similar.
Bank vole infection status was initially modelled using GLMs with binomial errors (Table S09 in the ESM). However, due to the lack of variation in infection status (most bank voles were infected), the standard errors (SE) estimated by the glm() function had very low precision (i.e., SE was ~1000 times bigger than the parameter estimate; Table S10 in the ESM). To avoid this problem with parameter estimation, we re-analysed the data using linear models with normal errors.
For the model selection analysis of the linear models of infection status of 100 bank voles, the top 6 models had 99.9% of the support; the remaining 111 models had 0.1% of the support (Table 3 and Table S11 in the ESM). The support for individual factors was as follows: number of infected nymphs (>99.9%), experiment (>99.9%), sex (55.6%), and TLR2 genotype (<0.001% for 98 models). The model-averaged parameter estimates found that infection increased significantly with the number of engorged infected nymphs per bank vole and that infection was significantly higher in the Finnish experiment compared to the Swiss experiment (Table S12 in the ESM).
Table 3.
Model selection table is shown for the linear models of bank vole infection status (infection).
| Model | Model structure | df | logLik | AICc | Delta | Weight (%) |
|---|---|---|---|---|---|---|
| model011 | infection~E + N + E:N | 5 | −12.287 | 35.212 | 0.000 | 44.374 |
| model007 | infection~E + S + N + E:N | 6 | −11.802 | 36.507 | 1.295 | 23.226 |
| model005 | infection~E + S + N + E:N + S:N | 7 | −11.164 | 37.546 | 2.334 | 13.817 |
| model003 | infection~E + S + N + E:S + E:N | 7 | −11.317 | 37.851 | 2.639 | 11.862 |
| model002 | infection ~E + S + N + E:S + E:N + S:N | 8 | −11.032 | 39.646 | 4.434 | 4.835 |
| model001 | infection~E + S + N + E:S + E:N + S:N + E:S:N | 9 | −10.826 | 41.651 | 6.439 | 1.774 |
| model014 | infection~E + N | 4 | −21.428 | 51.277 | 16.065 | 0.014 |
| model606 | infection~geno6 + S + N + geno6:S | 6 | −19.780 | 52.463 | 17.251 | 0.008 |
| model009 | infection ~E + X + N | 5 | −20.939 | 52.517 | 17.305 | 0.008 |
| model008 | infection ~E + X + N + S:N | 6 | −19.871 | 52.645 | 17.433 | 0.007 |
The analysis was done on the entire data set of 100 bank voles. Of the 117 models, the top 10 models are shown; the top 6 models have 99.9% of the support. Table S11 in the ESM shows all 117 models. The fixed factors are number of engorged B. afzelii-infected nymphs (N), experiment (E), sex (S), and TLR2 genotype. TLR2 genotype is modelled in 7 different ways (geno1, geno2, geno3, geno4, geno5, geno6, and geno7). For each model, the model structure, model degrees of freedom (df), log likelihood, corrected AIC value (AICc), difference in AICc from the top model (Delta), and the support (Weight) expressed as a percent are shown.
We do not present the model selection analysis for the subset of 88 bank voles that had been successfully challenged because there was no variation in infection status (84 infected/ 88 total).
We compared the susceptibility of bank voles to infection with B. afzelii between our study and the study by Tschirren et al.13 (for details, see section 4 in the ESM). For the study by Tschirren et al.13, the probability of encountering an infected tick in the field was not known, but by setting this probability to a maximum (100%) or a minimum (48.7%), we were able to estimate the susceptibility of each TLR2 genotype. This comparison found that the susceptibility was significantly different between the two studies (Fig. 1).
Figure 1.
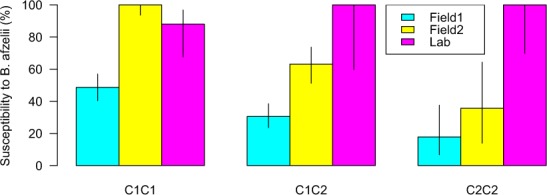
The susceptibility of the three bank vole TLR2 genotypes (C1C1, C1C2, and C2C2) to infection with B. afzelii is compared between our lab study (Lab) and the field study by Tschirren et al.13 (Field1, Field2). The field study by Tschirren et al.13 assumed that the exposure rates were identical for the three TLR2 genotypes and that bank voles do not clear the infection. Using these assumptions, the prevalence of infection can be separated into the probability of exposure and the susceptibility to infection following an infectious challenge. The Field1 estimates of susceptibility are based on the unrealistic assumption that 100% of the bank voles were exposed to an infected tick. The Field2 estimates of susceptibility are based on the assumption that 48.6% of the bank voles were exposed to an infected tick, which maximizes the susceptibility of the C1C1 genotype to 100.0% (for details, see section 4 in the ESM).
B. afzelii spirochete load of the bank vole tissue samples
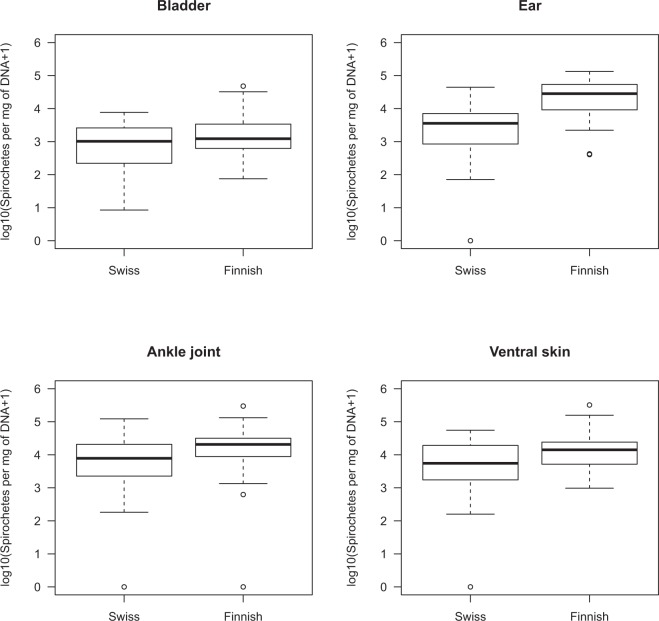
For each organ, the repeatability of the log10-transformed tissue spirochete loads was high (range = 71–75%; section 5 in the ESM). For the Swiss bank voles, the mean spirochete load per mg of DNA was lowest in the bladder (540 spirochetes per mg of DNA; Table 4), 4.2 times higher in the ear, 5.1 times higher in the dorsal skin, and 8.4 times higher in the ankle joint (Fig. 2; Table 4). For the Finnish bank voles, the mean spirochete load per mg of DNA was lowest in the bladder (1339 spirochetes per mg of DNA; Table 4), 9.4 times higher in the dorsal skin, 10.9 times higher in the ankle joint, and 15.2 times higher in the ear (Fig. 2; Table 4). Averaged across the four organs, the tissue spirochete loads in the Finnish bank voles were 4.2 times higher than the Swiss bank voles (Fig. 2; Table 4).
Table 4.
Mean tissue spirochete loads in the bank voles are shown for each organ in both the Swiss and Finnish infection experiments.
| Experiment | Organ | Mean | LL | UL |
|---|---|---|---|---|
| Switzerland | Bladder | 540 | 286 | 1017 |
| Switzerland | Ear | 2283 | 1211 | 4303 |
| Switzerland | Joint | 4531 | 2404 | 8541 |
| Switzerland | Skin | 2768 | 1469 | 5218 |
| Finland | Bladder | 1339 | 794 | 2259 |
| Finland | Ear | 20300 | 12036 | 34240 |
| Finland | Joint | 14533 | 8616 | 24512 |
| Finland | Skin | 12587 | 7463 | 21230 |
Bank vole organs were dissected at 49 days post-infection. The tissue spirochete load was standardized per mg of DNA (units are spirochetes per mg of DNA). Shown are the means, and the lower limits (LL) and upper limits (UL) of the 95% confidence interval.
Figure 2.
Organ and experiment had a significant effect on the tissue spirochete load of B. afzelii in the bank voles. The spirochete loads were standardized per mg of DNA and then log10-transformed. For the Finnish bank voles, the tissue spirochete loads were higher compared to the Swiss bank voles. The rank order of the organ spirochete loads (from lowest to highest) depended on the experiment: bladder, ear, ventral skin, ankle joint for the Swiss bank voles and bladder, ventral skin, ankle joint, ear for the Finnish bank voles. Shown are the medians (black line), the 25th and 75th percentiles (edges of the box), the minimum and maximum values (whiskers), and the outliers (circles).
For the model selection analysis of tissue spirochete load per mg of DNA, the top 5 models had 99.4% of the support; the remaining 112 models had 0.6% of the support (Table 5 and Table S13 in the ESM). The support for individual factors was as follows: experiment (99.99%), sex (11.4%), organ (100.0%), and TLR2 genotype (<0.001% for 98 models). The model-averaged parameter estimates found that the ear, joints, and ventral skin had significantly higher spirochete loads than the bladder (Fig. 2; Table S14 in the ESM). The Finnish bank voles had significantly higher spirochete loads in their tissues than the Swiss bank voles (Fig. 2; Table S14 in the ESM). Finally, the interaction between experiment and organ was significant because the difference in ear tissue spirochete load between Finnish and Swiss bank voles was higher than the difference for the other tissues (Fig. 2; Table S14 in the ESM).
Table 5.
Model selection table is shown for the linear mixed effects models of the B. afzelii spirochete load in bank vole tissues (spiro.per.mg.DNA).
| Model | Model structure | df | logLik | AICc | Delta | Weight (%) |
|---|---|---|---|---|---|---|
| lm.model1014 | log10(spiro.per.mg.DNA)~E + O | 7 | −408.954 | 832.250 | 0.000 | 76.959 |
| lm.model1011 | log10(spiro.per.mg.DNA)~E + O + E:O | 10 | −407.676 | 836.029 | 3.779 | 11.632 |
| lm.model1009 | log10(spiro.per.mg.DNA)~E + S + O | 8 | −410.178 | 836.796 | 4.547 | 7.925 |
| lm.model1006 | log10(spiro.per.mg.DNA)~E + S + O + E:S | 9 | −410.639 | 839.830 | 7.580 | 1.739 |
| lm.model1007 | log10(spiro.per.mg.DNA)~E + S + O + E:O | 11 | −408.900 | 840.614 | 8.364 | 1.175 |
| lm.model1003 | log10(spiro.per.mg.DNA)~E + S + O + E:S + E:O | 12 | −409.361 | 843.688 | 11.438 | 0.253 |
| lm.model1008 | log10(spiro.per.mg.DNA)~E + S + O + S:O | 11 | −410.562 | 843.939 | 11.689 | 0.223 |
| lm.model1004 | log10(spiro.per.mg.DNA)~E + S + O + E:S + S:O | 12 | −411.023 | 847.012 | 14.762 | 0.048 |
| lm.model1005 | log10(spiro.per.mg.DNA)~E + S + O + E:O + S:O | 14 | −409.259 | 847.826 | 15.576 | 0.032 |
| lm.model1002 | log10(spiro.per.mg.DNA)~E + S + O + E:S + E:O + S:O | 15 | −409.720 | 850.939 | 18.690 | 0.007 |
Spirochete load is standardized per mg of DNA and was log10-transformed to normalize the residuals (log10(spiro.per.mg.DNA)). The analysis was done on the subset of 84 bank voles infected with B. afzelii. Of the 117 models, the top 10 models are shown; the top 5 models have 99.4% of the support. Table S12 in the ESM shows all 117 models. The fixed factors are experiment (E), organ (O), sex (S), and TLR2 genotype. TLR2 genotype is modelled in 7 different ways (geno1, geno2, geno3, geno4, geno5, geno6, and geno7). For each model, the model structure, model degrees of freedom (df), log likelihood, corrected AIC value (AICc), difference in AICc from the top model (Delta), and the support (Weight) expressed as a percent are shown.
Strain-specific qPCR to determine infection success of the local strain
For the sample of infected Swiss bank voles, 88.2% (30/34) were infected with the local strain of B. afzelii (strain NE4049). For the sample of infected Finnish bank voles, 98.0% (49/50) were infected with the local strain of B. afzelii (strain Fin-Jyv-A3). Thus, 94.0% (79/84) of the bank voles became infected with their local (and intended) strain of B. afzelii following the infectious challenge.
Discussion
In this study, we standardized exposure to infected ticks and tested whether a genetic polymorphism at the TLR2 locus in the bank vole explained variation in susceptibility to B. afzelii, a common and important tick-borne pathogen. Most studies on the role of TLR2 polymorphisms in the susceptibility to B. burgdorferi sl pathogens have been association studies in humans and wild rodents4,13,14,26,39,40. To our knowledge, there are no experimental infection studies showing that a TLR2 polymorphism influences variation in susceptibility to infection with B. burgdorferi sl. Under the laboratory conditions of our study, ~100% of all bank voles become infected following exposure to B. afzelii-infected ticks. Thus, our study found no evidence that bank voles with different TLR2 genotypes (C1C1, C2C2, C3C3) differed in their susceptibility to infection with B. afzelii. One limitation of this study was the small sample size for the C2C2 genotypes (n = 12), which limits our power to detect differences in susceptibility to B. afzelii infection between the susceptible (C1C1) and resistant (C2C2) genotypes.
The prevalence of B. afzelii infection in our study (95.5%) was 2.5 times higher than the field study by Tschirren et al. (37.5%)13 as shown in Fig. 1. This discrepancy is due to differences in the risk of exposure between the two studies. In our study, the risk of exposure was 100.0% for the subset of 88 bank voles that had been successfully challenged (by definition). In the field study by Tschirren et al.13, the risk of exposure was unknown, but was assumed to be identical for the three TLR2 genotypes. Under this assumption and after minimizing the risk of exposure (subject to the assumption that bank voles cannot clear their infection), the susceptibility of the resistant C2C2 TLR2 genotype remained significantly lower in the study by Tschirren et al.13 compared to our study (Fig. 1). Why did we not find the expected differences in susceptibility to infection with B. afzelii between the TLR2 genotypes?
One explanation for the different infection prevalences in the field study by Tschirren et al.13 is that the three TLR2 genotypes differed in their risk of exposure to B. afzelii-infected nymphs. Fine-scale spatial studies have shown that the density of infected nymphs (DIN) and the risk of exposure can vary dramatically over very small distances41. If by chance, families of bank voles carrying the C2 allele live in areas of the sampling grid that have a low DIN compared to families of bank voles carrying the C1 allele, then observed differences in infection prevalence would be caused by these spatial differences in the risk of exposure and not by differences in susceptibility between the TLR2 genotypes. Differences in exposure can also explain why the prevalence of B. afzelii infection was 2.5 times higher in our lab study compared to the field study by Tschirren et al.13.
A second explanation involves grooming, which is an important behaviour that protects vertebrate hosts against ectoparasites such as ticks42. Previous studies have shown that the probability of nymph-to-host transmission of B. burgdorferi sl pathogens increases with the duration of attachment of the nymphal tick to the vertebrate host43,44. In nature, the normal grooming behaviour of the vertebrate host is a significant cause of tick mortality that would reduce the risk of pathogen transmission following nymphal attachment42,45. In the present study, we prevented the bank voles from grooming off the nymphs by fitting them with a collar and by placing the nymphs in a protective capsule. If C2C2 individuals have a more effective grooming response than C1C1 individuals, they would be less likely to become infected with B. afzelii following the attachment of an infected nymph. The link between the immune system and grooming behaviour is not so far-fetched because the former has itch-generation mechanisms (e.g. release of histamine by mast cells) that would stimulate the latter46. The absence of the grooming defence mechanism can also explain why the prevalence of B. afzelii infection was 2.5 times higher in our lab study compared to the field study by Tschirren et al.13.
A third explanation involves acquired immunity, which can influence susceptibility to infection with B. afzelii. We have recently shown that infected female bank voles can transmit maternal antibodies to their offspring that protect against infection with B. afzelii47–49. Bank voles can also develop acquired immunity against I. ricinus ticks50, and this anti-tick immunity protects against infection with B. burgdorferi sl51–53. Thus variation in maternal antibodies and/or anti-tick immunity in wild bank vole populations will influence variation in susceptibility to infection with B. burgdorferi sl. In our study, the bank voles did not have any antibodies to either B. afzelii or to I. ricinus ticks at the time of the infectious challenge (i.e. they were completey naive). In contrast, field studies can not control for this variation in acquired immunity13,14,26. The absence of maternal antibodies or anti-tick immunity can also explain why the prevalence of B. afzelii infection was 2.5 times higher in our lab study compared to the field study by Tschirren et al.13
A fourth explanation involves the ability of bank voles to clear B. afzelii. The consensus is that rodent reservoir hosts cannot clear B. burgdorferi sl18–20,23,27,54. In our study, there was no evidence of clearance at 49 days post-infection, as the four tissues in the 84 infected bank voles were all positive for B. afzelii. However, we recently found clearance of spirochetes from ear tissues in bank voles that had been experimentally infected with B. afzelii strain NE404948. Bank voles that had cleared the infection from their ear tissues still had very high spirochete loads in other tissues (bladder, heart, dorsal skin, ventral skin)48. This study suggests that ear tissue biopsies might underestimate the infection status of bank voles in the field48. In most field studies, the infection status of the bank voles is determined by taking ear tissue biopsies13,14,26. Thus, another explanation for the difference in prevalence of B. afzelii infection between the two studies, is that we determined infection status by testing for spirochetes in multiple internal organs.
A fifth explanation is that our laboratory environment was so stressful that it overwhelmed the variation in susceptibility to infection between the TLR2 genotypes. Physiological stress can suppress the host immune system and increase susceptibility to infection with pathogens55–58. Although we cannot rule out the stress-immunosuppression explanation, we believe that it is unlikely for the following reasons. First, there is a long history in Lyme disease research of performing experimental infections with wild animals in the lab, and this work has successfully determined the susceptibility of many vertebrate hosts to various B. burgdorferi sl pathogens18,19,59–65. Second, as mentioned previously, using the same experimental conditions described in the present study, female bank voles infected with B. afzelii transmitted antibodies to their offspring, which protected the offspring against infected ticks47–49. The creation of antibodies in the mothers and their ability to block infection in the offspring requires the coordinated action of both the adaptive and innate immune system. That study provides some evidence that the immune system of our bank voles is functioning as it should under our laboratory conditions47–49. Third, although the laboratory environment may be stressful, other stressors found in nature, such as predators, food shortage, competitive interactions, and other parasites, were absent in our lab environment. Fourth, lab mice (Mus musculus) are presumably not stressed by our lab conditions, but they are also 100% susceptible to B. afzelii29. The most parsimonious explanation is that competent rodent reservoir hosts are highly susceptible to infection with B. burgdorferi sl if Ixodes nymphs feed to completion.
A sixth explanation for the difference in susceptibility to B. afzelii infection between our laboratory study and the field study by Tschirren et al.13 is the presence of other parasites. In natural systems, hosts are often co-infected with multiple parasites including viruses, bacteria, protozoans, helminths, and ectoparasites66,67. Field studies have shown that the susceptibility of infection to any particular parasite depends on the presence of other parasites68. For example, in a wild population of the field vole (Microtus agrestis), infection with tick-borne Babesia microti increased and decreased the risk of acquiring infections with tick-borne Anaplasma phagocytophilum and flea-borne Bartonella spp., respectively68. A recent experimental infection study using lab mice found no evidence that infection with the nematode Heligmosomoides polygyrus influenced the susceptibility to tick-borne B. afzelii69. In our laboratory system, co-infection with other parasites cannot increase the susceptibility of naive bank voles to tick-borne B. afzelii because this susceptibility is already very high (95.5%). However, we cannot exclude that co-infection with other parasites in nature decreases the susceptibility to tick-borne B. afzelii, but to our knowledge, no such parasite has been identified.
A unique aspect of this study was our effort to expose the bank voles to a realistic infectious challenge. Field studies have shown that rodents are rarely infested with more than one I. ricinus nymph at a time13,23,24. In the present study, we collected between 1 and 2 engorged B. afzelii-infected nymphs from each bank vole indicating that they had been exposed to an ecologically relevant infectious tick bite challenge. We collected more engorged B. afzelii-infected nymphs per bank vole in the Finnish infection experiment (1.92) than the Swiss infection experiment (1.24). The reason for this difference was improved methodology; the consistent use of collars in the Finnish infection experiment prevented the bank voles from removing the capsules and from grooming off and killing the ticks. The improvement in the infestation methodology explains why the infection success was higher in the Finnish experiment than the Swiss experiment. However, when the infection success was restricted to the subset of 88 bank voles that had been successfully challenged, the infection success was similar between the two experiments. Most important for the purpose of this study was our demonstration that within each infection experiment, the TLR2 genotypes were exposed to the same infectious tick bite challenge.
Estimates of the probability of nymph-to-host transmission of B. burgdorferi sl pathogens for wild reservoir hosts are rare in the scientific literature29,31. Under the conditions of our study, we found that bank voles were highly susceptible to acquiring B. afzelii infection following an infected tick bite. Of the 88 voles that had been successfully challenged, 95.5% developed a systemic infection (95% CI = 88.1–98.5%). We recently showed for B. afzelii strain NE4049 that 96.4% of laboratory mice developed a systemic infection following an infected tick bite29. Taken together, these studies suggest that tick-to-host transmission of B. afzelii is ~100% when one or a few infected nymphs feed to completion on a competent rodent host.
The TLR2 receptor plays an important role in controlling the spirochete load in the tissues of laboratory mice (Mus musculus). Genetically modified mice that do not have TLR2 have spirochete loads that are 100-fold higher than wild-type mice9. We therefore expected resistant C2C2 genotypes to have lower spirochete loads than susceptible C1C1 genotypes. However, our study found no evidence that the TLR2 polymorphism influences variation in the tissue spirochete load of B. afzelii. The higher tissue spirochete loads in the Finnish versus the Swiss experiment may be due to differences between the two bank vole populations or between the strains of B. afzelii. Our recent finding that strain Fin-Jyv-A3 establishes a higher tissue spirochete load than strain NE4049 in inbred lab mice70 suggests that strain is part of the explanation.
We also found significant differences in spirochete load between organs (Fig. 2). In both infection experiments, the spirochete loads were significantly higher in the ear and the dorsal skin compared to the bladder. These results are similar to a study on B. afzelii strain NE4049 in lab mice, which found that the spirochete loads were 1.4 to 2.1 times higher in the skin compared to the bladder29. Others and we have shown that the probability of host-to-tick transmission of B. afzelii increases with the spirochete load in the skin30,71. B. burgdorferi sl must establish a persistent infection in the skin to achieve effective host-to-tick transmission71–74. B. burgdorferi sl also invades joint tissues, which causes swelling and arthritis in the ankle joints of lab mice35,36. We found no evidence that infection with B. afzelii caused swelling of the ankle joints in bank voles (see section 7 in the ESM).
We chose to work with B. afzelii strains NE4049 and Fin-Jyv-A3 because these strains occur in the Swiss and Finnish bank vole populations and because our previous work had shown that they are competent at establishing infection in laboratory mice29–32. While the original study by Tschirren et al.13 did not suggest that the variation in susceptibility to B. afzelii among TLR2 genotypes would be strain-dependent, we acknowledge that repeating our study with different strains of B. afzelii might reveal host variation in susceptibility. In each of the two infection experiments, the bank voles were challenged with nymphs that were co-infected with two different strains of B. afzelii. Co-infections are the norm in nature; 80% of wild I. ricinus nymphs are infected with multiple strains75–77. Likewise, small mammal hosts (including bank voles) are often infected with multiple strains of B. afzelii25,78–81. Thus, co-infected nymphs frequently bite bank voles in nature. In our study, the bank voles were simultaneously challenged with one local strain and one foreign strain. We showed that the local co-adapted strain was present in at least 94.0% (79/84) of the infected bank voles. One potential criticism of our study is that the presence of the foreign strain could have enhanced the ability of the local strain to establish infection. We believe that this explanation is unlikely for two reasons. First, we recently conducted two other studies where Swiss bank voles were experimentally infested with nymphs that were singly infected with either strain NE4049 (n = 22) or strain Fin-Jyv-A3 (n = 21) and found that 95.3% (41/43) of the bank voles became infected48. These studies suggest that the susceptibility of bank voles to strains NE4049 and Fin-Jyv-A3 is ~100% and does not depend on whether the nymph is infected with one strain or two strains. Second, we recently found that when lab mice are simultaneously infected with two strains (NE4049 and Fin-Jyv-A3), there can be interference but not facilitation32. In summary, we have consistently found that our bank voles and lab mice are ~100% susceptible to infection with B. afzelii when we successfully challenge them with at least 1 infected nymph.
In conclusion, after controlling for exposure to infected ticks, we did not find that bank voles with different TLR2 genotypes differed in their susceptibility to infection with B. afzelii under the laboratory conditions of our study. Bank voles were highly susceptible to acquiring B. afzelii infection when nymphs were allowed to feed to repletion. Future studies should investigate whether different strains of B. afzelii or different experimental conditions (e.g. semi-natural enclosures that reduce stress) could reveal the expected variation in susceptibility to B. afzelii infection among TLR2 genotypes. Our study emphasizes the importance of using controlled experimental infections to separate variation in exposure from variation in susceptibility in the study of candidate immune genes for resistance to pathogens.
Supplementary information
Acknowledgements
This work was supported by a grant from the Swiss National Science Foundation (SNSF) to Maarten Voordouw (FN 31003A_141153), as well as the Kone Foundation, the University of Jyväskylä, and the Academy of Finland. We thank Gaétan Pheulpin and Tom Fluri for help with capturing the bank voles at the site in Neuchâtel. We thank Barbara Tschirren for advice on where to capture the bank voles in Zurich and on how to use PHASE v2.1 to reconstruct the TLR2 haplotypes. Thanks to three anonymous reviewers whose comments improved this manuscript.
Author Contributions
A.G.-C. and M.J.V. designed the study. N.B. gave advice on the experimental design. A.G.-C. and F.B. captured bank voles at the field sites in Switzerland, bred the bank voles, performed the experimental infections and the molecular work. C.C., E.K. and T.M. helped with the experimental infections of the Finnish bank voles. D.G. and A.S. created the B. afzelii-infected nymphs. A.G.-C. analysed the data. A.G.-C. and M.J.V. wrote the manuscript. All authors read and approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43160-3.
References
- 1.Janeway CA, Medzhitov R. Introduction: The role of innate immunity in the adaptive immune response. Seminars in Immunology. 1998;10:349–350. doi: 10.1006/smim.1998.0142. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R. Toll-like receptors and innate immunity. Nature Reviews Immunology. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 3.Takeda K, Akira S. Toll-like receptors in innate immunity. International Immunology. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 4.Misch EA, Hawn TR. Toll-like receptor polymorphisms and susceptibility to human disease. Clinical Science. 2008;114:347–360. doi: 10.1042/CS20070214. [DOI] [PubMed] [Google Scholar]
- 5.Skevaki C, Pararas M, Kostelidou K, Tsakris A, Routsias JG. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious diseases. Clinical and Experimental Immunology. 2015;180:165–177. doi: 10.1111/cei.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oosting M, et al. TLR1/TLR2 heterodimers play an important role in the recognition of Borrelia spirochetes. Plos One. 2011;6:e25998. doi: 10.1371/journal.pone.0025998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirschfeld M, et al. Cutting edge: Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. Journal of Immunology. 1999;163:2382–2386. [PubMed] [Google Scholar]
- 8.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochemical and Biophysical Research Communications. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 9.Wooten RM, et al. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. Journal of Immunology. 2002;168:348–355. doi: 10.4049/jimmunol.168.1.348. [DOI] [PubMed] [Google Scholar]
- 10.Alexopoulou L, et al. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nature Medicine. 2002;8:878–884. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- 11.Rahman S, Shering M, Ogden NH, Lindsay R, Badawi A. Toll-like receptor cascade and gene polymorphism in host-pathogen interaction in Lyme disease. Journal of Inflammation Research. 2016;9:91–102. doi: 10.2147/JIR.S104790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Texereau J, et al. The importance of toll-like receptor 2 polymorphisms in severe infections. Clinical Infectious Diseases. 2005;41:S408–S415. doi: 10.1086/431990. [DOI] [PubMed] [Google Scholar]
- 13.Tschirren B, et al. Polymorphisms at the innate immune receptor TLR2 are associated with Borrelia infection in a wild rodent population. Proceedings of the Royal Society B-Biological Sciences. 2013;280:20130364. doi: 10.1098/rspb.2013.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornetti L, Hilfiker D, Lemoine M, Tschirren B. Small-scale spatial variation in infection risk shapes the evolution of a Borrelia resistance gene in wild rodents. Molecular Ecology. 2018;27:3515–3524. doi: 10.1111/mec.14812. [DOI] [PubMed] [Google Scholar]
- 15.Kurtenbach K, et al. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nature Reviews Microbiology. 2006;4:660–669. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- 16.Piesman J, Gern L. Lyme borreliosis in Europe and North America. Parasitology. 2004;129:S191–S220. doi: 10.1017/S0031182003004694. [DOI] [PubMed] [Google Scholar]
- 17.van Duijvendijk G, Sprong H, Takken W. Multi-trophic interactions driving the transmission cycle of Borrelia afzelii between Ixodes ricinus and rodents: a review. Parasites & Vectors. 2015;8:1–11. doi: 10.1186/s13071-014-0608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gern L, et al. Borrelia burgdorferi in rodents (Apodemus flavicollis and A. sylvaticus): Duration and enhancement of infectivity for Ixodes ricinus ticks. European Journal of Epidemiology. 1994;10:75–80. doi: 10.1007/BF01717456. [DOI] [PubMed] [Google Scholar]
- 19.Richter D, Klug B, Spielman A, Matuschka FR. Adaptation of diverse Lyme disease spirochetes in a natural rodent reservoir host. Infection and Immunity. 2004;72:2442–2444. doi: 10.1128/IAI.72.4.2442-2444.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacquet, M., Margos, G., Fingerle, V. & Voordouw, M. J. Comparison of the lifetime host-to-tick transmission between two strains of the Lyme disease pathogen Borrelia afzelii. Parasites & Vectors9 (2016). [DOI] [PMC free article] [PubMed]
- 21.Richter D, Debski A, Hubalek Z, Matuschka FR. Absence of Lyme disease spirochetes in larval Ixodes ricinus ticks. Vector-Borne and Zoonotic Diseases. 2012;12:21–27. doi: 10.1089/vbz.2011.0668. [DOI] [PubMed] [Google Scholar]
- 22.Rollend L, Fish D, Childs JE. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: A summary of the literature and recent observations. Ticks and Tick-borne. Diseases. 2013;4:46–51. doi: 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Humair PF, Rais O, Gern L. Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: differential transmission pattern and overwintering maintenance. Parasitology. 1999;118:33–42. doi: 10.1017/S0031182098003564. [DOI] [PubMed] [Google Scholar]
- 24.Randolph SE, Miklisova D, Lysy J, Rogers DJ, Labuda M. Incidence from coincidence: patterns of tick infestations on rodents facilitate transmission of tick-borne encephalitis virus. Parasitology. 1999;118:177–186. doi: 10.1017/S0031182098003643. [DOI] [PubMed] [Google Scholar]
- 25.Raberg L, et al. Evolution of antigenic diversity in the tick-transmitted bacterium Borrelia afzelii: a role for host specialization? Journal of Evolutionary Biology. 2017;30:1034–1041. doi: 10.1111/jeb.13075. [DOI] [PubMed] [Google Scholar]
- 26.Tschirren, B. Borrelia burgdorferi sensu lato infection pressure shapes innate immune gene evolution in natural rodent populations across Europe. Biology Letters11 (2015). [DOI] [PMC free article] [PubMed]
- 27.Talleklint L, Jaenson TGT. Is the small mammal (Clethrionomys glareolus) or the tick vector (Ixodes ricinus) the primary overwintering reservoir for the Lyme borreliosis spirochete in Sweden. Journal of Wildlife Diseases. 1995;31:537–540. doi: 10.7589/0090-3558-31.4.537. [DOI] [PubMed] [Google Scholar]
- 28.Tschirren B, et al. Contrasting patterns of diversity and population differentiation at the innate immunity gene toll-like receptor 2 (TLR2) in two sympatric rodent species. Evolution. 2012;66:720–731. doi: 10.1111/j.1558-5646.2011.01473.x. [DOI] [PubMed] [Google Scholar]
- 29.Belli A, Sarr A, Rais O, Rego ROM, Voordouw MJ. Ticks infected via co-feeding transmission can transmit Lyme borreliosis to vertebrate hosts. Scientific Reports. 2017;7:5006. doi: 10.1038/s41598-017-05231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacquet M, Durand J, Rais O, Voordouw MJ. Cross-reactive acquired immunity influences transmission success of the Lyme disease pathogen, Borrelia afzelii. Infection Genetics and Evolution. 2015;36:131–140. doi: 10.1016/j.meegid.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Tonetti N, Voordouw MJ, Durand J, Monnier S, Gern L. Genetic variation in transmission success of the Lyme borreliosis pathogen Borrelia afzelii. Ticks and Tick-borne. Diseases. 2015;6:334–343. doi: 10.1016/j.ttbdis.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Genné, D. et al. Competition between strains of Borrelia afzelii inside the rodent host and the tick vector. Proceedings of the Royal Society B: Biological Sciences285 (2018). [DOI] [PMC free article] [PubMed]
- 33.Hanincova K, et al. Association of Borrelia afzelii with rodents in Europe. Parasitology. 2003;126:11–20. doi: 10.1017/S0031182002002548. [DOI] [PubMed] [Google Scholar]
- 34.Barthold SW, Persing DH, Armstrong AL, Peeples RA. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. American Journal of Pathology. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G, et al. Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infection and Immunity. 2001;69:4303–4312. doi: 10.1128/IAI.69.7.4303-4312.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang GQ, et al. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. Journal of Infectious Diseases. 2002;186:782–791. doi: 10.1086/343043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weis JJ. Host-pathogen interactions and the pathogenesis of murine Lyme disease. Current Opinion in Rheumatology. 2002;14:399–403. doi: 10.1097/00002281-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Hodzic E, Feng SL, Freet KJ, Barthold SW. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infection and Immunity. 2003;71:5042–5055. doi: 10.1128/IAI.71.9.5042-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bramwell, K. K. C., Teuscher, C. & Weis, J. J. Forward genetic approaches for elucidation of novel regulators of Lyme arthritis severity. Frontiers in Cellular and Infection Microbiology4 (2014). [DOI] [PMC free article] [PubMed]
- 40.Schroder NWJ, et al. Heterozygous Arg753Gln polymorphism of human TLR-2 impairs immune activation by Borrelia burgdorferi and protects from late stage Lyme disease. Journal of Immunology. 2005;175:2534–2540. doi: 10.4049/jimmunol.175.4.2534. [DOI] [PubMed] [Google Scholar]
- 41.Vourc’h G, et al. Mapping human risk of infection with Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis, in a periurban forest in France. Ticks and Tick-borne Diseases. 2016;7:644–652. doi: 10.1016/j.ttbdis.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Keesing F, et al. Hosts as ecological traps for the vector of Lyme disease. Proceedings of the Royal Society Biological Sciences Series B. 2009;267:3911–3919. doi: 10.1098/rspb.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cook MJ. Lyme borreliosis: a review of data on transmission time after tick attachment. International journal of general medicine. 2015;8:1–8. doi: 10.2147/IJGM.S73791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eisen L. Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks and Tick-borne Diseases. 2018;9:535–542. doi: 10.1016/j.ttbdis.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paziewska A, Zwolinska L, Harris PD, Bajer A, Sinski E. Utilisation of rodent species by larvae and nymphs of hard ticks (Ixodidae) in two habitats in NE Poland. Experimental and Applied Acarology. 2010;50:79–91. doi: 10.1007/s10493-009-9269-8. [DOI] [PubMed] [Google Scholar]
- 46.Kupfer, T. R. & Fessler, D. M. T. Ectoparasite defence in humans: relationships to pathogen avoidance and clinical implications. Philosophical Transactions of the Royal Society B-Biological Sciences373 (2018). [DOI] [PMC free article] [PubMed]
- 47.Voordouw, M., Gomez-Chamorro, A., Heinrich, V. & Sarr, A. In 15th International Conference on Lyme Borreliosis and Other Tick-Borne Diseases (2018).
- 48.Gomez-Chamorro, A. Immunoecology of a rodent reservoir host of the Lyme disease pathogen: the bank vole – Borrelia afzelii model PhD thesis, University of Neuchâtel (2018).
- 49.Gomez-Chamorro, A. et al. Maternal antibodies provide strain-specific protection against infection with the Lyme disease pathogen in a wild rodent. BioRxiv, 522789 (2019). [DOI] [PMC free article] [PubMed]
- 50.Dizij A, Kurtenbach K. Clethrionomys glareolus, but not Apodemus flavicollis, acquires resistance to Ixodes ricinus L., the main European vector of Borrelia burgdorferi. Parasite Immunology (Oxford) 1995;17:177–183. doi: 10.1111/j.1365-3024.1995.tb00887.x. [DOI] [PubMed] [Google Scholar]
- 51.Narasimhan, S. et al. Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. Plos One2 (2007). [DOI] [PMC free article] [PubMed]
- 52.Nazario S, et al. Prevention of Borrelia burgdorferi transmission in guinea pigs by tick immunity. American Journal of Tropical Medicine and Hygiene. 1998;58:780–785. doi: 10.4269/ajtmh.1998.58.780. [DOI] [PubMed] [Google Scholar]
- 53.Wikel SK, Ramachandra RN, Bergman DK, Burkot TR, Piesman J. Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infection and Immunity. 1997;65:335–338. doi: 10.1128/iai.65.1.335-338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donahue JG, Piesman J, Spielman A. Reservoir competence of white-footed mice for Lyme disease spirochetes. American Journal of Tropical Medicine and Hygiene. 1987;36:92–96. doi: 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- 55.Costantini D. Oxidative stress in ecology and evolution: lessons from avian studies. Ecology Letters. 2008;11:1238–1251. doi: 10.1111/j.1461-0248.2008.01246.x. [DOI] [PubMed] [Google Scholar]
- 56.Davis AK, Maney DL, Maerz JC. The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Functional Ecology. 2008;22:760–772. doi: 10.1111/j.1365-2435.2008.01467.x. [DOI] [Google Scholar]
- 57.Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunologic Research. 2014;58:193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- 58.Sorci G, Faivre B. Inflammation and oxidative stress in vertebrate host-parasite systems. Philosophical Transactions of the Royal Society B-Biological Sciences. 2009;364:71–83. doi: 10.1098/rstb.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of host diversity and community composition on Lyme disease risk. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richter D, Spielman A, Komar N, Matuschka FR. Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerging Infectious Diseases. 2000;6:133–138. doi: 10.3201/eid0602.000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brisson D, Dykhuizen DE. ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics. 2004;168:713–722. doi: 10.1534/genetics.104.028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heylen D, Matthysen E, Fonville M, Sprong H. Songbirds as general transmitters but selective amplifiers of Borrelia burgdorferi sensu lato genotypes in Ixodes rinicus ticks. Environmental Microbiology. 2014;16:2859–2868. doi: 10.1111/1462-2920.12304. [DOI] [PubMed] [Google Scholar]
- 63.Norte AC, de Carvalho IL, Nuncio MS, Ramos JA, Gern L. Blackbirds Turdus merula as competent reservoirs for Borrelia turdi and Borrelia valaisiana in Portugal: evidence from a xenodiagnostic experiment. Environmental Microbiology Reports. 2013;5:604–607. doi: 10.1111/1758-2229.12058. [DOI] [PubMed] [Google Scholar]
- 64.Kurtenbach K, et al. Differential survival of Lyme borreliosis spirochetes in ticks that feed on birds. Infection and Immunity. 2002;70:5893–5895. doi: 10.1128/IAI.70.10.5893-5895.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matuschka FR, Spielman A. Loss of Lyme disease spirochetes from Ixodes ricinus ticks feeding on European blackbirds. Experimental Parasitology. 1992;74:151–158. doi: 10.1016/0014-4894(92)90042-9. [DOI] [PubMed] [Google Scholar]
- 66.Pedersen AB, Fenton A. Emphasizing the ecology in parasite community ecology. Trends in Ecology & Evolution. 2007;22:133–139. doi: 10.1016/j.tree.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 67.Rynkiewicz EC, Pedersen AB, Fenton A. An ecosystem approach to understanding and managing within-host parasite community dynamics. Trends in Parasitology. 2015;31:212–221. doi: 10.1016/j.pt.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 68.Telfer S, et al. Species interactions in a parasite community drive infection risk in a wildlife population. Science (Washington D C) 2010;330:243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maaz D, et al. Susceptibility to ticks and Lyme disease spirochetes is not affected in mice coinfected with nematodes. Infection and Immunity. 2016;84:1274–1286. doi: 10.1128/IAI.01309-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Fabritiis, M. Competition of two Borrelia afzelii strains in the rodent host Mus musculus Master of Science thesis, University of Neuchâtel (2018).
- 71.Raberg L. Infection intensity and infectivity of the tick-borne pathogen Borrelia afzelii. Journal of Evolutionary Biology. 2012;25:1448–1453. doi: 10.1111/j.1420-9101.2012.02515.x. [DOI] [PubMed] [Google Scholar]
- 72.Grillon A, et al. Identification of Borrelia protein candidates in mouse skin for potential diagnosis of disseminated Lyme borreliosis. Scientific Reports. 2017;7:16719. doi: 10.1038/s41598-017-16749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsao, J. Reviewing molecular adaptations of Lyme borreliosis spirochetes in the context of reproductive fitness in natural transmission cycles. Veterinary Research (Paris)40 (2009). [DOI] [PMC free article] [PubMed]
- 74.Voordouw MJ. Co-feeding transmission in Lyme disease pathogens. Parasitology. 2015;142:290–302. doi: 10.1017/S0031182014001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Durand, J. et al. Multistrain infections with Lyme borreliosis pathogens in the tick vector. Applied and Environmental Microbiology83 (2017). [DOI] [PMC free article] [PubMed]
- 76.Durand J, et al. Cross-immunity and community structure of a multiple-strain pathogen in the tick vector. Applied and Environmental Microbiology. 2015;81:7740–7752. doi: 10.1128/AEM.02296-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Durand J, Jacquet M, Rais O, Gern L, Voordouw MJ. Fitness estimates from experimental infections predict the long-term strain structure of a vector-borne pathogen in the field. Scientific Reports. 2017;7:1851. doi: 10.1038/s41598-017-01821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andersson M, Scherman K, Raberg L. Multiple-strain infections of Borrelia afzelii: a role for within-host interactions in the maintenance of antigenic diversity? American Naturalist. 2013;181:545–554. doi: 10.1086/669905. [DOI] [PubMed] [Google Scholar]
- 79.Jacquot M, et al. High-throughput sequence typing reveals genetic differentiation and host specialization among populations of the Borrelia burgdorferi species complex that infect rodents. Plos One. 2014;9:e88581. doi: 10.1371/journal.pone.0088581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pérez D, Kneubühler Y, Rais O, Jouda F, Gern L. Borrelia afzelii ospC genotype diversity in Ixodes ricinus questing ticks and ticks from rodents in two Lyme borreliosis endemic areas: Contribution of co-feeding ticks. Ticks and Tick-borne. Diseases. 2011;2:137–142. doi: 10.1016/j.ttbdis.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 81.Strandh, M. & Raberg, L. Within-host competition between Borrelia afzelii ospC strains in wild hosts as revealed by massively parallel amplicon sequencing. Philosophical transactions of the Royal Society of London. Series B, Biological sciences370 (2015). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.